Abstract
Background
This study investigated the clinical factors related to hospital-acquired disability (HAD) among 70 patients (median age, 78 years; interquartile range (IQR), 78 - 83) who were hospitalized for heart failure (HF) at Ayase Heart Hospital between December 2019 and October 2020.
Methods
HAD was defined as a ≥ 5-point decrease in Barthel Index (BI) scores from admission to discharge. Twenty-nine HF patients (41%) developed HAD after admission.
Results
Compared to the non-HAD group, the HAD group had higher Kihon Checklist scores (14 points (IQR, 11 - 17) vs. 9 points (IQR, 6 - 13); P < 0.01) and prevalence of multi-faceted frailty (90% vs. 29%; P < 0.01), a longer urinary-catheter-placement period (3 days (IQR, 1 - 5] vs. 1 day (IQR, 0 - 2), P < 0.05), less daily number of steps (457 steps (IQR, 301 - 997) vs. 1,692 steps (IQR, 1,227 - 2,418); P < 0.01), and moderate-intensity physical activity time (0 min (IQR, 0 - 2] vs. 1 min (IQR, 0 - 3); P < 0.05).
Conclusion
In conclusion, lower physical function and general physical activity and longer urinary-catheter-placement are associated with HAD.
Keywords: Older patients, Heart failure, Hospital-acquired disability, Clinical characteristics
Introduction
Japan is the first to become a super-aging country, with the highest aging rate worldwide at 28.7% as of September 15, 2020 [1]. The number of older patients with heart failure (HF) is significantly increasing [2], with approximately 1.3 million HF patients expected by 2030 [3].
HF is associated with not only cardiac dysfunction, but also reduced physical function and quality of life (QOL) [4]. Moreover, decreased physical function is a risk factor for mortality and re-hospitalization among older HF patients [5, 6].
Hospital-acquired disability (HAD) refers to either a new or worsened in-hospital functional decline and develops in approximately 30-60% of older patients [7, 8]. HAD is associated with clinical outcomes, including the in-hospital functional trajectory, in the older population [8-10]. However, few studies have investigated the determinants of HAD among older HF patients.
HAD requires reconsideration of rehabilitation or healthcare services, and a higher medical expenditure to prevent functional decline. There is little evidence whether a standard acute cardiac rehabilitation phase improves HAD among older HF patients. Hence, this study investigated the prevalence and clinical characteristics of HAD among older HF patients.
Materials and Methods
Subjects
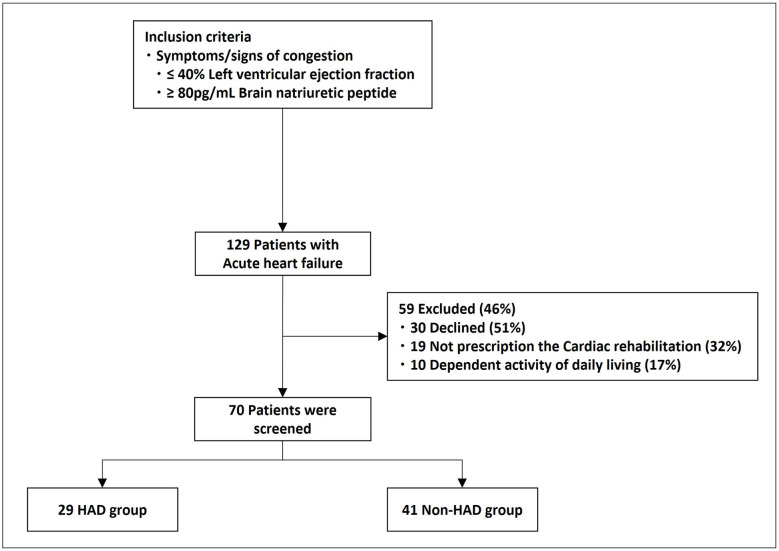
Details on the acute-phase index and clinical outcomes of 70 patients aged ≥ 65 years out of 129 patients hospitalized for acute HF in Ayase Heart Hospital between December 2019 and October 2020 were collected. The inclusion criteria, adopted from existing guidelines, were as follows: symptoms/signs of congestion, and left ventricular ejection fraction (LVEF) ≤ 40% or brain natriuretic peptide (BNP) level ≥ 80 pg/mL [4, 11].
HAD was defined as a ≥ 5-point decrease on the Barthel index (BI) score from admission to the day before discharge [9, 10]. Among the 70 patients, 29 (female, 48%; median age, 81 (interquartile range (IQR), 78 - 86) years) and 41 (female, 36%; median age, 78 (IQR, 72 - 83) years) patients were classified into HAD and non-HAD groups, respectively, 19 were not prescribed acute-phase rehabilitation because of unstable hemodynamics, 10 were dependent on activities of daily living (ADLs) (BI scores before hospital admission < 70 points), and 30 did not agree to participate and were excluded (Fig. 1).
Figure 1.
Flow chart with inclusion and exclusion criteria. HAD: hospital-acquired disability.
Ethical issues
All relevant information, including the purpose and methodology of the experiment, was explained beforehand to the study participants. All procedures were conducted in compliance with the Declaration of Helsinki. This study was approved by the Ayase Heart Hospital Ethics Review Committee.
Data collection and measurements
We assessed the clinical characteristics of the patients, including age, sex, body mass index (BMI), cohabitation status, long-term care, etiology of HF, New York Heart Association functional class, chronic comorbidities, Charlson Comorbidity Index, and history of HF-related hospitalization.
HF affects a wide range of patients, from those with normal LVEF (typically ≥ 50%; HF with preserved EF) to those with reduced LVEF (typically < 40%; HF with reduced EF). LVEFs within 40-49% represent a “gray area”, which we now define as HF with a mid-range LVEF [12].
Biochemical markers on admission, including the BNP level, hemoglobin, estimated glomerular filtration rate, C-reactive protein concentration, and sodium level, as well as data on inotrope use, mechanical circulatory support, progression of cardiac rehabilitation (time to initiation, walking exercise, and rehabilitation time), urinary-catheter-placement period, intravenous fluid therapy period, medication at discharge (beta-blocker, mineralocorticoid receptor antagonist, angiotensin-converting enzyme inhibitor, angiotensin II receptor blocker, and calcium-channel blocker), length of hospital stay, and return-to-home rate were collected.
HAD
The HAD was assessed using the BI, which is a simple and independent index for scoring the physical ability of patients with chronic diseases. The BI items can be categorized into those related to self-care (feeding, grooming, bathing, dressing, bowel and bladder care, and toilet use) and mobility (ambulation, transfers, and stair climbing). Each item was scored, with a total score of 100 points. A full BI score is not given for an activity if the patient needs any help and/or supervision [13]. Before hospitalization, ADL independence is defined as a ≥ 70 BI score [14]. The BI has more information about ADLs and is sensitive even to small changes in functional capacity [15]. It has also been demonstrated high inter-rater and test-retest reliability, and high correlations [16].
Recently, the BI among older HF patients is used for prognosis prediction and rehabilitation of acute-phase diseases [17, 18]; thus, we decided to use the BI for the older HF patients.
The BI scores were obtained by physiotherapists and nurses, who asked the subjects and their families about the BI items in the 2 weeks before admission, when the symptoms of HF had been stable. The BI scores at the time of discharge were also determined. The HAD was defined by a decrease in at least 5-point on the BI at the day before discharge.
Kihon checklist (KCL)
The KCL investigated physical function before admission using 25 yes-or-no questions related to living, mental, and physical functions. The KCL consists of seven categories aimed at assessing instrumental and social ADL, physical and cognitive function, nutritional status and oral function, and depressive mood. Higher scores indicated more functional problems [19]. The KCL is a comprehensive evaluation method that focuses on the social, psychological, physical aspects of frailty, making it an effective screening tool. Scores ≥ 8 points indicated multi-faceted frailty [20].
Short physical performance battery (SPPB)
The SPPB is used for evaluating lower extremity function, and consists of balance, gait, and stand-up tests. Each test is scored from 0 to 4 points, for a total score within 0 - 12 points. To assess balance, the participants attempted to hold the side-by-side, semi-tandem, and full-tandem positions for 10 seconds each, and were scored as follows: 1, unable to hold a semi-tandem stand; 2, held a semi-tandem stand but not a full-tandem stand for more than 2 seconds; 3, held the full-tandem stand for 3 - 9 s; and 4, held the full-tandem stand for 10 s. To assess gait, a usual-paced, 4-m walk was timed from the standing position, and the walking time was scored as follows: 1, ≥ 8.70 s (≤ 0.46 m/s); 2, 6.21 to < 8.70 s (0.46 to < 0.64 m/s); 3, 4.82 to < 6.20 s (0.65 to < 0.83 m/s); and 4, < 4.82 s (≥ 0.84 m/s). To assess stand-up performance, participants were asked to fold their arms across their chest and stand up once from a chair and stand up and sit down for five times as quickly as possible if successful. The participants’ total time was scored as follows: 1, 60 s or incomplete; 2, > 16.7 s; 3, 13.70 - 16.69 s; and 4, ≤ 11.19 s [21]. SPPB total scores < 10 indicate physical frailty and are associated with all-cause mortality [22].
Handgrip strength
The size of the dynamometer handle was turning the knob to adjust the grip width so that the second joint of the pointing finger makes a right angle. The patients were ask to stand upright, arm down naturally, clasp the grip with full force, and prevent the grip strength meter and upper limbs from touching the body side, and avoid swinging the grip parameter. Each limbs underwent two trials, and the better value was used for analysis [23].
Cognitive function
Cognitive function was evaluated using the mini-mental state examination (MMSE). The English-version MMSE is the most widely used dementia screening test [24].
In this study, the Japanese version was used. Faithful translation and cultural adaptation to the English version of MMSE was ensured, with confirmed validity and reproducibility. The MMSE was scored on a 30-point scale and comprises 11 items: time orientation, location orientation, immediate and delayed playback of three words, calculation, article designation, sentence repetition, three-step oral instruction, writing instruction, writing, and graphic copying [25].
Physical activity (PA)
The median number of steps and moderate-intensity PA time (≥ 3 metabolic equivalents; METs/min) were measured daily at the hospital from initial ambulation to discharge. The PA meter was measured using a waist-mounted triaxial accelerometer (Mediwalk; TERUMO, Tokyo, Japan). A single investigator instructed the patients in using the accelerometer and ensured that it was working properly and correctly positioned at the patient’s waist pocket in front of the right hip. The patients were instructed to wear the monitor for ≥ 8 h daily. The median number of steps walked and maximum value of moderate-intensity PA time during the in-patient days was calculated and used in the analyses. Once patients were able to walk independently, they were free to choose their level of daily activity.
Acute-phase cardiac hospital rehabilitation
Acute-phase cardiac hospital rehabilitation was performed according to the guidelines for acute and chronic HF [4]. The criteria for starting rehabilitation during hospitalization were as follows: absence of moderate or severe pulmonary congestion and overt low-output syndrome, and stable respiratory and circulatory dynamics on bed rest. The rehabilitation program progressed step-by-step with a multi-disciplinary team with reference to the cardiovascular disease rehabilitation guidelines, including work with a physiotherapist, stretching of eight large joints, resistance training centered on the lower limbs, and a bicycle ergometer for aerobic exercises [11].
Statistical analysis
The non-parametric data were expressed as medians with IQRs and compared using the Mann-Whitney U test. Categorical variables were expressed as numbers with percentages and compared using Chi-square test. A two-sided P-value of < 0.05 was considered statistically significant. All statistical analyses were performed with EZR. EZR is a modified version of R designed to add frequently used statistical functions in biostatistics [26].
Results
Baseline characteristics
Baseline demographics and characteristics are shown in Table 1. Among 70 patients, 29 (41%) developed HAD. Compared to patients without HAD, those with HAD were significantly older (age ≥ 80 years); had higher KCL scores and prevalence of multi-faceted frailty, had longer walking exercise and urinary-catheter-placement period; and had lower handgrip strength, SPPB scores, prevalence of physical frailty at discharge, MMSE scores, and return-to-home rates. In addition, the median daily number of steps and moderate-intensity PA time were related to HAD.
Table 1. Patient Clinical Characteristics.
| All (n = 70) | HAD (n = 29) | Non-HAD (n = 41) | P value | |
|---|---|---|---|---|
| Age, years | 78 (74 - 83) | 81 (78 - 86) | 78 (72 - 83) | 0.034 |
| Sex, female, n (%) | 29 (41) | 14 (48) | 15 (37) | 0.416 |
| BMI, kg/m2 | 22 (20, 25) | 21 (19, 25) | 24 (20, 26) | 0.110 |
| Living alone, n (%) | 24 (34) | 8 (28) | 16 (39) | 0.506 |
| Requiring care, n (%) | 14 (41) | 9 (31) | 5 (12) | 0.101 |
| Etiology, n (%) | ||||
| Ischemic heart disease, n (%) | 19 (27) | 8 (28) | 11 (27) | 0.999 |
| Hypertensive heart disease, n (%) | 25 (36) | 9 (31) | 16 (39) | 0.664 |
| Valvular heart disease, n (%) | 26 (37) | 12 (41) | 14 (34) | 0.714 |
| NYHA class III/IV, n (%) | 26 (37)/44 (63) | 8 (28)/21 (72) | 18 (44)/23 (56) | 0.254 |
| Hypertension, n (%) | 68 (97) | 28 (97) | 40 (98) | 0.999 |
| Dyslipidemia, n (%) | 28 (40) | 14 (48) | 14 (34) | 0.347 |
| Diabetes mellitus, n (%) | 20 (29) | 6 (21) | 14 (34) | 0.338 |
| Atrial fibrillation, n (%) | 26 (37) | 11 (38) | 15 (37) | 0.999 |
| Myocardial infarction, n (%) | 8 (11) | 4 (14) | 4 (10) | 0.887 |
| Charlson Comorbidity Index | 4 (3 - 5) | 4 (3 - 5) | 4 (3 - 5) | 0.950 |
| History of HF, n (%) | 37 (53) | 18 (62) | 19 (48) | 0.340 |
| LVEF, % | 43 (32 - 56) | 43 (36 - 58) | 42 (31 - 54) | 0.349 |
| HFrEF, n (%) | 29 (41) | 10 (35) | 19 (46) | 0.456 |
| HFmEF, n (%) | 15 (21) | 6 (21) | 9 (22) | 0.999 |
| HFpEF, n (%) | 26 (37) | 13 (45) | 13 (33) | 0.429 |
| BNP, pg/dL | 535 (344 - 927) | 538 (393 - 1267) | 527 (265 - 749) | 0.285 |
| Hb, g/dL | 11.8 (10.2- 14.1) | 11.5 (10.3- 12.7) | 11.9 (9.7 - 14.4) | 0.807 |
| eGFR, mL/min/1.73 m2 | 33.8 (25.4 - 45.9) | 32.4 (22.9 - 45.2) | 34.5 (25.9 - 50) | 0.788 |
| CRP, mg/dL | 0.6 (0.1 - 2.9) | 0.8 (0.2 - 2.9) | 0.5 (0.1 - 2.9) | 0.725 |
| Na, mEq/L | 140 (136 - 141) | 138 (138 - 141) | 140 (138 - 141) | 0.107 |
Data are presented as the median (interquartile range). HAD: hospital-acquired disability; BMI: body mass index; NYHA: New York Heart Association Functional Classification; LVEF: left ventricular ejection fraction; HFrEF: HF with reduced ejection fraction; HFmrEF: HF with midrange ejection fraction; HFpEF: HF with preserved ejection fraction; BNP: brain natriuretic peptide; Hb: hemoglobin; eGFR: estimated glomerular filtration rate; CRP: C-reactive protein; Na: serum sodium.
HAD and clinical outcome
Comparison of the baseline characteristics showed that only age (HAD, 81 (78 - 86) years vs. non-HAD, 78 (IQR, 72 - 83) years; P < 0.05) was associated with HAD among older HF patients (Table 1). In addition, patients with HAD had significantly longer urinary-catheter-placement periods (3 days (IQR, 1 - 5] vs. 1 days (IQR, 0 - 2); P < 0.05) and delayed initiation of walking exercise (3 days (IQR, 2 - 4] vs. 2 days (IQR, 1 - 4); P < 0.05) (Table 2).
Table 2. Progress of Acute Treatment.
| All (n = 70) | HAD (n = 29) | Non-HAD (n = 41) | P value | |
|---|---|---|---|---|
| Inotropes use, n (%) | 2 (4) | 2 (7) | 1 (2) | 0.758 |
| IABP, n (%) | 1 (1) | 0 (0) | 1 (2) | 0.999 |
| Ventilator, n (%) | 1 (1) | 1 (3) | 0 (0) | 0.999 |
| NPPV, n (%) | 15 (21) | 8 (28) | 7 (17) | 0.861 |
| CRRT, n (%) | 3 (4) | 2 (7) | 1 (2) | 0.447 |
| Patients admitted to the ICU, n (%) | 9 (13) | 6 (21) | 3 (7) | 0.199 |
| ICU length of stay, days | 0 (0 - 0) | 0 (0 - 0) | 0 (0 - 0) | 0.124 |
| Physical therapy start date | 2 (1 - 3) | 2 (1 - 3) | 1 (1 - 3) | 0.389 |
| Rehabilitation time, min | 180 (100 - 240) | 200 (80 - 260) | 160 (100 - 220) | 0.693 |
| Walking exercise, days | 2 (1 - 4) | 3 (2 - 4) | 2 (1 - 4) | 0.015 |
| Urinary-catheter-placement period, days | 2 (0 - 4) | 3 (1 - 5) | 1 (0 - 2) | 0.019 |
| Intravenous therapy started, days | 4 (2 - 6) | 4 (3 - 6) | 3 (1 - 6) | 0.244 |
| Medication at discharge | ||||
| Beta-blocker, n (%) | 52 (74) | 22 (76) | 30 (73) | 0.999 |
| MRA, n (%) | 36 (37) | 18 (62) | 18 (44) | 0.209 |
| ACEI/ARB, n (%) | 22 (31) | 9 (31) | 13 (32) | 0.999 |
| CCB, n (%) | 19 (27) | 7 (24) | 12 (29) | 0.839 |
| Length of stay, days | 11 (7 - 14) | 12 (7 - 15) | 10 (7 - 14) | 0.470 |
| Return to home rate, n (%) | 61 (87) | 20 (67) | 41 (100) | < 0.01 |
Data are presented as the median (interquartile range). HAD: hospital-acquired disability; IABP: intra-aortic balloon pumping; NPPV: non-invasive positive pressure ventilation; CRRT: continuous renal replacement therapy; ICU: intensive care unit; MRA: mineralocorticoid receptor antagonist; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blocker; CCB: calcium channel blocker.
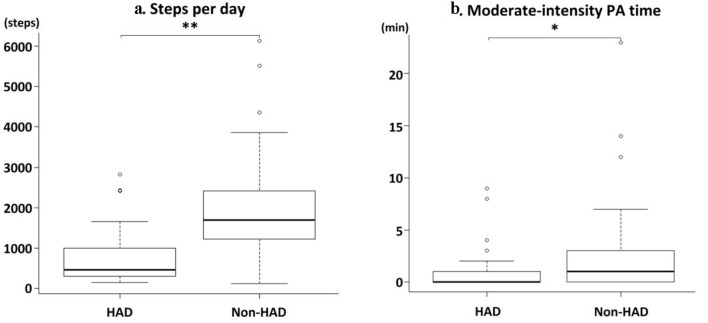
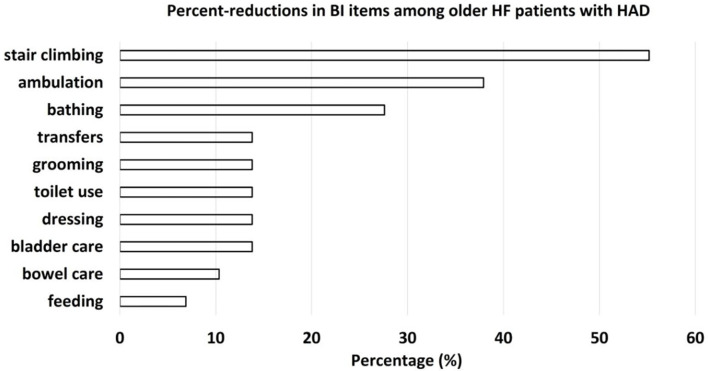
Comparing physical and cognitive activity, patients with HAD had higher KCL scores (14 points (IQR, 11 - 17) vs. 9 points (IQR, 6 - 13); P < 0.01) and prevalence of multi-faceted frailty (90% vs. 29%; P < 0.01), lower SPPB scores (6 points (IQR, 4 - 10) vs. 10 points (IQR, 8 - 12); P < 0.01), prevalence of physical frailty (76% vs. 42%; P < 0.01), grip strength (18 kg (IQR, 15 - 30) vs. 22 kg (IQR, 18 - 30); P < 0.01), MMSE scores (21 points (IQR, 17 - 24) vs. 24 points (IQR, 22 - 27); P < 0.05) (Table 3), number of steps per day (457 steps (IQR, 301 - 997) vs. 1,692 steps (IQR, 1,227 - 2,418); P < 0.01), and moderate-intensity PA time during hospitalization (0 min (IQR, 0 - 2) vs. 1 min (IQR, 0 - 3); P < 0.05) than patients without HAD (Fig. 2). No other significant differences were observed. Investigation of sub-items among participants with lower BI scores showed that patients with HAD showed a reduction of 55.2%, 37.9%, 27.6%, 13.8%, 10.4%, and 6.9% in stair-climbing, ambulation, bathing, hygiene-related activities, bowel control, and feeding, respectively (Fig. 3). HAD caused decreased self-care movements and reduced mobility.
Table 3. Physical Function and Cognitive Function, Physical Activity.
| All (n=70) | HAD (n=29) | Non-HAD (n=41) | P value | |
|---|---|---|---|---|
| Before admission | ||||
| BI, score | 100 (90 - 100) | 95 (90 - 100) | 100 (90 - 100) | 0.050 |
| KCL, score | 12 (7 - 15) | 14 (11 - 17) | 9 (6 - 13) | < 0.01 |
| Multi-faceted frailty, n (%) | 62 (89) | 26 (90) | 36 (29) | < 0.01 |
| At discharge | ||||
| SPPB, score | 9 (6 - 11) | 6 (4 - 10) | 10 (8 - 12) | < 0.01 |
| Physical frailty, n (%) | 39 (58) | 22 (76) | 17 (42) | < 0.01 |
| Handgrip strength, kg | 19 (16 - 26) | 18 (14 - 19) | 22 (18 - 30) | < 0.01 |
| MMSE, score | 26 (21 - 26) | 21 (17 - 24) | 24 (22 - 27) | 0.022 |
| BI, score | 95 (85 - 100) | 85 (70 - 90) | 100 (100 - 100) | < 0.01 |
| PA during hospitalization | ||||
| Steps per day, step | 1,239 (452 - 1,878) | 457 (301 - 997) | 1,692 (1,227 - 2,418) | < 0.01 |
| Moderate-intensity PA time, min | 0 (0 - 1) | 0 (0 - 1) | 1 (0 - 3) | 0.021 |
Data are presented as the median (interquartile range). HAD: hospital-acquired disability; BI: Barthel Index; KCL: Kihon checklist; Multi-faceted frailty: KCL scores ≥ 8; SPPB: Short physical performance battery; Physical frailty: SPPB scores < 10; MMSE: mini-mental state examination; PA: physical activity.
Figure 2.
Comparisons of PA between the HAD and non-HAD groups. Steps per day (a). Moderate-intensity physical activity time (b). *P < 0.05, **P < 0.01. PA: physical activity; HAD: hospital-acquired disability.
Figure 3.
Percent-reductions in BI items among patients with HAD. BI: Barthel Index; HAD: hospital-acquired disability.
Discussion
This study had several strengths. To the best of our knowledge, this is the first to report that HAD among older HF patients was associated with longer urinary-catheter-placement period, and lower physical and cognitive function. Moreover, HAD was associated with lower amounts of PA during hospitalization.
These findings suggest that assessment of functional status and PA during hospitalization is important for risk stratification in older HF patients.
In this study, the incidence of HAD among older HF patients was 41%, and the reduction in daily activities was particularly high. In the recent years, active engagement in rehabilitation from the early stages of hospitalization has been strongly suggested to prevent deconditioning in HF patients [4]. However, HAD is common among older patients. Palleschi et al [9] reported an incidence of HAD of 18-45% among older patients. A recently reported meta-analysis of HAD reported a prevalence of HAD of 30% [27]. In addition to age, disease severity, physical and cognitive decline before hospitalization, and the environment of medical care during hospitalization are important risk factors for HAD [7-9]. HAD is an important issue associated with all-cause mortality and increased risk of readmission among older HF patients [18].
Early active rehabilitation was performed in this study according to the guidelines. The association of the duration of urinary-catheter-placement and the amount of PA with the occurrence of HAD in HF patients suggests that the hospital environment is related to HAD. Therefore, longer catheter retention periods may be associated with low mobility.
The main purpose of urinary-catheter-placement is strict fluid management during acute medical care [28]. In their investigation of HAD-related factors in older patients with severe acute illness, Zisberg et al [29] reported that excretory function during hospitalization was associated with the incidence of HAD. In HF patients, prolonged urinary-catheter-placement may indicate acute treatment or poor excretion control. Our results suggested that while the use of a urinary-catheter-placement enabled proper fluid management, it also reduced opportunities for PA and movement associated with toilet movement. Therefore, a decrease in ADL was observed in BI items related to decreased PA and ability to move. In addition, the median number of steps per day and moderate-intensity PA time during hospitalization were lower in the HAD group than those in the non-HAD group.
Compared to the non-HAD group, there were no significant differences in the rehabilitation start date and rehabilitation implementation time in the HAD group, suggesting that the amount of PA during the period other than rehabilitation was the main factor.
The results of this study similar to several prior studies reported that ≥ 50% of hospitalized among older patients do not walk outside their room except during rehabilitation or medical examinations [30, 31]. Goto et al [32] reported that HAD occurred in several diseases associated with cognitive impairment, frailty, and low physical function. A previous study reported that improvement in ADL is not possible among older patients with frailty, even if early rehabilitation is performed during hospitalization, due to their living environment before hospitalization [33]. Thus, comprehensive frailty evaluation from the early stage of hospitalization is important for understanding the risk of developing HAD.
This study had several limitations. First, the survey period was short, and the number of cases was small; therefore, a detailed examination of the deduction and subordinate items was not possible. Second, the ADLs at discharge were determined based on BI calculated by physiotherapists and nurses, whereas the ADL before admission was based on interviews with the subjects and their families. Therefore, in severe HF patients, it was possible that the evaluation of ADL due to mixed signs of HF led to the results of this study. Additionally, mobility exercises were only physiotherapeutic interventions. It is still unclear whether the results will differ upon addition of other rehabilitation therapies. Further research is needed to determine whether this will help older patients with severe HF to return to baseline ADL levels. It seems optimal exercise progression PA management may be also required to prevent the HAD particularly among older HF patients prolonged hospitalization. This study showed that older and frail HF patients take time to return to baseline physical functions. Thus, HAD cases are needed to return the ADL levels of before admission early through continued rehabilitation after discharge. Finally, we hope that future studies would have a larger sample size and allow more robust analyses of the comorbidities, clinical characteristics, and incidence of HAD among older HF patients.
Conclusions
HAD occurred in 41% of older HF patients. In addition, less PA and a longer urinary-catheter-placement time were associated with HAD.
Acknowledgments
We thank the patients and their families for their understanding and cooperation in the purpose and content of this study. Moreover, we thank our medical staff in Ayase Heart Hospital.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Informed Consent
All patients provided written informed consent.
Author Contributions
YT and MS designed and performed the study. YT, NR, MS, MT, TT, and TF drafted the manuscript and performed critical editing. NR, NY, MS, and IT in Ayase Heart Hospital assisted and supported the sample collection. MS, TM, TT, and TF supervised the manuscript preparation and writing.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
References
- 1. Statistics Bureau, Ministry of Internal Affairs and Communications: elderly population. https://www.stat.go.jp/data/topics/topi1261.html. Retrieved December 1, 2020.
- 2.Okura Y, Ramadan MM, Ohno Y, Mitsuma W, Tanaka K, Ito M, Suzuki K. et al. Impending epidemic: future projection of heart failure in Japan to the year 2055. Circ J. 2008;72(3):489–491. doi: 10.1253/circj.72.489. [DOI] [PubMed] [Google Scholar]
- 3.Tsutsui H, Kousaka S, Saito A, Saito Y, Sakata Y, Matoba T, Mitani Y. et al. The Japanese Circulation Society. Cardiovascular disease medical care survey 2018 report. https://j-circ.or.jp/jittai_chosa/jittai_chosa2018web.pdf. Retrieved December 1, 2020.
- 4.Tsutsui H, Isobe M, Ito H, Saito Y, Sakata Y, Matoba T, Mitani Y. et al. Guidelines for diagnosis and treatment of acute and chronic heart failure (JCS 2017/JHFS 2017). https://www.j-circ.or.jp/old/guideline/pdf/JCS2017_tsutsui_h.pdf. Retrieved December 1, 2020.
- 5.Chiarantini D, Volpato S, Sioulis F, Bartalucci F, Del Bianco L, Mangani I, Pepe G. et al. Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J Card Fail. 2010;16(5):390–395. doi: 10.1016/j.cardfail.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Sze S, Zhang J, Pellicori P, Morgan D, Hoye A, Clark AL. Prognostic value of simple frailty and malnutrition screening tools in patients with acute heart failure due to left ventricular systolic dysfunction. Clin Res Cardiol. 2017;106(7):533–541. doi: 10.1007/s00392-017-1082-5. [DOI] [PubMed] [Google Scholar]
- 7.Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: "She was probably able to ambulate, but I'm not sure". JAMA. 2011;306(16):1782–1793. doi: 10.1001/jama.2011.1556. [DOI] [PubMed] [Google Scholar]
- 8.Hoogerduijn JG, Schuurmans MJ, Duijnstee MS, de Rooij SE, Grypdonck MF. A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs. 2007;16(1):46–57. doi: 10.1111/j.1365-2702.2006.01579.x. [DOI] [PubMed] [Google Scholar]
- 9.Palleschi L, De Alfieri W, Salani B, Fimognari FL, Marsilii A, Pierantozzi A, Di Cioccio L. et al. Functional recovery of elderly patients hospitalized in geriatric and general medicine units. The PROgetto DImissioni in GEriatria Study. J Am Geriatr Soc. 2011;59(2):193–199. doi: 10.1111/j.1532-5415.2010.03239.x. [DOI] [PubMed] [Google Scholar]
- 10.Fimognari FL, Pierantozzi A, De Alfieri W, Salani B, Zuccaro SM, Arone A, Palleschi G. et al. The severity of acute illness and functional trajectories in hospitalized older medical patients. J Gerontol A Biol Sci Med Sci. 2017;72(1):102–108. doi: 10.1093/gerona/glw096. [DOI] [PubMed] [Google Scholar]
- 11.Nohara T, Adati J, Ishihara S, Ito H, Ueshima K, Kimura J, Goto Y. et al. Guidelines for Rehabilitation in Patients with Cardiovascular Disease (JCS 2012). https://www.jcirc.or.jp/old/guideline/pdf/JCS2012_nohara_h.pdf. Retrieved December 1, 2020.
- 12.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 13.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–65. doi: 10.1037/t02366-000. [DOI] [PubMed] [Google Scholar]
- 14.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L. et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres Moreno B, Nunez Gonzalez E, Perez Hernandez Dde G, Simon Turriate JP, Alastuey Gimenez C, Diaz Melian J, Corujo Rodriguez E. et al. [Barthel and Charlson indexes for the prognosis of mortality and institutionalization in hospitalized geriatric patients] Rev Esp Geriatr Gerontol. 2009;44(4):209–212. doi: 10.1016/j.regg.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Sainsbury A, Seebass G, Bansal A, Young JB. Reliability of the Barthel Index when used with older people. Age Ageing. 2005;34(3):228–232. doi: 10.1093/ageing/afi063. [DOI] [PubMed] [Google Scholar]
- 17.Rossello X, Miro O, Llorens P, Jacob J, Herrero-Puente P, Gil V, Rizzi MA. et al. Effect of barthel index on the risk of thirty-day mortality in patients with acute heart failure attending the emergency department: a cohort study of nine thousand ninety-eight patients from the epidemiology of acute heart failure in emergency departments registry. Ann Emerg Med. 2019;73(6):589–598. doi: 10.1016/j.annemergmed.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Saitoh M, Takahashi Y, Okamura D, Akiho M, Suzuki H, Noguchi N, Yamaguchi Y. et al. Prognostic impact of hospital-acquired disability in elderly patients with heart failure. ESC Heart Fail. 2021;8(3):1767–1774. doi: 10.1002/ehf2.13356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki T. Manual on life function evaluation for care prevention (Revised Edition). https://www.mhlw.go.jp/topics/2009/05/dl/tp0501-1c.pdf. Retrieved December 1, 2020.
- 20.Satake S, Shimada H, Yamada M, Kim H, Yoshida H, Gondo Y, Matsubayashi K. et al. Prevalence of frailty among community-dwellers and outpatients in Japan as defined by the Japanese version of the Cardiovascular Health Study criteria. Geriatr Gerontol Int. 2017;17(12):2629–2634. doi: 10.1111/ggi.13129. [DOI] [PubMed] [Google Scholar]
- 21.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S. et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–231. doi: 10.1093/gerona/55.4.M221. [DOI] [PubMed] [Google Scholar]
- 22.Pavasini R, Guralnik J, Brown JC, di Bari M, Cesari M, Landi F, Vaes B. et al. Short Physical Performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med. 2016;14(1):215. doi: 10.1186/s12916-016-0763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ministry of Education, Culture Sports, Science and Technology. New Physical Fitness Test Implementation Guidelines. https://www.mext.go.jp/a_menu/sports/stamina/03040901.html. Retrieved December 1, 2020.
- 24.Perneczky R, Wagenpfeil S, Komossa K, Grimmer T, Diehl J, Kurz A. Mapping scores onto stages: mini-mental state examination and clinical dementia rating. Am J Geriatr Psychiatry. 2006;14(2):139–144. doi: 10.1097/01.JGP.0000192478.82189.a8. [DOI] [PubMed] [Google Scholar]
- 25.Sugishita M, Koshizuka Y, Sudou S, Sugishita K, Hemmi I, Karasawa H, Ihara M. et al. The validity and reliability of the Japanese version of the Mini-Mental State Examination (MMSE-J) with the original procedure of the Attention and Calculation Task (2001) Cognitive Neuroscience. 2018;20(2):91–110. [Google Scholar]
- 26.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loyd C, Markland AD, Zhang Y, Fowler M, Harper S, Wright NC, Carter CS. et al. Prevalence of hospital-associated disability in older adults: a meta-analysis. J Am Med Dir Assoc. 2020;21(4):455–461. doi: 10.1016/j.jamda.2019.09.015. e455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yano K. Guideline for prevention of catheter associated urinary tract infections, 2009. Centers for Disease Control and Prevention (CDC). https://www.info-cdcwatch.jp/views/pdf/CDC_guideline2009.pdf. Retrieved December 1, 2020.
- 29.Zisberg A, Shadmi E, Gur-Yaish N, Tonkikh O, Sinoff G. Hospital-associated functional decline: the role of hospitalization processes beyond individual risk factors. J Am Geriatr Soc. 2015;63(1):55–62. doi: 10.1111/jgs.13193. [DOI] [PubMed] [Google Scholar]
- 30.Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52(8):1263–1270. doi: 10.1111/j.1532-5415.2004.52354.x. [DOI] [PubMed] [Google Scholar]
- 31.Van Grootven B, Jeuris A, Jonckers M, Devriendt E, Dierckx de Casterle B, Dubois C, Fagard K. et al. Predicting hospitalisation-associated functional decline in older patients admitted to a cardiac care unit with cardiovascular disease: a prospective cohort study. BMC Geriatr. 2020;20(1):112. doi: 10.1186/s12877-020-01510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goto R, Watanabe H, Tsutsumi M, Kanamori T, Maeno T, Yanagi H. Factors associated with the recovery of activities of daily living after hospitalization for acute medical illness: a prospective cohort study. J Phys Ther Sci. 2016;28(10):2763–2768. doi: 10.1589/jpts.28.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldfarb M, Afilalo J, Chan A, Herscovici R, Cercek B. Early mobility in frail and non-frail older adults admitted to the cardiovascular intensive care unit. J Crit Care. 2018;47:9–14. doi: 10.1016/j.jcrc.2018.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.