ABSTRACT
Background
Childhood cognitive development is influenced by biological and environmental factors. One such factor, obesity, impairs cognitive development and is associated with sleep disturbances.
Objectives
We aimed to examine the mediating role of sleep disturbances on the relation between BMI and cognitive function in children.
Methods
A total of 9951 children aged 9–10 y were included in this cross-sectional study. Children were recruited from the longitudinal ABCD (Adolescent Brain Cognitive Development) Study. Cognitive development was assessed using metrics for fluid, crystallized, and total cognitive function. Mediation analyses were conducted via linear regression modeling, with adjustment for potential confounders (sex, age, ethnicity, household income, parental education, and self-reported physical activity) for each of the 3 outcomes. Mediation significance was determined by bootstrapping.
Results
A statistically significant inverse association was found between BMI and total (β = −0.41, P < 0.001) and fluid (β = −0.49, P < 0.001) cognition, but not for crystallized cognition. Total sleep disturbances partially mediated the association between BMI and fluid cognition (indirect effect: −0.02, P = 0.002; proportion of the total effect: 0.05, P = 0.002), but no mediation was found in the association between BMI and total cognition.
Conclusions
Sleep disturbances partially mediate the effect of childhood obesity on cognitive function, particularly in fluid cognitions. Future work is necessary to understand the effects of sleep disturbances and obesity on reduced childhood cognition throughout time, predominantly across the life course.
Keywords: BMI, obesity, childhood, sleep disturbances, cognitive function, fluid cognition, crystallized cognition, mediation analysis
The study explored the mediating role of sleep disturbances in the relation between obesity and cognitive function in children from a large longitudinal cohort sample.
Introduction
Neurocognitive development is critical in childhood. During this period of life, a dynamic restructuring of the neural structures takes place, where neurons undergo an initial overproduction, and later pruning of the synapses during puberty, resulting in more efficient neural processing (1). Optimal childhood cognitive development allows for the improvement and control of abilities within multiple domains, in addition to the successful foundation of the skills required in adulthood (2, 3). Within these domains, there are abilities associated to biological processes that comprise problem solving, immediate learning, and comprehension tasks, known as fluid cognition. Fluid cognition includes tasks related to executive function, attention, cognitive flexibility, working and episodic memory, and processing speed (4). The ability to utilize information that is acquired through experience, cultural exposure, and education, such as verbal knowledge and language skills, pertains to crystallized cognition (5). Thus, crystallized cognition is associated with long-term abilities, whereas fluid cognitive abilities serve as the building blocks of crystallized cognition.
Moreover, total cognition measures the composite of all neurocognitive abilities, which is important to understand adaptative functioning during childhood and across the life span (6).
Cognition is a developmental process that can be altered by the interaction of biological, genetic, and environmental factors (7–10). One such factor, obesity, has been associated with poorer cognitive performance and altered brain plasticity across the life span (11, 12). Obesity has also been linked to physiological and psychological consequences, including poor motor function, a higher risk of comorbidities (such as type 2 diabetes mellitus, hypertension, dyslipidemia, and obstructive sleep apnea), and emotional and social difficulties among children and adolescents (13–16). In the United States, ∼18.4% of children aged 6–11 y and 20.6% of children and adolescents aged 12–19 y have been classified with obesity (17). Owing to the deleterious effects of obesity on childhood cognitive development and the growing childhood obesity epidemic in the United States and worldwide, understanding the many elements of obesity's impact on cognition is needed.
Furthermore, obesity is associated with the development of sleep disorders (18). Poor sleep has increasingly become a common problem in children (19). Sleep is critical to supporting several biological functions, including cardiovascular, endocrine, and immune processes (20–22). Neural and cognitive processes, such as learning and memorizing, are directly related to healthy sleep patterns (18). Multiple studies have found that poor sleep patterns compromise cognition and mental health, as well as other developmental processes such as emotional control and metabolic regulation (23–25). Previous studies have examined the relation between obesity, obstructive sleep apnea, and executive function and attention, but findings have been inconclusive. This may be due to important study limitations including small sample sizes, lack of adjustment for confounders, as well as the inability to test for interaction and/or mediation effects (26–28).
Considering that previous studies have been limited to focusing on specific sleep disorders and cognitive tasks, this study examines the role of overall sleep disturbances in the association between obesity and 1) total, 2) crystallized, and 3) fluid cognition. Specifically, this study sought to understand the potential mediation effect of sleep disturbances on the obesity–cognition pathway.
Methods
Participants
This cross-sectional analysis included a total of 9951 children aged 9–10 y from the ABCD (Adolescent Brain Cognitive Development) Study, a longitudinal and demographically diverse data set with >11,800 children, recruited from 21 centers across the United States. Children were recruited through the school system, mailing lists at the catchment areas, enrollment of affiliates, referrals, and summer recruitment (29). The sampling scheme included considerations of age, gender, race, socioeconomic status, and urbanicity to reflect the distribution of the US population. The ABCD Study design and recruitment details are described elsewhere (29). For the analysis, children with missing data (n = 1924) and/or implausible values (BMI z score > 5, after reviewing height and weight values; n = 6) were excluded. This study received approval from the Indiana University Institutional Review Board.
Weight status
BMI (in kg/m2) was calculated for each child by dividing their weight in kilograms by their squared height in meters. WHO Anthro-plus was used to calculate the BMI-for-age and gender z scores (SD scores) (30, 31). Following the WHO recommendations, BMI-for-age was then categorized based on the z score cutoffs of <−2.0, >1.0, and >2.0 to classify children as underweight, overweight, and obese, respectively (32).
Sleep disturbances
Sleep disturbances were assessed using the Sleep Disturbance Scale for Children and were reported by parents. The Sleep Disturbance Scale for Children is a questionnaire designed to categorize sleep disorders. It includes 27 items, each rated on a 5-point Likert scale. These 27 items score 5 subdomains that include disorders of initiating and maintaining sleep, disorders of sleep somnolence, sleep breathing disorders, sleep-wake transition disorders, disorders of arousal, and sleep hyperhydrosis (33). The analysis focused on the sleep disturbance total score, which ranges from 26 to 130 points [based on the Sleep Disturbance Scale for Children punctuation system (1–5)], and summed the scores of all subdomains.
Cognitive function
The ABCD Study has a detailed neurocognitive battery that includes visual acuity, handedness, laterality, and neurocognitive development (34, 35). This study was focused on the neurocognitive development area, i.e., the scores of the cognitive tests that comprised the NIH Toolbox. The NIH Toolbox was used to determine cognitive functionality (36, 37). The NIH Toolbox consists of 7 cognitive tasks which cover the 6 cognitive domains: episodic memory (Picture Sequence Memory task), executive function (Flanker Inhibitory Control and Attention, Dimensional Change Card Sort tasks), attention (Flanker Inhibitory Control and Attention task), working memory (List Sorting Working Memory task), processing speed (Pattern Comparison Processing Speed task), and language abilities (Picture Vocabulary, Oral Reading Recognition tasks) (34, 35). The tasks were scored and used to generate 3 composite scores: fluid, crystallized, and total cognitive abilities (6). Fluid cognition includes 5 domains: episodic memory, executive function, attention, working memory, and processing speed; whereas crystallized cognition is particularly focused on language abilities.
For this analysis, the age-corrected cognitive scores available in the ABCD Study data set were used. The age-corrected standard scores, derived from a normative sample of 2917 children and adolescents, account for the performance of individuals in the same age group, and consider the effect of age, as the neurodevelopmental stages change during childhood (38).
Potential confounders
Based on previous literature, several potential confounders were included in the analysis (39, 40). More specifically, sex (female and male), age (as a continuous variable; mo), race [white, black, and other races (including Native American, Pacific Islanders, Asians, and others)], combined household income (categorized as ≤$15,999; $16,000–$34,999; $34,500–$49,999; $50,000–$74,999; $75,000–$99,999; $100,000–$199,999; and ≥$200,000), parental education (categorized as less than high school degree; completed high school/General Educational Development; some college; undergraduate/occupational degree; graduate degree), and self-reported physical activity (as the number of days that the participant was active for ≥60 min/d) were included in the analyses.
Statistical analysis
A mediation analysis was conducted to determine whether sleep disturbances mediate the relation between BMI and cognitive function. Figure 1 shows a direct acyclic graph of the hypothesized relation. Potential mediation effects were assessed using the multivariate linear regression methods described by Baron and Kenny (41). Modeling included 1) BMI on cognitive function (separately modeled for the total, fluid, and crystallized composite scores); 2) BMI on the sleep disturbances total score; and 3) a regression of the 3 cognitive composite scores on the sleep disturbances total score. In addition, it was determined whether the sleep disturbances total score was a significant mediator of cognitive function in the full model (including BMI). Covariate significance was assessed through a backward elimination stepwise regression. All potential confounders, including age, sex, physical activity, family income, parents’ education, and race, were included as significant covariates in the full-sample and stratified models. Descriptive statistical analyses were performed for these covariates. A chi-square test was applied for the categorical covariates and a one-way ANOVA was applied for the continuous covariates. All analyses were conducted in R version 3.6.0, using the “mediation” package (42, 43). The mediation significance was assessed with the Hayes and Preacher method by bootstrapping (44), with 1000 simulation samples used to generate 95% CIs for the indirect effect [average causal mediator effect (ACME)] and the average direct effect (ADE). Standardized regression coefficients and their corresponding 95% CIs were reported in this study.
FIGURE 1.
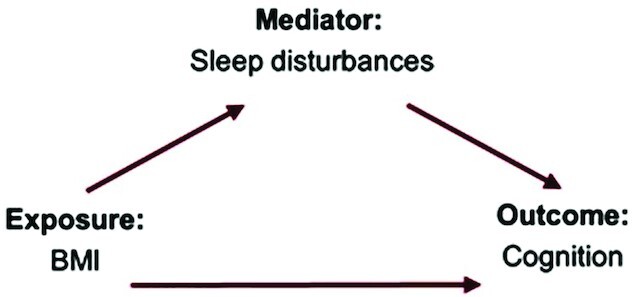
Direct acyclic graph of the potential mediation effect of sleep disturbances on the association between BMI and cognition composite scores.
Results
A total of 9951 children were included in the analysis. Table 1 shows the demographic characteristics of the sample. Mean ± SD age in the overall sample was 9.91 ± 0.62 y, with statistically significant differences between BMI categories. In this analysis, 52.23% of the participants were male and 66.2% of the children in the overall sample were identified as white; however, distribution by BMI categories varied between sex and race (P < 0.01). Children with normal BMI (≤1 SD) had a higher mean number of hours spent in physical activity than children with overweight or obesity (P < 0.01). Statistically significant differences were also found regarding family income and parents’ education between BMI categories (P = 0.69 and P = 0.37, respectively). Compared with children with a normal BMI, children with overweight (>1 SD to ≤2 SD) or obesity (>2 SD) had a statistically significantly higher total score in sleep disturbances. Cognition scores were statistically higher in children with normal BMI than in children with overweight and obesity (P < 0.01).
TABLE 1.
Descriptive characteristics of the ABCD (Adolescent Brain Cognitive Development) Study participants1
| BMI-for-age SD scores | |||||
|---|---|---|---|---|---|
| Overall (n = 9951) | ≤1 SD (n = 6208) | >1 SD to ≤2 SD (n = 2005) | >2 SD (n = 1738) | P value | |
| Age, mo | 118.96 ± 7.43 | 119.12 ± 7.45 | 118.78 ± 7.43 | 118.57 ± 7.35 | 0.01 |
| Physical activity, d | 3.55 ± 2.31 | 3.68 ± 2.31 | 3.46 ± 2.29 | 3.20 ± 2.29 | <0.01 |
| Sex | <0.01 | ||||
| Male | 5197 (52.23) | 3178 (51.19) | 1047 (52.22) | 972 (55.93) | |
| Female | 4754 (47.77) | 3030 (48.81) | 958 (47.78) | 766 (44.07) | |
| Race | <0.01 | ||||
| Black | 1434 (14.41) | 674 (10.86) | 329 (16.41) | 431 (24.80) | |
| Other white | 1928 (19.37) | 1132 (18.23) | 407 (20.30) | 389 (22.38) | |
| White | 6589 (66.21) | 4402 (70.91) | 1269 (63.29) | 918 (52.82) | |
| Family income, $ | <0.01 | ||||
| ≤15,999 | 960 (9.45) | 425 (6.85) | 218 (10.87) | 317 (18.24) | |
| 16,000–34,999 | 1047 (10.52) | 524 (8.44) | 244 (12.17) | 279 (16.05) | |
| 35,000–49,999 | 838 (8.43) | 442 (7.12) | 208 (10.37) | 188 (10.82) | |
| 50,000–74,999 | 1388 (13.95) | 823 (13.26) | 287 (14.31) | 278 (16.00) | |
| 75,000–99,999 | 1458 (14.65) | 930 (14.98) | 297 (14.81) | 231 (13.29) | |
| 100,000–199,999 | 3087 (31.03) | 2167 (34.91) | 560 (27.93) | 360 (20.71) | |
| ≥200,000 | 1173 (11.79) | 897 (14.45) | 191 (9.53) | 85 (4.89) | |
| Parental education | <0.01 | ||||
| Less than high school | 507 (5.09) | 224 (3.61) | 120 (5.98) | 163 (9.38) | |
| High school | 941 (9.46) | 451 (7.26) | 192 (9.58) | 298 (17.15) | |
| Some college | 1602 (16.10) | 886 (14.27) | 368 (18.35) | 348 (20.02) | |
| College degree | 4215 (42.36) | 2745 (44.22) | 820 (40.90) | 650 (37.40) | |
| Graduate degree | 2686 (26.99) | 1902 (30.64) | 505 (25.19) | 279 (16.05) | |
| Sleep disturbances total score | 36.47 ± 8.04 | 35.97 ± 7.39 | 37.17 ± 9.09 | 37.47 ± 8.82 | <0.01 |
| Total cognition score | 101.34 ± 17.80 | 103.20 ± 17.74 | 99.87 ± 17.46 | 96.40 ± 17.28 | <0.01 |
| Fluid cognition score | 96.24 ± 17.27 | 97.72 ± 17.23 | 95.10 ± 17.04 | 92.25 ± 16.96 | <0.01 |
| Crystallized cognition score | 106.46 ± 18.24 | 108.07 ± 18.40 | 105.14 ± 17.85 | 102.22 ± 17.25 | <0.01 |
Values are mean ± SD or n (%) unless indicated otherwise. P values are for any difference across race (white, African American, and other races) using ANOVA or chi-square test, as appropriate.
This analysis explored the association of BMI with total, fluid, and crystallized cognition, and the effect of sleep disturbances as a mediator. Table 2 shows these results. In the preliminary analysis, it was found that higher BMI was positively associated with sleep disturbances. After adjusting for potential confounders, attenuated inverse statistically significant associations of BMI with fluid cognition and total cognition were found. When sleep disturbances total score was included in the model, no mediation effect was found in the associations of BMI with total and crystallized cognition, but a small partial mediation effect of sleep disturbance was found in the association between BMI and fluid cognition. Sleep disturbances had an ACME of −0.02 (P < 0.01) and an ADE of −0.46 (P < 0.01) on the association between BMI and fluid cognition, and the proportion of the total effect caused by sleep disturbances was 0.05 (P < 0.01). Figure 2 shows the mediation significance of sleep disturbances in the overall sample.
TABLE 2.
β Coefficients from the linear regression approach for the mediation effect of sleep disturbances in the association of BMI and cognitive scores1
| Sample | Cognitive test | Exposure to mediator (β,α) | Exposure to outcome (β,α) | Mediator to outcome (β,α) | Exposure to outcome, adjusted by mediator (β,α) |
|---|---|---|---|---|---|
| Total sample | Fluid cognition | 0.38 (<0.001) | −0.49 (<0.01) | −0.07 (<0.01) | −0.47 (<0.01) |
| Crystallized cognition | −0.20 (0.12) | 0.05 (0.01) | −0.21 (0.09) | ||
| Total cognition | −0.41 (<0.01) | −0.01 (0.71) | −0.41 (<0.01) |
All models were adjusted for age, sex, physical activity, family income, parents’ education, and race.
FIGURE 2.
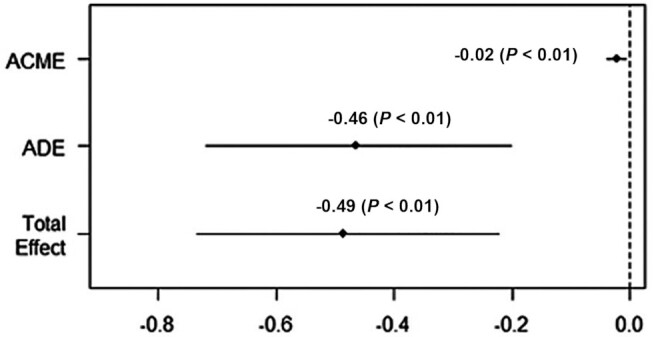
Sleep disturbances’ mediation significance in the association between BMI and fluid cognition, for the overall sample. ACME, average causal mediation effect/indirect effect; ADE, average direct effect.
Discussion
A statistically significant, although partial, mediation effect of sleep disturbances on the association between BMI and fluid cognition was found. There was no significant mediation for total or crystallized cognition. These results can be explained by the influence of biological and external factors on fluid cognition, which comprise a series of cognitive processes that develop during late childhood. Fluid cognition is sensitive to changes in neurobiological integrity and other factors, such as sleep quality and weight status, which can alter the brain structure and its function (6). Previous studies have shown that obesity was associated with the impairment of inhibition, cognitive flexibility, working memory, and planning. Incremental increases in BMI have been associated with a decline in individual cognitive performance among both children and adults (45–49). Moreover, neuroimaging literature has shown that an increased BMI is associated with reduced cortical thickness in the prefrontal region, which is involved in fluid cognition, and with lower executive function, processes involved in fluid cognition (39, 50). In addition, sleep quality and quantity are also linked to metabolic and endocrine disorders affected by obesity (39, 50–53). The quality and quantity of sleep are fundamental for memory consolidation, and sleep deprivation can affect the brain's reaction time and information processing (36). Poor sleep can negatively affect cognitive processes related to fluid cognition that include learning, cognitive performance, IQ measures, and self-regulatory abilities (54–56). These findings align with the results presented in the current study, suggesting the importance of sleep disturbances for fluid cognition. Moreover, the results in this analysis support and complement the evidence found in a recent study conducted with the ABCD data set, which found that fewer hours of sleep were related to reduced fluid cognition in children (57).
Finally, the results in this study are supported by previous studies that have explored the BMI, sleep, and cognition axis. A previous small-sample study showed that apnea, a specific sleep disturbance, can worsen the effect of obesity on cognitive function, particularly mental processing (58). In addition, other studies have shown that worse sleep behaviors are associated with reduced gray matter volume and cognitive performance (59, 60). One advantage of the present study is that it incorporates several types of sleep disturbances in the analysis, which strengthen the findings from previous studies.
This study has several limitations that may limit the interpretation of our findings. First, the cross-sectional study design does not permit the examination of causal relations owing to the lack of temporality, owing to measurement of the exposure and outcome at the same time point, and not measuring the exposure before the occurrence of the outcome. Second, sleep disturbances were collected through the widely used Sleep Disturbance Scale for Children. Nonetheless, the sleep outcomes might be underestimated because the scale was based on parental report and not clinical measurements. In addition, the use of gold-standard techniques for body composition, such as DXA, was unavailable. However, BMI is a valid surrogate estimate that allows a representation of the population weight distribution in large samples, like the ABCD Study. Furthermore, this analysis was developed with the baseline measurements of the ABCD Study. The baseline sample only included children aged 9–10 y, which limits the generalizability of the results beyond these ages. In addition, nutrient and energy intake measurements were not available in the baseline data set, which limits the potential adjustment for energy and nutrient intakes. Other potential confounders such as caffeine were not included in the study, because the available information was not detailed enough and could potentially have introduced bias into the analysis. Finally, physical activity was self-reported in this data release, which could potentially have created report bias.
This analysis suggests that sleep disturbances partially mediate the effect of childhood obesity on cognitive function, particularly as it pertains to fluid cognition. Future work is needed to understand the effects of sleep disturbances and obesity on reduced childhood cognition over time, predominantly across the life course, and particularly on fluid cognition.
Fluid cognition is critical for neurodevelopment. The fluid abilities developed during childhood support crystallized cognition. During childhood, individuals acquire the knowledge that is needed for long-term memory, message decoding, and comprehension, which are abilities that are fundamental in life. Understanding the effect of sleep disturbance patterns in the association between obesity and fluid cognition in childhood may lead to appropriate interventions to improve the developmental conditions that could enhance neurodevelopment in children.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—PPM-M: conducted the analysis and interpretation of the data and wrote the manuscript; EJN: critically reviewed all the stages and the final version of the manuscript; and both authors: read and approved the final manuscript.
The data utilized in this article were obtained in the National Institute of Mental Health Data Archive, from the ABCD (Adolescent Brain Cognitive Development) Study (61). The ABCD Study is supported by the NIH and additional federal partners under awards U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, and U24DA041147, listed at: https://abcdstudy.org/federal-partners.html. ABCD consortium investigators designed and implemented the longitudinal study and provided accessibility to the data but did not participate in the analysis and writing of this report. The ABCD data repository grows and changes over time. The ABCD data used in this study came from https://data-archive.nimh.nih.gov/s/sharedcontent/about/policy.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
This article reflects the views of the authors and may not reflect the opinions or views of the NIH or the Adolescent Brain Cognitive Development (ABCD) consortium investigators.
Abbreviations used: ABCD, Adolescent Brain Cognitive Development; ACME, average causal mediation effect; ADE, average direct effect.
Contributor Information
Paola P Mattey-Mora, Email: pamattey@iu.edu, Department of Epidemiology and Biostatistics, School of Public Health—Bloomington, Indiana University, Bloomington, IN, USA.
Erik J Nelson, Department of Public Health, Brigham Young University, Provo, UT, USA.
Data Availability
The data that support this study are openly available in the National Institute of Mental Health Data Archive (NDA). The ABCD Study data repository will grow and change over time.
References
- 1. Stiles J, Brown TT, Haist F, Jernigan TL. Brain and cognitive development. In: Liben LS, Müller U, editors. Handbook of child psychology and developmental science. Vol. 2, Cognitive processes. 7th ed. Hoboken, NJ: Wiley; 2015. p. 9–62. [Google Scholar]
- 2. Committee on the Science of Children Birth to Age 8: Deepening and Broadening the Foundation for Success, Board on Children, Youth, and Families, Institute of Medicine, National Research Council, Allen LR, Kelly BB, editors. Transforming the workforce for children birth through age 8: a unifying foundation. Washington (DC): National Academies Press (US); Child Development and Early Learning; 2015. [PubMed] [Google Scholar]
- 3. Carson V, Hunter S, Kuzik N, Wiebe SA, Spence JC, Friedman A, Tremblay MS, Slater L, Hinkley T. Systematic review of physical activity and cognitive development in early childhood. J Sci Med Sport. 2016;19(7):573–8. [DOI] [PubMed] [Google Scholar]
- 4. Garon N, Bryson SE, Smith IM. Executive function in preschoolers: a review using an integrative framework. Psychol Bull. 2008;134(1):31–60. [DOI] [PubMed] [Google Scholar]
- 5. Tomasello M. Language development. In: Goswami U, editor. The Wiley-Blackwell handbook of childhood cognitive development. 2nd ed. Hoboken, NJ: Wiley-Blackwell; 2010. p. 239–57. [Google Scholar]
- 6. Akshoomoff N, Beaumont JL, Bauer PJ, Dikmen SS, Gershon RC, Mungas D, Slotkin J, Tulsky D, Weintraub S, Zelazo PDet al. NIH Toolbox Cognition Battery (CB): composite scores of crystallized, fluid, and overall cognition. Monogr Soc Res Child Dev. 2013;78(4):119–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Briley DA, Tucker-Drob EM. Comparing the developmental genetics of cognition and personality over the life span. J Pers. 2017;85(1):51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mollon J, Knowles EEM, Mathias SR, Gur R, Peralta JM, Weiner DJ, Robinson EB, Gur RE, Blangero J, Almasy Let al. Genetic influence on cognitive development between childhood and adulthood. Mol Psychiatry. 2021;26(2):656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MAL-ED Network Investigators . Early childhood cognitive development is affected by interactions among illness, diet, enteropathogens and the home environment: findings from the MAL-ED birth cohort study. BMJ Glob Health. 2018;3(4):e000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr. 2007;85(2):614S–20S. [DOI] [PubMed] [Google Scholar]
- 11. Prickett C, Brennan L, Stolwyk R. Examining the relationship between obesity and cognitive function: a systematic literature review. Obes Res Clin Pract. 2015;9(2):93–113. [DOI] [PubMed] [Google Scholar]
- 12. Wang C, Chan JSY, Ren L, Yan JH. Obesity reduces cognitive and motor functions across the lifespan. Neural Plast. 2016:2473081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kyrou I, Randeva HS, Tsigos C, Kaltsas G, Weickert MO. Clinical problems caused by obesity. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Grossman A, Hershman JM, Hofland Jet al. et al.editors.Endotext. [Internet]. South Dartmouth, MA: MDText.com, Inc; 2000. [cited 20 June, 2021]Available from: https://www.ncbi.nlm.nih.gov/books/NBK278973/. [Google Scholar]
- 14. Rankin J, Matthews L, Cobley S, Han A, Sanders R, Wiltshire HD, Baker JS. Psychological consequences of childhood obesity: psychiatric comorbidity and prevention. Adolesc Health Med Ther. 2016;7:125–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pulgarón ER. Childhood obesity: a review of increased risk for physical and psychological comorbidities. Clin Ther. 2013;35(1):A18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kumar S, Kelly AS. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc. 2017;92(2):251–65. [DOI] [PubMed] [Google Scholar]
- 17. CDC . Promoting health for children and adolescents. [Internet]. Atlanta, GA: CDC; c2020; [cited 20 June, 2021]. Available from: . [Google Scholar]
- 18. Bonanno L, Metro D, Papa M, Finzi G, Maviglia A, Sottile F, Corallo F, Manasseri L. Assessment of sleep and obesity in adults and children: observational study. Medicine (Baltimore). 2019;98(46):e17642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller AL, Lumeng JC, LeBourgeois MK. Sleep patterns and obesity in childhood. Curr Opin Endocrinol Diabetes Obes. 2015;22(1):41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463(1):121–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thosar SS, Butler MP, Shea SA. Role of the circadian system in cardiovascular disease. J Clin Invest. 2018;128(6):2157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Mol Cell Endocrinol. 2012;349(1):91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Short MA, Blunden S, Rigney G, Matricciani L, Coussens S, Reynolds CM, Galland B. Cognition and objectively measured sleep duration in children: a systematic review and meta-analysis. Sleep Health. 2018;4(3):292–300. [DOI] [PubMed] [Google Scholar]
- 24. Philbrook LE, Hinnant JB, Elmore-Staton L, Buckhalt JA, El-Sheikh M. Sleep and cognitive functioning in childhood: ethnicity, socioeconomic status, and sex as moderators. Dev Psychol. 2017;53(7):1276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. El-Sheikh M, Philbrook LE, Kelly RJ, Hinnant JB, Buckhalt JA. What does a good night's sleep mean? Nonlinear relations between sleep and children's cognitive functioning and mental health. Sleep. 2019;42(6):zsz078. Erratum in: Sleep 2019;42(12):zsz195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watach AJ, Radcliffe J, Xanthopoulos MS, Novick MB, Sawyer AM. Executive function impairments in adolescents with obesity and obstructive sleep apnea syndrome. Biol Res Nurs. 2019;21(4):377–83. [DOI] [PubMed] [Google Scholar]
- 27. Xanthopoulos MS, Gallagher PR, Berkowitz RI, Radcliffe J, Bradford R, Marcus CL. Neurobehavioral functioning in adolescents with and without obesity and obstructive sleep apnea. Sleep. 2015;38(3):401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tan E, Healey D, Schaughency E, Dawes P, Galland B. Neurobehavioural correlates in older children and adolescents with obesity and obstructive sleep apnoea. J Paediatr Child Health. 2014;50(1):16–23. [DOI] [PubMed] [Google Scholar]
- 29. Garavan H, Bartsch H, Conway K, Decastro A, Goldstein RZ, Heeringa S, Jernigan T, Potter A, Thompson W, Zahs D. Recruiting the ABCD sample: design considerations and procedures. Dev Cogn Neurosci. 2018;32:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Health Organization . Growth reference data for 5-19 years. [Internet]. Geneva, Switzerland: WHO; 2009. [cited 20 June, 2021]. Available from: http://www.who.int/growthref/tools/en/. [Google Scholar]
- 31. de Onis M, Blössner M. The World Health Organization Global Database on Child Growth and Malnutrition: methodology and applications. Int J Epidemiol. 2003;32(4):518–26. [DOI] [PubMed] [Google Scholar]
- 32. de Onis M, Lobstein T. Defining obesity risk status in the general childhood population: which cut-offs should we use?. Int J Pediatr Obes. 2010;5(6):458–60. [DOI] [PubMed] [Google Scholar]
- 33. Bruni O, Ottaviano S, Guidetti V, Romoli M, Innocenzi M, Cortesi F, Giannotti F. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res. 1996;5(4):251–61. [DOI] [PubMed] [Google Scholar]
- 34. Luciana M, Bjork JM, Nagel BJ, Barch DM, Gonzalez R, Nixon SJ, Banich MT. Adolescent neurocognitive development and impacts of substance use: overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Dev Cogn Neurosci. 2018;32:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lisdahl KM, Sher KJ, Conway KP, Gonzalez R, Feldstein Ewing SW, Nixon SJ, Tapert S, Bartsch H, Goldstein RZ, Heitzeg M. Adolescent brain cognitive development (ABCD) study: overview of substance use assessment methods. Dev Cogn Neurosci. 2018;32:80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, Carlozzi NE, Slotkin J, Blitz D, Wallner-Allen Ket al. Cognition assessment using the NIH Toolbox. Neurology. 2013;80(11 Supplement 3):S54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Slotkin J, Carlozzi NE, Bauer PJ, Wallner-Allen K, Fox Net al. The cognition battery of the NIH toolbox for assessment of neurological and behavioral function: validation in an adult sample. J Int Neuropsychol Soc. 2014;20(6):567–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Casaletto KB, Umlauf A, Marquine M, Beaumont JL, Mungas D, Gershon R, Slotkin J, Akshoomoff N, Heaton RK. Demographically corrected normative standards for the Spanish language version of the NIH Toolbox Cognition Battery. J Int Neuropsychol Soc. 2016;22(3):364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ronan L, Alexander-Bloch A, Fletcher PC. Childhood obesity, cortical structure, and executive function in healthy children. Cereb Cortex. 2020;30(4):2519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pearce AL, Mackey E, Nadler EP, Vaidya CJ. Sleep health and psychopathology mediate executive deficits in pediatric obesity. Child Obes. 2018;14(3):189–96. [DOI] [PubMed] [Google Scholar]
- 41. Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–82. [DOI] [PubMed] [Google Scholar]
- 42. R Core Team . R: a language and environment for statistical computing. [Internet]. Version 3.6.0 [Software]. Vienna, Austria: R Foundation for Statistical Computing; 2020. Available from: https://www.R-project.org/. [Google Scholar]
- 43. Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R package for causal mediation analysis. Version 4.5.0 [Software]. J Stat Soft. 2014;59(5):1–38. [Google Scholar]
- 44. Hayes AF. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monogr. 2009;76(4):408–20. [Google Scholar]
- 45. Yang Y, Shields GS, Guo C, Liu Y. Executive function performance in obesity and overweight individuals: a meta-analysis and review. Neurosci Biobehav Rev. 2018;84:225–44. [DOI] [PubMed] [Google Scholar]
- 46. Liang J, Matheson BE, Kaye WH, Boutelle KN. Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. Int J Obes. 2014;38(4):494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bocarsly ME, Fasolino M, Kane GA, LaMarca EA, Kirschen GW, Karatsoreos IN, McEwen BS, Gould E. Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proc Natl Acad Sci U S A. 2015;112(51):15731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vainik U, Dagher A, Dubé L, Fellows LK. Neurobehavioural correlates of body mass index and eating behaviours in adults: a systematic review. Neurosci Biobehav Rev. 2013;37(3):279–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smith E, Hay P, Campbell L, Trollor JN. A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obes Rev. 2011;12(9):740–55. [DOI] [PubMed] [Google Scholar]
- 50. Laurent JS, Watts R, Adise S, Allgaier N, Chaarani B, Garavan H, Potter A, Mackey S. Associations among body mass index, cortical thickness, and executive function in children. JAMA Pediatr. 2020;174(2):170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Garaulet M, Ortega FB, Ruiz JR, Rey-López JP, Béghin L, Manios Y, Cuenca-García M, Plada M, Diethelm K, Kafatos Aet al. Short sleep duration is associated with increased obesity markers in European adolescents: effect of physical activity and dietary habits. The HELENA study. Int J Obes. 2011;35(10):1308–17. [DOI] [PubMed] [Google Scholar]
- 52. Yoong SL, Chai LK, Williams CM, Wiggers J, Finch M, Wolfenden L. Systematic review and meta-analysis of interventions targeting sleep and their impact on child body mass index, diet, and physical activity. Obesity. 2016;24(5):1140–7. [DOI] [PubMed] [Google Scholar]
- 53. Aldabal L, Bahammam AS. Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respir Med J. 2011;5(1):31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hill CM, Hogan AM, Karmiloff-Smith A. To sleep, perchance to enrich learning?. Arch Dis Child. 2007;92(7):637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bernier A, Beauchamp MH, Bouvette-Turcot AA, Carlson SM, Carrier J. Sleep and cognition in preschool years: specific links to executive functioning. Child Dev. 2013;84(5):1542–53. [DOI] [PubMed] [Google Scholar]
- 56. Ravid S, Afek I, Suraiya S, Shahar E, Pillar G. Sleep disturbances are associated with reduced school achievements in first-grade pupils. Dev Neuropsychol. 2009;34(5):574–87. [DOI] [PubMed] [Google Scholar]
- 57. Kirlic N, Colaizzi JM, Cosgrove KT, Cohen ZP, Yeh H-W, Breslin F, Morris AS, Aupperle RL, Singh MK, Paulus MP. Extracurricular activities, screen media activity, and sleep may be modifiable factors related to children's cognitive functioning: evidence from the ABCD Study®. Child Dev. 2021;92(5):2035–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Spruyt K, Gozal D. A mediation model linking body weight, cognition, and sleep-disordered breathing. Am J Respir Crit Care Med. 2012;185(2):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Migueles JH, Martinez-Nicolas A, Cadenas-Sanchez C, Esteban-Cornejo I, Muntaner-Mas A, Mora-Gonzalez J, Rodriguez-Ayllon M, Madrid JA, Rol MA, Hillman CHet al. Activity-rest circadian pattern and academic achievement, executive function, and intelligence in children with obesity. Scand J Med Sci Sports. 2021;31(3):653–64. [DOI] [PubMed] [Google Scholar]
- 60. Migueles JH, Cadenas-Sanchez C, Esteban-Cornejo I, Mora-Gonzalez J, Rodriguez-Ayllon M, Solis-Urra P, Erickson KI, Kramer AF, Hillman CH, Catena Aet al. Associations of sleep with gray matter volume and their implications for academic achievement, executive function and intelligence in children with overweight/obesity. Pediatr Obes. 2021;16(2):e12707. [DOI] [PubMed] [Google Scholar]
- 61. ABCD Consortium . Adolescent Brain Cognitive DevelopmentSM (ABCD) Study, ABCD consortium, 2019. [Internet]. Curated Annual Release 2.0, NIMH Data Archive. NIMH Data Archive; 2019; [cited 20 June, 2021]. Available from: https://nda.nih.gov/abcd [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support this study are openly available in the National Institute of Mental Health Data Archive (NDA). The ABCD Study data repository will grow and change over time.


