Abstract
Objective
Patient involvement in decision making is conditional for personalised treatment decisions. We aim to provide an up‐to‐date overview of patients’ preferred and perceived level of involvement in decision making for cancer treatment.
Methods
A systematic search was performed in PubMed, EMBASE, PsycINFO and CINAHL for articles published between January 2009 and January 2020. Search terms were ‘decision making’, ‘patient participation’, ‘oncology’, ‘perception’ and ‘treatment’. Inclusion criteria were: written in English, peer‐reviewed, reporting patients’ preferred and perceived level of involvement, including adult cancer patients and concerning decision making for cancer treatment. The percentages of patients preferring and perceiving an active, shared or passive decision role and the (dis)concordance are presented. Quality assessment was performed with a modified version of the New‐Castle Ottawa Scale.
Results
31 studies were included. The median percentage of patients preferring an active, shared or passive role in decision making was respectively 25%, 46%, and 27%. The median percentage of patients perceiving an active, shared or passive role was respectively 27%, 39%, and 34%. The median concordance in preferred and perceived role of all studies was 70%. Disconcordance was highest for a shared role; 42%.
Conclusions
Patients’ preferences for involvement in cancer treatment decision vary widely. A significant number of patients perceived a decisional role other than preferred. Improvements in patient involvement have been observed in the last decade. However, there is still room for improvement and physicians should explore patients’ preferences for involvement in decision making in order to truly deliver personalised cancer care.
Keywords: decision making, medical oncology, neoplasms, patient participation, patient preference, psycho‐oncology
1. BACKGROUND
As science continues to reveal the heterogeneity of tumors, the number of possible treatment options rises. This increases the potential for personalised cancer treatment and makes ‘the best’ treatment choice increasingly subject to preference. In the process of reviewing treatment options, evaluating them in the medical and psychosocial context of the patient and matching them with individual preferences and priorities is needed for personalised cancer care. 1 Patient involvement is therefore required to make a deliberate choice. 2 , 3 Through this process of shared decision making (SDM), patients are enabled to play an active role in composing their individual cancer care. 4 , 5 , 6 , 7
Patient involvement in decision making for cancer treatment has been shown to improve patient’s perception of quality of care, 8 physical functioning, 9 patient satisfaction, 10 and quality of life. 11 Hack et al. 11 showed that women experiencing active involvement in treatment decision for breast cancer reported a significantly higher quality of life than women experiencing passive involvement. Moreover, among these women, decision regret was reported significantly more by women who experienced less involvement in treatment decision than they would have preferred. A passive role in treatment decision making led to greater distress and lower quality of life among breast and prostate cancer patients. 12 Also, satisfaction with treatment decision was positively influenced by level of involvement, with greater patient involvement leading to higher decision satisfaction. 10 Furthermore, treatment adherence is higher for patients experiencing a level of involvement that corresponds to their preference in treatment decision for breast cancer. 13
In the last two decades, research in decision making for cancer treatment increasingly underlined the mismatch between patients’ preferred and perceived level of involvement in decision making. In a previous systematic review on this topic, Tariman et al. 14 concluded that there was disconcordance between the role that patients wanted to play in treatment decision making and the involvement they actually perceived. Hence, more attention for actively involving patients in the SDM process in clinical practice was recommended.
Since 2009, the number of possible treatment options has further increased, which results in even more complex treatment decisions for patients with cancer. In parallel, the rise of values such as autonomy and self‐determination intensify the societal demand for patient involvement in medical decision making. Consequently, the call for more patient‐centred care, boosts the uptake of shared decision making in health care policy. 15 Therefore, for this new era in which SDM seems more important, this systematic review aims to provide an up‐to‐date overview of patients’ preferred and perceived level of involvement in decision making for cancer treatment, the concordance between preferred and perceived involvement and whether these outcomes have improved as compared to a decade ago.
2. METHODS
The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) 2009 checklist was used to report this systematic review. 16 This review was registered in the International Prospective Register of Systematic Reviews (registration number: CRD42020166925).
2.1. Eligibility criteria
We included peer‐reviewed articles published in English. Furthermore, studies needed to (i) include data on adult cancer patients, (ii) report both the preferred and perceived level of involvement in decision making, and (iii) concern decision making for cancer treatment. We excluded studies that performed a qualitative exploration of the role in decision making. If multiple publications were based on data of one study, we only included the publication that best reported the data of all participants.
2.2. Sources and search strategy
A literature search was carried out in PubMed, EMBASE, PsycINFO and CINAHL for articles published between January 2009 and January 2020 (previous review included studies until January 2009). We based our search on the search performed by Tariman et al. 14 which included the medical subject heading terms ‘decision making’, ‘patient participation’ and ‘oncology’. To further detail the search strategy, we added two search terms ‘perception’ and ‘treatment’. Key words and relevant terminology were based on the search terms, index terms and relevant terminology in title/abstract used in so‐called ‘key publications’. These key publications were selected before constructing the search strategy, as publications that answer the research question and should be identifiable in the search results. We validated the final search (Supporting Information 1), by checking whether our ‘key publications’ would be identified in the results of the search. Finally, we performed backward and forward citation tracking to identify any potential relevant missed studies.
2.3. Study selection
Two researchers (EN & LP) independently performed title/abstract screening for eligibility with the use of the online tool ‘Rayyan’. Any discrepancies in the selection of eligible studies based on title/abstract were discussed with a third researcher (CH). Full‐text screening of selected papers was done by two researchers (EN & CH).
2.4. Data collection
The following data were extracted from the individual studies: (1) the percentage of participants preferring predefined levels of involvement, (2) the percentage of participants perceiving these levels of involvement, and ‐ if provided ‐ (3) the percentage of participants with a (within‐person) disconcordance between their preferred and perceived level of involvement.
2.5. Level of involvement
The most commonly used scale in the included studies to measure the preferred and perceived level of involvement, is ‘The Control Preference Scale’ (CPS) designed by Degner et al. 17 The CPS asks patients to reflect on a specific decision and to select one of the five responses (A–E), which best corresponds with their preferred level of involvement (Table 1). These five responses are categorised into either an active, shared or passive decision role.
TABLE 1.
The control preference scale and the translation to decision roles 17
| Response | Control preference scale | Decision role |
|---|---|---|
| A | I prefer to make the final selection about which treatment I will receive | Active |
| B | I prefer to make the final selection of my treatment after seriously considering my doctor's opinion | Active |
| C | I prefer that my doctor and I share responsibility for deciding which treatment is best for me | Shared |
| D | I prefer that my doctor makes the final decision about which treatment, but seriously considers my opinion | Passive |
| E | I prefer to leave all decisions regarding treatment to my doctor | Passive |
Abbreviation: CPS, control preference scale.
Other methods used in included studies to measure the level of involvement in decision making are the Shared Decision Making Questionnaire (SDM‐Q‐9), 18 the Patient Perception Scale (PPS) 19 and the Treatment Decision Making (TDM) examples, designed by Charles et al. 20 , 21 These measurements also allow making a distinction between an active, shared or passive role in decision making.
2.6. Data analysis
From the included individual studies the following data were extracted: the percentage of patients preferring and perceiving an active, shared or passive role and the percentage of (dis)concordance. For studies presenting the percentages for the levels of involvement in five categories (A–E, see Table 1), we calculated the percentage of A plus B for an active decision role, and of D plus E for a passive decisional role. Additionally, if the percentage of (dis)concordance was not provided and if the data allowed, we calculated the overall (dis)concordance and the disconcordance separately for the three levels of involvement. Supporting information 2 shows the presentation of the data of individual studies that allow and do not allow for calculation of the (dis)concordance. Also, if individual studies presented their data in subgroups (such as for different age groups or different types of treatment), we calculated the overall percentages.
Subsequently, we calculated the median percentage and interquartile range of all studies for the: (1) percentage preferred, (2) percentage perceived and (3) percentage disconcordance between preferred and perceived for an active, shared and passive role and 4) the percentage of overall (dis)concordance. We present these medians and interquartile ranges for all included studies together and for the following subgroups: cancer diagnoses (breast, haematologic, lung, (colo) rectal, prostate cancer), culture (Western, Asian), and stage of cancer (early, advanced).
2.7. Quality assessment
For all included studies the quality was independently assessed by two researchers (EN, LP). To assess the risk of bias we used the Newcastle‐Ottawa Scale (NOS). 22 The NOS was originally designed to assess the risk of bias on outcome and study level for cohort and case‐control studies. Previous studies tested 23 and used 24 , 25 a modified version of the NOS to fit cross‐sectional studies. We modified these scales to fit our research (Supporting Information 3). We used the modified version of the NOS for all included studies, as the measurement of the variables of interest (irrespective of study design) was comparable. Quality of studies was scored for the topics ‘selection of participants’ and ‘definition and assessment of the outcome’. Scores could range from 0–9 stars, with 0–3 stars corresponding with a poor quality, 4–6 with a fair quality and 7–9 with a good quality.
2.8. Comparison with Tariman et al.
The steps as described in the data collection and data analysis section were also performed for the individual studies included in the review by Tariman et al. The differences in median percentages of the present review and the review by Tariman et al. were tested for significance with a (non‐parametric) median test for two independent medians. All analyses were performed with IBM SPSS 26.0.0.1 and a p‐value < 0.05 was considered as statistically significant.
3. RESULTS
3.1. Study selection
After removal of duplicates, 4,738 records were identified and screened on title and abstract (Figure 1). Sixty‐eight studies were screened full‐text, of which 28 were eligible. Backward and forward citation tracking yielded three additional studies, resulting in 31 studies for analysis. The main reasons for exclusion was the focus on a diagnosis other than cancer and a focus on decision‐making for cancer care in general instead of cancer treatment specifically.
FIGURE 1.
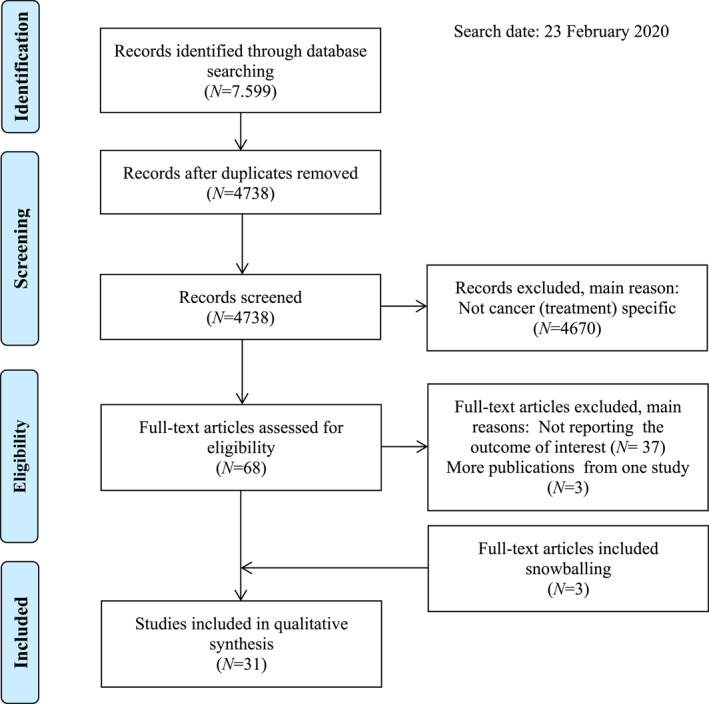
Flowchart for the selection of studies, based on preferred reporting items for systematic reviews and meta‐analyses 16
3.2. Study characteristics
In total, we included 31 studies, with 13,247 cancer patients participating. These patients reflected on 16,537 cancer treatment decisions. Table 2 provides an overview of the included studies. Most studies (N = 13) included breast cancer patients, 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 two studies included patients with haematologic cancer, 39 , 40 two studies lung cancer patients, 41 , 42 one study colorectal cancer patients, 43 two studies prostate cancer patients 44 , 45 and others included various cancers. 9 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 More studies were performed in Western countries, 9 , 27 , 28 , 29 , 30 , 31 , 33 , 35 , 36 , 39 , 40 , 42 , 44 , 45 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 as compared to Asian countries. 26 , 32 , 34 , 37 , 38 , 41 , 43 , 46 Most studies included early stage cancer patients. 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 36 , 37 , 42 , 44 Five studies included advanced stage cancer patients, 35 , 41 , 46 , 48 , 51 eight studies included all stages, 9 , 34 , 38 , 47 , 52 , 53 , 54 and for six studies cancer stage was not reported. 39 , 40 , 43 , 45 , 49 , 50 Most studies used a cross‐sectional design in which patients’ preferred and perceived decision role were measured after treatment decision. 9 , 26 , 27 , 29 , 30 , 32 , 33 , 35 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 Five studies used a prospective study design and measured patients’ preferred decision role before treatment decision and their perceived role afterwards. 28 , 31 , 34 , 36 , 44 Study characteristics of the studies included by Tariman et al. can be found in the original publication. 14
TABLE 2.
Overview of the included studies, presenting study characteristics, the reported level of preferred, perceived involvement and disconcordance between the preferred and perceived level of involvement
| Reference | Research design | Study population | Decision, moment, measurement | Preferred level of involvement | Perceived level of involvement | Disconcordance between preferred and perceived level of involvement | Disconcordance per level |
|---|---|---|---|---|---|---|---|
| N = number of participants, age, type of cancer, stage cancer, country |
|
|
|
||||
| Aminaie, 2019 26 | Cross‐sectional study | N = 328, mean 46 years, breast cancer, stage I‐II, Iran |
|
|
|
Not reported | |
| Atherton, 2013 9 | Cross‐sectional study | N = 594, mostly >60 years, various cancer, stage I‐IV, US |
|
|
|
Disconcordance: 12% | |
| Berger, 2018 27 | Cross‐sectional study | N = 873, mean 59 years, breast cancer, stage I‐III, US |
|
|
|
Disconcordance: 53% | |
| Bieber, 2018 53 | RCT | N = 107, mean 64 years, breast & colon cancer, stage I‐IV, Germany |
|
|
|
Disconcordance: 28% |
|
| Brown, 2012 28 | RCT | N = 683, mean 54 years/57 years, breast cancer, early stage, Australia, New Zealand, & Switzerland, Germany, Austria |
|
|
|
Disconcordance: 63% |
|
| Burton, 2017 29 | Cross‐sectional study | N = 101, included ≥75 years, breast cancer, early stage, UK |
|
|
|
Disconcordance: 26% |
|
| Carey, 2012 39 | Cross‐sectional study | N = 268, mean 60 years, haematologic cancer, stage unknown, Australia |
|
|
|
Disconcordance: 23% |
|
| Engelhardt, 2020 30 | Multicenter observational study | N = 101, mean 61 years, breast cancer, stage I‐III, The Netherlands |
|
|
|
Disconcordance: 51% |
|
| Ghoshal, 2019 46 | Cross‐sectional study | N = 150, median 47 years, various cancer, advanced stage, India |
|
|
|
Not reported | |
| Hamelinck, 2018 31 | Prospective study | N = 122, mean 60 years, breast cancer, early stage, The Netherlands |
|
|
|
Disconcordance: 60% |
|
| Herrmann, 2018 47 | Cross‐sectional study | N = 423, mean 64 years, various cancer, early & advanced stage, Australia |
|
|
|
Disconcordance: 20% |
|
| Hitz, 2013 48 | Cross‐sectional study | N = 480, median 67 years, various cancer, advanced, Switzerland |
|
|
|
Disconcordance: 29% |
|
| Hotta, 2010 41 | Substudy of RCT | N = 28, median 67 years, lung cancer, stage IIIb/IV, Japan |
|
|
|
Disconcordance: 32% |
|
| Hou, 2014 43 | Cross‐sectional study | N = 113, mean 63 years, colorectal cancer, stage unknown, China |
|
|
|
Disconcordance: 28% |
|
| Kehl, 2015 53 | Cross‐sectional study | N = 5315, included 18+, colon & lung cancer, stage I‐IV, US |
|
|
|
Disconcordance: 40% |
|
| Mack, 2019 49 | Cross‐sectional study |
|
|
|
|
Disconcordance: 34% | |
| Mansfield, 2019 50 | Cross‐sectional study | N = 355, mean 61 years, various cancer, stage unknown, Australia |
|
|
|
Disconcordance: 30% |
|
| Moth, 2016 42 | Observational cohort | N = 98, median 64 years, lung cancer, I‐IIIB, Australia & New Zealand |
|
|
|
Disconcordance: 19% |
|
| Moth, 2019 51 | Cross‐sectional study | N = 179, median 74 years, various cancer, advanced stage, Australia |
|
|
|
Disconcordance: 25% |
|
| Nakashima, 2012 32 | Cross‐sectional study | N = 104, majority >50 years, breast cancer, stage 0‐III, Japan |
|
|
|
Disconcordance: 41% |
|
| Nguyen, 2014 33 | Cross‐sectional Study | N = 238, mean 56 years, breast cancer, stage I‐II, France |
|
|
|
Not reported | |
| Nicolai, 2016 54 | Prospective parallel‐group cluster‐randomised controlled trial | N = 71, mean 64 years, breast & colon cancer, stage I‐IV, Germany |
|
|
|
Disconcordance: 34% | |
| Nies, 2017 34 | Cross‐sectional study | N = 204, mean 54 years, breast cancer, all stages, Malaysia |
|
|
|
Disconcordance: 9% |
|
| Palmer, 2013 45 | Cross‐sectional study | N = 181, mean 61 years, prostate cancer, stage unknown, US |
|
|
|
Disconcordance: 3% |
|
| Sepucha, 2009 35 | Pilot intervention study | N = 32, median 55 years, breast cancer, advanced stage, US |
|
|
|
Disconcordance: 62% | |
| Seror, 2013 36 | Cohort study | N = 415, mean 39 years, breast cancer, stage 0‐III, France |
|
|
|
Disconcordance: 46% |
|
| Stacey, 2010 52 | Descriptive study | N = 192, mean 60 years, various cancer and stages, Canada |
|
|
|
Not reported | |
| Van Stam, 2018 44 | Prospective, multicenter, observational study | N = 454, mean 67 years, prostate cancer, cT1‐cT2, Netherlands |
|
|
|
Disconcordance: 17% |
|
| Wang, 2018 37 | Cross‐sectional study | N = 154, mean 47 years, breast cancer, stage 0‐II, Taiwan |
|
|
|
Disconcordance: 31% |
|
| Yamauchi, 2017 38 | Cross‐sectional study | N = 650, included 20‐69 years, breast cancer, stage 0‐IV, Japan |
|
|
|
Disconcordance: 43% | |
| Yogaparan, 2009 40 | Cross‐sectional study | N = 31, mean 64 years, acute myeloid leukaemia, stage unknown, Canada |
|
|
|
Not reported |
Abbreviations: BCS, breast conversing surgey; CPS, control preference scale; CT, chemotherapy; PPS, patient perception scale; RT, radiotherapy; TDM, treatment decision making; SDM, shared decision making. *We only use data 18+.
3.3. Quality of studies
Quality of the included studies ranged from four to eight stars, with 12 studies having a good, 19 a fair and 0 a poor quality (Table 3). Most studies included a selected group of patients, lacked a sample size calculation and a description of the response rate and/or comparability with non‐responders. Also, in some studies the sample was not described clearly, in these cases cancer stage was not reported. Furthermore, in three studies timing of the measurement of patients’ preferred and perceived level of involvement was unclear. For retrospective studies, potential recall bias should be kept in mind.
TABLE 3.
Quality assessment of the individual study, based on a modified version of the NOS
| Selection | Outcome | Total stars | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Clear description sample | Representativeness sample | Sample size | Non responders | Clear variables | Outcome assessment | ||
| Aminaie | 2 | 0 | 0 | 0 | 2 | 2 | 6 |
| Atherton | 2 | 1 | 1 | 0 | 2 | 2 | 8 |
| Berger | 2 | 1 | 0 | 0 | 2 | 2 | 7 |
| Bieber | 2 | 0 | 0 | 0 | 2 | 2 | 6 |
| Brown | 2 | 0 | 0 | 0 | 2 | 2 | 6 |
| Burton | 2 | 0 | 0 | 0 | 2 | 2 | 6 |
| Carey | 1 | 1 | 0 | 0 | 2 | 2 | 6 |
| Engelhardt | 2 | 0 | 1 | 0 | 2 | 1 | 6 |
| Ghoshal | 1 | 0 | 1 | 0 | 1 | 2 | 5 |
| Hamelinck | 2 | 0 | 0 | 0 | 2 | 2 | 6 |
| Herrmann | 2 | 0 | 0 | 1 | 2 | 2 | 7 |
| Hitz | 2 | 1 | 1 | 0 | 2 | 2 | 8 |
| Hotta | 1 | 0 | 0 | 0 | 2 | 2 | 5 |
| Hou | 1 | 0 | 0 | 0 | 1 | 2 | 4 |
| Kehl | 2 | 1 | 0 | 0 | 2 | 2 | 7 |
| Mack | 1 | 0 | 1 | 0 | 2 | 2 | 6 |
| Mansfield | 1 | 0 | 1 | 1 | 2 | 2 | 7 |
| Moth 2016 | 2 | 1 | 0 | 0 | 2 | 2 | 7 |
| Moth 2019 | 2 | 1 | 0 | 0 | 2 | 2 | 7 |
| Nakashima | 2 | 0 | 0 | 0 | 2 | 2 | 6 |
| Nguyen | 2 | 0 | 0 | 0 | 1 | 1 | 4 |
| Nicolai | 2 | 0 | 0 | 0 | 2 | 2 | 6 |
| Nies | 2 | 1 | 0 | 0 | 2 | 2 | 7 |
| Palmer | 2 | 1 | 0 | 0 | 2 | 2 | 7 |
| Sepucha | 2 | 0 | 0 | 0 | 2 | 2 | 6 |
| Seror | 2 | 1 | 0 | 0 | 2 | 2 | 7 |
| Stacey | 1 | 0 | 0 | 0 | 2 | 2 | 5 |
| van Stam | 2 | 1 | 0 | 1 | 2 | 2 | 8 |
| Wang | 2 | 0 | 0 | 0 | 2 | 1 | 5 |
| Yamauchi | 2 | 0 | 0 | 0 | 2 | 2 | 6 |
| Yogaparan | 1 | 0 | 0 | 0 | 2 | 2 | 5 |
Note: Number of stars for ‘selection of participants’ and ‘definition and assessment of the outcome’. Maximum number of stars for selection = 5; Maximum number of stars for outcome = 4. Number of stars 0–3: poor quality, 4–6: fair quality, 7–9: good quality (note that this is based on an adapted scoring from the NOS).
Abbreviation: NOS, Newcastle‐Ottawa scale.
3.4. Preferred level of involvement
The median percentage of patients preferring a shared role for all studies was 46%, 25% for an active role and 27% for a passive role (Table 4 and Supporting Information 4). Subgroup analyses showed minor differences (Table 4). In both studies including haematologic cancer patients, the percentage of patients with a preference for a passive role was higher than for an active or shared role. For prostate cancer patients, the percentage of patients preferring active involvement was higher than for shared and passive involvement. The median percentage of patients preferring an active role was lower for Asian cancer patients (16%) than for Western cancer patients (31%). Patients with advanced cancer less often preferred an active role as compared to early stage cancer patients (median 14%, and 26%, respectively).
TABLE 4.
Comparison of the overall median of the included studies in the review of Tariman et al. and this review, concerning the percentage preferred and perceived active, shared and passive involvement for all studies and for subgroups 14
| Previous Review by Tariman et al. | Present review by Noteboom et al. | |||||||
|---|---|---|---|---|---|---|---|---|
| Preferred | Perceived | Preferred | Perceived | |||||
| N = number of studies, participants, decisions | Median % (IQR) | N = number of studies, participants, decisions | Median % (IQR) | N = number of studies, participants, decisions | Median % (IQR) | N = number of studies, participants, decisions | Median % (IQR) | |
| Active involvement in decision making | ||||||||
| All | N = 19, 5294, 5294 | 24 (19–39) | N = 18, 6079, 6332 | 32 (22–46) | N = 31, 13247, 16537 | 25 (14–36) | N = 31, 13247, 16537 | 27 (20–41) |
| Breast | N = 11, 3830, 3830 | 24 (20–35) | N = 10, 4667, 4667 | 35 (24–51) | N = 13, 4005, 4561 | 18 (9–36) | N = 13, 4005, 4561 | 27 (9–43) |
| Lung | N = 1 a , 2, 22 | 19 | N = 1 a , 22, 22 | 14 | N = 2 a , 126, 126 |
14 27 |
N = 2 a , 126, 126 | 29 |
| 24 | ||||||||
| Haematologic | N = 0 | ‐ | N = 0 | ‐ | N = 2 a , 299, 266 | 25 | N = 2 a , 299, 266 | 20 |
| 16 | 23 | |||||||
| Colorectal | N = 1 a , 55, 55 | 18 | N = 1 a , 55, 55 | 6 | N = 1, 113, 113 | 10 | N = 1 a , 113, 113 | 24 |
| Prostate | N = 4, 853, 853 | 41 (27–53) | N = 4, 853, 853 | 57 (35–78) | N = 2 a , 635, 635 | 89 | N = 2 a , 635, 635 | 87 |
| 45 | 39 | |||||||
| Western | N = 19, 5294, 5294 | 24 (19–39) | N = 18, 6079, 6332 | 32 (22–46) | N = 23, 11516, 14806 | 31 (18–38) | N = 23, 11516, 14806 | 28 (23‐42) |
| Asian | N = 0 | ‐ | N = 0 | ‐ | N = 8, 1731, 1731 | 16 (10–25) | N = 8, 1731, 1731 | 23 (10–29) |
| Early | N = 7, 2090, 2090 | 40 (31–53) | N = 7, 3076, 3076 | 62 (39–77) | N = 12, 3671, 4227 | 26 (15–37) | N = 12, 3671, 4227 | 28 (9–44) |
| Advanced | N = 1 a , 22, 22 | 19 | N = 1 a , 22, 22 | 14 | N = 5, 869, 845 | 14 (9–33) | N = 5, 869, 845 | 21 (13–36) |
| Shared involvement in decision making | ||||||||
| All | N = 19, 5294, 5294 | 42 (28–47) | N = 18, 6079, 6332 | 21 (17–34) | N = 30, 12793, 16083 | 46 (32–56) | N = 30, 12793, 16083 | 39 (22–47) |
| Breast | N = 11, 3830, 3830 | 42 (29–49) | N = 10, 4667, 4667 | 30 (18–36) | N = 13, 4005, 4561 | 48 (29–53) | N = 13, 4005, 4561 | 33 (17–48) |
| Lung | N = 1 a , 2, 22 | 24 | N = 1 a , 22, 22 | 9 | N = 2 a , 126, 126 | 61 | N = 2 a , 126, 126 | 46 |
| 47 | 48 | |||||||
| Haematologic | N = 0 | ‐ | N = 0 | ‐ | N = 2 a , 299, 266 | 30 | N = 2 a , 299, 266 | 22 |
| 32 | 39 | |||||||
| Colorectal | N = 1 a , 55, 55 | 47 | N = 1 a , 55, 55 | 18 | N = 1 a , 113, 113 | 35 | N = 1 a , 113, 113 | 18 |
| Prostate | N = 4, 853, 853 | 43 (38–48) | N = 4, 853, 853 | 30 (15–43) | N = 1 a , 181, 181 | 16 | N = 1 a , 181, 181 | 46 |
| Western | N = 19, 5294, 5294 | 42 (28–47) | N = 18, 6079, 6332 | 21 (17–34) | N = 22, 11062, 13632 | 43 (32–55) | N = 22, 11062, 13632 | 37 (22–43) |
| Asian | N = 0 | ‐ | N = 0 | ‐ | N = 8, 1731, 1731 | 49 (25–60) | N = 8, 1731, 1731 | 45 (22–60) |
| Early | N = 7, 2090, 2090 | 42 (29–48) | N = 7, 3076, 3076 | 17 (14–33) | N = 11, 3217, 3773 | 47 (27–51) | N = 12, 3671, 4227 | 33 (14–48) |
| Advanced | N = 1 a , 22, 22 | 24 | N = 1 a , 22, 22 | 9 | N = 5, 869, 845 | 45 (24–67) | N = 5, 869, 845 | 38 (21–44) |
| Passive involvement in decision making | ||||||||
| All | N = 19, 5294, 5294 | 34 (13–47) | N = 18, 6079, 6332 | 39 (21–76) | N = 31, 13247, 16537 | 27 (16‐44) | N = 31, 13247, 16537 | 34 (22‐46) |
| Breast | N = 11, 3830, 3830 | 34 (15–48) | N = 10, 4667, 4667 | 29 (20–55) | N = 13, 4005, 4561 | 27 (17‐51) | N = 13, 4005, 4561 | 36 (24‐46) |
| Lung | N = 1 a , 2, 22 | 57 | N = 1 a , 22, 22 | 76 | N = 2 a , 126, 126 | 25 | N = 2 a , 126, 126 | 25 |
| 27 | 28 | |||||||
| Haematologic | N = 0, 0, 0 | ‐ | N = 0, 0, 0 | ‐ | N = 2 a , 299, 266 | 46 | N = 2 a , 299, 266 | 58 |
| 52 | 39 | |||||||
| Colorectal | N = 1 a , 55, 55 | 35 | N = 1 a , 55, 55 | 76 | N = 1 a , 113, 113 | 54 | N = 1 a , 113, 113 | 59 |
| Prostate | N = 4, 853, 853 | 16 (8–29) | N = 4, 853, 853 | 15 (7–23) | N = 2 a , 635, 635 | 11 | N = 2 a , 635, 635 | 13 |
| 39 | 15 | |||||||
| Western | N = 19, 5294, 5294 | 34 (13–47) | N = 18, 6079, 6332 | 39 (21–76) | N = 23, 11516, 14806 | 23 (16–38) | N = 23, 11516, 14806 | 36 (21–46) |
| Asian | N = 0 | ‐ | N = 0 | ‐ | N = 8, 1731, 1731 | 35 (16–54) | N = 8, 1731, 1731 | 28 (23–54) |
| Early | N = 7, 2090, 2090 | 12 (10–17) | N = 7, 3076, 3076 | 18 (8–27) | N = 12, 3671, 4227 | 27 (16–54) | N = 12, 3671, 4227 | 31 (23–46) |
| Advanced | N = 1 a , 22, 22 | 57 | N = 1 a , 22, 22 | 76 | N = 5, 869, 845 | 35 (23–49) | N = 5, 869, 845 | 46 (31–55) |
For subgroups that include 1 or 2 studies, the individual percentage(s) is presented.
3.5. Perceived level of involvement
The median percentage of patients perceiving a shared role for all studies was 39%, 27% for an active role and 34% for a passive role (Table 4 and Supporting Information 4). Subgroup analyses showed minor differences (Table 4). For haematologic cancer patients, both studies showed that the percentage of patients perceiving a passive role was higher than those perceiving an active or shared role. In addition, the median percentage of cancer patients perceiving a passive role is somewhat higher for Western patients (36%) as compared to Asians (28%). Also, advanced stage cancer patients perceived a passive role more often when compared to early stage cancer patients (median 46% vs. 31%).
3.6. Concordance between the preferred and perceived level of involvement
Combining all studies, the median percentage of overall concordance between patients’ preferred and perceived level of involvement in decision making for cancer treatment was 70%. Disconcordance was highest for patients preferring a shared role (median 42%), as compared to patients preferring an active (median 26%) or a passive role (median 22%) (Table 5). In subgroup analyses, the overall disconcordance levels were the highest for studies in patients with early stage (44%) and breast cancer (46%).
TABLE 5.
Comparison of the overall median of the included studies in the review of Tariman et al. and this review, concerning the percentage disconcordance and the disconcordance per level presented for all studies and for subgroups 14
| Previous review by Tariman et al. | Present review by Noteboom et al. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Active | Shared | Passive | Overall | Active | Shared | Passive | |||
| Disconcordance | N = number of studies, participants, decisions a | Median % (IQR) | Median % (IQR) | Median % (IQR) | Median % (IQR) | N = number of studies, participants, decisions a | Median % (IQR) | Median % (IQR) | Median % (IQR) | Median % (IQR) |
| All | N = 14, 5054, 5255 | 38 (25–52) | 39 (22–63) | 67 (63–75) | 37 (27–56) | N = 26, 12308, 15598 | 31 (22–44) | 26 (18–41) | 42 (26–59) | 22 (14–40) |
| Breast | N = 10, 4443, 4644 | 38 (30–49) | 30 (13–41) | 65 (60–70) | 41 (19–85) | N = 11, 3439, 3995 | 46 (31–60) | 42 (20–67) | 62 (27–74) | 30 (16–53) |
| Lung | N = 1 b , 22, 22 | 29 | ‐ | ‐ | ‐ | N = 2 b , 126, 126 | 32 | 25 | 35 | 29 |
| 19 | 27 | 15 | 19 | |||||||
| Haematologic | N = 0 | ‐ | ‐ | ‐ | ‐ | N = 1 b , 268, 235 | 23 | 28 | 44 | 8 |
| Colorectal | N = 1 b , 55, 55 | 69 | 100 | 85 | 32 | N = 1 b , 113, 113 | 28 | 14 | 54 | 14 |
| Prostate | N = 0 | ‐ | ‐ | ‐ | ‐ | N = 2 b , 635, 635 | 17 | 11 | ‐ | 67 |
| 3 | 1 | 3 | 10 | |||||||
| Western | N = 14, 5054, 5255 | 38 (25–52) | 39 (22–63) | 67 (63–75) | 37 (27–56) | N = 20, 11055, 14345 | 30 (21‐50) | 27 (19‐42) | 42 (27‐64) | 21 (11‐52) |
| Asian | N = 0 | ‐ | ‐ | ‐ | ‐ | N = 6, 1253, 1253 | 32 (23–42) | 25 (12–48) | 35 (12–50) | 23 (14–33) |
| Early | N = 6, 2760, 2760 | 38 (31–51) | 26 (range 9–34) | 64 (range 58–66) | 41 (range 40–100) | N = 10, 3105, 3661 | 44 (24–55) | 37 (21–64) | 62 (27–74) | 36 (20–62) |
| Advanced | N = 1 b , 22, 22 | 29 | ‐ | ‐ | ‐ | N = 4, 719, 695 | 31 (26–55) | 25 | 37 | 20 |
Numbers are based on the overall disconcordance, numbers of studies, participants and decisions for the disconcordance of the active, shared or passive role might deviate from the numbers for the overall disconcordance, due to the incompleteness of the reported data in the individual studies.
For subgroups that include 1 or 2 studies, the individual percentage(s) is presented.
3.7. Comparison with Tariman et al.
Table 6 shows the difference in outcomes between the review by Tariman et al. and the present review. This table shows that compared to a decade ago the preference for active and shared involvement has somewhat increased, while the preference for passive involvement decreased. The perceived level of shared involvement is significantly higher than a decade ago (median review Tariman et al. 21%, median present review 39%, p = 0.036). The disconcordance between the preferred and perceived level of involvement decreased for all levels of involvement. Presently, the disconcordance in shared involvement is significantly lower than a decade ago (median review Tariman et al. 67%, median present review 42%, p = 0.005).
TABLE 6.
Differences of the overall median of the included studies in the review of Tariman et al. and the present review, concerning the percentage preferred and perceived active, shared and passive involvement for all studies and whether this difference is statistically significant 14
| Previous review by Tariman et al. | Present review by Noteboom et al. | |||
|---|---|---|---|---|
| Involvement | Median % (IQR) | Median % (IQR) | Difference (p‐value) | |
| Preferred | Active | 24 (19–39) | 25 (14–36) | +1 (1.0) |
| Shared | 42 (28–47) | 46 (32–56) | +4 (0.561) | |
| Passive | 34 (13–47) | 27 (16–44) | −7 (0.561) | |
| Perceived | Active | 32 (22–46) | 27 (20–41) | −5 (0.372) |
| Shared | 21 (17–34) | 39 (22–47) | +18 (0.036) a | |
| Passive | 39 (21–76) | 34 (22–46) | −5 (1.0) | |
| Disconcordance | Overall | 38 (25–52) | 31 (22–44) | −7 (0.198) |
| Active | 39 (22–63) | 26 (18–41) | −13 (0.645) | |
| Shared | 67 (63–75) | 42 (26–59) | −25 (0.005) a | |
| Passive | 37 (27–56) | 22 (14–40) | −15 (0.160) |
p‐value < 0.05 was considered as statistically significant.
4. DISCUSSION
This systematic review presents an overview of studies exploring cancer patients’ preferred and perceived level of involvement in decision making for cancer treatment and the (dis‐) concordance between these levels. Pooled results demonstrate that patients’ preferences for and perceptions of their decision role vary, but a majority of the patients preferred and perceived a shared role in decision making. About one in three patients perceived a decision role other than they preferred. Although the majority of cancer patients preferred a shared role in decision making, half of these patients perceived either an active or passive role.
In line with the previous systematic review, we found that patients’ preferences and perceptions for involvement in decision making vary and that disconcordance between preference and perception occurs frequently. 14 Tariman et al. 14 showed that the percentage of patients with prostate and breast cancer preferring a shared or active role is higher than for other cancer types (colorectal, lung, gynaecological). 10 years later this is still the case for breast and prostate cancer patients. For lung cancer, the limited number of new studies suggests a minor shift from both preference for and perception of a passive role, to a more active role. In addition, for breast cancer patients, it seems that the percentage of patients preferring and perceiving passive involvement has decreased. Also, for prostate cancer patients, the percentage of patients perceiving a passive role is now somewhat lower. This is likely to be due to the increased attention for SDM in this field, which together with the rising number of treatment options available with comparable efficacy, urges for more patient involvement in individual treatment decisions. 55 , 56
In summary, compared to the findings of Tariman et al., our review suggests that some progress in patient involvement has been made in the last decade. Patients are more involved in decision making than a decade ago and the disconcordance between the preferred and perceived level of involvement has decreased. Furthermore, although Tariman et al. 14 recommended to perform studies including patients with cancers other than breast cancer and to use a longitudinal design to measure patients’ level of involvement, the majority of studies in our review included breast cancer patients and used a cross‐sectional design. Hence, still longitudinal exploration of patients’ preferences and perceptions of involvement is needed, as preferences for involvement may change over time and since a prospective approach minimises the influence of recall bias on findings. 57 Also, studies should include more patients diagnosed with cancer other than breast cancer.
Our review highlights that even though most patients prefer shared or active involvement, some prefer a passive role more often. Haematologic cancer patients seem to be more likely to prefer and perceive a passive role in treatment decision making as compared to patients with other types of cancer. Ernst et al. 58 suggest that for haematologic cancer this might be due to the complex treatment plan and the perception of the physician as the expert, both impeding patient involvement.
Furthermore, in our results, the majority of the Asian cancer patients preferred and perceived a shared role. This is in contrast with the results of a review by Yilmaz et al. 59 which concluded that most studies including Asian cancer patients (living in Western countries) reported that these patients preferred a passive role in decision making. The difference in cultures between Asian countries might explain this difference, since our review included more Asian patients from Japan, whereas the review of Yilmaz et al. 59 included mostly patients of Chinese origin.
Although it seems that, in the past decade, some progress has been made in actively involving cancer patients in treatment decisions, the suboptimal concordance between patients’ preferred and perceived decision role shows that it remains challenging to involve patients to the level of their preference. Several potential explanations for the disconcordance between patients’ preferred and perceived level of involvement are described in literature. Insufficient creation of awareness among cancer patients that they do have choice 7 and inadequate exploration of patients’ values and preferences by physicians are mentioned as barriers for involvement in SDM. 60 Creating awareness of choice is difficult, since it has been reported that even when a choice in treatment is offered, cancer patients do not always experience having a treatment choice. 61 It is also suggested that physicians incorrectly estimate to what extent their cancer patients want to be involved in treatment decision making, without explicitly asking them. 62 This is further complicated by potential differences in the perception of the extent of involvement between cancer patients and physicians. 41 External factors might also influence the level of involvement. Keating et al. 63 showed that the more evidence based a specific treatment was, the more likely it was that decisions were shared. Also, lack of time during consultations is mentioned by physicians as a barrier for patient involvement. 64 , 65 All these internal and external factors could lead to the involvement of patients in decision making for cancer treatment at a level other than preferred.
4.1. Study limitations
This review has its strengths and limitations. A strength of this review is the large number of studies included and the completeness of the data we retrieved from the studies. A limitation of this review, similar to the review of Tariman et al. 14 is that the majority of the studies in our review included breast cancer patients. Therefore, the overall trends we show in our data might not be generalisable to other cancer diagnosis. In addition, even though the results of randomised trials showed similar results to those with a retrospective design, it should be taken into account that trials may have targeted level of decision involvement with an intervention which could influence results. Also, the data in the included studies does not allow to show the influence of important patient characteristics, such as socioeconomic status, race and health literacy.
4.2. Clinical implications
That said, our findings highlight the variety in preferences for involvement in treatment decision making and challenges of attempting to match the preferred with the perceived level of involvement. Consequently, the main implication for practice is that more actively tailoring of patient involvement to individual preference is needed. This active exploration of preference should be performed at an early stage of the treatment decision process, to enable patients to take their preferred roles in shaping their personalised cancer care. Attempts to create awareness of this among physicians have resulted in improvement, but still more effort is needed. The implementation of tools, such as the three question model, 66 could support physicians in exploring patients’ preferences and enable them to meet these preferences for involvement.
5. CONCLUSION
Patients’ preferences for involvement in cancer treatment decision making vary, but the majority of patients prefers to be involved. A significant number of patients perceive a decisional role other than preferred, especially when patients prefer a shared role. Improvements in patient involvement have been observed in the last decade. However, there is still room for improvement and physicians should be made more aware of the importance of exploring patients’ preferences for involvement in decision making to truly deliver personalised cancer care.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare that are relevant to the content of this article.
ETHICS STATEMENT
This is a systematic review. Ethics approval is not applicable.
Supporting information
Supporting Information 1
Supporting Information 2
Supporting Information 3
Supporting Information 4
ACKNOWLEDGEMENTS
We would like to thank our research assistant, Lianne Potters (LP) for her contribution to the screening and quality assessment of studies for this review. No funding was received for conducting this study.
Noteboom EA, May AM, van der Wall E, de Wit NJ, Helsper CW. Patients' preferred and perceived level of involvement in decision making for cancer treatment: a systematic review. Psychooncology. 2021;30(10):1663‐1679. doi: 10.1002/pon.5750
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
REFERENCES
- 1. Harter M, Moumjid N, Cornuz J, Elwyn G, Van der Weijden T. Shared decision making in 2017: international accomplishments in policy, research and implementation. Z Evidenz Fortbild Q. 2017;123‐124:1‐5. [DOI] [PubMed] [Google Scholar]
- 2. Entwistle VA, Watt IS. Patient involvement in treatment decision‐making: the case for a broader conceptual framework. Patient Educ Counsel. 2006;63(3):268‐278. [DOI] [PubMed] [Google Scholar]
- 3. Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients’ preferences matter. BMJ. 2012;345:e6572. [DOI] [PubMed] [Google Scholar]
- 4. Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Counsel. 2006;60(3):301‐312. [DOI] [PubMed] [Google Scholar]
- 5. Stiggelbout AM, van der Weijden T, De Wit MP, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256. [DOI] [PubMed] [Google Scholar]
- 6. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361‐1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brom L, De Snoo‐Trimp JC, Onwuteaka‐Philipsen BD, Widdershoven GA, Stiggelbout AM, Pasman HR. Challenges in shared decision making in advanced cancer care: a qualitative longitudinal observational and interview study. Health Expect 2017;20(1):69‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kehl KL, Landrum MB, Arora NK, et al. Association of actual and preferred decision roles with patient‐reported quality of care: shared decision making in cancer care. JAMA Oncol 2015;1(1):50‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Atherton PJ, Smith T, Singh JA, et al. The relation between cancer patient treatment decision‐making roles and quality of life. Cancer. 2013;119(12):2342‐2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Orom H, Biddle C, Underwood W III, Nelson CJ, Homish DL. What is a “good” treatment decision? Decisional control, knowledge, treatment decision making, and quality of life in men with clinically localized prostate cancer. Med Decis Mak. 2016;36(6):714‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hack TF, Degner LF, Watson P, Sinha L. Do patients benefit from participating in medical decision making? Longitudinal follow‐up of women with breast cancer. Psycho Oncol. 2006;15(1):9‐19. [DOI] [PubMed] [Google Scholar]
- 12. Hack TF, Pickles T, Ruether JD, et al. Predictors of distress and quality of life in patients undergoing cancer therapy: impact of treatment type and decisional role. Psycho Oncol. 2010;19(6):606‐616. [DOI] [PubMed] [Google Scholar]
- 13. Kahn KL, Schneider EC, Malin JL, Adams JL, Epstein AM. Patient centered experiences in breast cancer: predicting long‐term adherence to tamoxifen use. Medical care, 45. 2007:431‐439. [DOI] [PubMed] [Google Scholar]
- 14. Tariman JD, Berry D, Cochrane B, Doorenbos A, Schepp K. Preferred and actual participation roles during health care decision making in persons with cancer: a systematic review. Ann Oncol. 2010;21(6):1145‐1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elwyn G, Cochran N, Pignone M. Shared decision making‐the importance of diagnosing preferences. JAMA Intern Med. 2017;177(9):1239‐1240. [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264‐269. [DOI] [PubMed] [Google Scholar]
- 17. Degner LF, Sloan JA, Venkatesh P. The control preferences scale. Can J Nurs Res. 1997;29(3):21‐43. [PubMed] [Google Scholar]
- 18. Simon D, Schorr G, Wirtz M, et al. Development and first validation of the shared decision‐making questionnaire (SDM‐Q). Patient Educ Counsel. 2006;63(3):319‐327. [DOI] [PubMed] [Google Scholar]
- 19. Janz NK, Wren PA, Copeland LA, Lowery JC, Goldfarb SL, Wilkins EG. Patient‐physician concordance: preferences, perceptions, and factors influencing the breast cancer surgical decision. J Clin Oncol. 2004;22(15):3091‐3098. [DOI] [PubMed] [Google Scholar]
- 20. Charles C, Gafni A, Whelan T. Shared decision‐making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681‐692. [DOI] [PubMed] [Google Scholar]
- 21. Charles C, Gafni A, Whelan T. Decision‐making in the physician‐patient encounter: revisiting the shared treatment decision‐making model. Soc Sci Med. 1999;49(5):651‐661. [DOI] [PubMed] [Google Scholar]
- 22. Wells GA, Shea B, O’Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐Analyses. 2011. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 23. Moskalewicz A, Oremus M. No clear choice between Newcastle‐Ottawa Scale and Appraisal Tool for Cross‐Sectional Studies to assess methodological quality in cross‐sectional studies of health‐related quality of life and breast cancer. J Clin Epidemiol. 2020;120:94‐103. [DOI] [PubMed] [Google Scholar]
- 24. Modesti PA, Reboldi G, Cappuccio FP, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta‐analysis. PLoS One. 2016;11(1):e0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patra J, Bhatia M, Suraweera W, et al. Exposure to second‐hand smoke and the risk of tuberculosis in children and adults: a systematic review and meta‐analysis of 18 observational studies. PLoS Med. 2015;12(6):e1001835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aminaie N, Lehto RH, Negarandeh R. Iranian Women’s Decision Making Preferred roles, experienced involvement, and decisional conflict when undergoing surgery for early‐stage breast cancer. Clin J Oncol Nurs. 2019;23(5):529‐536. [DOI] [PubMed] [Google Scholar]
- 27. Berger AM, Buzalko RJ, Kupzyk KA, Gardner BJ, Djalilova DM, Otte JL. Preferences and actual chemotherapy decision‐making in the greater plains collaborative breast cancer study. Acta Oncol 2017;56(12):1690‐1697. [DOI] [PubMed] [Google Scholar]
- 28. Brown R, Butow P, Wilson‐Genderson M, Bernhard J, Ribi K, Juraskova I. Meeting the decision‐making preferences of patients with breast cancer in oncology consultations: impact on decision‐related outcomes. J Clin Oncol. 2012;30(8):857‐862. [DOI] [PubMed] [Google Scholar]
- 29. Burton M, Kilner K, Wyld L, et al. Information needs and decision‐making preferences of older women offered a choice between surgery and primary endocrine therapy for early breast cancer. Psycho Oncol. 2017;26(12):2094‐2100. [DOI] [PubMed] [Google Scholar]
- 30. Engelhardt EG, Smets EMA, Sorial I, Stiggelbout AM, Pieterse AH, Hillen MA. Is there a relationship between shared decision making and breast cancer patients’ trust in their medical oncologists? Med Decis Mak. 2020;40(1):52‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hamelinck VC, Bastiaannet E, Pieterse AH, van de Velde CJH, Liefers GJ, Stiggelbout AM. Preferred and perceived participation of younger and older patients in decision making about treatment for early breast cancer: a prospective study. Clin Breast Canc. 2018;18(2):e245‐e253. [DOI] [PubMed] [Google Scholar]
- 32. Nakashima M, Kuroki S, Shinkoda H, Suetsugu Y, Shimada K, Kaku T. Information‐seeking experiences and decision‐making roles of Japanese women with breast cancer. Fukuoka Igaku Zasshi. 2012;103(6):120‐130. [PubMed] [Google Scholar]
- 33. Nguyen F, Moumjid N, Charles C, Gafni A, Whelan T, Carrere MO. Treatment decision‐making in the medical encounter: comparing the attitudes of French surgeons and their patients in breast cancer care. Patient Educ Counsel. 2014;94(2):230‐237. [DOI] [PubMed] [Google Scholar]
- 34. Nies YH, Islahudin F, Chong WW, et al. Treatment decision‐making among breast cancer patients in Malaysia. Patient Prefer Adherence. 2017;11:1767‐1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sepucha KR, Ozanne EM, Partridge AH, Moy B. Is there a role for decision aids in advanced breast cancer? Med Decis Mak. 2009;29(4):475‐482. [DOI] [PubMed] [Google Scholar]
- 36. Seror V, Cortaredona S, Bouhnik AD, et al. Young breast cancer patients’ involvement in treatment decisions: the major role played by decision‐making about surgery. Psycho Oncol. 2013;22(11):2546‐2556. [DOI] [PubMed] [Google Scholar]
- 37. Wang AWT, Chang SM, Chang CS, et al. Regret about surgical decisions among early‐stage breast cancer patients: effects of the congruence between patients’ preferred and actual decision‐making roles. Psycho Oncol. 2018;27(2):508‐514. [DOI] [PubMed] [Google Scholar]
- 38. Yamauchi K, Nakao M, Nakashima M, Ishihara Y. Congruence between preferred and actual participation roles increases satisfaction with treatment decision making among Japanese women with breast cancer. Asian Pac J Cancer Prev. 2017;18(4):987‐994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carey M, Anderson A, Sanson‐Fisher R, Lynagh M, Paul C, Tzelepis F. How well are we meeting haematological cancer survivors’ preferences for involvement in treatment decision making? Patient Educ Counsel. 2012;88(1):87‐92. [DOI] [PubMed] [Google Scholar]
- 40. Yogaparan T, Panju A, Minden M, Brandwein J, Mohamedali HZ, Alibhai SM. Information needs of adult patients 50 or older with newly diagnosed acute myeloid leukemia. Leuk Res. 2009;33(9):1288‐1290. [DOI] [PubMed] [Google Scholar]
- 41. Hotta K, Kiura K, Takigawa N, et al. Desire for information and involvement in treatment decisions lung cancer patients’ preferences and their physicians’ perceptions: results from Okayama lung cancer study group trial 0705. J Thorac Oncol. 2010;5(10):1668‐1672. [DOI] [PubMed] [Google Scholar]
- 42. Moth E, McLachlan SA, Veillard AS, et al. Patients’ preferred and perceived roles in making decisions about adjuvant chemotherapy for non‐small‐cell lung cancer. Lung Canc. 2016;95:8‐14. [DOI] [PubMed] [Google Scholar]
- 43. Hou X‐T, Pang D, Lu Q, Xu Z, Zhou Y‐J. Preferred and actual participation roles in operation treatment decision making of patients with colorectal cancer. Int J Nurs Sci. 2014;1(4):376‐380. [Google Scholar]
- 44. Van Stam MA, Pieterse AH, van der Poel HG, et al. Shared decision making in prostate cancer care‐encouraging every patient to be actively involved in decision making or ensuring the patient preferred level of involvement? J Urol. 2018;200(3):582‐589. [DOI] [PubMed] [Google Scholar]
- 45. Palmer NR, Tooze JA, Turner AR, Xu J, Avis NE. African American prostate cancer survivors’ treatment decision‐making and quality of life. Patient Educ Counsel. 2013;90(1):61‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ghoshal A, Damani A, Muckaden MA, Yennurajalingam S, Salins N, Deodhar J. Patient’s decisional control preferences of a cohort of patients with advanced cancer receiving palliative care in India. J Palliat Care. 2019;34(3):175‐180. [DOI] [PubMed] [Google Scholar]
- 47. Herrmann A, Hall A, Sanson‐Fisher R, Zdenkowski N, Watson R, Turon H. Not asking cancer patients about their preferences does make a difference. A cross‐sectional study examining cancer patients’ preferred and perceived role in decision‐making regarding their last important cancer treatment. Eur J Canc Care. 2018;27(5). [DOI] [PubMed] [Google Scholar]
- 48. Hitz F, Ribi K, Li Q, Klingbiel D, Cerny T, Koeberle D. Predictors of satisfaction with treatment decision, decision‐making preferences, and main treatment goals in patients with advanced cancer. Support Care Canc. 2013;21(11):3085‐3093. [DOI] [PubMed] [Google Scholar]
- 49. Mack JW, Fasciano KM, Block SD. Adolescent and young adult cancer patients’ experiences with treatment decision‐making. Pediatrics. 2019;143(5). [DOI] [PubMed] [Google Scholar]
- 50. Mansfield E, Bryant J, Carey M, Turon H, Henskens F, Grady A. Getting the right fit: convergence between preferred and perceived involvement in treatment decision making among medical oncology outpatients. Health Sci Rep. 2019;2(1):e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moth E, Kiely BE, Martin AJ, et al. Older adults’ preferred and perceived roles in decision making about palliative chemotherapy: their decision priorities, and information preferences. J Geriatr Oncol. 2019;37(15). [DOI] [PubMed] [Google Scholar]
- 52. Stacey D, Paquet L, Samant R. Exploring cancer treatment decision‐making by patients: a descriptive study. Curr Oncol. 2010;17(4):85‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bieber C, Nicolai J, Gschwendtner K, et al. How does a shared decision‐making (SDM) intervention for oncologists affect participation style and preference matching in patients with breast and colon cancer? J Canc Educ. 2018;33(3):708‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nicolai J, Buchholz A, Seefried N, et al. When do cancer patients regret their treatment decision? A path analysis of the influence of clinicians’ communication styles and the match of decision‐making styles on decision regret. Patient Educ Counsel. 2016;99(5):739‐746. [DOI] [PubMed] [Google Scholar]
- 55. Jani AB, Hellman S. Early prostate cancer: clinical decision‐making. Lancet. 2003;361(9362):1045‐1053. [DOI] [PubMed] [Google Scholar]
- 56. Makarov DV, Chrouser K, Gore JL, et al. AUA white paper on implementation of shared decision making into urological practice. Urol Pract. 2016;3(5):355‐363. [DOI] [PubMed] [Google Scholar]
- 57. Butow PN, Maclean M, Dunn SM, Tattersall MH, Boyer MJ. The dynamics of change: cancer patients’ preferences for information, involvement and support. Ann Oncol. 1997;8(9):857‐863. [DOI] [PubMed] [Google Scholar]
- 58. Ernst J, Kuhnt S, Schwarzer A, et al. The desire for shared decision making among patients with solid and hematological cancer. Psycho Oncol. 2011;20(2):186‐193. [DOI] [PubMed] [Google Scholar]
- 59. Yilmaz NG, Schouten BC, Schinkel S, van Weert JCM. Information and participation preferences and needs of non‐Western ethnic minority cancer patients and survivors: a systematic review of the literature. Patient Educ Counsel. 2019;102(4):631‐650. [DOI] [PubMed] [Google Scholar]
- 60. Kunneman M, Marijnen CA, Baas‐Thijssen MC, et al. Considering patient values and treatment preferences enhances patient involvement in rectal cancer treatment decision making. Radiother Oncol. 2015;117(2):338‐342. [DOI] [PubMed] [Google Scholar]
- 61. Jansen SJT, Otten W, Stiggelbout AM. Factors affecting patients’ perceptions of choice regarding adjuvant chemotherapy for breast cancer. Breast Canc Res Treat. 2006;99(1):35‐45. [DOI] [PubMed] [Google Scholar]
- 62. Elit L, Charles CA, Gafni A. Oncologists’ perceptions of recurrent ovarian cancer patients’ preference for participation in treatment decision making and strategies for when and how to involve patients in this process. Int J Gynecol Canc. 2015;25(9):1717‐1723. [DOI] [PubMed] [Google Scholar]
- 63. Keating NL, Landrum MB, Arora NK, et al. Cancer patients’ roles in treatment decisions: do characteristics of the decision influence roles? J Clin Oncol. 2010;28(28):4364‐4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. O’Brien MA, Ellis PM, Whelan TJ, et al. Physician‐related facilitators and barriers to patient involvement in treatment decision making in early stage breast cancer: perspectives of physicians and patients. Health Expect 2013;16(4):373‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Legare F, Ratte S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision‐making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Counsel. 2008;73(3):526‐535. [DOI] [PubMed] [Google Scholar]
- 66. Shepherd HL, Barratt A, Trevena LJ, et al. Three questions that patients can ask to improve the quality of information physicians give about treatment options: a cross‐over trial. Patient Educ Counsel. 2011;84(3):379‐385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information 1
Supporting Information 2
Supporting Information 3
Supporting Information 4
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


