Abstract
Organisms have an evolutionarily conserved internal rhythm that helps them anticipate and adapt to daily changes in the environment. Synchronized to the light-dark cycle with a period of around 24 hours, the timing of the circadian clock is set by light-triggering signals sent from the retina to the suprachiasmatic nucleus. Other inputs, including food intake, exercise, and temperature, also affect clocks in peripheral tissues, including skin. Here, we review the intricate interplay between the core clock network and fundamental physiological processes in skin such as homeostasis, regeneration, immune and stress responses. We illustrate the effect of feeding time on the skin circadian clock and skin function, a previously overlooked area of research. We then discuss works that relate the circadian clock and its disruption to skin diseases, including skin cancer, sunburn, hair loss, aging, infections, inflammatory skin diseases, and wound healing. Finally, we highlight the promise of circadian medicine for skin disease prevention and management.
Keywords: Circadian clock, skin diseases, stem cells, feeding, cancer, wound healing, aging, psoriasis, stress and immune response, circadian medicine
Graphical Abstract
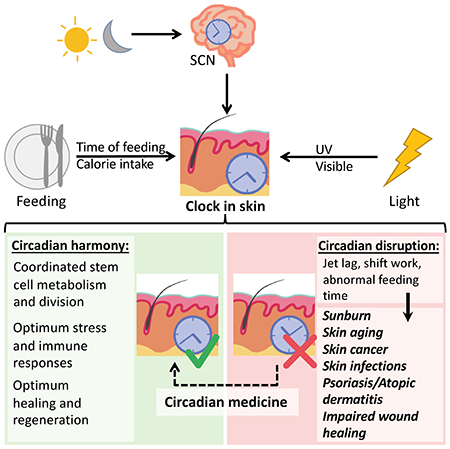
Introduction
As the largest organ of the body, skin provides primary defense against the environment, including toxins, microorganisms, and radiation [1,2]. It is also a critical part of organismal homeostasis through fat storage and the production of hormones, including vitamin D. Due to its interactions with external stimuli, the skin must be equipped to adapt to its environment.
The circadian clock, an intrinsic autonomous clock that controls the body’s divergent functions during day and night, affects the expression of multiple genes that mediate skin stem cell metabolism and proliferation, DNA repair, stimulus response, and immunity. Circadian clock research has elucidated fundamental regulatory mechanisms that contribute to skin cancer, aging, inflammatory and other skin diseases. Emerging clinical research has begun to employ these findings to refine treatment based on the principles of circadian medicine. While circadian dermatology is a nascent field, studies conducted on the circadian clock continue to unravel the mysteries of the skin and its complex system of regulation.
Understanding the role of the circadian clock in the skin may provide new insights into the pathogenesis of skin diseases and their treatment. To this end, we review the role of the circadian clock in skin, emphasizing the most recent findings in the field, and discuss the role of circadian clock disruption in skin diseases.
The circadian clock
From bacteria to plants and animals, all living organisms have intrinsic rhythms that are synchronized with the light-dark cycle with a period of around 24 hours. Controlled by internal biochemical oscillators, the circadian clock is autonomous and maintained at both organismal and cellular levels in the absence of outside stimuli [3,4]. In this section, we provide a brief overview of the mammalian circadian clock at the organismal and molecular level.
The SCN synchronizes peripheral clocks
In mammals, the circadian clock machinery is present in almost all cells. The suprachiasmatic nucleus (SCN), a small region in the hypothalamus of the brain, acts as the central pacemaker that synchronizes the circadian clocks in peripheral tissues through neuronal and hormonal signals [4,5].
The primary entrainment signal for the SCN is light. Upon light reception, intrinsically photosensitive retinal ganglion cells (ipRGCs), pivotal components of the retinohypothalamic tract, send signals to set the circadian time in the SCN. In the SCN, a subpopulation of cells produces neurotransmitters such as vasoactive intestinal peptide (VIP) and arginine vasopressin (AVP) to synchronize and stabilize the cellular circadian clocks within the SCN [4–6]. While the neurons in the SCN are traditionally believed to be the main contributor to circadian rhythm establishment, SCN astrocytes also have the ability to control the circadian rhythm in the SCN [4,5]. As the robustly autonomous SCN sends signals to peripheral tissues, peripheral clocks are synchronized with respect to the SCN and thus the ambient light-dark cycle (Fig. 1).
Figure 1: The SCN synchronizes peripheral clocks.
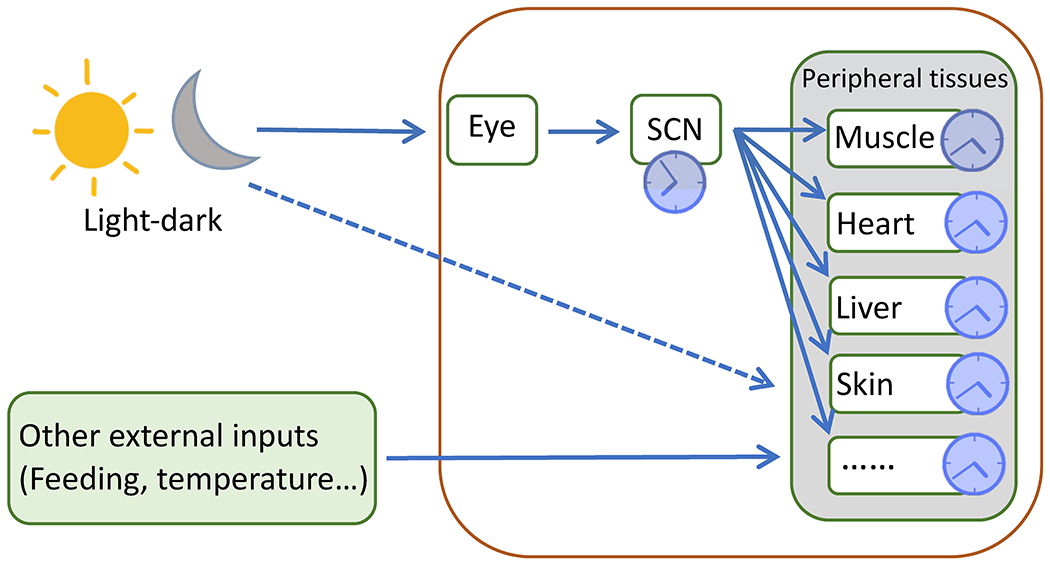
Light sends signals through the retina to set the clock in the SCN, which synchronizes peripheral clocks via neuronal and hormonal signals. As indicated, the phase of peripheral clocks is delayed by a few hours compared to the SCN clock. The SCN, however, is not required for the maintenance of peripheral clocks. It is also possible that light-dark cycles directly regulate clocks in the skin. Additionally, other external inputs such as feeding and temperature entrain peripheral clocks, including in the skin.
The SCN clock can synchronize peripheral clocks, but it is not necessary for circadian rhythm maintenance in peripheral organs [7,8]. Although ablation of the SCN in mice disturbs the circadian rhythms in peripheral tissues, the obliteration of BMAL1 in all tissues except the liver does not completely obliterate circadian rhythms in the liver, irrespective of whether light signals are available [7,8]. In addition, other signals such as food intake and temperature can set the clock in peripheral tissues independent of the SCN, causing phase shifts in the peripheral clocks relative to the central clock (Fig. 1) [9].
The molecular clock is a transcription-translation feedback loop
In each cell, the circadian clock is a biochemical oscillator formed with interlocked transcription-translation feedback loops [10]. The primary feedback loop, which is commonly referred to as the core clock gene network, consists of a positive and a negative arm. In the positive arm, transcription factors Brain and Muscle ARNT-Like 1 (BMAL1) and Circadian Locomotor Output Cycles Kaput (CLOCK) form heterodimers, which bind to the enhancer box (E-box) to activate transcription of Period 1, 2, and 3 (Per) and Cryptochrome 1 and 2 (Cry). PER and CRY drive the negative arm, inhibiting the expression of BMAL1-CLOCK and thus impeding their own transcription. With the levels of PER and CRY decreasing, the BMAL1-CLOCK dimer regains activation until PER and CRY accumulate and deactivate BMAL1-CLOCK again, completing the loop with a period of around 24 hours (Fig. 2) [10–12].
Figure 2: A transcription-translation feedback loop generates circadian rhythms.
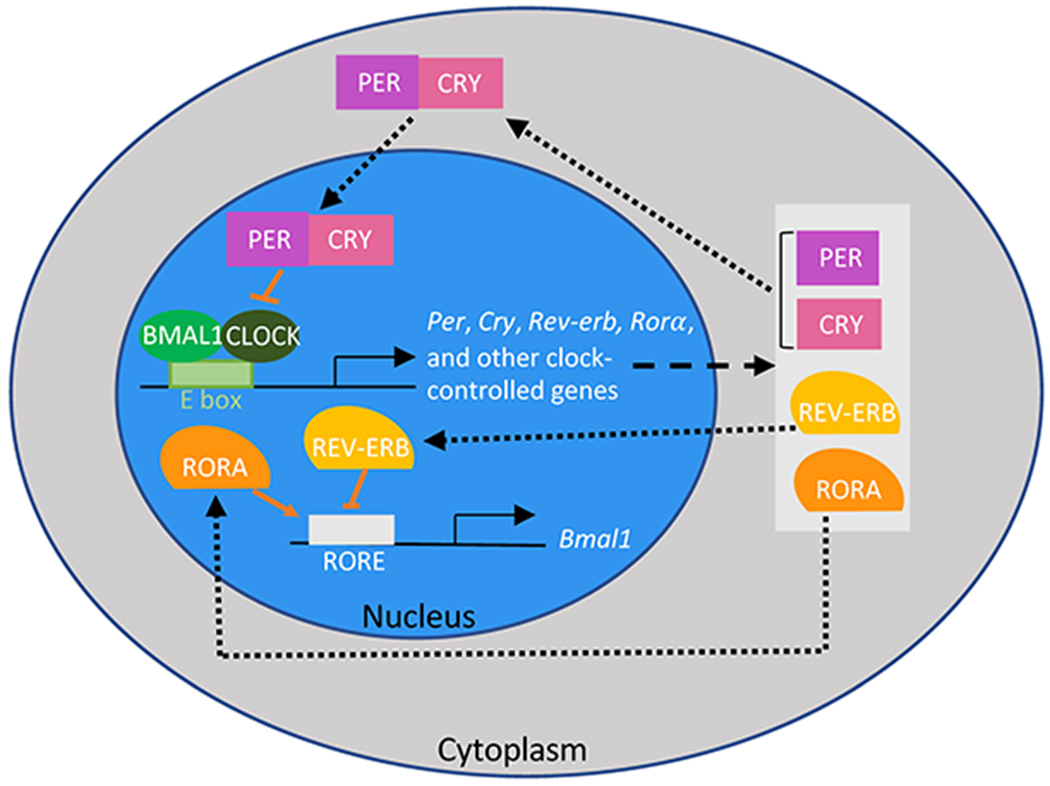
The core clock has a positive arm, consisting of BMAL1 and CLOCK, and a negative arm, consisting of PER and CRY, which inhibits the BMAL1-CLOCK heterodimer. RORs, REV-ERBS forms a secondary feedback loop to reinforce the oscillatory expression of Bmal1. The clock network directly and indirectly regulates the expression of about 10–20% of genes in each peripheral tissue.
In addition to the core clock gene network, a secondary feedback loop reinforces the 24-hour oscillation for Bmal1 expression. BMAL1-CLOCK activates transcriptions of not only Per and Cry but also other clock-controlled genes, including RAR-related Orphan Receptors (RORs) and REV-ERBs. While RORs bind to ROR/REV-ERB-response elements (RORE) to activate transcription of Bmal1, REV-ERBs bind to the same sites to inhibit Bmal1 transcription. With the level of BMAL1 decreasing, transcription of REV-ERBs stalls until transcription of Bmal1 is reestablished (Fig. 2) [10–12].
As simple as the network is, the core clock gene network directly or indirectly modulates the expression of multiple genes and biological processes in all organs, making those processes rhythmic as well. In mice, for example, around 16%, 13%, 12%, and 7% of protein coding genes are rhythmic in the liver, kidney, lung and skin, respectively [13,14]. While the same clock gene network is omnipresent in cells and the SCN acts as a synchronizer for the entire body, the set of genes that are circadian varies greatly between organs. This is because the circadian machinery regulates different biological processes in different organs to modulate their functions. In addition, there is variability in the amplitudes and the peak times of clock gene expressions between organs, as external signals such as temperature and food intake can selectively entrain peripheral clocks (Fig. 1) [9].
The circadian clock in the skin
Acting as a protective barrier against toxins and other external stressors, skin is a multi-layer organ harboring various cell types organized into several compartments to fulfill a range of tasks such as water loss prevention, sensation, and hormone synthesis [1,2]. In humans and mice, the skin comprises three main layers: the epidermis, the dermis, and the hypodermis; each layer has active circadian clocks. With the outside world changing throughout the 24-hour day, a mechanism has evolved to allow the skin to anticipate the environmental shifts and adjust accordingly. Indeed, diurnal rhythms are observed in multiple cell types across all layers of the skin [15].
As in other organs, the SCN synchronizes circadian clocks throughout the skin. This is supported by SCN ablation studies that abolished skin circadian rhythms [16]. More recent studies have brought to light that epidermal circadian clocks and time-setting mechanisms are independent of the SCN. Selective expression of Bmal1 only in the epidermis does not disturb the skin diurnal rhythm as long as the mice experience regular light-dark cycles [17], indicating that the epidermal clock does not require the SCN clock or clocks in other tissues. Possible contributors to the maintenance of such diurnality are light sensitive opsin proteins present in various skin cell types (such as melanocytes, keratinocytes, fibroblasts and hair follicle cells) that may be able to entrain the skin circadian clock [18,19].
The skin is a multi-layer organ with diurnal rhythms in every layer
The outermost layer of the skin is the epidermis (Fig. 3A). Hosting Merkel cells, melanocytes, T-cells, and Langerhans cells, the epidermis itself is organized into four layers (five on the palm and sole) consisting of keratinocytes at different differentiation stages: stratum basale, stratum spinosum, stratum granulosum, stratum lucidum (palm and sole specific) and stratum corneum (Fig. 3B) [1]. The journey of the keratinocytes in the epidermis starts in the stratum basale, where the intrinsic circadian clock is required for the diurnal rhythms in proliferation [14] as well as the coordination between cell division and intermediary metabolism [20] in epidermal stem cells. The keratinocyte progeny of stem cells is continuously pushed upwards until arriving in the stratum corneum at the top, where the dead keratin-filled cells slough off [1].
Figure 3: The skin is a multi-layer, compartmentalized organ.
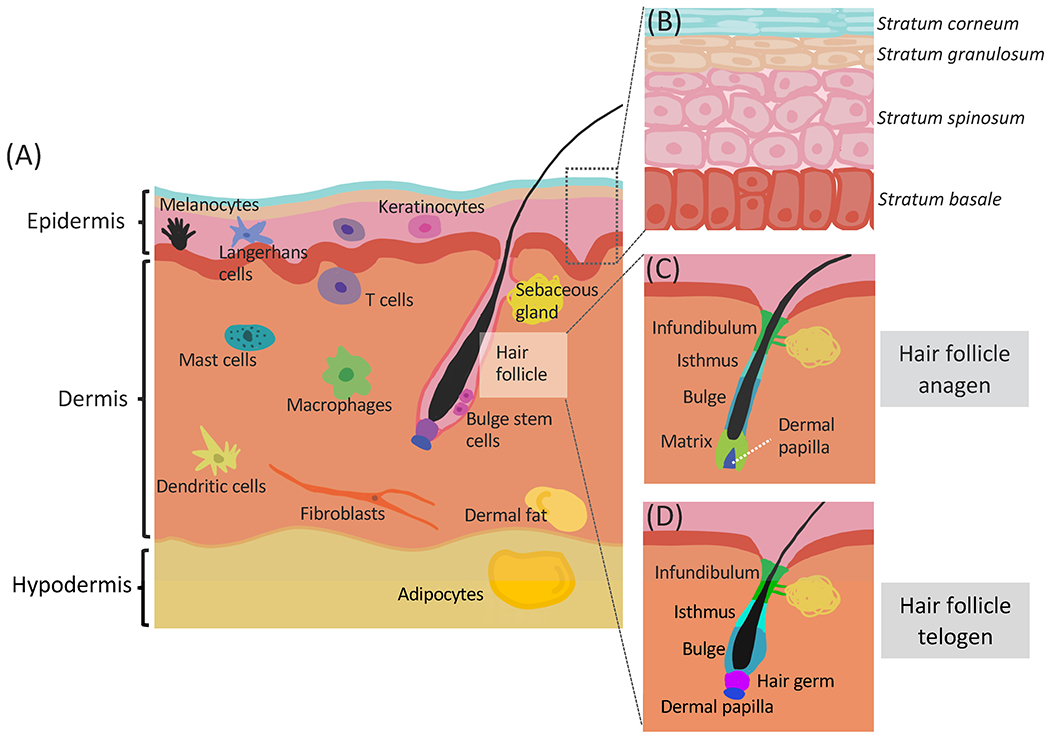
(A) There are three main layers in the skin: epidermis, dermis and hypodermis. Each layer contains multiple cell types. (B) The interfollicular epidermis contains mostly keratinocytes organized into four layers based on differentiation status. The epidermal stem cells reside in the stratum basale. (C) The anagen hair follicle. The matrix contains proliferating keratinocytes derived from the secondary hair germ, giving rise to the hair shaft. (D) The telogen hair follicle. The hair follicle bulge contains the slow cycling stem cells. The hair germ contains the progenitor cells for the hair. The dermal papilla is a mesenchymal structure that signals to the hair germ and stem cells.
Just below the epidermis, separated from it by a basement membrane, is the dermis (Fig. 3A). The dermis primarily consists of extracellular matrix with collagen fibers synthesized by fibroblasts. It is much thicker than the epidermis and contributes strength and elasticity to the skin [1]. The diverse cellular populations in the dermis include fibroblasts, multiple immune cells such as T cells, mast cells, macrophages, and dendritic cells [1], as well as dermal white adipocytes (Fig. 3A). All of these cell types participate in immune responses and wound healing [21]. Despite their spatial proximity, the dermis and the epidermis have distinct circadian behaviors. Computational analysis of data collected from skin biopsies from human forearm, buttock, and cheek revealed that oscillation of clock genes is more robust in the epidermis than in the dermis across all three sites [22]. This may be because the cell types in the dermis are more diverse than in the epidermis, leading to less uniform circadian gene expressions in the dermis.
The hypodermis is the layer just below the dermis (Fig. 3A); it mainly consists of adipocytes that form the subcutaneous fat, but also contains fibroblasts and macrophages [1,21]. As an energy reservoir, the adipose tissue has long been studied for its role in metabolic diseases, which are intertwined with the whole-body circadian network. The relationship between the adipose tissue and the circadian clock is reviewed in detail in recent articles [23–25].
The circadian clock in hair follicles
The hair, one of the defining characteristics of mammals, works together with the epidermis to sense and protect the body from environmental changes. Hair shafts are rooted in hair follicles, which are miniature organs consisting of concentric layers of keratinocytes. They are embedded in the skin, penetrating into the dermis (Fig. 3A). The infundibulum and isthmus are the two most superficial compartments of hair follicles. They are continuous with the epidermis and their circadian activities are similar to that of the epidermis (Fig. 3C, D) [15,26]. The remaining lower compartments of hair follicles, including the anagen-specific bulb, the stem cell-containing bulge, and the mesenchymal-derived dermal papilla, are more dynamic (Fig. 3C, D). In particular, the dermal papilla and the anagen-specific bulb undergo structural changes as the hair follicles move through the three main stages of the hair cycle: the growth stage anagen (Fig. 3C), the regression stage catagen, and the relatively quiescent stage telogen (Fig. 3D) [15,26]. Diurnal genes in telogen and anagen skin only partially overlap [14], suggesting that the circadian clock differentially regulates hair follicles at those two stages.
Anagen marks the beginning of hair renewal. During anagen, quiescent bulge stem cells migrate out of the bulge to become proliferating matrix keratinocytes within the anagen bulb. The dermal papilla directs the differentiation of those matrix keratinocytes to form the inner layers of the hair follicle and the hair shaft (Fig. 3C) [26,27]. In both the bulge and the anagen bulb of mouse hair follicles, the circadian clock is robustly rhythmic and gates mitotic rhythms by synchronizing the G2/M checkpoint. Due to this synchronization of mitosis, the extension of the hair shafts shows a diurnal pattern, with more growth in the morning than in the evening [28]. In vivo studies of the human hair cycle are difficult, but studies indicate that circadian genes are expressed rhythmically in human hair follicles as well [29,30]. Furthermore, ex vivo studies suggest that the circadian clock regulates the human hair cycle, as silencing BMAL1, CLOCK and PER1 extends anagen [30]. In addition to hair growth, the circadian clock also controls the pigmentation of the hair during anagen, as melanin production by melanocytes in the human anagen hair matrix increases when the core clock genes BMAL1 and PER1 are silenced [31].
Hair follicles cease to grow during catagen and eventually enter the resting stage telogen. During telogen, the hair follicles prepare for the next anagen by forming the secondary hair germ just above the dermal papilla (Fig. 3D) [27,32]. Interestingly, in mice, while the hair is not growing during this stage, the amplitude of clock gene expression is significantly higher than during anagen, with the strongest expression in the hair germ. This robust circadian clock in the hair germ regulates stem cell activation as well as anagen initiation [33]. Consistent with this finding, germline Bmal1 mutation in mice leads to a delay in anagen initiations. Knocking out Bmal1 specifically in keratinocytes, however, does not significantly delay anagen initiation [14,33]. This suggests that Bmal1 regulates anagen initiation through the central SCN clock or other non-epidermal cell types. In nature, hair cycles for most mammals are seasonal. Given the role of the clock in seasonal cycles, it is tempting to speculate that the circadian clock has a role in seasonal hair growth [34].
Among the bulge stem cells, circadian rhythms are heterogeneous during telogen, with cells expressing higher levels of Bmal1 being more prone to activation signals. As anagen begins, circadian heterogeneity gradually decreases with most of the stem cells expressing high levels of Bmal1 [15,35]. Consistently, overexpression of Bmal1 by knocking out its repressors Per1 and Per2 stimulates proliferation and reduces the number of dormant bulge stem cells [35]. These findings are consistent with a regulatory role of the circadian machinery in the hair cycle and indicate that robust clock output correlates with hair follicle stem cell activation and anagen initiation.
The skin immune system is circadian clock-regulated
In order to fulfill its infection control function, the skin harbors resident innate immune cells distributed throughout the epidermis and dermis (Fig. 3A) as well as in organized tertiary lymphoid structures [36–39]. The circadian clock modulates the activation of these cells, possibly to match the likelihood of infections and insults throughout the 24-hour day. By regulating the skin immune response in this way, the circadian clock may decrease the tendency for autoinflammatory and autoimmune skin diseases [40–42].
Epidermal-resident immune cells include specialized dendritic cells called Langerhans cells, as well as specialized T cells referred to as γ/δ+ T cells. γ/δ+ T cells are under clock regulation in that the CLOCK protein directly binds to the promoter of the interleukin 23 receptor (IL-23R) in γ/δ+ T cells, thereby playing a regulatory role in the development the inflammatory skin disease psoriasis [41]. Langerhans cells appear to be regulated by the circadian clock as well. For example, when exposed to a viral mimic, imiquimod (IMQ), Langerhans cells highly upregulate anti-viral genes, the expression of which is affected by Bmal1 deletion [40].
Other immune cells such as macrophages, mast cells, T cells, and dendritic cells reside in the dermis (Fig. 3A). Macrophages constantly monitor the skin microenvironment and innately respond to pattern-associated molecular patterns and damage-associated molecular patterns produced through infections and inflammation. Other key functions of macrophages, including cytokine production and phagocytosis, are diurnally gated [43,44]. Mast cells cause allergic responses by releasing histamine, which activates other immune cells to cause allergy-like symptoms such as itching, erythema, and edema. Clock deletion in murine mast cells specifically ablates temporal variations in IgE-mediated degranulation and thus passive cutaneous anaphylactic reactions [45,46], indicating direct regulation by the circadian clock.
Apart from its resident immune cells, the skin recruits adaptive immune cells through chemokine and cytokine release during infections and inflammation. Circadian expression of chemoattractants, and the subsequent rhythmic infiltration of neutrophils and macrophages into the skin, results in diurnal severity of parasitic infection in murine footpad models [47]. Moreover, all rhythms in infection are abolished in clock-deficient macrophages and mice lacking the circadian clock in immune cells [47]. Additionally, the diurnal pattern of T cell recruitment and function parallels nocturnal itching and exaggeration in atopic dermatitis (AD) [48].
Skin microbiome
A diverse community of microorganisms, termed the microbiome, colonizes the human body. Appropriate microbial composition and function is essential for proper host immune and metabolic activities [49,50]. Most human studies regarding potential diurnal rhythms in the microbiome have been conducted in the oral cavity [51] or the gut [49,50,52,53], showing that rhythms in the microbiome are primarily driven by food intake. On the skin, taxa abundance, mainly of the phylum Actinobacteria, and especially the families Propionibacteriaceae, Micrococcaceae, Gordoniaceae and Dermacoccaceae) varies diurnally. This pattern primarily correlates with human activity [54].
The effect of feeding on the skin circadian clock and skin functions
Although light entrains the SCN clock, additional inputs such as temperature, exercise, and food intake affect the clocks in peripheral tissues [9]. The effects of food intake on circadian clocks in metabolic organs such as liver and fat are extensively studied. In these organs, food intake has major roles in timing of the clock, which is unsurprising given the divergent types of whole-body metabolism required during the day (food intake and activity) and night (fast and rest). In contrast to extensive studies focusing on the liver, the connection between feeding and circadian rhythms in the skin remained unexplored until recently. Below we review some of the findings showing that feeding regulates the skin circadian clock and biology of the skin.
Feeding-induced gene expression changes in skin
In experiments with multiple time-restricted feeding schedules, it is possible to rearrange the transcriptome data into feeding time series covering the beginning of feeding to 8 hours after the beginning of feeding. This allows the study of the effect of feeding on skin independent of time of day. In total, the expression of around 2000 genes changes in response to food intake, indicating powerful effects of feeding on gene expression in the skin (Fig. 4, left). The feeding-affected upregulated genes are involved in lipid biosynthesis and protein synthesis, whereas the downregulated genes are involved in response to starvation, autophagy, negative regulation of cell proliferation, and response to oxidative stress. This suggests that the metabolism and cell cycle regulators in the skin respond to feeding. Additionally, the fact that half of the feeding-affected transcripts are diurnal in at least one of the feeding schedules suggests that feeding is a regulator of diurnal gene expression in the skin [55].
Figure 4: Food intake regulates skin functions.
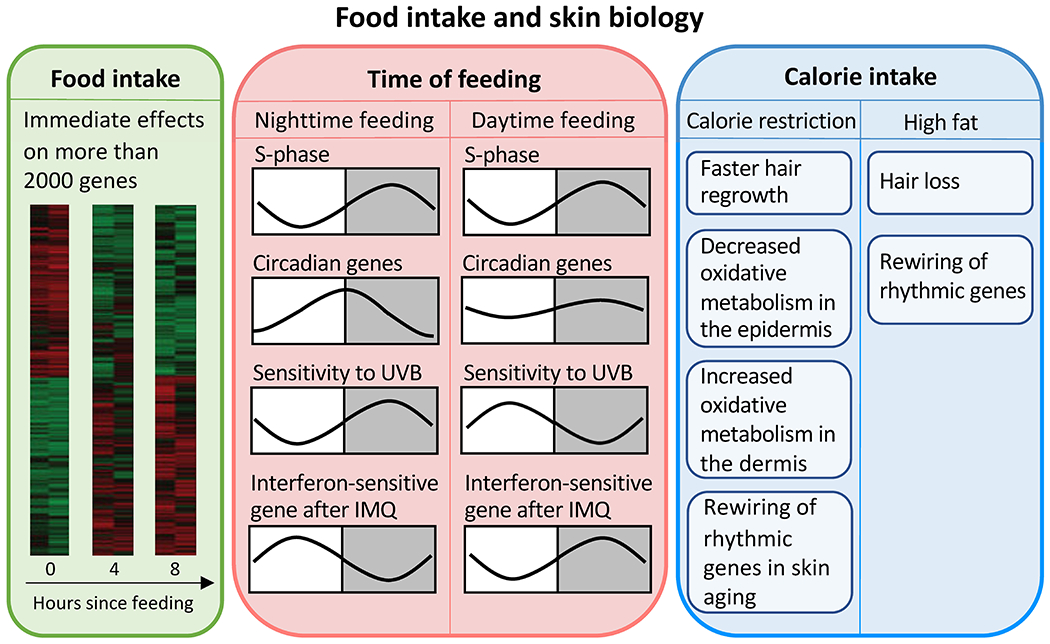
Left: Food intake immediately changes the expression of more than 2000 genes in the skin. Heat maps show gene expression before, four hours, and eight hours after food intake. Middle: Time of feeding does not affect the diurnal pattern of cell proliferation in the skin. However, daytime feeding shifts and dampens the expression of circadian genes. Daytime feeding also changes the diurnal variation in sensitivity to UVB-induced DNA damage and the IMQ-induced interferon response. Right: Calorie restriction and high fat diet affect metabolism and expression of rhythmic genes, hair follicle stem cell function, and skin aging. IMQ, imiquimod.
Daytime restricted feeding shifts the phase of the circadian clock in the skin
Daytime restricted feeding, as opposed to normal nighttime feeding schedules for mice, shifts the phase and amplitude of the circadian clock in skin (Fig. 4, middle) [55]. Compared to nighttime feeding groups, daytime-restricted feeding shifts the phase of the core circadian machinery in skin as indicated by Per2, Dbp and Per1 mRNA expression patterns. The magnitude and the direction of the shifts depend on the exact feeding time during the day. Additionally, the amplitude of Per2 mRNA expression decreases with daytime restricted feeding [55]. These changes in the skin circadian clock are different from the changes in the liver, where the phase of the clock is tightly coupled with feeding time, with phase advancements in all daytime-restricted feeding groups. Also, daytime-restricted feeding does not affect the amplitude of the liver clock [55]. These findings demonstrate a strong regulatory effect of feeding time over the skin circadian clock that is different from the effect on the liver clock.
The mechanisms whereby time of feeding shifts the phase and amplitude of the skin circadian clock are unknown. Since feeding regulates the expression of many systemic hormones including insulin, these hormones are potential mediators of the clock phase-shifting effects. Indeed, application of insulin to mouse whisker hair follicles induces activation of Per2, leading to circadian synchronization of hair follicles [56]. Interestingly, application of insulin before the peak of PER2 expression leads to phase advances, while application of insulin after the peak does not [56]. Similar patterns are observed in cultured human hair follicles [57], suggesting that the timing of insulin shifts; therefore, food intake, may disturb the circadian rhythm in human hair follicles. While insulin may be one mediator, the exact mechanisms connecting feeding time to circadian rhythms in the skin remain to be investigated.
Transcriptomic changes in skin are specific to the altered feeding schedules
RNA-seq of the exons, introns, and antisense RNA from the skin of mice under three different restricted feeding schedules shows that while around 2500 to 3000 exons oscillate in each of the schedules, only 147 transcripts are shared in all three groups, including several members of the core clock gene network [55]. Despite the transcriptomic diversity, gene ontology analysis of the diurnal genes indicates cell death, redox regulation, and circadian clock as enriched biological processes for all feeding groups. Significant correlations between the peak expression time of a gene’s intron and exon indicated that transcriptional regulation is responsible for the circadian phase shifts induced by restricted feeding. Some cytokinesis genes appear to be the exception to this rule and are regulated post-transcriptionally. Additionally, exons altered by food intake, regardless of the feeding schedule, are mostly related to metabolism, an indicator that feeding plays a role in skin metabolism status [55].
Time-restricted feeding does not affect diurnal rhythms in epidermal stem cell division but influences the sensitivity to UVB-induced DNA damage
Overall, daytime restricted feeding does not alter the structure of the skin with the exception that the dermis is thinner for the daytime-restricted feeding groups compared to the control [55]. Interestingly, although the cell cycle in epidermal stem cells shows a diurnal rhythm that depends on Bmal1, the phase of the circadian clock in skin does not control the cell cycle phase: the peak of S phase does not shift in the restricted feeding groups despite the shift in the phase of the clock (Fig. 4, middle). These experiments suggest that daytime restricted feeding desynchronizes intermediary metabolism and cell division in epidermal stem cells [55].
On the other hand, skin’s sensitivity to ultraviolet B (UVB) radiation-induced DNA damage is affected by daytime restricted feeding (Fig. 4, middle) [55]. More damage is caused by UVB applied during the night than during the day in the skin from the nighttime-feeding group, while more damage is caused during the day for the daytime-restricted feeding group. Furthermore, oscillation of xeroderma pigmentosum group A (Xpa), a gene responsible for UVB-induced DNA repair, is dampened and less robust in the restricted feeding groups, suggesting that feeding time affects the diurnal rhythms of genes responsible for UVB protection and DNA damage repair in skin [55].
Time-restricted feeding influences the skin immune response
Expression of interferon-sensitive genes in the skin is also shifted by time-restricted feeding (Fig. 4, middle) [55], suggesting that feeding time influences the skin immune response. Consistent with this idea, the expression pattern of interferon-sensitive genes in response to IMQ in the daytime-restricted feeding group is different from the one in the nighttime feeding group. In the daytime-restricted feeding group, interferon-sensitive genes are expressed at higher levels after IMQ application during the night than during the day, which is the opposite of the pattern shown in the nighttime feeding group (Fig. 4, middle). These findings suggest that time restricted feeding-induced circadian clock alterations affect the skin immune response [40].
The effect of calorie intake on diurnal gene expression and skin function
Besides food intake as such and time of feeding, the amount of calorie intake affects gene expression and function of the skin. Calorie restriction changes the structure of mouse skin by inducing faster hair regrowth after shaving, thicker epidermis, less dermal white adipose tissue, and greater vascular network [58]. Consistently, calorie-restricted mice have more interfollicular and bulge hair follicle stem cells that contribute to epidermal and hair growth. Additionally, calorie restriction alters the metabolism in mouse skin by decreasing oxidative metabolism in the epidermis but increasing it in the dermis [58]. Interestingly, calorie restriction can impede the aging-related reprogramming of clock-controlled genes, suggesting the connections between calorie restriction, circadian controlled genes and skin function (Fig. 4, right) [59].
On the other hand, high fat diet induces hair thinning and dermal adipose tissue expansion without significantly affecting epidermal thickness [60]. A closer look at the hair follicles in mice taking high fat diet reveals a decrease in hair follicle stem cells in the bulge as well as a fate shift of these stem cells from hair shaft to upper hair follicle components such as sebaceous gland and the epidermis-hair follicle junction zone [60]. On a transcriptomic level, high fat diet induces the expression of around 3000 rhythmic genes enriched for gene ontology terms such as fatty acid oxidation, responses to oxidative stress and mitochondrion organization [59]. This finding suggests a rewiring of the rhythmic genes to adjust skin metabolism in response to high fat diet (Fig. 4, right).
Taken together, food intake itself, the time of food intake, and the amount of calorie intake affect the expression of rhythmic genes in the skin, as well as the cellular composition and function of the skin. These findings suggest that feeding has powerful effects on the skin, in part through modulation of the rhythmic transcriptome (Fig. 4).
Circadian rhythms and skin diseases
Since the circadian machinery modulates many skin processes, including immunity, cell proliferation, metabolism, and DNA damage repair, it is plausible that circadian dysregulation can contribute to the development and progression of skin diseases.
The connection between circadian dysfunction and skin diseases is being studied both in the laboratory and in clinical settings. In the laboratory, chronic jet lag, prolonged light exposure, and flashlight treatment at night have successfully induced circadian disruption in mice. Additionally, mutation of core clock genes in mice and cultured tissues and cells provide insights into the role of the clock in disease. Human studies mostly focus on epidemiological data collected from shift workers, aiming to delineate the association between shift work-induced circadian disruption and diseases.
In this context, we focus on the role of the circadian clock in various skin diseases, including skin cancer, sunburn, aging, hair loss, inflammatory diseases, infections, and wound healing.
Skin cancer
The two main forms of skin cancers are carcinoma and melanoma. Carcinomas, such as squamous cell and basal cell carcinomas, are derived from keratinocytes. Melanomas, on the other hand, are derived from melanocytes [61]. Although carcinomas are more frequent, melanomas are more deadly by metastasizing through blood [62].
Ultraviolet (UV) radiation, either from the sun or tanning beds, is the major cause of melanomas and carcinomas. UV radiation from the sun can be classified into three types based on wavelength: UVA, UVB, and UVC. UVC is the most damaging of the three, but fortunately the majority of it is absorbed by the ozone layer. On the other hand, most of UVA and a small percentage of UVB still reach the earth. Those two wavelengths are absorbed by skin cells and cause DNA mutations through different mechanisms. UVB directly causes nucleotide changes while UVA raises the level of reactive oxygen species (ROS) that can lead to DNA damage. Additionally, while UVB is a thousand times more carcinogenic than UVA, UVA can penetrate deeper to cause DNA mutations [63,64].
Epidemiological studies linking work-related circadian disruption to cancer risk have highlighted the role of the circadian clock in several types of cancer, including melanoma [65].
The skin’s susceptibility to UV radiation-induced DNA damage is diurnal
Circadian rhythms are robustly present in the skin, an organ that directly experiences variations in UV radiation throughout the day. Indeed, the susceptibility of the skin to UV radiation-induced DNA damage is time-of-day dependent, and this diurnality is controlled by the circadian clock. There are at least two possible mechanisms contributing to the diurnal pattern of DNA damage. The first one relates to the susceptibility of the S phase of the cell cycle to UV-induced DNA damage [14] and the other one to the diurnality in DNA repair [66,67].
Exposing the dorsal skin of mice to UVB radiation at different times throughout the day revealed that more DNA damage occurs when the exposure takes place at ZT20 than at ZT8 [14]. Nucleotide excision repair (NER) is ordinarily capable of resolving UV damage, but rate-limiting subunits of the process such as XPA proteins are expressed in a rhythmic pattern due to circadian clock regulation. Because of this rhythmicity, mice that are exposed to UV radiation during dark hours experienced more severe and abundant squamous cell carcinomas compared to mice exposed to UV radiation during light hours [66]. Such time-dependent variation in DNA damage is replaced with a constant high level in Bmal1−/− mice, suggesting that the susceptibility to UVB radiation is controlled by the clock [14]. The cell cycle is also diurnal in mouse epidermal cells and controlled by the clock. More cells are in the S phase at night (ZT21) than during the day (ZT9). Similar to the DNA damage, the diurnal variation in the proportion of S phase cells disappeared in single knockout Bmal1−/− mice and double knockout Cry1−/−Cry2−/− mice [14,68]. It is noteworthy that the S phase is more prone to DNA damage, which may contribute to increased susceptibility to UVB radiation at night (ZT20) compared to the day (ZT8) [14].
XPA protein is expressed at high levels during the day and is at low levels at night [66,67]. P53 protein also accumulates to a higher level in mouse skin exposed to UV radiation at night (ZT21) than at during the day (ZT9). This variation could be explained by changes in the level of MDM2 protein, which stimulates the degradation of P53 protein and shows a diurnal expression pattern. During the night, the levels of both MDM2 and P53 proteins are low, resulting in less degradation of P53. Thus, when mouse skin is exposed to UV radiation at night, the P53 levels rise to a high level with minimum degradation, leading to more P53-dependent responses such as apoptosis. On the other hand, since the expression level of both P53 and MDM2 proteins are already high during the day, UV radiation does not cause much increase in the P53 level because the level of MDM2 is high enough to degrade most of the P53 proteins. Hence, the P53-dependent responses in mouse skin are less prominent when the radiation occurs during the day instead of at night [69].
The skin is protected from UV radiation by the pigment melanin produced by melanocytes [70]. The core clock component BMAL1 influences melanin production by associating with microphthalmia-associated transcription factor (MITF), the master regulator of melanogenesis. MITF has rhythmic expression levels alongside BMAL1. By overexpressing BMAL1, the resultant melanin from the melanogenesis pathway was correspondingly higher, conferring greater protection against UV radiation for skin cells [71].
For non-UV radiation induced carcinoma, which accounts for about 10% of carcinomas, the loss of Bmal1 results in decreased development of squamous tumors in mice with an oncogenic background [35]. Compared to the control, mice with Bmal1 knockout have lower percentages of tumor-initiating cells and increased expressions of tumor suppressors, thus decreasing the severity and abundance of carcinomas.
In sum, the clock affects UV-induced skin cancer initiation by regulating the cell cycle and DNA repair mechanisms in keratinocytes and through regulation of UV-protectant pigment from melanocytes.
The circadian clock regulates skin cancer progression
In addition to controlling susceptibility to UV-induced DNA damage and cancer initiation, the clock is involved in skin cancer progression in at least two ways. On the one hand, cancerous cells possess aberrantly expressed clock genes, resulting in impaired clock function [72]. On the other hand, mutations of core clock genes are associated with melanoma progression [73]. As a result, recent studies have targeted components of the circadian clock to restore tumor cell circadian rhythms in cancer treatment [72].
The clock network is dampened in cancer cells, demonstrating that an oncogenic landscape facilitates clock dysfunction [72]. A comparison of gene expression data from human melanoma and healthy skin samples reveals downregulation of clock genes in melanoma [74]. The upregulation of oncogenes such as Ras extends the period of the clock, demonstrating mechanisms in cancer progression that disturb the circadian machinery [75]. Consistently, mice injected with B16-F10 melanoma cells possess severely impaired clock gene regulation within and around the tumor site [76]. Core clock components can be indirectly regulated by proteins of the Melanoma Antigen (MAGE) family that bind to RORs or modify ubiquitination of clock proteins [77,78]. Similarly, circadian machinery is disrupted in oncogenic keratinocytes; ablation of the tumor suppressor gene Pten increases BMAL1 in hair follicle stem cells, highlighting the regulatory influence of tumor suppressors on core clock components [79].
In addition to disrupted circadian clock function in melanoma cells, circadian disruption contributes to other oncogenic mechanisms. Myeloid-specific Bmal1 knockouts lead to metabolic dysregulation in macrophages, thus promoting immune suppression and melanoma tumor burden [80]. In addition, chronically shifting light schedules alters the immune microenvironment in melanomas by inverting the ratio of M1 and M2 macrophages [81]. Thus, clock dysfunction can lead to tumor-promoting changes in the melanoma microenvironment.
Circadian disruption also promotes cell cycle transitions by upregulating cell cycle genes such as Ccna2 [81]. Clock proteins like PER can couple with nuclear proteins involved in the cell cycle, enabling circadian control of cell cycle checkpoint genes [82]. Normally, specific components of the clock, such as neuronal PAS domain 2 (NPAS2), also directly limit cellular proliferation by gatekeeping portions of the cell cycle, but mutations in these genes promote cell cycle transitions [83]. Overall, since clock proteins act as critical regulators of cell cycle checkpoint control, disruption of the clock leads to unregulated division and tumor proliferation [84].
Given that clock dysfunction enables tumor proliferation, targeting the clock to improve its function could facilitate cancer management. Inducing circadian rhythmicity in tumor cells by dexamethasone decreases in vivo melanoma growth in mice [85]. This response is clock-dependent, as knocking down Bmal1 in tumors prevents the growth-dampening effect from dexamethasone. Pharmacological studies also targeted components of the circadian clock, nuclear hormone receptors NR1D1 and NR1D2, to stimulate apoptosis in malignant cancer cells [86]. By applying agonists of these receptors, senescent cells like those in melanocytic nevi would undergo cell death, demonstrating that treatments targeting core clock machinery impair melanoma proliferation. In addition to targeting the circadian clock machinery, current cancer treatments can utilize the clock by modulating the time of treatment application. The toxicity of the chemotherapeutic cisplatin towards melanoma cells varies throughout the day, indicating that melanoma treatment is more beneficial in the morning (ZT0) for patients [87].
In sum, the circadian clock plays a role in the initiation and progression of skin cancer. The rhythmicity of the clock regulates DNA-damage repair, thus modulating the susceptibility to UV-induced DNA damage and cancer initiation. Carcinomas and melanomas often possess diminished clock function, promoting cell cycle transitions and cell proliferation. Also, dysregulation of the clock can alter the tumor microenvironment to make it more immunosuppressive. Targeting core clock genes therapeutically to restore clock function may be a promising approach to cancer treatment.
Sunburn
Short-term exposure to excessive UV radiation leads to sunburn. UV radiation-induced DNA damage leads to an inflammatory response characterized by skin redness, swelling, and pain [88]. Consistent with the diurnality in UV-induced DNA damage, the severity of sunburn symptoms also exhibits a diurnal pattern. While erythema severity saturates at high levels of UV exposure on the dorsal skin of mice, moderate levels of radiation applied during the night (ZT21) caused higher levels of inflammatory cytokines, apoptosis, and worse erythema than during the day (ZT9) [69].
Due to the opposite chronotypes of humans and mice, the diurnal pattern found in mice is speculated to be reversed in humans [14,63,69]. Indeed, radiation in the evening (between 7pm to 9pm) caused more serious erythema than in the morning (between 7am to 9am) on human skin, indicating that the human skin is more sensitive to radiation in the evening [89]. Interestingly, P53 protein accumulated more in skin samples collected 24 hours after morning irradiation, which is the same pattern in mice [89]. This could be explained by previous findings wherein UVB-induced accumulation of P53 is not correlated with severity of erythema in human skin [90].
In summary, UV-induced DNA damage directly links to skin carcinogenesis as well as acute inflammatory responses. Furthermore, the effect of UV radiation on skin is time-of-day dependent due to the diurnality of cell cycle and DNA damage repair.
Hair loss
Hair loss, also known as alopecia, is one of the most common skin concerns. While it usually occurs on the scalp, hair loss can affect any hair bearing part of the body. The most common form of alopecia is androgenetic alopecia, which affects both males and females, albeit with a different pattern of hair loss. There is also an aging-associated hair loss that may be independent of androgenetic alopecia. Telogen effluvium is another hair loss disease; it may be triggered by a number of stimuli, including stress, hormones, medications, and illness [91]. In this condition, hair follicles enter the shedding phase in a synchronized manner [92] whereas normally human hair follicles have desynchronized hair cycle stages. Anagen effluvium on the other hand is caused by cell death in the matrix of growing hair follicles in response to genotoxic stimuli such as ionizing radiation and chemotherapy [93]. Alopecia areata is an autoimmune disease characterized by hair loss. Finally, scarring alopecias are inflammatory conditions that lead to destruction of the hair follicle bulge stem cells and permanent loss of hair follicles [92].
Given the regulatory role of the circadian clock in hair follicles, it stands to reason that clock disruptions could contribute to hair loss, especially aging-related hair loss. Evidence for this, however, is missing from epidemiological studies. The clearest evidence for a role of the circadian clock in hair loss comes from mouse studies showing that the clock gates cell division in growing hair follicles. Thus, γ radiation causes more hair loss when applied to anagen hair follicles at the mitosis peak in the morning than in the evening when mitosis levels drop [28]. Based on this finding in mice, we speculate that radiation in the evening would causes more hair loss than radiation in the morning in humans. Hence, arranging radiotherapy in the morning may be better for managing hair follicle damage during the treatment.
Aging
At an organismal level, aging correlates with circadian rhythm alterations as indicated by features including advanced sleep pattern, flattened body temperature fluctuation, and decreased activity levels during the wake period [94]. Additionally, aged mice cannot adapt to light-dark schedule alterations as well as young adult mice [95]. One explanation for this is related to the impaired functions of retinal cells and the SCN. Since aging reduces the number of ipRGCs, the amount of SCN neurons expressing VIP, and neuron coupling, the circadian rhythm in aged individuals is not as robust as in young adults at the organismal level [96]. While the SCN function is compromised at a tissue level, the clock machinery evaluated by Per2 expression in the SCN is as robust as in young adult mice [95]. Similar to the case in the SCN, the molecular clock in mouse peripheral tissues such as kidney, liver, submandibular gland, and epidermis is robust despite the decrease in locomotor activity [59,97].
In the skin, features of aging include wrinkles and lines, dryness, a thin epidermis, hair loss, loss of elasticity, skin fragility with easy breaking, and slow wound healing. The causes of skin aging are on the one hand intrinsic, strongly correlated with chronological age, and on the other hand extrinsic. Intrinsic aging mechanisms include oxidative damage to DNA and proteins from metabolism-generated ROS, accumulating genetic mutations, stem cell dysfunction, and alterations in hormones. Extrinsic skin aging mechanisms involve primarily UV radiation, but also other insults such as cigarette smoking, and pollution [98].
While the exact mechanism is unknown, clock disruption contributes to intrinsic skin aging as mutations of the core clock genes Bmal1 and Clock in mice lead to premature aging in multiple organs, including the skin [99,100]. Conversely, aging influences the circadian function, as aging rewires rhythmic genes in the skin [59]. While the circadian machinery is robust in aged epidermal stem cells, other rhythmic genes in the young and aged groups are quite distinct [59]. Thousands of genes are diurnal in young and aged mouse epidermal stem cells, but only 750 genes overlap the two ages, including some genes from the core clock machinery. Additionally, the rhythmic genes present only in the young adult group are mainly related to homeostasis maintenance, while the genes that oscillate only in the aged group are enriched for stress response. This suggests a reprogramming of rhythmic genes in mouse epidermal stem cells as they age while maintaining the core circadian function [59]. Despite premature aging in core clock gene knockout mice [99,100], the failure to reproduce the transcriptional traits found in physiologically aged epidermal stem cells in Bmal1 knockout and Per1/Per2 double knockout mice suggests that the reprogramming of rhythmic genes is due to aging instead of loss of circadian function [59].
A similar observation was made in rat abdominal skin explants. There is no significant overall change in the average value of phase, amplitude and period lengths in rat skin explants as they age, although the phases are significantly more variable in the aged group [101]. However, when looking at the circadian rhythm in fibroblasts derived from these explants collected at different ages, Per1 expression amplitude attenuates uniformly as the age increases [101]. Along the same line, a microRNA miR-31 that is upregulated in aged mouse hair follicle stem cells targets at the core clock gene Clock, suggesting the potential effects of aging on the clocks in hair follicle stem cells [102]. While the differences in results could be caused by different experimental methods, the question about whether aging affects circadian clocks differently in different cell types warrants further investigation.
As discussed before, the rhythms in the skin regulate cell proliferation in a way to avoid exposing possible genotoxic and radiation stress to the vulnerable S phase [14,20]. Intriguingly, without affecting the core clock gene network, aging affects the cell cycle timing in mouse epidermal stem cells, which could be an explanation for the transcriptional enrichment of stress response and DNA damage repair in aged cells. While the S phase takes place mostly during the night in young adult epidermal stem cells [14,20,59], the peak DNA replication time is shifted to the day for the cells from the aged group [59]. Despite this delay, mitosis is rhythmic and still takes place at ZT20 in the aged group, which is the same as in the young adult group. Such delay may lead to the possible exposure of unwound DNA to oxidative stresses and increase the possibility of cells entering the mitosis with DNA damage [59]. This observation is consistent with some of the skin aging symptoms, as oxidative stress is one of the contributors to interfollicular epidermis stem cell senescence, which could lead to skin wound healing delay, thinning of the epidermis in aged skin, and age-related hair loss [15].
One way to lessen the effect of aging in mouse epidermal stem cells is through calorie restriction. For mice under calorie restriction (70% of the amount taken by the control group), both physiological and transcriptional traits found in young mice are maintained with rhythmic expressions preserved in genes related to homeostasis maintenance and lower level of oxidized DNA. Also, S phase peaks at ZT12, which is closer to the peaking time in young adult epidermis. Despite a 4-hour phase advancement for the calorie restriction group due to feeding anticipation, other circadian features such as the amplitude and the period length of some of the core clock genes are conserved in the calorie restriction group [59].
Since circadian rhythms in the skin may protect cells from UV-induced DNA damage by regulating cell proliferation [14], circadian reprogramming and cell cycle shifts caused by intrinsic aging may contribute to extrinsic skin aging. Furthermore, clock-related hormone function impairment caused by intrinsic aging can affect skin function and promote extrinsic skin aging. Melatonin is one of the hormones that regulates peripheral clocks and its receptor is present in the skin [103]. Interestingly, melatonin receptor MT1 plays a key role in DNA repair as it activates the P53-dependent DNA damage response [104]. In human skin, MT1 levels drop as we age, leading to increased sensitivity to UV radiation [105]. The receptor MT1 level is drastically lower in cultured dermal fibroblasts from a 67-year old donor when compared with cells from a 19-year donor. Furthermore, after UV radiation, the amount of UV-induced DNA damage and ROS generation is much higher in MT-1 knockdown human fibroblasts, suggesting that aged human dermal fibroblast are more susceptible to UV damage [105].
Much of the connections between skin aging and circadian rhythms remain to be explored. Questions such as whether aging affects the circadian rhythm differently in different cell types, what mechanisms cause the shifts in cell cycle while not affecting the core circadian clock in epidermal stem cells, and what kind of mechanisms contribute to the rescuing effect of calorie restriction on epidermal stem cell aging remain to be answered.
Skin infection
As a first line of protection against outside insults, the skin harbors various defensive strategies, including a physical barrier formed by corneocytes (terminally differentiated keratinocytes), resident innate immune cells, and antimicrobial peptides (AMPs), which are secreted to induce direct antimicrobial effects [37,38,106]. The immune system is circadian regulated, possibly to anticipate and prepare for outside insults when they are most likely to occur [15,107]. The circadian clock regulates immune responses to infections in the respiratory system [108,109], the liver [110], the digestive system [111], and other body sites [112]. Consistent with these findings, a growing body of animal research supports the hypothesis that immune responses and clinical outcomes of skin infections are influenced by the host’s circadian rhythms and time of infection [14,15,47,113].
Bacterial and parasite skin infections
The survival of bacteria on the skin depends on the time of infection. Staphylococcus aureus shows maximum survival on mouse skin when applied at ZT22 and minimum survival when applied at ZT16. This may be due to the diurnal expression of certain AMPs in the skin. The expression levels of Rarres2 (chemerin), Camp (cathelicidin CRAMP), and Defb1 (β-defensins) are diurnal, with the highest expression during the early night when mice are under 12:12 light-dark cycle. However, the rhythms for those genes in mouse skin are lost in constant darkness, although they are maintained in the liver. In contrast, Defb3 and Defb14 (β-defensins) aare circadian under both 12:12 light-dark cycles and in constant darkness, with expression peaking during the day [113].
The severity of parasite infections in skin is diurnal as well [47]. Leishmania parasites are transmitted through sandfly bites and use neutrophils and macrophages as host cells. Injection of Leishmania major parasite into mice at different times of the day revealed that inflammation is the mildest when the injection takes place at ZT3, with minimum macrophage recruitment six hours after injection. In vitro experiments confirm that the attachment of the parasite to mouse macrophages is circadian and dependent on the circadian machinery, which also regulates the time-dependent release of chemokines and cytokines [47].
Viral skin infections
Skin also has the important task of defending against many viruses, which are biological entities that can only thrive and multiply in a living cell. While there are few studies on circadian regulation of defenses against viral infections of the skin, studies in other organs suggest a role for the circadian clock in regulating this activity. Mice intranasally infected with herpes or influenza viruses during the daytime have greater infection loads and mortality than mice infected at nighttime. This time-of-day dependent difference may be clock controlled as deletion of Bmal1 exacerbates the infection [114]. In addition, disruption of circadian rhythms by Bmal1 deletion or altered sleep/wake patterns enhanced acute viral bronchiolitis after Sendai virus and influenza A viral infections [115].
Topical application of the viral mimic IMQ during the day results in greater expression of anti-viral defense response genes compared to application at night. Absence of Bmal1 results in heightened levels of those same genes [40]. This result suggests that the diurnal gating of sensitivity to viral infection is modulated by circadian clock control of anti-viral factors expressed in the skin. These results have implications for anti-cancer defenses as anti-viral mechanisms limit cancers as well. For example, IMQ is prescribed as an anti-tumor treatment for basal cell carcinomas, as it activates the immune system through type I interferon secretion.
Inflammatory skin diseases
Skin inflammation, which includes redness, heat, itching, sensitivity, and swelling serves to defend against invading pathogens and microbes. While controlled skin inflammation is necessary to regain homeostasis after infection, there are cases in which skin inflammation is triggered by self-peptides or persists following an infection, leading to skin diseases. Inflammatory responses are linked to circadian rhythms [40] and circadian disruption can lead to inflammatory diseases. In mice, for example, chronic jet lag induces ulcerative dermatitis [116]. For humans, psoriasis and AD are two of the most common autoimmune skin diseases. Both of these diseases, which can significantly impair patients’ life quality, are linked to circadian rhythms.
Psoriasis is caused in part by the immune system attacking healthy skin cells, resulting in intense inflammation. In humans, disruption of the circadian clock through night shift work is associated with psoriasis [117,118]. The symptoms of psoriasis also show a diurnal pattern, with more than 70% of patients reporting more severe itch occurring in the evening or at night [119]. With most of the itch occurring at night, AD and psoriasis symptoms compromise sleep quality [120], which in turn disrupts circadian rhythms and increases severity of the disease. This circadian disruption also contributes to the development of metabolic diseases such as obesity [121], which may be causally linked to psoriasis [122].
At a mechanistic level, the inflammatory response in psoriasis is controlled by the circadian machinery [40,41]. Mutation of Clock relieves the induced psoriasis-like symptoms significantly by controlling the transcription of IL-23R in γ/δ+ T cells while mutation of Per2, the inhibitor of Clock, promotes IMQ-induced symptoms [41]. Additionally, deletion of Bmal1 promotes IMQ-induced psoriasis [40]. Conversely, psoriasis inflammation disturbs the circadian clock locally in the skin lesions. In IMQ-induced psoriasis mouse model, the rhythmicity of keratinocyte proliferation is lost, and the expression of core clock genes is dampened [40]. Similar to the case in mice, bulk RNAseq studies using human patients skin samples show that circadian genes such as CRY2, PER3, NR1D1 and RORC are downregulated in the psoriasis lesion as well as the adjacent normal skin when compared to skin from non-psoriatic individuals [40,123]. These findings support the intricate connection between psoriasis and the circadian machinery and suggest that the circadian clock is a potential target in psoriasis treatment.
AD is another common inflammatory skin disease characterized by impaired barrier function, eczematous dermatitis, and chronic itching. The causes of AD are not fully understood, but may relate to barrier impairment, dysregulated immune system, and/or dysregulated commensal microbiota. AD symptoms tend to be greater at night and impair sleep quality [124,125]. There are several mouse models of AD. These typically apply an inflammatory insult (a chemical or hapten) to the skin to activate macrophage cytokine secretion, which in turn provokes release of histamines by mast cells that causes itch; the insult is then re-applied later to induce an adaptive immune response driven by T cells. Disruption in circadian rhythms by forcing mice to run during the day and rest during the night causes loss of rhythmicity in circadian gene expressions and more severe AD symptoms with increased expression of histamine and cytokines, including TNF-α and interleukins (IL-4, IL-10, IL-13). Additionally, more dendritic cells, mast cells and T helper cells are recruited to the skin in mice with the reversed chronotype [126]. Also, hapten induces more inflammation in the skin of mice living in constant light than in the skin of mice living in regular 12:12 light-dark cycle [127]. Implicating the circadian clock on the molecular level, deletion of Clock causes more severe delayed-type skin allergic reactions [128]. As the circadian clock regulates the immune responses that contribute to AD symptoms, studying the relationship between the circadian rhythm and AD will yield insights into symptom management.
Wound healing
Skin injuries invoke complex repair mechanisms that require regulation and coordination among cell types throughout the skin. These mechanisms go awry in chronic wounds, a major health problem sometimes caused by metabolic diseases like diabetes and obesity [129]. Several elements of skin wound healing have diurnal features and interact with the core clock machinery.
Wound healing progresses through five steps: hemostasis, inflammation, growth, re-epithelialization, and remodeling. Hemostasis stops the bleeding by forming a hemostatic plug at the injury site [130]. This very first step of wound healing displays diurnal rhythms in humans, with hemostasis being upregulated in the morning and fibrinolysis activation peaking in the afternoon [131]. As discussed earlier, elements of the inflammation step are under circadian control.
The growth stage of skin wound healing displays circadian rhythms. The actin polymeric state, as measured by the ratio of F:G actin produced by fibroblasts, is rhythmic and such rhythm is dependent on the cells’ internal circadian machinery [132]. Additionally, the circadian clock affects senescence of myofibroblasts through NONO, a binding partner of PER that regulates cell cycle inhibitors [82]. These findings support the idea that the circadian clock regulates both proliferation and migration of fibroblasts, key functions required for wound healing.
The time-of-day of wounding may affect the length of recovery [132]. Synchronized murine skin fibroblast monolayers scratch-wounded at the peak time of PER2 expression healed almost completely after 60 hours while those wounded at the PER2 trough time were far from closing. This can be explained by the significantly faster initial migration, stronger initial cell adhesion, and greater wound-oriented polarization of the most motile fibroblasts in monolayers wounded at the peak time of PER2 expression. Ex vivo wounding experiments done on 5-day-old murine skin explants harvested during the active phase and the resting phase show similar results, with twice as many fibroblasts invading the wound in the explants collected during the active phase when compared with the sample collected during the resting phase. This observation is reproduced in in vivo wounding experiments on adult mouse back skin, suggesting that the invasion of fibroblasts into the wounded area, and thus possibly the recovery of the wound, are dependent on the wounding time in mice [132].
A post hoc analysis of the datasets from the Burn Injury Database reveals that burns happening at night took around 60% more time to heal than the burns that took place during the day [132]. While wounds incurred during the active phase of humans and mice take less time to heal [132], the opposite pattern is true in nocturnal hamsters [133]. According to the wound sizes recorded daily, wounds incurred at ZT18 (night) take longer to recover by 50% than wounds incurred at ZT3 (day), even though wounds incurred at both times take the same number of days to heal completely [133].
Re-epithelization may also display circadian rhythms. While there is no ex vivo or in vivo wounding experiments done so far on human skin, scratch-wounds incurred on human keratinocyte monolayers at the peak time of fibroblast motility healed faster than the monolayers scratch-wounded at the trough time [132]. On the other hand, while the proliferation of mouse epidermal keratinocytes is circadian, with more cells at the S phase at night than during the day [14], the proliferation elevates and loses its rhythmicity when there is inflammation [40].
Circadian disruption delays skin wound healing. Cutaneous wounds in disruptive light-treated hamsters took longer to heal compared to hamsters with normal circadian rhythm, no matter the time of wound occurrence [133]. Similarly, dim light exposure to mice during dark hours before wounding slows down the healing process significantly [134].
Manipulation of the circadian machinery does not always impede wound healing. Partial or complete knock out of Npas2, whose protein is a paralog of CLOCK, significantly shortens the duration of dorsal skin wound healing in mice in vivo. In vitro experiments using the fibroblasts isolated from Npas2−/−, Npas2+/− and wild type mice uncovered accelerated proliferation of Npas2−/−, Npas2+/− fibroblasts without alternation in the rhythmic expression of most of the core clock genes except Per2. Additionally, Npas2−/− fibroblasts contract faster than wildtype and Npas2+/− fibroblasts. Intriguingly, the expression level of Acta2 and Actb, along with other integrin genes do not differ among the genotypes. Some collagen genes (Col12a1 and Col14a1) are upregulated in cultured Npas2−/− and Npas2+/− fibroblasts, in agreement with promoted collagen fiber formation observed in those knockout cells [135].
While more extensive studies are necessary to delineate the connection between the circadian clock and skin wound healing, current findings indicate that the time of wounding has an effect on the healing duration [132,133] and that the circadian machinery plays a role in many wound healing processes [133–135]. The field remains to be explored, especially the possibility of promoting wound healing via circadian clock manipulation [135].
The future of circadian medicine for skin health
Circadian medicine and chronotherapy are receiving increasing attention as important components of preventive medicine and patient care [136–138]. At its core, circadian medicine considers circadian rhythms in pre-clinical research and when developing disease management plans to improve clinical outcomes while minimizing side effects. For example, vaccination in the morning results in greater trained immunity and antibody responses than vaccination in the evening in humans [139–141]. Additionally, perioperative myocardial injury is more common after on-pump cardiac surgeries in the morning than in the afternoon [142]. In mice, non-steroidal anti-inflammatory drugs better manage pain and promote recovery with less adverse effects on healing after tibia fracture surgery when taken during the active phase than during the resting phase [143]. Such diurnal variation in treatment efficiency is also observed in other tissues and diseases [136–138], including cancer [144,145].
Circadian medicine uses three main approaches: lifestyle adjustments to regulate the internal clock, drugs targeting the clock machinery at a molecular level, and administration of drugs at the time of day that maximizes effectiveness and minimizes side effects (chronotherapy) [68,137]. Although the skin harbors a robust circadian clock, circadian medicine for skin diseases is a fledging field. Here, we discuss how circadian medicine can benefit skin health.
Managing the internal clock via lifestyle adjustment
Research in model animals and cultured human cells shows how disruptions of circadian rhythms via clock gene manipulations can contribute to the development and progression of various kinds of skin diseases including cancer, sunburns, radiation-induced hair loss, aging, infections, inflammatory diseases, and wounds. Consistent with these experimental studies, epidemiological studies on shift workers show that circadian disruption is associated with increased incidence of metabolic disease [146], cancer [65], and several skin diseases [68,117,118]. Therefore, it stands to reason that a healthy lifestyle aimed at maintaining normal circadian rhythms may improve skin health.
Currently, shift work, social jet lag, sleep disorder and excessive light exposure at night are common causes of circadian rhythm disruption in modern society. Shift work is extensively studied as it leads to disruption of the core clock machinery, including the skin [147,148]. Compared to normal daytime workers, shift workers possess altered circadian rhythms in the skin as indicated by significantly attenuated peak expression level of Per1 [147]] and altered expression patterns and levels of Per3, Nr1d1 and Nr1d2 [148] in hair follicles. Shift workers are more prone to skin diseases, including psoriasis, dry skin, itching, dandruff/seborrheic dermatitis, acne, and melanoma [65,68,117,118].
Conversely, skin diseases such as psoriasis and AD can affect the patients’ organismal circadian rhythm by disturbing sleep [120,125,149], which then adversely feeds back to disease progression. Besides being predisposed to greater disease risk, shift workers may experience worse side effects. In mice, circadian disruption via jet lag or clock gene mutations leads to more severe radiation-induced dermatitis [150]. Because of this, maintaining a normal sleeping pattern and avoiding shift work would be beneficial for minimizing the risk and severity of skin diseases.
As feeding can influence skin circadian rhythms in mice, and thus the immune response, adjusting feeding behavior in terms of both of the feeding time and the caloric intake may be a way to support circadian function in the skin [138,151]. In mice, time of feeding affects the skin immune response [40]. Additionally, calorie restriction delays the age-associated reprogramming of circadian controlled genes from homeostasis maintenance to stress response [59]. In contrast, high fat diet induces hair loss [60] and rewires the expression of rhythmic genes to favor oxidative metabolism [59]. While well-controlled experimental research in humans on the effect of feeding on skin health is lacking, a recent clinical study focusing on psoriasis patients before and after Ramadan fasting, which happens during daylight time for an entire month, showed alleviation of psoriasis after the fasting [152,153].
Targeting the clock machinery at a molecular level
Circadian rhythms are often disrupted in diseased tissues. Most often, this takes the form of dampened rhythms due to a loss of synchronization or suppressed circadian gene expression [40,85,144,154]. For example, expression of Bmal1 is dampened in the skin of IMQ-treated mice [40]. Therefore, it is possible that restoring or enhancing the circadian rhythm can control disease progression. Dexamethasone, for example, activates the circadian clock in B16 melanoma tumors, which decreases cell proliferation and tumor growth [85]. Indeed, the circadian machinery is a reservoir that may host promising targets for developing skin disease treatments.
For example, without harming normal cells, REV-ERBs agonists SR9009 and SR9011 adversely affect cancer cells derived from tumors, including melanoma [86]. In mice, mutation of Clock relieves psoriasis-like symptoms [41] and mutation of CLOCK protein paralog shortens wound healing duration [135]. Additionally, topical application of ROR inverse agonist SR1001 quenches inflammatory response in AD mouse models. For humans, melatonin supplement alleviates AD symptoms [155]. Future studies should explore drugs that target the circadian machinery in skin diseases, including inflammatory and hyperproliferative skin diseases.
Chronotherapy
Circadian rhythms regulate many metabolic enzymes that participate in drug metabolism in the skin [14]. Hence, more clinical studies should be conducted to assess how time of application impacts treatment efficacy and side effects. Experiments done in mice show that maxacalcitol, a vitamin D3 analogue for psoriasis symptom relief, is more effective when applied during the early to middle active phase of mice than during the early to middle resting phase [48]. For AD, application of topical treatment in the evening is proposed to improve symptoms [156].
For cancer, studies from the 1990’s describe more effective melanoma tumor shrinkage in mice when interferon was infused during the day as opposed to night [157]. Additionally, radiation chronotherapy in the morning for cancer treatment reduces its damaging effect on skin [158].
It may also be important to consider the time of diagnostic measures. For example, the time of diagnostic measures is critical for accurate diagnosis in endocrinology, as a number of hormones oscillate in a time-of-day dependent manner [159]. Similarly, it is possible that the time of measurement is important in dermatology, since features such as mitosis [14,28] trans-epidermal water loss [160], and immune components display diurnal rhythms [40,55,113].
Challenges
As promising as circadian medicine is, we face challenges. Most studies are done in vitro or in vivo using model organisms such as mice, rats and hamsters. Those animals are nocturnal while humans are diurnal, which can lead to difficulty in translating from mice to humans. Also, most pre-clinical intervention studies in mice are carried out during the day, the resting phase of mice. One possible step to reduce the gap between human and model nocturnal animals is to use inverted-light housing, so researchers can still make the measurements during the day while it will be the night for the nocturnal model animals.
Additionally, since circadian medicine works the best when it is correlated with the patient’s internal circadian rhythm, health professionals need a feasible method to accurately determine each patient’s circadian rhythm before establishing a treatment plan [161]. The most traditional and established way of measuring circadian phase of a patient includes a tedious process of collecting multiple saliva samples throughout the day in a dim room for the dim-light melatonin-onset assay [162–164]. For clinical applications, several approaches have been proposed to determine a patient’s clock phase [165], including body temperature [166], transcript biomarkers in blood [164,167,168], cortisol concentration in sweat [169], as well as volatile organic compounds and nitric oxide fraction in breath [170]. The accessibility of skin makes this organ a source to measure rhythms: indeed, transcripts from hair follicles [171], skin biopsies [163] and tape-stripped epidermis [172] can potentially be used for circadian time estimation [161]. However, the question as to which method is the best in terms of accuracy and accessibility remains unanswered. Another challenge to utilizing circadian medicine for skin diseases lies in the complexity of skin. The circadian rhythm in skin may not be as uniform as in other organs [15]. Additionally, two other clocks, hair cycle and natural aging, influence the circadian controlled gene expression, raising questions about whether these variables need to be considered [15,59].
Acknowledgement
This work was supported by NIH grants R01 AR056439 and P30AR075047, an NSF grant DMS1763272, a grant from the Simons Foundation (594598), and the Irving Weinstein Foundation (to B. Andersen).
Abbreviations:
- SCN
suprachiasmatic nucleus
- ipRGCs
intrinsically photosensitive retinal ganglion cells
- VIP
Vasoactive Intestinal Peptide
- AVP
Arginine Vasopressin
- BMAL1
Brain and Muscle ARNT-Like 1
- CLOCK
Circadian Locomotor Output Cycles Kaput
- E-box
Enhancer Box
- PER
Period
- CRY
Cryptochrome
- RORs
RAR-related Orphan Receptors
- RORE
ROR/REV-ERB-response element
- IL
Interleukin
- AD
Atopic Dermatitis
- IMQ
Imiquimod
- UV
Ultraviolet
- NER
Nucleotide Excision Repair
- XPA
DNA Damage Recognition and Repair Factor
- NPAS2
Neuronal PAS Domain Protein 2
- P53
Tumor Protein 53
- ROS
Reactive Oxygen Species
- MITF
Microphthalmia-associated Transcription Factor
- AMPs
Antimicrobial Peptides
References
- 1.Wong R, Geyer S, Weninger W, Guimberteau J-C & Wong JK (2016) The dynamic anatomy and patterning of skin. Experimental Dermatology 25, 92–98. [DOI] [PubMed] [Google Scholar]
- 2.Zouboulis CC (2009) The skin as an endocrine organ. Dermato-endocrinology 1, 250–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buhr ED & Takahashi JS (2013) Molecular components of the mammalian circadian clock. In Circadian clocks pp. 3–27. Springer; Berlin Heidelberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox KH & Takahashi JS (2019) Circadian clock genes and the transcriptional architecture of the clock mechanism. Journal of molecular endocrinology 63, R93–R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hastings MH, Maywood ES & Brancaccio M (2018) Generation of circadian rhythms in the suprachiasmatic nucleus. Nature Reviews Neuroscience 19, 453–469. [DOI] [PubMed] [Google Scholar]
- 6.Shan Y, Abel JH, Li Y, Izumo M, Cox KH, Jeong B, Yoo S-H, Olson DP, Doyle FJ & Takahashi JS (2020) Dual-color single-cell imaging of the suprachiasmatic nucleus reveals a circadian role in network synchrony. Neuron 108, 164–179.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koronowski KB, Kinouchi K, Welz P-S, Smith JG, Zinna VM, Shi J, Samad M, Chen S, Magnan CN, Kinchen JM, Li W, Baldi P, Benitah SA & Sassone-Corsi P (2019) Defining the independence of the liver circadian clock. Cell 177, 1448–1462.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinturel F, Gos P, Petrenko V, Hagedorn C, Kreppel F, Storch K-F, Knutti D, Liani A, Weitz C, Emmenegger Y, Franken P, Bonacina L, Dibner C & Schibler U (2021) Circadian hepatocyte clocks keep synchrony in the absence of a master pacemaker in the suprachiasmatic nucleus or other extrahepatic clocks. Genes & Development 35, 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buhr ED, Yoo S-H & Takahashi JS (2010) Temperature as a universal resetting cue for mammalian circadian oscillators. Science 330, 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi JS (2017) Transcriptional architecture of the mammalian circadian clock. Nature reviews Genetics 18, 164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sancar A & Van Gelder RN (2021) Clocks, cancer, and chronochemotherapy. Science 371. [DOI] [PubMed] [Google Scholar]
- 12.Crnko S, Du Pré BC, Sluijter JPG & Van Laake LW (2019) Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nature Reviews Cardiology 16, 437–447. [DOI] [PubMed] [Google Scholar]
- 13.Zhang R, Lahens NF, Ballance HI, Hughes ME & Hogenesch JB (2014) A circadian gene expression atlas in mammals: Implications for biology and medicine. Proceedings of the National Academy of Sciences 111, 16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geyfman M, Kumar V, Liu Q, Ruiz R, Gordon W, Espitia F, Cam E, Millar SE, Smyth P, Ihler A, Takahashi JS & Andersen B (2012) Brain and muscle arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proceedings of the National Academy of Sciences 109, 11758–11763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plikus MV, Van Spyk EN, Pham K, Geyfman M, Kumar V, Takahashi JS & Andersen B (2015) The circadian clock in skin: Implications for adult stem cells, tissue regeneration, cancer, aging, and immunity. Journal of biological rhythms 30, 163–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanioka M, Yamada H, Doi M, Bando H, Yamaguchi Y, Nishigori C & Okamura H (2009) Molecular clocks in mouse skin. Journal of Investigative Dermatology 129, 1225–1231. [DOI] [PubMed] [Google Scholar]
- 17.Welz P-S, Zinna VM, Symeonidi A, Koronowski KB, Kinouchi K, Smith JG, Guillén IM, Castellanos A, Furrow S, Aragón F, Crainiciuc G, Prats N, Caballero JM, Hidalgo A, Sassone-Corsi P & Benitah SA (2019) BMAL1-driven tissue clocks respond independently to light to maintain homeostasis. Cell 177, 1436–1447.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suh S, Choi EH & Mesinkovska NA (2020) The expression of opsins in the human skin and its implications for photobiomodulation: A systematic review. Photodermatology, Photoimmunology & Photomedicine 36, 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buhr ED, Vemaraju S, Diaz N, Lang RA & Gelder RNV (2019) Neuropsin (OPN5) mediates local light-dependent induction of circadian clock genes and circadian photoentrainment in exposed murine skin. Current Biology 29, 3478–3487.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stringari C, Wang H, Geyfman M, Crosignani V, Kumar V, Takahashi JS, Andersen B & Gratton E (2015) In vivo single-cell detection of metabolic oscillations in stem cells. Cell Reports 10, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Segalla L, Chirumbolo S & Sbarbati A (2021) Dermal white adipose tissue: Much more than a metabolic, lipid-storage organ? Tissue and Cell 71, 101583. [DOI] [PubMed] [Google Scholar]
- 22.Wu G, Ruben MD, Francey LJ, Smith DF, Sherrill JD, Oblong JE, Mills KJ & Hogenesch JB (2020) A population-based gene expression signature of molecular clock phase from a single epidermal sample. Genome Medicine 12, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Froy O & Garaulet M (2018) The circadian clock in white and brown adipose tissue: Mechanistic, endocrine, and clinical aspects. Endocrine reviews 39, 261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lekkas D & Paschos GK (2019) The circadian clock control of adipose tissue physiology and metabolism. Autonomic Neuroscience: Basic and Clinical 219, 66–70. [DOI] [PubMed] [Google Scholar]
- 25.Straat ME, Hogenboom R, Boon MR, Rensen PCN & Kooijman S (2021) Circadian control of brown adipose tissue. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 1866, 158961. [DOI] [PubMed] [Google Scholar]
- 26.Schneider MR, Schmidt-Ullrich R & Paus R (2009) The hair follicle as a dynamic miniorgan. Current Biology 19, R132–R142. [DOI] [PubMed] [Google Scholar]
- 27.Morgan BA (2014) The dermal papilla: An instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harbor perspectives in medicine 4, a015180–a015180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plikus MV, Vollmers C, Cruz D de la, Chaix A, Ramos R, Panda S & Chuong C-M (2013) Local circadian clock gates cell cycle progression of transient amplifying cells during regenerative hair cycling. Proceedings of the National Academy of Sciences 110, E2106–E2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe M, Hida A, Kitamura S, Enomoto M, Ohsawa Y, Katayose Y, Nozaki K, Moriguchi Y, Aritake S, Higuchi S, Tamura M, Kato M & Mishima K (2012) Rhythmic expression of circadian clock genes in human leukocytes and beard hair follicle cells. Biochemical and Biophysical Research Communications 425, 902–907. [DOI] [PubMed] [Google Scholar]
- 30.Al-Nuaimi Y, Hardman JA, Bíró T, Haslam IS, Philpott MP, Tóth BI, Farjo N, Farjo B, Baier G, Watson REB, Grimaldi B, Kloepper JE & Paus R (2014) A meeting of two chronobiological systems: Circadian proteins Period1 and BMAL1 modulate the human hair cycle clock. Journal of Investigative Dermatology 134, 610–619. [DOI] [PubMed] [Google Scholar]
- 31.Hardman JA, Tobin DJ, Haslam IS, Farjo N, Farjo B, Al-Nuaimi Y, Grimaldi B & Paus R (2015) The peripheral clock regulates human pigmentation. Journal of Investigative Dermatology 135, 1053–1064. [DOI] [PubMed] [Google Scholar]
- 32.Ali M, Zare M, Zarrintaj P, Alizadeh E, Taghiabadi E, Heidari-Kharaji M, Amirkhani MA, Saeb MR & Mozafari M (2019) Engineering the niche for hair regeneration a critical review. Nanomedicine: Nanotechnology, Biology and Medicine 15, 70–85. [DOI] [PubMed] [Google Scholar]
- 33.Lin KK, Kumar V, Geyfman M, Chudova D, Ihler AT, Smyth P, Paus R, Takahashi JS & Andersen B (2009) Circadian clock genes contribute to the regulation of hair follicle cycling. PLoS Genetics 5, e1000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geyfman M & Andersen B (2010) Clock genes, hair growth and aging. Aging 2, 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janich P, Pascual G, Merlos-Suárez A, Batlle E, Ripperger J, Albrecht U, Cheng H-YM, Obrietan K, Croce LD & Benitah SA (2011) The circadian molecular clock creates epidermal stem cell heterogeneity. Nature 480, 209–214. [DOI] [PubMed] [Google Scholar]
- 36.Pasparakis M, Haase I & Nestle FO (2014) Mechanisms regulating skin immunity and inflammation. Nature Reviews Immunology 14, 289–301. [DOI] [PubMed] [Google Scholar]
- 37.Schauber J & Gallo RL (2008) Antimicrobial peptides and the skin immune defense system. The Journal of allergy and clinical immunology 122, 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen AV & Soulika AM (2019) The dynamics of the skin’s immune system. International journal of molecular sciences 20, 1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdallah F, Mijouin L & Pichon C (2017) Skin immune landscape: Inside and outside the organism. Mediators of inflammation 2017, 5095293–5095293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenberg EN, Marshall ME, Jin S, Venkatesh S, Dragan M, Tsoi LC, Gudjonsson JE, Nie Q, Takahashi JS & Andersen B (2020) Circadian control of interferon-sensitive gene expression in murine skin. Proceedings of the National Academy of Sciences of the United States of America 117, 5761–5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ando N, Nakamura Y, Aoki R, Ishimaru K, Ogawa H, Okumura K, Shibata S, Shimada S & Nakao A (2015) Circadian gene clock regulates psoriasis-like skin inflammation in mice. The Journal of investigative dermatology 135, 3001–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamia KA, Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, Jonker JW, Downes M & Evans RM (2011) Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 480, 552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Labrecque N & Cermakian N (2015) Circadian clocks in the immune system. Journal of Biological Rhythms 30, 277–290. [DOI] [PubMed] [Google Scholar]
- 44.Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, Taniguchi N, Ohno H & Kizaki T (2014) A circadian clock gene, rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of <em>Ccl2</em> expression. The Journal of Immunology 192, 407. [DOI] [PubMed] [Google Scholar]
- 45.Baumann A, Gönnenwein S, Bischoff SC, Sherman H, Chapnik N, Froy O & Lorentz A (2013) The circadian clock is functional in eosinophils and mast cells. Immunology 140, 465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura Y, Nakano N, Ishimaru K, Hara M, Ikegami T, Tahara Y, Katoh R, Ogawa H, Okumura K, Shibata S, Nishiyama C & Nakao A (2014) Circadian regulation of allergic reactions by the mast cell clock in mice. Journal of Allergy and Clinical Immunology 133, 568–575.e12. [DOI] [PubMed] [Google Scholar]
- 47.Kiessling S, Dubeau-Laramée G, Ohm H, Labrecque N, Olivier M & Cermakian N (2017) The circadian clock in immune cells controls the magnitude of leishmania parasite infection. Scientific Reports 7, 10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshioka D, Ando H, Ushijima K, Kumazaki M & Fujimura A (2018) Chronotherapy of maxacalcitol on skin inflammation induced by topical 12-o-tetradecanoylphorbol-13-acetate in mice. Chronobiology International 35, 1269–1280. [DOI] [PubMed] [Google Scholar]
- 49.Liang X & FitzGerald GA (2017) Timing the microbes: The circadian rhythm of the gut microbiome. Journal of Biological Rhythms 32, 505–515. [DOI] [PubMed] [Google Scholar]
- 50.Voigt RM, Forsyth CB, Green SJ, Engen PA & Keshavarzian A (2016) Chapter nine - circadian rhythm and the gut microbiome. In International review of neurobiology pp. 193–205. Academic Press. [DOI] [PubMed] [Google Scholar]
- 51.Sarkar S, Porter KI, Dakup PP, Gajula RP, Koritala BSC, Hylton R, Kemp MG, Wakamatsu K & Gaddameedhi S (2021) Circadian clock protein BMAL1 regulates melanogenesis through MITF in melanoma cells. Pigment Cell & Melanoma Research n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng D, Ratiner K & Elinav E (2020) Circadian influences of diet on the microbiome and immunity. Trends in Immunology 41, 512–530. [DOI] [PubMed] [Google Scholar]
- 53.Zarrinpar A, Chaix A, Yooseph S & Panda S (2014) Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell metabolism 20, 1006–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilkins D, Tong X, Leung MHY, Mason CE & Lee PKH (2021) Diurnal variation in the human skin microbiome affects accuracy of forensic microbiome matching. Microbiome 9, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang H, Spyk E van, Liu Q, Geyfman M, Salmans ML, Kumar V, Ihler A, Li N, Takahashi JS & Andersen B (2017) Time-restricted feeding shifts the skin circadian clock and alters UVB-induced DNA damage. Cell reports 20, 1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crosby P, Hamnett R, Putker M, Hoyle NP, Reed M, Karam CJ, Maywood ES, Stangherlin A, Chesham JE, Hayter EA, Rosenbrier-Ribeiro L, Newham P, Clevers H, Bechtold DA & O’Neill JS (2019) Insulin/IGF-1 drives PERIOD synthesis to entrain circadian rhythms with feeding time. Cell 177, 896–909.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kajimoto J, Matsumura R, Node K & Akashi M (2018) Potential role of the pancreatic hormone insulin in resetting human peripheral clocks. Genes to Cells 23, 393–399. [DOI] [PubMed] [Google Scholar]
- 58.Forni MF, Peloggia J, Braga TT, Chinchilla JEO, Shinohara J, Navas CA, Camara NOS & Kowaltowski AJ (2017) Caloric restriction promotes structural and metabolic changes in the skin. Cell Reports 20, 2678–2692. [DOI] [PubMed] [Google Scholar]
- 59.Solanas G, Peixoto FO, Perdiguero E, Jardí M, Ruiz-Bonilla V, Datta D, Symeonidi A, Castellanos A, Welz P-S, Caballero JM, Sassone-Corsi P, Muñoz-Cánoves P & Benitah SA (2017) Aged stem cells reprogram their daily rhythmic functions to adapt to stress. Cell 170, 678–692.e20. [DOI] [PubMed] [Google Scholar]
- 60.Morinaga H, Mohri Y, Grachtchouk M, Asakawa K, Matsumura H, Oshima M, Takayama N, Kato T, Nishimori Y, Sorimachi Y, Takubo K, Suganami T, Iwama A, Iwakura Y, Dlugosz AA & Nishimura EK (2021) Obesity accelerates hair thinning by stem cell-centric converging mechanisms. Nature 595, 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simões MCF, Sousa JJS & Pais AACC (2015) Skin cancer and new treatment perspectives: A review. Cancer Letters 357, 8–42. [DOI] [PubMed] [Google Scholar]
- 62.Lubov J, Cvammen W & Kemp M (2021) The impact of the circadian clock on skin physiology and cancer development. International Journal of Molecular Sciences 22, 6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dakup P & Gaddameedhi S (2017) Impact of the circadian clock on UV-induced DNA damage response and photocarcinogenesis. Photochemistry and photobiology 93, 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen NT & Fisher DE (2019) MITF and UV responses in skin: From pigmentation to addiction. Pigment cell & melanoma research 32, 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yousef E, Mitwally N, Noufal N & Tahir MR (2020) Shift work and risk of skin cancer: A systematic review and meta-analysis. Scientific Reports 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gaddameedhi S, Selby CP, Kaufmann WK, Smart RC & Sancar A (2011) Control of skin cancer by the circadian rhythm. Proceedings of the National Academy of Sciences of the United States of America 108, 18790–18795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sancar A, Lindsey-Boltz LA, Kang T-H, Reardon JT, Lee JH & Ozturk N (2010) Circadian clock control of the cellular response to DNA damage. FEBS letters 584, 2618–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sawada Y, Saito-Sasaki N, Mashima E & Nakamura M (2021) Daily lifestyle and inflammatory skin diseases. International journal of molecular sciences 22, 5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gaddameedhi S, Selby CP, Kemp MG, Ye R & Sancar A (2015) The circadian clock controls sunburn apoptosis and erythema in mouse skin. The Journal of investigative dermatology 135, 1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sarkar S & Gaddameedhi S (2020) Solar ultraviolet-induced DNA damage response: Melanocytes story in transformation to environmental melanomagenesis. Environmental and Molecular Mutagenesis 61, 736–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sarkar S, Porter KI, Dakup PP, Gajula RP, Koritala BSC, Hylton R, Kemp MG, Wakamatsu K & Gaddameedhi S (2021) Circadian clock protein BMAL1 regulates melanogenesis through MITF in melanoma cells. Pigment Cell & Melanoma Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Masri S, Kinouchi K & Sassone-Corsi P (2015) Circadian clocks, epigenetics, and cancer. Current Opinion in Oncology 27, 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gutierrez D & Arbesman J (2016) Circadian dysrhythmias, physiological aberrations, and the link to skin cancer. International Journal of Molecular Sciences 17, 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Assis LVM de, Kinker GS, Moraes MN, Markus RP, Fernandes PA & Castrucci AM de L (2018) Expression of the circadian clock gene BMAL1 positively correlates with antitumor immunity and patient survival in metastatic melanoma. Frontiers in oncology 8, 185–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Relógio A, Thomas P, Medina-Pérez P, Reischl S, Bervoets S, Gloc E, Riemer P, Mang-Fatehi S, Maier B, Schäfer R, Leser U, Herzel H, Kramer A & Sers C (2014) Ras-mediated deregulation of the circadian clock in cancer. PLoS Genetics 10, e1004338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Assis LVM de, Moraes MN, Magalhães-Marques KK, Kinker GS, Silveira Cruz-Machado S da & Castrucci AM de L (2018) Non-metastatic cutaneous melanoma induces chronodisruption in central and peripheral circadian clocks. International journal of molecular sciences 19, 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang X, Tang J, Xing L, Shi G, Ruan H, Gu X, Liu Z, Wu X, Gao X & Xu Y (2010) Interaction of MAGED1 with nuclear receptors affects circadian clock function. The EMBO Journal 29, 1389–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carias KV, Zoeteman M, Seewald A, Sanderson MR, Bischof JM & Wevrick R (2020) A MAGEL2-deubiquitinase complex modulates the ubiquitination of circadian rhythm protein CRY1. PLOS ONE 15, e0230874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zagni C, Almeida LO, Balan T, Martins MT, Rosselli-Murai LK, Papagerakis P, Castilho RM & Squarize CH (2017) PTEN mediates activation of core clock protein BMAL1 and accumulation of epidermal stem cells. Stem cell reports 9, 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alexander RK, Liou Y-H, Knudsen NH, Starost KA, Xu C, Hyde AL, Liu S, Jacobi D, Liao N-S & Lee C-H (2020) Bmal1 integrates mitochondrial metabolism and macrophage activation. eLife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aiello I, Fedele MLM, Román F, Marpegan L, Caldart C, Chiesa JJ, Golombek DA, Finkielstein CV & Paladino N (2020) Circadian disruption promotes tumor-immune microenvironment remodeling favoring tumor cell proliferation. Science advances 6, eaaz4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kowalska E, Ripperger JA, Hoegger DC, Bruegger P, Buch T, Birchler T, Mueller A, Albrecht U, Contaldo C & Brown SA (2013) NONO couples the circadian clock to the cell cycle. Proceedings of the National Academy of Sciences of the United States of America 110, 1592–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Franzoni A, Markova-Car E, Dević-Pavlić S, Jurišić D, Puppin C, Mio C, Luca MD, Petruz G, Damante G & Pavelić SK (2017) A polymorphic GGC repeat in the NPAS2 gene and its association with melanoma. Experimental Biology and Medicine 242, 1553–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khapre RV, Kondratova AA, Susova O & Kondratov RV (2011) Circadian clock protein BMAL1 regulates cellular senescence in vivo. Cell Cycle 10, 4162–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kiessling S, Beaulieu-Laroche L, Blum ID, Landgraf D, Welsh DK, Storch K-F, Labrecque N & Cermakian N (2017) Enhancing circadian clock function in cancer cells inhibits tumor growth. BMC Biology 15, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sulli G, Rommel A, Wang X, Kolar MJ, Puca F, Saghatelian A, Plikus MV, Verma IM & Panda S (2018) Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 553, 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dakup PP, Porter KI, Little AA, Gajula RP, Zhang H, Skornyakov E, Kemp MG, Van Dongen HPA & Gaddameedhi S (2018) The circadian clock regulates cisplatin-induced toxicity and tumor regression in melanoma mouse and human models. Oncotarget 9, 14524–14538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Assis LVM de, Tonolli PN, Moraes MN, Baptista MS & Lauro Castrucci AM de (2021) How does the skin sense sun light? An integrative view of light sensing molecules. Journal of Photochemistry and Photobiology C: Photochemistry Reviews 47, 100403. [Google Scholar]
- 89.Nikkola V, Grönroos M, Huotari-Orava R, Kautiainen H, Ylianttila L, Karppinen T, Partonen T & Snellman E (2018) Circadian time effects on NB-UVB–induced erythema in human skin in vivo. Journal of Investigative Dermatology 138, 464–467. [DOI] [PubMed] [Google Scholar]
- 90.Healy E, Reynolds NJ, Smith MD, Campbell C, Farr PM & Rees JL (1994) Dissociation of erythema and p53 protein expression in human skin following UVB irradiation, and induction of p53 protein and mRNA following application of skin irritants. Journal of Investigative Dermatology 103, 493–499. [DOI] [PubMed] [Google Scholar]
- 91.Geyfman M, Plikus MV, Treffeisen E, Andersen B & Paus R (2015) Resting no more: Re-defining telogen, the maintenance stage of the hair growth cycle. Biological reviews of the Cambridge Philosophical Society 90, 1179–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vary JC (2015) Selected disorders of skin appendagesacne, alopecia, hyperhidrosis. Medical Clinics of North America 99, 1195–1211. [DOI] [PubMed] [Google Scholar]
- 93.Ramos-e-Silva M, Chaves Azevedo-e-Silva M & Carneiro SC (2008) Hair, nail, and pigment changes in major systemic disease. Clinics in Dermatology 26, 296–305. [DOI] [PubMed] [Google Scholar]
- 94.Hood S & Amir S (2017) The aging clock: Circadian rhythms and later life. Journal of Clinical Investigation 127, 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Buijink MR, Engberink AHOO, Wit CB, Almog A, Meijer JH, Rohling JHT & Michel S (2020) Aging affects the capacity of photoperiodic adaptation downstream from the central molecular clock. Journal of Biological Rhythms 35, 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Benitah SA & Welz P-S (2020) Circadian regulation of adult stem cell homeostasis and aging. Cell Stem Cell 26, 817–831. [DOI] [PubMed] [Google Scholar]
- 97.Tahara Y, Takatsu Y, Shiraishi T, Kikuchi Y, Yamazaki M, Motohashi H, Muto A, Sasaki H, Haraguchi A, Kuriki D, Nakamura TJ & Shibata S (2017) Age-related circadian disorganization caused by sympathetic dysfunction in peripheral clock regulation. npj Aging and Mechanisms of Disease 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bonté F, Girard D, Archambault J-C & Desmoulière A (2019) Skin changes during ageing. In Subcellular biochemistry pp. 249–280. Springer; Singapore. [DOI] [PubMed] [Google Scholar]
- 99.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV & Antoch MP (2006) Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes & development 20, 1868–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dubrovsky YV, Samsa WE & Kondratov RV (2010) Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging 2, 936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sandu C, Liu T, Malan A, Challet E, Pévet P & Felder-Schmittbuhl M-P (2015) Circadian clocks in rat skin and dermal fibroblasts: Differential effects of aging, temperature and melatonin. Cellular and Molecular Life Sciences 72, 2237–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu Y, Zhang X, Liu F, Zhu P, Zhang L, Peng Y, Yan X, Li Y, Hua P, Liu C, Li Q & Zhang L (2021) A stress-induced miR-31–CLOCK–ERK pathway is a key driver and therapeutic target for skin aging. Nature Aging. [DOI] [PubMed] [Google Scholar]
- 103.Slominski AT, Hardeland R, Zmijewski MA, Slominski RM, Reiter RJ & Paus R (2018) Melatonin: A cutaneous perspective on its production, metabolism, and functions. Journal of Investigative Dermatology 138, 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Santoro R, Mori F, Marani M, Grasso G, Cambria MA, Blandino G, Muti P & Strano S (2013) Blockage of melatonin receptors impairs p53-mediated prevention of DNA damage accumulation. Carcinogenesis 34, 1051–1061. [DOI] [PubMed] [Google Scholar]
- 105.Dong K, Goyarts E, Rella A, Pelle E, Wong YH & Pernodet N (2020) Age associated decrease of MT-1 melatonin receptor in human dermal skin fibroblasts impairs protection against UV-induced DNA damage. International journal of molecular sciences 21, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Di Meglio P, Perera GK & Nestle FO (2011) The multitasking organ: Recent insights into skin immune function. Immunity 35, 857–869. [DOI] [PubMed] [Google Scholar]
- 107.Scheiermann C, Kunisaki Y & Frenette PS (2013) Circadian control of the immune system. Nature reviews Immunology 13, 190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sundar IK, Yao H, Sellix MT & Rahman I (2015) Circadian molecular clock in lung pathophysiology. American journal of physiology Lung cellular and molecular physiology 309, L1056–L1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sengupta S, Tang SY, Devine JC, Anderson ST, Nayak S, Zhang SL, Valenzuela A, Fisher DG, Grant GR, López CB & FitzGerald GA (2019) Circadian control of lung inflammation in influenza infection. Nature communications 10, 4107–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen L, Li S, Nie J, Zhao J, Yu S, Li Y & Peng J (2020) Bmal1 regulates coagulation factor biosynthesis in mouse liver in streptococcus oralis infection. Frontiers in cellular and infection microbiology 10, 530190–530190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bellet MM, Deriu E, Liu JZ, Grimaldi B, Blaschitz C, Zeller M, Edwards RA, Sahar S, Dandekar S, Baldi P, George MD, Raffatellu M & Sassone-Corsi P (2013) Circadian clock regulates the host response to salmonella. Proceedings of the National Academy of Sciences of the United States of America 110, 9897–9902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhuang X, Rambhatla SB, Lai AG & McKeating JA (2017) Interplay between circadian clock and viral infection. Journal of molecular medicine (Berlin, Germany) 95, 1283–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bilska B, Zegar A, Slominski AT, Kleszczyński K, Cichy J & Pyza E (2020) Expression of antimicrobial peptide genes oscillates along day/night rhythm protecting mice skin from bacteria. Experimental Dermatology n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Edgar RS, Stangherlin A, Nagy AD, Nicoll MP, Efstathiou S, O’Neill JS & Reddy AB (2016) Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proceedings of the National Academy of Sciences 113, 10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ehlers A, Xie W, Agapov E, Brown S, Steinberg D, Tidwell R, Sajol G, Schutz R, Weaver R, Yu H, Castro M, Bacharier LB, Wang X, Holtzman MJ & Haspel JA (2018) BMAL1 links the circadian clock to viral airway pathology and asthma phenotypes. Mucosal immunology 11, 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kettner NM, Voicu H, Finegold MJ, Coarfa C, Sreekumar A, Putluri N, Katchy CA, Lee C, Moore DD & Fu L (2016) Circadian homeostasis of liver metabolism suppresses hepatocarcinogenesis. Cancer Cell 30, 909–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lu F, Suggs A, Ezaldein HH, Ya J, Fu P, Jamora J, Verallo-Rowel V & Baron ED (2019) The effect of shift work and poor sleep on self-reported skin conditions: A survey of call center agents in the philippines. Clocks & sleep 1, 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li W-Q, Qureshi AA, Schernhammer ES & Han J (2013) Rotating night-shift work and risk of psoriasis in US women. The Journal of investigative dermatology 133, 565–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ferguson FJ, Lada G, Hunter HJA, Bundy C, Henry AL, Griffiths CEM & Kleyn CE (2021) Diurnal and seasonal variation in psoriasis symptoms. Journal of the European Academy of Dermatology and Venereology 35, e45–e47. [DOI] [PubMed] [Google Scholar]
- 120.Tas B & Kabeloglu V (2021) Prevalence of metabolic syndrome and its parameters and their correlations with psoriasis duration, severity, and sleep quality in psoriasis patients: A cross-sectional study. Dermatology Practical & Conceptual, 2021049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rodrigues GD, Fiorelli EM, Furlan L, Montano N & Tobaldini E (2021) Obesity and sleep disturbances: The “chicken or the egg” question. European Journal of Internal Medicine. [DOI] [PubMed] [Google Scholar]
- 122.Nowowiejska J, Baran A & Flisiak I (2021) Aberrations in lipid expression and metabolism in psoriasis. International Journal of Molecular Sciences 22, 6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu Z, Gong Y, Cui L, Hu Y, Zhou Q, Chen Z, Yu Y, Chen Y, Xu P, Zhang X, Guo C & Shi Y (2020) High-throughput transcriptome and pathogenesis analysis of clinical psoriasis. Journal of Dermatological Science 98, 109–118. [DOI] [PubMed] [Google Scholar]
- 124.Yosipovitch G, Goon ATJ, Wee J, Chan YH, Zucker I & Goh CL (2002) Itch characteristics in chinese patients with atopic dermatitis using a new questionnaire for the assessment of pruritus. International Journal of Dermatology 41, 212–216. [DOI] [PubMed] [Google Scholar]
- 125.Chang Y-S & Chiang B-L (2016) Mechanism of sleep disturbance in children with atopic dermatitis and the role of the circadian rhythm and melatonin. International Journal of Molecular Sciences 17, 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hiramoto K, Orita K, Yamate Y, Kasahara E, Yokoyama S & Sato EF (2018) The clock genes are involved in the deterioration of atopic dermatitis after day-and-night reversed physical stress in NC/nga mice. The open biochemistry journal 12, 87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mizutani H, Tamagawa-Mineoka R, Minami Y, Yagita K & Katoh N (2017) Constant light exposure impairs immune tolerance development in mice. Journal of Dermatological Science 86, 63–70. [DOI] [PubMed] [Google Scholar]
- 128.Takita E, Yokota S, Tahara Y, Hirao A, Aoki N, Nakamura Y, Nakao A & Shibata S (2013) Biological clock dysfunction exacerbates contact hypersensitivity in mice. British Journal of Dermatology 168, 39–46. [DOI] [PubMed] [Google Scholar]
- 129.Martin P & Nunan R (2015) Cellular and molecular mechanisms of repair in acute and chronic wound healing. British Journal of Dermatology 173, 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rodrigues M, Kosaric N, Bonham CA & Gurtner GC (2019) Wound healing: A cellular perspective. Physiological reviews 99, 665–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Budkowska M, Lebiecka A, Marcinowska Z, Woźniak J, Jastrzębska M & Dołęgowska B (2019) The circadian rhythm of selected parameters of the hemostasis system in healthy people. Thrombosis Research 182, 79–88. [DOI] [PubMed] [Google Scholar]
- 132.Hoyle NP, Seinkmane E, Putker M, Feeney KA, Krogager TP, Chesham JE, Bray LK, Thomas JM, Dunn K, Blaikley J & O’Neill JS (2017) Circadian actin dynamics drive rhythmic fibroblast mobilization during wound healing. Science translational medicine 9, eaa12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cable EJ, Onishi KG & Prendergast BJ (2017) Circadian rhythms accelerate wound healing in female siberian hamsters. Physiology & behavior 171, 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Walker William H 2nd, Meléndez-Fernández OH & Nelson RJ (2019) Prior exposure to dim light at night impairs dermal wound healing in female C57BL/6 mice. Archives of dermatological research 311, 573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sasaki H, Hokugo A, Wang L, Morinaga K, Ngo JT, Okawa H & Nishimura I (2020) Neuronal PAS domain 2 (Npas2)-deficient fibroblasts accelerate skin wound healing and dermal collagen reconstruction. Anatomical record (Hoboken, NJ : 2007) 303, 1630–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jacob H, Curtis AM & Kearney CJ (2020) Therapeutics on the clock: Circadian medicine in the treatment of chronic inflammatory diseases. Biochemical Pharmacology 182, 114254. [DOI] [PubMed] [Google Scholar]
- 137.Sulli G, Manoogian ENC, Taub PR & Panda S (2018) Training the circadian clock, clocking the drugs, and drugging the clock to prevent, manage, and treat chronic diseases. Trends in pharmacological sciences 39, 812–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Deota S & Panda S (2021) New horizons: Circadian control of metabolism offers novel insight into the cause and treatment of metabolic diseases. The Journal of Clinical Endocrinology & Metabolism 106, e1488–e1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bree LCJ de, Mourits VP, Koeken VA, Moorlag SJ, Janssen R, Folkman L, Barreca D, Krausgruber T, Fife-Gernedl V, Novakovic B, Arts RJ, Dijkstra H, Lemmers H, Bock C, Joosten LA, Crevel R van, Benn CS & Netea MG (2020) Circadian rhythm influences induction of trained immunity by BCG vaccination. The Journal of clinical investigation 130, 5603–5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Long JE, Drayson MT, Taylor AE, Toellner KM, Lord JM & Phillips AC (2016) Morning vaccination enhances antibody response over afternoon vaccination: A cluster-randomised trial. Vaccine 34, 2679–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang H, Liu Y, Liu D, Zeng Q, Li L, Zhou Q, Li M, Mei J, Yang N, Mo S, Liu Q, Liu M, Peng S & Xiao H (2021) Time of day influences immune response to an inactivated vaccine against SARS-CoV-2. Cell Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Montaigne D, Marechal X, Modine T, Coisne A, Mouton S, Fayad G, Ninni S, Klein C, Ortmans S, Seunes C, Potelle C, Berthier A, Gheeraert C, Piveteau C, Deprez R, Eeckhoute J, Duez H, Lacroix D, Deprez B, Jegou B, Koussa M, Edme J-L, Lefebvre P & Staels B (2018) Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by rev-erbα antagonism: A single-centre propensity-matched cohort study and a randomised study. The Lancet 391, 59–69. [DOI] [PubMed] [Google Scholar]
- 143.Al-Waeli H, Nicolau B, Stone L, Abu Nada L, Gao Q, Abdallah MN, Abdulkader E, Suzuki M, Mansour A, Al Subaie A & Tamimi F (2020) Chronotherapy of non-steroidal anti-inflammatory drugs may enhance postoperative recovery. Scientific Reports 10, 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sulli G, Lam MTY & Panda S (2019) Interplay between circadian clock and cancer: New frontiers for cancer treatment. Trends in cancer 5, 475–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ashok Kumar PV, Dakup PP, Sarkar S, Modasia JB, Motzner MS & Gaddameedhi S (2019) It’s about time: Advances in understanding the circadian regulation of DNA damage and repair in carcinogenesis and cancer treatment outcomes. The Yale journal of biology and medicine 92, 305–316. [PMC free article] [PubMed] [Google Scholar]
- 146.Khosravipour M, Khanlari P, Khazaie S, Khosravipour H & Khazaie H (2021) A systematic review and meta-analysis of the association between shift work and metabolic syndrome: The roles of sleep, gender, and type of shift work. Sleep Medicine Reviews 57, 101427. [DOI] [PubMed] [Google Scholar]
- 147.Bracci M, Ciarapica V, Copertaro A, Barbaresi M, Manzella N, Tomasetti M, Gaetani S, Monaco F, Amati M, Valentino M, Rapisarda V & Santarelli L (2016) Peripheral skin temperature and circadian biological clock in shift nurses after a day off. International Journal of Molecular Sciences 17, 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hattammaru M, Tahara Y, Kikuchi T, Okajima K, Konishi K, Nakajima S, Sato K, Otsuka K, Sakura H, Shibata S & Nakaoka T (2019) The effect of night shift work on the expression of clock genes in beard hair follicle cells. Sleep Medicine 56, 164–170. [DOI] [PubMed] [Google Scholar]
- 149.Yu SH, Attarian H, Zee P & Silverberg JI (2016) Burden of sleep and fatigue in US adults with atopic dermatitis. Dermatitis 27, 50–58. [DOI] [PubMed] [Google Scholar]
- 150.Dakup PP, Porter KI & Gaddameedhi S (2020) The circadian clock protects against acute radiation-induced dermatitis. Toxicology and Applied Pharmacology 399, 115040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bragazzi NL, Sellami M, Salem I, Conic R, Kimak M, Pigatto PDM & Damiani G (2019) Fasting and its impact on skin anatomy, physiology, and physiopathology: A comprehensive review of the literature. Nutrients 11, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Damiani Mahroum, Pigatto Pacifico, Malagoli Tiodorovic, Conic Amital, Bragazzi Watad & Adawi (2019) The safety and impact of a model of intermittent, time-restricted circadian fasting (“ramadan fasting”) on hidradenitis suppurativa: Insights from a multicenter, observational, cross-over, pilot, exploratory study. Nutrients 11, 1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bragazzi NL, Trabelsi K, Garbarino S, Ammar A, Chtourou H, Pacifico A, Malagoli P, Kocic H, Conic RRZ, Kridin K, Pigatto PDM & and GD (2021) Can intermittent, time-restricted circadian fasting modulate cutaneous severity of dermatological disorders? Insights from a multicenter, observational, prospective study. Dermatologic Therapy 34. [DOI] [PubMed] [Google Scholar]
- 154.Davis K, Roden LC, Leaner VD & van der Watt PJ (2019) The tumour suppressing role of the circadian clock. IUBMB Life 71, 771–780. [DOI] [PubMed] [Google Scholar]
- 155.Chang Y-S, Chou Y-T, Lee J-H, Lee P-L, Dai Y-S, Sun C, Lin Y-T, Wang L-C, Yu H-H, Yang Y-H, Chen C-A, Wan K-S & Chiang B-L (2014) Atopic dermatitis, melatonin, and sleep disturbance. PEDIATRICS 134, e397–e405. [DOI] [PubMed] [Google Scholar]
- 156.Vaughn AR, Clark AK, Sivamani RK & Shi VY (2018) Circadian rhythm in atopic dermatitis—pathophysiology and implications for chronotherapy. Pediatric Dermatology 35, 152–157. [DOI] [PubMed] [Google Scholar]
- 157.Koren S, Whorton Elbert B. Jr. & Fleischmann W. Robert Jr. (1993) Circadian dependence of interferon antitumor activity in mice. JNCI: Journal of the National Cancer Institute 85, 1927–1932. [DOI] [PubMed] [Google Scholar]
- 158.Shuboni-Mulligan DD, Breton G, Smart D, Gilbert M & Armstrong TS (2019) Radiation chronotherapy-clinical impact of treatment time-of-day: A systematic review. Journal of neuro-oncology 145, 415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gamble KL, Berry R, Frank SJ & Young ME (2014) Circadian clock control of endocrine factors. Nature Reviews Endocrinology 10, 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Matsunaga N, Itcho K, Hamamura K, Ikeda E, Ikeyama H, Furuichi Y, Watanabe M, Koyanagi S & Ohdo S (2014) 24-hour rhythm of aquaporin-3 function in the epidermis is regulated by molecular clocks. Journal of Investigative Dermatology 134, 1636–1644. [DOI] [PubMed] [Google Scholar]
- 161.Plikus MV & Andersen B (2018) Skin as a window to body-clock time. Proceedings of the National Academy of Sciences 115, 12095–12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kennaway DJ (2019) A critical review of melatonin assays: Past and present. Journal of Pineal Research 67, e12572. [DOI] [PubMed] [Google Scholar]
- 163.Wu G, Ruben MD, Schmidt RE, Francey LJ, Smith DF, Anafi RC, Hughey JJ, Tasseff R, Sherrill JD, Oblong JE, Mills KJ & Hogenesch JB (2018) Population-level rhythms in human skin with implications for circadian medicine. Proceedings of the National Academy of Sciences 115, 12313–12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wittenbrink N, Ananthasubramaniam B, Münch M, Koller B, Maier B, Weschke C, Bes F, Zeeuw J de, Nowozin C, Wahnschaffe A, Wisniewski S, Zaleska M, Bartok O, Ashwal-Fluss R, Lammert H, Herzel H, Hummel M, Kadener S, Kunz D & Kramer A (2018) High-accuracy determination of internal circadian time from a single blood sample. The Journal of clinical investigation 128, 3826–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Matsumura R, Node K & Akashi M (2016) Estimation methods for human circadian phase by use of peripheral tissues. Hypertension Research 39, 623–627. [DOI] [PubMed] [Google Scholar]
- 166.Roche VP, Mohamad-Djafari A, Innominato PF, Karaboué A, Gorbach A & Lévi FA (2014) Thoracic surface temperature rhythms as circadian biomarkers for cancer chronotherapy. Chronobiology international 31, 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Laing EE, Möller-Levet CS, Poh N, Santhi N, Archer SN & Dijk DJ (2017) Blood transcriptome based biomarkers for human circadian phase. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Braun R, Kath WL, Iwanaszko M, Kula-Eversole E, Abbott SM, Reid KJ, Zee PC & Allada R (2018) Universal method for robust detection of circadian state from gene expression. Proceedings of the National Academy of Sciences 115, E9247–E9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Upasham S & Prasad S (2020) SLOCK (sensor for circadian clock): Passive sweat-based chronobiology tracker. Lab Chip 20, 1947–1960. [DOI] [PubMed] [Google Scholar]
- 170.Wilkinson M, Maidstone R, Loudon A, Blaikley J, White IR, Singh D, Ray DW, Goodacre R, Fowler SJ & Durrington HJ (2019) Circadian rhythm of exhaled biomarkers in health and asthma. The European respiratory journal 54, 1901068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Akashi M, Soma H, Yamamoto T, Tsugitomi A, Yamashita S, Yamamoto T, Nishida E, Yasuda A, Liao JK & Node K (2010) Noninvasive method for assessing the human circadian clock using hair follicle cells. Proceedings of the National Academy of Sciences 107, 15643–15648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wong R, Tran V, Morhenn V, Hung S, Andersen B, Ito E, Hatfield GW & Benson NR (2004) Use of RT-PCR and DNA microarrays to characterize RNA recovered by non-invasive tape harvesting of normal and inflamed skin. Journal of Investigative Dermatology 123, 159–167. [DOI] [PubMed] [Google Scholar]


