Abstract
There has been a significant interest in developing cell membrane-coated nanoparticles due to their unique abilities of biomimicry and biointerfacing. As the technology progresses, it becomes clear that the application of these nanoparticles can be drastically broadened if additional functions beyond those derived from the natural cell membranes can be integrated. Herein, we summarize the most recent advances in the functionalization of cell membrane-coated nanoparticles. In particular, we focus on the emerging methods, including (1) lipid insertion, (2) membrane hybridization, (3) metabolic engineering, and (4) genetic modification. These approaches contribute diverse functions in a non-disruptive fashion while preserving the natural function of the cell membranes. They also improve on the multi-functional and multi-tasking ability of cell membrane-coated nanoparticles, making them more adaptive to the complexity of biological systems. We hope that these approaches will serve as inspiration for more strategies and innovations to advance cell membrane coating technology.
Keywords: Nanotechnology, nanomedicine, nanoparticle, surface functionalization, cell membrane
Graphical Abstract
Introduction
Interest in developing therapeutic nanoparticles has grown for decades, motivated primarily by their potential applications to improve disease diagnosis, treatment, and prevention.1 Among various platforms, cell membrane-coated nanoparticles, made by wrapping natural cell membranes onto synthetic nanoparticulate cores, have attracted much attention.2 This platform stands out because of its ability to replicate the highly complex cellular functionalities to create new therapeutic modalities.3 For example, by inheriting ‘markers of self’ from the source cells, some cell membrane-coated nanoparticles effectively evade immune clearance, becoming superior long-circulating drug carriers.4, 5 Some inherit exquisite affinity ligands native to the parent cells, becoming capable of actively targeting the disease sites.6, 7 Some act as cell decoys to intercept harmful molecules or pathogens and protect source cells without the prior knowledge of the threat. This mechanism has allowed for a function-driven and broad-spectrum detoxification strategy.8 By mimicking parent cells, some cell membrane-coated nanoparticles offer faithful and more relevant antigen presentation.6, 9, 10 Some can also detain bacterial toxins to restrict their harm while preserving their structural integrity.11, 12 These unique abilities allow them to work as vaccines that elicit highly effective protective immunity.
Since their initial development, cell membrane-coated nanoparticles are increasingly applied to complex biological systems. This leads to an increased demand for multi-functionality and multitasking. In some scenarios, providing additional functions or functional ligands seems beneficial to boost the performance of these nanoparticles. For example, while the cell membrane coating offers impressive stealth and immune evasion, additional target-selectivity may further limit off-target side effects and enhance treatment efficacy.13, 14 While these nanoparticles can faithfully present antigenic information for immune uptake, additional control over the amplitude of the immune activation would be desirable to modulate immunity.15, 16 Furthermore, other functionalities such as those responsive to environmental stimuli, if available, would provide cell membrane-coated nanoparticles with a more dynamic and intelligent biointerfacing capability.17, 18 Clearly, functionalities beyond the natural properties of cell membranes, if added, would significantly expand the application of this novel class of nanoparticles.
To introduce additional functionalities, researchers have developed conjugation methods that employ amine-, carboxyl-, biotin-, or sulfhydryl-based reactions.19, 20 These methods are convenient to decorate the cell membrane with functional ligands. However, they lack control over the position and density of the linked ligands. Random chemical reactions tend to cause cell membrane damage such as membrane protein aggregation or undesirable exposure of phosphatidylserine to the outer leaflet of the membrane bilayers, which compromises immune integrity.21, 22 Sequential conjugations by first anchoring linkers onto the cell membrane followed next by ligand conjugation to the linkers have been developed.23 Although the method can minimize membrane damages, it may also limit ligand choices and density in the conjugation.
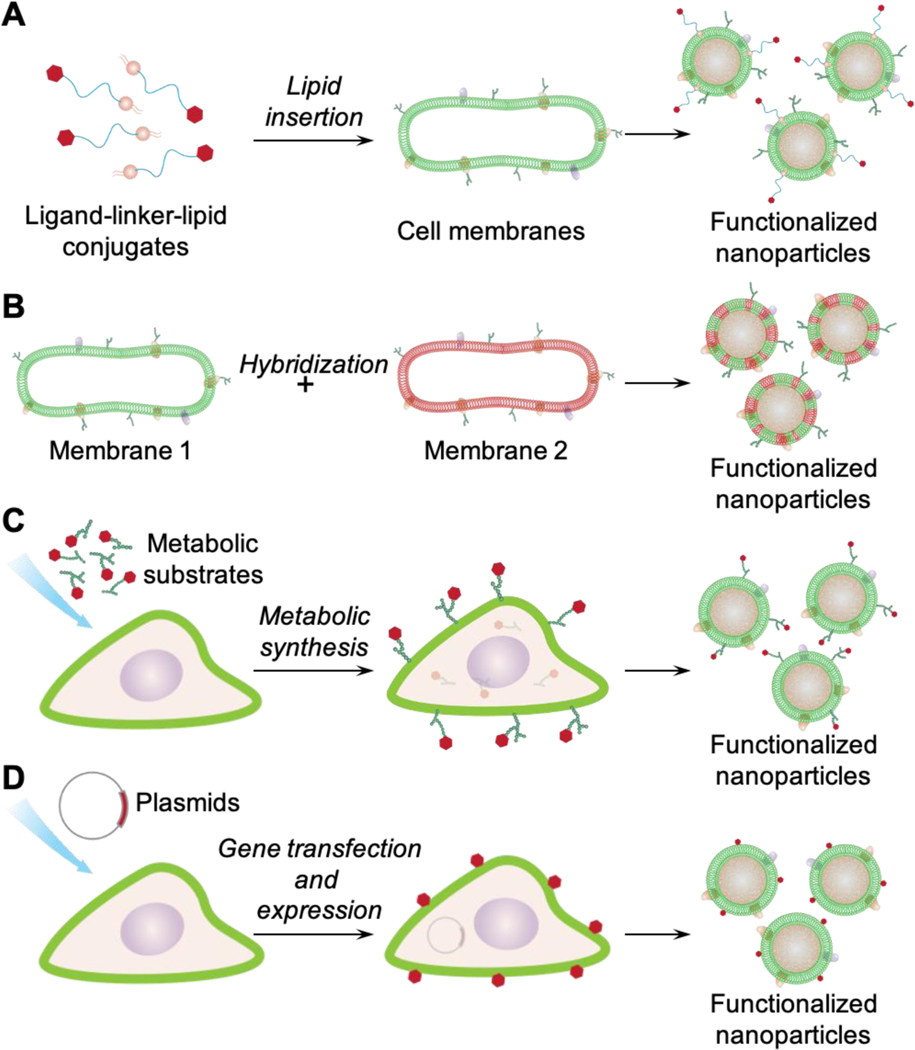
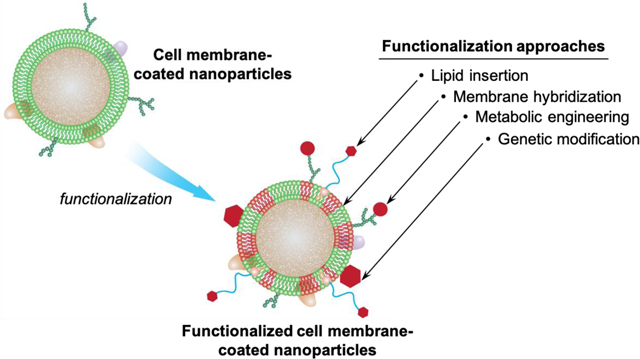
Challenges faced by traditional ligand conjugation have also motivated a few non-disruptive and straightforward strategies well suited for functionalizing cell membrane-coated nanoparticles (Figure 1). Specifically, these methods include (1) the lipid insertion method that incorporates functional ligands by first synthesizing a ligand-linker-lipid conjugate and then inserting the lipid tether into the membrane bilayers, (2) the membrane hybridization method that fuses membranes of different cell types to combine complementary ligands for functionalization, (3) the metabolic engineering method that allows the ligand to participate in natural oligosaccharide or lipid synthesis pathways for expression onto the cell membrane, and (4) the genetic modification method that expresses protein ligands onto the cell surface through gene editing. In this article, we discuss the principles of each method and summarize their recent development with an emphasis on how the introduced and native functionalities cooperate for a better therapeutic outcome. We conclude the article with a discussion on potential future development. As time progresses, these methods will undoubtedly inspire new functionalization approaches and broader applications of cell membrane-coated nanoparticles.
Figure 1.
Schematic showing different methods for functionalizing cell membrane-coated nanoparticles. (A) lipid insertion, (B) membrane hybridization, (C) metabolic engineering, and (D) genetic modification.
Lipid Insertion
Lipid insertion refers to a method that incorporates functional ligands onto natural cell membranes through a lipid anchor. Functional moieties can be conjugated to the anchor before mixing with the membrane.24 By exploiting the fluidity of bilayered lipid membranes, the insertion relies on physical rather than chemical interactions for membrane anchoring. Sonication or extrusion commonly used for membrane coating can facilitate the lipid insertion. In addition, ligand density can be precisely tailored by controlling its initial input, a property beneficial to formulation optimization. These advantages altogether make lipid insertion attractive for functionalizing cell membrane-coated nanoparticles.
Lipid insertion has been used to anchor a variety of affinity ligands onto cell membrane-coated nanoparticles to achieve targeting ability. For example, folate and aptamers were conjugated with 1,2-disteroyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)] (DSPE-PEG) and inserted into red blood cell (RBC) membranes for cancer targeting. The resulting membrane-coated nanoparticles bound to cancer cells in a ligand-specific manner, confirming the successful transfer of biological function onto the nanoparticles.25 Using lipid insertion to anchor small molecule ligands for functionalization has become popular due to the structural simplicity of the ligands and their easy conjugation to the lipid.26 Following this initial development, other small molecules have been used for functionalization, including mannose and binding peptides.27–37 In addition to the RBC membranes, cancer cell membranes have also been demonstrated for ligand insertion.38, 39 Targeted diseases have subsequently expanded to include more types of cancer, such as melanoma and glioblastoma, and other diseases, such as stroke. Lipid insertion further allowed nanoparticles to facilitate a two-step ‘pre-targeting’ strategy aimed towards enriching imaging agents at the tumor site. 40 In this work, RBC membranes were inserted with two ligands: folate and an azide. The membrane-coated nanoparticles were first directed to the tumor site by the folate, where they served as a homing agent to attract azide-reactive dibenzocyclooctyl (DBCO)-modified imaging agents.
In addition to small-molecule ligands, lipid insertion has been applied to anchor antibodies onto the surface of cell membrane-coated nanoparticles for targeting. In this case, lipid molecules are first linked with functional groups reactive to antibodies such as aldehyde, amine, thiol, and carboxyl groups, and used subsequently for insertion.19, 20 With this approach, antibodies were inserted to target human epidermal growth factor receptor 2 (HER2), epithelial growth factor receptor (EGFR), and epithelial cell adhesion molecule (EpCAM) on cancer cells.41–44 Notably, when compared with small-molecule ligands, antibodies are bulkier. Their geometric orientation is more challenging to control because available functional groups can randomly distribute over the protein surface. In this regard, modification of the reactivity across the antibody surfaces for site-selective conjugation can improve the control of antibody orientation.45 Meanwhile, antibody fragments could serve as an attractive alternative to replace full-size antibodies in lipid insertion for their smaller sizes and better control over the conjugation sites.46
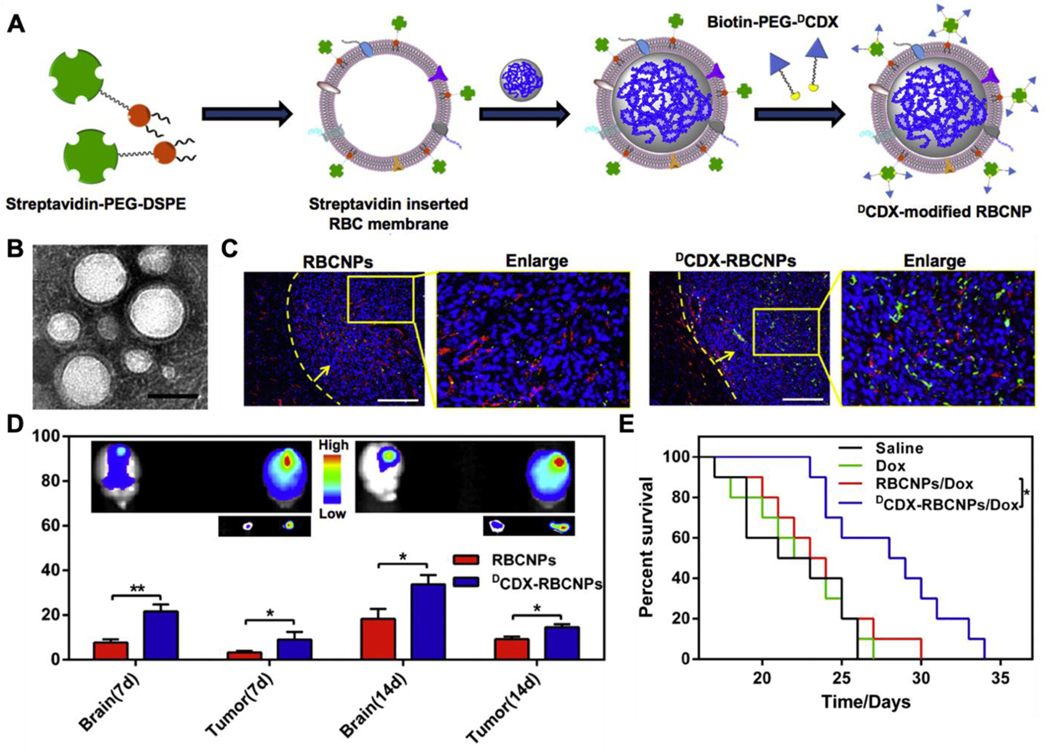
For lipid insertion, most applications have used 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000] (PEG-DSPE) as the lipid anchor, with a PEG spacer added to preserve the freedom of the ligand for bioactivity.23, 47 Streptavidin is often used as an additional linker between the lipid and the ligand. For the multivalency of streptavidin, each lipid could anchor up to four biotinylated ligands.48 With streptavidin-biotin chemistry, the lipid can be biotinylated for insertion, followed by linking with the streptavidin-conjugated ligands.43 On the other hand, the lipid can be linked with streptavidin first for insertion, followed by conjugation with biotinylated ligands.49 With a molecular weight of 60 kDa, streptavidin is relatively large. It also has a neutral charge at physiological pH. Therefore, when used as a linker, streptavidin blocks some interactions between the positively charged ligand and the negatively charged cell membranes that otherwise may restrict the freedom of the ligand and hinder its bioactivity. For example, DCDX peptide derived from candoxin targets the nicotinic acetylcholine receptors (nAChR) on the brain endothelial cells.50 However, with a strong positive charge, the peptide interacts with the cell membrane, making it unsuitable for direct lipid insertion. Using lipid with a streptavidin linker blocked such unwanted interactions and successfully targeted the nanoparticles to the brain (Figure 2).
Figure 2.
(A) Schematic of the preparation of nanoparticles coated with DCDX-modified RBC membranes (DCDX-RBCNPs). Streptavidin-PEG-DSPE is synthesized and then inserted into RBC membranes. After coating polymeric cores, biotin-PEG-DCDX binds to the streptavidin on the surface of the resulting RBCNPs to form DCDX-modified RBCNPs. (B) Transmission electron microscope image of DCDX-RBCNPs. (C) The distribution of nanoparticles in the brain of tumor-bearing mice 14 days post-implantation. Nuclei were stained with DAPI (blue), blood vessels were labeled with anti-CD31 (red), while green represents the DiI-loaded nanoparticles. The yellow dotted lines represent the margins of the glioma and the yellow arrows point to the glioma (scale bars, 200 μm). (D) Ex vivo images and average radiant efficacy of brains and tumors in tumor-bearing mice (7 or 14 days after implantation). Bars represent means with SD, n = 3, *p < 0.05, **p < 0.005. (E) Kaplan-Meier survival curves of nude mice bearing intracranial U87 glioma. Mice (n = 10) were injected at 7, 9, 11, 13 and 15 days after glioma implantation with saline, free Dox, Dox-loaded RBC-NPs (RBCNPs/Dox), and Dox-loaded DCDX-RBCNPs (DCDX-RBCNPs/Dox). Reproduced with permission from ref 50. Copyright 2017 Elsevier.
Besides serving as the anchor for the ligands, the lipid itself can also carry functions that, after insertion, alter cell membrane properties in response to environmental stimuli such as light, oxygen level, and pH for desirable purposes. For example, a lipid molecule, 1,1’-Dioctadecyl-3,3,3’,3’-Tetramethylindotricarbocyanine iodide (DiR), was inserted into RBC membranes to convert near-infrared (NIR) into heat and induce local hyperthermia (Figure 3).51 The nanoparticle core was prepared with a thermo-sensitive lipid 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) with a transition temperature around 41.5°C. Without NIR light irradiation, DiR did not generate heat, and the nanoparticle core remained intact. However, under NIR light, DiR produced thermal energy to trigger the phase transition of DPPC, which destroyed the nanoparticle core for drug release. In another example, DSPE-PEG was conjugated with a TGF β-neutralizing antibody through a hypoxia-sensitive azobenzene linker. In the normoxia environment, the nanoparticle retained the antibodies on its surface. However, in the hypoxic environment of the bone marrow, the azobenzene linker was cleaved, releasing the TGF β-neutralizing antibodies to block signaling between leukemia cells and adjacent niche cells.39 As another example, liposomes incorporating a pH-sensitive lipid, DSPE-polyethyloxazoline (PEOz), was co-extruded with platelet membrane to form DSPE-PEOz-inserted “platesomes”.52 The PEOz moiety can be rapidly protonated at endo-lysosomal pH, generating electrostatic repulsion to de-stabilize the membrane structure and release the therapeutic payload.
Figure 3.
(A) The near infrared light (NIR)-driven drug release of the red blood cell (RBC) membrane-coated nanoparticles (PTX-PN@DiR-RV). DiR dye was embedded in the RBC membrane (DiR-RV), and the thermosensitive lipid DPPC was added to the polymeric cores (PN). Under the 808 nm laser irradiation (+L), DiR provided strong thermal energy and then triggered the phase transition of DPPC, leading to the destruction of the cores and the release of paclitaxel (PTX). (B) The infrared thermographic images of mice after 4 h i.v. injection with PBS, free DiR, PN@DiR-RV, and PTX-PN@DiR-RV, respectively. (C) The temperature elevation profile of each group in (B). (D-E) In vivo antitumor and anti-metastasis efficacy by the synergetic chemo-photothermal therapy of PTX-PN@DiR-RV. (D) Tumor growth of mice after intravenous injection of different formulations. (E) Quantitative analysis of the lung metastatic nodules for each group. Data were presented as mean ± SD (n = 6), ** P < 0.01, *** P < 0.005. Reproduced with permission from ref 51. Copyright 2016 John Wiley and Sons.
Studies of using lipid insertion to functionalize cell membrane-coated nanoparticles are summarized in Table 1. Overall, this method is efficient and straightforward, offering tremendous versatility to functionalize cell membrane-coated nanoparticles. The lipids not only serve as anchors for the ligands but can also carry unique functionalities, especially those that are environment-responsive. To modulate ligand density, monovalent or multivalent linkers are available. The method has been proven successful for anchoring different ligands with varying physicochemical properties and biological functions. As the method becomes increasingly popular, fundamental understanding on ligand-membrane interactions has also improved the rationale selection of ligands for insertion toward in vivo applications. The lipid insertion method is expected to bring in tremendous opportunities for the development and use of functionalized cell membrane-coated nanoparticles.
Table 1.
Functionalization of cell membrane-coated nanoparticles by lipid insertion
| Ligand | Spacer | Membrane source | Target cell (receptor) and additional function | References |
|---|---|---|---|---|
| Small molecules | ||||
| AS1411 aptamer | PEG2000 | RBC | breast cancer cell (nucleolin) | 25 |
| Folate | PEG2000 | RBC | cervical cancer cell (folate receptor), | 25 |
| ovarian cancer cell (folate receptor) | 27, 29 | |||
| breast cancer cell (folate receptor) | 28, 30, 40 | |||
| Mannose | PEG2000 | RBC | antigen presenting cell (mannose receptor) | 34 |
| cancer cell | dendritic cell (mannose receptor) | 38 | ||
| cRGD | PEG2000 | RBC | melanoma cell (αvβ3 integrin) | 35, 37 |
| Angiopeptide 2 | PEG2000 | RBC | glioblastoma cell (LRP receptor) | 33, 36 |
| Stroke homing peptide | PEG2000 | RBC | apoptotic neuron cell | 31 |
| T7/NGR peptide | PEG2000 | RBC | brain endothelial cell (transferrin receptor), glioblastoma cell (CD13) | 32 |
| Biotinylated CDX peptide | PEG3400-streptavidin | RBC | brain endothelial cell (nAChR) | 50 |
| Biotinylated c(RGDyK) | PEG3400-streptavidin | RBC | tumor vasculature endothelial cell, glioma cell (αvβ3 integrin) | 49 |
| Antibodies | ||||
| Anti-HER2 | PEG2000 | RBC | ovarian cancer cell (HER2) | 44 |
| Anti-EGFR-iRGD | PEG3400 | RBC | gastric cancer cell (EGFR, αvβ3 integrin) | 41 |
| n.a. | RBC | colorectal cancer cell (EGFR, αvβ3 integrin) | 42 | |
| Biotinylated anti-EpCAM | PEG2000-biotin-avidin | RBC | breast cancer cell (EpCAM) | 43 |
| Anti-TGFβRII | PEG2000-azobenzene | Cancer cell | hypoxia-triggered release of TGFβ-neutralizing antibody | 39 |
| Lipid with responsive functions | ||||
| DiR | n.a. | RBC | NIR-triggered membrane disruption for drug release | 51 |
| PEOz | n.a. | Platelet | pH-sensitive membrane disruption for drug release | 52 |
Abbreviations: LRP—low-density lipoprotein receptor-related protein, nAChR—nicotinic acetylcholine receptor, n.a.—not applicable
Membrane Hybridization
A variety of cell membranes have been successfully utilized for nanoparticle coating.2 The success has also motivated the recent development of mixing multiple cell membranes to develop ‘hybrid membranes’ aimed at boosting the functional characteristics of coated nanoparticles.53 One way of making such hybrid membranes is to first derive the membrane from individual cell types and then fuse them through mechanical forces such as stirring, extrusion, or sonication.53–55 Alternatively, hybrid membranes can be made by first fusing different live cells, followed by deriving the membrane from the cell hybrids.56, 57 Nanoparticles coated with hybrid membranes inherit the virtues of each parent cell type and harness the complementary functionalities (Figure 4).53 In various applications, these nanoparticles have shown better performances when compared with their counterparts coated with the individual membrane.54–58
Figure 4.
Development of RBC–platelet hybrid membrane-coated nanoparticles ([RBC-P]NPs). (A) Schematic of membrane fusion and coating. Membrane material is derived from both RBCs and platelets and then fused together. The resulting fused membrane is used to coat poly(lactic-co-glycolic acid) (PLGA) polymeric cores to produce [RBC-P]NPs. (B) A representative TEM image of [RBC-P]NPs negatively stained with vanadium (scale bar = 100 nm). (C) Confocal fluorescent microscopy images of either a mixture of RBC membrane-coated nanoparticles (RBCNPs) and platelet membrane-coated nanoparticles [PNPs] or of the [RBC-P]NPs (red = RBC membrane, green = platelet membrane; scale bar = 10 μm). (D) Circulation time of fluorescently labeled RBCNPs, [RBC-P]NPs, and PNPs after intravenous administration to mice via the tail vein (n = 4; mean SEM; lines represent two-phase decay model) (E) Amount of free dichlorvos, a model organophosphate, remaining in solution after incubation with RBCNPs, [RBC-P]NPs, or PNPs (n = 3; mean SD). UD = undetectable. (F) Imaging of aortas from ApoE knockout mice fed with a high fat western diet, after intravenous administration with dye-labeled RBCNPs, [RBC-P]NPs, and PNPs (red = nanoparticles; scale bars = 1 mm). Oil Red O staining was used to confirm the presence of atherosclerotic plaque. Reproduced with permission from ref 53. Copyright 2017 John Wiley & Sons.
Membrane hybridization has been used to introduce affinity ligands unique to one cell type to another, therefore adding targeting ability to the hybrid membrane-coated nanoparticles. In this regard, the platelet membrane is a popular choice for platelet receptors such as P-selectin, glycoprotein IIb/IIIa, and C-type lectin-like receptor 2 (CLEC-2) for specific tumor targeting.59–61 For example, platelet membranes were hybridized with RBC membranes and coated onto synthetic liposomes for the co-delivery of a sonosensitizer and a cytotoxic compound for anti-cancer sonodynamic therapy.62 In tumor-bearing mice, the hybrid membrane endowed a tumor-targeting capability, leading to a higher level of drug accumulation at the tumor site. Platelet membranes were also hybridized with neutrophil membranes, and their hybrid membranes were coated onto gold nanocages for the delivery of cytotoxic drugs and photosensitizers.63 In this case, the neutrophil membrane contributed additional targeting ability by recognizing multiple adhesion molecules on circulating tumor cells (CTCs) such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1).63, 64 The nanocages coated with hybrid membranes showed greater cellular uptake, deeper tumor penetration, and higher cytotoxicity when compared to non-coated or single membrane-coated gold nanocages.63
Membrane hybridization has also been used to boost immune evasion of the nanoparticles by bringing in another membrane with a stronger stealth ability. For example, cancer cell membrane-coated nanoparticles (CCNPs) have become a popular delivery platform for tumor binding.6 However, CCNPs made from cancer cell membranes alone do not seem stealthy enough to evade immune surveillance, mostly attributed to their possession of tumor-specific antigens on the membrane surface.65 As a result, their efficacy is limited by the rapid phagocytic clearance in the circulation. To address this shortcoming, researchers hybridized cancer cell membranes with RBC membranes that brought in ‘markers of self’ such as CD47 to enhance the stealth capability of the hybrid membrane.66 Nanoparticles coated with such cancer cell-RBC hybrid membranes showed a prolonged circulation half-life and a higher level of accumulation at the tumor site. Besides RBCs, platelets and leukocytes are also known for their prominent immune evasion. Recently, their membranes were hybridized with cancer stem cell membranes. The resulting hybrid membrane-coated nanoparticles showed longer circulation times compared to their CCNP counterparts.65, 67
Membrane hybridization has also been used to bring in ‘homologous’ characteristics aimed at reducing undesirable cell-binding interactions. For example, CCNP were used to capture and isolate circulating tumor cells (CTCs) for their unique homotypic binding.68 However, the competitive binding between CCNP and white blood cells (WBCs) limited the detection sensitivity and capture efficiency. To overcome this challenge, researchers hybridized cancer cell membranes with membranes of WBCs.68 By being ‘homologous’ to WBCs, the hybrid-membrane coated nanoparticles had significantly reduced interference from WBCs. The capture efficiency and detection sensitivity toward CTCs were improved considerably. Similar to cancer cells, platelets also bind with CTCs specifically.59–61 Therefore, platelet membrane-coated nanoparticles (PNPs) were also applied for CTC capture and isolation.58 In this approach, leukocytes compete with CTCs to bind with PNPs, reducing the isolation efficiency. This challenge was addressed by hybridizing platelet membranes with leukocyte membranes. By possessing features homologous to the leucocytes, hybrid membrane-coated nanoparticles showed less undesirable binding, and the CTC isolation efficiency was improved.
Membrane hybridization has also been used to incorporate immune-stimulatory properties for improving the outcome of immunotherapy. In particular, CCNPs with an array of tumor antigens present on their surfaces have been explored as anti-cancer vaccines.6, 10 However, the anti-tumor response induced by CCNPs is often hampered by downregulated antigen expression and tumor heterogeneity. Hybridization of the cancer cell membrane with a secondary membrane has been used to enhance the immunogenicity. 56, 57 For this purpose, attenuated Salmonella outer membrane vesicles (OMVs) were hybridized with cancer cell membranes and coated onto nanoparticles, resulting in a tumor-specific antigenic nanoplatform with self-adjuvanting activities.69 In mouse melanoma models, the hybrid membrane-coated nanoparticles induced anti-cancer immunity boosted by both dendritic cells (DCs) and cytotoxic T cells. Somatic hybrids of DCs and cancer cells were also made to produce hybrid membranes (Figure 5).56 Nanoparticles coated with such hybrid membranes acquired the antigen-presenting ability of DCs and therefore enhanced both direct and DC-mediated T cell activation for better anti-cancer immunity. This platform also combined cancer immunotherapy with photodynamic therapy and showed promising efficacy.57
Figure 5.
Development of cancer-dendritic hybrid membrane-coated metal organic framework (MOF) nanoparticles as a cancer vaccine. (A) Schematic of the process for preparing MOF nanoparticles coated with the membrane of the fused cells (MOF@FM). (B) Dendritic cells (DCs, anti-MHC II-labeled, green), 4T1 cells (anti-CD44-APC labeled, magenta), and the fused cells (FC, double labeled) observed with the confocal laser scanning microscopy (CLSM). Scale bar = 10 μm. (C) TEM images of MOF@FM. Scale bar =100 nm. (D) Percentage of DC maturation based on the quantification of CD80 and CD86 expression after in vitro incubation of DCs with 4T1 cancer cell membrane (CM), DC membrane (DM), and fused cell membrane (FM) for 48 h. The mean values and s.d. were presented and measurements were taken from distinct samples (one-way ANOVA; **p<0.01, ***p<0.001, n = 3). (E) In vitro cytotoxicity of the T lymphocytes after incubation with above-pretreated DCs for 48 h to 3T3, 4T1, and CT26 cells. The mean values and s.d. were presented and measurements were taken from distinct samples (n = 5). (F) Percentage of tumor-free mice receiving immunization with MOF@FM vaccine followed by tumor challenge. Reproduced with permission from ref 56. Copyright 2019 Springer Nature.
Studies of using the hybrid membrane to functionalize nanoparticles are summarized in Table 2. This method provides nanoparticles with functionalities otherwise exclusive to individual cell membranes. A variety of membrane combinations have been studied, creating synergies by combining an array of functions such as long circulation with active targeting and antigen presentation with immune stimulation.54, 56, 57, 65, 67 With abundant cell membranes to choose from, this method provides great flexibility in designing tailored and personalized nanomedicines. Future development is increasingly focused on understanding the membrane composition-efficacy relationship while improving the precision and reproducibility of the membrane hybridization process.2, 70 With continuous development, hybrid membrane-coated nanoparticles are expected to make a more significant impact on future clinical applications.
Table 2.
Summary of the studies that made hybrid membranes to functionalize nanoparticles
| Membrane | Functionalities | Additional membrane | References |
|---|---|---|---|
| RBCs | Provide markers of self, neutralizing pore-forming toxins | Platelets | 53, 55, 62 |
| Cancer cells | 54, 66 | ||
| Platelets | Provide markers of self | RBCs | 53 |
| Cancer stem cells | 67 | ||
| Offer ligands for targeting circulating tumor cells (CTCs) | Leukocytes | 58 | |
| Neutrophils | 63 | ||
| Leukocytes | Confer homologous features to reduce the unintended cell-binding interactions | Platelets | 58 |
| Macrophages | Provide markers of self | Cancer cells | 65 |
| Confer homologous features to reduce the unintended cell-binding interactions | Cancer cells | 68 | |
| Neutrophils | Offer ligands for targeting CTCs | Platelets | 63 |
| Dendritic cells (DCs) | Provide immunological co-stimulatory molecules and lymph node-targeting | Cancer cells | 56, 57 |
| Cancer cells/Cancer stem cells | Offer homotypic targeting to tumors and CTCs | RBCs | 54 |
| Macrophages | 65, 68 | ||
| Dendritic cells | 56, 57 | ||
| Platelets | 67 | ||
| Bacterium (Salmonella) | Serve as an immunological adjuvant to induce DC maturation | Cancer cells | 69 |
Metabolic Engineering
Metabolic engineering aims to control cellular properties through manipulating cells’ natural biosynthetic pathways. For cell membrane modification, metabolic substrates are first conjugated with functional moieties and then incubated with cells for uptake and metabolism.71–73 These non-natural conjugates hijack natural biosynthesis pathways, participate in the relevant cellular metabolic processes, and subsequently anchor onto the cell surfaces.74–76 Based on this principle, glycoengineering relies on oligosaccharide and glycoconjugate productions, including fucose salvage, sialic acid, and N-acetylgalactosamine (GalNAc) salvage pathways, to modify cell membranes (Table 3).77–79 Monosaccharides substrates such as N-acetylmannosamine (ManNAc), N-acetylneuraminic acid (Neu5Ac), GalNAc, and fucose are commonly used to form conjugates with functional moieties for metabolism.80–83 Meanwhile, lipid engineering exploits natural lipid synthesis such as the cytidine 5′-diphosphocholine (CDP-choline) pathway for membrane modification, where moieties are commonly conjugated with choline analogs for metabolism.84–86 Through metabolic engineering, various functional moieties, especially bioorthogonal linkers, have been installed onto the membrane surface for desirable functionalities.87–89
Table 3.
Summary of metabolic engineering approaches used for functionalizing cell membrane-coated nanoparticles
| Metabolic Approaches | Biosynthesis Pathways | Metabolic Substrates | Chemical Structures |
|---|---|---|---|
|
| |||
| Glycoengineering | Sialic acid pathway | ManNAc |
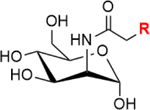
|
| Sialic acid pathway | Neu5Ac |

|
|
| GalNAc salvage pathway | GalNAc |
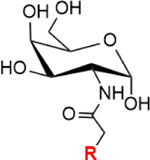
|
|
| Fucose salvage pathway | Fucose |
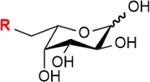
|
|
| Lipid-engineering | CDP-choline pathway | Choline |
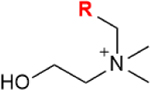
|
Note: GalNAc:N-acetylgalactosamine, ManNAc:N-acetylmannosamine, Neu5Ac:N-acetylneuraminic acid, CDP-choline: cytidine 5′-diphosphocholine. R= azide, alkynes, alkenes, ketone, thiol, isocyano, and diazirine groups.
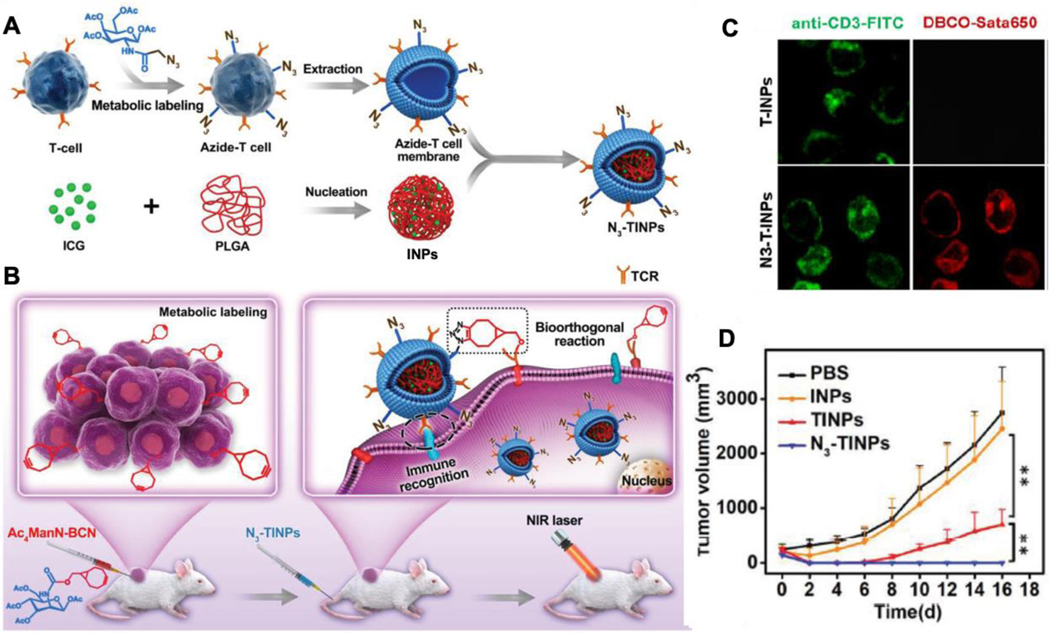
Recently, the glycoengineering was used to functionalize cell membrane-coated nanoparticles for tumor targeting in vivo. In this work, the tetraacetylated N-azidoacetylgalactosamine (Ac4GalNAz) was treated with T cells to introduce azide groups on cell membranes through the natural GalNAc salvage pathway (Figure 6).90 Following this modification, the N3-labeled T cell membranes were derived and coated onto PLGA cores pre-loaded with a photosensitizer (denoted ‘N3-TINPs’). Meanwhile, bicyclo [6.1.0] nonyne (BCN)-modified mannose substrate (Ac4ManN-BCN) was injected into the tumor region. Through the sialic acid pathway, the substrates were taken up by the tumor cells, and the BCN group was expressed onto the tumor surfaces. Following the injection of the N3-TINPs, the selective click reaction between BCN and N3 groups facilitated specific homing of the N3-TINPs to the tumor region. Such tumor-specific homing is further facilitated through the immune recognition of CD3 on the T cells membrane of N3-TINPs by the tumor cells. Equipped with such targeting mechanisms, N3-TINPs accumulated at a higher level in the tumor region after their intravenous administration when compared with nanoparticles coated with unmodified T cell membranes (denoted ‘TINP’). When tested for in vivo photothermal therapeutic efficacy, N3-TINPs also showed more significant tumor inhibition with negligible adverse effects compared with TINP.
Figure 6.
Metabolic glycoengineering approach for membrane modification. (A) Scheme of glycoengineered T cell membrane extraction and N3-labeled membrane-coated nanoparticles (N3-TINPs) construction. (B) Illustration of tumor-bearing mice with BCN group expression upon Ac4ManN-BCN injection. N3-TINPs could targeted anchor in tumor region through immune recognition of T cell membrane and bioorthogonal reaction between BCN and N3 groups, and effectively eliminate tumors based on ICG-mediated photothermal effects. (C) Identification of N3 group on the surface of N3-TINPs. Tumor cells were incubated with N3-TINPs or TINPs (control) for 1 h, and then stain with anti-CD3-FITC and DBCO-Sata650. (D) In vivo photothermal therapy efficacy based on tumor growth curves of different groups in Raji tumor-bearing mice (n = 5). Reproduced with permission from ref 90. Copyright 2019 John Wiley & Sons.
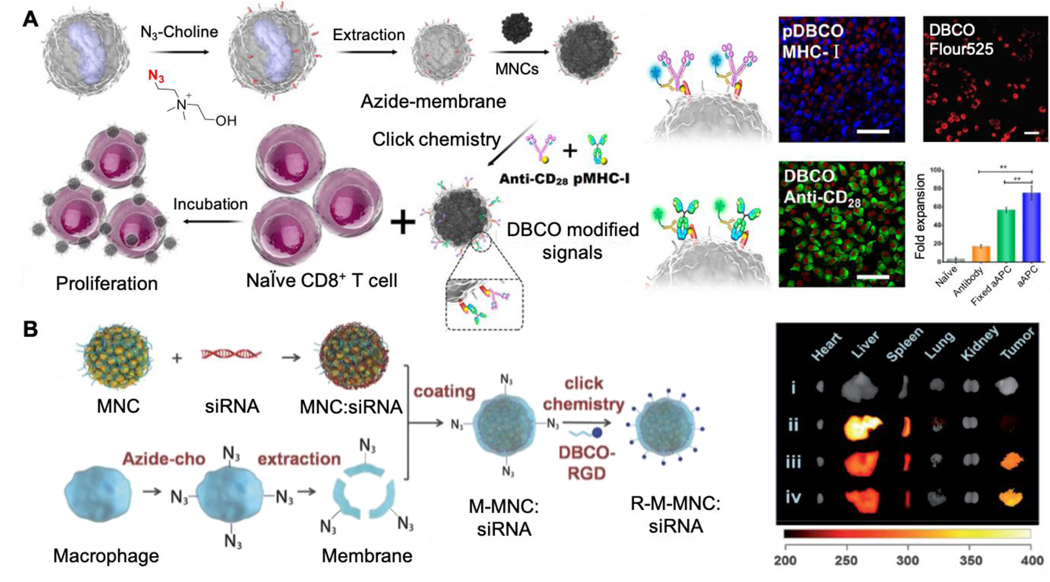
Phospholipid engineering was also utilized to introduce bioorthogonal linkers on the membrane-coated nanoparticles, which allowed for the further conjugation of immune stimulator ligands. In this study, an azide-choline substrate was applied to add N3 groups on the leukocyte membrane through the CDP-choline biosynthesis pathway (Figure 7A).91 Following the expression, N3-labeled membrane was then coated onto magnetic nanoclusters (MNCs). Through the click reaction, N3-tagged MNCs were further conjugated with major histocompatibility complex class-I (pMHC-I) and co-stimulatory ligand anti-CD28. With the presence of both ligands, the nanoclusters acted as artificial antigen-presenting cells (aAPCs) and induced a significant increase of CD8+ T cell proliferation when compared to free anti-CD28. The T cells activated by nanoclusters were intravenously injected into the EG7 tumor-bearing mice. These mice showed slower tumor growth and a better survival rate when compared with the control group injected with T cells activated by free antibodies. The versatility of phospholipid engineering for functionalizing membrane-coated nanoparticles was demonstrated in another study, where the same phospholipid pathway was used to express N3 groups on macrophage membranes (Figure 7B).92 Following the modification, the membrane was coated onto MNC-siRNA nanocomplex. Through click chemistry, the nanocomplex was further conjugated with an RGD peptide that targets integrin αvβ3 over-expressed on the tumor. When intravenously injected, the targeted nanocomplex showed a 2.7-fold increase of tumor accumulation as well as a significant inhibition of tumor growth compared to nanoparticles coated with unmodified membranes.
Figure 7.
Metabolic lipid-engineering approach for membrane modification. (A) Illustration of N3-tagged leukocyte membrane via lipid-engineering to develop biomimetic nanoplatform (MNCs) for enhanced CD8+ T cell proliferation. The T-cell stimuli conjugations were identified by immunostaining with the fluorescence-labeled secondary antibody of antiCD28 and pMHC-I. Then the N3 groups on cell membrane were confirmed by DBCO-Flour525. After incubation with CD8+ T cells for 7 days, the aAPCs presented the highest proliferation efficiency. Reproduced with permission from ref. 91. Copyright 2017 American Chemical Society. (B) Scheme of N3-labeled macrophage membrane-coated nanoplatform for targeted siRNA delivery. Following the modification via metabolic lipid-engineering, the N3-labeled membrane was coated onto MNC-siRNA nanocomplex and conjugated with DBCO-RGD for tumor targeting. The imaging of tumor and various organs were performed at 24 h after intravenous injection of different MNC-based nanoformulations. (i) PBS, (ii) MNC:siRNA, (iii) M-MNC:siRNA, (iv) R-M-MNC:siRNA. Reproduced with permission from ref. 92. Copyright 2018 John Wiley & Sons.
Overall, recent development has demonstrated metabolic engineering as an agile and versatile approach to harnessing natural biosynthesis pathways for ligand expression onto cell membrane-coated nanoparticles. Functionalization applications with metabolic engineering are expected to grow as novel ligands compatible with biosynthesis are continually discovered, and methods for enforced ligand expression are continually developed.93 Meanwhile, different ligands can be simultaneously installed by using substrates of non-overlapping pathways, potentially increasing the spectrum and capacity of drug targeting or detoxification.94 In addition to mammalian cells, metabolic engineering can also be applied to modify bacterial membranes. For example, modifying non-pathogenic bacteria to express surface glycans of pathogenic strains becomes attractive to modulate membrane self-adjuvanticity.95 Towards future development, the progress made in metabolic engineering will bring in new tools and strategies to functionalize cell membrane-coated nanoparticles for broader applications.
Genetic Modification
Genetic modification is a powerful method to acquire new functions by altering the protein expression on the cell surfaces. Through selective gene editing, genetically modified membranes (namely ‘GM membranes’) can be made and coated onto nanoparticles for functionalization. Genetic modification can use robust cell lines to express unique antigens native to sensitive cells, which may lower the cost for large-scale manufacturing.96 For gene modification, DNA or mRNA materials need to access the cytosol. Such intracellular delivery can be accomplished by using a variety of methods (Table 4). For example, viral vehicles, including those based on adenovirus, lentivirus, and adeno-associated virus, offer superior efficiency of the transfection.97–101 For better safety, synthetic materials such as cationic lipids or polymers have also been developed for intracellular delivery.102–105 Meanwhile, physical methods, including electroporation, gene gun, laser-irradiation, and microinjection, are popular.106–110 Recently, these methods were combined with CRISPR/Cas9 technology, resulting in faster, cheaper, more accurate, and more efficient gene editing capability.111, 112
Table 4.
Summary of common transfection methods for gene delivery
| Category (selective examples) | Pros | Cons |
|---|---|---|
| Viral based (lentivirus, adenovirus, adeno-associated virus) | • High transfection efficiency. • Easy to produce and use. |
• Risks of mutagenesis and immunogenicity • Limited space for packing the genomic materials. |
| Chemical (cationic lipid, cationic polymers) | • Easy to use and can be produced in large scales. • efficiency in vitro. • High capacity of packing genomic materials. |
• In vitro transfection efficiency varied by cell types. The in vivo efficiency is low. • Potential cytotoxicity depends on lipid or polymer used. • Lack of target specificity. |
| Physical (electroporation, laser-irradiation, gene gun, microinjection) | • High in vitro transfection efficiency, regardless of cell type. • Can achieve single-cell transfection |
• Need special instrument and training. • Most physical transfection methods cannot be applied in vivo. |
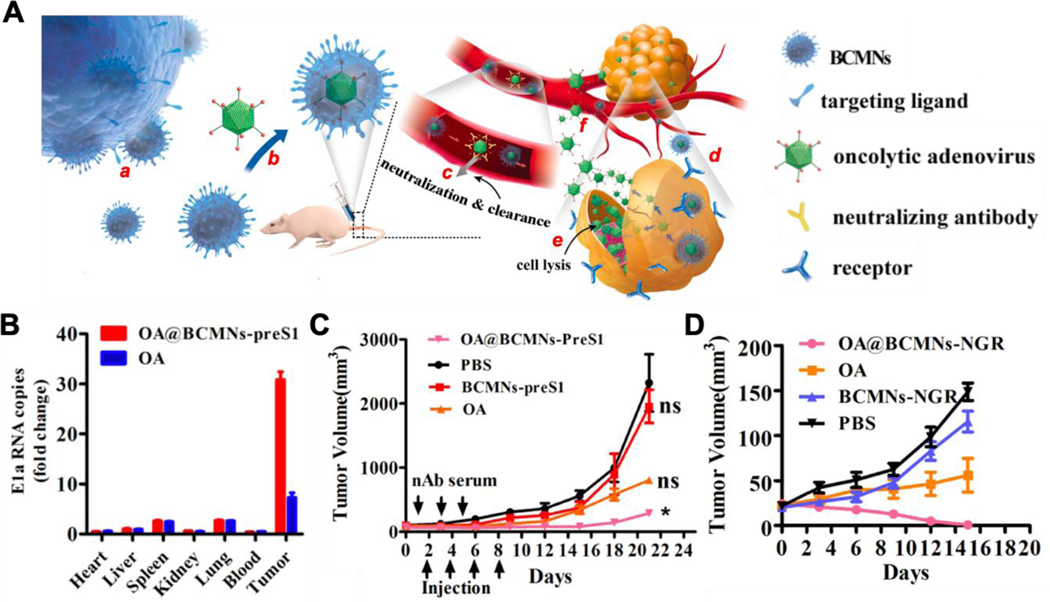
The genetic modification method can express highly specific affinity ligands to provide cell membrane-coated nanoparticles with targeting capability. For example, hepatitis B virus (HBV) preS1 ligand was expressed onto HepG2 cells, after which the membrane was derived and coated onto oncolytic adenoviruses (OAs, Figure 8A).113 Such membrane coating decreased the immunogenicity of OA without compromising their infectivity for tumor inhibition. It also allowed the GM-coated viruses to target tumors with active overexpression of preS1 receptor (NTCP). In the study, intravenous administration of GM-coated OAs resulted in a higher tumor accumulation and anti-cancer efficacy compared to uncoated viruses (Figure 8B and C). To further demonstrate the adaptability of using GM for viral targeting, a small peptide Asn-Gly-Arg (NGR), was expressed onto RBC membranes through in- body CRISPR technology. The peptide targets a specific isoform of aminopeptidase N (APN), a membrane metalloproteinase on a variety of cancer cells. Following the genetic modification, the GM-membranes were coated onto OAs. This time, GM membrane-coated OAs showed significant increases in tumor accumulation and tumor growth inhibition in APN receptor-expressing tumors, including PC13, U87, and HepG2 tumors, in comparison with uncoated OAs (Figure 8D).
Figure 8.
Schematic of bioengineered cell membrane nanovesicle coated oncolytic adenoviruses (OA@BCMNs) for OA delivery and in vivo antitumor efficacy of OA@BCMNs. (A) Design features and proposed mechanism of OA@BCMNs. The BCMNs encapsulated OA, protecting OAs from neutralizing antibodies and delivering them to tumors through receptor mediated endocytosis. Once entered tumor cells, OAs infect and amplify the tumor cells, causing the tumor cell lysis. (B) Viral genome copies in excised tumors and organs, after intravenous injection of OA and OA@BCMNs-preS1 into HepG2-NTCP bearing nude mouse model, were quantified using real time qPCR. (C) Tumor growth curve of HepG2-NTCP bearing nude mouse model after the indicated treatment. (D) Tumor growth curve of HepG2-APN bearing mouse model after the indicated treatment. Reproduced with permission from ref 113. Copyright 2019 American Chemical Society.
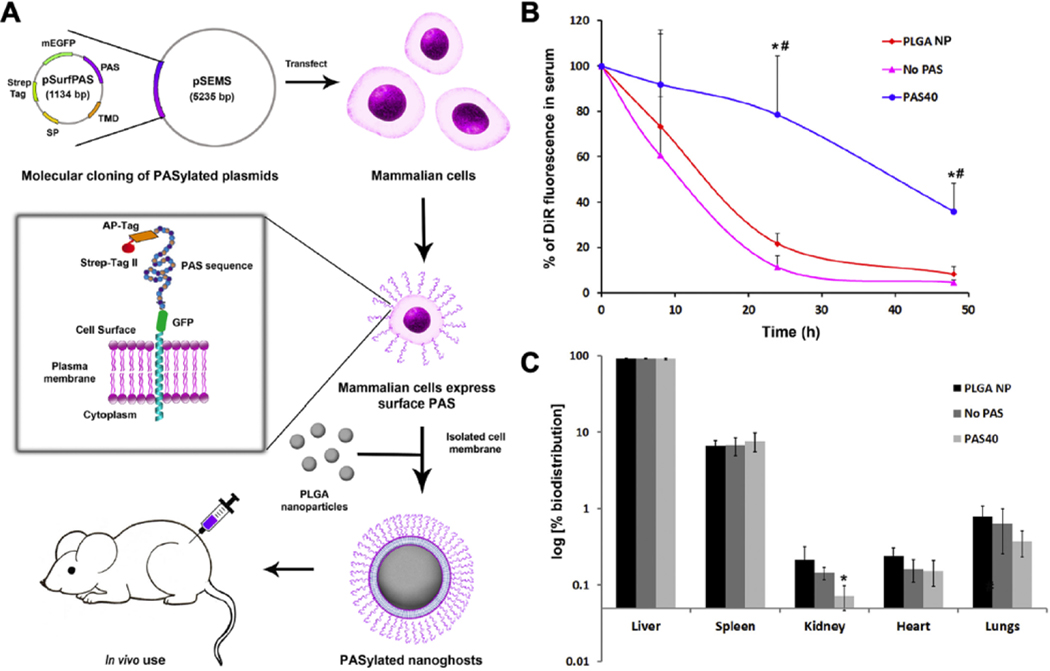
The genetic modification method can also express surface moieties aimed at prolonging nanoparticle in vivo circulation times. Traditionally, surface functionalization with PEG is a popular approach. However, concerns about PEG functionalization, such as the high cost of manufacturing and the secondary immunogenicity, are growing. As a potential alternative, peptide-based polymers such as natural L-amino acid chains containing Pro, Ala, and Ser (PAS) have shown stealth properties comparable to that of PEG.114 More compelling, these polymers can be produced by genetically encoded biosynthesis. Recently, PAS chains were expressed onto HEK293 cells with a plasmid encoding a fusion protein, PAS repeats, and a C-terminal transmembrane anchoring domain (Figure 9A).115 The GM membranes expressing PAS were derived and coated onto PLGA cores. The resulting nanoparticles (PASylated nanoghosts) showed a significant reduction in BSA adsorption and macrophage uptake when compared to those coated with wildtype HEK293 membranes. When tested in vivo, nanoparticles coated with GM membranes showed a three-fold increase in circulation half-life when compared to those coated with wild type membranes (Figure 9B). The percentage distribution of the sample groups in different organs at 48 h post-injection is shown in Figure 9C. All sample groups showed similar biodistribution profiles, with the majority of accumulation being observed in the liver.
Figure 9.
(A) Illustration of the steps involved in the synthesis of PASylated nanoghosts. A plasmid that expresses the proline-alanine-serine (PAS) peptides on the surface membrane is transfected into mammalian cells. PASylated cell membranes are then harvested and coated on PLGA nanoparticles (PLGA NPs) to form PASylated nanoghosts. (B) In vivo serum concentration of DiR dye from nanoparticle groups over 48 h. Sample groups are PLGA NPs, non-transfected nanoghosts (No PAS), and PASylated nanoghosts (PAS40). ⁎ and # denote statistical significance of PAS40 (P ≤ 0.005) in comparison to No PAS and PLGA NP. (C) Biodistribution of dye-loaded sample groups in the liver, spleen kidney, heart and lungs of mice at 48 h post treatment. * denotes statistical significance (P ≤ 0.001) in comparison to the PLGA NP control group. Reproduced with permission from ref 115. Copyright 2019 Elsevier.
Overall, cell membrane coating provides a technology platform that harnesses the merits in nanotechnology and genetic engineering. This advance is especially promising to make nanoparticles with ‘universal’ membranes, where membranes of allogeneic cells can be used for coating after selectively knocking out antigen-presenting proteins such as MHC I and II.116, 117 In addition, recent development in expressing viral antigens onto mammalian membranes and bacterial engineering for selective antigen expression on their outer membranes can be potentially used to make GM membrane-coated nanoparticles for better modulating anti-viral or anti-bacterial immunity.118–120 Overall, the combination of genetic engineering with cell membrane coating technology is expected to generate exciting innovations for future therapeutics.
Conclusions
As cell membrane-coated nanoparticles are increasingly developed for various biomedical applications, approaches to further functionalizing these biomimetic nanoparticles are emerging. In this article, we highlighted four unique methods, including lipid insertion, membrane hybridization, metabolic engineering, and genetic modification. Despite their different underlying principles, these methods all feature non-disruptive functionalization procedures compatible with existing membrane derivation and coating processes. We summarized the applications of each method with a specific emphasis on how the approach confers cell membrane-coated nanoparticles with more functions beyond those from the native cell membranes. Overall, these methods improve on the multi-functional and multi-tasking ability of cell membrane-coated nanoparticles, making them more adaptive to the complex biological systems.
As the nanoparticle functionalization strategies emerge, cell membrane coating technology has also made significant progress. For example, cell membrane-coated nanoparticles are increasingly combined with other materials such as hydrogels for local applications.121–123 Methods aimed at modifying the cores rather than the membranes have also been applied to enhance overall nanoparticle functionality.124 Meanwhile, cell membranes are increasingly used to coat self-propelled and autonomous nanomotors, opening a variety of in vivo applications.125, 126 Cell membranes have also been coated onto biomaterials with higher dimensions such as nanofibers and planary devices.127, 128 We believe that the strategies for functionalizing cell membrane-coated nanoparticles can also be applied to these new directions. For example, RBC-platelet hybrid membranes were recently coated onto nanomotors for concurrent removal and neutralization of pathogenic bacteria and toxins.129 Mechanistic studies have revealed that nanoparticle surface functionalization plays dynamic roles in altering the patterns and pathways of nanoparticle interactions with cells. Therefore, selecting an appropriate functionalization method may help to target specific intracellular pathways for better therapeutic interventions with reduced side effects.130, 131 Overall, we expect these emerging surface functionalization approaches discussed above to play significant roles as researchers continue to refine and expand cell membrane coating technology towards broader applications.
Acknowledgment
This work is supported by the National Institutes of Health under Award Number R01CA200574 and the National Science Foundation Grant DMR-1904702.
References
- (1).Ragelle H, Danhier F, Preat V, Langer R, and Anderson DG (2017) Nanoparticle-based drug delivery systems: a commercial and regulatory outlook as the field matures, Expert Opin. Drug Deliv 14, 851–864. [DOI] [PubMed] [Google Scholar]
- (2).Fang RH, Kroll AV, Gao W, and Zhang L. (2018) Cell Membrane Coating Nanotechnology, Adv. Mater 30, e1706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Hu CMJ, Fang RH, Luk BT, Chen KNH, Carpenter C, Gao W, Zhang K, and Zhang L. (2013) ‘Marker-of-self’ functionalization of nanoscale particles through a top-down cellular membrane coating approach, Nanoscale 5, 2664–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Hu CMJ, Zhang L, Aryal S, Cheung C, Fang RH, and Zhang L. (2011) Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform, Proc. Natl. Acad. Sci. U. S. A 108, 10980–10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Piao JG, Wang LM, Gao F, You YZ, Xiong YJ, and Yang LH (2014) Erythrocyte Membrane Is an Alternative Coating to Polyethylene Glycol for Prolonging the Circulation Lifetime of Gold Nanocages for Photothermal Therapy, ACS Nano 8, 10414–10425. [DOI] [PubMed] [Google Scholar]
- (6).Fang RH, Hu CMJ, Luk BT, Gao W, Copp JA, Tai YY, O’Connor DE, and Zhang L. (2014) Cancer Cell Membrane-Coated Nanoparticles for Anticancer Vaccination and Drug Delivery, Nano Lett. 14, 2181–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Hu CMJ, Fang RH, Wang KC, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, Carpenter C, Ramesh M, Qu V, Patel SH, Zhu J, Shi W, Hofman FM, Chen TC, Gao W, Zhang K, Chien S, and Zhang L. (2015) Nanoparticle biointerfacing by platelet membrane cloaking, Nature 526, 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Hu CMJ, Fang RH, Copp J, Luk BT, and Zhang L. (2013) A biomimetic nanosponge that absorbs pore-forming toxins, Nat. Nanotechnol 8, 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Gao W, Fang RH, Thamphiwatana S, Luk BT, Li JM, Angsantikul P, Zhang QZ, Hu CMJ, and Zhang L. (2015) Modulating Antibacterial Immunity via Bacterial Membrane-Coated Nanoparticles, Nano Lett. 15, 1403–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Kroll AV, Fang RH, Jiang Y, Zhou JR, Wei XL, Yu CL, Gao J, Luk BT, Dehaini D, Gao W, and Zhang L. (2017) Nanoparticulate Delivery of Cancer Cell Membrane Elicits Multiantigenic Antitumor Immunity, Adv. Mater 29, 1703969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Hu CMJ, Fang RH, Luk BT, and Zhang L. (2013) Nanoparticle-detained toxins for safe and effective vaccination, Nat. Nanotechnol 8, 933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Wei XL, Gao J, Wang F, Ying M, Angsantikul P, Kroll AV, Zhou JR, Gao W, Lu WY, Fang RH, and Zhang L. (2017) In Situ Capture of Bacterial Toxins for Antivirulence Vaccination, Adv. Mater 29, 1701644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Tietjen GT, Bracaglia LG, Saltzman WM, and Pober JS (2018) Focus on Fundamentals: Achieving Effective Nanoparticle Targeting, Trends Mol. Med 24, 598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Dehaini D, Fang RH, and Zhang L. (2016) Biomimetic strategies for targeted nanoparticle delivery, Bioeng. Transl. Med 1, 30–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Fang RNH, and Zhang L. (2016) Nanoparticle-Based Modulation of the Immune System, In Annu. Rev. Chem. Biomol. Eng (Prausnitz JM, Ed.), pp 305–326. [DOI] [PubMed] [Google Scholar]
- (16).Moyano DF, Liu YC, Peer D, and Rotello VM (2016) Modulation of Immune Response Using Engineered Nanoparticle Surfaces, Small 12, 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Deirram N, Zhang CH, Kermaniyan SS, Johnston APR, and Such GK (2019) pH-Responsive Polymer Nanoparticles for Drug Delivery, Macromol. Rapid Commun 40, 1800917. [DOI] [PubMed] [Google Scholar]
- (18).Karimi M, Ghasemi A, Zangabad PS, Rahighi R, Basri SMM, Mirshekari H, Amiri M, Pishabad ZS, Aslani A, Bozorgomid M, Ghosh D, Beyzavi A, Vaseghi A, Aref AR, Haghani L, Bahrami S, and Hamblin MR (2016) Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems, Chem. Soc. Rev 45, 1457–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Zhao WA, Teo GSL, Kumar N, and Karp JM (2010) Chemistry and material science at the cell surface, Mater. Today 13, 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Li PY, Fan ZY, and Cheng H. (2018) Cell Membrane Bioconjugation and Membrane-Derived Nanomaterials for Immunotherapy, Bioconj. Chem 29, 624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Spicer CD, Pashuck ET, and Stevens MM (2018) Achieving Controlled Biomolecule-Biomaterial Conjugation, Chem. Rev 118, 7702–7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Hu QY, Berti F, and Adamo R. (2016) Towards the next generation of biomedicines by site-selective conjugation, Chem. Soc. Rev 45, 1691–1719. [DOI] [PubMed] [Google Scholar]
- (23).Zhou H, Fan ZY, Lemons PK, and Cheng H. (2016) A Facile Approach to Functionalize Cell Membrane-Coated Nanoparticles, Theranostics 6, 1012–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Marques-Gallego P, and de Kroon A. (2014) Ligation Strategies for Targeting Liposomal Nanocarriers, Biomed Res. Int 2014, 129458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Fang RNH, Hu CMJ, Chen KNH, Luk BT, Carpenter CW, Gao W, Li SL, Zhang DE, Lu WY, and Zhang L. (2013) Lipid-insertion enables targeting functionalization of erythrocyte membrane-cloaked nanoparticles, Nanoscale 5, 8884–8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Kumar P, Huo PP, and Liu B. (2019) Formulation Strategies for Folate-Targeted Liposomes and Their Biomedical Applications, Pharmaceutics 11, 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Guliz AK, and Sanlier SH (2020) Erythrocyte membrane vesicles coated biomimetic and targeted doxorubicin nanocarrier: Development, characterization and in vitro studies, J. Mol. Struct 1205, 127664. [Google Scholar]
- (28).Rao L, Meng QF, Bu LL, Cai B, Huang QQ, Sun ZJ, Zhang WF, Li A, Guo SS, Liu W, Wang TH, and Zhao XZ (2017) Erythrocyte Membrane-Coated Upconversion Nanoparticles with Minimal Protein Adsorption for Enhanced Tumor Imaging, ACS Appl. Mater. Interfaces 9, 2159–2168. [DOI] [PubMed] [Google Scholar]
- (29).Ak G, Yilmaz H, Gunes A, and Sanlier SH (2018) In vitro and in vivo evaluation of folate receptor-targeted a novel magnetic drug delivery system for ovarian cancer therapy, Artif. Cells Nanomed. Biotechnol 46, S926–S937. [DOI] [PubMed] [Google Scholar]
- (30).Deng JJ, Xu SD, Hu WK, Xun XJ, Zheng LY, and Su M. (2018) Tumor targeted, stealthy and degradable bismuth nanoparticles for enhanced X-ray radiation therapy of breast cancer, Biomaterials 154, 24–33. [DOI] [PubMed] [Google Scholar]
- (31).Lv W, Xu JP, Wang XQ, Li XR, Xu QW, and Xin HL (2018) Bioengineered Boronic Ester Modified Dextran Polymer Nanoparticles as Reactive Oxygen Species Responsive Nanocarrier for Ischemic Stroke Treatment, ACS Nano 12, 5417–5426. [DOI] [PubMed] [Google Scholar]
- (32).Fu SY, Liang M, Wang YL, Cui L, Gao CH, Chu XY, Liu QQ, Feng Y, Gong W, Yang MY, Li ZP, Yang CR, Xie XY, Yang Y, and Gao CS (2019) Dual-Modified Novel Biomimetic Nanocarriers Improve Targeting and Therapeutic Efficacy in Glioma, ACS Appl. Mater. Interfaces 11, 1841–1854. [DOI] [PubMed] [Google Scholar]
- (33).Zou Y, Liu YJ, Yang ZP, Zhang DY, Lu YQ, Zheng M, Xue X, Geng J, Chung R, and Shi BY (2018) Effective and Targeted Human Orthotopic Glioblastoma Xenograft Therapy via a Multifunctional Biomimetic Nanomedicine, Adv. Mater 30, 1803717. [DOI] [PubMed] [Google Scholar]
- (34).Guo YY, Wang D, Song QL, Wu TT, Zhuang XT, Bao YL, Kong M, Qj Y, Tan SW, and Zhang ZP (2015) Erythrocyte Membrane-Enveloped Polymeric Nanoparticles as Nanovaccine for Induction of Antitumor Immunity against Melanoma, ACS Nano 9, 6918–6933. [DOI] [PubMed] [Google Scholar]
- (35).Liu W, Ruan ML, Wang YM, Song RG, Ji X, Xu JK, Dai J, and Xue W. (2018) Light-Triggered Biomimetic Nanoerythrocyte for Tumor-Targeted Lung Metastatic Combination Therapy of Malignant Melanoma, Small 14, 1801754. [DOI] [PubMed] [Google Scholar]
- (36).Liu W, Ruan ML, Liu LM, Ji X, Ma YD, Yuan PF, Tang GH, Lin HS, Dai J, and Xue W. (2020) Self-activated in vivo therapeutic cascade of erythrocyte membrane-cloaked iron-mineralized enzymes, Theranostics 10, 2201–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Wang YM, Ji X, Ruan ML, Liu W, Song RG, Dai J, and Xue W. (2018) Worm-Like Biomimetic Nanoerythrocyte Carrying siRNA for Melanoma Gene Therapy, Small 14, 1803002. [DOI] [PubMed] [Google Scholar]
- (38).Yang R, Xu J, Xu LG, Sun XQ, Chen Q, Zhao YH, Peng R, and Liu Z. (2018) Cancer Cell Membrane-Coated Adjuvant Nanoparticles with Mannose Modification for Effective Anticancer Vaccination, ACS Nano 12, 5121–5129. [DOI] [PubMed] [Google Scholar]
- (39).Dong X, Mu LL, Liu XL, Zhu H, Yang SC, Lai X, Liu HJ, Feng HY, Lu Q, Zhou BBS, Chen HZ, Chen GQ, Lovell JF, Hong DL, and Fang C. (2020) Biomimetic, Hypoxia-Responsive Nanoparticles Overcome Residual Chemoresistant Leukemic Cells with Co-Targeting of Therapy-Induced Bone Marrow Niches, Adv. Funct. Mater 30, 2000309. [Google Scholar]
- (40).Li M, Fang H, Liu Q, Gai Y, Yuan L, Wang S, Li H, Hou Y, Gao M, and Lan X. (2020) Red blood cell membrane-coated upconversion nanoparticles for pretargeted multimodality imaging of triple-negative breast cancer, Biomater. Sci 8, 1802–1814. [DOI] [PubMed] [Google Scholar]
- (41).Chen H, Sha HZ, Zhang LR, Qian HQ, Chen FJ, Ding NQ, Ji LL, Zhu AQ, Xu QP, Meng FY, Yu LX, Zhou Y, and Liu BR (2018) Lipid insertion enables targeted functionalization of paclitaxel-loaded erythrocyte membrane nanosystem by tumor-penetrating bispecific recombinant protein, Int. J. Nanomedicine 13, 5347–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Zhang Z, Qian HQ, Huang J, Sha HZ, Zhang H, Yu LX, Liu BR, Hua D, and Qian XP (2018) Anti-EGFR-iRGD recombinant protein modified biomimetic nanoparticles loaded with gambogic acid to enhance targeting and antitumor ability in colorectal cancer treatment, Int. J. Nanomedicine 13, 4961–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Zhu DM, Xie W, Xiao YS, Suo M, Zan MH, Liao QQ, Hu XJ, Chen LB, Chen B, Wu WT, Ji LW, Huang HM, Guo SS, Zhao XZ, Liu QY, and Liu W. (2018) Erythrocyte membrane-coated gold nanocages for targeted photothermal and chemical cancer therapy, Nanotechnology 29, 084002. [DOI] [PubMed] [Google Scholar]
- (44).Mac JT, Nunez V, Burns JM, Guerrero YA, Vullev VI, and Anvari B. (2016) Erythrocyte-derived nano-probes functionalized with antibodies for targeted near infrared fluorescence imaging of cancer cells, Biomed. Opt. Express 7, 1311–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Greene MK, Richards DA, Nogueira JCF, Campbell K, Smyth P, Fernandez M, Scott CJ, and Chudasama V. (2018) Forming next-generation antibody-nanoparticle conjugates through the oriented installation of non-engineered antibody fragments, Chem. Sci 9, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Richards DA, Maruani A, and Chudasama V. (2017) Antibody fragments as nanoparticle targeting ligands: a step in the right direction, Chem. Sci 8, 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Fan ZY, Zhou H, Li PY, Speer JE, and Cheng H. (2014) Structural elucidation of cell membrane-derived nanoparticles using molecular probes, J. Mater. Chem. B 2, 8231–8238. [DOI] [PubMed] [Google Scholar]
- (48).Hsu SM, Raine L, and Fanger H. (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques - a comparison between ABC and unlabeled antibody (PAP) procedures, J. Histochem. Cytochem 29, 577–580. [DOI] [PubMed] [Google Scholar]
- (49).Chai ZL, Ran DN, Lu LW, Zhan CY, Ruan HT, Hu XF, Xie C, Jiang K, Li JY, Zhou JF, Wang J, Zhang YY, Fang RH, Zhang L, and Lu WY (2019) Ligand-Modified Cell Membrane Enables the Targeted Delivery of Drug Nanocrystals to Glioma, ACS Nano 13, 5591–5601. [DOI] [PubMed] [Google Scholar]
- (50).Chai ZL, Hu XF, Wei XL, Zhan CY, Lu LW, Jiang K, Su BX, Ruan HT, Ran DN, Fang RH, Zhang L, and Lu WY (2017) A facile approach to functionalizing cell membrane-coated nanoparticles with neurotoxin-derived peptide for brain-targeted drug delivery, J. Control. Release 264, 102–111. [DOI] [PubMed] [Google Scholar]
- (51).Su JH, Sun HP, Meng QS, Yin Q, Zhang PC, Zhang ZW, Yu HJ, and Li YP (2016) Bioinspired Nanoparticles with NIR-Controlled Drug Release for Synergetic Chemophotothermal Therapy of Metastatic Breast Cancer, Adv. Funct. Mater 26, 7495–7506. [Google Scholar]
- (52).Liu GN, Zhao X, Zhang YL, Xu JC, Xu JQ, Li Y, Min H, Shi J, Zhao Y, Wei JY, Wang J, and Nie GJ (2019) Engineering Biomimetic Platesomes for pH-Responsive Drug Delivery and Enhanced Antitumor Activity, Adv. Mater 31, 1900795. [DOI] [PubMed] [Google Scholar]
- (53).Dehaini D, Wei XL, Fang RH, Masson S, Angsantikul P, Luk BT, Zhang Y, Ying M, Jiang Y, Kroll AV, Gao W, and Zhang L. (2017) Erythrocyte-Platelet Hybrid Membrane Coating for Enhanced Nanoparticle Functionalization, Adv. Mater 29, 1606209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Wang DD, Dong HF, Li M, Cao Y, Yang F, Zhang K, Dai WH, Wang CT, and Zhang XJ (2018) Erythrocyte-Cancer Hybrid Membrane Camouflaged Hollow Copper Sulfide Nanoparticles for Prolonged Circulation Life and Homotypic-Targeting Photothermal/Chemotherapy of Melanoma, ACS Nano 12, 5241–5252. [DOI] [PubMed] [Google Scholar]
- (55).Liu Y, Wang XJ, Ouyang BS, Liu XP, Du Y, Cai XZ, Guo HS, Pang ZQ, Yang WL, and Shen S. (2018) Erythrocyte-platelet hybrid membranes coating polypyrrol nanoparticles for enhanced delivery and photothermal therapy, J. Mater. Chem. B 6, 7033–7041. [DOI] [PubMed] [Google Scholar]
- (56).Liu WL, Zou MZ, Liu T, Zeng JY, Li X, Yu WY, Li CX, Ye JJ, Song W, Feng J, and Zhang XZ (2019) Cytomembrane nanovaccines show therapeutic effects by mimicking tumor cells and antigen presenting cells, Nat. Commun 10, 3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Liu WL, Zou MZ, Liu T, Zeng JY, Li X, Yu WY, Li CX, Ye JJ, Song W, Feng J, and Zhang XZ (2019) Expandable Immunotherapeutic Nanoplatforms Engineered from Cytomembranes of Hybrid Cells Derived from Cancer and Dendritic Cells, Adv. Mater 31, 1900499. [DOI] [PubMed] [Google Scholar]
- (58).Rao L, Meng QF, Huang QQ, Wang ZX, Yu GT, Li A, Ma WJ, Zhang NG, Guo SS, Zhao XZ, Liu K, Yuan YF, and Liu W. (2018) Platelet-Leukocyte Hybrid Membrane-Coated Immunomagnetic Beads for Highly Efficient and Highly Specific Isolation of Circulating Tumor Cells, Adv. Funct. Mater 28, 1803531. [Google Scholar]
- (59).Hu QY, Sun WJ, Qian CG, Wang C, Bomba HN, and Gu Z. (2015) Anticancer Platelet-Mimicking Nanovehicles, Adv. Mater 27, 7043–7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Gay LJ, and Felding-Habermann B. (2011) Contribution of platelets to tumour metastasis, Nat. Rev. Cancer 11, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Li NL (2016) Platelets in cancer metastasis: To help the “villain” to do evil, Int. J. Cancer 138, 2078–2087. [DOI] [PubMed] [Google Scholar]
- (62).Zhao HJ, Zhao BB, Li L, Ding KL, Xiao HF, Zheng CX, Sun LL, Zhang ZZ, and Wang L. (2020) Biomimetic Decoy Inhibits Tumor Growth and Lung Metastasis by Reversing the Drawbacks of Sonodynamic Therapy, Adv. Healthc. Mater 9, 1901335. [DOI] [PubMed] [Google Scholar]
- (63).Ye H, Wang K, Lu Q, Zhao J, Wang M, Kan Q, Zhang H, Wang Y, He Z, and Sun J. (2020) Nanosponges of circulating tumor-derived exosomes for breast cancer metastasis inhibition, Biomaterials 242, 119932. [DOI] [PubMed] [Google Scholar]
- (64).Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C, Scherrer R, Singer J, Beisel C, Kurzeder C, Heinzelmann-Schwarz V, Rochlitz C, Weber WP, Beerenwinkel N, and Aceto N. (2019) Neutrophils escort circulating tumour cells to enable cell cycle progression, Nature 566, 553–557. [DOI] [PubMed] [Google Scholar]
- (65).He HL, Guo CQ, Wang J, Korzun WJ, Wang XY, Ghosh S, and Yang H. (2018) Leutusome: A Biomimetic Nanoplatform Integrating Plasma Membrane Components of Leukocytes and Tumor Cells for Remarkably Enhanced Solid Tumor Homing, Nano Lett. 18, 6164–6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Jiang Q, Liu Y, Guo RR, Yao XX, Sung S, Pang ZQ, and Yang WL (2019) Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors, Biomaterials 192, 292–308. [DOI] [PubMed] [Google Scholar]
- (67).Bu LL, Rao L, Yu GT, Chen L, Deng WW, Liu JF, Wu H, Meng QF, Guo SS, Zhao XZ, Zhang WF, Chen GJ, Gu Z, Liu W, and Sun ZJ (2019) Cancer Stem Cell-Platelet Hybrid Membrane-Coated Magnetic Nanoparticles for Enhanced Photothermal Therapy of Head and Neck Squamous Cell Carcinoma, Adv. Funct. Mater 29, 1807733. [Google Scholar]
- (68).Ding CP, Zhang CL, Cheng SS, and Xian YZ (2020) Multivalent Aptamer Functionalized Ag2S Nanodots/Hybrid Cell Membrane-Coated Magnetic Nanobioprobe for the Ultrasensitive Isolation and Detection of Circulating Tumor Cells, Adv. Funct. Mater, DOI: 10.1002/adfm.201909781. [DOI] [Google Scholar]
- (69).Chen Q, Huang GJ, Wu WT, Wang JW, Hu JW, Mao JM, Chu PK, Bai HZ, and Tang GP (2020) A Hybrid Eukaryotic-Prokaryotic Nanoplatform with Photothermal Modality for Enhanced Antitumor Vaccination, Adv. Mater, 10.1002/adma.201908185. [DOI] [PubMed] [Google Scholar]
- (70).Vijayan V, Uthaman S, and Park IK (2018) Cell Membrane-Camouflaged Nanoparticles: A Promising Biomimetic Strategy for Cancer Theragnostics, Polymers 10, 983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Lee JW, Na D, Park JM, Lee J, Choi S, and Lee SY (2012) Systems metabolic engineering of microorganisms for natural and non-natural chemicals, Nat. Chem. Biol 8, 536–546. [DOI] [PubMed] [Google Scholar]
- (72).Agatemor C, Buettner MJ, Ariss R, Muthiah K, Saeui CT, and Yarema KJ (2019) Exploiting metabolic glycoengineering to advance healthcare, Nat. Rev. Chem 3, 605–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Garcia-Granados R, Lerma-Escalera JA, and Morones-Ramirez JR (2019) Metabolic Engineering and Synthetic Biology: Synergies, Future, and Challenges, Front. Bioeng. Biotechnol 7, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Woolston BM, Edgar S, and Stephanopoulos G. (2013) Metabolic Engineering: Past and Future, Annu. Rev. Chem. Biomol. Eng 4, 259–288. [DOI] [PubMed] [Google Scholar]
- (75).Agard NJ, and Bertozzi CR (2009) Chemical Approaches To Perturb, Profile, and Perceive Glycans, Acc. Chem. Res 42, 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Du J, Meledeo MA, Wang ZY, Khanna HS, Paruchuri VDP, and Yarema KJ (2009) Metabolic glycoengineering: Sialic acid and beyond, Glycobiology 19, 1382–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Pouilly S, Bourgeaux V, Piller F, and Piller V. (2012) Evaluation of Analogues of GalNAc as Substrates for Enzymes of the Mammalian GalNAc Salvage Pathway, ACS Chem. Biol 7, 753–760. [DOI] [PubMed] [Google Scholar]
- (78).Cheng B, Xie R, Dong L, and Chen X. (2016) Metabolic Remodeling of Cell-Surface Sialic Acids: Principles, Applications, and Recent Advances, ChemBioChem 17, 11–27. [DOI] [PubMed] [Google Scholar]
- (79).Li J, Hsu HC, Mountz JD, and Allen JG (2018) Unmasking Fucosylation: from Cell Adhesion to Immune System Regulation and Diseases, Cell Chem. Biol 25, 499–512. [DOI] [PubMed] [Google Scholar]
- (80).Pouilly S, Piller V, and Piller F. (2012) Metabolic glycoengineering through the mammalian GalNAc salvage pathway, FEBS J. 279, 586–598. [DOI] [PubMed] [Google Scholar]
- (81).Badr HA, AlSadek DMM, El-Houseini ME, Saeui CT, Mathew MP, Yarema KJ, and Ahmed H. (2017) Harnessing cancer cell metabolism for theranostic applications using metabolic glycoengineering of sialic acid in breast cancer as a pioneering example, Biomaterials 116, 158–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Rabuka D, Hubbard SC, Laughlin ST, Argade SP, and Bertozzi CR (2006) A chemical reporter strategy to probe glycoprotein fucosylation, J. Am. Chem. Soc 128, 12078–12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Wratil PR, Horstkorte R, and Reutter W. (2016) Metabolic Glycoengineering with N-Acyl Side Chain Modified Mannosamines, Angew. Chem. Int. Ed 55, 9482–9512. [DOI] [PubMed] [Google Scholar]
- (84).Vance JE (2015) Phospholipid Synthesis and Transport in Mammalian Cells, Traffic 16, 1–18. [DOI] [PubMed] [Google Scholar]
- (85).Jao CY, Roth M, Welti R, and Salic A. (2009) Metabolic labeling and direct imaging of choline phospholipids in vivo, Proc. Natl. Acad. Sci. U. S. A 106, 15332–15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Paper JM, Mukherjee T, and Schrick K. (2018) Bioorthogonal click chemistry for fluorescence imaging of choline phospholipids in plants, Plant Methods 14, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Lu G, Zuo L, Zhang J, Zhu H, Zhuang W, Wei W, and Xie H-Y (2020) Two-step tumor-targeting therapy via integrating metabolic lipid-engineering with in situ click chemistry, Biomater. Sci, doi: 10.1039/d1030bm00088d. [DOI] [PubMed] [Google Scholar]
- (88).Jao CY, Roth M, Welti R, and Salic A. (2015) Biosynthetic Labeling and Two-Color Imaging of Phospholipids in Cells, ChemBioChem 16, 472–476. [DOI] [PubMed] [Google Scholar]
- (89).Nilsson I, Lee SY, Sawyer WS, Rath CMB, Lapointe G, and Six D. (2020) Metabolic phospholipid labeling of intact bacteria enables a fluorescence assay that detects compromised outer membranes, J. Lipid Res, doi: 10.1194/jlr.RA120000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Han YT, Pan H, Li WJ, Chen Z, Ma AQ, Yin T, Liang RJ, Chen FM, Ma N, Jin Y, Zheng MB, Li BH, and Cai LT (2019) T Cell Membrane Mimicking Nanoparticles with Bioorthogonal Targeting and Immune Recognition for Enhanced Photothermal Therapy, Adv. Sci 6, 1900251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Zhang QB, Wei W, Wang PL, Zuo LP, Li F, Xu J, Xi XB, Gao XY, Ma GH, and Xie HY (2017) Biomimetic Magnetosomes as Versatile Artificial Antigen-Presenting Cells to Potentiate T-Cell-Based Anticancer Therapy, ACS Nano 11, 10724–10732. [DOI] [PubMed] [Google Scholar]
- (92).Zhang F, Zhao LJ, Wang SM, Yang J, Lu GH, Luo NN, Gao XY, Ma GH, Xie HY, and Wei W. (2018) Construction of a Biomimetic Magnetosome and Its Application as a SiRNA Carrier for High-Performance Anticancer Therapy, Adv. Funct. Mater 28, 1703326. [Google Scholar]
- (93).Sackstein R. (2012) Glycoengineering of HCELL, the Human Bone Marrow Homing Receptor: Sweetly Programming Cell Migration, Ann. Biomed. Eng 40, 766–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Devaraj NK (2018) The Future of Bioorthogonal Chemistry, ACS Cent. Sci 4, 952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Price NL, Goyette-Desjardins G, Nothaft H, Valguarnera E, Szymanski CM, Segura M, and Feldman MF (2016) Glycoengineered Outer Membrane Vesicles: A Novel Platform for Bacterial Vaccines, Sci. Rep 6, 24931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Stephan MT, and Irvine DJ (2011) Enhancing cell therapies from the outside in: Cell surface engineering using synthetic nanomaterials, Nano Today 6, 309–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Perez EE, Wang JB, Miller JC, Jouvenot Y, Kim KA, Liu O, Wang N, Lee G, Bartsevich VV, Lee YL, Guschin DY, Rupniewski I, Waite AJ, Carpenito C, Carroll RG, Orange JS, Urnov FD, Rebar EJ, Ando D, Gregory PD, Riley JL, Holmes MC, and June CH (2008) Establishment of HIV-1 resistance in CD4(+) T cells by genome editing using zinc-finger nucleases, Nat. Biotechnol 26, 808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Zhu J, Ming C, Fu X, Duan Y, Hoang DA, Rutgard J, Zhang RZ, Wang WQ, Hou R, Zhang D, Zhang E, Zhang C, Hao XK, Xiong WJ, Zhang K, and Consortium EGT (2017) Gene and mutation independent therapy via CRISPR-Cas9 mediated cellular reprogramming in rod photoreceptors, Cell Res. 27, 830–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay J, Thrasher AJ, Wulffraat N, Sorensen R, Dupuis-Girod S, Fischer A, Cavazzana-Calvo M, Davies EG, Kuis W, Lundlaan WHK, and Leiva L. (2002) Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy, New Engl. J. Med 346, 1185–1193. [DOI] [PubMed] [Google Scholar]
- (100).Zhang XJ, and Godbey WT (2006) Viral vectors for gene delivery in tissue engineering, Adv. Drug Del. Rev 58, 515–534. [DOI] [PubMed] [Google Scholar]
- (101).Davidson BL, and Breakefield XO (2003) Viral vectors for gene delivery to the nervous system, Nat. Rev. Neurol 4, 353–364. [DOI] [PubMed] [Google Scholar]
- (102).Pack DW, Hoffman AS, Pun S, and Stayton PS (2005) Design and development of polymers for gene delivery, Nat. Rev. Drug Discov 4, 581–593. [DOI] [PubMed] [Google Scholar]
- (103).Mahato RI (2005) Water insoluble and soluble lipids for gene delivery, Adv. Drug Del. Rev 57, 699–712. [DOI] [PubMed] [Google Scholar]
- (104).Zuris JA, Thompson DB, Shu Y, Guilinger JP, Bessen JL, Hu JH, Maeder ML, Joung JK, Chen ZY, and Liu DR (2015) Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo, Nat. Biotechnol 33, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Washbourne P, and McAllister AK (2002) Techniques for gene transfer into neurons, Curr. Opin. Neurobiol 12, 566–573. [DOI] [PubMed] [Google Scholar]
- (106).Kim TK, and Eberwine JH (2010) Mammalian cell transfection: the present and the future, Anal. Bioanal. Chem 397, 3173–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Mehier-Humbert S, and Guy RH (2005) Physical methods for gene transfer: Improving the kinetics of gene delivery into cells, Adv. Drug Del. Rev 57, 733–753. [DOI] [PubMed] [Google Scholar]
- (108).O’Brien JA, and Lummis SCR (2006) Biolistic transfection of neuronal cultures using a hand-held gene gun, Nat. Protoc 1, 977–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Shirahata Y, Ohkohchi N, Itagak H, and Satomi S. (2001) New technique for gene transfection using laser irradiation, J. Investig. Med 49, 184–190. [DOI] [PubMed] [Google Scholar]
- (110).Martinou I, Fernandez PA, Missotten M, White E, Allet B, Sadoul R, and Martinou JC (1995) Viral-Proteins E1b19k and P35 Protect Sympathetic Neurons from Cell-Death Induced by Ngf Deprivation, J. Cell Biol 128, 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Wang HF, La Russa M, and Qi LS (2016) CRISPR/Cas9 in Genome Editing and Beyond, Annu. Rev. Biochem 85, 227–264. [DOI] [PubMed] [Google Scholar]
- (112).Jakociunas T, Jensen MK, and Keasling JD (2016) CRISPR/Cas9 advances engineering of microbial cell factories, Metab. Eng 34, 44–59. [DOI] [PubMed] [Google Scholar]
- (113).Lv P, Liu X, Chen X, Liu C, Zhang Y, Chu C, Wang J, Wang X, Chen X, and Liu G. (2019) Genetically Engineered Cell Membrane Nanovesicles for Oncolytic Adenovirus Delivery: A Versatile Platform for Cancer Virotherapy, Nano Lett. 19, 2993–3001. [DOI] [PubMed] [Google Scholar]
- (114).Breibeck J, and Skerra A. (2018) The polypeptide biophysics of proline/alanine-rich sequences (PAS): Recombinant biopolymers with PEG-like properties, Biopolymers 109, e23069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Krishnamurthy S, Muthukumaran P, Jayakumar MKG, Lisse D, Masurkar ND, Xu C, Chan JM, and Drum CL (2019) Surface protein engineering increases the circulation time of a cell membrane-based nanotherapeutic, Nanomedicine 18, 169–178. [DOI] [PubMed] [Google Scholar]
- (116).Xu H, Wang B, Ono M, Kagita A, Fujii K, Sasakawa N, Ueda T, Gee P, Nishikawa M, Nomura M, Kitaoka F, Takahashi T, Okita K, Yoshida Y, Kaneko S, and Hotta A. (2019) Targeted Disruption of HLA Genes via CRISPR-Cas9 Generates iPSCs with Enhanced Immune Compatibility, Cell Stem Cell 24, 566–578 e567. [DOI] [PubMed] [Google Scholar]
- (117).Mattapally S, Pawlik KM, Fast VG, Zumaquero E, Lund FE, Randall TD, Townes TM, and Zhang JY (2018) Human Leukocyte Antigen Class I and II Knockout Human Induced Pluripotent Stem Cell-Derived Cells: Universal Donor for Cell Therapy, J. Am. Heart Assoc 7, e010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Gerritzen MJH, Martens DE, Wijffels RH, van der Pol L, and Stork M. (2017) Bioengineering bacterial outer membrane vesicles as vaccine platform, Biotechnol. Adv 35, 565–574. [DOI] [PubMed] [Google Scholar]
- (119).Zhang PF, Chen YX, Zeng Y, Shen CG, Li R, Guo ZD, Li SW, Zheng QB, Chu CC, Wang ZT, Zheng ZZ, Tian R, Ge SX, Zhang XZ, Xia NS, Liu G, and Chen XY (2015) Virus-mimetic nanovesicles as a versatile antigen-delivery system, Proc. Natl. Acad. Sci. U. S. A 112, E6129–E6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Huang Y, Liu H, Chen W, Nieh MP, and Lei Y. (2019) Genetically engineered bio-nanoparticles with co-expressed enzyme reporter and recognition element for IgG immunoassay, Sens. Actuators A 1, 1–7. [Google Scholar]
- (121).Chen MS, Zhang Y, and Zhang L. (2017) Fabrication and characterization of a 3D bioprinted nanoparticle-hydrogel hybrid device for biomimetic detoxification, Nanoscale 9, 14506–14511. [DOI] [PubMed] [Google Scholar]
- (122).Wang F, Gao W, Thamphiwatana S, Luk BT, Angsantikul P, Zhang QZ, Hu CMJ, Fang RH, Copp JA, Pornpattananangkul D, Lu WY, and Zhang L. (2015) Hydrogel Retaining Toxin-Absorbing Nanosponges for Local Treatment of Methicillin-Resistant Staphylococcus aureus Infection, Adv. Mater 27, 3437–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Zhang Y, Gao W, Chen YJ, Escajadillo T, Ungerleider J, Fang RH, Christman K, Nizet V, and Zhang L. (2017) Self-Assembled Colloidal Gel Using Cell Membrane-Coated Nanosponges as Building Blocks, ACS Nano 11, 11923–11930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Zhang Y, Zhang JH, Chen WS, Angsantikul P, Spiekermann KA, Fang RH, Gao W, and Zhang L. (2017) Erythrocyte membrane-coated nanogel for combinatorial antivirulence and responsive antimicrobial delivery against Staphylococcus aureus infection, J. Control. Release 263, 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Esteban-Fernandez de Avila B, Angsantikul P, Li JX, Lopez-Ramirez MA, Ramirez-Herrera DE, Thamphiwatana S, Chen CR, Delezuk J, Samakapiruk R, Ramez V, Zhang L, and Wang J. (2017) Micromotor-enabled active drug delivery for in vivo treatment of stomach infection, Nat. Commun 8, 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).de Avila BEF, Angsantikul P, Li JX, Gao W, Zhang L, and Wang J. (2018) Micromotors Go In Vivo: From Test Tubes to Live Animals, Adv. Funct. Mater 28, 1705640. [Google Scholar]
- (127).Chen WS, Zhang QZ, Luk BT, Fang RH, Liu YN, Gao W, and Zhang L. (2016) Coating nanofiber scaffolds with beta cell membrane to promote cell proliferation and function, Nanoscale 8, 10364–10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Gong H, Chen F, Huang ZL, Gu Y, Zhang QZ, Chen YJ, Zhang Y, Zhuang J, Cho YK, Fang RNH, Gao W, Xu S, and Zhang L. (2019) Biomembrane-Modified Field Effect Transistors for Sensitive and Quantitative Detection of Biological Toxins and Pathogens, ACS Nano 13, 3714–3722. [DOI] [PubMed] [Google Scholar]
- (129).de Avila BEF, Angsantikul P, Ramirez-Herrera DE, Soto F, Teymourian H, Dehaini D, Chen YJ, Zhang L, and Wang J. (2018) Hybrid biomembrane-functionalized nanorobots for concurrent removal of pathogenic bacteria and toxins, Sci. Robot 3, eaat0485. [DOI] [PubMed] [Google Scholar]
- (130).Bhattacharyya S, Bhattacharya R, Curley S, McNiven MA, and Mukherjee P. (2010) Nanoconjugation modulates the trafficking and mechanism of antibody induced receptor endocytosis, Proc. Natl. Acad. Sci. U. S. A 107, 14541–14546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Bhattacharyya S, Singh RD, Pagano R, Robertson JD, Bhattacharya R, and Mukherjee P. (2012) Switching the Targeting Pathways of a Therapeutic Antibody by Nanodesign, Angew. Chem. Int. Ed 51, 1563–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]