Abstract
As a common parasitic disease in animals, coccidiosis substantially affects the health of the host, even in the absence of clinical symptoms and intestinal tract colonization. Gut microbiota is an important part of organisms and is closely related to the parasite and host. Parasitic infections often have adverse effects on the host, and their pathogenic effects are related to the parasite species, parasitic site and host-parasite interactions. Coccidia-microbiota-host interactions represent a complex network in which changes in one link may affect the other two factors. Furthermore, coccidia-microbiota interactions are not well understood and require further research. Here, we discuss the mechanisms by which coccidia interact directly or indirectly with the gut microbiota and the effects on the host. Understanding the mechanisms underlying coccidia-microbiota-host interactions is important to identify new probiotic strategies for the prevention and control of coccidiosis.
Keywords: coccidia, gut microbiota, host, interaction, probiotics
Introduction
Coccidiosis is a self-limiting protozoal disease mainly caused by coccidia of the genus Eimeria (Kemp et al., 2013). Eimeria species are generally gastrointestinal parasites that cause different degrees of enteritis, such as diarrhea, dehydration, and weight loss. Eimeria is a large genus, with over 1,800 species identified to date (Duszynski, 2001). Compared with other genera and species related to coccidia, their life cycles are completed in a single host, and they have high host specificity. Generally, Eimeria are supposed not to spread between different host taxa (Bangoura and Bardsley, 2000), however, several of them are demonstrated to be able to infect among various species (Mácová et al., 2018; Trefancová et al., 2021). Furthermore, this genus has a highly diverse host range and affects all vertebrates (Duszynski, 2001).
All members of coccidia replicate and produce oocysts in the intestine of the final host, which enter into the environment with feces. Animals ingest sporulated oocysts from contaminated environments, which are transported to the intestine and then released as sporozoites (Chapman, 1978). Each sporozoite invades epithelial cells and remains within the parasitophorous vacuole during its development into trophozoites. The trophozoites begin asexual replication, at which point the parasite is referred to as a schizont. Each schizont forms thousands of first-generation merozoites. After a schizogony cycle is completed, the host cells are destroyed, and merozoites enter the intestinal lumen, where they infect new epithelial cells. After several generations of merogony, the parasite enters sexual replication, forming the dimorphic stages of macrogametes and microgametes. Microgametes enter the new host cell and fertilize the macrogametes to produce zygotes (Ferguson et al., 2003). After the zygote becomes an oocyst, it is released into the environment with feces (Shirley et al., 2005). Coccidia perform a series of life activities in the intestine of the host, including colonization, growth and reproduction, thereby disrupting the balance of the intestinal environment.
However, the mechanisms by which coccidia infect the organism and cause pathogenesis remain unknown. Most studies have focused on the pathogenesis of coccidia, mainly involving disruption of the intestinal mucosa and immunity. The gut microbiome is a complex network of symbiotic microorganisms with several functions that are beneficial to the host, including the absorption of nutrients, synthesis of essential organic compounds, protection from pathogens and development of the intestinal immune system. Coccidia and intestinal microbiota share an intestinal microenvironment. The composition of the gut microbiota is altered directly or indirectly via changes in the physiological characteristics, permeability, and antimicrobial peptide production in the intestine (Zaiss and Harris, 2016). In addition, alterations in the gut microbiota affect the colonization of the parasite in the host, infection status, and treatment of parasitic diseases (White et al., 2018). Therefore, this article describes the mechanisms underlying coccidia-microbiota-host interactions.
Eimeria species that cooperatively infect animals are usually referred to as coccidia based on the name of the group of unicellular parasites to which they belong. Although Cryptosporidium was formerly supposed to be closely related to coccidia, it now belongs to Gregarinasina (Adl et al., 2019); therefore it is described separately. This review concerns only Eimeria species.
Interactions Between Coccidia and Gut Microbiota
The intestinal mucosal interface is a large and complex three-dimensional defense system composed of mechanical, biological, chemical, and immune barriers. The function of the mucosal barrier is to prevent harmful substances from entering the systemic circulation. The number of intestinal microorganisms in animals is approximately 10 times the number of cells in the body, forming an interdependent and interactive micro-ecosystem. The source of gut microbiota in livestock is similar to that of humans. The sheep intestine is first colonized by Butyricicoccus and Lachnospiraceae, followed by Clostridiales, Lactobacillus, and Ruminococcaceae (Zhuang et al., 2020). In various stages of sheep development, the intestinal microorganisms mainly include Bacteroides, Lactobacillus, and Ruminococcus, similar to goats (Li et al., 2019), piglets (Kim et al., 2011), and calves (Dias et al., 2018). The main gut microbiomes in human are Actinobacteria, Bacteriodetes, Firmicutes, Proteobacteria (Davenport et al., 2017). The source of the initial gut microbiota is different between poultry and mammals, and Firmicutes is the main phylum in poultry intestines. Actinobacteria, Bacteroides, Proteobacteria and others have also been reported (Waite and Taylor, 2015). Coccidial infection affects the composition of the host’s gut microbiota directly or indirectly, and changes in the gut microbiota may also influence the infectivity of coccidia.
Infection with coccidia significantly decreases bacterial diversity in the small intestine. In chickens, Eimeria tenella is the most pathogenic Eimeria species. Animals infected with E. tenella showed a reduced abundance of most bacterial taxa, except for members of the family Enterobacteriaceae. (Kimura et al., 1976). E. tenella infection enriches hostile bacteria, including Bacillus, Enterococcus, Escherichia, Shigella, Staphylococcus, and others. Furthermore, Klebsiella, and Proteus were also enriched (Cui et al., 2017). It was previously observed that Eubacterium, Lactobacillus, and Ruminococcus were significantly decreased in the caecum of broiler chickens orally challenged with oocysts of Eimeria acervulina, Eimeria maxima, and Eimeria brunetti (Stanley et al., 2014) and the ileum of broiler chickens inoculated with E. maxima (Kim et al., 2015). Furthermore, the infection greatly decreased the frequency of the immune-modulating bacterium Candidatus arthromitus. In a similar study, Ruminococcaceae members were reduced, and three unknown Clostridium species were increased after infection with these three Eimeria species (Wu et al., 2014). Our previous work assessed the gut microbiota of Hu sheep naturally and artificially infected with coccidia and found that infection caused an increase in Firmicutes and Proteobacteria and a decrease in Bacteroidetes and Roseburia. The study also showed that coccidial infection had a greater effect on the gut microbiota of lactating lambs, causing a significant decrease in Christensenellaceae and Bifidobacteria (Zhou, 2020). This finding suggests that coccidial infection may cause more severe disorders in young sheep. In summary, coccidial infection dramatically decreased resident microbiome and enriched a large number of conditionally pathogenic bacteria. In contrast, the abundances of probiotics, including Alistipes, Blautia, Desulfovibrio, Lachnospiraceae, Lactobacillus, Roseburia, and Ruminococcus, were reduced in coccidia-infected mice (Huang et al., 2018).
Direct Interactions
The microbiome comprises bacteria, viruses, fungi, protozoa, and parasites, their comprehensive commensal, symbiotic, pathogenic, or parasitic relationship is important for health (Desselberger, 2018). The coexistence of microbiome and coccidia in the gut provides ample opportunities to interact with each other, both positive and negative (Leung et al., 2018). For example, supernatants of Lactobacillus had the inhibitory effects on the E. tenella (Tierney et al., 2004), meaning that some gut microbes have the capacity to directly attack sympatric coccidia. Certain probiotic bacteria have antimicrobial effects through their phagocytic antagonism and via the metabolism of acetic acid and other substances with broad-spectrum antimicrobial activity, which facilitates the inhibition of conditionally pathogenic bacteria (Biggs and Parsons, 2008). Now we have no evidence to demonstrate the mechanism about how the bacteria facilitate coccidia, while some research claimed that phagocytosis of pathogenic bacteria by Entamoeba histolytica induced virulence of parasite (Galván-Moroyoqui et al., 2008). Gaboriaud et al. (2021) compared the development of E. tenella in germ-free and conventional chickens, they observed the lower load of oocysts and the longer asexual phase in the absence of microbiota. Most likely this is because the digestive content and synthetizes metabolites synthetized by microbiota are crucial for the replication of coccidia (Gaboriaud et al., 2021). So it is important to identify the precise metabolites, and modulate the composition of the microbiota to inhibit the coccidia. Parasites and gut microbes may also interact by competing for the same nutrients or overlapping resource requirements. Following infection by coccidia, the balance between the organism and the microbiome is disrupted, resulting in dysbiosis of the gut microbiota. However, supplementation with beneficial microbiota protect against infection by competing with coccidia for space and resources (Butel, 2014).
Indirect Interactions
Interactions With the Intestinal Mechanical Barrier
Tight junctions play a crucial role in maintaining the intestinal epithelial cell barrier, protecting the host intestine from pathogens and preventing the transmission of macromolecules (Schneeberger and Lynch, 2004). Tight junction-related proteins include occludin, zonula occludens, and claudins. Eimeria vermiformis-infection inhibits the epithelial cell mRNA expression of zonula occludens-1 in mice (Farid et al., 2008), and zonula occludens-1 downregulation or reduced activity affects the formation of intercellular tight junctions. With higher concentrations of coccidia, the expression of tight junction proteins was dose-dependently upregulated, with a simultaneous increase in gastrointestinal permeability, indicating more severe intestinal damage (Teng et al., 2020). Combined with the disruption of the mucus layer, this damage profoundly alters the interactions between the host and its microflora, allowing for greater microbial contact with the epithelial barrier and even penetration across the interface. After treatment with probiotics, the expression levels of claudin-1 and zonula occludens-1 were increased in the E. tenella-infected chicken (Memon et al., 2020). Probiotics maintain tight junction integrity of intestinal epithelial cells, mainly through the bioactive substances produced by their metabolism, to protect against pathogenic bacteria-induced damage of intestinal epithelial cells. A mixture of Bacillus subtilis and Saccharomyces cerevisiae increased the expression of tight junction-associated proteins, such as occludin, claudin-2, and claudin-3, in broiler chickens (Rajput et al., 2013).
Due to the invasion and replication of coccidia, the host cells are under pressure, which may cause apoptosis. To grow and survive in host cells, coccidia inhibit apoptosis by regulating anti-apoptotic factors. In Eimeria intestinalis-infected rabbits, the percentage of apoptotic cells in the ileum was significantly higher compared with the control group (Abdel-Haleem et al., 2017). Before the development of second-generation schizonts is completed, E. tenella may directly activate the NF-κB pathway in host cells to further inhibit host cell apoptosis. After developmental completion, E. tenella prevent the expression of NF-κB response genes and further reduce the expression of the anti-apoptotic proteins Bcl-2 and Bcl-XL, thereby accelerating host cell apoptosis and promoting the release of merozoites (Del et al., 2004). During their early development, E. tenella inhibit pro-apoptotic proteins by inducing anti-apoptotic factors to protect their cells and ability to proliferate (Del et al., 2004). Using probiotics, including B. subtilis, Clostridium butyricum, and Lactobacillus, we observed upregulated Bax expression and downregulated Bcl-2 levels in the E. tenella-infected chicken (Memon et al., 2020). Zhang et al. (2015) demonstrated that E. tenella promoted the apoptosis of cecal epithelial cells in vitro, especially during the middle to late stages. The use of specific inhibitors significantly decreased DNA injury, apoptosis, and caspase-9 and caspase-3 activity in chick embryo cecal epithelial cells after E. tenella infection (Li et al., 2017). Most probiotics inhibit the NF-κB pathway by impairing epithelial cell protease function and preventing the degradation of NF-κB (IκB) negative regulators (Jiang et al., 2012). The induction of apoptosis may become a new direction in the treatment of coccidiosis. The use of probiotics during the early stage of coccidial infection promotes the apoptosis of intestinal epithelial cells and reduces coccidial colonization and development.
Interactions With the Intestinal Chemical Barrier
The chemical barrier of the intestine consists of mucin (MUC),antimicrobial peptides (AMPs), regenerating islet-derived protein 3, lysozymes, and other factors (Okumura and Takeda, 2017). Eimeria infection significantly downregulates the gene expression of MUC2 and MUC5ac (Jiang et al., 2013), resulting in a decrease in the content of MUC in the mucus layer. This prevents mucus layer replenishment and further disrupts the integrity of the intestinal mucosal chemical barrier. Mice infected with sporulated Eimeria papillata exhibit marked goblet cell hypoplasia and depleted mucus secretion (Dkhil et al., 2013). The number of colonic cup cells gradually decreases with the development of Eimeria pragensis endogenous life cycle stages (Yunus et al., 2005). Microorganisms, such as Actinobacteria, Bacteroidetes, Firmicutes, and Verrucomicrobia (Tailford et al., 2015), use mucus carbohydrates as a carbon source. Therefore, they may not gain a competitive advantage after a reduction in mucus production. It has been proposed that coccidia stimulate mucus production in vivo, leading to an increase in the relative abundance of MUC-utilizing bacteria, such as Clostridiales (Collier et al., 2008), whose growth in vitro was enhanced by the addition of MUC (Ramanan et al., 2016). The type and glycosylation of mucoproteins in the mucus layer covering the intestinal epithelium are different due to the various colonization sites of coccidia species in the intestine (Moncada et al., 2003), and both the MUC’s composition and glycosylation are known to affect the taxa that use the mucus (Sommer et al., 2014). Therefore, parasite-driven changes in mucus may alter the microbiota.
Host defense peptides exhibit direct antibacterial activity after coccidial infection and induce the expression of MUC and tight junctional proteins to enhance mucosal barrier function (Robinson et al., 2015). After infection with Eimeria praecox, several genes were downregulated, including those that encode antimicrobial peptide 2 and the cationic, anionic, and L-type amino acid transporters (Yin et al., 2015). Similar findings were reported for E. maxima (Casterlow et al., 2011) and E. acervulina (Su et al., 2014). E. acervulina and E. maxima challenge resulted in the downregulation of avian beta-defensin, which had antibacterial effects against Actinobacillus, Candida albicans, Escherichia coli, Listeria monocytogenes, and Salmonella typhimurium species (Elahi et al., 2005). The addition of moderate concentrations of quercetin to feed exerts a regulatory effect on the ileal avian beta-defensin and toll-like receptor (TLR) signaling pathways by reducing the abundance of Clostridium and increasing the levels of Bifidobacterium, thereby maintaining the ileal microecological balance and reducing mortality. In other words, increased host antimicrobial peptide production can improve the intestinal microbiota and subsequently ameliorate the symptoms of coccidia. Lactobacillus and some gram-positive bacteria enhance intestinal barrier function by inducing the NF-κB pathway and activating activator protein-1 and mitogen-activated protein kinase to upregulate β-defensin 2 (Schlee et al., 2008). Probiotics stimulate the host to produce active molecules, such as MUC and antimicrobial peptides, which may be one of their action mechanisms to enhance the body’s resistance to coccidial infection.
Interactions With the Immune System
Host anti-infectious strategies (including immune responses) are elicited following infection with parasites (Zhou et al., 2013). However, the immune system regulates the gut microbiota and their relative abundance to ensure a mutually beneficial host-microbe symbiosis. Eimeria species inhibit host immune responses to promote their invasion and colonization in hosts through negatively regulating the production of inflammatory cytokines (Zhao et al., 2018), thereby altering the gut microbiota.
The specific immune response to coccidiosis involves both cellular and humoral components. In infected animals, the humoral immune response indicates high titers of various antibody classes, beginning with the increase in IgM, followed by IgG, IgA, and others (Hughes et al., 1985). In an ovine model, increases in the IgG level and oocyst shedding occurred simultaneously during the primary infection and then decreased to baseline levels (Dalloul et al., 2005). Matos et al. (2018) demonstrated that Eimeria ninakohlyakimovae infected goats and revealed the increased levels of specific IgG, IgM, and IgA during the host immune response. By measuring the content of immunoglobulins and gut microbiota in inflammatory bowel disease patients, it was observed that IgG, IgM, and IgA had a positive correlation with Enterobacteriaceae and Enterococcus; while a negative correlation with Lactobacillus and Bifidobacterium. This indicates that IgM, IgG, and IgA are closely related to the imbalance in the gut microbiota, which may be caused by changes in the proportion and quantity of gut microbiota, leading to disruption of the intestinal mucosal microecological balance and abnormal immune responses. However, humoral immune reactions cannot eliminate primary coccidial infections (Daugschies and Najdrowski, 2005). Specific antibodies are reportedly produced in response to ruminant Eimeria infections, however, they are not protective. Although the specific mechanism of action by which intestinal IgA provides protection against coccidial infection remains unknown, it is hypothesized that IgA reduces the development of sporozoites or merozoites and prevents host cell invasion (Yun et al., 2000).
Although both cellular and humoral immunity are activated in response to coccidial infections (Daugschies and Najdrowski, 2005), several studies have shown that the cellular immune response mediated by T cells plays a key role in the protective immunity against coccidia. T-cell-mediated immune responses reduce the excretion of oocysts in animals infected with Eimeria bovis and mainly involve CD4+ and CD8+ lymphocytes (Sühwold et al., 2010). Matos et al. (2018) infected 3-, 4-, and 5-week-old goat kids with sporulated oocysts and subjected them to a homologous challenge 3 weeks later. The results demonstrated higher eosinophils and lymphocytes compared with challenged groups infected at 6, 7, and 8 weeks old. The activation of antigen-specific T cells from Eimeria-immune mice, cattle, and chickens has been demonstrated by lympho-proliferation assays (Lillehoj, 1986). In addition, the gut microbiota and its metabolites induce the differentiation of T cells by direct or indirect mechanisms, including T-bet+ Th1 cells, RORγt+ Th17 cells, Treg cells and GATA3+ Th2 cells (Lee and Kim, 2017), and coccidia colonization primarily mediates Th1 cell responses. E. bovis-mediated T cell activation was accompanied by increased levels of certain cytokines (such as IL2, IL4, and IFN-γ) known to participate in the regulation of complex networks, thereby activating the migration of immune cells to the site of infection (Taubert et al., 2008). E. tenella strongly induces an immune response and increases IL-8 and IL-6 expression in the cecum (Yu et al., 2020). Macrophages isolated from chickens infected with E. tenalla or E. maxima produced IL-1 in vitro and showed 80-fold increased mRNA levels of jejunal and cecum IL-1β after 7 days of culture. IL family members have a wide range of immunomodulatory functions and are highly beneficial for the host’s defense against coccidial infection. The administration of B. subtilis to chickens infected with coccidia increased the level of specific antibodies and regulated intestinal immunity by modulating the expression of IL-1β, IFN-γ, and CXCLi2 in the intestine (Lee et al., 2013). Lactobacillus-based feed products increased intestinal IFN-γ and IL-2 expression in chickens, resulting in a 14% reduction in fecal oocysts compared with the control group (Chaudhari et al., 2020).
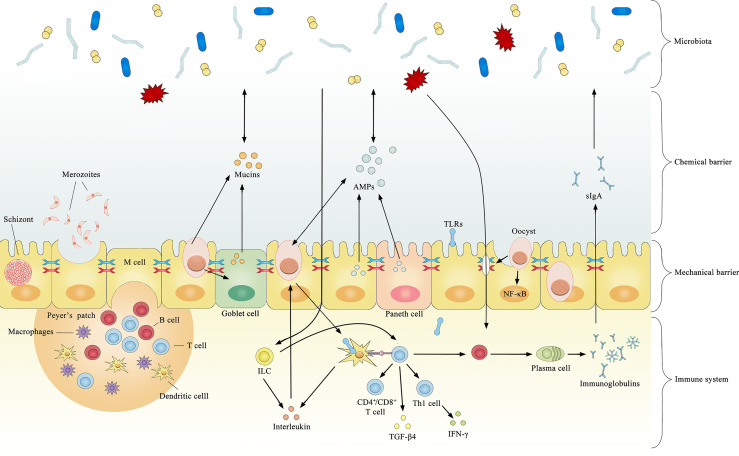
Several cytokines are produced after coccidial infection, most of which have a coccidial suppressive effect in vivo or in vitro. However, some may have both pathological and immunophysiological effects. Significantly increased TLR2, TLR4, and TLR15 expression is observed after infection by coccidia (Zhou et al., 2013), and the upregulation of TLRs typically induces pro-inflammatory cytokines that regulate the immune response against bacterial infections. TLR2 mediates intestinal repair and barrier function to prevent pathogenic microorganism invasion by recognizing the cell wall components of gram-positive bacteria. In chickens, the expression level of TGF-β4 in intestinal intraepithelial lymphocytes was increased by 5- to 8-fold after coccidial infection (Jakowlew et al., 1997), and the expression of TGF-β4 in the spleen and cecum tonsils was increased by 3-fold (Song et al., 2010). The increased expression of TGF-β4 decreases the expression of IFN-γ, preventing excessive inflammation from causing damage to the organism. This may be a potential mechanism regulating mucosal inflammatory responses against intestinal microbes to maintain intestinal immune homeostasis. The current literature on immune-mediated interactions between coccidia and the microbiota is limited, and most previous studies focused on how microorganisms enhance immunity against coccidia without considering the opposite circumstance. For example, Toxoplasma gondii was found to induce TLR2, TLR4, and TLR9 signaling through the stimulation of gut microbiota and indirectly stimulate dendritic cells to activate innate and adaptive immune responses (Benson et al., 2009). In healthy organisms, the gut microbiota activates B cell receptors or TLRs to promote antigen presentation and antibody production (Buchta and Bishop, 2014). Collectively, these results suggest that coccidia and the microbiota have a complex relationship and interact across the mechanical barrier, chemical barrier and immune system ( Figure 1 ).
Figure 1.
Summary of documented mechanisms by which infection with coccidia may indirectly interact with the gut microbiota.
Impact of Coccidia-Microbiota Interactions on the Host
Secondary Infection With Other Pathogens
Secondary Infection With Pathogenic Bacteria
Various studies have demonstrated the complex interactions of coccidia with bacteria, fungi, viruses or other intestinal parasites (Motha and Egerton, 1984; Fukata et al., 1984; Ruff and Rosenberger, 1985), which may lead to more severe clinical manifestations and economic losses. Changes in the gut microbiota caused by coccidial infection provide an environment that is conducive for the reproduction of pathogenic bacteria. Coccidial infections not only enhance the colonization of Campylobacter jejuni (Macdonald et al., 2019), Clostridium perfringens (Ficko-Blean et al., 2012), Salmonella (Kogut et al., 1994) and other bacteria but also increase their pathogenicity (Dykstra and Reid, 1978). This increases livestock and poultry diseases, thereby reducing animal performance, reproductive capacity and egg production and potentially leading to death. And coccidial infection causes a marked inflammatory response in the intestine, and the presence of inflammation favors the colonization of aerobic bacteria, especially Enterobacteriaceae (Lupp et al., 2007), which have been shown to exacerbate the increase in pathogenic bacteria. Enterobacteriaceae and Lactobacillus are antagonistic, and an increase in the number of Enterobacteriaceae may inhibit the intestinal colonization by Lactobacillus (Tortuero, 1973). A reduction in anaerobic bacteria in the intestine after coccidial infection in chickens was suggested to potentially decrease the concentration of volatile fatty acids in the cecum and induce changes in pH and oxidation-reduction potential in the intestine, which may directly lead to enhanced pathogenic infection (Qin et al., 1995). For example, a reduction in Lactobacillus after coccidial infection prevents the production of large amounts of lactic and acetic acid to effectively inhibit the invasion of Salmonella enteritidis (Bjerrum et al., 2006). The damage induced by coccidia appears to promote the spread and colonization of C. perfringens deep in the mucosa, and in some cases, this extends to the crypts and causes focal necrosis (Ficko-Blean et al., 2012), leading to secondary necrotic enteritis (Hofacre et al., 1998). The severity of necrotic enteritis has been reported to be associated with an increase in Proteobacteria and a decrease in Firmicutes (Xu et al., 2018), and these changes occurred during coccidial infection. Firmicutes were important for suppressing or eliminating C. perfringens and restoring intestinal homeostasis (Fasina et al., 2016). In addition, coccidial infections significantly increased Bacteroidetes, including Bacteroidaceae and Rikenellaceae. Bacteroidetes can damage intestinal epithelial cells and increase the invasion of other pathogens, thereby inducing or exacerbating enteritis. We speculate that the increase in Bacteroidetes and decrease in Firmicutes may be related to secondary infections with bacterial diseases.
Secondary Infections With Virus
Coccidial infection, which reduces the abundance of the microbes, is associated with low immunity. Virus-coccidial co-infection reportedly increased viral replication and delayed the clearance of viruses, such as avian leukosis virus (Cui et al., 2017), Marek’s disease virus (Biggs et al., 1968), infectious bursal disease virus (Giambrone et al., 1977), reticuloendotheliosis virus (Motha and Egerton, 1984) and reoviruses (Ruff and Rosenberger, 1985). Many conditionally pathogenic bacteria were significantly enriched in the intestine of coccidia-infected chickens, including Firmicutes and Proteobacteria. The significant enrichment of these conditionally pathogenic bacteria may be a key factor in the increased occurrence of secondary infections of avian leukosis virus (Dong et al., 2015). On the other hand, coccidia parasitize the intestinal epithelium and cause changes in the intestinal environment, like changes in metabolites such as SCFAs, which will influence the antiviral immune response (Chapman et al., 2013; Budden et al., 2017). Subdoligranulum, which decreases dramatically after coccidial infection, belongs to the subgroup of Clostridiales and is capable of butyrate production (Bjerrum et al., 2006). Butyrate reduces chronic inflammation by modulating the immune system, and its reduction may lead to increased chronic inflammation and immune disorders (Lund et al., 2010). Meanwhile, coccidial specific antigens can affect the activity of lymphocytes and suppress the immune response (Rose and Hesketh, 1984). When damaged the gut microbiota of chickens, we can observed higher cloacal and oropharyngeal shedding of avian influenza H9N2 in chickens, with the compromised type I IFNs and IL-22 expression (Yitbarek et al., 2018). So we may conclude that the coccidial infection may contribute the replication of virus. And dual infection of coccidia and virus will extend the replication time of the virus (Gao et al., 2015), which exacerbates clinical symptoms and leads the increased mortality (Giambrone et al., 1977).
Impact on Host Metabolism and Nutrition
Short-chain fatty acids (SCFAs), which are the most widely and intensively studied end product of intestinal metabolism, play an important role in metabolism (Ley et al., 2006). These mainly include acetic acid, propionic acid, and butyric acid. The common SCFA-producing bacteria are mainly anaerobic bacteria, including Bacillus, Bifidobacterium, Clostridium, Streptococcus, and others (Garcia et al., 2008). However, following stimulation by the external environment and pathogenic microorganisms, the gut microbiota is severely damaged, leading to changes in the contents of SCFAs. This subsequently disrupts the metabolism of SCFAs, energy efficiency of food intake, and metabolic homeostasis of the body, resulting in the development of intestinal and metabolic diseases. The concentration of SCFAs in the intestine of animals infected with coccidia markedly changes. In particular, the concentration of acetic acid decreases, the levels of butyric and isovaleric acids increase, and the concentration of isobutyric acid increases or is unaffected (Stanley et al., 2014). Acetic acid has broad-spectrum antibacterial effects, acting as an inhibitor against E. coli, Salmonella, Streptococcus, and Pseudomonas aeruginosa in the intestine (Liévin et al., 2000). A reduction in acetic acid often leads to secondary infection with coccidia. Infection with E. tenella drastically reduces butyrate-producing Subdoligranulum in the cecum (Bjerrum et al., 2006). In addition, butyrate plays an important role in animal health by regulating the immune system and reducing chronic inflammation. Therefore, a decrease in butyrate may lead to a high prevalence of chronic inflammation and immune disorders. Upon coccidial infection, SCFAs are reduced, and the pH is increased in the cecum (Leung et al., 2019). SCFAs are known to reduce intestinal pH, which promotes the growth and proliferation of probiotic bacteria and inhibits the colonization of specific pathogenic bacteria. Furthermore, SCFAs are an important mediator of signal transmission from microbiota to host cells, including enteroendocrine, immune, and nerve cells (Rhee et al., 2009), which indirectly influences homeostasis in the intestinal lumen.
Coccidial infection affects the amount of nutrients in the body’s tissues by disrupting the normal gut microbiota. Damage to the mucosa also leads to impaired digestion because the gut microbiota is involved in protein metabolism. Food and endogenous proteins are hydrolyzed into peptides and amino acids by proteases and peptidases produced by the host and bacteria, releasing amino acids (Macfarlane et al., 1988). The digestion and absorption of proteins were shown to be impaired after infection with Eimeria necatrix, Eimeria mitis, and E. maxima (Turk, 1972). In contrast, protein uptake was increased at some time points after infection with E. necatrix or E. acervulina (Turk, 1972), and infection with E. brunetti had no effect (Fetterer et al., 2014). This may be due to the differences in their pathogenicity and the degree of disruption of the normal gut microbiota. In chickens infected with E. acervulina, the total plasma lipid level was significantly decreased (Allen, 1988). This may be caused by the reduced relative abundance of the dominant microbes following coccidial infection-induced increases in oxidative stress in the intestine, which promotes the secretion of reactive oxygen species from the intestinal epithelium. Excess reactive oxygen species directly targets DNA, lipids, and proteins in the cells of the organism, causing changes in their function and structure, which subsequently induces oxidative stress, decreases host food intake and impairs energy metabolism (Cooke et al., 2003). Coccidial infection also alters carbohydrate metabolism and uptake. Downregulated sucrase-isomaltase (SI) and glucose transporter 2 (GLUT2) were observed in the duodenum of E. acervulina-challenged animals (Su et al., 2014). It has been shown that the activity of SI in the small intestinal mucosa was inhibited in rats following disruption of the gut microbiota (Nanthakumar et al., 2013). In addition, reduced expression of SI and GLUT2 may lead to inhibition of the carbohydrate supply in tissues (Treem, 2012), thereby preventing body weight gain. The results from studies on blood glucose levels have been inconsistent, but most have found that coccidiosis leads to a significant decrease in blood glucose levels. Therefore, we conclude that the interaction between coccidia and the microbiota alters proteins, lipids, glucose, and other factors.
Effects of Host Changes on Coccidia
The infection of animals with coccidia induces specific and long-term immune protection against coccidia and ameliorates the disruption of microbiota to a certain extent. It is generally accepted that coccidia has better immunogenicity in the early stages (endogamous stage) than in the later sexual stages. In coccidia-infected animals, the amount of sIgA is increased, which prevents microorganisms from residing and multiplying in the mucosal epithelium. sIgA can inhibit the invasion of bacteria in epithelial cells, increase the diversity of gut microbiota, and promote immune responses in intestinal epithelial cells (Hooper et al., 2012 and Mirpuri et al., 2014). IL-22 directly induces Reg IIIγ production in intestinal epithelial cells, thereby limiting the proliferation of C. arthromitus. The overgrowth of C. arthromitus not only increases the number of Th17 cells but also triggers Th17 cell-mediated intestinal inflammation, and T-bet expression in ILCs limits the accumulation of Klebsiella pneumoniae, and Proteus mirabilis to some extent (Kamada and Núñez, 2014). Reg IIIγ incubation with 105~106 CFU/mL Listeria monocytogenes or Enterococcus faecalis significantly decreases the bacterial survival rate and prevents the infection of the intestinal tract by pathogenic bacteria (Cash et al., 2006). The immune system regulates the structure of the intestinal microbiota through a variety of antimicrobial peptides secreted by intestinal epithelial cells, and defensins effectively kill several gram-positive and -negative bacteria, including C. albicans, E. coli, and Enterococcus, thereby restoring the normal microbial community composition.
The nutritional intake of the host also has a significant impact on the microflora composition and severity of coccidial infection. Similarly, the condition of the organism affects the infective ability of coccidia. Richter and Wiesner (1988) showed that increased levels of dietary crude protein from 11.3% to 12.4% reduced the mortality of chickens infected with coccidia by 16%. However, high protein contents were conducive to the development and reproduction of coccidia in the body. In addition, decreased dietary protein levels from 16% to 13% increased the abundance and diversity of ileal flora, including Lactobacillus and Megasphaera. In growing pigs, a 10% reduction in the protein level decreased the diversity of ileal and colonic flora (Fan et al., 2017). Therefore, the crude protein level in the diets of infected animals should not be too high or too low, and further research is necessary to determine the optimal diet composition.
The Anti-Coccidial Application of Probiotics
In the past, the treatment of coccidiosis mainly involved anti-coccidial drugs, which inhibit the asexual and sexual reproduction stages of coccidia (Odden et al., 2018). For example, diclazuril is the most common chemistry medicine in the coccidial infection, which can be used to reverse the microbial changes induced by Eimeria spp. (Wang et al., 2021). However, some research treatment of enrofloxacin and diclazuril altered the abundance of gut microbiota and their functional metabolite pathways, reducing bacterial diversity while expanding and collapsing composition of specific indigenous microbes, than formed a new microbial community (Elokil et al., 2020). So we may conclude that long-term chemical treatment caused irreversible movement to gut microbiota although the drugs are effective to coccidia. Furthermore, the genetic diversity of Eimeria species contributed to the development of anticoccidial drug resistance, severely limiting the long-term disease prevention ability of these agents (Tan et al., 2017). Probiotics are a new type of anticoccidial drug that take advantage of the mutually antagonistic relationship between the gut microbiota and coccidia. To treat coccidial infection, probiotics may manipulate the gastrointestinal tract by restoring balance to the intestinal microbial community, improving intestinal tissue morphology and stimulating specific and non-specific immunity. Probiotics are classified as autochthonous microbiota, allochthonous microbiota, and fungus according to the source and action mechanism of the strain.
The Function of Autochthonous Microbiota
Autochthonous microbiota come from the gut microbiota (Dubos et al., 1965), such as Bifidobacterium, C. butyricum, Lactobacillus, and Streptococcus faecalis. After obtaining the autochthonous microbiota, it can directly replenish the bacteria of origin and effectively colonize, reproduce and exert specific probiotic effects in animals (Mukai et al., 2002). The physiological and metabolic activities of autochthonous microbiota are closely related to the host. They can not only synthesize nutrients for the host and help maintain normal growth and life activities, but also form a biological barrier to prevent the invasion of pathogenic bacteria that compete for nutrients (Nava and Stappenbeck, 2011). In a previous study, the spent culture supernatant (SCS) of live and dead Lactobacilli was added to coccidia cultured in vitro, and the highest inhibition was found in the SCS of the live bacteria group, suggesting that the anticoccidial component is a secreted metabolite of lactic acid bacteria (Tierney et al., 2004). Exposure of E. acervulina, E. tenella, and E. maxima oocysts to the cell-free supernatant (corresponds to SCS) of Lactobacillus rhamnosus inhibited the sporulation of oocysts, which demonstrated the anti-coccidial activity of SCS (Biggs and Parsons, 2008). It has been shown that Lactobacillus salivarius produced antibacterial substances against Brachyspira hyodysenteriae, C. jejuni, C. perfringens, E. coli, and Salmonella choleraesuis (Klose et al., 2006). C. butyricum decreased the abundance of harmful bacteria, such as Brachybacterium, and Candidatus arthromitus, and increased the abundance of beneficial bacteria, such as Lactobacillus (Huang et al., 2019).
The Function of Allochthonous Microbiota
Allochthonous microbiota, such as Bacillus cereus, Bacillus licheniformis, B. subtilis, are not closely related to the host, and they either colonize the digestive tract for a short period or do not colonize it at all (Bäckhed et al., 2005). Allochthonous and autochthonous microbiota have symbiotic effects whereby allochthonous microbiota promote the growth and multiplication of autochthonous microbiota (Bortoluzzi et al., 2019; Whelan et al., 2019). Autochthonous microbiota generally induce the production of low antibody levels in the host, whereas allochthonous microbiota induce a strong immune response (Guo et al., 2021). B. subtilis clearly elevated serum nitric oxide levels in coccidia-infected chickens (Lee et al., 2014). Nitric oxide induced sporozoites to escape before maturity, which inhibited coccidia reproduction (Yan et al., 2021). Nitric oxide-induced sporozoites significantly decreased the invasive ability and reproductivity in chickens compared with fresh sporozoites. In coccidia-infected chickens, feed containing B. licheniformis significantly increased the expression of IL-10 and JAM2 (Chaudhari et al., 2020). We conclude that Bacillus eliminate coccidia by increasing immune factors that induce sporozoite escape before maturity. Bacillus spp. produce an antimicrobial factor that inhibits the colonization of gram-positive pathogens, such as B. cereus, Campylobacter coli, C. jejuni, Clostridium difficile, C. perfringens, L. monocytogenes, Micrococcus luteus, Staphylococcus aureus, and Streptococcus pneumoniae (Khochamit et al., 2015).
The Function of Fungus
Fungi commonly used include S. cerevisiae and Saccharomyces boulardii, which have specific mechanisms. In general, bacterial probiotics are generally sensitive to antibiotics. In contrast, fungal cell walls consist of two layers, forming a natural barrier. As a result, antibiotics cannot penetrate the cell wall to combine with nucleoproteins and interfere with the synthesis of nucleic acid, which makes yeast naturally resistant to antibiotics (Neut et al., 2017; Terciolo et al., 2019). Supplementation with Saccharomyces inhibited intestinal lesion formation and produced higher antibody titers (geomean titers), which provided protection against Eimeria infection in broilers (Awais et al., 2019). Meyerozyma guilliermondii isolated from chickens reduced E. tenella oocyst viability by damaging the resistant structure of oocysts, limiting their growth (Dantán-González et al., 2015). The action mode of yeasts in controlling intestinal diseases has not yet been elucidated, however, it is associated with the release of antimicrobial peptides, acidification of the surrounding environment, modification of inflammatory and immune responses and disruption of virulence factors (Hatoum et al., 2012). As immunomodulators, yeast cell wall components (β-glucans and mannans) are associated with immune system regulation, increasing local mucosal IgA secretion and cellular and humoral immune responses (Gómez-Verduzco et al., 2009). Dietary yeast cell wall (1 or 10 g/kg) reduced the severity of infection and oocyst shedding of a mixture of E. acervulina, E. maxima, and E. tenella (Elmusharaf et al., 2007) in broiler chickens. In addition, several investigations have shown that co-supplementation with yeast and bacterial probiotics improves survival and growth rates.
Several studies have confirmed the significant effect of probiotics on preventing coccidiosis, however, the exact mechanism has not yet been elucidated, and the following questions still need to be addressed. (1) How do probiotics regulate the gut microbiota to resist coccidia? (2) How do probiotics act on the intestinal biological barrier to exert anticoccidial effects? (3) What are the active ingredients of probiotics against coccidia? (4) Which probiotic has the best anticoccidial effect? In summary, a comprehensive understanding of the molecular mechanisms by which probiotics exert their beneficial effects on the host against coccidial infection is required for the development of highly effective probiotic formulations that can replace antibiotics for the prevention and control of coccidiosis.
Conclusion
In recent years, with the development of high-throughput sequencing technology, research on the interrelationship between the gut microbiota and diseases has progressed, and an increasing number of researchers have recognized the important role of gut microbiota in disease onset, progression, treatment, and prognosis. Although the mechanisms by which coccidia and intestinal microbiota interact are not well understood, this review analyzed the different aspects of their interactions. Coccidia share the intestinal environment with microbiota and directly antagonize commensal bacteria. In addition, coccidia indirectly affect the intestinal microbiota. Mechanical mucosal damage (impaired tight junctions and apoptosis of intestinal epithelial cells), chemical mucosal damage (increased mucus production and decreased antimicrobial peptides) and disruption of the immune system provide conditions suitable for the growth of conditionally pathogenic bacteria, leading to changes in the intestinal microbiota. The addition of probiotics directly or indirectly impair coccidia development by improving the intestinal microbiota.
Coccidia-microbiota-host interactions form a network of mutual constraints. For example, coccidial infection causes an imbalance in the intestinal microbiota, which not only leads to a decrease in food intake and impaired absorption but also increases the susceptibility of the organism to secondary infections. Conversely, the disruption of host health increases the number of pathogenic bacteria and impairs the intestinal mucosal barrier function and the ability of the immune system to target coccidia, resulting in a more serious coccidial infection. Coccidia, the microbiota and the host simultaneously interact, and a change in one factor may affect the entire network.
In conclusion, a holistic approach is needed to gain a better understanding of the mechanisms underlying coccidia development and infection. However, most studies on coccidiosis have focused on avian species, with limited studies on ruminants. With the development of intestinal microbiota sequencing technology in recent years, we can improve our understanding of the mechanisms contributing to coccidia-microbiota-host interactions and provide a theoretical basis for the control of coccidiosis.
Author Contributions
CL wrote the manuscript. CN and YY revised the manuscript. All authors read and approved the final version of the manuscript for publication.
Funding
This work was supported by the Earmarked Fund for China Modern Agro-industry Technology Research System (No. nycytx-38) and National Key R&D Program of China (No. 2018YFD0502100).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Melissa Crawford, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn/), for editing the English text of a draft of this manuscript.
References
- Abdel-Haleem H. M., Aboelhadid S. M., Sakran T., El-Shahawy G., El-Fayoumi H., Al-Quraishy S., et al. (2017). Gene Expression, Oxidative Stress and Apoptotic Changes in Rabbit Ileum Experimentally Infected With Eimeria Intestinalis. Folia Parasitol. (Praha). 64:2017.012. doi: 10.14411/fp.2017.012 [DOI] [PubMed] [Google Scholar]
- Adl S. M., Bass D., Lane C. E., Lukeš J., Schoch C. L., Smirnov A., et al. (2019). Revisions to the Classification, Nomenclature, and Diversity of Eukaryotes. J. Eukaryot. Microbiol. 66, 4–119. doi: 10.1111/jeu.12691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P. C. (1988). The Effect of Eimeria acervulina Infection on Plasmalipids and Lipoproteins in Young Broiler Chicks. Vet. Parasitol. 30, 17–30. doi: 10.1016/0304-4017(88)90139-2 [DOI] [PubMed] [Google Scholar]
- Awais M. M., Jamal M. A., Akhtar M., Hameed M. R., Anwar M. I., Ullah M. I. (2019). Immunomodulatory and Ameliorative Effects of Lactobacillus and Saccharomyces Based Probiotics on Pathological Effects of Eimeriasis in Broilers. Microb. Pathog. 126, 101–108. doi: 10.1016/j.micpath.2018.10.038 [DOI] [PubMed] [Google Scholar]
- Bäckhed F., Ley R. E., Sonnenburg J. L., Peterson D. A., Gordon J. I. (2005). Host-Bacterial Mutualism in the Human Intestine. Science 307, 1915–1920. doi: 10.1126/science.1104816 [DOI] [PubMed] [Google Scholar]
- Bangoura B., Bardsley K. D. (2020). Ruminant Coccidiosis. Vet. Clin. North Am. Food Anim. Pract. 36, 187–203. doi: 10.1016/j.cvfa.2019.12.006 [DOI] [PubMed] [Google Scholar]
- Benson A., Pifer R., Behrendt C. L., Hooper L. V., Yarovinsky F. (2009). Gut Commensal Bacteria Direct a Protective Immune Response Against Toxoplasma gondii . Cell Host Microbe 6, 187–196. doi: 10.1016/j.chom.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs P. M., Long P. L., Kenzy S. G., Rootes D. G. (1968). Relationship Between Marek’s Disease and Coccidiosis. II. The Effect of Marek’s Disease on the Susceptibility of Chickens to Coccidial Infection. Vet. Rec. 83, 284–289. doi: 10.1136/vr.83.12.284 [DOI] [PubMed] [Google Scholar]
- Biggs P., Parsons C. M. (2008). The Effects of Several Organic Acids on Growth Performance, Nutrient Digestibilities, and Cecal Microbial Populations in Young Chicks. Poult. Sci. 87, 2581–2589. doi: 10.3382/ps.2008-00080 [DOI] [PubMed] [Google Scholar]
- Bjerrum L., Engberg R. M., Leser T. D., Jensen B. B., Finster K., Pedersen K. (2006). Microbial Community Composition of the Ileum and Cecum of Broiler Chickens as Revealed by Molecular and Culture-Based Techniques. Poult. Sci. 85, 1151–1164. doi: 10.1093/ps/85.7.1151 [DOI] [PubMed] [Google Scholar]
- Bortoluzzi C., Serpa Vieira B., de Paula Dorigam J. C., Menconi A., Sokale A., Doranalli K., et al. (2019). Bacillus Subtilis DSM 32315 Supplementation Attenuates the Effects of Clostridium perfringens Challenge on the Growth Performance and Intestinal Microbiota of Broiler Chickens. Microorganisms 7:71. doi: 10.3390/microorganisms7030071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchta C. M., Bishop G. A.. (2014). Toll-Like Receptors and B Cells: Functions andMechanisms. Immunol. Res. 59, 12–22. doi: 10.1007/s12026-014-8523-2 [DOI] [PubMed] [Google Scholar]
- Budden K. F., Gellatly S. L., Wood D. L., Cooper M. A., Morrison M., Hugenholtz P., et al. (2017). Emerging Pathogenic Links Between Microbiota and the Gut-Lung Axis. Nat. Rev. Microbiol. 15, 55–63. doi: 10.1038/nrmicro.2016.142 [DOI] [PubMed] [Google Scholar]
- Butel M. J. (2014). Probiotics, Gut Microbiota and Health. Med. Mal. Infect. 44, 1–8. doi: 10.1016/j.medmal.2013.10.002 [DOI] [PubMed] [Google Scholar]
- Cash H. L., Whitham C. V., Behrendt C. L., Hooper L. V. (2006). Symbiotic Bacteria Direct Expression of an Intestinal Bactericidal Lectin. Science 313, 1126–1130. doi: 10.1126/science.1127119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casterlow S., Li H., Gilbert E. R., Dalloul R. A., McElroy A. P., Emmerson D. A., et al. (2011). An Antimicrobial Peptide is Downregulated in the Small Intestine of Eimeria maxima-Infected Chickens. Poult. Sci. 90, 1212–1219. doi: 10.3382/ps.2010-01110 [DOI] [PubMed] [Google Scholar]
- Chapman H. D. (1978). Studies on the Excystation of Different Species of Eimeria In Vitro . Z. Parasitenkd. 56, 115–121. doi: 10.1007/BF00930742 [DOI] [PubMed] [Google Scholar]
- Chapman H. D., Barta J. R., Blake D., Gruber A., Jenkins M., Smith N. C., et al. (2013). A Selective Review of Advances in Coccidiosis Research. Adv. Parasitol. 83, 93–171. doi: 10.1016/B978-0-12-407705-8.00002-1 [DOI] [PubMed] [Google Scholar]
- Chaudhari A. A., Lee Y., Lillehoj H. S. (2020). Beneficial Effects of Dietary Supplementation of Bacillus Strains on Growth Performance and Gut Health in Chickens With Mixed Coccidiosis Infection. Vet. Parasitol. 277, 109009. doi: 10.1016/j.vetpar.2019.109009 [DOI] [PubMed] [Google Scholar]
- Collier C. T., Hofacre C. L., Payne A. M., Anderson D. B., Kaiser P., Mackie R. I., et al. (2008). Coccidia-Induced Mucogenesis Promotes the Onset of Necrotic Enteritis by Supporting Clostridium Perfringens Growth. Vet. Immunol. Immunopathol. 122, 104–115. doi: 10.1016/j.vetimm.2007.10.014 [DOI] [PubMed] [Google Scholar]
- Cooke M. S., Evans M. D., Dizdaroglu M., Lunec J. (2003). Oxidative DNA Damage: Mechanisms, Mutation, and Disease. FASEB J. 17, 1195–1214. doi: 10.1096/fj.02-0752rev [DOI] [PubMed] [Google Scholar]
- Cui N., Wang X., Wang Q., Li H., Wang F., Zhao X. (2017). Effect of Dual Infection With Eimeria tenella and Subgroup J Avian Leukosis Virus on the Cecal Microbiome in Specific-Pathogen-Free Chicks. Front. Vet. Sci. 4, 177. doi: 10.3389/fvets.2017.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalloul R. A., Lillehoj H. S., Tamim N. M., Shellem T. A., Doerr J. A. (2005). Induction of Local Protective Immunity to Eimeria Acervulina by a Lactobacillus-Based Probiotic. Comp. Immunol. Microbiol. Infect. Dis. 28, 351–361. doi: 10.1016/j.cimid.2005.09.001 [DOI] [PubMed] [Google Scholar]
- Dantán-González E., Quiroz-Castañeda R. E., Cobaxin-Cárdenas M., Valle-Hernández J., Gama-Martínez Y., Tinoco-Valencia J. R., et al. (2015). Impact of Meyerozyma guilliermondii Isolated From Chickens Against Eimeria Sp. Protozoan, an In Vitro Analysis. BMC Vet. Res. 11, 278. doi: 10.1186/s12917-015-0589-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugschies A., Najdrowski M. (2005). Eimeriosis in Cattle: Current Understanding. J. Vet. Med. B. Infect. Dis. Vet. Public Health 52, 417–427. doi: 10.1111/j.1439-0450.2005.00894.x [DOI] [PubMed] [Google Scholar]
- Davenport E. R., Sanders J. G., Song S. J., Amato K. R., Clark A. G., Knight R. (2017). The Human Microbiome in Evolution. BMC Biol. 15, 127. doi: 10.1186/s12915-017-0454-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Cacho E., Gallego M., López-Bernad F., Quílez J., Sánchez-Acedo C. (2004). Expression of Anti-Apoptotic Factors in Cells Parasitized by Second-Generation Schizonts of Eimeria tenella and Eimeria necatrix . Vet. Parasitol. 125, 287–300. doi: 10.1016/j.vetpar.2004.07.017 [DOI] [PubMed] [Google Scholar]
- Desselberger U. (2018). The Mammalian Intestinal Microbiome: Composition, Interaction With the Immune System, Significance for Vaccine Efficacy, and Potential for Disease Therapy. Pathogens 7:57. doi: 10.3390/pathogens7030057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias J., Marcondes M. I., Motta de Souza S., Cardoso da Mata E Silva B., Fontes Noronha M., Tassinari Resende R., et al. (2018). Bacterial Community Dynamics Across the Gastrointestinal Tracts of Dairy Calves During Preweaning Development. Appl. Environ. Microbiol. 84, e02675–e02617. doi: 10.1128/AEM.02675-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dkhil M. A., Delic D., Al-Quraishy S. (2013). Goblet Cells and Mucin Related Gene Expression in Mice Infected With Eimeria papillata . ScientificWorldJournal 2013, 439865. doi: 10.1155/2013/439865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Zhao P., Chang S., Ju S., Li Y., Meng F., et al. (2015). Synergistic Pathogenic Effects of Co-Infection of Subgroup J Avian Leukosis Virus and Reticuloendotheliosis Virus in Broiler Chickens. Avian Pathol. 44, 43–49. doi: 10.1080/03079457.2014.993359 [DOI] [PubMed] [Google Scholar]
- Duszynski D. W. (2001). Eimeria. In: eLS. Chichester: John Wiley & Sons, Ltd. doi: 10.1002/9780470015902.a0001962.pub2 [DOI] [Google Scholar]
- Dubos R., Schaedler R. W., Costello R., Hoet ,. P. (1965). Indigenous, Normal, and Autochthonous Flora of the Gastrointestinal Tract. J. Exp. Med. 122, 67–76. doi: 10.1084/jem.122.1.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra D. D., Reid W. M. (1978). Monensin, Eimeria Tenella Infection, and Effects on the Bacterial Populations in the Ceca of Gnotobiotic Chickens. Poult. Sci. 57, 398–402. doi: 10.3382/ps.0570398 [DOI] [PubMed] [Google Scholar]
- Elahi S., Brownlie R., Korzeniowski J., Buchanan R., O’Connor B., Peppler M. S., et al. (2005). Infection of Newborn Piglets With Bordetella Pertussis: A New Model for Pertussis. Infect. Immun. 73, 3636–3645. doi: 10.1128/IAI.73.6.3636-3645.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmusharaf M. A., Peek H. W., Nollet L., Beynen A. C. (2007). The Effect of an in-Feed Mannanoligosaccharide Preparation (MOS) on a Coccidiosis Infection in Broilers. Anim. Feed. Sci. Technol. 134, 347–354. doi: 10.1016/j.anifeedsci.2006.11.022 [DOI] [Google Scholar]
- Elokil A. A., Abouelezz K., Ahmad H. I., Pan Y., Li S. (2020). Investigation of the Impacts of Antibiotic Exposure on the Diversity of the Gut Microbiota in Chicks. Anim. (Basel). 10, 896. doi: 10.3390/ani10050896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan P., Liu P., Song P., Chen X., Ma X. (2017). Moderate Dietary Protein Restriction Alters the Composition of Gut Microbiota and Improves Ileal Barrier Function in Adult Pig Model. Sci. Rep. 7, 43412. doi: 10.1038/srep43412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farid A. S., Jimi F., Inagaki-Ohara K., Horii Y. (2008). Increased Intestinal Endotoxin Absorption During Enteric Nematode But Not Protozoal Infections Through a Mast Cell-Mediated Mechanism. Shock 29, 709–716. doi: 10.1097/shk.0b013e31815c3f36 [DOI] [PubMed] [Google Scholar]
- Fasina Y. O., Newman M. M., Stough J. M., Liles M. R. (2016). Effect of Clostridium Perfringens Infection and Antibiotic Administration on Microbiota in the Small Intestine of Broiler Chickens. Poult. Sci. 95, 247–260. doi: 10.3382/ps/pev329 [DOI] [PubMed] [Google Scholar]
- Ferguson D. J., Belli S. I., Smith N. C., Wallach M. G. (2003). The Development of the Macrogamete and Oocyst Wall in Eimeria Maxima: Immuno-Light and Electron Microscopy. Int. J. Parasitol. 33, 1329–1340. doi: 10.1016/s0020-7519(03)00185-1 [DOI] [PubMed] [Google Scholar]
- Fetterer R. H., Miska K. B., Jenkins M. C., Wong E. A. (2014). Expression of Nutrient Transporters in Duodenum, Jejunum, and Ileum of Eimeria Maxima -Infected Broiler Chickens. Parasitol. Res. 113, 3891–3894. doi: 10.1007/s00436-014-4114-3 [DOI] [PubMed] [Google Scholar]
- Ficko-Blean E., Stuart C. P., Suits M. D., Cid M., Tessier M., Woods R. J., et al. (2012). Carbohydrate Recognition by an Architecturally Complex α-N-Acetylglucosaminidase From Clostridium Perfringens . PloS One 7, e33524. doi: 10.1371/journal.pone.0033524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata T., Baba E., Arakawa A. (1984). Growth of Salmonella typhimurium in the Caecum of Gnotobiotic Chickens With. Eimeria Tenella. Res. Vet. Sci. 37, 230–233. doi: 10.1016/s0034-5288(18)31911-8 [DOI] [PubMed] [Google Scholar]
- Gaboriaud P., Sadrin G., Guitton E., Fort G., Niepceron A., Lallier N., et al. (2021). The Absence of Gut Microbiota Alters the Development of the Apicomplexan Parasite Eimeria tenella . Front. Cell Infect. Microbiol. 10, 632556. doi: 10.3389/fcimb.2020.632556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván-Moroyoqui J. M., Del Carmen Domínguez-Robles M., Franco E., Meza I. (2008). The Interplay Between Entamoeba and Enteropathogenic Bacteria Modulates Epithelial Cell Damage. PloS Negl. Trop. Dis. 2, e266. doi: 10.1371/journal.pntd.0000266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Liu Y., Guan X., Li X., Yun B., Qi X., et al. (2015). Differential Expression of Immune-Related Cytokine Genes in Response to J Group Avian Leukosis Virus Infection. Vivo. Mol. Immunol. 64, 106–111. doi: 10.1016/j.molimm.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Garcia A., Olmo B., Lopez-Gonzalvez A., Cornejo L., Rupérez F. J., Barbas C. (2008). Capillary Electrophoresis for Short Chain Organic Acids in Faeces Reference Values in a Mediterranean Elderly Population. J. Pharm. Biomed. Anal. 46, 356–361. doi: 10.1016/j.jpba.2007.10.026 [DOI] [PubMed] [Google Scholar]
- Giambrone J. J., Anderson W. I., Reid W. M., Eidson C. S. (1977). Effect of Infectious Bursal Disease on the Severity of Eimeria Tenella Infections in Broiler Chicks. Poult. Sci. 56, 243–246. doi: 10.3382/ps.0560243 [DOI] [PubMed] [Google Scholar]
- Gómez-Verduzco G., Cortes-Cuevas A., López-Coello C., Avila-González E., Nava G. M. (2009). Dietary Supplementation of Mannan-Oligosaccharide Enhances Neonatal Immune Responses in Chickens During Natural Exposure to Eimeria Spp. Acta Vet. Scand. 51, 11. doi: 10.1186/1751-0147-51-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Xi Y., Xia Y., Wu T., Zhao D., Zhang Z., et al. (2021). Dietary Lactobacillus Fermentum and Bacillus Coagulans Supplementation Modulates Intestinal Immunity and Microbiota of Broiler Chickens Challenged by Clostridium Perfringens . Front. Vet. Sci. 8, 680742. doi: 10.3389/fvets.2021.680742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatoum R., Labrie S., Fliss I. (2012). Antimicrobial and Probiotic Properties of Yeasts: From Fundamental to Novel Applications. Front. Microbiol. 3, 421. doi: 10.3389/fmicb.2012.00421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofacre C. L., Froyman R., Gautrias B., George B., Goodwin M. A., Brown J. (1998). Use of Aviguard and Other Intestinal Bioproducts in Experimental Clostridium Perfringens-Associated Necrotizing Enteritis in Broiler Chickens. Avian. Dis. 42, 579–584. doi: 10.2307/1592685 [DOI] [PubMed] [Google Scholar]
- Hooper L. V., Littman D. R., Macpherson A. J. (2012). Interactions Between the Microbiota and the Immune System. Science 336, 1268–1273. doi: 10.1126/science.1223490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T., Peng X. Y., Gao B., Wei Q. L., Xiang R., Yuan M. G., et al. (2019). The Effect of Clostridium butyricum on Gut Microbiota, Immune Response and Intestinal Barrier Function During the Development of Necrotic Enteritis in Chickens. Front. Microbiol. 10, 2309. doi: 10.3389/fmicb.2019.02309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G., Zhang S., Zhou C., Tang X., Li C., Wang C., et al. (2018). Influence of Eimeria falciformis Infection on Gut Microbiota and Metabolic Pathways in Mice. Infect. Immun. 86, e00073–e00018. doi: 10.1128/IAI.00073-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes H. P., Gonzalez A., Guhl F., Hudson L. (1985). Antigen-Specific Lymphocyte Transformation in Congenital Toxoplasmosis. Immunol. Lett. 10, 95–98. doi: 10.1016/0165-2478(85)90182-8 [DOI] [PubMed] [Google Scholar]
- Jakowlew S. B., Mathias A., Lillehoj H. S. (1997). Transforming Growth Factor-Beta Isoforms in the Developing Chicken Intestine and Spleen: Increase in Transforming Growth Factor-Beta 4 With Coccidia Infection. Vet. Immunol. Immunopathol. 55, 321–339. doi: 10.1016/s0165-2427(96)05628-0 [DOI] [PubMed] [Google Scholar]
- Jiang Z., Applegate T. J., Lossie A. C. (2013). Cloning, Annotation and Developmental Expression of the Chicken Intestinal MUC2 Gene. PloS One 8, e53781. doi: 10.1371/journal.pone.0053781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Lü X., Man C., Han L., Shan Y., Qu X., et al. (2012). Lactobacillus Acidophilus Induces Cytokine and Chemokine Production via NF-κb and P38 Mitogen-Activated Protein Kinase Signaling Pathways in Intestinal Epithelial Cells. Clin. Vaccine Immunol. 19, 603–608. doi: 10.1128/CVI.05617-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N., Núñez G. (2014). Regulation of the Immune System by the Resident Intestinal Bacteria. Gastroenterology 146, 1477–1488. doi: 10.1053/j.gastro.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp L. E., Yamamoto M., Soldati-Favre D. (2013). Subversion of Host Cellular Functions by the Apicomplexan Parasites. FEMS Microbiol. Rev. 37, 607–631. doi: 10.1111/1574-6976.12013 [DOI] [PubMed] [Google Scholar]
- Khochamit N., Siripornadulsil S., Sukon P., Siripornadulsil W. (2015). Antibacterial Activity and Genotypic-Phenotypic Characteristics of Bacteriocin-Producing Bacillus Subtilis KKU213: Potential as a Probiotic Strain. Microbiol. Res. 170, 36–50. doi: 10.1016/j.micres.2014.09.004 [DOI] [PubMed] [Google Scholar]
- Kim H. B., Borewicz K., White B. A., Singer R. S., Sreevatsan S., Tu Z. J., et al. (2011). Longitudinal Investigation of the Age-Related Bacterial Diversity in the Feces of Commercial Pigs. Vet. Microbiol. 153, 124–133. doi: 10.1016/j.vetmic.2011.05.021 [DOI] [PubMed] [Google Scholar]
- Kim J. E., Lillehoj H. S., Hong Y. H., Kim G. B., Lee S. H., Lillehoj E. P., et al. (2015). Dietary Capsicum and Curcuma Longa Oleoresins Increase Intestinal Microbiome and Necrotic Enteritis in Three Commercial Broiler Breeds. Res. Vet. Sci. 102, 150–158. doi: 10.1016/j.rvsc.2015.07.022 [DOI] [PubMed] [Google Scholar]
- Kimura N., Mimura F., Nishida S., Kobayashi A. (1976). Studies on the Relationship Between Intestinal Flora and Cecal Coccidiosis in Chicken. Poult. Sci. 55, 1375–1383. doi: 10.3382/ps.0551375 [DOI] [PubMed] [Google Scholar]
- Klose V., Mohnl M., Plail R., Schatzmayr G., Loibner A. P. (2006). Development of a Competitive Exclusion Product for Poultry Meeting the Regulatory Requirements for Registration in the European Union. Mol. Nutr. Food Res. 50, 563–571. doi: 10.1002/mnfr.200500166 [DOI] [PubMed] [Google Scholar]
- Kogut M. H., Fukata T., Tellez G., Hargis B. M., Corrier D. E., DeLoach J. R. (1994). Effect of Eimeria Tenella Infection on Resistance to Salmonella Typhimurium Colonization in Broiler Chicks Inoculated With Anaerobic Cecal Flora and Fed Dietary Lactose. Avian Dis. 38, 59–64. doi: 10.2307/1591837 [DOI] [PubMed] [Google Scholar]
- Lee N., Kim W. U. (2017). Microbiota in T-Cell Homeostasis and Inflammatory Diseases. Exp. Mol. Med. 49, e340. doi: 10.1038/emm.2017.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. W., Lillehoj H. S., Jang S. I., Lee S. H. (2014). Effects of Salinomycin and Bacillus Subtilis on Growth Performance and Immune Responses in Broiler Chickens. Res. Vet. Sci. 97, 304–308. doi: 10.1016/j.rvsc.2014.07.021 [DOI] [PubMed] [Google Scholar]
- Lee K. W., Lillehoj H. S., Jang S. I., Lee S. H., Bautista D. A., Siragusa G. R. (2013). Effect of Bacillus Subtilis-Based Direct-Fed Microbials on Immune Status in Broiler Chickens Raised on Fresh or Used Litter. Asian-Australas. J. Anim. Sci. 26, 1592–1597. doi: 10.5713/ajas.2013.13178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J. M., Graham A. L., Knowles S. (2018). Parasite-Microbiota Interactions With the Vertebrate Gut: Synthesis Through an Ecological Lens. Front. Microbiol. 9, 843. doi: 10.3389/fmicb.2018.00843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung H., Yitbarek A., Snyder R., Patterson R., Barta J. R., Karrow N., et al. (2019). ). Responses of Broiler Chickens to Eimeria Challenge When Fed a Nucleotide-Rich Yeast Extract. Poult. Sci. 98, 1622–1633. doi: 10.3382/ps/pey533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R. E., Turnbaugh P. J., Klein S., Gordon J. I. (2006). Microbial Ecology: Human Gut Microbes Associated With Obesity. Nature 444, 1022–1023. doi: 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- Liévin V., Peiffer I., Hudault S., Rochat F., Brassart D., Neeser J. R., et al. (2000). Bifidobacterium Strains From Resident Infant Human Gastrointestinal Microflora Exert Antimicrobial Activity. Gut 47, 646–652. doi: 10.1136/gut.47.5.646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj H. S. (1986). Immune Response During Coccidiosis in SC and FP Chickens. I. In Vitro Assessment of T Cell Proliferation Response to Stage-Specific Parasite Antigens. Vet. Immunol. Immunopathol. 13, 321–330. doi: 10.1016/0165-2427(86)90025-5 [DOI] [PubMed] [Google Scholar]
- Li B., Zhang K., Li C., Wang X., Chen Y., Yang Y. (2019). Characterization and Comparison of Microbiota in the Gastrointestinal Tracts of the Goat (Capra Hircus) During Preweaning Development. Front. Microbiol. 10, 2125. doi: 10.3389/fmicb.2019.02125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zheng M. X., Xu H. C., Cui X. Z., Zhang Y., Zhang L., et al. (2017). Mitochondrial Pathways are Involved in Eimeria tenella-Induced Apoptosis of Chick Embryo Cecal Epithelial Cells. Parasito. Res. 116, 225–235. doi: 10.1007/s00436-016-5283-z [DOI] [PubMed] [Google Scholar]
- Lund M., Bjerrum L., Pedersen K. (2010). Quantification of Faecalibacterium prausnitzii and Subdoligranulum Variabile-Like Bacteria in the Cecum of Chickens by Real-Time PCR. Poult. Sci. 89, 1217–1224. doi: 10.3382/ps.2010-00653 [DOI] [PubMed] [Google Scholar]
- Lupp C., Robertson M. L., Wickham M. E., Sekirov I., Champion O. L., Gaynor E. C., et al. (2007). Host-Mediated Inflammation Disrupts the Intestinal Microbiota and Promotes the Overgrowth of Enterobacteriaceae. Cell Host Microbe 2, 204. doi: 10.1016/j.chom.2007.06.010 [DOI] [PubMed] [Google Scholar]
- Macdonald S. E., van Diemen P. M., Martineau H., Stevens M. P., Tomley F. M., Stabler R. A., et al. (2019). Impact of Eimeria tenella Coinfection on Campylobacter Jejuni Colonization of the Chicken. Infect. Immun. 87, e00772–e00718. doi: 10.1128/IAI.00772-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane G. T., Allison C., Gibson S. A., Cummings J. H. (1988). Contribution of the Microflora to Proteolysis in the Human Large Intestine. J. Appl. Bacteriol. 64, 37–46. doi: 10.1111/j.1365-2672.1988.tb02427.x [DOI] [PubMed] [Google Scholar]
- Mácová A., Hoblíková A., Hypša V., Stanko M., Martinů J., Kvičerová J. (2018). Mysteries of Host Switching: Diversification and Host Specificity in Rodent-Coccidia Associations. Mol. Phylogenet. Evol. 127, 179–189. doi: 10.1016/j.ympev.2018.05.009 [DOI] [PubMed] [Google Scholar]
- Matos L., Muñoz M. C., Molina J. M., Rodríguez F., Pérez D., López A. M., et al. (2018). Age-Related Immune Response to Experimental Infection With Eimeria Ninakohlyakimovae in Goat Kids. Res. Vet. Sci. 118, 155–163. doi: 10.1016/j.rvsc.2018.02.004 [DOI] [PubMed] [Google Scholar]
- Memon F. U., Yang Y., Lv F., Soliman A. M., Chen Y., Sun J., et al. (2020). Effects of Probiotic and Bidens Pilosa on the Performance and Gut Health of Chicken During Induced Eimeria Tenella Infection. J. Appl. Microbiol. 131, 425–434. doi: 10.1111/jam.14928 [DOI] [PubMed] [Google Scholar]
- Mirpuri J., Raetz M., Sturge C. R., Wilhelm C. L., Benson A., Savani R. C., et al. (2014). Proteobacteria-Specific IgA Regulates Maturation of the Intestinal Microbiota. Gut. Microbes 5, 28–39. doi: 10.4161/gmic.26489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada D. M., Kammanadiminti S. J., Chadee K. (2003). Mucin and Toll-Like Receptors in Host Defense Against Intestinal Parasites. Trends Parasitol. 19, 305–311. doi: 10.1016/s1471-4922(03)00122-3 [DOI] [PubMed] [Google Scholar]
- Motha M. X., Egerton J. R. (1984). Influence of Reticuloendotheliosis on the Severity of Eimeria tenella Infection in Broiler Chickens. Vet. Microbiol. 9, 121–129. doi: 10.1016/0378-1135(84)90027-0 [DOI] [PubMed] [Google Scholar]
- Mukai T., Asasaka T., Sato E., Mori K., Matsumoto M., Ohori H. (2002). Inhibition of Binding of Helicobacter Pylori to the Glycolipid Receptors by Probiotic Lactobacillus reuteri . FEMS Immunol. Med. Microbiol. 32, 105–110. doi: 10.1111/j.1574-695X.2002.tb00541.x [DOI] [PubMed] [Google Scholar]
- Nanthakumar N. N., Meng D., Newburg D. S. (2013). Glucocorticoids and Microbiota Regulate Ontogeny of Intestinal Fucosyltransferase 2 Requisite for Gut Homeostasis. Glycobiology 23, 1131–1141. doi: 10.1093/glycob/cwt050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava G. M., Stappenbeck T. S. (2011). Diversity of the Autochthonous Colonic Microbiota. Gut. Microbes 2, 99–104. doi: 10.4161/gmic.2.2.15416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neut C., Mahieux S., Dubreuil L. J. (2017). Antibiotic Susceptibility of Probiotic Strains: Is it Reasonable to Combine Probiotics With Antibiotics? Med. Mal. Infect. 47, 477–483. doi: 10.1016/j.medmal.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Odden A., Denwood M. J., Stuen S., Robertson L. J., Ruiz A., Hamnes I. S., et al. (2018). Field Evaluation of Anticoccidial Efficacy: A Novel Approach Demonstrates Reduced Efficacy of Toltrazuril Against Ovine Eimeria Spp. In Norway. Int. J. Parasitol. Drugs Drug Resist. 8, 304–311. doi: 10.1016/j.ijpddr.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura R., Takeda K. (2017). Roles of Intestinal Epithelial Cells in the Maintenance of Gut Homeostasis. Exp. Mol. Med. 49, e338. doi: 10.1038/emm.2017.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z. R., Arakawa A., Baba E., Fukata T., Miyamoto T., Sasai K., et al. (1995). Eimeria tenella Infection Induces Recrudescence of Previous Salmonella Enteritidis Infection in Chickens. Poult. Sci. 74, 1786–1792. doi: 10.3382/ps.0741786 [DOI] [PubMed] [Google Scholar]
- Rajput I. R., Li L. Y., Xin X., Wu B. B., Juan Z. L., Cui Z. W., et al. (2013). Effect of Saccharomyces Boulardii and Bacillus Subtilis B10 on Intestinal Ultrastructure Modulation and Mucosal Immunity Development Mechanism in Broiler Chickens. Poult. Sci. 92, 956–965. doi: 10.3382/ps.2012-02845 [DOI] [PubMed] [Google Scholar]
- Ramanan D., Bowcutt R., Lee S. C., Tang M. S., Kurtz Z. D., Ding Y., et al. (2016). Helminth Infection Promotes Colonization Resistance via Type 2 Immunity. Science 352, 608–612. doi: 10.1126/science.aaf3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. H., Pothoulakis C., Mayer E. A. (2009). Principles and Clinical Implications of the Brain-Gut-Enteric Microbiota Axis. Nat. Rev. Gastroenterol. Hepatol. 6, 306–314. doi: 10.1038/nrgastro.2009.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter G., Wiesner J. (1988). Relation Between the Protein Supply of Chicks and Disposition to Eimeria tenella Infections. Arch. Exp. Veterinarmed. 42, 147–153. [PubMed] [Google Scholar]
- Robinson K., Deng Z., Hou Y., Zhang G. (2015). Regulation of the Intestinal Barrier Function by Host Defense Peptides. Front. Vet. Sci. 2:57. doi: 10.3389/fvets.2015.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. E., Hesketh P. (1984). Infection With Eimeria tenella: Modulation of Lymphocyte Blastogenesis by Specific Antigen, and Evidence for Immunodepression. J. Protozool. 31, 549–553. doi: 10.1111/j.1550-7408.1984.tb05500.x [DOI] [PubMed] [Google Scholar]
- Ruff M. D., Rosenberger J. K. (1985). Interaction of Low-Pathogenicity Reoviruses and Low Levels of Infection With Several Coccidial Species. Avian Dis. 29, 1057–1065. doi: 10.2307/1590460 [DOI] [PubMed] [Google Scholar]
- Schlee M., Harder J., Köten B., Stange E. F., Wehkamp J., Fellermann K. (2008). Probiotic Lactobacilli and VSL3 Induce Enterocyte Beta-Defensin 2. Clin. Exp. Immunol. 151, 528–535. doi: 10.1111/j.1365-2249.2007.03587.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger E. E., Lynch R. D. (2004). The Tight Junction: A Multifunctional Complex. Am. J. Physiol. Cell Physiol. 286, C1213–C1228. doi: 10.1152/ajpcell.00558.2003 [DOI] [PubMed] [Google Scholar]
- Shirley M. W., Smith A. L., Tomley F. M. (2005). The Biology of Avian Eimeria With an Emphasis on Their Control by Vaccination. Adv. Parasitol. 60, 285–330. doi: 10.1016/S0065-308X(05)60005-X [DOI] [PubMed] [Google Scholar]
- Sommer F., Adam N., Johansson M. E., Xia L., Hansson G. C., Bäckhed F. (2014). Altered Mucus Glycosylation in Core 1 O-Glycan-Deficient Mice Affects Microbiota Composition and Intestinal Architecture. PloS One 9, e85254. doi: 10.1371/journal.pone.0085254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H., Song X., Xu L., Yan R., Shah M. A., Li X. (2010). Changes of Cytokines and IgG Antibody in Chickens Vaccinated With DNA Vaccines Encoding Eimeria acervulina Lactate Dehydrogenase. Vet. Parasitol. 173, 219–227. doi: 10.1016/j.vetpar.2010.06.030 [DOI] [PubMed] [Google Scholar]
- Stanley D., Wu S. B., Rodgers N., Swick R. A., Moore R. J. (2014). Differential Responses of Cecal Microbiota to Fishmeal, Eimeria and Clostridium Perfringens in a Necrotic Enteritis Challenge Model in Chickens. PloS One 9, e104739. doi: 10.1371/journal.pone.0104739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sühwold A., Hermosilla C., Seeger T., Zahner H., Taubert A. (2010). T Cell Reactions of Eimeria bovis Primary and Challenge-Infected Calves. Parasitol. Res. 106, 595–605. doi: 10.1007/s00436-009-1705-5 [DOI] [PubMed] [Google Scholar]
- Su S., Miska K. B., Fetterer R. H., Jenkins M. C., Wong E. A. (2014). Expression of Digestive Enzymes and Nutrient Transporters in Eimeria Acervulina-Challenged Layers and Broilers. Poult. Sci. 93, 1217–1226. doi: 10.3382/ps.2013-03807 [DOI] [PubMed] [Google Scholar]
- Tailford L. E., Crost E. H., Kavanaugh D., Juge N. (2015). Mucin Glycan Foraging in the Human Gut Microbiome. Front. Genet. 6:81. doi: 10.3389/fgene.2015.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L., Li Y., Yang X., Ke Q., Lei W., Mughal M. N., et al. (2017). Genetic Diversity and Drug Sensitivity Studies on Eimeria tenella Field Isolates From Hubei Province of China. Parasitol. Vectors 10, 137. doi: 10.1186/s13071-017-2067-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert A., Hermosilla C., Sühwold A., Zahner H. (2008). Antigen-Induced Cytokine Production in Lymphocytes of Eimeria bovis Primary and Challenge Infected Calves. Vet. Immunol. Immunopathol. 126, 309–320. doi: 10.1016/j.vetimm.2008.09.003 [DOI] [PubMed] [Google Scholar]
- Teng P. Y., Yadav S., Castro F., Tompkins Y. H., Fuller A. L., Kim W. K. (2020). Graded Eimeria Challenge Linearly Regulated Growth Performance, Dynamic Change of Gastrointestinal Permeability, Apparent Ileal Digestibility, Intestinal Morphology, and Tight Junctions of Broiler Chickens. Poult. Sci. 99, 4203–4216. doi: 10.1016/j.psj.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terciolo C., Dapoigny M., Andre F. (2019). Beneficial Effects of Saccharomyces Boulardii CNCM I-745 on Clinical Disorders Associated With Intestinal Barrier Disruption. Clin. Exp. Gastroenterol. 12, 67–82. doi: 10.2147/CEG.S181590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney J., Gowing H., Van Sinderen D., Flynn S., Stanley L., McHardy N., et al. (2004). In Vitro Inhibition of Eimeria tenella Invasion by Indigenous Chicken Lactobacillus Species. Vet. Parasitol. 122, 171–182. doi: 10.1016/j.vetpar.2004.05.001 [DOI] [PubMed] [Google Scholar]
- Tortuero F. (1973). Influence of the Implantation of Lactobacillus acidophilus in Chicks on the Growth, Feed Conversion, Malabsorption of Fats Syndrome and Intestinal Flora. Poult. Sci. 52, 197–203. doi: 10.3382/ps.0520197 [DOI] [PubMed] [Google Scholar]
- Treem W. R. (2012). Clinical Aspects and Treatment of Congenital Sucrase-Isomaltase Deficiency. J. Pediatr. Gastroenterol. Nutr. 55 Suppl 2, S7–S13. doi: 10.1097/01.mpg.0000421401.57633.90 [DOI] [PubMed] [Google Scholar]
- Trefancová A., Kvičerová J., Mácová A., Stanko M., Hofmannová L., Hypša V. (2021). Switch, Disperse, Repeat: Host Specificity is Highly Flexible in Rodent-Associated Eimeria . Int. J. Parasitol., S0020-7519(21)00195-8. doi: 10.1016/j.ijpara.2021.04.005 [DOI] [PubMed] [Google Scholar]
- Turk D. E. (1972). Protozoan Parasitic Infections of the Chick Intestine and Protein Digestion and Absorption. J. Nutr. 102, 1217–1221. doi: 10.1093/jn/102.9.1217 [DOI] [PubMed] [Google Scholar]
- Waite D. W., Taylor M. W. (2015). Exploring the Avian Gut Microbiota: Current Trends and Future Directions. Front. Microbiol. 6:673. doi: 10.3389/fmicb.2015.00673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lv X., Li X., Zhao J., Zhang K., Hao X., et al. (2021). Protective Effect of Lactobacillus plantarum P8 on Growth Performance, Intestinal Health, and Microbiota in Eimeria-Infected Broilers. Front. Microbiol. 12:705758. doi: 10.3389/fmicb.2021.705758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R. A., Doranalli K., Rinttilä T., Vienola K., Jurgens G., Apajalahti J. (2019). The Impact of Bacillus subtilis DSM 32315 on the Pathology, Performance, and Intestinal Microbiome of Broiler Chickens in a Necrotic Enteritis Challenge. Poult. Sci. 98, 3450–3463. doi: 10.3382/ps/pey500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E. C., Houlden A., Bancroft A. J., Hayes K. S., Goldrick M., Grencis R. K., et al. (2018). Manipulation of Host and Parasite Microbiotas: Survival Strategies During Chronic Nematode Infection. Sci. Adv. 4, eaap7399. doi: 10.1126/sciadv.aap7399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. B., Stanley D., Rodgers N., Swick R. A., Moore R. J. (2014). Two Necrotic Enteritis Predisposing Factors, Dietary Fishmeal and Eimeria Infection, Induce Large Changes in the Caecal Microbiota of Broiler Chickens. Vet. Microbiol. 169, 188–197. doi: 10.1016/j.vetmic.2014.01.007 [DOI] [PubMed] [Google Scholar]
- Xu S., Lin Y., Zeng D., Zhou M., Zeng Y., Wang H., et al. (2018). Bacillus Licheniformis Normalize the Ileum Microbiota of Chickens Infected With Necrotic Enteritis. Sci. Rep. 8, 1744. doi: 10.1038/s41598-018-20059-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Han W., Liu X., Suo X. (2021). Exogenous Nitric Oxide Stimulates Early Egress of Eimeria tenella Sporozoites From Primary Chicken Kidney Cells In Vitro. L’oxyde Nitrique Exogène Stimule In Vitro La Sortie Précoce Des Sporozoïtes D’ Eimeria Tenella Des Cellules Primaires De Rein De Poulet. Parasite 28, 11. doi: 10.1051/parasite/2021007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Sumners L. H., Dalloul R. A., Miska K. B., Fetterer R. H., Jenkins M. C., et al. (2015). Changes in Expression of an Antimicrobial Peptide, Digestive Enzymes, and Nutrient Transporters in the Intestine of E. Praecox-Infected Chickens. Poult. Sci. 94, 1521–1526. doi: 10.3382/ps/pev133 [DOI] [PubMed] [Google Scholar]
- Yitbarek A., Taha-Abdelaziz K., Hodgins D. C., Read L., Nagy É., Weese J. S., et al. (2018). Gut Microbiota-Mediated Protection Against Influenza Virus Subtype H9N2 in Chickens is Associated With Modulation of the Innate Responses. Sci. Rep. 8, 13189. doi: 10.1038/s41598-018-31613-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun C. H., Lillehoj H. S., Lillehoj E. P. (2000). Intestinal Immune Responses to Coccidiosis. Dev. Comp. Immunol. 24, 303–324. doi: 10.1016/s0145-305x(99)00080-4 [DOI] [PubMed] [Google Scholar]
- Yunus M., Horii Y., Makimura S., Smith A. L. (2005). Murine Goblet Cell Hypoplasia During Eimeria Pragensis Infection is Ameliorated by Clindamycin Treatment. J. Vet. Med. Sci. 67, 311–315. doi: 10.1292/jvms.67.311 [DOI] [PubMed] [Google Scholar]
- Yu H., Zou W., Wang X., Dai G., Zhang T., Zhang G., et al. (2020). Research Note: Correlation Analysis of Interleukin-6, Interleukin-8, and C-C Motif Chemokine Ligand 2 Gene Expression in Chicken Spleen and Cecal Tissues After Eimeria tenella Infection In Vivo . Poult. Sci. 99, 1326–1331. doi: 10.1016/j.psj.2019.10.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss M. M., Harris N. L. (2016). Interactions Between the Intestinal Microbiome and Helminth Parasites. Parasite. Immunol. 38, 5–11. doi: 10.1111/pim.12274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zheng M. X., Xu Z. Y., Xu H. C., Cui X. Z., Yang S. S., et al. (2015). Relationship Between Eimeria Tenella Development and Host Cell Apoptosis in Chickens. Poult. Sci. 94, 2970–2979. doi: 10.3382/ps/pev293 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhang K., Zou M., Sun Y., Peng X. (2018). gga-miR-451 Negatively Regulates Mycoplasma Gallisepticum (HS Strain)-Induced Inflammatory Cytokine Production via Targeting YWHAZ. Int. J. Mol. Sci. 19:1191. doi: 10.3390/ijms19041191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. C. (2020). Effects of Coccidia Infection on Intestinal Flora of Hu Sheep. [Master’s Thesis] (Zhengzhou (Henan: Henan Agriculture University (in Chinese; ). [Google Scholar]
- Zhou Z., Wang Z., Cao L., Hu S., Zhang Z., Qin B., et al. (2013). Upregulation of Chicken TLR4, TLR15 and MyD88 in Heterophils and Monocyte-Derived Macrophages Stimulated With Eimeria tenella In Vitro . Exp. Parasitol. 133, 427–433. doi: 10.1016/j.exppara.2013.01.002 [DOI] [PubMed] [Google Scholar]
- Zhuang Y., Chai J., Cui K., Bi Y., Diao Q., Huang W., et al. (2020). Longitudinal Investigation of the Gut Microbiota in Goat Kids From Birth to Postweaning. Microorganisms 8, 1111. doi: 10.3390/microorganisms8081111 [DOI] [PMC free article] [PubMed] [Google Scholar]