Abstract
Two factors intrinsic to health are diet and sleep. These two behaviors may well influence one another. Indeed, that insufficient sleep adversely impacts dietary intakes is well documented. On the other hand, diet may influence sleep via melatonin and its biosynthesis from tryptophan. Experimental data exist indicating that provision of specific foods rich in tryptophan or melatonin can improve sleep quality. Whole diets rich in fruits, vegetables, legumes, and other sources of dietary tryptophan and melatonin have been shown to predict favorable sleep outcomes. Although clinical trials are needed to confirm a causal impact of dietary patterns on sleep and elucidate underlying mechanisms, available data illustrate a cyclical relation between these lifestyle factors. We recommend adopting a healthful diet to improve sleep, which may further promote sustained favorable dietary practices.
Keywords: sleep, insomnia, melatonin, nutrition, diet, tryptophan
INTRODUCTION
Poor sleep health is endemic in the United States today, with over one-third of adults failing to achieve adequate sleep duration of ≥7 h/night (57) and a similar proportion (35%) reporting habitual sleep quality that is less than ideal (77). This is a growing public health concern given that both short and poor sleep are consistently linked with heightened risk for cardiometabolic diseases, including obesity (20, 29, 90), type 2 diabetes (50, 100), and cardiovascular disease (19, 46, 52). These findings have led to a statement from the American Heart Association recommending that sleep be highlighted in campaigns to improve cardiovascular health (108).
Diet quantity and quality undeniably influence the risk of cardiometabolic disorders and are the focus of lifestyle interventions to manage this risk. Findings of observational and experimental studies consistently support a role of sleep in determining dietary choices and intakes (26, 106). The influence of inadequate sleep on food intake results in a dietary profile that is linked with increased risk of cardiovascular and metabolic diseases (108). Indeed, short sleep duration and poor sleep quality have been associated with adverse dietary behaviors, including higher intakes of energy, fat, and sweets as well as lower intakes of foods shown to have health benefits, including vegetables, fruits, and whole grains (25, 26, 48, 72, 134).
Despite intervention studies highlighting a pathway by which sleep influences diet, one cannot ignore the fact that epidemiological findings of an association between sleep duration/quality and diet (26) are nondirectional. It is equally possible that associations between sleep and diet instead reflect a role of diet in modulating sleep quality. Thus, in this review, we aim to evaluate the cyclical nature of the sleep–diet relationship in adults. In the first instance, we provide an overview of the extensively reviewed adverse impact of inadequate sleep on dietary intakes (3, 18, 26, 106, 108). We then direct the focus of this review to the alternate proposition, that intake of healthful foods and high overall dietary quality improve sleep. To establish the biological plausibility of this relationship linking food and sleep, we illustrate the biological pathways by which dietary nutrients can impact sleep health. We then build from biochemistry to behavior by reviewing epidemiological and clinical intervention studies evaluating the effects of specific foods and complete dietary patterns on sleep, with an emphasis on sleep quality. Finally, we propose future research directions in this field and highlight public health implications of the bidirectional sleep–diet relationship.
THE IMPACT OF SLEEP ON DIETARY INTAKES
Numerous studies have experimentally curtailed nightly sleep time in individuals with adequate habitual sleep duration (≥7 h per night) to assess effects on ad libitum food and energy intakes; these studies have been the subject of several meta-analyses and systematic reviews (3, 18, 31), as well as descriptive reviews by our group (105, 106) and others (23, 26). Meta-analyses confirm that sleep restriction, generally limiting sleep to <5.5 h/night, increases energy intake relative to adequate habitual sleep (3, 18, 31). Beyond an impact on energy intake, there is also evidence of changes in the types of food consumed in response to short sleep. As summarized previously (3, 18, 31), trials show that sleep restriction leads to greater intake of fats, including saturated fat (110); promotes appetite for, and consumption of, carbohydrates (66, 103); and increases snacking (74). Taken together, these results suggest that reduced sleep decreases overall dietary quality.
Various mechanisms have been proposed to explain changes in dietary intakes in response to reduced sleep duration. These have ranged from increased opportunity to eat due to added wake time (83) to alterations in appetite-regulating hormones, mostly leptin and ghrelin but also adiponectin, glucagon-like peptide 1, and orexin (56); reward valuation of food (24); and taste perceptions (59, 114) (Figure 1). There is increasing consensus that data related to hormonal regulation of eating, via changes in leptin and ghrelin, following sleep restriction are less consistent than those related to reward valuation (24, 94).
Figure 1.
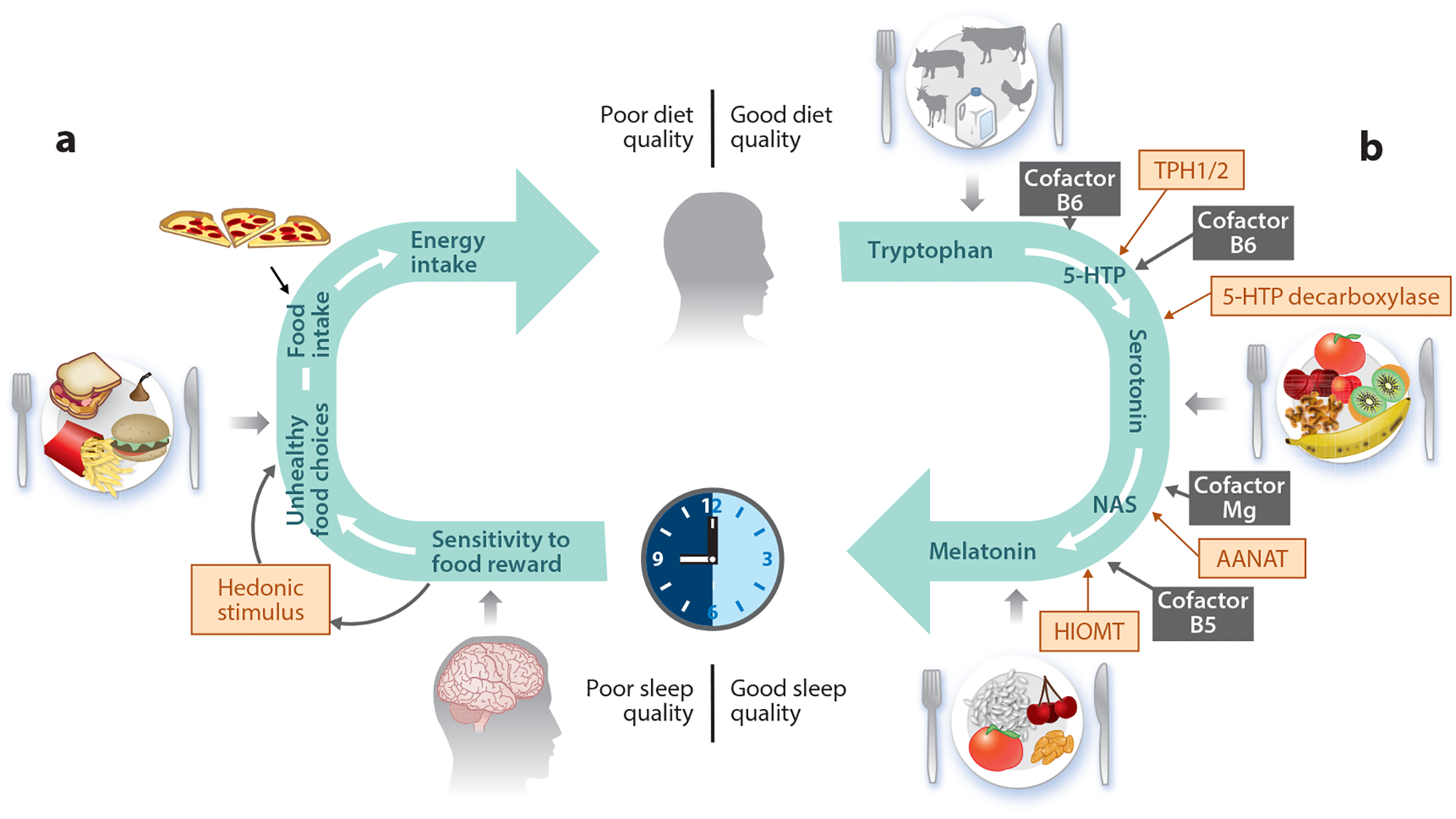
The cyclical nature of the sleep–diet relationship. (a) Mechanisms by which sleep influences food selection and energy intake. Poor sleep increases sensitivity to food reward and may alter taste sensitivity or perception as well as release of appetite-regulating hormones, leading to selection of energy-dense foods and increased snacking. (b) Pathway by which a healthful diet rich in food sources of tryptophan, serotonin, or melatonin leads to good-quality sleep. The correct functioning of the sleep–wake cycle is promoted by melatonin, exclusively synthesized from dietary tryptophan, via serotonin, obtained from ingested foods. Melatonin is synthesized enzymatically by the sequential actions of four enzymes (TPH1/2, 5-HTP decarboxylase, AANAT, HIOMT) and requires Mg and B vitamins as cofactors. Abbreviations: AANAT, arylalkylamine N-acetyltransferase; HIOMT, hydroxyindole-O-methyltransferase; 5-HTP, 5-hydroxytryptophan; Mg, magnesium; NAS, N-acetylserotonin; TPH1/2, tryptophan hydroxylase.
Indeed, studies of the influence of sleep restriction on hormonal regulation of food intake have yielded inconsistent results over the years (38, 105). In a recent extension of this body of work, Rihm et al. (95) failed to show evidence for a hormonal modulation of functional connectivity between reward and food regulatory centers of the brain (amygdala and hypothalamus) resulting from 1 night of total sleep deprivation. In that study, the authors reported a selective increase in subjective valuation of snack food rewards and amygdala and hypothalamus activity in response to food images after a night of total sleep deprivation compared to adequate sleep but no change in appetite-regulating hormones and metabolites (total and acyl ghrelin, leptin, insulin, cortisol). Increased reward valuation of food, reported as increased food craving scores, has been reported following 1 night of sleep restriction in women (129). Similarly, we found that 5 nights of sleep restriction led to increased activation in brain reward regions in response to energy-dense food stimuli (111). These findings suggest that, relative to physiological drivers of hunger and fullness, increased hedonic drive for foods may explain shifts toward poor diet quality following periods of inadequate sleep.
Changes in taste sensitivity have also been proposed to contribute to the impact of sleep restriction on food intake. One study reported an increase in preferred concentration of sucrose and sucralose with sleep restriction (114), while another reported a positive association between sleepiness, assessed by questionnaire, and intensity of umami and sour tastes (59). These studies suggest that sleep restriction could alter food choices by changing taste perceptions. For example, one might expect increased intakes of sweet foods to satisfy a desire for sweet tastes and lower intakes of foods with umami and sour tastes, which are often protein-rich foods, dairy, and fruits and vegetables, due to a lower intensity threshold for these tastes following periods of insufficient sleep. This supports early findings of increased ratings for desire to eat sweet foods but not meat and dairy after a period of sleep curtailment (103). However, these results must be interpreted cautiously because they were not paired with measures of food intake.
While the experimental studies reviewed to this point clearly demonstrate adverse dietary consequences of short sleep, it is also important to determine whether improvements in sleep can have favorable effects on diet. This is an evolving area of research, with most preliminary studies designed to extend sleep in short sleepers as a means to improve diet-related outcomes. Results of two pilot studies suggest a potential benefit of sleep extension to improve diet quality (2, 115). One study found that provision of a personalized sleep hygiene intervention, which extended sleep duration by 1.5 h for 2 weeks, led to significant reductions in appetite, highlighted by reduced desire for sweet and salty foods (115). The other study achieved an increase of 0.33 h of sleep over 4 weeks and reported reductions in total carbohydrate and free sugar intakes (2). Although further investigation into the effects of sleep extension on diet quantity and quality is needed, preliminary results are suggestive of improvements in dietary intakes with increases in sleep duration.
There is strong evidence that inadequate sleep has a detrimental effect on dietary choices and intakes, while improvements in sleep could ameliorate dietary outcomes. Recommendations to improve sleep quality and duration could therefore have clear public health relevance, since diet is a key predictor of cardiovascular health outcomes (4). In considering approaches to improve sleep health, it is pertinent to introduce the possibility of a cyclical relation of diet and sleep (Figure 1). While sleep is shown to impact dietary intakes, there is emerging interest in a role of diet in modulating sleep health, with recent reviews postulating various underlying mechanisms (23, 109, 112). Below, we highlight biological plausibility as well as epidemiological and clinical evidence for the diet-to-sleep directionality.
THE INFLUENCE OF DIETARY INTAKES ON SLEEP: BUILDING FROM THE BASICS
Serotonin and Melatonin in the Regulation of Sleep
Melatonin is often regarded as the main hormonal modulator of the sleep–wake cycle. Released by the pineal gland in response to darkness, melatonin acts as a circadian signal to systemic processes through its actions on the suprachiasmatic nucleus (104, 133). Melatonin secretion follows a circadian pattern with low levels during the day, which start to rise in the evening to promote sleep onset. Indeed, an acute rise in sleep propensity occurs approximately 2 h after the release of endogenous melatonin, increasing one’s ability to fall asleep (118).
Serotonin, unlike melatonin, is a neurotransmitter. Once thought to be the primary neurotransmitter of sleep (88), the role of serotonin in the sleep–wake cycle is now understood as more complex, being involved in various processes necessary for both sleep and wake. Serotonin has been hypothesized to regulate neuronal activity in the ventrolateral preoptic area of the brain, the main structure responsible for inducing slow-wave sleep (SWS) (91). High levels of neuronal activity in this region during sleep suggest a role of serotonin in the induction of the sleep cycle. On the other hand, the raphe nucleus, the epicenter of serotonin synthesis and release in the brain, is most active during wake, with activity reduced during SWS and stopped during rapid-eye movement (REM) sleep (71). This somewhat paradoxical relation between serotonin and raphe nucleus activity has contributed to the postulation that serotonin may participate in the homeostatic process of sleep–wake, marking the duration and intensity of wake, which then initiates a cascade of events that trigger sleep onset (119). These differential effects of serotonin on sleep–wake may be attributed to different receptor activities and brain regions. The two main serotonin sleep–wake receptors are 5-HT1A and 5-HT2. The 5-HT2 receptors, in the thalamic reticular nucleus, cortical cells, and reticular nucleus cells, promote wake. The 5-HT1A receptors, found postsynaptically on many neurons and somatodendritically on dorsal raphe neurons, also promote wake and reduce REM, while those receptors in the basal forebrain facilitate sleep and SWS (119). Furthermore, the position of serotonin in the biosynthesis pathway of melatonin, as highlighted in the next section, further supports a sleep-promoting effect of this neurotransmitter.
Synthesis of Serotonin and Melatonin from Nutrients
In humans, endogenous melatonin is exclusively produced from dietary tryptophan (132), but only a small portion (1–2%) of this dietary tryptophan is converted to melatonin via the serotonin pathway (8). Tryptophan is an essential amino acid found mostly in animal products, such as beef, lamb, pork, poultry, and dairy, as well as in nuts and seeds, whole grains, and legumes (Table 1). Protein breakdown during digestion releases amino acids for transport to the liver prior to their distribution to other organs. The majority of tryptophan is transported bound to albumin with trace amounts remaining free in the circulation (82). This ratio of free to bound tryptophan modulates crossing through the blood–brain barrier (81), which is critical for its role in serotonin and melatonin biosynthesis. Passing of tryptophan through the blood–brain barrier is a complex process, since the active transport of amino acids to the brain is available to all large neutral amino acids (LNAAs) and not specific to tryptophan. Tryptophan must therefore compete with other LNAAs, often more widely available in the food supply, for transport into the brain. A greater proportion of tryptophan would be available for serotonin and melatonin synthesis if these competing amino acids were shunted to peripheral tissues. This can be achieved by insulin release, which promotes protein synthesis in muscle. Indeed, insulin shunting effectively reduces the pool of LNAAs reaching the brain, freeing transporters for tryptophan binding (32). Therefore, in theory, consumption of carbohydrates along with tryptophan-rich foods could enhance tryptophan entry into the brain (81), consequently promoting the biosynthesis of serotonin and melatonin.
Table 1.
| Food source | Amount (per 100 g) | Amount (per serving) | Serving size | |
|---|---|---|---|---|
| Tryptophan | ||||
| Dairy | Cheddar | 574 mg | 233 mg | 1.5 oz |
| Parmesan, grated | 482 mg | 205 mg | 1.5 oz | |
| Fish and other seafood | Spiny lobster | 368 mg | 313 mg | 3 oz |
| Octopus | 334 mg | 284 mg | 3 oz | |
| Crab | 330 mg | 281 mg | 3 oz | |
| Tuna | 313 mg | 266 mg | 3 oz | |
| Snapper | 294 mg | 250 mg | 3 oz | |
| Clams | 286 mg | 243 mg | 3 oz | |
| Salmon | 285 mg | 242 mg | 3 oz | |
| Halibut | 283 mg | 241 mg | 3 oz | |
| Haddock | 260 mg | 221 mg | 3 oz | |
| Shrimp | 260 mg | 221 mg | 3 oz | |
| Trout | 257 mg | 218 mg | 3 oz | |
| Grains | Wheat germ | 398 mg | 113 mg | 1 oz |
| Oat bran | 335 mg | 315 mg | 1 cup | |
| Wheat bran | 282 mg | 82 mg | 0.5 cup | |
| Whole oats | 234 mg | 184 mg | 0.5 cup | |
| Buckwheat | 192 mg | 163 mg | 0.5 cup | |
| Legumes (including soy-based foods) | Soybeans, roasted | 575 mg | 535 mg | 8 oz |
| Tofu, firm | 235 mg | 200 mg | 3 oz | |
| Tempeh | 194 mg | 165 mg | 3 oz | |
| White beans | 115 mg | 103 mg | 0.5 cup | |
| Pinto beans | 108 mg | 92 mg | 0.5 cup | |
| Black beans | 105 mg | 90 mg | 0.5 cup | |
| Kidney beans | 104 mg | 92 mg | 0.5 cup | |
| Navy beans | 100 mg | 91 mg | 0.5 cup | |
| Lentils | 81 mg | 80 mg | 0.5 cup | |
| Nuts and seeds | Pumpkin and squash seeds | 576 mg | 162 mg | 1 oz |
| Chia seeds | 436 mg | 124 mg | 1 oz | |
| Sesame seeds | 371 mg | 105 mg | 1 oz | |
| Flaxseeds | 297 mg | 84 mg | 1 oz | |
| Sunflower seeds | 295 mg | 84 mg | 1 oz | |
| Pistachios | 251 mg | 71 mg | 1 oz | |
| Cashews | 237 mg | 67 mg | 1 oz | |
| Almonds | 209 mg | 59 mg | 1 oz | |
| Hazelnuts | 193 mg | 55 mg | 1 oz | |
| Red meats and poultry | Lamb shoulder | 415 mg | 353 mg | 3 oz |
| Chicken breast | 404 mg | 343 mg | 3 oz | |
| Goat | 403 mg | 343 mg | 3 oz | |
| Ground pork, lean | 326 mg | 277 mg | 3 oz | |
| Pork tenderloin | 376 mg | 320 mg | 3 oz | |
| Beef steak | 374 mg | 318 mg | 3 oz | |
| Turkey breast | 287 mg | 244 mg | 3 oz | |
| Egg, whole | 167 mg | 84 mg | 1 egg | |
| Serotonin | ||||
| Fruits and vegetables | Plantain | 3030 μg | 4545 μg | 1 cup |
| Pineapple | 1700 μg | 2805 μg | 1 cup | |
| Banana | 1500 μg | 2,250 μg | 1 cup, sliced | |
| Kiwi | 580 μg | 858 μg | 2 medium | |
| Plums | 470 μg | 705 μg | 2 medium | |
| Tomatoes | 320 μg | 576 μg | 1 cup, chopped/sliced | |
| Nuts | Black walnuts | 30400 μg | 9120 μg | 1 oz |
| English walnuts | 8700 μg | 2610 μg | 1 oz | |
| Pecans | 2900 μg | 870 μg | 1 oz | |
| Melatonin | ||||
| Grains and starches | Corn | 188 ng | 308 ng | 1 cup |
| Rice | 150 ng | 135 ng | 0.5 cup | |
| Barley | 87 ng | 137 ng | 1 cup | |
| Fruits and vegetables | Tomatoes (variety dependent) | 773 ng–11.45 μg | 1.39 μg–20.61 μg | 1 cup, chopped/sliced |
| Strawberries (variety dependent) | 210 ng–1.13 μg | 302 ng–1.63 μg | 1 cup, whole | |
| Ginger | 142 ng | 5.68 ng | 2 tsp | |
| Tart cherries | 135 ng | 186.3 ng | 1 cup | |
| Nuts | Almonds | 390 ng | 117 ng | 1 oz |
| Walnuts | 350 ng | 105 ng | 1 oz | |
Once in the brain, tryptophan conversion to serotonin is the first step toward melatonin synthesis (132) (Figure 2). Serotonin is then converted to melatonin via two enzymatic reactions (30, 132) that require B vitamins and magnesium as cofactors. Once melatonin is produced in the pineal gland, it is released into the blood for circulation (12, 86). Biosynthesized melatonin, as well as dietary melatonin, is then rapidly metabolized and undergoes urinary excretion as 6-sulfatoxymelatonin following hepatic deactivation. Expectedly, melatonin metabolism occurs primarily at night as evidenced by the high level of its metabolite, 6-sulfatoxymelatonin, in urine samples collected overnight (12).
Figure 2.
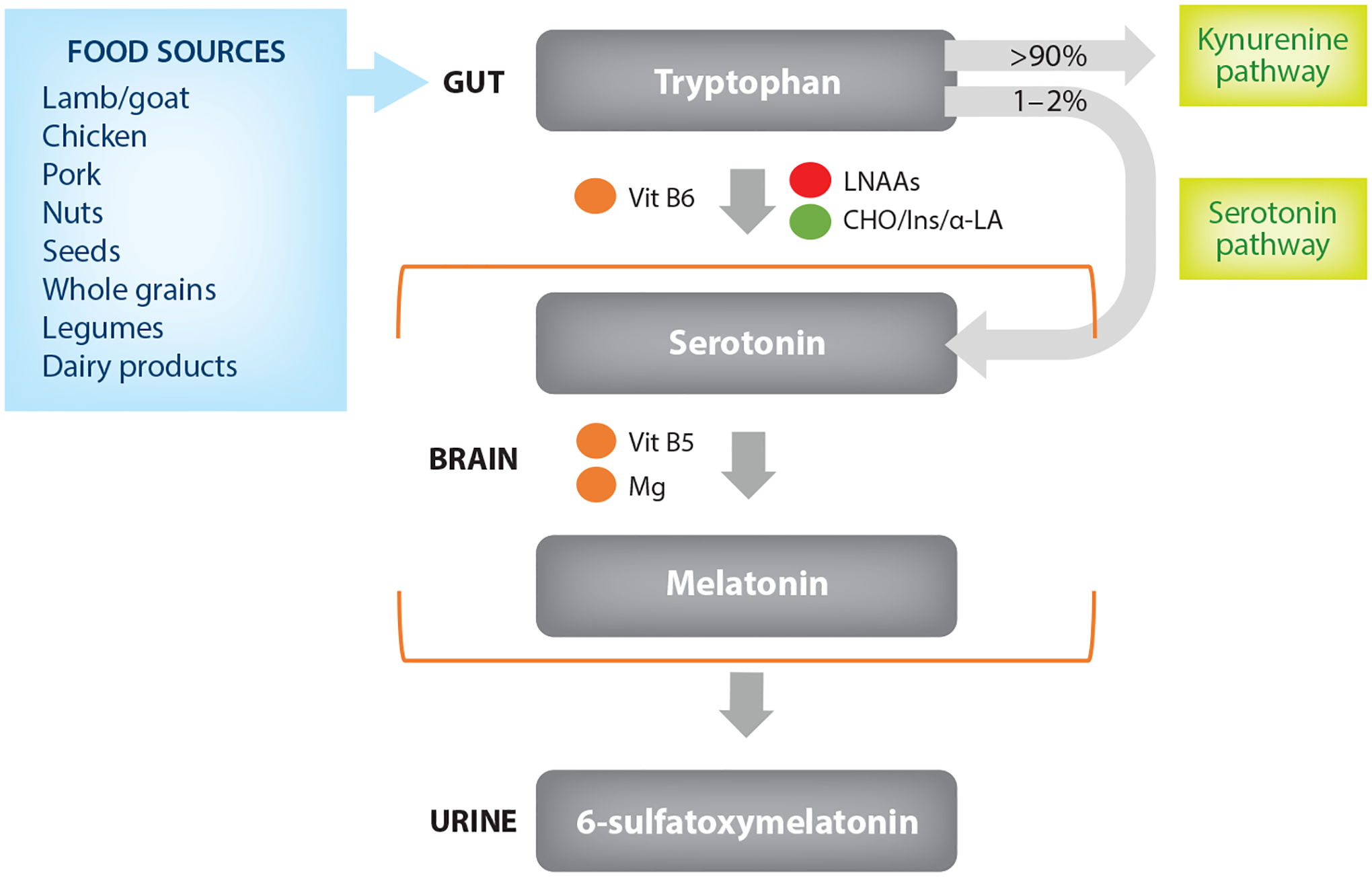
Synthesis of sleep-related hormones from dietary factors. Dietary tryptophan is degraded via two parallel pathways: the kynurenine pathway and the serotonin pathway. Only 1–2% of dietary tryptophan is converted to serotonin in the brain. Tryptophan competes in active transport with LNAAs, while carbohydrates may increase tryptophan concentrations by stimulating insulin-mediated amino acid uptake in muscles. Consumption of α-LA increases tryptophan uptake in the brain. Vitamins B5 and B6 and Mg act as cofactors in the synthesis of serotonin and melatonin. Finally, melatonin in blood circulation is metabolized and secreted in the urine in the form of 6-sulfatoxymelatonin. Abbreviations: α-LA, alpha-lactalbumin; CHO, carbohydrates; Ins, insulin; LNAAs, large neutral amino acids; Mg, magnesium; Vit, vitamin. Green circles signify positive effect; red circles represent inhibiting effect; orange circles represent cofactors.
DIETARY COMPONENTS INVOLVED IN THE BIOCHEMICAL PATHWAYS REGULATING SLEEP
Given the processes of serotonin and melatonin biosynthesis in the body, it is biologically plausible for foods to influence sleep quality. Tryptophan is an amino acid that is derived from food sources, and serotonin and melatonin have also been detected in foods (Table 1) (7, 14, 30, 93, 113). Therefore, consumption of foods rich in these dietary components may have a positive effect on sleep (Figure 1). Of interest are foods that can improve nightly sleep outcomes, such as longer sleep duration, greater sleep efficiency (percent of time in bed spent asleep), shorter sleep onset latency (time it takes to fall asleep), and fewer awakenings at night, as well as self-reports of better general sleep quality. These outcomes are assessed in research using subjective measures, such as questionnaires (used mostly in large-scale epidemiological studies), and objective measures, such as actigraphy (wrist-worn accelerometers that detect periods of inactivity and activity) and polysomnography (the gold-standard measure of sleep for diagnosis of sleep disorders such as sleep apnea and restless leg syndrome, which provides information on sleep stages such as REM and SWS). Clinical trials using objective measures of both sleep and diet can provide the most detailed insight into the causal impact of an exposure, such as diet, on sleep.
Effects of Tryptophan-Rich Foods on Sleep Parameters
Because LNAAs can impact tryptophan availability, consideration of the ratio of tryptophan to other amino acids is necessary in assessing the influence of protein consumption on sleep. Alpha-lactalbumin (α-LA), present in whey, a protein in milk, has been shown to increase plasma tryptophan-to-LNAA ratio (65) and increase tryptophan’s uptake into the brain (81). After a period of food restriction, which increased wakefulness and decreased SWS in rats, refeeding with diets varying in amount and/or quality of α-LA was found to impact sleep propensity (70). Refeeding with milk protein rich in α-LA (tryptophan-to-LNAA ratio of 8.95) decreased wakefulness and increased SWS within 1 day of refeeding. In contrast, it took 4–6 days to achieve these improvements in sleep when refeeding with diets providing lower tryptophan-to-LNAA ratios. These findings suggest that sleep disturbances resulting from dietary behaviors, such as fasting, can be attenuated by enriching diets with α-LA.
In humans, however, studies of α-LA supplementation provide conflicting results. In one study, the effect of α-LA on sleep was evaluated in university students, half of whom reported poor sleep and the other half reported good sleep. All participants completed two sessions in a counterbalanced order separated by a washout period (64). In one session, the evening meal was consumed with a milkshake rich in α-LA (20 g), which was followed by a second milkshake 1 h later. In the other session, placebo milkshakes were provided. Sleepiness was assessed by questionnaire prior to bedtime and in the morning after awakening. There was no difference in sleepiness ratings at bedtime between conditions. The following morning, all participants in the α-LA milkshake group reported being less sleepy compared to the placebo milkshake condition, and these results did not differ between those entering the study with poor versus good sleep. While this study obtained only subjective measures, a separate randomized, double-blind, placebo-controlled study assessed sleep using actigraphy following consumption of an α-LA-rich protein shake for 2 nights (79). Ten healthy young males with adequate sleep duration and no sleep complaint participated in this pilot study. Shakes containing 20 g α-LA or placebo (sodium caseinate) were consumed 1 h before bedtime. Total sleep time was longer and sleep efficiency higher following α-LA supplementation relative to placebo. Another short-term study tested the effect on sleep of consuming 40 g α-LA compared to collagen peptide 2 h before bedtime for 3 days each in trained cyclists (60). No differences in actigraphy-measured sleep outcomes were observed between conditions. Whether protein supplements in the form of nutritional shakes should be consumed to improve sleep remains to be determined. The studies reported herein were short in duration (1–3 days) and may not reflect effects of longer consumption periods. Moreover, participants are generally young and healthy, without sleep complaints. Such a population would not be the target of dietary interventions to improve sleep, and research is needed to establish efficacy in those who stand to benefit from such interventions.
Tryptophan supplementation in other food types has also been studied. The effect of tryptophan-enriched cereals on sleep was assessed in an elderly population (55–75 years) (11). In the first week, participants consumed control cereals providing 22.5 mg of tryptophan per 30 g, twice per day, as part of their usual diet, followed by 1 week of consuming 2 daily servings of 30 g of cereals enriched with 60 mg/serving of tryptophan and a final week in which participants returned to their habitual diet. Compared to the low-tryptophan cereal diet, consumption of a high-tryptophan cereal for 1 week increased actigraphy-measured sleep duration and efficiency and reduced sleep onset latency, wake bouts, total activity, and sleep fragmentation. Excretion metabolites of melatonin (6-sulfatoxymelatonin) and serotonin (5-hydroxyindoleacetic acid) were significantly increased after 1 week of consumption of the high-tryptophan cereal. The observed results were therefore attributed to increases in both melatonin and serotonin synthesis following increased tryptophan consumption within a carbohydrate-rich food.
Dairy products are natural sources of tryptophan (Table 1), and the influence of milk-based drinks on sleep parameters has been tested. For example, improvement in self-reported sleep quality was observed after twice-daily intake of a milk-honey beverage consisting of 150 mL of low-fat milk and 30 g of honey, compared to placebo, for 3 days in hospitalized acute coronary syndrome patients (28). It is possible that honey, a source of carbohydrates, improved tryptophan availability for uptake into the brain for melatonin synthesis, as explained in the section above. However, no objective measures of sleep or melatonin metabolites were obtained in this study. Another study showed that fermented milk consumption, at a level of 100 g/day for 3 weeks, improved sleep efficiency and number of awakenings (measured using wrist actigraphy) compared to placebo (acidified milk) with similar nutritional composition in healthy elderly participants (128). These findings suggest that milk-based drinks could have beneficial effects on sleep quality, although mechanisms have not yet been elucidated.
Effects of Serotonin-Rich Foods on Sleep Parameters
Very little information is available regarding the serotonin content of foods and, consequently, studies related to sleep-promoting effects of food sources of this neurotransmitter are limited. Kiwifruit has been noted as a dietary source of serotonin and studied in the context of poor sleep. In one study, participants ate two kiwifruits per day for 4 weeks, ~1 h before bedtime (55). Objective and subjective measures of sleep were taken. Total sleep time and sleep efficiency, measured with actigraphy, increased after the 4-week intervention whereas sleep onset latency and time awake after sleep onset decreased. Subjective measures of sleep also showed improvements with kiwifruit consumption. A key limitation of this study is the lack of a control group. However, this was corrected in another study that investigated the effects of kiwifruit consumption on chronic insomnia (75). Chronic insomnia is the presence of sleep complaints, such as difficulty falling asleep, difficulty maintaining sleep, or waking up too early despite ample opportunity for sleep, that occur ≥3 times per week for at least 3 months (15). Patients report nonrestorative sleep and daytime fatigue, sleepiness, poor attention/concentration, memory impairment, and/or physical pain (e.g., headaches, gastrointestinal symptoms). In this randomized, controlled trial (75), 74 students with chronic insomnia symptoms were instructed to eat either a kiwifruit or a pear (a fruit without serotonin) 1 h before bedtime every night for 4 weeks. When compared to the control group, the kiwifruit group experienced improvements in sleep quality and daytime functioning assessed by questionnaire, demonstrating possible sleep-promoting properties of kiwifruit. No differences in actigraphy-measured sleep outcomes were noted between groups.
Although these trials demonstrate favorable sleep outcomes in response to ingestion of kiwifruit (55, 75), with effects attributed to the serotonin content of the fruit, studies lacked measurement of metabolites of melatonin, the sleep-promoting product of serotonin. We are not aware of data showing that dietary serotonin serves as a substrate for melatonin synthesis. Therefore, the physiological plausibility of ascribing the effects of kiwifruit on sleep directly to its serotonin content is questionable. However, serotonin is metabolized in peripheral organs (78) and could have indirect effects on sleep via its metabolites or gut–brain interactions. This remains to be determined.
Effects of Melatonin-Rich Foods on Sleep Parameters
The melatonin content of foods has been better characterized than serotonin content (99). Some foods that are particularly rich sources of melatonin include fruits and nuts, specifically tart cherries, tomatoes, strawberries, almonds, and walnuts (Table 1). Tart cherries are of particular interest in sleep research because they contain tryptophan, serotonin, and melatonin.
One randomized, double-blind, placebo-controlled crossover study of healthy middle-aged (35–55 years) and elderly (65–85 years) men and women tested the effect of seven different Jerte Valley cherry cultivars (Ambrunés, Bourlat, Navalinda, Pico Limón, Pico Colorado, Pico Negro, and Van), consumed as fresh fruit, on total urinary melatonin (as 6-sulfatoxymelatonin) and sleep parameters (41). Intake of all cultivars, at a level of 200 g/day, twice daily for 3 days, increased urinary 6-sulfatoxymelatonin in both middle-aged and elderly adults. Additionally, in middle-aged adults, all cherry cultivars but one, Ambrunés, increased sleep duration, and some cultivars reduced nighttime awakenings (Pico Limón and Pico Colorado) and sleep onset latency (Navalinda and Pico Negro), measured using wrist actigraphy. Of note, this study did not include a non-cherry control period. In a separate study of young (20–30 years), middle-aged (35–55 years), and elderly (65–86 years) adults, the same research group found that consumption of a Jerte Valley cherry-derived beverage made from four different cultivars (equivalent to 141 g of fresh cherries) twice daily for 5 days increased 6-sulfatoxymelatonin for all age groups relative to baseline (40). Actigraphy-measured total sleep time increased, and number of nighttime awakenings decreased, relative to baseline in all age groups with consumption of the tart cherry beverage. Sleep onset latency was reduced relative to baseline only in middle-aged and older adults, while sleep efficiency increased only in older adults. No changes in 6-sulfatoxymelatonin or sleep were observed after consumption of the placebo. Notably, the authors failed to report whether changes in outcomes differed between cherry treatment and placebo in this placebo-controlled study.
Montmorency tart cherry juice has also been evaluated as a sleep aid. Montmorency cherries are the most commonly grown tart cherries in the United States (49). One study enrolled young healthy men and women (18–40 years) and randomized them to tart cherry juice concentrate (equivalent to 90–100 tart cherries) or placebo, twice daily, 30 min after awakening and 30 min prior to the evening meal, for periods of 7 days each (44). Sleep was measured using wrist actigraphy. Urinary 6-sulfatoxymelatonin increased with cherry juice consumption relative to baseline and placebo. Total sleep time and sleep efficiency increased more with tart cherry juice treatment than with placebo. Pigeon et al. (87) extended this work by testing the impact of Montmorency tart cherry juice on self-reported symptoms of insomnia in individuals over the age of 65 years. At the start of the study, all participants reported insomnia complaints >3 nights/week for at least 6 months, a score ≥10 on the Insomnia Severity Index, and time awake after sleep onset of ≥30 min. Participants consumed 8 oz tart cherry juice or placebo between 8:00 and 10:00 AM and again 1–2 h before bed every day for 2 weeks. Sleep was assessed by questionnaire and diary. Compared to placebo, tart cherry juice reduced insomnia ratings and time awake after sleep onset. No other improvements were noted in measures of sleep (duration, efficiency, latency) or fatigue, depression, and anxiety. Another study in middle-aged to older adults (≥50 years) with chronic insomnia tested the impact of Montmorency tart cherry juice consumption on sleep (58). Participants reported insomnia complaints >3 nights/week and a score ≥10 on the Insomnia Severity Index. Eight participants completed the study, in a randomized crossover design, which involved daily consumption of 240 mL of tart cherry juice or placebo in the morning and 1–2 h before bedtime for 2 weeks. Compared to placebo, tart cherry juice consumption increased total sleep time, measured by polysomnography, and improved self-reported ratings of sleep quality. These studies suggest that tart cherry products may improve sleep, with particular benefit to older adults and those with sleep problems.
Mammalian milk is also a source of melatonin, with its content varying with circadian time (89). In cows, for example, milking at night produces milk that is naturally higher in melatonin than milking during the day (69). A potential influence of consuming high-melatonin milk, produced via nighttime milking, on sleep was tested in patients with primary insomnia (17). In this randomized crossover study, patients consumed 30 g of high-melatonin milk (85.5 pg/mg) or low-melatonin milk (8.8 pg/mg) 30 min before bedtime for 3 weeks each. Minimal changes in objective sleep parameters were observed; moreover, only 8 of 19 patients reported that night milk improved their sleep. Another crossover study in an elderly population at varying levels of cognitive impairment showed no effect of nighttime milk consumption (5–20 ng melatonin daily) on sleep quality compared to placebo (normal commercial milk) (121). Results of these studies suggest limited efficacy of natural increases in melatonin content of milk in improving sleep quality, at least in populations with, or at risk for, disordered sleep.
Tomatoes are another rich source of melatonin, the content of which varies by season, year, and species. Beefsteak tomatoes are a particularly good source of dietary melatonin (130) and have been assessed for their influence on sleep. Postmenopausal women with poor sleep were randomized to consume 250 g of beefsteak tomatoes or no tomatoes, 2 h before bedtime, for 8 weeks (130). All women were counseled to consume five servings of fruits and vegetables per day. Sleep and diet were assessed at baseline and 8 weeks using questionnaires. No dietary differences, other than tomato consumption, were found between the two groups. Total sleep quality score and sleep onset latency, measured by the Pittsburgh Sleep Quality Index, were improved to a greater extent in women randomized to the tomato group compared to those in the control group. In addition, 6-sulfatoxymelatonin levels were ten-fold higher at 8 weeks in women in the tomato group. This study showed that increased dietary intake of melatonin from foods increases circulating melatonin concentrations and leads to improvements in sleep. However, sleep was assessed only by questionnaire and blinding could not be done, raising concerns about potential for bias.
Studies described to this point showed variable effects of consumption of different beverages and foods on sleep. It is important to note that the metabolism of proteins, serotonin, and melatonin is interrelated and dependent on the presence of other dietary components. These relationships pose challenges for extrapolating effects of these compounds from food sources. Therefore, many of the results observed were likely not due to a single dietary factor but rather represent the aggregate effects of the biochemical pathways. Furthermore, studies differ in their participant population (young/elderly, healthy/sleep disordered), treatment duration (days/weeks), treatment allocation (once/twice daily, morning/evening), and type of sleep measure (subjective/objective). These methodological differences hinder our ability to make definitive conclusions about the efficacy of these various foods to improve sleep health.
DIETARY PATTERNS AS PREDICTORS OF SLEEP QUALITY
The studies reviewed in the previous section establish proof of principle for an impact of diet on sleep outcomes. However, functional foods are often consumed alongside other foods comprising a broad range of nutrients within the context of a whole diet. Therefore, an important next step is to evaluate relationships between parameters of sleep quality and common dietary patterns that incorporate food sources of sleep-promoting compounds (highlighted in Table 1 and in the previous sections).
Our understanding of the biochemical pathways implicating dietary components in sleep regulation demonstrates mechanisms by which dietary patterns may impact sleep. Key to these mechanisms is the notion that the tryptophan-to-LNAA ratio, which determines brain uptake of tryptophan (126), relies on carbohydrate consumption (33). As mentioned above, insulin released as a result of carbohydrate consumption promotes LNAA uptake by cells, thereby raising the relative abundance of tryptophan that can cross into the brain and initiate the cascade of events toward melatonin synthesis. It is therefore plausible that sleep could be promoted by ingestion of foods with a macronutrient profile that, when metabolized, increases circulating tryptophan relative to LNAA, even if the dietary pattern itself is not rich in tryptophan. This is particularly relevant for diets that reflect a Mediterranean pattern (MedDiet) or that vary in carbohydrate quality (10, 127), for example, dietary glycemic index (GI).
Dietary Glycemic Index
The GI of a food is a standardized measure of circulating glucose levels following consumption of 50 g of that food, measured on a scale of 0–100 with 100 representing the GI of pure glucose or white bread (the reference foods) (47). A low-GI food is one for which postprandial glucose is much less than that of the reference food and usually scores <50, whereas high-GI foods have values of 70 and above; GI scores in between are considered intermediate. Dietary glycemic load is a composite measure of the GI of total carbohydrates consumed in the diet (6, 37) and is calculated as the product of GI and net carbohydrates (not including fiber) divided by 100.
A cross-sectional study conducted among nearly 2,000 Japanese men and women assessed habitual intakes of commonly consumed high-GI foods (rice, bread, and noodles) and overall dietary GI in relation to self-reported sleep quality (131). Results showed that higher intakes of rice and higher overall GI were associated with lower subjective sleep quality scores, indicative of better sleep quality, whereas the opposite was found for noodles (131). These findings highlight the complexity of GI. The GI of a food is evaluated when consumed in isolation, whereas meals contain multiple foods. The concomitant consumption of other foods, which can delay or hasten carbohydrate absorption, as well as the cooking or processing method and food ripeness influence GI. Furthermore, a food’s GI is established when 50 g of the food is consumed, which may not reflect usual amounts consumed.
In the only epidemiological study of dietary GI and sleep in a US population, findings revealed an association between high GI and insomnia (39). Using data collected from the Women’s Health Initiative, cross-sectional and prospective associations of dietary GI, glycemic load, and different forms of carbohydrate with insomnia were evaluated in over 70,000 and 50,000 women, respectively. Higher dietary GI was associated with higher odds of prevalent insomnia and incident insomnia at 3-year follow-up (39). Moreover, this study provided key insight into a role for carbohydrate quality in determining risk for disordered sleep patterns, as higher intakes of added sugars and refined grains predicted higher insomnia risk, while higher intakes of fruit and fiber were associated with favorable sleep quality outcomes.
The contrast between the two available observational studies is stark (39, 131); however, careful consideration of the differences between these studies leads to some possible explanations. First, the study conducted among Japanese adults was cross-sectional (131) and thus cannot indicate directionality. It could be the case that short sleep predisposes individuals to greater intakes of high-GI foods, and, consequently, to a higher dietary GI. Indeed, adolescents have been found to increase the GI of their diet in response to experimental sleep curtailment (9). Results of prospective analyses from the Women’s Health Initiative allow for determination of directionality; when diet is evaluated as the exposure, results clearly indicate that poor sleep is downstream of higher dietary GI in that sample of women (39). It is also noteworthy that the study populations differed from one another in numerous respects. For example, habitual dietary patterns in Japanese populations are distinct from Western diets. As noted by the authors, the GI of a typical Japanese diet is quite high and, in some cases, is nearly 1.5 times that of a common US diet (131). These differences in GI of the habitual diet may impact macronutrient metabolism in a way that differentially affects tryptophan availability. Another possibility implicates cross-cultural differences in habitual consumption of food sources rich in nutrients that serve as key cofactors in the metabolism of tryptophan to melatonin. Future epidemiological investigations of associations between GI and sleep quality would benefit from including assessment of dietary sources of foods containing these nutrient cofactors as well as measurement of melatonin metabolites.
Further insight into the GI–sleep relation can be gained from experimental studies. Two studies have evaluated effects of the GI of a meal on sleep quality (1, 124). In one study, healthy men were served an evening meal (4 h before their usual bedtime) that was either high or low in GI (1). The high-GI diet was characterized by 72% of energy from carbohydrates, 15.5% from protein, and 12.5% from fats, while the low-GI diet contained <1% of energy from carbohydrates, 38% from protein, and 61% from fats. Sleep onset latency, measured by polysomnography, was reduced to a greater extent following consumption of the high-GI meal. Although no differences were observed between the high- and low-GI conditions for most sleep parameters, subjective sleepiness prior to bedtime was higher in the high-GI condition. This study provided initial evidence of a sleep-promoting role of a high-GI meal consumed in the evening. However, it is notable that meal GI did not affect levels of 6-sulfatoxymelatonin (1), bringing into question whether results were driven by increased melatonin production in response to a high-GI meal. A subsequent study, which was also conducted among men, tested whether the GI of a postexercise evening meal (a low GI of 52 versus a high GI of 109) impacted sleep quality among recreational athletes (124). Overnight polysomnography demonstrated that total sleep time was longer following the high-GI meal compared with the low-GI meal. This difference in sleep duration was driven by longer sleep onset latency and greater time awake after sleep onset in the low-GI meal condition. Sleep stages, percent of time spent in light versus deep sleep, did not differ between conditions (124).
These experiments seem to confirm a role of GI in sleep promotion (1, 124), but the findings need further replication. First, both studies comprised only men, and both evaluated sleep only in response to variations in GI of a single meal. Moreover, in both studies, the test meal was served in the evening, 4 h (1) and 2 h (124), respectively, before usual bedtime. Meal timing is known to impact the circadian system and downstream physiological processes (84) and could be an important factor in determining the effects of low- and high-GI meals on sleep quality. These studies also provided limited insight into the mechanisms underlying GI effects on sleep parameters, as they lack data on the ratio of circulating tryptophan to LNAAs, and available data on melatonin metabolites yielded null results. More studies, both experimental and observational, are needed to evaluate a potential complex interplay between timing and GI content/glycemic load of meals, as well as the pathway linking dietary GI with sleep.
Mediterranean Dietary Patterns
While the potential impact of dietary GI on sleep relies on brain uptake of tryptophan and the mediating role of insulin (1, 10), dietary patterns that promote intakes of foods rich in melatonin could have a more direct influence on sleep quality. MedDiet is characterized by high intakes of fruits, nuts, and whole grains, which are notable sources of melatonin (Table 1), as well as fish, olive oil, red wine, and limited amounts of dairy, poultry, and red meat (117). We have previously suggested a potential sleep-promoting role of a MedDiet (107, 112), and the literature on this topic has grown substantially since. Despite increasing interest in a possible influence of this dietary pattern on sleep quality, the majority of available studies are cross-sectional in nature. While these studies cannot confirm that adherence to a MedDiet precedes good sleep quality, they do provide an essential foundation from which to build and test hypotheses of a causal relationship.
To our knowledge, the earliest evidence of a relation between adherence to a MedDiet and sleep quality came from an exploratory investigation into lifestyle behaviors that could lower the risk for insomnia in aging populations (45). In this study, conducted among nearly 6,000 French men and women, consumption of a MedDiet, versus not consuming a MedDiet, was associated with significantly lower odds of having one or two insomnia symptoms in women, but not in men (45). Another cross-sectional evaluation demonstrated that greater compliance with a MedDiet was associated with better subjective sleep quality in elderly adults from Greece (62). While sex differences were not observed in this study, results suggest that the MedDiet–sleep quality association may not persist beyond age 75. Interestingly, a study of 970 men aged >70 years also found no association between adherence to a traditional MedDiet and ability to initiate or maintain sleep (122). From these studies of elderly individuals, it is difficult to discern whether MedDiet adherence is linked with sleep quality, as results could be confounded by other health-related issues that occur with aging.
Cross-sectional associations of conformity with a MedDiet and general sleep quality are more consistently observed in middle-aged and younger adults. Observational data from 172 Italian men and women, with an average age of 52 years, showed that greater adherence to a MedDiet was related to better sleep quality (73). In addition, intakes of specific components of the MedDiet, including greater consumption of fruits, vegetables, and tree nuts, were related to better sleep quality. These favorable associations of fruits and vegetables with sleep quality have been corroborated in other cross-sectional investigations of diet and sleep (25, 48, 72, 76). Results of the study by Muscogiuri and colleagues (73) were extended in a cohort of 1,314 men and women aged 18 years and older from Italy, as associations of a MedDiet with both overall sleep quality and components of sleep quality were examined (42). Each one-point increase in MedDiet adherence score was related to 10% greater likelihood of having good overall sleep, including satisfactory sleep duration, sleep onset latency, and sleep efficiency. Investigation of individual foods in the diet revealed that high intakes of fruits and vegetables, among other components, were linked with higher odds of having good sleep quality, including shorter sleep onset latency and higher sleep efficiency. While both of these studies highlighted specific elements of the MedDiet linked with good sleep quality (42, 73), additional analyses from the study by Muscogiuri and colleagues suggested that overall adherence to the MedDiet pattern was a stronger correlate of sleep quality than any one particular component (73).
Results of cross-sectional studies seem to support a relation between MedDiet adherence and sleep quality; however, potential age-related differences cannot be ignored. Although there are limited data, null associations between this dietary pattern and sleep quality have been observed in very elderly men and women (62, 122), a population at heightened risk for insomnia and other sleep disorders (5). We can only speculate as to potential factors underlying these differences. One possibility is that synthesis of melatonin from dietary tryptophan is attenuated in older individuals, as reductions in melatonin production in the pineal gland have been observed with aging (116, 125). Alternatively, increased morbidity and medication use in older adults may adversely affect sleep and obscure any potential influence of diet.
In order to elucidate the directionality of associations between MedDiet patterns and sleep, data from longitudinal studies have been leveraged. The earliest prospective assessment of MedDiet adherence with sleep quality was from the Study on Nutrition and Cardiovascular Risk in Spain (16). Results showed that higher MedDiet adherence was associated with lower odds of large increase or decrease in self-reported sleep duration over a 3-year follow-up period, as well as lower odds of experiencing multiple symptoms of poor sleep quality. In addition, greater adherence to this MedDiet was related to reduced risk of experiencing the combination of large change in sleep duration with multiple symptoms of poor sleep (16).
Two studies from our group, the only available reports from US populations, confirm an association of a MedDiet with better sleep quality (21, 92). In the Multi-Ethnic Study of Atherosclerosis sleep ancillary study, higher adherence to a MedDiet at baseline was associated with 35% lower odds of having insomnia with short sleep (21), a combination consistently shown to increase risk for cardiovascular disease and mortality (123). Longitudinal analyses assessing the impact of an increase or no change in MedDiet adherence over time, compared to a decline in adherence, similarly showed a relation with lower insomnia risk at follow-up, 10 years later (21). In women representing all stages of adulthood, we evaluated for the first time the contribution of individual components of the MedDiet in predicting sleep quality (135). We found that greater adherence to a MedDiet at baseline was associated with better overall sleep quality, as well as higher sleep efficiency and fewer sleep disturbances after 1 year. Furthermore, greater baseline consumption of fruits, vegetables, and legumes, as well as fiber, was predictive of favorable sleep quality outcomes (135). Together, these prospective studies provide further evidence toward a causal influence of MedDiet adherence on sleep health and extend findings by demonstrating that this healthful dietary pattern, characterized by higher intakes of nutrient-rich fruits, vegetables, and legumes, may contribute to improvements in sleep quality (21, 135).
To our knowledge, only one experimental study has evaluated a potential causal effect of a MedDiet on measures of sleep quality (68). In this randomized, controlled trial, 22 women with fibromyalgia were assigned to 16 weeks of a standard MedDiet or MedDiet enhanced with walnuts (a source of dietary tryptophan and magnesium). Subjective sleep quality was measured pre- and postintervention. Improvements in sleep quality were noted in both groups, with no difference between groups, indicating an overall benefit of a MedDiet (68). However, these results must be interpreted with caution. No information was provided on baseline MedDiet adherence; therefore, whether conformity to a MedDiet was increased in response to the intervention is unknown. This restricts our ability to draw conclusions on the direct contribution of the MedDiet to observed changes in sleep quality. Moreover, this study lacked objective measures of sleep, which are needed for a more reliable and comprehensive assessment of changes in sleep quality and architecture, and a control group not following a MedDiet.
Findings from epidemiological studies provide support for the hypothesis that a MedDiet pattern could improve sleep quality or attenuate risk for developing or worsening disordered patterns of sleep. While prospective designs allow for establishment of a temporal relation, there is a dearth of experimental evidence at present. As was the case with the GI–sleep relation, more clinical trials are needed to address two of the major limitations of current cohort studies: lack of objective diet measures and inability to determine causality. Despite the noted limitations, the studies reviewed on MedDiet adherence and sleep provide an essential framework for the development of rigorous intervention studies to investigate whether increasing adherence to a MedDiet can enhance sleep quality or reduce disordered patterns of sleep.
Dietary Approaches to Stop Hypertension and Dietary Inflammatory Index
Other dietary patterns that include nutrients or food sources similar to the MedDiet or low-GI diets have been examined in relation to sleep outcomes. These are scored by the Dietary Approaches to Stop Hypertension (DASH) index (102) and Dietary Inflammatory Index (DII) (22). Investigation of these dietary patterns in relation to sleep is in the nascent stages, but initial data are supportive of an association.
Data from the National Health and Nutrition Examination Survey showed that higher DASH scores were associated with greater daytime dysfunction (a component of poor sleep), although the association was attenuated following further adjustment for health factors (54). This relation may be due to fiber, which was positively related with sleep quality in women and young adults. Two studies conducted among adolescent girls in Iran also revealed that greater adherence to a DASH diet was related to lower odds of having insomnia symptoms (96) and daytime sleepiness (80).
Only one study examined the association between DII score and sleep (43). That study, in nearly 2,000 Italian adults free of sleep disorders, showed that higher DII scores, indicative of greater inflammatory potential of the diet, were associated with higher odds of poor overall sleep quality as well as more frequent sleep disturbances.
These very preliminary data suggest that healthful dietary patterns beyond MedDiet and low GI may also contribute to good sleep health. Higher DASH scores and lower DII scores can be achieved in similar ways as higher MedDiet adherence and lower dietary GI, including through higher intakes of fruits, vegetables, and legumes; therefore, these dietary patterns could potentially impact sleep through shared mechanisms. However, interpretation of epidemiological findings is limited given the cross-sectional design of available studies. Longitudinal data are needed to determine whether adherence to a DASH diet or a diet with low inflammatory potential is predictive of good sleep, and potential mechanisms should be evaluated via intervention and translational studies.
FUTURE DIRECTIONS, PUBLIC HEALTH RELEVANCE, AND RECOMMENDATIONS
Findings of the studies reviewed here have potentially large public health implications. Short sleep duration, poor sleep quality, and insomnia are increasingly recognized as risk factors for cardiometabolic diseases (108). This recognition has led to consensus statements from various public health agencies and foundations encouraging the development and assessment of strategies to improve sleep (53, 108). Available evidence, reviewed here, highlights a potential role of dietary patterns as a modifiable lifestyle factor that could be leveraged to improve sleep health and also uncovers key avenues for future research.
Future Directions
The dietary pattern most consistently linked with favorable sleep outcomes is the MedDiet. Recent investigations using longitudinal data from large cohorts suggest that increasing adherence to a MedDiet may promote healthful patterns of sleep or reduce the risk for disordered patterns of sleep (16, 21, 135). However, an essential next step will be to develop clinical trials to test whether systematic changes in adherence to a MedDiet influence sleep patterns in comparison to a control group. Existing trials such as PREDIMED have demonstrated the feasibility of lifestyle changes to promote a MedDiet over sustained periods (27). Established protocols could be applied to populations with poor sleep health to evaluate downstream effects on sleep using both objective and subjective sleep measures.
As recommendations leveraging healthy dietary patterns to promote good sleep are developed, it will be important to identify the most potent drivers of effects. Several mechanistic pathways have been proposed, including the gut microbiome (112), inflammation (135), and dietary tryptophan and melatonin (107). However, few data are available on the biological underpinnings of the diet–sleep relation. Confirming these pathways in clinical trials will help to identify nutrients and food sources that should be promoted in whole diets. For example, fruits, vegetables, legumes, and nuts are often rich sources of tryptophan and melatonin (Table 1); are high in fiber, which is known to have favorable effects on gut microbial composition (61); and have anti-inflammatory properties. Indeed, intake of these foods is linked with good sleep quality in observational studies (42, 73, 135). As mechanisms are further elucidated, effects of different sources of nutrients can be tested, leading to reliable dietary approaches tailored to improve sleep health. There is a need for additional information on the sleep-promoting nutrient content of foods, including but not limited to those identified in this review.
Another important goal of research will be to identify individual differences in the effect of diet on sleep, enabling use of precision nutrition to make data-driven recommendations for healthy sleep. Data from existing epidemiological studies suggest that age and sex may moderate associations of diet with sleep outcomes (62, 122). Beyond other adverse health consequences, aging is linked with increased risk for poor sleep (5), and this may be particularly pronounced in women (85). This signifies the need to test whether diet can be leveraged to improve sleep, as well as downstream health outcomes, in these at-risk groups.
Public Health Relevance and Recommendations
Up to 55% of US adults struggle with insufficient sleep, poor sleep quality, and/or sleep disorders (57, 77), highlighting the need for novel interventions to improve sleep. Shifting patterns of intake to achieve recommendations common to all healthful diets, including increasing intakes of nutrient-dense fruits and vegetables, represents a potential novel strategy for individuals with poor sleep health. Although more work is needed to provide empirical evidence supporting efficacy of improving diet quality to ameliorate sleep, it is not premature for sleep health recommendations to encourage healthful dietary patterns.
While a variety of healthful diets could have beneficial effects on sleep health based on shared components, much of the current evidence, as noted above, is on the MedDiet. Estimates of associations between MedDiet adherence and sleep quality are often quite large (16, 21, 42, 73, 135), suggesting clinical significance. This is in contrast to more common interventions, including mindfulness meditation and melatonin supplementation, for which effect sizes are often small to moderate (13, 34, 97). Although a rather crude comparison of studies with very different designs does not necessarily suggest that dietary interventions should replace other approaches for sleep promotion, the comparison does demonstrate the potential utility of dietary strategies in the management of subclinical poor sleep. Furthermore, there is no risk to shifting dietary practices toward a MedDiet, as adherence to this dietary pattern is consistent with the dietary guidelines for Americans (120) and has been shown to promote other components of cardiovascular health (27, 67). In contrast, use of pharmaceutical agents to improve sleep can be accompanied by adverse side effects (35, 36). The potential benefits of this lifestyle modification are substantial, as effects on cardiovascular health (63, 67) could be augmented via promotion of healthful sleep.
An evidence-based recommendation for promoting sleep health would be to encourage individuals to increase intakes of fruits, vegetables, legumes, and other low-calorie-density foods, including fish and other lean animal protein sources, all of which are hallmarks of the MedDiet and other healthful dietary patterns (51, 98, 101, 117). In addition to being rich sources of sleep-promoting nutrients and contributing to better sleep, these foods have multiple cardiometabolic health benefits that can further enhance sleep quality.
The goal of these recommendations is to shift away from a vicious cycle of poor sleep prompting consumption of an undesirable diet that further propels poor sleep and overall health, toward a positive cycle where a healthful dietary pattern promotes good sleep and reduces the risk for chronic disease (Figure 1). Now is the time to replace the vicious cycle with a healthful one and leverage precision-nutrition approaches as we aim to improve the sleep health of the US population.
ACKNOWLEDGMENTS
Dr. St-Onge is funded by National Institute of Health (NIH) grants R01HL128226 and R01HL142648 and by American Heart Association (AHA) Go Red for Women grant 16SFRN27950012. Dr. Zuraikat is a Berrie Fellow in Diabetes Research and is supported by NIH grant T32HL007343.
Footnotes
DISCLOSURE STATEMENT
Dr. St-Onge has served as a consultant to PepsiCo and Nestle.
LITERATURE CITED
- 1.Afaghi A, O’Connor H, Chow CM. 2008. Acute effects of the very low carbohydrate diet on sleep indices. Nutr. Neurosci 11:146–54 [DOI] [PubMed] [Google Scholar]
- 2.Al Khatib HK, Hall WL, Creedon A, Ooi E, Masri T, et al. 2018. Sleep extension is a feasible lifestyle intervention in free-living adults who are habitually short sleepers: a potential strategy for decreasing intake of free sugars? A randomized controlled pilot study. Am. J. Clin. Nutr 107:43–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Khatib HK, Harding SV, Darzi J, Pot GK. 2017. The effects of partial sleep deprivation on energy balance: a systematic review and meta-analysis. Eur. J. Clin. Nutr 71:614–24 [DOI] [PubMed] [Google Scholar]
- 4.Anand SS, Hawkes C, de Souza RJ, Mente A, Dehghan M, et al. 2015. Food consumption and its impact on cardiovascular disease: importance of solutions focused on the globalized food system: a report from the workshop convened by the World Heart Federation. J. Am. Coll. Cardiol 66:1590–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ancoli-Israel S 2009. Sleep and its disorders in aging populations. Sleep Med 10(Suppl 1):S7–11 [DOI] [PubMed] [Google Scholar]
- 6.Augustin LS, Kendall CW, Jenkins DJ, Willett WC, Astrup A, et al. 2015. Glycemic index, glycemic load and glycemic response: an international scientific consensus summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis 25:795–815 [DOI] [PubMed] [Google Scholar]
- 7.Badria FA. 2002. Melatonin, serotonin, and tryptamine in some Egyptian food and medicinal plants. J. Med. Food 5:153–57 [DOI] [PubMed] [Google Scholar]
- 8.Barik S 2020. The uniqueness of tryptophan in biology: properties, metabolism, interactions and localization in proteins. Int. J. Mol. Sci 21:8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beebe DW, Simon S, Summer S, Hemmer S, Strotman D, Dolan LM. 2013. Dietary intake following experimentally restricted sleep in adolescents. Sleep 36:827–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry EM, Growdon JH, Wurtman JJ, Caballero B, Wurtman RJ. 1991. A balanced carbohydrate. Neurology 41:1295. [DOI] [PubMed] [Google Scholar]
- 11.Bravo R, Matito S, Cubero J, Paredes SD, Franco L, et al. 2013. Tryptophan-enriched cereal intake improves nocturnal sleep, melatonin, serotonin, and total antioxidant capacity levels and mood in elderly humans. Age 35:1277–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brzezinski A 1997. Melatonin in humans. N. Engl. J. Med 336:186–95 [DOI] [PubMed] [Google Scholar]
- 13.Brzezinski A, Vangel MG, Wurtman RJ, Norrie G, Zhdanova I, et al. 2005. Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med. Rev 9:41–50 [DOI] [PubMed] [Google Scholar]
- 14.Burkhardt S, Tan DX, Manchester LC, Hardeland R, Reiter RJ. 2001. Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus). J. Agric. Food Chem 49:4898–902 [DOI] [PubMed] [Google Scholar]
- 15.Buysse DJ. 2013. Insomnia. JAMA 309:706–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campanini MZ, Guallar-Castillón P, Rodríguez-Artalejo F, Lopez-Garcia E. 2017. Mediterranean diet and changes in sleep duration and indicators of sleep quality in older adults. Sleep 40:zsw083 [DOI] [PubMed] [Google Scholar]
- 17.Campbell A, Neill A. 2016. Melatonin-rich milk fortified with alpha s1 casein tryptic hydrolysate improves primary insomnia: a randomized placebo controlled trial. Sleep Biol. Rhythms 14:351–60 [Google Scholar]
- 18.Capers PL, Fobian AD, Kaiser KA, Borah R, Allison DB. 2015. A systematic review and meta-analysis of randomized controlled trials of the impact of sleep duration on adiposity and components of energy balance. Obes. Rev 16:771–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. 2011. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur. Heart J 32:1484–92 [DOI] [PubMed] [Google Scholar]
- 20.Cappuccio FP, Taggart FM, Kandala N-B, Currie A, Peile E, et al. 2008. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 31:619–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castro-Diehl C, Wood AC, Redline S, Reid M, Johnson DA, et al. 2018. Mediterranean diet pattern and sleep duration and insomnia symptoms in the Multi-Ethnic Study of Atherosclerosis. Sleep 41:zsy158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, et al. 2009. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J. Nutr 139:2365–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaput JP. 2014. Sleep patterns, diet quality and energy balance. Physiol. Behav 134:86–91 [DOI] [PubMed] [Google Scholar]
- 24.Chaput JP, St-Onge MP. 2014. Increased food intake by insufficient sleep in humans: Are we jumping the gun on the hormonal explanation? Front. Endocrinol 5:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng FW, Li Y, Winkelman JW, Hu FB, Rimm EB, Gao X. 2016. Probable insomnia is associated with future total energy intake and diet quality in men. Am. J. Clin. Nutr 104:462–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dashti HS, Scheer FA, Jacques PF, Lamon-Fava S, Ordovas JM. 2015. Short sleep duration and dietary intake: epidemiologic evidence, mechanisms, and health implications. Adv. Nutr 6:648–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, et al. 2018. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med 378:e34. [DOI] [PubMed] [Google Scholar]
- 28.Fakhr-Movahedi A, Mirmohammadkhani M, Ramezani H. 2018. Effect of milk-honey mixture on the sleep quality of coronary patients: a clinical trial study. Clin. Nutr. ESPEN 28:132–35 [DOI] [PubMed] [Google Scholar]
- 29.Fatima Y, Doi SA, Mamun AA. 2016. Sleep quality and obesity in young subjects: a meta-analysis. Obes. Rev 17:1154–66 [DOI] [PubMed] [Google Scholar]
- 30.Feldman JM, Lee EM. 1985. Serotonin content of foods: effect on urinary excretion of 5-hydroxyindoleacetic acid. Am. J. Clin. Nutr 42:639–43 [DOI] [PubMed] [Google Scholar]
- 31.Fenton S, Burrows TL, Skinner JA, Duncan MJ. 2021. The influence of sleep health on dietary intake: a systematic review and meta-analysis of intervention studies. J. Hum. Nutr. Diet 34:273–85 [DOI] [PubMed] [Google Scholar]
- 32.Fernstrom JD. 2013. Large neutral amino acids: dietary effects on brain neurochemistry and function. Amino Acids 45:419–30 [DOI] [PubMed] [Google Scholar]
- 33.Fernstrom JD, Wurtman RJ. 1972. Brain serotonin content: physiological regulation by plasma neutral amino acids. Science 178:414–16 [DOI] [PubMed] [Google Scholar]
- 34.Ferracioli-Oda E, Qawasmi A, Bloch MH. 2013. Meta-analysis: melatonin for the treatment of primary sleep disorders. PLOS ONE 8:e63773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzgerald T, Vietri J. 2015. Residual effects of sleep medications are commonly reported and associated with impaired patient-reported outcomes among insomnia patients in the United States. Sleep Disord 2015:607148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foley HM, Steel AE. 2019. Adverse events associated with oral administration of melatonin: a critical systematic review of clinical evidence. Complement Ther. Med 42:65–81 [DOI] [PubMed] [Google Scholar]
- 37.Foster-Powell K, Holt SH, Brand-Miller JC. 2002. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr 76:5–56 [DOI] [PubMed] [Google Scholar]
- 38.Gallegos JV, Boege HL, Zuraikat F, St-Onge M-P. 2021. Does sex influence the effects of experimental sleep curtailment and circadian misalignment on regulation of appetite? Curr. Opin. Endocr. Metab. Res 17:20–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gangwisch JE, Hale L, St-Onge MP, Choi L, LeBlanc ES, et al. 2020. High glycemic index and glycemic load diets as risk factors for insomnia: analyses from the Women’s Health Initiative. Am. J. Clin. Nutr 111:429–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garrido M, Gonzalez-Gomez D, Lozano M, Barriga C, Paredes SD, Rodriguez AB. 2013. A Jerte valley cherry product provides beneficial effects on sleep quality. Influence on aging. J. Nutr. Health Aging 17:553–60 [DOI] [PubMed] [Google Scholar]
- 41.Garrido M, Paredes SD, Cubero J, Lozano M, Toribio-Delgado AF, et al. 2010. Jerte Valley cherry-enriched diets improve nocturnal rest and increase 6-sulfatoxymelatonin and total antioxidant capacity in the urine of middle-aged and elderly humans. J. Gerontol. A 65:909–14 [DOI] [PubMed] [Google Scholar]
- 42.Godos J, Ferri R, Caraci F, Cosentino FII, Castellano S, et al. 2019. Adherence to the Mediterranean diet is associated with better sleep quality in Italian adults. Nutrients 11:976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Godos J, Ferri R, Caraci F, Cosentino FII, Castellano S, et al. 2019. Dietary inflammatory index and sleep quality in Southern Italian adults. Nutrients 11:1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howatson G, Bell PG, Tallent J, Middleton B, McHugh MP, Ellis J. 2012. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur. J. Nutr 51:909–16 [DOI] [PubMed] [Google Scholar]
- 45.Jaussent I, Dauvilliers Y, Ancelin ML, Dartigues JF, Tavernier B, et al. 2011. Insomnia symptoms in older adults: associated factors and gender differences. Am. J. Geriatr. Psychiatry 19:88–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Javaheri S, Redline S. 2017. Insomnia and risk of cardiovascular disease. Chest 152:435–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, et al. 1981. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am. J. Clin. Nutr 34:362–66 [DOI] [PubMed] [Google Scholar]
- 48.Katagiri R, Asakura K, Kobayashi S, Suga H, Sasaki S. 2014. Low intake of vegetables, high intake of confectionary, and unhealthy eating habits are associated with poor sleep quality among middle-aged female Japanese workers. J. Occup. Health 56:359–68 [DOI] [PubMed] [Google Scholar]
- 49.Kelley DS, Adkins Y, Laugero KD. 2018. A review of the health benefits of cherries. Nutrients 10:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knutson KL, Ryden AM, Mander BA, Van Cauter E. 2006. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch. Intern. Med 166:1768–74 [DOI] [PubMed] [Google Scholar]
- 51.Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, et al. 2018. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet 118:1591–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwok CS, Kontopantelis E, Kuligowski G, Gray M, Muhyaldeen A, et al. 2018. Self-reported sleep duration and quality and cardiovascular disease and mortality: a dose-response meta-analysis. J. Am. Heart Assoc 7:e008552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laposky AD, Van Cauter E, Diez-Roux AV. 2016. Reducing health disparities: the role of sleep deficiency and sleep disorders. Sleep. Med 18:3–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang H, Beydoun HA, Hossain S, Maldonado A, Zonderman AB, et al. 2020. Dietary Approaches to Stop Hypertension (DASH) score and its association with sleep quality in a national survey of middle-aged and older men and women. Nutrients 12:1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin HH, Tsai PS, Fang SC, Liu JF. 2011. Effect of kiwifruit consumption on sleep quality in adults with sleep problems. Asia Pac. J. Clin. Nutr 20:169–74 [PubMed] [Google Scholar]
- 56.Lin J, Jiang Y, Wang G, Meng M, Zhu Q, et al. 2020. Associations of short sleep duration with appetite-regulating hormones and adipokines: a systematic review and meta-analysis. Obes. Rev 21:e13051. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. 2016. Prevalence of healthy sleep duration among adults—United States, 2014. Morb. Mortal. Wkly. Rep 65:137–41 [DOI] [PubMed] [Google Scholar]
- 58.Losso JN, Finley JW, Karki N, Liu AG, Prudente A, et al. 2018. Pilot study of tart cherry juice for the treatment of insomnia and investigation of mechanisms. Am. J. Ther 25:e194–e201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lv W, Finlayson G, Dando R. 2018. Sleep, food cravings and taste. Appetite 125:210–16 [DOI] [PubMed] [Google Scholar]
- 60.MacInnis MJ, Dziedzic CE, Wood E, Oikawa SY, Phillips SM. 2020. Presleep alpha-lactalbumin consumption does not improve sleep quality or time-trial performance in cyclists. Int. J. Sport Nutr. Exerc. Metab 30:197–202 [DOI] [PubMed] [Google Scholar]
- 61.Makki K, Deehan EC, Walter J, Bäckhed F. 2018. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 23:705–15 [DOI] [PubMed] [Google Scholar]
- 62.Mamalaki E, Anastasiou CA, Ntanasi E, Tsapanou A, Kosmidis MH, et al. 2018. Associations between the Mediterranean diet and sleep in older adults: results from the Hellenic Longitudinal Investigation of Aging and Diet study. Geriatr. Gerontol. Int 18:1543–48 [DOI] [PubMed] [Google Scholar]
- 63.Mancini JG, Filion KB, Atallah R, Eisenberg MJ. 2016. Systematic review of the Mediterranean diet for long-term weight loss. Am. J. Med 129:407–15.e4 [DOI] [PubMed] [Google Scholar]
- 64.Markus CR, Jonkman LM, Lammers JH, Deutz NE, Messer MH, Rigtering N. 2005. Evening intake of alpha-lactalbumin increases plasma tryptophan availability and improves morning alertness and brain measures of attention. Am. J. Clin. Nutr 81:1026–33 [DOI] [PubMed] [Google Scholar]
- 65.Markus CR, Olivier B, Panhuysen GE, Van Der Gugten J, Alles MS, et al. 2000. The bovine protein alpha-lactalbumin increases the plasma ratio of tryptophan to the other large neutral amino acids, and in vulnerable subjects raises brain serotonin activity, reduces cortisol concentration, and improves mood under stress. Am. J. Clin. Nutr 71:1536–44 [DOI] [PubMed] [Google Scholar]
- 66.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, et al. 2013. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. PNAS 110:5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martinez-Gonzalez MA, Gea A, Ruiz-Canela M. 2019. The Mediterranean diet and cardiovascular health. Circ. Res 124:779–98 [DOI] [PubMed] [Google Scholar]
- 68.Martínez-Rodríguez A, Rubio-Arias JÁ, Ramos-Campo DJ, Reche-García C, Leyva-Vela B, Nadal-Nicolás Y. 2020. Psychological and sleep effects of tryptophan and magnesium-enriched Mediterranean diet in women with fibromyalgia. Int. J. Environ. Res. Public Health 17:2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Milagres MP, Minim VP, Minim LA, Simiqueli AA, Moraes LE, Martino HS. 2014. Night milking adds value to cow’s milk. J. Sci. Food Agric 94:1688–92 [DOI] [PubMed] [Google Scholar]
- 70.Minet-Ringuet J, Le Ruyet PM, Tomé D, Even PC. 2004. A tryptophan-rich protein diet efficiently restores sleep after food deprivation in the rat. Behav. Brain Res 152:335–40 [DOI] [PubMed] [Google Scholar]
- 71.Monti JM. 2011. Serotonin control of sleep-wake behavior. Sleep Med. Rev 15:269–81 [DOI] [PubMed] [Google Scholar]
- 72.Mossavar-Rahmani Y, Weng J, Wang R, Shaw PA, Jung M, et al. 2017. Actigraphic sleep measures and diet quality in the Hispanic Community Health Study/Study of Latinos Sueño ancillary study. J. Sleep Res 26:739–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muscogiuri G, Barrea L, Aprano S, Framondi L, Di Matteo R, et al. 2020. Sleep quality in obesity: Does adherence to the Mediterranean diet matter? Nutrients 12:1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. 2009. Sleep curtailment is accompanied by increased intake of calories from snacks. Am. J. Clin. Nutr 89:126–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nødtvedt ØO, Hansen AL, Bjorvatn B, Pallesen S. 2017. The effects of kiwi fruit consumption in students with chronic insomnia symptoms: a randomized controlled trial. Sleep Biol. Rhythms 15:159–66 [Google Scholar]
- 76.Noorwali EA, Cade JE, Burley VJ, Hardie LJ. 2018. The relationship between sleep duration and fruit/vegetable intakes in UK adults: a cross-sectional study from the National Diet and Nutrition Survey. BMJ Open 8:e020810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Knutson KL, Phelan J, Paskow MJ, Roach A, Whiton K, et al. 2017. The National Sleep Foundation’s Sleep Health Index. Sleep Health 3(4):234–40 [DOI] [PubMed] [Google Scholar]
- 78.O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. 2015. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res 277:32–48 [DOI] [PubMed] [Google Scholar]
- 79.Ong JN, Hackett DA, Chow CM. 2017. Sleep quality and duration following evening intake of alpha-lactalbumin: a pilot study. Biol. Rhythm. Res 48:507–17 [Google Scholar]
- 80.Pahlavani N, Khayyatzadeh SS, Banazadeh V, Bagherniya M, Tayefi M, et al. 2020. Adherence to a Dietary Approach to Stop Hypertension (DASH)-style in relation to daytime sleepiness. Nat. Sci. Sleep 12:325–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Palego L, Betti L, Rossi A, Giannaccini G. 2016. Tryptophan biochemistry: structural, nutritional, metabolic, and medical aspects in humans. J. Amino Acids 2016:8952520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paredes SD, Barriga C, Reiter RJ, Rodríguez AB. 2009. Assessment of the potential role of tryptophan as the precursor of serotonin and melatonin for the aged sleep-wake cycle and immune function: Streptopelia risoria as a model. Int. J. Tryptophan. Res 2:23–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patel SR, Hu FB. 2008. Short sleep duration and weight gain: a systematic review. Obesity 16:643–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patton DF, Mistlberger RE. 2013. Circadian adaptations to meal timing: neuroendocrine mechanisms. Front. Neurosci 7:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pengo MF, Won CH, Bourjeily G. 2018. Sleep in women across the life span. Chest 154:196–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peuhkuri K, Sihvola N, Korpela R. 2012. Dietary factors and fluctuating levels of melatonin. Food Nutr. Res 56: 10.3402/fnr.v56i0.17252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pigeon WR, Carr M, Gorman C, Perlis ML. 2010. Effects of a tart cherry juice beverage on the sleep of older adults with insomnia: a pilot study. J. Med. Food 13:579–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Portas CM, Bjorvatn B, Ursin R. 2000. Serotonin and the sleep/wake cycle: special emphasis on micro-dialysis studies. Progress Neurobiol 60:13–35 [DOI] [PubMed] [Google Scholar]
- 89.Qin Y, Shi W, Zhuang J, Liu Y, Tang L, et al. 2019. Variations in melatonin levels in preterm and term human breast milk during the first month after delivery. Sci. Rep 9:17984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rahe C, Czira ME, Teismann H, Berger K. 2015. Associations between poor sleep quality and different measures of obesity. Sleep Med 16:1225–28 [DOI] [PubMed] [Google Scholar]
- 91.Rancillac A 2016. Serotonin and sleep-promoting neurons. Oncotarget 7:78222–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reid M, Maras JE, Shea S, Wood AC, Castro-Diehl C, et al. 2019. Association between diet quality and sleep apnea in the Multi-Ethnic Study of Atherosclerosis. Sleep 42:zsy194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reiter RJ, Manchester LC, Tan D-x. 2005. Melatonin in walnuts: influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 21:920–24 [DOI] [PubMed] [Google Scholar]
- 94.Reutrakul S, Van Cauter E. 2018. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metab. Clin. Exp 84:56–66 [DOI] [PubMed] [Google Scholar]
- 95.Rihm JS, Menz MM, Schultz H, Bruder L, Schilbach L, et al. 2019. Sleep deprivation selectively upregulates an amygdala-hypothalamic circuit involved in food reward. J. Neurosci 39:888–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rostami H, Khayyatzadeh SS, Tavakoli H, Bagherniya M, Mirmousavi SJ, et al. 2019. The relationship between adherence to a Dietary Approach to Stop Hypertension (DASH) dietary pattern and insomnia. BMC Psychiatry 19:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rusch HL, Rosario M, Levison LM, Olivera A, Livingston WS, et al. 2019. The effect of mindfulness meditation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. Ann. N. Y. Acad. Sci 1445:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sacks FM, Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, et al. 1999. A dietary approach to prevent hypertension: a review of the Dietary Approaches to Stop Hypertension (DASH) study. Clin. Cardiol 22:III6–10 [DOI] [PubMed] [Google Scholar]
- 99.Salehi B, Sharopov F, Fokou PVT, Kobylinska A, Jonge L, et al. 2019. Melatonin in medicinal and food plants: occurrence, bioavailability, and health potential for humans. Cells 8:681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shan Z, Ma H, Xie M, Yan P, Guo Y, et al. 2015. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care 38:529–37 [DOI] [PubMed] [Google Scholar]
- 101.Smethers AD, Rolls BJ. 2018. Dietary management of obesity: cornerstones of healthy eating patterns. Med. Clin. North Am 102:107–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Soltani S, Chitsazi MJ, Salehi-Abargouei A. 2018. The effect of Dietary Approaches to Stop Hypertension (DASH) on serum inflammatory markers: a systematic review and meta-analysis of randomized trials. Clin. Nutr 37:542–50 [DOI] [PubMed] [Google Scholar]
- 103.Spiegel K, Tasali E, Penev P, Van Cauter E. 2004. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med 141:846–50 [DOI] [PubMed] [Google Scholar]
- 104.Srinivasan V, Pandi-Perumal SR, Trahkt I, Spence DW, Poeggeler B, et al. 2009. Melatonin and melatonergic drugs on sleep: possible mechanisms of action. Int. J. Neurosci 119:821–46 [DOI] [PubMed] [Google Scholar]
- 105.St-Onge MP. 2013. The role of sleep duration in the regulation of energy balance: effects on energy intakes and expenditure. J. Clin. Sleep Med 9:73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.St-Onge MP. 2017. Sleep-obesity relation: underlying mechanisms and consequences for treatment. Obes. Rev 18(Suppl. 1):34–39 [DOI] [PubMed] [Google Scholar]
- 107.St-Onge MP, Crawford A, Aggarwal B. 2018. Plant-based diets: reducing cardiovascular risk by improving sleep quality? Curr. Sleep Med. Rep 4:74–78 [PMC free article] [PubMed] [Google Scholar]
- 108.St-Onge MP, Grandner MA, Brown D, Conroy MB, Jean-Louis G, et al. 2016. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation 134:e367–e386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.St-Onge MP, Mikic A, Pietrolungo CE. 2016. Effects of diet on sleep quality. Adv. Nutr 7:938–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.St-Onge MP, Roberts AL, Chen J, Kelleman M, O’Keeffe M, et al. 2011. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am. J. Clin. Nutr 94:410–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.St-Onge MP, Wolfe S, Sy M, Shechter A, Hirsch J. 2014. Sleep restriction increases the neuronal response to unhealthy food in normal-weight individuals. Int. J. Obes 38:411–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.St-Onge MP, Zuraikat FM. 2019. Reciprocal roles of sleep and diet in cardiovascular health: a review of recent evidence and a potential mechanism. Curr. Atheroscler Rep 21:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stürtz M, Cerezo AB, Cantos-Villar E, Garcia-Parrilla MC. 2011. Determination of the melatonin content of different varieties of tomatoes (Lycopersicon esculentum) and strawberries (Fragaria ananassa). Food Chem 127:1329–34 [DOI] [PubMed] [Google Scholar]
- 114.Szczygiel EJ, Cho S, Tucker RM. 2019. Multiple dimensions of sweet taste perception altered after sleep curtailment. Nutrients 11:2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tasali E, Chapotot F, Wroblewski K, Schoeller D. 2014. The effects of extended bedtimes on sleep duration and food desire in overweight young adults: a home-based intervention. Appetite 80:220–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Touitou Y 2001. Human aging and melatonin. Clinical relevance. Exp. Gerontol 36:1083–100 [DOI] [PubMed] [Google Scholar]
- 117.Trichopoulou A, Martínez-González MA, Tong TY, Forouhi NG, Khandelwal S, et al. 2014. Definitions and potential health benefits of the Mediterranean diet: views from experts around the world. BMC Med 12:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tzischinsky O, Shlitner A, Lavie P. 1993. The association between the nocturnal sleep gate and nocturnal onset of urinary 6-sulfatoxymelatonin. J. Biol. Rhythms 8:199–209 [DOI] [PubMed] [Google Scholar]
- 119.Ursin R 2002. Serotonin and sleep. Sleep Med. Rev 6:55–69 [DOI] [PubMed] [Google Scholar]
- 120.Diet. Guidel. Advis. Comm. 2020. Scientific report of the 2020 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Agriculture and the Secretary of Health and Human Services. Rep., US Dep. Agric., Agric. Res. Serv., Washington, DC. https://www.dietaryguidelines.gov/sites/default/files/2020-07/ScientificReport_of_the_2020DietaryGuidelinesAdvisoryCommittee_first-print.pdf [Google Scholar]
- 121.Valtonen M, Niskanen L, Kangas AP, Koskinen T. 2005. Effect of melatonin-rich night-time milk on sleep and activity in elderly institutionalized subjects. Nord. J. Psychiatry 59:217–21 [DOI] [PubMed] [Google Scholar]
- 122.van Egmond L, Tan X, Sjögren P, Cederholm T, Benedict C. 2019. Association between healthy dietary patterns and self-reported sleep disturbances in older men: the ULSAM Study. Nutrients 11:1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. 2013. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med. Rev 17:241–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vlahoyiannis A, Aphamis G, Andreou E, Samoutis G, Sakkas GK, Giannaki CD. 2018. Effects of high versus low glycemic index of post-exercise meals on sleep and exercise performance: a randomized, double-blind, counterbalanced polysomnographic study. Nutrients 10:1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu Y-H, Swaab DF. 2005. The human pineal gland and melatonin in aging and Alzheimer’s disease. J. Pineal Res 38:145–52 [DOI] [PubMed] [Google Scholar]
- 126.Wurtman RJ, Hefti F, Melamed E. 1980. Precursor control of neurotransmitter synthesis. Pharmacol. Rev 32:315–35 [PubMed] [Google Scholar]
- 127.Wurtman RJ, Wurtman JJ, Regan MM, McDermott JM, Tsay RH, Breu JJ. 2003. Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios. Am. J. Clin. Nutr 77:128–32 [DOI] [PubMed] [Google Scholar]
- 128.Yamamura S, Morishima H, Kumano-go T, Suganuma N, Matsumoto H, et al. 2009. The effect of Lactobacillus helveticus fermented milk on sleep and health perception in elderly subjects. Eur. J. Clin. Nutr 63:100–5 [DOI] [PubMed] [Google Scholar]
- 129.Yang CL, Schnepp J, Tucker RM. 2019. Increased hunger, food cravings, food reward, and portion size selection after sleep curtailment in women without obesity. Nutrients 11:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang T-H, Chen YC, Ou T-H, Chien Y-W. 2020. Dietary supplement of tomato can accelerate urinary aMT6s level and improve sleep quality in obese postmenopausal women. Clin. Nutr 39:291–97 [DOI] [PubMed] [Google Scholar]
- 131.Yoneyama S, Sakurai M, Nakamura K, Morikawa Y, Miura K, et al. 2014. Associations between rice, noodle, and bread intake and sleep quality in Japanese men and women. PLOS ONE 9:e105198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhao D, Yu Y, Shen Y, Liu Q, Zhao Z, et al. 2019. Melatonin synthesis and function: evolutionary history in animals and plants. Front. Endocrinol 10:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zisapel N 2018. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol 175:3190–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zuraikat FM, Makarem N, Liao M, St-Onge MP, Aggarwal B. 2020. Measures of poor sleep quality are associated with higher energy intake and poor diet quality in a diverse sample of women from the Go Red for Women strategically focused research network. J. Am. Heart Assoc 9:e014587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zuraikat FM, Makarem N, St-Onge M-P, Xi H, Akkapeddi A, Aggarwal B. 2020. A Mediterranean dietary pattern predicts better sleep quality in US women from the American Heart Association Go Red for Women strategically focused research network. Nutrients 12:2830. [DOI] [PMC free article] [PubMed] [Google Scholar]


