Abstract
We have previously shown that nerve growth factor (NGF) withdrawal-induced death requires the activity of the small GTP-binding protein Cdc42 and that overexpression of an active form of Cdc42 is sufficient to mediate neuronal apoptosis via activation of the c-Jun pathway. Recently, a new mitogen-activated protein (MAP) kinase kinase kinase, apoptosis signal-regulating kinase 1 (ASK1) which activates both the c-Jun N-terminal kinase (JNK) and p38 MAP kinase pathways and plays pivotal roles in tumor necrosis factor- and Fas-induced apoptosis, has been identified. Therefore, we investigated the role of ASK1 in neuronal apoptosis by using rat pheochromocytoma (PC12) neuronal cells and primary rat sympathetic neurons (SCGs). Overexpression of ASK1-ΔN, a constitutively active mutant of ASK1, activated JNK and induced apoptosis in differentiated PC12 cells and SCG neurons. Moreover, in differentiated PC12 cells, NGF withdrawal induced a four- to fivefold increase in the activity of endogenous ASK1. Finally, expression of a kinase-inactive ASK1 significantly blocked both NGF withdrawal- and Cdc42-induced death and activation of c-jun. Taken together, these results demonstrate that ASK1 is a crucial element of NGF withdrawal-induced activation of the Cdc42–c-Jun pathway and neuronal apoptosis.
Programmed cell death is an active process occurring during both normal maturation of the nervous system and pathological situations such as neurodegenerative diseases and stroke (5, 15, 19, 25, 26). Various phenotypes of programmed cell death have been described, including apoptosis with chromatin and cytoplasmic condensation and fragmentation (7, 28, 29). Neuronal apoptosis and survival are regulated by two major kinds of cell surface molecules, which respond to external stimuli: the Trk molecules, which promote differentiation and survival of specific neuronal populations, and the p75 neurotrophin receptor, which can mediate apoptosis upon ligand binding. Ultimately, extracellular signals are transmitted into the activation of either anti- or proapoptotic genes. Some of the specific regulatory pathways involved in transducing these signals are begun to be understood. Indeed, activation of the stress-activated protein kinases (stress-activated protein kinase/c-Jun N-terminal kinase [SAPK/JNK] and/or p38 mitogen-activated protein kinase [MAPK]) pathway has been observed soon after induction of apoptosis by nerve growth factor (NGF) withdrawal in rat pheochromocytoma PC12 cells (38), in rat sympathetic neurons (superior cervical ganglion neurons [SCGs]) (8, 14), in rat cerebellar granule neurons (37), and in embryonic motoneurons (21). Moreover, phosphorylation of c-Jun and activation of SAPK/JNK have been observed after neuronal injury in the adult rat brain (16). Recently, we have shown that activated mutants of the Rho-like GTPases, Cdc42 and Rac1, induced apoptosis of SCGs by activating the c-Jun transcriptional pathway (1). More importantly, Cdc42 was shown to be required for NGF withdrawal-induced cell death (1). Therefore, a comprehensive knowledge of the upstream regulators of the JNK pathway is needed to provide insights about possible mediators of neuronal apoptosis induced by both NGF withdrawal and Cdc42.
Candidates for activation of the JNK and p38 MAPK pathways have been identified and include the p21-activated kinases (3, 12) mixed-lineage kinase 3 (23, 31), MEKK1 and MEKK4 (10, 13), POSH (30), and the recently identified apoptosis signal-regulating kinase 1 (ASK1) (18, 36). The ubiquitously expressed serine/threonine kinase ASK1 is a MAPK kinase kinase (MAPKKK) that activates both SEK1 (also termed MKK4 or JNKK1)-JNK and MKK3/MKK6-p38 kinase pathways. Overexpression of an activated ASK1 mutant in epithelial cells induced cell death, which exhibited the characteristics of apoptosis (18, 27). ASK1 was activated upon treatment with tumor necrosis factor (24) or with an anti-Fas antibody (4), and a kinase-inactive mutant of ASK1 blocked tumor necrosis factor- and Fas-induced apoptosis in Jurkat cells and HeLa cells, respectively (4, 18). These studies strongly suggested that ASK1 plays a central role in the mechanisms of stress- and cytokine-induced apoptosis and prompted us to look at its role in our models of neuronal cell death.
Herein, we report that an active ASK1 induces death by apoptosis of both a neuronal cell line (the rat pheochromocytoma PC12 cells) and rat primary sympathetic neurons (SCGs). Induction of cell death was accompanied by an increase in JNK activity in both systems. In addition, NGF deprivation led to a robust increase of ASK1 activity. We also found that a kinase-inactive mutant of ASK1, ASK1-KR, significantly blocked NGF withdrawal-mediated induction of cell death and of activation of c-jun. Finally, expression of ASK1-KR also blocked Cdc42-induced death and activation of JNK in SCGs, suggesting that ASK1 can act as a downstream mediator of the GTPase, at least in this cell type. Altogether these data demonstrate that ASK1 plays a central role in neurotrophic factor deprivation-induced death as well.
MATERIALS AND METHODS
Cell culture.
SCG neurons were isolated from 1-day-old Sprague-Dawley rats as described previously (14). The SCGs were plated on 13-mm glass coverslips, coated with poly-l-lysine and laminin (both from Sigma, Poole, United Kingdom), at a density of 8,000 to 10,000 cells per dish and cultured in SCG growth medium (Dulbecco's modified Eagle's medium [DMEM; Sigma]), 10% heat-inactivated fetal calf serum (FCS) (Globepharm, Surrey, United Kingdom], 2 mM glutamine, 2 mM penicillin-streptomycin [both from Life Technologies, Paisley, United Kingdom], 100 ng of NGF per ml, 20 mM antimitotic agents, 20 mM fluorodeoxyuridine, 20 mM uridine [all from Sigma]). The neurons were kept in culture in the presence of NGF for 5 to 8 days before being used for immunostaining and cell death experiments. NGF withdrawal was done by refeeding the cells with the SCG growth medium lacking NGF but supplemented with 100 ng of neutralizing anti-NGF antibody (Boehringer-Mannheim) per ml.
PC12 cells were plated at a density of 3 × 104 cells/ml in six-well cell culture plates coated with bovine type IV collagen (Cellmatrix; Nitta Gelatin Inc.). They were cultured in DMEM supplemented with 10% FCS, 10% heat-inactivated horse serum, and 100 U of penicillin G per ml. The PC12 cells were differentiated for 7 to 9 days in DMEM containing 1% heat-inactivated horse serum, 50 ng of 2.5S NGF (Takara) per ml, and 100 U of penicillin G per ml. For the NGF withdrawal experiments, the cells were washed twice with phosphate-buffered saline and refed with defined medium without NGF. Similar death-inducing activity was observed in the presence or absence of 100 ng of anti-NGF antibody per ml.
Antibodies.
The ASK1 antibody (DAV) is a rabbit polyclonal antibody raised against the peptide sequence DAVATSGVSTLSSTVSHDSQ (amino acids 1216 to 1235 of ASK1) as described previously (27). The JNK antibody (C-17), which recognizes the JNK1 and JNK3 isoforms, and the anti-phospho-JNK (G-7), which recognizes the phosphorylated JNK1, JNK2, and JNK3 isoforms, were purchased from Santa Cruz. The HA mouse monoclonal antibody (clone 12CA5) was from Boehringer Mannheim. The polyclonal anti-phospho–c-Jun (Ser 73) antibody was from New England Biolabs, and the monoclonal anti-phospho–c-Jun (Ser 63) antibody was raised against a phosphopeptide encompassing amino acids 57 to 68 and with phosphoserine 63. The anti-chloramphenicol acetyltransferase (CAT) antibody is a polyclonal antibody from 5 Prime → 3 Prime, Inc.
Preparation of total-cell lysates and Western blot analysis.
Cells were lysed in a lysis buffer containing 20 mM Tris-HCl (pH 7.5), 12 mM glycerophosphate, 150 mM NaCl, 5 mM EGTA, 10 mM NaF, 1% Triton X-100, 0.5% deoxycholate, 3 mM dithiothreitol, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and 1.5% aprotinin. The cell extracts were clarified by centrifugation, and the supernatants were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were then subjected to Western blot analysis, and the blots were developed with the enhanced chemiluminescence system (Amersham).
Kinase assay.
The human MKK6 was obtained by PCR and subcloned into the pGEX-4T-1 bacterial expression vector (Pharmacia Biotech, Inc.). Glutathione S-transferase (GST)-rat SAPK3 (6) and the ATF2 peptide (peptide 1–109) were kindly provided by Michel Goedert and Zhengbin Yao, respectively. To assess the ASK1 kinase activity, the cells were lysed on ice as described above. The cell extracts were clarified by centrifugation, and the supernatants were immunoprecipitated with ASK1 (DAV) antibody. The immunocomplexes were bound to protein A-Sepharose (Zymed Laboratories). The beads were washed twice with 500 mM NaCl–20 mM Tris-HCl (pH 7.5)–1% Triton X-100–5 mM EGTA–2 mM dithiothreitol–1 mM phenylmethylsulfonyl fluoride and subjected to the ASK1 kinase assay. To measure the ASK1 activity, 0.1 μg of GST-MKK6 was incubated first with the immune complex for 10 min at 30°C in a final volume of 10 μl of a solution containing 20 mM Tris-HCl (pH 7.5), 20 mM MgCl2, and 100 mM ATP and subsequently with 1 mg of GST-SAPK3/p38γ for 10 min at 30°C. Thereafter, the activated complex was incubated with 0.3 μCi of [γ-32P]ATP and 0.5 μg of ATF2 peptide in the same solution (final volume, 30 μl). The samples were resolved by SDS-PAGE (15% polyacrylamide), and the phosphorylation of ATF2 was analyzed by an image analyzer (Fujix BAS2000). For the JNK kinase assay, the cell lysates were immunoprecipitated with an antibody to JNK (C-17; Santa Cruz), bound to protein A-Sepharose beads, and washed as described above. The beads were then incubated with 0.3 μCi of [γ-32P]ATP and 1 μg of c-Jun. The samples were subjected to SDS-PAGE (12% polyacrylamide), and the phosphorylation of c-Jun was analyzed by an image analyzer.
Tetracycline system.
The hygromycin resistance gene derived from pBHMr and the neomycin resistance gene derived from pABWN were each subcloned into the pTet-tTAK plasmid (Gibco BRL) and the pTet-Splice (Gibco BRL) and named pTet-tTAK-hyg and pTet-Splice-neo, respectively. The cDNAs for HA-tagged ASK1-ΔN or HA-tagged ASK1-KM were then subcloned into pTet-Splice-neo. The pTet-tTAK-hyg plasmid was transfected into PC12 cells by using DMRIE-C (Gibco BRL) as specified by the manufacturer. Cells were selected with 240 U of hygromycin (Wako) per ml, and several hygromycin-resistant stable clones were established. A clone termed PC12-pTet-tTAK-hyg was further transfected with pTet-Splice-neo-based expression vectors for HA-ASK1-ΔN or HA-ASK1-KM. After selection with 400 μg of neomycin (Geneticin; Gibco BRL) per ml and 240 U of hygromycin per ml, the doubly resistant clones were established and analyzed for the expression of HA-tagged ASK1 mutant proteins by Western blot analysis. The cells were maintained in DMEM supplemented with 10% FCS, 10% heat-inactivated horse serum, 100 U of penicillin G per ml, 0.5 μg of tetracycline per ml, 120 U of hygromycin per ml, and 200 μg of neomycin per ml.
Adenovirus vectors.
Recombinant adenoviruses were constructed as described previously (27). Briefly, HA-tagged ASK1 cDNA mutants and β-galactosidase cDNA were subcloned into the SwaI site of the pAxCAwt cassette cosmid, which is defective in the adenovirus E1A, E1B, and E3 regions. Each cosmid, bearing the expression unit and the adenovirus DNA-terminal protein complex, were cotransfected into the E1 transcomplementing cell line 293. The recombinant adenoviruses generated by homologous recombination were isolated, and the insertion of ASK1 cDNAs was confirmed by restriction endonuclease digestion. High-titer stocks of recombinant adenoviruses were grown in 293 cells and purified. Infection of PC12 cells with the recombinant adenoviruses was done at a multiplicity of infection (MOI) of 100 PFU/cell (or as otherwise indicated); in these infections, adenovirus vectors can transduce nearly 100% of PC12 cells as determined by β-galactosidase (β-gal) staining (T. Kanamoto and H. Ichijo unpublished observation). The presence of a comparable level of protein expression for each ASK1 construct was confirmed by immunoblotting with anti-HA antibody (data not shown).
DNA fragmentation analysis.
NGF-differentiated PC12 cells were infected with adenovirus vectors expressing β-gal or ASK1-ΔN and were maintained in the presence of NGF. At given times, small fragmented cytoplasmic DNA was isolated as previously described (18). Briefly, cells were lysed with 200 μl of a buffer containing 20 mM Tris-HCl (pH 7.5), 10 mM EDTA, and 0.5% Triton X-100. Cell extracts were clarified by centrifugation at 15,000 × g for 5 min. The lysates were then incubated with 0.2 mg of proteinase K per ml–0.1 mg of RNase A per ml at 42°C for 1 h. The DNA was purified by ethanol precipitation after phenol-chloroform extraction and analyzed by agarose gel electrophoresis (2% agarose).
Microinjection and survival assay.
Sympathetic neurons were microinjected as described previously (1). All plasmids were purified on a double cesium chloride gradient and resuspended in H2O. The injection mix constituted of 50% phosphate-buffered saline and 5 mg of 70,000-Da Texas Red-dextran (Molecular Probes, Eugene, Oreg.) per ml (survival assay) or 2.5 mg of purified guinea pig immunoglobulin G (IgG) (Sigma) per ml (immunostaining analysis) to observe the injected cells. At 4 h after injection, the neurons were refed as described above. The percent survival was assessed as previously described (1).
Immunofluorescence staining.
For the c-jun–CAT reporter gene assay, SCG neurons were injected with 0.05 mg of c-jun CAT per ml in the presence or absence of a plasmid of interest, together with guinea pig IgG to detect the injected cells. At 24 h after injection, the cells were fixed in 3.7% formaldehyde, permeabilized with 0.5% Triton X-100 in PBS, and blocked with 50% goat serum. The neurons were stained with a polyclonal anti-CAT antibody diluted 1:100 and finally with a fluorescein isothiocyanate-conjugated secondary antibody and a rhodamine-conjugated anti-guinea pig IgG antibody (Stratech Scientific) to detect the injected cells. Only the cells showing a clear increase over the background staining were scored positive. To detect phospho–c-Jun, the cells were fixed with 3% paraformaldehyde, permeabilised with 0.5% Triton X-100, and blocked as described above. They were then stained with a specific phospho-c-Jun antibody and with the appropriate secondary antibodies as described above. To examine nuclear morphology, cells were stained with Hoechst dye (Hoechst 33342; Sigma) at 10 μg/ml.
RESULTS
Activated ASK1 induces neuronal apoptosis.
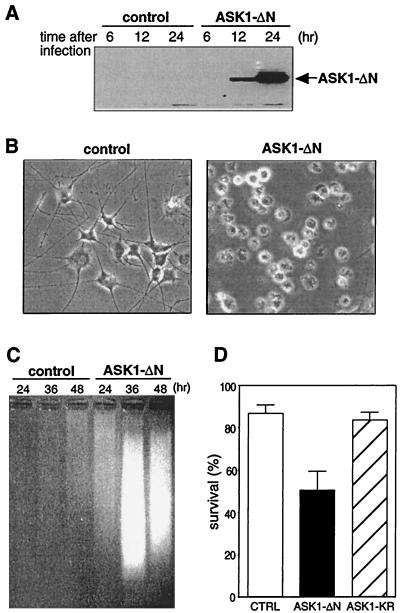
To investigate the role of ASK1 in neuronal cell death, we examined the effect of ASK1-ΔN, a constitutively active mutant of ASK1 (27), in differentiated PC12 cells and in rat primary sympathetic neurons. PC12 cells were cultured in the presence of 50 ng of NGF per ml for 7 days and then infected with recombinant adenoviruses encoding β-gal or HA-tagged ASK1-ΔN. The efficiency of the adenovirus infection was nearly 100% as determined by the control staining for β-gal infectant (data not shown), suggesting that recombinant adenoviruses provide a useful system to express exogenous genes in differentiated PC12 cells. The level of expression of ASK1-ΔN was determined by immunoblotting with a monoclonal anti-HA antibody and was first detected 12 h after infection (Fig. 1A). The viability of the infected cells was examined by phase-contrast microscopy 48 h after infection. Expression of ASK1-ΔN, but not of a control β-gal, clearly induced cell death, with morphologically apoptotic features including membrane blebbing, pyknosis, and cell body condensation even in the presence of NGF (Fig. 1B). To further characterize the death induced by ASK1-ΔN, we performed a DNA ladder analysis. The cytoplasmic small fragmented DNA was extracted after infection and analyzed by agarose gel electrophoresis. Genomic DNA fragmentation was first observed 24 h after infection (Fig. 1C) in the cells overexpressing ASK1-ΔN but not in those expressing the control β-gal.
FIG. 1.
Effect of constitutively active ASK1 on neuronal survival. (A) Expression of ASK1-ΔN in PC12 cells by using recombinant adenoviruses. NGF-differentiated PC12 cells were infected with 100 PFU of recombinant adenoviruses encoding β-gal or HA-tagged ASK1-ΔN per cell. At the indicated times after infection, the cells were lysed and immunoblotted with a monoclonal antibody to HA. (B) Induction of neuronal cell death by ASK1-ΔN in NGF-differentiated PC12 cells. The morphology of β-gal-infected cells (left) and ASK1-ΔN-infected cells (right) was examined by phase-contrast microscopy 48 h after infection in the presence of 50 ng of NGF per ml. Original magnification, ×82.5. (C) DNA fragmentation in ASK1-ΔN-infected cells. Soluble cytoplasmic DNA was extracted from 3 × 106 cells after infection with recombinant adenoviruses encoding β-gal or ASK1-ΔN and analyzed by agarose gel electrophoresis. (D) Induction of neuronal cell death by ASK1-ΔN in SCG neurons. SCG neurons, cultured for 5 to 7 days in the presence of NGF, were microinjected with 0.3 mg of ASK1-ΔN (solid box), ASK1-KR (hatched box), or an empty expression vector (empty box) per ml. The 70-kDa Texas Red-dextran was included to mark the injected cells. The number of injected cells was scored 4 to 18 h after injection to allow expression of the proteins of interest (100% value). At 48 h later, the percentage of surviving cells was assessed as described in Materials and Methods. In each experiment, 200 cells were injected. The results are the means of three independent experiments and standard error of the mean.
We next extended our study of the role of ASK1 to primary rat SCG neurons, cultured for 6 days in the presence of NGF, were microinjected at 0.3 mg/ml with an empty expression vector (control) or of the different ASK1 mutants including ASK1-ΔN and ASK1-KR, a kinase-inactive mutant in which Lys 709 was replaced by Arg. The percent survival was then assessed 48 h after injection. Figure 1D shows that ASK1-ΔN significantly decreased the viability of the SCG neurons whereas the empty vector and the kinase-inactive mutant ASK1-KR had no effect. The cell death induced by ASK1 exhibited the characteristics of an apoptotic mechanism where the nuclei were clearly pyknotic and stained positive for terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) analysis (data not shown). Taken together, these results demonstrate that ASK1 has a proapoptotic activity in neuronal cells and that its kinase activity is required for the induction of cell death.
ASK1 is required for NGF withdrawal-induced death.
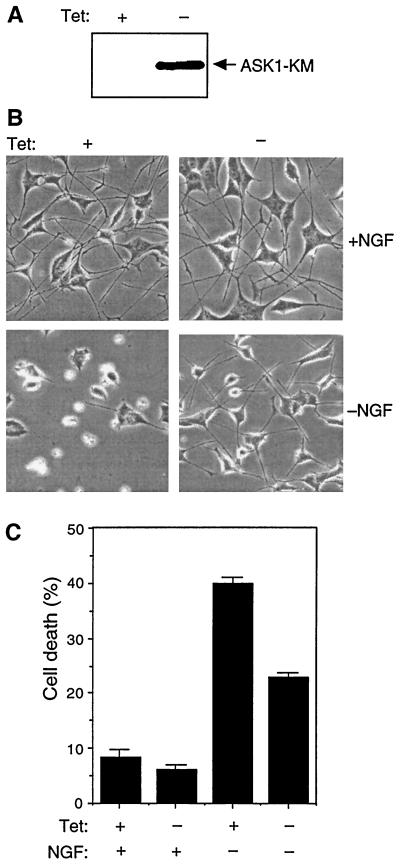
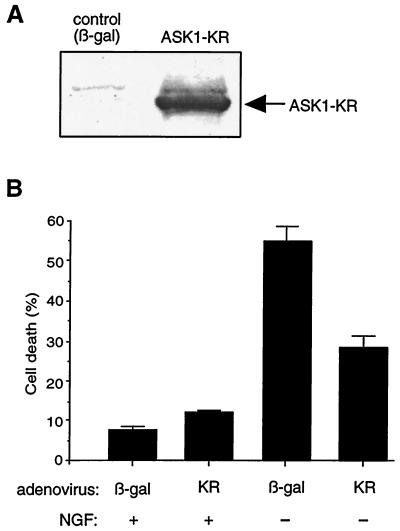
When differentiated, PC12 cells are dependent on NGF and undergo apoptosis following NGF deprivation. To examine whether ASK1 is involved in NGF withdrawal-induced death, we have established a stable PC12 cell line expressing HA-tagged ASK1-KM (PC12-ASK1-KM cells), another kinase-inactive mutant of ASK1, in which Lys 709 was replaced by Met. The expression of ASK1 is under the control of a tetracycline-repressible promoter (see Materials and Methods). After removal of tetracycline from the culture medium, the expression of ASK1-KM was turned on and was readily detectable by Western blot analysis with an anti-HA antibody (Fig. 2A). Notably, no leaky expression was detected in the presence of tetracycline, indicating that the tetracycline-repressible promoter was well controlled in this cell line. In the presence of tetracycline, PC12-ASK1-KM cells differentiated into sympathetic neuron-like cells by NGF treatment and were indistinguishable from the parental PC12 cells (Fig. 2B). When the differentiated PC12-ASK1-KM cells were deprived of NGF but maintained in the presence of tetracycline, their viability significantly decreased (Fig. 2B and C). In contrast, when NGF was withdrawn 24 h after induction of ASK1-KM by tetracycline removal, NGF withdrawal-induced cell death was partially but significantly inhibited at 48 h after NGF deprivation (Fig. 2B and C). Furthermore, to exclude the possibility that the effect of ASK1-KM in differentiated PC12-ASK1-KM cells was caused by a clonal variation, we also examined the effect of recombinant adenoviruses encoding HA-tagged ASK1-KR on parental PC12 cells as previously described. Briefly, the level of expression of ASK1-KR was determined by immunoblotting with a monoclonal anti-HA antibody (Fig. 3A) and the viability of the infected cells was examined by phase-contrast microscopy 48 h after infection. Similarly, expression of ASK1-KR, but not of β-gal, partially prevented cell death induced by NGF withdrawal (Fig. 3B). These results strongly suggest that ASK1 is an important component of the death-signaling pathway mediated by NGF withdrawal in neuronal cells.
FIG. 2.
Effect of ASK1-KM on NGF withdrawal-induced cell death. (A) Expression of ASK1-KM in PC12 cells stably transfected with ASK1-KM (PC12-ASK1-KM cells). PC12-ASK1-KM cells, stably expressing HA-tagged ASK1-KM under the control of a tetracycline-repressible promoter, were cultured in the presence of 50 ng of NGF per ml and 0.5 μg of tetracycline per ml for 9 days. After the additional 24 h of culture in the presence (+) or absence (−) of tetracycline (Tet), the cells were lysed and immunoblotted with anti-HA antibody. (B) Phase-contrast micrographs showing the morphology of PC12-ASK1-KM cells under various culture conditions. PC12-ASK1-KM cells were cultured in the presence of 50 ng of NGF per ml and 0.5 μg of tetracycline per ml for 9 days. After the additional 24 h of culture in the presence (left) or absence (right) of tetracycline (Tet), the medium was changed with (top) or without (bottom) NGF. Cell morphology was examined by phase-contrast microscopy after 48 h. Original magnification, ×82.5. (C) ASK1-KM prevents NGF withdrawal-induced cell death in PC12-ASK1-KM cells. The graph shows the data (mean and standard deviation) for the percentage of apoptotic cells in the experiments in panel B. Apoptotic cells were determined by observing morphological changes with cellular shrinkage or membrane blebbing. The data are averages of five random fields.
FIG. 3.
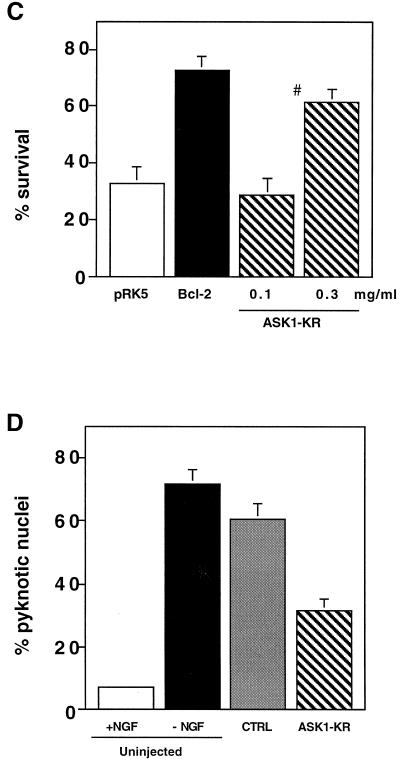
Effect of ASK1-KR on NGF withdrawal-induced cell death. (A) Expression of ASK1-KR in PC12 cells by using recombinant adenoviruses. NGF-differentiated PC12 cells were infected with 100 PFU of recombinant adenoviruses encoding β-gal or HA-tagged ASK1-KR per cell. The cells were lysed and immunoblotted with a monoclonal antibody to HA. (B) Protection of neuronal cell death by ASK1-KR in NGF-differentiated PC12 cells. The morphology of β-gal-infected cells and ASK1-KR-infected cells was examined by phase-contrast microscopy 48 h after infection in the presence of 50 ng of NGF per ml (controls) or in the absence of NGF. The graph shows a quantification of the percent cell death as determined by observing morphological changes with cellular shrinkage or membrane blebbing. The data are averages of five random fields. (C) ASK1-KR prevents NGF withdrawal-induced cell death in SCG neurons. SCG neurons, cultured for 5 to 7 days in the presence of NGF, were microinjected with 70-kDa Texas Red-dextran and increasing concentrations of ASK1-KR (hatched boxes), 0.6 mg of pRK5 (negative control; open box) per ml, or 0.05 mg of Bcl-2 (positive control; solid boxes) per ml. At 24 h later, the cells were deprived of NGF and the number of injected cells was scored (100% value). The percentage of surviving cells was assessed after 48 h as described in Materials and Methods. The results are the means of three independent experiments and standard errors of the mean. (D) ASK1-KR decreases the number of pyknotic nuclei after NGF withdrawal-induced cell death in SCG neurons. SCG neurons were injected with a control empty expression vector or with ASK1-KR and removed from NGF. Uninjected cells, maintained in the presence or the absence of NGF, were included as controls. After 24 h, the nuclear morphology was visualized by Hoechst 33342 staining. The results are the mean of three independent experiments and standard error of the mean. The P value of ASK1-KR at 0.3 mg/ml is <0.01.
Finally, we determined whether ASK1 is a required component of the NGF withdrawal-induced death of SCG neurons. We microinjected increasing concentrations of ASK1-KR DNA into SCG neurons as well as an empty vector (negative control) and Bcl-2, a potent antiapoptotic protein (positive control). At 4 to 5 h after injection, the cells were deprived of NGF and the percent surviving cells was assessed 48 h later as explained in Materials and Methods. Although slightly less effective than Bcl-2, ASK1-KR protected SCG neurons from NGF withdrawal-induced death in a dose-dependent manner (Fig. 3C). In addition, we looked at the effect of ASK1-KR on the nuclear morphology of the injected cells. SCGs were microinjected with 0.5 mg of either an empty expression vector or ASK1-KR per ml. The cells were removed from NGF and stained with Hoechst 33342 24 h later. Controls of uninjected cells, maintained in the presence or absence of NGF, were included. Figure 3D shows that blocking the ASK1 activity significantly decreased the number of cells displaying pyknotic nuclei. Taken together, these results confirm the involvement of ASK1 in mediating neuronal cell death induced by deprivation of trophic factors.
ASK1 activity increases following NGF withdrawal.
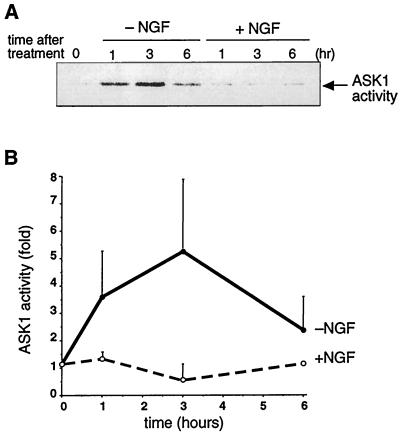
To determine whether ASK1 was activated upon NGF deprivation, we set out to measure the kinase activity of endogenous ASK1 in PC12 cells undergoing apoptosis. NGF-differentiated PC12 cells were deprived of NGF and further cultured in the presence or absence of NGF. The ASK1 kinase activity was determined by an immune complex-coupled kinase assay with GST-MKK6, GST-SAPK3/p38γ, and the ATF2 peptide as sequential substrates (27). When the medium was replaced with NGF-containing medium, the kinase activity was unchanged (Fig. 4). On the other hand, when the cells were deprived of NGF, a rapid increase in the kinase activity of ASK1, which peaked at 3 h after NGF deprivation, was observed (Fig. 4B). These results strongly suggest that ASK1 kinase activity can be regulated after induction of cell death by NGF withdrawal.
FIG. 4.
Activation of endogenous ASK1 by NGF withdrawal. (A) NGF-differentiated PC12 cells were deprived of NGF and further cultured in the absence (−NGF) or presence (+NGF) of NGF for the indicated times. Endogenous ASK1 was immunoprecipitated with anti-ASK1 antibody (DAV). The immune complex was incubated with GST-MKK6 and GST-SAPK3/p38γ, and the kinase activity was measured with ATF2 peptide as a substrate. (B) Relative changes of the kinase activity were calculated by standardizing the background signal as a basal level and plotted to give a graphic representation.
Involvement of the ASK1-JNK pathway in the NGF withdrawal-induced signal.
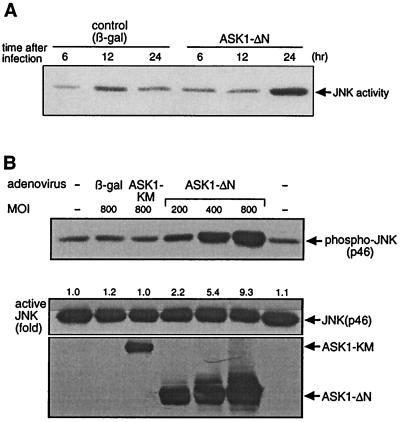
ASK1 has previously been shown to activate the JNK pathway in various cell types (4, 18, 35). To determine whether this was the case in neuronal cells, we infected NGF-differentiated PC12 cells with a recombinant adenovirus encoding ASK1-ΔN or the β-gal control and measured JNK activity by an immune-complex JNK kinase assay at 6, 12, and 24 h after infection with a GST–c-Jun substrate. An increase in JNK activity was observed 24 h after infection (Fig. 5A), which correlates with the kinetics of expression of ASK1-ΔN (Fig. 1A). In addition, we investigated the activation of JNK for up to 45 h after infection (Fig. 5B). Differentiated PC12 cells were infected with adenoviruses encoding control β-gal, ASK1-KM at 800 PFU/cell, and ASK1-ΔN at increasing MOIs. At 45 h later, the cells were lysed and subjected to Western blot analysis with specific phospho-JNK, JNK, and HA antibodies. The increase in JNK activation by ASK1-ΔN expression was dose dependent (Fig. 5B) whereas infection with the control β-gal or ASK1-KM, even at higher MOIs and for a longer period (45 h), had no effect on the level of JNK activation compared to the uninfected control (Fig. 5B).
FIG. 5.
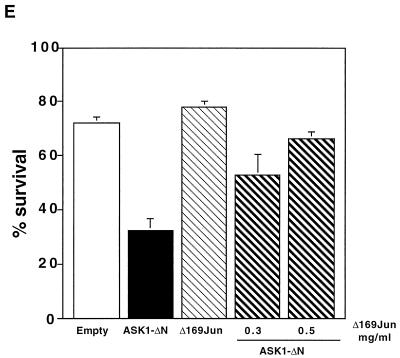
ASK1-dependent activation of JNK pathway. (A) ASK1-ΔN-dependent activation of endogenous JNK in PC12 cells. NGF-differentiated PC12 cells were infected with 100 PFU of recombinant adenoviruses encoding β-gal or HA-tagged ASK1-ΔN per cell. At 24 h after infection, immunoprecipitated endogenous JNK activity was measured with GST–c-Jun as a substrate. The samples were analysed by SDS-PAGE with an image analyzer. (B) Effect of ASK1-ΔN at 45 h. Differentiated PC12 cells were infected with adenoviruses encoding control β-gal, ASK1-KM at 800 PFU/cell, and ASK1-ΔN at the indicated MOIs. At 45 h later, the cells were lysed and subjected to Western blot analysis with specific phospho-JNK (top), JNK (middle), and HA (bottom) antibodies. The fold activation of JNK was standardized to the +NGF control (top panel, left lane). (C) ASK1-dependent phosphorylation of c-Jun in SCG neurons. SCG neurons were microinjected either with 0.3 mg of an empty expression vector or ASK1-ΔN per ml, together with 5 mg of guinea pig IgG per ml to detect the injected cells, and maintained in the presence of NGF or with 0.5 mg of the control empty vector per ml or various concentrations of ASK1-KR and removed from NGF. Controls of uninjected cells were included. After 24 h, the cells were fixed, permeabilized, and stained with a specific anti-phospho–c-Jun antibody. Only the cells in which phospho–c-Jun staining was clearly above background were scored positive. The results are represented as a bar graph and are the mean and standard error of the mean of four independent experiments, (D) ASK1-ΔN-dependent activation of c-jun promoter in SCG neurons. A 0.05-mg/ml concentration of the c-jun–CAT reporter gene was microinjected alone or with 0.3 mg of ASK1-ΔN per ml into 5 to 7-day-old SCG neurons, together with 5 mg of guinea pig IgG per ml to detect the injected cells. In the NGF withdrawal experiments, SCG neurons were microinjected with 0.05 mg of the c-jun–CAT reporter gene per ml alone or with increasing concentrations of ASK1-KR together with 5 mg of guinea pig IgG per ml to monitor the injected cells. The neurons were either maintained in the presence of NGF or removed from NGF for 24 h. The cells were then fixed, permeabilized, and stained as described in Materials and Methods. Only the cells in which CAT staining was clearly above background were scored positive. The results are presented as a bar graph. The data are the means and standard errors of the mean of three independent experiments. ASK1-ΔN is capable of inducing a twofold increase in the level of CAT expression, whereas ASK1-KR blocks the increase in c-jun activation that is normally observed after NGF withdrawal. (E) FLAGΔ169-Jun blocks ASK1-induced apoptosis. FLAGΔ169-Jun at the indicated concentrations and 0.3 mg of ASK1-ΔN per ml were microinjected into 5- to 7-day old SCG neurons and maintained in the presence of NGF. The percentage of surviving cells was assessed 48 h later. The results are the means and standard errors of the mean of three independent experiments.
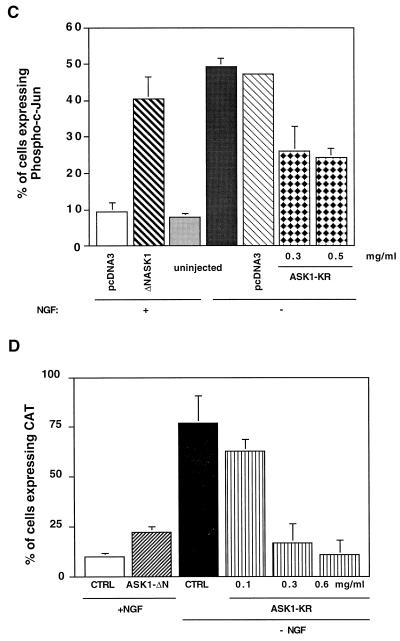
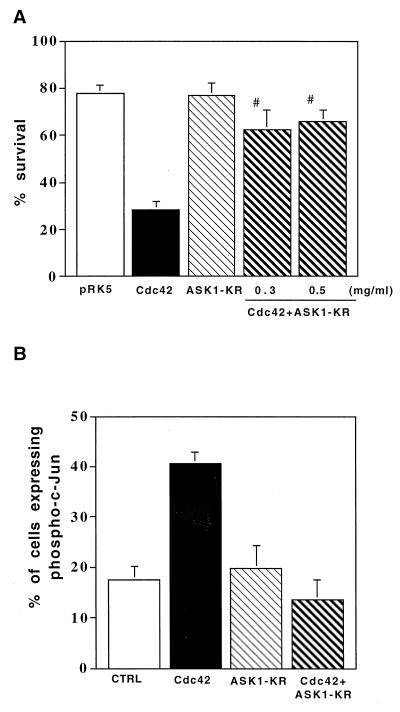
In parallel, we investigated the effect of ASK1 mutants on the level of phosphorylated c-Jun in SCG neurons in the presence or absence of NGF. Cells, microinjected with either the ASK1 mutants or an empty expression vector were labelled with a specific anti-phospho–c-Jun antibody. Cells injected with the empty control did not show any increase in phospho–c-Jun staining compared to the NGF control, whereas overexpression of ASK1-ΔN induced a 4.3-fold increase in the level of nuclearly phosphorylated c-Jun (Fig. 5C). In addition, we examined the effect of ASK1-KR on the increase in the level of phosphorylated c-Jun observed after NGF deprivation. We found that expression of a kinase dead mutant of ASK1 reduced dramatically the increase in phospho–c-Jun levels after NGF deprivation (Fig. 5C). ASK1-KR had no effect on the phospho–c-Jun level when cells were maintained in the presence of NGF (Fig. 6B). This suggests that ASK1 activity is crucial for the induction of phosphorylation of c-Jun after NGF withdrawal.
FIG. 6.
Effect of a dominant negative ASK1 on Cdc42-induced cell death. (A) Cdc42-induced apoptosis requires ASK1 activity. We coinjected 0.3 or 0.5 mg of ASK1-KR per ml, 0.1 mg of V12Cdc42 per ml, and 70-kDa Texas Red-dextran into SCG neurons. The cells were maintained in the presence of NGF, and the percentage of surviving cells was assessed 48 h after injection as previously described. The results are the means and standard errors of the mean of three independent experiments. ASK1-KR blocks Cdc42-induced death. The P value of ASK1-KR is <0.01 at 0.3 mg/ml and <0.001 at 0.5 mg/ml. (B) ASK1-KR blocks Cdc42-induced increase of phosphorylation of c-Jun. SCG neurons were coinjected with 0.5 mg of ASK1-KR per ml and 0.1 mg of V12Cdc42 per ml together with 5 mg of guinea pig IgG per ml to detect the injected cells and maintained in the presence of NGF. The cells were stained 24 h later with a specific anti-phospho–c-Jun antibody. The results are the means and standard errors of the mean of three independent experiments. The P value of ASK1-KR at 0.5 mg/ml is <0.01.
We also examined whether ASK1 activated c-jun in SCG neurons by using a c-jun–CAT reporter gene, in which c-jun promoter sequences from −1600 to +170 were subcloned upstream of the bacterial CAT gene (34). SCG neurons were coinjected with ASK1-ΔN and a c-jun–CAT reporter gene together with guinea pig IgG as a marker for the injected cells. The cells were maintained in the presence of NGF and stained with an anti-CAT antibody 24 h after injection. About 10 to 20% of the cells injected with the c-jun–CAT gene alone expressed the CAT protein, whereas withdrawal of NGF triggered a three- to fourfold increase in the number of cells expressing the CAT protein showing the activation of c-Jun after NGF deprivation (Fig. 5D) (8). Coinjection of ASK1-ΔN increased by twofold the percentage of cells expressing CAT (Fig. 5D). The relatively weak CAT-inducing activity mediated by ASK1-ΔN may be caused by the underscore of CAT-positive cells because of the decreased viability of ASK1-ΔN-injected cells. Thus, c-jun appears to be activated by ASK1 in SCG neurons.
To investigate the requirement for ASK1 in the activation of c-Jun transcriptional pathway, we coinjected increasing concentration of ASK1-KR with c-jun–CAT into SCG neurons. At 4 h after injection, the cells were deprived of NGF, and they were stained for CAT expression 20 h later. Overexpression of ASK1-KR blocked the increase in the CAT expression that was observed after NGF withdrawal (Fig. 5D). These results demonstrated not only that ASK1 is capable of activating the c-jun pathway in neuronal cells but also that it is required for the activation of this pathway by NGF withdrawal.
Finally, we investigated the effect of FLAGΔ169-Jun, a dominant negative mutant of c-Jun, that lacks the amino-terminal transactivation domain and also acts as a dominant inhibitor of AP-1 activity (14), on ASK1-induced apoptosis. SCG neurons were coinjected with 0.3 mg of ASK1-ΔN per ml and increasing concentrations of FLAGΔ169-Jun. Cell survival was assessed 48 h after the cells were refed with medium supplemented with NGF. Coexpression of FLAGΔ169-Jun efficiently blocked the induction of cell death by ASK1 (Fig. 5E).
These results, together with the data that kinase-negative form of ASK1 protected NGF withdrawal-induced death both in PC12 cells and in SCG neurons (Fig. 2), strongly suggest that the death signal induced by NGF withdrawal is mediated at least in part via the ASK1-JNK pathway in neurons.
ASK1 is required for Cdc42-induced death.
Recently, we have shown that NGF withdrawal-induced death requires the activity of the small GTP-binding protein Cdc42 and that overexpression of an active form of Cdc42 is sufficient to mediate neuronal apoptosis via activation of the c-Jun pathway (1). To determine whether ASK1 and Cdc42 lie on the same pathway, we coinjected an activated mutant of Cdc42 (V12Cdc42) and the kinase-inactive mutant ASK1-KR into SCG neurons. The cells were maintained in the presence of NGF, and the percent survival was determined 48 h after injection. Figure 6A shows that blocking ASK1 activity was sufficient to rescue SCG neurons from Cdc42-induced cell death. Furthermore, we investigated the effect of ASK1-KR on the activation of the JNK pathway by V12Cdc42. SCG neurons were injected as described above and stained with a specific anti-phospho–c-Jun antibody 24 h later. Coexpression of ASK1-KR significantly reversed the induction of phosphorylation of c-Jun by an activated mutant of Cdc42. These results suggest that ASK1 acts as a downstream mediator of Cdc42 in SCG neurons.
DISCUSSION
In sympathetic neurons and in differentiated PC12 cells, activation of the c-Jun pathway plays an important role in the induction of apoptosis by NGF deprivation (9, 14, 38). Recently, we showed that the small GTP-binding protein Cdc42 functions as an initiator of neuronal apoptosis via regulation of this pathway (1). In an attempt to identify a mediator of neuronal apoptosis induced by NGF withdrawal that would also lie on the Cdc42-JNK pathway, we examined the role of ASK1. ASK1 is a newly identified MAPKKK and has been reported to activate the JNK pathway and to induce apoptosis in a variety of cell types (4, 18, 27) for the tumor necrosis factor alpha- and Fas-induced JNK pathways (4, 24).
To study its role in NGF withdrawal and Cdc42-induced cell death, we have overexpressed a constitutively active mutant of ASK1 (ASK1-ΔN) in the PC12 cell line and rat SCG neurons. ASK1-ΔN induced a significant decrease in cell viability (Fig. 1), and this death exhibited the characteristics of apoptosis, with the cells displaying pyknotic nuclei, DNA fragmentation, shrinkage, and membrane blebbing (Fig. 1 and data not shown). More importantly, kinase-inactive mutants of ASK1, which act as dominant interfering mutants, significantly blocked NGF withdrawal-induced death (Fig. 2 and 3) demonstrating that ASK1 is a crucial element of neuronal apoptosis induced by neurotrophic factor deprivation.
ASK1 activity peaked at 3 h after NGF withdrawal in differentiated PC12 cells (Fig. 4); this would precede the peak of JNK activity and the increase in c-Jun protein levels. Indeed, JNK activity peaks between 4 and 8 h under the same conditions, at the time when the levels of c-Jun proteins are at their highest (8, 14). Consistent with this, we found that activated ASK1 induced an activation of JNK in PC12 cells (Fig. 5A and B) and an increase in the level of phosphorylated c-Jun and of its transcriptional activity in SCG neurons (Fig. 5C and D). More importantly, we found that a dominant negative mutant of ASK1, ASK1-KR, blocked the activation of JNK and of c-jun induced by NGF deprivation (Fig. 5C and D).
Although SEK1 has been shown to be activated by ASK1, a dominant negative mutant of SEK1, SEK-AL, could not block ASK1-induced death (data not shown) in SCGs. It has been previously shown that SEK-AL could not block NGF withdrawal- or Cdc42-induced death in SCG neurons (reference 8 and data not shown). Although we cannot rule out completely the involvement of SEK1 in neuronal apoptosis, it appears that there must be an additional JNKK activated by Cdc42 or by ASK1. In this regard, a recently identified JNKK termed MKK7 might be the target of ASK1 in SCGs (11, 17, 20, 22, 32, 33). This possibility remains to be confirmed. In addition, the p38 kinase pathway is not activated in SCG neurons after NGF deprivation (8), and therefore we believe that ASK1 is initiating cell death via regulation of the JNK pathway only in these cells. This is confirmed by the fact that a dominant negative mutant of c-Jun, FLAGΔ169, blocked the ASK1-induction of cell death in SCG neurons (Fig. 5E).
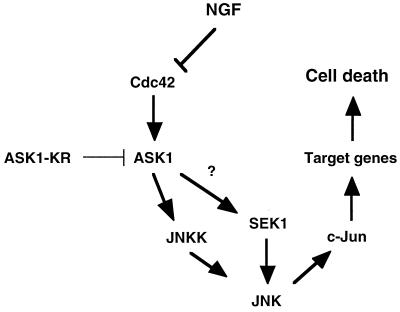
Figure 7 represents our current model of NGF withdrawal-induced cell death-signalling pathways in neurons. Although MEKK1 but not ASK1 is likely to be involved in Cdc42-induced JNK activation in COS-7 cells (2), ASK1 is required for Cdc42 to induce apoptosis in SCG neurons. Once activated by NGF withdrawal, ASK1 may induce neuronal apoptosis through the JNK–c-Jun pathway.
FIG. 7.
Model for the apoptotic signalling pathway mediated by NGF withdrawal. Removal of the survival agent, NGF, activates the small GTPase Cdc42. Activated Cdc42 leads to activation of ASK1 which induces an increase in JNK activity. Presumably, phosphorylation of c-Jun activates its transcriptional activity, which then turn on the transcription of genes necessary for neuronal apoptosis to proceed.
In conclusion, we have identified ASK1 as another crucial element of the signalling mechanism of neuronal cell death induced by neurotrophic factor withdrawal.
ACKNOWLEDGMENTS
We thank M. Kato and D. Goto for providing modified pTet vectors; A. Hall for the Cdc42 plasmid; and M. Fujii, I. Sato, and J. Miyazaki for the adenovirus vectors. We also thank M. Saitoh and H. Nishitoh for valuable discussions.
This work was supported by Grants-in-Aid for scientific Research from the Ministry of Education, Science and Culture of Japan, and grants provided by the Mochida Memorial Foundation for Medical and Pharmaceutical Research and the Ichiro Kanehara Foundation.
T.K., M.M., and K.T. contributed equally to this work.
REFERENCES
- 1.Bazenet C E, Mota M, Rubin L L. The small GTP-binding protein Cdc42 is required for nerve growth factor withdrawal-induced neuronal death. Proc Natl Acad Sci USA. 1998;95:3984–3989. doi: 10.1073/pnas.95.7.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berestetskaya Y V, Faure M P, Ichijo H, Voyno-Yasenetskaya T A. Regulation of apoptosis by alpha-subunits of G12 and G13 proteins via apoptosis signal-regulating kinase-1. J Biol Chem. 1998;273:27816–27823. doi: 10.1074/jbc.273.43.27816. [DOI] [PubMed] [Google Scholar]
- 3.Brown J L, Stowers L B, Trejo J A, Coughlin S, Chant J. Human Ste20 homologue hPAK1 links GTPases to the JNK MAP kinase pathway. Curr Biol. 1996;6:598–605. doi: 10.1016/s0960-9822(02)00546-8. [DOI] [PubMed] [Google Scholar]
- 4.Chang H Y, Nishitoh H, Yang X, Ichijo H, Baltimore D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science. 1998;281:1860–1863. doi: 10.1126/science.281.5384.1860. [DOI] [PubMed] [Google Scholar]
- 5.Clarke P G. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol. 1990;181:195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- 6.Cuenda A, Cohen P, Buée-Scherrer V, Goedert M. Activation of stress-activated protein kinase-3 (SAPK3) by cytokines and cellular stresses is mediated via SAPKK3 (MKK6); comparison of the specificities of SAPK3 and SAPK2 (RK/p38) EMBO J. 1997;16:295–305. doi: 10.1093/emboj/16.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duvall E, Wyllie A H. Death and the cell. Immunol Today. 1986;7:115–119. doi: 10.1016/0167-5699(86)90152-0. [DOI] [PubMed] [Google Scholar]
- 8.Eilers A, Whitfield J, Babij C, Rubin L L, Ham J. Role of the Jun kinase pathway in the regulation of c-Jun expression and apoptosis in sympathetic neurons. J Neurosci. 1998;18:1713–1724. doi: 10.1523/JNEUROSCI.18-05-01713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estus S, Zaks W J, Freeman R S, Gruda M, Bravo R, Johnson E M J. Altered gene expression in neurons during programmed cell death: identification of c-jun as necessary for neuronal apoptosis. J Cell Biol. 1994;127:1717–1727. doi: 10.1083/jcb.127.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanger G R, Johnson N L, Johnson G L. MEK kinases are regulated by EGF and selectively interact with Rac/Cdc42. EMBO J. 1997;16:4961–4972. doi: 10.1093/emboj/16.16.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foltz I N, Gerl R E, Wieler J S, Luckach M, Salmon R A, Schrader J W. Human mitogen-activated protein kinase kinase 7 (MKK7) is a highly conserved c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) activated by environmental stresses and physiological stimuli. J Biol Chem. 1998;273:9344–9351. doi: 10.1074/jbc.273.15.9344. [DOI] [PubMed] [Google Scholar]
- 12.Frost J A, Xu S, Hutchinson M R, Marcus S, Cobb M H. Actions of Rho family small G proteins and p21-activated protein kinases on mitogen-activated protein kinase family members. Mol Cell Biol. 1996;16:3707–3713. doi: 10.1128/mcb.16.7.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerwins P, Blank J, Johnson G L. Cloning of a novel mitogen-activated protein kinase kinase kinase, MEKK4, that selectively regulates the c-jun amino terminal kinase pathway. J Biol Chem. 1997;272:8288–8295. doi: 10.1074/jbc.272.13.8288. [DOI] [PubMed] [Google Scholar]
- 14.Ham J, Babij C, Whitfield J, Pfarr C M, Lallemand D, Yaniv M, Rubin L L. A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron. 1995;14:927–939. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- 15.Henderson C E. Programmed cell death in the developing nervous system. Neuron. 1996;17:579–585. doi: 10.1016/s0896-6273(00)80191-9. [DOI] [PubMed] [Google Scholar]
- 16.Herdegen T, Claret F X, Kallunki T, Martin-Villalba A, Winter C, Hunter T, Karin M. Lasting N-terminal phosphorylation of c-Jun and activation of c-Jun N-terminal kinases after neuronal injury. J Neurosci. 1998;18:5124–5135. doi: 10.1523/JNEUROSCI.18-14-05124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holland P M, Suzanne M, Campbell J S, Noselli S, Cooper J A. MKK7 is a stress-activated mitogen-activated protein kinase kinase functionally related to hemipterous. J Biol Chem. 1997;272:24994–24998. doi: 10.1074/jbc.272.40.24994. [DOI] [PubMed] [Google Scholar]
- 18.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 19.Linnik M D. Role of apoptosis in acute neurodegenerative disorders. Restor Neurol Neurosci. 1996;9:219–225. doi: 10.3233/RNN-1996-9404. [DOI] [PubMed] [Google Scholar]
- 20.Lu X, Nemoto S, Lin A. Identification of c-Jun NH2-terminal protein kinase (JNK)-activating kinase 2 as an activator of JNK but not p38. J Biol Chem. 1997;272:24751–24754. doi: 10.1074/jbc.272.40.24751. [DOI] [PubMed] [Google Scholar]
- 21.Maroney A C, Glicksman M A, Basma A N, Walton K M, Knight E, Jr, Murphy C A, Bartlett B A, Finn J P, Angeles T, Matsuda Y, Neff N T, Dionne C A. Motoneuron apoptosis is blocked by CEP-1347 (KT 7515), a novel inhibitor of the JNK signaling pathway. J Neurosci. 1998;18:104–111. doi: 10.1523/JNEUROSCI.18-01-00104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moriguchi T, Toyoshima F, Masuyama N, Hanafusa H, Gotoh Y, Nishida E. A novel SAPK/JNK kinase, MKK7, stimulated by TNFalpha and cellular stresses. EMBO J. 1997;16:7045–7053. doi: 10.1093/emboj/16.23.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagata K-I, Puls A, Futter C, Apenstrom P, Schaefer E, Nakata T, Hirokawa H, Hall A. The MAP kinase kinase kinase MLK2 co-localizes with activated JNK along microtubules and associates with kinesin superfamily motor KIF3. EMBO J. 1998;17:149–158. doi: 10.1093/emboj/17.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishitoh H, Saitoh M, Mochida Y, Takeda K, Nakano H, Rothe M, Miyazono K, Ichijo H. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol Cell. 1998;2:389–395. doi: 10.1016/s1097-2765(00)80283-x. [DOI] [PubMed] [Google Scholar]
- 25.Oppenheim R W. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 26.Pettmann B, Henderson C E. Neuronal cell death. Neuron. 1998;20:633–647. doi: 10.1016/s0896-6273(00)81004-1. [DOI] [PubMed] [Google Scholar]
- 27.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz L M, Osborne B A. Programmed cell death, apoptosis and killer genes. Immunol Today. 1993;14:582–590. doi: 10.1016/0167-5699(93)90197-S. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz L M, Smith S W, Jones M E, Osborne B A. Do all programmed cell deaths occur via apoptosis? Proc Natl Acad Sci USA. 1993;90:980–984. doi: 10.1073/pnas.90.3.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tapon N, Nagata K-I, Lamarche N, Hall A. A new Rac target POSH is an SH3-containing scaffold protein involved in the JNK and NF-kB signalling pathways. EMBO J. 1998;17:1395–1404. doi: 10.1093/emboj/17.5.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tibbles L A, Ing Y-L, Kiefer F, Chan J, Iscove N, Woodgett J R, Lassam N J. MLK-3 activates the SAPK/JNK and p38/RK pathways via SEK1 and MKK3/6. EMBO J. 1996;15:7026–7035. [PMC free article] [PubMed] [Google Scholar]
- 32.Tournier C, Whitmarsh A J, Cavanagh J, Barrett T, Davis R J. Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase. Proc Natl Acad Sci USA. 1997;94:7337–7342. doi: 10.1073/pnas.94.14.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tournier C, Whitmarsh A J, Cavanagh J, Barrett T, Davis R J. The MKK7 gene encodes a group of c-Jun NH2-terminal kinase kinases. Mol Cell Biol. 1999;19:1569–1581. doi: 10.1128/mcb.19.2.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van-Dam H, Wilhem D, Herr I, Steffen A, Herlich P, Angel P. ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotoxic agents. EMBO J. 1995;14:1798–1811. doi: 10.1002/j.1460-2075.1995.tb07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang T-H, Wang H-S, Ichijo H, Giannakakou P, Foster J S, Fojo T, Wimalasena J. Microtuble-interfering agents activate c-Jun N-terminal kinase/stress-activated kinase (JNK/SAPK) through both ras and apoptosis signal-regulating kinase (ASK1) pathways. J Biol Chem. 1998;273:4928–4936. doi: 10.1074/jbc.273.9.4928. [DOI] [PubMed] [Google Scholar]
- 36.Wang X S, Diener K, Jannuzzi D, Trollinger D, Tan T H, Lichenstein H, Zukowski M, Yao Z. Molecular cloning and characterization of a novel protein kinase with a catalytic domain homologous to mitogen-activated protein kinase kinase kinase. J Biol Chem. 1996;271:31607–31611. doi: 10.1074/jbc.271.49.31607. [DOI] [PubMed] [Google Scholar]
- 37.Watson A, Eilers A, Lallemand D, Kyriakis J, Rubin L L, Ham J. Phosphorylation of c-Jun is necessary for apoptosis induced by survival signmal withdrawal in cerebellar granule neurons. J Neurosci. 1998;18:751–762. doi: 10.1523/JNEUROSCI.18-02-00751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]