Exposure to noise of high intensities (> 80 dB) is considered a stressful event and might produce both auditory and extra-auditory damage, including different central nervous system (CNS) injuries. Within the CNS, the hippocampus (HC), a structure related to several cognitive functions, has shown to be particularly susceptible to the effects of noise. Human and animal studies have demonstrated that exposure to noise can generate different HC-related alterations, including morphological, functional and behavioral changes (Molina et al., 2016b, 2019; Nadhimi and Llano, 2020). Unfortunately, little is known about the mechanisms involved in noise-induced hippocampal damage and more research is needed to understand how the different alterations are caused. In addition, considering that noise exposure constitutes a public health problem that is increasing in urbanized societies, accurate knowledge of these mechanisms has clinical relevance as it can lead to develop preventive strategies. In this perspective, the possible mechanisms involved in noise-induced hippocampal cell damage will be discussed, as well as the pathways through which these mechanisms could be triggered by noise exposure.
Noise can be defined as an unpleasant sound, in general of moderate to loud intensity (Molina et al., 2016a). In particular, people that live in a noisy environment might be in a health-threatening situation, partly because they might be involuntarily exposed to high levels of noise coming from different sources such as traffic, home appliances as well as a variety of loud sounds generated in music shows and discotheques (Molina et al., 2016b, 2021). In humans, it has been reported that living in a noisy environment or being exposed to occupational noise can cause cardiovascular, autonomic and sleep pattern alterations, as well as hearing loss. In addition, exposure to noise can cause emotional reactions such as annoyance and stress, mental and physical fatigue and changes in behavior, including difficulties in reading comprehension, memory and concentration in children (Molina et al., 2016b). It should be noted that although some of the effects of noise exposure can be reverted in the short-term, others can cause long-lasting or even permanent damage. Importantly, animal studies have provided relevant information about the mechanisms involved in the negative effects of noise exposure on individual’s extra-auditory structures such as the HC. These studies have shown that acute and chronic noise exposure might produce different behavioral alterations which could be correlated to changes on hippocampal biomarkers, demonstrating that disturbances in behavior would be mediated by biochemical and histological modifications in this structure. Furthermore, it is important to highlight that noise-induced behavioral changes can also affect the HC, as has been shown in studies on sleep disturbances and other behavioral alterations (Hahad et al., 2019; Molina et al., 2019, 2021; Nadhimi and Llano, 2020). Unfortunately, the mechanisms of noise-induced biological damage are not fully understood, although many factors may account for this damage. In fact, it has been proposed that noise might induce hippocampal cell damage through different mechanisms that can be turned on mainly indirectly, either through the activation of the auditory pathway or the stimulation of hippocampal glucocorticoid receptors (GR; Molina et al., 2016b; Hahad et al., 2019; Nadhimi and Llano, 2020). In addition, we recently proposed the mechanical vibration of tissues as a direct mechanism of noise-induced damage in developing animals (Molina et al., 2016a, 2019). The GR activation might occur as a consequence of the stress response generated by the individual’s emotional perception of noise and might negatively affect different tissues of the body, including HC. Furthermore, the activation of all these pathways could favor the presence of hippocampal oxidative stress and aminoacidergic neurotransmission alterations.
Oxidative stress consists of a state of imbalance between oxidants and antioxidants in favor of oxidants, which can promote cell damage. Data from the literature have shown that noise can generate oxidative stress in different tissues of the auditory system as well as in extra-auditory areas of the brain, including HC. In these works, it was found that exposure to noise can increase the levels of reactive oxygen species (ROS) and peroxidative damage, as well as decrease the levels and/or activity of antioxidant defenses, generating oxidative stress. In addition, oxidative imbalance was associated to neural damage and behavioral alterations (Molina et al., 2019, 2021; Akefe et al., 2020; Nadhimi and Llano, 2020). Importantly, some factors such as intensity, frequency and exposure time have shown to be crucial in determining the effects of noise on HC oxidative status. In most cases, the greater the intensity and exposure sessions, the greater the oxidative stress and the neural damage generated. However, the age of exposure would also be essential as developing animals appear to be more susceptible to the long-term effects of noise after a single exposure rather than repeated sessions (Molina et al., 2019, 2021).
Furthermore, it has also been proposed that noise exposure can induce neurotransmission alterations in extra-auditory CNS structures (Molina et al., 2021). In particular, HC would be susceptible to noise-induced aminoacidergic neurotransmission changes, characterized by an increase in glutamatergic activity, which could favor excitotoxicity and neural damage. It has been reported that noise can increase extra-cellular glutamate levels, generate changes in glutamine synthetase activity as well as in the expression of N-methyl-D-aspartic acid receptors and the glutamate transporter EAAT-1, in different developmental stages. Moreover, these alterations have been related to neural damage and alterations in HC-related learning and memory (Nadhimi and Llano, 2020; Molina et al., 2021). It is important to highlight that there is a reciprocal relationship between excitotoxicity and ROS generation, in which one of the mechanisms favors the presence of the other. Firstly, when an excitotoxic process is ongoing and excessive activation of glutamate receptors occurs, several detrimental events might take place including impairment of calcium buffering and generation of ROS. On the other hand, if increased production of ROS is not counterbalanced by the antioxidant capacity of the endogenous system, ROS-induced overstimulation of glutamate receptor can, in turn, transform glutamatergic neurotransmission in a mediator of intracellular oxidative stress (Molina et al., 2021). Consequently, it is possible that exposure to noise stimulates one or both mechanisms and if they are not counteracted by endogenous defenses (e.g. antioxidant machinery and glutamate transporters), further neural damage might occur as a result of increased ROS levels and glutamatergic activity.
It should be noted that the auditory pathway is considered the main route through which loud noise can induce oxidative stress or excitotoxicity in HC; nevertheless, it is still not entirely clear how it occurs. On the one hand, it has been suggested that hearing loss would drive hippocampal dysfunctions (Nadhimi and Llano, 2020; Zhang et al., 2020). Indeed, in addition to HC damage, different alterations have been reported in the circuits that run between the auditory system and the HC. Moreover, it has been suggested that deprivation of sensory information to the HC could affect its normal operations. Additionally, some studies reported a correlation between increased hearing loss and behavioral disturbances (Hayes et al., 2019; Nadhimi and Llano, 2020). Unfortunately, few studies have evaluated both HC damage and hearing function. Importantly, afferent pathways to the HC coming from the auditory pathway are not entirely clear since multiple routes have been identified (Nadhimi and Llano, 2020). In fact, some studies reported hippocampal alterations without hearing loss. For example, in previous studies we have found hippocampal oxidative imbalance and neurotransmission alterations in noise-exposed developing animals without changes in the auditory function (Molina et al., 2019). In addition, the age of exposure seems to be a determining factor of how noise can affect the HC: it has been shown that noise-induced hippocampal oxidative alterations in postnatal day 7 animals, should be generated through the activation of an auditory-independent pathway, such as the mechanical vibration of the tissues (Molina et al., 2016a, 2019), given that the auditory system of rats matures and becomes susceptible to sounds at approximately postnatal day 12. Finally, Zhang et al. (2020) observed hippocampal and behavioral disturbances after chronic exposure to noise of about 65 dB, intensity considered non-stressful and not harmful to the auditory system. It should be noted that exposed animals were going through critical periods for development of the HC, which would make them more vulnerable to the noxious effects of noise. However, it is important to highlight that even moderate levels of noise, which are not considered stressful or harmful, could have negative effects on HC function. Considering that in these studies developing animals were used, it is possible that hippocampal damage without hearing loss could be associated with the age of exposure; however, more studies are necessary to confirm this hypothesis.
Last, it has also been postulated that noise can indirectly cause adverse effects in individuals when it interferes with daily activities, communication and disrupts sleep, leading to endocrine and sympathetic systems activation, as well as to cognitive and emotional reactions. Additionally, when exposure to noise is sustained over time (i.e., chronic exposure), cognitive and emotional reactions can induce a pathophysiological cascade, generating an increase in stress hormone levels, blood pressure, and heart rate, which promotes the development of cerebrovascular risk factors (Jafari et al., 2018; Hahad et al., 2019). It is well known that prolonged exposure to glucocorticoids can produce detrimental effects on HC, affecting its functions. It is important to highlight that HC is involved not only in behavioral and cognitive functions but also in the neuroendocrine regulation of stress hormones. When chronic stress occurs, there is an increase in the activation of hippocampal GR that promotes the appearance of different negative effects such as disturbances in neural metabolism, cell survival, physiological functions, neuronal morphology and neurogenesis (Gai et al., 2017; Jafari et al., 2018; Hayes et al., 2019). For example, adult neurogenesis is critical for spatial navigation, learning and memory. A decreased ability of HC to generate new cells limits its ability to mediate plastic changes in synaptic connections in response to environmental demands, generating a deficit in cognitive functions. It is important to note that chronic stress as well as noise exposure and hearing loss, have been associated to a reduction in hippocampal neurogenesis (Jafari et al., 2018; Hayes et al., 2019; Nadhimi and Llano, 2020). Finally, the decrease in hippocampal neurogenesis could be a consequence not only of the increase in stress hormones but also of the oxidative stress or excitotoxicity found in HC after noise exposure (Nadhimi and Llano, 2020). Furthermore, increased levels of circulating stress hormones can also promote oxidative stress together with the subsequent endothelial dysfunction and release of pro-inflammatory mediators in different tissues, including HC. Even more, prolonged upregulation of glucocorticoids can promote glutamate release and N-methyl-D-aspartic acid-receptor overstimulation in HC, favoring excitotoxicity and elevated ROS formation (Osborne et al., 2015; Nadhimi and Llano, 2020). In consequence, both oxidative stress and excitotoxicity can also be indirectly triggered by increased stress hormones, which could lead to hippocampal cells damage and decreased neurogenesis. Figure 1 summarizes the proposed mechanisms involved in noise-induced damage and the corresponding activation pathways.
Figure 1.
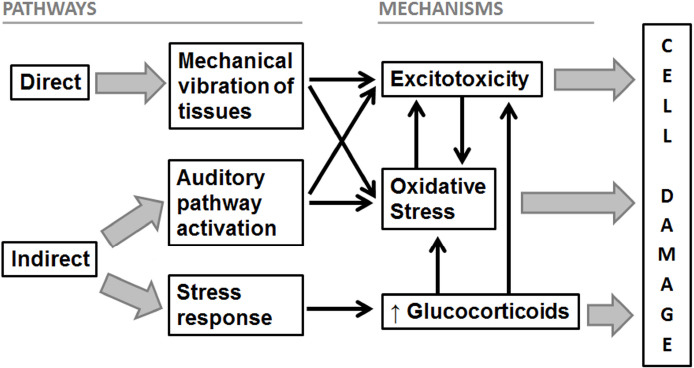
Schematic representation of the pathways and mechanisms involved in noise-induced cell damage.
Arrows mean a stimulating action (the activation of a pathway or mechanism promotes the activation of another mechanism).
In conclusion, different mechanisms have been postulated through which noise could induce hippocampal damage and affect its functions. Although these mechanisms are still not fully understood, there is evidence that noise could promote oxidative stress, excitotoxicity, and increased levels of circulating stress hormones, which would affect HC, either directly or indirectly. It is important to note that noise exposure could activate one or more mechanisms at a time, either because it is acting through different routes or because the activation of one mechanism promotes the presence of the other, which makes HC particularly vulnerable to noise-induced damage. The activation of these mechanisms would generate different alterations in hippocampal cells, affecting both cognitive functions and neuroendocrine regulation. As future research directions, it would be important to continue studying noise-induced hippocampal damage in relation to: auditory system injury; stress response; schemes and age of exposure; behavior; neurogenesis; gene expression; and aging. Finally, considering noise exposure as a public health problem that is increasing in urbanized societies, the knowledge about the mechanisms involved in noise-induced damage is essential to devise neuroprotective strategies designed to counteract and prevent CNS damage.
This work was supported by the Universidad de Buenos Aires (20020160100005BA and 20020190100222BA) (to LRG).
Additional file: Open peer review reports 1 (100.5KB, pdf) and 2 (89.5KB, pdf) .
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: R M Leão, University of Sao Paulo, Brazil; Bryan E. Kolb, University of Lethbridge, Canada.
P-Reviewers: Leão RM, Kolb BE; C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- 1.Akefe IO, Ayo JO, Sinkalu VO. Kaempferol and zinc gluconate mitigate neurobehavioral deficits and oxidative stress induced by noise exposure in Wistar rats. PLoS One. 2020;15:e0236251. doi: 10.1371/journal.pone.0236251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gai Z, Su D, Wang Y, Li W, Cui B, Li K, She X, Wang R. Effects of chronic noise on the corticotropin-releasing factor system in the rat hippocampus: relevance to Alzheimer’s disease-like tau hyperphosphorylation. Environ Health Prev Med. 2017;22:79. doi: 10.1186/s12199-017-0686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hahad O, Prochaska JH, Daiber A, Muenzel T. Environmental noise-induced effects on stress hormones, oxidative stress, and vascular dysfunction: key factors in the relationship between cerebrocardiovascular and psychological disorders. Oxid Med Cell Longev. 2019;2019:4623109. doi: 10.1155/2019/4623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayes SH, Manohar S, Majumdar A, Allman BL, Salvi R. Noise-induced hearing loss alters hippocampal glucocorticoid receptor expression in rats. Hear Res. 2019;379:43–51. doi: 10.1016/j.heares.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jafari Z, Kolb BE, Mohajerani MH. Chronic traffic noise stress accelerates brain impairment and cognitive decline in mice. Exp Neurol. 2018;308:1–12. doi: 10.1016/j.expneurol.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Molina SJ, Buján GE, Guelman LR. Noise-induced hippocampal oxidative imbalance and aminoacidergic neurotransmitters alterations in developing male rats: Influence of enriched environment during adolescence. Dev Neurobiol. 2021;81:164–188. doi: 10.1002/dneu.22806. [DOI] [PubMed] [Google Scholar]
- 7.Molina SJ, Buján GE, Rodriguez Gonzalez M, Capani F, Gómez-Casati ME, Guelman LR. Exposure of developing male rats to one or multiple noise sessions and different housing conditions: hippocampal thioredoxin changes and behavioral alterations. Front Behav Neurosci. 2019;13:182. doi: 10.3389/fnbeh.2019.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molina SJ, Capani F, Guelman LR. Noise exposure of immature rats can induce different age-dependent extra-auditory alterations that can be partially restored by rearing animals in an enriched environment. Brain Res. 2016a;1636:52–61. doi: 10.1016/j.brainres.2016.01.050. [DOI] [PubMed] [Google Scholar]
- 9.Molina SJ, Miceli M, Guelman LR. Noise exposure and oxidative balance in auditory and extra-auditory structures in adult and developing animals. Pharmacological approaches aimed to minimize its effects. Pharmacol Res. 2016b;109:86–91. doi: 10.1016/j.phrs.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Nadhimi Y, Llano DA. Does hearing loss lead to dementia? A review of the literature. Hear Res. 2020;402:108038. doi: 10.1016/j.heares.2020.108038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osborne DM, Pearson-Leary J, McNay EC. The neuroenergetics of stress hormones in the hippocampus and implications for memory. Front Neurosci. 2015;9:164. doi: 10.3389/fnins.2015.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Zhu M, Sun Y, Tang B, Zhang G, An P, Cheng Y, Shan Y, Merzenich MM, Zhou X. Environmental noise degrades hippocampus-related learning and memory. Proc Natl Acad Sci U S A. 2020;118:e2017841117. doi: 10.1073/pnas.2017841117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


