Abstract
Mantle cell lymphoma (MCL) accounts for approximately 3% of all cases of malignant lymphoma in Japan. The CLIMBER-DBR (Treatment practices and patient burden in chronic lymphocytic leukemia and mantle cell lymphoma patients in the real world: An observational database research in Japan) study examined the clinical characteristics, treatment patterns, and healthcare resource utilization of MCL in a real-world clinical setting in Japan. Using the Japanese Medical Data Vision database, we extracted data for 1130 patients with MCL (ICD-10 code C83.1) registered between March 1, 2013 and February 28, 2018. The date of first MCL diagnosis was taken as the index date. The mean (standard deviation) age, body weight, and modified Charlson Comorbidity Index were 71.4 (10.9) years, 58.3 (11.7) kg, and 1.9 (1.6), respectively, and 24.6% were ≤65 years old. The median follow-up period was 654 days (first–third quartile 290.5–1049 days). A total of 802 patients (71.0%) underwent first-line treatment. The most common first-line treatment was bendamustine/rituximab (BR; 27.8%), followed by rituximab/cyclophosphamide/doxorubicin/vincristine/prednisolone (R-CHOP; 15.6%) and rituximab/tetrahydropyranyl-adriamycin/cyclophosphamide/vincristine/prednisolone (R-THP-COP; 6.5%). The median (95% confidence interval) times to initial (first-line), second-line, and third-line treatments were 45 (36–62), 687 (624–734), and 1188 (1099–1444) days, respectively. Treatment practices for MCL in Japan are consistent with trends observed in Western countries. Our study can serve as a benchmark to assess future MCL treatments in Japan.
Keywords: Mantle cell lymphoma, Japan, real world, treatment patterns, resource utilization
INTRODUCTION
Mantle cell lymphoma (MCL) is a type of mature B-cell non-Hodgkin lymphoma (NHL) that is characterized by the CCND1-IGH translocation and overexpression of the cell cycle regulator cyclin D1.1 It is a rare disorder that accounts for approximately 3% of all cases of malignant lymphoma in Japan.2 Most patients with MCL present at an advanced stage and it is generally incurable by chemoimmunotherapy.1
The Japanese Society of Hematology (JSH) 2018 practice guidelines for MCL do not specify any standard or established treatments for MCL.3 First-line treatment options for MCL in clinical practice may include involved-field radiation therapy with or without chemotherapy for limited-stage MCL and combination chemotherapy with rituximab for advanced MCL. Patients aged ≤65 years may receive intensive chemotherapy with rituximab and high-dose cytarabine, and responders may be candidates for high-dose chemotherapy followed by autologous hematopoietic stem cell transplantation (HDC/AHSCT) as consolidation therapy in the first response phase. The guidelines also recommend bendamustine, bortezomib, and fludarabine. In addition, ibrutinib was recently introduced for patients with relapsed or refractory disease, and favorable treatment outcomes have been reported in these settings.4-6
Although clinical trials demonstrated promising efficacy and safety of these treatments, further studies using secondary data sources (e.g., hospital claims or healthcare insurance provider databases) are needed to elucidate whether the benefits of treatments observed in trial settings are consistent with those in real-world settings. This is important because, in real-world clinical practice, clinicians select the most appropriate treatment for their patients by taking their age, disease stage, general conditions, and comorbidities into consideration. Age, in particular, is an important factor because MCL predominantly affects elderly patients and under-representation of elderly patients in clinical trials may have negative implications in real-world clinical practice. Thus, clinical trials do not fully reflect the real-world situation. Although the JSH 2018 practice guidelines also suggest a “watch and wait” approach for MCL, there are no real-world data reflecting how many patients undergo this approach. We must also consider the impact of newly approved drugs, such as ibrutinib, on prescribing practices.
In recent years, increasing medical costs have become a problem in Japan. Thus, it is important to clarify the costs associated with MCL in Japan, including direct costs of treatment, management of adverse events (AEs), and clinical events that may be involved in healthcare resources utilized by patients with MCL.
Recently, real-world evidence of the treatment of MCL in the United States was reported in a study that used a commercial healthcare insurance claims database.7 The authors found that bendamustine/rituximab (BR; 35.4%) was the most common first-line treatment, followed by rituximab/cyclophosphamide/doxorubicin/vincristine/prednisolone (R-CHOP) (24.9%) and rituximab monotherapy (12.9%). The study also examined the treatment costs and impact of AEs on monthly healthcare expenditure, which were two-fold higher in patients with six or more AEs than in patients without AEs (US$13,650 vs $5131 per month).7
No real-world studies have used secondary data sources to elucidate the current treatment practices or healthcare resource utilization associated with MCL in Japan. Therefore, we performed the CLIMBER-DBR (Treatment practices and patient burden in chronic lymphocytic leukemia and mantle cell lymphoma patients in the real world: An observational database research in Japan) study in order to elucidate the characteristics of patients with MCL, the common treatment patterns, and healthcare resource utilization in real-world clinical settings in Japan. To achieve this, we performed retrospective analyses of the Medical Data Vision (MDV) database, a Japanese healthcare database.
METHODS
Ethics
The MDV database (Medical Data Vision Co., Ltd., Tokyo, Japan), the database used in this study, comprises data collected under contract from the participating institutions. All data regarding personal information were anonymized by each institution and the database does not include the location of institution. Therefore, patient information cannot be retrieved from the database. This complies with the Act on the Protection of Personal Information in Japan. The contracts between MDV and the institutions permit the MDV database to be used for research purposes. The study was also approved by the Institutional Review Board of the Clinical Research Promotion Network-Japan (Osaka, Japan; approval number: 7625-NIS-8326-00; date: June 20, 2019).
MDV database
The MDV database records healthcare claims and diagnostic procedure combination information under consent from hospitals across Japan, and is managed by Medical Data Vision Co., Ltd (Tokyo, Japan). As of February 2018, the database included data from 375 hospitals (including 187 designated cancer care hubs), and over 10 million patients treated in inpatient and outpatient settings. All data are recorded anonymously. Diseases are classified using the International Classification of Diseases, 10th Edition (ICD-10) codes and treatments are recorded using Anatomical Therapeutic Chemical Classification (ATC) codes. Data recorded between April 2008 and February 2019 were used as the dataset in this study.
Patients
For this study, we analyzed the MDV database for patients registered between March 1, 2013 to February 28, 2018 with an ICD-10 diagnosis code of C83 (non-follicular lymphoma) or C91 (lymphocytic leukemia), and retrieved all patients with a recorded diagnosis of C83.1 for MCL. The date of the first diagnosis of MCL was recorded as the index date and patients who were already diagnosed and/or receiving treatment before the follow-up period were excluded. We excluded patients aged <18 years at the index date, patients with an index date outside the study period, and patients who underwent AHSCT before the index date.
Measures
The clinical data for eligible patients were retrieved from the MDV database. Baseline data included (if recorded) age, sex, body weight, and modified Charlson Comorbidity Index (mCCI). The mCCI was derived from the following items: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, mild liver disease, diabetes without chronic complications, diabetes with chronic complications, hemiplegia or paraplegia, renal disease, moderate or severe liver disease, metastatic solid tumor, and acquired immune deficiency syndrome/human immunodeficiency virus infection. Malignant neoplasm of the skin was included in mCCI, but hematological malignancies (e.g. lymphoma and leukemia) were excluded. Follow-up data were recorded for all patients until February 28, 2019, or until their last recorded visit or death.
The prescription of antineoplastic agents and corticosteroids (when used in combination with antineoplastic drugs) was identified using the ATC codes L01 and H02AB, respectively. Antineoplastic medications were grouped by treatment and sequence for up to three lines of treatment per patient.
We assessed the frequencies of comorbidities and AEs that are commonly associated with MCL by considering the following clinical events: in-hospital death, diagnosis of comorbidities, emergency hospitalization, and surgical/diagnostic/medical procedures (including head and neck imaging, catheter ablation, and blood transfusion). We also assessed the prescription of medications used to treat associated AEs (e.g. systemic steroids not prescribed in combination with antineoplastic drugs, anti-infective agents, antiarrhythmic agents, antithrombotic agents, or urate-lowering agents).
Healthcare resource utilization was assessed in terms of the number of hospitalizations, duration of hospitalization, number of outpatient visits, number of emergency admissions, number of procedures, and number of variant medical examinations. The costs attributable to these events, in addition to costs of medications and other costs, were retrieved from the database.
Study outcomes
The primary study outcomes were the treatment pattern and the time to initial (i.e., first-line) treatment (TIT), which was calculated as the time from the index date (date of first MCL diagnosis) to the first prescribed antineoplastic treatment. Secondary outcomes included patient characteristics, the times to first-subsequent (i.e., second-line) treatment (TFST) and/or second-subsequent (i.e., third-line) treatment (TSST), clinical outcomes, and medical resource utilization expressed in millions of Japanese yen (JPY). TFST and TSST were calculated from the time of the first prescription to the prescription of second-line (TFST) or third-line (TSST) treatments. These outcomes were selected to understand the treatment choices and disease progression in real-world settings in Japan, in addition to the healthcare resources utilized for the treatment of MCL and some of its associated AEs.
Statistical analysis
Data were analyzed using SAS version 9.3 or later (SAS Inc., Cary, NC, USA). The median TIT, TFST, and TSST with 95% confidence intervals (CI) were determined using the Kaplan–Meier method. TIT, TFST, and TSST were also determined in patients stratified by age (≤65 or >65 years) in post-hoc analyses. Medications and treatment combinations were summarized descriptively for up to three lines of treatment per patient. All data were assessed descriptively, and the results are presented as the number (percent) of patients, mean ± standard deviation, or median (interquartile range), as appropriate. As the diagnosis code was classified as severe for some patients, sensitivity analysis was performed after excluding patients with other diagnoses of hematological malignancies.
RESULTS
Patients
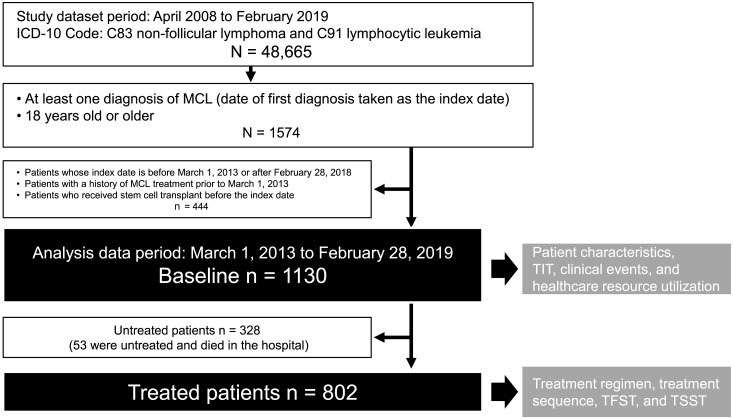
A total of 48,665 patients had documentation of C83 (non-follicular lymphoma) or C91 (lymphocytic leukemia) between April 2008 and February 2019. Of these, 1574 were ≥18 years old at the first diagnosis of MCL (index date). Four hundred and forty-four patients were subsequently excluded either because their index date was outside the period of March 1, 2013–February 28, 2018, they received treatment for MCL prior to March 1, 2013, or they underwent HSCT before the index date. Therefore, 1130 patients were included in the present analyses (Fig. 1).
Fig. 1.
Study population
A total of 802 patients (71.0%) were prescribed at least one treatment for MCL, whereas the other 328 (29.0%) were untreated, which included 53 patients who died in the hospital before starting treatment.
Baseline characteristics of the patients are presented in Table 1. Overall, approximately three-quarters of the patients were >65 years at diagnosis (75.4%) or male (73.2%). Their mean body weight at baseline was 58.3 kg and the mean mCCI was 1.9. The baseline characteristics of 802 treated and 328 untreated patients were similar (Table 1), except for mCCI, which was 2.1 ± 1.6 in the treated patients and 1.6 ± 1.5 in the untreated patients. The median follow-up was 654 days (Q1–Q3 290.5–1049 days) for all patients and 324.5 days (Q1–Q3 39–939.75 days) for untreated patients.
Table 1. Patient characteristics.
| Characteristic | Total N = 1130 |
Treated N = 802 |
Untreated N = 328 |
|---|---|---|---|
| Age, years, mean (SD) | 71.4 (10.9) | 71.4 (9.3) | 71.5 (12.7) |
| Age, years, median (range) | 73 (20–96) | 72 (25–96) | 73 (20–94) |
| Age >65 years, n (%) | 852 (75.4) | 615 (76.7) | 237 (72.3) |
| Male, n (%) | 827 (73.2) | 603 (75.2) | 224 (68.3) |
| Weight (kg), mean (SD) | 58.3 (11.7) | 59.3 (11.4) | 53.6 (11.7) |
| mCCI, mean (SD)* | 1.9 (1.6) | 2.1 (1.6) | 1.6 (1.5) |
| Radiation treatment, n (%) | 113 (10.0) | 99 (12.3) | 14 (4.3) |
*Derived from the following items: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, mild liver disease, diabetes without chronic complications, diabetes with chronic complications, hemiplegia or paraplegia, renal disease, moderate or severe liver disease, metastatic solid tumor, and acquired immune deficiency syndrome/human immunodeficiency virus, excluding hematological malignancies (lymphoma and leukemia)
SD, standard deviation; mCCI, modified Charlson Comorbidity Index
Sixty-eight patients (6.0%) underwent HSCT during the follow-up period. AHSCT was performed for 64 patients and cord blood transplantation for four. Their ages ranged from 25 to 72 years; 59 patients were ≤65 years old and 9 were >65 years old.
Treatments
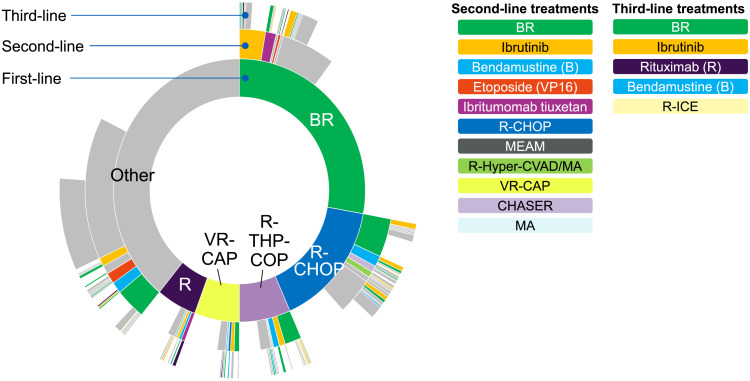
The regimens used in ≥2% of patients for each treatment line during the follow-up period are shown in Table S1. The frequent sequences of treatments prescribed are shown in Fig. 2. BR was frequently prescribed as first-line (27.8%), second-line (18.9%), or third-line (13.6%) treatment. R-CHOP was frequently prescribed as first-line treatment (15.6%). Other first-line treatments prescribed to ≥5% of patients were rituximab/tetrahydropyranyl-adriamycin/cyclophosphamide/vincristine/prednisolone (R-THP-COP) (6.5%), bortezomib/rituximab/cyclophosphamide/doxorubicin/prednisolone (VR-CAP) (5.9%), and rituximab monotherapy (5.1%).
Fig. 2.
Treatment sequence. The top five first-line treatments and the second-/third-line treatments prescribed to ≥2% of patients are shown in color. Inner, middle, and outer rings represent first-line, second-line, and third-line treatments, respectively. The individual treatments are shown in descending order of frequency. B, bendamustine; R, rituximab; CHOP, cyclophosphamide/doxorubicin/vincristine/prednisolone; MEAM, ranimustine/etoposide/cytarabine/melphalan; hyper-CVAD, hyperfractionated cyclophosphamide/vincristine/doxorubicin/dexamethasone; MA, methotrexate/cytarabine; VR-CAP, bortezomib/rituximab/cyclophosphamide/doxorubicin/prednisolone; CHASER, cyclophosphamide/cytarabine/dexamethasone/etoposide/rituximab; ICE, ifosfamide/carboplatin/etoposide
Ibrutinib monotherapy was prescribed to 2.5% of patients as first-line treatment, to 10.0% of patients as second-line treatment, and to 12.2% of patients as third-line treatment. Bendamustine monotherapy was prescribed to 2.4% of patients as first-line treatment, to 6.4% of patients as second-line treatment, and to 3.8% of patients as third-line treatment. Rituximab monotherapy was prescribed to only 1.2% of patients as second-line treatment and to 5.6% of patients as third-line treatment. Radiation therapy was performed for 12.3% of treated patients and for 4.3% of untreated patients.
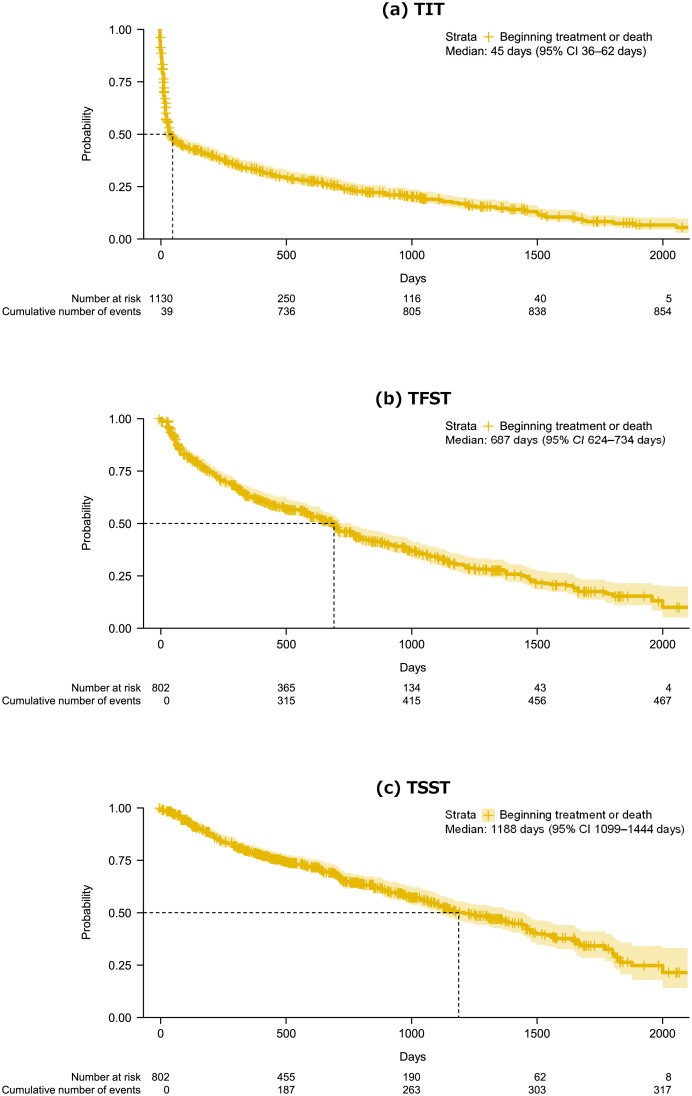
In the overall cohort, the median TIT was 45 days (95% CI 36–62 days; Fig. 3a) and 44% were untreated at 3 months. The median TFST was 687 days (95% CI 624–734 days; Fig. 3b) and the median TSST was 1188 days (95% CI 1099–1444; Fig. 3c).
Fig. 3.
Kaplan–Meier plots of TIT (a), TFST (b), and TSST (c) in the overall study cohort. TIT was calculated as the time from the index date (date of first MCL diagnosis) to the first prescribed antineoplastic treatment. TFST and TSST were calculated as the time from the first prescription to the prescription of second-line (TFST) or third-line (TSST) treatments. TIT, time to initial (i.e., first-line) treatment; TFST, time to first-subsequent (i.e., second-line) treatment; TSST, time to second-subsequent (i.e., third-line) treatment
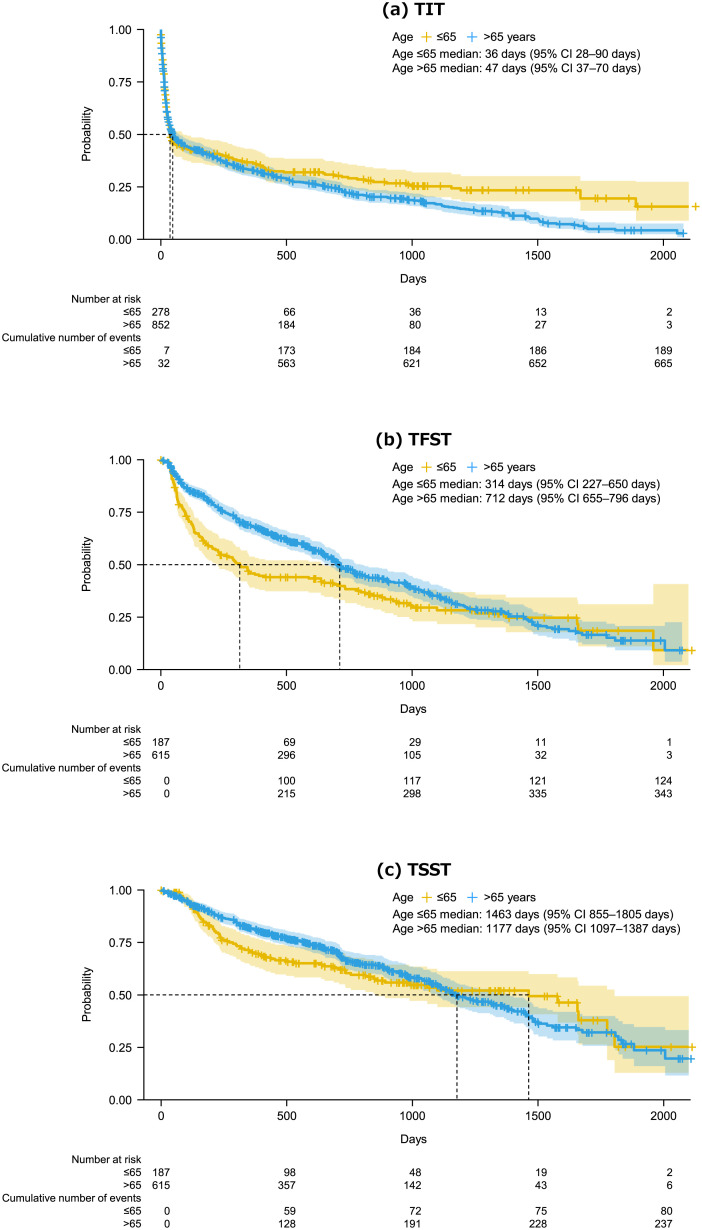
In post-hoc analyses, patients were divided into two age-groups (≤65 or >65 years at the index date) to calculate the TIT, TFST, and TSST. The median TIT was 36 days in patients aged ≤65 years (95% CI 28–90 days) and 47 days in patient aged >65 years (95% CI 37–70 days) (Fig. 4a). Among patients aged ≤65 years, the median TFST and TSST were 314 and 1463 days, respectively. The corresponding values in patients aged >65 years were 712 and 1177 days (Fig. 4b,c).
Fig. 4.
Kaplan–Meier plots of TIT (a), TFST (b), and TSST (c) treatments in patients divided by age at the study index (≤65 or >65 years). TIT was calculated as the time from the index date (date of first MCL diagnosis) to the first prescribed antineoplastic treatment. TFST and TSST were calculated as the time from the first prescription to the prescription of second-line (TFST) or third-line (TSST) treatments. TIT, time to initial (i.e., first-line) treatment; TFST time to first-subsequent (i.e., second-line) treatment; TSST, time to second-subsequent (i.e., third-line) treatment
HSCT was performed for 68 patients, 59 of whom were ≤65 years old and 9 were >65 years old. AHSCT was performed for 64 patients. In these patients, the most common first-line treatments were rituximab/hyperfractionated cyclophosphamide/vincristine/doxorubicin/dexamethasone/methotrexate/cytarabine (R-Hyper-CVAD/MA), which was prescribed to 15 patients (all ≤65 years old at the index date), and R-CHOP, which was prescribed to 14 patients (11 were ≤65 years old at the index date).
Among 1062 patients who did not undergo HSCT, the median TIT, TFST, and TSST were 56 (95% CI 43–90), 717 (675–812), and 1258 (95% CI 1122–1460) days, respectively (Fig. S1). Within this cohort of 1062 patients (excluding patients who underwent HSCT), the median TIT was 176 days in those aged ≤65 years and 49 days in those aged >65 years, whereas the median TFST was 734 and 414 days, and the median TSST was not reached and 1188 days, respectively (Fig. S2).
Clinical events and associated safety risks
The most common clinical events (based on the prescribed drugs) experienced during the follow-up period included bacterial infection (83.5%), tumor lysis syndrome (TLS)/hyperuricemia (44.2%), fungal infection (41.9%), cytopenia (37.4%), surgery (36.5%), and emergency admission (22.7%) (Table 2). Rasburicase, which is used to prevent TLS, was prescribed to 5.0% of patients. Antithrombotic drugs were prescribed to 61.9%, which decreased to 22.0% when we excluded heparin, which is often used as a flush to maintain venous line patency.
Table 2. Clinical events and associated safety risks.
| Event* | Associated safety risk | Total N = 1130 |
Treated N = 802 |
Untreated N = 328 |
|---|---|---|---|---|
| Systemic antimicrobial agents | Infection | 944 (83.5) | 772 (96.3) | 172 (52.4) |
| Intravenous antimicrobial agents | Infection | 676 (59.8) | 575 (71.7) | 101 (30.8) |
| Antithrombotic drug prescription | Thrombosis | 700 (61.9) | 607 (75.7) | 93 (28.4) |
| Antithrombotic drugs excluding heparin | Thrombosis | 249 (22.0) | 206 (25.7) | 43 (13.1) |
| Prevention or treatment of TLS/hyperuricemia | TLS/hyperuricemia | 499 (44.2) | 445 (55.5) | 54 (16.5) |
| Rasburicase | TLS | 57 (5.0) | 57 (7.1) | 0 |
| Systemic antifungal agents | Infection | 473 (41.9) | 431 (53.7) | 42 (12.8) |
| Intravenous antifungal agents | Infection | 169 (15.0) | 159 (19.8) | 10 (3.0) |
| Transfusion | Cytopenia | 423 (37.4) | 389 (48.5) | 34 (10.4) |
| Any surgery | Any surgery | 412 (36.5) | 347 (43.3) | 65 (19.8) |
| Emergency admission | Emergency admission | 257 (22.7) | 214 (26.7) | 43 (13.1) |
| In-hospital death | In-hospital death | 240 (21.2) | 187 (23.3) | 53 (16.2) |
| Antiarrhythmic drug prescription | Arrhythmia | 194 (17.2) | 160 (20.0) | 34 (10.4) |
| Steroids for systemic use only (before treatment) | Steroid administration | 180 (15.9) | 180 (22.4) | 0 |
| Diagnosis of ischemic heart disease | Ischemic heart disease | 161 (14.2) | 130 (16.2) | 31 (9.5) |
| Diagnosis of interstitial lung disease | Interstitial lung disease | 80 (7.1) | 62 (7.7) | 18 (5.5) |
| Stem cell transplantation | Transplantation | 68 (6.0) | 68 (8.5) | 0 |
| Diagnosis of intracranial hemorrhage | Intracranial hemorrhage | 12 (1.1) | 8 (1.0) | 4 (1.2) |
| Endoscopic hemostasis for gastrointestinal hemorrhage | Gastrointestinal hemorrhage | 8 (0.7) | 7 (0.9) | 1 (0.3) |
Values are n (%)
*Includes in-hospital death, diagnosis of comorbidities, emergency admission, surgical/diagnostic/medical procedures, and prescription of medications
TLS, tumor lysis syndrome
Healthcare resource utilization and expenditure
In terms of healthcare resource utilization during the follow-up period, most patients (79.7%) were hospitalized during follow-up for a median of three times per patient (Table 3). Patients spent a median of 56 days in the hospital in total during follow-up. In addition, nearly one-quarter of patients (22.7%) required emergency admission for a median of 0.7 times per patient per year. On the other hand, 94.7% of patients attended outpatient visits for a median of 15.8 times per patient per year.
Table 3. Healthcare resource utilization.
| Variable | Total N = 1130 |
Treated N = 802 |
Untreated N = 328 |
|---|---|---|---|
| Follow-up, days, median (Q1–Q3) | 654 (290.5–1049) | 728.5 (436.75–1147) | 324.5 (39–939.75) |
| Patients requiring hospitalization, n (%) | 901 (79.7) | 760 (94.8) | 141 (43.0) |
| Hospitalizations per patient, median (Q1–Q3) | 3 (1–6) | 3 (2–6) | 1 (1–2) |
| Total hospitalization days per patient, median (Q1–Q3) | 56 (21–124) | 72 (29–134) | 15 (6–35) |
| Patients requiring emergency admission, n (%) | 257 (22.7) | 214 (26.7) | 43 (13.1) |
| Emergency admissions per patient per year, median (Q1–Q3) | 0.7 (0.4–1.5) | 0.7 (0.4–1.3) | 1.1 (0.5–4.6) |
| Patients requiring outpatient visits, n (%) | 1070 (94.7) | 777 (96.9) | 293 (89.3) |
| Outpatient visits per patient per year, median (Q1–Q3) | 15.8 (9.7–22.9) | 17.0 (11.3–23.4) | 10.8 (6.4–18.6) |
Q1, quartile 1; Q3, quartile 3
The median healthcare expenditure for this cohort of 1130 patients with MCL was 5.7 million JPY, with a median annual cost per patient of 3.8 million JPY (Table 4). Inpatient treatments and prescriptions accounted for 4.0 and 3.2 million JPY per patient, respectively.
Table 4. Healthcare expenditure (in millions of Japanese yen).
| Variable | Total N = 1130 |
Treated N = 802 |
Untreated N = 328 |
|---|---|---|---|
| Total cost per patient | 5.7 (1.5–10.6) | 8.2 (5.0–12.5) | 0.6 (0.2–1.4) |
| Total cost per patient per year | 3.8 (1.6–7.9) | 4.5 (2.5–8.4) | 0.7 (0.2–3.9) |
| Inpatient cost per patient | 4.0 (1.4–7.9) | 5.1 (2.0–8.8) | 0.8 (0.4–1.5) |
| Outpatient cost per visit | 0.02 (0.01–0.08) | 0.03 (0.01–0.09) | 0.02 (0.01–0.04) |
| Prescription costs per patient | 3.2 (0.7–5.9) | 4.3 (2.6–7.0) | 0.09 (0.02–0.3) |
| Examination costs per patient | 0.6 (0.3–1.0) | 0.8 (0.5–1.2) | 0.2 (0.09–0.4) |
| Other costs per patient | 1.5 (0.4–3.7) | 2.4 (1.0–4.6) | 0.1 (0.03–0.7) |
Values are the median (quartile 1–quartile 3)
Sensitivity analysis
The sensitivity analysis excluding patients with concurrent diagnoses of hematological malignancies yielded results that were unchanged from the overall study cohort described above (data not shown).
DISCUSSION
The CLIMBER-DBR study was performed to investigate the current clinical practices and healthcare resource utilization associated with the treatment of MCL in Japan. To our knowledge, our study is the first in Japan to use a secondary database to capture claims and diagnostic procedure claims data for these purposes.
In this study of 1130 patients with a median follow-up period of 654 days (Q1–Q3 290–1049 days), 71.0% of patients were prescribed antineoplastic drugs to treat MCL, with a median TIT of 45 days, and many patients received at least one subsequent line of therapy. The top five regimens accounted for 60.8% of first-line treatments. This study included patients with multiple diagnoses, together with MCL; therefore, we may have included treatments for other diseases.
To date, few studies have examined the real-world treatment practices or healthcare resource utilization associated with MCL. However, the present results reflect those reported in several studies performed in countries other than Japan. In a similarly designed study involving 783 patients in a US commercial claims database,7 71.9% received one or more treatments, and BR (41.1%), R-CHOP (26.7%), rituximab monotherapy (20.4%), and ibrutinib monotherapy (14.2%) were the predominant treatments. In another US study examining treatments and healthcare expenditure for 2509 patients, the most common treatment was R-CHOP (26%), followed by rituximab monotherapy (19%), BR (15%), and ibrutinib (5%).8 In a smaller study of 335 patients in the UK, the prescription of first-line rituximab-containing regimens increased from 32% to 86% of patients over an 11-year period (2004–2015). This was accompanied by an increase in median survival from 2.0 years (2004–2011) to 3.5 years (2012–2015), with the overall survival doubling among patients aged >70 years.9 Rituximab-containing regimens were also commonly prescribed and associated with improved overall survival in a study of Swedish and Danish patients.10 That study also documented that AHSCT was frequently performed and associated with improved survival.10 Our study and those in other countries suggest that rituximab-based treatments are frequently used as first-line therapies for MCL. Ibrutinib was also used as a first-line treatment in 2.5% of patients versus 4.6% in US studies.7,8 In Japan, ibrutinib was approved for refractory or relapsed MCL in 2016, which suggests that it can be used off-label in some patients and explains its less frequent use as first-line treatment in our study. However, some patients may have received first-line treatment at another hospital not participating in the MDV database, and then transferred to a participating hospital where they may have been re-diagnosed with MCL and prescribed ibrutinib, which was assessed as the primary treatment in the present analyses. For these reasons and because ibrutinib was approved part-way through the follow-up period, our study may underestimate the current usage of ibrutinib.
MCL exhibits an indolent clinical course, enabling a watch and wait approach in some patients, with the aim of avoiding unnecessary cytotoxic or immune therapies and minimizing AEs or other clinically significant events.11-13 In the present study, 44% were untreated at 3 months based on the Kaplan–Meier curve of TIT, with a median follow-up of 324.5 days. If we exclude the 53 patients who died before starting treatment from the 328 untreated patients, it is likely that the 275 surviving patients (275/1130 = 24.3%) were managed by observation. In a retrospective study of 97 patients in the US, 31 (32%) were observed for more than 3 months before initial systemic therapy.14 Based on this, our study is comparable with those reported from the US. However, our study may overestimate the true proportion of patients managed with a watch and wait approach because some patients may have been transferred to another hospital or did not visit after a complete examination. Thus, some patients may have received appropriate pharmacological or radiation therapies that were not recorded in the MDV database.
Another objective of our study was to determine the healthcare resource utilization associated with MCL in Japan. Although we were unable to exclude the possibility that the costs in our study included the costs related to other diseases, we revealed considerable healthcare expenditure associated with MCL, and that the total cost per patient is driven by the number of hospitalizations and drug prescriptions for treated patients.
In addition, we noted a significant number of clinical events, especially infections, thrombosis, TLS/hyperuricemia, and transfusion in our MCL cohort. Clinical events, including AEs, make up a significant part of the costs associated with MCL treatment, as stated in previous US studies.7,8 Thus, prevention/management of clinical events, including AEs relevant to MCL itself and MCL therapies, may represent a key target for reducing the overall expenditure associated with MCL.
There are some limitations that should be considered when interpreting the results of this study. As we used a real-world database to identify clinical events using specific diagnostic/prescription codes, we may have included treatments for other comorbidities and conditions. In addition, it is possible that some patients moved to another hospital during the follow-up period and were lost to follow-up, preventing us from obtaining ongoing data on MCL treatment or outcomes. For these reasons, our results may not precisely reflect the treatment practices and healthcare resource utilization of MCL in Japan.
In conclusion, the CLIMBER-DBR study was the first database study focusing on the current treatment practices and healthcare resource utilization associated with MCL in Japan. In general, the described treatment practices are consistent with the trends observed in Western countries. In Japan and in other countries, MCL is associated with considerable healthcare resource utilization and associated expenditure. We believe that this study can serve as a broad benchmark to assess which treatment options are ideal in Japan by taking into consideration clinical and economic factors.
Supplemental Materials and Methods
ACKNOWLEDGMENTS
This study was funded by AstraZeneca K.K., Osaka, Japan. The authors thank IQVIA for performing the analyses and Nicholas D. Smith (EMC K.K.) for medical writing support, which were funded by AstraZeneca K.K.
Footnotes
CONFLICT OF INTEREST
KI received honoraria from Kyowa Kirin, and Eisai; and research funds from Celgene, Chugai, Novartis, Ono Pharmaceutical, Bayer, Daiichi Sankyo, Takeda Pharmaceutical, Zenyaku Kogyo, Kyowa Kirin, AstraZeneca, Incyte, Abbvie, HUYA Japan, Sanofi, SymBio, Solasia, Pfizer, Janssen, and Yakult.
JS received honoraria from AstraZeneca, Abbvie, Bristol-Myers Squibb, Celgene, Chugai, Eisai, Janssen, and Takeda Pharmaceutical; and research funds from AstraZeneca, Bayer, Celgene, Chugai, Eisai, Kyowa Kirin, Ono Pharmaceutical, SymBio, Takeda Pharmaceutical, and Yakult.
JT received research funds from Kyowa Kirin, Takeda Pharmaceutical, Eisai, and Ono Pharmaceutical.
KF, MN, and MJ are employees of AstraZeneca.
HN received honoraria from Celgene, Takeda Pharmaceutical, Eisai, and Mundipharma; and research funds from Bayer, IQVIA, AstraZeneca, Takeda Pharmaceutical, Chugai, Mundipharma, Zenyaku Kogyo, SymBio, Kyowa Kirin, Celgene, Chugai, and Nippon Shinyaku.
REFERENCES
- 1.Klener P. Advances in molecular biology and targeted therapy of mantle cell lymphoma. Int J Mol Sci. 2019; 20: 4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lymphoma Study Group of Japanese Pathologists . The World Health Organization classification of malignant lymphomas in Japan: incidence of recently recognized entities. Pathol Int. 2000; 50: 696-702. [DOI] [PubMed] [Google Scholar]
- 3.Japanese Society of Hematology. JSH practical guidelines for hematological malignancies, 2018, Chapter IV Lymphoma. II Lymphoma. 4 Mantle cell lymphoma. Available at: http://www.jshem.or.jp/gui-hemali/2_4.html. Accessed March 9, 2020 [in Japanese].
- 4.Maruyama D, Nagai H, Fukuhara N, et al. Final analysis of a phase II study of ibrutinib in Japanese patients with relapsed/refractory mantle cell lymphoma. J Clin Exp Hematop. 2019; 59: 98-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang ML, Blum KA, Martin P, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015; 126: 739-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013; 369: 507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goyal RK, Nagar SP, Kabadi SM, et al. Adverse events, resource use, and economic burden associated with mantle cell lymphoma: a real-world assessment of privately insured patients in the United States. Leuk Lymphoma. 2019; 60: 955-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kabadi SM, Near A, Wada K, Burudpakdee C. Treatment patterns, adverse events, healthcare resource use and costs among commercially insured patients with mantle cell lymphoma in the United States. Cancer Med. 2019; 8: 7174-7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith A, Roman E, Appleton S, et al. Impact of novel therapies for mantle cell lymphoma in the real world setting: a report from the UK’s Haematological Malignancy Research Network (HMRN). Br J Haematol. 2018; 181: 215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrahamsson A, Albertsson-Lindblad A, Brown PN, et al. Real world data on primary treatment for mantle cell lymphoma: a Nordic Lymphoma Group observational study. Blood. 2014; 124: 1288-1295. [DOI] [PubMed] [Google Scholar]
- 11.Abrisqueta P, Scott DW, Slack GW, et al. Observation as the initial management strategy in patients with mantle cell lymphoma. Ann Oncol. 2017; 28: 2489-2495. [DOI] [PubMed] [Google Scholar]
- 12.Cohen JB, Han X, Jemal A, Ward EM, Flowers CR. Deferred therapy is associated with improved overall survival in patients with newly diagnosed mantle cell lymphoma. Cancer. 2016; 122: 2356-2363. [DOI] [PubMed] [Google Scholar]
- 13.Ruan J, Martin P. Which patients with mantle cell lymphoma do not need aggressive therapy. Curr Hematol Malig Rep. 2016; 11: 234-240. [DOI] [PubMed] [Google Scholar]
- 14.Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol. 2009; 27: 1209-1213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.