Abstract
Background:
Our previous study revealed that plasma levels of a-2,6-sialyltransferase 1 (ST6GAL1) were increased in patients with IgA nephropathy (IgAN). ST6GAL1 catalyzes terminal sialylation of IgG to shift the antibody effector function to the anti-inflammatory pattern. However, the role of plasma ST6GAL1 in the progression of IgAN and underlying mechanisms are still unknown.
Methods:
A total of 180 IgAN patients were included. The kidney outcomes were defined as the eGFR decline or proteinuria remission. Peripheral blood mononuclear cells (PBMCs) were either stimulated with purified sialylated IgG (SA-IgG) or with non-sialylated IgG (NSA-IgG) from IgAN patients to detect the levels of interleukin (IL)-6 and tumor necrosis factor-α (TNF-α) in supernatant.
Results:
Compared with the lower ST6GAL1 (reference), the risk of eGFR decline decreased for the higher ST6GAL1 group after adjustment for baseline eGFR, systolic blood pressure (SBP), and proteinuria. The results showed that patients with higher ST6GAL1 levels had a higher rate of proteinuria remission. ST6GAL1, expressed as a continuous variable, was a protective factor for eGFR decline and proteinuria remission. An in vitro study showed that the administration of recombinant ST6GAL1 (rST6GAL1) decreased the levels of IL-6 and TNF-α in PBMCs. Furthermore, the administration of rST6GAL1 resulted in the enrichment of SA-IgG in a concentration-dependent manner. In addition, as compared to control, purified SA-IgG-treated PBMCs showed a significant decrease in the expression of IL-6 and TNF-α.
Conclusion:
Our study indicated that elevated ST6GAL1 was associated with a slower progression of IgAN, which may play a protective effect by increasing IgG sialylation to inhibit the production of proinflammatory cytokines in PBMCs.
Keywords: IgA nephropathy, IL-6 and TNF-α, kidney outcomes, sialylated IgG, ST6GAL1
Introduction
Immunoglobulin A nephropathy (IgAN) is the most common glomerulonephritis in the world, with 10–20% of patients progressing to end-stage kidney disease (ESKD) within 10 years after diagnosis. 1 A combination of four processes induces renal injury that culminates in IgAN: Hit 1: production of aberrant glycosylation of IgA1 (Gd-IgA1); Hit 2: synthesis of antibodies (mainly IgG) directed against Gd-IgA1; Hit 3: binding of the Gd-IgA1 with its antibodies to form circulation immune complexes (CICs); and Hit 4: accumulation of CIC in the glomerular mesangium to initiate renal injury.2–4 More and more evidence showed that abnormal release of proinflammatory cytokines, including interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) in peripheral blood mononuclear cells (PBMCs) play an important role in the pathogenesis and progression of IgAN.5–9 The risk of kidney failure under the current therapy regimen remains high; new interventions are urgently needed to slow the progression of the disease. 10
ST6GAL1 encoding β-galactoside α2,6-sialyltransferase mediates the transfer of sialic acid residue with an α-2,6-linkage to a terminal galactose. Genome-wide association studies (GWASs) were performed in the Chinese Han population, which identified the risk allele C at rs7634389 at the ST6GAL1 locus was associated with the susceptibility and slower progression of IgAN.11,12 Our previous results showed that administration of recombinant ST6GAL1 (rST6GAL1) inhibited the production of galactose-deficient IgA1 (Gd-IgA1) in cultured PBMCs from IgAN patients. 13 However, whether increased ST6GAL1 levels are associated with a slower disease progression in IgAN is unknown.
ST6GAL1 has a modulatory role in the anti-inflammatory activity of sialylated IgG (SA-IgG). 14 Engineered sialylation of IgG with ST6GAL1 shifts the antibody effector function to the anti-inflammatory pattern in a mice model of Goodpasture disease, thus effectively attenuating disease severity. 15 Elevated ST6GAL1 inhibits the production of the proinflammatory cytokines in patients with rheumatoid arthritis (RA) by inducing IgG-Fc sialylation. 16 Moreover, several clinical observations indicated that patients with progressive RA have poorly SA-IgG compared with patients with less-severe disease or those in remission.17,18 Whether ST6GAL1 can play anti-inflammatory effects by increasing sialylation of IgG in IgAN has not been investigated yet.
In this study, we examined the prognostic utility of plasma levels ST6GAL1 in 180 patients with IgAN. Furthermore, we determined whether the addition of ST6GAL1 played protective effects by an increase in IgG sialylation in vitro.
Materials and methods
Sample collection
In this retrospective case–control study, 180 patients with IgAN diagnosed by renal biopsy, and 50 age- and gender-matched healthy participants were included. Of these participants, 10 patients with IgAN and 10 healthy participants were randomly selected to isolate IgG. The diagnosis of IgAN was based on the presence of IgA deposition in the glomerular mesangium by immunofluorescence and electron-dense material deposition in the mesangium by electronic microscopy. The patients with Henoch-Schönlein purpura and other secondary IgAN were excluded. Plasma from all participants was collected. Written informed consent was obtained from each patient and healthy participant. Clinical informations, including age, gender, blood pressure, 24-h urine protein excretion, serum creatinine, estimated glomerular filtration rate (eGFR), total IgA levels, were collected at the time of renal biopsy. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. 19 The histological lesions were classified according to Oxford classification—MEST-C (M: mesangial hypercellularity; E: endocapillary hypercellularity; S: segmental glomerulosclerosis; T: tubular atrophy/interstitial fibrosis, and C: crescent). 20
Outcomes
The eGFR decline was evaluated as ΔeGFR, which was calculated as eGFR in the next year minus eGFR at baseline per month. 21 We defined proteinuria remission as a more than 50% reduction in proteinuria from baseline or proteinuria <300 mg/d.
Isolation of SA-IgG and non-sialylated IgG
The total serum immunoglobulin was separated and purified with a protein G affinity column (Nunc, Rochester, USA). Briefly, the phosphate-buffered saline (PBS)-diluted plasma were added to protein G affinity columns and eluted by washing the affinity columns with 10 mL of buffer B (0.2 M glycine/in chloride acid, pH 2.5). Purified IgG was desalted by PD-10 column. Draw the bulk of IgG into the prepared sambucus nigra lectin (SNA)-agarose column and let the buffer drain by gravity. The outflowing liquid was centrifuged to collected non-sialylated IgG (NSA-IgG). SA-IgG was eluted by adding the eluting solution (VECTOR Labs, USA), followed by washing with PBS. Fractions containing IgG were concentrated by a protein centrifugal filter unit (Millipore, USA). Enrichment of the eluate was verified by Western blotting (WB) with biotinylated SNA, IgG antibody, and horseradish peroxidase (HRP)-conjugated streptavidin.
ELISA assay for SA-IgG in cell supernatant
The level of SA-IgG in cell supernatant was determined using enzyme-linked immunosorbent assay (ELISA) according to the slightly modified procedure (ab195215; Abcam, USA). Assay diluent cell culture supernatants were added into appropriate wells and incubated for 2.5 h at room temperature. After washing with wash solution, the plate was added 100 μL of 1:50 diluted SNA (VECTOR, USA) for 2 h. HRP-Streptavidin solution was incubated for 45 mins. After TMB (3,3′, 5,5;-tetramethylbenzidine) solution incubation for 30 mins, the reaction was stopped with a stop solution. The optical densities (ODs) were determined at 450 nm with an EL312 Bio-Kinetics microplate reader (Bio-TekInstruments, Winooski, VT).
ELISA assay for ST6GAL1, IL-6, and TNF-α
The ST6GAL1 levels from plasma and IL-6 and TNF-α levels from cell supernatants were measured using commercial ELISA kits (R&D Systems, USA) according to the manufacturer’s specifications. At last, the absorbance was detected at 450 nm with an EL312 Bio-Kinetics microplate reader (Bio-TekInstruments, Winooski, VT).
PBMCs culture and treatment
About 5-mL peripheral blood was taken into ethylenediaminetetraacetic acid (EDTA)-anticoagulated tubes. PBMCs were separated by density-gradient centrifugation on Ficoll (TBD, China) and resuspended in PBS. In the in vitro experiment, PBMCs were seeded into 24-well plates. The cells were stimulated with lipopolysaccharide (LPS) (Sigma-Aldrich, USA) and incubated with 0, 500, 1000 ng/mL human recombinant ST6GAL1 (rST6GAL1, R&D Systems, USA) and CMP-sialic acid (Sigma, USA) at a final concentration of 1.0 mM for 24 h to detect SA-IgG levels. PBMCs were incubated with purified SA-IgG (0.5 mg/mL) and NSA-IgG (0.5 mg/mL) for 24 h to detect the levels of IL-6 and TNF-α in cell supernatants.
Statistical analysis
For continuous variables, data with a normal distribution was expressed as the mean ± SD and compared by an unpaired t-test. For nonnormally distributed variables, data were expressed as the median (first quartile and third quartile) and analyzed by the Mann–Whitney U-test. Categorical variables were summarized as proportions and were compared by a chi-square test. Correlation analysis was performed by Pearson’s correlation test. Linear regression analyses were used to evaluate levels of ST6GAL1 on the rate of eGFR decline. Kaplan–Meier analysis was used to compare proteinuria remission with different levels of ST6GAL1. We estimated the regression coefficient and the corresponding 95% confidence interval (CI) between ST6GAL1 and eGFR decline rates with unadjusted and multivariable adjusted linear regression. The odds ratio (OR) and the 95% CI between ST6GAL1 and proteinuria remission were examined in logistic models. Power calculations were used to determine the number of participants required with 0.90 power at an alpha level of 0.05. A two-tailed p value < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS, version 16.0 software.
Results
Baseline clinical and pathological data
The demography characteristics of the study population, clinical findings, and pathological lesions at the time of renal biopsy were listed in Supplement Table 1. There were 91 males (51%) with a mean age of 40 ± 13 years in patients with IgAN and were 22 males (44%) with a mean age of 38 ± 10 years in healthy controls. The average systolic blood pressure (SBP) was 132 ± 17 mmHg and diastolic blood pressure (DBP) was 82 ± 11 mmHg. The mean eGFR was 93.5 ± 44.29 mL/min per 1.73 m2, and the proteinuria level was 1763.35 ± 1721.48 mg/d. After a mean follow-up of 17 months, 85 (47%) participants reached the proteinuria remission.
Plasma ST6GAL1 levels were correlated with the severity of IgAN
The mean ST6GAL1 level in plasma in participants with IgAN at biopsy was 6914 ± 2624 pg/mL, significantly higher than that of healthy controls (4321 ± 827 pg/mL, p < 0.001, Supplement Figure 1). The patients were then divided into two equal subgroups according to the median level of plasma ST6GAL1 levels (low ST6GAL1 < 6412 pg/mL and high ST6GAL1 > 6412 pg/mL) to explore the association of plasma ST6GAL1 with clinical pathological lesions in patients with IgAN (Table 1). We found that patients with higher ST6GAL1 levels had higher levels of triglyceride, serum creatinine, uric acid, serum IgA, serum C3, serum C4 and proteinuria, and lower levels of eGFR and IgG compared with those patients with lower SA-IgG levels. As for the Oxford classification, patients with higher ST6GAL1 levels tend to have a higher proportion of glomerulosclerosis.
Table 1.
The baseline data for IgAN patients with lower and higher ST6GAL1 levels.
| Characters | Mean ± SD or n (%) | p | |
|---|---|---|---|
| Low ST6GAL1 (n < 6412 pg/mL) | High ST6GAL1 (n > 6412 pg/mL) | ||
| Gender (M/F) | 44 (49)/46 (51) | 47 (52)/43 (48) | 0.65 |
| Age (mean ± SD, year) | 40.01 ± 13.11 | 39.84 ± 13.63 | 0.93 |
| SBP (mmHg) | 130.43 ± 17.81 | 134.84 ± 17.4 | 0.09 |
| DBP (mmHg) | 80.59 ± 11.69 | 83.47 ± 11.72 | 0.1 |
| BMI (kg/m2) | 24.14 ± 4.21 | 25.28 ± 3.35 | 0.06 |
| Hemoglobin (g/L) | 131.58 ± 18.13 | 133.08 ± 20.13 | 0.6 |
| Serum albumin (g/L) | 37.04 ± 5.06 | 37.03 ± 5.03 | 0.98 |
| Triglyceride (mmol/L) | 1.69 ± 1 | 2.78 ± 2.66 | 0.001 |
| Serum creatinine (μmol/L) | 87.47 ± 47.81 | 100.96 ± 47.78 | 0.06 |
| eGFR (mL/min/1.73 m2) | 102.18 ± 51.62 | 84.81 ± 33.6 | 0.008 |
| Uric acid (μmol/L) | 357.21 ± 97.52 | 401.99 ± 107.09 | 0.004 |
| Serum IgA (mg/dL) | 313.22 ± 121.19 | 573.74 ± 418.8 | 0.001 |
| Serum IgG (mg/dL) | 1051.3 ± 261.4 | 889.42 ± 420.69 | 0.003 |
| Serum IgM (mg/dL) | 119.39 ± 64.23 | 113.31 ± 54.85 | 0.513 |
| Serum IgE (mg/dL) | 66.4 ± 167.19 | 158.64 ± 495.06 | 0.11 |
| Serum C3 (mg/dL) | 86.63 ± 15.6 | 94.47 ± 17.86 | 0.003 |
| Serum C4 (mg/dL) | 21.95 ± 5.95 | 25.15 ± 8.48 | 0.005 |
| Proteinuria (mg/d) | 1409.83 ± 1373.14 | 2116.87 ± 1955 | 0.006 |
| Urine RBC (/HP) | 35.93 ± 61.24 | 80.58 ± 257.26 | 0.11 |
| Oxford classification | |||
| M score (M0/M1) | 11 (12)/79 (88) | 10 (11)/80 (89) | 0.82 |
| E score (E0/E1) | 53 (59)/ 37 (41) | 52 (58)/38 (42) | 0.89 |
| S score (S0/S1) | 44 (49)/46 (51) | 28 (31)/62 (69) | 0.02 |
| T score (T0/T1/T2) | 35 (39)/50 (56)/5 (5) | 27 (30)/50 (55)/13 (15) | 0.15 |
| C score (C0/C1/C2) | 23 (26)/54 (60)/13 (14) | 32 (36)/50 (55)/8 (9) | 0.25 |
BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; IgAN, immunoglobulin A nephropathy; RBC, red blood cell; SBP, systolic blood pressure; SD, standard deviation.
Plasma ST6GAL1 levels were negatively correlated with kidney disease progression
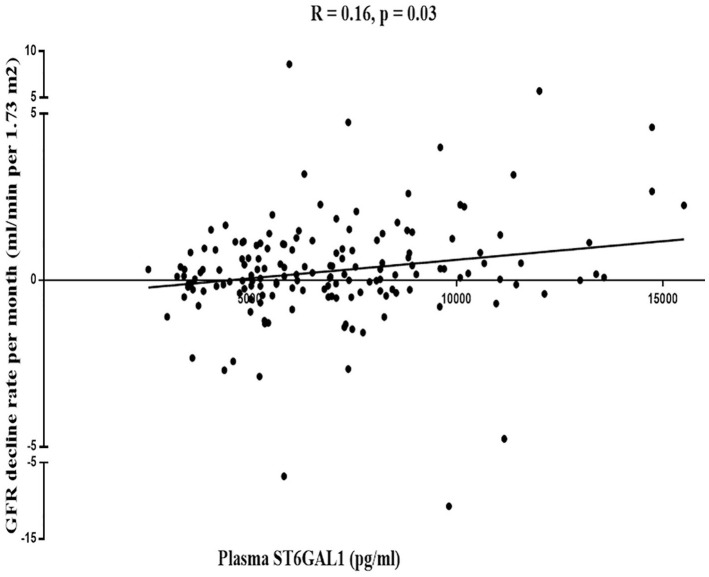
Mean rates of eGFR decline over 1 year in the higher and lower ST6GAL1 level groups, measured by the average of ΔeGFR, were 0.25 and −0.76 mL/min/1.73 m2, respectively. Patients with lower levels of ST6GAL1 had a more rapid decline in the eGFR (p = 0.03, Figure 1). Multiple linear regression analyses suggested a significant correlation of eGFR decline rate with ST6GAL1 after adjusting for baseline eGFR, SBP, and proteinuria (regression coefficient: 2.98 × 10−4; 95% CI: 4.45 × 10−4 to 1.51 × 10−4). Compared with the lower ST6GAL1 (reference), the risk of eGFR decline decreased for the higher ST6GAL1 group (regression coefficient: 1.27; 95% CI: 0.48–2.05, Table 2).
Figure 1.
The correlation between ST6GAL1 and eGFR decline rate.
Table 2.
Univariate and multivariate linear regression analysis of ST6GAL1 influencing eGFR decline rate.
| Characteristic | Mean ± SD (pg/mL) | Univariate analysis | Multivariate analysis a | ||
|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | ||
| ST6GAL1 | 6914 ± 2624 | 3.45 × 10−4 (1.92 × 10−4 to 4.98 × 10−4) | <0.001 | 2.98 × 10−4 (1.51 × 10−4to 4.45 × 10−4) | <0.001 |
| ST6GAL1 dichotomy | |||||
| Lower level | 5009 ± 951 | 1 (reference) | 1 (reference) | ||
| Higher level | 8365 ± 2079 | 1.56 (0.75–2.37) | <0.001 | 1.27 (0.48–2.05) | 0.002 |
CI, confidence interval; eGFR, estimated glomerular filtration rate; SD, standard deviation.
Adjusted for eGFR, proteinuria, and systolic pressure.
The plasma ST6GAL1 level was separately tested for association with the proteinuria remission. Thirty-five patients (38%) got proteinuria remission in the lower ST6GAL1 group, and 50 patients (56%) reached remission in the higher ST6GAL1 group. Kaplan–Meier analysis showed that the proteinuria remission rate in the high ST6GAL1 group was significantly better than that in the low group (p = 0.004, Figure 2).
Figure 2.
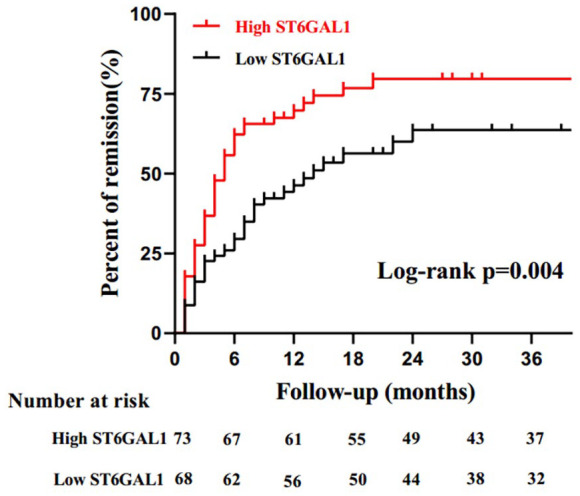
Kaplan–Meier analysis of proteinuria remission in IgA nephropathy (IgAN) patients with the median plasma of ST6GAL1.
The logistic regression analysis showed that compared with the low ST6GAL1 (reference), the rate of proteinuria remission increased for the high ST6GAL1 level after adjustment for baseline eGFR, SBP, and proteinuria (OR: 2.09; 95% CI: 1.01–4.31, Table 3).
Table 3.
Univariate and multivariate logistic regression analysis of ST6GAL1 at baseline influencing proteinuria remission.
| Characteristic | Univariate analysis | Multivariate analysis a | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| ST6GAL1 | 1.0001 (0.999–1.0004) | 0.11 | 1.0001 (0.9999–1.0002) | 0.1 |
| ST6GAL1 dichotomy | ||||
| Lower level | 1 (reference) | 1 (reference) | ||
| Higher level | 2.05 (1.03–4.06) | 0.04 | 2.09 (1.01–4.31) | 0.04 |
CI, confidence interval; OR, odds ratio.
Adjusted for eGFR, proteinuria, and systolic pressure.
Recombinant ST6GAL1 downregulated the secretion of IL-6 and TNF-α in PBMCs
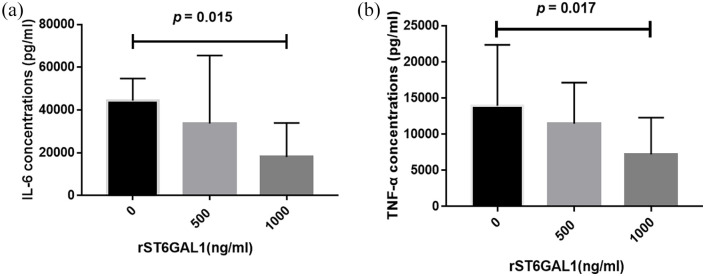
To address the possible influence of rST6GAL1 on the production of proinflammatory cytokines, IL-6, and TNF-α levels were analyzed in supernatant from PBMCs. As depicted in Figure 3, rST6GAL1 at a concentration of 1000 ng/mL significantly decreased the production of IL-6 (p = 0.015, Figure 3(a)) and TNF-α (p = 0.017, Figure 3(b)) in PBMCs.
Figure 3.
The expression of IL-6 (a) and TNF-α (b) after rST6GAL1 stimulation in supernatant of PBMCs.
Recombinant ST6GAL1 upregulated SA-IgG expression in PBMCs
We detected the SA-IgG levels in culture supernatant of PBMCs under rST6GAL1 challenge. Our results indicated that in the presence of the corresponding CMP-sialic acid, ST6GAL1 resulted in enrichment of SA-IgG (p = 0.025, Figure 4).
Figure 4.
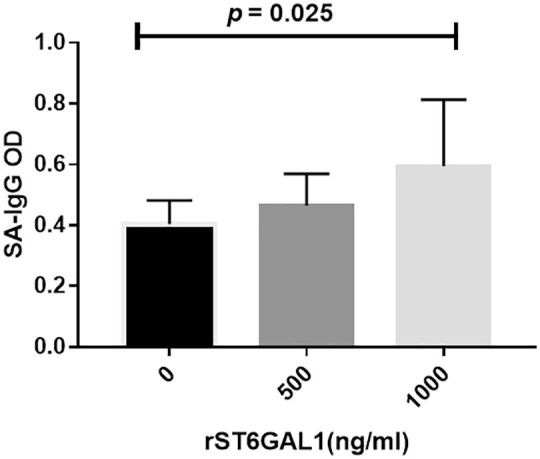
SA-IgG production after rST6GAL1 stimulation in supernatant of PBMCs.
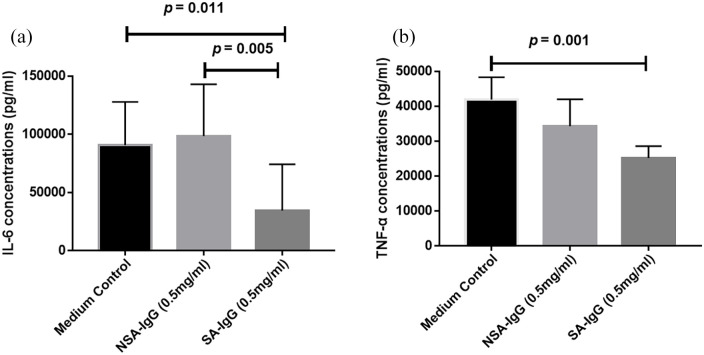
SA-IgG downregulated IL-6 and TNF-α secretion in PBMCs
As depicted in Figure 5, the releases of IL-6 and TNF-α were significantly decreased in PBMCs cultured with SA-IgG from patients with IgAN. The findings implied that SA-IgG protected against inflammatory response in PBMCs.
Figure 5.
The expression of IL-6 (a) and TNF-α (b) after SA-IgG and NSA-IgG stimulation in supernatant of PBMCs.
Discussion
Consistent with our previous findings, 13 the results of this study reconfirmed that increased plasma levels of ST6GAL1 were associated with the severity of IgAN. Most importantly, it demonstrated that high levels of ST6GAL1 predicted a slower eGFR decline rate and a higher proteinuria remission rate in IgAN. Furthermore, the data provided evidence that ST6GAL1 increased sialylation of IgG-Fc to counteract the actions of the proinflammatory cytokines IL-6 and TNF-α in IgAN. Preliminary observations indicated that ST6GAL1 played protective effects in IgAN by an increase in IgG sialylation. It lends support to the notion that ST6GAL1 can be used as a promising addition to improve existing strategies for the treatment of IgAN.
Recently, several genetic studies explored the association between ST6GAL1 and IgAN pathogenesis and progression. A GWAS follow-up study including 613 patients with IgAN revealed that the patients carrying the risk allele rs7634389-C at the ST6GAL1 locus had a decreased risk for the progression of IgAN. 12 Fu et al. 22 reported that rs7634389-C allele was associated with increased susceptibility to IgAN, higher risks of hyperuricaemia, and segmental glomerulosclerosis. Interestingly, they found patients carrying the risk allele rs7634389-C allele had a lower risk for disease progression. 22 The authors explained that this discrepancy might be caused by the complex interactions between genetic, epigenetic, and environmental factors during the progression of IgAN. 22 These findings indicated that genetic variation in ST6GAL1 participated in the development and progression of IgAN. However, the plasma levels of ST6GAL1 were not explored in these studies. In this study, we expanded on the findings of previous studies by showing that the elevated levels of ST6GAL1 were associated with disease severity in patients with IgAN with larger sample sizes. More importantly, compared with patients with lower ST6GAL1, those with higher levels of ST6GAL1 had a lower eGFR decline rate after adjusting for confounding factors. The results stressed the importance of ST6GAL1 in delaying renal function decline in IgAN. There is growing evidence that suggests proteinuria reduction can be used as a reasonably surrogate endpoint for a treatment’s effect on progression to end-stage renal disease (ESRD) in patients with IgAN. 23 We showed a higher proteinuria remission rate in IgAN patients with higher plasma levels of ST6GAL1 than those in patients with lower levels of ST6GAL1. Together, the data suggest that high levels of baseline ST6GAL1 have a prognostic utility in IgAN patients with better outcomes.
The underlying protective mechanism of ST6GAL1 should be discussed. Previous research showed that lots of proinflammatory cytokines, IL-6 and TNF-α, were triggered in the development of IgAN and associated with the pathogenesis, aggravation, and progression of IgAN.6,9,24 The present data confirmed that the administration of recombinant ST6GAL1 decreased IL-6 and TNF-α levels in PBMCs and advanced the idea that the ability of sialyltransferase ST6GAL1 to SA-IgG in vitro. Furthermore, we explored whether the anti-inflammatory effect of ST6GAL1 in IgAN was mediated by increasing sialylation of IgG. It showed that increased SA-IgG induced by ST6GAL1 decreased inflammation activation of PBMC with the downregulatory synthesis of IL-6 and TNF-α. The galactosylation is a prerequisite for sialylation of ST6GAL1 at the Asn297 site of IgG-Fc. In contrast to these findings in sialylation, we did not detect a difference in the expression of B4GALT1, the enzyme-mediating attachment of galactose to the IgG-Fc glycan, between IgAN patients and healthy control. The galactosylated IgG-Fc were then sialylated with 2,6 sialyltransferases The use of artificially sialylated IgG-Fc has been confirmed to increase the efficacy of anti-inflammatory treatment.25,26 The sialylation of IgG reduced the pathogenicity of autoantibodies and diminished the arthritogenic activity in the collagen-induced arthritis (CIA) model. 27 Immune complexes with antigen-specific SA-IgG inhibited the production of IL-6 in autoimmune mouse models of lupus nephritis and RA. 28 Troelsen et al. 29 showed that the reduction of IgG glycosylation in RA was correlated with IL-6 and C-reactive protein. These data suggested that upregulation of IgG sialylation modulating by ST6GAL1 was a potent approach to attenuate harmful autoantibody-mediated inflammation in autoimmune disease. In this study, we also observed consistent evidence for the anti-inflammation effect of SA-IgG. We speculate ST6GAL1 is actively upregulated in an effort to protect the body from damage. Plasma ST6GAL1 is increased in patients with an initial onset of IgAN and the more serious the disease, the higher the ST6GAL1 level. Furthermore, elevated ST6GAL1 catalyzes terminal sialylation of IgG to inhibit the production of inflammatory cytokines in PBMCs to delay the progression of IgAN. It indicated the therapeutic application of sialylation of IgG in IgAN.
Patients with IgAN are characterized by a highly variable clinical course ranging from a totally benign incidental condition to rapidly progressive renal failure. Many risk factors including high proteinuria, hypertension, low GFR, and high MEST-C score have been confirmed to predict the adverse kidney outcomes in IgAN. 1 However, the current therapy mainly focused on proteinuria reduction and antihypertension. No effective therapies are available. Disease-specific therapies which slow the progression of disease are urgently needed. Many pieces of clinical evidence suggested a pathogenic role of autoantibodies (mainly IgG) in IgAN. The serum levels of IgG autoantibodies at the time of biopsy were significantly associated with the clinical progression of IgAN.30,31 The modulation of sialylation patterns of IgAN-associated IgG autoantibodies with recombinant ST6GAL1 administration may be a possible novel potential candidate for therapeutic intervention of IgAN.
Limitations
There are several limitations in this study. First, the small sample size and the relatively short follow-up with fewer endpoints reduced the study power. On further expansion, the sample size and extension of the follow-up period would provide more accurate effect estimates of the associations between the levels of ST6GAL1 and disease severity and progression of IgAN. Second, we did not stimulate cells at different concentrations of SA-IgG because of the small amount of extraction. Third, the detailed mechanistic links of how cell surface sialylation affects cell behavior remain to be elucidated. Additional longitudinal studies of more diverse patient cohorts are needed to validate the protective role of ST6GAL1 in IgAN.
Conclusion
Our study indicated that elevated ST6GAL1 was associated with a slower progression of IgAN. It played a protective effect by increasing IgG sialylation to inhibit the production of proinflammatory cytokines in PBMCs. This was the first study to highlight the critical role of ST6GAL1 in delaying the progression of IgAN, which provided a potential therapeutic target for the treatment of IgAN.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223211048644 for Plasma ST6GAL1 regulates IgG sialylation to control IgA nephropathy progression by Youxia Liu, Huyan Yu, Sijing Wu, Xia Yang, Congcong Cao, Fanghao Wang, Junya Jia and Tiekun Yan in Therapeutic Advances in Chronic Disease
Supplemental material, sj-pptx-1-taj-10.1177_20406223211048644 for Plasma ST6GAL1 regulates IgG sialylation to control IgA nephropathy progression by Youxia Liu, Huyan Yu, Sijing Wu, Xia Yang, Congcong Cao, Fanghao Wang, Junya Jia and Tiekun Yan in Therapeutic Advances in Chronic Disease
Acknowledgments
The authors thank all the study subjects for their participation.
Footnotes
Author contributions: Y.X.L. and J.Y.J. conceived the study, Y.X.L., J.Y.J., and T.K.Y. participated in its design and coordination. H.Y.Y., C.C.C., and F.H.W. collected clinical data. H.Y.Y., X.Y., and S.J.W. performed the experiment. Y.X.L. contributed to the writing of the manuscript. All authors read and approved the final manuscript for submission.
Ethical approval: All subjects provided written informed consent. The study protocol was approved by the Institutional Ethical Committee of Tianjin Medical University General Hospital (approval id: ZYY-IRB-SOP-016(F)-002-04).
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported by the National Natural Science Foundation (grant nos. 81600553 and 82000669).
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Youxia Liu  https://orcid.org/0000-0002-9058-0218
https://orcid.org/0000-0002-9058-0218
Availability of data and materials: Raw data used during this study are available from the corresponding author on reasonable request for non-commercial use.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Youxia Liu, Department of Nephrology, Tianjin Medical University General Hospital, No. 154, Anshan Road, Heping District, Tianjin, P.R. China.
Huyan Yu, Department of Nephrology, Tianjin Medical University General Hospital, Tianjin, P.R. China.
Sijing Wu, Department of Nephrology, Tianjin Medical University General Hospital, Tianjin, P.R. China.
Xia Yang, Department of Nephrology, Tianjin Medical University General Hospital, Tianjin, P.R. China.
Congcong Cao, Hematology Department, The People’s Hospital of Pingyi County, Linyi, P.R. China.
Fanghao Wang, Department of Nephrology, Tianjin Medical University General Hospital, Tianjin, P.R. China.
Junya Jia, Department of Nephrology, Tianjin Medical University General Hospital, No. 154, Anshan Road, Heping District, Tianjin, P.R. China.
Tiekun Yan, Department of Nephrology, Tianjin Medical University General Hospital, Tianjin, P.R. China.
References
- 1. Li X, Liu Y, Lv J, et al. Progression of IgA nephropathy under current therapy regimen in a Chinese population. Clin J Am Soc Nephrol 2014; 9: 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hassler JR. IgA nephropathy: a brief review. Semin Diagn Pathol 2020; 37: 143–147. [DOI] [PubMed] [Google Scholar]
- 3. Rodrigues JC, Haas M, Reich HN. IgA nephropathy. Clin J Am Soc Nephrol 2017; 12: 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang K, Li Q, Zhang Y, et al. Clinical significance of galactose-deficient IgA1 by KM55 in patients with IgA nephropathy. Kidney Blood Press Res 2019; 44: 1196–1206. [DOI] [PubMed] [Google Scholar]
- 5. Mao X, Xu Z, Xu X, et al. TGF-beta1 inhibits the autophagy of podocytes by activating mTORC1 in IgA nephropathy. Exp Cell Res 2019; 385: 111670. [DOI] [PubMed] [Google Scholar]
- 6. Mattii L, Segnani C, Cupisti A, et al. Kidney expression of RhoA, TGF-beta1, and fibronectin in human IgA nephropathy. Nephron Exp Nephrol 2005; 101: e16–e23. [DOI] [PubMed] [Google Scholar]
- 7. Premuzic V, Padjen I, Cerovec M, et al. The association of TNF-alpha inhibitors and development of IgA nephropathy in patients with rheumatoid arthritis and diabetes. Case Rep Nephrol 2020; 2020: 9480860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suzuki H, Raska M, Yamada K, et al. Cytokines alter IgA1 O-glycosylation by dysregulating C1GalT1 and ST6GalNAc-II enzymes. J Biol Chem 2014; 289: 5330–5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taniguchi Y, Yorioka N, Kumagai J, et al. Interleukin-6 localization and the prognosis of IgA nephropathy. Nephron 1999; 81: 94–98. [DOI] [PubMed] [Google Scholar]
- 10. Lin Y, Jia J, Guo Y, et al. Corticosteroid for IgA nephropathy: are they really therapeutic? Am J Nephrol 2018; 47: 385–394. [DOI] [PubMed] [Google Scholar]
- 11. Li M, Foo JN, Wang JQ, et al. Identification of new susceptibility loci for IgA nephropathy in Han Chinese. Nat Commun 2015; 6: 7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi M, Ouyang Y, Yang M, et al. IgA nephropathy susceptibility loci and disease progression. Clin J Am Soc Nephrol 2018; 13: 1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu Y, Wang F, Zhang Y, et al. ST6Gal1 is up-regulated and associated with aberrant IgA1 glycosylation in IgA nephropathy: an integrated analysis of the transcriptome. J Cell Mol Med 2020; 24: 10493–10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barb AW, Brady EK, Prestegard JH. Branch-specific sialylation of IgG-Fc glycans by ST6Gal-I. Biochemistry 2009; 48: 9705–9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pagan JD, Kitaoka M, Anthony RM. Engineered sialylation of pathogenic antibodies in vivo attenuates autoimmune disease. Cell 2018; 172: 564–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Engdahl C, Bondt A, Harre U, et al. Estrogen induces St6gal1 expression and increases IgG sialylation in mice and patients with rheumatoid arthritis: a potential explanation for the increased risk of rheumatoid arthritis in postmenopausal women. Arthritis Res Ther 2018; 20: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gudelj I, Salo PP, Trbojevic-Akmacic I, et al. Low galactosylation of IgG associates with higher risk for future diagnosis of rheumatoid arthritis during 10years of follow-up. Biochim Biophys Acta Mol Basis Dis 2018; 1864: 2034–2039. [DOI] [PubMed] [Google Scholar]
- 18. Alavi A, Arden N, Spector TD, et al. Immunoglobulin G glycosylation and clinical outcome in rheumatoid arthritis during pregnancy. J Rheumatol 2000; 27: 1379–1385. [PubMed] [Google Scholar]
- 19. Wang J, Xie P, Huang JM, et al. The new Asian modified CKD-EPI equation leads to more accurate GFR estimation in Chinese patients with CKD. Int Urol Nephrol 2016; 48: 2077–2081. [DOI] [PubMed] [Google Scholar]
- 20. Trimarchi H, Barratt J, Cattran DC, et al. Oxford classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int 2017; 91: 1014–1021. [DOI] [PubMed] [Google Scholar]
- 21. Hayashi K, Takayama M, Abe T, et al. Investigation of metabolic factors associated with eGFR decline over 1 year in a Japanese Population WITHOUT CKD. J Atheroscler Thromb 2017; 24: 863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fu D, Zhong Z, Shi D, et al. ST6GAL1 polymorphisms influence susceptibility and progression of IgA nephropathy in a Chinese Han population. Immunobiology 2020; 225: 151973. [DOI] [PubMed] [Google Scholar]
- 23. Thompson A, Carroll K, Inker LA, et al. Proteinuria reduction as a surrogate end point in trials of IgA nephropathy. Clin J Am Soc Nephrol 2019; 14: 469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee TW, Ahn JH, Park JK, et al. Tumor necrosis factor alpha from peripheral blood mononuclear cells of IgA nephropathy and mesangial cell proliferation. Korean J Intern Med 1994; 9: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anthony RM, Ravetch JV. A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs. J Clin Immunol 2010; 30(Suppl. 1): S9–S14. [DOI] [PubMed] [Google Scholar]
- 26. Wong AH, Fukami Y, Sudo M, et al. Sialylated IgG-Fc: a novel biomarker of chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry 2016; 87: 275–279. [DOI] [PubMed] [Google Scholar]
- 27. Ohmi Y, Ise W, Harazono A, et al. Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis. Nat Commun 2016; 7: 11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bartsch YC, Rahmoller J, Mertes MMM, et al. Sialylated autoantigen-reactive IgG antibodies attenuate disease development in autoimmune mouse models of lupus nephritis and rheumatoid arthritis. Front Immunol 2018; 9: 1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Troelsen LN, Jacobsen S, Abrahams JL, et al. IgG glycosylation changes and MBL2 polymorphisms: associations with markers of systemic inflammation and joint destruction in rheumatoid arthritis. J Rheumatol 2012; 39: 463–469. [DOI] [PubMed] [Google Scholar]
- 30. Maixnerova D, Ling C, Hall S, et al. Galactose-deficient IgA1 and the corresponding IgG autoantibodies predict IgA nephropathy progression. PLoS One 2019; 14: e212254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moldoveanu Z, Suzuki H, Reily C, et al. Experimental evidence of pathogenic role of IgG autoantibodies in IgA nephropathy. J Autoimmun 2021; 118: 102593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223211048644 for Plasma ST6GAL1 regulates IgG sialylation to control IgA nephropathy progression by Youxia Liu, Huyan Yu, Sijing Wu, Xia Yang, Congcong Cao, Fanghao Wang, Junya Jia and Tiekun Yan in Therapeutic Advances in Chronic Disease
Supplemental material, sj-pptx-1-taj-10.1177_20406223211048644 for Plasma ST6GAL1 regulates IgG sialylation to control IgA nephropathy progression by Youxia Liu, Huyan Yu, Sijing Wu, Xia Yang, Congcong Cao, Fanghao Wang, Junya Jia and Tiekun Yan in Therapeutic Advances in Chronic Disease