Abstract
Background:
Food allergy (FA) is a growing global problem and can affect patients’ health related quality of life (HRQoL) due to increased anxiety as well as social and economic restrictions. Interventions such as oral food challenges (OFCs) and oral immunotherapy (OIT) have been shown to improve HRQoL, however, meta-analysis and systematic synthesis of these data are lacking.
Objective:
The objective of this study was to systematically review and quantitatively synthesize potential benefits of interventions (OIT and OFC) for addressing FA to a variety of foods.
Methods:
We conducted a systematic search through PubMed and Cochrane Medical Library databases and performed a meta-analysis focusing on studies assessing changes in HRQoL after OIT and/or OFCs in FA participants and caregivers from 2010 to July 2020. Random effects model and I2 statistics were used to assess the overall intervention effects and heterogeneity across studies.
Results:
We included 13 publications in this meta-analysis (OIT=7, OFCs=6). The mean change of HRQoL scores after OIT and OFCs were −1.25 (P<0.001) and −0.78 (P=0.052), with significant I2 of 87% (P<0.001) and 90% (P<0.001), respectively. Five OIT studies found significant improvements in HRQoL in the OIT group compared to the placebo group with an overall standardized mean difference of −0.56 (P=0.007; I2=42%, P=0.099).
Conclusion:
This meta-analysis showed that in FA patients, both OIT and OFCs are associated with an improvement in HRQoL. Well-designed and long-term HRQoL studies are necessary to ascertain sustained benefits of OIT and OFCs.
Keywords: Food allergy, oral immunotherapy, oral food challenge, quality of life questionnaires, meta-analysis
Introduction
Food Allergy (FA) is a public health burden affecting personal, social, nutritional and economic aspects of one’s life. Its prevalence is growing in the western world1, currently affecting about 6% of children and 10% of adults in the United States2. Currently, the only approved FA treatment is Palforzia3; for children aged 4–17 years old with peanut allergy; the standard of care for patients with other FA remains dietary avoidance of the implicated food allergen and administration of rescue medicine in case of accidental allergen exposure4,5. Dietary avoidance can be challenging and approximately 40% of patients with FAs present with reactions, ranging from mild allergic reactions and, in very rare cases, to fatal anaphylaxis, upon accidental exposure, even on very minimal exposures each year.6, 7 Studies suggest that higher levels of anxiety and stress are often found in FA patients and their caretakers, with resultant negative effects on health-related quality of life (HRQoL)8,9.
Although HRQoL and Quality of Life (QoL) are commonly used interchangeably in the literature, they represent two distinct constructs.10 QoL is a broad, multidimensional concept which covers all aspect of one’s life: physical wellbeing, material wellbeing, social wellbeing, emotional wellbeing, and development and activity. HRQoL measures disease-specific QoL (e.g., burden of treatment, symptoms, etc.), and it is a patient- and caregiver-perspective multidimensional questionnaire that evaluates physical, psychological and social aspects that may be impacted by a disease or medical condition.11 Awareness of risk of severe allergic reactions (including rare cases of fatal anaphylaxis) leads to anxiety and stress among FA patients and their caretakers12. Strategies for avoiding ingestion of allergens can prove burdensome to families (e.g., buying special foods, limiting social encounters, work and school absenteeism, changing careers, and frequent emergency room visits) and can lead to psychological distress (e.g., depression, anxiety, and social stress) 13. The Food Allergen Labelling and Consumer Protection Act (FALCPA) requires that every FDA-regulated packaged food labeled on or after January 1, 2006, lists the major allergens it contains (e.g., milk, eggs, fish, shellfish, peanuts, tree nuts, wheat, and soy); however, FALCPA does not apply to restaurant foods, which do not require precautionary “may contain” statements14. The overall HRQoL for FA patients and their caretakers has been found to be significantly impaired, even more than in other chronic childhood diseases, such asType-1 diabetes mellitus 15.
Longitudinal HRQoL changes in FA have been evaluated by numerous studies after allergen-specific oral immunotherapy (OIT) or after oral food challenge (OFC) and many of these studies have found substantial improvements in participants’ HRQoL after either of these interventions. Carraro et al. 16 found a significant improvement of HRQoL after OIT in milk-allergic children. A randomized controlled study by Reier-Nilsen et al.17 reported that both children’s self-reported and parental proxy-reported HRQoL scores significantly improved in the OIT-group at two years compared to baseline. In addition, DunnGalvin et al.18 assessed the longitudinal effect of OFC in caregivers of FA-children. The results showed a rapid improvement in caregivers’ HRQoL, irrespective of the outcome of the challenge.
Although a substantial number of published studies have reported improvements in HRQoL after OIT and OFC, in the field of FA immunotherapy, there is a lack of rigorous HRQoL data and quantitative synthesis.19, 20 Therefore, the primary objective of our study is to provide a systematic review and quantitative synthesis of published studies to identify the potential HRQoL benefits of OFCs for diagnosing and OIT as a therapeutic option for food allergic individuals.
Methods
Searching strategy
We performed a systematic search using several online publication databases, including PubMed, Cochrane Medical Library and Stanford Lane Medical Library. We limited our search to articles in English and did not restrict studies based on age or study type. Keywords such as “food allergies” and “quality of life questionnaires” were used. We focused on clinical studies conducted in children, adolescents, adults, and caregivers of patients with FA to milk, eggs, peanuts, tree nuts or other foods confirmed by positive skin prick, specific-IgE, and/or food challenge tests. Cochrane definitions and criteria were used to identify randomized clinical trials. This study focused on changes in HRQoL of patients and their caregivers (when evaluated) after allergen-specific immunotherapy and/or OFC. We restricted publications from 2010 to July 2020, prior to our manuscript preparation. Three reviewers independently scanned the literature, extracted the data, and met to review the studies that were included in this analysis, and achieved consensus. The senior author made the final decision on the inclusion of studies if there was any disagreement.
Outcome assessment
We analyzed both longitudinal prospective cohort studies and randomized clinical trials in which HRQoL was assessed with FA specific questionnaires (FAQLQ-PF, FAQLQ-PB, FAQLQ-CF, FAQLQ-AF, and FAQLQ-TF, children allergy-specific HRQoL by Avery). Our outcomes were to: i) evaluate any change in HRQoL scores in longitudinal studies from baseline to follow-up visit after the intervention, ii) compare the change in HRQoL scores from baseline between the active treatment and placebo groups in placebo-controlled studies.
Statistical analysis
HRQoL scores for each study were collected and summarized by time point and/or by treatment groups as mean difference (MD) and standard deviation (SD) of the difference. For the studies that only reported the median, range and sample size, we estimated the mean and SD using the formulas introduced by Hozo et al.21 and Wan et al.22 If the SD of the difference was not available from the publications, we imputed the missing SD difference using the methods introduced by Follmann et al.23 and Abrams et al.24 We followed the Cochrane Handbook for Systematic Reviews of Interventions version 5.1.025 for the statistical analysis. The effect of intervention on the HRQoL scores across studies was evaluated using meta-analysis approach implemented in the R software version 3.6 packages “meta” and “metaphor.” The random effect model was performed to quantify the average intervention effects using the inverse-variance approach. We also analyzed heterogeneity of effects across all studies using the I2 statistic, which describes the percentage of variation across studies that is due to heterogeneity rather than to chance.
Results
Study overview
Our online database searches resulted in 979 publications. After removal of duplicate publications, we screened 946 publications through titles and/or abstracts, reviewed 32 in full-text, and included 13 publications in our meta-analysis (Figure 1). The characteristics of each study are summarized in Table 1 and Table 2. Seven studies were focused on the HRQoL changes before and after OIT (Table 1) and 6 were focused on the food challenge (Table 2).
Figure 1.
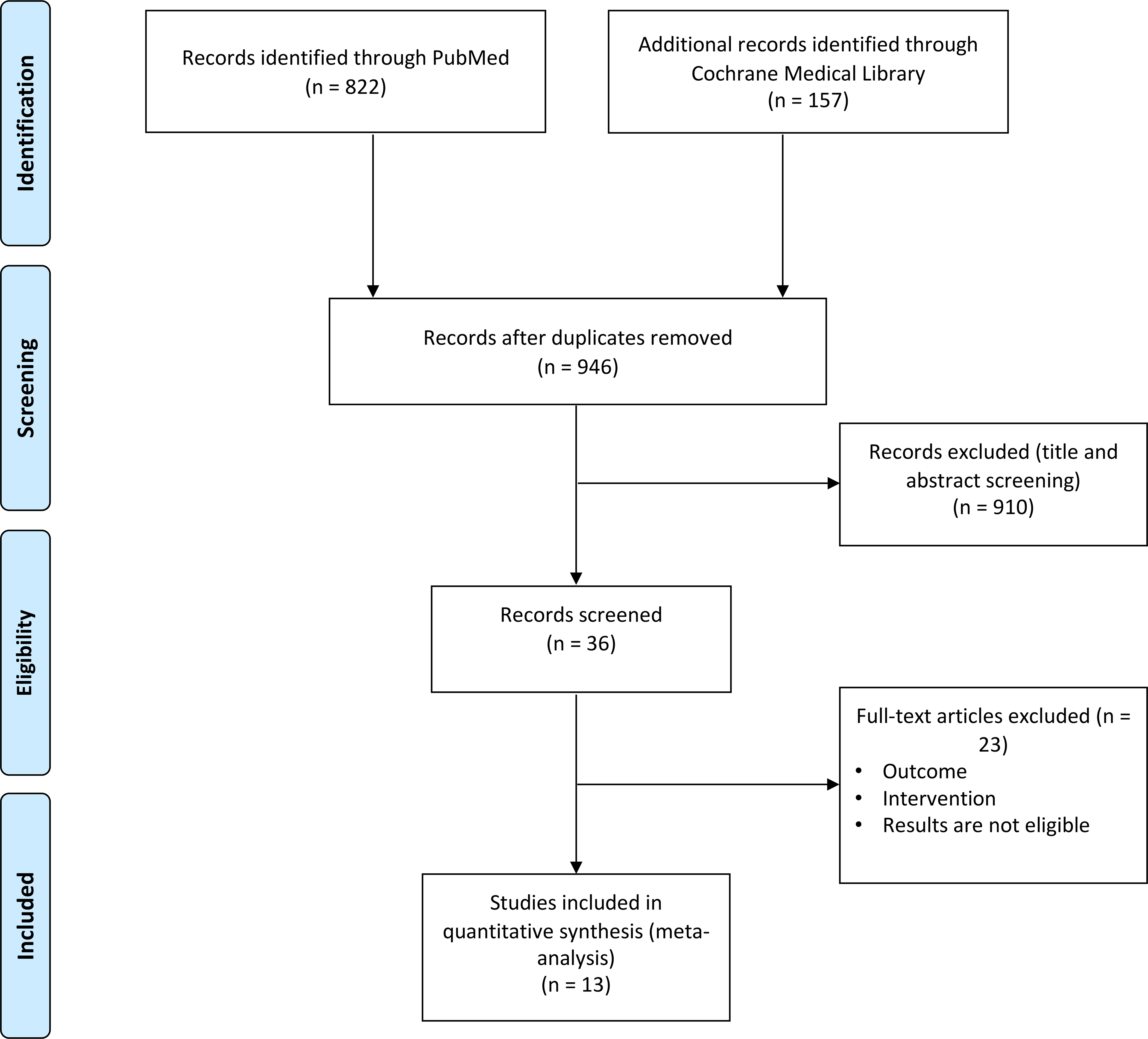
PRISMA flow diagram.
Table 1.
Characteristics of publications focused on OIT
| Publication | Study design | Participants (active group) | Food | Intervention | HRQoL form | Endpoints | Placebo arm | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Anagnostou, 2014 42 | Randomized controlled trial (UK) | 39 children 7–16 years old | Peanut | OIT | FAQLQ-PF | FAQLQ-PF scores pre- and post-OIT | Yes | Both active and controls groups showed clinical meaningful improvement in FAQLQ-PF overall scores post treatment. |
| Blumchen, 2019 38 | Randomized controlled trial (Germany) | 20 children 3–17 years old | Peanut | OIT | FAQLQ-PF FAQLQ-CF |
HRQoL scores pre- and post-OIT | Yes | Significant improvement on both FAQLQ-PF and FAQLQ-CF in the active OIT group 10 weeks after final OFC, not in the placebo group. |
| Carraro, 2012 16 | Prospective cohort study (Italy) | 30 children 0–12 years old | Milk | OIT | FAQLQ-PF | FAQLQ-PF scores pre- and post-OIT | No | Significant improvement on FAQLQ-PF scores and in each domain 2 months after OIT in all age groups. |
| Dunn Galvin, 2018 11 | Randomized controlled trial (Ireland) | 20 children 2–11 years old | Peanut | OIT | FAQLQ-PF FAIM |
FAQLQ-PF and FAIM scores pre- and post-OIT | Yes | Significant improvement on FAQLQ-PF and FAIM scores 3 and 12 months after OIT. No change for FAQLQ-PF in the placebo group. Furthermore, significant improvement was reported from 3-month to 12-month post OIT. |
| Epstein-Rigbi, 2018 35 | Prospective cohort study (Israel) | 175 children 4–12 years old | Milk, peanut, egg, sesame, or tree nuts | OIT | FAQLQ-PF | FAQLQ-PF scores pre- and post-OIT | Yes (data not shown) | Significant improvement on FAQLQ-PF overall scores and each domain in the OIT group from the start to the end of treatment. No changes on HRQoL scores in the control group. |
| Otani, 2014 37 | Two phase I clinical trials (USA) | 40 children 4–16 years old | Peanut, walnut, cashew, pecan, milk, egg, sesame, almond, hazelnut | OIT | FAQLQ-PB | FAQLQ-PB scores pre- and post-OIT | Yes | Significant improvement on FAQLQ-PB scores in the active OIT groups at 6-month and 18-month follow-up. HRQoL scores in the control group significantly worsened at 6-month follow-up. No changes on HRQoL scores in the control group at 18-month follow-up. |
| Reier- Nilsen, 2019 17 | Randomized controlled trial (Norway) | 37 children 5–15 years old | Peanut | OIT | PedsQL 4.0 FAQLQ-PB |
HRQoL scores pre- and post-OIT | Yes | Significant improvement in children and parents after up-dosing and maintenance phases in both OIT and control groups. The change in HRQoL was significantly different from the controls for the parental proxy-reports only |
Table 2.
Characteristics of publications focused on food challenge
| Publication | Study design | Participants (active group) | Food | Intervention | HRQoL form | Endpoints | Placebo arm | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Dunn Galvin, 2010 18 | Prospective cohort study (Ireland) | 82 children 0–12 years old | Peanut, nut, milk, egg, fish/shellfish | OFC | FAQLQ-PF FAIM |
FAQLQ-PF and FAIM scores pre- and post-OFC | No | Significant improvement in FAQLQ-PF total scores and each domain and FAIM 6 months after OFC. |
| Hourihane, 2017 43 | Prospective cohort study (Ireland, Australia, US) | 378 children 1–18 years old | Peanut | OFC | FAQLQ-PF FAQLQ-CF |
FAQLQ-PF and CF scores pre- and post- OFC | No | Significant improvement in FAQLQ-PF and CF 1 month after the single-dose OFC, irrespective of the OFC outcome. |
| Knibb, 2012 40 | Prospective cohort study (UK) | 31 children 6–16 years old | Peanut, tree nut | OFC | FAQLQ-PB FA-specific HRQoL by Avery PredsQL 4.0 |
HRQoL scores for children and parents pre- and post- OFC | Yes | Significant improvement of related-food HRQoL in children and mother 3–6 months following OFC. No improvement in the control group. |
| Soller, 2014 44 | Prospective cohort study (Ireland) | 54 children 0–12 years old | Peanut, tree nut, milk, egg, wheat, sesame | OFC | FAQLQ-PF | FAQLQ-PF scores pre- and post- OFC | No | FAHRQoL improved significantly from 2 months prechallenge to 2 months postchallenge for both groups, but began to decrease at 6 months postchallenge in allergic patients. |
| van der Valk, 2016 39 | Prospective cohort study (Netherlands) | 84 parents 33 children 2–17 years old 26 teanagers |
Cachew | DBPCFC | FAQLQ-PF FAQLQ-CF FAQLQ-TF FAIM |
HRQoL scores pre- and post- DBPCFC for each age group | No | No significant improment in FAQLQ-PF, FAQLQ-CF and FAQLQ-TF total and domain scores 6 months after the OFC compared to baseline. |
| van der Velde, 2012 45 | Prospective cohort study (Netherlands) | 53 adults ≥18 years old; 46 adolescents 13–17 years old; 57 children 8–12 years old |
peanut, nut, milk, egg, wheat, soy, sesame | DBPCFC | FAQLQ-AF FAQLQ-TF FAQLQ-CF FAIM |
HRQoL scores pre- and post- DBPCFC for each age group | Yes | Significant improvement in FAQLQ-AF and FAQLQ-CF in OFC group 6 months after challenge, irrespective of the OFC outcome. Significant improvement was found in FAQLQ-TF in participants with negative OFC outcome, and in the control group. |
Instruments used for Food Allergy Quality of Life Questionnaire (FAQLQ)
HRQoL questionnaires vary widely among studies. Questionnaires can be designed for completion by FA patients or by their caregivers. Data are collected relative to FA patient HRQoL (by age group) or relative to caregiver HRQoL. A summary of the various questionnaires is detailed below and in Table 3.
Table 3.
Summary of main questionnaire.
| Name | Target population | Respondent | Number of questions |
|---|---|---|---|
| FAQLQ – PF | Children 0–12 years old | Caregiver | 14 – 25 – 30 |
| FAQLQ - CF | Children 8–12 years old | Children 8–12 years old | 24 |
| FAQLQ – TF | Teenagers 13–17 years old | Teenagers 13–17 years old | 28 |
| FAQLQ – AF | Adult over 18 years old | Adult over 18 years old | 29 |
| FAQLQ – PB | Caregivers | Caregivers | 17 |
| FAIM | Patients | Patients | 6 |
| PedsQL 4.0 | Children (5–18) and parents | Children (5–18) and parents | 13 |
| FA-specific HRQoL by Avery | Children 8–17 years old | Children 8–17 years old | 25 |
The FAQLQ-PB (Parental Burden Form). This questionnaire was validated in 2004 and is designed to measure the parental burden associated with having a child (0 to 12 years old) with FAs. The questionnaire presents three different domains: emotional impact, food anxiety, and social and dietary limitations.26
The FAQLQ-PF (Parent Form) is a disease-specific HRQoL questionnaire presented to caregivers with excellent validity and reliability regarding patient HRQoL. It has been identified by the European Academy of Allergy and Clinical Immunology (EAACI) as the preferred tool for HRQoL-assessment of FA-patients aged between 0 and 8 years-old, but this can be extended up to 12 years of age. The number of items on the questionnaire varies with age: 14 items (0–3 years), 25 items (4–6 years) or 30 items (7–12 years) with a response scale ranging from 0 (minimal impairment in HRQoL) to 6 (maximal impairment in HRQoL).27
The FAQLQ-CF (Child Form) is completed by children themselves if they are between 8 and 12 years old and is comprised of 24 items with possible answers ranging on a 7-point-scale from not at all (1) to extremely (7). As in the -PF, a higher score means higher burden/poorer HRQoL and lower score reflects lower burden/better HRQoL.28
The FAQLQ-TF (Teenage Form) is the self-reported adolescent version of FAQLQ and targets patients between 13 to 17 years-old. Patients are asked to answer 28 questions.29
The FAQLQ-AF (Adult Form) was developed and validated in the Netherlands and addresses patients older than 18 years old. Every patient is asked to answer 29 items using a 7-point response scale.30
In addition to the main instruments listed above, additional tools were also implemented in many FA studies. Food Allergy Independent Measure (FAIM) is a tool used to evaluate the participant’s perceived chance of accidental exposure to allergens and perception of disease severity.31 Pediatric Quality of Life Inventory Version 4.0 (PedsQL 4.0) applies a 5-point Likert-scale (from 0 = never to 4 = almost always) for children and teenagers between 8–18 years, and a simplified 3-point scale to children between 5– 7-years. It consists of 13 items that can be answered with a reverse score scale with higher scores representing a better quality of life (0 = 100, 1 = 75, 2 = 50, 3 = 25, 4 = 0).32 Additionally, the HRQoL by Avery et al. is a non-validated 25 item self-report measure designed for peanut allergic children aged 8 and older.33 Children are asked to rate the frequencies of items on the questionnaire using a Likert scale, ranging from never (scored 1) to always (scored 4); scores range from 25 to 100 with a higher score indicating a poorer HRQoL.34
In 2014, the EAACI provided guidelines regarding the correct questionnaire to assess patient or caregiver HRQoL based on the patient’s age.27 It assesses three general domains, such as general emotional impact, food anxiety, and social and dietary limitations. The total score is calculated as the mean of the items. The purpose of the questionnaire, used mainly in clinical research, is to determine whether interventions have a benefit for the patient. In order to demonstrate a longitudinal effect, researchers look for a minimal clinically important difference (MCID) for the instrument used. The MCID is intended as the smallest increment of difference in the HRQoL score that patients find clinically meaningful. To date, there is not an established and validated MCID value, but most papers use a difference of greater than 0.5 point as a cut-off for significance.11,35–37
Results from OIT Studies
Among the OIT studies, 4 studies were randomized clinical trials, 2 studies were prospective cohort studies, and 1 study was combined with two phase I clinical trials (Table 1). A total of 361 children from 0 to 17 years old received active OIT treatment and had available longitudinal HRQoL results. Six studies investigated FA to peanut and included the comparisons between the active OIT and placebo-control arms; 2 of them were multi-FA studies. All of these studies reported FAQLQ-PF or FAQLQ-PB; one study also reported FAQLQ-CF. The follow-up time ranged from 1 month to 24 months post OIT.
All of the OIT studies reported a significant improvement of participants’ HRQoL scores for parent forms, parental burden forms, and child forms at the time of follow-up compared to baseline HRQoL scores, with overall mean changes of −1.25 (95% confidence interval [CI]: −1.77, −0.72; p<0.001) (Figure 2). High heterogeneity was also identified between studies (I2 =87%, p<0.001). Three studies reported HRQoL at different follow-up time points. Dunn Galvin 10 found a continuous improvement in FAQLQ-PF score from baseline to 3-month and 12-month follow-up (MD of 3-month and 12-month post-treatment compared to baseline: −0.80 vs −1.30, respectively). A similar effect was also found by Otani 37 (MD of 6-month and 18-month follow-up compared to baseline: −2.09 vs −2.67, respectively). In addition, Blumchen 38 reported higher improvement in CF scores compared to PF scores, which were completed after a median of 9.5 weeks and 11 weeks after the final OIT visit, respectively (MD: −1.4 vs −0.54). Six studies compared the effects of OIT between the active OIT group and the placebo group; 5 of them reported the available results. Two hundred and seventy-six (276) participants were included and reported HRQoL scores: One hundred and fifty-six (57%) were enrolled in the active OIT group. All of these studies found higher improvement of HRQoL scores in the active OIT group compared to the placebo group, with an overall standardized mean difference (SMD) of −0.56 (95% CI: −0.92, −0.20, p=0.007) (Figure 3). Studies with different follow-up time points were also included in this analysis, however, only Dunn Galvin 11 reported a higher SMD at the 12-month follow-up time point compared to the 3-month time point (−0.77 vs −0.23). The heterogeneity among these studies was not significant when comparing the active OIT group and the placebo group (I2=42%, p=0.099).
Figure 2. Forest plot of meta-analysis for studies focused on OIT.
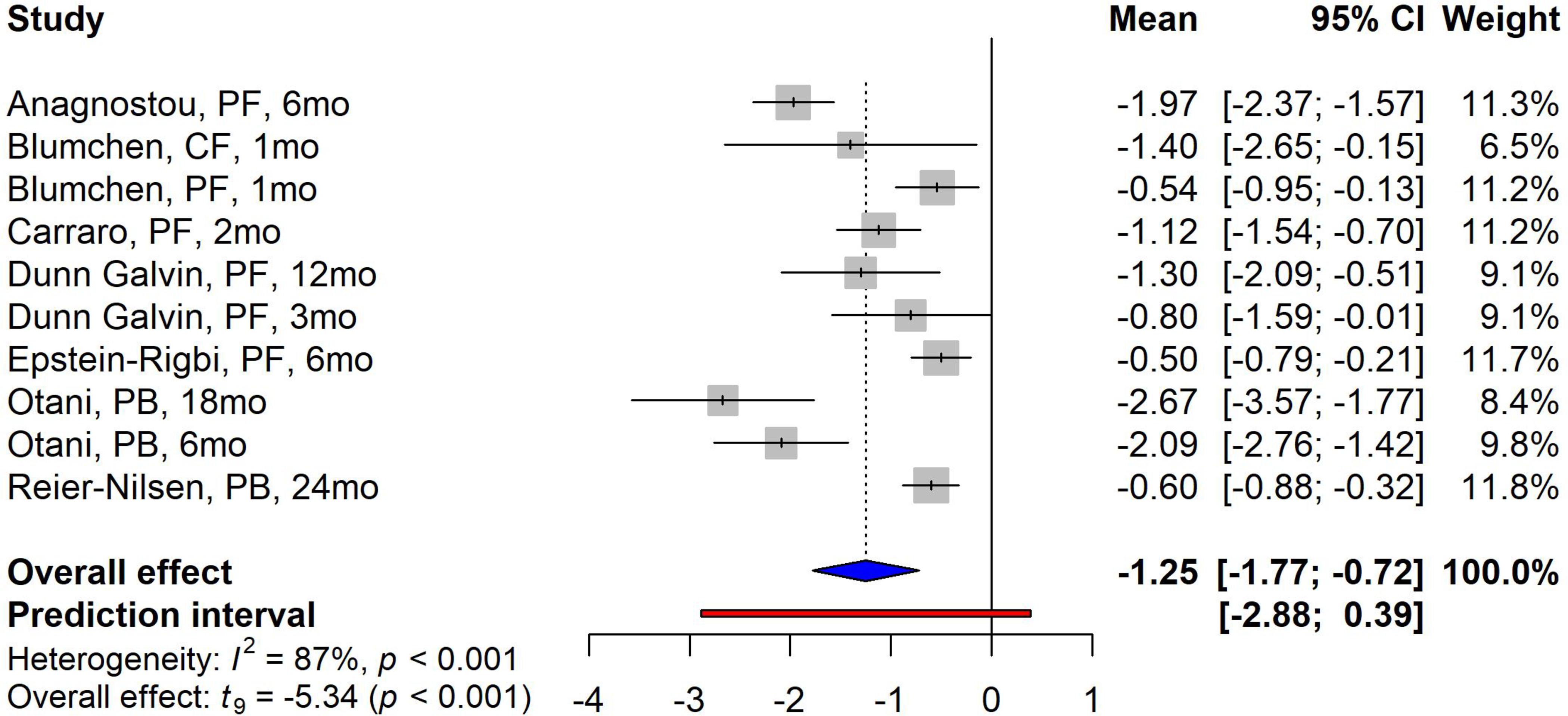
Study label includes first author’s last name, questionnaire forms, and follow-up time in months/weeks. PF=parental form, CF=child form, PB=parental burden form.
Figure 3. Forest plot of meta-analysis for studies comparing active OIT group and placebo group.
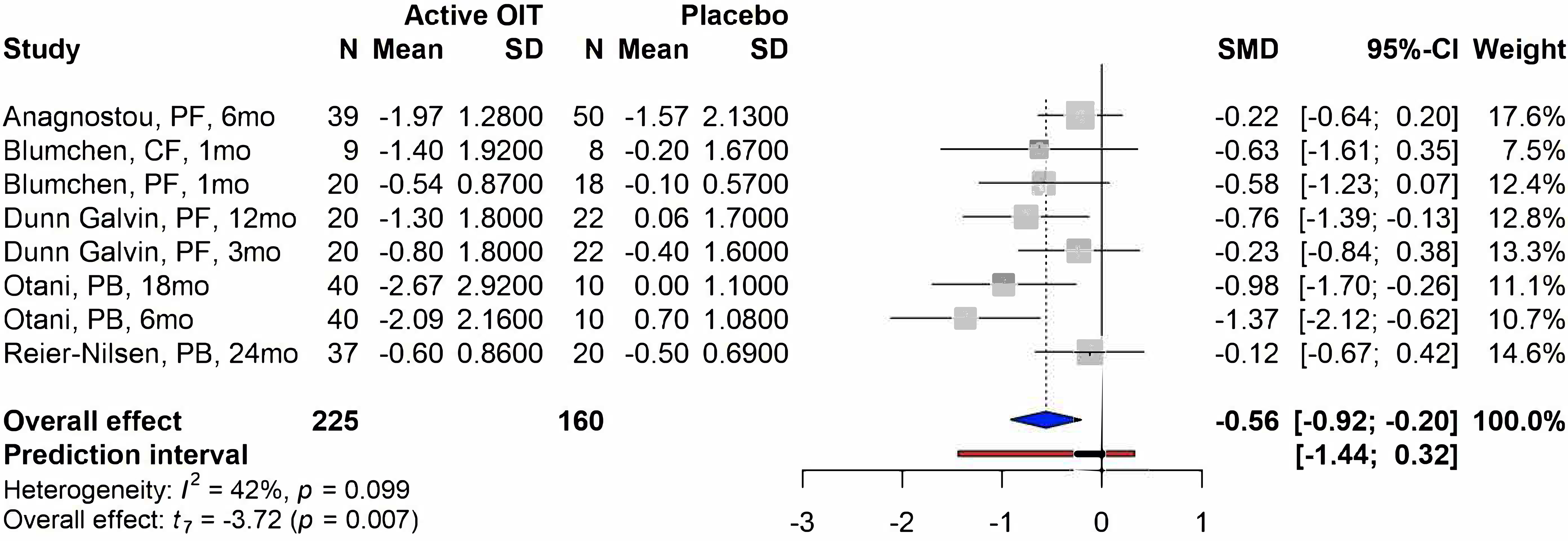
Study label includes first author’s last name, questionnaire forms, and follow-up time in months/weeks. PF=parental form, CF=child form, PB=parental burden form, SD=standard deviation, SMD=standardized mean difference.
Results from OFC studies
All of the 6 studies that investigated effects of food challenges were prospective cohort studies (total of 760 enrolled children and adults) reporting available HRQoL score changes before and after the food challenges. Four studies were multi-FA studies. Only one study included a placebo-controlled arm. The follow-up time for these studies ranged between 1 to 6 months after the food challenges, which was shorter than that of the OIT studies.
Five trials reported a significant improvement at the time of follow-up; the overall effect showing a trend towards improvement of the HRQoL scores after OFC (overall mean change: − 0.78, 95% CI: −1.56, 0.01, p=0.052) (Figure 4). Van der Valk 39 reported worse HRQoL scores at the 6-month follow-up time for child forms (0.17, 95% CI: −0.31, 0.65) and parent forms (0.06, 95% CI: −0.13, 0.25), whereas the scores improved for teenage forms (−0.28, 95% CI: − 0.78, 0.22). These studies also showed a significant heterogeneity with I2 of 44% (p<0.001). When comparing results between challenge and non-challenge groups, Knibb 40 found no change of HRQoL scores in the non-challenge group.
Figure 4. Forest plot of meta-analysis for studies focused on the OFC.
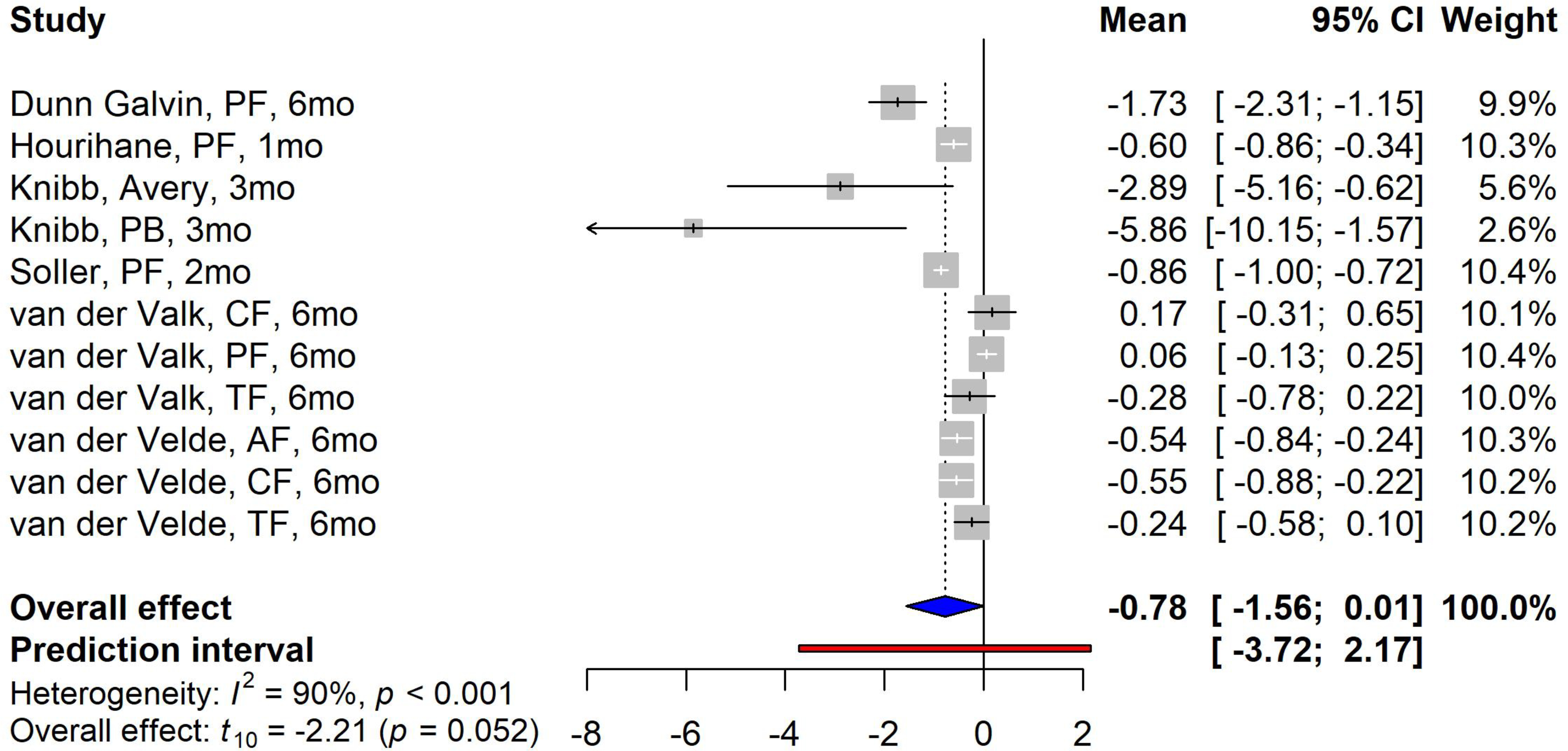
Study label includes first author’s last name, questionnaire forms, and follow-up time in months/weeks. PF=parental form, CF=child form, PB=parental burden form, TF=teenager form, AF=adult form, Avery=the food allergy specific QoL scale by Avery et al.
Among these 13 studies, the FAQLQ-PB form used in the Otani37 study showed higher improvements after the intervention compared to other forms. This study reported the highest HRQoL score change with a mean difference of −2.67 at 18-month follow-up and −2.09 at the 6-month follow-up. They also reported a higher SMD on comparing the active OIT group with the placebo group (SMD: −0.98 and −1.37 at 18-month follow-up and 6-month follow-up, respectively). In the OFC studies, Knibb 40 reported the FAQL-PB that showed the most significant improvement after the food challenge with a mean change of −5.86 (95% CI: −10.15, − 1.57).
Discussion
This study provides solid evidence evaluating the effect of clinical interventions, such as OIT and OFC, on participants’ and caregiver’s HRQoL assessed with age-specific iterations of the FAQoL Questionnaires. Over 1000 participants with FA enrolled in 13 studies were included in this systematic review and meta-analysis.
All OIT studies showed a significant improvement on HRQoL scores after OIT compared to baseline (p<0.0001). Additionally, on evaluating placebo-controlled studies, we found that participants who received active OIT had a significantly higher improvement on HRQoL scores compared to the placebo groups (p=0.007). Caregivers of pediatric participants also reported an improvement in QoL after OIT. These results demonstrate that OIT can be a promising intervention to reduce the psychosocial burden of FA for patients, caregivers, and families. This meta-analysis also shows a trend of improved HRQoL after OFC, although the overall effect did not reach statistical significance at the .05 level (p=0.052). A possible reason for this is the favorable HRQoL at baseline, when a caregiver considers an allergy to a specific food to be relatively easily managed (e.g., allergen is found relatively infrequently in packaged or restaurant foods). Therefore, the absolute increase in HRQoL following OFC will be relatively small compared to a patient or caregiver with very low HRQoL before OFC. Two studies support this assertion and posit that HRQoL may depend on the allergen. The study by van der Valk 39 reported that the lack of improvement of HRQoL after cashew OFC was probably due to a relatively good baseline HRQoL compared to other studies, which corresponded to the relatively benign perception of participants on the severity of their FA. The study by Warren 41 demonstrated that the caregivers of children with milk or egg allergy experienced a poorer HRQoL compared to caregivers of children with peanut or tree nut allergy. This may be because dietary avoidance of cashew is relatively easy to manage in some geographic areas, compared to milk or egg, and the risk of accidental ingestion of cashew is relatively low. As this study included results from multiple allergens, HRQoL may have been significantly improved for certain allergens and allergen combinations, but not others. Future studies of HRQoL in FA should recruit robust samples for subgroup analyses to determine whether response to OFC or OIT varies by allergen or is moderated by perceived severity of allergen(s).
A notable limitation of this meta-analysis is the high heterogeneities between studies, which is likely due to the different study designs, sample sizes, questionnaires, and follow-up intervals after treatment reported across studies. The varying age-specific iterations of FA HRQoL measures for patients and their caregivers may be a reason for the dearth of systematic syntheses for these data, as the many different assessments add difficulty for comparison. Even so, it is important to consider the differing effects of FA for individuals based on activities common to their age group. Among all these questionnaires, the parental burden form showed the most significant improvement after both OIT and OFC compared to the baseline scores. Additionally, it showed the highest difference between the active group and the placebo group. This may be attributable to parents of pediatric patients being more involved in their children’s meal and social activity planning than the children themselves and, thus, more cognizant of the burden of FA than their children, making them more attuned to HRQoL changes. Lastly, the follow-up period after the treatment interventions varied across studies, from 1 month to 2 years. However, when comparing the results from different follow-up time points within studies, the longer-term follow-up showed a higher improvement of HRQoL scores, although these findings were not statistically significant. In addition to the differences in study measures, it is noteworthy that our analysis included studies of varying FAs, and HRQoL might differ between allergens, further contributing to measurement heterogeneity. Although these data do not allow for quantitative conclusions for individual allergens, future research should determine whether changes in HRQoL after OIT or OFC differ between patients’ FAs.
The findings of this meta-analysis are consistent with the majority of the literature reviewed; however, results differ from the conclusion of a recent meta-analysis from Chu et al. 19, which found that there was no significant difference in HRQoL between OIT and dietary avoidance or placebo groups for peanut allergic trial participants. This meta-analysis relied on two placebo-controlled studies, which used FAQLQ-PF, and two studies which used FAQLQ-CF or PedsQL 4.0. Our meta-analysis differs from the Chu et al. study because our primary goal was to comprehensively access HRQoL for FA patients and caregivers, while Chu et al. focuses primarily on physiological benefits and harms of OIT for peanut allergy, looking at HRQoL as a secondary aim. Our analysis also includes HRQoL assessment after both negative and positive OFC, and participants who report FA to a wide variety of allergens, rather than exclusively to peanut as in the Chu study. Additionally, the heterogeneities on study designs might contribute to different conclusions across studies. Dunn Galvin et al. for example, concluded that the improvement in HRQoL related specifically to successful attainment of sustained unresponsiveness and not simply to having received OIT. Whereas, in a study by Knibb et al’s39, the authors concluded that the improvements in HRQoL were irrespective of the challenge outcome and despite co-existing FAs. Future studies stratified by different clinical outcomes are needed for a further understanding of OIT’s role in improving long-term HRQoL.
In conclusion, our findings suggest that interventions are associated with significant improvements in participants and caregivers’ HRQoL. These results are important for FA patients and their caregivers, as well as physicians and researchers, because they show that both OIT and OFCs can improve participant HRQoL spanning social and psychological domains. Given the lack of longitudinal HRQoL studies involving both FA patients and their caregivers, future studies should include long-term follow up that focuses on the effect of OIT and OFCs on the HRQoL for participants with peanut and other FAs.
Highlight Box:
What is already known about this topic?
Food allergy can affect patients’ HRQoL due to increased anxiety and social and economic restrictions. In recent studies, OIT and OFCs have been shown to be associated with improving patients’ health related quality of life.
What does this article add to our knowledge?
Both OIT and OFC were found to be significantly associated with improved HRQoL. Five OIT studies found a significant improvement of HRQoL in the OIT group compared to the placebo group.
How does this study impact current management guidelines?
Our study underscores the potential benefits of OIT and OFC in improving patients’ quality of life, which should be considered when balancing the pros and cons of treatment in clinical practice.
Acknowledgments
Conflicts of Interest:
Shu Cao, Matteo Borro, and Sarah Alonzi have nothing to report for conflicts of interest.
Sayantani Sindher reports that she has received funding for clinical trials from the following: DBV technologies, Aimmune Therapeutics, Regeneron and NIH-NIAID for research clinical trial funding
Kari Nadeau reports grants from National Institute of Allergy and Infectious Diseases (NIAID), National Heart, Lung, and Blood Institute (NHLBI), and National Institute of Environmental Health Sciences (NIEHS); Food Allergy Research & Education (FARE), Director of World Allergy Organization (WAO) Center of Excellence at Stanford; Advisor at Cour Pharma; Co-founder of Before Brands, Alladapt, Latitude, and IgGenix; National Scientific Committee member at Immune Tolerance Network (ITN) and National Institutes of Health (NIH) clinical research centers; DSMB member for NHLBI, US patents for basophil testing, multifood immunotherapy and prevention, monoclonal antibody from plasmoblasts, and device for diagnostics.
Dr. Chinthrajah receives grant support from CoFAR NIAID, Aimmune, DBV Technologies, Astellas, AnaptysBio, Novartis, and Regeneron, and is an advisory board member for Alladapt Immunotherapeutics, Novartis, Sanofi, and Genentech.
Abbreviations:
- EAACI
European Academy of Allergy and Clinical Immunology
- FA
Food Allergy
- FAIM
Food Allergy Independent Measure
- FALCPA
Food Allergen Labelling and Consumer Protection Act
- FAQLQ
Food Allergy Quality of Life Questionnaires
- FAQLQ-AF
Food Allergy Quality of Life Questionnaires-Adult Form
- FAQLQ-CF
Food Allergy Quality of Life Questionnaires-Child Form
- FAQLQ-PB
Food Allergy Quality of Life Questionnaires-Parental Burden Form
- FAQLQ-PF
Food Allergy Quality of Life Questionnaires-Parent Form
- FAQLQ-TF
Food Allergy Quality of Life Questionnaires-Teenage Form
- HRQoL
Health-related Quality of Life
- MCID
Minimal Clinically Important Difference
- MD
Mean Difference
- OFC
Oral Food Challenge
- OIT
Oral Immunotherapy
- PedsQL
Pediatric Quality of Life Inventory
- SD
Standard Deviation
- SMD
Standardized Mean Difference
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference:
- 1.Prescott SL, Pawankar R, Allen KJ, Campbell DE, Sinn J, Fiocchi A, et al. A global survey of changing patterns of food allergy burden in children. World Allergy Organ J 2013; 6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunlop JH, Keet CA. Epidemiology of Food Allergy. Immunol Allergy Clin North Am 2018; 38:13–25. [DOI] [PubMed] [Google Scholar]
- 3.therapeutics A. FDA approves Aimmune’s PALFORZIA as first treatment for peanut allergy. 2020.
- 4.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010; 126: S1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, Bindslev-Jensen C, et al. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy 2014; 69:1008–25. [DOI] [PubMed] [Google Scholar]
- 6.Rudders SA, Banerji A, Vassallo MF, Clark S, Camargo CA Jr. Trends in pediatric emergency department visits for food-induced anaphylaxis. J Allergy Clin Immunol 2010; 126:385–8. [DOI] [PubMed] [Google Scholar]
- 7.Greenhawt M Food allergy quality of life and living with food allergy. Curr Opin Allergy Clin Immunol 2016; 16:284–90. [DOI] [PubMed] [Google Scholar]
- 8.Antolín-Amérigo D, Manso L, Caminati M, de la Hoz Caballer B, Cerecedo I, Muriel A, et al. Quality of life in patients with food allergy. Clinical and molecular allergy : CMA 2016; 14:4-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DunnGalvin A, Dubois AE, Flokstra-de Blok BM, Hourihane JO. The effects of food allergy on quality of life. Chem Immunol Allergy 2015; 101:235–52. [DOI] [PubMed] [Google Scholar]
- 10.Felce D, Perry J. Quality of life: its definition and measurement. Res Dev Disabil 1995; 16:51–74. [DOI] [PubMed] [Google Scholar]
- 11.Dunn Galvin A, McMahon S, Ponsonby AL, Hsiao KC, Tang MLK. The longitudinal impact of probiotic and peanut oral immunotherapy on health-related quality of life. Allergy 2018; 73:560–8. [DOI] [PubMed] [Google Scholar]
- 12.Umasunthar T, Leonardi-Bee J, Hodes M, Turner PJ, Gore C, Habibi P, et al. Incidence of fatal food anaphylaxis in people with food allergy: a systematic review and meta-analysis. Clin Exp Allergy 2013; 43:1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, Meltzer D. The economic impact of childhood food allergy in the United States. JAMA Pediatr 2013; 167:1026–31. [DOI] [PubMed] [Google Scholar]
- 14.Shaker MS, Schwartz J, Ferguson M. An update on the impact of food allergy on anxiety and quality of life. Curr Opin Pediatr 2017; 29:497–502. [DOI] [PubMed] [Google Scholar]
- 15.Cummings AJ, Knibb RC, King RM, Lucas JS. The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: a review. Allergy 2010; 65:933–45. [DOI] [PubMed] [Google Scholar]
- 16.Carraro S, Frigo AC, Perin M, Stefani S, Cardarelli C, Bozzetto S, et al. Impact of oral immunotherapy on quality of life in children with cow milk allergy: a pilot study. Int J Immunopathol Pharmacol 2012; 25:793–8. [DOI] [PubMed] [Google Scholar]
- 17.Reier-Nilsen T, Carlsen KCL, Michelsen MM, Drottning S, Carlsen KH, Zhang C, et al. Parent and child perception of quality of life in a randomized controlled peanut oral immunotherapy trial. Pediatr Allergy Immunol 2019; 30:638–45. [DOI] [PubMed] [Google Scholar]
- 18.DunnGalvin A, Cullinane C, Daly DA, Flokstra-de Blok BM, Dubois AE, Hourihane JO. Longitudinal validity and responsiveness of the Food Allergy Quality of Life Questionnaire - Parent Form in children 0–12 years following positive and negative food challenges. Clin Exp Allergy 2010; 40:476–85. [DOI] [PubMed] [Google Scholar]
- 19.Kansen HM, Le TM, Meijer Y, Flokstra-de Blok BMJ, Welsing PMJ, van der Ent CK, et al. The impact of oral food challenges for food allergy on quality of life: A systematic review. Pediatr Allergy Immunol 2018; 29:527–37. [DOI] [PubMed] [Google Scholar]
- 20.Chu DK, Wood RA, French S, Fiocchi A, Jordana M, Waserman S, et al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. Lancet 2019; 393:2222–32. [DOI] [PubMed] [Google Scholar]
- 21.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 1992; 45:769–73. [DOI] [PubMed] [Google Scholar]
- 24.Abrams KR, Gillies CL, Lambert PC. Meta-analysis of heterogeneously reported trials assessing change from baseline. Stat Med 2005; 24:3823–44. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPTG Cochrane handbook for systematic reviews of interventions version 5.1. 0 [updated March 2011]. The cochrane collaboration 2011; 20011. [Google Scholar]
- 26.Cohen BL, Noone S, Muñoz-Furlong A, Sicherer SH. Development of a questionnaire to measure quality of life in families with a child with food allergy. J Allergy Clin Immunol 2004;114:1159–63. [DOI] [PubMed] [Google Scholar]
- 27.Muraro A, Dubois AE, DunnGalvin A, Hourihane JO, de Jong NW, Meyer R, et al. EAACI Food Allergy and Anaphylaxis Guidelines. Food allergy health-related quality of life measures. Allergy 2014; 69:845–53. [DOI] [PubMed] [Google Scholar]
- 28.Flokstra-de Blok BM, DunnGalvin A, Vlieg-Boerstra BJ, Oude Elberink JN, Duiverman EJ, Hourihane JO, et al. Development and validation of a self-administered Food Allergy Quality of Life Questionnaire for children. Clin Exp Allergy 2009; 39:127–37. [DOI] [PubMed] [Google Scholar]
- 29.Flokstra-de Blok BM, DunnGalvin A, Vlieg-Boerstra BJ, Oude Elberink JN, Duiverman EJ, Hourihane JO, et al. Development and validation of the self-administered Food Allergy Quality of Life Questionnaire for adolescents. J Allergy Clin Immunol 2008; 122:139–44, 44.e1–2. [DOI] [PubMed] [Google Scholar]
- 30.Flokstra-de Blok BM, van der Meulen GN, DunnGalvin A, Vlieg-Boerstra BJ, Oude Elberink JN, Duiverman EJ, et al. Development and validation of the Food Allergy Quality of Life Questionnaire - Adult Form. Allergy 2009; 64:1209–17. [DOI] [PubMed] [Google Scholar]
- 31.van der Velde JL, Flokstra-de Blok BM, Vlieg-Boerstra BJ, Oude Elberink JN, DunnGalvin A, Hourihane JO, et al. Development, validity and reliability of the food allergy independent measure (FAIM). Allergy 2010; 65:630–5. [DOI] [PubMed] [Google Scholar]
- 32.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care 2001; 39:800–12. [DOI] [PubMed] [Google Scholar]
- 33.Avery NJ, King RM, Knight S, Hourihane JO. Assessment of quality of life in children with peanut allergy. Pediatr Allergy Immunol 2003; 14:378–82. [DOI] [PubMed] [Google Scholar]
- 34.Cummings AJ, Knibb RC, Erlewyn-Lajeunesse M, King RM, Roberts G, Lucas JS. Management of nut allergy influences quality of life and anxiety in children and their mothers. Pediatr Allergy Immunol 2010; 21:586–94. [DOI] [PubMed] [Google Scholar]
- 35.Epstein RM, Duberstein PR, Fenton JJ, Fiscella K, Hoerger M, Tancredi DJ, et al. Effect of a patient-centered communication intervention on oncologist-patient communication, quality of life, and health care utilization in advanced cancer: the VOICE randomized clinical trial. JAMA oncology 2017; 3:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arasi S, Otani IM, Klingbeil E, Bégin P, Kearney C, Dominguez TLR, et al. Two year effects of food allergen immunotherapy on quality of life in caregivers of children with food allergies. Allergy, Asthma & Clinical Immunology 2014; 10:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otani IM, Bégin P, Kearney C, Dominguez TL, Mehrotra A, Bacal LR, et al. Multiple-allergen oral immunotherapy improves quality of life in caregivers of food-allergic pediatric subjects. Allergy Asthma Clin Immunol 2014; 10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blumchen K, Trendelenburg V, Ahrens F, Gruebl A, Hamelmann E, Hansen G, et al. Efficacy, Safety, and Quality of Life in a Multicenter, Randomized, Placebo-Controlled Trial of Low-Dose Peanut Oral Immunotherapy in Children with Peanut Allergy. J Allergy Clin Immunol Pract 2019; 7:479–91.e10. [DOI] [PubMed] [Google Scholar]
- 39.van der Valk JP, Gerth van Wijk R, Flokstra-de Blok BM, van der Velde JL, de Groot H, Wichers HJ, et al. No difference in health-related quality of life, after a food challenge with cashew nut in children participating in a clinical trial. Pediatr Allergy Immunol 2016; 27:812–7. [DOI] [PubMed] [Google Scholar]
- 40.Knibb RC, Ibrahim NF, Stiefel G, Petley R, Cummings AJ, King RM, et al. The psychological impact of diagnostic food challenges to confirm the resolution of peanut or tree nut allergy. Clin Exp Allergy 2012; 42:451–9. [DOI] [PubMed] [Google Scholar]
- 41.Warren CM, Gupta RS, Sohn MW, Oh EH, Lal N, Garfield CF, et al. Differences in empowerment and quality of life among parents of children with food allergy. Ann Allergy Asthma Immunol 2015; 114:117–25. [DOI] [PubMed] [Google Scholar]
- 42.Anagnostou K, Islam S, King Y, Foley L, Pasea L, Bond S, et al. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet 2014; 383:1297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hourihane JO, Allen KJ, Shreffler WG, Dunngalvin G, Nordlee JA, Zurzolo GA, et al. Peanut Allergen Threshold Study (PATS): Novel single-dose oral food challenge study to validate eliciting doses in children with peanut allergy. J Allergy Clin Immunol 2017; 139:1583–90. [DOI] [PubMed] [Google Scholar]
- 44.Soller L, Hourihane J, DunnGalvin A. The impact of oral food challenge tests on food allergy health-related quality of life. Allergy 2014; 69:1255–7. [DOI] [PubMed] [Google Scholar]
- 45.van der Velde JL, Flokstra-de Blok BM, de Groot H, Oude-Elberink JN, Kerkhof M, Duiverman EJ, et al. Food allergy-related quality of life after double-blind, placebo-controlled food challenges in adults, adolescents, and children. J Allergy Clin Immunol 2012; 130:1136–43.e2. [DOI] [PubMed] [Google Scholar]


