Abstract
Background
Severe coronavirus disease 2019 (COVID-19) has been increasingly recognized as a multisystem disease. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can infect literally any cell type that expresses its target receptor angiotensin-converting enzyme 2. However, COVID-19-associated organ dysfunction is not only mediated by direct viral effects but also by the interaction between the host’s immune response, endotheliopathy, and microvascular coagulopathy. It has been proposed that the activation of the complement system plays a central role in the pathophysiology of severe COVID-19 and the associated endotheliopathy.
Case summary
A 76-year-old male patient with indeterminate cardiogenic shock in the setting of confirmed SARS-CoV-2 infection was admitted to our intensive care unit. Coronary angiography did not reveal a plausible explanation for his symptoms. The patient developed renal failure, neurological symptoms, severe thrombocytopenia, and a Coombs-negative haemolytic anaemia with schistocytes. All together the clinical picture was highly suggestive of a thrombotic microangiopathy (TMA) with microvascular cardiac involvement. Conventional therapeutic strategies including high-dose steroids and seven sessions of therapeutic plasma exchange were all unsuccessful. Interestingly, complement inhibition with Eculizumab as rescue approach led to a rapid clinical and laboratory improvement and the patients were discharged with normalized organ functions at Day 36.
Conclusion
The aetiology of cardiogenic shock observed in this patient cannot simply be explained by his focal and chronic coronary findings. Although viral myocarditis was not formally excluded, both the clinical features of TMA and the rapid resolution of all clinical signs and symptoms after pharmacological complement inhibition suggest a SARS-CoV-2-driven microangiopathic origin of heart failure.
Keywords: Case report, Haemolytic anaemia, Thrombotic microangiopathy, Eculizumab, Complement, Plasma exchange, Acute heart failure, COVID-19
Learning points
Aetiology of myocardial injury related to coronavirus disease 2019 (COVID-19) is a consequence of either viral myocarditis or indirect injury via endotheliopathy.
Thrombotic microangiopathy (TMA) in the absence of shiga-toxin producing bacteria and with normal ADAMTS13 is suggestive for a complement-mediated pathology.
Eculizumab is a monoclonal antibody against the complement component C5 and blocks the formation of the terminal complement complex (C5b-9) that can cause endothelial injury.
Introduction
The disease caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) has been termed coronavirus disease 2019 (COVID-19) and the complexity of its pathophysiology has been just started to be understood. SARS-CoV-2 can infect literally any cell that expressed its entry receptor angiotensin-converting enzyme 2 (ACE2).1 Multiple organ failure is triggered by endothelial cell infection and endotheliitis2 inducing a global thromboinflammatory state. From the pathophysiological point of view, activation of the complement system together with a dysbalance between von Willebrand factor (vWF) antigen and its cleaving protease ADAMTS133 leads to a thrombotic microangiopathy (TMA)-like phenotype.4
The aim of this case is to illustrate that SARS-CoV-2 triggered TMA can lead to a microcirculatory syndrome that potentially involves many organs including the heart ultimately leading to organ failure. Furthermore, the case demonstrates that this specific entity was resistant to classical treatment approaches but highly responsive to a pharmacological complement inhibition.
Timeline
| Time | Events |
|---|---|
| Admission |
|
| Day 5 after admission |
|
| Day 7 [Day 2 intensive care unit (ICU)] |
|
| Day 8 (Day 3 ICU) |
|
| Day 15 (Day 10 ICU) |
|
| Day 16 (Day 11 ICU) |
|
| Day 17 (Day 12 ICU) |
|
| Day 18 (Day 13 ICU) |
|
| Day 19 (normal ward) |
|
| Day 32 |
|
Case presentation
A 74-year-old Caucasian male presented with malaise, dyspnoea, and cough at our hospital. Initially, the temperature was 37.6°C, blood pressure 124/70 mmHg, pulse rate 135 beats/min and irregular, and oxygen saturation 91% under 6 L/min oxygen. An electrocardiogram confirmed a known atrial fibrillation. No other comorbidities were known. He had bilateral crackles and basal interstitial pulmonary infiltrates. A nasopharyngeal swab confirmed a SARS-CoV-2 infection. The patient was hospitalized and treated with remdesivir and dexamethason. On the 5th day after admission, he developed acute chest pain with ST-elevation in leads II, III, aVF, and elevated cardiac biomarkers (troponin I: 116 ng/L). A coronary angiography was performed. Unexpectedly, the angiography did only reveal a chronic thrombotic occlusion of the marginal branches of the left coronary artery circumflex branch. The patient was admitted to the intensive care unit (ICU) due to cardiogenic shock. A cardiac index of 1.2 mL/(min*m2) with an elevated pulmonary capillary wedge pressure of 27 mmHg and a mean pulmonary artery pressure of 33 mmHg was found (Table 1). Echocardiography showed normal left ventricle cavity size with a severely reduced left ventricular ejection fraction (LVEF 15%) with akinesia of the apex and all apical segments, inferior and inferolateral to mid-ventricular, anterolateral and anterior to just mid-ventricular, hypokinesia of the remaining sections of the wall. Moreover, mild aortic, mitral, and tricuspid valve regurgitation was seen. The right ventricle showed a significantly reduced systolic function with akinesia of the free wall mid-ventricular to apical. Respiratory failure worsened and nasal high flow oxygen therapy was initiated (FiO2 100%, flow 60 L/min). Cardiogenic shock was initially treated with levosimendan and nitroglycerine in the later course. Clearly, the findings of the coronary angiogram with localized chronic changes did not deliver an explanation of this clinical condition.
Table 1.
Haemodynamic parameters and cardiogenic shock treatment during the ICU course
| Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | Day 13 | |
|---|---|---|---|---|---|---|---|---|
| ICU 1 | ICU 2 | ICU 3 | ICU 4 | ICU 5 | ICU 6 | ICU 7 | ICU 8 | |
| CI (L/min/m2) | 1.2 | 1.5 | 2.6 | 2.7 | 2.1 | 2.3 | 2.5 | 2.7 |
| sPAP (mmHg) | 39 | 32 | 29 | 33 | 34 | 31 | 33 | 27 |
| dPAP (mmHg) | 28 | 20 | 11 | 13 | 14 | 10 | 12 | 9 |
| mPAP (mmHg) | 33 | 24 | 16 | 20 | 21 | 16 | 19 | 14 |
| PAWP (mmHg) | 27 | 22 | 14 | 12 | 16 | 12 | 6 | 6 |
| CVP (mmHg) | 13 | 10 | 5 | 6 | 10 | 7 | 3 | 6 |
| SvO2 (%) | 42 | 50 | 65 | 63 | 54 | 54 | 60 | 64 |
| LVEF (%) | 15 | 41 | ||||||
| HFOT (FiO2/Flow) | 100/60 | 100/60 | 65/50 | 90/55 | 60/55 | |||
| Levosimendan (mcg/kg/min) | 0.1 | 0.1 | 0.1 | |||||
| Noradrenalin (mcg/min) | 10 | 15 | 5 | 4 | ||||
| Cordarone (mg/day) | 600 | 1200 | 200 | 200 | 200 | |||
| Nitroglycerine (mcg/min) | 150 | 110 | 50 |
CI, cardiac index; CVP, central venous pressure; dPAP, diastolic pulmonary pressure; HFOT, high flow oxygen therapy; LVEF, left ventricular ejection fraction; mPAP, mean pulmonary artery pressure; PAWP, pulmonary arterial wedge pressure; sPAP, systolic pulmonary pressure; SvO2, mixed venous oxygen saturation.
Within the first week, a rapid decrease of the platelet count from 265 to 12 × 109/L was recorded. Furthermore, progressive Coombs-negative haemolytic anaemia (haemoglobin drop from 153 to 98 g/dL, lactate dehydrogenase 2844 U/L, and haptoglobin <0.10 g/L) with 12% schistocytes on peripheral-blood smear was noticed. The patient further developed acute kidney injury requiring renal replacement therapy and intermittent neurological symptoms such as delirium, drowsiness, and a slowdown in movements. The combination of thrombocytopenia, Coombs-negative haemolytic anaemia with schistocytes, renal failure, and neurological symptoms is highly suggestive of a TMA. Nevertheless, he was not suffering from diarrhoea and no shigatoxin-producing bacteria were detectable in the stool. ADAMTS13 activity came back normal (52%). With pending ADAMTS13 activity testing, the decision was made to start (i) therapeutic plasma exchange (TPE) against fresh frozen plasma and (ii) high-dose methylprednisolone pulses (1 g/day) over 3 days followed by prednisone 1 mg/kg bodyweight daily. Despite mild neurological improvement, thrombocyte count did not rise above 24 × 109/L and haemolysis persisted. Complement levels were severely decreased [C3c <0.03 g/L (0.8–16 g/L), C4 < 0.01 (0.10–0.40 g/L)].
Cardiac biomarkers continuously increased after ICU admission up to a maximum troponin of 4814 ng/L the day before the first TPE. The daily plasma exchange then eliminated it to a level around 1000 ng/L where it reached a plateau (Figure 1, Tables 2 and 3). Of note, creatine kinase and myoglobin were within normal range. After 7 TPE procedures, the patient continued to require inotropic support and the troponin levels remained elevated so that TPE was stopped [note that circulating markers such as troponin, creatinine, and N-terminal pro-B-type natriuretic peptide rose the next day due to the simple lack of removal not due to a biological disease effect]. After an interdisciplinary discussion, a single dose of 900 mg Eculizumab was applied. In the following days, a continuous rise in thrombocyte counts and a decrease in lactate dehydrogenase, and troponin levels were detected (Figure 1). Renal replacement therapy could be discontinued and the estimated glomerular filtration rate reached a steady level around 40 mL/min/m2. Over the following days, schistocytes disappeared, haptoglobin increased to 1.21 g/L, and haemoglobin levels normalized. Importantly, cardiac function improved clinically, and the patient was successfully weaned from inotropic agents. Echocardiography showed an increase in LVEF to 41%. Steroids were tapered and the patient was finally discharged into rehabilitation after 36 days.
Figure 1.
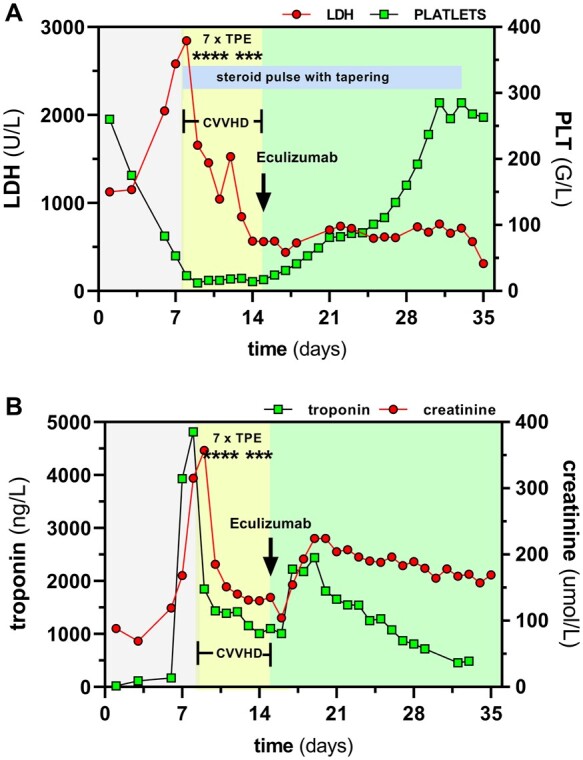
Course of thrombotic microangiopathy relevant biochemical and clinical parameters. Illustration of clinical course with regard to (A) lactate dehydrogenase and platelets and (B) creatinine and troponin. Therapeutic plasma exchange was immediately initiated at Day 8, but shortly after dialysis was necessary. As blood values did not improve rapidly, we finally administer Eculizumab and saw a rapid response in lactate dehydrogenase, platelet number, troponin, and creatinine. CVVHD, continuous veno-venous haemodialysis; LDH, lactate dehydrogenase; PLT, platelets; TPE, therapeutic plasma exchange
Table 2.
Baseline and follow-up serum parameters
|
Ref.
range |
D1 | D5 | D6 ICU 1 |
D7 ICU 2 |
D8 | D9 | D10 | D11 | D12 | D13 | D14 | D15 | D16 | D17 | D20 | D26 | D28 | D30 | D32 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICU 3 | ICU 4 | ICU 5 | ICU 6 | ICU 7 | ICU 8 | ICU 9 | ICU10 | ICU11 | ICU12 | |||||||||||
| Plasma exchange | x | x | x | x | x | x | x | |||||||||||||
| Eculizumab | x | |||||||||||||||||||
| Creatinine (umol/L) | 44–80 | 88 | 119 | 168 | 315 | 357 | 185 | 151 | 140 | 131 | 130 | 135 | 104 | 154 | 193 | 204 | 183 | 179 | 178 | 170 |
| LDH (U/L) | <250 | 1125 | 2046 | 2580 | 2844 | 1655 | 1455 | 1043 | 1527 | 844 | 565 | 560 | 565 | 439 | 546 | 693 | 607 | 729 | 762 | 714 |
| Haptoglobin | <0.10 | <0.10 | 0.1 | <0.10 | 0.72 | 1.21 | 1.35 | 1.81 | 1.94 | |||||||||||
| CK (U/L) | <170 | 108 | 101 | 703 | 450 | 165 | 144 | 131 | 85 | 60 | 56 | 45 | 56 | 47 | 40 | 47 | 23 | |||
| Myoglobin (ug/L) | 25–58 | 116 | 257 | 701 | 355 | 193 | 136 | 129 | 91 | 84 | 73 | 63 | 73 | 157 | 237 | 286 | ||||
| Troponin (ng/L) | <14 | 17 | 169 | 3933 | 4814 | 1848 | 1435 | 1391 | 1419 | 1158 | 1006 | 1102 | 1006 | 2224 | 2175 | 1655 | 871 | 718 | 484 | |
| NT-proBNP (ng/L) | <210 | 1908 | 12390 | 32280 | 44443 | 11791 | 11791 | 6695 | 6038 | 4535 | 5350 | 4863 | 5350 | 9714 | 10470 | 9400 | ||||
| Haemoglobin (g/L) | 134–170 | 153 | 157 | 130 | 119 | 98 | 109 | 104 | 93 | 76 | 87 | 76 | 86 | 83 | 81 | 93 | 75 | 80 | 89 | 85 |
| Platelets (G/L) | 143–400 | 260 | 83 | 53 | 23 | 12 | 16 | 16 | 18 | 19 | 14 | 17 | 24 | 31 | 41 | 81 | 134 | 192 | 285 | 285 |
| Schistocytes (%/BF) | 0 | 0 | 12 | 10 | 12 | 12 | 12 | 7 | 3 | 1 | ||||||||||
| Fibrinogen (mg/L) | 1.5–4.0 | 1.1 | 1.1 | 1.9 | 1.6 | 1.5 | 1.5 | 1 | 1.2 | 1.4 | 1.4 | 1.4 | 1.7 | 2.7 | 3.2 | 4.3 | 5 | |||
| D-dimers (mg/L) | <0.5 | >20 | >20 | >20 | >20 | >20 | 7.05 | 10.13 | 8.05 | 5.15 | 3.34 | 2.34 | 1.78 | 1.93 | 1.38 | 0.88 |
Before and after treatments with therapeutic plasma exchange (Days 8–9 and Days 11–15) and Eculizumab (Day 16).
BF, bright field; CK, creatine kinase; D-dimer, fibrin degradation product; LDH, lactate dehydrogenase; NT-proBNP, N-terminal pro-brain natriuretic peptide.
Table 3.
Autoantibodies, immunoglobulins, and complement activity lab results
| Reference range | Results | |
|---|---|---|
| Autoantibodies | ||
| ANA (titer) | <1:320 | 1:640 |
| Cytoplasmic ANA (titer) | <1:320 | negative |
| Anti-dsDNA (U/mL) | <1:320 | 1 |
| ANCA (titer) | <1:40 | negative |
| Anti-MPO (U/mL) | <6 | <1 |
| Anti-PR3 (U/mL) | <5 | <1 |
| Anti-Cardiolipin IgG (U/mL) | <40 | 1.4 |
| Anti-Cardiolipin IgA (U/mL) | <20 | 2.3 |
| Anti-Cardiolipin IgM (U/mL) | <40 | 2.5 |
| Anti-B2Glycoprotein I IgG (U/mL) | <10 | 1.5 |
| Anti-B2Glycoprotein I IgA (U/mL) | <10 | 1.6 |
| Anti-B2Glycoprotein I IgM (U/mL) | <10 | 0 |
| Anti-Scl-70 | Negative | Negative |
| Anti-Centromer (U/L) | <10 | 1 |
| Immunoglobulins | ||
| IgG (g/L) | 7.0–16.0 | 6.8 |
| IgA (g/L) | 0.7–4.0 | 1.39 |
| IgM (g/L) | 0.4–2.3 | 0.6 |
| Immunofixation electrophoresis | normal | normal |
| Complement activity | ||
| C3c (g/L) | 0.8–1.6 | <0.03 |
| C4 (g/L) | 0.10–0.40 | <0.01 |
| Factor H (mg/L) | 320–750 | 285 |
| Factor B (mg/L) | 200–400 | 269 |
| Classical pathway (%)* | 69–129 | 2 |
| Alternative pathway (%)* | 30–113 | 1 |
| MBL-Pathway* | 10–125 | 1 |
* after Eculizumab. ANA, antinuclear antibody; ANCA, antineutrophil cytoplasmic antibodies; B2Glycoprotein I, beta 2 gycoprotein 1 antibodies; C3c, complement component 3; C4, complement 4; dsDNA, double stranded DNA antibodies; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; MBL, Mannan-binding lectin; MPO, myeloperoxidase antibodies; PR3, proteinase 3 antibodies; Scl-70, topoisomerase I. Remarkable values are highlighted in bold.
Discussion
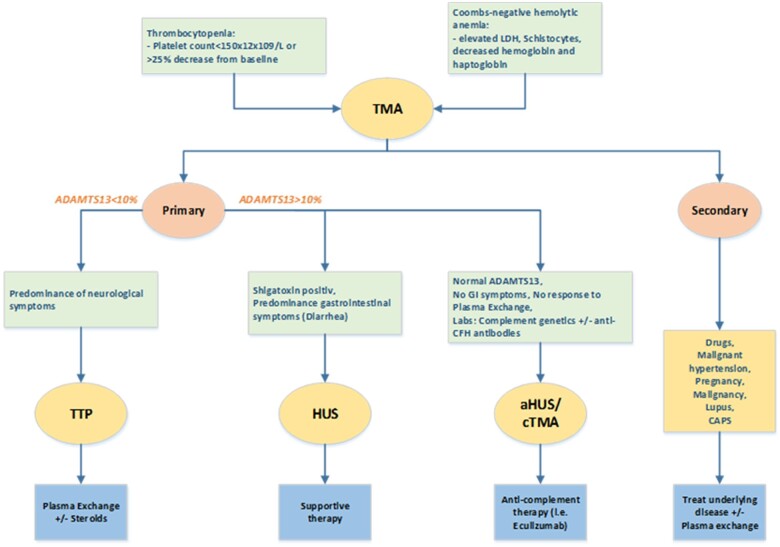
Myocardial injury related to COVID-19 has been described in case series reporting a broad aetiological range5 but acute heart failure and cardiogenic shock appears to be among the more rare complications. One can assume that a cardiac magnetic resonance imaging would have helped to narrow the differential diagnostic window6 in the absence of a clear trigger, in particular in myocarditis.7 However, this could initially not be done for practicality reasons (nasal high flow oxygen device, pulmonary artery catheter). In the later course, the clinical and biochemical surrogates were rather typical. Along the same line, a myocardial biopsy would have been helpful clinically but was not done in the context of progressive thrombocytopenia. Together with the haemolytic anaemia with schistocytes, renal failure, and neurological symptoms, the constellation is highly suggestive of TMA. In general, three forms are important to differentiate (Figure 2). In the absence of shiga-toxins (as seen in haemolytic uraemic syndrome, HUS) and normal ADAMTS13 activity (seen in thrombotic thrombocytopenic purpura) the underlying pathophysiology is usually complement mediated.8 This group has been re-named from atypical (a)HUS into complement-mediated TMA (cTMA).
Figure 2.
Diagnostic algorithm for thrombotic microangiopathies. Thrombocytopenia and Coombs-negative anaemia suggest for a diagnosis of thrombotic microangiopathy. Thrombotic microangiopathy is divided in primary and secondary. The first includes a spectrum of entities like thrombocytopenic purpura, haemolytic uraemic syndrome, and complement-mediated thrombotic microangiopathy. The main characteristics of thrombocytopenic purpura is ADAMTS13 reduction or deficiency and neurological syndrome. In haemolytic uraemic syndrome, the prominent organ injury is renal and ADAMTS13 activity is normal. Typical haemolytic uraemic syndrome is caused from enteric bacterial infection (Escherichia coli) that produces Shigatoxin that damages endothelial cells. More rarely, there are thrombotic microangiopathies cases with normal ADAMTS13 activity and in the absence of Shiga toxin-producing organisms or enteric symptoms. This group is referred to complement-mediated thrombotic microangiopathy (or atypical haemolytic uraemic syndrome) due to the dysregulation of the alternate complement pathway leading to complement overactivation and consequent endothelial damage. Secondary thrombotic microangiopathies are caused by other underlying conditions, such as infections, autoimmune disease, or medical treatment. ADAMTS13, a disintegrin and metalloprotease with thrombospondin type 1 motifs, member 13; CFH, complement factor H; cTMA, complement-mediated thrombotic microangiopathy; GI, gastrointestinal; HUS, haemolytic uraemic syndrome; TMA, thrombotic microangiopathy; TTP, thrombotic thrombocytopenic purpura.
Given that complement activation also plays a role in the endotheliopathy of COVID-19,9 these considerations built the rationale for the explorative use of Eculizumab10 that ultimately led to an immediate remission with normalization of thrombocytes, haemolytic features, and a recovery of affected organs including the heart.11 Of note, other therapeutic strategies in COVID-19 associated TMA have recently been discussed in more detail.12
Conclusion
This case highlights the importance of recognizing cTMA associated with COVID-19 as a treatable trigger of microangiopathic cardiogenic shock. After pharmacological complement inhibition a rapid clinical and laboratory improvement was observed. Prospective studies are needed to better understand the mechanisms of complement activation in SARS-CoV-2 infections,13 and the efficacy of complement inhibition.
Lead author biography

Didar Utebay was born in Locarno, Switzerland. She graduated from the Faculty of Medicine, University of Lausanne in 2013. Currently, she is a resident in Intensive care at the University Hospital of Zurich.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
References
- 1. Ge XY, Li JL, Yang XL, Chmura AA, Zhu G, Epstein JH. et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 2013;503:535–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS. et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Philippe A, Chocron R, Gendron N, Bory O, Beauvais A, Peron N. et al. Circulating Von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict COVID-19 in-hospital mortality. Angiogenesis 2021;24:505–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Falter T, Rossmann H, Menge P, Goetje J, Groenwoldt S, Weinmann A. et al. No evidence for classic thrombotic microangiopathy in COVID-19. J Clin Med 2021;10:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F. et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J. et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sawalha K, Abozenah M, Kadado AJ, Battisha A, Al-Akchar M, Salerno C. et al. Systematic review of COVID-19 related myocarditis: insights on management and outcome. Cardiovasc Revasc Med 2021;23:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akesson A, Martin M, Blom AM, Rossing M, Gabrielaite M, Zetterberg E, Klintman J.. Clinical characterization and identification of rare genetic variants in atypical hemolytic uremic syndrome: a Swedish retrospective observational study. Ther Apher Dial 2021; doi: 10.1111/1744-9987.13634. [DOI] [PubMed] [Google Scholar]
- 9. Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J. et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 2020;220:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diurno F, Numis FG, Porta G, Cirillo F, Maddaluno S, Ragozzino A. et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci 2020;24:4040–4047. [DOI] [PubMed] [Google Scholar]
- 11. Laurence J, Mulvey JJ, Seshadri M, Racanelli A, Harp J, Schenck EJ. et al. Anti-complement C5 therapy with eculizumab in three cases of critical COVID-19. Clin Immunol 2020;219:108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tiwari NR, Phatak S, Sharma VR, Agarwal SK.. COVID-19 and thrombotic microangiopathies. Thromb Res 2021;202:191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vitiello A, La Porta R, D'Aiuto V, Ferrara F.. Pharmacological approach for the reduction of inflammatory and prothrombotic hyperactive state in COVID-19 positive patients by acting on complement cascade. Hum Immunol 2021;82:264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.