Abstract
Background
Patient‐reported outcomes measures (PROMs) assess a patient’s subjective appraisal of health outcomes from their own perspective. Despite hypothesised benefits that feedback on PROMs can support decision‐making in clinical practice and improve outcomes, there is uncertainty surrounding the effectiveness of PROMs feedback.
Objectives
To assess the effects of PROMs feedback to patients, or healthcare workers, or both on patient‐reported health outcomes and processes of care.
Search methods
We searched MEDLINE, Embase, CENTRAL, two other databases and two clinical trial registries on 5 October 2020. We searched grey literature and consulted experts in the field.
Selection criteria
Two review authors independently screened and selected studies for inclusion. We included randomised trials directly comparing the effects on outcomes and processes of care of PROMs feedback to healthcare professionals and patients, or both with the impact of not providing such information.
Data collection and analysis
Two groups of two authors independently extracted data from the included studies and evaluated study quality. We followed standard methodological procedures expected by Cochrane and EPOC. We used the GRADE approach to assess the certainty of the evidence. We conducted meta‐analyses of the results where possible.
Main results
We identified 116 randomised trials which assessed the effectiveness of PROMs feedback in improving processes or outcomes of care, or both in a broad range of disciplines including psychiatry, primary care, and oncology. Studies were conducted across diverse ambulatory primary and secondary care settings in North America, Europe and Australasia. A total of 49,785 patients were included across all the studies.
The certainty of the evidence varied between very low and moderate. Many of the studies included in the review were at risk of performance and detection bias.
The evidence suggests moderate certainty that PROMs feedback probably improves quality of life (standardised mean difference (SMD) 0.15, 95% confidence interval (CI) 0.05 to 0.26; 11 studies; 2687 participants), and leads to an increase in patient‐physician communication (SMD 0.36, 95% CI 0.21 to 0.52; 5 studies; 658 participants), diagnosis and notation (risk ratio (RR) 1.73, 95% CI 1.44 to 2.08; 21 studies; 7223 participants), and disease control (RR 1.25, 95% CI 1.10 to 1.41; 14 studies; 2806 participants). The intervention probably makes little or no difference for general health perceptions (SMD 0.04, 95% CI ‐0.17 to 0.24; 2 studies, 552 participants; low‐certainty evidence), social functioning (SMD 0.02, 95% CI ‐0.06 to 0.09; 15 studies; 2632 participants; moderate‐certainty evidence), and pain (SMD 0.00, 95% CI ‐0.09 to 0.08; 9 studies; 2386 participants; moderate‐certainty evidence). We are uncertain about the effect of PROMs feedback on physical functioning (14 studies; 2788 participants) and mental functioning (34 studies; 7782 participants), as well as fatigue (4 studies; 741 participants), as the certainty of the evidence was very low. We did not find studies reporting on adverse effects defined as distress following or related to PROM completion.
Authors' conclusions
PROM feedback probably produces moderate improvements in communication between healthcare professionals and patients as well as in diagnosis and notation, and disease control, and small improvements to quality of life. Our confidence in the effects is limited by the risk of bias, heterogeneity and small number of trials conducted to assess outcomes of interest. It is unclear whether many of these improvements are clinically meaningful or sustainable in the long term. There is a need for more high‐quality studies in this area, particularly studies which employ cluster designs and utilise techniques to maintain allocation concealment.
Plain language summary
Using patient questionnaires for improving clinical management and outcomes
What is the aim of this review?
The aim of this Cochrane Review was to find out whether healthcare workers who receive information from questionnaires completed by their patients give better health care and whether their patients have better health. We collected and analysed all relevant studies.
Key messages
Patient questionnaire responses fed back to health workers and patients may result in moderate benefits for patient‐provider communication and small benefits for patients' quality of life. Healthcare workers probably make and record more diagnoses and take more notes. The intervention probably makes little or no difference for patient's general perceptions of their health, social functioning, and pain. There appears to be no impact on physical and mental functioning, and fatigue. Our confidence in these results is limited by the quality and number of included studies for each outcome.
What was studied in the review?
When receiving health care, patients are not always asked about how they feel, either about their physical, mental or social health. This can be a problem as knowing how the patient is feeling might help to make decisions about diagnosis and the course of the treatment. One possible solution is to ask the patients to complete questionnaires about their health, and then give that information to the healthcare workers and to patients.
What are the main results of the review?
We found 116 studies (49,785 participants), all of which were from high‐income countries. We found that feeding back patient questionnaire responses to healthcare workers and patients probably slightly improves quality of life and increases communication between patients and their doctors, but probably does not make a lot of difference to social functioning. We are not sure of the impact on physical and mental functioning or fatigue of feeding back patient questionnaire responses as the certainty of this evidence was assessed as very low. The intervention probably increases diagnosis and note‐taking. We did not find studies reporting on adverse effects defined as distress following or related to Patient‐reported outcomes measures (PROM) completion.
How up‐to‐date is this review?
The review authors searched for studies that had been published up to October 2020.
Summary of findings
Summary of findings 1. PROM feedback compared to usual care for improve processes and outcomes of care.
| PROM feedback compared to usual care for improve processes and outcomes of care | ||||||
| Patient or population: ambulatory adult patients. Setting: primary and secondary care settings in North America and Europe. Intervention: PROM feedback reported to physicians or both patients and physicians. Comparison: usual care. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with PROM feedback | |||||
| Quality of life | SMD 0.15
(0.05 to 0.26) favouring PROM feedback vs usual care |
‐ | 2687 (11 randomised trials) | ⊝⊕⊕⊕
Moderate 1 |
PROM feedback probably slightly improves quality of life. Quality of life was assessed using the EuroQoL‐5D (EQ‐5D) KIDSCREEN‐10, Manchester Short Assessment for Quality of Life (MSAQ) , Short Form‐36 (SF‐36), and the Functional Assessment of Cancer Therapy (FACT) PROMs. Three additional studies also measured overall quality of life; one favoured the intervention and for the other two there was little or no difference between groups. |
|
| General health perceptions | SMD 0.04
(‐0.17 lower to 0.24) indicating little or no difference between PROM feedback and usual care. |
‐ | 552 (2 randomised trials) | ⊕⊕⊝⊝ Low 1, 2 | PROM feedback may make little or no difference to general health perceptions. |
|
| Functioning | Physical functioning | |||||
| SMD ‐0.10 (‐0.30 to 0.10) indicating little or no difference between PROM feedback and usual care. | ‐ | 2788 (14 randomised trials) | ⊕⊝⊝⊝ Very low 3, 4 | The evidence is very uncertain about the effect of PROM feedback on physical functioning. Physical functioning was assessed using the physical functioning subscales of the Short Form‐12 (SF‐12), Short form‐36 (SF‐36) Patient‐Physican Communication on HRQOL, European Organization for Research and Treatment of Cancer (EORTC‐QLQ‐30) physical functioning, KIDSCREEN‐10, Functional Living Index ‐ Cancer (FLIC) PROMs. |
||
| Mental functioning | ||||||
| SMD 0.16 (0.06 to 0.27) favouring PROM feedback vs usual care | ‐ | 7782 (34 randomised trials) | ⊕⊝⊝⊝ Very low 1, 4 | The evidence is very uncertain about the effect of PROM feedback on mental functioning. Mental functioning was assessed using the Outcomes Questionnaire ‐ 45 (OQ‐45), the Outcomes Rating Scale (ORS), General Health Questionnaire (GHQ), Short Form ‐ 12 (SF‐12), Patient‐physician communication on HRQOL, European Organization for Research and Treatment of Cancer (EORTC‐QLQ‐30) mental functioning, World Health Organization ‐ 5 (WHO‐5), Beth Isreal‐UCLA Functional Status, Functional Living Index ‐ Cancer (FLIC) PROMs. Six other studies also reported mental functioning, for five studies there was little or no difference between groups and for the sixth study it was not possible to ascertain the direction of the effect. |
||
| Social functioning | ||||||
| SMD 0.02 (‐0.06 to 0.09) indicating little or no difference between PROM feedback and usual care. | ‐ | 2632 (15 randomised trials) | ⊕⊕⊕⊝ Moderate 1 | PROM feedback probably makes little or no difference to social functioning. Social functioning was assessed using the Community‐Oriented Programs Environment Scale (COPES), the Functional Assessment of Cancer Therapy (FACT), Work and Social Adjustment Scale (WSAS), Short Form‐12 (SF‐12), Short Form‐36 (SF‐36), KIDSCREEN‐27, Beth Isreal‐UCLA Functional Status, Functional Living Index ‐ Cancer (FLIC) PROMs. One study also reported social functioning, finding little or no difference between groups. |
||
| Symptoms | Pain | |||||
| SMD ‐0.00 (‐0.09 to 0.08) indicating little or no difference between PROM feedback and usual care. | ‐ | 2386 (9 randomised trials) | ⊕⊕⊕⊝ Moderate 1 | PROM feedback probably makes little or no difference for pain. Pain was assessed using the Short‐Form 36 (SF‐36), European Organization for Research and Treatment of Cancer (EORTC‐QLQ‐30) pain module, Symptom Monitor, and the Roland‐Morris Disability Questionnaire |
||
| Fatigue | ||||||
| SMD 0.03 (‐0.29 to 0.36) indicating little or no difference between PROM feedback and usual care. | ‐ | 741 (4 randomised trials) | ⊕⊝⊝⊝ Very low 1, 2, 4 | The evidence is very uncertain about the effect of PROM feedback on fatigue. Fatigue was assessed using the Chronic Heart Failure Questionnaire, Symptom Monitor, and the European Organization for Research and Treatment of Cancer (EORTC‐QLQ‐30) fatigue module. |
||
| Patient‐physician communication | SMD 0.36 (0.21 to 0.52) favouring PROM feedback vs usual care | ‐ | 658 (5 randomised trials) | ⊕⊕⊕⊝ Moderate 1 | PROM feedback probably increases patient‐physician communication. Communcation was assessed using patient‐physician communication on HRQOL, Consumer Assessment of Healthcare Providers and Systems Clinician and Group Survey (CAHPS) PROM, number of topics discussed. One study not included in the pooled analysis indicated that participants allocated to the intervention rated communication with their physician better than those allocated to usual care. |
|
| Diagnosis and notation | Study population | RR 1.73 (1.44 to 2.08) | 7223 (21 randomised trials) | ⊕⊕⊕⊝ Moderate 4 | PROM feedback probably increases diagnosis and notation. Diagnosis and notation was assessed using chart review. |
|
| 172 per 1,000 | 347 per 1,000 (278 to 423) | |||||
| Disease control | Study population | RR 1.25 (1.10 to 1.41) | 2806 (14 randomised trials) | ⊕⊕⊕⊝ Moderate1 | PROM feedback probably leads to an increase in disease control. Disease control was assessed using both PROMs and chart‐based assessments including Partners for Change Outcome Measurement System (PRCOMS), Outcomes Questionnaire ‐ 45 (OQ‐45), Outcomes Rating Scale (ORS), Primary Care Screener for Affective Disorders, Cutting down; Annoyance by criticism, Guilty feeling, and Eye‐openers (CAGE) questionnaire; New York Heart Association class, Geriatric Depression Scale (GDS), Beck Depression Inventory (BDI), and Diagnostic and Statistical Manual (DSM; depression symptoms >= 1). |
|
| 300 per 1,000 | 400 per 1,000 (345 to 458) | |||||
| Adverse effects | ‐‐ | ‐‐ | ‐‐ | ‐‐ | ‐‐ | We did not find studies reporting on adverse effects. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio;SMD: Standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 We downgraded one point for risk of unblinding due to the nature of the intervention in the majority of studies.
2 We downgraded one point for imprecision due to the small number of studies with wide confidence intervals included in meta‐analysis.
3 We downgraded one point for high risk of bias in multiple studies.
4 We downgraded one point for inconsistency due to statistical heterogeneity.
Background
Description of the condition
Definition of patient‐reported outcome measures
Patient‐reported outcomes measures (PROMs) assess patients' subjective appraisal of outcomes from their own perspective (Valderas 2008b). PROMs feedback offer complementary information to the objective measurements usually collected (Porter 2016).
Historically, the use of PROM information has been far less common in clinical practice than in research, where PROMs are often selected as outcome measures in clinical trials (FDA 2009;Fitzpatrick 1998; Nelson 2015; Valderas 2008c). At an individual level and within the clinician‐patient interface, PROMs have been used for screening and monitoring a condition, such as depression symptoms; for monitoring the progress of the patient during the course of treatment or throughout time; and for promoting patient‐centred care, by explicitly assessing the patient’s perspective (Basch 2016; Greenhalgh 2009).
Description of the intervention
Patient reported outcomes (PRO) have been defined as assessments of any aspect of a patient’s health status which are provided directly by the patient (FDA 2009; Valderas 2008b), usually through a questionnaire scale referred to as PROMs. Patient‐reported outcome is an umbrella term: it can be applied to an array of different outcomes, including symptoms, functioning, perceived health status and health‐related quality of life (Black 2013; McKenna 2011).
PROMs that measure aspects of health which are relevant to all people are referred to as generic. One such example is the Short Form 36, which assesses, alongside specific symptoms, physical functioning and psychological well‐being, as well as evaluating overall self‐reported health (Garratt 1993; Valderas 2008d). In theory, such generic measures can be used within and between populations, regardless of age, gender, and disease or condition. Concerns regarding the suitability of generic PROMs for patients and groups with specific conditions has led to the development of PROMs with a narrower focus on a single group of patients. (Garratt 2002). So called disease‐specific PROMs are widely available for common conditions such as diabetes (Bradley 1999), to less frequent ones, including amyotrophic lateral sclerosis (Gibbons 2011), and haemophilia (Arranz 2004).
When used in clinical practice at the level of the individual patient level, PROM feedback forms part of a complex intervention which can include a number of different components (Craig 2008). The fundamental components of a PROM intervention is that: a) patients complete one of more questionnaires and b) the results are fed back to the clinician, the patient, or both. The International Society for Quality of Life Research has defined a set of eight considerations which ought to be followed when implementing PROMs in clinical practice; establishing the goals;identifying patients and settings; selecting questionnaires; defining the administration and scoring procedures; reporting results; facilitating score interpretation; establishing protocols to address issues raised by the questionnaires; and assessing the eventual impact of the questionnaire in clinical practice (Snyder 2012).
While evidence can be found that these steps have been followed in many PROM feedback interventions, considerable variation is also apparent. For instance, instruments can be self‐completed (Rand 1988) or interviewer‐administered (German 1987); completed in the clinical setting (Christensen 2005) or posted to the patient’s home (Lewis 1996); and supported by an electronic format such as online or tablet administration (Basch 2016; Velikova 2004) or rely on pencil and paper (Trowbridge 1997). As for the feedback, discrepancies might exist between trials as to when the information is given to healthcare professionals, e.g. immediately before the visit (Berry 2011); and how it is given, e.g. printed form (Saitz 2003); and by whom, e.g. available in the notes (Linn 1980). More importantly, considerable differences occur regarding the amount of feedback provided. For example, in some studies the only information fed back to healthcare professionals were the scores each patient obtained in the PROM (Bergus 2005), whereas in other studies professionals were given information on how to apply interpretation guidelines for the scores (Rosenbloom 2007), or treatment guidelines for the conditions detected by the PROM (Saitz 2003). The number of times the patient completes the PROM can also vary considerably, from single responses (Hoeper 1984) to feedback at multiple points (Cleeland 2011; Klinkhammer‐Schalke 2012). Reflecting this, there is also variation in whether the clinician receives the PROM scores immediately or at given intervals (e.g. daily, weekly). Finally, the endpoints used to assess the impact of PROM feedback in clinical practice have also been a source of considerable variation, with trials inconsistently reporting on processes of healthcare (e.g. patient‐clinician communication), outcomes of healthcare (e.g. changes in the number or rate of symptoms or complaints), and patient experience (e.g. overall satisfaction with care).
How the intervention might work
The Feedback Intervention Theory (FIT) posits that behaviour is regulated through comparison with standards or goals, and that feedback can draw attention to existing gaps between current and ideal states (Kluger 1996). In the context of PROM feedback interventions, PROM scores are being presented to either patients or clinicians to highlight specific issues and, in some cases, are presented alongside information designed to help to address the highlighted issues (Greenhalgh 2017). For example, If a patient scores above the established cut‐off point in a depression screening PROM, then the healthcare professional will be made aware of this discrepancy between the desired state of psychological well‐being and the current distress experienced by the patient. In this case, the PROM feedback and the desired outcome may be measured by the same PROM. Other interventions may utilise PROM feedback to improve other outcomes, such as an intervention to feedback information relating to symptoms of cancer and its treatments with the goal of reducing emergency room visits. Whether the same PROMs are used to provide feedback and measure outcomes or not, FIT further postulates that once the gap has been identified, different methods can be followed in order to decrease this gap and attain the standard, including increasing the effort currently being made(Kluger 1996).
Feedback to patients and clinicians could be expected to modify a number of behaviours (Greenhalgh 2017; Greenhalgh 2018; Porter 2016). Feedback to clinicians could be substantiated by the professional using several strategies, including providing advice, referring to other services, or altering the patient's medication plan. All of these are processes of care that would, potentially, trigger improvements in outcomes, such as improved functioning and increased health‐related quality of life. Feedback given directly to patients could result in additional care being sought or implementing self‐management solutions relating to the PROM scores. However, whether these outcomes do materialise depends on a range of other contextual factors including the patient or clinician's willingness or ability to act on the provided feedback as well the patient’s acceptance of, and adherence to, any treatment changes and the effectiveness of that treatment.
Why it is important to do this review
In the UK, PROMs are one of the cornerstones of National Health Service reform for the transition towards a patient outcomes‐oriented performance model (Black 2016; Calvert 2019; Valderas 2012). In the USA, initiatives such as the Patient Reported Outcomes Measurement Information System (Alonso 2013; PROMIS 2007), funded by the National Institutes of Health, or the inclusion of PROMs in electronic health record software, such as EpicCare (EpicCare 2015) held by Group Health Cooperative, highlight the progressive relevance these outcome measures play in healthcare contexts. The US Department of Health and Human Services also plans to incorporate PROMs into meaningful use standards, which is likely to prompt more widespread use (Hostetter 2011).
The level of evidence for the impact of assessing outcome using PROM feedback in clinical practice has been mixed (Espallargues 2000; Gilbody 2001; Greenhalgh 1999; Marshall 2006; Valderas 2008a). Valderas 2008a found that there was more evidence for impact upon the processes rather than the outcomes of care. Specifically, there was an increase for the rate of diagnoses and chart notations for the conditions targeted by the interventions (e.g. diagnosis of depression in primary care). Similarly, there was also a positive effect on the advice and education provided by the healthcare professionals. Furthermore, Valderas 2008a identified a total of 36 endpoints for the 28 randomised trials included in their systematic review, which seems to reiterate the lack of consensus amongst researchers of how the intervention should work and thus what constitutes a relevant indicator when using PROMs in clinical practice.
Notwithstanding the potential benefits for clinical practice, several objections have been raised in relation to their routine use. Healthcare professionals have expressed doubts about the clinical utility of PROM feedback, as they consider that little value is added to their clinical judgement (Leydon 2011; Taylor 1996). Healthcare professionals have also described how burdensome the use of PROMs can be, as it requires time to administer the measures and time to learn how to analyse and interpret the results (Brown 2006) and also to integrate them into clinical practice in an efficient and non‐disruptive manner (Nelson 1990). Clinicians have voiced concerns that the PROMs might represent a threat to the holistic nature of the patient‐doctor relationship (Leydon 2011). It has also been suggested that PROMs increase the healthcare professional’s responsibility and burden of care, as they might detect problems that could otherwise go unnoticed (Tavabie 2009). Finally, the use of PROMs has been increasingly advocated for guiding the provision of care for people with multiple chronic conditionsValderas 2009; Smith 2021; Valderas 2019.
Taking both the potential benefits and risks and the current health policy initiatives into account, it becomes essential to ascertain to what extent the use of PROMs in clinical practice does actually improve processes and outcomes of care. Previous reviews have provided mixed evidence and a number of relevant studies have been subsequently published (Valderas 2010).
Objectives
To assess the effects of Patient‐reported outcomes measures (PROMs) feedback to patients, healthcare workers, or both on patient‐reported health outcomes and processes of care.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials and cluster‐randomised trials, where individuals (healthcare professionals or patients) or groups of individuals (including whole hospitals or practices) were randomly allocated to either a control or an intervention group. We did not include studies that follow a non‐randomised design, such as before‐after studies and interrupted time series. The protocol of this review is available on the Cochrane LibraryGonçalves‐Bradley 2015.
Types of participants
We only included studies where participants have been recruited in primary (e.g. health practitioner’s office) or secondary/tertiary (e.g. hospital) care settings in order to ensure that interventions were delivered as part of clinical care. We excluded studies conducted outside primary and secondary/tertiary healthcare settings (e.g. assisted living facilities) in order to ensure that PROM feedback was used for clinical purposes only. There were no age or gender restrictions, nor restrictions based on the presence or absence of any specific disease.
Types of interventions
We only included studies if they reported a replicable intervention, where standardised or individualised PROMs were administered to patients and the resulting information on each individual patient was subsequently fed back to healthcare providers or patients, or both. Patient‐reported outcome measures were defined as the assessment of any aspect of a patient’s health status which is provided directly by the patient (FDA 2009), usually through a questionnaire or scale. PROMs could be used for a number of different outcomes, including measurements of health status, quality of life, symptoms and functioning (McKenna 2011). A replicable intervention was defined as one where details of the content and timing of the assessment and feedback provision were clearly described. We included studies regardless of whether feedback was provided to patients only or to healthcare providers only, or to both. We included studies irrespective of whether the results were fed back along with guidelines regarding their optimal use, or other educational strategies. We included studies if they were conducted either during a specific procedure, for instance a surgical procedure; or during routine care, for example a primary‐care appointment. The comparison (control) condition consisted of routine clinical practice without the feedback of any information to the healthcare professionals.
When multiple control arms were included, we selected as control the arm that most closely reproduced standard care. For intervention arms, we selected the arm that included the least additional components (other than PROMs were fed‐back)Cochrane Handbook 5.1.0, Section 16.5.4.
Types of outcome measures
Our primary outcomes included generic or disease‐specific patient‐reported outcomes such as health‐related quality of life and functioning. Secondary outcome measures were considered to assess processes of care.
The intervention is hypothesised to increase the awareness of those receiving feedback of health problems as perceived and reported by patients. Since the additionally available information on health problems that is fed back is patient‐reported, the main benefit of the intervention can be anticipated to be on health status as appraised by patient themselves (Greenhalgh 2017; Greenhalgh 2018; Porter 2016; Porter 2021). In addition, increased awareness of existing health problems can also have the negative effect of creating anxiety and distress on patients (Porter 2016; Valderas 2012).
Awareness of a health problem can potentially impact on a cascade of effects on processes of health care involving the appraisal of the severity of problem and consideration of whether it meets diagnostic criteria for a specific condition, proposing, implementing and monitoring a management. In the case of the patient as a recipient of the information, increased awareness may also trigger self‐management activities, activation and concordance with the agreed management plan (Greenhalgh 2017; Greenhalgh 2018;Porter 2016; Porter 2021).
Primary outcomes
Our primary outcomes were:
patient‐reported outcomes: quality of life, general health perceptions, functioning, and symptoms, such as nausea, fatigue, and mental health‐related symptoms;
adverse effects: distress following or related to PROM completion.
Secondary outcomes
For the processes of health care, we considered the following endpoints:
patient‐physician communication (e.g. patients' ratings of the quality of the communication);
diagnosis and recognition (e.g. number of target diagnoses made);
treatment (e.g. changes to treatment);
health services and resource use (e.g. referral to specialist or social care);
patient behaviour (e.g. compliance with treatment);
patient empowerment (e.g. measured using available self‐reported instruments); and
healthcare professionals’ awareness of patients' quality of life.
Other outcomes included: patients' experiences (e.g. overall satisfaction with care) and healthcare professionals' perceptions (e.g. attitude and overall satisfaction with intervention); consultation length; healthcare costs.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Database of Systematic Reviews (CDSR; to 2018, Issue 9) and the Database of Abstracts of Reviews of Effects (DARE; to 2015, Issue 2) for primary studies in related systematic reviews. We searched the following databases on 5 October 2020:
MEDLINE Ovid (including in‐process and other non‐indexed citations; 1946 onwards)
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 10) in the Cochrane Library
Embase Ovid (1974 onwards)
PsycINFO Ovid (1806 onwards)
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1980 onwards)
The EPOC Cochrane Information Specialist (CIS) developed the search strategies in consultation with the authors. Search strategies are comprised of natural language and controlled vocabulary terms. We applied no language or date limits. All search strategies used are provided in Appendix 1.
Searching other resources
Trial Registries
We searched the following trials registries on 5 October 2020:
International Clinical Trials Registry Platform (ICTRP), Word Health Organization (WHO) www.who.int/ictrp/en/;
ClinicalTrials.gov, US National Institutes of Health (NIH) clinicaltrials.gov/.
We also conducted the following measures:
screened previously published reviews for potentially‐relevant references;
contacted authors of the included studies to request information about ongoing studies.
Data collection and analysis
Selection of studies
Two review authors independently assessed each reference in title and abstract form to ascertain whether they met the eligibility criteria. We piloted the eligibility criteria against a random sample of approximately 1% of all the documents received, after which two review authors independently screened all the references. Because we were aiming for maximum sensitivity at this stage, we included all references assessed as relevant by at least one team member, and only excluded references unanimously assessed as irrelevant.
We followed the same strategy for the full‐text documents selected for inclusion in the review. We conducted a sensitivity strategy with a random sample of approximately 1% of the records. As at this stage in order for maximum specificity to be achieved, we discussed disagreements between team members until consensus was reached, and we only include references rated as relevant by all the review authors. We involved a third review author where consensus was not achieved. Whenever pertinent and possible, we contacted authors for the documents that received a discrepant rating, in order to clarify any queries. We documented the selection process using a PRISMA flow diagram and described all the studies that fulfil the inclusion criteria in the Characteristics of included studies table.
Data extraction and management
We independently saved all the retrieved results to a bibliographic database using reference management software (Reuters 2011). We saved all the results and removed any duplicates. Two review authors independently extracted data from the studies assessed as relevant during the stage of study, and we resolved any disagreements through discussion. We designed the data extraction form according to aspects considered to be relevant for the present systematic review, including those suggested by the Cochrane Effective Practice and Organisation of Care Group (EPOC 2014), and covered the following domains.
a) Study features: clinical setting (type of setting, academic status, and country); method of randomisation (including allocation concealment and blinding); unit of randomisation and analysis (patient/healthcare professional or practice/hospital); number of arms.
b) Participants' features: inclusion and exclusion criteria; patients' characteristics (socio‐demographic information using the PROGRESS framework; health condition; and whether new or known to the healthcare professional); healthcare professionals' characteristics (profession; level of training; and previous experiences with PROM feedback).
c) Intervention features: design, which were either: single simple feedback (one PROM at a single time); multiple simple feedback (one PROM at multiple times); single complex feedback (multiple PROMs at a single time); multiple complex feedback (multiple PROMs at multiple times); and how PROMs were used (which may be for the intervention or for assessing outcomes, or both); constructs measured; PROM categories/domains.
d) Administration features: method for data collection (self‐reported; interviewer; other); support used (pencil and paper; computer‐assisted; other); setting of data collection (home; clinical; other); facilitator (no facilitator; clinical facilitator; research facilitator; other); other relevant administration‐related characteristics.
e) Feedback: timing (associated with visits or not; scores given before appointments, during or other); amount of information provided (last score; previous scores; application of interpretation guidelines; application of treatment guidelines; other); support used (printed form; computer‐assisted; other); method for feeding back the information (handed by patients; handed by research staff; available in notes; other).
f) Description of the intervention: narrative description as provided by authors.
g) Results: results as provided by authors, both for processes and outcomes of care.
h) Other features: study identifier; source of funding; ethical approval; sample size calculation; prospectively‐identified barriers to change; methodological quality.
Complex health interventions pose specific challenges to assessment (Craig 2008); and data synthesis (Shepperd 2009). Specific recommendations on how to overcome these limitations have now been suggested, including identifying key components of the interventions and categorising them according to those components (Shepperd 2009). Hence, when extracting data we also categorised the identified interventions according to their main components.
Given the heterogeneity of outcomes in this review, we handled the outcome results in a two‐stage approach. In the first stage, we carried out the following measures.
1) Collated data according to the headings outlined in the Types of outcome measures section.
2) Extracted the appropriate data for each arm according to the principle of intention‐to‐treat (i.e. according to the original random allocation). For dichotomous data: number of patients experiencing outcome/total patient number. For continuous data: total patient number, outcome mean and standard deviation (SD). We sought continuous data reported as mean and SD for change in outcome from baseline (adjusted for baseline score); and, where not available, mean absolute outcome and SD at follow‐up was recorded. For other outcome types (e.g. event rate, time to event) we extracted data appropriately.
3) Extracted outcome data for all follow‐up points.
4) Extracted outcome data by subgroups according to the characteristics of the intervention (straight feedback of the results to the healthcare professional; or feedback along with guidelines regarding how to interpret results or other educational strategies); and patient characteristics (educational level). When required and feasible, data were transformed in order to standardise outcomes, for instance for differences in the direction of the scales.
We piloted the data extraction form with a small sample of articles. The sample was purposively selected to ensure heterogeneity in terms of type of studies and interventions. All researchers who participated in the data extraction took part in this pilot. Extracted data were stored in an electronic database, which was created using RevMan 5 (RevMan 2012).
Assessment of risk of bias in included studies
We assessed risk of bias based on criteria suggested by Cochrane (Higgins 2011) and additional criteria proposed by EPOC (EPOC 2017c), assessing the following nine domains: random sequence generation; allocation concealment; participants' blinding (either patients or healthcare providers); blinding of outcome assessment; similarity of baseline measurement, both for outcome measures and participants’ characteristics; incomplete outcome data; protection against contamination; selective reporting; and other sources of bias, including whether the used PROMs have been previously validated for the specific setting and population. We classified each parameter as high risk of bias, low risk of bias, or unclear, and obtained information was summarised in tabulated form, using RevMan 5 (RevMan 2012). As a guide, we judged a study as at high risk of bias if more than three of the nine individual items were considered to be high risk. We expressed level of confidence in the evidence for each outcome using the GRADE criteria, by assessing the type of evidence, limitations in study design, indirectness of evidence, unexplained heterogeneity of findings, imprecision of results, and probability of publication bias in accordance with the guidance of Higgins 2011. We assessed publication bias by inspecting funnel plots for all analyses.
Measures of treatment effect
We calculated risk ratios with 95% confidence intervals (CIs) for dichotomous data. Where studies used continuous scales of measurement to assess the effects of the intervention, we used mean differences (MD) with 95% CIs; or, when studies used different scales or measurements, we used the standardised mean difference (SMD). Where studies used other outcome metrics, e.g. rates of events or time to event, we sought the appropriate overall measure of effect, e.g. rate RR, hazard ratio (HR). We used established guidelines to aid interpretation of effect sizes (Cohen 1988), and considered estimates <0.35 to represents a small effect, 0.35 to 0.65 a moderate effect, and d>0.65 a large effect. Similarly, we considered RR estimates to correspond to small (0.66>RR>1.5), moderate (between 033>RR>0.66 or 1.5>RR>3), and large effects (either RR<0.33 or RR>3).
Unit of analysis issues
Where included studies included a cluster design, we contacted the trial authors to obtain an estimate of the intra‐cluster correlation (ICC) where appropriate adjustments for the correlation between participants within clusters had not been made, or imputed it using estimates from the other included trials, or from similar external trials. Where necessary, we inflated the trial standard errors (SEs). We attempted to either reduce the size of trials to its ‘effective sample size’ or recalculate the effects using an approximately correct analysis and using design effect calculated from the ICC (Higgins 2011). Whenever studies included more than one intervention arm, we combined arms to create a single pair‐wise comparison or conducted pair‐wise comparisons by comparing each intervention arm to the control arm (splitting the control arm sample size).
Dealing with missing data
We attempted to obtain any missing information which was necessary to conduct our analyses by contacting the authors of the trials. Missing information included outcome data including estimates of distribution and number of patients included in each analysis.
For dichotomous outcomes, we carried out analyses according to the intention‐to‐treat (ITT) method (Higgins 2011), which includes all participants irrespective of compliance or follow‐up. For the primary analyses, we assumed that participants lost to follow‐up were alive, and had no serious adverse events. For continuous outcomes, we performed available patient analysis and included data only on those for whom results were known (Higgins 2011). Wherever it had not been possible to obtain SDs either from authors or by calculation, we planned for the missing data to be imputed by using SDs from other included trials, specifically trials with a low risk of bias (Furukawa 2006). However, heterogeneity in populations and measures prevented us from doing so in all the relevant cases.
Assessment of heterogeneity
We explored clinical heterogeneity across studies by comparing the population, intervention and control arms. We explored statistical heterogeneity observed in the trials both by visual inspection of a forest plot, and by using a standard Chi² value with a significance level of P = 0.10. We assessed heterogeneity using the I² statistic. An I² estimate greater than 50% was interpreted as evidence of a substantial problem with heterogeneity (Higgins 2011). Where this was the case, we explored reasons for heterogeneity.
Assessment of reporting biases
We did not assess reporting biases through visual inspection of funnel plots.
Data synthesis
We performed data synthesis according to recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using RevMan 5 (RevMan 2012) and STATA v13 (StataCorp 2013). Given the likely heterogeneity of data in this review, we handled the outcome results in a two‐stage approach. In the first stage, we: (1) collated data according to the headings outlined in the Types of outcome measures section; (2) according to outcome, extracted the appropriate data for each arm according to the principle of ITT (i.e. according to the original random allocation): for dichotomous data: number of patients experiencing outcome/total patient number; for continuous data: total patient number, outcome mean and SD. We sought continuous data reported as mean and SD for change in outcome from baseline (adjusted for baseline score) and where not available, we recorded mean absolute outcome and SD at follow‐up. For other outcome types (e.g. event rate, time to event) we extracted data appropriately; (3) we extracted outcome data at all follow‐up points; (4) where reported, we also extracted this outcome data by subgroups according to the characteristics of the intervention (straight feedback of the results to the healthcare professional; feedback along with guidelines regarding how to interpret results or other educational strategies) and patient characteristics (educational level).
In the second stage, based on the quality and consistency of outcome reporting, we decided to synthesise results across studies using either a formal quantitative meta‐analytic approach or a more descriptive approach that focused on summarising the size and direction of treatment effect separately for each individual study. Where sufficient information was provided by the studies included in the review, the potential impact of moderator variables was considered through meta‐regression analysis. When required and feasible, we transformed data in order to homogenise outcomes, for instance for differences in the direction of the scales. We assessed heterogeneity using the I² statistic (Higgins 2003). Due to the expected heterogeneity of the data, we employed random‐effects methods (Deeks 2008). Further specification of the methods for analysis, e.g. MD versus SMD, was tailored to the type of outcome data. When the heterogeneity of studies was found to be substantial, i.e. I² above 50%, we performed a meta‐analysis to quantify the results by calculating effect sizes (EPOC 2014b).
Subgroup analysis and investigation of heterogeneity
We did not hypothesise interactions or effect modifiers in this review, and therefore we did not pre‐specify stratified meta‐analysis or meta‐regression analyses (except for risk of bias — see Sensitivity analysis below). However, where conducted, we extracted data and reported trial‐level subgroup analyses to inform hypothetical models of subgroup analysis for future meta‐analyses.
Sensitivity analysis
We conducted a sensitivity analysis by verifying the impact that the exclusion of certain studies (e.g. those with high overall risk of bias (see definition above), and those with large samples) has on the overall results. We defined a large sample as having more than twice the number of patients than the second largest study in that analysis. Whenever relevant and possible we attempted to contact study authors in order to obtain missing information. Where authors failed to provide missing information, existing data were analysed and the hypothetical impact of the missing data examined as a sensitivity analysis. Finally, we undertook a sensitivity analysis to examine the impact varying the ICC for reanalysis of cluster‐randomised trials.
Summary of findings and assessment of the certainty of the evidence
Four review authors (CSG, IP, ET, JMV) worked in two groups to assess the certainty of the evidence (high, moderate, low, and very low) using the five GRADE considerations: risk of bias, inconsistency, imprecision, indirectness, and publication bias (Guyatt 2008). We used methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2019) and the EPOC worksheets (EPOC 2017), using GRADEpro software (GRADEpro GDT). We resolved disagreements on certainty ratings by discussion and provided justification for decisions to down‐ or upgrade the ratings using footnotes in the table, making comments to aid readers' understanding of the review where necessary. We used plain language statements to report these findings in the review (EPOC 2017b).
We created a summary of findings table with the following outcomes in order to draw conclusions about the certainty of the evidence within the text of the review: quality of life, general health perceptions, functioning (physical, mental, and social), symptoms (pain and fatigue), patient‐physician communication, diagnosis and notation, and adverse effects.
We considered whether there was any additional outcome information that we were not able to incorporate into meta‐analyses, noted this in the footnotes and stated if it supports or contradicts the information from the meta‐analyses. When it was not possible to meta‐analyse the data, we summarised the results in the text and in the comments section of the summary of findings tables.
Results
Description of studies
See Characteristics of included studies for more information.
Results of the search
The electronic searches yielded 30,191 references for screening, with an additional 27 references identified from other sources. After we removed duplicates, we screened 16,653 records against title and abstract. Of these, we excluded directly or indirectly 16,009 records following title and abstract screening (Figure 1). We retrieved full texts for 644 records which two independent reviewers assessed for eligibility. We excluded further 457 records (see Characteristics of excluded studies). We included 116 studies in this review, from 125 records. We identified 51 ongoing studies (see Characteristics of ongoing studies), and there is one study awaiting classification (see Characteristics of studies awaiting classification).
1.
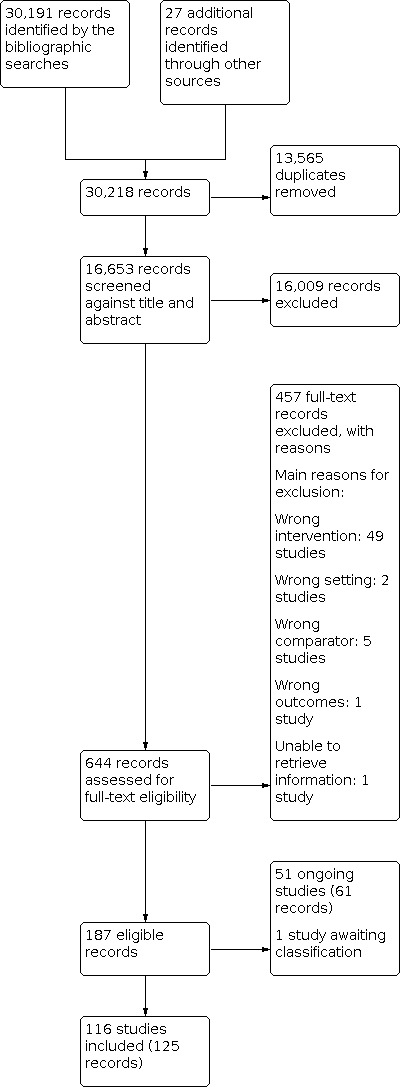
Study flow diagram.
Included studies
One hundred and sixteen studies met our inclusion criteria. The individual studies are described in detail in the Characteristics of included studies table.
Population/Participants
There was a total of 49,785 participants randomised across all the included studies. The number of patients randomised ranged from 30 (Blonigen 2015) to 2284 (Stuck 2015). There was a wide variation in the time to follow‐up between one month and two years. Studies were conducted across a broad range of settings including primary and secondary care clinics in North America and Europe.
Mean age varied between 22 (Lambert 2001) and 80 years (Hadjistavropoulos 2009). Seventy‐three studies recruited a higher percentage of women compared to men, including one study (Wheelock 2015) which recruited 100% women (breast cancer study). Twenty‐seven studies recruited more men than women, including two which had 100% male recruitment, one was a prostate cancer study (Davis 2013), the other too few women were recruited so were excluded from the sample altogether (Magruder‐Habib 1990). Three studies recruited an equal proportion of females and males (Anker 2009; Rand 1988van der Hout 2020). A minority of studies did not report exact or accurate figures for gender, e.g. ‘about two thirds female’ (German 1987) including six which did not report clearly enough to indicate whether more men or women were recruited.
Description of the interventions
Included studies were conducted in high‐income countries including the USA, Canada, Ireland, Spain, the UK, France, the Netherlands, Norway, Denmark, Sweden, Switzerland, Italy, Australia and New Zealand.
All interventions were designed to elicit information from patients using a standardised patient‐reported outcome measure (PROM )and fed that information back to either patients, clinicians, or both. Different types of PROMs as well as administration methods and timings were used. Studies either assessed PROM feedback once at a single visit, multiple times prior to or during scheduled ambulatory visits, or by assessing patients in the community at pre‐specified intervals. 27 studies utilised single simple feedback (one PROM at a single time); 37 studies utilised multiple simple feedback (one PROM at multiple times); 7 studies utilised single complex feedback (multiple PROMs at a single time); 45 studies utilised multiple complex feedback (multiple PROMs at multiple times). The majority of studies (84 studies) utilised a domain or disease‐specific PROM, 24 studies used both and generic and disease‐specific tool, and the remaining 8 studies reported the use of a generic PROM alone.
In total, 58 of the included studies used paper‐based PROMs, 47 studies used electronic administration methods, while 3 studies used a combination of both. The assessment method was unclear in the remaining 8 studies.
Information was most frequently fed‐back to clinicians alone (74 studies). Some studies reported feeding this information back to both patients and clinicians (35 studies). Only three studies fed the information back to patients alone (Gossec 2018; LeBlanc 2019; van der Hout 2020). In the Gossec 2018 study it was at patients’ discretion how many times they recorded information and received feedback, and in turn could share the feedback with clinicians at their instigation, while the LeBlanc 2019 and van der Hout 2020 studies both recommended professional health‐care options based upon symptoms.
Funding sources
Most studies were funded by governmental or academic grants. Some studies were partially or fully funded by pharmaceutical companies (Gilliam 2004; Lugtenberg 2020; Mathias 1994; Mazonson 1996; Moore 2019; Myasoedova 2019; Schriger 2001; Schriger 2005; van Os 2003), a health‐insurance fund (Scheidt 2012), a home‐assistance company (Mathias 1994), a contract research organisation (Gossec 2018), and a digital platform for early detection of disease (Denis 2017). Seventeen studies did not report funding sources.
Excluded studies
We excluded 58 studies (456 reports and present in the Characteristics of excluded studies the 58 studies for which we could not reach immediate consensus, or that readers might expect to see included in the review. The main reason for exclusion was wrong intervention, as PROMs feedback was not part of the intervention (49 studies).
Risk of bias in included studies
For a summary assessment of the risk of bias of the included studies see Figure 2 and Figure 3. Most studies were at high risk of bias for blinding of patients and personnel (performance bias) and blinding of outcomes assessment (detection bias). We did not find any evidence of publication bias in the funnel plots of the studies included in the meta‐analyses except for studies evaluating the impact of the intervention in dyspnoea, anxiety, and disease control, for which there seemed to be a fewer studies than expected with small sample sizes and negative results.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We assessed sequence generation as high risk of bias in 10 studies (Absolom 2021; Anker 2009; Berking 2006; Christensen 2005; Dowrick 1995b; German 1987; McCusker 2001; Scheidt 2012; Subramanian 2004; Zung 1983).
Thirty‐one studies had a high risk of allocation disclosure (Amble 2014; Berking 2006; Calkins 1994; Callahan 1994; Callahan 1996; Cherkin 2018; Detmar 2002; Goldsmith 1989; Gutteling 2008; Hadjistavropoulos 2009; Hoekstra 2006; Kendrick 2017; Mathias 1994; Mazonson 1996; Murillo 2017; Priebe 2007; Puschner 2009; Rand 1988; Rubenstein 1995; Ruland 2003; Saitz 2003; Scheidt 2012; Slade 2006b; Strasser 2016; Subramanian 2004; Trudeau 2001; Wasson 1992; White 1995; Whooley 2000; Wikberg 2017; Wolfe 2014).
Blinding
Due to the nature of the interventions in this review, which all included the routine administration and feedback of PROMs in clinical practice, it was not feasible to blind participants and personnel, hence we necessarily assessed this criterion as high risk in most studies. We did not have enough information to make a decision for Atreja 2018; Cooley 2016; Haas 2016. Similarly, we deemed the blinding of outcomes assessment as high risk in most studies, as due to the nature of the interventions blinding of outcomes was not possible, PROMS used for feedback were also used to assess outcome. For 17 studies (Absolom 2021; Atreja 2018; Bastiaansen 2018; Bryant 2020; Cooley 2016; Dueck 2015; Franco 2020; Haas 2016; Kuo 2020; LeBlanc 2019; Lugtenberg 2020; Moore 2019; Myasoedova 2019; Richardson 2019; Tolstrup 2020; Valles 2017; van der Hout 2020), there was not enough information to make a decision and we assessed those studies to have an unclear risk of detection bias. We assessed one study to be at low risk of detection bias as the main outcome was objective and directly collected from the health records (Sandheimer 2020).
Baseline characteristics and outcome measurements
We assessed differences in baseline characteristics between intervention and control groups as high risk in nine studies (Amble 2014; Hadjistavropoulos 2009; McCusker 2001; Puschner 2009; Simon 2012; Strasser 2016; Thomas 2016; Valles 2017; Wagner 1997).
Ten studies had a risk of bias for differences in baseline outcome measurements between intervention and control groups (Anderson 2015; Blonigen 2015; De Jong 2014; Denis 2017; Kendrick 2017; Nimako 2017; Puschner 2009; Trudeau 2001; Valles 2017; Zung 1983).
Incomplete outcome data
Inadequate strategies for addressing incomplete data leading to high risk of bias were evident in 26 studies (Anderson 2015; Anker 2009; Callahan 1994; Callahan 1996; De Jong 2012; De Jong 2014; Dowrick 1995a; Gossec 2018; Haas 2016; Hadjistavropoulos 2009; Hawkins 2004; Kazis 1990; Kendrick 2017; Lugtenberg 2020; Magruder‐Habib 1990; Moore 1978; Murphy 2012; Rubenstein 1995; Saitz 2003; Scheidt 2012; Schottke 2019; Simon 2012; Strasser 2016; Thomas 2016; Whipple 2003; Wikberg 2017).
Protected against contamination
Thirty‐four studies were at high risk of contamination (Absolom 2021; Amble 2014; Anker 2009; Bastiaansen 2018; Bryant 2020; Christensen 2005; Cleeland 2011; Detmar 2002; Dowrick 1995a; Dowrick 1995b; Franco 2020; Hoeper 1984; Kazis 1990; Lambert 2001; Linn 1980; Lugtenberg 2020; Magruder‐Habib 1990; McLachlan 2001; Nimako 2017; Nipp 2019; Pouwer 2001; Richardson 2019; Rubenstein 1995; Saitz 2003; Sandheimer 2020; Simons 2015; Slade 2006b; Stuck 2015; Thomas 2016; Tolstrup 2020; Wagner 1997; Whipple 2003; Wikberg 2017; Zung 1983).
Selective reporting
Six studies were at high risk for selective outcome reporting (Hoeper 1984; LeBlanc 2019; Lugtenberg 2020; Mazonson 1996; Reese 2009; Yager 1981).
Other potential sources of bias
We did not assess other potential sources of bias.
Effects of interventions
See: Table 1
Our comprehensive search of the literature identified 116 randomised studies which evaluated the impact of patient‐reported outcome assessment and feedback on either processes or patient‐reported outcomes of care. We conducted 37 analyses in 15 categories of Quality of Life, Health Perceptions, Functioning, Symptoms, Communication, Clinician‐rated severity, diagnosis and notation, Pharmacological treatment, Counselling, Referrals, Visits and sessions, Hospital admissions and length of stay, Disease control, Patient perceptions, Quality of care, and Costs. For specific details on the certainty of the evidence, refer to Table 1 and Table 2.
1. PROM feedback compared to usual care for improve processes and outcomes of care.
| PROM feedback compared to usual care for improve processes and outcomes of care: additional analyses not included in Summary of Findings. | ||||||
| Patient or population: Ambulatory adult patients. Setting: Primary and secondary care settings in North America and Europe. Intervention: PROM feedback reported to physicians or both patients and physicians. Comparison: Usual care. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with PROM feedback | |||||
| Symptoms | Dyspnoea | |||||
| SMD ‐0.11 (‐0.32 to 0.11) indicating no difference between PROM feedback and usual care | ‐ | 765 (5 randomised trials) | ⊕⊝⊝⊝ Very low 1, 2, 3 | We are uncertain about the effect of PROM feedback on dyspnoea. | ||
| Nausea | ||||||
| SMD ‐0.08 (‐0.76 to 0.59) indicating no difference between PROM feedback and usual care | ‐ | 239 (2 randomised trials) | ⊕⊝⊝⊝ Very low 1, 2, 3 | We are very uncertain about the effect of PROM feedback on nausea. | ||
| Cough | ||||||
| SMD ‐0.14 (‐0.75 to 0.48) indicating no difference between PROM feedback and usual care | ‐ | 122 (2 randomised trials) | ⊕⊝⊝⊝ Very low 1, 2, 3 | The evidence is very uncertain about the effect of PROM feedback on cough. | ||
| Depressive symptoms | ||||||
| SMD ‐0.12 (‐0.19 to ‐0.05) indicating no difference between PROM feedback and usual care | ‐ | 3449 (16 randomised trials) | ⊕⊕⊕⊝ Moderate 1 | PROM feedback probably results in a slight reduction in depressive symptoms. | ||
| Anxiety symptoms | ||||||
| SMD ‐0.17 (‐0.31 to ‐0.03) indicating no difference between PROM feedback and usual care | ‐ | 2334 (8 randomised trials) | ⊕⊝⊝⊝ Very low 1, 4 | We are very uncertain about the effect of PROM feedback on anxiety. | ||
| Clinician severity ratings | SMD 0.36 (0.12 to 0.6) favouring PROM feedback vs usual care. | ‐ | 312 (3 randomised trials) | ⊕⊝⊝⊝ Very low 1, 4 | We are very uncertain about the effect of PROM feedback on clinician severity ratings. | |
| Pharmacological treatment | Study population | RR 1.21 (0.91 to 1.59) | 2528 (10 randomised trials) | ⊕⊕⊕⊝ Moderate 3 | The evidence suggests that PROM feedback probably makes little or no difference for pharmacological treatment. Pharmacological treatment was assessed using chart review. Two additional studies reported little or no difference between groups, a third study reported that those allocated to the intervention were more Liley to have their pharmacological treatment changed. |
|
| 195 per 1,000 | 256 per 1,000 (171 to 365) | |||||
| Hospital admissions | Study population | RR 0.96 (0.82 to 1.11) | 1681 (4 randomised trials) | ⊕⊕⊕⊝ Moderate 1 | PROM feedback probably results in little to no difference in hospital admissions. | |
| 66 per 1,000 | 60 per 1,000 (45 to 79) | |||||
| Visits | Visits | |||||
| Study population | RR 1.09 (0.92 to 1.30) | 2777 (8 randomised trials) | ⊕⊝⊝⊝ Very low 1, 2, 3 | The evidence is very uncertain about the effect of PROM feedback on visits. | ||
| 502 per 1,000 | 514 per 1,000 (410 to 619) | |||||
| ER visits | ||||||
| Study population | RR 0.83 (0.68 to 1.01) | 812 (3 randomised trials) | ⊕⊕⊕⊝ Moderate 3 | PROM feedback may reduce ER visits slightly. | ||
| 434 per 1,000 | 359 per 1,000 (293 to 427) | |||||
| Unscheduled visits | ||||||
| Study population | RR 1.43 (0.55 to 3.74) | 333 (2 randomised trials) | ⊕⊕⊝⊝ Low 2, 3 | PROM feedback likely results in little to no difference in unscheduled visits. | ||
| 401 per 1,000 | 551 per 1,000 (194 to 862) | |||||
| Number of visits | ||||||
| SMD 0.02 (‐0.17 to 0.21) indicating no difference between PROM feedback and usual care. | ‐ | 2505 (7 randomised trials) | ⊕⊝⊝⊝ Very low 2, 4 | The evidence is very uncertain about the effect of PROM feedback on number of visits. | ||
| Referral | Study population | RR 2.00 (1.58 to 2.54) | 2519 (10 randomised trials) | ⊕⊝⊝⊝ Very low 1, 4 | The evidence is very uncertain about the effect of PROM feedback on referral. |
|
| 66 per 1,000 | 148 per 1,000 (113 to 190) | |||||
| Counselling (provided or referred to) | Study population | RR 1.61 (1.02 to 2.53) | 815 (4 randomised trials) | ⊕⊝⊝⊝ Very low 1, 4 | The evidence is very uncertain about the effect of PROM feedback on counselling (provided or referred to). | |
| 246 per 1,000 | 396 per 1,000 (251 to 622) | |||||
| Patient satisfaction | SMD 0.12 SD higher (0.12 lower to 0.36 higher) indicating no difference between PROM feedback and usual care. | ‐ | 2760 (10 randomised trials) | ⊕⊝⊝⊝ Very low 3, 4 | The evidence is very uncertain about the effect of PROM feedback on patient satisfaction (overall). | |
| Patient perceptions | Self efficacy | |||||
| SMD ‐0.05 (‐0.21 to 0.32) indicating no difference between PROM feedback and usual care. | ‐ | 349 (2 randomised trials) | ⊕⊕⊕⊝ Moderate 2 | PROM feedback likely results in little to no difference in self efficacy. | ||
| Unmet needs | ||||||
| SMD ‐0.10 (‐0.22 to 0.02) indicating no difference between PROM feedback and usual care. | ‐ | 1025 (3 randomised trials) | ⊕⊕⊕⊝ Moderate 2 | PROM feedback probably results in little to no difference in unmet needs. | ||
| Patient‐physician relationship | ||||||
| SMD 0.12 (‐0.12 to 0.36) indicating no difference between PROM feedback and usual care. | ‐ | 282 (2 randomised trials) | ⊕⊕⊝⊝ Low 1, 3 | PROM feedback may result in little to no difference in patient‐physician relationship. | ||
| Quality of care | SMD 1.47 (1.00 to 2.17) favouring PROM feedback vs usual care. | ‐ | 1403 (2 randomised trials) | ⊕⊕⊝⊝ Low 1, 2 | PROM feedback may increase the quality of care but the evidence is uncertain. | |
| Length of stay | SMD 0.18 (‐0.12 to 0.49) indicating no difference between PROM feedback and usual care. | ‐ | 174 (2 randomised trials) | ⊕⊕⊝⊝ Low 1, 2 | The evidence is very uncertain about the effect of PROM feedback on length of stay | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; SMD: standardised mean difference; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded one point for high risk of unblinding due to nature of intervention for most studies.
2 We downgraded one point for imprecision due to the small number of studies with wide confidence intervals included in meta‐analysis.
3 We downgraded one point for inconsistency due to high heterogeneity.
4 We downgraded two points for inconsistency due to very high heterogeneity.
1. Primary outcomes
1.1 Quality of Life
In total, 16 randomised trials assessed overall quality of life (QoL) using a generic PROM (Aardoom 2016; Basch 2016; Calkins 1994; Jha 2013; Kendrick 2017; LeBlanc 2019; Murillo 2017; Priebe 2007; Richardson 2008; Rosenbloom 2007; Santana 2010; Simons 2015; Slade 2006b; Strasser 2016; van der Hout 2020; Wikberg 2017).
Our meta‐analysis involving 11 studies including 2687 patients revealed a small improvement in QoL for patients receiving the intervention (standardised mean difference (SMD) = 0.15, 95% confidence interval (CI) 0.05 to 0.26; Analysis 1.1). It was not possible to include the studies by Calkins 1994, LeBlanc 2019, Slade 2006b, Strasser 2016 and Wikberg 2017 in the meta‐analysis because of missing information. There was little or no difference between groups in satisfaction with health status at 12 months for Calkins 1994. There were little or no differences between groups in mean follow‐up patient‐rated quality of life for Calkins 1994, LeBlanc 2019, Slade 2006b, and Wikberg 2017. For Strasser 2016 the between‐arm difference in global QoL scores was in favour of the intervention arm. We rated the certainty of the evidence as moderate for this analysis, downgrading one point for risk of bias in the included studies.
1.1. Analysis.

Comparison 1: Quality of Life, Outcome 1: Quality of life (all generic)
1.2 General health perceptions
We conducted a single meta‐analysis containing 552 patients from two randomised trials which had evaluated health perceptions (Mathias 1994; Richardson 2008). Our meta‐analysis revealed no effect of the intervention (SMD = 0.04, 95% CI = ‐0.17 to 0.24; Analysis 2.1). We could not include Stuck 2015 in the meta‐analyses because of how the data were reported. Patients in the intervention group of this study reported better health perceptions than those in the control group. We rated the certainty of the evidence as low; downgrading both for risk of bias arising from intervention design and imprecision due to the small number of studies available for analysis.
2.1. Analysis.

Comparison 2: General health perceptions, Outcome 1: General health perceptions (overall)
1.3 Functioning
In total, 17 studies assessed the impact of the intervention on physical functioning using different measures (Absolom 2021; Davis 2013; Detmar 2002; Girgis 2009; Gutteling 2008; Kornblith 2006; Lugtenberg 2020; Mathias 1994; Murillo 2017; Nimako 2017; Richardson 2008; Rosenbloom 2007; Scheidt 2012; Strasser 2016; Subramanian 2004; van Dijk‐de Vries 2015; Wolfe 2014). Our meta‐analysis of 14 studies included 2788 patients illustrated little or no effect of the intervention (SMD = ‐0.10, 95% CI ‐0.30 to 0.10; Analysis 3.1). We could not include in the meta‐analysis the studies by Absolom 2021 because of the way in which data had been reported, nor for Strasser 2016 or Wolfe 2014 due to missing information. There were little or no difference in physical functioning between intervention and control groups for any of these studies.
3.1. Analysis.

Comparison 3: Functioning, Outcome 1: Physical functioning
Forty studies evaluated the effect of the intervention on mental functioning (Amble 2014; Anker 2009; Berking 2006; Brody 1990; Calkins 1994; Davis 2013; De Jong 2012; Detmar 2002; Fann 2017; Girgis 2009; Gossec 2018; Gutteling 2008; Hansson 2013; Hawkins 2004; Jha 2013; Kornblith 2006; Lambert 2001; Lugtenberg 2020; Mathias 1994; Murillo 2017; Murphy 2012; Nimako 2017; Pouwer 2001; Probst 2013; Puschner 2009; Reese 2009; Richardson 2008; Rosenbloom 2007; Rubenstein 1995; Scheidt 2012; Schottke 2019; Simon 2012; Strasser 2016; Subramanian 2004; Trudeau 2001; van der Hout 2020; van Dijk‐de Vries 2015; Whipple 2003; Wikberg 2017; Wolfe 2014). Our meta‐analysis of thirty‐four studies, which included 7782 patients demonstrated a small positive benefit of the intervention (SMD = 0.16, 95% CI 0.06 to 0.27; Analysis 3.2). It was not possible to include studies by Calkins 1994, De Jong 2012, Strasser 2016, Trudeau 2001, Wikberg 2017 and Wolfe 2014 in the meta‐analysis because of missing information. In Calkins 1994 the number of patients in the intervention and usual care groups was not specified, and there was little or no difference between groups for De Jong 2012, Strasser 2016, Trudeau 2001, Wikberg 2017, and Wolfe 2014.
3.2. Analysis.

Comparison 3: Functioning, Outcome 2: Mental functioning
Social functioning was assessed by 16 studies (Bastiaansen 2018; Blonigen 2015; Calkins 1994; Davis 2013; Detmar 2002; Fann 2017; Girgis 2009; Kendrick 2017; Lugtenberg 2020; Mathias 1994; Murillo 2017; Nimako 2017; Richardson 2008; Rosenbloom 2007; Rubenstein 1995; van Dijk‐de Vries 2015). Meta‐analysis of 15 studies (Bastiaansen 2018; Blonigen 2015; Davis 2013; Detmar 2002; Fann 2017; Girgis 2009; Kendrick 2017; Lugtenberg 2020; Mathias 1994; Murillo 2017; Nimako 2017; Richardson 2008; Rosenbloom 2007; Rubenstein 1995; van Dijk‐de Vries 2015) including a total of 2632 patients revealed no effect of the intervention (SMD = 0.02, 95% CI ‐0.06 to 0.09; Analysis 3.3). It was not possible to include in the meta‐analysis the study by Calkins 1994 because of missing information. There was no difference between intervention and control participants in social function.
3.3. Analysis.

Comparison 3: Functioning, Outcome 3: Social Functioning
We rated the certainty of the evidence as very low for both the physical functioning and mental functioning meta‐analyses, downgrading the evidence for both risk of bias in the included studies and statistical heterogeneity (I2 = 85% and 80%, respectively). In both cases, the heterogeneity appeared to be driven by the inclusion of Subramanian 2004, a large study with high risk of bias that produced markedly different results to the majority of other studies. The evidence for social functioning was rated as moderate, with the evidence downgraded due to risk of bias of the included studies on the basis of un‐blinding due to the nature of the intervention.
1.4 Symptoms
We conducted 11 meta‐analyses which assessed the impact of PROM feedback on patient symptoms including pain, fatigue, insomnia, anorexia, nausea, diarrhoea, constipation, dyspnoea, cough, as well as symptoms of anxiety and depression.
We included nine studies (10 comparisons: Cherkin 2018; Detmar 2002; Hadjistavropoulos 2009; Hoekstra 2006; Kazis 1990; Lugtenberg 2020; Mathias 1994; Nimako 2017; Richardson 2008) in a meta‐analysis assessing the impact of the intervention on pain. Our analysis included 2386 participants and found little or no improvement in pain scores associated with the intervention (SMD = ‐0.00, 95% CI ‐0.09 to 0.08, Analysis 4.1). It was not possible to include in the meta‐analysis on pain the studies by Kroenke 2018 and Strasser 2016 because of missing information nor Bryant 2020 because of the nature of the categorical nature of the data. For Kroenke 2018, participants allocated to the intervention group had a slight improvement on the PROMIS pain scale (0.07; P > 0.10). There was little or no difference between groups in Strasser 2016 and Bryant 2020.
4.1. Analysis.

Comparison 4: Symptoms, Outcome 1: Pain
Seven studies evaluated fatigue (Bryant 2020; Hoekstra 2006; Kroenke 2018; Lugtenberg 2020; Nimako 2017; Strasser 2016; Subramanian 2004). Pooled analysis of four studies and 741 participants (Hoekstra 2006; Lugtenberg 2020; Nimako 2017; Subramanian 2004) revealed little or no improvement for participants in the intervention group (SMD = 0.03, 95% CI ‐0.29 to 0.36, Analysis 4.2). It was not possible to include the study by Bryant 2020 because of the categorical nature of the reported data, nor the studies by Kroenke 2018 and Strasser 2016 in the meta‐analysis on fatigue because of missing information. For Kroenke 2018 there was a slight improvement for participants allocated to the intervention and there were little or no differences in Bryant 2020 and Strasser 2016.
4.2. Analysis.

Comparison 4: Symptoms, Outcome 2: Fatigue
Dyspnoea was assessed in six studies (Hoekstra 2006; Lugtenberg 2020; Nimako 2017; Strasser 2016; Subramanian 2004; White 1995) and our meta‐analysis of five studies and 765 patients found no effect of the intervention (SMD = ‐0.11, 95% CI ‐0.32 to 0.11; Analysis 4.3). It was not possible to include the study by Strasser 2016 because of missing information (little or no differences reported for this study).
4.3. Analysis.

Comparison 4: Symptoms, Outcome 3: Dysponea
Cough was assessed in two studies (Hoekstra 2006, White 1995) and our meta‐analyses (N = 122) suggested that the intervention had little or no effect on cough (SMD = ‐0.14, 95% CI ‐0.75 to 0.480; Analysis 4.4).
4.4. Analysis.

Comparison 4: Symptoms, Outcome 4: Cough
Nausea was assessed in three studies (Hoekstra 2006; Rosenbloom 2007; Strasser 2016). Our meta‐analysis of two studies (239 patients) revealed little or no effect of the intervention (SMD = 0.08, 5% CI ‐0.76 to 0.59; Analysis 4.5). The meta‐analysis did not include the study by Strasser 2016 due to missing information (no differences reported between intervention and control group).
4.5. Analysis.

Comparison 4: Symptoms, Outcome 5: Nausea
Vomiting was assessed in the study by Hoekstra 2006. The severity scores for vomiting in the control group were lower than those in the intervention group (median 2 compared to median 4, P < 0.05).
Symptoms of depression were evaluated in 16 studies which included 3449 patients (Bastiaansen 2018; Boyer 2013; Brodey 2005; Cherkin 2018; Dowrick 1995a; Fann 2017; Hadjistavropoulos 2009; Jha 2013; Kazis 1990; Kendrick 2017; Kornblith 2006; Lugtenberg 2020; Picardi 2016; Scheidt 2012; Simons 2015; Whooley 2000). Our meta‐analysis revealed a small improvement in depression symptoms for patients receiving the intervention (SMD = ‐0.12, 95% CI ‐0.20 to ‐0.05; Analysis 4.6). Anxiety was evaluated in eight studies which included 2334 (Brodey 2005; Brody 1990; Cherkin 2018; Dailey 2002; Kazis 1990; Kornblith 2006; Lugtenberg 2020; Mathias 1994). Our meta‐analysis revealed a small improvement in anxiety symptoms (SMD = ‐0.17, 95% CI ‐0.31 to ‐0.03; Analysis 4.7).
4.6. Analysis.

Comparison 4: Symptoms, Outcome 6: Depressive symptoms
4.7. Analysis.

Comparison 4: Symptoms, Outcome 7: Anxiety symptoms
We graded the certainty of the evidence as moderate for the pain and depression analyses, downgrading the evidence for risk of bias. The certainty of the evidence for the fatigue, dyspnoea, cough, nausea, and anxiety symptoms was very low with studies being downgraded for risk of bias, inconsistency, and imprecision. The explaination for the heterogeneity was not found.
1.5 Adverse effects
We did not find studies reporting adverse effects defined as distress following or related to PROM completion. Some studies reported on outcomes associated with the intervention that can be perceived as adverse (e.g. anxiety and depression), however we reported those outcomes in other categories. Three studies studied the impact of the intervention on adverse events related to the usual management of the relevant diseases (Gilliam 2004; Murillo 2017; Wikberg 2017). Due to differences in the nature and reporting of data it was not possible to conduct a meta‐analysis. In the study by Gilliam 2004, patients in intervention group experienced a significantly higher improvement in a self‐reported adverse events scale than those in the control group. There were no differences between intervention and control groups in number of hypoglycaemic events in the study by Murillo 2017, and no adverse events were reported for any participant in the study by Wikberg 2017.
2. Secondary outcomes
2.1 Communication between patients and clinicians
Communication between patients and health professionals was evaluated in six studies (Davis 2013; Detmar 2002; Lugtenberg 2020; Santana 2010; van Os 2003; Velikova 2004). Our meta‐analysis of five studies included 658 patients, and demonstrated a moderate improvement in communication associated with the intervention (SMD = 0.36, 95% CI 0.21 to 0.52; Analysis 5.1). It was not possible to include van Os 2003 in the meta‐analysis on communication between patients and physicians because of missing information. In the study patients using the 2‐COM PROM feedback tool rated communication with their doctor as better than patients on ‘standard care’ (2‐COM group mean score 3.4, standard care group mean score 3.2; adjusted = 0.33, P = 0.03). The certainty of the evidence in support of patient‐physician communication was rated as moderate, downgraded for risk of bias.
5.1. Analysis.

Comparison 5: Communication, Outcome 1: Patient‐physician communication
2.2 Clinician assessed severity, diagnosis and notation
Three studies evaluated the impact of the intervention of ratings of patient severity as appraised by clinicians (Berking 2006; Brody 1990; Slade 2006b). We included three studies with a total of 312 patient in our meta‐analysis, which suggested a moderate improvement in severity ratings was associated with the intervention (SMD = 0.36, 95% CI 0.12 to 0.60; Analysis 6.1). We graded the certainty of the evidence as very low for clinician severity ratings due to risk and bias and imprecision.
6.1. Analysis.

Comparison 6: Clinician severity ratings, Outcome 1: Clinician severity ratings
Twenty‐one studies assessed the impact of PROM feedback on diagnosis and notation (Brody 1990; Callahan 1994; Callahan 1996; Christensen 2005; Dowrick 1995b; German 1987; Gold 1989; Hoeper 1984; Linn 1980; Magruder‐Habib 1990; Mazonson 1996; Moore 1978; Rand 1988; Rubenstein 1995; Schriger 2001; Schriger 2005; Shapiro 1987; Thomas 2016; Williams 1990; Yager 1981; Zung 1983). Our meta‐analysis included 21 comparisons (N = 7223) and found a medium‐sized effect in favour of the intervention (risk ratio (RR) = 1.73, 95% CI 1.44 to 2.08; Analysis 7.1). We downgraded the evidence for high statistical heterogeneity (I² = 67%) as we could find no clear explaination for the heterogeneity.
7.1. Analysis.

Comparison 7: Diagnosis, Outcome 1: Diagnosis and notations
2.3 Pharmacological treatment
Thirteen studies assessed the impact of PROM feedback on pharmacological treatment (Absolom 2021; Boyer 2013; Brody 1990; Callahan 1994; German 1987; Gilliam 2004; Kroenke 2018; Mazonson 1996; Rubenstein 1995; Shapiro 1987; Trowbridge 1997; van Os 2003; Wikberg 2017). Our meta‐analysis of 10 studies and 2528 patients revealed little or no effect of the intervention (RR = 1.21, 95% CI 0.91 to 1.59; Analysis 8.1). It was not possible to include studies by Kroenke 2018, Rubenstein 1995 and van Os 2003 in the meta‐analysis on pharmacological treatment because of missing information. For Kroenke 2018 there were little or no difference between feedback and control group patients (P > 0.10) in number of medications. The study by Rubenstein 1995 reported no dispersion data. In the van Os 2003 study patients in the 2‐COM group were more likely to have had their treatment changed, as reported by the doctor, than were those in the standard care group (2‐COM 74%, standard care 61%; adjusted odds ratio (OR) = 2.2, 95%CI 1.02–4.7; number needed to treat for an additional benefit (NNTB) = 8). We graded the quality of evidence as moderate, downgrading once for imprecision due to statistical heterogeneity (I2 = 67%). No explaination for the heterogeneity was found.
8.1. Analysis.

Comparison 8: Pharmacological treatment, Outcome 1: Pharmacological treatment
2.4 Counselling
We assessed four studies including 815 patients assessing referral or attendance at counselling (Detmar 2002; German 1987; Saitz 2003; Shapiro 1987). Our meta‐analysis revealed a small effect favouring the intervention (RR = 1.38, 95% CI 1.14 to 1.65; Analysis 9.1), however, we rated the certainty of the evidence as very low; downgrading two points for risk of bias and one point for inconsistency.
9.1. Analysis.

Comparison 9: Counselling, Outcome 1: Counseling (provided or referred to)
2.5 Referrals
Eleven studies evaluated changes to referral (Brody 1990; Callahan 1994; Callahan 1996; German 1987; Gold 1989; Kroenke 2018; Kuo 2020; Magruder‐Habib 1990; Mazonson 1996; Saitz 2003; Shapiro 1987) with a total population of 1938 patients. Our meta‐analysis estimated a moderate increase for PROM feedback on referrals (RR = 2.00, 95% CI 1.58 to 2.54; 10 studies, 2519 participants; Analysis 10.1). It was not possible to include Kroenke 2018 in the meta‐analysis on referrals because of missing information. The study reported little or no difference between feedback and control group patients (P > 0.10) in referrals. We graded the evidence as very low, downgrading twice for both risk of bias and statistical heterogeneity (i² = 56%). The reason for the heterogenity was not clear.
10.1. Analysis.

Comparison 10: Referral, Outcome 1: Referral
2.6 Number of visits
We conducted five meta‐analyses to evaluate the ability of the intervention to reduce the number of visits, we evaluated reduction of all visits, visits specifically to the emergency room (ER), and all unscheduled visits.
Eight studies evaluated the impact of the intervention on any visit (Absolom 2021; Basch 2016; Callahan 1996; Cherkin 2018; Denis 2017; Mazonson 1996; Sandheimer 2020; Tolstrup 2020). We conducted ameta‐analysis of eight studies (some studies provided multiple estimates) including 2777 patients and found no support for the intervention (RR = 1.09, 95% CI 0.92 to 1.30; Analysis 11.1).
11.1. Analysis.

Comparison 11: Visits, Outcome 1: Visits
Three studies evaluated the impact of the intervention on ER visits (Basch 2016; Callahan 1996; Cherkin 2018). Our meta‐analysis with 812 patients did not find support for the intervention on reducing ER visits (RR = 0.83, 95% CI 0.68 to 1.01; Analysis 11.2).
11.2. Analysis.

Comparison 11: Visits, Outcome 2: Emergency room visits
Two studies evaluated the impact of the intervention on unscheduled visits (Callahan 1996; Denis 2017). Our meta‐analysis consisted of 333 patients and did not reveal any support for the intervention (RR = 1.43, 95% CI 0.55 to 3.74; Analysis 11.3).
11.3. Analysis.

Comparison 11: Visits, Outcome 3: Unscheduled visits
In total, seven studies evaluated the impact of the intervention on the number of visits (Gilliam 2004; Kazis 1990; Slade 2006b; Subramanian 2004; Wheelock 2015; Whooley 2000; Wikberg 2017). Our meta‐analysis included 2505 patients and did not find any support for the intervention (SMD = 0.02, 95% CI ‐0.17 to 0.21; Analysis 11.4).
11.4. Analysis.

Comparison 11: Visits, Outcome 4: Number of visits
A meta‐analyses of the two studies (262 participants) that assessed the length of visits (Lugtenberg 2020; Velikova 2004) revealed little or no difference between intervention and control groups (SMD = 0.21, 95% CI ‐0.28 to 0.71; Analysis 11.5).
11.5. Analysis.

Comparison 11: Visits, Outcome 5: Length of visits
Four studies (1681 participants)which assessed the impact of the intervention on number of therapy sessions attended (Amble 2014; Callahan 1996; Hawkins 2004; Whipple 2003); our meta‐analysis found no evidence to support the intervention (SMD = 0.02, 95% CI ‐0.11 to 0.15; Analysis 12.1).
12.1. Analysis.

Comparison 12: Sessions, Outcome 1: Number of sessions
We graded the certainty of the evidence in support of reducing ER visits as moderate, downgrading once for risk of bias in the included studies. There was substantial heterogeneity between the two studies which evaluated unscheduled visits in terms of outcome as well as increased uncertainty due to wide confidence intervals, resulting in the certainty of the evidence being low. The analyses which evaluated any reduction of visits, the number of visits, and the length of visits were graded as very uncertain. We rated the certainty of the evidence for number of therapy sessions as very low, downgrading multiple times for risk of bias and once for imprecision.
2.8 Hospital admissions
Five studies measured the impact of the intervention on hospital admissions (Mazonson 1996; Slade 2006b; Basch 2016; Cherkin 2018; Absolom 2021). Our meta‐analysis of four studies with a total of 1681 patients revealed no effect of the intervention (RR = 0.96, 95% CI 0.82 to 1.11; moderate‐certainty evidence, Analysis 13.1). It was not possible to include Slade 2006b in the meta‐analysis on hospital admissions because of missing information. The study reported intervention‐group patients had reduced hospital admissions, and with fewer admissions in the six months before follow‐up (mean 0.13 versus 0.33, bootstrapped 95% CI ‐0.46 to ‐0.04). We graded the certainty of the evidence as high.
13.1. Analysis.

Comparison 13: Hospital admissions, Outcome 1: Hospital admissions (patients)
We included two studies in a single meta‐analysis of the impact of PROM feedback on length of stay (Anker 2009; Blonigen 2015). Our meta‐analysis did not reveal support for the intervention (SMD = 0.18, 95% CI ‐0.12 to 0.49; Analysis 14.1). We graded the certainty of the evidence as low, downgrading twice for risk of bias and once for imprecision due to the small number of studies included in the analysis.
14.1. Analysis.

Comparison 14: Length of stay, Outcome 1: Length of stay
2.9 Patient perceptions
We conducted three meta‐analysis concerning the ability of the intervention to positively alter patient's perceptions of themselves, their needs, their relationship with their physician and their overall satisfaction.
Four studies evaluated the impact of the intervention on self‐efficacy (Cherkin 2018; van Dijk‐de Vries 2015; Absolom 2021; Bastiaansen 2018); our meta‐analysis included 837 patients and did not support the intervention (SMD = ‐0.05, 95% CI ‐0.21 to 0.32; Analysis 15.1).
15.1. Analysis.

Comparison 15: Patient perceptions, Outcome 1: Self‐Efficacy
Unmet needs was evaluated by three studies (Priebe 2007; Slade 2006b; van der Hout 2020). We included 1025 patients in our meta‐analysis which revealed no support for the intervention (SMD = ‐0.10, 95% CI ‐0.22 to 0.02; Analysis 15.2). It was not possible to include Slade 2006a in the meta‐analysis on unmet needs because of missing information. The study reported no evidence for differences between groups in mean follow‐up patient‐rated unmet need (mean difference 0.15, 95% CI ‐1.20 to 1.49, P = 0.83).
15.2. Analysis.

Comparison 15: Patient perceptions, Outcome 2: Unmet needs
Two studies evaluated the effect of the intervention on patient‐physician relationship (Rosenbloom 2007; Slade 2006b). Our meta‐analysis or 282 patients did not support the intervention (SMD = 0.12, 95% CI ‐0.12 to 0.36; Analysis 15.3).
15.3. Analysis.

Comparison 15: Patient perceptions, Outcome 3: Patient‐physician relationship
We graded the certainty of the evidence self‐efficacy and unmet needs as moderate, downgrading once for inconsistency due to the small number of studies, and we ranked the evidence of patient‐physician relationship as low risk of bias.
We conducted a meta‐analysis of overall satisfaction (Blonigen 2015; Brody 1990; Davis 2013; Detmar 2002; Gossec 2018; Kazis 1990; Kendrick 2017; Priebe 2007; Rosenbloom 2007; Subramanian 2004). Our meta‐analysis of 2760 patients revealed no support for the intervention (SMD = 0.12, 95% CI ‐0.12 to 0.36; Analysis 16.1). It was not possible to include Ruland 2003 and Williams 1990 in the meta‐analysis on patient satisfaction because of missing information. Both Ruland 2003 and Williams 1990 reported little or no difference in patients satisfaction between intervention and control groups. We rated the certainty of the evidence as very low, downgrading multiple times for imprecision. The exaplaination for heterogenetiy was unclear.
16.1. Analysis.

Comparison 16: Patient satisfaction, Outcome 1: Patient satisfaction
2.10 Disease control
We conducted a single meta‐analysis including 14 studies which had evaluated the impact of the intervention on disease control (Anker 2009; De Jong 2014; Hawkins 2004; Murphy 2012; Picardi 2016; Probst 2013; Reese 2009; Saitz 2003; Simon 2012; Subramanian 2004; van Dijk‐de Vries 2015; Whooley 2000; Wikberg 2017; Williams 1990). We included 2806 patients in our meta‐analysis for which a small effect in favour of the intervention was observed RR = 1.25, 95% CI 1.10 to 1.41; Analysis 17.1). We were not able to include Murillo 2017 because of the nature of the data reported (no differences between intervention and control groups in disease control as measured by HbA1C).
17.1. Analysis.

Comparison 17: Disease control, Outcome 1: Disease control
The certainty of the evidence in support of disease control was rated as moderate, with a single downgrade for risk of bias.
2.11 Quality of care
We conducted a single meta‐analysis to evaluate the impact of the intervention on quality of care. Our meta‐analysis of two studies (Rosenbloom 2007; Subramanian 2004) and 1403 patients did not reveal an impact of the intervention for the intervention (RR = 1.47, 95% CI 1.00 to 2.17; Analysis 18.1). We graded the certainty of the evidence as low, downgrading twice for risk of bias.
18.1. Analysis.

Comparison 18: Quality of care, Outcome 1: Quality of care
2.12 Healthcare costs
We included three studies in a single meta‐analysis to evaluate the impact of the intervention on costs (Simons 2015; Slade 2006b; van der Hout 2020). Our meta‐analysis with 833 patients did not reveal support for the intervention (SMD = ‐0.12, 95% CI ‐0.34 to 0.09; Analysis 19.1). We graded the certainty of the evidence as low, downgrading twice for risk of bias and once for imprecision due to the small number of studies included in the analysis.
19.1. Analysis.

Comparison 19: Costs, Outcome 1: Overall costs
3. Other outcomes not reported in the included studies
We included the following outcomes in our protocol, but none of the included studies reported them: patient behaviour, patient empowerment, healthcare professionals awareness of patients' quality of life, and healthcare professionals perceptions.
Sensitivity Analyses
We conducted sensitivity analyses to investigate the impact of studies which were either large, at high overall risk of bias, or both. The majority of the analyses were unchanged however removal of studies with a high risk of bias from the clinicial severity ratings (Berking 2006, Slade 2006b) resulted in a single study remaining in the analysis. Additionally, removal of high risk of bias studies from analysis of referral or attendance counselling analysis (Detmar 2002, Saitz 2003) suggested no effect of the intervention (RR = 1.10 95% CI 0.70 to 1.75).
Discussion
Summary of main results
In this systematic review, we present a comprehensive compilation of the evidence on the feeding back of information from patient‐reported outcomes measures (PROMs) to inform clinical practice. We identified 116 randomised trials involving 49,785 participants) eligible for inclusion in the review, with an overall low risk of bias, although it was also frequently unclear, particularly for blinding of outcomes assessment, assessment of missing data, contamination, and adequate prevention of allocation knowledge.
There was considerable variation in participants, settings, interventions, and measures used to quantify outcomes. Studies evaluated the impact of feeding back information on a wide variety of condition‐specific and also generic PROMs in ambulatory primary care and specialised and in inpatient settings across a wide range of outcomes most frequently including other PROMs as well as health service processes. Given this variation, a meta‐analysis could be done only on a substantially reduced number of studies.
The most profound improvements were generally seen in processes of care, with PROM feedback leading to moderate improvements in diagnosis and notation (patients in the intervention arm almost twice more likely to receive a relevant diagnose or have a relevant notation in their medical records), based on both a large number of studies and a very large number of randomised participants. Moderate improvements were also apparent in patient's perceptions of communication with their providers, although the number of studies and randomised patients, although substantial, was relatively smaller. PROM feedback was associated with small improvements in both disease control and patient quality of life. There were little or no effects for general health perceptions, social functioning and pain, and we are uncertain of the effect of the intervention on physical and mental functioning and fatigue. No studies reported on adverse effects of the intervention, defined as distress as a result of completing the PROM.
These heterogeneous results provide partial support for a cascade of effects whereby PROMs feedback would be linked to changes in process of care (diagnosis, treatment, and quality of care and use of health services), which would then result in improvements in outcomes (symptoms, functioning, and quality of life). Such a cascade would anticipate decreasing effect sizes alongside the proximal‐distal continuum of effects of the intervention. Our findings confirm the largest impact for processes of care that are typically the results of decisions made by the physicians who would have received the information, and smaller effects for other variables. There is also consistency in a positive small impact across a number of health outcomes (mental symptoms and functioning and overall quality of life). We failed, however, to detect many positive impacts for many of the intermediate variables in the cascade of effects (treatments, use of health services, patient self‐efficacy).
Given the importance of developing identifying the best ways in which the routine measurement and feedback of PROMs could be implemented in clinical practice, our review demonstrates that the majority of interventions in included studies had multiple components incorporating different elements, making direct and accurate comparison of intervention effects difficult. In addition, many of the studies utilised different measures of the same construct.
Overall completeness and applicability of evidence
Most of the outcomes that were deemed relevant in our protocol were not reported in the majority of studies. The largest meta‐analysis (mental function) included less than a third or all studies. However, the large number different outcomes analysed allowed for the first comprehensive picture of the impact of the intervention and of the potential cascade of effects, from more proximal (a physician's impression of the severity of symptoms) to the more distal (quality of life). A limitation of the studies reviewed is the absence of any study using any techniques which could tailor the information provided within the questionnaire to the individual patient, such as individualised questionnaires or computerised adaptive testing.
This review has identified many more studies than previous reviews conducted by some co‐authors (Espallargues 2000, Valderas 2008a) and others (Kendrick 2016). Many of these additional studies are relatively recent reflecting the fact that this is an area of increasing interest, both reinforcing the need for this review and suggesting that this evaluation may benefit from being updated in near future.
The included studies do not provide evidence that the proposed interventions would reduce inequalities. All studies were conducted in high‐income countries, which makes inference on the generalisability of the observed effects of the interventions to lower‐/middle‐ income countries tentative. Studies did not take special measures to ensure the inclusion of disadvantaged populations, nor were attrition, adherence or results disaggregated by key characteristics relevant to the understanding of inequalities (socioeconomic status, ethnicity).
Quality of the evidence
All the included studies were randomised trials. Overall, they were of mixed quality with low proportion of studies with high risk of bias (except for risk of allocation concealment and contamination, which exceeded 25%), and a high proportion of studies unclear risk of bias (the most frequent category for sequence generation, attrition and reporting bias, contamination and prevention of knowledge of allocated interventions). Twenty‐eight of the included studies used a cluster design to reduce the risk of contamination. Low risk of bias could be established for over 50% of studies for only three out of 10 domains and as many as 16 studies met criteria for being at overall high risk of bias. Although none of these studies at high risk bias was included in the analyses because they did not consider the relevant outcomes, the quality of the included studies seriously limits our ability to evaluate the impact of feedback based on the available evidence.
Potential biases in the review process
The review was carried out in accordance with EPOC guidelines and using the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Potential limitations in the search process relate to the lack of MeSH term for either patient‐reported outcomes (PROs) or PROMs. This meant that we had to use broad search terms which led to a high yield of citations to be searched. The review authors are active researchers in the field of PROMs, many have contributed to a previous review and are unaware of any potentially eligible studies that were missed by the search. We were also unable to retrieve some missing data from authors.
Agreements and disagreements with other studies or reviews
This review substantially expands the scope and comprehensiveness of any previous reviews. Taking this into consideration, our results are broadly in agreement with the literature. A previous review conducted by co‐authors of this review (Valderas 2008a), which identified a great heterogeneity of impact and concluded that contexts and interventions that could yield important benefits remained to be clearly defined. Our results are also in agreement with a Cochrane Review on the use of PROMs feedback for common mental health disorders (Kendrick 2017), whose authors found insufficient evidence to support the use of routine outcome monitoring using PROMs in the treatment of common mental health disorders, in terms of improving patient outcomes or in improving management. They considered their findings subject to considerable uncertainty however, due to the high risk of bias in the large majority of trials meeting the inclusion criteria, which means further research is very likely to have an important impact on the estimate of effect and is likely to change the estimate. Both reviews highlighted the need for more research of better quality, particularly addressing issues of attrition and blinding, especially given the complex nature of the intervention.
Authors' conclusions
Implications for practice.
Patient‐reported outcomes measures (PROMs) feedback probably produces small to moderate improvements in communication between healthcare professionals and patients as well as in diagnosis and notation, and small improvements in quality of life. Limitations brought about by study design and heterogeneity of outcomes limited the quality of the results.
Despite the mixed certainty of the evidence, due mainly to issues with blinding and concealment which are difficult to overcome in trials of complex interventions that include feedback elements, the data suggest that routine use of PROM feedback in clinical practice could thus improve the quality of health care.
PROMs data can also inform clinical practice at a broader level by facilitating comparative effectiveness between different treatments, and supporting value‐based healthcare, and quality improvement initiatives and may have considerable value beyond their usefulness solely as a clinical tool. PROMs interventions do generate data which can be used not only at the individual patient level but also be aggregated and used to conduct comparative effectiveness research to inform continuous quality improvement.
When considering using PROMs in clinical practice, policymakers and clinicians should take opportunity costs into account in this context.
Implications for research.
Large cluster‐randomised trials are needed that evaluate the impact of feedback in different clinical contexts in which both clinicians and their patients are provided with sufficient training on the interpretation of PROM scores and tailored feedback on those scores. Such trials would also benefit from allowing sufficient time to both clinicians and patients to familiarise themselves with the administration methods, the scoring system and their interpretation, as to give a proper chance to the intervention to be fully integrated into the clinical context. Further work is required to assess the cost‐effectiveness of PROMs feedback interventions. In addition, collection and feedback may be best evaluated using the usual information systems available in the standard setting, rather than bespoke systems, wherever feasible. The widespread occurrence of electronic health records in many care settings now permits PROMs feedback interventions to be assessed within an infrastructure which is becoming more familiar to providers and is increasingly a standard component of care at many institutions, particularly as part of value based health care delivery. There are therefore new opportunities to design and implement interventions which may be more effective at translating increased detection of healthcare issues into tangible improvements in patient‐reported outcomes.
Further research on the mechanisms by which this complex intervention operates is needed as well as research on specific clinical applications and circumstances in which PROMs feedback can provide added value. In particular a paucity of research in supporting the management of people with multimorbidity was found.
The current review contains studies which span more than 30 years. During this period, the rise in popularity of personal computers and, more recently, smart devices and integrated systems such as electronic health records have fundamentally changed the way in which information is collected, scored, and displayed to doctors and patients. Studies included in the review typically do not provide rich details about how users interfaced with the interventions, which may have important effects on their usability, perceived usefulness and, resultantly, the probability that their use will successfully improve processes and outcomes of care. Though some notable studies have already been published in this area (Bantug 2016; Brundage 2015), further research is warranted to define standards for user interfaces in PROM assessment and feedback applications.
In the future, comparability of PROMs feedback interventions could be improved if an international consensus could be reached on which measures were most suitable for different constructs or if metrics were widely available to cross‐link different measures of the same construct. Of course, the type of PROM used is one part of a complex intervention. Other such initiatives could similarly be used to standardise the feedback gained from PROMS in terms of format and timing as well as structure the process by which salient information from PROMs are used to improve the processes and outcomes of care.
History
Protocol first published: Issue 4, 2015
Acknowledgements
The authors would like to thank the editorial support from the Cochrane Effective Practice and Organisation of Care (EPOC) Group, including Chris Cooper (managing editor), Paul Miller (information specialist), Michel Wensing/Luciana Balini (contact editors), Simon Lewin(internal editor), and Andrew Hutchings (statistical editor). The authors are also grateful to Michelle Peters and Kurt Kroenke (peer referees) for providing insightful commentaries on drafts of the review. The authors are also grateful to contributors to the protocol for this review, in particular Bobrovitz NJH, Fitzpatrick R, Rajmil L, Roberts NW, and Taylor RS.
This work was partly funded by Clinician Scientist Award NIHR/CS/010/024. National Institute for Health Research,UK. Improving the management of long term conditions with the clinical use of patient reported outcome measures in Primary Care UK. Period: 01/3/2011‐01/3/2016
We are also thankful to the National Institute for Health Research, via Cochrane Infrastructure funding to the Effective Practice and Organisation of Care Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
MEDLINE
| No. | Search terms | Results |
| 1 | patient reported outcome measures/ | 6425 |
| 2 | ((quality of life or wellbeing or well‐being or QoL or HRQoL or HRQL) adj5 (tool? or questionnaire? or scale? or instrument? or index or indices or measure? or profile? or assess*)).ti,ab. | 90539 |
| 3 | self administ*.ti,ab. | 45512 |
| 4 | ((patient? or self) adj2 (report* or apprais* or rate* or rating* or response* or evaluat*)).ti,ab. | 537609 |
| 5 | ((patient? or adult?) adj5 complet*).ti,ab. | 178035 |
| 6 | self‐assess*.ti,ab. | 16003 |
| 7 | patient questionnaire?.ti,ab. | 2312 |
| 8 | ((function* or health) adj2 status adj2 report*).ti,ab. | 2763 |
| 9 | (screen* adj2 (tool? or questionnaire? or instrument?)).ti,ab. | 35539 |
| 10 | or/1‐9 | 837800 |
| 11 | ((physician? or doctor? or nurse? or dentist? or practitioner? or clinician? or team? or anesthetist? or cardiologist? or dentist? or dermatologist? or gastroenterologist? or gp? or geriatrician? or gerontologist? or gynaecologist? or gynecologist? or hematologist? or haematologist? or intensivist? or neurologist? or obstetrician? or oncologist? or paediatrician? or pediatrician? or psychiatrist? or radiologist? or rheumatologist? or surgeon? or urologist?) adj5 (notif* or inform* or disclos* or report* or provid* or result* or recei* or summar* or availab*)).ti,ab. | 238678 |
| 12 | feedback/ | 29891 |
| 13 | (feedback or feed back or fed back).ti,ab. | 143160 |
| 14 | or/11‐13 | 388946 |
| 15 | ((routine* or regular*) adj2 (quality of life or wellbeing or well‐being or QoL or HRQoL or HRQL)).ti,ab. | 309 |
| 16 | (14 and 10) or 15 | 37618 |
| 17 | exp randomized controlled trial/ | 515015 |
| 18 | controlled clinical trial.pt. | 93863 |
| 19 | randomi#ed.ti,ab. | 644220 |
| 20 | placebo.ab. | 213528 |
| 21 | randomly.ti,ab. | 348255 |
| 22 | Clinical Trials as topic.sh. | 193083 |
| 23 | trial.ti. | 227648 |
| 24 | exp animals/ not humans/ | 4738847 |
| 25 | or/17‐23 | 1374896 |
| 26 | 25 not 24 | 1269096 |
| 27 | 16 and 26 | 5786 |
Embase
| No. | Search terms | Results |
| 1 | patient‐reported outcome/ | 24762 |
| 2 | ((quality of life or wellbeing or well‐being or QoL or HRQoL or HRQL) adj5 (tool? or questionnaire? or scale? or instrument? or index or indices or measure? or profile? or assess*)).ti,ab. | 143294 |
| 3 | self administ*.ti,ab. | 59000 |
| 4 | ((patient? or self) adj2 (report* or apprais* or rate* or rating* or response* or evaluat*)).ti,ab. | 804303 |
| 5 | ((patient? or adult?) adj5 complet*).ti,ab. | 292954 |
| 6 | self‐assess*.ti,ab. | 22600 |
| 7 | patient questionnaire?.ti,ab. | 3820 |
| 8 | ((function* or health) adj2 status adj2 report*).ti,ab. | 3617 |
| 9 | (screen* adj2 (tool? or questionnaire? or instrument?)).ti,ab. | 53444 |
| 10 | or/1‐9 | 1265437 |
| 11 | ((physician? or doctor? or nurse? or dentist? or practitioner? or clinician? or team? or anesthetist? or cardiologist? or dentist? or dermatologist? or gastroenterologist? or gp? or geriatrician? or gerontologist? or gynaecologist? or gynecologist? or hematologist? or haematologist? or intensivist? or neurologist? or obstetrician? or oncologist? or paediatrician? or pediatrician? or psychiatrist? or radiologist? or rheumatologist? or surgeon? or urologist?) adj5 (notif* or inform* or disclos* or report* or provid* or result* or recei* or summar* or availab*)).ti,ab. | 349639 |
| 12 | (feedback or feed back or fed back).ti,ab. | 183192 |
| 13 | *feedback system/ | 14588 |
| 14 | or/11‐13 | 526104 |
| 15 | ((routine* or regular*) adj2 (quality of life or wellbeing or well‐being or QoL or HRQoL or HRQL)).ti,ab. | 508 |
| 16 | (14 and 10) or 15 | 63642 |
| 17 | random*.ti,ab. | 1580844 |
| 18 | factorial*.ti,ab. | 39074 |
| 19 | (crossover* or cross over*).ti,ab. | 108687 |
| 20 | ((doubl* or singl*) adj blind*).ti,ab. | 236559 |
| 21 | (assign* or allocat* or volunteer* or placebo*).ti,ab. | 1060144 |
| 22 | crossover procedure/ | 64535 |
| 23 | single blind procedure/ | 40363 |
| 24 | randomized controlled trial/ | 622762 |
| 25 | double blind procedure/ | 176401 |
| 26 | or/17‐25 | 2387512 |
| 27 | exp animal/ not human/ | 4831880 |
| 28 | 26 not 27 | 2150229 |
| 29 | 16 and 28 | 11821 |
CENTRAL (Cochrane Library)
| No. | Search terms | Results |
| #1 | [mh "patient reported outcome measures"] | 575 |
| #2 | (("quality of life" or wellbeing or well‐being or QoL or HRQoL or HRQL) near/5 (tool* or questionnaire* or scale* or instrument* or index or indices or measure* or profile* or assess*)):ti,ab | 42929 |
| #3 | self next administ*:ti,ab | 6070 |
| #4 | ((patient* or self) near/2 (report* or apprais* or rate* or rating* or response* or evaluat*)):ti,ab | 98275 |
| #5 | ((patient* or adult*) near/5 complet*):ti,ab | 47638 |
| #6 | self next assess*:ti,ab | 3428 |
| #7 | patient next questionnaire*:ti,ab | 749 |
| #8 | ((function* or health) near/2 status near/2 report*):ti,ab | 374 |
| #9 | (screen* near/2 (tool* or questionnaire* or instrument*)):ti,ab | 2763 |
| #10 | {OR #1‐#9} | 178772 |
| #11 | ((physician* or doctor* or nurse* or dentist* or practitioner* or clinician* or team* or anesthetist* or cardiologist* or dentist* or dermatologist* or gastroenterologist* or gp* or geriatrician* or gerontologist* or gynaecologist* or gynecologist* or hematologist* or haematologist* or intensivist* or neurologist* or obstetrician* or oncologist* or paediatrician* or pediatrician* or psychiatrist* or radiologist* or rheumatologist* or surgeon* or urologist*) near/5 (notif* or inform* or disclos* or report* or provid* or result* or recei* or summar* or availab*)):ti,ab | 26466 |
| #12 | (feedback or feed back or "fed back"):ti,ab,kw | 17072 |
| #13 | {or #11‐#12} | 41898 |
| #14 | ((routine* or regular*) near/2 ("quality of life" or wellbeing or well‐being or QoL or HRQoL or HRQL)):ti,ab | 101 |
| #15 | (#10 and #13) or #14 | 10244 |
CINAHL (EBSCO)
| No. | Search terms | Results |
| S1 | (quality of life or wellbeing or well‐being or QoL or HRQoL or HRQL) N5 (tool? or questionnaire? or scale? or instrument? or index or indices or measure? or profile? or assess*) | 68,582 |
| S2 | self administ* | 20,543 |
| S3 | (patient? or self) N2 (report* or apprais* or rate* or rating* or response* or evaluat*) | 295,502 |
| S4 | (patient? or adult?) N5 complet*) | 51,068 |
| S5 | self‐assess* | 15,184 |
| S6 | patient questionnaire? | 20,684 |
| S7 | (function* or health) N2 status N2 report*) | 1,910 |
| S8 | (screen* N2 (tool? or questionnaire? or instrument?) | 15,594 |
| S9 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 | 430,899 |
| S10 | (physician? or doctor? or nurse? or dentist? or practitioner? or clinician? or team? or anesthetist? or cardiologist? or dentist? or dermatologist? or gastroenterologist? or gp? or geriatrician? or gerontologist? or gynaecologist? or gynecologist? or hematologist? or haematologist? or intensivist? or neurologist? or obstetrician? or oncologist? or paediatrician? or pediatrician? or psychiatrist? or radiologist? or rheumatologist? or surgeon? or urologist?) N5 (notif* or inform* or disclos* or report* or provid* or result* or recei* or summar* or availab*) | 142,257 |
| S11 | MH "Feedback" | 15,059 |
| S12 | (feedback or feed back or fed back) | 42,673 |
| S13 | S10 OR S11 OR S12 | 181,234 |
| S14 | (routine* or regular*) N2 (quality of life or wellbeing or well‐being or QoL or HRQoL or HRQL) | 528 |
| S15 | (MH "clinical trials+") | 305,113 |
| S16 | pt clinical trial | 106,213 |
| S17 | (clin* n25 trial*) | 278,842 |
| S18 | (singl* n25 blind*) or (doubl* n25 blind*) or (trebl* n25 blind*) or (tripl* n25 blind*) | 78,095 |
| S19 | (singl* n25 mask*) or (doubl* n25 mask*) or (trebl* n25 mask*) or (tripl* n25 mask*) | 1,289 |
| S20 | random* or placebo* | 437,377 |
| S21 | (MH "random assignment") | 63,755 |
| S22 | (MH "placebos") | 12,558 |
| S23 | (MH "quantitative studies") | 27,864 |
| S24 | control* or prospective* or volunteer* | 1,702,170 |
| S25 | S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 | 1,943,917 |
| S26 | (MH "Patient‐Reported Outcomes") | 2,654 |
| S27 | S9 OR S26 | 430,899 |
| S28 | S13 AND S27 | 29,365 |
| S29 | S28 or S14 | 29,864 |
| S30 | S25 AND S29 | 13,337 |
PsycINFO
| No. | Search terms | Results |
| 1 | ((quality of life or wellbeing or well‐being or QoL or HRQoL or HRQL) adj5 (tool? or questionnaire? or scale? or instrument? or index or indices or measure? or profile? or assess*)).ti,ab. | 31270 |
| 2 | self administ*.ti,ab. | 18536 |
| 3 | ((patient? or self) adj2 (report* or apprais* or rate* or rating* or response* or evaluat*)).ti,ab. | 191385 |
| 4 | ((patient? or adult?) adj5 complet*).ti,ab. | 23761 |
| 5 | self‐assess*.ti,ab. | 8452 |
| 6 | patient questionnaire?.ti,ab. | 251 |
| 7 | ((function* or health) adj2 status adj2 report*).ti,ab. | 1014 |
| 8 | (screen* adj2 (tool? or questionnaire? or instrument?)).ti,ab. | 12619 |
| 9 | or/1‐8 | 267663 |
| 10 | ((physician? or doctor? or nurse? or dentist? or practitioner? or clinician? or team? or anesthetist? or cardiologist? or dentist? or dermatologist? or gastroenterologist? or gp? or geriatrician? or gerontologist? or gynaecologist? or gynecologist? or hematologist? or haematologist? or intensivist? or neurologist? or obstetrician? or oncologist? or paediatrician? or pediatrician? or psychiatrist? or radiologist? or rheumatologist? or surgeon? or urologist?) adj5 (notif* or inform* or disclos* or report* or provid* or result* or recei* or summar* or availab*)).ti,ab. | 67442 |
| 11 | (feedback or feed back or fed back).ti,ab. | 67050 |
| 12 | feedback/ or "knowledge of results"/ | 18724 |
| 13 | or/10‐12 | 134676 |
| 14 | ((routine* or regular*) adj2 (quality of life or wellbeing or well‐being or QoL or HRQoL or HRQL)).ti,ab. | 94 |
| 15 | (13 and 9) or 14 | 13856 |
| 16 | exp clinical trial/ | 12439 |
| 17 | random*.ti,ab. | 203832 |
| 18 | ((clinical or control*) adj3 trial*).ti,ab. | 76256 |
| 19 | ((singl* or doubl* or trebl* or tripl*) adj5 (blind* or mask*)).ti,ab. | 26736 |
| 20 | (volunteer* or control group or controls).ti,ab. | 250010 |
| 21 | placebo/ or placebo*.ti,ab. | 40810 |
| 22 | or/16‐21 | 465835 |
| 23 | 15 and 22 | 2700 |
ClinicalTrials.gov
Interventional Studies | "patient reported outcome" OR "patient reported outcomes" OR "functional status" [INTERVENTION TERMS]
Interventional Studies | routine AND ("quality of life" OR well‐being OR wellbeing OR QoL OR HRQL OR HRQoL)
WHO ICTRP
patient reported OR functional status [intervention terms]
Data and analyses
Comparison 1. Quality of Life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Quality of life (all generic) | 11 | 2687 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [0.05, 0.26] |
Comparison 2. General health perceptions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 General health perceptions (overall) | 2 | 552 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.17, 0.24] |
Comparison 3. Functioning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Physical functioning | 14 | 2788 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.30, 0.10] |
| 3.2 Mental functioning | 34 | 7782 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [0.06, 0.27] |
| 3.3 Social Functioning | 15 | 2632 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.06, 0.09] |
Comparison 4. Symptoms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Pain | 9 | 2386 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.09, 0.08] |
| 4.2 Fatigue | 4 | 741 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.29, 0.36] |
| 4.3 Dysponea | 5 | 765 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.32, 0.11] |
| 4.4 Cough | 2 | 122 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.75, 0.48] |
| 4.5 Nausea | 2 | 239 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.76, 0.59] |
| 4.6 Depressive symptoms | 16 | 3449 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.19, ‐0.05] |
| 4.7 Anxiety symptoms | 8 | 2334 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.31, ‐0.03] |
| 4.8 Insomnia | 2 | 202 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐14.77, 8.57] |
| 4.9 Anorexia | 2 | 202 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.37, 0.19] |
| 4.10 Constipation | 2 | 202 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.14, 0.42] |
| 4.11 Diarrhoea | 2 | 202 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.33, 0.22] |
4.8. Analysis.

Comparison 4: Symptoms, Outcome 8: Insomnia
4.9. Analysis.

Comparison 4: Symptoms, Outcome 9: Anorexia
4.10. Analysis.

Comparison 4: Symptoms, Outcome 10: Constipation
4.11. Analysis.

Comparison 4: Symptoms, Outcome 11: Diarrhoea
Comparison 5. Communication.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Patient‐physician communication | 5 | 658 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [0.21, 0.52] |
Comparison 6. Clinician severity ratings.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Clinician severity ratings | 3 | 312 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [0.12, 0.60] |
Comparison 7. Diagnosis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Diagnosis and notations | 21 | 7223 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.44, 2.08] |
Comparison 8. Pharmacological treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Pharmacological treatment | 10 | 2528 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.91, 1.59] |
Comparison 9. Counselling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Counseling (provided or referred to) | 4 | 815 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.14, 1.65] |
Comparison 10. Referral.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Referral | 10 | 2519 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.58, 2.54] |
Comparison 11. Visits.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Visits | 8 | 2777 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.92, 1.30] |
| 11.2 Emergency room visits | 3 | 812 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.68, 1.01] |
| 11.3 Unscheduled visits | 2 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.55, 3.74] |
| 11.4 Number of visits | 7 | 2505 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.17, 0.21] |
| 11.5 Length of visits | 2 | 262 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.28, 0.71] |
Comparison 12. Sessions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Number of sessions | 4 | 1593 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.11, 0.15] |
Comparison 13. Hospital admissions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Hospital admissions (patients) | 4 | 1681 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.82, 1.11] |
Comparison 14. Length of stay.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Length of stay | 2 | 174 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.12, 0.49] |
Comparison 15. Patient perceptions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Self‐Efficacy | 4 | 837 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.21, 0.32] |
| 15.2 Unmet needs | 3 | 1025 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.22, 0.02] |
| 15.3 Patient‐physician relationship | 2 | 282 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.12, 0.36] |
Comparison 16. Patient satisfaction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Patient satisfaction | 10 | 2760 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.12, 0.36] |
Comparison 17. Disease control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 17.1 Disease control | 14 | 2806 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.10, 1.41] |
Comparison 18. Quality of care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 18.1 Quality of care | 2 | 1403 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.00, 2.17] |
Comparison 19. Costs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 19.1 Overall costs | 3 | 833 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.34, 0.09] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Absolom 2021.
| Study characteristics | ||
| Methods | Randomised trial, UK. | |
| Participants | 508 patients with colorectal, breast, or gynaecological cancer treated with curative intent. Median age 56 (18‐79) years, 79.9% female. | |
| Interventions | Patients who were commencing chemotherapy were randomly assigned to usual care (UC) or usual care with the addition of eRAPID (weekly online symptom reporting for 18 weeks). Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: FACT‐General, FACT‐PWB, EQ‐5D‐VAS, European Organisation for Research and Treatment of Cancer (EORTC QLQ‐C30) Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Generic, Domain/Disease specific Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: Realtime feedback over 18 weeks (at least weekly plus when having symptoms) Who information fed back to: Clinicians, Patients Information fed back: Scores, Management recommendations |
|
| Outcomes | Real‐time monitoring with electronic patient‐reported outcomes improved physical well‐being (6 and 12 weeks) and self‐efficacy (18 weeks) in a patient population predominantly treated with curative intent, without increasing hospital workload. | |
| Notes | Funded by the National Institute for Health Research (UK). The study ran from 29/09/2014 until 23/10/2018. The following conflicts of interest were declared. Julia Brown Research Funding: Roche Other Relationship: NIHR Galina Velikova Honoraria: Eisai, Novartis, Pfizer, Roche UK Consulting or Advisory Role: Roche UK, Eisai, Novartis Speakers' Bureau: Novartis Research Funding: Pfizer Travel, Accommodations, Expenses: Roche UK, Novartis, Eisai Other Relationship: University of Leeds No other potential conflicts of interest were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random assignment of clinicians not possible. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patient and provider aware of group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Baseline outcome measurements similar | Low risk | All measurements the same. |
| Baseline characteristics similar | Low risk | Characteristics similar (no statistical test performed). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Was study protected against contamination | High risk | Single‐site study. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
Amble 2014.
| Study characteristics | ||
| Methods | Multisite randomised trial, Norway | |
| Participants | 377 adult patients with moderate to severe dysfunction in and outpatient in Norwegian naturalistic psychiatric setting. mean age 35.8 years (SD: 11.66, Range: 18‐65), 68% female | |
| Interventions | All patients were asked to online fill out the OQ‐45 prior to each session. Both patients and physicians in the intervention arm received feedback about their OQ‐45 outcome. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: The Outcome Questionnaire‐45.2 Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific Administration features Where PROMs administered: Unclear How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: Before each session with therapist Who information fed back to: Clinicians, Patients Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Main outcomes: number of sessions; proportion of signal cases Other outcomes: recovery rate (OQ‐45 score) | |
| Notes | Funding information not stated. The study ran from June 2010 until September 2013. No conflicts of interest were reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomised in blocks of 8 and by gender |
| Allocation concealment (selection bias) | High risk | Patients notified of their status. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to study design (crossed design; study looking at feedback). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Table 2 provided similar outcome measurements for the first scores |
| Baseline characteristics similar | High risk | Table 1 provided the characteristics across the clinics and there were big differences between the number of feedback sessions, gender breakdown and other characteristics |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was no discussion on missing data. |
| Was study protected against contamination | High risk | No blinding. |
| Selective reporting (reporting bias) | Low risk | None apparent. |
Anderson 2015.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 60 low‐income African American and Latina women with breast cancer and cancer‐related pain recruited in the outpatient medical oncology clinic of a large public hospital in Houston, Texas, that treats underserved patients. | |
| Interventions | Pilot study of an automated, telephone‐based, interactive voice response (IVR) intervention. Women in the intervention group were called twice weekly by the IVR system and asked to rate the intensity of their pain and other symptoms. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: MD Anderson Symptom Inventory (MDASI), The Barriers Questionnaire II (BQ‐II), The Eastern Cooperative Oncology Group performance status scale, Pain management index Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: Patients called 2 times a week for 8 weeks. Clinicians provided IVR symptom ratings before clinics. Who information fed back to: Clinicians Information fed back: Scores |
|
| Outcomes | Main outcomes: severity of cancer‐related symptoms; patient beliefs that are barriers to optimal pain treatment | |
| Notes | Supported by American Cancer Society Grant RSGT‐05‐219‐01‐ CPPB and in part by the National Institutes of Health/National Cancer Institute through The University of Texas MD Anderson Cancer Center’s Support Grant P30 CA016672. The trial period is not reported. Reported conflicts of interest state: Dr. Cleeland has a patent for the MD Anderson Symptom Inventory (MDASI), which is licensed to The University of Texas MD Anderson Cancer Center and Charles Cleeland; he is a consultant to Astra Zeneca, Abbott, Genentech, Amgen, Bristol‐Myers Squibb, Pfizer, Estellas, Bayer, Acetylon, Johnson & Johnson, and Novartis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by an electronic protocol management system ‐ although it did not specify who ran this. |
| Allocation concealment (selection bias) | Unclear risk | Electronic protocol management system conducted the allocation ‐ but unclear who administered this. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Research staff knew which patients were in the intervention group ‐ but it was unclear whether patients knew. Physicians also knew the patients in the intervention group. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding outcomes. |
| Baseline outcome measurements similar | High risk | No comparisons were made at baseline between intervention and control groups. |
| Baseline characteristics similar | Low risk | No differences between characteristics of intervention and control groups. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No discussion in the analysis section on how missing data were handled. High attrition rate for outcome assessment completion for intervention group (> 80%) and lower for control (< 70%). |
| Was study protected against contamination | Low risk | Controls did not have access to the IVR system. |
| Selective reporting (reporting bias) | Low risk | All outcome measurements mentioned in methods section was reported in results. |
Anker 2009.
| Study characteristics | ||
| Methods | Randomised trial, Norway | |
| Participants | 906 adults (453 couples) who sought outpatient couple therapy services at a family counselling agency providing free government‐subsidised services in southern Norway | |
| Interventions | Investigated the effects of providing treatment progress and alliance information to both clients and therapists during couple therapy. Outpatients at a community family counselling clinic were randomly assigned to 1 of 2 groups: treatment as usual (TAU) or feedback. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: The Outcomes Rating Scale (ORS); The Locke‐Wallace (LW) Marital Adjustment Test Constructs measured: Functioning Instrument categories/domains: Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Each session Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcome: psychological functioning and distress | |
| Notes | Funding information not reported. The study ran from October 2005 to December 2007. No conflicts of interest are reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were randomised to one of two groups following phone intake. Intake forms were shuffled, and then a coin flip determined assignment to the feedback vs. TAU groups |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Only clients were not informed about the different conditions of feedback and TAU but not possible to blind therapists due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The PROM used for feedback was also used for outcome assessment. |
| Baseline outcome measurements similar | Low risk | Imbalanced but appropriate adjusted analysis was performed. |
| Baseline characteristics similar | Unclear risk | Not clear in the text and no table was provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A total of 245 (59.8%) out of 410 individuals, representing 149 couples, responded to 6‐month follow‐up. |
| Was study protected against contamination | High risk | Therapists was aware that the couples were participants in the study. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
Atreja 2018.
| Study characteristics | ||
| Methods | Randomised trial, USA. | |
| Participants | 320 patients with irritable bowel syndrome. 47% female. | |
| Interventions | Intervention patients update their information and receive a disease summary of quality of care metrics and IBD‐specific quality of life trends. Atreja 2018 Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: HealthPROMISE app measuring quality of care and quality of life Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Generic, Domain/Disease specific (IBD) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: Whenever HealthPROMISE patients updated their information Who information fed back to: Clinicians Information fed back: Scores |
|
| Outcomes | Primary outcome was change in quality of care. Secondary outcomes were disparities in IBD‐related emergency room visits and hospitalisations, change in quality of life score from baseline, and proportion of patients reporting controlled disease status. | |
| Notes | Funding information not provided. The study period is not reported. Conflicts of interest are not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract only. |
| Allocation concealment (selection bias) | Unclear risk | Abstract only. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Abstract only. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Abstract only. |
| Baseline outcome measurements similar | Unclear risk | Abstract only. |
| Baseline characteristics similar | Unclear risk | Abstract only. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only. |
| Was study protected against contamination | Unclear risk | Abstract only. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. |
Basch 2016.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 766 patients initiating chemotherapy at Memorial Sloan Kettering Cancer Center (MSK) in New York for metastatic breast, genitourinary, gynaecological, or lung cancers. | |
| Interventions | Nonblinded, randomised, controlled trial of web‐based self‐reporting of symptoms, compared with usual care. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: STAR (Symptom Tracking and Reporting) Constructs measured: Health related Quality of Life, Symptoms Instrument categories/domains: Generic, Domain/Disease specific (cancer) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) and non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic and paper (clinicians received symptom printouts, nurses received email alerts when patient symptoms worsening) How often information fed back: At each clinic visit Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance |
|
| Outcomes | Main outcome: change in HRQL at 6 months from baseline Other outcomes: survival at 1 year, quality‐adjusted survival |
|
| Notes | Funded by the Conquer Cancer Foundation of the American Society of Clinical Oncology. The study ran from March 2014 until January 2017. Two authors reported receiving funding from pharmaceutical companies (MGK; HIS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generation random. |
| Allocation concealment (selection bias) | Low risk | Allocations conducted by different service. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to study design (crossed design; study looking at feedback). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Baseline variables were well balanced between groups. |
| Baseline characteristics similar | Low risk | All baseline characteristics relatively balanced between sub groups within intervention and usual care. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Multiple sensitivity imputation analysis conducted for incomplete data past baseline measurements. |
| Was study protected against contamination | Low risk | Controls did not do intervention or had access to intervention system. |
| Selective reporting (reporting bias) | Low risk | All outcome measurements mentioned in methods section was reported in results. |
Bastiaansen 2018.
| Study characteristics | ||
| Methods | Pragmatic randomised trial, the Netherlands. | |
| Participants | 161 patient with a primary diagnosis of depression. Mean age 32 years (12), 54% female. | |
| Interventions | Systematic self‐monitoring in combination with digital feedback reports and face‐to‐face discussion. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Routine Outcome Monitoring web application (RoQua) Constructs measured: Symptoms, Functioning, Other (Empowerment) Instrument categories/domains: Generic Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: Weekly Who information fed back to: Clinicians, Patients Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcome: change in depression symptom severity. Other outcomes: psychological functioning, empowerment, and costs. |
|
| Notes | Funded by grants from the Gratama, Stichting tot Steun VCVGZ, and the Dutch Depression Foundation. The study ran from 1 March 2016 until 31 July 2018. The authors did not report any conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequential block allocation using randomisation tool. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding impossible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Baseline outcome measurements similar | Low risk | Baseline measurement identical. |
| Baseline characteristics similar | Low risk | Characteristics all similar. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis. Multiple imputation of missing data. |
| Was study protected against contamination | High risk | Multi‐site study with randomisation at the patient level. |
| Selective reporting (reporting bias) | Low risk | Published protocol. |
Berking 2006.
| Study characteristics | ||
| Methods | Individual randomised controlled trial, Germany | |
| Participants | 118 patients in a cognitive‐behavioural oriented impatient setting | |
| Interventions | Half of the therapists were provided with systematic feedback on their patients’ progress. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: 10‐Item‐Form des Emotionalitätsinventars (EMI‐B), 11‐Item‐Form des Brief Symptom Inventory (BSI), 12‐Item‐Form des Inventars Interpersonaler Probleme (IIP), 10‐Item‐Form des Inkongruenzfragebogens (INK). Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (Mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Routine systematic feedback Who information fed back to: Clinicians Information fed back: Scores, Previous scores |
|
| Outcomes | Main outcome: impact of psychotherapy measured with: 10‐Item‐Form des Emotionalitätsinventars (EMI‐B); 11‐Item‐Form des Brief Symptom Inventory (BSI); 12‐Item‐Form des Inventars Interpersonaler Probleme (IIP); 10‐Item‐Form des Inkongruenzfragebogens (INK). | |
| Notes | Funding information not reported. Study period not reported. Conflicts of interest not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Tossing a coin |
| Allocation concealment (selection bias) | High risk | Tossing a coin |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Could not occur given the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Unclear risk | Unclear |
| Baseline characteristics similar | Low risk | No significant differences between the control and experimental groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Pre and post data sets reported |
| Was study protected against contamination | Unclear risk | Not enough information to make a decision |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
Blonigen 2015.
| Study characteristics | ||
| Methods | Pilot randomised trial, USA | |
| Participants | 30 patients entering a 90‐day residential substance use disorder treatment program. Mean age 49 (range 26‐64) years, 93.3% male. | |
| Interventions | Patients completed assessments of sociodemographics, treatment history, substance‐ related functioning, and personality and worked with an Intervention Co‐ordinator (IC) to work on assessment questions. At patient‐centred feedback session (13.8 mean days after treatment entry) they received a summary of their personality profile and recommendation to help address problematic behavior tendencies. At 1‐month follow‐up sessions patient completed assessment regarding their adjustment to the residential program.
response time: 13.8 mean days via face‐to‐face Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: The Brief Addiction Monitor, The NEO PI‐R measure of normal‐range personality, The Assessment Questionnaire (AQ) measuring satisfaction with the patient‐centred assessment process. Constructs measured: Functioning, Other (Satisfaction) Instrument categories/domains: Domain/Disease specific (Mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Unclear Feedback features Format of PROMs feedback: Unclear How often information fed back: At feedback session and one month follow up Who information fed back to: Clinicians, Patients Information fed back: Scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcome: assessment Questionnaire (AQ) Other outcomes: length of stay in the program and whether or not patient dropped out of the program | |
| Notes | The study was supported by Career Development Award‐2, VA Office of Research and Development (Clinical Sciences R&D); Locally Initiated Project (LIP13DB1), VA Palo Alto Centre for Innovation to Implementation (Ci2i). The study period was not reported. The authors declared no competing interesting. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Numbers randomly added to an excel spreadsheet |
| Allocation concealment (selection bias) | Unclear risk | Patients notified of their status |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to study design (crossed design; study looking at feedback). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | High risk | No information on baseline measurements |
| Baseline characteristics similar | Low risk | None apparent |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No discussion on missing data |
| Was study protected against contamination | Unclear risk | No discussion on whether the clinician delivering the intervention interacted with the control group patients |
| Selective reporting (reporting bias) | Low risk | All outcome measurements mentioned in methods section was reported in results |
Boyer 2013.
| Study characteristics | ||
| Methods | Prospective, randomised open‐label trial, France | |
| Participants | 124 adult patients with the diagnosis schizophrenia and a stable disease status. Mean age 41.1 years (SD 11.8), 67.7% male. | |
| Interventions | Patients with schizophrenia were assigned to one of three groups: patients completed the standard face‐to‐face psychiatric assessment (PANSS, CDSS, ESRS, GAF), patients completed a QoL questionnaire (S‐QOL) in addition to the standard psychiatric assessment, feedback regarding the QoL scores was presented to clinicians in addition to the standard psychiatric assessment.
Evaluations were performed at three different time points: (a) at randomisation (baseline; T0) as well as 3 months (T1) and at 6 months (T2). The effect of QOL assessments and feedback on patient’s satisfaction was measured. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: S‐QoL (Schizophrenia Quality of Life) questionnaire Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Generic, Domain/Disease specific (Mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Completed on paper, item scores entered on computer by researcher Feedback features Format of PROMs feedback: Unclear How often information fed back: At each evaluation session Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcome: patient satisfaction (QSH‐45) Other outcomes: psychotic symptomatology (PANSS), depression (CDSS), drug‐induced movement disorder (ESRS), global Functioning (GAF). | |
| Notes | The study was supported by Institutional grants ‐ 2005 Programme Hospitalier Recherche Clinique National. Sponsor: Assistance Publique, Hopitaux de Marseille, France. The study period was not reported. The authors declared no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Computer generated randomisation using permuted block design |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to study design (crossed design; study looking at feedback). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | None apparent |
| Baseline characteristics similar | Low risk | No significant differences (table of sociodemographics and clinical characteristics provided) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 patients out of 124 did not complete follow up assessments |
| Was study protected against contamination | Low risk | None apparent |
| Selective reporting (reporting bias) | Low risk | All outcome measurements mentioned in methods section was reported in results |
Brodey 2005.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 1374 adult patients 87,5% white, 4,5% black, 4% Hispanic, 4% multiracial | |
| Interventions | Patients complete 11 items from the SCL‐90 at starting point and 6 weeks later. In the intervention group a report detailing survey results were given after the initial and 6‐week administration to the clinician. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: S‐QoL (Schizophrenia Quality of Life) questionnaire Constructs measured: Symptoms Instrument categories/domains: Generic, Domain/Disease specific (Mental health) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Paper, or via telephone system Feedback features Format of PROMs feedback: Paper How often information fed back: At intake and at 6 weeks Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance |
|
| Outcomes | Main outcomes: depression (SCL‐11), anxiety (SCL‐11) Other outcomes: clinician satisfaction | |
| Notes | National Institute of Mental Health grant (1 R43MH57614‐O1 A1). The study period was not reported. No conflicts of interest were reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not clear as randomisation method was not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to study design (crossed design; study looking at feedback). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | None apparent |
| Baseline characteristics similar | Low risk | None apparent |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None apparent |
| Was study protected against contamination | Unclear risk | Unclear as the patients were contacted via telephone or post |
| Selective reporting (reporting bias) | Unclear risk | Outcome measurements collected were reported |
Brody 1990.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 106 patients. Mean age 57.1 years. 77% female | |
| Interventions | Trial Residents received feedback about their patient’s mental health problem (GHQ and ad hoc questionnaire about life stress) prior to seeing that patient. Residents received this feedback + a counselling protocol. And a control group with no feedback. Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: GHQ 12 ‐ General Health Questionnaire Constructs measured: Symptoms Instrument categories/domains: Generic, Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Main outcome: assessment of patient mental health problem and types or amounts of mental health treatment provided Other outcomes: patient and physician evaluation of the care provided during the medical visit. | |
| Notes | The study was funded by the Robert Wood Johnson Foundation (Princeton, NJ) and Henry J. Kaiser Foundation (Meulo Park, CA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation of participating clinics not specified |
| Allocation concealment (selection bias) | Low risk | Cluster‐randomised design. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to study design (crossed design; study looking at feedback). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants of the intervention group received the outcomes as it was part of the protocol. |
| Baseline outcome measurements similar | Low risk | None apparent |
| Baseline characteristics similar | Low risk | No significant differences (table of baseline characteristics provided) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was no discussion on missing data |
| Was study protected against contamination | Low risk | Each clinic were either a control or intervention group and were provided with the relevant protocols |
| Selective reporting (reporting bias) | Low risk | All measures mentioned in the methods section were reported in the results |
Bryant 2020.
| Study characteristics | ||
| Methods | Pilot randomised trial, USA. | |
| Participants | 76 hospitalised haematopoietic stem cell transplantation patients. | |
| Interventions | Symptom monitoring using the PRO‐CTCAE with daily feedback to nurse. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: PRO‐CTCAE survey Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (cancer symptoms) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: 7, 10 and 14 days Who information fed back to: Clinicians Information fed back: Scores, Previous scores |
|
| Outcomes | Main outcome: symptom burden on days 7, 10, and 14 following hospitalisation. | |
| Notes | University of North Carolina Cancer Research Fund and Lineberger Comprehensive Cancer Center Core Grant. The study recruited between May 2015 and June 2017. Dr. Bill Wood reported funding support from Pfizer, Genetech, Koneksa Health, and Best Doctors. Dr. Wood did not have a financial relationship with the organisation that sponsored the research. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation process not described. |
| Allocation concealment (selection bias) | Low risk | Screening, enrolment, randomisation,and study orientation were conducted by a research coordinator who was not a member of the clinical care team. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Baseline outcome measurements similar | Low risk | Baseline measurements the same. |
| Baseline characteristics similar | Low risk | No apparent differences between groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data distributed evenly between both groups. |
| Was study protected against contamination | High risk | Single‐institution study, nurses saw patients in both groups. |
| Selective reporting (reporting bias) | Unclear risk | Reporting consistent with pre‐registration information. |
Calkins 1994.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 497 adults’ patients with a least 2 visits to the outpatient internal medicine department in the preceding year. Mean age 59 years. 77% female. | |
| Interventions | Patient had to fill out the Functional Status Questionnaire (FSQ) 4 times at 4‐month interval for 1 year. The clinician in the intervention group received report summarising the results of the questionnaire. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Functional Status Questionnaire (FSQ) Constructs measured: Functioning Instrument categories/domains: Generic Administration features Where PROMs administered: Unclear How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: 4 month intervals over a year Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcome: functional status (FSQ) | |
| Notes | The study was supported by the Robert Wood Johnson Foundation (Princeton, NJ). Study period was not reported. Conflicts of interest were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paper is 'brief report', lacking detail. |
| Allocation concealment (selection bias) | High risk | Due to cluster‐randomised design not possible to conceal allocation from clinicians. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | No significant differences between baseline scores on FSQ (Functional Status Questionnaire) |
| Baseline characteristics similar | Unclear risk | Paper is 'brief report', lacking detail. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Paper is 'brief report', lacking detail. |
| Was study protected against contamination | Unclear risk | Paper is 'brief report', lacking detail. |
| Selective reporting (reporting bias) | Unclear risk | Paper is 'brief report', lacking detail. |
Callahan 1994.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 175 patients who screened positive for depression on the CES‐D and the HAM‐D and were under the care of 103 physicians at a multi‐speciality ambulatory care clinic associated with an urban county hospital. Average age for participants was 65. | |
| Interventions | Physicians of intervention patients were provided with patient‐specific treatment recommendations during three visits to address the symptoms of depression. Guidelines for antidepressant prescription were provided. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Centers for Epidemiologic Studies Depression Scale (CES‐D), Hamilton Depression Rating Scale (HAM‐D), Short Portable Mental Status Questionnaire (SPMSQ), CAGE alcoholism questionnaire Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Unclear How administered: Interviewer‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Paper (added to medical record) How often information fed back: 3 times over 3 months Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcomes: frequency of recorded depression diagnosis, stopping medications associate with depression, initiating antidepressant medication, psychiatry referrals, mean changes in both HAM‐D and Sickness Impact Profile (SIP) scores. | |
| Notes | The study was supported by the John A. Hartford Foundation, Inc. New York. The study period is not reported. Conflicts of interest are not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only mentioned randomly assigned |
| Allocation concealment (selection bias) | High risk | Due to cluster‐randomised design not possible to conceal allocation from clinicians. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Unclear risk | Not clearly reported |
| Baseline characteristics similar | Low risk | There were no significant differences between these 2 groups in any of the baseline characteristics. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Among the 254 patients who completed the second stage assessment with the HAM‐D, 175 (68%) scored 15 or greater and comprise the study sample. |
| Was study protected against contamination | Low risk | Control group had no access to the intervention. 3 sessions were excluded because the investigators involved in this study practiced in them. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section |
Callahan 1996.
| Study characteristics | ||
| Methods | Cluster‐randomised trial | |
| Participants | 222 adults aged over 60 in primary care. | |
| Interventions | Feedback plus additional interventions for patients and clinicians Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Patient depression (measured with the HAM‐D); Patient function (measured with the SIP) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Unclear How administered: Interviewer‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcome: diagnosis of depression Other outcomes: discontinue medications associated with depression, initiate antidepressants, referral to psychiatry, patient depression (measured with the HAM‐D), patient function (measured with the SIP) | |
| Notes | The study was supported in part by a grant from the John A. Hartford Foundation, New York, New York. Dr. Callahan was supported by grant K08 AG00538‐02 from the National Institutes of Health. Dr. Tierney was supported by grants HS07632, HS07763, and HS07719 from the Agency for Health Care Policy and Research. The study period is not reported. Conflicts of interest are not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only mentioned randomly assigned |
| Allocation concealment (selection bias) | High risk | Due to cluster‐randomised design not possible to conceal allocation from clinicians. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | There were no statistically significant differences between the intervention and control groups |
| Baseline characteristics similar | Low risk | There were no significant differences by study group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | An ‘intention‐to‐treat’ analysis was performed |
| Was study protected against contamination | Low risk | Control group had no access to the intervention. No physician had both intervention and control patients. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section |
Cherkin 2018.
| Study characteristics | ||
| Methods | Cluster‐randomised trial, USA | |
| Participants | 2138 patients visited the intervention clinics and 2571 the control clinics. Six primary care clinics were pair randomised, three to training in the STarT Back strategy and three to serve as controls. | |
| Interventions | The STarT Back risk‐stratification strategy matches treatments for LBP to physical and psychosocial obstacles to recovery using patient‐reported data (the STarT Back Tool) to categorize patients’ risk of persistent disabling pain. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: STarT Back tool (back pain) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (back pain) Administration features Where PROMs administered: Non‐clinical setting How administered: Interviewer‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: 3 times (baseline, 2 months, and 6 months) Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcomes: back‐related physical function and pain severity Other outcomes: healthcare utilisation. |
|
| Notes | Funding for this trial was provided by the Patient Centered Care Research Institute (“Evaluation of a Patient‐Centered Risk Stratification Method for Improving Primary Care for Back Pain”: Contract #398) and by the National Center for Complementary and Integrative Health/NIH (“Implementing Evidence‐Based Treatments for Persistent Back Pain into Primary Care”: Grant No. R21AT0007326). Martin Levine, Diane Piekara, and Pam Rock received support to participate in the quality improvement activities from Group Health. Nadine E Foster, an NIHR Senior Investigator, and Jonathan C. Hill were supported through an NIHR Research Professorship (NIHR‐RP‐ 011‐015) awarded to Nadine Foster. The study recruited between March 2013 and December 2015. The authors do not report conflicts of interest information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial biostatistician randomly assigned participants using computer‐generated system. |
| Allocation concealment (selection bias) | High risk | Due to cluster‐randomised design not possible to conceal allocation from clinicians. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Similar results of baseline outcomes in Table 1. |
| Baseline characteristics similar | Low risk | Characteristics of both groups similar. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Interviewers used to collect outcome data via telephone to reduce missing data. |
| Was study protected against contamination | Low risk | Intervention was delivered in separate clinics. |
| Selective reporting (reporting bias) | Low risk | All outcome measurements mentioned in methods section was reported in results. |
Christensen 2005.
| Study characteristics | ||
| Methods | Randomised trial, Denmark | |
| Participants | 1785 adult patients with a new health problem consult their primary care doctor. Mean age (SD): IG: 39.3 years (12.9); CG: 38.2 years (12.9) Gender (% female): IG: 59%; CG: 61% |
|
| Interventions | Patient were screened before consultation using a screening questionnaire (SQ): including SCL‐90R, SCL‐SOM, Whiteley‐7, SCL‐8, CAGE and SF‐36). In the intervention group the questionnaires were disclosed and scored by GPs before consultation, and in the control group the results were not scored and thus blinded.
Immediately after the consultation, the GPs completed a questionnaire on their own assessment, subjects of conversation, actions taken, and self‐reported benefit from disclosed screening results, if any. Intervention features Single complex feedback (multiple PROMs at a single time) PROM(s) used as intervention: SCL‐90R somatisation subscale (SCL‐SOM), Whiteley‐7 scale, anxiety and depression (SCL‐8) subscale, alcohol abuse scale (CAGE), SF‐36 Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Generic, Domain/Disease specific (mental health, alcohol abuse) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Unclear How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Management recommendations |
|
| Outcomes | Main outcome: GPs recognition and provision of care Other outcomes: outline useful strategies for case‐finding | |
| Notes | Interdisciplinary Research Programme of the Danish National Research Council: Quote: “Sundhedsfremme og forebyggelsesforskning” (grant# 9801278). GPs training participation, data collection and use of SQs* by The Regional Health Assurance in Aarhus County through a local pay agreement (project# 0871). The study recruited from 3 March to 1 May 2000. The authors reported no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | How randomisation was done was not discussed |
| Allocation concealment (selection bias) | Low risk | Colour‐coded allocation used by medical secretaries only. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Medical secretaries aware of allocation arm. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible due to study design (disclosure of questionnaire versus not) |
| Baseline outcome measurements similar | Low risk | None apparent |
| Baseline characteristics similar | Low risk | No significant differences (table of baseline characteristics provided) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unanswered questions were scored zero and analysis was based on random allocation |
| Was study protected against contamination | High risk | Study design not optimal enough so contamination likely. |
| Selective reporting (reporting bias) | Low risk | None apparent. |
Cleeland 2011.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 100 adult patients receiving thoracotomy for lung cancer or lung metastasis Mean age intervention group: 59.2 years (SD 13.6) and 44.7% female. Mean age control group: 60.9 years (SD 11.8) and 48.8% female. | |
| Interventions | This study examines whether at‐home symptom monitoring plus feedback to clinicians about severe symptoms contributes to more effective postoperative symptom control.
After hospital discharge, patients rated symptoms twice weekly for 4 weeks via automated telephone calls. For intervention group patients, an e‐mail alert was forwarded to the patient’s clinical team for response if any of a subset of symptoms (pain, disturbed sleep, distress, shortness of breath, or constipation) reached a predetermined severity threshold using the M.D. Anderson Symptom Inventory (MDASI). Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: M.D. Anderson Symptom Inventory (MDASI) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (cancer) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered by telephone Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: Patients rated symptoms twice weekly for 4 weeks via automated telephone calls Who information fed back to: Clinicians Information fed back: Scores |
|
| Outcomes | Main outcomes: number of symptom threshold events (MDASI); mean symptom severity between discharge and follow‐up(MDASI) Other outcomes: mean symptom interference (MDASI), patient satisfaction (AD HOC) | |
| Notes | American Cancer Society, Atlanta, GA (grant# RSGPB‐03‐244‐01‐BBP); National Cancer Institute, Bethesda, MD (grant# R01CA026582). The study period was not reported. The authors indicated no potential conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paper only states randomisation was performed electronically using the medical centre's 'protocol management system'. |
| Allocation concealment (selection bias) | Unclear risk | Not clear whether allocation concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinicians knew about intervention patients due to their email alert system |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Clinicians and surgical nurses were informed of symptoms through the IVR‐alerts which was an outcome |
| Baseline outcome measurements similar | Low risk | None apparent |
| Baseline characteristics similar | Low risk | No significant differences (patient demographics provided) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None apparent |
| Was study protected against contamination | High risk | In the discussion the authors highlighted the possibility of the intervention patients knowing their symptoms were being monitored possibly affecting their results |
| Selective reporting (reporting bias) | Low risk | All the outcomes were reported in the results section |
Cooley 2016.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 179 patients. Mean age 63 years, 58% female. | |
| Interventions | Web‐based symptom assessment using the Treatment Outcome Index. Tailored report provides longitudinal symptoms and recommendations for management provided to clinicians. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Web‐based symptom assessment Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (cancer) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: Each visit Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Improvement in the Treatment Outcome Index, better management for depression, anxiety, and fatigue. More palliative care consults. | |
| Notes | No funding information provided. The study period was not reported. The authors did not report conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract only. |
| Allocation concealment (selection bias) | Unclear risk | Abstract only. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Abstract only. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Abstract only. |
| Baseline outcome measurements similar | Unclear risk | Abstract only. |
| Baseline characteristics similar | Unclear risk | Abstract only. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only. |
| Was study protected against contamination | Unclear risk | Abstract only. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. |
Dailey 2002.
| Study characteristics | ||
| Methods | Randomised trial, England | |
| Participants | 123 Adult patients going to their first dental treatment visit with an anxiety for the dentist measured with the Modified Dental Anxiety Scale (MDAS). Mean age intervention group: 40.1 years (SD 13.0, Range 19‐67). Mean age control group: 42.5 years (SD 15.0, Range 19‐51). | |
| Interventions | All patient fills out the MDAS prior to seeing the dentist. In the intervention group the dentist was informed about their patients MDAS score. Prior and after treatment patient completed the Spielberger State Anxiety Inventory for State Anxiety (STAI‐S). Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: State Anxiety Inventory Scores (STAI‐S) and Modified Dental Anxiety Scale (MDAS) Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Main outcome: change in state of anxiety (STAI‐S) | |
| Notes | The study was supported by the Department of Clinical Dental Sciences, The University of Liverpool, School of Dentistry, Liverpool, UK. The study period was not reported. The authors did not report any conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Paper states "Randomization was generated prior to the start of the study by means of a computerized stratified block design". |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes were used for the randomisation process |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to study design (disclosing questionnaire versus not) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | None apparent |
| Baseline characteristics similar | Low risk | No significant differences (table of baseline characteristics available) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Was study protected against contamination | Unclear risk | The dentist could have been aware of the intervention patients because of the screening forms but not sure if the patients were aware |
| Selective reporting (reporting bias) | Low risk | Outcomes mentioned in the methods were reported in results |
Davis 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial, the Netherlands | |
| Participants | 413 adult mental health (mood, anxiety, adjustment and personality disorder) patients in an outpatient setting Control group mean age 36.9 years (SD 11.8), female 60% Feedback group mean age 36.7 (SD 12.1) female 62% | |
| Interventions | Patient progress in terms of symptom distress, interpersonal relations, and social role (OQ‐45)
Feedback propensity (IEFPS and adaption of CFIT User Survey)
Use of feedback (post hoc question wether or not the therapist had used treatment and in what way) Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Prostate Cancer Subscale (PCS) of the Functional Assessment of Cancer Therapy‐Prostate (FACT‐P) Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (cancer) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Paper How often information fed back: Twice, participants completed a total of 2 monitoring interventions in approximately 7 months. Who information fed back to: Clinicians Information fed back: Scores |
|
| Outcomes | Primary Effect of feedback on the rate of change in patients Secondary Therapist characteristics | |
| Notes | The study was funded by the National Cancer Institute (grant# R03 ‐ CA119765‐01A1a). The study period was not reported. Potential conflicts of interest were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RandomiSation performed using a telephone‐based system. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was done over a telephone‐based system so unclear as to whether it was known to others |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | None apparent |
| Baseline characteristics similar | Low risk | No significant differences mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Was study protected against contamination | Low risk | Control group did not have access to the intervention system |
| Selective reporting (reporting bias) | Low risk | Outcomes mentioned in the methods were reported in results |
De Jong 2012.
| Study characteristics | ||
| Methods | Randomised trial, the Netherlands | |
| Participants | 413 adult mental health (mood, anxiety, adjustment and personality disorder) patients in an outpatient setting Control group mean age 36.9 YEARS (SD 11.8), female 60% Feedback group mean age 36.7 YEARS (SD 12.1) female 62% | |
| Interventions | Patient progress in terms of symptom distress, interpersonal relations, and social role (OQ‐45)
Feedback propensity (IEFPS and adaption of CFIT User Survey)
Use of feedback (post hoc question whether or not the therapist had used treatment and in what way) Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Outcome Questionnaire‐45 (OQ‐45), The Internal and External Feedback Propensity Scales, (an adaptation of) the CFIT* User Survey Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: At each of the first five sessions of therapy, and subsequently every fifth session for a maximum period of 1 year Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcome: effect of feedback on the rate of change in patients Other outcomes: therapist characteristics | |
| Notes | Funding information was not reported. The study period was not reported. Conflicts of interest were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients assigned to groups by software |
| Allocation concealment (selection bias) | Low risk | Allocation concealed to patients |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | None apparent |
| Baseline characteristics similar | Low risk | Paper states"'The groups did not differ on most variable". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rates of attrition in both groups which was not adequately addressed |
| Was study protected against contamination | Unclear risk | No mention as to what the control group did and whether they had access to the feedback for the intervention group |
| Selective reporting (reporting bias) | Low risk | Outcomes mentioned in the methods were discussed in the results section |
De Jong 2014.
| Study characteristics | ||
| Methods | Randomised trial, the Netherlands | |
| Participants | 475 adult patients, recruited from private psychotherapy practices and outpatient mental health institutions Mean age 38.2 YEARS (SD 12.0) Female 68% | |
| Interventions | Patient progress in terms of symptom distress, interpersonal relations, and social role (OQ‐45) Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Outcome Questionnaire‐45 (OQ‐45) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Patients could log in anywhere, but most completed on laptop provided in therapist's waiting room How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: Each therapy session Who information fed back to: One intervention group clinician only, one intervention group clinicians and patients Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcome: patient progress (OQ‐45) | |
| Notes | The study was supported by The Netherlands Organization for Health Research and Development (grant# 94506414). The study period was from 1 July 2006 to 31 June 2011. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using an online system |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was done online so unclear as to whether participants or therapists knew of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | High risk | Significant differences (P = 0.01) found between conditions. |
| Baseline characteristics similar | Low risk | None apparent. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rates of attrition in both groups which was not adequately addressed |
| Was study protected against contamination | Unclear risk | Not clear what the control group had access to |
| Selective reporting (reporting bias) | Low risk | Outcomes mentioned in the methods were discussed in the results section |
Denis 2017.
| Study characteristics | ||
| Methods | InvididualLY‐randomised controlled trial, France | |
| Participants | Five hospitals and clinics in France, advanced‐stage lung cancer patients without evidence of disease progression after or during initial treatment. | |
| Interventions | Patients were randomly assigned to be followed with either a web‐mediated prompting of follow‐up imaging or scheduled interval imaging. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: 5 self‐assessed symptoms (appetite loss, fatigue [asthenia], pain, cough, and breathlessness) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (cancer) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: Weekly Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance |
|
| Outcomes | Main outcome: overall survival | |
| Notes | The study was supported by Sivan Innovation Ltd. Ths study ran from June 1 2014 to January 9 2016. The funder had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. No further conflicts were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation sequence was generated by the study team |
| Allocation concealment (selection bias) | Low risk | Study team enrolled and assigned allocations to participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | High risk | Difference in FACIT score between groups |
| Baseline characteristics similar | Low risk | Baseline characteristics had no sig differences between groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary endpoint was overall survival in advanced lung cancer patients |
| Was study protected against contamination | Unclear risk | Unclear whether the study was protected from contamination |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
Detmar 2002.
| Study characteristics | ||
| Methods | Randomised trial, the Netherlands | |
| Participants | 214 adult patients undergoing outpatient palliative chemotherapy after at least 2 cycles of chemotherapy Mean age 57 years Female 76% | |
| Interventions | HRQL (QLQ‐C30 version 3.0) with feedback for physician and patient before consultation
Patient management (with audiotapes of consultations)
Physician's awareness of patients' health problems (comparing COOP and WONCA between physician and patient)
Patients self‐reported HRQL (SF‐36)
Patient and physician evaluation of intervention (questionnaire and telephone interview regarding their experience with the intervention) Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: European Organization for Research and Treatment of Cancer, Quality of Life Questionnaire‐Core 30 (QLQ‐C30) Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (cancer) Administration features Where PROMs administered: Clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: At 3 successive outpatient visits Who information fed back to: Clinicians, Patients Information fed back: Scores, Previous scores, Interpretation guidance |
|
| Outcomes | Main outcome: patient‐physician communication Other outcomes: physician awareness of patients' HRQL (agreement between physician and patients' reporting of problems) | |
| Notes | The study was supported by the Dutch Cancer Society. The study ran from June 1996 to June 1998. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cross‐over design and the physicians took part in both the intervention and control |
| Allocation concealment (selection bias) | High risk | Not possible to blind clinicians due to study design. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to study design (intervention group received graphical summary of questionnaire results) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Table 1 shows similar baseline results of the outcome measurements |
| Baseline characteristics similar | Low risk | Table provided and paper states: "The intervention and control groups were well‐balanced on variables except primary diagnosis, with the control group having proportionally more breast cancer patients than the intervention group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comparisons were made between those complete datasets and those who did not complete the follow‐ups |
| Was study protected against contamination | High risk | Cross‐over design so contamination likely |
| Selective reporting (reporting bias) | Low risk | None apparent. |
Dowrick 1995a.
| Study characteristics | ||
| Methods | Randomised trial, UK | |
| Participants | 116 patients with a score of at least 14 but below 35 on the Beck depression inventory (BDI) | |
| Interventions | Disclosure of depression scores to general practitioners for participants with an undetected depression. Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: Beck Depression Inventory; ICD‐10* Criteria for depression Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Main outcome: depression status (BDI) Other outcome: management of depression, intention to treat depression (no intention, possible intention, definite intention) | |
| Notes | The study did not receive external funding.The study ran from 1993‐1994. The authors reported no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Doctors were aware of patients scores but not sure for which group. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to study design (disclosure of questionnaire to GP versus not) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | None apparent |
| Baseline characteristics similar | Unclear risk | No characteristics presented in a table ‐ only mentioned that there were no sig differences between groups |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A total of 10,99 consented to take part but the numbers presented in the tables are of only 227 (table 4) |
| Was study protected against contamination | High risk | the clinical researcher was not blind to the group status of the subjects, and this could have led to selection bias at the diagnostic interview. |
| Selective reporting (reporting bias) | Low risk | None apparent. |
Dowrick 1995b.
| Study characteristics | ||
| Methods | Randomised trial, UK | |
| Participants | 179 adults with a positive depression screen attending primary care. | |
| Interventions | Feedback plus additional interventions (patients and clinicians). Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: Beck Depression Inventory (BDI) Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Main outcome: depression scores (measured with the BDI‐21) | |
| Notes | No funding declared. The study was conducted in 1993. The authors reported no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were randomly subdivided on a 6:5 ratio (to allow later diagnoses to be discounted in assessing changes in depressing status) |
| Allocation concealment (selection bias) | Low risk | Allocated using sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to study design (intervention group received graphical summary of questionnaire results) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | There were no statistically significant differences between the intervention and control groups |
| Baseline characteristics similar | Low risk | There were no significant differences between the two groups in terms of age, gender, civil, employment or physical health status. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | An ‘intention‐to‐treat’ analysis was performed |
| Was study protected against contamination | High risk | The researcher performing the interview and analysing the data were aware that the patient was a participant in the study |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section |
Dueck 2015.
| Study characteristics | ||
| Methods | Cluster‐randomised trial, USA. | |
| Participants | 269 patients with rectal cancer. | |
| Interventions | Symptom feedback to clinicians using the PRO‐CTCAE. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: National Cancer Institute’s Patient‐Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO‐CTCAE) Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (cancer) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: At time of toxicity assessments Who information fed back to: Clinicians Information fed back: Unclear |
|
| Outcomes | Clinician reporting of adverse events. | |
| Notes | The study was sponsored by the Alliance for Clinical Trials in collaboration with the National Cancer Institute (NCI) and the Canadian Cancer Trials Group. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded by nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided. |
| Baseline outcome measurements similar | Unclear risk | Insufficient information provided. |
| Baseline characteristics similar | Unclear risk | Insufficient information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information provided. |
| Was study protected against contamination | Unclear risk | Insufficient information provided. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided. |
Fann 2017.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | Adult patients starting cancer therapy | |
| Interventions | Adult patients starting cancer therapy were randomised to receive usual education about symptoms and quality of life (SxQOL) topics (control) or usual education plus self‐care instruction for SxQOL issues, communication coaching, and the opportunity to track SxQOL between clinic visits (intervention). Clinicians received summaries of participant reports at each time point in both groups. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: PHQ‐9=Patient Health Questionnaire‐9, EF=QLQ‐C30 emotional functioning, HSCT=hematopoietic stem cell transplant, RF=QLQ‐C30 role functioning, SF=QLQ‐C30 social functioning Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: PROMs were administered before treatment (T1), 3–6 weeks after starting treatment (T2), 2 weeks later (T3), and 2–4 weeks after treatment ended or at the next restaging visit for participants who continued to receive treatment (T4). Who information fed back to: Clinicians, Patients Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Secondary analysis of psychosocial outcomes of the ESRA‐C‐II study by examining the effects of the intervention on depression and on social, emotional and role functioning. | |
| Notes | The study was funded by the National Institute of Nursing Research R01 NR008726. The study recruited from October 2008 until December 2013. The authors reported no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Unclear risk | No information provided about who did allocations. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Mean scores were similar between groups. |
| Baseline characteristics similar | Low risk | No significant differences in characteristics. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Original paper stated that incomplete data was removed. |
| Was study protected against contamination | Low risk | Controls did not do intervention or had access to intervention system. |
| Selective reporting (reporting bias) | Low risk | Main outcome reported in the results. |
Franco 2020.
| Study characteristics | ||
| Methods | Randomised trial. Italy. | |
| Participants | 222 patients with uncontrolled epileptic seizures. | |
| Interventions | Assessment of adverse events using the Adverse Event Profile (AEP) and communication of patient scores to treating physicians. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: 31‐item epilepsy‐specific Quality of Life Inventory ‐ Epilepsy–31 (QOLIE‐31), 19‐item AEP questionnaire, Beck Depression Inventory II (BDI), 5‐digit Clinical Global Impression (CGI) scale Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Generic, Domain/Disease specific (epilepsy) Administration features Where PROMs administered: Clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Unclear How often information fed back: 0 (enrolment), 6, 12, and 18 months Who information fed back to: Clinicians Information fed back: Scores |
|
| Outcomes | Main outcome: adverse events measured by the AEP and quality of life measured by the Quality of Life Inventory for Epilepsy‐31 (QOLIE‐31). | |
| Notes | Funded by the Italian Medicines Agency (Agenzia Italiana del Farmaco [AIFA]) (FARM52K2WM_003) and the University Pavia. The study was conducted between 2006 and 2009. No conflicts of interest are reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Secure online system delivered allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Baseline outcome measurements similar | Low risk | Measurements the same. |
| Baseline characteristics similar | Low risk | Characteristics similar. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Was study protected against contamination | High risk | Multi‐site no cluster design. |
| Selective reporting (reporting bias) | Unclear risk | No published protocol. |
German 1987.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 809 adults recruited from general practices who had a 'positive' score on the GHQ. | |
| Interventions | Feedback of depression scores reported to the physician. Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: General Health Questionnaire (GHQ) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting How administered: Interviewer‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Main outcome: number of enrolled on treatment program. Other outcomes: percentage of patients attending counselling, percentage of patients attending social agency contact, percentage of patients with psychotropic drugs noted or prescribed, referral to mental health specialist or other agency. | |
| Notes | The study was supported in part by contract 278‐81‐0026(DB) from the National Institute of Mental Health to the Health Services Research and Development Center, Department of Health Policy and Management, School of Hygiene and Public Health, The Johns Hopkins University. The study took place between December 1981 and March 1982. No conflicts of interest are reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random samples were taken from each day's appointment list. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Unclear risk | Not clearly reported |
| Baseline characteristics similar | Unclear risk | Not clearly reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clearly reported |
| Was study protected against contamination | Low risk | Control group had no access to the intervention. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section |
Gilliam 2004.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 62 epilepsy patients with an AEP (Adverse Events Profile) score of at least 45 AEP provided group mean age 38.6 years (SD 9.5) female 68% AEP inaccessible group mean age 38.9 (SD 11.9) female 67% | |
| Interventions | Measuring drug side effects (AEP)
Measuring quality of life of epilepsy patients (QOLIE‐89) Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: The Adverse Events Profile (AEP) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (epilepsy) Administration features Where PROMs administered: Unclear How administered: Unclear Format of PROMs questionnaire(s): Unclear Feedback features Format of PROMs feedback: Unclear How often information fed back: Over 4 months. Mean clinic visits 2.2 (SD, 0.89), range 1 to 4. Who information fed back to: Clinicians Information fed back: Scores |
|
| Outcomes | Main outcome: improvement in drug side effects (AEP) Other outcomes: quality of life (QOLIE‐89) | |
| Notes | The study was funded by National Institutes of Health (grant NS01794), GlaxoSmithKline (unrestricted grant). The study took place between 1 February 2001 and 1 April 2001. No conflicts of interest are reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was performed with a computer program |
| Allocation concealment (selection bias) | Low risk | Participants were not informed of their randomisation status (stated in paper) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to study design |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | No significant differences in baseline |
| Baseline characteristics similar | Low risk | No significant differences in characteristics |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No discussion of how incomplete data was addressed ‐ attrition rate was between 63% and 78% |
| Was study protected against contamination | Low risk | Unlikely as standard practice was used for control patients when scores were not available |
| Selective reporting (reporting bias) | Low risk | All the outcome assessments reported in methods were presented in the results section |
Girgis 2009.
| Study characteristics | ||
| Methods | Randomised trial, Australia | |
| Participants | 356 patients with non‐localised breast or colorectal cancer within 6 months of diagnosis Usual care mean age 57.4 years female 71.8% O/GP mean age 58.3 years female 72.3% TCW mean age 57.8 years female 72.5% | |
| Interventions | Feedback of PROs via either a telephone caseworker or a oncologist/GP
Anxiety and depression (HADS)
Quality of Life (EORTC version 3)
Perceived needs (Suportive Needs Survey‐Short Form) Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: European Organisation for Research and Treatment of Cancer (EORTC QLQ‐C30), 34‐item Supportive Needs Survey‐SF (perceived needs), 10 items from the Needs Assessment for Advanced Cancer Patients Questionnaire (other prevalent needs) Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (cancer) Administration features Where PROMs administered: Non‐clinical setting How administered: Interviewer‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic, Paper How often information fed back: 3 times (baseline, 3 months, and 6 months) Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcome: impact of supportive care models Other outcomes: anxiety and depression (HADS), quality of Life (EORTC version 3), perceived needs (Suportive Needs Survey‐Short Form). The study period is not reported. The authors declare no conflicts of interest. | |
| Notes | The study was funded by National Health and Medical Research Council of Australia Palliative Care Research (grant# 300807; Medical Benefits Fund of Australia; Hunter Medical Research Institute (infrastructure support); Afaf Girgis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed quote: "using a computer‐generated algorithm". |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Unclear risk | No baseline outcome scores provided ‐ only at T2 and T3 in table 1 |
| Baseline characteristics similar | Low risk | Paper states quote: "All groups had similar baseline demographic and clinical characteristics" (table provided). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Was study protected against contamination | Unclear risk | GPs and oncologists nominated by control groups participants but intervention participants allocated case workers ‐ unclear as to whether either group could have had access to the other information |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
Gold 1989.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 599 non critical emergency department patients Mean age unknown Female 61.2% | |
| Interventions | Providing the results of a psychiatric screening instrument (GHQ) to emergency physicians. Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: The General Health Questionnaire‐28 (GHQ‐28) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Main outcome: patient judged to have a psychiatric problem (psychiatric diagnosis and/or psychosocial referral) Other outcomes: acceptance rate of psychosocial referral, medical management (number of laboratory test or medical/surgical referrals) | |
| Notes | The study was funded by National Institute of Mental Health (grant# RO 1 MH 3703). The study was conducted between 1 January 1983 and 1 February 1984. No statement was given concerning conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paper only states quote "Patients were assigned to the control or intervention group based on the time they presented to the ED." |
| Allocation concealment (selection bias) | Unclear risk | Not stated in text |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Unclear risk | There was only one measure ‐ i.e. no baseline and follow‐up. |
| Baseline characteristics similar | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Was study protected against contamination | Unclear risk | The physicians would have had a different process for intervention patients ‐ but it was unclear as to whether they knew what to expect |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
Goldsmith 1989.
| Study characteristics | ||
| Methods | Cluster‐randomised trial, USA | |
| Participants | 62 older adults (mean age 70 years) with at least one chronic illness attending a family physicians. | |
| Interventions | Feedback of score from the SIP immediately before a visit. Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: Sickness Impact Profile (SIP) Constructs measured: Functioning Instrument categories/domains: Domain/Disease specific (physical health) Administration features Where PROMs administered: Clinical setting How administered: Interviewer‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Main outcome: physician and patient agreement on the presence of disabilities. | |
| Notes | The study was funded by American Academy of Family Physicians. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Startified randomisation method |
| Allocation concealment (selection bias) | High risk | Due to cluster‐randomised design not possible to conceal allocation from clinicians. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Unclear risk | Not clearly reported |
| Baseline characteristics similar | Low risk | There were no statistically significant differences between the intervention and control groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Was study protected against contamination | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | All relevant outcomes in the methods section are reported in the results section |
Gossec 2018.
| Study characteristics | ||
| Methods | Randomised trial, France | |
| Participants | 320 rheumatoid arthritis (RA) patients in 13 rheumatology centres across France. | |
| Interventions | Online interactive electronic e‐health platform developed to allow patient self‐assessment and self‐monitoring. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: RAPID3 Health Assessment Questionnaire, RA Impact of Disease scores, as well as symptoms as free text Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Generic, Domain/Disease specific (rheumatoid arthritis) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: Patients not prompted, at their discretion how many times they recorded information and received feedback. Who information fed back to: Patients (who can share with clinicians at their instigation) Information fed back: Scores, Previous scores, Interpretation guidance |
|
| Outcomes | Main outcome: change in patient‐physician inter‐ actions, assessed using the Perceived Efficacy in Patient–Physician Interactions questionnaire (PEPPI‐5), over 12 months. | |
| Notes | The study was funded by UCB France and e‐Health Services Sanoia. The study period was between June 2014 and April 2016. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of how randomisation was done. |
| Allocation concealment (selection bias) | Unclear risk | No information about randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Outcome scores in Table 2 similar at baseline. |
| Baseline characteristics similar | Low risk | No sig differences between groups. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data were imputed using last observation carried forward. |
| Was study protected against contamination | Low risk | Control group not informed of the intervention. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes reported in the results. |
Gutteling 2008.
| Study characteristics | ||
| Methods | Randomised trial. the Netherlands | |
| Participants | 162 adults (mean age 48 years) | |
| Interventions | Computerized HRQOL assessment completed and feedback graphically to clinicians. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: 12‐Item Short Form Survey (SF‐12), PCS Physical Component Summary, MCS Mental Component Summary, LDSI 2.0 Liver Disease Symptom Index 2.0 Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Generic, Domain/Disease specific (liver disease, mental health) Administration features Where PROMs administered: Clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: Before each consultation for the duration of one year Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance |
|
| Outcomes | Main outcomes: generic HRQOL (measured with the SF‐12), disease‐specific HRQOL (measured with the LDSI 2.0) | |
| Notes | No funding declared. The study was initiated between September 2004 and September 2005. Conflicts of interest were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Restricted randomisation procedure through blocking |
| Allocation concealment (selection bias) | High risk | Due to cluster‐randomised design not possible to conceal allocation from clinicians. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the intervention, it was impossible to blind physicians to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The PROM used for feedback was also used for outcome assessment |
| Baseline outcome measurements similar | Low risk | Adjusted for analysis |
| Baseline characteristics similar | Low risk | There were statistically significant differences between the intervention and control groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Out of 327, 162 patients were included in the data analyses |
| Was study protected against contamination | Low risk | physicians rather than patients were randomly assigned to either the intervention or control group. |
| Selective reporting (reporting bias) | Low risk | None reported |
Haas 2016.
| Study characteristics | ||
| Methods | Randomised trial, USA. | |
| Participants | 117 patients with cancer beginning chemo, hormone, or radio therapy. | |
| Interventions | Assessment of symptoms using the FACT‐G bi‐weekly with feedback to clinical team vs usual care. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: SymptomCareAnywhere (SCA) Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (Cancer) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: At lease weekly Who information fed back to: Clinicians, Patients Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcome: FACT‐G scores. | |
| Notes | Funding source not reported. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported ‐ abstract only. |
| Allocation concealment (selection bias) | Unclear risk | Not reported ‐ abstract only. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported ‐ abstract only. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported ‐ abstract only. |
| Baseline outcome measurements similar | Unclear risk | Not reported ‐ abstract only. |
| Baseline characteristics similar | Unclear risk | Not reported ‐ abstract only. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only regular users of the interventions were included in the analysis (22 of 51 randomised). |
| Was study protected against contamination | Unclear risk | Not reported ‐ abstract only. |
| Selective reporting (reporting bias) | Unclear risk | Not reported. |
Hadjistavropoulos 2009.
| Study characteristics | ||
| Methods | Randomised trial, Canada | |
| Participants | 114 patients at least 65 years old with complex medical problems and who where being assessed by case coordinators working for the local health region Mean age 80.7 (SD 7.9) Female 70.4% | |
| Interventions | Integrate geriatric depression scale (GDS‐SF) and a Pain Assessment Battery (21‐point box scale, GPM, GDS‐SF and Pain drawing) into usual care
Quantify medications (MQS‐III). Intervention features Single complex feedback (multiple PROMs at a single time) PROM(s) used as intervention: The 21‐point box pain scale, the Geriatric Pain Measure (GPM), the Geriatric Depression Scale ‐ short form (GDS‐SF) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (geriatric health) Administration features Where PROMs administered: Clinical setting How administered: Interviewer‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians, Patients Information fed back: Scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcomes: change in medication practices, change in patient self‐reports of pain | |
| Notes | The study was funded by Canadian Institutes of Health Research. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not stated |
| Allocation concealment (selection bias) | High risk | Due to cluster‐randomised design not possible to conceal allocation from clinicians. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to study design |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Unclear risk | Only baseline outcomes presented for experimental group not the control group |
| Baseline characteristics similar | High risk | Baseline measurements not collected from control group participants. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No mention of how missing data would be handled but there were 30 dropouts from the experimental group from baseline to follow‐up |
| Was study protected against contamination | Unclear risk | The study was announced in the local clinicians meetings and mailed the study information ‐ although patients were recruited through case coordinators ‐ thus unclear who knew what information |
| Selective reporting (reporting bias) | Low risk | None apparent. |
Hansson 2013.
| Study characteristics | ||
| Methods | Randomised trial, Sweden | |
| Participants | 374 patients from a psychiatric outpatient clinic Mean age 39 years (SD 13) Female 73% | |
| Interventions | Feedback of treatment progress with OQ‐45 scores to the patient and therapist. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Outcome Questionnaire 45 (OQ‐45) Swedish version Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Electronic How often information fed back: Weekly Who information fed back to: Clinicians, Patients Information fed back: Scores, Previous scores |
|
| Outcomes | Main outcome: efficacy in patients regarding changes in the total OQ‐45 scale Other outcomes: changes in the OQ‐45 subscales of psychiatric symptoms, interpersonal problems and social functioning, frequency of OQ‐45 scores indicating alert status | |
| Notes | The study was funded by Improved process for reporting of illness (grant), Skåne, Skåne County Council; Skåne County Council’s Research and Development Foundation; Swedish Social Insurance Agency, Malmö. The study period was not reported. The authors reported no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation performed using a pre‐prepared list |
| Allocation concealment (selection bias) | Low risk | Paper reads:quote: 'Everyone involved—patient, receptionist, therapist and researcher—were blinded to the allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Table 2 presented similar baseline scores between groups |
| Baseline characteristics similar | Low risk | Table provided and paper states:quote: "'No significant differences were found between first visits and other study participants concerning the number of visits during the study year, sex and age." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysed performed with last value carried forward for missing data. |
| Was study protected against contamination | Unclear risk | Unsure whether patients were able to access information on intervention and control groups. All the therapists in the intervention group were trained but there was no info about the control group |
| Selective reporting (reporting bias) | Low risk | None apparent |
Hawkins 2004.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 201 outpatients at a hospital‐based psychotherapy clinics Mean age 30.8 years (SD 10.5) Female 68% | |
| Interventions | Feedback of treatment progress with the OQ‐45 to only therapists, and to both patients and therapists. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Outcome Questionnaire 45 (OQ‐45) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Each session Who information fed back to: One intervention group therapists only, one intervention group therapists and patients Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcome: effect of feedback on OQ‐45 scores Other outcomes: effect of feedback on amount of psychotherapy | |
| Notes | Funding source not disclosed. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomised block design was used. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | None apparent |
| Baseline characteristics similar | Unclear risk | Baseline measurements provided but significance in difference not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rate of attrition, 112 of 313 participants (35.8%) excluded from analysis |
| Was study protected against contamination | Unclear risk | Unsure what information was provided to controls ‐ although therapists were either intervention or control. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
Hoekstra 2006.
| Study characteristics | ||
| Methods | Randomised trial, the Netherlands | |
| Participants | 146 patients with cancer in the palliative phase | |
| Interventions | Symptom reporting with a systematic symptom monitoring instrument (Symptom Monitor). Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Symptom Monitor (assessing 10 symptoms) Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (cancer) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Weekly Who information fed back to: Clinicians Information fed back: Scores |
|
| Outcomes | Main outcome: prevalence and severity of symptoms (Symptom Monitor) | |
| Notes | The study was funded by the Dutch Cancer Society (grant). The study recruited between January 2000 and June 2002. Conflicts of interest were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block design (randomisation by GP practice) |
| Allocation concealment (selection bias) | High risk | Patients knew their allocation and so did the therapist in the intervention group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to study design. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Tables 2 and 3 compare the prevalence and symptom severity scores at baseline between groups which are similar |
| Baseline characteristics similar | Low risk | Table is provided and paper states that the baseline characteristics were quote "distributed equally in terms of age and gender between the two groups". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study group expected a high dropout rate due to death and analysis was done separately for complete datasets |
| Was study protected against contamination | Low risk | Randomisation by GP practice. |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
Hoeper 1984.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 1452 adult patients from a multi‐speciality group practice Mean age unknown Female 58.4% | |
| Interventions | Providing the results of GHQ mental disorder scores to the physician. Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: GHQ‐28 Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Main outcome: effect of mental disorder screening with GHQ on the rate of detection of mental disorders | |
| Notes | The study was funded by National Institute of Mental Health (contract 278‐79‐0013). Patients were recruited between 29th Oct 1979, and 1st April 1980.Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to study design (disclosure of questionnaire to physician versus not) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | None apparent |
| Baseline characteristics similar | Unclear risk | Paper states quote "There were only slight sociodemographic differences between groups." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Was study protected against contamination | High risk | Physicians saw participants from both the intervention and control groups. |
| Selective reporting (reporting bias) | High risk | Paper states quote: "analyses of several characteristics thought to influence physician diagnosis were done." |
Jha 2013.
| Study characteristics | ||
| Methods | Randomised trial, UK | |
| Participants | 48 patients (24 recovery, 24 on‐treatment) with early dementia visiting a specialist mental health team. The sample included mainly females (n = 37,77%) with a mean age of 78.4 years in the recovery group and 79 in the treatment (control) group | |
| Interventions | Recovery patients received pre‐diagnostic well‐being assessment and counselling, diagnostic consultation with written feedback and post‐diagnostic support over a period of 6 months. Single simple feedback (one PROM at a single time) PROM(s) used as intervention: Mini Wellness State Examination (MWeSE) ‐ adapted from WHO‐Five Well‐Being Index Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (Mental health) Administration features Where PROMs administered: Clinical setting How administered: Interviewer‐administered Format of PROMs questionnaire(s): Unclear Feedback features Format of PROMs feedback: Unclear How often information fed back: Once Who information fed back to: Clinicians, Patients Information fed back: Scores, Management recommendations |
|
| Outcomes | Main outcome: recovery‐focused pre‐diagnostic well‐being assessment and the WHO Wellbeing Index Other outcomes: mental state (Mini Mental State Examination), depression (Cornell Scale for Depression in Dementia, HRQOL (EUROQOL EQ‐5D), caregiver burden (Zarit Burden Interview) |
|
| Notes | Study funding not disclosed. The study period was not reported. The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomisation list, prepared by the study statistician, was used. |
| Allocation concealment (selection bias) | Low risk | Single‐blind design. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Unclear risk | Table 2 provided similar outcome measurements for baseline |
| Baseline characteristics similar | Unclear risk | Baseline characteristics provided but no indication of significance testing for differences. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Was study protected against contamination | Unclear risk | All intervention patients were allocated to a specific nurse and controls to other nurses ‐ possibly to limit contamination |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
Kazis 1990.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 1920 patients with rheumatoid arthritis treated at Boston University (BU) Arthritis Center and the Vanderbilt University (VU) Division of Rheumatology and Immunology. BU participants were mainly female (78%) with an average age of 56 years. VU participants were mostly female (78%) with an average age of 57 years. | |
| Interventions | The health status report of the intervention group involved quarterly patient assessments coupled with quarterly health status reports sent to the patients’ doctors every 3 months over 1 year. The attention placebo group completed quarterly assessments, but health status reports were not fed back to the doctors. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Arthritis Impact Measurement Scales (AIMS), Modified Health Assessment Questionnaire (MHAQ) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (arthritis) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Up to 5 administrations over a year Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance |
|
| Outcomes | Main outcomes: Arthritis Impact Measurement Scales (AIMS), Modified Health Assessment Questionnaire (MHAQ) | |
| Notes | The study was supported by Robert Wood Johnson Foundation Program on Functional Status, NIH Multipurpose Arthritis Centre (grant AR20613), NIH (grant AM‐21393) (ARAMIS), Arthritis Foundation, Jack C. Massey Foundation. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not stated. |
| Allocation concealment (selection bias) | Unclear risk | No mention of who knew about the allocations |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Physicians knew the patients in the intervention group because they were sent weekly reports |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Only placebo group physicians were sent outcome assessment discussion with their physician |
| Baseline outcome measurements similar | Low risk | Table 2 had no sig differences between groups for baseline data |
| Baseline characteristics similar | Low risk | First paragraph of the results showed similar characteristics of both groups |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No mention of how they would deal with missing data |
| Was study protected against contamination | High risk | Study completed at two sites, with 'similar study designs'. |
| Selective reporting (reporting bias) | Low risk | None apparent. |
Kendrick 2017.
| Study characteristics | ||
| Methods | Partly individually randomised, partly cluster‐randomised controlled trial, UK | |
| Participants | 47 adults with new episodes of depression, in 9 general practices in Southern England. | |
| Interventions | Patient Health Questionnaire, Distress Thermometer Analogue Scale and PSYCHLOPS problem profile for monitoring depression, following diagnosis and at 10–35 days later. Feedback of scores to patients was determined by practitioners. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: PHQ‐9 for depressive symptoms, Distress Thermometer Analogue Scale for distress, PSYCHLOPS profile rating of one or two problems individual to the patient Constructs measured: Health related Quality of Life, Symptoms, Functioning, Other (Rating of one or two problems individual to the patient ‐ PSYCHLOPS) Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical and non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Unclear How often information fed back: Twice (follow up consultation 10‐35 days later) Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Main outcome: Beck Depression Inventory (BDI‐II). Other outcomes: Work and Social Adjustment Scale (WSAS), EuroQol Five‐item, Five‐level (EQ‐5D‐5L), Scale for quality of life, modified Client Service Receipt Inventory for costs, Medical Informant Satisfaction Scale (MISS) |
|
| Notes | The study was supported by National Institute for Health Research (NIHR) Research for Patient Benefit (RfPB) Programme (grant number PB‐PG‐0613‐31004). The study period was not reported. The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial statistician used computer sequence generation. |
| Allocation concealment (selection bias) | High risk | Due to (part) cluster‐randomised design not possible to conceal allocation from clinicians. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | High risk | Control group patients had higher scores for depression, social functioning was whose and anxiety higher at baseline. |
| Baseline characteristics similar | Low risk | Reasonably balanced ‐ but there were more married/cohabiting patients in intervention group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Did not mention how they would deal with incomplete data ‐ but this was a feasibility study. |
| Was study protected against contamination | Low risk | Control did not complete any PROMs. |
| Selective reporting (reporting bias) | Low risk | All outcome measurements mentioned in methods section was reported in results. |
Kornblith 2006.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 192 older patients with breast, prostate, and colorectal cancers who had advanced disease and currently were receiving treatment (initiate 2 months or less prior to recruitment). Mean age was 73 years in the TM+EM group and 74 in the EM group. No significant differences in sociodemographic characteristics between treatment arms | |
| Interventions | Patients were randomised to receive either telephone monitoring (TM) + educational materials (EM) or EM alone. EM involved support for people with cancer and the people who care about them, eating hints for cancer patients, helping hand, as well as available resources that were specific to each disease site. TM involved 1 telephone call each month for 6 months from centralized, trained telephone monitors. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Hospital Anxiety and Depression Scale (HADS), European Organisation for Research and Treatment of Cancer (EORTC) QoL questionnaire (QLQ‐C30), Medical Outcomes Study (MOS) Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health, cancer) Administration features Where PROMs administered: Non‐clinical setting How administered: Interviewer‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: 3 times (study entry, 6 months, 9 months) Who information fed back to: Clinicians Information fed back: Scores |
|
| Outcomes | Main outcome: depression (HADS) Other outcomes: General physical symptoms (EORTC QLQ‐C30), general physical health (Older American Resources and Services Questionnaire), depression (GDS‐SF), social support (MOS Social Support Survey), mental health services (Utilisation of Mental Health and Psychosocial Services instrument). life events (Geriatric Schedule of Recent Experience (GSRE)), cognitive impairment (Patient Satisfaction with the Research Program BOMC test) |
|
| Notes | The study was supported by National Cancer Institute (grants# CA31946, CA33601); Cancer and Leukemia Group B Foundation. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not stated. |
| Allocation concealment (selection bias) | Unclear risk | Two researchers conducted telephone interviews with patients but it was unclear as to who knew which group they were in |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to study design (telephone monitoring + educational materials versus educational materials only) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Table 2 ‐ baseline outcome measurements were similar between the two groups |
| Baseline characteristics similar | Low risk | Paper reads: quote: No significant differences with regard to sociodemographic or disease characteristics were observed." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A plan to deal with missing data was put into place due to the potential low rates of attrition due to the population group |
| Was study protected against contamination | Low risk | Only the telephone monitor phoned the patients to collect the data and the patients had no contact with others who knew about the study |
| Selective reporting (reporting bias) | Unclear risk | None apparent. |
Kroenke 2018.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 300 patients in general internal medicine and family practice clinics in an academic healthcare system. | |
| Interventions | After completing the PROMIS symptom measures electronically immediately prior to their visit, the 300 study participants were randomised to a feedback group in which their clinician received a visual display of symptom scores or a control group in which scores were not provided to clinicians. Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: PROMIS (Patient‐Reported Outcome Measure Information System) Constructs measured: Symptoms Instrument categories/domains: Generic Administration features Where PROMs administered: Clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Main outcome: 3‐month change in composite SPADE score Other outcomes: individual symptom scores, symptom documentation in the clinic note, symptom‐specific clinician actions, and patient satisfaction |
|
| Notes | Supported by Patient‐Centered Outcomes Research Institute (PCORI) Contract ME‐1403‐12043. The study period was not reported. The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. participants were allocated to the feedback or control group in randomly alternating computer‐generated blocks of 2 and 4. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Baseline variables were well balanced between groups. |
| Baseline characteristics similar | Low risk | Baseline charactereistics reported in text and table. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 85.3% follow‐up at 3‐month period. |
| Was study protected against contamination | Unclear risk | Clinicians were allocated within a clinic or clinics and it is possible that communication between intervention and control professionals could have occurred. |
| Selective reporting (reporting bias) | Low risk | None apparent. |
Kuo 2020.
| Study characteristics | ||
| Methods | Randomised trial, Canada. | |
| Participants | 96 patients with advanced non‐small cell lung cancer. | |
| Interventions | Electronic Lung Cancer Symptom Scale scores delivered to clinicians at each visit vs. usual care. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Electronic Lung Cancer Symptom Scale (eLCSSl‐QL) Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (lung cancer) Administration features Where PROMs administered: In clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: Patients completed the elcss‐ql at baseline, before each chemotherapy cycle, and at subsequent follow‐up visits until disease progression. Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Management recommendations |
|
| Outcomes | Main outcome: palliative care referral rates. Secondary outcome: health‐related quality of life. |
|
| Notes | Funded by the Princess Margaret Research Foundation and the Ontario Cancer Research Network. Patients were recruited between November 2004 and May 2011. The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded by nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Baseline outcome measurements similar | Low risk | Baseline measurement the same. |
| Baseline characteristics similar | Low risk | Baseline characteristics similar. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Was study protected against contamination | Low risk | Cluster‐randomised design at the level of the oncologist. |
| Selective reporting (reporting bias) | Unclear risk | Not reported. |
Lambert 2001.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 609 clients treated in a university counselilng centre. Mean age of participants was 22.23 years and were mainly female (70%) | |
| Interventions | Participants were randomly assigned to the experimental (feedback) or control (no feedback) groups. Feedback was provided to participants weekly by a therapist and was based on participant scores on the Outcome Questionnaire. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Outcome Questionnaire (OQ) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Unclear How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Weekly Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcome: psychological dysfunction (Outcome Questionnaire) | |
| Notes | The study was supported by Brigham Young University; German‐American Academic Council Foundation. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not reported. |
| Allocation concealment (selection bias) | Unclear risk | Both control and experimental groups of therapists were given same information. Clients were unaware |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | All pretreatment OQ scores were similar at baseline |
| Baseline characteristics similar | Low risk | No sig differences were found between groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of how incomplete data was handled |
| Was study protected against contamination | High risk | Physicians saw participants from all groups so cross‐contamination possible. |
| Selective reporting (reporting bias) | Unclear risk | None apparent. |
LeBlanc 2019.
| Study characteristics | ||
| Methods | Randomised trial, USA. | |
| Participants | 50 patients with advanced cancer. | |
| Interventions | Assessment of symptoms using the Edinburgh Symptom Assessment Scale with feedback. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: App developed based on the Edmonton Symptom Assessment Scale, ESAS) Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (cancer) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: Repeated over 12 weeks Who information fed back to: Appears to be patients only Information fed back: Scores, Management recommendations |
|
| Outcomes | Main outcome: feasibility of using the app. Secondary outcomes: Knowledge of care programmes, usability, satisfaction, quality of life, and patient activation. | |
| Notes | The study was sponsored by Duke University Cancer Centre and AstraZeneca. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information reported. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded by nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information reported. |
| Baseline outcome measurements similar | Unclear risk | Insufficient information reported. |
| Baseline characteristics similar | Unclear risk | Insufficient information reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information reported. |
| Was study protected against contamination | Unclear risk | Insufficient information reported. |
| Selective reporting (reporting bias) | High risk | Reported outcomes differ to trial registration information. |
Linn 1980.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 150 ambulatory care patients. | |
| Interventions | Feedback of depression scores inserted into patient note. Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: Zung self‐rating depression scale (SDS) Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Main outcome: presence of depression notation in the patient's note at two weeks. | |
| Notes | The study was supported by grant 2177 from the Robert Wood Johnson Foundation and by U.S. Public Service training grant 1‐D28‐19157‐01. The study was conducted between August and October 1979. Conflicts of interest were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Unclear risk | Not clear |
| Baseline characteristics similar | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Was study protected against contamination | High risk | clinicians were allocated within a clinic or clinics and it is possible that communication between intervention and control professionals could have occurred |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
Lugtenberg 2020.
| Study characteristics | ||
| Methods | Randomised trial, the Netherlands. | |
| Participants | 113 patients with Stage I‐IIIB breast cancer treated with chemotherapy. | |
| Interventions | Scores from a PROM assessing quality of life, distress, and care needs fed back to clinicians before chemotherapy cycles vs. usual care. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: The European Organization for Research and Treatment of Cancer BR‐23 breast cancer questionnaire, The Care Notebook (CNB), The National Comprehensive Cancer Network (NCCN) Distress Thermometer (DT), One free text dialog box (patients were invited to list topics or specific questions they would like to discuss with their HCP during their next hospital visit), One question assessing additional supportive care needs. Constructs measured: Health related Quality of Life, Symptoms, Functioning, other (additional supportive care needs) Instrument categories/domains: Domain/Disease specific (cancer) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Unclear Feedback features Format of PROMs feedback: Electronic How often information fed back: 3 episodes of recording. Fed back on second and third visit Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Primary outcome: number of quality of life topics discussed prior to chemotherapy initiation. | |
| Notes | Funded by Dutch Pink Ribbon Foundation and Pfizer, Japan. The study period was not reported. The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation tool not described. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded by nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Baseline outcome measurements similar | Low risk | Baseline measurement the same. |
| Baseline characteristics similar | Low risk | Baseline characteristics similar. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data not dealt with. |
| Was study protected against contamination | High risk | Randomisation at the patient level. |
| Selective reporting (reporting bias) | High risk | No protocol. |
Magruder‐Habib 1990.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 100 depressed patients, both new and known. | |
| Interventions | Feedback of depression scores inserted into patient note. Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: Zung self‐rating depression scale (SDS) Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Interviewer‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores |
|
| Outcomes | Primary: percentage of patients treated for depression. | |
| Notes | The study was supported in part by a grant (R01MH39730) from the National Institute of Mental Health, and the A.W. Mellon Foundation. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomised by a personal computer in blocks of 10, however it is not clear who did this |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | No significant differences were found |
| Baseline characteristics similar | Low risk | There were no statistically significant differences between the intervention and control groups |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 880 eligible patients, 112 (12.7%) who met both screening criteria and were considered quote: "unrecognized" depressed patients |
| Was study protected against contamination | High risk | Hawthorne effect for the physicians, which would increase contamination of the control group |
| Selective reporting (reporting bias) | Low risk | None reported |
Mathias 1994.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 75 physicians and 573 primary care patients with unrecognised and untreated anxiety at TakeCare, a mixed‐ model health maintenance organisation (HMO) in central Colorado. Mean age of participants was 41.5 years for the demonstration group and 43.6 for the control and were mainly female (61.1% for the demonstration group and 54.6% for the control) | |
| Interventions | Participating physicians were randomised to either the demonstration or the control arm, and patients were assigned to a study arm based on the randomisation of their physicians.The patients were followed for change in outcome measures during the five‐month study period. The physician intervention was to providing an educational demonstration of anxiety in the primary care setting and to provide a reporting system for summarising the anxiety symptom levels and functioning status of the patients enrolled in the study. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Global Anxiety Score (GAS), Global Severity Index (GSI), Highest Anxiety Subscale Score (HASS) Constructs measured: Symptoms, Functioning Instrument categories/domains: Generic, Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Electronic and Paper Feedback features Format of PROMs feedback: Unclear How often information fed back: 3 times Who information fed back to: Clinicians Information fed back: Scores |
|
| Outcomes | Main outcomes: anxiety symptoms (GAS, HASS) Other outcomes: psychological distress (the Global Severity Index (GSI), functioning and well‐being (SF‐36), global improvement (perceived changes in anxiety level, functioning and well‐being, and perceived changes in communication with their physicians since the baseline survey). The study period was not reported. Conflicts of interest were not reported. |
|
| Notes | The study was supported by Upjohn Company, Kalamazoo, MI; TakeCare,CO. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Physicians were randomised by call group but no other information available about how that was done |
| Allocation concealment (selection bias) | High risk | Due to cluster‐randomised design not possible to conceal allocation from clinicians. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | None apparent |
| Baseline characteristics similar | Low risk | Table of baseline characteristics provided and no significant differences identified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Baseline characteristics of those lost to follow‐up were compared with those who completed the study |
| Was study protected against contamination | Low risk | None apparent |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
Mazonson 1996.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 573 adult patients with depression or anxiety. | |
| Interventions | Patient‐reported mental health information was fedback to clinicians. Intervention features Single complex feedback (multiple PROMs at a single time) PROM(s) used as intervention: Anxiety and Depression Symptom Checklist (SCL‐90‐R), Functioning and well‐being measures (SF‐36), Diagnostic Interview schedule (DIS) Constructs measured: Symptoms, Functioning Instrument categories/domains: Generic, Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcomes: notation in chart, mental health referral, psychotropic medications. Other outcomes: any hospitalisation, any office visit | |
| Notes | The study was supported by Upjohn Company, Kalamazoo, Mich. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned |
| Allocation concealment (selection bias) | High risk | Patients were assiigned based on the assignment of the primary care physicians practice group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Physician practices rather than patients were randomised |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Adjusted for analysis |
| Baseline characteristics similar | Low risk | There were no statistically significant differences between the intervention and control groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clearly reported |
| Was study protected against contamination | Low risk | To minimise contamination physicians and physician extenders were randomised to intervention or control by physician‐call group |
| Selective reporting (reporting bias) | High risk | None reported |
McCusker 2001.
| Study characteristics | ||
| Methods | Multicentre randomised trial, Canada | |
| Participants | 388 older adults (65.2% male). | |
| Interventions | Feedback and notification to primary care and home care teams. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Older American Resources and Services scale (OARS), Geriatric Depression Scale (GDS) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health, physical health ‐ functional decline) Administration features Where PROMs administered: Clinical setting (ED waiting room) and non clinical setting (by telephone) How administered: Interviewer‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Unclear How often information fed back: 3 times Who information fed back to: Clinicians Information fed back: Scores, Previous scores |
|
| Outcomes | Main outcomes: functional decline (OARS ADL), depressive symptoms (GDS‐SF) | |
| Notes | The study was supported by the Health Transition Fund, Health Canada. The study was conducted from 14th September 1998 to 1st April 1999. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were randomised to the intervention or usual care group by day of recruitment. |
| Allocation concealment (selection bias) | Low risk | Each of two intervention nurses was assigned to two hospitals and rotated between them on a schedule assigned by the statistician, using blocked randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Research assistants did not inform staff which patients were recruited into the study. However, the intervention nurses coordinated the intervention with other staff, who were therefore aware of certain intervention group patients. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Adjusted for analysis |
| Baseline characteristics similar | High risk | There was a significant difference by study group in the proportion of patients with a family caregiver: 76.4% in the intervention group and 65.2% in the control group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 2,166 eligible patients, 63 (2.9%) declined the screening and 11 could not be found to complete the screening. |
| Was study protected against contamination | Low risk | Research assistants did not inform staff which patients were recruited into the study. |
| Selective reporting (reporting bias) | Low risk | Not reported |
McLachlan 2001.
| Study characteristics | ||
| Methods | Randomised trial, Australia | |
| Participants | 450 patients with cancer from the ambulatory clinics at Peter MacCallum Cancer Institute. Median age of participants was 61 years (range, 18 to 92) and were mainly male (59%). | |
| Interventions | Self‐reported cancer needs, QOL, and psychosocial information was collected using standardized questionnaires via a touch‐screen computer. For a randomly chosen 2/3, the information was made available to the health care team who coordinated targeted psychosocial interventions. Information from the remaining 1/3 was not seen. Patients were assessed 2 and 6 months after randomisation for changes in their cancer needs, QOL, and psychosocial functioning and satisfaction with overall care received. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Cancer Needs Questionnaire–short form (CNQ), European Organisation for Research and Treatment of Cancer (EORTC QLQ‐C30), Beck Depression Inventory (BDI) Short Form Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health, cancer) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: 3 times Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcome: psychological and Information Scales of the Cancer Needs (Cancer Needs Questionnaire–short form, CNQ) Other outcomes: remaining domains of the CNQ, HRQOL (EORTC QLQ‐C30), depression (BDI) | |
| Notes | The study was supported by the Commonwealth of Australia; State Government of Victoria. Patients were recruited between March1999 and February 2000. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed using quote: "Computer‐generated randomization charts". |
| Allocation concealment (selection bias) | Low risk | All patients completed the same measurements at the same times ‐ although the intervention received the feedback ‐ unsure whether patients knew which group they were in |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to study design |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Table provided similar outcome scores at baseline for both groups |
| Baseline characteristics similar | Low risk | Paper states quote: "Patient demographics were well balanced in the two arms." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were high and similar across the groups |
| Was study protected against contamination | High risk | Doctors and clinic nurses were involved in seeing both intervention and control patients in the ambulatory care clinics. The health professionals’ behavior may have changed as a result of a heightened awareness of the study purposes and issues raised by patients in the intervention group |
| Selective reporting (reporting bias) | Low risk | None apparent |
Mellema 2015.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 136 orthopaedic patients. | |
| Interventions | Patients were randomly assigned to either receive feedback about the Patient‐Reported Outcomes Measurement Information System (PROMIS) Pain Interference computer‐adaptive test (CAT) prior to the visit with the hand surgeon or not. Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: (PROMIS) Pain Interference computer‐adaptive test (CAT) Constructs measured: Symptoms, Functioning Instrument categories/domains: Generic Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians, Patients Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Main outcome: patient satisfaction with the consultation Other outcomes: patient‐physician communication |
|
| Notes | No funding was reported for this study. The study period was not reported. The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated random numbers and using a permuted block approach |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patient and physician were not blinded to the assignment of intervention research fellows, and research fellows that have evaluated the patient‐physician communication were aware of the allocation of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome data collected by research fellows. And research fellows that have evaluated the patient‐physician communication were aware of the allocation of intervention. |
| Baseline outcome measurements similar | Low risk | The participants of the intervention and control groups also had similar baseline scores. |
| Baseline characteristics similar | Low risk | The intervention and control groups were well balanced. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clearly reported. |
| Was study protected against contamination | Unclear risk | Only one surgeon at a orthpaedic outpatient clinic participated in the study. |
| Selective reporting (reporting bias) | Low risk | None apparent. |
Moore 1978.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 212 adults attending family practices. | |
| Interventions | A note was attached to the patient's visit note indicating depression status as assessed with SDS. Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: Zung self‐rating depression scale (SDS) Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered and interviewer‐administered (patients unable to complete the self‐rating form were interviewed using the interviewer completed form) Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Main outcome: recognition of depression | |
| Notes | No funding was reported for this study. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Paper states quote:"For randomisation an on‐line random number generator was utilised." |
| Allocation concealment (selection bias) | Low risk | Discrete labelling of patient files. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | None apparent |
| Baseline characteristics similar | Low risk | T‐tests used to analyse demographics for differences ‐ no significance found. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rates of attrition, not adequately addressed |
| Was study protected against contamination | Low risk | All the clients had numbered files so did not know which group they were allocated |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
Moore 2019.
| Study characteristics | ||
| Methods | Pilot randomised trial, Australia. | |
| Participants | 32 patients with multiple myeloma | |
| Interventions | Quality of life assessment using the Myeloma Patient Outcome Scale and feedback to clinicians. Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: Myeloma Patient Outcome Scale (MyPOS6) Constructs measured: Health related Quality of Life, Symptoms Instrument categories/domains: Domain/Disease specific (multiple myeloma) Administration features Where PROMs administered: Unclear How administered: Both self‐administered and interviewer‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Non assessed. | |
| Notes | Funded by Gilead Australia Fellowship Research Grant and a grant from Takeda Pharmaceuticals Australia Pvt Ltd. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded by nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Baseline outcome measurements similar | Unclear risk | Not reported. |
| Baseline characteristics similar | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Was study protected against contamination | Low risk | Non‐cluster design but no risk to reported outcomes. |
| Selective reporting (reporting bias) | Low risk | Pilot study reporting acceptability and completion metrics. |
Murillo 2017.
| Study characteristics | ||
| Methods | Cluster‐randomised trial, Spain | |
| Participants | 136 patients recruited from five centres in Barcelona, Spain (72 girls, mean age 13.4 years). | |
| Interventions | The HRQOL intervention consisted of discussing the HRQOL scores between the doctor and the patient at each visit from visit 1 to visit 3, emphasising those points where the result was worse. The scores are reflected in a few simple graphics which the doctor showed the patient on a computer screen. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: KIDSCREEN‐27 Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (diabetes ‐ children) Administration features Where PROMs administered: Both clinical and non‐clinical setting. Questionnaires were completed online at home within 48 hours of visit 1 and 4, patients without home internet completed the questionnaire at hospital. How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: 4 times Who information fed back to: Clinicians, Patients Information fed back: Scores, Previous scores, Interpretation guidance |
|
| Outcomes | Main outcome: HRQOL assessed using KIDSCREEN‐27 collected online | |
| Notes | The study was funded by the Spanish Ministry of Health, contract No. PI12/01296. The study period was not reported. The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only stated quote: "patients were randomly allocated". |
| Allocation concealment (selection bias) | High risk | Due to cluster‐randomised design not possible to conceal allocation from clinicians. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patient and physician were not blinded to the assignment of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | HRQOL was assessed using KIDSCREEN‐27 collected online.The intervention group discussed the results of HRQOL face‐to‐face with the physician, quarterly over a year. |
| Baseline outcome measurements similar | Low risk | No statistically significant differences were found at baseline between HRQOL intervention and control group regarding age, sex, type of family, or the highest family education level. |
| Baseline characteristics similar | Low risk | baseline characteristics of the study and control providers are reported and similar. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 87.5% of participants at baseline completed data at follow‐up. |
| Was study protected against contamination | Unclear risk | Paediatrician randomisation was used rather than patients’, to avoid contamination at paediatricians’ level but the paediatricians could have communicated. |
| Selective reporting (reporting bias) | Low risk | None apparent. |
Murphy 2012.
| Study characteristics | ||
| Methods | Randomised trial between participants design, Ireland | |
| Participants | 60 clients attending an Irish university counselling service. Mean age of participants was 23.82 and were mainly female (58.2%) | |
| Interventions | Participants were randomly assigned to the feedback or no feedback groups. Feedback was provided to participants session‐by‐session progress feedback by a therapist that was based on participant scores on the Outcome Rating Scale. Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: A.S.I.S.T. for Agencies ‐ a PC‐based version of the Outcome Rating Scale (ORS) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians, Patients Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcome: ORS | |
| Notes | Funding information not reported. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Paper states quote: "For randomisation an on‐line random number generator was utilised." |
| Allocation concealment (selection bias) | Low risk | Discrete labelling of patient files. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | None apparent |
| Baseline characteristics similar | Low risk | T‐tests used to analyse demographics for differences ‐ no significance found. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rates of attrition, not adequately addressed |
| Was study protected against contamination | Low risk | All the clients had numbered files so did not know which group they were allocated |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
Myasoedova 2019.
| Study characteristics | ||
| Methods | Randomised trial, USA. | |
| Participants | Adult patients with rheumatoid arthritis. | |
| Interventions | Flare Assessment in Rheumatoid Arthritis (FLARE‐RA) PROM assessment with nurse‐led counselling or an expedited visit with a rheumatology provider offered to patients in the intervention arm who indicated they were in flare versus usual care. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: FLARE‐RA (devised and validated to improve the detection of current and recent flares in rheumatoid arthritis) Constructs measured: Health related Quality of Life, Symptoms, Functioning, other (social and emotional wellbeing) Instrument categories/domains: Generic, Domain/Disease specific (cancer) Administration features Where PROMs administered: Unclear How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Unclear How often information fed back: 4 times Who information fed back to: Clinicians Information fed back: Scores, Management recommendations |
|
| Outcomes | Primary outcome: Flare rate by OMERACT 9 definition. Secondary outcomes: disease activity, remission, flare by provider opinion, treatment change, patient satisfaction, musculoskeletal ultrasound. |
|
| Notes | This work was financially supported by a grant from Pfizer (Grant ID 15322005). The study period was not reported. Conflicts of interest were reported as follows: Disclosures Elena Myasoedova: no disclosures or COI Cynthia S. Crowson: no disclosures or COI Rachel E. Giblon: no disclosures or COI Kathleen McCarthy‐Fruin: no disclosures or COI Daniel E. Schaffer: no disclosures or COI Kerry Wright: no disclosures or COI Eric L. Matteson: Grant/Research/Clinical Trial Support (rheumatoid arthritis) Genentech, Mesoblast, Novartis, Pfizer, Sun Pharmaceutical Industries, Ltd Editorial functions: UpToDate John M. Davis, III: Grant/Research/Clinical Trial Support (rheumatoid arthritis) Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was by a computer‐generated random number algorithm prepared by a statistician |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded by nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Baseline outcome measurements similar | Low risk | Adjusted analysis. |
| Baseline characteristics similar | Low risk | Baseline characteristics similar. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat protocol. |
| Was study protected against contamination | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not published. |
Nimako 2017.
| Study characteristics | ||
| Methods | Randomised trial, UK | |
| Participants | 138 patients attending the Royal Marsden Hospital for cancer treatment. | |
| Interventions | Participants were randomised in equal numbers (1:1:1) to either one of the three groups (Intervention, Attention and Control groups). (1) an Intervention group that completed the European Organisation for Research and Treatment of Cancer–Core Quality of Life Questionnaire and Lung Cancer Module (EORTC QLQ‐C30 and LC13) at baseline and received feedback during a clinic, (2) an Attention group that completed the questionnaire at baseline without feedback and (3) a Control group that did not complete the questionnaire. Intervention features Single complex feedback (multiple PROMs at a single time) PROM(s) used as intervention: European Organisation for Research and Treatment of Cancer (EORTC QLQ‐C30), Lung Cancer Module (LC13) Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (cancer) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians, Patients Information fed back: Scores |
|
| Outcomes | Main outcome: cancer‐related symptoms | |
| Notes | There was no formal funding for this study but the authors acknowledge NHS funding to the Royal Marsden Hospital/Institute of Cancer Research NIHR Biomedical Research Centre and an academic grant from Philips Healthcare. Dr Popat is in receipt of a clinical senior lectureship award from the Higher Education Funding Council for England. The study period was not reported. The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified randomisation was used. |
| Allocation concealment (selection bias) | Low risk | The clinical trials unit carried out randomisation electronically. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The PROM used for feedback was also used for outcome assessment. |
| Baseline outcome measurements similar | High risk | There was a significant difference between the Intervention and Control groups for the mean number of QoL issues identified at baseline. |
| Baseline characteristics similar | Low risk | Baseline characteristics of the study and control providers are reported and similar. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Ninety‐five per cent (131/138) of the participants completed the outcome questionnaire at 6 weeks. |
| Was study protected against contamination | High risk | As the doctors performing the consultation were aware that the patient was a participant in the study, they may have raised the care that they gave to the patients. |
| Selective reporting (reporting bias) | Low risk | None apparent. all relevant outcomes in the methods section are reported in the results section. |
Nipp 2019.
| Study characteristics | ||
| Methods | Pilot randomised trial, USA. | |
| Participants | Hospitalised patients with cancer. | |
| Interventions | Daily symptom reports using the Edmonton Symptom Assessment System and Patient Health Questionnaire‐4 with graphical feedback including alerts to clinical team during daily rounds. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Edmonton Symptom Assessment System and Patient Health Questionnaire‐4 Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (cancer) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: Daily Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Primary outcome: feasibility defined as >75% of patients hospitalised for 3 days or longer completing >2 symptom reports. Secondary outcome: preliminary assessment of feasibility. |
|
| Notes | Funded by National Cancer Institute (USA), Massachusetts General Hospital Cancer Centre, and Schullen Centre for Cancer Data Analysis. The study period was not reported. The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation using a computer. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded by design. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded by design. |
| Baseline outcome measurements similar | Low risk | Adjusted for within analysis. |
| Baseline characteristics similar | Low risk | Baseline values were similar. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis. |
| Was study protected against contamination | High risk | Chance that control arm patients can report symptoms. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not published. |
Picardi 2016.
| Study characteristics | ||
| Methods | Randomised trial, Italy | |
| Participants | 115 patients in 13 primary care practices who screened positive for depression and did not report suicidal ideation. | |
| Interventions | Those who screened positive and did not report suicidal ideation were randomised to an intervention group (communication of the result and offer of psychiatric evaluation and treatment free of charge; 56) or a control group (no feedback on test result for 3 months; 59). Intervention features Single complex feedback (multiple PROMs at a single time) PROM(s) used as intervention: The 5‐item version of the PC‐SAD (PC‐SAD5), WHOQOL‐Bref Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Generic, Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Electronic How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores |
|
| Outcomes | Main outcomes: depression (PC‐SAD), QoL (WHOQOL‐Bref) | |
| Notes | The study was funded by Italian Ministry of Health in the framework of the ‘Programma Ricerca Finalizzata 2006’. The study ran from January2009 to June 2010. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated simple randomisation list. |
| Allocation concealment (selection bias) | Low risk | Participants were given an envelope containing a sociodemographic form and the Primary Care Screener for Affective Disorders (PC‐SAD) and WHOQOL‐Bref questionnaires to complete. Participants placed the completed questionnaires back in the envelope, and they put it in a transparent drop box located in the waiting room. The PC‐SAD was scored through an automated system by a researcher who was not involved in subsequent assessments. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | No significant differences in baseline. |
| Baseline characteristics similar | Low risk | Baseline characteristics of the study and control providers are reported and similar. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 87% of randomised patients (intervention group,N = 46; control group,N = 54) completed the 3‐month assessment. |
| Was study protected against contamination | Low risk | Control group had no access to the intervention. |
| Selective reporting (reporting bias) | Low risk | None apparent. |
Pouwer 2001.
| Study characteristics | ||
| Methods | Randomised trial, the Netherlands | |
| Participants | 400 outpatients with diabetes treated at the outpatient diabetes clinic of Vrije Universiteit Medical Center. Mean age of participants was 53 years for the monitoring group and 54 for the standard care group and were mainly female for the monitoring group (57%) and male for the standard care group (52%) | |
| Interventions | The standard care group had regular appointments with an internist (3‐ to 4‐month intervals) and, if needed, other members of the diabetes team, as well as at least two 15‐minute consultations with the diabetes nurse specialist (DNS) in which various topics related to diabetes were discussed (including psychosocial issues). No formal assessment of psychological well‐being was performed. The diabetes nurse specialist assessed and discussed psychological well‐being with the patient (with an interval of 6 months) in addition to standard care for patients in the monitoring group. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Computerized Well‐being Questionnaire (W‐BQ) Constructs measured: Functioning Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Interviewer‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Unclear How often information fed back: 3 times Who information fed back to: Clinicians Information fed back: Scores, Previous scores |
|
| Outcomes | Main outcome: mood, HbA1c, quality of diabetes care at 1‐year follow‐up (Well‐being Questionnaire). Other outcomes: number of referrals to the psychologist. The study was conducted between May 1997 and December 1999. Conflicts of interest were not reported. | |
| Notes | The study was funded by Dutch Diabetes Research Foundation (grant# 95.805). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers were used. |
| Allocation concealment (selection bias) | Unclear risk | It was unclear whether patients were aware of their allocation. Formal assessments were made in the intervention group by clinicians thus they may have known |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to study design (nurse discussion with patient about their psychological well‐being versus standard care). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | None apparent |
| Baseline characteristics similar | Low risk | Tables provided and paper states Quote:"The monitoring group did not differ signifi‐cantly from the standard care group with regard to demographic, clinical, and psy‐chological variables at baseline." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis was intention‐to‐treat and complete‐case analysis |
| Was study protected against contamination | High risk | Possible contamination by the nurses administering the measurements |
| Selective reporting (reporting bias) | Low risk | None apparent |
Priebe 2007.
| Study characteristics | ||
| Methods | Cluster‐randomised trial, Spain, the Netherlands, UK, Sweden, Germany, and Switzerland | |
| Participants | 134 clinicians and 507 patients from community psychiatric services in participating countries. Mean age of participants was 41.8 years for the treatment as usual group and 42.5 for the intervention group and were mainly male for both groups (64.8% and 67.5%, respectively) | |
| Interventions | Clinicians in the control group continued with standard treatment with their participating patients. Clinicians in the intervention group continued with standard treatment with their participating patients and implemented DIALOG to discuss satisfaction with 11 domains [life domains (mental health, physical health, accommodation, job situation, leisure activities, friendships, relationship with family/partner, personal safety) and treatment domains (practical help, psycho‐ logical help and medication)]. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: DIALOG covering 8 life domains and 3 treatment domains, Manchester Short Assessment of Quality of Life (MANSA), Camberwell Assessment of Need Short Appraisal Schedule, patient‐rated version (CANSA ‐ to assess unmet needs), Client Satisfaction Questionnaire (CSQ–8) Constructs measured: Health related Quality of Life, Symptoms, Functioning, Other (treatment satisfaction) Instrument categories/domains: Generic, Domain/Disease specific (mental health, life satisfaction, treatment satisfaction) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) and non‐clinical setting How administered: Interviewer‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: Every 2 months for 1 year Who information fed back to: Clinicians Information fed back: Scores, Previous scores |
|
| Outcomes | Main outcome: QoL (Manchester Short Assessment of Quality of Life (MANSA) Other outcomes: met needs (Camberwell Assessment of Need Short Appraisal Schedule), satisfaction with treatment at 12 months (Client Satisfaction Questionnaire (CSQ–8)) | |
| Notes | The study was funded by Research Directorate of the European Commission within Framework Programme 5 (QLG5‐CT‐2002‐01938). The study ran from December 2002 until May 2005. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using a quote: "computer‐generated random block number allocation sequence". |
| Allocation concealment (selection bias) | High risk | Cluster‐randomisation design was used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Table 1 presented similar baseline outcome measurements for both groups |
| Baseline characteristics similar | Low risk | Table provided and paper states 'quote: "There were no significant differences in the characteristics of participants in the control and intervention groups." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None apparent |
| Was study protected against contamination | Low risk | Separate clinicians used for control and intervention groups |
| Selective reporting (reporting bias) | Unclear risk | None apparent. |
Probst 2013.
| Study characteristics | ||
| Methods | Randomised trial, Germany | |
| Participants | 43 patients of two psychosomatic clinics. Mean age of participants was 43.45 years for the experimental group and 47.34 for the control. Participants in the experimental group were mainly female (60.9%), but for the control group there was an equal number of males and females. | |
| Interventions | Patients were randomised either into the experimental group or the control group. Both groups were tracked weekly with the Outcome Questionnaire 45 and the Assessment of Signal Cases tool. Therapists received feedback from both instruments for only the experimental group patients and the therapists could choose to discuss the feedback information with the patient, the clinic team and/or supervisors. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Outcome Questionnaire 45 (OQ‐45), Assessment of Signal Cases (ASC) ‐ clinical support tools instrument Constructs measured: Symptoms, Functioning, Other (therapeutic alliance, social support, motivation for change, life events) Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Weekly, at least 3 times Who information fed back to: Clinicians Information fed back: Scores, Previous scores |
|
| Outcomes | Main outcomes: patient progress (OQ‐45), clinical support (Assessment of Signal Cases, ASC) | |
| Notes | The study was funded by Susa Young Gates University (Professorship awarded to Michael J. Lambert). The study was conducted between 2010 and 2012. The authors declared no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified. |
| Allocation concealment (selection bias) | Unclear risk | No information how randomisation occurred although sealed envelopes of scores were given to therapists for their experimental group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | There were similar outcome scores for both groups at baseline (T1 measurement) |
| Baseline characteristics similar | Low risk | Table provided and no significant differences identified. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Incomplete data was excluded from the analysis |
| Was study protected against contamination | Unclear risk | Therapists received closed envelopes with experimental patients feedback, unclear as to whether patients knew or could find out |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
Puschner 2009.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 294 adults receiving inpatient mental health care. | |
| Interventions | Continous feedback of patient‐reported treatment outcome information to physicians in the intervention arm. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: EB‐45, the German version of the Outcome Questionnaire 45.2 (OQ‐45.2) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Paper How often information fed back: Administered weekly, feedback continuous until discharge Who information fed back to: Clinicians, Patients Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcome: measured by the (German version of OQ‐45) | |
| Notes | The study was funded by German Federal Ministry of Education and Research (grant number: 01GL0504). The study period was not reported. The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent unit (Ulm Universitys Institute for Biometrics) randomised all clinicians at the wards where the study took place to either intervention or control group. |
| Allocation concealment (selection bias) | High risk | Cluster‐randomisation with the therapists |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | High risk | Statistically significant differences were found for the outcomes |
| Baseline characteristics similar | High risk | Statistical differences were found for education and diagnosis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout |
| Was study protected against contamination | Low risk | Cluster‐randomisation with clinicians as the unit of randomisation. there were changes of patients between clinicians during inpatient treatment |
| Selective reporting (reporting bias) | Low risk | None reported |
Rand 1988.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 32 residents and 1040 patients visiting a family practice site. Participants were mainly between the ages of 18 to 40 years and female | |
| Interventions | Participants in the experimental group were provided feedback on the GHQ by the residents. Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: GHQ‐28 Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores |
|
| Outcomes | Main outcomes: psychiatric screening (GHQ), chart audit form (psychologic or psychiatric of condition, and patient demographics (sex, race, age)) | |
| Notes | The study was funded by University of Alabama and College of Community Health Sciences Research Grants Committees. The study period was not reported. The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not specified. |
| Allocation concealment (selection bias) | High risk | Allocation concealment not possible due to cluster‐randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind physicians |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Outcome measurements were similar at baseline. |
| Baseline characteristics similar | Low risk | Table is provided and paper states that no significant differences between groups were found. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data were not mentioned in terms of how it was handled statistically |
| Was study protected against contamination | Low risk | Sites were randomised but it was not clear whether the sites were in contact with each other |
| Selective reporting (reporting bias) | Low risk | None apparent. |
Reese 2009.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | Study 1: 74 clients that received individual therapy at a university counselling centre (UCC). Study 2: 74 clients receiving individual therapy at a graduate training clinic for a marriage and family therapy master’s program (MFC) | |
| Interventions | Study 1: Clients in the feedback condition completed the ORS at the beginning of each session and the SRS at the end of each session. Participants in the no‐feedback condition completed the ORS only at the beginning and end of treatment. Study 2: Clients in the feedback condition completed the ORS at the beginning of each session and the SRS at the end of each session. Clients in the no‐feedback condition completed the ORS at the beginning of each session, rather than just at the beginning and end of treatment. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Outcome Rating Scale (ORS) Constructs measured: Functioning Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Interviewer‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Each session over academic year Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcomes: outcomes (ORS), therapeutic alliance (Session Rating Scale, SRS) | |
| Notes | Funding not reported. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomised block design was used. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was not detailed enough to determine whether patients or clinicians knew of their allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Pretreatment mean differences were not statistically significant between groups |
| Baseline characteristics similar | Unclear risk | No mention of characteristics of the sample |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None apparent |
| Was study protected against contamination | Unclear risk | Unsure as it was not clear how therapists and clients were randomised |
| Selective reporting (reporting bias) | High risk | Not all results presented from feedback group |
Richardson 2008.
| Study characteristics | ||
| Methods | Randomised trial, Canada | |
| Participants | 265 community‐dwelling people from family practice units. Mean age of participants was 73.89 years for the control group and 73.61 for the intervention group and were mainly female for both groups (55.1% and 53.6%, respectively) | |
| Interventions | Participants in the intervention group attended a functional status lab at baseline, 9 months and 18 months post baseline. The intervention group received feedback (approximately 30 minutes) from a physiotherapist or occupational therapist about the results of their assessments. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Self‐Reported Task Modification and Disability Scale, Health Utilities Index – Mark III, Short Form‐36 (SF‐36) Constructs measured: Symptoms, Functioning Instrument categories/domains: Generic, Domain/Disease specific (physical health – older adults) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Unclear Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: 3 times: baseline, 9 months and 18 months post baseline Who information fed back to: Clinicians, Patients Information fed back: Scores, Previous scores, Interpretation guidance |
|
| Outcomes | Main outcomes: health status (SF‐36), functional status (Task Modification and Disability Scale) Other outcomes: utilisation of health services, number of falls or exercise programme attendance, equipment purchase or medication change and were recorded in the encounter log | |
| Notes | The study was funded by Change Foundation, Toronto, Ontario. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers were used to randomise participants to either intervention or control groups |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The assessors were not blinded to the group allocation as they were collecting the outcome measurements |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessments were collected by the therapist delivering the intervention |
| Baseline outcome measurements similar | Low risk | Table 2 in the study showed baseline measurements were similar between intervention and control |
| Baseline characteristics similar | Low risk | Paper states quote: "Participants were similar with respect to age, sex, education and income." Table provided and no significant differences identified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysed performed. |
| Was study protected against contamination | Low risk | The participants and physicians in the control group did not see the information about the intervention |
| Selective reporting (reporting bias) | Unclear risk | None apparent. |
Richardson 2019.
| Study characteristics | ||
| Methods | Randomised trial. | |
| Participants | 300 paediatric primary care patients. | |
| Interventions | Electronic screening and clinician feedback versus. usual care. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: HEADSS (home, education, activities, depression, sexual activity, safety, and substance use) framework Constructs measured: Functioning, Other (emotional wellbeing) Instrument categories/domains: Generic Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: On day 1 and 3 months Who information fed back to: Clinicians Information fed back: Scores |
|
| Outcomes | Main outcome: self‐report of counselling and risk behaviours | |
| Notes | Study was funded by Health Resources and Services Administration of the US Department of Health and Human Services. The study was conducted between 13th March 2015 and 8th August 2016. Conflicts were reported as: Drs Richardson and McCarty reported receiving grants from Health Resources and Services Administration Maternal Child Health Bureau during the conduct of the study. Drs Richardson and McCarty reported having a license agreement with Tickit Health Inc as inventors of the Check Yourself Tool whereby they will receive royalties from the future sale of the tool to other health care companies; Seattle Children’s Hospital has a management plan in place to oversee their interests with Tickit Health Inc. No other disclosures were reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded by nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Baseline outcome measurements similar | Low risk | Baseline outcome measurements the same. |
| Baseline characteristics similar | Low risk | Baseline characteristics similar. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐t0‐treat analysis. |
| Was study protected against contamination | High risk | Non‐cluster design. |
| Selective reporting (reporting bias) | Low risk | Reporting as per protocol. |
Rosenbloom 2007.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 213 adults with metastatic breast, lung or colorectal cancer | |
| Interventions | 3 arm: usual care, HRQL assessment, HRQL assessment + structured interview and discussion. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Functional Assessment of Cancer Therapy‐General (FACT‐G), Functional Living Index‐Cancer (FLIC), Brief Profile of Mood States (Brief POMS‐17) Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health, cancer) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered and interviewer‐administered Format of PROMs questionnaire(s): Unclear Feedback features Format of PROMs feedback: Unclear How often information fed back: Baseline and 1, 2, 3, 6 months Who information fed back to: Clinicians, Patients Information fed back: Scores, Previous scores |
|
| Outcomes | HRQL: Functional Living Index Cancer (FLIC); Brief Profile of Mood States (Brief POMS‐17) for distress outcomes; Medical Outcomes Study Patient Satisfaction Questionnaire‐ III (PSQ‐III) for satisfaction with medical treatment. Lastly a composite clinical treatment change variable was computed. | |
| Notes | Study was funded by American Cancer Society (grant #PBR 6132); National Cancer Institute (grant #R29 CA51926).The study was conducted between 1990 and 1992. Conflicts of interest were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not stated. |
| Allocation concealment (selection bias) | Unclear risk | No mention of who knew about the allocations |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to study design |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible due to study design |
| Baseline outcome measurements similar | Low risk | All baseline assessments were of similar levels |
| Baseline characteristics similar | Low risk | Table of patient demographics and clinical characteristics provided. No significant P values returned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data was examined using AUC and models created to which there were no significant differences found |
| Was study protected against contamination | Low risk | Data from the control group (non‐assessment control) were not shared with the treatment nurses |
| Selective reporting (reporting bias) | Low risk | Unclear whether selective reporting took place |
Rubenstein 1995.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 557 primary care patients | |
| Interventions | 1. Computer‐generated feedback about functional pt status; patient reported (complaint and problem specific resource) and management suggestion. 2. brief interactive educational sessions for physicians. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Beth Israel‐UCLA Functional Status Questionnaire (FSQ), CAGE alcoholism screening questionnaire Constructs measured: Symptoms, Functioning, Other (chief complaint) Instrument categories/domains: Generic, Domain/Disease specific (alcoholism) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered and interviewer‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: 2 times: Baseline and 6 months Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcomes: functional status (FSQ), management plans, physician attitude (scale 1‐5) | |
| Notes | The study was funded by Robert Wood Johnson Foundation. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation was used |
| Allocation concealment (selection bias) | High risk | Allocation concealment not possible due to cluster randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Adjusted for analysis |
| Baseline characteristics similar | Low risk | Statistical differences were found for all variables |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A total of 190 of 309 patients {61%) in the experimental group and 152 of 248 (61%) in the control group completed both baseline and six‐month postintervention functional status surveys. No mention of how missing data would be handled. |
| Was study protected against contamination | High risk | Clinicians were allocated within a clinic or clinics and it is possible that communication between intervention and control professionals could have occurred |
| Selective reporting (reporting bias) | Low risk | The outcomes reported in the methods section were presented in the results section |
Ruland 2003.
| Study characteristics | ||
| Methods | Clinician level randomised into two groups, USA | |
| Participants | 59 patients undergoing treatment for various cancer diagnoses; 27 experiment group, 25 control group | |
| Interventions | Participant reported symptoms and preferences prior to their consultation and in experimental group presented to clinician. Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: CHOICEs (to assess health problems, symptoms, and preferences) Constructs measured: Health related Quality of Life, Symptoms, Functioning, Other (priorities for treatment/care) Instrument categories/domains: Domain/Disease specific (cancer) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic and paper How often information fed back: Once Who information fed back to: Clinicians, Patients Information fed back: Scores |
|
| Outcomes | Main outcome: patient satisfaction (Patient Satisfaction with Decision Making) Other outcome: ease of use |
|
| Notes | The study was funded by Hitchcock Foundation (grant # 250‐442). The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done at clinician level not patient level so the clinician kept the same consultation style |
| Allocation concealment (selection bias) | High risk | Allocation concealment not possible due to cluster randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Tables 1 and 3 were similar |
| Baseline characteristics similar | Low risk | Similar numbers in each roup although the mean age was only reported for the whole sample |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was only one time point |
| Was study protected against contamination | Low risk | the researcher was on site helping the control and experimental group and the clinicians were randomised so there is a low likelihood of contamination |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
Ruland 2010.
| Study characteristics | ||
| Methods | Randomised trial, Norway | |
| Participants | 145 patients starting treatment for leukaemia or lymphoma | |
| Interventions | (Computer‐assisted) Interactive tailored patient assessment (ITPA) tool. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Interactive tailored patient assessment (ITPA) tool Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (cancer) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Paper How often information fed back: Each visit for up to a year Who information fed back to: Clinicians Information fed back: Scores, Previous scores |
|
| Outcomes | Main outcomes: number of patient symptoms and problems addressed by physicians and nurses in patient records, changes in symptom distress, changes in patients' need for symptom management support over time | |
| Notes | The study was funded by Norwegian Research Council (grant #154739/320). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated minimisation algorithm used to randomise clinicians |
| Allocation concealment (selection bias) | Low risk | Because the study’s intervention was to provide nurses and physicians with assessment summaries of patient symptoms, problems, and concerns, a patient’s group assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Table 1 presented similar baseline outcome measurements for both groups |
| Baseline characteristics similar | Low risk | Table 1 presented similar characteristics of patients in both groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Charts were assessed by a blinded rater for outcomes |
| Was study protected against contamination | Unclear risk | All patients interacted with a researcher and data collection was conducted with them |
| Selective reporting (reporting bias) | Unclear risk | All measures mentioned in the methods section were reported in the results |
Saitz 2003.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 301 patients described as hazardous drinkers. | |
| Interventions | Feedback and specific recommendations about management. Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: CAGE alcoholism questionnaire Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (alcoholism) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Management recommendations |
|
| Outcomes | Main outcomes: occurrence of physician discussions regarding alcohol problems, decrease in patient drinking (drinks per drinking day) | |
| Notes | The study was funded by Robert Wood Johnson Foundation (grant 031489), Princeton, New Jersey. The study enrolled patients between February 1998 and August 1999. The authors declared no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | High risk | Allocation concealment not possible due to cluster randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Baseline outcome measurements similar | Low risk | Adjusted for analysis |
| Baseline characteristics similar | Low risk | No statistically significant difference found |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 102 out of 146 (39 dropouts) in intervention group‐ 134 out of 162 (28 dropouts) |
| Was study protected against contamination | High risk | Cluster‐randomised trial at the physician level because randomisation at the patient level would have risked contamination. |
| Selective reporting (reporting bias) | Low risk | None reported |
Sandheimer 2020.
| Study characteristics | ||
| Methods | Cluster‐randomised trial, Sweden. | |
| Participants | 271 patients attending primary care clinics. | |
| Interventions | PRO assessment using the Work Stress Questionnaire and clinician feedback versus usual care. Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: Work stress questionnaire (WSQ) Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (work related stress) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Unclear Feedback features Format of PROMs feedback: Unclear How often information fed back: Once Who information fed back to: Clinicians, Patients Information fed back: Scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Primary outcome: perceived stress. Secondary outcomes: healthcare use. |
|
| Notes | The study was funded by the Swedish Research Council for Health, Working Life and Welfare (FORTE). The study period was not reported. The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random generation. |
| Allocation concealment (selection bias) | Low risk | Random allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded by the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Main outcome is objective and obtained from health records. |
| Baseline outcome measurements similar | Unclear risk | Not enough information. |
| Baseline characteristics similar | Low risk | Similar between groups. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Higher proportion of patient assigned to the control group declined to participate (10% versus 2%). |
| Was study protected against contamination | High risk | Clinicans were the cluster and could provide care to patient in either group. |
| Selective reporting (reporting bias) | Unclear risk | There are several publications associated with this trial; it is not made clear that some refer to secondary outcomes. |
Santana 2010.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 213 outpatient lung transplant patients in routine clinical care | |
| Interventions | Feedback to cliniicians (of Health utilities Index Mark 2 and 3) Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Health Utilities Index Mark 2 (HUI2) and Mark 3 (HUI3) Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Generic Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Paper How often information fed back: Every clinic visit for up to 6 months Who information fed back to: Clinicians Information fed back: Scores, Previous scores |
|
| Outcomes | Main outcomes: issues discussed, changes in clinical management (medication changes, number of referrals and test ordered), EQ‐5D | |
| Notes | The study was funded by Institute of Health Economics (IHE), Edmonton, AB, Canada. The study period was not reported. The authors reported that David Feeny has a proprietary interest in Health Utilities Incorporated, Dundas, Ontario, Canada. HUInc. distributes copyrighted Health Utilities Index (HUI) materials and provides methodological advice on the use of HUI. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization scheme was generated by using the Web site Randomization.com" |
| Allocation concealment (selection bias) | Low risk | Computer conducted the assignment and the patients were unaware of assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Table 2 presented similar baseline outcome measurements for both groups |
| Baseline characteristics similar | Low risk | Table 1 presented similar characteristics of patients in both groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | An intention to treat analysis where missing values were imputed using the last value carried forward |
| Was study protected against contamination | Unclear risk | Unlikely as the patients completed the touch screen questionnaire and went in the consultation immediately |
| Selective reporting (reporting bias) | Unclear risk | All measures mentioned in the methods section were reported in the results |
Scheidt 2012.
| Study characteristics | ||
| Methods | Cluster‐randomised controlled trial, Germany | |
| Participants | Outpatient psychotherapy patients | |
| Interventions | Therapist decisions based on feedback. 400 psychotherapists in private practice participated in a cluster‐randomised comparison study, 200 were allocated to the intervention group, and 200 to the control group. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Brief Symptom Inventory (BSI), Inventar für Interpersonale Probleme (IIP‐D), Beck Depressionsinventar (BDI), Fragebogen zu Körperbezogenen Ängsten, Kognitionen und Vermeidung (AKV), Hamburger Zwangsinventar (HZI), Eating Disorder Inventory (EDI), Screening für Somatoforme Störungen (SOMS), Helping Alliance Questionnaire (HAQ), 12‐Item Short Form Survey (SF‐12) Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Generic, Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) and non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Electronic, Paper Feedback features Format of PROMs feedback: Electronic How often information fed back: 3 points in time, one at the beginning of treatment, one at the end of treatment and one at follow‐up 12 months post‐treatment Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcomes: Brief Symptom Inventory (BSI), Inventar für Interpersonale Probleme (IIP‐D) Other outcomes: depression(BDI), Fragebogen zu Körperbezogenen Ängsten, Kognitionen und Vermeidung (AKV), Hamburger Zwangsinventar (HZI), Eating Disorder Inventory (EDI), Screening für Somatoforme Störungen (SOMS), Helping Alliance Questionnaire (HAQ), SF‐12 |
|
| Notes | The study was funded by Techniker Krankenkasse health insurance programme. The study period was not reported. The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Drawing lots |
| Allocation concealment (selection bias) | High risk | Therpists aware of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Unclear risk | Unclear |
| Baseline characteristics similar | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rates of attrition, not adequately addressed |
| Was study protected against contamination | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
Schmidt 2006.
| Study characteristics | ||
| Methods | Randomised trial, UK | |
| Participants | 61 patients with eating disorder who received 14 sessions of cognitive behavioural guided self‐care | |
| Interventions | Adding personalised feedback on current physical and psychological status, risk and problems, and variables facilitating or hindering change. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: TREAT‐EAT, Short Evaluation of Eating Disorders (SEED), Hospital Anxiety and Depression Scale (HADS) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered and interviewer‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic, Paper How often information fed back: 14 sessions (10 weekly, 4 monthly booster sessions) Who information fed back to: Clinicians, Patients Information fed back: Scores, Previous scores, Management recommendations |
|
| Outcomes | Main outcome: patient‐rated measures of bulimic symptoms at the end of treatment and at 6‐month follow‐up. | |
| Notes | Funding source not disclosed. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence was generated by an independent investigator using a random numbers table. |
| Allocation concealment (selection bias) | Low risk | Allocation sequences were contained in sequentially numbered, sealed opaque envelopes that were opened by the clinical assessor after the initial assessment during which eligibility and willingness to participate had been obtained. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Table 1 presented similar baseline outcme measurements for both groups |
| Baseline characteristics similar | Low risk | Table 1 presented similar characteristics of patients in both groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data was dealt with using bootstrapping methods |
| Was study protected against contamination | Unclear risk | Unclear as to whether the patients knew which group they were in |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
Schottke 2019.
| Study characteristics | ||
| Methods | Randomised trial, Germany. | |
| Participants | 230 adult patients receiving psychotherapy. | |
| Interventions | Psychotherapy progress monitoring with feedback. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: FEP‐2, OQ‐30, Beck Depression Inventory (BDI‐2), Symptom Checklist‐90 (SCL‐90‐R), Patient Health Questionnaire (PHQ‐D) Constructs measured: Health related Quality of Life, Symptoms Instrument categories/domains: Generic, Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Unclear Feedback features Format of PROMs feedback: Unclear How often information fed back: At the beginning of each calendar quarter Who information fed back to: Clinicians, Patients Information fed back: Scores, Previous scores, Interpretation guidance |
|
| Outcomes | Primary outcome: impairment measured using the Outcomes Questionnaire 30. | |
| Notes | The funding source was not reported. The study period was not reported. The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough information to make a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Not enough information to make a judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded by nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outcome from participants who were aware of group allocation. |
| Baseline outcome measurements similar | Low risk | Baseline outcome measurements are the same. |
| Baseline characteristics similar | Low risk | Baseline characteristics are similar. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1911 patients randomised but only baseline data collected for 1124 participants. |
| Was study protected against contamination | Unclear risk | Unclear if stratification meant cluster design. |
| Selective reporting (reporting bias) | Low risk | Pre‐registration information provided. |
Schriger 2001.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 95 Patients who presented at the EDept at daytime with diffuse somatic complaints not mandating acute care or somatically diagnosed. | |
| Interventions | Prior to seeing the physician. computerized PRIME‐MD to screen psychiatric domains. Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: PRIME‐MD Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Main outcome: diagnosis by the PRIME‐MD software leading to psychiatric consultation or referral / treatment. | |
| Notes | The study was funded by Pfizer Corporation and MedAmerica corporation (unrestricted gifts). The study ran from March 1998 through August 1999. Conflicts of interest were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | PRIME‐MD was programmed to create random assignments |
| Allocation concealment (selection bias) | Low risk | Patients were not informed of their assignment. Physicians caring for patients in the report group were provided with the results of the computer interview |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Table 2 presented similar outcome measurements |
| Baseline characteristics similar | Low risk | Baseline characteristics between the groups were similar |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Although the figure reported missing charts ‐ there was no discussion on how incomplete data was managed |
| Was study protected against contamination | Unclear risk | Unclear as to whether there was contamination |
| Selective reporting (reporting bias) | Low risk | All measures mentioned in the methods section were reported in the results |
Schriger 2005.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 190 nonspecific complaints potentially associated with occult psychiatric illness (e,g, long‐standing headache, abdominal or back pain), filling out the Primary Care Evaluation of Mental Disorders | |
| Interventions | Informing physicians of the PRIME‐MD results Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: PRIME‐MD Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Main outcome: influence of PRIME‐MD on treatment: a psychiatric diagnosis, consultation, or referral from the emergency physician | |
| Notes | The study was funded by Pfizer Corporation (unrestricted gift). The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number function used in STATA |
| Allocation concealment (selection bias) | Unclear risk | Unclear as to whether the patients knew of their allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Similar results of baseline outcomes in Table 2 |
| Baseline characteristics similar | Low risk | Similar characteristics in both groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of how incomplete data was managed although it seemed a one off data collection |
| Was study protected against contamination | Unclear risk | No mention of potential contamination ‐ all patients were attending ED departments |
| Selective reporting (reporting bias) | Low risk | All measures mentioned in the methods section were reported in the results |
Shapiro 1987.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | Adult patients that filled out the general health questionnaire (GHQ), home interview and DIS | |
| Interventions | Feedback of GHQ or DIS results Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: General Health Questionnaire (GHQ) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Main outcome: effect of feedback information on detection and management of psychiatric disorders | |
| Notes | The study was funded by National Institute of Mental health, USA (contract 278‐81‐0025). The study was run from 1st December 1981 until 31st March 1982. Conflicts of interest were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of how randomisation was done |
| Allocation concealment (selection bias) | Unclear risk | No mention of who knew about the allocations |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Baseline measurements were similar between groups |
| Baseline characteristics similar | Low risk | Characteristics of both groups similar |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | no mention of how missing data was managed |
| Was study protected against contamination | Unclear risk | Not sure whether there was contamination |
| Selective reporting (reporting bias) | Low risk | none apparent |
Simon 2012.
| Study characteristics | ||
| Methods | Cluster Randomised trial, USA | |
| Participants | 370 patients at outpatient psychotherapy clinic. | |
| Interventions | The primary purpose of this study was to investigate the effects of progress feedback interventions on (not on track) NOT patients’ outcomes in a psychiatric setting, using the OQ‐45 alert system, and the Clinical Support Tool intervention. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Outcome Questionnaire (OQ‐45) Constructs measured: Symptoms, Functioning, Other (Therapeutic Alliance, Social Support, Motivation for Therapy, and Life Events) Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper and electronic How often information fed back: Each session Who information fed back to: Clinicians, Patients Information fed back: Scores, Previous scores, Management recommendations |
|
| Outcomes | Main outcomes: OQ‐45, ASC‐40 | |
| Notes | The study was funded by the Funded by the Susa Young Gates University Professorship awarded to Michael J. Lambert. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation design using therapists as blocking variable. |
| Allocation concealment (selection bias) | Unclear risk | No mention how randomisation happened. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Baseline outcomes were balanced between therapists and between intervention/control groups. |
| Baseline characteristics similar | High risk | No characteristics presented. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rates of attrition, not adequately addressed |
| Was study protected against contamination | Unclear risk | No discussion of controls in methods section ‐ so unclear as to if there was a possibility of cross contamination. |
| Selective reporting (reporting bias) | Low risk | All outcomes were presented in results. |
Simons 2015.
| Study characteristics | ||
| Methods | Randomised trial, the Netherlands | |
| Participants | 102 depressed out‐patients receiving psychopharmacological treatment | |
| Interventions | Three arms: (i) an experimental group receiving six weeks of experience sampling method (ESM) self‐monitoring combined with weekly feedback sessions, (ii) a pseudo‐experimental group participating in six weeks of ESM self‐monitoring without feedback, and (iii) a control group (treatment as usual only). Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Experience sampling method (ESM) a validated, structured diary technique consisting of repeated in‐the‐moment micro‐measurements of affect and context Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Paper How often information fed back: 6 feedback sessions over 6 weeks Who information fed back to: Clinicians, Patients Information fed back: Scores, Previous scores, Interpretation guidance |
|
| Outcomes | Main outcomes: empowerment (Dutch Empowerment questionnaire, economic evaluation, depression (HDRS), quality adjusted life years (QALYs) | |
| Notes | The study was funded by the Dutch Health Research Council (ZON‐MW) (grants nos. 171001002 and 91501003). The study recruited between 2010 and February 2012. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation method. |
| Allocation concealment (selection bias) | Low risk | Allocation took place using opaque, sealed, sequentially numbered envelopes (prepared by an independent research coordinator) with a number sequence produced by an electronic random sequence generator (http://www.random.org), in blocks of six. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Owing to the nature of the intervention, it was not possible to blind participants. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Interviewers were not blind to the patients’ treatment allocation due to nature of the intervention. |
| Baseline outcome measurements similar | Low risk | There was no significant difference in baseline HDRS depressive symptoms between patients who fully completed the intervention period and those who did not. |
| Baseline characteristics similar | Low risk | Baseline characteristics of the study and control providers are reported and similar. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 69 patients allocated to the experimental or pseudo‐experimental group, 59 (85.5%) completed the six‐week intervention period; Pre‐intervention empowerment scores were available for respectively 32 of 33 (control), 35 of 36 (pseudo‐experimental), and 33 of 33 (experimental) participants. Post‐intervention empowerment scores were available for 30 (control), 32 (pseudo‐ experimental), and 27 (experimental) participants. Two participants had incomplete assessments of empowerment (front page only, i.e. 15 items), their total scores (mean item score 40) were retained in the analyses. |
| Was study protected against contamination | High risk | As the psychologist or psychiatrist performing the interview were aware that the patient was a participant in the study. |
| Selective reporting (reporting bias) | Low risk | None apparent. |
Slade 2006a.
| Study characteristics | ||
| Methods | Invididual randomised controlled trial, UK | |
| Participants | Patients attending one of eight Community Mental Health Teams in Croydon, South London, for at least 3 months, and were aged between 18 and 64 inclusive. | |
| Interventions | The hypothesis tested was that pre‐morbid IQ impacts on the response to the intervention of routine outcome assessment. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Camberwell Assessment of Need Short Appraisal Schedule patient version CANSAS‐P, Manchester Short Assessment (MANSA), Helping Alliance Scale patient version (HAS‐P) Constructs measured: Health related Quality of Life, Symptoms, Functioning, Other (patient’s met and unmet needs) Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Electronic, Paper How often information fed back: Monthly Who information fed back to: Clinicians, Patients Information fed back: Scores, Previous scores |
|
| Outcomes | Main outcomes: patient‐rated unmet need (CANSAS‐P) and quality of life (MANSA) | |
| Notes | The study was funded by the Dutch Health Research Council (ZON‐MW (grants nos. 171,001,002 and 91,501,003). The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified random sampling was used for sample selection using STATA |
| Allocation concealment (selection bias) | Unclear risk | Unclear as to whether the patients knew of their allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | No sig differences between groups for outcome measurements |
| Baseline characteristics similar | Low risk | More male and white participants n the intervention group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of how incomplete data was managed although some explanation for dropouts were discussed |
| Was study protected against contamination | Unclear risk | Patients and staff were posted questionnaires ‐ but unsure whether there was contamination |
| Selective reporting (reporting bias) | Low risk | None apparent ‐ all the measurements mentioned in methods reported |
Slade 2006b.
| Study characteristics | ||
| Methods | As Slade 2006a | |
| Participants | As Slade 2006a | |
| Interventions | As Slade 2006a | |
| Outcomes | As Slade 2006a | |
| Notes | As Slade 2006a | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Following baseline assessment, patients were allocated by an independent statistician who was masked to the results of the baseline assessment. |
| Allocation concealment (selection bias) | High risk | Staff and patients were aware of their allocation status |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Staff and patients were aware of their allocation status |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Baseline measurements were similar between groups |
| Baseline characteristics similar | Low risk | Characteristics of both groups similar (age, gender, education, diagnosis) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing values imputed from baseline data |
| Was study protected against contamination | High risk | Quote: “In the control group, 46 (78%) of the 59 patients had a member of staff who also had an intervention‐group patient, indicating that contamination was possible between the two groups.” |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes reported in the results |
Strasser 2016.
| Study characteristics | ||
| Methods | Randomised trial, Switzerland | |
| Participants | Patients with incurable, symptomatic, solid tumours, who received new outpatient chemotherapy with palliative intention, were eligible. In 8 centres, 82 oncologists treated 264 patients (median 66 years; overall survival intervention 6.3, control 5.4 months) with various tumours. | |
| Interventions | Real‐time monitoring of both symptoms and clinical syndromes to improve symptom management by oncologists and patient outcomes. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Edmonton Symptom Assessment System (ESAS), European Organisation for Research and Treatment of Cancer (EORTC QLQ‐C30) Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (cancer) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Paper How often information fed back: Weekly during oncology outpatient visits Who information fed back to: Clinicians Information fed back: Scores, Previous scores |
|
| Outcomes | Main outcome: Global Quality of Life (G‐QoL), measured as the difference in G‐QoL between baseline and after last study visit (6 weeks), QoL (EORTC‐QLQ‐C30) | |
| Notes | This work is supported by a scientific grant from Swiss Cancer League/Swiss Cancer Research foundation (formerly Oncosuisse, OSC 01696‐04‐2005), the Swiss State Secretariat for Education, Research and Innovation (SERI), unrestricted grants from Sanofi‐ Aventis and Amgen (no grant number) and an EURO IMPACT— Marie Curie PhD training grant for DB. The study was run from February 2007 until January 2012. The authors declared no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation procedure. |
| Allocation concealment (selection bias) | High risk | Allocation concealment not possible due to cluster randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Significant differences only in baseline G‐QoL |
| Baseline characteristics similar | High risk | Baseline characteristics of the study and control providers are reported but dissimilar. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | For the primary analysis, 102 (39%) patients were included. Main reasons for non‐inclusion were attrition (missing QoL measurement at week 6, 78 patients), <4 physician visits (44 patients) and insufficient cognitive function (58 patients). |
| Was study protected against contamination | Low risk | Cluster design |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
Stuck 2015.
| Study characteristics | ||
| Methods | Randomised trial, Switzerland | |
| Participants | Individuals aged 65 years or older registered with one of 19 primary care physician (PCP) practices in a mixed rural and urban area in Switzerland. A total of 4,115 patients aged 65 years and older were assessed for eligibility, 3,493 were eligible, and 2,284 were included in the study and underwent randomisation. In all, 874 participants were allocated to the intervention group, and 1,410 to the control group. | |
| Interventions | The intervention consisted of HRA based on self‐administered questionnaires and individualised computer‐generated feedback reports, combined with nurse and PCP counselling over a 2‐y period. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Health Risk Assessment for Older Persons (HRA‐O) Constructs measured: Functioning Instrument categories/domains: Domain/Disease specific (geriatric health) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Electronic How often information fed back: Twice (baseline and 1 year) Who information fed back to: Clinicians, Patients Information fed back: Scores, Management recommendations |
|
| Outcomes | Main outcomes: health behaviours, preventive care use (2 years), all‐cause mortality (8 years) | |
| Notes | The study was supported by a European Union (QLK6‐CT‐1999‐02205) (AS SI CS); the Federal Education and Science Ministry (Bern, Switzerland, BBW 990311.1) (AS); the Swiss National Science Foundation (32‐52804.97) (AS); the Swiss National Science Foundation Swiss National Cohort (projects 0071, 3347CO‐108806, 33CS30_134273 and 33CS30_148415) (ME); the Swiss Foundation for Health Promotion (Project No. 398) (AS); the Velux Foundation (AS); the Langley Research Institute (JCB). The study period was not reported. The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated method. |
| Allocation concealment (selection bias) | Low risk | Group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | There were no statistically significant differences between the intervention and control groups for self‐reported dependency in basic activities of daily living or for nursing home admissions. |
| Baseline characteristics similar | Low risk | There were no significant differences between the intervention and control groups in any of the baseline characteristics. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 94% intervention group and 93% control group of the participants completed the outcome questionnaire at 2 year follow‐up. |
| Was study protected against contamination | High risk | Primary care physicians received training and gained experience in preventive care, which likely resulted in improved care for individuals in the control group. |
| Selective reporting (reporting bias) | Low risk | None apparent. |
Subramanian 2004.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 720 heart failure patients | |
| Interventions | Care suggestions, generated with electronic medical record data (also in control group) and symptom data obtained from pre visit questionnaires (only intervention group). Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Kansas City Cardiomyopathy Questionnaire (KCCQ Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Generic, Domain/Disease specific (heart failure) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered, Interviewer administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Electronic How often information fed back: Each scheduled primary care visit over a year Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Management recommendations |
|
| Outcomes | Main outcomes: physician treatment decisions, QoL, satisfaction (NYAH, SF‐36 (rash), McMaster, Chronic Heart Failure Questionnaire’s five scales, patient satisfaction with doctor, Medical Outcomes Study Visit‐Specific Questionnaire) | |
| Notes | The study was supported by Department of Veterans Affairs Health Services Research and Development Service (CPG 97‐001‐B and REA 01‐098); Department of Veterans Affairs Health Services Research and Career Development Program. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Coin flip was used to randomise clinicians |
| Allocation concealment (selection bias) | High risk | Allocation concealment not possible due to cluster randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Unclear risk | Baseline outcome measurements were not presented |
| Baseline characteristics similar | Low risk | The only significant baseline difference between intervention and control patients was NYHA class, for which all comparative analyses were adjusted |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of how incomplete data was handled |
| Was study protected against contamination | Unclear risk | Unclear as patients were required to post back questionnaires, clinicians knew their allocation |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
Thomas 2016.
| Study characteristics | ||
| Methods | Randomised trial, Canada | |
| Participants | 54 families | |
| Interventions | This trial’s purpose is to: (1) compare identification rates of developmental problems by GPs/family physicians using four evidence‐based tools with non‐evidence based screening, and (2) ascertain whether the four tools can be completed in 10‐min pre‐visit on a computer. Intervention features Single complex feedback (multiple PROMs at a single time) PROM(s) used as intervention: Parents’ Evaluation of Developmental Status (PEDS), the PEDS‐Developmental Milestones (PEDS‐DM), the Modified Checklist for Autism in Toddlers (M‐CHAT) and PHQ9 (maternal depression) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (child development, autism, mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: Once Who information fed back to: Clinicians, Patients Information fed back: Scores |
|
| Outcomes | Main outcomes: Parents’ Evaluation of Developmental Status (PEDS), PEDS‐Developmental Milestones (PEDS‐DM), Modified Checklist for Autism in Toddlers (M‐CHAT), maternal depression (PHQ9). | |
| Notes | Funding not disclosed. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated method. |
| Allocation concealment (selection bias) | Low risk | Allocation was done by research assistant by computer to ‘usual care’ or evidence‐based screening. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither the participants nor family physicians could be blinded due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Unclear risk | Not clearly reported. |
| Baseline characteristics similar | High risk | The usual care and evidence based care groups were very similar in gestational age at birth and age at screening (17.84 and 17.59 months). They differed markedly in female gender (40%, 62%) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In the ‘usual care’ group four (16%) and in the evidence‐based tools group 18 (62%) were identified as having a possible developmental problem. |
| Was study protected against contamination | High risk | As the physician or research associate were aware that the patient was a participant in the study. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section are reported in the results section. |
Tolstrup 2020.
| Study characteristics | ||
| Methods | Randomised trial, Denmark. | |
| Participants | 146 patients with multiple myeloma receiving immunotherapy. | |
| Interventions | Symptom report using the PRO‐CTCAE with clinician feedback versus usual care. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Patient‐Reported Outcomes version of the Common Terminology Criteria for Adverse Event (PRO‐CTCAE) Constructs measured: Symptoms Instrument categories/domains: Generic, Domain/Disease specific (mental health) Administration features Where PROMs administered: Unclear How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Unclear How often information fed back: Patients reported symptoms weekly but not clear if they were also fed back weekly Who information fed back to: Clinicians Information fed back: Unclear |
|
| Outcomes | Main outcome: number of Grade 3 or 4 adverse events assessed by the Common Terminology for Cancer Adverse Events. | |
| Notes | The study was funded by the Danish Cancer Society. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded by nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information. |
| Baseline outcome measurements similar | Low risk | Baseline outcome measurements are similar. |
| Baseline characteristics similar | Unclear risk | Baseline characteristics are similar. Statistical tests conducted. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information. |
| Was study protected against contamination | High risk | Single‐centre study. Clinicians can treat patients in intervention and control group. |
| Selective reporting (reporting bias) | Low risk | Pre‐publication information available. |
Trowbridge 1997.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 320 cancer patients with oncological pain | |
| Interventions | Provide pain assessment forms to oncologist Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: Pain Management Index (PMI) Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (cancer) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) and non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Previous scores |
|
| Outcomes | Main outcomes: prescriptions, incidence of pain in follow‐up | |
| Notes | The study was supported by 1995 William Campbell Felch CME Research Award. The study ran from 5th July 1995 until 30th September 1995. Conflicts of interest were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of how randomisation was done |
| Allocation concealment (selection bias) | Unclear risk | Unsure as to whether patients knew, but clinicians in the intervention group were required to look at their patients' charts |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Similar outcomes at baseline between the groups |
| Baseline characteristics similar | Low risk | Similar characteristics although age ranges were different (means were similar) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of how incomplete data was managed ‐ only differences between groups were reported |
| Was study protected against contamination | Unclear risk | Unsure whether any contamination was possible |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
Trudeau 2001.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 127 clients at mental health centre in rural area. | |
| Interventions | Outcomes Questionnaire (OQ), a monitoring system measuring mental health symptoms and functioning. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Outcomes Questionnaire (OQ) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: 3 times Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance |
|
| Outcomes | Main outcomes: self‐esteem (Rosenberg self esteem scale ‐ RSE), mental heath (OQ, SF‐36) | |
| Notes | Funding source not reported. The study period was not reported. Conflicts of interest were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clients were randomly assigned by case number to either the control condition for case numbers ending in 3, 6 or 9, or one of the feedback conditions |
| Allocation concealment (selection bias) | High risk | Allocation concealment not possible due to cluster randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | High risk | Quasi‐experimental design of the study. quote: ''[...] even though randomization of therapists to the feedback conditions and clients to the control and experimental conditions was performed, the cells were unbalanced, and there was a significant difference between the assigned treatment groups on the initial measure of mental health status, the Total Mental Health composite.'' |
| Baseline characteristics similar | Low risk | No sig differences in characteristics |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Those participants who dropped out were compared with those in the study and no sig differences were found |
| Was study protected against contamination | Unclear risk | Unclear as to whether the study was protected from contamination |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
Valles 2017.
| Study characteristics | ||
| Methods | Randomised trial, Spain. | |
| Participants | 136 paediatric patients with Type 1 diabetes mellitus. | |
| Interventions | Health‐related quality of life assessed by the KIDSCREEN‐27 with feedback to clinician vs usual care. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: KIDSCREEN‐27 Constructs measured: Health related Quality of Life Instrument categories/domains: Generic Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: Quarterly Who information fed back to: Clinicians Information fed back: Scores, Previous scores |
|
| Outcomes | Primary outcome: change in health‐related quality of life. | |
| Notes | Funded partially by Spanish Ministry of Health. The study ran from July 2014 until December 2014. The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation tool not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded by nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information. |
| Baseline outcome measurements similar | High risk | Baseline outcome measurements the same |
| Baseline characteristics similar | High risk | Statistically significant differences in both family affluence and reported adherence between both groups. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information. |
| Was study protected against contamination | Unclear risk | Randomised at the level of the clinician but unclear if clinicians worked at different sites. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐registration information not available. |
van der Hout 2020.
| Study characteristics | ||
| Methods | Randomised trial, the Netherlands. | |
| Participants | 625 cancer survivors not on active treatment. | |
| Interventions | PRO assessment and feedback to patients using the Oncokompass tool versus usual care. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Oncokompas (an eHealth self‐management application) Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (cancer) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic How often information fed back: 3 and 6 months Who information fed back to: Patients (In case of seriously elevated well‐being risks, professional health‐care options are offered). Information fed back: Scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Primary outcome: patient activation (knowledge, skills, and confidence for self‐management) at 3‐ and 6‐month follow‐up. Secondary outcomes: health‐related quality of life (including tumour‐specific symptoms within the tumour groups), mental adjustment to cancer, supportive care needs, self‐efficacy, personal control, perceived efficacy in patient–physician interaction, cost‐effectiveness. |
|
| Notes | Funded by the Dutch Cancer Society. The study period was not reported. Conflicts of interest were reported as: IMV‐dL has received grants from the Dutch Cancer Society (KWF Kankerbestrijding), Pink Ribbon, the Netherlands Organization for Health Research and Development (ZonMW), the SAG Foundation–Zilveren Kruis Health Care Assurance Company, Danone Ecofund–Nutricia, Red‐kite (distributor of eHealth tools), and Bristol‐Myers Squibb, during the conduct of this study. CRL has received personal fees for global advisory board participation from MSD, during the conduct of this study. All other authors have no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded by nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information. |
| Baseline outcome measurements similar | Low risk | Baseline outcome measurements are the same. |
| Baseline characteristics similar | Low risk | Baseline characteristics are similar. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis. |
| Was study protected against contamination | Low risk | Feedback to individual patients. |
| Selective reporting (reporting bias) | Low risk | Pre‐registration information available. |
van Dijk‐de Vries 2015.
| Study characteristics | ||
| Methods | Pragmatic cluster‐randomised trial, the Netherlands | |
| Participants | 40 practice nurses specialised in diabetes mellitus in general practitioner practices (19 intervention versus 21 control). 264 patients (117 intervention, 147 usual care; 46% female patients, average age 65years). | |
| Interventions | Biopsychosocial self‐management support (SMS) Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Daily Functioning Thermometer (DFT), Distress Screener (DS), Four‐Dimensional Symptom Questionnaire (4DSQ) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health ‐ emotional distress, physical health ‐ diabetes) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) and non‐clinical setting How administered: Both self‐administered and interviewer‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: 3 times (baseline, 4 months, and 12 months) Who information fed back to: Clinicians, Patients Information fed back: Scores |
|
| Outcomes | Main outcome: dichotomised Visual Analog Scale on perceived effect of diabetes on daily functioning Other outcomes: patients’ diabetes‐related distress (PAID), quality of life (SF12), autonomy and participation (IPA), self‐efficacy (GSES‐12), self‐ management (PIH) |
|
| Notes | The study was supported by the Dutch Diabetes Research Foundation (Diabetes Fonds) (grant# 2010.13.1366). The study period was not reported. The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number seed computer program to assign PNs to study arms, assuming an allocation ratio of 1:1. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not possible due to cluster randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Patients of both groups were comparable for the primary and secondary outcomes at the baseline measurement except for the sum score on the PIH scale. |
| Baseline characteristics similar | Low risk | Table 1 had similar baseline characteristics for both groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing items were imputed using patients’ individual mean score if at least 50% of items were available. |
| Was study protected against contamination | Low risk | Risk of contamination was considered by the research team and practice was done to avoid it |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
van Os 2003.
| Study characteristics | ||
| Methods | Pragmatic randomised trial, international European study (the Netherlends, UK, Italy, Spain, Denmark, Germany, France) | |
| Participants | 134 patients with clinical diagnosis of schizophrenia or schizoaffective disorder 976 vs 67). Mean age 40.8 years, 61% women. | |
| Interventions | Two‐Way Communication Checklist (2‐COM). Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: Two‐Way Communication Checklist (2‐COM) Constructs measured: Symptoms, Functioning, Other (patient‐clinician communication) Instrument categories/domains: Domain/Disease specific (patient‐clinician communication) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians, Patients Information fed back: Scores |
|
| Outcomes | Main outcomes: patient‐reported quality of patient–clinician communication (self‐developed question: "How easy did you find it to discuss the problems and worries you have with your doctor at today’s clinic appointment?" on 4‐point scale), physician‐reported change in behaviour (dichotomous question) | |
| Notes | The study was supported by AstraZeneca (unrestricted grant). The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised centrally by an independent, non‐investigator agency using a predetermined random sequence |
| Allocation concealment (selection bias) | Unclear risk | No mention of who knew about the allocations |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Similar GAF scores between the groups |
| Baseline characteristics similar | Low risk | Similar patient characteristics between the groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There is no discussion around missing data |
| Was study protected against contamination | Unclear risk | Unsure about potential contamination between groups |
| Selective reporting (reporting bias) | Unclear risk | None apparent |
Velikova 2004.
| Study characteristics | ||
| Methods | Randomised trial, UK | |
| Participants | 286 cancer patients visiting the Leeds cancer centre. mean age participants 54.9years. 73% female. | |
| Interventions | Use of health‐related quality‐of‐life (HRQL) data in oncology practice. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: European Organisation for Research and Treatment of Cancer (EORTC QLQ‐C30), Hospital Anxiety and Depression Scale (HADS) Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health, cancer) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) and non‐clinical setting How administered: Self‐administered, Interviewer‐administered Format of PROMs questionnaire(s): Electronic, Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Before every encounter for approximately 6 months Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance |
|
| Outcomes | Main outcomes: HRQOL over time using FACT, physician‐patient communication, clinical management measured by content analysis of audiotaped‐recorded encounters. | |
| Notes | The study was supported by Cancer Research UK; National Lotteries Charities Board; National Health Service Research and Development. The study period was not reported. The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random assignment was stratified by site of cancer in random permuted blocks. Random assignment was carried out by telephone, by the Administrative Office at Cancer Research UK Centre |
| Allocation concealment (selection bias) | Unclear risk | No mention of who knew about the allocations |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | All baseline assessments were of similar levels |
| Baseline characteristics similar | Low risk | All baseline characteristics were similar between the groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis |
| Was study protected against contamination | Unclear risk | Unsure as to whether contamination could be possible |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
Wagner 1997.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 210 epilepsy patients visiting an outpatient neurology clinic. | |
| Interventions | Optically scanned versions of the SF‐36 were presented to physicians in the intervention group before their encounter with the patients. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: MOS SF‐36 Health Survey (SF‐36) Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Generic Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Each visit Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance |
|
| Outcomes | Main outcomes: physician's perceptions on the usefulness of SF‐36 assessment, patient perceptions about their satisfaction with care. | |
| Notes | The study was supported by Cancer Research UK; National Lotteries Charities Board; Department of Health. The study ran between January 1994 and June 1994. Conflicts of interest were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned to two groups using a random number table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Baseline outcome measurements similar | Low risk | No statistically significant differences were found for the outcomes |
| Baseline characteristics similar | High risk | Significant differences were found for most of the variables |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low number of dropouts |
| Was study protected against contamination | High risk | Clinicians were allocated within a clinic or clinics and it is possible that communication between intervention and control professionals could have occurred |
| Selective reporting (reporting bias) | Low risk | None reported |
Wasson 1992.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 56 clinicians were randomised (29 intervention vs 27 control). | |
| Interventions | Self‐developed health assessment form Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: Dartmouth COOP Charts Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Generic Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians, Patients Information fed back: Scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcomes: effect of short‐term health‐assessment on the process of care (self‐developed clinician form) and patients' satisfaction (self‐developed 10‐item patient satisfaction questionnaire) | |
| Notes | The study was supported by the Epilepsy Foundation of America. The study period was not reported. Conflicts of interest were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clinicians were randomised by blocks |
| Allocation concealment (selection bias) | High risk | Allocation concealment not possible due to cluster randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Baseline data on Table 1 were similar between chart and control groups |
| Baseline characteristics similar | Low risk | Little or no differences between the groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of how missing data were handled |
| Was study protected against contamination | Unclear risk | No mention about potential contamination |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
Wheelock 2015.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 102 patients with TNM stage I to III breast cancer, average age 53yrs, average time from diagnosis 3.2 years | |
| Interventions | SIS.NET (System for Individualized Survivorship Care, based on patient self‐reported data, with review by nurse practitioners, targeted Education, and Triage) vs usual care. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Web based application SIS.NET (System for Individualized Survivorship Care, based on patient self‐reported data, with review by Nurse practitioners, targeted Education, and Triage) including ‐ 36‐Item Short Form Survey (SF‐36), Personal Health Questionnaire Depression Scale (PHQ‐8), (modified questions from) Memorial Symptom Assessment Scale Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Generic, Domain/Disease specific (mental health, cancer) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic, Paper How often information fed back: Patients in the SIS.NET arm were scheduled for 3 breast cancer‐related clinic visits with the providers of their choice (breast surgeon, medical oncologist, and radiation oncologist) during the 18‐month duration of the study, with additional appointments scheduled later as needed. The SIS.NET intervention also included the integration of online health questionnaires at 3‐month intervals between clinic visits evaluating symptoms that were monitored and followed by telephone as necessary by a designated nurse practitioner (NP). Who information fed back to: Clinicians, Patients Information fed back: Scores, Previous scores |
|
| Outcomes | Primary endpoint: time in days between symptom reporting and remote evaluation of symptoms (i.e. time elapsed between completion of questionnaire (as documented automatically by the ISS software) and the NP’s documentation of attempts to contact the patient to evaluate the symptom and make treat‐ ment recommendations). Other outcome: use of healthcare resources (breast cancer‐related visits, total medical appointments, and laboratory and imaging studies) over an 18‐month period. |
|
| Notes | The study was supported by the Henry J. Kaiser Family Foundation (grant). The study period was not reported. Conflicts of interest were reported as: Ms. Wheelock, Dr. Melisko, Dr. Martin, Ms. Ernest, and Ms. Bock report that the Safeway Foundation provided financial support for the Athena Breast Health Network and Survivorship Programming for work performed as part of the current study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation occurred by block design developed by the statistician |
| Allocation concealment (selection bias) | Unclear risk | Allocation was done by the research coordinator, but unclear if patients/personnel aware |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Baseline data on Table 1 were similar between chart and control groups |
| Baseline characteristics similar | Low risk | No significant differences between the groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of how missing data were handled |
| Was study protected against contamination | Unclear risk | No mention about potential contamination |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
Whipple 2003.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | Participants were 981 clients of a possible 1,339 treated in a university counseling center. | |
| Interventions | The authors examined whether feedback regarding client progress and the use of clinical support tools (CSTs) affected client outcome and number of psychotherapy sessions attended. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Outcome Questionnaire–45 (OQ‐45) Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Every session Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcomes: psychological dysfunction (OQ‐45) | |
| Notes | The funding source was not reported. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The participants in the experimental and control groups were divided into groups based on random assignment |
| Allocation concealment (selection bias) | Low risk | An administrative employee blinded to the aim of the trial drew names. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | There were no statistically significant differences between the intervention and control groups. |
| Baseline characteristics similar | Low risk | No significant differences were found between the participants in the intervention and TAU groups at baseline. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At the 12 month follow up, participation rate was almost thesame in intervention and control groups; 70% and 69%, respectively. |
| Was study protected against contamination | High risk | The nurse was aware that the patient was a participant in the study. |
| Selective reporting (reporting bias) | Low risk | None apparent. |
White 1995.
| Study characteristics | ||
| Methods | Randomised trial, UK | |
| Participants | 23 general practices with at least 20 asthmatic patients in their practice | |
| Interventions | Receiving feedback on control of asthma versus no feedback. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Self‐developed asthma questionnaire Constructs measured: Symptoms, Functioning Instrument categories/domains: Domain/Disease specific (asthma) Administration features Where PROMs administered: Non‐clinical setting How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Electronic, Paper How often information fed back: First questionnaire then 4 further questionnaires mailed at 6 month intervals Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcomes: type/frequency asthma symptoms, use of health services, use of asthma drugs (i.e. self‐developed questionnaire) | |
| Notes | The study was supported by the Department of Health. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of how randomisation was done |
| Allocation concealment (selection bias) | High risk | Allocation concealment not possible due to cluster randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Table 5 presented similar outcomes for both groups |
| Baseline characteristics similar | Low risk | Baseline characteristics between the groups were similar |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of how incomplete data were addressed |
| Was study protected against contamination | Unclear risk | Intervention practices and control practices different ‐ although one control practice was paired with an intervention |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
Whooley 2000.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 13 primary care medical clinics (7 intervention, 6 control). | |
| Interventions | Feedback of Geriatric Depression Scale (GDS) Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: Geriatric Depression Scale (GDS‐15) Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (geriatric mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Interviewer‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcomes: patient‐reported GDS outcomes, physician diagnosis of depression, antidepressant use, prevalence of depression | |
| Notes | The study was supported by the Garfield Memorial Fund (grant). The study recruited between June 1994 and October 1995. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation occurred for practices |
| Allocation concealment (selection bias) | High risk | Allocation concealment not possible due to cluster randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | No sig difference in outcome scores between the groups |
| Baseline characteristics similar | Low risk | Only significant differences in income and education between the groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of missing data handling |
| Was study protected against contamination | Unclear risk | Practices were in different groups |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether selective reporting took place |
Wikberg 2017.
| Study characteristics | ||
| Methods | Randomised trial, Sweden | |
| Participants | The trial took place at 22 Swedish PHCCs between March 2010 and December 2013. All 98 PHCCs in the region were invited to participate in the intervention; 22 agreed to participate. 258 Study participants were patients aged 18 and up who visited the PHCCs and were identified and diagnosed by a GP with a new episode of mild/moderate depressive disorder. | |
| Interventions | The intervention consisted of using a patient depression self‐rating scale (MADRS‐S) in recurrent monthly consultations during the 3‐month intervention. Patients made 4 visits to their GPs, at which time they completed MADRS‐S to monitor changes in their depressive symptoms that were then discussed in the person‐centred consultation. MADRS‐S was used as a supplement to, rather than as a substitute for, TAU. Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: Beck Depression Inventory‐II (BDI‐II), EQ‐5D, 12‐ item General Health Questionnaire (GHQ‐12) Constructs measured: Health related Quality of Life, Symptoms, Functioning Instrument categories/domains: Generic, Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Unclear Feedback features Format of PROMs feedback: Unclear How often information fed back: 4 times over 3 months Who information fed back to: Clinicians, Patients Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcome: depression severity (BDI‐II), depression remission, quality of life (EQ‐5D), overall psychological well‐being (GHQ‐12), prescriptions for antidepressants, prescriptions for sedatives, sick leave, healthcare use. | |
| Notes | The study was supported by the Department of Health. The study period was not reported. The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The participants in the experimental and control groups were divided into groups based on random assignment. |
| Allocation concealment (selection bias) | High risk | Allocation concealment not possible due to cluster randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Unclear risk | Not reported. |
| Baseline characteristics similar | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1339 randomised, of whom 358 (26.7%) excluded due to not completing an outcome measure, or not returning for a second session. |
| Was study protected against contamination | High risk | Therapists was aware that the patient was a participant in the study. |
| Selective reporting (reporting bias) | Low risk | None apparent. |
Williams 1990.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 969 adults (mean age 58 years) | |
| Interventions | Diagnostic information relating to the patient's depression status was reported to the physician following either a single item assessment of the CES‐D. Intervention features Single simple feedback (one PROM at a single time) PROM(s) used as intervention: 20‐item Center for Epidemiologic Studies Depression Screen Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Main outcome: depression recognition Other outcomes: changes to treatment, recovery from depression symptoms, number of depressive symptoms | |
| Notes | The study was supported by Supported by a Robert Wood Johnson Generalist Physician Faculty Award (No. 22324) and the Hispanic Healthy Aging Center, NIA Grant No. IT20AG12044‐04. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment was stratified by site |
| Allocation concealment (selection bias) | Low risk | Computer‐generated, blocked randomisation log. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | No statistically significant differences were found for the outcomes |
| Baseline characteristics similar | Low risk | No statistically significant difference found |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intervention‐3 month follow‐up‐ 5/2 dropouts Control‐ 3 month follow‐up‐ 7dropouts |
| Was study protected against contamination | Low risk | Control group had no access to the intervention |
| Selective reporting (reporting bias) | Low risk | None reported |
Wolfe 2014.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 104 oncologists (51 intervention, 53 control) at paediatric cancer centres. | |
| Interventions | Summary feedback of PediQUEST data to oncologists and families Intervention features Multiple complex feedback (multiple PROMs at multiple times) PROM(s) used as intervention: PQ Memorial Symptom Assessment Scale (PQ‐MSAS), Pediatric Quality of Life Inventory 4.0 Generic Core Scales (PedsQL4.0), overall sickness question (Sickness) developed de novo Constructs measured: Health related Quality of Life, Symptoms Instrument categories/domains: Domain/Disease specific (cancer ‐ children) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) and non‐clinical setting How administered: Self‐administered and proxy (by families) Format of PROMs questionnaire(s): Electronic Feedback features Format of PROMs feedback: Electronic and paper How often information fed back: At most once a week over 3 month period Who information fed back to: Clinicians, Patients Information fed back: Scores, Previous scores, Interpretation guidance, Management recommendations |
|
| Outcomes | Main outcomes: child distress (PQ‐MSAS), HRQoL (PedsQL4.0), satisfaction with PediQUEST (self‐developed questionnaire) | |
| Notes | The study was supported by the National Institutes of Health/National Cancer Institute PediQUEST Study (Evaluation of Pediatric Quality of Life and Evaluation of Symptoms Technology); Charles H. Hood Foundation Child Health Research Award; American Cancer Society Pilot and Exploratory Project Award in Palliative Care of Cancer Patients and Their Families. The study ran between December 2004 and December 2009. The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence by site |
| Allocation concealment (selection bias) | High risk | Allocation not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | Low risk | Outcome measurements were similar at baseline. |
| Baseline characteristics similar | Low risk | Baseline characteristics were similar |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data approach for the trial |
| Was study protected against contamination | Unclear risk | Contamination effect could not be ruled out by authors |
| Selective reporting (reporting bias) | Low risk | None reported |
Yager 1981.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 150 patients from a University Medical Ambulatory Care Clinic, mostly related to chronic diseases (82%) Median age 56 years 71% female | |
| Interventions | Assessing depression screening scores (Zung SDS) to patients
Assessing Global Depression Index (GDI) to both patients and treating physician.
Providing the results of patients' depression screening scores (Zung SDS) to physicians. Intervention features Single complex feedback (multiple PROMs at a single time) PROM(s) used as intervention: Zung self rating depression scale (SDS), Global Depression Index (GDI) Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Self‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: Once Who information fed back to: Clinicians Information fed back: Scores, Interpretation guidance |
|
| Outcomes | Main outcomes: effects of screening, feedback, and sensitisation on notation and treatment, physician‐patient agreement about patient depression | |
| Notes | Funding source not reported. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paper only states quote:"Patients were randomly assigned to one of six groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to study design (physicians either given the global depression index and/or the Zung self rating depression scale or not). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Baseline outcome measurements similar | Unclear risk | No comparisons were made between groups |
| Baseline characteristics similar | Unclear risk | Some demographics provided but no indication of any significance testing for differences |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Was study protected against contamination | Unclear risk | Unclear as to whether the physicians knew which group they were in |
| Selective reporting (reporting bias) | High risk | Tables do not provide summaries for each of the intervention group rather blocked them together |
Zung 1983.
| Study characteristics | ||
| Methods | Randomised trial, USA | |
| Participants | 143 adults (mean age years) attending a family medical practice with a positive screen for depression | |
| Interventions | Feedback and prompts to evaluate further depending on need. Intervention features Multiple simple feedback (one PROM at multiple times) PROM(s) used as intervention: Zung self rating depression scale (SDS) Constructs measured: Symptoms Instrument categories/domains: Domain/Disease specific (mental health) Administration features Where PROMs administered: Clinical setting (e.g. waiting room, office, etc) How administered: Interviewer‐administered Format of PROMs questionnaire(s): Paper Feedback features Format of PROMs feedback: Paper How often information fed back: 2 times (second time after 4 weeks) Who information fed back to: Clinicians Information fed back: Scores, Previous scores |
|
| Outcomes | Main outcome: depression recognition | |
| Notes | Funding source not reported. The study period was not reported. Conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random assignment ‐ not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention not possible to blind patients and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to nature of the intervention blinding of outcomes not possible: PROM used for feedback also used to assess outcome, patients were aware they received the intervention. |
| Baseline outcome measurements similar | High risk | Not clear |
| Baseline characteristics similar | Low risk | Statistically significant differences were found for sex |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clearly reported |
| Was study protected against contamination | High risk | Clinicians were allocated within a clinic or clinics and it is possible that communication between intervention and control professionals could have occurred |
| Selective reporting (reporting bias) | Low risk | None reported |
AEP: Adverse Events Profile;ASC: Assessment for Signal Clients; AUC: area under the curve; BDI: Beck Depression Inventory; CAGE: cut‐annoyed‐guilty‐eye; CDSS: Calgary Depression Scale for Schizophrenia; CES‐D: Center for Epidemiologic Studies Depression Scale; CG: control group; COOP: Dartmouth Cooperative Functional Assessment Charts; DIS: Diagnostic Interview Schedule; ED: eating disorder; EDept: emergency department; EORTC: European Organisation for Research and Treatment of Cancer; EQ‐5D: EuroQol 5 Dimensions; ESRA‐C: Electronic Self‐Report Assessment‐Cancer; ESRS: Manual for the Extrapyramidal Symptom Rating Scale; FACIT: Functional Assessment of Chronic Illness Therapy; FACT: Functional Assessment of Cancer Therapy; FSQ: Functional Status Questionnaire; G‐QoL: Global Quality of Life; GAF: Global Assessment of Functioning; GAS: Global Anxiety Score; GDS‐SF: Geriatric Depression Scale, short form; GHQ: General Health Questionnaire; GPs: general practitioners; HADS: Hospital Anxiety and Depression Scale; HASS: Highest Anxiety Subscale Score; HAM‐D: Hamilton Depression Rating Scale; HbA1c: glycated haemoglobin; HRS: Health risk assessment; HRQL/HRQOL: health‐related quality of life; IG: intervention group; IVR: interactive voice response; MDAS: Modified Dental Anxiety Scale; MDASI: M.D. Anderson Symptom Inventory; MQS: Medication Quantification Scale; NYHA: New York Heart Association; O/GP: oncologist/general practitioner; OARS ADL: Older Americans Resources and Services Activities of Daily Living Scale; OQ‐45: Outcome questionnaire 45; ORS: Outcome Rating Scale; PANSS: Positive and Negative Syndrome Scale; PC‐SAD: Primary Care Screener for Affective Disorders; PCP: primary care physician; PHQ: Patient Health Questionnaire; PRIME‐MD: Primary Care Evaluation of Mental Disorders; PROM: patient‐reported outcome measure; PROMIS: Patient‐Reported Outcomes Measurement Information System; QLQ‐C30: Quality of Life Questionnaire‐Core 30; QoL: quality of life; QOLIE: Quality of LIfe in Epilepsy Inventory; R&D: Research and Development; S‐QOL: Schizophrenia Quality of Life Questionnaire; SCL: Symptom Checklist; SD: standard deviation; SDS: Zung Self‐rating Depression Scale; SF‐36: Short Form Health Survey; SIP: Sickness Impact Profile; SPADE: Sleep disturbance, pain, anxiety, depression and low energy/fatigue; STAI‐S: Spielberger State Anxiety Inventory for State Anxiety; TAU: treatment as usual; TC; W: telephone caseworker; WCL: wait control list; WHOQOL: World Health Organization Quality of Life; WONCA: World Organization of Colleges, Academies and Academic Associations of General Practitioners/Family Physicians.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aakhus 2016 | Wrong intervention |
| Aardoom 2016 | Wrong setting. Patients were recruited from the community through a website. |
| Adamowicz 2017 | Wrong intervention |
| Adams 2015 | Wrong intervention |
| Adams 2016 | Wrong intervention |
| Ahmed 2016 | Wrong intervention |
| Al Jundi 2016 | Wrong intervention |
| Anderson 2018 | Wrong intervention |
| Baron 2017 | Wrong intervention |
| Baron 2017a | Wrong intervention |
| Boogaard 2018 | Wrong intervention |
| Boyce 2018 | Wrong intervention. Aggregate PRO data are fed‐back to clinicians to improve their practice over time |
| Carlson 2010 | Wrong comparator |
| Cook 2016 | Wrong setting |
| Cruickshank 2015 | Wrong intervention |
| Curtis 2018 | Wrong intervention |
| daSilvaRibeiro 2015 | Wrong intervention |
| Davidson 2017 | Wrong intervention |
| Dougados 2015 | Wrong intervention |
| Freyer Adam 2018 | Wrong intervention |
| Friedly 2016 | Wrong intervention |
| Gallo 2016 | Wrong intervention |
| Gossec 2016 | Wrong intervention |
| Graetz 2018 | Wrong comparator |
| Hanling 2016 | Wrong intervention |
| Indovina 2016 | Wrong intervention |
| Jaeger 2017 | Abstract without eligible outcomes, tried to retrieve protocol for confirming eligibility: not found; messaged contact e‐mail on trial website, no response. |
| Janse 2015 | Wrong intervention |
| Janssens 2015 | Wrong intervention |
| Jones 2016 | Wrong intervention |
| Kesanen 2017 | Wrong intervention |
| Kwan 2017 | Wrong intervention |
| Liimatta 2017 | Wrong outcomes |
| Lowenstein 2018 | Wrong intervention |
| McCombie 2020 | Wrong intervention |
| Mooney 2015 | Wrong intervention |
| Murff 2017 | Wrong intervention |
| Olson 2017 | Wrong intervention |
| Paterson 2017 | Wrong intervention |
| Piette 2015 | Wrong comparator |
| Riese 2015 | Wrong comparator |
| Roberts 2017 | Wrong intervention |
| Sanchez 2018 | Wrong intervention |
| Sepucha 2019 | Wrong intervention. Information fed back was not a PRO measure |
| Skinner 2016 | Wrong intervention |
| Smith 2018 | Wrong comparator |
| Sonal Sekhar 2018 | Wrong intervention |
| Stump 2017 | Wrong intervention |
| Szots 2016 | Wrong intervention |
| Uchitomi 2015 | Wrong intervention |
| Valle 2018 | Wrong intervention |
| vanderWeegen 2015 | Wrong intervention |
| vanDijk 2015 | Wrong intervention |
| Voruganti 2017 | Wrong intervention |
| Weiss 2019 | Wrong intervention. Readiness to discharge (information fed back) is not a measure of health, hence not a PRO measure. |
| Williamson 2015 | Wrong intervention |
| Wright 2018 | Wrong intervention |
| Yee 2017 | Wrong intervention |
PRO: patient‐reported outcome
Characteristics of studies awaiting classification [ordered by study ID]
Castillo 2017.
| Methods | Randomised trial, Uruguay |
| Participants | 67 adults diagnosed with cancer |
| Interventions | Intervention: regular completion of touch‐screen health‐related quality of life questionnaires and feedback of results to physician; telephone follow‐up Comparison: telephone follow‐up |
| Outcomes | Main outcome: health‐related quality of life |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Absolom 2017.
| Study name | Electronic patient self‐Reporting of Adverse‐events: Patient Information and aDvice (eRAPID): a randomised controlled trial in systemic cancer treatment |
| Methods | Randomised trial, UK |
| Participants | 1) Adult patients (aged 18 years or over) attending St James’ Institute of Oncology, Leeds with breast cancer undertaking either neo‐adjuvant or adjuvant systemic treatment pathways, gynaecological or colorectal cancer requiring chemotherapy. 2) Prescribed at least 3 months of planned chemotherapy cycles at the time of study consent. 3) Able and willing to give informed consent. 4) Able to read and understand English. 5) Access to the Internet at home. |
| Interventions | eRAPID (electronic patient self‐Reporting of Adverse‐events: Patient Information and aDvice) is an Internet based system for patients to self‐report symptoms and side effects (adverse events or AE) of cancer treatments. Participants (adult patients with breast cancer on neo‐adjuvant or adjuvant chemotherapy, colorectal and gynaecological cancer receiving chemotherapy) are randomised to receive the eRAPID intervention or usual care over 18 weeks of treatment. Participants in the intervention arm receive training in using the eRAPID system to provide routine weekly adverse event reports from home. Hospital staff can access eRAPID reports via the EPR and use the information during consultations or phone calls with patients. |
| Outcomes | The primary outcome of the trial is quality of life (FACT‐G) with secondary outcomes including health economics (costs to patients and the NHS), process of care (e.g. contacts with the hospital, number of admissions, clinic appointments and changes to treatment/medications) and patient self‐efficacy. Outcome data is collected at baseline, 6, 12, 18 weeks and 12 months. The intervention is also being evaluated via end of study interviews with patient participants and clinical staff. |
| Starting date | May 2016 |
| Contact information | Galina Velikova, Section of Patient Centred Outcomes Research (PCOR), Leeds Institute of Cancer and Pathology, University of Leeds, Leeds, UK. Email: g.velikova@leeds.ac.uk |
| Notes |
ACTRN12619001126101.
| Study name | PROpatient: Can symptom monitoring and care coordination improve the quality of life of people with upper gastrointestinal cancer |
| Methods | Randomised parallel trial, Australia |
| Participants | Inclusion criteria: patients aged 18 years and older newly diagnosed with pancreatic, oesophageal and gastric cancer |
| Interventions | Participants allocated to the intervention group complete a self‐report questionnaire on their smartphone, tablet or computer every two‐weeks, severe or worsening symptoms are automatically flagged, in which case care coordinators contact the participant. Participants allocated to the control group receive usual care. |
| Outcomes | Main outcome: health‐related quality of life (European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire) at 3, 6, and 12 months post‐baseline Other outcomes: patient information needs, health services use, emergency department visits, median survival, referral to palliative care |
| Starting date | June 2020 (estimated completion date June 2022) |
| Contact information | John Zalcberg (john.zalcberg@monash.edu) |
| Notes |
ACTRN12620000174987.
| Study name | Using patient‐reported outcome measures for children with life‐altering skin conditions in routine clinical practice: A pilot randomised effectiveness‐implementation study (PEDS‐ePROM) |
| Methods | Randomised parallel trial, Australia |
| Participants | Inclusion criteria: children and adolescents aged <16 years, with burn scars and infantile haemangiomas, receiving outpatient treatment at eligible hospital Exclusion criteria: inability to provide consent or understanding written English, involvement with Child Safety |
| Interventions | Intervention: prior to each appointment generic and disease‐specific health‐related quality of life measures will be complete by the children or their caregivers. A summary will be printed and given to the children and the parents, as well as the attending physicians, and an electronic copy will be added to the medical records. Comparison: children and their caregivers will also complete the same questionnaires prior to each appointment, and results will be available at the end of the follow‐up period (6 months after baseline) |
| Outcomes | Main outcomes: generic child overall health‐related quality of life (Pediatric Quality of Life Evaluation ‐ total score) Other outcomes: health‐related quality of life, quality of life, disease‐specific health‐related quality of life, number and type of referrals |
| Starting date | January 2020 (estimated completion date February 2021) |
| Contact information | Zephanie Tyack (z.tyack@uq.edu.au) |
| Notes |
Arts 2017.
| Study name | Lymphoma InterVEntion (LIVE) – patient‐reported outcome feedback and a web‐based self‐management intervention for patients with lymphoma: study protocol for a randomised controlled trial |
| Methods | Randomised trial, Netherlands |
| Participants | Patients who have been diagnosed with Hodgkin lymphoma, non‐Hodgkin lymphoma, including chronic lymphocytic leukaemia, as registered in the Netherlands Cancer Registry in various hospitals will be selected for participation. Patients are invited via their haemato‐oncologist 6 to 15 months after diagnosis. |
| Interventions | The LIVE randomised trial consists of three arms: (1) standard care, (2) PRO feedback, and (3) PRO feedback and the Living with lymphoma intervention. Patients with lymphoma from various hospitals in the Netherlands will be included and asked to complete questionnaires at four points in time: baseline (T0; 6 to 15 months after diagnosis), after 16 weeks (T1; post intervention), after 12 months (T2), and after 24 months (T3). The PRO feedback includes a graphical overview of patients ’ own symptom and functioning scores and an option to compare their scores with those of other patients with lymphoma and a normative population of the same age and sex. The Living with lymphoma intervention is based on cognitive behavioural therapy components and includes information, assignments, assessments, and videos. |
| Outcomes | To examine whether PRO feedback and the Living with lymphoma intervention will increase self‐management skills and satisfaction with information and reduce psychological distress. |
| Starting date | Not available |
| Contact information | Lindy P. J. Arts, Department of Research, Netherlands Comprehensive Cancer Organisation, PO Box 190793501 DB Utrecht, the Netherlands. |
| Notes | Trial registry NTR5953 |
Atreja 2016.
| Study name | Impact of the Mobile HealthPROMISE Platform on the Quality of Care and Quality of Life in Patients With Inflammatory Bowel Disease: Study Protocol of a Pragmatic Randomised trial. |
| Methods | Randomised trial, USA |
| Participants | This study will prospectively enrol about 300 patients with Crohn's Disease or Ulcerative Colitis presenting at the Mount Sinai Health System. |
| Interventions | Patients using HealthPROMISE will be asked to use the application once every two weeks at a minimum to provide updates on health information. Providers can use the data entered by patients in real time. Patients will get alerts requesting them to contact their providers if their quality of life scores fall below a certain threshold or their symptoms scores are worrisome. Both patients and physicians are also sent regular notifications with data about their own health or health of their patient panel respectively. Both patients and providers are encouraged to use existing communication tools (phone, office visits, personal health records) since direct patient‐physician messaging is not provided in the HealthPROMISE platform. Reminders through app, email and SMS will be used to facilitate patient engagement. Physicians will also be encouraged to check the physician panel to see how patients are doing through weekly updates and monthly quality improvement meetings. |
| Outcomes | Primary Outcome Measure: Improvement in Quality Indicators (adapted from the American Gastroenterological Association (AGA) outpatient IBD quality metrics and other consensus recommendations) [Time Frame: up to 2 years]. Quality metrics for primary end‐point will be adapted from the American Gastroenterological Association (AGA) outpatient IBD quality metrics and other consensus recommendations. |
| Starting date | December 2014 |
| Contact information | Ashish Atreja, Icahn School of Medicine at Mount Sinai |
| Notes |
Bansback 2019.
| Study name | A PROMs Based Educational Tool (PROM‐DA) for Patients Considering Total Knee Arthroplasty: Development and a Pilot Randomized Controlled Trial |
| Methods | Randomised parallel trial, Canada |
| Participants | Adults aged >=30 years, with knee osteoarthritis |
| Interventions | Intervention: participants complete the Patient Reported Outcome Measure informed Decision Aid Comparison: usual care |
| Outcomes | Main outcome: decision quality Other outcomes: quality of life, depression, satisfaction, other outcomes |
| Starting date | June 2017 (estimated completion date March 2020) |
| Contact information | Nick Bansback |
| Notes | Trial registry NCT03240913 |
Bubb 2018.
| Study name | Incorporation of Patient Reported Outcomes Data in the Care of US Veterans With Rheumatoid Arthritis |
| Methods | Randomised parallel trial, USA |
| Participants | Adults aged >=18 years, US veterans |
| Interventions | Intervention: participants will complete patient‐reported outcome measures and their scores will be provided to the treating physician Comparison: participants will complete patient‐reported outcome measures and their scores will not be provided to the treating physician |
| Outcomes | Main outcome: physician/lab‐derived instruments of clinical efficacy Other outcomes: patient satisfaction, medication compliance |
| Starting date | February 2015 (estimated completion date June 2021) |
| Contact information | Michael R Bubb |
| Notes | Trial registry NCT02326532 |
ChiCTR1800018769.
| Study name | The application of patient reported outcomes in preventing relapse of depression |
| Methods | Randomised parallel trial, China |
| Participants | Inclusion criteria: patients aged 18 to 65 years, diagnosed with current major depressive disorder, who have been treated for 6‐12 weeks in acute phase Exclusion criteria: presence of other mental health conditions, suicidal ideation, pregnancy |
| Interventions | Quote: "The patients in PRO treatment group were required to complete the self‐evaluation of PHQ‐9, GAD‐7, AIS, MARS, Q‐LES‐Q‐SF and other related symptoms, medication compliance and quality of life according to the regulations. The patients in PRO treatment group were asked to complete the self‐evaluation periodically based on the way of Wechat public signal platform, and then the doctors made a comprehensive evaluation according to the results of self‐evaluation and adjusted the treatment according to the research plan. At the same time, the patients were given health education. The self‐evaluation, feedback and patient education were all pushed on the platform of Wecaht public number. Finally, the differences between the two groups were compared." |
| Outcomes | Main outcome: sustained response time (measured with Hamilton Rating Scale for Depression) Other outcomes: relapse rate, compliance |
| Starting date | April 2018 |
| Contact information | Hu Chang‐Qing (coannhu@126.com) |
| Notes |
ChiCTR1900020846.
| Study name | A study for perioperative symptom management in patients with lung cancer based on patient‐reported outcomes |
| Methods | Randomised parallel trial, China |
| Participants | Inclusion criteria: people aged 18 to 75 years, diagnosed with primary lung cancer (clinical stage I‐IIIA), waiting to receive surgery and willing to answer a repeated electronic questionnaire on a smartphone or tablet. Exclusion criteria: neoadjuvant therapy, other malignant tumours, inability to understand the study requirements. |
| Interventions | Quote: "After enrolment, all the patients will use their WeChat app to connect with the participating specialists’ WeChat app via a mini programme (ePRO Cell). Then, they will be taught how to use the programme. The ePRO questionnaires will be set to send to the patients’ WeChat app automatically after randomisation. Patients are required to complete the ePRO questionnaires on their smartphones or tablets before surgery (baseline, typically 1–3 days before the operation), daily after surgery (in‐hospital, typically 1 to 7 days after the operation) and twice a week after discharge until 4 weeks or the start of postoperative oncological treatment (typically collecting PRO data six to eight times after discharge). In a hospital setting, if the patients do not complete the ePRO questionnaires within the scheduled time, an electronic reminder (e‐reminder) and up to two bedside reminders will be delivered at the same day. After discharge, if the patients fail to complete the ePRO questionnaires within the scheduled time, an e‐reminder and up to two phone reminders will be delivered with 24 hours." (Protocol) |
| Outcomes | Main outcome: mean symptom threshold events using the MDASI lung cancer‐specific scale Other outcomes: symptom severity, daily functioning and quality of life, revisit rate after discharge |
| Starting date | 1 December 2018 (estimated completion date 31 December 2020) |
| Contact information | Qiang Li (liqiang@sichuancancer.org) |
| Notes |
Gorini 2016.
| Study name | A web‐based interactive tool to improve breast cancer patient centredness |
| Methods | Randomised trial, Italy |
| Participants | Women with breast cancer aged 18 to 75 years diagnosed with primary breast cancer who undergo a radical surgery. Patients with recurrent breast cancer or overt psychiatric illness that could interfere with the measurement of psychological variables will be excluded from the study. The study will be conducted at the European Institute of Oncology (IEO) in Milan, Italy, and patients will be recruited via medical oncologists operating in the same Institute. |
| Interventions | The study will be implemented as a two‐arm randomised trial with 100 adult breast cancer patients who fill in the ALGA‐BC questionnaire, a computerised validated instrument to evaluate the patient’s physical and psychological characteristics following a breast cancer diagnosis. The IEm tool will collect and analyse the patient’s answers in real time and send them, together with specific recommendations to the physician’s computer immediately before physician’s first encounter with the patient. Patients will be randomised to either the intervention group using the IEm tool or to a control group who will only fill in the questionnaire without taking advantage of the tool (physicians will not receive the patient’s profile). |
| Outcomes | To evaluate the effect of an interactive empowerment tool (IEm) on enhancing the breast cancer patient–physician experience, in terms of increasing empowerment, i.e. by providing physicians with a personalised patient’s profile, accompanied by specific recommendations to advise them how to interact with each individual patient on the basis of her personal profile. |
| Starting date | Not available |
| Contact information | Alessandra Gorini. Email: alessandra.gorini@unimi.it |
| Notes |
Grove 2018.
| Study name | |
| Methods | Randomized trial, Denmark. |
| Participants | Outpatients with chronic kidney disease. |
| Interventions | Assessment and feedback of PROM information. |
| Outcomes | Main outcome: loss of renal function evaluated by estimated glomerule filtration rate. Secondary outcomes: intiation of acute dialysis, hospitalisation, mortality, utilisation of healthcare resources, quality of life, and illness perceptions. |
| Starting date | |
| Contact information | |
| Notes | Funded by Karen Elise Jensen foundation, Helsefonden and Trygfonden. |
Grove 2019.
| Study name | PROKID study |
| Methods | Parallel randomised trial, Denmark |
| Participants | Adults age >=18 years, referred to renal care services at eligible sites |
| Interventions | Arm 1: participants complete a questionnaire every 3 months, which is used as a decision aid alongside other clinical information to decide whether the participant needs an appointment or not. Arm 2: participants complete a questionnaire every 3 months prior to a telephone appointment, the information is used during the appointment. Arm 3: comparison (usual care) |
| Outcomes | Main outcome: Change from baseline Estimated Glomerular Filtration Rate (eGFR) at 18 months Other outcomes: mortality, hospital admission, kidney transplant, health‐related quality of life (among others) |
| Starting date | December 2018 |
| Contact information | Birgith Grove |
| Notes | Trial registry NCT03847766 |
Holch 2018.
| Study name | eRAPID electronic patient self‐Reporting of Adverse‐events: Patient Information and aDvice: a pilot study protocol in pelvic radiotherapy |
| Methods | Randomised trial, UK |
| Participants | Patients attending St James ’ s University hospital cancer centre and The Christie Hospital Manchester undergoing pelvic radiotherapy+/ − chemotherapy/hormonotherapy for prostate, lower gastrointestinal and gynaecological cancers. |
| Interventions | Prospective 1:1 randomised (intervention or usual care) parallel group design with repeated measures and mixed methods will be employed. Aim is to recruit 168 patients following recommendations for sample size estimates for pilot studies. Participants using eRAPID will report AE (at least weekly) from home weekly for 6 weeks and 6 weeks post‐treatment (12‐week total) then at 18 and 24 weeks.Hospital staff will review eRAPID reports and use information during consultations. Notifications will be sent to the relevant clinical team when severe symptoms are reported. |
| Outcomes | The objectives are to establish feasibility, recruitment, integrity of the system and attrition rates, determine effect sizes and aid selection of the primary outcome measure for a future randomised trial. |
| Starting date | September 2016 |
| Contact information | Trish Holch, Department of Psychology, School of Social Sciences, Leeds Beckett University, Calverley Building, Room CL 815 City Campus, Leeds LS1 9HE, UK. Email: T.Holch@Leedsbeckett.ac.uk |
| Notes |
ISRCTN82172279.
| Study name | Reconceptualising patient‐reported outcome measures for back pain |
| Methods | Cluster randomised controlled trial (cRCT) and process evaluation |
| Participants | Private patient at least 16 years old presenting to the musculoskeletal clinic with self‐reported back pain |
| Interventions | Patients will be asked to complete PROMs at various stages during their treatment. The PROMs will be the Musculoskeletal Health Questionnaire (MSK‐HQ) and the Patient Global Impression of Change Scale (PGIC). The chiropractors recruited into the study will be randomly allocated to one of the three groups using a randomisation generator. Patients booking in with these chiropractors will be asked if they would like to take part in the study and those who consent to take part to the study will be allocated to that chiropractor’s group in the trial. Depending whether patients have booked in with chiropractors in the routine PROM group or the intensive PROM group, they will be asked to complete PROMs at various stages during their treatment. Patients in the routine PROM group will be asked to complete PROMs three times. Patients in the intensive PROM group will be asked to complete PROMs seven times. Those in the control group will not complete PROMs. Chiropractors in the routine and intensive PROM groups will be asked to discuss PROMs with their patients at every session after a PROM has been completed. The follow up will be 90 days. |
| Outcomes | Back pain (physical functioning and disability) measured with the Roland‐Morris Questionnaire at baseline and 90 days |
| Starting date | 31/01/2018 |
| Contact information | University of Southampton, University Road, Southampton, SO17 1PS United Kingdom |
| Notes |
Kendrick 2020.
| Study name | Patient‐reported outcome measures for monitoring primary care patients with depression: PROMDEP randomised controlled trial |
| Methods | Randomised cluster trial, UK |
| Participants | Adults aged >=18 years who attended their general practices within the last 2 weeks and assigned Read computerised medical record codes by GPs or nurse practitioners (NPs) for new presentations with diagnoses or symptoms of depression |
| Interventions | Intervention: participants will complete patient‐reported outcome measure and receive their score as well as treatment recommendations to discuss with their general practitioner Comparison: usual care |
| Outcomes | Main outcome: symptoms of depression (12 weeks) Other outcomes: symptoms of depression (26 weeks), social functioning, quality of life, costs of consultations, quality of life |
| Starting date | November 2021 (estimated completion date October 2021) |
| Contact information | Rachel Dewar‐Haggart (r.v.dewar‐haggart@soton.ac.uk) |
| Notes | Trial registry ISRCTN17299295 |
Klinkhammer‐Schalke 2015.
| Study name | Direct improvement of quality of life in colorectal cancer patients using a tailored pathway with quality of life diagnosis and therapy (DIQOL): study protocol for a randomised controlled trial |
| Methods | Randomised trial, Germany |
| Participants | Patients are included under broad inclusion criteria: (1) diagnosis of primary colorectal cancer and (2) surgery in one of the four participating hospitals. |
| Interventions | In the intervention group, QoL scores are transformed into a QoL profile. This is sent to the coordinating practitioner (general practitioner, internist, or oncologist) with an expert report including treatment recommendations for QoL deficits. The control group receives routine follow‐up care attending the guideline recommendations for colorectal cancer without profile or expert report. At the primary endpoint (12 months), the rates of patients with diseased QoL in both groups are compared. |
| Outcomes | The primary objective of the study is to improve QoL of colorectal cancer patients during follow‐up care with systematic QoL diagnosis and targeted treatment. |
| Starting date | December 2014 |
| Contact information | Monika Klinkhammer‐Schalke, Tumor Center Regensburg e.V., An‐Institute of the University of Regensburg, Josef‐Engert‐Straße 9, 93053 Regensburg, Germany. Email: Monika.Klinkhammer‐Schalke@ukr.de |
| Notes |
Kuklinski 2020.
| Study name | PROMoting Quality ‐ Intersectoral use of Patient Reported Outcome Measures to increase patient‐relevant outcome quality |
| Methods | Randomised parallel trial, Germany |
| Participants | Adults aged >= 18 years, awaiting for primary elective surgery for total knee replacement and total hip replacement |
| Interventions | Intervention: participants will complete an electronic patient‐reported outcome measure at regular intervals and the results will be shared with treating healthcare professional and study staff Comparison: usual care |
| Outcomes | Main outcome: composite measure of PROMs and clinical outcome measures; direct and follow‐up health care cost of the procedures; cost of implementing the designed intervention Other outcomes: functionality, health‐related quality of life, patient satisfaction |
| Starting date | November 2019 |
| Contact information | David Kuklinski |
| Notes | Trial registry DRKS00019916 |
Kyte 2018.
| Study name | RePROM pilot/feasibility study in chronic kidney disease |
| Methods | Parallel randomised trial, UK |
| Participants | Adults aged >= 18 with advanced chronic kidney disease |
| Interventions | Intervention: participants provide monthly reports on their health status using an online electronic Patient‐Reported Outcome Measure (ePROM) system. Comparison: usual care |
| Outcomes | Main outcomes: recruitment and retention rates, data collection processes, data completeness and adherence to the ePROM intervention Other outcomes: health‐related quality of life, clinical condition, clinical event data, health resource use |
| Starting date | January 2017 |
| Contact information | Derek Kyte (kytedg@bham.ac.uk) |
| Notes | Trial registry ISRCTN12669006 |
Mamguem Kanga 2020.
| Study name | |
| Methods | Randomised trial, France. |
| Participants | Women with non‐metastatic hormone receptor‐positive breast cancer. |
| Interventions | Health‐related quality of life assessment with delivery of scores to clinicians vs usual care. |
| Outcomes | Primary outcome: compliance with endocrine therapy at 12 months. |
| Starting date | |
| Contact information | |
| Notes | The study was funded by the Georges François LeClerc Center, Burgundy, France. |
Matsuda 2018.
| Study name | |
| Methods | Randomised trial, Japan. |
| Participants | Patients with cancer receiving palliative care. |
| Interventions | Quality of life assessment and feedback to clinicians using the Care Notebook |
| Outcomes | Main outcome: global health status measured by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 15 Palliative PROM |
| Starting date | |
| Contact information | |
| Notes | Study is funded by the Japanese Ministry of Education, Culture, Sports, Science and Technology. |
Morton 2019.
| Study name | Symptom Monitoring with Feedback Trial (SWIFT) |
| Methods | Cluster randomised trial, Australia and New Zealand |
| Participants | Adults aged >= 18 years with kidney disease receiving in‐centre haemodialysis or haemodiafiltration |
| Interventions | Intervention: regular symptom monitoring with feedback to the renal team Comparison: collection of health‐related quality of life at baseline and follow‐up |
| Outcomes | Main outcomes: overall response rate, barriers and facilitators to using patient‐reported outcome measures Other outcomes: time taken to complete measures, patient representativeness and retention |
| Starting date | December 2018 |
| Contact information | Rachael Morton (rachael.morton@ctc.usyd.edu.au) |
| Notes | Trial registry ACTRN12618001976279 |
NCT02591472.
| Study name | An integrated‐delivery‐of‐care approach to improve patient outcomes, safety, well‐being after orthopaedic trauma. |
| Methods | Randomised trial, USA |
| Participants | 111 participants with serious musculoskeletal injury, being treated at to the University of Florida's (UF) Orthopaedic Trauma service at UF Health at Shands Hospital, randomised between the two groups (intervention and usual care). |
| Interventions | The research study will determine whether the Usual Care or Integrated Care (which is Usual Care plus emotional support, and education/information during the hospital stay) helps patients feel better about their physical function and emotional well‐being. Participants with serious musculoskeletal injury, being treated at to the University of Florida's (UF) Orthopaedic Trauma service at UF Health at Shands Hospital, will be randomised (like tossing a coin) between the two groups. Usual Care will follow all the highest standards for injury treatment. Integrated Care will include medical care and emotional support. Study Staff are trained to provide emotional support and teach patients the skills for goal setting, taking ownership of journey, establishing lifelines, mobilizing resources and reducing stressors. In addition, questionnaires and simple functional tests will be collected at the hospital and at normal follow‐up visits at weeks 2, 6 and 12 and months 6 and 12. |
| Outcomes | Primary outcome 1: Change in baseline, at weeks 2, 6 and 12 and months 6 and 12 on the Patient Reported Outcome Measurement Information System (PROMIS) ‐ Physical Function between the groups. [ Time Frame: Change in Baseline, at weeks 2, 6 and 12 and months 6 and 12 ] Survey questionnaire measures the perception of Physical Function. Physical Function Average: T score = 50±10 Min: 10 Max: 90 Primary outcome 2: Change in baseline, at weeks 2, 6 and 12 and months 6 and 12 on the Patient Reported Outcome Measurement Information System (PROMIS) ‐ Social Roles between the groups. [ Time Frame: Change in Baseline, at weeks 2, 6 and 12 and months 6 and 12 ] Survey questionnaire measures the perception of Social Roles. Social Roles Average: T score = 50±10 Min: 10 Max: 90 Primary outcome 3: Change in baseline, at weeks 2, 6 and 12 and months 6 and 12 on the Patient Reported Outcome Measurement Information System (PROMIS) ‐ ‐ Psychosocial Illness Impact‐positive between the groups. [ Time Frame: Change in Baseline, at weeks 2, 6 and 12 and months 6 and 12 ] Survey questionnaire measures the perception of Psychosocial Illness Impact. Psychosocial Average: T score = 50±10 Min: 13.8 Max: 68.7 |
| Starting date | January 2016 |
| Contact information | Heather K Vincent, Ph.D. University of Florida Department of Orthopaedics. |
| Notes |
NCT02673580.
| Study name | Tele‐patient‐reported Outcomes (telePRO) in clinical practice |
| Methods | Randomised trial, Denmark |
| Participants | 593 participants. Inclusion Criteria: Males and females from Age 15 years Diagnosis of epilepsy Referred to standard telePRO by a clinician Access to Internet (web‐responders in standard telePRO) Can speak and understand Danish |
| Interventions | To compare quality of care and patient experiences in two outpatients follow‐up activities: 1) Standard telePRO (fixed interval telePRO follow‐up) and 2) Open Access telePRO (patient‐initiated telePRO follow‐up) |
| Outcomes | Primary outcome: Number of contacts [Time Frame: 18 months] includes all contacts with the outpatient clinic in the study follow‐up period |
| Starting date | January 2016 |
| Contact information | Niels Henrik Hjollund, Professor Regional Hospital West Jutland |
| Notes |
NCT02818478.
| Study name | Patient reported outcomes reported via PC/ tablet home versus touch screen at hospital among patients with arthritis (PRO) |
| Methods | Randomised trial, Denmark |
| Participants | Inclusion Criteria Rheumatoid arthritis OR axial spondyloarthritis Active treatment and monitoring of the Knowledge Center for Rheumatology and Spine diseases, Rigshospitalet, Denmark Patients must have reported patient reported outcome measures via DANBIOs touch‐screen solution ≥ 3 times Exclusion Criteria Impaired vision Non‐Danish speaking No electronic device at home,, tablet or computer |
| Interventions | To investigate if electronic reporting of patient reported outcome measures from home is comparable to the traditional touch‐screen solution to hospital among patients with rheumatoid arthritis and axial spondyloarthritis |
| Outcomes | Primary outcome: The Health Assessment Questionnaire (HAQ) developed to retrieve quantitative information on outcomes among patients with rheumatoid arthritis |
| Starting date | May 2016 |
| Contact information | Merete M Hetland, Rigshospitalet, Denmark |
| Notes |
NCT02917954.
| Study name | Electronic patient reported outcome (ePRO) mobile application pragmatic trial |
| Methods | Randomised trial, Canada |
| Participants | A FHT patient at one of the FHT sites selected and is 60 years or older; Physical capability to use a tablet and/or a caregiver who can use the tablet on their behalf; Ability to read and write in English and/or the availability of a caregiver who can do so on their behalf; Has complex care needs defined as two or more chronic conditions and 10 or more visits to their primary health care provider within the last 12 months; and Be thinking about or ready to make changes to support their self‐management. |
| Interventions | During the ePRO Tool intervention participants will complete surveys at every 3 months intervals starting month at 4 or month 7, for study duration. Surveys capture patient demographics, assessment of quality‐of‐life, chronic disease management, primary care experience, and Electronic Patient Reported Outcome (ePRO) Mobile Application tool usability. Participants will also meet with their provider to setup and monitor a health goal to track during the study via the ePRO application. During the study, participants will meet with their primary care providers 4‐5 times to discuss their health goal monitoring. Post‐study participants will discuss their experience using the ePRO app in an interview or focus group setting. |
| Outcomes | Primary outcome: Change from baseline Assessment of Quality‐of‐Life at 3 month intervals for 15 months [ Time Frame: Baseline, 3 months, 6 months, 9 months, 12 months, and study end (15‐months)] |
| Starting date | May 2017 |
| Contact information | Carolyn Steele Gray. Email: Carolyn.SteeleGray@sinaihealthsystem.ca |
| Notes |
NCT02949167.
| Study name | MyHealth: Follow‐up after breast cancer treatment |
| Methods | Randomised trial, Denmark |
| Participants | 494 primary BC patients will be recruited from the Departments of Oncology at Naestved and Roskilde Hospital. Inclusion criteria: Complete remission following primary treatment for loco‐regional BC (stage I‐II) ‐ No confirmed genetic predisposition to BC Female gender Performance status ≤3 Read, understand and speak Danish No severe cognitive problems No severe psychiatric disease requiring treatment or any substance abuse. |
| Interventions | The MyHealth intervention is a nurse‐led individually tailored symptom management program, focused on patient education and regularly collection of Patient Reported Outcomes (PRO) subsequently evaluated by specialist nurses and navigation to health care service. The nurse will meet with the patient on three‐five planned appointments focused on adjustment of life after breast cancer treatment including information on symptoms of relapse or late effects and how to react on these. Close relatives are invited if patients accept. Patients will report PRO´s on symptoms of recurrence and late effects every three months during the first year and thereafter every six months.The appointments with the nurse are finalized within 3‐6 month and patients will be followed with PRO for three years. |
| Outcomes | Primary outcome: Changes in breast cancer specific symptom burden (TOI‐PFB) [ Time Frame: at inclusion, 6 months,12 months, 24 months, 36 months and 60 months] |
| Starting date | November 2016 |
| Contact information | Christoffer Johansen, The Cancer Society Research Center, Survivorship |
| Notes |
NCT02996201.
| Study name | Electronic patient reporting of side effects to chemotherapy: A cluster randomised trial |
| Methods | Randomised trial, Denmark |
| Participants | Breast cancer patients starting adjuvant chemotherapy in the period November 1, 2015 ‐ September 1, 2016 in Danish oncology clinics |
| Interventions | To determine whether the use of breast cancer patients' own electronic reporting of side effects to chemotherapy in a treatment setting has an impact on the handling of side effects and on the number of hospitalizations, febrile neutropenia and dose adjustments. Study uses the Patient‐Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO‐CTCAE) for the patients' reporting of side effects. Patients report PRO‐CTCAE symptoms on a tablet computer before each cycle of chemotherapy. |
| Outcomes | Primary outcome: Dose adjustments reported in the medication treatment sheet before each cycle of chemotherapy (5 time points with three weeks interval) [ Time Frame: up to 18 weeks of treatment in the period between November 1, 2015 and January 31, 2017 ] |
| Starting date | November 2015 |
| Contact information | Helle Pappot, Rigshospitalet, Denmark |
| Notes |
NCT03056469.
| Study name | Patient‐reported outcomes integrated in the follow‐up of patients with hematological cancer |
| Methods | Randomised trial, New Zealand |
| Participants | Patients newly diagnosed with not curable, chronic hematological cancer |
| Interventions | This study investigates, if use of the patient‐reported outcome (PRO) questionnaires are useful in the assessment of the patients needs and health care providers decision making regarding supportive care interventions. It investigates, if completion of PRO questionnaires changes the number and kind of supportive care interventions. In one randomisation arm the participants submit patient‐reported outcomes, and the care providers have access to the patient‐reported outcomes. In another randomisation arm the participants submit patient‐reported outcomes, but the care providers do not have access to the patient‐reported outcomes. In the last randomisation arm the participants are randomised to standard follow‐up, do not complete PRO questionnaires and are thus controls. |
| Outcomes | Primary outcome: Number and kind of supportive care interventions are registered. Supportive care actions are defined as: a) a plan for rehabilitation, b) an intervention by a physiotherapist, occupational therapist, dietician, or social worker, c) consultation with a psychologist or talk with a priest, d) an intervention done by a general practitioner because of the hematological cancer after contact between the hematological department and the general practitioner, e) use of offers like group talks etc offered by the Danish Cancer Society, or f) other supportive care interventions |
| Starting date | September 2016 |
| Contact information | Nana Brochmann. Email: nmor@regionsjaelland.dk |
| Notes |
NCT03093649.
| Study name | Measuring patient‐reported adverse events in oncology practice improves quality of life in nasopharyngeal carcinoma |
| Methods | Randomised trial, China |
| Participants | Patients with newly histologically confirmed non‐keratinizing carcinoma (according to WHO histological type) |
| Interventions | Patients report adverse events using patient reported outcomes version of common terminology criteria for adverse events (PRO‐CTCAE) through Application (APP) during the treatment. The summary report is transferred to their clinician immediately. Oncologists alarmed if patients reports exceed the pre‐defined threshold. |
| Outcomes | Primary outcome: Score of physical functioning in quality of life |
| Starting date | July 2017 |
| Contact information | Ying Sun, Sun Yat‐sen University |
| Notes |
NCT03202732.
| Study name | DiabetesFlex ‐ Patient involvement and patient‐reported outcome measures in Type 1 Diabetes |
| Methods | Randomised trial, Denmark |
| Participants | Adults with T1DM for more than 2 years |
| Interventions | DiabetesFlex consists of one mandatory and two optional consultations. Before the consultations patients receive the AmbuFlex Diabetes questionnaire. The AmbuFlex Diabetes questionnaire is based on both validated questionnaires and clinical consensus. The AmbuFlex Diabetes questionnaire consists of: SF36 well‐being question, WHO‐5 Well‐being Index. Questions concerning: HgA1c, home‐based blood pressure monitoring, incidents of hypoglycaemia, diabetes complications, regular eye check, regular food check, erectile dysfunction and peripheral neuropathy, The PAID scale, Topics patients may want to talk with the health care professional about, the patient's evaluation of the need for diabetes care. View the AmbuFlex Diabetes questionnaire at the homepage: www.diabetesflex.auh.dk. |
| Outcomes | Primary outcome: Non‐inferiority with respect to HbA1c |
| Starting date | October 2017 |
| Contact information | Annesofie L. Jensen. Email: anejns@rm.dk |
| Notes |
NCT03240913.
| Study name | A PROMs based educational tool (PROM‐DA) for patients considering total knee arthroplasty |
| Methods | Randomised trial, Canada |
| Participants | Inclusion Criteria: Adult (age≥30) patients with knee osteoarthritis (OA) Have an appointment with a surgeon for consultation about Total Knee Arthroplasty at the Edmonton Bone and Joint Centre Understands, speaks and reads English; and Able to provide informed consent. |
| Interventions | 1) develop an educational tool known as the Patient Reported Outcome Measure informed Decision Aid (PROM‐DA) that will describe the options for patients considering total knee arthroplasty (TKA) surgery, and help them imagine what to expect if they choose either option; 2) assess the extent that the PROM‐DA improves patients decision quality; 3) determine the feasibility of a larger trial to test the PROM‐DA in multiple sites and more patients. |
| Outcomes | Primary outcome: Decision quality [ Time Frame: 40 to 52 weeks after baseline ] Hip and Knee Decision Quality Instrument (HK‐DQI) |
| Starting date | June 2017 |
| Contact information | |
| Notes |
NCT03249090.
| Study name | Electronic patient reporting of symptoms during cancer treatment (PRO‐TECT) |
| Methods | Randomised trial, USA |
| Participants | Inclusion Criteria: Adults (21+) with advanced/metastatic cancer of any type (EXCEPT leukaemia or indolent [slow growing] lymphoma) Receiving outpatient systemic cancer treatment for non‐curative/palliative intent, including chemotherapy, targeted therapy, or immunotherapy. Enrolled at any point in their treatment trajectory, meaning during any line of treatment, and at any point during a course or cycle of treatment. Can understand English, Spanish, and/or Mandarin Chinese. |
| Interventions | At baseline, CRAs will train patients to self‐report symptoms and physical functioning weekly for up to a year, with a choice to do so online or via an automated telephone system. Whenever a concerning symptom is reported, an automated "email alert" notification will be sent to the site CRA. The CRA will forward the alert to the responsible clinical nurse (or other covering clinician) and CC the site's Nurse Champion. Within 72 hours, the CRA will document what action(s), if any, were taken by the nurse in response to the alert (entered by the CRA into a form in the PRO‐Core system). A symptom report will be printed/generated by the site CRA whenever the patient has a clinic visit and will be given to the oncologist and nurse caring for the patient. |
| Outcomes | Primary outcomes: 1) Physical Functioning [Time Frame: 3 months] Physical functioning will be measured via the QLQ‐C30 2) Overall Survival [Time Frame: Up to 24 months] Based on the number of events observed. Overall survival will be compared between arms using a stratified log‐rank rest. |
| Starting date | October 2017 |
| Contact information | Sydney Henson. Email: seriggsb@email.unc.edu |
| Notes |
NCT03471104.
| Study name | Patient‐reported outcome measures in diabetes care (DiaPROM) |
| Methods | Randomised trial, Norway |
| Participants | Inclusion Criteria: type 1 diabetes for more than one year |
| Interventions | The intervention starts when participants complete PROMs before an annual consultation. The physician reviews the PAID (problem areas in diabetes scale) scores with the participant. Participants with one or more single PAID item(s) scored 3 or 4, or a PAID score ≥30, will be referred to extra follow‐up which will consist of at least two diabetes nurse consultations. The nurses will follow a communication manual based on key elements from empowerment theory and self‐determination theory. The participants then complete the PROMs prior to the next annual consultation with the physician. |
| Outcomes | Primary outcome: Change in Diabetes Distress Scale (DDS) [ Time Frame: Baseline, 12 months and 24 months. ] Self reported diabetes‐related distress. 17 items are scored on a 6 point Likert scale from 1 "not a problem" to 6 "very serious problem". Scores are summated and divided by 17 to form a mean/average score. There are also four subscales; emotional burden (5 items), physician‐related distress (4 items), regimen‐related distress (5 items) and interpersonal distress (3 items). The subscales scores are calculated similar to the total score except for dividing by the number of items for each subscale. A total DDS‐score or subscale score of more than 3 is regarded as high degree of diabetes distress. Whilst a score of 2 indicate moderate diabetes distress and a score of 1 is considered as low degree of diabetes distress. |
| Starting date | September 2020 |
| Contact information | Lars Birger Nesje, Haukeland University Hospital, Bergen, Hordaland, Norway, 5021. Email: lars.birger.nesje@helse‐bergen.no |
| Notes |
NCT03535922.
| Study name | Evaluation of routinely measured patient‐reported outcomes in haemodialysis care |
| Methods | Randomised trial, Canada |
| Participants | Inclusion criteria: Undergoing haemodialysis within an eligible in‐centre dialysis unit in Alberta or Ontario 18 years or older at the start of the study Willing and able to complete the PROMs as part of the trial |
| Interventions | In the trial, patients will be invited to complete the PROMs, and results of the measures will be linked to treatment aids for clinicians, providing specific information on how symptoms can best be managed. These care pathways will also be available to patients not receiving PROMs. The main outcome of this study will be patient‐clinician communication, which will be assessed using a questionnaire called the "Communication Assessment Tool". In addition to assessing the effect of using these questionnaires on patient‐provider communication, this study will allow us to explore whether their use affects patient management and symptoms, use of healthcare services, and the overall cost of implementing these questionnaires in clinical practice. Each dialysis unit (including all patients) will be randomised to one of four study groups: 1) Patients will complete the disease‐specific PROM; 2) Patients will complete the generic PROM; 3) Patients will complete both the disease‐specific and generic PROM; 4) Patients will receive usual care. Clinicians (in dialysis units randomised to PROMs, groups 1‐3) will receive the results of the questionnaires completed by the patients. This is intended to trigger the clinician to ask the patient about certain symptoms if any exist. All clinicians in all study groups will have access to the clinical "treatment aids", which are tools that help identify and manage certain symptoms that patients might have. For example, people with severe itching will be cared for based on a step‐wise treatment algorithm. Patients will also receive a report of their questionnaire(s) results, with an explanation of what it means. |
| Outcomes | Primary outcome: Change in Communication Assessment Tool (CAT) scores over 12 months [ Time Frame: Measured at baseline, 6 months, and 12 months ] The CAT assesses patient perceptions of clinicians' interpersonal and communication skills. 'Communication' refers to the interactions between members of the healthcare team (i.e., nurses, nephrologists) and the patient. |
| Starting date | September 2018 |
| Contact information | Jeffrey Johnson, University of Alberta |
| Notes |
NCT03608410.
| Study name | Intensified follow‐up of lung cancer using weekly questionnaires via the Internet (ProWide) |
| Methods | Randomised trial, Denmark |
| Participants | Inclusion Criteria: Patients with lung cancer (NSCLC and SCLC), who have received 1st line induction treatment* for lung cancer and have no sign of progressive disease at first evaluation CT scan. Patients diagnosed with stage III treated with palliative intention, and stage IV, regardless of treatment intention. |
| Interventions | Patients will be asked to fill in a web‐based Patient Reported Outcome (PRO) questionnaire every week. If one of the reported symptoms worsens and exceed a predefined threshold of severity, a notification is automatically sent to the hospital. A nurse will review the questionnaire and contact the patient for verification of symptoms. If progression of disease is suspected, a CT scan will be made. Otherwise, the nurse will schedule a visit at the clinic for physical examination and evaluation by a clinician. If progressive disease is not suspected, supportive care will be adjusted and the patient will continue follow up according to the usual schedule. |
| Outcomes | Primary outcome: Overall survival [ Time Frame: 2 years ] |
| Starting date | September 2018 |
| Contact information | Rasmus Friis. Email: rasfri@rm.dk |
| Notes |
NCT03850912.
| Study name | SIMPRO Research Center: Integration and Implementation of PROs for Symptom Management in Oncology Practice |
| Methods | Randomised parallel trial, USA |
| Participants | Adults aged >= 18 years, who meet one of the following:
|
| Interventions | Intervention: participants will receive patient‐reported outcome measures and receive feedback Comparison: participants will receive patient‐reported outcome measures and not receive feedback |
| Outcomes | Main outcome: 'Emergency Department ‐ Treat and Release' (EDTR) Rate at 30‐days Other outcomes: symptom burden, patient satisfaction, initiation of adjuvant chemotherapy, sustainability of the intervention |
| Starting date | July 2019 (estimated completion date September 2023) |
| Contact information | Deborah Schrag |
| Notes |
NCT03995082.
| Study name | A Randomized Study of Breast Cancer Patient Engagement With Patient Reported Outcome Measure Survey Results |
| Methods | Parallel randomised trial, USA |
| Participants | Women aged >=18, diagnosed with breast cancer |
| Interventions | Intervention: participants complete a patient‐reported outcome measure and receive a graphic depiction of their score Comparison: participants complete a patient‐reported outcome measure but are not provided feedback |
| Outcomes | Main outcome: patient satisfaction with patient‐provider communication Other outcomes: patient satisfaction (other domains), healthcare use |
| Starting date | October 2019 (estimated completion date July 2021) |
| Contact information | Sarah Tevis (sarah.tevis@ucdenver.edu) |
| Notes |
NCT04066868.
| Study name | Evaluating the Use of Patient‐Reported Outcome Measures for Improving the Inter‐Rater Reliability of Common Terminology Criteria for Adverse Event Ratings |
| Methods | Randomised parallel trial, Austria |
| Participants | Inclusion criteria: patients >=18 years with a cancer diagnosis, currently receiving chemotherapy or immunotherapy, with symptom burden equal or greater score 3 of the screening question "On a scale of 0 to 10, to what degree did you experience physical or emotional symptoms/problems during the last week?" Exclusion criteria: psychiatric diagnosis or mental health problems |
| Interventions | All participants complete patient‐reported outcome measures, the physicians of patients allocated to the intervention group receive feedback about their scores |
| Outcomes | Main outcomes: patient‐reported quality of life, physician‐rated quality of life |
| Starting date | February 2020 (estimated completion date August 2021) |
| Contact information | Bernhard Holzner (bernhard.holzner@tirol‐kliniken.at |
| Notes |
NCT04069455.
| Study name | A Randomized, Multi‐center, Prospective Study Evaluating e‐Patient Report Outcomes (ePRO) for Adjuvant Chemotherapy in Chinese Patients With Colorectal Cancers |
| Methods | Randomised parallel trial, China |
| Participants | Adults aged 18 to 75 years old, diagnosed with colorectal cancer, who underwent radical surgery for cancer |
| Interventions | Intervention: participants will report their symptoms using a we‐based system, and will receive severity‐based advice Comparison: usual care |
| Outcomes | Main outcomes: global health and functional scores; health‐related quality of life Other outcomes: adverse events; proportion of completed chemotherapy; overall survival |
| Starting date | October 2019 (estimated completion date September 2024) |
| Contact information | Ding Ke‐Feng |
| Notes |
NCT04164004.
| Study name | Randomized Trial of Patient‐Reported Outcome Measurement in Heart Failure Clinic |
| Methods | Randomised parallel trial, USA |
| Participants | Adults aged >= years, attending Heart Failure clinic |
| Interventions | Intervention: participants will complete a condition‐specific patient‐reported outcome measures and results will be made available to the treating clinicians Comparison: usual care |
| Outcomes | Main outcome: health status, completion of patient‐reported outcome measure Other outcomes: percentage of participants on different medications, percentage of participants with other therapies, referral to other clinics, medication adjustment, healthcare use |
| Starting date | May 2020 (estimated completion date May 2022) |
| Contact information | Alexander T Sandhu |
| Notes |
NCT04342260.
| Study name | Improving Quality of Life After Thoracic Surgery Using Patient‐Reported Outcomes |
| Methods | Randomised parallel trial, USA |
| Participants | Adults aged >= 18 years, presenting for inpatient thoracic surgery |
| Interventions | Intervention: participants will complete patient‐reported outcome measures, treating clinicians will be alerted when the scores exceed baseline postoperative scores by 2 points or more, or when 'severe' or 'very severe' symptoms are reported Comparison: participants will complete patient‐reported outcome measures, however treating clinicians will not be alerted |
| Outcomes | Main outcomes: quality of life, disease‐specific quality of life, percentage of completed surveys, percentage of surveys that trigger a response, barriers and facilitators of using patient‐reported outcome measures Other outcomes: readmission, overall survival, percentage of quality of life surveys completed |
| Starting date | April 2020 (estimated completion date May 2024) |
| Contact information | Gita Mody |
| Notes |
NCT04356209.
| Study name | Improving Theempowerment in Patients With Severe Breast Fibrosis Radio‐induced Treated by Pravastatin: Benefit of e‐PROs (Electronic " Patient Reported Outcome ") on Breast‐related Quality of Life |
| Methods | Randomised parallel trial, France |
| Participants | Adults aged >=18 years, treated by conserving surgery followed by adjuvant RT |
| Interventions | Intervention: symptoms and health status will be collected using patient‐reported outcome measures, supported by a web interface Comparison: usual care |
| Outcomes | Main outcome: breast‐related quality of life Other outcomes: health‐related quality of life; use of antidepressants, analgesics and anxiolytics; other outcomes |
| Starting date | September 2020 (estimated completion date June 2025) |
| Contact information | Celine Bourgier (celine.bourgier@ivm.unicancer.fr) |
| Notes |
NCT04393571.
| Study name | The Utility of Mobile Based Patient Reported Outcome Measures(PROMS) in Patients With Acetabular Fractures: A Randomized Controlled Trial. |
| Methods | Randomised parallel trial, Egypt |
| Participants | Adults aged >= 18 years scheduled for surgery for an acetabular fracture |
| Interventions | Intervention: participant's quality of life will be collected using a mobile application Comparison: unclear |
| Outcomes | Main outcome: percentage of missed follow up data Other outcome: ability of the mobile application to rapidly detect the occurrence of serious postoperative complication |
| Starting date | October 2020 (estimated completion date October 2023) |
| Contact information | Mohammed Kamal Abdelnasser |
| Notes |
NCT04401332.
| Study name | Integrating Patient‐Reported Outcomes Into Routine Primary Care: Monitoring Asthma Between Visits |
| Methods | Randomised parallel trial, USA |
| Participants | Inclusion criteria: patients attending primary care appointments at one of eligible outpatient clinics, with at least one asthma‐related visit in the past 12 months, aged >=18 years, able to provide consent, speak English and use a compatible smartphone |
| Interventions | Patients allocated to intervention will have access to asthma symptom monitoring via a clinically integrated mobile health (mHealth) app installed on their smartphones. Comparison will be usual care. |
| Outcomes | Main outcome: asthma‐related quality of life (6 and 12 months, using Mini Asthma Quality of life Questionnaire) Other outcome: asthma‐related healthcare use |
| Starting date | |
| Contact information | Robert S Rudin (rrudin@rand.org) |
| Notes |
NCT04492007.
| Study name | Feasibility Testing of Patient Reported Outcomes ‐ Informed Symptom Management System (PRISMS) |
| Methods | Randomised parallel trial, USA |
| Participants | Inclusion criteria: patients aged >=40 years who have underwent surgery for colorectal, bladder, ovarian, cervical, or uterine cancer with curative intent; be within 2 weeks of hospital discharge of a newly created ostomy with curative intent; have a caregiver. Caregivers: >= 18 years, without a previous diagnosis of cancer. Exclusion criteria: additional cancer diagnosis, unable to understand English or provide consent |
| Interventions | Participants allocated to the intervention have access to PRISMS, an online portal where they can complete questionnaires, receive personalised feedback and guidance based on their symptoms and signs, and access a peer support online forum. Those allocated to the comparison group receive usual care. |
| Outcomes | Main outcomes: recruitment rate, enrolment rate, retention rate, satisfaction with the programme, perceived ease of use of the programme Other outcomes: quality of life, healthcare use |
| Starting date | November 2020 (estimated completion rate June 2021) |
| Contact information | Shenmeng Xu (shenmeng@email.unc.edu) |
| Notes |
Paladino 2019.
| Study name | THRIVE Breast Cancer App Study (THRIVE) |
| Methods | Randomised parallel trial. USA |
| Participants | Inclusion criteria: Adult female patients (age≥18) diagnosed with ductal carcinoma in situ or Stage I‐III hormone receptor‐positive breast cancer, who have been prescribed an aromatase inhibitor or tamoxifen and have access to a mobile device or home computer and an email address. |
| Interventions | Intervention arm 1: patients will access an application to report medication adherence and symptoms; concerning changes or symptoms will trigger an automatic alert to the oncology team. Intervention arm 2: similar to arm 1; additionally, patients will receive weekly tailored feedback messages and/or images. Arm 3: "Usual Care" group |
| Outcomes | Main outcome: medication adherence Other outcomes: change in Functional Assessment Of Cancer Therapy‐Endocrine Subscale (FACT‐ES) Score; Short Form Health Survey (SF‐12) Score; Patient‐Reported Outcomes Measurement Information System (PROMIS) Self‐Efficacy for Managing Symptoms Score |
| Starting date | Novermber 2018 (expected completion date September 2022) |
| Contact information | Andrew Paladino |
| Notes | Trial registry NCT03592771 |
Roberts 2019.
| Study name | Patient Reported Outcomes in the Medical Oncology Setting (iPROMOS) |
| Methods | Cluster randomised trial, Australia |
| Participants | Adults aged >= years, attending for medical review in oncology outpatient settings |
| Interventions | Intervention: when attending clinic, participants will be asked to complete a patient‐reported outcome measure; feedback will be given to the participant and a copy put into their medical record Comnparison: usual care |
| Outcomes | Main outcome: successful implementation of the intervention Other outcomes: hospital admissions, emergency room presentations, survival |
| Starting date | March 2018 |
| Contact information | Natasha Roberts |
| Notes | Trial registry ACTRN12618000398202 |
Rogers 2018.
| Study name | Improving Quality of Life Through the Routine Use of the Patient Concerns Inventory for Head and Neck Cancer Patients |
| Methods | Parallel randomised trial, UK |
| Participants | Adults aged between 18 and 90 years, with head and neck cancer |
| Interventions | Intervention: participants complete the patient‐reported outcome measure during clinics Comparison: usual care |
| Outcomes | Main outcome: overall quality of life Other outcomes: distress, health economics |
| Starting date | January 2017 |
| Contact information | Simon N Rogers |
| Notes | Trial registry NCT03086629 |
Rogers 2019.
| Study name | |
| Methods | Randomised trial, United Kingdom. |
| Participants | Patients with head and neck cancer. |
| Interventions | Assessment and feedback using the Patient Concerns Inventory (PCI) vs usual care. |
| Outcomes | Main outcome: percentage of patients with less than good overall quality of life at one year. Secondary outcomes: Social‐emotional quality of life, distress thermometer, and health economic measures including quality‐adjusted life years. |
| Starting date | |
| Contact information | |
| Notes | Funded by the National Institute for Health Research (UK). |
Seppen 2020.
| Study name | SeMoRa‐3 study. Self‐monitoring in rheumatoid arthritis |
| Methods | Randomised parallel trial. The Netherlands |
| Participants | Inclusion criteria: Adults who own a smartphone, diagnosed with rheumatoid arthritis by a rheumatologist for >=2 years, with low disease activity and taking a disease‐modifying anti‐rheumatic drug |
| Interventions | Patients allocated to the intervention group will have access to the MyRheumatism application, which collects weekly self‐assessed questionnaire data (up to three reminders). Data will be transmitted through secure servers to the electronic medical record, where it will be revised by healthcare professionals. |
| Outcomes | Main outcomes: disease activity, number of outpatient clinic visits |
| Starting date | 1 June 2019 |
| Contact information | Bart Seppen. Email: b.seppen@reade.nl |
| Notes | Trial registry NL7715 |
Serrano‐Ripoll 2019.
| Study name | Improving Patient Safety in Spanish Primary Care (PC) Centres (SinergiAPS) |
| Methods | Randomised cluster trial, Spain |
| Participants | Inclusion criteria: Spanish speaking patients who have visited their primary care centre at least once in the previous 12 months Exclusion criteria: overt psychosis/critically ill/altered mental status, unable to provide written informed consent |
| Interventions | Patients attending practices allocated to the intervention complete a questionnaire about their safety experiences, which are immediately fed back to the primary healthcare providers. Patients in control practices complete the same questionnaire, which are fed back to the primary healthcare providers after post‐intervention data have been collected. |
| Outcomes | Main outcome: change in the Patient Safety Climate Synthetic Index Other outcomes: patient safety experiences in Primary Care settings (PREOS‐PC), rate of avoidable hospitalisations |
| Starting date | May 2019 (estimated completion date December 2020) |
| Contact information | Ignacio Ricci‐Cabello (ignacio.ricci@ssib.es) |
| Notes | Trial registry NCT03837912 |
Differences between protocol and review
We modified the search strategies based on editorial feedback received while conducting the review.
For the main outcomes, we specified the patient‐reported outcomes as quality of life, general health perceptions, functioning (physical, mental, and social), and symptoms (pain, fatigue, nausea, vomiting, cough, anxiety, and depression).
Contributions of authors
Conceiving and designing the review: CG, DBG, JMV
Coordinating the review: CG, JMV
Data collection for the review: CG, IP, IRC, DGB, EJG, AK, PB, JA, JG, PJvdW, EK, ET, JG, AD, SS, JE
Data management for the review: CG, IP, JMV
Analysis of data: CG, IP, JMV
Interpretation of data: CG, IP, DGB, JMV
Writing the review: CG, IP, DGB, JMV
Providing general advice on the review: CG, IP, IRC, DGB, EJG, AK, PB, JA, JG, PJvdW, EK, ET, JG, AD, SS, JE, JMV
All the authors approved the final version submitted for publication.
Contributions of the editorial base (since 2019)
Managing editor (EPOC): Organised and contributed to one round of peer review and organised two rounds of corrections with the editorial staff and editors.
Contact editor: contributed to one round of peer review and subsequent rounds of corrections. Led sign‐off of the review for publication (Michel Wensing).
Information specialist (EPOC): undertook two rounds of searching and contributed to peer review (Paul Miller); and
Statistical Editor (EPOC): contributed to peer review, provided feedback to the authors to support revisions, and provided input to two rounds of further corrections. Provided sign‐off for the statistical elements of the review (Andrew Hutchings)
Sources of support
Internal sources
-
University of Exeter, UK
Provided salary for Jose M Valderas throught the period
External sources
-
National Institutes of Health Research (NIHR) grant, UK
“Improving the management of long term conditions with the clinical use of patient reported outcome measures in Primary Care”, awarded to Jose M Valderas (NIHR/CS/010/024)
Declarations of interest
All of the authors have completed deceleration of interest forms as standard procedure for undertaking a Cochrane review. These decelerations were updated and re‐checked prior to publication. With the exception of the following authors, all authors declared no known conflicts interest. The authors declaring below are not perceived to hold any conflict of interest related to the review and its work.
Chris Gibbons: Chris holds an National Institute for Health Research (UK) ‐ Post‐doctoral and career development Fellowship. This grant is designed to foster his development as an independent researcher. As part of this work, he will develop a computerised questionnaire administration system with the goal of improving patient‐provider communication in primary care.
Joanne Greenhalgh is currently President‐Elect of the International Society for Quality of Life Research (ISOQOL) and from 2021‐2023 will be president of ISOQOL.
Anna Kotzeva has not received any payment or services from a third party for any aspect of the submitted work. Ann would like to note the following, albeit that this does not constitute a conflict of interest with this review: In the beginning of the review Anna was employed by the Agencia de Qualitat i Avaluacio Sanitaries de Catalunya (Spain) and this institution has received grants from the Spanish Ministry of Science and Innovation and the European commission (ICT PSP as part of the Competitiveness and Innovation Framework Programme). Currently, Anna is employed by F. Hoffmann ‐ La Roche (Basel, Switzerland) but has not received any payment or funding for the work on this manuscript. The author has had financial relationships with: the Universitat Oberta de Catalonia (UOC) receiving remuneration for online lectures and development of educational materials; the European Patient Forum covering travel and accommodations expenses for speaking at conference workshops.
Jose Valderas has undertaken consultancy for the WHO but this is unrelated to this review.
New
References
References to studies included in this review
Absolom 2021 {published data only}
- Absolom K, Holch P, Warrington L, Salmy F, Hulme C, Gibson A, et al. ERAPID electronic patient self-reporting of adverse events-patient information and advice: results from pilot phase of an RCT in systemic cancer treatment. Journal of Clinical Oncology 2016;25(1):178-9. [Google Scholar]
Amble 2014 {published data only}
- Amble I, Gude T, Stubdal S, Andersen BJ, Wampold BE. The effect of implementing the Outcome Questionnaire-45.2 feedback system in Norway: a multisite randomized clinical trial in a naturalistic setting. Psychotherapy Research 2014;25(6):669-77. [DOI] [PubMed] [Google Scholar]
Anderson 2015 {published data only}
- Anderson KO, Palos GR, Mendoza TR, Cleeland CS, Liao KP, Fisch MJ, et al. Automated pain intervention for underserved minority women with breast cancer. Cancer 2015;121(11):1882-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anker 2009 {published data only}
- Anker MG, Duncan BL, Sparks JA. Using client feedback to improve couple therapy outcomes: a randomized clinical trial in a naturalistic setting. Journal of Consulting and Clinical Psychology 2009;77(4):693–704. [DOI] [PubMed] [Google Scholar]
Atreja 2018 {published data only}
- Atreja A, Otobo E, Szigethy E, Kohli A, Shroff H, Chang H, et al. Improved quality of care and quality of life for IBD patients using mobile based remote monitoring platform: a randomized control trial. Journal of Crohn's and Colitis 2018;12(1):S30‐. [Google Scholar]
Basch 2016 {published data only}
- Basch E, Deal A, Dueck AC, Bennett AV, Atkinson TM, Rogak LJ, et al. Overall survival results of a randomized trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318(2):197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch E, Deal AM, Kris MG, Scher H I, Hudis CA, Sabbatini P, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial.[Erratum appears in J Clin Oncol. 2016 Jun 20;34(18):2198; PMID: 27281229]. Clinical Oncology 2016;34(6):557-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bastiaansen 2018 {published data only}
- Bastiaansen JA, Meurs M, Stelwagen R, Wunderink L, Schoevers RA, Wichers M, et al. Self-monitoring and personalized feedback based on the experiencing sampling method as a tool to boost depression treatment: a protocol of a pragmatic randomized controlled trial (ZELF-i). BMC Psychiatry 2018;18(1):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen JA, Ornee DA, Meurs M, Oldehinkel AJ. An evaluation of the efficacy of two add-on ecological momentary intervention modules for depression in a pragmatic randomized controlled trial (ZELF-i). Psychological Medicine 17th November 2020;Online:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berking 2006 {published data only}
- Berking M, Ulrich O, Wolfgang L. How effective is systematic feedback of treatment progress to the therapist? An empirical study in a cognitive-behavioural-oriented inpatient setting [[Wie effektiv sind systematische Rückmeldungen des Therapieverlaufs an den Therapeuten?]]. Zeitschrift für Klinische Psychologie und Psychotherapie 2006;35(1):21-9. [Google Scholar]
Blonigen 2015 {published data only}
- Blonigen DM, Timko C, Jacob T, Moos RH. Patient-centered feedback on the results of personality testing increases early engagement in residential substance use disorder treatment: a pilot randomized controlled trial. Addiction Science & Clinical Practice 2015;10(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyer 2013 {published data only}
- Boyer L, Lançon C, Baumstarck K, Parola N, Berbis J, Auquier P. Evaluating the impact of a quality of life assessment with feedback to clinicians in patients with schizophrenia: randomised controlled trial. British Journal of Psychiatry 2013;202:447-53. [DOI] [PubMed] [Google Scholar]
Brodey 2005 {published data only}
- Brodey BB, Cuffel B, McCulloch J, Tani S, Maruish M, Brodey I, et al. The acceptability and effectiveness of patient-reported assessments and feedback in a managed behavioral healthcare setting. American Journal of Managed Care 2005;11(12):774-80. [PubMed] [Google Scholar]
Brody 1990 {published data only}
- Brody DS, Lerman CE, Wolfson HG, Caputo GC. Improvement in physicians' counseling of patients with mental health problems. Archives of Internal Medicine 1990;150:993–8. [PubMed] [Google Scholar]
Bryant 2020 {published data only}
- Bryant AL, Coffman E, Phillips B, Tan X, Bullard E, Hirschey R, et al. Pilot randomized trial of an electronic symptom monitoring and reporting intervention for hospitalized adults undergoing hematopoietic stem cell transplantation. Supportive Care in Cancer 2020;28(3):1223-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calkins 1994 {published data only}
- Calkins DR, Rubenstein LV, Cleary PD, Davies AR, Jette AM, Fink A, et al. Functional disability screening of ambulatory patients: a randomized controlled trial in a hospital-based group practice. Journal of General Internal Medicine 1994;9(10):590-2. [DOI] [PubMed] [Google Scholar]
Callahan 1994 {published data only}
- Callahan CM, Hendrie HC, Dittus RS, Brater DC, Hui SL, Tierney WM. Improving treatment of late life depression in primary care: a randomized clinical trial. Journal of the American Geriatrics Society 1994;42(8):839-46. [DOI] [PubMed] [Google Scholar]
Callahan 1996 {published data only}
- Callahan CM, Dittus RS, Tierney WM. Primary care physicians’ medical decision making for late-life depression. Journal of General Internal Medicine 1996;11:218–9. [DOI] [PubMed] [Google Scholar]
Cherkin 2018 {published data only}
- Cherkin D, Balderson B, Wellman R, Hsu C, Sherman KJ, Evers SC, et al. Effect of low back pain risk-stratification strategy on patient outcomes and care processes: the MATCH randomized trial in primary care. Journal of General Internal Medicine 2018;33(8):1324-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Christensen 2005 {published data only}
- Christensen KS, Toft T, Frostholm L, Ornbøl E, Fink P, Olesen F. Screening for common mental disorders: who will benefit? Results from a randomised clinical trial. Family Practice 2005;22(4):428-34. [DOI] [PubMed] [Google Scholar]
Cleeland 2011 {published data only}
- Cleeland CS, Wang XS, Shi Q, Mendoza TR, Wright SL, Berry MD, et al. Automated symptom alerts reduce postoperative symptom severity after cancer surgery: a randomized controlled clinical trial. Journal of Clinical Oncology 2011;29(8):994-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooley 2016 {published data only}
- Cooley ME, Blonquist T, Catalano P, Lobach D, Braun I, Halpenny B, et al. Point of care clinical decision support for cancer symptom management: results of a group randomized trial. Journal of Clinical Oncology 2016;24(1):S45‐. [Google Scholar]
Dailey 2002 {published data only}
- Dailey YM, Humphris GM, Lennon MA. Reducing patients' state anxiety in general dental practice: a randomized controlled trial. Journal of Dental Research 2002;81(5):319-22. [DOI] [PubMed] [Google Scholar]
Davis 2013 {published data only}
- Davis KM, Dawson D, Kelly S, Red S, Penek S, Lynch J, et al. Monitoring of health-related quality of life and symptoms in prostate cancer survivors: a randomized trial. Journal of Supportive Oncology 2013;11(4):174-82. [DOI] [PubMed] [Google Scholar]
De Jong 2012 {published data only}
- De Jong K, Sluis P, Nugter MA, Heiser WJ, Spinhoven P. Understanding the differential impact of outcome monitoring: therapist variables that moderate feedback effects in a randomized controlled trial. Psychotherapy Research 2012;22(4):464-74. [DOI] [PubMed] [Google Scholar]
De Jong 2014 {published data only}
- De Jong K, Timman R, Hakkaart-Van Roijen L, Vermeulen P, Kooiman K, Passchier J, et al. The effect of outcome monitoring feedback to clinicians and patients in short and long-term psychotherapy: a randomized controlled trial. Psychotherapy Research 2014;24(6):629-39. [DOI] [PubMed] [Google Scholar]
Denis 2017 {published data only}
- Denis F, Lethrosne C, Pourel N, Molinier O, Pointreau Y, Domont J, et al. Randomized trial comparing a web-mediated follow-up with routine surveillance in lung cancer patients. Journal of the National Cancer Institute 2017;109(9):djx029. [DOI] [PubMed] [Google Scholar]
Detmar 2002 {published data only}
- Detmar SB, Muller MJ, Schornagel JH, Wever LD, Aaronson NK. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA 2002;288(23):3027-34. [DOI] [PubMed] [Google Scholar]
Dowrick 1995a {published data only}
- Dowrick C, Buchan I. Twelve month outcome of depression in general practice: does detection or disclosure make a difference? BMJ 1995;311:1274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dowrick 1995b {published data only}
- Dowrick C. Does testing for depression influence diagnosis or management by general practitioners? Family Practice 1995;12:461-5. [DOI] [PubMed] [Google Scholar]
Dueck 2015 {published data only}
- Dueck AC, Mitchell SA, Rogak LJ, Ginos B, Sargent DJ, Shi Q, et al. A cluster-randomized study of clinician-patient shared vs standard reporting of symptomatic adverse events using PROCTCAE nested in a multicenter trial of multimodal therapy for rectal cancer (Alliance N1048 PROSPECT). Quality of Life Research 2015;24(1):1-2. [Google Scholar]
Fann 2017 {published data only}
- Fann JR, Hong F, Halpenny B, Blonquist TM, Berry DL. Psychosocial outcomes of an electronic self-report assessment and self-care intervention for patients with cancer: a randomized controlled trial. Psychooncology 2017;26(11):1866-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Franco 2020 {published data only}
- Franco V, Canevini MP, De Sarro G, Fattore C, Fedele G, Galimberti CA, et al. Does screening for adverse effects improve health outcomes in epilepsy? A randomized trial. Neurology 2020;95(3):e239-e246. [DOI] [PubMed] [Google Scholar]
German 1987 {published data only}
- German PS, Shapiro S, Skinner EA, Korff MV, Klein LE, Turner RW, et al. Detection and management of mental health problems of older patients by primary care providers. JAMA 1987;257(4):489-93. [PubMed] [Google Scholar]
Gilliam 2004 {published data only}
- Gilliam FG, Fessler AJ, Baker G, Vahle V, Carter J, Attarian H. Systematic screening allows reduction of adverse antiepileptic drug effects: a randomized trial. Neurology 2004;62(1):23-7. [DOI] [PubMed] [Google Scholar]
Girgis 2009 {published data only}
- Girgis A, Breen S, Stacey F, Lecathelinais C. Impact of two supportive care interventions on anxiety, depression, quality of life, and unmet needs in patients with nonlocalized breast and colorectal cancers. Journal of Clinical Oncology 2009;27(36):6180-90. [DOI] [PubMed] [Google Scholar]
Gold 1989 {published data only}
- Gold I, Baraff LJ. Psychiatric screening in the emergency department: Its effect on physician behavior. Annals of Emergency Medicine 1989;18(8):875-80. [DOI] [PubMed] [Google Scholar]
Goldsmith 1989 {published data only}
- Goldsmith G, Brodwick M. Assessing the functional status of older patients with chronic illness. Family Medicine 1989;21(1):38-41. [PubMed] [Google Scholar]
Gossec 2018 {published data only}
- Gossec L, Cantagrel A, Soubrier M, Berthelot JM, Joubert J M, Combe B, et al. An e-health interactive self-assessment website (Sanoia) in rheumatoid arthritis. A 12-month randomized controlled trial in 320 patients. Joint Bone Spine 2018;85(6):709-14. [DOI] [PubMed] [Google Scholar]
Gutteling 2008 {published data only}
- Gutteling JJ, Darlington AS, Janssen HL, Duivenvoorden HJ, Busschbach JJ, Man RA. Effectiveness of health-related quality-of-life measurementin clinical practice: a prospective, randomized controlledtrial in patients with chronic liver disease and their physicians. Quality of Life Research 2008;2:195-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haas 2016 {published data only}
- Haas NM, Yang DS, Lopez AM, Kallen MA. SymptomCareAnywhere: cancer symptom self-management at home. Quality of Life Research 2016;25(1):74. [Google Scholar]
Hadjistavropoulos 2009 {published data only}
- Hadjistavropoulos T, MacNab Y, Lints-Martindale A, Martin R, Hadjistavropoulos H. Does routine pain assessment result in better care? Pain Research & Management 2009;14(3):211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansson 2013 {published data only}
- Hansson H, Rundberg J, Ö sterling A, Ö jehagen A, Berglund M. Intervention with feedback using Outcome Questionnaire 45 (OQ-45) in a Swedish psychiatric outpatient population. A randomized controlled trial. Nordic Journal of Psychiatry 2013;67(4):274-81. [DOI] [PubMed] [Google Scholar]
Hawkins 2004 {published data only}
- Hawkins EJ, Lambert MJ, Vermeersch DA, Slade KL, Tuttle KC. The therapeutic effects of providing patient progress information to therapists and patients. Psychotherapy Research 2004;14(3):308-27. [Google Scholar]
Hoekstra 2006 {published data only}
- Hoekstra J, Vos R, Duijn NP, Schadé E, Bindels PJ. Using the symptom monitor in a randomized controlled trial: The effect on symptom prevalence and severity. Journal of Pain and Symptom Management 2006;31(1):22-30. [DOI] [PubMed] [Google Scholar]
Hoeper 1984 {published data only}
- Hoeper EW, Kessler LG, Nycz GR, Burke JD JR, Pierce WE. The usefulness of screening for mental illness. Lancet 1984;323(8367):33-5. [DOI] [PubMed] [Google Scholar]
Jha 2013 {published data only}
- Jha A, Jan F, Gale T, Newman C. Effectiveness of a recovery-orientated psychiatric intervention package on the wellbeing of people with early dementia: a preliminary randomised controlled trial. International Journal of Geriatric Psychiatry 2013;28(6):589-96. [DOI] [PubMed] [Google Scholar]
Kazis 1990 {published data only}
- Kazis LE, Callahan LF, Meenan RF, Pincus T. Health status reports in the care of patients with rheumatoid arthritis. Journal of Clinical Epidemiology 1990;43(11):1243-53. [DOI] [PubMed] [Google Scholar]
Kendrick 2017 {published data only}
- Kendrick T, Moore M, Leydon G, Stuart B, Geraghty AW, Yao G, et al. Patient-reported outcome measures for monitoring primary care patients with depression (PROMDEP): study protocol for a randomised controlled trial.. Trials 2020;21:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick T, Stuart B, Leydon GM, Geraghty AW, Yao L, Ryves R, et al. Patient-reported outcome measures for monitoring primary care patients with depression: PROMDEP feasibility randomised trial. BMJ Open 2017;7(3):e015266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kornblith 2006 {published data only}
- Kornblith AB, Dowell JM, Herndon JE 2nd, Engelman BJ, Bauer-Wu S, Small EJ, et al. Telephone monitoring of distress in patients aged 65 years or older with advanced stage cancer. Cancer 2006;107(11):2706-14. [DOI] [PubMed] [Google Scholar]
Kroenke 2018 {published data only}
- Kroenke K, Talib TL, Stump TE, Kean J, Haggstrom DA, DeChant P, et al. Incorporating PROMIS symptom measures into primary care practice-a randomized clinical trial. Journal of General Internal Medicine 2018;33(8):1245-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuo 2020 {published data only}
- Kuo JC, Graham DM, Salvarrey A, Kassam F, Le LW, Shepherd FA, et al. A randomized trial of the electronic Lung Cancer Symptom Scale for quality-of-life assessment in patients with advanced non-small-cell lung cancer. Current Oncology 2020;27(2):e156-e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lambert 2001 {published data only}
- Lambert MJ, Whipple JL, Smart DW, Vermeersch DA, Nielsen SL, Hawkins EJ. The effects of providing therapists with feedback on patient progress during psychotherapy: are outcomes enhanced? Psychotherapy Research 2001;11(1):49-68. [DOI] [PubMed] [Google Scholar]
LeBlanc 2019 {published data only}
- LeBlanc TW, Corbett C, Davis DM, Herring K, Locke SC, Troy JD, et al. Digital Supportive Care Awareness and Navigation (DSCAN): results of a pilot randomized trial. Journal of Clinical Oncology 2019;37:31. [Google Scholar]
Linn 1980 {published data only}
- Linn LS, Yager J. The effect of screening, sensitization, and feedback on notation of depression. Journal of Medical Education 1980;55(11):942-9. [DOI] [PubMed] [Google Scholar]
Lugtenberg 2020 {published data only}
- Lugtenberg RT, Fischer MJ, De Jongh F, Inoue K, Matsuda A, Ramai SRS, et al. Monitoring quality of life in Dutch women with breast cancer: the care notebook. Annals of Oncology 2018;29(viii):625. [Google Scholar]
- Lugtenberg RT, Fischer MJ, Jongh F, Kobayashi K, Inoue K, Matsuda A, et al. Using a quality of life (QoL)-monitor: preliminary results of a randomized trial in Dutch patients with early breast cancer. Quality of Life Research 2020;29(11):2961-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magruder‐Habib 1990 {published data only}
- Magruder-Habib K, Zung WW, Feussner JR. Improving physicians' recognition and treatment of depression in general medical care: results from a randomized clinical trial. Medical Care 1990;28(3):239-50. [DOI] [PubMed] [Google Scholar]
Mathias 1994 {published data only}
- Mathias SD, Fifer SK, Mazonson PD, Lubeck DP, Buesching DP, Patrick DL. Necessary but not sufficient: the effect of screening and feedback on outcomes of primary-care patients with untreated anxiety. Journal of General Internal Medicine 1994;9(11):606-15. [DOI] [PubMed] [Google Scholar]
Mazonson 1996 {published data only}
- Mazonson PD, Mathias SD, Fifer SK, Buesching DP, Malek P, Patrick DL. The mental health patient profile: does it change primary care physicians' practice patterns? Journal of the American Board of Family Medicine 1996;9(5):336-45. [PubMed] [Google Scholar]
McCusker 2001 {published data only}
- McCusker J, Verdon J, Tousignant P, Courval LP, Dendukuri N, Belzile E. Rapid emergency department intervention for older people reduces risk of functional decline: results of a multicenter randomized trial. Journal of the American Geriatrics Society 2001;49(10):1272-81. [DOI] [PubMed] [Google Scholar]
McLachlan 2001 {published data only}
- McLachlan SA, Allenby A, Matthews J, Wirth A, Kissane D, Bishop M, et al. Randomized trial of coordinated psychosocial interventions based on patient self-assessments versus standard care to improve the psychosocial functioning of patients with cancer. Journal of Clinical Oncology 2001;19(21):4117-25. [DOI] [PubMed] [Google Scholar]
Mellema 2015 {published data only}
- Mellema JJ, O'Connor CM, Overbeek CL, Hageman MG, Ring D. The effect of feedback regarding coping strategies and illness behavior on hand surgery patient satisfaction and communication: a randomized controlled trial. HAND 2015;10(3):503-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 1978 {published data only}
- Moore JT, Silimperi DR, Bobula JA. Recogition of depression by family medicine residents: the Impact of screening. Journal of Family Practice 1978;7(3):509-13. [PubMed] [Google Scholar]
Moore 2019 {published data only}
- Moore E, King T, Ho PJ, Klarica D, Prince HM, Quach H, et al. Patient Reported Outcome Measures in multiple myeloma: real-time rePorTing to improve care (My-PROMPT) - a pilot randomised controlled trial. American Journal of Hematology 2019;19(10):e340‐e341. [DOI] [PubMed] [Google Scholar]
Murillo 2017 {published data only}
- Murillo M, Bel J, Perez J, Corripio R, Carreras G, Herrero X, et al. Impact of monitoring health-related quality of life in clinical practice in children with type 1 diabetes mellitus. Quality of Life Research 2017;26(12):3267-77. [DOI] [PubMed] [Google Scholar]
Murphy 2012 {published data only}
- Murphy KP, Rashleigh CM, Timulak L. The relationship between progress feedback and therapeutic outcome in student counselling: a randomised control trial. Counselling Psychology Quarterly 2012;25(1):1-18. [Google Scholar]
Myasoedova 2019 {published data only}
- Myasoedova E, Crowson CS, Giblon RE, McCarthy-Fruin K, Schaffer DE, Wright K, etal. Optimization of flare management in patients with rheumatoid arthritis: results of a randomized controlled trial. Clinical Rheumatology 2019;38(11):3025-32. [DOI] [PubMed] [Google Scholar]
Nimako 2017 {published data only}
- Nimako K, Ayite B, Priest K, Severn J, Fries HM, Gunapala R, et al. A randomised assessment of the use of a quality of life questionnaire with or without intervention in patients attending a thoracic cancer clinic. European Journal of Cancer Care 2017;26(4):n/a-N.PAG. [DOI] [PubMed] [Google Scholar]
Nipp 2019 {published data only}
- Nipp RD, El-Jawahri A, Ruddy M, Fuh C, Temel B, D'Arpino SM, et al. Pilot randomized trial of an electronic symptom monitoring intervention for hospitalized patients with cancer. Annals of Oncology 2019;30(2):274-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Picardi 2016 {published data only}
- Picardi A, Lega I, Tarsitani L, Caredda M, Matteucci G, Zerella MP, et al. A randomised controlled trial of the effectiveness of a program for early detection and treatment of depression in primary care. Journal of Affective Disorders 2016;198:96-101. [DOI] [PubMed] [Google Scholar]
Pouwer 2001 {published data only}
- Pouwer F, Snoek FJ, Ploeg HM, Adèr HJ, Heine RJ. Monitoring of psychological well-being in outpatients with diabetes: effects on mood, HbA(1c), and the patient's evaluation of the quality of diabetes care: a randomized controlled trial. Diabetes Care 2001;24(11):1929-35. [DOI] [PubMed] [Google Scholar]
Priebe 2007 {published data only}
- Priebe S, McCabe R, Bullenkamp J, Hansson L, Lauber C, Martinez-Leal R, et al. Structured patient-clinician communication and 1-year outcome in community mental healthcare: cluster randomised controlled trial. British Journal of Psychiatry 2007;191:420-6. [DOI] [PubMed] [Google Scholar]
Probst 2013 {published data only}
- Probst T, Lambert MJ, Loew TH, Dahlbender RW, Göllner R, Tritt K. Feedback on patient progress and clinical support tools for therapists: improved outcome for patients at risk of treatment failure in psychosomatic in-patient therapy under the conditions of routine practice. Journal of Psychosomatic Research 2013;75(3):255-61. [DOI] [PubMed] [Google Scholar]
Puschner 2009 {published data only}
- Puschner B, Scho¨fer D, Knaup C, Becker T. Outcome management in in-patientpsychiatric care. Acta Psychiatrica Scandinavica 2009;120:308–19. [DOI] [PubMed] [Google Scholar]
Rand 1988 {published data only}
- Rand EH, Badger LW, Coggins DR. Toward a resolution of contradictions. Utility of feedback from the GHQ. General Hospital Psychiatry 1998;10(3):189-96. [DOI] [PubMed] [Google Scholar]
Reese 2009 {published data only}
- Reese RJ, Norsworthy LA, Rowlands SR. Does a continuous feedback system improve psychotherapy outcome? Psychotherapy 2009;46(4):418-31. [DOI] [PubMed] [Google Scholar]
Richardson 2008 {published data only}
- Richardson J, Chan D, Risdon K, Giles C, Cripps D. Does monitoring change in functioncommunity-dwelling older adults? A randomized controlled trial. Clinical Rehabilitation 2008;22:1061–70. [DOI] [PubMed] [Google Scholar]
Richardson 2019 {published data only}
- Richardson L, Zhou C, Gersh E, Spielvogle H, McCarty C. Check Yourself: a randomized trial of electronic screening and personalized feedback for adolescent health risk behaviors in primary care. Journal of Adolescent Health 2019;64(2):S41‐S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson L, Zhou C, Gersh E, Spielvogle H, McCarty C. Effect of electronic screening with personalized feedback on adolescent health risk behaviors in a primary care setting: a randomized clinical trial. JAMA Network Open 2019;2(5):e193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenbloom 2007 {published data only}
- Rosenbloom SK, Victorson DE, Hahn EA, Peterman AH, Cella D. Assessment is not enough: a randomized controlled trial of the effects of HRQL assessment on quality of life and satisfaction in oncology clinical practice. Psycho-Oncology 2007;16(12):1069-79. [DOI] [PubMed] [Google Scholar]
Rubenstein 1995 {published data only}
- Rubenstein LV, McCoy JM, Cope DW, Barrett PA, Hirsch SH, Messer KS, et al. Improving patient quality of life with feedback to physicians about functional status. Journal of General Internal Medicine 1995;10(11):607-14. [DOI] [PubMed] [Google Scholar]
Ruland 2003 {published data only}
- Ruland CM, White T, Stevens M, Fanciullo G, Khilani SM. Effects of a computerized system to support shared decision making in symptom management of cancer patients: preliminary results. Journal of the American Medical Informatics Association 2003;10(6):573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruland 2010 {published data only}
- Ruland CM, Holte HH, Røislien J, Heaven C, Hamilton GA, Kristiansen J, et al. Effects of a computer-supported interactive tailored patient assessment tool on patient care, symptom distress, and patients' need for symptom management support: a randomized clinical trial. Journal of the American Medical Informatics Association 2010;17(4):403-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saitz 2003 {published data only}
- Saitz R, Horton NJ, Sullivan LM, Moskowitz MA, Samet JH. Addressing alcohol problems in primary care: a cluster randomized, controlled trial of a systems intervention. Annals of Internal Medicine 2003;138(5):372-82. [DOI] [PubMed] [Google Scholar]
Sandheimer 2020 {published data only}
- Sandheimer C, Hedenrud T, Hensing G, Holmgren K. Effects of a work stress intervention on healthcare use and treatment compared to treatment as usual: a randomised controlled trial in Swedish primary healthcare. BMC Family Practice 2020;21(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Santana 2010 {published data only}
- Santana MJ, Feeny D, Johnson JA, McAlister FA, Kim D, Weinkauf J, et al. Assessing the use of health-related quality of life measures in the routine clinical care of lung-transplant patients. Quality of Life Research 2010;19(3):371-9. [DOI] [PubMed] [Google Scholar]
Scheidt 2012 {published data only}
- Scheidt CE, Brockmann J, Caspar F, Rudolf G, Stangier U, Vogel H. The project of the Techniker-Krankenkasse: some comments on the results by the project's scientific advisory board [Das modellprojekt der Techniker–Krankenkasse:eine kommentierung der ergebnisse aus der aicht deswissenschaftlichen projektbeirates]. PsychotherapiePsychosomatik Medizinische Psychologie 2012;62(11):405-12. [DOI] [PubMed] [Google Scholar]
Schmidt 2006 {published data only}
- Schmidt U, Landau S, Pombo-Carril MG, Bara-Carril N, Reid Y, Murray K, et al. Does personalized feedback improve the outcome of cognitive-behavioural guided self-care in bulimia nervosa? A preliminary randomized controlled trial. British Journal of Clinical Psychology 2006;45:111–21. [DOI] [PubMed] [Google Scholar]
Schottke 2019 {published data only}
- Schottke H, Unrath M, Uhlmann C. The effect of patient progress feedback on psychotherapy outcome. Verhaltenstherapie 2019;Online first. [DOI: 10.1159/000501176] [DOI]
Schriger 2001 {published data only}
- Schriger DL, Gibbons PS, Langone CA, Lee S, Altshuler LL. Enabling the diagnosis of occult psychiatric illness in the emergency department: a randomized, controlled trial of the computerized, self-administered PRIME-MD diagnostic system. Annals of Emergency Medicine 2001;37(2):132-40. [DOI] [PubMed] [Google Scholar]
Schriger 2005 {published data only}
- Schriger DL, Gibbons PS, Nezami WA, Langone CA. Failure of a patient-centered intervention to substantially increase the identification and referral for-treatment of ambulatory emergency department patients with occult psychiatric conditions: a randomized trial. BMC Emergency Medicine 2005;5(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shapiro 1987 {published data only}
- Shapiro S, German PS, Skinner EA, VonKorff M, Turner RW, Klein LE, et al. An experiment to change detection and management of mental morbidity in primary care. Medical Care 1987;25(4):327-39. [DOI] [PubMed] [Google Scholar]
Simon 2012 {published data only}
- Simon W, Lambert MJ, Harris MW, Busath G, Vazquez A. Providing patient progress information and clinical support tools to therapists: effects on patients at risk of treatment failure. Psychotherapy Research 2012;22(6):638-47. [DOI] [PubMed] [Google Scholar]
Simons 2015 {published data only}
- Simons CJ, Hartmann JA, Kramer I, Menne-Lothmann C, Hohn P, Bemmel AL, et al. Effects of momentary self-monitoring on empowerment in a randomized controlled trial in patients with depression. European Psychiatry 2015;30(8):900-6. [DOI] [PubMed] [Google Scholar]
- Simons CJP, Drukker M, Evers S, van Mastrigt Gapg, Hohn P, Kramer I, et al. Economic evaluation of an experience sampling method intervention in depression compared with treatment as usual using data from a randomized controlled trial. BMC Psychiatry 2017;17(1):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Slade 2006a {published data only}
- Slade M, Leese M, Gilldard M, Kuipers E, Thornicroft G. Pre-morbid IQ and response to routine outcome assessment. Psychological Medicine 2006;36:1183–91. [DOI] [PubMed] [Google Scholar]
Slade 2006b {published data only}
- Slade M, McCrone P, Kuipers E, Leese M, Cahill S, Parabiaghi A, et al. Use of standardised outcome measures in adult mental health services: randomised controlled trial. British Journal of Psychiatry 2006;189:330-6. [DOI] [PubMed] [Google Scholar]
Strasser 2016 {published data only}
- Strasser F, Blum D, Moos R, Cathomas R, Ribi K, Aebi S, et al. The effect of real-time electronic monitoring of patient-reported symptoms and clinical syndromes in outpatient workflow of medical oncologists: E-MOSAIC, a multicenter cluster-randomized phase III study (SAKK 95/06). Annals of Oncology 2016;27(2):324-32. [DOI] [PubMed] [Google Scholar]
Stuck 2015 {published data only}
- Stuck AE, Moser A, Morf U, Wirz U, Wyser J, Gillmann G, et al. Effect of health risk assessment and counselling on health behaviour and survival in older people: a pragmatic randomised trial. PLOS Medicine 2015;12(10). [DOI] [PMC free article] [PubMed]
Subramanian 2004 {published data only}
- Subramanian U, Fihn SD, Weinberger M, Plue L, Smith FE, Udris EM, et al. A controlled trial of including symptom data in computer-based care suggestions for managing patients with chronic heart failure. American Journal of Medicine 2004;116(6):375-84. [DOI] [PubMed] [Google Scholar]
Thomas 2016 {published data only}
- Thomas RE, Spragins W, Mazloum G, Cronkhite M, Maru G. Rates of detection of developmental problems at the 18-month well-baby visit by family physicians' using four evidence-based screening tools compared to usual care: a randomized controlled trial. Child: Care, health and Development 2016;42(3):382-93. [DOI] [PubMed] [Google Scholar]
Tolstrup 2020 {published data only}
- Tolstrup LK, Bastholt L, Dieperink KB, Möller S, Zwisler AD, Pappot H. The use of patient-reported outcomes to detect adverse events in metastatic melanoma patients receiving immunotherapy: a randomized controlled pilot trial. Journal of Patient Reported Outcomes. 2020;4:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstrup LK, Pappot H, Dieperink KB, Zwisler A-DO, Bastholt L. PRO-CTCAE in the management of adverse effects in patients receiving immunotherapy for metastatic melanoma-an ongoing randomized clinical trial. ISOQOL International Conference. 2018;27:S76‐S77. [Google Scholar]
Trowbridge 1997 {published data only}
- Trowbridge R, Dugan W, Jay SJ, Littrell D, Casebeer LL, Edgerton S, et al. Determining the effectiveness of a clinical-practice intervention in improving the control of pain in outpatients with cancer. Academic Medicine 1997;72(9):798-800. [DOI] [PubMed] [Google Scholar]
Trudeau 2001 {published data only}
- Trudeau LS. Effects of a clinical feedback system on client and therapist outcomes in a rural community mental health center. Retrospective Theses and Dissertations 2000;Paper 12367.
Valles 2017 {published data only}
- Valles MM, Comos JB, Perez J, Corripio R, Carreras G, Herrero X, et al. 'Impact of monitoring health-related quality of life in clinical practice in children with type 1 diabetes mellitus'. Quality of Life Research 2017;26(2):3267-77. [DOI] [PubMed] [Google Scholar]
van der Hout 2020 {published data only}
- Hout A, Jansen F, Uden-Kraan CF, Coupe VM, Holtmaat K, Nieuwenhuijzen GA, et al. Cost-utility of an eHealth application 'Oncokompas' that supports cancer survivors in self-management: results of a randomised controlled trial. Journal of Cancer Survivorship 2021;15:77-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hout A, Uden-Kraan CF, Holtmaat K, Jansen F, Lissenberg-Witte BI, Nieuwenhuijzen GA, et al. Role of eHealth application Oncokompas in supporting self-management of symptoms and health-related quality of life in cancer survivors: a randomised, controlled trial. Lancet Oncology 2020;21(1):80-94. [DOI] [PubMed] [Google Scholar]
van Dijk‐de Vries 2015 {published data only}
- Dijk-de Vries A, Bokhoven MA, Winkens B, Terluin B, Knottnerus JA, Weijden T, et al. Lessons learnt from a cluster-randomised trial evaluating the effectiveness of Self-Management Support (SMS) delivered by practice nurses in routine diabetes care. BMJ Open 2015;5:e007014. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Os 2003 {published data only}
- Van Os J, Altamura AC, Bobes J, Gerlach J, Hellewell JS, Kasper S, et al. Evaluation of the Two-Way Comminication Checklist as a clinical intervention. British Journal of Psychiatry 2004;184:79-83. [DOI] [PubMed] [Google Scholar]
Velikova 2004 {published data only}
- Velikova G, Booth L, Smith AB, Brown PM, Lynch P, Brown JM, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. Journal of Clinical Oncology 2004;22(4):714-24. [DOI] [PubMed] [Google Scholar]
- Velikova G, Keding A, Harley C, Cocks K, Booth L, Smith AB, et al. Patients report improvements in continuity of care when quality of life assessments are used routinely in oncology practice: secondary outcomes of a randomised controlled trial. European Journal of Cancer 2010;13:2381-8. [DOI] [PubMed] [Google Scholar]
Wagner 1997 {published data only}
- Wagner AK, Ehrenberg BL, Tran TA, Bungay KM, Cynn DJ, Rogers WH. Patient-based health status measurement in clinical practice: A study of its impact on epilepsy patients’ care. Quality of Life Research 1997;6(4):329-41. [DOI] [PubMed] [Google Scholar]
Wasson 1992 {published data only}
- Wasson J, Hays R, Rubenstein L, Nelson E, Leaning J, Johnson D, et al. The short-term effect of patient health status assessment in a health maintenance organization. Quality of Life Research 1992;1(2):99-106. [DOI] [PubMed] [Google Scholar]
Wheelock 2015 {published data only}
- Wheelock AE, Bock MA, Martin EL, Hwang J, Ernest ML, Rugo HS, et al. SIS.NET: a randomized controlled trial evaluating a web-based system for symptom management after treatment of breast cancer. Cancer 2015;121(6):893-9. [DOI] [PubMed] [Google Scholar]
Whipple 2003 {published data only}
- Whipple JL, Lambert MJ, Vermeersch DA, Smart DW, Nielsen SL, Hawkins EJ. Improving the effects of psychotherapy: The use of early identification of treatment and problem-solving strategies in routine practice. Journal of Counseling Psychology 2003;50(1):59-68. [Google Scholar]
White 1995 {published data only}
- White P, Atherton A, Hewett G, Howells K. Using information from asthma patients: a trial of information feedback in primary care. BMJ 1995;311(7012):1065–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whooley 2000 {published data only}
- Whooley MA, Stone B, Soghikian K. Randomized trial of case-finding for depression in elderly primary care patients. Journal of General Internal Medicine 2000;15(5):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wikberg 2017 {published data only}
- Wikberg C, Westman J, Petersson EL, Larsson ME, Andre M, Eggertsen R, et al. Use of a self-rating scale to monitor depression severity in recurrent GP consultations in primary care - does it really make a difference? A randomised controlled study. BMC Family Practice 2017;18(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 1990 {published data only}
- Williams JW, Mulrow CD, Kroenke K, Dhanda R, Badgett RG, Omori D, et al. Case-finding for depression in primary care: A randomized trial. American Journal of Medicine 1999;106(1):36-43. [DOI] [PubMed] [Google Scholar]
Wolfe 2014 {published data only}
- Wolfe J, Orellana L, Cook EF, Ullrich C, Kang T, Geyer JR, et al. Improving the care of children with advanced cancer by using an electronic patient-reported feedback intervention: results from the PediQUEST randomized controlled trial. Journal of Clinical Oncology 2014;32(11):1119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yager 1981 {published data only}
- Yager J, Linn LS. Physician-patient agreement about depression: notation in medical records. General Hospital Psychiatry 1981;3(4):271-6. [DOI] [PubMed] [Google Scholar]
Zung 1983 {published data only}
- Zung WW, Magill M, Moore JT, George DT. Recogntion and treatment of depression in a family medical practice. Journal of Clinical Psychiatry 1983;44(1):3-6. [PubMed] [Google Scholar]
References to studies excluded from this review
Aakhus 2016 {published data only}
- Aakhus E, Granlund I, Odgaard-Jensen J, Oxman AD, Flottorp SA. A tailored intervention to implement guideline recommendations for elderly patients with depression in primary care: a pragmatic cluster randomised trial. Implementation Science 2016;11:32. [DOI: 10.1186/s13012-016-0397-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aardoom 2016 {published data only}
- Aardoom JJ, Dingemans AE, Spinhoven P, Ginkel JR, Rooij M, Furth EF. Web-based fully automated self-help with different levels of therapist support for individuals with eating disorder symptoms: a randomized controlled trial. Journal of Medical Internet Research 2016;18(6):e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aardoom JJ, Dingemans AE, Ginkel JR, Spinhoven P, Van Furth EF, Van den Akker-van Marle ME. Cost-utility of an internet-based intervention with or without therapist support in comparison with a waiting list for individuals with eating disorder symptoms: a randomized controlled trial. International Journal of Eating Disorders 2016;49(12):1068-76. [DOI] [PubMed] [Google Scholar]
Adamowicz 2017 {published data only}
- Adamowicz K. Assessment of quality of life in advanced, metastatic prostate cancer: an overview of randomized phase III trials. Quality of Life Research 2017;26(4):813-22. [DOI: 10.1007/s11136-016-1429-9] [DOI] [PubMed] [Google Scholar]
Adams 2015 {published data only}
- Adams J, Cymbala AA, Delate T, Kurz D, Olson KL, Zadvorny E. Cluster-randomized trial of clinical pharmacist tobacco cessation counseling among patients with cardiovascular disease. Population Health Management 2015;18(4):300‐6. [DOI: 10.1089/pop.2014.0106] [DOI] [PubMed] [Google Scholar]
Adams 2016 {published data only}
- Adams AS, Bayliss EA, Schmittdiel JA, Altschuler A, Dyer W, Neugebauer R, et al, Diabetes Telephone Study Team. The Diabetes Telephone Study: Design and challenges of a pragmatic cluster randomized trial to improve diabetic peripheral neuropathy treatment. Clinical Trials 2016;13(3):286-93. [DOI: 10.1177/1740774516631530] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahmed 2016 {published data only}
- Ahmed S, Ernst P, Bartlett SJ, Valois MF, Zaihra T, Pare G, et al. The effectiveness of web-based asthma self-management system, My aAsthma Portal (MAP): A pilot randomized controlled trial. Journal of Medical Internet Research 2016;18(12):e313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Al Jundi 2016 {published data only}
- Al-Jundi W, Elsharif M, Anderson M, Chan P, Beard J, Nawaz S. A randomized controlled trial to compare e-feedback versus standard face-to-face verbal feedback to improve the acquisition of procedural skill. Journal of Surgical Education 2016;(no pagination). [DOI: 10.1016/j.jsurg.2016.11.011] [DOI] [PubMed]
Anderson 2018 {published data only}
- Anderson M, Rallapalli V, Schoof T, Souza P, Arehart K. The use of self-report measures to examine changes in perception in response to fittings using different signal processing parameters. International Journal of Audiology 2018;57(11):1-7. [DOI: 10.1080/14992027.2018.1490035] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baron 2017 {published data only}
- Baron JS, Hirani SP, Newman SP. Investigating the behavioural effects of a mobile-phone based home telehealth intervention in people with insulin-requiring diabetes: results of a randomized controlled trial with patient interviews. Journal of Telemedicine and Telecare 2017;23(5):503-12. [DOI: 10.1177/1357633X16655911] [DOI] [PubMed] [Google Scholar]
Baron 2017a {published data only}
- Baron JS, Hirani S, Newman SP. A randomised, controlled trial of the effects of a mobile telehealth intervention on clinical and patient-reported outcomes in people with poorly controlled diabetes. Journal of Telemedicine and Telecare 2017;23(2):207-216. [DOI: 10.1177/1357633X16631628] [DOI] [PubMed] [Google Scholar]
Boogaard 2018 {published data only}
- Boogaard JA, Vet HC, Soest-Poortvliet MC, Anema JR, Achterberg WP, Steen JT. Effects of two feedback interventions on end-of-life outcomes in nursing home residents with dementia: a cluster-randomized controlled three-armed trial. Palliative Medicine 2018;32(3):693-702. [DOI: 10.1177/0269216317750071] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyce 2018 {published data only}
- Boyce MB, Browne JP. The effectiveness of providing peer benchmarked feedback to hip replacement surgeons based on patient-reported outcome measures—results from thePROFILE (Patient-ReportedOutcomes: Feedback Interpretation and LearningExperiment) trial: a cluster randomised controlled study.. BMJ Open 2015;5:e008325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carlson 2010 {published data only}
- Carlson LE, Groff SL, Maciejewski O, Bultz BD. Screening for distress in lung and breast cancer outpatients: a randomized controlled trial. Journal of Clinical Oncology 2010;28(33):4884-91. [DOI] [PubMed] [Google Scholar]
Cook 2016 {published data only}
- Cook CM, Suhr JA. Effects of screening for postconcussive syndrome (PCS) on PCS symptom self-report and neuropsychological test performance. Clinical Neuropsychologist 2016;30(2):284-300. [DOI: 10.1080/13854046.2016.1139186] [DOI] [PubMed] [Google Scholar]
Cruickshank 2015 {published data only}
- Cruickshank S, Barber M, Donaldson J, Raeside R, Gray M. A randomised controlled trial to assess the effectivensss of using patient reported needs and psychological information to guide care in a breast cancer follow-up clinic. Cancer Nursing 2015;38(4 SUPPL. 1):S34‐S35. [DOI: 10.1097/NCC.0000000000000287] [DOI] [Google Scholar]
Curtis 2018 {published data only}
- Curtis JR, Downey L, Back AL, Nielsen EL, Paul S, Lahdya AZ, et al. Effect of a patient and clinician communication-priming intervention on patient-reported goals-of-care discussions between patients with serious illness and clinicians: a randomized clinical trial. JAMA Internal Medicine 2018;178(7):930-40. [DOI: 10.1001/jamainternmed.2018.2317] [DOI] [PMC free article] [PubMed] [Google Scholar]
daSilvaRibeiro 2015 {published data only}
- da Silva Ribeiro NM, Ferraz DD, Pedreira E, Pinheiro I, da Silva Pinto AC, Neto MG, et al. Virtual rehabilitation via Nintendo Wii and conventional physical therapy effectively treat post-stroke hemiparetic patients. Topics in Stroke Rehabilitation 2015;22(4):299-305. [DOI: 10.1179/1074935714Z.0000000017] [DOI] [PubMed] [Google Scholar]
Davidson 2017 {published data only}
- Davidson KM, Rankin ML, Begley A, Lloyd S, Barry SJ, McSkimming P, et al. Assessing patient progress in psychological therapy through feedback in supervision: the MeMOS randomized controlled trial (Measuring and Monitoring clinical Outcomes in Supervision: MeMOS). Behavioural and Cognitive Psychotherapy 2017;45(3):209-24. [DOI: 10.1017/S1352465817000029] [DOI] [PubMed] [Google Scholar]
Dougados 2015 {published data only}
- Dougados M, Soubrier M, Perrodeau E, Gossec L, Fayet F, Gilson M, et al. Impact of a nurse-led programme on comorbidity management and impact of a patient self-assessment of disease activity on the management of rheumatoid arthritis: results of a prospective, multicentre, randomised, controlled trial (COMEDRA). Annals of the Rheumatic Diseases 2015;74(9):1725‐33. [DOI: 10.1136/annrheumdis-2013-204733] [DOI] [PMC free article] [PubMed] [Google Scholar]
Freyer Adam 2018 {published data only}
- Freyer-Adam J, Baumann S, Haberecht K, Tobschall S, Bischof G, John U, Gaertner B. In-person alcohol counseling versus computer-generated feedback: results from a randomized controlled trial. Health Psychology 2018;37(1):70-80. [DOI: 10.1037/hea0000556] [DOI] [PubMed] [Google Scholar]
Friedly 2016 {published data only}
- Friedly JL, Edwards T, Lavallee D, Bauer Z, Makris U, Nedeljkovic S, et al. Does provision of individualized reports showing changes in pain and function after epidural steroid injections (ESI) alter decision-making about future ESI use? PM & R 2016;8(9 Supplement):S309. [DOI] [PubMed] [Google Scholar]
Gallo 2016 {published data only}
- Gallo JJ, Hwang S, Joo JH, Bogner HR, Morales KH, Bruce ML, et al. Multimorbidity, depression, and mortality in primary care: Randomized clinical trial of an evidence-based depression care management program on mortality risk. Journal of General Internal Medicine 2016;31(4):380-6. [DOI: 10.1007/s11606-015-3524-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gossec 2016 {published data only}
- Gossec L, Servy H, Soubrier M, Joubert J-M, Czarlewski W, Combe B, et al. Access to an active, interactive self-assessment e-health platform improves patient-physician communication in rheumatoid arthritis: results of a randomized controlled trial including 320 patients over 1 year. Arthritis and Rheumatology 2016;68:1770‐1. [DOI: 10.1002/art.39977] [DOI] [Google Scholar]
Graetz 2018 {published data only}
- Graetz I, Anderson JN, McKillop CN, Stepanski EJ, Paladino AJ, Tillmanns TD. Use of a web-based app to improve postoperative outcomes for patients receiving gynecological oncology care: a randomized controlled feasibility trial. Gynecologic Oncology 2018;150(2):311-7. [DOI: ] [DOI] [PubMed] [Google Scholar]
Hanling 2016 {published data only}
- Hanling SR, Hickey A, Lesnik I, Hackworth RJ, Stedje-Larsen E, Drastal CA, et al. Stellate ganglion block for the treatment of Posttraumatic Stress Disorder: a randomized, double-blind, controlled trial. Regional Anesthesia and Pain Medicine 2016;41(4):494‐500. [DOI: 10.1097/AAP.0000000000000402] [DOI] [PubMed] [Google Scholar]
Indovina 2016 {published data only}
- Indovina K, Keniston A, Reid M, Sachs K, Zheng C, Tong A, et al. Real-time patient experience surveys of hospitalized medical patients. Journal of Hospital Medicine 2016;11(4):251-6. [DOI: 10.1002/jhm.2533] [DOI] [PubMed] [Google Scholar]
Jaeger 2017 {published data only}
- Jaeger VK, Schiffer P, Zufferey P, Pichler L, Badaracco A, Walder M, et al. Patient's self-monitoring of disease activity of rheumatic diseases via webapp-study design, patient's perspective and recruitment in the first 11 months of the Swiss multicentre, longitudinal compass ii study. Annals of the Rheumatic Diseases 2017;76(Suppl 2):1445. [Google Scholar]
Janse 2015 {published data only}
- Janse A, Worm-Smeitink M, Bussel-Lagarde J, Bleijenberg G, Nikolaus S, Knoo H. Testing the efficacy of web-based cognitive behavioural therapy for adult patients with chronic fatigue syndrome (CBIT): study protocol for a randomized controlled trial. BMC Neurology 2015;15(1). [DOI: 10.1186/s12883-015-0392-3] [DOI] [PMC free article] [PubMed]
Janssens 2015 {published data only}
- Janssens T, Harver A. Effects of symptom perception interventions on trigger identification and quality of life in children with asthma. Pulmonary Medicine 2015;2015(no pagination). [DOI: 10.1155/2015/825137] [DOI] [PMC free article] [PubMed]
Jones 2016 {published data only}
- Jones F, Gage H, Drummond A, Bhalla A, Grant R, Lennon S, et al. Feasibility study of an integrated stroke self-management programme: a cluster-randomised controlled trial. BMJ Open 2016;6 (1) (no pagination)(e008900). [DOI: 10.1136/bmjopen-2015-008900] [DOI] [PMC free article] [PubMed]
Kesanen 2017 {published data only}
- Kesanen J, Leino-Kilpi H, Lund T, Montin L, Puukka P, Valkeapaa K. Increased preoperative knowledge reduces surgery-related anxiety: a randomised clinical trial in 100 spinal stenosis patients. European Spine Journal 2017;26(10):1‐9. [DOI: 10.1007/s00586-017-4963-4] [DOI] [PubMed] [Google Scholar]
Kwan 2017 {unpublished data only}
- Kwan WMC. A randomized control trial to evaluate the effectiveness of short-term life review intervention in enhancing spiritual well-being and lowering anxiety and depression in palliative patients. ProQuest Dissertations Publishing 2017.
Liimatta 2017 {published data only}
- Liimatta H, Lampela P, Laitinen-Parkkonen P, Pitkala KH. Preventive home visits to promote the health-related quality of life of home-dwelling older people: baseline findings and feasibility of a randomized, controlled trial. European Geriatric Medicine 2017;8:440-5. [DOI: 10.1016/j.eurger.2017.06.003] [DOI] [Google Scholar]
Lowenstein 2018 {published data only}
- Lowenstein LM, Escoto KH, Leal VB, Bailey L, Bevers TB, Cantor SB, et al. Randomized trial of a patient-centered decision aid for promoting informed decisions about lung cancer screening: Implementation of a PCORI study protocol and lessons learned. Contemporary Clinical Trials 2018;72:26-34. [DOI: 10.1016/j.cct.2018.07.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCombie 2020 {published data only}
- McCombie A, Walmsley R, Barclay M, Ho C, Langlotz T, Regenbrecht H, et al. A noninferiority randomized clinical trial of the use of the smartphone-based health applications IBDsmart and IBDoc in the care of inflammatory bowel disease patients. Inflamatory Bowel Diseases 2020;26(7):1098-109. [DOI] [PubMed] [Google Scholar]
Mooney 2015 {published data only}
- Mooney K, Blaz J, Donaldson G. Reducing family caregiver psychosocial distress through automated remote home monitoring and self-care coaching. Psycho-oncology 2015;24:194. [DOI: 10.1002/pon.3874] [DOI] [Google Scholar]
Murff 2017 {published data only}
- Murff HJ. Self-monitoring of blood glucose did not improve HbA1c or QoL at 1 year in non-insulin-treated type 2 diabetes. Annals of Internal Medicine 2017;167(8):JC46. [DOI: 10.7326/ACPJC-2017-167-8-046] [DOI] [PubMed] [Google Scholar]
Olson 2017 {published data only}
- Olson D, Lamb M, Lopez MR, Colborn K, Paniagua-Avila A, Zacarias A, et al. Performance of a mobile phone app-based participatory syndromic surveillance system for acute febrile illness and acute gastroenteritis in rural Guatemala. Journal of Medical Internet Research 2017;19(11):e368. [DOI: 10.2196/jmir.8041] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paterson 2017 {published data only}
- Paterson C, Primeau C, Nabi G. A multimodal supportive care intervention in men and their partners/carers affected by metastatic prostate cancer: a randomised controlled feasibility study. European Urology 2017;16(3):e1641. [DOI: 10.1016/S1569-9056%2817%2930992-2] [DOI] [Google Scholar]
Piette 2015 {published data only}
- Piette JD, Striplin D, Marinec N, Chen J, Trivedi RB, Aron DC, et al. A mobile health intervention supporting heart failure patients and their informal caregivers: a randomized comparative effectiveness trial. Journal of Medical Internet Research 2015;17(6):e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Riese 2015 {published data only}
- Riese A, Mello MJ, Baird J, Steele DW, Ranney ML. Prompting discussions of youth violence using electronic previsit questionnaires in primary care: a cluster randomized trial. Academic Pediatrics 2015;15(3):345-52. [DOI] [PubMed] [Google Scholar]
Roberts 2017 {published data only}
- Roberts W, Fillmore MT. Curbing the DUI offender's self-efficacy to drink and drive: a laboratory study. Drug and Alcohol Dependence 2017;172:73-9. [DOI: 10.1016/j.drugalcdep.2016.12.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanchez 2018 {published data only}
- Sanchez R, Mateo KF. Survey-based priming intervention linked to improved communication with the seriously Ill. Journal of Clinical Outcomes Management 2018;25(7):300-3. [Google Scholar]
Sepucha 2019 {published data only}
- Sepucha K, Bedair H, Yu L, Dorrwachter JM, Dwyer M, Talmo CT, et al. Decision support strategies for hip and knee osteoarthritis: less is more: a randomized comparative effectiveness trial (DECIDE-OA Study). Journal of Bone and Joint Surgery: American volume. 2019;101(18):1645-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skinner 2016 {published data only}
- Skinner CS, Gupta S, Bishop WP, Ahn C, Tiro JA, Halm EA, et al. Tailored information increases patient/physician discussion of colon cancer risk and testing: the Cancer Risk Intake System trial. Preventive Medicine Reports 2016;4:6-10. [DOI: 10.1016/j.pmedr.2016.04.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2018 {published data only}
- Smith A, Pennington A, Carter B, Gething S, Price M, White J, et al. Acceptability of the method of administration of a patient-reported outcome measure (PROM) with stroke survivors, a randomised controlled trial protocol. Trials 2018;19(1):349. [DOI: 10.1186/s13063-018-2694-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sonal Sekhar 2018 {published data only}
- Sonal Sekhar M, Unnikrishnan MK, Vijayanarayana K, Rodrigues GS. Impact of patient-education on health related quality of life of diabetic foot ulcer patients: a randomized study. Clinical Epidemiology and Global Health 2018. [DOI: 10.1016/j.cegh.2018.07.009] [DOI]
Stump 2017 {published data only}
- Stump TK. Daily feedback improves sun protection among patients with an elevated risk of skin cancer. Dissertation Abstracts International: Section B: The Sciences and Engineering 2017;78(4-B(E)):No Pagination Specified. [Google Scholar]
Szots 2016 {published data only}
- Szots K, Konradsen H, Solgaard S, Ostergaard B. Telephone follow-up by nurse after total knee arthroplasty: results of a randomized clinical trial. Orthopaedic Nursing 2016;35(6):411-20. [DOI] [PubMed] [Google Scholar]
Uchitomi 2015 {published data only}
- Uchitomi Y, Fujimori M. Effect of communication skills training program for oncologists based on patient preferences for communication when receiving bad news: a randomized controlled trial. Supportive Care in Cancer 2015;23(1 SUPPL. 1):S239. [DOI: 10.1007/s00520-015-2712-y] [DOI] [PubMed] [Google Scholar]
Valle 2018 {published data only}
- Valle CG, Queen TL, Martin BA, Ribisl KM, Mayer DK, Tate DF. Optimizing tailored communications for health risk assessment: a randomized factorial experiment of the effects of expectancy priming, autonomy support, and exemplification. Journal of Medical Internet Research 2018;20(3):e63. [DOI: 10.2196/jmir.7613] [DOI] [PMC free article] [PubMed] [Google Scholar]
vanderWeegen 2015 {published data only}
- Weegen S, Verwey R, Spreeuwenberg M, Tange H, Weijden T, Witte L. It's LiFe! Mobile and web-based monitoring and feedback tool embedded in primary care increases physical activity: a cluster randomized controlled trial. Journal of Medical Internet Research 2015;17(7):e184. [DOI: 10.2196/jmir.4579] [DOI] [PMC free article] [PubMed] [Google Scholar]
vanDijk 2015 {published data only}
- Dijk ET, Westerink JHD, Beute F, Ijsselsteijn WA. In Sync: the effect of physiology feedback on the match between heart rate and self-reported stress. BioMed Research International 2015;2015:1-9. [DOI: 10.1155/2015/134606] [DOI] [PMC free article] [PubMed] [Google Scholar]
Voruganti 2017 {published data only}
- Voruganti T, Grunfeld E, Jamieson T, Kurahashi AM, Lokuge B, Krzyzanowska MK, et al. My Team of Care Study: a pilot randomized controlled trial of a web-based communication tool for collaborative care in patients with advanced cancer. Journal of Medical Internet Research 2017;19(7):e219. [DOI: 10.2196/jmir.7421] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2019 {published data only}
- Weiss ME, Yakusheva O, Bobay KL, Costa L, Hughes RG, Nuccio S, et al, READI Site Investigators. Effect of implementing discharge readiness assessment in adult medical-surgical units on 30-day return to hospital: the READI randomized clinical trial. JAMA Network Open 2019;2(1):e187387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williamson 2015 {published data only}
- Williamson S, Beaver K, Martin-Hirsch P, Patrick K, Anne T, Sutton C. Comparing hospital and telephone follow-up for women treated for endometrial cancer (ENDCAT trial). European Journal of Cancer 2015;51:S251. [Google Scholar]
Wright 2018 {published data only}
- Wright C, Dietze PM, Agius PA, Kuntsche E, Livingston M, Black OC, et al. Mobile phone-based ecological momentary intervention to reduce young adults' alcohol use in the event: a three-armed randomized controlled trial. JMIR Mhealth and Uhealth 2018;6(7):e149. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yee 2017 {published data only}
- Yee R, Jones L, Hosey M. What the child "SAID" to the dentist: a UK randomized controlled trial. Child: Care, Health and Development 2017;43(6):926-32. [DOI: 10.1111/cch.12510] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Castillo 2017 {published data only}
- Castillo C, Alfonzo AL, Camejo N, Schiavone A, Diaz M, Camano I, et al. Impact of measuring quality of life in oncology practice: a prospective study. Journal of Cinical Oncology 2017;35(15 Supplement 1):(no pagination). [Google Scholar]
References to ongoing studies
Absolom 2017 {published data only}
- Absolom K, Holch P, Warrington L, Samy F, Hulme C, Hewison J, et al, Rapid Systemic Treatment Work Group. Electronic patient self-Reporting of Adverse-events: patient Information and aDvice (eRAPID): a randomised controlled trial in systemic cancer treatment. BMC Cancer 2017;17(1):318. [DOI: 10.1186/s12885-017-3303-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
ACTRN12619001126101 {published data only}
- ACTRN12619001126101. PROpatient: Can symptom monitoring and care coordination improve the quality of life of people with upper gastrointestinal cancer? anzctr.org.au/Trial/Registration/TrialReview.aspx?id=378002 (first received 18 July 2019).
ACTRN12620000174987 {published data only}
- ACTRN12620000174987. Using responses to child and caregiver questionnaires about quality of life in hospital consultations. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12620000174987 (first received 23 January 2020).
Arts 2017 {published data only}
- Arts LP van de Poll-Franse LV, den Berg SW, Prins JB, Husson O, Mols F, et al. Lymphoma InterVEntion (LIVE) - patient-reported outcome feedback and a web-based self-management intervention for patients with lymphoma: study protocol for a randomised controlled trial. Trials 2017;18(1) (no pagination). [DOI: 10.1186/s13063-017-1943-2] [DOI] [PMC free article] [PubMed]
Atreja 2016 {published data only}
- Atreja A, Khan S, Rogers JD, Otobo E, Patel NP, Ullman T, et al. Impact of the Mobile HealthPROMISE Platform on the quality of care and quality of life in patients with inflammatory bowel disease: study protocol of a pragmatic randomized controlled trial. JMIR Research Protocols 2015;150(4 SUPPL. 1):S1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bansback 2019 {published data only}
- Bansback N, Trenaman L, MacDonald KV, Hawker G, Johnson JA, Stacey D, et al. An individualized patient-reported outcome measure (PROM) based patient decision aid and surgeon report for patients considering total knee arthroplasty: protocol for a pragmatic randomized controlled trial. BMC Musculoskeletal Disorders 2019;20(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bubb 2018 {published data only}
- Bubb MR, Judd R, Chauffe AD. Incorporation of patient reported outcomes data in the care of us veterans with rheumatoid arthritis: a randomized, controlled trial. Arthritis and Rheumatology 2018;70:3228-9. [Google Scholar]
ChiCTR1800018769 {published data only}
- ChiCTR1800018769. The application of patient reported outcomes in preventing relapse of depression. www.chictr.org.cn/showprojen.aspx?proj=31602 (first received 09 October 2018).
ChiCTR1900020846 {published data only}
- ChiCTR1900020846. Using patient-reported outcomes to manage postoperative symptoms in patients with lung cancer: a multicentre, randomised controlled trial. www.chictr.org.cn/hvshowproject.aspx?id=62679 (first received 22 November 2018).
- Dai W, Zhang Y, Feng W, Liao X, Mu Y, Zhang R, et al. Using patient-reported outcomes to manage postoperative symptoms in patients with lung cancer: protocol for a multicentre, randomised controlled trial. BMJ Open 2019;9:e030041. [DOI: 10.1136/bmjopen-2019-030041] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gorini 2016 {published data only}
- Gorini A, Mazzocco K, Kondylakis H, McVie G, Pravettoni G. A web-based interactive tool to improve breast cancer patient centredness. ecancermedicalscience 2016;10 (no pagination)(659). [DOI: 10.3332/ecancer.2016.659] [DOI] [PMC free article] [PubMed]
Grove 2018 {published data only}
- Grove B, Ivarsen P, De Thurah A, Kyte D, Henrik HN. Remote follow-up using patient-reported outcome measures in patients with chronic kidney disease: the PROKID study – study protocol for a non-inferiority pragmatic randomised controlled trial. BMC Health Services Research 2019;19:S143‐S144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grove 2019 {published data only}
- Grove B, Ivarsen P, De Thurah A, Kyte D, Henrik HN. Tele follow-up using patient-reported outcomes (PRO) measures in patients with chronic kidney disease-the PRO-KID study: a study protocol for a non-inferiority randomised controlled trial in Denmark. Quality of Life Research 2018;27:S143-4. [Google Scholar]
- Grove BE, Ivarsen P, Thurah A, Schougaard LM, Kyte D, Hjollund NH. Remote follow-up using patient-reported outcome measures in patients with chronic kidney disease: the PROKID study – study protocol for a non-inferiority pragmatic randomised controlled trial. BMC Health Services Research 2019;19:631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holch 2018 {published data only}
- Holch P, Pini S, Henry AM, Davidson S, Routledge J, Brown J, et al, Rapid radiotherapy work group. eRAPID electronic patient self-Reporting of Adverse-events: Patient Information and aDvice: a pilot study protocol in pelvic radiotherapy. Pilot and Feasibility Studies 2018;4:110. [DOI: 10.1186/s40814-018-0304-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN82172279 {published data only}
- ISRCTN82172279 Reconceptualising patient-reported outcome measures for back pain. ISRCTN Registry. [DOI: ]
Kendrick 2020 {published data only}
- ISRCTN17299295. Patient-reported outcome measures for monitoring primary care patients with depression: PROMDEP randomised controlled trial. www.isrctn.com/ISRCTN17299295 (first received 1 January 2018).
- Kendrick T, Moore M, Leydon G, Stuart B, Geraghty AW, Yao G, et al. Patient-reported outcome measures for monitoring primary care patients with depression (PROMDEP): study protocol for a randomised controlled trial. Trials 2020;21(1):441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klinkhammer‐Schalke 2015 {published data only}
- Klinkhammer-Schalke M, Lindberg P, Koller M, Wyatt JC, Hofstädter F, Lorenz W, et al. Direct improvement of quality of life in colorectal cancer patients using a tailored pathway with quality of life diagnosis and therapy (DIQOL): study protocol for a randomised controlled trial. Trials 2015;16:460. [DOI: 10.1186/s13063-015-0972-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg P, Steinger B, Koller M, Furst A, Kastel M, Obermaier R, et al. Implementing a clinical pathway with quality of life diagnosis and therapy of patients with primary colorectal cancer: A randomised controlled trial. Oncology Research and Treatment 2016;39:137. [DOI: 10.1159/000444354] [DOI] [Google Scholar]
Kuklinski 2020 {published data only}
- DRKS00019916. PROMoting Quality - Intersectoral use of Patient Reported Outcome Measures to increase patient-relevant outcome quality. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00019916 2019.
- Kuklinski D, Oschmann L, Pross C, Busse R, Geissler A. 2020. Trials 2020;21:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kyte 2018 {published data only}
- Kyte D, Nishop J, Brettell E, Calvert M, Cockwell P, Dutton M, et al. Use of an electronic patient-reported outcome measure in the management of patients with advanced chronic kidney disease: the RePROM pilot trial protocol. BMJ Open 2018;8(10):e026080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mamguem Kanga 2020 {published data only}
- Mamguem Kamga A, Di Martino C, Anota A, Paget-Bailly S, Coutant C, Arveux P, et al. Impact of routine assessment of health-related quality of life coupled with therapeutic information on compliance with endocrine therapy in patients with non-metastatic breast cancer: protocol for a randomized controlled trial. Trials 2020;21(1):527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsuda 2018 {published data only}
- Matsuda A, Yamada Y, Ishizuka N, Matsushima E, Kobayashi K, Ohkubo T, et al. Effects of a self-monitoring quality of life intervention for patients with cancer receiving palliative care in Japan: study protocol for a randomized controlled trial. Asian Pacific Journal of Cancer Prevention 2018;19(11):3027-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morton 2019 {published data only}
- Morton R, Jose M, Brown C, Bennett P, Palmer S, Caskey F, et al. The symptom monitoring with feedback trial (Swift): a novel registry-based cluster randomised controlled trial among Australian and New Zealand adults with end-stage kidney disease managed on haemodialysis. Nephrology Dialysis Transplantation 2019;34(Suppl. 1):a13. [Google Scholar]
NCT02591472 {published data only}
- NCT02591472. An integrated-delivery-of-care approach to improve patient outcomes, safety, well-being after orthopaedic trauma. clinicaltrials.gov/ct2/show/NCT02591472 (first received 29 October 2015).
NCT02673580 {published data only}
- NCT02673580. Tele-patient-reported Outcomes (telePRO) in clinical practice. clinicaltrials.gov/ct2/show/NCT02673580 (first received 4 February 2016).
NCT02818478 {published data only}
- NCT02818478. Patient reported outcomes reported via PC/ tablet home versus touch screen at hospital among patients with arthritis (PRO). clinicaltrials.gov/ct2/show/NCT02818478 (first received 29 June 2016).
NCT02917954 {published data only}
- NCT02917954. Electronic patient reported outcome (ePRO) mobile application pragmatic trial. clinicaltrials.gov/ct2/show/NCT02917954 (first received 28 September 2016).
NCT02949167 {published data only}
- NCT02949167. MyHealth: follow-up after breast cancer treatment. clinicaltrials.gov/ct2/show/NCT02949167 (first received 31 October 2016).
NCT02996201 {published data only}
- NCT02996201. Electronic patient reporting of side effects to chemotherapy: a cluster randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02996201 (first received 19 December 2016).
- Pappot H, Baeksted C, Knoop A, Mitchell SA, Nissen A, Johansen C. Routine surveillance for symptomatic toxicities with real-time clinician reporting in Danish breast cancer patients-Organization and design of the first national, cluster randomized trial using the Patient-Reported Outcomes version of Common Terminology C. Breast Journal 2019;25(2):269-272. [DOI] [PubMed] [Google Scholar]
NCT03056469 {published data only}
- NCT03056469. Patient-reported outcomes integrated in the follow-up of patients with hematological cancer. clinicaltrials.gov/ct2/show/NCT03056469 (first received 17 February 2017).
NCT03093649 {published data only}
- NCT03093649. Measuring patient-reported adverse events in oncology practice improves quality of life in nasopharyngeal carcinoma. clinicaltrials.gov/ct2/show/NCT03093649 (first received 28 March 2017).
NCT03202732 {published data only}
- NCT03202732. DiabetesFlex - Patient involvement and patient-reported outcome measures in Type 1 diabetes. clinicaltrials.gov/ct2/show/NCT03202732 (first received 29 June 2017).
NCT03240913 {published data only}
- NCT03240913. A PROMs based educational tool (PROM-DA) for patients considering total knee arthroplasty. clinicaltrials.gov/ct2/show/NCT03240913 (first received 7 August 2017).
NCT03249090 {published data only}
- NCT03249090. Electronic patient reporting of symptoms during cancer treatment (PRO-TECT). clinicaltrials.gov/ct2/show/NCT03249090 (first received 15 August 2017).
- Stover AM, Henson S, Deal AM, Stricker CT, Hammelef KJ, Bennett AV, et al. Methods for alerting clinicians to concerning symptom questionnaire responses during cancer care: approaches from two randomized trials (STAR, AFT-39 PRO-TECT). Journal of Clinical Oncology 2018;36:30. [Google Scholar]
NCT03471104 {published data only}
- NCT03471104. Patient-reported outcome measures in diabetes care (DiaPROM). clinicaltrials.gov/ct2/show/NCT03471104 (first received 20 March 2018).
NCT03535922 {published data only}
- NCT03535922. Evaluation of routinely measured patient-reported outcomes in hemodialysis care. clinicaltrials.gov/ct2/show/NCT03535922 (first received 24 May 2018).
NCT03608410 {published data only}
- NCT03608410. Intensified follow-up of lung cancer using weekly questionnaires via the Internet (ProWide). clinicaltrials.gov/ct2/show/NCT03608410 (first received 1 Augus 2018).
NCT03850912 {published data only}
- NCT03850912. Symptom Management Implementation of Patient Reported Outcomes in Oncology (SIMPRO). clinicaltrials.gov/ct2/show/NCT03850912 (first received 22 February 2019).
NCT03995082 {published data only}
- NCT03995082. Breast cancer patient engagement with patient reported outcome measure survey. clinicaltrials.gov/ct2/show/NCT03995082 (first received 21 June 2019).
NCT04066868 {published data only}
- NCT04066868. Evaluating the use of patient-reported outcome measures for improving the inter-rater reliability of common terminology criteria for adverse event ratings. clinicaltrials.gov/ct2/show/NCT04066868 (first received 26 August 2019).
NCT04069455 {published data only}
- NCT04069455. ePRO for adjuvant therapy of colorectal adenocarcinoma. clinicaltrials.gov/ct2/show/NCT04069455 (first received 28 August 2019).
NCT04164004 {published data only}
- NCT04164004. Patient-Reported Outcome Measurement in Heart Failure Clinic (PRO-HF). clinicaltrials.gov/ct2/show/NCT04164004 (first received 15 November 2019).
NCT04342260 {published data only}
- NCT04342260. Improving quality of life after thoracic surgery using patient-reported outcomes. clinicaltrials.gov/ct2/show/NCT04342260 (first received 10 April 2020).
NCT04356209 {published data only}
- NCT04356209. Improving The empowerment inpatients with severe breast fibrosis radio-induced treated by pravastatin: benefit of e-PROs (Electronic " Patient Reported Outcome ") on breast-related quality of life (PRAVACUR-02). clinicaltrials.gov/ct2/show/NCT04356209 (first received April 2020).
NCT04393571 {published data only}
- NCT04393571. The utility of mobile based patient reported outcome measures in patients with acetabular fractures: a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT04393571 (first received 19 May 2020).
NCT04401332 {published data only}
- NCT04401332. A randomized controlled trial of AppS To Home Monitor your Asthma (ASTHMA). clinicaltrials.gov/ct2/show/NCT04401332 (first received 26 May 2020).
NCT04492007 {published data only}
- NCT04492007. Feasibility Testing of Patient Reported Outcomes - Informed Symptom Management System (PRISMS). clinicaltrials.gov/ct2/show/NCT04492007 (firs received 30 July 2020).
Paladino 2019 {published data only}
- Paladino AJ, Anderson JN, Krukowski RA, Waters T, Kocak M, Graff C, et al. THRIVE study protocol: a randomized controlled trial evaluating a web-based app and tailored messages to improve adherence to adjuvant endocrine therapy among women with breast cancer. BMC Health Services Research 2019;19(1):977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 2019 {published data only}
- Roberts NA, Mudge A, Alexander K, Wyld D, Janda M. The iPROMOS protocol: a stepped-wedge study to implement routine patient-reported outcomes in a medical oncology outpatient setting. BMJ Open 2019;9(2):e027046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rogers 2018 {published data only}
- Rogers S, Lowe D, Lowies C, Tien Yeo S, Allmark C, Mcavery D. Improving quality of life through routine use of the Patient Concerns Inventory (PCI) for head and neck cancer patients: a cluster preference randomized controlled trial. Psycho-oncology 2019;28(Suppl 2):10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SN, Lowe D, Lowies C, Yeo ST, Allmark C, Mcavery D, et al. Improving quality of life through the routine use of the patient concerns inventory for head and neck cancer patients: a cluster preference randomized controlled trial. BMC Cancer 2018;18:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rogers 2019 {published data only}
- Rogers S, Lowe D, Lowies C, Tien Yeo S, Allmark C, Mcavery D, et al. Improving quality of life through routine use of the Patient Concerns Inventory (PCI) for head and neck cancer patients: a cluster preference randomized controlled trial. Psycho-oncology 2019;28(Suppl 2):10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SN, Allmark C, Bekiroglu F, Edwards RT, Fabbroni G, Flavel R, et al. Improving quality of life through the routine use of the patient concerns inventory for head and neck cancer patients: baseline results in a cluster preference randomised controlled trial.. European Archives of Oto-Rhino-Laryngology. 2020;277:3435-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SN, Lowe D, Lowies C, Yeo ST, Allmark C, Mcavery D, et al. Improving quality of life through the routine use of the patient concerns inventory for head and neck cancer patients: a cluster preference randomized controlled trial. BMC Cancer 2018;18:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seppen 2020 {published data only}
- Seppen BF, L'ami MJ, Duarte Dos Santos Rico S, Ter Wee MM, Turkstra F, Roorda LD, et al. A smartphone app for self-monitoring of rheumatoid arthritis disease activity to assist patient-initiated care: protocol for a randomized controlled trial. JMIR Research Protocols 2020;9(2):e15105. [DOI: 10.2196/15105] [DOI] [PMC free article] [PubMed] [Google Scholar]
Serrano‐Ripoll 2019 {published data only}
- Serrano-Ripoll MJ, Ripoll J, Valderas JM, Pastor-Moreno G, Labry Lima A, Ricci-Cabello I. Development and evaluation of an intervention based on the provision of patient feedback to improve patient safety in Spanish primary healthcare centres: study protocol. BMJ Open 2019;9(12):e031367. [DOI: 10.1136/bmjopen-2019-031367] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Alonso 2013
- Alonso J, Bartlett SJ, Rose M, Aaronson NK, Chaplin JE, Efficace D, et al, PROMIS International Group. The case for an international patient-reported outcomes measurement information system (PROMIS®) initiative. Health and Quality of Life Outcomes 2013;11(210):1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arranz 2004
- Arranz P, Remor E, Quintana M, Villar A, Díaz JL, Moreno M, et al, Hemofilia-QoL Group. Development of a new disease-specific quality-of-life questionnaire to adults living with haemophilia. Haemophilia 2004;10(4):376-82. [DOI] [PubMed] [Google Scholar]
Bantug 2016
- Bantug ET, Coles T, Smith KC, Snyder CF, Rouette J, Brundage MD, et al. Graphical displays of patient-reported outcomes (PRO) for use in clinical practice: what makes a pro picture worth a thousand words? Patient Education and Counseling 2016;99(4):483-90. [DOI] [PubMed] [Google Scholar]
Bergus 2005
- Bergus GR, Hartz AJ, Noyes R Jr, Ward MM, James PA, Vaughn T, et al. The limited effect of screening for depressive symptoms with the PHQ-9 in rural family practices. Journal of Rural Health 2005;21(4):303-9. [DOI] [PubMed] [Google Scholar]
Berry 2011
- Berry DL, Blumenstein BA, Halpenny B, Wolpin S, Fann JR, Austin-Seymour M, et al. Enhancing patient-provider communication with the electronic self-report assessment for cancer: a randomized trial. Journal of Clinical Oncology 2011;29(8):1029-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Black 2013
- Black N. Patient reported outcome measures could help transform healthcare. BMJ 2013;346:f167. [DOI] [PubMed] [Google Scholar]
Black 2016
- Black N, Burke L, Forrest CB, Sieberer UH Ravens, Ahmed S, et al. Patient-reported outcomes: pathways to better health, better services, and better societies. Quality of Life Research : an international journal of quality of life aspects of treatment, care and rehabilitation 2016/05;25(5):1103-12. [DOI] [PubMed] [Google Scholar]
Bradley 1999
- Bradley C, Todd C, Gorton T, Symonds E, Martin A, Plowright R. The development of an individualized questionnaire measure of perceived impact of diabetes on quality of life: the ADDQoL. Quality of Life Research 1999;8(1-2):79-91. [DOI] [PubMed] [Google Scholar]
Brown 2006
- Brown H, Bacigalupo R. Health visitors and postnatal depression: identification and practice. Community Practitioner: the Journal of the Community Practitioners' & Health Visitors' Association 2006;79(2):49-52. [PubMed] [Google Scholar]
Brundage 2015
- Brundage MD, Smith KC, Little EA, Bantug ET, Snyder CF, PRO Data Presentation Stakeholder Advisory Board. Communicating patient-reported outcome scores using graphic formats: results from a mixed-methods evaluation. Quality of Life Research 2015;24(10):2457-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calvert 2019
- Calvert M, Kyte D, Price G, Valderas Jose M, Hjollund NH. Maximising the impact of patient reported outcome assessment for patients and society. BMJ (Clinical research ed.) 2019/01//;364:k5267-k5267. [DOI] [PubMed] [Google Scholar]
Cochrane Handbook 5.1.0, Section 16.5.4
- Julian Higgins, Sally Green. How to include multiple groups from one study. [How to include multiple groups from one study.]. In: Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0.. Cochrane Collaboration, 2011. [HTTPS://HANDBOOK-5-1.COCHRANE.ORG/: https://handbook-5-1.cochrane.org/] [Google Scholar]
Cohen 1988
- Cohen, J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum, 1988. [Google Scholar]
Craig 2008
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2008
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
EpicCare 2015 [Computer program]
- EpicCare EMR. Verona: Epic, 2015.
EPOC 2014
- Effective Practice and Organisation of Care (EPOC). Good practice data extraction form. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services;2014. Available at: http://epoc.cochrane.org/epoc-resources (accessed 14 March 2014).
EPOC 2014b
- Effective Practice and Organisation of Care (EPOC). Analysis in EPOC reviews. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services;2014. Available at: http://epocoslo.cochrane.org/epoc-specific-resources-review-authors (accessed 27 October 2014).
EPOC 2017
- Cochrane Effective Practice and Organisation of Care (EPOC). EPOC worksheets for preparing a 'Summary of findings' table using GRADE. EPOC resources for review authors, 2017. epoc.cochrane.org/resources/epoc-resources-review-authors (accessed 31 August 2017).
EPOC 2017b
- Cochrane Effective Practice and Organisation of Care (EPOC). Reporting the effects of an intervention in EPOC reviews. EPOC Resources for review authors, 2017. epoc.cochrane.org/resources/epoc-resources-review-authors (accessed 31 August 2017).
EPOC 2017c
- Cochrane Effective Practice and Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors, 2017. epoc.cochrane.org/resources/epoc-resources-review-authors (accessed 9 December 2019).
Espallargues 2000
- Espallargues M, Valderas JM, Alonso J. Provision of feedback on perceived health status to health care professionals: a systematic review of its impact. Medical Care 2000;38(2):175-86. [DOI] [PubMed] [Google Scholar]
FDA 2009
- US Department of Health and Human Services Food and Drug Administration. Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf (accessed prior to 26 September 2019).
Fitzpatrick 1998
- Fitzpatrick R, Davey C, Buxton MJ, Jones DR. Evaluating patient-based outcome measures for use in clinical trials. Health Technology Assessment 1998;2(14):i-86. [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006;51(1):7-10. [DOI] [PubMed] [Google Scholar]
Garratt 1993
- Garratt AM, Ruta DA, Abdalla MI, Buckingham JK, Russell IT. The SF-36 Health Survey Questionnaire: an outcome measure suitable for routine use within the NHS? BMJ 1993;306(6890):1440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garratt 2002
- Garratt A, Schmidt L, Mackintosh A, Fitzpatrick R. Quality of life measurement: bibliographic study of patient assessed health outcome measures. BMJ 2002;324(7351):1417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibbons 2011
- Gibbons CJ, Mills RJ, Thornton EW, Ealing J, Mitchell JD, Shaw PJ, et al. Development of a patient-reported outcome measure for fatigue in motor neurone disease: the Neurological Fatigue Index (NFI-MND). Health and Quality of Life Outcomes 2011;9:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilbody 2001
- Gilbody SM, House AO, Sheldon TA. Routinely administered questionnaires for depression and anxiety: Systematic review. BMJ 2001;322(7283):406-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), Version accessed prior to 1 August 2019.
Greenhalgh 1999
- Greenhalgh J, Meadows K. The effectiveness of the use of patient-based measures of health in routine practice in improving the process and outcomes of patient care: a literature review. Journal of Evaluation in Clinical Practice 1999;5(4):401-16. [DOI] [PubMed] [Google Scholar]
Greenhalgh 2009
- Greenhalgh J. The applications of PROs in clinical practice: what are they, do they work, and why? Quality of Life Research 2009;18(1):115-23. [DOI] [PubMed] [Google Scholar]
Greenhalgh 2017
- Greenhalgh J, Dalkin S, Gooding K, Gibbons E, Wright J, Meads D, et al. Health Services and Delivery Research. 1 edition. Vol. 5.2. Southampton (UK): NIHR Journals Library, 2017. [PubMed] [Google Scholar]
Greenhalgh 2018
- Greenhalgh J, Gooding K, Gibbons E, Dalkin S, Wright J, Valderas J, et al. How do patient reported outcome measures (PROMs) support clinician-patient communication and patient care? A realist synthesis. Journal of Patient Reported Outcomes 2018;2(42):1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al, GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DF, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hostetter 2011
- Hostetter M, Klein S. Using patient-reported outcomes to improve health care quality. the Commonwealth Fund. www.commonwealthfund.org/Newsletters/Quality-Matters/2011/December-January-2012/In-Focus.aspx (accessed prior 26 September 2019).
Kendrick 2016
- Kendrick T, El‐Gohary M, Stuart B, Gilbody S, Churchill R, Aiken L, et al. Routine use of patient reported outcome measures (PROMs) for improving treatment of common mental health disorders in adults. Cochrane Database of Systematic Reviews 2016, Issue 7. Art. No: CD011119. [DOI: 10.1002/14651858.CD011119.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klinkhammer‐Schalke 2012
- Klinkhammer-Schalke M, Koller M, Steinger B, Ehret C, Ernst B, Wyatt JC, et al, Regensburg QoL Study Group. Direct improvement of quality of life using a tailored quality of life diagnosis and therapy pathway: randomised trial in 200 women with breast cancer. British Journal of Cancer 2012;106(5):826-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kluger 1996
- Kluger AN, DeNisi A. The effects of feedback interventions on performance: a historical review, a meta-analysis, and a preliminary feedback intervention theory. Psychological Bulletin 1996;119(2):254-84. [Google Scholar]
Lewis 1996
- Lewis G, Sharp D, Bartholomew J, Pelosi AJ. Computerized assessment of common mental disorders in primary care: effect on clinical outcome. Family Practice 1996;13(2):120-6. [DOI] [PubMed] [Google Scholar]
Leydon 2011
- Leydon GM, Dowrick CF, McBride AS, Burgess HJ, Howe AC, Clarke PD, et al, QOF Depression Study Team. Questionnaire severity measures for depression: a threat to the doctor-patient relationship? British Journal of General Practice 2011;61(583):117-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2006
- Marshall S, Haywood K, Fitzpatrick R. Impact of patient-reported outcome measures on routine practice: a structured review. Journal of Evaluation in Clinical Practice 2006;12(5):559-68. [DOI] [PubMed] [Google Scholar]
McKenna 2011
- McKenna SP. Measuring patient-reported outcomes: moving beyond misplaced common sense to hard science. BMC Medicine 2011;9(86):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nelson 1990
- Nelson EC, Landgraf JM, Hays RD, Wasson JH, Kirk JW. The functional status of patients. How can it be measured in physicians' offices? Medical Care 1990;28(12):1111-26. [PubMed] [Google Scholar]
Nelson 2015
- Nelson EC, Eftimovska E, Lind C, Hager A, Wasson JH, Lindblad S. Patient reported outcome measures in practice. BMJ 2015;350:g7818. [DOI] [PubMed] [Google Scholar]
Porter 2016
- Porter I, Gonçalves-Bradley D, Ricci-Cabello I, Gibbons C, Gangannagaripalli J, Fitzpatrick R, et al. Framework and guidance for implementing patient-reported outcomes in clinical practice: evidence, challenges and opportunities. Journal of Comparative Effectiveness Research 2016;5(5):507-19. [DOI] [PubMed] [Google Scholar]
Porter 2021
- Porter I, Davey A, Gangannagaripalli J, Evans J, Bramwell C, Evans P, et al. Integrating Patient Reported Outcome Measures (PROMs) into routine nurse-led primary care for patients with multimorbidity: a feasibility and acceptability study. Health and Quality of Life Outcomes 2021;19(133):1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
PROMIS 2007
- Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Medical Care 2007;45(5 supply 1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reuters 2011 [Computer program]
- Endnote X5. Reuters T. New York, NY: Thompson Reuters, 2011.
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2019
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al, on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editors(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. [Google Scholar]
Shepperd 2009
- Shepperd S, Lewin S, Straus S, Clarke M, Eccles MP, Fitzpatrick R, et al. Can we systematically review studies that evaluate complex interventions? PLOS Medicine 2009;6(8):e1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2021
- Smith SM, Wallace E, O'Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings.. Cochrane Database of Systematic Reviews 2016, Issue 3. Art. No: CD006560. [DOI: 10.1002/14651858.CD006560.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Snyder 2012
- Snyder CF, Aaronson NK, Choucair AK, Elliott TE, Greenhalgh J, Halyard MY, et al. Implementing patient-reported outcomes assessment in clinical practice: a review of the options and considerations. Quality of Life Research 2012;21(8):1305-14. [DOI] [PubMed] [Google Scholar]
StataCorp 2013 [Computer program]
- Stata Statistical Software. StataCorp, Version Release 13. College Station, TX: StataCorp LP, 2013.
Tavabie 2009
- Tavabie JA, Tavabie OD. Improving care in depression: qualitative study investigating the effects of using a mental health questionnaire. Quality in Primary Care 2009;17(4):251-61. [PubMed] [Google Scholar]
Taylor 1996
- Taylor KM, Macdonald KG, Bezjak A, Ng P, DePetrillo AD. Physicians' perspective on quality of life: an exploratory study of oncologists. Quality of Life Research 1996;5(1):5-14. [DOI] [PubMed] [Google Scholar]
Valderas 2008a
- Valderas JM, Kotzeva A, Espallargues M, Guyatt G, Ferrans CE, Halyard MY, et al. The impact of measuring patient-reported outcomes in clinical practice: A systematic review of the literature. Quality of Life Research 2008;17(2):179-93. [DOI] [PubMed] [Google Scholar]
Valderas 2008b
- Valderas JM, Alonso J. Patient reported outcome measures: a model-based classification system for research and clinical practice. Quality of Life Research 2008;17(9):1125-35. [DOI] [PubMed] [Google Scholar]
Valderas 2008c
Valderas 2008d
- Valderas JM, Ferrer M, Mendivil J, Garin O, Rajmil L, Herdman M, Alonso J, Scientific Committee on "Patient-Reported Outcomes" of the Iryss Network. Development of EMPRO: a tool for the standardized assessment of patient-reported outcome measures. Value Health 2008;11(4):700-8. [DOI] [PubMed] [Google Scholar]
Valderas 2009
- Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services [Defining comorbidity: implications for understanding health and health services]. The Annals of Family Medicine 1/7/2009;7(4):357-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valderas 2010
- Valderas JM, Espallargues M, Kotzeva A, Alonso J. Assessing the impact of routinely measuring patient-reported outcomes in clinical practice: critical appraisal of 34 randomized clinical trials. Quality of Life Research 2010;17(Suppl 1):11. [Google Scholar]
Valderas 2012
- Valderas JM, Fitzpatrick R, Roland M. Using health status to measure NHS performance: another step into the dark for the health reform in England. BMJ Quality & Safety 2012;21(4):352-3. [DOI] [PubMed] [Google Scholar]
Valderas 2019
- Valderas JM, Gangannagaripalli J, Nolte E, Boyd CM, Roland M, Sarria‐Santamera A, Jones E, Rijken M . Quality of care assessment for people with multimorbidity.. Journal of internal medicine. 2019 Mar;285(3):289-300. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gonçalves‐Bradley 2015
- Gonçalves-Bradley DC, Gibbons C, Ricci-Cabello I, Bobrovitz NJ, Gibbons EJ, Kotzeva A, et al. Routine provision of information on patient-reported outcome measures to healthcare providers and patients in clinical practice. Cochrane Database of Systematic Reviews 2015, Issue 4. Art. No: CD011589. [DOI: 10.1002/14651858.CD011589] [DOI] [PMC free article] [PubMed] [Google Scholar]


