Abstract
In many types of apoptosis, the proapoptotic protein Bax undergoes a change in conformation at the level of the mitochondria. This event always precedes the release of mitochondrial cytochrome c, which, in the cytosol, activates caspases through binding to Apaf-1. The mechanisms by which Bax triggers cytochrome c release are unknown. Here we show that following binding to the BH3-domain-only proapoptotic protein Bid, Bax oligomerizes and then integrates in the outer mitochondrial membrane, where it triggers cytochrome c release. Bax mitochondrial membrane insertion triggered by Bid may represent a key step in pathways leading to apoptosis.
Bcl-2 family members play a key role in processes underlying programmed cell death or apoptosis (17, 25, 36). The Bcl-2 family is composed of both antiapoptotic (Bcl-2, Bcl-xL, Bcl-w, Mcl-1, A1, NR-13, BHRF1, LMW5-HL, ORF16, KS-Bcl-2, E1B-19K, and CED-9) and proapoptotic (Bax, Bak, Bok, Bik, Blk, Hrk, BNIP3, BimL, Bad, Bid, and EGL-1) molecules (1). These proteins can form homo- and heterodimers that involve amino acid sequences known as Bcl-2 homology (BH) domains. Four of these domains (BH1 to BH4) have been identified (20, 25, 36, 44). The BH3 domain of the proapoptotic members appears to be required for the interaction between anti- and proapoptotic molecules (5).
The principal site of action of some of the Bcl-2 family members appears to be the mitochondrion (9, 19, 21, 37, 41). Mitochondria play a major role in many types of apoptosis. In particular, this organelle releases apoptosis-inducing factor (40) and cytochrome c (3, 9, 19, 21, 37, 45). The latter triggers caspase 9 activation through Apaf-1–caspase 9 complex formation (26). Bcl-2 family members play a key role in regulating cytochrome c release. While Bcl-2 and Bcl-xL suppress cytochrome c release (3, 21, 45), Bax stimulates this event both in vitro in isolated mitochondria and in intact cells following heterologous expression (3, 9, 19). The mechanisms by which these proteins perform their function are currently unknown.
The three-dimensional structures of Bcl-xL (34) and Bid (6, 31) revealed structural similarities between these proteins and the channel-forming domains of the bacterial toxins colicins and diphtheria toxins. Consistent with such structural similarity, some members of this family including Bax, Bcl-2, and Bcl-xL are also able to form ion channels in synthetic lipid membranes (2, 33, 38, 39). The channel-forming activity of these proteins has not yet been demonstrated in vivo. However, it is now clear that, at least during apoptosis, these proteins are associated with intracellular membranes, in particular with mitochondrial membranes. The events that trigger membrane association of the Bcl-2 family members are still poorly understood, although it is probable that, like colicins and diphtheria toxins, Bcl-2 family members may have to undergo a change in conformation before undertaking membrane insertion.
We have previously shown that at an early stage of apoptosis in cerebellar granule cells deprived of serum and potassium or in HeLa cells exposed to staurosporine, Bax undergoes a change in conformation (8). Similarly, the structure of Bak, another proapoptotic member of the Bcl-2 family, was reported to undergo conformational change during various types of apoptosis well before cytochrome c release from mitochondria (8, 12). For both Bax and Bak, this change in conformation appears to expose the N-terminal domain, which otherwise is cryptic and nonaccessible to antibodies. Interestingly, Bid, a BH3-domain-only protein which interacts with Bax, was able to trigger this conformational change in Bax (8). The goal of our present experiments was to understand the role of the switch in Bax conformation and how this key event could be related to cytochrome c release from mitochondria. Here we report that following Bid-induced conformational change, Bax oligomerizes and inserts tightly within the outer mitochondrial membrane without a requirement for any proteolytic event. The integration of Bax in the outer mitochondrial membrane is followed by cytochrome c release, which, in contrast to Bax membrane integration, is highly dependent on magnesium.
MATERIALS AND METHODS
Cell culture.
HeLa cells and the stable HeLa cell line that constitutively overexpresses Bcl-2 (HeLa–Bcl-2) (10) were cultured in a 1:1 mixture of basal Iscove medium and Ham's F-12 medium (Seromed) supplemented with 10% fetal calf serum and 2 mM l-glutamine.
Subcellular fractionation.
At different times after the induction of apoptosis, HeLa cells were harvested in isotonic mitochondrial buffer (MB) (210 mM mannitol, 70 mM sucrose, 1 mM EDTA, 10 mM HEPES [pH 7.5]) supplemented with complete protease inhibitor cocktail (Boehringer Mannheim). The cells were broken by six passages through a 25G1 0.5- by 25-mm needle fitted on a 5-ml syringe, and the suspension was centrifuged at 2,000 × g in an Eppendorf centrifuge at 4°C. This procedure was repeated twice, and supernatants from each step were pooled before being subjected to centrifugation at 13,000 × g at 4°C for 10 min. The supernatant was further centrifuged at 600,000 × g for 10 min at 4°C to yield the light membrane pellet (not analyzed) and the final soluble fraction (S100). The heavy membrane material was pooled and resuspended in MB-EGTA (MB with 0.5 mM EGTA instead of EDTA) and centrifuged at 500 × g for 3 min at 4°C to eliminate residual nuclei. The resulting supernatant was centrifuged at 10,000 × g for 10 min at 4°C to further purify the mitochondrial fraction. The protein concentration was estimated by the method of Bradford (4) with bovine serum albumin as the standard.
In vitro assay for Bax insertion and cytochrome c release.
Mitochondria (100 μg of proteins) were incubated in the presence or absence of various recombinant proteins in 100 μl of MBC buffer (MB with EGTA supplemented with 4 mM MgCl2, 5 mM Na2HPO4, 5 mM succinate, and 5 μM rotenone) for 15 min at 30°C and then centrifuged for 5 min at 13,000 × g and 4°C. The supernatants and the pellets were used for the determination of cytochrome c release. For alkali extraction, the mitochondrial pellets were resuspended (1 mg of protein/ml) in freshly prepared 0.1 M Na2CO3 (pH 11.5) and incubated for 20 min on ice. The membranes were then pelleted by centrifugation (600,000 × g for 20 min at 4°C). Mitochondrial membrane pellets corresponding to 10 μg of proteins (the alkali-resistant fractions) and the corresponding volume of supernatants (the alkali-sensitive fractions) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 4 to 20% Tris-Gly gels (NOVEX). Their respective contents of cytochrome c and Bax were estimated by Western blotting with a polyclonal anti-cytochrome c antibody (dilution 1:2,500) or a polyclonal antibody directed against Bax (Upstate Biotechnology). Equal loading of the mitochondrial pellet was verified by using an antibody either against cytochrome c oxidase subunit IV (Cox IV) or against cytochrome c oxidase subunit II (Cox II) (both from Molecular Probes) or an antibody against the voltage-dependent anion channel VDAC (Calbiochem). Antigen-antibody complexes were detected by using horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G and enhanced chemiluminescence detection reagents.
Cross-linking.
The mitochondria (0.5 mg of proteins) were incubated with the recombinant proteins, then pelleted by centrifugation, and resuspended in MB-EGTA, and disuccinimidyl subernate (DSS) (in dimethyl sulfoxide DMSO; Pierce) or bis(sulfosuccinimidyl) subernate (BS3) (in 5 mM sodium citrate buffer [pH 5.0]; Pierce) was added from a 10-fold stock solution to a final concentration of 2 mM. After incubation for 30 min at room temperature, the cross-linker was quenched by the addition of 1 M Tris-HCl (pH 7.5) to a final concentration of 20 mM. After quenching, the membranes were dissolved in RIPA buffer and cleared by centrifugation at 12,000 × g. The lysate was immunoprecipitated with anti-Bax 2D2 monoclonal antibody (Genzyme) and then analyzed by Western blotting with the polyclonal anti-Bax antibody.
Digitonin treatment of mitochondria.
Following incubation with Bid, mitochondria (100 μg) were pelleted, dissolved in 100 μl of digitonin (1.2 mg/ml), and incubated for 25 min on ice. The mitoplasts were pelleted by centrifugation and dissolved in 100 μl of RIPA buffer. The fractions were analyzed by Western blotting as mentioned above.
Production of recombinant proteins.
His-tagged Bid, Bid mutants, human Bcl-xL, and human mutant Bcl-xL (Bcl-xLm: G138→A), both Bcl-xL protins lacking 24 amino acids at the COOH terminus, were produced as described previously (8).
RESULTS
Bax integration into the mitochondrial membrane in staurosporine-treated HeLa cells.
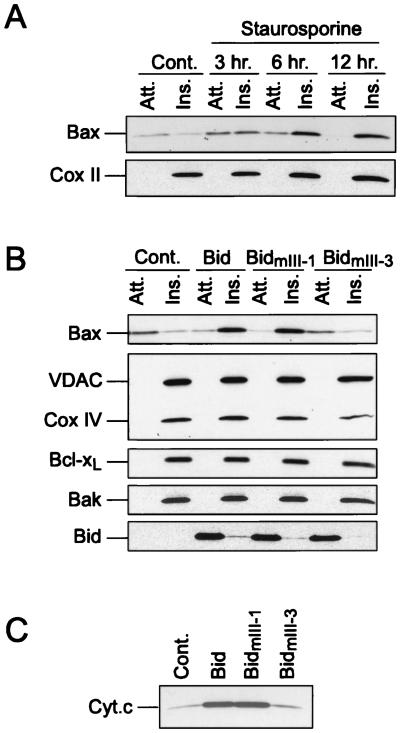
In many cultured cells, Bax is found in both the cytosolic and mitochondrial fractions (15, 43; unpublished observation). To test whether mitochondrial Bax was integrated in the mitochondrial membranes, we performed an alkali extraction of proteins from mitochondria isolated from HeLa cells. Bcl-xL, Bak, and Cox II were analyzed in parallel. In contrast to Bcl-xL or Bak, which were resistant to the alkali extraction, most Bax was lost during this treatment, indicating that under normal conditions Bax is loosely attached to mitochondria while most Bcl-xL and Bak proteins are inserted in the membrane (Fig. 1A and B). When HeLa cells were treated with staurosporine, Bax became resistant to alkali extraction in a time-dependent manner. After 12 h of incubation with staurosporine, almost all the Bax was found to be inserted in the mitochondrial membranes (Fig. 1A). These results, in agreement with previous results reported by Goping et al. (11), demonstrate that in response to a death stimulus, Bax becomes inserted in the mitochondrial membranes.
FIG. 1.
Bax integration into the mitochondrial membranes during apoptosis and after Bid treatment. (A) Mitochondria from HeLa cells cultured in the absence or presence of 1 μM staurosporine for increasing times were isolated and treated with 0.1 M Na2CO3 to produce alkali-sensitive (Att [attached]) and -resistant (Ins [inserted]) fractions. Both fractions were analyzed by Western blotting for the presence of Bax. Cox II was used as a gel-loading control. (B) Mitochondria from HeLa cells were incubated with 100 nM recombinant wild-type Bid and two Bid mutants (BidmIII-1 and BidmIII-3) for 15 min at 30°C, recovered by centrifugation, and treated with 0.1 M Na2CO3 as above. Various proteins were analyzed by Western blotting in alkali-sensitive (Att.) and -resistant fractions (Ins.). (C) Cytochrome c was analyzed by Western blotting in the mitochondrial suspension following incubation with wild-type and mutant Bid.
The major objective of this study was to determine the mechanisms that are responsible for Bax insertion in mitochondrial membranes. We reported previously that during staurosporine-triggered apoptosis of HeLa cells, mitochondrial Bax undergoes a change in conformation, rendering its N terminus accessible to antibodies (8). This event was accompanied by a release of cytochrome c. Moreover, we reported that Bid, which translocates to mitochondria during apoptosis, was able to trigger a change in Bax conformation when added directly to isolated mitochondria. Bid was therefore a likely candidate as the protein responsible for driving the Bax integration into the mitochondrial membrane. Figure 1B shows that addition of 100 nM Bid to mitochondria isolated from HeLa cells rendered Bax resistant to alkali extraction, indicating that it had undergone membrane integration. Similar results were obtained when mitochondria were incubated in the presence of 100 nM Bid and 100 μM z-VAD-fmk, a broad-spectrum caspase peptide inhibitor, indicating that Bid-induced Bax membrane insertion was independent of caspase activation (results not shown). The mitochondrial levels of Bcl-xL, Bak, Cox IV, and VDAC, all membrane-integrated proteins, did not change following Bid treatment (Fig. 1B). Bid association with the mitochondria remained sensitive to alkali treatment, suggesting that in contrast to Bax, this protein does not itself integrate into the mitochondrial membranes (Fig. 1B).
Bid interacts with other Bcl-2 family members via its BH3 domain (42). To determine if binding of Bid to Bax is a prerequisite for Bax insertion, two Bid BH3 mutants with selectively lowered affinity for either Bax (BidmIII-3: G94→A) (8, 42) or Bcl-2 (BidmIII-1: M97D98→AA) (8, 42) were tested. While BidmIII-1 was as effective as wild-type Bid, BidmIII-3 was unable to stimulate Bax insertion (Fig. 1B) and cytochrome c release from mitochondria (Fig. 1C). Together, these results strongly indicate that Bid induces the insertion of Bax by interacting directly with Bax.
Bax integration in the outer mitochondrial membrane.
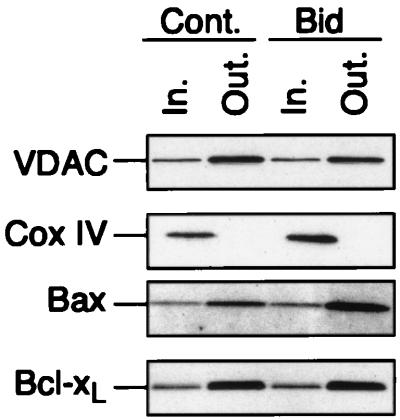
We next tested whether Bax inserts into the outer or the inner mitochondrial membrane by using digitonin to selectively dissolve the outer mitochondrial membrane. VDAC and Cox IV, which are present in the outer and inner mitochondrial membranes, respectively, were used to set up the conditions for optimal extraction. In the presence of 1.2 mg of digitonin/mg of protein, mitochondria from HeLa cells lost most of the VDAC protein while Cox IV remained attached to the mitochondria, indicating conditions for selective extraction of proteins from the outer mitochondrial membrane (Fig. 2). Like VDAC, Bax was found to be sensitive to digitonin extraction, confirming that in nonapoptotic cells Bax is attached to the outer mitochondrial membrane. A small amount of Bax remained insensitive to digitonin extraction, which could reflect Bax present at contact sites, although we cannot exclude the possibility that a minor portion of the protein is also localized in the inner mitochondrial membrane. Importantly, however, the fraction of digitonin-resistant Bax was unaltered following incubation with Bid for 15 min. In contrast, an increase in the amount of digitonin-sensitive Bax was detected (Fig. 2). The increase in Bax levels detected in the presence of Bid reflects the fact that Bax becomes tightly attached to mitochondrial membranes, while in the absence of Bid, substantial amounts of Bax can be easily lost from mitochondria during repetitive washes. Bcl-xL was also present mainly in the outer mitochondrial membrane, and its distribution remained unchanged following treatment with Bid (Fig. 2). We conclude from these experiments that following its interaction with Bid, Bax integrates into the outer mitochondrial membrane.
FIG. 2.
Bid induces the insertion of Bax into the outer mitochondrial membrane. Isolated mitochondria (100 μg) from HeLa cells were incubated with 100 nM Bid for 15 min at 30°C and treated with digitonin (1.2 mg/ml) for 25 min at 4°C. The digitonin-sensitive (Out. [outer mitochondrial membrane]) and resistant (In. [inner mitochondrial membrane]) fractions were analyzed by Western blotting for the presence of VDAC, Cox IV, Bax, and Bcl-xL.
Bax dimerization.
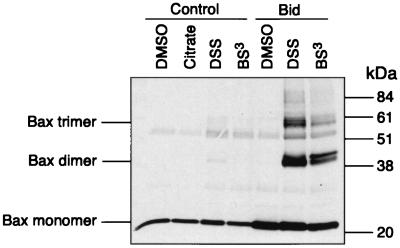
It was reported previously that Bax is present in a monomeric form in the cytoplasm (16) and that dimerization is a prerequisite for mitochondrial translocation (13). This prompted us to test whether Bid was able to trigger Bax dimerization and whether this precedes membrane integration. Intact mitochondria were treated with either the membrane-permeable DSS or the non-membrane-permeable BS3 cross-linking agents. In the absence of Bid, Bax was detected as a monomer. However, in the presence of 100 nM Bid, two Bax-immunoreactive bands of approximately 40 and 60 kDa were clearly detectable (Fig. 3). These are likely to correspond either to Bax homodimers and trimers or to Bax heterodimers. We also detected an additional Bax-immunoreactive band running at ∼40 kDa that could correspond to a Bax monomer linked to an unknown protein (Fig. 3). Similar results were obtained with two different anti-Bax antibodies. No immunoreactivity of these protein bands was detected with antibodies directed against Bcl-2, Bcl-xL, Bag-1, Bid, or VDAC.
FIG. 3.
Bid induces Bax oligomerization. Isolated mitochondria from HeLa cells were incubated with 1 μM Bid for 15 min at 30°C, and the mitochondrial pellet was treated with two different cross-linkers as described in Materials and Methods. After cross-linking, the mixture was immunoprecipitated with anti-Bax monoclonal antibody and analyzed by Western blotting with an anti-Bax polyclonal antibody.
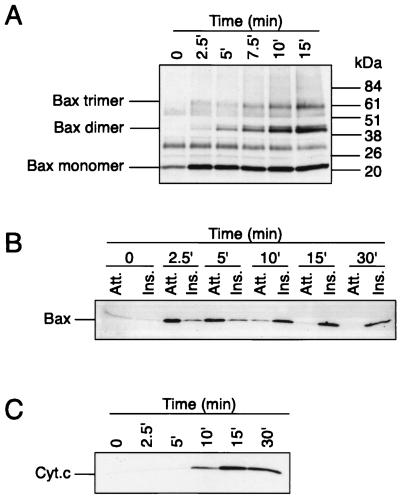
We next examined the temporal relationship between Bax oligomerization, its membrane insertion, and mitochondrial cytochrome c release. Figure 4A shows that upon addition of Bid to isolated mitochondria, Bax dimers and trimers were observed within 2.5 to 5 min and their levels peaked at 10 min. Importantly, Bax oligomerization preceded Bax insertion into the membrane, which appeared evident only between 5 and 10 min after Bid addition (Fig. 4B). Release of cytochrome c from mitochondria also occurred between 5 and 10 min after Bid addition, although maximal levels were not observed until after 15 min (Fig. 4C). These results suggest that Bax dimerization (or oligomerization) precedes its membrane integration and the efflux of cytochrome c from mitochondria.
FIG. 4.
Time course study of Bax oligomerization, Bax membrane insertion, and cytochrome c release after addition of Bid to isolated mitochondria. Isolated mitochondria from HeLa cells were incubated with recombinant Bid at 30°C for increasing times and analyzed for Bax oligomerization (A), Bax membrane insertion (B), and cytochrome c release (C). Att., attached (alkali sensitive); Ins., inserted (alkali resistant); Cyt.c, cytochrome c.
Regulation of Bid-induced Bax integration in the outer mitochondrial membrane by Bcl-xL and Bcl-2.
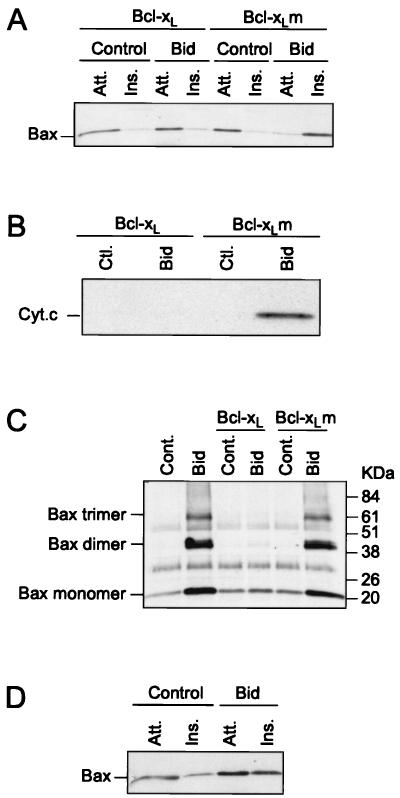
We have shown previously that Bcl-2 and Bcl-xL are able to inhibit the change in conformation of Bax in cells exposed to an apoptotic stimulus or following the addition of Bid to isolated mitochondria. Here we tested whether Bcl-xL could prevent Bid-induced Bax oligomerization and insertion into the membrane. We found that in the presence of Bcl-xL, Bid was unable to trigger Bax membrane integration, cytochrome c release, or Bax oligomerization (Fig. 5A to C). In contrast, a mutant of Bcl-xL (Bcl-xLm), which fails to bind Bax, did not inhibit these sequential events. Bid was also unable to trigger Bax integration when added to mitochondria from HeLa cells overexpressing Bcl-2 (Fig. 5D).
FIG. 5.
Bcl-xL and Bcl-2 inhibit Bid-induced oligomerization and insertion of Bax into the outer mitochondrial membrane. (A to C) Mitochondria isolated from HeLa cells were incubated with 100 nM Bid in the presence of 1 μM recombinant Bcl-xL or Bcl-xLm at 30°C for 15 min. Mitochondria were used to study Bax insertion into the outer mitochondrial membrane (A), cytochrome c release (B), and Bax dimerization (C). (D) Mitochondria from HeLa cells overexpressing Bcl-2 were isolated and incubated with 100 nM Bid at 30°C for 15 min and used to analyze Bax insertion into the outer mitochondrial membrane. Att., attached (alkali sensitive); Ins., inserted (alkali resistant); Ctl., Cont., control; Cyt.c, cytochrome c.
Membrane insertion of Bax without cytochrome c release in the absence of magnesium ions.
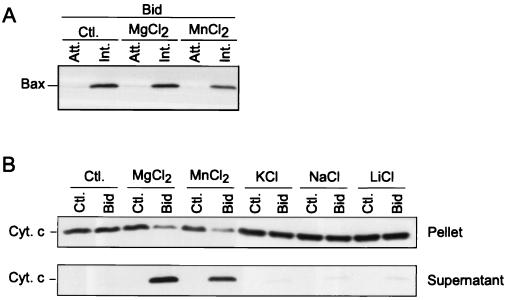
We have reported previously that the ability of Bax to trigger cytochrome c release from mitochondria was highly dependent on magnesium ions but independent of the opening of the permeability transition pore (9). We have now extended these observations by testing whether magnesium is important for membrane insertion of Bax. Figure 6A shows that Bax was resistant to alkali extraction following Bid addition to mitochondria in both the presence and absence of 2.5 mM Mg2+, indicating that Bax integration in the membrane occurs independently of Mg2+. However, only mitochondria incubated with Bid in the presence of 2.5 mM Mg2+ were found to release cytochrome c (Fig. 6B). Similar results were obtained with 2.5 mM MnCl2, which can substitute for MgCl2 in many cases (Fig. 6). However, other ions such as sodium, potassium, or lithium, all tested at 5 mM, were much less effective (Fig. 6B). We conclude that the presence of magnesium is important for Bax-induced cytochrome c release only after Bax is inserted in the mitochondria.
FIG. 6.
In contrast to cytochrome c release, Bid-induced insertion of Bax does not require the presence of Mg2+. (A) Mitochondria from HeLa cells were incubated with 100 nM Bid for 15 min at 30°C in the presence or absence of 2.5 mM MgCl2 or MnCl2 before analysis of Bax insertion into membranes. (B) Mitochondria were incubated in the presence or absence of 100 nM Bid and various salts including MgCl2 (2.5 mM), MnCl2 (2.5 mM), KCl (5 mM), NaCl (5 mM), and LiCl (5 mM). Both supernatants and mitochondrial pellets were analyzed for cytochrome c release. Ctl., control; Att., attached (alkali sensitive); Ins., inserted (alkali resistant); Cyt. c, cytochrome c.
DISCUSSION
Bax, a proapoptotic protein that is absolutely required for apoptosis in many neuronal cell types and in ovarian follicles (7, 32), exerts at least part of its activity by triggering cytochrome c release from mitochondria (9, 19, 21, 37, 41). The mechanisms by which Bax stimulates cytochrome c efflux are still unclear. In previous reports, several events occurring during apoptosis at the level of the Bax protein, including translocation to mitochondria (15, 43), change in conformation (8), dimerization (11, 13), and membrane integration (11, 13), have been described. Despite this, the mechanisms responsible for Bax insertion into the mitochondrial membrane and the localization of Bax within mitochondria are still unclear. Here we show that Bid, a BH3-only Bax-interacting protein, is able to trigger Bax integration in the outer mitochondrial membrane. We have reported previously that Bid is able to induce a change in Bax conformation leading to the exposure of its N-terminal domain. Interestingly, the N-terminal domain of Bax exerts a repressing activity on the targeting of Bax to mitochondrial membranes, possibly by interfering with the hydrophobic C-terminal membrane-anchoring domain (11). A Bid-induced change in Bax conformation may therefore represent the first of a series of events leading to Bax insertion into membranes. Bax dimerization appears to be another critical event for Bax integration in membranes, since enforced dimers of Bax specifically target mitochondria and trigger some mitochondrial dysfunction (13). However, in this study, the Bax-enforced dimers failed to trigger cytochrome c release from mitochondria, suggesting that Bax dimers may not represent the correct quaternary structure either for membrane insertion or for cytochrome c release (13). Consistent with the data reported by Gross et al. (13), our results suggest that active Bax homodimers or oligomers form following interaction with Bid. However, we cannot exclude the possibility that Bax also forms large complexes with other proteins. Both Bcl-2 and Bcl-xL were able to prevent Bax oligomerization and insertion, which is consistent with our previous results that both antiapoptotic proteins counteract the Bid-induced change of conformation of Bax by binding directly with Bax. Nevertheless, it remains possible that Bcl-xL or Bcl-2 inhibits Bax-induced cytochrome c release independently of binding to Bax (22), by forming channels which would counterbalance Bax action.
The mechanisms by which Bax, following its membrane insertion, triggers cytochrome c release from mitochondria are still unclear. It has been proposed that Bax may stimulate the opening of the permeability transition pore (PTP) through interaction with the adenine nucleotide translocator (30). As a result of PTP opening, mitochondria would swell, leading to rupture of the outer mitochondrial membrane and passive release of cytochrome c (23, 24). There are, however, types of apoptosis in which a shrinkage rather than a swelling of mitochondria has been reported (18, 27–29, 46), and in sympathetic neurons deprived of nerve growth factor, the release of cytochrome c can be reversed if caspase activation is prevented (29). Moreover, we have found that cyclosporin A, an inhibitor of the PTP, was unable to inhibit Bid-induced Bax insertion into mitochondrial membranes (results not shown) and Bax-induced cytochrome c release from mitochondria (9). Together, these studies suggest that the release of cytochrome c from mitochondria may be a well-controlled event, occurring, under certain circumstances, independently of the opening of the PTP. One possible mechanism is that Bax forms a channel large enough to allow the release of cytochrome c as well as other proteins of a similar size. The integration of Bax within membranes would be the first of a series of events leading to channel formation. Nonetheless, we report that Bax insertion into mitochondria is not sufficient to trigger cytochrome c release. Indeed, in the absence of Mg2+, cytochrome c was not released despite Bax insertion into the mitochondrial membranes.
All the experiments described above were performed with full-length Bid, which has been reported previously to translocate to mitochondria during staurosporine-induced apoptosis of HeLa cells (8). In contrast to Bax, we found that Bid does not integrate in the mitochondrial membrane. This is in contrast to what was found after cleavage of Bid by caspase 8 during interleukin-3 deprivation of FL5.12 cells. In these studies, the cleaved form of Bid (p15 BID) translocated to mitochondria and became an integral membrane protein (14). We have found that Bid cleaved by caspase 8 is at least 10-fold more efficient in triggering Bax insertion into the mitochondrial membrane and cytochrome c efflux from mitochondria (R. Eskes et al., unpublished data).
Bax integration was shown to occur in the outer mitochondrial membrane as assessed by digitonin extraction. We have not been able to detect any translocation of Bax from the outer to the inner mitochondrial membrane during the process of cytochrome c release. This contrasts with the results of others, who reported Bax redistribution from the outer to the inner mitochondrial membrane following treatment of mitochondria with atractyloside (30). Our results suggest strongly that Bax exerts its apoptotic function by being physically present in the outer mitochondrial membrane. This localization is compatible with the ability of Bax to interact with the outer mitochondrial membrane channel VDAC, as previously reported (35). This localization would also be consistent with the hypothesis that Bax itself may form a channel to allow the release of cytochrome c. Nevertheless, a fraction of Bax, as well as Bak and Bcl-xL, was found to be integrated in the outer mitochondrial membrane of mitochondria isolated from nonapoptotic cells. It is possible that in the absence of an apoptotic stimulus, both Bax and Bak are inactivated in the membrane by forming Bax–Bcl-xL or Bak–Bcl-xL heterodimers, although we cannot exclude the possibility that these proteins are also functional and play a physiological role in nonapoptotic cells.
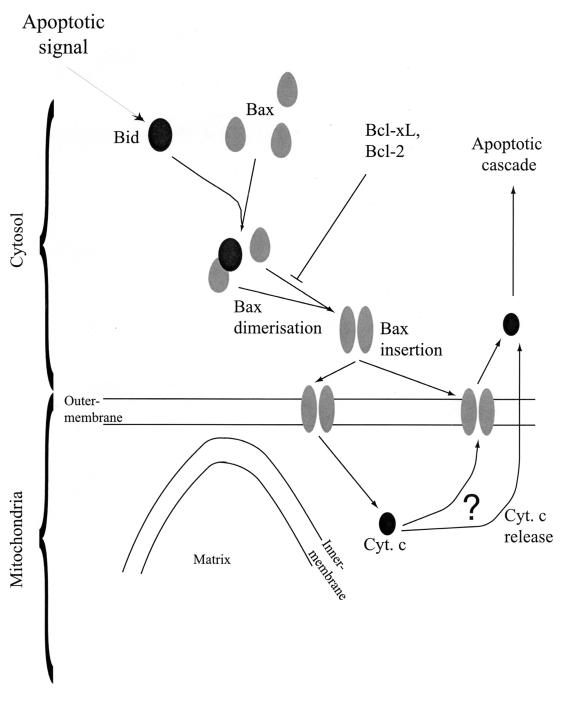
In summary, our results are consistent with a model of cellular apoptosis in which Bid interacts with Bax to trigger a change in Bax conformation leading to dimerization (or oligomerization) and integration into the outer mitochondrial membrane (Fig. 7).
FIG. 7.
Model for the activation of Bax by Bid during apoptosis. Following an apoptotic stimulus, Bid binds to Bax and triggers a change in the conformation of Bax. As a result, Bax dimerizes (or oligomerizes) and inserts into the outer mitochondrial membrane, which results in cytochrome c (Cyt. c) release from mitochondria.
ACKNOWLEDGMENTS
We thank S. Arkinstall and K. Maundrell for critical reading of the manuscript, C. Herbert for artwork, and T. Wells for encouraging support.
Part of this work was also supported by grants from the European Community (Biotech grant BIO4CT96 0774 to J.-C. Martinou).
REFERENCES
- 1.Adams J M, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Antonsson B, Conti F, Ciavatta A M, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod J-J, Mazzei G, Maundrell K, Gambale F, Sadoul R, Martinou J-C. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 3.Bossy-Wetzel E, Newmeyer D D, Green D R. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 1998;17:37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Chittenden T, Flemington C, Houghton A B, Ebb R G, Gallo G J, Elangovan B, Chinnadurai G, Lutz R J. A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO J. 1995;14:5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou J J, Honglin L, Salvesen G S, Yuan J, Wagner G. Solution structure of Bid, an intracellular amplifier of apoptotic signaling. Cell. 1999;96:615–624. doi: 10.1016/s0092-8674(00)80572-3. [DOI] [PubMed] [Google Scholar]
- 7.Deckwerth T L, Elliott J L, Knudson C M, Johnson E M J, Snider W D, Korsmeyer S J. BAX is required for neuronal death after trophic factor deprivation and during development. Neuron. 1996;17:401–411. doi: 10.1016/s0896-6273(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 8.Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou J-C. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eskes R, Antonsson B, Osen-Sand A, Montessuit S, Richter C, Sadoul R, Mazzei G, Nichols A, Martinou J-C. Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J Cell Biol. 1998;143:217–224. doi: 10.1083/jcb.143.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estoppey S, Rodriguez I, Sadoul R, Martinou J-C. Bcl-2 prevents activation of CPP32 cysteine protease and cleavage of poly (ADP-ribose) polymerase and U1-70 kD proteins in staurosporine-mediated apoptosis. Cell Death Differ. 1997;4:34–38. doi: 10.1038/sj.cdd.4400205. [DOI] [PubMed] [Google Scholar]
- 11.Goping I S, Gross A, Lavoie J N, Nguyen M, Jemmerson R, Roth K, Korsmeyer S J, Shore G C. Regulated targeting of BAX to mitochondria. J Cell Biol. 1998;143:207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffiths G J, Dubrez L, Morgan C P, Jones N A, Whitehouse J, Corfe B M, Dive C, Hickman J A. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J Cell Biol. 1999;144:903–914. doi: 10.1083/jcb.144.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross A, Jockel J, Wei M C, Korsmeyer S J. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross A, Yin X-M, Wang K, Wei M C, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer S J. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 15.Hsu Y-T, Wolter K G, Youle R J. Cytosol-to-membrane redistribution of Bax and Bcl-XL during apoptosis. Proc Natl Acad Sci USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu Y-T, Youle R J. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson M D. Bcl-2-related proteins get connected. Curr Biol. 1997;7:R277–R281. doi: 10.1016/s0960-9822(06)00136-9. [DOI] [PubMed] [Google Scholar]
- 18.James T N, Terasaki F, Pavlovich E R, Vikhert A M. Apoptosis and pleomorphic micromitochondriosis in the sinus nodes surgically excised from five patients with the long QT syndrome. J Lab Clin Med. 1993;122:309–323. [PubMed] [Google Scholar]
- 19.Jürgensmeier J M, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed J C. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelekar A, Thompson C B. Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 1998;8:324–330. doi: 10.1016/s0962-8924(98)01321-x. [DOI] [PubMed] [Google Scholar]
- 21.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 22.Knudson C M, Korsmeyer S J. Bcl-2 and Bax function independently to regulate cell death. Nat Genet. 1997;16:358–363. doi: 10.1038/ng0897-358. [DOI] [PubMed] [Google Scholar]
- 23.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 24.Kroemer G, Zamzami N, Susin S A. Mitochondrial control of apoptosis. Immunol Today. 1997;18:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 25.Kroemer G C. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 26.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cytochrome c and dATP dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 27.Lia J, Dourmashkin R R, Newland A C, Kelsey S M. Mitochondrial ultracondensation, but not swelling, is involved in TNF alpha-induced apoptosis in human T-lymphoblastic leukaemic cells. Leuk Res. 1997;21:973–983. doi: 10.1016/s0145-2126(97)00078-7. [DOI] [PubMed] [Google Scholar]
- 28.Mancini M, Anderson B O, Caldwell E, Sedghinasab M, Paty P B, Hockenbery D M. Mitochondrial proliferation and paradoxical membrane depolarization during terminal differenciation and apoptosis in a human colon carcinoma cell line. J Cell Biol. 1997;138:449–469. doi: 10.1083/jcb.138.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinou I, Desagher S, Eskes R, Antonsson B, André E, Fakan S, Martinou J-C. The release of cytochrome c from mitochondria during apoptosis of NGF-deprived sympathetic neurons is a reversible event. J Cell Biol. 1999;144:883–889. doi: 10.1083/jcb.144.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marzo I, Brenner C, Zamzami N, Jürgensmeier J M, Susin S A, Vieira H L A, Prévost M-C, Xie Z, Matsuyama S, Reed J C, Kroemer G. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- 31.McDonnell J M, Fushman D, Milliman C L, Korsmeyer S J, Cowburn D. Solution structure of the proapoptotic molecule Bid: a structural basis for apoptotic agonists and antagonists. Cell. 1999;96:625–634. doi: 10.1016/s0092-8674(00)80573-5. [DOI] [PubMed] [Google Scholar]
- 32.Miller T M, Moulder K L, Knudson C M, Creedon D J, Deshmukh M, Korsmeyer S J, Johnson E M J. Bax deletion further orders the cell death pathway in cerebellar granule cells and suggests a caspase-independent pathway to cell death. J Cell Biol. 1997;139:205–217. doi: 10.1083/jcb.139.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minn A J, Vélez P, Schendel S L, Liang H, Muchmore S W, Fesik S W, Fill M, Thompson C B. Bcl-xL forms an ion channel in synthetic lipid membranes. Nature. 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- 34.Muchmore S W, Sattler M, Liang H, Meadows R P, Harlan J E, Yoon H S, Nettesheim D, Chang B S, Thompson C B, Wong S-L, Ng S-C, Fesik S W. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 35.Narita M, Shimizu S, Ito T, Chittenden T, Lutz R, Matsuda H, Tsujimoto Y. Bax interacts with the prermeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:14681–14686. doi: 10.1073/pnas.95.25.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed J C. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 37.Rossé T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 38.Schendel S L, Xie Z, Montal M O, Matsuyama S, Montal M, Reed J C. Channel formation by antiapoptotic protein Bcl-2. Proc Natl Acad Sci USA. 1997;94:5113–5118. doi: 10.1073/pnas.94.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlesinger P H, Gross A, Yin X-M, Yamamoto K, Saito M, Waksman G, Korsmeyer S J. Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2. Proc Natl Acad Sci USA. 1997;94:11357–11362. doi: 10.1073/pnas.94.21.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Susin S A, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med. 1996;184:1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vander Heiden M G, Chandel N S, Williamson E K, Schumacker P T, Thompson C B. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 42.Wang K, Yian X-M, Chao D T, Milliman C L, Korsmeyer S J. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 43.Wolter K G, Hsu Y-T, Smith C L, Nechushtan A, Xi X-G, Youle R J. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang E, Korsmeyer S J. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- 45.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T-I, Jones D P, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 46.Zhuang J, Dinsdale D, Cohen G M. Apoptosis, in human monocytic THP.1 cells, results in the release of cytochrome c from mitochondria prior to their ultracondensation, formation of outer membrane discontinuities and reduction in inner membrane potential. Cell Death Differ. 1998;5:953–962. doi: 10.1038/sj.cdd.4400440. [DOI] [PubMed] [Google Scholar]