Abstract
In patients suffering from alcohol use disorder (AUD), stress and environmental stimuli associated with alcohol availability are important triggers of relapse. Activation of the nociceptin opioid peptide (NOP) receptor by its endogenous ligand Nociceptin/Orphanin FQ (N/OFQ) attenuates alcohol drinking and relapse in rodents, suggesting that NOP agonists may be efficacious in treating AUD. Intriguingly, recent data demonstrated that also blockade of NOP receptor reduced alcohol drinking in rodents. To explore further the potential of NOP antagonism, we investigated its effects on the reinstatement of alcohol-seeking elicited by administration of the α2 antagonist yohimbine (1.25 mg/kg, i.p.) or by environmental conditioning factors in male and female genetically selected alcohol-preferring Marchigian Sardinian (msP) rats. The selective NOP receptor antagonist LY2817412 (0.0, 3.0, 10.0, and 30.0 mg/kg) was first tested following oral (p.o.) administration. We then investigated the effects of LY2817412 (1.0, 3.0, 6.0 μg/μl/rat) microinjected into three candidate mesolimbic brain regions: the ventral tegmental area (VTA), the central nucleus of the amygdala (CeA), and the nucleus accumbens (NAc). We found that relapse to alcohol seeking was generally stronger in female than in male rats and oral administration of LY2817412 reduced yohimbine- and cue-induced reinstatement in both sexes. Following site-specific microinjections, LY2817412 reduced yohimbine-induced reinstatement of alcohol-seeking when administered into the VTA and the CeA, but not in the NAc. Cue-induced reinstatement was suppressed only when LY2817412 was microinjected into the VTA. Infusions of LY2817412 into the VTA and the CeA did not alter saccharin self-administration. These results demonstrate that NOP receptor blockade prevents the reinstatement of alcohol-seeking through modulation of mesolimbic system circuitry, providing further evidence of the therapeutic potential of NOP receptor antagonism in AUD.
Subject terms: Reward, Pharmacology, Motivation
Introduction
Alcohol use disorder (AUD) is a chronic relapsing disease characterized by compulsive drinking and emergence of a negative emotional state when access to alcohol is prevented, heightening the risk of relapse to pathological drinking [1, 2]. In the last WHO report on the impact of alcohol on global health, it was reported that in 2016 alone more than 3 million deaths and 132.6 million disability-adjusted life years were caused by AUD (2018). This placed AUD as the fifth major risk factor for premature death and disability worldwide. Environmental factors such as drug-paired stimuli and stress are important elements that heighten vulnerability to relapse in abstinent detoxified alcoholics and present a major difficulty for the development of effective therapies to manage AUD [3–5]. Neurobiological mechanisms underlying relapse to alcohol-seeking are linked to profound counteradaptive changes in neuronal circuitries mediating motivation, emotions, and reward processing [6, 7]. Untangling these neuroadaptations is complex but essential to uncover the mechanisms of relapse to alcohol-seeking and to develop more efficacious therapies.
The nociceptin opioid peptide (NOP) receptor is the fourth member of the opioid subfamily of G-protein coupled receptors whose natural ligand is the 17 amino acid peptide Nociceptin/Orphanin FQ (N/OFQ) [8, 9]. Over the 25 years since receptor deorphanization, substantial progresses have been made to demonstrate that NOP can be a valuable therapeutic target for various pathological conditions. Clinical and preclinical studies have shown that NOP receptor agonists and antagonists attenuate pain and show promising effects in various psychiatric disorders such as major depression, anxiety, and addiction [10–12].
Preclinical data showing the efficacy of NOP agonism in preventing alcohol-seeking and relapse are particularly significant. In previous works we have demonstrated that activation of NOP by intracerebroventricular (i.c.v.) infusion of N/OFQ reduced stress-induced reinstatement of alcohol-seeking both in genetically selected Marchigian Sardinian alcohol-preferring (msP) rats and in post-dependent Wistar rats [13–15]. Moreover, we have showed that i.c.v. administration of N/OFQ significantly inhibited cue-induced reinstatement of extinguished alcohol-seeking in msP rats [13]. Recently, we found that blockade of NOP receptors by selective antagonists, also reduced alcohol drinking in rats and mice [16–18]. Furthermore, in an initial study we found that NOP blockade attenuated stress-induced reinstatement of alcohol-seeking in msP rats [16]. The mechanism through which both NOP receptor agonists and antagonists reduce alcohol drinking and reinstatement of alcohol-seeking is still unclear. Several hypotheses could be raised in the attempt to explain this paradoxical effect. For instance, NOP receptor agonists may depress N/OFQ signaling through receptor desensitization, leading to functional blockade of NOP receptors [16, 19]. Alternatively, it is possible that the effects of NOP receptor agonists and antagonists are mediated by different neurocircuitries or they may act at different levels within the same neural systems.
These findings prompted us to further investigate the pharmacological properties of NOP antagonists by exploring the efficacy of LY2817412, a potent and selective NOP blocker, on alcohol-seeking elicited by yohimbine or by environmental conditioning factors in an alcohol-preferring rat line. Moreover, to gather information on the action of NOP antagonists at the neurocircuitry level, we studied the effects of brain site-specific microinjection of LY2817412 on both yohimbine and cue-induced reinstatement. Guided by the role of the mesolimbic circuitry in mediating reinstatement and the distribution of NOP receptors in the brain [20, 21], we focused our attention on the ventral tegmental area (VTA), the central nucleus of the amygdala (CeA), and the nucleus accumbens (NAc). Finally, to determine the effect of sex in response to NOP antagonists, male and female msP rats were used.
Materials and methods
Animals
Male (n = 101) and female (n = 102) genetically selected alcohol-preferring msP rats were used. Experimental procedures were performed in accordance with the guidelines of the European Community Council Directive for Care and Use of Laboratory Animals and European legislation (2010/63/EU). Formal approval to conduct the experiments was obtained from the Italian Ministry of Health and the Organism Responsible for Animal Welfare of the University of Camerino (protocol no. 1D580.1). For details, see Supplementary Information.
Drugs
The following reagents and drugs were used: alcohol (10% v/v) prepared from alcohol 95% (FL Carsetti SNC, Camerino, Italy); saccharin (0.2% w/v; Sigma-Aldrich, Milan, Italy); yohimbine (Sigma-Aldrich, Milan, Italy); LY2817412 kindly provided by Eli Lilly (Indianapolis, IN, USA). For details, see Supplementary Information.
Intracranial surgery and infusion procedure
Bilateral guide cannulas (0.65 mm outside diameter) were aimed at the VTA, CeA and NAc with the following coordinates: [VTA: anterior/posterior (AP): −5.7 mm; medial/lateral (ML): ± 2.2 mm; dorsal/ventral (DV): −7.4 mm, 12° angle; CeA: AP: −2.3 mm, ML: ± 4.2 mm, DV: −6.5 mm; NAc: AP: + 1.5 mm; ML: ± 1.1 mm, DV: −5.5 mm] and female: [VTA: AP: −5.6 mm, ML: ± 2.0 mm, DV: −7.2 mm, 10° angle; CeA: AP: −1.8 mm, ML: ± 4.0 mm, DV: −6.4 mm; NAc: AP: + 1.40 mm, ML: ± 1.0 mm, DV: −5.2 mm]. For details see, Supplementary Information.
Alcohol and saccharin self-administration training
Operant training and testing were performed in standard self-administration operant chambers (Med Associate, Inc.). Male and female msP rats were trained to self-administer 10% (v/v) alcohol or 0.2% (w/v) saccharin for 5 days a week, in 30 min daily sessions under a fixed-ratio one schedule of reinforcement as previously described [22]. Alcohol and saccharin self-administration training was continued until animals reached a stable baseline of responding. After the acquisition phase for alcohol rats have been subjected to an extinction procedure followed by the relapse tests, whereas after saccharin training animals underwent directly to the testing phase. For details, see Supplementary Information.
Experimental procedures
Effect of systemic administration of LY2817412 on yohimbine-induced reinstatement of alcohol seeking in male and female msP rats
The experimental procedure consisted of three phases: operant training, extinction and reinstatement (for details, see the experimental timeline in Fig. 1A and Supplementary Information). Briefly, male (n = 10) and female (n = 10) msP rats was trained to self-administer 10% (v/v) alcohol in 30 min daily sessions. Training phase (total number of daily sessions: 20) continued until animals reached a stable baseline of responding. The mean of g/kg/30 min of alcohol consumed in the last 3 self-administration days was 1.30 for female rats and 0.86 for males. Rats were then subjected to 15 daily 30 min extinction sessions, followed by the reinstatement test. On the test days, animals were injected with either vehicle or LY2817412 (3.0 and 30.0 mg/kg; p.o.) 30 min prior to yohimbine (1.25 mg/kg; i.p.). Reinstatement sessions started 30 min after yohimbine administration. Experiments were carried out in a Latin square within-subjects counterbalanced design with a 3-day interval between drug tests during which animals were subjected to extinction sessions.
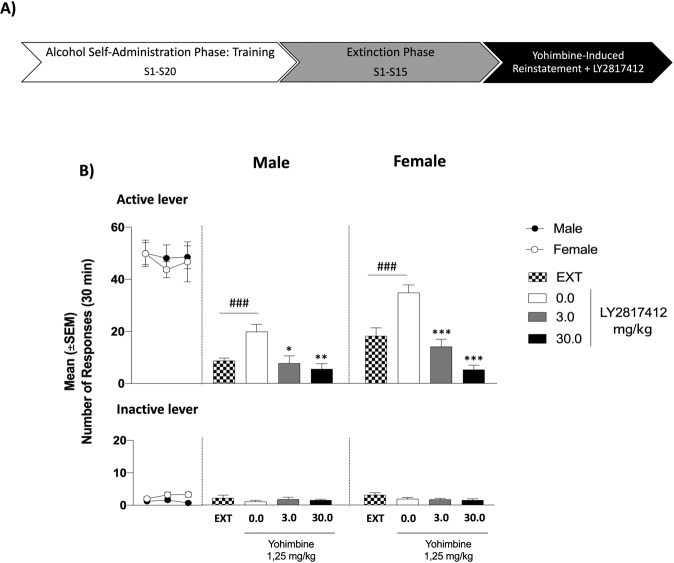
Fig. 1. Effect of Systemic Administration of LY2817412 on Yohimbine-Induced Reinstatement of Alcohol Seeking in Male and Female msP Rats.
A Schematic representation of the experimental timeline. B Self-administration: black circles (male) and white circles (female) represent mean number of the responses during the last 3 days of alcohol self-administration sessions. No differences were denoted in the number of active or inactive lever presses during this phase. Extinction: mean number of lever presses during the last 3 days of extinction (EXT). Compared to extinction, male (n = 10) and female (n = 10) msP rats treated with yohimbine (1.25 mg/kg; i.p.) and LY2817412 vehicle (0.0) showed a significant reinstatement of responding. Administration of LY2817412 significantly reduced yohimbine-induced reinstatement both in males and females. Previously alcohol paired active and inactive lever presses are presented in the upper and lower panels, respectively. Values represent the mean (±SEM). ###p < 0.001, difference between EXT and rats treated with yohimbine plus LY2817412 vehicle (0.0); *p < 0.05, **p < 0.01, ***p < 0.001, differences between rats treated with yohimbine and LY2817412 vehicle (0.0) and rats treated different doses of the antagonist.
Effect of systemic administration of LY2817412 on cue-induced reinstatement of alcohol-seeking in male and female msP rats
The experimental procedure consisted of four phases: operant training, conditioning, extinction, and reinstatement (for details, see the experimental timeline in Fig. 2B and Supplementary Information). Briefly, male (n = 8) and female (n = 10) msP rats were subjected to a self-administration/discrimination training procedure. During training the mean alcohol intake (g/kg/30 min) of the last 3 alcohol self-administration days was 1.44 for females and 1.01 for males. During the discrimination phase rats received a total of ten alcohol and ten water sessions. Discriminative stimuli (SD) predictive of alcohol (CS+, odor of an orange extract) versus water availability (CS−, odor of an anise extract) were presented during alcohol and water self-administration sessions, respectively. In addition, each lever press resulting in the delivery of alcohol was followed by a 5 s time-out period contingently paired with the illumination of the chamber’s house light, while lever presses resulting in water delivery were accompanied by a 5 s time-out period paired with a 70 dB tone.
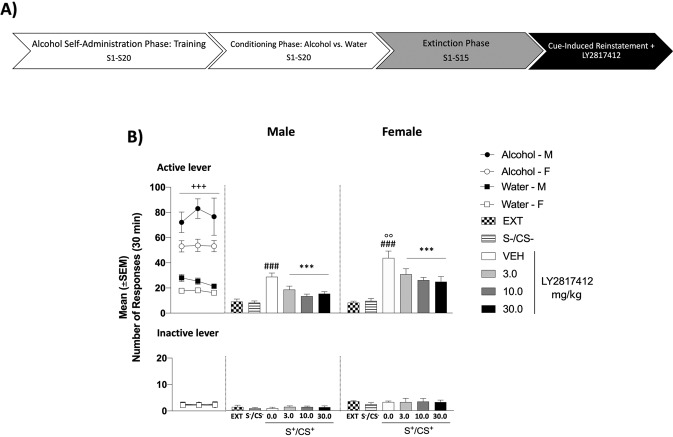
Fig. 2. Effect of Systemic Administration of LY2817412 on Cue-Induced Reinstatement of Alcohol-Seeking in Male and Female msP Rats.
A Schematic representation of the experimental timeline. B Conditioning phase: black circles (male) and white circles (female) represent the responses during the last 3 days of alcohol self-administration sessions; black squares (male) and white squares (female) represents the responses during the last 3 days of water self-administration sessions during the discrimination phases. Analysis of this phase showed a significant time × drugs interaction for the active lever presses. No differences were denoted for the inactive lever. Extinction: mean number of lever presses during the last 3 days of extinction (EXT). Compared to extinction, male (n = 8) and female (n = 10) msP rats showed a significant reinstatement of lever pressing in response to alcohol cues (S+/CS+) but not to water (S−/CS−). Administration of LY2817412 significantly reduced cue (S+/CS+) induced reinstatement of alcohol seeking. Previously alcohol paired active and inactive lever presses are presented in the upper and lower panels, respectively. Values represent the mean (±SEM). ###p < 0.001, difference between EXT and rats exposed to alcohol paired cues (S+/CS+) treated with LY2817412 vehicle (0.0); °°p < 0.01, difference in the reinstatement between male and female msP rats; ***p < 0.001, difference between rats presented with S+/CS+ and LY2817412 vehicle (0.0) and rats treated different doses of the antagonist.
After completion of the conditioning phase, rats were subjected to a 15 daily 30 min extinction sessions during which lever pressing was no longer reinforced and cues were not present.
Followed the reinstatement tests in which the effect of LY2817412 (0.0, 3.0, 10.0, and 30.0 mg/kg; p.o.) was evaluated. The experiment was carried out in a Latin square within-subjects counterbalanced design with the drug given 1 h prior to the beginning of the sessions. Between reinstatement tests, animals remained confined in their home cages.
Effect of intracranial administration of LY2817412 on yohimbine- and cue-induced reinstatement of alcohol-seeking in male and female msP rats
For both yohimbine (1.25 mg/kg, i.p.) and cue-induced reinstatement, male and female msP rats were injected bilaterally with LY2817412 (1.0, 3.0, 6.0 μg/0.5 μl/rat) or vehicle into the VTA (yohimbine: male/female n = 12/9; cue: male/female n = 10/10) the CeA (yohimbine: male/female n = 9/9; cues: male/female n = 10/10) or the NAc (yohimbine: male/female n = 8/9; cue: male/female n = 9/10). Experiments were carried out in a Latin square within-subjects counterbalanced design. During the last 3 self-administration training days the mean values of alcohol intake (g/kg/30 min) for yohimbine experiments were: male, 0.92 for VTA, 0.96 for CeA, 1.24 for NAc; female: 1.06 for VTA, 1.15 for CeA, 1.31 for NAc. For cue-experiments were: male, 0.92 for VTA, 0.93 for CeA, 0.95 for NAc; female: 1.58 for VTA, 1.53 for CeA, 1.44 for NAc. For details, see Supplementary Information (see also Supplementary Fig. 3 and Fig.4).
Effect of intracranial administration of LY2817412 on saccharin self-administration in male and female msP rats
To investigate the effect of NOP receptor blockade on 0.2% (w/v) saccharin self-administration, LY2817412 (1.0, 3.0, 6.0 μg/0.5 μl/rat) or its vehicle were bilaterally microinjected into the VTA (male/female n = 13/12) or the CeA (male/female n = 12/13). Experiments were carried out in a Latin square within-subjects counterbalanced design. A 3-day interval between drug tests was employed. Training data are reported as Supplementary Information (Supplementary Fig. 5).
Histological verification of correct cannula placement
Only data from rats with correct cannula placements were included in the statistical analysis [yohimbine: (male: n = 9 for VTA, n = 7 for CeA, n = 7 for NAc; female: n = 7 for VTA, n = 7 for CeA, n = 8 for NAc); cue: (male: n = 7 for VTA, n = 7 for CeA, n = 8 for NAc; female: n = 7 for VTA, n = 8 for CeA, n = 8 for NAc); saccharin: (male: n = 10 for VTA; n = 10 for CeA; female: n = 9 for VTA; n = 9 for CeA)]. For details, see Supplementary Information.
Statistical analysis
Appropriate ANOVAs were used with ‘sex’ as a between-subjects factor, treatment and ‘time’ as a within-subject factors. Where needed the Newman–Keuls post-hoc test was used. For details, see Supplementary Information.
Results
Systemic administration of LY2817412 reduces yohimbine-induced reinstatement of alcohol seeking in male and female msP rats
During training the mean value of the last 3 days of alcohol self-administration was 48.87 ± 3.98 in males and 46.77 ± 3.98 in females. No significant differences were denoted in alcohol self-administration training both in the active lever [(sex: F(1, 18) = 0.14, p > 0.05; time: F(2, 36) = 0.53, p > 0.05; sex x time: F(2, 36) = 0.15, p > 0.05)] and the inactive lever presses [(sex: F(1, 18) = 7.52, p < 0.05; time: F(2, 36) = 1.06, p > 0.05; sex x time: F(2, 36) = 1.35, p > 0.05)]. During the extinction phase, the number of responding at the active lever progressively decreased to 8.73 ± 0.98 in male and to 18.23 ± 3.07 in female msP rats. As shown in Fig. 1B, overall ANOVA revealed a main effect of treatment, suggesting that administration of yohimbine reinstated the extinguished operant responding for alcohol (sex: F(1, 18) = 22.72, p < 0.001; reinstatement: F(1, 18) = 28.52, p < 0.001; sex × reinstatement: F(1, 18) = 1.08, p > 0.05). A two-way ANOVA denoted that this effect was prevented by treatment with LY2817412 in both sexes (sex: F(1, 18) = 23.05, p < 0.001; treatment: F(2, 36) = 31.68, p < 0.001; sex × treatment: F(2, 36) = 3.48, p < 0.05). Post-hoc analysis showed that both doses of LY2817412 tested, 3.0 and 30.0 mg/kg, were able to reduce yohimbine-induced reinstatement in male (3.0, p < 0.05, 30.0, p < 0.01) and in female (p < 0.001) msP rats. Responding at the inactive lever was negligible and unchanged either by yohimbine (sex: F(1,18) = 2.69, p > 0.05; relapse: F(1, 18) = 3.05, p > 0.05; sex × relapse: F(1, 18) = 0.02, p > 0.05) or LY2817412 (sex: F(1, 18) = 0.57, p > 0.05; treatment: F(2, 36) = 0.13, p > 0.05; sex × treatment: F(2, 36) = 0.60, p > 0.05) (Fig. 1B).
Systemic administration of LY2817412 reduces cue-induced reinstatement of alcohol seeking in male and female msP rats
During the conditioning phase, both male and female msP rats learned to discriminate between alcohol and water availability. At the end of this phase, the number of alcohol-reinforced responses was significantly higher compared to water-reinforced responses [(sex: F(1, 16) = 7.63, p < 0.05; time: F(2, 32) = 68.31, p < 0.001; time × sex: F(2, 32) = 4.13, p < 0.05; drugs: F(1, 16) = 19.88, p < 0.001; drugs × sex: F(1, 16) = 0.54, p > 0.05; time × drugs: F(2, 32) = 32.54, p < 0.001; time × drugs × sex F(2, 32) = 2.48, p > 0.05)]. Alcohol-reinforced responses progressively diminished throughout the extinction phase from 77.25 ± 9.38 to 9.17 ± 1.98 in males, and from 55.37 ± 3.44 to 8.33 ± 1.10 in female msP rats. Two-way ANOVA revealed that presentation of cues predictive of alcohol availability significantly increased alcohol seeking [(sex: F(1, 16) = 3.74, p > 0.05; reinstatement: F(2, 32) = 57.23, p < 0.001; sex × reinstatement: F(2, 32) = 4.17, p < 0.05)]. Post-hoc analysis revealed higher level of reinstatement in female msP rats compared to their male counterpart (p < 0.01, Fig. 2B). In addition, ANOVA revealed a main effect of treatment, with a decreased reinstatement elicited by alcohol-paired cues after systemic administration of LY2817412 [(sex: F(1, 16) = 10.06, p < 0.01; treatment: F(3, 48) = 15.15, p < 0.001; sex × treatment: F(3, 48) = 0.32, p > 0.05)] (Fig. 2B).
Responses at the inactive lever were negligible and not significantly affected by the presentation of cues during the conditioning phase [(sex: F(1, 16) = 0.31, p > 0.05; time: F(2, 32) = 0.00, p > 0.05; time × sex: F(2, 32) = 0.02, p > 0.05; drugs: F(1, 16) = 0.17, p > 0.05; drugs × sex: F(1, 16) = 0.17, p > 0.05; time × drugs: F(2, 32) = 0.05, p > 0.05; time × drugs × sex F(2, 32) = 0.05, p > 0.05)], reinstatement [(sex: F(1, 16) = 30.80, p < 0.001; reinstatement: F(2, 32) = 1.32, p > 0.05; sex × reinstatement: F(2, 32) = 0.36, p > 0.05)] and LY2817412 treatment [(sex: F(1, 16) = 8.62, p < 0.01; treatment: F(3, 48) = 0.11, p > 0.05; sex × treatment: F(3, 48) = 0.05, p > 0.05)] (Fig. 2B).
Intracranial administration of LY2817412 into the VTA and CeA but not into the NAc reduces yohimbine-induced reinstatement of alcohol seeking in male and female msP rats
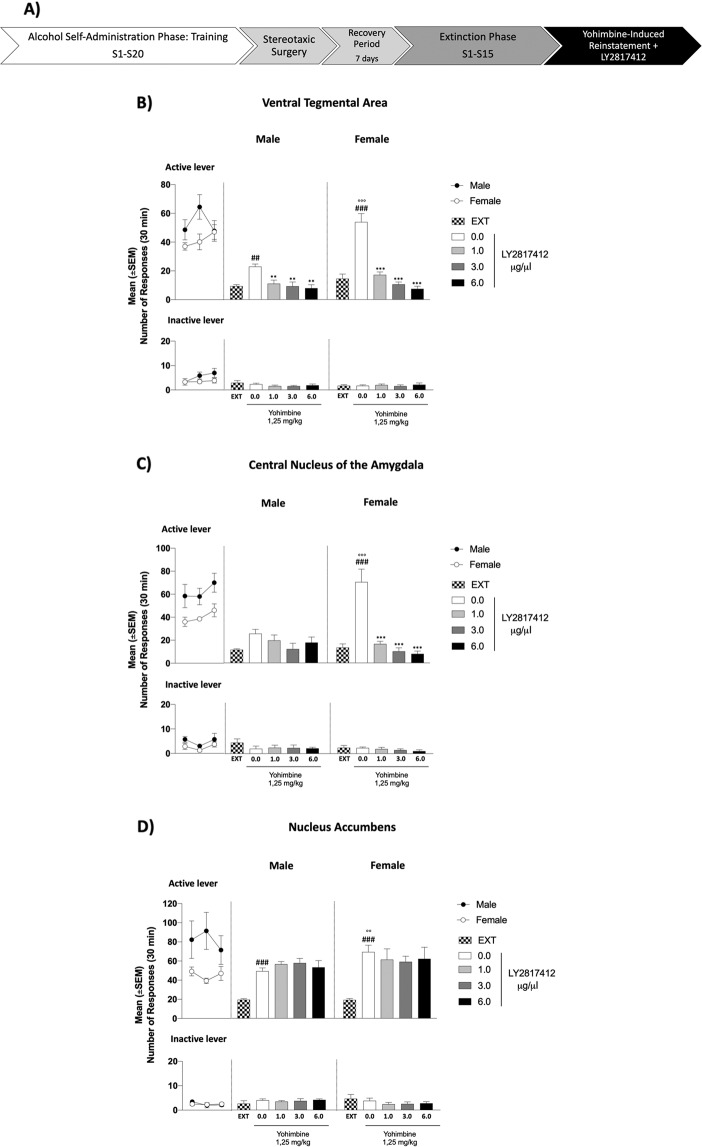
To better investigate the neural substrates involved in the effect of the systemic LY2817412 treatment, we evaluated the effects of LY2817412 microinjections in the VTA, CeA, and NAc on yohimbine-induced reinstatement of alcohol seeking. During the training phase, all experimental groups acquired alcohol self-administration and reached a stable baseline of active lever responses, which was progressively decreased during the extinction phase [(VTA, male: from 53.59 ± 6.42 to 9.30 ± 1.05; female: from 42.0 ± 3.90 to 14.48 ± 3.22; CeA, male: from 62.14 ± 6.53 to 11.48 ± 0.79; female: from 40.19 ± 3.36 to 13.38 ± 3.17; NAc, male: from 81.71 ± 17.12 to 19.48 ± 1.05; female: from 45.17 ± 3.13 to 19.29 ± 1.92)] (Fig. 3B, D). No significant differences were denoted in alcohol self-administration training both in the active lever [VTA: (sex: F(1, 14) = 2.28, p > 0.05; time: F(2, 28) = 2.29, p > 0.05; sex × time: F(2, 28) = 3.51, p > 0.05); CeA: (sex: F(1, 12) = 8.92, p > 0.05; time: F(2, 24) = 2.58, p > 0.05; sex × time: F(2, 24) = 0.10, p > 0.05); NAc: (sex: F(1, 13) = 5.04, p > 0.05; time: F(2, 26) = 0.73, p > 0.05; sex × time: F(2, 26) = 2.71, p > 0.05)] and the inactive lever presses [VTA: (sex: F(1, 14) = 1.96, p > 0.05; time: F(2, 28) = 1.67, p > 0.05; sex × time: F(2, 28) = 0.96, p > 0.05); CeA: (sex: F(1, 12) = 3.94, p > 0.05; time: F(2, 24) = 2.19, p > 0.05; sex × time: F(2, 24) = 0.10, p > 0.05); NAc: (sex: F(1, 13) = 0.02, p > 0.05; time: F(2, 26) = 1.24, p > 0.05; sex × time: F(2, 26) = 0.82, p > 0.05)]. As revealed by two-way ANOVA, administration of yohimbine significantly reinstated the operant response for alcohol in all the experimental groups [VTA: (sex: F(1, 14) = 23.62, p < 0.001, reinstatement: F(1,14) = 118.60, p < 0.001, sex × reinstatement: F(1, 14) = 28.11, p < 0.001); CeA: (sex: F(1, 12) = 11.76, p < 0.01; reinstatement: F(1, 12) = 44.24, p < 0.001; sex × reinstatement: F(1, 12) = 16.26, p < 0.01); NAc: (sex: F(1, 13) = 4.99, p < 0.05, reinstatement: F(1, 13) = 101.51, p < 0.001, sex × reinstatement: F(1, 13) = 6.46, p < 0.05)] (Fig. 3). Post-hoc analysis revealed that female msP rats showed a more pronounced reinstatement than males following yohimbine treatment in all the groups (VTA: p < 0.001; CeA: p < 0.001; NAc: p < 0.01). Yohimbine did not significantly increase active lever presses in male rats microinjected into the CeA (p > 0.05). When the effect of LY2817412 was evaluated, overall ANOVA showed a main effect of intra-VTA (Fig. 3B) and intra-CeA (Fig. 3C) treatments in preventing yohimbine-induced reinstatement of alcohol seeking behavior [VTA: (sex: F(1, 14) = 12.37, p < 0.01, treatment: F(3, 42) = 67.46, p < 0.001, sex × treatment: F(3, 42) = 17.86, p < 0.001); CeA: (sex: F(1, 12) = 2.44, p > 0.05, treatment: F(3, 36) = 25.25, p < 0.001, sex × treatment: F(3, 36) = 13.48, p < 0.001)]. Post-hoc analysis revealed that all doses of LY2817412 (1.0, 3.0, 6.0 μg/0.5 μl/rat) infused in the VTA attenuated yohimbine-induced reinstatement both in male (p < 0.01) and female (p < 0.001) rats. When injected into the CeA LY2817412 reduced yohimbine-induced alcohol-seeking only in female (p < 0.001). Microinjection of LY2817412 into the NAc (Fig. 3D) did not significantly affect yohimbine-induced reinstatement of alcohol seeking in both sexes [(NAc, sex: F(1, 13 = 0.98, p > 0.05, treatment: F(3, 39) = 0.03, p > 0.05, sex × treatment: F(3, 39) = 1.08, p > 0.05)].
Fig. 3. Effect of Intra-VTA, Intra-CeA and Intra-NAc Administration of LY2817412 on Yohimbine‐Induced Reinstatement of Alcohol Seeking in Male and Female msP rats.
A Schematic representation of the experimental timeline. B–D Self-administration: black circles (male) and white circles (female) represent the mean number of responses during the last 3 days of alcohol self-administration sessions. Self‐administration: black (male) and white (female) circle represents the responses during the last 3 days of alcohol self‐administration sessions. No differences were denoted in the number of active or inactive lever presses during this phase in all brain regions. Extinction: mean number of lever presses during the last 3 days of extinction (EXT). B Male (n = 9) and female (n = 7) msP rats were implanted with bilateral cannulas aimed at the VTA. Compared with EXT, yohimbine elicited a significant reinstatement of responding, both in male and in female rats. Intra-VTA administration of LY2817412 reduced the active lever responses elicited by yohimbine treatment in both sexes. C Male (n = 7) and female (n = 7) msP rats were implanted with bilateral cannulas aimed at the CeA. Compared with EXT, yohimbine elicited a significant reinstatement of responding in female but not in male subjects. Intra-CeA administration of LY2817412 reduced the active lever responses elicited by yohimbine treatment only in female rats. D Male (n = 7) and female (n = 8) msP rats were implanted with bilateral cannulas aimed at the NAc. Compared with EXT, yohimbine elicited a significant reinstatement of responding both in male and female msP rats. Values represent the mean (±SEM). ##p < 0.01, ###p < 0.001, difference between EXT and rats treated with yohimbine plus LY2817412 vehicle (0.0); °°p < 0.01, °°°p < 0.001, difference in the reinstatement between male and female msP rats; **p < 0.01, ***p < 0.001, difference between rats treated with yohimbine and LY2817412 vehicle (0.0) and rats treated with different doses of the antagonist.
The number of responses at the inactive control lever was very low throughout all the experiments and was not influenced by yohimbine [VTA: (sex: F(1, 14) = 2.15, p > 0.05, reinstatement: F(1,14) = 0.23, p > 0.05, sex × reinstatement: F(1, 14) = 0.23, p > 0.05); CeA: (sex: F(1, 12) = 0.72, p > 0.05; reinstatement: F(1, 12) = 1.44, p > 0,05; sex × reinstatement: F(1, 12) = 1.14, p > 0.05); NAc: (sex: F(1, 13) = 0.53, p > 0.05, reinstatement: F(1, 13) = 0.04, p > 0.05, sex × reinstatement: F(1, 13) = 0.74, p > 0.05)] nor it was affected by LY2817412 [VTA: (sex: F(1, 14) = 0.00, p > 0.05, treatment: F(3, 42) = 0.49, p > 0.05, sex × treatment: F(3, 42) = 0.55, p > 0.05); CeA: (sex: F(1, 12) = 0.41 p > 0.05; treatment: F(3,36) = 0.48, p > 0,05; sex × treatment: F(3, 36) = 0.57, p > 0.05); NAc: (sex: F(1, 13) = 1.95, p > 0.05, treatment: F(3, 39) = 0.84, p > 0.05, sex × treatment: F(3, 39) = 0.29, p > 0.05)] (Fig. 3).
Intracranial administration of LY2817412 into the VTA but not into the CeA and the NAc reduces cue-induced reinstatement of alcohol seeking in male and female msP rats
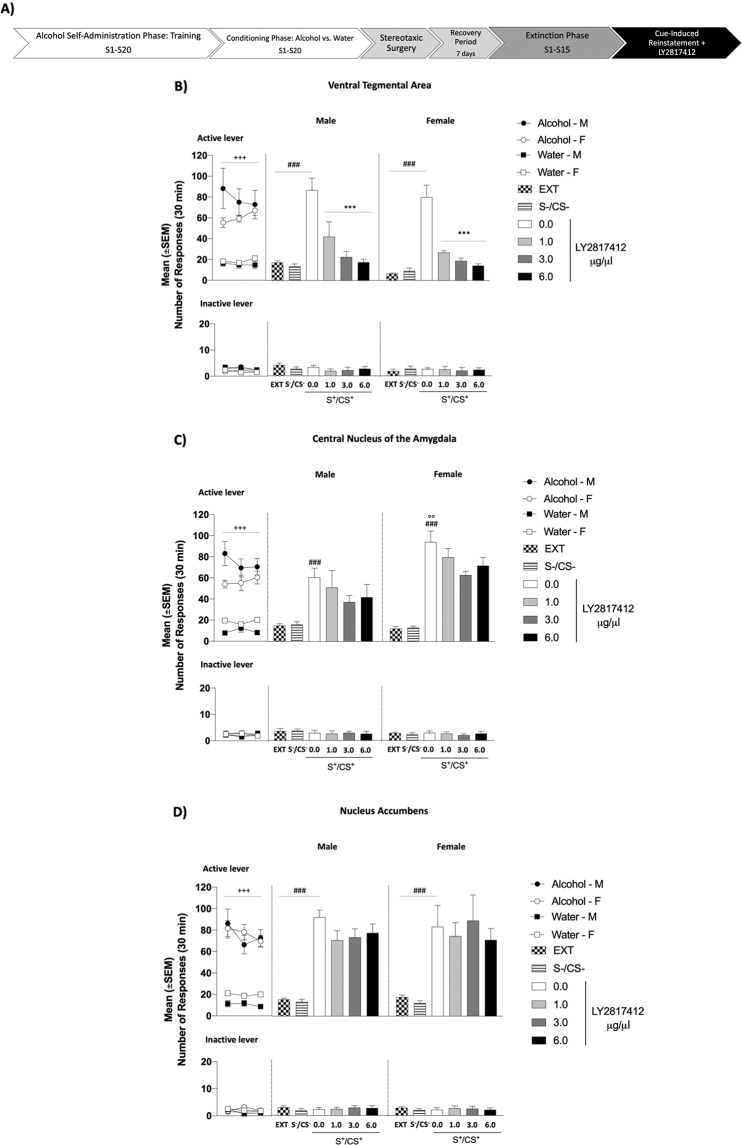
During the conditioning phase, all experimental groups learned to discriminate between alcohol and water availability (Fig. 4A–F) and showed a significant higher number of alcohol-reinforced responses when compared to water-reinforced responses [VTA: (sex: F(1, 12) = 0.95, p > 0.05; time: F(2, 24) = 40.00, p < 0.001; time × sex: F(2, 24) = 3.26, p < 0.05; drugs: F(1, 12) = 23.26, p < 0.001; drugs × sex: F(1, 12) = 1.66, p > 0.05; time × drugs: F(2, 24) = 24.43, p < 0.001; time × drugs × sex: F(2, 24) = 0.29, p > 0.05); CeA: (sex: F(1, 13) = 1.14, p > 0.05; time: F(2, 26) = 93.30, p < 0.001; time × sex: F(2, 26) = 8.41, p < 0.01; drugs: F(1, 13) = 41.62, p < 0.001; drugs × sex: F(1, 13) = 7.43, p; time × drugs: F(2, 26) = 24.24, p < 0.001; time × drugs × sex: F(2, 26) = 5.03, p > 0.05); NAc: (sex: F(1, 14) = 0.82, p > 0.05; time: F(2, 28) = 93.87, p < 0.001; time × sex: F(2, 28) = 0.26, p > 0.05; drugs: F(1, 14) = 52.17, p < 0.001; drugs × sex: F(1, 14) = 3.19, p > 0.05; time × drugs: F(2, 28) = 29.89, p < 0.001; time × drugs × sex: F(2, 28) = 0.34, p > 0.05)]. Alcohol-reinforced responses progressively decreased during the extinction phase [VTA, male: from 78.71 ± 13.90 to 17.33 ± 1.72; female: from 60.57 ± 2.87 to 6.52 ± 0.77; CeA, male: from 74.33 ± 5.68 to 14.90 ± 1.82; female: from 56.54 ± 4.19 to 12.17 ± 2.02; NAc, male: from 75.04 ± 9.11 to 15.67 ± 1.17; female: from 76.42 ± 3.98 to 18.21 ± 1.96]. ANOVA revealed that presentation of cues predictive of alcohol availability significantly increased alcohol seeking behavior in all the experimental groups [VTA: (sex: F(1, 12) = 1.91, p > 0.05, reinstatement: F(2, 24) = 70.38, p < 0.001; sex × reinstatement: F(2, 24) = 0.10, p > 0.05); CeA: (sex: F(1, 13) = 3.95, p > 0.05; reinstatement: F(2, 26) = 74.55, p < 0.001; sex × reinstatement: F(2, 26) = 6.18, p < 0.01); NAc: (sex: F(1, 14) = 0.12, p > 0.05; reinstatement: F(2, 28) = 49.95, p < 0.001; sex × reinstatement: F(2, 28) = 0.23, p > 0.05)]. Post-hoc analysis showed that female rats with cannulas implanted in the CeA reinstated higher than male counterparts (p < 0.01, Fig. 4C). In addition, ANOVA revealed a main effect of treatment when LY2817412 was administered in the VTA [(sex: F(1, 12) = 1.13, p > 0.05; treatment: F(3, 36) = 33.84, p < 0.001; sex × treatment: F(3, 36) = 0.27, p > 0.05)] (Fig. 4B), but not into the CeA [(sex: F(1, 13) = 12.32, p < 0.01; treatment: F(3, 39) = 2.55, p > 0.05; sex × treatment: F(3, 39) = 0.07, p > 0.05)] (Fig. 4C) and the NAc [(sex: F(1, 14) = 0.00, p > 0.05; treatment: F(3, 42) = 1.66, p > 0.05; sex × treatment: F(3, 42) = 1.08, p > 0.05)] (Fig. 4D).
Fig. 4. Effect of Intra-VTA, Intra-CeA and Intra-NAc Administration of LY2817412 on Cue‐Induced Reinstatement of Alcohol Seeking in Male and Female msP rats.
A Schematic representation of the experimental timeline. B–D Conditioning phase: black circles (male) and white circles (female) represent the responses during the last 3 days of alcohol self‐administration; black squares (male) and white squares (female) represent the responses during the last 3 days of water self-administration during the discrimination phases. Analysis of this phase showed a significant time × drugs interaction in all brain regions for the active lever presses. No differences were denoted for the inactive lever. Extinction: mean number of lever presses during the last 3 days of extinction (EXT). B Male (n = 7) and female (n = 7) rats were implanted with bilateral cannulas aimed at the VTA. Compared to EXT, rats exposed to alcohol-predictive of discriminative stimuli (S+/CS+) and treated with LY2817412 vehicle (0.0) reinstated active lever pressing. Intra-VTA administration of the drug attenuated the reinstatement elicited by the alcohol-predictive discriminative stimuli. C Male (n = 7) and female (n = 8) rats were implanted with bilateral cannulas aimed at the CeA. Compared to EXT, rats exposed to alcohol-predictive of discriminative stimuli (S+/CS+) and treated with LY2817412 vehicle (0.0) reinstated active lever pressing. Intra-CeA administration of the NOP antagonist did not prevent the effect of S+/CS+. D Male (n = 8) and female (n = 8) msP rats were implanted with bilateral cannulas aimed at the NAc. Compared to EXT, rats exposed to the alcohol-predictive stimuli (S+/CS+) elicited a significant reinstatement of responding. Intra-NAc administration of the NOP antagonist did not prevent the effect of S+/CS+. Presentation of water paired cues (S−/CS−) never affected operant responding that in all groups remained at extinction level. Values represent the mean (±SEM). ###p < 0.001, difference between EXT and rats exposed to alcohol paired cues (S+/CS+) treated with LY2817412 vehicle (0.0); °°p < 0.001, difference in the reinstatement between male and female msP rats; ***p < 0.001, difference between rats presented with S+/CS+ and LY2817412 vehicle (0.0) and rats treated different doses of the antagonist.
Responses at the inactive lever were negligible and not significantly affected either by cues presentation during the conditioning phase (data not shown), reinstatement [VTA: (sex: F(1, 12) = 1.43, p > 0.05; reinstatement: F(2, 24) = 0.10, p > 0.05; sex × reinstatement: F(2, 24) = 2.10, p > 0.05); CeA: (sex: F(1, 13) = 1.37, p > 0.0l5; reinstatement: F(2, 26) = 0.11, p > 0.05; sex × reinstatement: F(2, 26) = 0.45, p > 0.05); NAc: (sex: F(1, 14) = 0.00, p > 0.05; reinstatement: F(2, 28) = 1.94, p > 0.05; sex × reinstatement: F(2, 28) = 0.04, p > 0.05)] or by LY2817412 [VTA: (sex: F(1, 12) = 0.05, p > 0.05; treatment: F(3, 36) = 0.43, p > 0.05; sex × treatment: F(3, 36) = 0.21, p > 0.05); CeA: (sex: F(1, 13) = 0.10, p > 0.05; treatment: F(3, 39) = 0.11, p > 0.05; sex × treatment: F(3, 39) = 0.16, p > 0.05); NAc: (sex: F(1, 14) = 0.08, p > 0.05; treatment: F(3, 42) = 0.21, p > 0.05; sex × treatment: F(3, 42) = 0.17, p > 0.05)] (Fig. 4).
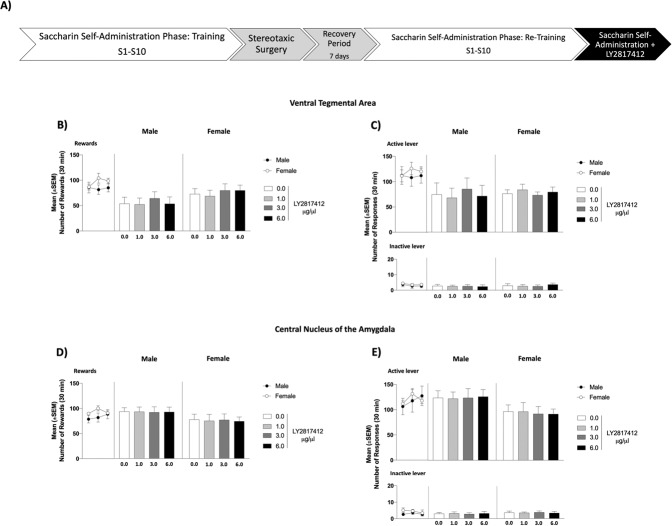
Intracranial administration of LY2817412 into the VTA and the CeA does not reduce saccharin self-administration in male and female msP rats
During the training phase, all experimental groups acquired saccharin self-administration and reached a stable baseline of rewards [(VTA, male: 84.03 ± 8.55; female: 96.37 ± 6.09; CeA, male: 82.97 ± 7.32; female: 93.56 ± 3.04)] and active lever presses [(VTA, male: 110 ± 15.98; female: 119 ± 9.59; CeA, male: 117 ± 15.81; female: 120.44 ± 5.57)] (Fig. 5). No significant differences were denoted in saccharin self-administration training both in the number of rewards [VTA: (sex: F(1, 17) = 1.33, p > 0.05; time: F(2, 34) = 1.24, p > 0.05; sex × time: F(2, 34) = 2.83, p > 0.05); CeA: (sex: F(1, 17) = 1.64, p > 0.05; time: F(2, 34) = 1.22, p > 0.05; sex × time: F(2, 34) = 1.48, p > 0.05)] active lever [VTA: (sex: F(1, 17) = 0.20, p > 0.05; time: F(2, 34) = 0.40, p > 0.05; sex × time: F(2, 34) = 1.19, p > 0.05); CeA: (sex: F(1, 17) = 0.03, p > 0.05; time: F(2, 24) = 1.13, p > 0.05; sex × time: F(2, 34) = 0.57, p > 0.05)] and inactive lever presses [VTA: (sex: F(1, 17) = 1.02, p > 0.05; time: F(2, 34) = 0.60, p > 0.05; sex × time: F(2, 34) = 0.00, p > 0.05); CeA: (sex: F(1, 17) = 1.44, p > 0.05; time: F(2, 34) = 0.73, p > 0.05; sex × time: F(2, 34) = 0.45, p > 0.05)]. As revealed by two-way ANOVA, administration of LY2817412 did not modify the operant response for saccharin in all the experimental groups [rewards: VTA: (sex: F(1, 17) = 1.45, p > 0.05, treatment: F(3, 51) = 1.62, p > 0.05, sex × treatment: F(3, 51) = 0.40, p > 0.05 (Fig. B); CeA: (sex: F(1, 17) = 1.97, p > 0.05; treatment: F(3, 51) = 0.04, p > 0.05; sex × treatment: F(3, 51) = 0.03, p > 0.05 (Fig. D); active lever: VTA: (sex: F(1, 17) = 0.79, p > 0.05, treatment: F(3, 51) = 0.23, p > 0.05, sex × treatment: F(3, 51) = 2.68, p > 0.05 (Fig. C); CeA: (sex: F(1, 17) = 3.30, p > 0.05; treatment: F(3, 51) = 0.01, p > 0.05; sex × treatment: F(3, 51) = 0.08, p > 0.05 (Fig. E)]. Responding at the inactive lever was negligible and unchanged by LY2817412 [VTA: (sex: F(1, 17) = 0.29, p > 0.05, treatment: F(3, 51) = 0.01, p > 0.05, sex x treatment: F(3, 51) = 0.19, p > 0.05); CeA: (sex: F(1, 17) = 0.58, p > 0.05; treatment: F(3, 51) = 0.00, p > 0.05; sex × treatment: F(3, 51) = 0.10, p > 0.05] (Fig. 5).
Fig. 5. Effect of Intra-VTA and Intra-CeA Administration of LY2817412 on Saccharin Self-Administration in Male and Female msP rats.
A Schematic representation of the experimental timeline. B–E Black circles (male) and white circles (female) represent the mean number of rewards (B, D), active (C, E upper panel) and inactive (C, E lower panel) levers during the last 3 days of saccharin self‐administration sessions. B, C Male (n = 10) and female (n = 9) msP rats microinjected into the VTA with LY2817412 did not show changes in saccharin reward or in the total number of lever pressing at both the active and inactive levers. D, E Male (n = 10) and female (n = 9) msP rats microinjected into the CeA with LY2817412 did not show changes in saccharin reward or in the total number of lever pressing at both the active and inactive levers. Values represent the mean (±SEM).
Discussion
The results demonstrated that systemic NOP receptor blockade by LY2817412 significantly attenuated yohimbine- and cue-induced reinstatement of alcohol-seeking in male and female msP rats. These data strengthen recent evidence indicating a role of NOP receptor antagonists in reducing alcohol drinking behavior [16–18]. Here LY2817412 produced a significant decrease of yohimbine-induced reinstatement, replicating the result of earlier work in which a similar effect was obtained with LY2940094, another selective NOP receptor antagonist [16, 23]. With the present work, we also discovered for the first time that yohimbine-induced reinstatement is reduced following blockade of NOP receptors in the VTA and in the CeA but not in the NAc. When we studied the effect of LY2817412 on cue-induced reinstatement, we again observed a significant reduction of lever pressing in msP rats. In this case, the effect was replicated following microinjection of the NOP antagonist into the VTA, but not into the CeA and the NAc. The effect of LY2817412 was specific for alcohol as intra-VTA and intra-CeA infusions of the compound did not modify lever pressing for saccharin. Consistent with this finding, in an earlier study, we found that LY2817412 reduced the intake of alcohol but not that of food and water in the rat [17]. In addition, when rats were treated with the NOP antagonist during the extinction phase (Supplementary Information), it did not affect lever pressing. This finding suggests that blockade of NOP receptors no longer produces its effects if operant behavior is decontextualized from alcohol drinking or is not triggered by yohimbine.
Administration of NOP receptor antagonist LY2817412 in the VTA reduces yohimbine- and cue-induced reinstatement
Noteworthy, previous studies showed that activation of NOP receptors prevents stress- and cue-induced reinstatement of alcohol seeking [14, 24–27]. For instance, i.c.v. administration of N/OFQ markedly inhibited footshock stress- and cue-induced reinstatement of alcohol-seeking in msP rats [13, 14], and subchronic treatment with MT-7716, a selective NOP receptor agonist, reduced yohimbine- and cue-induced reinstatement in msPs and in post-dependent Wistar rats [15]. Finally, SR-8993, another selective NOP receptor agonist, prevented yohimbine-induced reinstatement in Wistar rats [27]. These effects observed following NOP receptor activation might depend on their ability to negatively modulate the activity of the mesolimbic dopamine (DA) system, which is known to play a crucial role in the regulation of stress- and cue-induced reinstatement [28, 29]. Notably, NOP receptors are widely expressed (~75%) on VTA tyrosine hydroxylase positive neurons [30] and N/OFQ inhibits DA neuronal activity in this region [31]. In agreement with these data, intra-VTA administration of N/OFQ attenuated basal DA release in the NAc [32]. Moreover, it was demonstrated that i.c.v. administration of the peptide dampened morphine- and cocaine-induced increases in extracellular DA levels in the NAc [33, 34]. Finally, retrodialysis of N/OFQ into the NAc attenuated the ability of cocaine to enhance local extracellular DA levels into the NAc [35]. Intriguingly, NOP receptor antagonism, produced similar effects as it resulted in a significant decrease of alcohol-induced enhancement of DA outflow in the NAc [16]. Stemming from this latter finding and considering the prominent role of the VTA in modulating alcohol reward and reinforcement, we sought to determine the effect of LY2817412 microinjection into this region [36–38]. Results revealed that intra-VTA infusions of this NOP antagonist reduced both yohimbine- and cue- induced reinstatement, making therefore plausible the hypothesis that receptor blockade could modulate alcohol-seeking via modulation of DA transmission in the VTA. An intriguing finding is that NOP receptor activation and blockade may produce overlapping neurochemical and behavioral effects and that the brain regions (i.e., the VTA and the CeA) mediating these actions are the same. The mechanisms through which this may occur are not yet clear, and to disentangle this complex phenomenon may not be easy, however, several hypotheses can be suggested. For example, it is possible that administration of non-physiological doses of NOP agonists may produce paradoxical antagonistic effects by depressing N/OFQ transmission through receptor desensitization. This possibility is supported by data showing that NOP receptors are subject to desensitization within minutes following administration of an agonist [39]. Consistent with the desensitization hypothesis there are also data showing that the efficacy of NOP agonists on alcohol drinking increases during repeated administration and is maintained for several days after treatment discontinuation [24]. An alternative hypothesis is that, despite having an effect within the same regions, NOP agonists and antagonists may act at different neurocircuitry levels. For instance, electrophysiological data from VTA slices suggested that NOP receptors are located both on DA cells and in presynaptic GABA and glutamate neurons. Hence, they can modulate DA function through both presynaptic and postsynaptic mechanisms, leading to sophisticated modulation of the activity of this catecholaminergic system [31, 40].
Administration of NOP receptor antagonist LY2817412 in the CeA, but not in the NAc, reduces yohimbine-induced reinstatement
A wealth of studies has shown that the α2-adrenergic receptor antagonist yohimbine induces alcohol craving in humans [41] and reinstates extinguished alcohol-seeking in rodents previously trained to self-administer alcohol [42–45]. The mechanism through which yohimbine evokes drug-seeking is complex and likely consists of a concomitant activation of the stress system and the invigoration of responding triggered by exposure to sensory cues [28, 46–49]. As a result of this intricate mechanism, corticotropin-releasing factor-1 receptor antagonism and blockade of DA transmission both reduced yohimbine-induced reinstatement of drug-seeking behavior [50–52]. The amygdala is known to play a pivotal role in mediating stress-related effects on alcohol [1, 53, 54]. Whereas, NOP receptor agonists exert marked anxiolytic and anti-stress effects [55, 56] through modulation of N/OFQ transmission in this region [57, 58]. The impact of NOP antagonists on such behaviors is under intense scrutiny and although in most of the studies receptor blockade has been found ineffective, in some reports, anxiolytic- and antistress-like activities have been reported [59, 60]. For instance, i.c.v. administration of the NOP antagonist UFP-101 reduced the latency of inhibitory avoidance [61]. In addition, the NOP antagonist LY2940094, attenuated fear-evoke immobility in mice, stress-induced hyperthermia in rats [60] and showed antidepressant activity in depressed alcoholics [62]. Furthermore, administration of JTC-801 and J-113397, other two NOP receptor antagonists, reversed anxiety-like behavior and modulated HPA axis activity following traumatic stress [63, 64]. More recently, in a model of inescapable electric foot-shock, administration of the NOP receptor antagonist SB-612111 reversed helpless-induced anxiety-like behaviors, whereas it increased anxiety levels in non-helpless mice, and was ineffective in non-stressed animals tested in the elevated plus-maze (EPM) test [65]. Together these data suggest that NOP receptor blockade may be particularly effective in exerting anxiolytic actions when this condition is associated with stress exposure. In msP rats, excessive alcohol drinking, anxiety-like predispositions, and hypersensitivity to stress have been co-segregated through genetic selection [39]. This multifaceted behavioral trait has been linked to an innate upregulation of the CRF1 and the NOP receptor systems in several stress-related regions, including the amygdala [66–68]. A tempting hypothesis is that intra-CeA administration of LY2817412 attenuated yohimbine-induced reinstatement of alcohol-seeking by alleviating the negative emotional load associated with the over-reactive stress system of msP rats [69, 70].
Electrophysiological studies revealed a significant role of NOP agonists in orchestrating both basal CeA synaptic functions and responsiveness to alcohol. For instance, activation of NOP receptors in this brain region reduced basal GABAergic synaptic transmission and counteracted the facilitatory effect of alcohol in both naïve and post-dependent rats [71, 72]. Activation of NOP receptors also diminished CeA basal glutamatergic transmission and at the same time reduced the inhibitory effects of alcohol on this neurotransmission [73]. Moreover, incubation with N/OFQ evoked a direct postsynaptic inhibition in all neurons recorded from the centromedial amygdala (CeM) [74] and in a subset of the total cells recorded from the CeA [71, 75]. NOP receptor blockade instead produced little effects in naïve control rats, but significantly increased both CeA GABAergic and glutamatergic neurotransmission in alcohol-dependent and chronic stress-exposed rats [57, 73]. Noteworthy, the antagonist prevented the effects of N/OFQ on both GABA and glutamate transmission but failed to prevent the effects of alcohol on glutamate [57, 71–73]. These data indicate that under basal condition CeA output activity is regulated in an opposite direction by NOP agonists and antagonists as expected. However, a history of alcohol exposure likely leads to adaptive changes of the system so that the effects of alcohol on glutamate remain sensitive to NOP agonists but is no longer influenced by receptor blockade. How these adaptive changes influence the effects of NOP agonists and antagonists on reinstatement behavior is unknown at present. But the fact that the CeA has been identified as an important neuroanatomical substrate mediating this action guarantee further investigations.
In conclusion, the present study demonstrates that the pharmacological blockade of NOP receptors by LY2817412 prevents relapse elicited by stress and environmental stimuli predictive of alcohol availability in msP rats. Furthermore, these effects were specifically mediated by the recruitment of the VTA and the CeA, but not the NAc. More studies are needed to better clarify the mechanisms through which NOP receptor blockade prevents reinstatement of alcohol-seeking behavior. The evidence established so far demonstrate that NOP receptor antagonists attenuate alcohol drinking and seeking opening to the possibility of developing these agents for AUD treatments.
Supplementary information
Acknowledgements
We wish thank Linda M. Rorick-Kehn for the scientific inputs and thoughtful comments on the work. We also thank Rina Righi, Agostino Marchi, Mariangela Fiorelli for animal care as well as Alfredo Fiorelli for his excellent technical support. Authors gratefully thank Federica Benvenuti, Marina Antonini, Martina Mondaini, and Maria Sole Centanni for their support in behaviorally testing the animals. This work was supported by the National Institutes of Health, grant RO1 AA014351 (to FW and RC) from the National Institute on Alcohol Abuse and Alcoholism.
Author contributions
AMB and RC were responsible for the study concept and design. AMB performed surgeries, behavioral testing, data analysis and wrote the paper. YF performed surgeries and behavioral tests. SS, AD, and SDC performed behavioral tests. MP, MU, FW, and RC provided critical revision of the paper for important intellectual content. RC and MP contributed to write the paper. All authors critically reviewed the content and approved the final version for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-01096-1.
References
- 1.Koob GF. Neurocircuitry of alcohol addiction: synthesis from animal models. Handb Clin Neurol. 2014;125:33–54. doi: 10.1016/B978-0-444-62619-6.00003-3. [DOI] [PubMed] [Google Scholar]
- 2.Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–4. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- 3.Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol. 1987;96:122–6. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- 4.Martin-Fardon R, Weiss F. Modeling relapse in animals. Curr Top Behav Neurosci. 2013;13:403–32. doi: 10.1007/7854_2012_202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106:243–50. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, et al. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13:372–80. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zilverstand A, Huang AS, Alia-Klein N, Goldstein RZ. Neuroimaging Impaired Response Inhibition and Salience Attribution in Human Drug Addiction: a Systematic Review. Neuron. 2018;98:886–903. doi: 10.1016/j.neuron.2018.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, et al. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–4. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- 9.Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–5. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- 10.Ciccocioppo R, Borruto AM, Domi A, Teshima K, Cannella N, Weiss F. NOP-Related Mechanisms in Substance Use Disorders. Handb Exp Pharm. 2019;254:187–212. doi: 10.1007/164_2019_209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaveri NT. Nociceptin Opioid Receptor (NOP) as a Therapeutic Target: progress in Translation from Preclinical Research to Clinical Utility. J Med Chem. 2016;59:7011–28. doi: 10.1021/acs.jmedchem.5b01499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witkin JM, Statnick MA, Rorick-Kehn LM, Pintar JE, Ansonoff M, Chen Y, et al. The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharm Ther. 2014;141:283–99. doi: 10.1016/j.pharmthera.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, et al. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacol (Berl) 2004;172:170–8. doi: 10.1007/s00213-003-1645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin-Fardon R, Ciccocioppo R, Massi M, Weiss F. Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport. 2000;11:1939–43. doi: 10.1097/00001756-200006260-00026. [DOI] [PubMed] [Google Scholar]
- 15.de Guglielmo G, Martin-Fardon R, Teshima K, Ciccocioppo R, Weiss F. MT-7716, a potent NOP receptor agonist, preferentially reduces ethanol seeking and reinforcement in post-dependent rats. Addict Biol. 2015;20:643–51. doi: 10.1111/adb.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rorick-Kehn LM, Ciccocioppo R, Wong CJ, Witkin JM, Martinez-Grau MA, Stopponi S, et al. A Novel, Orally Bioavailable Nociceptin Receptor Antagonist, LY2940094, Reduces Ethanol Self-Administration and Ethanol Seeking in Animal Models. Alcohol Clin Exp Res. 2016;40:945–54. doi: 10.1111/acer.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borruto AM, Fotio Y, Stopponi S, Brunori G, Petrella M, Caputi FF, et al. NOP receptor antagonism reduces alcohol drinking in male and female rats through mechanisms involving the central amygdala and ventral tegmental area. Br J Pharm. 2020;177:1525–37. doi: 10.1111/bph.14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunori G, Weger M, Schoch J, Targowska-Duda K, Barnes M, Borruto AM, et al. NOP Receptor Antagonists Decrease Alcohol Drinking in the Dark in C57BL/6J Mice. Alcohol Clin Exp Res. 2019;43:2167–78. doi: 10.1111/acer.14165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cippitelli A, Schoch J, Debevec G, Brunori G, Zaveri NT, Toll L. A key role for the N/OFQ-NOP receptor system in modulating nicotine taking in a model of nicotine and alcohol co-administration. Sci Rep. 2016;6:26594. doi: 10.1038/srep26594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999;406:503–47. [PubMed] [Google Scholar]
- 21.Letchworth SR, Mathis JP, Rossi GC, Bodnar RJ, Pasternak GW. Autoradiographic localization of (125)I[Tyr(14)]orphanin FQ/nociceptin and (125)I[Tyr(10)]orphanin FQ/nociceptin(1-11) binding sites in rat brain. J Comp Neurol. 2000;423:319–29. [PubMed] [Google Scholar]
- 22.Economidou D, Mattioli L, Cifani C, Perfumi M, Massi M, Cuomo V, et al. Effect of the cannabinoid CB1 receptor antagonist SR-141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacol (Berl) 2006;183:394–403. doi: 10.1007/s00213-005-0199-9. [DOI] [PubMed] [Google Scholar]
- 23.Toledo MA, Pedregal C, Lafuente C, Diaz N, Martinez-Grau MA, Jiménez A, et al. Discovery of a novel series of orally active nociceptin/orphanin FQ (NOP) receptor antagonists based on a dihydrospiro(piperidine-4,7’-thieno[2,3-c]pyran) scaffold. J Med Chem. 2014;57:3418–29. doi: 10.1021/jm500117r. [DOI] [PubMed] [Google Scholar]
- 24.Ciccocioppo R, Stopponi S, Economidou D, Kuriyama M, Kinoshita H, Heilig M, et al. Chronic treatment with novel brain-penetrating selective NOP receptor agonist MT-7716 reduces alcohol drinking and seeking in the rat. Neuropsychopharmacology. 2014;39:2601–10. doi: 10.1038/npp.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciccocioppo R, Martin-Fardon R, Weiss F, Massi M. Nociceptin/orphanin FQ inhibits stress- and CRF-induced anorexia in rats. Neuroreport. 2001;12:1145–9. doi: 10.1097/00001756-200105080-00019. [DOI] [PubMed] [Google Scholar]
- 26.Ciccocioppo R, Biondini M, Antonelli L, Wichmann J, Jenck F, Massi M. Reversal of stress- and CRF-induced anorexia in rats by the synthetic nociceptin/orphanin FQ receptor agonist, Ro 64-6198. Psychopharmacol (Berl) 2002;161:113–9. doi: 10.1007/s00213-002-1020-7. [DOI] [PubMed] [Google Scholar]
- 27.Aziz AM, Brothers S, Sartor G, Holm L, Heilig M, Wahlestedt C, et al. The nociceptin/orphanin FQ receptor agonist SR-8993 as a candidate therapeutic for alcohol use disorders: validation in rat models. Psychopharmacol (Berl) 2016;233:3553–63. doi: 10.1007/s00213-016-4385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology. 2016;41:335–56. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Namba MD, Tomek SE, Olive MF, Beckmann JS, Gipson CD. The Winding Road to Relapse: forging a New Understanding of Cue-Induced Reinstatement Models and Their Associated Neural Mechanisms. Front Behav Neurosci. 2018;12:17. doi: 10.3389/fnbeh.2018.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norton CS, Neal CR, Kumar S, Akil H, Watson SJ. Nociceptin/orphanin FQ and opioid receptor-like receptor mRNA expression in dopamine systems. J Comp Neurol. 2002;444:358–68. doi: 10.1002/cne.10154. [DOI] [PubMed] [Google Scholar]
- 31.Zheng F, Grandy DK, Johnson SW. Actions of orphanin FQ/nociceptin on rat ventral tegmental area neurons in vitro. Br J Pharm. 2002;136:1065–71. doi: 10.1038/sj.bjp.0704806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy NP, Maidment NT. Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J Neurochem. 1999;73:179–86. doi: 10.1046/j.1471-4159.1999.0730179.x. [DOI] [PubMed] [Google Scholar]
- 33.Lutfy K, Do T, Maidment NT. Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacol (Berl) 2001;154:1–7. doi: 10.1007/s002130000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Giannuario A, Pieretti S. Nociceptin differentially affects morphine-induced dopamine release from the nucleus accumbens and nucleus caudate in rats. Peptides. 2000;21:1125–30. doi: 10.1016/s0196-9781(00)00250-3. [DOI] [PubMed] [Google Scholar]
- 35.Vazquez-DeRose J, Stauber G, Khroyan TV, Xie XS, Zaveri NT, Toll L. Retrodialysis of N/OFQ into the nucleus accumbens shell blocks cocaine-induced increases in extracellular dopamine and locomotor activity. Eur J Pharm. 2013;699:200–6. doi: 10.1016/j.ejphar.2012.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brodie MS, Scholz A, Weiger TM, Dopico AM. Ethanol interactions with calcium-dependent potassium channels. Alcohol Clin Exp Res. 2007;31:1625–32. doi: 10.1111/j.1530-0277.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 37.Budygin EA, Bass CE, Grinevich VP, Deal AL, Bonin KD, Weiner JL. Opposite Consequences of Tonic and Phasic Increases in Accumbal Dopamine on Alcohol-Seeking Behavior. iScience. 2020;23:100877. doi: 10.1016/j.isci.2020.100877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.You C, Vandegrift B, Brodie MS. Ethanol actions on the ventral tegmental area: novel potential targets on reward pathway neurons. Psychopharmacol (Berl) 2018;235:1711–26. doi: 10.1007/s00213-018-4875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toll L, Bruchas MR, Calo' G, Cox BM, Zaveri NT. Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems. Pharm Rev. 2016;68:419–57. doi: 10.1124/pr.114.009209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Driscoll JR, Wallace TL, Mansourian. KA, Martin WJ, Margolis EB. Differential Modulation of Ventral Tegmental Area Circuits by the Nociceptin/Orphanin FQ System. eNeuro. 2020;7:1–15. doi: 10.1523/ENEURO.0376-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George DT, et al. Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropsychopharmacology. 2011;36:1178–86. doi: 10.1038/npp.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simms JA, Bito-Onon JJ, Chatterjee S, Bartlett SE. Long-Evans rats acquire operant self-administration of 20% ethanol without sucrose fading. Neuropsychopharmacology. 2010;35:1453–63. doi: 10.1038/npp.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lê AD, Funk D, Coen K, Li Z, Shaham Y. Role of corticotropin-releasing factor in the median raphe nucleus in yohimbine-induced reinstatement of alcohol seeking in rats. Addict Biol. 2013;18:448–51. doi: 10.1111/j.1369-1600.2011.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, et al. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacol (Berl) 2008;199:109–17. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cippitelli A, Damadzic R, Hansson AC, Singley E, Sommer WH, Eskay R, et al. Neuropeptide Y (NPY) suppresses yohimbine-induced reinstatement of alcohol seeking. Psychopharmacol (Berl) 2010;208:417–26. doi: 10.1007/s00213-009-1741-y. [DOI] [PubMed] [Google Scholar]
- 46.Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacol (Berl) 2013;229:453–76. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen YW, Fiscella KA, Bacharach SZ, Tanda G, Shaham Y, Calu DJ. Effect of yohimbine on reinstatement of operant responding in rats is dependent on cue contingency but not food reward history. Addict Biol. 2015;20:690–700. doi: 10.1111/adb.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabbara RI, Rahbarnia A, Lê AD, Fletcher PJ. The pharmacological stressor yohimbine, but not U50,488, increases responding for conditioned reinforcers paired with ethanol or sucrose. Psychopharmacol (Berl) 2020;237:3689–702. doi: 10.1007/s00213-020-05647-0. [DOI] [PubMed] [Google Scholar]
- 49.Mahler SV, Moorman DE, Feltenstein MW, Cox BM, Ogburn KB, Bachar M, et al. A rodent “self-report” measure of methamphetamine craving? Rat ultrasonic vocalizations during methamphetamine self-administration, extinction, and reinstatement. Behav Brain Res. 2013;236:78–89. doi: 10.1016/j.bbr.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ball KT, Miller L, Sullivan C, Wells A, Best O, Cavanaugh B, et al. Effects of repeated yohimbine administration on reinstatement of palatable food seeking: involvement of dopamine D1 -like receptors and food-associated cues. Addict Biol. 2016;21:1140–50. doi: 10.1111/adb.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown ZJ, Kupferschmidt DA, Erb S. Reinstatement of cocaine seeking in rats by the pharmacological stressors, corticotropin-releasing factor and yohimbine: role for D1/5 dopamine receptors. Psychopharmacol (Berl) 2012;224:431–40. doi: 10.1007/s00213-012-2772-3. [DOI] [PubMed] [Google Scholar]
- 52.Nair SG, Navarre BM, Cifani C, Pickens CL, Bossert JM, Shaham Y. Role of dorsal medial prefrontal cortex dopamine D1-family receptors in relapse to high-fat food seeking induced by the anxiogenic drug yohimbine. Neuropsychopharmacology. 2011;36:497–510. doi: 10.1038/npp.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Becker HC. Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Res. 2012;34:448–58. [PMC free article] [PubMed] [Google Scholar]
- 55.Jenck F, Moreau JL, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJ, Jr, et al. Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc Natl Acad Sci USA. 1997;94:14854–8. doi: 10.1073/pnas.94.26.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jenck F, Ouagazzal AM, Pauly-Evers M, Moreau JL. OrphaninFQ: role in behavioral fear responses and vulnerability to stress? Mol Psychiatry. 2000;5:572–4. doi: 10.1038/sj.mp.4000793. [DOI] [PubMed] [Google Scholar]
- 57.Ciccocioppo R, de Guglielmo G, Hansson AC, Ubaldi M, Kallupi M, Cruz MT, et al. Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: significance for anxiety-like behaviors. J Neurosci. 2014;34:363–72. doi: 10.1523/JNEUROSCI.2400-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andero R, Brothers SP, Jovanovic T, Chen YT, Salah-Uddin H, Cameron M, et al. Amygdala-dependent fear is regulated by Oprl1 in mice and humans with PTSD. Sci Transl Med. 2013;5:188ra73. doi: 10.1126/scitranslmed.3005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gavioli EC, Holanda VAD, Ruzza C. NOP Ligands for the Treatment of Anxiety and Mood Disorders. Handb Exp Pharm. 2019;254:233–57. doi: 10.1007/164_2018_188. [DOI] [PubMed] [Google Scholar]
- 60.Witkin JM, Rorick-Kehn LM, Benvenga MJ, Adams BL, Gleason SD, Knitowski KM, et al. Preclinical findings predicting efficacy and side-effect profile of LY2940094, an antagonist of nociceptin receptors. Pharm Res Perspect. 2016;4:e00275. doi: 10.1002/prp2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duzzioni M, Duarte FS, Leme LR, Gavioli EC, De Lima TC. Anxiolytic-like effect of central administration of NOP receptor antagonist UFP-101 in rats submitted to the elevated T-maze. Behav Brain Res. 2011;222:206–11. doi: 10.1016/j.bbr.2011.03.056. [DOI] [PubMed] [Google Scholar]
- 62.Post A, Smart TS, Krikke-Workel J, Dawson GR, Harmer CJ, Browning M, et al. A Selective Nociceptin Receptor Antagonist to Treat Depression: evidence from Preclinical and Clinical Studies. Neuropsychopharmacology. 2016;41:1803–12. doi: 10.1038/npp.2015.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Genovese RF, Dobre S. Mitigation of adverse behavioral impact from predator exposure by the nociceptin/orphanin FQ peptide antagonist J-113397 in rats. Behav Pharm. 2017;28:521–30. doi: 10.1097/FBP.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Schalo I, Durand C, Standifer KM. Sex Differences in Nociceptin/Orphanin FQ Peptide Receptor-Mediated Pain and Anxiety Symptoms in a Preclinical Model of Post-traumatic Stress Disorder. Front Psychiatry. 2018;9:731. doi: 10.3389/fpsyt.2018.00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silva AI, Holanda V, Azevedo Neto JG, Silva Junior ED, Soares-Rachetti VP, Calo G, et al. Blockade of NOP receptor modulates anxiety-related behaviors in mice exposed to inescapable stress. Psychopharmacol (Berl) 2020;237:1633–42. doi: 10.1007/s00213-020-05487-y. [DOI] [PubMed] [Google Scholar]
- 66.Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Björk K, Soverchia L, et al. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci USA. 2006;103:15236–41. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M. Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addict Biol. 2007;12:30–4. doi: 10.1111/j.1369-1600.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- 68.Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, et al. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol Psychiatry. 2008;64:211–8. doi: 10.1016/j.biopsych.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, et al. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol. 2006;11:339–55. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Borruto AM, Stopponi S, Li H, Weiss F, Roberto M, Ciccocioppo R, et al. Genetically Selected Alcohol-Preferring msP Rats to Study Alcohol Use Disorder: Anything Lost in Translation? Neuropharmacology. 2021;186:108446. doi: 10.1016/j.neuropharm.2020.108446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roberto M, Siggins GR. Nociceptin/orphanin FQ presynaptically decreases GABAergic transmission and blocks the ethanol-induced increase of GABA release in central amygdala. Proc Natl Acad Sci USA. 2006;103:9715–20. doi: 10.1073/pnas.0601899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cruz MT, Herman MA, Kallupi M, Roberto M. Nociceptin/orphanin FQ blockade of corticotropin-releasing factor-induced gamma-aminobutyric acid release in central amygdala is enhanced after chronic ethanol exposure. Biol Psychiatry. 2012;71:666–76. doi: 10.1016/j.biopsych.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kallupi M, Varodayan FP, Oleata CS, Correia D, Luu G, Roberto M. Nociceptin/orphanin FQ decreases glutamate transmission and blocks ethanol-induced effects in the central amygdala of naive and ethanol-dependent rats. Neuropsychopharmacology. 2014;39:1081–92. doi: 10.1038/npp.2013.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chieng B, Christie MJ. Somatostatin and nociceptin inhibit neurons in the central nucleus of amygdala that project to the periaqueductal grey. Neuropharmacology. 2010;59:425–30. doi: 10.1016/j.neuropharm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 75.Meis S, Pape HC. Postsynaptic mechanisms underlying responsiveness of amygdaloid neurons to nociceptin/orphanin FQ. J Neurosci. 1998;18:8133–44. doi: 10.1523/JNEUROSCI.18-20-08133.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.