Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the COVID-19 pandemic. Neutralizing antibodies target the receptor binding domain of the spike (S) protein, a focus of successful vaccine efforts. Concerns have arisen that S-specific vaccine immunity may fail to neutralize emerging variants. We show that vaccination with a human adenovirus type 5 vector expressing the SARS-CoV-2 nucleocapsid (N) protein can establish protective immunity, defined by reduced weight loss and viral load, in both Syrian hamsters and K18-hACE2 mice. Challenge of vaccinated mice was associated with rapid N-specific T cell recall responses in the respiratory mucosa. This study supports the rationale for including additional viral antigens in SARS-CoV-2 vaccines, even if they are not a target of neutralizing antibodies, to broaden epitope coverage and immune effector mechanisms.
Introduction
Vaccine candidates targeting SARS-CoV-2 S protein, which is essential for cell entry, were designed based on viral sequences reported in January 2020. Studies of the highly efficacious Pfizer/BioNTech and Moderna mRNA vaccines show a strong correlation between the establishment of neutralizing antibodies (NAb) and protection from disease (1, 2). As the virus continues to spread globally, variants have emerged that partly evade neutralization by vaccine elicited S antibodies (3), which may require continued vaccine adaptation and boosting.
T cells contribute to immunity against respiratory pathogens, including serological variants of influenza virus(4). T cells may target conserved epitopes that have an unfavorable fitness barrier to mutation (5). Unlike NAb, T cell immunity is not limited to surface antigens and immunodominant epitopes vary considerably between individuals due to recognition in the context of genetically diverse MHC molecules. Accordingly, T cell immunity may be less vulnerable to immune selection pressure and viral escape mutations. We immunized mice and hamsters against SARS-CoV-2-N to address whether protection could be conferred independently of spike-specific responses.
Materials and Methods
Hamster immunizations and SARS-CoV-2 infection
Male Syrian hamsters (Charles River Laboratories) were maintained in a BSL-2 containment facility under specific-pathogen-free conditions at the University of Minnesota in accordance with the Institutional Animal Care and Use Committees guidelines.
Eighteen- to nineteen-week-old hamsters were anesthetized with isoflurane and immunized using a single intravenous (IV) dose of 1.8×10^11 viral particles of either a replication-deficient (E1 and E3 deleted) human adenovirus type 5 (Ad5) vectors expressing SARS- CoV-2-N (Ad5-N) or a control Ad-5 vector (Ad5-NULL). Ad5 vectors were constructed as described (6). Hamsters were moved to BSL-3 containment for SARS-CoV-2 challenge studies, anesthetized with isoflurane and challenged intranasally (IN) with a total of 6.75×10^5 PFU in a 100ul volume of either the 2019-nCoV/USA_WA1/2020 (WA) strain or the B.1.1.7 variant SARS-CoV- 2/human/USA/CA_CDC_5574/2020 (provided by World Reference Center for Emerging Viruses and Arboviruses at the University of Texas Medical Branch). Animals were weighed daily and terminated thirteen days after the challenge. Hamsters challenged for determining viral titers were euthanized three days after challenge.
Mouse immunizations, T cell depletion, and SARS-CoV-2 infection
Six- to ten-week-old female C57Bl/6 or hemizygous K18.hACE2 (K18) mice (stock no. 034860, Jackson Laboratories) were maintained in specific-pathogen-free conditions at the University of Minnesota in accordance with the Institutional Animal Care and Use Committees guidelines (7). Littermates were immunized IV with 5×10^10 viral particles of Ad5-N vector or Ad5-Null.
Immunized K18 mice were challenged with SARS-CoV-2 in the BSL-3 containment facility. For depletion experiments, mice received intraperitoneal injections of 300ug of both CD4 (GK1.5) and CD8b (Lyt 3.2) or isotype controls (all from InVivoMab) on days 2, 3, 5, and 6 before challenge. Animals were anesthetized with ketamine/xylazine, laid supine, and challenged IN with 300 PFU of WA SARS-CoV-2 in a 30 ul volume. Infected animals were monitored daily for health, weight loss and morbidity and were deemed terminal if they reached 75% of starting weight. All weight loss experiments were terminated 13 days after infection. Mice assigned for flow cytometry analysis and the concurrent determination of viral titers were euthanized four days after challenge.
Intravascular labeling, cell isolation, and flow cytometry
To discriminate extravascular cells from intravascular cells, mice were injected IV with BV605-conjugated anti-CD8a Ab, sacrificed after 3 min, and tissues were harvested (8). Mouse lungs were mechanically minced into small pieces and further dissociated in PBS using gentleMACS tubes. For SARS CoV-2 challenge studies, dissociated tissue was split equally into two parts, one for quantification of SARS CoV-2 viral titers and the other for isolation of lymphocytes for flow cytometric analyses. Isolation of cells for flow cytometric analysis was performed as previously described (9). Cells were stained with Abs to CD8a (53-6.7), CD62L (MEL-14), Ly6C (HK1.4), CD44 (IM7), CD69 (H1.2F3), and CD103 (M290) (all from BD Biosciences, Tonbo Biosciences, BioLegend, or Affymetrix eBiosciences) and H2-Db/N219–227 MHC class I tetramers (made in-house) and ghost dye 780 (Tonbo Biosciences). For monomer preparation, the N219–227 peptide sequence was LALLLLDRL. Stained samples were acquired on LSRII or LSR Fortessa flow cytometers (BD Biosciences) and analyzed with FlowJo software (BD Biosciences). Cells were gated on singlets, live lymphocytes, CD8 T cells, and/or tetramer-specific cells as indicated.
Hamster and mouse lung lysate preparation
Hamsters and mice were euthanized 3 and 4 days after SARS-CoV-2 infection, respectively. Lungs were harvested from hamsters, weighed, and homogenized in PBS using gentleMACS M tubes and a gentleMACS dissociator on the protein setting. Mouse lungs were weighed and processed as described above before being further homogenized in PBS using gentleMACS M tubes (9). Tubes were centrifuged to pellet debris. The supernatants were collected, aliquoted, and stored at −80°C.
Cell Culture and Plaque Assays
Vero E6 cells (ATCC) were maintained in DMEM with 10% FBS and 1% penicillin-streptomycin. Infections were carried out in an infection medium (DMEM with 1% penicillin-streptomycin, 1% Non-Essential Amino Acids, 10mM HEPES, and 2% FBS). The plaque assay was adapted from Mendoza et al. (10). Ten-fold dilutions of the homogenized lung samples were adsorbed to Vero E6 cells. The cells were incubated for 1 hour at 37°C in a 5% CO2 incubator, rocking every 10 minutes. Cells were washed with PBS and overlaid with a carboxymethylcellulose (CMC) overlay media (MEM, 1.5% CMC) and incubated at 37°C in a 5% CO2 incubator for 3 days. The overlay media was removed, cells were washed with PBS, and fixed for 1 hour with 4% paraformaldehyde at room temperature. The fixative was discarded, and the cells were stained with crystal violet (0.5%) for 5–15 minutes. Cells were washed with distilled water 2–4 times, blotted dry, and plaques were enumerated.
Results
Ad5-N vaccine confers protection against SARS-CoV-2 infection
Outbred Syrian hamsters are permissive to SARS-CoV-2 infection. We vaccinated hamsters IV with Ad5-N, which expresses the N sequence derived from WA, or a control Ad5 vector lacking a SARS-CoV2 sequence, Ad5-NULL. 7–8 weeks later, animals were challenged IN with 6.8×104 PFU WA SARS-CoV-2. Vaccination reduced weight loss (Fig. 1A, B). Vaccinated hamsters were also challenged with WA or a variant strain B.1.1.7, which contains two amino acid substitutions in N that do not occur within the N219–227 immunodominant epitope that we previously defined in C57Bl/6 mice(6). Vaccination elicited a significant 30-fold (WA) and 12-fold (B.1.1.7) reduction in median lung viral titer three days following challenge, though the latter was not significant. We next immunized transgenic K18 mice that are highly susceptible to SARS-CoV-2 (11). 30 days following IV vaccination with Ad5-N or Ad5-NULL, K18 mice were challenged with 300 PFU SARS-CoV-2. Ad5-N vaccinated mice experienced significantly reduced weight loss (Fig. 1D, E) and mortality, with 75% surviving challenge vs. 0% in the Ad5-NULL vaccinated group (Fig. 1F).
Figure 1. Ad5-N vaccine confers protection against SARS-CoV-2 infection in Syrian hamsters and K18 mice.
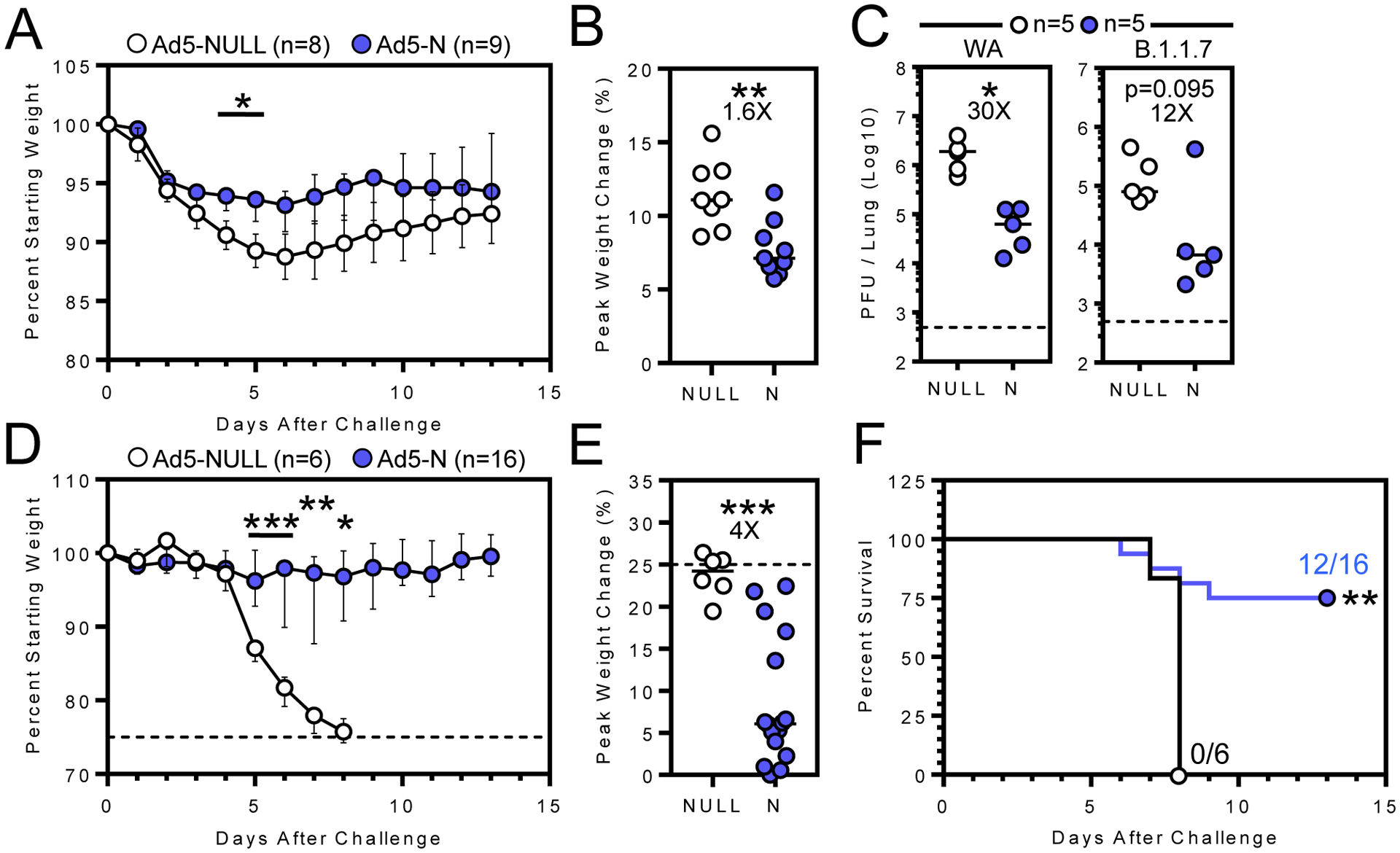
A) Median and B) peak weight change after vaccinated hamsters were challenged 7–8 weeks later with WA SARS-CoV-2. C) Lung viral titers three days post-challenge with WA (left) or B.1.1.7 (right) SARS-CoV-2. D) Median and E) peak weight change (dashed lines represent humane endpoint), and F) survival of vaccinated K18 mice following IN WA SARS-CoV-2 challenge. Bars denote median and interquartile range *, P < 0.05; **, P < 0.01; ***, P < 0.001. Individual comparisons were analyzed using two-sided Mann–Whitney tests (A-E) using Bonferroni-Dunn correction for multiple comparisons (A, D) and survival by the log-rank test (F).
Memory T cells respond to SARS-CoV-2 challenge
Vaccination established SARS-CoV-2-N219–227-specific memory CD8 T cells that persisted in lung, airways, and spleen from 40 to 86 days, and increased in the lung draining mediastinal lymph node (Fig. 2A) (12). We examined whether T cells in vaccinated mice participated in the response four days after WA SARS-CoV-2 challenge using a combined antibody-mediated CD8b and CD4 depletion strategy (Fig 2B, 2C). Vaccinated mice showed a ~3.8-log10 reduction in lung viral load (Fig. 2D). Combined CD8b and CD4 depletion prior to challenge partially abrogated protection, although T cell depletion was not absolute, (Fig. 2B) and residual N219–227 -specific CD8 T cells were still observed in vaccinated mice (Fig. 2C).
Figure 2. Memory T cells respond to SARS-CoV-2 challenge.
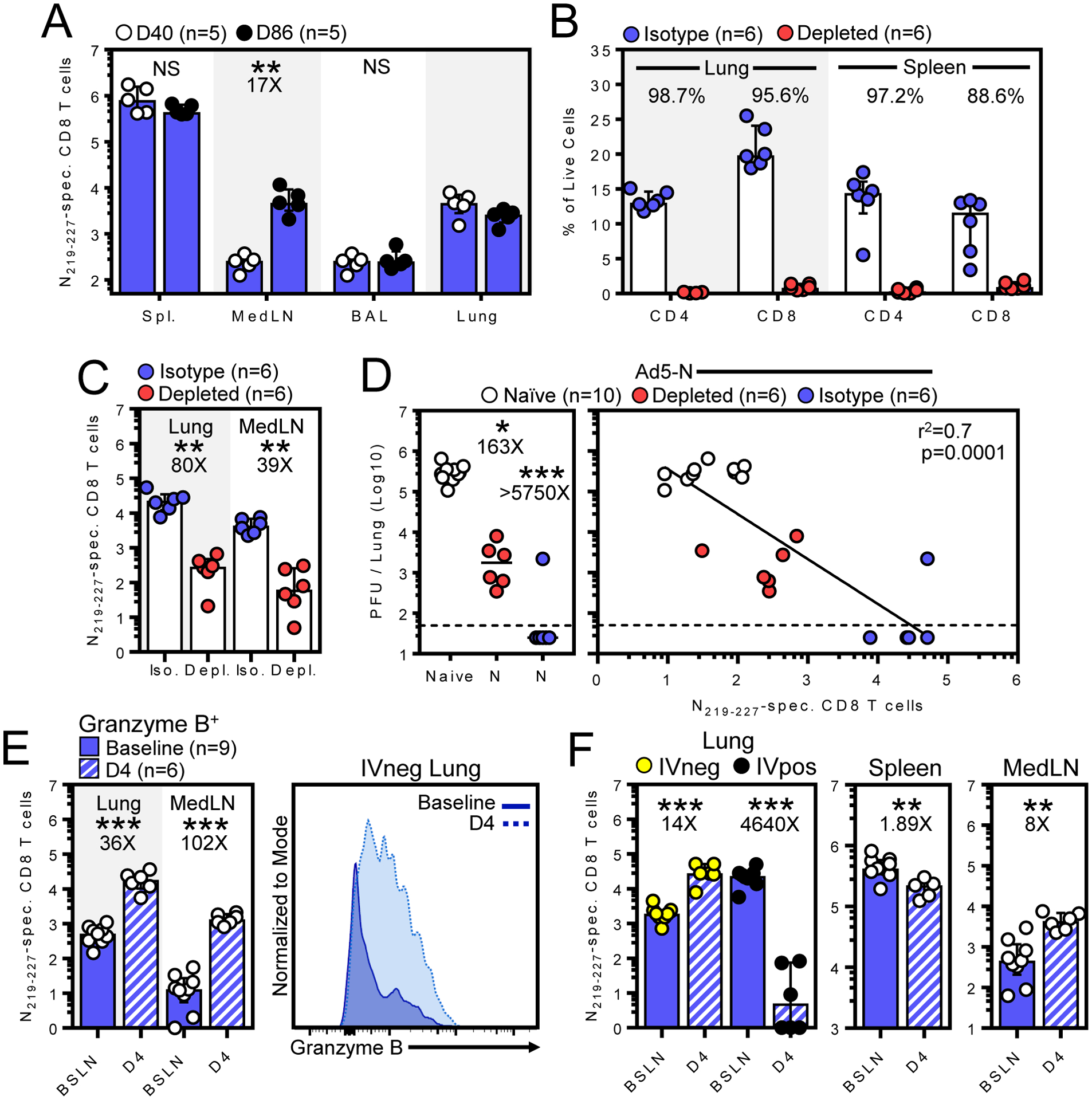
A) Number of N219–227-specific CD8 T cells 40 to 86 days after Ad5-N vaccination. B) Percent CD4 and CD8 T cells among IVneg lung or total splenocytes in isotype or T cell depleted Ad5-N vaccinated mice. C) Total N219–227-specific CD8 T cells in IVneg lung or MedLN of Ad5-N vaccinated mice following T cell depletion. D) Four days after WA SARS-CoV-2 challenge, viral titers in lungs of naive and Ad5-N vaccinated K18 mice ± T cell depletion (left) and viral titers plotted against N219–227-specific CD8 T cells within IVneg lung (right). E) Abundance of granzyme B+ N219–227-specific CD8 T cells in IVneg lung or mediastinal LN and F) of total N219–227-specific CD8 T cells within indicated compartments immediately prior (baseline) and four days after challenge. Bars denote median and interquartile range. Flow plots were gated on lymphocytes, single cells, live, CD8a IVneg cells followed by the gates indicated in each panel, except in (D) where IVpos cells were also independently evaluated*, P < 0.05; **, P < 0.01; ***, P < 0.001 as determined by a two-sided Mann–Whitney test (A-F).
IN challenge increased granzyme B+ N219–227-specific CD8 T cells in the pulmonary mucosa (Fig. 2E). Total N219–227-specific CD8 T cells substantially increased in both lung parenchyma (IVneg, defined by the absence of intravascular anti-CD8a staining, as previously described (8)) and draining mediastinal lymph node, moderately decreased in spleen, and substantially decreased in the lung vasculature (IVpos, Fig. 2F). These data indicate that vaccine-elicited memory CD8 T cells underwent rapid reactivation and migration to the site of viral challenge, and that T cells may have contributed to viral control.
Discussion
The rapidity and success of SARS-CoV-2 vaccine development and deployment is unprecedented. Most strategies, including all vaccines licensed for emergency use in the USA, immunize only against the viral spike. Spike is a logical choice because it contains the receptor binding domain that is the main target of NAb. Nevertheless, SARS-CoV-2 is likely to become endemic. Viral variants have emerged that reduce vaccine-elicited NAb efficacy (12), suggesting partial escape, and SARS-CoV-2 may continue to evolve with or without selection pressure from increased global immunity. While vaccination strategies often rely on NAbs for efficacy, engaging other arms of the immune system, such as cellular immune memory, have been shown to offer protection against viral infections and/or reduce the threshold of NAbs needed for protection (13, 14). This study provides evidence in rodents that immunological memory to additional antigens could provide protection, which may involve memory T cells and other non-neutralizing effector mechanisms. It appears likely that viral evolution will necessitate vaccine evolution and booster immunizations that address emergent variants. This study supports the rationale for including additional viral antigens into future vaccine candidates to broaden epitope diversity and protection mechanisms while limiting opportunities for viral escape.
Key points:
Vaccine elicited N-specific immunity confers protection against SARS-CoV-2
Memory CD8 T cells respond rapidly upon intranasal SARS-CoV-2 challenge
Acknowledgments
We thank the Biosafety Level 3 Program at the University of Minnesota. SARS-CoV-2 viruses were obtained through WRCEVA at UTMB.
Grant support:
Supported by the Office of the Dean of the University of Minnesota Medical School and UMN-Mayo Partnership for Biotechnology and Medical Genomics. W.E.M., V.J., J.M.S., S.W., and J.M.T. were supported NIH T32 HL007741, the Canadian Institutes of Health Research Fellowship, NIH T90 DE 022732, F30 DK114942 and NIH T32 AI055433, respectively.
Abbreviations used in this article:
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- S
spike protein
- N
nucleocapsid
- Ad5
human adenovirus type 5
- NAb
neutralizing antibodies
- IV
intravenous
- IN
intranasal
- WA
2019-nCoV/USA_WA1/2020 isolate
- CMC
carboxymethyl cellulose
- K18
K18-hACE2
References
- 1.Widge AT, et al. , Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. New Engl J Med 384, 80–82 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, et al. , Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New Engl J Med 383, 2603–2615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Beltran WF, et al. , Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell (2021) 10.1016/j.cell.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altenburg AF, Rimmelzwaan GF, de Vries RD, Virus-specific T cells as correlate of (cross-)protective immunity against influenza. Vaccine 33, 500–506 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Goulder PJR, Watkins DI, HIV and SIV CTL escape: implications for vaccine design. Nat Rev Immunol 4, 630–640 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Joag V, et al. , Cutting Edge: Mouse SARS-CoV-2 Epitope Reveals Infection and Vaccine-Elicited CD8 T Cell Responses. J Immunol, ji2001400 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCray PB, et al. , Lethal Infection of K18-hACE2 Mice Infected with Severe Acute Respiratory Syndrome Coronavirus▿. J Virol 81, 813–821 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson KG, et al. , Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc 9, 209–222 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinert EM, et al. , Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell 161, 737–749 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendoza EJ, Manguiat K, Wood H, Drebot M, Two Detailed Plaque Assay Protocols for the Quantification of Infectious SARS-CoV-2. Curr Protoc Microbiol 57, ecpmc105(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winkler ES, et al. , SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol 21, 1327–1335 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Planas D, et al. , Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med, 1–8 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Pleguezuelos O, et al. , Efficacy of FLU-v, a broad-spectrum influenza vaccine, in a randomized phase IIb human influenza challenge study. Npj Vaccines 5, 22(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arunachalam PS, et al. , T cell-inducing vaccine durably prevents mucosal SHIV infection even with lower neutralizing antibody titers. Nat Med 26, 932–940 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]


