Abstract
Background
Factors affecting outcomes of SARS-CoV-2 infection in people living with HIV are unclear. We assessed the factors associated with SARS-CoV-2 diagnosis and severe outcomes among people living with HIV.
Methods
We did a retrospective cohort study using data from the PISCIS cohort of people with HIV in Catalonia (Spain) between March 1 and Dec 15, 2020. We linked PISCIS data with integrated health-care, clinical, and surveillance registries through the Public Data Analysis for Health Research and Innovation Program of Catalonia (PADRIS) to obtain data on SARS-CoV-2 diagnosis, chronic comorbidities, as well as clinical and mortality outcomes. Participants were aged at least 16 years in care at 16 hospitals in Catalonia. Factors associated with SARS-CoV-2 diagnoses and severe outcomes were assessed using univariable and multivariable Cox regression models. We estimated the effect of immunosuppression on severe outcomes (hospital admission for >24 h with dyspnoea, tachypnoea, hypoxaemia, asphyxia, or hyperventilation; or death) using Kaplan-Meier survival analysis.
Findings
We linked 20 847 (72·8%) of 28 666 participants in the PISCIS cohort with PADRIS data; 13 142 people had HIV. 749 (5·7%) people with HIV were diagnosed with SARS-CoV-2: their median age was 43·5 years (IQR 37·0–52·7), 131 (17·5%) were female, and 618 (82·5%) were male. 103 people with HIV (13·8%) were hospitalised, seven (0·9%) admitted to intensive care, and 13 (1·7%) died. SARS-CoV-2 diagnosis was more common among migrants (adjusted hazard ratio 1·55, 95% CI 1·31–1·83), men who have sex with men (1·42, 1·09–1·86), and those with four or more chronic comorbidities (1·46, 1·09–1·97). Age at least 75 years (5·2, 1·8–15·3), non-Spanish origin (2·1, 1·3–3·4), and neuropsychiatric (1·69, 1·07–2·69), autoimmune disease (1·92, 1·14–3·23), respiratory disease (1·84, 1·09–3·09), and metabolic disease (2·59, 1·59–4·23) chronic comorbidities were associated with increased risk of severe outcomes. A Kaplan-Meier estimator showed differences in the risk of severe outcomes according to CD4 cell count in patients with detectable HIV RNA (p=0·039) but no differences were observed in patients with undetectable HIV RNA (p=0·15).
Interpretation
People living with HIV with detectable HIV viraemia, chronic comorbidities, and some subpopulations could be at increased risk of severe outcomes from COVID-19. These groups should be prioritised in clinical management and SARS-CoV-2 vaccination programmes.
Funding
Fundació “la Caixa”.
Translations
For the Catalan, Spanish and Russian translations of the Summary see Supplementary Materials section.
Introduction
At the beginning of the COVID-19 pandemic, people living with HIV, particularly those with low CD4 cell count, detectable HIV RNA viral load, chronic comorbidities, and not receiving antiretroviral therapy (ART), were speculated to be at an increased risk of severe disease from the co-infection.1, 2, 3 The first published case series and cohort studies on the incidence of COVID-19 or adverse outcomes among people with HIV, however, showed contradictory results and remains a matter of debate.4 In fact, reports suggested that high HIV viraemia and low CD4 cell count might restrict COVID-19-associated immune dysregulation and diminish the development of a cytokine storm, hence, minimising disease severity.5 The potential protection from some anti-HIV agents has also been postulated by different studies.6 The therapeutic effects of these medications against COVID-19 are, however, inconclusive.6
Nevertheless, a report from the WHO Global Clinical Platform for COVID-19 involving 37 countries indicated that HIV is an independent risk factor for severe or critical presentation at hospital admission and in-hospital mortality.7 This finding is similar to those from the UK8 and South Africa,9 reporting increased mortality risk for patients co-infected with HIV and SARS-CoV-2.
The potential effect of immunosuppression and unsuppressed HIV RNA on severe COVID-19 outcomes has become a matter of concern.10 A pooled analysis of a combined group of 175 patients with both HIV and SARS-CoV-2 from Germany, Italy, and Spain identified an association between low CD4 count (<350 cells per μL) and severe COVID-19 (adjusted odds ratio 2·85, 95% CI 1·26–6·44; p=0·01).11 In this study, a lower nadir CD4 cell count was associated with increased mortality risk.11 The study from South Africa reported a similar increased risk of mortality for co-infected patients with CD4 counts less than 200 cells per μL.9 This study did not, however, capture data on socioeconomic status and obesity, both of which can be associated with increased mortality. Little is known about the effect of detectable HIV viral load on patients with severe COVID-19. An analysis by Tesoriero and colleagues in New York (NY, USA) showed that co-infected patients with detectable HIV viraemia were 30% more likely to be hospitalised, compared with those not living with an HIV diagnosis.12
Research in context.
Evidence before this study
We searched PubMed, Embase, MEDLINE, medRxiv, and Google Scholar using the search terms “SARS-CoV-2” or “COVID-19” or “coronavirus disease 2019” and “HIV” or “AIDS” or “human immunodeficiency virus” from Jan 1 to Oct 30, 2020, without language restrictions. The published data was inconclusive regarding an increased risk of people living with HIV to SARS-CoV-2 infection. The largest cohort study of SARS-CoV-2 infection among people with HIV in Europe (77 590 people with HIV) found a higher incidence than in the general population (3·7 per 10 000 vs 2·1 per 10 000). Factors associated with SARS-CoV-2 diagnoses have not, however, been assessed in large cohort studies of people with HIV. Three large studies subsequently suggested an increased incidence of severe COVID-19 outcomes for people with HIV. There were, however, common limitations, such as an inadequate adjustment for potentially confounding variables including socioeconomic factors, comorbidities, and immunological markers (CD4 cell count and plasma HIV RNA viral load). Whether immunosuppression and unsuppressed HIV viraemia are associated with severe COVID-19 outcomes is unknown. In a large study from South Africa, lower CD4 cell count was associated with higher mortality, but CD4 cell count was only measured during the presence of SARSCoV-2 co-infection and could be misleading.
Added value of the study
In this observational cohort study, we investigated SARS-CoV-2 diagnosis and severe outcomes in a cohort of people with HIV. To the best of our knowledge, this study of 749 people with HIV with SARS-CoV-2 co-infection is the largest in Europe. 103 (13·8%) co-infected patients were hospitalised and 13 (1·7%) died. All SARS-CoV-2 diagnoses were laboratory confirmed. SARS-Cov-2 diagnoses were increased among migrants, men who have sex with men (MSM), and those with four or more comorbidities. Being aged 75 years or older, of non-Spanish origin, with chronic comorbidities, and detectable HIV viraemia were associated with severe COVID-19.
Implications of all the available evidence
Our findings are incongruous with those of previous studies, which observed reduced SARS-CoV-2 diagnosis rates among people with HIV. Factors such as non-Spanish origin, multimorbidity, and MSM were associated with SARS-CoV-2 diagnosis, and studies are needed to further explore this association. Our results also show that subpopulations (eg, migrants, people aged 75 years or older, and people with HIV with chronic comorbidities and unsuppressed HIV RNA viral load) could be at increased risk of severe COVID-19 outcomes and should be prioritised in terms of clinical management and SARS-CoV-2 vaccination programmes.
Moreover, COVID-19 might cause more adverse outcomes among people with HIV because of the increasing prevalence of comorbidities such as diabetes, chronic obstructive pulmonary disease, and chronic kidney disease in this population.13 The high presence of overlapping social determinants of health such as low socioeconomic status, sex, gender, racial and minority ethnic groups, and mental health burden might also contribute to more severe outcomes of COVID-19 among people with HIV.14
The existing controversy on the effect of SARS-CoV-2 on people with HIV is partly a result of study limitations, including inadequate sample sizes and an absence of data on key variables that potentially confound results (eg, socioeconomic factors, CD4 cell count, plasma HIV RNA concentrations, ART, and chronic comorbidities). Large population-based studies are imperative to assess whether people with HIV or subpopulations thereof are at increased risk of SARS-CoV-2 infection and severe COVID-19 disease after adjusting for potential confounders. Additional studies are also crucially needed to investigate whether immunosuppression and detectable HIV RNA are associated with severe COVID-19. To date, data available are inconclusive on these matters.10
In this study, we aimed to describe the sociodemographic and clinical characteristics associated with SARS-CoV-2 diagnosis and COVID-19 outcomes among people living with HIV in Catalonia (Spain), and assess the risk factors for severe disease prognosis in a multicentre population-based cohort of people with HIV (Populational HIV Cohort from Catalonia and Balearic Islands [PISCIS]).
Methods
Study design and population
We did an observational retrospective cohort study using the PISCIS cohort of people with HIV in Catalonia, Spain, between March 1 and Dec 15, 2020. The design of the PISCIS cohort has been described elsewhere.15 Briefly, PISCIS is a population-based cohort of people with HIV aged at least 16 years in care at 16 hospitals in Catalonia. The cohort has collected sociodemographic and clinical data since Jan 1, 1998.
We linked PISCIS data with integrated health-care, clinical, and surveillance registries through the Public Data Analysis for Health Research and Innovation Program of Catalonia (PADRIS) to obtain data on SARS-CoV-2 diagnosis, chronic comorbidities, as well as clinical and mortality outcomes (appendix 4 p 1). PADRIS encompasses patient-level information on overall public health-care usage including primary, emergency, outpatient, and inpatient care, laboratory and diagnostic tests, pharmacy, and disease surveillance registries.16 We excluded patients who were recorded dead before Feb 1, 2020.
The study was done in accordance with the Declaration of Helsinki and adhered to existing legislation on data protection. The PISCIS cohort study has been approved by the Institutional Review Board of Germans Trias i Pujol Hospital, Badalona, Spain. All participating patients have signed informed consent forms. Patient information obtained from PADRIS was anonymised and de-identified before analysis.
Procedures
SARS-CoV-2 diagnoses, hospital admissions, and mortality were assessed among people with HIV in the PISCIS-PADRIS cohort. SARS-CoV-2 diagnosis was defined by a nucleic acid amplification test or antigen detection from respiratory samples, or antibodies detection according to the Spanish Ministry of Health guidelines.17
Severe COVID-19 outcome was defined as hospitalisation (admission for >24 h with any of the following signs: dyspnoea, tachypnoea, hypoxaemia, asphyxia, or hyperventilation), admission to an intensive care unit (ICU), or death.
We extracted the most recent data on sociodemographic characteristics (age, sex, country of origin, and socioeconomic status), HIV-associated variables, chronic comorbidities, SARS-CoV-2 diagnosis, hospitalisation, and mortality. The HIV-associated variables analysed were years since diagnosis; transmission group (people who inject drugs [PWID]; men who have sex with men [MSM]; male heterosexual; female homosexual, heterosexual, or bisexual); years on ART; current ART; most recent CD4 count (<200 cells per μL, 200–499 cells per μL, or ≥500 cells per μL), and HIV RNA viral load (detectable and undetectable). Undetectable plasma viral load was defined as HIV RNA of 50 copies per mL or lower. Immunosuppression was defined as CD4 count of less than 200 cells per μL.
We used the International Classification of Diseases (ICD), ninth and tenth revisions, to extract chronic comorbidities. The ICD classification system was modified to group the most prevalent chronic comorbidities in our population (appendix 4 p 2).
Socioeconomic status was classified according to the socioeconomic deprivation level index created by the Catalan Government according to the basic health area of residence.18 This index is based on five indicators: proportion of manual workers, proportion of residents with low education level, proportion with low income, rate of premature mortality, and rate of avoidable hospitalisation.18 The index produces a continuous variable of 0 to 100, with zero being the lowest level and 100 being the highest.18 We divided our study population into a tertile, with the highest socioeconomic group being in the first tertile (least deprived) and the third tertile being the lowest (most deprived).
Statistical analysis
Categorical variables were expressed as counts and percentages and continuous variables were expressed as medians and IQRs. Proportions for categorical variables were compared using the χ2 or Fisher's exact test when appropriate. Continuous variables were compared with the Mann-Whitney U test. Univariable and multivariable Cox proportional hazards regression models were estimated to identify risk factors associated with SARS-CoV-2 diagnosis and severe COVID-19, providing hazard ratios (HRs) and unadjusted HRs with 95% CIs.
We assessed the effect of immunosuppression on severe outcomes stratifying by unsuppressed and suppressed plasma HIV viral load, using survival analysis techniques. We reported the survival probability during the study period using the Kaplan-Meier estimator. We censored patients who did not have a severe outcome by the end of the follow-up period. The differences between the curves were assessed with the log-rank test. Statistical significance was set at a p value of less than 0·05 (two-sided). All analyses were done with R (version 4.0.0).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
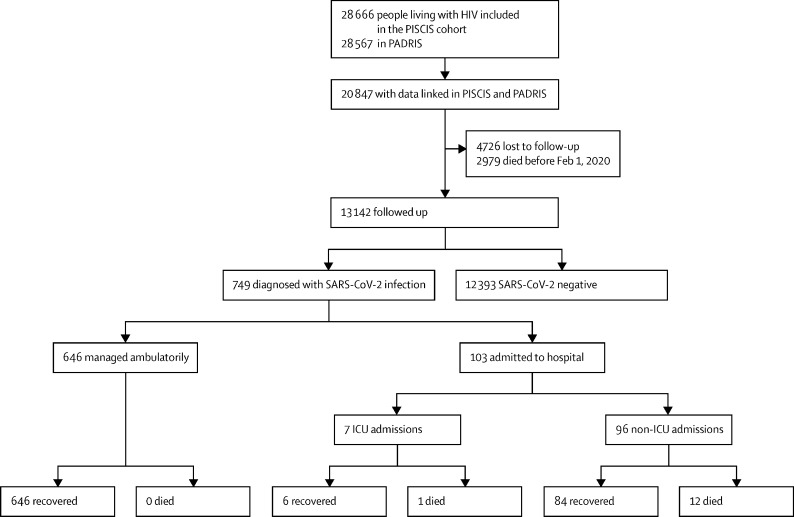
We linked 20 847 (72·8%) of 28 666 participants in the PISCIS cohort with PADRIS data. 749 (5·7%) of the 13 142 people with HIV who were followed up were diagnosed with SARS-CoV-2. 103 (13·8%) were hospitalised, seven (0·9%) were admitted to the ICU, and 13 (1·7%) died (figure 1 ).
Figure 1.
Patient flow diagram
ICU=intensive care unit. PADRIS=Public Data Analysis for Health Research and Innovation Program of Catalonia.
The median age was 43·5 years (IQR 37·0–52·7) for co-infected patients and 47·6 years (39·6–54·9) for the overall cohort. Among co-infected patients, 618 (82·5%) were male, 131 (17·5%) were female, 347 (46·3%) had non-Spanish origin, and 373 (49·8%) were in the least deprived socioeconomic stratum.
The median time since HIV diagnosis in the co-infected population was 10·3 years (IQR 6·1–15·7), 14·4 years (9·4–20·7) among hospitalised patients, and 17·4 years (12·7–21·7) among patients who died (table 1 ). The most frequently reported HIV transmission group among co-infected patients was MSM (table 1). 529 (70·9%) co-infected patients had a CD4 count of 500 cells per μL or greater and 622 (83·0%) had HIV viral suppression (table 1).
Table 1.
Characteristics of people with HIV with and without SARS-CoV-2 diagnosis, hospitalisation, and death
| People living with HIV (n=13 142) | SARS-CoV-2 negative (n=12 393) | SARS-CoV-2 positive (n=749) | Hospital admission (n=103) | Death (n=13) | ||
|---|---|---|---|---|---|---|
| Sex* | ||||||
| Male | 10 739 (81·7%) | 10 121 (81·7%) | 618 (82·5%) | 81 (78·6%) | 10 (76·9%) | |
| Female | 2402 (18·3%) | 2271 (18·2%) | 131 (17·5%) | 22 (21·4%) | 3 (23·1%) | |
| Unknown | 1 (<1%) | 1 (<1%) | 0 | 0 | 0 | |
| Age, years† | ||||||
| Median (IQR) | 47·6 (39·6–54·9) | 47·8 (39·8–55·0) | 43·5 (37·0–52·7) | 52·3 (43·4–59·8) | 60·2 (53·6–67·5) | |
| 16–39 | 3442 (26·2%) | 3181 (25·7%) | 261 (34·9%) | 16 (15·5%) | 1 (7·7%) | |
| 40–64 | 8870 (67·5%) | 8426 (68·0%) | 444 (59·3%) | 71 (68·9%) | 7 (53·9%) | |
| 65–74 | 643 (4·9%) | 610 (4·9%) | 33 (4·4%) | 10 (9·7%) | 3 (23·1%) | |
| ≥75 | 187 (1·4%) | 176 (1·4%) | 11 (1·5%) | 6 (5·8%) | 2 (15·4%) | |
| Place of birth‡ | ||||||
| Spain | 8564 (65·2%) | 8162 (65·9%) | 402 (53·7%) | 59 (57·3%) | 11 (84·6%) | |
| Outside Spain | 4576 (34·8%) | 4229 (34·1%) | 347 (46·3%) | 44 (42·7%) | 2 (15·4%) | |
| Unknown | 2 (<0·1%) | 2 (<0·1%) | 0 | 0 | 0 | |
| Socioeconomic status | ||||||
| Least deprived | 6377 (48·5%) | 6004 (48·5%) | 373 (49·8%) | 54 (52·4%) | 5 (38·5%) | |
| Mildly deprived | 2523 (19·2%) | 2409 (19·4%) | 114 (15·2%) | 12 (11·7%) | 3 (23·1%) | |
| Most deprived | 3941 (30·0%) | 3696 (29·8%) | 245 (32·7%) | 34 (33·0%) | 5 (38·5%) | |
| Missing | 301 (2·3%) | 284 (2·3%) | 17 (2·3%) | 3 (2·9%) | 0 | |
| HIV transmission route | ||||||
| PWID | 1876 (14·3%) | 1817 (14·7%) | 59 (7·9%) | 19 (18·5%) | 2 (15·4%) | |
| MSM | 6870 (52·3%) | 6425 (51·8%) | 445 (59·4%) | 48 (46·6%) | 4 (30·8%) | |
| Male heterosexual | 1768 (13·5%) | 1680 (13·6%) | 88 (11·8%) | 14 (13·6%) | 4 (30·8%) | |
| Female heterosexual, homosexual, or bisexual | 1714 (13·0%) | 1609 (13·0%) | 105 (14·0%) | 15 (14·6%) | 2 (15·4%) | |
| Other | 787 (6·0%) | 741 (6·0%) | 46 (6·1%) | 7 (6·8%) | 1 (7·7%) | |
| Missing | 127 (1·0%) | 121 (1·0%) | 6 (0·8%) | 0 | 0 | |
| Years since HIV diagnosis | 12·0 (7·0–18·7) | 12·1 (7·1–18·9) | 10·3 (6·1–15·7) | 14·4 (9·4–20·7) | 17·4 (12·7–21·7) | |
| CD4 count (cells per μL) | ||||||
| <200 | 413 (3·1%) | 388 (3·1%) | 25 (3·3%) | 8 (7·8%) | 2 (15·4%) | |
| 200–499 | 2716 (20·7%) | 2552 (20·6%) | 164 (21·9%) | 30 (29·1%) | 6 (46·2%) | |
| ≥500 | 9451 (71·9%) | 8922 (72·0%) | 529 (70·6%) | 60 (58·3%) | 5 (38·5%) | |
| Missing | 562 (4·3%) | 531 (4·3%) | 31 (4·1%) | 5 (4·9%) | 0 | |
| Median (IQR) | 692·5 (500·0–917·0) | 692·0 (500·0–917·0) | 695·5 (483·5–912·3) | 611·0 (387·5–865·8) | 469·0 (250·0–587·0) | |
| CD4/CD8 ratio | 0·9 (0·6–1·2) | 0·9 (0·6–1·2) | 0·9 (0·6–1·2) | 0·8 (0·6–1·1) | 0·7 (0·5–1·0) | |
| Plasma HIV RNA viral load | ||||||
| Detectable | 1068 (8·1%) | 1017 (8·2%) | 51 (6·8%) | 14 (13·6%) | 1 (7·7%) | |
| Undetectable | 10758 (81·9%) | 10136 (81·8%) | 622 (83·0%) | 76 (73·8%) | 10 (76·9%) | |
| Missing | 1316 (10·0%) | 1240 (10·0%) | 76 (10·2%) | 13 (12·6%) | 2 (15·4%) | |
| Number of chronic comorbidities | ||||||
| 0 | 4489 (34·2%) | 4231 (34·1%) | 258 (34·5%) | 5 (4·9%) | 0 | |
| 1 | 3434 (26·2%) | 3247 (26·2%) | 187 (25·0%) | 21 (20·4%) | 2 (15·4%) | |
| 2 | 2244 (17·1%) | 2106 (17·0%) | 138 (18·4%) | 19 (18·5%) | 1 (7·7%) | |
| 3 | 1325 (10·1%) | 1253 (10·1%) | 72 (9·6%) | 22 (21·4%) | 3 (23·1%) | |
| ≥4 | 1650 (12·6%) | 1556 (12·6%) | 94 (12·6%) | 36 (35·0%) | 7 (53·9%) | |
| Type of chronic comorbidities | ||||||
| Respiratory disease | 1665 (12·7%) | 1553 (12·5%) | 112 (15·0%) | 33 (32·0%) | 6 (46·2%) | |
| Cardiovascular disease | 1912 (14·6%) | 1800 (14·5%) | 112 (15·0%) | 31 (30·1%) | 4 (30·8%) | |
| Autoimmune disease | 1376 (10·5%) | 1282 (10·3%) | 94 (12·6%) | 22 (21·4%) | 2 (15·4%) | |
| Chronic kidney disease | 569 (4·3%) | 535 (4·3%) | 34 (4·6%) | 9 (8·7%) | 2 (15·4%) | |
| Chronic liver disease | 2144 (16·3%) | 2038 (16·4%) | 106 (14·2%) | 33 (32·0%) | 4 (30·7%) | |
| Neuropsychiatric | 3458 (26·3%) | 3272 (26·4%) | 186 (24·8%) | 48 (46·6%) | 10 (76·9%) | |
| Diabetes (any type) | 741 (5·6%) | 706 (5·7%) | 35 (4·7%) | 11 (10·7%) | 0 | |
| Metabolic disease | 2945 (22·4%) | 2781 (22·4%) | 164 (22·0%) | 53 (51·5%) | 5 (38·5%) | |
| Cancer | 1198 (9·1%) | 1133 (9·1%) | 65 (8·7%) | 15 (14·6%) | 5 (38·5%) | |
| Hypertension | 2752 (20·9%) | 2585 (20·9%) | 167 (22·3%) | 42 (40·8%) | 6 (46·2%) | |
| Obesity | 1114 (8·5%) | 1042 (8·4%) | 72 (9·6%) | 19 (18·5%) | 0 | |
| Years on ART§ | 9·4 (5·1–15·0) | 9·5 (5·1–15·2) | 7·8 (4·6–12·9) | 11·7 (6·9–17·7) | 15·4 (11·2–17·1) | |
| Backbone ART | ||||||
| Tenofovir alafenamide | 6606 (50·3%) | 6215 (50·2%) | 391 (52·2%) | 57 (55·3%) | 7 (53·9%) | |
| Tenofovir disoproxil fumarate | 939 (7·2%) | 891 (7·2%) | 48 (6·4%) | 7 (6·8%) | 0 | |
| Abacavir plus lamivudine | 3820 (29·1%) | 3594 (29·0%) | 226 (30·2%) | 26 (25·2%) | 3 (23·1%) | |
| Other | 670 (5·1%) | 630 (5·1%) | 40 (5·3%) | 6 (5·8%) | 1 (7·7%) | |
| Missing | 1107 (8·4%) | 1063 (8·6%) | 44 (5·9%) | 7 (6·8%) | 2 (15·4%) | |
| Third ART | ||||||
| Integrase inhibitors | 8048 (61·2%) | 7565 (61·0%) | 483 (64·5%) | 57 (55·3%) | 7 (53·9%) | |
| Protease inhibitors | 2699 (20·5%) | 2574 (20·8%) | 125 (16·7%) | 29 (28·2%) | 3 (23·1%) | |
| NNRTIs | 3167 (24·1%) | 2993 (24·4%) | 174 (23·2%) | 28 (27·2%) | 4 (30·8%) | |
| Other | 68 (0·5%) | 66 (0·5%) | 2 (0·3%) | 1 (1·0%) | 0 | |
| Missing | 255 (1·9%) | 244 (2·0%) | 11 (1·5%) | 1 (1·0%) | 0 | |
Data are n (%) or median (IQR). ART=antiretroviral therapy. MSM=men who have sex with men. NNRTI=non-nucleoside reverse transcriptase inhibitor. PWID=people who inject drugs.
Sex at birth as registered in the health registries of the participating hospitals was used. One participant in the overall cohort had unknown sex.
Age for all patients was as at Feb 1, 2020.
Country of origin was as indicated by the Public Data Analysis for Health Research and Innovation Program of Catalonia, recorded as Spanish or non-Spanish.
Years on ART was defined as the difference in time between the first treatment administration date to the latest treatment date or the latest hospital visit if the latest treatment date was missing.
Among co-infected patients, 491 (65·5%) had chronic comorbidities, which was similar to the proportion in the overall cohort (table 1). The most prevalent comorbidities among co-infected patients were neuropsychiatric conditions, hypertension, and metabolic disease (table 1). All patients who died had at least one chronic comorbidity.
705 (94·1%) co-infected patients were on ART before a SARS-CoV-2 diagnosis (table 1). The median time on ART was 7·8 years (IQR 4·6–12·9; table 1). Most co-infected patients and those without SARS-CoV-2 diagnosis (57·3%) were receiving tenofovir-based ART (table 1).
Patient characteristics in terms of age, country of birth, HIV transmission group, years since HIV diagnosis, and years on ART varied significantly between people living with HIV with and without SARS-CoV-2 diagnosis (appendix 4 p 29).
In a multivariable Cox regression model, non-Spanish origin, being MSM, and having four or more chronic comorbidities were associated with an increased risk of SARS-CoV-2 diagnosis (table 2 ). Being a PWID and aged 40–64 years were negatively associated with SARS-CoV-2 diagnosis in adjusted analysis (table 2).
Table 2.
Factors associated with SARS-CoV-2 diagnosis and severe COVID-19 outcomes among people living with HIV
|
SARS-CoV-2 diagnosis |
Severe COVID-19 outcomes |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI); n=13 142 | p value | Adjusted HR (95% CI); n=10 227* | p value | HR (95% CI); n=13 142 | p value | Adjusted HR (95% CI); n=10 227* | p value | ||
| Sex | |||||||||
| Female | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| Male | 1·06 (0·87–1·27) | 0·58 | 1·15 (0·68–1·95) | 0·61 | 0·82 (0·51–1·32) | 0·42 | 0·70 (0·26–1·87) | 0·47 | |
| Age, years | |||||||||
| 16–39 | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| 40–64 | 0·65 (0·56–0·76) | <0·0001 | 0·70 (0·58–0·85) | <0·0001 | 1·73 (1·00–2·97) | 0·05 | 1·03 (0·54–1·95) | 0·94 | |
| 65–74 | 0·68 (0·47–0·98) | 0·036 | 0·65 (0·42–1·02) | 0·059 | 3·38 (1·54–7·46) | <0·0001 | 1·53 (0·58–4·00) | 0·39 | |
| ≥75 | 0·79 (0·43–1·45) | 0·45 | 1·00 (0·53–1·90) | >0·99 | 7·10 (2·78–18·14) | <0·0001 | 3·57 (1·21–10·51) | 0·021 | |
| Place of birth | |||||||||
| Spain | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| Outside Spain | 1·64 (1·42–1·89) | <0·0001 | 1·55 (1·31–1·83) | <0·0001 | 1·40 (0·95–2·06) | 0·09 | 2·34 (1·46–3·75) | <0·0001 | |
| Socioeconomic status | |||||||||
| Least deprived | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| Mildly deprived | 0·76 (0·62–0·94) | 0·012 | 0·87 (0·69–1·10) | 0·26 | 0·56 (0·30–1·05) | 0·07 | 0·58 (0·29–1·16) | 0·13 | |
| Most deprived | 1·06 (0·91–1·25) | 0·45 | 1·20 (0·99–1·45) | 0·057 | 1·02 (0·66–1·57) | 0·92 | 1·13 (0·69–1·84) | 0·63 | |
| HIV transmission group | |||||||||
| Male heterosexual | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| PWID | 0·63 (0·45–0·88) | 0·0001 | 0·66 (0·44–0·98) | 0·040 | 1·28 (0·64–2·55) | 0·48 | 0·90 (0·36–2·21) | 0·81 | |
| MSM | 1·31 (1·04–1·65) | 0·021 | 1·42 (1·09–1·86) | 0·0098 | 0·88 (0·48–1·59) | 0·67 | 1·49 (0·75–3·00) | 0·26 | |
| Female heterosexual, homosexual, or bisexual | 1·24 (0·93–1·64) | 0·14 | 1·47 (0·79–2·71) | 0·22 | 1·10 (0·53–2·28) | 0·79 | 0·75 (0·21–2·70) | 0·66 | |
| Other | 1·18 (0·82–1·68) | 0·37 | 1·12 (0·73–1·73) | 0·59 | 1·12 (0·45–2·77) | 0·81 | 1·00 (0·33–3·00) | 0·99 | |
| CD4 count, cells per μL | |||||||||
| ≥500 | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| 200–499 | 1·09 (0·91–1·29) | 0·36 | 1·14 (0·94–1·39) | 0·19 | 1·75 (1·13–2·71) | 0·01 | 1·41 (0·86–2·31) | 0·17 | |
| <200 | 1·11 (0·74–1·66) | 0·60 | 1·34 (0·84–2·13) | 0·21 | 3·11 (1·49–6·50) | <0·0001 | 1·87 (0·76–4·62) | 0·17 | |
| Plasma HIV RNA viral load | |||||||||
| Undetectable | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| Detectable | 0·83 (0·62–1·10) | 0·20 | 0·80 (0·59–1·08) | 0·15 | 1·87 (1·06–3·30) | 0·03 | 1·73 (0·93–3·23) | 0·083 | |
| Backbone ART | |||||||||
| Other | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| Tenofovir alafenamide | 0·99 (0·72–1·37) | 0·96 | 0·97 (0·68–1·38) | 0·86 | 0·96 (0·42–2·24) | 0·93 | 1·42 (0·51–3·99) | 0·50 | |
| Tenofovir disoproxil fumarate | 0·85 (0·56–1·30) | 0·45 | 0·83 (0·52–1·33) | 0·45 | 0·83 (0·28–2·48) | 0·74 | 1·47 (0·41–5·26) | 0·56 | |
| Abacavir plus lamivudine | 0·99 (0·71–1·39) | 0·96 | 1·04 (0·72–1·50) | 0·82 | 0·76 (0·31–1·85) | 0·55 | 0·99 (0·34–2·87) | 0·98 | |
| Number of comorbidities | |||||||||
| 0 | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| 1 | 0·95 (0·79–1·15) | 0·60 | 1·04 (0·84–1·28) | 0·74 | 5·51 (2·08–14·61) | <0·0001 | 5·90 (1·97–17·65) | <0·0001 | |
| 2 | 1·08 (0·88–1·33) | 0·47 | 1·25 (0·98–1·59) | 0·07 | 7·64 (2·85–20·47) | <0·0001 | 8·48 (2·76–26·01) | <0·0001 | |
| 3 | 0·96 (0·74–1·24) | 0·74 | 1·23 (0·90–1·68) | 0·19 | 15·09 (5·71–39·84) | <0·0001 | 18·77 (6·15–57·33) | <0·0001 | |
| ≥4 | 1·02 (0·80–1·29) | 0·89 | 1·46 (1·09–1·97) | 0·01 | 20·01 (7·85–50·99) | <0·0001 | 22·63 (7·42–68·97) | <0·0001 | |
HRs were calculated using Cox proportional hazards models. ART=antiretroviral therapy. HR=hazard ratio. MSM=men who have sex with men. PWID=people who inject drugs.
Model adjusted for sex, age, country of origin, socioeconomic status, HIV transmission group, backbone ART, plasma HIV RNA viral load (detectable or undetectable), CD4 cell count (<200 cells per μL, 200–499 cells per μL, and ≥500 cells per μL), and number of comorbidities.
In a multivariable Cox regression analysis, we found that age 75 years or older, non-Spanish origin, and having one or more chronic comorbidities were associated with severe COVID-19 outcomes (table 2). Our adjusted analysis also showed an increased risk of severe COVID-19 outcomes for people living with HIV with neuropsychiatric conditions, autoimmune disease, respiratory disease, and metabolic disease (figure 2 ).
Figure 2.
Estimated hazard ratios for each chronic comorbidity for SARS-CoV-2 diagnosis and severe COVID-19 outcomes, from a multivariable Cox model
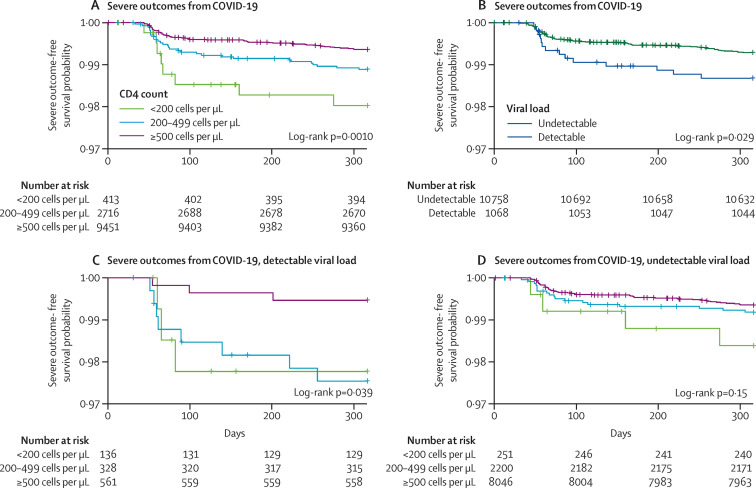
Kaplan-Meier survival analysis revealed that, compared with higher CD4 cell counts, a CD4 count of less than 200 cells per μL was significantly associated with severe COVID-19 outcomes during the study period (p=0·0010). A detectable HIV RNA viral load was also associated with severe COVID-19 outcomes (p=0·029; figure 3 ). We then did a Kaplan-Meier survival analysis, stratifying patients into detectable and undetectable HIV RNA. We found differences in the risk of severe outcomes according to CD4 counts in patients with detectable HIV RNA (p=0·039) but no differences were observed in patients with undetectable HIV RNA (p=0·15; figure 3).
Figure 3.
Kaplan-Meier survival curves for people living with HIV in Catalonia, Spain, from March 1 to Dec 15, 2020
Discussion
In a multicentre population-based cohort of people with HIV, SARS-CoV-2 diagnosis was more common among migrants, MSM, and those with four or more comorbidities; whereas, severe COVID-19 was associated with older age and increased numbers of chronic comorbidities. Notably, we observed differences in the risk of severe outcomes according to CD4 cell counts in patients with detectable HIV RNA viral loads. Our results show that people with HIV with chronic comorbidities and unsuppressed HIV RNA viral load could be at increased risk of severe outcomes and should be prioritised in terms of testing strategies, clinical management, and SARS-CoV-2 vaccination programmes.
The proportion of patients who tested positive for SARS-CoV-2 was higher in our study than in the general population in Catalonia during the same period. Existing data on the incidence of COVID-19 do not suggest that the incidence is higher among people living with HIV compared with the worldwide general population.4 In a large study from New York (NY, USA), the rate of SARS-CoV-2 diagnosis was higher among people living with HIV compared with that of the general population (2·8% vs 1·9%) but, when the analysis was adjusted for sex, age, and region, the relative risk (RR) between HIV-positive and HIV-negative individuals was almost the same (RR 0·94, 95% CI 0·91–0·97).12 The testing protocols in Spain might, however, mask the true diagnosis rate, because people living with HIV are not prioritised for SARS-CoV-2 testing.19 With emerging data suggesting an increased risk of severe COVID-19 outcomes for people living with HIV, testing strategies should be revised to target this population.
The mortality rate from our study was lower than the reported mortality rates from other studies of patients co-infected with HIV and SARS-Cov-2.4 The mortality rate in the general Catalan population was approximately double what we found in our population during the same period. Also, the rate of hospitalisation in our study was similar to the reported hospitalisation rates in the general Catalan population during the same period. In our cohort, patients were younger than in the general Catalan population and 34·5% of co-infected patients were without chronic comorbidities. Both older age and chronic comorbidities have been identified as major risk factors for severe COVID-19, which partially explains the lower incidence of poorer outcomes in our population.4, 20
The COVID-19 pandemic has highlighted the existing health inequities affecting specific subpopulations because of their social background. After adjusting for potential confounders, SARS-CoV-2 diagnosis was more common among individuals born outside of Spain. A study from Catalonia has shown that precarious employment is more prevalent among migrants than among non-migrants and the positive association between precarious employment and poor health is well known.21 During the COVID-19 pandemic, people in precarious employment might find it difficult to work from home or follow physical-distancing guidelines. This situation might partly explain why SARS-CoV-2 diagnosis is more prevalent in people of non-Spanish origin. Similar results were observed in the ENE-COVID study assessing the seroprevalence of SARS-CoV-2 in the overall Spanish population.22 Intuitively, we would expect socioeconomic deprivation to have similar effects on SARS-CoV-2 diagnosis and severe outcomes as country of origin, but we did not find this association in our analysis. This finding could partly be explained by the fact that socioeconomic deprivation in our analysis was an ecological variable based on place of residence. Place of residence might not, however, always reflect the socioeconomic level of an individual.
Our results suggest the possibility of an increased SARS-CoV-2 diagnosis rate among MSM. Studies from London, UK,23 Brazil,24 and Portugal24 showed that, despite implemented isolation measures to control COVID-19 transmission, a large proportion of MSM continued to engage in sex outside their homes that could potentially increase their risk of SARS-CoV-2 diagnosis. We, however, do not have data to support this finding among MSM in Catalonia. Surprisingly, the decreased risk of SARS-CoV-2 diagnosis among PWID was unanticipated. PWID face additional risk for COVID-19 infection due to potential difficulties in adhering to physical distancing and isolation measures, poor hygiene, stigmatisation, and reduced access to key services during the pandemic. These social health disparities could also hamper SARS-CoV-2 testing opportunities in this population and could contribute, albeit marginally, to explaining the decreased risk in our analysis. Our findings call for further studies to understand the increased risk of SARS-CoV-2 diagnosis among MSM and the reduced risk among PWID.
People living with HIV with four or more comorbidities were also identified to have an increased risk of SARS-CoV-2 diagnosis. We conceive that people with more comorbidities are more likely to become ill and visit health-care centres more frequently. This explanation means that such patients are more likely to be tested for SARS-CoV-2 and hence their increased risk of SARS-CoV-2 diagnosis in our analysis.
As expected, older age (≥75 years) was associated with a higher risk of severe COVID-19 outcomes. This finding is similar to that of a large study on the risk factors of severe COVID-19 showing that people older than 80 years have at least a 20-times risk of severe COVID-19 compared with the 50–59 years age group.25 We also observed an increased risk of severe outcomes for migrants. This finding is similar to findings from other studies in the general population that have reported an increased risk of severe outcomes for migrants and minority ethnic groups.25, 26 This finding also reflects the existing vulnerabilities among migrants including reduced patronage of health-care services, unequal socioeconomic factors, and an increased prevalence of comorbidities.27
The effect of comorbidities on severe COVID-19 has been established in different studies.20 Our study shows an increasing risk of severe COVID-19 with increasing number of comorbidities. Neuropsychiatric conditions, autoimmune, respiratory, and metabolic disease were associated with an increased risk of severe COVID-19 outcomes compared with the group without chronic diseases. Similar findings have been reported in other studies.25, 28 Clinical outcomes for people with HIV with SARS-CoV-2 infection and comorbidities could be detrimental and therefore special consideration must be given in the clinical management of such individuals.
Early reports indicated that advanced HIV infection, immunosuppression, and high viraemia might restrict COVID-19-associated immune dysregulation and the development of cytokine storm and therefore might reduce disease severity.5 However, our findings show that low CD4 count (<200 cells per μL) and detectable plasma HIV RNA viral load were associated with worse outcomes from HIV-COVID-19 co-infection. Notably, low CD4 cell count was not associated with severe outcomes among patients with HIV virological suppression. These findings are similar to those of a study by Hoffmann and colleagues who showed that low CD4 count (<350 cells per μL) or low nadir CD4 cell count were predictors of worse outcomes.11 However, a multicentre study from the USA showed that CD4 count less than 200 cells per μL, regardless of virological suppression, was associated with worse outcomes of HIV-COVID-19 co-infection.29 Immunosuppression was not associated with worse outcomes among patients with virological suppression in our study.
This study has some limitations. First, because our overall cohort has not been tested for SARS-CoV-2, we cannot precisely assess the incidence of the infection in our study population. The fact that we found a higher diagnosis rate than did reports from other cohorts of people with HIV suggests that, at least during the first year of the pandemic in Spain, HIV-positive patients were not specifically targeted for SARS-CoV-2 screening, but rather testing was done on the basis of presenting symptoms, existing comorbidities, or age.19 Therefore, we might have overestimated the diagnosis rate in this population. Second, our data do not include information on smoking and body-mass index. Both factors are associated with worse COVID-19 outcomes. Third, we could not link patient-level information for some PISCIS cohort participants with the PADRIS data. We were able to link 20 847 out of the 28 666 (72·8%) participants of the PISCIS cohort with the PADRIS data. But the proportion of participants alive as of Feb 1, 2020, and followed up was still a good representable sample size to be analysed and make deductions.
In conclusion, our results show that among people living with HIV, migrants, MSM, and those with comorbidities were at an increased risk of SARS-CoV-2 diagnosis; however, the diagnosis rate of SARS-CoV-2 infection could be masked because of the absence of specific testing policies addressing this population, because testing in Spain has been based on presenting signs and symptoms and contact tracing. The fact that people with HIV were not prioritised for SARS-CoV-2 testing could disguise the real picture of the pandemic in this population. Finally, our findings also suggest that people with HIV with immunosuppression, detectable HIV viraemia, chronic comorbidities, and subpopulations (eg, older people and migrants) could be more susceptible to severe outcomes from COVID-19 and should be prioritised in testing strategies, clinical management, and considered a target group for SARS-CoV-2 vaccination programmes.
Data sharing
The study protocol is available from JR-U (jmreyes@iconcologia.net). Statistical code for the analysis can be requested from YD, SM, or JA (ydiaz@igtp.cat, smorenof@iconcologia.net, oe jaceiton@igtp.cat). The data for this study are available from the Centre for Epidemiological Studies of Sexually Transmitted Diseases and HIV/AIDS in Catalonia (CEEISCAT), the coordinating centre of the PISCIS cohort study and from each of the collaborating hospitals upon request. Requests can be made via https://pisciscohort.org/contacte/.
Declaration of interests
JMM reports receiving a personal 80:20 research grant from Institut d'Investigacions Biomèdiques August Pi I Sunyer, Barcelona, Spain, during 2017–21. EL reports receiving honoraria for lectures and presentations from ViiV Healthcare, Gilead Sciences, and Jansen Therapeutics; travel support for attending meetings from ViiV Healthcare and Jansen Therapeutics; payments for participating in the data safety monitoring and advisory board of ViiV Healthcare; being a full time employee of ViiV Healthcare since May 3, 2020; and payments made from Juan Rodés to the Spanish Government on his behalf. PD reports that his institution received grants from Gilead Sciences, Janssen-Cilag, and ViiV Healthcare; and he personally received honoraria from Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, ViiV Healthcare, Roche, and Thera Technologies. AI reports that his institution received grants from Gilead Sciences and Merck Sharp & Dohme; and he personally received consultation fees from Gilead Sciences, ViiV Healthcare, and Thera Technologies; honoraria for lectures and presentations from Gilead Sciences, Merck Sharp & Dohme, Jansen Therapeutics, and ViiV Healthcare; and travel support for attending meetings from Gilead Sciences, Jansen, and ViiV Healthcare. VF reports that his institution received grants from Gilead Sciences, ViiV Healthcare, and Merck Sharp & Dohme; and he personally received consultation fees from ViiV Healthcare; honoraria for lectures and presentations from ViiV Healthcare, Gilead Sciences, Jansen Therapeutics, and Merck Sharp & Dohme; and travel support for attending meetings from Gilead Sciences. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This study was funded by the COVIHCAT grant from Fundació “la Caixa”. We also want to thank PADRIS and the Programme for the Prevention, Control and Care for HIV, Sexually Transmitted Diseases and Viral Hepatitis of the Ministry of Health of the Government of Catalonia for their support.
Contributors
DKN, JR-U, YD, and JMM conceived and designed the study. DKN, JR-U, and YD had full access to all of the study data, verified the data, and take responsibility for the integrity of the data and the accuracy of the data analysis. DKN, JR-U, YD, SM, and JA did the analyses. DKN and JRU wrote the first draft of the paper and incorporated revisions. All authors contributed in the interpretation of results. All authors critically revised and approved the final manuscript.
Contributor Information
PISCIS study group:
Esteve Muntada, Anna Esteve, Melchor Riera, Gemma Navarro, Hernando Knobel, Josep Mallolas, Daniel Podzamczer, Adrià Curran, Joaquín Burgos, Maria Gracia Mateo, Maria del Mar Gutierrez, Javier Murillas, Francisco Homar, Jose Vicente Fernández-Montero, Eva González, Joaquim Peraire, Francesc Vidal, Elena Leon, Àngels Masabeu, Amat-Joaquim Orti, David Dalmau, Àngels Jaen, Elisabet Deig, Elisa De Lazzari, Leire Berrocal, Guillem Fernandez, Lucía Rodríguez, Freya Gargoulas, Toni Vanrell, Jose Carlos Rubia, Josep Vilà, Marina Martínez, Bibiana Morell, Maribel Tamayo, Jorge Palacio, Juan Ambrosioni, Montse Laguno, María Martínez-Rebollar, José Luis Blanco, Felipe Garcia, Esteban Martínez, Berta Torres, Lorena de la Mora, Alexy Inciarte, Ainoa Ugarte, Iván Chivite, Ana González-Cordon, Lorna Leal, Antoni Jou, Maria Saumoy, Ana Silva, Sofia Scévola, Jordi Navarro, Paula Suanzes, Isabel Mur, Maria Àngels Ribas, Antoni A Campins, Francisco Fanjul, María Leyes, María Peñaranda, María Luisa Martin, Helem Haydee Vilchez, Sònia Calzado, Manel Cervantes, M. José Amengual, Marta Navarro, Antoni Payeras, Carmen Cifuentes, Aroa Villoslada, Patrícia Sorní, Marta Molero, Nadia Abdulghani, Thaïs Comella, Rocio Sola, Montserrat Vargas, Consuleo Viladés, Anna Martí, Pilar Barrufet, Laia Arbones, Elena Chamarro, Mireia Cairó, Xavier Martinez-Lacas, Roser Font, and Lizza Macorigh
Supplementary Materials
References
- 1.Vizcarra P, Pérez-Elías MJMJMJ, Quereda C, et al. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV. 2020;3018:1–11. doi: 10.1016/S2352-3018(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu F, Cao Y, Xu S, Zhou M. Co-infection of SARS-CoV-2 and HIV in a patient in Wuhan city, China. J Med Virol. 2020;92:529–530. doi: 10.1002/jmv.25732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Centers for Disease Control and Prevention What to know about HIV and COVID-19. 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/hiv.html
- 4.Cooper T, Woodward B, Alom S, Harky A. Coronavirus disease 2019 (COVID-19) outcomes in HIV/AIDS patients: a systematic review. HIV Med. 2020;21:567–577. doi: 10.1111/hiv.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo W, Ming F, Dong Y, et al. A survey for COVID-19 among HIV/AIDS patients in two districts of Wuhan, China. SSRN. 2020 https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3550029 published online March 13. (preprint). [Google Scholar]
- 6.Ford N, Vitoria M, Rangaraj A, Norris SL, Calmy A, Doherty M. Systematic review of the efficacy and safety of antiretroviral drugs against SARS, MERS or COVID-19: initial assessment. J Int AIDS Soc. 2020;23:1–9. doi: 10.1002/jia2.25489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertagnolio S, Thwin S, Silva R, et al. Clinical characteristics and prognostic factors in people living with HIV hospitalized with COVID-19: findings from the WHO Global Clinical Platform. 11th International AIDS Society Conference on HIV Science; Berlin and virtual; July 18–21, 2021 (abstr PEBLB20).
- 8.Bhaskaran K, Rentsch CT, MacKenna B, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV. 2021;8:e24–e32. doi: 10.1016/S2352-3018(20)30305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulle A, Davies M-A, Hussey H, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1198. published online Aug 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambrosioni J, Blanco JL, Reyes-urueña JM, et al. Overview of SARS-CoV-2 infection in adults living with HIV. Lancet HIV. 2021;8:294–305. doi: 10.1016/S2352-3018(21)00070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann C, Casado JL, Härter G, et al. Immune deficiency is a risk factor for severe COVID-19 in people living with HIV. HIV Med. 2021;22:372–378. doi: 10.1111/hiv.13037. [DOI] [PubMed] [Google Scholar]
- 12.Tesoriero JM, Swain C-AE, Pierce JL, et al. COVID-19 outcomes among persons living with or without diagnosed HIV Infection in New York State. JAMA Netw Open. 2021;4:e2037069. doi: 10.1001/jamanetworkopen.2020.37069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Francesco D, Verboeket SO, Underwood J, et al. Patterns of co-occurring comorbidities in people living with HIV. Open Forum Infect Dis. 2018;5:ofy272. doi: 10.1093/ofid/ofy272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiau S, Krause KD, Valera P, Swaminathan S, Halkitis PN. The burden of COVID-19 in people living with HIV: a syndemic perspective. AIDS Behav. 2020;24:2244–2249. doi: 10.1007/s10461-020-02871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaén Á, Casabona J, Esteve A, et al. Clinical-epidemiological characteristics and antiretroviral treatment trends in a cohort of HIV infected patients. The PISCIS Project. Med Clin. 2005;124:525–531. doi: 10.1157/13073938. [DOI] [PubMed] [Google Scholar]
- 16.AQuAS Programa públic d'analítica de dades per a la recerca i la innovació en salut a Catalunya. 2017. https://aquas.gencat.cat/web/.content/minisite/aquas/publicacions/2017/Programa_analitica_dades_PADRIS_aquas2017.pdf
- 17.Ministerio de Sanidad Gobierno de España. Estrategia de detección precoz, vigilancia y control de COVID-19. 2020. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/COVID19_Estrategia_vigilancia_y_control_e_indicadores.pdf
- 18.García-Altés A, Ruiz-Muñoz D, Colls C, Mias M, Bassols NM. Socioeconomic inequalities in health and the use of healthcare services in Catalonia: analysis of the individual data of 7·5 million residents. J Epidemiol Community Heal. 2018;72:871–879. doi: 10.1136/jech-2018-210817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ministerio de Sanidad Gobierno de España. Procedimiento de actuación frente a casos de infección por el nuevo coronavirus (SARS-CoV-2). Actualizado a 31 de marzo de 2020. 2020. https://www.semg.es/images/2020/Coronavirus/20200331_Procedimiento_actuacion_COVID_19.pdf
- 20.Gold MS, Sehayek D, Gabrielli S, Zhang X, McCusker C, Ben-Shoshan M. COVID-19 and comorbidities: a systematic review and meta-analysis. Postgrad Med. 2020;132:749–755. doi: 10.1080/00325481.2020.1786964. [DOI] [PubMed] [Google Scholar]
- 21.Benach J, Julià M, Tarafa G, Mir J, Molinero E, Vives A. Multidimensional measurement of precarious employment: social distribution and its association with health in Catalonia (Spain) Gac Sanit. 2015;29:375–378. doi: 10.1016/j.gaceta.2015.04.002. (in Spanish). [DOI] [PubMed] [Google Scholar]
- 22.Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyndman I, Nugent D, Whitlock GG, McOwan A, Girometti N. COVID-19 restrictions and changing sexual behaviours in HIV-negative MSM at high risk of HIV infection in London, UK. Sex Transm Infect. 2021 doi: 10.1136/sextrans-2020-054768. published online Jan 18. [DOI] [PubMed] [Google Scholar]
- 24.de Sousa AFL, de Oliveira LB, Queiroz AAFLN, et al. Casual sex among MSM during the period of sheltering in place to prevent the spread of COVID-19: results of national online surveys in Brazil and Portugal. medRxiv. 2020 doi: 10.1101/2020.06.07.20113142. published online Sept 23. (preprint). [DOI] [Google Scholar]
- 25.Williamson EJ, Walker AJ, Bhaskaran K, et al. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tai DBG, Shah A, Doubeni CA, Sia IG, Wieland ML. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72:703–706. doi: 10.1093/cid/ciaa815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vazquez M-L, Vargas I, Jaramillo DLL, et al. Was access to health care easy for immigrants in Spain? The perspectives of health personnel in Catalonia and Andalusia. Health Policy. 2016;120:396–405. doi: 10.1016/j.healthpol.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Guan W, Ni Z, Hu YYH, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dandachi D, Geiger G, Montgomery MW, et al. Characteristics, comorbidities, and outcomes in a multicenter registry of patients with HIV and coronavirus disease-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1339. published online Sept 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study protocol is available from JR-U (jmreyes@iconcologia.net). Statistical code for the analysis can be requested from YD, SM, or JA (ydiaz@igtp.cat, smorenof@iconcologia.net, oe jaceiton@igtp.cat). The data for this study are available from the Centre for Epidemiological Studies of Sexually Transmitted Diseases and HIV/AIDS in Catalonia (CEEISCAT), the coordinating centre of the PISCIS cohort study and from each of the collaborating hospitals upon request. Requests can be made via https://pisciscohort.org/contacte/.