ABSTRACT
Phytopathogenic oomycetes are known to successfully infect their hosts due to their ability to secrete effector proteins. Of interest to many researchers are effectors with the N-terminal RxLR motif (Arginine-any amino acid-Leucine-Arginine). Owing to advances in genome sequencing, we can now comprehend the high level of diversity among oomycete effectors, and similarly, their conservation within and among species referred to here as “core” RxLR effectors (CREs). Currently, there is a considerable number of CREs that have been identified in oomycetes. Functional characterization of these CREs propose their virulence role with the potential of targeting central cellular processes that are conserved across diverse plant species. We reason that effectors that are highly conserved and recognized by the host, could be harnessed in engineering plants for durable as well as broad-spectrum resistance.
KEYWORDS: Oomycetes, durable-resistance, virulence, “core” RxLR effectors (CRE), phytophthora spp
Introduction
The world’s population is growing, and it is expected that by 2050, there will be an additional 2.3 billion people, putting pressure on our agricultural systems [1,2]. With increasing food demand worldwide, it is of the utmost importance to maintain food security and increase productivity to meet these demands. On the other hand, plant diseases are a constant and devastating threat to sustainable crop production worldwide. Amongst the most notorious and economically important pathogens of crop species are plant pathogenic oomycetes.
Plants are “motionless but not defenceless”. They have developed two sophisticated immune systems, that are intertwined, to perceive as well as respond to pathogens [3–7]. The first line of defense employs cell surface pattern-recognition receptors (PRRs) to recognize microbe-associated molecular patterns (MAMPs). This leads to the activation of defenses against invading pathogens, known as pattern-triggered immunity (PTI) [3,8]. The second line of defense uses disease resistance (R)-gene products to respond to effector molecules that are secreted by pathogens to establish successful infections and suppress plant immunity [9]. Effectors are recognized by plant intracellular nucleotide binding-site leucine-rich repeat (NLR) proteins, resulting in effector-triggered immunity (ETI) responses [3,10]. Contrary to PTI, ETI is specific, more amplified, faster and leads to constant immune responses, which are revealed by a hypersensitive response (HR) or cell death [11]. Despite these advanced defense and severe selective forces by their host immunity, successful pathogens such as phyto-oomycetes alter their effector repertoire and avoid host resistance. This co-evolutionary arms race between plants and their pathogens reveals the potential of employing pathogen effector proteins to breed for durable resistance in plants [12].
Although crops can be protected from oomycetes through various management strategies including the use of “fungicides” [13,14], this leads to inflated costs of production and adverse environmental effects. In addition, oomycetes may complete several infection cycles a week on a susceptible host under optimal weather conditions, with pathogen control failure leading to rapid epidemics and crop loss. Globally, efforts aimed at sustainable agriculture are geared toward the use of environmentally friendly mechanisms such as protection through crop resistance to improve crop production.
Undisputedly, deployment of R-genes is the most effective, environmentally sound, and widely used strategy for providing disease resistance to crop plants. Although this approach has been actively used for over a century, it is unfortunate that some R-genes have been overpowered in a single season due to the evolution of new virulence traits within pathogen populations (resistance-breaking strains) [15,16]. Stacking multiple R-genes in one genotype is a promising strategy for breeding more stable and durable resistance [17,18]. Nonetheless, this is a very long and tedious process, hence most current agro-ecosystems lack this R-gene assortment to improve the durability of resistance genes. In order to effectively control plant diseases, new strategies and techniques in terms of R-gene identification, introgression, functional characterization, and field deployment are needed [19]. One of the strategies is to identify R-gene products that can recognize effectors that are highly conserved among strains of a pathogen, “core” effectors. Although the concept of “core” effectors has been well documented in bacteria [20–22] and fungi [23–27], it is yet to be formally described for oomycetes (Table 1). Therefore, this review explores the concept of highly conserved effectors, referred to here as “core” RxLR effectors (CREs) in oomycetes, their role in virulence as well as their potential application in breeding for durable resistance.
Table 1.
“Core” effectors in different groups of plant pathogens
| Group | Organism | Identified core effectors | Virulence role | Reference |
|---|---|---|---|---|
| Oomycete | Plasmopara halstedii | 354 [30] | Suppress pattern-triggered immunity and some induce hypersensitive responses | [78] |
| Hyaloperonospora arabidopsidis | 18 [4] | RXLR29 was shown to suppress pathogen-induced callose deposition | [153] | |
| Bacteria | Ralstonia solanacearum | 60–75 [32] | - | [154–156] |
| Xanthomonas arboricola | 57 [11] | - | [157] | |
| Fungi | Ustilaginoidea virens | 193 [18] | UV_1261 suppress host plant hypersensitive responses | [158–160] |
| Zymoseptoria tritici | 591 [153] | - | [161] | |
| Ustilago maydis | 467 (202) | Pep1 inhibits the activity of the apoplastic maize peroxidase POX12 Cce1 hypothesized to inhibit early plant defense responses in the apoplast Rsp3 has a conserved virulence role of protecting the fungal hyphae from maize antifungal proteins activity. Sta1 a novel core effector with virulence role through host cell-wall modification for disease progression |
[27,162–167] | |
| Colletotrichum orbiculare | necrosis-inducing secreted protein 1 (NIS1), targets conserved immune kinases hence interfering with PTI signaling | [115] |
Oomycetes RxLR effectors
Oomycetes comprise a group of successful filamentous microorganisms that threaten not only global food security but also natural ecosystems [28,29]. Most notorious among oomycete species is the hemibitrophic genus Phytophthora, also known as “the plant destroyers” [30–33]. Another group of plant devastating oomycete species is the obligate biotrophs including downy mildews, Bremia lactucae and Plasmopara viticola [34,35]. The success of these pathogens is attributed to their ability to secrete an arsenal of effectors. Oomycete genomes encode both extracellular (apoplastic) and intracellular (cytoplasmic) effectors [29]. Apoplastic effectors comprise of cell-wall degrading enzymes [36,37], elicitins [38], and protease inhibitors [39,40]. Contrary to apoplastic effectors that are secreted by the pathogen and execute their pathogenic activity outside of the host cell, cytoplasmic effectors are secreted and translocated into host cells [41,42]. To date, Arginine-any amino acid-Leucine-Arginine (RxLR), Crinkler (CRN) and cysteine, histidine, x, cycteine (CHXC) are the three classes of oomycete cytoplasmic effectors that have been identified [29,31,43].
RxLR-containing effectors represent a rapidly evolving class of effectors that are associated with the biotrophic phase of oomycetes infection [43]. This could be true since most of these effectors have been shown to be highly expressed at the early infection stage and are required for suppression of host immunity [44–49]. In addition, necrotrophic oomycetes, including Pythium species were previously thought to be lacking any RxLR-encoding genes [50–52]. Yet, Ai, Yang [53] predicted a total of 359 putative RxLR effectors from nine Pythium species. Therefore, it is possible that RxLR effectors in oomycetes share a common ancestor.
Owing to the tremendous advancements in next-generation sequencing technologies, several genomes of phytopathogenic oomycetes have been sequenced [29,31,35,54,55]. This allows a detailed analysis of existing trench-warfare scenario between pathogens through secretion of effectors and host plant-elicited defenses. To date, there are several reviews on the role of RxLR effectors in pathogen–host interaction [56–62]. We can now comprehend the high level of diversity among oomycete effectors, and similarly, their conservation within and among species also known as “core” effectors”. For us to have a better understanding on how “core” RxLR effectors can be utilized in breeding for durable resistance, it is crucial to answer the following questions: What are “core” effectors? Do the available sequenced genomes of oomycetes encode “core” RxLR effectors (CREs)? Do these CREs play a crucial role in virulence activity? Can these CREs be harnessed for durable resistance breeding? What is the future of CREs?
What are “core” effectors?
Operationally, a core can be defined as a set of all genes shared as orthologs by all members of an evolutionarily coherent group [63]. In the context of effector genes from phytopathogens, the term emerged from high-throughput genomic sequencing study of cassava bacterial pathogen, Xanthomonas axonopodis pv. manihotis [21]. The study reported a set of conserved effectors (core effectors) that were preserved over three continents, 11 different countries, and seven decades of evolution. This gave birth to a vague definition of “core” effectors as effector proteins that are widely distributed across a population of a particular pathogen [22]. Since effector genes are crucial in pathogen virulence [64], a bona fide core effector must be [1]: highly conserved among diverse strains [2], highly expressed during infection, and [3] indispensable for virulence activity. Based on existing studies, it is evident that several conserved RxLR effectors of oomycetes have been identified however, only a few of these have been functionally characterized. Thus, in this review, we define “core” RxLR effectors as those that are either conserved among strains of a pathogen or different pathogen species, with the potential of playing a virulence role during infection process as well as those that have been validated to play a role in virulence. We argue that a pathogen cannot afford to lose “core” effectors since they are indispensable for virulence activity. Therefore, in the absence of functional redundancy, “core” effectors can be key drivers in search for durable resistance considering that; despite different selection pressures coming from diverse hosts and ecosystems, these effectors lack the freedom to mutate. This could probably be due to their location in the gene-dense/repeat-poor regions of the genome [65,66].
Do genomes of oomycetes encode “core” RxLR effectors (CREs)?
Over the last decade, the genomes of over 65 oomycete species have been sequenced [29,67]. Analyses of these genomes revealed that oomycete genome sizes vary from 32.1 to 295.3 Mb in Peronospora effuse and Plasmopara obducens, respectively [29,35]. In addition, RxLR secretomes of oomycetes vary significantly. For instance, predictions of RxLR effector genes in the genomes of P. multivora, P. infestans, P. palmivora, and P. megakarya encode 84, 500–563, 991 and 1181 RxLR effectors, respectively [31,68,69]. This effector content variation has been largely attributed to their location in repeat rich gene sparse regions of the genome [31,70,71]. This promotes genome plasticity as well as genetic variation of effector genes. In addition, expansion of RxLR effector family in Phytophthora species was suggested to be through gene duplication and rapid divergence, which could have resulted from illegitimate recombinations [72–74].
Despite the gain and loss of RxLR effectors in oomycetes due to various selection pressures from plant hosts as well as ecosystems, a small number of these are conserved across the population of a particular species and/or the genus. To date, a considerable number of CREs have been identified. This milestone is attributed to the availability of sequenced genomes of various species of oomycetes. In addition, the presence of N-terminal signature motifs mainly the RxLR-ERR and signal peptide [75] has enabled the identification of various CREs using in silico bioinformatics-based approaches. These approaches allow large-scale identification of oomycete RxLR effector arsenals [76–79]. A typical pipeline used in mining CREs in oomycete species is illustrated in Figure 1 leading to identification of putative CREs in some oomycete species (Figure 2). A general approach of the pipeline begins with mining of the genome for effectors by determining their ability to be secreted (presence of a signal peptide and lack of transmembrane domains) and finally, authentication of these effectors through in planta expression patterns as depicted in Figure 1.
Figure 1.
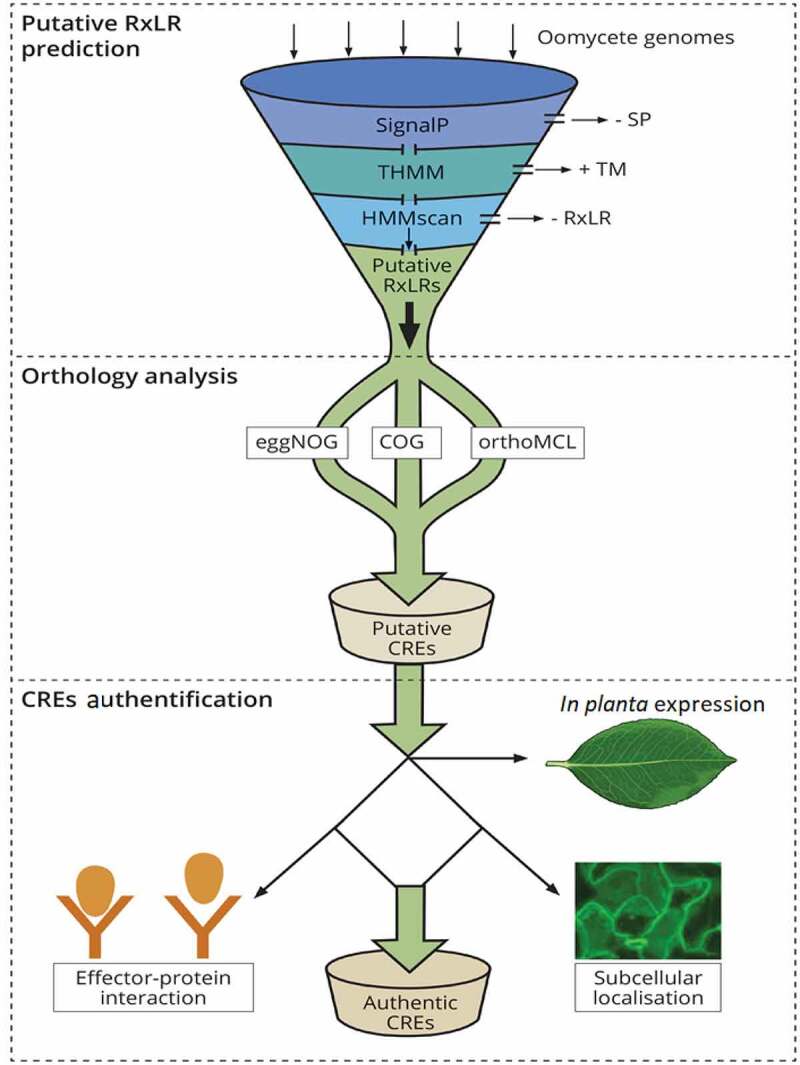
A schematic representation of in silico prediction and validation of putative CREs in oomycetes. The secretome prediction pipeline begins with the removal of proteins without a signal peptide (SP) while retaining those with a transmembrane domain (TM), by use of signalP tool and THMM, respectively. Effector proteins with a TM are discarded after signal peptide cleavage as these proteins are not likely to be retained in the plasma membrane. This is followed by removing effector proteins without the signature RxLR motif using HMMscan tool. Orthology analysis is performed to determine RxLR effectors that are conserved within strains or within species of a pathogen (CREs) using orthology analyses tools like COG, eggNOG or orthofinder. The final output is composed of putative secreted CREs with a SP, RxLR motif and without a TM. This output is further authenticated through in planta expression to ascertain their role in virulence for instance, their role in enhancing/suppressing host immunity, localization in planta using confocal microscopy as well as interacting proteins within host partners
Figure 2.
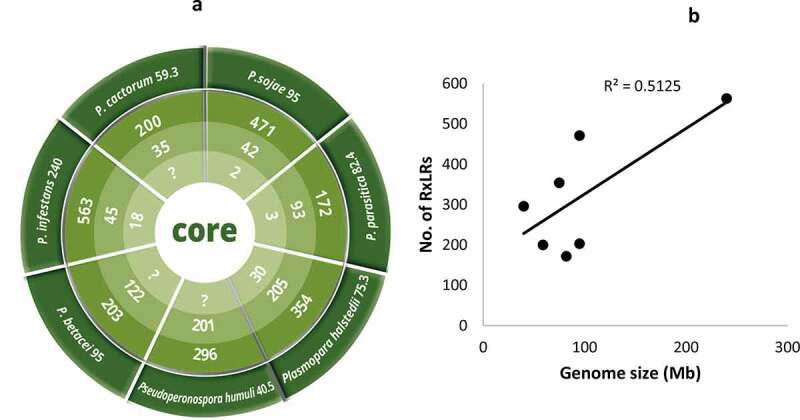
Illustration of phytopathogenic oomycetes with their respective genome sizes (Mb) on the outermost ring. Most of the genomes are Phytophthora spp (p). In terms of genome size, P. infestans and Pseudoperonospora humulis recorded the highest (240Mb) and lowest (40.5Mb) genome sizes, respectively. Counting from the outside, the second ring is the total number of predicted RxLR effectors ranging from 172 in P. parasitica to 563 in P. infestans. The third ring is the total number of putative CREs while the fourth ring is the total number of authentic CREs with Plasmopara halstedii recording a total of 30 CREs. In terms of association between genome size and the number of predicted RxLR effectors in oomycetes, insignificant positive correlation (P = 0,07;R2 = 0.51, at 95% confidence level) was recorded (b)
Besides bioinformatics prediction of putative CREs, genome comparisons can be employed to identify these effectors [80]. For instance, comparing genomes of strains of a species can aid in the identification of sequence polymorphisms, particularly single nucleotide polymorphisms (SNPs) in the protein-coding regions of effectors [64,81]. Generally, effectors are under strong selection pressure, and they are typified by high dN/dS (ratio of non-synonymous and synonymous substitutions) (Bos et al., 2009). However, conserved effectors are well-marked with low ratios of substitutions that change amino acids (non-synonymous) to substitutions that do not change amino acids (synonymous) coupled with significantly low or no copy number variation amongst effector genes, while the opposite applies to non-conserved RxLR effectors [64,82]. This is the case since conserved effectors are believed to be ancestral and because of their obligate role in pathogen virulence, they are subject to purifying selection [83]. This clearly demonstrates how important “core” effectors are since mutations could be detrimental to fitness and subsequently have low fixation probabilities.
Genomic comparison of the potato late blight pathogen P. infestans identified about 563 RxLR effectors, with 45 of these being shared among the three strains (06_3924A, NL07434, and T30-4) and expressed in planta as CREs [64]. Among these 45 CREs, five Avr genes (Avr2, Avr3a, Avrblb1, Avrblb2, and Avrvnt1) are known gain-of-virulence variants [19]. It was further revealed that Avr2 and Avr3a contain sequence polymorphisms that potentially enable them to evade recognition by cognate R–gene products in plants. Likewise, CREs Avrblb1, Avrblb2, and Avrvnt1 have intact coding sequences that are induced during infection [64]. These three Avr effectors are therefore predicted to be recognized by their cognate immunoreceptors. Yin, Gu [46] employed next-generation transcriptome deep sequencing strategy coupled with sequence polymorphisms to identify 18 candidate CREs in P. infestans. A recent study on genome re-sequencing of P. sojae identified a set of 471 RxLR effectors across 26 genomes with 42 of these being conserved as well as expressed in planta. Among the 42 “core” effectors, two RxLR effectors, PsAvh241 and PsAvh23 have been demonstrated as essential for full virulence of P. sojae [84,85]. This insinuates that the remaining 40 effectors could be critical in the infection process.
To further characterize the level of allelic diversity in RxLR effectors of the oomycete Phytophthora, pathogen enrichment sequencing (PenSeq) method has been devised [86]. The method enables the identification of either the presence or absence of variations as well as sequence polymorphisms in important genes of a pathogen, which is a criterion for the effective deployment of host resistance genes [86]. At this point, it is evident that bioinformatics-based approaches have been successfully used in CREs identification. However, in this success, therein lies a trap. With this approach, effector proteins lacking a signal peptide (SP) are discarded. However, these SP-lacking RxLR effector proteins have been shown to be secreted unconventionally [87]. Specifically, RxLR candidate effectors of P. infestans were detected in pelleted samples of culture filtrates, providing compelling evidence that these effectors could be delivered using the extracellular vesicles (EVs) [88]. Therefore, most CREs could be overlooked when using bioinformatics approaches only. In addition, bioinformatic identification of RxLR effectors in phytopathogen oomycetes largely depends on the signature motif RxLR. Nonetheless, this motif has been found to be degenerate [89,90]. To circumvent this enigma, EffectorO pipeline was recently developed [91]. The pipeline can predict novel effectors in oomycete genomes independent of motif-based searches. This approach is intended to expand the candidate effector repertoire of narrow host range oomycete plant pathogens.
Mass spectrometry is a powerful technique that can be used to solve deficiencies of in silico prediction of RxLR effectors. It has been previously employed in validating computationally predicted RxLR effector proteins to be secreted as well as identifying extracellular proteins that lack typical SP, which would then be overlooked [92–94].
Taken together, coupling in silico-based approaches with experimental techniques such as mass spectrometry could be the gold standard for identifying CREs and more so, novel CREs that have no matches in public databases. In addition, there is a need to verify whether indeed RxLR effectors form part of the cargo that is being delivered to the extracellular environment of the pathogen using EVs. More importantly, the association of RxLR effectors with EVs during their biogenesis is worth investigating.
Do “core” RxLR effectors (CREs) play a role in virulence?
Despite the presence of a multi-layered immune response in plants [7], CREs subdue host immune responses by targeting key components leading to disease proliferation. In most cases, these targeted components are central cellular processes/proteins that are conserved across diverse plant species. The last two decades have witnessed the identification of conserved RxLR Avr3a, in P. infestans with its cognate R-gene in the host cell [95]. Subsequent characterization of this effector revealed its crucial role in preventing host cell death during the biotrophic phase of infection by interacting with and stabilizing the host ubiquitin E3-ligase CMPG1 [96]. Since then, several other studies have been carried out. For instance, P. sojae RxLR effector, PsPSR2, that is conserved among eight Phytophthora spp, suppresses RNA silencing activity in various plants [97], an activity that has been reported in novel RxLR effectors [98]. Another P. sojae RxLR effector (PsAvh73) homologous to oomycete Hyaloperonospora arabidopsidis effector (HaRxL23) was reported to suppress PTI responses in Nicotiana benthamiana and ETI in Glycine max [48]. Phytophthora brassicae effector, RxLR24, was showed to be highly conserved among most successful species of Phytophthora such as P. infestans, P. sojae, and P. parasitica var. nicotianae [99]. Further, characterization of this effector and its close homolog in P. infestans, PiRxLR24, revealed that the two effectors localize to the plasma and vesicular membranes, where they associate with members of the RABA GTPase subfamily, hence interfering with vesicle tracking of the host plant [99]. In a separate study, three “core” effectors of P. parasitica, PpRxLR2, PpRxLR3, and PpRxLR5 were highly expressed in N. benthamiana leaves during infection [76]. Further analysis showed that effector PpRxLR2 enhanced the virulence of P. parasitica via complete suppression of the INF-1 induced PCD, while effectors PpRxLR3 and PpRxLR5 partially suppressed host plant defenses. Two CREs, REX3 and REX2, of the broad host-range oomycete P. palmivora were demonstrated to promote disease development upon expression, where effector REX3 enhanced virulence of the pathogen by interfering with host secretion pathways [100]. The well-studied oomycete P. infestans was reported to harbor a total of 18 CREs that are not only expressed during the early phase of infection, but also contribute to disease development by inhibiting plant defense responses induced by both PTI and ETI [46]. Although CREs are known to target positive regulators of host immunity, it is fascinating that they also target negative regulators of host immunity called susceptibility factors (SFs). A good example is Avr3a-related RxLR effectors that are distributed across diverse Phytophthora species. These effectors target the family of cinnamyl alcohol dehydrogenase 7 (CAD7) leading to downstream suppression of PTI [101].
We believe that evolutionary conservation is good since motif occurrences that are unlikely to have functional importance are eliminated hence retaining those motifs/domains that are crucial for the pathogen to successfully infect the host. Although CREs are said to be highly conserved, their essentiality is not likely to be retained through the conservation of overall proteins but through specific protein domains. This is driven by the specificity of substrate recognition, and it is therefore anticipated that active site residues are preserved better compared to the overall protein conservation. From the few existing studies on CREs of oomycetes, there is no specific domain that has been implicated in effector virulence activity [97,99,101]. Interestingly, alignment analyses of these CREs reveal the presence of C-terminal W (Trp) and Y (Tyr) motifs [102,103]. We therefore hypothesize that these motifs could be crucial in virulence activity of CREs of oomycetes since these motifs have been implicated in effector function [96,102,104,105]. Studies have shown that approximately 44% of Phytophthora RxLR effectors and 26% of H. arabidopsidis possess a highly conserved W and Y motif at the C-terminal [102,103]. Structural analyses of WY motif(s) have revealed the presence of more than one α-helix bundle formed by each motif [106]. It is hypothesized that the α-helical-domain, which is the “WY-domain”, enhances effector adaptation through mutations, while the hydrophobic core fold provides stability and flexibility therefore, implicated in virulence activities of the effector [103]. Following this hypothesis, studies have reported that this hydrophobic core is crucial in effector-host target protein interaction [105,107,108], cell-death induction [109,110] RNA silencing suppression activity and suppression of PTI and ETI events [48],101.
Although the WY motifs have been associated with effector virulence activity, P. infestans RxLR effector PexRD54, was shown to have a total of five WY repeats but surprisingly, the virulence activity of the effector was dependent on a C-terminal ATG8-interaction motif (AIM) that binds proteins related to autophagy (ATG8) [111,112]. Further analysis of PexRD54 revealed that the AIM motif, at the C-terminus of the effector, is linked to the last WY domain by a short helix [112]. Therefore, we can hypothesize that the main function of WY motifs in RxLR effectors is to act as a “dais” to introduce functional motifs or domains for interaction with host plant proteins. Recently, the highly conserved Avr3a-like effectors from Phytophthora species showed a conserved function by targeting plant CAD7 subfamily [101]. Amazingly, this function was independent of a putative enzyme active site of these effectors. Since the sequence conservation of these proteins revealed the presence of conserved WY motif at the C-terminal, we can therefore propose that WY motif could be responsible for Avr3a-like effectors-CAD7 interaction.
Although it appears that W-Y motifs are crucial in virulence activity of most RxLR effectors in oomycetes, it is not apparent whether these motifs are key players in CREs activity. Therefore, dissecting the structure of CREs using experimental and computational approaches is encouraged. This will inform not only the functional motifs or domains but also the host immune proteins or processes that these CREs target.
Do CREs target “core” host proteins/processes?
Since “core” effectors are maintained in effector repertories over a long evolutionary time [66], they are likely to target conserved elements in the plant immune system or metabolism that facilitate host colonization [113,114]. It is also important to know that these targeted host proteins and processes are “the candy liked by many” since they are crucial processes that cannot be altered or eliminated without complete damage to plant fitness. The concept of “core” effector targeting a conserved host protein has been witnessed in plant fungal effectors [27,115] and also in effectors (AvrB, AvrPto, HopAI1) of bacteria such as Pseudomonas syringae [116–118]. In oomycetes, this concept has not been sufficiently exploited. A recent study revealed that RxLR effectors target various plant processes with vesicle trafficking being a major targeted process [119]. A few studies have explored whether RxLR effector proteins target conserved process. For instance, evolutionarily conserved RxLR effectors in oomycetes H. arabidopsidis and P. sojae were shown to suppress immunity in plant species that are divergent from the source pathogen’s host [48,81]. In the same token, several conserved RxLR effectors from oomycete P. agathidicida, a pathogen of gymnosperms, were revealed to interact with the immune system of model angiosperm plants (Nicotiana spp), in a similar way to that of angiosperm pathogens [120]. These findings provide a hint of possible interaction of conserved effectors with conserved host targets. Recently, Avr3a-like conserved effectors from Phytophthora pathogens were reported to target a negative regulator of immunity, CAD7 in both Arabidopsis thaliana and N. benthamiana leading to disease development [101]. The notion of oomycetes’ effectors targeting negative regulators/susceptibility factors is currently a fertile ground for potential “core” effectors as reviewed by [121].
At this point in time, we cannot confidently conclude that CREs of oomycetes target broadly conserved plant proteins, nonetheless, the presence of “core” effectors in these pathogens could explain the success of most broad host-range oomycetes, notably Phytophthora species. To fully understand this concept, functional characterization of “core” effectors is key. This can be achieved through screening for protein–protein interactions using a yeast two-hybrid system (Y2H) [122,123], followed by validation of the interaction through co-immunoprecipitation [124]. Other validation methods include biotinylation [125] and bimolecular fluorescence complementation (BiFC) [126,127]. Further, mutation analyses like site-directed mutagenesis [128,129] and virus induced gene silencing, VIGs [130,131] can be performed to gain more insight on effector-host protein interaction.
Can “core” effectors be useful in breeding for durable resistance?
Currently, there is a paradigm shift from conventional to breeding for durable resistance. Effectoromics is a high-throughput functional genomics approach that employs the use of effectors to probe plant germplasm [132,133]. Here, we reason that since “core” effectors are present in most strains or species of a pathogen as well as playing an important role in virulence, a pathogen cannot easily lose them even after a new resistance gene is deployed in the host. Consequently, R-gene products that recognize such effectors are anticipated to be more durable than resistance gene products that perceive non conserved effectors.
The journey to durable resistance using “core” effectors starts with employing next-generation sequencing technologies to sequence and assemble genomes of various pathogens that are responsible for disease in different fields. Using computational approaches, “core” effectors in these strains can be identified. Consequently, these “core” effectors can be employed as probes in screening for cognate R proteins from wild germplasm using mainly transient co-expression assays [134] followed by either marker-assisted breeding or transgene deployment [12,22,135]. Validation of these new R-genes could be enhanced by new genome-editing methods like clustered regulatory interspaced short palindromic repeat (CRISPR) technologies [136].
One fascinating fact about RxLR effectors is their ability to operate as “double edge swords”, where on one side they suppress host immune responses, while on the other side they act as avirulence (Avr) factors leading to R protein mediated defenses in plants [11]. Screening for potential R proteins that recognize Avr RxLR effectors of oomycetes has been attempted [95,137–141]. Nonetheless, efforts have been directed toward the well-conserved P. infestans RxLR effector Avr3a [95,142]. This effector exists in two alleles (Avr3aKI and Avr3aEM), and this translates to a difference of two amino acids in the mature protein where AVR3aKI is recognized by R3a while AVR3aEM evades R3a recognition [95]. The study marked Avr3a as a potential candidate in breeding for durable resistance, however, there was a need to produce potato plants with an enhanced resistance spectrum and durability by integrating naturally occurring R-genes or engineered, synthetic R-genes with extended pathogen recognition precisions that comprises Avr3aEM recognition. Nine years later, a random mutagenesis study was conducted to generate a mutant version of R3a that recognized AVR3aEM [143]. Intriguingly, mutation of I2 gene, a close homologue of the R3a gene in tomato, made the gene product more responsive to AVR3a hence conferring resistance not only to P. infestans but also to the fungal pathogen Fusarium oxysporum [144].
Although “core” effectors appear to be the perfect targets in breeding for durable resistance, some studies have reported that due to the long evolution of plant–pathogen interaction, there is a possibility of complex mechanisms coming into place to shield conserved effectors from recognition [145–147]. For instance, the virulence activity of the conserved pathogen-secreted xyloglucan-specific endoglucanase (PsXEG1), an apoplastic effector of P. sojae, was shown to be protected by its paralog that is enzymatically inactive by binding more tightly to the host apoplastic glucanase inhibitor GmGIP1 than PsXEG1 [40]. Whether this is also the case in intracellular effectors like those with RxLR motifs needs to be investigated. We therefore suggest that deploying multiple, stacked R-genes that recognize “core” effectors can be of importance in reducing chances of a pathogen to overcome resistance.
Another potential way of exploiting CREs for durable resistance and broad spectrum breeding is capitalizing on their ability to target susceptibility (S)-genes [101]. Although resistance and susceptibility appear to be opposite sides of the same coin, the two have “resistance” as the focal point. S-genes are recessively inherited, with resistance being achieved through the loss of function of a host factor required by the pathogen. On the other hand, R-genes are dominantly inherited, and resistance is triggered when a pathogen-derived avirulence determinant is recognized by the R protein [148]. S-genes come in two “flavours”: Those that are independent of immunity as they directly serve to promote disease (genuine S-genes) and those that promote disease indirectly also termed as negative regulators of immunity [149]. Pathogens may indirectly benefit from the activity of S-gene products or directly by forcing plants to cooperate by activating or stabilizing S genes or their products, with the help of effectors [148]. A review by He, McLellan [121] documents several RxLR effectors of plant pathogenic oomycetes that target host S-genes and among these are “core” RxLR effectors [101,123]. We therefore reason that durable as well as broad-spectrum resistance can be attained by identifying those susceptibility genes that are targeted by CREs, using protein–protein interaction methods such as yeast-two hybrid screening [96,123]. After the identification, inactivation of these S-genes by mutations or genome editing [150] is performed with the aim of interfering with the ability of the effectors to associate with their host partners [148,151]. For instance, potato plants showed complete resistance to P. infestans after successful knockdown of six S-genes [152]. Targeting S-genes seems to be an avenue to breeding for durable resistance using “core” effectors, however, there is a cause for alarm since introduction of mutations to susceptibility genes has been linked to pleiotropic effects, specifically dwarfism and sensitivity to stress [148,151]. This limits the utilization of these genes in agriculture. Therefore, for an S-gene mutant to be practical in crop breeding, the following questions should be considered: (i) Does mutation or editing of an S-gene have undesirable side effects? (ii) In a scenario where an S-gene is redundant, is it possible to target multiple genes? (iii) Will targeting an S-gene for mutation result in sufficiently improved resistance?
What is the future of CREs research?
There is a clear potential for “core” effectors to target conserved processes in diverse host plants [115]. However, studies on the ability of CREs in oomycetes to target conserved host processes/protein have not been fully explored. Therefore, functional studies on these effectors are highly encouraged. The emerging reports that genomes of oomycetes species encode CREs shed light on important virulence roles played by these effectors [46,48,81,99]. Nevertheless, some key questions remain to be answered: Why do oomycetes conserve some RxLR effectors? Do these effectors play conserved roles in targeting host plant defenses? Do these effectors act as probes in screening for cognate R- genes in search for durable resistance in plants? Providing answers to these questions can potentially further advance the field. In addition, to gain further insight of the biology of “core” effectors in oomycetes, biochemical, genetic as well as biophysical studies are highly encouraged.
Although less has been documented on “core” RxLR effectors in oomycetes, the few existing studies have identified putative CREs through in silico prediction-based approaches. The task ahead is to validate the expression of these effectors in planta to have consensus in defining the term “core effectors”. In addition, functional characterization is worth undertaking to dissect these effectors and hence identifying specific domains that are conserved as well as important in virulence roles of these effectors. Therefore, harnessing CREs as future breeding tools to increase host resistance requires extensive collaborations between plant breeders, geneticists, and phythologists.
Figures in and outside the parenthesis are the “core” and potentially secreted effectors respectively
Acknowledgments
The authors extend their gratitude to Glenda Brits for doing the graphics.
Funding Statement
This work was supported by the NRF, South Africa [120585].
Disclosure statement
No potential conflict of interest was reported by the authors.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and freely available, under a license allowing re-use by any third party for any lawful purpose. Data shall be findable and fully accessible.
References
- [1].Rahman MH. Exploring sustainability to feed the world in 2050. J Food Microbiol. 2016;1(1). [Google Scholar]
- [2].Röös E, Bajželj B, Smith P, et al. Greedy or needy? Land use and climate impacts of food in 2050 under different livestock futures. Glob Environ Change. 2017;47:1–12. [Google Scholar]
- [3].Jones JD, Dangl JL. The plant immune system. nature. 2006;444(7117):323–329. [DOI] [PubMed] [Google Scholar]
- [4].Boller T, Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60(1):379–406. [DOI] [PubMed] [Google Scholar]
- [5].Thomma BP, Nürnberger T, Joosten MH. Of PAMPs and effectors: the blurred PTI-ETI dichotomy. Plant Cell. 2011;23(1):4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Spoel SH, Dong X. How do plants achieve immunity? Defence without specialized immune cells. Nat Rev Immunol. 2012;12(2):89–100. [DOI] [PubMed] [Google Scholar]
- [7].Saijo Y, Loo E. Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 2020;225(1):87–104. [DOI] [PubMed] [Google Scholar]
- [8].Zipfel C. Plant pattern-recognition receptors. Trends Immunol. 2014;35(7):345–351. [DOI] [PubMed] [Google Scholar]
- [9].Upson JL, Zess EK, Białas A, et al. The coming of age of EvoMPMI: evolutionary molecular plant–microbe interactions across multiple timescales. Curr Opin Plant Biol. 2018;44:108–116. [DOI] [PubMed] [Google Scholar]
- [10].Turnbull D, Yang L, Naqvi S, et al. RXLR effector AVR2 up-regulates a brassinosteroid-responsive bHLH transcription factor to suppress immunity. Plant Physiol. 2017;174(1):356–369. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Naveed ZA, Wei X, Chen J, et al. The PTI to ETI Continuum in Phytophthora-Plant Interactions. Front Plant Sci. 2020;11:2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vleeshouwers VG, Finkers R, Budding D, et al. SolRgene: an online database to explore disease resistance genes in tuber-bearing Solanumspecies. BMC Plant Biol. 2011;11(1):116. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gisi U, Sierotzki H. Springer: Fungicide modes of action and resistance in downy mildews. The Downy Mildews-Genetics, Molecular Biology and Control; 2008. 157–167. [Google Scholar]
- [14].Gray MA, Hao W, Förster H, et al. Baseline sensitivities of new fungicides and their toxicity to selected life stages of Phytophthora species from citrus in California. Plant Dis. 2018;102(4):734–742. [DOI] [PubMed] [Google Scholar]
- [15].Fry W. Phytophthora infestans: the plant (and R gene) destroyer. Mol Plant Pathol. 2008;9(3):385–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Haverkort A, Boonekamp P, Hutten R, et al. Durable late blight resistance in potato through dynamic varieties obtained by cisgenesis: scientific and societal advances in the DuRPh project. Potato Res. 2016;59(1):35–66. . [Google Scholar]
- [17].Douglas E, Halpin C. Gene stacking. Mol Tech Crop Improve. 2010;p. 613–29. [Google Scholar]
- [18].Zhu S, Li Y, Vossen JH, et al. Functional stacking of three resistance genes against Phytophthora infestans in potato. Transgenic Res. 2012;21(1):89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vleeshouwers VG, Oliver RP. Effectors as tools in disease resistance breeding against biotrophic, hemibiotrophic, and necrotrophic plant pathogens. Mol Plant-Microbe Interact. 2014;27(3):196–206. [DOI] [PubMed] [Google Scholar]
- [20].Baltrus DA, Nishimura MT, Romanchuk A, et al. Dynamic evolution of pathogenicity revealed by sequencing and comparative genomics of 19 Pseudomonas syringae isolates. PLoS Pathog. 2011;7(7):e1002132. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bart R, Cohn M, Kassen A, et al. High-throughput genomic sequencing of cassava bacterial blight strains identifies conserved effectors to target for durable resistance. Proc Nat Acad Sci. 2012;109(28):E1972–E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dangl JL, Horvath DM, Staskawicz BJ. Pivoting the plant immune system from dissection to deployment. Science. 2013;341(6147):746–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].De Jonge R, Van Esse HP, Kombrink A, et al. Conserved fungal LysM effector Ecp6 prevents chitin-triggered immunity in plants. science. 2010;329(5994):953–955. . [DOI] [PubMed] [Google Scholar]
- [24].Marshall R, Kombrink A, Motteram J, et al. Analysis of two in planta expressed LysM effector homologs from the fungus Mycosphaerella graminicola reveals novel functional properties and varying contributions to virulence on wheat. Plant Physiol. 2011;156(2):756–769. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mentlak TA, Kombrink A, Shinya T, et al. Effector-mediated suppression of chitin-triggered immunity by Magnaporthe oryzae is necessary for rice blast disease. Plant Cell. 2012;24(1):322–335. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Saitoh H, Fujisawa S, Mitsuoka C, et al. Large-scale gene disruption in Magnaporthe oryzae identifies MC69, a secreted protein required for infection by monocot and dicot fungal pathogens. PLoS Pathog. 2012;8(5):e1002711. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hemetsberger C, Mueller AN, Matei A, et al. The fungal core effector P ep1 is conserved across smuts of dicots and monocots. New Phytol. 2015;206(3):1116–1126. . [DOI] [PubMed] [Google Scholar]
- [28].Thines M, Kamoun S. Oomycete–plant coevolution: recent advances and future prospects. Curr Opin Plant Biol. 2010;13(4):427–433. [DOI] [PubMed] [Google Scholar]
- [29].McGowan J, Fitzpatrick DA. Recent advances in oomycete genomics. Adv Genet. 2020;105:175–228. [DOI] [PubMed] [Google Scholar]
- [30].Rizzo DM, Garbelotto M, Hansen EM. Phytophthora ramorum: integrative research and management of an emerging pathogen in California and Oregon forests. Annu Rev Phytopathol. 2005;43(1):309–335. [DOI] [PubMed] [Google Scholar]
- [31].Haas BJ, Kamoun S, Zody MC, et al. Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature. 2009;461(7262):393–398. . [DOI] [PubMed] [Google Scholar]
- [32].Tyler BM. Phytophthora sojae: root rot pathogen of soybean and model oomycete. Mol Plant Pathol. 2007;8(1):1–8. [DOI] [PubMed] [Google Scholar]
- [33].Hardham AR. Phytophthora cinnamomi. Mol Plant Pathol. 2005;6(6):589–604. [DOI] [PubMed] [Google Scholar]
- [34].Dussert Y, Mazet ID, Couture C, et al. A high-quality grapevine downy mildew genome assembly reveals rapidly evolving and lineage-specific putative host adaptation genes. Genome Biol Evol. 2019;11(3):954–969. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fletcher K, Gil J, Bertier LD, et al. Genomic signatures of heterokaryosis in the oomycete pathogen Bremia lactucae. Nat Commun. 2019;10(1):1–13. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Blackman LM, Cullerne DP, Torrena P, et al. RNA-Seq analysis of the expression of genes encoding cell wall degrading enzymes during infection of lupin (Lupinus angustifolius) by Phytophthora parasitica. PLoS One. 2015;10(9):e0136899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McGowan J, Fitzpatrick DA. Genomic, network, and phylogenetic analysis of the oomycete effector arsenal. MSphere 2017;2(6):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kamoun S. A catalogue of the effector secretome of plant pathogenic oomycetes. Ann Rev Phytopathol. 2006;44. [DOI] [PubMed] [Google Scholar]
- [39].Tian M, Win J, Song J, et al. A Phytophthora infestans cystatin-like protein targets a novel tomato papain-like apoplastic protease. Plant Physiol. 2007;143(1):364–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ma Z, Zhu L, Song T, et al. A paralogous decoy protects Phytophthora sojae apoplastic effector PsXEG1 from a host inhibitor. Science. 2017;355(6326):710–714. . [DOI] [PubMed] [Google Scholar]
- [41].Wawra S, Belmonte R, Löbach L, et al. Secretion, delivery and function of oomycete effector proteins. Curr Opin Microbiol. 2012;15(6):685–691. [DOI] [PubMed] [Google Scholar]
- [42].Whisson SC, Boevink PC, Moleleki L, et al. A translocation signal for delivery of oomycete effector proteins into host plant cells. Nature. 2007;450(7166):115–118. . [DOI] [PubMed] [Google Scholar]
- [43].Schornack S, van Damme M, Bozkurt TO, et al. Ancient class of translocated oomycete effectors targets the host nucleus. Proc Nat Acad Sci. 2010;107(40):17421–17426. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang Q, Han C, Ferreira AO, et al. Transcriptional programming and functional interactions within the Phytophthora sojae RXLR effector repertoire. Plant Cell. 2011;23(6):2064–2086. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zheng X, McLellan H, Fraiture M, et al. Functionally redundant RXLR effectors from Phytophthora infestans act at different steps to suppress early flg22-triggered immunity. PLoS Pathog. 2014;10(4):e1004057. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yin J, Gu B, Huang G, et al. Conserved RXLR effector genes of Phytophthora infestans expressed at the early stage of potato infection are suppressive to host defense. Front Plant Sci. 2017;8:2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lei X, Lan X, Ye W, et al. Plasmopara viticola effector PvRXLR159 suppresses immune responses in Nicotiana benthamiana. Plant Signal Behav. 2019;14(12):1682220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Deb D, Anderson RG, How-Yew-Kin T, et al. Conserved RxLR effectors from oomycetes Hyaloperonospora arabidopsidis and Phytophthora sojae suppress PAMP-and effector-triggered immunity in diverse plants. Mol Plant-Microbe Interact. 2018;31(3):374–385. [DOI] [PubMed] [Google Scholar]
- [49].Anderson R, Deb D, Withers J, et al. An oomycete RXLR effector triggers antagonistic plant hormone crosstalk to suppress host immunity. bioRxiv 2019;561605. [Google Scholar]
- [50].Lévesque CA, Brouwer H, Cano L, et al. Genome sequence of the necrotrophic plant pathogen Pythium ultimum reveals original pathogenicity mechanisms and effector repertoire. Genome Biol. 2010;11(7):R73. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Adhikari BN, Hamilton JP, Zerillo MM, et al. Comparative genomics reveals insight into virulence strategies of plant pathogenic oomycetes. PloS One. 2013;8(10):e75072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rujirawat T, Patumcharoenpol P, Lohnoo T, et al. Probing the phylogenomics and putative pathogenicity genes of Pythium insidiosum by oomycete genome analyses. Sci Rep. 2018;8(1):1–14. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ai G, Yang K, Ye W, et al. Prediction and Characterization of RXLR Effectors in Pythium Species. Mol Plant-Microbe Interact. 2020;33(ja):1046–1058. . [DOI] [PubMed] [Google Scholar]
- [54].Kemen E, Gardiner A, Schultz-Larsen T, et al. Gene gain and loss during evolution of obligate parasitism in the white rust pathogen of Arabidopsis thaliana. PLoS Biol. 2011;9(7):e1001094. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Thines M, Sharma R, Rodenburg SY, et al. The genome of Peronospora belbahrii reveals high heterozygosity, a low number of canonical effectors, and TC-rich promoters. Mol Plant-Microbe Interact. 2020;33(5):742–753. . [DOI] [PubMed] [Google Scholar]
- [56].Birch PR, Armstrong M, Bos J, et al. Towards understanding the virulence functions of RXLR effectors of the oomycete plant pathogen Phytophthora infestans. J Exp Bot. 2009;60(4):1133–1140. . [DOI] [PubMed] [Google Scholar]
- [57].Anderson RG, Deb D, Fedkenheuer K, et al. Recent progress in RXLR effector research. Mol Plant-Microbe Interact. 2015;28(10):1063–1072. [DOI] [PubMed] [Google Scholar]
- [58].Whisson SC, Boevink PC, Wang S, et al. The cell biology of late blight disease. Curr Opin Microbiol. 2016;34:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Krishnan A, Joseph L, Roy CB. An insight into Hevea-Phytophthora interaction: the story of Hevea defense and Phytophthora counter defense mediated through molecular signalling. Curr Plant Biol. 2019;17:33–41. [Google Scholar]
- [60].Wang J, Gao C, Li L, et al. Transgenic RXLR effector PITG_15718. 2 suppresses immunity and reduces vegetative growth in potato. Int J Mol Sci. 2019;20(12):3031. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Chepsergon J, Motaung TE, Bellieny-Rabelo D, et al. Organize, Don’t Agonize: strategic Success of Phytophthora Species. Microorganisms. 2020;8(6):917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Boevink PC, Birch PR, Turnbull D, et al. Devastating intimacy: the cell biology of plant–Phytophthora interactions. In: New Phytologist. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Charlebois RL, Doolittle WF. Computing prokaryotic gene ubiquity: rescuing the core from extinction. Genome Res. 2004;14(12):2469–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Cooke DE, Cano LM, Raffaele S, et al. Genome analyses of an aggressive and invasive lineage of the Irish potato famine pathogen. PLoS Pathog. 2012;8(10):e1002940. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Santhanam P, Van Esse HP, Albert I, et al. Evidence for functional diversification within a fungal NEP1-like protein family. Mol Plant-Microbe Interact. 2013;26(3):278–286. [DOI] [PubMed] [Google Scholar]
- [66].Depotter JR, Doehlemann G. Target the core: durable plant resistance against filamentous plant pathogens through effector recognition. Pest Manag Sci. 2020;76(2):426–431. [DOI] [PubMed] [Google Scholar]
- [67].Benson DA, Cavanaugh M, Clark K, et al. GenBank. Nucleic Acids Res. 2012;41(D1):D36–D42. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ali SS, Shao J, Lary DJ, et al. Phytophthora megakarya and Phytophthora palmivora, closely related causal agents of cacao black pod rot, underwent increases in genome sizes and gene numbers by different mechanisms. Genome Biol Evol. 2017;9(3):536–557. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Vetukuri RR, Tripathy S, Malar CM, et al. Draft genome sequence for the tree pathogen Phytophthora plurivora. Genome Biol Evol. 2018;10(9):2432–2442. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Raffaele S, Win J, Cano LM, et al. Analyses of genome architecture and gene expression reveal novel candidate virulence factors in the secretome of Phytophthora infestans. BMC Genomics. 2010;11(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Engelbrecht J, Duong TA, Prabhu SA, et al. van den Berg N. Genome of the destructive oomycete Phytophthora cinnamomi provides insights into its pathogenicity and adaptive potential. BMC Genomics. 2021;22(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Jiang RH, Tripathy S, Govers F, et al. RXLR effector reservoir in two Phytophthora species is dominated by a single rapidly evolving superfamily with more than 700 members. Proceedings of the National Academy of Sciences. 2008;105(12):4874–4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Prince VE, Pickett FB. Splitting pairs: the diverging fates of duplicated genes. Nat Rev Genet. 2002;3(11):827–837. [DOI] [PubMed] [Google Scholar]
- [74].Yang L, Ouyang HB, Fang ZG, et al. Evidence for intragenic recombination and selective sweep in an effector gene of Phytophthora infestans. Evol Appl. 2018;11(8):1342–1353. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Armenteros JJA, Tsirigos KD, Sønderby CK, et al. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat Biotechnol. 2019;37(4):420–423. . [DOI] [PubMed] [Google Scholar]
- [76].Dalio R, Maximo H, Oliveira T, et al. Phytophthora parasitica effector PpRxLR2 suppresses Nicotiana benthamiana immunity. Mol Plant-Microbe Interact. 2018;31(4):481–493. . [DOI] [PubMed] [Google Scholar]
- [77].Armitage AD, Lysøe E, Nellist CF, et al. Bioinformatic characterisation of the effector repertoire of the strawberry pathogen Phytophthora cactorum. PloS One. 2018;13(10):e0202305. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Pecrix Y, Buendia L, Penouilh‐Suzette C, et al. Sunflower resistance to multiple downy mildew pathotypes revealed by recognition of conserved effectors of the oomycete Plasmopara halstedii. Plant J. 2019;97(4):730–748. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Rojas-Estevez P, Urbina-Gómez DA, Ayala-Usma DA, et al. Effector Repertoire of Phytophthora betacei: in Search of Possible Virulence Factors Responsible for Its Host Specificity. Front Genet. 2020;11:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mestre P, Carrere S, Gouzy J, et al. Comparative analysis of expressed CRN and RXLR effectors from two Plasmopara species causing grapevine and sunflower downy mildew. Plant Pathol. 2016;65(5):767–781. . [Google Scholar]
- [81].Anderson RG, Casady MS, Fee RA, et al. Homologous RXLR effectors from Hyaloperonospora arabidopsidis and Phytophthora sojae suppress immunity in distantly related plants. Plant J. 2012;72(6):882–893. . [DOI] [PubMed] [Google Scholar]
- [82].Win J, Morgan W, Bos J, et al. Adaptive evolution has targeted the C-terminal domain of the RXLR effectors of plant pathogenic oomycetes. Plant Cell. 2007;19(8):2349–2369. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lindeberg M, Cunnac S, Collmer A. Pseudomonas syringae type III effector repertoires: last words in endless arguments. Trends Microbiol. 2012;20(4):199–208. [DOI] [PubMed] [Google Scholar]
- [84].Yu X, Tang J, Wang Q, et al. The RxLR effector Avh241 from Phytophthora sojae requires plasma membrane localization to induce plant cell death. New Phytol. 2012;196(1):247–260. . [DOI] [PubMed] [Google Scholar]
- [85].Kong L, Qiu X, Kang J, et al. A Phytophthora effector manipulates host histone acetylation and reprograms defense gene expression to promote infection. Curr Biol. 2017;27(7):981–991. . [DOI] [PubMed] [Google Scholar]
- [86].Thilliez GJ, Armstrong MR, Lim TY, et al. Pathogen enrichment sequencing (PenSeq) enables population genomic studies in oomycetes. New Phytol. 2019;221(3):1634–1648. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Liu T, Song T, Zhang X, et al. Unconventionally secreted effectors of two filamentous pathogens target plant salicylate biosynthesis. Nat Commun. 2014;5(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Wang S, Welsh L, Thorpe P, Whisson SC, Boevink PC, Birch PR . The Phytophthora infestans haustorium is a site for secretion of diverse classes of infection-associated proteins. MBio. 2018;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Gu B, Kale SD, Wang Q, et al. Rust secreted protein Ps87 is conserved in diverse fungal pathogens and contains a RXLR-like motif sufficient for translocation into plant cells. PLoS One. 2011;6(11):e27217. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kale SD, Gu B, Capelluto DG, et al. External lipid PI3P mediates entry of eukaryotic pathogen effectors into plant and animal host cells. Cell. 2010;142(2):284–295. . [DOI] [PubMed] [Google Scholar]
- [91].Nur M, Wood K, Michelmore R. EffectorO: motif-independent prediction of effectors in oomycete genomes using machine learning and lineage specificity. In bioRxiv. 2021. [DOI] [PubMed] [Google Scholar]
- [92].Meijer HJ, Mancuso FM, Espadas G, et al. Profiling the secretome and extracellular proteome of the potato late blight pathogen Phytophthora infestans. Mol Cell Proteomics. 2014;13(8):2101–2113. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Severino V, Farina A, Fleischmann F, et al. Molecular profiling of the Phytophthora plurivora secretome: a step towards understanding the cross-talk between plant pathogenic oomycetes and their hosts. PloS One. 2014;9(11):e112317. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].McGowan J, O’Hanlon R, Owens RA, et al. Comparative Genomic and Proteomic Analyses of Three Widespread Phytophthora Species: phytophthora chlamydospora, Phytophthora gonapodyides and Phytophthora pseudosyringae. Microorganisms. 2020;8(5):653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Armstrong MR, Whisson SC, Pritchard L, et al. An ancestral oomycete locus contains late blight avirulence gene Avr3a, encoding a protein that is recognized in the host cytoplasm. Proceedings of the National Academy of Sciences. 2005;102(21):7766–7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Bos JI, Armstrong MR, Gilroy EM, et al. Phytophthora infestans effector AVR3a is essential for virulence and manipulates plant immunity by stabilizing host E3 ligase CMPG1. Proceedings of the National Academy of Sciences. 2010;107(21):9909–9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Xiong Q, Ye W, Choi D, et al. Phytophthora suppressor of RNA silencing 2 is a conserved RxLR effector that promotes infection in soybean and Arabidopsis thaliana. Mol Plant-Microbe Interact. 2014;27(12):1379–1389. . [DOI] [PubMed] [Google Scholar]
- [98].Vetukuri RR, Whisson SC, Grenville-Briggs LJ. Phytophthora infestans effector Pi14054 is a novel candidate suppressor of host silencing mechanisms. EurJ Plant Pathol. 2017;149(3):771–777. [Google Scholar]
- [99].Tomczynska I, Stumpe M, Mauch F. A conserved Rx LR effector interacts with host RABA‐type GTP ases to inhibit vesicle‐mediated secretion of antimicrobial proteins. Plant J. 2018;95(2):187–203. [DOI] [PubMed] [Google Scholar]
- [100].Evangelisti E, Gogleva A, Hainaux T, et al. Time-resolved dual transcriptomics reveal early induced Nicotiana benthamiana root genes and conserved infection-promoting Phytophthora palmivora effectors. BMC Biol. 2017;15(1):39. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Li T, Wang Q, Feng R, et al. Negative regulators of plant immunity derived from cinnamyl alcohol dehydrogenases are targeted by multiple Phytophthora Avr3a‐like effectors. New Phytol. 2019. DOI: 10.1111/nph.16139. [DOI] [PubMed] [Google Scholar]
- [102].He J, Ye W, Choi DS, et al. Structural analysis of Phytophthora suppressor of RNA silencing 2 (PSR2) reveals a conserved modular fold contributing to virulence. Proceedings of the National Academy of Sciences. 2019;116(16):8054–8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Boutemy LS, King SR, Win J, et al. Structures of Phytophthora RXLR effector proteins a conserved but adaptable fold underpins functional diversity. J Biol Chem. 2011;286(41):35834–35842. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Dou D, Kale SD, Wang X, et al. RXLR-mediated entry of Phytophthora sojae effector Avr1b into soybean cells does not require pathogen-encoded machinery. Plant Cell. 2008;20(7):1930–1947. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].King SR, McLellan H, Boevink PC, et al. Phytophthora infestans RXLR effector PexRD2 interacts with host MAPKKKε to suppress plant immune signaling. Plant Cell. 2014;26(3):1345–1359. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Win J, Krasileva KV, Kamoun S, et al. Sequence divergent RXLR effectors share a structural fold conserved across plant pathogenic oomycete species. PLoS Pathog. 2012;8(1):e1002400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Du Y, Mpina MH, Birch PR, et al. Phytophthora infestans RXLR effector AVR1 interacts with exocyst component Sec5 to manipulate plant immunity. Plant Physiol. 2015;169(3):1975–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Qiao Y, Shi J, Zhai Y, et al. Phytophthora effector targets a novel component of small RNA pathway in plants to promote infection. Proceedings of the National Academy of Sciences. 2015;112(18):5850–5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Xiang J, Li X, Yin L, et al. A candidate RxLR effector from Plasmopara viticola can elicit immune responses in Nicotiana benthamiana. BMC Plant Biol. 2017;17(1):75. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Combier M, Evangelisti E, Piron M-C, et al. A secreted WY-domain-containing protein present in European isolates of the oomycete Plasmopara viticola induces cell death in grapevine and tobacco species. PloS One. 2019;14(7):e0220184. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Maqbool A, Hughes RK, Dagdas YF, et al. Structural basis of host autophagy-related protein 8 (ATG8) binding by the Irish potato famine pathogen effector protein PexRD54. J Biol Chem. 2016;291(38):20270–20282. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Dagdas YF, Belhaj K, Maqbool A, et al. An effector of the Irish potato famine pathogen antagonizes a host autophagy cargo receptor. Elife. 2016;5:e10856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Ai G, Xia Q, Song T, et al. A Phytophthora sojae CRN effector mediates phosphorylation and degradation of plant aquaporin proteins to suppress host immune signaling. PLoS Pathog. 2021;17(3):e1009388. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Carella P, Evangelisti E, Schornack S. Sticking to it: phytopathogen effector molecules may converge on evolutionarily conserved host targets in green plants. Curr Opin Plant Biol. 2018;44:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Irieda H, Inoue Y, Mori M, et al. Conserved fungal effector suppresses PAMP-triggered immunity by targeting plant immune kinases. Proceedings of the National Academy of Sciences. 2019;116(2):496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Alfano JR, Charkowski AO, Deng W-L, et al. The Pseudomonas syringae Hrp pathogenicity island has a tripartite mosaic structure composed of a cluster of type III secretion genes bounded by exchangeable effector and conserved effector loci that contribute to parasitic fitness and pathogenicity in plants. Proceedings of the national Academy of Sciences. 2000;97(9):4856–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].DebRoy S, Thilmony R, Kwack Y-B, et al. A family of conserved bacterial effectors inhibits salicylic acid-mediated basal immunity and promotes disease necrosis in plants. Proceedings of the National Academy of Sciences. 2004;101(26):9927–9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Badel JL, Shimizu R, Oh H-S, et al. A Pseudomonas syringae pv. tomato avrE1/hopM1 mutant is severely reduced in growth and lesion formation in tomato. Mol Plant-microbe Interactions. 2006;19(2):99–111. [DOI] [PubMed] [Google Scholar]
- [119].Vossenll H, Robatzek S, Kamoun S, et al. Host-interactor screens of Phytophthora infestans RXLR proteins reveal vesicle trafficking as a major effector-targeted process. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Guo Y, Dupont PY, Mesarich CH, et al. Functional analysis of RXLR effectors from the New Zealand kauri dieback pathogen Phytophthora agathidicida. Mol Plant Pathol. 2020;21(9):1131–1148. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].He Q, McLellan H, Boevink PC, et al. All roads lead to susceptibility: the many modes-of-action of fungal and oomycete intracellular effectors. Plant Commun. 2020;1(4):100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Mukhtar MS, Carvunis A-R, Dreze M, et al. Independently evolved virulence effectors converge onto hubs in a plant immune system network. science. 2011;333(6042):596–601. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Boevink PC, Wang X, McLellan H, et al. A Phytophthora infestans RXLR effector targets plant PP1c isoforms that promote late blight disease. Nat Commun. 2016;7(1):1–14. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Petre B, Saunders DG, Sklenar J, et al. Candidate effector proteins of the rust pathogen Melampsora larici-populina target diverse plant cell compartments. Mol Plant-Microbe Interact. 2015;28(6):689–700. . [DOI] [PubMed] [Google Scholar]
- [125].Roux KJ, Kim DI, Burke B. BioID: a screen for protein‐protein interactions. Curr Protoc Protein Sci. 2013;74(1):19.23.1–19.23. 14. [DOI] [PubMed] [Google Scholar]
- [126].Miller KE, Kim Y, Huh W-K, et al. Bimolecular fluorescence complementation (BiFC) analysis: advances and recent applications for genome-wide interaction studies. J Mol Biol. 2015;427(11):2039–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Graciet E, Wellmer F. The plant N-end rule pathway: structure and functions. Trends Plant Sci. 2010;15(8):447–453. [DOI] [PubMed] [Google Scholar]
- [128].Whigham E, Qi S, Mistry D, et al. Broadly Conserved Fungal Effector BEC1019 Suppresses Host Cell Death and Enhances Pathogen Virulence in Powdery Mildew of Barley (Hordeum vulgare L.)(Retracted). Mol Plant-Microbe Interact. 2015;28(9):968–983. . [DOI] [PubMed] [Google Scholar]
- [129].Sang Q, Pajoro A, Sun H, et al. Mutagenesis of a Quintuple Mutant Impaired in Environmental Responses Reveals Roles for CHROMATIN REMODELING4 in the Arabidopsis Floral Transition. Plant Cell. 2020;32(5):1479–1500. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Yang L, McLellan H, Naqvi S, et al. Potato NPH3/RPT2-like protein StNRL1, targeted by a Phytophthora infestans RXLR effector, is a susceptibility factor. Plant Physiol. 2016;171(1):645–657. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Ren Y, Armstrong M, Qi Y, et al. Phytophthora infestans RXLR effectors target parallel steps in an immune signal transduction pathway. Plant Physiol. 2019;180(4):2227–2239. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Vleeshouwers VG, Rietman H, Krenek P, et al. Effector genomics accelerates discovery and functional profiling of potato disease resistance and Phytophthora infestans avirulence genes. PLoS One. 2008;3(8):e2875. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Pais M, Win J, Yoshida K, et al. From pathogen genomes to host plant processes: the power of plant parasitic oomycetes. Genome Biol. 2013;14(6):1–10. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Rietman H, Bijsterbosch G, Cano LM, et al. Qualitative and quantitative late blight resistance in the potato cultivar Sarpo Mira is determined by the perception of five distinct RXLR effectors. Mol Plant-Microbe Interact. 2012;25(7):910–919. . [DOI] [PubMed] [Google Scholar]
- [135].Yang H, Tao Y, Zheng Z, et al. Application of next-generation sequencing for rapid marker development in molecular plant breeding: a case study on anthracnose disease resistance in Lupinus angustifolius L. BMC Genomics. 2012;13(1):318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Wang W, Pan Q, He F, et al. Transgenerational CRISPR-Cas9 activity facilitates multiplex gene editing in allopolyploid wheat. CRISPR J. 2018;1(1):65–74. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Rehmany AP, Gordon A, Rose LE, et al. Differential recognition of highly divergent downy mildew avirulence gene alleles by RPP1 resistance genes from two Arabidopsis lines. Plant Cell. 2005;17(6):1839–1850. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Champouret N, Bouwmeester K, Rietman H, et al. Phytophthora infestans isolates lacking class I ipiO variants are virulent on Rpi-blb1 potato. Mol Plant-Microbe Interact. 2009;22(12):1535–1545. . [DOI] [PubMed] [Google Scholar]
- [139].Oh S-K, Young C, Lee M, et al. In planta expression screens of Phytophthora infestans RXLR effectors reveal diverse phenotypes, including activation of the Solanum bulbocastanum disease resistance protein Rpi-blb2. Plant Cell. 2009;21(9):2928–2947. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Gilroy EM, Taylor RM, Hein I, et al. CMPG1‐dependent cell death follows perception of diverse pathogen elicitors at the host plasma membrane and is suppressed by Phytophthora infestans RXLR effector AVR3a. New Phytol. 2011;190(3):653–666. [DOI] [PubMed] [Google Scholar]
- [141].Vleeshouwers VG, Raffaele S, Vossen JH, et al. Understanding and exploiting late blight resistance in the age of effectors. Annu Rev Phytopathol. 2011;49(1):507–531. . [DOI] [PubMed] [Google Scholar]
- [142].Cárdenas M, Grajales A, Sierra R, et al. Genetic diversity of Phytophthora infestans in the Northern Andean region. BMC Genet. 2011;12(1):23. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Chapman S, Stevens LJ, Boevink PC, et al. Detection of the virulent form of AVR3a from Phytophthora infestans following artificial evolution of potato resistance gene R3a. PLoS One. 2014;9(10):e110158. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Giannakopoulou A, Steele JF, Segretin ME, et al. Tomato I2 immune receptor can be engineered to confer partial resistance to the oomycete Phytophthora infestans in addition to the fungus Fusarium oxysporum. Mol Plant-Microbe Interact. 2015;28(12):1316–1329. . [DOI] [PubMed] [Google Scholar]
- [145].Kombrink A, Thomma BP. LysM effectors: secreted proteins supporting fungal life. PLoS Pathog. 2013;9(12):e1003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Bourras S, McNally KE, Ben-David R, et al. Multiple avirulence loci and allele-specific effector recognition control the Pm3 race-specific resistance of wheat to powdery mildew. Plant Cell. 2015;27(10):2991–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Plissonneau C, Daverdin G, Ollivier B, et al. A game of hide and seek between avirulence genes AvrLm4‐7 and AvrLm3 in Leptosphaeria maculans. New Phytol. 2016;209(4):1613–1624. . [DOI] [PubMed] [Google Scholar]
- [148].van Schie CC, Takken FL. Susceptibility genes 101: how to be a good host. Annu Rev Phytopathol. 2014;52(1):551–581. [DOI] [PubMed] [Google Scholar]
- [149].Thordal-Christensen H. A holistic view on plant effector-triggered immunity presented as an iceberg model. In: Cellular and Molecular Life Sciences. 2020. p. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Zaidi SS-E-A, Mukhtar MS, Mansoor S. Genome editing: targeting susceptibility genes for plant disease resistance. Trends Biotechnol. 2018;36(9):898–906. [DOI] [PubMed] [Google Scholar]
- [151].Gawehns F, Cornelissen BJ, Takken FL. The potential of effector‐target genes in breeding for plant innate immunity. Microb Biotechnol. 2013;6(3):223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Sun K, Wolters A-MA, Vossen JH, et al. Silencing of six susceptibility genes results in potato late blight resistance. Transgenic Res. 2016;25(5):731–742. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Cabral A, Stassen JH, Seidl MF, et al. Van den Ackerveken G. Identification of Hyaloperonospora arabidopsidis transcript sequences expressed during infection reveals isolate-specific effectors. PLoS One. 2011;6(5):e19328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Peeters N, Guidot A, Vailleau F, et al. R alstonia solanacearum, a widespread bacterial plant pathogen in the post‐genomic era. Mol Plant Pathol. 2013;14(7):651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Deslandes L, Genin S. Opening the Ralstonia solanacearum type III effector tool box: insights into host cell subversion mechanisms. Curr Opin Plant Biol. 2014;20:110–117. [DOI] [PubMed] [Google Scholar]
- [156].Clarke CR, Studholme DJ, Hayes B, et al. Genome-enabled phylogeographic investigation of the quarantine pathogen Ralstonia solanacearum race 3 biovar 2 and screening for sources of resistance against its core effectors. Phytopathology. 2015;105(5):597–607. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Merda D, Briand M, Bosis E, et al. Ancestral acquisitions, gene flow and multiple evolutionary trajectories of the type three secretion system and effectors in Xanthomonas plant pathogens. Mol Ecol. 2017;26(21):5939–5952. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Zhang Y, Zhang K, Fang A, et al. Specific adaptation of Ustilaginoidea virens in occupying host florets revealed by comparative and functional genomics. Nat Commun. 2014;5(1):1–12. [DOI] [PubMed] [Google Scholar]
- [159].Fang A, Han Y, Zhang N, et al. Identification and characterization of plant cell death–inducing secreted proteins from Ustilaginoidea virens. Mol Plant-Microbe Interact. 2016;29(5):405–416. . [DOI] [PubMed] [Google Scholar]
- [160].Fang A, Gao H, Zhang N, et al. A novel effector gene SCRE2 contributes to full virulence of Ustilaginoidea virens to rice. Front Microbiol. 2019;10:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Plissonneau C, Hartmann FE, Croll D. Pangenome analyses of the wheat pathogen Zymoseptoria tritici reveal the structural basis of a highly plastic eukaryotic genome. BMC Biol. 2018;16(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Hemetsberger C, Herrberger C, Zechmann B, et al. The Ustilago maydis effector Pep1 suppresses plant immunity by inhibition of host peroxidase activity. PLoS Pathog. 2012;8(5):e1002684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Lanver D, Tollot M, Schweizer G, et al. Ustilago maydis effectors and their impact on virulence. Nature Rev Microbiol. 2017;15(7):409. . [DOI] [PubMed] [Google Scholar]
- [164].Schuster M, Schweizer G, Kahmann R. Comparative analyses of secreted proteins in plant pathogenic smut fungi and related basidiomycetes. Fungal Genet Biol. 2018;112:21–30. [DOI] [PubMed] [Google Scholar]
- [165].Seitner D, Uhse S, Gallei M, et al. The core effector Cce1 is required for early infection of maize by Ustilago maydis. Mol Plant Pathol. 2018;19(10):2277–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Ma L-S, Wang L, Trippel C, et al. The Ustilago maydis repetitive effector Rsp3 blocks the antifungal activity of mannose-binding maize proteins. Nat Commun. 2018;9(1):1–15. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Tanaka S, Gollin I, Rössel N, et al. The functionally conserved effector Sta1 is a fungal cell wall protein required for virulence in Ustilago maydis. New Phytol. 2020;227(1):185–199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and freely available, under a license allowing re-use by any third party for any lawful purpose. Data shall be findable and fully accessible.


