Abstract
Nutrient composition and caloric intake have traditionally been used to devise optimized diets for various phases of life. Adjustment of meal size and frequency have emerged as powerful tools to ameliorate and postpone the onset of disease and delay aging, whereas periods of fasting, with or without reduced energy intake, can have profound health benefits. The underlying physiological processes involve periodic shifts of metabolic fuel sources, promotion of repair mechanisms, and the optimization of energy utilization for cellular and organismal health. Future research endeavors should be directed to the integration of a balanced nutritious diet with controlled meal size and patterns and periods of fasting to develop better strategies to prevent, postpone, and treat the socioeconomical burden of chronic diseases associated with aging.
The worldwide increase in life expectancy has not been paralleled by an equivalent increase in healthy aging. Developed and developing countries are facing social and economic challenges caused by disproportional increases in their elderly populations and the accompanying burden of chronic diseases (1). Geriatricians and gerontologists have contributed greatly to our understanding of the consequences and processes that underlie aging from clinical, social, mental, physical, and biological perspectives. The primary goal of aging research is to improve the health of older persons and to design and test interventions that may prevent or delay age-related diseases. Besides socioeconomic status, energy, environmental quality, and genetics are the most powerful determinants of health and longevity. Although environmental quality and genetics are not under our direct control, energy intake is. The consumption of food provides energy and nutrients necessary to sustain life and allows growth, repair, and reproduction. Proper nutrition can influence health and survival and delay or, in some cases, prevent the onset and progression of chronic diseases. However, both hypo- and hypernutrition have the potential to increase the risk of chronic disease and premature death. Furthermore, manipulation of a nutritionally balanced diet, whether by altering caloric intake or meal timing, can lead to a delay of the onset and progression of diseases and to a healthier and longer life in most organisms (2–4). In general, both prolonged reduction in daily caloric intake and periodic fasting cycles have the power to delay the onset of disease and increase longevity. Data from experimental studies in short-lived species and emerging clinical and epidemiological observations indicate that dietary interventions are valuable strategies that can be applied to promote healthy aging. In model organisms, caloric restriction (CR) provides beneficial effects on health and survival, and there is an extensive literature that provides in-sights into its molecular mechanisms of action (4). However, chronic CR has been reported to exert adverse effects for a number of mouse strains (5) and could push humans to what one may consider a near anorexic state (6), underlying some cautionary negative outcomes pointed out by the eating-disorders field as well (7–9).
An emerging area of research is the investigation of the independent consequences of variations in meal size (through the control of energy intake) and meal frequency (by controlling the time of feeding and fasting) on the incidence or amelioration of multiple age-related diseases, including cardiovascular disease, diabetes, cancer, and dementia (2, 10). These studies are starting to reveal that health-span and life-span extension can be achieved by interventions that do not require an overall reduction in caloric intake. Major challenges for the future include the design of well-controlled and randomized clinical trials to test whether these observations can be translated to humans, determination of the importance of individual genetic variant medicine, and implementation of health care policies in which new eating regimens can be integrated into clinical practice. We discuss four experimental strategies aimed at altering energy intake or the duration of fasting and feeding periods that result in improved aspects of health in mammals. These are (i) classical CR, in which daily caloric intake is typically decreased by 15 to 40%; (ii) time-restricted feeding (TRF), which limits daily intake of food to a 4- to 12-hour window; (iii) intermittent or periodic full or partial fasting, that is a periodic, full- or multiday decrease in food intake; and (iv) fasting-mimicking diets (FMDs) that use a strategy to maintain a physiological fasting-like state by reducing caloric intake and modifying diet composition but not necessarily fasting (Fig. 1). We also summarize the metabolic and cellular responses triggered by these feeding regimens and their impact on physiology, focusing on studies in rodents, monkeys, and humans. Finally, we discuss how these studies may provide a basis for future investigations on the role of various dietary interventions in the prevention of diseases and promotion of health throughout the life span.
Fig. 1. Experimental approaches used to improve fitness and promote health span.
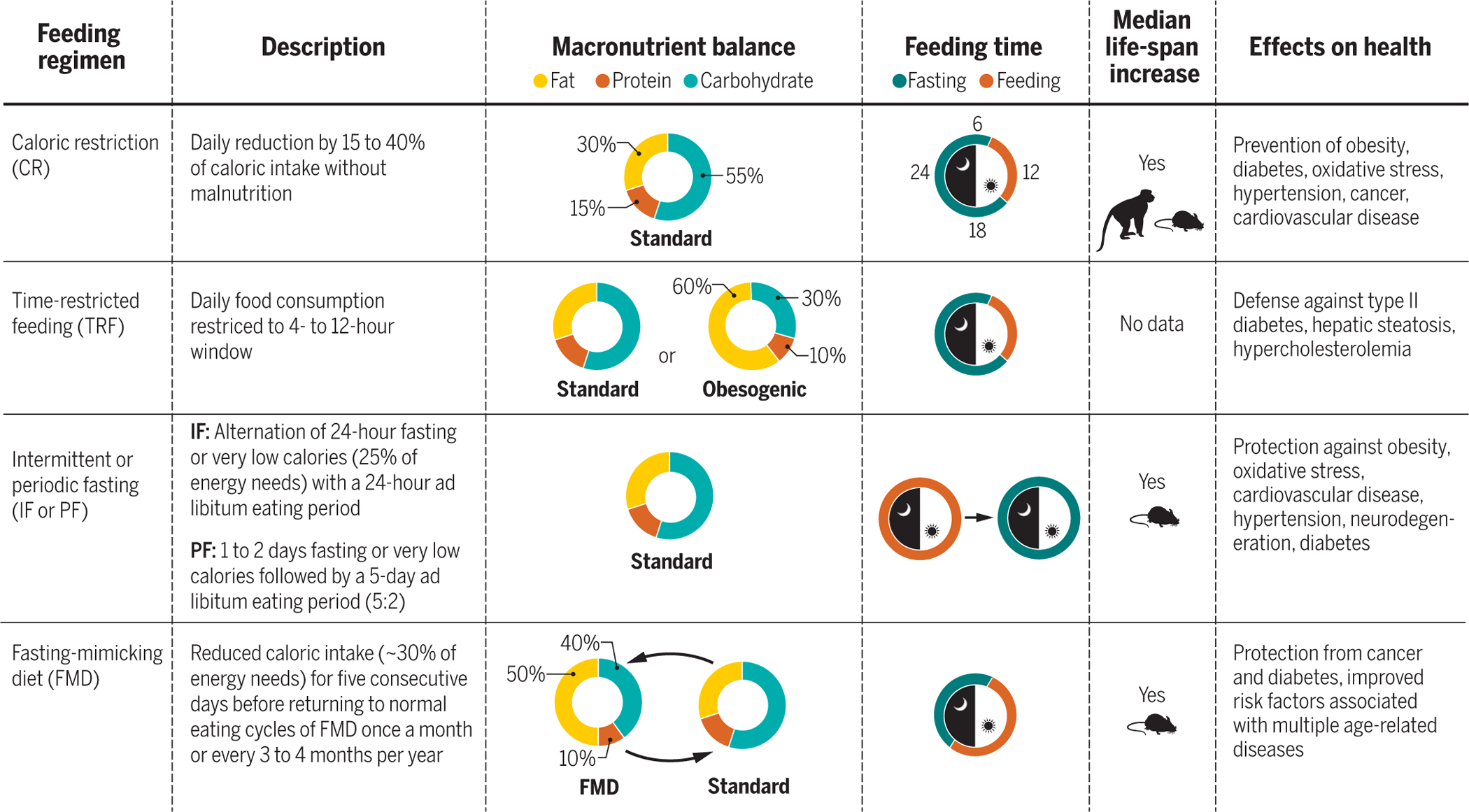
Description of different feeding regimens, their macronutrient balance, and feeding time during a 24-hour period. The feeding time represented in the day-night diagrams refers to eating time in humans and nonhuman primates. Mice undergoing a CR regimen tend to finish their food allotment quickly, self-imposing a continuous form of TRF. In human studies, people voluntarily reduce their food intake and mostly adhere to a three-meals-per-day schedule. Mean life-span extension is documented for all the treatments in the species depicted in the box, whereas maximum life span is only achieved after CR and IF or PF.
Caloric restriction
Early observations linking reduced food intake to improvements on health and survival are now a century old. Rous discovered that limiting food intake had an impact on cancer development (11), and Osborne et al. reported growth retardation and life-span extension by a reduction in food intake (12). McCay and colleagues later published a seminal paper showing that rats fed a limited amount of food lived much longer than their ad libitum (AL)–fed littermates (13). Nearly a century after these initial studies, the positive impact of CR on health span and life span has been documented in many model organisms that include unicellular yeast, nematode worms, fruitflies, mice, and primates, suggesting a strong evolutionary conservation of common mechanisms connecting food intake to longevity (4). CR interventions oppose several age-associated pathophysiological changes, including reduction of metabolic rate and oxidative damage (14), enhanced cellular turnover and protein homeostasis, and improvement of age-related metabolic disorders that include central obesity, insulin resistance, dyslipidemia, and hypertension. Studies seeking to unravel the link between metabolism and aging have demonstrated that CR induces profound metabolic and molecular changes in components of the nutrient-sensing and stress-responsive pathways, such as growth hormone, insulin and insulin-like growth factor (IGF) signaling, mechanistic target of rapamycin (mTOR), adenosine 5′-monophosphate–activated protein kinase (AMPK), forkhead box protein O (FOXO), sirtuins, and nuclear factor erythroid 2–related factor 2 (NRF2) (15, 16) (Fig. 2). The identification of longevity-regulatory pathways led to studies of pharmacological interventions with U.S. Food and Drug Administration–approved drugs (for example, rapalogs and metformin) and other naturally occurring compounds (such as resveratrol and spermidine) that mimic changes observed in CR without the requirement of changes in food intake (17, 18). We focus on the most relevant molecular and metabolic alterations elicited by dietary interventions that involve a reduction in caloric intake and fasting periods.
Fig. 2. Fasting time and energy restriction share biological responses implicated in metabolite-controlled longevity pathways.
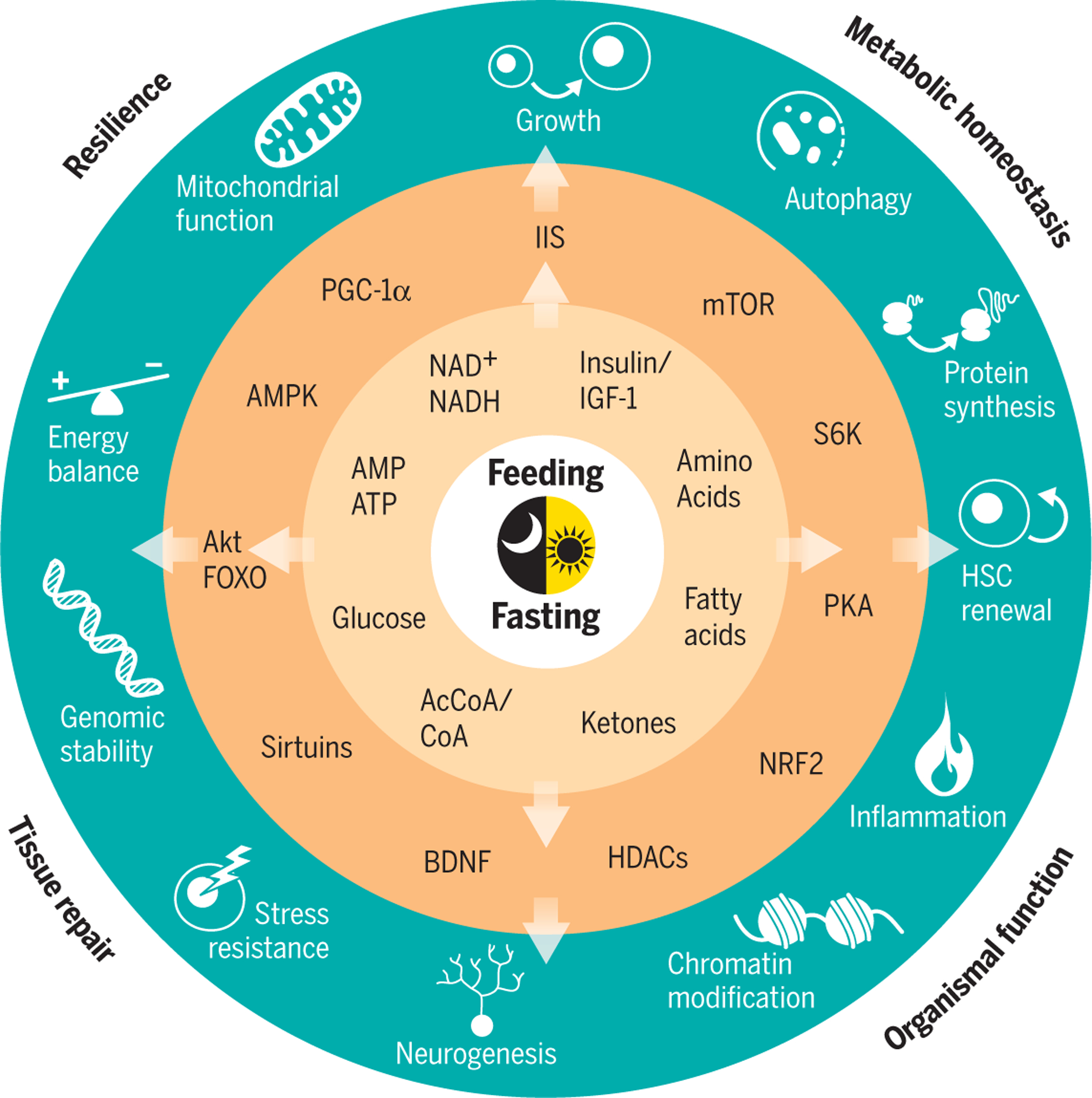
Reduction of calories by continuous energy restriction or prolonged fasting periods trigger metabolic adaptations characterized by increased amounts of circulating ketones, whereas circulating fatty acids, amino acids, glucose, and insulin are maintained at low concentrations. Adaptive cellular responses involve alterations in the ratios of adenosine monophosphate (AMP) to adenosine triphosphate (ATP), of oxidized nicotinamide adenine dinucleotide (NAD+) to the reduced form NADH, and of acetyl-CoA to CoA. After a few hours of fasting, increased AMP to ATP ratios activate AMPK, which triggers repair and inhibits anabolic processes. Acetyl-CoA and NAD+ serve as cofactors for epigenetic modifiers such as histone acetyltransferases and NAD+-dependent deacetylases, the sirtuins, thus linking nutrition, energy metabolism, and post-translational modifications of histone proteins. Sirtuins deacetylate FOXOs and peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), factors respectively involved in stress resistance and mitochondrial biogenesis. Production of ketone bodies such as β-hydroxybutyrate from fatty-acid catabolism may operate as endogenous histone deacetylase (HDAC) inhibitors and may contribute to epigenetic control of gene expression, DNA repair, and genome stability. Ketogenesis also promotes synaptic plasticity and neurogenesis by increasing the expression of brain-derived neurotrophic factor (BDNF). Periodic cycles of fasting have systemic anti-inflammatory effects and increase progenitor stem cells. Down-regulation of the insulin–IGF-1 signaling (IIS) pathway and reduction of circulating amino acids repress the activity of mTOR and its downstream effector, the ribosomal protein S6 kinase beta-1 (S6K). This mechanism inhibits global protein synthesis and promotes recycling of macromolecules by stimulation of autophagy. CR promotes the expression and activity of NRF2, which induces a number of antioxidative and carcinogen-detoxifying enzymes. Collectively, the organism responds to a low-energy challenge by minimizing anabolic processes (synthesis, growth, and reproduction), favoring maintenance systems, and enhancing stress resistance, tissue repair, and recycling of damaged molecules. Improvement in resilience, metabolic homeostasis, tissue repair, and organismal function can act as direct modifiers of the four domains of the aging phenotype: body composition (1); balance between energy availability and energy demand (27); signaling networks that maintain homeostasis (81); and neurodegeneration (4). Each of these domains can be assessed readily by routine clinical tests.
Numerous strides have been made to understand how the number of calories, diet composition, and timing of the intervention can be manipulated to effectively extend longevity and to translate these observations into feeding paradigms that can be applied to humans, with the goal of delaying the onset of age-associated conditions and promoting healthy aging. CR without malnutrition can be accomplished by chronically reducing energy intake by 15 to 40% from AL conditions, while maintaining adequate intake of vitamins and minerals. In rodents, under certain circumstances, CR can extend life span by up to 50% (19). Traditionally, diet composition and genetic background have been thought to have a marginal impact on life-span extension elicited by CR. More recently, studies in mice and nonhuman primates have revealed that the effect of CR on life-span extension is not universal (20). Indeed, certain mouse recombinant inbred strains show either little increase or deleterious effects on life span after CR (5). Analysis of body composition revealed that the best outcomes on survival were obtained in mice that preserved their fat stores during the second year of life, suggesting the necessity of a minimum level of adiposity for the full benefit of CR (21, 22). In CR regimens, sex, age, and genetic background contribute to outcomes regarding health and survival in mice (22), and this may also be true for long-lived organisms, including humans. Data from two independent nonhuman primate studies, one at the National Institute on Aging (NIA) and the other at the University of Wisconsin–Madison (UW), challenged the association between life-span extension and health span by reporting similar improvements in health but contrasting survival benefits in response to CR (23, 24). Possible explanations for the divergent outcomes emanating from these two studies have revealed important differences in genetic background, onset of the intervention, feeding practices, and diet composition (25).
In humans, short-term trials such as the multi-center CALERIE (Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy) study (26–29), the observational studies of centenarians residing in Okinawa who have been exposed to CR for most of their lives (30), and observations of the members of the Calorie Restriction Society (CRONies) who self-impose CR (31) have shown the occurrence of many of the same physiological, metabolic, and molecular benefits typically associated with long-lived animals on CR. These studies support the observation that long-term CR preserves a more youthful functionality by improving several markers of health, including decreases in body weight, metabolic rate, and oxidative damage (14); lower incidence of cardiovascular disease (31) and cancer; and decreased activity of the insulin-Akt-FOXO signaling pathway (32, 33) (Fig. 2).
Although these findings clearly indicate that a reduction of caloric intake could be an effective intervention to improve health and prevent disease during aging in humans, there are several obstacles that halt the transition from experimental studies into standard medical practice: (i) the lack of clinical data supporting consistent effects of CR in older populations and the incomplete understanding of the age-specific effects of these interventions (4, 10); (ii) safety concerns related to lack of reserve capacity upon exposure to infection (34), injury, or surgery (35) and about bone thinning that could lead to the development of osteoporosis in older individuals (36); (iii) the difficulty of compliance to extreme restriction; and (iv) the interindividual variability in body mass, especially lean mass, which strongly correlates with frailty (23). The current “obesogenic” social environment makes it difficult for individuals to adhere to strict dietary regimens and lifestyle modifications for long periods of time. Thus, there is interest in alternative feeding regimens that may recapitulate at least some of the beneficial effects of CR by controlling feeding-fasting patterns with little or no reduction in caloric intake.
Time-restricted feeding
Recent evidence indicates that the benefits of CR may not be entirely related to a reduction in calories. In many experimental models of CR, the reduction in energy intake encourages the animals to consume their entire daily food allowance in a very short interval, thus promoting a longer fasting period than when consuming standard or hypercaloric diets AL (37). Although (nocturnal) rodents with free access to food eat predominantly at night, they also tend to feed during the day, which correlates with gains in body weight (37). These observations raise the question of whether the timing of food consumption (either feeding duration or circadian timing) is a determinant of metabolic health, independent of total caloric intake and quality of calories. Thus, it is possible that triggering the fasting response on a daily basis or at specific times is in itself beneficial. This would explain why dietary dilution, a form of CR in which mice eat all day to compensate for the low density of energy in their diet, does not result in life-span extension (38, 39). Hence, chronic CR may improve health, at least in part, through an extended period of fasting.
TRF refers to daily limitations in the timing of food intake, spanning from 4 to 12 hours, without reduction in caloric intake (Fig. 1). Although data on the effects of TRF on longevity are not yet available, studies in rodents have shown that TRF can confer protection against several detrimental metabolic consequences of a typical western diet (high fat and high carbohydrates, particularly refined sugars) through reduction in body weight, increase in energy expenditure, improved glycemic control and lower insulin levels, decrease in hepatic fat and hyperlipidemia, and attenuated inflammatory outcomes, even when food intake or body weight or both are matched to the control group (40–43). The molecular mechanisms responsible for the effects of altered meal patterns on metabolic health appears to be related, at least in part, to the synchronization between the time of fasting-feeding and the circadian rhythm (3) (Fig. 3). The circadian clock provides a conserved mechanism that allows organisms to anticipate and respond to environmental changes. This perpetual rhythm leads to the timely expression of clock-controlled genes, especially those encompassing enzymes and regulatory molecules that mediate physiological and metabolic functions. A strong relation exists between the circadian clock and metabolism, as they share some common regulators. Indeed, TRF can restore cycling of metabolic regulators, such as nicotinamide phosphoribosyltransferase (NAMPT), cAMP response element–binding protein, mTOR, AMPK, or the insulin signaling pathway, all of which take part in the life-span and health-span benefits of CR (40) (Fig. 3).
Fig. 3. Integration of the circadian rhythms and feeding-fasting cycles with metabolism.
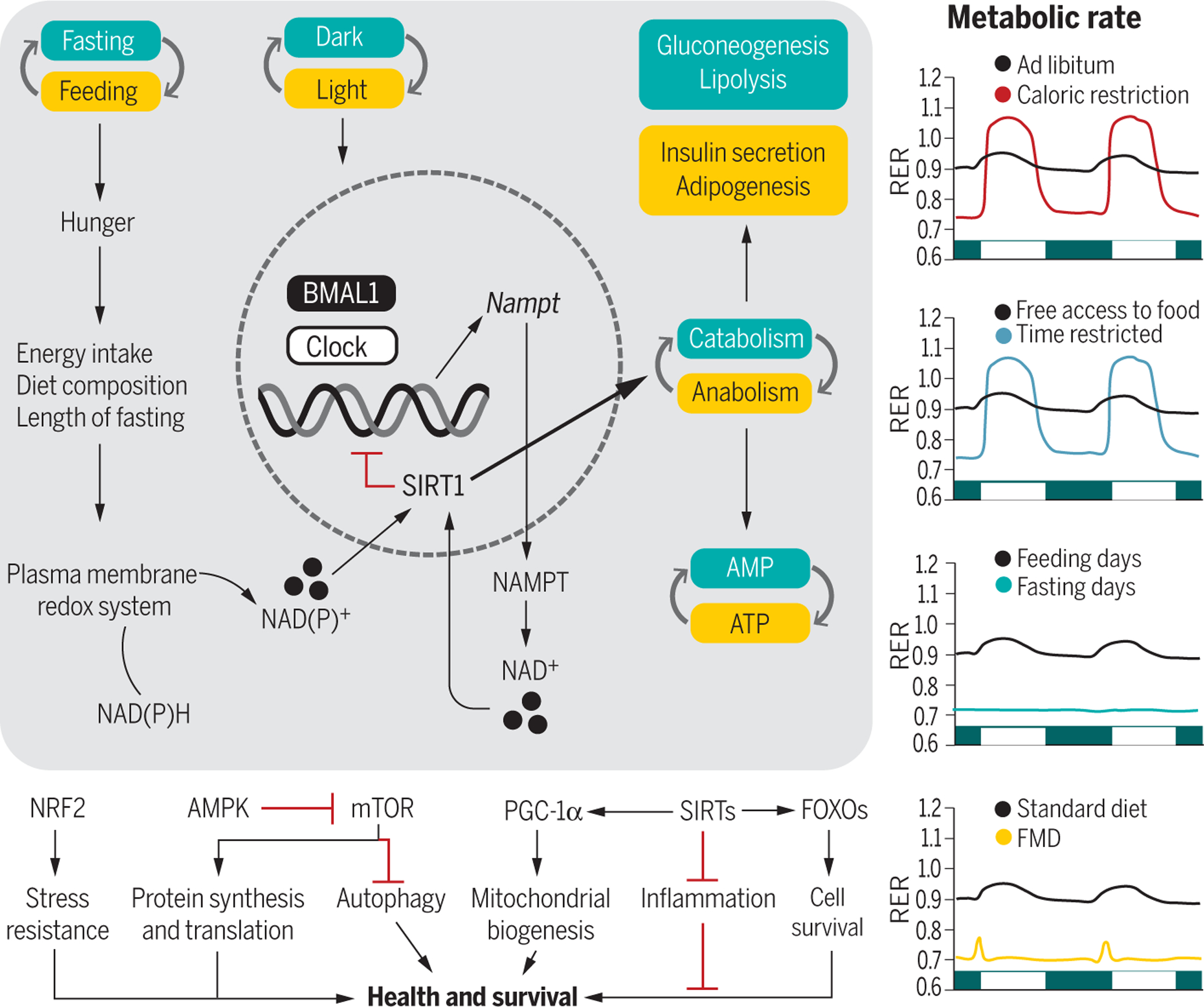
(Left) The transcriptional activators BMAL1 and CLOCK are at the core of a cell-autonomous molecular circuit that governs circadian rhythms. Fasting increases hunger, the extent of which depends on the overall energy intake, diet composition, and length of fasting. The internal circadian clock also increases hunger independent of food intake and other behaviors. Intermittent energy restriction increases concentrations of the plasma membrane redox system enzymes, NADH-cytochrome b5 reductase and NAD(P)H-quinone oxidoreductase, contributing to oscillations in the NAD(P)H [reduced form of NAD(P)+] to NAD(P)+ (nicotinamide adenine dinucleotide phosphate) ratio (82). The circadian rhythmicity of CLOCK and BMAL1 expression regulates the transcription of NAMPT, a key regulatory enzyme involved in the generation of NAD+, a metabolite required for the deacetylase activity of SIRT1. Active SIRT1 influences metabolism through its effects on catabolic and anabolic reactions and mediates BMAL1 deacetylation, which inhibits the circadian clock machinery. During the active phase (yellow boxes), increased production of ATP sustains anabolic pathways. During the resting phase (green boxes), a shift toward AMP gears metabolism toward catabolic processes. These intermediate energy carriers activate downstream transcription factors, kinases, and deacetylases like NRF2, AMPK, PGC-1α, sirtuins, and FOXOs, whose activation influences health and survival. (Right) At the organismal level, fasting or feeding states are paralleled by changes in the metabolic rate. AL–fed animals set their RERs at around 0.9, showing an intermediate preference between fat and carbohydrate metabolism. Both CR and TRF regimens increase the amplitude of RER oscillations, characterized by higher RER (utilization of carbohydrates) during feeding and lower RER (utilization of lipids) during fasting. Under prolonged fasting, lipids are the only source of energy, as opposed to feeding time. The FMD diet results in lower RER with a slight peak after the meal. The RER traces are idealized and may be close to what is seen in nocturnal rodents.
The NIA and UW CR monkey studies showed that the genetic background, age of onset of the intervention, and diet composition per se were not sufficient to explain the differences observed in longevity under both control diet and CR. Upon completion of the studies, the two research teams came to realize the notable differences in the feeding regimen (25), whereby UW monkeys were fed in the morning and the food was removed in the afternoon when another small treat, such as a piece of fruit, was offered. This protocol caused the animals to eat during the day and fast overnight. By contrast, NIA animals were fed twice daily, without removal of the second meal, thus virtually excluding the possibility of an overnight fast. To further shed light on the interaction between diet composition and eating patterns in a genetically homogeneous animal model, we recently compared the survival of mice fed the same diets used in the two nonhuman primate studies (NIA and UW) under AL, 30% CR, or a daily single meal feeding (MF) to match the calories consumed by the AL animals. Although both CR and MF mice showed increased life span compared with the AL groups, the effect was independent of the diet composition. Both the CR and MF mice self-imposed a TRF paradigm, and the life-span and health-span extension seen in those groups appeared to be directly proportional to the time spent fasting (44). Similar behavior was reported in mice under CR, which voluntarily adopted a TRF paradigm, as measured by an automated system that recorded time of food availability and consumption (37).
Outcomes from TRF trials in humans also appear to depend on the distribution of meals during the day and the duration of fasting (45–49). Limiting food intake to the middle of the day decreased body weight or body fat, fasting glucose and insulin levels, insulin resistance, hyperlipidemia, and inflammation and produced mild caloric restriction and weight loss, without calorie counting (46, 47, 50, 51). Similarly, metabolic markers were improved in a group of people eating an isocaloric diet with a bigger breakfast and a smaller dinner (52, 53), and type 2 diabetic patients under hypocaloric diet obtained better metabolic outcome by eating most of their daily allotment in the first half of the day rather than divided into six meals throughout the day (54). On the contrary, restricting food intake to the late afternoon or evening either produced mostly null results or worsened glucose levels after eating, β cell responsiveness, blood pressure, and lipid levels (45, 48, 49). A strictly controlled feeding trial tested prediabetic subjects that were allowed to eat their meals in either a 6-hour time window in the morning (before 3 p.m.) or a 12-hour time window for 5 weeks. Early TRF ameliorated the metabolic markers of diabetes without a significant reduction of body weight (55). Individuals on a hypocaloric, three-meal-per-day diet lost more weight when the majority of the food was consumed in the morning, as opposed to the evening (51). However, no significant changes in glycemia, insulin sensitivity, or respiratory exchange ratio (RER, defined as the ratio between the amount of carbon dioxide produced and oxygen used during breathing) were observed when obese, insulin-resistant men were exposed to a hypocaloric diet with food provided in the morning (56). In the context of cancer, two studies found that a fasting period of more than 13 hours resulted in lower risk of breast cancer recurrence than that in subjects who fasted less than 13 hours (57, 58). The discrepancies remain to be explained, but collectively, given the present body of knowledge, these studies indicate that both the amount of time spent eating during each day and the time at which food is consumed relative to the circadian rhythm are critically important to the effects of diet on health and longevity.
Intermittent and periodic fasting
An increasingly popular alternative to both continuous CR and TRF is intermittent fasting (IF), an eating pattern in which no or few calories are consumed for periods of time that range from one to several days, followed by AL feeding on the remaining days (10) (Fig. 1). One example of IF is the 24-hour water fast without solid food followed by a normal feeding period of 24 hours. This alternate-day fasting differs somewhat from the alternate-day modified fasting in which participants consume very few calories one day (e.g., 25% of usual intake) followed by a day without restrictions.
Both CR and fasting promote stress resistance in model organisms ranging from unicellular yeast to mammals, presumably by shifting energy from growth and reproduction to maintenance, recycling, and repair in order to increase cellular protection and survival (Fig. 2). There is an abundance of data that supports this hypothesis (59). From an evolutionary perspective, fasting is a natural phenomenon to which both humans and lower organisms were regularly exposed. Although many animals in the wild still encounter prolonged periods of time with little or no food, humans have rapidly transitioned into a sedentary lifestyle accompanied by a continuous and abundant supply of food. In a bygone era, the postabsorptive, or fasting, state triggered hunger and food-seeking behavior that took hours, sometimes days, to satisfy. Mechanisms to survive such periods of fasting are believed to have pleiotropic benefits. However, in our present-day civilization, hunger and food-seeking behavior result in instant gratification and alterations in eating patterns characterized by the consumption of high-energy meals as soon as the urge arises, thus negating the putative benefits that periods without food provide. This behavior might be a major contributor to the emergence of the obesity epidemic and obesity-related morbidities (2).
In rodents, IF extends life span (60) and protects against obesity, cardiovascular disease, hypertension, diabetes, and neurodegenerative diseases (2). It also retards the growth of tumors (61) and sensitizes a range of cancer cell types to chemotherapy (62). Additional benefits of IF include improvement in insulin sensitivity, independently of both total food intake and weight loss (63), and enhancement in brain function as evidenced by better performance on behavioral tests that assess motor responses to sensory stimuli (64). The behavioral responses to IF are associated with increased synaptic plasticity and increased production of new neurons from neural stem cells (2). Similar results were achieved when animals were fed a ketogenic diet (65) that is composed almost exclusively of fat. In mice, a ketogenic diet improved health span and delayed age-associated neurological decline, without an effect on longevity (66, 67). In the study of Verdin and colleagues, feeding a ketogenic diet to mice caused weight gain without life-span extension when compared to the controls, but a cyclic ketogenic diet reduced midlife mortality, whereas in the second study, mice consuming a ketogenic diet isocaloric to that of the control group had increased life span despite having similar body weight (66, 67). The benefits of fasting may be mediated by a highly conserved stress-response or nutrient-sensing pathway, whereby organismal metabolism switches from storage to mobilization, in part, by increasing autophagy and recycling at the cellular level. The ensuing production and utilization of fatty acid–derived ketones serve to preserve the brain and muscle function and allow the organism to withstand extended periods of food shortage. Production of ketone bodies (β-hydroxybutyrate and aceto-acetate) by the liver and gut epithelial cells (68) during β-oxidation of fatty acids to acetyl–coenzyme A (CoA) or conversion of ketogenic amino acids or both, are released into the bloodstream to provide metabolic fuel to various organs (Fig. 4).
Fig. 4. Systemic effects of caloric restriction or intermittent fasting.
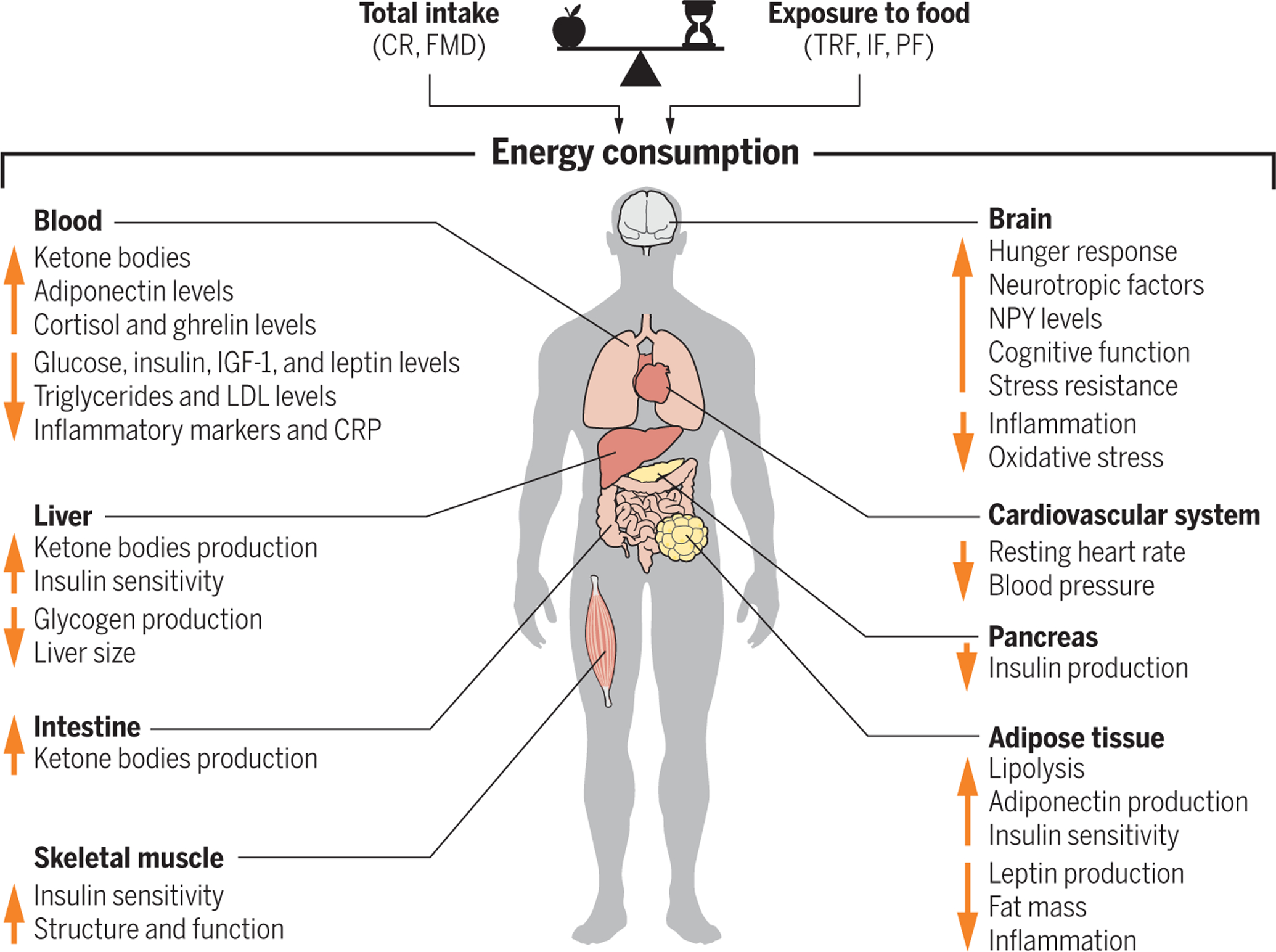
The balance between reduction in total food intake and timing contributes to differences in energy consumption, leading to changes in circulating factors and organ function. The height of each arrow does not reflect the intensity level but instead highlights a grouping. Down arrows indicate decreased levels, and up arrows indicate increased levels.
Several short-term human clinical trials have shown that alternate-day fasting can deliver benefits similar to CR in terms of weight loss and cardiometabolic health, including reduction in body weight and improved lipid profiles, lower blood pressure, and increased insulin sensitivity (69–71). In cancer patients, fasting selectively protects normal cells, but not cancerous cells, against toxicity related to chemotherapeutic agents, and fasting for up to 5 days followed by a normal diet appear to be a safe, feasible, and effective strategy in reducing common side effects associated with chemotherapy (72). Nevertheless, the challenges of implementing alternate-day fasting or similar interventions are real and can be a major burden by causing difficulties reminiscent to those encountered by individuals on chronic CR.
Fasting-mimicking diets
Implementing a long-term calorie restriction or complete fasting can be challenging in humans, and compliance tends to decrease with time. In a human study, the withdrawal rates of CR and periodic fasting (PF) participants from a 12-month study were about 40 and 30%, respectively (73). In the 2-year CALERIE clinical trial, the compliance rate was reduced to 82% with the restriction achieved averaging −12%, which was half the targeted reduction (28). The success of the long-term CRONies study in which individuals of the CR group ate nearly half of the calories compared to the AL subjects (1112 to 1958 kcal/day compared with 1976 to 3537 kcal/day) relied upon the strong motivation of its participants (31). There are side effects associated with prolonged periods of daily fasting (>15 hours) for human health. Studies associating such extended fasting periods and skipping breakfast with mortality and disease in humans have been reported (74, 75), although most long-lived populations from around the globe do practice 12- to 13-hour TRF with little to no adverse effects (5).
To make fasting acceptable to most people, Longo and colleagues conceived a low-carbohydrate, high-fat diet that enhances compliance by avoiding complete deprivation of food. The diet coined as FMD (Fig. 1) has low calories and provides for plant-based soups, herbal tea, energy bars, nut-based snacks, and supplements to be gradually implemented in a 5-day cycle each month for 3 months (76). For the first day, the caloric amount is about 1090 kcal (10% protein, 56% fat, and 34% carbohydrate) and, for days 2 to 5, only 725 kcal are provided (9% protein, 44% fat, and 47% carbohydrate) (76). A similar diet was also designed for laboratory mice, whereby animals were allowed to consume 50% of the AL food supplied as a vegetable-based powder mixed with hydro-gel on day 1 and reduced to 10% of AL on days 2 through 4. The main goal of the FMD is to maintain low circulating concentrations of IGF-1, insulin, and glucose, while increasing plasma concentrations of IGF-binding protein 1 and ketone bodies. Rejuvenating effects of FMD were initially reported through an increased number of progenitor stem cells (77). The FMD regimen also leads to improvement of markers of diseases and metabolic dysfunction, lowers cancer incidence, and extends health span in rodents, but without affecting maximum longevity (76, 78). More recently, FMD has been proposed to exert a therapeutic, antidiabetic effect by fostering regeneration of pancreatic β cells and restoring insulin secretion and glucose homeostasis in mice through the regulation of protein kinase A (PKA) and mTOR pathways (77). Furthermore, this dietary intervention improves the control of autoimmune disease—for example, multiple sclerosis—through regulation of the immune system (79). Despite the positive results on health span, FMD did not increase maximum longevity in mice, and, when administered to very old animals, it may have been detrimental (76).
Conclusions
Pharmacological interventions with proven effectiveness are often accompanied by a range of unwanted side effects. Many elderly people take multiple medications, which can cause adverse geriatric outcomes linked to increases in morbidity and mortality (80). Dietary interventions that are accompanied by long fasting periods have emerged as promising strategies to target a myriad of clinical parameters that constitute the foundation for metabolic syndrome, cardiovascular disease, cancer, and even neurodegenerative diseases (2). Although the specific mechanisms are far from being fully understood, this periodic absence of energy intake appears to improve multiple risk factors and, in some cases, reverse disease progression in mice and humans. Thus, the time is ripe to add to our understanding of the molecular mechanisms of action and efficacy of these dietary interventions to the foundation for future clinical trials. It will be important to understand and consider the differences in metabolic rate, equivalence of fasting times, role of diet composition, and the duration of the intervention as they relate to a particular disease or clinical target. Although current mouse models have not been very useful in identifying translatable therapies for some of the most common chronic diseases (e.g., Alzheimer’s and cardiovascular disease), it should be acknowleged that most experiments have been limited to a few inbred stains. It may be important to move to other model organisms closer to human physiology and etiology—such as pigs, dogs, and nonhuman primates—to test, develop, and translate interventions. Strategies could use different forms of CR, FMD, PF, and TRF in combination with known pharmacological interventions and CR mimetics. We expect that these dietary interventions combined with classical pharmacology and clinical practice will yield interventions that will improve human health and enhance health span and quality of life as we grow old. Although promising, these approaches are still experimental in nature and should not be initiated without medical supervision.
ACKNOWLEDGMENTS
We wish to thank L. Brick for excellent assistance with the art production of figures. We apologize for the omission of relevant works owing to space constraints.
Funding:
This work was supported by the Intramural Research Program of the NIA-NIH.
Footnotes
Competing interests: The authors declare no competing interests, financial or otherwise.
REFERENCES AND NOTES
- 1.World Health Organization (WHO), “World health statistics 2018: Monitoring health for the SDGs” (WHO Report, WHO, 2018). [Google Scholar]
- 2.Mattson MP et al. , Proc. Natl. Acad. Sci. U.S.A 111, 16647–16653 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panda S, Science 354, 1008–1015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speakman JR, Mitchell SE, Mol. Aspects Med 32, 159–221 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF, Aging Cell 9, 92–95 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walford RL, Mock D, Verdery R, MacCallum T, J. Gerontol. A Biol. Sci. Med. Sci 57, B211–B224 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Vitousek KM, Manke FP, Gray JA, Vitousek MN, Eur. Eat. Disord. Rev 12, 338–360 (2004). [Google Scholar]
- 8.Vitousek KM, Gray JA, Grubbs KM, Eur. Eat. Disord. Rev 12, 279–299 (2004). [Google Scholar]
- 9.Vitousek KM, Eur. Eat. Disord. Rev 12, 275–278 (2004). [Google Scholar]
- 10.Longo VD, Panda S, Cell Metab 23, 1048–1059 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rous P, J. Exp. Med 20, 433–451 (1914). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osborne TB, Mendel LB, Ferry EL, Science 45, 294–295 (1917). [DOI] [PubMed] [Google Scholar]
- 13.McCay CM, Crowell MF, Maynard LA, J. Nutr 10, 63–79 (1935). [Google Scholar]
- 14.Redman LM et al. , Cell Metab 27, 805–815.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontana L, Partridge L, Longo VD, Science 328, 321–326 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G, Cell 153, 1194–1217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingram DK, Roth GS, Ageing Res. Rev 20, 46–62 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Madeo F, Pietrocola F, Eisenberg T, Kroemer G, Nat. Rev. Drug Discov 13, 727–740 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Speakman JR, Hambly C, J. Nutr 137, 1078–1086 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Ingram DK, de Cabo R, Ageing Res. Rev 39, 15–28 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao CY et al. , Aging Cell 10, 629–639 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell SJ et al. , Cell Metab 23, 1093–1112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colman RJ et al. , Science 325, 201–204 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattison JA et al. , Nature 489, 318–321 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattison JA et al. , Nat. Commun 8, 14063 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heilbronn LK et al. , JAMA 295, 1539–1548 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin CK et al. , JAMA Intern. Med 176, 743–752 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravussin E et al. , J. Gerontol. A Biol. Sci. Med. Sci 70, 1097–1104 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rochon J et al. , J. Gerontol. A Biol. Sci. Med. Sci 66, 97–108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willcox DC, Willcox BJ, Hsueh W-C, Suzuki M, Age (Dordr.) 28, 313–332 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fontana L, Meyer TE, Klein S, Holloszy JO, Proc. Natl. Acad. Sci. U.S.A 101, 6659–6663 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fontana L, Klein S, JAMA 297, 986–994 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Mercken EM et al. , Aging Cell 12, 645–651 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kristan DM, Age (Dordr.) 30, 147–156 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunt ND et al. , Age (Dordr.) 34, 1453–1458 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dirks AJ, Leeuwenburgh C, Mech. Ageing Dev 127, 1–7 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Acosta-Rodríguez VA, de Groot MHM, Rijo-Ferreira F, Green CB, Takahashi JS, Cell Metab 26, 267–277.e2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roe FJ et al. , Food Chem. Toxicol 33 (suppl. 1), S1–S100 (1995). [DOI] [PubMed] [Google Scholar]
- 39.Solon-Biet SM et al. , Cell Metab 19, 418–430 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hatori M et al. , Cell Metab 15, 848–860 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherman H et al. , J. Cell. Mol. Med 15, 2745–2759 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodie LN et al. , Metabolism 82, 1–13 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Chaix A, Zarrinpar A, Miu P, Panda S, Cell Metab 20, 991–1005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchell SJ et al. , Cell Metab S1550-4131(18)30512-6 (2018).
- 45.Carlson O et al. , Metabolism 56, 1729–1734 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gill S, Panda S, Cell Metab 22, 789–798 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moro T et al. , J. Transl. Med 14, 290 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stote KS et al. , Am. J. Clin. Nutr 85, 981–988 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tinsley GM et al. , Eur. J. Sport Sci 17, 200–207 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Gabel K et al. , Nutr. Healthy Aging 4, 345–353 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raynor HA, Li F, Cardoso C, Physiol. Behav 192, 167–172 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Jakubowicz D, Barnea M, Wainstein J, Froy O, Obesity (Silver Spring) 21, 2504–2512 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Yoshizaki T et al. , Eur. J. Appl. Physiol 113, 2603–2611 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Kahleova H et al. , Diabetologia 57, 1552–1560 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sutton EF et al. , Cell Metab 27, 1212–1221.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Versteeg RI et al. , J. Biol. Rhythms 32, 130–142 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marinac CR et al. , Cancer Epidemiol. Biomarkers Prev 24, 783–789 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marinac CR et al. , JAMA Oncol 2, 1049–1055 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Finkel T, Nat. Med 21, 1416–1423 (2015). [DOI] [PubMed] [Google Scholar]
- 60.Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N, Mech. Ageing Dev 55, 69–87 (1990). [DOI] [PubMed] [Google Scholar]
- 61.Xie K et al. , Nat. Commun 8, 155 (2017).28761067 [Google Scholar]
- 62.Lee C et al. , Sci. Transl. Med 4, 124ra27 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anson RM et al. , Proc. Natl. Acad. Sci. U.S.A 100, 6216–6220 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singh R et al. , Age (Dordr.) 34, 917–933 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baur JA et al. , Nature 444, 337–342 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Newman JC et al. , Cell Metab 26, 547–557.e8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roberts MN et al. , Cell Metab 27, 1156 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Puchalska P, Crawford PA, Cell Metab 25, 262–284 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Catenacci VA et al. , Obesity (Silver Spring) 24, 1874–1883 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoddy KK et al. , Obesity (Silver Spring) 22, 2524–2531 (2014). [DOI] [PubMed] [Google Scholar]
- 71.Varady KA, Bhutani S, Church EC, Klempel MC, Am. J. Clin. Nutr 90, 1138–1143 (2009). [DOI] [PubMed] [Google Scholar]
- 72.Safdie FM et al. , Aging 1, 988–1007 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trepanowski JF et al. , JAMA Intern. Med 177, 930–938 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yokoyama Y et al. , Yonago Acta Med 59, 55–60 (2016). [PMC free article] [PubMed] [Google Scholar]
- 75.Uzhova I et al. , J. Am. Coll. Cardiol 70, 1833–1842 (2017). [DOI] [PubMed] [Google Scholar]
- 76.Brandhorst S et al. , Cell Metab 22, 86–99 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng C-W et al. , Cell 168, 775–788.e12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei M et al. , Sci. Transl. Med 9, eaai8700 (2017).28202779 [Google Scholar]
- 79.Choi IY et al. , Cell Reports 15, 2136–2146 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McLean AJ, Le Couteur DG, Pharmacol. Rev 56, 163–184 (2004). [DOI] [PubMed] [Google Scholar]
- 81.Rikke BA et al. , Mech. Ageing Dev 124, 663–678 (2003). [DOI] [PubMed] [Google Scholar]
- 82.Diaz-Ruiz A et al. , Aging Cell 17, e12767 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


