Abstract
The Johnson and Johnson Ad26.COV2.S single-dose vaccine represents an attractive option for coronavirus disease 2019 (COVID-19) vaccination in countries with limited resources. We examined the effect of prior infection with different SARS-CoV-2 variants on Ad26.COV2.S immunogenicity. We compared participants who were SARS-CoV-2 naive with those either infected with the ancestral D614G virus or infected in the second wave when Beta predominated. Prior infection significantly boosts spike-binding antibodies, antibody-dependent cellular cytotoxicity, and neutralizing antibodies against D614G, Beta, and Delta; however, neutralization cross-reactivity varied by wave. Robust CD4 and CD8 T cell responses are induced after vaccination, regardless of prior infection. T cell recognition of variants is largely preserved, apart from some reduction in CD8 recognition of Delta. Thus, Ad26.COV2.S vaccination after infection could result in enhanced protection against COVID-19. The impact of the infecting variant on neutralization breadth after vaccination has implications for the design of second-generation vaccines based on variants of concern.
Keywords: neutralization, Fc effector function, vaccines, SARS-CoV-2, Ad26CoV2.S, variants of concern, hybrid immunity
Graphical abstract
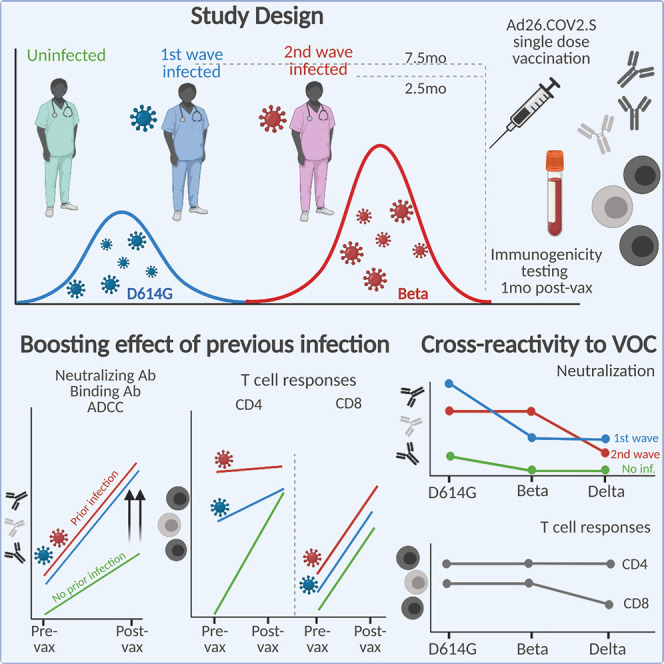
Keeton, Richardson, Moyo-Gwete, et al. show that SARS-CoV-2 infection prior to Ad26CoV2.S vaccination significantly boosts cross-reactive ADCC and binding and neutralizing antibodies and moderately boosts T cell responses against variants of concern. Furthermore, the infecting virus spike sequence determines the cross-reactivity of neutralizing responses, with implications for second-generation vaccine design.
Introduction
The Johnson and Johnson Ad26.COV2.S vaccine is a single-dose adenovirus 26-vectored vaccine expressing the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Wuhan-1 stabilized spike. A phase 3 clinical trial of Ad26.COV2.S on three continents demonstrated 66% efficacy against moderate disease and 85% protection against severe disease 28 days after vaccination (Sadoff et al., 2021). Moreover, the South African arm of the trial showed similar levels of efficacy despite the emergence of the neutralization-resistant SARS-CoV-2 Beta variant. Vaccination with Ad26.COV2.S triggers neutralizing responses that gradually increase in magnitude and breadth, as well as potent antibody Fc effector functions and T cell activity, both of which retain activity against variants of concern (VOCs) (Moore et al., 2021; Barouch et al., 2021; Stephenson et al., 2021; Alter et al., 2021).
Prior infection boosts titers of binding and neutralizing antibodies elicited by mRNA vaccines (Manisty et al., 2021; Saadat et al., 2021; Stamatatos et al., 2021; Vanshylla et al., 2021; Wang et al., 2021b). These increased titers conferred the ability to neutralize SARS-CoV-2 VOCs, illustrating that only one dose of these vaccines might be sufficient to protect previously infected individuals. Similarly, a single dose of the BNT162b2 vaccine boosted antibody-dependent cellular cytotoxicity (ADCC) in previously infected individuals, and T cell cross-reactivity was largely retained (Geers et al., 2021; Reynolds et al., 2021; Tauzin et al., 2021). The impact of prior infection on immune responses elicited by vectored vaccines is less well defined (Havervall et al., 2021), as is the impact of the duration between infection and vaccination, or the genotype of the infecting virus.
Results
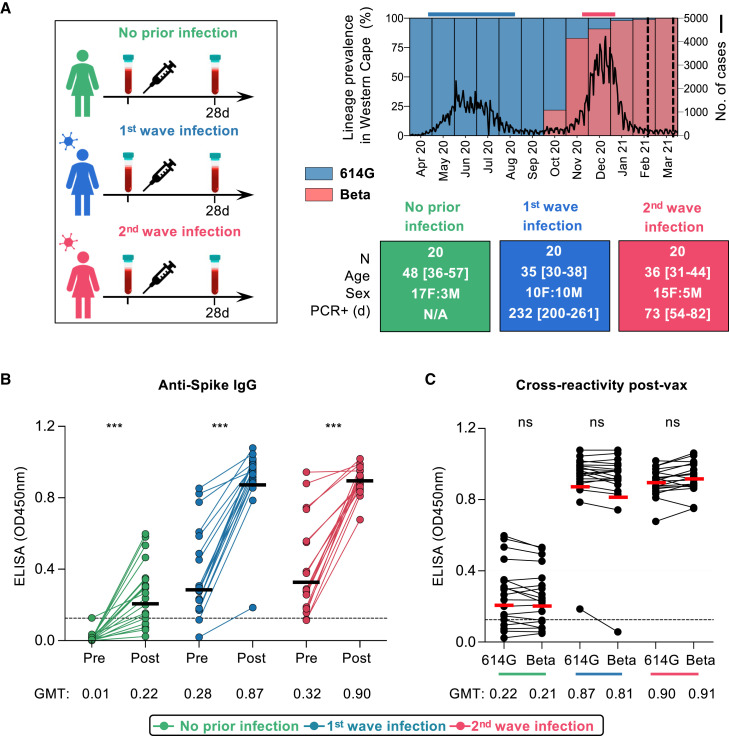
South Africa experienced a first wave of infections in mid-2020, dominated by the ancestral SARS-CoV-2 D614G variant. From November 2020 to February 2021, a second wave of infections was dominated by the Beta variant (Tegally et al., 2021; Wibmer et al., 2021; Cele et al., 2021; Wang et al., 2021a). We established an observational study of 400 healthcare workers (HCWs) with serial sampling since the first wave. We studied 60 HCWs who were vaccinated in a phase 3b implementation trial of single-dose Ad26.COV2.S vaccine (Takuva et al., 2021). HCWs were recruited into three groups, namely those never infected with SARS-CoV-2 (n = 20) and those with PCR-confirmed infection during the first wave (n = 20) or second wave (n = 20) (Figure 1A; Table S1). The Beta variant accounted for >90% of infections in the Western Cape in the second wave (Figure 1A), making it likely that this variant was responsible for infections in the latter group. Indeed, whole-genome sequencing of 8/20 second-wave participants confirmed infection with Beta. Serological profiles were generated for each participant by measuring nucleocapsid and spike antibodies since July 2020 (3–8 monthly visits) (Figures S1A–S1C). These data confirmed the absence of infection (or re-infection), and the timing of first- or second-wave infection. We identified one potential vaccine breakthrough infection and one suspected re-infection, both excluded from subsequent analyses.
Figure 1.
Spike-specific antibody responses in Ad26.COV2.S-vaccinated healthcare workers
(A) Study design showing three groups (left panel), either with no prior infection or infection in the first wave (May–August 2020) and infection in the second wave (November 2020–January 2021). Samples were taken pre-vaccination and one month after vaccination. SARS-CoV-2 epidemiological dynamics in the Western Cape (South Africa) are shown (top right panel). Prevalence of SARS-CoV-2 lineages is shown on the left y axis. The ancestral strain (D614G) is depicted in blue, and Beta is depicted in red. The number of COVID-19 cases is represented on the right y axis. The bars on top of the graph indicate the periods when participants were infected in the first and second waves. Vertical dotted lines indicate when vaccination occurred. Characteristics of participants in the three groups (bottom right panel). Sex, age (median and IQR), and days since PCR-confirmed infection.
(B) Plasma samples from participants with no prior infection (green, n = 19), first-wave infection (blue, n = 20), or second-wave infection (red, n = 19) were tested for binding to D614G spike protein pre- and post-vaccination (OD450nm).
(C) Cross-reactivity of vaccine-induced antibody responses to D614G and Beta spike. The colored lines below the graph correspond with the key. The threshold for positivity is indicated by a dotted line. Horizontal bars indicate GMT, with values shown. Statistical analyses were performed with the Mann-Whitney test between groups, and the Wilcoxon test was performed for pre- and post-vaccine time points or D614G in comparison with Beta responses. ∗∗∗p < 0.001.
Peripheral blood mononuclear cells (PBMCs) and plasma were collected prior to vaccination (median 22 days, interquartile range (IQR) 14–29) and approximately one month after vaccination (median 29 days, IQR 28–34). We tested pre- and post-vaccination plasma for immunoglobulin (Ig)G binding antibodies to the ancestral D614G spike. Binding antibodies elicited by vaccination in the absence of infection (geometric mean titer [GMT]: 0.22) were comparable with those in both infected groups prior to vaccination (GMT: 0.28 and 0.32 for first and second wave, respectively). However, vaccination in HCWs with prior infection in both waves resulted in binding responses being boosted 3-fold, to a GMT of 0.87 or 0.9 for the first and second waves, respectively (Figure 1B). In all HCWs, regardless of prior infection, spike-specific binding antibodies were cross-reactive, with no significant difference in binding between the D614G and Beta spike (Figure 1C).
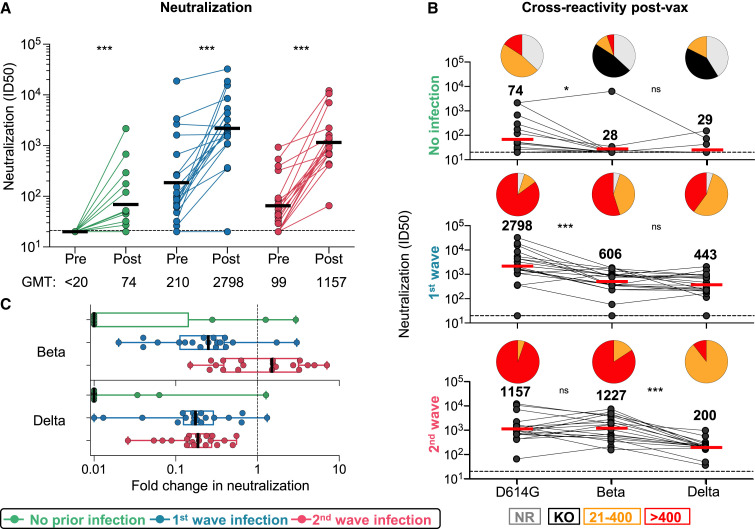
By using a SARS-CoV-2 pseudovirus assay with the D614G spike, we tested neutralizing antibodies elicited by vaccination alone. Consistent with previous studies (Moore et al., 2021), we saw low titers post-vaccination in the infection-naive group (GMT: 74). In both groups with prior infection, we observed a significant boost in neutralization after vaccination against D614G and Beta (Figure 2 A; Figure S2A). For first-wave HCWs, titers were boosted 13-fold from a GMT of 210 to 2,798 (Figure 2A). Second-wave HCWs were boosted 12-fold from a GMT of 99 to 1,157. To determine cross-reactivity of neutralizing antibodies, we compared neutralization of D614G with Beta and Delta. For antibodies induced by vaccination alone, all participants showed significantly lower titers against Beta (85% showing no neutralization, GMT: 28) and Delta (78% showing no neutralization, GMT: 29). In both groups of previously infected HCWs, we saw cross-neutralization of Beta and Delta, but the degree of cross-reactivity varied by wave of infection (Figures 2B and 2C). For HCWs infected in the first wave, although neutralization of Beta and Delta was maintained, titers were significantly lower for both VOCs (a reduction in GMT from 2,798 to 606 and 443, respectively, compared to those for D614G). In contrast, plasma from those infected in the second wave with Beta showed no significant difference in neutralization of D614G (GMT: 1157) but 6-fold lower neutralization of Delta (GMT: 200, p < 0.001) (Figures 2B and 2C). Overall, prior infection followed by vaccination triggered high-titer neutralizing antibodies able to neutralize VOCs. However, the pattern of neutralization varied by wave, suggesting that the neutralizing antibody repertoire was shaped by the genotype of the infecting variant.
Figure 2.
Neutralizing antibody responses to Ad26.COV2.S vaccination
(A) Neutralization of the SARS-CoV-2 D614G pseudovirus by plasma pre- and post-vaccination from participants with no prior infection (green, n = 19) and those infected in the first (blue, n = 20) and second waves (red, n = 19). Neutralization is reflected as an ID50 titer. The threshold for positivity is indicated by a dotted line
(B) Cross-reactive neutralization post-vaccination against D614G, Beta, and Delta. Pie charts show the proportion of vaccine non-responders (NR; gray), knockout of neutralization of Beta or Delta (KO; black), and the titer of 20–400 (orange), or >400 (red). The horizontal bars indicate GMT, with values indicated. Statistical analyses were performed with the Friedman test between groups and the Wilcoxon test for paired analyses. ∗p < 0.05, ∗∗∗p < 0.001.
(C) Fold change of post-vaccination D614G neutralization titers relative to Beta or Delta. The vertical bars indicate median fold change with error bars for IQR.
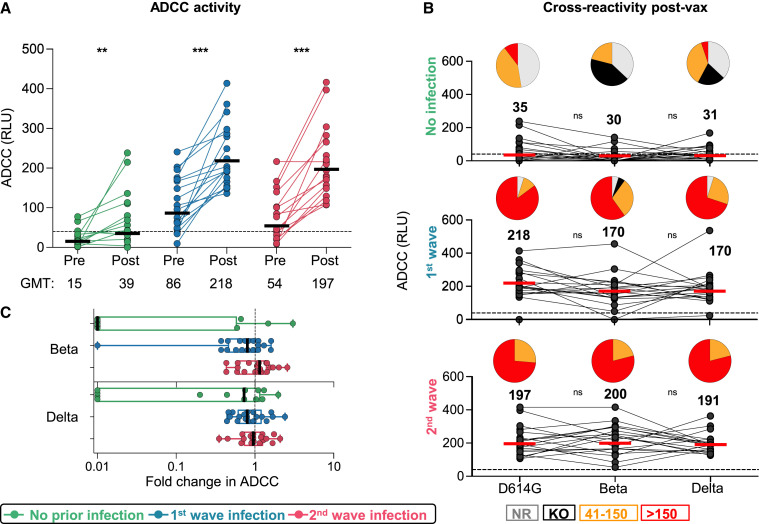
To assess the impact of prior infection on Fc effector responses to vectored vaccines is unknown. We measured the ability of plasma antibodies to cross-link FcγRIIIa (CD16)-expressing cells and cell surface D614G, Beta, or Delta spikes on target cells, as a surrogate for ADCC. In previously infected individuals, post-vaccination responses after both waves were significantly higher against D614G, Beta, and Delta (Figure 3 A; Figure S2B), closely mirroring the fold increases of spike binding titers (Figure 1B). However, responses to D614G elicited by vaccination alone (GMT: 39) were similar to those elicited by infection (GMT: 86 for first wave and 54 for second wave) (Figure 3A; Figure S2B). ADCC assays performed with the Beta and Delta variants showed no significant loss in activity when compared to D614G in the vaccine-only group (Figure 3B) or in individuals with prior infection (Figures 3B and 3C), demonstrating cross-reactive ADCC responses to VOCs.
Figure 3.
ADCC responses to Ad26.COV2.S vaccination
(A) ADCC activity represented as relative light units (RLU).
(B) Cross-reactive ADCC activity 28 days post-vaccination against D614G, Beta, and Delta. Pie charts show the proportion of vaccine non-responders (NR; gray), knockout of Beta/Delta neutralization (KO; black), or detectable ADCC activity (41–150, orange; >150, red). Statistical analyses were performed with the Friedman test between groups and the Wilcoxon test for pre- and post-vaccine time points or D614G in comparison with Beta/Delta responses. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
(C) Fold change of post-vaccination D614G ADCC levels relative to those of the Beta/Delta variants. The vertical bars indicate median fold difference and error bars the IQR.
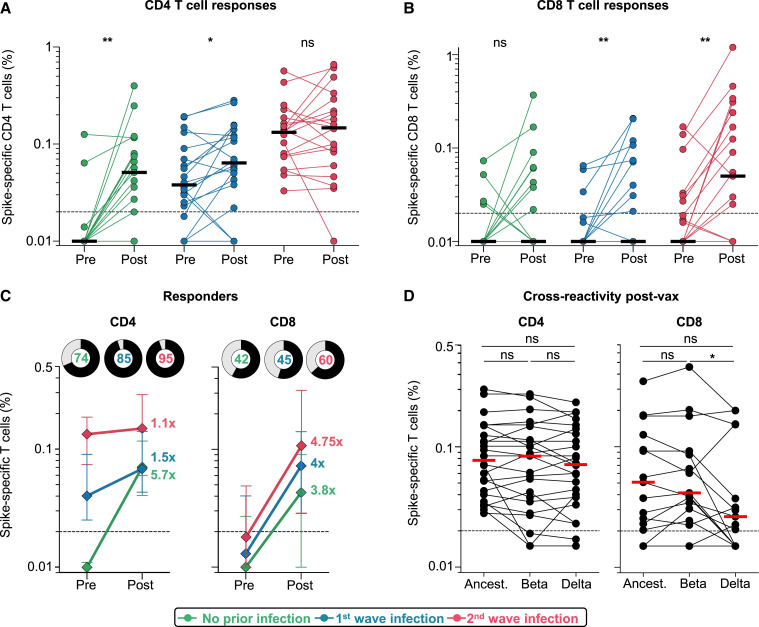
We examined the effect of prior SARS-CoV-2 infection on vaccine T cell responses. We measured intracellular cytokine production (interferon [IFN]-γ, tumor necrosis factor [TNF]-α, and interleukin [IL]-2) in response to peptides covering the Wuhan-1 spike (Figure S3A). Vaccination induced spike-specific CD4 and CD8 T cell responses in all groups (Figures 4A and 4B). Infection-naive participants or those infected in the first wave had significantly higher CD4 T cell responses after vaccination (median: 0.051 and 0.064, respectively). The second-wave group had pre-existing responses that were significantly higher than the first-wave baseline infection responses and mounted a more modest response to vaccination, with similar medians (0.132% and 0.147%, p = not significant [ns]). CD8 responses were present in fewer individuals prior to vaccination (15 and 32%, versus 85 and 100% for CD4 responses in first- and second-wave groups, respectively) and did not differ significantly between the groups (Figure 4B; Figure S3B). Median CD4 T cell frequencies from pre- to post-vaccination decreased with higher magnitude of pre-existing responses, with a 5.7-fold change in the infection-naive group, 1.5-fold in the first-wave group, and 1.1-fold in the second-wave group. For CD8 responses, the fold increase was similar for the three groups (Figure 4C). A greater proportion of individuals mounted a CD4 response than to a CD8 response (Figure 4C). Polyfunctional profiles of vaccine responses demonstrated that CD4 T cells had the capacity to produce multiple cytokines simultaneously, whereas CD8 T cells produced predominantly IFN-γ alone (Figure S3C), with no significant difference in the profiles after vaccination for those who were infection naive to those with prior infection.
Figure 4.
T cell responses to Ad26.COV2.S vaccination
(A and B) Frequency of total cytokine-producing spike-specific CD4 T cells (A) and CD8 T cells (B) in those with no prior infection (green, n = 19), infection in the first wave (blue, n = 20), and infection in the second wave (red, n = 19), in PBMCs stimulated with peptides based on Wuhan spike.
(C) Median fold change of CD4 and CD8 T cell frequencies after vaccination in responders. Error bars indicate IQR. Pie charts show responders (black) and non-responders (gray), with the percentage of responders indicated.
(D) Cross-reactivity of T cell responses post-vaccination (n = 24) after peptide stimulation with spike from the ancestral strain, Beta, or Delta is shown. Horizontal bars indicate medians. The dotted line indicates the threshold for positivity and values are background subtracted. Statistical analyses were performed with the Wilcoxon test. ∗p < 0.05, ∗∗p < 0.01.
Finally, we assessed whether T cells induced by vaccination recognized Beta and Delta. We tested spike peptides corresponding to the viral sequences of the ancestral strain, Beta, or Delta in 24 vaccinees. CD4 T cell recognition of Beta or Delta was fully preserved, compared to the ancestral strain (Figure 4D). Spike-specific CD8 T cells (in 15/24 participants) cross-recognized Beta spike in 14/15 responders. In contrast, the median magnitude of the cytokine response was significantly lower against Delta than against Beta (p = 0.041), and 8/15 (53%) of CD8 responders had a 2-fold or greater reduction in the response to Delta, including five with complete loss of recognition (Figure 4D). Overall, these results demonstrate that robust CD4 and CD8 T cell responses are generated after vaccination, regardless of prior infection. Vaccine T cell cross-recognition of variants is largely preserved, with the exception of a reduced ability for CD8 recognition of Delta in some vaccinees.
Discussion
Several mRNA vaccines have demonstrated a boosting effect of prior infection (Manisty et al., 2021; Reynolds et al., 2021; Saadat et al., 2021; Stamatatos et al., 2021; Wang et al., 2021b). However, whether this is true of viral vectors, including the single-dose Johnson and Johnson vaccine, is unclear. We show that infection prior to vaccination with Ad26.COV2.S significantly boosts the magnitude and cross-reactivity of binding antibodies, neutralizing antibodies, and Fc effector function. T cell responses were robustly generated even in the absence of prior infection and were preserved against Beta. These data have particular significance in countries like South Africa, where SARS-CoV-2 seropositivity is 20%–40% (Mutevedzi et al., 2021; Hsiao et al., 2020; Sykes et al., 2021). Thus, prior infection could enhance the protective efficacy of this vaccine, which is frequently used in settings with limited resources.
Neutralization breadth was shaped by the variant responsible for infection. Prior exposure to D614G resulted in reduced titers against both Beta and Delta, consistent with previous studies (Liu et al., 2021). However, although Beta infection resulted in the preserved neutralization of D614G, we, like others (Liu et al., 2021), noted significant loss of activity against Delta. Therefore, although all participants were exposed to the same vaccine, the genotype of the infecting virus determined the specificity of the responses, prior to vaccine boosting. These findings have important implications for vaccine design, because the sequence of VOC spikes in second-generation vaccines could impact the repertoire of vaccine-induced antibodies.
Fc effector functions are important in vaccine-elicited protection against many viruses (Richardson and Moore, 2021). Reduced SARS-CoV-2 severity/mortality correlates with Fc effector activity (Zohar et al., 2020), and monoclonal antibodies could require Fc function for optimal protection (Winkler et al., 2021; Schäfer et al., 2021). We show that Ad26.COV.2 vaccination in SARS-CoV-2-naive HCWs elicits significant ADCC responses, consistent with previous data (Stephenson et al., 2021). In addition, prior infection significantly enhanced vaccine-elicited ADCC responses, independent of time post-infection, as for the BNT162b2 vaccine (Geers et al., 2021; Tauzin et al., 2021). Finally, unlike neutralizing antibodies, ADCC activity through vaccination alone, or boosted by prior infection, was cross-reactive for Beta and Delta. This is consistent with previous findings and suggests that ADCC-mediating antibodies target regions of the spike beyond the major neutralization epitopes (Alter et al., 2021).
SARS-CoV-2-specific T cells play a key role in modulating coronavirus disease 2019 (COVID-19) disease severity (Rydyznski Moderbacher et al., 2020) and provide protective immunity in the context of low antibody titers (McMahan et al., 2021). We show that robust spike-specific CD4 and CD8 memory T cell responses were induced by AD26.COV2.S vaccination, consistent with the findings of Alter et al. (2021). The magnitude of vaccine-induced T cell responses was similar to that of convalescent responses. The effect of prior infection was distinct from the antibody response, with CD4 responses in the infection naive group induced to a similar magnitude as the first-wave group, and existing CD4 T cells were only moderately boosted, if at all, in the second-wave group. There was an increase in the magnitude and proportion of CD8 responses to spike induced de novo after vaccination, in comparison with that seen in infection, similar for the three study groups. Four participants in the infection naive group displayed spike-specific CD8 T cells prior to vaccination, as described elsewhere, likely through exposure to endemic human coronaviruses (Braun et al., 2020; Grifoni et al., 2020; Mateus et al., 2020). Of note, most CD8 responses declined after vaccination, suggesting a lack of cognate cross-reactivity.
Vaccination induced T cells that largely cross-recognized peptides based on Beta and Delta spike, suggesting that most vaccinees target conserved epitopes in spike, as previously described (Reynolds et al., 2021; Riou et al., 2021; Gallagher et al., 2021; Geers et al., 2021; Tarke et al., 2021). However, a third of vaccinees showed reduced CD8 recognition of Delta, which harbors the L452R mutation that confers resistance to human leukocyte antigen (HLA)-A∗24:02 recognition (Motozono et al., 2021). Thus, reduced CD8 recognition of Delta might be attributed to the HLA repertoire of individuals.
Overall, we show a dramatic effect of recent or distant infection on the magnitude and breadth of neutralizing responses and ADCC. Ad26.COV2.S vaccination alone drives continued maturation of B cell responses, conferring enhanced neutralization of variants and durability (Barouch et al., 2021). It is still unknown whether prior infection will enhance maturation of neutralizing antibodies and extend durability further. T cell responses, though more modestly impacted by prior infection, were robust and largely cross-reactive. This suggests that an infection “prime” boosts Ad26.COV2.S immunogenicity and, in areas of high seroprevalence, could positively impact the effectiveness of this single-dose vaccine. Most significantly, we show that breadth of neutralization after vaccination is dictated by the infecting variant, with important implications for adapted vaccines based on VOCs.
Limitations of the study
This study focused on variant-specific responses post-vaccination, and we did not fully assess neutralizing breadth prior to vaccination. However, several studies have now characterized the neutralizing activity against VOCs, including Delta, which now dominates globally and shows approximately 4- to 6-fold reduced sensitivity to convalescent plasma, in comparison with D614G (Edara et al., 2021; Liu et al., 2021; Planas et al., 2021). Furthermore, whether vectored vaccines predominantly elicit or boost pre-existing receptor binding domain responses, like mRNA vaccines (Stamatatos et al., 2021), remains to be defined. Finally, further analyses to identify the HLA alleles associated with reduced CD8 T cell cross-reactivity to Delta, and confirmation of the mutation(s) that are responsible for epitope loss, would shed light on the significance of cellular immune evasion by Delta.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| purified NA/LE mouse anti-human CD28 (clone 28.2) | BD PharMingen | Cat# 555725; RRID: AB_2130052 |
| purified NA/LE mouse anti-human CD49d (clone L25) | BD PharMingen | Cat# 555501; RRID: AB_396068 |
| LIVE/DEAD™ Fixable VIVID Stain | Invitrogen | Cat# L34955 |
| CD14 Pac Blue (clone TuK4) | Invitrogen Thermofisher Scientific | Cat# MHCD1428; RRID: AB_10373537 |
| CD19 Pac Blue (clone SJ25-C1) | Invitrogen Thermofisher Scientific | Cat# MHCD1928; RRID: AB_10373689 |
| CD4 PERCP-Cy5.5 (clone L200) | BD Biosciences | Cat# 552838; RRID: AB_394488 |
| CD8 BV510 (clone RPA-8) | Biolegend | Cat# 301048; RRID: AB_2561942 |
| PD-1 BV711 (clone EH12.2H7) | Biolegend | Cat# 329928; RRID: AB_11218612 |
| CD27 PE-Cy5 (clone 1A4) | Beckman Coulter | Cat# 6607107; RRID: AB_10641617) |
| CD45RA BV570 (clone HI100) | Biolegend | Cat# 304132; RRID: AB_2563813 |
| CD3 BV650 (clone OKT3) | Biolegend | Cat# 317324; RRID: AB_2563352 |
| IFN-g Alexo 700 (clone B27) | BD Biosciences | Cat# 557995; RRID: AB_396977 |
| TNF BV786 (clone Mab11) | Biolegend | Cat# 502948; RRID: AB_2565858 |
| IL-2 APC (clone MQ1-17H12) | Biolegend | Cat# 500310; RRID: AB_315097 |
| CR3022 | Genscript (https://www.genscript.com) | N/A |
| BD23 | Dr Nicole Doria-Rose, VRC, USA | N/A |
| P2B-2F6 | Dr Nicole Doria-Rose, VRC, USA | N/A |
| anti-IgG APC (clone QA19A42) | Biolegend | Cat#366905 RRID: AB_2888847 |
| Palivizumab | Medimmune | Synagis; RRID: AB_2459638 |
| Bacterial and virus strains | ||
| SARS-CoV-2 pseudoviruses for ancestral, Beta and Delta |
Wibmer et al., 2021; This paper |
N/A |
| Biological samples | ||
| Convalescent health care worker blood samples | Groote Schuur Hospital | https://www.gsh.co.za |
| Chemicals, peptides, and recombinant proteins | ||
| PepTivator® SARS-CoV-2 Prot_S | Miltenyi Biotech | Cat #130-126-701 |
| PepTivator® SARS-CoV-2 Prot_S1 | Miltenyi Biotech | Cat# 130-127-048 |
| SARS-CoV-2 Original Wuhan, Beta and Delta spike synthetic peptides | TC Peptide Lab | https://tcpeptidelab.com |
| SARS-CoV-2 original and Beta variant spike proteins | Original: Hsieh et al., 2020; Beta: Moyo-Gwete et al., 2021 | N/A |
| Critical commercial assays | ||
| Superscript IV Reverse Transcriptase | Life Technologies, Carlsbad, CA | Cat # 18090200 |
| Qubit dsDNA High Sensitivity assay | Life Technologies Carlsbad, CA | Cat # Q32854 |
| AMPure XP magnetic beads | Beckman Coulter | Cat # A63881 |
| Illumina® DNA Prep kit | Illumina, San Diego | Cat # 20018705 |
| Nextera™ DNA CD Indexes (96 Indexes, 96 Samples) | Illumina, San Diego | Cat # 20018708 |
| Elecsys anti-SARS-CoV-2 Spike immunoassay | Roche Diagnostics | CAT# 09 289 275 190 |
| Elecys anti-SARS-CoV-2 electrochemiluminescent immunoassay | Roche Diagnostics | CAT# 09 203 095 190 |
| PEI-MAX 40,000 | Polysciences | Cat # 24765-1 |
| LIVE/DEAD™ Fixable VIVID Stain | Invitrogen | Cat # L34955 |
| Cytofix/Cyto perm buffer | BD Biosciences | Cat # 554722 |
| CellFIX | BD Biosciences | Cat # 340181 |
| QUANTI-Luc luciferase | Invivogen | Cat# rep-qlc2 |
| Luciferase for neuts | Promega | Cat# PRE263B-C |
| Deposited data | ||
| PID1054 SARS-CoV-2 Viral sequence | GISAID EpiCoV database | GISAID Accession # EPI_ISL_2621106 |
| PID1127 SARS-CoV-2 Viral sequence | GISAID EpiCoV database | GISAID Accession # EPI_ISL_2621118 |
| PID1128 SARS-CoV-2 Viral sequence | GISAID EpiCoV database | GSAID Accession # EPI_ISL_2621122 |
| PID1134 SARS-CoV-2 Viral sequence | GISAID EpiCoV database | GISAID Accession # EPI_ISL_1534413 |
| PID1169 SARS-CoV-2 Viral sequence | GISAID EpiCoV database | GISAID Accession # EPI_ISL_2621117 |
| PID1319 SARS-CoV-2 Viral sequence | GISAID EpiCoV database | GISAID Accession # EPI_ISL_2621127 |
| PID1337 SARS-CoV-2 Viral sequence | GISAID EpiCoV database | GISAID Accession # EPI_ISL_2621124 |
| PID1399 SARS-CoV-2 Viral sequence | GISAID EpiCoV database | GISAID Accession # EPI_ISL_2621110 |
| Ancestral (Wuhan) | www.ncbi.nlm.nih.gov | NC_045512.2 |
| B.1.351 (Beta) | www.GISAID.org | EPI_ISL_660629 |
| B.1.351 (Beta) | www.GISAID.org | EPI_ISL_736930 |
| B.1.351 (Beta) | www.GISAID.org | EPI_ISL_736932 |
| B.1.351 (Beta) | www.GISAID.org | EPI_ISL_736944 |
| B.1.351 (Beta) | www.GISAID.org | EPI_ISL_736971 |
| B.1.351 (Beta) | www.GISAID.org | EPI_ISL_736966 |
| B.1.351 (Beta) | www.GISAID.org | EPI_ISL_736973 |
| B.1.351 (Beta) | www.GISAID.org | EPI_ISL_825104 |
| B.1.351 (Beta) | www.GISAID.org | EPI_ISL_825120 |
| B.1.351 (Beta) | www.GISAID.org | EPI_ISL_825131 |
| B.1.617.2 (Delta) | www.GISAID.org | EPI_ISL_2020950 |
| Experimental models: Cell lines | ||
| Human Embryonic Kidney (HEK) 293F | Dr Nicole Doria-Rose, VRC, USA | N/A |
| HEK293T-ACE2 cells | Dr Michael Farzan, Scripps, USA | N/A |
| Jurkat-Lucia™ NFAT-CD16 cells | Invivogen | Cat # jktl-nfat-cd16 |
| Recombinant DNA | ||
| Spike Hexapro plasmid | Original: Hsieh et al., 2020Beta: Moyo-Gwete et al., 2021 | N/A |
| SARS-CoV-2 ancestral variant spike (D614G) plasmid | Wibmer et al., 2021 | N/A |
| Beta spike (L18F, D80A, D215G, K417N, E484K, N501Y, D614G, A701V, 242-244 del) plasmid | Wibmer et al., 2021 | N/A |
| Delta spike (T19R, R158G L452R, T478K, D614G, P681R, D950N, 156-157 del) plasmid | This paper | N/A |
| Firefly luciferase encoding lentivirus backbone plasmid | Dr Michael Farzan, Scripps | N/A |
| Software and algorithms | ||
| Genome Detective 1.132 | Genome Detective | https://www.genomedetective.com |
| Coronavirus Typing Tool | Cleemput et al., 2020 | N/A |
| Geneious software | Biomatters Ltd | N/A |
| NextStrain | Hadfield et al., 2018 | https://github.com/nextstrain/ncov |
| FACSDiva 9 | BD Biosciences | https://www.bdbiosciences.com |
| FlowJo 10 | FlowJo, LLC | https://www.flowjo.com |
| Graphpad Prism 9 | Graphpad | https://graphpad.com |
| BioRender | BioRender | https://biorender.com |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Wendy Burgers (wendy.burgers@uct.ac.za).
Materials availability
Materials will be made available by request to Wendy Burgers (wendy.burgers@uct.ac.za).
Experimental model and subject details
Human subjects
Participants were recruited from a longitudinal study of healthcare workers (HCW; n = 400) enrolled from Groote Schuur Hospital (Cape Town, Western Cape, South Africa). HCW in this cohort were recruited between July 2020 and January 2021, and vaccination with single dose Johnson and Johnson Ad26.COV2.S in the Sisonke Phase 3b trial took place between 17 February and 26 March 2021. Sixty participants were selected for inclusion in this study, based on the availability of PBMC and plasma prior to vaccination and approximately one month after vaccination, and who fell into one of three groups: (1) No evidence of previous SARS-CoV-2 infection by diagnostic PCR test or serial serology; (2) infection during the ‘first wave’ of the pandemic in South Africa, prior to 1 September 2020, with known date of laboratory (PCR)-confirmed SARS-CoV-2 infection; and (3) infection during the ‘second wave’, with known date of laboratory (PCR)-confirmed SARS-CoV-2 infection between 1 November 2020 and 31 January 2021. Full demographic and clinical characteristics of participants are summarized in Table S1. The study was approved by the University of Cape Town Human Research Ethics Committee (HREC 190/2020 and 209/2020) and the University of the Witwatersrand Human Research Ethics Committee (Medical) (no M210429). Written informed consent was obtained from all participants.
Cell lines
Human embryo kidney HEK293T cells were cultured at 37°C, 5% CO2, in DMEM containing 10% heat-inactivated fetal bovine serum (GIBCO BRL Life Technologies) and supplemented with 50 μg/mL gentamicin (Sigma). Cells were disrupted at confluence with 0.25% trypsin in 1 mM EDTA (Sigma) every 48–72 h. HEK293T-ACE2 cells were maintained in the same way as HEK293T cells but were supplemented with 3 μg/mL puromycin for selection of stably transduced cells. Jurkat-Lucia NFAT-CD16 cells were maintained in IMDM media with 10% heat-inactivated fetal bovine serum (GIBCO, Gaithersburg, MD), 1% Penicillin Streptomycin (GIBCO, Gaithersburg, MD) and 10 μg/mL of Blasticidin and 100 μg/mL of Zeocin was added to the growth medium every other passage.
Method details
SARS-CoV-2 spike WGS and phylogenetic analysis
Whole genome sequencing (WGS) of SARS-CoV-2 was performed using nasopharyngeal swabs obtained from 19 of the hospitalized patients recruited during the second COVID-19 wave. Sequencing was performed as previously published (Moyo-Gwete et al., 2021). Briefly, cDNA was synthesized from RNA extracted from the nasopharyngeal swabs using the Superscript IV First Strand synthesis system (Life Technologies, Carlsbad, CA) and random hexamer primers. Whole genome amplification was then performed by multiplex PCR using the ARTIC V3 protocol (https://www.protocols.io/view/ncov-2019-sequencing-protocol-v3-locost-bh42j8ye). PCR products were purified with AMPure XP magnetic beads (Beckman Coulter, CA) and quantified using the Qubit dsDNA High Sensitivity assay on the Qubit 3.0 instrument (Life Technologies Carlsbad, CA). The Illumina® DNA Prep kit was used to prepare indexed paired end libraries of genomic DNA. Sequencing libraries were normalized to 4 nM, pooled, and denatured with 0.2 N sodium hydroxide. Libraries were sequenced on the Illumina MiSeq instrument (Illumina, San Diego, CA, USA). The quality control checks on raw sequence data and the genome assembly were performed using Genome Detective 1.132 (https://www.genomedetective.com) and the Coronavirus Typing Tool (Cleemput et al., 2020). The initial assembly obtained from Genome Detective was polished by aligning mapped reads to the references and filtering out low-quality mutations using bcftools 1.7-2 mpileup method. Mutations were confirmed visually with bam files using Geneious software (Biomatters Ltd, New Zealand). Phylogenetic clade classification of the genomes in this study consisted of analyzing them against a global reference dataset using a custom pipeline based on a local version of NextStrain (https://github.com/nextstrain/ncov) (Hadfield et al., 2018).
Roche serology
Serial serum samples were analyzed from longitudinal study visits from enrolment to post-vaccination (3-8 time points per participant) at Public Health England, Porton Down. The Elecsys anti-SARS-CoV-2 Spike and the Elecys anti-SARS-CoV-2 electrochemiluminescent immunoassays were performed (Roche Diagnostics, GmbH), which enable detection of total antibodies against the SARS-CoV-2 spike (S) receptor binding domain (RBD) and nucleocapsid (N) proteins, respectively. Samples were analyzed on a Cobas e801 instrument and a result ≥0.8 U/mL was considered positive in the S assay, and ≥1.0 U/mL positive in the N assay, according to the manufacturer’s instructions.
Isolation of PBMC
Blood was collected in heparin tubes and processed within 3 h of collection. Peripheral blood mononuclear cells (PBMC) were isolated by density gradient sedimentation using Ficoll-Paque (Amersham Biosciences, Little Chalfont, UK) as per the manufacturer’s instructions and cryopreserved in freezing media consisting of heat-inactivated fetal bovine serum (FBS, Thermofisher Scientific) containing 10% DMSO and stored in liquid nitrogen until use.
SARS-CoV-2 antigens
For serology assays, SARS-CoV-2 original and Beta variant spike proteins were expressed in Human Embryonic Kidney (HEK) 293F suspension cells by transfecting the cells with the spike plasmid. After incubating for six days at 37°C, 70% humidity and 10% CO2, proteins were first purified using a nickel resin followed by size-exclusion chromatography. Relevant fractions were collected and frozen at −80°C until use.
For T cell assays, we used peptides covering the full length SARS-CoV-2 spike protein, by combining two commercially available peptide pools of 15-mer sequences with 11 amino acids (aa) overlap (PepTivator®, Miltenyi Biotech, Bergisch Gladbach, Germany). These peptides are based on the Wuhan-1 strain and cover the N-terminal S1 domain of SARS-CoV-2 from aa 1 to 692, as well as the majority of the C-terminal S2 domain. Pools were resuspended in distilled water at a concentration of 50 μg/mL and used at a final concentration of 1 μg/mL. To determine T cell responses to SARS-CoV-2 variants, peptides were synthesized that spanned the entire SARS-CoV-2 spike protein and corresponded to the ancestral Wuhan sequence (GenBank: MN908947) or the Beta (B.1.351; GISAID: EPI_ISL_736932, EPI_ISL_736944, EPI_ISL_736971, EPI_ISL_736966, EPI_ISL_736973, EPI_ISL_825104, EPI_ISL_825120,EPI_ISL_825131), as previously reported (Tarke et al., 2021) or to the Delta SARS-CoV-2 variants (B.1.617.2; GISAID: EPI_ISL_2020950). Peptides were 15-mers overlapping by 10 amino acids and were synthesized as crude material (TC Peptide Lab, San Diego, CA). All peptides were individually resuspended in dimethyl sulfoxide (DMSO) at a concentration of 10–20 mg/mL. Megapools for each antigen were created by pooling aliquots of these individual peptides in the respective SARS-CoV-2 spike sequences, followed by sequential lyophilization steps, and resuspension in DMSO at 1 mg/mL. Pools were used at a final concentration of 1 μg/mL with an equimolar DMSO concentration in the non-stimulated control.
SARS-CoV-2 spike ELISA
Two μg/mL of spike protein were used to coat 96-well, high-binding plates and incubated overnight at 4°C. The plates were incubated in a blocking buffer consisting of 5% skimmed milk powder, 0.05% Tween 20, 1x PBS. Plasma samples were diluted to 1:100 starting dilution in a blocking buffer and added to the plates. Secondary antibody was diluted to 1:3000 in blocking buffer and added to the plates followed by TMB substrate (Thermofisher Scientific). Upon stopping the reaction with 1 M H2SO4, absorbance was measured at a 450nm wavelength. In all instances, mAbs CR3022 and BD23 were used as positive controls and palivizumab was used as a negative control. All values were normalized with the CR3022 mAb.
Pseudovirus neutralization assay
SARS-CoV-2 pseudotyped lentiviruses were prepared by co-transfecting the HEK293T cell line with either the SARS-CoV-2 ancestral variant spike (D614G), the Beta spike (L18F, D80A, D215G, K417N, E484K, N501Y, D614G, A701V, 242-244 del) or the Delta spike (T19R, R158G L452R, T478K, D614G, P681R, D950N, 156-157 del) plasmids in conjunction with a firefly luciferase encoding lentivirus backbone plasmid. For the neutralization assay, heat-inactivated plasma samples from vaccine recipients were incubated with the SARS- CoV-2 pseudotyped virus for 1 h at 37°C, 5% CO2. Subsequently, 1x104 HEK293T cells engineered to overexpress ACE-2 were added and incubated at 37°C, 5% CO2 for 72 h upon which the luminescence of the luciferase gene was measured. CB6 was used as a positive control.
ADCC assay
The ability of plasma antibodies to cross-link FcγRIIIa (CD16) and spike expressing cells was measured as a proxy for antibody-dependent cellular cytotoxicity (ADCC). HEK293T cells were transfected with 5 μg of SARS-CoV-2 original variant spike (D614G), Beta or Delta spike plasmids using PEI-MAX 40,000 (Polysciences) and incubated for 2 days at 37°C. Expression of spike was confirmed by binding of CR3022 and P2B-2F6 and their detection by anti-IgG APC staining measured by flow cytometry. Subsequently, 1x105 spike transfected cells per well were incubated with heat inactivated plasma (1:100 final dilution) or control mAbs (final concentration of 100 μg/mL) in RPMI 1640 media supplemented with 10% FBS 1% Pen/Strep (GIBCO, Gaithersburg, MD) for 1 h at 37°C. Jurkat-Lucia NFAT-CD16 cells (Invivogen) (2x105 cells/well) were added and incubated for 24 h at 37°C, 5% CO2. Twenty μl of supernatant was then transferred to a white 96-well plate with 50 μL of reconstituted QUANTI-Luc secreted luciferase and read immediately on a Victor 3 luminometer with 1 s integration time. Relative light units (RLU) of a no antibody control were subtracted as background. Palivizumab was used as a negative control, while CR3022 was used as a positive control, and P2B-2F6 to differentiate the Beta from the D614G variant. To induce the transgene 1x cell stimulation cocktail (Thermofisher Scientific, Oslo, Norway) and 2 μg/mL ionomycin in R10 was added as a positive control.
Cell stimulation and flow cytometry staining
Cryopreserved PBMC were thawed, washed and rested in RPMI 1640 containing 10% heat-inactivated FCS for 4 h prior to stimulation. PBMC were seeded in a 96-well V-bottom plate at ∼2 × 106 PBMC per well and stimulated with SARS-CoV-2 spike peptide pools: full spike pool (Miltenyi), and ancestral and Beta mutated S1 and S2 pools (1 μg/mL). All stimulations were performed in the presence of Brefeldin A (10 μg/mL, Sigma-Aldrich, St Louis, MO, USA) and co-stimulatory antibodies against CD28 (clone 28.2) and CD49d (clone L25) (1 μg/mL each; BD Biosciences, San Jose, CA, USA). As a negative control, PBMC were incubated with co-stimulatory antibodies, Brefeldin A and an equimolar amount of DMSO.
After 16 h of stimulation, cells were washed, stained with LIVE/DEAD Fixable VIVID Stain (Invitrogen, Carlsbad, CA, USA) and subsequently surface stained with the following antibodies: CD14 Pac Blue (TuK4, Invitrogen Thermofisher Scientific), CD19 Pac Blue (SJ25-C1, Invitrogen Thermofisher Scientific), CD4 PERCP-Cy5.5 (L200, BD Biosciences, San Jose, CA, USA), CD8 BV510 (RPA-8, Biolegend, San Diego, CA, USA), PD-1 BV711 (EH12.2H7, Biolegend, San Diego, CA, USA), CD27 PE-Cy5 (1A4, Beckman Coulter), CD45RA BV570 (HI100, Biolegend, San Diego, CA, USA). Cells were then fixed and permeabilized using a Cytofix/Cyto perm buffer (BD Biosciences) and stained with CD3 BV650 (OKT3) IFN-g Alexo 700 (B27), TNF BV786 (Mab11) and IL-2 APC (MQ1-17H12) from Biolegend. Finally, cells were washed and fixed in CellFIX (BD Biosciences). Samples were acquired on a BD LSR-II flow cytometer and analyzed using FlowJo (v10, FlowJo LLC, Ashland, OR, USA). A median of 282 848 CD4 events (IQR:216 796 - 355 414) and 153 192 CD8 events (IQR 109 697 - 202 204) were acquired. Cells were gated on singlets, CD14- CD19-, live lymphocytes and memory cells (excluding naive CD27+ CD45RA+ population). Results are expressed as the frequency of CD4+ or CD8+ T cells expressing IFN-g, TNF-a or IL-2. Due to high TNF-a backgrounds, cells producing TNF-a alone were excluded from the analysis. Cytokine responses presented are background subtracted values (from the frequency of cytokine produced in unstimulated cells), and the threshold for a positive cytokine response was defined as > 0.02%.
Quantification and statistical analysis
Statistical analyses were performed in Prism (v9; GraphPad Software Inc, San Diego, CA, USA). Non-parametric tests were used for all comparisons. The Mann-Whitney, Friedman and Wilcoxon tests were used for unmatched and paired samples, respectively. All correlations reported are non-parametric Spearman’s correlations. P values less than 0.05 were considered statistically significant. Details of analysis performed for each experiment are described in the figure legends.
Acknowledgments
We thank the study participants and the clinical staff and personnel at Groote Schuur Hospital, Cape Town for their dedication. We thank F. Ayres, D. Mhlanga, B. Oosthuysen, and B. Lambson for production of protein and pseudoviruses. The parental soluble spike was provided by J. McLellan. The parental pseudovirus plasmids were kindly provided by Drs E. Landais and D. Sok. We thank the Variant Consortium of South African scientists. The graphical abstract was created with BioRender.com. This research was supported by the South African Medical Research Council, with funds received from the South African Department of Science and Innovation (DSI), including grants 96825, SHIPNCD 76756, and DST/CON 0250/2012. This work was supported by the Poliomyelitis Research Foundation (21/65) and the Wellcome Centre for Infectious Diseases Research in Africa (CIDRI-Africa), which is supported by core funding from the Wellcome Trust (203135/Z/16/Z and 222754). Funding support was received from NIH NIAID with the SARS-CoV-2 Assessment of Viral Evolution program and contract no. 75N9301900065 to A.S. and D.W. P.L.M. and S.I.R. are supported by the South African Research Chairs Initiative of DSI and the National Research Foundation (NRF; no. 98341). S.I.R. is a L’Oreal/UNESCO Women in Science South Africa Young Talents awardee. W.A.B. and C.R. are supported by the EDCTP2 program of the European Union’s Horizon 2020 program (TMA2017SF-1951-TB-SPEC and TMA2016SF-1535-CaTCH-22). N.A.B.N. acknowledges funding from the SA-MRC, MRC UK, NRF, and the Lily and Ernst Hausmann Trust. M.N. is supported by the Wellcome Trust (207511/Z/17/Z) and by NIHR Biomedical Research Funding to University College London Hospitals. For the purposes of open access, the authors have applied a CC BY public copyright license to any author-accepted version arising from this submission.
Author contributions
W.A.B., P.L.M., and N.A.B.N. designed the study. W.A.B. and P.L.M. analyzed the data and wrote the manuscript. R.K., S.I.R., and T.M.G. generated and analyzed the data and wrote the manuscript. S.I.R., T.M.G., T.H., N.P.M., Z.M., and T.M. performed antibody assays. R.K., M.B.T., N.B., R.B., and A.N. performed T cell assays. M.M., S.S., and L.R.C. managed the HCW cohort and contributed clinical samples. A.O. and T.B. characterized the serological profiles. N.Y.H. contributed samples, and A.G., D.W., and A.S. provided variant peptide pools. D.D., A.I., and C.W. performed viral sequencing. H.M. and J.B. contributed to cohort characterization. C.R. contributed to data analysis. A.G., N.G., L.G.B., and G.G. established and led the Sisonke vaccine study. N.A.B.N., J.M., and M.N. established and led the HCW cohort. All authors reviewed and edited the manuscript.
Declaration of interests
A.S. is a consultant for Gritstone, Flow Pharma, CellCarta, Arcturus, Oxford Immunotech, and Avalia. All of the other authors declare no competing interests. LJI has filed for patent protection for various aspects of vaccine design and identification of specific epitopes.
Published: October 13, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.chom.2021.10.003.
Supplemental information
Data and code availability
The published article includes all data generated or analyzed during this study, and summarized in the accompanying tables, figures and supplemental information. The following sequences were deposited onto the GISAID EpiCov database (https://www.gisaid.org): PID1054 SARS-CoV-2 Viral sequence (GISAID Accession # EPI_ISL_2621106), PID1127 SARS-CoV-2 Viral sequence (GISAID Accession # EPI_ISL_2621118), PID1128 SARS-CoV-2 Viral sequence (GSAID Accession # EPI_ISL_2621122), PID1134 SARS-CoV-2 Viral sequence (GISAID Accession # EPI_ISL_1534413), PID1169 SARS-CoV-2 Viral sequence (GISAID Accession # EPI_ISL_2621117), PID1319 SARS-CoV-2 Viral sequence (GISAID Accession # EPI_ISL_2621127), PID1337 SARS-CoV-2 Viral sequence (GISAID Accession # EPI_ISL_2621124), PID1399 SARS-CoV-2 Viral sequence (GISAID Accession # EPI_ISL_2621110).
References
- Alter G., Yu J., Liu J., Chandrashekar A., Borducchi E.N., Tostanoski L.H., McMahan K., Jacob-Dolan C., Martinez D.R., Chang A., et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature. 2021;596:268–272. doi: 10.1038/s41586-021-03681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch D.H., Stephenson K.E., Sadoff J., Yu J., Chang A., Gebre M., McMahan K., Liu J., Chandrashekar A., Patel S., et al. Durable Humoral and Cellular Immune Responses 8 Months after Ad26.COV2.S Vaccination. N. Engl. J. Med. 2021;385:951–953. doi: 10.1056/NEJMc2108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F., Hippenstiel S., Dingeldey M., Kruse B., Fauchere F., et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587:270–274. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- Cele S., Gazy I., Jackson L., Hwa S.H., Tegally H., Lustig G., Giandhari J., Pillay S., Wilkinson E., Naidoo Y., et al. Network for Genomic Surveillance in South Africa. COMMIT-KZN Team Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma. Nature. 2021;593:142–146. doi: 10.1038/s41586-021-03471-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleemput S., Dumon W., Fonseca V., Abdool Karim W., Giovanetti M., Alcantara L.C., Deforche K., de Oliveira T. Genome Detective Coronavirus Typing Tool for rapid identification and characterization of novel coronavirus genomes. Bioinformatics. 2020;36:3552–3555. doi: 10.1093/bioinformatics/btaa145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edara V.V., Pinsky B.A., Suthar M.S., Lai L., Davis-Gardner M.E., Floyd K., Flowers M.W., Wrammert J., Hussaini L., Ciric C.R., et al. Infection and Vaccine-Induced Neutralizing-Antibody Responses to the SARS-CoV-2 B.1.617 Variants. N. Engl. J. Med. 2021;385:664–666. doi: 10.1056/nejmc2107799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher K.M.E., Leick M.B., Larson R.C., Berger T.R., Katsis K., Yam J.Y., Brini G., Grauwet K., Maus M.V., MGH COVID-19 Collection & Processing Team SARS -CoV-2 T-cell immunity to variants of concern following vaccination. bioRxiv. 2021 doi: 10.1101/2021.05.03.442455. [DOI] [Google Scholar]
- Geers D., Shamier M.C., Bogers S., den Hartog G., Gommers L., Nieuwkoop N.N., Schmitz K.S., Rijsbergen L.C., van Osch J.A.T., Dijkhuizen E., et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci. Immunol. 2021;6:eabj1750. doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield J., Megill C., Bell S.M., Huddleston J., Potter B., Callender C., Sagulenko P., Bedford T., Neher R.A. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018;34:4121–4123. doi: 10.1093/bioinformatics/bty407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havervall S., Marking U., Greilert-Norin N., Ng H., Gordon M., Salomonsson A.C., Hellström C., Pin E., Blom K., Mangsbo S., et al. Antibody responses after a single dose of ChAdOx1 nCoV-19 vaccine in healthcare workers previously infected with SARS-CoV-2. EBioMedicine. 2021;70:103523. doi: 10.1101/2021.05.08.21256866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao M., Davies M., Kalk E. SARS-CoV-2 seroprevalence in the Cape Town Metropolitan sub-districts after the peak of infections. NICD COVID-19 Special Public Health Surveill Bull. 2020;18:1–9. [Google Scholar]
- Hsieh C.-L., Goldsmith J.A., Schaub J.M., DiVenere A.M., Kuo H.-C., Javanmardi K., Le K.C., Wrapp D., Lee A.G., Liu Y., et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science. 2020;369:1501–1505. doi: 10.1126/science.abd0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Ginn H.M., Dejnirattisai W., Supasa P., Wang B., Tuekprakhon A., Nutalai R., Zhou D., Mentzer A.J., Zhao Y., et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184:4220–4236.e13. doi: 10.1016/j.cell.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manisty C., Otter A.D., Treibel T.A., McKnight Á., Altmann D.M., Brooks T., Noursadeghi M., Boyton R.J., Semper A., Moon J.C. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397:1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateus J., Grifoni A., Tarke A., Sidney J., Ramirez S.I., Dan J.M., Burger Z.C., Rawlings S.A., Smith D.M., Phillips E., et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370:89–94. doi: 10.1126/science.abd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan K., Yu J., Mercado N.B., Loos C., Tostanoski L.H., Chandrashekar A., Liu J., Peter L., Atyeo C., Zhu A., et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P., Moyo-Gwete T., Hermanus T., Kgagudi P., Ayres F., Makhado Z., Sadoff J., Le Gars M., van Roey G., Crowther C., et al. Neutralizing antibodies elicited by the Ad26.COV2.S COVID-19 vaccine show reduced activity against 501Y.V2 (B. 1.351), despite protection against severe disease by this variant. bioRxiv. 2021 doi: 10.1101/2021.06.09.447722. [DOI] [Google Scholar]
- Motozono C., Toyoda M., Zahradnik J., Saito A., Nasser H., Tan T.S., Ngare I., Kimura I., Uriu K., Kosugi Y., et al. Genotype to Phenotype Japan (G2P-Japan) Consortium SARS-CoV-2 spike L452R variant evades cellular immunity and increases infectivity. Cell Host Microbe. 2021;29:1124–1136.e11. doi: 10.1016/j.chom.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyo-Gwete T., Madzivhandila M., Makhado Z., Ayres F., Mhlanga D., Oosthuysen B., Lambson B.E., Kgagudi P., Tegally H., Iranzadeh A., et al. Cross-Reactive Neutralizing Antibody Responses Elicited by SARS-CoV-2 501Y.V2 (B.1.351) N. Engl. J. Med. 2021;384:2161–2163. doi: 10.1056/NEJMc2104192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutevedzi P.C., Kawonga M., Kwatra G., Moultrie A., Baillie V.L., Mabhena N., Mathibe M.N., Rafuma M.M., Maposa I., Abbott G. Population Based SARS-CoV-2 Sero-Epidemiological Survey and Estimated Infection Incidence and Fatality Risk in Gauteng Province, South Africa. 2021. https://ssrn.com/abstract=3855442 [DOI] [PMC free article] [PubMed]
- Planas D., Veyer D., Baidaliuk A., Staropoli I., Guivel-Benhassine F., Rajah M.M., Planchais C., Porrot F., Robillard N., Puech J., et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- Reynolds C.J., Pade C., Gibbons J.M., Butler D.K., Otter A.D., Menacho K., Fontana M., Smit A., Sackville-West J.E., Cutino-Moguel T., et al. UK COVIDsortium Immune Correlates Network. UK COVIDsortium Investigators Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science. 2021;372:1418–1423. doi: 10.1126/science.abh1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S.I., Moore P.L. Targeting Fc effector function in vaccine design. Expert Opin. Ther. Targets. 2021;25:467–477. doi: 10.1080/14728222.2021.1907343. [DOI] [PubMed] [Google Scholar]
- Riou C., Keeton R., Moyo-Gwete T., Hermanus T., Kgagudi P., Baguma R., Tegally H., Doolabh D., Iranzadeh A., Tyers L., et al. Loss of recognition of SARS-CoV-2 B.1.351 variant spike epitopes but overall preservation of T cell immunity. medRxiv. 2021 doi: 10.1101/2021.06.03.21258307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydyznski Moderbacher C., Ramirez S.I., Dan J.M., Grifoni A., Hastie K.M., Weiskopf D., Belanger S., Abbott R.K., Kim C., Choi J., et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell. 2020;183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat S., Rikhtegaran Tehrani Z., Logue J., Newman M., Frieman M.B., Harris A.D., Sajadi M.M. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA. 2021;325:1467–1469. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., Goepfert P.A., Truyers C., Fennema H., Spiessens B., et al. ENSEMBLE Study Group Safety and efficacy of single-dose Ad26. COV2. S vaccine against Covid-19. N. Engl. J. Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer A., Muecksch F., Lorenzi J.C.C., Leist S.R., Cipolla M., Bournazos S., Schmidt F., Maison R.M., Gazumyan A., Martinez D.R., et al. Antibody potency, effector function, and combinations in protection and therapy for SARS-CoV-2 infection in vivo. J. Exp. Med. 2021;218:e20201993. doi: 10.1084/jem.20201993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatatos L., Czartoski J., Wan Y.H., Homad L.J., Rubin V., Glantz H., Neradilek M., Seydoux E., Jennewein M.F., MacCamy A.J., et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021:eabg9175. doi: 10.1126/science.abg9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson K.E., Le Gars M., Sadoff J., de Groot A.M., Heerwegh D., Truyers C., Atyeo C., Loos C., Chandrashekar A., McMahan K., et al. Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19. JAMA. 2021;325:1535–1544. doi: 10.1001/jama.2021.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes W., Mhlanga L., Swanevelder R., Glatt T.N., Grebe E., Coleman C., Pieterson N., Cable R., Welte A., van den Berg K., Vermeulen M. Prevalence of anti-SARS-CoV-2 antibodies among blood donors in Northern Cape, KwaZulu-Natal, Eastern Cape, and Free State provinces of South Africa in January 2021. Res Sq. 2021 doi: 10.21203/rs.3.rs-233375/v1. rs.3.rs-233375. [DOI] [Google Scholar]
- Takuva S., Takalani A., Garrett N., Goga A., Peter J., Louw V., Opie J., Jacobson B., Sanne I., Gail-Bekker L., Gray G. Thromboembolic Events in the South African Ad26.COV2.S Vaccine Study. N. Engl. J. Med. 2021;385:570–571. doi: 10.1056/NEJMc2107920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarke A., Sidney J., Methot N., Yu E.D., Zhang Y., Dan J.M., Goodwin B., Rubiro P., Sutherland A., Wang E., et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Rep Med. 2021;2:100355. doi: 10.1016/j.xcrm.2021.100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauzin A., Nayrac M., Benlarbi M., Gong S.Y., Gasser R., Beaudoin-Bussières G., Brassard N., Laumaea A., Vézina D., Prévost J., et al. A single dose of the SARS-CoV-2 vaccine BNT162b2 elicits Fc-mediated antibody effector functions and T cell responses. Cell Host Microbe. 2021;29:1137–1150.e6. doi: 10.1016/j.chom.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegally H., Wilkinson E., Giovanetti M., Iranzadeh A., Fonseca V., Giandhari J., Doolabh D., Pillay S., San E.J., Msomi N., et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592:438–443. doi: 10.1038/s41586-021-03402-9. [DOI] [PubMed] [Google Scholar]
- Vanshylla K., Di Cristanziano V., Kleipass F., Dewald F., Schommers P., Gieselmann L., Gruell H., Schlotz M., Ercanoglu M.S., Stumpf R., et al. Kinetics and correlates of the neutralizing antibody response to SARS-CoV-2 infection in humans. Cell Host Microbe. 2021;29:917–929.e4. doi: 10.1016/j.chom.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., Wang M., Yu J., Zhang B., Kwong P.D., et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- Wang Z., Muecksch F., Schaefer-Babajew D., Finkin S., Viant C., Gaebler C., Hoffmann H.-H., Barnes C.O., Cipolla M., Ramos V., et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595:426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibmer C.K., Ayres F., Hermanus T., Madzivhandila M., Kgagudi P., Oosthuysen B., Lambson B.E., de Oliveira T., Vermeulen M., van der Berg K., et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat. Med. 2021;27:622–625. doi: 10.1038/s41591-021-01285-x. [DOI] [PubMed] [Google Scholar]
- Winkler E.S., Gilchuk P., Yu J., Bailey A.L., Chen R.E., Chong Z., Zost S.J., Jang H., Huang Y., Allen J.D., et al. Human neutralizing antibodies against SARS-CoV-2 require intact Fc effector functions for optimal therapeutic protection. Cell. 2021;184:1804–1820.e16. doi: 10.1016/j.cell.2021.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar T., Loos C., Fischinger S., Atyeo C., Wang C., Slein M.D., Burke J., Yu J., Feldman J., Hauser B.M., et al. Compromised humoral functional evolution tracks with SARS-CoV-2 mortality. Cell. 2020;183:1508–1519.e12. doi: 10.1016/j.cell.2020.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article includes all data generated or analyzed during this study, and summarized in the accompanying tables, figures and supplemental information. The following sequences were deposited onto the GISAID EpiCov database (https://www.gisaid.org): PID1054 SARS-CoV-2 Viral sequence (GISAID Accession # EPI_ISL_2621106), PID1127 SARS-CoV-2 Viral sequence (GISAID Accession # EPI_ISL_2621118), PID1128 SARS-CoV-2 Viral sequence (GSAID Accession # EPI_ISL_2621122), PID1134 SARS-CoV-2 Viral sequence (GISAID Accession # EPI_ISL_1534413), PID1169 SARS-CoV-2 Viral sequence (GISAID Accession # EPI_ISL_2621117), PID1319 SARS-CoV-2 Viral sequence (GISAID Accession # EPI_ISL_2621127), PID1337 SARS-CoV-2 Viral sequence (GISAID Accession # EPI_ISL_2621124), PID1399 SARS-CoV-2 Viral sequence (GISAID Accession # EPI_ISL_2621110).