Abstract
Introduction
Approved pharmacological treatments for smoking cessation are modestly effective, underscoring the need for improved pharmacotherapies. Glucagon-like peptide-1 receptor (GLP-1R) agonists attenuate the rewarding effects of nicotine in preclinical studies. We examined the efficacy of extended-release exenatide, a GLP-1R agonist, combined with nicotine replacement therapy (NRT, patch) for smoking cessation, craving, and withdrawal symptoms, with post-cessation body weight as a secondary outcome.
Methods
Eighty-four prediabetic and/or overweight smokers were randomized (1 : 1) to once-weekly placebo or exenatide, 2 mg, subcutaneously. All participants received NRT (21 mg) and brief smoking cessation counseling. Seven-day point prevalence abstinence (expired CO level ≤5 ppm), craving, withdrawal, and post-cessation body weight were assessed following 6 weeks of treatment. A Bayesian approach for analyzing generalized linear models yielded posterior probabilities (PP) to quantify the evidence favoring hypothesized effects of treatment on the study outcomes.
Results
Exenatide increased the risk for smoking abstinence compared to placebo (46.3% and 26.8%, respectively), (risk ratio [RR] = 1.70; 95% credible interval = [0.96, 3.27]; PP = 96.5%). Exenatide reduced end-of-treatment craving in the overall sample and withdrawal among abstainers. Post-cessation body weight was 5.6 pounds lower in the exenatide group compared to placebo (PP = 97.4%). Adverse events were reported in 9.5% and 2.3% of participants in the exenatide and placebo groups, respectively.
Conclusions
Exenatide, in combination with the NRT improved smoking abstinence, reduced craving and withdrawal symptoms, and decreased weight gain among abstainers. Findings suggest that the GLP-1R agonist strategy is worthy of further research in larger, longer duration studies.
Implications
Despite considerable progress in tobacco control, cigarette smoking remains the leading cause of preventable disease, disability, and death. In this pilot study, we showed that extended-release exenatide, a glucagon-like peptide-1 receptor agonist, added to the nicotine patch, improved abstinence and mitigated post-cessation body weight gain compared to patch alone. Further research is needed to confirm these initial positive results.
Introduction
The prevalence of cigarette smoking in the U.S. has decreased substantially, from 42.4% in 1965 to 14% in 2018, since the 1964 surgeon general’s report on smoking and health. Despite tremendous progress, cigarette smoking remains the leading cause of preventable disease, disability, and death in the U.S.1 Approximately 400,000 Americans still die each year from smoking-related causes, and 16 million Americans are living today with a smoking-related disease. In addition to the human costs, cigarette smoking places a substantial burden on society, costing the U.S. $170 billion in direct medical care and over $156 billion in lost productivity annually.1
First-line smoking cessation treatments include nicotine replacement therapy (NRT), varenicline, and bupropion. NRT is the most commonly used smoking cessation therapy with demonstrated effectiveness and safety for virtually all smokers. Current evidence suggests that all of the available forms of NRT can increase the chances of successful cessation by 50%; 2 however, the long-term success of NRT is variable, prompting research efforts to improve the efficacy of NRT. Many of these efforts have focused on combining different forms of NRT,3 or combining NRT with non-nicotine medications, including other first-line smoking cessation agents, such as bupropion4 or varenicline.5 In the latter case, the goal is to achieve synergy by the simultaneous use of medications with different mechanisms of action. Studies of combination therapy have demonstrated increased abstinence rates compared to NRT only,5 thereby supporting the search for other adjunctive agents to enhance the efficacy of NRT. Combining NRT with glucagon-like peptide-1 (GLP-1) receptor (GLP-1R) agonists, medications that are currently used for the treatment of type 2 diabetes (DM) and obesity, may provide one novel approach for improving the efficacy of NRT.
An incretin hormone, GLP-1 is secreted from intestinal L-cells and hindbrain nucleus tractus solitarius neurons in response to nutrient ingestion.6,7 GLP-1 enhances glucose-dependent insulin secretion and exhibits other antihyperglycemic actions.8 GLP-1 also reduces food intake in part by reducing appetite.9,10 Centrally, GLP-1 receptors are expressed in areas associated with drug- and food-induced reinforcement, such as the ventral tegmental area and the nucleus accumbens.11 Consistent with this, GLP-1R agonists attenuate the rewarding effects of alcohol,12 cocaine,13 amphetamine,13 and most relevant here, nicotine14,15 in animal models. For example, administration of a GLP-1 agonist selectively attenuated nicotine-induced locomotor stimulation, dopamine release in the nucleus accumbens, and conditioned place preference in mice.14 Collectively, these data suggest that pharmacologically targeting the GLP1-R might reduce the reinforcing effects of nicotine.
The aims of this parallel group, two-arm, double-blind, randomized, controlled clinical trial were to determine the effects of extended-release exenatide, a GLP-1R agonist, on 7-day point prevalence abstinence, craving, and withdrawal among prediabetic and/or overweight treatment-seeking adult smokers. The study was initiated at the Michael E. DeBakey VA Medical Center (MEDVAMC); however, following the PI’s (LY) relocation to the University of Texas Health Science Center at Houston (UTHealth) Center for Neurobehavioral Research on Addiction (CNRA), CNRA became the recruitment site for the remainder of the study. At the time of the study transfer, post-cessation body weight was added as a secondary outcome.
Methods
Study Design and Procedures
Participants (n = 84) were enrolled at MEDVAMC (n = 32) and CNRA (n = 52), two University-affiliated research sites located in Houston, TX between July 2016 and December 2019. Print advertisements were used to recruit participants. Individuals were screened initially for eligibility by phone and, if eligible, completed informed consent at a subsequent in-person assessment visit. Institutional Review Boards affiliated with each of the participating sites approved the protocol.
Eligible participants were males and females between 18 and 75 years of age who smoked for at least one year, currently smoked ≥10 cigarettes per day, and desired to quit smoking. Participants had to have glycosylated hemoglobin levels between 5.7 and 6.4% and/or a body mass index of ≥25 kg/m2. The exclusion criteria were: (1) psychotic or bipolar disorder, or mood disorder with psychotic features (existing diagnosis or as determined by the structured interview); (2) moderate to high risk of suicidality, (3) psychoactive substance abuse or dependence (excluding nicotine dependence) within the past 3 months; (4) personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2; (5) type 1 diabetes mellitus; (6) current use of oral or injectable glucose-lowering medications; (7) severe cardiovascular disease (history of myocardial infarction, life-threatening arrhythmia, or worsening angina pectoris); (8) active temporomandibular joint disease; (9) severe gastrointestinal disease (i.e., severe gastroparesis); (10) previous history of pancreatitis or risk of pancreatitis; (11) creatinine clearance <30; (12) previous medically adverse reaction to study the medication, nicotine, or menthol; and (13) women who were currently pregnant or lactating, or of childbearing potential and were not using medically accepted forms of contraception.
Participants were randomized (1 : 1) via a computer-generated random number sequence, using blocks of four to receive either placebo or exenatide, 2 mg subcutaneously (SC), once a week for 6 weeks. Randomization was performed by the hospital pharmacist (MEDVAMC) or project coordinator (CNRA). Exenatide purchased commercially as Bydureon for SC injection is supplied as a powder with solvent for once-weekly injection. Each single-dose dual-chamber pen contains 0.65 mg of diluent and 2 mg of exenatide, which remain isolated until mixed. Placebo (normal saline) was administered in the same manner and volume as exenatide using insulin syringes. Patients were blindfolded while receiving the injections. Medication preparation and administration were performed by an unblinded research nurse who was not involved in the behavioral counseling, data collection, or outcome measurement. The care providers, data collectors, outcome assessors, and the statistician performing the analysis remained blinded to the participants’ assigned conditions (exenatide versus placebo) throughout the study.
Participants in both groups received nicotine patches (generic, 21 mg) for daily use during the 6-week treatment period, along with brief weekly individual smoking cessation counseling (10 to 20 minutes) from a trained smoking cessation counselor. Target quit date was set following 2 weeks of treatment, allowing for extended-release exenatide to reach the minimally effective concentration (~50 pg/mL) to reduce fasting plasma glucose levels.16,17 Participants attended weekly clinic visits to complete measures and receive study medication, NRT, and smoking cessation counseling. Weekly measures included assessment of cigarettes smoked per day, using timeline follow-back procedures,18 breath carbon monoxide (CO) levels (assessed by breath CO monitor, Micro+ Smokerlyzer, Williamsburg, VA), craving for cigarettes, and withdrawal symptoms. Craving for cigarettes was assessed using the Questionnaire of Smoking Urges (QSU),19 a 10-item scale (total score range = 10–70) that evaluates the intention and desire to smoke and anticipation of relief from withdrawal-associated negative affect. Withdrawal symptoms were assessed using the Wisconsin Scale of Withdrawal Symptoms (WSWS),20 a 28-item questionnaire that evaluates different aspects of the smoking withdrawal syndrome, including anger, anxiety, concentration, craving, hunger, sadness, and sleep (total score range = 0–112). Post-cessation body weight was added as a secondary outcome of interest when enrollment moved to the second site and was collected weekly on all CNRA participants (n = 52) using a digital medical scale (SECA 644, seca GmbH & Co, Hamburg, Germany). For descriptive purposes, Positive and Negative Affect Schedule (PANAS)21 and the Patient Health Questionnaire (PHQ)-822 were also administered weekly.
To promote retention, compensation for attending weekly study visits followed a progressive schedule, starting at $10 (Week 1) and increasing to $50 (Week 6). The total amount of compensation, including the screen and weekly visits, was $160.
Statistical Modeling
Generalized linear modeling (GLM) was used to perform all statistical analyses. GLM is a flexible abstraction of ordinary linear regression that allows for modeling non-normally distributed outcomes (e.g., dichotomous outcomes, as in logistic regression). Specific analyses then accounted for the effect of site and/or baseline levels of a given outcome where necessary by inclusion as a covariate; these are described in greater detail below for abstinence, craving, withdrawal, and post-cessation weight outcomes.
Bayesian Analysis
Bayesian statistical inference was used as a recommended approach for this early-phase pilot study to directly estimate the probability that the alternative hypothesis is true (i.e., exenatide confers benefit on treatment outcomes).23–25 Detailed descriptions of Bayesian inference exist elsewhere; 26 however, in brief, Bayesian inference incorporates a prior distribution of plausible parameter values with observed data to form a posterior distribution. In the present trial, weakly informative priors (b ~ Normal [µ = 0,σ 2 = 1 × 105]; sigma ~Student-t [µ = 0,df = 0,σ 2=1 × 105]) were used to maximize the influence of the present data on the posterior distribution. Assumptions of Bayesian analyses were evaluated via effective sample size, scale convergence factors (i.e., “rhat”), and posterior predictive checking (graphically confirming that the observed data fell entirely within the range of distributions produced by 1,000 replications of the posterior predictive distribution). Assumptions were satisfied for most analyses, save the craving outcome (deviations from the posterior predictive distribution, described below).
Probabilities that parameter estimates exist were quantified as the extent to which the density of the posterior distribution was less or greater than zero; this is hereafter referred to as the posterior probability (PP). Rather than depending on the p-value, as in null hypothesis statistical testing, decision-making regarding the PP relies on establishing a probability threshold of interest concerning the efficacy of the treatment. This threshold is set based on expertise; in the viewpoint of the current investigators, a 3 in 4 chance or greater that the treatment confers benefit (i.e., PP ≥ 75%) would provide evidence in favor of that treatment and would support committing resources to future investigation. Disparate researchers may then establish their own subjective probability threshold when evaluating the results of a Bayesian analysis. The current threshold PP ≥75% was a pre-specified decision threshold indicating sufficient belief in the reliability of estimates given the data and is consistent with thresholds set for decision-making in other medication trials.27,28 Further, this threshold is equivalent to a Bayes factor = 3.0, which has been described elsewhere as “moderate evidence” in favor of the alternative hypothesis.29
Sample Size and Power Considerations
The current pilot study was designed to attain the largest sample size possible given the fiscal and temporal constraints of the funding mechanism. Although Bayesian methods do not conceive of statistical power in the same fashion as frequentist inference, the methodology may provide probabilistic estimates of the relative effect of the treatment as compared to placebo even in the context of small sample sizes.30 Power consideration for a larger trial will be based upon the outcomes of this investigation.
Abstinence
Seven-day point prevalence abstinence (defined as no smoking, not even a puff, in the preceding 7 days), was assessed at Week 6 (end of treatment) via self-report and confirmed via breath CO measurement of ≤5 parts per million (ppm). We used generalized linear modeling (GLM) to model 7-day point prevalence abstinence as a function of treatment condition, adjusted for site, via the binomial distribution with a log link function. Point estimates and 95% credible intervals (CrI) were estimated from the posterior distribution and exponentiated to provide risk ratios (RRs). While the frequentist 95% confidence interval does not permit estimation of relative probabilities that values within the 95% range represent the true value (i.e., a value in the middle of the confidence interval cannot be distinguished from another value in either extreme in terms of the relative probability that it represents the true parameter), the Bayesian 95% CrI permits just this. Because Bayesian posterior distributions are by definition probability densities, the relative probability that one estimate in the CrI is more or less likely than another can be ascertained from the differential heights. Hence, even if the Bayesian 95% CrI includes the null value stipulated by the research question, one can still estimate that the true parameter surpasses the null value.
Craving, Withdrawal, and Post-Cessation Body Weight
End-of-treatment craving and withdrawal were modeled as a function of treatment condition with adjustment for the baseline level of each outcome (via inclusion as a covariate). Craving and withdrawal were adjusted for site and fit as truncated processes (i.e., values constrained to the minimum and maximum possible values of each total score) via the Student-t distribution. Follow-up analyses for craving and withdrawal evaluated effects by abstinence subgroup (abstainers vs. non-abstainers). The model for craving within abstainers would not converge under the primary specification (priors and distributional family); this model was respecified to use more conservative priors (i.e., b ~ Normal [µ = 0,σ 2 = 25]) and fit via the Gaussian distribution. This respecification provided a better fit; however, the posterior predictive check maintained some deviance from the observed distribution of the data whereby values around the median were slightly under-represented (QSU total scores 10 to 20) and over-represented some of the higher values (QSU total scores 30 to 40). Model fit for the rest of the observed distribution was adequately represented by the posterior predictive check, and no other models demonstrated a problematic fit of this nature. Post-cessation body weight was modeled via the lognormal distribution, and the resulting parameter estimates were exponentiated to provide percentage change in the outcome. Data regarding weight were only collected at one site; as such, analyses for this outcome did not require covariate adjustment for the site. Analyses were conducted in the R Statistical Computing Environment31 via packages brms32 and rstan.33 Before scoring, missing items for the craving and withdrawal measures at baseline (<1% of responses) were imputed using the bagged trees approach (bagImpute method in R package caret).34 Retention was evaluated via Bayesian Cox proportional hazards regression in SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Enrollment and Randomization
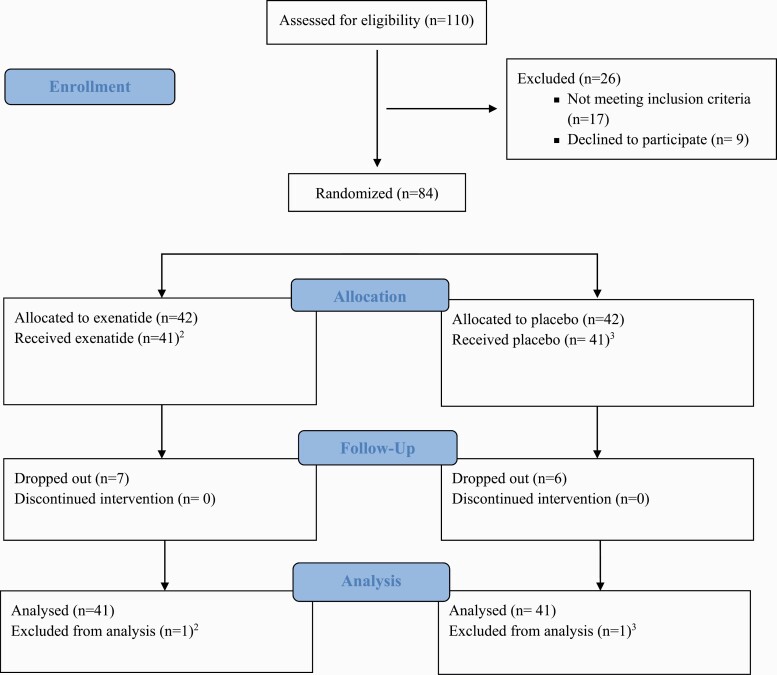
As shown in Figure 1, a total of 84 eligible participants were enrolled and randomized. Two individuals did not receive any medication (Figure 1, footnotes); thus, we utilized a modified intent-to-treat approach, with an analyzable sample size of 82. In this study, the modified intention-to-treat population was defined as a subset of the intention-to-treat population excluding randomized subjects who were deemed ineligible post-randomization and/or never started treatment. There were no differences in any of the baseline characteristics between the two sites (i.e., MEDVAMC and CNRA). As shown in Table 1, the two treatment groups had similar sociodemographic and smoking characteristics. The majority (92%) reported at least one previous quit attempt. Almost half (45%) of the sample reported living with other tobacco users.
Figure 1.
CONSORT Flow Diagram1. 1Consolidated Standards of Reporting Trials diagram of the progress through the phases (enrollment, intervention allocation, follow-up, and data analysis) of a parallel group, two-arm, double-blind, randomized, controlled clinical trial in prediabetic and/or overweight treatment seeking smokers randomized to receive once-weekly placebo or exenatide, 2 mg, subcutaneously. 2Participant was on the way to research clinic for visit 1 (randomization and first dosing) but had family emergency and was forced to cancel the visit. She did not receive the study treatment. 3Participant reported to research clinic for visit 1 (randomization and first dosing), however, he stated that he had been trying to quit smoking on his own and had not smoked at all during the preceding 5 days (breath CO was 2 ppm). The participant was discharged; he did not receive the study treatment.
Table 1.
Socio-demographic characteristics and tobacco use history (n = 82)
| Characteristic | Total Sample (n = 82) | Exenatide (n = 41) | Placebo (n = 41) |
|---|---|---|---|
| Age, years ± SD | 51.1 ± 9.2 | 51.0 ± 9.1 | 51.2 ± 9.4 |
| Sex, n (%) | |||
| Male | 57 (69.5) | 28 (68.3) | 29 (70.7) |
| Female | 25 (30.5) | 13 (31.7) | 12 (29.3) |
| Race/Ethnicity, n (%) | |||
| White | 27 (32.9) | 14 (34.1) | 13 (31.7) |
| Black/African American | 52 (63.4) | 25 (61) | 27 (65.9) |
| Hispanic/Latino | 1 (1.2) | 0 | 1 (2.4) |
| Other | 2 (2.4) | 2 (4.9) | 0 |
| Marital Status, n (%) | |||
| Never Married | 31 (37.8) | 17 (41.5) | 14 (34.1) |
| Divorced/Separated | 37 (45.1) | 17 (41.5) | 20 (48.8) |
| Married | 10 (12.2) | 6 (14.6) | 4 (9.8) |
| Widowed | 4 (4.9) | 1 (2.4) | 3 (7.3) |
| Highest Level of Education, n (%) | |||
| 8th Grade or Less | 3 (3.7) | 1 (2.4) | 2 (4.9) |
| High School | 51 (62.2) | 27 (65.9) | 24 (58.5) |
| College Degree | 23 (28) | 11 (26.8) | 12 (29.3) |
| Graduate Degree | 5 (6.1) | 2 (4.9) | 3 (7.3) |
| Household Income, n (%) | |||
| <$19,999 | 49 (59.8) | 23 (56.1) | 26 (63.4) |
| $20,000–39,999 | 22 (26.8) | 12 (29.3) | 10 (24.4) |
| $40,000–59,999 | 4 (4.9) | 2 (4.9) | 2 (4.9) |
| $60,000–79,999 | 4 (4.9) | 4 (9.8) | 0 |
| $80,000–99,999 | 1 (1.2) | 0 | 1 (2.4) |
| >$100,000 | 1 (1.2) | 0 | 1 (2.4) |
| Not reported | 1 (1.2) | 0 | 1 (2.4) |
| Cigarettes smoked per day, mean ± SD | 18.4 ± 8.9 | 18.5 ± 8.7 | 18.4 ± 9.3 |
| Years of regular smoking, mean ± SD | 27.0 ± 11.8 | 27.4 ± 11 | 26.6 ± 12.7 |
| FTND, mean ± SD | 6.2 ± 2.2 | 6.3 ± 1.9 | 6 ± 2.5 |
| Positive and Negative Affect (PANAS), mean ± SD | |||
| Positive Affect | 34.2 ± 10 | 35.9 ± 10.1 | 32.5 ± 9.8 |
| Negative Affect | 19.3 ± 7.8 | 18.6 ± 7.6 | 20.1 ± 8.0 |
| Depressive Symptoms (PHQ-8), mean ± SD | 8.5 ± 6.1 | 8.3 ± 6.4 | 8.6 ± 5.9 |
Treatment Retention
The percentage of participants who completed the trial was 83% (34/41) in the exenatide group and 85% (35/41) in the placebo group. Bayesian Cox proportional hazards regression (HR) did not support differential time to dropout across groups (HR = 1.19 95% CrI [0.38, 3.59], PP = 62.0%).
Seven-Day Point Prevalence Abstinence
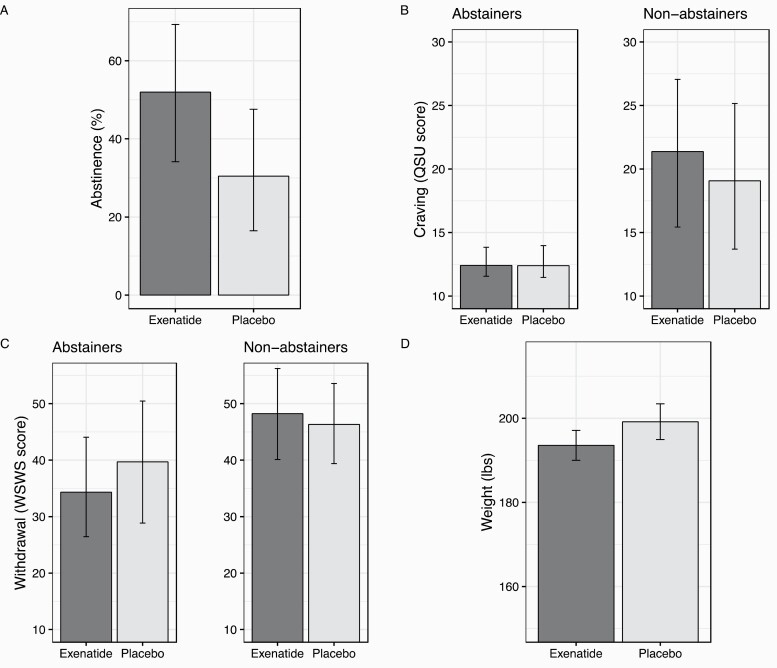
The raw proportion of participants who achieved abstinence at end-of-treatment was 46.3% (19/41) for exenatide and 26.8% (11/41) for placebo. Conditional on the effect of site, Bayesian GLM found that exenatide (relative to placebo) demonstrated a high posterior probability (PP = 96.5%) of a greater risk of abstinence (RR = 1.70; 95% CrI [0.96, 3.27]). Exenatide yielded 21.5% higher abstinence than placebo when adjusting for site (exenatide: 51.95% [34.14%, 69.30%]; placebo: 30.43% [16.47%, 47.59%] (Figure 2A).
Figure 2.
Bar graphs depicting means by treatment condition for the study outcomes. QSU = Questionnaire of Smoking Urges; WSWS = Wisconsin Scale of Withdrawal Symptoms. Number of participants: A (n = 82), B (Abstainers n = 30; Non-abstainers n = 39), C (Abstainers n = 30; Non-abstainers n = 39), D (n = 21). Figure 2A depicts 7-day point prevalence abstinence. Figures 2B and 2C depict end-of-treatment craving (QSU) and withdrawal (WSWS), controlling for baseline scores. Figure 2D depicts post-cessation weight, controlled for baseline weight among participants who were abstinent from smoking at the end of treatment. Error bars depict lower and upper bounds of the uncertainty level of the outcome.
Craving
QSU scores decreased more among exenatide-treated participants compared to placebo (b = −1.25; 95% CrI [−4.34, 2.26], PP = 79.7%). Conditional on the effect of site and baseline scores, exenatide yielded 1.1 points lower end-of-treatment QSU total score than placebo (exenatide: 13.6 [11.7,16.8]; placebo: 14.7 [12.7,17.3]). Follow-up models within abstinence subgroups found that although exenatide did not affect end-of-treatment craving among abstainers (b = 0.21, 95% CrI [−7.86, 8.83], PP = 51.9%), it did increase craving among non-abstinent participants (b = 4.71, 95% CrI [−11.39, 18.61], PP = 75.6%) (Figure 2B). As noted, models within abstinence subgroups had posterior predictive checks that were problematic (even with more constrained priors); some values in the distribution were poorly represented (QSU total scores 10–20 were slightly under-represented in the model, and scores 30–40 were slightly over-represented). Subsequent attempts to respecify the model did not improve model fit.
Withdrawal
Across all participants, WSWS scores did not reveal differential change over time between groups (PP = 67.1%). Follow-up models within abstinence subgroups found that exenatide lowered withdrawal among abstainers (b =−5.93, 95% CrI [−16.22, 6.69], PP = 84.9%) but not among those that were not abstinent (b = 1.96, 95% CrI [−6.50, 10.54], PP = 67.9%) (Figure 2C).
Post-Cessation Weight
Baseline body weights for each group were M = 218.9 lbs (SD = 48.0) in placebo and M = 184.9 lbs (SD = 40.2) in exenatide. Raw values indicated a 2.96-lb increase in placebo and a 0.49-lb decrease in the exenatide group from baseline to 6 weeks of treatment. Exenatide reduced post-cessation weight more than placebo (−2.81%, 95% CrI [−5.60%, +0.04%]) after adjusting for baseline (PP = 97.4%). Conditional on baseline weight, end-of-treatment weight was 5.6 pounds lower for the exenatide group (193.6, 95% CrI [190.0, 197.1]) than the placebo group (199.2, 95% CrI [194.9, 203.4]) (Figure 2D).
Safety
Adverse events (AEs) were reported in 4 (9.5%) participants in the exenatide group, and 1 (2.3%) participant in the placebo group. There was no difference in the percentage of AEs noted between the groups (estimated using risk difference RD = 0.073, 95% CI [−0.03, 0.18], p = 0.16). All AEs were treatment-related but mild in severity, and there were no AE-related discontinuations. In the exenatide group, the AEs reported were injection site nodules. The nodules were <5 mm in diameter; there was no accompanying skin discoloration or any signs and/or symptoms of infection. The nodules resolved within 1–2 weeks. In the placebo group, one participant experienced localized pruritus (without rash) at the site of the nicotine patch application. Pruritus resolved after reminding the participant to alternate patch application sites. There were no reports of hypoglycemia, injection site pruritus, severe injection site reactions, nausea, vomiting, dyspepsia, diarrhea, constipation, dizziness, headache, rapid heartbeat, or any other AEs in either group.
Discussion
We found that treatment with exenatide, as an adjunct to the nicotine patch, improved abstinence rates, decreased craving and withdrawal symptoms, and mitigated post-cessation weight gain compared to treatment with nicotine patch alone. Participants who received exenatide demonstrated a 19.5% higher rate of abstinence and 5.6 lbs. lower weight following 6 weeks of exenatide treatment compared to those who received placebo. Participants who received exenatide had lower (but not clinically significant) cravings at the end of treatment compared to those who received placebo. End-of-treatment withdrawal symptoms were lower among abstinent participants who received exenatide than among abstinent participants who received placebo. Adverse events were mild in severity and did not result in treatment discontinuation.
Posterior probabilities favoring exenatide over placebo for abstinence and post-cessation weight were 96.5% and 97.4%, respectively. These probabilities demonstrate that exenatide plus NRT confers a greater than 9 in 10 chance of producing abstinence and lower post-cessation weight gain compared to NRT alone. While several adjunctive or alternative therapies to NRT may provide comparable or superior improvements in abstinence rates, exenatide’s improvements in post-cessation weight provide a novel benefit for treatment-seeking smokers. These effects are compelling, given the favorable tolerability profile of this medication. Further, these effects occurred in overweight/prediabetic individuals for whom post-cessation weight gain may be of particular concern.35
Our data, which show the effect of exenatide on abstinence from smoking, are consistent with the hypothesis that GLP-1R agonists influence the mesolimbic dopamine system and reward-seeking behaviors.9 Centrally, the preproglucagon neurons are located in the nucleus tractus solitarius (NTS) and produce brain-derived GLP-1.7,36,37 In addition to the hypothalamus and NTS, GLP-1Rs are expressed throughout the mesolimbic dopamine system, including in both the ventral tegmental area38 and nucleus accumbens,11,39 brain areas critical for reward regulation.9,40,41 For example, data from rodent studies demonstrate that GLP-1R agonists significantly decrease nicotine intake in self-administration experiments15 and attenuate nicotine conditioned place preference.14 In fact, activation of GLP-1Rs in these areas also reduces the intake of highly-palatable foods in rodents,42 suggesting that GLP-1 regulates reward through the mesolimbic dopamine system.9
Most (80–90%) people who quit smoking gain weight. On average, former smokers gain 5–15 pounds within the first few months of quitting, notwithstanding considerable inter-individual variability. Many who quit (13–14%) gain over 20 pounds.43 Post-cessation weight gain increases the incidence of obesity and type 2 diabetes mellitus, precipitates relapse, and is one of the most frequently cited barriers to smoking cessation.43 Thus, limiting post-cessation weight gain has significant public health implications because it should encourage more smokers to make a quit attempt, help quitters to maintain abstinence, and mitigate weight-related medical conditions. A 2009 Cochrane systematic review44 reported that some treatments (dexfenfluramine, phenylpropanolamine, naltrexone) resulted in a significant reduction of weight gain at the end of treatment; however, no pharmacological intervention significantly affected smoking cessation rates or provided sustained effects on post-cessation weight gain at 6 or 12 months. Naltrexone with NRT has been studied for smoking cessation and post-cessation weight gain, showing reductions in smoking among men and reductions of cessation-related weight gain among women.45 The impact of first-line smoking cessation treatments (NRT, bupropion, varenicline) on post-cessation weight gain is modest, with effects disappearing after treatment discontinuation.44 Although the sample size and design of the current pilot study did not allow us to examine potential sex differences or long-term effects on either abstinence rates or post-cessation weight, the results support further investigation of extended-release exenatide, including continuation for months rather than weeks, as a medication to uniquely target both smoking cessation and post-cessation weight gain. Combining exenatide with a more potent smoking cessation treatment may yield a “package” that confers maximum benefit for facilitating smoking cessation while minimizing post-cessation weight gain.
A strength of the current study includes examining a GLP-1R agonist as a novel treatment for facilitating smoking cessation with the potential of attenuating post-cessation weight gain. Although added after study initiation, weight assessments were conducted on all participants (n = 52) enrolled at one of the two participating sites, which decreases the possibility of selection bias. We also used extended-release exenatide, a once-weekly formulation administered during clinic visits, which assured 100% treatment adherence.
However, there were also several limitations. First, the duration of treatment was relatively short (6 weeks). Given that most of the weight gain occurs during the first three to six months of abstinence,46 the impact of exenatide on reducing post-cessation weight gain should be examined following a longer treatment period. Second, the sample size was relatively small and primarily Black (63%) males (70%). These findings should be replicated with a more diverse sample to increase the generalizability of the results to females and persons of other races/ethnicities. Third, examining the potential mechanisms underlying the effects of exenatide on smoking and weight was beyond the scope of this early-phase project. Given that exenatide is likely to impact post-cessation weight via attenuating the reinforcing effects of nicotine and food, future studies should include neurobehavioral assessments to explore potential mechanisms by which exenatide affects these outcomes. Finally, the models for the craving outcome within groups demonstrated small deviations from the posterior predictive distribution; as such, model inferences may be correspondingly less accurate for individuals with total scores in the over-and under-represented ranges of the observed data.
Our sample was limited to individuals with prediabetes and/or overweight. The latter can be viewed as a strength but also as a limitation of the current study. We focused on this population because smokers with higher baseline weight and metabolic abnormalities are at higher risk of worsening metabolic control after smoking cessation.35 Identifying therapeutic approaches to facilitate smoking cessation in this vulnerable subgroup of smokers has substantial individual and public health implications. At the same time, the focus on prediabetic and overweight persons limits the generalizability of the study findings. Future studies should consider including other subgroups of smokers, such as persons with type 2 diabetes, given that exenatide is an indicated treatment for this disorder and could thus be used as a therapeutic approach for controlling blood glucose, facilitating smoking cessation, and mitigating post-cessation weight gain in this at-risk population.35
In summary, we found that extended-release exenatide improved abstinence and decreased post-cessation weight gain in a sample of treatment-seeking prediabetic and/or overweight smokers. Despite the limitations, this study provides initial evidence that GLP-1R agonist therapy, used as an adjunct to NRT, holds the potential for improving both smoking and post-cessation weight outcomes. The findings of this study contribute to the critical line of research aimed to identify practical approaches for reducing post-cessation weight gain, a significant obstacle to successful smoking cessation, and a risk factor for adverse weight-related health outcomes.
Supplementary Material
Acknowledgments
Part of this work was conducted at, and supported by the Michael E. DeBakey VA Medical Center, Houston, TX.
Funding
Funding for this study has been provided by The University of Texas Health Science Center at Houston Center for Clinical and Translational Sciences (LY) and PARTNERS Research Awards (LY). These sources of support had no role in the study design, collection, management, analysis, and interpretation of the data, the preparation of the materials, and/or the ideas presented in the manuscript.
Study Registration
The study was registered on clinicaltrials.gov (ID: NCT 02975297). The study protocol was previously published.47
Declaration of Interests
The authors have no competing interests to declare.
References:
- 1. (CDC) CfDCaP. Smoking Cessation: A Report of the Surgeon General.https://www.cdc.gov/tobacco/data_statistics/sgr/2020-smoking-cessation/?s_cid=OSH_misc_m180. Accessed July 1, 2020.
- 2. Mills EJ, Wu P, Lockhart I, Thorlund K, Puhan M, Ebbert JO. Comparisons of high-dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: A systematic review and multiple treatment meta-analysis. Ann Med. 2012;44(6):588–597. [DOI] [PubMed] [Google Scholar]
- 3. Lindson N, Chepkin SC, Ye W, Fanshawe TR, Bullen C, Hartmann-Boyce J. Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2019;4(4):CD013308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stapleton J, West R, Hajek P, et al. Randomized trial of nicotine replacement therapy (NRT), bupropion and NRT plus bupropion for smoking cessation: Effectiveness in clinical practice. Addiction. 2013;108(12):2193–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang PH, Chiang CH, Ho WC, Wu PZ, Tsai JS, Guo FR. Combination therapy of varenicline with nicotine replacement therapy is better than varenicline alone: A systematic review and meta-analysis of randomized controlled trials. BMC Public Health. 2015;15:689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reimann F. Molecular mechanisms underlying nutrient detection by incretin-secreting cells. Int Dairy J. 2010;20(4):236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jin SL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund PK. Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: An immunocytochemical study. J Comp Neurol. 1988;271(4):519–532. [DOI] [PubMed] [Google Scholar]
- 8. Matsuyama T, Komatsu R, Namba M, Watanabe N, Itoh H, Tarui S. Glucagon-like peptide-1 (7-36 amide): A potent glucagonostatic and insulinotropic hormone. Diabetes Res Clin Pract. 1988;5(4):281–284. [DOI] [PubMed] [Google Scholar]
- 9. Skibicka KP. The central GLP-1: Implications for food and drug reward. Front Neurosci. 2013;7:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Näslund E, Schmidt PT, Hellström PM. Gut peptide hormones: Importance for food intake. Scand J Gastroenterol. 2005;40(3):250–258. [DOI] [PubMed] [Google Scholar]
- 11. Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153(2):647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shirazi RH, Dickson SL, Skibicka KP. Gut peptide GLP-1 and its analogue, Exendin-4, decrease alcohol intake and reward. PLoS One. 2013;8(4):e61965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Egecioglu E, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS One. 2013;8(7):e69010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Egecioglu E, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue Exendin-4 attenuates the nicotine-induced locomotor stimulation, accumbal dopamine release, conditioned place preference as well as the expression of locomotor sensitization in mice. PLoS One. 2013;8(10):e77284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tuesta LM, Chen Z, Duncan A, et al. GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nat Neurosci. 2017;20(5):708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fineman M, Flanagan S, Taylor K, et al. Pharmacokinetics and pharmacodynamics of exenatide extended-release after single and multiple dosing. Clin Pharmacokinet. 2011;50(1):65–74. [DOI] [PubMed] [Google Scholar]
- 17. Parkes DG, Mace KF, Trautmann ME. Discovery and development of exenatide: The first antidiabetic agent to leverage the multiple benefits of the incretin hormone, GLP-1. Expert Opin Drug Discov. 2013;8(2):219–244. [DOI] [PubMed] [Google Scholar]
- 18. Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12(2):101–112. [Google Scholar]
- 19. Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. [DOI] [PubMed] [Google Scholar]
- 20. Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol. 1999;7(4):354–361. [DOI] [PubMed] [Google Scholar]
- 21. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. [DOI] [PubMed] [Google Scholar]
- 22. Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1– 3):163–173. [DOI] [PubMed] [Google Scholar]
- 23. O’Neill RT. FDA’s critical path initiative: A perspective on contributions of biostatistics. Biom J. 2006;48(4):559–564. [DOI] [PubMed] [Google Scholar]
- 24. Woodcock J. FDA introductory comments: Clinical studies design and evaluation issues. Clin Trials. 2005;2(4):273–275. [DOI] [PubMed] [Google Scholar]
- 25. Temple R. How FDA currently makes decisions on clinical studies. Clin Trials. 2005;2(4):276–81; discussion 364. [DOI] [PubMed] [Google Scholar]
- 26. McElreath R. Statistical rethinking: A Bayesian course with examples in R and Stan. Chapman & Hall/CRC; 2018. [Google Scholar]
- 27. Schmitz JM, Green CE, Hasan KM, et al. PPAR-gamma agonist pioglitazone modifies craving intensity and brain white matter integrity in patients with primary cocaine use disorder: A double-blind randomized controlled pilot trial. Addiction. 2017;112(10):1861–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cinciripini PM, Green CE, Robinson JD, et al. Benefits of varenicline vs. bupropion for smoking cessation: A Bayesian analysis of the interaction of reward sensitivity and treatment. Psychopharmacology. 2017;234(11):1769–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee MD, Wagenmakers EJ.. Bayesian Cognitive Modeling: A Practical Course. Cambridge: Cambridge University Press; 2014. [Google Scholar]
- 30. van de Schoot R, Broere JJ, Perryck KH, Zondervan-Zwijnenburg M, van Loey NE. Analyzing small data sets using Bayesian estimation: The case of posttraumatic stress symptoms following mechanical ventilation in burn survivors. Eur J Psychotraumatol. 2015;6:25216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: : R Foundation for Statistical Computing.2020. https://www.R-project.org/. [Google Scholar]
- 32. Bürkner PC. brms: An R Package for Bayesian Multilevel Models Using Stan. 2017;80(1):28. [Google Scholar]
- 33. Stan Development Team. RStan: The R interface to Stan. R package version 2.26.0. 2020. https://mc-stan.org. [Google Scholar]
- 34. Kuhn M. Building Predictive Models in R Using the caret Package. 2008; 28(5):26. [Google Scholar]
- 35. Yammine L, Kosten TR, Pimenova M, Schmitz JM. Cigarette smoking, type 2 diabetes mellitus, and glucagon-like peptide-1 receptor agonists as a potential treatment for smokers with diabetes: An integrative review. Diabetes Res Clin Pract. 2019;149:78–88. [DOI] [PubMed] [Google Scholar]
- 36. Han VK, Hynes MA, Jin C, Towle AC, Lauder JM, Lund PK. Cellular localization of proglucagon/glucagon-like peptide I messenger RNAs in rat brain. J Neurosci Res. 1986;16(1):97–107. [DOI] [PubMed] [Google Scholar]
- 37. Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77(1):257–270. [DOI] [PubMed] [Google Scholar]
- 38. Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010;1350:18–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011;31(41):14453–14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wise RA, Bozarth MA. Brain reward circuitry: Four circuit elements “wired” in apparent series. Brain Res Bull. 1984;12(2):203–208. [DOI] [PubMed] [Google Scholar]
- 41. Koob GF. Drugs of abuse: Anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13(5):177–184. [DOI] [PubMed] [Google Scholar]
- 42. Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: A new role for mesolimbic GLP-1 receptors. J Neurosci. 2012;32(14):4812–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bush T, Lovejoy JC, Deprey M, Carpenter KM. The effect of tobacco cessation on weight gain, obesity, and diabetes risk. Obesity. 2016;24(9):1834–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Farley AC, Hajek P, Lycett D, Aveyard P. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst Rev. 2012;1:CD006219. [DOI] [PubMed] [Google Scholar]
- 45. King AC, Cao D, O’Malley SS, et al. Effects of naltrexone on smoking cessation outcomes and weight gain in nicotine-dependent men and women. J Clin Psychopharmacol. 2012;32(5):630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: Meta-analysis. BMJ. 2012;345:e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yammine L, Kosten TR, Cinciripini PM, et al. Exenatide once weekly for smoking cessation: Study protocol for a randomized clinical trial. Medicine. 2018;97(2):e9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.