Abstract
The diversity of the cytotoxin-associated gene (cagA) of Helicobacter pylori was analyzed in 45 isolates obtained from nine countries. We examined variation in the 5′ end of the cagA open reading frame as determined by PCR and sequencing. Phylogenetic analysis revealed the existence of at least two distinct types of cagA. One variant (cagA1) was found exclusively in strains from Europe, the United States, and Australia, whereas a novel variant (cagA2) was found in strains from East Asia. The greatest diversity between cagA1 and cagA2 was found in the first 20 amino acids of the cagA open reading frame, where several consistent insertions or deletions were observed. Additional cagA sequence variants that could be classified as separate subtypes were found in two of three Peruvian and in five of seven U.S. strains tested. The calculated isoelectric point of the first 154 amino acids of the cagA1 variants (7.52 ± 1.54) was significantly higher than that of the first 154 amino acids of the cagA2 variants (5.61 ± 0.94; P < 0.001). Most cagA2 strains contained vacA subtype s1c (P < 0.001), and in vacA m1 strains cagA1 was more frequently observed than cagA2. These results show the epidemiological relationship between cagA and vacA at the subtype level and indicate the existence of distinct H. pylori lineages that are not uniformly distributed over the globe.
Helicobacter pylori is a medically important bacterium that is involved in the pathogenesis of peptic ulcer disease and that is associated with gastric carcinoma. The ecological niche of H. pylori is the human stomach, where it establishes a long-term colonization of the mucosa (8). During the past decade, products of several H. pylori genes that are markers for differences in clinical outcomes in colonized persons have been identified (7).
A cytotoxin that may damage epithelial cells by inducing the formation of vacuoles is encoded by vacA (22). Although vacA is present in all H. pylori strains, it contains at least two variable parts (4). The s region (which encodes the signal peptide) exists as s1 or s2 allelic types. Among type s1 strains, subtypes s1a, s1b, and s1c have been identified, and the m region (the middle region) occurs as m1, m2a, or m2b allelic types (43). The particular vacA s/m genotype is a marker of the pathogenicity of an individual strain, since in vitro production of the cytotoxin, in vivo epithelial damage, and development of peptic ulcer disease are all related to the vacA genotype (4, 5).
The cytotoxin-associated gene (cagA), which is not present in every H. pylori strain (11, 12, 37), is a marker for a genomic pathogenicity (cag) island of about 40 kbp (2, 35). The presence of this island is associated with more severe clinical outcomes (6, 21, 28) as well as with protection from esophageal diseases (10, 46). The function of CagA has not yet been determined. The cag island contains other genes that encode proteins that enhance the interaction of the strain with host cells, for example, by induction of cytokine production (2, 9, 38). There is a close association between the presence of cagA and vacA type s1, because most s1 strains are cagA positive (4).
The use of DNA fingerprinting techniques has revealed substantial genetic heterogeneity among different clinical isolates (1, 24, 41), and the total genetic variation within H. pylori is greater than that in other bacteria that have been studied (14). However, there is heterogeneity in the degree of variation; e.g., the variability of single-copy genes encoding enzymes may be restricted by functional constraints on the encoded proteins. Since cagA is an important marker for H. pylori strains that are highly interactive with the host, the present study aimed to investigate the existence of distinct cagA variants. Earlier studies indicated that the 5′ part of cagA is more conserved than the middle and 3′ end of the gene (11, 37). Since cagA is considered an important pathogenicity marker, the sequence heterogeneity of the 5′ end of cagA as well as the relationship with vacA variants was investigated among H. pylori strains of various geographic origins. We found that distinct cagA variants exist, with specific geographic distributions and particular associations with vacA genotypes.
MATERIALS AND METHODS
H. pylori isolates.
A total of 45 H. pylori isolates were obtained from different countries, including Australia (n = 5), China (n = 4), Hong Kong (n = 9), Japan (n = 5), The Netherlands (n = 6), Peru (n = 3), Portugal (n = 3), Thailand (n = 3), and the United States (n = 7). These were randomly selected from a large number of cultures representing cultures of isolates from each country. Each isolate was obtained from a different patient who underwent gastroscopy and gastric biopsy for dyspeptic symptoms. After primary isolation and identification of the gastric organisms as H. pylori, the strains were frozen at −70°C until their use in these studies. Subsequently, bacteria were cultured on Trypticase soy agar plates containing 5% sheep blood (Becton Dickinson) for 3 to 5 days at 37°C under microaerobic conditions (5% O2, 10% CO2, 85% N2). The H. pylori cells were harvested from plates by suspension in 2 ml of sterile 0.9% NaCl solution and were pelleted by centrifugation at 10,000 × g for 2 min. The cells were resuspended in 400 μl of 10 mM Tris-HCl (pH 8.0), 5 mM EDTA, 0.1% sodium dodecyl sulfate, and 0.1 mg of proteinase K per ml and were incubated for 2 to 4 h at 55°C. Proteinase K was inactivated by incubation at 95°C for 10 min. The lysate was clarified by centrifugation at 14,000 × g for 2 min. The clarified supernatant was diluted 1/100 in sterile water and was directly used for PCR.
PCRs.
All primers used in this study are shown in Table 1. Primer set cagP1-B1 and primer set cagF2-B1 were used to amplify the 5′ 450 bp of the cagA open reading frame (ORF). Primers cagAF and cagAR were used as a universal cagA detection primer set. Separate PCRs were used to detect the presence of cagE and cagT in the cag pathogenicity island.
TABLE 1.
PCR primers for amplification of cagA used in this study
| Primer designation (polarity) | Primer sequence (5′→3′) | Position |
|---|---|---|
| cagP1 (+) | CCATTTTAAGCAACTCCATAAACC | 552–575a |
| cagF1 (+) | TGGGTAAAAATGTGAATCGT | 858–877a |
| cagF2 (+) | AAGATACCGATAGGTATGAA | 1032–1051a |
| B1 (−)b | TCTGCCAAACAATCTTTTGCAG | 1557–1578a |
| cagAF (+) | Bioc-TTGACCAACAACCACAAACCGAAG | 1090–1113a |
| cagAR (−) | Bio-CTTCCCTTAATTGCGAGATTCC | 1251–1272a |
| cagEF (+)d | GCGATTGTTATTGTGCTTGTAG | 16891–16870e |
| cagER (−)d | GAAGTGGTTAAAAAATCAATGCCCC | 16563–16587e |
| cagTF (+)d | CCATGTTTATACGCCTGTGT | 442–461e |
| cagTR (−)d | CATCACCACACCCTTTTGAT | 723–742e |
All PCR mixtures consisted of 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 200 μM deoxynucleotides, 25 pmol each of the forward and reverse primers, and 1.5 U of AmpliTaq Gold DNA polymerase (Perkin-Elmer) in a final volume of 50 μl. One microliter of DNA from a culture lysate was used in each PCR. The mixture was covered with mineral oil to prevent evaporation. The PCR program comprised 9 min of predenaturation at 94°C to activate the AmpliTaq Gold DNA polymerase, followed by 40 cycles of 30 s at 94°C, 45 s at 50°C, 45 s at 72°C, and a final incubation at 72°C for 5 min.
Sequence analysis of cagA.
The PCR products, synthesized with either primers cagP1 and B1 or primers cagF2 and B1, were inspected by electrophoresis on 2% agarose gels. The PCR products were sequenced with the Thermo-Sequenase cycle sequencing kit (Amersham) by using Cy-labeled primers, followed by electrophoresis on an ALF-express automatic sequencer (Pharmacia Biotech). Sequences were analyzed with PC-Gene software (Intelligenetics Inc.) and ClustalX alignment software. Phylogenetic analyses were performed with Windows Easy Tree software (version 1.31) (12a). Pairwise sequence comparisons were made by using the Jukes and Cantor parameters, and matrices of sequence distances were produced. Phylogenetic relationships were further analyzed by the neighbor-joining method with 500 bootstrap steps, and the program Treecon (39) was used to create a graphic output.
Reverse hybridization LiPA.
The presence of cagA and the subtypes of the vacA s and m regions were determined by multiplex PCR followed by reverse hybridization by a line probe assay (LiPA), as described earlier (32, 42). This assay consists of a nitrocellulose strip that contains poly(dT)-tailed oligonucleotide probes for cagA and the vacA s and m region genotypes (s1a, s1b, s1c, m1, m2a, and m2b) immobilized as parallel lines. Briefly, PCR products from cagA and the vacA s and m regions (containing biotin at the 5′ end of each primer) were mixed and denatured by addition of 400 mM NaOH and 10 mM EDTA in a plastic trough. Hybridization buffer and a LiPA strip were added, hybridization was performed, and the strip was stringently washed. Hybrids were detected by addition of conjugate (streptavidin-alkaline phosphatase) and substrate (4-nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolylphosphate). Hybrids are visible as purple probe lines. Interpretation of the hybridization patterns was performed visually.
Statistical analysis.
Data were analyzed by the χ2 test or Fisher’s exact test.
RESULTS
Nucleotide sequence analysis and distinct variants of cagA.
The sequence heterogeneity of cagA was analyzed for 45 H. pylori isolates randomly selected from a larger panel of cultures obtained from nine different countries in Europe, North America, South America, and Asia by PCR and direct sequencing. Primer set cagP1 and B1 (1,026 bp) or primer set cagF2 and B1 (546 bp) was used to amplify the 5′ end of the cagA ORF. Neither of the two primer sets permitted amplification of cagA sequences for all 45 strains. However, with both primer sets, the sequences encoding the first 154 amino acids of the cagA ORF from all strains could be determined. cagE and cagT sequences also could be amplified from each of the 45 strains, suggesting that instances when the cagA pathogenicity island is not intact are uncommon.
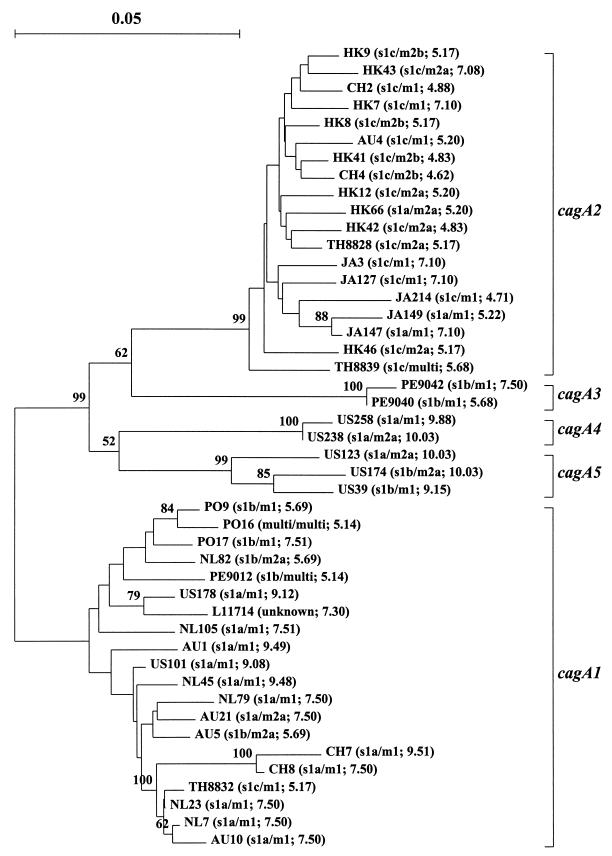
Phylogenetic analyses of cagA sequences revealed the existence of several distinct groups (Fig. 1). The first group, designated cagA1, comprised 19 strains, mainly from Europe and Australia, and the reference sequence from U.S. strain ATCC 53726 (GenBank accession no. L11714), as well as a reference strain from Italy (GenBank accession no. X70039), which is virtually identical to ATCC 53726 in that region. The second group was designated cagA2 and exclusively comprised 19 strains from East Asia. The patient from whom strain AU4 was obtained lived in Australia but also originated from East Asia.
FIG. 1.
Phylogenetic tree of cagA nucleotide sequences encoding the N-terminal 149 to 154 amino acids of the cagA ORF, including one reference sequence (GenBank accession no. L11714). Clusters of subtypes cagA1 to cagA5 are indicated, and bootstrap values greater than 50 are shown. Letters in the names of the isolates indicate the country of origin: Australia (AU), Costa Rica (CR), China (CH), Hong Kong (HK), Japan (JA), The Netherlands (NL), Peru (PE), Portugal (PO), Thailand (TH), and the United States (US). The vacA s and m subtypes and the isoelectric point of the deduced protein sequence are shown in parentheses after each sequence name (Multi indicates the presence of multiple vacA subtypes). A reference bar is shown for molecular distance.
In two of four Peruvian and in five of seven U.S. strains, variant cagA sequences that belonged to neither cagA1 nor cagA2 were found. On the basis of the distribution of molecular distances, as shown in the phylogenetic tree (Fig. 1), three possible additional cagA subtypes comprising sequences from Peru (cagA3) and the United States (cagA4 and cagA5) could be distinguished. Formal classification of these sequences would require a larger number of strains from these parts of the world.
Analyses of the cagA sequences.
The average G+C content was 36.3% and was not significantly different between the cagA1 (37.3%) and cagA2 (36.0%) variants. These values are similar to the 35% G+C content of the cag pathogenicity island and lower than the average 39% G+C content of the entire genome, as reported earlier (36).
On the basis of the alignment of the 45 novel and 1 reference cagA sequences, a set of general PCR primers (cagAF and cagAR) which permitted detection of cagA in all strains studied was designed. Strains that were negative with various other combinations of the cagA primer sets also remained negative with the novel cagAF and cagAR primer set (data not shown).
Pairwise comparisons of all sequences showed that nucleotide sequences were more than 95% conserved within these two groups (Table 2). Transitions were on average 2.2-fold more frequent than transversions. As expected, nonsynonymous substitutions (Ka) were less common than synonymous substitutions (Ks). That the mean synonymous substitution rate between cagA1 and cagA2 strains was greater than that within each group is consistent with the phylogenetic tree in Fig. 1, indicating that the deep branching represents the most fundamental dichotomy in the populations. That within each branch the synonymous substitution rates are similar suggests that the two branches are about the same age. Furthermore, the Ka/Ks ratio serves as an index of the functional constraints on the protein. The similarity of these ratios within cagA1 and cagA2 indicates that they are subject to similar constraints. That comparison between cagA1 and cagA2 yields a higher ratio indicates that to a certain degree the constraints are different.
TABLE 2.
Phylogenetic analysis of 46 cagA sequencesa
| Sequence compared | Distanceb | Homologyc | Transitions (Ts)d | Transversions (Tv)e | Ts:Tv | Ksf | Kag | Ka/Ks ratio |
|---|---|---|---|---|---|---|---|---|
| cagA1(n = 20) | 0.032 ± 0.012 | 96.2 ± 2.5 (94.2 ± 2.3) | 0.024 ± 0.009 | 0.008 ± 0.006 | 3.0 | 0.060 ± 0.024 | 0.023 ± 0.011 | 0.38 |
| cagA2(n = 19) | 0.027 ± 0.011 | 95.9 ± 1.8 (94.8 ± 2.7) | 0.020 ± 0.006 | 0.007 ± 0.003 | 2.9 | 0.064 ± 0.026 | 0.018 ± 0.007 | 0.28 |
| cagA1 + cagA2(n = 39) | 0.068 ± 0.039 | 93.6 ± 3.5 (90.5 ± 5.6) | 0.045 ± 0.023 | 0.024 ± 0.016 | 1.9 | 0.110 ± 0.052 | 0.053 ± 0.033 | 0.48 |
| All cagA sequences (n = 46) | 0.077 ± 0.037 | 92.8 ± 3.3 (83.1 ± 5.5) | 0.053 ± 0.025 | 0.025 ± 0.014 | 2.1 | 0.121 ± 0.051 | 0.060 ± 0.032 | 0.50 |
Based on the 5′ 462 bp from the cagA ORF in each of the 46 H. pylori strains.
Average substitution rates (corrected with the Jukes and Cantor parameters) ± standard deviation.
Average percentage similarity ± standard deviation based on pairwise comparisons of sequences of the corresponding subtypes. Homologies are based on nucleotide sequences; values in parentheses are based on deduced amino acid sequences.
Transitions are nucleotide replacements of one purine by another purine.
Transversions are nucleotide replacements of a purine by a pyrimidine or vice versa.
Synonymous substitutions do not result in an amino acid change in the encoded open reading frame.
Nonsynonymous substitutions result in an amino acid change.
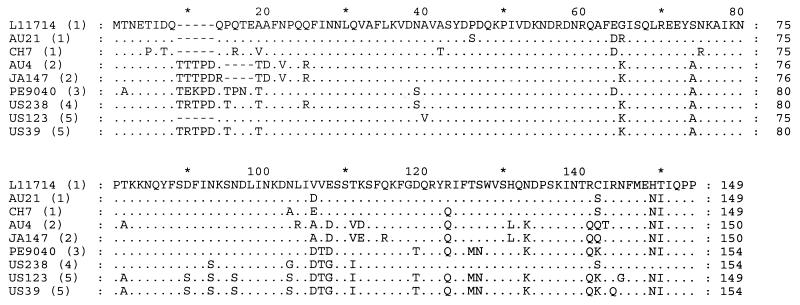
Taking all 46 cagA sequences together, the nucleotide and amino acid sequences were 92.8 and 83.1% conserved, respectively (Table 2). The deduced amino acid sequences of cagA1 and cagA2 were more than 94% conserved within each group. Analysis of representative amino acid sequences of the major cagA variants confirmed that cagA does not have a cleaved signal sequence (Fig. 2). The ATG start codon was completely conserved in all 46 sequences studied. No conserved cysteine residues are present in this part of the CagA protein. A potential N-linked glycosylation site at Asn3 was conserved in 43 (93%) of the 46 sequences. Potential phosphorylation sites were found in several sequences, but none of these was conserved.
FIG. 2.
Alignment of representative N-terminal amino acid sequences (residue 1 to residues 149 to 154) of the distinct cagA variants, as deduced from nucleotide sequences. To obtain proper alignment, a hyphen indicates the absence of an amino acid residue. Identical amino acids are indicated by a dot. The deduced amino acid sequence from GenBank accession no. L11714 is also shown as a reference. The numbers in parentheses indicate the cagA subgroup.
Compared to sequences from the cagA1 group, cagA2 sequences contain an insertion of five amino acids (TXTPD in 13 of 19 strains) between residues 9 and 10. Four strains (JA127, JA147, JA214, and AU4) show a deletion of four amino acids (PQTQ in L11714) between residues 14 and 19. Thus, the first 20 amino acids of the cagA ORF are highly polymorphic. There were no consistent differences between the hydrophilicity/hydrophobicity patterns of the deduced CagA protein sequences. However, the calculated isoelectric points of the deduced 154 N-terminal amino acids of CagA ranged from 4.62 (CH4) to 10.03 (US174 and US123), as indicated in Fig. 1. The average isoelectric point of the sequences from the cagA1 group (7.52 ± 1.54) was significantly higher (P < 0.001 by the t test) than that of the sequences from the cagA2 group (5.61 ± 0.94). The deduced protein sequences from the seven strains of subtypes cagA3 to cagA5 had even higher isoelectric points (8.88 ± 1.68).
Association of cagA variants with vacA types or subtypes.
All 45 strains were analyzed for subtypes of cagA (cagA1 to cagA5), the vacA s region (s1a, s1b, s1c, s2), and the vacA m region (m1, m2a, m2b) by PCR-LiPA (Table 3). Multiple vacA genotypes were observed in three strains (PE9012, PO16, and TH8839) and were excluded from the analysis. Of the 17 cagA1 strains with a single vacA genotype, 16 (94%) were s1a or s1b and only 1 (6%) was s1c. Conversely, of the 18 cagA2 strains with a single vacA genotype, 15 (83%) were s1c, whereas only 3 (17%) were s1a or s1b. This relationship between the vacA s-region subtype and the cagA subtype was highly significant (P < 0.001). Of the 17 cagA1 strains, 14 were m1 and 3 were m2. Conversely, of the 18 cagA2 strains, 8 were m1 and 10 were m2 (P = 0.035). The numbers of strains of the cagA3 to cagA5 genotypes were too small for association analysis.
TABLE 3.
Relationship between cagA and vacA subtypes in 45 H. pylori strains
| cagA genotype | No. of strains with the following vacA genotype:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| s1a
|
s1b
|
s1c
|
Multiplesa | Total | ||||
| m1 | m2 | m1 | m2 | m1 | m2 | |||
| cagA1b | 11 | 1 | 2 | 2 | 1 | 0 | 2 | 19 |
| cagA2c | 2 | 1 | 0 | 0 | 6 | 9 | 1 | 19 |
| cagA3 to cagA5 | 1 | 2 | 3 | 1 | 0 | 0 | 0 | 7 |
| Total | 14 | 4 | 5 | 3 | 7 | 9 | 3 | 45 |
Strains containing multiple vacA subtypes (PE9012, PO16, and TH8839).
The presence of cagA1 is associated with vacA type s1a or s1b (P < 0.001) and type m1 (P = 0.035).
The presence of cagA2 is associated with vacA type s1c (P < 0.001) and type m2 (P = 0.035).
DISCUSSION
Knowledge of the existence of different H. pylori genotypes may become clinically important. In Western populations, strains that contain cagA (indicating the presence of the pathogenicity island) are more strongly associated with more severe disease than strains that lack cagA (7, 12, 30, 34). Similarly, type s1 vacA strains are more often associated with disease than type s2 strains (3, 5), and responses to anti-Helicobacter therapy also may vary for strains of different genotypes (45). Adhesion of H. pylori to human gastric epithelium, mediated by histo-blood group antigens, also appears to be related to distinct allelic variants of the bacterial babA gene (18). In contrast, in Asian populations the association of cagA positivity and disease risk is much weaker or not present (13, 26, 27, 47). The existence of distinct variants of certain genes of the cag pathogenicity island may offer an explanation for this discrepancy. Recently, analysis of H. pylori strains from East Asian patients suggested that in some strains, only part of the cag pathogenicity island is present (17, 23). Some strains contained cagA but appeared to lack cagE and/or cagT, which was located in other parts of the pathogenicity island. That each of the 45 strains analyzed in the present study contained cagA and cagE, as well as cagT, suggests the presence of a complete pathogenicity island in these strains. Polymorphisms may have been responsible for failure to detect these other cag island genes in the previous studies (17).
The present study extended previous analyses of cagA heterogeneity (25, 40) and examined the relationship with vacA subtypes. The overall genetic heterogeneity of H. pylori is very high (14), and several studies reveal extreme variability, even within a single patient (19, 35, 41). The existence of distinct cagA variants that appear to be highly conserved (>95% at the nucleotide level and >94% at the amino acid level) confirms the observations of van der Ende et al. (40), who analyzed a more downstream portion of cagA. That the 5′ end of the cagA ORF was in frame in all 45 strains is consistent with a functional CagA protein. The nucleotide substitution rates (transitions to transversions and nonsynonymous to synonymous substitution rates) observed in cagA also are consistent with functional constraints at the protein level, implying that the cagA gene product has an in vivo role. The Ka/Ks ratio for cagA was much higher than the average value of 0.04 for Escherichia coli and Salmonella genes (31). One explanation could be the strong immunogenicity of the CagA protein, since most patients colonized by a cagA-positive H. pylori strain show high titers of anti-CagA antibodies (12, 29). A rabbit antiserum raised to a single CagA protein permitted detection of cagA-positive strains from several geographic regions (15), but whether variant-specific antibodies to CagA exist remains unknown. However, the efficacy of detection of anti-CagA antibodies by a single CagA variant should be evaluated separately with strains from patients from different geographic areas.
That the substitution rates among cagA1 and cagA2 were very similar suggests that the evolutionary ages for these variants are similar. On the basis of phylogenetic analyses, the number of recombinants between the distinct cagA subtypes appears to be limited. The heterogeneity of the single-copy genes that encode enzymes is usually restricted due to functional constraints on the encoded proteins, and substitutions tend to be synonymous. Virulence-associated genes may be subjected to selection for differences in biological specificity, resulting in phenotypically distinct variants distinguishable at the genetic level. Thus, the 5′ heterogeneity of cagA may be related to differences in biological activity of the CagA protein.
Although only the 154 N-terminal amino acids of the cagA ORF were analyzed, there were considerable differences in the isoelectric points of the deduced peptides. cagA transcription can be increased after exposure of H. pylori cells to acid pH, and CagA surface exposure also increased (20). Since H. pylori colonizes within the acidic environment of the gastric mucus layer, the isoelectric point may be important, especially if the protein is secreted or surface exposed, as CagA is. Differences in the CagA isoelectric points also may reflect particular adaptations to gastric acidity present in host populations.
Although several sets of PCR primers for cagA amplification have been described (13, 16, 37, 48), the utility of specific primers should be evaluated for each geographic area. The new cagA primers that we designed were deduced from the alignment of 46 sequences and permitted efficient amplification from all tested strains, indicating their broad applicability for analysis of H. pylori isolates of various geographic origins.
Analysis of H. pylori isolates of diverse geographic origins permitted a comprehensive description of the cagA and vacA variants. We confirmed the association between the presence of cagA and vacA s1 (44) and now show that there is also a highly significant association between the distinct cagA and vacA s1 subtypes. The different vacA s1-region subtypes are related to the level of cytotoxin production (4), but the evolutionary and functional relationships between vacA and cagA have not been elucidated. That the association between cagA and vacA variants was strong, but not perfect, is consistent with the recombinatorial genetic population structure of H. pylori (33). The observed relationship between variants of cagA and vacA provides further evidence for the existence of distinct clonal groupings of H. pylori. These data appear to be in contrast to those from studies of other genetic loci that showed that H. pylori does not have a strong clonal structure (14) and that recombination is frequent (33). That there are distinct East Asian and Western branches could indicate that cagA has been present in H. pylori for at least tens of thousands of years, before human populations segregated in Europe and Asia. On the basis of earlier studies we speculate that recombination at the cagA locus has been ongoing within each isolated geographic pool of strains. Further subtyping of H. pylori strains should facilitate insights into the evolution, epidemiology, and clinical significance of particular variants.
ACKNOWLEDGMENTS
C. Figueiredo is supported by PRAXIS XXI. This work was supported in part by R01 DK 53707 from the National Institutes of Health.
REFERENCES
- 1.Akopyants N S, Bukanov T U, Westblom T U, Kresovich S, Berg D E. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD profile. Nucleic Acids Res. 1992;20:S137–S142. doi: 10.1093/nar/20.19.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akopyants N S, Clifton S W, Kersulyte D, Crabtree J E, Youree B E, Reece C A, Bukanov N O, Drazek E S, Roe B A, Berg D E. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37–54. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 3.Atherton J C. The clinical relevance of strain types of Helicobacter pylori. Gut. 1997;40:701–703. doi: 10.1136/gut.40.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atherton J C, Cao P, Peek R M J, Tummuru M K, Blaser M J, Cover T L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 5.Atherton J C, Peek R M J, Tham K T, Cover T L, Blaser M J. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology. 1997;112:92–99. doi: 10.1016/s0016-5085(97)70223-3. [DOI] [PubMed] [Google Scholar]
- 6.Beales I L, Crabtree J E, Scunes D, Covacci A, Calam J. Antibodies to cagA protein are associated with gastric atrophy in Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1996;8:645–649. [PubMed] [Google Scholar]
- 7.Blaser M J. Intrastrain differences in Helicobacter pylori: a key question in mucosal damage? Ann Med. 1995;27:559–563. doi: 10.3109/07853899509002469. [DOI] [PubMed] [Google Scholar]
- 8.Blaser M J. Ecology of Helicobacter pylori in the human stomach. J Clin Invest. 1997;100:759–762. doi: 10.1172/JCI119588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow W H, Blaser M J, Blot W J, Gammon M D, Vaughan T L, Risch H A, Perez-Perez G I, Schoenberg J B, Stanford J L, Rotterdam H, et al. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res. 1998;58:588–590. [PubMed] [Google Scholar]
- 11.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cover T L, Glupczynski Y, Lage A P, Burette A, Tummuru M K, Perez-Perez G I, Blaser M J. Serologic detection of infection with cagA+Helicobacter pylori strains. J Clin Microbiol. 1995;33:1496–1500. doi: 10.1128/jcm.33.6.1496-1500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Dopazo, J. 1997. Windows Easy Tree software, version 1.31. [Online.] http://www.tdi.es. [30 April 1999, last date accessed.]
- 13.Go M F, Graham D Y. Presence of the cagA gene in the majority of Helicobacter pylori strains is independent of whether the individual has duodenal ulcer or asymptomatic gastritis. Helicobacter. 1996;1:107–111. doi: 10.1111/j.1523-5378.1996.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 14.Go M F, Kapur V, Graham D Y, Musser J M. Population genetic analysis of Helicobacter pylori by multilocus enzyme electrophoresis: extensive allelic diversity and recombinational population structure. J Bacteriol. 1996;178:3934–3938. doi: 10.1128/jb.178.13.3934-3938.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hook-Nikanne J, Perez-Perez G I, Blaser M J. Antigenic characterization of Helicobacter pylori strains from different parts of the world. Clin Diagn Lab Immunol. 1997;4:592–597. doi: 10.1128/cdli.4.5.592-597.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Husson M O, Gottrand F, Vachee A, Dhaenens L, De La Salla E M, Turclk D, Houcke M L H. Importance in diagnosis of gastritis of detection by PCR of the cagA gene in Helicobacter pylori strains isolated from children. J Clin Microbiol. 1995;33:3300–3303. doi: 10.1128/jcm.33.12.3300-3303.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikenoue T, Maeda S, Ogura K, Yoshida H, Kanai F, Shiratori Y, Omata M. Simple and practical methods to study cag pathogenicity island: usage of cagE and cagT gene amplifications. Gastroenterology. 1998;114:A157. (abstr. G0641). [Google Scholar]
- 18.Ilver D, Arnqvist A, Ogren J, Frick I M, Kersulyte D, Incecik E T, Berg D E, Covacci A, Engstrand L, Boren T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 19.Jorgensen M, Daskalopoulos G, Warburton V, Mitchell H M, Hazell S L. Multiple strain colonization and metronidazole resistance in Helicobacter pylori-infected patients: identification from sequential and multiple biopsy specimens. J Infect Dis. 1996;174:631–635. doi: 10.1093/infdis/174.3.631. [DOI] [PubMed] [Google Scholar]
- 20.Karita M, Tummuru M K, Wirth H P, Blaser M J. Effect of growth phase and acid shock on Helicobacter pylori cagA expression. Infect Immun. 1996;64:4501–4507. doi: 10.1128/iai.64.11.4501-4507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuipers E J, Perez-Perez G I, Meuwissen S G, Blaser M J. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995;87:1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- 22.Leunk R D, Johnson P T, David B S, Kraft W G, Morgan D R. Cytotoxic activity in broth culture filtrates of Campylobacter pylori. J Med Microbiol. 1988;26:93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- 23.Maeda, S., T. Ikenoue, K. Ogura, H. Yoshida, F. Kanai, N. Kato, Y. Shiratori, and M. Omata. Structure of cag pathogenicity island in Japanese and Western Helicobacter pylori isolates. Gastroenterology 114:A210 (abstr. G0861). [DOI] [PMC free article] [PubMed]
- 24.Marshall D G, Coleman D C, Sullivan D J, Xia H, O’Morain C A, Smyth C J. Genomic DNA fingerprinting of clinical isolates of Helicobacter pylori using short oligonucleotide probes containing repetitive sequences. J Appl Bacteriol. 1996;81:509–517. doi: 10.1111/j.1365-2672.1996.tb03540.x. [DOI] [PubMed] [Google Scholar]
- 25.Miehlke S, Kibler K, Kim J G, Figura N, Small S M, Graham D Y, Go M F. Allelic variation in the cagA gene of Helicobacter pylori obtained from Korea compared to the United States. Am J Gastroenterol. 1996;91:1322–1325. [PubMed] [Google Scholar]
- 26.Ogura K, Kanai F, Maeda S, Yoshida H, Ogura M, Lan K H, Hirota K, Kawabe T, Shiratori Y, Omata M. High prevalence of cytotoxin positive Helicobacter pylori in patients unrelated to the presence of peptic ulcers in Japan. Gut. 1997;41:463–468. doi: 10.1136/gut.41.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan Z J, van der Hulst R W, Feller M, Xiao S D, Tytgat G N, Dankert J, van der Ende A. Equally high prevalences of infection with cagA-positive Helicobacter pylori in Chinese patients with peptic ulcer disease and those with chronic gastritis-associated dyspepsia. J Clin Microbiol. 1997;35:1344–1347. doi: 10.1128/jcm.35.6.1344-1347.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peek R M, Miller G G, Tham K T, Perez-Perez G, Zhao X, Atherton J C, Blaser M J. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest. 1995;73:760–770. [PubMed] [Google Scholar]
- 29.Perez-Perez G I, Bhat N, Gaensbauer J, Fraser A, Taylor D N, Kuipers E J, Zhang L, You W C, Blaser M J. Country-specific constancy by age in cagA+ proportion of Helicobacter pylori infections. Int J Cancer. 1997;72:453–456. doi: 10.1002/(sici)1097-0215(19970729)72:3<453::aid-ijc13>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 30.Rudi J, Kolb C, Maiwald M, Zuna I, von Herbay A, Galle P R, Stremmel W. Serum antibodies against Helicobacter pylori proteins VacA and CagA are associated with increased risk for gastric adenocarcinoma. Dig Dis Sci. 1997;42:1652–1659. doi: 10.1023/a:1018849112533. [DOI] [PubMed] [Google Scholar]
- 31.Sharp P M. Determinants of DNA sequence divergence between Escherichia coli and Salmonella typhimurium: codon usage, map position, and concerted evolution. J Mol Evol. 1991;33:23–33. doi: 10.1007/BF02100192. [DOI] [PubMed] [Google Scholar]
- 32.Stuyver L, Rossau R, Wyseur A, Duhamel M, Vandenborght B, van Heuverswyn H, Maertens G. Typing of HCV isolates and characterization of new (sub)types using a line probe assay. J Gen Virol. 1993;74:1093–1102. doi: 10.1099/0022-1317-74-6-1093. [DOI] [PubMed] [Google Scholar]
- 33.Suerbaum S, Smith J M, Bapumia K, Morelli G, Smith N H, Kunstmann E, Dyrek I, Achtman M. Free recombination within Helicobacter pylori. Proc Natl Acad Sci USA. 1998;95:12619–12624. doi: 10.1073/pnas.95.21.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takata T, Fujimoto S, Anzai K, Shirotani T, Okada M, Sawae Y, Ono J. Analysis of the expression of cagA and vacA and the vacuolating activity in 167 Helicobacter pylori isolates from patients with either peptic ulcers or non-ulcer dyspepsia. Am J Gastroenterol. 1998;93:30–34. doi: 10.1111/j.1572-0241.1998.030_c.x. [DOI] [PubMed] [Google Scholar]
- 35.Taylor N S, Fox J G, Akopyants N S, Berg D E, Thompson N, Shames B, Yan L, Fontham E, Janney F, Hunter F M, Correa P. Long-term colonization with single and multiple strains of Helicobacter pylori assessed by DNA fingerprinting. J Clin Microbiol. 1995;33:918–923. doi: 10.1128/jcm.33.4.918-923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzegerald L M, Lee N, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidman J M, Fujii C, Bowman C, Wattey L, Wallin E, Hayes W S, Borodovsky M, Karp P D, Smith H O, Fraser C M, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 37.Tummuru M K, Cover T L, Blaser M J. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993;61:1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tummuru M K, Sharma S A, Blaser M J. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995;18:867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]
- 39.Van de Peer Y, De Wachter R. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci. 1994;10:569–570. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- 40.van der Ende A, Pan Z J, Bart A, van der Hulst R W, Feller M, Xiao S D, Tytgat G N J, Dankert J. cagA-positive Helicobacter pylori populations in China and The Netherlands are distinct. Infect Immun. 1998;66:1822–1826. doi: 10.1128/iai.66.5.1822-1826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Ende A, Rauws E A, Feller M, Mulder C J, Tytgat G N, Dankert J. Heterogeneous Helicobacter pylori isolates from members of a family with a history of peptic ulcer disease. Gastroenterology. 1996;111:638–647. doi: 10.1053/gast.1996.v111.pm8780568. [DOI] [PubMed] [Google Scholar]
- 42.van Doorn L J, Figueiredo C, Rossau R, Jannes G, van Asbroeck M, Sousa J C, Carneiro F, Quint W. Typing of the Helicobacter pylori vacA gene and detection of the cagA gene by PCR and reverse hybridization. J Clin Microbiol. 1998;36:1271–1276. doi: 10.1128/jcm.36.5.1271-1276.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Doorn L J, Figueiredo C, Sanna R, Pena A S, Midolo P, Ng E K, Atherton J C, Blaser M J, Quint W. Expanding allelic diversity of Helicobacter pylori vacA. J Clin Microbiol. 1998;36:2597–2603. doi: 10.1128/jcm.36.9.2597-2603.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Doorn L J, Figueiredo C, Sanna R, Plaisier A, Schneeberger P, de Boer W A, Quint W. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology. 1998;115:58–66. doi: 10.1016/s0016-5085(98)70365-8. [DOI] [PubMed] [Google Scholar]
- 45.van Doorn L J, Quint W G V, Schneeberger P M, Tytgat G N J, de Boer W A. Association between vacA and cagA status of Helicobacter pylori and the efficacy of a 1-day quadruple therapy. Lancet. 1997;350:71–72. doi: 10.1016/s0140-6736(05)66280-0. [DOI] [PubMed] [Google Scholar]
- 46.Vicari J J, Peek R M, Falk G W, Goldblum J R, Easley K A, Schnell J, Perez-Perez G I, Halter S A, Rice T W, Blaser M J, Richter J E. The seroprevalence of cagA-positive Helicobacter pylori strains in the spectrum of gastroesophageal reflux disease. Gastroenterology. 1998;115:50–57. doi: 10.1016/s0016-5085(98)70364-6. [DOI] [PubMed] [Google Scholar]
- 47.Webb P M, Yu M C, Forman D, Henderson B, Newell D G, Yuan J M, Gao Y T, Ross R K. An apparent lack of association between Helicobacter pylori infection and risk of gastric cancer in China. Int J Cancer. 1996;67:603–607. doi: 10.1002/(SICI)1097-0215(19960904)67:5<603::AID-IJC2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 48.Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology. 1996;110:1744–1752. doi: 10.1053/gast.1996.v110.pm8964399. [DOI] [PubMed] [Google Scholar]