Abstract
Current glaucoma treatments aim to lower intraocular pressure, often with topical ocular hypotensive medications. Unfortunately, the effectiveness of these medications depends on sustained patient adherence to regimens which may involve instilling multiple medications several times daily. Patient adherence to glaucoma medications is often low. Recent innovations in digital sensor technologies have been leveraged to confirm eyedrop medication usage in real-time and relay this information back to providers. Some sensors have also been designed to deliver medication reminders and notifications as well as assist with correct eyedrop administration technique. Here, we review recent innovations targeted at improving glaucoma medication adherence and discuss their limitations.
INTRODUCTION
Current glaucoma treatments lower intraocular pressure (IOP), the only proven modifiable risk factor which has been shown in multiple landmark clinical trials to reduce the risk of disease progression.1–5 Despite increasing evidence to support the use of selective laser trabeculoplasty as first-line treatment,6–8 lowering IOP with eyedrops remains the most common first-line therapy.9 However, their effectiveness is based on sustained patient adherence to treatment regimens, which can involve instilling multiple medications several times daily.
Medication adherence is a major issue for all of medicine, and glaucoma management is no exception. One of the reasons why patients with chronic asymptomatic diseases like glaucoma are more prone to non-adherence is because they may not notice the benefits of medications.10 Adherence rates for topical glaucoma medications range from 30% to 80%.11–16 In a study of newly treated patients with glaucoma, more than 20% failed to obtain follow-up visits within at least 18 months of diagnosis,17 as recommended by the American Academy of Ophthalmology’s Preferred Practice Pattern guidelines.18 Moreover, only 10% of patients refilled their prescription within 12 months, suggesting they are not taking glaucoma medications as prescribed.1 17 19 Because poor medication adherence can hasten glaucoma progression and vision loss,20 it is crucial to understand reasons for poor adherence, develop robust methods of measuring adherence and deploy interventions to improve adherence. Here, we have conducted a narrative review of recent advances in monitoring glaucoma medication adherence and novel patient engagement strategies that leverage innovations in digital health technologies.
REASONS FOR NON-ADHERENCE IN GLAUCOMA
Non-adherence to glaucoma medication is multifactorial. Robin and Muir grouped non-adherence to topical glaucoma medication into five dimensions: patient-related, social and economic, health-care system, therapy-related, and condition-related dimensions.1 Patient-related barriers that negatively influence glaucoma adherence include insufficient knowledge about the risks of disease progression and the benefits of treatment, lack of self-efficacy and forgetfulness. In a study by Newman-Casey et al of both adherent and non-adherent patients, forgetfulness was ranked as the number one barrier to adherence, although among non-adherent patients, each of 11 commonly cited barriers was salient to greater than 30% of participants.11 Other studies have also found that difficulties with glaucoma drop administration, costs and complex medication schedules were additional barriers.21–24 Therefore, patients with glaucoma often face multiple barriers to medication adherence.
TRADITIONAL METHODS OF MONITORING ADHERENCE
Some traditional methods of monitoring medication adherence include patient self-report and analysing health insurance claims data or pharmacy claims data. Here, we review examples of how these methods are used.
Patient self-report
Patient self-report is one of the most commonly used measures of adherence.25 Self-reported adherence is assessed through self-administered questionnaires or interviews by trained personnel. While simple and inexpensive, patient self-report commonly overestimates adherence when compared with electronic monitoring results.26 According to the Glaucoma Adherence and Persistency Study, 95% of patients claimed they never missed taking their glaucoma medications despite clear evidence of poor adherence.23 One potential factor cited by Sayner et al10 in a study demonstrating patients over-reporting adherence compared with electronic monitors was social desirability bias, where patients’ desire to please their physicians influences them to unconsciously over-report adherence.27 28 Patient self-report is also subject to various biases like inaccurate memory and assumptions.29
Claims data
Health insurance claims data provide a complete list of reimbursed services for a large insured population, and pharmacy claims data are a subset of health insurance claims data specifically related to medications. In an analysis of claims data by Nordstrom et al, nearly half of patients who filled a glaucoma prescription discontinued all topical hypotensive treatments within 6 months.30 Three years after the initial prescription, only 37% of patients had recently refilled their medication.21 30 Several other studies have also used claims data to understand adherence rates in patients with glaucoma.17 31 32 However, having a prescription alone does not equate to the patient successfully getting the medication onto the eye. Furthermore, some patients may have more than one health insurance, which leads to discrepancies in adherence using this method.
Claims data allow evaluation of large sample populations, but they demand a complex approach to measurement and interpretation. Pharmacy records have difficulty capturing the difference between the addition of a new medication and the switch to a new medication. In a study by Friedman et al, 17% of 174 patients were given a second medication for glaucoma, but only 10% of the added medications were correctly identified using pharmacy claims data. Moreover, 66.7% of the added medications were misclassified as switched medications, and 23.3% were misclassified as no change.19 This suggests that misclassifications from pharmacy claims data can inflate adherence rates and obscure poor adherence. Additionally, pharmacy claims data cannot ensure patient usage of glaucoma medications at home, again resulting in overstatements of true adherence.
Pharmacy claims data can be used to assess the medication possession ratio (MPR), defined as the ‘days of prescription supply dispensed divided by the number of days between the first and last prescription refill’.19 MPR assesses the adherence of patients who are prescribed multiple medications and distinguishes between added and switched medications. Thus, MPR may be more comprehensive for patients who cycle off and back on medications.19 However, MPR does not provide insight into timeliness and consistency of refilling. For example, a patient who struggles to properly instil an eyedrop will use more drops than prescribed and may request multiple bottles in early months of therapy, leading to a high MPR.26
In summary, traditional methods of monitoring can provide rough estimations of medication adherence but have major limitations, including mischaracterisation of adherence and lack of granularity. Thus, these methods are limited in accurately identifying non-adherent patients and facilitating interventions in real-time.
INNOVATIONS IN SENSOR TECHNOLOGY FOR ADHERENCE MONITORING
Given the potential inaccuracy of traditional methods of adherence monitoring, several digital sensor monitoring systems have been developed. Digital sensor monitoring systems have been referred to as the ‘gold standard’ for assessing medication adherence as they objectively quantify adherence, often in real-time, allowing clinicians to deliver personalised interventions and reminders targeting individual patient behaviours.33–36 First, we will summarise developments for oral medications, where this technology is more well-developed. Then, we will specifically describe recent innovations in sensors for eye medication adherence monitoring.
Smart pill bottles
Several smart pill bottles have been developed to track medication usage. One example is the widely used Medication Event Monitoring Systems (MEMS) Smart Cap (AARDEX Group, rue bois St Jean, Seraing, Belgium), which is embedded with a sensor that records every time the pill bottle is opened and has been used in the field of ophthalmology among others.10 37–42 This is assumed to correspond to patient consumption of a medication dosage. The MEMS Smart Cap can also alert a patient if he/she has already taken a dose that day. Similarly, the MedTracker is an instrumented 7-day pill reminder box that records each time the pillbox has been opened and closed.43 Other smart pill bottles are able to detect when pills are removed and generate weekly patient feedback reports regarding adherence; they also glow, light up, buzz, sound alarms, and even send text and voice messages to remind patients to take medications (Vitality GlowCap, Vitality, Los Angeles, California, USA; AdhereTech, New York, New York, USA).44 A randomised controlled trial using one such smart bottle, the Vitality GlowCap, found that daily alarms combined with individual or partner feedback reports improved statin medication adherence.45
Sensors for confirming medication ingestion
A notable limitation of smart pill bottles is that the sensors only measure when the bottle has been opened or when a pill has been removed, but not actual ingestion of the medication. One proposed way to overcome this is to supplement the smart bottle with a smart necklace capable of determining if medication ingestion has occurred based on skin movement of the neck during a swallow.46 This smart necklace was able to correctly differentiate between chewable vitamins, saliva swallows, medication capsules, speaking and drinking water, with average precision and recall of 90.17% and 88.9%, respectively.46 Alternatively, inertial sensors can be used to recognise the ‘twist cap’ and the ‘hand to mouth’ motions associated with pill taking.47 Technology for inertial sensors has also been leveraged to measure adherence to home physiotherapy regimens.48 49
Ingestible biosensors are another digital method to directly assess medication ingestion. One of the most notable prototypes, Proteus Discover,50 consists of ingestible sensors composed of copper, magnesium and silicon that are embedded within medication tablets. A signal is transmitted to the patient’s mobile phone when the tablets dissolve in the stomach, with an overall accuracy of 97.6%. Clinicians can then analyse patients’ adherence data through a secure web portal. Over 100 studies have investigated the use of these ingestible sensors to improve medication adherence for tuberculosis, diabetes, hypertension, psychiatric and post-transplant treatments, among others.33 51–57 Other ingestible sensors like MyTMed and ID-Cap System (eTectRx, Newbury, Florida, USA) offer similar methods of monitoring adherence.58–60
Sensors for eye medications
The majority of digital sensor technologies have been studied and implemented to assist with oral medication adherence. In the early 1990s, C Cap (C Cap Compliance Cap, Allergan), was developed as the first medication cap with a memory aid to assess glaucoma medication adherence.61 An early commercial device for monitoring eye drop adherence was the Travatan Dosing Aid (figure 1A), designed to assist patients in taking travoprost and record when drops were administered.19 62 In a multifaceted programme to enhance glaucoma eyedrop usage, which included the device and patient education and reminders, mean adherence rate improved from 54% to 73% (p<0.001) in patients with <75% initial baseline drop-taking.19 63 However, this dosing aid could not be used for other eye medications and was not widely adopted by patients or eyecare providers. In addition, the device was only available for a limited time.
Figure 1.
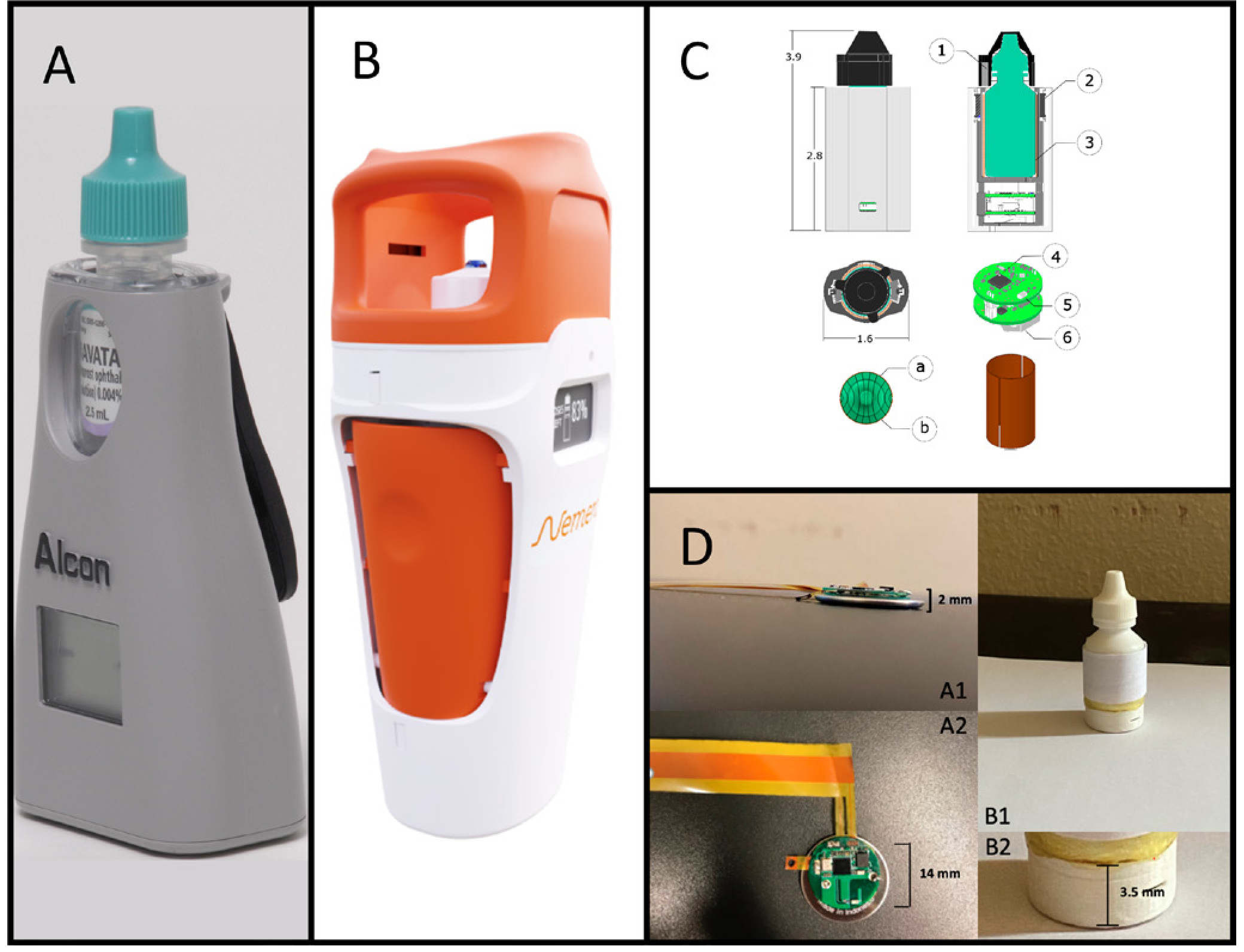
Digital sensor technologies for glaucoma medication adherence monitoring. (A) The Travatan Dosing Aid. Reprinted from Friedman et al108, Copyright (2007), with permission from Elsevier. (B) The e-Novelia Smart add-on for ophthalmic droppers. Available from: https://www.nemera.net/smart-ophthalmic-add-on-e-novelia/. (C) An intelligent sleeve with embedded sensors to quantify adherence and fluid level for glaucoma medication. Bar magnets (1) are placed in the cap, reed switches (2) in the sleeve sense cap removal, the two-part capacitive sensor (3) has two copper sheets (a) and (b) surrounding the bottle, electronics are embedded in an nRF51422 system-on-a-chip (4), Bluetooth low energy (5) is used for data transfer, and the system is powered with a single rechargeable coin cell battery (6). Reproduced with permission from Payne et al.68 (D) A smart drop sensor system comprised a thin conductive pressure-sensitive electronic sensor, for bottle squeezing detection and an electronic circuit (<2 mm thick) for signal processing and wireless transmission, in lateral view (A1) and top view (A2). The flexible sensor is contained underneath the label (B1), with electronics at the base (B2). Reproduced with permission from Aguilar-Rivera et al.69
Recently, others have innovated new ‘smart’ technologies designed for eyedrop bottles that are applicable across different medications. For example, E-Novelia (figure 1B) developed a custom, large bottle that encases eyedrop medication bottles which can detect wrong usage via location tracking and an inclination light used for electronic guidance, remaining volume of medication and drop dispensing while delivering reminders.64 E-Novelia also contains sensors that can wirelessly transfer data to mobile phones. However, its large size makes it difficult for patients to carry and use, potentially posing a barrier to adoption. Kali Drop (Kali Care, Santa Clara, California, USA) is another smart eyedrop bottle designed to collect and record objective medication use data (ie, number of drops dispensed and the time and date taken), which can be analysed by patients and providers alike to track adherence. The Kali Drop device is compact and has 3G wireless sensors embedded in the base of the device capable of detecting whether the device was inverted at the time of use.65 66 Embedded coloured lights are also employed to signal when a successful dose is recorded and transmitted. In a recent pilot study of the Kali Drop, the majority of patients found the device easy to use, that it did not interfere with medication-taking or their normal activities, and that they were not bothered by physicians tracking their adherence data.65
Several groups have developed less obtrusive sensors that can be adapted to various eyedrop medications. For example, Nishimura et al recently developed an eyedrop bottle motion sensor system to measure patient adherence with topical glaucoma medications.67 Waveform data are automatically collected from the sensor and judged as a complete instillation via a deep learning model.67 When evaluated, the sensor system successfully collected instillation data of 20 patients with glaucoma for 3 days with 100% accuracy.67
Similarly, Payne et al developed an intelligent sleeve for eyedrop bottles with a sensor capable of detecting eyedrop use, measuring fluid level and sending user information to providers to facilitate intervention (figure 1C). When tested, the smart sleeve successfully identified and time-stamped 94% of use events.68
Finally, Aguilar-Rivera et al developed a ‘smart drop’ bottle containing a thin electronic force sensor located under the label of the eyedrop medication bottle (figure 1D). This sensor detects and wirelessly transmits drop deliveries to a smartphone app with a 100% success rate of wireless communication over 75 feet and <1% false positive and false negative rates of single drop deliveries.69
The Hawthorne effect applies to the majority of the smart technology studies reviewed, given that patients were aware that their adherence to medication would be monitored while using the smart devices.70 71 As a result, medication adherence may be overestimated with the use of smart technologies given the design of most of the studies. However, these eyedrop specific sensors offer a promising new avenue for generating glaucoma medication adherence data in real-time, which can help clinicians customise patient-specific interventions to improve adherence.
Similar to the use of smart pill bottles for monitoring oral medications, these sensors are focused on monitoring the medication dispensing event—that is, the transfer of medication from within the bottle to outside the bottle. They do not directly measure the instillation of medication in the eye itself. It is also important to note that storing of eyedrop medications outside of the smart sensor devices can lead to underestimations of adherence. Although there have been several studies regarding the use of contact lens biosensors to measure IOP72–74 or to detect glucose levels,73 75 our literature search did not yield formal assessments of using these devices for measuring medication adherence specifically. Evaluating the application of these devices for monitoring medication adherence in a more direct fashion (ie, instillation into the tear film) would be a promising avenue of future investigation. However, the current medication adherence sensors that are based on monitoring eyedrop bottles may have potential for broader deployment and acceptability due to their non-invasive design and reduced risk for side effects compared with devices that come into contact with the eye. Therefore, these sensors focused on medication dispensing still represent a worthwhile initial approach for measuring and monitoring eye medication adherence.
INNOVATIONS IN DELIVERY OF MEDICATION REMINDERS
Traditionally, patients are reminded to adhere to medication regimens during clinical encounters or phone calls. Some studies have shown that intensive educational and coaching programmes can improve adherence.76–85 However, these approaches are labour-intensive and not easily scalable. Innovations in digital health technology—particularly the widespread adoption of smartphones, mobile apps and electronic health record (EHR) systems and their associated patient portals—offer novel modes of patient engagement around medication adherence.
Smartphones and mobile apps
Smartphone-based mobile apps are increasingly used to track adherence and deliver reminders, with 704 medication adherence-related apps available as of 2019.86 The vast majority of sensor technologies for medication adherence monitoring are linked to mobile apps. In general, smartphones have been widely adopted, even among older adults who are more likely to be affected by glaucoma.87 88 Lee et al found that patients with glaucoma were receptive to smartphone technology being used to monitor and improve glaucoma outcomes, with 26/43 (60%) study respondents reporting that they would use a medication adherence app.89
EHR systems and patient portals
EHR systems have also been widely adopted across the USA.90 91 Boland et al92 demonstrated that automated telecommunication-based reminders linked to medication data in an EHR improved patient adherence with once daily glaucoma medications. Patient portals linked to EHR systems have also recently become increasingly used, allowing patients to message clinicians, view laboratory testing and imaging results, and track medications.93–95 These portals can also facilitate communication with patients and deliver automated medication reminders.
For instance, Varadaraj et al examined the feasibility and effectiveness of automated dosing reminders using an EHR-linked patient portal for glaucoma medication adherence and to assess patient satisfaction with EHR-linked reminders.96 A web-based application was linked to the EHR patient portal, allowing patients to select medications from their medication list, format (via text or voice call), and timing of their automated, EHR-linked dosing reminders. Three months after initiation, 74% of participants found the reminders useful, and 42% reported they were very likely or likely to continue using EHR-linked reminders.96
Regardless of the source, long-term effects of automated reminders on patient behaviours require further study, as ‘alert fatigue’ may hinder effectiveness. Alert fatigue is a well-documented phenomenon among clinicians receiving alerts from EHR systems97; it is foreseeable patients may develop similar behaviours.
DISCUSSION
Glaucoma medication adherence is often undermined by the need for frequent and long-term use of medications which can be difficult to administer. Increased efforts are underway to better identify, monitor and address adherence (figure 2).
Figure 2.
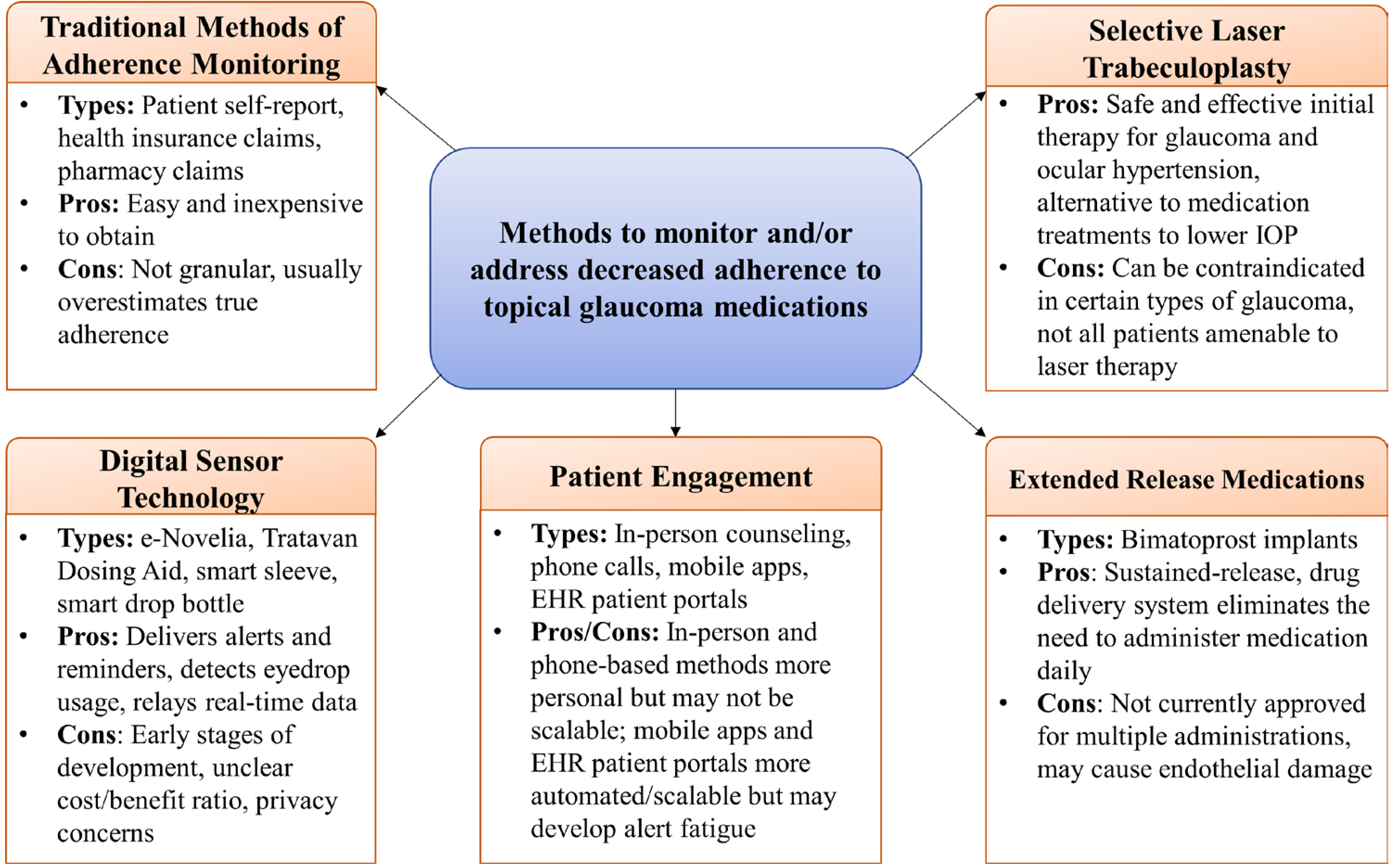
Methods to monitor and/or address decreased adherence to topical glaucoma medications. Overview of developments in adherence monitoring and approaches. EHR, electronic health records; IOP, intraocular pressure.
Innovations in sensor technology designed for eyedrop bottles offer a novel approach for assessing adherence. Connecting these data to smartphone-based apps and EHR systems can facilitate relaying adherence information back to both patients and clinicians in real-time. Despite the increasing use of alternative treatment to eyedrops, including selective laser trabeculoplasty,6–8 minimally invasive glaucoma surgery,98–100 and sustained release implants,101 102 measuring and improving adherence to topical glaucoma medications remains an ongoing challenge.
There are several limitations of current strategies to enhance adherence with eyedrop administration. First, while several sensors detect eyedrop medication administration, they do not actually measure medication instillation into the eye itself. Similar to sensors specifically targeted at confirming oral medication ingestion, more work is needed to develop ocular sensors to confirm instillation of medications into the eye. Second, medication adherence affects racial minorities and individuals with less socioeconomic resources to a greater extent.29 85 103 In a study by Sleath et al, African Americans were less likely to be educated about glaucoma by their providers and they were significantly less likely to be adherent to their glaucoma medications.104 White patients were three times more likely to be 80% adherent or more to their glaucoma medications than non-white patients, mostly of whom were African Americans.105 Thus, improving patient education and understanding of glaucoma and glaucoma medications could improve patient adherence and outcomes, particularly for racial minorities. Few studies have looked specifically at whether these new technological advancements can effectively be leveraged to address this health disparity. For example, the majority of participants in the study of EHR-linked reminders were white men with low risk for non-adherence.106 Future studies should aim to have greater representation of women and minorities, who are disproportionately affected by glaucoma. Additionally, patients’ digital literacy should be considered when designing and deploying these interventions.
Moreover, the majority of existing studies reviewed here had relatively small sample sizes and short follow-up periods. Because glaucoma is a chronic disease, longer follow-up periods are needed to establish whether patients can tolerate long-term granular medication adherence monitoring. Bias from the Hawthorne effect,70 71 can also confound results, as adherence may have appeared to improve simply because patients knew they were being monitored. To establish efficacy, randomised controlled trials and masking procedures should be prioritised. Additionally, financial and economic considerations need to be addressed if these technologies are to be sustainable. Although decreased medication adherence incurs increased costs,21 the vast majority of adherence technologies are still in development phases, and currently the published literature regarding novel sensors for eye medication adherence has not included detailed cost information. This may be due to these technologies being in very early stages of development, and costs might be anticipated to decrease as the technologies reach more widespread manufacturing and deployment that can leverage economies of scale. In the future, more rigorous economic analyses for the implementation of these sensor technologies will need to be conducted. Evaluating cost-effectiveness will be important given that low-cost alternatives such as educational videos to help patients improve their eyedrop administration technique have already been shown to be efficient avenues to address some of the barriers patients face in glaucoma medication adherence.107
Finally, ethical considerations must be at the forefront of all decisions to incorporate medication adherence technology into practice. Concerns about patient privacy, data sharing and adherence data becoming ‘weaponised’ to deny patients insurance coverage or increase premiums highlight some of the ethical issues that must be addressed.
In conclusion, the digital health era has brought multiple advancements in monitoring glaucoma medication adherence, such as improved sensor technologies and expanded modalities for communicating and engaging with patients in a widely scalable fashion. Increased focus on establishing efficacy with randomised controlled trials with long follow-up periods, rigorous economic analyses and addressing patient concerns about privacy and data sharing will be necessary to translate these technological innovations into clinical practice.
Acknowledgments
Funding This study was supported by the National Institutes of Health (Bethesda, MD, USA, grants T15LM011271, DP5OD029610 and 1R01MD014850) and by an unrestricted departmental grant from Research to Prevent Blindness (New York, NY; no award number available).
Footnotes
Competing interests RNW is a co-founder and officer of Toromodes, which has licensed the patent for commercialisation of the ’Smart Drop’ electronic sensor device described in the article.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Robin AL, Muir KW. Medication adherence in patients with ocular hypertension or glaucoma. Expert Rev Ophthalmol 2019;14:199–210. [Google Scholar]
- 2.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial. Arch Ophthalmol 2002;120:1268–79. [DOI] [PubMed] [Google Scholar]
- 3.The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension glaucoma Study Group. Am J Ophthalmol 1998;126:498–505. [DOI] [PubMed] [Google Scholar]
- 4.Musch DC, Gillespie BW, Lichter PR, et al. Visual field progression in the Collaborative initial glaucoma treatment study the impact of treatment and other baseline factors. Ophthalmology 2009;116:200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120:701–13. [DOI] [PubMed] [Google Scholar]
- 6.Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Laser in glaucoma and ocular hypertension (light) trial. A multicentre, randomised controlled trial: design and methodology. Br J Ophthalmol 2018;102:593–8. [DOI] [PubMed] [Google Scholar]
- 7.Katz LJ, Steinmann WC, Kabir A, et al. Selective laser trabeculoplasty versus medical therapy as initial treatment of glaucoma: a prospective, randomized trial. J Glaucoma 2012;21:460–8. [DOI] [PubMed] [Google Scholar]
- 8.Garg A, Vickerstaff V, Nathwani N, et al. Efficacy of repeat selective laser trabeculoplasty in Medication-Naive open-angle glaucoma and ocular hypertension during the light trial. Ophthalmology 2020;127:467–76. [DOI] [PubMed] [Google Scholar]
- 9.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma. JAMA 2014;311:1901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sayner R, Carpenter DM, Blalock SJ, et al. Accuracy of patient-reported adherence to glaucoma medications on a visual analog scale compared with electronic monitors. Clin Ther 2015;37:1975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology 2015;122:1308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deokule S, Sadiq S, Shah S. Chronic open angle glaucoma: patient awareness of the nature of the disease, topical medication, compliance and the prevalence of systemic symptoms. Ophthalmic Physiol Opt 2004;24:9–15. [DOI] [PubMed] [Google Scholar]
- 13.Taylor SA, Galbraith SM, Mills RP. Causes of non-compliance with drug regimens in glaucoma patients: a qualitative study. J Ocul Pharmacol Ther 2002;18:401–9. [DOI] [PubMed] [Google Scholar]
- 14.Rotchford AP, Murphy KM. Compliance with timolol treatment in glaucoma. Eye 1998;12:234–6. [DOI] [PubMed] [Google Scholar]
- 15.Patel SC, Spaeth GL. Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic Surg 1995;26:233–6. [PubMed] [Google Scholar]
- 16.Konstas AGP, Maskaleris G, Gratsonidis S, et al. Compliance and viewpoint of glaucoma patients in Greece. Eye 2000;14:752–6. [DOI] [PubMed] [Google Scholar]
- 17.Friedman DS, Nordstrom B, Mozaffari E, et al. Glaucoma management among individuals enrolled in a single comprehensive insurance plan. Ophthalmology 2005;112:1500–4. [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Ophthalmology. Glaucoma Summary Benchmarks - 2019 [Internet], 2017. Available: https://www.aao.org/summary-benchmark-detail/glaucoma-summary-benchmarks-2019 [Accessed 17 Aug 2020].
- 19.Friedman DS, Quigley HA, Gelb L, et al. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the glaucoma adherence and persistency study (gaps). Invest Ophthalmol Vis Sci 2007;48:5052–7. [DOI] [PubMed] [Google Scholar]
- 20.Newman-Casey PA, Niziol LM, Gillespie BW, et al. The association between medication adherence and visual field progression in the Collaborative initial glaucoma treatment study. Ophthalmology 2020;127:477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol 2008;53 Suppl 1:S57–68. [DOI] [PubMed] [Google Scholar]
- 22.Tsai JC, McClure CA, Ramos SE, et al. Compliance barriers in glaucoma: a systematic classification. J Glaucoma 2003;12:393–8. [DOI] [PubMed] [Google Scholar]
- 23.Friedman DS, Hahn SR, Gelb L, et al. Doctor-Patient communication, health-related beliefs, and adherence in glaucoma results from the glaucoma adherence and persistency study. Ophthalmology 2008;115:1320–7. [DOI] [PubMed] [Google Scholar]
- 24.Sleath B, Robin AL, Covert D, et al. Patient-Reported behavior and problems in using glaucoma medications. Ophthalmology 2006;113:431–6. [DOI] [PubMed] [Google Scholar]
- 25.Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-Report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med 2015;5:470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muir KW, Lee PP. Glaucoma medication adherence. Arch Ophthal 2011;129:243–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krumpal I. Determinants of social desirability bias in sensitive surveys: a literature review. Qual Quant 2013;47:2025–47. [Google Scholar]
- 28.Edwards AL. The relationship between the judged desirability of a trait and the probability that the trait will be endorsed. J Appl Psychol 1953;37:90–3. [Google Scholar]
- 29.Dreer LE, Girkin C, Mansberger SL. Determinants of medication adherence to topical glaucoma therapy. J Glaucoma 2012;21:234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nordstrom BL, Friedman DS, Mozaffari E, et al. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol 2005;140:598.e1–598.e11. [DOI] [PubMed] [Google Scholar]
- 31.Frech S, Kreft D, Guthoff RF, et al. Pharmacoepidemiological assessment of adherence and influencing co-factors among primary open-angle glaucoma patients—An observational cohort study. PLoS One 2018;13:e0191185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheer R, Bunniran S, Uribe C, et al. Predictors of nonadherence to topical intraocular pressure reduction medications among Medicare members: a Claims-Based retrospective cohort study. JMCP 2016;22:808–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frias J, Virdi N, Raja P, et al. Effectiveness of digital medicines to improve clinical outcomes in patients with uncontrolled hypertension and type 2 diabetes: prospective, open-label, cluster-randomized pilot clinical trial. J Med Internet Res 2017;19:e246. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riekert KA, Rand CS. Electronic monitoring of medication adherence: when is high-tech best? J Clin Psychol Med Settings 2002;9:25–34. [Google Scholar]
- 35.Ingerski LM, Hente EA, Modi AC, et al. Electronic measurement of medication adherence in pediatric chronic illness: a review of measures. J Pediatr 2011;159:528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henriksson J, Tydén G, Höijer J, et al. A prospective randomized trial on the effect of using an electronic monitoring drug dispensing device to improve adherence and compliance. Transplantation 2016;100:203–9. [DOI] [PubMed] [Google Scholar]
- 37.Vrijens B, Vincze G, Kristanto P, et al. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ 2008;336:1114–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson AC, Woolson S, Olsen MK, et al. Relationship between electronically measured medication adherence and vision-related quality of life in a cohort of patients with open-angle glaucoma. BMJ Open Ophthalmol 2018;3:e000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cook PF, Schmiege SJ, Mansberger SL, et al. Predictors of adherence to glaucoma treatment in a multisite study. Ann Behav Med 2015;49:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sleath B, Blalock S, Covert D, et al. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology 2011;118:2398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boland MV, Chang DS, Frazier T, et al. Electronic monitoring to assess adherence with once-daily glaucoma medications and risk factors for nonadherence: the automated dosing reminder study. JAMA Ophthalmol 2014;132:838–44. [DOI] [PubMed] [Google Scholar]
- 42.Robin AL, Novack GD, Covert DW, et al. Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol 2007;144:533–40. [DOI] [PubMed] [Google Scholar]
- 43.Hayes TL, Hunt JM, Adami A, et al. An electronic pillbox for continuous monitoring of medication adherence. Conf Proc IEEE Eng Med Biol Soc 2006;2006:6400–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zijp TR, Touw DJ, van Boven JFM. User acceptability and technical robustness evaluation of a novel smart pill bottle prototype designed to support medication adherence. Patient Prefer Adherence 2020;14:625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reddy A, Huseman TL, Canamucio A, et al. Patient and partner feedback reports to improve statin medication adherence: a randomized control trial. J Gen Intern Med 2017;32:256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalantarian H, Motamed B, Alshurafa N, et al. A wearable sensor system for medication adherence prediction. Artif Intell Med 2016;69:43–52. [DOI] [PubMed] [Google Scholar]
- 47.Chen C, Kehtarnavaz N, Jafari R. A medication adherence monitoring system for pill bottles based on a wearable inertial sensor. Annu Int Conf IEEE Eng Med Biol Soc 2014;2014:4983–6. [DOI] [PubMed] [Google Scholar]
- 48.Burns DM, Leung N, Hardisty M, et al. Shoulder physiotherapy exercise recognition: machine learning the inertial signals from a smartwatch. Physiol Meas 2018;39:075007. [DOI] [PubMed] [Google Scholar]
- 49.Burns D, Razmjou H, Shaw J, et al. Adherence tracking with smart watches for shoulder physiotherapy in rotator cuff pathology: protocol for a longitudinal cohort study. JMIR Res Protoc 2020;9:e17841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noble K, Brown K, Medina M, et al. Medication adherence and activity patterns underlying uncontrolled hypertension: assessment and recommendations by practicing pharmacists using digital health care. J Am Pharm Assoc 2016;56:310–5. [DOI] [PubMed] [Google Scholar]
- 51.Belknap R, Weis S, Brookens A, et al. Feasibility of an ingestible sensor-based system for monitoring adherence to tuberculosis therapy. PLoS One 2013;8:e53373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eisenberger U, Wüthrich RP, Bock A, et al. Medication adherence assessment: high accuracy of the new Ingestible sensor system in kidney transplants. Transplantation 2013;96:245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kane JM, Perlis RH, DiCarlo LA, et al. First experience with a wireless system incorporating physiologic assessments and direct confirmation of digital tablet ingestions in ambulatory patients with schizophrenia or bipolar disorder. J Clin Psychiatry 2013;74:e533–40. [DOI] [PubMed] [Google Scholar]
- 54.Naik R, Macey N, West R. First use of an Ingestible sensor to manage uncontrolled blood pressure in primary practice: the UK hypertension registry. J Community Med Health Educ 2017;7:1–5. [Google Scholar]
- 55.Browne SH, Peloquin C, Santillo F, et al. Digitizing medicines for remote capture of oral medication adherence using Co-encapsulation. Clin Pharmacol Ther 2018;103:502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alipour A, Gabrielson S, Patel PB. Ingestible sensors and medication adherence: focus on use in serious mental illness. Pharmacy 2020;8:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Triplett KN, El-Behadli AF, Masood SS, et al. Digital medicine program with pediatric solid organ transplant patients: perceived benefits and challenges. Pediatr Transplant 2019;23:e13555. [DOI] [PubMed] [Google Scholar]
- 58.Chai PR, Rosen RK, Boyer EW. Ingestible biosensors for real-time medical adherence monitoring: MyTMed. Proc Annu Hawaii Int Conf Syst Sci 2016;2016:3416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chai PR, Carreiro S, Innes BJ, et al. Oxycodone ingestion patterns in acute fracture pain with digital pills. Anesth Analg 2017;125:2105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chai PR, Carreiro S, Innes BJ, et al. Digital pills to measure opioid ingestion patterns in emergency department patients with acute fracture pain: a pilot study. J Med Internet Res 2017;19:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang JS, Lee DA, Petursson G, et al. The effect of a glaucoma medication reminder cap on patient compliance and intraocular pressure. J Ocul Pharmacol 1991;7:117–24. [DOI] [PubMed] [Google Scholar]
- 62.O’Dell L, Hennessy A, Robin A. Evaluating adherence to ocular hypotensives using the Travatan dosing aid. OPTO 2012:1. [Google Scholar]
- 63.Okeke CO, Quigley HA, Jampel HD, et al. Interventions improve poor adherence with once daily glaucoma medications in electronically monitored patients. Ophthalmology 2009;116:2286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nemera. e-Novelia® - Smart add-on for ophthalmic droppers [Internet] Available: https://www.nemera.net/smart-ophthalmic-add-on-e-novelia/ [Accessed 05 Nov 2019].
- 65.Gatwood JD, Johnson J, Jerkins B. Comparisons of self-reported glaucoma medication adherence with a new wireless device. J Glaucoma 2017;26:1056–61. [DOI] [PubMed] [Google Scholar]
- 66.Kali. Solutions [Internet] Available: https://www.kali.care/solutions [Accessed 16 Feb 2021].
- 67.Nishimura K, Tabuchi H, Nakakura S, et al. Evaluation of automatic monitoring of instillation adherence using eye Dropper bottle sensor and deep learning in patients with glaucoma. Transl Vis Sci Technol 2019;8:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Payne N, Gangwani R, Barton K, et al. Medication adherence and liquid level tracking system for healthcare provider feedback. Sensors 2020;20:2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aguilar-Rivera M, Erudaitius DT, Wu VM, et al. Smart electronic eyedrop bottle for Unobtrusive monitoring of glaucoma medication adherence. Sensors 2020;20:2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wickström G, Bendix T. The "Hawthorne effect"--what did the original Hawthorne studies actually show? Scand J Work Environ Health 2000;26:363–7. [PubMed] [Google Scholar]
- 71.Parsons HM. What happened at Hawthorne?: new evidence suggests the Hawthorne effect resulted from operant reinforcement contingencies. Science 1974;183:922–32. [DOI] [PubMed] [Google Scholar]
- 72.Carnero E, Bragard J, Urrestarazu E, et al. Continuous intraocular pressure monitoring in patients with obstructive sleep apnea syndrome using a contact lens sensor. PLoS One 2020;15:e0229856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim J, Kim M, Lee M-S, et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat Commun 2017;8:14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bertsch A, Leonardi M, Renaud P. The sensing contact lens. Med Device Technol 2006;17:19–21. [PubMed] [Google Scholar]
- 75.Elsherif M, Hassan MU, Yetisen AK, et al. Wearable contact lens biosensors for continuous glucose monitoring using Smartphones. ACS Nano 2018;12:5452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pradipta IS, Houtsma D, van Boven JFM, et al. Interventions to improve medication adherence in tuberculosis patients: a systematic review of randomized controlled studies. NPJ Prim Care Respir Med 2020;30:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lonie JM, Austin Z, Nguyen R, et al. Pharmacist-based health coaching: a new model of pharmacist-patient care. Res Social Adm Pharm 2017;13:644–52. [DOI] [PubMed] [Google Scholar]
- 78.Murray CM, Shah BR. Diabetes self-management education improves medication utilization and retinopathy screening in the elderly. Prim Care Diabetes 2016;10:179–85. [DOI] [PubMed] [Google Scholar]
- 79.Killeen OJ, MacKenzie C, Heisler M, et al. User-centered design of the eyeGuide: a tailored glaucoma behavior change program. J Glaucoma 2016;25:815–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Newman-Casey PA, Niziol LM, Mackenzie CK, et al. Personalized behavior change program for glaucoma patients with poor adherence: a pilot interventional cohort study with a pre-post design. Pilot Feasibility Stud 2018;4:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Newman-Casey PA, Dayno M, Robin AL. Systematic review of educational interventions to improve glaucoma medication adherence: an update in 2015. Expert Rev Ophthalmol 2016;11:5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zomahoun HTV, Guénette L, Grégoire J-P. Effectiveness of motivational interviewing interventions on medication adherence in adults with chronic diseases: a systematic review and meta-analysis. Int J Epidemiol 2017;46:589–602. [DOI] [PubMed] [Google Scholar]
- 83.Zhang NJ, Terry A, McHorney CA. Impact of health literacy on medication adherence: a systematic review and meta-analysis. Ann Pharmacother 2014;48:741–51. [DOI] [PubMed] [Google Scholar]
- 84.Davis SA, Carpenter DM, Blalock SJ, et al. A randomized controlled trial of an online educational video intervention to improve glaucoma eye drop technique. Patient Educ Couns 2019;102:937–43. [DOI] [PubMed] [Google Scholar]
- 85.Rees G, Chong X-L, Cheung CY, et al. Beliefs and adherence to glaucoma treatment: a comparison of patients from diverse cultures. J Glaucoma 2014;23:293–8. [DOI] [PubMed] [Google Scholar]
- 86.Park JYE, Li J, Howren A, et al. Mobile phone Apps targeting medication adherence: quality assessment and content analysis of user reviews. JMIR Mhealth Uhealth 2019;7:e11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Herrmann M, Boehme P, Hansen A, et al. Digital competencies and attitudes toward digital adherence solutions among elderly patients treated with novel anticoagulants: qualitative study. J Med Internet Res 2020;22:e13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ali ZC, Shakir S, Aslam TM. Perceptions and use of technology in older people with ophthalmic conditions. F1000Research 2019;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee RMH, Oleszczuk JD, Hyer JN, et al. Patient acceptance to smartphone technology to monitor and improve glaucoma health-care outcomes. Eye 2014;28:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.2017_NEHRS_Web_Table_EHR_State.pdf [Internet] Available: https://www.cdc.gov/nchs/data/nehrs/2017_NEHRS_Web_Table_EHR_State.pdf [Accessed 16 Aug 2020].
- 91.Office-based Physician Health IT Adoption and Use [Internet] Available: https://dashboard.healthit.gov/datadashboard/documentation/physician-health-it-adoption-use-data-documentation.php [Accessed 16 Aug 2020].
- 92.Boland MV, Chang DS, Frazier T, et al. Automated telecommunication-based reminders and adherence with once-daily glaucoma medication dosing: the automated dosing reminder study. JAMA Ophthalmol 2014;132:845–50. [DOI] [PubMed] [Google Scholar]
- 93.Griffin A, Skinner A, Thornhill J, et al. Patient portals: who uses them? what features do they use? and do they reduce hospital readmissions? Appl Clin Inform 2016;7:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oest SER, Hightower M, Krasowski MD. Activation and utilization of an electronic health record patient portal at an academic medical Center—Impact of patient demographics and geographic location. Acad Pathol 2018;5:237428951879757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Measuring the Impact of Patient Portals: What the Literature Tells Us.:22.
- 96.Varadaraj V, Friedman DS, Boland MV. Association of an electronic health Record—Linked glaucoma medical reminder with patient satisfaction. JAMA Ophthalmol 2019;137:240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Embi PJ, Leonard AC. Evaluating alert fatigue over time to EHR-based clinical trial alerts: findings from a randomized controlled study. Journal of the American Medical Informatics Association 2012;19:e145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ansari E. An update on implants for minimally invasive glaucoma surgery (MIGS). Ophthalmol Ther 2017;6:233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dhingra D, Bhartiya S. Evaluating glaucoma surgeries in the MIGS context. Rjo 2020;64:85–95. [PMC free article] [PubMed] [Google Scholar]
- 100.Lavia C, Dallorto L, Maule M, et al. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One 2017;12:e0183142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shirley M. Bimatoprost implant: first approval. Drugs Aging 2020;37:457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Medeiros FA, Walters TR, Kolko M, et al. Phase 3, Randomized, 20-Month Study of Bimatoprost Implant in Open-Angle Glaucoma and Ocular Hypertension (ARTEMIS 1). Ophthalmology 2020;127:1627–41. [DOI] [PubMed] [Google Scholar]
- 103.Murakami Y, Lee BW, Duncan M, et al. Racial and ethnic disparities in adherence to glaucoma follow-up visits in a County hospital population. Arch Ophthalmol 2011;129:872–8. [DOI] [PubMed] [Google Scholar]
- 104.Sleath B, Davis S, Sayner R, et al. African American patient preferences for glaucoma education. Optom Vis Sci 2017;94:482–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sleath B, Blalock SJ, Covert D, et al. Patient race, reported problems in using glaucoma medications, and adherence. ISRN Ophthalmol 2012;2012:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Varadaraj V, Friedman DS, Boland MV. Association of an electronic health Record-Linked glaucoma medical reminder with patient satisfaction. JAMA Ophthalmol 2019;137:240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Davis SA, Carpenter DM, Blalock SJ, et al. Glaucoma patient preferences for video education on eye drop technique. Optom Vis Sci 2019;96:325–30. [DOI] [PubMed] [Google Scholar]
- 108.Friedman DS, Jampel HD, Congdon NG, et al. The TRAVATAN dosing aid accurately records when drops are taken. Am J Ophthalmol 2007;143:699–701. [DOI] [PubMed] [Google Scholar]


