Abstract
Aims
Mental stress substantially contributes to the initiation and progression of human disease, including cardiovascular conditions. We aim to investigate the underlying mechanisms of these contributions since they remain largely unclear.
Methods and results
Here, we show in humans and mice that leucocytes deplete rapidly from the blood after a single episode of acute mental stress. Using cell-tracking experiments in animal models of acute mental stress, we found that stress exposure leads to prompt uptake of inflammatory leucocytes from the blood to distinct tissues including heart, lung, skin, and, if present, atherosclerotic plaques. Mechanistically, we found that acute stress enhances leucocyte influx into mouse atherosclerotic plaques by modulating endothelial cells. Specifically, acute stress increases adhesion molecule expression and chemokine release through locally derived norepinephrine. Either chemical or surgical disruption of norepinephrine signalling diminished stress-induced leucocyte migration into mouse atherosclerotic plaques.
Conclusion
Our data show that acute mental stress rapidly amplifies inflammatory leucocyte expansion inside mouse atherosclerotic lesions and promotes plaque vulnerability.
Keywords: Acute mental stress, Atherosclerosis, Vascular inflammation, Leucocyte recruitment, Sympathetic nervous system, Neuroimmune interaction
Graphical Abstract
See page 4089 for the editorial comment for this article ‘The endothelium is a key player in the vascular response to acute mental stress’, by J.D.S. Sara et al., https://doi.org/10.1093/eurheartj/ehab510.
Introduction
Mental or psychological stress is a well-established contributor to human morbidity and mortality.1 The term stress describes the body’s response to environmental challenges that exceed an individual’s ability to cope.1 Stress is comprised of a stimulus (stressor) that precipitates a reaction in the brain (stress perception). This, in turn, activates physiologic ‘fight-or-flight’ systems in the body (stress response).2
Experiencing a stressful situation, as perceived by the cerebral cortex, stimulates the hypothalamic-pituitary-adrenal axis.1 The pituitary gland liberates adrenocorticotropic hormone, triggering the systemic release of glucocorticoid hormones from the adrenal cortex.1 , 3 Additionally, the sympathetic-adrenal-medullary axis activates when the adrenal medulla releases catecholamines systemically (predominantly epinephrine).1 Stress can also activate local sympathetic neurons in end organs, leading to the local release of the neurotransmitter norepinephrine.3
Clinical and experimental studies have associated mental stressors with the development of diseases and infections.1 , 4 Specifically, social stressors increase the incidence of cardiovascular diseases, including atherosclerosis, myocardial infarction (MI), and stroke.5–10 Stress at work and in private life associates with a 40–50% increase in coronary artery disease, as observed in prospective studies.3 Mental stressors can be chronic or acute, depending on the length and severity of the exposure. Chronic mental stress (such as job stress, marital unhappiness, burden of caregiving, or perceptions of being treated unfairly) refers to long-term, repetitive stress exposure.11 We showed that chronic variable stressors promote leucocyte production through increased haematopoietic stem cell proliferation.12 , 13 In contrast, acute mental stress is short-term exposure to severe stressors.14 Acute mental stress (for instance, anger or emotional upset) is more common and associates with an even greater incidence of cardiovascular events than chronic stress.2 Indeed, the incidence of sudden deaths from cardiac causes acutely quintupled after an earthquake struck the Los Angeles area in 1994.15 Likewise, the day of the first missile strikes during the Gulf War in 1990 witnessed a steep increase in mortality due to MI and sudden cardiac death in Israel.16 More moderate stressors, including the type we routinely face in daily life, also raise the incidence of cardiovascular events. When, for instance, the German soccer team played during the 2006 World Cup, monitored sites in Germany reported 2.7 more cardiac emergencies than when other teams played.17 The majority of these events happened during the first 2 h after the game started. Moreover, MI patients reported that anger or emotional upset was common in the hour before the onset of MI symptoms.18 , 19
Despite its huge impact on human health, precisely how acute mental stress precipitates disease remains unclear. Here, we report that acute mental stress exposure activates endothelial cells via the local release of norepinephrine, leading to increased blood inflammatory leucocyte influx into tissues, including atherosclerotic plaques.
Results
Acute mental stress exposure promotes leucocyte trafficking into tissues
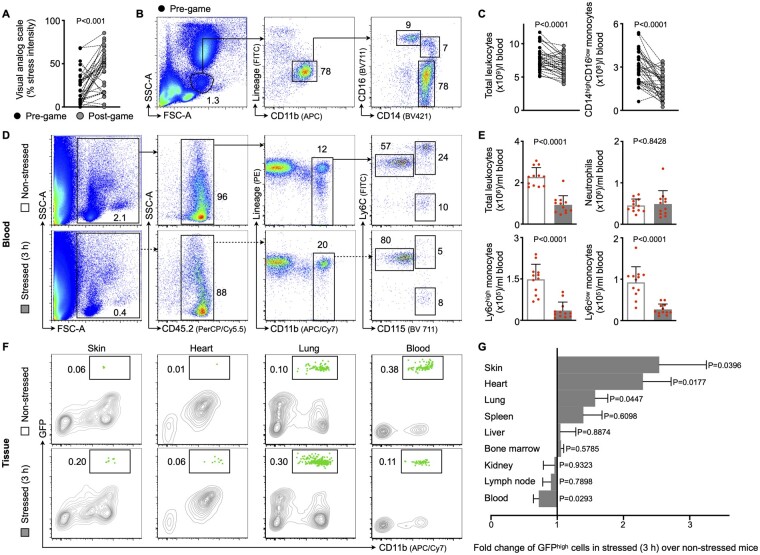
Since the psyche is closely linked to the immune system, we first explored the immune response in humans who were exposed to acute mental stress. A previous study showed that watching exciting soccer matches raises cardiovascular events acutely and transiently.17 Hence, we performed an experiment in which 35 soccer fans watched a thrilling key soccer match. Study participants were emotionally involved because their national team played. Accordingly, when we assessed stress levels 24 h before and immediately after the game ended, we found that watching the match augmented stress perception, as measured by the visual analogue scale (Figure 1A, experimental design is outlined in Supplementary material online, Figure S1A). Further, we obtained two blood samples to analyse the numbers of blood leucocytes, the effector cells of the immune system. A first sample was collected 24 h before the game ended and a second sample immediately after the game, so that the samples were taken at the same time of day. Using flow cytometry, we found that total leucocyte—and specifically inflammatory monocyte—numbers decreased rapidly after stress (Figure 1B and C). Further, lymphocyte numbers also declined upon stress, while neutrophil numbers remained unchanged (Supplementary material online, Figure S1B and C). Of note, systolic and diastolic blood pressure did not change and only a slight increase in heart rate followed acute mental stress exposure (Supplementary material online, Figure S1D).
Figure 1.
Acute mental stress exposure promotes inflammatory blood leucocyte uptake into tissues. (A) Visual analogue scales for stress intensity in soccer spectators either 24 h before or immediately after watching a key soccer match (n = 35 healthy individuals, aged 26.1 ± 1.3, 46% female, Wilcoxon matched-pairs test). (B, C) Gating and quantification of human blood leucocytes and subsets in soccer spectators (paired t-test). (D, E) Gating and quantification of murine blood leucocytes and subsets in non-stressed vs. stressed wild-type mice (immediately after a single 3 h episode of restraint stress, n = 12 per group, Mann–Whitney U-test). (F, G) Gating and quantification of green fluorescent protein (GFP)high myeloid cells in various organs after adoptive transfer of GFPhigh monocytes and neutrophils into non-stressed vs. stressed wild-type mice (21 h after a single 3 h episode of restraint stress, n = 11–18 per group, Student’s t-test for lung, bone marrow, and blood; Mann–Whitney U-test for skin, heart, spleen, liver, kidney, and lymph node). Numbers next to gates indicate frequencies (%). Data are presented as mean ± SD.
To follow-up on the observed drop in leucocyte numbers, we subjected mice to acute mental stress by restraint/immobilization, the most widely used procedure in rodents to evoke acute mental stress.20 Flow cytometry showed lower blood leucocyte levels, specifically for inflammatory monocytes, immediately after a single 3 h episode of mental stress, a result that agrees with our human data (Figure 1D and E). Similarly, lymphocyte numbers dropped after stress, while neutrophil numbers were not altered at this distinct time point (Figure 1E and Supplementary material online, Figure S1E and F). Kinetic experiments revealed that blood leucocyte levels started to drop just 15 min after stress initiation and rebounded rapidly once stress ended (Supplementary material online, Figure S1G –I). Cell-tracking experiments using adoptive transfer explored the fate of these vanishing leucocytes. We retrieved GFPhigh myeloid cells (monocytes admixed with neutrophils) from naïve transgenic Ubc-GFP mice (all leucocytes express green fluorescent protein, GFP), injected the cells intravenously into wild-type mice (all cells are GFPnegative), and began stressing them for 3 h immediately thereafter. A control cohort also underwent adoptive cell transfer but was not exposed to stress. We harvested several tissues and tracked the GFPhigh myeloid cells in tissues using flow cytometry 21 h after we stopped stressing the mice (Supplementary material online, Figure S2A). While GFPhigh myeloid cell numbers decreased in the blood, we found more numerous GFPhigh myeloid cells in the skin, heart, and lung of stressed mice than in those of unstressed controls, indicating that acute mental stress promotes leucocyte migration to these tissues (Figure 1F and G and Supplementary material online, Figure S2B –D). To exclude the possibility that stress simply enhances leucocyte adhesion to endothelial cells without subsequent transmigration into tissues (i.e. marginalization), we labelled the blood pool using an intravenously injected, fluorescently tagged anti-CD45 (pan-leucocyte marker) antibody 5 min before we harvested tissues, as described previously.21 Blood pool labelling was highly efficient (Supplementary material online, Figure S2E) and we used this technique in every flow cytometry experiment to exclude blood cell contamination. Taken together, our data indicate that mental stress exposure promotes the rapid recruitment of inflammatory blood leucocytes into distinct tissues.
Acute stress promotes atherosclerotic plaque inflammation through increased leucocyte influx
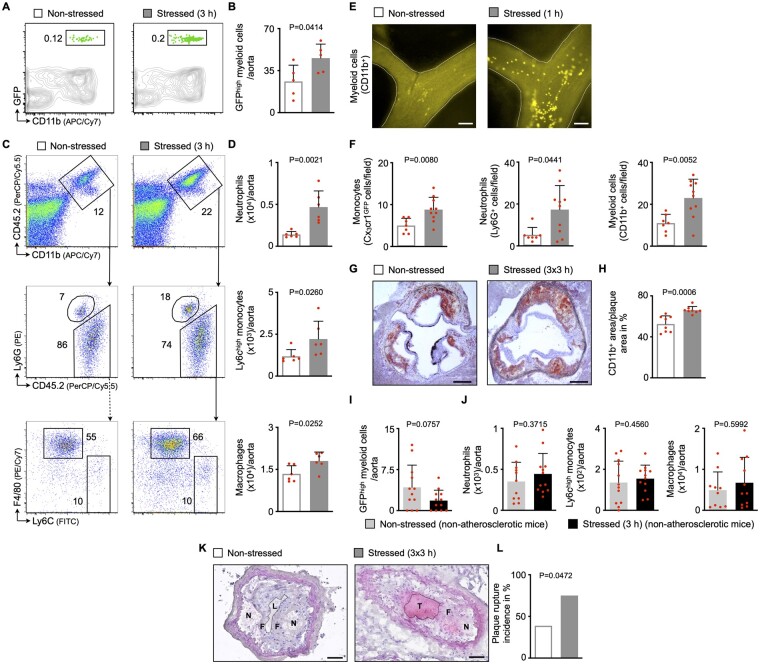
Since observational studies show that acute stress preferentially associates with cardiovascular disease and its complications, such as acute coronary syndromes,2 , 3 , 5 , 13 we tested the hypothesis that acute mental stress exposure promotes plaque progression and destabilization via enhanced vascular inflammation triggered by inflammatory leucocyte recruitment. We adoptively transferred GFPhigh myeloid cells into atherosclerosis-prone mice (ApoE−/− mice fed a high-cholesterol diet for 8 weeks) and applied mental stress for 3 h (Supplementary material online, Figure S3A). In accordance with the data obtained in other tissues (Figure 1F and G), recruited GFPhigh myeloid cell numbers rose inside atherosclerotic aortas in stressed compared with non-stressed ApoE−/− mice (Figure 2A and B). Consequently, acute stress rapidly expanded inflammatory aortic leucocyte numbers (Figure 2C and D). Moreover, intravital microscopy of the carotid artery in atherosclerotic mice revealed that myeloid cell adhesion to carotid endothelial cells increased after 1 h of stress (Figure 2E and F). Immunohistochemical observations corroborated our findings from flow cytometry and intravital microscopy experiments and confirmed a surge in intimal myeloid cell—but not lymphocyte—levels after stress exposure (Figure 2G and H and Supplementary material online, Figure S3B –G). Of note, enhanced recruitment of myeloid cells did not occur in non-atherosclerotic aortas (Figure 2I and J).
Figure 2.
Acute mental stress exposure aggravates inflammatory blood leucocyte migration into atherosclerotic plaques. (A, B) Gating and quantification of GFPhigh myeloid cells in atherosclerotic aortas after adoptive transfer of GFPhigh monocytes and neutrophils into non-stressed vs. stressed ApoE−/− mice (21 h after a single 3 h episode of restraint stress, n = 5 per group, Student’s t-test). (C, D) Gating and quantification of aortic myeloid cells from non-stressed vs. stressed ApoE−/− mice (n = 6 per group, Mann–Whitney U-test for Ly6Chigh monocytes, Student’s t-test for neutrophils and macrophages). (E, F) Representative images (myeloid cells, CD11bhigh) and quantification of adherent myeloid cells in non-stressed vs. stressed ApoE−/− mice using intravital microscopy on carotid arteries (immediately after a single 1 h episode of restraint stress, n = 7–10 per group, Student’s t-test, Mann–Whitney U-test for neutrophils). Scale bars represent 100 µm. (G, H) Representative immunohistochemical staining of aortic roots from non-stressed vs. stressed ApoE−/− mice (after three episodes of 3 h restraint stress once daily) for myeloid cells (CD11b). Scale bars represent 200 µm. Bar graphs show the percentage of positive area per plaque area (n = 8 per group, Student’s t-test). (I) Quantification of GFPhigh myeloid cells in aortas after adoptive transfer of GFPhigh monocytes and neutrophils into non-stressed vs. stressed non-atherosclerotic wild-type mice (21 h after a single 3 h episode of restraint stress, n = 12 per group, Mann–Whitney U-test). (J) Quantification of aortic myeloid cells from non-stressed vs. stressed non-atherosclerotic wild-type mice (n = 10–12 per group, Student’s t-test). (K, L) Representative images and quantification of plaque rupture in carotid arteries from non-stressed vs. stressed ApoE−/− mice (after three episodes of 3 h restraint stress once daily; N, necrotic core; F, fibrous cap; L, arterial lumen; T, thrombus). Thrombus formation is considered an indicator for plaque rupture. Scale bars represent 50 µm. Bar graphs show the incidence of plaque rupture (n = 18–20 per group, Fisher’s exact test). Numbers next to gates indicate frequencies (%). Data are presented as mean ± SD.
Next, we assessed how this rapid stress-induced leucocyte expansion affects the plaque character. Assessment of aortic mRNA using qPCR showed more matrix metalloproteinase and fewer procollagen transcripts, indicating that stress quickly shifts plaque functions towards extracellular matrix breakdown (Supplementary material online, Figure S3H), features associated with thinning of the fibrous cap.22 High leucocyte content in plaques combined with shrinking fibrous caps increase the risk of plaque rupture.23 Thus, we tested whether stress-induced alterations of the plaque character lead to plaque destabilization. Since plaque rupture does not often occur spontaneously at the early stages of atherosclerosis in mice, we used a plaque rupture model in which ApoE−/− mice were subjected to partial carotid ligation followed by cuff placement around the carotid artery (Supplementary material online, Figure S3I).24 Here, we found that intimal myeloid cell numbers increased, while smooth muscle cell and collagen content decreased (Supplementary material online, Figure S3J –O). Consequently, plaque rupture, as assessed by histology, occurred more often after acute stress exposure (Figure 2K and L).
Importantly, our findings were not limited to restraint/immobilization stress and could be replicated using predator-prey stress as an alternative stress model. We distributed fox odour using trimethylthiazoline (a component of fox faeces/urine) in mouse cages for 3 h. Acute predator-prey stress had similar effects on leucocyte numbers in blood and atherosclerotic aortas as restraint stress (Supplementary material online, Figure S3P). Of note, acute stress exposure did not alter the lipid profile in atherosclerotic mice (Supplementary material online, Figure S3Q). Taken together, our data indicate that acute stress exposure promotes vascular inflammation and hence plaque destabilization by enhancing inflammatory blood leucocyte uptake into atherosclerotic aortas.
Stress-induced modulation of endothelial cells depends on norepinephrine
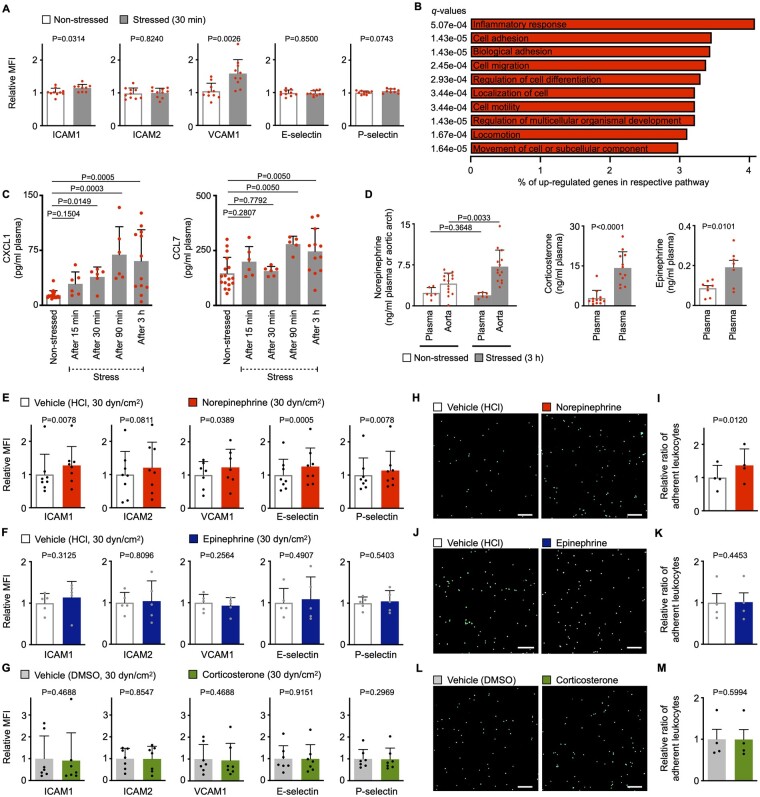
Leucocyte recruitment is a process in which circulating blood leucocytes and endothelial cells act jointly to mediate rolling, adhesion, and transmigration.25 , 26 Thus, we next explored mechanistically how stress promotes leucocyte recruitment into tissues. We first tested whether stress activates endothelial cells and consequently promotes leucocyte influx. We applied acute mental stress to wild-type mice for 30 min, excised aortas to identify aortic endothelial cells using flow cytometry, and assessed classical cell adhesion molecule levels on the endothelial cell surfaces (Supplementary material online, Figure S4A). Protein levels of ICAM1 and VCAM1 rose after 30 min of acute mental stress (Figure 3A). To better understand the changes endothelial cells undergo in response to acute stress, we isolated by FACS (fluorescence-activated cell sorting) aortic endothelial cells from stressed vs. non-stressed mice for RNA sequencing. Out of 15 054 detectable transcripts, 533 genes were expressed differentially, of which 335 (63%) rose and 198 (37%) fell (log2 fold change ≥ ±0.58, adj. P < 0.05, Supplementary material online, Figure S4B). A gene set enrichment analysis found that up-regulated genes were preferentially enriched in pathways related to cell ‘movement’, ‘locomotion’, ‘adhesion’, ‘localization’, ‘motility’, and ‘migration’ (Figure 3B and Supplementary material online, Figure S4C and Tables S1 and S2), all critical to leucocyte recruitment. Apart from expressing cell adhesion molecules, endothelial cells—among other cells that reside inside plaques—can also release cytokines/chemokines that function as chemoattractants for circulating leucocytes.27 Thus, we used a proteome profiler to screen plasma from non-stressed and stressed wild-type mice for 36 cytokines and chemokines. We found that only CXCL1 [chemokine (C-X-C motif) ligand 1] and CCL7 [chemokine (C-C motif) ligand 7] were differentially regulated upon stress indicating a rather specific response (Supplementary material online, Figure S4D). We confirmed these results using ELISA and further found that CXCL1 levels expanded as early as 30 min after stress, while CCL7 levels increased after 90 min of stress (Figure 3C). Other classical chemokines such as CCL2 were not affected (Supplementary material online, Figure S4E). To determine the cellular source of CXCL1 and CCL7, we first FACS-isolated endothelial cells, monocytes/macrophages, neutrophils, and fibroblasts from atherosclerotic aortas (Supplementary material online, Figure S4F). Using qPCR, we found that all cell fractions express CXCL1 and CCL7 in reasonable amounts (Supplementary material online, Figure S4G and H). In line, cultured endothelial cells, macrophages, vascular smooth muscle cells, and fibroblasts release substantial amounts of CXCL1 and CCL7 (Supplementary material online, Figure S4I).
Figure 3.
Acute mental stress exposure promotes leucocyte recruitment by modulating endothelial cells. (A) Protein levels as mean fluorescent intensities of adhesion molecules expressed by aortic endothelial cells from non-stressed vs. stressed wild-type mice (immediately after 30 min of restraint stress, n = 9–10 per group, Student’s t-test). (B) Pathway analysis of up-regulated genes as assessed by RNA sequencing from fluorescence-activated cell sorting-isolated aortic endothelial cells from non-stressed vs. stressed ApoE−/− mice (after three episodes of 3 h restraint stress once daily, n = 3 per group). Percentage of up-regulated genes per respective pathway and q-values as assessed by Benjamini–Hochberg correction are shown for the top 10 pathways as defined by P-values. (C) Quantification of plasma chemokine levels measured by ELISA in non-stressed vs. stressed wild-type mice (before, during, and immediately after 3 h of restraint stress, n = 6–18 per group, one-way ANOVA for CCL7, Kruskal–Wallis test for CXCL1, only P-values for comparisons with a control group are annotated). (D) Quantification of stress hormones measured by ELISA in plasma and aortic arches from non-stressed vs. stressed ApoE−/− mice (immediately after a single 3 h episode of restraint stress, n = 6–14 per group, Student’s t-test, Mann–Whitney U-test for corticosterone). (E–G) Protein levels as mean fluorescent intensities of adhesion molecules expressed by primary murine aortic endothelial cells cultured under high flow conditions (high shear stress, 30 dyn/cm2) incubated with either a stress hormone or respective vehicle [n = 5–8 per group, paired t-test, Wilcoxon test for ICAM1 (all hormones), VCAM1 (corticosterone) and P-selectin (norepinephrine and corticosterone)]. (H–M) Representative images and quantification of monocyte and neutrophil adherence to primary murine aortic endothelial cells cultured under high flow conditions (high shear stress, 30 dyn/cm2) and primed with either a stress hormone or respective vehicle (n = 4 per group, paired t-test). Scale bars represent 100 µm. Data are presented as mean ± SD.
Next, we investigated how acute stress activates endothelial cells. It is known that stress exposure leads to release of stress hormones (norepinephrine, epinephrine, and corticosterone), followed by elevated blood pressure and heart rate.2 Norepinephrine levels rose locally inside vessel walls, where it is released from sympathetic fibres and acts as their neurotransmitter, but not systemically (i.e. in plasma). Moreover, when we assessed the tunica intima separately, we detected a substantial amount of norepinephrine in human intima samples obtained surgically during coronary (2.32 ± 0.4 pg/mg tissue, n = 3, all male, age 69 ± 14.2, mean ± SD) and carotid endarterectomy (0.78 ± 0.9 pg/mg tissue, n = 12, 75% male, age 72 ± 8.4, mean ± SD). In contrast, levels of corticosterone (the murine analogue of human cortisol) and epinephrine rose systemically, as they are mainly released into the circulation from the adrenal gland (Figure 3D). We also found that acute stress leads to aortic tyrosine hydroxylase phosphorylation that activates this rate-limiting enzyme in norepinephrine synthesis (Supplementary material online, Figure S4J). In line with this stress-induced hormone release, we found that blood pressure and heart rate surged transiently during stress, as assessed by continuous invasive measurements (Supplementary material online, Figure S4K). In conclusion, our findings indicate that acute stress promotes leucocyte recruitment to tissues through the modulation of endothelial cells.
Next, we explored whether norepinephrine, epinephrine, or corticosterone directly activate endothelial cells, independently of secondary haemodynamic changes. We cultured primary murine aortic endothelial cells under elevated flow conditions (30 dyn/cm2, mimicking increased blood pressure) and incubated them with either norepinephrine, epinephrine, or corticosterone (Supplementary material online, Figure S5A and B). In contrast to epinephrine and corticosterone, norepinephrine increased protein levels of ICAM1, VCAM1, E-, and P-Selectin, indicating that norepinephrine directly stimulates endothelial cells (Figure 3E–G). We also tested the functional correlates of the altered adhesion molecules by monitoring leucocyte adherence to either norepinephrine-, epinephrine-, or corticosterone-exposed endothelial cells. For this purpose, we repeated the experiment described above and added naïve neutrophils/monocytes retrieved from wild-type mice and fluorescently labelled ex vivo (Supplementary material online, Figure S5C). We detected more neutrophil/monocyte adherence to the norepinephrine-primed endothelial cell layer, but not in epinephrine- or corticosterone-primed endothelial cells (Figure 3H–M). Further experiments revealed that this norepinephrine effect is dose-dependent (Supplementary material online, Figure S5D). Similar to our murine data, we found more adherent monocytes when human endothelial cells were primed with norepinephrine (Supplementary material online, Figure S5E and F). Next, we wondered whether adhesion can increase further when not only endothelial cells but also neutrophils/monocytes are primed with norepinephrine. To address this, we repeated the experiments described above and added neutrophils/monocytes while norepinephrine was still present in the culture medium (Supplementary material online, Figure S5G). Exposing neutrophils/monocytes to norepinephrine did not further boost adherence (Supplementary material online, Figure S5H). Next, we probed via which adrenoreceptor norepinephrine exerts its actions on endothelial cells. Treatment with phentolamine, a pan alpha-blocker, but not with propranolol, a pan beta-blocker, reduced leucocyte adherence to a norepinephrine-stimulated endothelial cell layer (Supplementary material online, Figure S5I and J). Taken together, these results indicate that norepinephrine, but not epinephrine or corticosterone, increases adhesion molecule levels on endothelial cells and thereby promotes neutrophil and monocyte adherence. Unlike epinephrine and corticosterone, norepinephrine led to a similar endothelial cell activation pattern under both low and high shear stress conditions, suggesting this effect occurs independently of altered haemodynamic/shear forces (Figure 3E– M and Supplementary material online, Figure S5K –P). Next, we explored whether norepinephrine also elevates the release of CXCL1 and CCL7 and found in cell culture experiments that only endothelial cells and macrophages secrete surplus CXCL1 and CCL7 upon norepinephrine stimulation (Supplementary material online, Figure S4I). In line, neutralizing endothelial cell secreted CCL7 dampened leucocyte adhesion after norepinephrine stimulation in cell culture experiments (Supplementary material online, Figure S5Q and R).
Although our experiments suggest a direct effect of norepinephrine on endothelial cells in terms of adhesion molecule levels and chemokine release (Figure 3E, H, and I and Supplementary material online, Figures S4I and S5R), indirect effects may also occur. Other adrenoreceptor carrying cells inside plaques, such as plaque macrophages, vascular smooth muscle cells, and fibroblasts may also respond to norepinephrine and secrete pro-inflammatory cytokines such as tumour necrosis factor-alpha, interleukin-1 beta (IL-1ß), and interleukin-6 (IL-6). These cytokines may then signal to endothelial cells and further fuel their activation. We were indeed able to detect a rise in IL-1β and IL-6 levels in the supernatant of norepinephrine-stimulated macrophages and vascular smooth muscle cells (Supplementary material online, Figure S5S –U).
Taken together, our findings demonstrate that norepinephrine activates endothelial cells directly. However, norepinephrine may also indirectly affect endothelial cell phenotypes: macrophages and vascular smooth muscle cells release more pro-inflammatory cytokines upon norepinephrine stimulation that may signal to endothelial cells and promote further activation.
Disrupting norepinephrine signalling dampens stress-induced leucocyte recruitment
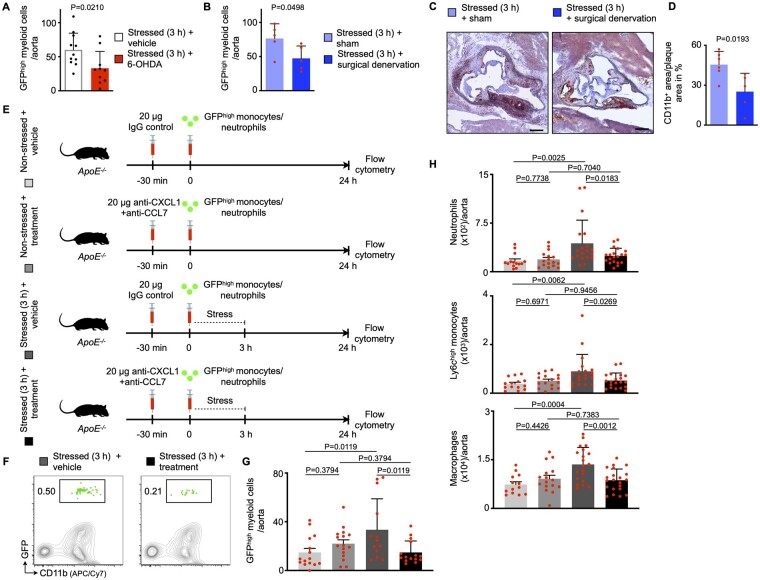
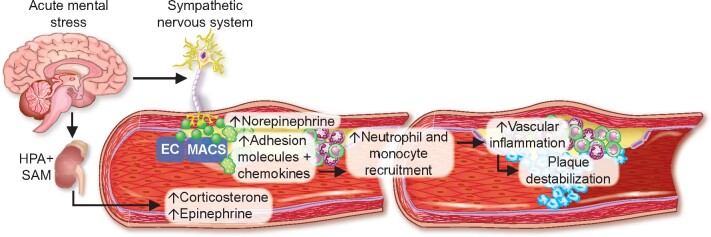
Based on our findings, we sought to determine in vivo whether stress-induced leucocyte recruitment relies on norepinephrine. Hence, we disrupted norepinephrine signalling in atherosclerotic mice by administering 6-hydroxydopamine (6-OHDA), a neurotoxin that ablates peripheral sympathetic nerves.28 Four days thereafter, we adoptively transferred GFPhigh neutrophils and monocytes retrieved from naïve Ubc-GFP mice and stressed the recipients immediately after the transfer (Supplementary material online, Figure S6A). We found that leucocyte recruitment was dampened in stressed mice with prior 6-OHDA treatment (Figure 4A). In line, levels of CXCL1 and CCL7 did not rise in stressed mice that were pre-treated with 6-OHDA (Supplementary material online, Figure S6B). In addition to this chemical depletion approach, we disrupted norepinephrine signalling by subjecting atherosclerotic mice to surgical bilateral removal of the superior cervical ganglion (Supplementary material online, Figure S6C). The superior cervical ganglion is part of the sympathetic nervous system and provides sympathetic innervation to the aortic arch/cardiac plexus.29–31 As a proof of concept, surgical treatment lowered the amount of norepinephrine locally (inside the vessel wall), but not systemically (in plasma) (Supplementary material online, Figure S6D). Four weeks after either surgical superior cervical ganglion removal or sham surgery, we adoptively transferred GFPhigh neutrophils and monocytes retrieved from naïve Ubc-GFP mice and stressed the recipients immediately after the transfer (Supplementary material online, Figure S6C). In accordance with the data produced by chemical norepinephrine depletion, surgical norepinephrine depletion lowered uptake and expansion of myeloid cells in atherosclerotic aortas (Figure 4B–D). Next, we lowered the systemic supply of stress hormones using bilateral adrenalectomy and found that endothelial leucocyte adhesion molecules and blood leucocyte levels were unchanged in stressed adrenalectomized mice in comparison to stressed sham-operated mice (Supplementary material online, Figure S6E and F). These experiments indicate that locally rather than systemically sourced norepinephrine promotes stress-induced plaque leucocyte recruitment. Since acute stress leads to a surplus release of CXCL1 and CCL7, we conducted an intervention experiment in which we neutralized these chemokines using anti-CXCL1 and -CCL7 antibodies (Figure 4E). Anti-CXCL1 and -CCL7 treatment curtailed plaque GFPhigh cell recruitment compared with controls (Figure 4F and G) and hence lowered aortic myeloid cell numbers (Figure 4H). In summary, after exposure to acute mental stress, norepinephrine acts on endothelial cells and macrophages, leading to increased expression of endothelial adhesion molecules and augmented release of the chemokines CXCL1 and CCL7. As a consequence, monocyte and neutrophil recruitment occurs in susceptible tissues, particularly atherosclerotic aortas. These data provide new insights into how acute mental stress elevates vascular inflammation and promotes plaque rupture (Graphical a bstract).
Figure 4.
Depleting norepinephrine signalling reverts stress-induced endothelial cell activation. (A) Quantification of GFPhigh myeloid cells in atherosclerotic aortas after adoptive transfer of GFPhigh monocytes and neutrophils into stressed ApoE−/− mice with or without prior chemical norepinephrine depletion (21 h after a single 3 h episode of restraint stress, n = 10–11 per group, Mann–Whitney U-test). 6-OHDA, 6-Hydroxydopamine. (B) Quantification of GFPhigh myeloid cells in atherosclerotic aortas after adoptive transfer of GFPhigh monocytes and neutrophils into stressed ApoE−/− mice with or without prior surgical norepinephrine depletion (21 h after a single 3 h episode of restraint stress, n = 5 per group, Student’s t-test). (C, D) Representative immunohistochemical staining and quantification of aortic roots from stressed ApoE−/− mice with or without prior surgical norepinephrine depletion (after three episodes of 3 h restraint stress once daily) for myeloid cells (CD11b). Scale bars represent 200 µm. Bar graphs show the percentage of positive area per plaque area (n = 5–6 per group, Students t-test). (E) Experimental setup for panels F–H. (F, G) Gating and quantification of GFPhigh myeloid cells in atherosclerotic aortas after adoptive transfer of GFPhigh monocytes and neutrophils into non-stressed and stressed ApoE-/- mice treated with either vehicle or anti-CXCL1 and anti-CCL7 antibodies (21 h after a single 3 h episode of restraint stress, n = 13–16 per group, one-way ANOVA, only P-values from planned comparisons are annotated). (H) Quantification of aortic myeloid cells from non-stressed and stressed ApoE−/− mice treated with either vehicle or anti-CXCL1 and anti-CCL7 antibodies (21 h after a single 3 h episode of restraint stress, n = 14–21 per group, one-way ANOVA, only P-values from planned comparisons are annotated). Numbers next to gates indicate frequencies (%). Data are presented as mean ± SD.
This study provides novel mechanistic insights into how acute mental stress fuels vascular inflammation and promotes plaque rupture. EC, endothelial cells; HPA, hypothalamic-pituitary-adrenal axis; MACS, macrophages; SAM, sympathetic-adrenal-medullary axis.
Discussion
Observational studies identified mental stress as a risk and prognostic factor for several diseases. Specifically, mental stress associates with cardiovascular disease, diabetes mellitus, and mental health disorders.2 In the INTERHEART study, the authors report that mental stress is as strongly associated with the occurrence of MI (odds ratio 2.67) than other classical risk factors, such as diabetes (odds ratio 2.37) or hypertension (odds ratio 1.91).32 However, this association may specifically affect stress-susceptible individuals.19 Taken together, these studies indicate that mental stress is a potentially modifiable risk factor that substantially contributes to cardiovascular mortality and morbidity. However, we do not fully understand the mechanisms by which stress exacerbates cardiovascular disease, and current state-of-the-art treatment does not address this risk. Our study provides evidence that acute stress exposure promotes the rapid expansion of inflammatory leucocytes in hearts, lungs, skin, and, if present, atherosclerotic plaques. Expansion occurs through a stress-induced increase in blood leucocyte recruitment. Mechanistically, stress promotes leucocyte recruitment through norepinephrine-mediated modulation of endothelial cells. Our observations propose a possible mechanism through which people with acute stress exposure (for instance soccer spectators) experience cardiovascular events such as acute coronary syndrome, including MI. Further, we provide evidence that acute stress-induced leucocyte recruitment can be therapeutically targeted.
Neuroimmune interactions upon acute mental stress
Stress-induced activation of endothelial cells
Stress activates the neuroendocrine axis, which typically leads to systemic release of epinephrine and glucocorticoids from the adrenal gland and local release of norepinephrine from direct sympathetic tissue innervation.1 In cell culture experiments, we addressed which of these three stress hormones predominantly modulated endothelial cell phenotypes. Our data suggest that norepinephrine contributes more to stress-induced endothelial activation than epinephrine or corticosterone and aligns with other previous reports.12 , 33
Norepinephrine which is typically released locally from sympathetic nervous fibres may reach endothelial cells either by diffusion from adjacent layers/compartments or by direct wiring of the tunica intima.34 , 35 In that light, we detected substantial amounts of norepinephrine in the intima in human endarterectomy samples. We next examined how endothelial cells sense incoming norepinephrine. Since endothelial cells are known to express both alpha- and beta-adrenoreceptors, we incubated endothelial cells with different adrenoreceptor blockers before stimulating them with norepinephrine. Our findings indicate that norepinephrine exerts its actions on endothelial cells via alpha- and not beta-adrenoreceptors.
Norepinephrine typically signals to smooth muscle cells of the tunica media and can induce vasoconstriction resulting in elevated blood pressure. Since luminal endothelial cells are particularly sensitive to varying haemodynamics with alterations in shear stress, we addressed whether norepinephrine-induced endothelial cell activation is shear stress-dependent. Comparing high vs. low shear forces using a flow chamber, we found that norepinephrine increased levels of cell adhesion molecules and adherence of leucocytes in both conditions equally. Taken together, our data indicate that locally released norepinephrine activated endothelial cells via alpha-adrenoreceptors. This activation is direct and independent of altered haemodynamics (vasoconstriction with altered shear stress).
Stress-induced migration of inflammatory leucocytes to tissues
Endothelial cells respond to norepinephrine stimulation with an increase in cell adhesion molecule levels and chemokine release. Both groups of molecules are critically involved in the process of leucocyte recruitment and mediate leucocyte attraction and adhesion.27 Since differences between non-stress and stress were more pronounced in chemokines, our data may indicate that stress-induced leucocyte recruitment may predominately be driven by chemokines rather than adhesion molecules. In addition to endothelial cells, macrophages also respond to norepinephrine and increase the release of CXCL1 and CCL7. Of note, another study also identified CXCL1 to be significantly altered by stress.36 Our study rather focused on endothelial cells, but the effect of acute stress/norepinephrine on leucocyte recruitment is most likely multifactorial affecting circulating leucocytes37 and resident endothelial cells and macrophages at the same time. Our blood leucocyte subset kinetics reveal that stress-induced leucocyte recruitment may involve neutrophils and monocytes serially and not in parallel. Blood neutrophil numbers seem to decrease earlier in the blood and may hence be earlier recruited to plaques than blood Ly6Chigh monocytes. This is in line with our finding that CXCL1—a strong chemoattractant for neutrophils—rises early in response to stress.
Moreover, our adoptive transfer experiments suggest that blood Ly6Chigh monocyte (the murine equivalent to human inflammatory monocytes) numbers decline because they are taken up by susceptible tissues. Since classical monocytes also decreased in humans after stress exposure, we believe that a similar mechanism (recruitment to susceptible tissues) also occurs in humans, but cannot exclude other potential causes for the observed decline, like altered conversion from classical to intermediate or non-classical monocyte subsets.38 , 39 Further studies are needed to fully explore the fate of the non-classical monocyte subset in mice and humans upon stress.
Further, our findings indicate that uptake of neutrophils and monocytes does not occur equally among several tissues but rather seems to be restricted to lungs, heart, skin, and, if present, atherosclerotic plaques. Our results in the skin agree with a previous study in which researchers stressed mice once for 2.5 h (restraint stress) immediately before subcutaneously implanting a saline-treated gelatine sponge.40 They explanted the sponge 6, 24, 48, or 72 h later and assessed the leucocyte content. The authors found that leucocyte numbers in the sponge peaked 24 h after stress, indicating that acute stress exposure promoted influx into this subcutaneously implanted foreign body.
Furthermore, we provide evidence that stress-induced leucocyte recruitment can be pharmacologically modulated. Data from our intervention experiments suggest that targeting the stress-induced release of CXCL1 and CCL7 may represent a novel therapeutic strategy to reduce cardiovascular complications of stress.
Stress-induced destabilization of atherosclerotic plaques
Vulnerable, rupture-prone plaques typically show a high content of inflammatory leucocytes, large necrotic cores, and thin fibrous caps.23 Our data reveal that acute stress rapidly rendered plaques more vulnerable as documented by an expansion of plaque inflammatory leucocytes, a decrease in intimal smooth muscle cell numbers, and a shift towards increased extracellular matrix breakdown. Consequently, plaque rupture occurred more often than in non-stressed counterparts. This finding aligns with a study where exposure to mental stress also led to acute myocardial ischaemia in atherosclerotic mice.41
Our experiments show an association of acute stress with a higher incidence of plaque rupture, but whether plaque rupture is caused by the involvement of local stress-induced norepinephrine signalling remains to be clarified. Future experiments are needed to fully determine the link of norepinephrine/endothelial cell interactions and plaque rupture. Among soccer spectators, the incidence of ST-elevation myocardial infarction (STEMI) rose transiently during key games.17 STEMI is typically caused by obstructive coronary artery disease with plaque rupture/erosion. These observations align with our findings that stress triggers plaque rupture and may hence lead to coronary obstruction resulting in MI. However, stress may trigger myocardial ischaemia also in the absence of plaque rupture and obstructive coronary arteries (MINOCA, myocardial infarction with non-obstructive coronary arteries). Stress leads to a transient catecholamine excess which may, for instance, cause coronary artery spasm, microvascular dysfunction, Takotsubo cardiomyopathy, and a supply/demand mismatch (elevated blood pressure and heart rate).
Biological basis of stress-induced leucocyte recruitment
One may speculate about the purpose of enhanced leucocyte recruitment in a ‘fight-or-flight’ situation. In general, this ‘fight-or-flight’ stress response likely evolved to bolster survival.42 In hunter-gatherers thousands of years ago, stress-induced leucocyte recruitment to lungs, heart, and skin may have rapidly and beneficially supported the cardio-pulmonary system, since enhanced physical activity demands increased oxygen supply from the lungs and higher cardiac output from the heart, while the skin is likely the first organ that could be injured in a ‘fight-or-flight’ situation. Maybe the body prepares distinct tissues to face challenges even before a wound or infection occurs to enhance function or regeneration after injury. In modern times, we more often acquire diseases that were not relevant for our ancestors. One example is atherosclerosis, which is now the leading cause of death worldwide. When concomitant atherosclerosis exists, stress-induced leucocyte recruitment also occurs in atherosclerotic plaques, where these inflammatory cells drive disease progression resulting in plaque destabilization with subsequent erosion/rupture, as documented here and described in humans.17 Thus, mental stress seems to be acutely harmful if atherosclerotic plaques are present. Further, contemporary humans do not exercise after stress exposure like our hunter-gatherer ancestors did and hence we do not counterbalance the stress reaction via physical activity. In addition to being beneficial in cardiovascular disease,43 physical activity may be a valuable outlet for stress.
Acute mental stress vs. other non-classical risk factors
Our study reveals that mental stressor type (intense vs. mild) and exposure time (acute vs. chronic exposure) contribute differentially to disease progression. Chronic mild stress (6 weeks of repetitive exposure to mild stressors) activates haematopoietic stem cells in the bone marrow, leading to increased leucocyte production, and consequently resulting in blood leukocytosis.12 Since leucocyte supply was higher, increased leucocyte numbers accumulated inside plaques. In contrast, acute stress (short-term exposure to an intense stressor) decreases blood leucocyte levels, promotes leucocyte recruitment, and thus results in inflammatory cell accumulation inside tissues.
Moreover, our study has to be interpreted in the context of other studies, which mechanistically link non-classical risk factors, such as sleep deprivation, sedentary lifestyle, air pollution, and environmental noise to cardiovascular disease.43–46
Limitations
Because our human study is associative by nature, interpretation of our findings merits consideration of confounding factors that may have influenced the results. Further, an additional control group is lacking. This could have been, for instance (i) the same study group watching a soccer game in which they are not emotionally involved or (ii) a group of matched individuals with no interest in soccer watching the match side by side with the soccer fans. Moreover, unlike the mice we used (pre-existing atherosclerotic plaques), our study population was healthy and did not show any comorbidities. Consequently, it was not possible to address recruitment to human plaques. In addition, we did not account for instrumental variability and cannot exclude an effect of chance due to a small sample size. Also, since blood pressure and heart rate were measured twice and not continuously, we may have missed a transient elevation during the game. Nevertheless, we chose to perform this experiment because observational studies strongly linked watching exciting soccer games to cardiovascular event occurence.17 In our pre-clinical experiments, restraint stress may represent a rather severe form of stress and may therefore generate a strong stress response. Thus, our findings may indicate one extreme form of stress-induced responses. Yet, the finding that blood leucocyte levels also decreased after stress exposure in humans possibly suggests the operation of similar mechanisms, even if the humans have less pronounced differences.
Summary and clinical perspective
In summary, stress is a crucial risk factor for atherosclerosis and substantially contributes to cardiovascular morbidity and mortality. Here, we show that acute stress enhances leucocyte recruitment to tissues, including atherosclerotic plaques, through norepinephrine-dependent modulation of endothelial cells and macrophages. As a consequence, stress promotes the accumulation of inflammatory plaque leucocytes, known drivers of vascular inflammation and plaque progression. From a clinical perspective, our findings indicate that (i) acute mental stress exposure may trigger plaque rupture/erosion by promoting vascular inflammation, (ii) particularly stress-susceptible patients with pre-existing atherosclerosis are at risk and should be identified early, and (iii) targeting stress-induced uptake of inflammatory cells may present a novel therapeutic avenue to prevent stress-related cardiovascular complications.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The authors thank Kaley Joyes, PhD, for editing the article. They thank Servier Medical Art (https://smart.servier.com) for providing free illustrations for figures and the cartoon.
Funding
H.B.S. has received funding from the European Research Council under the European Union’s Horizon 2020 Research and Innovation Programme (STRATO, grant agreement No. 759272), the ‘Else-Kröner-Fresenius-Stiftung’ (2020_EKSE.07), ‘Else-Kröner-Forschungskolleg München’, Technical University Munich, and the ‘Deutsche Herzstiftung’ (F/28/17). This study was further supported by the ‘Deutsche Forschungsgemeinschaft (DFG)’ [H.B.S.: SA 1668/5-1 and T.K.: CRC 1123 (B02)]. T.K. is supported by the Corona Foundation (Junior Research Group Translational Cardiovascular Genomics). Additionally, we gratefully acknowledge the support of the German Federal Ministry of Education and Research (BMBF), to H.S., within the framework of ERA-NET on Cardiovascular Disease (Druggable-MI-genes: 01KL1802) and the scheme of target validation (BlockCAD: 16GW0198K), as well as the German Centre of Cardiovascular Research (DZHK) Munich Heart Alliance, within the framework of the e:Med research and funding concept (AbCD-Net: 01ZX1706C). The British Heart Foundation (BHF)/German Centre of Cardiovascular Research (DZHK) Collaboration, for which we are co-applicants, have also provided support and funding. H.S. has received further support from the German Research Foundation (DFG) as part of the ‘Sonderforschungsbereich’ 1123 (B02) and the ‘Sonderforschungsbereich’ TRR 267 (B05). Bavarian State Ministry of Health and Care also funded this work as part of ‘DigiMed Bayern’ (grant No: DMB-1805-0001). The German Federal Ministry of Economics and Energy has supported this work within ModulMax (grant No: ZF4590201BA8). C.W. is a Van de Laar professor of atherosclerosis and supported by the European Research Council AdG°692511, DFG SFB1123-A1/A10/Z1 and TRR267-B02, and German Centre for Cardiovascular Research (DZHK; 81Z0600202). P.W. received funding from the German Federal Ministry of Education and Research (BMBF 01EO1503) and DFG (INST 371/47-1 FUGG) in support of this study. C.S. received support from the European Research Council (Starting grant 635872, CIRCODE). P.L. receives funding support from the National Heart, Lung, and Blood Institute (R01HL080472 and 1R01HL134892), the American Heart Association (18CSA34080399), and the RRM Charitable Fund. P.L. and O.S. are members of the Leducq transatlantic network on clonal haematopoiesis. I.H. is funded by the German Research Foundation (HI1573/2).
Conflict of interest: B.V. is the CEO of net-OMICS, a bioinformatics company. P.W. reports grants from ‘Bundesministerium für Bildung und Forschung’, grants from ‘Deutsche Forschungsgemeinschaft’, during the conduct of the study. P.L. is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Esperion Therapeutics, Ionis Pharmaceuticals, Kowa Pharmaceuticals, Novartis, Pfizer, Sanofi-Regeneron, and XBiotech, Inc. P.L. is a member of the scientific advisory board for Amgen, Corvidia Therapeutics, DalCor Pharmaceuticals, IFM Therapeutics, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, and XBiotech, Inc. P.L. serves on the Board of XBiotech, Inc. P.L.’s laboratory has received research funding in the last 2 years from Novartis. P.L. has a financial interest in Xbiotech, a company developing therapeutic human antibodies. P.L.'s interests were reviewed and are managed by Brigham and Women's Hospital and Partners HealthCare, Boston, USA in accordance with their conflict of interest policies. M.N. reports grants from NHLBI, during the conduct of the study; personal fees from IFM Therapeutics, Verseau, Gimv, and Sigilon, outside the submitted work. H.S. reports personal fees from MSD Sharp & Dohme, AMGEN, Bayer Vital GmbH, Boehringer Ingelheim, Daiichi-Sankyo, Novartis, Servier, Brahms, Bristol-Myers Squibb, Medtronic, sanofi-aventis, Synlab, Pfizer, and Vifor, grants and personal fees from Astra-Zeneca, outside the submitted work. H.B.S. reports grants from European Research Council, grants from ‘Else-Kröner-Fresenius-Stiftung’, ‘Deutsche Herzstiftung’, and ‘Deutsche Forschungsgemeinschaft’, during the conduct of the study. All other authors have nothing to disclose.
Data availability
Materials used in this paper are commercially available and suppliers are listed in the respective methods section. Upon request, samples can be provided or a detailed description of how to obtain the respective samples will be given. All relevant data are included in this paper and its supplementary file. Raw RNA sequencing data are available at the Gene Expression Omnibus repository under the accession number GSE155396.
Contributor Information
Julia Hinterdobler, Department of Cardiology, German Heart Centre Munich, Technical University Munich, Munich, Germany; DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany.
, Simin Schott, Department of Cardiology, German Heart Centre Munich, Technical University Munich, Munich, Germany; DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany.
Hong Jin, Department of Medicine, Karolinska Institute, Stockholm, Sweden.
Almut Meesmann, Department of Cardiology, German Heart Centre Munich, Technical University Munich, Munich, Germany; DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany.
Anna-Lena Steinsiek, Department of Cardiology, German Heart Centre Munich, Technical University Munich, Munich, Germany.
Anna-Sophia Zimmermann, Department of Cardiology, German Heart Centre Munich, Technical University Munich, Munich, Germany; DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany.
Jana Wobst, Department of Cardiology, German Heart Centre Munich, Technical University Munich, Munich, Germany; DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany.
Philipp Müller, Department of Cardiology, German Heart Centre Munich, Technical University Munich, Munich, Germany; DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany.
Carina Mauersberger, Department of Cardiology, German Heart Centre Munich, Technical University Munich, Munich, Germany; DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany.
Baiba Vilne, Department of Cardiology, German Heart Centre Munich, Technical University Munich, Munich, Germany; Bioinformatics Unit, Riga Stradiņš University, Riga, Latvia; SIA net-OMICS, Riga, Latvia.
Alexandra Baecklund, Department of Medicine, Karolinska Institute, Stockholm, Sweden.
Chien-Sin Chen, Walter Brendel Centre of Experimental Medicine, Ludwig Maximilians University Munich, BioMedical Centre, Planegg-Martinsried, Germany.
Aldo Moggio, Department of Cardiology, German Heart Centre Munich, Technical University Munich, Munich, Germany.
Quinte Braster, Institute for Cardiovascular Prevention (IPEK), Ludwig Maximilians University Munich, Munich, Germany.
Michael Molitor, Center for Thrombosis and Hemostasis and Department of Cardiology, University Medical Center, Mainz, Germany; DZHK (German Centre for Cardiovascular Research), partner site Rhine-Main, Mainz, Germany.
Markus Krane, DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany; Department of Cardiac Surgery, German Heart Centre Munich, Technical University Munich, Munich, Germany.
Wolfgang E Kempf, Department of Vascular and Endovascular Surgery, Klinikum rechts der Isar, Technical University Munich, Munich, Germany.
Karl-Heinz Ladwig, DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany; Institute of Epidemiology Mental Health Research Unit, Helmholtz Zentrum Munich, German Research Center for Environmental Health, Munich, Germany.
Michael Hristov, DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany; Institute for Cardiovascular Prevention (IPEK), Ludwig Maximilians University Munich, Munich, Germany.
Maarten Hulsmans, Center for Systems Biology and Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Ingo Hilgendorf, Department of Cardiology and Angiology I, University Heart Center Freiburg-Bad Krozingen, Faculty of Medicine, University of Freiburg, Freiburg, Germany.
Christian Weber, DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany; Institute for Cardiovascular Prevention (IPEK), Ludwig Maximilians University Munich, Munich, Germany; Munich Cluster for Systems Neurology (SyNergy), Munich, Germany; Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands.
Philip Wenzel, Center for Thrombosis and Hemostasis and Department of Cardiology, University Medical Center, Mainz, Germany; DZHK (German Centre for Cardiovascular Research), partner site Rhine-Main, Mainz, Germany.
Christoph Scheiermann, DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany; Walter Brendel Centre of Experimental Medicine, Ludwig Maximilians University Munich, BioMedical Centre, Planegg-Martinsried, Germany; Department of Pathology and Immunology, University of Geneva, Geneva, Switzerland.
Lars Maegdefessel, DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany; Department of Medicine, Karolinska Institute, Stockholm, Sweden; Department of Vascular and Endovascular Surgery, Klinikum rechts der Isar, Technical University Munich, Munich, Germany.
Oliver Soehnlein, DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany; Institute for Cardiovascular Prevention (IPEK), Ludwig Maximilians University Munich, Munich, Germany; Department of Physiology and Pharmacology (FyFa), Karolinska Institute, Stockholm, Sweden; Institute for Experimental Pathology, University of Münster, Münster, Germany.
Peter Libby, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
Matthias Nahrendorf, Center for Systems Biology and Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA; Cardiovascular Research Center, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Heribert Schunkert, Department of Cardiology, German Heart Centre Munich, Technical University Munich, Munich, Germany; DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany.
Thorsten Kessler, Department of Cardiology, German Heart Centre Munich, Technical University Munich, Munich, Germany; DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany.
Hendrik B Sager, Department of Cardiology, German Heart Centre Munich, Technical University Munich, Munich, Germany; DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany.
Translational perspective
Mental stress is comparably associated with the occurrence of myocardial infarction, a major contributor to worldwide mortality, as other classical risk factors, such as diabetes or hypertension. Despite this huge impact on public health, we do not yet fully understand the mechanisms nor does state-of-the-art treatment reduce stress-related risk.
Our study addresses this gap in mechanistic knowledge and reveals that after exposure to acute mental stress, norepinephrine acts on endothelial cells and macrophages, leading to increased expression of adhesion molecules and augmented release of the chemokines CXCL1 and CCL7. As a consequence, monocyte and neutrophil recruitment occurs in susceptible tissues, particularly atherosclerotic plaques. We further provide evidence that these pre-clinical findings may be translated to humans with stress exposure and that specific therapeutic targeting of the stress response can beneficially alter the course of cardiovascular disease. In summary, our data provide new insights into how acute mental stress elevates vascular inflammation and may promote acute coronary syndromes.
References
- 1. Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 2005;5:243–251. [DOI] [PubMed] [Google Scholar]
- 2. Kivimäki M, Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol 2018;15:215–229. [DOI] [PubMed] [Google Scholar]
- 3. Steptoe A, Kivimäki M. Stress and cardiovascular disease. Nat Rev Cardiol 2012;9:360–370. [DOI] [PubMed] [Google Scholar]
- 4. Wieduwild E, Girard-Madoux MJ, Quatrini L, Laprie C, Chasson L, Rossignol R, Bernat C, Guia S, Ugolini S. β2-adrenergic signals downregulate the innate immune response and reduce host resistance to viral infection. J Exp Medicine 2020;217:e20190554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 1999;99:2192–2217. [DOI] [PubMed] [Google Scholar]
- 6. Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, Truong QA, Solomon CJ, Calcagno C, Mani V, Tang CY, Mulder WJ, Murrough JW, Hoffmann U, Nahrendorf M, Shin LM, Fayad ZA, Pitman RK. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet 2017;389:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nahrendorf M. Myeloid cell contributions to cardiovascular health and disease. Nat Med 2018;24:711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schloss MJ, Swirski FK, Nahrendorf M. Modifiable cardiovascular risk, hematopoiesis, and innate immunity. Circ Res 2020;126:1242–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kang DO, Eo JS, Park EJ, Nam HS, Song JW, Park YH, Park SY, Na JO, Choi CU, Kim EJ, Rha S-W, Park CG, Seo HS, Kim CK, Yoo H, Kim JW. Stress-associated neurobiological activity is linked with acute plaque instability via enhanced macrophage activity: a prospective serial 18F-FDG-PET/CT imaging assessment. Eur Heart J 2021;42:1883–1895. [DOI] [PubMed] [Google Scholar]
- 10. Kuebler U, Zuccarella-Hackl C, Arpagaus A, Wolf JM, Farahmand F, Känel RV, Ehlert U, Wirtz PH. Stress-induced modulation of NF-κB activation, inflammation-associated gene expression, and cytokine levels in blood of healthy men. Brain Behav Immun 2015;46:87–95. [DOI] [PubMed] [Google Scholar]
- 11. Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol 2008;51:1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, C von Zur M, Bode C, Fricchione GL, Denninger J, Lin CP, Vinegoni C, Libby P, Swirski FK, Weissleder R, Nahrendorf M. Chronic variable stress activates hematopoietic stem cells. Nat Med 2014;20:754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hanna RN, Hedrick CC. Stressing out stem cells: linking stress and hematopoiesis in cardiovascular disease. Nat Med 2014;20:707–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mostofsky E, Penner EA, Mittleman MA. Outbursts of anger as a trigger of acute cardiovascular events: a systematic review and meta-analysis. Eur Heart J 2014;35:1404–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med 1996;334:413–419. [DOI] [PubMed] [Google Scholar]
- 16. Meisel SR, Dayan KI, Pauzner H, Chetboun I, Arbel Y, David D, Kutz I. Effect of Iraqi missile war on incidence of acute myocardial infarction and sudden death in Israeli civilians. Lancet 1991;338:660–661. [DOI] [PubMed] [Google Scholar]
- 17. Wilbert-Lampen U, Leistner D, Greven S, Pohl T, Sper S, Völker C, Güthlin D, Plasse A, Knez A, Küchenhoff H, Steinbeck G. Cardiovascular events during world cup soccer. N Engl J Med 2008;358:475–483. [DOI] [PubMed] [Google Scholar]
- 18. Smyth A, O’Donnell M, Lamelas P, Teo K, Rangarajan S, Yusuf S; INTERHEART Investigators. Physical activity and anger or emotional upset as triggers of acute myocardial infarction: the INTERHEART study. Circulation 2016;134:1059–1067. [DOI] [PubMed] [Google Scholar]
- 19. Edmondson D, Newman JD, Whang W, Davidson KW. Emotional triggers in myocardial infarction: do they matter? Eur Heart J 2012;34:300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glavin GB, Paré WP, Sandbak T, Bakke H-K, Murison R. Restraint stress in biomedical research: an update. Neurosci Biobehav Rev 1994;18:223–249. [DOI] [PubMed] [Google Scholar]
- 21. Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 2014;40:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med 2013;368:2004–2013. [DOI] [PubMed] [Google Scholar]
- 23. Silvestre-Roig C, de Winther MP, Weber C, Daemen MJ, Lutgens E, Soehnlein O. Atherosclerotic plaque destabilization. Circ Res 2014;114:214–226. [DOI] [PubMed] [Google Scholar]
- 24. Jin H, Li DY, Chernogubova E, Sun CY, Busch A, Eken SM, Saliba-Gustafsson P, Winter H, Winski G, Raaz U, Schellinger IN, Simon N, Hegenloh R, Matic LP, Jagodic M, Ehrenborg E, Pelisek J, Eckstein HH, Hedin U, Backlund A, Maegdefessel L. Local delivery of miR-21 stabilizes fibrous caps in vulnerable atherosclerotic lesions. Mol Ther 2018;26:1040–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol 2010;7:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007;7:678–689. [DOI] [PubMed] [Google Scholar]
- 27. Gerhardt T, Ley K. Monocyte trafficking across the vessel wall. Cardiovasc Res 2015;107:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sager HB, Dutta P, Dahlman JE, Hulsmans M, Courties G, Sun Y, Heidt T, Vinegoni C, Borodovsky A, Fitzgerald K, Wojtkiewicz GR, Iwamoto Y, Tricot B, Khan OF, Kauffman KJ, Xing Y, Shaw TE, Libby P, Langer R, Weissleder R, Swirski FK, Anderson DG, Nahrendorf M. RNAi targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction. Sci Transl Med 2016;8:342ra80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scheiermann C, Kunisaki Y, Lucas D, Chow A, Zhang JE, Zhang D, Hashimoto D, Frenette PS. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 2012;37:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Malliani A, Pagani M. Afferent sympathetic nerve fibres with aortic endings. J Physiol 1976;263:157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Juan A D, Ince LM, Pick R, Chen C-S, Molica F, Zuchtriegel G, Wang C, Zhang D, Druzd D, Hessenauer MET, Pelli G, Kolbe I, Oster H, Prophete C, Hergenhan SM, Albrecht U, Ripperger J, Montanez E, Reichel CA, Soehnlein O, Kwak BR, Frenette PS, Scheiermann C. Artery-associated sympathetic innervation drives rhythmic vascular inflammation of arteries and veins. Circulation 2019;140:1100–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L; INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 33. Zhang B, Ma S, Rachmin I, He M, Baral P, Choi S, Gonçalves WA, Shwartz Y, Fast EM, Su Y, Zon LI, Regev A, Buenrostro JD, Cunha TM, Chiu IM, Fisher DE, Hsu Y-C. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature 2020;577:676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eichmann A, Brunet I. Arterial innervation in development and disease. Sci Transl Med 2014;6:252ps9. [DOI] [PubMed] [Google Scholar]
- 35. Sheng Y, Zhu L. The crosstalk between autonomic nervous system and blood vessels. Int J Physiol Pathophysiol Pharmacol 2018;10:17–28. [PMC free article] [PubMed] [Google Scholar]
- 36. Qing H, Desrouleaux R, Israni-Winger K, Mineur YS, Fogelman N, Zhang C, Rashed S, Palm NW, Sinha R, Picciotto MR, Perry RJ, Wang A. Origin and function of stress-induced IL-6 in murine models. Cell 2020;182:372–387.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heijden CVD, Groh L, Keating ST, Kaffa C, Noz MP, Kersten S, Herwaarden AE, van, Hoischen A, Joosten LAB, Timmers HJLM, Netea MG, Riksen NP. Catecholamines induce trained immunity in monocytes in vitro and in vivo. Circ Res 2020;127:269–283. [DOI] [PubMed] [Google Scholar]
- 38. Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, Bigley V, Flavell RA, Gilroy DW, Asquith B, Macallan D, Yona S. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med 2017;214:1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yona S, Kim K-W, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013;38:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Viswanathan K, Dhabhar FS. Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. Proc Natl Acad Sci USA 2005;102:5808–5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Caligiuri G, Levy B, Pernow J, Thorén P, Hansson GK. Myocardial infarction mediated by endothelin receptor signaling in hypercholesterolemic mice. Proc Natl Acad Sci USA 1999;96:6920–6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nahrendorf M. Multiorgan imaging of comorbidity and cardiovascular risk. JACC Cardiovasc Imaging 2020;13:478–480. [DOI] [PubMed] [Google Scholar]
- 43. Frodermann V, Rohde D, Courties G, Severe N, Schloss MJ, Amatullah H, McAlpine CS, Cremer S, Hoyer FF, Ji F, Koeverden IV, Herisson F, Honold L, Masson GS, Zhang S, Grune J, Iwamoto Y, Schmidt SP, Wojtkiewicz GR, Lee I-H, Gustafsson K, Pasterkamp G, Jager S. D, Sadreyev RI, MacFadyen J, Libby P, Ridker P, Scadden DT, Naxerova K, Jeffrey KL. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat Med 2019;25:1761–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McAlpine CS, Kiss MG, Rattik S, He S, Vassalli A, Valet C, Anzai A, Chan CT, Mindur JE, Kahles F, Poller WC, Frodermann V, Fenn AM, Gregory AF, Halle L, Iwamoto Y, Hoyer FF, Binder CJ, Libby P, Tafti M, Scammell TE, Nahrendorf M, Swirski FK. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 2019;566:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lelieveld J, Klingmüller K, Pozzer A, Pöschl U, Fnais M, Daiber A, Münzel T. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur Heart J 2019;40:1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Münzel T, Herzog J, Schmidt FP, Sørensen M. Environmental stressors and cardiovascular disease: the evidence is growing. Eur Heart J 2017;38:2297–2299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Materials used in this paper are commercially available and suppliers are listed in the respective methods section. Upon request, samples can be provided or a detailed description of how to obtain the respective samples will be given. All relevant data are included in this paper and its supplementary file. Raw RNA sequencing data are available at the Gene Expression Omnibus repository under the accession number GSE155396.