Abstract
Changes over the last decade in overt proficiency testing (OPT) regulations have been ostensibly directed at improving laboratory performance on patient samples. However, the overt (unblinded) format of the tests and regulatory penalties associated with incorrect values allow and encourage laboratorians to take extra precautions with OPT analytes. As a result OPT may measure optimal laboratory performance instead of the intended target of typical performance attained during routine patient testing. This study addresses this issue by evaluating medical mycology OPT and comparing its fungal specimen identification error rates to those obtained in a covert (blinded) proficiency testing (CPT) program. Identifications from 188 laboratories participating in the New York State mycology OPT from 1982 to 1994 were compared with the identifications of the same fungi recovered from patient specimens in 1989 and 1994 as part of the routine procedures of 88 of these laboratories. The consistency in the identification of OPT specimens was sufficient to make accurate predictions of OPT error rates. However, while the error rates in OPT and CPT were similar for Candida albicans, significantly higher error rates were found in CPT for Candida tropicalis, Candida glabrata, and other common pathogenic fungi. These differences may, in part, be due to OPT’s use of ideal organism representatives cultured under optimum growth conditions. This difference, as well as the organism-dependent error rate differences, reflects the limitations of OPT as a means of assessing the quality of routine laboratory performance in medical mycology.
The New York State Public Health Law requires all clinical laboratories and blood banks conducting tests on specimens collected within New York State to hold a state clinical laboratory permit, unless the tests are conducted as an adjunct to a physician’s medical practice (physician office laboratory). In response to a 1964 legislative mandate, the New York State Department of Health initiated an overt proficiency testing (OPT) program as one method of monitoring the overall quality of testing performed by state permit-holding clinical facilities. The program, as of 1998, has grown to include almost 1,000 clinical facilities, both within New York State and in over 30 other states. Of these, 188 hold permits in medical mycology to isolate and identify either yeast-like potential pathogens only (limited permit) or all fungi and aerobic actinomycetes (general permit) which may be recovered from clinical specimens.
After learning of widely publicized problems found in the screening of Papanicolaou smears in cytopathology laboratories, Congress enacted the Clinical Laboratory Improvement Amendments of 1988 (CLIA ’88). The statute and enabling regulations initially required, in part, that all sites conducting laboratory tests on human specimens must participate in federally approved OPT programs. While the regulatory burden has been lightened over the years, current CLIA ’88 regulations require approximately 40% of all federally registered test facilities to participate in one of 20 OPT programs approved by the Health Care Financing Administration. Proficiency testing has gained wide acceptance as a regulatory tool; the maintenance of clinical laboratory operating licenses at state and federal levels are chiefly dependent upon successful OPT performance.
There have been few investigations of the ability of OPT to evaluate a laboratory’s routine testing capability. Previous studies primarily described the effects of the number and type of test specimens, laboratory personnel qualifications, and test methodology, etc., on proficiency test results (7, 15, 17, 18, 21, 30). This investigation was undertaken to compare the identification of fungi reported by laboratories which participated in the New York State mycology OPT to those reported by these facilities for the same fungi isolated from routine clinical specimens that had been obtained during unanticipated surveyor collections (covert proficiency testing [CPT]).
MATERIALS AND METHODS
Laboratories studied.
The OPT portion of the study includes all 188 laboratories holding mycology permits whose test-specific results are stored in the department’s electronic records for tests between September 1989 and May 1994. In order to enhance the assessment of long-term trends, these data were augmented with results of semiannual OPT events conducted between January 1982 and September 1989, even though only aggregate, rather than laboratory-specific, electronic records were available from these earlier tests (Table 1).
TABLE 1.
Proficiency test event timings for three types of data
| OPT
|
CPT
|
||||
|---|---|---|---|---|---|
| Time period | No. of participant laboratories | Remarks | Time period | No. of participant laboratories | Remarks |
| 1982–1987 | 110–122 | Laboratory-specific results not computerizeda; 10 specimens sent semiannually | |||
| 1988–1991 | 108–120 | Laboratory-specific results computerized in 1988b | 1988–1989 | 66 | |
| 1992–1993 | 122–131 | 5 specimens sent quarterly | |||
| 1994 | 182–188 | 5 specimens sent every 4 moc | 1993–1994 | 57 | 35 laboratories from 1988–1989 CPT and 22 additional laboratories |
| Total | 188 | 2 OPT periods, before and after 1988 computerization | Total | 88 | 2 periods of CPT |
Paper records were not computerized for this study; rather, summary event error rates were used as auxiliary data to estimate longitudinal changes in error rates prior to 1988 (Fig. 2 to 4).
Computerized records of laboratory-specific performances permit estimates of organism-specific Poisson error rates after 1988 (Table 4).
Reduction to three events yearly as mandated by CLIA ’88.
The CPT portion of the study includes isolates from routine patient specimens and laboratory identifications of them obtained by state surveyors during regular semiannual inspections. The isolates were analyzed by both the New York State Laboratories for Mycology and a reference laboratory. CPT was a double-blinded investigation, in that laboratories did not know which fungal isolates would be selected for confirmatory testing during the on-site inspections. Additionally, during reanalysis both confirmatory laboratories were unaware of the isolates’ sources and original identifications.
OPT.
From 1982 to 1991, 10 test specimens were sent twice each year (total of 20 specimens) to each participating laboratory. In the fall of 1991, the content and number of test events were modified to five test specimens shipped four times each year (20 specimens). To comply with CLIA ’88 requirements, the frequency of test events and number of test specimens were altered in the fall of 1993 to five test specimens sent three times each year (15 specimens; Table 1).
Prior to OPT, a minimum of three strains of each of the proposed five specimens were obtained from the Laboratories for Mycology culture collection or recent clinical specimens. The morphological features of each mold were evaluated. Physiological characteristics, e.g., temperature optimum and growth on cycloheximide-containing media, etc., were examined through the use of appropriate supplemental tests (23). The morphologies of all yeasts were studied with yeast grown on cornmeal agar plus Tween 80 in 100-mm-diameter petri dishes inoculated by the Dalmau or streak-cut methods. Carbohydrate assimilation characteristics were determined by using the API 20C system (Biomerieux-Vitek, Hazelwood, Mo.) and other commercial yeast identification kits. Supplemental physiological characteristics, e.g., nitrate assimilation, urease activity, and temperature optimum, etc., were studied with appropriate test media (23). Based upon the results of these tests on each strain, the strains that demonstrated the best morphological and physiologic characteristics were selected for use in the test event.
CPT.
The 88 laboratories in the CPT portion of the study were a subset of the OPT participants selected on the basis of sample availability and the surveyor’s inspection schedules.
Specimens were obtained from 66 laboratories in 1988 and 1989 and 57 laboratories in 1993 and 1994. Isolate availability led to 22 of these 57 not being included among the 1988 and 1989 inspections (Table 1). The collected isolates (nominally three yeasts and two molds or aerobic actinomycetes) were recently recovered from clinical specimens and identified by the laboratory as part of its routine workload, i.e., CPT.
Isolates were immediately shipped to the Laboratories for Mycology by the surveyors where they were accessioned, subcultured, and reidentified by standard methods (23). To independently validate the identifications done by the permit-holding laboratories and Wadsworth Center’s Laboratories of Mycology, additional subcultures of all isolates were prepared and submitted to the Mycology Reference Laboratory at the University of Texas Medical Branch, Galveston. All CPT results refer only to 452 of 466 viable specimens (97%) on which both confirmatory laboratories agreed on the identification.
Statistical methods.
The sparseness of available OPT and CPT data necessitated pooling results from all participating laboratories. These aggregated data were examined for the possibility of combining test and collection events and again pooled. Because many of the fungi were infrequently seen in either a clinical setting or OPT, the doubly pooled data were coalesced further into four groups of related molds (I to IV) and two groups of yeasts (V and VI). However, the test results for individual organisms were retained for analysis whenever sufficient data had been collected to make statistical comparisons, i.e., for Aspergillus fumigatus, Trichophyton tonsurans, Candida albicans, Candida parapsilosis, Candida tropicalis, Candida glabrata, and Cryptococcus neoformans.
Error rates were estimated by maximum likelihood (11) with truncated Poisson distributions (22), which account for laboratories’ multiple encounters with the same organism. The resulting error rate parameter estimates, which vary directly with the ease with which an organism or group could be identified, were tested for statistically significant OPT and CPT differences by using a likelihood ratio goodness of fit test (4).
The time trends from the more voluminous historical OPT data were assessed by using logistic regression maximum likelihood estimation of the changes in error rates with respect to time (8). The significance of the rate of change (slope) was assessed with a chi-square test. All programming, which is available upon request, was done in APL (29).
RESULTS
Over 105 laboratories participated in each of the semiannual OPT events between 1982 and 1989. Laboratory participation during the 1989 to 1994 period was stable, with less than 7% turnover in the three or four events per year (data not shown). However, due to the merger of the separate OPT programs operated by the New York City and New York State Departments of Health, 28 general- and 54 limited-permit laboratories joined the program in 1994.
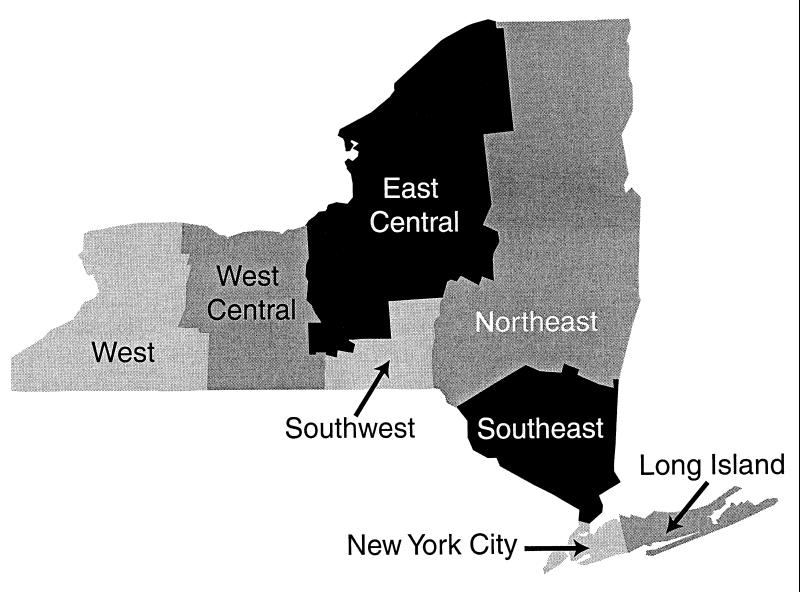
The laboratories participating in the post-1988 OPT and CPT portions of the study were distributed throughout the state in proportion to the size of the populations of each region, except for New York City (Table 2 and Fig. 1). Approximately half (44 to 63%) of the laboratories in each region held general permits. However, since facilities in New York City were not totally integrated into the state OPT program until 1995, there were significantly fewer (P < 0.0001) New York City laboratories that participated in the CPT. CPT samples were collected from fewer non-hospital-based laboratories, 16 of 88 (18%), than OPT samples, 49 of 188 (26%), but this difference was not statistically significant (P > 0.05).
TABLE 2.
Geographical distribution of participants in OPT and CPT
| Region of New York | % of total popula-tiona | Laboratories participating in:
|
|||||
|---|---|---|---|---|---|---|---|
| OPT
|
CPT
|
||||||
| No.b | %c | General permits (%)d | No.b | %c | General permits (%)d | ||
| West | 8.9 | 16 | 10.1 | 43.8 | 11 | 13.8 | 54.5 |
| Southwest | 1.7 | 2 | 1.3 | 50.0 | 2 | 2.5 | 50.0 |
| East central | 6.9 | 8 | 5.1 | 62.5 | 8 | 10.0 | 62.5 |
| West central | 8.1 | 12 | 7.6 | 58.3 | 11 | 13.8 | 54.5 |
| Northeast | 8.0 | 11 | 7.0 | 54.5 | 10 | 12.5 | 50.0 |
| Southeast | 11.4 | 21 | 13.3 | 47.6 | 16 | 20.0 | 56.3 |
| New York City | 40.7 | 61 | 38.6 | 67.2 | 4 | 5.0 | 100.0 |
| Long Island | 14.3 | 27 | 17.1 | 55.6 | 18 | 22.5 | 77.8 |
| Out of statee | NA | 30 | 19.0 | 76.7 | 8 | 10.0 | 100.0 |
Percent of New York State population. NA, not applicable.
Number of laboratories participating in the mycology proficiency testing program.
Percent of laboratories participating in the mycology proficiency testing program.
Percent of laboratories participating in the mycology proficiency testing program and holding general permits.
Includes laboratories participating in the mycology proficiency testing program located in 20 states and 1 province of Canada.
FIG. 1.
Geographical locations of New York State health service areas (Table 2).
The error rates for identifying mold and yeast specimens (Table 3) in OPT events over the combined 1982 to 1994 period are shown in Fig. 2 to 5. Although the identification errors varied considerably among events, logistic regression with respect to time revealed significant longitudinal decreases. This effect was pronounced in the error rates found in the identifications of selected mold and yeast test specimens (Fig. 2 and 4). For example, while more than 50% of the participating laboratories could not identify Cunninghamella spp. and about 30% misidentified Cladophialophora carrionii (syn. Cladosporium carrionii) in 1982, less than 5% of the laboratories holding general permits erroneously identified either of these two molds in 1994. Similarly, 25% or more of the permit-holding laboratories did not appropriately identify Candida rugosa and Candida krusei when first seen as proficiency test specimens in the early 1980s. Less than 2% of the laboratories misidentified these two yeast-like potential pathogens in OPT events in 1993 and 1994, respectively.
TABLE 3.
Identification and grouping of OPT and CPT organisms
| Group | Type of organism | Type of test in which organism appeared
|
||
|---|---|---|---|---|
| Both OPT and CPTa | OPT onlyb | CPT onlyc | ||
| I | Agents of aspergillosis and closely related hyalohyphomycosis | Aspergillus fumigatus (4%), A. flavus, A. niger | Penicillium spp., Aspergillus spp., A. glaucus gr., A. versicolor gr., A. terreus, A. ustus gr.0, Paecilomyces spp., P. variotii, A. nidulans | |
| II | Dermatophytes | Trichophyton tonsurans (4%), Epidermophyton floccosum, Microsporum canis, T. rubrum, T. mentagrophytes | Microsporum audouinii, M. gypseum, M. nanum, Trichophyton terrestre | Microsporum ferrugineum, M. persicolor0, Trichophyton soudanense, Philophora verrucosum0 |
| III | Agents of phaeohyphomycosis or hyalohyphomycosis | Chrysosporium spp., Cladosporium spp., Exophilia jeanselmei, Fusarium spp., Scopulariopsis spp., Sporothrix schenckii | Cladosporium carrionii, Exserohilum rostratum, Pseudallescheria boydii, Phialophora verrucosa, Scopulariopsis brumptii, Wangiella dermatiditis | Acremonium spp., Alternaria spp., Curvularia spp., Neurospora spp., Fonsecaea pedrosoi0, Scytalidium spp., Scedosporium apiospermum0, Scopulariopsis brevicaulis, Exserohilum bantiana |
| IV | All Candida species and closely related forms | Candida albicans (31%), C. parapsilosis (6%), C. tropicalis (9%), C. glabrata (11%), C. krusei, C. guilliermondii, C. lusitaniae, C. rugosa, C. lipolytica | Candida lambica, C. paratropicalis | Candida spp., C. famata, C. albicans and C. parapsilosis, C. humicola, C. kefyr |
| V | Miscellaneous yeast, basidomycetes, and ascomycetes | Cryptococcus neoformans (5%), Blastoschizomyces capitatus, C. albidus, Hansenula anomala, Trichosporon beigelii, Rhodotorula rubra, R. glutinis, Saccharomyces cerevisiae | Cryptococcus laurentii, Candida zeylanoides, Geotrichum candidum, Malassezia pachydermatis | Rhodotorula pilimanae, Geotrichumspp., Candida guilliermondii, Cryptococcus terreus0, C. uniguttulatus0 |
| VI | Miscellaneous mold and algae | Mucor spp., Nocardia asteroides, N. brasiliensis, Prototheca wickerhamii | Chaetomium spp., Cunninghamella spp., Nocardia otitidiscaviarum, P. zopfii, Streptomyces spp., Syncephalastrum spp., Sporobolomyces salmonicolor, Trichoderma spp., Trichothecium spp., Ulocladium spp. | Absidia spp.0, Auerobasidium pullulans0, Histoplasma capsulatum, Nocardia caviae, Rhizomucor spp.0, Rhizopus spp. |
Percentages of some organisms in the CPT samples are shown in parentheses to indicate the degree to which OPT organism selection mimics the frequency of recovery of these organisms from patient samples.
Organisms not observed among the CPT specimens were included in OPT as an educational tool for enhancing the identification capabilities of laboratories.
Some organisms are listed because they were identified only to the genus level by the laboratories. Superscript zeros indicate that the organism was misidentified in CPT data, suggesting that further education is needed.
FIG. 2.
OPT error rates observed in 1982 to 1994 for defined mold groups (Table 3). Dashed lines, associated logistic regression curves.
FIG. 5.
OPT error rates for the five individual yeasts showing the greatest decreases between 1982 and 1994. Symbols indicate the observed percentages of errors committed at each event. Dashed lines, associated logistic regression curves. All decreases were statistically significant (P < 0.007).
FIG. 4.
OPT error rates observed in 1982 to 1994 for defined yeast groups (Table 3). Dashed lines, associated logistic regression curves.
Error rates estimates for the OPT and CPT data after September 1989 were derived from the number of times laboratories were tested with a given organism. For example, Table 4 shows that 125 of the 188 permit-holding laboratories were mailed isolates of Hansenula anomala at least once and that of the 52 laboratories that received the organism in three events, six misidentified it once. The maximum likelihood truncated Poisson error rate estimate is therefore 7.5%. Multiple recoveries of the same organism, instead of multiple test events, in the CPT portion of this study led to similar data for which the same estimation techniques were employed. Goodness of fit testing for each organism (not shown, except in Table 4) failed to reject the truncated Poisson distribution as an adequate model for the errors.
TABLE 4.
Truncated Poisson parameters estimated from OPT events, 1988 to 1994
| Organism | Estimated Poisson error rate | Goodness of fita | Cumulative performance
|
Total no. of laboratories | Event date (mo/day/yr) | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. of test eventsb | No. of times misidentifiedc
|
||||||||
| 0 | 1 | 2 | 3 | ||||||
| Cryptococcus laurentii | 0.061656 | 0.41 | 1 | 18 | 0 | —d | — | 18 | 9/14/90 |
| 2 | 105 | 8 | 0 | — | 113 | 5/20/93 | |||
| Malassezia pachydermatis | 0.175258 | 1.00 | 1 | 97 | 17 | — | — | 114 | 2/15/91 |
| Hansenula anomala | 0.074587 | 0.75 | 1 | 60 | 2 | — | — | 62 | 9/15/89 |
| 2 | 10 | 1 | 0 | — | 11 | 1/09/92 | |||
| 3 | 46 | 6 | 0 | 0 | 52 | 9/23/93 | |||
| Candida rugosa | 0.140923 | 0.83 | 1 | 4 | 1 | — | — | 5 | 1/09/92 |
| 2 | 53 | 6 | 1 | — | 60 | 0/10/92 | |||
| Torulopsis candida | 0.201835 | 1.00 | 1 | 109 | 22 | — | — | 131 | 5/20/93 |
P values indicating the truncated Poisson distribution fits with historical specific error rates, i.e., values of <0.05 indicate poor agreement with the modeled error rates.
Number of test events in which the organism was used from 1988 to 1994, e.g., C. laurentii was used in two test events (18 laboratories received it in 1990 only and 113 laboratories received it in both 1990 and 1993).
Number of laboratories misidentifying the organism the stated number of times, e.g., eight laboratories misidentified C. laurentii in one test event, but none of the laboratories misidentified the organism twice. Of the 244 C. laurentii identifications eight were incorrect.
—, no labs were sent the organism the indicated number of times.
Figures 2 to 5 display initially rapid decreases in error rates that generally tended to stabilize after 1988. A further assessment of stability was achieved by utilizing the 1988 to 1994 OPT results to predict the expected number of errors in post-1994 OPT events, before the results were observed. The predicted and observed numbers of errors are shown in Table 5 for representative 1995 and 1998 events. In the 1995 prediction the organisms listed in Table 4 were used as the test samples. The P values of 0.93 for 1995 and 0.72 for 1998 indicate that the predictions cannot be differentiated statistically from the observed results.
TABLE 5.
Predicted results of representative OPT eventsa
| No. of errorsb | Total no. of errors
|
|||
|---|---|---|---|---|
| February 1995
|
June 1998
|
|||
| Predictedc | Observedd | Predictedc | Observedd | |
| 0 | 96.28 | 103 | 110.85 | 125 |
| 1 | 62.99 | 56 | 52.26 | 38 |
| 2 | 15.76 | 16 | 9.13 | 10 |
| 3 | 1.87 | 2 | 0.73 | 0 |
| 4 | 0.10 | 0 | 0.03 | 0 |
| 5 | 0.00 | 0 | 0.00 | 0 |
Overall goodness of fit involves combining the organism-specific Poisson distributions to provide the predicted distribution of total errors. These can be compared to the observed errors by using a chi-square statistic to assess if this procedure successfully predicts OPT event outcomes (P = 0.93 and 0.72, each on 5 degrees of freedom, for 1995 and 1998, respectively).
Total number of misidentifications in the test event.
Total number of laboratories predicted to make the indicated number of misidentifications in the test event.
Total number of laboratories which actually made the indicated number of misidentifications in the test event.
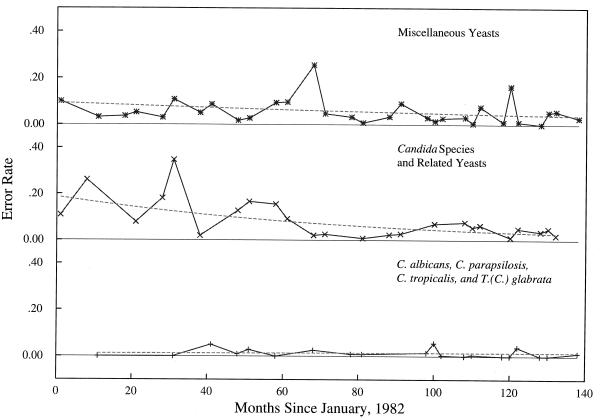
Thirteen organism-specific and grouped error rates estimated from CPT samples collected in 1988 and 1989 were compared to those collected in 1993 and 1994. No significant differences were detected between the two time periods (Fig. 6).
FIG. 6.
Grouped and organism-specific comparison of CPT error rates from 1988 to 1989 and 1993 to 1994 (Table 3). No errors were observed in 1990 data for some groups (a). C. albicans had only one error in 81 (1%) specimens from 1988 to 1989 but seven errors in 77 (9%) specimens from 1994 to 1995 (P = 0.02) (b). However, the figure displays 13 simultaneous comparisons so an appropriate significance level is calculated by the following equation: 0.05/13 = 0.003. No errors were observed in data for some groups (c).
Error rates in the identification of the majority of those individual fungi for which sufficient data were collected for comparative analysis were found to be statistically significantly greater in CPT than in OPT (Fig. 7). While none of the participating laboratories misidentified A. fumigatus in OPT specimens, greater than 25% (P < 0.002) of the isolates of this clinically significant mold were not correctly identified when recovered from routine clinical specimens (CPT). Similarly, almost all laboratories appropriately identified C. parapsilosis, C. tropicalis, and C. glabrata in OPT events, but about 15% (P < 0.005, P < 0.004, and P < 0.005, respectively) of these yeasts were misidentified when isolated as part of routine workloads. The miscellaneous yeasts placed into group V were also significantly more difficult to identify in CPT than as part of an OPT event (P < 0.008).
FIG. 7.
Grouped and organism-specific comparison of OPT and combined CPT error rates from 1988 to 1994 (Table 3). For some groups no errors were observed in OPT (a), in CPT (b), or in all data (c).
In contrast, there were no differences in the error rates found in OPT and CPT in the identification of C. albicans (11 versus 10%) and T. tonsurans (10 versus 12%). While there were differences from CPT in the capability of participating laboratories to identify members of groups I to IV (13 to 25 CPT isolates) and group VI (three CPT isolates) when presented in OPT events, small sample sizes prevented these differences from becoming statistically significant.
DISCUSSION
The inferences drawn from this observational study are influenced by its design, which did not randomly select laboratories and compare their matched OPT and CPT performance through time. There is therefore a possibility that differential selection biases and participant turnover may have influenced the results. While the 188 OPT participants are a census, the 88 CPT participants were haphazardly selected, except that New York City laboratories were underrepresented. This would be more troubling if there was not a high correlation of laboratory location with regional population size to suggest that urban laboratories continue to be represented in the CPT in other regions of the state. The failure to find differences between the two time periods in which CPT samples were collected is an indicator that the inclusion of 22 new laboratories among the 1993 and 1994 CPT participants did not introduce biases into the estimated error rates. Similarly, the low number of participating laboratory changes in the OPT data suggests that this contribution to bias was likely to be small. Finally, the maximal OPT turnover was due to the introduction of new laboratories in the last two events, and these have the smallest influence on the error rate estimates, which are largely controlled by laboratories with multiple-event organism-specific errors.
Permit holders must identify 80% of test specimens in two of three consecutive OPT test events in order to qualify for and maintain their permits. In contrast, for the purposes of this study, species-specific identification errors were used to compare laboratory performances in OPT and CPT. Ideally, precise species-specific error rates would be estimated from a large data pool collected under both testing modes from each laboratory on a single date. The effect of pooling the data, required by the small numbers of samples, can be examined by noting that of the 125 laboratories who participated in at least five OPT events from 1988 to 1994, 79 (63%) had less than 10% misidentifications, 39 (31%) misidentified 10 to 15%, and 7 (6%) had more than 15% misidentifications. Compared to a 10% misidentification rate for the majority of facilities four of the seven with more than 15% errors displayed significantly higher errors (P < 0.05). Nevertheless, the distribution of errors displayed by all the laboratories is a continuum starting from a minimum of 1.3% and ending at the maximum of 22.4% (data not shown). Since all laboratories were pooled, the degree of the observed differences therefore depends on the inclusion of a few laboratories with noticeably poor performance.
Although it is possible that grouping could obscure differential performance with a particular organism, segregating out such candidates in these data will not identify additional significant differences due to the small sample sizes. Coalescing the data into six groups, in addition to selected individual organisms, has resulted in detecting differences between CPT and OPT performances only for group V, miscellaneous yeast. In group V the OPT organism most difficult to identify was Geotrichum candidum, with 38 of 131 (29%) samples misidentified. No confirmed identified isolates of this organism were recovered in CPT. The corresponding group V CPT error estimates are therefore presumably smaller than if G. candidum had been recovered. Grouping, by retaining a difficult organism in only the OPT part of the comparison, has therefore introduced a bias diminishing the estimated OPT-CPT differences.
The significantly decreasing error rates in identification of mold and yeast test specimens found in the longitudinal OPT portion of the study provide evidence that OPT is useful as an educational tool and thereby successfully fulfills one of its goals (25). Coupling this with the low laboratory turnover suggests that the presentation of clinically significant fungi in OPT events is correlated with improved OPT performance over time. Many of the OPT events were composed of organisms rarely seen in patient samples (Table 3), encouraging participants to enhance their identification capabilities. This contributed to more than 95% of the laboratories correctly identifying mold and/or yeast specimens in each of the later OPT events. Error rates for many of the test organisms employed in OPT were found to be so consistent over the later portion of the study period, 1988 to 1994, that 1995 to 1998 event results could be predicted prior to the shipment of test specimens. A control group of laboratories sampled over the same time period but not subjected to OPT is needed to confirm that the improvement was due solely to the state OPT. Although such control laboratories do not exist, participants indicated that greater efforts were expended to correctly identify isolates, particularly after failures in the early 1980s.
Improved OPT performance may or may not indicate that the diagnostic capabilities of participating laboratories simultaneously improve. The CPT study examined patient specimens from permitted laboratories during two periods of recent performance and found no significant changes in error rates. The differences between the OPT and CPT performances, discovered by putting the analysis on an organism- or group-specific basis, correspond to the relative difficulty of the identification. Commonly seen and easily identified fungi such as C. albicans and T. tonsurans were misidentified about 10% of the time under both protocols. The differential misidentification rates of A. fumigatus, C. parapsilosis, C. tropicalis, C. glabrata, and group V organisms are disquieting. These differences may, in part, be due to OPT’s use of ideal representatives monocultured under optimum growth conditions. Alternatively, laboratories have the motivation and opportunity to expend greater efforts examining OPT isolates. For example, the 25% higher misidentification rate for CPT isolates of A. fumigatus could be due to some laboratories’ omitting parts of the standard techniques used in the identification of this organism when it is recovered from patient specimens.
There are several other ways that laboratories can favorably affect OPT performance. For administration reasons schedules of OPT events are announced at the beginning of each year. This information permits participants to optimize their performance by coincidentally scheduling reagent renewals and maintenance activities. Additionally, there is sufficient volume in some test categories’ OPT samples for laboratories to perform multiple tests and report either the best result or eliminate outliers and report an average of the remainder. Finally, the most proficient medical mycology staff may be specifically assigned to process the OPT samples and may spend extra time and care in executing each analysis.
This intensive study of the value of OPT in clinical mycology relies on detailed comparisons to error rates found in blind covert retrotesting, i.e., to laboratory performances achieved on actual patient samples. Previous reports on mycology OPT have described the fungi used as test samples and the relationship of the volume of samples processed by laboratories with their OPT performances (3, 10, 12, 13, 31). Published analyses of OPTs in mycobacteriology have discussed the effects of the types of identification methods on the results reported by the participating facilities, as well as described test results over time (33). Investigations in other areas of microbiology (14, 32) have dealt solely with the evaluation of OPT test specimens, internal laboratory quality control monitoring (2), or, as here, comparisons of OPT and blinded specimens (5, 6, 24). Richardson et al. (24) compared OPT results with routine work on patient specimens and found that laboratories performing poorly on OPT lacked effective quality control, used nonstandard methods, and failed to follow in-house procedure manuals during analyses.
The selection of the current retrospective design was partially motivated by the infeasibility of splitting fungal samples. However, Black and Dorse (5, 6) report on a bacteriology study that used a simulated blind split sample submission design. This design allowed the examination of performance at all stages of normal specimen handling. Their results revealed that some laboratories were unable to isolate organisms from the test specimens confirmed positive. Additionally, they found correct identifications of mucoid Escherichia coli decreased from 94% under OPT to 47% under CPT, as well as large decreases for Yersinia enterocolitica (45%) and streptococcal Lancefield group C (13%). Significant variation was also found depending on whether the isolate was from urine, pus, or feces. Additionally, in contrast to the results of this investigation, the most commonly encountered organisms were among the worst handled. These authors suggested that a laboratory’s OPT ability to identify (lyophylized) bacteria has little to do with its performance on normal patient specimens.
Studies of OPTs in quantitative clinical areas, e.g., clinical chemistry, diagnostic immunology, and hematology, etc., have concentrated on its use in evaluating interlaboratory variations associated with specific diagnostic techniques, establishing grading criteria and reviewing personnel qualifications (1, 9, 18–20, 30). Hansen et al. (16) found greater than 50% decreases in correct analyses under CPT for barbiturates, amphetamines, cocaine, codeine, and morphine. The authors speculated that the 13 laboratories under study used a higher minimum reporting level under CPT than under OPT. Inferences about drug testing sensitivity obtained via OPT were therefore artificially inflated. Sargent et al. (26) submitted 53 blinded specimens with known blood lead concentrations to 18 laboratories. The samples had concentrations more than 30% higher or lower than the Centers for Disease Control and Prevention threshold of 10 μg/dl. With respect to this threshold they estimated the correct classification rate (95% confidence interval) to be in the range of 79 to 95%. OPT of about 100 laboratories with similar samples in the New York State Blood Lead Proficiency Testing Program typically leads to more than 97% of samples being correctly classified. In contrast, Schalla et al. (27) found no difference between OPT and CPT in examining the results of split samples sent to 262 participants testing for HIV antibodies.
The results of this and previous studies point to limitations on the effectiveness of evaluating a laboratory’s capability in medical mycology under the OPT paradigm. The scheme mandated by CLIA ’88 introduces further limitations. Fifteen specimens, presuming they were all of a single species whose nominal error rate is 0, are sufficient to identify only laboratories with greater than a 25% error rate (at P = 0.05). However, test events are actually composed of a mixture of specimens of differing difficulties (Table 3), where the average nominal error rate is closer to 10% than to 0. In this case 15 specimens, three events with nominal 10% rates, are sufficient to identify only laboratories with greater than a 40% error rate. Various adjustments to the scoring scheme further inflate the error rate at which poor performers are identified. For example, the OPT error rates found for Torulopsis candida, Blastoschizomyces capitatus, G. candidum, and Candida lipolytica exceed 20%, but all laboratories received passing scores under the rule requiring specimens with less than 80% correct responses to be declared invalid.
We have considered in detail the open aspect of the CLIA ’88 testing scheme by comparing OPT species-specific results to double-blind covert testing. For easily identified species, such as C. albicans, blinding the testing appears to make little difference. For “difficult” species, such as A. fumigatus, whose nominal error rate in OPT is close to 0, the open testing protocol fails to identify laboratories whose usual error rate in the identification of patient specimens exceeds 65%. These results, as well as the cited concordant literature, clearly indicate the need for improving proficiency testing schemes for some analytes.
OPT is only one of the tools used to maintain and improve the quality of clinical laboratory services. In addition to laboratories’ routine internal quality control practices, they are regularly inspected to check adherence to testing protocols, validation procedures, and schedules for the maintenance and calibration of instrumentation. Additionally, regulators provide consultation services to laboratories actively seeking to improve the quality of their tests, changing testing procedures, or failing OPT. Nevertheless, open testing’s primary importance derives from the CLIA ’88 specification that the issuance and maintenance of state and federal licenses to conduct clinical testing depend upon laboratories meeting the specified OPT passing criteria. The CPT portion of this study suggests that improvements to OPT could be achieved by supplementing normal open testing with a sentinel system of blind testing with difficult specimens. The obstacles and extra costs associated with submissions and retrospective reanalyses are not insurmountable. One solution is to administer CPTs to sequential participant subsets preferentially constructed from laboratories with marginal performances. Even if only a portion of the participants are to be tested, simply announcing the implementation of such a sentinel system is likely to favorably impact performances on routine patient specimens at all laboratories.
The data presented suggest that (i) additional studies should be conducted to enhance efficient statistical comparisons of fungal identification error rates between OPT and CPT, (ii) OPT is not an effective mechanism for evaluating the ability of clinical mycology laboratories to identify all medically important fungi, and (iii) CPT, despite its high costs and administrative problems, should be used on a selective basis to augment OPT to obtain clearer perspectives of the performance of clinical mycology laboratories (25, 28).
FIG. 3.
OPT error rates for the five individual molds showing the greatest decreases between 1982 and 1994. Symbols indicate the observed percentages of errors committed at each event. Dashed lines, associated logistic regression curves. All decreases were statistically significant (P < 0.002).
ACKNOWLEDGMENTS
This project was supported under a cooperative agreement from the Centers for Disease Control and Prevention through the Association of Schools of Public Health.
Special thanks go to Vishnu Chaturvedi, current director of the New York State Laboratories for Mycology and the Mycology Proficiency Testing Program, for supporting and reviewing the manuscript. Additional thanks go to John Qualia and Teresa Wilson for assisting with database management and computer program development.
REFERENCES
- 1.Bakken L L, Case K L, Callister S M, Bourdeau N J, Schell R F. Performance of laboratories participating in a proficiency testing program for Lyme disease serology. JAMA. 1992;268:891–895. [PubMed] [Google Scholar]
- 2.Bartlett R C, Mazens-Sullivan M, Tetreault J Z, Lobel S, Nivard J. Evolving approaches to management of quality in clinical microbiology. Clin Microbiol Rev. 1994;7:55–88. doi: 10.1128/cmr.7.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartola J. The importance of an effective proficiency testing program to the regulation of clinical laboratories. The view from one state. Arch Pathol Lab Med. 1988;112:368–370. [PubMed] [Google Scholar]
- 4.Bishop Y M M, Fienberg S E, Holland P W. Discrete multivariate analysis: theory and practice. Cambridge, Mass: MIT Press; 1975. pp. 123–155. [Google Scholar]
- 5.Black W A, Dorse S E. A regional quality control program in microbiology. I. Administrative aspects. Am J Clin Pathol. 1976;66:401–406. doi: 10.1093/ajcp/66.2.401. [DOI] [PubMed] [Google Scholar]
- 6.Black W A, Dorse S E. A regional quality control program in microbiology. II. Advantages of simulated clinical specimens. Am J Clin Pathol. 1976;66:407–415. doi: 10.1093/ajcp/66.2.407. [DOI] [PubMed] [Google Scholar]
- 7.Boone D J, Hansen H J, Hearn T L, Lewis D S, Dudley D. Laboratory evaluation and assistance efforts: mailed, on-site and blind proficiency testing surveys conducted by the Centers for Disease Control. Am J Public Health. 1982;72:1364–1368. doi: 10.2105/ajph.72.12.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox D R. Analysis of binary data. London, England: Methuen & Co., Ltd.; 1970. pp. 87–94. [Google Scholar]
- 9.Dayian G, Morse D L, Schryver G D, Stevens R W, Birkhead G S, White D J, Hechemy K E. Implementation of a proficiency testing program for Lyme disease in New York State. Arch Pathol Lab Med. 1994;118:501–505. [PubMed] [Google Scholar]
- 10.Dolan C T. A summary of the 1972 mycology proficiency testing survey of the College of American Pathologists. Am J Clin Pathol. 1974;61:990–993. [PubMed] [Google Scholar]
- 11.Edwards A W F. Likelihood: an account of the statistical concept of likelihood and its application to scientific inference, rev. ed. Baltimore, Md: Johns Hopkins University Press; 1992. [Google Scholar]
- 12.Fuchs P C, Dolan C T. Summary and analysis of the mycology proficiency testing survey results of the College of American Pathologists. Am J Clin Pathol. 1981;76:538–543. [PubMed] [Google Scholar]
- 13.Griffin C W, III, Mehaffey M A, Cook E C, Blumer S O, Podeszwik P A. Relationship between performance in three of the Centers for Disease Control microbiology proficiency testing programs and the number of actual patient specimens tested by participating laboratories. J Clin Microbiol. 1986;23:246–250. doi: 10.1128/jcm.23.2.246-250.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin C W, III, Cook E C, Mehaffey M A. Centers for Disease Control performance evaluation program in bacteriology: 1980 to 1985 review. J Clin Microbiol. 1986;24:1004–1012. doi: 10.1128/jcm.24.6.1004-1012.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamlin W. Proficiency testing as a regulatory device: a CAP perspective. Clin Chem. 1992;38:1234–1236. [PubMed] [Google Scholar]
- 16.Hansen H J, Caudill S P, Boone D J. Crisis in drug testing. Results of CDC blind study. JAMA. 1985;253:2382–2387. [PubMed] [Google Scholar]
- 17.Howanitz P J. Use of proficiency test performance to determine clinical laboratory director qualifications. Arch Pathol Lab Med. 1988;112:349–353. [PubMed] [Google Scholar]
- 18.Hurst J, Nickel K, Hilborne L H. Are physicians’ office laboratory results of comparable quality to those produced in other laboratory settings? JAMA. 1998;279:468–471. doi: 10.1001/jama.279.6.468. [DOI] [PubMed] [Google Scholar]
- 19.Jenny R W, Jackson K Y. Evaluation of the rigor and appropriateness of CLIA ’88 toxicology proficiency testing standards. Clin Chem. 1992;38:496–500. [PubMed] [Google Scholar]
- 20.Jenny R W, Jackson K Y. Proficiency test performance as a predictor of accuracy of routine patient testing for theophylline. Clin Chem. 1993;39:76–81. [PubMed] [Google Scholar]
- 21.Luntz M E, Castleberry B M, James K. Laboratory staff qualifications and accuracy of proficiency test results. Arch Pathol Lab Med. 1992;116:820–824. [PubMed] [Google Scholar]
- 22.Matsunawa T. Poisson distribution. In: Kotz S, Johnson N L, editors. Encyclopedia of statistical science. Vol. 7. New York, N.Y: John Wiley & Sons; 1986. pp. 20–25. [Google Scholar]
- 23.Merz W G, Roberts G D. Detection and recovery of fungi from clinical specimens. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 709–722. [Google Scholar]
- 24.Richardson H, Wood D, Whitby J, Lannigan R, Fleming C. Quality improvement of diagnostic microbiology through a peer-group proficiency assessment program. A 20-year experience in Ontario. The Microbiology Committee. Arch Pathol Lab Med. 1996;120:445–455. [PubMed] [Google Scholar]
- 25.Salkin I F, Limberger R J, Stasik D. Commentary on the objectives and efficacy of proficiency testing in microbiology. J Clin Microbiol. 1997;35:1921–1923. doi: 10.1128/jcm.35.8.1921-1923.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sargent J D, Johnson L, Roda S. Disparities in clinical laboratory performance for blood lead analysis. Arch Pediatr Adolesc Med. 1996;150:609–614. doi: 10.1001/archpedi.1996.02170310043008. [DOI] [PubMed] [Google Scholar]
- 27.Schalla W O, Blumer S O, Taylor R N, Muir H W, Hancock J S, Gaunt E E, Seilkop S K, Huges S S, Holzworth D A, Hearn T L. HIV blind performance evaluation: a method for assessing HIV-antibody testing performance. In: Krolak J M, O’Connor A, Thompson P, editors. Institute on critical issues in health laboratory practice: frontiers in laboratory practice research. Atlanta, Ga: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention; 1995. p. 409. [Google Scholar]
- 28.Shahangian S. Proficiency testing in laboratory medicine. Arch Pathol Lab Med. 1998;122:15–30. [PubMed] [Google Scholar]
- 29.STSC, Inc. APL*PLUS II system for Unix, version 4. Rockville, Md: STSC, Inc.; 1991. [Google Scholar]
- 30.Stull T M, Hearn T L, Hancock J S, Handsfield J H, Collins C L. Variation in proficiency testing performance by testing site. JAMA. 1998;279:463–467. doi: 10.1001/jama.279.6.463. [DOI] [PubMed] [Google Scholar]
- 31.Wilson M E, Faur Y C, Schaefler S, Weitzman I, Weisburd M H, Schaeffer M. Proficiency testing in clinical microbiology: the New York City program. Mt Sinai J Med. 1977;44:142–163. [PubMed] [Google Scholar]
- 32.Woods G L, Bryan J A. Detection of Chlamydia trachomatis by direct fluorescent antibody staining. Results of the College of American Pathologists proficiency testing program, 1986–1992. Arch Pathol Lab Med. 1994;118:483–488. [PubMed] [Google Scholar]
- 33.Woods G L, Long T A, Witebsky F G. Mycobacterial testing in clinical laboratories that participate in the College of American Pathologists mycobacteriology surveys—changes in practices based on responses to 1992, 1993, and 1995 questionnaires. Arch Pathol Lab Med. 1996;120:429–435. [PubMed] [Google Scholar]