ABSTRACT
Aim
This study aimed to determine whether there is an association between influenza and new‐onset type 1 diabetes.
Materials and methods
This population‐based retrospective cohort study used data from the National Database of Health Insurance Claims and Specific Health Check‐ups of Japan. Influenza was defined based on drug prescriptions and the onset of type 1 diabetes was defined using specific medical codes indicating a diagnosis of type 1 diabetes. The incidence rate ratio of new‐onset type 1 diabetes within 180 days after an influenza diagnosis was calculated and it was compared with that at other times using Poisson regression and generalized estimating equations. Sensitivity analyses were performed to confirm the robustness of this finding.
Results
The data of 10,400 patients with new‐onset type 1 diabetes were analyzed, including 2,196 (952 male 1,244 female) patients diagnosed with influenza between 1 September 2014 and 31 August 2017. Although only patients with type 1 diabetes were included, adjusted analysis showed that individuals had a 1.3‐fold (95% confidence interval: 1.15–1.46) higher risk of developing type 1 diabetes in the first 180 days after influenza diagnosis than that at other times.
Conclusions
In this Japanese population‐based cohort, the risk of new‐onset type 1 diabetes may increase after the diagnosis of influenza. These results, which must be confirmed in other populations, suggest that influenza may be a causal factor for new‐onset type 1 diabetes. The molecular mechanisms underlying the potential etiological relationship between influenza and type 1 diabetes should be elucidated.
Keywords: Diabetes mellitus type 1, Influenza
In this Japanese population‐based cohort, the risk of new‐onset type 1 diabetes increased after being diagnosed with influenza. These results, which must be confirmed in other populations, suggest that influenza may be a causal factor for new‐onset type 1 diabetes. The molecular mechanisms underlying the potential etiological relationship between influenza and type 1 diabetes should be elucidated.

INTRODUCTION
Type 1 diabetes is usually mediated by immune mechanisms 1 Influenza A viruses have been shown to cause pancreatitis and diabetes using an animal model 2 Additionally, a previous study reported the development of fulminant type 1 diabetes after influenza B virus infection 3 The association between type 1 diabetes and influenza has been attracting attention over the past decade 4 , 5 , 6 , 7 In Norway, the incidence of new‐onset type 1 diabetes doubled after a national epidemic of influenza A (H1N1) 7 Although numerous environmental factors are known to trigger type 1 diabetes, the etiology of type 1 diabetes has not yet been elucidated 8 Adjustments for unknown confounding factors, such as the individual’s genetic background, must be considered in epidemiological studies of type 1 diabetes. No previous studies on the association between influenza and the development of type 1 diabetes were adjusted for unknown confounding factors.
The National Database of Health Insurance Claims and Specific Health Check‐ups of Japan (National Database) is a comprehensive database of health insurance claims covered by the Japanese National Health Insurance system. We have previously used the National Database to create retrospective sample cohorts of > 100 million individuals with a very small selection bias, and thus, good generalizability 9 The National Database contains health data on an extremely large population, which enables epidemiological studies of relatively rare conditions to be conducted with good statistical power. The National Database has been used to obtain real‐world health‐related evidence of global relevance 10 , 11 , 12 , 13 , 14
Using a large national database, this population‐based study aimed to determine whether there is an association between the onset of type 1 diabetes and a diagnosis of influenza. To this end, we used a self‐controlled case series method that enabled us to evaluate the effects of specific environmental factors on the onset of type 1 diabetes.
MATERIALS AND METHODS
Data source
Japan has a universal health coverage system, and the National Database includes all individuals using any type of insurance program that covers Japan’s 127 million citizens. The National Database provides the following information: personal identifier (ID0 variable 9 ), date, age group, sex, description of the procedures performed, diagnosis codes according to the International Classification of Diseases (ICD‐10), medical care received, medical examinations conducted (without the results of these examinations), and drugs prescribed. These data are independent of doctor and patient reports. Drug information includes the brand name, generic name, dosage, and the number of days prescribed.
Study design
The present study was a population‐based, retrospective cohort study conducted in circumstances of standard medical care using data from the National Database. The study cohort consisted of individuals recorded in the National Database. A self‐controlled case series method was used for the analysis.
Study population
In this study, we used data of individuals who were diagnosed with type 1 diabetes between 1 September 2014 and 31 August 2017. To guarantee that they were at risk during the period and to increase the reproducibility of our database study 15 , we excluded from our analysis patients who did not use health insurance between 1 April 2013 and 31 August 2014, and who did not use health insurance between 1 October 2017 and 31 March 2018. The primary exposure was a diagnosis of influenza. The outcome was the occurrence of new‐onset type 1 diabetes.
We designed the study cohort using data collected for all individuals between 1 April 2013 and 31 March 2018. We defined the individual’s age as the age at the last visit between 1 September 2017 and 31 March 2018.
Definition of type 1 diabetes (primary outcome)
Individuals who had already been prescribed a drug for diabetes between 1 April 2013 and 31 August 2014 were classified as having pre‐existing diabetes and were excluded from the analysis. Individuals who were diagnosed with type 1 diabetes and who were prescribed insulin and who were advised to self‐monitor their blood glucose from 1 September 2014 to 31 August 2017 were classified as cases of new‐onset type 1 diabetes and were included in the study 16 . Individuals with gestational diabetes had their onset date of type 1 diabetes defined differently because in women with gestational diabetes, insulin may be prescribed before the onset of type 1 diabetes. In women with diagnosis codes of both type 1 diabetes and gestational diabetes, the onset date of type 1 diabetes was defined as the first date on which a type 1 diabetes‐related diagnosis code was recorded. In women with a diagnosis code of type 1 diabetes without a diagnosis code of gestational diabetes, the onset date of type 1 diabetes was defined as the first date of being prescribed insulin. The codes used for the analysis are shown in Tables S1–S4.
Definition of influenza
In Japan, 11–12% of the population have at least one episode of influenza per year, and 85% of individuals with influenza are prescribed anti‐influenza drugs 17 We defined the onset of influenza as the date of prescription of an anti‐influenza drug, namely oseltamivir (medicine codes: 610443074, 610462002, 622638801, 622638901), laninamivir (medicine codes: 622012101, 622688601), zanamivir (medicine code: 660443018), and peramivir (medicine codes: 621972101, 621972102, 621972201, 621972202). Amantadine was not included in the definition because it is mainly used to treat Parkinson’s disease, and baloxavir (medicine codes: 622622501, 622622601) was not included in the definition because it had not yet been released at the time of the study. The definition did not include the prescription of anti‐influenza drugs for prophylaxis because these are not covered by national insurance in Japan. In Japan, anti‐influenza drug prescribing trends, estimated based on medical records, are similar to influenza incidence trends reported by medical institutions. Furthermore, the Pearson correlation coefficients of time series data were 0.972 and 0.992 in 2009–2010 and 2010–2011, respectively 18 Although evidence, such as the prescription ratio in Japan, is limited, it is clinically considered that most patients with confirmed influenza diagnosis were prescribed an anti‐influenza drug. Even with an unexpectedly low prescription ratio, as long as a certain number of prescriptions is available, this poses little challenge for the statistical adjustment.
Statistical analysis
Seasons were defined in 3 month blocks (September to November: fall; December to February: winter; March to May: spring; June to August: summer, according to the definition used by the Japanese Meteorological Agency).
We calculated the incidence rate ratio of the risk period after influenza diagnosis (180 days) relative to the other period (control period) using a self‐controlled case series method (generalized estimating equations, Poisson regression model) 19 , 20 (Figure S1) because the onset of type 1 diabetes (outcome) could only occur once in each individual. We defined the risk period as 180 days after a diagnosis of influenza. The definition of the risk period was based on the time difference between pancreatic insulitis in female non‐obese diabetic (NOD) mice and the onset of type 1 diabetes 21 , 22 The model accounted for influenza (exposure). Independent variables in the model included the years after observation (0, 1, or 2) and the seasonal categories (fall, winter, spring, summer), to adjust for the effect of time dependent variables, such as other seasonal infections.
Several types of sensitivity analyses were performed to assess the robustness of the results: Analysis 1 used multiple risk periods; Analysis 2 was stratified by anti‐influenza drug; Analysis 3 used varying lengths (days) of the risk period; Analysis 4 was stratified by age group; and Analysis 5 used varying definitions of exposure based on ICD‐10 codes. The different definitions of exposure based on ICD‐10 codes were grouped to form negative comparison groups. ICD‐10 codes I–XXII served as a neutral exposure group, except for code IV (diabetes and endocrine disease). Many of the diseases associated with ICD‐10 codes I–XXII are not directly related to type 1 diabetes, and thus, would not be expected to be associated with the onset of type 1 diabetes.
All statistical tests were two tailed, and P‐values < 0.05 were considered statistically significant. All statistical analyses were performed with the Microsoft SQL Server 2016 Standard (Microsoft Corp., Redmond, WA, USA) and IBM SPSS Statistics for Windows, version 25.0 (IBM, Armonk, NY, USA).
Ethical information
This study was approved by the Ethics Committee of Nara Medical University (1123‐5). The need for informed consent was waived because this study was based on a retrospective analysis of already anonymized, routinely collected data that do not allow for the identification of specific individuals and for which a correspondence table has not been created.
RESULTS
Number of individuals in the study cohort
There were 150,328,339 individuals enrolled in the National Database who used health insurance from April 2013 to March 2018. Of these patients, we excluded 30,661,464 individuals because they did not use medical insurance from 1 April 2013 to 31 August 2014. Further, we excluded 47,207,831 individuals because they did not use medical insurance from 1 September 2017 to 31 March 2018. Of the remaining 79,173,553 individuals, within the 3 year period from 1 September 2014 to 31 August 2017, 10,400 individuals who were diagnosed with type 1 diabetes were included in the study. Among these, there were 2,196 individuals who were diagnosed with influenza during the 3 year observation period (Figure 1).
Figure 1.
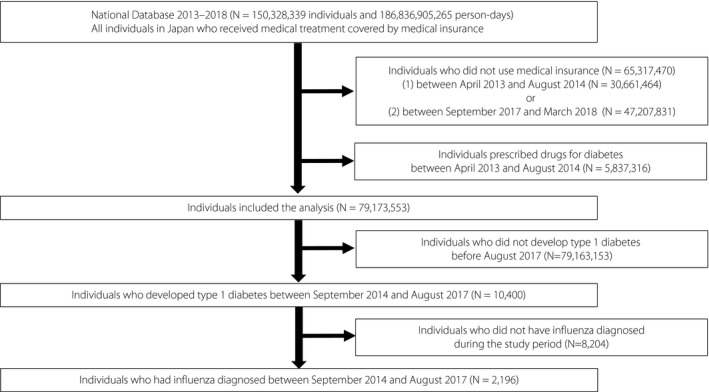
Flowchart showing the process for selecting individuals for inclusion in the study. Of 150,328,339 individuals in the National Database, 10,400 met the criteria for inclusion in the analysis and 2,196 were treated for influenza and developed type 1 diabetes during the follow‐up period.
Characteristics of individuals who were diagnosed with influenza and subsequently developed type 1 diabetes
Table 1 shows the age and sex of individuals who were diagnosed with influenza and subsequently developed type 1 diabetes. Of the 2,196 individuals included in the analysis, there were 952 male patients and 1,244 female patients, and 43.9% of the male patients and 45.6% of the female patients were aged < 20 years.
Table 1.
The age and sex of patients who developed both influenza and type 1 diabetes during the study period (n = 2,196)
| Age | Male | Female | ||
|---|---|---|---|---|
| Number | percentage | Number | percentage | |
| 00–04 | 24 | 2.5% | 22 | 1.8% |
| 05–09 | 120 | 12.6% | 185 | 14.9% |
| 10–14 | 147 | 15.4% | 227 | 18.2% |
| 15–19 | 127 | 13.3% | 133 | 10.7% |
| 20–24 | 38 | 4.0% | 39 | 3.1% |
| 25–29 | 26 | 2.7% | 46 | 3.7% |
| 30–34 | 55 | 5.8% | 78 | 6.3% |
| 35–39 | 61 | 6.4% | 78 | 6.3% |
| 40–44 | 87 | 9.1% | 96 | 7.7% |
| 45–49 | 79 | 8.3% | 69 | 5.5% |
| 50–54 | 51 | 5.4% | 67 | 5.4% |
| 55–59 | 41 | 4.3% | 54 | 4.3% |
| 60–64 | 22 | 2.3% | 53 | 4.3% |
| 65–69 | 31 | 3.3% | 36 | 2.9% |
| 70–74 | 20 | 2.1% | 22 | 1.8% |
| 75–79 | 13 | 1.4% | 23 | 1.8% |
| ≥ 80 | 10 | 1.1% | 16 | 1.3% |
| Total | 952 | 100.0% | 1244 | 100.0% |
Risk ratio of type 1 diabetes developing within 180 days after influenza diagnosis to that developing during the control period
In our study, 441 individuals developed type 1 diabetes within 180 days after an influenza diagnosis (2.4 cases/day), and 1,755 individuals developed diabetes outside the 180‐day period after influenza diagnosis (1.9 cases/day). Table 2 shows that individuals had a 1.30 times greater risk of developing type 1 diabetes during the risk period (≤ 180 days) after influenza diagnosis (95% confidence interval [CI]: 1.15–1.46) than that at other times, after adjusting for season and year of observation.
Table 2.
Risk ratio of developing type 1 diabetes within 180 days after influenza infection to developing type 1 diabetes within the control period (n = 10,400)
| Within 180 days | Risk ratio of type 1 diabetes | 95% confidence interval | P‐value | |
|---|---|---|---|---|
| 1.30 | 1.15 | 1.46 | <0.001 | |
| Control period | Reference | – | – | – |
| The first year of observation | 1.03 | 0.93 | 1.14 | 0.53 |
| The second year of observation | 0.98 | 0.89 | 1.09 | 0.75 |
| The third year of observation | Reference | – | – | – |
| Seasons | ||||
| Fall | 1.05 | 0.92 | 1.20 | 0.45 |
| Winter | 1.29 | 1.15 | 1.46 | <0.001 |
| Spring | 1.26 | 1.11 | 1.42 | <0.001 |
| Summer | Reference | – | – | – |
We analyzed the data of 10,400 patients with new‐onset type 1 diabetes, including 2,196 (952 male 1,244 female) patients diagnosed with influenza between 1 September 2014 and 31 August 2017.
Table 3 shows the results of the sensitivity analyses. Analysis 1 showed that there was no significant increase in the risk of developing type 1 diabetes from 1 to 180 days before influenza diagnosis or from 181 to 360 days after influenza diagnosis compared with 0–180 days after influenza diagnosis. Analysis 2 showed no difference in the increased risk of developing type 1 diabetes associated with specific types of anti‐influenza drugs, and the risk ratio of developing type 1 diabetes during the risk period was significantly higher than 1 for all drugs, except for peramivir. In Analysis 3, the risk ratio of type 1 diabetes development did not change during the risk period, even if the risk period was changed from 180 days to 90 days or 360 days. The risk ratio of type 1 diabetes seemed to have slightly reduced at 90 and 360 days compared with that at 180 days. Analysis 4, an analysis by age group, showed an increase in the incidence of type 1 diabetes after influenza diagnosis for the 0–19, 20–39, and 60–79 year age groups, but no significant association was found for the 40–59 and ≥ 80 year age groups. In Analysis 5, which considered other ICD‐10 codes as exposures, there were some significant differences, but the relative risk increases were within 11% (I–XXII).
Table 3.
Sensitivity analyses of the risk ratio of type 1 diabetes
| Analyses | Incidence rate ratios | 95% confidence intervals | P‐value | |
|---|---|---|---|---|
| Analysis 1: setting multiple risk periods (n = 10,400) | ||||
| Before influenza infection (within 180 days) [n = 2,196] | 1.05 | 0.90 | 1.22 | 0.515 |
| After influenza infection (000–180 days) [n = 2,196] | 1.30 | 1.16 | 1.46 | <0.001 |
| After influenza infection (181–360 days) [n = 2,196] | 1.05 | 0.92 | 1.20 | 0.484 |
| Other periods | Reference | ‐ | ‐ | ‐ |
| Analysis 2: by anti‐influenza drug (n = 10,400) | ||||
| All [n = 2,196: Table 2] | 1.30 | 1.15 | 1.46 | <0.001 |
| Oseltamivir [n = 904] | 1.44 | 1.20 | 1.73 | 0.000 |
| Laninamivir [n = 1,151] | 1.26 | 1.07 | 1.48 | 0.006 |
| Zanamivir [n = 499] | 1.39 | 1.05 | 1.82 | 0.020 |
| Peramivir [n = 120] | 1.30 | 0.81 | 2.08 | 0.280 |
| Analysis 3: Changing risk periods from 180 days (n = 10,400) | ||||
| 30 days [n = 2,196] | 0.90 | 0.58 | 1.42 | 0.660 |
| 60 days [n = 2,196] | 1.13 | 0.89 | 1.45 | 0.308 |
| 90 days [n = 2,196] | 1.19 | 1.01 | 1.41 | 0.037 |
| 180 days [n = 2,196: Table 2] | 1.30 | 1.15 | 1.46 | <0.001 |
| 360 days [n = 2,196] | 1.15 | 1.05 | 1.27 | 0.003 |
| Analysis 4: by age group (years) (n = 10,400) | ||||
| 00–19 [n = 985] | 1.44 | 1.21 | 1.71 | <0.001 |
| 20–39 [n = 421] | 1.31 | 1.00 | 1.72 | 0.049 |
| 40–59 [n = 544] | 1.05 | 0.82 | 1.35 | 0.695 |
| 60–79 [n = 220] | 1.50 | 1.03 | 2.16 | 0.033 |
| ≥80 [n = 26] | 0.38 | 0.08 | 1.80 | 0.226 |
| Analysis 5: Changing exposure, from influenza medication to others (ICD‐10 classification codes) (n = 10,400) | ||||
| I (A00–B99; [n = 7,014]) | 1.03 | 0.98 | 1.09 | 0.177 |
| II (C00–D48; [n = 6,029]) | 1.11 | 1.05 | 1.17 | <0.001 |
| III (D50–D89; [n = 3,674]) | 1.04 | 0.97 | 1.12 | 0.274 |
| V (F00–F99; [n = 1,752]) | 1.01 | 0.91 | 1.13 | 0.800 |
| VI (G00–G99; [n = 4,001]) | 1.06 | 0.99 | 1.14 | 0.091 |
| VII (H00–H59; [n = 8,706]) | 1.01 | 0.97 | 1.05 | 0.683 |
| VIII (H60–H95; [n = 2,290]) | 0.99 | 0.90 | 1.09 | 0.885 |
| IX (I00–I99; [n = 6,406]) | 1.09 | 1.03 | 1.15 | 0.003 |
| X (J00–J99; [n = 8,784]) | 1.04 | 0.99 | 1.09 | 0.085 |
| XI (K00–K93; [n = 8,327]) | 1.06 | 1.01 | 1.11 | 0.020 |
| XII (L00–L99; [n = 6,239]) | 1.06 | 1.00 | 1.12 | 0.038 |
| XIII (M00–M99; [n = 5,806]) | 1.04 | 0.98 | 1.10 | 0.160 |
| XIV (N00–N99; [n = 7,682]) | 1.07 | 1.01 | 1.12 | 0.012 |
| XV (O00–O99; [n = 427]) | 1.10 | 0.88 | 1.38 | 0.385 |
| XVI (P00–P96; [n = 65]) | 1.08 | 0.55 | 2.12 | 0.815 |
| XVII (Q00–Q99; [n = 682]) | 1.02 | 0.85 | 1.22 | 0.865 |
| XVIII (R00–R99; [n = 7,029]) | 1.08 | 1.02 | 1.13 | 0.006 |
| XIX (S00–T98; [n = 4,101]) | 1.04 | 0.97 | 1.13 | 0.271 |
| XX (V01–Y98; [n = 160]) | 0.99 | 0.67 | 1.47 | 0.975 |
| XXI (Z00–Z99; [n = 985]) | 1.08 | 0.93 | 1.26 | 0.326 |
| XXII (U00–U89; [n = 130]) | 1.10 | 0.70 | 1.74 | 0.673 |
(The number of all cases analyzed).
[The number of patients experienced both exposures and outcome].
DISCUSSION
We found that the risk of new‐onset type 1 diabetes was 30% higher during the 180 days after the diagnosis of influenza than at other times. No previous epidemiological study has shown a link between influenza and the onset of type 1 diabetes. The findings of this study contribute to elucidating one of the possible triggers of type 1 diabetes. Although the definitions of influenza and of type 1 diabetes are imperfect because this study used data from the claims database, the study design, that is, a self‐control case series, allowed for determining the association between type 1 diabetes and influenza in individuals for the first time.
It is not uncommon for islet‐associated antibodies to be present many years before the onset of type 1 diabetes 23 In addition, epidemiological adjustment is generally difficult in type 1 diabetes because both genetic and environmental factors contribute to its onset. However, using a self‐controlled case series method, we could control for unmeasured genetic factors and time independent environmental factors. This enabled us to assess whether influenza is associated with the incidence of type 1 diabetes. A previous study showed that influenza A virus causes pancreatitis in human pancreatic cells in an animal model 2 Our study showed that individuals had an increased risk of developing type 1 diabetes after influenza relative to other periods. Female NOD mice spontaneously developed isletitis from 4 weeks of age, and almost 80–100% were diabetic by 30 weeks of age 21 Diabetes in NOD mice has similarities to human type 1 diabetes because both types are characterized by isletitis at disease onset 22 Although our findings suggest that pancreatic insulitis was caused by influenza, leading to the development of type 1 diabetes over a few months, further research is required to conclusively prove this hypothesis.
In the sensitivity analysis shown in Table 3, the incidence of type 1 diabetes was not increased compared with that in the control period when the risk period was set within 30 days or within 60 days. Considering that NOD mice become diabetic by 30 weeks of age 21 we considered the 180 day risk period to be reasonable. However, in Analysis 4 shown in Table 3, the differences in association between influenza and type 1 diabetes depending on age cannot be ruled out.
Our results suggested that influenza may cause type 1 diabetes, as also seen in previous studies 2 , 3 , 7 Valdes et al. 7 also showed a two‐fold higher incidence of new‐onset diabetes in the subgroup younger than 30 years among the entire Norwegian population exposed to pandemic influenza A (H1N1), after adjusting for year of birth, sex, place of birth, and education (adjusted hazard ratio: 2.26, 95% CI 1.51–3.38). Although they could not demonstrate an association between influenza and the onset of type 1 diabetes, we showed a clear association between these diseases, with appropriate adjustment, including all time independent comparisons such as sex.
Our findings, combined with previous evidence that influenza vaccination reduces the risk of islet autoimmunity and type 1 diabetes 24 may support the international guidelines encouraging annual influenza vaccination 25 However, our results should not be interpreted as evidence on vaccine ineffectiveness: our study was not designed to evaluate the effectiveness of influenza vaccines, as it was not known whether individuals in the study cohort had received influenza vaccination.
There are some limitations of this study. First, we defined individuals with type 1 diabetes in the National Database as those associated with any of the type 1 diabetes diagnosis codes, who were prescribed medication for type 1 diabetes (insulin), and who had medical examination codes for self‐monitoring of blood glucose. We did not include patients with diabetes whose management did not include self‐monitoring of blood glucose. The Information Center for Specific Pediatric Chronic Diseases in Japan (https://www.ncchd.go.jp/en/center/activity/diseases/) reported that the number of individuals who developed type 1 diabetes between the ages of 0 and 15 years was 500–600 per year. This is consistent with the results of previous studies. According to Table S5, the number of individuals who developed type 1 diabetes between the ages of 0 and 19 years in our study was 1,895 in the 3 year period, which agreed well with the report of 500–600 children between the ages of 0 and 15 years with type 1 diabetes of the Information Center for Specific Pediatric Chronic Diseases in Japan. Thus, we consider that our cohort selection procedure was appropriate. Second, we defined influenza by the prescription of anti‐influenza drugs, rather than by laboratory confirmation of influenza virus infection. In Japan, 85% of all individuals diagnosed with influenza are currently prescribed anti‐influenza drugs 17 and thus, our definition was considered appropriate 18 However, individuals with influenza who were not prescribed anti‐influenza drugs or who were prescribed anti‐influenza drugs before the observation period would not have been included in this study. Although these effects may bias the results, the impact of individuals with influenza not being prescribed anti‐influenza drugs or being prescribed anti‐influenza drugs before the observation period, would bias the association between influenza and type 1 diabetes toward null and the risk ratio toward 1. Third, the National Database did not include any laboratory data. Therefore, we could not confirm the blood glucose level, hemoglobin A1c level, or levels of any types of antibodies, such as the anti‐glutamic acid decarboxylase antibody, in cases of type 1 diabetes. We defined type 1 diabetes in the study dataset using certain codes as an alternative indicator, and the prevalence of type 1 diabetes among children was similar to that previously reported. Fourth, the self‐controlled case series method only assessed patients who developed type 1 diabetes; such approaches cannot control time dependent confounding factors unless they are included in the regression model. Although we made all possible adjustments for all patients with type 1 diabetes who were recorded in the National Database, unmeasured time dependent confounding factors could have affected the results. Fifth, our study did not include data of patients who did not use medical insurance between 1 April 2013 and 31 August 2014, to increase the reproducibility of our database study. Though comparability is not affected because this study comprises intra‐individual comparisons, generalization of these findings, especially for those who have not used insurance for a certain period of time, should be exercised with caution. Finally, we could not review the medical records of each individual in detail, and thus, were unable to consider body weight, smoking history, and family history in the analysis. Using a self‐controlled case series study design, it was possible to adjust for time dependent confounding variables, including unmeasured confounding variables; however, as this was an observational study, adjustment for confounding factors may not have been complete. Thus, it is possible that certain diseases that might have increased within the 180 days after influenza infection, might exist as confounding factors.
In conclusion, we found that the onset of type 1 diabetes within 180 days after an influenza diagnosis was 30% higher than outside of this period. This is an important epidemiological discovery that can contribute to determining the potential etiology of type 1 diabetes. The molecular mechanisms underlying the etiological relationship between influenza and type 1 diabetes require further research.
DISCLOSURE
Dr Nishioka reports receiving consultant fees from Novo Nordisk; Dr Okada reports receiving lecturer’s fees from Novo Nordisk, Mitsubishi Tanabe, Sumitomo Dainippon, MSD, Bayer, Eli Lilly, Boehringer Ingelheim, Ono, AstraZeneca, Sanofi, Takeda, and ARKRAY; Dr Ishii reports receiving lecture fees and consultant fees from Takeda, Eli Lilly Japan, Sanofi, Merck & Co., Astellas, Mitsubishi Tanabe, Daiichi Sankyo, Ono, AstraZeneca, Taisho Toyama, Shionogi, Kowa, Boehringer Ingelheim, Novo Nordisk, Sumitomo Dainippon, and Kyowa Hakko Kirin. No other potential conflicts of interest relevant to this article are reported.
Supporting information
Figure S1 | A self‐controlled case series method.
Table S1 | Diagnosis codes related to type 1 diabetes.
Table S2 | Diagnosis codes related to gestational diabetes patients.
Table S3 | Medical examinations codes related to the self‐monitoring of blood glucose by type 1 diabetes patients.
Table S4 | The medicine codes for insulin.
Table S5 | The number of patients who were suffering from type 1 diabetes, by sex and age.
Table S6 | The number of the populations at risk by sex and age.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.com) for English language editing. This study was supported by the Japanese Society for the Promotion of Science KAKENHI (grant numbers JP18K17390 and 18H04126).
J Diabetes Investig. 2021; 12: 1797–1804
REFERENCES
- 1. Kerner W, Bruckel J. German Diabetes Association Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes 2014; 122: 384–386. [DOI] [PubMed] [Google Scholar]
- 2. Capua I, Mercalli A, Pizzuto MS, et al. Influenza A viruses grow in human pancreatic cells and cause pancreatitis and diabetes in an animal model. J Virol 2013; 87: 597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sano H, Terasaki J, Tsutsumi C, et al. A case of fulminant type 1 diabetes mellitus after influenza B infection. Diabetes Res Clin Pract 2008; 79: e8–e9. [DOI] [PubMed] [Google Scholar]
- 4. Ruiz PL, Tapia G, Bakken IJ, et al. Pandemic influenza and subsequent risk of type 1 diabetes: a nationwide cohort study. Diabetologia 2018; 61: 1996–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patterson CC, Harjutsalo V, Rosenbauer J, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989–2013: a multicentre prospective registration study. Diabetologia 2019; 62: 408–417. [DOI] [PubMed] [Google Scholar]
- 6. Nenna R, Papoff P, Moretti C, et al. Detection of respiratory viruses in the 2009 winter season in Rome: 2009 influenza A (H1N1) complications in children and concomitant type 1 diabetes onset. Int J Immunopathol Pharmacol 2011; 24: 651–659. [DOI] [PubMed] [Google Scholar]
- 7. Valdes C, Unanue N, Hernandez M, et al. Is there a link between influenza and type I diabetes? Increased incidence of TID during the pandemic H1N1 influenza of 2009 in Chile. Pediatr Endocrinol Rev 2013; 11: 161–166. [PubMed] [Google Scholar]
- 8. Gale EA. The rise of childhood type 1 diabetes in the 20th century. Diabetes 2002; 51: 3353–3361. [DOI] [PubMed] [Google Scholar]
- 9. Shinichiro K, Tatsuya N, Tomoya M, et al. National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB): Outline and patient‐matching technique. bioRxiv 2018. 10.1101/280008 [DOI] [Google Scholar]
- 10. Nishioka Y, Okada S, Noda T, et al. Absolute risk of acute coronary syndrome after severe hypoglycemia: a population‐based 2‐year cohort study using the National Database in Japan. J Diabetes Investig 2020; 11: 426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sugiyama T, Imai K, Ihana‐Sugiyama N, et al. Variation in process quality measures of diabetes care by region and institution in Japan during 2015–2016: an observational study of nationwide claims data. Diabetes Res Clin Pract 2019; 155: 107750. [DOI] [PubMed] [Google Scholar]
- 12. Hayashi S, Noda T, Kubo S, et al. Data regarding fracture incidence according to fracture site, month, and age group obtained from the large public health insurance claim database in Japan. Data Brief 2019; 23: 103780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayashi S, Noda T, Kubo S, et al. Variation in fracture risk by season and weather: a comprehensive analysis across age and fracture site using a National Database of Health Insurance Claims in Japan. Bone 2019; 120: 512–518. [DOI] [PubMed] [Google Scholar]
- 14. Okumura Y, Sugiyama N, Noda T, et al. Psychiatric admissions and length of stay during fiscal years 2014 and 2015 in Japan: a retrospective cohort study using a nationwide claims database. J Epidemiol 2019; 29: 288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang SV, Schneeweiss S, Berger ML, et al. Reporting to improve reproducibility and facilitate validity assessment for healthcare database studies V1. 0. Value Health 2017; 20: 1009–1022. [DOI] [PubMed] [Google Scholar]
- 16. Nishioka Y, Noda T, Okada S, et al. Incidence and seasonality of type 1 diabetes: a population‐based 3‐year cohort study using the National Database in Japan. BMJ Open Diabetes Res Care 2020; 8: e001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fujibayashi K, Takahashi H, Tanei M, et al. A new influenza‐tracking smartphone app (flu‐report) based on a self‐administered questionnaire: cross‐sectional study. JMIR MHealth UHealth 2018; 6: e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sugawara T, Ohkusa Y, Ibuka Y, et al. Real‐time prescription surveillance and its application to monitoring seasonal influenza activity in Japan. J Med Internet Res 2012; 14: e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Whitaker HJ, Paddy Farrington C, Spiessens B, et al. Tutorial in biostatistics: the self‐controlled case series method. Stat Med 2006; 25: 1768–1797. [DOI] [PubMed] [Google Scholar]
- 20. Escolano S, Hill C, Tubert‐Bitter P. A new self‐controlled case series method for analyzing spontaneous reports of adverse events after vaccination. Am J Epidemiol 2013; 178: 1496–1504. [DOI] [PubMed] [Google Scholar]
- 21. Kikutani H, Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol 1992; 51: 285–322. [DOI] [PubMed] [Google Scholar]
- 22. Pearson JA, Wong FS. Wen L The importance of the non obese diabetic (NOD) mouse model in autoimmune diabetes. J Autoimmun 2016; 66: 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hummel M, Bonifacio E, Schmid S, et al. Brief communication: early appearance of islet autoantibodies predicts childhood type 1 diabetes in offspring of diabetic parents. Ann Intern Med 2004; 140: 882–886. [DOI] [PubMed] [Google Scholar]
- 24. Larsson HE, Lynch KF, Lönnrot M, et al. Pandemrix® vaccination is not associated with increased risk of islet autoimmunity or type 1 diabetes in the TEDDY study children. Diabetologia 2018; 61: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grohskopf LA, Olsen SJ, Sokolow LZ, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep 2014; 63: 691–697. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | A self‐controlled case series method.
Table S1 | Diagnosis codes related to type 1 diabetes.
Table S2 | Diagnosis codes related to gestational diabetes patients.
Table S3 | Medical examinations codes related to the self‐monitoring of blood glucose by type 1 diabetes patients.
Table S4 | The medicine codes for insulin.
Table S5 | The number of patients who were suffering from type 1 diabetes, by sex and age.
Table S6 | The number of the populations at risk by sex and age.


