Abstract
Autoinjectors are self-injectable devices; they are important class of medical devices which can deliver drugs through subcutaneous or intramuscular route. They enclose prefilled syringes or cartridges which are driven by a spring system. The major benefits of this device are easy self-administration, improved patient compliance, reduced anxiety, and dosage accuracy. Immediate treatment during emergency conditions such as anaphylaxis, migraine, and status epilepticus or for chronic conditions like psoriasis, diabetes, multiple sclerosis, and rheumatoid arthritis, Reformulation of first-generation biologics, technical advancements, innovative designs, patient compliance, overwhelming interest for self-administration all these made entry of more and more autoinjectors into use. In this review, intensive efforts have been made for exploring the different types of currently available autoinjectors for the management of emergency and chronic diseases.
Keyword: Autoinjector, Chronic diseases, Emergency condition, Medical device
1. Introduction
Autoinjectors are self-injectable devices; they are important class of medical devices which can deliver drugs through subcutaneous or intramuscular route. They enclose prefilled syringes or cartridges which are driven by a spring system. The major benefits of this device are easy self-administration, improved patient compliance, reduced anxiety, and dosage accuracy. The autoinjectors were initially introduced in 1960s for military purpose to inject the antidote for nerve agents in chemical warfare (Vijayaraghavan, 2012). Later in 1980s EpiPen got approval for administration of epinephrine for anaphylaxis (Posner and Camargo, 2017). Immediate treatment during emergency conditions, prevalence of many chronic diseases, reformulation of first-generation biologics, technical advancements, innovative designs, patient compliance, overwhelming interest for self-administration all these made entry of more and more autoinjectors into use (Vijayaraghavan, 2012).
More than 20 pharmaceutical companies have developed nearly about 80 autoinjectors until today. Nearly 50 drugs are developed as combination products for administration using autoinjectors. Among this, 62% of the autoinjectors are disposable. There is a tremendous growth in the development of reusable autoinjectors with multiple dosing. This highly competent developments in device manufacture, give importance to user friendly devices for emergency use as well as for chronic disease conditions. Hence, devices with push on skin, one handed delivery, twist and mix mechanism, sleek and handy design, automatic reconstitution, devices with blue tooth connection, devices for large volume with high viscosity are the new challenges met with advancement of these gadgets (Global Autoinjectors Market, 2016).
2. Drug delivery through autoinjector
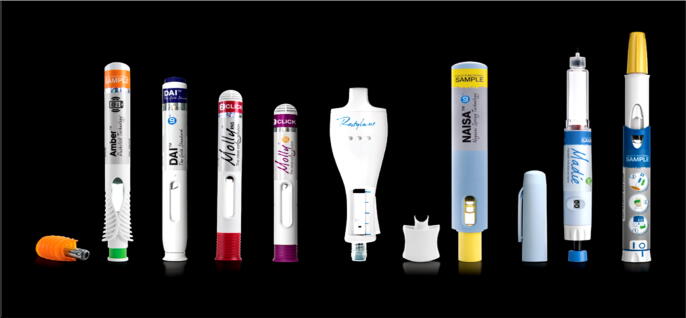
Autoinjectors (Fig. 1) are devices for self-administration of drugs in emergency situations such as anaphylaxis, migraine, and status epilepticus or for chronic conditions like psoriasis, multiple sclerosis and rheumatoid arthritis (Vijayaraghavan, 2012). Autoinjectors can be used either subcutaneously or by intramuscular route (Table 1). There are many advantages for autoinjectors. They work with different principles. There are autoinjectors with simple push- on- skin devices to fully automated button activated technologies. There are many autoinjectors as alternative to manual syringe for subcutaneous administration of drugs for different conditions (French and Collins, 2010). These devices have increased patient compliance and adherence because the drugs can be self-injected. Several antidotes for toxic and poisonous chemicals, disease modifying drugs and monoclonal antibodies have been proven to be very effective, easy to use and tolerable in humans (Vijayaraghavan, 2020).
Fig. 1.
Commercially available autoinjectors for drug delivery. (Adopted from https://www.ondrugdelivery.com, Accessed on 01 March 2021.)
Table 1.
List of autoinjector devices for the administration of some drugs in use and under clinical trials (Vijayaraghavan, 2020).
| Clinical condition | Autoinjector device | Route of administration |
Dosage forms Strength |
Dose |
|---|---|---|---|---|
| Anaphylaxis | Epinephrine (EpiPen) Omalizumab (Xolair**) |
i.m. s.c. |
0.3 mg & 0.15 mg 75 mg/0.5 mL |
0.3 mg 150 mg/mL |
| Seizures | Diazepam Midazolam |
i.m. i.m. |
10 mg 10 mg/2 mL or 5 mg/mL |
10 mg-20 mg 5 mg-10 mg |
| Organophosphorus poisoning | Atropine (AtroPen®) Pralidoxime Pralidoxime + Atropine (DuoDote® autoinjector) |
i.m. i.m. i.m |
0.5 mg/1 mg/2 mg 600 mg/2 mL (Pralidoxime 600 mg/2 mL + Atropine 2.1 mg/0.7 mL) |
1–2 g |
| Migraine | Sumatriptan (ZEMBRACE® SymTouch®) | s.c. | 3 mg | 3–12 mg/day |
| Opioid overdose | Naloxone (Evzio) | i.m./s.c. | 0.4 mg & 2 mg/0.4 mL | 0.4 mg-2 mg |
| Psoriasis | Ixekizumab (TALTZ) Secukinumab (Cosentyx) Certolizumab pegol (cimzia) |
s.c. s.c. s.c. |
80 mg/mL 150 mg or 300 mg 200 mg/mL |
|
| Rheumatoid arthritis | Sirukumab (PLIVENSIA™) Sarilumab (KEVZARA) Tocilizumab (ACTpen) Certolizumab pegol Golimumab (SIMPONI) Adalimumab (Humira pen) Methotrexate (Otrexup) Etanercept (Enbrel®) |
s.c. s.c. s.c. s.c. s.c. s.c. s.c. s.c |
50 mg, 100 mg 200 mg 62 mg/0.9 mL 200 mg/mL 40 mg/0.8 mL 10, 15, 20, and 25 mg 50 mg/mL |
Q4W Q2W Q2W 50 mg once a month (7.5–25 mg/week) 50 mg/week |
| Multiple sclerosis | Interferon β-1a (Avonex) Interferon β-1b (ExtaviPro™) |
i.m s.c |
30 μg 0.3 mg. |
30 μg/week 0.0625 mg alternate day |
| Systemic lupus erythematosus | Belimumab (Benlysta) | s.c. | 200 mg/mL | 200 mg/week |
| Erectile dysfunction | Aviptadil/phentolamine | i. c. | 2 μg/2 mg | |
| Analgesic | Buprenorphine* | i.m. | ||
| Diabetes | Insulin (KwikPen) Exenatide (Exenatide QWS AI) |
s.c. s.c. |
100 unit/200 units/3 mL pen 2 mg |
10 units/day 2 mg/week |
| Antibacterial | Amikacin* Amikacin + Cefazolin* |
i.m. i.m. |
500 mg 250 mg + 250 mg 250 mg + |
500 mg/day 250 mg/day |
| Hypercholesterolaemia | Evolocumab (SureClick) Alirocumab |
s.c. s.c. |
140 mg/mL 75 |
Q2W mg/mL single-dose pre-filled pen |
i.m. - intramuscular, s.c. - subcutaneous, i.c. – intracorporeal, * only in animal model.
** Pre-filled syringe-s.
A study has been conducted for the safe and effective use of the autoinjector devices on 43 participants in 5 groups, received training and performed simulated injections either into an injection pad or a mannequin. 93% of the user reported comfort, 98% expressed confidence in using the device and 98% expressed comprehensibility in instruction for use and training (Lange et al., 2015). Another study with 48 participants compared the usability and patient preference of different brands of epinephrine- epipen, twinject, INT01 and INT02, and the study concluded that correct epinephrine autoinjector use and patient preference depends on the user-centered device design. The study included preference among the different preparations, task completion time, usability, and device malfunction (Guerlain et al., 2010). In another study on 118 patients with multiple sclerosis, 90% of them preferred fully automatic interferon beta-1b autoinjector to their previous injection method and expressed high level of satisfaction. It has many features in reducing injection site pain, promoting comfort, smooth injections, and ease of use (Ziemssen et al., 2015).
3. Autoinjectors available currently
There is a fast growth in global autoinjectors due to increase in prevalence and incidence of anaphylaxis and food allergies. Anaphylaxis is increasing in developed countries (Poulos et al., 2007, Rudders et al., 2014). One of the reasons for anaphylaxis is the prevalence of food allergy (Branum and Lukacs, 2009). In 2015, 15 million people in the U.S suffered from food allergies as per American Academy of Allergy Asthma and Immunology.
European Academy of Allergy and Clinical Immunology reported that 3.5 million children suffered from food allergy in the same year. 200–250 million people in the world suffered from food allergy in 2015 as per WHO reports. Being the drug of choice for anaphylaxis, there was an overwhelming growth in the development of epinephrine autoinjectors. There are many chronic diseases which otherwise require a comfortable drug delivery by the patient or caregiver, further brought fueling demand for autoinjectors. Many studies support the preference of autoinjectors by patients in chronic disease such as rheumatoid arthritis, multiple sclerosis, and psoriasis, as they are more convenient and easier to use compared to syringes and vials; moreover, they are less painful than prefilled syringes (Kivitz et al., 2006, Lugaresi et al., 2008, Berteau et al., 2010, Lim et al., 2012, Demary et al., 2014). Autoinjectors not only help in the quality of life but also can improve self-esteem, ease of use, time, and cost. They also help in better treatment adherence, reduced costs for society and could reduce frequency of health care visit.
4. Autoinjectors for emergency
4.1. Epinephrine autoinjectors for anaphylaxis
Epinephrine (also known as adrenaline) is the physiological antagonist for histamine. Adrenaline is not effective orally (Simons et al., 2014) and administration through subcutaneous route takes nearly 30 min. to reach optimal level, while intramuscular injection can give a rapid peak (Simons et al., 2001). However, taste masked sublingual tablets are produced for pediatric use, also subcutaneous adrenaline reaches peak plasma concentration in 30 min. for an emergency critical situation IM administration will be convenient and effective (Simons et al., 2001, Rachid et al., 2018).
It is used for anaphylaxis, i.e, sudden allergic reactions which are potentially life threatening. The salient features of anaphylaxis include itching, erythema, pruritus, urticaria, angioedema, bronchospasm, laryngeal edema, cough, and respiratory arrest. Anaphylaxis is rapid in onset and may be mild, serious or fatal (Kemp et al., 2008, Simons et al., 2014, Simons et al., 2011). Allergic reactions can be due to many reasons – drug induced, or food induced. It can be associated with exercise, latex, and insect stings or with any unknown factors. Rapid response is needed to prevent the complications because the affected person becomes panic. There are many risk factors for anaphylaxis like age, disease - asthma or medications like beta- blockers and angiotensin – converting enzyme inhibitors (Simons et al., 2015). Recurrence after treatment also can occur in case of anaphylaxis (Lieberman et al., 2015).
The itching and hives can be treated with antihistamines like diphenhydramine, but their effect will be slow when taken orally. Bronchodilators can control cough and wheezing. The most serious and deleterious symptoms of anaphylaxis – throat swelling, and low blood pressure can be reversed immediately by parenteral epinephrine only. Injection of epinephrine i.m. is life saving and first line drug for severe anaphylactic reactions as per World Allergy Guidelines. In rare cases, death occurs soon after contact with the allergen. Respiratory arrest can occur with food allergens within half an hour. Insect stings are more dangerous and can lead to collapse from shock even within 15 min. Same is the case of allergic reactions to parenteral injection of medicine which may cause death within 5 min. (Soar et al., 2008, Boyce et al., 2010, Simons et al., 2011). Hence, spontaneous treatment of anaphylactic reaction is necessary.
Delayed epinephrine administration may lead to worse outcomes and fatalities (Fleming et al., 2015). To prevent hospitalization and fatal complications, it is necessary to administer epinephrine immediately after diagnosis (Fromer, 2016). Epinephrine autoinjector is classified as a grade A or B preventive medicine by the United States Preventive Services Task Force (USPSTF). It is included in the preventive medication lists as a first line drug for anaphylaxis treatment which may reduce fatalities from anaphylaxis. Many branded autoinjectors are available for epinephrine – epipen, twinject, INT01 and INT02 (Guerlain et al., 2010). Epipen or epipen jr contain one dose per autoinjector for adults and the young children, respectively. As the injector works through clothing no need to undress while using the autoinjectors. Auvi-Q or allerject is also available as adult and child dose with a recording for its self-injecting process. Adrenaclick - is another autoinjector that contains one dose of epinephrine. 5–10 µg/kg is the recommended dose. The recommended dose through autoinjector is 150 µg for children weighing between 15 and 30 kg and adults 300–500 µg. There were different studies to optimise the route of administration of adrenaline in both adult and children. It was found that the intramuscular injection in thighs could achieve greater levels compared to subcutaneous route. In children, the subcutaneous injection was done with needle and syringe and the intramuscular injection with autoinjector- EpiPen. This study concluded that the average time to get maximum level through intramuscular was far better than subcutaneous injection mentioning the rapid action of adrenaline through autoinjector- EpiPen by intramuscular route (Simons et al., 2001).
The highest risk for food allergy and fatality is associated with adolescents (Bock et al., 2007). In modern life, more people are depending upon catering service for routine meals. In Spain, around two million children depend on catering service and prone to potentially life – threatening food allergies. At least one food allergy is reported in approximately 18% of the school children (Nowak-Wegrzyn et al., 2001). There are many reports from Spain showing a great increase in the food anaphylaxis (Alonso et al., 2012, Alonso et al., 2015). Sesame is an important allergen and a major cause of anaphylaxis in the Middle East and is the third most common food allergen in Israel. The management of food allergy will be managed by providing education about allergen avoidance, and prompt and correct use of epinephrine in case of an emergency (Adatia et al., 2017). Anaphylaxis and allergic conditions are managed by i.m epinephrine (Campbell et al., 2016).
4.2. Diazepam autoinjectors for acute status epilepticus
Diazepam is a benzodiazepine derivative. Diazepam autoinjector can deliver the contents automatically upon activation. Studies on healthy volunteers prove that diazepam autoinjector can provide 100% mean percent availability as that obtained from the syringe injection. It is indicated for the management of anxiety disorders and short-term relief of the symptoms of anxiety. It has great value in status epilepticus, severe recurrent convulsive seizures. Diazepam is useful premedication for relief of anxiety and tension in patients who are to undergo surgical procedures. In1990s, diazepam autoinjectors were used in military service for nerve agent intoxication (Carroll et al., 2012).
Acute repetitive seizure is also known as cluster or serial seizure that typically lasts for several minutes to one or two days (Haut, 2006). In the absence of prompt intervention with drug, it can be progressed to status epilepticus or prolonged seizures. Such episodes limit social gathering because of the unavailability of immediate medical attention. Oral preparations can be used for cooperative and conscious patients. Otherwise, intravenous administration of benzodiazepines or antiepileptic drugs in an emergency department is mandatory. Previous reports claim that i.m. administration of diazepam is inconsistent. Recent studies revealed that i.m. administration through autoinjectors could provide rapid and accurate drug delivery and dosing, rapid and reliable drug absorption (Garnett et al., 2011, Lamson et al., 2011, Silbergleit et al., 2012).
A randomized double blinded multicentric study with 234 participants on stable antiepileptic drugs who required intermittent medical intervention for acute status epilepticus were treated with diazepam and placebo using autoinjectors. The study concluded the effectiveness, tolerance, and capacity to delay next seizure capacity of the diazepam autoinjectors and its usefulness and convenience by the trained care givers in emergency situations (Abou-Khalil et al., 2013).
4.3. Midazolam autoinjectors acute seizures
Midazolam is a rapidly acting benzodiazepine with sedative, anxiolytic, anticonvulsant and amnestic properties (Mandrioli et al., 2008). It is the drug of choice for acute seizures and status epilepticus and nerve agent–induced seizures. It modulates and potentiates the synaptic – GABAa receptors and bring about its anti-seizure activity. GABAa receptor are pentameric in structure with five subunits enclosing the central chloride ion channel (Möhler et al., 2002, Kucken et al., 2003). They are able to inhibit neuronal excitability. Midazolam can be administered through different routes – i.v., i.m. and intranasal route. Seizures can be suppressed rapidly by administering midazolam by intranasal route, but it is not advisable in case of casualty due to nerve agent as they can produce copious nasal secretions (Thakker and Shanbag, 2013).
Immediate control of nerve agent induced seizure is very important for neuroprotection and survival (Shih et al., 2003). Among the chemical agents, nerve agents are the most toxic which act by irreversible inhibition of acetylcholinsterase leading to hyper secretion, respiratory distress, tremor, convulsions, and status epilepticus. Japan and Syria witnessed extreme neurotoxic potential of Sarin (Yanagisawa et al., 2006, Dolgin, 2013, Rosman et al., 2014). The status epilepticus caused by nerve agents leads to profound brain damage which may be fatal or can precipitate long term neuronal dysfunction. Long lasting seizures usually result increased brain damage so for immediate neuroprotection and survival, its rapid control is critical (Shih et al., 2003).
Pharmacokinetic properties of midazolam make it a better choice to diazepam to treat status epilepticus and persistent acute seizures caused by nerve agents and organophosphorus compounds. Rapid anticonvulsant medication (Rampart) prior to arrival trial with i.m. midazolam was found to be very effective compared to i.v. lorazepam. Intramuscular autoinjector delivery can be accomplished faster than i.v. administration with comparable effectiveness. It has more social acceptance, easiness in use and convenience. It is found to be a reliable option for acute seizure by caregivers (Reddy and Reddy, 2015).
4.4. Autoinjectors for nerve agent and organophosphorus intoxication
Organophosphorus compounds are extremely toxic and some of them are used as chemical weapons. These agents have the potential to inhibit acetyl cholinesterase enzyme responsible to hydrolyse acetyl choline irreversibly leading to accumulation of acetyl choline. The excessive acetyl choline leads to hyper secretions, miosis, tremors, convulsion and respiratory depression which may be fatal. Immediate administration of atropine is advisable. Organophosphorus compounds are also used for pesticide purpose. Atropine is administered intravenously in a hospital set up in the case of poisoning. In case of terrorist attack or chemical war or casualty, i.m. administration of atropine using autoinjector is very useful. However, atropine can reverse only the muscarinic symptoms of acetyl choline. Pralidoxime (PAM), is used within twenty-four hours to reverse the cholinesterase activity. It combines with the free anionic site of the phosphorylated enzyme complex and dephosphorylates the enzyme and makes it free to release the enzyme. Thus, the freed enzyme could hydrolyse all the excessive acetyl choline and reverse the symptoms of poisoning. Traditional indigenous designed autoinjector contains PAM chloride and atropine sulphate drugs for nerve agent poisoning. Here, both the drugs are separately injected but to avoid such a delay, PAM chloride (300 mg/mL) in combination with atropine sulphate (1 mg/mL) autoinjectors were developed by research and development laboratories for organophosphorous poisoning to replace the traditional autoinjector containing PAM chloride and atropine sulphate (Jain et al., 2012). A three drug combination of atropine sulphate, pralidoxime chloride and diazepam are available in the brand name chempacks for nerve agent and organophosphorous poisoning (Nolin et al., 2006). This could additionally manage the acute severe convulsions produced by some of the nerve agents.
4.5. Sumatriptan autoinjectors for migraine
Sumatriptan is a drug used for migraine headaches. It is available as s.c. injection, rectal suppositories, nasal spray, and oral tablets. Migraine can cause severe impairment and reduction of cognitive function. The condition is not only debilitating but also associated with severe pain. It also impairs hand – eye coordination. DFN-11, a two-step autoinjector of sumatriptan was found to be very effective for cognitive and physical impairments. It allows easy, safe, and simple use of the drug by the patient or untrained people. It was found to be easier than s.c. injection from a vial. The DFN-11 autoinjector was designed for easy use during cognitive impairments, to have clear visual and audible feedback indicators. Studies on patients have validated the usability and safety of autoinjector to treat migraine. Moreover DFN-11 autoinjector was with lower incidence of triptan sensation especially without chest pain compared to normal s.c. injections (Cady et al., 2017).
A study was conducted to assess the ability of patients to self-administer sumatriptan using autoinjector, Alsuma for acute migraine. The effectiveness, tolerability and safety were also evaluated. This phase 3 trial was conducted in 10 different sites in USA with 63 patients who were asked to administer 6 mg sumatriptan by autoinjector during the onset of moderate to severe migraine and assessed after 72 h. All the patients agreed that the instruction for use in the label was easy to follow and clear. More than 95% agreed its easiness of use and 65% reported their preference for the new device over the traditional autoinjector which they were using prior to this study. The study concluded that the autoinjector is safe and well tolerated with mild and infrequent injection site reactions (Landy et al., 2013).
4.6. Naloxone autoinjector for opioid overdosage
Naloxone is a short-acting, competitive opioid antagonist used for respiratory or central nervous system depression from opioid overdose. Unlike opioids, it is non-addictive. It is used for the rapid reversal of opioid overdose. In emergency, it is the standard first line drug. In 2014, FDA has approved naloxone injector for adults and children (Merlin et al., 2015). The autoinjector ezvioTM was considered as an extremely important innovation. It is very handy and small. It is with auditory and visual commands for easy administration. It is a prefilled device which is battery operated. It has a 23 gauge retractable needle which can be used to inject the drug into the lateral thigh even through the dress (Berteau et al., 2010). The rapid dissemination of naloxone is the only effective way to combat the crisis of opioid overdose around the world (Merlin et al., 2015).
5. Autoinjectors for chronic disease
5.1. Insulin pen for diabetes
The first reusable insulin pen was launched in 1984 and was commercialised by Novo Nordisk. The development of 1.5 mL insulin cartridges, 3 mL insulin cartridges made them easy to use. The first disposable insulin pen was introduced in 1990s. Now nearly 1.3 billion insulin cartridges in the form of reusable or disposable pens are available. Pen devices have a high level of convenience with features including large display and dose correction, geared or spring driven injection mechanism, and simple cartridge exchange for reusable pens (Pearson, 2010). The most available self injection drug may be insulin as the diabetic burden is very high in global level. So much research is going on with the devices for minimizing long term costs and improved diabetic care with less complication. So much digital health advancements are seen with insulin pen currently (Sangave et al., 2019).
5.2. Exenatide autoinjector for diabetes
Exenatide is a glucagon-like peptide-1 agonist. Administration of insulin and oral hypoglycaemic agents are to be on everyday basis in diabetes mellitus. The efficacy and safety of exenatide autoinjector was compared with sitagliptin and placebo in a multicentric study with 365 patients who were on metformin. This 28 week study showed that exenatide autoinjector could significantly reduce HbA1c compared to sitagliptin. There was no significant difference in reduction of fasting sugar in both exenatide group and sitagliptin treated group, but the former group showed better HbA1c level, less than 7% than latter group and placebo treated group. The efficacy and safety of exenatide was proved in this study (Gadde et al., 2017). In another open label randomized study with 375 patients, the efficacy and safety of exenatide autoinjector once in a week was compared to exenatide twice daily preparation. Exenatide autoinjector could reduce HbA1c to a greater extent with minimum adverse effects (Wysham et al., 2018).
5.3. Ixekizumab autoinjector for psoriasis
Ixekizumab is an anti-interleukin 17A monoclonal antibody, used in the treatment of moderate-to-severe plaque psoriasis, which is a chronic, immune-mediated inflammatory skin disease. It requires continuous long-term systemic treatment. Ixekizumab is given subcutaneous injection with an autoinjector or a prefilled syringe. Ixekizumab delivered through an autoinjector or prefilled syringe is found to be equivalent in their pharmacokinetic profile. The ixekizumab autoinjector – Uncover - A has an ergonomic shape, grip, and dose with a button wider base and has a lock to prevent the device from misfiring. It has audible clicks to confirm the start and end of drug delivery. Studies show that this autoinjector can facilitate patient compliance and satisfaction on long term therapy (Duffin et al., 2017).
5.4. Certolizumab pegol autoinjector for rheumatoid arthritis
Certolizumab pegol (CZP), a polyethylene glycosylated, Fc-free anti-TNF, for moderate-to-severe rheumatoid arthritis which is a systemic disease with persistant synovial inflammation causing pain, stiffness and swelling of joints and progresses to loss of function and mobility (Kvien, 2004). The combination of antitumour necrotizing factor with disease modifying drugs is a breakthrough in the treatment of rheumatoid arthritis (Smolen et al., 2007, Smolen et al., 2014). However, for short- and long-term benefits of this anti tumour necrotising factor therapy depends on adherence to treatment. The patient factors like needle phobia, lack of confidence, deformities, pain, swelling in the joints, limited manual dexterity may complicate self-administration of drugs (Keininger and Coteur, 2011). In such a scenario, self-administration devices can provide many advantages like portability, flexibility in the timing of injection and ease of use which can further improve adherence to regular therapy (Anderson and Redondo, 2011). Certolizumab pegol autoinjector has an ergonomic design intended to facilitate self-administration. Two identical studies with 76 patients were performed in 2013 and 2016 to assess the comparative usability of this autoinjector with the other three autoinjectors adalimumab, etanercept, and golimumab devices. 67% of 2013 studies and 59% of 2016 studies preferred Certolizumab pegol autoinjector. The patients found it very easy to use and favoured with low rate of error and high level of satisfaction (Domańska et al., 2017).
Xiao et al has demonstrated the feasibility of performance of simulated self-injection and found that even patients with rheumatoid arthritis with severe hand disability could perform self-injection with autoinjector in similar level as that of healthy volunteers concluding that autoinjectors not only have convenience and easiness of use but also have high level of device acceptance and compliance (Xiao et al., 2018)
5.5. Methotrexate autoinjector for rheumatoid arthritis
Methotrexate (MTX), a disease modifying drug for the pharmacological management of rheumatoid arthritis is recommended by the American College of Rheumatology (Singh et al., 2012). It has got anti-inflammatory, immunosuppressant, and cytotoxic effect. MTX may be used orally but in case of inadequate clinical response or intolerance, the multinational recommendation is parenteral methotrexate as its bioavailability is optimum and administered by s.c. route to treat rheumatoid arthritis (Visser et al., 2009). This route was found to be difficult by the patients and caregivers as it is a cytotoxic drug; careful handling of the drug is a must with vials, syringes, and needles (Freundlich et al., 2014). Moreover, in rheumatoid arthritis, self-injection is a challenge as they have impaired hand strength. Such functional limitations could be overcome by the use of comfortable devices which are easy to handle to improve therapy (Domańska et al., 2017). Later FDA has approved otrexup, single use, prefilled, pressure assisted autoinjector for severe rheumatoid arthritis (Gaurav, 2013). A phase 2, multi-centric, clinical trial was conducted with 102 participants with rheumatoid arthritis to evaluate the human use of the methotrexate autoinjector (MTXAI). The patients were trained and evaluated for pain after self-administration of methotrexate. 98% of the patients rated it as easy to use. All of them (100%) reported its instructions were easy and clear to follow while 92.3% reported no erythema at the injection site. The study concluded that the subcutaneous MTXAI was easy to use by rheumatoid arthritis patients and was well tolerated with improved delivery (Freundlich et al., 2014).
In another study, evaluation of the MTX usability by self-administration as per user instruction was carried out in 43 subjects. This multicentric study was conducted in two phases with three different groups of subjects. Two groups were with rheumatoid arthritis and the third group was care givers. This trial revealed the robustness and reliability of the use of autoinjectors by rheumatoid arthritis patients. The results proved high level of acceptance, usability, and intuitive handling of the new methotrexate autoinjector irrespective of hand disability. More than 75% showed correct performance in the first phase just by reading the instructions and in second phase, all subjects showed 100% correct performance. Rheumatoid arthritis patients with high hand disability also showed 100% performance (Hudry et al., 2017). A two-week study was conducted in five different centres in the U.S. during 2012–2013 to assess the usability, device robustness, pharmacokinetic profile and safety outcome on 103 subjects. The study also evaluated the label comprehension and safety of s.c. self-administration of MTX using the MTX pen in patients with rheumatoid arthritis who required MTX treatment (Pachon et al., 2014).
A study conducted in Germany in 12 different centres assessed the preference of methotrexate prefilled pen to the prefilled syringes. It was a prospective and two sequence crossover study. Among the study group 76% preferred prefilled pen over prefilled syringes. The usability, satisfaction and acceptability of the self-injecting pen were appreciated by 73% − 76% of the total 120 patients participated in the study. The prefilled pen of methotrexate was introduced for easy use and patient’s convenience. In the prefilled pen, the needle covers automatically which could protect the patient from accidental needle prick and secondary infections (Demary et al., 2014). In another study with 130 patients on methotrexate therapy, reported the preference and comfort of high concentration of methotrexate, 50 mg/mL over 10 mg/mL as this could reduce injected volume with prefilled syringe (Hoekstra et al., 2004, Cronstein, 2005).
In a recent study to compare the acceptability and compliance of injection of methotrexate with pre-filled syringe and new auto-injector in rheumatoid arthritis patients, the conclusion was that though the compliance the users expressed was excellent with both the devices they have used but the preference showed was for autoinjectors (Saraux et al., 2019).
5.6. Interferon autoinjector for multiple sclerosis
Interferon beta -1b is a drug used in the management of multiple sclerosis which is a chronic autoimmune disease. It affects all parts of the central nervous system. In the early stages of the disease, most of the patients experience periods of neurological relapses and remissions which progress into neurological deterioration later (Barone et al., 2016). Though complete cure is not possible for multiple sclerosis, the disease exacerbation could be reduced with disease modifying drugs like interferon β-1a so that the disability progression may be delayed and produce effective improvement. A common feature of patients with multiple sclerosis is the deterioration of fine motor skills which reduce their capacity to administer injections (Lugaresi et al., 2014). As these patients will be on chronic medication the drug administration will be a task on regular basis. In addition to the deterioration of motor skills, needle phobia and anxiety to injection make the patient to skip or discontinue medication (Tremlett et al., 2008, Devonshire et al., 2011). Hence, ease of use is very important for self-administration of the drug, which can be achieved by improvements in the autoinjector. In such cases electronic autoinjector may be a better option.
Bayer Pharma AG has introduced BetaconnectTM, an electronic autoinjector of interferon beta-1b/betaseron for multiple sclerosis. This autoinjector is with an ergonomic design which makes the device easy to handle and allows one-handed injections with hidden needle. It has audibled and visible indicators of battery status, safety release and dose reminder functions. The syringe for interferon beta-1b release is placed within the autoinjector, and the interior features injection setting controls (Ziemssen et al., 2015). The most valued unique features are its self-check function, built in dwell time, greater ability to customize injections, low injection noise, adjustment of injection speed and automatic needle retraction. A survey conducted on 90 multiple sclerosis patients for their preference of betaconnect over their current mechanical autoinjectors, 83 percent preferred betaconnect over their current autoinjector (Barone et al., 2016).
Using Rebismart – a retrospective observational study was reported for the administration of interferon beta-1a to assess the adherence and relapses. This cohort study was conducted between 2010 and 2015 with 110 Spanish patients. The study concluded that 96.5% had median adherence and 77.3% had relapse free. The study also concluded that, with every percentage unit increase, there was 4.7% decrease in relapse supporting the acceptability of self-administered rebismart for interferon beta-1a (Solsona et al., 2017).
Kleiter et al. (2017) has conducted a study among patients with relapsing remitting multiple sclerosis or with a clinically isolated syndrome were treated IFN beta-1b, BETACONNECT® autoinjector to assess the adherence and satisfaction BETACONNECT® autoinjector therapy. The study could conclude that the autoinjectors showed high compliance, adherence, persistence being a user friendly. It also boosted up their confidence in using the device. Hence, BETACONNECT may be a useful tool to use IFN beta-1b in patients with multiple sclerosis.
5.7. Belimumab autoinjector for systemic lupus erythematosus
Systemic lupus erythematosus is an autoimmune disease with chronic inflammation and associated manifestations like fatigue, joint pain, and wrist and fingers swelling can make difficulty in the regular life (Tench et al., 2000, Lau and Mak, 2009, Pettersson et al., 2012, Holloway et al., 2014).
B-lymphocyte stimulator is associated with systemic lupus erythematosus, which is a potent B- cell survival and differentiation factor (Thompson et al., 2000, Do et al., 2000, Batten et al., 2000, Cheema et al., 2001, Zhang et al. 2001, Stohl et al., 2017). Belimumab is a human, monoclonal antibody with B-lymphocyte stimulator inhibitory action (Baker et al., 2003). Intravenous infusion of belimumab is used as an add on drug to the normal therapy of systemic lupus erythematosus, which can be done only in hospital set up and this gave way to the development of subcutaneous injection of belimumab with autoinjector, so that the drug can be easily and safely administered.
The bioavailability of belimumab autoinjector was studied in healthy subjects on s.c. administration (Struemper et al., 2016). The safety profile of s.c. belimumab was compared with i.v. belimumab and found to be safe. A randomized, placebo-controlled 52 weeks study explored the efficacy and safety of s.c. belimumab in systemic lupus erythematosus. Its safety profile was similar to that of belimumab as reported earlier. As it could be administered by the patient, it has become a viable treatment choice (Stohl et al., 2017). Self-administration is a criterion especially in conditions like rheumatoid arthritis as it could decrease the travel and expense to take an infusion in the hospital and hence patients prefer to get the drug administered by themselves (Chilton and Collett, 2008). Self-administration of belimumab 200 mg as single dose s.c. injection using prefilled syringes or autoinjector in healthy volunteers was found to be reliable and safe with good usability profile (Struemper et al., 2016). This dose could give a steady state area under curve similar to 10 mg/kg i.v. every four weeks (Cai et al., 2013, Yapa et al., 2016).
The suitability of a novel autoinjector for self-administration of belimumab by patients with active systemic lupus erythematosus and the changes in pharmacokinetic trough concentrations when switching from i.v. to s.c. administration was assessed in patients. It was an open-label multi-dose study in 95 patients. The safety and tolerability of belimumab administered via the autoinjector was also evaluated. Patients have self-administered belimumab 200 mg s.c. through autoinjector device for 8 weekly doses which was well tolerated and showed good level of reliability and usability. The pharmacokinetic data strongly supported a time interval of 1 – 4 weeks, when switching between the i.v. and s.c. administration (Sheikh et al., 2016).
5.8. Golimumab autoinjector for ulcerative colitis
Golimumab is available as 50 mg and 100 mg solution, in prefilled syringe and prefilled Smartject autoinjector (Flamant et al., 2017). It can be used for moderate to severe ulcerative colitis (Strik et al., 2017), which is a chronic inflammatory bowel disease characterized by symptoms of bloody diarrhea, abdominal pain, and asthenia.
Golimumab is a drug used for the treatment rheumatoid arthritis. In a study, golimumab was administered by s.c. route using autoinjector and needle with syringe. This study could prove a comparable tolerability and pharmacokinetic property of the two drug delivery systems. The patient evaluation of golimumab autoinjector was carried out in a prospective, open label study with 3280 patients and found 67.7% people were comfortable to use autoinjector and had less baseline disease activity (Schulze-Koops et al., 2015).
5.9. Phentolamine autoinjector for erectile dysfunction
Phentolamine is an adrenergic drug. It is available in autoinjector with vasoactive intestinal polypeptide for erectile dysfunction. The availability of this autoinjector could overcome the difficulty of drug administration and different side effects associated with most of the preparations available for this condition. Reliaject is a patented autoinjector which is used for the safe, easy, and accurate administration of this combination (Keijzers, 2001).
5.10. Buprenorphine autoinjector for severe pain
Buprenorphine is used for the management of severe pain including cancer pain. It is an opioid analgesic which is 25 times more potent than morphine in its analgesic activity. It is having long duration of action due to its tight binding to tissues. Buprenorphine is found to be safe in pregnancy and is preferred to be formulated in autoinjector for its long action. Buprenorphine administered through autoinjector was compared with manual injection and found to be safe in rat model (Sheela et al., 2015). The buprenorphine cartridges for analgesic purpose were prepared and safety evaluation and quality control tests were carried out and found to be safe for further research The haematological and biochemical parameters were evaluated after administering buprenorphine for 7 and 14 days and there was no significant difference between the autoinjector and manual injection (Sheela et al., 2017).
5.11. Amikacin alone and in combination with cefazolin autoinjector for microbial infection
The aminoglycoside antibiotic amikacin sulphate is effective on aerobic gram-negative bacteria, a few gram-positive microorganisms and gentamycin-resistant organisms (Gonzalez and Spencer, 1998). Vijayaraghavan et al. (2014) studied and reported the stress induced changes in haematological and biochemical responses by the administration of amikacin injection using autoinjector in animals and found to be safe and tolerable. An autoinjector with 500 mg amikacin sulphate has been developed with dual dose adjustment and a dilution facility for child and also for veterinary use (Anitha et al., 2016). Geetha et al has reported the combination of amikacin with cefazolin (250 mg + 500 mg/2 mL) for bio-threat organisms (Geetha et al., 2016).
6. Conclusions
Autoinjectors has many benefits for the user and health care professionals in case of emergency as well as in chronic diseases. It is recommended for anaphylaxis, migraine, status epilepticus, psoriasis, diabetes, multiple sclerosis, and rheumatoid arthritis. The administration of drugs using autoinjectors device provides multiple benefits like safety, efficacy, and fast absorption. The drug cartridge can also be replaced and the autoinjector device can be made reusable. Autoinjector device could be used to deliver emergency drugs to children and it may be used interchangeably. Autoinjector device is used to deliver antidotes and vaccines for veterinary purposes in farm and pet animals. Natural and manmade disasters are sudden and rapid, many times it is not possible to mobilize the exposed casualties for medical treatment. An autoinjector would be very useful in such situations since it can deliver the drugs within seconds. Autoinjectors are proven safe for many lifesaving drugs. Looking to the future, these needs will continue to be important in device development and selection. In conclusion, we recommend that the new autoinjector entering the market should have the following requirements: 1. Safety, 2. Ease of use, 3. Convenience, 4. Sustainability, 5. Interchangeable use, 6. Provide additional off-device services, and 7. Reusable.
7. Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable for this article.
Funding
This study was not supported by any funding.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Authors are thankful to Saveetha Institute of Medical and Technical Sciences, India and AIMST University, Malaysia for providing the necessary facilities to write this review.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abou-Khalil B., Wheless J., Rogin J., Wolter K.D., Pixton G.C., Shukla R.B., Sherman N.A., Sommerville K., Goli V., Roland C.L. A double-blind, randomized, placebo-controlled trial of a diazepam auto-injector administered by caregivers to patients with epilepsy who require intermittent intervention for acute repetitive seizures. Epilepsia. 2013;54:1968–1976. doi: 10.1111/epi.12373. [DOI] [PubMed] [Google Scholar]
- Adatia A., Clarke A.E., Yanishevsky Y., Ben-Shoshan M. Sesame allergy: current perspectives. J. Asthma Allergy. 2017;10:141. doi: 10.2147/JAA.S113612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso T.M.A., Moro Moro M., Mosquera González M., Rodriguez-Alvarez M., Pérez Fernández E., Latasa Zamalloa P., Farias Aquino E., Gil Prieto R., Gil de Miguel A. Increased incidence of admissions for anaphylaxis in Spain 1998–2011. Allergy. 2015;70(7):880–883. doi: 10.1111/all.12613. [DOI] [PubMed] [Google Scholar]
- Alonso T.M.A., Moro Moro M., Mugica Garcia M.V., Esteban Hernandez J., Rosado Ingelmo A., Vila Albelda C., Gomez Traseira C., Cardenas Contreras R., Sanz Sacristan J., Hernandez Merino A. Incidence of anaphylaxis in the city of Alcorcon (Spain): a population-based study. Clin. Exp. Allergy. 2012;42(4):578–589. doi: 10.1111/j.1365-2222.2012.03930.x. [DOI] [PubMed] [Google Scholar]
- Anitha R., Vijayaraghavan R., Geetha R.V., Anitha M., Vishnu Priya S., Anusha R. A comparative study of the effect of amikacin administered through autoinjector and manual injection on biochemical parameters in rats. J. App. Pharm. Sci. 2016;6(02):109–114. [Google Scholar]
- Anderson B.J., Redondo M.J. What can we learn from patient-reported outcomes of insulin pen devices? J. Diabetes Sci. Tech. 2011;5(6):1563–1571. doi: 10.1177/193229681100500633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K.P., Edwards B.M., Main S.H., Choi G.H., Wager R.E., Halpern W.G., Albert V.R. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum. 2003;48(11):3253–3265. doi: 10.1002/art.11299. [DOI] [PubMed] [Google Scholar]
- Barone D.A., Singer B.A., Merkov L., Rametta M., Suarez G. Survey of US patients with multiple sclerosis: comparison of the new electronic interferon beta-1b autoinjector (BETACONNECTTM) with mechanical autoinjectors. Neurol. Ther. 2016;5:155–167. doi: 10.1007/s40120-016-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batten M., Groom J., Cachero T.G., Qian F., Schneider P., Tschopp J., Browning J.L., Mackay F. BAFF mediates survival of peripheral immature B Lymphocytes. J. Exp. Med. 2000;192(10):1453–1466. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berteau C., Schwarzenbach F., Donazzolo Y., Latreille M., Berube J., Abry H., Cotten J., Feger C., Laurent P.E. Evaluation of performance, safety, subject acceptance, and compliance of a disposable autoinjector for subcutaneous injections in healthy volunteers. Patient Prefer. Adherence. 2010;4:379–388. doi: 10.2147/ppa.s13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock S.A., Muñoz-Furlong A., Sampson H.A. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J. Allergy Clin. Immunol. 2007;119(4):1016–1018. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- Boyce, J.A., Assa’ad, A., Burks, A.W., Jones, S.M., Sampson, H.A., Wood, R.A., Plaut, M., Cooper, S.F., Fenton, M.J., Arshad, S. H., Bahna, S.L., Beck, L.A., Byrd-Bredbenner, C., Camargo, C.A., Jr, Eichenfield, L., Furuta, G.T., Hanifin, J.M., Jones, C., Kraft, M., Levy, B.D., Lieberman, P., Luccioli, S., McCall, K.M., Schneider, L.C., Simon, R.A., Simons, F.E.R., Teach, S.J., Yawn, B.P., Schwaninger, J.M., 2010. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J. Allergy Clin. Immunol. 126 (6 Suppl), S1-58. [DOI] [PMC free article] [PubMed]
- Branum A.M., Lukacs S.L. Food allergy among children in the United States. Pediatrics. 2009;124:1549–1555. doi: 10.1542/peds.2009-1210. [DOI] [PubMed] [Google Scholar]
- Cady R.K., Munjal S., Cady R.J., Manley H.R., Brand-Schieber E. Randomized, double-blind, crossover study comparing DFN-11 injection (3 mg subcutaneous sumatriptan) with 6 mg subcutaneous sumatriptan for the treatment of rapidly escalating attacks of episodic migraine. J. Headache Pain. 2017;18(1):17. doi: 10.1186/s10194-016-0717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W.W., Fiscella M., Chen C., Zhong Z.J., Freimuth W.W., Subich D.C. Bioavailability, pharmacokinetics, and safety of belimumab administered subcutaneously in healthy subjects: Clinical pharmacology in drug development. Clin. Pharmacol. Drug Dev. 2013;2:349–357. doi: 10.1002/cpdd.54. [DOI] [PubMed] [Google Scholar]
- Duffin C.K., Bagel J., Bukhalo M., Mercado Clement I.J., Choi S.L., Zhao F., Gill A., Pangallo B., Shuler C., Mallbris L., Jackson K. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A) J. Eur. Acad. Dermatol. Venereol. 2017;31:107–113. doi: 10.1111/jdv.13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R.L., Bellolio M.F., Motosue M.S., Sunga K.L., Lohse C.M., Rudis M.I. Autoinjectors preferred for intramuscular epinephrine in anaphylaxis and allergic reactions. West. J. Emerg. Med. 2016;17:775–782. doi: 10.5811/westjem.2016.8.30505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J.K., Cullinan E., Clarke L., Davis N.F. The role of anxiolytic premedication in reducing preoperative anxiety. Br. J. Nurs. 2012;21:479–483. doi: 10.12968/bjon.2012.21.8.479. [DOI] [PubMed] [Google Scholar]
- Cheema G.S., Roschke V., Hilbert D.M., Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001;44:1313–1319. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Chilton F., Collett R.A. Treatment choices, preferences, and decision-making by patients with rheumatoid arthritis: Treatment choices in patients with RA. Musculoskeletal Care. 2008;6:1–14. doi: 10.1002/msc.110. [DOI] [PubMed] [Google Scholar]
- Cronstein B.N. Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol. Rev. 2005;57:163–172. doi: 10.1124/pr.57.2.3. [DOI] [PubMed] [Google Scholar]
- Demary W., Schwenke H., Rockwitz K., Kästner P., Liebhaber A., Schoo U., Hübner G., Pichlmeier U., Guimbal-Schmolck C., Müller-Ladner U. Subcutaneously administered methotrexate for rheumatoid arthritis, by prefilled syringes versus prefilled pens: patient preference and comparison of the self-injection experience. Patient Prefer. Adherence. 2014;8:1061–1071. doi: 10.2147/PPA.S64111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devonshire, V., Lapierre, Y., Macdonell, R., Ramo-Tello, C., Patti, F., Fontoura, P., Suchet, L., Hyde, R., Balla, I., Frohman, E.M., Kieseier, B.C., GAP Study Group, 2011. The Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis: Global Adherence Project: adherence to DMTs in MS. Eur. J. Neurol. 18, 69–77. [DOI] [PubMed]
- Do R.K., Hatada E., Lee H., Tourigny M.R., Hilbert D., Chen-Kiang S. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J. Exp. Med. 2000;192:953–964. doi: 10.1084/jem.192.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgin E. Syrian gas attack reinforces need for better anti-sarin drugs. Nat. Med. 2013;19:1194–1195. doi: 10.1038/nm1013-1194. [DOI] [PubMed] [Google Scholar]
- Domańska B., VanLunen B., Peterson L., Mountian I., Schiff M. Comparative usability study for a certolizumab pegol autoinjection device in patients with rheumatoid arthritis. Expert Opin. Drug Deliv. 2017;14:15–22. doi: 10.1080/17425247.2016.1256283. [DOI] [PubMed] [Google Scholar]
- Flamant M., Paul S., Roblin X. Golimumab for the treatment of ulcerative colitis. Expert Opin. Biol. Ther. 2017;17:879–886. doi: 10.1080/14712598.2017.1327576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J.T., Clark S., Camargo C.A., Jr, Rudders S.A. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J. Allergy Clin. Immunol. Pract. 2015;3:57–62. doi: 10.1016/j.jaip.2014.07.004. [DOI] [PubMed] [Google Scholar]
- French, D.L., Collins, J.J., 2010. Advances in parenteral injection devices and aids. In: Pharmaceutical dosage forms, parenteral medications, third ed. Informa Healthcare, London, UK, vol. 3, pp. 71–75.
- Freundlich, B., Kivitz, A., Jaffe, J.S., 2014. Nearly pain-free self-administration of subcutaneous methotrexate with an autoinjector: results of a phase 2 clinical trial in patients with rheumatoid arthritis who have functional limitations: Results of a phase 2 clinical trial in patients with rheumatoid arthritis who have functional limitations. J. Clin. Rheumatol. 20, 256–260. [DOI] [PMC free article] [PubMed]
- Fromer L. Prevention of anaphylaxis: The role of the epinephrine auto-injector. Am. J. Med. 2016;129:1244–1250. doi: 10.1016/j.amjmed.2016.07.018. [DOI] [PubMed] [Google Scholar]
- Gadde, K.M., Vetter, M.L., Iqbal, N., Hardy, E., Öhman, P., DURATION-NEO-2 study investigators, 2017. Efficacy and safety of autoinjected exenatide once-weekly suspension versus sitagliptin or placebo with metformin in patients with type 2 diabetes: The DURATION-NEO-2 randomized clinical study. Diabetes Obes. Metab. 19, 979–988. [DOI] [PMC free article] [PubMed]
- Garnett W.R., Barr W.H., Edinboro L.E., Karnes H.T., Mesa M., Wannarka G.L. Diazepam autoinjector intramuscular delivery system versus diazepam rectal gel: A pharmacokinetic comparison. Epilepsy Res. 2011;93:11–16. doi: 10.1016/j.eplepsyres.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Gaurav, 2013. Otrexup methotrexate self injection device receives FDA approval to treat rheumatoid arthritis [WWW Document]. Medgadget.com. URL https://www.medgadget.com/2013/10/otrexup-self-injection-device-receives-fda-approval-to-treat-rheumatoid-arthritis.html (accessed 3.1.21).
- Geetha R.V., Roy A., Senthilkumar S., Bhaskar A.S.B., Vijayaraghavan R. A concept of a probable autoinjector for bio-threat agents. Def. Sci. J. 2016;66(5):464–470. [Google Scholar]
- Gonzalez L.S., 3rd, Spencer J.P. Aminoglycosides: a practical review. Am. Fam. Physician. 1998;58:1811–1820. [PubMed] [Google Scholar]
- Guerlain S., Hugine A., Wang L. A comparison of 4 epinephrine autoinjector delivery systems: usability and patient preference. Ann. Allergy Asthma Immunol. 2010;104:172–177. doi: 10.1016/j.anai.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haut S.R. Seizure clustering. Epilepsy Behav. 2006;8:50–55. doi: 10.1016/j.yebeh.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Hoekstra M., Haagsma C., Neef C., Proost J., Knuif A., van de Laar M. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J. Rheumatol. 2004;31:645–648. [PubMed] [Google Scholar]
- Holloway L., Humphrey L., Heron L., Pilling C., Kitchen H., Højbjerre L., Strandberg-Larsen M., Hansen B.B. Patient-reported outcome measures for systemic lupus erythematosus clinical trials: a review of content validity, face validity and psychometric performance. Health Qual. Life Outcomes. 2014;12:116. doi: 10.1186/s12955-014-0116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudry C., Lebrun A., Moura B., Zinovieva E., Backers O., Herman-Demars H. Evaluation of usability and acceptance of a new autoinjector intended for methotrexate subcutaneous self-administration in the management of rheumatoid arthritis. Rheumatol. Ther. 2017;4:183–194. doi: 10.1007/s40744-017-0057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N., Kumar P., Kumar D., Mavai Y., Vijayaraghavan R. Development and evaluation of combined drug formulation for autoject-injector, for emergency application in organophosphate poisoning. Def. Sci. J. 2012;62:105–111. [Google Scholar]
- Keijzers G.B. Aviptadil (senatek) Curr. Opin. Invest. Drugs. 2001;2:545–549. [PubMed] [Google Scholar]
- Keininger D., Coteur G. Assessment of self-injection experience in patients with rheumatoid arthritis: psychometric validation of the Self-Injection Assessment Questionnaire (SIAQ) Health Qual. Life Outcomes. 2011;9:2. doi: 10.1186/1477-7525-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp, S.F., Lockey, R.F., Simons, F.E.R., World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis, 2008. Epinephrine: the drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy 63, 1061–1070. [DOI] [PubMed]
- Kivitz A., Cohen S., Dowd J.E., Edwards W., Thakker S., Wellborne F.R., Renz C.L., Segurado O.G. Clinical assessment of pain, tolerability, and preference of an autoinjection pen versus a prefilled syringe for patient self-administration of the fully human, monoclonal antibody adalimumab: the TOUCH trial. Clin. Ther. 2006;28:1619–1629. doi: 10.1016/j.clinthera.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Kleiter I., Lang M., Jeske J., Norenberg C., Stollfu B., Schurks M. Adherence, satisfaction, and functional health status among patients with multiple sclerosis using the BETACONNECT® autoinjector: a prospective observational cohort study. BMC Neurol. 2017;17:174. doi: 10.1186/s12883-017-0953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucken A.M., Teissére J.A., Seffinga-Clark J., Wagner D.A., Czajkowski C. Structural requirements for imidazobenzodiazepine binding to GABA(A) receptors. Mol. Pharmacol. 2003;63:289–296. doi: 10.1124/mol.63.2.289. [DOI] [PubMed] [Google Scholar]
- Kvien T.K. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics. 2004;22:1–12. doi: 10.2165/00019053-200422001-00002. [DOI] [PubMed] [Google Scholar]
- Lamson M.J., Sitki-Green D., Wannarka G.L., Mesa M., Andrews P., Pellock J. Pharmacokinetics of diazepam administered intramuscularly by autoinjector versus rectal gel in healthy subjects: a phase I, randomized, open-label, single-dose, crossover, single-centre study: A phase I, randomized, open-label, single-dose, crossover, single-centre study. Clin. Drug Investig. 2011;31:585–597. doi: 10.2165/11590250-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Landy S.H., Tepper S.J., Wein T., Schweizer E., Ramos E. An open-label trial of a sumatriptan auto-injector for migraine in patients currently treated with subcutaneous sumatriptan. Headache. 2013;53:118–125. doi: 10.1111/j.1526-4610.2012.02295.x. [DOI] [PubMed] [Google Scholar]
- Lange J., Richard P., Bradley N. Usability of a new disposable autoinjector platform device: results of a formative study conducted with a broad user population. Med. Devices (Auckl.) 2015;8:255–264. doi: 10.2147/MDER.S85938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C.S., Mak A. The socioeconomic burden of SLE. Nat. Rev. Rheumatol. 2009;5:400–404. doi: 10.1038/nrrheum.2009.106. [DOI] [PubMed] [Google Scholar]
- Lieberman, P., Nicklas, R.A., Randolph, C., Oppenheimer, J., Bernstein, D., Bernstein, J., Ellis, A., Golden, D.B.K., Greenberger, P., Kemp, S., Khan, D., Ledford, D., Lieberman, J., Metcalfe, D., Nowak-Wegrzyn, A., Sicherer, S., Wallace, D., Blessing-Moore, J., Lang, D., Portnoy, J.M., Schuller, D., Spector, S., Tilles, S.A., 2015. Anaphylaxis--a practice parameter update 2015. Ann. Allergy Asthma Immunol. 115, 341–384. [DOI] [PubMed]
- Lim, W.H., Chan, D., Boudville, N., Pellicano, S., Herson, H., Moody, H., Hutchison, B., M, S., Dogra, G., 2012. Patient’s perceptions of subcutaneous delivery of darbepoetin alfa by autoinjector prefilled pen versus prefilled syringe: a randomized, crossover study. Clin. Ther. 34, 1948–1953. [DOI] [PubMed]
- Lugaresi, A., Durastanti, V., Gasperini, C., Lai, M., Pozzilli, C., Orefice, G., Sotgiu, S., Pucci, E., Ardito, B., Millefiorini, E., CoSa Study Group, 2008. Safety and tolerability in relapsing-remitting multiple sclerosis patients treated with high-dose subcutaneous interferon-beta by Rebiject autoinjection over a 1-year period: the CoSa study: The CoSa study. Clin. Neuropharmacol. 31, 167–172. [DOI] [PubMed]
- Lugaresi A., Rottoli M.R., Patti F. Fostering adherence to injectable disease-modifying therapies in multiple sclerosis. Expert Rev. Neurother. 2014;14:1029–1042. doi: 10.1586/14737175.2014.945523. [DOI] [PubMed] [Google Scholar]
- Mandrioli R., Mercolini L., Raggi M.A. Benzodiazepine metabolism: an analytical perspective. Curr. Drug Metab. 2008;9:827–844. doi: 10.2174/138920008786049258. [DOI] [PubMed] [Google Scholar]
- Merlin M.A., Ariyaprakai N., Arshad F.H. Assessment of the safety and ease of use of the naloxone auto-injector for the reversal of opioid overdose. Open Access Emerg. Med. 2015;7:21–24. doi: 10.2147/OAEM.S82133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak-Wegrzyn A., Conover-Walker M.K., Wood R.A. Food allergic reactions in schools and preschools. Arch. Pediatr. Adolesc. Med. 2001;155:790–795. doi: 10.1001/archpedi.155.7.790. [DOI] [PubMed] [Google Scholar]
- Möhler H., Fritschy J.M., Rudolph U. A new benzodiazepine pharmacology. J. Pharmacol. Exp. Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Nolin K., Murphy C., Ahern J.W., McBride K., Corriveau M., Morgan J. Chempack program: role of the health-system pharmacist. Am. J. Health Syst. Pharm. 2006;63(2188):2190. doi: 10.2146/ajhp060255. [DOI] [PubMed] [Google Scholar]
- Pachon J.A., Kivitz A.J., Ku H., Pichlmeier U. Assessing usability, label comprehension, pen robustness and pharmacokinetics of a self administered prefilled autoinjector pen of Methotrexate in patients with rheumatoid arthritis. SAGE Open Medicine. 2014;2:1–12. doi: 10.1177/2050312114564241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson T.L. Practical aspects of insulin pen devices. J. Diabetes Sci. Technol. 2010;4:522–531. doi: 10.1177/193229681000400304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson S., Lövgren M., Eriksson L.E., Moberg C., Svenungsson E., Gunnarsson I., Welin Henriksson E. An exploration of patient-reported symptoms in systemic lupus erythematosus and the relationship to health-related quality of life. Scand. J. Rheumatol. 2012;41:383–390. doi: 10.3109/03009742.2012.677857. [DOI] [PubMed] [Google Scholar]
- Posner L.S, Camargo C.A., Jr. Update on the usage and safety of epinephrine auto-injectors, 2017. Drug, Healthcare and Patient Safety. 2017;9:9–18. doi: 10.2147/DHPS.S121733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos L.M., Waters A.M., Correll P.K., Loblay R.H., Marks G.B. Trends in hospitalizations for anaphylaxis, angioedema, and urticaria in Australia, 1993–1994 to 2004–2005. J. Allergy Clin. Immunol. 2007;120:878–884. doi: 10.1016/j.jaci.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Rachid O., Rawas-Qalaji M., Simons K.J. Epinephrine in anaphylaxis: Preclinical study of pharmacokinetics after sublingual administration of taste-masked tablets for potential paediatric use. Pharmaceutics. 2018;10(1):24. doi: 10.3390/pharmaceutics10010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S.D., Reddy D.S. Midazolam as an anticonvulsant antidote for organophosphate intoxication–A pharmacotherapeutic appraisal. Epilepsia. 2015;56:813–821. doi: 10.1111/epi.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosman Y., Eisenkraft A., Milk N., Shiyovich A., Ophir N., Shrot S., Kreiss Y., Kassirer M. Lessons learned from the Syrian sarin attack: evaluation of a clinical syndrome through social media. Ann. Intern. Med. 2014;160:644–648. doi: 10.7326/M13-2799. [DOI] [PubMed] [Google Scholar]
- Rudders S.A., Arias S.A., Camargo C.A., Jr Trends in hospitalizations for food-induced anaphylaxis in US children, 2000–2009. J. Allergy Clin. Immunol. 2014;134 doi: 10.1016/j.jaci.2014.06.018. 960–2.e3. [DOI] [PubMed] [Google Scholar]
- Saraux A., Hudry C., Zinovieva E., Herman-Demars H. Use of auto-injector for methotrexate subcutaneous self-injections: High satisfaction level and good compliance in self-I study, a randomized, open-label, parallel group study. Rheumatol. Ther. 2019;6:47–60. doi: 10.1007/s40744-018-0134-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangave N.A., Aungst T.D., Patel D.K. Smart connected insulin pens, caps, and attachments: A review of the future of diabetes technology. Fall. 2019;32(4):378–384. doi: 10.2337/ds18-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Koops H., Giacomelli R., Samborski W., Rednic S., Herold M., Yao R., Govoni M., Vastesaeger N., Weng H.H. Factors influencing the patient evaluation of injection experience with the SmartJect autoinjector in rheumatoid arthritis. Clin. Exp. Rheumatol. 2015;33:201–208. [PubMed] [Google Scholar]
- Sheela, D., Geetha, R.V., S, K.M., Vijayaraghavan, R., 2015. A concept on the development of buprenorphine autoinjector for self and emergency administration. Int J Pharm Pharm Sci 7, 253–257.
- Sheela D., Vijayaraghavan R., Senthilkumar S. A study on the safety evaluation of buphrenorphine administered through an autoinjector compared with manual injection using haematological and biochemical variables in rats. Hum. Exp. Toxicol. 2017;36:901–909. doi: 10.1177/0960327116674528. [DOI] [PubMed] [Google Scholar]
- Shih T.M., Sm D., McDonough J.H. Control of nerve agent induced seizures is critical for neuroprotection and survival. Toxicol. Appl. Pharmacol. 2003;188:69–80. doi: 10.1016/s0041-008x(03)00019-x. [DOI] [PubMed] [Google Scholar]
- Silbergleit R., Durkalski V., Lowenstein D., Conwit R., Pancioli A., Palesch Y., Barsan W., Investigators N.E.T.T. Intramuscular versus intravenous therapy for prehospital status epilepticus. N. Engl. J. Med. 2012;366:591–600. doi: 10.1056/NEJMoa1107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons F.E., Gu X., Simons K.J. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J. Allergy Clin. Immunol. 2001;108:871–873. doi: 10.1067/mai.2001.119409. [DOI] [PubMed] [Google Scholar]
- Simons F.E.R., Ardusso L.R., Bilò M.B., Cardona V., Ebisawa M., El-Gamal Y.M., Lieberman P., Lockey R.F., Muraro A., Roberts G., Sanchez-Borges M., Sheikh A., Shek L.P., Wallace D.V., Worm M. International consensus on (ICON) anaphylaxis. World Allergy Organ. J. 2014;7:9. doi: 10.1186/1939-4551-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, F.E.R., Ardusso, L.R.F., Bilò, M.B., El-Gamal, Y.M., Ledford, D.K., Ring, J., Sanchez-Borges, M., Senna, G.E., Sheikh, A., Thong, B.Y., World Allergy Organization, 2011. World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ. J. 4, 13–37. [DOI] [PMC free article] [PubMed]
- Simons F.E.R., Ebisawa M., Sanchez-Borges M., Thong B.Y., Worm M., Tanno L.K., Lockey R.F., El-Gamal Y.M., Brown S.G., Park H.-S., Sheikh A. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ. J. 2015;8:32. doi: 10.1186/s40413-015-0080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J.A., Furst D.E., Bharat A., Curtis J.R., Kavanaugh A.F., Kremer J.M., Moreland L.W., O’Dell J., Winthrop K.L., Beukelman T., Bridges S.L., Jr, Chatham W.W., Paulus H.E., Suarez-Almazor M., Bombardier C., Dougados M., Khanna D., King C.M., Leong A.L., Matteson E.L., Schousboe J.T., Moynihan E., Kolba K.S., Jain A., Volkmann E.R., Agrawal H., Bae S., Mudano A.S., Patkar N.M., Saag K.G. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis: 2012 ACR RA Treatment Recommendations. Arthritis Care Res. (Hoboken) 2012;64:625–639. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen J.S., Aletaha D., Koeller M., Weisman M.H., Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861–1874. doi: 10.1016/S0140-6736(07)60784-3. [DOI] [PubMed] [Google Scholar]
- Smolen J.S., Emery P., Fleischmann R., van Vollenhoven R.F., Pavelka K., Durez P., Guérette B., Kupper H., Redden L., Arora V., Kavanaugh A. Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. Lancet. 2014;383:321–332. doi: 10.1016/S0140-6736(13)61751-1. [DOI] [PubMed] [Google Scholar]
- Soar J., Pumphrey R., Cant A., Clarke S., Corbett A., Dawson P., Ewan P., Foëx B., Gabbott D., Griffiths M., Hall J., Harper N., Jewkes F., Maconochie I., Mitchell S., Nasser S., Nolan J., Rylance G., Sheikh A., Unsworth D.J., Warrell D. Working Group of the Resuscitation Council (UK. Resuscitation. 2008;77:157–169. doi: 10.1016/j.resuscitation.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Solsona M.D., Boquet E.M., Bc E., Andres J.L. Impact of adherence on subcutaneous interferon beta-1a effectiveness administered by Rebismart® in patients with multiple sclerosis. Patient Preference and Adherence. 2017;11:415–421. doi: 10.2147/PPA.S127508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl W., Schwarting A., Okada M., Scheinberg M., Doria A., Hammer A.E., Kleoudis C., Groark J., Bass D., Fox N.L., Roth D., Gordon D. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: A fifty-two-week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 2017;69:1016–1027. doi: 10.1002/art.40049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strik A.S., Berends S.E., Mathôt R.A., D’Haens G.R., Löwenberg M. Golimumab for moderate to severe ulcerative colitis. Expert Rev. Gastroenterol. Hepatol. 2017;11:401–406. doi: 10.1080/17474124.2017.1303376. [DOI] [PubMed] [Google Scholar]
- Struemper H., Murtaugh T., Gilbert J., Barton M.E., Fire J., Groark J., Fox N.L., Roth D., Gordon D. Relative bioavailability of a single dose of belimumab administered subcutaneously by prefilled syringe or autoinjector in healthy subjects. Clin. Pharmacol. Drug Dev. 2016;5:208–215. doi: 10.1002/cpdd.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tench C.M., McCurdie I., White P.D., D’Cruz D.P. The prevalence and associations of fatigue in systemic lupus erythematosus. Rheumatology (Oxford) 2000;39:1249–1254. doi: 10.1093/rheumatology/39.11.1249. [DOI] [PubMed] [Google Scholar]
- Thakker A., Shanbag P. A randomized controlled trial of intranasal-midazolam versus intravenous-diazepam for acute childhood seizures. J. Neurol. 2013;260:470–474. doi: 10.1007/s00415-012-6659-3. [DOI] [PubMed] [Google Scholar]
- Thompson J.S., Schneider P., Kalled S.L., Wang L., Lefevre E.A., Cachero T.G., MacKay F., Bixler S.A., Zafari M., Liu Z.Y., Woodcock S.A., Qian F., Batten M., Madry C., Richard Y., Benjamin C.D., Browning J.L., Tsapis A., Tschopp J., Ambrose C. BAFF binds to the tumor necrosis factor receptor-like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. J. Exp. Med. 2000;192:129–135. doi: 10.1084/jem.192.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremlett H., Van der Mei I., Pittas F., Blizzard L., Paley G., Dwyer T., Taylor B., Ponsonby A.-L. Adherence to the immunomodulatory drugs for multiple sclerosis: contrasting factors affect stopping drug and missing doses. Pharmacoepidemiol. Drug Saf. 2008;17:565–576. doi: 10.1002/pds.1593. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan R. Autoinjector device for rapid administration of life saving drugs in emergency. Def. Sci. J. 2012;62:307–314. [Google Scholar]
- Vijayaraghavan R., Selvaraj R., Krishna Mohan S., Gopi P. Haematological and biochemical changes in response to stress induced by the administration of amikacin injection by autoinjector in animals. Def. Sci. J. 2014;64:99–105. [Google Scholar]
- Vijayaraghavan R. Autoinjector device for rapid administration of drugs and antidotes in emergency situations and in mass casualty management. J. Inter. Med. Res. 2020;48(5):1–12. doi: 10.1177/0300060520926019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser K., Katchamart W., Loza E., Martinez-Lopez J.A., Salliot C., Trudeau J., Bombardier C., Carmona L., van der Heijde D., Bijlsma J.W.J., Boumpas D.T., Canhao H., Edwards C.J., Hamuryudan V., Kvien T.K., Leeb B.F., Martín-Mola E.M., Mielants H., Müller-Ladner U., Murphy G., Østergaard M., Pereira I.A., Ramos-Remus C., Valentini G., Zochling J., Dougados M. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann. Rheum. Dis. 2009;68:1086–1093. doi: 10.1136/ard.2008.094474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysham C.H., Rosenstock J., Vetter M.L., Dong F., Öhman P., Iqbal N. Efficacy and tolerability of the new autoinjected suspension of exenatide once weekly versus exenatide twice daily in patients with type 2 diabetes. Diabetes Obes. Metab. 2018;20:165–172. doi: 10.1111/dom.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa N., Morita H., Nakajima T. Sarin experiences in Japan: acute toxicity and long-term effects. J. Neurol. Sci. 2006;249:76–85. doi: 10.1016/j.jns.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Yapa S.W.S., Roth D., Gordon D., Struemper H. Comparison of intravenous and subcutaneous exposure supporting dose selection of subcutaneous belimumab systemic lupus erythematosus Phase 3 program. Lupus. 2016;25:1448–1455. doi: 10.1177/0961203316642309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Li W., Clawson C., Karvani D., Sondag P., Hahn J.K. Evaluation of performance, acceptance, and compliance of an auto-injector in healthy and rheumatoid arthritic subjects measured by a motion capture system. Patient Preference Adherence. 2018;12:515–526. doi: 10.2147/PPA.S160394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Roschke V., Baker K.P., Wang Z., Alarcon G.S., Fessler B.J., Bastian H., Rp K., Zhou T. Cutting edge:a role for B lymphocyte stimulator in systemic lupus erythematosus. J. Immunol. 2001;166:6–10. doi: 10.4049/jimmunol.166.1.6. [DOI] [PubMed] [Google Scholar]
- Ziemssen T., Sylvester L., Rametta M., Ross A.P. Patient satisfaction with the new interferon beta-1b autoinjector (BETACONNECTTM) Neurol. Ther. 2015;4:125–136. doi: 10.1007/s40120-015-0036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Autoinjectors Market, 2016 (Accessed 01 August 2021)
Further Reading
- Schwarzenbach F., Dao Trong M., Grange L., Laurent P.E., Abry H., Cotten J., Granger C. Results of a human factors experiment of the usability and patient acceptance of a new autoinjector in patients with rheumatoid arthritis. Patient Prefer Adherence. 2014:199. doi: 10.2147/PPA.S50583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh S.Z., Hammer A.E., Fox N.L., Groark J., Struemper H., Roth D., Gordon D. Evaluation of a novel autoinjector for subcutaneous self-administration of belimumab in systemic lupus erythematosus. Int. J. Clin. Pharmacol. Ther. 2016;54:914–922. doi: 10.5414/CP202623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl, W., Metyas, S., Tan, S.M., Cheema, G.S., Oamar, B., Xu, D., Roschke, V., Y, W., Hilbert, B.K.P., D M, 2003. B-lymphocyte stimulator over expression in patients with systemic lupus erythematosus: longitudinal observations. Arthritis 3475–86. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable for this article.