Abstract
A juvenile form of paroxysmal dyskinesia segregated in the Markiesje dog breed. Affected pups exhibited clinical signs of a severe tetraparesis, dystonia, cramping and falling over when trying to walk. In most cases, the presentation deteriorated within weeks and elective euthanasia was performed. Pedigree analysis indicated autosomal recessive inheritance. Genome-wide association and homozygosity mapping of 5 affected dogs from 3 litters identified the associated locus on chromosome 31 in the region of SOD1. The DNA sequence analysis of SOD1 showed that the patients were homozygous for a frameshift mutation in the fourth codon. None of the other analyzed dogs of the breed was homozygous for the mutation, indicating full penetrance of the genetic defect. Mutations in SOD1 are known to cause recessive degenerative myelopathy in middle-aged dogs with low penetrance and dominant amyotrophic lateral sclerosis in humans with variable age of onset. Our findings are similar to recent observations in human patients that a loss of function mutation in SOD1 leads to a juvenile neurologic disease distinct from amyotrophic lateral sclerosis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00439-021-02271-6.
Introduction
Paroxysmal dyskinesias (PDs) are a group of diseases of the central nervous system that produce abnormal movements occurring involuntary and in episodes (Urkasemsin et al. 2014; Lowrie et al. 2017). The duration of these episodes can vary from seconds to hours with a sudden start and ending (Lowrie et al. 2017). The observed abnormal and involuntary movements can comprise athetosis, dystonia, ballismus/hemi-ballismus and chorea (Lowrie et al. 2017). Between the episodes, motor functions are normal and there are no neurological deficits, despite the visible dramatic clinical signs during an attack. Paroxysmal dyskinesias have been described in several dog breeds with variable clinical presentations and outcomes (Packer et al. 2010; Black et al. 2014; Gill et al. 2012; Lowrie et al. 2016; Kolicheski et al. 2017; Polidoro et al. 2020). Breed predisposition points to an inherited origin and causative mutations have been found in BCAN in the Cavalier King Charles Spaniel with episodic falling (OMIA 001592–9615) and in PIGN in Soft Coated Wheaten Terriers with PD (OMIA 002084–9615; Gill et al. 2012; Kolicheski et al. 2016). In Malteser dogs and Border terriers, PD is most likely induced by gluten and hence called paroxysmal gluten-sensitive dyskinesia (Stassen et al. 2017; Lowrie et al. 2018; Polidoro et al. 2020). The Markiesje is a small Dutch breed of middle-sized dogs that is only recognized by the Dutch Kennel Club. Since 2003 occasionally litters were born with pups that exhibited clinical signs of a severe, rapidly progressing PD. Any effort to move seemed to trigger an episode. Here we describe the clinical presentation of the disorder and the molecular genetic study to identify its origin.
Materials and methods
Dogs
Routine physical and neurological examinations by a board-certified European Specialist in Veterinary Neurology (PJJM) were performed on 13 Markiesje pups that were presented to the Utrecht University Clinic for Companion Animals with signs of a paroxysmal dyskinesia. Additional examinations pertained to hematology, clinical chemistry, urinalysis, and radiography of the vertebral column. If possible an EMG (electromyogram), tensilon test and additional blood and urine examination (for myasthenia gravis, toxoplasmosis, neospora, and organic acid analysis) was performed as well. The tensilon test consists of the intravenous injection of the drug edrophonium that blocks the degradation of acetylcholine in the neuromuscular junction. If the clinical signs diminish or vanish, the disease most likely is myasthenia gravis.
If the owners elected euthanasia a post-mortem examination was performed by a board-certified European Specialist in Veterinary Pathology (WB). DNA was available of 6 affected Markiesje dogs from 4 litters, 8 unaffected siblings, and 7 parents and 1 grandparent (Supplementary Figure S1). Unrelated dogs from the same breed were sampled at a club match and samples were submitted for DNA testing by individual owners. DNA was isolated from EDTA blood samples using a Chemagic MSM1 robot (Perkin Elmer).
Molecular genetic analysis
Five affected and 18 unrelated Markiesje dogs were genotyped with the Illumina CanineHD SNP array with over 170,000 SNPs. Genome-wide association study was performed with PLINK version 1.07 software (Purcell et al. 2007). The region that displayed strong association was inspected in the output file allelicassoc.assoc for shared homozygosity by the cases. Four SNPs were selected from the region to genotype a sixth patient, healthy siblings, parents and a grandparent. The DNA sequences of oligonucleotides used for PCR and chain termination reactions are listed in Supplementary Table S1. Coincidently, additional informative SNPs were located on two of the PCR fragments, at CanFam3.1 positions g.31:25160489 and g.31:25644241. The PCR conditions and procedures for DNA sequence analysis were as described elsewhere (Wu et al. 2020a).
Enrichment of protein-coding gene exons of the chromosome region of interest from genomic DNA and library preparation for next-generation sequencing (NGS) was as described (Wu et al. 2020b). The positions of the exon sequences used for enrichment are listed in Supplementary Table S2. The NGS was performed on a MiSeq according to the protocol of the manufacturer (Illumina). Reads of 151 bp were collected as mate pairs. The NGS data quality control was as described (Wu et al. 2020b) and variants were annotated using SIFT (Kumar et al. 2009).
The gene of interest, SOD1, was further analyzed by conventional DNA sequencing using BigDye v.3.1 of Applied Biosystems (Thermo Fisher). The exons of the gene were amplified and subsequently sequenced with primers described by others (Zeng et al. 2014). The DNA sequences were generated by a Genetic Analyzer 3130xL (Applied Biosystems) and analyzed with Seqman Pro 14 software of the Lasergene package (DNASTAR). LOD scores for linkage were calculated using Superlink version 1.7 (Fishelson and Geiger 2004).
Results
Clinical presentation
From 2003 to 2016, 14 young Markiesje dogs, born in 7 litters that consisted of a total of 36 pups, were presented with clinical signs of a severe tetraparesis, dystonia, and cramping. All cases but one were examined by one of us (PJJM). The first signs became manifest at an age of around 10 weeks. They fell over when the owners tried walking them (Supplementary Video S1). At rest, a physical and neurological examination did not reveal any abnormalities but when stressed or walked the pups started to show cramping and dystonic features of all legs. In all pups, routine hematology and clinical chemistry and urinalysis did not reveal any significant abnormalities. In two pups the complete vertebral column was radiographically examined, in four pups an EMG (electromyogram) performed and these did not reveal any abnormalities. On four pups a so-called tensilon test was performed that ruled out myasthenia gravis. In two pups the acetylcholinesterase receptor titer for myasthenia gravis was measured and was found not to be abnormal. In seven pups titers for toxoplasmosis and neospora revealed no increase. In three pups an additional organic acid analysis was performed, which did not reveal any abnormality. All 7 mothers of affected dogs and 10 siblings were examined and none of these displayed clinical signs of a movement disorder.
In attempts to alleviate the clinical signs, the pups were treated with drugs such as non-steroidal anti-inflammatory drugs, phenobarbital, phenytoin, benzodiazepines, acetazolamide, pyridostigmine, and corticosteroids, but none of the pups showed any improvement. Although at rest the pups showed limited clinical signs their ability to function normally was extremely limited and twelve owners elected euthanasia before the age of 3 months. Routine post-mortem examinations were possible in 8 of these 12 pups. These revealed no morphological abnormalities macroscopically. Histologically, the most consistent finding was mild, random Wallerian-like degeneration in the brain stem and spinal cord with mild denervation atrophy of the skeletal muscle. Two owners continued to take care of the affected pups and although they were severely handicapped, one dog lived for three years after diagnosis and the other for 10 years after diagnosis.
Gene mapping and analysis
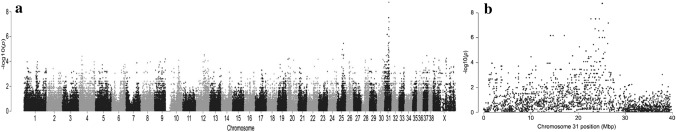
Markiesje dogs with PD were born from healthy parents that often were related within a limited number of generations (Supplementary Figure S1). Analysis of the pedigrees of all affected dogs suggested that the disease followed Mendelian inheritance and was autosomal recessive. The genome-wide association analysis of 5 affected Markiesje dogs and 18 unrelated controls of the same breed indicated the involvement of a region on canine chromosome 31 (Fig. 1). The p-value of 1.8 × 10–9 of the three top SNPs was 3 orders of magnitude smaller than that of the next best peak. The top SNPs were located between positions 25.1–25.2 Mbp of CFA31 (CanFam3.1). Inspection of the SNP allele frequencies in the group of affected dogs showed that these shared a region of homozygosity from positions 24.7–26.7 Mbp. A newly presented sixth patient and available siblings and parents were genotyped for six SNPs in the region (Supplementary Figure S1). Only the affected dogs were homozygous for the associated haplotype.
Fig. 1.
Genome-wide association study of paroxysmal dyskinesia in Markiesje dogs. a Manhattan plot of the SNP-data of 5 cases and 18 unrelated controls indicated the involvement of chromosome 31. b Zoom of chromosome 31 points to the region around position 25 Mbp
The 2 Mbp region contained few notable protein-coding genes including GRIK1, CLDN8, and CLDN17, 15 genes for keratin-associated proteins, TIAM1, SOD1, and SCAF4. Of these, only mutations in SOD1 were known for well-established neurologic phenotypes in humans and dogs: amyotrophic lateral sclerosis type 1 (ALS1, OMIM 105400) and progressive spastic tetraplegia and axial hypotonia (OMIM 618598), and degenerative myelopathy (DM, OMIA 000263–9615), respectively. We considered this gene, located at 26.5 Mbp of CFA31, the prime candidate gene for involvement in PD in the Markiesje dogs.
Targeted NGS analysis of the exons of protein-coding genes in the CFA31 region of 24.9–26.7 Mbp of 3 cases and 2 unrelated controls did not yield a variant as the likely cause of PD in Markiesje dogs (Supplementary Table S3). A predicted high impact variant was detected at position 31:g.26539786 in the annotated exon 1 of SOD1 of a control dog. However, this exon does not align with the reference cDNA sequence of canine SOD1. The proper exon 1 is located in a gap of the reference genome sequence and was, therefore, not included in the NGS analysis.
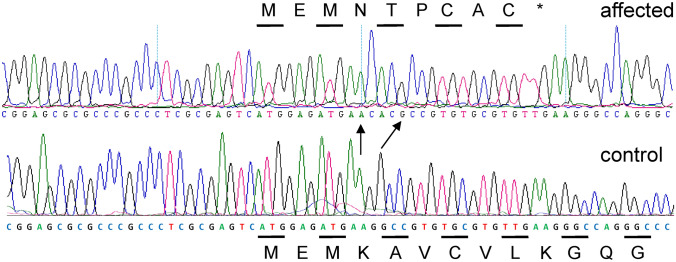
The coding DNA sequence of SOD1, including the correct exon 1 sequence, was analyzed by conventional techniques and we identified a frameshift mutation in the analyzed affected Markiesje dogs (Fig. 2). A G-nucleotide of the fourth codon of the gene is replaced by a CAC-trinucleotide. The shifted coding sequence runs into a stop codon at the tenth codon. The annotation of the indel mutation is NM001003035.1:c.12delinsCAC. The protein annotation is NP_001003035.1:p.Lys4Aspfs*6. The genomic annotation cannot be given because exon 1 of SOD1 is not represented in the reference genome CanFam3.1. Genotyping of available Markiesje dogs from the pedigree showed that only the affected dogs were homozygous for the mutation (Supplementary Figure S1). The LOD score for linkage between the mutation and PD in the pedigree of the six affected dogs was 4.5.
Fig. 2.
DNA sequence of the translation start region of exon 1 of SOD1 in Markiesje dogs with and without paroxysmal dyskinesia. In the affected dog, the first of two G-residues is replaced by the trinucleotide CAC. The arrows indicate unaltered A- and G-residues. The mutation leads to a frameshift at the 4th codon of the gene. The codons are indicated by alternating lines with the encoded amino acid in one letter code. *Denotes the TGA stop codon
Of the Markiesje dogs from the general population, 31 out of 184 dogs were heterozygous and none were homozygous for the SOD1 frameshift mutation, corresponding to an allele frequency of 0.08, which is not unusually high in dog genetics.
Discussion
We report a new inherited neurologic disorder that occurred in juvenile dogs of the Markiesje breed. Based on the clinical presentation, the disorder is classified as a paroxysmal dyskinesia. Never before has PD been described in pups of this age with such a poor clinical outcome. Typical characteristics of PDs are its sudden presentation, its sudden disappearance and the absence of autonomic signs. During the events, that can, in contrast with epilepsy, last for minutes to hours, all pups remained conscious and for this reason a (focal) epilepsy was ruled out. Most dogs with a PD have a low frequency of events and can be clinically managed rather reasonably. Dogs that show many PD events are treated accordingly and rarely euthanasia is elected. In contrast, in the affected Markiesje pups the PD was almost always present when the dogs were stressed or walked. For this reason, the possibility of disorders such as myopathies and myasthenia gravis were carefully examined and although the outcome was negative, the treatment was based on these disorders. None of the pups responded to the treatment, suggesting that this group of differential diagnosis was also not applicable.
Paroxysmal dyskinesias are by itself rarely lethal. Maybe this is also the case in this breed as two of the 14 pups were not euthanatized after diagnosis and these pups continued to live, although severely handicapped, for several years. For all these reasons we classify the neurological disorder in these pups as a PD. In human medicine, PDs are classified into Paroxysmal Kinesigenic Dyskinesia in which episodes are provoked by movements that occur suddenly; Paroxysmal Non-Kinesigenic Dyskinesia in which movements are not the cause of the episodes but alcohol, stress, caffeine and fatigue may be triggers, and Paroxysmal Exertion- Induced Dyskinesia with continuous exercise as precipitating factor (Lowrie et al. 2017). In veterinary medicine most PDs are either classified as a Paroxysmal Gluten-Sensitive Dyskinesia or as an unspecified Paroxysmal Dyskinesia. It is difficult to differentiate the disease in dogs further because often they show signs that warrant classification in each of the human types of PD. In the Markiesje breed one could argue that the disorder resembles a Paroxysmal Kinesigenic Dyskinesia.
We were able to identify the gene mutation that most likely causes the disease. The LOD score of 4.5 for linkage, well above the threshold value of 3, is proof for the involvement of the SOD1 gene region. The mutation identified in SOD1 leads to a frameshift after the third codon, effectively knocking out the gene. In dogs, two point mutations in SOD1 have been established as the cause of DM in a variety of breeds (Zeng et al. 2014). This disease is characterized by late-onset paralysis due to degeneration of white matter of the spinal cord. The degeneration is accompanied and probably triggered by aggregates of mutant SOD1 protein (Awano et al. 2009). In this respect, DM is a model for ALS1 in humans which is characterized by the death of motor neurons in the brain, brainstem and spinal cord due to SOD1 mutations, often with SOD1 aggregates. Most forms of ALS1 follow dominant inheritance, although recessive forms have also been described. In general, DM inherits recessively and the frequency of the causative E40K mutation of SOD1 can be quite high in some breeds. The penetrance of DM in dogs with the homozygous genotype is incomplete, however, and varies per breed (Awano et al. 2009; Zeng et al. 2014). Aggregate formation has been found in heterozygous dogs without clinical signs and affected heterozygous cases have been identified (Awano et al. 2009; Zeng et al. 2014). So, DM, such as ALS1, is caused by a gain of function mutation of SOD1, the function gained being aggregate formation (Awano et al. 2009). In contrast, PD in Markiesje dogs is caused by a loss of function mutation of SOD1 with full penetrance, and it presents as a distinct disease entity. The gene encodes superoxide dismutase 1, one of two scavengers of free superoxide radicals, which are damaging by nature.
Recently, two independent human case reports reached a similar conclusion of pleiotropy of the SOD1 gene due to distinct pathogenic effects of aggregate formation and loss of superoxide dismutase 1 activity. Two unrelated patients of Afghan descent displayed clinical signs of motor neuron deterioration at ages 6 and 9 months. The disease was termed progressive spastic tetraplegia and axial hypotonia (STAHP, OMIM 618598) and no similarities with ALS1 were recognized. (Andersen et al. 2019; Park et al. 2019). Both patients were homozygous for the same frameshift mutation in SOD1 after codon 112 and deficiency of SOD1 activity was demonstrated.
The phenotype of the SOD1 knockout Markiesje dogs is similar to that of the SOD1 deficient human patients. Both diseases manifest at an early age when the juveniles should start moving around. In dog and human patients there is a severe and progressive loss of motor abilities, tetraspasticity predominantly in the lower extremities, and dystonia (Park et al. 2019). The few fasciculations and myokymia observed in one of the human patients were not seen in the Markiesje dogs (Andersen et al. 2019). Cognitive functions seem to be spared.
It can be concluded that SOD1 mutations are associated with distinct neurologic disease entities in humans and dogs. Deficiency of SOD1 leads to early-onset movement disorders with full penetrance, while mutations that lead to SOD1 aggregation cause motor neuron death with variable and age-dependent penetrance.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was funded in part by a gift from the ‘Nederlandse Markiesjes Vereniging’. We are especially grateful to the owners of the dogs that participated in this study.
Funding
The research was supported in part by the Dutch breed club ‘Nederlandse Markiesjes Vereniging’.
Data availability
The NGS data have been deposited in the GEO database and are accessible through GEO Series accession number GSE166712 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE166712).
Declarations
Conflict of interest
The results have been used to develop a DNA test that is offered to owners and breeders of Maskiesje dogs for a fee. The test is performed at the research laboratory of the Department of Clinical Sciences, Faculty of Veterinary Medicine, Utrecht University. The test results are interpreted by the author PAL.
Ethical approval
All dogs were privately owned and included in the study with the informed consent of their owners. Thus, we complied with the conditions set forth in the Dutch ‘Wet op de Uitoefening van de Diergeneeskunde’ (Law on the Practice of Veterinary Medicine) of 21 March 1990 and approval by an Animal Ethics Committee for the use of samples was not necessary.
Consent to participate
All dogs were privately owned and included in the study with the informed consent of their owners.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Andersen PM, Nordström U, Tsiakas K, Johannsen J, Volk AE, Bierhals T, Zetterström P, Marklund SL, Hempel M, Santer R. Phenotype in an infant with SOD1 homozygous truncating mutation. N Engl J Med. 2019;381:486–488. doi: 10.1056/NEJMc1905039. [DOI] [PubMed] [Google Scholar]
- Awano T, Johnson GS, Wade CM, Katz ML, Johnson GC, Taylor JF, Perloski M, Biagi T, Baranowska I, Long S, March PA, Olby NJ, Shelton GD, Khan S, O'Brien DP, Lindblad-Toh K, Coates JR. Genome-wide association analysis reveals a SOD1 mutation in canine degenerative myelopathy that resembles amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2009;106:2794–2799. doi: 10.1073/pnas.0812297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black V, Garosi L, Lowrie M, Harvey RJ, Gale J. Phenotypic characterisation of canine epileptoid cramping syndrome in the border terrier. J Small Anim Pract. 2014;55:102–107. doi: 10.1111/jsap.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland DW, Rothstein JD. From charcot to lou gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- Fishelson M, Geiger D. Optimizing exact genetic linkage computations. J Comput Biol. 2004;11:263–275. doi: 10.1089/1066527041410409. [DOI] [PubMed] [Google Scholar]
- Gill JL, Tsai KL, Krey C, Noorai RE, Vanbellinghen JF, Garosi LS, Shelton GD, Clark LA, Harvey RJ. A canine BCAN microdeletion associated with episodic falling syndrome. Neurobiol Dis. 2012;45:130–136. doi: 10.1016/j.nbd.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolicheski AL, Johnson GS, Mhlanga-Mutangadura T, Taylor JF, Schnabel RD, Kinoshita T, Murakami Y, O'Brien DP. A homozygous PIGN missense mutation in soft-coated wheaten terriers with a canine paroxysmal dyskinesia. Neurogenetics. 2017;18:39–47. doi: 10.1007/s10048-016-0502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- Lowrie M, Garosi L. Natural history of canine paroxysmal movement disorders in labrador retrievers and jack russell terriers. Vet J. 2016;213:33–37. doi: 10.1016/j.tvjl.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Lowrie M, Garosi L. Classification of involuntary movements in dogs: paroxysmal dyskinesias. Vet J. 2017;220:65–71. doi: 10.1016/j.tvjl.2016.12.017. [DOI] [PubMed] [Google Scholar]
- Lowrie M, Garden OA, Hadjivassiliou M, Sanders DS, Powell R, Garosi L. Characterization of paroxysmal gluten-sensitive dyskinesia in border terriers using serological markers. J Vet Intern Med. 2018;32:775–781. doi: 10.1111/jvim.15038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer RA, Patterson EE, Taylor JF, Coates JR, Schnabel RD, O'Brien DP. Characterization and mode of inheritance of a paroxysmal dyskinesia in chinook dogs. J Vet Intern Med. 2010;24:1305–1313. doi: 10.1111/j.1939-1676.2010.0629.x. [DOI] [PubMed] [Google Scholar]
- Park JH, Elpers C, Reunert J, McCormick ML, Mohr J, Biskup S, Schwartz O, Rust S, Grüneberg M, Seelhöfer A, Schara U, Boltshauser E, Spitz DR, Marquardt T. SOD1 deficiency: a novel syndrome distinct from amyotrophic lateral sclerosis. Brain. 2019;142:2230–2237. doi: 10.1093/brain/awz182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polidoro D, Van Ham L, Santens P, Cornelis I, Charalambous M, Broeckx BJG, Bhatti SFM. Phenotypic characterization of paroxysmal dyskinesia in Maltese dogs. J Vet Intern Med. 2020;34:1541–1546. doi: 10.1111/jvim.15804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, Sham PC. PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassen QEM, Koskinen LLE, van Steenbeek FG, Seppälä EH, Jokinen TS, Prins PGM, Bok HGJ, Zandvliet MMJM, Vos-Loohuis M, Leegwater PAJ, Lohi H. Paroxysmal dyskinesia in border terriers: clinical, epidemiological, and genetic investigations. J Vet Intern Med. 2017;31:1123–1131. doi: 10.1111/jvim.14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urkasemsin G, Olby NJ. Canine paroxysmal movement disorders. Vet Clin North Am Small Anim Pract. 2014;44:1091–1102. doi: 10.1016/j.cvsm.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Wu X, Mandigers PJJ, Fieten H, Leegwater PA. Evaluation of COMMD1 in copper toxicosis in Labrador retrievers and Dobermans. Vet J. 2020;265:105561. doi: 10.1016/j.tvjl.2020.105561. [DOI] [PubMed] [Google Scholar]
- Wu X, den Boer ER, Vos-Loohuis M, van Steenbeek FG, Monroe GR, Nijman IJ, Leegwater PAJ, Fieten H. Investigation of genetic modifiers of copper toxicosis in Labrador retrievers. Life (Basel) 2020;10:266. doi: 10.3390/life10110266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng R, Coates JR, Johnson GC, Hansen L, Awano T, Kolicheski A, Ivansson E, Perloski M, Lindblad-Toh K, O'Brien DP, Guo J, Katz ML, Johnson GS. Breed distribution of SOD1 alleles previously associated with canine degenerative myelopathy. J Vet Intern Med. 2014;28:515–521. doi: 10.1111/jvim.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The NGS data have been deposited in the GEO database and are accessible through GEO Series accession number GSE166712 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE166712).