Abstract
During the perinatal period in mammals when active sleep predominates, skeletal muscles twitch throughout the body. We have hypothesized that myoclonic twitches provide unique insight into the functional status of the human infant’s nervous system. However, assessments of the rate and patterning of twitching have largely been restricted to infant rodents. Thus, here we analyze twitching in human infants over the first seven postnatal months. Using videography and behavioral measures of twitching during bouts of daytime sleep, we find at all ages that twitching across the body occurs predominantly in bursts at intervals of 10 seconds or less. We also find that twitching is expressed differentially across the body and with age. For example, twitching of the face and head is most prevalent shortly after birth and decreases over the first several months. In addition, twitching of the hands and feet occurs at a consistently higher rate than does twitching elsewhere in the body. Finally, the patterning of twitching becomes more structured with age, with twitches of the left and right hands and feet exhibiting the strongest coupling. Altogether, these findings support the notion that twitches can provide a unique source of information about typical and atypical sensorimotor development.
Keywords: development, REM sleep, active sleep, behavior, sensorimotor development, rapid eye movements, rat, physiological time, neurodevelopmental disorders
Myoclonic twitches are spontaneous, jerky, discrete, low-amplitude movements that occur throughout the body during active (or REM) sleep. In turn, active sleep is a prominent behavioral state in mammalian infants (Blumberg & Rattenborg, 2017; Holditch-Davis, Scher, Schwartz, & Barr, 2004; Jouvet-Mounier, Astic, & Lacote, 1970; Roffwarg, Muzio, & Dement, 1966; Shimizu & Himwich, 1968). In particular, human newborns sleep 16 hours each day, with half of their sleep time spent in active sleep and the other half in quiet (or non-REM) sleep.
Contrary to the impression gleaned from casual observation, twitches are not random and uncoordinated; rather, as demonstrated in infant rats and mice, twitches exhibit spatiotemporal structure that is fundamentally related to the development of sensorimotor systems in the brain and spinal cord (Blumberg, Coleman, Gerth, & McMurray, 2013a; Blumberg et al., 2015). However, in contrast with spontaneous wake movements (Einspieler & Prechtl, 2005; Jensen, Schneider, Ulrich, Zernicke, & Thelen, 2010; Piek & Carman, 1994; Thelen, 1979), few studies have focused on twitching in human infants as a behavioral phenomenon unto itself (Hadders-Algra, Nakae, Van Eykern, Klip-Van den Nieuwendijk, & Prechtl, 1993; Kohyama, 1996).
Studies with infant rats established that twitches are produced predominantly by motor structures in the brainstem (Blumberg & Plumeau, 2016; Del Rio-Bermudez, Sokoloff, & Blumberg, 2015). When a twitch occurs, the resulting sensory feedback (i.e., reafference) triggers a cascade of activity throughout the sensorimotor system, from spinal cord to cortex, and this activity drives neural plasticity to enable precise somatotopic organization (Blumberg & Dooley, 2017; Inácio, Nasretdinov, Lebedeva, & Khazipov, 2016; Khazipov et al., 2004; Petersson, Waldenström, Fåhraeus, & Schouenborg, 2003). More broadly, twitches provide opportunities for infants to embody their growing limbs, that is, to assimilate them into their nervous system (Blumberg & Dooley, 2017). Although most of what researchers know about twitch-related reafference derives from work in rat pups, similar movement-related activity has been detected in the somatosensory cortex of premature human infants as early as 29-31 weeks postconceptional age (Milh et al., 2007; Whitehead, Meek, & Fabrizi, 2018). The abundance of twitching and the robustness with which it activates neural circuits provide ample opportunity for twitches to contribute to the processes by which the sensorimotor system maps and assimilates growing limbs into the developing nervous system (Blumberg & Dooley, 2017; Blumberg, Marques, & Iida, 2013b).
Here we describe developmental changes in the rate and patterning of twitching in human infants across the first seven postnatal months during daytime sleep. We find that twitches are abundant in early infancy and exhibit spatiotemporal features that are similar in several ways to those documented previously in infant rats (Blumberg, Coleman, Gerth, & McMurray, 2013a). In addition, consistent with the notion that twitches provide a behavioral readout of the functional status of the nervous system, we find that rates of twitching are higher in some body segments (e.g., hands and feet) than in others, and that twitching exhibits distinct age-related patterns of activity that highlight organizational features of the human infant’s nervous system.
METHODS
Participants
Sixteen term infants (10 boys, 6 girls) between 2 weeks and 7 months of age participated. Families received $30 for each sleep session. Infants contributed a total of 41 sleep session recordings; 5 infants contributed one session and the remaining 11 infants contributed 2-4 sessions (Figure 1A). All study procedures were approved by the institutional review board at the University of Iowa.
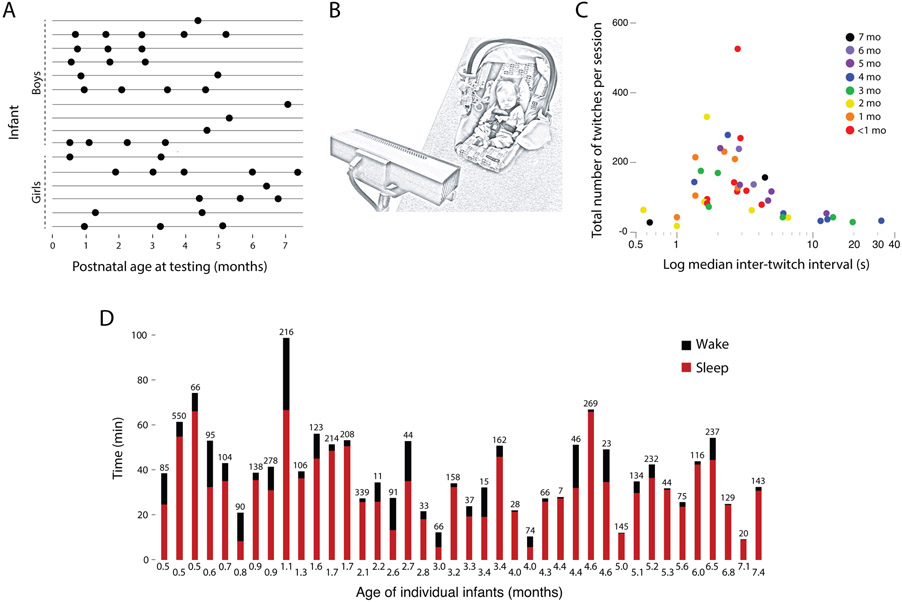
Figure 1.
(A) Infants’ age at each sleep session by sex. Each row represents one infant. Ten infants were observed 2-4 times and 6 were observed once. (B) Illustration of the experimental set-up with infant asleep in car seat as behavior was recorded. This illustration shows a Kinect sensor in the foreground, although 11 of 41 sessions were recorded using a standard video camera. (C) Scatterplot of median inter-twitch intervals (ITIs) across all infants at each age and as a function of the total number of twitches detected in the sleep session. Median ITIs are shown on a log scale to reveal each data point more clearly. (D) Histograms of amount of time spent awake (black) and asleep (red) during the sleep sessions for each infant at each age; the number of twitches detected during the session is shown at the top of each bar.
Procedure and Data Acquisition
We recorded infants’ sleep during the daytime in the laboratory. In all sessions, infants slept in a supine, semi-reclined position (Figure 1B). In every session, infants’ movements were recorded using a single video camera from a frontal view. In addition, in 9 sleep sessions (distributed over 4 infants), Bluetooth accelerometers (Kinesia, Great Lakes NeuroTechnologies, Valley View, OH) were attached to both wrists and ankles using Velcro elastic bracelets. However, data collected using accelerometry were not sufficiently sensitive to detect movements in each limb, so they are not discussed further. Caregivers sat in the recording room with their infants or in a nearby waiting room equipped with a baby video monitor. While infants slept, caregivers reported demographic information.
For 30 sleep sessions, videos were acquired using a Kinect sensor (30 frames/s; Microsoft, Redmond, WA); for the remaining 11 sessions, videos were recorded with a digital camera (30 frames/s; Blackfly; FLIR, British Columbia, CA). Each recording continued for as long as infants slept with only brief interposed periods of wake.
Data Coding and Reliability
We relied on established behavioral criteria to define wake and active sleep (Thoman, 1990; Thoman, Davis, & Denenberg, 1987). During wake, infants’ eyes are often open for sustained periods, muscle tone is high, and the limbs exhibit high-amplitude and often synchronous movements. During active sleep, the eyes are closed (although they can open briefly), muscle tone is low (e.g., the head droops and the arms and legs are relaxed), and rapid eye movements and myoclonic twitches are evident (Supplemental Video 1). Similar to infant rats (Blumberg, Coleman, Gerth, & McMurray, 2013a), twitch movements in human infants differ from wake movements in that they are relatively brief, low in amplitude, and discrete; although rare, twitches can occur nearly simultaneously in different body segments.
Three coders scored the video data, with at least two of them scoring 100% of each session. For each session, we first determined periods when infants were awake or asleep. Next, coders scored the data in multiple passes to identify twitches of the face, head, and limbs. Startles, defined as the simultaneous extension of all limbs, were scored but not analyzed because they are phenomenologically distinct from twitches and are not closely associated with active sleep (Karlsson, Mohns, Vianna di Prisco, & Blumberg, 2006; Prechtl, 1974). Throughout the scoring process, coders determined whether sleep movements were produced secondarily to another movement (e.g., deep respiration, one limb moving into another one). For videos acquired using the Kinect sensor, data were analyzed using the Sleep Motion Analyzer (SMA; Austrian Institute of Technology, Vienna, AT); for the videos acquired using the Blackfly video camera, data were analyzed using Spike2 (Cambridge Electronic Design, Cambridge, UK). Regardless of the technology, the same coding procedures were used.
We scored twitches that resulted in movements of the head, face (i.e., mouth, cheeks, and brow), arms (i.e., shoulders and elbows), hands (i.e., wrists and fingers), legs (i.e., hips and knees), and feet (i.e., ankles and toes). These categories were chosen because, without multiple camera views, it can be difficult to distinguish, for example, shoulder from elbow movements (see Blumberg, Coleman, Gerth, & McMurray, 2013a); for face twitches, we combined across mouth, cheeks, and brow because overall rates of face twitching were relatively low and these twitches tended not to occur in isolation. In most sessions, infants wore socks so that twitches of individual toes were obscured. Rapid eye movements were scored in a subset of infants in which those movements were detectible.
To assess inter-observer reliability, we determined the time of occurrence and duration of twitches detected by each coder across all movement categories (face, head, and left and right arms, hands, legs, and feet). For files analyzed using Spike2, twitch duration was set at 500 ms (the approximate average duration of a twitch). For those twitches in which the two coders disagreed, the times of these events were noted and each coder individually reviewed these events and recoded them; these recoded events were incorporated into the individual records. To calculate Cohen’s kappa, the time series data (comprising values for twitch onset and duration) were first down-sampled to 15 samples/s. Cohen’s kappa was calculated and at this stage ranged from 0.42-0.85 (median: 0.69).
Next, the data were assessed to determine when the two coders disagreed about the movement of a particular body segment; coders usually disagreed by no more than 1.5 s as to when the twitch began and ended. In addition, we ensured that twitches from other segments of the body were not the source of the disagreement. When these conditions were met, the timing and duration of the twitches were aligned across the two coders. After this corrective step, kappa increased to 0.75-0.96 (median: 0.85), which indicates >92.4% agreement between the two coders. Finally, the coders together made a final pass through the data record and all remaining discrepancies were resolved by mutual agreement.
Data Analysis
Sleep-wake transitions.
For each sleep session, the onset times of sleep and wake periods were determined. An arousal from sleep was only considered a transition to wake if it lasted more than 10 s (arousals lasting less than 10 s are hereafter referred to as microarousals). The total number of sleep-wake transitions was divided by the session time to provide a rate of sleep-wake transitions per min. Age-related changes in sleep-wake transitions were analyzed using bivariate correlation in SPSS (IBM, Armonk, NY). For this analysis, one outlier exceeded the mean +3 standard deviations and was removed.
Inter-twitch intervals.
The time between two successive twitches within a sleep period was defined as an inter-twitch interval (ITI). ITIs were determined for all twitches within each sleep session. ITIs were not included if they were interrupted by a microarousal or startle. The median ITI was less than 30 s in all but one infant (Figure 1C). Across all 41 sessions, 85-95% of twitches were associated with ITIs less than 30 s. These ITI distributions informed the criteria described below. Log-survivor ITI distributions were determined and analyzed. To test for differences in the survival distributions across age, the Mantel-Cox log-rank test was used.
Active sleep bouts:
We defined the onset of a bout of active sleep as the first twitch during a period of behaviorally defined sleep. A minimum bout of active sleep was comprised of at least three twitches with ITIs less than 30 s each; the bout continued as long as each subsequent twitch occurred within 30 s of the previous one. If an ITI exceeded 30 s, the bout was terminated at the last twitch associated with an ITI that was less than 30 s. Termination of a bout was also marked by a transition to wake or the occurrence of a microarousal or startle. The duration of an active sleep bout was calculated as the time from the first twitch to the time of wake onset or the occurrence of a microarousal or startle; in those cases where an ITI exceeded 30 s without one of those events occurring, the duration of the bout was calculated as the time from the first twitch to the last twitch plus 30 s.
Twitch rate and patterning:
To calculate the rate of twitching for each sleep session, we first calculated the sum of the duration of all active sleep bouts. Then, the total number of twitches across all active sleep bouts was divided by the duration of active sleep to provide a measure of twitches per minute of active sleep. We also calculated the rates of twitching separately for the face, head, arms, hands, legs, and feet for each session; data for the right and left sides of the body were combined in these measures. For the mixed effects model analyses of twitch rate described below, we determined the rate of twitching for each bout of active sleep within each session.
Individual twitch rates for each bout of active sleep within each session were calculated for face, head, arms, hands, legs, and feet. There was a total of 422 sleep bouts across infants and an average of 26.4 sleep bouts for each infant (range: 1-77). Data were analyzed using a linear mixed effects approach implemented using the lme4 package in R (version 1.1-18-1). This approach was ideal for several reasons. First, it treats age as a continuous variable. Second, the use of random slopes (in conjunction with a continuous age term) allowed us to account for the overall effect of age while dealing with the fact that not all infants were tested at all ages.
The model included fixed effects for age and body segment. Age was scored in months and centered. Effectors were deviation coded into five variables. Each variable scored whether a given effector differed from the average twitch rate across all limbs (once age and differences among subjects were accounted for). The legs were used as a reference level. The dependent measure was twitch rate (twitches/minute within an active sleep bout). Twitch rate was 0-bounded, as it is not possible to have a twitch-rate below 0. This tends to lead to skewed distributions that violate the Gaussian assumptions of regression, ANOVA, and linear mixed models. To address this, we log-scaled the twitch rates for each individual limb in each bout. Some body segments had a twitch rate of 0 for some bouts, which can lead to invalid logs. To avoid this, we added one half of the minimum non-zero twitch rate (i.e., 0.092) to all rates for all bouts before log scaling.
Potential random effects included age and body segment as potential slopes on subject, which was further nested within bouts. Random effects were selected via a variant of the model space selection procedure advocated by Seedorff et al. (submitted), and further constrained to avoid high collinearity among the random effects. The final model included a random slope of age on subject, and random slopes of each body segment variable on bout nested within subject. Covariance terms were dropped. For estimating the significance of fixed effects, the degrees of freedom for t statistics were estimated using the Satterthwaite approximation (Satterthwaite, 1978), as implemented in the lmerTest package in R (version 3.0-1).
To assess the patterning of twitching, hierarchical cluster analysis with seriation was performed using PermutMatrix software (Caraux & Pinloche, 2005). The settings used were Manhattan distance and Ward’s Minimum Variance Method. For seriation, the multiple-fragment heuristic was used. For this analysis, we created sequential 10-s windows of twitch “events” within each sleep session. Each window began with the first twitch and ended 10 s later; the windowed event was composed of all twitches throughout the body. The subsequent 10-s window began with the very next twitch, whenever it occurred. Single-twitch bouts were excluded; bouts comprising more than one twitch from the same body segment were included. For these analyses, to attain adequate sample sizes and similar numbers of events per age group, data were pooled as follows: <1 month, 1-2 months, 3-4 months, and 5-7 months. Each infant contributed only one session to each pooled dataset. Two sessions in the <1-month group were excluded from this analysis because one hand was obscured for a majority of the session and could not be scored.
Unless otherwise stated, alpha was set at p<.05 for all tests.
RESULTS
Across all sessions, the duration of sleep ranged from 5.8 to 66.7 minutes and the duration of active sleep ranged from 0.7 to 32.2 minutes (Figure 1D). We identified a total of 5015 twitches, with a range of 7 to 550 twitches per session (median: 100 twitches).
As one validation of our behavioral scoring method, we quantified the rate of sleep-wake transitions within each age group. Consistent with numerous reports of the organization of sleep and wake bouts across the early postnatal period (e.g., Kleitman & Engelmann, 1953; Parmelee, 1961), we observed greater bout fragmentation at the earlier ages (Figure 2A). The rate of these transitions decreased significantly and monotonically across age, r2=.44, n=40, p<0.0001 (Figure 2B).
Figure 2.
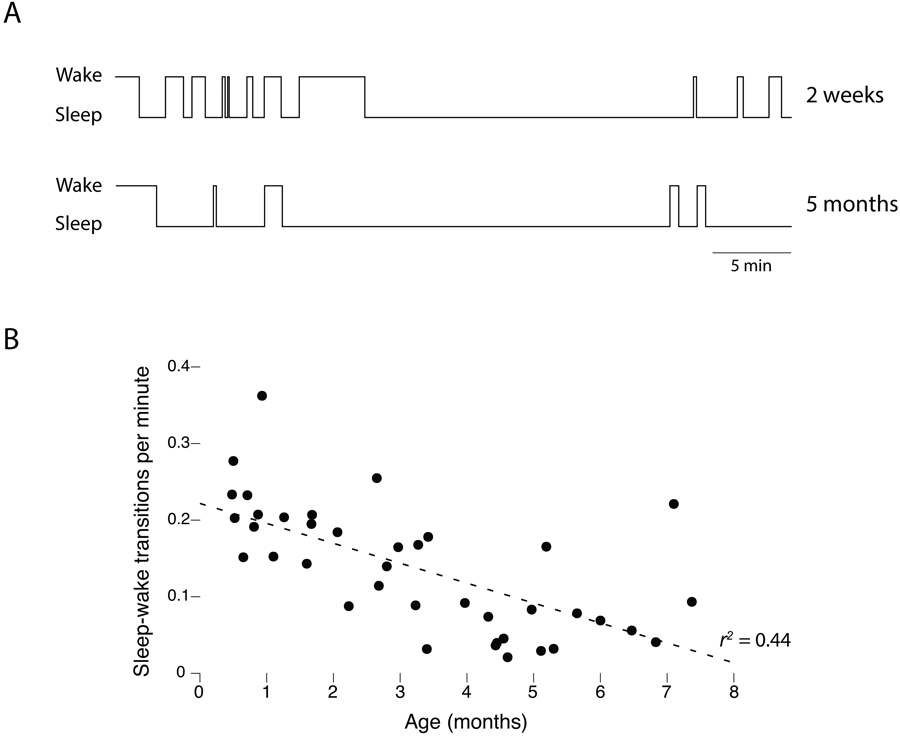
Consolidation of wake and sleep bouts with age. (A) Wake and sleep bout durations for one representative infant at 2 weeks and 5 months of age. Note the age-related reduction in the number of state transitions. (B) Scatterplot showing a monotonic reduction in the rate of sleep-wake transitions across age.
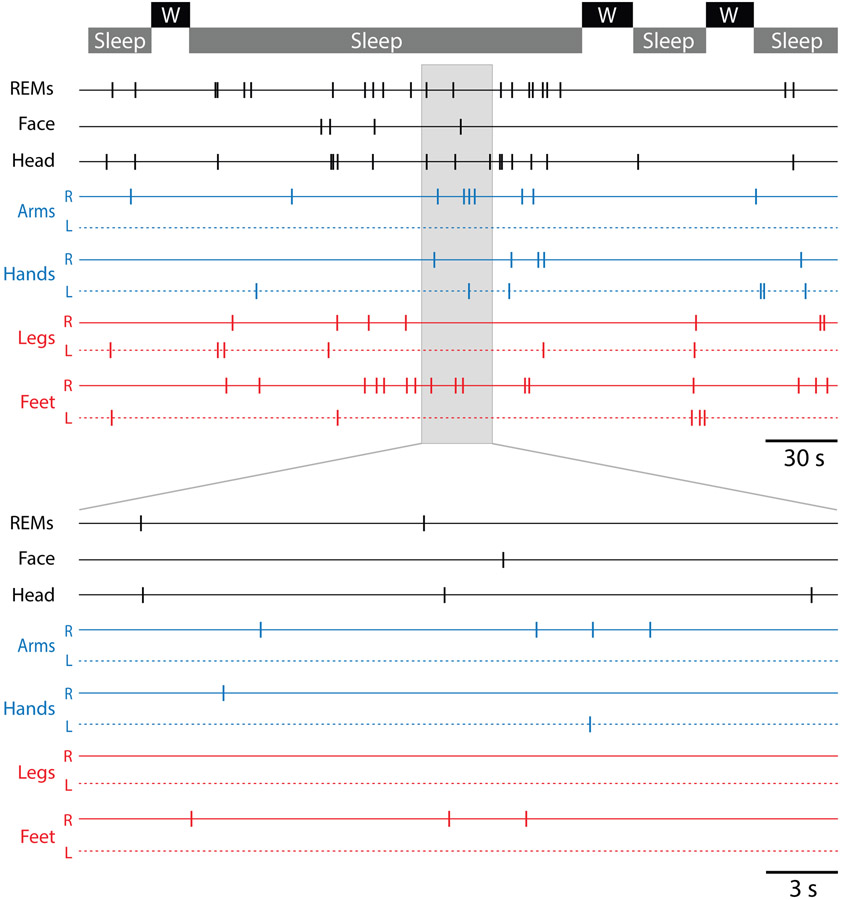
Turning now to the expression of twitching, Figure 3 (top) shows a representative segment of data for a 2-week-old infant across several sleep-wake cycles over a 6.7-minute period. Each tick mark denotes a scored twitch in the identified body part: rapid eye movements (REMs), face, head, arms, and legs. The clustering of REMs with twitches elsewhere in the body is evident. The sleep period within the grey box is expanded below to better reveal the clustering of twitches interposed between periods of quiescence, indicating a relative abundance of twitches at short intervals.
Figure 3.
Top: Spatiotemporal distribution of twitching over a 6.7-minute period comprising several sleep and wake (W) bouts for a representative infant at 2 weeks of age. The sleep period highlighted in gray is expanded below. REMs = rapid eye movements; R = right; L = left.
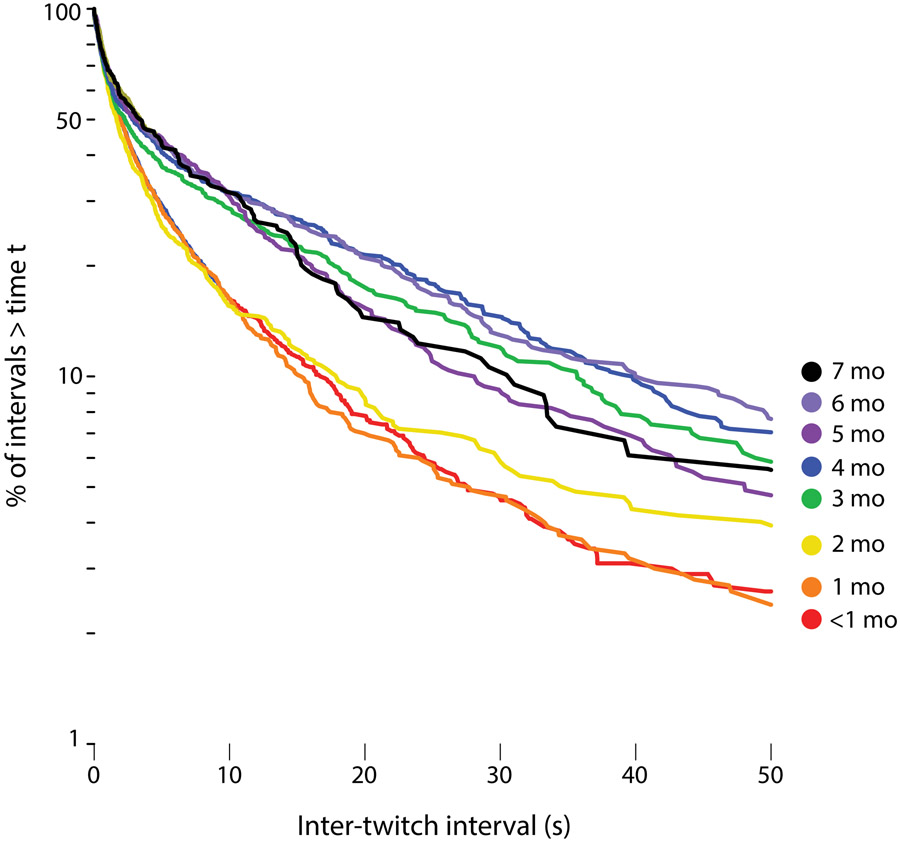
To analyze the temporal structure of twitching, we constructed log-survivor plots for ITIs pooled within 8 age groups (Figure 4). Linearity on such semi-log plots denotes an exponential distribution, with the slope of the line proportional to the probability that, as time passes after one event (e.g., a twitch), a second event will occur; steeper slopes indicate higher event rates than shallower slopes. As shown in Figure 4, across all ages, the ITIs exhibit overlapping distributions with steep slopes at values less than 1-2 s. ITIs at these short intervals account for approximately 50% of all twitches. At ITIs greater than 2 s, the distributions bifurcate, with the rate of twitching slowing considerably for infants 3 months and older in relation to the younger infants. The survival distributions were significantly different for these age groups (Mantel-Cox log-rank text, X2=105.67, df = 7, p<.0001).
Figure 4.
Log-survivor plots of inter-twitch intervals pooled across sleep sessions within the following age groups: <1 month (8 sessions; 1,420 ITIs), 1 month (6 sessions; 923 ITIs), 2 months (6 sessions; 599 ITIs), 3 months (6 sessions; 530 ITIs), 4 months (6 sessions; 617 ITIs), 5 months (4 sessions; 518 ITIs), 6 months (3 sessions; 354 ITIs), and 7 months (2 sessions; 183 ITIs).
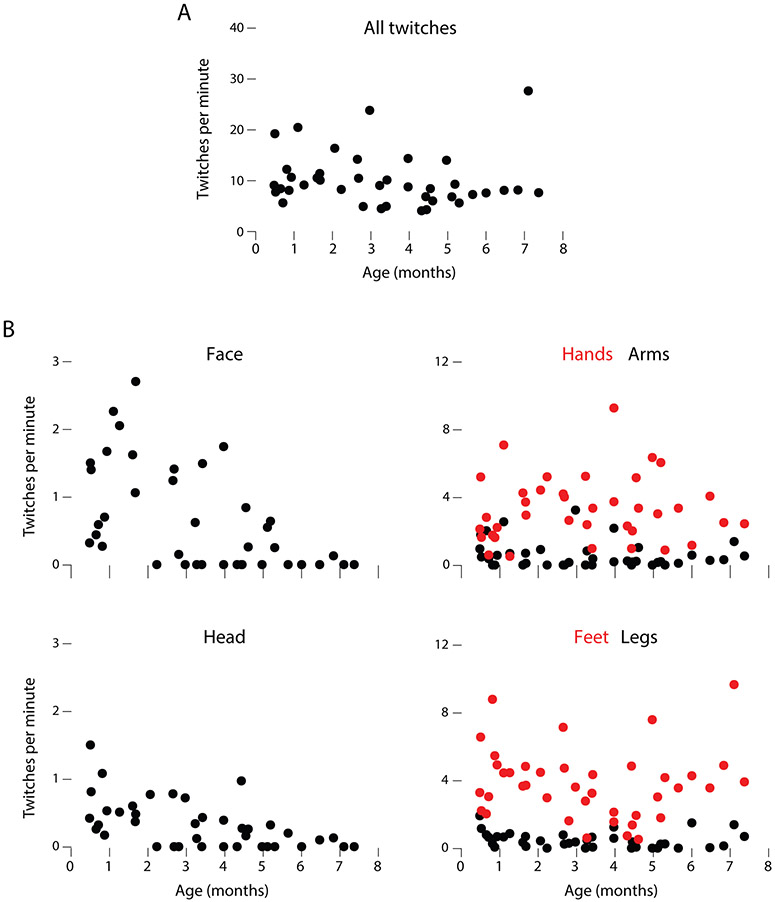
We next analyzed twitch rate for each individual bout of active sleep within each sleep session. (Although the mixed effects model analysis was performed on individual bouts, the twitch-rate data in Figure 5 are averaged for each sleep session.) First, the overall rate of twitching decreased significantly with age (B=−0.109, SE=0.04, t11.25=2.50, p=.029; Figure 5A). Second, individual body segments exhibited significantly different twitch rates relative to the average twitch rate across all limbs at a given age (Figure 5B). Specifically, the face (B=−0.554, SE=0.12, t16.03=4.62, p<.0001), head (B=−0.901, SE=0.06, t2417.4=−14.76, p<.0001), and arms (B=−0.691, SE=0.10, t13.23=6.63, p<.0001) all showed significantly less twitching than average. In contrast, the hands (B=1.126, SE=0.13, t10.82=8.61, p<.0001) and feet (B=1.618, SE=0.17, t11.85=9.26, p<.0001) showed significantly more twitching than average. Finally, there were three significant interactions with age: Whereas the face (B=−0.136, SE=0.04, t208.5=3.65, p<0.001) and head (B=−0.069, SE=0.03, t2417.4=2.36, p=0.019) showed net decreases in twitch rate with age, the hands showed large net increases (B=0.315, SE=0.04, t183.6=8.28, p<0.0001).
Figure 5.
(A) Scatterplot of age-related changes in mean rates of twitching per minute of active sleep across all body segments. (B) Scatterplots of age-related changes in twitching across the six body segments. Data from left and right limbs were pooled. For clarity in these plots, five outliers were removed (one each for face, head, and legs; two for hands). Note different scales on y-axes. See text for statistical analyses.
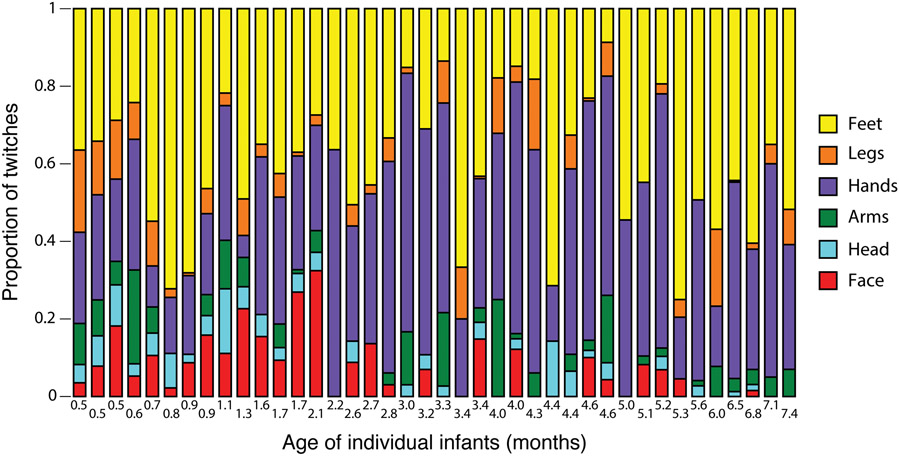
To visualize the proportional relations of twitching among body segments, we created stacked plots for each sleep session (Figure 6). These plots show the relative and consistent predominance of twitching in the hands and feet and the relative preponderance of head and face twitching at the earliest ages.
Figure 6.
Proportion of twitches across six body segments for each sleep session ordered by infant age. Data for right and left limbs are combined.
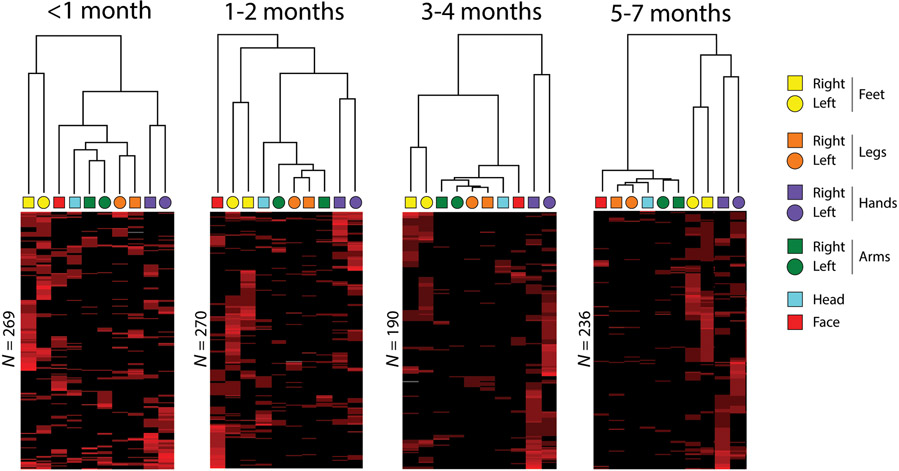
To determine whether twitching across body segments exhibits organized structure and whether that structure changes with age, we performed hierarchical cluster analysis with seriation (Caraux & Pinloche, 2005) on sequential 10-s windows of twitching. This analysis reveals structure in twitching among the body segments (dendrograms at the top of Figure 7). In addition, structure among the events (i.e., rows) is revealed using seriation, in which the events are reordered to maximally reveal their structure. (Although seriation yields a second dendrogram along the rows, only the dendrograms along the columns in Figure 7 are shown to make it easier to directly compare the patterning of twitching across age.)
Figure 7.
Hierarchical cluster analyses, with seriation, of twitching pooled across four age groups. These analyses were performed on 10-s windowed “events” composed of bouts of twitches during sleep. Each 10-s event is represented as a row in the cluster diagram, with red corresponding to the presence of a twitch and black to its absence. The intensity of each red bar is proportional to the number of twitches in the associated body segment that occurred within that row (i.e., the same body segment could twitch multiple times within a 10-s window). Seriation of the rows reorders them such that events with similar patterns are placed closer together. Each infant contributed only once to the data within each pooled dataset. The number of rows for each age group is indicated.
The cluster analyses in Figure 7 reveal aspects of organizational structure that change with age. At all ages, twitches often occurred repeatedly within the same body segment, as evidenced by the variable intensity of the red bars in the figure. At <1 month of age, several clusters are apparent that indicate strong linkages for twitching of the left and right limb segments. Already at this age, the two most distinct clusters are for the (1) left and right hands and (2) left and right feet. At 1-2 months of age, the same two clusters of hands and feet are still the strongest, as the organization of twitches elsewhere in the body exhibits even less structure than at the earlier age. In addition, facial twitches at this age are not strongly associated with any other twitches. By 3-4 months of age, the clusters for the left and right hands and feet are even more distinct from the clusters for twitching elsewhere in the body. This trend continues such that, at 5-7 months, the clusters for the left and right hands and feet account for nearly all of the structure in the data. Also, for the first time at these older ages, the hands and feet are clustered together under a higher-order structure.
DISCUSSION
A large literature describes how sleep is expressed and how it develops in premature and full-term human infants (e.g., Coons & Guilleminault, 1982; Curzi-Dascalova, Peirano, & Morel-Kahn, 1988; Grieve et al., 2008; Jenni, Borbély, & Achermann, 2004; Kleitman & Engelmann, 1953; Parmelee, Wenner, Akiyama, Schultz, & Stern, 1967; Prechtl, 1974; Roffwarg et al., 1966; Thoman & Whitney, 1989; Vanhatalo & Kaila, 2006). Two broad traditions characterize this literature—one that focuses on the cortical electroencephalogram (EEG) and its state-dependent differentiation, and another that focuses on behavior. Within both traditions, myoclonic twitching is viewed primarily as a defining feature of active sleep (Blumberg, 2010), with one exception in which the features of twitches themselves were a primary focus (Kohyama, 1996). In addition, several recent studies addressed the neural correlates and behavioral significance of head and limb twitching in the early postnatal period (Denisova, 2019; Denisova & Zhao, 2017; Whitehead et al., 2018; Whitehead, Slobodina, Meek, & Fabrizi, 2019).
The current study is the first to assess the rate and patterning of twitching across the body in human infants. We were motivated by the notion that twitching, as a distinct class of behavior, can provide unique insights into the typical and atypical development of the sensorimotor system (Blumberg, 2015). Building on insights gleaned from rat pups using similar methods (Blumberg, Coleman, Gerth, & McMurray, 2013a), we identified several features of the spatiotemporal organization of twitching over the first seven postnatal months. The most striking findings relate to developmental changes in the temporal organization of twitching (Figure 4) and the relative distribution of twitching across the body (Figures 5-7).
Functional aspects of twitching
High levels of active sleep in early development inspired, over 50 years ago, the hypothesis that active sleep is critical for brain development (Roffwarg et al., 1966). In the intervening years, developmental scientists have sought to identify the functions of active sleep and the mediating mechanisms involved (Blumberg, 2015; Del Rio-Bermudez & Blumberg, 2018; Frank, Issa, & Stryker, 2001; Marks, Shaffery, Oksenberg, Speciale, & Roffwarg, 1995; Mirmiran, 1995; Tarullo, Balsam, & Fifer, 2010). We posited that twitches exhibit features that, in contrast to wake movements, make them well suited to contribute to certain aspects of sensorimotor development (Blumberg, Marques, & Iida, 2013b). In addition, whereas sensory feedback (reafference) from twitches reliably triggers brain activity in rat pups, reafference from even the most vigorous wake-related movements typically does not (Tiriac, Del Rio-Bermudez, & Blumberg, 2014). Subsequent work identified a specific gating mechanism in the medulla that selectively blocks wake-related reafference (Dooley & Blumberg, 2018; Tiriac & Blumberg, 2016). It will be important to determine whether human infants possess similar mechanisms for differentially processing twitch- and wake-related reafference.
Developmental changes in the rate and patterning of twitching
The log-survivor plots in Figure 4 indicate a sharp developmental transition in the temporal structure of twitching after 2 months of age, suggesting a qualitative shift in the mechanisms that produce twitching. This transition may be directly or indirectly associated with developmental increases in sleep consolidation (see Figure 2), changes in the microstructure of sleep, and/or the developmental emergence of circadian modulation of sleep processes (Jenni et al., 2004; Jenni, Deboer, & Achermann, 2006; Kleitman & Engelmann, 1953).
Although twitching across the body might have increased and decreased in unison, it did not. Instead, twitching in different segments of the body—even within a limb—exhibited segment-specific patterns. Specifically, rates of twitching in the head and face declined over the first few postnatal months; the decrease in head twitches appears to coincide with the increased ability of infants to control head movements (Lee & Galloway, 2012; Prechtl, 2001). Moreover, whereas rates of twitching of the arms and legs remained relatively low across age, rates of twitching of the hands and feet occurred at much higher rates. In part, higher rates of twitching in the distal parts of infants’ limbs could reflect the fact that there are more movable parts in the hands and feet than arms and legs. Alternatively, increased rates of twitching in the hands might reflect the increased need to develop and maintain fine neural control of fingers. That feet exhibit similar rates of twitching as hands may relate to such extreme forms of developmental plasticity whereby infants born without arms develop hand-like motor skills with their feet and associated somatotopic “toe maps” (Blumberg, 2009; Dempsey-Jones, Wesselink, Friedman, & Makin, 2019).
We also found that, within a 10-s window, twitches tended to recur within the same body segment (e.g., multiple twitches of the right hand) or co-occur in contralateral body segments (e.g., twitches of the right and left hand; see Figure 7). In previous studies using infant rats and mice, we saw similar biases toward repeated twitching within a limb segment and strong coupling across the right and left sides of the body, both of which suggest the contribution of spinal circuitry to bouts of twitches (Blumberg et al., 2015; Blumberg, Coleman, Gerth, & McMurray, 2013a).
Finally, the present data indicate that the vast majority of twitches occur in bursts (with ITIs less than 10 s) and that these bursts often occur in conjunction with rapid eye movements, suggestive of active sleep. But many twitches also occur at much longer ITIs, with the proportion of such twitches increasing after 2 months of age (see Figure 4); these less-clustered movements may be less closely associated with active sleep. By combining precise assessments of twitching with electrographic measures of sleep, we expect that the relation between twitching and the sleep substate will be clarified.
Twitching in the craniofacial system
Twitching during active sleep is a characteristic of all skeletal muscle. This includes REMs, which are produced by twitches of the extraocular muscles (Chase & Morales, 1983; Seelke, Karlsson, Gall, & Blumberg, 2005). Although we did not quantify REMs for all infants in this study, we often observed them occurring during periods of twitching elsewhere in the face and body (see Figure 3), which is consistent with early observations of active sleep in human infants (Aserinsky & Kleitman, 1955). REMs are often interpreted as evidence of people “scanning” their dreams (Leclair-Visonneau, Oudiette, Gaymard, Leu-Semenescu, & Arnulf, 2010), similar to how limb twitches have been interpreted as by-products of dreams ((Blumberg & Plumeau, 2016).
Twitches of the face—the mouth, cheeks, and brows—were most prevalent at earlier ages. Occasionally, facial twitches occur bilaterally to give the impression of smiles or frowns (Dondi et al., 2007; Emde & Koenig, 1969; Emde, McCartney, & Harmon, 1971; Messinger et al., 2002); similarly, some unilateral twitches can give the impression of a smirks. However, the fact that various kinds of facial twitches occur contemporaneously with twitches elsewhere in the body suggests that all these movements belong together within the broader category of phasic activity during active sleep. Thus, although facial twitches may reflect a sensorimotor system that is developing the capacity to make emotional expressions, categorizing these infant movements in terms of emotional expression may not be ideal for understanding them.
Comparative aspects: Physiological time and the quantity of input
Although behavioral states can be defined similarly in human infants and rat pups, the two species exhibit pronounced differences in the temporal structure of their sleep and wake activities. For example, whereas a 2-week-old human infant might transition from wake to sleep once every four minutes (see Figure 2B), 2- to 8-day-old rats transition from wake to sleep several times per minute (Blumberg, Seelke, Lowen, & Karlsson, 2005).
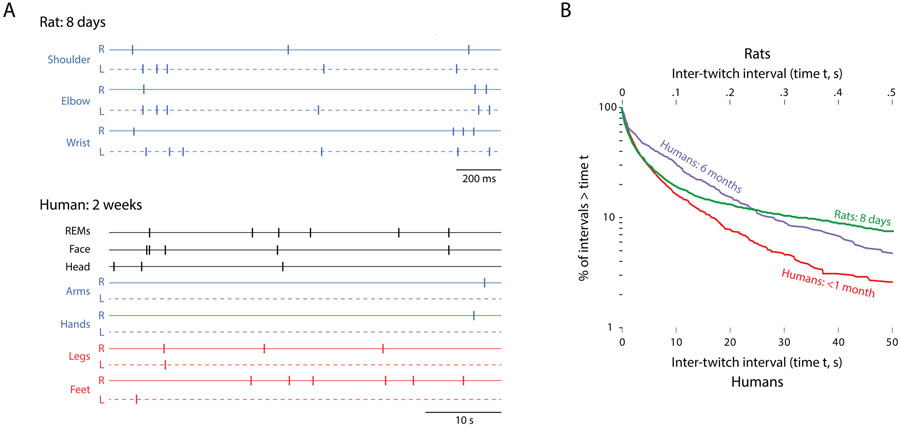
Differences in the temporal scale of behavior are even more striking when twitching in human infants (Supplemental Video 1) is compared with twitching in newborn rats (Blumberg, Coleman, Gerth, & McMurray, 2013a). Figure 8A compares 53 s of twitching in a two-week-old human infant with 3.5 s of twitching in an 8-day-old rat (from (Blumberg, Coleman, Gerth, & McMurray, 2013a). At these different time scales, twitching occurs at roughly the same rate. To provide a more quantitative comparison, in Figure 8B we have replotted the log-survivor analysis of ITIs for infants at <1 month and at 6 months of age (from Figure 4) along with a similar plot of ITI data for 8-day-old rats from a previous study (Blumberg, Coleman, Gerth, & McMurray, 2013a). Note how the ITI distributions for the rat and the human infant at 2 weeks of age almost perfectly overlap for over 80% of the intervals before they begin to diverge.
Figure 8.
(A) Top: Spatiotemporal patterning of twitching in an 8-day-old rat pup. Data for right (R) and left (L) limbs are shown. Bottom: Same as in (A) but for twitches across more areas of the body in a 2-week-old human infant. Note the different time scales: The duration of the entire segment for the rat is 3.5 s and that for the human is 53 s. (B) Log-survivor plots of inter-twitch intervals (ITIs) for rats at 8-days of age (5,139 twitches) and humans at <1 month and 6 months of age (replotted from Figure 4). Note that the x-axis values differ by two orders of magnitude for the two species. The distribution patterns for the rats and humans at <1-month are nearly identical over 80-90% of ITIs. Rat data are from Blumberg et al., 2013a.
In addition to the lower absolute rate of twitching in human infants, individual twitches exhibit longer durations in humans (~500 ms) than in rat pups (~50 ms). Differences in twitch duration help to account for the perception that twitching in human infants more closely resembles that in rat pups when playback speed is increased (Supplemental Video 2).
These collective observations are consistent with the concept of physiological time, which captures the notion that small animals with high metabolic rates live faster lives than large animals with low metabolic rates (Lindstedt & Calder, 1981; Schmidt-Nielsen, 1984). Accordingly, if twitches provide opportunities for infants to learn about their bodies (Blumberg & Dooley, 2017), and if large-bodied human infants pace their lives more slowly than small-bodied rat pups, then we would expect human infants to accumulate twitch-related experience more slowly than rat pups; after all, humans reach critical developmental milestones much more slowly than rats (Clancy, Darlington, & Finlay, 2001; Workman, Charvet, Clancy, Darlington, & Finlay, 2013).
We estimated that infant rats produce hundreds of thousands of twitches each day (Blumberg, Marques, & Iida, 2013b). Given the rate of twitching found here (~10/minute for the face, head, and limbs), and the number of hours of active sleep per day in newborns (8), we estimate that human newborns exhibit ~4800 twitches per day. Accordingly, twitches could comprise an abundant source of sensory experience to the developing sensorimotor system, akin to the abundant quantity of input that developmental psychologists point to when explaining the emergence of such skills as walking (Adolph et al., 2012) and word learning (Hart & Risley, 1995).
Limitations of this study
This study had several important limitations. First, although we relied on twitching to define bouts of active sleep, the presence of twitching alone may not be sufficient to identify that state. Accordingly, to better understand the association between twitching and active sleep across early human development, future studies should seek to associate the rate and patterning of twitching with such physiological measures as EEG activity and respiration (Isler, Thai, Myers, & Fifer, 2016).
Second, infants in our study slept in a semi-reclined supine position. This particular posture could have biased the types of proximal and distal limb movements that could be detected and perhaps altered the relative rates of twitching in various body segments. The effect of posture on limb twitches should also be examined in future studies. Finally, sleep was monitored exclusively during the day for relatively brief periods of time: Longer-term monitoring of twitches across the day and night and across ages when sleep bouts consolidate during the night will broaden our perspective on the regulation and functions of twitch movements.
Broader implications and future directions
This investigation of twitching in human infants, combined with recent advances in understanding the spatiotemporal dynamics and neurophysiological causes and consequences of twitching in infant rodents (Blumberg & Dooley, 2017; Del Rio-Bermudez & Blumberg, 2018), leads us to believe that increased attention to sleep and sleep-related twitching has the potential to open new avenues to understanding typical development. Such attention may also facilitate improved practices in neonatal intensive care units (NICUs), in which ambient conditions (e.g., lighting, sound) are often not conducive to sleep (Lai & Bearer, 2008). Given that sleep provides a critical context for sensory processing and functional connectivity in early development (Del Rio-Bermudez & Blumberg, 2018; Del Rio-Bermudez, Kim, Sokoloff, & Blumberg, 2020), minimizing sleep disturbances in the NICU could mitigate the negative effects of premature birth on sensory processing (Ryckman, Hilton, Rogers, & Pineda, 2017) and functional connectivity (Rogers, Lean, Wheelock, & Smyser, 2018).
The present findings may also open new paths to understanding the close links between cognitive and motor development (Diamond, 2000; Mittal, Neumann, Saczawa, & Walker, 2008) and autism (Dawson et al., 2018; Diamond, 2000; Esposito & Paşca, 2013; Green et al., 2009; Jansiewicz et al., 2006; Mittal & Walker, 2007; Nebel et al., 2014; Whyatt & Craig, 2013). Indeed, movements produced spontaneously by awake infants can provide a diagnostic tool for the functional assessment of the infant nervous system (Einspieler & Prechtl, 2005; Ferrari et al., 2002; Hadders-Algra, 2007; P. Teitelbaum, Teitelbaum, Nye, Fryman, & Maurer, 1998). We propose that measures of twitching can complement and broaden such efforts by providing a sensitive tool for assessing sensorimotor development in typical and atypical human populations.
Many questions remain: Are there reliable individual differences in the rate and patterning of twitching and are they associated with individual differences in emerging motor skills? Does twitching vary substantially across the day and night, especially over the first several postnatal months as circadian sleep rhythmicity emerges? Is premature birth associated with decrements in twitching? Can we detect cortical or subcortical activity associated with twitching beyond the early postnatal period? Answering these and other questions will be necessary to realize the diagnostic and explanatory potential of myoclonic twitching in early development and across the lifespan.
Supplementary Material
Supplemental Video 1. Twitching in a 2-week-old infant played back in real-time and at 3x and 10x speeds.
Supplemental Video 2. Comparison between twitching in a 2-week-old human played back at 10x speed and an 8-day-old rat played back in real-time. The white dots on the rat pup were used to track limb movements (see Blumberg et al., 2013a).
Acknowledgments.
We thank the infants and parents who participated in this study, which was supported by a Grand Challenges Exploration Grant from the Bill & Melinda Gates Foundation and a grant from National Institute of Child Health and Human Development (HD095153) to M.S.B. We thank Karen Adolph, Jimmy Dooley, and Kim Whitehead for many helpful comments, and Jimmy Dooley and Toby Mordkoff for technical assistance.
Data availability.
Behaviorally coded data and a subset of videos are shared on Databrary (https://nyu.databrary.org/volume/174).
References
- Adolph KE, Cole WG, Komati MM, Garciaguirre JS, Badaly DD, Lingeman JM, Chan GLY, et al. (2012). How do you learn to walk? Thousands of steps and dozens of falls per day. Psychological Science, 23, 1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aserinsky E, & Kleitman N (1955). A motility cycle in sleeping infants as manifested by ocular and gross bodily activity. Journal of Applied Physiology, 8, 11–18. [DOI] [PubMed] [Google Scholar]
- Blumberg MS (2009). Freaks of nature: What anomalies tell us about development and evolution. New York: Oxford University Press. [Google Scholar]
- Blumberg MS (2010). Beyond dreams: Do sleep-related movements contribute to brain development? Frontiers in Neurology, 1, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS (2015). Developing sensorimotor systems in our sleep. Current Directions in Psychological Science, 24, 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg M (2015). Spatiotemporal organization of myoclonic twitching in sleeping human infants. Databrary. Retrieved January 15, 2020 from https://nyu.databrary.org/volume/174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, & Dooley JC (2017). Phantom limbs, neuroprosthetics, and the developmental origins of embodiment. Trends in Neurosciences, 40, 603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, & Plumeau AM (2016). A new view of “dream enactment” in REM sleep behavior disorder. Sleep Medicine Reviews, 39, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, & Rattenborg NC (2017). Decomposing the evolution of sleep: Comparative and developmental approaches. In Kaas JH (Ed.), Evolution of Nervous Systems (Vol. 3, pp. 523–545). Oxford: Elsevier. [Google Scholar]
- Blumberg MS, Coleman CM, Gerth AI, & McMurray B (2013a). Spatiotemporal structure of REM sleep twitching reveals developmental origins of motor synergies. Current Biology, 23, 2100–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Coleman CM, Sokoloff G, Weiner JA, Fritzsch B, & McMurray B (2015). Development of twitching in sleeping infant mice depends on sensory experience. Current Biology, 25, 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Marques HG, & Iida F (2013b). Twitching in sensorimotor development from sleeping rats to robots. Current Biology, 23, R532–R537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Seelke AMH, Lowen SB, & Karlsson KE (2005). Dynamics of sleep-wake cyclicity in developing rats. Proceedings of the National Academy of Sciences of the United States of America, 102, 14860–14864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraux G, & Pinloche S (2005). PermutMatrix: a graphical environment to arrange gene expression profiles in optimal linear order. Bioinformatics, 21, 1280–1281. [DOI] [PubMed] [Google Scholar]
- Chase M, & Morales F (1983). Subthreshold excitatory activity and motoneuron discharge during REM periods of active sleep. Science, 221, 1195–1198. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, & Finlay BL (2001). Translating developmental time across mammalian species. Neuroscience, 105, 7–17. [DOI] [PubMed] [Google Scholar]
- Coons S, & Guilleminault C (1982). Development of sleep-wake patterns and non-rapid eye movement sleep stages during the first six months of life in normal infants. Pediatrics, 69, 793–798. [PubMed] [Google Scholar]
- Curzi-Dascalova L, Peirano P, & Morel-Kahn F (1988). Development of sleep states in normal premature and full-term newborns. Developmental Psychobiology, 21, 431–444. [DOI] [PubMed] [Google Scholar]
- Dawson G, Campbell K, Hashemi J, Lippmann SJ, Smith V, Carpenter K, Egger H, et al. (2018). Atypical postural control can be detected via computer vision analysis in toddlers with autism spectrum disorder. Scientific Reports, 8, 17008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio-Bermudez C, & Blumberg MS (2018). Active sleep promotes functional connectivity in developing sensorimotor networks. BioEssays, 304, 1700234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio-Bermudez C, Kim J, Sokoloff G, & Blumberg MS (2020). Active sleep promotes coherent oscillatory activity in the cortico-hippocampal system of infant rats. Cerebral Cortex, 23, R774–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio-Bermudez C, Sokoloff G, & Blumberg MS (2015). Sensorimotor processing in the newborn rat red nucleus during active sleep. Journal of Neuroscience, 35, 8322–8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey-Jones H, Wesselink DB, Friedman J, & Makin TR (2019). Organized toe maps in extreme foot users. Cell Reports, 28, 2748–2756.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisova K (2019). Age attenuates noise and increases symmetry of head movements during sleep resting-state fMRI in healthy neonates, infants, and toddlers. Infant Behavior and Development, 57, 101317–10. [DOI] [PubMed] [Google Scholar]
- Denisova K, & Zhao G (2017). Inflexible neurobiological signatures precede atypical development in infants at high risk for autism. Scientific Reports, 7, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A (2000). Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Development, 71, 44–56. [DOI] [PubMed] [Google Scholar]
- Dondi M, Messinger D, Colle M, Tabasso A, Simion F, Barba BD, & Fogel A (2007). A new perspective on neonatal smiling: differences between the judgments of expert coders and naive observers. Infancy, 12, 235–255. [Google Scholar]
- Dooley JC, & Blumberg MS (2018). Developmental “awakening” of primary motor cortex to the sensory consequences of movement. eLife, 7, e41841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einspieler C, & Prechtl H (2005). Prechtl's assessment of general movements: A diagnostic tool for the functional assessment of the young nervous system. Mental Retardation and Developmental Disabilities Research Reviews, 11, 61–67. [DOI] [PubMed] [Google Scholar]
- Emde RN, & Koenig KL (1969). Neonatal smiling, frowning, and rapid eye movement states. Journal of the American Academy of Child Psychiatry, 8(4), 637–656. [DOI] [PubMed] [Google Scholar]
- Emde RN, McCartney RD, & Harmon RJ (1971). Neonatal smiling in REM states, IV: Premature study. Child Development, 42, 1657–1661. [PubMed] [Google Scholar]
- Esposito G, & Paşca SP (2013). Motor abnormalities as a putative endophenotype for Autism Spectrum Disorders. Frontiers in Integrative Neuroscience, 7, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari F, Cioni G, Einspieler C, Roversi MF, Bos AF, Paolicelli PB, Ranzi A, et al. (2002). Cramped synchronized general movements in preterm infants as an early marker for cerebral palsy. Archives of Pediatrics and Adolescent Medicine, 156, 460–467. [DOI] [PubMed] [Google Scholar]
- Frank MG, Issa NP, & Stryker MP (2001). Sleep enhances plasticity in the developing visual cortex. Neuron, 30, 275–287. [DOI] [PubMed] [Google Scholar]
- Green D, Charman T, Pickles A, Chandler S, Loucas T, Simonoff E, & Baird G (2009). Impairment in movement skills of children with autistic spectrum disorders. Developmental Medicine and Child Neurology, 51, 311–316. [DOI] [PubMed] [Google Scholar]
- Grieve PG, Isler JR, Izraelit A, Peterson BS, Fifer WP, Myers MM, & Stark RI (2008). EEG functional connectivity in term age extremely low birth weight infants. Clinical Neurophysiology, 119, 2712–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadders-Algra M (2007). Putative neural substrate of normal and abnormal general movements. Neuroscience & Biobehavioral Reviews, 31, 1181–1190. [DOI] [PubMed] [Google Scholar]
- Hadders-Algra M, Nakae Y, Van Eykern LA, Klip-Van den Nieuwendijk AW, & Prechtl HF (1993). The effect of behavioural state on general movements in healthy full-term newborns. A polymyographic study. Early Human Development, 35, 63–79. [DOI] [PubMed] [Google Scholar]
- Hart B, & Risley TR (1995). Meaningful differences in the everyday experience of young American children. Baltimore: Paul H. Brookes Publishing Company. [Google Scholar]
- Holditch-Davis D, Scher M, Schwartz T, & Barr DH (2004). Sleeping and waking state development in preterm infants. Early Human Development, 80, 43–64. [DOI] [PubMed] [Google Scholar]
- Inácio AR, Nasretdinov A, Lebedeva J, & Khazipov R (2016). Sensory feedback synchronizes motor and sensory neuronal networks in the neonatal rat spinal cord. Nature Communications, 7, 13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isler JR, Thai T, Myers MM, & Fifer WP (2016). An automated method for coding sleep states in human infants based on respiratory rate variability. Developmental Psychobiology, 58, 1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla MB, Landa R, & Mostofsky SH (2006). Motor signs distinguish children with high functioning autism and Asperger’s Syndrome from controls. Journal of Autism and Developmental Disorders, 36, 613–621. [DOI] [PubMed] [Google Scholar]
- Jenni OG, Borbély AA, & Achermann P (2004). Development of the nocturnal sleep electroencephalogram in human infants. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 286, R528–38. [DOI] [PubMed] [Google Scholar]
- Jenni OG, Deboer T, & Achermann P (2006). Development of the 24-h rest-activity pattern in human infants. Infant Behavior and Development, 29, 143–152. [DOI] [PubMed] [Google Scholar]
- Jensen JL, Schneider K, Ulrich BD, Zernicke RF, & Thelen E (2010). Adaptive dynamics of the leg movement patterns of human infants: I. The effects of posture on spontaneous kicking. Journal of Motor Behavior, 26, 303–312. [DOI] [PubMed] [Google Scholar]
- Jouvet-Mounier D, Astic L, & Lacote D (1970). Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Developmental Psychobiology, 2, 216–239. [DOI] [PubMed] [Google Scholar]
- Karlsson KE, Mohns EJ, Vianna di Prisco G, & Blumberg MS (2006). On the co-occurrence of startles and hippocampal sharp waves in newborn rats. Hippocampus, 16, 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, & Buzsáki G (2004). Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature, 432, 758–761. [DOI] [PubMed] [Google Scholar]
- Kleitman N, & Engelmann T (1953). Sleep characteristics of infants, 6, 269–282. [DOI] [PubMed] [Google Scholar]
- Kohyama J (1996). A quantitative assessment of the maturation of phasic motor inhibition during REM sleep. Journal of the Neurological Sciences, 143, 150–155. [DOI] [PubMed] [Google Scholar]
- Lai TT, & Bearer CF (2008). Iatrogenic environmental hazards in the neonatal intensive care unit. Clinics in Perinatology, 35, 163–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclair-Visonneau L, Oudiette D, Gaymard B, Leu-Semenescu S, & Arnulf I (2010). Do the eyes scan dream images during rapid eye movement sleep? Evidence from the rapid eye movement sleep behaviour disorder model. Brain, 133, 1737–1746. [DOI] [PubMed] [Google Scholar]
- Lee HM, & Galloway JC (2012). Early intensive postural and movement training advances head control in very young infants. Physical Therapy, 92, 935–947. [DOI] [PubMed] [Google Scholar]
- Lindstedt S, & Calder WA III. (1981). Body size, physiological time, and longevity of homeothermic animals, 56, 1–16. [Google Scholar]
- Marks G, Shaffery J, Oksenberg A, Speciale S, & Roffwarg H (1995). A functional role for REM sleep in brain maturation. Behavioural Brain Research, 69, 1–11. [DOI] [PubMed] [Google Scholar]
- Messinger D, Dondi M, Nelson-Goens G, Beghi A, Fogel A, & Simion F (2002). How sleeping neonates smile. Developmental Neuropsychology, 5, 48–54. [Google Scholar]
- Milh M, Kaminska A, Huon C, Lapillonne A, Ben-Ari Y, & Khazipov R (2007). Rapid cortical oscillations and early motor activity in premature human neonate. Cerebral Cortex, 17, 1582–1594. [DOI] [PubMed] [Google Scholar]
- Mirmiran M (1995). The function of fetal/neonatal rapid eye movement sleep. Behavioural Brain Research, 69, 13–22. [DOI] [PubMed] [Google Scholar]
- Mittal VA, & Walker EF (2007). Movement abnormalities predict conversion to Axis I psychosis among prodromal adolescents. Journal of Abnormal Psychology, 116, 796–803. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Neumann C, Saczawa M, & Walker EF (2008). Longitudinal progression of movement abnormalities in relation to psychotic symptoms in adolescents at high risk of schizophrenia. Archives of General Psychiatry, 65, 165–171. [DOI] [PubMed] [Google Scholar]
- Nebel MB, Joel SE, Muschelli J, Barber AD, Caffo BS, Pekar JJ, & Mostofsky SH (2014). Disruption of functional organization within the primary motor cortex in children with autism. Human brain mapping, 35, 567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmelee AH (1961). Sleep patterns in infancy: A study of one infant from birth to 8 months of age. Acta Pædiatrica, 50, 160–170. [DOI] [PubMed] [Google Scholar]
- Parmelee A, Wenner W, Akiyama Y, Schultz M, & Stern E (1967). Sleep states in premature infants. Developmental Medicine and Child Neurology, 9, 70–77. [DOI] [PubMed] [Google Scholar]
- Petersson P, Waldenström A, Fåhraeus C, & Schouenborg J (2003). Spontaneous muscle twitches during sleep guide spinal self-organization. Nature, 424, 72–75. [DOI] [PubMed] [Google Scholar]
- Piek JP, & Carman R (1994). Developmental profiles of spontaneous movements in infants. Early Human Development, 39, 109–126. [DOI] [PubMed] [Google Scholar]
- Prechtl HFR (1974). The behavioural states of the newborn infant. Brain Research, 76, 185–212. [DOI] [PubMed] [Google Scholar]
- Prechtl HFR (2001). Prenatal and early postnatal development of human motor behavior. In Kalverboer A & Gramsbergen A (Eds.), Handbook of brain and behaviour in human development (pp. 415–428). Norwell, MA. [Google Scholar]
- Roffwarg HP, Muzio JN, & Dement WC (1966). Ontogenetic development of the human sleep-dream cycle. Science, 152, 604–619. [DOI] [PubMed] [Google Scholar]
- Rogers CE, Lean RE, Wheelock MD, & Smyser CD (2018). Aberrant structural and functional connectivity and neurodevelopmental impairment in preterm children. Journal of Neurodevelopmental Disorders, 10, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryckman J, Hilton C, Rogers C, & Pineda R (2017). Sensory processing disorder in preterm infants during early childhood and relationships to early neurobehavior. Early Human Development, 113, 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite FE (1978). Tests of hypotheses in fixed-effects linear models. In SAS Technical Report. Cary, NC: SAS Institute, Inc. [Google Scholar]
- Schmidt-Nielsen K (1984). Scaling: Why is animal size so important? Cambridge: Cambridge University Press. [Google Scholar]
- Seelke AMH, Karlsson KE, Gall AJ, & Blumberg MS (2005). Extraocular muscle activity, rapid eye movements and the development of active and quiet sleep. European Journal of Neuroscience, 22, 911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu A, & Himwich HE (1968). The ontogeny of sleep in kittens and young rabbits. Electroencephalography and Clinical Neurophysiology, 24(4), 307–318. [PubMed] [Google Scholar]
- Tarullo AR, Balsam PD, & Fifer WP (2010). Sleep and infant learning. Infant and Child Development, 20, 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum P, Teitelbaum O, Nye J, Fryman J, & Maurer RG (1998). Movement analysis in infancy may be useful for early diagnosis of autism. Proceedings of the National Academy of Sciences of the United States of America, 95, 13982–13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen E (1979). Rhythmical stereotypies in normal human infants. Animal Behaviour, 27, 699–715. [DOI] [PubMed] [Google Scholar]
- Thoman EB (1990). Sleeping and waking states in infants: a functional perspective. Neuroscience & Biobehavioral Reviews, 14, 93–107. [DOI] [PubMed] [Google Scholar]
- Thoman EB, & Whitney MP (1989). Sleep states of infants monitored in the home: Individual differences, developmental trends, and origins of diurnal cyclicity. Infant Behavior and Development, 12, 59–75. [Google Scholar]
- Thoman EB, Davis DH, & Denenberg VH (1987). The sleeping and waking states of infants: correlations across time and person. Physiology and Behavior, 41, 531–537. [DOI] [PubMed] [Google Scholar]
- Tiriac A, & Blumberg MS (2016). Gating of reafference in the external cuneate nucleus during self-generated movements in wake but not sleep. eLife, 5, e18749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiriac A, Del Rio-Bermudez C, & Blumberg MS (2014). Self-generated movements with “unexpected” sensory consequences. Current Biology, 24, 2136–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhatalo S, & Kaila K (2006). Development of neonatal EEG activity: from phenomenology to physiology. Seminars in fetal & neonatal medicine, 11, 471–478. [DOI] [PubMed] [Google Scholar]
- Whitehead K, Meek J, & Fabrizi L (2018). Developmental trajectory of movement-related cortical oscillations during active sleep in a cross-sectional cohort of pre-term and full-term human infants. Scientific Reports, 8, 111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead K, Slobodina M, Meek J, & Fabrizi L (2019). Fronto-central slow cortical activity is attenuated during phasic events in rapid eye movement sleep at full-term birth. Early Human Development, 136, 45–48. Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt C, & Craig C (2013). Sensory-motor problems in Autism. Frontiers in Integrative Neuroscience, 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman AD, Charvet CJ, Clancy B, Darlington RB, & Finlay BL (2013). Modeling transformations of neurodevelopmental sequences across mammalian species. Journal of Neuroscience, 33, 7368–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video 1. Twitching in a 2-week-old infant played back in real-time and at 3x and 10x speeds.
Supplemental Video 2. Comparison between twitching in a 2-week-old human played back at 10x speed and an 8-day-old rat played back in real-time. The white dots on the rat pup were used to track limb movements (see Blumberg et al., 2013a).
Data Availability Statement
Behaviorally coded data and a subset of videos are shared on Databrary (https://nyu.databrary.org/volume/174).