Abstract
Pain is a fundamental experience that promotes survival. In humans, pain stands at the intersection of multiple health crises: chronic pain, the opioid epidemic, and health disparities. The study of placebo analgesia highlights how social, cognitive, and affective processes can directly shape pain and identifies potential paths for mitigating these crises. In this review, I examine recent progress in the study of placebo analgesia through affective science. I focus on how placebo effects are shaped by expectations, affect, and the social context surrounding treatment, and discuss neurobiological mechanisms of placebo, highlighting unanswered questions and implications for health. Collaborations between clinicians and social and affective scientists can address outstanding questions and leverage placebo to reduce pain and improve human health.
Keywords: Pain, affect, analgesia, placebo, social
Pain: A biopsychosocial phenomenon that requires integrative research
Pain lies at the core of multiple health crises. It is central to the opioid epidemic [1,2], there are substantial health disparities in pain [3–5], over 11% of American adults report experiencing daily pain [6], and chronic pain is highly comorbid with other central nervous system conditions, including mental health illnesses [7] and substance use disorders [8]. Health care practitioners have reduced opioid prescriptions in response to the opioid epidemic [9], yet we lack effective alternatives, including non-addictive and nonpharmacological therapies.
Hope can be found in the progress the field has made in isolating mechanisms that contribute to pain through animal models, as the systems that process pain ([10]; see Glossary) and nociception are highly conserved across species. Yet in humans, pain is ultimately a subjective, conscious experience that involves the integration of sensory processing, emotion, context, and decision making. Understanding human pain requires an integrative approach that draws on psychology, neuroscience, and medicine. In this review, I focus on placebo analgesia, a phenomenon that lies at the intersection of these diverse fields. In the following sections, I review the critical influences of expectations, affect, and the social context surrounding treatment (see Figure 1, Key Figure). I then discuss the brain mechanisms underlying placebo effects and the extent to which placebo analgesia provides insights on domains other than pain. Throughout, I emphasize that placebo research has important implications for the urgent crises surrounding opioid use disorder and health disparities in pain.
Figure 1, Key Figure: Expectations, affect, and social context shape pain and lead to placebo analgesia or nocebo hyperalgesia.
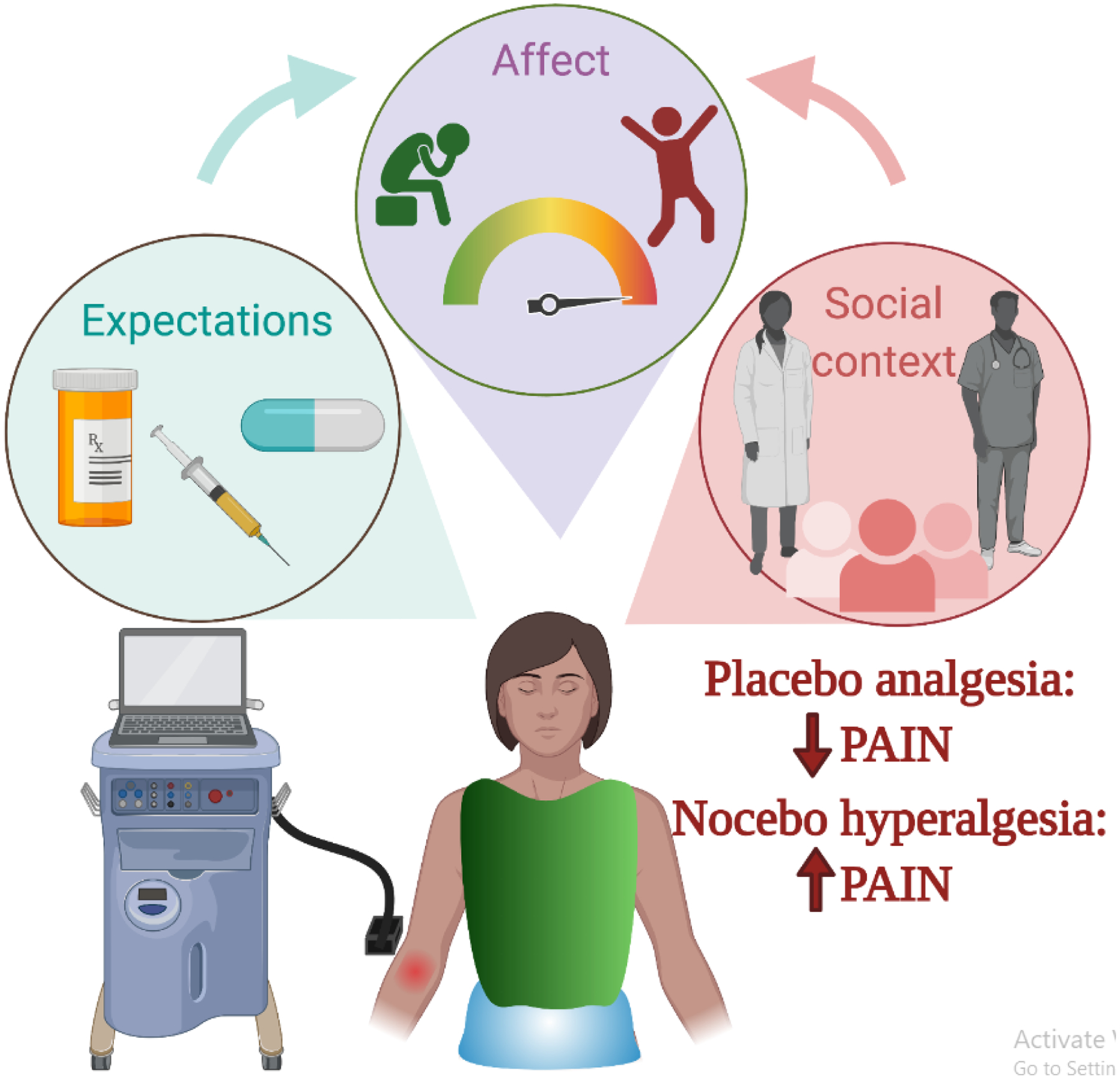
Laboratory models of placebo analgesia combine acute noxious stimuli (e.g. heat administered through a thermode) with experimental manipulations to measure how expectations, affect, and social factors influence perceived pain and pain-related neurobiological responses. Expectations may be driven by associative learning from cues associated with treatment or from verbal instructions about the anticipated effects of treatment. Placebos may influence affect by reducing anxiety or increasing positive emotion, whereas nocebo effects are associated with increases in anxiety. Social factors also shape placebo effects through the patient-provider interaction. Placebo analgesia refers to reduced pain as a result of expectations, affect, and the social context surrounding treatment, whereas nocebo hyperalgesia refers to the exacerbation of pain due to these psychosocial processes.
Placebo analgesia: A window on social, cognitive, and affective influences on pain
The placebo effect refers to a positive treatment outcome that is attributable to the psychosocial context surrounding treatment, rather than pharmacological or biological properties of the treatment itself. In clinical trials, placebo effects can be differentiated from placebo responses by comparing placebo administration to a natural history or waitlist control, which allows researchers to differentiate between true placebo effects and other non-specific factors. Meta-analyses of clinical trials including natural history control groups indicate that placebo effects are strongest in pain and subjective outcomes [11,12]. This finding has motivated researchers across diverse fields to move beyond self-report and study the mechanisms of placebo. The placebo effect’s negative counterpart, the nocebo effect, can also be measured by studying increases in pain or side effects or pain in response to inert treatments.
Laboratory experiments can isolate mechanisms of placebo analgesia and nocebo hyperalgesia by pairing acute pain stimulation (e.g. heat administered through a device called a thermode; see Figure 1) with an inert substance (e.g. skin cream) that a participant believes is a potent analgesic treatment and measuring associations between pain and neurobiological responses (see Figure 2). Studies implicate the critical role of expectations that are driven by instructed knowledge or learned through experience [13,14]. But expectations about treatment are not cold cognitions; they are laden with meaning and emotional significance, leading to the proposal that the placebo effect be called a “meaning” response [15]. The placebo effect also depends on the unique psychosocial context surrounding treatment, including patient-provider interactions. In the following sections, I review recent progress on affective, social, and cognitive influences on placebo analgesia.
Figure 2. Brain mechanisms of placebo analgesia and relationship with salience, interoception, and nociceptive processing.

Placebo analgesia is associated with variations in brain responses to noxious stimuli that can be visualized in humans using FMRI or PET imaging. Left: The regions that are most commonly activated by painful stimuli include the anterior cingulate cortex (ACC), insula, primary and secondary somatosensory cortex (S1 and S2), and thalamus. Each of these are targeted by afferent nociceptive pathways and contain nociceptive neurons. FMRI studies that compare responses to placebo administration with a control treatment (i.e. no expected pain relief) indicate that pain-related responses in the ACC, thalamus, and anterior insula are reduced with placebo (blue), whereas placebos elicit increases in activation in modulatory regions (gold) including the dorsolateral prefrontal cortex, rostral ACC, and the opioid-rich periaqueductal gray. Upper middle: Meta-analysis of studies of placebo analgesia indicate reliable reductions in the dorsal ACC, anterior insula, and thalamus [75]. Lower middle: Despite placebo-induced reductions in pain-evoked responses in a subset of pain-related regions, placebos do not elicit reliable modulation of the Neurologic Pain Signature [30,92], which is a brain-based pattern that can reliably distinguish responses to painful and non-painful stimuli and is sensitive and specific to pain. This suggests that placebos might modulate non-specific affective and cognitive processes rather than affecting nociception. Right: Studies of placebo analgesia and other forms of pain modulation must carefully distinguish pain from salience processing (upper right) and interoception (lower right) since pain is highly salient and requires interoception, and the brain networks that process pain overlap substantially with the salience network and interoceptive processing. Maps depict term-based meta-analyses from Neurosynth [98] (red and yellow = association tests, green and purple = uniformity tests). Images of placebo-induced reductions (upper middle) were adapted with permissions from [75] and images of the Neurologic Pain Signature (lower middle) were adapted with permissions from [30].
Expectations and the predictive coding model of placebo analgesia
Much work has focused on the role of expectation and prediction in placebo analgesia. Expectancy-based models of placebo assume that individuals develop expectations about clinical outcomes through experience (i.e. conditioning or associative learning) and explicit knowledge (e.g. through verbal suggestion or instruction), and that these expectations lead to placebo effects. Studies suggest that conditioning and verbal suggestion may have differential impacts on different outcomes. For example, one team of researchers treated different groups of participants with active treatments over several days [16]. Participants who underwent a tourniquet pain challenge received the analgesic ketorolac tromethamine, patients with Parkinson’s Disease received subthalamic nucleus stimulation, and healthy volunteers received sumatriptan, which decreases cortisol and increases growth hormone. After this conditioning phase, subsets of participants were instructed that they would receive a new treatment with opposite effects from the conditioned treatment. All participants received placebo, and researchers measured whether placebo effects mimicked instructions (i.e. reversed with the placebo) or conditioning (no reversal). Placebo effects on pain and motor performance reversed with instructions, while growth hormone and cortisol mirrored conditioning. This provides evidence that a) placebo effects depend on both prior experience and explicit beliefs; and b) outcomes may differ in their sensitivity to explicit expectations mediated by verbal instruction. We recently combined this type of instructed reversal with computational models of aversive learning and identified dissociable effects of instructions on brain regions involved in learning, such that the striatum and orbitofrontal cortex updated with instructions, whereas the amygdala learned purely from experience [17,18]. Future work should test whether similar mechanisms underlie dissociable effects of instructions and learning on placebo effects across domains.
Computational frameworks can indeed provide insights on the mechanisms of placebo. Some researchers have argued that placebo effects and pain modulation can be explained through predictive coding [19–23], which draws on Bayesian integration [24]. This framework predicts that the extent to which perception is biased toward prior expectations depends on the expectation’s precision: The more certain the expectation, the stronger the influence on perception. Conversely, if an expectation is vague or variable, perception will be driven by the experience itself (in the case of experimental pain, the noxious stimulus). This framework links placebo analgesia with other areas of perception and new interest in computational psychiatry and provides theoretical models that can be formally tested. How well do these models account for placebo effects in practice?
The predictive coding model has been directly tested in a series of studies by Büchel and colleagues [25–27]. In one task [25], individuals saw cues that predicted different intensities of noxious heat with different probabilities. Pain-predictive cues often serve as a model of placebo analgesia since they easily isolate effects of learning and instructions on pain (see Box 1). The researchers measured whether responses to noxious stimuli tracked a) stimulus intensity alone; b) additive effects of expectation & stimulus intensity; or c) a weighted sum of prediction and prediction errors. Autonomic responses and responses in the anterior insula integrated predictions and prediction errors consistent with predictive coding, while the posterior insula, a region thought to be fundamental for pain perception [28,29], and the neurologic pain signature, a pain-predictive brain classification pattern (NPS; [30]) tracked stimulus intensity. Büchel’s team subsequently built on this approach in a placebo analgesia experiment by manipulating the precision, or variability, of the expectation [26]. During conditioning, healthy volunteers either experienced consistent relief (i.e. temperature reduction) on the placebo-treated skin site, or experienced variable relief, which reduces the precision of the expectation. Placebo effects were larger in the group with high precision, and pain ratings conformed to Bayesian integration. In addition, activation in the opioid-rich periaqueductal gray (PAG) correlated with the attraction weight, a quantity that is sensitive to the precision of both the prior and the likelihood. This builds on work demonstrating that the PAG tracks uncertainty [31] and prediction errors [32] during pain prediction and plays a key role in descending modulation of pain [33,34], as discussed later in this review. Together, this work indicates that instructions and experiential learning combine to shape expectations, which in turn directly impact pain and pain-related processing.
Box 1. Stimulus expectancies vs treatment expectancies.
Both pain-predictive cues and placebo analgesia paradigms can lead to pain modulation through predictive coding. However, it is unknown how well cue-based pain modulation paradigms capture the construct of placebo analgesia, or whether these approaches should be evaluated independently. Pain-predictive cues provide information about the intensity of an upcoming noxious stimulus, inducing stimulus expectancies. Both the cue and the stimulus are usually brief phasic signals. In contrast, a typical analgesic treatment is long lasting, has a variable onset, and makes people less sensitive to the noxious stimulus itself. For example, a patient on morphine cares little about whether they will receive an innocuous finger prick, a shot, or a more invasive procedure. We refer to cue-based expectations as stimulus expectancies and placebo-related expectations as treatment expectancies, similar to Kirsch’s distinction between stimulus and response expectancies [118], although both stimulus and treatment expectancies are likely to induce response expectancies (i.e. expectations for reduced pain).
We have previously shown that cue-based pain modulation is additive with placebo and opioid analgesia [119], supporting the idea that stimulus and treatment expectancies engage independent mechanisms. We have hypothesized that the two are mediated by separable neuromodulatory mechanisms [13], whereby placebo analgesia engages tonic μ-opioid release to engage long-lasting descending inhibition [120], while cue-based pain modulation is likely to involve phasic dopamine prediction errors to signal the expected value of the upcoming stimulus [121]. Consistent with this hypothesis, placebo analgesia is intact during haloperidol dopamine blockade [122], while cue-based pain modulation is not influenced by the μ-opioid receptor antagonist naltrexone [123]. One study that directly compared treatment and stimulus expectancies indicated that the framing did indeed influence both prediction error processing and expectancy-related brain responses, such that prediction errors were only observed in response to stimulus expectancies, while the striatum and prefrontal cortex showed greater coupling in response to treatment expectancies [124]. We also conducted a meta-analysis that indicated preliminary distinctions between the brain mechanisms of stimulus and treatment expectancy [75], such that treatment expectancies were more likely to elicit decreases in the left insula and increases in the ventral striatum, rACC, PAG, and other regions, while stimulus expectancies were more associated with increases in the cerebellum, inferior parietal lobule, and lateral prefrontal cortex. To determine the validity of our paradigms, researchers should continue to assess whether stimulus and treatment expectancies do indeed engage the same mechanisms, or whether they reflect distinct pain-modulatory processes.
The role of affect in placebo analgesia and nocebo hyperalgesia
While most studies of placebo mechanisms have focused on manipulating expectations and learning, placebo effects do not depend strictly on expectations, and expectations are not purely cognitive. Patients seek treatment when they are suffering, and regardless of the mechanisms of treatment or even the specific disease or condition, the treatment ritual might relieve suffering by a) reducing negative affect and emotions such as anxiety, fear, and stress and b) increasing positive affect through feelings of trust, hope, and being supported. Likewise fear or anxiety about side effects or negative outcomes might directly lead to nocebo effects, since negative emotions can exacerbate pain [35,36]. Thus, emotion and affect may be as important as beliefs and expectations, yet relatively few studies have measured the relationships between placebo effects and mood or emotion. Do placebos act by reducing negative affect and increasing positive affect?
Several studies indicate that placebos do reduce negative emotion [37]. For example, placebo analgesia is associated with reduced fear and anxiety [38,39] relative to no-treatment control conditions in healthy volunteers, and placebo analgesia is inversely related to negative affect in patients with neuropathic pain [40]. Brain imaging studies also support links between placebo analgesia and affective processing. Individual differences in the magnitude of placebo analgesia can be predicted based on anticipatory activity in emotion-related brain networks, but not regions involved in pain or cognitive control [41], and placebo effects for pain and anxiety are associated with overlapping activity in the rostral anterior cingulate cortex (rACC), lateral orbitofrontal cortex (OFC), and ventrolateral prefrontal cortex (VLPFC) [42], suggesting common mechanisms of action in value-related circuits.
Studies of nocebo hyperalgesia also implicate anxiety. Nocebo administration enhances anticipatory anxiety [39] and nocebo analgesia can be blocked by the anxiolytic diazepam [43] and the cholecystokinin (CCK) antagonist proglumide [43]. CCK mediates anxiety-induced hyperalgesia and stress-induced hyperalgesia primarily through the PAG, where it also can inhibit opioid-related analgesia [44]. Interestingly, pre-existing anxiety does not seem to be a risk factor for nocebo effects [45–47], suggesting anxiety influences outcomes insofar as it emerges in response to the treatment ritual itself. Recent behavioral work also indicates that positive mood inductions can reduce the nocebo effect for headache [48,49], indicating a direct link between affect and nocebo. Fewer studies have examined the neural and psychological mechanisms of nocebo, relative to the large literature on placebo. We need more research on the specific factors that give rise to nocebo effects so that clinicians can mitigate nocebo effects in clinical practice. For a comprehensive review of the role of affect in placebo and nocebo, see [50].
Social influences on placebo analgesia
The role of affect in placebo and nocebo likely stems not only from internal shifts in mood and expectations, but also through the psychosocial treatment context, including patient-provider interactions. Having a positive therapeutic alliance is associated with beneficial outcomes, including in chronic pain [51,52]. Studies of the placebo effect indicate that the physician’s attitude and behavior directly influence clinical outcomes. Patients with irritable bowel syndrome show improvements with placebo acupuncture when administered by a warm, empathic provider, relative to a neutral doctor or waitlist control [53], and experimental models indicate that placebo effects on allergic reactions are largest when a provider is both warm and competent [54]. Qualities of the health practitioner are likely to induce shifts in patients’ mood and emotions that in turn maximize placebo, although studies rarely measure how providers influence affect per se. Social factors may also influence expectations and health outcomes even before interactions. Recent work indicates that first impressions of health care providers based on facial features alone can influence pain and expectations about pain [55,56].
Of course, actual clinical encounters involve bidirectional interactions between patient and provider, and these may be critical for determining whether a patient will show placebo or nocebo effects [57]. Simulated clinical interactions indicate that perceived similarity [58] and perceived empathy [59] influence patients’ responses. Importantly, patient-provider interactions and the social context surrounding treatment are not likely to be the same for all patients. For instance, experienced discrimination is associated with increased stress and reduced physical and mental health [60], and individuals who have experienced negative outcomes or discrimination in a healthcare environment have different expectations than individuals who have had positive experiences [61] (see also Box 2).
Box 2. Leveraging the study of placebo to mitigate health disparities.
While we have learned a great deal about mechanisms of placebo, not all placebo effects are equal, and not all individuals are placebo responders. Individual differences have been studied from a psychological and neurobiological perspective, yet social determinants of health are likely to play a critical role. For instance, in the US, non-White individuals are less likely to receive adequate treatment for their pain [4,125], despite the fact that they experience more pain in both clinical and laboratory settings [3,126], and even health care practitioners endorse false beliefs about Black people’s pain [127]. Disparities are present even in simple decision-making tasks: White perceivers are slower to identify pain in Black faces than White faces [128] and are less likely to prescribe pain treatment for the same level of pain in Black relative to White faces. Inequities may also directly influence pain perception in racial and ethnic minority patients: A recent fMRI study demonstrated that African Americans experienced increased pain and increased heat-related activation in the VMPFC and VS relative to White and Hispanic Americans [129], and these associations were larger in participants who had experienced higher levels of discrimination.
These inequities are likely to directly impact placebo responses, but placebo research also indicates that leveraging the patient-provider interaction might help reduce disparities. Individuals whose racialized groups have suffered trauma and unfair treatment are less likely to report trust in their physician [130,131], but reported satisfaction is higher when patients see providers from their own background [132,133]. Consistent with this, in a simulated clinical experiment, concordance between experimenter and participant reduced pain within Black participants, but not Hispanic or non-Hispanic White Participants [134], and the protective effect of concordance was largest for participants who reported higher levels of experienced discrimination and worry about discrimination. Across multiple studies, perceived similarity is associated with stronger placebo analgesia [58,134] and lower expected pain [55], whereas greater experienced discrimination has been linked to higher pain and stronger brain responses to pain in Black / African American participants in a study of acute pain [129]. Non-Hispanic Black participants have also been shown to exhibit reduced μ-opioid receptor binding during painful stimulation [135], which might contribute to these effects. Placebo researchers and health disparities researchers should work together to understand how placebo and nocebo effects might differ across sociocultural groups, while taking a cultural neuroscience perspective into account [136]. For further perspectives on paths to racial equality in pain science, see [137].
A recent study of acupuncture analgesia makes an important first step toward illuminating behavioral and neurobiological processes that underlie patient-provider interactions [62]. Researchers used fMRI hyper-scanning to simultaneously measure neural responses in fibromyalgia patients undergoing experimental acute pain and clinicians. Half the dyads had a preliminary interaction to establish rapport, and researchers measured the patient-provider coherence of both facial and neural responses while clinicians applied real or sham electroacupuncture in response to patients’ pain. Analgesic effects of real and sham electroacupuncture were similar, indicating the study provides more insight into placebo effects than acupuncture per se. Patient analgesia was associated with numerous indicators of the therapeutic alliance, including: 1) the strength of the self-reported relationship with the provider; 2) providers’ estimated treatment efficacy; 3) how much patients and providers mirrored one another’s facial expressions during pain anticipation; and 4) patient-provider concordance during pain anticipation in the right TPJ and other regions of the mentalizing network [63]. These exciting findings provide initial insight on links between therapeutic rapport and acute pain relief in chronic pain. Future work should extend this approach to other patient populations and measure interpersonal processes in the context of active treatments that differ from placebo.
Brain mechanisms of placebo analgesia and specificity to pain
The potency of placebo analgesia provides evidence that pain is modulated by the joint influences of expectation, affect, and the social context surrounding treatment. How are these effects mediated, and are these mechanisms unique to pain? Placebo analgesia is likely to engage similar psychological processes as other placebo effects, since most treatments should influence expectations, shape mood, and occur in the clinic’s unique psychosocial context. Neural mechanisms that support these psychological processes may be similar across clinical outcomes, although downstream mediators of placebo effects are likely to differ across conditions. While only a few studies have compared placebo effects across modalities [42,64–67], a larger literature focuses on whether brain mechanisms that process pain (and show reductions with placebo) are unique to pain. Here, I review brain mechanisms of placebo analgesia and evaluate evidence regarding pain specificity.
Brain mechanisms of placebo analgesia
The first evidence that placebo analgesia is accompanied by real biological processes came when the μ-opioid receptor (MOR) antagonist naloxone was administered along with placebo in patients who underwent wisdom tooth extraction [68]. Patients who received naloxone reported higher post-surgical pain than the those who received only placebo, suggesting that naloxone blocked the placebo effect. This implied that the placebo effect reduced pain by engaging endogenous MORs, mimicking the effects of pharmacological opioid analgesics. Findings were later confirmed with meta-analysis [69] and PET studies that measured MOR binding under placebo [38,70,71] or showed that placebo analgesia and opioid analgesia engaged similar rACC glucose release during painful stimulation [72]. In addition, fMRI studies demonstrated that brain responses to noxious stimuli in pain-related regions were diminished with placebo [73] (see Figure 2), and that these reductions were abolished with naloxone administration [74]. Furthermore, the regions that show consistent placebo-induced increases in fMRI activation, including the dorsolateral prefrontal cortex (DLPFC), rACC, and PAG [75], have high MOR density [70,71]. Together, this work provides strong evidence for the involvement of endogenous MOR binding in placebo analgesia.
MOR engagement indicates that placebo analgesia engages some of the same mechanisms as pharmacological analgesics (see Box 3). MORs are critical for descending pain modulation, i.e. inhibiting nociceptive signals from reaching the brain [33,34]. Thus placebos might block otherwise painful signals from reaching the brain, consistent with the gate-control theory of pain [76,77]. Importantly, the effects of MOR binding are not specific to pain. MOR agonists can produce euphoria and affective shifts [78] and directly influence social processing [79,80]. Preclinical models indicate that chronic pain reduces opioid receptor availability, and this is associated with reduced sucrose preference [81]. Therefore, simply showing MOR involvement in placebo analgesia is not sufficient for proving that placebo blocks nociceptive signals through descending modulation. Instead, MORs might be more closely associated with affective and social influences discussed above. A meta-analysis of brain imaging studies of placebo analgesia indicates reliable reductions in responses to noxious stimuli within the anterior cingulate, insula, and thalamus [75] (see Figure 2). These regions have been associated with pain’s affective components (i.e. pain unpleasantness [82,83]). However, they are also collectively known as the “salience network”, due to their reliable recruitment by any salient stimulus, including pain [84–86], and are implicated generally in interoception [87–89] (see Figure 2). Thus, placebos and other forms of pain modulation might reduce the salience or aversiveness of the stimulus without changing nociception per se [85]. To determine whether placebos modulate the earliest pain signals, we need tests that are specific to pain.
Box 3. Combining placebos with pharmacological treatment: Implications for reducing opioid dependence.
Studies of placebo analgesia isolate the psychological factors that shape pain in response to inert treatment. However, these same factors also directly influence responses to active treatment, which is why clinical trials compare a treatment arm with a placebo arm to isolate the pure effects of the treatment. This subtraction logic assumes that placebo effects and treatment effects are additive and independent. We and others have directly tested these assumptions using a balanced placebo design [138], in which instructions about treatment – which manipulate expectations – are crossed with actual drug delivery in a classic 2 × 2 factorial design. Thus, participants receive an active treatment under both open (expect drug) and hidden (expect placebo) administration and receive an inert treatment under both placebo (expect drug) and control (expect placebo) conditions. This allows researchers to directly test for pure drug effects, placebo/instruction effects, and whether they interact to affect clinical outcomes. We previously observed additive effects of placebo and the μ-opioid analgesic remifentanil on subjective pain [93,119], but pure drug effects on responses to heat in pain-related regions [30,93], regardless of instruction. Importantly, different patterns of placebo-drug combinations are likely with different doses, pain measures, and analgesics. For instance, expectancy-drug interactions have been observed with the peripherally acting analgesic lidocaine [139], while cannabidiol shows additive effects on conditioned pain modulation and interactive effects on offset analgesia, pain threshold, and pain tolerance [140]. Continued research using the balanced placebo design can provide a path forward to understanding how to leverage psychological factors that underlie placebo and effectively combine them with active treatments to enhance patient outcomes and perhaps even reduce the need for pharmacological treatments.
As we learn how placebos combine with active analgesics, we may be able to safely integrate these approaches to lessen our reliance on pharmacological interventions. If we can continue to enhance the psychosocial context surrounding pain by providing a supportive patient-provider relationship, target patients’ expectations by avoiding nocebo effects and enhancing placebo effects, and reduce anxiety in patients, we may ultimately enhance pain relief non-pharmacologically and reduce the quantities of pharmacological treatments necessary to relieve suffering.
Fortunately, we can now use advanced neuroimaging analysis approaches and machine learning to develop reliable pain classifiers and evaluate pain specificity. In particular, the Neurologic Pain Signature [30] is a fMRI-based brain pattern (see Figure 2) that predicts whether a statistical brain map (i.e. a standard fMRI analysis result) represents pain or not, and which of two conditions is more painful. It is sensitive and specific to pain, highly reliable [90], and can predict acute pain accurately even in new studies [30] and in different types of noxious stimuli [91]. The NPS is strongly modulated by opioid analgesics [30]. Does it show similar reductions with placebo?
This was tested in a meta-analysis of twenty placebo analgesia fMRI studies [92]. NPS differences between painful stimulation and baseline had a large effect size, with activation in all studies and over 95% of individual participants. Across studies, placebo effects on subjective pain ratings were moderate. However, placebo analgesia was not accompanied by robust reductions in the NPS; effects were small even when restricted to placebo responders (i.e. individuals who reported large reductions in pain with placebo). Two studies in the meta-analysis permitted direct comparisons of placebo and the μ-opioid analgesic remifentanil [93,94]. While the magnitude of remifentanil analgesia and placebo analgesia was similar, the drug effect on the NPS was about ten times larger than the placebo effect [92].
Taken together, these findings suggest that placebo effects on acute pain are mediated at least in part by non-nociceptive networks. A follow up meta-analysis [95] indicated that the only reliable reductions across subjects were in the insula, habenula, and the cerebellum, as well as the ventral attention and somatomotor networks. Interestingly, most pain-related regions did not show consistent effects across studies, but were instead correlated with the magnitude of the placebo effect across participants. Most regions that had previously been implicated as showing increases with placebo analgesia, including the DLPFC and rACC, showed large heterogeneity across studies. This suggests that these modulatory regions may be sensitive to the various contextual factors reviewed earlier, such as expectation, affect, and the social context surrounding treatment, since these factors varied across studies.
Placebo effects across modalities
To what extent do studies of placebo analgesia generalize to other modalities? We can consider both the neural and psychological processes identified in placebo analgesia and studies that directly measured placebo and expectancy effects across modalities.
As mentioned in the previous section, placebos engage MOR binding, which is associated with descending pain modulation [96] as well as affective shifts [33] and social processing [97]. Rather than being specific to placebo analgesia, placebo effects on MOR binding might mediate the affective effects that accompany placebo administration. As mentioned above, placebos do not elicit reliable reductions in the NPS, and the strongest reductions are observed in regions that are not specific to pain but are instead part of the so-called “salience network.” In fact, the brain regions that make up the so-called “pain matrix” and show the strongest evidence of pain-related responses during neuroimaging include the insula, dACC, and thalamus [98,99], as depicted in Figure 2, leading to a wide debate on whether activation of these regions is indicative of pain [100,101]. Pain is highly salient and arousing, and therefore placebo effects on regions like the insula and cingulate may not be specific to pain but may instead reflect reductions in the aversiveness or salience of the noxious stimulus. If this is the case, alterations in salience responses should be seen in domains other than pain, and the brain mechanisms of placebo analgesia should generalize across domains.
Several recent studies compared noxious stimuli to salient stimuli from other sensory modalities [102–105] to measure the specificity of pain-related brain responses. When stimuli were matched on subjective intensity/salience [102], spatial patterns of brain activation distinguished painful stimuli from non-nociceptive stimuli, and nearly all nociceptive regions, with the exception of the ACC, showed stronger activity for painful stimuli relative to equally salient touch, sound, or vision. Similarly, when painful heat and unpleasant sounds were matched on the basis of physiological arousal (a proxy for salience), brain responses in the posterior parietal operculum distinguished painful stimuli from salient auditory stimuli [105]. Thus, even though pain is highly salient and activates the salience network, some brain responses are unique to pain.
Do we see similar specificity when it comes to placebo analgesia? To determine whether neural and psychological mechanisms of pain modulation are specific to placebo analgesia or whether they support placebo effects more generally, we must compare placebo analgesia with other modalities. Unfortunately, few studies have directly compared placebo effects across domains. In two studies that measured whether placebo analgesia can transfer across domains, participants were instructed that a treatment would not only reduce pain, but also that it would have effects on a second modality – either to improve motor performance [106] or to reduce anxiety [65]. Participants in the key conditions then underwent a standard placebo analgesia conditioning manipulation in which noxious stimuli were surreptitiously reduced. The researchers measured subjective and objective outcomes for both pain and the secondary modality. In each study, the researchers observed placebo analgesia as well as placebo effects in the secondary modality even though subjects were never conditioned in the secondary domain. Interestingly, placebo effects on objective outcomes for the secondary domain (i.e. improved motor performance [106] or altered P2 and N2 amplitude [65]) were only observed when instructions were paired with placebo conditioning, whereas placebo effects on subjective outcomes (fatigue [106] and unpleasantness ratings [65]) were observed whether or not subjects underwent conditioning.
Thus placebo-related expectations can transfer across modalities with expectations for cross-modal effects, although experiential learning may be necessary to reinforce expectations based on instructions. Interestingly, instructions elicited cross modal effects even without conditioning when researchers tested whether placebos could modulate both physical pain and so-called “social pain”, or feelings of rejection [107]: Relative to a control condition, individuals who received a placebo that was described as an “effective painkiller, reducing emotional distress” reported less pain in response to noxious heat and less negative affect when viewing images of partners who had rejected them. However, even within a modality, individuals show different responses to different placebo interventions [108], which might relate to individuals’ distinct expectations about different treatments. Together, studies of placebo generalization and transfer suggest that placebo effects can generalize across modalities when instructions induce expectations about this type of cross-modal reaction. However, under basic conditions individuals have different expectations about different outcomes, which may impact the extent to which expectations transfer in natural situations.
To what extent are the brain mechanisms of placebo conserved across modalities? Petrovic and colleagues [42] compared brain imaging data from a study of placebo analgesia [72] with a study of placebo anxiolytics [109] in which participants viewed emotional images when they believed they had received an anxiolytic treatment. In both studies, the magnitude of the placebo effect on subjective outcomes was correlated with the magnitude of the placebo effect on the right lateral OFC, a region that has been implicated in value processing and affective regulation in other studies. Though the lateral OFC was not identified in placebo analgesia meta-analyses [75,95], it is an important candidate to consider in placebo studies in other domains, as this region and the adjacent right VLPFC have been implicated in placebo analgesia [73,110], emotion regulation [111], and social exclusion [112], and may play a general role in regulating negative affect [113] and defensive responses [114] based on lesion models. More recently, Koban and colleagues directly compared placebo effects on pain and negative affect when participants viewed images of partners who had rejected them [107]. Activation in the right DLPFC mediated placebo effects in each domain, although the responses were distinct both in location and when tested using a classifier. The DLPFC has been identified in meta-analyses of placebo analgesia [75] and plays a causal role in placebo based on studies using transcranial magnetic stimulation [115] or measuring prefrontal degradation in Alzheimer’s Disease [116]. These findings are in line with a large body of work on the DLPFC’s general role in higher order knowledge and goal directed behavior [117], and thus it is perhaps the strongest candidate to play a modulatory role in placebo effects across domains.
Studies of cue-based expectations provide further insight on the brain mechanisms of cross-modal expectancy effects. Several recent studies [103,104] compared cue-based expectancy effects on painful and non-painful stimuli and measured the specificity of brain responses. Researchers compared pain with a different aversive outcome (disgusting odors [104] or aversive images [103]) that was matched in perceived unpleasantness. To test whether predictive cues induce cross-modal modulation, modality-specific predictive cues were presented prior to stimulation and occasionally followed by outcomes from the opposite modality (i.e. pain cues were followed by odors or images). In these studies, predictive cues modulated perception in a domain-specific manner (i.e. predictions about pain only modulated subjective pain and predictions about odors or images only modulated responses to the non-painful stimuli) and the anterior insula mediated domain-specific expectancy effects on perception in each domain. Both studies also found support for pain-specific processing in the posterior insula, similar to results mentioned earlier with respect to predictive coding [25]. As with the DLPFC’s role in placebo effects on physical and social pain [107], these studies suggest that even if the same brain region (i.e. the anterior insula) is associated with unpleasantness across modalities, it still functions in a largely modality-specific manner. Extrapolating from these findings, placebo effects may indeed engage the same circuits across modalities to support expectations, learning, and affect, but ultimately downstream mechanisms are likely to differ for distinct clinical outcomes.
Concluding remarks
In this paper I have reviewed the four main areas of research in placebo analgesia: expectancy/learning, affect, social influences, and pain specificity. I argue that we can leverage this active area of research to make headway in addressing the urgent crises surrounding health disparities in pain (see Box 2) and opioid addiction (see Box 3). While decades of research have isolated neural circuits that support pain and introduced potent pharmacological interventions that reduce pain, we are still building our knowledge base regarding the mechanisms of placebo (see Outstanding Questions). Understanding how expectations, emotion, and the social context surrounding treatment shape pain and clinical outcomes will allow us to directly target these psychological processes to reduce pain and related conditions and improve well-being. When pain researchers and clinicians join forces with social and affective neuroscientists, we may make true strides in our ability to address the opioid epidemic, reduce health disparities in pain, and improve health by reducing the burden of pain.
OUTSTANDING QUESTIONS:
Experimental studies of placebo separately manipulate and measure cognitive processes and neural mechanisms that contribute to clinical placebo effects, including learning, instruction, affect, and social interactions, but rarely consider interactions between these different aspects of placebo. For example, do caring providers alter patients’ expectations, or do they reduce anxiety? How do expectations about pain shape emotion, mood, and affect?
How reliable is the placebo effect across contexts or treatments? If biological factors determine an individual’s propensity to respond to placebo, responses should be stable across time within individuals, and we may be able to predict whether someone will be a “placebo responder”. Alternatively, since placebo effects depend on contextual factors, person × situation interactions might be more likely to determine responses to placebo.
How well do experimental models of placebo analgesia generalize to clinical populations and clinical trials? Although most experimental models have manipulated acute noxious stimuli and measured placebo and mechanisms of expectancy-based pain modulation, acute experimental pain is a poor model for chronic pain. Basic researchers and clinicians should continue to combine efforts to understand whether and how placebo mechanisms shape outcomes for specific patients, specific pain conditions, and specific treatment contexts.
Highlights.
The study of placebo analgesia provides insights on how pain and clinical outcomes are shaped by social, cognitive, and affective processes.
Treatments for pain are highly influenced by the clinical context and the patient-provider relationship, which modulate pain by altering expectations, shifting mood, or providing social support.
Placebo effects on pain depend on similar types of psychological mechanisms as other placebo effects, and underlying brain mechanisms contain both shared and distinct processes.
The study of placebo has implications for mitigating health disparities and addressing the opioid crisis.
To improve human health, pain researchers should work together with social and affective neuroscientists to understand links between pain, affect, and social processing.
Acknowledgments.
LYA is supported by the Intramural Research Program of the National Center for Complementary and Integrative Health (ZIA-AT03000030, ZIA-AT03000036), as well as the National Institute of Mental Health and the National Institute on Drug Abuse. Thank you to Drs. Massieh Moayedi and M. Catherine Bushnell and anonymous reviewers for helpful comments on earlier versions of this manuscript. Figures were created with BioRender.com.
Glossary
- Acute pain
Brief pain that is caused by something specific (e.g. an injury or brief noxious stimulus), in contrast to chronic pain. Pain administered in the lab is usually acute pain, as it is controlled by a device that administers brief noxious stimuli such as heat, laser, or electrical pulses. Post-surgical pain or short-term pain due to injury are other examples of acute pain.
- Nocebo effect
Negative health outcomes that reflect from the psychosocial treatment context, such as side effects or enhanced pain (see nocebo hyperalgesia).
- Nocebo hyperalgesia
A phenomenon in which pain is exacerbated in response to an inert treatment due to expectations, emotions, and the context surrounding treatment.
- Nociception
The neurobiological response to a noxious (i.e. potentially damaging) stimulus, regardless of how it is ultimately perceived.
- Pain
A subjective feeling that is associated with discomfort, suffering, and either actual injury or the potential for physical damage.
- Placebo
An inert substance that is administered instead of an active treatment. May be used in clinical trials to differentiate between active effects of a treatment and so-called “non-specific” factors (expectations and psychological effects, regression to the mean, and natural history).
- Placebo analgesia
Pain reduction in response to an inert treatment that is due to expectations, emotions, and the context surrounding treatment.
- Placebo effect
A positive treatment outcome that is attributable to the psychosocial context surrounding treatment, rather than pharmacological or biological properties of the treatment itself. Can be distinguished from placebo response in clinical trials by comparing placebo arm with waitlist control group.
- Placebo response
Health outcomes in response to an inert treatment, including regression to the mean and natural fluctuations in clinical outcomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Volkow N et al. (2018) Use and Misuse of Opioids in Chronic Pain. Annual Review of Medicine 69, annurev-med-011817–044739 [DOI] [PubMed] [Google Scholar]
- 2.Marshall B et al. (2019) Considerations in addressing the opioid epidemic and chronic pain within the USA. Pain Management 9, 131–138 [DOI] [PubMed] [Google Scholar]
- 3.Kim HJ et al. (2016) Racial and ethnic differences in experimental pain sensitivity, [DOI] [PubMed]
- 4.Burgess DJ et al. (2014) Racial differences in prescription of opioid analgesics for chronic noncancer pain in a national sample of veterans. Journal of Pain 15, 447–455 [DOI] [PubMed] [Google Scholar]
- 5.Rahim-Williams B et al. (2012) A quantitative review of ethnic group differences in experimental pain response: do biology, psychology, and culture matter? Pain medicine (Malden, Mass.) 13, 522–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nahin RL (2015) Estimates of Pain Prevalence and Severity in Adults: United States, 2012. The Journal of Pain 16, 769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lerman SF et al. (2015) Longitudinal Associations Between Depression, Anxiety, Pain, and Pain-Related Disability in Chronic Pain Patients: Psychosomatic Medicine 77, 333–341 [DOI] [PubMed] [Google Scholar]
- 8.Vowles KE et al. (2015) Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. PAIN 156, 569–576 [DOI] [PubMed] [Google Scholar]
- 9.García MC et al. (2019) Opioid Prescribing Rates in Nonmetropolitan and Metropolitan Counties Among Primary Care Providers Using an Electronic Health Record System — United States, 2014–2017. MMWR. Morbidity and Mortality Weekly Report 68, 25–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raja SN et al. (2020) The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain 161, 1976–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hróbjartsson A and Gøtzsche PC (2004) Is the placebo powerless? Update of a systematic review with 52 new randomized trials comparing placebo with no treatment. Journal of Internal Medicine 256, 91–100 [DOI] [PubMed] [Google Scholar]
- 12.Hróbjartsson A and Gøtzsche PC (2001) Is the Placebo Powerless? The New England Journal of Medicine 344, 1594–1602 [DOI] [PubMed] [Google Scholar]
- 13.Atlas LY and Wager TD (2012) How expectations shape pain. Neuroscience Letters 520, [DOI] [PubMed] [Google Scholar]
- 14.Colloca L et al. (2008) The role of learning in nocebo and placebo effects. PAIN 136, 211–218 [DOI] [PubMed] [Google Scholar]
- 15.Moerman DE and Jonas WB (2002) Deconstructing the placebo effect and finding the meaning response. Annals of internal medicine 136, 471–476 [DOI] [PubMed] [Google Scholar]
- 16.Benedetti F et al. (2003) Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. The Journal of neuroscience 23, 4315–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atlas LY et al. (2016) Instructed knowledge shapes feedback-driven aversive learning in striatum and orbitofrontal cortex, but not the amygdala. eLife 5, e15192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atlas LY (2019) How instructions shape aversive learning: higher order knowledge, reversal learning, and the role of the amygdala. Current Opinion in Behavioral Sciences 26, 121–129 [Google Scholar]
- 19.Ongaro G and Kaptchuk TJ (2018) Symptom perception, placebo effects, and the Bayesian brain. Pain 00, 0–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Büchel C et al. (2014) Placebo Analgesia: A Predictive Coding Perspective. Neuron 81, 1223–1239 [DOI] [PubMed] [Google Scholar]
- 21.Tabor A and Burr C (2019) Bayesian Learning Models of Pain: A Call to Action. Current Opinion in Behavioral Sciences 26, 54–61 [Google Scholar]
- 22.Seymour B and Mancini F (2020) Hierarchical models of pain: Inference, information-seeking, and adaptive control. NeuroImage 222, 117212. [DOI] [PubMed] [Google Scholar]
- 23.Anchisi D and Zanon M (2015) A Bayesian Perspective on Sensory and Cognitive Integration in Pain Perception and Placebo Analgesia. PLoS ONE 10, e0117270–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Körding KP and Wolpert DM (2004) Bayesian integration in sensorimotor learning. Nature 427, 244–247 [DOI] [PubMed] [Google Scholar]
- 25.Geuter S et al. (2017) Functional dissociation of stimulus intensity encoding and predictive coding of pain in the insula. DOI: 10.7554/eLife.24770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grahl A et al. (2018) The periaqueductal gray and Bayesian integration in placebo analgesia. eLife 7, e32930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strube A et al. (2021) The temporal and spectral characteristics of expectations and prediction errors in pain and thermoception. eLife 10, e62809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segerdahl AR et al. (2015) The dorsal posterior insula subserves a fundamental role in human pain. Nature Neuroscience 18, 499–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craig AD et al. (2000) Thermosensory activation of insular cortex. Nature Neuroscience 3, 184–190 [DOI] [PubMed] [Google Scholar]
- 30.Wager TD et al. (2013) An fMRI-Based Neurologic Signature of Physical Pain. New England Journal of Medicine 368, 1388–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida W et al. (2013) Uncertainty Increases Pain: Evidence for a Novel Mechanism of Pain Modulation Involving the Periaqueductal Gray. Journal of Neuroscience 33, 5638–5646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy M et al. (2014) Representation of aversive prediction errors in the human periaqueductal gray. Nature Neuroscience 17, 1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fields HL (2006) A Motivation-Decision Model of Pain: The Role of Opioids. In Proceedings of the 11th World Congress on Pain (Flor H et al. , eds), pp. 11, IASP Press [Google Scholar]
- 34.Fields HL (2018) How expectations influence pain: PAIN 159, S3–S10 [DOI] [PubMed] [Google Scholar]
- 35.Bushnell MC et al. (2013) Cognitive and emotional control of pain and its disruption in chronic pain. Nature Reviews Neuroscience 14, 502–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vlaeyen JWS et al. (2016) The fear-avoidance model of pain: PAIN 157, 1588–1589 [DOI] [PubMed] [Google Scholar]
- 37.Flaten MA (2014) Pain-Related Negative Emotions and Placebo Analgesia BT - Placebo. In Placebo 225pp. 81–96, Springer; Berlin Heidelberg: [DOI] [PubMed] [Google Scholar]
- 38.Scott DJ et al. (2007) Individual Differences in Reward Responding Explain Placebo-Induced Expectations and Effects. Neuron 55, 325–336 [DOI] [PubMed] [Google Scholar]
- 39.Colagiuri B and Quinn VF (2018) Autonomic Arousal as a Mechanism of the Persistence of Nocebo Hyperalgesia. The Journal of Pain 19, 476–486 [DOI] [PubMed] [Google Scholar]
- 40.Petersen GL et al. (2012) Placebo manipulations reduce hyperalgesia in neuropathic pain. PAIN 153, 1292–1300 [DOI] [PubMed] [Google Scholar]
- 41.Wager TD et al. (2011) Predicting individual differences in placebo analgesia: Contributions of brain activity during anticipation and pain experience. Journal of Neuroscience 31, 439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petrovic P et al. (2010) A prefrontal non-opioid mechanism in placebo analgesia. PAIN DOI: 10.1016/j.pain.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 43.Benedetti F et al. (2006) The Biochemical and Neuroendocrine Bases of the Hyperalgesic Nocebo Effect. Journal of Neuroscience 26, 12014–12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lovick TA (2008) Pro-nociceptive action of cholecystokinin in the periaqueductal grey: A role in neuropathic and anxiety-induced hyperalgesic states. Neuroscience & Biobehavioral Reviews 32, 852–862 [DOI] [PubMed] [Google Scholar]
- 45.Webster RK et al. (2016) A systematic review of factors that contribute to nocebo effects. Health Psychology 35, 1334–1355 [DOI] [PubMed] [Google Scholar]
- 46.Michalska KJ et al. (2018) Anticipatory effects on perceived pain: Associations with development and anxiety, Psychosom Med, 80(9):853–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abend R et al. (2021) Threat-anticipatory psychophysiological response is enhanced in youth with anxiety disorders and correlates with prefrontal cortex neuroanatomy. J Psychiatry Neurosci 46, E212–E221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geers AL et al. (2019) Testing a positive-affect induction to reduce verbally induced nocebo hyperalgesia in an experimental pain paradigm. Pain 160, 2290–2297 [DOI] [PubMed] [Google Scholar]
- 49.Geers AL et al. (2019) A positive mood induction for reducing the formation of nocebo effects from side effect information. Annals of Behavioral Medicine 53, 999–1008 [DOI] [PubMed] [Google Scholar]
- 50.Geers AL et al. (2021) Affect and emotions in placebo and nocebo effects: What do we know so far? Social and Personality Psychology Compass 15, e12575 [Google Scholar]
- 51.Ferreira PH et al. (2013) The Therapeutic Alliance Between Clinicians and Patients Predicts Outcome in Chronic Low Back Pain. Physical Therapy 93, 470–478 [DOI] [PubMed] [Google Scholar]
- 52.Farin E et al. (2013) The patient–physician relationship in patients with chronic low back pain as a predictor of outcomes after rehabilitation. Journal of Behavioral Medicine 36, 246–258 [DOI] [PubMed] [Google Scholar]
- 53.Kelley JM et al. (2009) Patient and Practitioner Influences on the Placebo Effect in Irritable Bowel Syndrome: Psychosomatic Medicine 71, 789–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howe LC et al. (2017) Harnessing the Placebo Effect: Exploring the Influence of Physician Characteristics on Placebo Response. Health Psychology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Necka EA et al. (2021) Expectations about pain and analgesic treatment are shaped by medical providers’ facial appearances: Evidence from five online clinical simulation experiments. Social Science & Medicine 281, 114091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mattarozzi K et al. (2021) Pain and satisfaction: healthcare providers’ facial appearance matters. Psychological research 85, 1706–1712 [DOI] [PubMed] [Google Scholar]
- 57.Necka EA and Atlas LY (2018) The Role of Social and Interpersonal Factors in Placebo Analgesia. In International Review of Neurobiology 138pp. 161–179, Elsevier; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Losin EAR et al. (2017) Feelings of Clinician-Patient Similarity and Trust Influence Pain: Evidence From Simulated Clinical Interactions. The Journal of Pain 18, 787–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen P-HA et al. (2019) Socially transmitted placebo effects. Nature Human Behaviour 3, 1295–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pascoe EA and Smart Richman L (2009) Perceived discrimination and health: A meta-analytic review. Psychological Bulletin 135, 531–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hall OT et al. (2021) Experiences of racial discrimination in the medical setting and associations with medical mistrust and expectations of care among black patients seeking addiction treatment. Journal of Substance Abuse Treatment DOI: 10.1016/j.jsat.2021.108551 [DOI] [PubMed] [Google Scholar]
- 62.Ellingsen D-M et al. (2020) Dynamic brain-to-brain concordance and behavioral mirroring as a mechanism of the patient-clinician interaction. Science advances 6, eabc1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mar RA (2011) The neural bases of social cognition and story comprehension. Annual review of psychology 62, 103–134 [DOI] [PubMed] [Google Scholar]
- 64.Weng L et al. (2021) Can placebo and nocebo effects generalize within pain modalities and across somatosensory sensations? Pain. doi: 10.1097/j.pain.0000000000002390 [DOI] [PubMed] [Google Scholar]
- 65.Zhang W and Luo J (2009) The transferable placebo effect from pain to emotion: Changes in behavior and EEG activity. Psychophysiology 46, 626–634 [DOI] [PubMed] [Google Scholar]
- 66.Zhang W et al. (2011) A follow-up fMRI study of a transferable placebo anxiolytic effect. Psychophysiology 48, 1119–1128 [DOI] [PubMed] [Google Scholar]
- 67.Zhao Y et al. (2015) A transferable anxiolytic placebo effect from noise to negative effect. Journal of Mental Health 00, 1–6 [DOI] [PubMed] [Google Scholar]
- 68.Levine JD et al. (1978) The mechanism of placebo analgesia. The Lancet 2, 654–657 [DOI] [PubMed] [Google Scholar]
- 69.Sauro MD and Greenberg RP (2005) Endogenous opiates and the placebo effect. Journal of Psychosomatic Research 58, 115–120 [DOI] [PubMed] [Google Scholar]
- 70.Zubieta J-K (2005) Placebo Effects Mediated by Endogenous Opioid Activity on μ-Opioid Receptors. Journal of Neuroscience 25, 7754–7762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wager TD et al. (2007) Placebo effects on human μ-opioid activity during pain. Proceedings of the National Academy of Sciences 104, 11056–11061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petrovic P (2002) Placebo and Opioid Analgesia– Imaging a Shared Neuronal Network. Science 295, 1737–1740 [DOI] [PubMed] [Google Scholar]
- 73.Wager TD (2004) Placebo-Induced Changes in fMRI in the Anticipation and Experience of Pain. Science 303, 1162–1167 [DOI] [PubMed] [Google Scholar]
- 74.Eippert F et al. (2009) Activation of the Opioidergic Descending Pain Control System Underlies Placebo Analgesia. Neuron 63, 533–543 [DOI] [PubMed] [Google Scholar]
- 75.Atlas LY and Wager TD (2014) A Meta-analysis of Brain Mechanisms of Placebo Analgesia: Consistent Findings and Unanswered Questions. In Handbook of Experimental Pharmacology 225pp. 37–69, Springer; Berlin Heidelberg: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Melzack R (1999) From the gate to the neuromatrix. PAIN Suppl 6, S121–6 [DOI] [PubMed] [Google Scholar]
- 77.Moayedi M and Davis KD (2012) Theories of pain: from specificity to gate control. Journal of Neurophysiology 109, 5–12 [DOI] [PubMed] [Google Scholar]
- 78.Nummenmaa L and Tuominen L (2018) Opioid system and human emotions. British Journal of Pharmacology 175, 2737–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chelnokova O et al. (2014) Rewards of beauty: the opioid system mediates social motivation in humans. Mol Psychiatry 19, 746–747 [DOI] [PubMed] [Google Scholar]
- 80.Loseth GE (2014) State-dependent μ-opioid modulation of social motivation. Front. Behav. Neurosci DOI: 10.3389/fnbeh.2014.00430/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thompson SJ et al. (2018) Chronic neuropathic pain reduces opioid receptor availability with associated anhedonia in rat. Pain 159, 1856–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rainville P et al. (1999) Dissociation of sensory and affective dimensions of pain using hypnotic modulation. PAIN 82, 159–171 [DOI] [PubMed] [Google Scholar]
- 83.Rainville P et al. (1997) Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277, 968–971 [DOI] [PubMed] [Google Scholar]
- 84.Uddin LQ (2014) Salience processing and insular cortical function and dysfunction. Nature Publishing Group 16, 55–61 [DOI] [PubMed] [Google Scholar]
- 85.Legrain V et al. (2011) The pain matrix reloaded: A salience detection system for the body. Progress in Neurobiology 93, 111–124 [DOI] [PubMed] [Google Scholar]
- 86.Lee I-S et al. (2019) Distinguishing pain from nociception, salience, and arousal: How autonomic nervous system activity can improve neuroimaging tests of specificity. NeuroImage DOI: 10.1016/j.neuroimage.2019.116254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barrett LF and Simmons WK (2015) Interoceptive predictions in the brain. Nature Publishing Group; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bud Craig AD (2003) Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology 13, 500–505 [DOI] [PubMed] [Google Scholar]
- 89.Critchley HD et al. (2004) Neural systems supporting interoceptive awareness. Nature Neuroscience 7, 189–195 [DOI] [PubMed] [Google Scholar]
- 90.Han X et al. (2021) Effect sizes and test-retest reliability of the fMRI-based Neurologic Pain Signature. bioRxiv DOI: 10.1101/2021.05.29.445964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van Oudenhove L et al. (2020) Common and distinct neural representations of aversive somatic and visceral stimulation in healthy individuals. Nat Commun 11, 5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zunhammer M et al. (2018) Placebo Effects on the Neurologic Pain Signature. JAMA Neurology DOI: 10.1001/jamaneurol.2018.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Atlas LY et al. (2012) Dissociable influences of opiates and expectations on pain. Journal of Neuroscience 32, 8053–8064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bingel U et al. (2011) The Effect of Treatment Expectation on Drug Efficacy: Imaging the Analgesic Benefit of the Opioid Remifentanil. Science Translational Medicine 3, 70ra14–70ra14 [DOI] [PubMed] [Google Scholar]
- 95.Zunhammer M et al. (2021) Meta-analysis of neural systems underlying placebo analgesia from individual participant fMRI data. Nat Commun 12, 1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fields H (2004) State-dependent opioid control of pain. Nature Reviews Neuroscience 5, 565–575 [DOI] [PubMed] [Google Scholar]
- 97.Loseth GE et al. (2014) State-dependent μ-opioid modulation of social motivation. Front. Behav. Neurosci 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yarkoni T et al. (2011) Large-scale automated synthesis of human functional neuroimaging data. Nature Methods 8, 665–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Duerden EG and Albanese M-C (2013) Localization of pain-related brain activation: A meta-analysis of neuroimaging data. Human brain mapping 34, 109–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wager TD et al. (2016) Pain in the ACC? Proceedings of the National Academy of Sciences of the United States of America 113, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lieberman MD and Eisenberger NI (2015) The dorsal anterior cingulate cortex is selective for pain: Results from large-scale reverse inference. Proceedings of the National Academy of Sciences 112, 15250–15255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liang M et al. (2019) Spatial Patterns of Brain Activity Preferentially Reflecting Transient Pain and Stimulus Intensity. Cerebral Cortex 29, 2211–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fazeli S and Büchel C (2018) Pain related expectation and prediction error signals in the anterior insula are not related to aversiveness. The Journal of Neuroscience 38, 0671–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sharvit G et al. (2018) Modality-specific effects of aversive expectancy in the anterior insula and medial prefrontal cortex: PAIN 159, 1529–1542 [DOI] [PubMed] [Google Scholar]
- 105.Horing B et al. (2019) The parietal operculum preferentially encodes heat pain and not salience. PLoS Biology DOI: 10.1101/581504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carlino E and Benedetti F (2016) Different contexts, different pains, different experiences. Neuroscience 338, 19–26. [DOI] [PubMed] [Google Scholar]
- 107.Koban L et al. (2017) Frontal-Brainstem Pathways Mediating Placebo Effects on Social Rejection. J. Neurosci 37, 3621–3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kong J et al. (2013) Are All Placebo Effects Equal? Placebo Pills, Sham Acupuncture, Cue Conditioning and Their Association. PLoS ONE 8, e67485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Petrovic P et al. (2005) Placebo in Emotional Processing— Induced Expectations of Anxiety Relief Activate a Generalized Modulatory Network. Neuron 46, 957–969 [DOI] [PubMed] [Google Scholar]
- 110.Lieberman MD et al. (2004) The neural correlates of placebo effects: a disruption account. NeuroImage 22, 447–455 [DOI] [PubMed] [Google Scholar]
- 111.Lieberman MD (2007) Social Cognitive Neuroscience: A Review of Core Processes. Annual Review of Psychology 58, 259–289 [DOI] [PubMed] [Google Scholar]
- 112.Eisenberger NI (2003) Does Rejection Hurt? An fMRI Study of Social Exclusion. Science 302, 290–292 [DOI] [PubMed] [Google Scholar]
- 113.Hooker CI and Knight RT (2006) The role of lateral orbitofrontal cortex in the inhibitory control of emotion. In The Orbitofrontal Cortex (Zald D and Rauch S, eds), pp. 307–324, Oxford University Press [Google Scholar]
- 114.Pujara MS et al. (2019) Heightened Defensive Responses Following Subtotal Lesions of Macaque Orbitofrontal Cortex. J. Neurosci 39, 4133–4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Krummenacher P et al. (2010) Prefrontal cortex modulates placebo analgesia. PAIN 148, 368–374 [DOI] [PubMed] [Google Scholar]
- 116.Benedetti F et al. (2006) Loss of expectation-related mechanisms in Alzheimer’s disease makes analgesic therapies less effective. PAIN 121, 133–144 [DOI] [PubMed] [Google Scholar]
- 117.Miller EK and Cohen JD (2001) An integrative theory of prefrontal cortex function. Annual Review of Neuroscience 24, 167–202 [DOI] [PubMed] [Google Scholar]
- 118.Kirsch I (1997) Response expectancy theory and application: A decennial review. Applied and preventive Psychology 6, 69–79 [Google Scholar]
- 119.Atlas LY et al. (2013) Specifying the non-specific factors underlying opioid analgesia: expectancy, attention, and affect. Psychopharmacology 231, 813–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Watkins LR and Mayer DJ (1982) Involvement of spinal opioid systems in footshock-induced analgesia: antagonism by naloxone is possible only before induction of analgesia. Brain Research 242, 309–326 [DOI] [PubMed] [Google Scholar]
- 121.Schultz W et al. (1997) A neural substrate of prediction and reward. Science 275, 1593–1599 [DOI] [PubMed] [Google Scholar]
- 122.Wrobel N et al. (2014) Haloperidol blocks dorsal striatum activity but not analgesia in a placebo paradigm. CORTEX 57, 60–73 [DOI] [PubMed] [Google Scholar]
- 123.Pontén M et al. (2020) Naltrexone during pain conditioning: A double-blind placebo-controlled experimental trial. Mol Pain 16, 174480692092762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schenk LA et al. (2017) Suppression of striatal prediction errors by the prefrontal cortex in placebo hypoalgesia. The Journal of Neuroscience DOI: 10.1523/JNEUROSCI.1101-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Goyal MK et al. (2015) Racial Disparities in Pain Management of Children With Appendicitis in Emergency Departments. JAMA Pediatrics 169, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Riskowski JL (2014) Associations of Socioeconomic Position and Pain Prevalence in the United States: Findings from the National Health and Nutrition Examination Survey. Pain Medicine 15, 1508–1521 [DOI] [PubMed] [Google Scholar]
- 127.Hoffman KM et al. (2016) Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proceedings of the National Academy of Sciences 113, 201516047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mende-Siedlecki P et al. (2019) Perceptual Contributions to Racial Bias in Pain Recognition. Journal of Experimental Psychology: General 148, 863–889 [DOI] [PubMed] [Google Scholar]
- 129.Losin EAR et al. (2020) Neural and sociocultural mediators of ethnic differences in pain. Nature Human Behaviour DOI: 10.1038/s41562-020-0819-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Doescher MP et al. (2000) Racial and ethnic disparities in perceptions of physician style and trust. Arch Fam Med, 9, 1156–63. [DOI] [PubMed] [Google Scholar]
- 131.Sewell AA (2015) Disaggregating ethnoracial disparities in physician trust. Social Science Research 54, 1–20 [DOI] [PubMed] [Google Scholar]
- 132.Cooper-Patrick L et al. (1999) Race, gender, and partnership in the patient-physician relationship. Jama 282, 583–589 [DOI] [PubMed] [Google Scholar]
- 133.LaVeist TA and Nuru-Jeter A (2002) Is doctor-patient race concordance associated with greater satisfaction with care? Journal of health and social behavior, 43, 296–306 [PubMed] [Google Scholar]
- 134.Anderson SR et al. (2020) Clinician–Patient Racial/Ethnic Concordance Influences Racial/Ethnic Minority Pain: Evidence from Simulated Clinical Interactions. Pain Medicine 21, 3109–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Letzen JE et al. (2020) Ethnic disparities in pain processing among healthy adults: μ-opioid receptor binding potential as a putative mechanism. Pain 161, 810–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Anderson SR and Losin EAR (2017) A sociocultural neuroscience approach to pain. Cult. Brain 5, 14–35 [Google Scholar]
- 137.Booker SQ et al. (2021) The Imperative for Racial Equality in Pain Science: A Way Forward. The Journal of Pain. DOI: 10.1016/j.jpain.2021.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rohsenow DJ and Marlatt GA (1981) The balanced placebo design: methodological considerations. Addictive behaviors 6, 107–122 [DOI] [PubMed] [Google Scholar]
- 139.Schenk LA et al. (2013) Expectation requires treatment to boost pain relief. An fMRI study. PAIN DOI: 10.1016/j.pain.2013.09.024 [DOI] [PubMed] [Google Scholar]
- 140.De Vita MJ et al. (2021) The effects of cannabidiol and analgesic expectancies on experimental pain reactivity in healthy adults: A balanced placebo design trial. Experimental and Clinical Psychopharmacology DOI: 10.1037/pha0000465 [DOI] [PMC free article] [PubMed] [Google Scholar]


