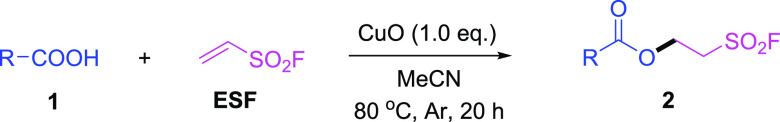
Abstract
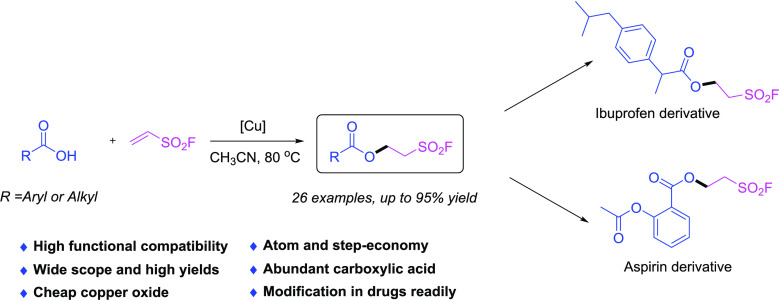
A CuO-promoted direct hydrocarboxylation of ethenesulfonyl fluoride (ESF) was developed using carboxylic acid as a nucleophile under mild conditions. A variety of molecules containing both ester group and aliphatic sulfonyl fluoride moiety exhibit great potential in medicinal chemistry and chemical biology. Furthermore, the modification of the known drugs Ibuprofen and Aspirin was also demonstrated.
Introduction
Since the seminal work reported by Sharpless group in 2014,1 sulfur fluoride exchange (SuFEx), another representative exemplification of click chemistry,2 has triggered immense interest among the community of researchers due to its extraordinary efficiency, unique reactivity, and absolute reliability for achieving the molecular diversity through a sulfur hub.3 Furthermore, sulfonyl fluoride is one of the irreplaceable members of SuFEx chemistry family4 and has been considerably applied to various productive fields based on its unique prominent features of stability–reactivity.5
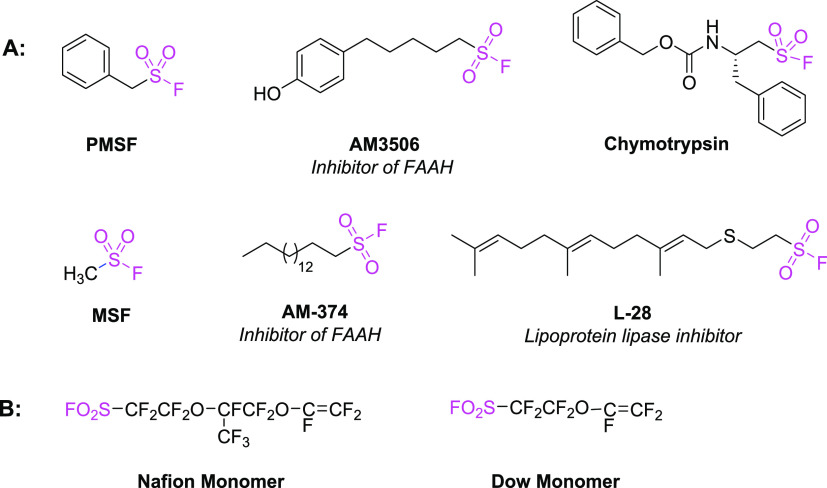
Aliphatic sulfonyl fluorides, bearing the attractive sulfonyl fluoride motifs, have been considered as one of the most prominent covalent inhibitors or privileged drug warheads along with numerous other applications in the development of chemical biology and medicinal chemistry in recent decades.6 Phenylmethanesulfonyl fluoride (PMSF), was found to act as a covalent enzyme inhibitor of serine proteases and also as a reactive probe in the routine preparation of cell lysates.7 In addition, AM3506 and AM-374, effective inhibitors of fatty acid amide hydrolase (FAAH) and lipoprotein lipase inhibitor (L-28), were all functionalized with valuable aliphatic sulfonyl fluorides as core pharmacophores and showed excellent activity for treating the corresponding diseases.8 In addition, molecules bearing sulfonyl fluoride moieties have been identified as inhibitors of esterase (Figure 1A).7d7e Moreover, substrates with sulfonyl fluoride moiety have also gained significant attention in polymer chemistry.9 For instance, some well-known sulfonyl fluoride-containing monomers including “Nafion” and “Dow monomer” have been widely utilized to make sulfonic end-group fluorocarbon-based ion-exchange membrane via polymerization with various perfluoro alkenes and hydrolysis of sulfonyl fluoride functionality (Figure 1B).10 Because of the great importance and omnipresent applications of sulfonyl fluorides, the development of reliable methods for the installation of sulfonyl fluoride moiety to the scaffolds of valuable industrial applications is highly desirable.
Figure 1.
Representative enzyme inhibitors or covalent drugs (A) and perfluorofunctional polymers (B) containing aliphatic sulfonyl fluoride.
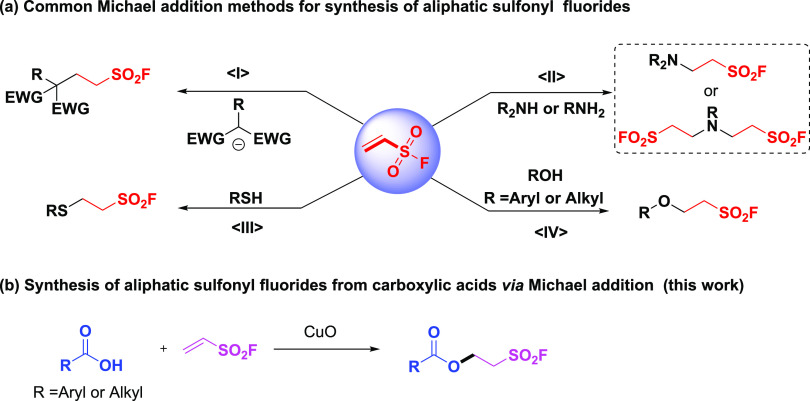
Additionally, ethenesulfonyl fluoride (ESF), an excellent Michael acceptor,11 has been demonstrated as one of the most effective reagents for incorporating the sulfonyl fluoride group into the molecules via the classic Michael addition using carbon, nitrogen, sulfur, and oxygen nucleophiles (Scheme 1a).1,12,13 On the other hand, carboxylic acids are among the most common and available chemical compounds categories. Meanwhile, they are essential in organic chemistry and industry, among which various (hetero)-aromatic carboxylic acids are good structurally diverse, inexpensive, readily available, and bench-stable candidates for medicinal chemistry and agrochemistry.14 Carboxylic acids occur widely in natural products and common chemicals, and the specific characteristics of this carboxylic acid functional group enable them to serve as central building blocks for the preparation of derivatives like carboxylate salts, anhydrides, esters, nitriles, and amides.15 Also, the corresponding products find numerous applications for polymers, cosmetics, pharmaceuticals, agrochemicals, and other manufactured chemicals.16 Notably, to the best of our knowledge, esters are commonly constructed through the coupling of alcohols with acids while the direct Michael addition of acids to electron-deficient alkenes for the synthesis of esters has been rarely reported due to their paucity of nucleophilic ability.17 Therefore, nucleophilic activation of carboxylic acids has always been a challenging task in organic transformations.
Scheme 1. Approaches to Aliphatic Sulfonyl Fluorides via Michael Addition of Various Nucleophiles.
The nucleophilic conjugate additions of oxygen-centered nucleophiles to conjugate acceptors are one of the most potent strategies for the C–O bond formation.18 Inspired by the difunctionalization of terminal alkynes by Cu(III)–CF3 complex in the combination of carboxylic acids,19 and considering the significance of ester and aliphatic sulfonyl fluoride as the structurally irreplaceable scaffolds of the various molecules, we envision that the synthesis of this category of molecules bearing both these motifs will be very attractive in organic synthesis, chemical biology, drug discovery, and ionic membrane chemistry.20 Herein, we reported the Michael addition of ESF using diverse carboxylic acids as the nucleophiles in the presence of CuO, affording a family of 2-fluorosulfonylethyl esters for further utilization (Scheme 1b).
Results and Discussion
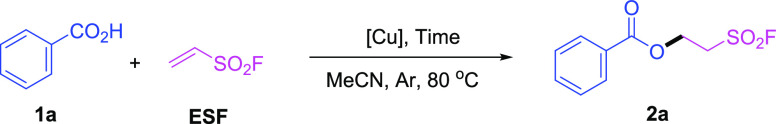
Originally, benzoic acid (1a) was selected as a model substrate to investigate the feasibility of synthesizing the desired 2-(fluorosulfonyl)ethyl benzoate (2a). When the reaction of benzoic acid (1a) with ESF was carried out in the presence of CuO using dry acetonitrile as a solvent under air, the desired product 2-(fluorosulfonyl)ethyl benzoate (2a) was obtained in 20% yield (Table 1, entry 1, see the Supporting Information for details). When the reaction was carried under an argon atmosphere, the yield of 2a was improved to 74% (Table 1, entry 2). The use of dry solvent under an argon atmosphere (Table 1, entry 3, 89%) was found to be the best condition for the desired transformation. Besides, reducing the loading of CuO from 2.0 to 1.0 equiv afforded a similar yield of 2a. However, the reaction was completely frustrated in the absence of CuO (Table 1, entries 4 and 5). The loading of ESF was also assessed and 2.0 equiv of ESF turned out to be the most effective for the formation of 2-(fluorosulfonyl)ethyl benzoate (2a) (Table 1, entries 4, 6, and 7). It was worth noting that when the reactions were operated at an elevated temperature of 90 °C or a reduced temperature of 70 °C, the desired product was obtained in relatively lower yields, indicating that 80 °C was the most suitable reaction temperature (Table 1, entries 4, 8, and 9). Additionally, prolonging the reaction time to 48 h or decreasing the reaction time to 5 h diminished the yield of 2a (Table 1, entries 10 and 11). Accordingly, the reaction condition of Table 1, entry 4 was chosen as the standard condition for further examination of substrate scope and functional group compatibility.
Table 1. Optimization of the Reaction Conditionsa.
| entry | [Cu] (X equiv) | ESF (Y equiv) | time (h) | yield (2a, %)b |
|---|---|---|---|---|
| 1c | CuO (2.0) | 2.0 | 20 | 20 |
| 2d | CuO (2.0) | 2.0 | 20 | 74 |
| 3 | CuO (2.0) | 2.0 | 20 | 89 |
| 4 | CuO (1.0) | 2.0 | 20 | 91 |
| 5 | / | 2.0 | 20 | trace |
| 6 | CuO (1.0) | 4.0 | 20 | 73 |
| 7 | CuO (1.0) | 1.5 | 20 | 70 |
| 8e | CuO (1.0) | 1.5 | 20 | 80 |
| 9f | CuO (1.0) | 2.0 | 20 | 87 |
| 10 | CuO (1.0) | 2.0 | 5 | N.D. |
| 11 | CuO (1.0) | 2.0 | 48 | 89 |
Reaction conditions: a mixture of benzoic acid (1a, 0.1 mmol), [Cu], ESF, and anhydrous MeCN (2 mL) reacted at 80 °C under argon atmosphere for the corresponding time.
The yields were determined by HPLC using 2a as the external standard (tR = 7.5 min, λmax = 229.9 nm, water/acetonitrile = 50:50 (v/v)).
In the air.
Undried MeCN (2 mL).
Reacted at 70 °C.
Reacted at 90 °C.
With the optimized reaction conditions in hand, we next evaluated the substrate scope and functional group tolerance of this oxa-Michael addition of ESF using various substituted carboxylic acids 1 as the reaction partners. As the results illustrated in Table 2, carboxylic acids functionalized with electron-withdrawing groups, such as the nitro group (1b and 1c), halogen (1d–1l), the cyano group (1m), or electron-donating groups, such as the methoxyl group (1n), the methyl group (1o–1r) at ortho-, meta-, and para-positions of the aromatic rings tolerated this process smoothly to deliver the corresponding products 2b–2r in moderate to excellent isolated yields (34–95%). Note that the aromatic carboxylic acids (1g and 1r) bearing multisubstituted aromatic rings were also smoothly converted into the Michael addition products 2g and 2r in 63 and 60% yield, respectively. The acetal moiety in the piperic acid (1s) remained intact during the transformation and the targeted product 2s was successfully isolated in 60% yield. Besides, the polycyclic molecule naphthyl (1t) turned out to be the suitable substrate as well under the standard conditions. In addition, the heterocyclic carboxylic acids furan (1u), benzofuran (1v), benzindole (1w), and benzothiophene (1x) can also be applied for this direct transformation with the additional 5 mol % ruthenium catalyst, which indicated the potential authentic value of this method for the synthesis of bioactive heterocycle-containing molecules. The heteroatoms (X = O, N, and S) in the heterocyclic carboxylic acids can coordinate with CuO easily to deactivate its reactivity in this transformation. In contrast, the metal complex Ru(bpy)3(PF6)2 with a large steric hindrance can activate the carboxylic acid group more readily. Remarkably, the aliphatic carboxylic acids (1y and 1z) were also compatible with this reaction system, albeit moderate yields of the anticipated products (2y and 2z) were obtained. With regard to the cases of aliphatic carboxylic acids, the alkyl carboxylic acid group was relatively difficult to activate, while metal ruthenium was widely used to activate the carbon–hydrogen bonds,21 can probably form metal complexes with benzoic acids in our reaction that served as key reaction intermediates. To demonstrate the practicality of this method, a gram-scale reaction of 1d (1.00 g, 5 mmol) was also performed under identical conditions, affording 2d in 76% yield (1.18 g).
Table 2. Scope of the Addition of Carboxylic Acids to Ethenesulfonyl Fluoride (ESF)ab.
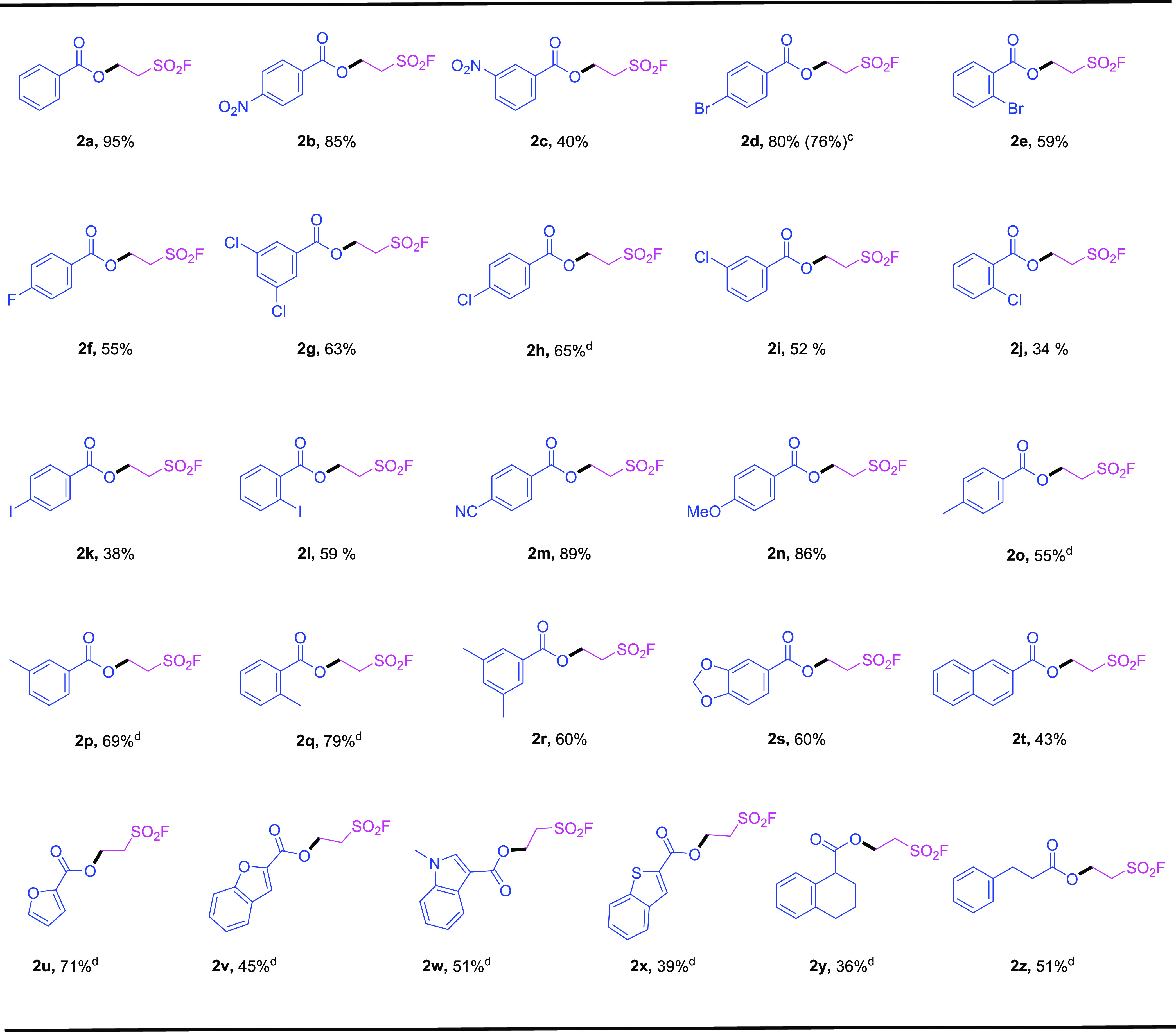
Reaction condition: a mixture of carboxylic acid (1, 1.0 mmol), ESF (2.0 mmol), CuO (1.0 equiv), and MeCN (0.2 M, 5.0 mL) reacted at 80 °C under an argon atmosphere for 20 h.
Isolated yield.
The reaction was conducted on a 5 mmol scale (1d, 1.00 g).
Additional 5 mol % Ru(bpy)3(PF6)2 was added.
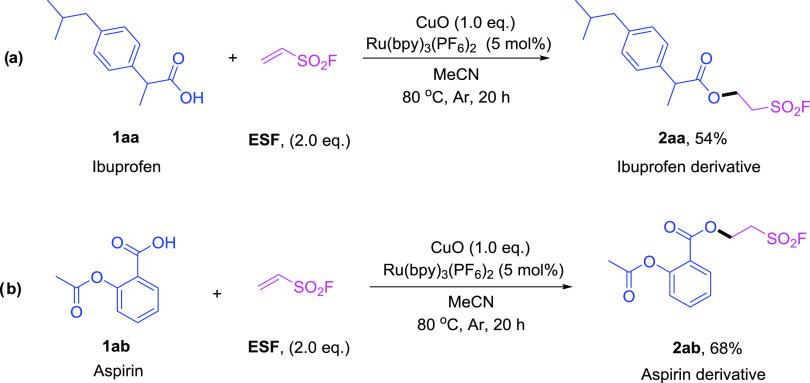
Ibuprofen (1aa) and Aspirin (1ab, acetylsalicylic acid), both over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs), are the most widely used analgesic–antipyretic and anti-inflammatory drugs in many countries.22 Also, the carboxylic acid groups in these two molecules can also react with ESF smoothly to afford their corresponding aliphatic sulfonyl fluoride derivatives (Table 3, 2aa and 2ab) in good yields (54 and 68%, respectively).
Table 3. Modifications of Ibuprofen and Aspirin.
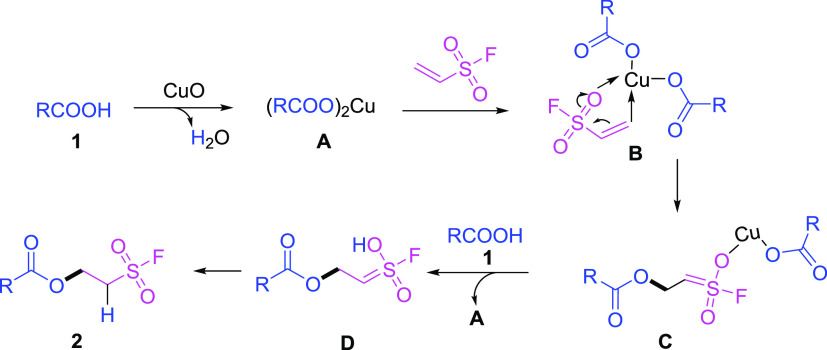
On the basis of the previous studies,23 a plausible reaction mechanism for the oxa-Michael addition of carboxylic acid 1 to electron-deficient alkene ESF with the promotion of CuO is postulated in Scheme 2. Initially, the treatment of carboxylic acid 1 with alkaline metal oxide CuO generated intermediate A, which can further coordinate with ESF to form a Cu complex B. The subsequent Michael addition of the thioxyl anion to ESF led to the formation of intermediate C, and in the presence of another molecule of carboxylic acid 1, the intermediate D was successfully obtained. The regenerated copper–alkoxyl complex A was applied for the next catalytic cycle. Finally, the expected product 2 was achieved through isomerization of D. The deuterated experiment indicated that the proton of benzoic acid group (−CO2H) was transferred to the α-carbon adjacent to sulfonyl fluoride group, which clearly revealed that the reactions of ESF and carboxylic acids proceeded through an oxa-Michael addition (see the Supporting Information for details).
Scheme 2. Proposed Reaction Mechanism.
In conclusion, we have developed a method for a direct transformation of carboxylic acids to a class of novel 2-(fluorosulfonyl)ethyl benzoate in the promotion of CuO. This protocol featured with broad substrate scope and sufficient structural diversity including the drugs Ibuprofen and Aspirin. Further studies on applications of these molecules in chemical biology and medicinal chemistry are underway in our laboratory.
Experimental Section
General Considerations
All reactions were carried out under an argon atmosphere unless otherwise specified. The NMR spectra were recorded in CDCl3 on 500 MHz (for 1H), 471 MHz (for 19F), and 126 MHz (for 13C) spectrometers. All chemical shifts were reported in ppm relative to TMS (1H NMR, 0 ppm) as internal standards. The HPLC experiments were carried out on a Waters e2695 instrument (column: J&K, RP-C18, 5 μm, 4.6 × 150 mm2), and the yields of the products were determined using the corresponding pure compounds as the external standards. The coupling constants were reported in Hertz (Hz). The following abbreviations were used to explain the multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, and br = broad. Melting points were measured and uncorrected. The MS experiments were performed on a TOF-Q ESI or CI/EI instrument. Reagents used in the reactions were all purchased from commercial sources and used without further purification.
General Procedures for Synthesis of 2-(Fluorosulfonyl)ethyl benzoate with ESF and Carboxylic Acids
Procedure A
An oven-dried reaction tube (30 mL) was charged with (hetero) aromatic carboxylic acids or aliphatic carboxylic acids (1, 1.0 mmol), CuO (1.0 equiv, 80 mg), ESF (2.0 equiv, 2.0 mmol), and anhydrous acetonitrile (5 mL). The resulting mixture was stirred at 80 °C under an argon atmosphere for 18–22 h and monitored by TLC. The crude mixture was purified by column chromatography on a silica gel to give the desired product 2 and recycle the starting material 1.
Procedure B
2h, 2o–2q, 2u–2z, and 2aa–2ab were prepared according to the procedure to furnish better yields. An oven-dried reaction tube (30 mL) was charged with (hetero) aromatic carboxylic acids or aliphatic carboxylic acids (1, 1.0 mmol), CuO (1.0 equiv, 80 mg), Ru(bpy)3(PF6)2 (5 mol %, 43 mg), ESF (2.0 equiv, 2.0 mmol), and anhydrous acetonitrile (5 mL). The resulting mixture was stirred at 80 °C under an argon atmosphere for 18–22 h and monitored by TLC. The crude mixture was purified by column chromatography on silica gel to give the desired product 2 and recycle the starting material 1.
2-(Fluorosulfonyl)ethyl benzoate (2a)
Procedure A was followed, with petroleum ether/ethyl acetate = 10:1 (v/v) as an eluent for column chromatography. White soild, mp 47–48 °C, 221 mg, 95% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 8.05 (d, J = 7.3 Hz, 2H), 7.62–7.59 (m, 1H), 7.48–7.45 (m, 2H), 4.81 (t, J = 5.3 Hz, 2H), 3.85 (q, J = 5.3 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ 59.0 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 166.0, 133.9, 130.0, 128.8, 57.5, 50.3 (d, J = 18.2 Hz). ESI-MS HRMS calculated for C9H10FO4S [M + H]+: 233.0278, found 233.0276.
2-(Fluorosulfonyl)ethyl 4-nitrobenzoate (2b)
Procedure A was followed, with petroleum ether/ethyl acetate = 5:1 (v/v) as an eluent for column chromatography. White solid, mp 86–88 °C, 237 mg, 85% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 8.32–8.31 (m, 2H), 8.24–8.22 (m, 2H), 4.87 (t, J = 5.1 Hz, 2H), 3.89 (q, J = 5.1 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ 59.3 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 164.1, 151.1, 134.2, 131.2, 123.9, 58.3, 50.1 (d, J = 19.0 Hz). ESI-MS HRMS calculated for C9H9FNO6S [M + H]+: 278.0129, found 278.0129.
2-(Fluorosulfonyl)ethyl 3-nitrobenzoate (2c)
Procedure A was followed, with petroleum ether/ethyl acetate = 5:1 (v/v) as an eluent for column chromatography. Light yellow solid, mp 71–72 °C, 111 mg, 40% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 8.85 (s, 1H), 8.45 (d, J = 8.2 Hz, 1H), 8.37 (d, J = 7.7 Hz, 1H), 7.69 (t, J = 8.0 Hz, 1H), 4.88 (t, J = 5.7 Hz, 2H), 3.91 (q, J = 5.2 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ 59.1 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 163.9, 148.5, 135.5, 130.6, 130.1, 128.2, 124.9, 58.2, 50.1 (d, J = 18.1 Hz). ESI-MS HRMS calculated for C9H9FNO6S [M + H]+: 278.0129, found 278.0127.
2-(Fluorosulfonyl)ethyl 4-bromobenzoate (2d)
Procedure A was followed, with petroleum ether/ethyl acetate = 5:1 (v/v) as an eluent for column chromatography. Light yellow solid, mp 82–83 °C, 248 mg, 80% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 7.90 (d, J = 8.4 Hz, 2H), 7.60 (d, J = 8.4 Hz, 2H), 4.80 (t, J = 5.3 Hz, 2H), 3.85 (q, J = 5.2 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ 59.1 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 165.3 132.2, 131.5, 129.2, 127.8, 57.7, 50.3 (d, J = 18.2 Hz). ESI-MS HRMS calculated for C9H9BrFO4S [M + H]+: 310.9383, found 310.9379.
2-(Fluorosulfonyl)ethyl 2-bromobenzoate (2e)
Procedure A was followed, with petroleum ether/ethyl acetate = 5:1 (v/v) as an eluent for column chromatography. Light yellow oil, 185 mg, 59% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 7.86 (d, J = 8.9 Hz, 1H), 7.69 (d, J = 7.1 Hz, 1H), 7.41–7.36 (m, 2H), 4.81 (t, J = 5.7 Hz, 2H), 3.87 (q, J = 5.3 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ 58.9 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 165.1, 134.8, 133.5, 131.9, 130.3, 127.5, 122.3, 57.9, 50.1(d, J = 17.2 Hz). ESI-MS HRMS calculated for C9H9BrFO4S [M + H]+: 310.9383, found 310.9376.
2-(Fluorosulfonyl)ethyl 4-fluorobenzoate (2f)
Procedure A was followed, with petroleum ether/ethyl acetate = 10:1 (v/v) as an eluent for column chromatography. White solid, mp 82–83 °C, 138 mg, 55% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 8.07 (q, J = 4.7 Hz, 2H), 7.14 (t, J = 8.6 Hz, 2H), 4.81 (t, J = 5.6 Hz, 2H), 3.85 (q, J = 5.2 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ 59.1 (s, 1F), −104.0 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 167.4, 164.9, 132.6 (d, J = 10.0 Hz), 125.1, 116.0 (d, J = 21.8 Hz), 57.6, 50.3 (d, J = 17.3 Hz). ESI-MS HRMS calculated for C9H9F2O4S [M + H]+: 251.0184, found 251.0176.
2-(Fluorosulfonyl)ethyl 3,5-dichlorobenzoate (2g)
Procedure A was followed, with petroleum ether/ethyl acetate = 5:1 (v/v) as an eluent for column chromatography. White solid, mp 61–62 °C, 191 mg, 63% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 7.91 (d, J = 1.9 Hz, 2H), 7.59 (t, J = 2.0 Hz, 1H), 4.82 (t, J = 6.3 Hz, 2H), 3.85 (q, J = 5.3 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ 59.1 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 163.7, 135.8, 133.8, 131.7, 128.4, 58.2, 50.1 (d, J = 19.1 Hz). ESI-MS HRMS calculated for C9H8Cl2FO4S [M + H]+ 300.9499, found 300.9493.
2-(Fluorosulfonyl)ethyl 4-chlorobenzoate (2h)
Procedure B was followed, with petroleum ether/ethyl acetate = 5:1 (v/v) as an eluent for column chromatography. Light yellow solid, mp 89–91 °C, 173 mg, 65% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 7.99 (d, J = 8.6 Hz, 2H), 7.45 (d, J = 8.6 Hz, 2H), 4.81 (t, J = 6.2 Hz, 2H), 3.85 (q, J = 5.2 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ 59.1 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 165.1, 140.5, 131.4, 129.2, 127.3, 57.7, 50.3 (d, J = 18.2 Hz). ESI-MS HRMS calculated for C9H9ClFO4S [M + H]+ 266.9889, found 266.9882.
2-(Fluorosulfonyl)ethyl 3-chlorobenzoate (2i)
Procedure A was followed, with petroleum ether/ethyl acetate = 5:1 (v/v) as an eluent for column chromatography. Light yellow oil, 137 mg, 52% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 7.96 (s, 1H), 7.89 (d, J = 7.6 Hz, 1H), 7.52 (d, J = 8.1 Hz, 1H), 7.36 (t, J = 8.0 Hz, 1H), 4.78 (t, J = 5.7 Hz, 2H), 3.87 (q, J = 5.1 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ 59.1 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 164.6, 134.7, 133.7, 130.5, 130.0, 129.8, 127.9, 57.7, 50.0 (d, J = 17.3 Hz). ESI-MS HRMS calculated for C9H9ClFO4S [M + H]+ 266.9889, found 266.9885.
2-(Fluorosulfonyl)ethyl 2-chlorobenzoate (2j)
Procedure A was followed, with petroleum ether/ethyl acetate = 5:1 (v/v) as an eluent for column chromatography. Colorless oil, 91 mg, 34% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 7.89 (d, J = 7.6 Hz, 1H), 7.48–7.47 (m, 2H), 7.36–7.33 (m, 1H), 4.82 (t, J = 5.8 Hz, 2H), 3.87 (q, J = 5.3 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ 58.9 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 164.6, 134.4, 133.5, 131.9, 131.5, 128.3, 126.9, 57.8, 50.1(d, J = 18.2 Hz). ESI-MS HRMS calculated for C9H9ClFO4S [M + H]+ 266.9889, found 266.9883.
2-(Fluorosulfonyl)ethyl 4-iodobenzoate (2k)
Procedure A was followed, with petroleum ether/ethyl acetate = 5:1 (v/v) as an eluent for column chromatography. White solid, mp 76–77 °C, 134 mg, 38% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 7.84 (d, J = 8.4 Hz, 2H), 7.75 (d, J = 8.4 Hz, 2H), 4.80 (t, J = 5.5 Hz, 2H), 3.84 (q, J = 5.2 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ 59.1(s, 1F). 13C NMR (126 MHz, CDCl3) δ 165.5, 138.2, 131.3, 128.4, 102.0, 57.7, 50.3 (d, J = 18.2 Hz). ESI-MS HRMS calculated for C9H9FIO4S [M + H]+ 358.9245, found 358.9239.
2-(Fluorosulfonyl)ethyl 2-iodobenzoate (2l)
Procedure A was followed, with petroleum ether/ethyl acetate = 5:1 (v/v) as an eluent for column chromatography. Light yellow solid, mp 66–68 °C, 211 mg, 59% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 8.03 (d, J = 8.1 Hz, 1H), 7.88 (dd, J = 7.8 Hz, J = 1.2 Hz, 1H), 7.44 (t, J = 7.6 Hz, 1H), 7.20 (td, J = 7.7 Hz, J = 1.3 Hz, 1H), 4.82 (t, J = 5.7 Hz, 2H), 3.88 (q, J = 5.3 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ 58.9 (s, 1F), 13C NMR (126 MHz, CDCl3) δ 165.5, 141.9, 133.6, 133.1, 131.6, 128.3, 94.7, 58.0, 50.1(d, J = 18.2 Hz). ESI-MS HRMS calculated for C9H9FIO4S [M + H]+ 358.9245, found 358.9240.
2-(Fluorosulfonyl)ethyl 4-cyanobenzoate (2m)
Procedure A was followed, with petroleum ether/ethyl acetate = 3:1 (v/v) as an eluent for column chromatography. White solid, mp 121–122 °C, 229 mg, 89% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 8.16 (d, J = 7.9 Hz, 2H), 7.78 (d, J = 8.1 Hz, 2H), 4.85 (t, J = 5.6 Hz, 2H), 3.87 (q, J = 5.1 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ 59.3 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 164.3, 132.6, 130.5, 117.9, 117.4, 58.2, 50.1 (d, J = 18.1 Hz). ESI-MS HRMS calculated for C19H9FNO4S [M + H]+ 258.0231, found 258.0232.
2-(Fluorosulfonyl)ethyl 4-methoxybenzoate (2n)
Procedure A was followed, with petroleum ether/ethyl acetate = 5:1 (v/v) as an eluent for column chromatography. White solid, mp 50–51 °C, 226 mg, 86% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 8.00 (d, J = 8.8 Hz, 2H), 6.94 (d, J = 9.0 Hz, 2H), 4.77 (t, J = 5.2 Hz, 2H), 3.87 (s, 3H), 3.83 (q, J = 5.3 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ 59.0 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 165.6, 164.1, 132.1, 121.2, 114.0, 57.2, 55.6, 50.4 (d, J = 18.1 Hz). ESI-MS HRMS calculated for C10H12FO5S [M + H]+ 263.0384, found 263.0382.
2-(Fluorosulfonyl)ethyl 4-methylbenzoate (2o)
Procedure B was followed, with petroleum ether/ethyl acetate = 10:1 (v/v) as an eluent for column chromatography. White solid, mp 58–60 °C, 135 mg, 55% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 7.93 (d, J = 8.3 Hz, 2H), 7.26 (d, J = 8.1 Hz, 2H), 4.78 (t, J = 5.7 Hz, 2H), 3.84 (q, J = 5.3 Hz, 2H), 2.42 (s, 3H). 19F NMR (471 MHz, CDCl3) δ 59.0 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 166.0, 144.8, 130.0, 129.5, 126.1, 57.3, 50.4 (d, J = 18.1 Hz), 29.8. ESI-MS HRMS calculated for C10H12FO4S [M + H]+ 247.0435, found 247.0445.
2-(Fluorosulfonyl)ethyl 3-methylbenzoate (2p)
Procedure B was followed, with petroleum ether/ethyl acetate = 10:1 (v/v) as an eluent for column chromatography. Light yellow oil, 169 mg, 69% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 7.92–7.84 (m, 2H), 7.42–7.41 (m, 1H), 7.36–7.33 (m, 1H), 4.80 (t, J = 5.7 Hz, 2H), 3.85 (q, J = 5.3 Hz, 2H), 2.41(s, 3H). 19F NMR (471 MHz, CDCl3) δ 59.0 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 166.1, 138.6, 134.6, 130.4, 128.8, 128.6, 127.1, 57.4, 50.3(d, J = 17.3 Hz), 21.3. ESI-MS HRMS calculated for C10H12FO4S [M + H]+ 247.0435, found 247.0444.
2-(Fluorosulfonyl)ethyl 2-methylbenzoate (2q)
Procedure B was followed, with petroleum ether/ethyl acetate = 10:1 (v/v) as an eluent for column chromatography. Light yellow oil, 194 mg, 79% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 7.96 (d, J = 8.3 Hz, 1H), 7.46–7.43 (m, 1H), 7.28–7.26 (m, 2H), 4.78 (t, J = 5.8 Hz, 2H), 3.85 (q, J = 5.3 Hz, 2H), 2.62 (s, 3H). 19F NMR (471 MHz, CDCl3) δ 58.9 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 166.5, 141.2, 133.0, 132.0, 131.1, 127.9, 126.1, 57.2, 50.4 (d, J = 18.2 Hz), 22.4 (d, J = 116.2 Hz). ESI-MS HRMS calculated for C10H12FO4S [M + H]+ 247.0435, found 247.0441.
2-(Fluorosulfonyl)ethyl 3,5-dimethylbenzoate (2r)
Procedure A was followed, with petroleum ether/ethyl acetate = 10:1 (v/v) as an eluent for column chromatography. White solid, mp 95–97 °C, 156 mg, 60% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 7.66 (s, 2H), 7.23 (s, 1H), 4.79 (t, J = 5.8 Hz, 2H), 3.84 (q, J = 5.3 Hz, 2H), 2.37 (s, 6H). 19F NMR (471 MHz, CDCl3) δ 58.9 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 166.3, 138.5, 135.6, 128.7, 127.7, 57.4, 50.3 (d, J = 17.2 Hz), 21.3. ESI-MS HRMS calculated for C11H14FO4S [M + H]+ 261.0591, found 264.0585.
2-(Fluorosulfonyl)ethyl benzo[d][1,3]dioxole-5-carboxylate (2s)
Procedure A was followed, with petroleum ether/ethyl acetate = 5:1 (v/v) as an eluent for column chromatography. White solid, mp 100–101 °C, 164 mg, 60% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 7.67 (dd, J = 8.1 Hz, J = 1.3 Hz, 1H), 7.46 (d, J = 1.1 Hz, 1H), 6.86 (d, J = 8.3 Hz, 1H), 6.06 (s, 2H), 4.77 (t, J = 5.5 Hz, 2H), 3.83 (q, J = 5.3 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ 59.0 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 165.3, 152.5, 148.1, 126.1, 122.8, 109.7, 108.3, 102.1, 57.4, 50.4 (d, J = 17.3 Hz). ESI-MS HRMS calculated for C10H10FO6S [M + H]+ 277.0177, found 277.0170.
2-(Fluorosulfonyl)ethyl 2-naphthoate (2t)
Procedure A was followed, with petroleum ether/ethyl acetate = 10:1 (v/v) as an eluent for column chromatography. Yellow solid, mp 73–75 °C, 124 mg, 43% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 8.63 (s, 1H), 8.06–8.04 (m, 1H), 7.97 (d, J = 8.1 Hz, 1H), 7.92–7.89 (m, 2H), 7.63 (t, J = 7.3 Hz, 1H), 7.57 (t, J = 7.4 Hz, 1H), 4.88 (t, J = 5.5 Hz, 2H), 3.90 (q, J = 5.3 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ 59.1 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 166.1, 136.0, 132.6, 131.9, 129.6, 128.9, 128.6, 128.0, 127.1, 126.1, 125.1, 57.6, 50.4 (d, J = 18.2 Hz). ESI-MS HRMS calculated for C13H12FO4S [M + H]+: 283.0435, found 283.0439.
2-(Fluorosulfonyl)ethyl furan-2-carboxylate (2u)
Procedure B was followed, with petroleum ether/ethyl acetate = 5:1 (v/v) as an eluent for column chromatography. Yellow oil, 157 mg, 71% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 7.64 (s, 1H), 7.27 (s, 1H), 6.56–6.55 (m, 1H), 4.79 (t, J = 5.9 Hz, 2H), 3.84 (q, J = 5.5 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ 59.0 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 157.8, 147.5, 143.4, 119.6, 112.3, 57.3, 50.2 (d, J = 18.2 Hz). ESI-MS (m/z) HRMS calculated for C7H8FO5S: 223.0071, found 223.0071.
2-(Fluorosulfonyl)ethyl 2,3-dihydrobenzofuran-2-carboxylate (2v)
Procedure B was followed, with petroleum ether/ethyl acetate = 5:1 (v/v) as an eluent for column chromatography. Yellow oil, 123 mg, 45% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 7.71 (d, J = 7.8 Hz, 1H), 7.61–7.59 (m, 2H), 7.49 (t, J = 7.9 Hz, 1H), 7.33 (t, J = 7.5 Hz, 1H), 4.86 (t, J = 5.8 Hz, 2H), 3.88 (q, J = 5.4 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ 59.1 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 158.7, 156.2, 144.1, 128.4, 126.8, 124.2, 123.3, 115.6, 112.6, 57.7, 50.1 (d, J = 18.1 Hz). ESI-MS (m/z) HRMS calculated for C11H10FO5S: 273.0227, found 273.0276.
2-(Fluorosulfonyl)ethyl 1-methyl-1H-indole-3-carboxylate (2w)
Procedure B was followed, with petroleum ether/ethyl acetate = 3:1 (v/v) as an eluent for column chromatography. Light yellow solid, mp 105–106 °C, 145 mg, 51% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 8.16–8.14 (m, 1H), 7.82 (s, 1H), 7.38–7.31 (m, 3H), 4.81 (t, J = 5.6 Hz, 2H), 3.87–3.85 (m, 5H). 19F NMR (471 MHz, CDCl3) δ 59.0 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 163.8, 137.4, 136.1, 126.6, 123.2, 122.4, 121.5, 110.1, 105.5, 56.3, 50.7 (d, J = 17.3 Hz), 33.6. ESI-MS HRMS calculated for C12H13FNO4S [M + H]+ 286.0544, found 286.0543.
2-(Fluorosulfonyl)ethyl benzo[b]thiophene-2-carboxylate (2x)
Procedure B was followed, with petroleum ether/ethyl acetate = 3:1 (v/v) as an eluent for column chromatography. Brown oil, 113 mg, 39% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 8.12 (s, 1H), 7.91–7.87 (m, 2H), 7.51–7.48 (m, 1H), 7.45–7.42 (m, 1H), 4.84 (t, J = 5.8 Hz, 2H), 3.87 (q, J = 5.3 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ 59.2 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 162.1, 142.7, 138.7, 132.0, 131.8, 127.6, 126.0, 125.3, 122.9, 57.9, 50.2 (d, J = 18.2 Hz). ESI-MS HRMS calculated for C11H10FO4S2 [M + H]+ 288.9999, found 288.9991.
2-(Fluorosulfonyl)ethyl 1,2,3,4-tetrahydronaphthalene-1-carboxylate (2y)
Procedure B was followed, with petroleum ether/ethyl acetate = 5:1 (v/v) as an eluent for column chromatography. Yellow oil, 103 mg, 36% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 7.19–7.13 (m, 4H), 4.58 (t, J = 5.7 Hz, 2H), 3.90 (t, J = 5.8 Hz, 1H), 3.70 (q, J = 5.3 Hz, 2H), 2.88–2.78 (m, 2H), 2.20–2.16 (m, 1H), 2.06–1.96 (m, 2H), 1.83–1.80 (m, 1H). 19F NMR (471 MHz, CDCl3) δ 59.0 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 174.3, 137.4, 132.4, 129.6 (d, J = 20.0 Hz), 127.3, 126.0, 57.3, 50.1 (d, J = 18.2 Hz), 44.5, 29.1, 26.4, 20.5. ESI-MS HRMS calculated for C13H16FO4S [M + H]+ 287.0748, found 287.0770.
2-(Fluorosulfonyl)ethyl 3-phenylpropanoate (2z)
Procedure B was followed, with petroleum ether/ethyl acetate = 5:1 (v/v) as an eluent for column chromatography. Yellow oil, 133 mg, 51% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 7.32–7.29 (m, 2H), 7.24–7.20 (m, 3H), 4.55 (t, J = 5.9 Hz, 2H), 3.65 (q, J = 5.4 Hz, 2H), 2.97 (t, J = 7.7 Hz, 2H), 2.71 (t, J = 7.8 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ 58.7 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 172.3, 140.0, 128.7, 128.4, 126.6, 56.9, 50.1 (d, J = 17.2 Hz), 35.5, 30.8. ESI-MS (m/z) HRMS calculated for C11H14FO4S: 261.0591, found 261.0607.
2-(Fluorosulfonyl)ethyl 2-(4-isobutylphenyl)propanoate (2aa)
Procedure B was followed, with petroleum ether/ethyl acetate = 10:1 (v/v) as an eluent for column chromatography. Light yellow oil, 171 mg, 54% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 7.20–7.19 (m, 2H), 7.12–7.11 (m, 2H), 4.57–4.50 (m, 2H), 3.75 (q, J = 7.2 Hz, 1H), 3.68–3.63 (m, 2H), 2.46 (d, J = 7.2 Hz, 2H), 1.89–1.83 (m, 1H), 1.52 (d, J = 7.2 Hz, 3H), 0.91 (d, J = 6.5 Hz, 6H). 19F NMR (471 MHz, CDCl3) δ 58.8 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 174.2, 141.1, 136.8, 129.6, 127.3, 57.2, 50.0 (d, J = 18.2 Hz), 45.0 (d, J = 22.7 Hz), 30.3, 22.5, 18.3. ESI-MS HRMS calculated for C15H22FO4S [M + H]+ 317.1217, found 317.1223.
2-(Fluorosulfonyl)ethyl 2-acetoxybenzoate (2ab)
Procedure B was followed, with petroleum ether/ethyl acetate = 3:1 (v/v) as an eluent for column chromatography. Light yellow oil, 197 mg, 68% (isolated yield), 1H NMR (500 MHz, CDCl3) δ 8.04 (d, J = 7.8 Hz, 1H), 7.62 (t, J = 7.8 Hz, 1H), 7.34 (t, J = 7.6 Hz, 1H), 7.14 (d, J = 8.1 Hz, 1H), 4.76 (t, J = 5.7 Hz, 2H), 3.82 (q, J = 5.2 Hz, 2H), 2.36 (s, 3H). 19F NMR (471 MHz, CDCl3) δ 58.9 (s, 1F). 13C NMR (126 MHz, CDCl3) δ 169.7, 163.6, 151.2, 134.8, 131.9, 126.3, 124.1, 121.9, 57.5, 50.2 (d, J = 17.2 Hz), 21.1. ESI-MS HRMS calculated for C11H12FO6S [M + H]+ 291.0333, found 291.0268.
Acknowledgments
We are grateful to the National Natural Science Foundation of China (Grant Nos. 51976143, 21772150, and 22071190), the National Key Research and Development Program of China (2018YFA0702001), the Fundamental Research Funds for the Central Universities (Grant No. 2020-YB-013), and the Wuhan University of Technology for the financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c02804.
Reaction conditions screening, deuterated experiment, and characterization data of the products and NMR spectra (PDF)
Author Contributions
§ X.Z. and Y.-M.H. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- a Dong J.; Krasnova L.; Finn M. G.; Sharpless K. B. Sulfur (VI) fluoride exchange (SuFEx): another good reaction for click chemistry. Angew. Chem., Int. Ed. 2014, 53, 9430. 10.1002/anie.201309399. [DOI] [PubMed] [Google Scholar]; b Dong J.; Sharpless K. B.; Kwisnek L.; Oakdale J. S.; Fokin V. V. SuFEx-Based Synthesis of Polysulfates. Angew. Chem., Int. Ed. 2014, 53, 9466. 10.1002/anie.201403758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Sharpless K. B.; Kolb H. C. In Book of Abstracts, 217th ACS National Meeting, Anaheim, CA, March 21–25, 1999; ORGN-105, Accession Number 199:145537.; b Kolb H. C.; Finn M. G.; Sharpless K. B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem., Int. Ed. 2001, 40, 2004.. [DOI] [PubMed] [Google Scholar]
- a Li S.; Wu P.; Moses J. E.; Sharpless K. B. Multidimensional SuFEx click chemistry: sequential sulfur (VI) fluoride exchange connections of diverse modules launched from an SOF4 hub. Angew. Chem., Int. Ed. 2017, 56, 2903. 10.1002/anie.201611048. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Yatvin J.; Brooks K.; Locklin J. SuFEx on the surface: a flexible platform for postpolymerization modification of polymer brushes. Angew. Chem., Int. Ed. 2015, 54, 13370. 10.1002/anie.201506253. [DOI] [PubMed] [Google Scholar]
- a Meng Y.-P.; Wang S.-M.; Fang W.-Y.; Xie Z.-Z.; Leng J.; Alsulami H.; Qin H.-L. Ethenesulfonyl Fluoride (ESF) and Its Derivatives in SuFEx Click Chemistry and More. Synthesis 2020, 52, 673. 10.1055/s-0039-1690038. [DOI] [Google Scholar]; b Chinthakindi P. K.; Arvidsson P. I. Sulfonyl fluorides (SFs): more than click reagents?. Eur. J. Org. Chem. 2018, 2018, 3648. 10.1002/ejoc.201800464. [DOI] [Google Scholar]; c Fattah T. A.; Saeed A.; Albericio F. Recent advances towards sulfur (VI) fluoride exchange (SuFEx) click chemistry. J. Fluorine Chem. 2018, 213, 87. 10.1016/j.jfluchem.2018.07.008. [DOI] [Google Scholar]
- a Liu Z.; Li J.; Li S.; Li G.; Sharpless K. B.; Wu P. SuFEx click chemistry enabled late-stage drug functionalization. J. Am. Chem. Soc. 2018, 140, 2919. 10.1021/jacs.7b12788. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zheng Q.; Woehl J. L.; Kitamura S.; Santos-Martins D. C.; Smedley J.; Li G.; Forli S.; Moses J. E.; Wolan D. W.; Sharpless K. B. SuFEx-enabled, agnostic discovery of covalent inhibitors of human neutrophil elastase. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 18808. 10.1073/pnas.1909972116. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Barrow A. S.; Smedley C. J.; Zheng Q.; Li S.; Dong J.; Moses J. E. The growing applications of SuFEx click chemistry. Chem. Soc. Rev. 2019, 48, 4731. 10.1039/C8CS00960K. [DOI] [PubMed] [Google Scholar]
- a Hett E. C.; Xu H.; Geoghegan K. F.; Gopalsamy A. Jr.; Kyne R. E.; Menard C. A.; Narayanan A.; Parikh M. D.; Liu S.; Roberts L.; Robinson R. P.; Tones M. A.; Jones L. H. Rational targeting of active-site tyrosine residues using sulfonyl fluoride probes. ACS Chem. Biol. 2015, 10, 1094. 10.1021/cb5009475. [DOI] [PubMed] [Google Scholar]; b Chen W.; Dong J.; Plate L.; Mortenson D. E.; Brighty G. J.; Li S.; Liu Y.; Galmozzi A.; Lee P. S.; Hulce J. J.; Cravatt B. F.; Saez E.; Powers E. T.; Wilson I. A.; Sharpless K. B.; Kelly J. W. Arylfluorosulfates inactivate intracellular lipid binding protein (s) through chemoselective SuFEx reaction with a binding site Tyr residue. J. Am. Chem. Soc. 2016, 138, 7353. 10.1021/jacs.6b02960. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Shishido Y.; Tomoike F.; Kimura Y.; Kuwata K.; Yano T.; Fukui K.; Fujikawa H.; Sekido Y.; Murakami-Tonami Y.; Kameda T.; Shuto S.; Abe H. A covalent G-site inhibitor for glutathione S-transferase Pi (GSTP 1-1). Chem. Commun. 2017, 53, 11138. 10.1039/C7CC05829B. [DOI] [PubMed] [Google Scholar]; d Alvarez N. H.; van de Langemheen H.; Brouwer A. J.; Liskamp R. M. J. Potential peptidic proteasome inhibitors by incorporation of an electrophilic trap based on amino acid derived α-substituted sulfonyl fluorides. Bioorg. Med. Chem. 2017, 25, 5055. 10.1016/j.bmc.2017.07.019. [DOI] [PubMed] [Google Scholar]; e Gehringer M.; Laufer S. A. Emerging and re-emerging warheads for targeted covalent inhibitors: applications in medicinal chemistry and chemical biology. J. Med. Chem. 2019, 62, 5673. 10.1021/acs.jmedchem.8b01153. [DOI] [PubMed] [Google Scholar]
- a Gold A. M. Sulfonylation with sulfonyl halides. Methods Enzymol. 1967, 11, 706. [Google Scholar]; b Narayanan A.; Jones L. H. Sulfonyl fluorides as privileged warheads in chemical biology. Chem. Sci. 2015, 6, 2650. 10.1039/C5SC00408J. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Powers J. C.; Asgian J. L.; Ekici O. D.; James K. E. Irreversible inhibitors of serine, cysteine, and threonine proteases. Chem. Rev. 2002, 102, 4639. 10.1021/cr010182v. [DOI] [PubMed] [Google Scholar]; d Gold A. M.; Fahrney D. E. Sulfonyl fluorides as inhibitors of esterases. II. Formation and reactions of phenylmethanesulfonyl α-chymotrypsin. Biochemistry 1964, 3, 783. 10.1021/bi00894a009. [DOI] [PubMed] [Google Scholar]; e Fahrney D. E.; Gold A. M. Sulfonyl fluorides as inhibitors of esterases. I. Rates of reaction with acetylcholinesterase, α-chymotrypsin, and trypsin. J. Am. Chem. Soc. 1963, 85, 997. 10.1021/ja00890a037. [DOI] [Google Scholar]
- a Alapafuja S. O.; Nikas S. P.; Bharathan I. T.; Shukla V. G.; Nasr M. L.; Bowman A. L.; Zvonok N.; Li J.; Shi X.; Engen J. R.; Makriyannis A. Sulfonyl fluoride inhibitors of fatty acid amide hydrolase. J. Med. Chem. 2012, 55, 10074. 10.1021/jm301205j. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Karanian D. A.; Brown Q. B.; Makriyannis A.; Kosten T. A.; Bahr B. A. Dual modulation of endocannabinoid transport and fatty acid amide hydrolase protects against excitotoxicity. J. Neurosci. 2005, 25, 7813. 10.1523/JNEUROSCI.2347-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Aguilar B.; Amissah F.; Duverna R.; Lamango N. S. Polyisoprenylation potentiates the inhibition of polyisoprenylated methylated protein methyl esterase and the cell degenerative effects of sulfonyl fluorides. Curr. Cancer Drug Targets 2011, 11, 752. 10.2174/156800911796191015. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Huang Y.-M.; Wang S.-M.; Leng J.; Moku B.; Zhao C.; Alharbi N. S.; Qin H.-L. Converting (E)-(Hetero) arylethanesulfonyl Fluorides to (Z)-(Hetero) arylethanesulfonyl Fluorides Under Light Irradiation. Eur. J. Org. Chem. 2019, 2019, 4597. 10.1002/ejoc.201900799. [DOI] [Google Scholar]
- a Oakdale J. S.; Kwisnek L.; Fokin V. V. Selective and Orthogonal Post-Polymerization Modification using Sulfur (VI) Fluoride Exchange (SuFEx) and Copper-Catalyzed Azide-Alkyne Cycloaddition (CuAAC) Reactions. Macromolecules 2016, 49, 4473. 10.1021/acs.macromol.6b00101. [DOI] [Google Scholar]; b Yatvin J.; Brooks K.; Locklin J. SuFEx click: new materials from SOxF and Silyl ethers. Chem. - Eur. J. 2016, 22, 16348. 10.1002/chem.201602926. [DOI] [PubMed] [Google Scholar]; c Gao B.; Zhang L.; Zheng Q.; Zhou F.; Klivansky L. M.; Lu J.; Liu Y.; Dong J.; Wu P.; Sharpless K. B. Bifluoride-catalysed sulfur (VI) fluoride exchange reaction for the synthesis of polysulfates and polysulfonates. Nat. Chem. 2017, 9, 1083. 10.1038/nchem.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Wang H.; Zhou F.; Ren G.; Zheng Q.; Chen H.; Gao B.; Klivansky L.; Liu Y.; Wu B.; Xu Q.; Lu J.; Sharpless K. B.; Wu P. SuFEx-Based Polysulfonate Formation from Ethenesulfonyl Fluoride-Amine Adducts. Angew. Chem., Int. Ed. 2017, 56, 11203. 10.1002/anie.201701160. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Yang C.; Flynn J. P.; Niu J. Facile Synthesis of Sequence-Regulated Synthetic Polymers Using Orthogonal SuFEx and CuAAC Click Reactions. Angew. Chem., Int. Ed. 2018, 57, 16194. 10.1002/anie.201811051. [DOI] [PubMed] [Google Scholar]; f Xu L.; Wu P.; Dong J.. New Polymers from SuFEx Click Chemistry: Syntheses and Perspectives. In Synthetic Polymer Chemistry: Innovations and Outlook; 2019, 1–31.. [Google Scholar]
- a Brett D. J. L.; Kucernak A. R.; Aguiar P.; Atkins S.; Brandon N. P.; Clague R.; Cohen L. F.; Hinds G.; Kalyvas C.; Offer G. J.; Ladewig B.; Maher R.; Marquis A.; Shearing P.; Vasileiadis N.; Vesovic V. What happens inside a fuel cell? Developing an experimental functional map of fuel cell performance. Chem. Phys. Chem. 2010, 11, 2714. 10.1002/cphc.201000487. [DOI] [PubMed] [Google Scholar]; b Chandan A.; Hattenberger M.; El-kharouf A.; Du S.; Dhir A.; Self V.; Pollet B. G.; Ingram A.; Bujalski W. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC) - A review. J. Power Sources 2013, 231, 264. 10.1016/j.jpowsour.2012.11.126. [DOI] [Google Scholar]; c Comprehensive Membrane Science and Engineering; Drioli E.; Giorno L., Eds.; Elsevier Science: Amsterdam, 2010; p 1570. [Google Scholar]; d Souzy R.; Ameduri B. Functional fluoropolymers for fuel cell membranes. Prog. Polym. Sci. 2005, 30, 644. 10.1016/j.progpolymsci.2005.03.004. [DOI] [Google Scholar]; e Mauritz K. A.; Moore R. B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535. 10.1021/cr0207123. [DOI] [PubMed] [Google Scholar]; f Souzy R.; Ameduri B. Functional fluoropolymers for fuel cell membranes. Prog. Polym. Sci. 2005, 30, 644. 10.1016/j.progpolymsci.2005.03.004. [DOI] [Google Scholar]; g Curtin D. E.; Lousenberg R. D.; Henry T. J.; Tangeman P. C.; Tisack M. E. Advance materials for improved PEMFC performance and life. J. Power Sources 2004, 131, 41. 10.1016/j.jpowsour.2004.01.023. [DOI] [Google Scholar]; h Cui Z.; Drioli E.; Lee Y. M. Recent progress in fluoropolymers for membranes. Prog. Polym. Sci. 2014, 39, 164. 10.1016/j.progpolymsci.2013.07.008. [DOI] [Google Scholar]; i Arcella V.; Ghielmi A.; Tommasi G. High performance perfluoropolymer films for membranes. Ann. N. Y. Acad. Sci. 2003, 984, 226. 10.1111/j.1749-6632.2003.tb06002.x. [DOI] [PubMed] [Google Scholar]; j Gibbs H. H.; Vienna V. W.; Griffin R. N. U.S. Patent US3,041,317, 1962. (DuPont de Nemours).; k Connolly D. J.; Gresham W. F.. Sulfo derivatives of perfluorovinyl ether monomers. U.S. Patent US3,282,875, 1966. (E.I. DuPont de Nemours and Company).; l Carl W. P.; Ezzel B. R.. Low equivalent weight sulfonic fluoropolymers. U.S. Patent US4,940,525, 1990. (Dow Chemical Co.).
- Chen Q.; Mayer P.; Mayr H. Ethenesulfonyl fluoride: the most perfect Michael acceptor ever found?. Angew. Chem., Int. Ed. 2016, 55, 12664. 10.1002/anie.201601875. [DOI] [PubMed] [Google Scholar]
- Krutak J. J.; Burpitt R. D.; Moore W. H.; Hyatt J. A. Chemistry of ethenesulfonyl fluoride. Fluorosulfonylethylation of organic compounds. J. Org. Chem. 1979, 44, 3847. 10.1021/jo01336a022. [DOI] [Google Scholar]
- a Jimonet P.; Audiau F.; Barreau M.; Blanchard J.-C.; Boireau A.; Bour Y.; Coleno M.-A.; Doble A.; Doerflinger G.; Do Huu C.; Donat M.-H.; Duchesne J. M.; Ganil P.; Gueremy C.; Honoré E.; Just B.; Kerphirique R.; Gontier S.; Hubert P.; Laduron P. M.; Le Blevec J.; Meunier M.; Miquet J.-M.; Nemecek C.; Pasquet M.; Piot O.; Pratt J.; Rataud J.; Reibaud M.; Stutzmann J.-M.; Mignani S. Riluzole series. Synthesis and in vivo “antiglutamate” activity of 6-substituted-2-benzothiazolamines and 3-substituted-2-imino-benzothiazolines. J. Med. Chem. 1999, 42, 2828. 10.1021/jm980202u. [DOI] [PubMed] [Google Scholar]; b Kreimeyer A.; Laube B.; Sturgess M.; Goeldner M.; Foucaud B. Evaluation and biological properties of reactive ligands for the mapping of the glycine site on the N-methyl-D-aspartate (NMDA) receptor. J. Med. Chem. 1999, 42, 4394. 10.1021/jm9910730. [DOI] [PubMed] [Google Scholar]; c Aguilar B.; Amissah F.; Duverna R.; Lamango N. S. Polyisoprenylation potentiates the inhibition of polyisoprenylated methylated protein methyl esterase and the cell degenerative effects of sulfonyl fluorides. Curr. Cancer Drug Targets 2011, 11, 752. 10.2174/156800911796191015. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Krutak J. J.; Burpitt R. D. U.S. Patent US3,952,029, 1976.; e Fujigaya T.; Sibasaki Y.; Ando S.; Kishimura S.; Endo M.; Sasago M.; Ueda M. New photoresist materials for 157-nm lithography. poly [vinylsulfonyl fluoride-co-4-(1, 1, 1, 3, 3, 3-hexafluoro-2-hydroxypropyl)-styrene] partially protected with tert-butoxycarbonyl. Chem. Mater. 2003, 15, 1512. 10.1021/cm020198h. [DOI] [Google Scholar]; f Vries L. U.S. Patent US4,269,790, 1981.; g Hedrick R. M. U.S. Patent US2,653,973, 1953.
- Trofymchuk S.; Bugera M. Y.; Klipkov A. A.; Razhyk B.; Semenov S.; Tarasenko K.; Starova V. S.; Zaporozhets O. A.; Tananaiko O. Y.; Alekseenko A. N.; Pustovit Y.; Kiriakov O.; Gerus I. I.; Tolmachev A. A.; Mykhailiuk P. K. Deoxofluorination of (Hetero) aromatic Acids. J. Org. Chem. 2020, 85, 3110. 10.1021/acs.joc.9b03011. [DOI] [PubMed] [Google Scholar]
- a Wang S.-M.; Zhao C.; Zhang X.; Qin H.-L. Clickable coupling of carboxylic acids and amines at room temperature mediated by SO2F2: a significant breakthrough for the construction of amides and peptide linkages. Org. Biomol. Chem. 2019, 17, 4087. 10.1039/C9OB00699K. [DOI] [PubMed] [Google Scholar]; b Steglich W.; Neises B. Simple method for the esterification of carboxylic acids. Angew. Chem., Int. Ed. 1978, 17, 522. 10.1002/anie.197805221. [DOI] [Google Scholar]; c Korstanje T. J.; van der Vlugt J. I.; Elsevier C. J.; de Bruin B. Hydrogenation of carboxylic acids with a homogeneous cobalt catalyst. Science 2015, 350, 298. 10.1126/science.aaa8938. [DOI] [PubMed] [Google Scholar]; d Russo R.; de Caro C.; Avagliano C.; Cristiano C.; la Rana G.; Raso G. M.; Canani R. B.; Meli R.; Calignano A. Sodium butyrate and its synthetic amide derivative modulate nociceptive behaviors in mice. Pharmacol. Res. 2016, 103, 279. 10.1016/j.phrs.2015.11.026. [DOI] [PubMed] [Google Scholar]; e Kerenkan A. E.; Béland F.; Do T.-O. Chemically catalyzed oxidative cleavage of unsaturated fatty acids and their derivatives into valuable products for industrial applications: a review and perspective. Catal. Sci. Technol. 2016, 6, 971. 10.1039/C5CY01118C. [DOI] [Google Scholar]; f Liu W.; Liu T.; Liu T.; Liu T.; Xin J.; Hiscox W. C.; Liu H.; Liu L.; Zhang J. Improving grafting efficiency of dicarboxylic anhydride monomer on polylactic acid by manipulating monomer structure and using comonomer and reducing agent. Ind. Eng. Chem. Res. 2017, 56, 3920. 10.1021/acs.iecr.6b05051. [DOI] [Google Scholar]; g Sharmin E.; Zafar F.; Akram D.; Alam M.; Ahmad S. Recent advances in vegetable oils based environment friendly coatings: A review. Ind. Crops Prod. 2015, 76, 215. 10.1016/j.indcrop.2015.06.022. [DOI] [Google Scholar]; h Doucet H.; Martin-Vaca B.; Bruneau C.; Dixneuf P. H. General synthesis of (Z)-alk-1-en-1-yl esters via ruthenium-catalyzed anti-Markovnikov trans-addition of carboxylic acids to terminal alkynes. J. Org. Chem. 1995, 60, 7247. 10.1021/jo00127a033. [DOI] [Google Scholar]; i Jørgensen M.; Lee S.; Liu X.; Wolkowski J. P.; Hartwig J. F. Efficient synthesis of α-aryl esters by room-temperature palladium-catalyzed coupling of aryl halides with ester enolates. J. Am. Chem. Soc. 2002, 124, 12557. 10.1021/ja027643u. [DOI] [PubMed] [Google Scholar]; j Wang S.-M.; Alharbi N. S.; Qin H.-L. Construction of Esters through Sulfuryl Fluoride (SO2F2) Mediated Dehydrative Coupling of Carboxylic Acids with Alcohols at Room Temperature. Synthesis 2019, 51, 3901. 10.1055/s-0039-1690017. [DOI] [Google Scholar]
- a Sang R.; Kucmierczyk P.; Dghren R.; Razzaq R.; Dong K.; Liu J.; Franke R.; Jackstell R.; Beller M. Synthesis of Carboxylic Acids by Palladium-Catalyzed Hydroxycarbonylation. Angew. Chem., Int. Ed. 2019, 58, 14365. 10.1002/anie.201908451. [DOI] [PubMed] [Google Scholar]; b Zhang X.; Fang W.; Ravindar L.; Tang W.; Qin H.-L. An Easy, General and Practical Method for the Construction of Alkyl Sulfonyl Fluorides. Adv. Synth. Catal. 2020, 362, 3358. 10.1002/adsc.202000515. [DOI] [Google Scholar]; c Ostermann T.Building Blocks for the Feed Industry: Oxea Expands Production Capabilities for Butyric Acid and Propionic Acid; OXEA GmbH: Monheim am Rhein, 2017. [Google Scholar]; d Burdock G. A.Fenaroli’s Handbook of Flavor Ingredients; CRC Press: Boca Raton, 2009. [Google Scholar]; e Hoseinifar S. H.; Sun Y.-Z.; Caipang C. M. Short-chain fatty acids as feed supplements for sustainable aquaculture: An updated view. Aquacult. Res. 2017, 48, 1380. 10.1111/are.13239. [DOI] [Google Scholar]; f Ruiz-Lopez N.; Haslam R. P.; Napier J. A.; Sayanova O. Successful high-level accumulation of fish oil omega-3 long-chain polyunsaturated fatty acids in a transgenic oilseed crop. Plant J. 2014, 77, 198. 10.1111/tpj.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Polycarpo G. V.; Andretta I.; Kipper M.; Cruz-Polycarpo V. C.; Dadalt J. C.; Rodrigues P. H. M.; Albuquerque R. Meta-analytic study of organic acids as an alternative performance-enhancing feed additive to antibiotics for broiler chickens. Poult. Sci. 2017, 96, 3645. 10.3382/ps/pex178. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Bagal V. L.; Khatta V. K.; Tewatia B. S.; Sangwan S. K.; Raut S. S. Relative efficacy of organic acids and antibiotics as growth promoters in broiler chicken. Vet. World 2016, 9, 377. 10.14202/vetworld.2016.377-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Zeng L.; Lai Z.; Cui S. One-Pot Reaction of Carboxylic Acids and Ynol Ethers for The Synthesis of β-Keto Esters. J. Org. Chem. 2018, 83, 14834. 10.1021/acs.joc.8b02715. [DOI] [PubMed] [Google Scholar]; b Rostovtsev V. V.; Green L. G.; Fokin V. V.; Sharpless K. B. A stepwise huisgen cycloaddition process: copper (I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem., Int. Ed. 2002, 41, 2596.. [DOI] [PubMed] [Google Scholar]; c Monaco M. R.; Poladura B.; Diaz de Los Bernardos M.; Leutzsch M.; Goddard R.; List B. Activation of carboxylic acids in asymmetric organocatalysis. Angew. Chem., Int. Ed. 2014, 53, 7063. 10.1002/anie.201400169. [DOI] [PubMed] [Google Scholar]; d Mitsunobu O.; Yamada M. Preparation of esters of carboxylic and phosphoric acid via quaternary phosphonium salts. Bull. Chem. Soc. Jpn. 1967, 40, 2380. 10.1246/bcsj.40.2380. [DOI] [Google Scholar]
- a Luan Z.-H.; Qu J.-P.; Kang Y.-B. Discovery of Oxygen α-Nucleophilic Addition to α, β-Unsaturated Amides Catalyzed by Redox-Neutral Organic Photoreductant. J. Am. Chem. Soc. 2020, 142, 20942. 10.1021/jacs.0c10707. [DOI] [PubMed] [Google Scholar]; b Nising C. F.; Brase S. The oxa-Michael reaction: from recent developments to applications in natural product synthesis. Chem. Soc. Rev. 2008, 37, 1218. 10.1039/b718357g. [DOI] [PubMed] [Google Scholar]; c Resch V.; Hanefeld U. The selective addition of water. Catal. Sci. Technol. 2015, 5, 1385. 10.1039/C4CY00692E. [DOI] [Google Scholar]
- Zhang S.-L.; Xiao C.; Wan H.-X.; Zhang X. General and selective syn-carboxylation-trifluoromethylation of terminal alkynes: application to the late-stage modification of dehydrocholic acid. Chem. Commun. 2019, 55, 4099. 10.1039/C9CC01173K. [DOI] [PubMed] [Google Scholar]
- Deininger M. W. N.; Druker B. J. Specific targeted therapy of chronic myelogenous leukemia with imatinib. Pharmacol. Rev. 2003, 55, 401. 10.1124/pr.55.3.4. [DOI] [PubMed] [Google Scholar]
- a Han W.-J.; Pu F.; Li C.-J.; Liu Z.-W.; Fan J.; Shi X.-Y. Carboxyl-Directed Conjugate Addition of C-H Bonds to α,β-Unsaturated Ketones in Air and Water. Adv. Synth. Catal. 2018, 360, 1358. 10.1002/adsc.201701468. [DOI] [Google Scholar]; b Ackermann L.; Pospech J. Ruthenium-Catalyzed Oxidative C-H Bond Alkenylations in Water: Expedient Synthesis of Annulated Lactones,. Org. Lett. 2011, 13, 4153. 10.1021/ol201563r. [DOI] [PubMed] [Google Scholar]; c Yi C. S.; Yun S. Y.; He Z. Conjugate Addition of Alcohols to Acrylic Compounds Catalyzed by a Bifunctional Ruthenium-Acetamido Complex. Organometallics 2003, 22, 3031. 10.1021/om030418g. [DOI] [Google Scholar]
- a Davies N. M. Clinical pharmacokinetics of ibuprofen. Clin. Pharmacokinet. 1998, 34, 101. 10.2165/00003088-199834020-00002. [DOI] [PubMed] [Google Scholar]; b Rainsford K. D. Ibuprofen: pharmacology, efficacy and safety. Inflammopharmacology 2009, 17, 275. 10.1007/s10787-009-0016-x. [DOI] [PubMed] [Google Scholar]; c Awtry E. H.; Loscalzo J. Aspirin. Circulation 2000, 101, 1206. 10.1161/01.CIR.101.10.1206. [DOI] [PubMed] [Google Scholar]
- a Wang F.; Yang H.; Fu H.; Pei Z. Efficient copper-catalyzed Michael addition of acrylic derivatives with primary alcohols in the presence of base. Chem. Commun. 2013, 49, 517. 10.1039/C2CC37595H. [DOI] [PubMed] [Google Scholar]; b Sequeira F. C.; Chemler S. R. Stereoselective synthesis of morpholines via copper-promoted oxyamination of alkenes. Org. Lett. 2012, 14, 4482. 10.1021/ol301984b. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Munro-Leighton C.; Delp S. A.; Blue E. D.; Gunnoe T. B. Addition of N-H and O-H Bonds of Amines and Alcohols to Electron-Deficient Olefins Catalyzed by Monomeric Copper (I) Systems: Reaction Scope, Mechanistic Details, and Comparison of Catalyst Efficiency. Organometallics 2007, 26, 1483. 10.1021/om061133h. [DOI] [Google Scholar]; d Rostamnia S.; Alamgholiloo H. Synthesis and Catalytic Application of Mixed Valence Iron (Fell/Fell)-Based OMS-MIL-100(Fe) as an Efficient Green Catalyst for the aza-Michael Reaction. Catal. Lett. 2018, 148, 2918. 10.1007/s10562-018-2490-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.