Abstract
Background
Growing consideration is emerging regarding the burden of persisting sequelae after SARS-CoV-2 infection. Out-patients exhibiting long Covid may benefit from ambulatory rehabilitation which is, to date, poorly documented.
Methods
A longitudinal follow-up over a one-year period was conducted in two ambulatory rehabilitation structures in order to describe the characteristics of real-life patients referred with Covid-19 sequelae and their evolution over the course of rehabilitation.
Results
39 consecutive patients were included from April 1st, 2020 to April 1st, 2021. Patients were middle-aged (48 ± 15yr), without comorbidities, and mostly mild to moderate SARS-CoV-2 infection (25(64%) not requiring hospitalisation). Rehabilitation referral was considered with a median delay of 73[34–178] days after disease onset. Most prevalent symptoms were dyspnoea (n = 35(90%)) and fatigue (n = 30(77%)). Hyperventilation syndrome was highly frequent (n = 12(34%)). 29(74%) patients presented with prolonged functional sequelae, which was associated with younger age (43 ± 14 vs. 50 ± 10yr; p = 0.002), greater prevalence of hyperventilation syndrome (n = 12(41%) vs. 0(0%); p = 0.255) and poorer quality of life (VQ-11; 31 ± 10 vs. 23 ± 9; p = 0.030). Over the course of rehabilitation, exertional dyspnoea, 6-min walking distance, 3-min sit-to-stand test, hyperventilation syndrome prevalence and quality of life significantly improved.
Conclusion
Hyperventilation is frequent in long Covid and may explain persistent dyspnoea as well as altered quality of life. Our data support screening of hyperventilation syndrome and functional impairment in mild Covid-19 out-patients as both of these components may improve with ambulatory rehabilitation.
Keywords: Post-Covid-19 syndrome, Long Covid, Hyperventilation syndrome, Ambulatory physiotherapy, Respiratory rehabilitation
1. Introduction
Since the outbreak of the current Covid-19 pandemic, first descriptions of cohorts of patients infected with SARS-CoV-2 suggested that this disease would yield a wide spectrum of clinical manifestations, ranging from asymptomatic to severe and critical presentation requiring hospital admission [1,2]. Early clinical guidelines as well as public health policies naturally focused on this severe population in order to reduce pressure on hospital facilities and especially intensive care units [[3], [4], [5]]. Besides, long-lasting symptoms in patients discharged from hospital after severe SARS-CoV-2 infection have been reported [[6], [7], [8]]. However, it is noteworthy that more than 80% of patients infected with SARS-CoV-2 did not exhibit severe or critical manifestations and were managed as outpatients [2,9]. Furthermore, there are few data about persistent symptoms after mild-to-moderate SARS-CoV-2 infection. In this latter population, increasing evidences from long-term follow-up report substantial prevalence of persisting symptoms after the acute episode, including chronic cough, shortness of breath, chest tightness, cognitive dysfunction, and extreme fatigue [8,[10], [11], [12]]. This so-called “long Covid” which can be defined as the presence of symptoms for more than 3 months after the initial onset of the disease raises new public health concerns as it is reported to potentially affect from 10% to 51% of patients [10,11,13].
In this context and despite little evidence, several patients infected at the beginning of the pandemic were referred to ambulatory respiratory rehabilitation with the aim of improving functional impairment and/or persisting dyspnoea [4,10,[14], [15], [16], [17]]. From one-year experience, we thus designed the current study in order 1) to describe the clinical and functional characteristics of real-life patients referred to ambulatory respiratory rehabilitation following SARS-CoV-2 infection, 2) to describe the evolution of these patients over the course of rehabilitation.
2. Material and methods
2.1. Study design and participants
This longitudinal observational study was conducted in two ambulatory physiotherapy structures specialised in outpatient respiratory rehabilitation in Bordeaux area, France. The study protocol was registered under number F20210419101248 on Health Data Hub (www.heath-data-hub.fr) and conformity declaration was provided to the Commission Nationale Informatique et Libertés (CNIL; www.cnil.fr) under registration number 2221978v0. Written informed consent was obtained from all participants. Study protocol was conducted according to STROBE guidelines.
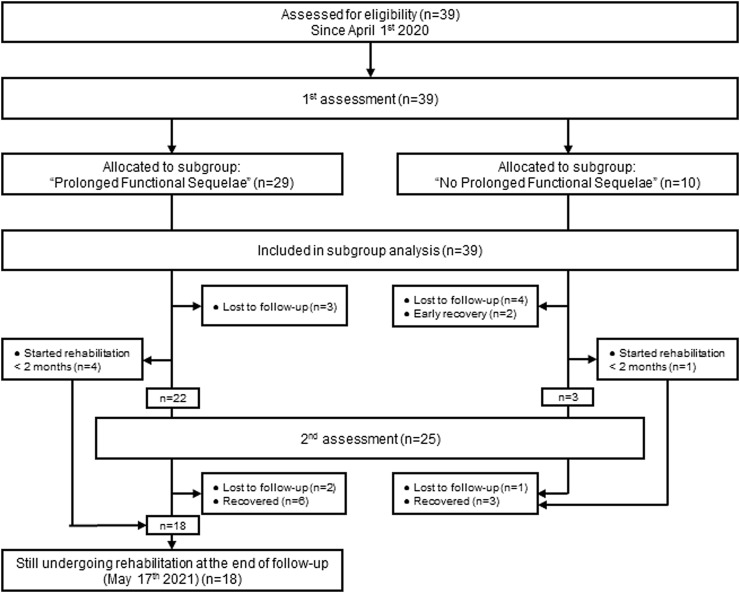
All patients referred in a participating centre with a medical prescription of ambulatory respiratory rehabilitation following suspected or confirmed SARS-CoV-2 infection were consecutively enrolled in this study. The referral decision was made in case of unusual dyspnoea and/or functional impairment deemed likely to benefit from rehabilitation at the discretion of the attending pulmonologist. Patients were included from April 1st, 2020 to April 1st, 2021. The presence of prolonged functional sequelae (PFS) was defined as persistence of rehabilitation requirement more than three months after disease onset. Follow-up therefore continued until participants with the latest disease onset date achieved this 3-months threshold, i.e. May 17th, 2021 (Fig. 1 ).
Fig. 2.
Flow diagram of the study.
2.2. Data collection
Consistently with usual care, a first evaluation was conducted at the time of rehabilitation referral, and a second after two months of rehabilitation. The second evaluation was anticipated when patients recovered within this period. Recovery was defined as normalisation of functional deficiencies (6- minutes walking distance >95% of the predicted normal value, mMRC = 0, resolution of hyperventilation syndrome).
Standardised evaluation included collection of anthropometric data, work situation, comorbidities and clinical symptoms. Disease onset date was defined as the self-reported date of first symptoms. Time before rehabilitation was defined as the duration between disease onset and beginning of rehabilitation. For patients who were still undergoing rehabilitation at the end of the follow-up period, rehabilitation duration was censored and defined as the difference between the beginning of rehabilitation and May 17th, 2021 (n = 18).
Functional assessment included forced spirometry parameters (i.e., forced expiratory volume in 1 s(FEV1), forced vital capacity (FVC) and FEV1/FVC ratio) [18], 3 min sit-to-stand test (3-STS) and 6 min walking test (6-MWT) [19] with baseline recording of pulsed oxygen saturation (SpO2) at rest and at the end of the 6-MWT. Dyspnoea was assessed using modified Medical Research Council scale (mMRC) (0–4) and modified Borg dyspnoea scale (0-10) at rest and at the end of the 6-MWT. Quality of life was evaluated by the VQ-11 questionnaire [20,21]. We were soon confronted with the fact that a substantial number of patients presented with a hyperventilation pattern associated for some of them with anxiety symptoms and therefore included later a systematic screening for hyperventilation syndrome (complete cases: n = 35) and assessment of the Hospital Anxiety and Depression scale (HAD) [22] (complete cases: n = 29). Hyperventilation syndrome was defined as the combination of a Nijmegen score >22 and a positive hyperventilation provocation test [23,24]. Provocation test was conducted with end-tidal CO2 monitoring (PEt-CO2) (LifeSense® II, Nonin Medical Inc, Plymouth, MN, USA) and was considered positive when, after a 3-min voluntary hyperventilation, recovery of baseline PEt-CO2 (±10%) was >5 min [24].
Rehabilitation program was conducted as sessions of 1.5 h three times a week, and included aerobic exercise and strength training combined with specific controlled ventilation techniques when necessary.
2.3. Statistical analysis
Continuous data are presented according to their distribution as mean and standard deviations (mean ± SD) or median and interquartile range (median[IQR]). Categorical data are presented as n(%). For variables including missing data, in the population characteristics before rehabilitation (Table 1 ) analysis was performed on available-cases without imputing data, in the evolution over the course of rehabilitation analysis was performed on complete-cases.
Table 1.
Patient's characteristics at first assessment.
| Total | Prolonged functional sequelae | No prolonged functional sequelae | p-value | ||
|---|---|---|---|---|---|
| Population characteristics; n | 39 | 29 | 10 | ||
| Age (yr) | 48 ± 15 | 43 ± 14 | 60 ± 10 | 0.002 | |
| Male; no.(%) | 17(44%) | 11(38%) | 6(60%) | 0.281 | |
| Body mass index (kg.m−2) | 25 ± 4 | 25 ± 5 | 25 ± 2 | 0.946 | |
| Work situation | |||||
| Off work; no.(%) | 25(64%) | 20(69%) | 5(50%) | 0.446 | |
| No occupation, retirement; no.(%) | 8(21%) | 4(14%) | 4(40%) | 0.167 | |
| Working: no.(%) | 6(15%) | 5(17%) | 1(10%) | 1 | |
| Initial hospitalisation >48h; no.(%) | 14(36%) | 8(28%) | 6(60%) | 0.123 | |
| Time before rehabilitation (d); (median[IQR]) | 73[34–178] | 107[56–240] | 32 [26–41] | <.001 | |
| Rehabilitation duration (d); (median[IQR]) | 66[26–110] | 94[63–142] | 24 [13–35] | <.001 | |
| PCR + diagnostic; no.(%) | 34(87%) | 25(86%) | 9(90%) | 1 | |
| Symptoms; n | 39 | 29 | 10 | ||
| Dyspnoea (modified MRC scale) | 2 ± 1 | 2 ± 1 | 1 ± 1 | 0.051 | |
| Dyspnoea (modified MRC ≥ 1); no.(%) | 35(90%) | 26(90%) | 9(90%) | 1 | |
| Fatigue; no.(%) | 30(77%) | 24(83%) | 6(60%) | 0.197 | |
| Chest pain; no.(%) | 21(54%) | 18(62%) | 3(30%) | 0.140 | |
| Headache; no.(%) | 15(38%) | 14(48%) | 1(10%) | 0.040 | |
| Muscular or articular pain; no.(%) | 15(38%) | 12(41%) | 3(30%) | 0.711 | |
| Cough; no.(%) | 13(33%) | 12(41%) | 1(10%) | 0.120 | |
| Lung function; n | 34 | 28 | 6 | ||
| FVC (% pred.) | 96 ± 20 | 96 ± 19 | 97 ± 25 | 0.952 | |
| FEV1 (% pred.) | 95 ± 19 | 95 ± 18 | 97 ± 24 | 0.796 | |
| FEV1/FVC (%) | 83 ± 8 | 83 ± 9 | 80 ± 3 | 0.466 | |
| Functional assessment; n | 33 | 28 | 5 | ||
| 6-MWT distance (% pred.) | 84 ± 17 | 83 ± 17 | 89 ± 17 | 0.420 | |
| 6-MWT dyspnoea at rest (modified Börg scale) | 1 ± 2 | 2 ± 2 | 0 ± 0 | 0.002 | |
| 6-MWT end-test dyspnoea (modified Börg scale) | 6 ± 2 | 6 ± 2 | 4 ± 3 | 0.083 | |
| 6-MWT SpO2 at rest (%) | 98 ± 1 | 98 ± 1 | 98 ± 2 | 0.874 | |
| 6-MWT SpO2 min (%) | 95 ± 4 | 95 ± 4 | 93 ± 6 | 0.741 | |
| 3 min sit-to-stand test (n rep.) | 57 ± 26 | 60 ± 27 | 47 ± 21 | 0.253 | |
| Hyperventilation syndrome | 35 | 29 | 6 | ||
| Nijmegen score* | 25 ± 10 | 27 ± 10 | 17 ± 11 | 0.020 | |
| Nijmegen >22; no.(%) | 17(49%) | 16(55%) | 1(17%) | 0.177 | |
| Provocation test >5 min; no. (%) | 21(70%) | 21(72%) | 0(0%) | 0.020 | |
| Nijmegen >22 and Provocation test >5min; no.(%) | 12(34%) | 12(41%) | 0(0%) | 0.255 | |
| Hospital Anxiety and Depression; n | 29 | 23 | 6 | ||
| Anxiety scorea | 7 ± 4 | 8 ± 4 | 6 ± 4 | 0.468 | |
| Anxiety >7; no.(%) | 13(45%) | 11(48%) | 2(33%) | 0.662 | |
| Depression scorea | 6 ± 4 | 7 ± 4 | 4 ± 3 | 0.106 | |
| Depression >7; no.(%) | 11(38%) | 10(43%) | 1(17%) | 0.362 | |
| Quality of life (VQ-11 questionnaire); n | 35 | 27 | 8 | ||
| Total scoreb | 29 ± 10 | 31 ± 10 | 23 ± 9 | 0.030 | |
| VQ-11 > 21; no.(%) | 24(69%) | 20(74%) | 4(50%) | 0.225 | |
| Functional dimension | 10 ± 3 | 11 ± 3 | 7 ± 3 | 0.008 | |
| Relational dimension | 9 ± 4 | 9 ± 4 | 7 ± 3 | 0.156 | |
| Psychological dimension | 11 ± 4 | 11 ± 4 | 9 ± 3 | 0.124 |
FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; 6-MWT: 6-min walking test; SpO2: pulsed oxygen saturation; PCR+: positive SARS-CoV-2 Polymerase Chain Reaction test.
* Nijmegen score is considered positive when >22.
Anxiety or depression components of the HAD are considered positive when >7.
VQ-11 is considered altered when >21.
For statistical analysis, between-group differences for continuous variables were assessed using independent group t-tests or Mann-Whitney test depending on data distribution. Categorical variables were compared using Fisher's exact test. Between-assessment differences for paired continuous variables were assessed using paired sample t-tests or Wilcoxon's signed rank test depending on data distribution. The McNemar test was used for categorial variables. Statistical analyses were performed using GraphPad Prism (v. 6.01, La Jolla, CA) A two-tailed p value < 0.05 was considered statistically significant.
3. Results
3.1. Population characteristics before rehabilitation
This study included a total of 39 consecutive patients whose baseline characteristics at first assessment are displayed in Table 1. The flow diagram of the study is depicted in Fig. 2 .
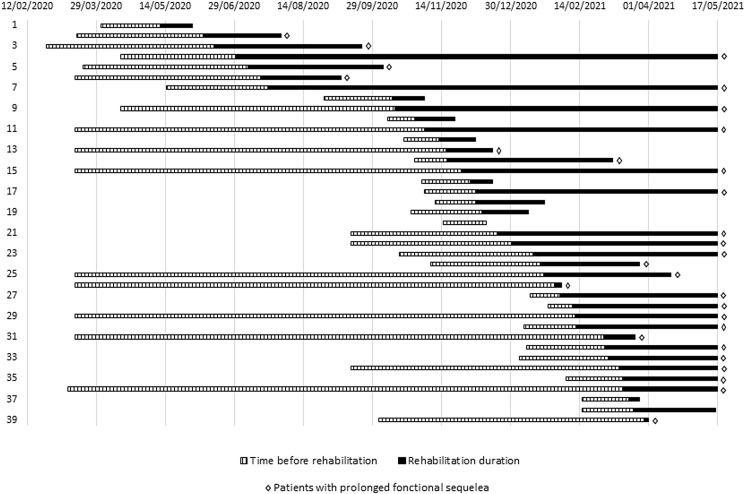
Fig. 1.
Chronology of inclusions for each individual from April 1st, 2020 to April 1st, 2021.
Diagnosis was confirmed by quantitative PCR positive test (PCR+) in 34(87%) participants. Mean age was 48 ± 15 years and 22(56%) were females. Patients had no comorbidities, 14(36%) of them required initial hospitalisation of more than 48 h and 25(64%) were off work. Duration between initial onset of symptoms and rehabilitation referral was 73[34–178] days and individual data are depicted in supplementary Figure S1. Chronology of inclusions for each individual from April 1st, 2020 to April 1st, 2021 is reported in Fig. 1.
The most common symptoms were exertional dyspnoea (mean mMRC: 2±1) and fatigue, which were reported respectively by 35(90%) and 30(77%) participants (Table 1). Respiratory function was preserved (mean FEV1: 95 ± 19%, mean FVC: 96 ± 20%). Functional evaluation revealed a mean 6-MWT distance at 84 ± 17% of theoretical values. At the end of 6-MWT, 3(8%) patients had a SpO2 <90%, including 1(3%) <85%.
Regarding hyperventilation syndrome assessment, the average Nijmegen score was 25 ± 10, with 17(49%) patients exhibiting a positive score. The hyperventilation provocation test was positive for 21(70%) patients. 12(34%) patients had a combination of a positive Nijmegen score and a positive provocation test, and were therefore diagnosed with hyperventilation syndrome. Quality of life was impaired in 24(69%) patients, with a VQ-11 score at 29 ± 10. The anxiety and depression components of the HAD were assessed positive (>7) in 13(45%) and 11(38%) patients, respectively.
During the 6-MWT, resting and end-test SpO2 values were 98 ± 1% and 95 ± 3% for patients with hyperventilation syndrome, compared to 98 ± 1% (p = 0.129) and 94 ± 4% (p = 0.558) for patients without hyperventilation syndrome. Similarly, no difference was evidenced for heart rate values during 6-MWT, with a resting and end-test HR of 86 ± 10 bpm and 119 ± 23 bpm vs. 85 ± 13 bpm (p = 0.829) and 127 ± 19 bpm (p = 0.340) respectively for patients with and without hyperventilation syndrome.
3.2. Variables associated with prolonged functional sequelae
Out of the whole cohort, 29(74%) patients had PFS (Table 1). They were younger (43 ± 14 vs. 60 ± 10yr, p = 0.002), had a non-significantly lower rate of initial hospital admission (28% vs. 60%, p = 0.123), a later rehabilitation referral (107[56–240] vs. 32[26–41] days, p < 0.001) and a longer rehabilitation duration (94[63–142] vs. 24[13–35] days, p < 0.001) than those without PFS. PFS were associated with more severe rest and exertional dyspnoea (respectively, Börg scale at rest: 2 ± 2 vs. 0 ± 0; p = 0.002, mMRC: 2 ± 1 vs. 1 ± 1; p = 0.051). Nijmegen score as well as prevalence of positive provocation test were significantly higher in this subgroup (respectively 27 ± 10 vs. 17 ± 11; p = 0.02; and 72% vs. 0%; p = 0.02). Moreover, although the difference was not statistically significant, all patients presenting with hyperventilation syndrome were in this subgroup (12(41%) vs. 0(0%); p = 0.255). Patients with PFS also reported more severely impaired quality of life (VQ-11 score: 31 ± 10 vs. 23 ± 9; p = 0.030), mainly according to functional dimension (11 ± 3 vs. 7 ± 3; p = 0.008). Importantly, objective parameters such as lung function, 6-MWT distance or 3-STS did not significantly differ between both groups (Table 1).
3.3. Evolution over the course of rehabilitation
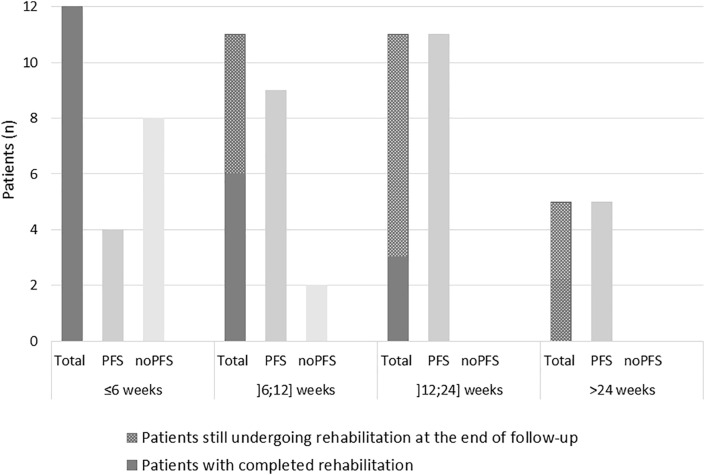
The duration of rehabilitation was highly variable. The number of patients according to the rehabilitation duration (<6 weeks, 6–12 weeks, 12–24 weeks, >24 weeks) was evenly distributed (Fig. 3 ). Of note, 18 patients were still undergoing rehabilitation at the end of the follow-up. The actual proportion of patients requiring prolonged rehabilitation may have therefore been underestimated.
Fig. 3.
Patients' distribution according rehabilitation duration.
64% of patients included in the initial cohort (n = 25) underwent both first and second assessments. Reasons for absence of second assessment were: recent start of rehabilitation (<2 months; n = 5), early recovery irrelevant for a second evaluation (n = 2) and loss of follow-up (n = 7) (Fig. 2). A total of 11(28%) patients recovered before the end of the current follow-up. Characteristics of patients who underwent both assessments did not significantly differ from the whole cohort (supplementary Table S1), except for rehabilitation duration which was significantly longer (97[60–154] vs. 66[26–110]; p < 0.001). The average duration between first and second assessments was 61 ± 20 days.
Data from patients who underwent both assessments are displayed in Table 2 . Among them, 22(88%) required rehabilitation >3 months after disease onset and were therefore integrated in the PFS subgroup. From first to second assessment, mean exertional dyspnoea (mMRC) improved from 2 ± 1 to 1 ± 1 (p < 0.001), consistently with improved functional capacities as evaluated by the 6-MWT distance and the 3-STS which respectively increased from 83% ± 19% to 93% ± 18% and from 61 ± 30 to 80 ± 29 repetitions (p < 0.001). Hyperventilation also improved: the number of patients with both Nijmegen score>22 and positive provocation test reduced from 10(50%) to 3(15%) (p = 0.023). This result was mainly driven by a reduction in the prevalence of positive provocation test which decreased from 17(89%) to 5(25%) (p = 0.001). Importantly, despite both functional and respiratory improvements between first and second assessments, most patients still reported at second assessment unusual fatigue (n = 15(60%)), anxiety symptoms (n = 10(59%)) and altered quality of life (n = 12(57%)).
Table 2.
Evolution between first and second rehabilitation assessments.
| First assessment | Second assessment | p-value | ||
|---|---|---|---|---|
| Symptoms; n | 25 | |||
| Dyspnoea (modified MRC scale) | 2 ± 1 | 1 ± 1 | <.001 | |
| Dyspnoea (modified MRC ≥ 1); no.(%) | 22(88%) | 13(52%) | 0.007 | |
| Fatigue; no.(%) | 19(76%) | 15(60%) | 0.133 | |
| Chest pain; no.(%) | 13(52%) | 10(40%) | 0.371 | |
| Headache; no.(%) | 9(36%) | 9(36%) | 1 | |
| Muscular or articular pain; no.(%) | 10(40%) | 9(36%) | 1 | |
| Cough; no.(%) | 8(32%) | 5(20%) | 0.449 | |
| Lung function; n | 22 | |||
| FVC (% pred.) | 97 ± 17 | 104 ± 22 | 0.006 | |
| FEV1 (% pred.) | 96 ± 17 | 101 ± 23 | 0.052 | |
| FEV1/FVC (%) | 82 ± 9 | 80 ± 8 | 0.290 | |
| Functional assessment; n | 22 | |||
| 6-MWT distance (m) | 510 ± 89 | 569 ± 98 | <.001 | |
| 6-MWT distance (% pred.) | 83 ± 19 | 93 ± 18 | <.001 | |
| 6-MWT dyspnoea at rest (modified Börg scale) | 1 ± 2 | 1 ± 1 | 0.305 | |
| 6-MWT end-test dyspnoea (modified Börg scale) | 6 ± 2 | 5 ± 3 | 0.104 | |
| 6-MWT SpO2 at rest (%) | 98 ± 1 | 98 ± 1 | 0.042 | |
| 6-MWT SpO2 min (%) | 96 ± 2 | 96 ± 2 | 0.110 | |
| 3 min sit-to-stand test (n rep.) | 61 ± 30 | 80 ± 29 | <.001 | |
| Hyperventilation syndrome | 20 | |||
| Nijmegen score* | 27 ± 10 | 24 ± 11 | 0.709 | |
| Nijmegen >22; no.(%) | 11(55%) | 10(50%) | 1 | |
| Provocation test >5min; no. (%) | 17(89%) | 5(25%) | 0.001 | |
| Nijmegen >22 and Provocation test >5min; no.(%) | 10(50%) | 3(15%) | 0.023 | |
| Hospital Anxiety and Depression; n | 17 | |||
| Anxiety scorea | 8 ± 3 | 8 ± 0 | 0.941 | |
| Anxiety >7; no.(%) | 8(47%) | 10(59%) | 0.683 | |
| Depression scorea | 6 ± 4 | 5 ± 0 | 0.306 | |
| Depression >7; no.(%) | 6(35%) | 5(29%) | 1 | |
| Quality of life (VQ-11 questionnaire); n | 21 | |||
| Total scoreb | 29 ± 10 | 25 ± 10 | 0.041 | |
| VQ-11 > 21; no.(%) | 15(71%) | 12(57%) | 0.789 | |
| Functional dimension | 10 ± 3 | 8 ± 3 | <.001 | |
| Relational dimension | 9 ± 4 | 8 ± 4 | 0.162 | |
| Psychological dimension | 11 ± 4 | 10 ± 4 | 0.264 | |
FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; 6-MWT: 6-min walking test; SpO2: pulsed oxygen saturation. PCR+: positive SARS-CoV-2 Polymerase Chain Reaction test.
Duration between first and second assessments was 61 ± 20 days.
* Nijmegen score is considered positive when >22.
Anxiety or depression components of the HAD are considered positive when >7.
VQ-11 is considered altered when >21.
4. Discussion
This study reports original data from a long-term longitudinal follow-up in patients presenting with clinical sequelae of Covid-19, mostly after mild-to-moderate Sars-CoV-2 infection, referred to ambulatory rehabilitation. In fact, 64% of our cohort did not require hospital admission. Despite their middle age, the lack of significant medical past-history, and a mild severity at disease onset, patients included in this study exhibited multiple clinical and functional sequelae more than two months after SARS-CoV-2 infection. Striking was the prevalence of hyperventilation syndrome, with 34% of the whole cohort presenting with both positive Nijmegen score and positive provocation test. On the other hand, according to our data, the heterogeneity of rehabilitation duration does not support that a fixed duration program may apply in such population.
Of note, formal diagnosis was not available for 5 patients (13%). However, ambulatory access to diagnosis was highly limited in the early beginning of the current pandemic and recent guidelines support including in Covid-19 cohorts patients in which diagnosis was based on clinical symptoms [25]. Characteristics of our population are nevertheless consistent with data recently reported by Trinkmann et al. and Sonnweber et al. in patients presenting with long-lasting Covid-19 symptoms [6,13,[26], [27], [28]].
In our population, rehabilitation referral was considered because of the presence of disabling functional sequelae. In the subgroup of patients with PFS, 70% were still off-work four months after disease onset. A recent survey found that approximately 45% of patients may require a reduced work schedule at 6 months from disease onset, highlighting the substantial socio-economic burden of persisting symptoms following SARS-CoV-2 infection [29].
Beyond this social dimension, Jacobs et al. already underlined in patients following hospitalisation that quality of life might still be altered 35 days after discharge [30]. Our data support that this impairment may last even longer, as 69% of our patients exhibited altered VQ-11 at initial assessment (73[34–178] days after disease onset) although most of our patients were not initially hospitalised. It should be noted that we evaluated quality of life with the VQ-11 questionnaire, which is mainly validated in COPD patients and is not intended for being used in the general population [20,21]. However, this questionnaire is routinely used in our structures, easy administered, and appears consistent with the indication of rehabilitation in the current study as it specifically evaluates the alteration of quality of life related to respiratory disability.
Underlying mechanisms promoting long lasting respiratory disorders and especially exertional dyspnoea in Covid-19 population remain to be clarified. Our data suggest respiratory function was almost preserved with respect to spirometric measurements, consistently with previous reports in a similar population [8,13,26]. Data from Sonnweber et al. support that impaired lung function might poorly be described based on spirometry solely, as diffusing capacity is the most frequently encountered alteration in Covid-19 population [26]. However these authors also described favourable evolution of lung function at 60 and 100 days which, along with our data demonstrating persistence of dyspnoea more than 4 months after infection, suggests an absence of correlation between respiratory function and symptoms [26].
Indeed, several symptoms described in “long Covid” manifestations do not appear to be closely related to lung function [10,11,13]. In our study, we systematically searched for dyspnoea, fatigue, headaches, muscular, articular or chest pain and cough. A more complete evaluation might have yielded different results as attention disorders, hair loss, ageusia, anosmia and depression have also been documented as highly prevalent in long Covid [31,32]. We determined this list based on our experience and our approach as physiotherapists facing this population referred for an indication of rehabilitation. From our data and from a functional perspective, dyspnoea and fatigue were the most prevalent symptoms in our population, consistently with previous reports [8,12,33].
Mohr's study supports the observation that dyspnoea cannot be explained by cardiac, pulmonary or ventilatory limitation in all patients [34]. Of the 10 patients included, 6 of whom were hospitalised, this work showed that muscular deficiency and thus metabolic limitation might contribute to dyspnoea [34]. Our study does not support the fact that muscular limitation might explain dyspnoea-related functional impairment. Indeed, in the group with PFS, 6-MWT distance as well as end-test dyspnoea were more severely altered than in the other group while the 3-STS, which evaluates muscle function, was 50% higher.
Another component which may explain persistence of symptoms in our population, and especially dyspnoea-related functional limitation, is the presence of hyperventilation syndrome as previously suggested by Motiejunaite et al. [6,14,27,28]. In our study, 41% of patients with PFS presented with both positive Nijmegen score and positive provocation test. 72% exhibited a positive provocation test. We recognize the lack of specificity of this diagnostic modality and that a standardised cardiopulmonary exercise testing would have more accurately prevented from capturing other aetiologies of dysfunctional breathing [[35], [36], [37], [38]]. However, hyperventilation syndrome in our population was improved between first and second assessments with the implementation of specific treatment (from 10(50%) to 3(15%); p = 0.023) [39]. Additionally, HR and SpO2 variations during the 6-MWT were similar between patients considered with and without hyperventilation syndrome, supporting that the observed dyspnoea was not related to another physiological explanation that would not have been explored. This suggests a low proportion of false positives and supports that, when all other causes have been eliminated, the screening for hyperventilation syndrome should be included in the evaluation of patients referred to rehabilitation with persistent dyspnoea related to Covid-19.
Over the course of rehabilitation, most clinical symptoms decreased, exertional dyspnoea as well as hyperventilation prevalence significantly decreased and 6-MWT, 3-STS and quality of life significantly improved. Regarding hyperventilation syndrome, Nijmegen score remained quite high while provocation test was significantly improved. We believe this might be explained by a lack of specificity of Nijmegen questionnaire in Covid-19 population, with an overlap of symptoms. We cannot conclude from the design of this study [40] whether these improvements were promoted by rehabilitation program itself or by the natural evolution of the disease. However, patients were referred with a median delay of 84 days after disease onset and it seems reasonable to state that rehabilitation probably played a role in this directional change. Early referral to rehabilitation might anticipate this directional change, and in that way our data confirm recent guidance from the ERS-ATS task force which strongly encourages identifying rehabilitation needs in Covid-19 patients regardless of whether they required hospitalisation or not [4,16,41]. Further studies with comparative design could help understanding the contribution of rehabilitation to exertional limitation and hyperventilation syndrome in this population.
Finally, it should be noted that our results cannot be generalised to all Covid-19 patients. Our cohort included patients specifically referred because of their functional complaints. Nevertheless, the prevalence of prolonged Covid-19 sequelae is likely to be substantial, with data suggesting from 10% to 51% of the infected population [10,11,13]. This may represent a “large hidden iceberg” of self-confined people who may suffer from these sequelae without being taken care of [16].
5. Conclusion
This study suggests that long-lasting clinical and functional sequelae qualifying patients for ambulatory rehabilitation after SARS-CoV-2 infection may be unexpectedly encountered in a population of patients without severe initial manifestation of the disease. Lung function tests seem insufficient to clearly decipher long-lasting breathlessness after Covid-19. Our data therefore support screening of hyperventilation syndrome in Covid-19 outpatients referred for rehabilitation in relation to their dyspnoea. This component as well as the associated functional disorders can improve with early rehabilitation referral.
Funding
Devices and consumables used for EtCO2measurements were provided by L3 Medical SARL, which was not involved in the current study.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
BB, PH, CR, MZ and MD contributed substantially to study conception and design; BB, RE and MD contributed to data acquisition; BB, PH, LG, FB, MZ and MD contributed to data analysis and interpretation; BB, PH, MZ and MD drafted the manuscript. All authors revised the manuscript for important intellectual content, approved the final version, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
CRediT authorship contribution statement
Benoit Bouteleux: Conceptualization, Methodology, Investigation, Writing – original draft, writing. Pauline Henrot: Validation, Visualization. Rachel Ernst: Investigation. Léo Grassion: Visualization. Chantal Raherison-Semjen: Supervision. Fabien Beaufils: Resources. Maéva Zysman: Supervision, Visualization, Validation, Resources, Methodology. Mathieu Delorme: Methodology, Investigation, Supervision, Visualization, Conceptualization.
Declaration of competing interest
MZ reports grants and personal fees from Boehringer Ingelheim, personal fees from Novartis, personal fees from Chiesi, personal fees from Astra Zeneca and personal fees from GSK outside the submitted work. FB reports personal fees and non-financial support from Novartis, personal fees and non-financial support from Chiesi, grants, personal fees and non-financial support from AstraZeneca, outside the submitted work.PH reports non-financial support from Avad, outside the submitted work. Other authors have no conflicts of interest to disclose.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmed.2021.106648.
Each line represents one participant. Hatched lines represent the duration between disease onset and rehabilitation referral (time before rehabilitation; days). Solid lines represent rehabilitation duration (days). Patients were classified chronologically based on rehabilitation start date. Note that follow-up was continued up to May 17th, 2021 so that all participants could be categorised according to a 3-months threshold defining “prolonged functional sequelae” as persistence of rehabilitation requirement more than 3 months after disease onset.Patients with prolonged functional sequalae are marked with ◊. Note that 18 participants were still undergoing rehabilitation on May 17th, 2021. Rehabilitation duration is therefore censored for these participants.
The graph displays the number of patients according to rehabilitation duration. For rehabilitation duration of ≤6, ]6; 12], ]12; 24] and >24 weeks, respectively 5, 8 and 5 patients were still undergoing rehabilitation at the end of follow-up.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 Hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. J. Am. Med. Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. J. Am. Med. Assoc. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Thomas P., Baldwin C., Bissett B., Boden I., Gosselink R., Granger C.L., Hodgson C., Jones A.Y., Kho M.E., Moses R., Ntoumenopoulos G., Parry S.M., Patman S., van der Lee L. Physiotherapy management for COVID-19 in the acute hospital setting: clinical practice recommendations. J. Physiother. 2020;66:73–82. doi: 10.1016/j.jphys.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spruit M.A., Holland A.E., Singh S.J., Tonia T., Wilson K.C., Troosters T. COVID-19: interim guidance on rehabilitation in the hospital and post-hospital phase from a European respiratory society and American thoracic society-coordinated international task force. Eur. Respir. J. 2020 doi: 10.1183/13993003.02197-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalmers J.D., Crichton M.L., Goeminne P.C., Cao B., Humbert M., Shteinberg M., Antoniou K.M., Ulrik C.S., Parks H., Wang C., Vandendriessche T., Qu J., Stolz D., Brightling C., Welte T., Aliberti S., Simonds A.K., Tonia T., Roche N. Management of hospitalised adults with coronavirus disease-19 (COVID-19): a European Respiratory Society living guideline. Eur. Respir. J. 2021 doi: 10.1183/13993003.00048-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lerum T.V., Aaløkken T.M., Brønstad E., Aarli B., Ikdahl E., Lund K.M.A., Durheim M.T., Rodriguez J.R., Meltzer C., Tonby K., Stavem K., Skjønsberg O.H., Ashraf H., Einvik G. Dyspnoea, lung function and CT findings 3monthsafter hospital admission for COVID -19. Eur. Respir. J. 2021;57:2003448. doi: 10.1183/13993003.03448-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu X., Liu X., Zhou Y., Yu H., Li R., Zhan Q., Ni F., Fang S., Lu Y., Ding X., Liu H., Ewing R.M., Jones M.G., Hu Y., Nie H., Wang Y. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir. Med. 2021;9:747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Froidure A., Mahsouli A., Liistro G., De Greef J., Belkhir L., Gérard L., Bertrand A., Koenig S., Pothen L., Yildiz H., Mwenge B., Aboubakar F., Gohy S., Pilette C., Reychler G., Coche E., Yombi J.-C., Ghaye B. Integrative respiratory follow-up of severe COVID-19 reveals common functional and lung imaging sequelae. Respir. Med. 2021;181:106383. doi: 10.1016/j.rmed.2021.106383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bajwah S., Wilcock A., Towers R., Costantini M., Bausewein C., Simon S.T., Bendstrup E., Prentice W., Johnson M.J., Currow D.C., Kreuter M., Wells A.U., Birring S.S., Edmonds P., Higginson I.J. Managing the supportive care needs of those affected by COVID-19. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00815-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkatesan P. NICE guideline on long COVID. Lancet Respir. Med. 2021;9:129. doi: 10.1016/S2213-2600(21)00031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Writing Committee for the COMEBAC Study Group. Morin L., Savale L., Pham T., Colle R., Figueiredo S., Harrois A., Gasnier M., Lecoq A.-L., Meyrignac O., Noel N., Baudry E., Bellin M.-F., Beurnier A., Choucha W., Corruble E., Dortet L., Hardy-Leger I., Radiguer F., Sportouch S., Verny C., Wyplosz B., Zaidan M., Becquemont L., Montani D., Monnet X. Four-Month clinical status of a cohort of patients after hospitalization for COVID-19. J. Am. Med. Assoc. 2021 doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortés-Telles A., López-Romero S., Figueroa-Hurtado E., Pou-Aguilar Y.N., Wong A.W., Milne K.M., Ryerson C.J., Guenette J.A. Pulmonary function and functional capacity in COVID-19 survivors with persistent dyspnoea. Respir. Physiol. Neurobiol. 2021;288:103644. doi: 10.1016/j.resp.2021.103644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trinkmann F., Müller M., Reif A., Kahn N., Kreuter M., Trudzinski F., Eichinger M., Heussel C.-P., Herth F.J.F. Lung Network Rhine-Neckar-Region, Residual symptoms and lower lung function in patients recovering from SARS-CoV-2 infection. Eur. Respir. J. 2021;57 doi: 10.1183/13993003.03002-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motiejunaite J., Balagny P., Arnoult F., Mangin L., Bancal C., d'Ortho M.-P., Frija-Masson J., Hyperventilation A possible explanation for long-lasting exercise intolerance in mild COVID-19 survivors? Front. Physiol. 2020;11:614590. doi: 10.3389/fphys.2020.614590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrejak C., Cottin V., Crestani B., Debieuvre D., Gonzalez-Bermejo J., Morelot-Panzini C., Stach B., Uzunhan Y., Maitre B., Raherison C. [Guide for management of patients with possible respiratory sequelae after a SARS-CoV-2 pneumonia. Rev. Mal. Respir. 2020 doi: 10.1016/j.rmr.2020.11.009. Support proposals developed by the French-speaking Respiratory Medicine Society. Version of 10 November 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wise J. Long covid: WHO calls on countries to offer patients more rehabilitation. BMJ. 2021;372:n405. doi: 10.1136/bmj.n405. [DOI] [PubMed] [Google Scholar]
- 17.Médrinal C., Combret Y., Bonnevie T., Smondack P., Gravier F.E., Lebret M., Prieur G. Physiotherapy during the Covid-19 pandemic: management of critically ill patients in the ICU and follow-up care. Eur. Rehabil. J. 2021:1–8. doi: 10.52057/erj.v1i1.2. 01. [DOI] [Google Scholar]
- 18.Knudson R.J., Slatin R.C., Lebowitz M.D., Burrows B. The Maximal Expiratory Flow-Volume Curve. Normal standards, variability, and effects of age. Am. Rev. Respir. Dis. 1976;113:587–600. doi: 10.1164/arrd.1976.113.5.587. [DOI] [PubMed] [Google Scholar]
- 19.Enright P.L., Sherrill D.L. Reference equations for the six-minute walk in healthy adults. Am. J. Respir. Crit. Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 20.Coquart J.B., Heutte N., Terce G., Grosbois J.-M. Convergent validity and minimal clinically important difference of the maugeri foundation respiratory failure questionnaire (MRF-28) and the chronic obstructive pulmonary disease-specific health-related quality of life questionnaire (VQ11) Int. J. Chron. Obstruct. Pulmon. Dis. 2019;14:2895–2903. doi: 10.2147/COPD.S222165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ninot G., Soyez F., Préfaut C. A short questionnaire for the assessment of quality of life in patients with chronic obstructive pulmonary disease: psychometric properties of VQ11. Health Qual. Life Outcome. 2013;11:179. doi: 10.1186/1477-7525-11-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beekman E., Verhagen A. Clinimetrics: hospital anxiety and depression scale. J. Physiother. 2018;64:198. doi: 10.1016/j.jphys.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 23.van Dixhoorn J., Duivenvoorden H.J. Efficacy of Nijmegen Questionnaire in recognition of the hyperventilation syndrome. J. Psychosom. Res. 1985;29:199–206. doi: 10.1016/0022-3999(85)90042-x. [DOI] [PubMed] [Google Scholar]
- 24.Vansteenkiste J., Rochette F., Demedts M. Diagnostic tests of hyperventilation syndrome. Eur. Respir. J. 1991;4:393–399. [PubMed] [Google Scholar]
- 25.Sivan M., Taylor S. NICE guideline on long covid. BMJ. 2020;371:m4938. doi: 10.1136/bmj.m4938. [DOI] [PubMed] [Google Scholar]
- 26.Sonnweber T., Sahanic S., Pizzini A., Luger A., Schwabl C., Sonnweber B., Kurz K., Koppelstätter S., Haschka D., Petzer V., Boehm A., Aichner M., Tymoszuk P., Lener D., Theurl M., Lorsbach-Köhler A., Tancevski A., Schapfl A., Schaber M., Hilbe R., Nairz M., Puchner B., Hüttenberger D., Tschurtschenthaler C., Aßhoff M., Peer A., Hartig F., Bellmann R., Joannidis M., Gollmann-Tepeköylü C., Holfeld J., Feuchtner G., Egger A., Hoermann G., Schroll A., Fritsche G., Wildner S., Bellmann-Weiler R., Kirchmair R., Helbok R., Prosch H., Rieder D., Trajanoski Z., Kronenberg F., Wöll E., Weiss G., Widmann G., Löffler-Ragg J., Tancevski I. Cardiopulmonary recovery after COVID-19 - an observational prospective multi-center trial. Eur. Respir. J. 2020 doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taverne J., Salvator H., Leboulch C., Barizien N., Ballester M., Imhaus E., Chabi-Charvillat M.-L., Boulin A., Goyard C., Chabrol A., Catherinot E., Givel C., Couderc L.-J., Tcherakian C. High incidence of hyperventilation syndrome after COVID-19. J. Thorac. Dis. 2021;13:3918–3922. doi: 10.21037/jtd-20-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motiejunaite J., Balagny P., Arnoult F., Mangin L., Bancal C., Vidal-Petiot E., Flamant M., Jondeau G., Cohen-Solal A., d'Ortho M.-P., Frija-Masson J. Hyperventilation as one of the mechanisms of persistent dyspnoea in SARS-CoV-2 survivors. Eur. Respir. J. 2021;58:2101578. doi: 10.1183/13993003.01578-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis H.E. Characterizing long COVID in an international Cohort: 7 months of symptoms and their impact | medRxiv. https://www.medrxiv.org/content/10.1101/2020.12.24.20248802v1.full n.d. (accessed April 5, 2021) [DOI] [PMC free article] [PubMed]
- 30.Jacobs L.G., Gourna Paleoudis E., Lesky-Di Bari D., Nyirenda T., Friedman T., Gupta A., Rasouli L., Zetkulic M., Balani B., Ogedegbe C., Bawa H., Berrol L., Qureshi N., Aschner J.L. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez-Leon S., Wegman-Ostrosky T., Perelman C., Sepulveda R., Rebolledo P.A., Cuapio A., Villapol S. More than 50 Long-term effects of COVID-19: a systematic review and meta-analysis. MedRxiv Prepr. Serv. Health Sci. 2021 doi: 10.1101/2021.01.27.21250617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandal S., Barnett J., Brill S.E., Brown J.S., Denneny E.K., Hare S.S., Heightman M., Hillman T.E., Jacob J., Jarvis H.C., Lipman M.C.I., Naidu S.B., Nair A., Porter J.C., Tomlinson G.S., Hurst J.R. Thorax; 2020. ARC Study Group, “Long-COVID”: a Cross-Sectional Study of Persisting Symptoms, Biomarker and Imaging Abnormalities Following Hospitalisation for COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goërtz Y.M.J., Van Herck M., Delbressine J.M., Vaes A.W., Meys R., Machado F.V.C., Houben-Wilke S., Burtin C., Posthuma R., Franssen F.M.E., van Loon N., Hajian B., Spies Y., Vijlbrief H., van ’t Hul A.J., Janssen D.J.A., Spruit M.A. Persistent symptoms 3monthsafter a SARS -CoV-2infection :the post -COVID-19syndrome. ERJ Open Res. 2020;6 doi: 10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohr A., Dannerbeck L., Lange T.J., Pfeifer M., Blaas S., Salzberger B., Hitzenbichler F., Koch M. Cardiopulmonary exercise pattern in patients with persistent dyspnoea after recovery from COVID-19, Multidiscip. Respir. Med. 2021;16:732. doi: 10.4081/mrm.2021.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmed M., Withana K., Stephenson J.B. Hyperventilation syndrome. Lancet Lond. Engl. 1996;348:65634–65636. doi: 10.1016/s0140-6736(05. 750. [DOI] [PubMed] [Google Scholar]
- 36.Brat K., Stastna N., Merta Z., Olson L.J., Johnson B.D., Cundrle I. Cardiopulmonary exercise testing for identification of patients with hyperventilation syndrome. PLoS One. 2019;14 doi: 10.1371/journal.pone.0215997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hornsveld H.K., Garssen B., Dop M.J., van Spiegel P.I., de Haes J.C. Double-blind placebo-controlled study of the hyperventilation provocation test and the validity of the hyperventilation syndrome. Lancet Lond. Engl. 1996;348:154–158. doi: 10.1016/s0140-6736(96)02024-7. [DOI] [PubMed] [Google Scholar]
- 38.Boulding R., Stacey R., Niven R., Fowler S.J. Dysfunctional breathing: a review of the literature and proposal for classification. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2016;25:287–294. doi: 10.1183/16000617.0088-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ristiniemi H., Perski A., Lyskov E., Emtner M. Hyperventilation and exhaustion syndrome. Scand. J. Caring Sci. 2014;28:657–664. doi: 10.1111/scs.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zampogna E., Paneroni M., Belli S., Aliani M., Gandolfo A., Visca D., Bellanti M.T., Ambrosino N., Vitacca M. Pulmonary rehabilitation in patients recovering from COVID-19, respir. Int. Rev. Thorac. Dis. 2021;100:416–422. doi: 10.1159/000514387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemhöfer C., Gutenbrunner C., Schiller J., Loudovici-Krug D., Best N., Bökel A., Sturm C. Assessment of rehabilitation needs in patients after COVID-19: development of the COVID-19-rehabilitation needs survey. J. Rehabil. Med. 2021;53 doi: 10.2340/16501977-2818. jrm00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.