Abstract
Clinical and translational studies suggest that prefrontal cortex (PFC) dysregulation is a hallmark feature of several affective disorders. Thus, investigating the mechanisms involved in the regulation of PFC function and synaptic plasticity could aid in developing new medications. In recent years, the mGlu2 and mGlu3 subtypes of metabotropic glutamate (mGlu) receptors have emerged as exciting potential targets for the treatment of affective disorders, as mGlu2/3 antagonists exert antidepressant-like effects across many rodent models. Several recent studies suggest that presynaptic mGlu2 receptors may contribute to these effects by regulating excitatory transmission at synapses from the thalamus to the PFC. Interestingly, we found that mGlu3 receptors also inhibit excitatory drive to the PFC but act by inducing long-term depression (LTD) at amygdala-PFC synapses. It remains unclear, however, whether blockade of presynaptic, postsynaptic, or glial mGlu3 receptors contribute to long-term effects on PFC circuit function and antidepressant-like effects of mGlu2/3 antagonists. To address these outstanding questions, we leveraged transgenic Grm3fl/fl mice and viral-mediated gene transfer to genetically ablate mGlu3 receptors from pyramidal cells in the frontal cortex of adult mice of all sexes. Consistent with a role for mGlu3 in PFC pyramidal cells, mGlu3-dependent amygdala-cortical LTD was eliminated following mGlu3 receptor knockdown. Furthermore, knockdown mice displayed a modest, task-specific anxiolytic phenotype and decreased passive coping behaviors. These studies reveal that postsynaptic mGlu3 receptors are critical for mGlu3-dependent LTD and provide convergent genetic evidence suggesting that modulating cortical mGlu3 receptors may provide a promising new approach for the treatment of mood disorders.
Subject terms: Long-term depression, Neurotransmitters, Target validation, Electrophysiology
Introduction
Available medications for mood disorders suffer from several major limitations. Conventional antidepressants require several weeks of treatment, display high rates of discontinuation, and do not improve symptoms in nearly one-third of patients [1–4]. The unsatisfactory nature of antidepressant and anxiolytic treatments contributes to the large socioeconomic burden and negative impact on quality of life imparted by affective disorders [5]. Thus, continued translational research to scrutinize novel treatment approaches is imperative to alleviate individual suffering and societal burdens stemming from the psychiatric disease.
Prefrontal cortex (PFC) dysregulation is a hallmark feature of several affective disorders [6, 7]. Alterations to synaptic proteins and glutamate signaling have been widely implicated in human studies and in animal models of chronic stress [7–10]. Thus, molecules that regulate PFC glutamate transmission, such as metabotropic glutamate (mGlu) receptors, have emerged as exciting targets for the development of novel psychiatric medications. Extensive preclinical studies have demonstrated that non-selective antagonists for the mGlu2 and mGlu3 receptor subtypes exert antidepressant-like effects by enhancing PFC glutamate transmission [11–15]. Several studies suggested that PFC mGlu2 and mGlu3 receptors might serve as presynaptic autoreceptors, as acute administration of mGlu2/3 agonists and antagonists modulates parameters that often reflect glutamate release probability [16–20]. However, recent findings using contemporary, selective pharmacological tools suggest these acute presynaptic effects may be strictly attributable to mGlu2 receptors [21]. By contrast, studies from our laboratory and others indicate that mGlu receptor long-term depression (LTD) of excitatory transmission is blocked by mGlu3 negative allosteric modulators (NAMs), which involves postsynaptic signaling cascades, and is manifested via AMPA receptor internalization [22–26]. Thus, mGlu2 receptors transiently depress glutamate release while mGlu3 receptors facilitate persistent postsynaptic LTD. However, mGlu3 receptors are expressed across several PFC cell types [27, 28] and it remains unclear whether presynaptic, postsynaptic, or glial mGlu3 receptors confer long-term effects on PFC circuit function.
Based on previous pharmacological studies, we hypothesized that postsynaptic mGlu3 receptors modulate synaptic physiology and affective behaviors. To test this hypothesis, we leveraged newly developed transgenic Grm3fl/fl mice and viral-mediated gene transfer to genetically delete mGlu3 receptor expression from pyramidal cells in the frontal cortex of adult female mice and male mice. While mGlu3 receptor ablation had modest effects on pyramidal cell physiology, postsynaptic mGlu3 receptors were critical for mGlu3-dependent LTD, particularly at synapses arising from the basolateral amygdala (BLA). Furthermore, cortical mGlu3 receptor knockdown mice displayed decreased passive coping behavior in multiple assays.
Materials and methods
Animals
Adult (>10 weeks old) mice were used for all experiments and were not excluded by sex. No differences related to external genitalia were detected. Grm3fl/fl mice were developed as described [29]. In brief, loxP recombination sites were introduced to flank the third exon within the Grm3 gene (Grm3fl/fl). Cre-mediated recombination, therefore, generates several frameshift mutations and ablates mGlu3 receptor protein. Upon arrival, Grm3fl/wt mice on a C57BL/6N background were crossed once with C57BL/6J mice. That generation was interbred, and mice were maintained on the mixed F2 C57BL/6N ×6J background. All experiments were conducted with litter- and cage-matched controls. Mice were group-housed (3–5 per cage) on a 12-h light cycle (lights on at 6:00 a.m.) with all experiments conducted during the light phase across multiple days. Food and water were available ad libitum. All experimental protocols were approved by the Vanderbilt Institutional Animal Care and Use Committee. We performed behavioral studies across three cohorts. A total of 18 GFP mice (10 ♀, 8 ♂) and 16 Cre mice (8 ♀, 8 ♂) were used. Two cohorts underwent the open field test before further testing. All three cohorts underwent the elevated plus maze, light-dark box (LDB), tail suspension test (TST), and forced swim test (FST), in that order. Only one cohort underwent the second FST. Mice used for biochemistry and electrophysiology were not included in behavioral experiments.
Stereotaxic injections
At 4–5 weeks of age, Grm3fl/fl mice underwent surgery under 1.5–2% isoflurane anesthesia for viral-mediated expression of green fluorescent protein (GFP) or Cre recombinase (800 nL, AAV5-CaMKII-GFP or AAV5-CaMKII-Cre-GFP, ≥1 × 1011 vg/mL, UNC Viral Vector Core) in the PFC [M/L: ±0.3, A/P: +1.9, D/V: −2.3]. In some experiments, the red-shifted opsin Chrimson (400 nL, AAV5-Syn-ChrimsonR-tdTomato, ≥7 × 1012 vg/mL, Addgene viral prep # 59171-AAV5) was expressed in the BLA [M/L: −3.2, A/P: −1.6, D/V: −4.4] during the same procedure as PFC virus delivery. Viruses were infused at 100 nL/min. AAV5-Syn-ChrimsonR-tdTomato was a gift from Edward Boyden. Experiments were conducted at least 6 weeks following viral-mediated Cre expression.
Synaptosome preparation and SDS-PAGE Western blotting
The PFC was microdissected and synaptosome preparations were prepared as described [30]. Briefly, brain tissue samples were homogenized in ice-cold buffer (320 mM sucrose, 4.2 mM HEPES, pH 7.4) with Roche cOmpleteTM EDTA-free protease inhibitor cocktail (Sigma) using a glass homogenizer (Wheaton). After homogenization, samples were centrifuged at 1000 g for 5 min at 4 °C and the resulting supernatant was subsequently centrifuged at 12,000 g for 15 min. The P2 pellet was resuspended in radioimmunoprecipitation assay buffer (10 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 0.1% sodium dodecyl sulfate, 1% Triton X-100, and 1% deoxycholate) (Sigma) containing protease inhibitors Roche cOmpleteTM EDTA-free and phenylmethylsulfonyl fluoride, and phosphatase inhibitors cocktails 1 and 2 (Sigma). Protein concentration was determined using a bicinchoninic acid protein assay (Pierce). As previously described [31], 50 μg of total protein was electrophoretically separated using a 4–20% SDS polyacrylamide gel and transferred onto a nitrocellulose membrane (iBlot2, ThermoFisher). Membranes were blocked in TBS Odyssey blocking buffer (LI-COR) for 1 hr at room temperature. Membranes were probed with primary antibodies overnight at 4 °C: rabbit anti-mGlu3 (1:1000, Alomone, AGC_012) and mouse anti-Gapdh (1:1000, ThermoFisher, MA5-15738), followed by the fluorescent secondary antibodies: goat anti-rabbit (800 nm, 1:5000, LI-COR) and goat anti-mouse (680 nm, 1:10,000, LI-COR). Fluorescence was then quantified using the Image Studio Lite software (LI-COR). Values were normalized to GAPDH and compared relative to controls (Grm3fl/fl GFP).
Whole-cell electrophysiology
Slices were prepared and recordings made as described [32]. Mice were anesthetized with isoflurane and decapitated. Coronal slices (300 μm) were prepared using an N-Methyl-D-glucamine-based cutting and recovery solution. The artificial cerebrospinal fluid solute concentrations were (in mM): 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgCl2, 1 NaH2PO4, 11 glucose, and 26 NaHCO3. Recordings were performed in a heated (30 ± 1°C), oxygenated (95% O2/5% CO2) bath, perfused at 2 mL/min. Pyramidal cells in layer 5 were filled with a potassium-based solution (in mM): 125 K-gluconate, 4 NaCl, 10 HEPES, 4 MgATP, 0.3 NaGTP, 10 Tris-phosphocreatine. Electrical or optical excitatory postsynaptic currents (EPSCs or op-EPSCs) were elicited by 0.1–0.2 ms, 5–50 µA electrical stimulation within layer 5, or by 1–3 ms, 620 nm light stimulation passed through the microscope objective. Recordings were made at −80 mV and LTD was induced by applying 200 nM LY379268 (Abcam) for 10 min [22, 24].
Open-field test
The Activity Monitor Program was used to track mouse movement and activity within an open field chamber housed inside a sound-attenuating cabinet (ENV-510 and MED-OFA-022, MedAssociates). Experiments were conducted over 90 min without any prior habituation to the chamber.
Elevated zero-maze (EZM)
The EZM was performed as previously described [33]. ANYmaze software tracked mouse movement on the elevated zero maze apparatus. Percent time in open arms, open arm entries and distance traveled were analyzed.
Light-dark box (LDB)
The LDB was performed for 10 min using a tinted insert that covers half of an open field chamber (ENV-511, MedAssociates). A zoning analysis was conducted to assess the amount of time spent on the light side and the number of entries/crossings into the light side.
Tail suspension test (TST)
Mice were suspended by their tails onto a computer-monitored load cell. The Tail Suspension Program in conjunction with the Tail Suspension Starter Package (MED-TSS-MS, MedAssociates) was used to detect movement, and immobility was defined as the lack of movements below a set threshold. Each session lasted 6 min and the total time of immobility and the latency to enter the first 10-s immobile bout were recorded.
Forced swim test (FST)
Mice were placed in a Plexiglas cylinder (diameter 22 cm, height 26 cm) with ~10 cm of water (23 ± 1 °C). Six-minute swim sessions were manually scored by a blinded observer. Total time of immobility and latency to enter the first 10-s immobile bout was recorded. A subset of mice underwent a second forced swim one day after the initial test.
Statistics
The number of cells and mice for each experiment is denoted by “n” and “N”, respectively. No overt sex differences were observed so all data were pooled and analyzed together. Data are presented as mean ± standard error. For individual data points in behavioral experiments, circles indicate data obtained from female mice, and squares indicate values from male mice. Analyses were performed using GraphPad Prism. Statistical outliers were detected and removed using the ROUT method and a maximum false discovery rate of 5%. Two-tailed Student’s t-test, two-way ANOVA with Sidak post-tests, or the Log-rank (Mantel–Cox) test were used to detect significance.
Results
Frontal cortex mGlu3 receptor ablation
Acute inhibition of mGlu3 receptors generates antidepressant-like effects in animal models [34–36], but the requisite cell types mediating the responses remain unclear. Here, we leveraged Grm3fl/fl transgenic mice recently developed by our laboratory [29] to assess how mGlu3 receptors modulate PFC function. We implemented a viral approach to regionally express Cre recombinase in pyramidal cells in adult mice (Figs. 1a and S1).
Fig. 1. Viral-mediated Cre expression in Grm3f/f mouse frontal cortex attenuates neuronal mGlu3 expression and function.
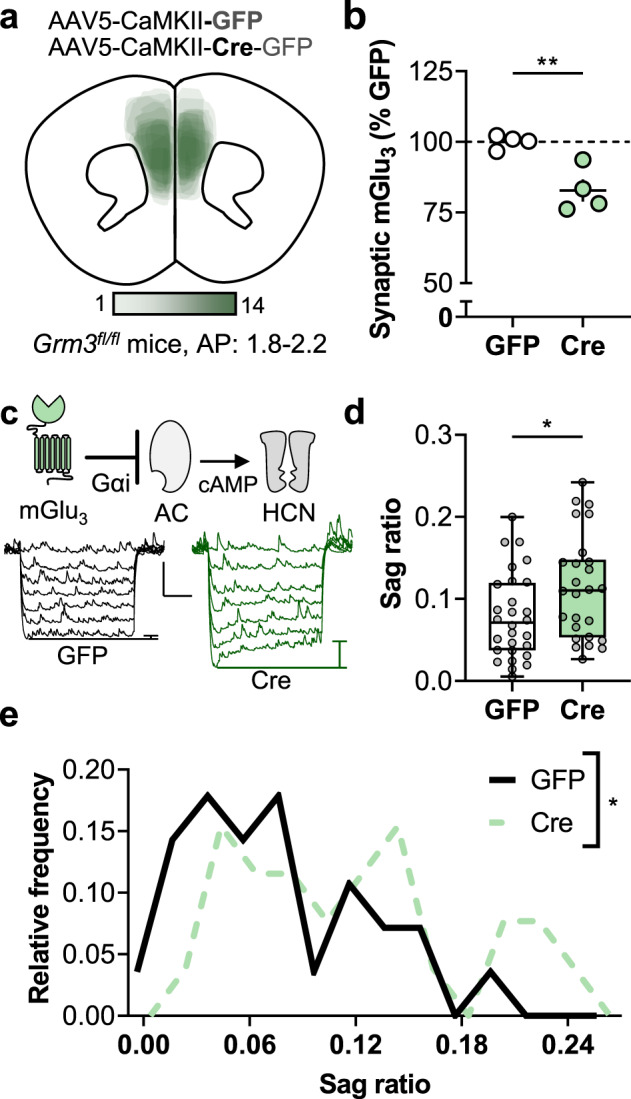
a The adeno-associated virus was delivered to express Cre recombinase or GFP alone as a control. The illustration depicts the location of fluorophore expression in the mouse frontal cortex from the Cre-expressing mice used in the behavioral experiments (Figs. 4 and 5). b Crude synaptosomes were prepared from the frontal cortices of mice that underwent viral-mediated Cre expression. mGlu3 receptor expression was assessed via Western blot. Cre samples contained less mGlu3 receptor protein than GFP controls (82.8 ± 3.9% GFP, **p < 0.01, t6 = 4.196, N = 4 mice per group). c Hyperpolarization sag ratio was assessed as a downstream measurement related to mGlu3 receptor signaling. In layer 5 prelimbic pyramidal cells, hyperpolarization-activated cation (HCN) currents are regulated by intracellular cAMP and adenylate cyclase (AC) activity, processes inhibited by Gαi protein signaling. The representative traces depict how HCN channel currents are detected as upward, depolarizing rebounds following negative current injections (−150…0, Δ25 pA). Scale bars indicate 5 mV, 250 ms. d Cre expression increased the hyperpolarization sag ratio in layer 5 pyramidal cells (0.114 ± 0.012 Cre vs 0.079 ± 0.010 GFP, *p < 0.05, t52 = 2.256, n/N = 26–28 cells from N = 12–13 mice per group). e Layer 5 pyramidal cells displayed a broad, biphasic distribution in sag ratio. The Cre-expressing neuron sag ratio distribution was significantly different than GFP controls (*p < 0.05, Log-rank test).
We have previously shown that genetic CaMKII-Cre expression reduces Grm3 expression in hippocampal pyramidal cells but not in glia [29]. To validate that viral-mediated expression of Cre recombinase ablates mGlu3 receptors, we isolated crude synaptosomes and performed Western blots to assess protein expression. Grm3fl/fl mice displayed comparable basal levels of synaptic mGlu3 receptor protein to congenic C57BL/6J mice (94.9 ± 7.5% C57BL/6J, n.s., t6 = 0.6212, N = 4 mice per group, data not shown). As expected, cortical synaptosomes from Cre-expressing tissues displayed decreased mGlu3 receptor protein relative to matched controls (Fig. 1b).
As synaptosomes contain pre- and postsynaptic membranes, receptor location cannot be inferred from these data alone. In addition, residual mGlu3 expression in Cre mice might result from (1) presynaptic receptors from long-range afferents (e.g., BLA), (2) receptors not yet degraded following Grm3 excision, (3) receptors on PFC cell types not targeted by the virus (e.g., glial receptors that may have contaminated the crude synaptosome preparation), and/or (4) receptors on PFC pyramidal cells outside the range of virus spread. Based on these limitations, we functionally assessed how reduced mGlu3 expression affects the physiology of PFC pyramidal cells. Guided by GFP fluorescence, we targeted Cre-expressing neurons for whole-cell patch-clamp electrophysiology in acute PFC slices. All fluorescent cells (n = 56) exhibited the characteristic firing properties of pyramidal cells, including large capacitance, spike-firing adaptation, and hyperpolarization rebound sag. The rebound sag in PFC pyramidal cells is mediated by hyperpolarization-activated cyclic nucleotide-gated (HCN) channels [37, 38], which are activated by cAMP and other related molecules (Fig. 1c). mGlu3 receptors canonically couple with Gαi proteins [39]; therefore, we predicted that reduced mGlu3 receptor expression would elevate basal cAMP levels and facilitate HCN channel function. Indeed, Cre-expressing pyramidal cells displayed enhanced hyperpolarization sag relative to GFP controls, consistent with reduced mGlu3 receptor expression (Fig. 1d, e). Otherwise, Cre-expressing neurons exhibited comparable intrinsic physiology relative to GFP controls (Fig. S2a–c), with the sole exception of the reduced medium afterhyperpolarization (Fig. S2d).
Pyramidal cell expression of mGlu3 gates synaptic plasticity in the mouse PFC
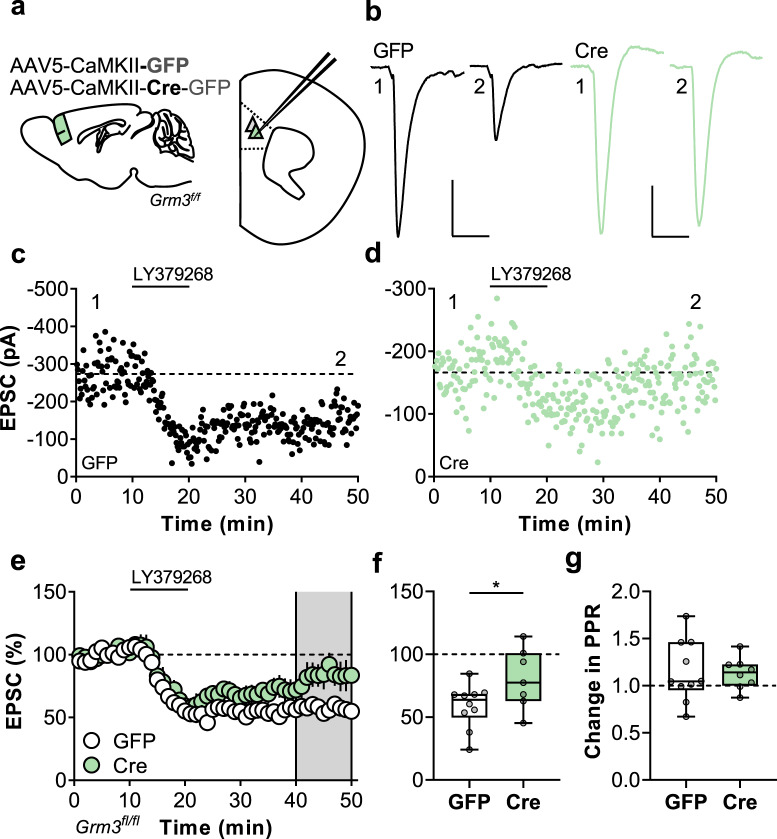
We next aimed to assess how genetic ablation of neuronal mGlu3 receptors affects synaptic physiology and plasticity. Cre- and GFP-expressing pyramidal cells did not differ regarding basal excitatory synaptic strength (Fig. S2e, f) and we proceeded to assess synaptic plasticity. mGlu3 receptor activation induces postsynaptically-maintained LTD of excitatory transmission in the mouse PFC [22–24]. This LTD is blocked by bath application of highly selective mGlu3 NAMs but the cellular location of receptor expression is not known. We evoked EPSCs with electrical stimulation of medial layer 5 (Fig. 2a, b). In the PFC, mGlu3 receptors gate postsynaptically-maintained synaptic plasticity, while mGlu2 receptors modulate presynaptic glutamate release at thalamocortical synapses [34]. Thus, non-selective mGlu2/3 agonists exert several effects on PFC neurotransmission. As reported previously, bath application of the mGlu2/3 receptor agonist LY379268 (200 nM) generated an acute depression and a persistent LTD in control pyramidal cells (Fig. 2c, e, f). We also observed the acute depression of synaptic transmission in Cre-expressing cells, consistent with the retained function of presynaptic mGlu2 receptors (Fig. 2d, e). By contrast, Cre-expressing neurons displayed an impairment of LTD (Fig. 2e, f), indicating that mGlu3 receptors expressed in pyramidal cells are required for this form of PFC plasticity. Consistent with the presynaptic expression of mGlu2 receptors, LY379268 transiently increased the paired-pulse ratio (PPR) in GFP- and Cre-expressing mice (Fig. S3) without having any long-term effect (Fig. 2g), consistent with a postsynaptic locus for LTD expression.
Fig. 2. Neuronal mGlu3 receptors mediate synaptic plasticity in the prefrontal cortex (PFC).
a Schematic depicting viral-mediated expression of Cre recombinase in mouse PFC. At least four weeks following the procedure, acute slices containing PFC were prepared and whole-cell patch-clamp recordings were made from fluorescent pyramidal cells in layer 5 prelimbic cortex. b Excitatory postsynaptic currents (EPSCs) were elicited via local electrical stimulation and long-term depression (LTD) of synaptic transmission was evoked by bath applying the mGlu2/3 agonist LY379268 (200 nm, 10 min). Representative traces depict EPSCs during baseline (1) and after LTD (2) from neurons expressing GFP alone (left, black) or Cre (right, green). Scale bars indicate 100/50 pA, 25 ms. c Representative LTD experiment from GFP control pyramidal cell. d Representative LTD experiment from Cre-expressing pyramidal cell. e Summarized time-courses from multiple LTD experiments. The EPSC amplitude in each experiment is normalized to its baseline value (n/N = 7–10 cells from 5–6 mice per group). f Average relative change in EPSC amplitude following LTD. Expression of Cre recombinase attenuated the induction of LTD (80.3 ± 9.1% Cre vs 58.9 ± 5.5% GFP, *p < 0.05, t15 = 2.153). g LTD was not associated with a change in the paired-pulse ratio (PPR) in either GFP or Cre cells.
Postsynaptic mGlu3 receptors are essential for PFC synaptic plasticity
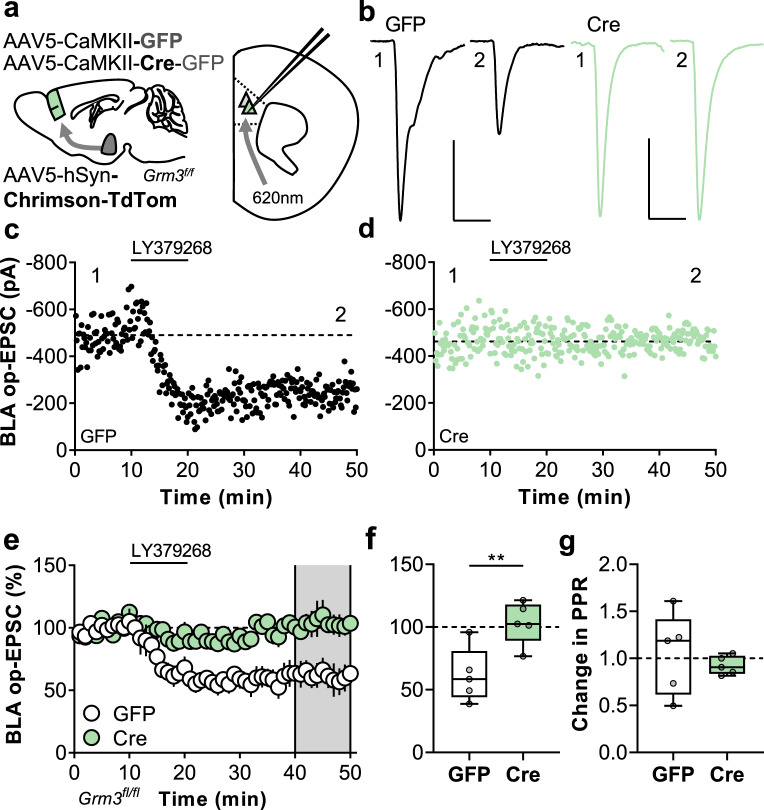
The previous experiments clearly implicated pyramidal cell mGlu3 receptors as necessary for the expression of mGlu3-dependent LTD, yet a non-negligible LTD remained. This finding suggests that the residual component is mediated by: (1) incomplete ablation of mGlu3 protein and/or (2) presynaptic mGlu2 receptors (likely at thalamic terminals). Moreover, the experiments using electrical stimulation did not definitively implicate postsynaptic mGlu3 receptors, as LTD could potentially involve mGlu3 receptors expressed in presynaptic terminals from superficial cortical layers [27, 28]. To address all these outstanding questions, we targeted long-range afferents arising from the BLA, which display mGlu3-LTD without detectable effects following mGlu2 receptor activation [24]. In these studies, we virally expressed Chrimson to optogenetically isolate BLA-PFC synapses in GFP- and Cre-expressing slices (Fig. 3a, b). Consistent with previous studies, control neurons displayed slow-onset BLA-PFC LTD following LY379268 application (Fig. 3c, e, f). Cre-expressing cells, however, exhibited no LTD whatsoever (Fig. 3d–f). As expected, changes in BLA op-EPSC PPR were not observed at any timepoint in GFP- or Cre-expressing cells (Figs. S3 and 3g). While an occlusion effect in Cre-expressing cells is not inconsistent with the present findings, these data indicate that postsynaptic mGlu3 receptors mediate synaptic plasticity in PFC.
Fig. 3. Postsynaptic mGlu3 receptors are indispensable for synaptic plasticity at amygdalo-cortical synapses.
a Schematic depicting viral-mediated expression of Cre recombinase in mouse prefrontal cortex (PFC) and Chrimson expression in mouse basolateral amygdala (BLA). Acute slices containing PFC were prepared and whole-cell patch-clamp recordings were made from fluorescent pyramidal cells in layer 5 prelimbic cortex. b Optical excitatory postsynaptic currents (op-EPSCs) were elicited via red light stimulation (620 nm, 1–3 ms) and long-term depression (LTD) of synaptic transmission was evoked with the mGlu2/3 agonist LY379268 (200 nm, 10 min). Representative traces depict op-EPSCs during baseline (1) and after LTD (2) from neurons expressing GFP alone (left, black) or Cre (right, green). Scale bars indicate 200 pA, 25 ms. c Representative LTD experiment from GFP control pyramidal cell. d Representative LTD experiment from Cre-expressing pyramidal cell. e Summarized time-courses from multiple LTD experiments where the op-EPSC amplitude in each experiment is normalized to its baseline value (n/N = 5 cells from 2–4 mice per group). f Average relative change in op-EPSC amplitude following LTD. Expression of Cre recombinase attenuated the induction of LTD (103.3 ± 7.6% Cre vs 61.6 ± 9.7% GFP, **p < 0.01, t8 = 3.382). g LTD was not associated with a change in the paired-pulse ratio (PPR) in either GFP or Cre cells.
Cortical ablation of mGlu3 receptors induces a modest anxiolytic phenotype
Acute inhibition of mGlu3 receptors attenuates behavioral responses to stress and alters cortical synaptic plasticity [24]. Based on this, we predicted that cortical mGlu3 receptor downregulation might confer anxiolytic-like effects in rodent models. Prior to examining affective behaviors in specialized tasks, we performed an open field test in GFP and Cre mice to test whether genetic deletion of cortical mGlu3 receptors overtly alters locomotor activity. Similar to previous studies in global Grm3−/− mice [40–42], we observed comparable patterns of locomotor activity in a novel environment (Fig S4) and therefore felt confident in our ability to interpret data from studies that involve mouse locomotion.
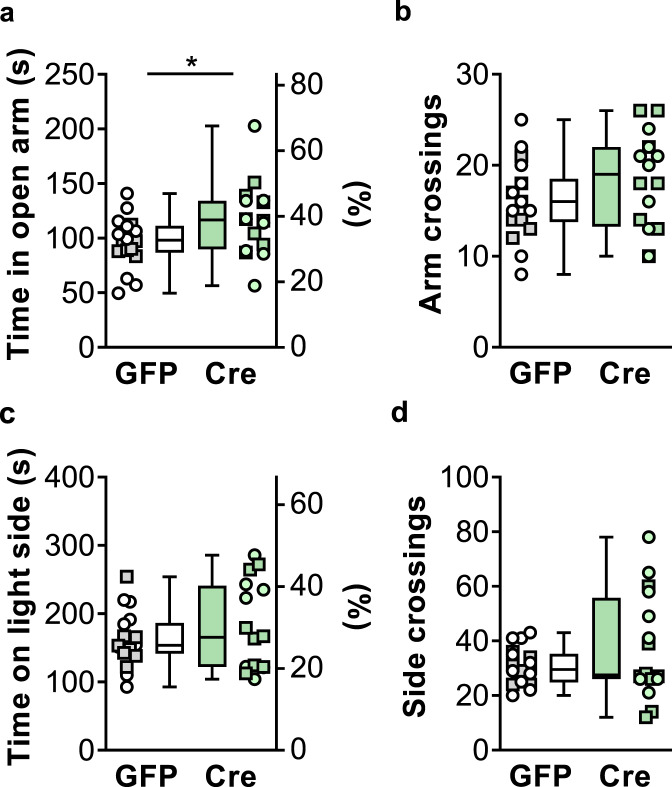
We assessed anxiety-like behavior in two tasks that take advantage of approach-avoidance conflicts. The EZM consists of a platform with brightly lit open arms and sheltered closed arms flanked by raised walls. A rodent’s innate tendency to explore a new environment is counteracted by its instinct to avoid exposed spaces, such that anxiolytic manipulations generally increase the amount of time spent in the open arms. Cre mice displayed more time in the open arms of the EZM than GFP littermate controls (Fig. 4a), consistent with a potential anxiolytic phenotype following mGlu3 receptor knockdown. No differences were observed in the number of arm crossings (Fig. 4b), suggesting that changes in locomotor activity did not contribute to the amount of time spent in the open arms. We also assessed anxiety-like behavior using the LDB. The LDB is operationally similar to the EZM, in that mice face a conflict to approach the novel area but avoid the light-exposed environment. In this task, no differences were observed with respect to the total time spent in the light zone (Fig. 4c) or the number of zone crossings (Fig. 4d). In addition, GFP- and Cre-expressing mice displayed comparable center time when placed in a novel open field (Fig. S4). Collectively, these data suggest that cortical mGlu3 knockdown may generate a modest and/or task-dependent anxiolytic phenotype in mice. These data are consistent with previous studies [40, 41] that showed modest differences in a subset of anxiety-like in global Grm3−/− mice.
Fig. 4. Frontal cortex mGlu3 receptor knockdown induces a modest anxiolytic phenotype.
a Cre-expressing mice (green circles ♀, green squares ♂) spent more time in the open components of the elevated-zero maze than GFP controls (white circles ♀, gray squares ♂) (117.2 ± 8.4 s Cre vs 96.4 ± 5.5 s GFP, *p < 0.05, t32 = 2.115, N = 16–18 mice/group). b No difference in the total number of arm crossings between GFP and Cre mice. c No difference in the proportion of time spent in the brightly lit side of a light-dark box between GFP and Cre mice. d No group difference in the number of side crossings.
Cortical knockdown of mGlu3 decreases passive coping behavior
Conventional, novel, and experimental antidepressant treatments, including mGlu3 NAMs [34–36], decrease passive coping behavior in preclinical models. By contrast, studies involving constitutive mGlu3 receptor knockout mice have yielded mixed findings related to escape behavior [11, 42]. We, therefore, performed the TST and FST in mice with mGlu3 receptors ablated from PFC. In these assays, mice initially attempt to free themselves from noxious yet inescapable positions but eventually submit to an immobile posture, reminiscent of defeat. Consistent with an antidepressant-like manipulation, Cre mice displayed a longer latency to immobility than controls in the TST (Fig. 5a), although total immobility did not reach statistical significance between groups (Fig. 5b). We next assessed passive coping behavior in the FST. In this task, Cre mice took longer to first enter an immobile posture than GFP mice (Fig. 5c) and spent more total time immobile throughout the task (Fig. 5d). In a subset of mice, we examined the FST on the second day of testing when rodents spend more time immobile than during their first exposure. Both GFP and Cre mice displayed rapid transitions into immobility on the second day and no difference between groups was observed (Fig. 5e). Nonetheless, Cre mice exhibited decreased total time immobile on day 2 relative to GFP controls (Fig. 5f). We did not find evidence for correlated behaviors across assays in either Cre or GFP mice (Fig. S5). Taken together, these data indicate that decreased expression of cortical mGlu3 receptors decreases passive coping behavior, consistent with an antidepressant-like effect.
Fig. 5. Frontal cortex mGlu3 receptor knockdown decreases passive coping behaviors.
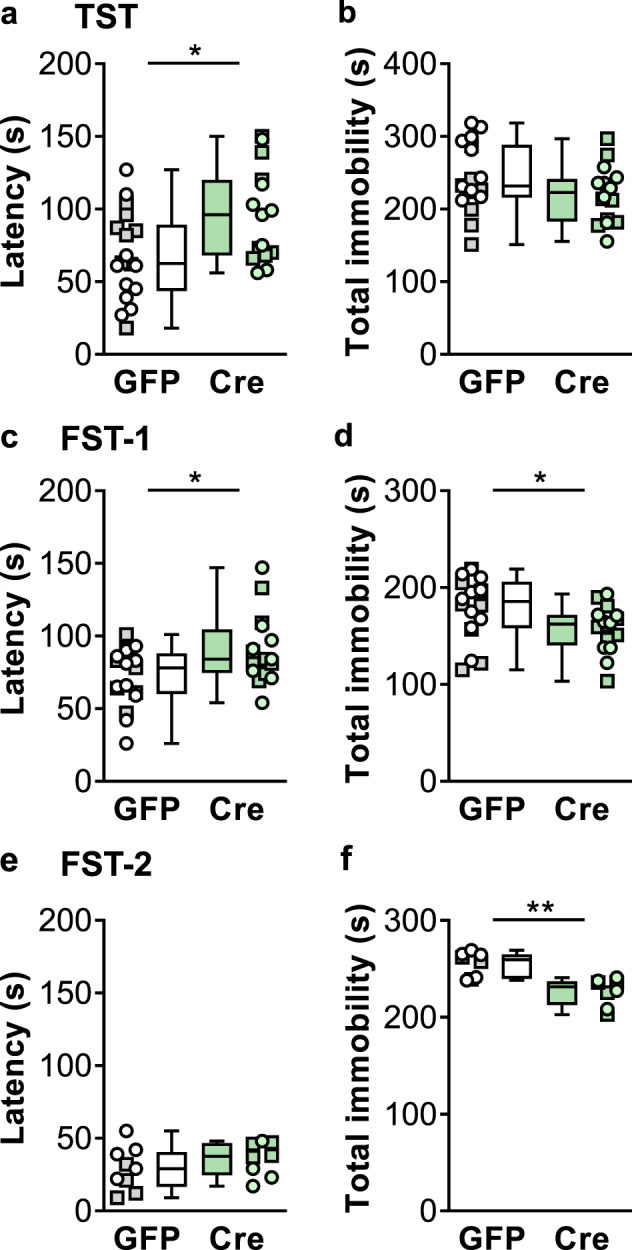
a Cre-expressing mice (green circles ♀, green squares ♂) exhibited increased latency to enter an immobile posture in the tail-suspension test relative to GFP controls (white circles ♀, gray squares ♂) (95.9 ± 8.4 s Cre vs 67.4 ± 7.2 s GFP, *p < 0.05, t31 = 2.589, N = 15–18 mice/group). b No difference in the total time of immobility during the tail suspension test between GFP and Cre mice. c Cre-expressing mice displayed a longer latency to immobility on the first exposure to the forced swim test (90.4 ± 6.0 s Cre vs 71.5 ± 4.9 s GFP, *p < 0.05, t31 = 2.453, N = 16–17). d Decreased total immobility in the first forced to swim in Cre mice relative to GFP controls (157.7 ± 6.0 s Cre vs 179.4 ± 7.8 s GFP, *p < 0.05, t32 = 2.172, N = 16–18). e No difference in latency to immobility on the second exposure to a forced swim between GFP- and Cre-expressing mice. f Cre expression decreased the total immobility on the second exposure to a forced swim (227.0. ± 5.0 s Cre vs 254.7 ± 4.6 s GFP, **p < 0.01, t32 = 4.109, N = 8).
Discussion
Acute inhibition of mGlu3 receptors confers antidepressant-like effects in preclinical models [34–36], but the effects of Grm3 manipulation and the neurocircuits recruited have not been adequately characterized. Here, we leveraged the recent development of transgenic mice to manipulate mGlu3 receptor expression in neocortical neurons. Physiological studies provided the first concrete evidence that mGlu3 receptors expressed at a postsynaptic location are indispensable for synaptic plasticity. Furthermore, the genetic deletion of mGlu3 receptors from adult mice precipitated effective behavioral changes. Together, these studies provide valuable mechanistic insight and translational evidence that bolster the therapeutic potential for mGlu3 receptor inhibition as a novel approach to treat affective disorders.
Pharmacological manipulations that stimulate PFC neurotransmission can exert robust antidepressant activity [43, 44]. Based on this hypothesis, non-selective mGlu2/3 antagonists have been proposed as a potential antidepressant approach [12], with conventional wisdom dictating that these compounds act by inhibiting autoreceptors to acutely facilitate glutamate release. Increasing evidence, however, suggests that mGlu2 and mGlu3 receptors serve vastly different roles in the central nervous system. Within the neocortex [21], hippocampus [45], and striatum [46], mGlu2 receptors do, in fact, function as autoreceptors to regulate neurotransmitter release probability. In contrast, mGlu3 receptors have been recently implicated in several forms of postsynaptic plasticity. Within the PFC, mGlu3 receptors gate the internalization of AMPA receptors through a PI3K/Akt-dependent pathway [22, 24]. Despite the clear postsynaptic locus for these downstream signaling cascades, the precise cellular location of the requisite mGlu3 receptor molecules has remained debatable. Now, by regionally demarcating Cre expression within the PFC and opsin expression in a distal glutamate afferent, the present studies provide clear evidence that the postsynaptic mGlu3 receptors guide synaptic plasticity. This critical experiment culminates in a series of recent studies that have fundamentally reshaped our understanding of how mGlu3 receptors regulate central nervous system function.
The prototypical fast-acting antidepressant ketamine attenuates depressive-like behavior in rodent models through cortical disinhibition and exerts efficacy in treatment-resistant clinical populations within hours of administration [47–49]. Unfortunately, ketamine and other fast-acting antidepressants display several limitations that hinder their utility in broad clinical populations. In addition to concerns related to abuse liability and its burdensome route of administration, ketamine displays disconcerting on-target side effects [50–52]. Like other NMDA receptor antagonists, ketamine administration can alter oscillatory activity within the gamma range [53, 54] and induce delirious side effects by inhibiting glutamate receptors expressed on inhibitory interneurons [55]. This disinhibitory motif is also essential for ketamine’s antidepressant effects [56], thus presenting challenges in tailoring ketamine-based therapies to retain efficacy while mitigating the risk of disruptive side effects. In contrast, mGlu3 receptors do not appear to regulate gamma power [15], and the present findings suggest that modulating mGlu3 receptor signaling on pyramidal cells is sufficient to confer antidepressant-like effects. By avoiding direct modulation of cortical interneurons, mGlu3 NAMs may confer lower risks of psychotomimetic side effects. Indeed, studies using global knockout mice, mGlu2/3 antagonists, and mGlu3 NAMs have revealed minimal evidence of psychotomimetic-like phenotypes [40–42]. On the other hand, mGlu3 NAMs and ketamine may confer antidepressant-like effects through overlapping neurocircuits. Ketamine induces its antidepressant-like effects in mice by disinhibiting BLA-projecting PFC pyramidal cells [57], which receive strong reciprocal glutamate projections from the BLA [58, 59]. As these synapses express mGlu3 receptor-dependent LTD, in vivo mGlu3 NAM administration may therefore potentiate similar PFC circuits as ketamine and other fast-acting antidepressants. Nonetheless, the current studies certainly do not exclude the possibility that mGlu3 NAMs also promote antidepressant-like effects through extracortical loci. Several brain regions involved in affective behaviors and mood disorder etiology, such as the hippocampus and nucleus accumbens, highly express mGlu3 receptors and represent intriguing substrates for continued investigation [60]. Future studies involving chronic pharmacological inhibition will also be needed to translate mGlu3 receptor NAMs as potential breakthrough antidepressants.
In addition to regulating synaptic plasticity, our findings showed that mGlu3 receptors regulate PFC pyramidal cell membrane physiology to some extent. An interesting hypothesis for future studies is that mGlu3 receptors regulate coping and anxiety-like behaviors by potentiating HCN channels, which have been shown to regulate depressive-like behavior in rodent models [37, 61]. The antidepressant-like effects of mGlu3 receptor modulation might also involve changes to other pathways related to cAMP and/or pathways related to crosstalk with mGlu5 receptors [23, 62]. Indeed, considering that mGlu5 receptors inhibit small-conductance potassium channels [22, 63, 64], the decreased mAHP we observed in Cre-expressing cells suggests that mGlu3 receptor ablation affects multiple modes of mGlu5 receptor signaling. Nonetheless, several pieces of evidence suggest the signaling mechanisms involved in mGlu3-LTD are necessary for the concomitant anxiolytic and antidepressant-like behavioral ramifications following receptor inhibition. For one, mGlu3-LTD ultimately proceeds through the internalization of AMPA receptors [24], and PFC infusions of AMPA receptor antagonists block the antidepressant-like effects of mGlu2/3 antagonists [13, 14]. These findings suggest that functional changes in PFC AMPA receptors are intimately linked with coping behaviors and other antidepressant-like outcomes. In addition, clinical associations with mood disorders have been observed with several signaling molecules that are critical for mGlu3-LTD, including mGlu receptor subtype 5 (GRM5) [65], Homer1 (HOMER1) [66], and glycogen synthase kinase 3β (GSK3B) [67]. Conversely, manipulations that target these molecules modulate affective behaviors in preclinical models [68–74]. Together, these findings suggest that antidepressant-like manipulations that inhibit cortical mGlu3 receptors exert their efficacy by inhibiting LTD and altering how information from the limbic system is processed within the PFC.
Collectively, the current studies conclusively demonstrate that postsynaptic mGlu3 receptors are essential for LTD. Decreased expression of cortical mGlu3 receptors modulates affective behaviors, an important translational finding considering that the efficacy of many antidepressant and anxiolytic medications wanes following chronic treatment. Means to inhibit cortical mGlu3 receptors thus represent a promising novel approach for the treatment of mood disorders.
Funding and disclosures
This work was supported by the National Institutes of Health (NIH) grants R01MH062646 and R37NS031373 (PJC). MEJ was supported by NIH grant K99AA027806 and a postdoctoral fellowship through the Pharmaceutical Research and Manufacturers of America Foundation. CIS was supported by the SyBBURE Searle Undergraduate Research Program. SADV was supported by NIH grants T32GM007628 and F31MH119699. NMF was supported by NIH grants T32GM007628 and F31MH113259. Behavioral experiments were performed through the Murine Neurobehavioral Core lab at the Vanderbilt University Medical Center. PJC and CMN receive research support from Acadia Pharmaceuticals and Boehringer Ingelheim and are inventors on multiple patents for allosteric modulators of metabotropic glutamate receptors. No other authors declare any potential conflicts of interest.
Supplementary information
Acknowledgements
The authors thank members of the Conn and Niswender laboratories and the WCNDD for their technical assistance and feedback.
Author contributions
Conceptualization, MEJ and CIS; investigation, MEJ, CIS, SADV, NMF, and SD; supervision, MEJ, CMN, and PJC; writing–original draft, MEJ and CIS; writing–review & editing—all authors.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Max E. Joffe, Chiaki I. Santiago.
Contributor Information
Max E. Joffe, Email: max.joffe@vanderbilt.edu
P. Jeffrey Conn, Email: jeff.conn@vanderbilt.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-01041-2.
References
- 1.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–66. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 3.Machado-Vieira R, Baumann J, Wheeler-Castillo C, Latov D, Henter ID, Salvadore G, et al. The timing of antidepressant effects: a comparison of diverse pharmacological and somatic treatments. Pharmacy. 2010;3:19–41. doi: 10.3390/ph3010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rush AJ, South C, Jha MK, Jain SB, Trivedi MH. What to expect when switching to a second antidepressant medication following an ineffective initial SSRI: a report from the randomized clinical STAR*D study. J Clin Psychiatry. 2020;81. [DOI] [PubMed]
- 5.DALYs, G.B.D. and H. Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1603–58. [DOI] [PMC free article] [PubMed]
- 6.Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–87. doi: 10.1016/S0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- 7.Murray EA, Wise SP, Drevets WC. Localization of dysfunction in major depressive disorder: prefrontal cortex and amygdala. Biol Psychiatry. 2011;69:e43–54. doi: 10.1016/j.biopsych.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdallah CG, Averill CL, Salas R, Averill LA, Baldwin PR, Krystal JH, et al. Prefrontal connectivity and glutamate transmission: relevance to depression pathophysiology and ketamine treatment. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:566–74. [DOI] [PMC free article] [PubMed]
- 10.Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X. An excitatory synapse hypothesis of depression. Trends Neurosci. 2015;38:279–94. doi: 10.1016/j.tins.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Highland JN, Zanos P, Georgiou P, Gould TD Group II metabotropic glutamate receptor blockade promotes stress resilience in mice. Neuropsychopharmacology, 2019. [DOI] [PMC free article] [PubMed]
- 12.Chaki S. mGlu2/3 receptor antagonists as novel antidepressants. Trends Pharm Sci. 2017;38:569–80. doi: 10.1016/j.tips.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Fukumoto K, Iijima M, Chaki S. The antidepressant effects of an mGlu2/3 receptor antagonist and ketamine require AMPA receptor stimulation in the mPFC and subsequent activation of the 5-HT neurons in the DRN. Neuropsychopharmacology. 2016;41:1046–56. doi: 10.1038/npp.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koike H, Chaki S. Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behav Brain Res. 2014;271:111–5. doi: 10.1016/j.bbr.2014.05.065. [DOI] [PubMed] [Google Scholar]
- 15.Zanos P, Highland JN, Stewart BW, Georgiou P, Jenne CE, Lovett J, et al. (2R,6R)-hydroxynorketamine exerts mGlu2 receptor-dependent antidepressant actions. Proc Natl Acad Sci USA. 2019. [DOI] [PMC free article] [PubMed]
- 16.Marek GJ. Metabotropic glutamate2/3 (mGlu2/3) receptors, schizophrenia and cognition. Eur J Pharm. 2010;639:81–90. doi: 10.1016/j.ejphar.2010.02.058. [DOI] [PubMed] [Google Scholar]
- 17.Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharm Exp Ther. 2000;292:76–87. [PubMed] [Google Scholar]
- 18.Barbara JG, Auclair N, Roisin MP, Otani S, Valjent E, Caboche J, et al. Direct and indirect interactions between cannabinoid CB1 receptor and group II metabotropic glutamate receptor signalling in layer V pyramidal neurons from the rat prefrontal cortex. Eur J Neurosci. 2003;17:981–90. doi: 10.1046/j.1460-9568.2003.02533.x. [DOI] [PubMed] [Google Scholar]
- 19.Klodzinska A, Bijak M, Tokarski K, Pilc A. Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of DOI in mice. Pharm Biochem Behav. 2002;73:327–32. doi: 10.1016/S0091-3057(02)00845-6. [DOI] [PubMed] [Google Scholar]
- 20.Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–52. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 21.Joffe ME, Santiago CI, Oliver KH, Maksymetz J, Harris NA, Engers JL, mGlu2 and mGlu3 negative allosteric modulators divergently enhance thalamocortical transmission and exert rapid antidepressant-like effects. Neuron. 2019. [DOI] [PMC free article] [PubMed]
- 22.Joffe ME, Santiago CI, Stansley BJ, Maksymetz J, Gogliotti RG, Engers JL, et al. Mechanisms underlying prelimbic prefrontal cortex mGlu3/mGlu5-dependent plasticity and reversal learning deficits following acute stress. Neuropharmacology. 2019;144:19–28. doi: 10.1016/j.neuropharm.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Menna L, Joffe ME, Iacovelli L, Orlando R, Lindsley CW, Mairesse J, et al. Functional partnership between mGlu3 and mGlu5 metabotropic glutamate receptors in the central nervous system. Neuropharmacology. 2018;128:301–13. doi: 10.1016/j.neuropharm.2017.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joffe ME, Santiago CI, Engers JL, Lindsley CW, Conn PJ Metabotropic glutamate receptor subtype 3 gates acute stress-induced dysregulation of amygdalo-cortical function. Mol Psychiatry. 2017. [DOI] [PMC free article] [PubMed]
- 25.Otani S, Daniel H, Takita M, Crepel F. Long-term depression induced by postsynaptic group II metabotropic glutamate receptors linked to phospholipase C and intracellular calcium rises in rat prefrontal cortex. J Neurosci. 2002;22:3434–44. doi: 10.1523/JNEUROSCI.22-09-03434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otani S, Auclair N, Desce JM, Roisin MP, Crepel F. Dopamine receptors and groups I and II mGluRs cooperate for long-term depression induction in rat prefrontal cortex through converging postsynaptic activation of MAP kinases. J Neurosci. 1999;19:9788–802. doi: 10.1523/JNEUROSCI.19-22-09788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, Munguba H, Gutzeit VA, Singh DR, Kristt M, Dittman JS, et al. Defining the homo- and heterodimerization propensities of metabotropic glutamate receptors. Cell Rep. 2020;31:107605. doi: 10.1016/j.celrep.2020.107605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin LE, Wang M, Galvin VC, Lightbourne TC, Conn PJ, Arnsten AFT, et al. mGluR2 versus mGluR3 metabotropic glutamate receptors in primate dorsolateral prefrontal cortex: postsynaptic mGluR3 strengthen working memory networks. Cereb Cortex. 2018;28:974–87. doi: 10.1093/cercor/bhx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dogra S, Stansley BJ, Xiang Z, Qian W, Gogliotti RG, Nicoletti F, et al., Activating mGlu3 metabotropic glutamate receptors rescues schizophrenia-like cognitive deficits through metaplastic adaptations within the hippocampus. BioRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 30.Fentress HM, Klar R, Krueger JJ, Sabb T, Redmon SN, Wallace NM, et al. Norepinephrine transporter heterozygous knockout mice exhibit altered transport and behavior. Genes Brain Behav. 2013;12:749–59. doi: 10.1111/gbb.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher NM, Gogliotti RG, Vermudez SAD, Stansley BJ, Conn PJ, Niswender CM, et al. Genetic reduction or negative modulation of mGlu7 does not impact anxiety and fear learning phenotypes in a mouse model of MECP2 duplication syndrome. ACS Chem Neurosci. 2018;9:2210–17. doi: 10.1021/acschemneuro.7b00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joffe ME, Winder DG, Conn PJ. Contrasting sex-dependent adaptations to synaptic physiology and membrane properties of prefrontal cortex interneuron subtypes in a mouse model of binge drinking. Neuropharmacology. 2020;178:108126. doi: 10.1016/j.neuropharm.2020.108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gogliotti RG, Fisher NM, Stansley BJ, Jones CK, Lindsley CW, Conn PJ, et al. Total RNA sequencing of rett syndrome autopsy samples identifies the M4 muscarinic receptor as a novel therapeutic target. J Pharm Exp Ther. 2018;365:291–300. doi: 10.1124/jpet.117.246991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joffe ME, Santiago CI, Oliver KH, Maksymetz J, Harris NA, Engers JL, et al. mGlu2 and mGlu3 negative allosteric modulators divergently enhance thalamocortical transmission and exert rapid antidepressant-like effects. Neuron. 2020;105:46–59 e3. doi: 10.1016/j.neuron.2019.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engers JL, Bollinger KA, Weiner RL, Rodriguez AL, Long MF, Breiner MM, et al. Design and synthesis of N-aryl phenoxyethoxy pyridinones as highly selective and CNS penetrant mGlu3 NAMs. ACS Med Chem Lett. 2017;8:925–30. doi: 10.1021/acsmedchemlett.7b00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engers JL, Rodriguez AL, Konkol LC, Morrison RD, Thompson AD, Byers FW, et al. Discovery of a selective and CNS penetrant negative allosteric modulator of metabotropic glutamate receptor subtype 3 with antidepressant and anxiolytic activity in rodents. J Med Chem. 2015;58:7485–500. doi: 10.1021/acs.jmedchem.5b01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis AS, Vaidya SP, Blaiss CA, Liu Z, Stoub TR, Brager DH, et al. Deletion of the hyperpolarization-activated cyclic nucleotide-gated channel auxiliary subunit TRIP8b impairs hippocampal Ih localization and function and promotes antidepressant behavior in mice. J Neurosci. 2011;31:7424–40. doi: 10.1523/JNEUROSCI.0936-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salling MC, Jane Skelly M, Avegno E, Regan S, Zeric T, Nichols E, et al., Alcohol consumption during adolescence in a mouse model of binge drinking alters the intrinsic excitability and function of the prefrontal cortex through a reduction in the hyperpolarization-activated cation current. J Neurosci. 2018. [DOI] [PMC free article] [PubMed]
- 39.Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharm Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Filippis B, Lyon L, Taylor A, Lane T, Burnet PW, Harrison PJ, et al. Therole of group II metabotropic glutamate receptors in cognition and anxiety: comparative studies in GRM2(−/−), GRM3(−/−) and GRM2/3(−/−) knockout mice. Neuropharmacology. 2015;89:19–32. doi: 10.1016/j.neuropharm.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujioka R, Nii T, Iwaki A, Shibata A, Ito I, Kitaichi K, et al. Comprehensive behavioral study of mGluR3 knockout mice: implication in schizophrenia related endophenotypes. Mol Brain. 2014;7:31. doi: 10.1186/1756-6606-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woolley ML, Pemberton DJ, Bate S, Corti C, Jones DN. The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology. 2008;196:431–40. doi: 10.1007/s00213-007-0974-x. [DOI] [PubMed] [Google Scholar]
- 43.Duman RS, Shinohara R, Fogaca MV, Hare B Neurobiology of rapid-acting antidepressants: convergent effects on GluA1-synaptic function. Mol Psychiatry. 2019. [DOI] [PMC free article] [PubMed]
- 44.Chowdhury GM, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry. 2017;22:120–26. doi: 10.1038/mp.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scholler P, Nevoltris D, de Bundel D, Bossi S, Moreno-Delgado D, Rovira X, et al. Allosteric nanobodies uncover a role of hippocampal mGlu2 receptor homodimers in contextual fear consolidation. Nat Commun. 2017;8:1967. doi: 10.1038/s41467-017-01489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson KA, Mateo Y, Lovinger DM. Metabotropic glutamate receptor 2 inhibits thalamically-driven glutamate and dopamine release in the dorsal striatum. Neuropharmacology. 2017;117:114–23. doi: 10.1016/j.neuropharm.2017.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gould TD, Zarate CA, Jr, Thompson SM. Molecular pharmacology and neurobiology of rapid-acting antidepressants. Annu Rev Pharm Toxicol. 2019;59:213–36. doi: 10.1146/annurev-pharmtox-010617-052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krystal JH, Abdallah CG, Sanacora G, Charney DS, Duman RS. Ketamine: a paradigm shift for depression research and treatment. Neuron. 2019;101:774–78. doi: 10.1016/j.neuron.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monteggia LM, Zarate C., Jr Antidepressant actions of ketamine: from molecular mechanisms to clinical practice. Curr Opin Neurobiol. 2015;30:139–43. doi: 10.1016/j.conb.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Short B, Fong J, Galvez V, Shelker W, Loo CK. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65–78. doi: 10.1016/S2215-0366(17)30272-9. [DOI] [PubMed] [Google Scholar]
- 51.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 52.Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74:399–405. doi: 10.1001/jamapsychiatry.2017.0080. [DOI] [PubMed] [Google Scholar]
- 53.Hong LE, Summerfelt A, Buchanan RW, O'Donnell P, Thaker GK, Weiler MA, et al. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology. 2010;35:632–40. doi: 10.1038/npp.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muthukumaraswamy SD, Shaw AD, Jackson LE, Hall J, Moran R, Saxena N. Evidence that subanesthetic doses of ketamine cause sustained disruptions of NMDA and AMPA-mediated frontoparietal connectivity in humans. J Neurosci. 2015;35:11694–706. doi: 10.1523/JNEUROSCI.0903-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ali F, Gerhard DM, Sweasy K, Pothula S, Pittenger C, Duman RS, et al. Ketamine disinhibits dendrites and enhances calcium signals in prefrontal dendritic spines. Nat Commun. 2020;11:72. doi: 10.1038/s41467-019-13809-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerhard DM, Pothula S, Liu RJ, Wu M, Li XY, Girgenti MJ, et al., GABA interneurons are the cellular trigger for ketamine’s rapid antidepressant actions. J Clin Invest, 2019. [DOI] [PMC free article] [PubMed]
- 57.Hare BD, Shinohara R, Liu RJ, Pothula S, DiLeone RJ, Duman RS. Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nat Commun. 2019;10:223. doi: 10.1038/s41467-018-08168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Little JP, Carter AG. Synaptic mechanisms underlying strong reciprocal connectivity between the medial prefrontal cortex and basolateral amygdala. J Neurosci. 2013;33:15333–42. doi: 10.1523/JNEUROSCI.2385-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGarry LM, Carter AG. Inhibitory gating of basolateral amygdala inputs to the prefrontal cortex. J Neurosci. 2016;36:9391–406. doi: 10.1523/JNEUROSCI.0874-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993;335:252–66. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- 61.Lyman KA, Han Y, Chetkovich DM. Animal models suggest the TRIP8b-HCN interaction is a therapeutic target for major depressive disorder. Expert Opin Ther Targets. 2017;21:235–37. doi: 10.1080/14728222.2017.1287899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cho K, Brown MW, Bashir ZI. Mechanisms and physiological role of enhancement of mGlu5 receptor function by group II mGlu receptor activation in rat perirhinal cortex. J Physiol. 2002;540:895–906. doi: 10.1113/jphysiol.2001.013920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cannady R, McGonigal JT, Newsom RJ, Woodward JJ, Mulholland PJ, Gass JT. Prefrontal cortex KCa2 channels regulate mGlu5-dependent plasticity and extinction of alcohol-seeking behavior. J Neurosci. 2017;37:4359–369. doi: 10.1523/JNEUROSCI.2873-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci. 2001;21:5925–34. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22:343–52. doi: 10.1038/s41593-018-0326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rietschel M, Mattheisen M, Frank J, Treutlein J, Degenhardt F, Breuer R, et al. Genome-wide association-, replication-, and neuroimaging study implicates HOMER1 in the etiology of major depression. Biol Psychiatry. 2010;68:578–85. doi: 10.1016/j.biopsych.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 67.Jope RS. Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Front Mol Neurosci. 2011;4:16. doi: 10.3389/fnmol.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chaki S, Ago Y, Palucha-Paniewiera A, Matrisciano F, Pilc A. mGlu2/3 and mGlu5 receptors: potential targets for novel antidepressants. Neuropharmacology. 2013;66:40–52. doi: 10.1016/j.neuropharm.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 69.Wagner KV, Hartmann J, Labermaier C, Hausl AS, Zhao G, Harbich D, et al. Homer1/mGluR5 activity moderates vulnerability to chronic social stress. Neuropsychopharmacology. 2015;40:1222–33. doi: 10.1038/npp.2014.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holz A, Mulsch F, Schwarz MK, Hollmann M, Dobrossy MD, Coenen VA, et al. Enhanced mGlu5 signaling in excitatory neurons promotes rapid antidepressant effects via AMPA receptor activation. Neuron. 2019;104:338–352 e7. doi: 10.1016/j.neuron.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 71.Lominac KD, Oleson EB, Pava M, Klugmann M, Schwarz MK, Seeburg PH, et al. Distinct roles for different Homer1 isoforms in behaviors and associated prefrontal cortex function. J Neurosci. 2005;25:11586–94. doi: 10.1523/JNEUROSCI.3764-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klugmann M, Szumlinski KK. Targeting Homer genes using adeno-associated viral vector: lessons learned from behavioural and neurochemical studies. Behav Pharm. 2008;19:485–500. doi: 10.1097/FBP.0b013e32830c369f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, et al. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci USA. 2008;105:1333–8. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharm Ther. 2015;148:114–31. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.