To the Editor:
A common sequela of prematurity is bronchopulmonary dysplasia (BPD), characterized by impaired alveolar growth, airway inflammation, and airflow obstruction (1), which may affect up to 50,000 U.S. infants annually (2, 3). Pulmonary hypertension (PH) is an increasingly recognized comorbidity of BPD. Cohort studies estimate that 14–43% of infants with BPD will develop PH, which is associated with increased mortality (14–38%) (4). Few studies describe the natural history of PH in infants with BPD after neonatal intensive care unit (NICU) discharge. Two retrospective studies found that 24–34% of survivors still had PH at ∼3 years of age (5, 6), but it has not been observed in school-age children with BPD (7, 8). Given these studies, our first objective was to characterize preterm infants at risk for prolonged resolution of PH after 1 year of age.
Published guidelines recommend screening echocardiograms at 36 weeks postmenstrual age (PMA) for infants with moderate or severe BPD (9). One subsequent study did not identify any cases of PH after 40 weeks PMA (10), but another study found that 48% of PH cases were diagnosed after NICU discharge (6). Our second objective was to identify infants with negative screening echocardiograms who were subsequently diagnosed with PH.
Methods
Charts for 758 subjects enrolled in an outpatient BPD clinical registry between 2008 and 2018 were retrospectively reviewed. Inclusion criteria included birth at <32 weeks gestation and a diagnosis of BPD (all severities) (2, 11). Caregivers were consented per the Johns Hopkins University Institutional Review Board. Echocardiogram findings were abstracted from the medical record. Subjects were classified as having PH if PH was present on any clinically obtained echocardiogram in the screening period (34–38 wk PMA) and/or follow-up period (>38 wk PMA). The diagnosis of PH was based on elevated right ventricular pressures defined by tricuspid regurgitation jet, patent ductus arteriosus (PDA) gradient, or systolic interventricular septal position. Of the 758 subjects, 57 subjects had echocardiograms only in the screening period, 197 only had them in the follow-up period, and 168 had them in both periods. For this study, we arbitrarily examined children who had PH resolve after 1 year of chronological age versus before 1 year of chronological age. Late-onset PH was defined as PH found in the follow-up period that was not observed during the screening period; infants were only included in this analysis if they had echocardiograms during both the screening and follow-up periods. Patient characteristics were compared using Chi-square tests, Wilcoxon rank-sum tests, and t tests (Tables 1 and 2). Censored data for time to PH resolution and time to late-onset PH were described using Kaplan-Meier analysis.
Table 1.
Resolution of pulmonary hypertension
| Any Pulmonary Hypertension (n = 143) | Resolution after 1 yr of Age (n = 25) | Resolution before 1 yr of Age (n = 118) | P Value | |
|---|---|---|---|---|
| Sex, % female | 46.9 | 52.0 | 45.8 | 0.57 |
| Race/ethnicity, % non-White | 69.2 | 60.0 | 71.2 | 0.27 |
| Gestational age, mean ± SD (range), wk | 26.0 ± 2.2 (23–32) | 26.8 ± 2.9 (23–32) | 25.9 ± 2.0 (23–30.7) | 0.07 |
| Birthweight, mean ± SD (range), grams | 815 ± 295 (380–1,900) | 957 ± 447 (420–1,900) | 785 ± 243 (380–1,650) | 0.008 |
| Birthweight percentile, mean ± SD (range), % | 40 ± 24 (1–94) | 36 ± 23 (2–88) | 41 ± 24 (1–94) | 0.37 |
| G-tube, % yes | 55.9 | 92.0 | 48.3 | <0.001 |
| Nissen fundoplication, % yes | 37.8 | 72.0 | 30.5 | <0.001 |
| Supplemental oxygen at discharge, % yes | 65.0 | 72.0 | 63.6 | 0.42 |
| Initial oxygen amount, mean ± SD (range), LPM | 0.53 ± 0.43 (0.13–2.00) (n = 93) | 0.94 ± 0.59 (0.13–2.00) (n = 18) | 0.43 ± 0.31 (0.13–1.50) (n = 75) | <0.001 |
| Age at home oxygen discontinuation, median (95% CI), yr | 1.18 (1.12–1.34) (n = 93) | 1.97 (1.59–3.95) (n = 18) | 1.13 (1.02–1.18) (n = 75) | <0.001* |
| Diuretics, % yes | 79.0 | 96.0 | 75.4 | 0.022 |
| Tracheostomy, % yes | 11.9 | 32.0 | 7.6 | 0.001 |
| Home ventilator, % yes | 10.5 | 32.0 | 5.9 | <0.001 |
| PDA requiring procedure, % yes | 38.5 | 48.0 | 36.4 | 0.28 |
| CSF shunt, % yes | 10.5 | 20.0 | 8.5 | 0.09 |
| Initial length of stay, mean ± SD (range), d | 195 ± 125 (53–806) | 296 ± 189 (92–806) | 173 ± 95 (53–744) | <0.001 |
| Outpatient vasodilator therapy, % yes† | 24.5 | 44.0 | 20.3 | 0.012 |
Definition of abbreviations: CI = confidence interval; CSF = cerebrospinal fluid; LPM = liters per minute; PDA = patent ductus arteriosus; SD = standard deviation.
P value generated via Wilcoxon rank-sum test.
Out of the 143 subjects, 30 subjects were on sildenafil alone, 4 were on sildenafil and bosentan, and 1 was on sildenafil and treprostinil.
Table 2.
Late onset of pulmonary hypertension
| Negative Screen; Positive in Follow-Up (n = 27) | Negative Screen; Negative in Follow-Up (n = 133) | P Value | |
|---|---|---|---|
| Sex, % female | 40.7 | 42.1 | 0.90 |
| Race/ethnicity, % non-White | 59.3 | 62.4 | 0.76 |
| Gestational age, mean ± SD (range), wk | 25.7 ± 2.1 (23.1–32) | 26.4 ± 2.2 (22.7–32) | 0.14 |
| Birthweight, mean ± SD (range), grams | 787 ± 277 (470–1,590) | 880 ± 332 (400–2,310) | 0.17 |
| Birthweight percentile, mean ± SD (range), % | 41 ± 25 (4–89) | 44 ± 25 (1–95) | 0.70 |
| G-tube, % yes | 66.7 | 33.8 | 0.001 |
| Nissen fundoplication, % yes | 44.4 | 19.6 | 0.006 |
| Supplemental oxygen at discharge, % yes | 74.1 | 40.6 | 0.001 |
| Initial oxygen amount, mean ± SD (range), LPM | 0.48 ± 0.24 (0.13–1.00) (n = 20) | 0.32 ± 0.23 (0.03–1.00) (n = 54) | 0.010 |
| Age at home oxygen discontinuation, median (95% CI), yr | 1.31 (1.06–2.10) (n = 20) | 0.81 (0.70–1.00) (n = 54) | 0.034* |
| Diuretics, % yes | 88.9 | 72.2 | 0.07 |
| Tracheostomy, % yes | 7.4 | 3.0 | 0.27 |
| Home ventilator, % yes | 7.4 | 3.0 | 0.27 |
| PDA requiring procedure, % yes | 48.2 | 27.1 | 0.030 |
| CSF shunt, % yes | 11.1 | 12.0 | 0.89 |
| Initial length of stay, mean ± SD (range), d | 211 ± 80 (95–452) | 139 ± 63 (49–507) | <0.001 |
Definition of abbreviations: CI = confidence interval; CSF = cerebrospinal fluid; LPM = liters per minute; PDA = patent ductus arteriosus; SD = standard deviation.
P value generated via Wilcoxon rank-sum test.
Results
Timing of resolution of PH
Of the 758 infants with BPD in the registry, 143 (18.9%) had PH during the screening period and/or during the follow-up period (Table 1). Of those with PH, the median age of resolution was 20 weeks after birth (Figure 1A). However, 25 infants (17.5%) had PH that resolved after 1 year of age. This prolonged resolution of PH was not associated with sex, race and/or ethnicity, gestational age, or birthweight percentile but was associated with heavier birthweight. In addition, those with PH that persisted after 1 year of age were more likely to receive gastrostomy tube placement and Nissen fundoplication than those with resolution of PH before 1 year of age. Although this prolonged PH was not associated with home supplemental oxygen, if supplemental oxygen was required, those with PH persisting after 1 year of age required a greater mean amount of oxygen with longer duration of use and were more likely to require tracheostomy and home ventilation. This prolonged PH was associated with increased diuretic and vasodilator use and a longer NICU admission (296 d vs. 173 d).
Figure 1.
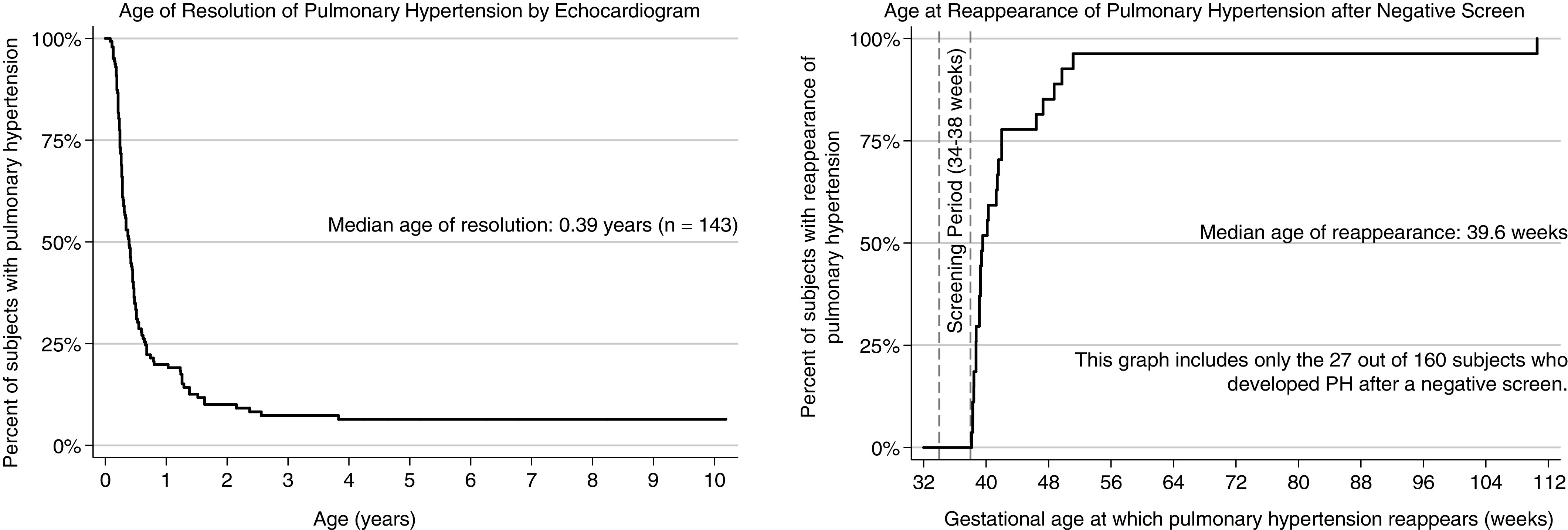
Resolution and reappearance of PH. PH = pulmonary hypertension.
Subpopulation with late-onset PH
A total of 160 patients had negative or inconclusive echocardiograms during the screening period, 27 (16.9%) of whom developed echocardiographic evidence of PH in the follow-up period (Table 2). The median age of first positive echocardiogram was 39.6 weeks PMA (range, 38.1–110.6 wk PMA; Figure 1B). Late-onset PH was not associated with any demographic characteristics. Infants with late-onset PH were more likely to receive gastrostomy tube placement and Nissen fundoplication than infants with persistently negative echocardiograms. In addition, infants with late-onset PH were more likely to have received a procedural intervention for a PDA, have home respiratory support needs with longer duration of oxygen use, and have a longer initial NICU admission (211 d vs. 139 d). Time to resolution of PH did not differ between infants with early- versus late-onset PH (P = 0.65).
Discussion
This retrospective cohort study describes the natural history of PH in BPD, including subpopulations with more prolonged resolution of PH (after 1 yr of age) and late-onset PH. Our findings suggest that these subpopulations cannot be readily identified based on demographic characteristics, as there were no significant differences in gestational age, etc., and surprisingly, infants with more prolonged PH were counterintuitively heavier at birth. However, infants with PH that persisted after 1 year of age and those with late-onset PH did require increased home respiratory support, suggesting more severe lung disease. Although it is assumed that respiratory support requirements are a surrogate marker of lung disease, supplemental oxygen may also be a concomitant PH therapy. Gastrostomy tube placement and Nissen fundoplication are a common sequela of prematurity. Both were seen with increased frequency in PH persisting after 1 year of age and late-onset PH, which again likely reflects a marker of more severe disease. We found that PDAs receiving procedural intervention were more common with late-onset PH, and this may reflect that infants with hemodynamically significant PDAs may be at risk for the development of PH due to ongoing systemic-to-pulmonary shunting (12). Lastly, infants with late-onset PH had longer initial hospitalizations than infants with persistently negative echocardiograms. Therefore, it is reasonable to consider repeating screening echocardiograms in infants with BPD who remain hospitalized beyond the standard screening age of 36 weeks PMA, even if initially negative.
This study was limited by its retrospective nature, echocardiograms obtained for clinical reasons, and absence of control subjects without BPD. In addition, as resolution of PH likely occurred between echocardiograms, bias secondary to interval censoring may be present (13). Also, using echocardiograms to determine the presence of PH is a potential limitation, as cardiac catheterization is more accurate in determining presence as well as severity (14). In addition, during the study period, there was no standardized protocol for who was screened for PH at ∼36 weeks PMA, so the overall prevalence, and particularly mild disease, may be underestimated.
Although PH affects a minority of premature infants with BPD, improving screening and follow-up guidelines to identify those at highest risk and better understanding of PH resolution and recurrence may improve outcomes and anticipatory guidance. Future prospective studies should focus on further clarifying risk factors for the development of disease as well as the ideal screening window to identify patients.
Acknowledgments
Acknowledgment
The authors thank the patients and their families who participated in this study.
Footnotes
Supported in part by a Johns Hopkins All Children’s Foundation Institutional Research Grant (Principal investigator: N. A. Goldenberg; Johns Hopkins “iPICS” prospective multicohort and biobanking study of pediatric acute and chronic health conditions). S.A.M.-M. receives grant funding from the National Institutes of Health (R01-HL114800).
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 2. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 3. Collaco JM, McGrath-Morrow SA. Respiratory phenotypes for preterm infants, children, and adults: bronchopulmonary dysplasia and more. Ann Am Thorac Soc. 2018;15:530–538. doi: 10.1513/AnnalsATS.201709-756FR. [DOI] [PubMed] [Google Scholar]
- 4. Collaco JM, Romer LH, Stuart BD, Coulson JD, Everett AD, Lawson EE, et al. Frontiers in pulmonary hypertension in infants and children with bronchopulmonary dysplasia. Pediatr Pulmonol. 2012;47:1042–1053. doi: 10.1002/ppul.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Altit G, Bhombal S, Hopper RK, Tacy TA, Feinstein J. Death or resolution: the "natural history" of pulmonary hypertension in bronchopulmonary dysplasia. J Perinatol. 2019;39:415–425. doi: 10.1038/s41372-018-0303-8. [DOI] [PubMed] [Google Scholar]
- 6. del Cerro MJ, Sabaté Rotés A, Cartón A, Deiros L, Bret M, Cordeiro M, et al. Pulmonary hypertension in bronchopulmonary dysplasia: clinical findings, cardiovascular anomalies and outcomes. Pediatr Pulmonol. 2014;49:49–59. doi: 10.1002/ppul.22797. [DOI] [PubMed] [Google Scholar]
- 7. Korhonen P, Hyödynmaa E, Lautamatti V, Iivainen T, Tammela O. Cardiovascular findings in very low birthweight schoolchildren with and without bronchopulmonary dysplasia. Early Hum Dev. 2005;81:497–505. doi: 10.1016/j.earlhumdev.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 8. Joshi S, Wilson DG, Kotecha S, Pickerd N, Fraser AG, Kotecha S. Cardiovascular function in children who had chronic lung disease of prematurity. Arch Dis Child Fetal Neonatal Ed. 2014;99:F373–F379. doi: 10.1136/archdischild-2013-305185. [DOI] [PubMed] [Google Scholar]
- 9. Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, et al. American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; and the American Thoracic Society. Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation. 2015;132:2037–2099. doi: 10.1161/CIR.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 10. Vayalthrikkovil S, Vorhies E, Stritzke A, Bashir RA, Mohammad K, Kamaluddeen M, et al. Prospective study of pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 2019;54:171–178. doi: 10.1002/ppul.24211. [DOI] [PubMed] [Google Scholar]
- 11. McGrath-Morrow SA, Lee G, Stewart BH, McGinley BM, Lefton-Greif MA, Okelo SO, et al. Day care increases the risk of respiratory morbidity in chronic lung disease of prematurity. Pediatrics. 2010;126:632–637. doi: 10.1542/peds.2010-0844. [DOI] [PubMed] [Google Scholar]
- 12. Philip R, Nathaniel Johnson J, Naik R, Kimura D, Boston U, Chilakala S, et al. Effect of patent ductus arteriosus on pulmonary vascular disease. Congenit Heart Dis. 2019;14:37–41. doi: 10.1111/chd.12702. [DOI] [PubMed] [Google Scholar]
- 13. Zhang Z, Sun J. Interval censoring. Stat Methods Med Res. 2010;19:53–70. doi: 10.1177/0962280209105023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mourani PM, Sontag MK, Younoszai A, Ivy DD, Abman SH. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics. 2008;121:317–325. doi: 10.1542/peds.2007-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]


