Abstract
Optoacoustic (photoacoustic) imaging has demonstrated versatile applications in biomedical research, visualizing the disease pathophysiology and monitoring the treatment effect in an animal model, as well as toward applications in the clinical setting. Given the complex disease mechanism, multimodal imaging provides important etiological insights with different molecular, structural, and functional readouts in vivo. Various multimodal optoacoustic molecular imaging approaches have been applied in preclinical brain imaging studies, including optoacoustic/fluorescence imaging, optoacoustic imaging/magnetic resonance imaging (MRI), optoacoustic imaging/MRI/Raman, optoacoustic imaging/positron emission tomography, and optoacoustic/computed tomography. There is a rapid development in molecular imaging contrast agents employing a multimodal imaging strategy for pathological targets involved in brain diseases. Many chemical dyes for optoacoustic imaging have fluorescence properties and have been applied in hybrid optoacoustic/fluorescence imaging. Nanoparticles are widely used as hybrid contrast agents for their capability to incorporate different imaging components, tunable spectrum, and photostability. In this review, we summarize contrast agents including chemical dyes and nanoparticles applied in multimodal optoacoustic brain imaging integrated with other modalities in small animals, and provide outlook for further research.
Keywords: optoacoustic (photoacoustic) imaging, animal model, brain imaging, multimodal imaging, contrast agent, fluorescence imaging, nanoparticle, magnetic resonance imaging
Introduction
Optoacoustic Imaging
The multimodal imaging strategy across different scales has gained huge interest in recent years. The advances in neuroimaging such as positron emission tomography (PET) and magnetic resonance imaging (MRI) have provided valuable tools for understanding brain function, for early and differential diagnosis of brain disorders and for monitoring treatment effect (Fox and Raichle, 2007; Langen et al., 2017; Hansson, 2021; Kreisl et al., 2021). Optoacoustic (photoacoustic; OA) imaging is an emerging imaging tool and has demonstrated versatile applications in biomedical research (Deán-Ben et al., 2016; Knieling et al., 2017; Masthoff et al., 2018; Neuschmelting et al., 2018; Gottschalk et al., 2019; Qian et al., 2019; Karlas et al., 2021; Na et al., 2021; Razansky et al., 2021). OA imaging utilizes absorption of light as a source of contrast, while the emitted ultrasound (US) is used for image formation (Razansky et al., 2009; Wang and Yao, 2016). As the spatial resolution of OA imaging is not changed by photon scattering, it thus exhibits a unique combination of high sensitivity and high spatial resolution. The detection depth of OA ranges from millimeters to centimeters, which associates with spatial resolution (from <1 μm in OA microscopy to 100 μm in OA tomography) (Li et al., 2017; Zhang et al., 2019a; Li et al., 2020). Recent OA tomography has allowed imaging the whole mouse brain with <100 µm spatial resolution in vivo (Deán-Ben et al., 2016; Vaas et al., 2017; Gottschalk et al., 2019; Ni et al., 2020a; Ni et al., 2021) which is around 10 times higher than the resolution achievable by using commercial small-animal microPET scanners (Lancelot and Zimmer, 2010).
Multimodal Optoacoustic Brain Imaging
Assessing the brain function under whole brain complex network dynamics is the key for understanding physiology of the brain and deciphering brain disorders. While PET provides excellent accuracy in quantification, the limited spatial resolution (approximately 1 mm) relative to the small mouse brain and the signal spillover hinders accurate mapping of the target (Lancelot and Zimmer, 2010). Optical two-photon microscopy offers excellent spatial resolution, however limited by depth penetration and small (sub-mm) field-of-view (Ntziachristos, 2010). Macroscopic imaging with MRI provides high resolution however has the limitations in sensitivity and temporal resolution (Razansky et al., 2021). The use of hybrid contrast agents in multimodal imaging has enabled to detect the targets with different sensitivity and provide comprehensive molecular information, as well as better soft tissue contrast, facilitating accurate quantification (e.g., combined with MRI and computed tomography (CT) (Ren et al., 2019). Various multimodal OA molecular imaging techniques have been applied in preclinical brain imaging studies, including OA/fluorescence imaging, OA/MRI, OA/US, OA/MRI/Raman, OA/PET, OA/single-photon emission computerized tomography (SPECT), and OA/CT. (Kircher et al., 2012; Fan et al., 2014; Zhu et al., 2015; Qiao et al., 2018) (Table 1).
TABLE 1.
Summary of hybrid contrast agents for multimodal optoacoustic brain imaging.
| Modality | Contrast agent | Abs | Target |
|---|---|---|---|
| OA/FL | AOI987 Ni et al. (2020a) | 650 | Amyloid-β, AD |
| CRANAD-2 Ni et al. (2021) | 640 | ||
| Congo red Zhou et al. (2021) | 500 | ||
| PBB5 Vagenknecht et al. (2021) | 635 | Tau, AD, FTD | |
| CDnir7 Park et al. (2019) | 806 | Microglia, macrophage, AD | |
| IRDye 800CW–conjugated CAIX-800 Huang et al. (2020) | 800 | Hypoxia, nasopharyngeal tumor | |
| MMPsense Ni et al. (2018a) | 680 | MMP, stroke | |
| Cu2-xSe NPs Zhang et al. (2019b) | NIR II | ROS, glioblastoma | |
| Conjugated polymer NP Guo et al. (2017) | NIR II | Glioblastoma | |
| Semiconducting polymer NP Yang et al. (2019) | NIR II | ||
| Cu2-xSe NPs, DOX‐HCu Wu M. et al., (2018) | 808 | ||
| Single-layer MoS2 Nanosheets Chen et al. (2016) | 675 | ||
| P1RGD NP Guo et al. (2018) | NIR II | ||
| PBT NP Guo et al. (2019a) | NIR II | ||
| PTD NP Guo et al. (2019b) | NIR II | ||
| IRDye800-H-ferritin NP Jia et al. (2020) | 800 | ||
| Aggregation-induced emission dots Sheng et al. (2018) | NIR II | ||
| CR780RGD-NPs Liu et al. (2021b) | 780 | ||
| ICG/AuNR@BCNP Yang et al. (2020) | 808 | ||
| ICG-holo-transferrin NP Zhu et al. (2017) | 780 | ||
| SPN-OT, SPN-PT, and SPN-DT Jiang et al. (2019) | NIR II | Metabolizable | |
| QC-1/BSA/BODIPY Cardinell et al. (2021) | 750 | Lymphatic drainage | |
| Prussian blue particle–labeled MSC Li et al. (2018a) | 701 | Brain injury | |
| Polymer-blend dot-chlorotoxin Wu et al. (2011) | 488 | Medulloblastoma | |
| OA/SPECT | CPMSN@[125I]SD Yao et al. (2020) | 680 | MSC, stroke |
| OA/SPECT/FL | [131I]A1094@RGD-HBc Liu et al. (2019a) | NIR II | Glioblastoma |
| [99mTc]UCS Zhang et al. (2018a) | 633 | Blood–brain barrier | |
| OA/PET/FL | [18F]CDA-3 Liu et al. (2017) | 798 | Amyloid-β, AD |
| OA/PET | [64Cu]RGD-Au-tripod Cheng et al. (2014) | 710 | Glioblastoma |
| OA/PET/MRTI | [64Cu]c(KRGDf)-PEG-HAuNS Lu et al. (2011) | 800 | |
| OA/PET/MRI/FL | IRDye78-α-LA-DFO-[89Zr] Yang et al. (2021) | 770 | |
| OA/MRI | Prussian blue–poly(l-lysine) NP Kim et al. (2017) | 715 | MSC |
| Prussian blue nanocubes (PBNCs) Kubelick and Emelianov, (2020) | 734 | MSC, spinal cord | |
| Magneto-plasmonic MNP@Au nanostars Tomitaka et al. (2020) | 710 | Drug delivery | |
| gM-Luc-GRMNBs Chen et al. (2015) | 810 | MSC, stroke | |
| SPIO@Au-labeled MSC Qiao et al. (2018) | 810 | MSC | |
| Gd-PEG-polypyrrole NPs Liang et al. (2015) | 808 | Glioblastoma | |
| cRGD-CM-CPIO Duan et al. (2020a) | 730 | ||
| HALF-cRGD Duan et al. (2020b) | 685 | ||
| Mn2+-doped Prussian blue Zhu et al. (2015) | 808 | ||
| OA/CT/MRI | Core-shell Au nanorod@metal-organic NP Shang et al. (2017) | 720 | |
| OA/SWIR/CT/UCL | NaErF4:Tm@NaYF4:Yb@NaLuF4:Nd,Yb-ZnPc Lv et al. (2019) | 808 | |
| OA/US | PDI NP Fan et al. (2015) | 700 | |
| OA/Raman | SERRS-MSOT-nanostar Neuschmelting et al. (2018) | 770 | |
| OA/MRI/Raman | Maleimide-DOTA-Gd @Au-silica–based SERS Kircher et al. (2012) | 540 |
Abs, absorbance; AD, Alzheimer’s disease; Au, gold; CT, computed tomography; FL, fluorescence imaging; FTD, frontotemporal dementia; Gd, gadolinium; MRI, magnetic resonance imaging; MRTI, magnetic resonance thermal imaging; MSC, mesenchymal stem cells; MMP, matrix metalloproteinases; NP, nanoparticle; OA, optoacoustic imaging; PDI, perylene-diimide; PEG, polyethylene glycol; PET, positron emission tomography; ROS, reactive oxygen species; SPECT, single-photon emission computed tomography; SPIO, superparamagnetic iron oxide; SWIR, short-wavelength infrared; US, ultrasound imaging; UCL, upconversion luminescence;
Hybrid Contrast Agents for Multimodal OA Brain Imaging
The contrast of OA imaging comes from endogenous tissue contrasts or chromophores (e.g., oxyhemoglobin (HbO)/deoxyhemoglobin (Hb), melanin, and lipids), as well as from the administrated spectrally distinctive exogenous contrast agents (Weber et al., 2016). The majority of preclinical OA molecular imaging in the brain has been focused on detecting the pathological changes in a glioblastoma model, and applications have also emerged in animal models of stroke, epilepsy, Alzheimer’s disease (AD), and neuroinflammation (Ni et al., 2017; Xi et al., 2017; Ni et al., 2018a; Ni et al., 2018b; Ishikawa et al., 2018; Ni et al., 2020a; Kasten et al., 2020; Razansky et al., 2021). Different types of exogenous contrast agents have been developed, including synthetic (chemical dyes or nanoparticles (NPs)), semi-genetic, and genetic contrast agents (e.g., genetically encoded calcium indicators and reversibly switchable OA proteins (Roberts et al., 2018; Qian et al., 2019; Mishra et al., 2020; Farhadi et al., 2021; Qu et al., 2021; Shemetov et al., 2021)). The criteria for contrast agent applied in OA brain imaging include a suitable absorbance spectrum (>600 nm wavelength) to allow unmixing with endogenous signals (e.g., Hb/HbO and melanin) and sufficient brain penetration depth, high affinity and specific binding to the target, sufficient blood–brain barrier entrance, photostability, solubility, low toxicity, high thermodynamics for MRI probes, and optimal pharmacokinetics (Weber et al., 2016). Chemical dyes are mainly used for OA/fluorescence imaging and have the advantage of low toxicity, sufficient blood–brain barrier entrance due to the small molecular weight, fast metabolism, and clearance; however, they have limited adjustment potential. The NPs utilized for OA imaging are mainly carbon-based NPs, for example, single-walled carbon nanotubes; metal-based NPs, for example, gold NPs; bismuth-based NPs; polymer-encapsulated organic NPs; semiconducting polymer NPs (SPNs); conjugated polymer; and novel DNA-based nanocarriers. (Pu et al., 2014; Li and Chen, 2015; Weber et al., 2016; Yang et al., 2018; Yu et al., 2019; Zhan et al., 2019; Xu et al., 2020; Cheng et al., 2021; Fan et al., 2021; Joseph et al., 2021; Qi et al., 2021a; Tuo et al., 2021; Wang et al., 2021a; Wang et al., 2021b; Zhen et al., 2021). NPs have the advantage of versatile multimodal imaging capacity, a favorable signal/noise ratio, high photothermal conversion, deep penetration depth with near-infrared (NIR) II probes, and diverse structure and types (activable, turnable, and metabolizable). However, the stability, biodegradability, biocompatibility, clearance toxicity, nanostructural control, and blood–brain barrier entrance of NPs require careful designing (Liu et al., 2021a).
OA/Fluorescence Imaging
Chemical Dyes
Many chemical dyes for OA imaging have fluorescence properties and have been widely used in hybrid OA/fluorescence imaging (Li et al., 2021a); the OA/fluorescence dye ideally has a distinct absorption peak and a relatively low quantum yield to allow OA detection, for example, IRDye 800CW (Attia et al., 2016) and naphthalocyanine (Bézière and Ntziachristos, 2015), indocyanine green (ICG) (Mokrousov et al., 2021), and Prussian blue. Administration of ICG visualizes blood vessels and enables OA/fluorescence imaging of cerebral perfusion in glioblastoma mouse models (Burton et al., 2013). Neuroinflammation and glial activation–related molecular changes are implicated in many brain disorders, such as stroke, multiple sclerosis, and AD (Leng and Edison, 2021; McAlpine et al., 2021). The change in the levels of endogenous oxygen saturation (calculated based on hemoglobin readouts) has been used as an indicator for neuroinflammation in rats with stereotaxic injection of lipopolysaccharides (LPS) (Guevara et al., 2013). Targeted probes for molecular changes including matrix metalloproteinases and nitric oxide production have been employed for visualizing neuroinflammation in animal models (McQuade et al., 2010; Qi et al., 2021b). Upregulated levels of matrix metalloproteinases (MMPs) were detected using an MMPsense probe (e.g., 680 nm) with OA/fluorescence imaging approaches in the cerebral ischemic lesion region of a mouse model at 48 h after transient middle cerebral artery occlusion (Ni et al., 2018a). In addition, recent OA/fluorescence imaging studies reported using NIR cyanine derivative CDnir7 to detect microglia and astroglia activation in the brain of triple transgenic AD mice (Park et al., 2019). CDnir7 has previously been utilized to detect macrophage uptake in the peripheral organs using fluorescence molecular tomography and OA tomography (Kang et al., 2014).
The cerebral accumulation and spreading of proteiopathies are central to neurodegenerative diseases including AD and Parkinson’s disease. Previous studies have utilized two-photon imaging and near-infrared imaging with probes BF-158, BODIPY derivative, HS-84, HS-169, methoxy-X04, and fluorescent-labeled antibodies (Krishnaswamy et al., 2014; Kuchibhotla et al., 2014; Verwilst et al., 2017; Wu Q. et al., 2018; Calvo-Rodriguez et al., 2019; Detrez et al., 2019; Voigt et al., 2019; Zhou et al., 2019; Fung et al., 2020; Ni et al., 2020b) for amyloid-β and tau detection at cellular resolution in animal models. Several studies have employed β-sheet binding OA/fluorescence hybrid dyes with an NIR range absorbance spectrum peak for in vivo imaging of the proteinopathy accumulation in the brain. OA tomography using oxazine derivative AOI987 has been shown to provide transcranial visualization of the bio-distribution of amyloid-β deposits in mouse models of AD amyloidosis (arcAβ and APP/PS1 model) (Ni et al., 2020a). A similar design using OA tomography with curcumin derivative CRANAD-2 in has been used in an arcAβ mouse model (Ni et al., 2021). OA microscopy with Congo red has been used for the detection of amyloid-β plaques and cerebral amyloid angiopathy in the APP/PS1 mouse model (Hu et al., 2009; Zhou et al., 2021). OA tomography with chemical dye PBB5 (PBB3 derivative) for detection of β-sheet–containing tau deposits in the P301L 4-repeat tau mouse model has been reported (Ono et al., 2017; Vagenknecht et al., 2021). It is foreseeable that OA tomography pipeline with the deep brain region detection capability will be applied together with OA/fluorescence β-sheet–binding dyes to image other proteiopathy disease models, such as Parkinson’s disease mouse model with α-synuclein accumulation and the amyotrophic lateral sclerosis animal model with TAR DNA-binding protein 43 deposits.
Nanoparticles
NPs are widely used as hybrid contrast agents for their capability to incorporate different imaging components, tunable spectrum, and photostability. OA/fluorescence imaging has been reported for monitoring lymphatic drainage using QC-1/bovine serum albumin/BODIPY (Cardinell et al., 2021) and brain injury with Prussian blue particle–labeled mesenchymal stem cells (Li et al., 2018a). Many OA/fluorescence NPs are targeted toward integrin α(v)β(3) which is overexpressed in endothelial cells in the glioblastoma mouse model. Near-infrared (NIR) I range NPs in glioblastoma imaging include quantum dots (Zhao et al., 2020), gold NPs (Lu et al., 2011; Kircher et al., 2012; Lozano et al., 2012; Shang et al., 2017), copper/iron-based NPs (Wu M. et al., 2018; Zhou et al., 2018; Zhang et al., 2019b), carbon nanorods (Pramanik et al., 2009; Qian et al., 2018), MoS2 nanosheets (Chen et al., 2016; Guo et al., 2017), semiconducting polymeric NPs (Yang et al., 2019), nanodot–chlorotoxin conjugates (Wu et al., 2011), polymer-encapsulated organic NPs (Li and Liu, 2014), ICG-holo-transferrin NPs (Zhu et al., 2017), and liposomes (Miranda et al., 2019). The circulating dyes and NPs accumulate in brain tumors due to a disruption of the blood–brain barrier (Kircher et al., 2012; Burton et al., 2013; Neuschmelting et al., 2018) or enhanced permeability and retention effect (Li et al., 2018b). To enhance the brain uptake and OA signal, one strategy is to load the chemical dyes into NPs, for example, a recent study utilized CR780RGD-NPs, formed by conjugating the croconaine dye, NH2–polyethylene glycol (PEG) 2000-MAL, and the cancer-targeting c(RGDyC) peptide, to detect the tumor in the deep brain region in a glioblastoma mouse model (Liu et al., 2021b). Another strategy is to use activable hybrid OA/fluorescence probes for detection with higher specificity. The activable probes that have been reported mainly target at tumor-related hypoxia, glutathione, pH changes, and reactive oxygen species (Liu et al., 2021c). Hypoxia plays an important role in tumor metastasis and resistance to chemoradiotherapy and has been an important target for tumor imaging (Rankin and Giaccia, 2016). IRDye800-H-ferritin nanocarrier (IRDye800-HFn) (Jia et al., 2020) was applied in imaging hypoxia in glioma, and albumin-based gold (Au) NP, ICG/AuNR@BCNP, was used as theranostics for glioma- and hypoxia-alleviating treatment (Yang et al., 2020). In addition, an IRDye 800CW–conjugated probe CAIX-800 in imaging changes in carbonic anhydrase IX (CAIX) in nasopharyngeal carcinomas in a mouse model has been reported with excellent signal/noise ratios (Huang et al., 2020).
In the NIR I range, light scattering, hemoglobin absorbance, and skull attenuation interferes in the signal/noise ratio, unmixing, and penetration depth in the small animal brain (Wan et al., 2018; Liang et al., 2019). The skull attenuation positively associates with increasing age, which makes imaging in aged disease animal models difficult. Efforts are thus made to develop NIR II (>1,000 nm) hybrid OA/fluorescence probes (He et al., 2018; Huang and Pu, 2020; Dai et al., 2021). Several NIR II range NPs with excellent photothermal conversion efficiency have been applied for in vivo glioblastoma imaging in the mouse brain, for example, Cu2-xSe NPs for detecting reactive oxygen species (Zhang et al., 2019b), aggregation-induced emission dots A1094@RGD-HBc (Sheng et al., 2018), excitable semiconducting polymer NPs (Yang et al., 2019), and P1RGD NP conjugated polymers. (Guo et al., 2018). Jiang et al. (2019) reported metabolizable NIR II SPN for mouse brain imaging such as SPN-OT, SPN-PT, or SPN-DT of high photothermal conversion efficiencies and effective clearance with minimum toxicity.
OA/Positron Emission Tomography; OA/Single-Photon Emission Computerized Tomography
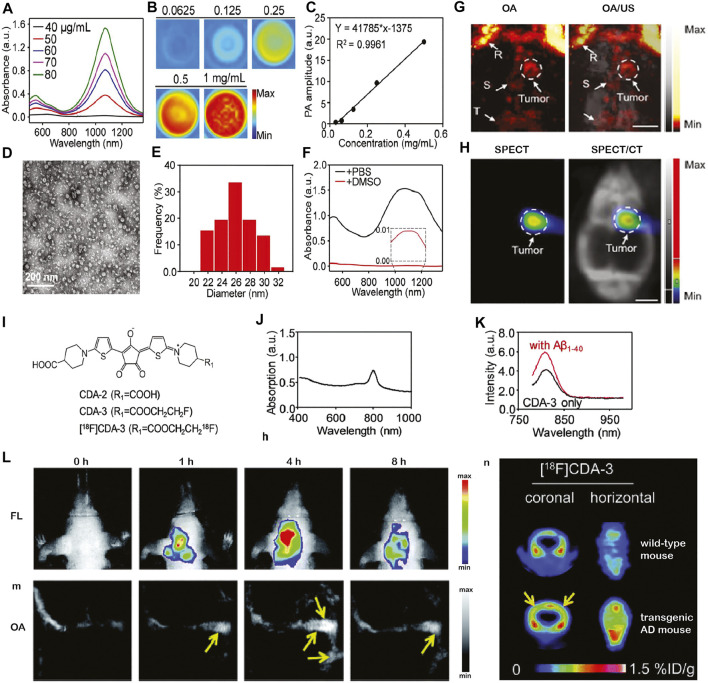
Signal spillover is a known issue in small animal PET mainly due to the size of the small animal brain (Lancelot and Zimmer, 2010). The rational for integrating OA and PET/SPECT imaging is that OA imaging provides a higher resolution tomographic/or microscopic imaging, while PET/SPECT provides higher detection sensitivity. Several studies reported using single-walled carbon nanotubes at NIR II conjugated probes that target integrin for OA/PET or OA/SPECT tumor imaging in animal models, such as [64Cu]RGD-Au-tripod for OA/PET (Cheng et al., 2014) and [131I]A1094@RGD-HBc for OA/SPECT/CT in an U87MG tumor‐bearing glioblastoma mouse model (Liu et al., 2019a) (Figures 1A–H). The OA data correlated well with PET data as well as SPECT data in both studies. In addition to imaging brain tumor in animal models, OA/PET and OA/SPECT applications in AD and in the stroke mouse model as theragnostic agents have been reported (Liu et al., 2017; Yao et al., 2020). OA/PET/fluorescence triple-modality imaging of brain amyloid-β plaques has been demonstrated using functionalized croconium dye [18F]CDA-3 (Liu et al., 2017), showing cortical accumulation of amyloid-β deposits in mice with AD amyloidosis (Figures 1I–K). OA/SPECT imaging using CPMSN@[125I]SD, formed by cobalt protoporphyrin IX–loaded mesoporous silica NPs labeled with [125I]-conjugated/spermine-modified dextran polymer, was reported for tracking mesenchymal stem cells, exerting antioxidant effects, and improving the recovery in a mouse model of cerebral ischemia (Yao et al., 2020). Moreover, biodegradable ultrasmall Cu2–xSe NPs (diameter 3.0 nm) labeled with [99mTc] has been demonstrated to monitor the opening and recovery of the blood–brain barrier induced by focused ultrasound using OA/SPECT/CT triple-modality imaging in the mouse model (Zhang et al., 2018a).
FIGURE 1.
In vivo multimodal optoacoustic (OA) brain imaging in small disease animal models. (A–H) Multimodal optoacoustic (OA) imaging in the brain of U87MG tumor-bearing mice using [131I]A1094@RGD‐HBc. (A–F) Characterization of the A1094@RGD‐HBc; (A) absorption spectra of A1094; (B) OA images of A1094 at different concentrations at 950 nm in DMSO; and (C) relation between a OA signal and A1094 concentrations in DMSO. (D) Transmission electron microscope of A1094@RGD-HBc; (E) dynamic light scattering of A1094@RGD-HBc; (F) absorption spectra of A1094@RGD-HBc before/after 90% DMSO destroying the protein; (G) In vivo OA tomography/ultrasound images, and (H) microSPECT/CT images of the brain of U87MG tumor‐bearing mice at 2 h after injection of [131I]A1094@RGD‐HBc; Scale bar = 2 mm. R, rostral rhinal vein; S, sagittal sinus; T, transverse sinus; reproduced from ref. Liu et al. (2019a) with permission from the WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim; (I–N) multimodal imaging of cerebral amyloid-β plaque in an Alzheimer’s disease mouse model. (I) Chemical structure of [18F]CDA-3; (J) UV-vis absorption curve of CDA-3; (K) fluorescence intensity of CDA-3 with/without Aβ1–40 aggregates; (L–N) in vivo OA tomography/positron emission tomography/near-infrared fluorescence imaging of brain amyloid-beta plaque detection in the Alzheimer amyloidosis mouse model. Reproduced from ref. Liu et al. (2017) with permission from the Royal Society of Chemistry.
OA/Magnetic Resonance Imaging
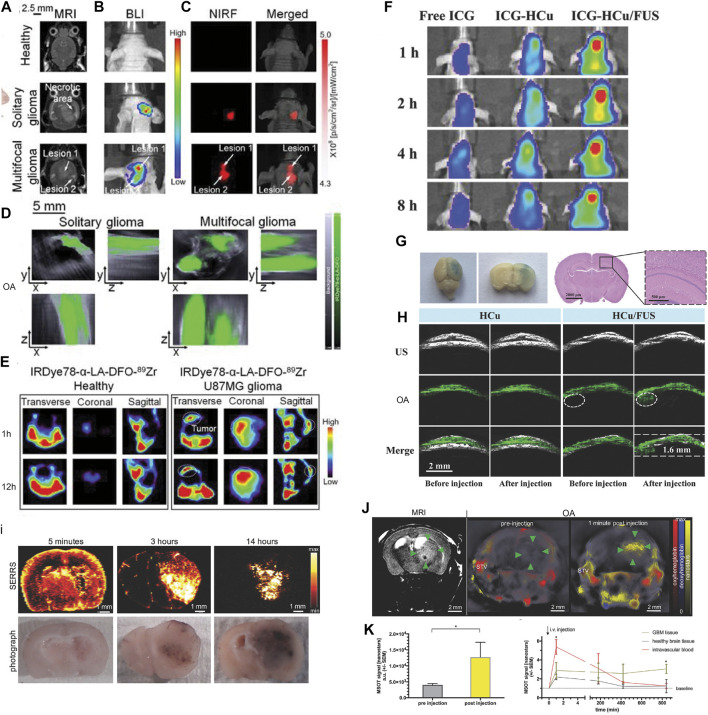
Magnetic resonance imaging (MRI) provides versatile high-resolution structural, functional, and molecular image data with high soft tissue contrast such as T1, T2 anatomical scans, functional connectivity by using fMRI, white matter integrity assessed by diffusion tensor imaging, blood–brain barrier integrity assessed by dynamic contrast enhanced MRI, cerebral perfusion measured by arterial spin labeling sequence, and molecular imaging using contrast agents (Judenhofer et al., 2008; Ni et al., 2019; Ni et al., 2020c; Massalimova et al., 2021). The structural information derived from MRI helps locate specific molecular information provided by OA tomography after registration. However, the sensitivity of molecular imaging MRI is lower than that of OA imaging and PET. Chen et al. (2015) reported gM-Luc-GRMNBs, multi-theragnostic multi-GNR crystal-seeded magnetic nanoseaurchin, which labeled the injected mesenchymal stem cells in the stroke mouse model for tracking and therapeutic purpose. Many other MR/OA imaging hybrid NPs have been utilized in brain tumor imaging in mouse/rat models, such as Mn2+-doped Prussian blue nanocubes, cobalt NPs, PEGylated polypyrrole NPs conjugating gadolinium (Gd) chelates, Gd(III)-phthalocyaninate probes, superparamagnetic iron oxide@Au–labeled stem cells, and copper manganese sulfide nanoplates (Park et al., 2012; Zhang et al., 2012; Gao et al., 2015; Liang et al., 2015; Song et al., 2015; Zhu et al., 2015; Ke et al., 2017; Kim et al., 2017; Qiao et al., 2018; Zhou et al., 2018; Kubelick and Emelianov, 2020; Tomitaka et al., 2020; Yang et al., 2021; Zhang et al., 2021a) (Table 1). Ni et al. (2014) reported ANG/PEG-UCNPs for simultaneous MR/NIR/ upconversion luminescence bimodal imaging target glioblastoma for the efficient tumor surgery. Song et al. (2019) demonstrated using triple-modality MRI/fluorescence/OA imaging probe Fe3O4@semiconducting polymer NPs for imaging the orthotopic brain U87 tumor mouse model. This NP showed photostability, long-term blood circulation time (t1/2 49 h), and specific tumor uptake (Song et al., 2019). Yang et al. (2021) showed in vivo OA/MR/PET/FL imaging using heptamethine sulfoindocyanine IRDye78-α-LA-DFO-[89Zr] of glioblastoma in the mouse brain with a low–molecular weight protein alpha-lactalbumin (α-LA) as the carrier to allow efficient hepatic clearance (Figures 2A–E).
FIGURE 2.
(A–C) Imaging of U-87MG orthotopic brain tumors using IRDye78-α-LA-DFO. (A) Coronal T2-weighted MRI, (B) bioluminescence imaging, (C) NIR fluorescence imaging, and (D) OA imaging of healthy mice and mice with the solitary and bilobed orthotopic gliomas after i.v. injection of IRDye78-α-LA-DFO. (E) PET imaging of U-87MG glioma after i.v. injection of IRDye78-α-LA-DFO[89Zr] compared to a healthy control. Reproduced from ref. Yang et al. (2021) with permission from the Ivyspring International Publisher. (F–H) Focused ultrasound (FUS)–augmented delivery of biodegradable inorganic hybrid hollow mesoporous organosilica nanoparticles-ss-Cu2−x Se (HCu) nanosystems for brain tumor detection; (F) In vivo fluorescence images of U87 glioma‐bearing mice after i.v. injection with free indocyanine green (ICG), ICG‐HCu, and ICG‐HCu/FUS; (G) Evans Blue and hematoxylin and eosin staining of the mouse brain after FUS‐induced blood–brain barrier opening; (H) ultrasound (US), OA, and overlay images of orthotopic brain tumors acquired before and after i.v. injection of HCu without or with FUS‐induced blood–brain barrier opening. Reproduced from ref. Wu M. et al. (2018) with permission from the WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (I–L) Multimodal OA/MRI/Raman imaging using SERRS-MSOT nanostars in glioblastoma-bearing mice. (I) The pharmacokinetic profile of the nanostars over the course of 14 h after i.v. injection confirmed by ex vivo SERRS Raman imaging; (J,K) T2-weighted MRI and OA imaging showing a significantly increased OA signal unmixed for the nanostars (yellow) in the tumorous area (green arrowheads) and blood circulation (STV, superficial temporal artery and vein, *p < 0.05); (L) the peak signal in the blood stream gradually declined over time. No remnant signal deriving from the nanostars was detectable by OA in the blood or the healthy brain tissue area on the contralateral hemisphere. Reproduced from ref. Neuschmelting et al. (2018) with permission from the WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
OA/Raman
Few studies have so far utilized hybrid OA/Raman imaging for detection of the molecular and structural changes in the small animal brain. Surface-enhanced resonance Raman (SERS) harbors features such as high sensitivity, brightness, low photobleaching, high resolution, and availability of various tags (Langer et al., 2020). Kircher et al. (2012) demonstrated using an indocyanine green derivative (IRDye-800-c(KRGDf) for triple-modality OA/MRI/Raman imaging of brain tumor detection in the glioblastoma mouse model (Kircher et al., 2012). Neuschmelting et al. (2018) reported OA/SERS dual-modality imaging using SERRS-MSOT-nanostar (absorption peak 770 nm, composed of a gold nanostar core, and encapsulated with IR780-embedded silica layer) for brain tumor delineation in Nestin-tv-a;Ink4a/Arf −/− ;Pten fl/fl glioblastoma mice (Figures 2I–L). Li et al. (2021b) reported ratiometric core-satellite structure AuNNR@MSi-AuNPs for NIR II OA/SERS dual detecting of hydrogen peroxide in inflammation and subcutaneous tumors in the limb of the animal.
OA/Ultrasound
US imaging is a most frequent combination with OA imaging (Wang and Hu, 2012; Gottschalk et al., 2019; Qian et al., 2019) and provides brain structure information and tumor boundaries, while OA imaging provides molecular or functional readouts (Guo et al., 2017). Encapsulated dye PLGA, methylene blue microbubbles, or nanobubbles have been reported for enhancing the US and OA signals in imaging (Das et al., 2018; Frinking et al., 2020). Santiesteban et al. (2017) showed that copper sulfide perfluorocarbon nanodroplets (CuS–PFCnDs) enhanced contrast in OA/US imaging of the lymph node in mice. Meng et al. (2019) demonstrated US-responsive OA imaging probe Au@lip MBs based on microbubbles (MBs) containing AuNPs for in vivo “background-free” OA imaging. In addition, functional US has been developed for imaging microvasculature dynamics at whole brain scale in rodents (Macé et al., 2011; Rabut et al., 2019). Wu M. et al. (2018) reported using focused US-augmented delivery of biodegradable multifunctional inorganic hybrid hollow mesoporous organosilica nanoparticle-ss-Cu2−x Se (HCu) ICG-HCu for brain glioblastoma imaging and treatment (Figures 2F–H); Park et al. (2021) recently reported using quadruple OA/US/optical coherence/fluorescence fusion imaging with a transparent US transducer for in vivo monitoring of rat eyes after injuries.
OA/Computed Tomography
CT is widely used for providing structural information in combination with PET, SPECT, or fluorescence imaging studies in small animals (Polatoglu et al., 2019; Herfert et al., 2020). A few studies have been reported using NPs such as porous MnO@Au nanocomposites and Pdots@hydrogel nanoplatform for MR/OA/CT tumor imaging in the peripheral (Liu et al., 2018; Men et al., 2020). A recent study by Shang et al. (2017) demonstrated an OA/CT/MRI triple-modality core-shell Au nanorod@metal–organic NP for imaging U87MG gliomas in mice with low toxicity, strong X-ray attenuation, and high contrast and penetration depth. Lv et al. (2019) showed OA/CT/upconversion luminescence/short-wavelength infrared luminescence imaging using a UCNP@mSiO2-ZnPc NP (using NaErF4 as host) for brain glioblastoma imaging. Biocompatible conjugated polymer nanoparticles for highly efficient photoacoustic imaging of orthotopic brain tumors in the second near-infrared window. The CT intensity and the OA signal intensities correlated with different concentrations of this NP with a high signal-to-noise ratio.
Discussion
The multiplex molecular, structural, and functional imaging readouts using OA imaging provide important etiological insights into brain function and disease pathophysiology in small animal models. There is a rapid development in molecular imaging contrast agents employing a multimodal imaging strategy for pathological targets involved in brain diseases. Hybrid imaging systems such as SPECT/PET/CT and PET/MRI have greatly improved the workflow and data analysis (Lancelot and Zimmer, 2010; Rodriguez-Vieitez et al., 2015). Fluorescence imaging/MRI hybrid imaging enables to answer the longstanding research questions such as the link between MRI blood-oxygen-level-dependent readout and calcium recording (Schulz et al., 2012; Schlegel et al., 2018; Lake et al., 2020). We propose the following aspects of particular interest for the development in small-animal OA hybrid brain imaging.
1) Registration and analysis: In most small-animal OA imaging studies, the data from different imaging modalities were acquired sequentially (Ni et al., 2018c). Co-registration and post-processing of small-animal neuroimage datasets acquired sequentially using OA imaging and other modalities have been performed for the region/volume of interest analysis (Attia et al., 2016; Ren et al., 2019). For this, manual/semi-automatic atlas–based analysis and algorithms have been developed (Ren et al., 2019; Ren et al., 2021; Zhang et al., 2021b). Further studies to develop a deep learning–based method for fully automatic segmentation and registration are needed, for example, between OA/MRI or OA/CT brain imaging data and for position-dependent light fluence correction hold great promise (Sarah et al., 2019; Waterhouse et al., 2019; Ni et al., 2020a; Dean-Ben et al., 2020; Hu et al., 2021). Additionally, bimodal animal holder (Gehrung et al., 2020; Zhang et al., 2021b) or concurrent imaging acquisition OA tomography–MRI, OA–fluorescence confocal microscopy, and OA tomography–fluorescence imaging have already been developed (Chen et al., 2017; Zhang et al., 2018b; Liu et al., 2019b; Ren et al., 2021; Zhang et al., 2021c; Dadkhah and Jiao, 2021; Deán-Ben et al., 2021). Further development in synchronized OA-MR platforms for small-animal brain imaging for simultaneous detection will further improve the workflow (Ren et al., 2021).
2) Modeling of pharmacokinetics: One compartment fluence independent model has been reported for OA imaging in the tumor tissue of the animal model (Hupple et al., 2018). So far, no kinetic modeling has been developed and validated for OA brain imaging. For the OA tomographic imaging data, pharmacokinetic modeling will facilitate the interpretation of results more and improve accuracy and the further development of imaging probes.
3) Standardization: Various aspects can impact on in vivo OA imaging data quality in small animals, such as an imaging protocol, anesthesia and animal handling, OA signal calibration, an image analysis method, and data processing and sharing tools. Standardization on the phantom OA imaging data has been initiated (Bohndiek et al., 2019), and further image acquisition and post-processing regarding small animal brain imaging data are essential.
4) New multimodal NIR II probes: There is a rapid development in hybrid OA imaging probes, especially NIR II probes for brain imaging. NIR II probes allow for deeper penetration, improved signal/noise ratio, and more reliable unmixing from strong endogenous hemoglobin background. Many NIR II OA/fluorescence probes, such as novel NIR II OA probes with DNA-based nanocarriers, PEGylated Au nanoparticles, and SPNs, that are of high chemical stability, low toxicity, and a high signal-to-noise ratio showed great promise for multimodal imaging and photothermal therapy (Jin et al., 2010; Ding et al., 2019; Meng et al., 2019; Sun et al., 2019; Zhang et al., 2019c; Feng et al., 2020; Xu et al., 2020; Joseph et al., 2021; Miyasato et al., 2021).
5) Toward clinical translation: For fluorescence imaging, the U.S. Food and Drug Administration (FDA) approved several probes such as ICG (Mokrousov et al., 2021), methylene blue, fluorescein, Prussian blue, 5-aminolevulinic acid (Stummer et al., 2006), and Evans blue. A few fluorescence imaging contrast agents are in clinical trials at different 0stages including ONM-100 (pH-activable NP), second window ICG or SWIG, BLZ-100, Tumor Paint™, TumorGlow™, ABY-029, LUM015, SMG-101, OTL38, and Cornell dots (Phillips et al., 2014; Whitley et al., 2016; Gutowski et al., 2017; Randall et al., 2019; Samkoe et al., 2019; Wahsner et al., 2019; Voskuil et al., 2020; Teng et al., 2021). For MRI, eight Gd-based probes such as Gd-DOTA and superparamagnetic iron oxide agents, such as ferumoxytol, ferucarbotran, and ferumoxtran‐10 (Combidex/Sinerem) have been approved by the FDA. For US imaging, Definity (perflutren lipid microspheres), Optison (human serum albumin stabilized perflutren microspheres), SonoVue (phospholipid-stabilized microbubble), and Sonazoid (F‐butane encapsulated in a lipid shell) have been approved by the FDA for clinical usage; further clinical studies with OA imaging contrast agents are needed. Applications of OA imaging in the clinical research have shown promising results mainly in the peripheral with endogenous contrast (melanin, Hb, and HbO) such as in inflammatory bowl, dermatology, and breast cancer (Garcia-Uribe et al., 2015; Knieling et al., 2017; Masthoff et al., 2018; Nyayapathi et al., 2021). Na et al. (2021); Na and Wang (2021) recently demonstrated the first OA imaging in a living human brain. Significant challenges need to be overcome for OA human brain imaging due to the thickness of the human skull, the acoustic distortions, and penetration depth.
To conclude, multimodal OA brain imaging assisted with contrast agents in small animals has facilitated the understanding of brain physiology and disease-related mechanisms. As OA imaging is a rapidly evolving technique, many outstanding challenges need to be tackled to further improve the quantitativeness and achieve even wider applications.
Author Contributions
XS and RN wrote the draft manuscript. All authors contributed to the manuscript.
Funding
RN received funding from Helmut Horten Stiftung, Jubiläumsstiftung von SwissLife, Vontobel Stiftung, and UZH Entrepreneur Fellowship (reference no. MEDEF-20-021).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Attia A. B. E., Ho C. J. H., Chandrasekharan P., Balasundaram G., Tay H. C., Burton N. C., et al. (2016). Multispectral Optoacoustic and MRI Coregistration for Molecular Imaging of Orthotopic Model of Human Glioblastoma. J. Biophoton 9 (7), 701–708. 10.1002/jbio.201500321 [DOI] [PubMed] [Google Scholar]
- Bézière N., Ntziachristos V. (2015). Optoacoustic Imaging of Naphthalocyanine: Potential for Contrast Enhancement and Therapy Monitoring. J. Nucl. Med. 56 (2), 323–328. 10.2967/jnumed.114.147157 [DOI] [PubMed] [Google Scholar]
- Bohndiek S., Brunker J., Gröhl J., Hacker L., Joseph J., Vogt W. C., et al. (2019). International Photoacoustic Standardisation Consortium (IPASC): Overview (Conference Presentation). 10.1117/12.2506044 [DOI] [Google Scholar]
- Burton N. C., Patel M., Morscher S., Driessen W. H. P., Claussen J., Beziere N., et al. (2013). Multispectral Opto-Acoustic Tomography (MSOT) of the Brain and Glioblastoma Characterization. Neuroimage 65, 522–528. 10.1016/j.neuroimage.2012.09.053 [DOI] [PubMed] [Google Scholar]
- Calvo-Rodriguez M., Hou S. S., Snyder A. C., Dujardin S., Shirani H., Nilsson K. P. R., et al. (2019). In Vivo detection of Tau Fibrils and Amyloid β Aggregates with Luminescent Conjugated Oligothiophenes and Multiphoton Microscopy. Acta Neuropathol. Commun. 7 (1), 171. 10.1186/s40478-019-0832-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinell K., Gupta N., Koivisto B. D., Kumaradas J. C., Zhou X., Irving H., et al. (2021). A Novel Photoacoustic-Fluorescent Contrast Agent for Quantitative Imaging of Lymphatic Drainage. Photoacoustics 21, 100239. 10.1016/j.pacs.2021.100239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.-J., Kang Y.-D., Lin C.-H., Chen S.-Y., Hsieh C.-H., Chen Y.-Y., et al. (2015). Multitheragnostic Multi-GNRs Crystal-Seeded Magnetic Nanoseaurchin for Enhanced In Vivo Mesenchymal-Stem-Cell Homing, Multimodal Imaging, and Stroke Therapy. Adv. Mater. 27 (41), 6488–6495. 10.1002/adma.201502784 [DOI] [PubMed] [Google Scholar]
- Chen J., Liu C., Hu D., Wang F., Wu H., Gong X., et al. (2016). Single-Layer MoS2Nanosheets with Amplified Photoacoustic Effect for Highly Sensitive Photoacoustic Imaging of Orthotopic Brain Tumors. Adv. Funct. Mater. 26 (47), 8715–8725. 10.1002/adfm.201603758 [DOI] [Google Scholar]
- Chen Z., Deán-Ben X. L., Gottschalk S., Razansky D. (2017). Hybrid System for In Vivo Epifluorescence and 4D Optoacoustic Imaging. Opt. Lett. 42 (22), 4577–4580. 10.1364/ol.42.004577 [DOI] [PubMed] [Google Scholar]
- Cheng K., Kothapalli S.-R., Liu H., Koh A. L., Jokerst J. V., Jiang H., et al. (2014). Construction and Validation of Nano Gold Tripods for Molecular Imaging of Living Subjects. J. Am. Chem. Soc. 136 (9), 3560–3571. 10.1021/ja412001e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Wang X., Liu X., Wang X., Wen H., Cheng Y., et al. (2021). An Effective NIR Laser/tumor-Microenvironment Co-responsive Cancer Theranostic Nanoplatform with Multi-Modal Imaging and Therapies. Nanoscale 13, 10816–10828. 10.1039/d1nr01645h [DOI] [PubMed] [Google Scholar]
- Dadkhah A., Jiao S. (2021). Integrating Photoacoustic Microscopy with Other Imaging Technologies for Multimodal Imaging. Exp. Biol. Med. (Maywood) 246 (7), 771–777. 10.1177/1535370220977176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H., Shen Q., Shao J., Wang W., Gao F., Dong X. (2021). Small Molecular NIR-II Fluorophores for Cancer Phototheranostics. The Innovation 2 (1), 100082. 10.1016/j.xinn.2021.100082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D., Sivasubramanian K., Yang C., Pramanik M. (2018). On-chip Generation of Microbubbles in Photoacoustic Contrast Agents for Dual Modal Ultrasound/photoacoustic In Vivo Animal Imaging. Sci. Rep. 8 (1), 6401. 10.1038/s41598-018-24713-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deán-Ben X. L., Sela G., Lauri A., Kneipp M., Ntziachristos V., Westmeyer G. G., et al. (2016). Functional Optoacoustic Neuro-Tomography for Scalable Whole-Brain Monitoring of Calcium Indicators. Light Sci. Appl. 5 (12), e16201. 10.1038/lsa.2016.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean-Ben X. L., Robin J., Ni R., Razansky D. (2020). Noninvasive Three-Dimensional Optoacoustic Localization Microangiography of Deep Tissues. Available at: https://arxiv.org/abs/2007.00372 (Accessed July 1, 2020). [Google Scholar]
- Deán-Ben X. L., Robin J., Razansky R. D., Daniel R. (2021). In Vivo localization Optoacoustic Tomography (LOT) with Particles Smaller Than Red Blood Cells. Proc.SPIE 11642. 10.1117/12.2578191 [DOI] [Google Scholar]
- Detrez J. R., Maurin H., Van Kolen K., Willems R., Colombelli J., Lechat B., et al. (2019). Regional Vulnerability and Spreading of Hyperphosphorylated Tau in Seeded Mouse Brain. Neurobiol. Dis. 127, 398–409. 10.1016/j.nbd.2019.03.010 [DOI] [PubMed] [Google Scholar]
- Ding F., Chen Z., Kim W. Y., Sharma A., Li C., Ouyang Q., et al. (2019). A Nano-Cocktail of an NIR-II Emissive Fluorophore and Organoplatinum(ii) Metallacycle for Efficient Cancer Imaging and Therapy. Chem. Sci. 10, 7023–7028. 10.1039/C9SC02466B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y., Wu M., Hu D., Pan Y., Hu F., Liu X., et al. (2020). Biomimetic Nanocomposites Cloaked with Bioorthogonally Labeled Glioblastoma Cell Membrane for Targeted Multimodal Imaging of Brain Tumors. Adv. Funct. Mater. 30 (38), 2004346. 10.1002/adfm.202004346 [DOI] [Google Scholar]
- Duan Y., Hu D., Guo B., Shi Q., Wu M., Xu S., et al. (2020). Nanostructural Control Enables Optimized Photoacoustic-Fluorescence-Magnetic Resonance Multimodal Imaging and Photothermal Therapy of Brain Tumor. Adv. Funct. Mater. 30 (1), 1907077. 10.1002/adfm.201907077 [DOI] [Google Scholar]
- Fan Q., Cheng K., Hu X., Ma X., Zhang R., Yang M., et al. (2014). Transferring Biomarker into Molecular Probe: Melanin Nanoparticle as a Naturally Active Platform for Multimodality Imaging. J. Am. Chem. Soc. 136 (43), 15185–15194. 10.1021/ja505412p [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q., Cheng K., Yang Z., Zhang R., Yang M., Hu X., et al. (2015). Perylene-diimide-based Nanoparticles as Highly Efficient Photoacoustic Agents for Deep Brain Tumor Imaging in Living Mice. Adv. Mater. 27 (5), 843–847. 10.1002/adma.201402972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z., Liu H., Xue Y., Lin J., Fu Y., Xia Z., et al. (2021). Reversing Cold Tumors to Hot: An Immunoadjuvant-Functionalized Metal-Organic Framework for Multimodal Imaging-Guided Synergistic Photo-Immunotherapy. Bioactive Mater. 6 (2), 312–325. 10.1016/j.bioactmat.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadi A., Sigmund F., Westmeyer G. G., Shapiro M. G. (2021). Genetically Encodable Materials for Non-invasive Biological Imaging. Nat. Mater. 20 (5), 585–592. 10.1038/s41563-020-00883-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G., Zhang G.-Q., Ding D. (2020). Design of Superior Phototheranostic Agents Guided by Jablonski Diagrams. Chem. Soc. Rev. 49 (22), 8179–8234. 10.1039/d0cs00671h [DOI] [PubMed] [Google Scholar]
- Fox M. D., Raichle M. E. (2007). Spontaneous Fluctuations in Brain Activity Observed with Functional Magnetic Resonance Imaging. Nat. Rev. Neurosci. 8 (9), 700–711. 10.1038/nrn2201 [DOI] [PubMed] [Google Scholar]
- Frinking P., Segers T., Luan Y., Tranquart F. (2020). Three Decades of Ultrasound Contrast Agents: A Review of the Past, Present and Future Improvements. Ultrasound Med. Biol. 46 (4), 892–908. 10.1016/j.ultrasmedbio.2019.12.008 [DOI] [PubMed] [Google Scholar]
- Fung C. W., Guo J., Fu H., Figueroa H. Y., Konofagou E. E., Duff K. E. (2020). Atrophy Associated with Tau Pathology Precedes Overt Cell Death in a Mouse Model of Progressive Tauopathy. Sci. Adv. 6 (42), eabc8098. 10.1126/sciadv.abc8098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D., Zhang P., Liu C., Chen C., Gao G., Wu Y., et al. (2015). Compact Chelator-free Ni-Integrated CuS Nanoparticles with Tunable Near-Infrared Absorption and Enhanced Relaxivity for In Vivo Dual-Modal Photoacoustic/MR Imaging. Nanoscale 7 (42), 17631–17636. 10.1039/C5NR05237H [DOI] [PubMed] [Google Scholar]
- Garcia-Uribe A., Erpelding T. N., Krumholz A., Ke H., Maslov K., Appleton C., et al. (2015). Dual-Modality Photoacoustic and Ultrasound Imaging System for Noninvasive Sentinel Lymph Node Detection in Patients with Breast Cancer. Sci. Rep. 5 (1), 15748. 10.1038/srep15748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrung M., Tomaszewski M., McIntyre D., Disselhorst J., Bohndiek S. (2020). Co-registration of Optoacoustic Tomography and Magnetic Resonance Imaging Data from Murine Tumour Models. Photoacoustics 18, 100147. 10.1016/j.pacs.2019.100147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk S., Degtyaruk O., Mc Larney B., Rebling J., Hutter M. A., Deán-Ben X. L., et al. (2019). Rapid Volumetric Optoacoustic Imaging of Neural Dynamics across the Mouse Brain. Nat. Biomed. Eng. 3 (5), 392–401. 10.1038/s41551-019-0372-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara E., Berti R., Londono I., Xie N., Bellec P., Lesage F., et al. (2013). Imaging of an Inflammatory Injury in the Newborn Rat Brain with Photoacoustic Tomography. PLoS One 8 (12), e83045. 10.1371/journal.pone.0083045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B., Sheng Z., Kenry K., Hu D., Lin X., Xu S., et al. (2017). Biocompatible Conjugated Polymer Nanoparticles for Highly Efficient Photoacoustic Imaging of Orthotopic Brain Tumors in the Second Near-Infrared Window. Mater. Horiz. 4 (6), 1151–1156. 10.1039/C7MH00672A [DOI] [Google Scholar]
- Guo B., Sheng Z., Hu D., Liu C., Zheng H., Liu B. (2018). Through Scalp and Skull NIR‐II Photothermal Therapy of Deep Orthotopic Brain Tumors with Precise Photoacoustic Imaging Guidance. Adv. Mater. 30 (35), 1802591. 10.1002/adma.201802591 [DOI] [PubMed] [Google Scholar]
- Guo B., Feng Z., Hu D., Xu S., Middha E., Pan Y., et al. (2019). Precise Deciphering of Brain Vasculatures and Microscopic Tumors with Dual NIR‐II Fluorescence and Photoacoustic Imaging. Adv. Mater. 31 (30), 1902504. 10.1002/adma.201902504 [DOI] [PubMed] [Google Scholar]
- Guo B., Chen J., Chen N., Middha E., Xu S., Pan Y., et al. (2019). High‐Resolution 3D NIR‐II Photoacoustic Imaging of Cerebral and Tumor Vasculatures Using Conjugated Polymer Nanoparticles as Contrast Agent. Adv. Mater. 31 (25), 1808355. 10.1002/adma.201808355 [DOI] [PubMed] [Google Scholar]
- Gutowski M., Framery B., Boonstra M. C., Garambois V., Quenet F., Dumas K., et al. (2017). SGM-101: An Innovative Near-Infrared Dye-Antibody Conjugate that Targets CEA for Fluorescence-Guided Surgery. Surg. Oncol. 26 (2), 153–162. 10.1016/j.suronc.2017.03.002 [DOI] [PubMed] [Google Scholar]
- Hansson O. (2021). Biomarkers for Neurodegenerative Diseases. Nat. Med. 27 (6), 954–963. 10.1038/s41591-021-01382-x [DOI] [PubMed] [Google Scholar]
- He S., Song J., Qu J., Cheng Z. (2018). Crucial Breakthrough of Second Near-Infrared Biological Window Fluorophores: Design and Synthesis toward Multimodal Imaging and Theranostics. Chem. Soc. Rev. 47 (12), 4258–4278. 10.1039/C8CS00234G [DOI] [PubMed] [Google Scholar]
- Herfert K., Mannheim J. G., Kuebler L., Marciano S., Amend M., Parl C., et al. (2020). Quantitative Rodent Brain Receptor Imaging. Mol. Imaging Biol. 22 (2), 223–244. 10.1007/s11307-019-01368-9 [DOI] [PubMed] [Google Scholar]
- Hu S., Yan P., Maslov K., Lee J.-M., Wang L. V. (2009). Intravital Imaging of Amyloid Plaques in a Transgenic Mouse Model Using Optical-Resolution Photoacoustic Microscopy. Opt. Lett. 34 (24), 3899–3901. 10.1364/OL.34.003899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Lafci B., Luzgin A., Wang H., Klohs J., Dean-Ben X. L. (2021). Deep Learning Facilitates Fully Automated Brain Image Registration of Optoacoustic Tomography and Magnetic Resonance Imaging. arXiv. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Pu K. (2020). Activatable Molecular Probes for Second Near‐Infrared Fluorescence, Chemiluminescence, and Photoacoustic Imaging. Angew. Chem. Int. Ed. 59 (29), 11717–11731. 10.1002/anie.202001783 [DOI] [PubMed] [Google Scholar]
- Huang W., Wang K., An Y., Meng H., Gao Y., Xiong Z., et al. (2020). In Vivo three-dimensional Evaluation of Tumour Hypoxia in Nasopharyngeal Carcinomas Using FMT-CT and MSOT. Eur. J. Nucl. Med. Mol. Imaging 47 (5), 1027–1038. 10.1007/s00259-019-04526-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupple C. W., Morscher S., Burton N. C., Pagel M. D., McNally L. R., Cárdenas-Rodríguez J. (2018). A Light-fluence-independent Method for the Quantitative Analysis of Dynamic Contrast-Enhanced Multispectral Optoacoustic Tomography (DCE MSOT). Photoacoustics 10, 54–64. 10.1016/j.pacs.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A., Tokunaga M., Maeda J., Minamihisamatsu T., Shimojo M., Takuwa H., et al. (2018). In Vivo Visualization of Tau Accumulation, Microglial Activation, and Brain Atrophy in a Mouse Model of Tauopathy rTg4510. Jad 61 (3), 1037–1052. 10.3233/jad-170509 [DOI] [PubMed] [Google Scholar]
- Jia X., Fan K., Zhang R., Zhang D., Zhang J., Gao Y., et al. (2020). Precise Visual Distinction of Brain Glioma from normal Tissues via Targeted Photoacoustic and Fluorescence Navigation. Nanomedicine: Nanotechnology, Biol. Med. 27, 102204. 10.1016/j.nano.2020.102204 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Upputuri P. K., Xie C., Zeng Z., Sharma A., Zhen X., et al. (2019). Adv. Mater. 31 (11), 1808166. 10.1002/adma.201808166 [DOI] [PubMed] [Google Scholar]
- Jin Y., Jia C., Huang S.-W., O'Donnell M., Gao X. (2010). Multifunctional Nanoparticles as Coupled Contrast Agents. Nat. Commun. 1, 41. 10.1038/ncomms1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J., Baumann K. N., Postigo A., Bollepalli L., Bohndiek S. E., Hernández‐Ainsa S. (2021). DNA‐Based Nanocarriers to Enhance the Optoacoustic Contrast of Tumors In Vivo . Adv. Healthc. Mater. 10 (2), 2001739. 10.1002/adhm.202001739 [DOI] [PubMed] [Google Scholar]
- Judenhofer M. S., Wehrl H. F., Newport D. F., Catana C., Siegel S. B., Becker M., et al. (2008). Simultaneous PET-MRI: a New Approach for Functional and Morphological Imaging. Nat. Med. 14 (4), 459–465. 10.1038/nm1700 [DOI] [PubMed] [Google Scholar]
- Kang N.-Y., Park S.-J., Ang X. W. E., Samanta A., Driessen W. H. P., Ntziachristos V., et al. (2014). A Macrophage Uptaking Near-Infrared Chemical Probe CDnir7 for In Vivo Imaging of Inflammation. Chem. Commun. 50 (50), 6589–6591. 10.1039/c4cc02038c [DOI] [PubMed] [Google Scholar]
- Karlas A., Pleitez M. A., Aguirre J., Ntziachristos V. (2021). Optoacoustic Imaging in Endocrinology and Metabolism. Nat. Rev. Endocrinol. 17 (6), 323–335. 10.1038/s41574-021-00482-5 [DOI] [PubMed] [Google Scholar]
- Kasten B. B., Jiang K., Cole D., Jani A., Udayakumar N., Gillespie G. Y., et al. (2020). Targeting MMP-14 for Dual PET and Fluorescence Imaging of Glioma in Preclinical Models. Eur. J. Nucl. Med. Mol. Imaging 47 (6), 1412–1426. 10.1007/s00259-019-04607-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke K., Yang W., Xie X., Liu R., Wang L.-L., Lin W.-W., et al. (2017). Copper Manganese Sulfide Nanoplates: A New Two-Dimensional Theranostic Nanoplatform for MRI/MSOT Dual-Modal Imaging-Guided Photothermal Therapy in the Second Near-Infrared Window. Theranostics 7 (19), 4763–4776. 10.7150/thno.21694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T., Lemaster J. E., Chen F., Li J., Jokerst J. V. (2017). Photoacoustic Imaging of Human Mesenchymal Stem Cells Labeled with Prussian Blue-Poly(l-Lysine) Nanocomplexes. ACS nano 11 (9), 9022–9032. 10.1021/acsnano.7b03519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher M. F., de la Zerda A., Jokerst J. V., Zavaleta C. L., Kempen P. J., Mittra E., et al. (2012). A Brain Tumor Molecular Imaging Strategy Using a New Triple-Modality MRI-Photoacoustic-Raman Nanoparticle. Nat. Med. 18 (5), 829–834. 10.1038/nm.2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knieling F., Neufert C., Hartmann A., Claussen J., Urich A., Egger C., et al. (2017). Multispectral Optoacoustic Tomography for Assessment of Crohn's Disease Activity. N. Engl. J. Med. 376 (13), 1292–1294. 10.1056/NEJMc1612455 [DOI] [PubMed] [Google Scholar]
- Kreisl W. C., Lao P. J., Johnson A., Tomljanovic Z., Klein J., Polly K., et al. (2021). Patterns of Tau Pathology Identified with 18 F‐MK‐6240 PET Imaging. Alzheimer's Demen. 10.1002/alz.12384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaswamy S., Lin Y., Rajamohamedsait W. J., Rajamohamedsait H. B., Krishnamurthy P., Sigurdsson E. M. (2014). Antibody-derived In Vivo Imaging of Tau Pathology. J. Neurosci. 34 (50), 16835–16850. 10.1523/jneurosci.2755-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubelick K. P., Emelianov S. Y. (2020). Prussian Blue Nanocubes as a Multimodal Contrast Agent for Image-Guided Stem Cell Therapy of the Spinal Cord. Photoacoustics 18, 100166. 10.1016/j.pacs.2020.100166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchibhotla K. V., Wegmann S., Kopeikina K. J., Hawkes J., Rudinskiy N., Andermann M. L., et al. (2014). Neurofibrillary Tangle-Bearing Neurons Are Functionally Integrated in Cortical Circuits In Vivo . Proc. Natl. Acad. Sci. 111 (1), 510–514. 10.1073/pnas.1318807111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake E. M. R., Ge X., Shen X., Herman P., Hyder F., Cardin J. A., et al. (2020). Simultaneous Cortex-wide Fluorescence Ca2+ Imaging and Whole-Brain fMRI. Nat. Methods 17 (12), 1262–1271. 10.1038/s41592-020-00984-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancelot S., Zimmer L. (2010). Small-animal Positron Emission Tomography as a Tool for Neuropharmacology. Trends Pharmacol. Sci. 31 (9), 411–417. 10.1016/j.tips.2010.06.002 [DOI] [PubMed] [Google Scholar]
- Langen K.-J., Galldiks N., Hattingen E., Shah N. J. (2017). Advances in Neuro-Oncology Imaging. Nat. Rev. Neurol. 13 (5), 279–289. 10.1038/nrneurol.2017.44 [DOI] [PubMed] [Google Scholar]
- Langer J., Jimenez de Aberasturi D., Aizpurua J., Alvarez-Puebla R. A., Auguié B., Baumberg J. J., et al. (2020). Present and Future of Surface-Enhanced Raman Scattering. ACS nano 14 (1), 28–117. 10.1021/acsnano.9b04224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng F., Edison P. (2021). Neuroinflammation and Microglial Activation in Alzheimer Disease: where Do We Go from Here?. Nat. Rev. Neurol. 17 (3), 157–172. 10.1038/s41582-020-00435-y [DOI] [PubMed] [Google Scholar]
- Li W., Chen X. (2015). Gold Nanoparticles for Photoacoustic Imaging. Nanomedicine 10 (2), 299–320. 10.2217/nnm.14.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Liu B. (2014). Polymer-encapsulated Organic Nanoparticles for Fluorescence and Photoacoustic Imaging. Chem. Soc. Rev. 43 (18), 6570–6597. 10.1039/C4CS00014E [DOI] [PubMed] [Google Scholar]
- Li L., Zhu L., Ma C., Lin L., Yao J., Wang L., et al. (2017). Single-impulse Panoramic Photoacoustic Computed Tomography of Small-Animal Whole-Body Dynamics at High Spatiotemporal Resolution. Nat. Biomed. Eng. 1 (5), 0071. 10.1038/s41551-017-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Chen R., Lv J., Wang H., Liu Y., Peng Y., et al. (2018). In Vivo Photoacoustic Imaging of Brain Injury and Rehabilitation by High-Efficient Near-Infrared Dye Labeled Mesenchymal Stem Cells with Enhanced Brain Barrier Permeability. Adv. Sci. 5 (2), 1700277. 10.1002/advs.201700277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Shemetov A. A., Baloban M., Hu P., Zhu L., Shcherbakova D. M., et al. (2018). Small Near-Infrared Photochromic Protein for Photoacoustic Multi-Contrast Imaging and Detection of Protein Interactions In Vivo . Nat. Commun. 9 (1), 2734. 10.1038/s41467-018-05231-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li L., Zhu L., Maslov K., Shi J., Hu P., et al. (2020). Snapshot Photoacoustic Topography through an Ergodic Relay for High-Throughput Imaging of Optical Absorption. Nat. Photon. 14 (3), 164–170. 10.1038/s41566-019-0576-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Liu C., Fan Y., Ma X., Zhan Y., Lu X., et al. (2021). Recent Development of Near-Infrared Photoacoustic Probes Based on Small-Molecule Organic Dye. RSC Chem. Biol. 2 (3), 743–758. 10.1039/D0CB00225A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Ge X., Ye J., Li Z., Su L., Wu Y., et al. (2021). Dual Ratiometric SERS and Photoacoustic Core-Satellite Nanoprobe for Quantitatively Visualizing Hydrogen Peroxide in Inflammation and Cancer. Angew. Chem. Int. Ed. 60 (13), 7323–7332. 10.1002/anie.202015451 [DOI] [PubMed] [Google Scholar]
- Liang X., Li Y., Li X., Jing L., Deng Z., Yue X., et al. (2015). PEGylated Polypyrrole Nanoparticles Conjugating Gadolinium Chelates for Dual-Modal MRI/Photoacoustic Imaging Guided Photothermal Therapy of Cancer. Adv. Funct. Mater. 25 (9), 1451–1462. 10.1002/adfm.201402338 [DOI] [Google Scholar]
- Liang B., Liu W., Zhan Q., Li M., Zhuang M., Liu Q. H., et al. (2019). Impacts of the Murine Skull on High‐frequency Transcranial Photoacoustic Brain Imaging. J. Biophotonics 12 (7), e201800466. 10.1002/jbio.201800466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yang Y., Sun M., Cui M., Fu Y., Lin Y., et al. (2017). Highly Specific Noninvasive Photoacoustic and Positron Emission Tomography of Brain Plaque with Functionalized Croconium Dye Labeled by a Radiotracer. Chem. Sci. 8 (4), 2710–2716. 10.1039/c6sc04798j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Lv X., Liu H., Zhou Z., Huang J., Lei S., et al. (2018). Porous Gold Nanocluster-Decorated Manganese Monoxide Nanocomposites for Microenvironment-Activatable MR/photoacoustic/CT Tumor Imaging. Nanoscale 10 (8), 3631–3638. 10.1039/c7nr08535d [DOI] [PubMed] [Google Scholar]
- Liu Y., Liu H., Yan H., Liu Y., Zhang J., Shan W., et al. (2019). Aggregation‐Induced Absorption Enhancement for Deep Near‐Infrared II Photoacoustic Imaging of Brain Gliomas In Vivo . Adv. Sci. 6 (8), 1801615. 10.1002/advs.201801615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Liao J., Chen L., Chen J., Ding R., Gong X., et al. (2019). The Integrated High-Resolution Reflection-Mode Photoacoustic and Fluorescence Confocal Microscopy. Photoacoustics 14, 12–18. 10.1016/j.pacs.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Duan Y., Liu B. (2021). Nanoparticles as Contrast Agents for Photoacoustic Brain Imaging. Aggregate 2 (1), 4–19. 10.1002/agt2.26 [DOI] [Google Scholar]
- Liu N., Gujrati V., Malekzadeh-Najafabadi J., Werner J. P. F., Klemm U., Tang L., et al. (2021). Croconaine-based Nanoparticles Enable Efficient Optoacoustic Imaging of Murine Brain Tumors. Photoacoustics 22, 100263. 10.1016/j.pacs.2021.100263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Gong X., Yuan J., Fan X., Zhang X., Ren T., et al. (2021). Dual-Stimulus Responsive Near-Infrared Reversible Ratiometric Fluorescent and Photoacoustic Probe for In Vivo Tumor Imaging. Anal. Chem. 93 (13), 5420–5429. 10.1021/acs.analchem.0c04804 [DOI] [PubMed] [Google Scholar]
- Lozano N., Al-Jamal W. T., Taruttis A., Beziere N., Burton N. C., Van den Bossche J., et al. (2012). Liposome-Gold Nanorod Hybrids for High-Resolution Visualization Deep in Tissues. J. Am. Chem. Soc. 134 (32), 13256–13258. 10.1021/ja304499q [DOI] [PubMed] [Google Scholar]
- Lu W., Melancon M. P., Xiong C., Huang Q., Elliott A., Song S., et al. (2011). Effects of Photoacoustic Imaging and Photothermal Ablation Therapy Mediated by Targeted Hollow Gold Nanospheres in an Orthotopic Mouse Xenograft Model of Glioma. Cancer Res. 71 (19), 6116–6121. 10.1158/0008-5472.can-10-4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv R., Feng M., Liu J., Jiang X., Yuan H., Yan R., et al. (2019). Improved Red Emission and Short-Wavelength Infrared Luminescence under 808 Nm Laser for Tumor Theranostics. ACS Biomater. Sci. Eng. 5 (9), 4683–4691. 10.1021/acsbiomaterials.9b00688 [DOI] [PubMed] [Google Scholar]
- Macé E., Montaldo G., Cohen I., Baulac M., Fink M., Tanter M. (2011). Functional Ultrasound Imaging of the Brain. Nat. Methods 8 (8), 662–664. 10.1038/nmeth.1641 [DOI] [PubMed] [Google Scholar]
- Massalimova A., Ni R., Nitsch R. M., Reisert M., von Elverfeldt D., Klohs J. (2021). Diffusion Tensor Imaging Reveals Whole-Brain Microstructural Changes in the P301L Mouse Model of Tauopathy. Neurodegener Dis., 1–12. 10.1159/000515754 [DOI] [PubMed] [Google Scholar]
- Masthoff M., Helfen A., Claussen J., Karlas A., Markwardt N. A., Ntziachristos V., et al. (2018). Use of Multispectral Optoacoustic Tomography to Diagnose Vascular Malformations. JAMA Dermatol. 154 (12), 1457–1462. 10.1001/jamadermatol.2018.3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlpine C. S., Park J., Griciuc A., Kim E., Choi S. H., Iwamoto Y., et al. (2021). Astrocytic Interleukin-3 Programs Microglia and Limits Alzheimer's Disease. Nature 595, 701–706. 10.1038/s41586-021-03734-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade L. E., Ma J., Lowe G., Ghatpande A., Gelperin A., Lippard S. J. (2010). Visualization of Nitric Oxide Production in the Mouse Main Olfactory Bulb by a Cell-Trappable Copper(II) Fluorescent Probe. Proc. Natl. Acad. Sci. 107 (19), 8525–8530. 10.1073/pnas.0914794107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Men X., Chen H., Sun C., Liu Y., Wang R., Zhang X., et al. (2020). Thermosensitive Polymer Dot Nanocomposites for Trimodal Computed Tomography/Photoacoustic/Fluorescence Imaging-Guided Synergistic Chemo-Photothermal Therapy. ACS Appl. Mater. Inter. 12 (46), 51174–51184. 10.1021/acsami.0c13252 [DOI] [PubMed] [Google Scholar]
- Meng Z., Zhou X., She J., Zhang Y., Feng L., Liu Z. (2019). Ultrasound-Responsive Conversion of Microbubbles to Nanoparticles to Enable Background-free In Vivo Photoacoustic Imaging. Nano Lett. 19 (11), 8109–8117. 10.1021/acs.nanolett.9b03331 [DOI] [PubMed] [Google Scholar]
- Miranda D., Huang H., Kang H., Zhan Y., Wang D., Zhou Y., et al. (2019). Highly-Soluble Cyanine J-Aggregates Entrapped by Liposomes for In Vivo Optical Imaging Around 930 Nm. Theranostics 9 (2), 381–390. 10.7150/thno.28376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra K., Stankevych M., Fuenzalida-Werner J. P., Grassmann S., Gujrati V., Huang Y., et al. (2020). Multiplexed Whole-Animal Imaging with Reversibly Switchable Optoacoustic Proteins. Sci. Adv. 6 (24), eaaz6293. 10.1126/sciadv.aaz6293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasato D. L., Mohamed A. W., Zavaleta C. (2021). A Path toward the Clinical Translation of Nano‐based Imaging Contrast Agents. WIREs Nanomed Nanobiotechnol. e1721. 10.1002/wnan.1721 [DOI] [PubMed] [Google Scholar]
- Mokrousov M. D., Thompson W., Ermilov S. A., Abakumova T., Novoselova M. V., Inozemtseva O. A., et al. (2021). Indocyanine green Dye Based Bimodal Contrast Agent Tested by Photoacoustic/fluorescence Tomography Setup. Biomed. Opt. Express 12 (6), 3181–3195. 10.1364/BOE.419461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na S., Wang L. V. (2021). Photoacoustic Computed Tomography for Functional Human Brain Imaging [Invited]. Biomed. Opt. Express 12 (7), 4056–4083. 10.1364/BOE.423707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na S., Russin J. J., Lin L., Yuan X., Hu P., Jann K. B., et al. (2021). Massively Parallel Functional Photoacoustic Computed Tomography of the Human Brain. Nat. Biomed. Eng. 10.1038/s41551-021-00735-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuschmelting V., Harmsen S., Beziere N., Lockau H., Hsu H.-T., Huang R., et al. (2018). Dual-Modality Surface-Enhanced Resonance Raman Scattering and Multispectral Optoacoustic Tomography Nanoparticle Approach for Brain Tumor Delineation. Small 14 (23), 1800740. 10.1002/smll.201800740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni D., Zhang J., Bu W., Xing H., Han F., Xiao Q., et al. (2014). Dual-targeting Upconversion Nanoprobes across the Blood-Brain Barrier for Magnetic Resonance/fluorescence Imaging of Intracranial Glioblastoma. ACS Nano 8 (2), 1231–1242. 10.1021/nn406197c [DOI] [PubMed] [Google Scholar]
- Ni R., Gillberg P.-G., Bogdanovic N., Viitanen M., Myllykangas L., Nennesmo I., et al. (2017). Amyloid Tracers Binding Sites in Autosomal Dominant and Sporadic Alzheimer's Disease. Alzheimer's Demen. 13 (4), 419–430. 10.1016/j.jalz.2016.08.006 [DOI] [PubMed] [Google Scholar]
- Ni R., Vaas M., Ren W., Klohs J. (2018). Noninvasive Detection of Acute Cerebral Hypoxia and Subsequent Matrix-Metalloproteinase Activity in a ? Model of Cerebral Ischemia Using Multispectral-Optoacoustic-Tomography. Neurophoton. 5 (1), 1–15010. 10.1117/1.NPh.5.1.015005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni R., Ji B., Ono M., Sahara N., Zhang M.-R., Aoki I., et al. (2018). Comparative In Vitro and In Vivo Quantifications of Pathologic Tau Deposits and Their Association with Neurodegeneration in Tauopathy Mouse Models. J. Nucl. Med. 59 (6), 960–966. 10.2967/jnumed.117.201632 [DOI] [PubMed] [Google Scholar]
- Ni R., Rudin M., Klohs J. (2018). Cortical Hypoperfusion and Reduced Cerebral Metabolic Rate of Oxygen in the arcAβ Mouse Model of Alzheimer's Disease. Photoacoustics 10, 38–47. 10.1016/j.pacs.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni R., Kindler D. R., Waag R., Rouault M., Ravikumar P., Nitsch R., et al. (2019). fMRI Reveals Mitigation of Cerebrovascular Dysfunction by Bradykinin Receptors 1 and 2 Inhibitor Noscapine in a Mouse Model of Cerebral Amyloidosis. Front. Aging Neurosci. 11, 27. 10.3389/fnagi.2019.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni R., Dean-Ben X. L., Kirschenbaum D., Rudin M., Chen Z., Crimi A., et al. (2020). Whole Brain Optoacoustic Tomography Reveals Strain-specific Regional Beta-Amyloid Densities in Alzheimer's Disease Amyloidosis Models. bioRxiv. 10.1101/2020.02.25.96406410.1101/2020.02.25.964064 [DOI] [Google Scholar]
- Ni R., Chen Z., Shi G., Villois A., Zhou Q., Arosio P., et al. (2020). Transcranial In Vivo Detection of Amyloid-Beta at Single Plaque Resolution with Large-Field Multifocal Illumination Fluorescence Microscopy. bioRxiv, 929844. 10.1101/2020.02.01.929844 [DOI] [Google Scholar]
- Ni R., Zarb Y., Kuhn G. A., Müller R., Yundung Y., Nitsch R. M., et al. (2020). SWI and Phase Imaging Reveal Intracranial Calcifications in the P301L Mouse Model of Human Tauopathy. Magn. Reson. Mater. Phy 33 (6), 769–781. 10.1007/s10334-020-00855-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni R., Villois A., Dean-Ben X. L., Chen Z., Vaas M., Stavrakis S., et al. (2021). In-vitro and Iin-Vvivo Characterization of CRANAD-2 for Multi-Spectral Optoacoustic Tomography and Fluorescence Imaging of Amyloid-Beta Deposits in Alzheimer Mice. Photoacoustics 23, 100285. 10.1016/j.pacs.2021.100285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntziachristos V. (2010). Going Deeper Than Microscopy: the Optical Imaging Frontier in Biology. Nat. Methods 7 (8), 603–614. 10.1038/nmeth.1483 [DOI] [PubMed] [Google Scholar]
- Nyayapathi N., Zhang H., Zheng E., Nagarajan S., Bonaccio E., Takabe K., et al. (2021). Photoacoustic Dual-Scan Mammoscope: Results from 38 Patients. Biomed. Opt. Express 12 (4), 2054–2063. 10.1364/boe.420679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Sahara N., Kumata K., Ji B., Ni R., Koga S., et al. (2017). Distinct Binding of PET Ligands PBB3 and AV-1451 to Tau Fibril Strains in Neurodegenerative Tauopathies. Brain 140, aww339–780. 10.1093/brain/aww339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. I., Kim H. M., Kim J. H., Moon K. C., Yoo B., Lee K. T., et al. (2012). Theranostic Probe Based on Lanthanide-Doped Nanoparticles for Simultaneous In Vivo Dual-Modal Imaging and Photodynamic Therapy. Adv. Mater. 24 (42), 5755–5761. 10.1002/adma.201202433 [DOI] [PubMed] [Google Scholar]
- Park S.-J., Ho C. J. H., Arai S., Samanta A., Olivo M., Chang Y.-T. (2019). Visualizing Alzheimer's Disease Mouse Brain with Multispectral Optoacoustic Tomography Using a Fluorescent Probe, CDnir7. Sci. Rep. 9 (1), 12052. 10.1038/s41598-019-48329-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Park B., Kim T. Y., Jung S., Choi W. J., Ahn J., et al. (2021). Quadruple Ultrasound, Photoacoustic, Optical Coherence, and Fluorescence Fusion Imaging with a Transparent Ultrasound Transducer. Proc. Natl. Acad. Sci. USA 118 (11), e1920879118. 10.1073/pnas.1920879118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips E., Penate-Medina O., Zanzonico P. B., Carvajal R. D., Mohan P., Ye Y., et al. (2014). Clinical Translation of an Ultrasmall Inorganic Optical-PET Imaging Nanoparticle Probe. Sci. translational Med. 6 (260), 260ra149. 10.1126/scitranslmed.3009524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polatoglu M. N., Liu Y., Ni R., Ripoll J., Rudin M., Wolf M., et al. (2019). Simulation of Fluorescence Molecular Tomography Using a Registered Digital Mouse Atlas. In: Clinical and Preclinical Optical Diagnostics II. Munich: Optical Society of America. 10.1117/12.2525366 [DOI] [Google Scholar]
- Pramanik M., Swierczewska M., Green D., Sitharaman B., Wang L. V. (2009). Single-walled Carbon Nanotubes as a Multimodal-Thermoacoustic and Photoacoustic-Contrast Agent. J. Biomed. Opt. 14 (3), 034018. 10.1117/1.3147407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu K., Shuhendler A. J., Jokerst J. V., Mei J., Gambhir S. S., Bao Z., et al. (2014). Semiconducting Polymer Nanoparticles as Photoacoustic Molecular Imaging Probes in Living Mice. Nat. Nanotech 9 (3), 233–239. 10.1038/nnano.2013.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi S., Zhang Y., Liu G., Chen J., Li X., Zhu Q., et al. (2021). Plasmonic-doped Melanin-Mimic for CXCR4-Targeted NIR-II Photoacoustic Computed Tomography-Guided Photothermal Ablation of Orthotopic Hepatocellular Carcinoma. Acta Biomater. 129, 245–257. 10.1016/j.actbio.2021.05.034 [DOI] [PubMed] [Google Scholar]
- Qi J., Feng L., Zhang X., Zhang H., Huang L., Zhou Y., et al. (2021). Facilitation of Molecular Motion to Develop Turn-On Photoacoustic Bioprobe for Detecting Nitric Oxide in Encephalitis. Nat. Commun. 12 (1), 960. 10.1038/s41467-021-21208-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian M., Du Y., Wang S., Li C., Jiang H., Shi W., et al. (2018). Highly Crystalline Multicolor Carbon Nanodots for Dual-Modal Imaging-Guided Photothermal Therapy of Glioma. ACS Appl. Mater. Inter. 10 (4), 4031–4040. 10.1021/acsami.7b19716 [DOI] [PubMed] [Google Scholar]
- Qian Y., Piatkevich K. D., Mc Larney B., Abdelfattah A. S., Mehta S., Murdock M. H., et al. (2019). A Genetically Encoded Near-Infrared Fluorescent Calcium Ion Indicator. Nat. Methods 16 (2), 171–174. 10.1038/s41592-018-0294-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y., Gumin J., MacLellan C. J., Gao F., Bouchard R., Lang F. F., et al. (2018). Magnetic Resonance and Photoacoustic Imaging of Brain Tumor Mediated by Mesenchymal Stem Cell Labeled with Multifunctional Nanoparticle Introduced via Carotid Artery Injection. Nanotechnology 29 (16), 165101. 10.1088/1361-6528/aaaf16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X., Hu Q., Song Z., Sun Z., Zhang B., Zhong J., et al. (2021). Adsorption and Desorption Mechanisms on Graphene Oxide Nanosheets: Kinetics and Tuning. The Innovation 2 (3), 100137. 10.1016/j.xinn.2021.100137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabut C., Correia M., Finel V., Pezet S., Pernot M., Deffieux T., et al. (2019). 4D Functional Ultrasound Imaging of Whole-Brain Activity in Rodents. Nat. Methods 16 (10), 994–997. 10.1038/s41592-019-0572-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. M., Wenham R. M., Low P. S., Dowdy S. C., Tanyi J. L. (2019). A Phase II, Multicenter, Open-Label Trial of OTL38 Injection for the Intra-operative Imaging of Folate Receptor-Alpha Positive Ovarian Cancer. Gynecol. Oncol. 155 (1), 63–68. 10.1016/j.ygyno.2019.07.010 [DOI] [PubMed] [Google Scholar]
- Rankin E. B., Giaccia A. J. (2016). Hypoxic Control of Metastasis. Science 352 (6282), 175–180. 10.1126/science.aaf4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razansky D., Distel M., Vinegoni C., Ma R., Perrimon N., Köster R. W., et al. (2009). Multispectral Opto-Acoustic Tomography of Deep-Seated Fluorescent Proteins In Vivo . Nat. Photon 3, 412–417. https://www.nature.com/articles/nphoton.2009.98#supplementary-information 10.1038/nphoton.2009.98 [DOI] [Google Scholar]
- Razansky D., Klohs J., Ni R. (2021). Multi-scale Optoacoustic Molecular Imaging of Brain Diseases. Eur. J. Nucl. Med. Mol. Imaging. 10.1007/s00259-021-05207-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W., Skulason H., Schlegel F., Rudin M., Klohs J., Ni R. (2019). Automated Registration of Magnetic Resonance Imaging and Optoacoustic Tomography Data for Experimental Studies. Neurophoton. 6 (2), 1–10. 10.1117/1.NPh.6.2.025001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W., Deán‐Ben X. L., Augath M. A., Razansky D. (2021). Development of Concurrent Magnetic Resonance Imaging and Volumetric Optoacoustic Tomography: A Phantom Feasibility Study. J. Biophotonics 14 (2), e202000293. 10.1002/jbio.202000293 [DOI] [PubMed] [Google Scholar]
- Roberts S., Seeger M., Jiang Y., Mishra A., Sigmund F., Stelzl A., et al. (2018). Calcium Sensor for Photoacoustic Imaging. J. Am. Chem. Soc. 140 (8), 2718–2721. 10.1021/jacs.7b03064 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Vieitez E., Ni R., Gulyás B., Tóth M., Häggkvist J., Halldin C., et al. (2015). Astrocytosis Precedes Amyloid Plaque Deposition in Alzheimer APPswe Transgenic Mouse Brain: a Correlative Positron Emission Tomography and In Vitro Imaging Study. Eur. J. Nucl. Med. Mol. Imaging 42 (7), 1119–1132. 10.1007/s00259-015-3047-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samkoe K. S., Sardar H. S., Bates B. D., Tselepidakis N. N., Gunn J. R., Hoffer‐Hawlik K. A., et al. (2019). Preclinical Imaging of Epidermal Growth Factor Receptor with ABY‐029 in Soft‐tissue Sarcoma for Fluorescence‐guided Surgery and Tumor Detection. J. Surg. Oncol. 119 (8), 1077–1086. 10.1002/jso.25468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiesteban D. Y., Dumani D. S., Profili D., Emelianov S. Y. (2017). Copper Sulfide Perfluorocarbon Nanodroplets as Clinically Relevant Photoacoustic/Ultrasound Imaging Agents. Nano Lett. 17 (10), 5984–5989. 10.1021/acs.nanolett.7b02105 [DOI] [PubMed] [Google Scholar]
- Sarah B., Joanna B., Janek G., Lina H., James J., William C. V., et al. (2019). International Photoacoustic Standardisation Consortium (IPASC): Overview (Conference Presentation). Proc.SPIE 10878. 10.1117/12.2506044 [DOI] [Google Scholar]
- Schlegel F., Sych Y., Schroeter A., Stobart J., Weber B., Helmchen F., et al. (2018). Fiber-optic Implant for Simultaneous Fluorescence-Based Calcium Recordings and BOLD fMRI in Mice. Nat. Protoc. 13 (5), 840–855. 10.1038/nprot.2018.003 [DOI] [PubMed] [Google Scholar]
- Schulz K., Sydekum E., Krueppel R., Engelbrecht C. J., Schlegel F., Schröter A., et al. (2012). Simultaneous BOLD fMRI and Fiber-Optic Calcium Recording in Rat Neocortex. Nat. Methods 9 (6), 597–602. 10.1038/nmeth.2013 [DOI] [PubMed] [Google Scholar]
- Shang W., Zeng C., Du Y., Hui H., Liang X., Chi C., et al. (2017). Core-Shell Gold Nanorod@Metal-Organic Framework Nanoprobes for Multimodality Diagnosis of Glioma. Adv. Mater. 29 (3), 1604381. 10.1002/adma.201604381 [DOI] [PubMed] [Google Scholar]
- Shemetov A. A., Monakhov M. V., Zhang Q., Canton-Josh J. E., Kumar M., Chen M., et al. (2021). A Near-Infrared Genetically Encoded Calcium Indicator for In Vivo Imaging. Nat. Biotechnol. 39 (3), 368–377. 10.1038/s41587-020-0710-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z., Guo B., Hu D., Xu S., Wu W., Liew W. H., et al. (2018). Bright Aggregation-Induced-Emission Dots for Targeted Synergetic NIR-II Fluorescence and NIR-I Photoacoustic Imaging of Orthotopic Brain Tumors. Adv. Mater. 30, 1800766. 10.1002/adma.201800766 [DOI] [PubMed] [Google Scholar]
- Song X.-R., Wang X., Yu S.-X., Cao J., Li S.-H., Li J., et al. (2015). Co9Se8Nanoplates as a New Theranostic Platform for Photoacoustic/Magnetic Resonance Dual-Modal-Imaging-Guided Chemo-Photothermal Combination Therapy. Adv. Mater. 27 (21), 3285–3291. 10.1002/adma.201405634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G., Zheng X., Wang Y., Xia X., Chu S., Rao J. (2019). A Magneto-Optical Nanoplatform for Multimodality Imaging of Tumors in Mice. ACS Nano 13 (7), 7750–7758. 10.1021/acsnano.9b01436 [DOI] [PubMed] [Google Scholar]
- Stummer W., Pichlmeier U., Meinel T., Wiestler O. D., Zanella F., Reulen H.-J. (2006). Fluorescence-guided Surgery with 5-aminolevulinic Acid for Resection of Malignant Glioma: a Randomised Controlled Multicentre Phase III Trial. Lancet Oncol. 7 (5), 392–401. 10.1016/s1470-2045(06)70665-9 [DOI] [PubMed] [Google Scholar]
- Sun Y., Ding F., Chen Z., Zhang R., Li C., Xu Y., et al. (2019). Melanin-dot-mediated Delivery of Metallacycle for NIR-II/photoacoustic Dual-Modal Imaging-Guided Chemo-Photothermal Synergistic Therapy. Proc. Natl. Acad. Sci. USA 116 (34), 16729–16735. 10.1073/pnas.1908761116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C. W., Cho S. S., Singh Y., De Ravin E., Somers K., Buch L., et al. (2021). Second Window ICG Predicts Gross-Total Resection and Progression-free Survival during Brain Metastasis Surgery. J. Neurosurg., 1–10. 10.3171/2020.8.jns201810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomitaka A., Arami H., Ahmadivand A., Pala N., McGoron A. J., Takemura Y., et al. (2020). Magneto-plasmonic Nanostars for Image-Guided and NIR-Triggered Drug Delivery. Sci. Rep. 10 (1), 10115. 10.1038/s41598-020-66706-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo W., Xu Y., Fan Y., Li J., Qiu M., Xiong X., et al. (2021). Biomedical Applications of Pt(II) Metallacycle/metallacage-Based Agents: From Mono-Chemotherapy to Versatile Imaging Contrasts and Theranostic Platforms. Coord. Chem. Rev. 443, 214017. 10.1016/j.ccr.2021.214017 [DOI] [Google Scholar]
- Vaas M., Ni R., Rudin M., Kipar A., Klohs J. (2017). Extracerebral Tissue Damage in the Intraluminal Filament Mouse Model of Middle Cerebral Artery Occlusion. Front. Neurol. 8, 85. 10.3389/fneur.2017.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagenknecht P., Ono M., Luzgin A., Ji B., Higuchi M., Noain D., et al. (2021). Non-invasive Imaging of Tau-Targeted Probe Uptake by Whole Brain Multi-Spectral Optoacoustic Tomography. bioRxiv, 451626. 10.1101/2021.07.10.451626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwilst P., Kim H.-R., Seo J., Sohn N.-W., Cha S.-Y., Kim Y., et al. (2017). Rational Design Ofin VivoTau Tangle-Selective Near-Infrared Fluorophores: Expanding the BODIPY Universe. J. Am. Chem. Soc. 139 (38), 13393–13403. 10.1021/jacs.7b05878 [DOI] [PubMed] [Google Scholar]
- Voigt F. F., Kirschenbaum D., Platonova E., Pagès S., Campbell R. A. A., Kastli R., et al. (2019). The mesoSPIM Initiative: Open-Source Light-Sheet Microscopes for Imaging Cleared Tissue. Nat. Methods 16 (11), 1105–1108. 10.1038/s41592-019-0554-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuil F. J., Steinkamp P. J., Zhao T., van der Vegt B., Koller M., Doff J. J., et al. (2020). Exploiting Metabolic Acidosis in Solid Cancers Using a Tumor-Agnostic pH-Activatable Nanoprobe for Fluorescence-Guided Surgery. Nat. Commun. 11 (1), 3257. 10.1038/s41467-020-16814-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahsner J., Gale E. M., Rodríguez-Rodríguez A., Caravan P. (2019). Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 119 (2), 957–1057. 10.1021/acs.chemrev.8b00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H., Yue J., Zhu S., Uno T., Zhang X., Yang Q., et al. (2018). A Bright Organic NIR-II Nanofluorophore for Three-Dimensional Imaging into Biological Tissues. Nat. Commun. 9 (1), 1171. 10.1038/s41467-018-03505-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. V., Hu S. (2012). Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science 335 (6075), 1458–1462. 10.1126/science.1216210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. V., Yao J. (2016). A Practical Guide to Photoacoustic Tomography in the Life Sciences. Nat. Methods 13 (8), 627–638. 10.1038/nmeth.3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Yang H., Liu C., Qiu M., Ma X., Mao Z., et al. (2021). Recent Advances in the Development of Activatable Multifunctional Probes for In Vivo Imaging of Caspase-3. Chin. Chem. Lett. 32 (1), 168–178. 10.1016/j.cclet.2020.11.056 [DOI] [Google Scholar]
- Wang T., Wang S., Liu Z., He Z., Yu P., Zhao M., et al. (2021). A Hybrid Erbium(III)-bacteriochlorin Near-Infrared Probe for Multiplexed Biomedical Imaging. Nat. Mater. 10.1038/s41563-021-01063-7 [DOI] [PubMed] [Google Scholar]
- Waterhouse D. J., Fitzpatrick C. R. M., Pogue B. W., O’Connor J. P. B., Bohndiek S. E. (2019). A Roadmap for the Clinical Implementation of Optical-Imaging Biomarkers. Nat. Biomed. Eng. 3 (5), 339–353. 10.1038/s41551-019-0392-5 [DOI] [PubMed] [Google Scholar]
- Weber J., Beard P. C., Bohndiek S. E. (2016). Contrast Agents for Molecular Photoacoustic Imaging. Nat. Methods 13 (8), 639–650. 10.1038/nmeth.3929 [DOI] [PubMed] [Google Scholar]
- Whitley M. J., Cardona D. M., Lazarides A. L., Spasojevic I., Ferrer J. M., Cahill J., et al. (2016). A Mouse-Human Phase 1 Co-clinical Trial of a Protease-Activated Fluorescent Probe for Imaging Cancer. Sci. Transl. Med. 8 (320), 320ra4. 10.1126/scitranslmed.aad0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Hansen S. J., Hou Q., Yu J., Zeigler M., Jin Y., et al. (2011). Design of Highly Emissive Polymer Dot Bioconjugates for In Vivo Tumor Targeting. Angew. Chem. Int. Ed. 50 (15), 3430–3434. 10.1002/anie.201007461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Chen W., Chen Y., Zhang H., Liu C., Deng Z., et al. (2018). Focused Ultrasound-Augmented Delivery of Biodegradable Multifunctional Nanoplatforms for Imaging-Guided Brain Tumor Treatment. Adv. Sci. 5 (4), 1700474. 10.1002/advs.201700474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Lin Y., Gu J., Sigurdsson E. M. (2018). Dynamic Assessment of Tau Immunotherapies in the Brains of Live Animals by Two-Photon Imaging. EBioMedicine 35, 270–278. 10.1016/j.ebiom.2018.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi L., Jin T., Zhou J., Carney P., Jiang H. (2017). Hybrid Photoacoustic and Electrophysiological Recording of Neurovascular Communications in Freely-Moving Rats. Neuroimage 161, 232–240. 10.1016/j.neuroimage.2017.08.037 [DOI] [PubMed] [Google Scholar]
- Xu Y., Zhang Y., Li J., An J., Li C., Bai S., et al. (2020). NIR-II Emissive Multifunctional AIEgen with Single Laser-Activated Synergistic Photodynamic/photothermal Therapy of Cancers and Pathogens. Biomaterials 259, 120315. 10.1016/j.biomaterials.2020.120315 [DOI] [PubMed] [Google Scholar]
- Yang C., Guo C., Guo W., Zhao X., Liu S., Han X. (2018). Multifunctional Bismuth Nanoparticles as Theranostic Agent for PA/CT Imaging and NIR Laser-Driven Photothermal Therapy. ACS Appl. Nano Mater. 1 (2), 820–830. 10.1021/acsanm.7b00255 [DOI] [Google Scholar]
- Yang Y., Chen J., Yang Y., Xie Z., Song L., Zhang P., et al. (2019). A 1064 Nm Excitable Semiconducting Polymer Nanoparticle for Photoacoustic Imaging of Gliomas. Nanoscale 11 (16), 7754–7760. 10.1039/C9NR00552H [DOI] [PubMed] [Google Scholar]
- Yang Z., Du Y., Sun Q., Peng Y., Wang R., Zhou Y., et al. (2020). Albumin-Based Nanotheranostic Probe with Hypoxia Alleviating Potentiates Synchronous Multimodal Imaging and Phototherapy for Glioma. ACS Nano 14 (5), 6191–6212. 10.1021/acsnano.0c02249 [DOI] [PubMed] [Google Scholar]
- Yang J., Zhao C., Lim J., Zhao L., Tourneau R. L., Zhang Q., et al. (2021). Structurally Symmetric Near-Infrared Fluorophore IRDye78-Protein Complex Enables Multimodal Cancer Imaging. Theranostics 11 (6), 2534–2549. 10.7150/thno.54928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M., Shi X., Zuo C., Ma M., Zhang L., Zhang H., et al. (2020). Engineering of SPECT/Photoacoustic Imaging/Antioxidative Stress Triple-Function Nanoprobe for Advanced Mesenchymal Stem Cell Therapy of Cerebral Ischemia. ACS Appl. Mater. Inter. 12 (34), 37885–37895. 10.1021/acsami.0c10500 [DOI] [PubMed] [Google Scholar]
- Yu J., Wang X. H., Feng J., Meng X., Bu X., Li Y., et al. (2019). Antimonene Nanoflakes: Extraordinary Photoacoustic Performance for High‐Contrast Imaging of Small Volume Tumors. Adv. Healthc. Mater. 8 (17), 1900378. 10.1002/adhm.201900378 [DOI] [PubMed] [Google Scholar]
- Zhan C., Huang Y., Lin G., Huang S., Zeng F., Wu S. (2019). A Gold Nanocage/Cluster Hybrid Structure for Whole-Body Multispectral Optoacoustic Tomography Imaging, EGFR Inhibitor Delivery, and Photothermal Therapy. Small 15 (33), 1900309. 10.1002/smll.201900309 [DOI] [PubMed] [Google Scholar]
- Zhang F., Huang X., Zhu L., Guo N., Niu G., Swierczewska M., et al. (2012). Noninvasive Monitoring of Orthotopic Glioblastoma Therapy Response Using RGD-Conjugated Iron Oxide Nanoparticles. Biomaterials 33 (21), 5414–5422. 10.1016/j.biomaterials.2012.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Wang T., Qiu W., Han Y., Sun Q., Zeng J., et al. (2018). Monitoring the Opening and Recovery of the Blood-Brain Barrier with Noninvasive Molecular Imaging by Biodegradable Ultrasmall Cu2-xSe Nanoparticles. Nano Lett. 18 (8), 4985–4992. 10.1021/acs.nanolett.8b01818 [DOI] [PubMed] [Google Scholar]
- Zhang W., Li Y., Nguyen V. P., Huang Z., Liu Z., Wang X., et al. (2018). High-resolution, In Vivo Multimodal Photoacoustic Microscopy, Optical Coherence Tomography, and Fluorescence Microscopy Imaging of Rabbit Retinal Neovascularization. Light Sci. Appl. 7 (1), 103. 10.1038/s41377-018-0093-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Li L., Lin L., Shi J., Wang L. V. (2019). In Vivo superresolution Photoacoustic Computed Tomography by Localization of Single Dyed Droplets. Light Sci. Appl. 8, 36. 10.1038/s41377-019-0147-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Wang T., Liu H., Ren F., Qiu W., Sun Q., et al. (2019). Second Near-Infrared Photodynamic Therapy and Chemotherapy of Orthotopic Malignant Glioblastoma with Ultra-small Cu2−xSe Nanoparticles. Nanoscale 11 (16), 7600–7608. 10.1039/c9nr01789e [DOI] [PubMed] [Google Scholar]
- Zhang R., Xu Y., Zhang Y., Kim H. S., Sharma A., Gao J., et al. (2019). Rational Design of a Multifunctional Molecular Dye for Dual-Modal NIR-II/photoacoustic Imaging and Photothermal Therapy. Chem. Sci. 10 (36), 8348–8353. 10.1039/c9sc03504d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Zeng Q., Li X., Xing D., Zhang T. (2021). Versatile Gadolinium(III)-phthalocyaninate Photoagent for MR/PA Imaging-Guided Parallel Photocavitation and Photodynamic Oxidation at Single-Laser Irradiation. Biomaterials 275, 120993. 10.1016/j.biomaterials.2021.120993 [DOI] [PubMed] [Google Scholar]
- Zhang S., Qi L., Li X., Liang Z., Wu J., Huang S., et al. (2021). In Vivo co-registered Hybrid-Contrast Imaging by Successive Photoacoustic Tomography and Magnetic Resonance Imaging. bioRxiv preprint. 10.1101/2021.03.06.434031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Song M., Yang X., Yang J., Du C., Wang G., et al. (2020). Amorphous Ag2-xCuxS Quantum Dots: "All-In-One" Theranostic Nanomedicines for Near-Infrared Fluorescence/photoacoustics Dual-Modal-Imaging-Guided Photothermal Therapy. Chem. Eng. J. 399, 125777. 10.1016/j.cej.2020.125777 [DOI] [Google Scholar]
- Zhen X., Pu K., Jiang X. (2021). Photoacoustic Imaging and Photothermal Therapy of Semiconducting Polymer Nanoparticles: Signal Amplification and Second Near‐Infrared Construction. Small 17 (6), 2004723. 10.1002/smll.202004723 [DOI] [PubMed] [Google Scholar]
- Zhou P., Zhao H., Wang Q., Zhou Z., Wang J., Deng G., et al. (2018). Photoacoustic-Enabled Self-Guidance in Magnetic-Hyperthermia Fe@Fe3 O4 Nanoparticles for Theranostics In Vivo . Adv. Healthc. Mater. 7 (9), 1701201. 10.1002/adhm.201701201 [DOI] [PubMed] [Google Scholar]
- Zhou K., Yuan C., Dai B., Wang K., Chen Y., Ma D., et al. (2019). Environment-Sensitive Near-Infrared Probe for Fluorescent Discrimination of Aβ and Tau Fibrils in AD Brain. J. Med. Chem. 62 (14), 6694–6704. 10.1021/acs.jmedchem.9b00672 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Zhong F., Yan P., Lee J.-M., Hu S. (2021). Simultaneous Imaging of Amyloid Deposition and Cerebrovascular Function Using Dual-Contrast Photoacoustic Microscopy. Opt. Lett. 46 (11), 2561–2564. 10.1364/ol.419817 [DOI] [PubMed] [Google Scholar]
- Zhu W., Liu K., Sun X., Wang X., Li Y., Cheng L., et al. (2015). Mn2+-Doped Prussian Blue Nanocubes for Bimodal Imaging and Photothermal Therapy with Enhanced Performance. ACS Appl. Mater. Inter. 7 (21), 11575–11582. 10.1021/acsami.5b02510 [DOI] [PubMed] [Google Scholar]
- Zhu M., Sheng Z., Jia Y., Hu D., Liu X., Xia X., et al. (2017). Indocyanine Green-holo-Transferrin Nanoassemblies for Tumor-Targeted Dual-Modal Imaging and Photothermal Therapy of Glioma. ACS Appl. Mater. Inter. 9 (45), 39249–39258. 10.1021/acsami.7b14076 [DOI] [PubMed] [Google Scholar]