Abstract
Rationale & Objective
The 2018 American Heart Association/American College of Cardiology (AHA/ACC) cholesterol guideline uses risk stratification to guide the decision to initiate nonstatin lipid-lowering medication among adults with atherosclerotic cardiovascular disease (CVD). We determined atherosclerotic CVD (ASCVD) event rates among adults with chronic kidney disease (CKD) taking statin therapy within 2018 AHA/ACC cholesterol guideline risk categories.
Study Design
Observational cohort study.
Setting & Participants
Adults with CKD not on dialysis in the Chronic Renal Insufficiency Cohort (CRIC) study who were taking a moderate/high-intensity statin 1 year after enrollment (baseline for the current analysis, n = 1,753).
Exposure
2018 AHA/ACC cholesterol guideline risk categories: without a history of ASCVD, a history of 1 major ASCVD event and multiple high-risk conditions, and a history of ≥2 major ASCVD events.
Outcome
Adjudicated ASCVD events after the year 1 study visit.
Analytical Approach
We calculated age-sex standardized rates for ASCVD events and age-sex adjusted hazard ratios for ASCVD events accounting for the competing risk of death.
Results
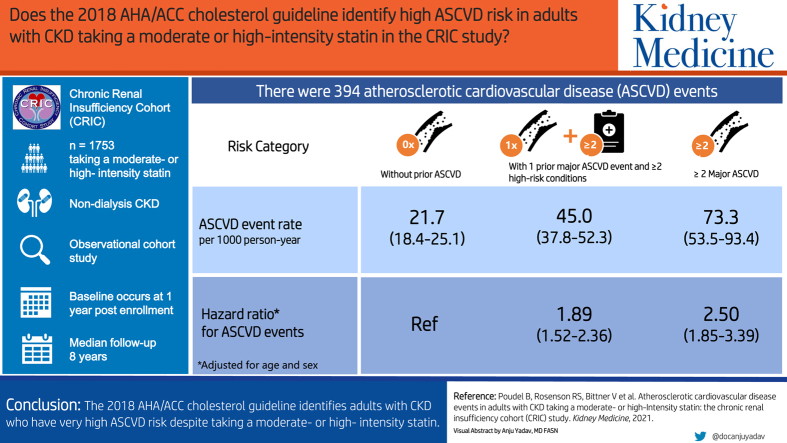
There were 394 ASCVD events over a median follow-up period of 8 years. The ASCVD event rates (with 95% CI) per 1,000 person-years among participants without a history of ASCVD, with a history of 1 major ASCVD event and multiple high-risk conditions, and with a history of ≥2 major ASCVD events were 21.7 (18.4-25.1), 45.0 (37.8-52.3), and 73.3 (53.3-93.4), respectively. Compared with participants without a history of ASCVD, the HR (95% CI) rates for ASCVD events among those with a history of 1 major ASCVD event and multiple high-risk conditions, and with a history of ≥2 major ASCVD events were 1.89 (1.52-2.36) and 2.50 (1.85-3.39), respectively.
Limitations
Data on whether participants were taking a maximally tolerated statin dosage were unavailable.
Conclusions
The 2018 AHA/ACC cholesterol guideline identifies adults with CKD who have very high ASCVD risk despite taking a moderate/high-intensity statin.
Index Words: American Heart Association/American College of Cardiology cholesterol guideline, atherosclerotic cardiovascular disease, chronic kidney disease, moderate- or high-intensity statin, risk assessment
Graphical abstract
Plain-Language Summary.
We analyzed the risk for atherosclerotic cardiovascular disease (CVD) events among 1,753 adults with chronic kidney disease (CKD) and not on dialysis taking a moderate- or high-intensity statin according to risk categories in the 2018 AHA/ACC cholesterol guideline. Compared with individuals without a history of ASCVD, those with a history of 1 major ASCVD event and multiple high-risk conditions and with a history of ≥2 major ASCVD events had a higher age-sex adjusted risk for ASCVD events. These results indicate that the risk stratification algorithm in the 2018 AHA/ACC cholesterol guideline identifies adults with CKD who have a very high ASCVD risk despite taking a moderate- or high-intensity statin.
Statins reduce the risk for atherosclerotic cardiovascular disease (CVD) events in the general population and among adults with chronic kidney disease (CKD) and not on dialysis.1, 2, 3 The 2018 American Heart Association/American College of Cardiology (AHA/ACC) guideline on the management of blood cholesterol recommends maximally tolerated statin therapy for adults with CKD who have a history of atherosclerotic CVD (ASCVD).4 This guideline also recommends ezetimibe and/or a proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9I) for adults with ASCVD who have very high ASCVD risk and low-density lipoprotein (LDL) cholesterol levels of ≥70 mg/dL despite taking a maximally tolerated statin dose.4 Very high ASCVD risk is defined in this guideline as a history of 1 major ASCVD event with multiple high-risk conditions or a history of ≥ 2 major ASCVD events (Table S1).
As CKD is considered a high-risk condition by the 2018 AHA/ACC cholesterol guideline and most adults with CKD have other ASCVD risk factors,5,6 a high proportion of adults with CKD and a history of a major ASCVD event may meet the definition of very high ASCVD risk. Determining the absolute risk for ASCVD events among adults with CKD taking a moderate- or high-intensity statin who meet the definition of very high ASCVD risk in the 2018 AHA/ACC cholesterol guideline may inform the need for, and potential benefit of, additional interventions to prevent cardiovascular events in this population.
We determined the rates of ASCVD events among adults with CKD taking a moderate- or high-intensity statin who met the definition of very high ASCVD risk according to the 2018 AHA/ACC cholesterol guideline. For comparison, we also calculated the rate of ASCVD events among adults with CKD taking a moderate- or high-intensity statin who did not meet the AHA/ACC guideline definition for very high risk. To accomplish this objective, we analyzed data from the Chronic Renal Insufficiency Cohort (CRIC) study.7
Methods
Study Population
The CRIC study enrolled 3,939 adults aged 21 to 74 years with mild-to-moderate CKD and not on dialysis between May 2003 and August 2008 at 7 centers in the United States.8 Mild-to-moderate CKD was defined by an estimated glomerular filtration rate (eGFR) of 20-70 mL/min/1.73 m2 for adults aged 21-44 years, 20-60 mL/min/1.73 m2 for adults aged 45-64 years, and 20-50 mL/min/1.73 m2 for adults aged 65-74 years. The CRIC study protocol was approved by the institutional review boards at the participating centers, and all participants provided written informed consent.
All CRIC study participants completed an in-person study visit upon enrollment (ie, the year 0 study visit). Participants were asked to return for annual in-person follow-up study visits.9 For the current analysis, we included 3,520 CRIC study participants who completed the follow-up study visit conducted 1 year after enrollment (ie, the year 1 study visit), which served as baseline for the current analysis (Fig S1). This restriction was applied so that we could identify participants who had an acute coronary syndrome (ACS) in the prior year (ie, a recent ACS), one of the components of the definition of major ASCVD events in the 2018 AHA/ACC cholesterol guideline (Table S1). We restricted the analysis to CRIC study participants with valid data on statin use and dosage based on the medication inventory conducted at the year 1 study visit (n = 3,400). We excluded 1,425 participants who were not taking a statin and 218 who were taking a low-intensity statin. Finally, we excluded 4 participants who did not have follow-up data for cardiovascular outcomes, resulting in 1,753 participants being included in the current analysis.
Participant Characteristics and Statin Use
Data on the participants’ sex, race/ethnicity, education, and self-reported history of myocardial infarction (MI), stroke, and peripheral artery disease (PAD) were collected at the year 0 study visit (ie, their time of enrollment into the CRIC study).10 We used the data on the participants’ age, body mass index, eGFR, and LDL cholesterol obtained at their year 1 follow-up study visit (ie, the baseline for the current analysis). Estimated GFR was assessed using serum creatinine, serum cystatin-C, age, sex, and race and the CRIC-eGFR equation.11 LDL cholesterol was measured from blood samples using β quantification.12 We used data on physical activity, serum high-sensitive C-reactive protein, fibroblast growth factor 23, and urinary albumin-to-creatinine ratio obtained through study procedures during the year 0 study visit because these variables were not assessed at the year 1 follow-up study visit. We used data from the medication inventory at the year 1 follow-up study visit to identify the use and intensity of statin therapy (Table S2). We used the medication inventory data of participants who attended follow-up study visits after the year 1 study visit to identify changes in statin use.
Identification of Cardiovascular Hospitalizations and All-Cause Mortality
Participants were asked about hospitalizations possibly related to cardiovascular events, including MI, ischemic stroke, PAD and heart failure, at every annual follow-up study visit and through phone calls at 6-month intervals between visits. Selected hospitals and health care systems were also queried for possible cardiovascular hospitalizations. Medical records were retrieved and adjudicated by at least 2 study clinicians to confirm the occurrence of MI, ischemic stroke, PAD, or heart failure event. For the current analysis, ASCVD events included MI, ischemic stroke, or PAD, and total CVD events included ASCVD events or heart failure. The definitions of these events are provided in Table S3. Deaths were identified from reports of relatives, retrieval of death certificates or obituaries, hospital and outpatient records, and the Social Security Death Master File.
We used CRIC study follow-up between the year 0 and year 1 study visits (ie, before baseline for the current analysis) to define the participants’ baseline ASCVD risk categories, as described in the next section. We used data after the year 1 study visit to identify MI, ischemic stroke, PAD, and heart failure hospitalizations and all-cause mortality as outcome events. For this analysis, the participants were censored if they were lost to follow-up observation or on December 31, 2016, whichever occurred first.
Atherosclerotic CVD Risk Assessment
We used data on the participants’ characteristics collected at the year 0 and year 1 study visits, and data on adjudicated MI, ischemic stroke, and PAD hospitalizations between the year 0 and year 1 study visits (ie, before baseline for the current analysis) to determine each participant’s ASCVD risk category according to the 2018 AHA/ACC cholesterol guideline. The participants were categorized into 3 mutually exclusive groups: (1) not having a history of major ASCVD events; (2) having a history of major ASCVD events (ie, history of coronary heart disease, stroke, or PAD, recent ACS, or an acute ischemic stroke or PAD event, Table S1, top panel) and very high ASCVD risk, including the following 2 groups: having a history of 1 major ASCVD event and multiple (ie, 2 or more) high-risk conditions (ie, CKD and age ≥ 65 years, prior coronary artery bypass grafting, or percutaneous coronary intervention, diabetes, hypertension, current smoking, LDL cholesterol ≥ 100 mg/dL while taking a statin, and history of heart failure [Table S1, bottom panel]); and (3) having a history of ≥ 2 major ASCVD events.
All CRIC study participants eligible for the current analysis with a history of a major ASCVD event met the definition for very high ASCVD risk.
Statistical Analysis
We calculated separately the summary statistics for characteristics and the cumulative incidence of ASCVD events among participants without a history of major ASCVD events, with a history of 1 major ASCVD event and multiple high-risk conditions, and with a history of ≥ 2 major ASCVD events. We also calculated the unadjusted rate and the age-sex adjusted rate, and rate difference and hazard ratio for ASCVD events among participants without a history of major ASCVD events, with a history of 1 major ASCVD event and multiple high-risk conditions, and with a history of ≥ 2 major ASCVD events. Adjusted rates, rate differences, and hazard ratios included adjustment for age and sex as these are non-modifiable risk factors.
The adjusted rates were calculated using direct standardization to represent the age-sex distribution of participants with a history of 1 major ASCVD event and multiple high-risk conditions and with a history of ≥ 2 major ASCVD events combined (ie, those at very high ASCVD risk). Rate differences were calculated using Poisson regression. The analyses described previously were repeated to calculate the cumulative incidence, unadjusted rate and the age-sex adjusted rate, and rate difference and hazard ratio for MI, ischemic stroke, PAD, total CVD events, heart failure hospitalizations, and all-cause mortality, separately. For all outcomes except all-cause mortality, the cumulative incidence and hazard ratio were calculated accounting for the competing risk of death as described by Fine and Gray.13 No competing risk was considered for the analysis of all-cause mortality.
The calculation of unadjusted rates and age-sex-adjusted rates, rate differences, and hazard ratios for ASCVD, MI, ischemic stroke, PAD, total CVD and heart failure events, and all-cause mortality was repeated within subgroups defined by eGFR (ie, <30, 30 to <45, and ≥45 mL/min/1.73 m2), and LDL cholesterol (ie, <70, 70 to <100, and ≥100 mg/dL). In a sensitivity analysis, we calculated unadjusted rates and age-sex-adjusted rates, rate differences and hazard ratios in the overall study population censoring CRIC study participants if they down-titrated to a low-intensity statin or discontinued statin therapy during the follow-up period.
Results
Among the participants included in the current analysis, 1,106 (63.1%) did not have a history of major ASCVD events, 488 (27.8%) had a history of 1 major ASCVD event and multiple high-risk conditions, and 159 (9.1%) had a history of ≥2 major ASCVD events. The participants who had a history of 1 major ASCVD event with multiple high-risk conditions or ≥ 2 major ASCVD events were older and more likely to be Black versus their counterparts without a history of major ASCVD events (Table 1). A higher percentage of participants with a history of 1 major ASCVD event and multiple high-risk conditions and with a history of ≥2 major ASCVD events had a prior coronary artery bypass grafting or percutaneous coronary intervention, diabetes, hypertension, or history of heart failure compared with their counterparts without a history of major ASCVD events.
Table 1.
Characteristics of Chronic Renal Insufficiency Cohort Study Participants Included in the Current Analysis
| Participant Characteristicsa | 2018 AHA/ACC Blood Cholesterol Guideline ASCVD Risk Category |
||
|---|---|---|---|
| No History of Major ASCVD Events | History of 1 Major ASCVD Event With Multiple High-Risk Conditionsb | History of ≥2 Major ASCVD Eventsb | |
| No. of participants | 1,106 | 488 | 159 |
| Age, y | 59.4 ± 10.4 | 62.8 ± 8.0 | 63.5 ± 6.9 |
| Sex, male | 616 (55.7%) | 310 (63.5%) | 94 (59.1%) |
| Race/ethnicity | |||
| White | 489 (44.2%) | 205 (42.0%) | 68 (42.8%) |
| Black | 416 (37.6%) | 211 (43.2%) | 77 (48.4%) |
| Hispanic | 147 (13.3%) | 54 (11.1%) | 11 (6.9%) |
| Other | 54 (4.9%) | 18 (3.7%) | 3 (1.9%) |
| Less than high school education | 230 (20.8%) | 109 (22.3%) | 39 (24.5%) |
| Body mass index, kg/m2 | 32.8 ± 7.8 | 32.5 ± 7.2 | 33.4 ± 6.8 |
| Physical activity, total MET scorec,d | 163.5 (110.1, 244.5) | 146.5 (97.8, 214.8) | 133.4 (94.5, 215.8) |
| Major ASCVD Events | |||
| History of coronary heart diseasec | 0 (0) | 301 (61.7%) | 139 (87.4%) |
| History of strokec | 0 (0) | 114 (23.4%) | 85 (53.5%) |
| History of PADc | 0 (0) | 60 (12.3%) | 89 (56.0%) |
| Between year 0 and year 1 study visits | |||
| Recent acute coronary syndromee | 0 (0) | 7 (1.4%) | 10 (6.3%) |
| Ischemic stroke | 0 (0) | 5 (1.0%) | 9 (5.7%) |
| Peripheral artery disease | 0 (0) | 1 (0.2%) | 14 (8.8%) |
| High-Risk Conditions | |||
| Age ≥ 65 y | 386 (34.9%) | 230 (47.1%) | 70 (44.0%) |
| History of CABG/PCI | 78 (7.1%) | 216 (44.3%) | 107 (67.3%) |
| Diabetes | 646 (58.4%) | 332 (68.0%) | 111 (69.8%) |
| Hypertension | 1,042 (94.3%) | 466 (95.5%) | 155 (97.5%) |
| Current smoking | 126 (11.4%) | 70 (14.3%) | 20 (12.6%) |
| LDL cholesterol ≥ 100 mg/dL while taking a statinf | 367 (35.6%) | 109 (23.5%) | 39 (25.3%) |
| History of heart failure | 81 (7.3%) | 102 (20.9%) | 64 (40.3%) |
| Laboratory Measurements | |||
| eGFR, mL/min/1.73 m2 | |||
| <30 | 279 (26.1%) | 135 (28.7%) | 61 (38.6%) |
| 30-44 | 380 (35.6%) | 169 (36.0%) | 57 (36.1%) |
| 45-59 | 273 (25.6%) | 126 (26.8%) | 32 (20.2%) |
| ≥60 | 136 (12.7%) | 40 (8.5%) | 8 (5.1%) |
| High sensitivity C-reactive protein > 3 mg/Lc | 465 (42.2%) | 204 (42.0%) | 95 (59.8%) |
| Albuminuria, mg/gc | |||
| <30 | 458 (43.0%) | 186 (39.6%) | 59 (38.1%) |
| 30-300 | 261 (24.5%) | 128 (27.2%) | 42 (27.1%) |
| >300 | 347 (32.5%) | 156 (33.2%) | 54 (34.8%) |
| FGF-23, RU/mLc | 144.9 (96.3, 230.2) | 161.6 (109.6, 257.1) | 184.0 (109.6, 279.0) |
| Uric acid, mg/dLc | 7.4 ± 1.9 | 7.6 ± 1.9 | 7.7 ± 2.0 |
| LDL cholesterol, mg/dL | 88.0 (72.0, 107.0) | 82.0 (65.0, 98.0) | 83.5 (66.0, 102.0) |
| LDL cholesterol, mg/dL | |||
| <70 | 220 (21.4%) | 148 (31.9%) | 45 (29.2%) |
| 70-<100 | 443 (43.0%) | 207 (44.6%) | 70 (45.5%) |
| ≥100 | 367 (35.6%) | 109 (23.5%) | 39 (25.3%) |
The values in the table are number (percentage) or mean ± SD except for physical activity, FGF-23, and LDL cholesterol, which are expressed as median (25th, 75th percentile).
Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; ASCVD, atherosclerotic cardiovascular disease; CABG, coronary artery bypass grafting; CRIC, Chronic Renal Insufficiency Cohort; eGFR, estimated glomerular filtration rate; FGF, fibroblast growth factor; LDL, low-density lipoprotein; MET, metabolic equivalent of task; PAD, peripheral artery disease; PCI, percutaneous coronary interventions.
Participant characteristics were calculated at the CRIC year 1 study visit (ie, baseline for the current analysis), unless otherwise indicated.
Adults with a history of 1 major ASCVD event with multiple high-risk conditions or with a history of ≥2 major ASCVD events are considered to be at a very high risk for ASCVD events in the 2018 AHA/ACC cholesterol guideline. Major ASCVD events include history of coronary heart disease, stroke or PAD, recent acute coronary syndrome, or an acute ischemic stroke or PAD event. Multiple high-risk conditions include chronic kidney disease and one or more of the following: age ≥ 65 years, history of prior CABG or PCI, diabetes, hypertension, current smoking, low-density lipoprotein cholesterol ≥ 100 mg/dL or history of heart failure.
Calculated at the CRIC year 0 study visit.
Physical activity was assessed using total MET from Typical Week Physical Activity Survey (TWPAS).
Recent acute coronary syndrome is defined by a myocardial infarction hospitalization between year 0 and year 1 study visits (ie, before baseline for the current study).
All the participants included in this analysis were taking a moderate- or high-intensity statin.
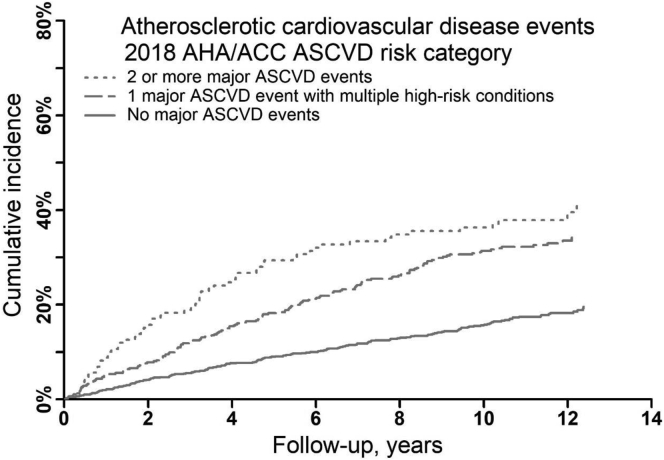
Over a median of 8 years of follow-up observation, there were 394 ASCVD events, including 255 MI events, 89 ischemic strokes, and 120 PAD events. The cumulative incidence and rate of ASCVD events were higher among participants with a history of 1 major ASCVD event and multiple high-risk conditions and among participants with a history of ≥2 major ASCVD events compared with their counterparts without a history of major ASCVD events (Fig 1, Fig S2, and Table 2).
Figure 1.
Cumulative incidence of atherosclerotic cardiovascular disease events by the 2018 AHA/ACC atherosclerotic cardiovascular disease risk categories. ASCVD events include myocardial infarction, ischemic stroke, or peripheral artery disease. Cumulative incidence was calculated accounting for the competing risk of death. Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; ASCVD, atherosclerotic cardiovascular disease.
Table 2.
Atherosclerotic Cardiovascular Disease Event Rates and Hazard Ratios by the 2018 AHA/ACC Atherosclerotic Cardiovascular Disease Risk Categories
| Outcomes | ASCVD Risk Category |
||
|---|---|---|---|
| No History of Major ASCVD Events | History of 1 major ASCVD Event With Multiple High-Risk Conditions | History of ≥2 major ASCVD Events | |
| No. of participants | 1,106 | 488 | 159 |
| ASCVD events | |||
| Events/person-years | 182/9,292 | 152/3,426 | 60/900 |
| Unadjusted rate | 19.6 (16.7-22.4) | 44.4 (37.3-51.4) | 66.7 (49.8-83.5) |
| Adjusted rate | 21.7 (18.4-25.1) | 45.0 (37.8-52.3) | 73.3 (53.3-93.4) |
| Adjusted rate difference | 0.0 (reference) | 23.3 (15.4-31.3) | 51.6 (31.3-71.9) |
| Hazard ratio | 1.0 (reference) | 1.89 (1.52-2.36) | 2.50 (1.85-3.39) |
| Myocardial infarction | |||
| Events/person-years | 121/9,519 | 103/3,631 | 31/1,029 |
| Unadjusted rate | 12.7 (10.4-15.0) | 28.4 (22.9-33.8) | 30.1 (19.5-40.7) |
| Adjusted rate | 14.5 (11.8-17.3) | 28.8 (23.2-34.4) | 32.4 (20.3-44.4) |
| Adjusted rate difference | 0.0 (reference) | 14.3 (8.0-20.5) | 17.9 (5.5-30.2) |
| Hazard ratio | 1.0 (reference) | 1.84 (1.40-2.40) | 1.72 (1.15-2.58) |
| Ischemic stroke | |||
| Events/person-years | 42/9,875 | 30/3,863 | 17/1,071 |
| Unadjusted rate | 4.3 (3.0-5.5) | 7.8 (5.0-10.5) | 15.9 (8.3-23.4) |
| Adjusted rate | 4.4 (3.0-5.8) | 7.8 (5.0-10.6) | 16.4 (8.1-24.7) |
| Adjusted rate difference | 0.0 (reference) | 3.4 (0.2-6.5) | 12.0 (3.7-20.4) |
| Hazard ratio | 1.0 (reference) | 1.54 (0.96-2.46) | 2.71 (1.55-4.74) |
| Peripheral artery disease | |||
| Events/person-years | 49/9,772 | 48/3,811 | 23/1,039 |
| Unadjusted rate | 5.0 (3.6-6.4) | 12.6 (9.0-16.2) | 22.1 (13.1-31.2) |
| Adjusted rate | 5.3 (3.7-6.9) | 12.8 (9.2-16.4) | 25.3 (14.2-36.5) |
| Adjusted rate difference | 0.0 (reference) | 7.5 (3.5-11.4) | 20.0 (8.7-31.3) |
| Hazard ratio | 1.0 (reference) | 2.21 (1.47-3.33) | 3.53 (2.10-5.92) |
Values between parenthesis indicate 95% CI. Hazard ratios were adjusted for age and sex, and account for the competing risk of death. ASCVD events include myocardial infarction, ischemic stroke, or peripheral artery disease. Rates and rate differences are expressed per 1,000 person-years. Adjusted rates were calculated using direct standardization, with the standard population being all participants with very high ASCVD risk: men <55 years (8.7%), 55-65 years (24.4%), 65-70 years (15.1%), and ≥70 years (14.2%); and women <55 years (5.9%), 55-65 years (14.7%), 65-70 years (8.7%), and ≥70 years (8.3%).
Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval.
The age-sex-adjusted hazard ratios for ASCVD events among the participants with a history of 1 major ASCVD event and multiple high-risk conditions and a history of ≥2 major ASCVD events compared with those without a history of major ASCVD events were 1.89 (95% CI, 1.52-2.36) and 2.50 (95% CI, 1.85-3.39), respectively. The ASCVD event rates were higher among participants with a history of 1 major ASCVD event and multiple high-risk conditions and with ≥ 2 major ASCVD events versus those without a history of major ASCVD events within each eGFR subgroup (Table 3) and LDL cholesterol subgroup (Table S4) and in a sensitivity analysis that censored participants upon their titrating to a low-intensity statin or discontinuing statin therapy (Table S5).
Table 3.
Atherosclerotic Cardiovascular Disease Event Rates and Hazard Ratios by the 2018 AHA/ACC Atherosclerotic Cardiovascular Disease Risk Categories and Estimated Glomerular Filtration Rate Levels
| Outcomes | ASCVD Risk Category |
||
|---|---|---|---|
| No History of Major ASCVD Events | History of 1 Major ASCVD Event With Multiple High-Risk Conditions | History of ≥2 Major ASCVD Events | |
| eGFR < 30 mL/min/1.73 m2 | n=279 | n=135 | n=61 |
| ASCVD events | |||
| Events/person-years | 59/2,017 | 53/718 | 22/284 |
| Unadjusted rate | 29.3 (21.8-36.7) | 73.8 (53.9-93.7) | 77.5 (53.9-93.7) |
| Adjusted rate | 38.8 (27.9-49.8) | 83.0 (59.5-106.5) | 81.8 (41.3-122.3) |
| Adjusted rate difference | 0.0 (reference) | 44.2 (18.2-70.1) | 43.0 (1.03-84.9) |
| Hazard ratio | 1.0 (reference) | 1.85 (1.26-2.72) | 1.76 (1.07-2.92) |
| Myocardial infarction | |||
| Events/person-years | 43/2,097 | 35/783 | 14/318 |
| Unadjusted rate | 20.5 (14.4-26.6) | 44.7 (29.9-59.5) | 44.0 (20.9-67.0) |
| Adjusted rate | 26.1 (17.5-34.8) | 50.9 (33.4-68.4) | 49.1 (17.6-80.6) |
| Adjusted rate difference | 0.0 (reference) | 24.8 (5.2-44.3) | 23.0 (−9.7-55.6) |
| Hazard ratio | 1.0 (reference) | 1.56 (0.98-2.49) | 1.41 (0.77-2.58) |
| Ischemic stroke | |||
| Events/person-years | 8/2,196 | 11/867 | 3/336 |
| Unadjusted rate | 3.6 (1.1-6.2) | 12.7 (5.2-20.2) | 8.9 (0.0-19.0)a |
| Adjusted rate | 5.0 (1.2-8.4) | 13.2 (5.0-21.5) | 16.7 (0.0-42.7)a |
| Adjusted rate difference | 0.0 (reference) | 8.2 (−0.9-17.3) | 11.7 (−14.6-37.9) |
| Hazard ratio | 1.0 (reference) | 2.69 (1.03-7.03) | 1.64 (0.44-6.16) |
| Peripheral artery disease | |||
| Events/person-years | 20/2,132 | 19/848 | 8/317 |
| Unadjusted rate | 9.4 (5.3-13.5) | 22.4 (12.3-32.5) | 25.2 (7.8-42.8) |
| Adjusted rate | 12.0 (6.3-17.8) | 24.6 (13.1-36.2) | 25.7 (6.7-44.8) |
| Adjusted rate difference | 0.0 (reference) | 12.6 (−0.3-25.5) | 13.7 (−6.2-33.6) |
| Hazard ratio | 1.0 (reference) | 2.07 (1.06-4.03) | 2.07 (0.87-4.95) |
| eGFR 30 to <45 mL/min/1.73 m2 | n=380 | n=169 | n=57 |
| ASCVD events | |||
| Events/person-years | 61/3,221 | 57/1,205 | 20/326 |
| Unadjusted rate | 18.9 (14.2-23.7) | 47.3 (35.0-59.6) | 61.3 (34.5-88.3) |
| Adjusted rate | 20.2 (14.9-25.4) | 50.4 (37.0-63.7) | 70.7 (37.6-103.8) |
| Adjusted rate difference | 0.0 (reference) | 30.2 (15.8-44.6) | 50.5 (17.0-84.0) |
| Hazard ratio | 1.0 (reference) | 2.18 (1.52-3.14) | 2.37 (1.40-4.02) |
| Myocardial infarction | |||
| Events/person-years | 38/3,276 | 41/1,273 | 10/374 |
| Unadjusted rate | 11.6 (7.9-15.3) | 32.2 (22.3-42.1) | 26.7 (10.2-43.3) |
| Adjusted rate | 12.9 (8.7-17.1) | 34.1 (23.5-44.7) | 28.0 (9.9-46.1) |
| Adjusted rate difference | 0.0 (reference) | 21.2 (9.8-32.6) | 15.1 (−3.5-33.7) |
| Hazard ratio | 1.0 (reference) | 2.37 (1.51-3.73) | 1.70 (0.82-3.52) |
| Ischemic stroke | |||
| Events/person-years | 21/3,369 | 13/1,349 | 5/401 |
| Unadjusted rate | 6.2 (3.6-8.9) | 9.6 (4.4-14.9) | 12.5 (1.5-23.4) |
| Adjusted rate | 6.0 (3.3-8.7) | 10.7 (4.7-16.6) | 12.2 (1.1-23.3) |
| Adjusted rate difference | 0.0 (reference) | 4.7 (−1.8-11.2) | 6.2 (−5.2-17.6) |
| Hazard ratio | 1.0 (reference) | 1.35 (0.67-2.70) | 1.49 (0.57-3.88) |
| Peripheral artery disease | |||
| Events/person-years | 11/3,379 | 19/1,342 | 7/384 |
| Unadjusted rate | 3.3 (1.3-5.2) | 14.2 (7.8-20.5) | 18.2 (4.7-31.8) |
| Adjusted rate | 3.9 (1.6-6.3) | 15.1 (8.1-22.0) | 18.4 (4.0-32.8) |
| Adjusted rate difference | 0.0 (reference) | 11.2 (3.8-18.5) | 14.5 (−0.1-29.1) |
| Hazard ratio | 1.0 (reference) | 3.87 (1.84-8.15) | 4.24 (1.60-11.20) |
| eGFR ≥45 mL/min/1.73 m2 | n=409 | n=166 | n=40 |
| ASCVD events | |||
| Events/person-years | 56/3,782 | 38/1,387 | 17/289 |
| Unadjusted rate | 14.8 (10.9-18.7) | 27.4 (18.7-36.1) | 58.8 (30.8-86.6) |
| Adjusted rate | 15.0 (10.6-19.4) | 26.6 (17.8-35.5) | 67.5 (30.8-104.1) |
| Adjusted rate difference | 0.0 (reference) | 11.6 (1.8-21.5) | 52.5 (15.6-89.4) |
| Hazard ratio | 1.0 (reference) | 1.66 (1.10-2.51) | 3.69 (2.10-6.48) |
| Myocardial infarction | |||
| Events/person-years | 36/3,867 | 25/1,448 | 7/335 |
| Unadjusted rate | 9.3 (6.3-12.4) | 17.3 (10.5-24.0) | 20.9 (5.4-36.4) |
| Adjusted rate | 10.2 (6.5-13.9) | 18.1 (10.7-25.5) | 20.7 (4.5-37.0) |
| Adjusted rate difference | 0.0 (reference) | 7.9 (−0.3-16.2) | 10.5 (−6.1-27.2) |
| Hazard ratio | 1.0 (reference) | 1.66 (0.98-2.79) | 1.98 (0.85-4.60) |
| Ischemic stroke | |||
| Events/person-years | 12/4,013 | 6/1,516 | 9/332 |
| Unadjusted rate | 3.0 (1.3-4.7) | 4.0 (0.8-7.1) | 27.1 (9.4-44.8) |
| Adjusted rate | 2.7 (1.0-4.3) | 3.6 (0.6-6.6) | 31.1 (8.0-54.2) |
| Adjusted rate difference | 0.0 (reference) | 0.9 (−2.5-4.3) | 28.4 (5.3-51.6) |
| Hazard ratio | 1.0 (reference) | 1.19 (0.46-3.13) | 8.30 (3.59-19.22) |
| Peripheral artery disease | |||
| Events/person-years | 14/3,971 | 8/1,501 | 7/338 |
| Unadjusted rate | 3.5 (1.7-9.0) | 5.3 (1.6-9.0) | 20.7 (5.4-36.0) |
| Adjusted rate | 3.5 (1.4-5.5) | 4.4 (1.3-7.4) | 25.4 (4.9-45.8) |
| Adjusted rate difference | 0.0 (reference) | 0.9 (−2.8-4.6) | 21.9 (1.3-42.5) |
| Hazard ratio | 1.0 (reference) | 1.27 (0.54-2.99) | 5.34 (2.15-13.28) |
Values between parenthesis indicate 95% CI. Rates and rate differences are expressed per 1,000 person-years. Adjusted rates were calculated using direct standardization, with the standard population being all participants with very high ASCVD risk: men <55 years (8.7%), 55 to 65 years (24.4%), 65 to 70 years (15.1%), and ≥70 years (14.2%); women <55 years (5.9%), 55 to 65 years (14.7%), 65 to 70 years (8.7%), ≥70 years (8.3%). Hazard ratios were adjusted for age and sex, and account for the competing risk of death. There were 57 participants with missing eGFR who were not included in this table. ASCVD events include myocardial infarction, ischemic stroke, or peripheral artery disease.
Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; eGFR, estimated glomerular filtration rate.
A lower 95% CI <0 was calculated. However, we reported a lower 95% CI of zero instead as rates cannot be negative.
The total CVD, heart failure, and all-cause mortality event rates and hazard ratios were each higher overall among participants with 1 major ASCVD event and multiple high-risk conditions and with a history of ≥2 major ASCVD events versus those without a history of major ASCVD events (Fig S3 and Table S6) and within each subgroup defined by eGFR (Table S7) and LDL cholesterol (Tables S8) levels, and when censoring participants if they titrated to a low-intensity statin or discontinued statin therapy (Table S9).
Discussion
In the current study of adults with CKD taking a moderate- or high-intensity statin, all participants with a history of ASCVD had multiple high-risk conditions or a history of ≥2 major ASCVD events and, therefore, met the 2018 AHA/ACC cholesterol guideline definition of very high ASCVD risk. Despite taking a moderate- or high-intensity statin, the ASCVD event rates were high among participants meeting the definition of very high risk. The ASCVD risk reported in the current study could be used to determine the potential benefit of additional interventions to prevent cardiovascular events among adults with CKD at very high ASCVD risk who are taking a statin.
The 2018 AHA/ACC cholesterol guideline recommends a moderate- or high-intensity statin for adults with a history of ASCVD.4 The guideline also recommends initiation of ezetimibe or a PCSK9I among adults with a history of ASCVD who meet the definition of very high ASCVD risk and have an LDL cholesterol level of ≥70 mg/dL despite taking a maximally tolerated statin dosage.4 As ezetimibe is available as generic drug, the guidelines suggest taking into account cost-effectiveness when considering the initiation of a nonstatin lipid-lowering therapy.4,14
Prior studies of patients with a history of ASCVD, most of whom did not have CKD, suggest that a high proportion of this population meets the definition of very high ASCVD risk in the 2018 AHA/ACC cholesterol guideline.15,16 In a study of adults with commercial health insurance and established ASCVD, 55.3% of patients met the definition of very high ASCVD risk.15 In another study of adults with ACS and dyslipidemia, 63.1% of participants met the definition of very high risk.16 Results from the current study suggest that the vast majority of adults with CKD who have a history of ASCVD can be expected to meet the definition of very high risk in the 2018 AHA/ACC cholesterol guideline and may be considered for ezetimibe and/or PCSK9I initiation.
The Study of Heart and Renal Protection (SHARP) trial demonstrated a 17% reduction in major vascular events among participants with CKD and not on dialysis randomized to simvastatin plus ezetimibe compared with placebo (risk ratio, 0.83; 95% CI, 0.74-0.94).17 In a post hoc analysis of the randomized trial IMPROVE-IT, which compared participants randomized to simvastatin and ezetimibe versus simvastatin alone, the hazard ratios for the primary end point of cardiovascular death, major coronary event, or nonfatal stroke were 0.88 (95% CI, 0.82-0.95) and 0.87 (95% CI, 0.78-0.98) at eGFR levels of 60 and 45 mL/min/1.73 m2, respectively.18 In the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial, participants taking a statin who were randomized to PCSK9I had a lower risk for the primary end point of cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization compared with their counterparts randomized to placebo (hazard ratio, 0.85; 95% CI: 0.79, 0.92).19 There was no evidence supporting a difference in the hazard ratios between participants with eGFR ≥ 90, 60 to 89, and < 60 mL/min/1.73 m2: 0.82 (95% CI, 0.71-0.94), 0.85 (95% CI, 0.77-0.94), and 0.89 (95% CI, 0.76-1.05), respectively (P interaction = 0.75).20 Participants meeting the definition of very high-risk in the current study had a high ASCVD event rate, so the absolute risk reduction conferred by ezetimibe or a PCSK9I in addition to statin therapy in these individuals may be substantial.
Other interventions, in addition to a maximally tolerated statin therapy and nonstatin lipid-lowering medication, may further reduce the risk for ASCVD events in adults with CKD at very high ASCVD risk. According to the 2018 AHA/ACC cholesterol guideline, all adults with a history of ASCVD should engage in healthy lifestyles, including eating a healthy diet, participating in physical activity, and not smoking.4 Blood pressure control can also reduce the risk for ASCVD events in this population. In a prespecified subgroup analysis of the Systolic Blood Pressure Intervention Trial (SPRINT) among adults with CKD, the hazard ratio for the composite cardiovascular outcome and all-cause mortality with more intensive versus less intensive blood pressure control (ie, systolic blood pressure < 120 versus < 140 mm Hg) was 0.81 (95% CI, 0.63-1.05) and 0.72 (95% CI, 0.53-0.99), respectively.21 The nonsteroidal selective mineralocorticoid receptor antagonist finerenone has also shown to reduce the risk for cardiovascular events in adults with CKD who have diabetes.22 Sodium-glucose cotransporter-2 inhibitors have been shown to reduce the risk for ASCVD events in adults with CKD, both with and without diabetes.23,24
The current study has several strengths. The CRIC study consists of a diverse population with CKD with a broad range of eGFR and LDL cholesterol levels. A long follow-up period was available to ascertain outcomes. The current study used data collected before the publication of the 2018 AHA/ACC blood cholesterol guideline, so the ASCVD event rates could be interpreted as the expected rate without treatment intensification through the addition of nonstatin lipid-lowering therapy, as recommended in the guideline.
Results from this study should be interpreted in the context of some limitations. The CRIC study did not have information on familial hypercholesterolemia, which is one of the very high-risk conditions in the 2018 AHA/ACC cholesterol guideline. Some of the comorbidities assessed were self-reported, and some participant characteristics were only available at the year 0 study visit (ie, 1 year before baseline for the current analysis). The CRIC study included adults with CKD who were 21 to 74 years of age, so results from the current analysis cannot be extrapolated to adults ≥ 75 years of age with CKD. Data on the duration of statin therapy were not available. We could not determine whether participants were taking a maximally tolerated statin dosage as data on statin intolerance were not available. Results from subgroup analyses need to be considered with caution, as some included a small number of events.
In conclusion, in the current study of adults with established CKD taking moderate- to high-intensity statins, the ASCVD event rates were substantially higher among participants with a history of 1 major ASCVD event and multiple high-risk conditions and with a history of ≥2 major ASCVD events compared with those without a history of major ASCVD events. These data support the ASCVD risk stratification algorithm in the 2018 AHA/ACC cholesterol guideline for adults with CKD. Additional interventions to prevent cardiovascular events among adults with CKD at very high ASCVD risk according to the 2018 AHA/ACC cholesterol guideline who are already taking a statin may be warranted.
Article Information
CRIC Study Investigators
Lawrence J. Appel, MD, MPH, Harold I. Feldman, MD, MSCE, Alan S. Go, MD, Jiang He, MD, PhD, James P. Lash, MD, Robert G. Nelson, MD, PhD, MS, Mahboob Rahman, MD, Panduranga S. Rao, MD, Vallabh O. Shah, PhD, MS, Raymond R. Townsend, MD, and Mark L. Unruh, MD, MS.
Authors’ Full Names and Academic Degrees
Bharat Poudel, MSPH, Robert S. Rosenson, MD, Vera Bittner, MD, MSPH, Orlando M. Gutiérrez, MD, Amanda H. Anderson, PhD, MPH, Mark Woodward, PhD, Rajat Deo, MD, April P. Carson, PhD, Katherine E. Mues, PhD, Paul J. Dluzniewski, PhD, Bernard G. Jaar, MD, Claudia M. Lora, MD, Jonathan Taliercio, MD, Paul Muntner, PhD, and Lisandro D. Colantonio, MD, PhD, on behalf of the Chronic Renal Insufficiency Cohort (CRIC) Study Investigators.
Authors’ Contributions
Statistical analysis and draft of manuscript: BP; assisted with statistical analysis and draft of manuscript: PM, LDC. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, U01DK060902, and U24DK060990). In addition, this work was supported in part by the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131, Department of Internal Medicine, University of New Mexico School of Medicine Albuquerque, NM R01DK119199. Further support for the secondary analysis of CRIC data was obtained through a research grant from Amgen, Inc. (Thousand Oaks, CA). The funders had no role in the study design, analysis, reporting, or decision to submit the manuscript for publication.
Financial Disclosure
Dr Rosenson reports research support to his institution from Amgen, Medicines Company, Novartis, and Regeneron; he serves on advisory boards for Amgen, C5, CVS Caremark, and Corvidia; receives honoraria for noncommercial educational activities from Amgen, Kowa, Pfizer, and Regeneron, has stock ownership in MediMergent LLC; and receives royalties from UpToDate. Dr Bittner serves on the executive steering committee of the ODYSSEY OUTCOMES trial (Sanofi), as National Coordinator for STRENGTH (Astra Zeneca), DalGene (Dalcor), and CLEAR (Esperion), and as local site investigator for ORION IV (Novartis), all contracted through the University of Alabama at Birmingham; she has received research support from Amgen as coinvestigator of a collaboration between Amgen and the UAB School of Public Health; and served as a consultant for Sanofi in 2018; and is currently serving as a consultant for Pfizer. Dr Gutiérrez reports grant funding and consulting fees from Akebia Therapeutics; grant funding and consulting fees from Amgen; grant funding from GSK; and consulting fees from QED Therapeutics. Dr Anderson reports research support from NIH; NIH honoraria; reports receiving a consultant fee and travel reimbursement from Kyowa Hakko Kirin. Dr Muntner reports research support and consulting fees from Amgen Inc. Drs Mues and Dluzniewski are employees and stockholders of Amgen Inc. Dr Woodward serves as a consultant for Amgen Inc., and Kyowa Kirin. Dr Carson reports grant support from Amgen. Dr Colantonio reports research support from Amgen Inc. All other authors report no disclosures. The remaining authors declare that they have no relevant financial interests.
Acknowledgements
We would like to express our appreciation to the CRIC participants for their commitment to this study.
Peer Review
Received September 27, 2020, as a submission to the expedited consideration track with 4 external peer reviews. Direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form April 4, 2021.
Footnotes
Complete author and article information provided before references.
Figure S1: Cascade of Chronic Renal Insufficiency Cohort study participants included in the current analysis.
Figure S2: Cumulative incidence of myocardial infarction, ischemic stroke, and peripheral artery disease by the 2018 AHA/ACC atherosclerotic cardiovascular disease risk categories.
Figure S3: Cumulative incidence of total cardiovascular disease, heart failure, and all-cause mortality by the 2018 AHA/ACC atherosclerotic cardiovascular disease risk categories.
Table S1: Major atherosclerotic cardiovascular disease events and high-risk conditions in the 2018 AHA/ACC cholesterol guideline and their definitions as operationalized using the Chronic Renal Insufficiency Cohort study data.
Table S2: Dosages used to define high-, moderate-, and low-intensity statin therapy by statin type.
Table S3: Definitions of outcome events including myocardial infarction, ischemic stroke, peripheral artery disease, and heart failure.
Table S4: Atherosclerotic cardiovascular disease event rates by the 2018 AHA/ACC atherosclerotic cardiovascular disease risk categories and on-treatment LDL cholesterol levels.
Table S5: Atherosclerotic cardiovascular disease event rates and hazard ratios by the 2018 AHA/ACC atherosclerotic cardiovascular disease risk categories censoring participants when they titrated to a low-intensity statin or discontinued statin treatment.
Table S6: Total cardiovascular disease, heart failure, and all-cause mortality event rates and hazard ratios by the 2018 AHA/ACC atherosclerotic cardiovascular disease risk categories.
Table S7: Total cardiovascular disease, heart failure, and all-cause mortality event rates and hazard ratios by the 2018 AHA/ACC atherosclerotic cardiovascular disease risk categories and on levels of estimated glomerular filtration rate levels.
Table S8: Total cardiovascular disease, heart failure, and all-cause mortality event rates and hazard ratios by the 2018 AHA/ACC atherosclerotic cardiovascular disease risk categories and on-treatment LDL cholesterol levels.
Table S9: Total cardiovascular disease, heart failure, and all-cause mortality event rates and hazard ratios by the 2018 AHA/ACC atherosclerotic cardiovascular disease risk categories censoring participants when they titrated to a low-intensity statin or discontinued statin treatment.
Supplementary Material
Figures S1-S3. Tables S1-S9.
References
- 1.Taylor F., Huffman M.D., Macedo A.F. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD004816. doi: 10.1002/14651858.CD004816.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Upadhyay A., Earley A., Lamont J.L., Haynes S., Wanner C., Balk E.M. Lipid-lowering therapy in persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2012;157:251–262. doi: 10.7326/0003-4819-157-4-201208210-00005. [DOI] [PubMed] [Google Scholar]
- 3.Strippoli G.F., Navaneethan S.D., Johnson D.W. Effects of statins in patients with chronic kidney disease: meta-analysis and meta-regression of randomised controlled trials. BMJ. 2008;336:645–651. doi: 10.1136/bmj.39472.580984.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grundy S.M., Stone N.J., Bailey A.L. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Muntner P., He J., Astor B.C., Folsom A.R., Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol. 2005;16:529–538. doi: 10.1681/ASN.2004080656. [DOI] [PubMed] [Google Scholar]
- 6.Foley R.N., Wang C., Collins A.J. Cardiovascular risk factor profiles and kidney function stage in the US general population: the NHANES III study. Mayo Clin Proc. 2005;80:1270–1277. doi: 10.4065/80.10.1270. [DOI] [PubMed] [Google Scholar]
- 7.Feldman H.I., Appel L.J., Chertow G.M. The Chronic Renal Insufficiency Cohort (CRIC) study: design and methods. J Am Soc Nephrol. 2003;14:S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 8.Lash J.P., Go A.S., Appel L.J. Chronic Renal Insufficiency Cohort (CRIC) study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denker M., Boyle S., Anderson A.H., Appel L.J., Chen J., Fink J.C. Chronic Renal Insufficiency Cohort Study (CRIC): overview and summary of selected findings. Clin J Am Soc Nephrol. 2015;10:2073–2083. doi: 10.2215/CJN.04260415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harhay M.N., Xie D., Zhang X. Cognitive impairment in non-dialysis-dependent CKD and the transition to dialysis: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2018;72:499–508. doi: 10.1053/j.ajkd.2018.02.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson A.H., Yang W., Hsu C.Y. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2012;60:250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bajaj A., Xie D., Cedillo-Couvert E. Lipids, apolipoproteins, and risk of atherosclerotic cardiovascular disease in persons with CKD. Am J Kidney Dis. 2019;73:827–836. doi: 10.1053/j.ajkd.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 14.Grundy S.M., Stone N.J. 2018 American Heart Association/American College of Cardiology/Multisociety guideline on the management of blood cholesterol-secondary prevention. JAMA Cardiol. 2019;4:589–591. doi: 10.1001/jamacardio.2019.0911. [DOI] [PubMed] [Google Scholar]
- 15.Colantonio L.D., Shannon E.D., Orroth K.K. Ischemic event rates in very-high-risk adults. J Am Coll Cardiol. 2019;74:2496–2507. doi: 10.1016/j.jacc.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Roe M.T., Li Q.H., Bhatt D.L. Risk categorization using new American College of Cardiology/American Heart Association guidelines for cholesterol management and its relation to alirocumab treatment following acute coronary syndromes. Circulation. 2019;140:1578–1589. doi: 10.1161/CIRCULATIONAHA.119.042551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baigent C., Landray M.J., Reith C. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanifer J.W., Charytan D.M., White J. Benefit of ezetimibe added to simvastatin in reduced kidney function. J Am Soc Nephrol. 2017;28:3034–3043. doi: 10.1681/ASN.2016090957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabatine M.S., Giugliano R.P., Keech A.C. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 20.Charytan D.M., Sabatine M.S., Pedersen T.R. Efficacy and safety of evolocumab in chronic kidney disease in the FOURIER Trial. J Am Coll Cardiol. 2019;73:2961–2970. doi: 10.1016/j.jacc.2019.03.513. [DOI] [PubMed] [Google Scholar]
- 21.Cheung A.K., Rahman M., Reboussin D.M. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28:2812–2823. doi: 10.1681/ASN.2017020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakris G.L., Agarwal R., Anker S.D. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 23.McMurray J.J.V., Wheeler D.C., Stefánsson B.V. Effect of dapagliflozin on clinical outcomes in patients with chronic kidney disease, with and without cardiovascular disease. Circulation. 2020;143(5):438–448. doi: 10.1161/CIRCULATIONAHA.120.051675. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Fernandez B., Sarafidis P., Kanbay M. SGLT2 inhibitors for non-diabetic kidney disease: drugs to treat CKD that also improve glycaemia. Clin Kidney J. 2020;13:728–733. doi: 10.1093/ckj/sfaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1-S3. Tables S1-S9.