Abstract
Background
Lung cancer is the major cause of mortality in tumor patients. While its incidence rate has recently declined, it is still far from satisfactory and its potential modifiable risk factors should be explored.
Methods
We performed a two-sample Mendelian randomization (MR) study to investigate the causal relationship between potentially modifiable risk factors (namely smoking behavior, alcohol intake, anthropometric traits, blood pressure, lipidemic traits, glycemic traits, and fasting insulin) and lung cancer. Besides, a bi-directional MR analysis was carried out to disentangle the complex relationship between different risk factors. Inverse-variance weighted (IVW) was utilized to combine the estimation for each SNP. Cochrane’s Q value was used to evaluate heterogeneity and two methods, including MR-Egger intercept and MR-PRESSO, were adopted to detect horizontal pleiotropy.
Results
Three kinds of smoking behavior were all causally associated with lung cancer. Overall, smokers were more likely to suffer from lung cancer compared with non-smokers (OR = 2.58 [1.95, 3.40], p-value = 2.07 x 10−11), and quitting smoking could reduce the risk (OR = 4.29[2.60, 7.07], p-value = 1.23 x 10−8). Furthermore, we found a dose-response relationship between the number of cigarettes and lung cancer (OR = 6.10 [5.35, 6.96], p-value = 4.43x10-161). Lower HDL cholesterol could marginally increase the risk of lung cancer, but become insignificant after Bonferroni correction (OR = 0.82 [0.68, 1.00], p-value = 0.045). In addition, we noted no direct causal relationship between other risk factors and lung cancer. Neither heterogeneity nor pleiotropy was observed in this study. However, when treating the smoking behavior as the outcome, we found the increased BMI could elevate the number of cigarettes per day (beta = 0.139[0.104, 0.175], p-value = 1.99x10-14) and a similar effect was observed for the waist circumference and hip circumference. Additionally, the elevation of SBP could also marginally increase the number of cigarettes per day (beta = 0.001 [0.0002, 0.002], p-value = 0.018).
Conclusion
Smoking behavior might be the most direct and effective modifiable way to reduce the risk of lung cancer. Meanwhile, smoking behavior can be affected by other risk factors, especially obesity.
Introduction
Although its mortality rate has declined rapidly, lung cancer continues to be the leading cause of cancer death in the world [1]. Both unfavorable environmental factors (tobacco smoking, high intake of meat, alcohol intake, and air pollution, et al) and genetic susceptibility (CHRNA3, CHRNA5, CHRNB4, TERT and CLPTM1L, et al) contribute to its initiation and progression [1], and tobacco consumption has been well recognized as the most important risk factor [2]. Furthermore, two SNPs of CLPTM1L (rs401681 and rs402710) can influence susceptibility to lung cancer by regulating TERT expression in East Asian populations [3]. Meanwhile, the body mass index (BMI) is also inversely associated with lung cancer [4]. However, it was still far from satisfactory in terms of how we could reduce the incidence rate of lung cancer. Changes in metabolic patterns have been characterized in lung cancer, but it is still not known if endogenous metabolic biomarkers could increase lung cancer. Some observational studies reported that changes in lipid biomarkers could lead to the increased risk of lung cancer; and a U-shaped association was observed between total cholesterol (TC), triglycerides (TG), and lung cancer [5]. However, a recent meta-analysis indicated the null association between cholesterol intake and lung cancer [6]. Meanwhile, elevated insulin may increase the risk of lung cancer, but no other study has explored the impact of other glycemic traits on lung cancer [7]. Besides, it should be noted that observational studies can be easily biased by the confounders such as socioeconomic status and education attainment; and its results should be interpreted as association instead of causation. Thus, it is necessary to screen for risk factors of lung cancer using a relatively robust method.
Mendelian randomization (MR) is a causal inference method using genetic variants (usually single nucleotide polymorphism, SNP) as the instrumental variables to appraise the causation between exposure and outcome; and has achieved great success in determining the risk factors of diseases [8]. The genetic variants are randomly allocated at conception based on Mendel’s law and cannot be biased by potential confounders to some extent. Thus, it should be suitable for causal inference. With the rapid development of genome-wide association study (GWAS), the association between the genetic variant and human phenotype can be conveniently available. Nowadays, many MR studies (mainly two-sample MR studies) have been performed to detect the risk factors of lung cancer; and these risk factors included education [9], BMI [10], and some metabolic risk factors [11]. Therein, lower education might increase the risk of lung cancer [9], and a higher BMI could directly elevate the risk of small-cell lung cancer and squamous cell carcinoma while decreasing the risk of lung adenocarcinoma [10]. Meanwhile, a previous study has reported that higher fasting insulin could increase the risk of lung cancer [7,11]. These studies filled the gap between association and causation since MR studies could reduce these biases caused by unrecognized confounders, reverse causation, and measurement error [12]. However, these results still show a discrepancy and further analysis should be conducted. For instance, there has been disagreement on whether BMI was causally associated with lung adenocarcinoma Additionally, the relationship between blood pressure and lung cancer is inconsistent because the association between high blood pressure and lung cancer is either positive [13] or null [14]. Therefore, blood pressure should be included as a risk factor in our study and whether it can affect the risk of lung cancer should be determined.
Furthermore, we hope to include some new potential risk factors (blood pressure) that have not been investigated in MR studies considering that Shen et al has demonstrated the potential risk factors of lung cancer, including socioeconomic status, lifestyle, dietary, and obesity [15]. Meanwhile, we selected three types of smoking behavior (smoking initiation, smoking cessation, and the number of cigarettes per day) and tried to give novel insights into smoking’s impact on lung cancer. Here, we relied on publicly available GWAS summary statistics to detect modifiable factors that might cause lung cancer. These modifiable factors include tobacco consumption, alcohol intake, BMI, waist-hip-ratio (WHR), lipidemic and glycemic traits. The two-sample MR method was mainly implemented to detect causal factors and a bi-directional MR analysis was carried out to assess other risk factors’ effects on smoking, hoping to account for the inconsistency in observational studies and clarify the relationship between different types of smoking behavior and lung cancer.
Methods
Genetic instrumental variables for modifiable risk factors
All genetic instrumental variables were extracted from non-UK Biobank GWAS results with the largest sample size and they were clumped to get independent instrumental variables (linkage disequilibrium r2 < 0.01) using 1000 genome Phase 3 European samples as the reference panel (https://www.internationalgenome.org/) [16]. All instrumental variables hit the genome-wide significance (GWAS p-value < 5x10-8) except those from GWAS of 2 h after glucose challenge (2-h glucose) during an oral glucose tolerance test (OGTT) (GWAS p-value < 1x10-5) and each minor allele frequency was more than 0.01. Genetic variants associated with smoking behavior, namely smoking initiation, the number of cigarettes per day, and smoking cessation, were all obtained from the recent GWAS result derived from GWAS & Sequencing Consortium of Alcohol and Nicotine use (GSCAN) and the participants of GCSAN GWAS were all Europeans [17]. Smoking initiation phenotype was defined as having smoked (past or current) versus never smoked (non-smokers) and smoking cessation phenotype was defined as current versus past smokers. The number of cigarettes per day phenotype was classified into 5 categories: (1) less than a half pack, (2) a half pack, (3) 1 pack, (4) 2 packs, and (5) more than 2 packs. The alcohol instrumental variables were extracted from a habitual alcohol intake GWAS where the maximum habitual alcohol intake was defined as the largest number of drinks of alcohol (beer, wine, and/or liquor) one may have had in one day in a typical month [18]. GWAS results from the Genetic Investigation of ANthropometric Traits (GIANT) consortium were used to identify genetic variants associated with BMI, waist circumference, hip circumference, and waist-to-hip ratio (WHR) [19]. Since the hip circumference, waist circumference and WHR are closely correlated with BMI, we hope to explore their causal effect on lung cancer with adjustment of BMI. The adjustment has been performed by GINAT consortium and we used the summary statistics of hip circumference adjusted by BMI, waist circumference adjusted by BMI and WHR adjusted by BMI. The genetic variants associated with systolic blood pressure (SBP) and diastolic blood pressure (DBP) were extracted from a recent GWAS meta-analysis [20]. Genetic variants associated with lipid traits, including HDL, LDL, total cholesterol, and triglycerides, were obtained from the Global Lipids Genetics Consortium (GLGC) [21]. Results from the Meta-Analyses of Glucose and Insulin-related traits Consortium were used to extract instrumental variables for HbA1c [22], 2 hour glucose after oral glucose tolerance test (2-h glucose) [23], and fasting glucose and insulin [24]. All these GWASs have genomic control. Further details have been displayed in Table 1.
Table 1. A brief description of GWAS summary statistics for modifiable risk factors.
| Risk factor | Consortium | Sample size | Covariates | PMID |
|---|---|---|---|---|
| Alcohol intake | GSCAN | 126,936 Europeans + 17,029 non-Europeans | age, sex and first 10 genetic principal components | 31151762 |
| Smoking initiation | GSCAN | 842,717 Europeans | age, gender, and the first 10 genetic principal components | 33082346 |
| Smoking cessation | GSCAN | 842,717 Europeans | age, gender, and the first 10 genetic principal components | 33082346 |
| Cigarettes per day | GSCAN | 842,717 Europeans | age, gender, and the first 10 genetic principal components | 33082346 |
| BMI | GIANT | 344,369 Europeans | age, the first 15 genetic principal components, assessment center, and the genotyping chip | 30778226 |
| Hip circumference | GIANT | 344,369 Europeans | age, the first 15 genetic principal components, assessment center, and the genotyping chip | 30778226 |
| Waist circumference | GIANT | 344,369 Europeans | age, the first 15 genetic principal components, assessment center, and the genotyping chip | 30778226 |
| WHR | GIANT | 344,369 Europeans | age, the first 15 genetic principal components, assessment center, and the genotyping chip | 30778226 |
| DBP | --- | 757,601 Europeans | sex, age, age2, BMI, and genotyping chips. | 30224653 |
| SBP | --- | 757,601 Europeans | sex, age, age2, BMI, and genotyping chips. | 30224653 |
| HDL cholesterol | GLGC | 169,899 Europeans and 18,678 non-Europeans | age, age2, and sex | 24097068 |
| LDL cholesterol | GLGC | 169,899 Europeans and 18,678 non-Europeans | age, age2, and sex | 24097068 |
| Total cholesterol | GLGC | 169,899 Europeans and 18,678 non-Europeans | age, age2, and sex | 24097068 |
| Triglycerides | GLGC | 169,899 Europeans and 18,678 non-Europeans | age, age2, and sex | 24097068 |
| HbA1c | MAGIC | 159,940 Europeans | study-specific covariates | 28898252 |
| Fasting glucose | MAGIC | 151,188 Europeans | age, sex, study site, and principal components | 33402679 |
| 2-h glucose | MAGIC | 15,234 Europeans | BMI, age, sex and study-specific covariates | 20081857 |
| Fasting insulin | MAGIC | 105,056 Europeans | age, sex, study site, and principal components | 33402679 |
Notes: PMID is the publication ID in PubMed. BMI is the body mass index; WHR is the waist-to-hip ratio; DBP is the diastolic blood pressure; SBP is the systolic blood pressure; HDL is the high-density lipoprotein; LDL is the low-density lipoprotein; 2-h glucose is the 2-hour glucose level of the oral glucose tolerance test.
GWAS summary statistics for lung cancer
The genetic instrumental variable information on lung cancer was obtained from the most recent pan-cancer GWAS results with 2,485 European ancestry lung cancer cases and 410,350 European ancestry controls from two large cohorts the UK Biobank and Kaiser Permanente Genetic Epidemiology Research on Adult Health and Aging (GERA) cohorts genotyped by Affymetrix [7846216] [25]. With the Haplotype Reference Consortium (HRC) as the main reference panel and the merged UK 10K and 1000 Genomes phase 3 reference panels for additional data, the genotype imputation was performed and 93,095,623 SNPs were obtained. SNPs with low imputation quality (INFO < 0.3) and low allele frequency (MAF < 0.01) were excluded from the study. They included age, sex, the first 10 genetic principal components, and genotyping chips as the covariates, and this GWAS has genomic control.
Mendelian randomization
Primarily, each modifiable risk factor was treated as the exposure to appraise its causal effect on lung cancer. Then, another MR analysis was carried out to estimate other risk factors’ effect on smoking, and a bi-directional MR was carried out to disentangle the complex relationship between obesity, alcohol intake and smoking considering their complicated effects on lung cancer.
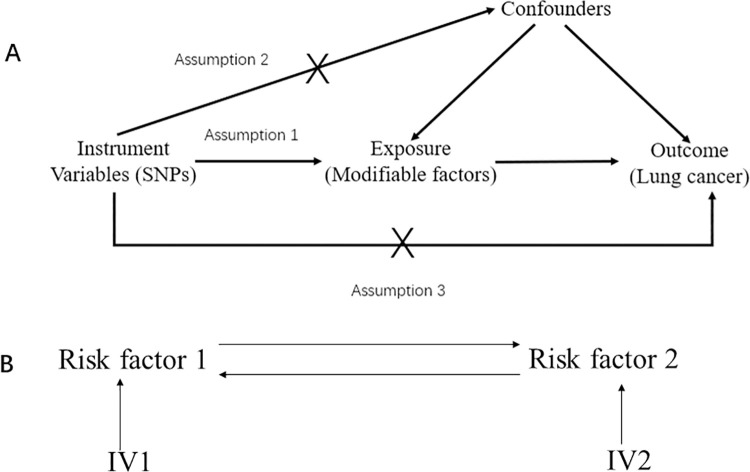
MR is performed based on its three main principles: 1) The genetic variant was closely associated with the exposure; 2) The genetic variant was not associated with potential confounders; 3) The genetic variant was not associated with the outcome except via the way of exposure [8] (Fig 1). The first assumption can be tested by F statistics with the formula F = β2/se2. However, it is difficult to test the last two assumptions; and we can only use pleiotropy test to detect the violation of these two assumptions. When estimating the causal effect with IV analysis, additional assumptions should be satisfied, including linearity and no interactions between exposures and mediator [26]. Generally, the inverse-variance weighted (IVW) method was mainly used in estimating the genetic-driven causal effect of exposure on the outcome. Initially, we adopted the “MR-PRESSO” method to detect the outliers in the instrumental variables and removed them [27]; and then causal estimation was conducted. Bonferroni correction was used to adjust multiple testing (Bonferroni p-value < 0.05/18 = 2.77 x 10−3). Cochrane’s Q value was utilized to detect the heterogeneity in the linear regression and the MR-Egger intercept was used to test the horizontal pleiotropy [28], as a supplement to the “MR-PRESSO” global test [27]. MR Steiger test was employed to judge whether the SNP was more likely to be associated with the exposure than the outcome and we removed the SNP more associated with the outcome than the exposure [29]. Also, a leave-one-out sensitivity analysis was performed to find the driving genetic variants. If there was no heterogeneity or pleiotropy, the IVW estimation was adopted; and a random effect model was used when heterogeneity existed. The causal estimation would be the causal effect size if pleiotropy existed. Besides, the weighted median method was also performed as a supplement to IVW and MR-Egger methods implemented in R package “TwoSampleMR”.
Fig 1. The basic principles underlying Mendelian randomization.
Fig 1A is the three assumptions for Mendelian randomization analysis. Fig 1B is the basic principles of bi-directional Mendelian randomization. SNP is the single nucleotide polymorphism and IV is the instrumental variable.
All statistical analyses were conducted by R programming language (https://www.r-project.org/, version 4.0.0). The Power calculation was performed in mRnd (https://cnsgenomics.shinyapps.io/mRnd/).
Results
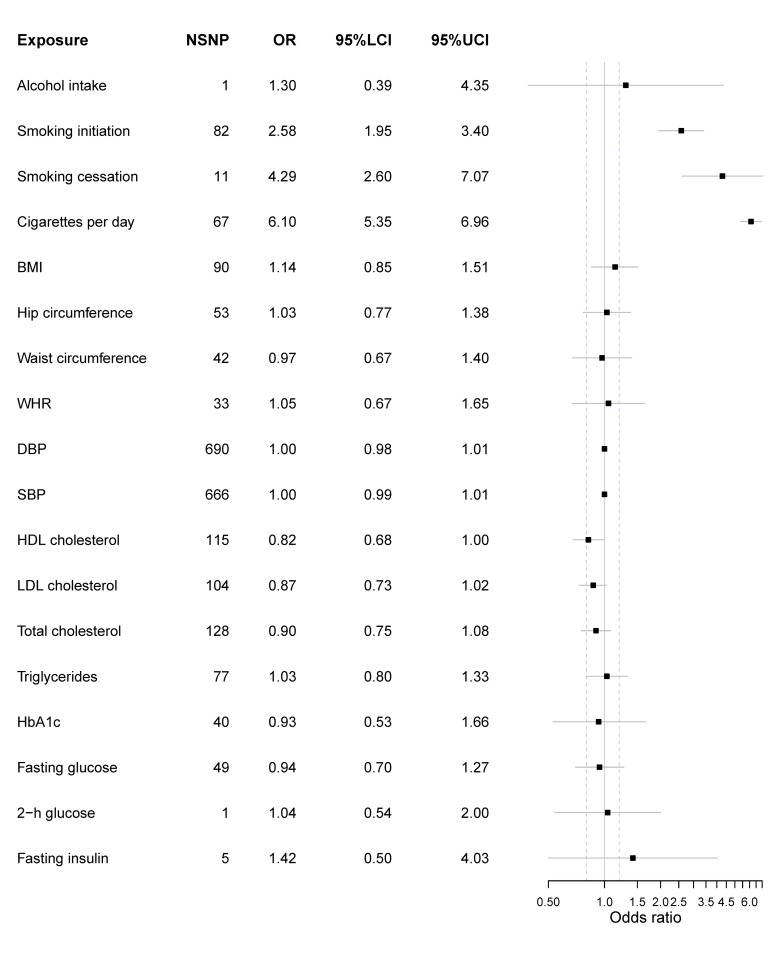
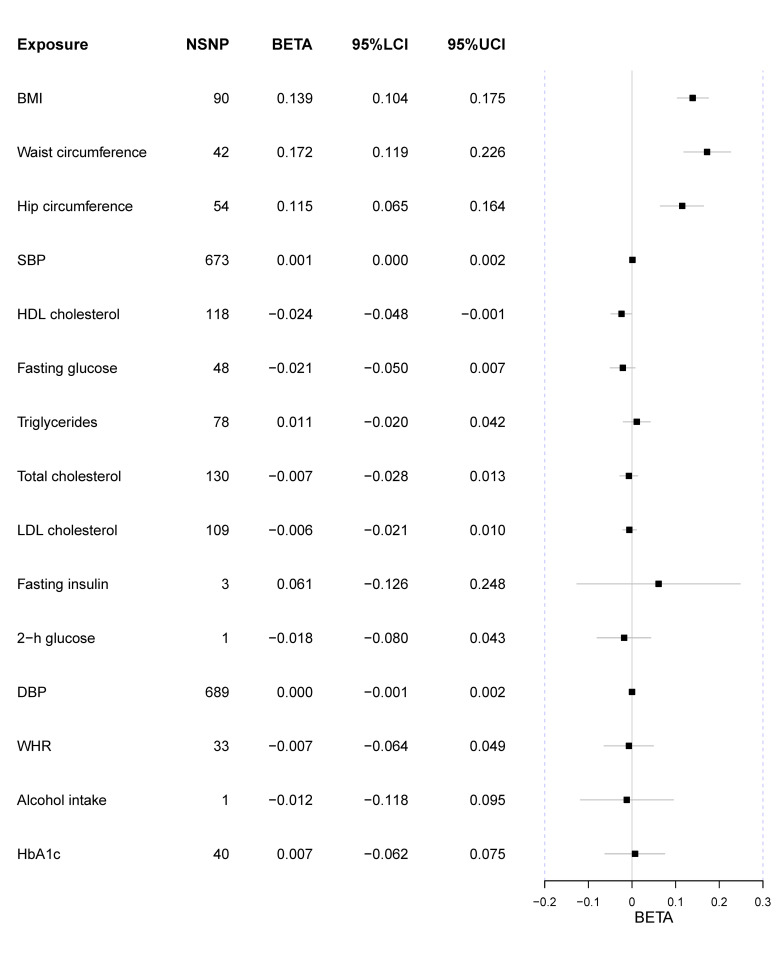
All genetic variants’ F statistics were more than 10, indicating there was less bias caused by potential weak instruments (S1–S18 Tables). Overall, only three kinds of smoking behavior were observed to directly causally increase the risk of lung cancer after Bonferroni correction (Fig 2). And a lower HDL level could marginally increase the risk of lung cancer. Besides, genetically-elevated BMI could increase the number of cigarettes per day (Fig 3).
Fig 2. Forest plot of main MR results with lung cancer as the outcome.
Exposure represents risk factors; NSNP is the number of SNPs used to estimate the causal effect size; OR is the odds ratio; 95%LCI is the lower limit of 95% confidence interval; 95%UCL is the upper limit of 95% confidence interval. BMI is the body mass index; WHR is the waist-to-hip ratio; DBP is the diastolic blood pressure; SBP is the systolic blood pressure; HDL is the high-density lipoprotein; LDL is the low-density lipoprotein; 2-h glucose is the 2-hour glucose level of the oral glucose tolerance test. The units of effect measures are per 1-SD increase for continuous exposures and 1-unit increase in log OR of binary exposures.
Fig 3. Forest plot of MR results treating the number of cigarettes per day as the outcome.
Exposure represents risk factors; NSNP is the number of SNPs used to estimate the causal effect size; BETA is the effect size; 95%LCI is the lower limit of 95% confidence interval; 95%UCL is the upper limit of 95% confidence interval; BMI is the body mass index; WHR is the waist-to-hip ratio; DBP is the diastolic blood pressure; SBP is the systolic blood pressure; HDL is the high-density lipoprotein; LDL is the low-density lipoprotein; 2-h glucose is the 2-hour glucose level of the oral glucose tolerance test. The units of effect measures are per 1-SD increase for continuous exposures and 1-unit increase in log OR of binary exposures.
Alcohol intake & smoking-related exposures
Only 1 SNP rs1229984 located in ADH1B was qualified as the instrumental variable. No causal relationship was observed between habitual alcohol intake and lung cancer (OR = 1.30 [0.39, 4.35], p-value = 0.674). Also, no direct evidence supported that alcohol intake could alter the number of cigarettes per day (beta = -0.012 [-0.118, 0.095], p-value = 0.829).
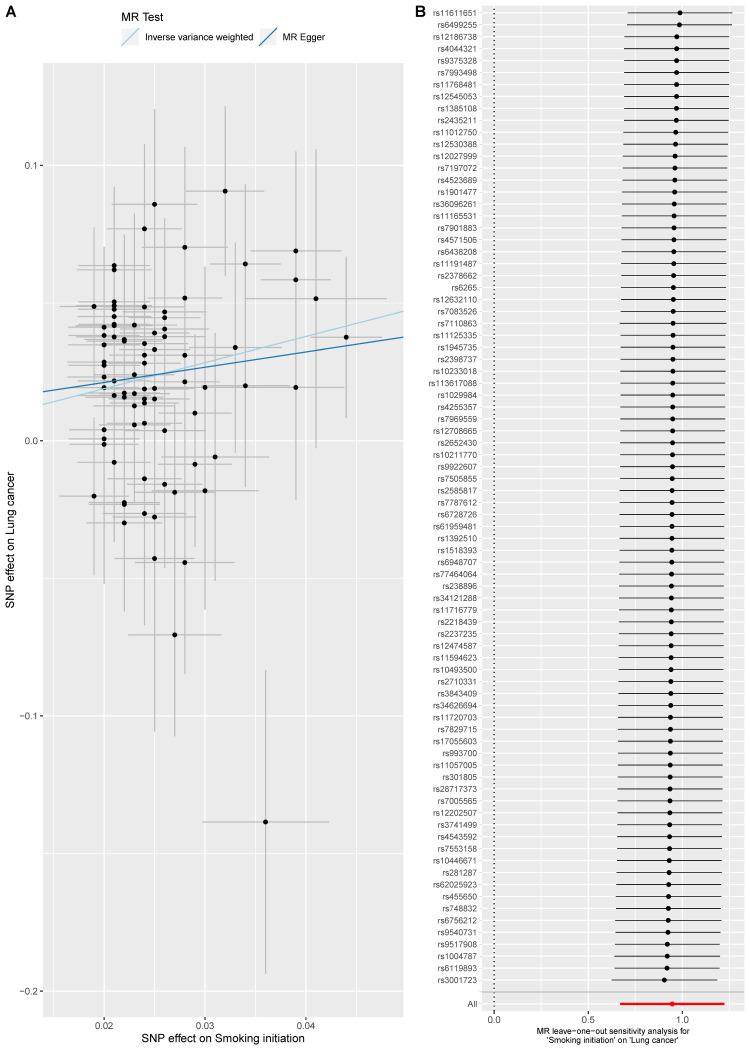
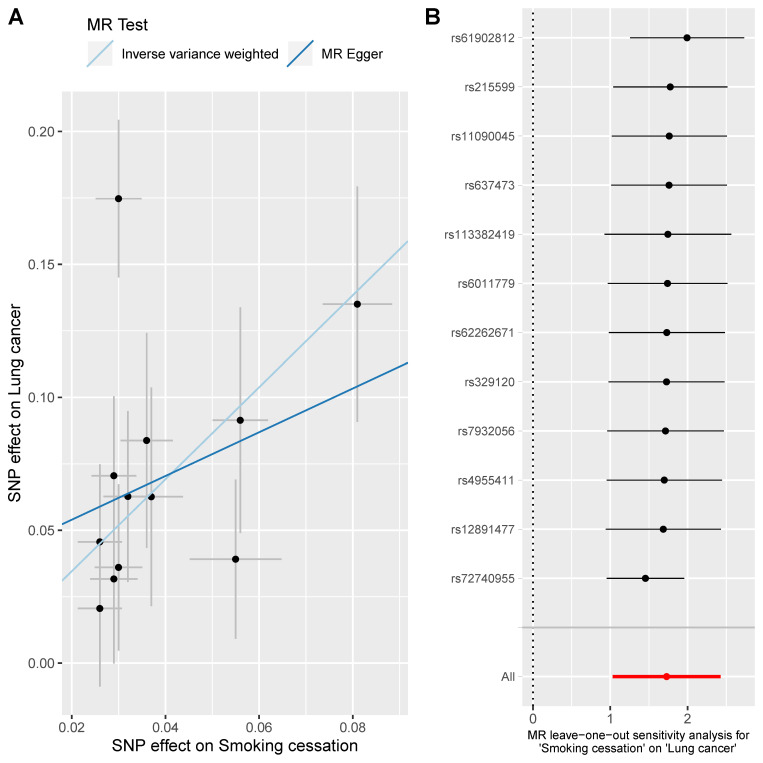
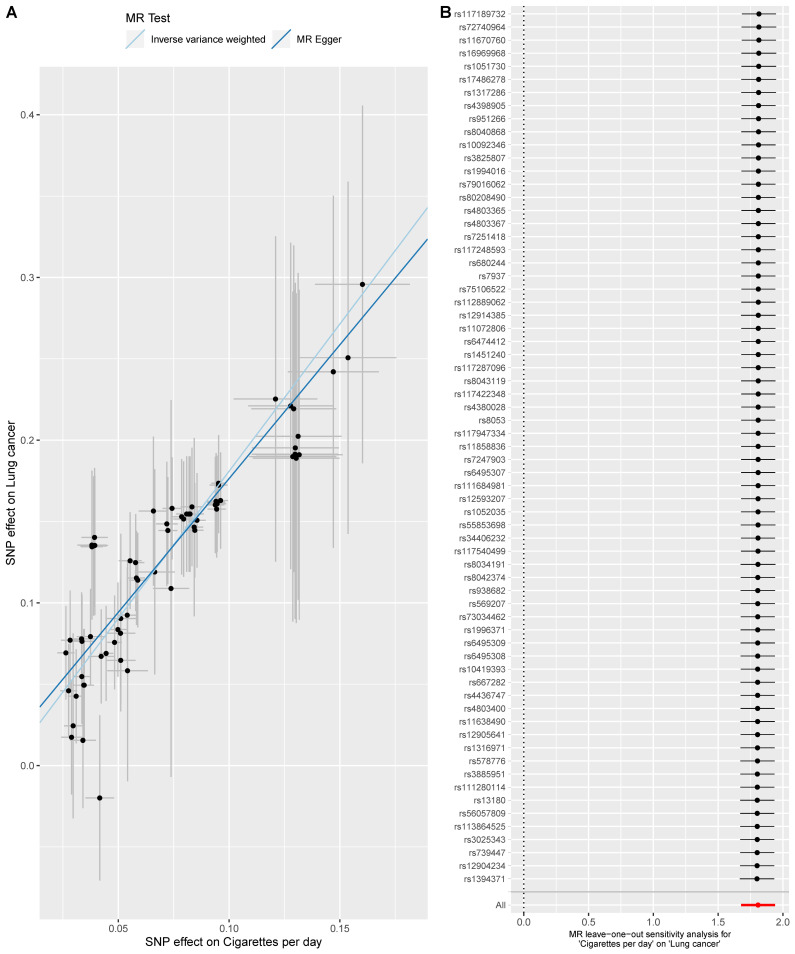
Here, three smoking-related exposures were selected to appraise the causal effect of smoking on lung cancer. Overall, three exposures were all causally associated with lung cancer. For smoking initiation, the smokers were more likely to suffer from lung cancer compared with non-smokers (OR = 2.58 [1.95, 3.40], p-value = 2.07 x 10−11) (Fig 4). When analyzing smoking cessation, 2 SNPs (rs56113850 & rs72740955) were removed since they did not pass the outlier test and 1 SNP rs56113850 was excluded since it did not pass the leave-one-out sensitivity analysis; and the results indicated that those tended to have a higher risk of lung cancer if not quitting smoking (OR = 4.29 [2.60, 7.07], p-value = 1.23x10-8) (Fig 5). Besides, we found a dose-response relationship between the number of cigarettes and lung cancer (OR = 6.10 [5.35, 6.96], p-value = 4.43x10-161) (Fig 6). These results added evidence to the well-established causal relationship between smoking and lung cancer, indicating a necessity for quitting smoking. All MR-Egger intercept p-value and Cochrane’s p-value were more than 0.05.
Fig 4. MR analysis of the effect of smoking initiation on lung cancer.
Fig 4A is the scatter plot of the MR result. Fig 4B is the forest plot of the leave-one-out sensitivity result.
Fig 5. MR analysis of the effect of smoking cessation on lung cancer.
Fig 5A is the scatter plot of the MR result. Fig 5B is the forest plot of the leave-one-out sensitivity result.
Fig 6. MR analysis of the effect of cigarettes per day of lung cancer.
Fig 6A is the scatter plot of the MR result. Fig 6B is the forest plot of the leave-one-out sensitivity result.
Anthropometric traits
In this study, we did not observe the causal relationship between BMI and lung cancer (OR = 1.14 [0.85, 1.51], p-value = 0.385) (S1 Fig). Also, neither heterogeneity nor pleiotropy was observed (Cochrane’s Q and MR-Egger intercept p-value > 0.05). For hip circumference, it might not lead to the change of lung cancer risk (OR = 1.03 [0.77, 1.38], p-value = 0.840) (S2 Fig) and the result was still insignificant after the adjustment of BMI (OR = 1.21 [0.93, 1.57], p-value = 0.151). Meanwhile, an increased waist circumference could not decrease the risk of lung cancer (OR = 0.97 [0.67, 1.40], p-value = 0.878) (S3 Fig), even after the adjustment of BMI (OR = 1.28 [0.90, 1.82], p-value = 0.177). Similarly, WHR was not causally associated with lung cancer, whether it was adjusted by BMI (OR = 1.11 [0.68, 1.83], p-value = 0.662) (S4 Fig) or not (OR = 1.05 [0.67, 1.65], p-value = 0.825).
However, we found BMI could increase the number of cigarettes per day (beta = 0.139 [0.104, 0.175], p-value = 1.99x10-14). Additionally, an increase of waist circumference and hip circumference could elevate the number of cigarettes per day as well (waist circumference beta = 0.172 [0.119, 0.226], p-value = 1.92x10-10; hip circumference beta = 0.115 [0.065, 0.164], p-value = 5.16x10-6).
Neither heterogeneity nor pleiotropy was detected in all these MR results (Cochrane’s Q p-value > 0.05; MR-Egger intercept p-value > 0.05; MR-PRESSO global test p-value > 0.05).
Overall, we did not observe the evidence that anthropometric traits, including BMI, waist circumference, hip circumference, and WHR, could directly affect the risk of lung cancer.
Blood pressure
In our MR analysis, both SBP (OR = 1.00 [0.99, 1.01], p-value = 0.638) and DBP (OR = 1.00 [0.98, 1.01], p-value = 0.586) could not lead to the change of lung cancer risk (S5 and S6 Figs). When exploring the causal effect on smoking behavior, no significant results were observed after Bonferroni correction. However, the result indicated the elevation of SBP could increase the number of cigarettes per day (beta = 0.001 [0.0002, 0.002], p-value = 0.018). There was marginal heterogeneity in DBP (Cochrane’s p-value = 0.045) and we adopted the random effect model. Besides, we did not observe any heterogeneity and pleiotropy in other results (Cochrane’s Q p-value > 0.05; MR-Egger intercept p-value > 0.05; MR-PRESSO global test p-value > 0.05).
Lipid traits
Four kinds of blood lipid levels were treated as exposures and the decreased HDL level was likely to increase the risk of lung cancer with a relatively marginal statistical significance (OR = 0.82 [0.68, 1.00], p-value = 0.045) (S7 Fig). Besides, LDL (OR = 0.87 [0.73, 1.02], p-value = 0.092), total cholesterol (OR = 0.90 [0.75, 1.08], p-value = 0.260) and triglycerides (OR = 1.03 [0.80,1.33], p-value = 0.824) were not causally associated with lung cancer (p-value > 0.05) (S8–S10 Figs). We did not observe significant results when treating the smoking behavior as the outcome after Bonferroni correction, but the HDL could marginally reduce the number of cigarettes per day (beta = -0.024 [-0.048, -0.001], p-value = 0.043). There was no heterogeneity and pleiotropy in these analyses (Cochrane’s Q p-value > 0.05; MR-Egger intercept p-value > 0.05; MR-PRESSO global test p-value > 0.05).
Glycemic traits & fasting insulin
No causation was observed between glycemic traits and lung cancer, including HbA1c (OR = 0.93 [0.53, 1.66], p-value = 0.817) (S11 Fig) and fasting glucose (OR = 0.94 [0.70, 1.27], p-value = 0.681) (S12 Fig). Furthermore, a higher fasting insulin level could not lead to lung cancer (OR = 1.42 [0.50, 4.03], p-value = 0.514) (S13 Fig). Also, 2-h glucose could not change the risk of lung cancer (OR = 1.04 [0.54, 2.00], p-value = 0.911). No significant results were observed when treating the smoking behavior as the outcome. The fasting glucose result indicated the existence of heterogeneity (Cochrane’s Q p-value = 0.019) and a random effect model was employed to it. Besides, there was no pleiotropy or heterogeneity of other results (Cochrane’s Q p-value > 0.05; MR-Egger intercept p-value > 0.05; MR-PRESSO global test p-value > 0.05).
Bidirectional MR between smoking, alcohol intake, and BMI
Considering the complex relationship between smoking, alcohol intake, and BMI, we have performed a bi-directional MR analysis for them. Our bi-directional MR suggested that alcohol intake could cause a higher BMI (beta = 0.197[0.135, 0.258], p-value = 4.59 x 10−10) and a higher BMI can increase the number of cigarettes per day (beta = 0.139[0.104, 0.175], p-value = 1.99 x 10−14). Besides, no other significant relationship was observed in this bi-directional analysis between these three risk factors. There was no pleiotropy or heterogeneity of other results (Cochrane’s Q p-value > 0.05; MR-Egger intercept p-value > 0.05; MR-PRESSO global test p-value > 0.05).
Power calculation
The power of smoking initiation, smoking cessation, and the number of cigarettes per day were all 100%, indicating a sufficient statistical power for smoking behavior’s causal effect on lung cancer. The power for HDL is 0.69 and LDL is 0.57. Besides, no other exposure’s power was greater than 0.5.
Discussion
In this MR study, we assessed the causal relationship between modifiable risk factors and lung cancer, hoping to help control this common cancer. These MR results indicated smoking was still the most risk factor for lung cancer. Besides, decreased level of HDL cholesterol level could marginally elevate the risk of lung cancer, but become insignificant after Bonferroni correction; and no other direct causal relationship was observed. Therein, we excluded the causal effect of blood pressure on lung cancer. When treating smoking behavior as the outcome, we found the BMI, waist circumference and hip circumference could increase the number of cigarettes per day, suggesting that genetic predisposition to obesity could increase the risk of smoking and further elevate the risk of lung cancer. However, we should take caution of it as there was no true total effect of risk factors on lung cancer except smoking. Additionally, SBP could marginally increase the number of cigarettes per day while HDL cholesterol might slightly decrease it.
The alcohol intake behavior is usually in strong correlation with smoking behavior and we are likely to observe the positive association between alcohol intake and lung cancer [1]. However, the association between alcohol consumption and lung cancer is still under debate for never-smokers [30,31]; and our results lend support for it that habitual alcohol intake cannot elevate the risk of lung cancer. Two reasons might help to account for it: (1) Only 1 genetic variant is available for this MR analysis and the statistical power is not sufficient; (2) The habitual alcohol intake cannot affect the risk of lung cancer if removing the impact of smoking. In our bi-directional MR analysis, we ruled out the causal relationship between alcohol intake and smoking, and alcohol intake cannot directly affect the risk of lung cancer. The previously reported association between alcohol intake and lung cancer might be confounded considering that alcohol intake could lead to a higher BMI.
As for smoking behavior, three subtypes of such behavior indicated smoking is the most perilous factor for lung cancer and there is a dose-response relationship between the number of cigarettes per day and lung cancer. Our results were consistent with previous MR studies [10,15]. Considering that Shen et al included the number of cigarettes per day in their study, the causal relationship between smoking cessation and lung cancer from our study indicated quitting smoking might be effective in reducing the risk of lung cancer. Thus, it is never late to quit smoking.
Obesity and related phenotypes were reported to be inversely associated with lung cancer and such phenomenon was called the “obesity paradox” [32]. Previous MR results unveiled that a higher BMI could increase the risk of lung squamous cell carcinoma, while not adenocarcinoma; and it could not increase the overall risk of lung cancer [11]. Our MR results lend strong support to it that a higher BMI could not affect the risk of lung cancer in enlarged sample size, but we could not validate the causal effect in subtypes of lung cancer due to data limits. BMI is an indicator for general obesity while WHR is for central obesity. Our results demonstrate that obesity might not directly elevate the risk of lung cancer no matter what the obesity type is. Since MR utilized genetic variants to evaluate the effect of risk factors on lung cancer, it could reduce the potential confounders to the largest extent; and the observed “obesity paradox” might be biased by potential confounders. In our bi-directional MR analysis between smoking, alcohol intake, and smoking, we found that obesity might alter smoking behavior and increase the number of cigarettes per day after Bonferroni correction. This conclusion was confirmed by Taylor et al where there was a positive causal effect of BMI on smoking [33]. Thus, we deemed that previously observed associations between obesity and lung cancer might be confounded by smoking.
A previous study indicated the association between increased blood pressure and lung cancer in men [34] while the most recent observational challenged this conclusion where blood pressure was not associated with lung cancer [14]. Our study firstly lent support to the latter using Mendelian randomization. We observed that the elevation of SBP could increase the number of cigarettes per day but it turned insignificant after the Bonferroni correction. Thus, we deemed that the previously observed association between blood pressure and lung cancer might be confounded by smoking behavior. Also, the conclusion should not be definite since we did not take sex differences in our MR study. Furthermore, these observational results might be biased due to their relatively small sample size. Overall, genetically elevated blood pressure might not increase the risk of lung cancer, but its sex-specific differences should be further investigated.
Our results indicate a very weak causal relationship between HDL cholesterol and lung cancer, and add evidence in previous observational studies where reduced lipid levels could elevate the risk of lung cancer [35]. Meanwhile, the elevation of HDL cholesterol could slightly decrease the number of cigarettes per day. However, such MR causal estimation becomes insignificant after Bonferroni correction. Our MR results for glycemic traits and fasting insulin did not observe any causal relationship. Such results seem to be inconsistent with previous observational and MR studies where the increased fasting insulin level could elevate the risk of lung cancer [11,36]. However, the direction of OR suggested high fasting insulin level could increase the risk of lung cancer, though not significant, suggesting a lack of statistical power of IVs for fasting insulin in our study. Thus, the association between fasting insulin and lung cancer should be further explored. The metabolic profile in lung cancer is much heterogenous and tumor cells can alter the original human metabolism [37]. Our MR results show that alteration of both lipid and glucose metabolism cannot increase the risk of lung cancer while not vice versa.
In our study, we performed a comprehensive MR to explore the risk factors for lung cancer and further identified risk factors for smoking behavior. Here, we strictly followed 3 assumptions for MR analysis and guaranteed the IV’s validity and power. Of the three MR assumptions, only the first 1 can be well satisfied and assumptions 2 and 3 cannot be fully met. We selected SNP reaching the genome-wide significance (p-value < 5x10-8) as the IV for the exposure and calculated the F statistics to appraise the power. However, assumption 2 cannot be fully tested since we cannot thoroughly rule out the association between IV and confounders. Thus, we performed the heterogeneity and pleiotropy test using Cochrane’s Q value, MR-Egger intercept, and MR-PRESSO, hoping to reduce the bias caused by assumption 2. As for assumption 3, we cannot judge whether a SNP can directly affect the outcome. Thus, we simply removed SNPs more likely to be associated with the outcome than the exposure using the MR Steiger test. However, several limitations should be pointed: (1) We cannot perform MR analysis on different subtypes of lung cancer due to data limitation; (2) We strictly selected the IVs, which might lower the statistical power; (3) We could not avoid the potential selection bias in evaluating lung cancer since individuals with multiple cancer diagnoses were classified as a case only for their first cancer. Thus, a case might suffer from more than 1 cancer and the lung cancer might be later than other cancer. Due to data limitation, we cannot obtain the individual-level data and cannot exactly appraise the potential selection bias in evaluating lung cancer due to its competing risk factors or missing genetic makeup during recruitment for exposures of interest; (4) Assumption 3 might be violated for binary exposures, but our conclusion might be robust considering that the number of cigarettes per day is a continuous variable for smoking behavior. It should be noted that the MR analysis tended to obtain more negative results than traditional observational studies since the 3 assumptions usually excluded more SNPs and lowered the statistical power. Thus, we still cannot rule out other risk factors’ effects on lung cancer.
In conclusion, our MR results indicate smoking behavior might be the sole effective modifiable way to reduce the risk of lung cancer considering three types of smoking behavior were all causally associated with lung cancer, and quitting smoking could lower the risk. Besides, the genetic liability to obesity can increase the risk of smoking. This MR study suggested the effects of other risk factors on smoking, indicating previously observed associations between risk factors and lung cancer might be confounded by smoking, such as blood pressure and HbA1c. Our study indicated smoking is the most perilous factor for lung cancer, further strengthening the need for tobacco control.
Supporting information
A is the scatter plot of MR result. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result of the effect of hip circumference on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result of the effect of waist circumference on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result of the effect of WHR on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result for the effect of DBP on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result for the effect of SBP on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result for the effect of HDL cholesterol on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result for the effect of LDL cholesterol on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result for the effect of total cholesterol on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result for the effect of triglycerides on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result for the effect of HbA1c on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result for the effect of fasting glucose on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result for the effect of fasting insulin on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
Acknowledgments
J.D proposed the idea, elaborated the research, and wrote the draft of the manuscript. Both J.D and Z.T contributed to the data analysis and manuscript revision. H.C revised the manuscript. Z.L supervised the whole research and is responsible for the integrity of the study.
Data Availability
The accession number of data from https://www.ebi.ac.uk/gwas/ is: GCST90011812.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Malhotra J, Malvezzi M, Negri E, La Vecchia C, Boffetta P. Risk factors for lung cancer worldwide. The European respiratory journal. 2016;48(3):889–902. Epub 2016/05/14. doi: 10.1183/13993003.00359-2016 . [DOI] [PubMed] [Google Scholar]
- 2.Proctor RN. Tobacco and the global lung cancer epidemic. Nature reviews Cancer. 2001;1(1):82–6. Epub 2002/03/20. doi: 10.1038/35094091 . [DOI] [PubMed] [Google Scholar]
- 3.Yang YC, Fu WP, Zhang J, Zhong L, Cai SX, Sun C. rs401681 and rs402710 confer lung cancer susceptibility by regulating TERT expression instead of CLPTM1L in East Asian populations. Carcinogenesis. 2018;39(10):1216–21. Epub 2018/06/26. doi: 10.1093/carcin/bgy084 . [DOI] [PubMed] [Google Scholar]
- 4.Yu D, Zheng W, Johansson M, Lan Q, Park Y, White E, et al. Overall and Central Obesity and Risk of Lung Cancer: A Pooled Analysis. Journal of the National Cancer Institute. 2018;110(8):831–42. Epub 2018/03/09. doi: 10.1093/jnci/djx286 ; PubMed Central PMCID: PMC6093439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyu Z, Li N, Wang G, Feng X, Chen S, Su K, et al. Independent and joint associations of blood lipids and lipoproteins with lung cancer risk in Chinese males: A prospective cohort study. International journal of cancer. 2019;144(12):2972–84. Epub 2018/12/12. doi: 10.1002/ijc.32051 . [DOI] [PubMed] [Google Scholar]
- 6.Lin X, Liu L, Fu Y, Gao J, He Y, Wu Y, et al. Dietary Cholesterol Intake and Risk of Lung Cancer: A Meta-Analysis. Nutrients. 2018;10(2). Epub 2018/02/09. doi: 10.3390/nu10020185 ; PubMed Central PMCID: PMC5852761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan S, Kar S, Carter P, Vithayathil M, Mason AM, Burgess S, et al. Is Type 2 Diabetes Causally Associated With Cancer Risk? Evidence From a Two-Sample Mendelian Randomization Study. Diabetes. 2020;69(7):1588–96. Epub 2020/05/01. doi: 10.2337/db20-0084 ; PubMed Central PMCID: PMC7306131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization. Jama. 2017;318(19):1925–6. Epub 2017/11/23. doi: 10.1001/jama.2017.17219 . [DOI] [PubMed] [Google Scholar]
- 9.Zhou H, Zhang Y, Liu J, Yang Y, Fang W, Hong S, et al. Education and lung cancer: a Mendelian randomization study. International journal of epidemiology. 2019;48(3):743–50. Epub 2019/06/21. doi: 10.1093/ije/dyz121 . [DOI] [PubMed] [Google Scholar]
- 10.Zhou W, Liu G, Hung RJ, Haycock PC, Aldrich MC, Andrew AS, et al. Causal relationships between body mass index, smoking and lung cancer: Univariable and multivariable Mendelian randomization. International journal of cancer. 2021;148(5):1077–86. Epub 2020/09/12. doi: 10.1002/ijc.33292 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carreras-Torres R, Johansson M, Haycock PC, Wade KH, Relton CL, Martin RM, et al. Obesity, metabolic factors and risk of different histological types of lung cancer: A Mendelian randomization study. PLoS One. 2017;12(6):e0177875. Epub 2017/06/09. doi: 10.1371/journal.pone.0177875 ; PubMed Central PMCID: PMC5464539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyko EJ. Observational research—opportunities and limitations. Journal of Diabetes and its Complications. 2013;27(6):642–8. doi: 10.1016/j.jdiacomp.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindgren A, Pukkala E, Nissinen A, Tuomilehto J. Blood pressure, smoking, and the incidence of lung cancer in hypertensive men in North Karelia, Finland. Am J Epidemiol. 2003;158(5):442–7. Epub 2003/08/26. doi: 10.1093/aje/kwg179 . [DOI] [PubMed] [Google Scholar]
- 14.Christakoudi S, Kakourou A, Markozannes G, Tzoulaki I, Weiderpass E, Brennan P, et al. Blood pressure and risk of cancer in the European Prospective Investigation into Cancer and Nutrition. International journal of cancer. 2020;146(10):2680–93. Epub 2019/07/19. doi: 10.1002/ijc.32576 ; PubMed Central PMCID: PMC7115826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen J, Zhou H, Liu J, Zhang Y, Zhou T, Yang Y, et al. A modifiable risk factors atlas of lung cancer: A Mendelian randomization study. Cancer Medicine. 2021;10(13):4587–603. doi: 10.1002/cam4.4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sudmant PH, Rausch T, Gardner EJ, Handsaker RE, Abyzov A, Huddleston J, et al. An integrated map of structural variation in 2,504 human genomes. Nature. 2015;526(7571):75–81. Epub 2015/10/04. doi: 10.1038/nature15394 ; PubMed Central PMCID: PMC4617611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu K, Li B, McGinnis KA, Vickers-Smith R, Dao C, Sun N, et al. Genome-wide association study of smoking trajectory and meta-analysis of smoking status in 842,000 individuals. Nature communications. 2020;11(1):5302. Epub 2020/10/22. doi: 10.1038/s41467-020-18489-3 ; PubMed Central PMCID: PMC7598939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelernter J, Sun N, Polimanti R, Pietrzak RH, Levey DF, Lu Q, et al. Genome-wide Association Study of Maximum Habitual Alcohol Intake in >140,000 U.S. European and African American Veterans Yields Novel Risk Loci. Biological psychiatry. 2019;86(5):365–76. Epub 2019/06/04. doi: 10.1016/j.biopsych.2019.03.984 ; PubMed Central PMCID: PMC6919570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Justice AE, Karaderi T, Highland HM, Young KL, Graff M, Lu Y, et al. Protein-coding variants implicate novel genes related to lipid homeostasis contributing to body-fat distribution. Nature genetics. 2019;51(3):452–69. Epub 2019/02/20. doi: 10.1038/s41588-018-0334-2 ; PubMed Central PMCID: PMC6560635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H, et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nature genetics. 2018;50(10):1412–25. Epub 2018/09/19. doi: 10.1038/s41588-018-0205-x ; PubMed Central PMCID: PMC6284793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, et al. Discovery and refinement of loci associated with lipid levels. Nature genetics. 2013;45(11):1274–83. Epub 2013/10/08. doi: 10.1038/ng.2797 ; PubMed Central PMCID: PMC3838666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wheeler E, Leong A, Liu CT, Hivert MF, Strawbridge RJ, Podmore C, et al. Impact of common genetic determinants of Hemoglobin A1c on type 2 diabetes risk and diagnosis in ancestrally diverse populations: A transethnic genome-wide meta-analysis. PLoS medicine. 2017;14(9):e1002383. Epub 2017/09/13. doi: 10.1371/journal.pmed.1002383 ; PubMed Central PMCID: PMC5595282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saxena R, Hivert MF, Langenberg C, Tanaka T, Pankow JS, Vollenweider P, et al. Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nature genetics. 2010;42(2):142–8. Epub 2010/01/19. doi: 10.1038/ng.521 ; PubMed Central PMCID: PMC2922003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagou V, Mägi R, Hottenga JJ, Grallert H, Perry JRB, Bouatia-Naji N, et al. Sex-dimorphic genetic effects and novel loci for fasting glucose and insulin variability. Nature communications. 2021;12(1):24. Epub 2021/01/07. doi: 10.1038/s41467-020-19366-9 ; PubMed Central PMCID: PMC7785747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rashkin SR, Graff RE, Kachuri L, Thai KK, Alexeeff SE, Blatchins MA, et al. Pan-cancer study detects genetic risk variants and shared genetic basis in two large cohorts. Nature communications. 2020;11(1):4423. Epub 2020/09/06. doi: 10.1038/s41467-020-18246-6 ; PubMed Central PMCID: PMC7473862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Statistics in medicine. 2008;27(8):1133–63. Epub 2007/09/22. doi: 10.1002/sim.3034 . [DOI] [PubMed] [Google Scholar]
- 27.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nature genetics. 2018;50(5):693–8. Epub 2018/04/25. doi: 10.1038/s41588-018-0099-7 ; PubMed Central PMCID: PMC6083837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. European journal of epidemiology. 2017;32(5):377–89. Epub 2017/05/21. doi: 10.1007/s10654-017-0255-x ; PubMed Central PMCID: PMC5506233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS genetics. 2017;13(11):e1007081. Epub 2017/11/18. doi: 10.1371/journal.pgen.1007081 ; PubMed Central PMCID: PMC5711033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boffetta P, Hashibe M. Alcohol and cancer. The Lancet Oncology. 2006;7(2):149–56. Epub 2006/02/04. doi: 10.1016/S1470-2045(06)70577-0 . [DOI] [PubMed] [Google Scholar]
- 31.García-Lavandeira JA, Ruano-Ravina A, Barros-Dios JM. Alcohol consumption and lung cancer risk in never smokers. Gaceta sanitaria. 2016;30(4):311–7. Epub 2016/06/09. doi: 10.1016/j.gaceta.2016.03.017 . [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Liu Y, Shao H, Zheng X. Obesity Paradox in Lung Cancer Prognosis: Evolving Biological Insights and Clinical Implications. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2017;12(10):1478–88. Epub 2017/08/02. doi: 10.1016/j.jtho.2017.07.022 . [DOI] [PubMed] [Google Scholar]
- 33.Taylor AE, Richmond RC, Palviainen T, Loukola A, Wootton RE, Kaprio J, et al. The effect of body mass index on smoking behaviour and nicotine metabolism: a Mendelian randomization study. Human molecular genetics. 2019;28(8):1322–30. Epub 2018/12/19. doi: 10.1093/hmg/ddy434 ; PubMed Central PMCID: PMC6452214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stocks T, Van Hemelrijck M, Manjer J, Bjørge T, Ulmer H, Hallmans G, et al. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension (Dallas, Tex: 1979). 2012;59(4):802–10. Epub 2012/02/23. doi: 10.1161/HYPERTENSIONAHA.111.189258 . [DOI] [PubMed] [Google Scholar]
- 35.Puchades-Carrasco L, Jantus-Lewintre E, Pérez-Rambla C, García-García F, Lucas R, Calabuig S, et al. Serum metabolomic profiling facilitates the non-invasive identification of metabolic biomarkers associated with the onset and progression of non-small cell lung cancer. Oncotarget. 2016;7(11):12904–16. Epub 2016/02/18. doi: 10.18632/oncotarget.7354 ; PubMed Central PMCID: PMC4914330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Argirion I, Weinstein SJ, Männistö S, Albanes D, Mondul AM. Serum Insulin, Glucose, Indices of Insulin Resistance, and Risk of Lung Cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2017;26(10):1519–24. Epub 2017/07/13. doi: 10.1158/1055-9965.EPI-17-0293 ; PubMed Central PMCID: PMC5626607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hensley CT, Faubert B, Yuan Q, Lev-Cohain N, Jin E, Kim J, et al. Metabolic Heterogeneity in Human Lung Tumors. Cell. 2016;164(4):681–94. Epub 2016/02/09. doi: 10.1016/j.cell.2015.12.034 ; PubMed Central PMCID: PMC4752889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A is the scatter plot of MR result. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result of the effect of hip circumference on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result of the effect of waist circumference on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result of the effect of WHR on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result for the effect of DBP on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result for the effect of SBP on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result for the effect of HDL cholesterol on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result for the effect of LDL cholesterol on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result for the effect of total cholesterol on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result for the effect of triglycerides on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result for the effect of HbA1c on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result for the effect of fasting glucose on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
A is the scatter plot of MR result for the effect of fasting insulin on lung cancer. B is the forest plot of leave-one-out sensitivity result.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
The SNP is the result of genetic variants; A1 is the effect allele; A2 is the other allele; beta is the effect size of A1 on the exposure; she is the standard error of beta; pval is the p-value of beta; F is the F statistics.
(PDF)
Data Availability Statement
The accession number of data from https://www.ebi.ac.uk/gwas/ is: GCST90011812.