Abstract
Background
The predictive power of extubation failure diagnosed by cough strength varies by study. Here we summarise the diagnostic power of extubation failure tested by cough strength.
Methods
A comprehensive online search was performed to select potentially eligible studies that evaluated the predictive power of extubation failure tested by cough strength. A manual search was also performed to identify additional studies. Data were extracted to calculate the pooled sensitivity, specificity, positive likelihood ratio (LR), negative LR, diagnostic odds ratio (DOR), and area under the receiver operating characteristic curve (AUC) to evaluate the predictive power of extubation failure.
Results
A total of 34 studies involving 45 study arms were enrolled, and 7329 patients involving 8684 tests were analysed. In all, 23 study arms involving 3018 tests measured cough peak flow before extubation. The pooled extubation failure was 36.2% and 6.3% in patients with weak and strong cough assessed by cough peak flow, respectively. The pooled sensitivity, specificity, positive LR, negative LR, DOR, and AUC were 0.76 (95% confidence interval [CI]: 0.72–0.80), 0.75 (0.69–0.81), 2.89 (2.36–3.54), 0.37 (0.30–0.45), 8.91 (5.96–13.32), and 0.79 (0.75–0.82), respectively. Moreover, 22 study arms involving 5666 tests measured the semiquantitative cough strength score (SCSS) before extubation. The pooled extubation failure was 37.1% and 11.3%, respectively, in patients with weak and strong cough assessed by the SCSS. The pooled sensitivity, specificity, positive LR, negative LR, DOR, and AUC were 0.53 (95% CI: 0.41–0.64), 0.83 (0.74–0.89), 2.50 (1.93–3.25), 0.65 (0.56–0.76), 4.61 (3.03–7.01), and 0.74 (0.70–0.78), respectively.
Conclusions
Weak cough is associated with increased extubation failure. Cough peak flow is superior to the SCSS for predicting extubation failure. However, both show moderate power for predicting extubation failure.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-021-03781-5.
Keywords: Ventilator weaning, Weak cough, Sensitivity, Specificity
Background
The use of a spontaneous breathing trial (SBT) has been recommended to help determine whether a patient can be weaned from mechanical ventilation (MV) [1–3]. After a successful SBT, extubation is recommended. However, 10–20% of patients who successfully complete an SBT experience extubation failure [4]. Compared to patients experience successful extubation, those who experience extubation failure are more likely to die in hospital [5, 6]. Evidence shows that early identification of patients at high risk for extubation failure and early application of preventive strategies (e.g. noninvasive ventilation or the use of a high-flow nasal cannula) can reduce hospital mortality [7, 8]. Therefore, the key question is how to identify patients at high risk for extubation failure.
Weak cough is a predictor of extubation failure. It can be measured by cough peak flow [9–17]. In some studies, patients with successful extubation had a higher cough peak flow than those who experienced extubation failure [9–16]. However, another study reported that cough peak flow did not differ between patients who experienced extubation success and failure [17]. In addition, cough strength can also be measured by the semiquantitative cough strength score (SCSS) [18–21]. Given the inconsistent results found by different studies and the use of multiple methods to measure cough strength, we reviewed the literature systematically and performed a meta-analysis to assess the efficacy of diagnostic tests that use cough strength for the early detection of extubation failure.
Methods
PICO statement
P-patient: adult patients were under MV through endotracheal intubation. I-index test: cough strength was measured in all included patients. C-complement: an SBT was given to all included patients who were deemed ready to be liberated from MV. O-outcome: the efficacy of cough strength for predicting extubation failure was estimated.
Search techniques and selection criteria
This systematic review and meta-analysis was performed in conformance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement [22]. We searched pertinent research published before June 2021 in PubMed, Web of Science, the Cochrane library, and some Chinese databases (CBM, Wanfang Data, and CNKI) without any language limitations. We also did manual searches of the reference lists of included articles to identify additional relevant articles. The studies were searched with the following key words: (“weak cough” OR “ineffective cough” OR “cough peak flow” OR “cough peak expiratory flow” OR “cough strength”) and (“ventilator weaning” OR “wean from mechanical ventilation” OR “weaning from mechanical ventilation” OR “liberation from mechanical ventilation” OR “liberate from mechanical ventilation” OR “withdrawal of mechanical ventilation” OR “extubation failure” OR “postextubation failure” OR “postextubation respiratory failure” OR “reintubation”).
Studies were enrolled based on the following inclusion criteria: (1) only adult patients with an endotracheal tube were involved, (2) an SBT was completed before extubation, (3) cough strength was assessed before extubation, and (4) data were available for calculating outcomes (true positive [TP], false positive [FP], false negative [FN], and true negative [TN]). The following works were excluded: (1) reviews, case reports, editorials, letters, and conference abstracts; (2) articles with no available data for patients with weak cough; and (3) articles without a definition of extubation failure. Extubation failure included reintubation, death, or the use of noninvasive ventilation due to postextubation respiratory failure.
Data extraction and evaluation of quality
All studies were independently selected by two investigators (JD and XFZ). Any discrepancies were resolved by consensus. If the researchers failed to reach a consensus, a third investigator (JPS) reviewed the data in question. The first author’s name; publication year; study region; sample size; methods of assessing cough strength; cut-off value; definition of weak cough; and number of patients with TP, FP, FN, and TN were collected. If numbers of TP, FP, FN, and TN were unavailable, we communicated with the corresponding author to obtain these data. The Quality Assessment of Diagnostic Accuracy Studies 2 was used to assess the quality of the enrolled articles [23].
Statistical analysis
The data were analysed with RevMan 5.3, Meta-Disc 1.4, and Stata SE 15.0. The pooled diagnostic odds ratio (DOR), sensitivity, specificity, positive likelihood ratio (LR), negative LR, and area under the receiver operating characteristic curve (AUC) were calculated by TP, FP, FN, TN. Sensitivity = true positives/(true positives + false negatives). Specificity = true negatives/(true negatives + false positives). True positives were patients with ineffective cough who failed extubation. False negatives were patients with effective cough who failed extubation. True negatives were patients with effective cough who were successfully extubated. False positives were patients with ineffective cough who were successfully extubated. Diagnostic power was good, moderate, and poor if the AUC was more than 0.8, between 0.7 and 0.8, and less than 0.7, respectively [24]. Deeks’ funnel plot was used to detect publication bias. If publication bias was present, a sensitivity analysis was performed to explore why.
Spearman’s correlation coefficient is used to detect threshold effects. I2 is used to describe heterogeneity. I2 ≥ 50% represents significant heterogeneity. A fixed effects model was used if no heterogeneity was observed. A random effects model was selected if significant heterogeneity was observed. Possible sources of heterogeneity were explored through a meta-regression analysis.
Results
Characteristics of the included studies
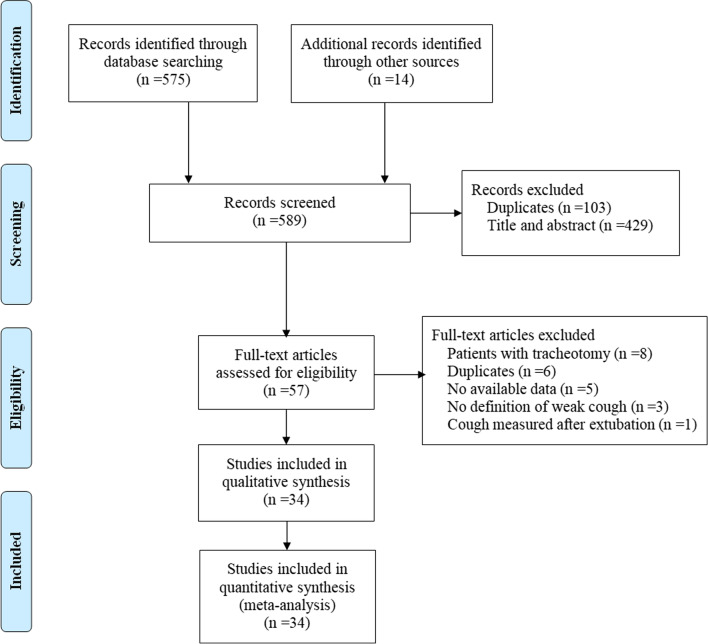
A total of 575 studies were obtained using the search strategy, and 14 studies were identified from other sources (Fig. 1). After screening titles and abstracts and reviewing full papers, we enrolled 34 studies involving 45 study arms in this meta-analysis [9–21, 25–45]. A total of 7329 patients involving 8684 tests were analysed. The characteristics of the study arms are summarised in Table 1. A total of 23 study arms involving 3018 tests measured cough peak flow before extubation. The pooled extubation failure was 36.2% and 6.3%, respectively, among patients with weak and strong cough assessed by cough peak flow (Additional file 1: Figure 1). Spearman’s correlation coefficient was 0.034 (p = 0.88), indicating no threshold effect. Four subgroups of studies measured cough peak flow. Details are reported in Table 2 and Additional file 12: Text 1.
Fig. 1.
Flowchart of study selection
Table 1.
Characteristics of the included studies
| Author | Year | Country | Design | Method of SBT | Measurement of cough strength | Definition of weak cough | Total tests | TP | FP | FN | TN | Time and definition of extubation failure | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beuret | 2009 | France | Prospective | T-piece | Voluntary CPF tested with an external flowmeter | CPF ≤ 35 L/min | 130 | 11 | 34 | 3 | 82 | 48 h | Reintubation |
| Duan | 2014a | China | Prospective | PSV | Voluntary CPF tested with an external flowmeter | CPF ≤ 62.4 L/min | 115 | 17 | 34 | 3 | 61 | 72 h | Reintubation |
| Duan | 2014b | China | Prospective | PSV | #Involuntary CPF tested with an external flowmeter | CPF ≤ 49.8 L/min | 115 | 14 | 32 | 6 | 63 | 72 h | Reintubation |
| Gao | 2009a | China | Prospective | PSV/CPAP | Voluntary CPF tested with a ventilator | CPF ≤ 58.5 L/min | 200 | 20 | 55 | 8 | 117 | 72 h | Reintubation/death |
| Gao | 2009b | China | Prospective | PSV/CPAP | SCSS (strong, moderate, weak) | Weak | 200 | 13 | 3 | 15 | 169 | 72 h | Reintubation/death |
| Salam | 2004a | USA | Prospective | T-piece/PSV | Voluntary CPF tested with an external flowmeter | CPF ≤ 60 L/min | 88 | 11 | 25 | 3 | 49 | 72 h | Reintubation |
| Salam | 2004b | USA | Prospective | T-piece/PSV | WCT | Negative | 88 | 10 | 36 | 4 | 38 | 72 h | Reintubation |
| Smailes | 2013 | UK | Prospective | T-piece | Voluntary CPF tested with an external flowmeter | CPF ≤ 60 L/min | 125 | 10 | 7 | 7 | 101 | 48 h | Reintubation |
| Smina | 2003 | USA | Prospective | T-piece/PSV | Voluntary CPF tested with an external flowmeter | CPF ≤ 60 L/min | 111 | 9 | 25 | 4 | 73 | 72 h | Reintubation |
| Su | 2010a | China | Prospective | T-piece/PSV/CPAP | #Involuntary CPF tested with an external flowmeter | CPF ≤ 58.5 L/min | 150 | 25 | 25 | 7 | 93 | Hospital stay | Reintubation |
| Su | 2010b | China | Prospective | T-piece/PSV/CPAP | SCSS (strong, weak, no cough) | Weak or no cough | 150 | 25 | 47 | 7 | 71 | Hospital stay | Reintubation |
| Khamiees | 2001a | China | Prospective | T-piece/PSV/CPAP | SCSS (grade 0 to 5) | Grade 0 to 2 | 100 | 10 | 14 | 8 | 68 | 72 h | Reintubation |
| Khamiees | 2001b | China | Prospective | T-piece/PSV/CPAP | WCT | Negative | 100 | 9 | 16 | 9 | 66 | 72 h | Reintubation |
| Huang | 2013 | China | Retrospective | T-piece/PSV | SCSS (effective and ineffective) | Ineffective | 119 | 23 | 27 | 4 | 65 | 7 d | Reintubation |
| Gobert | 2017 | France | Prospective | PSV | Voluntary CPF tested with a ventilator | CPF ≤ 60 L/min | 92 | 7 | 24 | 4 | 57 | 48 h | Reintubation/death |
| Liu | 2014 | China | Prospective | PSV | Voluntary CPF tested with a ventilator | CPF ≤ 60 L/min | 102 | 8 | 36 | 7 | 51 | 48 h | Reintubation |
| Duan | 2015a | China | Prospective | PSV | SCSS (grade 0 to 5) | Grade 0 to 2 | 186 | 16 | 52 | 12 | 106 | 72 h | Reintubation |
| Duan | 2015b | China | Prospective | PSV | Voluntary CPF tested with an external flowmeter | CPF ≤ 60 L/min | 186 | 23 | 71 | 5 | 87 | 72 h | Reintubation |
| Bai | 2017a | China | Prospective | PSV | Voluntary CPF tested with an external flowmeter | CPF ≤ 56.4 L/min | 126 | 11 | 14 | 4 | 97 | 72 h | Reintubation |
| Bai | 2017b | China | Prospective | PSV | Voluntary CPF tested with an external ventilator | CPF ≤ 56 L/min | 126 | 11 | 16 | 4 | 95 | 72 h | Reintubation |
| Xiao | 2018 | China | Prospective | PSV | Voluntary CPF tested with an external flowmeter | CPF ≤ 60 L/min | 139 | 15 | 36 | 7 | 81 | 72 h | Reintubation |
| Duan | 2017 | China | Prospective | PSV | Voluntary CPF tested with an external flowmeter | CPF ≤ 70 L/min | 356 | 61 | 119 | 15 | 161 | 7 d | Reintubation |
| Thille | 2015 | France | Prospective | PSV | SCSS (grade 0 to 4) | Grade 0 to 2 | 223 | 10 | 15 | 20 | 178 | 7 d | Reintubation |
| Kutchak | 2015 | Brazil | Prospective | T-piece | $Involuntary CPF tested with an external flowmeter | CPF ≤ 80 L/min | 135 | 35 | 20 | 10 | 70 | 48 h | Reintubation |
| Almeida | 2020a | Brazil | Prospective | T-piece/PSV | Voluntary CPF tested with an external flowmeter | CPF ≤ 45 L/min | 81 | 23 | 3 | 10 | 45 | 48 h | Reintubation |
| Almeida | 2020b | Brazil | Prospective | T-piece/PSV | #Involuntary CPF tested with an external flowmeter | CPF ≤ 60 L/min | 81 | 29 | 4 | 4 | 44 | 48 h | Reintubation |
| Almeida | 2020c | Brazil | Prospective | T-piece/PSV | $Involuntary CPF tested with an external flowmeter | CPF ≤ 55 L/min | 81 | 30 | 7 | 3 | 41 | 48 h | Reintubation |
| Aziz | 2018 | Egypt | Prospective | Not reported | SCSS (grade 0 to 5) | Grade 0 to 2 | 80 | 18 | 3 | 19 | 40 | 72 h | Reintubation |
| Vivier | 2019a | France | Prospective | T-piece | SCSS (ineffective, moderate, and effective) | Ineffective | 181 | 6 | 7 | 27 | 141 | 7 d | Reintubation/death |
| Vivier | 2019b | France | Prospective | T-piece | Voluntary CPF tested by an external flowmeter | CPF ≤ 60L/min | 160 | 18 | 74 | 10 | 58 | 7 d | Reintubation/death |
| Wang | 2019 | China | Retrospective | Not reported | SCSS (with or without spontaneous cough) | Without spontaneous cough | 86 | 19 | 0 | 6 | 61 | Hospital stay | Reintubation |
| Ma | 2018 | China | Retrospective | Not reported | SCSS (strong, weak, no cough) | Weak or no cough | 108 | 11 | 6 | 8 | 83 | 48 h | Reintubation |
| Frutos-Vivar | 2006 | Canada | Prospective | T-piece/PSV/CPAP | SCSS (poor, moderate, or excellent) | Poor | 900 | 33 | 178 | 88 | 601 | 72 h | Reintubation |
| Jaber | 2018 | France | Prospective | T-piece/PSV/CPAP | SCSS (weak and strong) | Weak | 1505 | 116 | 811 | 32 | 546 | 48 h | Reintubation |
| Dos | 2017 | Brazil | Prospective | T-piece/PSV | SCSS (grade 0 to 5) | Grade 0 to 2 | 311 | 8 | 21 | 35 | 247 | 48 h | Reintubation |
| Michetti | 2018 | USA | Prospective | PSV/CPAP | SCSS (not strong and strong) | Not strong | 464 | 11 | 142 | 24 | 287 | 96 h | Reintubation |
| Abbas | 2018 | Egypt | Prospective | Not reported | SCSS (grade 0 to 5) | Grade 0 to 2 | 90 | 7 | 11 | 19 | 53 | 48 h | Reintubation/NIV |
| Norisue | 2020 | Japan | Prospective | PSV | Voluntary CPF tested with a ventilator | CPF ≤ 50 L/min | 252 | 8 | 62 | 4 | 178 | 72 h | Reintubation |
| Sanson | 2018 | Italy | Prospective | Not reported | SCSS (strong, weak, no cough) | Weak or no cough | 205 | 21 | 121 | 5 | 58 | ICU stay | Reintubation/NIV |
| Wang | 2009a | China | Prospective | PSV | SCSS (grade 0 to 5) | Grade 0 to 2 | 68 | 9 | 9 | 11 | 39 | 72 h | Reintubation |
| Wang | 2009b | China | Prospective | PSV | WCT | Negative | 68 | 10 | 11 | 10 | 37 | 72 h | Reintubation |
| Elkholy | 2021 | Egypt | Prospective | PSV | WCT | Negative | 150 | 21 | 24 | 1 | 104 | 72 h | Reintubation |
| Lu | 2010 | China | Prospective | PSV | Voluntary CPF tested with an external flowmeter | CPF ≤ 29.35 L/min | 19 | 7 | 4 | 0 | 8 | 72 h | Reintubation |
| Liang | 2019 | China | Prospective | PSV | #Involuntary CPF tested with a ventilator | CPF ≤ 71.15 L/min | 48 | 8 | 4 | 2 | 34 | 48 h | Reintubation/death |
| Thille | 2020 | France | Prospective | T-piece/PSV | SCSS (grade 0 to 4) | Grade 0 to 1 | 284 | 11 | 16 | 45 | 212 | 7 d | Reintubation/death |
Letters a, b, and c after the year (e.g. 2014a and 2014b) represent different arms of a given study
#Cough was stimulated with 2 mL normal saline
$Cough was stimulated with suction catheter
CPF = cough peak flow, SCSS = semiquantitative cough strength score, WCT = white card test, TP = true positive, FP = false positive, FN = false negative, TN = true negative, NIV = noninvasive ventilation, SBT = spontaneous breathing trial, PSV = pressure support ventilation, CPAP = continuous positive airway pressure
Sensitivity = true positives/(true positives + false negatives). Specificity = true negatives/(true negatives + false positives)
True positives were patients with ineffective cough who failed extubation. False negatives were patients with effective cough who failed extubation
True negatives were patients with effective cough who were successfully extubated. False positives were patients with ineffective cough who were successfully extubated
Table 2.
Summary of the outcomes of different subgroups
| Measurement of cough peak flow | Measurement of semiquantitative cough strength score | ||||||
|---|---|---|---|---|---|---|---|
| Voluntary CPF | Involuntary CPF | CPF measured with an external flowmeter | CPF measured with a ventilator | SCSS (grade 0 to 4/5) | WCT | Other# | |
| No. of study arms | 17 | 6 | 18 | 5 | 8 | 4 | 10 |
| Total cases | 2282 | 529 | 2023 | 718 | 1342 | 406 | 3918 |
| Total tests | 2408 | 610 | 2300 | 718 | 1342 | 406 | 3918 |
| Pooled sensitivity | 0.73 (0.68–0.78) | 0.82 (0.73–0.88) | 0.77 (0.72–0.81) | 0.72 (0.60–0.81) | 0.36 (0.26–0.48) | 0.70 (0.44–0.88) | 0.59 (0.41–0.47) |
| Pooled specificity | 0.72 (0.65–0.79) | 0.82 (0.74–0.88) | 0.74 (0.67–0.81) | 0.77 (0.69–0.84) | 0.87 (0.80–0.91) | 0.74 (0.61–0.84) | 0.83 (0.62–0.64) |
| Pooled positive LR | 2.7 (2.1–3.4) | 4.5 (2.9–7.0) | 3.0 (2.3–4.0) | 3.1 (2.1–4.6) | 2.7 (2.1–3.6) | 2.7 (1.5–4.8) | 3.5 (1.5–8.2) |
| Pooled negative LR | 0.37 (0.31–0.45) | 0.22 (0.14–0.35) | 0.31 (0.25–0.39) | 0.37 (0.25–0.55) | 0.73 (0.64–0.84) | 0.40 (0.18–0.90) | 0.49 (0.33–0.73) |
| Pooled DOR | 7 (5–10) | 21 (9–48) | 10 (6–15) | 9 (4–18) | 4 (3–5) | 7 (2–25) | 7 (2–21) |
| Pooled AUC | 0.76 (0.72–0.79) | 0.89 (0.86–0.91) | 0.80 (0.77–0.84) | 0.77 (0.73–0.81) | 0.70 (0.65–0.73) | 0.78 (0.74–0.82) | 0.75 (0.71–0.79) |
CPF = cough peak flow, SCSS = semiquantitative cough strength score, WCT = white card test, LR = likelihood ratio, DOR = diagnostic odds ratio, AUC = area under the receiver operating characteristic curve
#Includes strong, moderate, and weak; strong, weak, and no cough; effective and ineffective; with or without spontaneous cough; excellent, moderate, and poor; strong and weak; strong and not strong; and effective, moderate, and ineffective
Assessment of the SCSS before extubation was performed in 22 study arms involving 5666 tests. The pooled extubation failure was 37.1% and 11.3%, respectively, among patients with weak and strong cough assessed by the SCSS (Additional file 2: Figure 2). Spearman’s correlation coefficient was 0.450 (p = 0.04), indicating the presence of a threshold effect. Three subgroups of studies measured the SCSS. Details are reported in Table 2 and Additional file 12: Text 1.
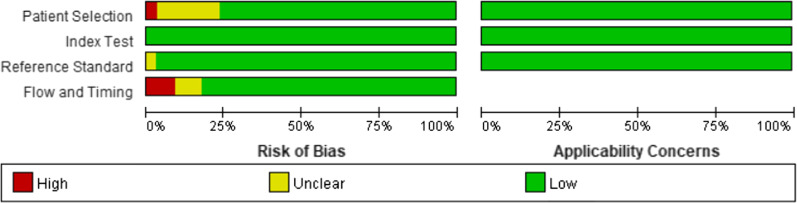
Quality assessment and publication bias
The quality of the included studies is summarised in Fig. 2. The main high risk of bias was the time between the removal of the endotracheal tube and extubation failure. The majority of studies judged extubation failure at a prespecified time after extubation, detailed in Table 1, except for four studies. Three study arms collected data on extubation failure during hospitalisation after extubation. And one study arm collected data on extubation failure during the ICU stay after extubation. Additional file 3: Figure 3 shows the lack of publication bias among studies that used cough peak flow to predict extubation failure (p = 0.41). Additional file 4: Figure 4 shows the presence of publication bias among studies that used the SCSS to predict extubation failure (p = 0.02). The sensitivity analysis showed that excluding Frutos–Vivar et al.’s study [34] negated the publication bias (p = 0.07). The sensitivity analysis also showed that the pooled DOR ranged from 4.08 to 5.02 and the pooled AUC ranged from 0.71 to 0.75 when one study was omitted (Additional file 5: Figure 5).
Fig. 2.
Quality Assessment of Diagnostic Accuracy Studies criteria for the included studies
Accuracy of extubation failure diagnosed by cough peak flow
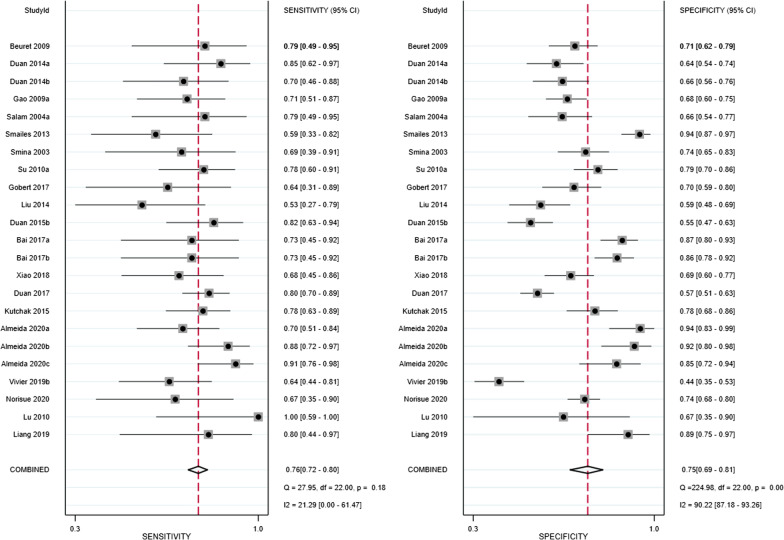
The pooled sensitivity and specificity were 0.76 (95% confidence interval [CI]: 0.72–0.80) and 0.75 (0.69–0.81), respectively (Fig. 3). Meta-regression analyses indicated that sensitivity and specificity did not vary by publication year, country, assessment of voluntary or involuntary cough peak flow, assessment of cough peak flow with an external flowmeter or a ventilator, different cut-off values, number of cases in the study arm, time to extubation failure after the removal of the endotracheal tube, or definition of extubation failure (Additional file 6: Figure 6). The pooled positive LR and negative LR were 2.89 (95% CI: 2.36–3.54) and 0.37 (0.30–0.45), respectively (Additional file 7: Figure 7). The pooled DOR was 8.91 (95% CI: 5.96–13.32; Additional file 8: Figure 8). The AUC was 0.79 (95% CI: 0.75–0.82) when cough peak flow was used to predict extubation failure (Fig. 4). The results of subgroup analyses are summarised in Table 2.
Fig. 3.
Forest plot of sensitivity and specificity in the diagnosis of extubation failure tested by cough peak flow. CI = confidence interval
Fig. 4.
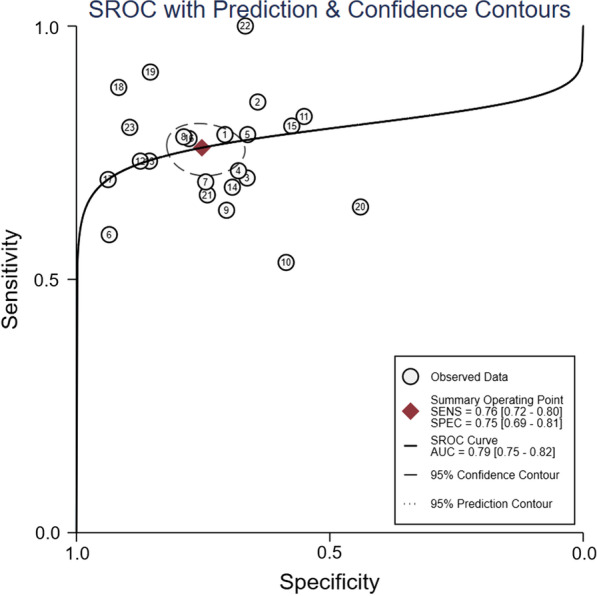
Summary receiver operator characteristic (SROC) curve in the prediction of extubation failure tested by cough peak flow. SENS = sensitivity, SPEC = specificity, AUC = area under the receiver operating characteristic curve. Numbers 1 to 23 represent the study arms (Beuret 2009, Duan 2014a, Duan 2014b, Gao 2009a, Salam 2004a, Smailes 2013, Smina 2003, Su 2010a, Gobert 2017, Liu 2014, Duan 2015b, Bai 2017a, Bai 2017b, Xiao 2018, Duan 2017, Kutchak 2015, Almeida 2020a, Almeida 2020b, Almeida 2020c, Vivier 2019b, Norisue 2020, Lu 2010, and Liang 2019)
Accuracy of extubation failure diagnosed by the SCSS
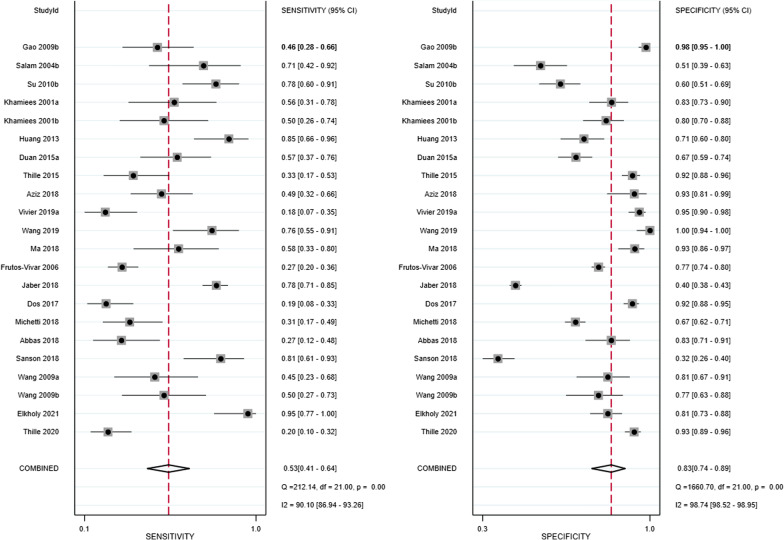
The pooled sensitivity and specificity were 0.53 (95% CI: 0.41–0.64) and 0.83 (0.74–0.89), respectively (Fig. 5). Meta-regression analyses indicated that sensitivity and specificity did not vary by publication year, country, study design, method used to assess the SCSS, number of cases in the study arm, time to extubation failure after the removal of the endotracheal tube, or definition of extubation failure (Additional file 9: Figure 9). The pooled positive LR and negative LR were 2.50 (95% CI: 1.93–3.25) and 0.65 (0.56–0.76), respectively (Additional file 10: Figure 10). The pooled DOR was 4.61 (95% CI: 3.03–7.01; Additional file 11: Figure 11). The AUC was 0.74 (95% CI: 0.70–0.78) when the SCSS was used to predict extubation failure (Fig. 6). The results for cough strength assessed by the SCSS graded from 0 to 4/5, the white card test (WCT), and other semiquantitative scales are summarised in Table 2.
Fig. 5.
Forest plot of sensitivity and specificity in the diagnosis of extubation failure tested by the semiquantitative cough strength score. CI = confidence interval
Fig. 6.
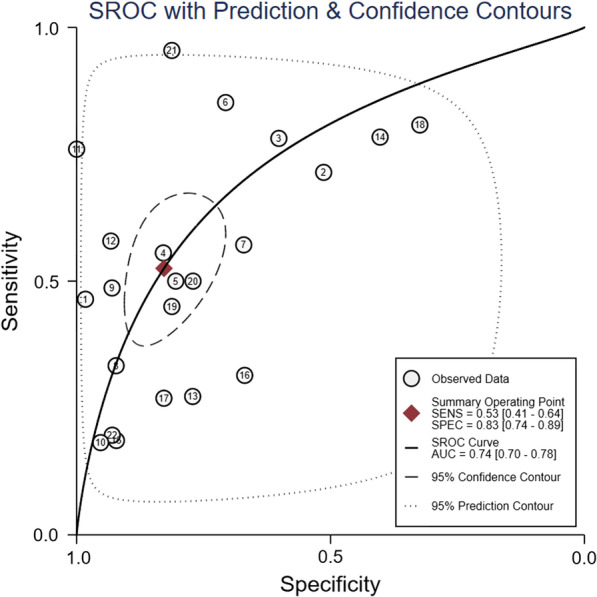
Summary receiver operator characteristic (SROC) curve in the prediction of extubation failure tested by the semiquantitative cough strength score. SENS = sensitivity, SPEC = specificity, AUC = area under the receiver operating characteristic curve. Numbers 1 to 22 represent the study arms (Gao 2009b, Salam 2004b, Su 2010b, Khamiees 2001a, Khamiees 2001b, Huang 2013, Duan 2015a, Thille 2015, Aziz 2018, Vivier 2019a, Wang 2019, Ma 2018, Frutos-Vivar 2006, Jaber 2018, Dos 2017, Michetti 2018, Abbas 2018, Sanson 2018, Wang 2009a, Wang 2009b, Elkholy 2021, and Thille 2020)
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis to explore the prediction of extubation failure diagnosed by cough strength. Cough peak flow includes voluntary and involuntary peak flow and can be measured with an external flowmeter or a ventilator. The SCSS can be measured with a scale from 0 to 4/5, the WCT, or other semiquantitative scales. Both cough peak flow and the SCSS show moderate diagnostic power for predicting extubation failure. However, cough peak flow is superior to the SCSS for predicting extubation failure.
Cough strength is strongly associated with maximal inspiratory and expiratory pressure [46], which in turn can reflect respiratory muscle function. Better respiratory muscle function is associated with lower extubation failure [47]. Therefore, weaker cough strength is associated with higher extubation failure. The current study with its large sample size demonstrates that both cough peak flow and the SCSS have moderate diagnostic power for predicting extubation failure. Therefore, cough strength can be commonly used to predict extubation failure in clinical practice.
Cough peak flow includes voluntary and involuntary peak flow. Voluntary peak flow can be measured when the investigator coaches the patient to cough. Involuntary peak flow can be stimulated with an injection of 2 mL normal saline or with a suction catheter. Two studies measured both voluntary and involuntary peak flow. One showed that voluntary peak flow was better than involuntary peak flow at predicting extubation failure [11]. However, the other showed no difference between the two methods in predicting extubation failure [30]. The current meta-analysis, which enrolled 17 study arms that measured voluntary peak flow and 6 that measured involuntary peak flow, found that involuntary peak flow had much higher predictive power than voluntary peak flow. Voluntary peak flow can only be measured in cooperative patients, as it requires the patient to cough on command. However, involuntary peak flow can be measured in all patients, even unconscious patients, as it does not require the patient’s cooperation. Thus, involuntary peak flow may be more suitable for predicting extubation failure in patients who are ready for extubation.
Cough peak flow can be measured with an external flowmeter or a ventilator. Only one study with 126 cases measured cough peak flow using both methods [26]. And both methods showed similar predictive accuracy. However, given the small sample size in that study, its power is inadequate. Our meta-analysis, which enrolled 18 study arms that measured cough peak flow with an external flowmeter and 5 that measured it with a ventilator, found that the AUC was higher when cough peak flow was measured with an external flowmeter than a ventilator. This indicates that predictive accuracy is greater when cough peak flow is measured with an external flowmeter. However, measuring cough peak flow with an external flowmeter requires a dedicated device. This may limit the use of this method. As the AUC was 0.77 when cough peak flow was measured with a ventilator, indicating moderate accuracy for predicting extubation failure, it can be used to predict extubation failure if an external flowmeter is unavailable. However, cut-off values differ among studies. This may be related to the different devices used in the studies. Therefore, the generalisation of the measure of cough peak flow is limited by the variability in cut-off values by study, even when the method is the same.
The SCSS, which ranges from 0 to 4/5, was the most common semiquantitative method of measuring cough strength in this meta-analysis. A score of 0 indicates the weakest cough, and a score of 4/5 indicates the strongest cough [18, 21]. The WCT was another semiquantitative method used to measure cough strength [13]. However, no studies compared the two methods on their predictive accuracy for extubation failure. This study found that the WCT is more accurate than an SCSS score of 0–4/5 for predicting extubation failure. The SCSS graded 0–4/5 is subjectively rated by the investigators. However, the WCT, which is scored based on the moisture on a card when the investigator coaches the patient to cough, is less likely to be influenced by the investigator’s experience. Thus, the WCT can be given priority over the SCSS for predicting extubation failure.
Sensitivity was lower but specificity was higher when the SCSS (vs. cough peak flow) was used to assess cough strength. This might suggest that weak cough identified using the SCSS is actually very weak with a very low peak flow (if performed) and consequently associated with more false negatives but fewer false positives. When patients are identified as having weak cough using the SCSS, their risk of extubation failure is very high. In contrast, patients identified as having weak cough using peak flow may have a stronger cough than those identified as having weak cough using the SCSS and consequently fewer false negatives and more false positives. It may be that the SCSS is unable to detect weak cough in patients with moderately decreased peak flow (around 60 L/min).
This study has several limitations. First, the time between the removal of the endotracheal tube and extubation failure was the main high risk of quality evaluation on included studies. However, we analysed studies that defined extubation failure within and beyond 72 h. The meta-regression showed that this factor did not influence sensitivity and specificity. Second, publication bias was observed among studies that measured the SCSS. We performed a sensitivity analysis and found that the pooled DOR ranged from 4.08 to 5.02 and the pooled AUC ranged from 0.71 to 0.75. This indicates that the results were stable despite the presence of publication bias. Third, judging weak cough is difficult, as the definition of weak cough varies by study. A consensus on the definition of weak cough based on cough peak flow or the SCSS would be helpful for improving operability. Fourth, different types of SBTs were performed in the enrolled studies. The rate of successful SBTs was higher when they were performed under pressure support ventilation than under T-piece or continuous positive airway pressure [48]. However, extubation failure did not vary by type of SBT [49, 50]. Therefore, type of SBT is unlikely to influence results for the association between cough strength and extubation failure.
Conclusions
Weak cough is associated with increased extubation failure. It can be assessed by cough peak flow and the SCSS. The predictive power of cough peak flow may be better than that of the SCSS for diagnosing extubation failure.
Supplementary Information
Additional file 1: Figure 1. Pooled extubation failure in patients with weak and strong cough tested by cough peak flow (CPF). CI = confidence interval.
Additional file 2: Figure 2. Pooled extubation failure in patients with weak and strong cough tested by the semiquantitative cough strength score (SCSS). CI = confidence interval.
Additional file 3: Figure 3. Deeks’ funnel plot of publication bias among studies that assessed cough peak flow. ESS = effective sample size. Numbers 1 to 23 represent the study arms (Beuret 2009, Duan 2014a, Duan 2014b, Gao 2009a, Salam 2004a, Smailes 2013, Smina 2003, Su 2010a, Gobert 2017, Liu 2014, Duan 2015b, Bai 2017a, Bai 2017b, Xiao 2018, Duan 2017, Kutchak 2015, Almeida 2020a, Almeida 2020b, Almeida 2020c, Vivier 2019b, Norisue 2020, Lu 2010, and Liang 2019).
Additional file 4: Figure 4. Deeks’ funnel plot of publication bias among studies that assessed the semiquantitative cough strength score. ESS = effective sample size. Numbers 1 to 22 represent the study arms (Gao 2009b, Salam 2004b, Su 2010b, Khamiees 2001a, Khamiees 2001b, Huang 2013, Duan 2015a, Thille 2015, Aziz 2018, Vivier 2019a, Wang 2019, Ma 2018, Frutos-Vivar 2006, Jaber 2018, Dos 2017, Michetti 2018, Abbas 2018, Sanson 2018, Wang 2009a, Wang 2009b, Elkholy 2021, and Thille 2020).
Additional file 5: Figure 5. Sensitivity analysis of the diagnostic odds ratio (DOR) and area under the receiver operating characteristic curve (AUC) among studies that assessed the semiquantitative cough strength score when one study arm was omitted.
Additional file 6: Figure 6. Meta-regression analysis of studies that assessed cough peak flow (CPF). CI = confidence interval. Meta-regression was performed by publication year, country (China, France, USA, or other), voluntary or involuntary CPF, assessment of CPF with an external flowmeter or a ventilator, different cut-off values, number of cases in the study arm, time to extubation failure (EF) after the removal of the endotracheal tube (≤72 h or >72 h), and definition of EF (reintubation, death, or noninvasive ventilation).
Additional file 7: Figure 7. Forest plot of the positive likelihood ratio (LR) and negative LR in the diagnosis of extubation failure tested by cough peak flow. CI = confidence interval.
Additional file 8: Figure 8. Forest plot of the diagnostic odds ratio (OR) in the prediction of extubation failure tested by cough peak flow. CI = confidence interval.
Additional file 9: Figure 9. Meta-regression analysis of studies that assessed the semiquantitative cough strength score (SCSS). CI = confidence interval. Meta-regression was performed by publication year, country (China, France, USA, or other), study design (prospective or retrospective), method of measuring the SCSS (white card test or not), number of cases in the study arm, time to extubation failure (EF) after the removal of the endotracheal tube (≤72 h or >72 h), and definition of EF (reintubation, death, or noninvasive ventilation).
Additional file 10: Figure 10. Forest plot of the positive likelihood ratio (LR) and negative LR in the diagnosis of extubation failure tested by the semiquantitative cough strength score. CI = confidence interval.
Additional file 11: Figure 11. Forest plot of the diagnostic odds ratio (OR) in the prediction of extubation failure tested by the semiquantitative cough strength score. CI = confidence interval.
Additional file 12. Details on the different subgroups.
Acknowledgements
We thank all authors of the included studies to help us get the data required in this meta-analysis.
Abbreviations
- MV
Mechanical ventilation
- SBT
Spontaneous breathing trial
- CPF
Cough peak flow
- SCSS
Semiquantitative cough strength score
- WCT
White card test
- TP
True positive
- FP
False positive
- FN
False negative
- TN
True negative
- LR
Likelihood ratio
- DOR
Diagnostic odds ratio
- AUC
Area under the receiver operating characteristic curves
- CI
Confidence interval
- SENS
Sensitivity
- SPEC
Specificity
- SROC
Summary receiver operator characteristic
- ESS
Effective sample size
Authors' contributions
JD conceived this study. JD and XFZ participated in study design, literature research, article selection, and data extraction. JD and JPS participated in data analysis and interpretation. JPS also participated in article selection. All authors participated in manuscript preparation and revision, and approved the final version.
Funding
None.
Availability of data and materials
All data generated and/or analysed during the current study are included within the published article and its additional files.
Declarations
Ethical approval and consent to participate
Not applicable.
Competing interests
We declare that we have no competing interests.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jun Duan and Xiaofang Zhang have contributed equally to this work.
Contributor Information
Jun Duan, Email: duanjun412589@163.com.
Jianping Song, Email: 125718258@qq.com.
References
- 1.Schönhofer B, Geiseler J, Dellweg D, Fuchs H, Moerer O, Weber-Carstens S, et al. Prolonged weaning: S2k guideline published by the german respiratory society. Respiration. 2020:1–102. [DOI] [PubMed]
- 2.Fan E, Zakhary B, Amaral A, McCannon J, Girard TD, Morris PE, et al. Liberation from mechanical ventilation in critically ill adults. An official ATS/ACCP clinical practice guideline. Ann Am Thorac Soc. 2017;14(3):441–443. doi: 10.1513/AnnalsATS.201612-993CME. [DOI] [PubMed] [Google Scholar]
- 3.Macintyre NR. Evidence-based assessments in the ventilator discontinuation process. Respir Care. 2012;57(10):1611–1618. doi: 10.4187/respcare.02055. [DOI] [PubMed] [Google Scholar]
- 4.Thille AW, Richard JC, Brochard L. The decision to extubate in the intensive care unit. Am J Respir Crit Care Med. 2013;187(12):1294–1302. doi: 10.1164/rccm.201208-1523CI. [DOI] [PubMed] [Google Scholar]
- 5.Kaur R, Vines DL, Patel AD, Lugo-Robles R, Balk RA. Early identification of extubation failure using integrated pulmonary index and high-risk factors. Respir Care. 2021 doi: 10.4187/respcare.08656. [DOI] [PubMed] [Google Scholar]
- 6.Ionescu F, Zimmer MS, Petrescu I, Castillo E, Bozyk P, Abbas A, et al. Extubation failure in critically ill COVID-19 patients: risk factors and impact on in-hospital mortality. J Intensive Care Med. 2021:8850666211020281. [DOI] [PMC free article] [PubMed]
- 7.Ferrer M, Valencia M, Nicolas JM, Bernadich O, Badia JR, Torres A. Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med. 2006;173(2):164–170. doi: 10.1164/rccm.200505-718OC. [DOI] [PubMed] [Google Scholar]
- 8.Hernández G, Vaquero C, Colinas L, Cuena R, González P, Canabal A, et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA. 2016;316(15):1565–1574. doi: 10.1001/jama.2016.14194. [DOI] [PubMed] [Google Scholar]
- 9.Su WL, Chen YH, Chen CW, Yang SH, Su CL, Perng WC, et al. Involuntary cough strength and extubation outcomes for patients in an ICU. Chest. 2010;137(4):777–782. doi: 10.1378/chest.07-2808. [DOI] [PubMed] [Google Scholar]
- 10.Beuret P, Roux C, Auclair A, Nourdine K, Kaaki M, Carton M-J. Interest of an objective evaluation of cough during weaning from mechanical ventilation. Intensive Care Med. 2009;35(6):1090–1093. doi: 10.1007/s00134-009-1404-9. [DOI] [PubMed] [Google Scholar]
- 11.Duan J, Liu J, Xiao M, Yang X, Wu J, Zhou L. Voluntary is better than involuntary cough peak flow for predicting re-intubation after scheduled extubation in cooperative subjects. Respir Care. 2014;59(11):1643–1651. doi: 10.4187/respcare.03045. [DOI] [PubMed] [Google Scholar]
- 12.Gao XJ, Qin YZ. A study of cough peak expiratory flow in predicting extubation outcome. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2009;21(7):390–393. [PubMed] [Google Scholar]
- 13.Salam A, Tilluckdharry L, Amoateng-Adjepong Y, Manthous CA. Neurologic status, cough, secretions and extubation outcomes. Intensive Care Med. 2004;30(7):1334–1339. doi: 10.1007/s00134-004-2231-7. [DOI] [PubMed] [Google Scholar]
- 14.Smailes ST, McVicar AJ, Martin R. Cough strength, secretions and extubation outcome in burn patients who have passed a spontaneous breathing trial. Burns. 2013;39(2):236–242. doi: 10.1016/j.burns.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Smina M, Salam A, Khamiees M, Gada P, Amoateng-Adjepong Y, Manthous CA. Cough peak flows and extubation outcomes. Chest. 2003;124(1):262–268. doi: 10.1378/chest.124.1.262. [DOI] [PubMed] [Google Scholar]
- 16.Gobert F, Yonis H, Tapponnier R, Fernandez R, Labaune MA, Burle JF, et al. Predicting extubation outcome by cough peak flow measured using a built-in ventilator flow meter. Respir Care. 2017;62(12):1505–1519. doi: 10.4187/respcare.05460. [DOI] [PubMed] [Google Scholar]
- 17.Vivier E, Muller M, Putegnat JB, Steyer J, Barrau S, Boissier F, et al. Inability of diaphragm ultrasound to predict extubation failure: a multicenter study. Chest. 2019;155(6):1131–1139. doi: 10.1016/j.chest.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Khamiees M, Raju P, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA. Predictors of extubation outcome in patients who have successfully completed a spontaneous breathing trial. Chest. 2001;120(4):1262–1270. doi: 10.1378/chest.120.4.1262. [DOI] [PubMed] [Google Scholar]
- 19.Huang CT, Yu CJ. Conventional weaning parameters do not predict extubation outcome in intubated subjects requiring prolonged mechanical ventilation. Respir Care. 2013;58(8):1307–1314. doi: 10.4187/respcare.01773. [DOI] [PubMed] [Google Scholar]
- 20.Duan J, Zhou L, Xiao M, Liu J, Yang X. Semiquantitative cough strength score for predicting reintubation after planned extubation. Am J Crit Care. 2015;24(6):e86–90. doi: 10.4037/ajcc2015172. [DOI] [PubMed] [Google Scholar]
- 21.Thille AW, Boissier F, Ben Ghezala H, Razazi K, Mekontso-Dessap A, Brun-Buisson C. Risk factors for and prediction by caregivers of extubation failure in ICU patients: a prospective study. Crit Care Med. 2015;43(3):613–620. doi: 10.1097/CCM.0000000000000748. [DOI] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed]
- 23.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 24.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5(9):1315–1316. doi: 10.1097/JTO.0b013e3181ec173d. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Li Y, He W, Xu Y, Sang L. The application of fibrobronchoscopy in extubation for patients suffering from acute exacerbation of chronic obstructive pulmonary disease with low cough peak expiratory flow. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014;26(12):855–859. doi: 10.3760/cma.j.issn.2095-4352.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Bai L, Duan J. Use of cough peak flow measured by a ventilator to predict re-intubation when a spirometer is unavailable. Respir Care. 2017;62(5):566–571. doi: 10.4187/respcare.05260. [DOI] [PubMed] [Google Scholar]
- 27.Xiao M, Duan J. Weaning attempts, cough strength and albumin are independent risk factors of reintubation in medical patients. Clin Respir J. 2018;12(3):1240–1246. doi: 10.1111/crj.12657. [DOI] [PubMed] [Google Scholar]
- 28.Duan J, Han X, Huang S, Bai L. Noninvasive ventilation for avoidance of reintubation in patients with various cough strength. Crit Care. 2016;20(1):316. doi: 10.1186/s13054-016-1493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kutchak FM, Debesaitys AM, Rieder Mde M, Meneguzzi C, Skueresky AS, Forgiarini Junior LA, et al. Reflex cough PEF as a predictor of successful extubation in neurological patients. J Bras Pneumol. 2015;41(4):358–364. doi: 10.1590/S1806-37132015000004453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almeida CM, Lopes AJ, Guimarães FS. Cough peak flow to predict the extubation outcome: comparison between three cough stimulation methods. Can J Respir Ther. 2020;56:58–64. doi: 10.29390/cjrt-2020-037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aziz EM, Ebrahim AE-RS, Mohammed MA, Mehany MM. Factors affecting extubation outcomes after using semi-quantitative cough strength scale. Assiut Sci Nurs J. 2018;6(13):20–28. [Google Scholar]
- 32.Wang K. Predictors of tracheal intubation in patients with non-coma nervous critical illness. Syst Med. 2019;4(14):40–42. [Google Scholar]
- 33.Ma SJ, Cheng WT, Xu YJ. Related factors of planned extubation failure in patients with severe brain injury. Neural Injury Funct Reconst. 2018;4(13):184–189. [Google Scholar]
- 34.Frutos-Vivar F, Ferguson ND, Esteban A, Epstein SK, Arabi Y, Apezteguía C, et al. Risk factors for extubation failure in patients following a successful spontaneous breathing trial. Chest. 2006;130(6):1664–1671. doi: 10.1378/chest.130.6.1664. [DOI] [PubMed] [Google Scholar]
- 35.Jaber S, Quintard H, Cinotti R, Asehnoune K, Arnal JM, Guitton C, et al. Risk factors and outcomes for airway failure versus non-airway failure in the intensive care unit: a multicenter observational study of 1514 extubation procedures. Crit Care. 2018;22(1):236. doi: 10.1186/s13054-018-2150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dos Reis HFC, Gomes-Neto M, Almeida MLO, da Silva MF, Guedes LBA, Martinez BP, et al. Development of a risk score to predict extubation failure in patients with traumatic brain injury. J Crit Care. 2017;42:218–222. doi: 10.1016/j.jcrc.2017.07.051. [DOI] [PubMed] [Google Scholar]
- 37.Michetti CP, Griffen MM, Teicher EJ, Rodriguez JL, Seoudi H, Liu C, et al. FRIEND or FOE: a prospective evaluation of risk factors for reintubation in surgical and trauma patients. Am J Surg. 2018;216(6):1056–1062. doi: 10.1016/j.amjsurg.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Abbas A, Shehata SM. Dead space addition test and swallowing assessment as new predictors of extubation outcome in mechanically ventilated patients with chronic obstructive pulmonary disease. Egypt J Chest Dis Tubercul. 2018;67(2):156–163. doi: 10.4103/ejcdt.ejcdt_25_18. [DOI] [Google Scholar]
- 39.Norisue Y, Santanda T, Nabeshima T, Tomita S, Saito S, Kataoka J, et al. Ultrasonographic measurement of the diaphragm movement during cough and extubation outcomes. Res Square. 2020. 10.21203/rs.3.rs-15791/v1
- 40.Sanson G, Sartori M, Dreas L, Ciraolo R, Fabiani A. Predictors of extubation failure after open-chest cardiac surgery based on routinely collected data. The importance of a shared interprofessional clinical assessment. Eur J Cardiovasc Nurs. 2018;17(8):751–759. doi: 10.1177/1474515118782103. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Huang JA, Jin J, Lv SQ, Huang F, Guo Q, et al. Risk factors of extubation failure in AECOPD patients complicated by respiratory failure with invasive mechanical ventilation. Chin J Crit Care Med. 2009;29(9):800–803. [Google Scholar]
- 42.Elkholy MM, Sadek SH, Elmorshedy RM, Abdulmoez MS. Predictors of extubation failure in mechanically ventilated patients with chronic obstructive pulmonary disease. Egypt J Chest Dis Tubercul. 2021;70(2):288–294. [Google Scholar]
- 43.Lu SB, Lv WH. Cough peak flow in COPD patients with mechanical ventilation weaning correlation. Med Innov China. 2010;7(9):6–8. [Google Scholar]
- 44.Liang J. Predictive value of involuntary cough peak flow on reintubation in ICU patients. Hebei Medical University, 2019: 1–46.
- 45.Thille AW, Boissier F, Muller M, Levrat A, Bourdin G, Rosselli S, et al. Role of ICU-acquired weakness on extubation outcome among patients at high risk of reintubation. Crit Care. 2020;24(1):86. doi: 10.1186/s13054-020-2807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang SW, Shin JC, Park CI, Moon JH, Rha DW, Cho DH. Relationship between inspiratory muscle strength and cough capacity in cervical spinal cord injured patients. Spinal Cord. 2006;44(4):242–248. doi: 10.1038/sj.sc.3101835. [DOI] [PubMed] [Google Scholar]
- 47.Khemani RG, Sekayan T, Hotz J, Flink RC, Rafferty GF, Iyer N, et al. Risk factors for pediatric extubation failure: the importance of respiratory muscle strength. Crit Care Med. 2017;45(8):e798–e805. doi: 10.1097/CCM.0000000000002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ouellette DR, Patel S, Girard TD, Morris PE, Schmidt GA, Truwit JD, et al. Liberation from mechanical ventilation in critically Ill adults: an Official American College of Chest Physicians/American Thoracic Society Clinical Practice Guideline: inspiratory pressure augmentation during spontaneous breathing trials, protocols minimizing sedation, and noninvasive ventilation immediately after extubation. Chest. 2017;151(1):166–180. doi: 10.1016/j.chest.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 49.Subirà C, Hernández G, Vázquez A, Rodríguez-García R, González-Castro A, García C, et al. Effect of pressure support vs T-piece ventilation strategies during spontaneous breathing trials on successful extubation among patients receiving mechanical ventilation: a randomized clinical trial. JAMA. 2019;321(22):2175–2182. doi: 10.1001/jama.2019.7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thille AW, Coudroy R, Nay MA, Gacouin A, Demoule A, Sonneville R, et al. Pressure-support ventilation vs T-piece during spontaneous breathing trials before extubation among patients at high risk of extubation failure: a post-hoc analysis of a clinical trial. Chest. 2020;158(4):1446–1455. doi: 10.1016/j.chest.2020.04.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure 1. Pooled extubation failure in patients with weak and strong cough tested by cough peak flow (CPF). CI = confidence interval.
Additional file 2: Figure 2. Pooled extubation failure in patients with weak and strong cough tested by the semiquantitative cough strength score (SCSS). CI = confidence interval.
Additional file 3: Figure 3. Deeks’ funnel plot of publication bias among studies that assessed cough peak flow. ESS = effective sample size. Numbers 1 to 23 represent the study arms (Beuret 2009, Duan 2014a, Duan 2014b, Gao 2009a, Salam 2004a, Smailes 2013, Smina 2003, Su 2010a, Gobert 2017, Liu 2014, Duan 2015b, Bai 2017a, Bai 2017b, Xiao 2018, Duan 2017, Kutchak 2015, Almeida 2020a, Almeida 2020b, Almeida 2020c, Vivier 2019b, Norisue 2020, Lu 2010, and Liang 2019).
Additional file 4: Figure 4. Deeks’ funnel plot of publication bias among studies that assessed the semiquantitative cough strength score. ESS = effective sample size. Numbers 1 to 22 represent the study arms (Gao 2009b, Salam 2004b, Su 2010b, Khamiees 2001a, Khamiees 2001b, Huang 2013, Duan 2015a, Thille 2015, Aziz 2018, Vivier 2019a, Wang 2019, Ma 2018, Frutos-Vivar 2006, Jaber 2018, Dos 2017, Michetti 2018, Abbas 2018, Sanson 2018, Wang 2009a, Wang 2009b, Elkholy 2021, and Thille 2020).
Additional file 5: Figure 5. Sensitivity analysis of the diagnostic odds ratio (DOR) and area under the receiver operating characteristic curve (AUC) among studies that assessed the semiquantitative cough strength score when one study arm was omitted.
Additional file 6: Figure 6. Meta-regression analysis of studies that assessed cough peak flow (CPF). CI = confidence interval. Meta-regression was performed by publication year, country (China, France, USA, or other), voluntary or involuntary CPF, assessment of CPF with an external flowmeter or a ventilator, different cut-off values, number of cases in the study arm, time to extubation failure (EF) after the removal of the endotracheal tube (≤72 h or >72 h), and definition of EF (reintubation, death, or noninvasive ventilation).
Additional file 7: Figure 7. Forest plot of the positive likelihood ratio (LR) and negative LR in the diagnosis of extubation failure tested by cough peak flow. CI = confidence interval.
Additional file 8: Figure 8. Forest plot of the diagnostic odds ratio (OR) in the prediction of extubation failure tested by cough peak flow. CI = confidence interval.
Additional file 9: Figure 9. Meta-regression analysis of studies that assessed the semiquantitative cough strength score (SCSS). CI = confidence interval. Meta-regression was performed by publication year, country (China, France, USA, or other), study design (prospective or retrospective), method of measuring the SCSS (white card test or not), number of cases in the study arm, time to extubation failure (EF) after the removal of the endotracheal tube (≤72 h or >72 h), and definition of EF (reintubation, death, or noninvasive ventilation).
Additional file 10: Figure 10. Forest plot of the positive likelihood ratio (LR) and negative LR in the diagnosis of extubation failure tested by the semiquantitative cough strength score. CI = confidence interval.
Additional file 11: Figure 11. Forest plot of the diagnostic odds ratio (OR) in the prediction of extubation failure tested by the semiquantitative cough strength score. CI = confidence interval.
Additional file 12. Details on the different subgroups.
Data Availability Statement
All data generated and/or analysed during the current study are included within the published article and its additional files.