Abstract
It has been almost two decades since the first link between microRNAs and cancer was established. In the ensuing years, this abundant class of short noncoding regulatory RNAs has been studied in virtually all cancer types. This tremendously large body of research has generated innovative technological advances for detection of microRNAs in tissue and bodily fluids, identified the diagnostic, prognostic, and/or predictive value of individual microRNAs or microRNA signatures as potential biomarkers for patient management, shed light on regulatory mechanisms of RNA–RNA interactions that modulate gene expression, uncovered cell‐autonomous and cell‐to‐cell communication roles of specific microRNAs, and developed a battery of viral and nonviral delivery approaches for therapeutic intervention. Despite these intense and prolific research efforts in preclinical and clinical settings, there are a limited number of microRNA‐based applications that have been incorporated into clinical practice. We review recent literature and ongoing clinical trials that highlight most promising approaches and standing challenges to translate these findings into viable microRNA‐based clinical tools for cancer medicine.
This article is categorized under:
RNA in Disease and Development > RNA in Disease
Keywords: cancer, clinical trials, diagnostics, microRNA, miR, miRNA, noncoding RNA, therapeutic, tumor
microRNAs are short noncoding regulatory RNAs with important roles in cancer biology. microRNAs are informative biomarkers for early cancer detection and treatment decisions. microRNAs are being evaluated in clinical trials as new drugs for personalized cancer medicine.
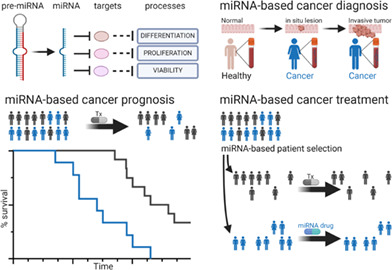
1. INTRODUCTION
microRNAs (miRNAs) are an evolutionarily conserved gene class of short noncoding regulatory RNAs (Bartel, 2018; Fromm et al., 2015; Gebert & MacRae, 2019; Sempere, 2019). The discovery, detection, biology, and clinical applications of miRNAs are intertwined with those of small interfering RNAs (siRNAs) (Sempere, 2019; Titze‐de‐Almeida et al., 2017). Accolades to these classes of short noncoding RNAs include Breakthrough of the Year 2002 for new roles for RNAs from Science magazine; the Nobel Prize in Physiology or Medicine 2006 to Andrew Fire and Craig Mello for their discovery of RNA interference—gene silencing by double‐stranded RNA; the 2008 Albert Lasker Award for Basic Medical Research to Victor Ambros, Gary Ruvkun, and David Baulcome for their discovery of an unanticipated world of tiny RNAs that regulate gene activity; and the 2015 Breakthrough Prize to Victor Ambros and Gary Ruvkun for their co‐discovery of microRNAs. The recent clinical success and FDA approval of two siRNA drugs, patisiran, and givosiran (Roberts et al., 2020), have paved the way for similar miRNA‐based drugs to reach the clinic in the near future. Unlike exogenous siRNAs, miRNAs are single stranded RNAs that result from the transcription and processing of endogenous longer precursor RNAs. Briefly, most primary miRNA transcripts are transcribed by RNA polymerase II from which a stereotypical stem‐loop hairpin precursor RNA is cleaved in the nucleus by the RNAse III enzyme Drosha, a core component of the microprocessor complex (Bartel, 2018; Gebert & MacRae, 2019). Once in the cytoplasm, the RNAse III enzyme Dicer cleaves the pre‐miRNA releasing the mature and biologically active miRNA strand, which is loaded in the Argonaute‐containing miRNA‐induced silencing complex (miRISC) (Bartel, 2018; Gebert & MacRae, 2019). In humans and other mammals, the mature miRNA guides the miRISC to partially complementary site(s) on the 3′UTR of target mRNAs, which leads to Argonaute‐mediated recruitment of adaptor protein TNRC6 and subsequent recruitment of deadenylase complexes such as CCR4‐NOT, that primarily triggers mRNA decay and secondarily, and to a lesser extent, translational repression (Bartel, 2018; Gebert & MacRae, 2019). Generally, miRNAs can interact with hundreds of target mRNAs and more abundant miRNAs more significantly decrease protein output of a larger number of their target genes (Bartel, 2018; Thomson & Dinger, 2016). Collectively, miRNA‐mediated regulation can affect up to 60% of the transcriptome of a given cell having a global influence on protein output and cell function (Bartel, 2018; Gebert & MacRae, 2019; Thomson & Dinger, 2016).
A myriad of regulatory mechanisms that affect miRNA activity have been associated with carcinogenic processes. Many of these regulatory mechanisms disrupt miRNA biogenesis at different stages that ultimately alter the levels of mature and biologically active miRNA molecules: copy number alterations at the chromosomal or region‐specific levels, epigenetic and transcriptional regulation, nuclear export, RNA processing and stability (Gebert & MacRae, 2019; Peng & Croce, 2016; Rupaimoole & Slack, 2017; Sempere & Kauppinen, 2009). Generally, levels of mature miRNA expression correlate well with miRNA activity (Anfossi et al., 2018; Gebert & MacRae, 2019; Thomson & Dinger, 2016). Hence, profiling of miRNA expression in cancer has served not only to identify differentially expressed miRNAs that could have diagnostic, prognostic, and/or predictive value, but also to uncover the etiological role of specific miRNAs relevant to cancer initiation, progression, and/or metastasis (Anfossi et al., 2018; Graveel et al., 2015; Peng & Croce, 2016; Rupaimoole & Slack, 2017; Sempere, 2014b; Sempere & Kauppinen, 2009). Nonetheless, it is worth mentioning some regulatory mechanisms that can affect miRNA activity without significantly altering miRNA expression such as isomer formation (Bartel, 2018; Gebert & MacRae, 2019), post‐translational modifications of miRISC components (Gebert & MacRae, 2019), 3′UTR shortening of target mRNAs (Hoffman et al., 2016; Mao et al., 2020; Mayr & Bartel, 2009), and RNA–RNA interactions (Anfossi et al., 2018; Gebert & MacRae, 2019; Thomson & Dinger, 2016). These diverse classes of noncoding RNAs can modulate the activity of miRNAs by either serving as precursors, stabilizers, decoys or sponges (Anfossi et al., 2018; Gebert & MacRae, 2019; Thomson & Dinger, 2016). Several studies provide experimental evidence that specific RNA–RNA interactions can have a profound effect on miRNA activity and cancer phenotype (Anfossi et al., 2018; Gebert & MacRae, 2019; Thomson & Dinger, 2016) via miRNA sequestration and target displacement as postulated by the endogenous competing RNA (ceRNA) hypothesis (Poliseno et al., 2010; Salmena et al., 2011). Additional mechanisms may be at play since a single ceRNA may not be able to displace a sufficiently large number of miRNA molecules to have a biologically detectable effect on a particular cognate target gene (Bartel, 2018; Gebert & MacRae, 2019; Thomson & Dinger, 2016).
This advanced review focuses on the most promising approaches and standing challenges to translate recent cancer research findings into viable microRNA‐based clinical tools for cancer medicine. We conducted a systematic PubMed search for articles that included the terms microRNA, Cancer, or Tumor, Diagnostic or Therapeutic in their Title or Abstract sections (microRNA[Title/Abstract] AND (Cancer[Title/Abstract] OR Tumor[Title/Abstract]) AND (Diagnostics[Title/Abstract] OR Therapeutic[Title/Abstract]) AND “2015/08/01”[Date–Entry]: “2020/11/01”[Date–Entry]) as well as active clinical trials registered at clinicaltrials.gov. Earlier studies of great significance may be cited directly via reference to original publication or indirectly via recent reviews in that topic area.
2. MICRORNA‐BASED DIAGNOSTICS
Seminal studies (Lu et al., 2005; Volinia et al., 2006) of miRNA expression profiling in healthy control and tumor tissues identified a core set of miRNAs (let‐7, miR‐10b, ‐15, ‐16, ‐17‐5p, ‐20a, ‐21, ‐29b, ‐34, ‐126, ‐145, ‐155, ‐221) with altered expression in multiple hematological and solid tumors, whereas altered expression of other miRNAs was specific to a particular cancer type or subtype (Barbarotto et al., 2008; Peng & Croce, 2016; Sempere, 2014b; Sempere et al., 2010). These initial discoveries were followed by more refined and focused studies to exploit the differential expression of miRNAs as diagnostic, prognostic, and/or predictive indicators. We review some of the largest retrospective studies conducted to illustrate the potential of specific miRNAs or miRNA signatures to improve patient management and how these studies have been received and could be implemented in the clinic based on current clinical trials registered at clinicantrials.gov. There is a clear trend in the recent literature and on‐going clinical trials to develop miRNA‐based noninvasive clinical assays using blood as starting material, whereas initial discovery, diagnostic, and prognostic studies mainly used tissues from diagnostic biopsies or surgical procedures.
2.1. Technological advances for microRNA detection
The specific and sensitive detection of the short sequence of a mature miRNA presents some challenges that have been overcome by different technological advances (Figure 1). Classic hybridization‐based detection methods, such as northern blot and RNA protection assays, require a large amount of total RNA (>1 μg) for analysis and are low‐throughput. Capture‐probe microarray and bead platforms were major technological advances in the mid 2000s for high‐throughput miRNA expression analysis in bulk tumor tissue samples (Liu et al., 2004; Lu et al., 2005; Nelson et al., 2004; Volinia et al., 2006). A reverse transcription quantitative‐polymerase chain reaction (RT‐qPCR) assay emerged shortly after as a highly sensitive method for detection of miRNA expression from a small amount of starting materials (>25 pg; Chen et al., 2005) and quickly became an orthogonal and gold‐standard method for validation of differential miRNA expression. This RT‐qPCR assay overcame short size challenge by providing an extended sequence in a stem‐loop primer with a secondary structure conformation that enables only reverse transcription of the miRNA sequence with a complementary and size‐match 3′ ending (C. Chen et al., 2005), which was followed by real‐time qPCR with a miRNA‐specific TaqMan® probe (Chen et al., 2005). Other RT‐qPCR assays overcame the short size challenge by extending the miRNA sequence via enzymatic addition of a poly(A) tail or another known sequences, two‐tailed RT primer with partial complementarity to the miRNAs at both ends and an internal hairpin to extend the sequence; miRNA levels are quantitated by qPCR assay with a TaqMan® probe or a DNA intercalating dye such as SYBR® Green (Forero et al., 2019). In general, these different RT‐qPCR assays produce similar readouts, but there are some discrepancies due to the terminal sequence of the miRNA (e.g., tolerance for isomers) and enzymatic preference for some bases at specific positions (Anfossi et al., 2018; Forero et al., 2019; Graveel et al., 2015). The original stem‐loop and other RT‐qPCR assays on an array card format can be used as a high‐throughput and sensitive method to analyze the expression of up to 384 known miRNAs. There are other methods such as NanoString nCounter microRNA assay with high‐throughput capability up to 800 miRNAs and high sensitivity (Foye et al., 2017). Next generation RNA sequencing (RNAseq) analysis is a more recent technology that has some advantages in the discovery setting since it provides deep expression analysis of well‐annotated miRNAs and their variants (e.g., isomers) as well as unknown miRNAs, but it is also a more resource‐intensive technology (Andres‐Leon et al., 2016; Aparicio‐Puerta et al., 2020; Lu et al., 2018). Likely technical advances in spatial transcriptomics, single‐cell RNAseq, and microfluidic sorting (Drula et al., 2020; Eng et al., 2019; Nagarajan et al., 2020; Rodriques et al., 2019; Wang et al., 2019; Yoosuf et al., 2020) will soon enable detection of miRNA expression at an unprecedented level of single‐cell, and even single‐extracellular vesicle, resolution.
FIGURE 1.
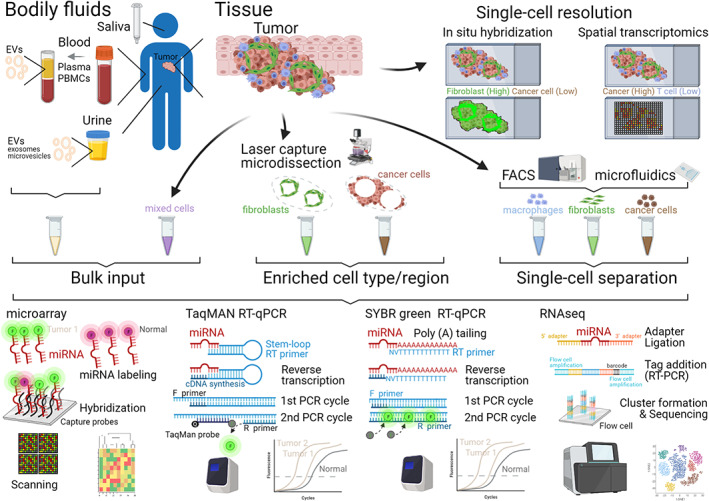
Biological source and detection technologies for miRNA expression analysis. Sensitive and specific detection technologies enable detection of miRNAs from tumor tissue or bodily fluids. These biological samples can be used as bulk input for miRNA analysis or can be further processed with different methodologies to refine the cellular (e.g., cancer cells vs. immune cells in tissue samples) or circulating source (e.g., extracellular vesicles vs. cell‐free in plasma samples) of miRNAs. Key steps of detection and/or readout of each detection technology are shown (see Tables 1 and 2 for more details on studies applying these technologies). Molecules and constructs not drawn to scale. EVs, extracellular vesicles; FACS, fluorescence‐activated cell sorting; F or R primer, forward or reserve primer; PBMCs, peripheral blood mononuclear cells
Independent studies with similar intent for clinical application of altered miRNA expression have often reported discordant results (Graveel et al., 2015; Jarry et al., 2014). The detection method (e.g., RNAseq, microarray chips, and RT‐qPCR), normalization and analytical tools, sample preparation (e.g., fresh, frozen, or fixed), source and quality of starting RNA material (e.g., bulk tissue vs. sorted cells, serum vs. plasma), patient characteristics (e.g., sex, age, ethnicity, stage, treatment history), sample size, study design (e.g., single cohort, vs. training and validation set), and statistical tools can affect the miRNA expression readout (Anfossi et al., 2018; Bahnassy et al., 2018; Drula et al., 2020; Foye et al., 2017; Graveel et al., 2015; Jarry et al., 2014; Nik Mohamed Kamal & Shahidan, 2019; Sempere et al., 2017; Skjefstad et al., 2018). Thus, it is important to understand the influence of all these variables in experimental design and analysis among studies to identify and translate the most robust protocols and informative miRNAs into clinical assays.
2.1.1. Considerations for microRNA detection in tissues
Total RNA extraction from bulk normal or tumor tissue is the most common approach for analysis of miRNA expression using standard detection methods (Figure 1). When RNA is extracted from snap‐frozen tissue, it is typically difficult to estimate the content of cancer cell and other cell types of the tumor microenvironment (TME) vis‐a‐vis immune cells and reactive stroma or remaining residual normal tissue. Improved methods for extracting RNAs from formalin‐fixed paraffin embedded (FFPE) tissues not only opened the possibility for conducting large retrospective studies with archival tissue blocks, but also for selecting tissue sections with higher content of cancer cells. However, these bulk tissue analyses cannot determine the specific cell source(s) of altered miRNA expression, which may lead to result misinterpretation (Kent et al., 2014; Nielsen, 2012; Sempere, 2014b; Sempere et al., 2010, 2020; Svoronos et al., 2016). Intra‐ and intertumoral heterogeneity can affect detected levels of a miRNA without a true change in miRNA expression but rather a change in the number of miRNA‐expressing cells present. Lower detected levels of a miRNA can reflect an etiological relevant downregulation in cancer cells or simply a loss or decreased representation of an expressing cell type in the tumor mass (e.g., adipocytes in breast cancer, acinar cells in pancreatic ductal adenocarcinoma, smooth muscle cells of the muscularis mucosa in colorectal cancer; Andrew et al., 2014; Kent et al., 2014; Kjaer‐Frifeldt et al., 2012; Nielsen et al., 2011; Sempere et al., 2007, 2010). Similarly, higher detected levels of a miRNA can reflect an etiologically relevant upregulation in cancer cells or simply a gain or increased representation of an expressing cell type in the tumor mass (e.g., tumor‐associated macrophages in breast cancer, myofibroblasts in pancreatic ductal adenocarcinoma and colorectal cancer; Preis et al., 2011; Sempere et al., 2007, 2010). Different methods have been deployed to detect miRNA expression more precisely at a single‐cell level. Laser capture microdissection of discrete tissue regions followed by quantitative analysis revealed that altered expression of some miRNA in neoplastic cells correlates with the stage of malignant transformation (Caponi et al., 2013; du Rieu et al., 2010; Han et al., 2017; Paterson et al., 2013; Smith et al., 2015), whereas altered expression of other miRNAs occurs in fibroblasts or other noncancer cell types of the TME (Bumrungthai et al., 2015; Han et al., 2017; Kent et al., 2014; Nielsen et al., 2011). In situ hybridization (ISH) assays with locked nucleic acid (LNA)‐modified DNA probes enables detection of miRNA expression at single‐cell resolution (Figure 1). While ISH assay is not as sensitive or quantitative as other methods, it uses workflow compatible with clinical immunohistochemical assays and can provide compartment‐specific diagnostic and prognostic information (Nielsen, 2012; Sempere, 2014a, 2014b; Sempere et al., 2020; Warford, 2016). Other approaches to determine miRNA expression in individual cells or cell pools enriched for specific cell‐type markers require physical separation methods (Figure 1) such as magnetic bead pull down, fluorescence‐activated cell sorting (FACS) or microfluidics (Hoefig & Heissmeyer, 2010; Li et al., 2019; Petriv et al., 2010; Wu et al., 2013).
2.1.2. Considerations for microRNA detection in bodily fluids
Blood and other bodily fluids (e.g., stool, urine, and saliva) are readily accessible by noninvasive means and thus can provide convenient and longitudinal measurements of circulating miRNA levels. Blood has been more frequently used as starting material than other bodily fluids to detect changes in miRNA levels associated with a specific cancer condition (Tables 1 and 2). miRNAs can be present in a variety of forms in blood: free, in protein complexes, or encapsulated in extracellular vesicles, but also in circulating immune cells, erythrocytes or platelets (Nik Mohamed Kamal & Shahidan, 2019; Sempere et al., 2017; Wu et al., 2017). Cell‐free circulating miRNAs may derive from lysed cancer cells or other cells in the TME due to inflammation and immune responses. Extracellular vesicle‐bound miRNAs may derive from cell‐to‐cell communications between cancer cells and TME or between immune cells mounting response against the tumor, may be shed from cancer cells as unwanted content (e.g., tumor suppressive miRNAs; Anfossi et al., 2018; Nik Mohamed Kamal & Shahidan, 2019; Sempere et al., 2017). miRNA levels can also vary in peripheral blood mononuclear cells (PBMCs) either as a change in the representation of lymphocytes and monocytes and/or activation of these immune cell types in response to the tumor (Ma, Lin, et al., 2015; Mishra et al., 2015; Mosallaei et al., 2020; Wang et al., 2013). Thus, blood samples need to be processed in different ways depending on the informative source of miRNA origin, and promptly and consistently to avoid unintended contamination from other sources such as lysed erythrocytes (haemolysis; Anfossi et al., 2018; Graveel et al., 2015; Jarry et al., 2014; Schwarzenbach, 2017). Plasma or serum has been used as starting material for miRNA analysis, but their content of cell‐free and EV‐bound miRNAs varies significantly (Graveel et al., 2015; Jarry et al., 2014; Nik Mohamed Kamal & Shahidan, 2019; Wang et al., 2012). Data normalization presents a greater challenge in blood and other bodily fluid studies than in tissues because the origin of the testing miRNAs and reference miRNA or other RNAs may be different and unrelated in this cell‐free medium (Anfossi et al., 2018; Graveel et al., 2015; Jarry et al., 2014). A generally accepted approach to address this challenge is to start with an identical sample volume, use an exogenous spike‐in RNA such as Cel‐miR‐39, and use a panel of relatively stable miRNAs for that diseased state rather than a single gene reference such as small nuclear U6 RNA (Anfossi et al., 2018; Jarry et al., 2014). Even with this best practice approach, some patient‐to‐patient variables can affect normalization. For example, hydration status of the patient and fluid retention due to medications or other conditions can alter miRNA concentration (e.g., blood and urine) and provide an inaccurate readout based on volume normalization. Similarly, there is no universal set of stable housekeeping miRNAs that can be used for normalization in all cancer types or that remain stable regardless of age, sex, or other variables of each individual patient.
TABLE 1.
Retrospective studies evaluating microRNAs as diagnostic, prognostic, and/or predictive indicators
| miRNA(s) | Source | Technology | Cancer site | Study design | Clinical application (s) | References |
|---|---|---|---|---|---|---|
| miR‐34a | Tissue (TMA) | LNA ISH | Bladder | Population‐based (n = 229) | Prognostic value of epithelial cell expression | Andrew et al. (2014) |
| miR‐21 | Tissue (TMA) | LNA ISH | Bladder | Testing (n = 232) | Prognostic value of both epithelial and stroma cell expression in urothelial carcinoma (80% of the bladder) | Ohno et al. (2016) |
| miR‐222 | Tissue (Frozen) | RT‐qPCR | Bladder | Testing (n = 387) | Diagnostic value for separating from malignant from normal tissue and prognostic value | Tsikrika et al. (2018) |
| miR‐658,‐762,‐4281,‐4649‐5p,‐4665‐3p,‐4736,‐6836‐3p | Blood (Serum) | 3D‐Gene® Human miRNA Oligo Chip | Bone and soft tissue | Discovery (n = 161), training (n = 276), validation (n = 356) | Diagnostic value of miRNA signature for early disease detection (sarcomas) and for separating malignant tumors from benign conditions | Asano et al. (2019) |
| miR‐1908 | Tissue (Frozen) | RT‐qPCR | Bone | Testing (n = 424) | Prognostic value in osteosarcoma | Lian et al. (2016) |
| miR‐1915‐3p,‐3679‐5p,‐4763‐3p | Blood (Serum) | 3D‐Gene® Human miRNA Oligo Chip | Brain | Training (n = 300), validation (n = 280) | Diagnostic value of miRNA signature for early disease detection (diffuse glioma) | Ohno et al. (2019) |
| miR‐1246,‐1307‐3p,‐1364,‐6861‐5p,‐6875‐5p | Blood (Serum) | 3D‐Gene® Human miRNA Oligo Chip | Breast | Training (n = 2081), validation (n = 2549) | Diagnostic value of miRNA signature for early disease detection | Shimomura et al. (2016) |
| let‐7b‐5p,miR‐16‐5p,‐19a‐3p,‐19b‐3p,‐20a‐5p,‐25‐3p,‐92a‐3p,‐93‐5p,‐106a‐5p,‐223‐3p,‐425‐5p,‐451a | Blood (Serum) | qRT‐PCR | Breast | Discovery (n = 48), training (n = 64), testing (n = 246), validation (n = 72) | Diagnostic value of miRNA signature for early disease detection | Zou, Xia, et al. (2020) |
| miR‐629‐3p,‐4710 | Blood (Serum) | 3D‐Gene® Human miRNA Oligo Chip | Breast | Training (n = 460) and validation (n = 461) | Diagnostic value of miRNA signature in combination with clinicopathological factors for detection of lymph node metastasis | Shiino et al. (2019) |
| miR‐26a‐5p,‐29c‐3p,‐29c‐5p,‐30b‐5p,‐148a‐3p,‐361‐3p,‐645,‐652‐5p,‐934 | Tissue (FFPE) | qRT‐PCR | Breast | Training (n = 318), testing (n = 318), validation (n = 204) | Diagnostic value of miRNA signature in combination with clinicopathological factors for detection of lymph node metastasis | Xie et al. (2018) |
| miR‐21,‐96,‐139,‐141,‐145,‐182,‐183,‐200a,‐429 | Tissue (TCGA) | Bioinformatics (RNAseq) | Breast | TCGA data (n = 1214) | Diagnostic value of miRNA signature for early disease detection and for separating malignant tumors from normal tissue | Xiong et al. (2017) |
| miR‐24‐3p | Blood (Plasma), Tissue (TCGA) | Nanostring, Bioinformatics (miRNAseq) | Breast | Testing (n = 230), TCGA validation (n = 1024) | Prognostic value and predictive value of metastatic disease | Khodadadi‐Jamayran et al. (2018) |
| miR‐21 | Tissue (TMA) | LNA ISH | Breast | Validation (n = 901) | Prognostic value of tumor‐associated fibroblast expression based on ER/PR/HER2 tumor classification | MacKenzie et al. (2014) |
| Let‐7b | Tissue (TMA) | LNA ISH | Breast | Population‐based (n = 2033) | Prognostic value of epithelial cell expression based on ER/PR/HER2 tumor classification | Quesne et al. (2012) |
| miR‐148b‐3p,‐190b,‐429 | Tissue (TCGA) | Bioinformatics (miRNAseq) | Breast | TCGA data (n = 1187) | Prognostic value of miRNA signature | Dai et al. (2019) |
| miR‐205 | Tissue, TMA | LNA ISH | Breast | Population‐based (n = 1686) | Prognostic value of epithelial cell expression based on histological tumor classification | Quesne et al. (2012) |
| miR‐210 | Tissue, Frozen | TaqMan RT‐qPCR | Breast | Validation (n = 219) | Prognostic value of miRNA expression | Camps et al. (2008) |
| miR‐221 | Tissue | ISH | Breast | Validation (n = 377) | Prognostic value of epithelial cell expression | Hanna, Wimberly, et al. (2012) |
| miR‐301 | Tissue (TMA, TCGA/METABRIC) | LNA ISH, bioinformatics (miRNAseq, microarrays) | Breast | Testing (n = 380), TCGA validation (n = 634), METABRIC validation (n = 1262) | Prognostic value of epithelial cell expression | Zheng, Huang, et al. (2018) |
| miR‐493 | Tissue, TMA | LNA ISH | Breast | Validation (n = 382) | Prognostic value of epithelial expression in triple negative breast cancer | Yao et al. (2018) |
| miR‐145,‐200c,‐218 | Tissue, TCGA | Bioinformatics | Cervix | Validation (n = 254) | Prognostic value of miRNA signature | Liang et al. (2017) |
| miR‐144, −200b‐3p,‐451 | Stool | RT‐qPCR | Colon, rectum | Training (n = 60), testing (n = 175) | Diagnostic value of erythrocyte‐specific miRNAs in the normalizer for early disease detection as a modified fecal occult blood test. | Wu et al. (2017) |
| miR‐18a,‐221 | Stool | TaqMan RT‐qPCR | Colon, rectum | Testing (n = 595) | Diagnostic value of miRNA signature for early disease detection | Yau et al. (2014) |
| miR‐21,‐320,‐498 | Tissue | TaqMan RT‐qPCR | Colon, rectum | Testing (n = 224) | Prognostic value of miRNA signature | Bahnassy et al. (2018) |
| miR‐10b | Tissue (Frozen/FFPE) | RT‐qPCR | Colon, rectum | Testing (n = 492) | Prognostic value and predictive value of metastatic disease | Jiang et al. (2016) |
| miR‐21 | Tissue, FFPE | LNA ISH | Colon, rectum | Population‐based (n = 764) | Prognostic value of tumor‐associated fibroblast expression | Kjaer‐Frifeldt et al. (2012) |
| miR‐21 | Tissue, TMA | LNA ISH | Colon, rectum | Population‐based (n = 277) | Prognostic value of tumor‐associated fibroblast expression | Kang et al. (2015) |
| miR‐181c | Tissue (Frozen, FFPE) | TaqMan RT‐qPCR | Colon, rectum | Discovery (n = 10), training (n = 80 × 2[matched Frozen and FFPE]), validation (n = 57) | Concordant prognostic value in frozen and FFPE tumor tissues of stage II cases | Yamazaki et al. (2017) |
| let‐7a‐2, miR‐32,‐181a‐1, ‐197,‐328,‐505,‐652 | Tissue (TCGA) | Bioinformatics (RNAseq) | Colon, rectum | TCGA data (n = 337) | Prognostic value of miRNA signature | Xu et al. (2017) |
| miR‐99a,‐137,‐499a,‐548k,‐654,‐3619,‐3170 | Tissue (TCGA) | Bioinformatics (miRNAseq) | Head and Neck | TCGA data (n = 569) | Prognostic value of miRNA signature in head and neck squamous cell carcinoma | Lu et al. (2019) |
| let‐7b‐5p, miR‐24‐3p,‐140‐3p,‐192‐5p,‐223‐3p | Blood, Serum | RT‐qPCR | Nasopharyngeal | Discovery (n = 60), testing (n = 304), validation (n = 82) | Diagnostic value of miRNA signature for early disease detection | Zou, Zhu, et al. (2020) |
| miR‐20a‐5p,‐25‐3p,‐30a‐5p,‐92a‐3p,‐132‐3p,‐185‐5p,‐320a,‐324‐3p | Blood (Plasma) | TaqMan RT‐qPCR | Liver | Training (n = 85), testing (n = 64), validation (n = 149) | Diagnostic value of miRNA signature for early disease detection | Wen, Han, et al. (2015) |
| miR‐21 | Blood (Serum) | RT‐qPCR | Liver | Training (n = 80), validation (n = 453) | Diagnostic value of miRNA signature for early disease detection | Guo et al. (2017) |
| miR‐26a,‐26b | Tissue (FFPE) | Multiplex RT‐qPCR | Liver | Training (n = 129), testing (n = 119) | Predictive value of treatment response to interpheron‐alpha | Ji et al. (2013) |
| miR‐101‐3p,‐101‐5p | Tissue (TCGA) | Bioinformatics (miRNAseq) | Liver | TCGA data (n = 414) | Diagnostic value for early disease detection and for separating hepatocellular carcinoma from normal liver tissue | Yang, Pang, et al. (2018) |
| miR‐10b,‐195 | Tissue (TCGA, GEO datasets) | Bioinformatics (miRNAseq, microarrays) | Liver | TCGA data (n = 421), GEO datasets (n = 803) | Prognostic value of miRNA signature | Nagy et al. (2018)) |
| miR‐17‐5p | Blood, Serum | RT‐qPCR | Lung | Validation (n = 275) | Diagnostic value for early disease detection and prognostic value | Chen et al. (2013) |
| miR‐20a,‐24,‐25,‐145,‐152,‐199a‐5p,‐221,‐222,‐223,‐320 | Blood, Serum | TaqMan RT‐qPCR | Lung | Training (n = 310), validation (n = 310) | Diagnostic value of miRNA signature for early disease detection | Chen et al. (2012) |
| miR‐21 | Tissue, TMA | LNA ISH | Lung | Validation (n = 335) | Prognostic value of tumor‐associated fibroblast and epithelial cell expression | Stenvold, Donnem, Andersen, Al‐Saad, Valkov, et al. (2014) |
| miR‐21‐5p,‐30d‐5p | Tissue (TCGA, GEO datasets) | Integrated bioinformatics | Lung | Training (n = 2251), TCGA validation (n = 423) | Prognostic value of miRNA signature | Li et al. (2017) |
| miR‐451 | Blood (Plasma) | 3D‐Gene® Human miRNA Oligo Chip | Lung | Discovery (n = 6), validation (n = 309) | Prognostic value of exosomal miRNA levels | Kanaoka et al. (2018) |
| miR‐126 | Tissue | LNA ISH | Lung | Validation (n = 312) | Prognostic value of epithelial cell expression | Donnem et al. (2011) |
| miR‐143,‐145 | Tissue, TMA | LNA ISH | Lung | Validation (n = 553) | Sex‐specific prognostic value of stromal expression (miR‐143 in females and miR‐145 in males) | Skjefstad et al. (2018) |
| miR‐155 | Tissue, TMA | LNA ISH | Lung | Validation (n = 320) | Prognostic value of epithelial cell expression | Donnem et al. (2011) |
| miR‐182 | Tissue, TMA | LNA ISH | Lung | Validation (n = 305) | Prognostic value of epithelial cell expression | Stenvold, Donnem, Andersen, Al‐Saad, Busund, et al. (2014) |
| miR‐205 | Tissue, Blood | Meta‐analysis | Lung | Aggregate (n = 1231) | Diagnostic value for separating malignant tumors from normal lung tissue | Li, Sun, et al. (2017) |
| miR‐205 | Tissue, Blood | Meta‐analysis | Lung | Aggregate (n = 756) | Prognostic value |
Li, Sun, et al. (2017) |
| miR‐210 | Tissue, TMA | LNA ISH | Lung | Validation (n = 259) | Prognostic value of stromal cell expression | Eilertsen et al. (2020) |
| miR‐873,‐1293,‐1914 | Tissue (TCGA) | Bioinformatics (miRNAseq) | Lung | TCGA data (n = 528) | Prognostic value of miRNA signature in lung adenocarcinoma | Zheng, Mao, et al. (2018) |
| miR‐10b | Blood (PBMC) | RT‐qPCR | Lung | Testing (n = 393) | Prognostic and predictive value of treatment response to chemotherapy | Yang, Wang, et al. (2018) |
| miR‐16,‐17,‐20a,‐21,‐29b,‐32,‐101,‐125b,‐155‐5p,‐181,‐196a,‐204,‐372,‐373,‐455‐5p,‐1246 | Tissue (FFPE, Frozen | Meta‐analysis | Mouth | Aggregate (n = 1200) | Prognostic value of miRNA signature in oral squamous cell carcinoma | Troiano et al. (2018) |
| miR‐744‐5p,‐3196,‐6794‐5p,‐6799‐5p,‐6820‐5p,‐8073 | Blood, Serum | 3D‐Gene® Human miRNA Oligo Chip | Esophagus | Training (n = 566), validation (n = 4965) | Diagnostic value of miRNA signature for early disease detection | Sudo et al. (2019) |
| miR‐18a,‐20b,‐106a,‐223‐3p,‐486‐5p,‐584 | Blood (Plasma) | RT‐qPCR | Esophagus | Discovery (n = 30), training (n = 84), testing (n = 214), validation (n = 99) | Diagnostic value of miRNA signature in exosomes for early disease detection | Zhou et al. (2017) |
| miR‐375 | Tissue, TMA | LNA ISH | Esophagus | Validation (n = 249) | Prognostic value of epithelial cell expression | Li et al. (2013) |
| miR‐27‐3p | Blood (PBMC) | TaqMan RT‐qPCR | Pancreas | Discovery (n = 60) and validation (n = 292) | Diagnostic value of miRNA expression in PBMCs in combination with CA‐19‐9 in serum for early disease detection | Wang et al. (2013) |
| let‐7b‐5p,miR‐19a‐3p,‐19b‐3p,‐25‐3p,‐192‐5p,‐223‐3p | Blood, Serum | RT‐qPCR | Pancreas | Training (n = 72), testing (n = 164), validation (n = 60) | Diagnostic value of miRNA signature for early disease detection | Zou et al. (2019) |
| miR‐24,‐130b,‐135b,‐148a,‐196 | Tissue (FFPE, FNA) | RT‐qPCR | Pancreas | Training (n = 95), validation (n = 228) | Diagnostic value of miRNA signature for separating PDAC from chronic pancreatitis and benign conditions | Brand et al. (2014) |
| miR‐182‐5p,‐375‐3p | Blood (Plasma) | RT‐qPCR | Prostate | Training (n = 113), validation (n = 304) | Diagnostic value for early disease detection and prognostic value | Bidarra et al. (2019) |
| miR‐27a‐3p,‐29b‐3p,‐4286 | Blood (Serum) | RT‐qPCR | Prostate | Training (n = 155), validation (n = 100) | Diagnostic value of miRNA signature in combination with clinicopathological factors for early disease detection | Lyu et al. (2019) |
| miR‐148,‐375 | Urine | TaqMan RT‐qPCR | Prostate | Training (n = 166), testing (n = 134) | Diagnostic value for early disease detection in combination with PSA levels | Stuopelyte et al. (2016) |
| miR‐21 | Tissue (TMA) | LNA ISH | Prostate | Validation (n = 478) | Prognostic value of stromal cell expression | Melbo‐Jorgensen et al. (2014) |
| miR‐182 | Tissue (TMA) | Prostate | Validation (n = 461) | Prognostic value of epithelial cell expression | Baumann et al. (2019) | |
| let‐7i‐3p,miR‐30d‐3p,‐106a‐5p,‐133a‐3p, ‐185‐5p,‐210‐3p, ‐221‐3p,‐222‐3p, ‐615‐3p | Tissue (FFPE,TCGA) | RT‐qPCR, bioinformatics (miRNAseq) | Prostate | Training (n = 198), validation (n = 159), TCGA validation (n = 350) | Prognostic value of miRNA signature in combination with DNA methylation status of AOX1, COL4A6, and PROM1 in prostate cancer | Strand et al. (2019) |
| miR‐205 | Tissue (TMA) | LNA ISH | Skin | Validation (n = 297) | Prognostic value of melanoma cell expression | Hanna, Hahn, et al. (2012) |
| miR‐15b‐5p,‐16‐5p,‐150‐5p,‐374b‐3p | Tissue (FFPE) | RT‐qPCR | Skin | Training (n = 92), testing (n = 119), validation (n = 45) | Prognostic value and predictive value for metastatic disease | Hanniford et al. (2015) |
| miR‐25 | Blood (serum) | RT‐qPCR | Stomach | Testing (n = 318) | Diagnostic and value for early disease detection and prognostic value | Kong et al. (2019) |
| miR‐200c | Blood, tissue | Meta‐analysis | Stomach | Aggregate (n = 452) | Diagnostic value for early disease detection | Huang et al. (2019) |
| miR‐200c | Blood, Tissue | Meta‐analysis | Stomach | Aggregate (n = 935) | Prognostic value | Huang et al. (2019) |
| let‐7e, miR‐21,‐26a,‐125b,‐126,‐148a,‐222 | Blood (plasma) | TaqMan RT‐qPCR | Stomach | Discovery (n = 27), training (n = 170), validation (n = 169) | Prognostic value of miRNA signature in Stage II and III cases | Liu, Zhang, et al. (2017) |
| miR‐29b‐1‐5p,‐31‐5p,‐138‐1‐3p,‐139‐5p,‐146b‐5p,‐155,‐204‐5p,‐222‐3p,‐375,‐551b‐3p | Tissue (FNA) | RT‐qPCR (miRInform Thyroid Test) | Thyroid | Training (n = 240), validation (n = 109) | Diagnostic value of miRNA signature for separating thyroid cancer from benign conditions | Labourier et al. (2015) |
| miR‐23a‐3p,‐31‐5p,‐125b‐5p,‐138‐5p,‐146b‐5p,‐152‐3p,‐181c‐5p,‐200c‐3p,‐222‐3p,‐342‐3p,‐345‐5p,‐346,‐375,‐424‐3p,‐486‐5p,‐551b‐3p,‐3074‐5p,‐574‐3p,‐5701,MID‐16582,‐20094,‐50969,‐50971,‐50976 | Tissue (FNA) | RT‐qPCR (RosettaGX Reveal assay) | Thyroid | Discovery (n = 82), training (n = 375), validation (n = 201) | Diagnostic value of miRNA signature for separating thyroid cancer from benign conditions | Lithwick‐Yanai et al. (2017) |
| miRNA classifier (64 miRNA signature) | Tissue (FFPE) | Microarrays (miRview mets2 assay) | Various | Training (n = 1282), validation (n = 509) | Diagnostic value of miRNA classifier for organ site assignation of cancers of unknown primary origin | Meiri et al. (2012) |
| miRNA classifier (up to 32 miRNA signature) | Tissue (TCGA, GEO datasets) | Bioinformatics (miRNAseq, microarrays) | Various | Training (n = 3578), testing (n = 3024), validation (n = 504) | Diagnostic value of miRNA classifier for organ site assignation of cancers of unknown primary origin | Tang et al. (2018) |
Note: Unless otherwise noted, cancer type arising from cancer site is as follows: urothelial carcinoma from bladder, breast carcinoma from breast, colorectal adenocarcinoma from colon and/or rectum, endometrial carcinoma from endometrium, esophageal squamous cell carcinoma from esophagus, nonsmall cell lung cancer (adenocarcinoma or squamous cell carcinoma) from lung, pancreatic adenocarcinoma from pancreas, prostatic adenocarcinoma from prostate, hepatocellular carcinoma from liver, and cutaneous melanoma from skin. Total number of analyzed samples (n) from healthy controls, site‐specific cancer, benign conditions, and/or (matched) normal sample of same organ site, and/or other‐organ site(s) cancer. Samples from same case analyzed in different forms (e.g., frozen vs. FFPE tissue, serum vs. exosomal fraction from blood) were counted as independent samples. To harmonize cohort/population studies, we used definitions that mostly matched those attributed in the original study: Discovery set is designed for selection of candidate miRNAs after high‐throughput profiling; Training set is designed for refining number of miRNAs in test and/or scoring system; Testing set is designed for assessing performance of miRNA(s), may include cases from Discovery and/or Training set; Validation set is designed for validating performance of miRNA(s) test in an independent cohort of patients not included in the Discovery, Training and/or Testing set(s). Population‐based set consists of consecutive cancer cases for a determined period of time.
Abbreviations: FFPE, formalin‐fixed paraffin‐embedded tissue; FNA, fine‐needle aspirate tissue biopsy; LNA ISH, in situ hybridization assay with locked nucleic acid‐modified oligonucleotide probes; METABRIC, Molecular Taxonomy of Breast Cancer International Consortium; miRNAseq, small RNA sequencing; PBMC, peripheral blood mononuclear cells; RT‐qPCR, reverse transcription quantitative‐polymerase chain reaction; TCGA, the cancer genome atlas; TMA, tissue microarray.
TABLE 2.
On‐going clinical trials evaluating microRNAs as diagnostic, prognostic, and/or predictive indicators
| miRNA(s) | Source | Technology | Cancer site | Study design | Clinical application(s) | Trial ID |
|---|---|---|---|---|---|---|
| Unspecified | Tissue (frozen) | Microarrays, miRNAseq | Bone and soft tissue | Discovery and testing (n = 1000 estimated) | Prognostic value in pediatric sarcomas, osteosarcomas, neuroblastomas | NCT01050296 |
| Unspecified | Tissue, blood | Microarrays | Bone marrow | Discovery and testing (n = 529 actual) | Diagnostic value of miRNA signatures in acute myeloid leukemia | NCT00900224 |
| Unspecified | Tissue, blood | Microarrays | Bone marrow | Discovery and testing (n = 735 estimated) | Diagnostic value of miRNA signatures in acute myeloid leukemia | NCT00898092 |
| miR‐10b | Tissue, Blood, CSF | RT‐qPCR? | Brain | Testing (n = 200 estimated) | Prognostic and predictive value in gliomas | NCT01849952 |
| Unspecified | Tissue (Frozen) | miRNAseq | Brain | Discovery and testing (n = 640 estimated) | Prognostic value in glioblastomas | NCT03770468 |
| Unspecified | Blood | Unspecified | Breast | Discovery and testing (n = 506 estimated) | Predictive value for recurrence risk reduction by interventional diet, exercise and vitamin D regimen | NCT02786875 |
| Unspecified | Blood | Unspecified | Breast | Discovery and testing (n = 3500 estimated) | Prognostic value in metastatic disease | NCT02338167 |
| Unspecified | Blood (Extracellular vesicles) | Unspecified | Breast | Discovery and testing (n = 370 estimated) | Prognostic and prediction for treatment response to anti‐HER2 therapy | NCT02514681 |
| Unspecified | Tissue (FFPE) | RT‐qPCR | Breast | Discovery and testing (n = 1000 estimated) | Prognostic value in early stage hormone‐receptor positive breast cancer cases | NCT02918084 |
| Unspecified | Tissue, Blood | Unspecified | Breast | Discovery (n = 217 actual) | Predictive value for treatment response to anti‐HER2 therapy in combination with PET imaging (scheduled) | NCT01957332 |
| Undisclosed | Blood (Plasma) | RT‐qPCR | Breast | Validation (n = 300 estimated) | Predictive value for treatment response to anti‐HER2 therapy | NCT02656589 |
| Unspecified | Tissue | Microarrays | Breast | Discovery and testing (n = 550 estimated) | Predictive value of miRNAs for risk stratification of lobular cancer in situ | NCT00581750 |
| 5‐miRNA, 8‐miRNA signatures | Tissue | RT‐qPCR? | Colon, rectum | Validation (n = 200 estimated) | Diagnostic value of miRNA signature for detection of lymph node metastasis | NCT04150081 |
| Unspecified | Blood (Plasma) | RT‐qPCR | Colon, rectum | Discovery and testing (n = 200 estimated) | Predictive value of miRNAs in neoadjuvant treatment of rectal cancer | NCT03962088 |
| miR‐20a‐5p,‐21,‐103a‐3p,‐106b‐5p,‐143‐5p,‐215 | Tissue | RT‐qPCR | Colon, rectum | Interventional validation (n = 430 estimated) | Prognostic value of miRNAs in Stage II colorectal cancer | NCT02466113 |
| miR‐20a‐5p,‐21,‐103a‐3p,‐106b‐5p,‐143‐5p,‐215 | Tissue | RT‐qPCR | Colon, rectum | Validation (n = 630 estimated) | Prognostic value of miRNAs in Stage II colorectal cancer | NCT02635087 |
| Unspecified | Tissue? | Unspecified | Colon, rectum | Discovery and testing (n = 1000 estimated) | Diagnostic and prognostic value of miRNAs | NCT03309722 |
| miR‐34b‐5p,‐34c‐5p,‐34c‐3p,‐184,‐375, | Blood (Plasma) | RT‐qPCR? | Endometrium | Validation (n = 443 estimated) | Diagnostic value of miRNA signature for detection of lymph node metastasis | NCT03776630 |
| Unspecified | Blood (Serum, Plasma) | Unspecified | Gastrointestinal tract | Discovery and testing (n = 6300 estimated) | Diagnostic and prognostic value in colorectal, pancreatic, and gastroesophageal cancer | NCT04363983 |
| miR‐371 | Blood (Plasma) | Unspecified | Germ cell | Testing (n = 956 estimated) | Diagnostic value for early disease detection of testicular cancer | NCT04435756 |
| Unspecified | Tissue | NGS | Germ cell | Discovery and testing (n = 200 estimated) | Diagnostic and prognostic value in testicular stromal tumors | NCT01970696 |
| Unspecified | Saliva, Blood, Tissue (FNA) | RT‐qPCR | Head and Neck | Discovery and testing (n = 462 estimated) | Diagnostic or prognostic value of miRNA signature in matched bodily fluids or tissue samples (squamous cell carcinoma) | NCT04305366 |
| Unspecified | Blood (Serum) | Unspecified | Head and Neck | Discovery (n = 370 estimated) | Prognostic value of miRNA signature in oral cancer | NCT03202810 |
| Unspecified | Blood (Exosomes) | Unspecified | Lung | Discovery (n = 200 estimated) | Predictive value of miRNA signature for treatment response to immunotherapy treatment (scheduled) | NCT04427475 |
| Unspecified | Blood (Exosomes) | Unspecified | Lung | Discovery (n = 800) | First line of screening for lung cancer detection in combination with high‐resolution CT imaging (scheduled) | NCT04629079 |
| Unspecified | Blood | Unspecified | Lung | Discovery and testing (n = 286) | Prognostic and predictive value in metastatic lung cancer | NCT03721120 |
| Unspecified | Blood | Unspecified | Lung | Discovery and testing (n = 1000 estimated) | Diagnostic and prognostic value in early stage cases | NCT03397355 |
| 24‐miRNA signature | Blood (Plasma) | Microfluidic card | Lung | Interventional, active surveillance of smokers (n = 4119 actual) | First line of screening for lung cancer detection | NCT02247453 |
| Hummingbird microRNA profile | Blood | HMBDx microRNA Test | Lung | Testing (n = 479 actual) | First line of screening for lung cancer detection in combination with low‐dose CT imaging (scheduled) | NCT03452514 |
| Unspecified | Blood (Plasma) | HTG EdgeSeq miRNA Assay | Lung | Discovery and testing (n = 900 estimated) | Predictive value of treatment response to targetable molecular alteration or immunotherapy | NCT02511288 |
| 16‐miRNA and 45‐miRNA signatures | Blood (Plasma) | Unspecified | Lung | Testing (n = 2000 estimated) | First line of screening for lung cancer detection in combination with low‐dose CT imaging | NCT04323579 |
| Unspecified | Blood (Plasma) | Unspecified | Esophagus | Discovery and testing (n = 200 estimated) | Predictive value of miRNA signature in combination with number of circulating tumor cells | NCT02812680 |
| miR‐192‐5p,‐194‐5p,‐215‐5p | Exfoliated cells, Blood (Serum) | Digital RT‐qPCR | Esophagus | Testing (n = 220 estimated) | Diagnostic value of miRNA signature for Barrett's Esophagus | NCT02464930 |
| Unspecified | Bile (Exosomes) | Digital RT‐qPCR | Esophagus | Testing (n = 220 estimated) | Diagnostic value of miRNAs for early cancer detection | NCT02464930 |
| Unspecified | Blood | Unspecified | Pancreas | Discovery and testing (n = 5000 estimated) | Diagnostic, prognostic and predictive value | NCT03311776 |
| Unspecified | Blood | Unspecified | Pancreas and Gall Bladder | Discovery and testing (n = 500 estimated) | Diagnostic value of miRNA signature compared to or in combination with other biomarkers (e.g., CA 19‐9) | NCT02531607 |
| Unspecified | Unspecified | RNAseq, nanostring, RT‐qPCR | Pancreas | Discovery and testing (n = 200) | Prognostic and predictive value in pancreatic ductal adenocarcinoma and/or neuroendocrine tumors | NCT03840460 |
| Unspecified | Blood (Serum) | miRNAseq | Pancreas | Discovery and testing (n = 629 estimated) | Predictive value of miRNA signature for malignant progression from Multiple Endocrine Neoplasia to Neuroendocrine Tumor | NCT03048266 |
| Unspecified | Tissue, Blood, Urine, Saliva | miRNAseq | Prostate | Discovery (n = 3000 estimated) | Exploratory molecular profiling studies | NCT02594202 |
| Unspecified | Blood | Unspecified | Prostate | Discovery and testing (n = 330 estimated) | Prognostic value for clinical relapse‐free survival | NCT02745587 |
| Unspecified | Blood (Exosomes) | miRNAseq | Prostate | Discovery and testing (n = 600 estimated) | Diagnostic value of exosomal miRNAs for early disease detection in combination with PSA and MR imaging | NCT03694483 |
| X chromosome‐linked miRNAs | Unspecified | Unspecified | Prostate | Discovery and testing (n = 329 actual) | Prognostic and predictive value for treatment response to hypofractionated radiotherapy | NCT01444820 |
| let‐7c,miR‐16,‐25,‐141,‐151‐3p ‐187,‐188‐5p,‐196b, ‐200c,‐200b,‐375 | Tissue | Unspecified | Prostate | Interventional, scheduled MR imaging and biopsy (n = 628 estimated) | Diagnostic value for early disease detection in combination with PSA and PHI | NCT04283032 |
| Unspecified | Urine (Exosomes) | NGS | Prostate | Discovery and validation (n = 240 estimated) | Diagnostic value of exosomal miRNAs for separating pathological significant and insignificant prostate cancer | NCT03911999 |
| miR‐200b | Blood (Plasma) | RT‐qPCR? | Ovary | Validation (n = 443 estimated) | Prognostic value of miR‐200b changes in matched pre‐ and post‐treatment samples | NCT03776630 |
| Unspecified | Tissue | NGS | Ovary | Discovery and testing (n = 200 estimated) | Diagnostic and prognostic value in ovarian stromal tumors | NCT01970696 |
| Unspecified | Blood | Unspecified | Pelvis | Discovery and testing (n = 500 estimated) | Prognostic and predictive value in cervical, endometrial, and ovarian cancer | NCT03622983 |
| Unspecified | Tissue (Frozen) | NGS, RT‐qPCR | Stomach | Discovery and validation (n = 800 estimated) | Predictive value of miRNA signature to chemotherapy treatment | NCT03253107 |
| KRAS variants (DNA) | Saliva | DNA sequencing | Various | Validation (n = 15,000 estimated) | Role of KRAS‐variant and miRNA binding site mutation in cancer risk, prevention, and treatment | NCT02253251 |
| Unspecified | Blood (Exosomes) | Unspecified | Various | Discovery and testing (n = 1000 estimated) | Diagnostic, prognostic and/or predictive value of miRNAs in combination with circulating DNA | NCT04530890 |
| Unspecified | Blood (Plasma) | Unspecified | Various | Discovery and testing (n = 388 estimated) | Diagnostic value for cancer detection in patients with serious illness but who have no organ specific symptoms | NCT01709539 |
| Unspecified | Tissue | NGS | Various | Discovery and testing (n = 300 estimated) | Predictive value of treatment response in patients who are exceptional responders | NCT02243592 |
Note: This table includes only clinical studies with a status of “Active, not recruiting,” “Recruiting,” or “Enrolling by invitation,” and with at least 200 participants by study design (estimated or actual participants). Unless otherwise noted, these are all observational prospective studies. Interventional study indicates that participants receive a treatment and/or other procedure (e.g., imaging) that is related to intended clinical application of miRNA(s) test. Unless otherwise indicated cancer type arising from cancer site is as follows: breast carcinoma from breast, colorectal adenocarcinoma from colon and/or rectum, esophageal squamous cell carcinoma from esophagus, nonsmall cell lung cancer (adenocarcinoma or squamous cell carcinoma) from lung, pancreatic adenocarcinoma from pancreas, prostatic adenocarcinoma from prostate.
Abbreviations: CSF, cerebrospinal fluid; CT, computed tomography; MR, magnetic resonance; NGS, next generation sequencing; RT‐qPCR, reverse transcription quantitative‐polymerase chain reaction; miRNAseq, small RNA sequencing; PET, positron emission tomography; PHI, prostate health index; PSA, prostate‐specific antigen.
2.2. Large retrospective studies
We highlight in broad strokes some of the largest retrospective studies that illustrate the versatility and potential of altered expression of specific miRNAs or miRNA signatures to provide actionable information to improve patient management. Table 1 provides a comprehensive summary of large retrospective studies that included more than 200 individuals.
2.2.1. Diagnostic value of microRNA for disease classification
The utility of differential miRNA expression for separating malignant from benign conditions, cancer staging, and/or cancer typing has been extensively evaluated in tissue samples. Differential diagnosis based only on visual examination of tissue material obtained by tissue biopsy of fine needle aspirate (FNA) can often be challenging, especially when tissue sample is limited and/or lacks architectural context. Differential diagnosis studies based on miRNA expression in FNA samples from thyroid (Labourier et al., 2015; Lithwick‐Yanai et al., 2017) and pancreas (Brand et al., 2014; Szafranska‐Schwarzbach et al., 2011) led to the first commercialization of miRNA‐based laboratory‐developed tests (see Section 2.3). miRNA signatures have also shown diagnostic value in assigning organ site to cancers of unknown primary origin, which can improve patient management and outcome by matching patients with treatment options for that organ site (Meiri et al., 2012; Tang et al., 2018). miRNA signatures correlate with clinical parameters such as tumor size, lymph node involvement, and stage (Table 1). A 9‐miRNA signature (miR‐26a‐5p, ‐29c‐3p, ‐29c‐5p, ‐30b‐5p, ‐148a‐3p, ‐361‐3p, ‐645, ‐652‐5p, ‐934) is associated with regional metastatic disease in early stage breast cancer patients (Xie et al., 2018). This miRNA signature along with other clinical parameters could be useful to guide what patients may elect to not have an axillary (sentinel) lymph node biopsy if at low risk of having metastatic spread to the lymph nodes and what patients should have said biopsy if at high risk. More recently, blood‐based analyses have been used to determine the diagnostic utility of miRNAs for separating benign from malignant tumors or diagnosing a specific cancer type or subtypes (Table 1). A two‐circulating miRNA signature (miR‐629‐3p, ‐4710) is associated with regional metastatic disease in breast cancer patients (Shiino et al., 2019). Curiously, there is no overlap between these diagnostic tissue‐based (Xie et al., 2018) and serum‐based miRNA signatures (Shiino et al., 2019), which may arise from technical (e.g., RT‐qPCR vs. microarray) or biological differences (e.g., most differentially expressed miRNA in tissues may not be proportionally represented in circulation). Diagnosis of sarcomas represent a challenge due to their relative rarity and diversity of histological subtypes. A seven‐circulating miRNA signature (miR‐658, ‐762, ‐4281, ‐4649‐5p, ‐4665‐3p, ‐4736, ‐6836‐3p) detected in serum samples can distinguish sarcoma patients regardless of subtype from other bone and soft tissue benign conditions as well as healthy controls (Asano et al., 2019).
2.2.2. Diagnostic value of microRNA for early cancer detection
The utility of differential miRNA expression for early cancer detection has been extensively evaluated in blood as starting material. There is a recent trend to specifically interrogate miRNA levels within the exosomal/EV fraction of blood, but there is not a clear consensus as to whether serum or plasma is a more appropriate source for miRNA analysis. Tissue analysis from previous studies or from matched individuals in concurrent study has often guided the selection of most informative miRNAs to be evaluated (Table 1). A five‐circulating miRNA (miR‐1246, ‐1307‐3p, ‐1364, ‐6861‐5p, ‐6875‐5p) and a 12‐circulating miRNA (let‐7b‐5p, miR‐16‐5p, ‐19a‐3p, ‐19b‐3p, ‐20a‐5p, ‐25‐3p, ‐92a‐3p, ‐93‐5p, ‐106a‐5p, ‐223‐3p, ‐425‐5p, ‐451a) signature analyzed in serum samples can distinguish breast cancer patients from individuals with benign breast conditions as well as healthy controls (Shimomura et al., 2016; Zou, Xia, et al., 2020). A five‐circulating miRNA (miR‐744‐5p, ‐3196, ‐6794‐5p, ‐6799‐5p,‐6820‐5p, ‐8073) signature analyzed in serum samples (Sudo et al., 2019) and a six‐circulating miRNA (miR‐18a, ‐20b, ‐106a, ‐223‐3p, ‐486‐5p, ‐584) signature analyzed in plasma samples (Zhou et al., 2017) can distinguish esophageal cancer patients from healthy controls. Concordant levels of miR‐223‐3p and miR‐584 in matched tissue, plasma, and the exosomal fraction of plasma from the same patients suggest that these two miRNAs are actively secreted by tumor cells (Zhou et al., 2017). Puzzlingly, there is more overlap between the 12‐circulating miRNA breast cancer signature and the six‐circulating miRNA esophageal cancer signature than there is between organ site–specific signatures.
Other bodily fluids have been used to study the diagnostic value of miRNA expression. The choice of these bodily fluids is informed and restricted by anatomic location of the organ(s) being interrogated such as saliva for upper digestive system (Setti et al., 2020) and stool for lower digestive system (Rashid et al., 2020), sputum for upper respiratory system (X. Zhang et al., 2019), and urine for urinary system (Kutwin et al., 2018; Paiva et al., 2020). A three‐miRNA (miR‐144‐5p, miR‐200b‐3p, miR‐451a) and a two‐miRNA (miR‐18a and ‐221) signature detected in stool samples can distinguish colorectal cancer patients from healthy controls (C. W. Wu et al., 2017; Yau et al., 2014). The lack of overlap between these signatures is expected since the three‐miRNA signature is tailored to detect erythrocyte‐expressed miRNAs (miR144‐5p and ‐451a) as a modified fecal occult blood test whereas the other signature interrogates tumor‐induced miRNA changes. A two‐miRNA (miR‐148 and ‐375) signature detected in urine samples can distinguish prostate cancer patients from individual with benign prostate conditions and healthy controls (Stuopelyte et al., 2016). The combination of this miRNA signature and serum levels of prostate‐specific antigen (PSA) provides a better diagnostic performance; this is good example of how integrating existing clinical indicators and new miRNA biomarkers can enhance diagnostic power.
2.2.3. Prognostic value of microRNA for treatment selection and patient management
The utility of differential miRNA expression for identifying patients with a worse clinical outcome regardless of treatment regimen has been most extensively studied in tissue samples. Especially in the setting of prognostic studies, ISH detection has flourished as a powerful research tool to extract contextual information based on altered miRNA expression at single‐cell resolution within tumor lesions. Moreover, an ISH detection assay can obtain information from hundreds of tumor samples at a time in rapid and cost‐effective fashion using tissue microarrays (Table 1). Depending on the cancer type and the specific miRNA(s), altered expression in cancer cells has been reported to be more informative than in other cell types of the TME or vice versa (Table 1). Using similar ISH assays, several groups have reported that stromal (mostly cancer‐associated fibroblast) expression of miR‐21 carries more prognostic information than cancer cell expression in breast, colorectal, pancreatic, and prostate cancer (Kadera et al., 2013; Kang et al., 2015; Kjaer‐Frifeldt et al., 2012; MacKenzie et al., 2014; Melbo‐Jorgensen et al., 2014; Nielsen et al., 2011). miR‐143 and miR‐145 are closely linked in a gene cluster, but they can be differentially expressed in some cell types and contexts (Kent et al., 2014; Sempere et al., 2004). Stromal expression of miR‐143 in female lung cancer patients and miR‐145 in male lung cancer patients is associated with clinical outcome (Skjefstad et al., 2018). This sex‐specific survival effect of stromal expression is highly correlated with steroid hormone receptor status in the tumor tissues, suggesting a regulatory interaction and crosstalk between these two miRNAs and sex hormones (Skjefstad et al., 2018). There are some limitations with these retrospective studies, either using ISH or other detection methods. miRNA expression may be correlated with known prognostic indicators (e.g., tumor size) and stratification of cases to match clinical parameters decreases statistical power. Similarly, a patient with a worse prognosis determined by standard clinical parameters would likely have received a more aggressive treatment, which may confound the interpretation of altered miRNA expression.
2.2.4. Predictive value of microRNA for treatment response and treatment selection
The utility of differential miRNA expression for identifying responders has been evaluated in tissue samples collected prior to or in blood samples before and after specific treatment. While many miRNA‐based predictive studies have been reported, we find only a few meet our inclusion criterion of participant number (Table 1). This type of study is more challenging due to the need to include a large sample size for each specific treatment arm to have robust statistical power for analysis. A case in point is the association of stromal expression of miR‐21 with poor treatment response to 5‐fluorouracil, but not gemcitabine in pancreatic cancer patients (Donahue et al., 2014). This study started with 538 patients recruited into RTOG‐9704 clinical trial comparing 5‐fluorouracil to gemcitabine before and after chemoradiation in an adjuvant setting (NCT00003216). Attrition occurred at different levels, from not meeting inclusion criteria, to lack of tissue materials for analysis or poor tissue preservation determined postanalysis, and thereby the final analysis was limited to about 90 cases in each treatment arm (Donahue et al., 2014). Some of these studies also blur the line between a prognostic and predictive indication based on miRNA expression. For example, if miRNA expression is associated with metastatic disease, it is difficult to dissociate a direct effect on tumor response from expected poorer clinical performance of more advanced cases with more extended tumor burden. A case in point is the association of miR‐10b expression in PBMCs with treatment response to chemotherapy in advanced stage lung cancer (Yang, Wang, et al., 2018). Lower levels of miR‐10b expression in PBMCs in pretreatment samples correlated with complete or partial response to treatment. In both responders and nonresponders miR‐10b levels were lower in PBMCs in post‐treatment relative to pretreatment samples (Yang, Wang, et al., 2018). miR‐10b levels were higher in patients with adenocarcinomas vs. other subtypes, with lymph node metastasis versus regional metastasis‐free, and without distant metastasis versus metastatic disease (Yang, Wang, et al., 2018). Thus, these associations may confound the pure predictive value of the miR‐10b assay.
2.3. Laboratory developed tests for miRNA‐based diagnosis
A laboratory develop test (LDT) is a type of in vitro diagnostic that is designed and performed by a single laboratory under Clinical Laboratory Improvement Amendments (CLIA) regulations. Asuragen and Rossetta Genomics were first companies in bringing miRNA‐based LDTs to market (Bonneau et al., 2019). These LDTs were rigorously validated in clinical trials (Brand et al., 2014; Labourier et al., 2015; Lithwick‐Yanai et al., 2017; Szafranska‐Schwarzbach et al., 2011). Asuragen miRInform Pancreas LDT has an improved sensitivity and specificity compared to cytological analysis of the same fine‐needle aspiration (FNA) specimens (82.6 vs. 78.8% and 96.1 vs. 69.2%, respectively) to correctly identify PDAC or benign conditions based on altered expression of miR‐24,‐130b,‐135b,‐148a,‐196 (Brand et al., 2014). The most successful miRNA‐based LDT is a 10‐miRNA signature (now TyraMIR®, Table 1) used in combination with Asuragen miRInform Thyroid test (now ThyGeNEXT®) that detects a DNA/mRNA mutation panel (Labourier et al., 2015). The combined information of miRNA expression and mutation status provides an 89% sensitivity and 85% specificity to correctly identify malignant or benign cases from FNAs of solid thyroid nodules, improving preoperative diagnosis based on cytology alone (Labourier et al., 2015). Similarly, Rosetta Genomics developed RosettaGX Reveal™ LDT that can correctly identify malignant and benign cases from FNAs of solid thyroid nodules with an 85% sensitivity and 72% specificity solely based on a 24‐miRNA signature (Lithwick‐Yanai et al., 2017; Table 1). Rosetta Genomics miRView™ mets is a LDT based on the differential expression of 64 miRNAs to assign most likely organ site for cancers of unknown primary. In a validation set of 509 independent cases, miRView™ correctly identified the tissue of origin for up to 90% of the cases (Meiri et al., 2012). These early successes seemed to have paved the way for miRNA‐based diagnosis in the clinic, however, this has not yet been realized. There are probably many contributing factors to this, including, but not limited to, competition with already established clinical biomarkers, coverage by insurance companies, the lack of mechanistic link or incomplete understanding between altered miRNA expression and the underlying disease. Rosetta Genomics and Asuragen have been since acquired or merged with other companies (Bonneau et al., 2019) reflecting the challenges of LDT profitability. TyraMIR® + ThyGeNEXT® LDT is being offered by Interpace Diagnostics based on Asuragen's assets for differential diagnosis of thyroid cancer or benign condition (ThyGeNEXT+ThyraMIR, 2020). Similarly, Genoptix acquired some assets of Rosetta Genomics in 2018, but miRView™ mets and Reveal™ tests are not currently being offered (Genoptix, 2019); Genoptix was subsequently acquired by NeoGenomics.
2.4. On‐going clinical trials
More than 150 clinical studies are registered at clinicaltrials.gov in which the value of a miRNA or miRNA signature is being investigated for a variety of clinical applications from early disease detection to treatment response (ClinicalTrials.gov, 2020). These clinical trials are different in scope, cancer site, and clinical applications, but there is a general trend for the desirable use and perceived value of miRNA analysis in bodily fluids, mainly blood, as a noninvasive tool to inform clinical decisions. We only consider for detailed discussion studies that include more than 200 participants (Table 2). Many of these clinical trials are exploratory or have combined discovery and validation phases, whereas some have a more advanced design to validate the clinical utility of a published miRNA signature. Hummingbird diagnostics (Hummingbird, 2020) and Toray Molecular Laboratory (Toray, 2020) are two of the companies conducting validation trials with the hope to bring to market miRNA‐based blood test for early detection of lung, breast, and other cancer types.
2.4.1. Diagnostic value of microRNA for early cancer detection
In several clinical studies (Table 2), the utility of miRNA‐based blood analysis for early detection is conducted in conjunction with a primary screening tool such as imaging (e.g., breast and lung cancer) or a more established biomarker (e.g., PSA in prostate cancer and CA 19‐9 for pancreatic cancer). In 2017, Toray launched a multi‐institutional prospective clinical trial with 2000 estimated participants to determine if serum miRNA signature can be used to stratify the risk of the individual to be diagnosed with breast cancer or a benign condition in subsequent tissue biopsies after receiving an abnormal breast imaging finding classified as BI‐RADS® 3, 4, or 5 (Barke, 2019). While the primary goal is to complement information of screening mammography and minimize the number of unwarranted call backs for diagnostic imaging and biopsies, implicit in this study is the possibility of using this serum miRNA signature for early detection of breast cancer either alone or by enhancing imaging findings. Hummingbird diagnostics and other sponsors have similar on‐going clinical trials to assess the utility of circulating miRNAs to assist or complement the information of low dose computed tomography (CT) scans for early detection and diagnosis of lung cancer.
2.4.2. Prognostic and predictive value of microRNA for patient management, treatment selection, and/or treatment response
While there are several clinical trials investigating the prognostic or predictive value of miRNAs, the potential use of these miRNAs is exploratory and not integral to the clinical study. This likely reflects the fact that known mechanisms of action of current treatments (e.g., chemotherapy, targeted therapies, immunotherapy) are not directly linked or dependent on miRNA activity. Several miRNAs have been shown to regulate cellular processes such as apoptosis, proliferation, migration, epithelial‐to‐mesenchymal transition, antigen presentation, and immunosuppression (see Section 3) that ultimately affect treatment response and clinical outcome. Because the same miRNA can be implicated in multiple cellular processes and lead to different regulatory outcomes depending on the disease context, this can create challenges for correctly interpreting and acting on the information provided by these miRNA biomarkers.
3. MICRORNA‐BASED THERAPEUTICS
The frequent expression dysregulation of a core set of cancer‐associated miRNAs (e.g., let‐7, miR‐10b, ‐15, ‐16, ‐17‐5p, ‐20a, ‐21, ‐29b, ‐34, ‐155, ‐221) lends support to their etiological involvement, but are these miRNAs drivers, mediators, or mere passengers in the processes of tumor formation, progression, and metastasis? A large body of literature of in vitro functional studies supports a phenotypic contribution of specific miRNA activities. We will focus our discussion on rigorous in vivo studies that complement or expand on the type of questions and mechanistic insights that can be addressed with in vitro cell models. These in vivo studies utilize genetically engineered, chemically induced, and/or transplantable cancer models in which miRNA activity can be modulated by genetic or pharmacological approaches (Bajan & Hutvagner, 2020; Fornari et al., 2019; Forterre et al., 2020; Pal & Kasinski, 2017). Genetic approaches include genetic engineering of the host animals or viral delivery systems (Fornari et al., 2019; Pal & Kasinski, 2017). Typically, the range of activity manipulation afforded by genetic engineering can be more sophisticated and precise than viral delivery systems. Retroviruses and lentiviruses are powerful research tools but with a limited translational potential, whereas adeno‐associated viruses have favorable properties to make it into the clinic. While none of the on‐going clinical trials with miRNA drugs uses a viral delivery system, viral approaches to replenish miRNA expression may be a viable option. Viral approaches to inhibit miRNA activity are more cumbersome, requiring a squelching strategy such as miRNA sponge to sequester miRNA molecules away from target sites or a combination with CRISPR system to delete miRNA locus, which currently do not seem viable in a clinical setting. Pharmacological approaches typically include chemically modified oligonucleotides that can be administered unconjugated, or conjugated to or encapsulated in a nanoparticle carrier (Bajan & Hutvagner, 2020; Forterre et al., 2020), but there are also some efforts to develop small molecule modulators with drug‐like properties (Fan et al., 2019; Monroig‐Bosque et al., 2018; Wen, Danquah, et al., 2015). Currently, chemically modified oligonucleotide approaches are the only ones that have been or are being evaluated in clinical trials.
3.1. Mouse models causally link miRNA activity to carcinogenesis
Global or conditional modulation of the activity of a miRNA or multiple miRNAs by genetic deletion of a miRNA or miRNA gene cluster for loss‐of‐function studies and enforced or inducible expression of a miRNA or miRNA gene cluster for gain‐of‐function studies have causally linked specific miRNAs to the initiation and/or progression of cancer in hematological and solid tumors (Table 3). The majority of global KO mouse lines of cancer‐associated miRNA or miRNA gene clusters are viable and do not exhibit overt developmental defects (Bartel, 2018; Park et al., 2010). Nonetheless, there are some notable exceptions to organismal viability, and many of these global KO mice have a spectrum of mild to severe phenotypes related to immune cell function (Bartel, 2018; Park et al., 2010). It is important to note that miRNA‐mediated regulation in immune cells may lead to different outcomes in the context of hematological malignancies in which a specific immune cell subtype constitutes the neoplastic cells versus solid tumors in which specific immune cell subtype(s) and their interactions with the neoplastic cells can be tumor‐promoting (tumorigenic) or tumor‐restraining (tumoricidal). We describe for some of the most studied miRNAs, their intrinsic role as tumor suppressive or oncogenic (oncomiR) actors in the neoplastic cells as well as their potential contribution or compounded effect in other cell types of the TME.
TABLE 3.
Genetic or viral modulation of miRNA activity in in vivo cancer models
| miRNA modulation | Approach | Model | Cancer site | Overall phenotype | miRNA role | References |
|---|---|---|---|---|---|---|
| miR‐20a loss | Ex vivo viral transduction, enforced expression of inhibitory sequence | Intravenous injection of LM7 osteosarcoma cells expressing BLOCK‐iT™ Pol II miR‐20a RNAi Expression Vector | Bone | Decrease in metastatic nodules in the lung | Oncogenic, promotes distant colonization | Huang et al. (2012) |
| miR‐15,‐16 loss | Genetic, global KO | Mir‐15a~‐16‐1−/− | Bone marrow, spleen | Develops lymphoproliferative disorders, including CLL | Tumor suppressive, B‐cell intrinsic function | Klein et al. (2010) |
| miR‐15,‐16 loss | Genetic, global KO | Mir‐15b~‐16‐2−/− | Bone marrow, spleen | Develops B cell malignancies, including CLL | Tumor suppressive, B‐cell intrinsic function | Lovat et al. (2015) |
| mir‐15,‐16 loss | Genetic, global KO | Mir‐15a~‐16‐1−/−; Mir‐15b~‐16‐2−/− | Bone marrow, spleen | Develops myeloid proliferative disorders, including AML | Tumor suppressive, Myeloid cell‐intrinsic function. Complete loss of activity of miR‐15/‐16 family members shifts cancer type susceptibility | Lovat et al. (2018) |
| miR‐15,‐16 gain | In vivo viral transduction, enforced expression | Systemic delivery lentiviral vector expressing miR‐15 and miR‐16 in NZB de novo model of CLL | Bone marrow, spleen | Decrease in tumor burden | Tumor suppressive, reduced viability of transduced malignant B‐1 cells | Kasar et al. (2012) |
| miR‐17,‐18,‐19a,‐20,‐19‐1b gain | Ex vivo viral transduction, enforced expression | Engrafted Eμ‐Myc cells expressing Mir‐17~‐19‐1b | Bone marrow | Increase in overall tumor burden and shorter overall survival | Oncogenic, accelerates Myc‐driven B‐cell lymphoma formation, and progression | He et al. (2005) |
| miR‐19a,‐19b‐1 gain | Ex vivo viral transduction, enforced expression | Engrafted Eμ‐Myc cells transduced with minigene expressing Mir‐19a~‐19‐1b | Bone marrow | Decrease in overall survival | Oncogenic, accelerates Myc‐driven B‐cell lymphoma formation and progression | Olive et al. (2009) |
| miR‐19a,‐19b‐1 loss | Genetic, global KO | Eμ‐Myc;miR‐19a~‐19b‐1−/− | Bone marrow | Increase in overall survival | Oncogenic, accelerates Myc‐driven B‐cell lymphoma formation, and progression | Han et al. (2015) |
| miR‐34a,‐34b,‐34c loss | Genetic, global KO | Eμ‐Myc; Mir‐34a−/−;Mir‐34b~‐34c−/− | Bone marrow | No difference in tumor incidence | Neutral, miR‐34 activity dispensable in cancer model known to be restrained by p53 function | Concepcion et al. (2012) |
| miR‐155 gain | Genetic, enforced expression in B cell | Eμ‐Mir‐155 | Bone marrow, spleen | Develops B cell malignancies, including ALL | Oncogenic, B‐cell intrinsic function | Costinean et al. (2006) |
| miR‐155 loss | Genetic, global KO | FLT3‐ITD;Mir‐155−/− | Bone marrow | Decrease in tumor burden | Oncogenic, likely myeloid cell‐intrinsic function | Wallace et al. (2017) |
| miR‐10b loss | Genetic, global KO | MMTV‐PyMT;Mir‐10b−/− | Breast | Delay in tumor formation, decreased in distant metastasis | Oncogenic, phenotype presumed to be driven by miR‐10b activity in epithelial cells | Kim, Diverly, et al. (2016) |
| miR‐21 loss | Genetic, global KO | MMTV‐PyMT;Mir‐21−/−, C3(1)‐TAg;Mir‐21−/− | Breast | Significant but slight delay in tumor formation, no change in tumor multiplicity | Tumor promoting, modest effect; cell type‐specific activity not determined | Sempere LF, unpublished observations |
| miR‐155 loss | Genetic, global KO | Brca1L/L;Trp53L/L;Mir‐155−/− ;K14‐Cre | Breast | No change in overall survival | Oncogenic activity in epithelial cells is negated by tumor suppressive activity in the TME | Kim, Song, et al. (2016) |
| miR‐155 loss | Ex vivo viral transduction, enforced expression of inhibitory sequence | Bone marrow engraftment of HSP cells expressing miRT against miR‐155 in MMTV‐PyMT | Breast | Increase in tumor growth and overall tumor burden | Tumor suppressive in the TME, miR‐155 activity induces tumoricidal phenotype of macrophages | Zonari et al. (2013) |
| miR‐17,‐18,‐19a,‐20,‐19‐1b,‐92 gain | Ex vivo viral transduction, enforced expression | Orthotopic implant of Ink4c−/−;Ptch1+/− granule neuron progenitors cells expressing Mir‐17~‐92 | Brain | Increase in tumor initiation, progression | Oncogenic, accelerates medulloblastoma | Uziel et al. (2009) |
| miR‐31 loss | Genetic, conditional KO in epithelial cells | ApcMin/+;Mir‐31L/L;Villin‐Cre | Colon | Increase in tumor multiplicity and tumor burden | Tumor suppressive, cell‐autonomous role in epithelial cells | Liu, Bai, et al. (2017) |
| miR‐34a and/or miR‐34b–34c loss | Genetic, global KO | ApcMin/+;Mir‐34a−/− and/or ApcMin/+;Mir‐34b~‐34c−/− | Colon | Increase in tumor burden, decreased in overall survival | Tumor suppressive, phenotype is enhanced when all miR‐34 family members are deleted | Jiang and Hermeking (2017) |
| miR‐155 loss | Genetic, global KO | Chemically‐induced carcinogenesis in Mir‐155−/− | Colon | Decrease in tumor multiplicity and tumor growth | Tumor‐promoting, miR‐155 activity likely tumorigenic in the TME | Chen et al. (2015) |
| miR‐155 loss | Genetic, global KO | Subcutaneous implant of MC38 colon cancer cells in Mir‐155−/− | Colon | Decrease in tumor multiplicity and tumor growth | Tumor‐promoting in the TME, miR‐155 activity dampens anti‐tumor T cell‐mediated responses | Chen et al. (2015) |
| miR‐17,‐18,‐19a,‐20,‐19‐1b,‐92 gain | Genetic, conditional overexpression in retinal cells | RbL/L;p107−/−;CAG‐LSL‐Mir‐17–92;Pax6α‐Cre | Eye | Increase in tumor initiation, progression and metastatic spread to the brain | Oncogenic, accelerates retinoblastoma | Conkrite et al. (2011) |
| miR‐15,‐16 loss | Genetic, global KO | Subcutaneous implant of H‐22 liver cancer cells in Mir‐15a~‐16‐1−/− | Liver | Decrease in tumor growth | Tumor promoting in the TME, macrophage‐mediated tumorigenic response | Jia et al. (2018) |
| miR‐15,‐16 loss | Genetic, global KO | Subcutaneous implant of H‐22 liver cancer cells in Mir‐15a~‐16‐1−/− | Liver | Increase in tumor growth | Tumor suppressive in the TME, B‐cell mediated anti‐tumor response | Jia et al. (2019) |
| miR‐21 loss | Genetic, global KO | Subcutaneous implant of Heps liver cancer cells in Mir‐21−/− | Liver | Increase in tumor growth | Tumor suppressive in the TME, T cell‐mediated anti‐tumor response | He et al. (2017) |
| Let‐7b gain | In vivo viral transduction, enforced expression | LSL‐KrasG12D/+ plus nasal inhalation of adenoviral vectors expressing let‐7b and Cre | Lung | Decrease in tumor growth and tumor burden | Tumor suppressive, likely cell‐autonomous role in epithelial cells based on adenoviral delivery | Esquela‐Kerscher et al. (2008) |
| Let‐7g gain | In vivo viral transduction, enforced expression | LSL‐KrasG12D/+;Tp53L/L plus intratracheal lentiviral vectors expressing let‐7 g and Cre | Lung | Decrease in tumor multiplicity and tumor burden | Tumor suppressive, likely cell‐autonomous role in epithelial cells based on lentiviral delivery | Kumar et al. (2008) |
| Let‐7g gain | In vivo viral transduction, enforced expression | LSL‐KrasG12D/+ plus nasal inhalation of lentiviral vector expressing let‐7g and Adeno‐Cre | Lung | Decrease in tumor growth and tumor burden | Tumor suppressive, likely cell‐autonomous role in epithelial cells based on lentiviral delivery | Trang et al. (2010) |
| miR‐21 loss | Genetic, global KO | Latent 2 KrasG12D;Mir‐21−/− | Lung | Delay in tumor initiation, decrease in tumor multiplicity and overall tumor burden | Oncogenic, phenotype presumed to be driven by miR‐10b activity in epithelial cells | Hatley et al. (2010) |
| miR‐21 gain | Genetic, global overexpression | Latent 2 KrasG12D; CAG‐Mir‐21 | Lung | Increase in tumor multiplicity and overall tumor burden | Oncogenic, phenotype presumed to be driven by miR‐10b activity in epithelial cells | Hatley et al. (2010) |
| miR‐21 loss | Genetic, conditional KO in macrophages | Orthotopic implant of LLC lung cancer cells in Mir‐21L/L;LysM‐Cre | Lung | Decrease in tumor growth | Tumor promoting in the TME, macrophage‐mediated tumorigenic response | Sahraei et al. (2019) |
| miR‐21 loss | Genetic, global KO | Subcutaneous implant of LLC1 lung cancer cells in Mir‐21−/− | Lung | Decrease in tumor growth | Tumor promoting in the TME, macrophage‐mediated tumorigenic response | Xi et al. (2018) |
| miR‐30b loss | In vivo CRISPR/Cas9‐mediated KO via viral transduction | LSL‐KrasG12D/+;Tp53L/L plus nasal inhalation of lentiviral CRISPR/Cas‐Cre vector | Lung | Increase in tumor multiplicity and overall tumor burden | Tumor suppressive, likely cell‐autonomous role in epithelial cells based on lentiviral delivery |
Hong, Yao, et al. (2020) |
| miR‐34a gain | In vivo viral transduction, enforced expression | LSL‐KrasG12D/+;LSL‐Tp53R172H/+ plus intratracheal lentiviral miR‐34a minigene and Adeno‐Cre | Lung | Delay in tumor initiation and progression | Tumor suppressive, likely cell‐autonomous role in epithelial cells based on lentiviral delivery | Kasinski and Slack (2012) |
| miR‐34a loss | Genetic, global knockout | LSL‐KrasG12D/+;Tp53L/+;Mir‐34a−/− plus nasal inhalation of Adeno‐Cre | Lung | Increase in tumor burden | Tumor suppressive | Okada et al. (2014) |
| miR‐146a loss | In vivo CRISPR/Cas9‐mediated KO via viral transduction | LSL‐KrasG12D/+;Tp53L/L plus nasal inhalation of lentiviral CRISPR/Cas‐Cre vector | Lung | Increase in tumor multiplicity and overall tumor burden | Tumor suppressive, likely cell‐autonomous role in epithelial cells based on lentiviral delivery |
Hong, Yao, et al. (2020) |
| miR‐155 loss | Genetic, global KO | Subcutaneous implant of LLC lung cancer cells in Mir‐155−/− | Lung | Increase in tumor growth | Tumor suppressive in the TME, miR‐155 activity reduces MDSC infiltration | Wang et al. (2015) |
| miR‐155 loss | Genetic, global KO | Subcutaneous implant of LLC1 or LLC1‐OVA lung cancer cells in Mir‐155−/− | Lung | Decrease in tumor growth | Tumor‐promoting in the TME, miR‐155 activity required for MDSC‐mediated Treg induction and dampening anti‐tumor T cell‐mediated responses | Chen et al. (2015) |
| miR‐190b loss | In vivo CRISPR/Cas9‐mediated KO via viral transduction | LSL‐KrasG12D/+;Tp53L/L plus nasal inhalation of lentiviral CRISPR/Cas9– Cre vector | Lung | Increase in tumor multiplicity and overall tumor burden | Tumor suppressive, likely cell‐autonomous role in epithelial cells based on lentiviral delivery |
Hong, Yao, et al. (2020) |
| miR‐190b gain | In vivo viral transduction, enforced expression | LSL‐KrasG12D/+;Trp53L/L plus nasal inhalation of lentiviral miR‐190b/Cre vector | Lung | Decrease in tumor multiplicity and overall tumor burden | Tumor suppressive, likely cell‐autonomous role in epithelial cells based on lentiviral delivery | Hong, Yao, et al. (2020) |
| miR‐21 gain | Genetic, Inducible expression in Nestin‐expressing cells | LSL‐TetOFFMir‐21;Nestin‐Cre | Lymph nodes, spleen | Develops pre‐B‐cell lymphoma | Oncogenic, oncogenic addition (tumor regresses when enforced expression is discontinued) | Medina et al. (2010) |
| miR‐31 loss | Genetic, global KO (Rat) | Chemically‐induced carcinogenesis in Mir‐31−/− | Esophagus | Prevents tumor formation | Tumor promoting | Fong et al. (2020) |
| miR‐17,‐18,‐19a,‐20,‐19‐1b, ‐92 loss | Genetic, conditional KO in pancreatic epithelia | LSL‐KrasG12D/+;Tp53L/+;Mir‐17–92L/L;Pft1a‐Cre | Pancreas | Delay in tumor formation, no change in overall survival | Neutral to oncogenic, epithelial activity may affect MAPK signaling, and metastatic spread | Quattrochi et al. (2017) |
| miR‐21 loss | Genetic, global KO | LSL‐KrasG12D;Tp53L/+;Mir‐21−/‐Pdx1‐Cre | Pancreas | Faster tumor initiation and progression, and shorter overall survival | Tumor suppressive in the TME, stromal activity required for myofibroblast formation (presumed tumor‐restraining) | Schipper et al. (2020) |
| Mir‐29a loss | Genetic, global KO | Ela1‐TAg;Mir‐29a−/− | Pancreas | No change in tumor initiation and overall survival | Neutral | Dooley et al. (2017) |
| miR‐34a loss | Genetic, conditional KO in pancreatic epithelia | LSL‐KrasG12D/+;Mir‐34aL/L;Pft1a‐Cre | Pancreas | Increase in tumor initiation and progression | Tumor suppressive | Hidalgo‐Sastre et al. (2020) |
| miR‐216 or miR‐217 loss | Genetic, global KO | Ela1‐KrasG12D/+;Mir‐216a−/− or Mir‐217−/− | Pancreas | No change in tumor growth | Neutral | Sutaria et al. (2019) |
| miR‐17,‐18,‐19a,‐20,‐19‐1b, ‐92 gain | Ex vivo viral transduction, enforced expression | Subcutaneous implant of PC‐3 or M12 prostate cancer cells transduced with minigene expressing Mir‐17~‐92 | Prostate | Decrease in tumor growth | Tumor suppressive | Ottman et al. (2016) |
| miR‐21 loss | Genetic, global KO | Chemical‐induced carcinogenesis in Mir‐21−/− | Skin | Delay in tumor formation and decrease in tumor multiplicity | Oncogenic, phenotype presumed to be driven by miR‐21 activity in keranocytes | Ma et al. (2011) |
| miR‐21 loss | Genetic, global KO | Subcutaneous implant of B16 melanoma cells in Mir‐21−/− | Skin | Decrease in tumor growth | Tumor promoting in the TME, macrophage‐mediated tumorigenic response | Xi et al. (2018) |
| miR‐155 loss | Genetic, conditional KO in T cells | Subcutaneous implant of B16F10 or B16F10‐OVA melanoma cells in Mir‐155−/− | Skin | Increase in tumor growth | Tumor suppressive in the TME, miR‐155 activity in T cells is required for anti‐tumor response, reduces MDSC infiltration | Ekiz et al. (2019) and Huffaker et al. (2017) |
| miR‐155 loss | Genetic, adoptative transfer of KO T cells | Subcutaneous implant of B16F10 melanoma cells in mice with Mir‐155−/− T‐cells | Skin | Increase in tumor growth, decrease in overall survival | Tumor suppressive in the TME, miR‐155 activity in T cells is required for anti‐tumor response | Dudda et al. (2013) |
| miR‐155 gain | Ex vivo viral transduction, enforced expression in T cells | Subcutaneous implant of B16F10 melanoma cells in animals overexpressing miR‐155 in T cells | Skin | Decrease in tumor growth, increase in overall survival | Tumor suppressive in the TME, miR‐155 activity in T cells is required for anti‐tumor response | Dudda et al. (2013) and Martinez‐Usatorre et al. (2019) |
| miR‐155 loss | Genetic, global KO | Subcutaneous implant of B16F10 melanoma cells in Mir‐155−/− | Skin | Increase in tumor growth | Tumor suppressive in the TME, miR‐155 activity reduces MDSC infiltration | Wang et al. (2015) |
| miR‐21 loss | Genetic, global KO | Subcutaneous implant of S180 fibrosarcoma cells in Mir‐21−/− | Soft tissue | Increase in tumor growth | Tumor suppressive in the TME, T cell‐mediated anti‐tumor response | He et al. (2017) |
| miR‐21 loss | Genetic, global KO | Tp53−/−;Mir‐21−/− | Various | Slight delay in tumor formation, no change in overall survival | Neutral to slightly oncogenic. Slight change in incidence of p53‐depedent cancer spectrum | Ma et al. (2013) |
| miR‐34a,‐34b,‐34c loss | Genetic, global KO | Mir‐34a−/−;Mir‐34b~‐34c−/− | Various | No spontaneous tumor formation | Neutral, lymphomas, and sarcomas arise in p53‐deficient mice | Concepcion et al. (2012) |
| miR‐34a,‐34b,‐34c loss | Genetic, global KO | 1‐Gy irradiation of Mir‐34a−/−;Mir‐34b~‐34c−/− | Various | No radiation‐induced tumor formation | Neutral, lymphomas and sarcomas arise in p53‐deficient mice | Concepcion et al. (2012) |
Note: Unless otherwise noted, experiments were conducted in mice.
Abbreviations: ALL, acute lymphoblastic leukemia or lymphoblastic lymphoma; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; HSP, hematopoietic stem and progenitor; L, conditional “floxed” allele, flanked by Lox P sites; LSL, Lox‐STOP‐Lox cassette; MDSC, myeloid‐derived suppressive cells; miRT, miRNA target sequence complementary to an miRNA seed region, acts as miRNA sponge; NZB, New Zealand Black; TME, tumor microenvironment.
3.1.1. Mir‐15~Mir‐16 gene family
Chromosomal deletion of MIR15A–MIR16‐1 gene cluster in chronic lymphocytic leukemia (CLL) was the first causal link between miRNA function and human cancer (Calin et al., 2002). MIR15A–MIR16‐1 gene cluster maps to the minimal region at chromosome 13q14.3 which is frequently deleted in CLL cases and expression of miR‐15 and miR‐16 is low in these cases (Calin et al., 2002). Enforced expression of miR‐15a and miR‐16‐1 in MEG‐01 leukemic cells completely inhibits tumor formation in a xenograft model (Calin et al., 2008). Similarly, enforced expression of miR‐15 and miR‐16 in B‐1 cells derived from New Zealand Black mouse model of CLL or systemically in this de novo CLL model significantly reduced tumor growth (Kasar et al., 2012; Salerno et al., 2009). Global KO of the Mir‐15a~Mir‐16‐1 gene cluster in mice leads to CLL (Klein et al., 2010). Similarly, global KO of the Mir‐15b~Mir‐16‐2 gene cluster in mice leads to CLL (Lovat et al., 2015). These studies indicate the requirement of minimal tumor suppressive activity miR‐15 and miR‐16 family members to keep CLL in check. Intriguingly and somewhat puzzlingly, global double KO of Mir‐15a~Mir‐16‐1 and Mir‐15b~Mir‐16‐2 gene clusters and consequently complete loss of activity of any miR‐15 or miR‐16 family members leads to acute myeloid leukemia (AML; Lovat et al., 2018). This mechanistic link to myeloproliferative disorders prompted the authors to analyze miR‐15 and miR‐16 expression in AML patients and their results suggest that restoring miR‐15 and miR‐16 activity could be an attractive therapeutic option in AML (Lovat et al., 2020). Collectively, these studies identified anti‐apoptotic BCL2 and MCL1, and proliferative Cyclin D1 as key targets of miR‐15 and miR‐16 family members (Lovat et al., 2018; Pekarsky et al., 2018). Enforced expression of miR‐15/‐16 in cell lines derived from an array of solid tumors consistently inhibits cell growth and viability in vitro through these key targets (Bonci et al., 2008; Cai et al., 2012; Reid et al., 2013). In in vivo mouse models of solid tumors using the global KO of Mir‐15a~Mir‐16‐1 strain, the intrinsic tumor suppressive role of these miRNAs in neoplastic cells is less obvious and their activity in immune cells seems to drive tumor initiation and progression. A role of miR‐15 and miR‐16 in macrophage polarization and regulatory B cells influences tumor growth in transplantable hepatic cancer and chemically‐induced colitis (prompt to develop colon cancer) models (Jia et al., 2018; Jia et al., 2019). A similar role of miR‐15 and miR‐16 in macrophage polarization has been observed in ex vivo experiments with multiple myeloma models (Khalife et al., 2019).
3.1.2. Mir‐17~Mir‐92 gene cluster (OncomiR‐1)
The MIR17~MIR92 gene cluster was first identified due to frequent amplification of this chromosomal region in B‐cell lymphomas (Mogilyansky & Rigoutsos, 2013; Olive et al., 2013). MIR17~MIR92 gene cluster contains six miRNAs: miR‐17, ‐18a, ‐19a, ‐20a, ‐19b‐1, and 92a‐1. Enforced expression of this miRNA gene cluster (lacking miR‐92) in conjunction with c‐Myc expression enhanced tumor development in a transplantable B‐cell lymphoma model demonstrating for the first time the oncogenic activity of miRNA(s) and hence its coining as oncomiR‐1 (L. He et al., 2005). Similarly, gain‐of‐function approaches show that enforced expression of all or specific miRNAs in this cluster can initiate or enhance tumor development of B‐cell and T‐cell malignancies as well as retinoblastoma and medulloblastoma in mouse models (Mogilyansky & Rigoutsos, 2013; Olive et al., 2013). Global KO of Mir‐17~‐92 gene cluster results in perinatal lethality with severe skeletal abnormalities resembling the clinical manifestation of patients with autosomal dominant Feingold syndrome due to heterozygous microdeletion of the MIR17~MIR92 gene cluster (Mogilyansky & Rigoutsos, 2013; Olive et al., 2013). Thus, the therapeutic window for inhibiting all members of this cluster is a concern due to developmental and physiological defects. Exquisite genetic dissection of individual miRNAs in this cluster reveals distinct roles for individual miRNAs in B cell development and Myc‐driven tumorigenesis (Han et al., 2015). Transcriptional regulation by Myc and E2F family members are major drivers of gene cluster expression. Several gene cluster members target tumor suppressive BIM, PTEN, p21, and/or p57, while some of these miRNAs and other miRNAs in the cluster seemingly restrain its oncogenic activity by downregulating expression of E2Fs and other transcription activators (Mogilyansky & Rigoutsos, 2013; Olive et al., 2013). Previous gain‐of‐function experiments of enforced expression suggested that miR‐19a and miR‐19‐1b activity was the main oncogenic driver in the gene cluster to cooperate with Myc oncogene in B‐cell lymphoma; genetic ablation of Mir‐19a and Mir‐19b‐1 loci significantly extends overall survival in the aggressive Eμ‐Myc mouse model of B‐cell lymphoma (Han et al., 2015). This study further demonstrates the oncogenic role of miR‐19a and miR‐19b in B‐cell lymphoma pointing to the possibility that their selective inhibition may be a viable option in Myc‐driven lymphoma and in other Myc‐driven cancers. Context of the activity for Mir‐17~‐92 gene cluster members is important and therapeutic intervention may lead to ineffective or paradoxical outcomes. Pancreatic ductal adenocarcinoma (PDAC) is among the most aggressive and lethal types of solid tumors. Deletion of the Mir‐17~‐92 gene cluster within the pancreatic epithelial cell lineages in the well‐established KRas‐driven p53‐deleted mouse model of PDAC (KPC model; Table 3) has little impact on overall survival, but may influence metastatic spread to the liver (Quattrochi et al., 2017). Enforced expression of all Mir‐17~‐92 gene cluster members in several human prostatic cancer cell lines induces an epithelial phenotype and increases drug sensitivity in in vitro studies, and inhibits tumor growth in in vivo xenograft mouse models (Ottman et al., 2016).
3.1.3. Mir‐21 gene
miR‐21 is among the most highly expressed miRNAs and is frequently upregulated in most cancer types (Bautista‐Sanchez et al., 2020). miR‐21 has a role in the regulation of carcinogenesis, tissue fibrosis, and inflammatory responses (Bautista‐Sanchez et al., 2020; Sheedy, 2015). Oncogenic activity of miR‐21 is mainly driven within cancer cells by downregulation of key anti‐proliferative and/or pro‐apoptotic target genes such as PTEN, PDCD4, RECK, and Sprouty1/2 (Bautista‐Sanchez et al., 2020). However, miR‐21 also engages these targets in other cell types of the TME such as fibroblasts, T cells, and macrophages. Thus, the regulatory interactions of miR‐21 with these and additional targets depending on signaling inputs, predominant cell type(s) at play, and mutational landscape can have distinct and somewhat opposite effects in tumor formation and evolution in different cancer types. Enforced expression of miR‐21 enhances tumor formation and increases tumor burden in a KRas‐driven lung cancer model (Hatley et al., 2010). While this enforced expression of miR‐21 under a ubiquitous promoter alone is not sufficient to cause lung cancer or other cancers (Hatley et al., 2010), enforced expression of miR‐21 under Nestin promoter induces pre‐B cell lymphoma and the malignant cells are addicted to continuous miR‐21 expression (Medina et al., 2010). Interestingly, enforced expression of miR‐21 in this model is not sufficient to induce neuroblastomas, glioblastomas, or other Nestin‐expressing brain tumors; MIR21 gene is locally amplified in a proportion of these tumor types in human patients (Calin et al., 2004). Thus, the difference in dose and timing of expression in different cell types may explain the unique susceptibility of B cells to become malignant in these mouse models. Global Mir‐21KO animals are viable and have been crossed into several genetic models, used as recipients of transplantable tumor, and subjected to chemically induced protocols of carcinogenesis. Global loss of miR‐21 activity significantly delays tumor formation and decreases tumor burden in a KRas‐driven lung cancer model (Hatley et al., 2010) and a chemically induced skin cancer model (X. Ma et al., 2011), but has a modest effect in lymphoma‐ and sarcoma‐prone Tpr53KO model (X. Ma et al., 2013) as well as in viral protein‐driven breast cancer models (Table 3). In human breast cancer tumors, miR‐21 expression is predominantly upregulated in the cancer‐associated fibrobasts (CAFs) and carries more robust prognostic information, especially in triple‐negative breast cancer (MacKenzie et al., 2014). It is possible that miR‐21 has a CAF‐specific tumor‐promoting role that is not accurately captured or required in these viral protein‐driven mouse models, known to have a relatively low desmoplastic reaction. In contrast, the KRas‐driven KPC model recapitulates several aspects of human PDAC including a profound desmoplastic reaction. Global loss of miR‐21 activity in this KPC model somewhat paradoxically accelerates tumor initiation and progression that results in a significantly much shorter overall survival (Schipper et al., 2020). While the effects could be compounded by the activity of miR‐21 in different cell types, an obvious absence in this profoundly remodeled TME are myofibrotic CAFs (Schipper et al., 2020), which have recently emerged as a tumor‐restraining barrier in PDAC (Helms et al., 2020). Tumor transplantation experiments demonstrate the requirement of stromal miR‐21 activity for formation of myofibroblasts (Schipper et al., 2020). This pro‐fibrotic role of miR‐21 has also been observed in chemically induced models of colitis (Shi et al., 2013) and pancreatitis (Ma, Conklin, et al., 2015) as well as in a diet‐induced model of nonalcoholic steatohepatitis (Rodrigues et al., 2017). Studies in other cancer models have also shown a range of immune cell–dependent tumoricidal to tumor promoting effects of miR‐21 activity. Global loss of miR‐21 activity reduces tumor growth by polarizing macrophages toward a tumoricidal phenotype in transplantable melanoma and lung cancer models (Sahraei et al., 2019; Xi et al., 2018). In contrast, global loss of miR‐21 activity enhances tumor burden in transplantable hepatocarcinoma and fibrosarcoma models due to diminished anti‐tumoral T responses (W. He et al., 2017). Collectively, these mouse model studies suggest a complex, cell type‐specific, and organ site‐dependent role of miR‐21 and that indiscriminate inhibition of miR‐21 activity may lead to unintended overall tumor promoting effects.
3.1.4. Mir‐34 gene family
miR‐34 family members are located at two chromosomal locations. In humans, miR‐34a transcript is processed from 1p36, a region frequently deleted in tumors, and miR‐34b~miR‐34c polycistronic transcript is processed from 11q23 (Jain & Barton, 2012; Slabakova et al., 2017). miR‐34 family members are transcriptionally upregulated by p53 and originally identified as important mediators of p53‐dependent DNA damage response (Agostini & Knight, 2014; Jain & Barton, 2012). Cell line studies uncovered a strong tumor suppressive role of miR‐34 via coordinated inhibition of a large number of (proto)oncogenic target genes involved in cell cycle regulation and proliferation (Cyclin D1, CDK4), apoptosis (BCL2, SIRT1), cancer cell stemness (e.g., CD44, Nanog), and oncogenic signaling (e.g., MET, MYC) (Agostini & Knight, 2014; Bader, 2012; Slabakova et al., 2017). Intratracheal delivery of lentiviral particles carrying miR‐34a‐expressing minigene in an aggressive KRas‐driven p53‐mutated mouse model of lung cancer was among the first studies to show therapeutic efficacy in vivo (Kasinski & Slack, 2012). Most of these in vitro and in vivo studies relied on enforced expression or replenishment of miR‐34 molecules, and collectively provided overwhelming support for the clinical development and evaluation of miR‐34 replacement therapy in cancer (Agostini & Knight, 2014; Bader, 2012). Indeed, a phase I clinical study started in 2013 to evaluate the safety of miR‐34 mimic drug, MRX34 ([Agostini & Knight, 2014]; see Section 3.4.3 for more details). Soon after, independent groups showed that mice with complete loss of miR‐34 activity in a global double KO of Mir‐34a and Mir‐34b~‐34c genes have virtually no phenotypic consequences and p53‐depedent tumor suppressive pathways were functioning robustly in several in vitro transformation assays and in vivo cancer models (Choi et al., 2011; Concepcion et al., 2012; Jiang & Hermeking, 2017). Functional redundancy with miR‐449 might in part offer an explanation, though miR‐34 family members are expressed at higher levels than miR‐449 family members in most tissue types (Concepcion et al., 2012; Song et al., 2014; J. Wu et al., 2014). Mice with complete loss of miR‐34 and miR‐449 activity in a global triple KO of Mir‐34a, Mir‐34b~‐34c, and Mir‐449a~‐449c genes die perinatally and a minimum activity level of miR‐34 and miR‐449 is required for proper brain development, ciliogenesis, and spermatogenesis (Song et al., 2014; J. Wu et al., 2014). The requirement of a minimum combined activity of miR‐34 and miR‐449 has not yet been formally tested in cancer models, and this may prove difficult due to organismal viability issues. More‐complex‐than‐anticipated regulatory loops between miR‐34 family members and p53 with the involvement of distinct inputs in different cancer contexts and cell types may be at play (Navarro & Lieberman, 2015; Slabakova et al., 2017). A point in case is the requirement of miR‐34a activity in a KRas‐driven lung cancer model to enhance p53 via downregulation of p53 repressor HDM4 (Okada et al., 2014). Global loss of miR‐34a activity has no effect in tumor initiation or progression in this KRas‐driven lung cancer model with intact p53 activity. However, reduced p53 activity by genetic deletion of one of the Tpr53 alleles (haploinsufficiency) exposes a requirement of miR‐34a activity in potentiating p53‐depedent tumor‐suppressive functions (Okada et al., 2014). Global KO of Mir‐34a or Mir‐34b~‐34c genes moderately increases tumor burden and decreases overall survival of the ApcMin genetic mouse model of colon cancer (Jiang & Hermeking, 2017). Global double KO of Mir‐34a and Mir‐34b~‐34c genes further enhances these phenotypes indicating that a collective tumor‐suppressive function of miR‐34 family members is at play in this model, but whether cooperation or interaction with p53 is required for this was not directly investigated (Jiang & Hermeking, 2017).
3.1.5. Mir‐155/BIC gene
miR‐155 is located at 21q21.3 region in the human genome and it is processed from host gene MIR155HG also known as BIC (B cell integration cluster). The BIC gene is evolutionarily conserved and is a common integration site for avian leucosis virus in chickens that leads to B cell lymphomas (Bayraktar & Van Roosbroeck, 2018). Enforced expression of miR‐155 in B cells under the Eμ promoter is sufficient to cause B cell malignancies, including acute lymphoblastic leukemia and high‐grade lymphomas, in transgenic mice (Costinean et al., 2006). Global KO of Mir‐155 gene in mice leads to impaired B and T cell responses (Thai et al., 2007), which indicate important physiological roles in development and differentiation of hematopoietic cell lineages, inflammation and protective immunity against pathogens (Bayraktar & Van Roosbroeck, 2018; O'Connell et al., 2012). These impaired B and T cell responses can in part be due to an intrinsic role of miR‐155 in these cell types, but also by a role of miR‐155 in other immune cell types. Global KO of Mir‐155 gene significantly diminishes overall tumor burden of AML in the FLT3‐ITD mouse model (Wallace et al., 2017). The extent of miR‐155 activity in myeloid cells can paradoxically lead to overall tumor suppressive or oncogenic effects in AML cell line models (Narayan et al., 2017). The role of miR‐155 in solid tumors may be also compounded by effects in the neoplastic cells as well as in immune cells. Global KO of Mir‐155 gene in a Brca1‐deficient mouse model of breast cancer has no impact on overall survival (Kim, Song, et al., 2016). Allograft transplantation reveals an oncogenic role of miR‐155 in the cancer cells that is counteracted by a tumoricidal role in the TME, which limits the recruitment and infiltration of tumor‐promoting myeloid‐derived suppressive cells (MDSCs) (S. Kim et al., 2018; Kim, Song, et al., 2016). While not addressed with a genetic approach, miR‐155 can have a cancer cell–intrinsic role in chemoresistance and/or radioresistance in breast, colorectal, lung, and other cancer types (Bayraktar & Van Roosbroeck, 2018). Opposite tumor‐promoting or tumoricidal roles of miR‐155 in MDSCs or tumor‐associated macrophages have been observed in different solid tumor models (Chen et al., 2015; Dueck et al., 2014; Li et al., 2014; Wang et al., 2015; Zonari et al., 2013). Moreover, conditional deletion of Mir‐155 gene exclusively within the T cell lineages uncovered a T‐cell specific activity of miR‐155 for mounting an anti‐tumor response in transplantable melanoma models (Dudda et al., 2013; Ekiz et al., 2019; Huffaker et al., 2017). In a complementary approach, enforced expression of miR‐155 in CD8+ T‐cells enhanced their in situ antigen‐specific response in similar transplantable melanoma mouse models (Dudda et al., 2013; Martinez‐Usatorre et al., 2019).
3.2. Therapeutic strategies and considerations for modulating miRNA activity in cancer
Two main features make miRNAs attractive candidates for cancer therapy: the ability of influencing protein output and function of many direct miRNA target genes with a single miRNA drug and the chemical synthesis of RNA‐based miRNA activity modulators (Figure 2). There are functional and technical considerations and challenges for replenishing the activity of a tumor suppressive miRNA or inhibiting the activity of an oncogenic miRNA. Replenishing miRNA activity or even supplementing the activity above physiological levels can have a potent effect in the downregulation of many direct target genes, but it could also have off‐target effects similar to siRNAs in the downregulation of unintended mRNAs and/or ectopic effects in unintended cell types. In contrast, inhibiting miRNA activity can be more precise since an inhibitor will bind with high affinity to the intended miRNA sequence, but the extent of downregulation of target genes may be more modest due to redundant regulation by other miRNAs or mechanisms. Mimic compounds used to replenish miRNA activity are generally double‐stranded RNA (dsRNA) molecules (Figure 2) that need to interact with and be processed by the cellular machinery as siRNAs do, which limits the type of chemical modifications that they can incorporate and may unintentionally affect processing of other miRNAs by saturating the miRISC processing capacity (Bajan & Hutvagner, 2020; Lee et al., 2020; Roberts et al., 2020). In contrast, anti‐miRNA inhibitors are typically single‐stranded molecules that directly bind to the complementary miRNA sequence in the cytoplasm, which allows them to have more types of chemical modifications and utilize more cell internalization routes without the need of nanoparticle conjugation or encapsulation (Bajan & Hutvagner, 2020; Lee et al., 2020). Recent FDA approval of two RNA‐based drugs, patisiran and givosiran, provides a confidence boost for clinical development and evaluation of similar miRNA‐based drugs (Figure 2). Patisiran is an siRNA targeting mutant transthyretin (TTR) mRNA to treat transthyretin amyloidosis and is delivered intravenously as cargo in a liposomal nanoparticle (Urits et al., 2020). Givosiran is an siRNA targeting delta‐aminolaevulinic acid‐synthase 1 (ALAS1) mRNA to treat acute hepatic porphyria and is delivered subcutaneously as an N‐acetylgalactosamine (GalNAc) conjugate (Neeleman et al., 2020; Springer & Dowdy, 2018). Lessons learned from these and other clinical experiences with siRNAs provide valuable information for the design of chemical modifications, complex formation, and delivery strategies aimed at increasing the stability, biodistribution, targeted delivery, and cell penetration of miRNA‐based drugs (Bajan & Hutvagner, 2020; Forterre et al., 2020; Lee et al., 2020; O'Neill & Dwyer, 2020; Roberts et al., 2020; Springer & Dowdy, 2018) .
FIGURE 2.
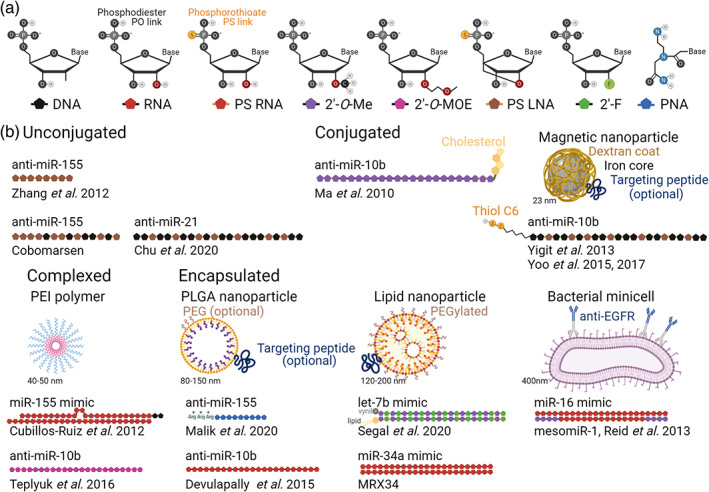
Chemical modifications and delivery technologies. (a) Chemical structure of most common RNA analogue modifications incorporated in antisense miRNA inhibitor or double‐stranded mimics. (b) Representative examples of different chemical modification and delivery technologies (see Tables 4 and 5 for more details). Pattern and location of chemical modifications are representative of that particular approach and are approximation (in some cases the exact modified sequence is not fully disclosed). For miRNA mimics, top strand depicts the guide strand (mature miRNA) and bottom strand the passenger strand. Molecules and constructs not drawn to scale. 2′‐F, 2′‐Fluoro; 2′‐O‐me, 2′‐O‐methyl; 2′‐O‐MOE, 2′‐O‐methoxyethyl; Arg, arginine; LNA, locked nucleic acid; PEG, polyethylene glycol; PLGA, poly(lactic‐co‐glycolic acid); PNA, peptide nucleic acid; PO, phosphodiester; PS, phosphorothioate
3.3. microRNA‐based therapy in preclinical models
Table 4 provides a comprehensive list of approaches describing the development of miRNA‐based drugs systemically delivered in in vivo models. We highlight pros and cons of some of these approaches and their therapeutic efficacy. Chemical modifications (Figure 2) to stabilize and protect from RNAse degradation include replacement of the labile 2′OH group by 2′‐O‐methyl, 2′‐O‐methoxyethyl, or 2′‐Fluoro and/or replacement or mixing of the phosphodiester backbone with phosphorothioates (Bajan & Hutvagner, 2020; Roberts et al., 2020). In addition to increasing stability, chemical modifications can increase affinity for complementary sequence including locked nucleic acid (LNA) and peptide nucleic acid (PNA; Bajan & Hutvagner, 2020; Roberts et al., 2020). The chemically modified oligonucleotide or the nanoparticle vehicle can be decorated with targeting peptides (e.g., iRGD, penetratin) and other moieties (e.g., folate, cholesterol) for preferential interaction and accumulation at tumor sites (Forterre et al., 2020; Lee et al., 2020).
TABLE 4.
Pharmacological modulation of miRNA activity in in vivo cancer models
| miRNA modulation | Chemistry | Delivery technology | Model | Cancer site(s) | Therapeutic benefit | References |
|---|---|---|---|---|---|---|
| miR‐155 inhibition | 8‐mer AMO (Fully LNA, fully PS backbone) | Unconjugated | Bone marrow engraftment, BCWM1 B‐cell lymphoma cells | Bone marrow | Inhibits tumor growth, decreases overall tumor burden, upregulates expression of direct target genes CEBPβ, MAFB, SOCS1, and others | Zhang et al. (2012) |
| miR‐10b inhibition | AMO (2′‐O‐MOE, partial PS backbone) | in vivo‐jetPEI® complex (intracranial injection) or unconjugated (IV injection) | Orthotopic, GBM8 glioblastoma cells | Brain | Inhibits primary tumor growth and increases overall survival. Effect is enhanced by combination treatment with doxorubicin | Teplyuk et al. (2016) |
| miR‐10b and miR‐21 inhibition | AMO (?) | PLGA nanoparticle with tumor‐penetrating cRGD | Subcutaneous, U87‐MG glioblastoma cells | Brain | Inhibits tumor growth. Effect is enhanced by combination treatment with temozolomide | Malhotra et al. (2018) |
| miR‐21 inhibition | AMO (2′‐O‐Me) | Exosome with tumor‐penetrating T7 peptide | Orthotopic, C6 glioblastoma cells | Brain | Inhibits tumor growth, upregulates expression of direct target genes PTEN and PDCD4 | G. Kim et al. (2020) |
| miR‐21 inhibition, miR‐100 replacement | AMO for miR‐21, ssRNA? for miR‐100 | Gold‐iron oxide nanoparticles with PEG‐T7 peptide (intranasal delivery) | Orthotopic, U87‐MG glioblastoma cells | Brain | Inhibits tumor growth, extends overall survival. Effect is enhanced by combination treatment with temozolomide | Sukumar et al. (2019) |
| miR‐21 inhibition | AMO (2′‐O‐Me) | Star‐branched PLA/PDMAEMA copolymers | Subcutaneous, LN229 glioma cells | Brain | Inhibits tumor growth, upregulates expression of direct target gene PTEN. Effect is enhanced by combination treatment with doxorubicin | Qian et al. (2014) |
| miR‐21 inhibition | AMO (partial LNA) | Lipid nanoparticle with chlorotoxin peptide | Orthotopic, GL261 glioma cell | Brain | Inhibits tumor growth in combination treatment with sunitinib | Costa et al. (2015) |
| miR‐21 inhibition | AMO (partial LNA) | Three‐way junction‐based RNA (2′‐F modified) nanoparticle with folate | Orthotopic, patient‐derived GBM30 glioblastoma cells | Brain | Inhibits tumor growth, upregulates expression of direct target genes PTEN and PDCD4, and increases overall survival | Lee et al. (2017) |
| miR‐34a replacement | Invitrogen™ Pre‐miR™ miRNA mimic | Silica nanoparticle with anti‐GD2 antibody | Subcutaneous, NB1691 or SK‐N‐AS neuroblastoma cells | Brain | Inhibits tumor growth | Tivnan et al. (2012) |
| miR‐10b inhibition | AMO (2′‐O‐Me, partial PS backbone) | Liposomal nanoparticle | Orthotopic, MDA‐MB‐231 breast cancer cells | Breast | Inhibits primary tumor growth | Monroig‐Bosque et al. (2018) |
| miR‐10b inhibition | AMO (2′‐O‐Me, partial PS backbone) | Cholesterol conjugation | Orthotopic, 4T1 breast cancer cells | Breast, Lung (distant metastasis) | Prevents formation of metastatic lung lesions. No effect on primary tumor growth or established lung metastasis | Ma et al. (2010) |
| miR‐10b inhibition | AMO (Partial LNA) | Dextran‐coated iron oxide magnetic nanoparticle with tumor targeting cRGD peptide (IV injection) | Orthotopic, MDA‐MB‐231 breast cancer cells | Breast, lymph node (regional metastasis) | Prevents formation of lymph node metastases, inhibits growth of established lymph node metastases. No effect on primary tumor growth | Yigit et al. (2013) |
| miR‐10b inhibition | AMO (Partial LNA) | Dextran‐coated iron oxide magnetic nanoparticle | Orthotopic, MDA‐MB‐231 breast cancer cells | Breast (primary tumor removed), lymph nodes and lung | Inhibits growth of established lymph node metastases, prevents lung metastases, increases overall survival. Effect is enhanced by combination treatment with low‐dose doxorubicin | Yoo et al. (2015) |
| miR‐10b inhibition | AMO (Partial LNA) | Dextran‐coated iron oxide magnetic nanoparticle | Orthotopic, 4T1 breast cancer cells | Breast (primary tumor removed), lymph nodes and lung | Inhibits growth of established lymph node and lung metastases, increases overall survival. Effect is enhanced by combination treatment with low dose doxorubicin | Yoo et al. (2017) |
| miR‐10b inhibition | AMO (partial PS backbone) | PLGA‐b‐PEG nanoparticle with targeting uPA peptide | Subcutaneous, MDA‐MB‐231 breast cancer cells | Breast | Inhibits tumor growth. Effect is enhanced by combination treatment with anti‐miR‐21 AMO | Devulapally et al. (2015) |
| miR‐21 inhibition | AMO (partial PS backbone) | PLGA‐b‐PEG nanoparticle with targeting uPA peptide | Subcutaneous, MDA‐MB‐231 breast cancer cells | Breast | Inhibits tumor growth. Effect is enhanced by combination treatment with anti‐miR‐10b AMO | Devulapally et al. (2015) |
| miR‐21 inhibition | AMO (partial LNA) | Three‐way junction‐based RNA (2′‐F modified) nanoparticle with EGFR or CD133 targeting aptamer | Orthotopic, MDA‐MB‐231 breast cancer cells | Breast | Inhibits tumor growth and upregulates expression of direct target genes PTEN and PDCD4 | Shu et al. (2015) and Yin et al. (2019) |
| miR‐210 inhibition | AMO (miniPEG‐γPNA) | PLGA nanoparticle | Subcutaneous, HeLa cervical cancer cells | Cervix |
Inhibits tumor growth and increases overall survival |
Gupta et al. (2017) |
| miR‐34a replacement | dsRNA mimic (RNA?) | Amphoteric liposome | Orthotopic, Hep3B or HuH7 liver cancer cells | Liver | Inhibits tumor growth, downregulates expression of direct target genes MET, MYCN, TRA2A, and others | Daige et al. (2014) |
| miR‐122 replacement | ssRNA (?) | Megamer‐based nanoparticles (PEG, PAMAM) | Subcutaneous, HuH7 liver cancer cells | Liver | Inhibits tumor growth | Wu et al. (2019) |
| miR‐122 replacement, miR‐21 inhibition | ssRNA (?) for miR‐122, AMO for miR‐21 | PLGA‐b‐PEG nanoparticle with ultrasound‐guided delivery | Subcutaneous, HepG2 or Hepa1–6 liver cancer cells | Liver | Inhibits tumor growth. Effect is enhanced by combination treatment with doxorubicin | Chowdhury et al. (2018), Mullick Chowdhury et al. (2016), and Wischhusen et al. (2020) |
| miR‐34a replacement | Dharmacon mimic | Lipid emulsion | Subcutaneous, U2932 lymphoma cells | Lymph node | Inhibits tumor growth | Craig et al. (2012) |
| miR‐155 inhibition | AMO (partial LNA, full PS backbone; Cobomarsen) | Unconjugated? | Subcutaneous, U2932 lymphoma cells | Lymph node | Inhibits tumor growth and upregulates expression of direct targets SOCS1, WEE1, and others | Anastasiadou et al. (2020) |
| Mir‐155 inhibition | Short AMO (PNAs) | PLGA nanoparticle | Subcutaneous, U2932 lymphoma cells | Lymph node | Inhibits tumor growth | Malik et al. (2020) |
| Let‐7b replacement | Invitrogen™ Pre‐miR™ miRNA mimic | siPORTamine complex | Subcutaneous, H460 lung cancer cells | Lung | Inhibits tumor growth | Trang et al. (2010) |
| Let‐7b replacement | Dharmacon mimic | Neutral lipid emulsion |
Genetic, LSL‐KrasG12D /+ plus intranasal Adeno‐Cre |
Lung | Inhibits tumor growth and decreases overall tumor burden | Trang et al. (2011) |
| Let‐7b replacement | AccuTarget miRNA mimic | Cationic copolymer (PEG5K, VE, DET) | Subcutaneous, A549 lung cancer cells | Lung | Inhibits tumor growth. Effect is enhanced by combination treatment with paclitaxel | Dai et al. (2016) |
| Let‐7b replacement | Invitrogen™ Pre‐miR™ miRNA mimic | Neutral lipid emulsion or lipid‐based NOV340 |
Genetic, LSL‐KrasG12D /+;LSL‐Tp53L/L plus intratracheal Adeno‐Cre |
Lung | Inhibits tumor growth, decreases overall tumor burden, and increases overall survival. Effect is enhanced by combination treatment with miR‐34a mimics |
Kasinski et al. (2015) |
| Let‐7b replacement | dsRNA mimic (2′‐F, 2′‐O‐Me, partial PS backbone) | Lipid (DCA, EPA or cholesterol) conjugation | Subcutaneous, HCC827 lung cancer cells | Lung | No effect on tumor growth, but decreases proliferation rate and downregulates direct target HMGA2 | Segal et al. (2020) |
| miR‐16 replacement | dsRNA mimic (2′‐O‐Me on passenger strand only) | Minicells with anti‐EGFR antibody | Subcutaneous, MSTO‐211H mesothelioma cells | Lung | Inhibits tumor growth | Reid et al. (2013) |
| miR‐34a replacement | Invitrogen™ Pre‐miR™ miRNA mimic | Lipid nanoparticle | Subcutaneous, H460 or A549 lung cancer | Lung | Inhibits tumor growth, downregulates expression of direct target genes CDK4 and MET | Wiggins et al. (2010) |
| miR‐34a replacement | Dharmacon mimic | Neutral lipid emulsion |
Genetic, LSL‐KrasG12D /+ plus intranasal Adeno‐Cre |
Lung | Inhibits tumor growth and decreases overall tumor burden. | Trang et al. (2011) |
| miR‐34a replacement | Invitrogen™ Pre‐miR™ miRNA mimic | Neutral lipid emulsion or NOV340 lipid‐based delivery agent |
Genetic, LSL‐KrasG12D /+;LSL‐Tp53L/L plus intratracheal Adeno‐Cre |
Lung | Inhibits tumor growth, decreases overall tumor burden, and increases overall survival. Effect is enhanced by combination treatment with let‐7b mimic |
Kasinski et al. (2015) |
| miR‐21 inhibition | AMO (partial LNA) | PEGylated porous silicon nanoparticle with tumor‐penetrating CGKRK peptide | Subcutaneous, COV‐318 ovarian cancer cells | Ovary | Inhibits tumor growth | Bertucci et al. (2019) |
| Mir‐155 replacement | dsRNA Dicer substrate | in vivo‐jetPEI® complex | Peritoneal, ID8 or ID8‐Defb29/Vegf‐A ovarian cancer cells | Ovary (metastatic) | Inhibits tumor growth, reprograms tumor‐associated dendritic cells, and increases overall survival. Effect is enhanced by combination treatment with anti‐CD40 agonistic antibody | Cubillos‐Ruiz et al. (2012) |
| miR‐21 inhibition | AMO (Partial LNA, fully PS backbone) | Unconjugated | Genetic, LSL‐KrasG12D/+;LSL‐Tp53R172H/+;Pdx1‐Cre | Pancreas | Prevents or delays the formation of advanced intraepithelial lesion and invasive carcinoma | Chu et al. (2020) |
| miR‐21 inhibition | miRVana® anti‐miR‐21 inhibitor | PEGylated lipid nanoparticle with tandem tumor‐penetrating iRG and cell‐penetrating transportan peptides | Subcutaneous, mouse or patient‐derived cancer cells | Pancreas | Inhibits tumor growth | Gilles et al. (2018) and Gilles et al. (2019) |
| miR‐634 replacement | miRVana® miRNA mimic | Lipid nanoparticle | Subcutaneous, BxPC‐3 pancreatic cancer cells | Pancreas | Inhibits tumor growth | Gokita et al. (2020) |
| miR‐34a replacement | Invitrogen™ Pre‐miR™ miRNA mimic | Chitosan nanoparticle | Subcutaneous, PC3MM2 prostate cancer cells | Prostate | Inhibits tumor growth, downregulates expression of direct target genes AXL, MET, MYC | Gaur et al. (2015) |
| miR‐34a replacement | Dharmacon mimic | LPH nanoparticle with tumor targeting scFv | Lung metastasis, B16F10 melanoma cells | Skin (metastatic) | Inhibits tumor growth | Chen et al. (2010) |
| miR‐155 inhibition | AMO (PNAs) | PLGA nanoparticle with cell‐penetrating penetratin peptide | Subcutaneous, LSL‐Mir‐155tTA; NesCre8 B‐cell lymphoma cells | Spleen | Inhibits tumor growth | Babar et al. (2012) |
Note: Unless otherwise noted, therapeutic agent was intravenously delivered.
Abbreviations: 2′‐F, 2′‐Fluoro; 2′‐O‐Me, 2′‐O‐methyl; 2′‐O‐MOE, 2′‐O‐methoxyethyl; AMO, antisense miRNA oligonucleotide; cRGD, Cyclic 3‐aminoacid (RGD) peptide has high affinity for αVβ3 integrin; DCA, dososanoic acid; dsRNA, Double‐stranded RNA; DET, diethylenetriamine; EPA, eicosapentaenic acid; GD2, disialoganglioside GD2 glycolipid; iRGD, Cyclic 9‐aminoacid (CRGDKGPDC) peptide has high affinity for αVβ3 integrin; LNA, locked nucleic acid; LPH, liposome‐polycation‐hyaluronic acid; mini‐PEG, Hydrophilic diethylene glycol moiety; PDMAEMA, poly‐dimethylaminoethyl methacrylate; PAMAM, polyamidoamine; PEG, polyethylene glycol; PEG5K, polyethylene glycol 5000; PLA, polylactic acid; PLGA, Poly(lactic‐co‐glycolic acid); PNA, peptide nucleic acid; PS, phosphorothioate; scFv, single‐chain antibody fragment; T7, TfR‐binding 7‐aminoacid (HAIYPRH) peptide; uPA, urokinase plasminogen activator; VE, vitamin E.
3.3.1. anti‐miR‐10b inhibitors
miR‐10b was first described as an important mediator of pro‐invasion and pro‐metastatic programs in cell line models of breast cancer via downregulation of HOXD10, a homeobox family transcription factor that represses expression of pro‐metastatic RHOC gene (Ma et al., 2007). Follow‐up studies focused on the pro‐invasion and pro‐metastatic function of miR‐10b and uncovered other key target genes, including BIM, KLF4, PTEN, TBX5, and TIAM1 (Sheedy & Medarova, 2018). Treatment with anti‐miR‐10b antisense chemically‐modified oligonucleotide (AMO) conjugated to cholesterol prevented seeding and initial formation of metastatic lung lesions, but had no effect in the growth of the primary tumor or established metastatic lesions in an orthotopically implanted mouse model of breast cancer (Ma et al., 2010). In contrast, another study using a very similar approach and therapeutic agent observed a significant inhibition of primary tumor growth (Monroig‐Bosque et al., 2018). Treatment with anti‐miR‐10b AMO conjugated to dextran‐coated iron oxide magnetic nanoparticles shows therapeutic efficacy against established regional and distant metastatic lesions in immunocompetent and immunocompromised orthotopically implanted mouse models of breast cancer (Yigit et al., 2013; Yoo et al., 2015; Yoo et al., 2017). These magnetic nanoparticles have intrinsic imaging capabilities; the biodistribution and tumor accumulation of this anti‐miR‐10b nanodrug can be monitored in vivo by magnetic resonance imaging. While this nanodrug accumulates both in primary tumor and metastatic lesions, it has a minimal effect on growth kinetics of the primary tumor (Yigit et al., 2013). These studies have led to the clinical development of the anti‐miR‐10b nanodrug, TTX‐MC138, by Transcode Therapeutics (Table 5). The timing, dose, tumor penetration, and/or potency of these different formulations may explain in part discordant effects of tumor growth inhibition at primary and distant metastatic sites. Global KO of Mir‐10b gene in an aggressive viral protein‐driven genetic model of breast cancer delays and reduces primary tumor growth in addition to decreasing multiplicity of metastatic lung lesions (Kim, Siverly, et al., 2016). Thus, this result suggests perhaps a different requirement for miR‐10b activity depending on the driver mutations of breast cancer tumor subtypes.
TABLE 5.
Clinical candidates and clinical trials evaluating microRNAs as therapeutic agents against cancer
| miRNA modulation | Drug name | Sponsor | Chemistry | Delivery technology | Phase | Clinical application(s) | Trial identifier |
|---|---|---|---|---|---|---|---|
| miR‐10b inhibition | RGLS5579 | Regulus Therapeutics | AMO (2′‐O‐MOE?, partial PS backbone?) | ? | Safety and dose escalation | Treatment of glioblastoma | Clinical candidate nomination (Regulus, 2019) |
| miR‐10b inhibition | TTX‐MC138 | Transcode Therapeutics | AMO (partial LNA, partial? PS backbone | Dextran‐coated iron oxide magnetic nanoparticle | Safety and dose escalation | Treatment of metastatic breast cancer | Scheduled in 2021–2022 (Transcode, 2020) |
| miR‐16 replacement | mesomiR1 (TargomiR) | Asbestos Diseases Research Foundation | dsRNA mimic (2′‐O‐Me on passenger strand only)) | Bacterial minicells with anti‐EGFR bispecific antibody | Safety, Tolerability and early signs of efficacy (phase 1) | Treatment of recurrent malignant pleural mesothelioma and nonsmall cell lung cancer | NCT02369198 (completed) |
| miR‐34a replacement |
MRX34 |
Mirna Therapeutics |
dsRNA mimic (RNA?) |
Liposome |
Safety, Tolerability and Pharmacokinetics (phase 1) | Treatment of primary liver cancer or other selected solid tumors or hematologic malignancies | NCT01829971 (terminated) |
| miR‐34a replacement | Pharmacodynamics and pharmacokinetics (phase 1b) | Treatment of advanced melanoma | NCT02862145 (withdrawn) | ||||
| miR‐155 inhibition |
Cobomarsen (MRG‐106) |
miRagen Therapeutics |
AMO (partial LNA, full PS backbone) |
Unconjugated? |
Safety, Tolerability and Pharmacokinetics (phase 1) | Treatment of certain lymphomas and leukemias, including CTCL mycosis fungoides subtype, CLL,D DLBCL, and ATLL | NCT02580552 (completed) |
| miR‐155 inhibition | Efficacy and Safety against active comparator (phase 2) |
Treatment of mycosis fungoides subtype CTCL, SOLAR clinical trial |
NCT03713320 (active, not recruiting) | ||||
| miR‐155 inhibition | Efficacy and Safety (phase 2) | Treatment of mycosis fungoides subtype CTCL, PRISM clinical trial | NCT03837457 (terminated, study no longer needed) |
Abbreviations: 2′‐O‐Me, 2′‐O‐methyl; 2′‐O‐MOE, 2′‐O‐methoxyethyl; AMO, antisense miRNA oligonucleotide; ATLL, adult T‐cell leukemia/lymphoma; CLL, chronic lymphocytic leukemia; CTCL, cutaneous T‐cell lymphoma; DLBCL, diffuse large B‐cell lymphoma; LNA, locked nucleic acid; PS, phosphorothioate.
miR‐10b is also highly expressed in glioblastoma, the most aggressive and lethal form of brain cancer. Anti‐miR‐10b AMO treatment or genetic ablation of Mir‐10b gene by CRISPR editing technology in in vitro cell models and in vivo orthotopic models reduces cancer cell growth and viability, increases apoptotic rate, and/or reduces migratory and invasive behavior (El Fatimy et al., 2017; Gabriely et al., 2011; Guessous et al., 2013; Teplyuk et al., 2016). Systemic delivery of an unconjugated anti‐miR‐10b AMO achieves a similar therapeutic efficacy as the same AMO complexed with in vivo‐jetPEI® transfection agent injected intracranially in an orthotopic mouse model of glioblastoma (Teplyuk et al., 2016). This study has led to the clinical development of RGLS5579 by Regulus Therapeutics (Table 5).
3.3.2. anti‐miR‐21 inhibitors
Several groups have used different encapsulation (e.g., exosomes and liposomes) approaches to effectively deliver anti‐miR‐21 AMO to the brain to treat glioblastoma in mouse and rat models (Costa et al., 2015; Kim et al., 2020; Qian et al., 2014). Decoration of the delivery vehicle with folate or tumor‐penetrating peptides (i.e., T7 and chlorotoxin) enhances the tumor accumulation of the anti‐miR‐21 AMO (Costa et al., 2015; Kim et al., 2020; Lee et al., 2017). Folate and anti‐miR‐21 AMO are directly conjugated to different strand ends of a three‐way junction‐based RNA nanoparticle (Lee et al., 2017). These three‐way junction‐based RNA nanoparticles can be readily functionalized with other targeting moieties. For example, functionalization with EGFR or CD133 aptamers (instead of folate) enhances the delivery of anti‐miR‐21 AMO to the tumor site in an orthotopic mouse model of breast cancer (Shu et al., 2015; Yin et al., 2019). Treatment with nanocomplex loaded with MirVana® miR‐21 inhibitor and decorated with tumor‐ and cell‐penetrating tandem peptides suppresses tumor growth in immunocompetent and immunocompromised subcutaneously implanted mouse models of PDAC (Gilles et al., 2018; Gilles et al., 2019). Treatment with an unconjugated anti‐miR‐21 AMO in KRas‐driven p53‐mutated genetically engineered model of PDAC (variant KPC model) at a young age, when only precursor lesions (pancreatic intraepithelial neoplasia) have formed, prevents the development of more advanced and invasive lesions (Chu et al., 2020). This is one of the few studies showing tumor delivery of miRNA‐based therapeutic agent in a genetic model, which more closely approximates to the TME of human tumors than do transplantable models with a less stiff stroma and more leaky vasculature. This study also introduces the interesting concept of using miRNA drugs for cancer interception. This study evaluated the therapeutic efficacy for cancer prevention after only 6 weeks of treatment by analyzing the pancreata from euthanized animals; longer treatment course or the potential consequences of prolonged miR‐21 inhibition were not examined. There could be some challenges with identifying individuals at high‐risk and when to optimally start such preventative treatment as well as unintended consequences of a prolonged period of miRNA inhibition for cancer interception. Unintended tumor promoting effects due to prolonged period of miRNA inhibition has been reported in particular for miR‐21 in PDAC and in general for other miRNAs (e.g., miR‐122 in liver cancer) using miRNA KO strains (Hsu et al., 2012; Schipper et al., 2020). Thus, more research is needed in this emerging area to understand if the dose and frequency of miRNA activity modulation for cancer interception may need to be adjusted with respect to the use of the same miRNA‐based drug(s) for cancer treatment in which maximal potency in an acute shorter period would likely be required and desirable.
3.3.3. miR‐34 mimics
Several groups used different liposomal formulations to effectively deliver chemically‐modified dsRNA miR‐34 mimics to the lung to treat lung cancer in KRas‐driven genetic models (Kasinski et al., 2015; Trang et al., 2011) as well as metastatic melanoma in an immunocompetent mouse model (Chen et al., 2010). These studies show the capability of these liposomal formulations to deliver the therapeutic agent to a more challenging and complex tumor context than that of orthotopically or subcutaneously implanted models of lung cancer and other cancer types (Table 4). Decoration of the delivery vehicle with tumor targeting single‐chain antibody fragments further enhances accumulation of the miR‐34 mimic in distant melanoma metastatic lesions to the lung (Y. Chen et al., 2010).
3.4. Clinical trials with miRNA drugs and promising clinical candidates
Table 5 provides a comprehensive list of clinical trials evaluating miRNA‐based drugs for cancer treatment. We highlight key findings, challenges, successes, and failures. We also touch briefly on other clinical trials with miRNA drugs for noncancer applications (Bonneau et al., 2019) that may inform and have implications for cancer treatment. miR‐122 exemplifies the promises and challenges of clinical implementation of a robust miRNA candidate (Bonneau et al., 2019). miR‐122 is expressed at high levels and almost exclusively in hepatocytes. Mechanistic studies in mouse and nonhuman primate models strongly supported targeting miR‐122 as therapeutic strategy against Hepatitis C virus (HCV). An unconjugated anti‐miR‐122 LNA‐modified AMO (miravirsen) developed by Santaris Pharma was successful at eliminating viral load of HCV genotype 1b as monotherapy in a phase 2b clinical trial (Janssen et al., 2013). Santaris Pharma was acquired by Roche in 2014. Further clinical development of miravirsen is uncertain, at least in part, due to concerns of sequence variants with innate and acquired resistance (Li, Van Pham, et al., 2016; Ottosen et al., 2015), broader activity spectrum of competitors such as HARVONI®, and, to a lesser extent, the risk of cancer development (Hsu et al., 2012; Li et al., 2016).
3.4.1. miR‐16 mimic
The majority (60%) of malignant pleural mesotheliomas do not respond to current chemotherapy treatments. A dsRNA miR‐16 mimic loaded in bacterial minicells (EnGeneIC Dream Vectors) showed therapeutic efficacy in a subcutaneously implanted xenograft mouse model of mesothelioma (Reid et al., 2013). The minicells were decorated with bispecific anti‐EGFR antibody to enhance delivery to mesothelioma tumors, which highly expressed EGFR (Reid et al., 2013). These positive results with this innovative nanoparticle (targomiR) led to a phase 1 clinical trial (NCT02369198) designed to evaluate the safety and tolerability in patients with malignant pleural mesothelioma refractory to chemotherapy (van Zandwijk et al., 2017). This miR‐16 mimic targomiR (mesomiR‐1) has an acceptable safety profile and weekly treatment with 5 × 109 mesomiR‐1 nanoparticles was established as the maximum tolerated dose (van Zandwijk et al., 2017). Preliminary assessment of treatment efficacy indicates an overall objective response of 5% (1/22 participants had a partial response). These results are encouraging and supportive of a phase 2 clinical trial designed to assess treatment efficacy of mesomiR‐1 as monotherapy or in combination with standard chemotherapy.
3.4.2. anti‐miR‐21 inhibitor
Despite the numerous preclinical studies supporting an oncogenic role of miR‐21 in many cancer contexts, there are no on‐going clinical trials evaluating miR‐21‐based therapies for cancer treatment. Dichotomy of the role(s) of miR‐21 in cancer cells and distinct elements of the TME and the complexity and context of these roles are clearly a challenge for identifying tumor types and subtypes that could benefit from miR‐21 activity modulation. Strategies and technologies to impart compartment or cell type‐specific modulation should be considered to overcome this challenge. An on‐going phase 2 clinical trial with an anti‐miR‐21 AMO (RG‐102 also known as lademirsen) for amelioration of fibrosis and renal function in patients with Alport syndrome (NCT02855268) could provide valuable information for its application in cancer treatment in contexts where fibroblast reprogramming could be beneficial.
3.4.3. miR‐34 mimic
The miR‐34a mimic, MRX34, was the first miRNA replacement drug to enter the clinic (Agostini & Knight, 2014; Bader, 2012). MRX34 was administered intravenously as cargo in a liposomal nanoparticle in patients with primary tumor or metastatic lesions in the liver, other solid tumors, or hematological malignancies to investigate its safety, pharmacokinetics and pharmacodynamics (NCT01829971). This study was halted and eventually terminated due to five severe immune reactions that resulted in the death of four participants (Hong, Kang, et al., 2020). A contemporaneous second clinical trial (NCT02862145) was designed to mitigate immune‐mediated toxicity by pretreatment with dexamethasone (Hong, Kang, et al., 2020), but it was withdrawn due to early termination of the other clinical trial. Evaluation of clinical data suggests that miR‐34a mimic specifically caused this immune‐mediated toxicity in patients since the same liposome formulation and other dsRNA compounds were well tolerated in other clinical trials (Hong, Kang, et al., 2020). This was an unexpected finding given the rigorous toxicology studies conducted in mouse and other animal models, including nonhuman primates (Hong, Kang, et al., 2020). Nonetheless, animal models may not fully recapitulate all aspects of human disease at molecular (e.g., repertoire of target genes, signaling pathways, and ligands), cellular (e.g., TME composition and immune response), and/or physiological (e.g., desmoplastic reaction, hypoxia, enhanced permeability and retention [EPR] effect) levels. Immune‐mediated toxicities of MRX34 are similar to those caused by immune checkpoint inhibitors (Hong, Kang, et al., 2020), suggesting an on‐target effect of miR‐34a mimic on immune cells that puts them on overdrive. In this heavily pretreated and diverse patient cohort the overall objective response was 4% (3/85 participants had a partial response to treatment; Hong, Kang, et al., 2020). A better understanding of on‐target effects of miR‐34a in cancer and immune cells could inform a more selective application of this miRNA replacement therapy in a more focused subset of cancer types. Mirna Therapeutics Inc canceled further development of MRX34 and was subsequently acquired by Synlogic Therapeutics in 2017. MRX34 or a modified formulation does not appear to be in the pipeline of Synlogic Therapeutics (Synlogic, 2020).
3.4.4. anti‐miR‐155 inhibitor
Cutaneous T cell lymphoma (CTCL) is a relatively rare cancer. Mycosis fungoides (MF) is the most common subtype of CTCL. In vitro functional studies and altered expression data in patient samples supported an etiological role of miR‐155 in MF (Moyal et al., 2013; Seto et al., 2018). Cobomarsen (also known as MRG‐06) is a chemically‐modified anti‐miR‐155 AMO functionalized for preferential uptake by MF and CD4+ T cells (Seto et al., 2018). Safety and tolerability of cobomarsen was evaluated in a successfully completed phase I clinical trial (NCT02580552) that included patients with MF, adult T‐cell leukemia/lymphoma, CLL, and diffuse large B‐cell lymphoma (DLBCL). A phase 2 clinical trial was initiated to evaluate the efficacy of cobomarsen for the treatment of MF (NCT03713320). In July 2020, cobomarsen received orphan drug designation for treatment of CTCL (miRagen, 2020a). However, preliminary analysis of this phase 2 clinical trial failed to show a treatment effect of cobomarsen over control arm receiving vorinostat treatment. miRagen Therapeutics recently decided to discontinue further internal development of cobomarsen (miRagen, 2020b). Genetic studies of Mir‐155KO mice (Table 3), therapeutic efficacy of cobomarsen and other anti‐miR‐155 AMOs in in vivo animal models (Table 4), and good safety profile of cobomarsen in patients (Table 5) supports its clinical evaluation in other hematological malignancies, especially B cell lymphomas. Anecdotal evidence from a relapsing DLBCL patient enrolled in the phase 1 study (NCT02580552) further supports a therapeutic effect of cobomarsen (Anastasiadou et al., 2020).
4. CONCLUSION
PubMed queries on studies from the last 15 years focusing on diagnostic (about 2700 entries) or therapeutic development (about 6660 entries) of miRNA‐based applications indicate great interest and activity on understanding the molecular mechanism of miRNA action and implementing delivery methods for patient treatment. However, these efforts have not yet fully translated to the clinical setting, in which the overwhelming number of current clinical trials are investigating miRNA‐based diagnostic applications (about 150 registered trials at clinicaltrials.gov; Table 2) and only a handful are directly evaluating miRNA‐based therapeutic applications (Table 5) in cancer medicine. Some challenges with the diagnostic application of circulating miRNA signatures are related to their specificity for a particular cancer type or even cancer per se since these miRNAs can be altered in other physiological (e.g., pregnancy) and pathological conditions (e.g., chronic inflammation, COVID‐19 comorbidities), and their diagnostic performance compared to other investigational and clinically established biomarkers. We provided some examples of added value of miRNA biomarkers and strategies to combine or complement existing clinical indicators rather than attempting to replace them. Clinical success of drug treatment has been greatly enhanced by the use of companion diagnostic tests for patient selection (e.g., HER‐2 overexpression for anti‐HER2 antibody treatment in breast cancer, BRAF mutations for BRAF inhibitor treatment in colon cancer and melanoma, cancer cell‐expressing PD‐L1 for immune checkpoint therapy in lung cancer and others). We described robust tissue‐based technologies for rapid and sensitive detection of miRNA expression at a single‐cell resolution that could serve as companion diagnostic tests for miRNA‐based drugs. These miRNA‐based companion diagnostic tests could provide clinically actionable information based on not only significant changes of miRNA expression, but also the specific cell type(s) with altered expression. miRNA‐based drugs in clinical trials have shown activity in the kidney, lung, and other organ sites demonstrating the therapeutic potential of various systemic delivery technologies to go beyond the liver. Some of these systemic delivery technologies such as gold nanoparticles and iron oxide magnetic nanoparticles have intrinsic imaging capabilities, whereas others can gain imaging capabilities via conjugation of an imaging agent; image guidance could be instrumental for noninvasive monitoring of miRNA‐based drug accumulation at tumor sites. Gained knowledge from cell type–specific mechanistic studies in in vivo mouse models (Tables 3 and 4) should guide clinical evaluation of miRNA modulation in cancer cells or other cells in the TME depending on the cancer type. To this end, targeting peptides and other moieties should be further exploited in future clinical studies to deliver the miRNA drug to the most etiologically relevant cellular compartment in order to maximize therapeutic benefit.
RESEARCH RESOURCES
Figures were created with BioRender.com.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
AUTHOR CONTRIBUTIONS
Lorenzo Sempere: Conceptualization; data curation; methodology; project administration; resources; visualization; writing‐original draft; writing‐review & editing. Asfar Azmi: Data curation; validation; writing‐review & editing. Anna Moore: Data curation; funding acquisition; methodology; resources; writing‐review & editing.
RELATED WIREs ARTICLES
MicroRNAs as therapeutic targets in human cancers
ACKNOWLEDGMENTS
The authors would like to thank members of the Sempere laboratory: Brooke Jackson, Beth Kenyon, and Katie Powell for critical reading and suggestions. This work was supported, in part, by National Cancer Institute R21 CA226579 grant to Lorenzo F. Sempere, R37CA215427 and R01CA240607 grants to Asfar S. Azmi, and R01CA221771 grant to Anna Moore.
Sempere, L. F. , Azmi, A. S. , & Moore, A. (2021). microRNA‐based diagnostic and therapeutic applications in cancer medicine. Wiley Interdisciplinary Reviews: RNA, 12 (6), e1662. 10.1002/wrna.1662
Edited by: Jeff Wilusz, Editor‐in‐Chief
Funding information National Cancer Institute, Grant/Award Numbers: R01 CA221771, R21 CA226579, R37CA215427, R01CA240607
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Agostini, M. , & Knight, R. A. (2014). miR‐34: From bench to bedside. Oncotarget, 5(4), 872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiadou, E. , Seto, A. , Beatty, X. , Hermreck, M. , Gilles, M. E. , Stroopinsky, D. , Pinter‐Brown, L. C. , Pestano, L. , Marchese, C. , Avigan, D. , Trivedi, P. , Escolar, D. , Jackson, A. , & Slack, F. J. (2020). Cobomarsen, an oligonucleotide inhibitor of miR‐155, slows DLBCL tumor cell growth in vitro and in vivo. Clinical Cancer Research, 27, 1139–1149. 10.1158/1078-0432.CCR-20-3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres‐Leon, E. , Nunez‐Torres, R. , & Rojas, A. M. (2016). miARma‐Seq: A comprehensive tool for miRNA, mRNA and circRNA analysis. Scientific Reports, 6, 25749. 10.1038/srep25749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew, A. S. , Marsit, C. J. , Schned, A. R. , Seigne, J. D. , Kelsey, K. T. , Moore, J. H. , Perreard, L. , Karagas, M. R. , & Sempere, L. F. (2014). Expression of tumor suppressive microRNA‐34a is associated with a reduced risk of bladder cancer recurrence. International Journal of Cancer, 137, 1158–1166. 10.1002/ijc.29413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anfossi, S. , Babayan, A. , Pantel, K. , & Calin, G. A. (2018). Clinical utility of circulating non‐coding RNAs—An update. Nature Reviews. Clinical Oncology, 15(9), 541–563. 10.1038/s41571-018-0035-x [DOI] [PubMed] [Google Scholar]
- Aparicio‐Puerta, E. , Gomez‐Martin, C. , Giannoukakos, S. , Medina, J. M. , Marchal, J. A. , & Hackenberg, M. (2020). mirnaQC: A webserver for comparative quality control of miRNA‐seq data. Nucleic Acids Research, 48(W1), W262–W267. 10.1093/nar/gkaa452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano, N. , Matsuzaki, J. , Ichikawa, M. , Kawauchi, J. , Takizawa, S. , Aoki, Y. , Sakamoto, H. , Yoshida, A. , Kobayashi, E. , Tanzawa, Y. , Nakayama, R. , Morioka, H. , Matsumoto, M. , Nakamura, M. , Kondo, T. , Kato, K. , Tsuchiya, N. , Kawai, A. , & Ochiya, T. (2019). A serum microRNA classifier for the diagnosis of sarcomas of various histological subtypes. Nature Communications, 10(1), 1299. 10.1038/s41467-019-09143-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babar, I. A. , Cheng, C. J. , Booth, C. J. , Liang, X. , Weidhaas, J. B. , Saltzman, W. M. , & Slack, F. J. (2012). Nanoparticle‐based therapy in an in vivo microRNA‐155 (miR‐155)‐dependent mouse model of lymphoma. Proceedings of the National Academy of Sciences of the United States of America, 109(26), E1695–E1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader, A. G. (2012). miR‐34 ‐ A microRNA replacement therapy is headed to the clinic. Frontiers in Genetics, 3, 120. 10.3389/fgene.2012.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahnassy, A. A. , Salem, S. E. , El‐Sayed, M. , Khorshid, O. , Abdellateif, M. S. , Youssef, A. S. , Mohanad, M. , Hussein, M. , Zekri, A. N. , & Ali, N. M. (2018). MiRNAs as molecular biomarkers in stage II egyptian colorectal cancer patients. Experimental and Molecular Pathology, 105(3), 260–271. 10.1016/j.yexmp.2018.09.002 [DOI] [PubMed] [Google Scholar]
- Bajan, S. , & Hutvagner, G. (2020). RNA‐based therapeutics: From antisense oligonucleotides to miRNAs. Cells, 9(1), 137. 10.3390/cells9010137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarotto, E. , Schmittgen, T. D. , & Calin, G. A. (2008). MicroRNAs and cancer: Profile, profile, profile. International Journal of Cancer, 122(5), 969–977. [DOI] [PubMed] [Google Scholar]
- Barke, L. (2019). Analysis of serum miRNA from women referred for breast biopsy.
- Bartel, D. P. (2018). Metazoan MicroRNAs. Cell, 173(1), 20–51. 10.1016/j.cell.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann, B. , Acosta, A. M. , Richards, Z. , Deaton, R. , Sapatynska, A. , Murphy, A. , Kajdacsy‐Balla, A. , Gann, P. H. , & Nonn, L. (2019). Association of High miR‐182 levels with low‐risk prostate cancer. The American Journal of Pathology, 189(4), 911–923. 10.1016/j.ajpath.2018.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista‐Sanchez, D. , Arriaga‐Canon, C. , Pedroza‐Torres, A. , De La Rosa‐Velazquez, I. A. , Gonzalez‐Barrios, R. , Contreras‐Espinosa, L. , Montiel‐Manriquez, R. , Castro‐Hernandez, C. , Fragoso‐Ontiveros, V. , Alvarez‐Gomez, R. M. , & Herrera, L. A. (2020). The promising role of miR‐21 as a cancer biomarker and its importance in RNA‐based therapeutics. Molecular Therapy ‐ Nucleic Acids, 20, 409–420. 10.1016/j.omtn.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayraktar, R. , & Van Roosbroeck, K. (2018). miR‐155 in cancer drug resistance and as target for miRNA‐based therapeutics. Cancer Metastasis Reviews, 37(1), 33–44. 10.1007/s10555-017-9724-7 [DOI] [PubMed] [Google Scholar]
- Bertucci, A. , Kim, K. H. , Kang, J. , Zuidema, J. M. , Lee, S. H. , Kwon, E. J. , Kim, D. , Howell, S. B. , Ricci, F. , Ruoslahti, E. , Jang, H. J. , & Sailor, M. J. (2019). Tumor‐targeting, MicroRNA‐silencing porous silicon nanoparticles for ovarian cancer therapy. ACS Applied Materials & Interfaces, 11(27), 23926–23937. 10.1021/acsami.9b07980 [DOI] [PubMed] [Google Scholar]
- Bidarra, D. , Constancio, V. , Barros‐Silva, D. , Ramalho‐Carvalho, J. , Moreira‐Barbosa, C. , Antunes, L. , Mauricio, J. , Oliveira, J. , Henrique, R. , & Jeronimo, C. (2019). Circulating MicroRNAs as biomarkers for prostate Cancer detection and metastasis development prediction. Frontiers in Oncology, 9, 900. 10.3389/fonc.2019.00900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci, D. , Coppola, V. , Musumeci, M. , Addario, A. , Giuffrida, R. , Memeo, L. , D'Urso, L. , Pagliuca, A. , Biffoni, M. , Labbaye, C. , Bartucci, M. , Muto, G. , Peschle, C. , & De Maria, R. (2008). The miR‐15a‐miR‐16‐1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nature Medicine, 14(11), 1271–1277. 10.1038/nm.1880 [DOI] [PubMed] [Google Scholar]
- Bonneau, E. , Neveu, B. , Kostantin, E. , Tsongalis, G. J. , & De Guire, V. (2019). How close are miRNAs from clinical practice? A perspective on the diagnostic and therapeutic market. EJIFCC, 30(2), 114–127. [PMC free article] [PubMed] [Google Scholar]
- Brand, R. E. , Adai, A. T. , Centeno, B. A. , Lee, L. S. , Rateb, G. , Vignesh, S. , Menard, C. , Wiechowska‐Kozlowska, A. , Boldys, H. , Hartleb, M. , Sanders, M. K. , Munding, J. B. , Tannapfel, A. , Hahn, S. A. , Stefanczyk, L. , Tsongalis, G. J. , Whitcomb, D. C. , Conwell, D. L. , Morisset, J. A. , … Szafranska‐Schwarzbach, A. E. (2014). A microRNA‐based test improves endoscopic ultrasound‐guided cytologic diagnosis of pancreatic cancer. Clinical Gastroenterology and Hepatology, 12(10), 1717–1723. 10.1016/j.cgh.2014.02.038 [DOI] [PubMed] [Google Scholar]
- Bumrungthai, S. , Ekalaksananan, T. , Evans, M. F. , Chopjitt, P. , Tangsiriwatthana, T. , Patarapadungkit, N. , Kleebkaow, P. , Luanratanakorn, S. , Kongyingyoes, B. , Worawichawong, S. , & Pientong, C. (2015). Up‐regulation of miR‐21 is associated with cervicitis and human papillomavirus infection in cervical tissues. PLoS One, 10(5), e0127109. 10.1371/journal.pone.0127109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, C. K. , Zhao, G. Y. , Tian, L. Y. , Liu, L. , Yan, K. , Ma, Y. L. , Ji, Z. W. , Li, X. X. , Han, K. , Gao, J. , Qiu, X. C. , Fan, Q. Y. , Yang, T. T. , & Ma, B. A. (2012). miR‐15a and miR‐16‐1 downregulate CCND1 and induce apoptosis and cell cycle arrest in osteosarcoma. Oncology Reports, 28(5), 1764–1770. 10.3892/or.2012.1995 [DOI] [PubMed] [Google Scholar]
- Calin, G. A. , Cimmino, A. , Fabbri, M. , Ferracin, M. , Wojcik, S. E. , Shimizu, M. , Taccioli, C. , Zanesi, N. , Garzon, R. , Aqeilan, R. I. , Alder, H. , Volinia, S. , Rassenti, L. , Liu, X. , Liu, C. G. , Kipps, T. J. , Negrini, M. , & Croce, C. M. (2008). MiR‐15a and miR‐16‐1 cluster functions in human leukemia. Proceedings of the National Academy of Sciences of the United States of America, 105(13), 5166–5171. 10.1073/pnas.0800121105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin, G. A. , Dumitru, C. D. , Shimizu, M. , Bichi, R. , Zupo, S. , Noch, E. , Aldler, H. , Rattan, S. , Keating, M. , Rai, K. , Rassenti, L. , Kipps, T. , Negrini, M. , Bullrich, F. , & Croce, C. M. (2002). Frequent deletions and down‐regulation of micro‐ RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proceedings of the National Academy of Sciences of the United States of America, 99(24), 15524–15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin, G. A. , Sevignani, C. , Dumitru, C. D. , Hyslop, T. , Noch, E. , Yendamuri, S. , Shimizu, M. , Rattan, S. , Bullrich, F. , Negrini, M. , & Croce, C. M. (2004). Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proceedings of the National Academy of Sciences of the United States of America, 101(9), 2999–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps, C. , Buffa, F. M. , Colella, S. , Moore, J. , Sotiriou, C. , Sheldon, H. , Harris, A. L. , Gleadle, J. M. , & Ragoussis, J. (2008). Hsa‐miR‐210 is induced by hypoxia and is an independent prognostic factor in breast cancer. Clinical Cancer Research., 14(5), 1340–1348. [DOI] [PubMed] [Google Scholar]
- Caponi, S. , Funel, N. , Frampton, A. E. , Mosca, F. , Santarpia, L. , Van der Velde, A. G. , Jiao, L. R. , De Lio, N. , Falcone, A. , Kazemier, G. , Meijer, G. A. , Verheul, H. M. , Vasile, E. , Peters, G. J. , Boggi, U. , & Giovannetti, E. (2013). The good, the bad and the ugly: A tale of miR‐101, miR‐21 and miR‐155 in pancreatic intraductal papillary mucinous neoplasms. Annals of Oncology, 24(3), 734–741. 10.1093/annonc/mds513 [DOI] [PubMed] [Google Scholar]
- Chen, C. , Ridzon, D. A. , Broomer, A. J. , Zhou, Z. , Lee, D. H. , Nguyen, J. T. , Barbisin, M. , Xu, N. L. , Mahuvakar, V. R. , Andersen, M. R. , Lao, K. Q. , Livak, K. J. , & Guegler, K. J. (2005). Real‐time quantification of microRNAs by stem‐loop RT‐PCR. Nucleic Acids Research, 33(20), e179. 10.1093/nar/gni178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Si, Q. , Xiao, S. , Xie, Q. , Lin, J. , Wang, C. , Chen, L. , Chen, Q. , & Wang, L. (2013). Prognostic significance of serum miR‐17‐5p in lung cancer. Medical Oncology, 30(1), 353. 10.1007/s12032-012-0353-2 [DOI] [PubMed] [Google Scholar]
- Chen, S. , Wang, L. , Fan, J. , Ye, C. , Dominguez, D. , Zhang, Y. , Curiel, T. J. , Fang, D. , Kuzel, T. M. , & Zhang, B. (2015). Host miR155 promotes tumor growth through a myeloid‐derived suppressor cell‐dependent mechanism. Cancer Research, 75(3), 519–531. 10.1158/0008-5472.CAN-14-2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. , Hu, Z. , Wang, W. , Ba, Y. , Ma, L. , Zhang, C. , Wang, C. , Ren, Z. , Zhao, Y. , Wu, S. , Zhuang, R. , Zhang, Y. , Hu, H. , Liu, C. , Xu, L. , Wang, J. , Shen, H. , Zhang, J. , Zen, K. , & Zhang, C. Y. (2012). Identification of ten serum microRNAs from a genome‐wide serum microRNA expression profile as novel noninvasive biomarkers for nonsmall cell lung cancer diagnosis. International Journal of Cancer, 130(7), 1620–1628. 10.1002/ijc.26177 [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Zhu, X. , Zhang, X. , Liu, B. , & Huang, L. (2010). Nanoparticles modified with tumor‐targeting scFv deliver siRNA and miRNA for cancer therapy. Molecular Therapy, 18(9), 1650–1656. 10.1038/mt.2010.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, Y. J. , Lin, C. P. , Ho, J. J. , He, X. , Okada, N. , Bu, P. , Zhong, Y. , Kim, S. Y. , Bennett, M. J. , Chen, C. , Ozturk, A. , Hicks, G. G. , Hannon, G. J. , & He, L. (2011). miR‐34 miRNAs provide a barrier for somatic cell reprogramming. Nature Cell Biology, 13(11), 1353–1360. 10.1038/ncb2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury, S. M. , Lee, T. , Bachawal, S. V. , Devulapally, R. , Abou‐Elkacem, L. , Yeung, T. A. , Wischhusen, J. , Tian, L. , Dahl, J. , Paulmurugan, R. , & Willmann, J. K. (2018). Longitudinal assessment of ultrasound‐guided complementary microRNA therapy of hepatocellular carcinoma. Journal of Controlled Release, 281, 19–28. 10.1016/j.jconrel.2018.05.009 [DOI] [PubMed] [Google Scholar]
- Chu, N. J. , Anders, R. A. , Fertig, E. J. , Cao, M. , Hopkins, A. C. , Keenan, B. P. , Popovic, A. , Armstrong, T. D. , Jaffee, E. M. , & Zimmerman, J. W. (2020). Inhibition of miR‐21 regulates mutant KRAS effector pathways and intercepts pancreatic ductal adenocarcinoma development. Cancer Prevention Research (Philadelphia, Pa.), 13(7), 569–582. 10.1158/1940-6207.CAPR-20-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials.gov . (2020). microRNA studies at ClinicalTrials.gov. Retrieved from https://www.clinicaltrials.gov/ct2/results?term=microrna&cond=cancer&Search=Apply&recrs=b&recrs=a&recrs=f&recrs=d&age_v=&gndr=&type=&rslt=
- Concepcion, C. P. , Han, Y. C. , Mu, P. , Bonetti, C. , Yao, E. , D'Andrea, A. , Vidigal, J. A. , Maughan, W. P. , Ogrodowski, P. , & Ventura, A. (2012). Intact p53‐dependent responses in miR‐34‐deficient mice. PLoS Genetics, 8(7), e1002797. 10.1371/journal.pgen.1002797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conkrite, K. , Sundby, M. , Mukai, S. , Thomson, J. M. , Mu, D. , Hammond, S. M. , & MacPherson, D. (2011). miR‐17~92 cooperates with RB pathway mutations to promote retinoblastoma. Genes & Development, 25(16), 1734–1745. 10.1101/gad.17027411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, P. M. , Cardoso, A. L. , Custodia, C. , Cunha, P. , Pereira de Almeida, L. , & Pedroso de Lima, M. C. (2015). MiRNA‐21 silencing mediated by tumor‐targeted nanoparticles combined with sunitinib: A new multimodal gene therapy approach for glioblastoma. Journal of Controlled Release, 207, 31–39. 10.1016/j.jconrel.2015.04.002 [DOI] [PubMed] [Google Scholar]
- Costinean, S. , Zanesi, N. , Pekarsky, Y. , Tili, E. , Volinia, S. , Heerema, N. , & Croce, C. M. (2006). Pre‐B cell proliferation and lymphoblastic leukemia/high‐grade lymphoma in E{micro}‐miR155 transgenic mice. Proceedings of the National Academy of Sciences of the United States of America, 103(18), 7024–7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, V. J. , Tzankov, A. , Flori, M. , Schmid, C. A. , Bader, A. G. , & Muller, A. (2012). Systemic microRNA‐34a delivery induces apoptosis and abrogates growth of diffuse large B‐cell lymphoma in vivo. Leukemia, 26(11), 2421–2424. 10.1038/leu.2012.110 [DOI] [PubMed] [Google Scholar]
- Cubillos‐Ruiz, J. R. , Baird, J. R. , Tesone, A. J. , Rutkowski, M. R. , Scarlett, U. K. , Camposeco‐Jacobs, A. L. , Nadon‐Arnillas, J. , Harwood, N. M. , Korc, M. , Fiering, S. N. , Sempere, L. F. , & Conejo‐Garcia, J. R. (2012). Reprogramming tumor‐associated dendritic cells in vivo using miRNA mimetics triggers protective immunity against ovarian cancer. Cancer Research, 72(7), 1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, W. , He, J. , Zheng, L. , Bi, M. , Hu, F. , Chen, M. , Niu, H. , Yang, J. , Luo, Y. , Tang, W. , & Sheng, M. (2019). miR‐148b‐3p, miR‐190b, and miR‐429 regulate cell progression and act as potential biomarkers for breast Cancer. Journal of Breast Cancer, 22(2), 219–236. 10.4048/jbc.2019.22.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, X. , Fan, W. , Wang, Y. , Huang, L. , Jiang, Y. , Shi, L. , McKinley, D. , Tan, W. , & Tan, C. (2016). Combined delivery of let‐7b MicroRNA and paclitaxel via biodegradable Nanoassemblies for the treatment of KRAS mutant Cancer. Molecular Pharmaceutics, 13(2), 520–533. 10.1021/acs.molpharmaceut.5b00756 [DOI] [PubMed] [Google Scholar]
- Daige, C. L. , Wiggins, J. F. , Priddy, L. , Nelligan‐Davis, T. , Zhao, J. , & Brown, D. (2014). Systemic delivery of a miR34a mimic as a potential therapeutic for liver cancer. Molecular Cancer Therapeutics, 13(10), 2352–2360. 10.1158/1535-7163.MCT-14-0209 [DOI] [PubMed] [Google Scholar]
- Devulapally, R. , Sekar, N. M. , Sekar, T. V. , Foygel, K. , Massoud, T. F. , Willmann, J. K. , & Paulmurugan, R. (2015). Polymer nanoparticles mediated codelivery of antimiR‐10b and antimiR‐21 for achieving triple negative breast cancer therapy. ACS Nano, 9(3), 2290–2302. 10.1021/nn507465d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue, T. R. , Nguyen, A. H. , Moughan, J. , Li, L. , Tatishchev, S. , Toste, P. , & Farrell, J. J. (2014). Stromal microRNA‐21 levels predict response to 5‐fluorouracil in patients with pancreatic cancer. Journal of Surgical Oncology, 110(8), 952–959. 10.1002/jso.23750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnem, T. , Eklo, K. , Berg, T. , Sorbye, S. W. , Lonvik, K. , Al‐Saad, S. , Al‐Shibli, K. , Andersen, S. , Stenvold, H. , Bremnes, R. M. , & Busund, L. T. (2011). Prognostic impact of MiR‐155 in non‐small cell lung cancer evaluated by in situ hybridization. Journal of Translational Medicine, 9, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley, J. , Lagou, V. , Garcia‐Perez, J. E. , Himmelreich, U. , & Liston, A. (2017). miR‐29a‐deficiency does not modify the course of murine pancreatic acinar carcinoma. Oncotarget, 8(16), 26911–26917. 10.18632/oncotarget.15850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drula, R. , Ott, L. F. , Berindan‐Neagoe, I. , Pantel, K. , & Calin, G. A. (2020). MicroRNAs from liquid biopsy derived extracellular vesicles: Recent advances in detection and characterization methods. Cancers (Basel), 12(8), 2009. 10.3390/cancers12082009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Rieu, M. C. , Torrisani, J. , Selves, J. , Al, S. T. , Souque, A. , Dufresne, M. , Tsongalis, G. J. , Suriawinata, A. A. , Carrere, N. , Buscail, L. , & Cordelier, P. (2010). MicroRNA‐21 is induced early in pancreatic ductal adenocarcinoma precursor lesions. Clinical Chemistry, 56(4), 603–612. [DOI] [PubMed] [Google Scholar]
- Dudda, J. C. , Salaun, B. , Ji, Y. , Palmer, D. C. , Monnot, G. C. , Merck, E. , Boudousquie, C. , Utzschneider, D. T. , Escobar, T. M. , Perret, R. , Muljo, S. A. , Hebeisen, M. , Rufer, N. , Zehn, D. , Donda, A. , Restifo, N. P. , Held, W. , Gattinoni, L. , & Romero, P. (2013). MicroRNA‐155 is required for effector CD8+ T cell responses to virus infection and cancer. Immunity, 38(4), 742–753. 10.1016/j.immuni.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dueck, A. , Eichner, A. , Sixt, M. , & Meister, G. (2014). A miR‐155‐dependent microRNA hierarchy in dendritic cell maturation and macrophage activation. FEBS Letters, 588(4), 632–640. 10.1016/j.febslet.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Eilertsen, M. , Andersen, S. , Al‐Saad, S. , Richardsen, E. , Stenvold, H. , Hald, S. M. , Al‐Shibli, K. , Donnem, T. , Busund, L. T. , & Bremnes, R. M. (2014). Positive prognostic impact of miR‐210 in non‐small cell lung cancer. Lung Cancer, 83(2), 272–278. 10.1016/j.lungcan.2013.11.005 [DOI] [PubMed] [Google Scholar]
- Ekiz, H. A. , Huffaker, T. B. , Grossmann, A. H. , Stephens, W. Z. , Williams, M. A. , Round, J. L. , & O'Connell, R. M. (2019). MicroRNA‐155 coordinates the immunological landscape within murine melanoma and correlates with immunity in human cancers. JCI Insight, 4(6), e126543. 10.1172/jci.insight.126543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Fatimy, R. , Subramanian, S. , Uhlmann, E. J. , & Krichevsky, A. M. (2017). Genome editing reveals glioblastoma addiction to MicroRNA‐10b. Molecular Therapy, 25(2), 368–378. 10.1016/j.ymthe.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng, C. L. , Lawson, M. , Zhu, Q. , Dries, R. , Koulena, N. , Takei, Y. , Yun, J. , Cronin, C. , Karp, C. , Yuan, G. C. , & Cai, L. (2019). Transcriptome‐scale super‐resolved imaging in tissues by RNA seqFISH. Nature, 568(7751), 235–239. 10.1038/s41586-019-1049-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquela‐Kerscher, A. , Trang, P. , Wiggins, J. F. , Patrawala, L. , Cheng, A. , Ford, L. , Weidhaas, J. B. , Brown, D. , Bader, A. G. , & Slack, F. J. (2008). The let‐7 microRNA reduces tumor growth in mouse models of lung cancer. Cell Cycle, 7(6), 759–764. [DOI] [PubMed] [Google Scholar]
- Fan, R. , Xiao, C. , Wan, X. , Cha, W. , Miao, Y. , Zhou, Y. , Qin, C. , Cui, T. , Su, F. , & Shan, X. (2019). Small molecules with big roles in microRNA chemical biology and microRNA‐targeted therapeutics. RNA Biology, 16(6), 707–718. 10.1080/15476286.2019.1593094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong, L. Y. , Taccioli, C. , Palamarchuk, A. , Tagliazucchi, G. M. , Jing, R. , Smalley, K. J. , Fan, S. , Altemus, J. , Fiehn, O. , Huebner, K. , Farber, J. L. , & Croce, C. M. (2020). Abrogation of esophageal carcinoma development in miR‐31 knockout rats. Proceedings of the National Academy of Sciences of the United States of America, 117(11), 6075–6085. 10.1073/pnas.1920333117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forero, D. A. , Gonzalez‐Giraldo, Y. , Castro‐Vega, L. J. , & Barreto, G. E. (2019). qPCR‐based methods for expression analysis of miRNAs. BioTechniques, 67(4), 192–199. 10.2144/btn-2019-0065 [DOI] [PubMed] [Google Scholar]
- Fornari, F. , Gramantieri, L. , Callegari, E. , Shankaraiah, R. C. , Piscaglia, F. , Negrini, M. , & Giovannini, C. (2019). MicroRNAs in animal models of HCC. Cancers (Basel), 11(12), 1906. 10.3390/cancers11121906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forterre, A. , Komuro, H. , Aminova, S. , & Harada, M. (2020). A comprehensive review of Cancer MicroRNA therapeutic delivery strategies. Cancers (Basel), 12(7), 1852. 10.3390/cancers12071852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foye, C. , Yan, I. K. , David, W. , Shukla, N. , Habboush, Y. , Chase, L. , Ryland, K. , Kesari, V. , & Patel, T. (2017). Comparison of miRNA quantitation by Nanostring in serum and plasma samples. PLoS One, 12(12), e0189165. 10.1371/journal.pone.0189165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm, B. , Billipp, T. , Peck, L. E. , Johansen, M. , Tarver, J. E. , King, B. L. , Newcomb, J. M. , Sempere, L. F. , Flatmark, K. , Hovig, E. , & Peterson, K. J. (2015). A uniform system for the annotation of vertebrate microRNA genes and the evolution of the human microRNAome. Annual Review of Genetics, 49, 213–242. 10.1146/annurev-genet-120213-092023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriely, G. , Yi, M. , Narayan, R. S. , Niers, J. M. , Wurdinger, T. , Imitola, J. , Ligon, K. L. , Kesari, S. , Esau, C. , Stephens, R. M. , Tannous, B. A. , & Krichevsky, A. M. (2011). Human glioma growth is controlled by microRNA‐10b. Cancer Research, 71(10), 3563–3572. 10.1158/0008-5472.CAN-10-3568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur, S. , Wen, Y. , Song, J. H. , Parikh, N. U. , Mangala, L. S. , Blessing, A. M. , Ivan, C. , Wu, S. Y. , Varkaris, A. , Shi, Y. , Lopez‐Berestein, G. , Frigo, D. E. , Sood, A. K. , & Gallick, G. E. (2015). Chitosan nanoparticle‐mediated delivery of miRNA‐34a decreases prostate tumor growth in the bone and its expression induces non‐canonical autophagy. Oncotarget, 6(30), 29161–29177. 10.18632/oncotarget.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebert, L. F. R. , & MacRae, I. J. (2019). Regulation of microRNA function in animals. Nature Reviews. Molecular Cell Biology, 20(1), 21–37. 10.1038/s41580-018-0045-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoptix . (2019). Test Menu—Search by test name alphabetically. Retrieved from https://genoptix.com/test-menu-search-test-name-alphabetically/
- Gilles, M. E. , Hao, L. , Brown, K. , Lim, J. , Bhatia, S. N. , & Slack, F. J. (2019). Tumor penetrating nanomedicine targeting both an oncomiR and an oncogene in pancreatic cancer. Oncotarget, 10(51), 5349–5358. 10.18632/oncotarget.27160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles, M. E. , Hao, L. , Huang, L. , Rupaimoole, R. , Lopez‐Casas, P. P. , Pulver, E. , Jeong, J. C. , Muthuswamy, S. K. , Hidalgo, M. , Bhatia, S. N. , & Slack, F. J. (2018). Personalized RNA medicine for pancreatic Cancer. Clinical Cancer Research, 24(7), 1734–1747. 10.1158/1078-0432.CCR-17-2733 [DOI] [PubMed] [Google Scholar]
- Gokita, K. , Inoue, J. , Ishihara, H. , Kojima, K. , & Inazawa, J. (2020). Therapeutic potential of LNP‐mediated delivery of miR‐634 for Cancer therapy. Molecular Therapy – Nucleic Acids, 19, 330–338. 10.1016/j.omtn.2019.10.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveel, C. R. , Calderone, H. M. , Westerhuis, J. J. , Winn, M. E. , & Sempere, L. F. (2015). Critical analysis of the potential for microRNA biomarkers in breast cancer management. Breast Cancer (Dove Med Press), 7, 59–79. 10.2147/BCTT.S43799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guessous, F. , Alvarado‐Velez, M. , Marcinkiewicz, L. , Zhang, Y. , Kim, J. , Heister, S. , Kefas, B. , Godlewski, J. , Schiff, D. , Purow, B. , & Abounader, R. (2013). Oncogenic effects of miR‐10b in glioblastoma stem cells. Journal of Neuro‐Oncology, 112(2), 153–163. 10.1007/s11060-013-1047-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, X. , Lv, X. , Lv, X. , Ma, Y. , Chen, L. , & Chen, Y. (2017). Circulating miR‐21 serves as a serum biomarker for hepatocellular carcinoma and correlated with distant metastasis. Oncotarget, 8(27), 44050–44058. 10.18632/oncotarget.17211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, A. , Quijano, E. , Liu, Y. , Bahal, R. , Scanlon, S. E. , Song, E. , Hsieh, W. C. , Braddock, D. E. , Ly, D. H. , Saltzman, W. M. , & Glazer, P. M. (2017). Anti‐tumor activity of miniPEG‐gamma‐modified PNAs to inhibit MicroRNA‐210 for cancer therapy. Molecular Therapy ‐ Nucleic Acids, 9, 111–119. 10.1016/j.omtn.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, S. , Gonzalo, D. H. , Feely, M. , Delitto, D. , Behrns, K. E. , Beveridge, M. , Zhang, D. , Thomas, R. , Trevino, J. G. , Schmittgen, T. D. , & Hughes, S. J. (2017). The pancreatic tumor microenvironment drives changes in miRNA expression that promote cytokine production and inhibit migration by the tumor associated stroma. Oncotarget, 8(33), 54054–54067. 10.18632/oncotarget.10722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Y. C. , Vidigal, J. A. , Mu, P. , Yao, E. , Singh, I. , Gonzalez, A. J. , Concepcion, C. P. , Bonetti, C. , Ogrodowski, P. , Carver, B. , Selleri, L. , Betel, D. , Leslie, C. , & Ventura, A. (2015). An allelic series of miR‐17 approximately 92‐mutant mice uncovers functional specialization and cooperation among members of a microRNA polycistron. Nature Genetics, 47(7), 766–775. 10.1038/ng.3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna, J. A. , Hahn, L. , Agarwal, S. , & Rimm, D. L. (2012). In situ measurement of miR‐205 in malignant melanoma tissue supports its role as a tumor suppressor microRNA. Laboratory Investigation, 92(10), 1390–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna, J. A. , Wimberly, H. , Kumar, S. , Slack, F. , Agarwal, S. , & Rimm, D. L. (2012). Quantitative analysis of microRNAs in tissue microarrays by in situ hybridization. BioTechniques, 52(4), 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanniford, D. , Zhong, J. , Koetz, L. , Gaziel‐Sovran, A. , Lackaye, D. J. , Shang, S. , Pavlick, A. , Shapiro, R. , Berman, R. , Darvishian, F. , Shao, Y. , Osman, I. , & Hernando, E. (2015). A miRNA‐based signature detected in primary melanoma tissue predicts development of brain metastasis. Clinical Cancer Research, 21(21), 4903–4912. 10.1158/1078-0432.CCR-14-2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatley, M. E. , Patrick, D. M. , Garcia, M. R. , Richardson, J. A. , Bassel‐Duby, R. , van Rooij, E. , & Olson, E. N. (2010). Modulation of K‐Ras‐dependent lung tumorigenesis by MicroRNA‐21. Cancer Cell, 18(3), 282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, L. , Thomson, J. M. , Hemann, M. T. , Hernando‐Monge, E. , Mu, D. , Goodson, S. , Powers, S. , Cordon‐Cardo, C. , Lowe, S. W. , Hannon, G. J. , & Hammond, S. M. (2005). A microRNA polycistron as a potential human oncogene. Nature, 435(7043), 828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, W. , Wang, C. , Mu, R. , Liang, P. , Huang, Z. , Zhang, J. , & Dong, L. (2017). MiR‐21 is required for anti‐tumor immune response in mice: An implication for its bi‐directional roles. Oncogene, 36(29), 4212–4223. 10.1038/onc.2017.62 [DOI] [PubMed] [Google Scholar]
- Helms, E. , Onate, M. K. , & Sherman, M. H. (2020). Fibroblast heterogeneity in the pancreatic tumor microenvironment. Cancer Discovery, 10(5), 648–656. 10.1158/2159-8290.CD-19-1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo‐Sastre, A. , Lubeseder‐Martellato, C. , Engleitner, T. , Steiger, K. , Zhong, S. , Desztics, J. , Ollinger, R. , Rad, R. , Schmid, R. M. , Hermeking, H. , Siveke, J. T. , & von Figura, G. (2020). Mir34a constrains pancreatic carcinogenesis. Scientific Reports, 10(1), 9654. 10.1038/s41598-020-66561-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefig, K. P. , & Heissmeyer, V. (2010). Measuring microRNA expression in size‐limited FACS‐sorted and microdissected samples. Methods in Molecular Biology, 667, 47–63. 10.1007/978-1-60761-811-9_4 [DOI] [PubMed] [Google Scholar]
- Hoffman, Y. , Bublik, D. R. , Ugalde, A. P. , Elkon, R. , Biniashvili, T. , Agami, R. , Oren, M. , & Pilpel, Y. (2016). 3′UTR shortening potentiates MicroRNA‐based repression of pro‐differentiation genes in proliferating human cells. PLoS Genetics, 12(2), e1005879. 10.1371/journal.pgen.1005879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, D. S. , Kang, Y. K. , Borad, M. , Sachdev, J. , Ejadi, S. , Lim, H. Y. , Brenner, A. J. , Park, K. , Lee, J. L. , Kim, T. Y. , Shin, S. , Becerra, C. R. , Falchook, G. , Stoudemire, J. , Martin, D. , Kelnar, K. , Peltier, H. , Bonato, V. , Bader, A. G. , … Beg, M. S. (2020). Phase 1 study of MRX34, a liposomal miR‐34a mimic, in patients with advanced solid tumours. British Journal of Cancer, 122(11), 1630–1637. 10.1038/s41416-020-0802-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, H. , Yao, S. , Zhang, Y. , Ye, Y. , Li, C. , Hu, L. , Sun, Y. , Huang, H. Y. , & Ji, H. (2020). In vivo miRNA knockout screening identifies miR‐190b as a novel tumor suppressor. PLoS Genetics, 16(11), e1009168. 10.1371/journal.pgen.1009168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, S. H. , Wang, B. , Kota, J. , Yu, J. , Costinean, S. , Kutay, H. , Yu, L. , Bai, S. , La Perle, K. , Chivukula, R. R. , Mao, H. , Wei, M. , Clark, K. R. , Mendell, J. R. , Caligiuri, M. A. , Jacob, S. T. , Mendell, J. T. , & Ghoshal, K. (2012). Essential metabolic, anti‐inflammatory, and anti‐tumorigenic functions of miR‐122 in liver. The Journal of Clinical Investigation, 122(8), 2871–2883. 10.1172/JCI63539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, G. , Nishimoto, K. , Zhou, Z. , Hughes, D. , & Kleinerman, E. S. (2012). miR‐20a encoded by the miR‐17‐92 cluster increases the metastatic potential of osteosarcoma cells by regulating Fas expression. Cancer Research, 72(4), 908–916. 10.1158/0008-5472.CAN-11-1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Z. S. , Guo, X. W. , Zhang, G. , Liang, L. X. , & Nong, B. (2019). The diagnostic and prognostic value of miR‐200c in gastric Cancer: A meta‐analysis. Disease Markers, 2019, 8949618. 10.1155/2019/8949618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker, T. B. , Lee, S. H. , Tang, W. W. , Wallace, J. A. , Alexander, M. , Runtsch, M. C. , Larsen, D. K. , Thompson, J. , Ramstead, A. G. , Voth, W. P. , Hu, R. , Round, J. L. , Williams, M. A. , & O'Connell, R. M. (2017). Antitumor immunity is defective in T cell‐specific microRNA‐155‐deficient mice and is rescued by immune checkpoint blockade. The Journal of Biological Chemistry, 292(45), 18530–18541. 10.1074/jbc.M117.808121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummingbird . (2020). Pipeline of Hummingbird Diagnostics.
- Jain, A. K. , & Barton, M. C. (2012). Unmet expectations: miR‐34 plays no role in p53‐mediated tumor suppression in vivo. PLoS Genetics, 8(7), e1002859. 10.1371/journal.pgen.1002859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen, H. L. , Reesink, H. W. , Lawitz, E. J. , Zeuzem, S. , Rodriguez‐Torres, M. , Patel, K. , van der Meer, A. J. , Patick, A. K. , Chen, A. , Zhou, Y. , Persson, R. , King, B. D. , Kauppinen, S. , Levin, A. A. , & Hodges, M. R. (2013). Treatment of HCV infection by targeting microRNA. The New England Journal of Medicine, 368(18), 1685–1694. 10.1056/NEJMoa1209026 [DOI] [PubMed] [Google Scholar]
- Jarry, J. , Schadendorf, D. , Greenwood, C. , Spatz, A. , & van Kempen, L. C. (2014). The validity of circulating microRNAs in oncology: Five years of challenges and contradictions. Molecular Oncology, 8(4), 819–829. 10.1016/j.molonc.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, J. , Yu, L. , Yu, Z. , Forgues, M. , Uenishi, T. , Kubo, S. , Wakasa, K. , Zhou, J. , Fan, J. , Tang, Z. Y. , Fu, S. , Zhu, H. , Jin, J. G. , Sun, H. C. , & Wang, X. W. (2013). Development of a miR‐26 companion diagnostic test for adjuvant interferon‐alpha therapy in hepatocellular carcinoma. International Journal of Biological Sciences, 9(3), 303–312. 10.7150/ijbs.6214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, X. , Hu, X. , Han, S. , Miao, X. , Liu, H. , Li, X. , Lin, Z. , Wang, Z. , & Gong, W. (2018). Increased M1 macrophages in young miR‐15a/16(−/−) mice with tumour grafts or dextran sulphate sodium‐induced colitis. Scandinavian Journal of Immunology, 88(3), e12703. 10.1111/sji.12703 [DOI] [PubMed] [Google Scholar]
- Jia, X. , Liu, H. , Xu, C. , Han, S. , Shen, Y. , Miao, X. , Hu, X. , Lin, Z. , Qian, L. , Wang, Z. , & Gong, W. (2019). MiR‐15a/16‐1 deficiency induces IL‐10‐producing CD19(+) TIM‐1(+) cells in tumor microenvironment. Journal of Cellular and Molecular Medicine, 23(2), 1343–1353. 10.1111/jcmm.14037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, H. , Liu, J. , Chen, Y. , Ma, C. , Li, B. , & Hao, T. (2016). Up‐regulation of mir‐10b predicate advanced clinicopathological features and liver metastasis in colorectal cancer. Cancer Medicine, 5(10), 2932–2941. 10.1002/cam4.789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, L. , & Hermeking, H. (2017). miR‐34a and miR‐34b/c suppress intestinal tumorigenesis. Cancer Research, 77(10), 2746–2758. 10.1158/0008-5472.CAN-16-2183 [DOI] [PubMed] [Google Scholar]
- Kadera, B. E. , Li, L. , Toste, P. A. , Wu, N. , Adams, C. , Dawson, D. W. , & Donahue, T. R. (2013). MicroRNA‐21 in pancreatic ductal adenocarcinoma tumor‐associated fibroblasts promotes metastasis. PLoS.ONE, 8(8), e71978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaoka, R. , Iinuma, H. , Dejima, H. , Sakai, T. , Uehara, H. , Matsutani, N. , & Kawamura, M. (2018). Usefulness of plasma Exosomal MicroRNA‐451a as a noninvasive biomarker for early prediction of recurrence and prognosis of non‐small cell lung Cancer. Oncology, 94(5), 311–323. 10.1159/000487006 [DOI] [PubMed] [Google Scholar]
- Kang, W. K. , Lee, J. K. , Oh, S. T. , Lee, S. H. , & Jung, C. K. (2015). Stromal expression of miR‐21 in T3‐4a colorectal cancer is an independent predictor of early tumor relapse. BMC Gastroenterology, 15, 2. 10.1186/s12876-015-0227-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasar, S. , Salerno, E. , Yuan, Y. , Underbayev, C. , Vollenweider, D. , Laurindo, M. F. , Fernandes, H. , Bonci, D. , Addario, A. , Mazzella, F. , & Raveche, E. (2012). Systemic in vivo lentiviral delivery of miR‐15a/16 reduces malignancy in the NZB de novo mouse model of chronic lymphocytic leukemia. Genes and Immunity, 13(2), 109–119. 10.1038/gene.2011.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasinski, A. L. , Kelnar, K. , Stahlhut, C. , Orellana, E. , Zhao, J. , Shimer, E. , Dysart, S. , Chen, X. , Bader, A. G. , & Slack, F. J. (2015). A combinatorial microRNA therapeutics approach to suppressing non‐small cell lung cancer. Oncogene, 34(27), 3547–3555. 10.1038/onc.2014.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasinski, A. L. , & Slack, F. J. (2012). miRNA‐34 prevents cancer initiation and progression in a therapeutically resistant K‐ras and p53‐induced mouse model of lung adenocarcinoma. Cancer Research, 72(21), 5576–5587. 10.1158/0008-5472.CAN-12-2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent, O. A. , McCall, M. N. , Cornish, T. C. , & Halushka, M. K. (2014). Lessons from miR‐143/145: The importance of cell‐type localization of miRNAs. Nucleic Acids Research, 42(12), 7528–7538. 10.1093/nar/gku461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalife, J. , Ghose, J. , Martella, M. , Viola, D. , Rocci, A. , Troadec, E. , Terrazas, C. , Satoskar, A. R. , Gunes, E. G. , Dona, A. , Sanchez, J. F. , Bergsagel, P. L. , Chesi, M. , Pozhitkov, A. , Rosen, S. , Marcucci, G. , Keats, J. J. , Hofmeister, C. C. , Krishnan, A. , … Pichiorri, F. (2019). MiR‐16 regulates crosstalk in NF‐kappaB tolerogenic inflammatory signaling between myeloma cells and bone marrow macrophages. JCI Insight, 4(21), e129348. 10.1172/jci.insight.129348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodadadi‐Jamayran, A. , Akgol‐Oksuz, B. , Afanasyeva, Y. , Heguy, A. , Thompson, M. , Ray, K. , Giro‐Perafita, A. , Sanchez, I. , Wu, X. , Tripathy, D. , Zeleniuch‐Jacquotte, A. , Tsirigos, A. , & Esteva, F. J. (2018). Prognostic role of elevated mir‐24‐3p in breast cancer and its association with the metastatic process. Oncotarget, 9(16), 12868–12878. 10.18632/oncotarget.24403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, G. , Kim, M. , Lee, Y. , Byun, J. W. , Hwang, D. W. , & Lee, M. (2020). Systemic delivery of microRNA‐21 antisense oligonucleotides to the brain using T7‐peptide decorated exosomes. Journal of Controlled Release, 317, 273–281. 10.1016/j.jconrel.2019.11.009 [DOI] [PubMed] [Google Scholar]
- Kim, J. , Siverly, A. N. , Chen, D. , Wang, M. , Yuan, Y. , Wang, Y. , Lee, H. , Zhang, J. , Muller, W. J. , Liang, H. , Gan, B. , Yang, X. , Sun, Y. , You, M. J. , & Ma, L. (2016). Ablation of miR‐10b suppresses oncogene‐induced mammary tumorigenesis and metastasis and reactivates tumor‐suppressive pathways. Cancer Research, 76(21), 6424–6435. 10.1158/0008-5472.CAN-16-1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. , Lee, E. , Jung, J. , Lee, J. W. , Kim, H. J. , Kim, J. , Yoo, H. J. , Lee, H. J. , Chae, S. Y. , Jeon, S. M. , Son, B. H. , Gong, G. , Sharan, S. K. , & Chang, S. (2018). microRNA‐155 positively regulates glucose metabolism via PIK3R1‐FOXO3a‐cMYC axis in breast cancer. Oncogene, 37(22), 2982–2991. 10.1038/s41388-018-0124-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. , Song, J. H. , Kim, S. , Qu, P. , Martin, B. K. , Sehareen, W. S. , Haines, D. C. , Lin, P. C. , Sharan, S. K. , & Chang, S. (2016). Loss of oncogenic miR‐155 in tumor cells promotes tumor growth by enhancing C/EBP‐beta‐mediated MDSC infiltration. Oncotarget, 7(10), 11094–11112. 10.18632/oncotarget.7150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer‐Frifeldt, S. , Hansen, T. F. , Nielsen, B. S. , Joergensen, S. , Lindebjerg, J. , Soerensen, F. B. , de Pont, C. R. , & Jakobsen, A. (2012). The prognostic importance of miR‐21 in stage II colon cancer: A population‐based study. British Journal of Cancer, 107(7), 1169–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, U. , Lia, M. , Crespo, M. , Siegel, R. , Shen, Q. , Mo, T. , Ambesi‐Impiombato, A. , Califano, A. , Migliazza, A. , Bhagat, G. , & Dalla‐Favera, R. (2010). The DLEU2/miR‐15a/16‐1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell, 17(1), 28–40. 10.1016/j.ccr.2009.11.019 [DOI] [PubMed] [Google Scholar]
- Kong, Y. , Ning, L. , Qiu, F. , Yu, Q. , & Cao, B. (2019). Clinical significance of serum miR‐25 as a diagnostic and prognostic biomarker in human gastric cancer. Cancer Biomarkers, 24(4), 477–483. 10.3233/CBM-182213 [DOI] [PubMed] [Google Scholar]
- Kumar, M. S. , Erkeland, S. J. , Pester, R. E. , Chen, C. Y. , Ebert, M. S. , Sharp, P. A. , & Jacks, T. (2008). Suppression of non‐small cell lung tumor development by the let‐7 microRNA family. Proceedings of the National Academy of Sciences of the United States of America, 105(10), 3903–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutwin, P. , Konecki, T. , Borkowska, E. M. , Traczyk‐Borszynska, M. , & Jablonowski, Z. (2018). Urine miRNA as a potential biomarker for bladder cancer detection ‐ a meta‐analysis. Central European Journal of Urology, 71(2), 177–185. 10.5173/ceju.2018.1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labourier, E. , Shifrin, A. , Busseniers, A. E. , Lupo, M. A. , Manganelli, M. L. , Andruss, B. , Wylie, D. , & Beaudenon‐Huibregtse, S. (2015). Molecular testing for miRNA, mRNA, and DNA on fine‐needle aspiration improves the preoperative diagnosis of thyroid nodules with indeterminate cytology. The Journal of Clinical Endocrinology and Metabolism, 100(7), 2743–2750. 10.1210/jc.2015-1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, T. J. , Yoo, J. Y. , Shu, D. , Li, H. , Zhang, J. , Yu, J. G. , Jaime‐Ramirez, A. C. , Acunzo, M. , Romano, G. , Cui, R. , Sun, H. L. , Luo, Z. , Old, M. , Kaur, B. , Guo, P. , & Croce, C. M. (2017). RNA nanoparticle‐based targeted therapy for glioblastoma through inhibition of oncogenic miR‐21. Molecular Therapy, 25(7), 1544–1555. 10.1016/j.ymthe.2016.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, T. J. , Yuan, X. , Kerr, K. , Yoo, J. Y. , Kim, D. H. , Kaur, B. , & Eltzschig, H. K. (2020). Strategies to modulate MicroRNA functions for the treatment of Cancer or organ injury. Pharmacological Reviews, 72(3), 639–667. 10.1124/pr.119.019026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. , Deng, M. , Hu, J. , Li, X. , Chen, L. , Ju, Y. , Hao, J. , & Meng, S. (2016). Chronic inflammation contributes to the development of hepatocellular carcinoma by decreasing miR‐122 levels. Oncotarget, 7(13), 17021–17034. 10.18632/oncotarget.7740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. , Yin, Y. , Liu, X. , Xi, X. , Xue, W. , & Qu, Y. (2017). Non‐small cell lung cancer associated microRNA expression signature: Integrated bioinformatics analysis, validation and clinical significance. Oncotarget, 8(15), 24564–24578. 10.18632/oncotarget.15596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Li, X. , Li, Y. , Yang, H. , Wang, L. , Qin, Y. , Liu, H. , Fu, L. , & Guan, X. Y. (2013). Cell‐specific detection of miR‐375 downregulation for predicting the prognosis of esophageal squamous cell carcinoma by miRNA in situ hybridization. PLoS One, 8(1), e53582. 10.1371/journal.pone.0053582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. H. , Sun, S. S. , Li, N. , Lv, P. , Xie, S. Y. , & Wang, P. Y. (2017). MiR‐205 as a promising biomarker in the diagnosis and prognosis of lung cancer. Oncotarget, 8(54), 91938–91949. 10.18632/oncotarget.20262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Lu, M. , Fan, Y. , Shui, L. , Xie, S. , Sheng, R. , Si, H. , Li, Q. , Wang, Y. , & Tang, B. (2019). High‐throughput and ultra‐sensitive single‐cell profiling of multiple microRNAs and identification of human cancer. Chemical Communications, 55(70), 10404–10407. 10.1039/c9cc05553c [DOI] [PubMed] [Google Scholar]
- Li, L. , Zhang, J. , Diao, W. , Wang, D. , Wei, Y. , Zhang, C. Y. , & Zen, K. (2014). MicroRNA‐155 and MicroRNA‐21 promote the expansion of functional myeloid‐derived suppressor cells. Journal of Immunology, 192(3), 1034–1043. 10.4049/jimmunol.1301309 [DOI] [PubMed] [Google Scholar]
- Li, Y. P. , Van Pham, L. , Uzcategui, N. , & Bukh, J. (2016). Functional analysis of microRNA‐122 binding sequences of hepatitis C virus and identification of variants with high resistance against a specific antagomir. The Journal of General Virology, 97(6), 1381–1394. 10.1099/jgv.0.000445 [DOI] [PubMed] [Google Scholar]
- Lian, D. , Wang, Z. Z. , & Liu, N. S. (2016). MicroRNA‐1908 is a biomarker for poor prognosis in human osteosarcoma. European Review for Medical and Pharmacological Sciences, 20(7), 1258–1262. [PubMed] [Google Scholar]
- Liang, B. , Li, Y. , & Wang, T. (2017). A three miRNAs signature predicts survival in cervical cancer using bioinformatics analysis. Scientific Reports, 7(1), 5624. 10.1038/s41598-017-06032-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithwick‐Yanai, G. , Dromi, N. , Shtabsky, A. , Morgenstern, S. , Strenov, Y. , Feinmesser, M. , Kravtsov, V. , Leon, M. E. , Hajduch, M. , Ali, S. Z. , VandenBussche, C. J. , Zhang, X. , Leider‐Trejo, L. , Zubkov, A. , Vorobyov, S. , Kushnir, M. , Goren, Y. , Tabak, S. , Kadosh, E. , … Meiri, E. (2017). Multicentre validation of a microRNA‐based assay for diagnosing indeterminate thyroid nodules utilising fine needle aspirate smears. Journal of Clinical Pathology, 70(6), 500–507. 10.1136/jclinpath-2016-204089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C. G. , Calin, G. A. , Meloon, B. , Gamliel, N. , Sevignani, C. , Ferracin, M. , Dumitru, C. D. , Shimizu, M. , Zupo, S. , Dono, M. , Alder, H. , Bullrich, F. , Negrini, M. , & Croce, C. M. (2004). An oligonucleotide microchip for genome‐wide microRNA profiling in human and mouse tissues. Proceedings of the National Academy of Sciences of the United States of America, 101(26), 9740–9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Zhang, X. , Zhang, Z. , Chang, J. , Wang, Z. , Wu, Z. , Wang, C. , Sun, Z. , Ge, X. , Geng, R. , Tang, W. , Dai, C. , Lin, Y. , Lin, F. , Sun, M. , Jia, W. , Xue, W. , Ji, J. , Hu, Y. , … Li, J. (2017). Plasma microRNA‐based signatures to predict 3‐year postoperative recurrence risk for stage II and III gastric cancer. International Journal of Cancer, 141(10), 2093–2102. 10.1002/ijc.30895 [DOI] [PubMed] [Google Scholar]
- Liu, Z. , Bai, J. , Zhang, L. , Lou, F. , Ke, F. , Cai, W. , & Wang, H. (2017). Conditional knockout of microRNA‐31 promotes the development of colitis associated cancer. Biochemical and Biophysical Research Communications, 490(1), 62–68. 10.1016/j.bbrc.2017.06.012 [DOI] [PubMed] [Google Scholar]
- Lovat, F. , Fassan, M. , Gasparini, P. , Rizzotto, L. , Cascione, L. , Pizzi, M. , Vicentini, C. , Balatti, V. , Palmieri, D. , Costinean, S. , & Croce, C. M. (2015). miR‐15b/16‐2 deletion promotes B‐cell malignancies. Proceedings of the National Academy of Sciences of the United States of America, 112(37), 11636–11641. 10.1073/pnas.1514954112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovat, F. , Fassan, M. , Sacchi, D. , Ranganathan, P. , Palamarchuk, A. , Bill, M. , Karunasiri, M. , Gasparini, P. , Nigita, G. , Distefano, R. , Veneziano, D. , Dorrance, A. M. , Garzon, R. , & Croce, C. M. (2018). Knockout of both miR‐15/16 loci induces acute myeloid leukemia. Proceedings of the National Academy of Sciences of the United States of America, 115(51), 13069–13074. 10.1073/pnas.1814980115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovat, F. , Nigita, G. , Distefano, R. , Nakamura, T. , Gasparini, P. , Tomasello, L. , Fadda, P. , Ibrahimova, N. , Catricala, S. , Palamarchuk, A. , Caligiuri, M. A. , Galli, A. , Malcovati, L. , Minden, M. D. , & Croce, C. M. (2020). Combined loss of function of two different loci of miR‐15/16 drives the pathogenesis of acute myeloid leukemia. Proceedings of the National Academy of Sciences of the United States of America, 117(22), 12332–12340. 10.1073/pnas.2003597117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, J. , Getz, G. , Miska, E. A. , varez‐Saavedra, E. , Lamb, J. , Peck, D. , Sweet‐Cordero, A. , Ebert, B. L. , Mak, R. H. , Ferrando, A. A. , Downing, J. R. , Jacks, T. , Horvitz, H. R. , & Golub, T. R. (2005). MicroRNA expression profiles classify human cancers. Nature, 435(7043), 834–838. [DOI] [PubMed] [Google Scholar]
- Lu, L. , Wu, Y. , Feng, M. , Xue, X. , & Fan, Y. (2019). A novel sevenmiRNA prognostic model to predict overall survival in head and neck squamous cell carcinoma patients. Molecular Medicine Reports, 20(5), 4340–4348. 10.3892/mmr.2019.10665 [DOI] [PubMed] [Google Scholar]
- Lu, Y. , Baras, A. S. , & Halushka, M. K. (2018). miRge 2.0 for comprehensive analysis of microRNA sequencing data. BMC Bioinformatics, 19(1), 275. 10.1186/s12859-018-2287-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu, J. , Zhao, L. , Wang, F. , Ji, J. , Cao, Z. , Xu, H. , Shi, X. , Zhu, Y. , Zhang, C. , Guo, F. , Yang, B. , & Sun, Y. (2019). Discovery and validation of serum MicroRNAs as early diagnostic biomarkers for prostate Cancer in Chinese population. BioMed Research International, 2019, 9306803. 10.1155/2019/9306803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J. , Lin, Y. , Zhan, M. , Mann, D. L. , Stass, S. A. , & Jiang, F. (2015). Differential miRNA expressions in peripheral blood mononuclear cells for diagnosis of lung cancer. Laboratory Investigation, 95(10), 1197–1206. 10.1038/labinvest.2015.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L. , Reinhardt, F. , Pan, E. , Soutschek, J. , Bhat, B. , Marcusson, E. G. , Teruya‐Feldstein, J. , Bell, G. W. , & Weinberg, R. A. (2010). Therapeutic silencing of miR‐10b inhibits metastasis in a mouse mammary tumor model. Nature Biotechnology, 28(4), 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L. , Teruya‐Feldstein, J. , & Weinberg, R. A. (2007). Tumour invasion and metastasis initiated by microRNA‐10b in breast cancer. Nature, 449(7163), 682–688. [DOI] [PubMed] [Google Scholar]
- Ma, X. , Choudhury, S. N. , Hua, X. , Dai, Z. , & Li, Y. (2013). Interaction of the oncogenic miR‐21 microRNA and the p53 tumor suppressor pathway. Carcinogenesis, 34(6), 1216–1223. 10.1093/carcin/bgt044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, X. , Conklin, D. J. , Li, F. , Dai, Z. , Hua, X. , Li, Y. , Xu‐Monette, Z. Y. , Young, K. H. , Xiong, W. , Wysoczynski, M. , Sithu, S. D. , Srivastava, S. , Bhatnagar, A. , & Li, Y. (2015). The oncogenic microRNA miR‐21 promotes regulated necrosis in mice. Nature Communications, 6, 7151. 10.1038/ncomms8151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, X. , Kumar, M. , Choudhury, S. N. , Becker Buscaglia, L. E. , Barker, J. R. , Kanakamedala, K. , Liu, M. F. , & Li, Y. (2011). Loss of the miR‐21 allele elevates the expression of its target genes and reduces tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America, 108(25), 10144–10149. 10.1073/pnas.1103735108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie, T. A. , Schwartz, G. N. , Calderone, H. M. , Graveel, C. R. , Winn, M. E. , Hostetter, G. , Wells, W. A. , & Sempere, L. F. (2014). Stromal expression of miR‐21 identifies high‐risk group in triple‐negative breast cancer. The American Journal of Pathology, 184(12), 3217–3225. 10.1016/j.ajpath.2014.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra, M. , Sekar, T. V. , Ananta, J. S. , Devulapally, R. , Afjei, R. , Babikir, H. A. , Paulmurugan, R. , & Massoud, T. F. (2018). Targeted nanoparticle delivery of therapeutic antisense microRNAs presensitizes glioblastoma cells to lower effective doses of temozolomide in vitro and in a mouse model. Oncotarget, 9(30), 21478–21494. 10.18632/oncotarget.25135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik, S. , Lim, J. , Slack, F. J. , Braddock, D. T. , & Bahal, R. (2020). Next generation miRNA inhibition using short anti‐seed PNAs encapsulated in PLGA nanoparticles. Journal of Controlled Release, 327, 406–419. 10.1016/j.jconrel.2020.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, Z. , Zhao, H. , Qin, Y. , Wei, J. , Sun, J. , Zhang, W. , & Kang, Y. (2020). Post‐transcriptional dysregulation of microRNA and alternative polyadenylation in colorectal Cancer. Frontiers in Genetics, 11, 64. 10.3389/fgene.2020.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Usatorre, A. , Sempere, L. F. , Carmona, S. J. , Carretero‐Iglesia, L. , Monnot, G. , Speiser, D. E. , Rufer, N. , Donda, A. , Zehn, D. , Jandus, C. , & Romero, P. (2019). MicroRNA‐155 expression is enhanced by T‐cell receptor stimulation strength and correlates with improved tumor control in melanoma. Cancer Immunology Research, 7(6), 1013–1024. 10.1158/2326-6066.CIR-18-0504 [DOI] [PubMed] [Google Scholar]
- Mayr, C. , & Bartel, D. P. (2009). Widespread shortening of 3′UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell, 138(4), 673–684. 10.1016/j.cell.2009.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina, P. P. , Nolde, M. , & Slack, F. J. (2010). OncomiR addiction in an in vivo model of microRNA‐21‐induced pre‐B‐cell lymphoma. Nature, 467(7311), 86–90. [DOI] [PubMed] [Google Scholar]
- Meiri, E. , Mueller, W. C. , Rosenwald, S. , Zepeniuk, M. , Klinke, E. , Edmonston, T. B. , Werner, M. , Lass, U. , Barshack, I. , Feinmesser, M. , Huszar, M. , Fogt, F. , Ashkenazi, K. , Sanden, M. , Goren, E. , Dromi, N. , Zion, O. , Burnstein, I. , Chajut, A. , … Aharonov, R. (2012). A second‐generation microRNA‐based assay for diagnosing tumor tissue origin. The Oncologist, 17(6), 801–812. 10.1634/theoncologist.2011-0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melbo‐Jorgensen, C. , Ness, N. , Andersen, S. , Valkov, A. , Donnem, T. , Al‐Saad, S. , Kiselev, Y. , Berg, T. , Nordby, Y. , Bremnes, R. M. , Busund, L. T. , & Richardsen, E. (2014). Stromal expression of MiR‐21 predicts biochemical failure in prostate cancer patients with Gleason score 6. PLoS One, 9(11), e113039. 10.1371/journal.pone.0113039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- miRagen . (2020a). Cobomarsen receives orphan drug designation from the U.S. FDA for the treatment oF T‐cell lymphoma. Retrieved from http://investors.miragen.com/press-releases/press-release/2020/Cobomarsen-Receives-Orphan-Drug-Designation-From-the-U.S.-FDA-for-the-Treatment-of-T-cell-Lymphoma/default.aspx
- miRagen . (2020b). miragen announces internal review of preliminary topline data for the phase 2 solar clinical trial of cobomarsen in patients with cutaneous T‐cell lymphoma (CTCL). Retrieved from http://investors.miragen.com/press-releases/press-release/2020/miRagen-Announces-Internal-Review-of-Preliminary-Topline-Data-for-the-Phase-2-SOLAR-Clinical-Trial-of-Cobomarsen-in-Patients-with-Cutaneous-T-Cell-Lymphoma-CTCL/default.aspx
- Mishra, S. , Srivastava, A. K. , Suman, S. , Kumar, V. , & Shukla, Y. (2015). Circulating miRNAs revealed as surrogate molecular signatures for the early detection of breast cancer. Cancer Letters, 369(1), 67–75. 10.1016/j.canlet.2015.07.045 [DOI] [PubMed] [Google Scholar]
- Mogilyansky, E. , & Rigoutsos, I. (2013). The miR‐17/92 cluster: A comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death and Differentiation, 20(12), 1603–1614. 10.1038/cdd.2013.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroig‐Bosque, P. D. C. , Shah, M. Y. , Fu, X. , Fuentes‐Mattei, E. , Ling, H. , Ivan, C. , Nouraee, N. , Huang, B. , Chen, L. , Pileczki, V. , Redis, R. S. , Jung, E. J. , Zhang, X. , Lehrer, M. , Nagvekar, R. , Mafra, A. C. P. , Monroig‐Bosque, M. D. M. , Irimie, A. , Rivera, C. , … Calin, G. A. (2018). OncomiR‐10b hijacks the small molecule inhibitor linifanib in human cancers. Scientific Reports, 8(1), 13106. 10.1038/s41598-018-30989-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosallaei, M. , Ehtesham, N. , Rahimirad, S. , Saghi, M. , Vatandoost, N. , & Khosravi, S. (2020). PBMCs: A new source of diagnostic and prognostic biomarkers. Archives of Physiology and Biochemistry (Apr 15), 1–7. 10.1080/13813455.2020.1752257 [DOI] [PubMed] [Google Scholar]
- Moyal, L. , Barzilai, A. , Gorovitz, B. , Hirshberg, A. , Amariglio, N. , Jacob‐Hirsch, J. , Maron, L. , Feinmesser, M. , & Hodak, E. (2013). miR‐155 is involved in tumor progression of mycosis fungoides. Experimental Dermatology, 22(6), 431–433. 10.1111/exd.12161 [DOI] [PubMed] [Google Scholar]
- Mullick Chowdhury, S. , Wang, T. Y. , Bachawal, S. , Devulapally, R. , Choe, J. W. , Abou Elkacem, L. , Yakub, B. K. , Wang, D. S. , Tian, L. , Paulmurugan, R. , & Willmann, J. K. (2016). Ultrasound‐guided therapeutic modulation of hepatocellular carcinoma using complementary microRNAs. Journal of Controlled Release, 238, 272–280. 10.1016/j.jconrel.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan, M. B. , Tentori, A. M. , Zhang, W. C. , Slack, F. J. , & Doyle, P. S. (2020). Spatially resolved and multiplexed MicroRNA quantification from tissue using nanoliter well arrays. Microsystems & Nanoengineering, 6, 51. 10.1038/s41378-020-0169-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy, A. , Lanczky, A. , Menyhart, O. , & Gyorffy, B. (2018). Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Scientific Reports, 8(1), 9227. 10.1038/s41598-018-27521-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan, N. , Morenos, L. , Phipson, B. , Willis, S. N. , Brumatti, G. , Eggers, S. , Lalaoui, N. , Brown, L. M. , Kosasih, H. J. , Bartolo, R. C. , Zhou, L. , Catchpoole, D. , Saffery, R. , Oshlack, A. , Goodall, G. J. , & Ekert, P. G. (2017). Functionally distinct roles for different miR‐155 expression levels through contrasting effects on gene expression, in acute myeloid leukaemia. Leukemia, 31(4), 808–820. 10.1038/leu.2016.279 [DOI] [PubMed] [Google Scholar]
- Navarro, F. , & Lieberman, J. (2015). miR‐34 and p53: New insights into a complex functional relationship. PLoS One, 10(7), e0132767. 10.1371/journal.pone.0132767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeleman, R. A. , Wensink, D. , Wagenmakers, M. , Mijnhout, G. S. , Friesema, E. C. H. , & Langendonk, J. G. (2020). Diagnostic and therapeutic strategies for porphyrias. The Netherlands Journal of Medicine, 78(4), 149–160. [PubMed] [Google Scholar]
- Nelson, P. T. , Baldwin, D. A. , Scearce, L. M. , Oberholtzer, J. C. , Tobias, J. W. , & Mourelatos, Z. (2004). Microarray‐based, high‐throughput gene expression profiling of microRNAs. Nature Methods, 1(2), 155–161. [DOI] [PubMed] [Google Scholar]
- Nielsen, B. S. (2012). MicroRNA in situ hybridization. Methods in Molecular Biology, 822, 67–84. [DOI] [PubMed] [Google Scholar]
- Nielsen, B. S. , Jorgensen, S. , Fog, J. U. , Sokilde, R. , Christensen, I. J. , Hansen, U. , Brunner, N. , Baker, A. , Moller, S. , & Nielsen, H. J. (2011). High levels of microRNA‐21 in the stroma of colorectal cancers predict short disease‐free survival in stage II colon cancer patients. Clinical & Experimental Metastasis, 28(1), 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nik Mohamed Kamal, N. , & Shahidan, W. N. S. (2019). Non‐Exosomal and Exosomal circulatory MicroRNAs: Which are more valid as biomarkers? Frontiers in Pharmacology, 10, 1500. 10.3389/fphar.2019.01500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell, R. M. , Rao, D. S. , & Baltimore, D. (2012). microRNA regulation of inflammatory responses. Annual Review of Immunology, 30, 295–312. 10.1146/annurev-immunol-020711-075013 [DOI] [PubMed] [Google Scholar]
- Ohno, M. , Matsuzaki, J. , Kawauchi, J. , Aoki, Y. , Miura, J. , Takizawa, S. , Kato, K. , Sakamoto, H. , Matsushita, Y. , Takahashi, M. , Miyakita, Y. , Ichimura, K. , Narita, Y. , & Ochiya, T. (2019). Assessment of the diagnostic utility of serum MicroRNA classification in patients with diffuse glioma. JAMA Network Open, 2(12), e1916953. 10.1001/jamanetworkopen.2019.16953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno, R. , Uozaki, H. , Kikuchi, Y. , Kumagai, A. , Aso, T. , Watanabe, M. , Watabe, S. , Muto, S. , & Yamaguchi, R. (2016). Both cancerous miR‐21 and stromal miR‐21 in urothelial carcinoma are related to tumour progression. Histopathology, 69(6), 993–999. 10.1111/his.13032 [DOI] [PubMed] [Google Scholar]
- Okada, N. , Lin, C. P. , Ribeiro, M. C. , Biton, A. , Lai, G. , He, X. , Bu, P. , Vogel, H. , Jablons, D. M. , Keller, A. C. , Wilkinson, J. E. , He, B. , Speed, T. P. , & He, L. (2014). A positive feedback between p53 and miR‐34 miRNAs mediates tumor suppression. Genes & Development, 28(5), 438–450. 10.1101/gad.233585.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive, V. , Bennett, M. J. , Walker, J. C. , Ma, C. , Jiang, I. , Cordon‐Cardo, C. , Li, Q. J. , Lowe, S. W. , Hannon, G. J. , & He, L. (2009). miR‐19 is a key oncogenic component of mir‐17‐92. Genes & Development, 23(24), 2839–2849. 10.1101/gad.1861409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive, V. , Li, Q. , & He, L. (2013). Mir‐17‐92: A polycistronic oncomir with pleiotropic functions. Immunological Reviews, 253(1), 158–166. 10.1111/imr.12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, C. P. , & Dwyer, R. M. (2020). Nanoparticle‐based delivery of tumor suppressor microRNA for Cancer therapy. Cells, 9(2), 521. 10.3390/cells9020521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottman, R. , Levy, J. , Grizzle, W. E. , & Chakrabarti, R. (2016). The other face of miR‐17‐92a cluster, exhibiting tumor suppressor effects in prostate cancer. Oncotarget, 7(45), 73739–73753. 10.18632/oncotarget.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottosen, S. , Parsley, T. B. , Yang, L. , Zeh, K. , van Doorn, L. J. , van der Veer, E. , Raney, A. K. , Hodges, M. R. , & Patick, A. K. (2015). In vitro antiviral activity and preclinical and clinical resistance profile of miravirsen, a novel anti‐hepatitis C virus therapeutic targeting the human factor miR‐122. Antimicrobial Agents and Chemotherapy, 59(1), 599–608. 10.1128/AAC.04220-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva, R. M. , Zauli, D. A. G. , Neto, B. S. , & Brum, I. S. (2020). Urinary microRNAs expression in prostate cancer diagnosis: A systematic review. Clinical & Translational Oncology, 22(11), 2061–2073. 10.1007/s12094-020-02349-z [DOI] [PubMed] [Google Scholar]
- Pal, A. S. , & Kasinski, A. L. (2017). Animal models to study MicroRNA function. Advances in Cancer Research, 135, 53–118. 10.1016/bs.acr.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, C. Y. , Choi, Y. S. , & McManus, M. T. (2010). Analysis of microRNA knockouts in mice. Human Molecular Genetics, 19(R2), R169–R175. 10.1093/hmg/ddq367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson, E. L. , Kazenwadel, J. , Bert, A. G. , Khew‐Goodall, Y. , Ruszkiewicz, A. , & Goodall, G. J. (2013). Down‐regulation of the miRNA‐200 family at the invasive front of colorectal cancers with degraded basement membrane indicates EMT is involved in cancer progression. Neoplasia, 15(2), 180–191. 10.1593/neo.121828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarsky, Y. , Balatti, V. , & Croce, C. M. (2018). BCL2 and miR‐15/16: From gene discovery to treatment. Cell Death and Differentiation, 25(1), 21–26. 10.1038/cdd.2017.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Y. , & Croce, C. M. (2016). The role of MicroRNAs in human cancer. Signal Transduction and Targeted Therapy, 1, 15004. 10.1038/sigtrans.2015.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petriv, O. I. , Kuchenbauer, F. , Delaney, A. D. , Lecault, V. , White, A. , Kent, D. , Marmolejo, L. , Heuser, M. , Berg, T. , Copley, M. , Ruschmann, J. , Sekulovic, S. , Benz, C. , Kuroda, E. , Ho, V. , Antignano, F. , Halim, T. , Giambra, V. , Krystal, G. , … Hansen, C. L. (2010). Comprehensive microRNA expression profiling of the hematopoietic hierarchy. Proceedings of the National Academy of Sciences of the United States of America, 107(35), 15443–15448. 10.1073/pnas.1009320107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliseno, L. , Salmena, L. , Zhang, J. , Carver, B. , Haveman, W. J. , & Pandolfi, P. P. (2010). A coding‐independent function of gene and pseudogene mRNAs regulates tumour biology. Nature, 465(7301), 1033–1038. 10.1038/nature09144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preis, M. , Gardner, T. B. , Gordon, S. R. , Pipas, M. J. , Mackenzie, T. A. , Klein, E. E. , Longnecker, D. S. , Gutmann, E. J. , Sempere, L. F. , & Korc, M. (2011). microRNA‐10b expression correlates with response to neoadjuvant therapy and survival in pancreatic ductal adenocarcinoma. Clinical Cancer Research, 17, 5812–5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, X. , Long, L. , Shi, Z. , Liu, C. , Qiu, M. , Sheng, J. , Pu, P. , Yuan, X. , Ren, Y. , & Kang, C. (2014). Star‐branched amphiphilic PLA‐b‐PDMAEMA copolymers for co‐delivery of miR‐21 inhibitor and doxorubicin to treat glioma. Biomaterials, 35(7), 2322–2335. 10.1016/j.biomaterials.2013.11.039 [DOI] [PubMed] [Google Scholar]
- Quattrochi, B. , Gulvady, A. , Driscoll, D. R. , Sano, M. , Klimstra, D. S. , Turner, C. E. , & Lewis, B. C. (2017). MicroRNAs of the mir‐17~92 cluster regulate multiple aspects of pancreatic tumor development and progression. Oncotarget, 8(22), 35902–35918. 10.18632/oncotarget.16277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesne, J. L. , Jones, J. , Warren, J. , Dawson, S. J. , Ali, H. R. , Bardwell, H. , Blows, F. , Pharoah, P. , & Caldas, C. (2012). Biological and prognostic associations of miR‐205 and let‐7b in breast cancer revealed by in situ hybridization analysis of micro‐RNA expression in arrays of archival tumour tissue. The Journal of Pathology, 227(3), 306–314. [DOI] [PubMed] [Google Scholar]
- Rashid, H. , Hossain, B. , Siddiqua, T. , Kabir, M. , Noor, Z. , Ahmed, M. , & Haque, R. (2020). Fecal MicroRNAs as potential biomarkers for screening and diagnosis of intestinal diseases. Frontiers in Molecular Biosciences, 7, 181. 10.3389/fmolb.2020.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regulus . (2019). Regulus announces clinical candidate nomination for the treatment of glioblastoma multiforme. Retrieved from http://ir.regulusrx.com/news-releases/news-release-details/regulus-announces-clinical-candidate-nomination-treatment
- Reid, G. , Pel, M. E. , Kirschner, M. B. , Cheng, Y. Y. , Mugridge, N. , Weiss, J. , Williams, M. , Wright, C. , Edelman, J. J. , Vallely, M. P. , McCaughan, B. C. , Klebe, S. , Brahmbhatt, H. , MacDiarmid, J. A. , & van Zandwijk, N. (2013). Restoring expression of miR‐16: A novel approach to therapy for malignant pleural mesothelioma. Annals of Oncology, 24(12), 3128–3135. 10.1093/annonc/mdt412 [DOI] [PubMed] [Google Scholar]
- Roberts, T. C. , Langer, R. , & Wood, M. J. A. (2020). Advances in oligonucleotide drug delivery. Nature Reviews. Drug Discovery, 19(10), 673–694. 10.1038/s41573-020-0075-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues, P. M. , Afonso, M. B. , Simao, A. L. , Carvalho, C. C. , Trindade, A. , Duarte, A. , Borralho, P. M. , Machado, M. V. , Cortez‐Pinto, H. , Rodrigues, C. M. , & Castro, R. E. (2017). miR‐21 ablation and obeticholic acid ameliorate nonalcoholic steatohepatitis in mice. Cell Death & Disease, 8(5), e2825. 10.1038/cddis.2017.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriques, S. G. , Stickels, R. R. , Goeva, A. , Martin, C. A. , Murray, E. , Vanderburg, C. R. , Welch, J. , Chen, L. M. , Chen, F. , & Macosko, E. Z. (2019). Slide‐seq: A scalable technology for measuring genome‐wide expression at high spatial resolution. Science, 363(6434), 1463–1467. 10.1126/science.aaw1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupaimoole, R. , & Slack, F. J. (2017). MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nature Reviews. Drug Discovery, 16(3), 203–222. 10.1038/nrd.2016.246 [DOI] [PubMed] [Google Scholar]
- Sahraei, M. , Chaube, B. , Liu, Y. , Sun, J. , Kaplan, A. , Price, N. L. , Ding, W. , Oyaghire, S. , Garcia‐Milian, R. , Mehta, S. , Reshetnyak, Y. K. , Bahal, R. , Fiorina, P. , Glazer, P. M. , Rimm, D. L. , Fernandez‐Hernando, C. , & Suarez, Y. (2019). Suppressing miR‐21 activity in tumor‐associated macrophages promotes an antitumor immune response. The Journal of Clinical Investigation, 129(12), 5518–5536. 10.1172/JCI127125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno, E. , Scaglione, B. J. , Coffman, F. D. , Brown, B. D. , Baccarini, A. , Fernandes, H. , Marti, G. , & Raveche, E. S. (2009). Correcting miR‐15a/16 genetic defect in New Zealand Black mouse model of CLL enhances drug sensitivity. Molecular Cancer Therapeutics, 8(9), 2684–2692. 10.1158/1535-7163.MCT-09-0127 [DOI] [PubMed] [Google Scholar]
- Salmena, L. , Poliseno, L. , Tay, Y. , Kats, L. , & Pandolfi, P. P. (2011). A ceRNA hypothesis: The Rosetta stone of a hidden RNA language? Cell, 146(3), 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper, J. , Westerhuis, J. J. , Beddows, I. , Madaj, Z. , Monsma, D. , Hostetter, G. , Kiupel, M. , Conejo‐Garcia, J. R. , & Sempere, L. F. (2020). Loss of microRNA‐21 leads to profound stromal remodeling and short survival in K‐Ras‐driven mouse models of pancreatic cancer. International Journal of Cancer, 147(8), 2265–2278. 10.1002/ijc.33041 [DOI] [PubMed] [Google Scholar]
- Schwarzenbach, H. (2017). Clinical relevance of circulating, cell‐free and exosomal microRNAs in plasma and serum of breast cancer patients. Oncology Research and Treatment, 40(7–8), 423–429. 10.1159/000478019 [DOI] [PubMed] [Google Scholar]
- Segal, M. , Biscans, A. , Gilles, M. E. , Anastasiadou, E. , De Luca, R. , Lim, J. , Khvorova, A. , & Slack, F. J. (2020). Hydrophobically modified let‐7b miRNA enhances biodistribution to NSCLC and downregulates HMGA2 in vivo. Molecular Therapy ‐ Nucleic Acids, 19, 267–277. 10.1016/j.omtn.2019.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempere, L. F. (2014a). Fully automated fluorescence‐based four‐color multiplex assay for co‐detection of microRNA and protein biomarkers in clinical tissue specimens. Methods in Molecular Biology, 1211, 151–170. 10.1007/978-1-4939-1459-3_13 [DOI] [PubMed] [Google Scholar]
- Sempere, L. F. (2014b). Tissue slide‐based microRNA characterization of tumors: How detailed could diagnosis become for cancer medicine? Expert Review of Molecular Diagnostics, 14(7), 853–869. 10.1586/14737159.2014.944507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempere, L. F. (2019). Celebrating 25years of MicroRNA research: From discovery to clinical application. International Journal of Molecular Sciences, 20(8), 1987. 10.3390/ijms20081987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempere, L. F. , Christensen, M. , Silahtaroglu, A. , Bak, M. , Heath, C. V. , Schwartz, G. , Wells, W. , Kauppinen, S. , & Cole, C. N. (2007). Altered MicroRNA expression confined to specific epithelial cell subpopulations in breast cancer. Cancer Research, 67(24), 11612–11620. [DOI] [PubMed] [Google Scholar]
- Sempere, L. F. , Freemantle, S. , Pitha‐Rowe, I. , Moss, E. , Dmitrovsky, E. , & Ambros, V. (2004). Expression profiling of mammalian microRNAs uncovers a subset of brain‐expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biology, 5(3), R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempere, L. F. , & Kauppinen, S. (2009). Translational implications of MicroRNAs in clinical diagnostics and therapeutics. In Bradshaw R. A. & Dennis E. A. (Eds.), Handbook of cell signaling (Vol. 2, pp. 2965–2981). Academic Press; (Reprinted from: IN FILE). [Google Scholar]
- Sempere, L. F. , Keto, J. , & Fabbri, M. (2017). Exosomal MicroRNAs in breast Cancer towards diagnostic and therapeutic applications. Cancers (Basel), 9(7), 71. 10.3390/cancers9070071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempere, L. F. , Preis, M. , Yezefski, T. , Ouyang, H. , Suriawinata, A. A. , Silahtaroglu, A. , Conejo‐Garcia, J. R. , Kauppinen, S. , Wells, W. , & Korc, M. (2010). Fluorescence‐based codetection with protein markers reveals distinct cellular compartments for altered MicroRNA expression in solid tumors. Clinical Cancer Research, 16(16), 4246–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempere, L. F. , Zaluzec, E. , Kenyon, E. , Kiupel, M. , & Moore, A. (2020). Automated five‐color multiplex co‐detection of MicroRNA and protein expression in fixed tissue specimens. Methods in Molecular Biology, 2148, 257–276. 10.1007/978-1-0716-0623-0_17 [DOI] [PubMed] [Google Scholar]
- Seto, A. G. , Beatty, X. , Lynch, J. M. , Hermreck, M. , Tetzlaff, M. , Duvic, M. , & Jackson, A. L. (2018). Cobomarsen, an oligonucleotide inhibitor of miR‐155, co‐ordinately regulates multiple survival pathways to reduce cellular proliferation and survival in cutaneous T‐cell lymphoma. British Journal of Haematology, 183(3), 428–444. 10.1111/bjh.15547 [DOI] [PubMed] [Google Scholar]
- Setti, G. , Pezzi, M. E. , Viani, M. V. , Pertinhez, T. A. , Cassi, D. , Magnoni, C. , Bellini, P. , Musolino, A. , Vescovi, P. , & Meleti, M. (2020). Salivary MicroRNA for diagnosis of cancer and systemic diseases: A systematic review. International Journal of Molecular Sciences, 21(3), 903. 10.3390/ijms21030907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedy, F. J. (2015). Turning 21: Induction of miR‐21 as a key switch in the inflammatory response. Frontiers in Immunology, 6, 19. 10.3389/fimmu.2015.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedy, P. , & Medarova, Z. (2018). The fundamental role of miR‐10b in metastatic cancer. American Journal of Cancer Research, 8(9), 1674–1688. [PMC free article] [PubMed] [Google Scholar]
- Shi, C. , Liang, Y. , Yang, J. , Xia, Y. , Chen, H. , Han, H. , Yang, Y. , Wu, W. , Gao, R. , & Qin, H. (2013). MicroRNA‐21 knockout improve the survival rate in DSS induced fatal colitis through protecting against inflammation and tissue injury. PLoS One, 8(6), e66814. 10.1371/journal.pone.0066814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiino, S. , Matsuzaki, J. , Shimomura, A. , Kawauchi, J. , Takizawa, S. , Sakamoto, H. , Aoki, Y. , Yoshida, M. , Tamura, K. , Kato, K. , Kinoshita, T. , Kitagawa, Y. , & Ochiya, T. (2019). Serum miRNA‐based prediction of axillary lymph node metastasis in breast Cancer. Clinical Cancer Research, 25(6), 1817–1827. 10.1158/1078-0432.CCR-18-1414 [DOI] [PubMed] [Google Scholar]
- Shimomura, A. , Shiino, S. , Kawauchi, J. , Takizawa, S. , Sakamoto, H. , Matsuzaki, J. , Ono, M. , Takeshita, F. , Niida, S. , Shimizu, C. , Fujiwara, Y. , Kinoshita, T. , Tamura, K. , & Ochiya, T. (2016). Novel combination of serum microRNA for detecting breast cancer in the early stage. Cancer Science, 107(3), 326–334. 10.1111/cas.12880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu, D. , Li, H. , Shu, Y. , Xiong, G. , Carson, W. E., 3rd , Haque, F. , Xu, R. , & Guo, P. (2015). Systemic delivery of anti‐miRNA for suppression of triple negative breast Cancer utilizing RNA nanotechnology. ACS Nano, 9(10), 9731–9740. 10.1021/acsnano.5b02471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjefstad, K. , Johannessen, C. , Grindstad, T. , Kilvaer, T. , Paulsen, E. E. , Pedersen, M. , Donnem, T. , Andersen, S. , Bremnes, R. , Richardsen, E. , Al‐Saad, S. , & Busund, L. T. (2018). A gender specific improved survival related to stromal miR‐143 and miR‐145 expression in non‐small cell lung cancer. Scientific Reports, 8(1), 8549. 10.1038/s41598-018-26864-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabakova, E. , Culig, Z. , Remsik, J. , & Soucek, K. (2017). Alternative mechanisms of miR‐34a regulation in cancer. Cell Death & Disease, 8(10), e3100. 10.1038/cddis.2017.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, L. , Baxter, E. W. , Chambers, P. A. , Green, C. A. , Hanby, A. M. , Hughes, T. A. , Nash, C. E. , Millican‐Slater, R. A. , Stead, L. F. , Verghese, E. T. , & Speirs, V. (2015). Down‐regulation of miR‐92 in breast epithelial cells and in Normal but not tumour fibroblasts contributes to breast carcinogenesis. PLoS One, 10(10), e0139698. 10.1371/journal.pone.0139698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, R. , Walentek, P. , Sponer, N. , Klimke, A. , Lee, J. S. , Dixon, G. , Harland, R. , Wan, Y. , Lishko, P. , Lize, M. , Kessel, M. , & He, L. (2014). miR‐34/449 miRNAs are required for motile ciliogenesis by repressing cp110. Nature, 510(7503), 115–120. 10.1038/nature13413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer, A. D. , & Dowdy, S. F. (2018). GalNAc‐siRNA conjugates: Leading the way for delivery of RNAi therapeutics. Nucleic Acid Therapeutics, 28(3), 109–118. 10.1089/nat.2018.0736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvold, H. , Donnem, T. , Andersen, S. , Al‐Saad, S. , Busund, L. T. , & Bremnes, R. M. (2014). Stage and tissue‐specific prognostic impact of miR‐182 in NSCLC. BMC Cancer, 14, 138. 10.1186/1471-2407-14-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvold, H. , Donnem, T. , Andersen, S. , Al‐Saad, S. , Valkov, A. , Pedersen, M. I. , Busund, L. T. , & Bremnes, R. M. (2014). High tumor cell expression of microRNA‐21 in node positive non‐small cell lung cancer predicts a favorable clinical outcome. BMC Clinical Pathology, 14(1), 9. 10.1186/1472-6890-14-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand, S. H. , Bavafaye‐Haghighi, E. , Kristensen, H. , Rasmussen, A. K. , Hoyer, S. , Borre, M. , Mouritzen, P. , Besenbacher, S. , Orntoft, T. F. , & Sorensen, K. D. (2019). A novel combined miRNA and methylation marker panel (miMe) for prediction of prostate cancer outcome after radical prostatectomy. International Journal of Cancer, 145(12), 3445–3452. 10.1002/ijc.32427 [DOI] [PubMed] [Google Scholar]
- Stuopelyte, K. , Daniunaite, K. , Bakavicius, A. , Lazutka, J. R. , Jankevicius, F. , & Jarmalaite, S. (2016). The utility of urine‐circulating miRNAs for detection of prostate cancer. British Journal of Cancer, 115(6), 707–715. 10.1038/bjc.2016.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo, K. , Kato, K. , Matsuzaki, J. , Boku, N. , Abe, S. , Saito, Y. , Daiko, H. , Takizawa, S. , Aoki, Y. , Sakamoto, H. , Niida, S. , Takeshita, F. , Fukuda, T. , & Ochiya, T. (2019). Development and validation of an esophageal squamous cell carcinoma detection model by large‐scale MicroRNA profiling. JAMA Network Open, 2(5), e194573. 10.1001/jamanetworkopen.2019.4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar, U. K. , Bose, R. J. C. , Malhotra, M. , Babikir, H. A. , Afjei, R. , Robinson, E. , Zeng, Y. , Chang, E. , Habte, F. , Sinclair, R. , Gambhir, S. S. , Massoud, T. F. , & Paulmurugan, R. (2019). Intranasal delivery of targeted polyfunctional gold‐iron oxide nanoparticles loaded with therapeutic microRNAs for combined theranostic multimodality imaging and presensitization of glioblastoma to temozolomide. Biomaterials, 218, 119342. 10.1016/j.biomaterials.2019.119342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutaria, D. S. , Jiang, J. , Azevedo‐Pouly, A. C. , Wright, L. , Bray, J. A. , Fredenburg, K. , Liu, X. , Lu, J. , Torres, C. , Mancinelli, G. , Grippo, P. J. , Coppola, V. , & Schmittgen, T. D. (2019). Knockout of acinar enriched microRNAs in mice promote duct formation but not pancreatic Cancer. Scientific Reports, 9(1), 11147. 10.1038/s41598-019-47566-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoronos, A. A. , Engelman, D. M. , & Slack, F. J. (2016). OncomiR or tumor suppressor? The duplicity of MicroRNAs in Cancer. Cancer Research, 76(13), 3666–3670. 10.1158/0008-5472.CAN-16-0359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synlogic . (2020). Pipeline + Programs. Retrieved from https://www.synlogictx.com/pipeline-programs/
- Szafranska‐Schwarzbach, A. E. , Adai, A. T. , Lee, L. S. , Conwell, D. L. , & Andruss, B. F. (2011). Development of a miRNA‐based diagnostic assay for pancreatic ductal adenocarcinoma. Expert Review of Molecular Diagnostics, 11(3), 249–257. [DOI] [PubMed] [Google Scholar]
- Tang, W. , Wan, S. , Yang, Z. , Teschendorff, A. E. , & Zou, Q. (2018). Tumor origin detection with tissue‐specific miRNA and DNA methylation markers. Bioinformatics, 34(3), 398–406. 10.1093/bioinformatics/btx622 [DOI] [PubMed] [Google Scholar]
- Teplyuk, N. M. , Uhlmann, E. J. , Gabriely, G. , Volfovsky, N. , Wang, Y. , Teng, J. , Karmali, P. , Marcusson, E. , Peter, M. , Mohan, A. , Kraytsberg, Y. , Cialic, R. , Chiocca, E. A. , Godlewski, J. , Tannous, B. , & Krichevsky, A. M. (2016). Therapeutic potential of targeting microRNA‐10b in established intracranial glioblastoma: First steps toward the clinic. EMBO Molecular Medicine, 8(3), 268–287. 10.15252/emmm.201505495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai, T. H. , Calado, D. P. , Casola, S. , Ansel, K. M. , Xiao, C. , Xue, Y. , Murphy, A. , Frendewey, D. , Valenzuela, D. , Kutok, J. L. , Schmidt‐Supprian, M. , Rajewsky, N. , Yancopoulos, G. , Rao, A. , & Rajewsky, K. (2007). Regulation of the germinal center response by microRNA‐155. Science, 316(5824), 604–608. [DOI] [PubMed] [Google Scholar]
- Thomson, D. W. , & Dinger, M. E. (2016). Endogenous microRNA sponges: Evidence and controversy. Nature Reviews. Genetics, 17(5), 272–283. 10.1038/nrg.2016.20 [DOI] [PubMed] [Google Scholar]
- ThyGeNEXT+ThyraMIR . (2020). Combination testing. Retrieved from https://thygenext-thyramir.com/combination-testing/
- Titze‐de‐Almeida, R. , David, C. , & Titze‐de‐Almeida, S. S. (2017). The race of 10 synthetic RNAi‐based drugs to the pharmaceutical market. Pharmaceutical Research, 34(7), 1339–1363. 10.1007/s11095-017-2134-2 [DOI] [PubMed] [Google Scholar]
- Tivnan, A. , Orr, W. S. , Gubala, V. , Nooney, R. , Williams, D. E. , McDonagh, C. , Prenter, S. , Harvey, H. , Domingo‐Fernandez, R. , Bray, I. M. , Piskareva, O. , Ng, C. Y. , Lode, H. N. , Davidoff, A. M. , & Stallings, R. L. (2012). Inhibition of neuroblastoma tumor growth by targeted delivery of microRNA‐34a using anti‐disialoganglioside GD2 coated nanoparticles. PLoS One, 7(5), e38129. 10.1371/journal.pone.0038129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toray . (2020). Analysis of miRNA in plasma or serum. Retrieved from https://www.3d-gene.com/en/case/paper/pap_001.html#/
- Trang, P. , Medina, P. P. , Wiggins, J. F. , Ruffino, L. , Kelnar, K. , Omotola, M. , Homer, R. , Brown, D. , Bader, A. G. , Weidhaas, J. B. , & Slack, F. J. (2010). Regression of murine lung tumors by the let‐7 microRNA. Oncogene, 29(11), 1580–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang, P. , Wiggins, J. F. , Daige, C. L. , Cho, C. , Omotola, M. , Brown, D. , Weidhaas, J. B. , Bader, A. G. , & Slack, F. J. (2011). Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Molecular Therapy, 19, 1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Transcode . (2020). Targeting microRNA‐10b. Retrieved from https://www.transcodetherapeutics.com/ttx-mc138.html
- Troiano, G. , Mastrangelo, F. , Caponio, V. C. A. , Laino, L. , Cirillo, N. , & Lo Muzio, L. (2018). Predictive prognostic value of tissue‐based MicroRNA expression in oral squamous cell carcinoma: A systematic review and meta‐analysis. Journal of Dental Research, 97(7), 759–766. 10.1177/0022034518762090 [DOI] [PubMed] [Google Scholar]
- Tsikrika, F. D. , Avgeris, M. , Levis, P. K. , Tokas, T. , Stravodimos, K. , & Scorilas, A. (2018). miR‐221/222 cluster expression improves clinical stratification of non‐muscle invasive bladder cancer (TaT1) patients' risk for short‐term relapse and progression. Genes, Chromosomes & Cancer, 57(3), 150–161. 10.1002/gcc.22516 [DOI] [PubMed] [Google Scholar]
- Urits, I. , Swanson, D. , Swett, M. C. , Patel, A. , Berardino, K. , Amgalan, A. , Berger, A. A. , Kassem, H. , Kaye, A. , & Viswanath, O. (2020). A review of Patisiran (ONPATTRO[R]) for the treatment of polyneuropathy in people with hereditary transthyretin amyloidosis. Neurology and Therapy, 9(2), 301–315. 10.1007/s40120-020-00208-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uziel, T. , Karginov, F. V. , Xie, S. , Parker, J. S. , Wang, Y. D. , Gajjar, A. , He, L. , Ellison, D. , Gilbertson, R. J. , Hannon, G. , & Roussel, M. F. (2009). The miR‐17~92 cluster collaborates with the sonic hedgehog pathway in medulloblastoma. Proceedings of the National Academy of Sciences of the United States of America, 106(8), 2812–2817. 10.1073/pnas.0809579106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zandwijk, N. , Pavlakis, N. , Kao, S. C. , Linton, A. , Boyer, M. J. , Clarke, S. , Huynh, Y. , Chrzanowska, A. , Fulham, M. J. , Bailey, D. L. , Cooper, W. A. , Kritharides, L. , Ridley, L. , Pattison, S. T. , MacDiarmid, J. , Brahmbhatt, H. , & Reid, G. (2017). Safety and activity of microRNA‐loaded minicells in patients with recurrent malignant pleural mesothelioma: A first‐in‐man, phase 1, open‐label, dose‐escalation study. The Lancet Oncology, 18(10), 1386–1396. 10.1016/S1470-2045(17)30621-6 [DOI] [PubMed] [Google Scholar]
- Volinia, S. , Calin, G. A. , Liu, C. G. , Ambs, S. , Cimmino, A. , Petrocca, F. , Visone, R. , Iorio, M. , Roldo, C. , Ferracin, M. , Prueitt, R. L. , Yanaihara, N. , Lanza, G. , Scarpa, A. , Vecchione, A. , Negrini, M. , Harris, C. C. , & Croce, C. M. (2006). A microRNA expression signature of human solid tumors defines cancer gene targets. Proceedings of the National Academy of Sciences of the United States of America., 103(7), 2257–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace, J. A. , Kagele, D. A. , Eiring, A. M. , Kim, C. N. , Hu, R. , Runtsch, M. C. , Alexander, M. , Huffaker, T. B. , Lee, S. H. , Patel, A. B. , Mosbruger, T. L. , Voth, W. P. , Rao, D. S. , Miles, R. R. , Round, J. L. , Deininger, M. W. , & O'Connell, R. M. (2017). miR‐155 promotes FLT3‐ITD‐induced myeloproliferative disease through inhibition of the interferon response. Blood, 129(23), 3074–3086. 10.1182/blood-2016-09-740209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Yu, F. , Jia, X. , Iwanowycz, S. , Wang, Y. , Huang, S. , Ai, W. , & Fan, D. (2015). MicroRNA‐155 deficiency enhances the recruitment and functions of myeloid‐derived suppressor cells in tumor microenvironment and promotes solid tumor growth. International Journal of Cancer, 136(6), E602–E613. 10.1002/ijc.29151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, K. , Yuan, Y. , Cho, J. H. , McClarty, S. , Baxter, D. , & Galas, D. J. (2012). Comparing the MicroRNA spectrum between serum and plasma. PLoS One, 7(7), e41561. 10.1371/journal.pone.0041561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, N. , Zheng, J. , Chen, Z. , Liu, Y. , Dura, B. , Kwak, M. , Xavier‐Ferrucio, J. , Lu, Y. C. , Zhang, M. , Roden, C. , Cheng, J. , Krause, D. S. , Ding, Y. , Fan, R. , & Lu, J. (2019). Single‐cell microRNA‐mRNA co‐sequencing reveals non‐genetic heterogeneity and mechanisms of microRNA regulation. Nature Communications, 10(1), 95. 10.1038/s41467-018-07981-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. S. , Liu, L. X. , Li, G. P. , Chen, Y. , Li, C. Y. , Jin, D. Y. , & Wang, X. L. (2013). Combined serum CA19‐9 and miR‐27a‐3p in peripheral blood mononuclear cells to diagnose pancreatic cancer. Cancer Prevention Research (Philadelphia, Pa.), 6(4), 331–338. 10.1158/1940-6207.CAPR-12-0307 [DOI] [PubMed] [Google Scholar]
- Warford, A. (2016). In situ hybridisation: Technologies and their application to understanding disease. Progress in Histochemistry and Cytochemistry, 50(4), 37–48. 10.1016/j.proghi.2015.12.001 [DOI] [PubMed] [Google Scholar]
- Wen, D. , Danquah, M. , Chaudhary, A. K. , & Mahato, R. I. (2015). Small molecules targeting microRNA for cancer therapy: Promises and obstacles. Journal of Controlled Release, 219, 237–247. 10.1016/j.jconrel.2015.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, Y. , Han, J. , Chen, J. , Dong, J. , Xia, Y. , Liu, J. , Jiang, Y. , Dai, J. , Lu, J. , Jin, G. , Han, J. , Wei, Q. , Shen, H. , Sun, B. , & Hu, Z. (2015). Plasma miRNAs as early biomarkers for detecting hepatocellular carcinoma. International Journal of Cancer, 137(7), 1679–1690. 10.1002/ijc.29544 [DOI] [PubMed] [Google Scholar]
- Wiggins, J. F. , Ruffino, L. , Kelnar, K. , Omotola, M. , Patrawala, L. , Brown, D. , & Bader, A. G. (2010). Development of a lung cancer therapeutic based on the tumor suppressor microRNA‐34. Cancer Research, 70(14), 5923–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischhusen, J. C. , Chowdhury, S. M. , Lee, T. , Wang, H. , Bachawal, S. , Devulapally, R. , Afjei, R. , Sukumar, U. K. , & Paulmurugan, R. (2020). Ultrasound‐mediated delivery of miRNA‐122 and anti‐miRNA‐21 therapeutically immunomodulates murine hepatocellular carcinoma in vivo. Journal of Controlled Release, 321, 272–284. 10.1016/j.jconrel.2020.01.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C. W. , Cao, X. , Berger, C. K. , Foote, P. H. , Mahoney, D. W. , Simonson, J. A. , Anderson, B. W. , Yab, T. C. , Taylor, W. R. , Boardman, L. A. , Kisiel, J. B. , & Ahlquist, D. A. (2017). Novel approach to fecal occult blood testing by assay of erythrocyte‐specific microRNA markers. Digestive Diseases and Sciences, 62(8), 1985–1994. 10.1007/s10620-017-4627-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. , Bao, J. , Kim, M. , Yuan, S. , Tang, C. , Zheng, H. , Mastick, G. S. , Xu, C. , & Yan, W. (2014). Two miRNA clusters, miR‐34b/c and miR‐449, are essential for normal brain development, motile ciliogenesis, and spermatogenesis. Proceedings of the National Academy of Sciences of the United States of America, 111(28), E2851–E2857. 10.1073/pnas.1407777111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, M. , Piccini, M. , Koh, C. Y. , Lam, K. S. , & Singh, A. K. (2013). Single cell microRNA analysis using microfluidic flow cytometry. PLoS One, 8(1), e55044. 10.1371/journal.pone.0055044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, W. , Hu, Q. , Wang, M. , Shao, S. , Zhao, X. , Bai, H. , Huang, J. , Tang, G. , & Liang, T. (2019). A PEGylated megamer‐based microRNA delivery system activatable by stepwise microenvironment stimulation. Chemical Communications, 55(63), 9363–9366. 10.1039/c9cc03846a [DOI] [PubMed] [Google Scholar]
- Xi, J. , Huang, Q. , Wang, L. , Ma, X. , Deng, Q. , Kumar, M. , Zhou, Z. , Li, L. , Zeng, Z. , Young, K. H. , Zhang, M. , & Li, Y. (2018). miR‐21 depletion in macrophages promotes tumoricidal polarization and enhances PD‐1 immunotherapy. Oncogene, 37(23), 3151–3165. 10.1038/s41388-018-0178-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, X. , Tan, W. , Chen, B. , Huang, X. , Peng, C. , Yan, S. , Yang, L. , Song, C. , Wang, J. , Zheng, W. , Tang, H. , & Xie, X. (2018). Preoperative prediction nomogram based on primary tumor miRNAs signature and clinical‐related features for axillary lymph node metastasis in early‐stage invasive breast cancer. International Journal of Cancer, 142(9), 1901–1910. 10.1002/ijc.31208 [DOI] [PubMed] [Google Scholar]
- Xiong, D. D. , Lv, J. , Wei, K. L. , Feng, Z. B. , Chen, J. T. , Liu, K. C. , Chen, G. , & Luo, D. Z. (2017). A nine‐miRNA signature as a potential diagnostic marker for breast carcinoma: An integrated study of 1,110 cases. Oncology Reports, 37(6), 3297–3304. 10.3892/or.2017.5600 [DOI] [PubMed] [Google Scholar]
- Xu, M. , Kuang, Y. , Wang, M. , Han, X. , & Yang, Q. (2017). A microRNA expression signature as a predictor of survival for colon adenocarcinoma. Neoplasma, 64(1), 56–64. 10.4149/neo_2017_107 [DOI] [PubMed] [Google Scholar]
- Yamazaki, N. , Koga, Y. , Taniguchi, H. , Kojima, M. , Kanemitsu, Y. , Saito, N. , & Matsumura, Y. (2017). High expression of miR‐181c as a predictive marker of recurrence in stage II colorectal cancer. Oncotarget, 8(4), 6970–6983. 10.18632/oncotarget.14344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. , Pang, Y. Y. , He, R. Q. , Lin, P. , Cen, J. M. , Yang, H. , Ma, J. , & Chen, G. (2018). Diagnostic value of strand‐specific miRNA‐101‐3p and miRNA‐101‐5p for hepatocellular carcinoma and a bioinformatic analysis of their possible mechanism of action. FEBS Open Bio, 8(1), 64–84. 10.1002/2211-5463.12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. L. , Wang, W. , & Xu, L. P. (2018). Predictive value of microRNA‐10b expression in peripheral blood mononuclear cells in evaluating short‐ and long‐term efficacy of chemotherapy for patients with advanced non‐small‐cell lung cancer. Neoplasma, 65(4), 610–619. 10.4149/neo_2018_170110N20 [DOI] [PubMed] [Google Scholar]
- Yao, L. , Liu, Y. , Cao, Z. , Li, J. , Huang, Y. , Hu, X. , & Shao, Z. (2018). MicroRNA‐493 is a prognostic factor in triple‐negative breast cancer. Cancer Science, 109(7), 2294–2301. 10.1111/cas.13644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau, T. O. , Wu, C. W. , Dong, Y. , Tang, C. M. , Ng, S. S. , Chan, F. K. , Sung, J. J. , & Yu, J. (2014). microRNA‐221 and microRNA‐18a identification in stool as potential biomarkers for the non‐invasive diagnosis of colorectal carcinoma. British Journal of Cancer, 111(9), 1765–1771. 10.1038/bjc.2014.484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yigit, M. V. , Ghosh, S. K. , Kumar, M. , Petkova, V. , Kavishwar, A. , Moore, A. , & Medarova, Z. (2013). Context‐dependent differences in miR‐10b breast oncogenesis can be targeted for the prevention and arrest of lymph node metastasis. Oncogene, 32(12), 1530–1538. 10.1038/onc.2012.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, H. , Xiong, G. , Guo, S. , Xu, C. , Xu, R. , Guo, P. , & Shu, D. (2019). Delivery of anti‐miRNA for triple‐negative breast Cancer therapy using RNA nanoparticles targeting stem cell marker CD133. Molecular Therapy, 27(7), 1252–1261. 10.1016/j.ymthe.2019.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo, B. , Kavishwar, A. , Ross, A. , Wang, P. , Tabassum, D. P. , Polyak, K. , Barteneva, N. , Petkova, V. , Pantazopoulos, P. , Tena, A. , Moore, A. , & Medarova, Z. (2015). Combining miR‐10b‐targeted Nanotherapy with low‐dose doxorubicin elicits durable regressions of metastatic breast Cancer. Cancer Research, 75(20), 4407–4415. 10.1158/0008-5472.CAN-15-0888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo, B. , Kavishwar, A. , Wang, P. , Ross, A. , Pantazopoulos, P. , Dudley, M. , Moore, A. , & Medarova, Z. (2017). Therapy targeted to the metastatic niche is effective in a model of stage IV breast cancer. Scientific Reports, 7, 45060. 10.1038/srep45060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoosuf, N. , Navarro, J. F. , Salmen, F. , Stahl, P. L. , & Daub, C. O. (2020). Identification and transfer of spatial transcriptomics signatures for cancer diagnosis. Breast Cancer Research, 22(1), 6. 10.1186/s13058-019-1242-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Wang, Q. , & Zhang, S. (2019). MicroRNAs in sputum specimen as noninvasive biomarkers for the diagnosis of nonsmall cell lung cancer: An updated meta‐analysis. Medicine (Baltimore), 98(6), e14337. 10.1097/MD.0000000000014337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Roccaro, A. M. , Rombaoa, C. , Flores, L. , Obad, S. , Fernandes, S. M. , Sacco, A. , Liu, Y. , Ngo, H. , Quang, P. , Azab, A. K. , Azab, F. , Maiso, P. , Reagan, M. , Brown, J. R. , Thai, T. H. , Kauppinen, S. , & Ghobrial, I. M. (2012). LNA‐mediated anti‐miR‐155 silencing in low‐grade B‐cell lymphomas. Blood, 120(8), 1678–1686. 10.1182/blood-2012-02-410647 [DOI] [PubMed] [Google Scholar]
- Zheng, J. Z. , Huang, Y. N. , Yao, L. , Liu, Y. R. , Liu, S. , Hu, X. , Liu, Z. B. , & Shao, Z. M. (2018). Elevated miR‐301a expression indicates a poor prognosis for breast cancer patients. Scientific Reports, 8(1), 2225. 10.1038/s41598-018-20680-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, R. , Mao, W. , Du, Z. , Zhang, J. , Wang, M. , & Hu, M. (2018). Three differential expression profiles of miRNAs as potential biomarkers for lung adenocarcinoma. Biochemical and Biophysical Research Communications, 507(1–4), 377–382. 10.1016/j.bbrc.2018.11.046 [DOI] [PubMed] [Google Scholar]
- Zhou, X. , Wen, W. , Zhu, J. , Huang, Z. , Zhang, L. , Zhang, H. , Qi, L. W. , Shan, X. , Wang, T. , Cheng, W. , Zhu, D. , Yin, Y. , Chen, Y. , Zhu, W. , Shu, Y. , & Liu, P. (2017). A six‐microRNA signature in plasma was identified as a potential biomarker in diagnosis of esophageal squamous cell carcinoma. Oncotarget, 8(21), 34468–34480. 10.18632/oncotarget.16519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonari, E. , Pucci, F. , Saini, M. , Mazzieri, R. , Politi, L. S. , Gentner, B. , & Naldini, L. (2013). A role for miR‐155 in enabling tumor‐infiltrating innate immune cells to mount effective antitumor responses in mice. Blood, 122(2), 243–252. [DOI] [PubMed] [Google Scholar]
- Zou, X. , Wei, J. , Huang, Z. , Zhou, X. , Lu, Z. , Zhu, W. , & Miao, Y. (2019). Identification of a six‐miRNA panel in serum benefiting pancreatic cancer diagnosis. Cancer Medicine, 8(6), 2810–2822. 10.1002/cam4.2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, X. , Xia, T. , Li, M. , Wang, T. , Liu, P. , Zhou, X. , Huang, Z. , & Zhu, W. (2020). MicroRNA profiling in serum: Potential signatures for breast cancer diagnosis. Cancer Biomarkers, 30, 41–53. 10.3233/CBM-201547 [DOI] [PubMed] [Google Scholar]
- Zou, X. , Zhu, D. , Zhang, H. , Zhang, S. , Zhou, X. , He, X. , Zhu, J. , & Zhu, W. (2020). MicroRNA expression profiling analysis in serum for nasopharyngeal carcinoma diagnosis. Gene, 727, 144243. 10.1016/j.gene.2019.144243 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


