Abstract
Time-of-day is a crucial, yet often overlooked, biological variable in biomedical research. We examined the top 25 most cited papers in several domains of behavioral phenotyping to determine whether time-of-day information was reported. The majority of studies report behavioral testing conducted during the day, which does not coincide with the optimal time to perform the testing from an functional perspective of the animals being tested. The majority of animal models used in biomedical research are nocturnal rodents; thus, testing during the light phase (i.e. animals’ rest period) may alter the results and introduce variability across studies. Time-of-day is rarely considered in analyses or reported in publications; the majority of publications fail to include temporal details when describing their experimental methods, and those few that report testing during the dark rarely report whether measures are in place to protect from exposure to extraneous light. We propose that failing to account for time-of-day may compromise replication of findings across behavioral studies and reduce their value when extrapolating results to diurnal humans.
Keywords: Circadian rhythms, diel, learning, memory, attention, food intake, mating behavior, aggression, drug seeking behaviors
Introduction
The United States National Institutes of Health (NIH) has underscored rigorous experimental design and unbiased analyses, as well as transparent reporting of results, as essential to improve reproducibility within the biomedical scientific enterprise (Baker, 2016; Collins and Tabak, 2014). Consideration of relevant biological variables including sex, age, body mass, and underlying health conditions that may contribute to health or disease is now compulsory for individuals seeking research funding through the NIH. The NIH has emphasized a focus on sex as a biological variable, in part, to remedy a long history of male-dominated studies in preclinical research (Beery and Zucker, 2011) that was apparent even in some models of female-prevalent diseases (Yoon et al., 2014). Not surprising, given the many sex differences in physiology and behavior, the exclusion of females from clinical and non-clinical research has likely had negative consequences for women’s health (Beery and Zucker, 2011; Correa-De-Araujo, 2006). Similarly, there are well-documented temporal differences in physiology and behavior that should be considered across all biological studies.
We propose that time-of-day is a crucial biological variable in biomedical research, generally, and behavioral neuroscience, specifically. Time-of-day should be considered in analyses and reported in publications to improve reproducibility of studies and to provide the appropriate context to the conclusions. Virtually, all physiological and behavioral processes display daily fluctuations driven by endogenous circadian clocks; circadian rhythms (i.e. circadian cycles) are internal representations of the external solar day that optimize biological function. Despite these strong daily fluctuations in physiology and behavior, the majority of behavioral neuroscience studies we reviewed conduct behavioral testing during the light phase, which may not produce optimal performance for nocturnal rodents because it is typically their less active period. As noted below, there is little consistency across studies; some labs place rodents on a reverse light-dark cycle and test during the animals’ active period, others maintain animals in a light-dark cycle similar to the external environment, but test the animals during the dark phase of the daily illumination cycle, whereas others test either early or late during the active phase. Unfortunately, the majority of publications fail to include sufficient temporal details when describing their experimental methods, and those that report testing during the active phase rarely report whether measures were in place to protect circadian rhythms by preventing exposure to extraneous light on route to or in the testing environment. However, even a brief pulse of light (~2 seconds of 100 lux) is sufficient to have biological effects in nocturnal rodents (Gorman et al., 2006). Furthermore, few of the studies we examined included time-of-day factors when interpreting their data.
We believe that failing to account for time-of-day as a key biological variable could hamper reproducibility across behavioral neuroscience laboratories, complicate interpretation of the results, and reduce the value of the data when extrapolating results based on animal studies to diurnal humans. Below, we (1) briefly review daily rhythms and circadian regulation of a wide range of behaviors, (2) review the importance of critical biological variables that should be considered in the design and conduct of biomedical research, and then (3) illustrate, using highly cited publications from several subdisciplines in behavioral neuroscience, the lack of consistency in accounting for time-of-day. Our goal is to raise awareness of the importance in time-of-day as a biological variable that influences reproducibility, reliability, and validity in behavioral phenotyping across biomedical disciplines.
Circadian Rhythms
As eminent circadian biologist Colin Pittendrigh noted, “A rose is not necessarily and unqualifiedly a rose…it is a very different biochemical system at noon and at midnight” (Pittendrigh, 1965). The same can be said of virtually all vertebrate animals, including humans. Accounting for time-of-day as a biological variable begins with the rigorous design and conduct of research studies and should include consideration of temporal factors in data collection and analysis of results, as well as reporting of findings. Consideration of time-of-day of all experimental manipulations (including behavioral training, testing, time of pharmacological, or surgical interventions, etc.) and sample collection may be critical to the interpretation, validation, and replication of research results.
Life on our planet is adapted to the 24-hour solar day. Essentially all animals display daily rhythms in physiology and behavior. Internal circadian (circa = about; dies = day) rhythms allow synchronization of biological and behavioral processes to the external temporal environment. Endogenous circadian rhythms have a period of ~24 h and are reset on a daily basis to precisely 24 h, typically via exposure to light during the day.
The suprachiasmatic nuclei (SCN) serve as the master circadian clock in vertebrates (Moore and Eichler, 1972; Stephan and Zucker, 1972). External light information is relayed from specialized non-image forming retinal ganglion cells via the retinohypothalamic tract (RHT) to the paired SCN in the hypothalamus. In turn, neural and humoral signals emit from the SCN to synchronize circadian rhythms throughout the body (Bedrosian and Nelson, 2017; Roenneberg and Merrow, 2016).
The SCN molecular clock comprises a set of transcriptional–translational feedback loops that drive rhythmic 24-hour expression of the core canonical clock components (Partch et al., 2014; Takahashi, 2015). In the primary feedback loop, circadian locomotor output cycle kaput (CLOCK) and brain and muscle ARNT-like protein 1 (BMAL1) proteins form heterodimers outside of the nucleus of SCN cells. The CLOCK-BMAL1 complex translocates to the nucleus and binds to DNA regulatory elements containing E-boxes, which in turn activate expression of period (per1, per2, and per3) and cryptochrome (cry1 and cry2) genes. PER and CRY proteins then heterodimerize and translocate into the nucleus, where they repress their own transcription by acting on the CLOCK-BMAL1 complexes. In mice, activation of CLOCK-BMAL1 occurs during early morning (onset of rest period) leading to peak transcription of PER and CRY protein in the early afternoon and subsequent repression of CLOCK-BMAL1 transcription during the evening/night (active period) (Takahashi, 2017). In an interacting feedback loop, CLOCK-BMAL1 complexes also activate expression of nuclear receptors, REV-ERB α and RORα. Their protein products feedback to regulate BMAL1 by competitively binding retinoic acid-related orphan receptor response elements to the BMAL1 promoter (Takahashi, 2017). REV-ERBs repress the transcription of BMAL1, whereas RORs activate BMAL1 transcription. These two loops form the basis of the molecular clock, but a complex network of interacting genes and post-translational modifications ensure that the process takes ~24 h to complete (Takahashi, 2015; Takahashi, 2017). This molecular transcriptional–translational feedback loop forms the basis of the intrinsic daily circadian rhythm that provides temporal organization throughout the brain and rest of the body.
It is easy to overlook time of day as a critical biological variable. For example, during an early phenotyping study of mice in which the neuronal nitric oxide synthase (nNOS or NOS1) gene was knocked out, we initially reported that balance and motor coordination was unaffected (Nelson et al., 1995). This outcome seemed inconsistent with a subsequent report that the cerebellum possesses the highest numbers of nNOS neurons in the brain (Eliasson et al., 1997). Notably, our original behavioral phenotyping study (Nelson et al., 1995) was conducted during the day (between 1400 and 1600; lights on at 0700). In contrast, when locomotor behavior was examined during the dark phase, we observed striking differences in citrulline, a marker for nitric oxide production, as well as abnormalities in balance and motor coordination among nNOS mice (Figure 1) (Kriegsfeld et al., 1999). When considered together, the data suggest that the reason we observed an effect of nNOS on motor behavior during the dark phase, but not the light phase, is that a “floor effect” on balance and coordination was observed among both genotypes of mice when tested during the middle of their rest phase, which may be analogous to waking and testing humans for balance and coordination at 0300 h (Hines, 2004).
Figure 1.
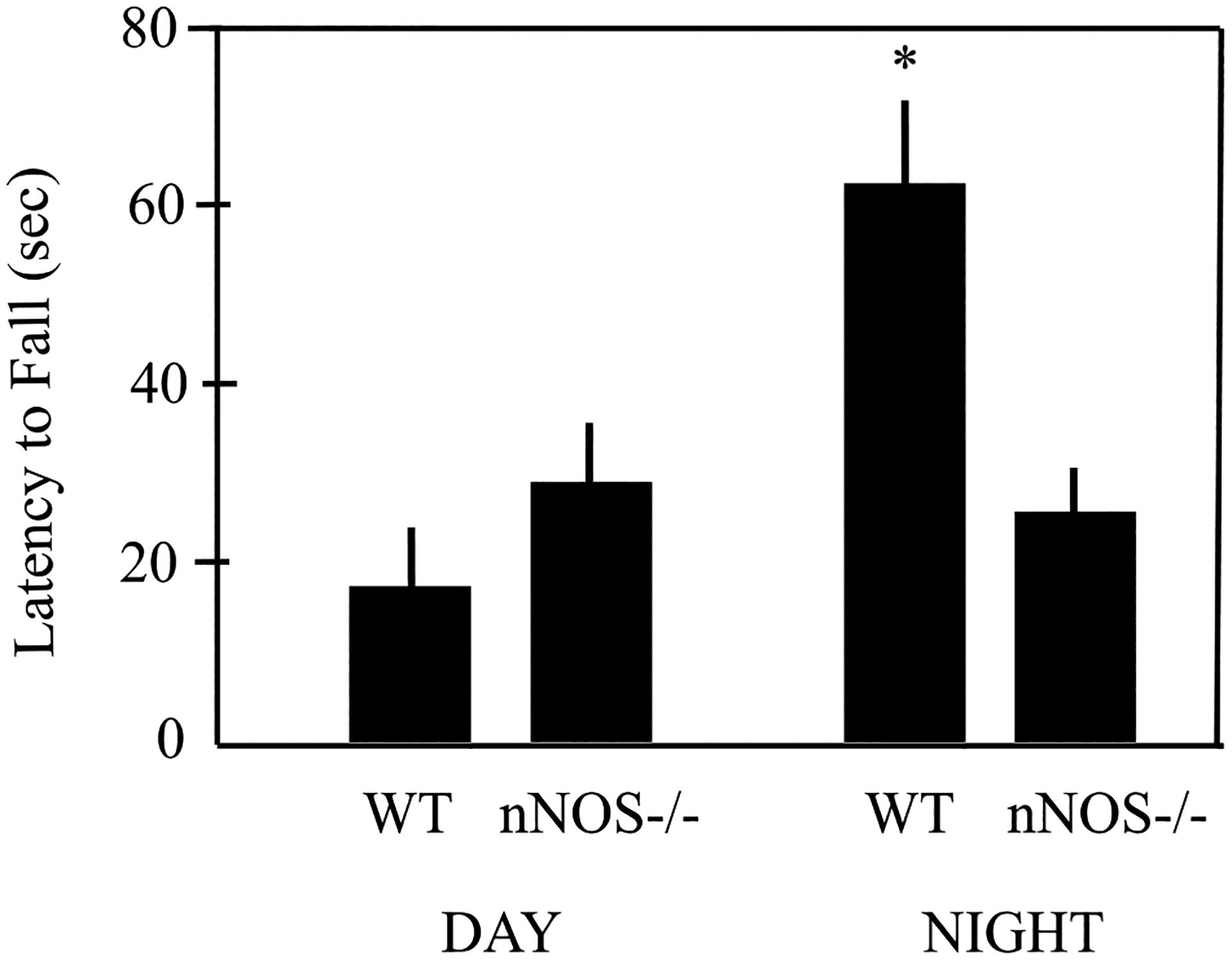
Mean latency (sec) to fall from a 2 cm pole during the day (rest phase) did not differ between wild-type (WT) and nNOS−/− mice (Nelson et al., 1995). However, latency to fall was increased for WT mice when tested during the night (active phase) suggesting a floor effect when tested during the day (Kriegsfeld et al., 1999).
We have examined the top cited 25 papers in several domains of behavioral neuroscience to determine when behavioral testing was conducted or whether time of day was even reported. Despite documented significant daily rhythms in the behaviors under study, the majority of publications fail to include sufficient temporal details when describing their experimental methods. The majority of studies report testing during the day (rest period), and only a few tested during the animals’ active phase at night (or during the dark in reverse light-dark schedules). In the latter cases, it was rarely reported what measures were in place to protect circadian rhythms by preventing exposure to extraneous light during the dark periods. Although our focus is on behavioral neuroscience, a similar lack of attention of time-of-day as a key biological variable likely occurs during behavioral phenotyping in animals with altered gene expression or in rodent models of disease, as well as in physiological studies examining immunology, metabolism, and endocrinology.
Learning and Memory
Circadian rhythms in learning and memory are well established (Krishnan and Lyons, 2015; Smarr et al., 2014). Typically, peak memory formation for intermediate and long-term memory tasks coincides with individuals’ active periods. Nocturnal rodents such as rats (Rattus) and mice (Mus) display peak performance in the Morris water maze test of spatial memory during the dark phase when they are active (Valentinuzzi et al., 2001; Valentinuzzi et al., 2004). Similarly, diurnal grass rats (Arvicanthis niloticus) display peak Morris water maze performance during the light phase, their active period (Martin-Fairey and Nunez, 2014). Expression of the core circadian clock genes is similar among diurnal and nocturnal rodents in the SCN, but vary in phase downstream in the amygdala and hippocampus (Martin-Fairey and Nunez, 2014; Otalora et al., 2013; Ramanathan et al., 2010; Wang et al., 2009). Persistence of memory depends on the time of training; nocturnal rodents display increased retention when trained during the night in both spatial learning and operant learning tasks (Gritton et al., 2012). This effect appears to be dependent on daily corticosterone rhythms, as adrenalectomy abolishes circadian variations in conditioned fear extinction learning (Woodruff et al., 2015). In contrast, mice trained during their inactive phase on cued fear conditioning acquired the conditioning faster and recalled it longer than mice trained during the active phase (Snider and Obrietan, 2018; Chaudhury and Colwell, 2002). However, extinction was similarly achieved more readily during the dark phase (Chaudhury and Colwell, 2002). It is possible that there are ecological reasons underlying this difference in learning and memory outcomes or that the fear learning, which is measured as freezing behavior in rodents, may reflect circadian differences in locomotion, sensory perception, or motivation.
The methodology of our search is detailed in Table 1. We examined the top 25 cited research (non-review) papers within a behavioral neuroscience domain (e.g., “Learning and Memory in Rodents”) and examined the temporal dimensions of behavioral testing. Examination of the top 25 cited research in learning and memory articles (Supplemental Table 1) revealed that five papers described behavioral testing occurring during the light phase of the daily light-dark cycle, 17 papers did not report time of day of behavioral assessment (but presumably during the light phase given that the learning tests required visual cues), and three papers provided ambiguous time of day reporting. We classified papers as ‘ambiguous’ if they reported the time of behavior testing without providing timing of the lighting cycle, provided timing for some, but not all behavioral tests, or when timing was not explicitly stated but cited published methods that reported time of day for behavioral testing. Only one paper in this domain suggested, but did not explicitly state, that they tested the animals during the dark (used infrared cameras under low light illumination). Several of these studies were conducted on mouse models of Alzheimer disease; others examined aspects of hippocampal-dependent spatial learning and memory.
TABLE 1.
We examined the timing of behavioral testing in the top 25 cited research (non-review) papers in several domains of behavioral neuroscience from 2000–2020 using Web of Science.
| Search Terms | Hits |
|---|---|
| Learning and Memory in Rodents | 2,636 |
| Sensation and Perception in Rodents | 1123 |
| Attention Behavior in Rodents | 617 |
| Food Intake Behavior in Rodents | 717 |
| Mating Behavior in Rodents | 548 |
| Maternal Behavior in Rodents | 1253 |
| Aggression in Rodents | 451 |
| Drug Seeking Behavior in Rodents | 388 |
Sensation and Perception
Both sensation and perception vary across the day among rodents. For example, pain sensitivity and responsiveness display daily rhythms (Segal et al., 2018; Palada et al., 2020), with higher pain thresholds typically observed during the night (i.e., dark phase) when nocturnal animals are most likely to encounter painful stimuli. Furthermore, endorphin levels and virtually all metabolites involved in the pain pathway vary across the day (Finlay, 2019; Segal et al., 2018). Olfactory stimuli provoke neural activity based on the phase of the circadian clock (Miller et al., 2014). Fos expression, a marker of neural activities, in the main olfactory bulb, anterior olfactory nucleus, and piriform cortex of rats were higher during the subjective night compared to the subjective day in response to neutral and predatory odors (Amir et al., 1999; Funk and Amir, 2000). These rhythms may be regulated by a circadian clock in the olfactory bulbs (Granados-Fuentes et al., 2006) that may be entrained by timing of food intake (Pavlovski et al., 2018). Visual perception (Finlay and Sengelaub, 1981) and auditory perception (Basinou et al., 2017; Meltser et al., 2014) also vary across the day among nocturnal rodents.
Examination of the top 25 cited empirical research articles (Supplemental Table 2) in sensation and perception revealed that 21 of these top-cited papers did not report the time-of-day during which behavioral tests were performed, but were presumably conducted during the light phase given other described experimental activities. Two studies explicitly reported behavioral testing during the day, and two studies performed behavioral testing in the dark (animals were housed in a reverse light-dark cycle).
Attention
A relationship exists between attention and time-of-day in mammals (Schmidt et al., 2007; Valdez, 2019; Valdez et al., 2005). Though attentional processes and arousal are intrinsically linked, the two should not be conflated. Various attentional processes appear to be differently affected by time-of-day; this can be observed by the varying results of different attention tests in humans. Performance on the psychomotor vigilance test remains consistent across the day in subjects with 8-hours of sleep, yet performance on the same task worsens across the waking phase in sleep deprived subjects (Mollicone et al., 2010). In a “two letter search” visual attention task, a time-of-day effect on performance was observed in the ‘simpler’ search portions of the test, but not the exhaustive search portion (Natale et al., 2003). Similarly, time-of-day effects on performance were observed in some – but not all – components of a continuous performance task (Valdez et al., 2010). Use of the attentional network test revealed different time-of-day variations within its individual subtests (Matchock and Mordkoff, 2009).
Time of day should be considered in the context of short-term and long-term attention behavioral experiments. For example, rats trained on a sustained attention task in the dark phase displayed better acquisition and performance in comparison to rats trained in the light phase (Gritton et al., 2012). Shifting the time of testing within the light phase can reduce performance on the sustained attention task in rats (Paolone et al., 2012). Further, attentional processes can also reciprocally influence circadian rhythms. Training on a sustained attention task in the light phase can induce a diurnal locomotor activity pattern in typically nocturnal rats (Gritton et al., 2009).
Of the top 25-cited relevant research articles on the topic of attention behavior in rodents (Supplemental Table 3), three performed experiments during the light phase, seven performed experiments during the dark phase, two performed experiments during both phases, 11 did not report timing of experiments, and two had ambiguous reporting. Additionally, one paper did not explicitly report time of day, but cited the methods of a previous publication that provided this information.
Motivated Behaviors
Food Intake.
Food intake patterns in rodents follow marked circadian rhythms (Challet, 2019; Johnston et al., 2016). Rodents eat the majority of their food near the beginning and end of their active phase and consume small amounts of food throughout the inactive phase (Rosenwasser et al., 1981; ter Haar, 1972; Possidente and Birnbaum, 1979). Daily patterns of food intake are driven by both orexigenic/anorexigenic homeostatic signaling and by circadian processes (Challet, 2019). Not only are food intake patterns driven by circadian rhythms, but the timing of food intake can also reciprocally influence circadian rhythms via central and peripheral food-entrainable circadian oscillators (Damiola et al., 2000; Mistlberger, 2011). Because of the interaction between these systems, mis-timed consumption has behavioral and physiological consequences (Loh et al., 2015; Ramirez-Plascencia et al., 2017). Accordingly, studies examining food intake behaviors or factors affected by food intake need to consider the time of day at which the experiments are performed to enhance rigor and reproducibility. Further, studies examining the effects of injected hormones or compounds on food intake should consider the timing of injections in relation to the typical circadian rhythms of the rodents.
Of the top 25 cited research articles on rodent food intake behavior (Supplemental Table 4), five papers conducted experiments during the light phase, two papers conducted experiments during the dark phase, four papers conducted experiments during both phases, two papers did not report timing of experiments, and 12 papers provided ambiguous time-of-day information.
Mating Behavior.
In many mammals, mating behavior is coordinated by circadian rhythms (Antle and Silver, 2016; Goldman, 1999; Rusak and Zucker, 1975); rhythmicity of mating behaviors has been observed and studied in spontaneously ovulating rodents (e.g., mice, rats, and hamsters). Male rodents preferentially engage in sexual behaviors during the dark phase (Beach and Levinson, 1949; Logan and Leavitt, 1992; Mahoney and Smale, 2005; McElhinny et al., 1997). Sexual receptivity in female rodents is regulated by estrous cycles driven by a complex cycle of gonadotrophin releasing hormones, gonadotropins, and sex steroid hormones (Goldman and Sheridan, 1974), all of which are directly regulated by circadian rhythms (de la Iglesia and Schwartz, 2006). Because of this, female rodents tend to be most sexually receptive during the dark phase (Reuter et al., 1970).
Of the top 25-cited research articles on rodent mating behavior (Supplemental Table 5), five performed experiments during the light phase, six performed experiments during the dark phase, one tested during both phases, eight did not report the timing of experiments, and five had partial or ambiguous reporting.
Maternal Behavior.
In contrast to most of the other commonly studied motivated behaviors, natural variation in the expression of maternal behavior in laboratory rodents favors increased pup contact and pup-oriented behaviors during the light phase (Grota and Ader, 1969; Ivy et al., 2008; Jensen Peña and Champagne, 2013; Leon et al., 1984; Toki et al., 2007). For example, among rats the amount of time spent in arched-back (kyphosis) nursing is nearly two-fold higher during the light period than during the dark period (Jensen Peña and Champagne, 2013). Duration of nursing is also elevated during the light phase in guinea pig dams (Hennessy and Jenkins, 1994). In contrast to most other behaviors examined, the amount of pup licking/grooming (LG) is relatively stable across the day in rats, although differences between subpopulations of high-LG versus low-LG rats are more apparent toward the end of the light-phase and during the dark-phase (Jensen Peña and Champagne, 2013).
Of the top 25 cited relevant research articles on maternal behavior among rodents (Supplemental Table 6), four reported performed behavioral tests during the dark phase, four performed tests during the light phase, five performed tests during both light and dark phases, nine did not report timing of tests, and three had inadequate reporting to determine conclusively the timing of the testing.
Aggressive Behavior.
Nocturnal rodents are more active during the night and aggressive behaviors display a predictable daily pattern of occurrence (Todd and Machado, 2019). Among male Syrian hamsters, aggressive behaviors peak shortly after the onset of dark and this rhythm persists in constant lighting conditions (Landau, 1975b). Intra-cage rat aggression and muricide peak during the early hours of dark in standard light-dark cycles (Kane and Knutson, 1976; Russell and Singer, 1983). In male mice, aggression towards an intruder similarly peaks one hour after the onset of darkness and displays a nadir one hour after the onset of light (Todd et al., 2018). Daily rhythms in aggressive behavior in rodents may be facilitated by daily rhythms in glucocorticoids (Haller et al., 2000; Landau, 1975a) that are associated with aggressive behavior (Nelson and Trainor, 2007).
Mutations of canonical clock genes also increase the likelihood of aggression in mice (Hood and Amir, 2018). For instance, mice that do not express the clock gene rev-erb display more aggression towards an intruder compared to wild type mice (Chung et al., 2014; Jager et al., 2014).
Humans also display daily rhythms in aggression. People tend to display elevated anger and aggression during the afternoon (Hood and Amir, 2018). Indeed, this pattern is exaggerated in people with Sundowning Syndrome where patterns of angry and aggressive outbursts peak late afternoon or early in the evening (Bedrosian and Nelson, 2013). There is no sex difference in temporal behavioral patterns among patients in a psychiatric facility who also display daily peaks of aggression during the afternoon (Manfredini et al., 2001).
Of the top 25 cited relevant research articles on aggressive behaviors among rodents (Supplemental Table 7), eight did not report the timing of aggression behavioral tests, whereas five conducted the tests during the light phase. Twelve studies were conducted during the dark phase, although six of these did not mention strategies used to protect the animals from extraneous lighting or other techniques to minimize disruption to circadian rhythms (e.g., testing under dim red illumination).
Drug Seeking Behavior.
Drug-seeking behaviors are influenced by circadian rhythms (Falcón and McClung, 2009; McClung, 2007; Webb, 2017). Time-of-day can influence drug-seeking behavior and behavioral sensitization to drugs in rodents (Arvanitogiannis et al., 2000; Sleipness et al., 2007); drug sensitization is influenced by the circadian system (Abarca et al., 2002; Baird and Gauvin, 2000; Sleipness et al., 2005) and drugs of abuse can also feedback to modulate circadian rhythms (Kosobud et al., 1998; Uz et al., 2005). For example, using locomotor output as a behavioral measure of cocaine sensitization, time-of-day influences sensitization. Rodents become more sensitized to cocaine when treated with the drug during the light phase (inactive phase) than during the dark phase (active phase) (Akhisaroglu et al., 2004; Uz et al., 2002). Further, conditioned place preference tests are influenced by the timing of tests; cocaine-induced conditioned place preference occurs more reliably during the light phase than the dark phase (Abarca et al., 2002).
Of the top 25 cited relevant research articles on rodent drug seeking behaviors (Supplemental Table 8), seven performed behavioral tests during the dark phase, five performed tests during the light phase, three performed tests during both phases, eight did not report timing of tests, and two provided ambiguous reporting.
Discussion
Significant variation occurred across the day for all of the behaviors examined for this report. With the exception of rodent maternal behaviors that peak during the light hours, the vast majority of rodent behaviors including learning and memory, sensation and perception, attention, as well as motivated behaviors such food intake, mating, and drug seeking behaviors peak during the dark (active) period. These time-of-day effects are likely driven by the circadian system; however, for some there may be some direct effects of light and dark on the behavior itself. Our analyses of the most cited papers in several domains of behavioral neuroscience revealed that time-of-day is rarely considered in either the design or analysis of experiments despite the potential significant effect on behavioral outcomes. Indeed, many behavioral tests were conducted during the day, although the majority of papers were not explicit about the time of behavioral testing. Importantly, the vast majority of animal models (i.e., mice and rats) used in behavioral research are nocturnal rodents (Supplemental Table 9); thus, testing during the light phase and the nocturnal animals’ rest period may alter the results and introduce unnecessary variability across studies and laboratories.
Very few of the studies we examined included time-of-day factors when interpreting their data. Failing to account for time-of-day as a key biological variable may contribute to reproducibility issues and inconsistent behavioral results across laboratories. Disregarding time-of-day when nocturnal animal models are examined may reduce the value of their data when extrapolating results to diurnal humans, in turn compromising translation of rodent studies to humans. The current review focused primarily on variations in rodent behaviors across the day. However, humans also demonstrate variations in behaviors throughout the day.
Of course, there are many sources of variability in animal behavioral phenotyping tests ranging from variation in animal testers’ odors, animal handling styles, and other gene-environment interactions (Bohlen et al., 2014; Chesler et al., 2002; Gouveia and Hurst, 2017; Nelson and Young, 1998). Some of these factors are difficult to control or interpret (e.g., gene-environment interactions), whereas others are less so (e.g., no perfumes or body sprays when interacting with animals). However, time-of-day is a controllable and critical biological factor that should be considered in the design, implementation, and analyses of experimental data. Importantly, time-of-day of animal testing, as well as lighting conditions should be tightly controlled and described in detail. In some cases, it may be necessary to test during the light phase. For example the use of some automated behavioral testing tools often requires animals to be tracked in the light. Nonetheless, details regarding photoperiod, time of testing (either clock time or zeitgeber (ZT), and whether testing occurred during the dark or light should always be reported. If testing occurs during the dark, then methods for protecting circadian rhythms such as using dim red lighting or night vision goggles should be described.
In sum, consideration of circadian rhythms in physiology and behavior is paramount to enhancing experimental rigor and reproducibility, and crucial for the interpretation of study results. Life on our planet is adapted to the 24-hour solar day and adaptations to temporal niches have shaped physiology and behavior over evolutionary time to increase fitness. Ignoring these temporal influences during the conduct of animal studies unnecessarily skews the data and muddles interpretation. Together, evidence based decision-making in the timing of data collection, protection against exposure to extraneous light during dark phase testing, incorporation of temporal factors in data analysis and interpretation, and meticulous reporting of temporal factors in publications, have the potential to improve experimental rigor and reproducibility across studies.
Supplementary Material
Figure 2. Time-of-day reporting in eight behavioral neuroscience domains.
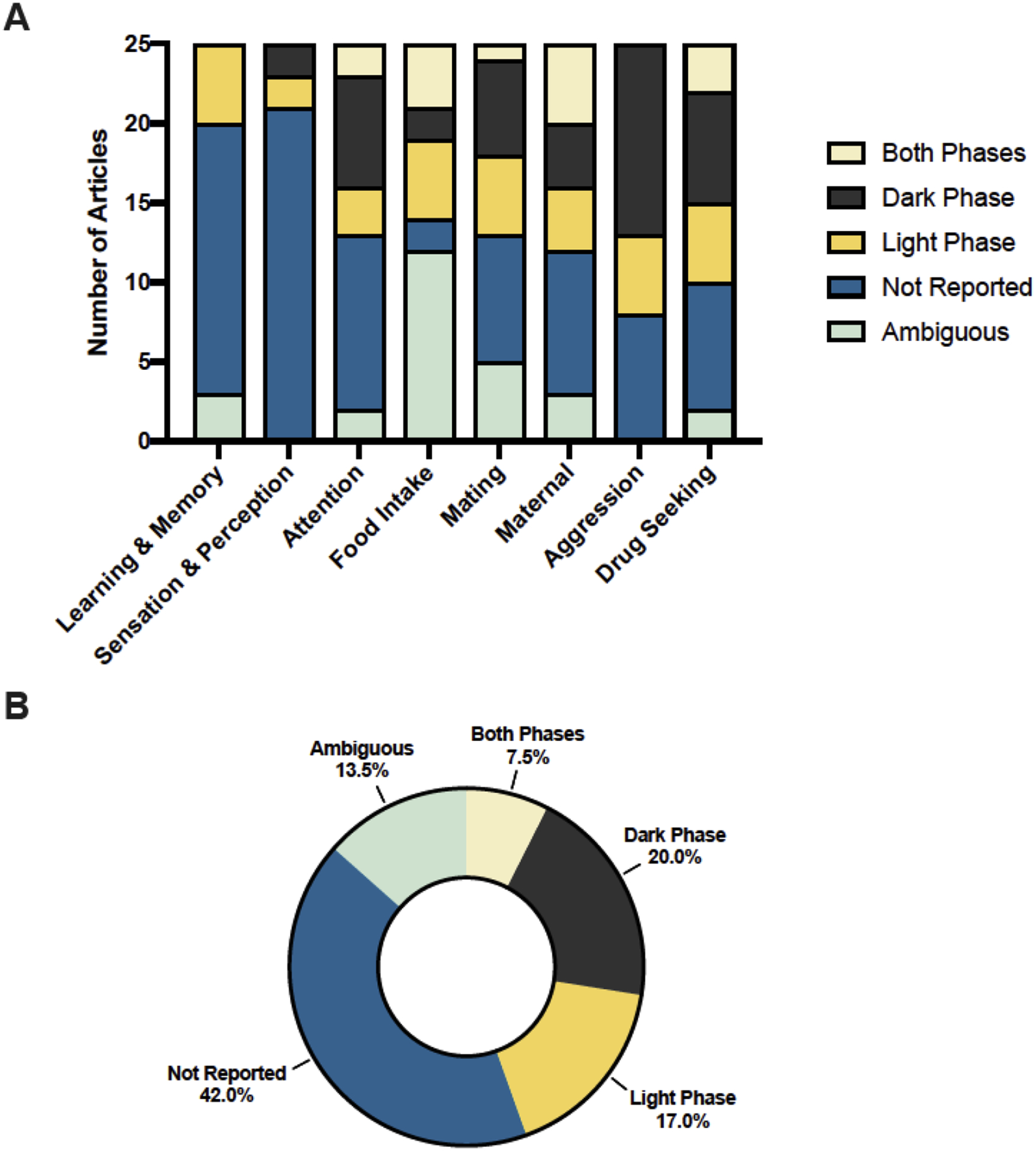
(A) Frequencies of behavioral testing timing reports from the top 25 cited articles in eight domains of behavioral neuroscience. Non-review articles from 2000–2020 were identified using Web of Science search queries and were cataloged according to reporting within the manuscript. (B) A pooled summary of reporting percentages across the eight examined domains reveals that over half of the 200 examined articles either failed to report timing of behavioral testing or had ambiguous reporting.
Highlights.
Time-of-day (TOD) is a crucial biological variable in biomedical research
The majority of publications fail to report TOD in experimental methods
Lack of TOD reporting may compromise replication across behavioral studies
Absence of TOD reporting may jeopardize translation of rodent studies to humans
Acknowledgements
Preparation of this review was supported by National Institute of Health grants R01NS092388 (RJN; ACD), R01CA 194924(ACD), and National Institute of General Medical Sciences of the National Institutes of Health under Award Number 5U54GM104942-03. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors do not have any conflicts of interest to report.
DATA AVAILABILITY
The data that support the findings of this study are available in the Supplemental Information.
References
- Abarca C, Albrecht U, and Spanagel R, 2002. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc. Natl. Acad. Sci. U S A 13, 9026–9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhisaroglu M, Ahmed R, Kurtuncu M, Manev H, and Uz T, 2004. Diurnal rhythms in cocaine sensitization and in period1 levels are common across rodent species. Pharmacol. Biochem. Behav 1, 37–42. [DOI] [PubMed] [Google Scholar]
- Amir S, Cain S, Sullivan J, Robinson B, and Stewart J, 1999. In rats, odor-induced fos in the olfactory pathways depends on the phase of the circadian clock. Neurosci. Lett 3, 175–178. [DOI] [PubMed] [Google Scholar]
- Antle MC, and Silver R, 2016. Circadian insights into motivated behavior. Curr. Top. Behav. Neurosci 137–169. [DOI] [PubMed] [Google Scholar]
- Arvanitogiannis A, Sullivan J, and Amir S, 2000. Time acts as a conditioned stimulus to control behavioral sensitization to amphetamine in rats. Neuroscience 1, 1–3. [DOI] [PubMed] [Google Scholar]
- Baird TJ, and Gauvin D, 2000. Characterization of cocaine self-administration and pharmacokinetics as a function of time of day in the rat. Pharmacol. Biochem. Behav 2, 289–299. [DOI] [PubMed] [Google Scholar]
- Baker M, 2016. Reproducibility: Seek out stronger science. Nature 7622, 703–704. [Google Scholar]
- Basinou V, Park J.-s., Cederroth CR, and Canlon B, 2017. Circadian regulation of auditory function. Hearing research 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach FA, and Levinson G, 1949. Diurnal variations in the mating behavior of male rats. Proc. Soc. Exp. Biol. Med 1, 78–80. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, and Nelson RJ, 2013. Sundowning syndrome in aging and dementia: Research in mouse models. Exp. Neurol 67–73. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, and Nelson RJ, 2017. Timing of light exposure affects mood and brain circuits. Transl. Psychiatry 1, e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, and Zucker I, 2011. Sex bias in neuroscience and biomedical research. Transl. Psychiatry 3, 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen M, Hayes ER, Bohlen B, Bailoo JD, Crabbe JC, and Wahlsten D, 2014. Experimenter effects on behavioral test scores of eight inbred mouse strains under the influence of ethanol. Behav. Brain Res 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challet E, 2019. The circadian regulation of food intake. J. Neurosci. Res 7, 393–405. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, and Colwell CS, 2002. Circadian modulation of learning and memory in fear-conditioned mice. Behav. Brain Res 1, 95–108. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, and Mogil JS, 2002. Influences of laboratory environment on behavior. Nat. Neurosci 11, 1101–1102. [DOI] [PubMed] [Google Scholar]
- Chung S, Lee EJ, Yun S, Choe HK, Park S-B, Son HJ, Kim K-S, Dluzen DE, Lee I, and Hwang O, 2014. Impact of circadian nuclear receptor rev-erbα on midbrain dopamine production and mood regulation. Cell 4, 858–868. [DOI] [PubMed] [Google Scholar]
- Collins FS, and Tabak LA, 2014. Policy: Nih plans to enhance reproducibility. Nature 7485, 612–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-De-Araujo R, 2006. Serious gaps: How the lack of sex/gender-based research impairs health. J Womens Health (Larchmt) 10, 1116–1122. [DOI] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, and Schibler U, 2000. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 23, 2950–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Iglesia HO, and Schwartz WJ, 2006. Minireview: Timely ovulation: Circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology 3, 1148–1153. [DOI] [PubMed] [Google Scholar]
- Eliasson MJ, Blackshaw S, Schell MJ, and Snyder SH, 1997. Neuronal nitric oxide synthase alternatively spliced forms: Prominent functional localizations in the brain. Proc. Natl. Acad. Sci. U S A 7, 3396–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcón E, and McClung CA, 2009. A role for the circadian genes in drug addiction. Neuropharmacology 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay BL, and Sengelaub DR, 1981. Toward a neuroethology of mammalian vision: Ecology and anatomy of rodent visuomotor behavior. Behav. Brain Res 2, 133–149. [DOI] [PubMed] [Google Scholar]
- Finlay BL, 2019. The neuroscience of vision and pain: Evolution of two disciplines. Philos. Trans. R. Soc. Lond. B. Biol. Sci 1785, 10.1098/rstb.2019.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, and Amir S, 2000. Circadian modulation of fos responses to odor of the red fox, a rodent predator, in the rat olfactory system. Brain Res 1–2, 262–267. [DOI] [PubMed] [Google Scholar]
- Goldman BD, 1999. The circadian timing system and reproduction in mammals. Steroids 9, 679–685. [DOI] [PubMed] [Google Scholar]
- Goldman BD, and Sheridan PJ, 1974. The ovulatory surge of gonadotropin and sexual receptivity in the female golden hamster. Physiol. Behav 6, 991–995. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Evans JA, and Elliott JA, 2006. Potent circadian effects of dim illumination at night in hamsters. Chronobiol. Int 1–2, 245–250. [DOI] [PubMed] [Google Scholar]
- Gouveia K, and Hurst JL, 2017. Optimising reliability of mouse performance in behavioural testing: The major role of non-aversive handling. Sci. Rep 44999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Fuentes D, Tseng A, and Herzog ED, 2006. A circadian clock in the olfactory bulb controls olfactory responsivity. J. Neurosci 47, 12219–12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritton HJ, Kantorowski A, Sarter M, and Lee TM, 2012. Bidirectional interactions between circadian entrainment and cognitive performance. Learn. Mem 3, 126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritton HJ, Sutton BC, Martinez V, Sarter M, and Lee TM, 2009. Interactions between cognition and circadian rhythms: Attentional demands modify circadian entrainment. Behav. Neurosci 5, 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grota LJ, and Ader R, 1969. Continuous recording of maternal behaviour in rattus norvegicus. Anim. Behav 4, 722–729. [DOI] [PubMed] [Google Scholar]
- Haller J, Millar S, van de Schraaf J, de Kloet RE, and Kruk MR, 2000. The active phase-related increase in corticosterone and aggression are linked. J. Neuroendocrinol 5, 431–436. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, and Jenkins R, 1994. A descriptive analysis of nursing behavior in the guinea pig (cavia porcellus). J. Comp. Psychol 1, 23–28. [DOI] [PubMed] [Google Scholar]
- Hines CB, 2004. Time-of-day effects on human performance. J. Cathol. Educ 3, 390–413. [Google Scholar]
- Hood S, and Amir S, 2018. Biological clocks and rhythms of anger and aggression. Front. Behav. Neurosci 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, and Baram TZ, 2008. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: A clinically relevant model for early-life stress. Neuroscience 3, 1132–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager J, O’Brien WT, Manlove J, Krizman EN, Fang B, Gerhart-Hines Z, Robinson MB, Klein PS, and Lazar MA, 2014. Behavioral changes and dopaminergic dysregulation in mice lacking the nuclear receptor rev-erbα. Mol. Endocrinol 4, 490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen Peña C, and Champagne FA, 2013. Implications of temporal variation in maternal care for the prediction of neurobiological and behavioral outcomes in offspring. Behav. Neurosci 1, 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JD, Ordovás JM, Scheer FA, and Turek FW, 2016. Circadian rhythms, metabolism, and chrononutrition in rodents and humans. Adv. Nutr 2, 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane NL, and Knutson JF, 1976. Influence of colony lighting conditions on home-cage spontaneous aggression. J. Comp. Physiol. Psychol 9, 889–897. [DOI] [PubMed] [Google Scholar]
- Kosobud AE, Pecoraro NC, Rebec GV, and Timberlake W, 1998. Circadian activity precedes daily methamphetamine injections in the rat. Neurosci. Lett 2, 99–102. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Eliasson MJ, Demas GE, Blackshaw S, Dawson TM, Nelson RJ, and Snyder SH, 1999. Nocturnal motor coordination deficits in neuronal nitric oxide synthase knock-out mice. Neuroscience 2, 311–315. [DOI] [PubMed] [Google Scholar]
- Krishnan HC, and Lyons LC, 2015. Synchrony and desynchrony in circadian clocks: Impacts on learning and memory. Learn. Mem 9, 426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau IT, 1975a. Effects of adrenalectomy on rhythmic and non-rhythmic aggressive behavior in the male golden hamster. Physiol. Behav 6, 775–780. [DOI] [PubMed] [Google Scholar]
- Landau IT, 1975b. Light-dark rhythms in aggressive behavior of the male golden hamster. Physiol. Behav 6, 767–774. [DOI] [PubMed] [Google Scholar]
- Leon M, Adels L, Coopersmith R, and Woodside B, 1984. Diurnal cycle of mother-young contact in norway rats. Physiol. Behav 6, 999–1003. [DOI] [PubMed] [Google Scholar]
- Logan FA, and Leavitt F, 1992. Sexual free behavior in male rats (rattus norvegicus). J. Comp. Psychol 1, 37–42. [DOI] [PubMed] [Google Scholar]
- Loh DH, Jami SA, Flores RE, Truong D, Ghiani CA, O’Dell TJ, and Colwell CS, 2015. Misaligned feeding impairs memories. Genes Dev [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney MM, and Smale L, 2005. A daily rhythm in mating behavior in a diurnal murid rodent arvicanthis niloticus. Horm. Behav 1, 8–13. [DOI] [PubMed] [Google Scholar]
- Manfredini R, Vanni A, Peron L, La Cecilia O, Smolensky MH, and Grassi L, 2001. Day-night variation in aggressive behavior among psychiatric inpatients. Chronobiol. Int 3, 503–511. [DOI] [PubMed] [Google Scholar]
- Martin-Fairey CA, and Nunez AA, 2014. Circadian modulation of memory and plasticity gene products in a diurnal species. Brain Res 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchock RL, and Mordkoff JT, 2009. Chronotype and time-of-day influences on the alerting, orienting, and executive components of attention. Exp. Brain Res 2, 189–198. [DOI] [PubMed] [Google Scholar]
- McClung CA, 2007. Circadian rhythms, the mesolimbic dopaminergic circuit, and drug addiction. Sci. World J 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhinny TL, Smale L, and Holekamp KE, 1997. Patterns of body temperature, activity, and reproductive behavior in a tropical murid rodent, arvicanthis niloticus. Physiol. Behav 1, 91–96. [DOI] [PubMed] [Google Scholar]
- Meltser I, Cederroth CR, Basinou V, Savelyev S, Lundkvist GS, and Canlon B, 2014. Trkb-mediated protection against circadian sensitivity to noise trauma in the murine cochlea. Curr. Biol 6, 658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.-e. K., Granados-Fuentes D, Wang T, Marpegan L, Holy TE, and Herzog ED, 2014. Vasoactive intestinal polypeptide mediates circadian rhythms in mammalian olfactory bulb and olfaction. J. Neurosci 17, 6040–6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE, 2011. Neurobiology of food anticipatory circadian rhythms. Physiol. Behav 4, 535–545. [DOI] [PubMed] [Google Scholar]
- Mollicone DJ, Van Dongen HP, Rogers NL, Banks S, and Dinges DF, 2010. Time of day effects on neurobehavioral performance during chronic sleep restriction. Yale J. Biol. Med 8, 735–744. [DOI] [PubMed] [Google Scholar]
- Moore RY, and Eichler VB, 1972. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 1, 201–206. [DOI] [PubMed] [Google Scholar]
- Natale V, Alzani A, and Cicogna P, 2003. Cognitive efficiency and circadian typologies: A diurnal study. Yale J. Biol. Med 5, 1089–1105. [Google Scholar]
- Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, and Snyder SH, 1995. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature 6555, 383–386. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, and Trainor BC, 2007. Neural mechanisms of aggression. Nat. Rev. Neurosci 536–546. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, and Young KA, 1998. Behavior in mice with targeted disruption of single genes. Neurosci. Biobehav. Rev 3, 453–462. [DOI] [PubMed] [Google Scholar]
- Otalora BB, Hagenauer MH, Rol MA, Madrid JA, and Lee TM, 2013. Period gene expression in the brain of a dual-phasing rodent, the octodon degus. J. Biol. Rhythms 4, 249–261. [DOI] [PubMed] [Google Scholar]
- Palada V, Gilron I, Canlon B, Svensson CI, and Kalso E, 2020. The circadian clock at the intercept of sleep and pain. Pain 5, 894–900. [DOI] [PubMed] [Google Scholar]
- Paolone G, Lee TM, and Sarter M, 2012. Time to pay attention: Attentional performance time-stamped prefrontal cholinergic activation, diurnality, and performance. J. Neurosci 35, 12115–12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partch CL, Green CB, and Takahashi JS, 2014. Molecular architecture of the mammalian circadian clock. PLoS One 2, 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovski I, Evans JA, and Mistlberger RE, 2018. Feeding time entrains the olfactory bulb circadian clock in anosmic per2::luc mice. Neuroscience 175–184. [DOI] [PubMed] [Google Scholar]
- Pittendrigh C, 1965. “Biological clocks: The functions, ancient and modern, of circadian oscillations.” Science and the Sixties, 96–111. [Google Scholar]
- Possidente B, and Birnbaum S, 1979. Circadian rhythms for food and water consumption in the mouse, mus musculus. Physiol. Behav 4, 657–660. [DOI] [PubMed] [Google Scholar]
- Ramanathan C, Stowie A, Smale L, and Nunez A, 2010. Per2 rhythms in the amygdala and bed nucleus of the stria terminalis of the diurnal grass rat (arvicanthis niloticus). Neurosci. Lett 3, 220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Plascencia OD, Saderi N, Escobar C, and Salgado-Delgado RC, 2017. Feeding during the rest phase promotes circadian conflict in nuclei that control energy homeostasis and sleep-wake cycle in rats. Eur. J. Neurosci 10, 1325–1332. [DOI] [PubMed] [Google Scholar]
- Reuter LA, Ciaccio LA, and Lisk RD, 1970. Progesterone: Regulation of estrous cycle, ovulion and estrous behavior in the golden hamster. Endocrinology 6, 1287–1297. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, and Merrow M, 2016. The circadian clock and human health. Curr. Biol 10, R432–43. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Boulos Z, and Terman M, 1981. Circadian organization of food intake and meal patterns in the rat. Physiol. Behav 1, 33–39. [DOI] [PubMed] [Google Scholar]
- Rusak B, and Zucker I, 1975. Biological rhythms and animal behavior. Annu. Rev. Psychol 137–171. [DOI] [PubMed] [Google Scholar]
- Russell JW, and Singer G, 1983. Relations between muricide, circadian rhythm and consummatory behavior. Physiol. Behav 1, 23–27. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Collette F, Cajochen C, and Peigneux P, 2007. A time to think: Circadian rhythms in human cognition. Cogn. Neuropsychol 7, 755–789. [DOI] [PubMed] [Google Scholar]
- Segal JP, Tresidder KA, Bhatt C, Gilron I, and Ghasemlou N, 2018. Circadian control of pain and neuroinflammation. J. Neurosci. Res 6, 1002–1020. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, and Jansen HT, 2005. Time of day alters long-term sensitization to cocaine in rats. Brain Res 1–2, 132–137. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, and Jansen HT, 2007. Contribution of the suprachiasmatic nucleus to day:night variation in cocaine-seeking behavior. Physiol. Behav 5, 523–530. [DOI] [PubMed] [Google Scholar]
- Smarr BL, Jennings KJ, Driscoll JR, and Kriegsfeld LJ, 2014. A time to remember: The role of circadian clocks in learning and memory. Behav. Neurosci 3, 283–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider KH, and Obrietan K, 2018. Modulation of learning and memory by the genetic disruption of circadian oscillator populations. Physiol. Behav 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan FK, and Zucker I, 1972. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci. U S A 6, 1583–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS, 2015. Molecular components of the circadian clock in mammals. Proc. Natl. Acad. Sci. U S A 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS, 2017. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet 3, 164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Haar MB, 1972. Circadian and estrual rhythms in food intake in the rat. Horm. Behav 213–219. [DOI] [PubMed] [Google Scholar]
- Todd WD, Fenselau H, Wang JL, Zhang R, Machado NL, Venner A, Broadhurst RY, Kaur S, Lynagh T, Olson DP, Lowell BB, Fuller PM, and Saper CB, 2018. A hypothalamic circuit for the circadian control of aggression. Nat. Neurosci 5, 717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd WD, and Machado NL, 2019. A time to fight: Circadian control of aggression and associated autonomic support. Auton. Neurosci 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toki S, Morinobu S, Imanaka A, Yamamoto S, Yamawaki S, and Honma K, 2007. Importance of early lighting conditions in maternal care by dam as well as anxiety and memory later in life of offspring. Eur. J. Neurosci 3, 815–829. [DOI] [PubMed] [Google Scholar]
- Uz T, Ahmed R, Akhisaroglu M, Kurtuncu M, Imbesi M, Dirim Arslan A, and Manev H, 2005. Effect of fluoxetine and cocaine on the expression of clock genes in the mouse hippocampus and striatum. Neuroscience 4, 1309–1316. [DOI] [PubMed] [Google Scholar]
- Uz T, Javaid JI, and Manev H, 2002. Circadian differences in behavioral sensitization to cocaine: Putative role of arylalkylamine n-acetyltransferase. Life Sci 25, 3069–3075. [DOI] [PubMed] [Google Scholar]
- Valdez P, 2019. Circadian rhythms in attention. Yale J. Biol. Med 1, 81–92. [PMC free article] [PubMed] [Google Scholar]
- Valdez P, Ramírez C, García A, Talamantes J, and Cortez J, 2010. Circadian and homeostatic variation in sustained attention. Chronobiol. Int 2, 393–416. [DOI] [PubMed] [Google Scholar]
- Valdez P, Ramírez C, García A, Talamantes J, Armijo P, and Borrani J, 2005. Circadian rhythms in components of attention. Biol. Rhythm Res 1–2, 57–65. [Google Scholar]
- Valentinuzzi VS, Kolker DE, Vitaterna MH, Ferrari EAM, Takahashi JS, and Turek FW, 2001. Effect of circadian phase on context and cued fear conditioning in c57bl/6j mice. Anim. Learn. Behav 2, 133–142. [Google Scholar]
- Valentinuzzi VS, Menna-Barreto L, and Xavier GF, 2004. Effect of circadian phase on performance of rats in the morris water maze task. J. Biol. Rhythms 4, 312–324. [DOI] [PubMed] [Google Scholar]
- Wang LM, Dragich JM, Kudo T, Odom IH, Welsh DK, O’Dell TJ, and Colwell CS, 2009. Expression of the circadian clock gene period2 in the hippocampus: Possible implications for synaptic plasticity and learned behaviour. ASN Neuro 3, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb IC, 2017. Circadian rhythms and substance abuse: Chronobiological considerations for the treatment of addiction. Curr. Psychiatry Rep 2, 12. [DOI] [PubMed] [Google Scholar]
- Woodruff ER, Greenwood BN, Chun LE, Fardi S, Hinds LR, and Spencer RL, 2015. Adrenal-dependent diurnal modulation of conditioned fear extinction learning. Behav. Brain Res 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon DY, Mansukhani NA, Stubbs VC, Helenowski IB, Woodruff TK, and Kibbe MR, 2014. Sex bias exists in basic science and translational surgical research. Surgery 3, 508–516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


