Abstract
The transgender adult population is growing globally, but clinical pharmacology has lagged behind other areas of transgender medicine. Medical care for transgender adults may include long‐term testosterone or estrogen treatment to align secondary sex characteristics with gender identity. Clinicians often use drug–drug interaction data from the general adult population to predict medication disposition or safety among transgender adults. However, this approach does not address the complex pharmacodynamic effects of hormone therapy in transgender adults. In this review, we critically examine sex‐related and gender‐related differences in clinical pharmacology and apply these data to discuss current gaps in transgender medicine.
Transgender adults have a gender identity that differs from their sex assigned at birth 1 (Table 1 ), but clinical pharmacologic data are lacking for this population. Sex and gender influence drug safety and effectiveness in adults. In the general adult population, medication‐related adverse event rates are nearly twofold higher among cisgender (nontransgender) women compared with cisgender men. 2 , 3 Based on a national database of US hospital emergency department data, cisgender women accounted for more than 60% of adverse drug event–related emergency department visits. 4 Sex and gender may also influence medication effectiveness. In an experimental cohort of adults (either healthy or living with coronary artery disease or risk factors), Friede et al. 5 reported lower rates of platelet inhibition among cisgender women randomized to low‐dose and high‐dose oral aspirin compared with cisgender men. Despite this finding, cisgender women had higher plasma concentrations of salicylate, aspirin’s active metabolite, compared with cisgender men. 5 Whether these medication safety and effectiveness outcomes are generalizable to the transgender population has not been investigated.
Table 1.
Terminology
| Term | Definition |
|---|---|
| Sex | Chromosomal, hormonal, or biologic factors associated with a person’s status as male, female, or intersex. Referred to as “sex assigned at birth” to indicate that sex is typically determined based on appearance of external genitalia at birth. 2 |
| Intersex person | Individual born with variations in sex characteristics such as genitalia, gonads, chromosomes, or endogenous hormone production that fall outside of the typical definitions of the binary sex categories of male and female. |
| Gender Identity, or Gender | One’s internal sense of being male, female, both, or neither. There is a spectrum of gender identities, and the concept of gender is a socially constructed category. |
| Transgender person | Individual whose gender identity differs from their sex assigned at birth. This includes trans men, trans women, and nonbinary people. |
| Transgender man, trans man, transmasculine adult | Individual with a male or masculine gender identity who was assigned female at birth. |
| Transgender woman, trans woman, transfeminine adult | Individual with a female or feminine gender identity who was assigned male at birth. |
| Nonbinary person | Individual with a gender identity outside of the binary of man or woman. Nonbinary is an umbrella term to describe genders that are neither male nor female. |
| Cisgender person | Individual whose gender identity aligns with their sex assigned at birth; a person who is not transgender. |
| Gender dysphoria | Discomfort associated with a disconnect between one’s gender identity and primary and/or secondary sex characteristics or gender assigned at birth. Not all transgender people experience gender dysphoria. Though the DSM‐5 uses the term "gender dysphoria," the ICD‐11 uses the term "gender incongruence" to describe this. |
DSM‐5, The Diagnostic and Statistical Manual of Mental Disorders; ICD‐11, International Classification of Diseases, 11th Revision.
Body composition, drug metabolizing enzyme activity, and kidney function may influence sex‐related differences in drug disposition. 6 Medical care for transgender adults may include long‐term testosterone or estrogen treatment to align secondary sex characteristics with gender identity. 7 For transgender adults, these interventions may reduce gender dysphoria, a discomfort associated with a disconnect from one’s primary and/or secondary sex characteristics or sex assigned at birth. Hormone therapy is a cost‐effective medical intervention for transgender adults, 8 and it is associated with improved psychological outcomes and quality of life. 1 , 9 This medical intervention causes marked physiologic and hormonal changes in transgender adults, 10 , 11 but its effect on the disposition of other prescribed medications is poorly understood.
Sex‐related differences affect drug safety and effectiveness in the general adult population. 12 , 13 , 14 , 15 , 16 , 17 However no studies have explored how these differences may influence clinical pharmacology in transgender adults undergoing hormone therapy or gonadectomy. Because clinicians are providing medical care to increasing numbers of transgender patients, 18 this review applies sex‐related and gender‐related differences in clinical pharmacology to transgender health.
A comment on language throughout this manuscript
We use “transgender” as an umbrella term for adults whose gender does not align with the sex they were assigned at birth. This includes transgender men, transgender women, and nonbinary people. Transgender individuals with a binary gender identity (e.g., transgender men, transgender women) may undergo hormone therapy with a goal of masculinization (testosterone treatment) or feminization (estrogen treatment). However, individuals with a nonbinary gender identity also may take hormone therapy without identifying as either a transgender man or transgender woman. To be sensitive to the diversity of individuals who may undergo hormone therapy, we used language that avoids associating hormone therapy with one specific gender identity where possible. When referring to “transgender adults undergoing hormone therapy,” this includes nonbinary adults undergoing hormone therapy. Additional details about terminology in this manuscript are included in Table 1 .
GLOBAL TRANSGENDER ADULT POPULATION
Twenty‐five million people aged 15 years and older worldwide are transgender, 19 and this population is growing. 20 US population‐based estimates suggest 0.7% of adults aged 18–24 years are transgender, compared with 0.6% and 0.5% of adults aged 25–64 and ≥65 years, respectively. 21 The European Network for the Investigation of Gender Incongruence (ENIGI), a multicenter prospective study of transgender adults among European gender clinics, reported adult patients were between 23 and 38 years of age on average when diagnosed with gender incongruence (i.e., incongruence between an individual’s own gender identity and their assigned gender). 22 At one ENIGI clinical site, Vrije Universiteit (VU) University Medical Centre gender clinic in Amsterdam, the Netherlands, Gooren et al. 23 reported nearly 7,000 transgender patients underwent hormone therapy and surgery (gonadectomy) between 1972 and 2015. The majority of patients identified as transgender women (65%, i.e., individuals with a female gender identity who were assigned male at birth) and were on average 25 to 40 years of age when presenting for initial care. Transgender men (i.e., individuals with a male gender identity and were assigned female at birth) comprised 35% of this cohort and were on average 20 to 35 years of age when presenting for care. Approximately 15% of this clinical cohort is currently ≥60 years of age. 23
In the United States, experts anticipate the transgender older adult population will grow over the next thirty years. 24 In a cross‐sectional US survey of more than 2,500 lesbian, gay, bisexual, and transgender older adults (≥50 years of age), Fredriksen‐Goldsen et al. 24 observed 2.2‐higher odds of self‐reported depressive symptomatology (P < 0.001), 5.5‐lower odds of perceived good physical health (P < 0.001), and 1.5‐higher odds of disability (P < 0.05) among transgender respondents compared with cisgender lesbian, gay, or bisexual counterparts. Based on Medicare claims data from US beneficiaries (≥65 years of age), Progovac et al. 25 reported gender minority beneficiaries (identified using International Classification of Diseases (ICD)–9 diagnosis codes associated with transgender‐related health services) had higher use of mental health care, including psychotropic medication use, than other beneficiaries. Psychotropic medication use increased more rapidly over a five‐year period among gender minority beneficiaries compared with other beneficiaries (17.9% to 29.2% vs. 16.5% to 21.7%, respectively, P < 0.0001). 25 Because older transgender adults may present for hormone therapy or gonadectomy, 23 clinicians must be aware of co‐occurring medical conditions experienced by this population and potential drug–drug interactions between chronic medications and hormone therapy.
Although global estimates are limited, US population‐based data suggest the transgender adult population is ethnically and racially diverse. 26 Among 1.4 million transgender adults in the United States, 16% identify as African American or Black people, more than 20% identify as Latino or Hispanic people, and 8% identify as other non‐White, non‐Hispanic races or ethnicities. 26 Age and race are important social determinants influencing the health status of transgender adults, 27 and both modify the strength of the association between sex and drug disposition. 17 For instance, genetic polymorphisms affect the activities of drug‐metabolizing enzymes and contribute to differences in the extent of drug metabolism across racial groups. 15
Nonhormone therapy–related prescription medication use
Few studies have characterized patterns of prescribed medication use among transgender adults. Most data on nonhormone therapy‐related medications focus on topics related to antiretroviral therapy for HIV treatment or prevention within the transgender population. 28 , 29 Metabolic and endocrine disorders, cerebro‐cardiovascular disease, and mental health contribute to the chronic disease burden among transgender adults. 30 Non‐HIV–related chronic disease management, including use of antidiabetic, antihypertensive, and psychotropic medications, remains an important yet understudied topic for this population.
HORMONE THERAPY
Based on findings from the US Transgender Health Survey, a nonprobability survey of ~ 30,000 transgender adults, more than 70% of transgender adults reported ever taking hormone therapy. 31 As part of hormone therapy, clinicians may prescribe either testosterone or estrogen treatment 7 (Table 2 ). The World Professional Association for Transgender Health and other professional organizations endorse individualized hormone regimens, 7 and several sex hormone preparations, administration routes, and doses are available based on patient preference, affordability, and individual drug safety profiles. 32 , 33 Changes in laboratory parameters during hormone therapy are listed in Table 3 . 10 , 34 , 35 , 36 , 37 , 38 , 39
Table 2.
Current hormone therapy regimens for transgender adults
| Regimen | Typical dose range(s) |
|---|---|
| Testosterone treatment | |
| Injectable (short‐acting: cypionate, enanthate; long‐acting: undecanoate) | 50–100 mg weekly (or 100–200 mg every 2 weeks) |
| 1,000 mg every 12 weeks a (or 750 mg every 4 weeks (initial) then every 10 weeks) | |
| Patches, gel | 2.5–7.5 mg/daily (patches); 50–100 mg daily (gel) |
| Estrogen treatment | |
| Oral tablets: micronized estradiol, estradiol valerate a | 1–8 mg daily, total (divided) |
| Estradiol patches, gel a | 25–200 mcg/daily 1–2 times weekly (patch); 1–2 mg daily (gel) |
| Injectable (estradiol valerate or cypionate) | 2–10 mg weekly (or 5–30 mg every 2 weeks) |
| Adjunctive agents | |
| Cyproterone acetate a | 25–50 mg daily |
| Spironolactone tablets | 100–400 mg daily, total (divided) |
| GnRH agonists | 3.75 mg monthly or 11.25 mg every 3 months (leuprolide acetate); 3.6 mg monthly (goserelin acetate) |
Table 3.
Laboratory parameters during first year of hormone therapy in transgender adults
| Laboratory parameter | Testosterone treatment | Cisgender Men | Estrogen treatment | Cisgender Women | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow‐up | Change from baseline | Reference limits | Baseline | Follow‐up | Change from baseline | Reference limits | ||
| Serum estradiol, pg/mL | 45–61 | 29–53 | ↓ 13–42% | 11–43 | 19–29 | 57–258 | ↑ 3–9‐fold | 12–233 | 10, 34, 36 |
| Serum total testosterone, ng/dL | 30–46 | 545–854 | ↑ 14.7–19.7‐fold | 249–836 | 405–567 | 11–42 | ↓ 90–98% | 0–75 | 10, 34, 36 |
| Serum free testosterone, ng/dL | 0.7 | 11.0 | ↑ 15.7‐fold | 32–168 | 11.4 | 0.8 | ↓ 93% | 0.8–5.3 | 36 |
| Serum albumin, g/dL | 4.3–4.6 | 4.1–4.6 | ↔ | 3.5–5.2 | 4.3–4.9 | 4.2–4.6 | ↔ | 3.5–5.2 | 34, 36, 38 |
| SHBG, nmol/L | 52–57 | 25–26 | ↓ 51‐54% | 10–80 | 31–50 | 42–48 | ↔/↑ 1.3‐fold | 20–130 | 10, 34 |
| CBG, mg/dL | 50 | 40 | ↔ | Not available | 46 | 50 | ↔ | Not available | 39 |
| Alpha‐1 acid glycoprotein, mg/mL | No data | No data | |||||||
Laboratory data are reported as the range of mean (or median) values from literature. Change from baseline is the mean (or median) percent or fold change from hormone therapy‐naïve values. Follow‐up was between 4 and 12 months for all studies. Sex hormone preparations, doses, and adjunctive agents varied across cohorts. Cisgender reference intervals were unavailable for all studies; we included reference intervals from Greene et al. 35 for reader interest, although interlaboratory variability may influence comparisons among referenced studies.
BMI, body mass index; CBG, corticosteroid binding globulin; CI, confidence interval; SHBG, sex hormone binding globulin; ↑, increase; ↓, decrease; ↔, no significant change.
Some transgender adults, such as some nonbinary people, may take hormone therapy at low doses or decreased dosing frequency to limit the effects of sex hormones on secondary sex characteristics based on individual goals for their gender expression. 40 Absolute contraindications for hormone therapy are similar to those for cisgender adults and include hormone‐sensitive cancer, pregnancy, or impaired kidney function (for adjunctive spironolactone use, described below). 33 Because hormone therapy is a medically necessary intervention associated with improved quality of life outcomes, 1 experts recommend a harm‐reduction approach for patients who may have risk factors for cardiovascular disease (e.g., tobacco use), which may include prescribing transdermal hormone preparations instead of oral or injectable preparations. 33
Several articles discuss endocrinologic management of transgender patients in detail. 1 , 23 Although beyond the scope of this review, gonadectomy (oophorectomy/hysterectomy or orchiectomy) can be another part of the standard of gender‐affirming medical care. 7 Like hormone therapy, gonadectomy suppresses endogenous sex hormone production. The Endocrine Society and World Professional Association for Transgender Health recommend continuous hormone therapy for at least one year in patients before undergoing gonadectomy. 7 , 32 Medical care, including hormone therapy, for transgender youth or for intersex adults (individuals with variations in sex characteristics outside binary sex categories) will not be addressed in this review.
TESTOSTERONE TREATMENT
Testosterone treatment commonly includes injectable, patch, or gel testosterone preparations 32 , 33 (Table 2 ). Other preparations may include nasal gel or subcutaneous testosterone pellets. Clinicians may adjust testosterone doses to achieve desired therapeutic effects during the first year of treatment. 33 Before initiating testosterone treatment, sex hormone concentrations are generally within reference ranges for premenopausal cisgender females (total testosterone, 0–75 ng/dL; estradiol (follicular phase), 12–233 pg/mL) 35 (Table 3 ). In a prospective clinical cohort of transgender men taking at least 12 months of testosterone treatment in the United States (n = 82), the upper limit of the total testosterone reference range was numerically higher than the cisgender male reference interval (e.g., total testosterone: 199–1149 vs. 249–836 ng/dL, respectively). 35 Individual testosterone concentrations may vary based on the route of hormone administration, hormone dose, and timing of blood sample relative to the last administered hormone dose. Clinicians may adjust testosterone dosing to achieve desired therapeutic effects during the first year of treatment or as needed thereafter.
Aromatase, an enzyme localized in adipose and gonadal tissue, may metabolize exogenous testosterone preparations to estradiol. During testosterone treatment, mean estradiol concentrations decreased among 17 to 53 transgender men in several prospective studies during the first year of testosterone treatment compared with baseline estradiol concentrations (mean percent decreases: 13–42%) 10 , 34 , 36 (Table 3 ). Based on data from 53 healthy cisgender men taking injectable testosterone, estradiol concentrations increased significantly following supraphysiologic intramuscular doses of 300–600 mg weekly compared with testosterone‐naive baseline estradiol concentrations (43.0‐55.7 vs. 19.5‐27.1 pg/mL, respectively, P = 0.0012) but did not change significantly at lower testosterone doses. 41 Because this was more than three times the maximum recommended weekly dose for transgender adults (100 mg weekly injection), 33 statistically significant increases in estradiol concentrations among transgender adults taking testosterone treatment are unlikely.
ESTROGEN TREATMENT
Clinicians prescribe one of several 17β‐estradiol preparations for transgender adults undergoing estrogen treatment 32 , 33 (Table 2 ). The Endocrine Society recommends avoiding synthetic or conjugated equine estrogens due to venous thromboembolism risk and a lack of clinically available assays for these preparations. 32 Before initiating hormone therapy, transgender adults undergoing estrogen treatment generally have serum sex hormone concentrations within cisgender male laboratory reference ranges (total testosterone: 249–836 ng/dL; estradiol: 11–43 pg/mL) 42 (Table 3 ). In a prospective clinical cohort of transgender women taking at least 12 months of estrogen treatment in the United States (n = 93), estradiol reference ranges were numerically higher than reference limits for cisgender women (20.7–505.0 vs. 12–233 pg/mL, respectively). 42 Clinicians may adjust estrogen dosing to achieve desired therapeutic effects during the first year of treatment or as needed thereafter.
Drugs that suppress androgen synthesis and activity
During estrogen treatment, clinicians may prescribe adjunctive medications to suppress endogenous androgen activity 32 , 33 (Table 2 ). Availability of these agents differs by country, 43 and clinicians currently prescribe cyproterone acetate (Europe, Canada, and Australia), spironolactone (United States, Australia), or gonadotropin‐releasing hormone agonists (United Kingdom). 43 , 44 Bicalutamide, a nonsteroidal androgen receptor antagonist, is available in certain settings, although limited data from clinics in Sweden and Norway suggest it is used less frequently than other antiandrogens. 45 Other adjunctive agents such as progestogens (oral medroxyprogesterone, micronized progesterone) or 5‐alpha reductase inhibitors (e.g., finasteride) may also be prescribed in certain settings. Angus et al. 44 discuss mechanisms of androgen suppression for these agents in detail. Despite the widespread use of adjunctive agents, no prospective studies have compared safety or effectiveness among these agents during estrogen treatment.
PHARMACOKINETICS AND PHARMACODYNAMICS
During hormone therapy, high‐dose exogenous sex hormones replace the endogenous sex hormone profile in transgender adults. Clinicians may extrapolate drug–drug interaction data from the general adult population to predict the effect of hormone therapy on other prescribed medications. Transgender adults take pharmacologic doses of testosterone or estrogen, which cause significant physiologic changes and bidirectional changes in sex hormone concentrations. The following sections review sex‐related and gender‐related differences in major drug‐metabolizing and transport proteins, in addition to available sex‐hormone data, to address these complex outcomes and identify potential mechanisms of altered drug disposition in transgender adults. Where available, we also discuss pharmacokinetic data during pregnancy to examine the extent to which physiologic and hormonal changes may influence drug disposition.
ABSORPTION
Cisgender women have slower gastrointestinal transit time and lower gastric acidity than cisgender men. 12 , 46 Although clinical examples are limited, several investigators discuss two compounds that exhibit sex‐related differences in oral absorption and bioavailability: ethanol and salicylate formulations (i.e., aspirin). Ethanol bioavailability is higher in cisgender women than cisgender men. Gastric enzyme activity (e.g., alcohol dehydrogenase), which is lower among cisgender women, contributes to these findings. 15 Age diminishes the strength of this association. 46 In a cohort of more than 100 adults, middle‐aged cisgender women had higher alcohol dehydrogenase activity than cisgender men, but sex‐related differences disappeared in older adults. 46
Aspirin is one of the most commonly used nonsteroidal anti‐inflammatory drugs globally. Small pharmacokinetic studies have reported faster oral absorption or higher oral bioavailability of aspirin and its active salicylate metabolite in cisgender women, although several conflicting studies report no sex‐related differences in aspirin absorption or bioavailability. 14 , 16 In a small clinical study among cisgender adults (n = 8), enteric‐coated aspirin absorption lag time was significantly longer in cisgender women following a meal compared with cisgender men (10.8 vs. 5.0 hours, respectively, P < 0.01). 15 However, experts have not issued sex‐specific guidance for administering drugs on an empty stomach in cisgender women.
Non‐oral drug administration routes may exhibit sex‐related absorption differences, although clinical examples are limited. When aspirin was administered as an intramuscular injection (as its lysine salt), one small study (n = 18) observed slower absorption among cisgender women compared with cisgender men. 16 Investigators suggested higher fat content in cisgender women influences this finding.
DISTRIBUTION
Protein binding
Major plasma binding proteins include albumin, alpha 1‐acid glycoprotein, and globulins. Changes in plasma drug binding can either increase or decrease free drug exposure. Protein‐binding interactions contribute to clinically significant changes in free drug exposure for non‐oral, high extraction ratio drugs (e.g., intravenous lidocaine). 15 Most medications are not susceptible to clinically significant protein‐binding interactions. 47 Clinical examples of sex‐related differences in protein‐binding interactions are lacking. 14
Although no studies have characterized changes in tissue or plasma drug binding for any medication taken by transgender adults, several have summarized changes in plasma protein concentrations during hormone therapy (Table 3 ). In two small studies of 17 and 30 transgender men undergoing testosterone treatment, serum albumin concentrations were unchanged relative to testosterone‐naïve baseline concentrations. 34 , 37 Conversely, corticosteroid‐binding globulin concentrations decreased in a small study of 15 transgender men taking testosterone therapy (20%, P < 0.01). 39 Sex hormone binding globulin concentrations decreased in several prospective studies of between 10 to 53 transgender men taking testosterone therapy (42–54%, respectively, P < 0.01). 10 , 39 , 48 , 49 , 50 , 51
During estrogen treatment, a prospective study of 29 transgender women reported a slight decrease in serum albumin concentrations (4%, P < 0.01 vs. estrogen‐naïve baseline concentrations), 37 although a separate study of 15 transgender women taking transdermal estradiol reported no significant change (P = 0.12). 38 In several prospective studies of up to ~ 50 transgender women, concentrations of sex hormone binding globulin increased threefold during either oral or injectable estradiol treatment (P < 0.001), 10 , 52 , 53 , 54 whereas investigators observed no change in individuals taking transdermal gel or patch preparations. 10 , 55 Finally, in a small study of 10 transgender women, corticosteroid‐binding globulin concentrations were similar before and during estrogen treatment, although most participants took non‐oral 17β‐estradiol preparations. 39 Drug protein binding data in transgender adults may be important for pharmacokinetic modeling, 47 but clinically significant protein‐mediated drug interactions during hormone therapy may be unlikely.
Body composition
Body composition underpins sex‐related and gender‐related differences in drug disposition; 17 however, the clinical impact of these differences is modest, 16 not requiring sex‐specific dose modifications beyond mere weight adjustments for certain medications (e.g., low‐molecular‐weight heparins). Cisgender women typically have a higher percentage of total body fat than cisgender men, increasing the volume of distribution of lipophilic drugs (e.g., benzodiazepines). 56 Conversely, lean body mass is on average higher in cisgender men, 13 increasing the volume of distribution of hydrophilic medications like beta‐adrenergic blocking agents atenolol or sotalol.
Hormone therapy alters body composition 11 , 57 , 58 , 59 (Figure 1 ). However, no studies have characterized the effect of compositional changes on drug disposition in transgender adults. In a meta‐analysis of 10 studies, Klaver et al. 11 reported testosterone treatment significantly altered body composition within 12 months of initiation, increasing lean body mass and decreasing total body fat in transgender men (both P < 0.01 vs. testosterone‐naïve baseline). In small cohorts of 10 and 17 transgender adults undergoing testosterone treatment, regional fat was redistributed, as characterized by decreased subcutaneous abdominal fat and increased visceral fat area (both P < 0.05 vs. testosterone‐naïve baseline). 58 , 59 This is consistent with the higher visceral fat area observed in cisgender men than cisgender women. In a systematic review of 13 studies among transgender men, body mass index significantly increased during testosterone treatment compared with hormone‐naive baseline (P < 0.05). 57
Figure 1.
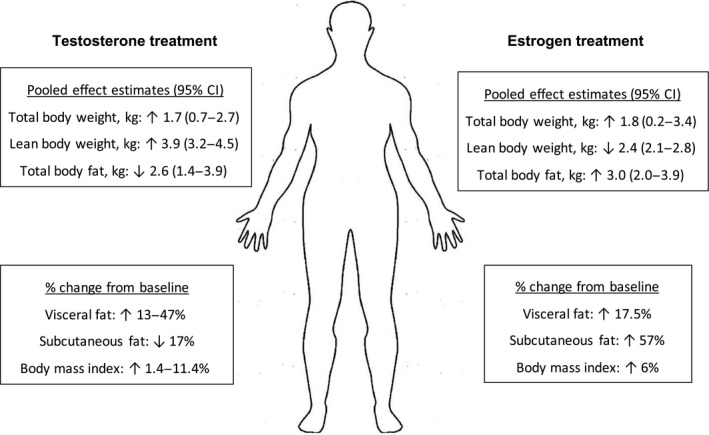
Reported changes in body composition parameters during the first year of testosterone or estrogen treatment in transgender adults. 11 , 57 , 58 , 59 , 60 CI, confidence interval.
During estrogen treatment, Klaver et al. 11 reported increased total body fat in transgender women (P < 0.05 vs. estrogen‐naïve baseline) and decreased lean body mass (P < 0.01). In two small prospective clinical studies of 20 and 28 transgender women, visceral and abdominal subcutaneous fat increased (both P = 0.01 vs. estrogen‐naïve baseline) and body mass index increased after initiating estrogen treatment. 59 , 60
Most published clinical studies include transgender participants from European gender identity clinics. Generalizability of these findings to other geographic locations is unclear. For instance, based on US population‐based survey data, nearly 75% of transgender respondents (n = 691 total) reported being overweight or obese and had twofold higher odds (age‐adjusted) of being overweight compared with cisgender respondents. 61 Not all respondents underwent hormone therapy, and it is unknown whether baseline body weight may influence the extent of hormone therapy–mediated body composition changes. Although hormone therapy may modify body composition parameters toward those measured in the opposite sex, clinically significant changes in drug distribution based on this factor alone are unlikely. However, retrospective analyses that disaggregate specific hormone regimens among participants (administration route, dose, and adjunctive agent) are needed to characterize the extent of body composition changes during hormone therapy in geographically diverse transgender adult populations.
METABOLISM
No studies have characterized cytochrome P450 (CYP) activity using validated probe substrates in transgender adults. In the general adult population, model CYP substrates (described below) reveal potential sex‐related differences in CYP metabolic activity, although conflicting data exist for many CYPs. The following examples are limited to clinical studies using model CYP substrates in the general adult population. In addition to sex‐related and gender‐related data, this section includes pregnancy‐related data, as hormonal and physiologic parameters change markedly from nonpregnant values and are associated with altered drug disposition. 62 Predicted changes in the drug‐metabolizing enzymes and transport protein activities during hormone therapy are summarized in Table 4 .
Table 4.
Predicted changes in major drug‐metabolizing / transport protein activities during hormone therapy
| Enzyme / transporter protein | Testosterone treatment | Estrogen treatment | Substrates (examples of medications potentially taken by transgender adults) |
|---|---|---|---|
| CYP1A2 | ? | ↓ | Duloxetine, clozapine, mirtazapine, olanzapine, ondansetron, theophylline |
| CYP2B6 | ↔ | ↔ | Bupropion, efavirenz |
| CYP2C9 | ↔ | ↔ | Celecoxib, diclofenac, ibuprofen, naproxen, glyburide, phenytoin, warfarin |
| CYP2C19 | ? | ↓ | Citalopram, escitalopram, sertraline, diazepam, omeprazole, pantoprazole |
| CYP2D6 | ? | ↔ | Citalopram, duloxetine, fluoxetine, paroxetine, metoprolol, dextromethorphan |
| CYP3A4 | ? | ↔/↑ | Protease inhibitors, midazolam, repaglinide |
| UGT1A1 | ? | ↑ | Lorazepam, oxazepam, bictegravir, cabotegravir, dolutegravir, elvitegravir, raltegravir |
| UGT1A4 | ? | ↑ | Lamotrigine |
| P‐glycoprotein | ? | ↑ | Atazanavir, darunavir, ritonavir, bictegravir, dolutegravir, verapamil, dabigatran etexilate |
CYP, cytochrome P450; NSAIDs, nonsteroidal anti‐inflammatory drugs; UGT, uridine 5'‐diphospho‐glucuronosyltransferase; ↑, increase; ↓, decrease; ↔, no significant change; ?, unknown.
CYP1A2
CYP1A2 metabolizes several important drugs, including caffeine, ondansetron, and olanzapine. Limited data suggest CYP1A2 activity is lower in cisgender women than cisgender men in studies using caffeine as a model CYP1A2 probe substrate. 14 Similarly, pregnancy decreases apparent CYP1A2 activity as determined by caffeine (up to 65%). 62 Sex hormones (estrogen replacement therapy and oral contraceptive pills) inhibit CYP1A2 activity in the general population. 63
Transgender adults may take several important medications metabolized by CYP1A2, including psychotropic medications (e.g., duloxetine and olanzapine). 25 Estrogen treatment may inhibit CYP1A2 activity. The effect of testosterone treatment on CYP1A2 activity is unclear. Lifestyle factors may influence CYP1A2 activity in transgender adults, independent of any hormonal influence. For instance, cigarette smoking induces CYP1A2 activity. 64 , 65 Transgender adults may have higher use of tobacco‐containing products than the general adult population, although data are conflicting. 66 , 67 In a United States–based national probability sample, Wheldon et al. 66 observed no significant difference in tobacco or cigarette use among transgender men, transgender women, or nonbinary adults. 66 However, data from the US Transgender Health Survey reported transgender men had 1.3 times higher odds of smoking cigarettes than transgender women (95% confidence interval, 1.2–1.5; P < 0.001). 67
CYP2B6
Clinical examples of sex‐related differences in CYP2B6 activity are limited. The bupropion metabolic ratio, a validated CYP2B6 biomarker, exhibited no difference between sexes in a study among cisgender men and cisgender women. 68 However, sex hormones (hormone replacement therapy and combined oral contraceptives) inhibited bupropion hydroxylation in a small clinical study of 12 cisgender women. 69
In addition to bupropion, transgender adults may take other medications metabolized by CYP2B6, including the antiretroviral medication efavirenz. In a prospective, single‐arm study among 20 Thai transgender women living with HIV and initiating efavirenz‐based antiretroviral therapy, Hiransuthikul et al. 70 reported a slight decrease in the plasma efavirenz concentration at 24 hours during estrogen treatment vs. without estrogen (geometric mean ratio, 90% confidence interval, 0.91, 0.85–0.97; P = 0.02). No other efavirenz exposure parameters (area under the plasma‐concentration time curve over 0–24 hours, maximum plasma concentration, time to maximum plasma concentration) changed significantly during estrogen treatment. 70 It is unclear whether testosterone treatment exerts an independent effect on CYP2B6 activity in transgender adults. Based on limited sex‐related clinical data in cisgender adults, we suspect clinically significant changes in CYP2B6 activity are unlikely among transgender adults undergoing hormone therapy.
CYP2C9 AND 2C19
Apparent CYP2C9 activity is similar between cisgender men and cisgender women, whereas data for CYP2C19 activity are conflicting. 15 CYP2C19 is polymorphic, and several factors contribute to interindividual variability in its metabolic activity in adults, including race/ethnicity, and concomitant medications like combined oral contraceptives. 15 Pregnancy may decrease CYP2C19 activity compared with post partum, although available data are conflicting. 62 Combined oral contraceptives may inhibit CYP2C19 activity, as cisgender women taking combined oral contraceptives had lower omeprazole and mephenytoin metabolism compared with cisgender women not taking oral contraceptives (60%, P < 0.01). 71 , 72 , 73
Transgender adults may take medications metabolized by CYP2C9, including nonsteroidal anti‐inflammatory drugs and oral antidiabetic agents. CYP2C19 also metabolizes several medications taken by transgender adults, including proton pump inhibitors and antidepressants. Although available sex‐related or hormone‐related data are conflicting, given the number of medications that transgender adults may take that are metabolized by these pathways, studies using model CYP2C9 and 2C19 substrates (e.g., warfarin and omeprazole, respectively) may be important to identify and characterize the direct role of sex hormones on these CYPs in transgender adults.
CYP2D6
CYP2D6 metabolizes ~ 25% of prescribed medications. 74 Data describing the effect of sex and gender on CYP2D6 activity are conflicting. Among CYP2D6 extensive metabolizers, the dextromethorphan urinary metabolic ratio is lower among cisgender women than men (20–40%, P < 0.05). 71 , 73 , 75 Similarly, CYP2D6 activity increases during pregnancy among intermediate and extensive CYP2D6 metabolizers (vs. post partum). 62 However, one study observed no association between sex and the urinary dextromethorphan metabolic ratio after adjusting for the extensive metabolizer phenotype and other CYP activity (CYP3A4). 76 Similarly, the debrisoquine recovery ratio exhibits no sex‐related differences in adults. 77 , 78 CYP2D6 genetic polymorphisms contribute to wide interindividual variability in CYP2D6 activity, 17 but sex does not influence this polymorphism. 16 Investigators observed no effect of sex hormones (estrogen or oral contraceptive pills) on CYP2D6 probe drugs, dextromethorphan or sparteine, in cisgender adults. 75 , 79 , 80 , 81
Transgender adults may take several medications metabolized by CY2D6. These include antidepressants and common antihypertensives like metoprolol. 25 Based on available data in cisgender adults, intermediate or extensive CYP2D6 metabolizer status is more likely to impact drug disposition in transgender adults rather than gender‐affirming medical care.
CYP3A
CYP3A metabolizes more than 50% of prescribed medications. 82 In the general adult population younger than 50 years of age, cisgender women have higher weight‐normalized clearance of oral and parenteral CYP3A substrates than cisgender men, although this difference is modest (up to 35%). 17 , 83 Investigators hypothesized that sex‐related differences in CYP3A activity are associated with P‐glycoprotein activity, 84 complicating our ability to determine the effect of sex hormones on CYP3A activity directly. During pregnancy, CYP3A activity is higher compared with postpartum activity. 62 Sex hormones (estrogen replacement therapy or combined oral contraceptives) do not alter systemic or oral midazolam clearance. 85 , 86 In addition to hormone therapy, transgender adults may take several medications metabolized by CYP3A, including antiretroviral therapy protease inhibitors. 25 , 28
Phase II metabolism and conjugation enzymes
In the general adult population, weight‐adjusted oral clearance of several nonspecific uridine diphosphate (UDP)‐glucuronosyltransferase (UGT) substrates is higher in cisgender men than cisgender women: benzodiazepines (oxazepam, 40% higher, P < 0.05), 87 and antipyretics (acetaminophen (paracetamol), 22% higher, P < 0.001). 88 During pregnancy, apparent UGT1A4 activity increases compared with post partum, demonstrated by decreased lamotrigine concentrations. 62 Sex hormones (combined oral contraceptives) similarly increase clearance of UGT substrates. For example, Christensen et al. 89 reported an 84% increase (95% confidence interval, 45–134%) in dose‐corrected lamotrigine concentrations in a small placebo‐controlled trial among 13 cisgender women when participants received placebo versus a combined oral contraceptive . 89 Acetaminophen clearance (via glucuronidation) was nearly 50% higher in 8 cisgender women taking combined oral contraceptives compared with 8 cisgender women who were not (P < 0.01). 88 Similarly, testosterone replacement therapy was positively correlated with oral clearance of the beta‐adrenergic receptor blocking agent propranolol in 11 cisgender men via the glucuronidation pathway (P < 0.002). 90
DRUG TRANSPORT PROTEINS
P‐glycoprotein
P‐glycoprotein is a membrane efflux transporter involved in absorbing, distributing, and excreting drugs. 91 Several tissues express P‐glycoprotein throughout the body, including the intestines, liver, and kidneys. In a post hoc subgroup analysis of more than 2,000 randomly selected adults enrolled in a randomized, placebo‐controlled digoxin efficacy trial, cisgender women had higher serum concentrations of digoxin, a model P‐glycoprotein substrate, 91 than cisgender men within the first month of daily digoxin therapy (P = 0.007), although this difference disappeared after 12 months of digoxin treatment. 92 Fexofenadine, another well‐characterized P‐glycoprotein substrate, exhibited no sex‐related differences in plasma exposure among adults. 93 During pregnancy, apparent P‐glycoprotein activity increases compared with postpartum activity when using net tubular secretion clearance of digoxin as a biomarker for P‐glycoprotein activity. 62
One limitation of these studies is that digoxin and fexofenadine are substrates of organic anion transporter polypeptides (OATPs), 91 complicating their use as P‐glycoprotein probes. Investigators suggest sex‐related or gender‐related differences in P‐glycoprotein activity modulate intracellular drug concentrations and contribute to observed differences in CYP3A activity between sexes. 84 , 94 Oral drugs that are both CYP3A and P‐glycoprotein substrates (e.g., verapamil) support this hypothesis. 13 , 84
The effect of hormone therapy on P‐glycoprotein activity is unclear. Transgender adults may take important medications that are transported by P‐glycoprotein, including certain antiviral medications. Studies using model P‐glycoprotein substrates are needed to characterize P‐glycoprotein activity in transgender adults.
KIDNEY ELIMINATION
Kidney drug clearance pathways involve glomerular filtration, tubular secretion, and tubular reabsorption. Measured glomerular filtration rate (GFR), an index of overall kidney function, is slightly lower in cisgender women after adjusting for body surface area than cisgender men (10%). 94 Digoxin is predominantly eliminated by the kidneys as unchanged drug (80%). Although a post hoc subgroup analysis of more than 2,000 randomly selected adults enrolled in a digoxin efficacy trial reported higher serum digoxin concentrations among cisgender women within the first month of therapy than among cisgender men, 92 because urinary digoxin excretion data were unavailable for this analysis, we cannot conclude whether this outcome was associated with sex‐related differences in kidney drug clearance. During pregnancy, GFR is nearly 50% higher than post partum. Kidney drug clearance of unbound digoxin was more than 50% higher during pregnancy than post partum in one study (n = 14), 62 and kidney drug clearance of atenolol, a beta‐adrenergic blocking agent predominantly eliminated in the urine as unchanged drug (>85%), was 11–12% higher during pregnancy in a separate study (n = 17). 62
Few prospective studies have characterized changes in kidney function in transgender adults. In a small prospective study, investigators observed no change in measured creatinine clearance from nine transgender women undergoing an average of 10 weeks of estrogen treatment (vs. estrogen‐naïve baseline). 95 In a retrospective cohort of 66 transgender adults undergoing estrogen treatment for at least one year, Humble et al. 96 observed a similar proportion of adults with estimated GFR (eGFR) ≥ 90 mL/min/1.73 m 2 before and during estrogen treatment (P value not reported). 96 In the same cohort, among 25 transgender adults undergoing testosterone treatment, the proportion of adults with eGFR ≥ 90 mL/min/1.73 m2 was numerically lower during testosterone treatment compared with testosterone‐naïve baseline (47% vs. 67%, respectively, P value not reported). 96
Humble et al. 96 estimated kidney function using a serum creatinine‐based estimating equation (4‐variable Modification of Diet in Renal Disease (MDRD) formula with the sex assigned at birth). 96 In a prospective study including more than 100 transgender adults, serum creatinine concentrations increased during testosterone treatment (n = 53: 0.74 to 0.84 mg/dL, P < 0.001), whereas serum creatinine decreased during estrogen treatment (n = 40, oral 17β‐estradiol: 0.90 to 0.80, P = 0.001; n = 13, transdermal 17β‐estradiol: 0.93 to 0.85 mg/dL, P = 0.011). 10 Because serum creatinine‐based kidney function estimating equations require steady‐state serum creatinine concentrations for reliable clinical use, best practices for eGFR determination in transgender adults are yet to be determined. Despite limitations in available eGFR data, we anticipate clinically significant changes in kidney function that impact drug clearance in healthy transgender adults are unlikely, although the impact of longer‐term hormone use, and considerations for transgender adults with chronic kidney disease, are unclear at this time. Although data on sex‐differences in tubular secretion clearance are lacking, the apparent activities of several protein transporters increase during pregnancy (organic anion transporter 1; organic cation transporter 2; P‐glycoprotein), increasing net secretion clearance of amoxicillin, metformin, and digoxin, respectively. 62
PHARMACODYNAMIC DIFFERENCES
Pharmacodynamic studies of prescription medications in transgender adults are lacking. Pharmacodynamic interactions may impact safety or effectiveness and involve either antagonistic, synergistic, or additive effects with other drugs or co‐occurring medical conditions. Although potential pharmacodynamic interactions may occur in transgender adults living with HIV and taking antiretroviral therapy, 28 clinical data to support these proposed outcomes are lacking. In the general population, cisgender women have higher, and more serious, medication‐related adverse event rates than cisgender men. 12 Exact mechanisms behind these differences are unclear.
CONSIDERATIONS FOR FUTURE RESEARCH
We suggest using pharmacokinetic studies with model probe substrates to investigate the activities of most major CYP enzymes in transgender adults. Based on available sex, gender, and hormonal data from the general population, CYP1A2 activity may be lower in transgender adults undergoing estrogen treatment. Because CYP1A2 metabolizes several medications that may be taken by transgender adults (e.g., duloxetine and olanzapine), we recommend further studies should characterize CYP1A2 activity in transgender adults before and during hormone therapy. Although sex‐related and gender‐related data regarding CYP3A activity are conflicting, because this major enzyme system metabolizes several drug classes that may be taken by transgender adults (protease inhibitors, benzodiazepines like alprazolam), appropriate intravenous and oral probe drug studies should characterize CYP3A activity in transgender adults before and during hormone therapy, as well as in older transgender adults. Because transgender adults may take important medications metabolized via UGT1A4 (lamotrigine) or UGT1A1/6/9 (acetaminophen), and acetaminophen is oxidized to an active toxic metabolite, consideration should be given to investigating the disposition of these drugs in transgender adults.
Aspirin may have either faster oral absorption or higher bioavailability based on sex assigned at birth among transgender adults. Although experts do not recommend routine venous thromboembolism prophylaxis (i.e., low‐dose aspirin) during hormone therapy, 33 transgender adults may take aspirin‐containing products for analgesia or low‐dose aspirin as secondary prevention for atherosclerotic cardiovascular disease. Future studies should examine the absorption kinetics and bioavailability of aspirin in transgender adults before and during hormone therapy to determine how therapy may influence its pharmacokinetic and pharmacodynamic profile.
Although sex‐related and gender‐related data regarding kidney drug clearance are lacking, pregnancy‐based data suggest net secretion clearance of antibiotics (amoxicillin) and digoxin may be influenced by supraphysiologic hormonal environments, which suggests this may require further investigation in transgender adults. Additional studies should examine net tubular secretion clearance of appropriate agents. These agents may include model probe substrates for P‐glycoprotein (digoxin) or organic cation transporter 2 (metformin).
Agencies like the National Institutes of Health do not recognize transgender adults formally as a special population in clinical research. However, investigators must be sensitive toward the demands of intensive pharmacokinetic sampling. For this reason, a systems pharmacology approach, including physiologically‐based pharmacokinetic modeling, may be useful for predicting changes in drug disposition, and implications for dosing modifications, for transgender adults across the lifespan. Novel in vitro technologies include microphysiological models of organs and tissues, like organ‐on‐a‐chip. This is an emerging tool that can model pharmacokinetic processes such intestinal absorption or drug transport in relevant hormonal environments. Investigators have suggested this technology has potential to model complex sex‐related differences influencing pharmacokinetic processes. 97
Available research regarding sex‐related and gender‐related differences in clinical pharmacology includes only cisgender male and female populations and is therefore binary in its approach. This framework may limit our ability to extrapolate established sex‐related and gender‐related pharmacologic data from the general population to transgender and nonbinary populations. Further research is necessary to better understand the intersection between low‐dose hormone therapy used by transgender and nonbinary adults and the influence on the pharmacokinetics and pharmacodynamics of the prescribed medications discussed in this article.
SUMMARY
Clinical pharmacology data are lacking in transgender adults. Most clinical data from the general adult population suggest minimal sex‐related or gender‐related differences in pathways of drug handling. However, the activities of certain CYPs (1A2, 3A4), kidney transporter proteins, and absorption kinetics of drugs like aspirin may require further study in transgender adults undergoing hormone therapy.
FUNDING
No funding was received for this work.
CONFLICT OF INTEREST
All authors declared no competing interests for this work.
ACKNOWLEDGMENTS
Kai J. Huang uses they/them/theirs, he/him/his, and ze/zir/zirs pronouns. Lauren R. Cirrincione uses she/her pronouns.
Linked article: This article is linked to Commentary on: “Sex and Gender Differences in Clinical Pharmacology: Implications for Transgender Medicine” by Cotreau, M.M., Clin. Pharmacol. Ther. 110, 863–865 (2021).
References
- 1. Wylie, K. , Knudson, G. , Khan, S.I. , Bonierbale, M. , Watanyusakul, S. & Baral, S. Serving transgender people: clinical care considerations and service delivery models in transgender health. Lancet 388, 401–411 (2016). [DOI] [PubMed] [Google Scholar]
- 2. Zucker, I. & Prendergast, B.J. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol. Sex Differ. 11, 32 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moyer, A.M. , Matey, E.T. & Miller, V.M. Individualized medicine: Sex, hormones, genetics, and adverse drug reactions. Pharmacol. Res. Perspect. 7, e00541‐e (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Budnitz, D.S. , Pollock, D.A. , Weidenbach, K.N. , Mendelsohn, A.B. , Schroeder, T.J. & Annest, J.L. National surveillance of emergency department visits for outpatient adverse drug events. JAMA 296, 1858–1866 (2006). [DOI] [PubMed] [Google Scholar]
- 5. Friede, K.A. et al. Influence of sex on platelet reactivity in response to aspirin. J. Am. Heart Assoc. 9, e014726 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soldin, O.P. , Chung, S.H. & Mattison, D.R. Sex differences in drug disposition. J. Biomed. Biotechnol. 2011, 187103 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coleman, E. et al. Standards of care for the health of transsexual, transgender, and gender‐nonconforming people, version 7. Int. J. Transgend. 13, 165–232 (2012). [Google Scholar]
- 8. Padula, W.V. , Heru, S. & Campbell, J.D. Societal implications of health insurance coverage for medically necessary services in the U.S. transgender population: a cost‐effectiveness analysis. J. Gen. Intern. Med. 31, 394–401 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baker, K.E. , Wilson, L.M. , Sharma, R. , Dukhanin, V. , McArthur, K. & Robinson, K.A. Hormone therapy, mental health, and quality of life among transgender people: a systematic review. J. Endocr. Soc. 5, bvab011 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wierckx, K. et al. Cross‐sex hormone therapy in trans persons is safe and effective at short‐time follow‐up: results from the european network for the investigation of gender incongruence. J. Sex. Med. 11, 1999–2011 (2014). [DOI] [PubMed] [Google Scholar]
- 11. Klaver, M. , Dekker, M.J.H.J. , de Mutsert, R. , Twisk, J.W.R. & den Heijer, M. Cross‐sex hormone therapy in transgender persons affects total body weight, body fat and lean body mass: a meta‐analysis. Andrologia 49, e12660 (2017). [DOI] [PubMed] [Google Scholar]
- 12. Soldin, O.P. & Mattison, D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 48, 143–157 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwartz, J.B. The influence of sex on pharmacokinetics. Clin. pharmacokinet. 42, 107–121 (2003). [DOI] [PubMed] [Google Scholar]
- 14. Gandhi, M. , Aweeka, F. , Greenblatt, R.M. & Blaschke, T.F. Sex differences in pharmacokinetics and pharmacodynamics. Annu. Rev. Pharmacol. Toxicol. 44, 499–523 (2004). [DOI] [PubMed] [Google Scholar]
- 15. Anderson, G.D. Sex and racial differences in pharmacological response: where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J. Womens Health (Larchmt) 14, 19–29 (2005). [DOI] [PubMed] [Google Scholar]
- 16. Harris, R.Z. , Benet, L.Z. & Schwartz, J.B. Gender effects in pharmacokinetics and pharmacodynamics. Drugs 50, 222–239 (1995). [DOI] [PubMed] [Google Scholar]
- 17. Schwartz, J.B. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin. Pharmacol. Ther. 82, 87–96 (2007). [DOI] [PubMed] [Google Scholar]
- 18. Aitken, M. et al. Evidence for an altered sex ratio in clinic‐referred adolescents with gender dysphoria. J. Sex. Med. 12, 756–763 (2015). [DOI] [PubMed] [Google Scholar]
- 19. Winter, S. et al. Transgender people: health at the margins of society. Lancet 388, 390–400 (2016). [DOI] [PubMed] [Google Scholar]
- 20. Arcelus, J. , Bouman, W.P. , Van Den Noortgate, W. , Claes, L. , Witcomb, G. & Fernandez‐Aranda, F. Systematic review and meta‐analysis of prevalence studies in transsexualism. Eur. Psychiatry. 30, 807–815 (2015). [DOI] [PubMed] [Google Scholar]
- 21. Herman, J.L. , Flores, A.R. , Brown, T.N.T. , Wilson, B.D.M. & Conron, K.J. Age of individuals who identify as transgender in the United States. University of California <https://williamsinstitute.law.ucla.edu/publications/age‐trans‐individuals‐us> (2017). Accessed October 30, 2020.
- 22. Kreukels, B.P.C. , Haraldsen, I.R. , De Cuypere, G. , Richter‐Appelt, H. , Gijs, L. & Cohen‐Kettenis, P.T. A European network for the investigation of gender incongruence: the ENIGI initiative. Eur. Psychiatry 27, 445–450 (2012). [DOI] [PubMed] [Google Scholar]
- 23. Gooren, L.J. & T’Sjoen, G. Endocrine treatment of aging transgender people. Rev. Endocr. Metab. Disord. 19, 253–262 (2018). [DOI] [PubMed] [Google Scholar]
- 24. Fredriksen‐Goldsen, K.I. et al. Physical and mental health of transgender older adults: an at‐risk and underserved population. Gerontologist 54, 488–500 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Progovac, A.M. et al. Trends in mental health care use in medicare from 2009 to 2014 by gender minority and disability status. LGBT Health 6, 297–305 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flores, A.R. , Brown, T.N.T. & Herman, J.L. Race and ethnicity of adults who identify as transgender in the United States. Williams Institute, UCLA School of Law Los Angeles <https://williamsinstitute.law.ucla.edu/publications/race‐ethnicity‐trans‐adults‐us> (October 2016). Accessed February 11, 2021.
- 27. Reisner, S.L. et al. Global health burden and needs of transgender populations: a review. Lancet 388, 412–436 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cirrincione, L.R. , Senneker, T. , Scarsi, K. & Tseng, A. Drug interactions with gender‐affirming hormone therapy: focus on antiretrovirals and direct acting antivirals. Expert Opin. Drug Metab. Toxicol. 16, 565–582 (2020). [DOI] [PubMed] [Google Scholar]
- 29. Radix, A. , Sevelius, J. & Deutsch, M.B. Transgender women, hormonal therapy and HIV treatment: a comprehensive review of the literature and recommendations for best practices. J. Int. AIDS Soc. 19, 20810 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rich, A.J. , Scheim, A.I. , Koehoorn, M. & Poteat, T. Non‐HIV chronic disease burden among transgender populations globally: a systematic review and narrative synthesis. Prev. Med. Rep. 20, 101259 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grant, J.M. , Mottet, L.A. & Tanis, J. Injustice at every turn: a report of the National Transgender Discrimination Survey <https://transequality.org/sites/default/files/docs/resources/NTDS_Report.pdf> (2010). Accessed February 10, 2021.
- 32. Hembree, W.C. et al. Endocrine treatment of gender‐dysphoric/gender‐incongruent persons: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 102, 3869–3903 (2017). [DOI] [PubMed] [Google Scholar]
- 33. Deutsch, M.B. Guidelines for the primary and gender‐affirming care of transgender and gender nonbinary people. University of California, San Francisco <https://transcare.ucsf.edu/guidelines> (2016). Accessed November 18, 2020.
- 34. Giltay, E.J. , Hoogeveen, E.K. , Elbers, J.M. , Gooren, L.J. , Asscheman, H. & Stehouwer, C.D. Effects of sex steroids on plasma total homocysteine levels: a study in transsexual males and females. J. Clin. Endocrinol. Metab. 83, 550–553 (1998). [DOI] [PubMed] [Google Scholar]
- 35. Greene, D.N. et al. Reproductive endocrinology reference intervals for transgender men on stable hormone therapy. J. Appl. Lab. Med. 6, 41–50 (2021). [DOI] [PubMed] [Google Scholar]
- 36. Deutsch, M.B. , Bhakri, V. & Kubicek, K. Effects of cross‐sex hormone treatment on transgender women and men. Obstet. Gynecol. 125, 605–610 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen, H. et al. Changes of vitamin D‐binding protein, and total, bioavailable, and free 25‐hydroxyvitamin D in transgender people. J. Clin. Endocrinol. Metab. 104, 2728–2734 (2019). [DOI] [PubMed] [Google Scholar]
- 38. Giltay, E.J. , Verhoef, P. , Gooren, L.J.G. , Geleijnse, J.M. , Schouten, E.G. & Stehouwer, C.D.A. Oral and transdermal estrogens both lower plasma total homocysteine in male‐to‐female transsexuals. Atherosclerosis 168, 139–146 (2003). [DOI] [PubMed] [Google Scholar]
- 39. Fuss, J. et al. Does sex hormone treatment reverse the sex‐dependent stress regulation? A longitudinal study on hypothalamus‐pituitary‐adrenal (HPA) axis activity in transgender individuals. Psychoneuroendocrinology 104, 228–237 (2019). [DOI] [PubMed] [Google Scholar]
- 40. Cocchetti, C. , Ristori, J. , Romani, A. , Maggi, M. & Fisher, A.D. Hormonal treatment strategies tailored to non‐binary transgender individuals. J. Clin. Med. 9, 1609 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Singh, A.B. et al. The effects of varying doses of T on insulin sensitivity, plasma lipids, apolipoproteins, and C‐reactive protein in healthy young men. J. Clin. Endocrinol. Metab. 87, 136–143 (2002). [DOI] [PubMed] [Google Scholar]
- 42. Greene, D.N. et al. Reproductive endocrinology reference intervals for transgender women on stable hormone therapy. J. Appl. Lab. Med. 6, 15–26 (2021). [DOI] [PubMed] [Google Scholar]
- 43. Tangpricha, V. & den Heijer, M. Oestrogen and anti‐androgen therapy for transgender women. Lancet Diabetes Endocrinol. 5, 291–300 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Angus, L.M. , Nolan, B.J. , Zajac, J.D. & Cheung, A.S. A systematic review of antiandrogens and feminization in transgender women. Clin. Endocrinol. (Oxf.) (2020). https://doi.org/10.1111/cen.14329. [DOI] [PubMed] [Google Scholar]
- 45. Hojbjerg, J.A. , Saini, S.L. , Hvas, A.‐M. & Hojgaard, A.D. Current treatment regimens for transfeminine individuals in the Nordic countries. J. Sex. Med. 18, 656– 663 (2021). [DOI] [PubMed] [Google Scholar]
- 46. Freire, A.C. , Basit, A.W. , Choudhary, R. , Piong, C.W. & Merchant, H.A. Does sex matter? The influence of gender on gastrointestinal physiology and drug delivery. Int. J. Pharm. 415, 15–28 (2011). [DOI] [PubMed] [Google Scholar]
- 47. Benet, L.Z. & Hoener, B.A. Changes in plasma protein binding have little clinical relevance. Clin. Pharmacol. Ther. 71, 115–121 (2002). [DOI] [PubMed] [Google Scholar]
- 48. Pelusi, C. et al. Effects of three different testosterone formulations in female‐to‐male transsexual persons. J. Sex. Med. 11, 3002–3011 (2014). [DOI] [PubMed] [Google Scholar]
- 49. Haraldsen, I.R. , Haug, E. , Falch, J. , Egeland, T. & Opjordsmoen, S. Cross‐sex pattern of bone mineral density in early onset gender identity disorder. Horm. Behav. 52, 334–343 (2007). [DOI] [PubMed] [Google Scholar]
- 50. Mueller, A. et al. Effects of intramuscular testosterone undecanoate on body composition and bone mineral density in female‐to‐male transsexuals. J. Sex. Med. 7, 3190–3198 (2010). [DOI] [PubMed] [Google Scholar]
- 51. van Kesteren, P. et al. The effect of one‐year cross‐sex hormonal treatment on bone metabolism and serum insulin‐like growth factor‐1 in transsexuals. J. Clin. Endocrinol. Metab. 81, 2227–2232 (1996). [DOI] [PubMed] [Google Scholar]
- 52. Mueller, A. et al. Body composition and bone mineral density in male‐to‐female transsexuals during cross‐sex hormone therapy using gonadotrophin‐releasing hormone agonist. Exp. Clin. Endocrinol. Diabetes 119, 95–100 (2011). [DOI] [PubMed] [Google Scholar]
- 53. Dittrich, R. , Binder, H. , Cupisti, S. , Hoffmann, I. , Beckmann, M.W. & Mueller, A. Endocrine treatment of male‐to‐female transsexuals using gonadotropin‐releasing hormone agonist. Exp. Clin. Endocrinol. Diabetes 113, 586–592 (2005). [DOI] [PubMed] [Google Scholar]
- 54. Mueller, A. et al. High dose estrogen treatment increases bone mineral density in male‐to‐female transsexuals receiving gonadotropin‐releasing hormone agonist in the absence of testosterone. Eur. J. Endocrinol. 153, 107–113 (2005). [DOI] [PubMed] [Google Scholar]
- 55. Gava, G. , Cerpolini, S. , Martelli, V. , Battista, G. , Seracchioli, R. & Meriggiola, M.C. Cyproterone acetate vs leuprolide acetate in combination with transdermal oestradiol in transwomen: a comparison of safety and effectiveness. Clin. Endocrinol. (Oxf.) 85, 239–246 (2016). [DOI] [PubMed] [Google Scholar]
- 56. Hanley, M.J. , Abernethy, D.R. & Greenblatt, D.J. Effect of obesity on the pharmacokinetics of drugs in humans. Clin. Pharmacokinet. 49, 71–87 (2010). [DOI] [PubMed] [Google Scholar]
- 57. Velho, I. , Fighera, T.M. , Ziegelmann, P.K. & Spritzer, P.M. Effects of testosterone therapy on BMI, blood pressure, and laboratory profile of transgender men: a systematic review. Andrology 5, 881–888 (2017). [DOI] [PubMed] [Google Scholar]
- 58. Elbers, J.M. , Asscheman, H. , Seidell, J.C. , Megens, J.A. & Gooren, L.J. Long‐term testosterone administration increases visceral fat in female to male transsexuals. J. Clin. Endocrinol. Metab. 82, 2044–2047 (1997). [DOI] [PubMed] [Google Scholar]
- 59. Elbers, J.M. , Asscheman, H. , Seidell, J.C. & Gooren, L.J. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am. J. Physiol. 276, E317–E325 (1999). [DOI] [PubMed] [Google Scholar]
- 60. Fisher, A.D. et al. Cross‐sex hormone treatment and psychobiological changes in transsexual persons: two‐year follow‐up data. J. Clin. Endocrinol. Metab. 101, 4260–4269 (2016). [DOI] [PubMed] [Google Scholar]
- 61. Meyer, I.H. , Brown, T.N.T. , Herman, J.L. , Reisner, S.L. & Bockting, W.O. Demographic characteristics and health status of transgender adults in select US regions: behavioral risk factor surveillance system, 2014. Am. J. Public Health 107, 582–589 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tasnif, Y. , Morado, J. & Hebert, M.F. Pregnancy‐related pharmacokinetic changes. Clin. Pharmacol. Ther. 100, 53–62 (2016). [DOI] [PubMed] [Google Scholar]
- 63. Pollock, B.G. et al. Inhibition of caffeine metabolism by estrogen replacement therapy in postmenopausal women. J. Clin. Pharmacol. 39, 936–940 (1999). [DOI] [PubMed] [Google Scholar]
- 64. Benowitz, N.L. , Peng, M. & Jacob, P. 3rd Effects of cigarette smoking and carbon monoxide on chlorzoxazone and caffeine metabolism. Clin. Pharmacol. Ther. 74, 468–474 (2003). [DOI] [PubMed] [Google Scholar]
- 65. Hukkanen, J. , Jacob, P. 3rd , Peng, M. , Dempsey, D. & Benowitz, N.L. Effect of nicotine on cytochrome P450 1A2 activity. Br. J. Clin. Pharmacol. 72, 836–838 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wheldon, C.W. & Wiseman, K.P. Tobacco use among transgender and gender non‐conforming adults in the United States. Tob. Use Insights 12, 1179173X19849419 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kcomt, L. , Evans‐Polce, R.J. , Veliz, P.T. , Boyd, C.J. & McCabe, S.E. Use of cigarettes and e‐cigarettes/vaping among transgender people: results from the 2015 U.S. Transgender Survey. Am. J. Prev. Med. 59, 538–547 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ilic, K. et al. The influence of sex, ethnicity, and CYP2B6 genotype on bupropion metabolism as an index of hepatic CYP2B6 activity in humans. Drug Metab. Dispos. 41, 575–581 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Palovaara, S. , Pelkonen, O. , Uusitalo, J. , Lundgren, S. & Laine, K. Inhibition of cytochrome P450 2B6 activity by hormone replacement therapy and oral contraceptive as measured by bupropion hydroxylation. Clin. Pharmacol. Ther. 74, 326–333 (2003). [DOI] [PubMed] [Google Scholar]
- 70. Hiransuthikul, A. et al. Drug–drug interactions among Thai transgender women living with human immunodeficiency undergoing feminizing hormone therapy and antiretroviral therapy: the iFACT study. Clin. Infect. Dis. 72, 396–402 (2021). [DOI] [PubMed] [Google Scholar]
- 71. Hägg, S. , Spigset, O. & Dahlqvist, R. Influence of gender and oral contraceptives on CYP2D6 and CYP2C19 activity in healthy volunteers. Br. J. Clin. Pharmacol. 51, 169–173 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Laine, K. , Tybring, G. & Bertilsson, L. No sex‐related differences but significant inhibition by oral contraceptives of CYP2C19 activity as measured by the probe drugs mephenytoin and omeprazole in healthy Swedish white subjects. Clin. Pharmacol. Ther. 68, 151–159 (2000). [DOI] [PubMed] [Google Scholar]
- 73. Tamminga, W.J. et al. CYP2D6 and CYP2C19 activity in a large population of Dutch healthy volunteers: indications for oral contraceptive‐related gender differences. Eur. J. Clin. Pharmacol. 55, 177–184 (1999). [DOI] [PubMed] [Google Scholar]
- 74. Ingelman‐Sundberg, M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J. 5, 6–13 (2005). [DOI] [PubMed] [Google Scholar]
- 75. Labbé, L. et al. Effect of gender, sex hormones, time variables and physiological urinary pH on apparent CYP2D6 activity as assessed by metabolic ratios of marker substrates. Pharmacogenetics 10, 425–438 (2000). [DOI] [PubMed] [Google Scholar]
- 76. Funck‐Brentano, C. , Boëlle, P.‐Y. , Verstuyft, C. , Bornert, C. , Becquemont, L. & Poirier, J.‐M. Measurement of CYP2D6 and CYP3A4 activity in vivo with dextromethorphan: sources of variability and predictors of adverse effects in 419 healthy subjects. Eur. J. Clin. Pharmacol. 61, 821–829 (2005). [DOI] [PubMed] [Google Scholar]
- 77. Bebia, Z. et al. Bioequivalence revisited: influence of age and sex on CYP enzymes. Clin. Pharmacol. Ther. 76, 618–627 (2004). [DOI] [PubMed] [Google Scholar]
- 78. Kallio, J. , Lindberg, R. , Huupponen, R. & Iisalo, E. Debrisoquine oxidation in a Finnish population: the effect of oral contraceptives on the metabolic ratio. Br. J. Clin. Pharmacol. 26, 791–795 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kashuba, A.D. et al. Quantification of intraindividual variability and the influence of menstrual cycle phase on CYP2D6 activity as measured by dextromethorphan phenotyping. Pharmacogenetics 8, 403–410 (1998). [DOI] [PubMed] [Google Scholar]
- 80. Bock, K.W. et al. The influence of environmental and genetic factors on CYP2D6, CYP1A2 and UDP‐glucuronosyltransferases in man using sparteine, caffeine, and paracetamol as probes. Pharmacogenetics 4, 209–218 (1994). [DOI] [PubMed] [Google Scholar]
- 81. McCune, J.S. et al. Lack of gender differences and large intrasubject variability in cytochrome P450 activity measured by phenotyping with dextromethorphan. J. Clin. Pharmacol. 41, 723–731 (2001). [DOI] [PubMed] [Google Scholar]
- 82. Wilkinson, G.R. Drug metabolism and variability among patients in drug response. N. Engl. J. Med. 352, 2211–2221 (2005). [DOI] [PubMed] [Google Scholar]
- 83. Greenblatt, D.J. & von Moltke, L.L. Gender has a small but statistically significant effect on clearance of CYP3A substrate drugs. J. Clin. Pharmacol. 48, 1350–1355 (2008). [DOI] [PubMed] [Google Scholar]
- 84. Cummins, C.L. , Wu, C.‐Y. & Benet, L.Z. Sex‐related differences in the clearance of cytochrome P450 3A4 substrates may be caused by P‐glycoprotein. Clin. Pharmacol. Ther. 72, 474–489 (2002). [DOI] [PubMed] [Google Scholar]
- 85. Gorski, J.C. , Wang, Z. , Haehner‐Daniels, B.D. , Wrighton, S.A. & Hall, S.D. The effect of hormone replacement therapy on CYP3A activity. Clin. Pharmacol. Ther. 68, 412–417 (2000). [DOI] [PubMed] [Google Scholar]
- 86. Kashuba, A.D. , Bertino, J.S. Jr , Rocci, M.L. Jr , Kulawy, R.W. , Beck, D.J. & Nafziger, A.N. Quantification of 3‐month intraindividual variability and the influence of sex and menstrual cycle phase on CYP3A activity as measured by phenotyping with intravenous midazolam. Clin. Pharmacol. Ther. 64, 269–277 (1998). [DOI] [PubMed] [Google Scholar]
- 87. Greenblatt, D.J. , Divoll, M. , Harmatz, J.S. & Shader, R.I. Oxazepam kinetics: effects of age and sex. J. Pharmacol. Exp. Ther. 215, 86–91 (1980). [PubMed] [Google Scholar]
- 88. Miners, J.O. , Attwood, J. & Birkett, D.J. Influence of sex and oral contraceptive steroids on paracetamol metabolism. Br. J. Clin. Pharmacol. 16, 503–509 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Christensen, J. et al. Oral contraceptives induce lamotrigine metabolism: evidence from a double‐blind, placebo‐controlled trial. Epilepsia 48, 484–489 (2007). [DOI] [PubMed] [Google Scholar]
- 90. Walle, T. , Walle, U.K. , Mathur, R.S. , Palesch, Y.Y. & Conradi, E.C. Propranolol metabolism in normal subjects: association with sex steroid hormones. Clin. Pharmacol. Ther. 56, 127–132 (1994). [DOI] [PubMed] [Google Scholar]
- 91. Ma, J.D. , Tsunoda, S.M. , Bertino, J.S. Jr , Trivedi, M. , Beale, K.K. & Nafziger, A.N. Evaluation of in vivo P‐glycoprotein phenotyping probes: a need for validation. Clin. Pharmacokinet. 49, 223–237 (2010). [DOI] [PubMed] [Google Scholar]
- 92. Rathore, S.S. , Wang, Y. & Krumholz, H.M. Sex‐based differences in the effect of digoxin for the treatment of heart failure. N. Engl. J. Med. 347, 1403–1411 (2002). [DOI] [PubMed] [Google Scholar]
- 93. Kim, R.B. et al. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin. Pharmacol. Ther. 70, 189–199 (2001). [DOI] [PubMed] [Google Scholar]
- 94. Meibohm, B. , Beierle, I. & Derendorf, H. How important are gender differences in pharmacokinetics? Clin. Pharmacokinet. 41, 329–342 (2002). [DOI] [PubMed] [Google Scholar]
- 95. Nicholls, A. , Snaith, M.L. & Scott, J.T. Effect of oestrogen therapy on plasma and urinary levels of uric acid. Br. Med. J. 1, 449–451 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Humble, R.M. , Imborek, K.L. , Nisly, N. , Greene, D.N. & Krasowski, M.D. Common hormone therapies used to care for transgender patients influence laboratory results. J. Appl. Lab. Med. 3, 799–814 (2019). [DOI] [PubMed] [Google Scholar]
- 97. Nawroth, J. , Rogal, J. , Weiss, M. , Brucker, S.Y. & Loskill, P. Organ‐on‐a‐chip systems for women's health applications. Adv. Healthc. Mater. 7, 1700550 (2018). [DOI] [PubMed] [Google Scholar]


