Abstract
Rationale: Androgens are potentially beneficial in asthma, but AR (androgen receptor) has not been studied in human airways.
Objectives: To measure whether AR and its ligands are associated with human asthma outcomes.
Methods: We compared the effects of AR expression on lung function, symptom scores, and fractional exhaled nitric oxide (FeNO) in adults enrolled in SARP (Severe Asthma Research Program). The impact of sex and of androgens on asthma outcomes was also evaluated in the SARP with validation studies in the Cleveland Clinic Health System and the NHANES (U.S. National Health and Nutrition Examination Survey).
Measurements and Main Results: In SARP (n = 128), AR gene expression from bronchoscopic epithelial brushings was positively associated with both FEV1/FVC ratio (R2 = 0.135, P = 0.0002) and the total Asthma Quality of Life Questionnaire score (R2 = 0.056, P = 0.016) and was negatively associated with FeNO (R2 = 0.178, P = 9.8 × 10−6) and NOS2 (nitric oxide synthase gene) expression (R2 = 0.281, P = 1.2 × 10−10). In SARP (n = 1,659), the Cleveland Clinic Health System (n = 32,527), and the NHANES (n = 2,629), women had more asthma exacerbations and emergency department visits than men. The levels of the AR ligand precursor dehydroepiandrosterone sulfate correlated positively with the FEV1 in both women and men.
Conclusions: Higher bronchial AR expression and higher androgen levels are associated with better lung function, fewer symptoms, and a lower FeNO in human asthma. The role of androgens should be considered in asthma management.
Keywords: asthma, androgens, airflow obstruction, airway inflammation
At a Glance Commentary
Scientific Knowledge on the Subject
AR (androgen receptor) gene expression has not previously been well studied in the human airway in general, or in asthma in particular, although AR ligands are beneficial in asthma.
What This Study Adds to the Field
In addition to our newly published data, which describe AR protein expression in the human airway, we show that increased AR gene expression is strongly associated with improved airflow, fewer symptoms, and less nitrosative inflammation in human asthma. We also confirm that male sex is associated with fewer asthma exacerbations and admissions and that AR ligands are associated with better lung function in three large cohorts of adult human asthma. Androgens are a major factor to be considered in planning future asthma research and management strategies.
Asthma is a common chronic airway disease that results in a significant health and financial burden to individuals and society (1–3). Epidemiologic evidence demonstrates a sex-based dimorphism in asthma prevalence and severity. For example, asthma is more common and severe in boys than in girls, but the prevalence and severity of asthma is higher in adult women before menopause than in men (1–3). These effects correspond to changes in sex hormones and suggest that sex hormones play an important role in the pathogenesis of asthma (2, 3). Indeed, the airway effects of estrogen and progesterone on asthma pathophysiology have been studied extensively (4–6).
However, the effects of androgens in asthma have not been as well characterized. Recent studies using data from the UK Biobank and NHANES (U.S. National Health and Nutrition Examination Survey) showed that, compared with free serum testosterone levels in the first quartile, free serum testosterone levels in the fourth quartile were associated with lower odds of current asthma in men (7–9) and women (7–10). We have also recently shown that higher levels of androgens are associated with better lung function in children (11). In a follow-up study with adults with severe asthma who were enrolled in the NIH–NHLBI SARP (Severe Asthma Research Program), we found that serum testosterone levels positively correlated with the prebronchodilator percent predicted FEV1 (FEV1PP) in men. Testosterone levels were also found to be lower in men with severe asthma compared with men with nonsevere asthma (12). Our findings are consistent with established data showing that testosterone levels are positively correlated with better lung function in two large cohorts of healthy men. Indeed, both boys and girls, followed longitudinally, often outgrow severe asthma during the period of increased systemic androgen levels during adolescence (13). Furthermore, we have recently shown that a missense-encoding variant in the androgen synthesis gene HSD3B1 associated with high tissue androgen levels is also associated with better lung function in patients with severe, corticosteroid-dependent asthma in particular. In a pilot study, dehydroepiandrosterone (DHEA) supplementation in women with asthma with low DHEA sulfate (DHEA-S) levels may benefit lung function. These data are consistent with emerging mechanistic data from our group and others suggesting that there are antiinflammatory airway effects of androgens (14–16).
Here, we report not only that the AR (androgen receptor) gene is expressed in the human airway epithelium, as recently reported by our group (17), but also that AR gene expression is strongly associated with better lung function and fewer symptoms in subjects with asthma in the SARP. Strikingly, AR gene expression is negatively associated with fractional exhaled nitric oxide (FeNO) in these subjects and with the expression of inducible NOS2 (nitric oxide synthase gene). Although the expression of estrogen receptors and the expression of other airway epithelial genes have been previously queried with regard to the determinants of FeNO (18, 19), AR expression has not previously been considered, and the association between epithelial AR expression and FeNO is, to our knowledge, the strongest association reported to date. In addition, we confirm that circulating androgen levels are associated with improved lung function in both men and women and, in three separate cohorts, that men have fewer asthma exacerbations and hospital admissions than women. Coupled with other recent mechanistic and epidemiologic discoveries regarding androgens in asthma (16, 17, 20–22), these data strongly suggest an important beneficial role for androgens in the pathophysiology of asthma in general and of nitric oxide signaling in asthma in particular. Here, we test the hypothesis that AR plays an important role in asthma and that its action is mediated by iNOS (inducible nitric oxide synthase).
Methods
SARP
Clinical and bronchial epithelial cell gene expression data were obtained from 128 patients with asthma enrolled in the SARP I and II between 2009 and 2011. Sponsored by the NIH/NHLBI, SARP is a multicenter study designed to study severe asthma to develop better treatments (23). SARP I and II recruited 1,644 patients with asthma between 2001 and 2012 from nine sites across the United States and one site in the United Kingdom. Thorough descriptions of the SARP I, II, and III network have been published previously (23, 24). The clinical characteristics of patients with asthma enrolled in SARP I and II, on whom gene expression data from the bronchial epithelium were available, are listed in Table E1 in the online supplement.
Spirometry and FeNO measurements were performed at SARP centers (25), and the airway epithelial cells of patients with asthma (n = 128) enrolled in SARP I and II were obtained with bronchoscopy and epithelial brushings (18). The description of sample preparation and microarray experiments has been previously reported (18), and data were made available online in the U.S. National Center for Biotechnology Information Gene Expression Omnibus (26) and can be accessed using the Gene Expression Omnibus series accession number GSE63142 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE63142). Gene expression was normalized using the cyclic, locally estimated scatterplot smoothing method by cyclically implementing locally estimated scatterplot smoothing to normalize any possible pairwise combination of arrays. SARP was approved by each network center’s institutional review board.
Immunoblot and Immunohistochemistry Studies of AR in Human Airway Epithelial Cells
Healthy subjects underwent bronchoscopy as described for the SARP population in a subsequent study, and samples were used to test whether the AR protein was expressed in the human airway epithelium. Airway brush biopsy specimens were used to grow well-differentiated primary human airway epithelial cells at an air–liquid interface as previously described (27, 28). Protein was extracted from primary airway epithelial cells grown at an air–liquid interface (three wells each from two healthy subjects), from LNCaP cells (prostate cancer positive control; American Type Culture Collection) lysed in radioimmunoprecipitation assay buffer, or from PC-3 cells (negative control; American Type Culture Collection) lysed in radioimmunoprecipitationassay buffer. Lysates underwent immunoblotting using our Jess (ProteinSimple) capillary-based system with Compass software for data analysis. The primary antibodies were mouse anti-AR (1:50; catalog number 66747-1-lg, ProteinTech Group) and rabbit anti–β-actin (1:100; catalog number 4970, Cell Signaling). All secondary antibodies were purchased from ProteinSimple (catalog numbers 042-205 and 042-206, respectively).
At the time of bronchoscopy, subjects also underwent endobronchial biopsy. Using two healthy male volunteers’ endobronchial biopsy specimens (and prostate controls), we performed immunohistochemistry. Formalin-preserved, paraffin-embedded biopsy specimens were sectioned (10 μm), immunostained for AR using AR monoclonal mouse anti–human antibodies (clone AR441; catalog number M356201-2, Agilent) and visualized by using an Invitrogen EVOS M5000 microscope (Thermo Fisher Scientific) at 40× magnification.
Asthma-related Healthcare Use
Data on healthcare use from the well-characterized, cross-sectional NIH–NHLBI SARP I and II cohorts (n = 1,361) and the (longitudinal) SARP III cohort (n = 714) were replicated using two additional cohorts: The Cleveland Clinic Health System (CCHS) cohort and the NHANES cohort (Table E2). In the CCHS cohort, asthma was identified using International Statistical Classification of Diseases, Tenth Revision, codes, and data were extracted from electronic health records of 32,527 patients with asthma who met the inclusion criteria (71.1% of all patients included in the CCHS cohort). The CCHS cohort constitutes a real-world sample of patients with asthma from the Cleveland region and Northeast Ohio seen at the CCHS in 2018. In NHANES, a population-based survey was designed to examine a representative sample across the United States (29) (Table E2). In NHANES, asthma is self-reported by the survey participants when they are asked if they have ever been told they have asthma by a doctor or other healthcare professional.
These three cohorts represent three diverse populations. SARP data included the highest proportion of patients with severe asthma (23, 30), and CCHS data included the lowest proportion of patients with severe asthma. Severe asthma was defined according to national and international guidelines in SARP (31, 32) and by the need for an inhaled corticosteroid (ICS)–long-acting β-agonist combination and/or therapy with oral corticosteroids (oCS) in NHANES. In CCHS, severe asthma was identified by the International Statistical Classification of Diseases, Tenth Revision, code of J45.5 (Table E2).
Sex Hormones, Lung Function, and Severe Asthma
We studied the relationship both between serum androgen levels and asthma severity and between androgen levels and FEV1PP in patients enrolled in SARP I, II, and III for whom androgen level measurements were available. We analyzed data on patients with asthma, age 18–80 years, and excluded pregnant women. To exclude effects of exogenous sex hormones, women receiving hormone-based contraceptives or hormone replacements were also excluded. Patients with other concurrent chronic lung diseases, such as chronic obstructive pulmonary disease, and current smokers and ex-smokers with a smoking history of 10 or more pack-years were also excluded.
Sex Hormone Assays
DHEA-S, testosterone, and SHBG (sex hormone–binding globulin) levels were analyzed at the University of Virginia’s Center for Research in the Reproduction Ligand Core Laboratory as previously described (11) using the Immulite 2000 assay (Siemens Healthcare Diagnostic). Free testosterone was calculated from total testosterone and SHBG, as previously described (33). The lower limits of detection (LODs) for assays were as follows: testosterone, 20 ng/dl; DHEA-S, 15 μg/dl; and SHBG, 10 nmol/L. Individuals with measurements below the LOD were counted as having a value just below that limit (LOD, 0.1). The intraassay and interassay coefficients of variation (percentages) were as follows: testosterone, 4.4% and 7.5%; DHEA-S, 5.4% and 6.5%; and SHBG, 2.7% and 5.2%. All analyses were run simultaneously to avoid the batch effect.
Total Testosterone versus Free Testosterone
We analyzed our data using the total testosterone in men and the free testosterone in women. In men, the total testosterone levels are used to assess testosterone deficiency according to the guidelines of the American Urologic Association (34). In women, we calculated free testosterone values as previously described (33) because the total testosterone cannot predict free testosterone levels (i.e., the active component). In fact, 66% of total testosterone is bound to SHBG and may simply reflect higher SHBG levels in women. Because SHBG levels are further related to characteristics such as obesity, free testosterone is used in clinical and research settings more often than the free androgen index (35).
Statistical analysis.
To account for significant sex differences in sex hormone levels, we analyzed the associations between androgens and lung function separately in men and women. Categorical data are presented as the count and percentage, and continuous data are presented as the mean (SD). We compared categorical variables using a chi-square test and compared continuous variables with the Kruskal-Wallis test. Multivariate linear regression models tested associations of clinical outcomes (prebronchodilator FEV1PP) with age, body mass index (BMI), and sex androgen levels as covariates. Using a stepwise (backward elimination) procedure, the covariate “race” was removed from the initial model on the basis of Akaike Information Criterion. Models were fit under the assumption of a normal distribution for the FEV1PP.
Expression levels of the AR in human bronchial epithelial biopsy were correlated with different outcome variables: FEV1PP, FEV1/FVC ratios, FeNO, and total Asthma Quality of Life Questionnaire scores, with higher scores indicating better quality of life. They were also correlated with expression levels of inducible NOS2 because of the association between AR gene expression and FeNO. Multivariate linear regression models were used to evaluate the association between sputum gene expression and different outcomes, adjusting for age, sex, BMI, and race. Bootstrapped 95% confidence intervals [CIs] for R2 in linear regressions were based on 1,000 replications and were calculated using the library “boot” in R (R Foundation for Statistical Computing). The unadjusted R2 value was used to represent the proportion of the variance in the dependent variable that is explained by the predictor variables, and a P value < 0.05 was considered to indicate statistical significance. Of note, we did not account for low socioeconomic status, environmental exposures, upper respiratory tract infections, and/or medication compliance in our analyses.
We also performed three sets of mediation analyses to further evaluate the association between AR expression and outcomes such as lung function and FeNO and adjust for confounding factors such as age and sex by using 1) sex as the “exposure” and AR expression as the “mediator,” 2) age as the exposure and AR expression as the mediator, and 3) AR expression as the exposure and NOS2 as the mediator. In both analysis 1 and analysis 2, the FEV1 (%) is a primary outcome of interest. Note, of course, that age cannot be an effect of AR; it can only be the other way around. In analysis 3, FeNO is the outcome studied. For analysis 1, age and sex are included as the covariates; for analysis 2, sex and race are the adjusted variables; and for analysis 3, sex, age, and race are the confounding factors.
Analyses were performed using R 4.0.2 statistical software.
Results
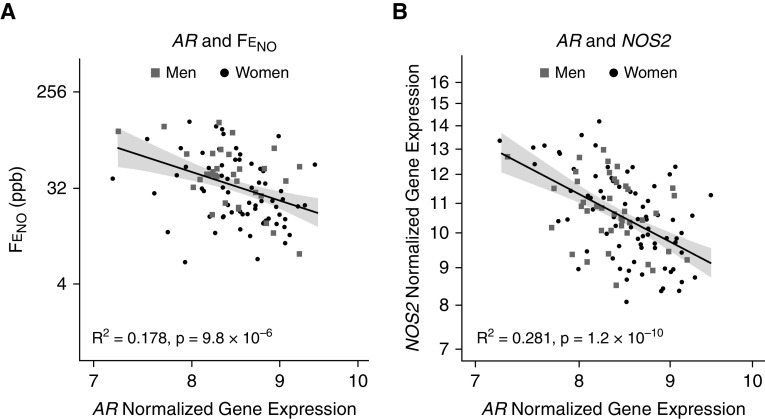
In bronchial epithelial cells obtained from subjects with asthma enrolled in SARP I and II (n = 128), AR gene expression was positively associated with the FEV1PP (R2 = 0.085 [95% CI, 0.011–0.211], P = 0.003), the FEV1/FVC ratio (R2 = 0.135 [95% CI, 0.052–0.259], P = 0.0002), and the total Asthma Quality of Life Questionnaire score (R2 = 0.056 [95% CI, 0.001–0.176]; P = 0.016) (Figure 1); and it was negatively associated with FeNO (R2 = 0.178 [95% CI, 0.056–0.351], P = 9.8 × 10−6) (Figure 2). The significant negative correlation between AR gene expression and FeNO was mirrored by an even stronger negative correlation between AR gene expression and iNOS (gene symbol NOS2) gene expression (R2 = 0.281 [95% CI, 163–0.414], P = 1.2 × 10−10) (Figure 2). These associations continued to be significant even after adjustment for age, sex, race, and BMI (Table E3). Interestingly, both FeNO and NOS2 expression positively correlated with the absolute eosinophil count in blood but not with BAL fluid eosinophilia (as a percentage of the BAL fluid total cell count) in SARP I and II using a Bonferroni-corrected P value threshold of α = 0.05/4 = 0.0125 (Figure E1). Of note, although the AR gene expression did not correlate with the dose of daily oCS (R2 = 0.063 [95% CI, 0.005–0.178]; P = 0.261; n = 22), AR expression was lower among SARP patients with asthma and treated with oCS (n = 22 out of 128) as compared with those not receiving oCS therapy (mean [SD], 8.32 [0.44] vs. 8.59 [0.46]; P = 0.01). AR gene expression did not vary by sex (P = 0.39). Note that AR protein was also expressed in human airway epithelial cells in culture, as well as in human airway endobronchial biopsy samples (Figure E2), which is consistent with our recent coronavirus disease (COVID-19) publication (17).
Figure 1.
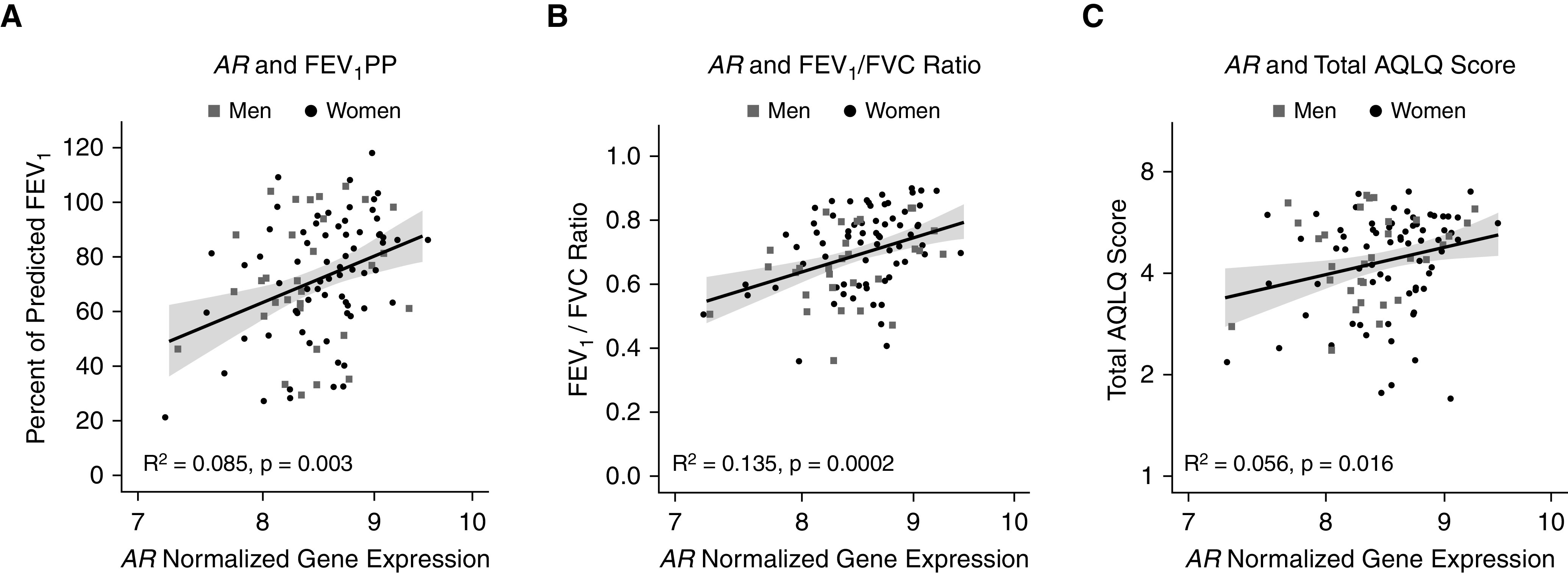
AR (androgen receptor) gene expression in bronchial epithelial cells from subjects with asthma enrolled in the Severe Asthma Research Program I and II is positively associated with the (A) FEV1PP, (B) FEV1/FVC ratio, and (C) total Asthma Quality of Life Questionnaire (AQLQ) score. The AR normalized gene expression and the total AQLQ score are plotted on a log-2 scale. The gray shaded regions represent SEs. FEV1PP = percent predicted FEV1.
Figure 2.
AR (androgen receptor) gene expression in bronchial epithelial cells from subjects with asthma enrolled in the Severe Asthma Research Program is positively associated with (A) FeNO and with (B) inducible NOS2 (nitric oxide synthase gene) expression. AR NOS2, and the fractional exhaled nitric oxide are plotted on a log-2 scale. The gray shaded regions represent SEs. FeNO = fractional exhaled nitric oxide.
AR expression was not statistically different between individuals who reported an asthma exacerbation in the year before SARP I and II enrollment (n = 51) versus those who did not report an asthma exacerbation (mean [SD], 8.43 [0.52] vs. 8.53 [0.39], respectively; P = 0.28). Furthermore, AR gene expression was not significantly associated with the expression of genes encoding type 2 (T2)-high asthma targets, IL-5 receptor, or IL-4/IL-13 receptor.
Our mediation analysis using sex as the exposure and AR as the mediator shows that sex has neither a direct nor an indirect effect on FEV1 (%). In contrast, we found that 11.3% of the effect of age on FEV1 (%) is mediated by AR. In the context of mediation analysis, this is a very small effect, and age did not impact the association between AR and lung function in multivariate analysis. In contrast, mediation analysis showed that 80.9% of the effect of AR expression on FeNO is mediated by NOS2 expression (Table E4).
Consistent with previous work from our group (11, 14) and others (8, 9), we found that female sex was associated with higher odds of having asthma exacerbations and asthma-related emergency room visits despite women having higher FEV1PP in all three cohorts (SARP: odds ratio [OR], 1.61 [95% CI, 1.29–2.02] and 1.41 [95% CI, 1.12–1.71], respectively; CCHS: OR, 1.27 [95% CI, 1.21–1.35] and OR, 1.31 [95% CI, 1.13–1.51], respectively; and NHANES: OR, 1.51 [95% CI, 1.23–1.87] and OR, 1.71 [95% CI, 1.26–2.31], respectively) (Figure 3). Furthermore, higher circulating levels of the adrenal androgen precursor steroid DHEA-S were associated with better lung function in men (n = 241) and women (n = 423) with asthma (Figure 4) enrolled in SARP I, II, and III. In women, the FEV1PP positively correlated with DHEA-S (R2 = 0.156 [95% CI, 0.094–0.227], P < 2.2 × 10−16) but not with free testosterone (R2 = 0.005 [95% CI, 0.002–0.024], P = 0.15) (Figure 4A). In men with asthma enrolled in SARP, the FEV1PP correlated positively with both DHEA-S and testosterone (R2 = 0.158 [95% CI, 0.089–0.239], P = 1.5 × 10−10 vs. R2 = 0.034 [95% CI, 0.002–0.101], P = 0.004, respectively) (Figure 4B). We built a multivariable linear regression model to predict the effect of sex hormones on the FEV1PP, adjusting for age and BMI in both men and women separately. In the final model, the FEV1PP continued to correlate positively with DHEA-S (P < 0.001) in both sexes. To account for the drop in testosterone in men and the drop in ovarian sex hormones from menopause in women, we stratified the correlation between DHEA-S and the FEV1PP by age category (age >50 yr vs. age ⩽50 yr) and found the correlation to be significant among individuals aged 50 years and younger in both men and women, but this correlation was not significant among older individuals using a Bonferroni-corrected P value threshold of α = 0.05/4 = 0.0125 (Table E5).
Figure 3.
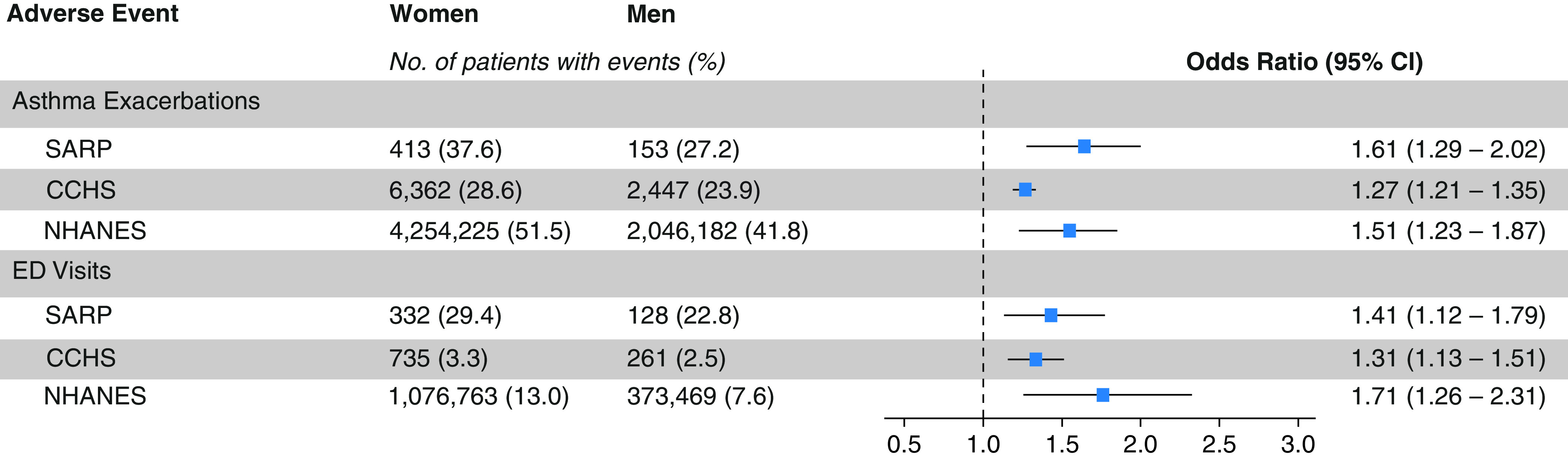
Asthma exacerbation and asthma-related emergency department (ED) visits in adults stratified by sex. The risk for asthma exacerbation and ED visits for a respiratory problem the year before SARP and NHANES participation and in 2018 in CCHS was higher in adult women than in adult men in all three cohorts. The sampling weights are used to produce the correct population estimates because each sample person does not have an equal probability of selection. CCHS = Cleveland Clinic Health System; CI = confidence interval; NHANES = U.S. National Health and Nutrition Examination Survey; SARP = Severe Asthma Research Program.
Figure 4.
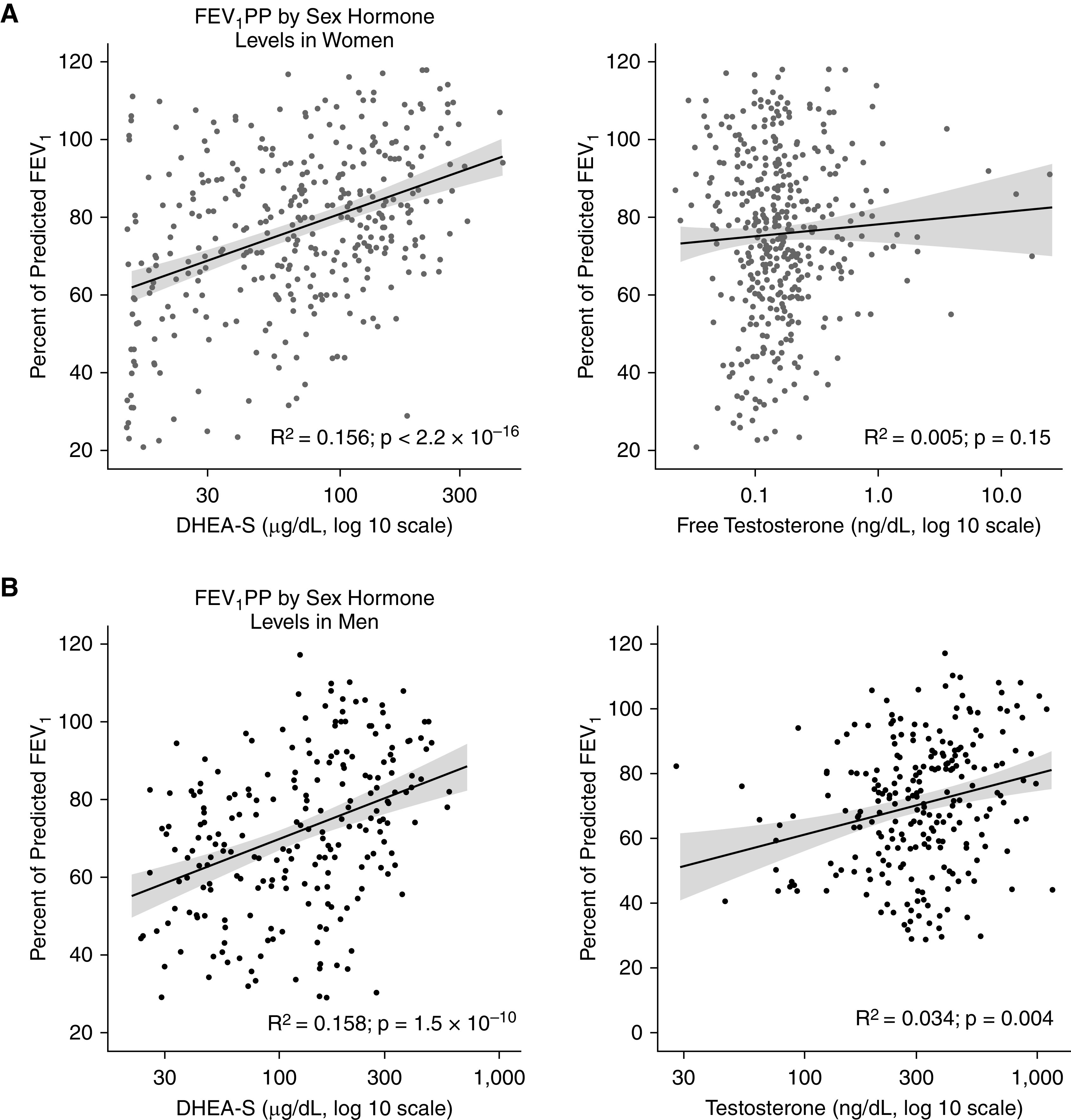
Prebronchodilator percent predicted FEV1 (FEV1PP) by sex hormone levels in SARP (Severe Asthma Research Program) I, II, and III adult participants. (A) In women enrolled in SARP I, II, and III, the FEV1PP correlated positively with dehydroepiandrosterone sulfate (DHEA-S) (R2 = 0.156; <2.2 × 10−16) but not with free testosterone (P = 0.15). (B) In adult men, the FEV1PP correlated positively with DHEA-S (R2 = 0.158; P = 1.5 × 10−10) and testosterone (R2 = 0.034; P = 0.004). The gray shaded regions represent SEs.
In SARP, subjects with asthma were classified as having severe asthma on the basis of the standard guidelines (31, 32). DHEA-S was lower in both women (median, 31.50 [interquartile range (IQR), 15.00–74.40]) and men (median, 59.70 [IQR, 34.47–136.50]) with severe asthma when compared with women (median, 99.50 [IQR, 48.65–152.25]) and men (median, 186.00 [IQR, 118.00–283.00]) with nonsevere asthma (P < 2.2 × 10−16) (Figure E3). Furthermore, testosterone levels were also lower in men with severe asthma as compared with their counterparts with nonsevere asthma (P = 4.2 × 10−6). Severe asthma was not associated with lower free testosterone in women (P = 0.008) (Figure E3).
Discussion
Asthma is a heterogeneous disease (25, 36). Androgens can affect the asthmatic airway. For example, androgens can directly cause airway smooth muscle relaxation mediated by decreased cellular calcium (Ca2+) influx through L-type Ca2+ channels (37), or androgens can indirectly attenuate inflammation and, consequently, improve bronchodilation (20). Airway inflammation, which results in airway hyperresponsiveness and excess mucus production, is mediated by the activation of a variety of immunomodulatory cells (6, 16, 20, 36). The role of sex hormones in asthma pathobiology has been studied in animal models. In general, immune activation underlying airway inflammation in asthma is increased by female sex hormones and suppressed by androgens (6, 15, 16). In one study, IL-33 stimulation of lung group 2 innate lymphoid cells (ILC2s) induced a larger increase in IL-5 and IL-13 in female mice than in male mice (38). Furthermore, 5α-dihydrotestosterone (DHT), a potent metabolite of testosterone, was shown to negatively regulate ILC2 proliferation and decrease IL-5 and IL-13 expression from ILC2s. Consequently, DHT resulted in the reduction of lung eosinophil activation and proliferation and the attenuation of T2-mediated allergic airway inflammation (15). Attenuation of T2 inflammation has also been reported with the adrenal androgen DHEA, which is a hormone upstream of testosterone (39). Mice that received DHEA supplementation in their diet had lower house dust mite–induced allergic airway inflammation than mice fed with a control diet (39). Similarly, DHEA inhibited bronchial epithelial-to-mesenchymal transition through inhibition of the PI3K/Akt-dependent signal pathway stimulated by TGF-β1. Theoretically, DHEA could have a beneficial effect on airway remodeling and fibrosis in asthma (22). In addition to their role in T2 inflammation, sex hormones also modulate T-helper cell type 17 (Th17)-mediated airway inflammation in severe asthma, such that the number of IL-17+ memory Th17 cells and Th17 cells mediating IL-17A production were found to be higher in women than in men (6, 40); these findings are supported by murine studies (6, 40). In contrast to ovarian sex hormones, androgens had the opposite effect on Th2 and Th17 airway inflammation (16). In a murine model of gonadectomized female and male mice intranasally challenged with house dust mites, testosterone decreased, and ovarian hormones increased, IL-13+ CD4 Th2 cells and IL-17A+ CD4 Th17 cells in the lung (16). Taken together, these data suggest a beneficial role of androgens in asthma.
Determinants of FeNO levels are multifactorial, but an important upstream cause of increased FENO is NOS2 expression (41, 42). Notably, our mediation analysis has shown that 80.9% of the effect of increased AR expression that decreases the FeNO is caused by an effect of AR expression to decrease NOS2 expression. It has not previously been reported that NOS2 expression is related to AR expression in the airway. Note in this regard that FeNO levels do not vary dramatically between men and women; if anything, they are lower in men than in women. This is despite previous correlative studies suggesting that sex hormone levels are related to FeNO levels and to the expression and activity of NOS isoforms (42–46). We speculate that it is tissue sex hormone metabolism and AR expression, more than sex and circulating sex hormone levels, that determines FeNO; and that AR expression has a protective, antiinflammatory effect. Additional mechanistic studies will be required.
Even though the role of ESR (estrogen receptor genes) and ESR signaling in lung diseases has been extensively studied, very little is known about the functional importance of the AR and androgen signaling in lung physiology or pathobiology beyond the embryonic period (47). The AR mediates the function of male sex hormones such as testosterone, DHT, and DHEA in males and females (48). The AR gene is a ligand-dependent nuclear transcription factor and member of the steroid hormone nuclear receptor family. It plays an important role in the biology of the reproductive, hemopoietic, musculoskeletal, cardiovascular, neuronal, and immune systems (48). In addition to its role in the physiologic function of many reproductive and nonreproductive organs, AR and androgen signaling have also been involved in the pathophysiology of many diseases across the life span and in prostate, liver, urologic, and lung cancer (48, 49). The AR protein has been demonstrated in a variety of human tissues obtained at surgery, including lung tissue (17, 50). Yet very limited data exist on their role in lung function and pathology (47). In the murine lung, AR is mainly expressed in T2 pneumocytes and bronchial epithelial cells, and AR protein levels are higher in intact male mice than in castrated male mice (51). Testosterone supplementation administered to castrated mice increases AR protein levels in lung cells and results in the upregulation of genes involved in oxygen transport and the downregulation of DNA repair and DNA recombination pathways (51). Furthermore, a significant sexual dimorphism exists between male and female mice. Some of the genes that are increased in male lungs compared with female lungs are involved in muscle development and contraction. In contrast, genes that are decreased in male lungs are involved in the acute inflammatory response and regulation of translation (51). Of note, the response of androgen-regulated genes may differ between organs and is tissue specific because gene expression depends on local hormone metabolism, chromatin modifications, activators, and repressors characteristic of a specific cellular milieu (14, 48, 52).
Note that we and others have shown that ICS use can affect DHEA-S levels (11, 14, 53). However, it is unlikely that ICS use accounts primarily for the associations between increased androgens and increased asthma severity for several reasons. These reasons include genetic data (14); pharmacologic data showing improvements in asthma with androgen supplementation (54, 55) in animal models, including the AR-deficient mouse (15, 16); the associations between human AR levels and asthmatic airflow obstruction, and symptoms reported here; and human data regarding the association between AR ligands and better lung function, even in the absence of ICS use (8).
Our analysis has several limitations. As compared with the SARP cohort, in which individuals with asthma are well characterized, clinical data were incomplete in the NHANES and CCHS cohorts. For example, asthma was diagnosed in 13,773 patients in the NHANES cohort between 1999 and 2017, out of which 73 men and 57 women had concurrent spirometry and testosterone-level measurement. None of the NHANES participants had spirometry performed simultaneously with measurement of estradiol and progesterone levels. DHEA-S levels were not measured in NHANES. In addition, although gene expression data were available on 128 well-characterized subjects with asthma from SARP I and II, this small sample size makes it difficult to stratify the analysis further by asthma phenotypes or endotypes (25, 56). The correlations reported here were statistically significant between lung function and androgens and between AR and asthma biomarkers and lung function under Bonferroni correction controlling for a family-wise error rate (α = 0.05/4 = 0.125 for associations with serum androgen levels and α = 0.05/5 = 0.01 for associations with AR gene expression). Asthma is a complex disease, in which many variables, such as age, BMI, race, exposures, and socioeconomic status, contribute to asthma severity and airway obstruction. Adjusting for age, sex, BMI, and race, androgens and AR expression are still significantly associated with lung function, FeNO, and asthma-related quality of life. This is true both in direct multivariate analysis and in mediation analysis. Our data are compatible with murine-knockout data, which show that a lack of AR is associated with an increased risk of murine airway inflammation and bronchoconstriction (4). Our work is the first to use human data to support these animal data.
Additional limitations related to SARP methodology could theoretically have biased our findings. Androgen levels can cyclically vary significantly in premenopausal women who are not receiving exogenous sex hormones (57). Similarly, circadian rhythms in testosterone and DHEA-S have been previously reported in men and women (58, 59). These variations may result in inaccurate correlations between androgens levels and outcomes. Furthermore, androgen metabolism is affected by obesity. In women, obesity is associated with significantly elevated free estradiol and free testosterone levels as a consequence of subnormal levels of SHBG (60). In men, obesity is associated with increased aromatase activity that converts testosterone to estradiol, which leads to low testosterone and high estrogen levels (61). The limited sample size prohibits us from stratifying our analysis by obesity category as compared with recent analyses using data from the UK Biobank (8). However, even after adjustment for the BMI using multivariate analysis, androgens continued to significantly correlate with the FEV1PP in both men and women. Our study was underpowered to study the association between systemic corticosteroids and AR gene expression, and prospective studies will need to be done in that regard. Finally, mechanisms underlying the benefit of AR expression in asthma are beginning to be understood (62) but were not the focus of this study.
Conclusions
Taken together, these data demonstrate that ARs are expressed in the human airways and that high levels of expression, together with high levels of AR ligands, are overall associated with better lung function, a better quality of life, and low FeNO in patients with asthma. These data are the first human data to support the murine observation that AR expression attenuates asthma.
Acknowledgments
Acknowledgment
The authors thank Mr. Bradly Souder for his valuable input and help. AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Sanofi-Genzyme-Regeneron, and TEVA have supported the Severe Asthma Research Program. However, they did not play any role in the conception or design of the work, analysis of data, interpretation of results, drafting of the manuscript, or critical revision of the manuscript for important intellectual content.
Footnotes
Supported by the NHLBI Severe Asthma Research Program grants U10 HL109250, P01 HL128192, P01 HL101871, R01 HL69170, U10 HL109250, K08 HL133381, P01 HL103453, P01 HL081064, R01CA172382, and R01CA190289.
Author Contributions: J.G.Z., S.C.E., T.L., and B.G. made substantial contributions to the conception or design of the work. J.G.Z., J.M.M., N.M., O.G., M.D.D., and B.G. acquired, analyzed, and interpreted the data for the work. S.C.E., M.D.D., W.C., K.F.C., J.V.F., E.I., N.N.J., B.D.L., D.T.M., W.C.M., V.E.O., M.P., E.R.B., D.A.M., S.E.W., and B.G. collected clinical data in the Severe Asthma Research Program network. J.G.Z., J.M.M., N.S., S.C.E., M.D.D., S.A.C., P.B., H.J.K, W.B., W.C., M.C., K.F.C., J.V.F., E.I., N.N.J, B.D.L., D.T.M., W.C.M., V.E.O., M.P., E.R.B., D.A.M., S.E.W., and B.G. contributed to drafting the work or revising it critically for important intellectual content. J.G.Z. agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. J.G.Z., S.C.E., M.D.D., S.A.C., P.B., H.J.K., W.B., W.C., M.C., K.F.C., J.V.F., E.I., N.N.J, B.D.L., D.T.M., W.C.M., V.E.O., M.P., E.R.B., D.A.M., S.E.W., and B.G. gave the final approval of the version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202009-3720OC on March 22, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the NHLBI Severe Asthma Research Program
References
- 1. Centers for Disease Control and Prevention (CDC) Vital signs: asthma prevalence, disease characteristics, and self-management education: United States, 2001–2009. MMWR Morb Mortal Wkly Rep. 2011;60:547–552. [PubMed] [Google Scholar]
- 2. Zein JG, Denson JL, Wechsler ME. Asthma over the adult life course: gender and hormonal influences. Clin Chest Med. 2019;40:149–161. doi: 10.1016/j.ccm.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 3. Zein JG, Erzurum SC. Asthma is different in women. Curr Allergy Asthma Rep. 2015;15:28. doi: 10.1007/s11882-015-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jain R, Ray JM, Pan JH, Brody SL. Sex hormone-dependent regulation of cilia beat frequency in airway epithelium. Am J Respir Cell Mol Biol. 2012;46:446–453. doi: 10.1165/rcmb.2011-0107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keselman A, Heller N. Estrogen signaling modulates allergic inflammation and contributes to sex differences in asthma. Front Immunol. 2015;6:568. doi: 10.3389/fimmu.2015.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Newcomb DC, Cephus JY, Boswell MG, Fahrenholz JM, Langley EW, Feldman AS, et al. Estrogen and progesterone decrease let-7f microRNA expression and increase IL-23/IL-23 receptor signaling and IL-17A production in patients with severe asthma. J Allergy Clin Immunol. 2015;136:1025–1034, e11. doi: 10.1016/j.jaci.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Han YY, Forno E, Celedón JC. Sex steroid hormones and asthma in a nationwide study of U.S. adults. Am J Respir Crit Care Med. 2020;201:158–166. doi: 10.1164/rccm.201905-0996OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Han YY, Yan Q, Yang G, Chen W, Forno E, Celedon JC. Serum free testosterone and asthma, asthma hospitalisations and lung function in British adults. Thorax. 2020;75:849–854. doi: 10.1136/thoraxjnl-2020-214875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bulkhi AA, Shepard KV, II, Casale TB, Cardet JC. Elevated testosterone is associated with decreased likelihood of current asthma regardless of sex. J Allergy Clin Immunol Pract. 2020;8:3029–3035, e4. doi: 10.1016/j.jaip.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Y, Zhou Y. Association of sex steroid hormones with adult asthma in the United States, 2013-2016. Am J Respir Crit Care Med. 2020;201:618–619. doi: 10.1164/rccm.201910-2044LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeBoer MD, Phillips BR, Mauger DT, Zein J, Erzurum SC, Fitzpatrick AM, et al. Effects of endogenous sex hormones on lung function and symptom control in adolescents with asthma. BMC Pulm Med. 2018;18:58. doi: 10.1186/s12890-018-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zein JG, DeBoer MD, Teague WG, Comhair SA, Castro M, Busse WW, et al. Sex hormones impact asthma severity and lung function in adult men and women [abstract] Am J Respir Crit Care Med. 2016;193:A1436. [Google Scholar]
- 13. Ross KR, Gupta R, DeBoer MD, Zein J, Phillips BR, Mauger DT, et al. Severe asthma during childhood and adolescence: a longitudinal study. J Allergy Clin Immunol. 2020;145:140–146, e9. doi: 10.1016/j.jaci.2019.09.030. [DOI] [PubMed] [Google Scholar]
- 14. Zein J, Gaston B, Bazeley P, DeBoer MD, Igo RP, Jr, Bleecker ER, et al. HSD3B1 genotype identifies glucocorticoid responsiveness in severe asthma. Proc Natl Acad Sci USA. 2020;117:2187–2193. doi: 10.1073/pnas.1918819117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cephus JY, Stier MT, Fuseini H, Yung JA, Toki S, Bloodworth MH, et al. Testosterone attenuates group 2 innate lymphoid cell-mediated airway inflammation. Cell Rep. 2017;21:2487–2499. doi: 10.1016/j.celrep.2017.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fuseini H, Yung JA, Cephus JY, Zhang J, Goleniewska K, Polosukhin VV, et al. Testosterone decreases house dust mite-induced type 2 and IL-17A-mediated airway inflammation. J Immunol. 2018;201:1843–1854. doi: 10.4049/jimmunol.1800293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baratchian M, McManus JM, Berk M, Nakamura F, Mukhopadhyay S, Xu W, et al. Sex, androgens and regulation of pulmonary AR, TMPRSS2 and ACE2 [preprint]. 2020. https://www.biorxiv.org/content/10.1101/2020.04.21.051201v2 [DOI] [PMC free article] [PubMed]
- 18. Modena BD, Tedrow JR, Milosevic J, Bleecker ER, Meyers DA, Wu W, et al. Gene expression in relation to exhaled nitric oxide identifies novel asthma phenotypes with unique biomolecular pathways. Am J Respir Crit Care Med. 2014;190:1363–1372. doi: 10.1164/rccm.201406-1099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Townsend EA, Meuchel LW, Thompson MA, Pabelick CM, Prakash YS. Estrogen increases nitric-oxide production in human bronchial epithelium. J Pharmacol Exp Ther. 2011;339:815–824. doi: 10.1124/jpet.111.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fuseini H, Newcomb DC. Mechanisms driving gender differences in asthma. Curr Allergy Asthma Rep. 2017;17:19. doi: 10.1007/s11882-017-0686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koziol-White CJ, Goncharova EA, Cao G, Johnson M, Krymskaya VP, Panettieri RA., Jr DHEA-S inhibits human neutrophil and human airway smooth muscle migration. Biochim Biophys Acta. 2012;1822:1638–1642. doi: 10.1016/j.bbadis.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu L, Xiang X, Ji X, Wang W, Luo M, Luo S, et al. Effects and mechanism of dehydroepiandrosterone on epithelial-mesenchymal transition in bronchial epithelial cells. Exp Lung Res. 2014;40:211–221. doi: 10.3109/01902148.2013.879966. [DOI] [PubMed] [Google Scholar]
- 23. Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. NHLBI’s Severe Asthma Research Program. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SA, et al. NHLBI Severe Asthma Research Program (SARP) Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med. 2012;185:356–362. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. NHLBI’s Severe Asthma Research Program. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- 28. Davis MD, Clemente TM, Giddings OK, Ross K, Cunningham RS, Smith L, et al. A treatment to eliminate SARS-CoV-2 replication in human airway epithelial cells is safe for inhalation as an aerosol in healthy human subjects. Respir Care. 2021;66:113–119. doi: 10.4187/respcare.08425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Center for Health Statistics. Atlanta, GA: Centers for Disease Control and Prevention; 2019. https://www.cdc.gov/nchs/nhanes/index.htm [Google Scholar]
- 30. Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, et al. Baseline features of the Severe Asthma Research Program (SARP III) cohort: differences with age. J Allergy Clin Immunol Pract. 2018;6:545–554, e4. doi: 10.1016/j.jaip.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. American Thoracic Society. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 32. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 33. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 34. Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, Chiles KA, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200:423–432. doi: 10.1016/j.juro.2018.03.115. [DOI] [PubMed] [Google Scholar]
- 35. Wiencek JR, McCartney CR, Chang AY, Straseski JA, Auchus RJ, Woodworth A. Challenges in the assessment and diagnosis of polycystic ovary syndrome. Clin Chem. 2019;65:370–377. doi: 10.1373/clinchem.2017.284331. [DOI] [PubMed] [Google Scholar]
- 36. Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160:1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 37. Montaño LM, Espinoza J, Flores-Soto E, Chávez J, Perusquía M. Androgens are bronchoactive drugs that act by relaxing airway smooth muscle and preventing bronchospasm. J Endocrinol. 2014;222:1–13. doi: 10.1530/JOE-14-0074. [DOI] [PubMed] [Google Scholar]
- 38. Warren KJ, Sweeter JM, Pavlik JA, Nelson AJ, Devasure JM, Dickinson JD, et al. Sex differences in activation of lung-related type 2 innate lymphoid cells in experimental asthma. Ann Allergy Asthma Immunol. 2017;118:233–234. doi: 10.1016/j.anai.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yu CK, Liu YH, Chen CL. Dehydroepiandrosterone attenuates allergic airway inflammation in Dermatophagoides farinae-sensitized mice. J Microbiol Immunol Infect. 2002;35:199–202. [PubMed] [Google Scholar]
- 40. Newcomb DC, Peebles RS., Jr Th17-mediated inflammation in asthma. Curr Opin Immunol. 2013;25:755–760. doi: 10.1016/j.coi.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dweik RA, Comhair SA, Gaston B, Thunnissen FB, Farver C, Thomassen MJ, et al. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci USA. 2001;98:2622–2627. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marozkina NV, Gaston B. Nitrogen chemistry and lung physiology. Annu Rev Physiol. 2015;77:431–452. doi: 10.1146/annurev-physiol-021113-170352. [DOI] [PubMed] [Google Scholar]
- 43. Duckles SP, Miller VM. Hormonal modulation of endothelial NO production. Pflugers Arch. 2010;459:841–851. doi: 10.1007/s00424-010-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McCredie RJ, McCrohon JA, Turner L, Griffiths KA, Handelsman DJ, Celermajer DS. Vascular reactivity is impaired in genetic females taking high-dose androgens. J Am Coll Cardiol. 1998;32:1331–1335. doi: 10.1016/s0735-1097(98)00416-1. [DOI] [PubMed] [Google Scholar]
- 45. Karpuzoglu E, Fenaux JB, Phillips RA, Lengi AJ, Elvinger F, Ansar Ahmed S. Estrogen up-regulates inducible nitric oxide synthase, nitric oxide, and cyclooxygenase-2 in splenocytes activated with T cell stimulants: role of interferon-gamma. Endocrinology. 2006;147:662–671. doi: 10.1210/en.2005-0829. [DOI] [PubMed] [Google Scholar]
- 46. Calippe B, Douin-Echinard V, Delpy L, Laffargue M, Lélu K, Krust A, et al. 17β-Estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J Immunol. 2010;185:1169–1176. doi: 10.4049/jimmunol.0902383. [DOI] [PubMed] [Google Scholar]
- 47. Sathish V, Martin YN, Prakash YS. Sex steroid signaling: implications for lung diseases. Pharmacol Ther. 2015;150:94–108. doi: 10.1016/j.pharmthera.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Davey RA, Grossmann M. Androgen receptor structure, function and biology: from bench to bedside. Clin Biochem Rev. 2016;37:3–15. [PMC free article] [PubMed] [Google Scholar]
- 49. Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28:778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- 50. Wilson CM, McPhaul MJ. A and B forms of the androgen receptor are expressed in a variety of human tissues. Mol Cell Endocrinol. 1996;120:51–57. doi: 10.1016/0303-7207(96)03819-1. [DOI] [PubMed] [Google Scholar]
- 51. Mikkonen L, Pihlajamaa P, Sahu B, Zhang FP, Jänne OA. Androgen receptor and androgen-dependent gene expression in lung. Mol Cell Endocrinol. 2010;317:14–24. doi: 10.1016/j.mce.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 52. Bolton EC, So AY, Chaivorapol C, Haqq CM, Li H, Yamamoto KR. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev. 2007;21:2005–2017. doi: 10.1101/gad.1564207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dorsey MJ, Cohen LE, Phipatanakul W, Denufrio D, Schneider LC. Assessment of adrenal suppression in children with asthma treated with inhaled corticosteroids: use of dehydroepiandrosterone sulfate as a screening test. Ann Allergy Asthma Immunol. 2006;97:182–186. doi: 10.1016/S1081-1206(10)60010-5. [DOI] [PubMed] [Google Scholar]
- 54. Marozkina N, Zein J, DeBoer MD, Logan L, Veri L, Ross K, et al. Dehydroepiandrosterone supplementation may benefit women with asthma who have low androgen levels: a pilot study. Pulm Ther. 2019;5:213–220. doi: 10.1007/s41030-019-00101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wenzel SE, Robinson CB, Leonard JM, Panettieri RA., Jr Nebulized dehydroepiandrosterone-3-sulfate improves asthma control in the moderate-to-severe asthma results of a 6-week, randomized, double-blind, placebo-controlled study. Allergy Asthma Proc. 2010;31:461–471. doi: 10.2500/aap.2010.31.3384. [DOI] [PubMed] [Google Scholar]
- 56. Carr TF, Kraft M. Use of biomarkers to identify phenotypes and endotypes of severe asthma. Ann Allergy Asthma Immunol. 2018;121:414–420. doi: 10.1016/j.anai.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 57. Vermeulen A, Verdonck L. Plasma androgen levels during the menstrual cycle. Am J Obstet Gynecol. 1976;125:491–494. doi: 10.1016/0002-9378(76)90363-x. [DOI] [PubMed] [Google Scholar]
- 58. Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907–913. doi: 10.1210/jc.2008-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nicolau GY, Haus E, Lakatua DJ, Bogdan C, Sackett-Lundeen L, Popescu M, et al. Circadian and circannual variations of FSH, LH, testosterone, dehydroepiandrosterone-sulfate (DHEA-S) and 17-hydroxy progesterone (17 OH-Prog) in elderly men and women. Endocrinologie. 1985;23:223–246. [PubMed] [Google Scholar]
- 60. Zumoff B. Hormonal abnormalities in obesity. Acta Med Scand Suppl. 1988;723:153–160. doi: 10.1111/j.0954-6820.1987.tb05939.x. [DOI] [PubMed] [Google Scholar]
- 61.Cohen PG. Obesity in men: the hypogonadal-estrogen receptor relationship and its effect on glucose homeostasis. Med Hypotheses. 2008;70:358–360. doi: 10.1016/j.mehy.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 62. Becerra-Díaz M, Strickland AB, Keselman A, Heller NM. Androgen and androgen receptor as enhancers of M2 macrophage polarization in allergic lung inflammation. J Immunol. 2018;201:2923–2933. doi: 10.4049/jimmunol.1800352. [DOI] [PMC free article] [PubMed] [Google Scholar]