Abstract
Fibroblasts are important cells for the support of homeostatic tissue function. In inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease, fibroblasts take on different roles (a) as inflammatory cells themselves and (b) in recruiting leukocytes, driving angiogenesis, and enabling chronic inflammation in tissues. Recent advances in single-cell profiling techniques have transformed the ability to examine fibroblast states and populations in inflamed tissues, providing evidence of previously underappreciated heterogeneity and disease-associated fibroblast populations. These studies challenge the preconceived notion that fibroblasts are homogeneous and provide new insights into the role of fibroblasts in inflammatory pathology. In addition, new molecular insights into the mechanisms of fibroblast activation reveal powerful cell-intrinsic amplification loops that synergize with primary fibroblast stimuli to result in striking responses. In this Review, we focus on recent developments in our understanding of fibroblast heterogeneity and fibroblast pathology across tissues and diseases in rheumatoid arthritis and inflammatory bowel diseases. We highlight new approaches to, and applications of, single-cell profiling techniques and what they teach us about fibroblast biology. Finally, we address how these insights could lead to the development of novel therapeutic approaches to targeting fibroblasts in disease.
Introduction
Fibroblasts in health and disease
Fibroblasts are mesenchymal cells that make up the stroma in organ tissues. This Review uses the term “fibroblasts” to collectively describe non-epithelial, non-hematopoietic, and non-endothelial mesenchymal cells that make up tissues throughout the body. In general, fibroblasts can be collectively defined as cells expressing genes encoding collagen I α chain (COL1A) (1), PDGFRα (CD140a), or THY-1 (CD90) in the resting state (2). Previously, fibroblasts were considered as a homogeneous cell population, but emerging evidence indicates that fibroblasts include diverse cell types based on developmental origin (3, 4), anatomic location (5), and function (4, 6, 7). Since the full extent of fibroblast heterogeneity in different tissue compartments is outside of the scope of this Review, we refer readers to excellent recent reviews discussing fibroblast heterogeneity in the skin and other tissue compartments (8–10).
Identifying disease-specific changes in fibroblasts is the key step toward elucidating molecular pathways underlying pathological alterations. Here, we focus on fibroblasts in the context of chronic inflammatory diseases. We begin by discussing technological advances in the field of single-cell profiling and the application of these techniques to query fibroblast heterogeneity in inflammatory diseases. We highlight recent studies in which single-cell techniques were applied to two inflammatory conditions: rheumatoid arthritis (RA) and inflammatory bowel diseases (IBDs). Fibroblasts play an important role in physiological processes including wound healing, extracellular matrix remodeling, immune response, and support for stem cell compartments. In particular, in inflammatory disease research, considerable efforts over the past decades have been devoted to identifying a therapeutic strategy targeting fibroblasts (11–15). For example, in inflammatory diseases such as RA and IBDs, it is now known that fibroblasts are the key cellular source of inflammatory cytokines and chemokines that enable chronic tissue inflammation.
While biologic therapies that block fibroblast-derived cytokines are being used to treat inflammatory diseases (e.g., tocilizumab, targeting IL-6 receptor), currently there are no FDA-approved therapies that directly target fibroblasts in inflammatory diseases. Yet several rationales justify targeting fibroblasts. First, although targeting immune cells in inflammatory disease inevitably compromises immune response to infections, targeting stromal cells may circumvent immunosuppression while abrogating pathology in the involved tissues. Second, fibroblast-targeted therapy may represent an alternative strategy for patients resistant to conventional immunosuppressive therapies and may be combined with targeting of immune cells and factors. While outside the scope of this Review on inflammatory diseases, cancer-associated fibroblasts represent an area of intense research focus, and we refer readers to several recent reviews (10, 16).
Challenges in studying fibroblast pathology in diseases
It is difficult to specifically define and accurately identify fibroblasts. While several genes and protein markers, such as COL1A2 and PDGFRA, have been used to identify cells of non-hematopoietic, non-epithelial, and non-endothelial lineages, there are few or no fibroblast-specific markers. Moreover, the function of many fibroblast markers is incompletely understood; therefore, most markers serve simply as a way to identify fibroblasts in tissue, rather than explain their pathological behavior. Further, different terms are used to describe specific populations of fibroblasts in different tissues.
Advances in single-cell profiling technologies
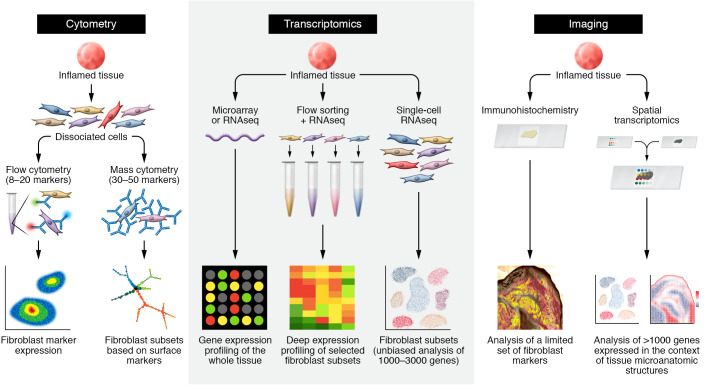
Until recently, the application of cellular profiling techniques to fibroblasts in pathological tissues has been mostly limited to low-dimensionality approaches such as immunohistochemistry techniques and flow cytometry with limited numbers of fibroblast markers. Over the past decade, rapid advances in RNA sequencing, tissue single-cell profiling, and spatial transcriptomic techniques have enabled the unprecedented examination of fibroblasts in pathological tissues (Figure 1). Here we highlight technologies employed in recent studies of fibroblasts in inflammatory diseases.
Figure 1. Approaches to examining fibroblast heterogeneity.
Schematics for studies using cytometry (left), transcriptomic (middle), and imaging (right) techniques, illustrating fibroblast heterogeneity in patient-derived tissues. Left: cytometric analysis of fibroblasts starts with staining of cells from dissociated tissues, followed by antibody staining against surface proteins expressed by fibroblasts. Whereas traditional flow cytometry discerns fibroblasts based on several surface markers — typically three to seven surface protein markers — mass cytometry studies can leverage 30 to 50 surface protein markers. In both approaches, putative fibroblast subsets can be nominated by differential expression of surface markers. Middle: transcriptomic analysis. Bulk transcriptomic analysis by microarray or RNA-Seq measures RNA from whole tissue, but does not discern cellular sources of gene expression. Purification of fibroblast or fibroblast subsets from the tissue by FACS or other cell-purification techniques followed by RNA-Seq enables deep expression profiling of selected fibroblast subsets. Single-cell RNA-Seq obviates the need to identify tissue fibroblasts by specific markers through gene expression profiling across diverse cell populations. Fibroblast subsets and states are determined through unbiased analysis of differentially expressed genes at a single-cell level. Imaging analysis: traditional imaging techniques, such as immunohistochemistry and RNA in situ hybridization, when applied to tissues, are limited to examining a few proteins or genes of interest. Spatial transcriptomic techniques capture gene expression profiles from intact tissue sections, enabling simultaneous gene expression profiling in the context of tissue architecture.
Mass cytometry.
Cytometry by time of flight (CyTOF) overcomes the limitations of conventional fluorescence-based cytometry (where marker use is limited by spectral overlap) by using antibodies conjugated to rare-earth metals rather than fluorophores (17, 18). Cells can be labeled with about 50 antibody markers simultaneously, in addition to metal tags to detect viability, DNA, and barcodes for batch processing. The resulting data represent high-dimensional single-cell phenotyping, including surface markers, intracellular proteins, phosphorylated signal transduction molecules, cytokines, and epigenetic markers, for high-resolution cell state determination (17).
Transcriptomic analysis of purified cell populations.
Transcriptomic profiling through RNA sequencing (RNA-Seq; ref. 19) has enabled a highly detailed examination of genes and pathways enriched in disease tissues. Recently developed, low-input RNA-Seq analysis of cell populations purified by fluorescence-activated cell sorting (FACS) enables the examination of distinct gene expression profiles of distinct populations within diseased tissues. Applied to RA synovia, this approach was recently used to identify distinct fibroblasts (7), T cells (20), and macrophages (21, 22) based on differential expression of surface markers (23).
Single-cell RNA-Seq.
Recently, the advent of single-cell RNA-Seq (scRNA-Seq) has revolutionized our ability to examine gene expression at the single-cell level. In particular, droplet-based scRNA-Seq platforms have further enabled unbiased cell profiling without a priori knowledge of cell markers (24). This approach is particularly helpful for studying fibroblasts in an unbiased manner in various organ tissues where fibroblasts were found to be heterogeneous and to vary across tissues.
High-dimensional imaging analysis.
A rapidly evolving field of technical development is high-dimensional imaging. Whereas traditional immunomicroscopy detects a limited number of genes or proteins, spatial transcriptomic techniques such as Multiplexed Error-Robust Fluorescence In Situ Hybridization (MERFISH; ref. 25) and Slide-seq (26) measure thousands of genes simultaneously across a tissue section while retaining tissue architecture.
Fibroblast heterogeneity in inflammatory diseases
Here, we highlight recent applications of high-dimensional cellular and transcriptomic analysis to fibroblasts to allow unbiased identification of fibroblast subsets. Using gene expression as a surrogate for phenotype and function, we focus on scRNA-Seq to uncover fibroblast heterogeneity and pathology in RA and IBDs.
Fibroblast heterogeneity in RA
RA is an autoimmune disease that causes chronic joint inflammation and results in cartilage and bone erosion in untreated patients (27, 28). Despite advances in treatment over the past several decades, many patients do not achieve sustained disease remission (28). In RA, the site of chronic inflammation is the synovium (29), a mesenchymal tissue organized by cadherin-mediated adhesion connecting fibroblasts into a lining and sublining structure that surrounds the joints (30). In healthy joints, the joint lining layer is one to three cells thick and consists of synovial fibroblasts and macrophages (12–14). Synovial fibroblasts are the parenchymal cells of the joint lining membrane that secrete proteoglycans (lubricin and the mucin hyaluronan) to support normal joint movement (13). Deep to the lining, fibroblasts and endothelial cells make up the loosely connected fibrovascular compartment termed the synovial sublining. In RA, synovial fibroblasts play critical roles in driving chronic inflammation and mediating joint damage in arthritis (13). It has long been appreciated that lining and sublining fibroblasts are morphologically distinct: lining fibroblasts appear more rounded and compacted compared with extended processes coming from sublining fibroblasts (31, 32). Studies using immunohistochemistry and immunofluorescence microscopy have demonstrated differential expression of surface protein markers where expression of lubricin (encoded by PRG4) is higher in the synovial lining (33) and CD90 (encoded by THY1) is higher in the synovial sublining (34), suggesting the existence of different fibroblast populations in the synovial lining and sublining regions (33–35).
To advance our understanding of the heterogeneity of synovial fibroblasts and to gain insight into their function, our group applied flow cytometry using the stromal markers PDPN, CDH11, CD34, and CD90 to discern putative fibroblast subsets (7). Using microarray and low-input RNA-Seq followed by principal component analysis, we identified three synovial fibroblast subsets with unique transcriptomic profiles: PDPN+CD34–CD90– lining fibroblasts, PDPN+CD34+CD90+ sublining fibroblasts, and PDPN+CD34–CD90+ sublining fibroblasts (7). Among the three fibroblast subsets identified in this study, fibroblasts characterized by high expression of CD90 were highly expanded in RA and correlated with the degree of joint tissue inflammation (7). In contrast, synovia in osteoarthritis was characterized by the expansion of lining (PDPN+CD34–CD90–) fibroblasts. Further, we found that sublining fibroblasts exhibit a secretory phenotype characterized by high expression of the chemokines IL-6, CXCL12, and CCL2 when exposed to TNF in vitro, reflected by enhanced ability to recruit monocytes in vitro (7). In contrast, lining fibroblasts are the source of the metalloproteinases MMP-1 and MMP-3, consistent with lining fibroblasts being the main driver of invasion and cartilage degradation (7, 36). A separate study using scRNA-Seq to examine synovial cellular heterogeneity independently confirmed the presence of distinct lining and sublining fibroblast subsets in RA synovia (37).
The observation that sublining rather than lining fibroblasts are the predominantly expanded population in inflamed RA synovia was later confirmed in three independent studies (6, 23, 38). In a study from the Accelerating Medicines Partnership RA/Systemic Lupus Erythematosus (AMP RA/SLE) Consortium, the parallel application of mass cytometry and unbiased scRNA-Seq clustering identified four synovial fibroblast populations with distinct transcriptomic profiles: a CD34+ sublining fibroblast population (F1 cluster); a fibroblast subset characterized by expression of CD90 and HLA-DRA (F2 cluster) that is markedly expanded in inflamed RA synovia (ref. 23 and Figure 2); a population of fibroblasts characterized by DKK3 expression (F3 cluster), a negative regulator of Wnt signaling (39); and PRG4+ lining fibroblasts (F4 cluster). Interestingly, gene expression profiling by scRNA-Seq confirmed that among fibroblasts, CD90+HLA-DR+ fibroblasts express most of the IL-6, high levels of CXCL12, and interferon-stimulated genes (ISGs), suggesting that these fibroblasts are highly inflammatory in RA (23). An interesting observation regarding CD34+ synovial fibroblasts is that mesenchymal CD34 expression has been described in a unique cell type termed telocytes (40, 41). Telocytes are characterized by the presence of telopodes that create contact with nearby cells and have been found in many organ tissues (40, 41), including in the human synovium (42). Additional imaging and functional studies are required to determine whether the CD34+ fibroblasts observed in the transcriptomic analysis represent a synovial telocyte equivalent.
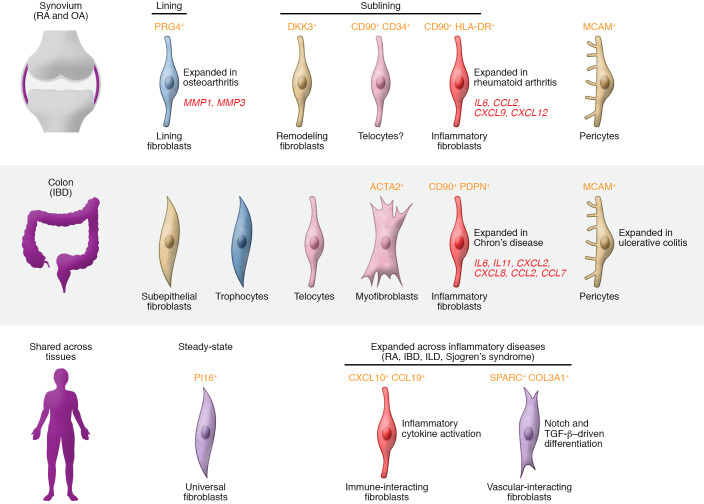
Figure 2. Fibroblast subsets in inflammatory diseases.
Fibroblast subsets in chronic inflammatory diseases revealed by single-cell RNA-Seq. In RA, four synovial fibroblast subsets were recently described. In RA, CD90+ sublining fibroblasts are expanded, whereas PRG4+ synovial lining fibroblasts are expanded in osteoarthritis. Among the sublining fibroblasts, an inflammatory fibroblast subset (CD90+HLA-DR+) expressing high levels of IL-6 and chemokines is highly expanded in patients with active RA. In IBDs, several fibroblast subsets have been described, including subepithelial, trophocytes, and telocytes. An inflammatory fibroblast subset characterized by high expression of IL6, IL11, and CXCL8 was found to be expanded in Crohn’s disease. PI16+ fibroblasts represent a universal fibroblast subset found across organ tissues in mice and humans (64). Two fibroblast subsets — CXCL10+CCL19+ immune-interacting fibroblasts and SPARC+COL3A1+ vascular-interacting fibroblasts — were identified across four inflammatory diseases.
Whether or not transcriptional differences in synovial fibroblasts represent functionally distinct cell types in the context of inflammatory arthritis was recently addressed (6). Using a mouse model of inflammatory arthritis in which the transfer of serum containing arthritogenic autoantibody leads to severe joint inflammation and joint destruction (43), the authors found that, like in RA in humans, FAP+CD90+ fibroblasts undergo significant expansion in response to inflammation. Remarkably, adoptive transfer of synovial CD90+ sublining fibroblasts, but not CD90– lining fibroblasts, worsened joint inflammation. In contrast, adoptive transfer of CD90– lining fibroblasts caused worsened cartilage and bone erosion (6).
Recognizing the important transcriptomic and functional differences in lining and sublining fibroblasts, and the sublining expansion in RA, we asked what might drive the differentiation of CD90+ sublining fibroblasts. Imaging analysis of RA synovia suggested that CD90+ fibroblasts surround synovial vasculature, leading to a hypothesis that CD90+ fibroblasts may be driven by vascular endothelium–derived signals (7, 35, 38). We found that endothelium-derived Notch signaling drives a key step in CD90+ fibroblast differentiation (38). The Notch pathway represented an attractive candidate in governing sublining fibroblast differentiation given its well-documented role in promoting mural cell differentiation during development (44–46). For details on the Notch pathway, we refer readers to excellent reviews on Notch in development (47, 48), cancer (49), and inflammatory arthritis (50). Among the four Notch receptors, we identified Notch3 expression to be mostly restricted to perivascular fibroblasts. We demonstrated a critical role for Notch3 signaling in inflammatory arthritis, since Notch3–/– mice and mice treated with anti-Notch3 mAb showed attenuated serum-transfer arthritis (38). While the role of Notch signaling through Notch1 in synovial fibroblast activation and proliferation was demonstrated previously (51, 52), our study identified Notch3 receptor signaling as a critical signal in driving CD90+ fibroblast differentiation and implicated Notch3 receptor as a therapeutic target in inflammatory arthritis.
Fibroblast remodeling in IBDs
IBDs are chronic inflammatory conditions affecting the gastrointestinal tract (53). Like RA, IBD is characterized by chronic tissue inflammation driven by the interaction between fibroblasts and local immune cells (53). Intestinal fibroblast heterogeneity is well characterized because of the appreciation that intestinal fibroblasts are instrumental in gut homeostasis and disease. For an in-depth discussion of the biology and functions of intestinal fibroblasts, we refer readers to recent reviews (54–56).
The major stromal cell types are fibroblasts, myofibroblasts, and perivascular fibroblasts/pericytes (ref. 54 and Figure 2). It is known that intestinal fibroblasts exhibit functional zonation characterized by distinct phenotypes along the crypt to the villus axis (55). Consistent with anatomical zonation as a key determinant of intestinal fibroblast heterogeneity, one of the first studies to examine human colonic fibroblasts by scRNA-Seq identified multiple fibroblast subsets characterized by differential expression of Wnt and BMP signaling genes, reflecting distinct positions along the crypt-villus anatomical axis (57). In another study of IBDs, Kinchen et al. (58) performed scRNA-Seq on colonic mesenchymal cells from patients with ulcerative colitis by flow sorting to enrich for CD90+ cells. They identified four colonic stromal fibroblast populations (referred to as S1–S4), myofibroblasts, and two pericyte clusters. Among the fibroblast subtypes, S1 fibroblasts were distributed throughout the lamina propria and exhibited elevated expression of TNF-responsive genes. S2 fibroblasts were restricted to areas close to the epithelium and displayed high expression of BMPs (BMP2 and BMP5) and noncanonical Wnt ligands (WNT5a and WNT5b). The localization of S2 fibroblasts near the colonic epithelium and their expression of morphogens suggest this population’s role in epithelial maintenance and regeneration. Interestingly, the authors found the emergence of an activated fibroblast subset (S4) that is highly expanded in ulcerative colitis and characterized by expression of IL-6, MHC class II invariant chain (CD74), IL-33, and homeostatic cytokines mediating lymphocyte recruitment and retention (CCL19 and CCL21), suggesting that S4 fibroblasts acquired at least some lymph node fibroblastic reticular cell–like features.
Another study described inflammation-associated fibroblasts that were drastically expanded in the inflamed tissues of some IBD patients. These fibroblasts were characterized by the expression of IL-24, IL-11, and IL-13RA2, implicating a role in inflammation and fibrosis. Interestingly, the authors identified high expression of OSMR, the receptor for oncostatin M (OSM), and noted that OSM expression correlated with patients’ failure to respond to anti-TNF therapy (59). Using bulk expression data to define TNF resistance, the authors found a strong enrichment of the TNF-resistance signature in inflammation-associated fibroblasts, suggesting a role for inflammation-associated fibroblasts in mediating the TNF-inadequate response.
In a separate scRNA-Seq study, Martin et al. (60) identified pericytes, vascular smooth muscle cells, and two subtypes of fibroblasts in patients with Crohn’s disease. One of the fibroblast populations exhibited an activated phenotype, characterized by high expression of CD90 and PDPN (podoplanin); IL-6; IL-11; neutrophil-attracting CXCL2, CXCL8, CXCL1, and CXCL5; and monocyte-recruiting CCL2 and CCL7. Using a unique signature that incorporated the activated fibroblast gene expression profile, the authors found an association between the enrichment of this signature in patients before treatment and resistance to anti-TNF therapy.
Emerging themes in fibroblast pathology across inflammatory diseases
Fibroblast subsets and inflammatory states
Fibroblasts are a major source of IL-6, and many cytokines and mediators of inflammation have been found to significantly induce IL-6 expression in fibroblasts, including TNF, IL-17, IL-1β, LPS, and IFN-α, -β, and -γ (61). Observations from in vitro studies of fibroblast activation suggest that fibroblasts in inflamed tissues are capable of responding to a variety of inflammatory cytokines and activators. Therefore, it stands to reason that the specific nature of inflammatory fibroblast phenotype observed in inflammatory diseases is likely shaped by the tissue- and disease-specific inflammatory milieu.
In RA synovium, expansion of CD90+HLA+ fibroblasts correlates with the degree of infiltration of IFN-γ–producing lymphocytes (23). This observation is congruent with the knowledge that lymphocyte infiltration in synovial tissue is a hallmark of RA pathology phenotype. Indeed, the shape of the inflammatory fibroblast is primarily an interferon-activated phenotype, characterized by high expression of interferon-stimulated genes (ISGs) such as CXCL9, CXCL10, HLA-DR, and CD74 as well as the NF-κB target genes IL-6 and CCL2 (23). The enrichment of ISGs in RA inflammatory fibroblasts suggests a model in which lymphocyte-derived IFN-γ activates surrounding fibroblasts, which leads to reprogramming of native sublining fibroblasts toward an inflammatory phenotype. In this study, the majority of samples analyzed were obtained from patients with early, treatment-naive RA. It remains to be determined whether the inflammatory fibroblast state identified here is altered at a later stage of the disease, in patients with RA refractory to conventional treatments, or in RA patients in whom tissue inflammation is driven by myeloid-dominant phenotype (23, 62, 63).
In IBDs, inflammatory fibroblasts are characterized by the expression of the NF-κB downstream targets IL-6, IL-11, and neutrophil-attracting CXCL2, CXCL8, and CCL2 (58, 59). In contrast to studies in RA, the inflammatory fibroblast gene signatures identified in IBDs are mostly devoid of typical ISGs, such as HLA-DR and CD74.
Recently, cross-tissue examination of fibroblasts by scRNA-Seq has highlighted shared fibroblast phenotypes across a spectrum of inflammatory and fibrotic diseases (64, 65). In the first study, Buechler et al. identified two common, universal fibroblast subsets in steady-state tissues that can give rise to activated fibroblast states in diseases, including an Lrrc15+ fibroblast subset that is expanded in mouse models of arthritis, skin wounds, fibrosis, and pancreatic cancer (64). In the second study (65), at least two shared fibroblast phenotypes were identified across human tissues from four inflammatory diseases: RA, IBD, interstitial lung disease, and Sjögren’s syndrome (Figure 2). The first shared fibroblast subset in inflammatory diseases is characterized by high expression of the chemokines CXCL10 and CCL19, suggesting an inflammatory fibroblast phenotype induced by cytokine activation. A second shared fibroblast subset expanded in inflammatory diseases is characterized by the expression of the extracellular matrix–related genes SPARC and COL3A1 (65). These fibroblasts exhibit high expression of genes downstream of the Notch and TGF-β pathway, suggesting a morphogen-driven differentiation. In summary, the inflammatory fibroblast phenotype is a shared phenotype in inflammatory diseases where fibroblasts, upon activation, become a dominant producer of inflammatory cytokines in affected tissues.
Fibroblast zonation
Functional zonation is a central concept in the structural design of tissues and organs and represents a division of labor among specialized cell types along with a defined spatial orientation that together supports specific tissue functions. While functional zonation is perhaps best appreciated in the epithelial compartments, spatial and functional compartmentalization of fibroblasts by morphogen signals, and alteration of these signals in disease, are emerging as the second theme in single-cell profiling of inflammatory diseases.
In the synovium, functional compartmentalization of fibroblasts has long been suspected based on differential expression of proteoglycans (34) and chemokines (7) with regard to their position within the lining or sublining compartment, respectively. We found that synovial fibroblast positional identity is maintained by local niche signals from the microenvironment and that endothelium-derived Notch signaling is a key positional signal for perivascular fibroblast identity (38).
In a healthy intestine, the fibroblast compartment is conceived to exhibit functional zonation based on a cell’s anatomical position relative to the intestinal crypt and villus lamina propria. Pericryptal fibroblasts in the crypt provide a source of Wnts and R-spondins, while fibroblasts near the villus lamina propria predominantly produce BMPs, providing a gradient of morphogen signals to ensure proper epithelial differentiation (54, 66–69). A recent study using scRNA-Seq elegantly demonstrated that the BMP activity level in the intestine follows a spatial gradient at a single-cell level (68, 69). Another study leveraging spatial transcriptomics and scRNA-Seq demonstrated the presence of a BMP morphogen gradient along the crypt-villus axis, and that disruption of this gradient using a transgenic approach in animals disrupts proper intestinal epithelial formation (69), highlighting the importance of fibroblast functional zonation in intestinal homeostasis. Given the importance of the morphogen gradients in establishing and maintaining the intestinal epithelium, it remains to be determined whether the fibroblast-derived morphogen gradients are perturbed in the context of IBD, in which the intestinal epithelium undergoes remodeling due to injury and chronic inflammation (53).
Fibroblast activation and the role of amplification loops
Fibroblasts may be synergistically activated by combinations of primary inflammatory cytokines. For example, TNF and IL-17A synergistically activate fibroblasts (70, 71). Subsequently, the transcriptional regulation of this synergistic activation was found to depend on the expression of cut-like homeobox 1 (CUX1) and IκBζ (also known as NFKBIZ, an atypical member of the IκB family), which in turn controls the amplitude of a program of chemokine expression (72). Specifically, CUX1 is involved in CXCL1, CXCL2, CXCL3, and CXCL8 regulation, while IκBζ, despite its name, instead of inhibiting NF-κB, helps sustain NF-κB transcriptional activity to upregulate striking levels of IL-6, CXCL8, and MMP-3. In another example, OSM is mainly derived from myeloid cells and T lymphocytes (73) and provides a paracrine activation signal for inflammatory fibroblasts. OSM alone, and more potently the combination of OSM with other inflammatory cytokines, such as TNF, drives an enhanced transcriptional response with inflammatory cytokine activation, including expression of IL-6, MCP-1, ICAM-1, VEGF, and CXCL9 (73–75) (59). Prominent fibroblast activation through OSMR signaling has been implicated in TNF-inadequate responses to therapy for IBDs (59).
Fibroblast activation appears to be a two-step process in which the primary, typically exogenous activators like TNF, IL-17, IL-1, and TLR agonists then trigger powerful fibroblast cell-autonomous autocrine amplification loops such as via leukemia inhibitory factor (LIF)/LIF receptor (LIFR). Thus, for fibroblasts to achieve maximal transcriptional responses to exogenous inflammatory cytokines, they use autocrine signaling involving the gp130 coreceptor to reinforce the primary inflammatory cytokine activation. The gp130 coreceptor binds to cytokine-specific receptor chains that recognize the IL-6 family of ligands including IL-6, LIF, and IL-11, all of which are produced by fibroblasts and can act in an autocrine manner, as well as OSM, which can act in a paracrine manner (61, 76). Nguyen et al. (61) first demonstrated that expression of IL-6 along with a module of other inflammatory cytokines and chemokines is regulated by an amplification feedback loop involving LIF, LIFR, and STAT4 (61). Since fibroblasts produce LIF rapidly after primary stimulation, LIF then acts in an autocrine manner via LIFR to profoundly upregulate and sustain the production of IL-6 and an array of other inflammatory effector genes. Secreted IL-6 also can interact with soluble IL-6 receptor to form a complex with gp130 to drive IL-6, CXCL1 (also known as KC), and MIP2 expression in a positive-feedback autocrine signaling loop in murine fibroblasts (77). OSM shares 22% sequence homology with LIF, and these two ligands are thought to arise through gene duplication (78). OSMR signaling is mostly STAT3 dependent, while LIFR signaling can activate both STAT3 and STAT4 in fibroblasts (72).
Epigenetic control of pathogenic fibroblast behavior
Epigenetic imprinting is an important contributor to aggressive fibroblast behavior, and abnormal DNA methylation patterns in fibroblasts are associated with joint damage and inflammation (13). In a large-scale epigenetic study that used whole-genome DNA methylation, chromatin accessibility, and transcriptome analysis of synovial fibroblasts, Huntingtin-interacting protein 1 was identified as an unexpected regulator of fibroblast invasion and migration (79). More recently, it was shown that pathogenic mechanisms are not just within joints but also include location-specific changes in gene expression, DNA methylation, and response to cytokine activation (79–81). For example, compared with fibroblasts derived from upper extremities, fibroblasts derived from knee joints exhibited increased MMP-1 production in response to TNF stimulation in vitro (81). Advances in epigenome profiling techniques such as the assay for transposase-accessible chromatin using sequencing (ATAC-seq; ref. 82) have now enabled assessment of chromatic accessibility of fibroblast subpopulations in healthy and disease states (64). The ability to examine the chromatin landscape of individual fibroblasts at a single-cell level by scATAC-seq (83) will be an exciting new direction for understanding fibroblast pathology in inflammatory diseases.
Therapeutic approaches to target fibroblasts
in inflammatory diseases
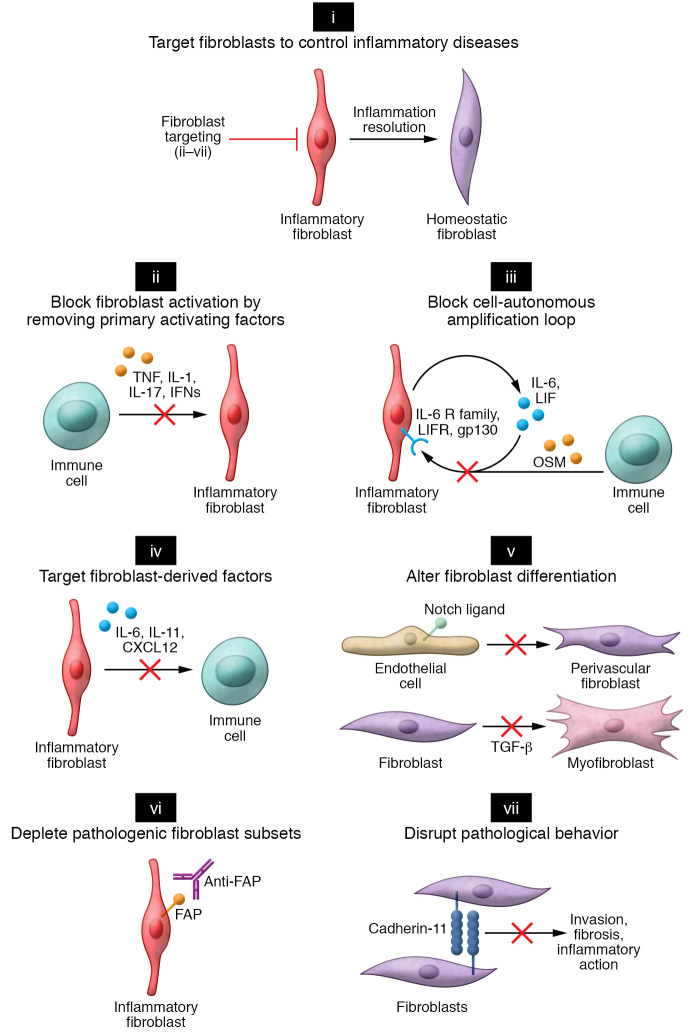
Here, we discuss how new insights into fibroblast-mediated pathology help to inform therapeutic paradigms (Figure 3).
Figure 3. Approaches to targeting fibroblasts in inflammatory diseases.
(i) Strategies to target fibroblasts in inflammatory disease can be categorized into several broad categories. (ii) Preventing fibroblast activation by neutralizing key cytokines such as TNF, IL-1, IL-17, and IFNs that lead to fibroblast inflammatory activation. (iii) Primary activating factors. The full extent of fibroblast inflammatory activation requires secreted autocrine factors (LIF) and paracrine factors (OSM). Strategies to block fibroblast amplification factors could result in attenuating the fibroblast inflammatory response. (iv) Targeting key fibroblast-derived factors crucial for immune cell activation or recruitment. (v) Preventing pathogenic fibroblast differentiation by blocking morphogen signaling pathways. In RA, Notch-mediated vascular fibroblasts could be targeted by blockade of Notch3 receptor signaling. In fibrotic disease, myofibroblast differentiation driven by TGF-β could be targeted by blockade of TGF-β signaling. (vi) Depleting pathogenic fibroblast subsets. Conceptually, pathogenic fibroblasts in inflammatory disease expressing distinct surface markers, such as fibroblast activation protein (FAP), could be targeted therapeutically through an antibody-mediated depletion strategy. Markers specifically expressed in disease-associated fibroblasts would enable depletion of pathogenic subsets without disruption of normal fibroblast functions in noninvolved organ tissues. (vii) Disrupting pathological processes mediated by fibroblasts. In this example, an inhibitory antibody against cadherin-11 could potentially prevent fibroblast-mediated invasion and blunt cytokine-induced inflammation.
Preventing inflammatory fibroblast activation
Considering that fibroblasts respond to diverse cytokines and activation signals, understanding those pathways and blocking them may be a key approach to targeting fibroblasts. The idea of preventing fibroblast activation by blocking specific fibroblast-activating cytokines is consistent with clinically proven therapies such as anti-TNF in RA and IBDs. Blocking TNF prevents fibroblast activation but is not specific; it also blocks activation of many other cell types, such as endothelial cells, epithelial cells, and leukocytes, and reshapes the disease response. However, given the large number of primary factors that can activate fibroblasts (TNF, IL-1, TLR agonists), blocking a single factor may fail to stop fibroblast activation when other stimuli persist. Thus, it would be appealing to find an approach that could block fibroblast activation endogenously no matter what external stimuli are present. Targeting fibroblast amplification loops might be an appealing approach since this can block activation across a range of primary activators (59, 61, 72). The identification of LIF and OSM as potent amplifiers of inflammatory fibroblast activation in RA (59, 61) and IBDs (59), respectively, highlights the importance of the gp130 receptor cytokine family in mediating a sustained fibroblast inflammatory response. A therapeutic strategy targeting LIF, OSM, or gp130 signaling in inflammatory diseases may attenuate inflammation by blocking critical amplifying signals necessary for sustained inflammatory fibroblast activation.
Targeting fibroblast-derived effector molecules
Fibroblasts are a key source of inflammatory cytokines and chemokines in inflammatory diseases such as RA (13). In fact, fibroblasts may produce larger amounts of inflammatory factors than leukocytes, as they are the dominant source of inflammatory cytokines like IL-6 (61). One approach to target inflammatory fibroblasts is to block the key cytokines and chemokines they secrete. As an example of this approach, the development of IL-6 receptor inhibitory antibodies has transformed the treatment of inflammatory diseases. IL-6 receptor blockade through monoclonal antibodies is routinely used in the treatment of chronic inflammatory conditions including RA, juvenile idiopathic arthritis, adult-onset Still’s disease, giant cell arteritis, Takayasu arteritis, and cytokine release syndrome (84). In addition to IL-6, the IL-6 family member IL-11 is highly expressed in fibroblasts (85). IL-11 was recently discovered to be a central driver of fibrotic conditions involving the cardiovascular system (86), lung (87, 88), and skin (89). It remains to be determined whether therapeutic targeting of IL-11 will successfully attenuate fibroblast-mediated fibrotic diseases. Leukocyte-recruiting chemokines such as CCL2 and CXCL12 are another class of fibroblast-derived factors critical for tissue inflammation, although the clinical experience targeting chemokine–chemokine receptor signaling in RA has generally been less successful (90, 91).
Disrupting pathological fibroblast behavior
Another approach to targeting fibroblasts could be directed at regulators of pathological fibroblast behavior. Cadherin-11 is a type II cadherin that mediates cell-cell contact and is essential for morphogenesis and architecture of the synovium (30, 32, 92, 93). However, cadherin-11 also regulates fibroblast migration and invasion (94) and augments fibroblast responses to activating cytokines (95). Further, cadherin-11 expression is upregulated by inflammation such as by TNF stimulation (96), and is localized to the pannus-cartilage junction (97). Importantly, in mouse models, genetic deletion of Cdh11 (encoding cadherin-11) or antibody-mediated blockade reduced arthritis severity and partially reversed established arthritis (30, 94). Interestingly, cadherin-11’s role in regulating fibroblast inflammation extends beyond inflammatory arthritis, as it has been reported that cadherin-11 regulated adipose tissue inflammation and was a crucial mediator of fibrosis in a bleomycin-induced model of skin fibrosis (98), pulmonary fibrosis (99), and cardiac fibrosis (100–102). Recently, a monoclonal antibody against cadherin-11 was evaluated in a phase II trial involving RA patients with inadequate response to anti-TNF therapy, representing the first time a fibroblast-targeted therapy has been tested in inflammatory diseases (103). While this study did not show a therapeutic benefit of anti–cadherin-11 treatment, it was examined in patients using ongoing anti-TNF therapy, which is known to downregulate cadherin-11 expression, limiting the anti–cadherin-11 treatment’s ability to alter disease pathology.
Modulating fibroblast differentiation
An emerging concept from fibroblast studies in inflammatory and fibrotic diseases is that fibroblast differentiation is maintained by active, local microenvironment–derived morphogens (38, 104). Morphogens are conserved signaling molecules that induce specific cell fate and function (105). These include members of the Hedgehog, Wnt, Notch, and TGF-β families. Identification of key morphogen signals that regulate fibroblast differentiation in diseases provides a therapeutic opportunity to block differentiation that is required to drive pathology.
TGF-β is perhaps the most well-studied morphogen in mediating pathological fibroblast differentiation. The role of TGF-β signaling in myofibroblast differentiation and its role in fibrosis have been studied extensively in the context of fibrotic diseases (105–107). Further, studies of the tumor microenvironment have recently revealed TGF-β’s critical role in the differentiation of cancer-associated fibroblasts and immune evasion (104, 108, 109). Several anti–TGF-β agents are being developed and are currently in clinical trials (110).
In addition to TGF-β signaling, Notch signaling has been implicated in fibroblast activation in pulmonary fibrosis (111, 112), kidney inflammation and fibrosis (113, 114), and inflammatory arthritis (38, 115). Importantly, several Notch receptor inhibitory antibodies (116–118) are being developed as a cancer therapy, highlighting the possibility of repurposing Notch receptor inhibition for the treatment of inflammatory and fibrotic diseases.
Beyond the study of Notch and TGF-β signaling, the role of Wnt, Hedgehog, and other morphogens in shaping fibroblast response in inflammatory or fibrotic diseases remains to be studied.
Depleting pathological fibroblasts
Targeting of pathological immune cells by selective depletion is the basis for the use of therapies such as rituximab (targeting CD20 on B cells) and alemtuzumab (targeting CD52 on lymphocytes). Studies using mAbs and scRNA-Seq are implicating a growing number of fibroblast subsets and states in driving pathology in inflamed tissues, which could enable therapeutics aimed at the selective deletion of pathological fibroblast subsets. In a mouse model, selective depletion of stromal cells expressing fibroblast activation protein (FAP) (119) using diphtheria toxin abrogated inflammatory arthritis (6). Several challenges need to be addressed for the clinical development of this approach, including identifying disease- and tissue-specific fibroblast markers and determining whether they can abrogate disease with minimal unwanted effects on normal tissue function. The ability of fibroblasts to evade apoptosis through activation of survival signals poses a further challenge to selective depletion strategies (120).
Future success in targeting fibroblasts will rely on a clearer understanding of which patient population(s) will benefit from a fibroblast-targeted therapy. In RA, for example, a recent study examining synovial tissue transcriptomic profiles from untreated patients suggested that synovial tissue pathotype signatures in a subset of RA patients whose synovial tissues are enriched with a fibroblast gene signature showed the poorest subsequent response to conventional disease-modifying anti-rheumatic drug (DMARD) treatment (62, 63). It would be interesting to determine whether the fibroid signature defined in this study represents a specific fibroblast activation state or expansion of a particular fibroblast subset. Elucidating molecular pathways associated with failure to respond to traditional oral DMARDs or biologic therapies and determining how fibroblasts in treatment-resistant patients may contribute to this escape will be crucial for development of novel treatment options. Indeed, fibroblast-targeted therapy may be most relevant in treatment-resistant patients who have failed standard immunosuppressive treatments. As studies of fibroblasts in IBDs point to a role of inflammatory fibroblasts in TNF-inadequate responders (57, 60), it is tempting to speculate that therapies targeting inflammatory fibroblasts in such patients could overcome the inadequate responses.
Targeting leukocytes and inflammatory cytokines has resulted in a wide range of important targeted therapeutics that have changed the medical approach to inflammatory disease. Yet for nearly all diseases, these therapeutics result in a partial reduction of disease activity with no cures and only a fraction of patients who achieve remission. Further, virtually all antiinflammatory therapeutics are immunosuppressive. Recognizing the important role of the fibroblastic stroma in producing inflammatory factors draws attention to the value of targeting it. Besides their role as inflammatory cells, the fibroblastic stromal cells may play a critical role in enabling chronic inflammation in tissues, where their production of chemokines and growth factors may be critical to perpetuating the recruitment, retention, and survival of leukocytes. Studies in mouse models now make it clear that blockade of fibroblast differentiation (38) or selective deletion (6, 119) can cause the entire immunopathological tissue reaction to unravel. Thus, targeting the fibroblastic stroma may provide a powerful new therapeutic approach in end-organ inflammatory diseases.
Conclusions and future directions
Our knowledge of fibroblast biology and fibroblast-mediated pathology is undergoing tremendous growth as a result of new, powerful single-cell and imaging techniques that enable an unprecedented examination of fibroblast heterogeneity in pathology. Applied to inflammatory diseases, this “disease deconstruction” approach has prompted a renewed appreciation for a remarkable diversity of fibroblast phenotypes and putative functions in both healthy and pathological states. Defining the markers, mechanisms, and pathways that drive activation and differentiation of pathological fibroblast states will enable the identification of targets for fibroblast therapeutics that may impact a range of inflammatory, fibrotic, and malignant diseases.
Acknowledgments
We thank members of the Brenner laboratory for their helpful discussion. KW is supported by a National Institute of Arthritis and Musculoskeletal and Skin Diseases award (K08AR077037), a Rheumatology Research Foundation Innovative Research award, and a Burroughs Wellcome Fund Career Award for Medical Scientists.
Version 1. 10/15/2021
Electronic publication
Footnotes
Conflict of interest: MBB has received consulting fees from GSK and 4FO Ventures, owns stock options in Mestag Therapeutics, and has conducted research sponsored by Celgene/Bristol Myers Squibb. KW serves as a consultant for Mestag Therapeutics.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(20):e149538.https://doi.org/10.1172/JCI149538.
Contributor Information
Kevin Wei, Email: kwei@bwh.harvard.edu.
Hung N. Nguyen, Email: hnguyen25@bwh.harvard.edu.
Michael B. Brenner, Email: MBRENNER@research.bwh.harvard.edu.
References
- 1.Lynch MD, Watt FM. Fibroblast heterogeneity: implications for human disease. J Clin Invest. 2018;128(1):26–35. doi: 10.1172/JCI93555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buechler MB, Turley SJ. A short field guide to fibroblast function in immunity. Semin Immunol. 2018;35:48–58. doi: 10.1016/j.smim.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Roelofs AJ, et al. Joint morphogenetic cells in the adult mammalian synovium. Nat Commun. 2017;8:15040. doi: 10.1038/ncomms15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driskell RR, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504(7479):277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinn JL, et al. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006;2(7):e119. doi: 10.1371/journal.pgen.0020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croft AP, et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature. 2019;570(7760):246–251. doi: 10.1038/s41586-019-1263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizoguchi F, et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat Commun. 2018;9(1):789. doi: 10.1038/s41467-018-02892-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plikus MV, et al. Fibroblasts: origins, definitions, and functions in health and disease. Cell. 2021;184(15):3852–3872. doi: 10.1016/j.cell.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson S, et al. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat Rev Immunol. doi: 10.1038/s41577.021. [published online April 28, 2021]. [DOI] [PubMed] [Google Scholar]
- 10.Koliaraki V, et al. The mesenchymal context in inflammation, immunity and cancer. Nat Immunol. 2020;21(9):974–982. doi: 10.1038/s41590-020-0741-2. [DOI] [PubMed] [Google Scholar]
- 11.Filer A, et al. Targeting the stromal microenvironment in chronic inflammation. Curr Opin Pharmacol. 2006;6(4):393–400. doi: 10.1016/j.coph.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dakin SG, et al. Pathogenic stromal cells as therapeutic targets in joint inflammation. Nat Rev Rheumatol. 2018;14(12):714–726. doi: 10.1038/s41584-018-0112-7. [DOI] [PubMed] [Google Scholar]
- 13.Nygaard G, Firestein GS. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat Rev Rheumatol. 2020;16(6):316–333. doi: 10.1038/s41584-020-0413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noss EH, Brenner MB. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol Rev. 2008;223(1):252–270. doi: 10.1111/j.1600-065X.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- 15.Pradhan RN, et al. A bird’s eye view of fibroblast heterogeneity: A pan-disease, pan-cancer perspective. Immunol Rev. 2021;302(1):299–320. doi: 10.1111/imr.12990. [DOI] [PubMed] [Google Scholar]
- 16.Derynck R, et al. TGFβ biology in cancer progression and immunotherapy. Nat Rev Clin Oncol. 2021;18(1):9–34. doi: 10.1038/s41571-020-0403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spitzer MH, Nolan GP. Mass cytometry: single cells, many features. Cell. 2016;165(4):780–791. doi: 10.1016/j.cell.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ermann J, et al. Immune cell profiling to guide therapeutic decisions in rheumatic diseases. Nat Rev Rheumatol. 2015;11(9):541–551. doi: 10.1038/nrrheum.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, et al. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10(1):57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao DA, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature. 2017;542(7639):110–114. doi: 10.1038/nature20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Culemann S, et al. Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature. 2019;572(7771):670–675. doi: 10.1038/s41586-019-1471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo D, et al. HBEGF+ macrophages in rheumatoid arthritis induce fibroblast invasiveness. Sci Transl Med. 2019;11(491):eaau8587. doi: 10.1126/scitranslmed.aau8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang F, et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat Immunol. 2019;20(7):928–942. doi: 10.1038/s41590-019-0378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macosko EZ, et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161(5):1202–1214. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moffitt JR, Zhuang X. RNA Imaging with Multiplexed Error-Robust Fluorescence In Situ Hybridization (MERFISH) Methods Enzymol. 2016;572:1–49. doi: 10.1016/bs.mie.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriques SG, et al. Slide-seq: a scalable technology for measuring genome-wide expression at high spatial resolution. Science. 2019;363(6434):1463–1467. doi: 10.1126/science.aaw1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7(6):429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 28.Smolen JS, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. doi: 10.1038/nrdp.2018.1. [DOI] [PubMed] [Google Scholar]
- 29.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 30.Lee DM, et al. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315(5814):1006–1010. doi: 10.1126/science.1137306. [DOI] [PubMed] [Google Scholar]
- 31.Fassbender HG. Histomorphological basis of articular cartilage destruction in rheumatoid arthritis. Coll Relat Res. 1983;3(2):141–155. doi: 10.1016/S0174-173X(83)80040-5. [DOI] [PubMed] [Google Scholar]
- 32.Kiener HP, et al. Cadherin-11 induces rheumatoid arthritis fibroblast-like synoviocytes to form lining layers in vitro. Am J Pathol. 2006;168(5):1486–1499. doi: 10.2353/ajpath.2006.050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhee DK, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115(3):622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer DG, et al. Features of synovial membrane identified with monoclonal antibodies. Clin Exp Immunol. 1985;59(3):529–538. [PMC free article] [PubMed] [Google Scholar]
- 35.Choi IY, et al. Stromal cell markers are differentially expressed in the synovial tissue of patients with early arthritis. PLoS One. 2017;12(8):e0182751. doi: 10.1371/journal.pone.0182751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Müller-Ladner U, et al. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am J Pathol. 1996;149(5):1607–1615. [PMC free article] [PubMed] [Google Scholar]
- 37.Stephenson W, et al. Single-cell RNA-seq of rheumatoid arthritis synovial tissue using low-cost microfluidic instrumentation. Nat Commun. 2018;9(1):791. doi: 10.1038/s41467-017-02659-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei K, et al. Notch signalling drives synovial fibroblast identity and arthritis pathology. Nature. 2020;582(7811):259–264. doi: 10.1038/s41586-020-2222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25(57):7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 40.Kondo A, Kaestner KH. Emerging diverse roles of telocytes. Development. 2019;146(14):dev175018. doi: 10.1242/dev.175018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosa I, et al. Impairment in the telocyte/CD34+ stromal cell network in human rheumatoid arthritis synovium. J Cell Mol Med. 2021;25(4):2274–2278. doi: 10.1111/jcmm.16225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosa I, et al. Morphological evidence of telocytes in human synovium. Sci Rep. 2018;8(1):3581. doi: 10.1038/s41598-018-22067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monach P, et al. The K/BxN mouse model of inflammatory arthritis: theory and practice. Methods Mol Med. 2007;136:269–282. doi: 10.1007/978-1-59745-402-5_20. [DOI] [PubMed] [Google Scholar]
- 44.High FA, et al. An essential role for Notch in neural crest during cardiovascular development and smooth muscle differentiation. J Clin Invest. 2007;117(2):353–363. doi: 10.1172/JCI30070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ando K, et al. Peri-arterial specification of vascular mural cells from naïve mesenchyme requires Notch signaling. Development. 2019;146(2):dev165589. doi: 10.1242/dev.165589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu H, et al. Notch3 is critical for proper angiogenesis and mural cell investment. Circ Res. 2010;107(7):860–870. doi: 10.1161/CIRCRESAHA.110.218271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bray SJ. Notch signalling in context. Nat Rev Mol Cell Biol. 2016;17(11):722–735. doi: 10.1038/nrm.2016.94. [DOI] [PubMed] [Google Scholar]
- 48.Andersson ER, et al. Notch signaling: simplicity in design, versatility in function. Development. 2011;138(17):3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 49.Andersson ER, Lendahl U. Therapeutic modulation of Notch signalling--are we there yet? Nat Rev Drug Discov. 2014;13(5):357–378. doi: 10.1038/nrd4252. [DOI] [PubMed] [Google Scholar]
- 50.Šućur A, et al. Notch receptors and ligands in inflammatory arthritis — a systematic review. Immunol Lett. 2020;223:106–114. doi: 10.1016/j.imlet.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 51.Nakazawa M, et al. Role of Notch-1 intracellular domain in activation of rheumatoid synoviocytes. Arthritis Rheum. 2001;44(7):1545–1554. doi: 10.1002/1529-0131(200107)44:7<1545::AID-ART278>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 52.Park JS, et al. Inhibition of notch signalling ameliorates experimental inflammatory arthritis. Ann Rheum Dis. 2015;74(1):267–274. doi: 10.1136/annrheumdis-2013-203467. [DOI] [PubMed] [Google Scholar]
- 53.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 54.Roulis M, Flavell RA. Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease. Differentiation. 2016;92(3):116–131. doi: 10.1016/j.diff.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 55.Powell DW, et al. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol. 2011;73:213–237. doi: 10.1146/annurev.physiol.70.113006.100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mifflin RC, et al. Intestinal myofibroblasts: targets for stem cell therapy. Am J Physiol Gastrointest Liver Physiol. 2011;300(5):684–696. doi: 10.1152/ajpgi.00474.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smillie CS, et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell. 2019;178(3):714–730. doi: 10.1016/j.cell.2019.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kinchen J, et al. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell. 2018;175(2):372–386. doi: 10.1016/j.cell.2018.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.West NR, et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med. 2017;23(5):579–589. doi: 10.1038/nm.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin JC, et al. Single-cell analysis of crohn’s disease lesions identifies a pathogenic cellular module associated with resistance to anti-TNF therapy. Cell. 2019;178(6):1493–1508. doi: 10.1016/j.cell.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen HN, et al. Autocrine loop involving IL-6 family member LIF, LIF receptor, and STAT4 drives sustained fibroblast production of inflammatory mediators. Immunity. 2017;46(2):220–232. doi: 10.1016/j.immuni.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Humby F, et al. Synovial cellular and molecular signatures stratify clinical response to csDMARD therapy and predict radiographic progression in early rheumatoid arthritis patients. Ann Rheum Dis. 2019;78(6):761–772. doi: 10.1136/annrheumdis-2018-214539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lliso-Ribera G, et al. Synovial tissue signatures enhance clinical classification and prognostic/treatment response algorithms in early inflammatory arthritis and predict requirement for subsequent biological therapy: results from the pathobiology of early arthritis cohort (PEAC) Ann Rheum Dis. 2019;78(12):1642–1652. doi: 10.1136/annrheumdis-2019-215751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buechler MB, et al. Cross-tissue organization of the fibroblast lineage. Nature. 2021;593(7860):575–579. doi: 10.1038/s41586-021-03549-5. [DOI] [PubMed] [Google Scholar]
- 65. doi: 10.1101/2021.01.11.426253. Korsunsky I, et al. Cross-tissue, single-cell stromal atlas identifies shared pathological fibroblast phenotypes in four chronic inflammatory diseases [preprint]. Posted on bioRxiv February 18, 2021. [DOI] [PMC free article] [PubMed]
- 66.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154(2):274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 67.Farin HF, et al. Redundant sources of Wnt Regulate intestinal stem cells and promote formation of paneth cells. Gastroenterology. 2012;143(6):1518–1529. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 68.McCarthy N, et al. Distinct mesenchymal cell populations generate the essential intestinal BMP signaling gradient. Cell Stem Cell. 2020;26(3):391–402. doi: 10.1016/j.stem.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Halpern KB, et al. Lgr5 telocytes are a signaling hub at the intestinal villus tip. Nature. 2020;11(1):1936. doi: 10.1038/s41467-020-15714-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chabaud M, et al. Enhancing effect of IL-1, IL-17, and TNF-alpha on macrophage inflammatory protein-3alpha production in rheumatoid arthritis: regulation by soluble receptors and Th2 cytokines. J Immunol. 2001;167(10):6015–6020. doi: 10.4049/jimmunol.167.10.6015. [DOI] [PubMed] [Google Scholar]
- 71.Ruddy MJ, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279(4):2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 72.Slowikowski K, et al. CUX1 and IκBζ (NFKBIZ) mediate the synergistic inflammatory response to TNF and IL-17A in stromal fibroblasts. Proc Natl Acad Sci U S A. 2020;117(10):5532–5541. doi: 10.1073/pnas.1912702117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malik N, et al. Molecular cloning, sequence analysis, and functional expression of a novel growth regulator, oncostatin M. Mol Cell Biol. 1989;9(7):2847–2853. doi: 10.1128/mcb.9.7.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gearing DP, et al. The IL-6 signal transducer, gp130: an oncostatin M receptor and affinity converter for the LIF receptor. Science. 1992;255(5050):1434–1437. doi: 10.1126/science.1542794. [DOI] [PubMed] [Google Scholar]
- 75.Hanlon MM, et al. STAT3 mediates the differential effects of oncostatin M and TNFα on RA Synovial fibroblast and endothelial cell function. Front Immunol. 2019;10:2056. doi: 10.3389/fimmu.2019.02056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumar J, Ward AC. Role of the interleukin 6 receptor family in epithelial ovarian cancer and its clinical implications. Biochim Biophys Acta. 2014;1845(2):117–125. doi: 10.1016/j.bbcan.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 77.Ogura H, et al. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 2008;29(4):628–636. doi: 10.1016/j.immuni.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 78.Rose TM, et al. The genes for oncostatin M (OSM) and leukemia inhibitory factor (LIF) are tightly linked on human chromosome 22. Genomics. 1993;17(1):136–140. doi: 10.1006/geno.1993.1294. [DOI] [PubMed] [Google Scholar]
- 79.Ai R, et al. Comprehensive epigenetic landscape of rheumatoid arthritis fibroblast-like synoviocytes. Nat Commun. 2018;9(1):1921. doi: 10.1038/s41467-018-04310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hammaker D, et al. Joint location-specific JAK-STAT signaling in rheumatoid arthritis fibroblast-like synoviocytes. ACR Open Rheumatol. 2019;1(10):640–648. doi: 10.1002/acr2.11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frank-Bertoncelj M, et al. Epigenetically-driven anatomical diversity of synovial fibroblasts guides joint-specific fibroblast functions. Nat Commun. 2017;8:14852. doi: 10.1038/ncomms14852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buenrostro JD, et al. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10(12):1213–1218. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Satpathy AT, et al. Massively parallel single-cell chromatin landscapes of human immune cell development and intratumoral T cell exhaustion. Nat Biotechnol. 2019;37(8):925–936. doi: 10.1038/s41587-019-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choy EH, et al. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol. 2020;16(6):335–345. doi: 10.1038/s41584-020-0419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cook SA, Schafer S. Hiding in plain sight: interleukin-11 emerges as a master regulator of fibrosis, tissue integrity, and stromal inflammation. Annu Rev Med. 2020;71:263–276. doi: 10.1146/annurev-med-041818-011649. [DOI] [PubMed] [Google Scholar]
- 86.Schafer S, et al. IL-11 is a crucial determinant of cardiovascular fibrosis. Nature. 2017;552(7683):110–115. doi: 10.1038/nature24676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ng B, et al. Interleukin-11 is a therapeutic target in idiopathic pulmonary fibrosis. Sci Transl Med. 2019;11(511):eaaw1237. doi: 10.1126/scitranslmed.aaw1237. [DOI] [PubMed] [Google Scholar]
- 88.Ng B, et al. Fibroblast-specific IL11 signaling drives chronic inflammation in murine fibrotic lung disease. FASEB J. 2020;34(9):11802–11815. doi: 10.1096/fj.202001045RR. [DOI] [PubMed] [Google Scholar]
- 89.Denton CP, et al. Therapeutic interleukin-6 blockade reverses transforming growth factor-beta pathway activation in dermal fibroblasts: insights from the faSScinate clinical trial in systemic sclerosis. Ann Rheum Dis. 2018;77(9):1362–1371. doi: 10.1136/annrheumdis-2018-213031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Szekanecz Z, Koch AE. Successes and failures of chemokine-pathway targeting in rheumatoid arthritis. Nat Rev Rheumatol. 2016;12(1):5–13. doi: 10.1038/nrrheum.2015.157. [DOI] [PubMed] [Google Scholar]
- 91.Haringman JJ, et al. A randomized controlled trial with an anti-CCL2 (anti-monocyte chemotactic protein 1) monoclonal antibody in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54(8):2387–2392. doi: 10.1002/art.21975. [DOI] [PubMed] [Google Scholar]
- 92.Okazaki M, et al. Molecular cloning and characterization of OB-cadherin, a new member of cadherin family expressed in osteoblasts. J Biol Chem. 1994;269(16):12092–12098. doi: 10.1016/S0021-9258(17)32685-6. [DOI] [PubMed] [Google Scholar]
- 93.Kimura Y, et al. Cadherin-11 expressed in association with mesenchymal morphogenesis in the head, somite, and limb bud of early mouse embryos. Dev Biol. 1995;169(1):347–358. doi: 10.1006/dbio.1995.1149. [DOI] [PubMed] [Google Scholar]
- 94.Kiener HP, et al. Cadherin 11 promotes invasive behavior of fibroblast-like synoviocytes. Arthritis Rheum. 2009;60(5):1305–1310. doi: 10.1002/art.24453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang SK, et al. Cadherin-11 regulates fibroblast inflammation. Proc Natl Acad Sci U S A. 2011;108(20):8402–8407. doi: 10.1073/pnas.1019437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vandooren B, et al. Tumor necrosis factor alpha drives cadherin 11 expression in rheumatoid inflammation. Arthritis Rheum. 2008;58(10):3051–3062. doi: 10.1002/art.23886. [DOI] [PubMed] [Google Scholar]
- 97.Valencia X, et al. Cadherin-11 provides specific cellular adhesion between fibroblast-like synoviocytes. J Exp Med. 2004;200(12):1673–1679. doi: 10.1084/jem.20041545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu M, et al. Identification of cadherin 11 as a mediator of dermal fibrosis and possible role in systemic sclerosis. Arthritis Rheumatol. 2014;66(4):1010–1021. doi: 10.1002/art.38275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schneider DJ, et al. Cadherin-11 contributes to pulmonary fibrosis: potential role in TGF-β production and epithelial to mesenchymal transition. FASEB J. 2012;26(2):503–512. doi: 10.1096/fj.11-186098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Riley LA, Merryman WD. Cadherin-11 and cardiac fibrosis: A common target for a common pathology. Cell Signal. 2021;78:109876. doi: 10.1016/j.cellsig.2020.109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schroer AK, et al. Cadherin-11 blockade reduces inflammation-driven fibrotic remodeling and improves outcomes after myocardial infarction. JCI Insight. 2019;4(18):e131545. doi: 10.1172/jci.insight.131545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Clark CR, et al. Targeting cadherin-11 prevents Notch1-mediated calcific aortic valve disease. Circulation. 2017;135(24):2448–2450. doi: 10.1161/CIRCULATIONAHA.117.027771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Finch R, et al. Op0224 Results of a phase 2 study of Rg6125, an anti-cadherin-11 monoclonal antibody, in rheumatoid arthritis patients with an inadequate response to anti-TNF-alpha therapy. Ann Rheum Dis. 2019;78:189 [Google Scholar]
- 104.Mariathasan S, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554(7693):544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tabata T, Takei Y. Morphogens, their identification and regulation. Development. 2004;131(4):703–712. doi: 10.1242/dev.01043. [DOI] [PubMed] [Google Scholar]
- 106.Györfi AH, et al. Targeting TGF-β signaling for the treatment of fibrosis. Matrix Biol. 2018;68-69:8–27. doi: 10.1016/j.matbio.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 107.Pohlers D, et al. TGF-beta and fibrosis in different organs - molecular pathway imprints. Biochim Biophys Acta. 2009;1792(8):746–756. doi: 10.1016/j.bbadis.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 108.Tauriello DVF, et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554(7693):538–543. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 109.Chakravarthy A, et al. TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat Commun. 2018;9(1):4692. doi: 10.1038/s41467-018-06654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ciardiello D, et al. Clinical development of therapies targeting TGFβ: current knowledge and future perspectives. Ann Oncol. 2020;31(10):1336–1349. doi: 10.1016/j.annonc.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 111.Vera L, et al. Notch3 deficiency attenuates pulmonary fibrosis and impedes lung-function decline. Am J Respir Cell Mol Biol. 2021;64(4):465–476. doi: 10.1165/rcmb.2020-0516OC. [DOI] [PubMed] [Google Scholar]
- 112.Cao Z, et al. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat Med. 2016;22(2):154–162. doi: 10.1038/nm.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brandt S, et al. Fibrosis and immune cell infiltration are separate events regulated by cell-specific receptor Notch3 expression. J Am Soc Nephrol. 2020;31(11):2589–2608. doi: 10.1681/ASN.2019121289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Djudjaj S, et al. Notch-3 receptor activation drives inflammation and fibrosis following tubulointerstitial kidney injury. J Pathol. 2012;228(3):286–299. doi: 10.1002/path.4076. [DOI] [PubMed] [Google Scholar]
- 115.Chen J, et al. Treatment of collagen-induced arthritis rat model by using Notch signalling inhibitor. J Orthop Translat. 2021;28:100–107. doi: 10.1016/j.jot.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Choy L, et al. Constitutive NOTCH3 signaling promotes the growth of basal breast cancers. Cancer Res. 2017;77(6):1439–1452. doi: 10.1158/0008-5472.CAN-16-1022. [DOI] [PubMed] [Google Scholar]
- 117.Bernasconi-Elias P, et al. Characterization of activating mutations of NOTCH3 in T-cell acute lymphoblastic leukemia and anti-leukemic activity of NOTCH3 inhibitory antibodies. Oncogene. 2016;35(47):6077–6086. doi: 10.1038/onc.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Varga J, et al. AKT-dependent NOTCH3 activation drives tumor progression in a model of mesenchymal colorectal cancer. J Exp Med. 2020;217(10):e20191515. doi: 10.1084/jem.20191515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kraman M, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330(6005):827–830. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 120.Hinz B, Lagares D. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat Rev Rheumatol. 2020;16(1):11–31. doi: 10.1038/s41584-019-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]