Abstract
Introduction
BRAF V600 E mutations have been identified in a subset of patients with primary brain tumors. Combination therapy with BRAF and Mitogen-activated protein kinase (MEK) inhibitors (BRAF/MEKi) targeting sequential steps in the MAPK pathway has replaced BRAFi monotherapy as the standard of care in multiple tumors with BRAF V600 E mutations, and clinical evidence for this strategy continues to grow in primary brain tumors.
Case series
We describe four patients with BRAF V600 E mutated gliomas, including a 21-year-old woman with a ganglioglioma WHO grade I, a 19-year-old man with a pleomorphic xanthoastrocytoma WHO grade III, and 21-year-old and 33-year-old women with epithelioid GBM WHO grade IV, who achieved durable progression-free survival with combination BRAF/MEKi.
Conclusion
Combination of BRAF/MEK inhibition can be a novel, promising approach as targeted therapy in gliomas with BRAF V600 E mutations, especially those that are resistant to standard therapy. Our cases, along with other early reports utilizing dabrafenib/trametinib, highlight the importance of somatic next-generation sequencing, particularly in younger patients. Interim results from clinical trials utilizing dabrafenib/trametinib have been promising thus far, and our case series suggests that durable clinical benefit is possible, even in the setting of glioblastoma, WHO grade IV.
Keywords: glioblastoma, BRAF, CNS tumor, brain tumor, cancer, treatment
Introduction
BRAF V600 E mutations have been identified in a subset of patients with primary brain tumors in both pediatric and adult populations, including but not limited to 18% of gangliogliomas (GG), 66% of pleomorphic xanthoastrocytomas (PXA), and 1–2% of glioblastomas (GBM).1-3 Targeted therapy for BRAF V600E-mutated tumors was first attempted with BRAF inhibition (BRAFi) monotherapy in the setting of melanoma in 2010. 4 Over the last decade, combination therapy with BRAF and Mitogen-activated protein kinase (MEK) inhibitors (BRAF/MEKi) targeting sequential steps in the MAPK pathway has replaced BRAFi monotherapy as the standard of care in melanoma following improvements in the 12-month overall survival rate (72% vs 65%) and median progression-free survival (11.4 months vs 7.3 months) with the combination of dabrafenib/trametinib vs vemurafenib monotherapy. 5 In the setting of primary brain tumors, vemurafenib monotherapy has been active in low-grade tumors (eg, PXA, WHO grade II), but has been less successful in GBM. Treatment with vemurafenib in the VE-BASKET study resulted in a best response of stable disease in three GBM patients, with two experiencing progression at 3.6 months (censored at the last assessment) and 3.7 months, and one with prolonged stable disease (SD) for 12.9 months. 6 Clinical trials are ongoing to assess the combination BRAF/MEKi for the treatment of low- and high-grade gliomas with encouraging interim data presented at the Society of Neuro-Oncology 2019 Annual Meeting. 7 Among a database of 469 primary brain tumor patients with genomic data entered between April 1, 2013 and November 1, 2018, we identified a cohort of 12 primary glioma patients with BRAF V600 E mutations. BRAF V600E-positivity was identified by immunohistochemistry (IHC) in 92% (n=11) of patients. All patients had confirmation of BRAF V600 E positivity by either next-generation sequencing (n=11) or pyrosequencing (n=1). Among the cohort of 12 BRAF V600E-mutated gliomas, we identified four patients treated with dabrafenib/trametinib. Herein, we describe those four patients with BRAF V600 E mutated gliomas who achieved durable progression-free survival (PFS) utilizing targeting therapy with combination BRAF/MEKi.
Case #1
A 21-year-old woman was found to have an enhancing mass with a cystic component in the upper cervical cord involving the medulla oblongata on Magnetic Resonance Imaging (MRI) after presenting with a syncopal episode. She underwent biopsy of the lesion at an outside hospital. Pathology showed mild chronic inflammatory disease; however, upon review at Moffitt Cancer Center (MCC), pathology revealed ganglioglioma, World Health Organization (WHO) grade I (Figure 1), IDH1 wild type by IHC, ATRX-retained, and positive for BRAF V600 E mutation by IHC. She remained stable radiographically on surveillance scans. However, 12 months after initial diagnosis, a repeat MRI scan showed increase in tumor growth both in the enhancing and the non-enhancing regions involving both the brainstem and the upper cervical cord (Figure 2). The enhancing part of the upper largest diameter of the brainstem lesion increased to 1.48 cm (compared to prior measuring 1.17 cm) on axial view (Figures 2A and 2B) and to 1.42 42 x 1.43 cm (compared to prior measuring 1.06 ×06 x .83 cm) on sagittal view (Figures 2G and 2H).
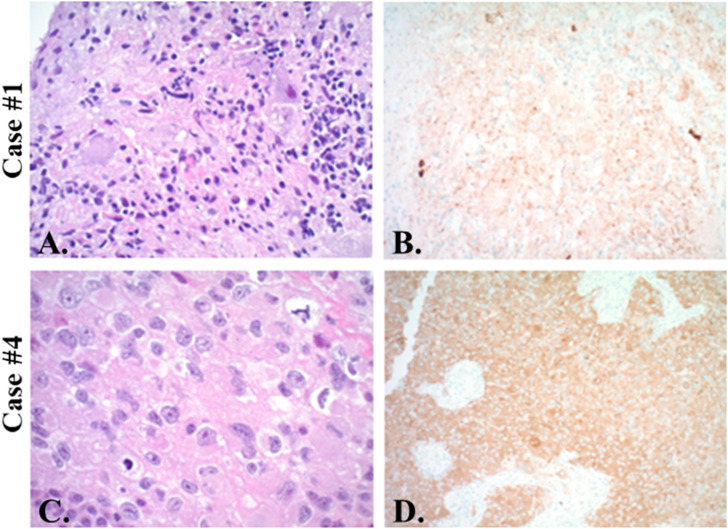
Figure 1.
Histopathology. Case 1 shows atypical ganglion cells immersed in disorganized glia (A. H&E, original magnification ×200) with positive immunohistochemistry for mutant BRAF V600 E (B. x200). Case 2 has been published in greater detail previously. 29 Case 4 shows mitotically active atypical epithelioid cells (C. H&E x400) which strongly express mutant BRAF V600 E (D. x200). Case 3 not shown.
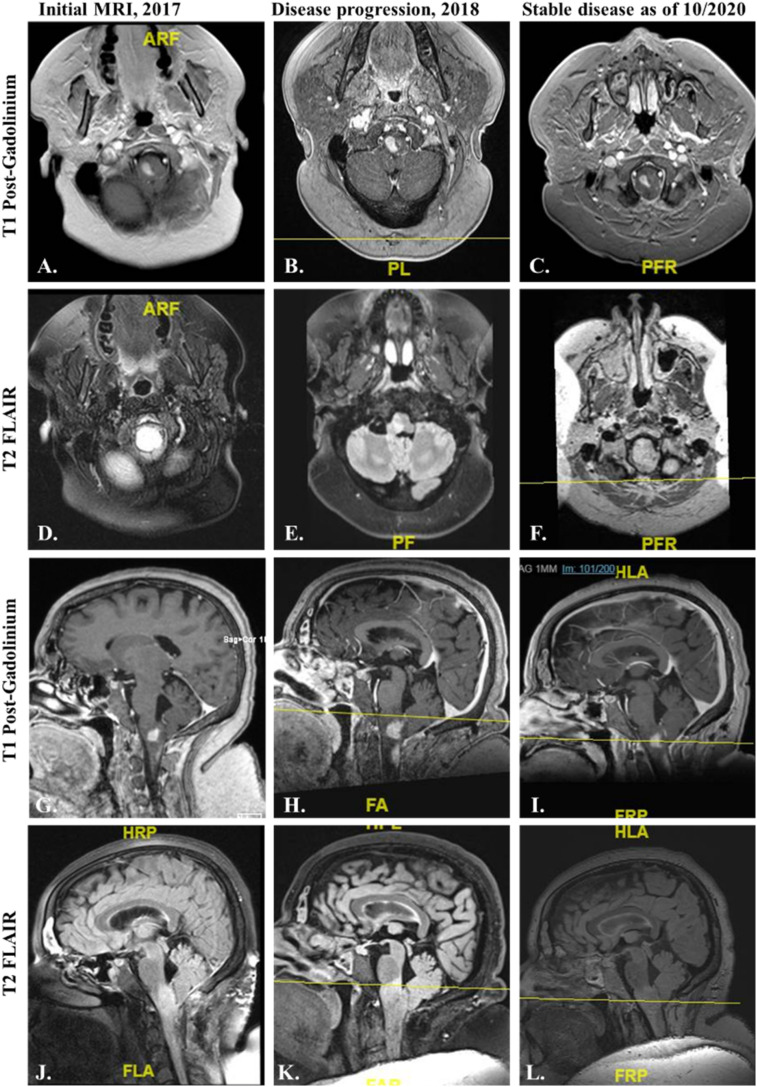
Figure 2.
Magnetic Resonance Imaging of the brain of case #1 at initial diagnosis and before and after treatment with BRAF/MEK inhibitors. On 05/2017, initial MRI brain showed interval tumor growth of the ganglioglioma involving brainstem and upper cervical spinal cord as seen with increased enhancing tumor and T2 signal (A, D, G, J.). The enhancing part of the upper largest brainstem lesions measures 1.17 cm on axial view (A.) and 1.06 x 0.83 cm on sagittal view (G.). On 05/2018, MRIs showed disease progression with increase in the size of the lesions, with the enhancing part of the upper brainstem lesion now measuring 1.48 cm in axial view (B.) and 1.42 x 1.43 cm in sagittal view (H.) (B, E, H, K.). BRAF/MEK inhibitors were added. Most current MRIs as of 10/2020 show overall smaller in the size of the lesions, with the enhancing part of the upper brainstem lesion now measuring 1.32 cm in axial view (C.) and 1.10 x 0.80 cm in sagittal view (L.) (C, E, I, L.). A–C and G–L = T1 Post-Gadolinium. D–F and J–L = T2 FLAIR sequences.
Given the superior efficacy of concurrent BRAF/MEKi therapy in other cancer types and gliomas with BRAF V600 E mutation,8–26 she was immediately started on dabrafenib 150 mg per oral (PO) twice a day (BID) with trametinib 2 mg PO daily added one month later due to insurance issues. MRI performed 3 months later showed a decrease in the size of the enhancing tumor involving the right brainstem, right cervical medullary junction, and dorsal aspect of the cervical cord at the level of C2. Serial imaging showed SD was maintained for 9 months after the initiation of BRAF/MEKi targeted therapy.
Unfortunately, soon after there was radiographic evidence of disease progression and therapy was changed to encorafenib 450 mg PO daily and binimetinib 45 mg PO BID, based upon higher dosing and potentially greater CNS penetrance, while minimizing toxicity. Chloroquine 500 mg PO daily was also added based on early evidence supporting inhibition of autophagy as a potential strategy for overcoming MAPK-pathway resistance.27,28 She had a good response to treatment. After 5 months, the treatment was held due to hyperglycemia. She remained on surveillance with serial scans. Since the treatment was held, she has remained with clinical and radiographic SD ongoing at 29 months after initial start of BRAF/MEKi and 18 months after addition of chloroquine to BRAF/MEKi therapy (Figure 2).
Case #2
A 19-year-old, previously healthy man was found on MRI of the brain to have a large right-sided lesion in the parieto-temporal lobes, with surrounding vasogenic edema and a right-to-left midline shift. He underwent a subtotal surgical resection and was diagnosed with GBM at an outside hospital. He was started on standard treatment with radiation therapy (RT) and concomitant temozolomide (TMZ); however, given tumor recurrence 4 months later, he underwent a second maximal safe gross total resection. Pathology was consistent with a glioma resembling an anaplastic PXA, WHO grade III–IV, non-infiltrating, with a high mitotic index, IDH1 R132H-negative by IHC, negative for EGFRviii and 1p/19q co-deletion, and positive for BRAF V600 E mutation by IHC. He was monitored with serial MRI scans and had SD on radiographic surveillance.
Seventeen months following his second resection, an MRI of the brain identified a small increase in the size of his tumor. MRI perfusion scan showed hyperperfusion supporting the diagnosis of recurrent tumor. Based on this, combination therapy with dabrafenib 150 mg PO BID and trametinib 2 mg PO daily was initiated. Imaging remained stable on serial MRIs for 14 months, at which point slight progression was noted on MRI and chloroquine 500 mg PO daily was added to his therapy regimen. 29 The patient remained stable for an additional 21 months with the addition of chloroquine to his BRAF/MEKi regimen. Recently, the patient began to progress (as confirmed on MRI brain with MRI perfusion scans) and underwent resection of his anaplastic PXA, WHO grade III-IV, after approximately 35 months on targeted therapy. This is an update to a case that has been published in greater detail previously. 29
Case #3
A 21-year-old woman presented with complaints of dizziness and severe headaches. Her primary care physician ordered an MRI brain, which showed a left frontal 1.2 cm size lesion. Due to these findings, she was advised to go the emergency department. She was admitted at an outside hospital, where an MRI brain/spectroscopy was performed, confirming the 1.2 cm left frontal rim enhancing cystic lesion with restricted diffusion, abutting the superior margin of the insula. She was evaluated by a neurosurgeon who recommended repeat MRI brain in 1 month. The repeat scans showed an increase in the size of the lesion. A left frontal craniotomy with gross total tumor resection was performed at MCC. Pathology showed an epithelioid GBM, WHO grade IV, negative for IDH1 R132H by IHC, ATRX-retained, negative for MGMT promoter methylation, and positive for BRAF V600 E confirmed by FoundationOne® assay.
She completed treatment with RT and TMZ followed by maintenance TMZ. After 4 cycles of maintenance TMZ, MRI brain showed two new subcentimeter enhancing nodules around the resection cavity with a mild to moderate increased in vasogenic edema on T2 FLAIR sequence, and no mass effect. These findings were concerning for tumor recurrence. TMZ was switched to BRAF/MEKi with dabrafenib 150 mg PO BID and trametinib 2 mg PO daily. She was not on steroids and given no mass effect or concern for treatment-related changes was not started on steroids or any other treatment for radiation necrosis. Subsequent MRI brain 2 months later showed a reduction in the size of the tumor. She has remained clinically and radiographically stable on this treatment at 16 months after initiation of targeted therapy.
Case #4
A 33-year-old woman with a past medical history of Graves’ disease, presented with a 3-year history of worsening episodes of left eye vision loss. An MRI of the brain showed a cystic right parietal mass with peripheral enhancement measuring 5.5 x 4.4 x 5.5 cm and a nodular enhancing region. She underwent a right parietal craniotomy and pathology confirmed an epithelioid GBM, WHO grade IV (Figure 1), negative for IDH1 R132H by IHC and confirmed IDH1/2 wild-type by NGS, ATRX-retained, MGMT promoter-methylated, negative for 1p/19q co-deletion, and positive for BRAF V600 E mutation by FoundationOne® assay (see supplementary Table 1 for complete NGS results from FoundationOne®). She was started on standard therapy with RT and TMZ for six weeks. Shortly thereafter, she developed a symptomatic increase in the size of the cystic region of the lesion and was taken back to the operating room for drainage, with quick refilling requiring a second drainage in the operating room a month later (Figure 3). She had been on dexamethasone 4 mg total a day during this time to help control her symptoms.
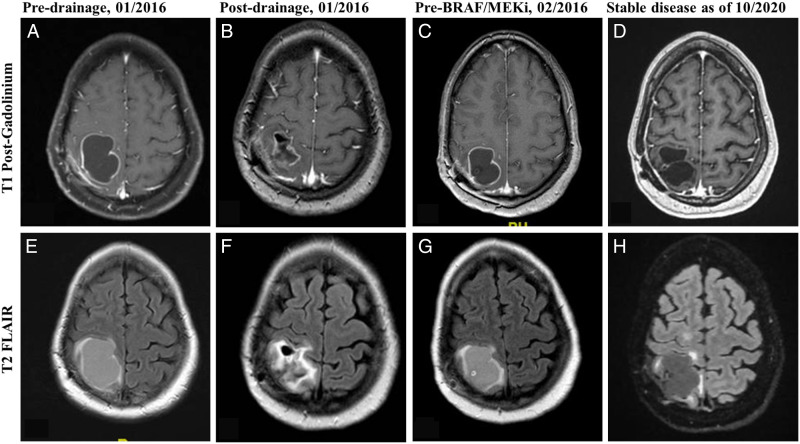
Figure 3.
Magnetic Resonance Imaging of the brain of case #4 pre- and post-cystic drainage, and before and after treatment with BRAF/MEK inhibitors. On 01/2016, MRI brain performed pre- (A. and E.) and post-surgical drainage of the cystic component of the lesion and before starting treatment with maintenance temozolomide (B. and F.). On 02/2016, MRI with rapid refilling of the cystic component of the lesion and before starting treatment with BRAF/MEK inhibitors (C. and G.). Most current MRIs as of 10/2020 show stable disease since started on BRAF/MEK inhibitors, without requiring further surgical drainage (D. and H.). A–D = T1 Post-Gadolinium. E–H = T2 FLAIR sequences.
She subsequently progressed after 2 cycles of maintenance TMZ, with MRI brain showing interval increase size of bilobed or 2 adjacent areas of ring enhancement in the right parietal lobe deep to the craniotomy site measuring now 5.0 x 3.3 cm compared to previous MRI measuring 4.2 x 2.7 cm, with persistent surrounding increased T2 FLAIR signal consistent with vasogenic edema, which was consistent with recurrent GBM. TMZ was discontinued and she was started on dabrafenib 150 mg PO BID and trametinib 2 mg PO daily. Dexamethasone was tapered off. She had a subsequent immediate response to therapy with interval decrease in the size of the right frontoparietal epithelioid GBM compared to prior MRI brain a month earlier, consistent with interval response to therapy. The patient remains clinically and radiographically stable at this time, 53 months after initiating treatment with BRAF/MEKi.
Discussion
Primary brain tumors are a heterogeneous group of neoplasms that range from relatively slow-growing (ie, pilocytic astrocytoma) to aggressive and invasive tumors (ie, GBM WHO grade IV) with an overall poor prognosis. 30 The standard of care for GBMs remains limited, which includes maximal safe surgical resection with subsequent RT and concomitant TMZ, followed by 6 cycles of adjuvant TMZ.31,32 Second-line therapy with bevacizumab was shown to improve PFS, but no improvement in overall survival was achieved. 33 Other therapies following the standard first-line of TMZ have provided only modest benefit with much need remaining for improved therapy options. 34
BRAF V600 E mutations have been identified in a subset of patients with melanoma (35 to 50% of cases), 35 non-small cell lung cancer (NSCLC, 1% to 2%), 36 colorectal cancer (8% to 15%), 37 and anaplastic thyroid cancer (20% to 50%). 38 This mutation has also been found in primary brain tumors among distinct histological subtypes including 9% of pilocytic astrocytomas, 18% of gangliogliomas, 66% of PXA, 90% of papillary craniopharyngiomas, and 1% to 2% of GBM IDH wild type.1-3 Notably, there is an enrichment for BRAF V600 E alterations in approximately 50% of the GBM epithelioid subtype, 39 which typically carry a poorer prognosis with a median survival of 6.3 months. 40 The potential predictive value of BRAF alterations in this GBM subtype underscores the importance of genomic testing and the critical need to find more effective therapeutic options.
In the setting of primary brain tumors, the VE-basket trial recently demonstrated the feasibility of targeting BRAF V600E-mutated gliomas with vemurafenib monotherapy. 6 The objective response rate (ORR) for the PXA patients treated with vemurafenib monotherapy was 42.9%. In contrast, the ORR for the malignant glioma subgroups (ie, anaplastic astrocytoma and GBM) was only 9.1%. The authors concluded that responses to vemurafenib monotherapy were observed across all glioma subsets, with the strongest signal observed in patients with lower-grade gliomas, particularly the PXA subgroup. 6
Other reports have also suggested that high grade gliomas may not achieve the same level of disease control from BRAFi monotherapy that has been seen in low-grade gliomas.8–26 In contrast, there are multiple documented cases with BRAF V600 E mutant low-grade gliomas and high-grade gliomas resistant to RT and TMZ with durable responses to concurrent BRAF/MEKi therapy (see Table 1). The superiority of combination BRAF/MEKi treatment has been clearly demonstrated in other cancer types and our cases, along with other small case series reported (Table 1), suggest that combination BRAF/MEKi may provide the additional MAPK pathway inhibition needed to see durable responses in patients with grade III and Grade IV gliomas (Figure 4).
Table 1.
Literature review of low-grade and high-grade gliomas treated with combination therapy with BRAF/MEKi or monotherapy with BRAFi.
| Citation | Age (years) | Sex | Diagnosis | WHO grade | Line of therapy | Treatment | Best response | Rx duration (months) |
|---|---|---|---|---|---|---|---|---|
| Patient case #1 | 21 | F | Ganglioglioma | I | 2nd | Dabraf+tramet | SD | 14 a |
| Del Bufalo et al 20 | 2.5 | M | Ganglioglioma | I | 2nd | Vemuraf | PR | 54 |
| Del Bufalo et al 20 | 4.5 | NR | Ganglioglioma | I | 1st | Vemuraf | CR | 40 |
| Aguilera et al 22 | 8 | M | Ganglioglioma | I | 1st | Vemuraf | PR | 14 a |
| Del Bufalo et al 20 | 7.4 | NR | Ganglioglioma | I | 1st | Vemuraf | SD | 13 |
| Chamberlain et al 23 | 45 | M | Ganglioglioma | I | 2nd | Dabraf | PR | 10 |
| Lassaletta et al 24 | 2 mo | F | Hypothalamic chiasmatic glioma | I | 2nd | Dabraf | PR | 10 |
| Chamberlain et al 23 | 34 | M | Ganglioglioma | I | 2nd | Dabraf | SD | 7 |
| Chamberlain et al 19 | 26 | F | Ganglioglioma | I | 2nd | Dabraf | SD | 4 |
| Del Bufalo et al 20 | 1 mo | NR | Ganglioneurocytoma | I | 1st | Vemuraf | PD | 3 |
| Del Bufalo et al 20 | 10 | NR | Ganglioglioma | I | 1st | Vemuraf | Insuf. F/up | 2 |
| Del Bufalo et al 20 | 9 | NR | PXA | II | 1st | Vemuraf | PR | 30 |
| Chamberlain et al 19 | 53 | M | PXA | II | 3rd | Vemuraf | PR | 10 |
| Chamberlain et al 19 | 47 | F | PXA | II | 3rd | Vemuraf | SD | 6 |
| Chamberlain et al 19 | 34 | F | PXA | II | 3rd | Vemuraf | SD | 4 |
| Usubalieva et al 21 | 35 | F | PXA | II | 1st | Dabraf | PR | 3 |
| Patient case #2 | 19 | M | Anaplastic PXA | III | 2nd | Dabraf+tramet | SD | 35 a |
| Toll et al 8 | 4 | F | Anaplastic ganglioma | III | 1st | Dabraf+tramet | PR | 23 a |
| Brown et al 11 | 21 | F | Anaplastic PXA | III | 1st | Dabraf+tramet | PR | 22 a |
| Toll et al 11 | 13 | M | Anaplastic astroblastoma | III | 2nd | Dabraf+tramet | CR | 20 |
| Brown et al 11 | 48 | F | Anaplastic PXA | III | 4th | Dabraf+tramet | PR | 8 a |
| Smith-Cohn et al 26 | 23 | F | Anaplastic PXA | III | 2nd | Dabraf+tramet | PR | 3 |
| Burger et al 15 | 24 | M | Anaplastic PXA | III | 2nd | Dabraf | CR | 27 a |
| Bautista et al 17 | 1.5 | F | Anaplastic ganglioma | III | 5th | Vemuraf | PR | 20 a |
| Burger et al 15 | 50 | M | Anaplastic PXA | III | 3rd | Dabraf | PR | 8 a |
| Bautista et al 17 | 6 | M | Anaplastic ganglioma | III | 2nd | Vemuraf | PR | 3 |
| Lee et al 18 | 41 | M | Anaplastic PXA | III | 2nd | Vemuraf | PR | 3 a |
| Leaver et al 16 | 39 | M | Anaplastic PXA | III | 1st | Vemuraf | PR | 2 |
| Chamberlain et al 19 | 43 | M | Anaplastic PXA | III | 3rd | Vemuraf | PD | 2 |
| Bautista et al 17 | 9 | F | Anaplastic astrocytoma | III | 4th | Vemuraf | PD | 0.5 |
| Patient case #4 | 33 | F | Epithelioid GBM | IV | 2nd | Dabraf+tramet | SD | 53 a |
| Toll et al 8 | 12 | F | HGG with epithelioid morphology | IV | 2nd | Dabraf+tramet | PR | 32 a |
| Johanns et al 9 | 28 | F | Epithelioid GBM | IV | 1st | Dabraf+tramet | PR | 11 |
| Patient case #3 | 21 | F | Epithelioid GBM | IV | 2nd | Dabraf+tramet | SD | 16 a |
| Woo et al 10 | 22 | F | Epithelioid GBM | IV | 1st | Dabraf+tramet | PR | 7 |
| Woo et al 10 | 22 | F | Epithelioid GBM | IV | 1st | Vemuraf+cobimet | SD | 5.5 |
| Johanns et al 9 | 24 | M | Epithelioid GBM | IV | 2nd | Dabraf+tramet | PR | 3 b |
| Smith-Cohn et al 26 | 47 | M | Epithelioid GBM | IV | 3rd | Dabraf+tramet | PD | 1 |
| Beba Abadal et al 12 | 34 | F | GBM | IV | 3rd | Vemuraf | SD | 11 |
| Ceccon et al 13 | 27 | M | Epithelioid GBM | IV | 4th | Dabraf | SD | 10 |
| Robinson et al 14 | 9 | M | Epithelioid GBM | IV | 3rd | Vemuraf | PR | 6 a |
| Burger et al 15 | 25 | M | Glioblastoma, IDH-wt | IV | 3rd | Dabraf | PR | 3 a |
| Leaver et al 16 | 26 | M | Epithelioid GBM | IV | 1st | Vemuraf | PD | 0.5 |
aTreatment ongoing
bNon-adherent to treatment.
Abbreviations: F = female, M = male, GBM = glioblastoma, PXA = pleomorphic xanthoastrocytoma, Dabraf = dabrafenib, dabraf+tramet = dabrafenib/trametinib, vemuraf = vemurafenib, vemuraf+vobemet = vemurafenib/vobimetinib, CR = complete response, PR = partial response, SD = stable disease, PD = progressive disease, NR = not reported, insuf. f/up = insufficient follow-up.
Keywords searched: glioma, primary brain tumor, BRAF, MEK, targeted, vemurafenib, dabrafenib, encorafenib, cobimetinib, trametinib, and binimetinib (Date range: January 2000 to December 2019).
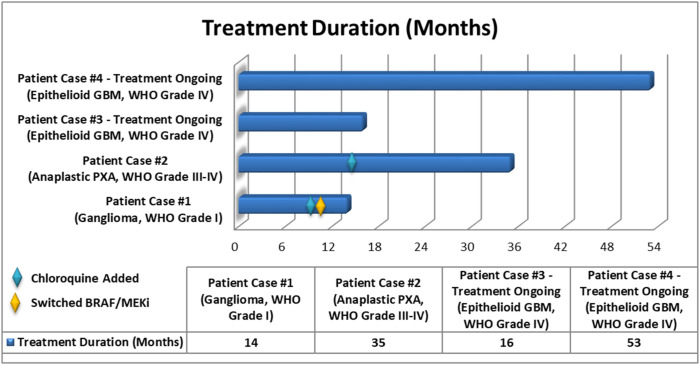
Figure 4.
Treatment duration for BRAF V600E-mutant gliomas treated with targeted therapy. Patients were treated with dabrafenib plus trametinib. Patient cases #1 and #2 had chloroquine added to their regimen at approximately 9 months and 14 months, respectively. Patient cases #3 and #4 are ongoing with treatment at 16 months and 53 months, respectively.
The potential for publication-bias remains with described clinical case series of benefit with targeted treatment, but in the setting of rare molecular subsets of cancer they offer important early evidence of clinical activity in rare molecular subsets of cancer. Clinical trials assessing combination dabrafenib/trametinib for both low- and high-grade gliomas are ongoing (NCT02034110) and interim reports have been promising, with an ORR of 62% and 29% in low- and high-grade gliomas, respectively. Durable clinical benefit was also seen in subset of patients with a median treatment duration of 19.5 months (range, 1 to 55 months) and 10.6 months (range, 1 to 43 months) in patients with low- and high-grade gliomas, respectively. 7
Patients with epithelioid GBM have historically had a poor prognosis; however, subsets of epithelioid GBM may have a more favorable prognosis than others. 41 Additional work is needed to characterize the response to combination BRAF/MEKi in each of these subsets. The patients with epithelioid GBM in our small series, treated with BRAF/MEKi, have both demonstrated stable clinical and radiographic disease, with a duration of response of 16 months and 53 months, respectively, both ongoing at the time of publication.8-24 To the best of our knowledge, case 4 in this small series represents the longest duration of clinical benefit from BRAF/MEKi that has been reported to date. Although of unclear significance, the two patients with epithelioid GBM share genetic alterations that may be relevant in the context of a favorable response to treatment with combination BRAF/MEKi and should be explored in future studies (Table 2). Our cases, along with other early reports utilizing dabrafenib/tramentinib, provide evidence of sustained clinical benefit from combined BRAF/MEKi therapy even in the setting of high-grade gliomas.
Table 2.
Molecular profile of BRAF V600E-mutant GBM cases treated with BRAF/MEKi. Highlighted are the mutations present in the two patients with epithelioid GBM WHO grade IV.
| Patient case #3—ongoing with treatment at 16 months | Patient case #4—Ongoing with treatment at 53 months | ||||||
|---|---|---|---|---|---|---|---|
| Gene | Alteration | MAF/Copy number | Location | Gene | Alteration | MAF/Copy number | Location |
| BRAF | V600 E | 38.3% | 7q34 | BRAF | V600 E | 32.0% | 7q34 |
| CDKN2A | Loss | 0 | 9p21 | CDKN2A | Loss | 0 | 9p21 |
| CDKN2B | Loss | 0 | 9p21 | CDKN2B | Loss | 0 | 9p21 |
| PIK3CG | R49S | 40.6% | 7q22.3 | PIK3CG | Amplification | 7 | 7q22.3 |
| BRD4 | P482 L | 61.3% | 19p13.1 | CDK6 | Amplification | 8 | 7q21-q22 |
| ZNF703 | A401_H402insPTHLGGSSCSTCSA | 34.9% | 8p11.23 | PIK3R1 | T576_L581del | 28.0% | 5q13.1 |
| CREBBP | A1907 T | 48.1% | 16p13.3 | HGF | Amplification | 8 | 7q21.1 |
| CREBBP | Q771 R | 49.0% | 16p13.3 | U2AF1 | Amplification | 8 | 21q22.3 |
| FANCA | V121 L | 51.1% | 16q24.3 | SNCAIP | Amplification | 8 | 5q23.2 |
| PREX2 | G382S | 44.2% | 8q13.2 | SMO | Amplification | 7 | 7q32.3 |
| PRKDC | Q947 L | 45.1% | 8q11 | RUNX1 | Amplification | 8 | 21q22.3 |
| RICTOR | Amplification | 8 | 5p13.1 | ||||
| RAC1 | Amplification | 7 | 7p22 | ||||
| PIK3R1 | Amplification | 8 | 5q13.1 | ||||
| PIK3CG | Q134 L | 7 | 7q22.3 | ||||
| MSH6 | K1358fs*2 | 29.0% | 2p16 | ||||
| MLL3 | P3468 L | 58.0% | 7q36.1 | ||||
| MET | Amplification | 7 | 7q31 | ||||
| MAP3K1 | Amplification | 8 | 5q11.2 | ||||
| MAGI2 | Amplification | 8 | 7q21 | ||||
| MLL3 | Amplification | 7 | 7q36.1 | ||||
| KEL | Amplification | 7 | 7q33 | ||||
| INHBA | Amplification | 7 | 7p15-p13 | ||||
| IL7R | Amplification | 8 | 5p13 | ||||
| IKZF1 | Amplification | 7 | 7p12.2 | ||||
| GRM3 | Amplification | 8 | 7q21.1-q21.2 | ||||
| GABRA6 | Amplification | 8 | 5q34 | ||||
| FGFR1 | S134D | 49.0% | 8p11.23-p11.22 | ||||
| FGF10 | Amplification | 8 | 5p13-p12 | ||||
| EZH2 | Amplification | 7 | 7q35-q36 | ||||
| ERG | Amplification | 8 | 21q22.3 | ||||
| EPHA3 | E265 G | 48.0% | 3p11.2 | ||||
| EGFR | Amplification | 7 | 7p12 | ||||
| BRCA2 | R118 C | 58% | 13q12.3 | ||||
| BRAF | Amplification | 7 | 7q34 | ||||
| APC | Amplification | 7 | 5q21-q22 | ||||
All patients who underwent RT, except case 4, had MRI perfusion performed in addition to the MRI brain to differentiate tumor progression vs treatment-related changes. Case 4 was deemed to have tumor progression based on radiographic appearance, which was further supported by her immediate response clinically and radiographically following treatment with BRAF/MEKi, without the need for dose appropriate glucocorticoids or other therapy for radiation necrosis (ie, bevacizumab). MRI changes observed prior to starting BRAF/MEKi being due to treatment-related changes remain a possibility. Nevertheless, the fact that a patient with epithelioid GBM has remained stable for up to 53 months after starting BRAF/MEKi is noteworthy.
It is worth noting that the ganglioglioma and anaplastic PXA patients had chloroquine added to their regimen of BRAF/MEKi at early signs of progression. Chloroquine was added based upon what was perceived as a relatively low risk of adverse events and promising early clinical data suggesting that autophagy inhibition may help to overcome resistance to targeted treatment with BRAFi.29,42-44
This small case report series has several limitations. These include being unable to generalize to a larger cohort of patients with similar diseases. In addition, it is difficult to determine a cause and effect relationship in these independent, single cases with different histopathological features and WHO grades. Prospective clinical trials are needed to better evaluate the effectiveness of BRAF/MEKi in patients with gliomas. Nevertheless, the positive responses to treatment with BRAF/MEKi seen in these patients can lead to the generation of new hypotheses regarding the pathophysiology of these diseases, and allow the expansion of successful new therapies, when prospective studies are not feasible.
Conclusion
The combination of BRAF/MEK inhibition has the potential to offer clinical benefit in both low-grade and high-grade gliomas that historically have not responded as well to BRAFi monotherapy. Our cases, along with other early reports utilizing dabrafenib/trametinib, highlight the importance of somatic next-generation sequencing, particularly in younger patients. Interim results from clinical trials of dabrafenib/trametinib have been promising thus far, and our case series suggests that durable clinical benefit is possible, even in the setting of glioblastoma, WHO grade IV.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by an NCI Comprehensive Cancer Center support grant (P30 CA076292), Moffitt Pinellas Partners, and the Collins Family Foundation.
Ethical Approval
Our study was approved by the Advarra Ethics Committee (no. Pro00031026, IRB protocol MCC 19859). A waiver of consent was granted for retrospective chart review in accordance with the Declaration of Helsinki and the 21st Century Cures Act.
Informed Consent
All of the patients provided a written informed consent for their information to be published in the current study, except for case #3 who moved out of state and was lost to follow-up. MRI brain and pathology imaging from case #3 were excluded from this study given inability to obtain written informed consent.
Data Availability
All data analyzed during this study are included in this article.
ORCID iDs
Michael J. Fusco https://orcid.org/0000-0002-5883-7466
Yolanda Piña https://orcid.org/0000-0003-1383-5704
References
- 1.Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121:397-405. [DOI] [PubMed] [Google Scholar]
- 2.Behling F, Barrantes-Freer A, Skardelly M, et al. Frequency of BRAF V600E mutations in 969 central nervous system neoplasms. Diagn Pathol. 2016;11:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brastianos PK, Santagata S. Endocrine tumors: BRAF V600E mutations in papillary craniopharyngioma. Eur J Endocrinol. 2016;174:R139-R144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2014;372:30-39. [DOI] [PubMed] [Google Scholar]
- 6.Kaley T, Touat M, Subbiah V, et al. BRAF Inhibition in BRAFV600-Mutant Gliomas: results from the VE-BASKET study. J Clin Oncol. 2018;36:3477-3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen P, Stein A, van den Bent M, et al. Updated Efficacy and Safety of Dabrafenib + Trametinib in Patients with Recurrent or Refractory BRAF V600E–Mutated High-Grade Glioma (HGG) and Low-Grade Glioma (LGG). Phoenix, AZ: Society for Neuro-Oncology; 2019. [Google Scholar]
- 8.Toll SA, Tran HN, Cotter J, et al. Sustained response of three pediatric BRAFV600E mutated high-grade gliomas to combined BRAF and MEK inhibitor therapy. Oncotarget. 2019;10:551-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johanns TM, Ferguson CJ, Grierson PM, Dahiya S, Ansstas G. Rapid clinical and radiographic response with combined dabrafenib and trametinib in adults with BRAF -mutated high-grade glioma. J Natl Compr Canc Netw. 2018;16:4-10. [DOI] [PubMed] [Google Scholar]
- 10.Woo PYM, Lam T-C, Pu JKS, et al. Regression of BRAFV600E mutant adult glioblastoma after primary combined BRAF-MEK inhibitor targeted therapy: a report of two cases. Oncotarget. 2019;10:3818-3826. [PMC free article] [PubMed] [Google Scholar]
- 11.Brown NF, Carter T, Kitchen N, Mulholland P. Dabrafenib and trametinib in BRAFV600E mutated glioma. CNS Oncology. 2017;6:291-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beba Abadal K, Walsh MA, Yachnis AT, et al. Eleven month progression–free survival on vemurafenib monotherapy in a patient with recurrent and metastatic BRAF V600E–mutated glioblastoma WHO Grade 4. JCO Precision Oncology. 2017;1:5. [DOI] [PubMed] [Google Scholar]
- 13.Ceccon G, Werner J-M, Dunkl V, et al. Dabrafenib treatment in a patient with an epithelioid glioblastoma and BRAF V600E mutation. Int J Mol Sci. 2018;19:1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson GW, Orr BA, Gajjar A. Complete clinical regression of a BRAF V600E-mutant pediatric glioblastoma multiforme after BRAF inhibitor therapy. BMC Canc. 2014;14:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burger MC, Ronellenfitsch MW, Lorenz NI, et al. Dabrafenib in patients with recurrent, BRAF V600E mutated malignant glioma and leptomeningeal disease. Oncol Rep. 2017;38:3291-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leaver KE, Zhang N, Ziskin JL, Vogel H, Recht L, Thomas RP. Response of metastatic glioma to vemurafenib. Neuro-Oncology Practice. 2015;3:268-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bautista F, Paci A, Minard-Colin V, et al. Vemurafenib in pediatric patients with BRAFV 600E mutated high-grade gliomas. Pediatr Blood Canc. 2014;61:1101-1103. [DOI] [PubMed] [Google Scholar]
- 18.Lee EQ, Ruland S, LeBoeuf NR, Wen PY, Santagata S. Successful treatment of a progressive BRAF V600E-mutated anaplastic pleomorphic xanthoastrocytoma with vemurafenib monotherapy. J Clin Oncol : Official Journal of the American Society of Clinical Oncology. 2014;34:e87-9. [DOI] [PubMed] [Google Scholar]
- 19.Chamberlain MC. Salvage therapy with BRAF inhibitors for recurrent pleomorphic xanthoastrocytoma: a retrospective case series. J Neuro Oncol. 2013;114:237-240. [DOI] [PubMed] [Google Scholar]
- 20.Del Bufalo F, Ceglie G, Cacchione A, et al. BRAF V600E Inhibitor (Vemurafenib) for BRAF V600E Mutated Low Grade Gliomas. Frontiers in Oncology. 2018;8:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Usubalieva A, Pierson CR, Kavran CA, et al. Primary meningeal pleomorphic xanthoastrocytoma with anaplastic features: a report of 2 cases, one with BRAFV600E mutation and clinical response to theBRAFInhibitor Dabrafenib. J Neuropathol Exp Neurol. 2015;74:960-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aguilera D, Janss A, Mazewski C, et al. Successful retreatment of a child with a refractory brainstem ganglioglioma with vemurafenib. Pediatr Blood Canc. 2016;63:541-543. [DOI] [PubMed] [Google Scholar]
- 23.Chamberlain MC. Recurrent ganglioglioma in adults treated with BRAF inhibitors. CNS Oncology. 2016;5:27-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lassaletta A, Guerreiro Stucklin A, Ramaswamy V, et al. Profound clinical and radiological response to BRAF inhibition in a 2-month-old diencephalic child with hypothalamic/chiasmatic glioma. Pediatr Blood Canc. 2016;63:2038-2041. [DOI] [PubMed] [Google Scholar]
- 25.Schreck KC, Guajardo A, Lin DDM, Eberhart CG, Grossman SA. Concurrent BRAF/MEK inhibitors in BRAF V600-mutant high-grade primary brain tumors. J Natl Compr Canc Netw. 2018;16:343-347. [DOI] [PubMed] [Google Scholar]
- 26.Smith-Cohn M, Davidson C, Colman H, Cohen AL. Challenges of targeting BRAF V600E mutations in adult primary brain tumor patients: a report of two cases. CNS Oncology. 2019;8:CNS48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy JMM, Thompson JC, Griesinger AM, et al. Autophagy inhibition improves chemosensitivity in BRAFV600E brain tumors. Canc Discov. 2014;4:773-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zahedi S, Fitzwalter BE, Morin A, et al. Effect of early-stage autophagy inhibition in BRAFV600E autophagy-dependent brain tumor cells. Cell Death Dis. 2019;10:679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piña Y, Fusco MJ, Macaulay RJ, et al. Using personalized medicine in gliomas: a genomic approach to diagnosis and overcoming treatment resistance in a case with pleomorphic xanthoastrocytoma. J Neurol. 2020;267:783-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reifenberger G, Wirsching H-G, Knobbe-Thomsen CB, Weller M. Advances in the molecular genetics of gliomas - implications for classification and therapy. Nat Rev Clin Oncol. 2016;14:434-452. [DOI] [PubMed] [Google Scholar]
- 31.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med. 2005;352:987-996. [DOI] [PubMed] [Google Scholar]
- 32.Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma. Jama. 2017;318:2306-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong ET, Gautam S, Malchow C, Lun M, Pan E, Brem S. Bevacizumab for recurrent glioblastoma multiforme: a meta-analysis. J Natl Compr Canc Netw. 2011;9:403-407. [DOI] [PubMed] [Google Scholar]
- 34.Lombardi G, De Salvo GL, Brandes AA, et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019;20:110-119. [DOI] [PubMed] [Google Scholar]
- 35.Cancer Genome Atlas N. Genomic Classification of Cutaneous Melanoma. Cell. 2015;161:1681-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18:1307-1316. [DOI] [PubMed] [Google Scholar]
- 37.Van Cutsem E, Huijberts S, Grothey A, et al. Binimetinib, encorafenib, and cetuximab triplet therapy for patients with BRAF V600E-mutant metastatic colorectal cancer: safety lead-in results from the phase III BEACON colorectal cancer study. J Clin Oncol : Official Journal of the American Society of Clinical Oncology. 2019;37:1460-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol. 2018;36:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleinschmidt-DeMasters BK, Aisner DL, Birks DK, Foreman NK. Epithelioid GBMs show a high percentage of BRAF V600E mutation. Am J Surg Pathol. 2013;37:685-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Louis DN. WHO Classification of Tumours of the Central Nervous System. 4th Edition. Switzerland: WHO Press and LLC, USA: Stylus Publishing; 2016. [Google Scholar]
- 41.Korshunov A, Chavez L, Sharma T, et al. Epithelioid glioblastomas stratify into established diagnostic subsets upon integrated molecular analysis. Brain Pathol. 2018;28:656-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levy JMM, Thompson JC, Griesinger AM, et al. Autophagy Inhibition Improves Chemosensitivity in BRAFV600E Brain Tumors. Canc Discov. 2014;4:773-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mulcahy Levy JM, Zahedi S, Griesinger AM, et al. Autophagy inhibition overcomes multiple mechanisms of resistance to BRAF inhibition in brain tumors. eLife. 2017;6:e19671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Canc. 2017;17:528-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed during this study are included in this article.