Abstract
Objectives
The global COVID-19 pandemic produced large-scale health and economic complications. Older people and those with comorbidities are particularly vulnerable to this virus, with nursing homes and long term care facilities (LTCF) experiencing significant morbidity and mortality associated with COVID-19 outbreaks. The aim of this rapid systematic review was to investigate measures implemented in LTCF to reduce transmission of COVID-19 and their effect on morbidity and mortality of residents, staff and visitors.
Setting
Long-term care facilities.
Participants
Residents, staff and visitors of facilities.
Primary and secondary outcome measures
Databases (PubMed, EMBASE, CINAHL, Cochrane Databases and repositories and MedRXiv prepublished database) were systematically searched from inception to 27 July 2020 to identify studies reporting assessment of interventions to reduce transmission of COVID-19 in nursing homes among residents, staff or visitors. Outcome measures include facility characteristics, morbidity data, case fatalities and transmission rates. Due to study quality and heterogeneity, no meta-analysis was conducted.
Results
The search yielded 1414 articles, with 38 studies included. Reported interventions include mass testing, use of personal protective equipment, symptom screening, visitor restrictions, hand hygiene and droplet/contact precautions, and resident cohorting. Prevalence rates ranged from 1.2% to 85.4% in residents and 0.6% to 62.6% in staff. Mortality rates ranged from 5.3% to 55.3% in residents.
Conclusions
Novel evidence in this review details the impact of facility size, availability of staff and practices of operating between multiple facilities, and for-profit status of facilities as factors contributing to the size and number of COVID-19 outbreaks. No causative relationships can be determined; however, this review provides evidence of interventions that reduce transmission of COVID-19 in LTCF.
PROSPERO registration number
CRD42020191569.
Keywords: COVID-19, public health, geriatric medicine
Strengths and limitations of this study.
Evidence from 38 studies identifies the measures taken to reduce transmission of COVID-19 in long-term care facilities.
No limitations were placed on study type, and all languages were eligible for inclusion.
Study quality was formally examined using the Mixed Methods Assessment Tool.
Due to the heterogeneity of included studies, meta-analysis was not able to be performed.
Introduction
SARS-CoV-2 is a novel virus, first identified in China in 2019, resulting in the current global pandemic in 2020.1 The ensuing disease associated with infection from SARS-CoV-2, termed COVID-19, has produced large-scale public health and worldwide economic effects.2
The virus spreads between people through close contact and droplet transmission (coughs and sneezes). While most infected people will experience mild influenza‐like symptoms, others may become seriously ill and die.3 At-risk groups include older people and those with underlying medical conditions, while men appear to have more susceptibility than women. Symptom severity varies; several individuals remain asymptomatic. Others experience fever, cough, sore throat, general weakness and fatigue, while more severe respiratory illnesses and infections may result, which can be fatal.4 5 Deterioration in clinical presentations can occur rapidly, leading to poorer health outcomes. Anosmia and ageusia are reported in evidence from South Korea, China and Italy in patients with confirmed SARS-CoV-2 infection, in some cases in the absence of other symptoms.6
The WHO declared the COVID-19 outbreak constituted a Public Health Emergency of International Concern on 30 January 2020.5 Two primary goals of action were (1) to accelerate innovative research to help contain the spread and facilitate care for all affected and (2) to support research priorities globally the learning from the pandemic response for preparedness. Globally, up to 25 March 2021, there are 123 636 852 cases of COVID-19 (following the applied case definitions and testing strategies in the affected countries) including 2 721 891 deaths.7 Within Europe, over 25 220 376 cases are reported, with 592 929 deaths.7
Given the infection and mortality figures noted, preventing and limiting transmission of the SARS-CoV-2 virus is advocated. International and national evidence mandates physical distancing, regular hand hygiene and cough etiquette, and limiting touching eyes, nose or mouth; in addition to regular cleaning of surfaces.8
As noted, older people are an at-risk group for COVID-19, and throughout the pandemic, the impact on this population has resulted in increased mortality, specifically those living in long term care facilities (LTCF) where a high proportion of outbreaks with increased rates of morbidity and case fatality in residents are recorded.9 In several European Union/European Economic Area countries, LTCF deaths among residents, associated with COVID-19, account for 37%–66% of all COVID-19-related fatalities.9 The specific rationale for their increased susceptibility is less clear. Comorbidities including cardiovascular disease and diabetes may increase the chances of fatal disease, but they alone do not explain why age is an independent risk factor.10 Molecular, biological and immunological changes inform emergent viable hypotheses.10 The United Nations (UN) (2020) acknowledge that COVID-19 exposes the inequalities in society and the failures expressed in the 2030 Agenda for Sustainable Development. The UN report the disproportionate fatality rates in those aged over 80 years as five times the global average11 and suggest a need for a more inclusive, equitable and age-friendly society, anchored in human rights (p16).12
The aim of this rapid review of the literature was to assess the extent to which measures implemented in LTCF reduced transmission of COVID-19 (SARS-CoV-2) among residents, staff and visitors, and the effect of these measures on morbidity and mortality outcomes.
Methods
The protocol is registered on PROSPERO13 and reporting follows Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.14 Ethical approval was not required for this systematic review.
Search strategy
Search strategies comprised search terms both for keywords and controlled-vocabulary search terms MESH and EMTREE (see online supplemental table 1 for full search terms). EMBASE (via OVID), PubMed (via OVID), Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane Database and Repository and MedRXiv prepublished databases were searched. No time limits were imposed, and databases were searched up to 27 July 2020. Reference lists of included evidence were checked for further articles.
bmjopen-2020-047012supp001.pdf (143.4KB, pdf)
Eligibility criteria
All study designs (experimental, observational and qualitative) are included, and no exclusions are placed on language. Included studies report an assessment of measures to reduce transmission of COVID-19 (including SARS or Middle Eastern Resipratory Virus (MERS)) in residents, employees or visitors of LTCF. To provide as comprehensive a review of the evidence we included any intervention implemented to reduce the transmission of COVID-19 in LTCF, including facility measures, social distancing, use of personal protective equipment (PPE) and hand hygiene.
A broad definition of LTCF was adopted for this review noting European Centre for Disease Prevention and Control (ECDC) guidance8 including institutions such as nursing homes, skilled nursing facilities, retirement homes, assisted-living facilities, residential care homes or other facilities providing care in a congregated setting for older aged adults.
Primary outcome measures
Primary outcome measures are morbidity data, case fatality rates and reductions in reported transmission rates.
Secondary outcomes
Secondary outcomes reported are facility characteristics associated with COVID-19 transmission.
Selection of studies and data extraction
Two authors developed search strings (DS and KF); all database searches were completed by one author (DS) (online supplemental table 1). Following de-duplication, references were uploaded into Covidence management platform (LM), and two authors independently screened all titles and abstracts (LM and KF). Full texts of all potentially eligible studies were independently reviewed by two authors (LM and KF). Disagreements were resolved by discussion with a third author (CCK). Data from included studies were independently extracted in duplicate (LM and KF). A data extraction form was developed and modified from documents used previously by authors (KF and CCK). Extracted data included study characteristics (title, lead author, year of publication, country, study setting, study design), description of the intervention, number and characteristics of participants, outcomes, duration of follow-up, sources of funding, peer review status. Study design (required for review of quality) was independently assessed by two authors (LM and KF), with disagreements resolved by a third author (CCK).
Assessment of quality
Two review authors (LM and EL) independently assessed the quality of included studies using Mixed Methods Assessment Tool (MMAT),15 with disagreements resolved by a third author (KF) and discussed with the lead author (CCK) (online supplemental table 2). The MMAT is used widely and considered a valid indicator of methodological quality using instruments for non-randomised and descriptive studies.
bmjopen-2020-047012supp002.pdf (68KB, pdf)
Data synthesis
Meta-analysis was not possible due to heterogeneity in study designs, participants, outcomes and nature of the interventions and no attempt was made to transform statistical data. The Synthesis without meta-analysis (SWiM) criteria16 guide a narrative summary, with data presented in tabular format and subgroup reporting of population groups.
Patient and public involvement
No patients were involved in this study.
Results
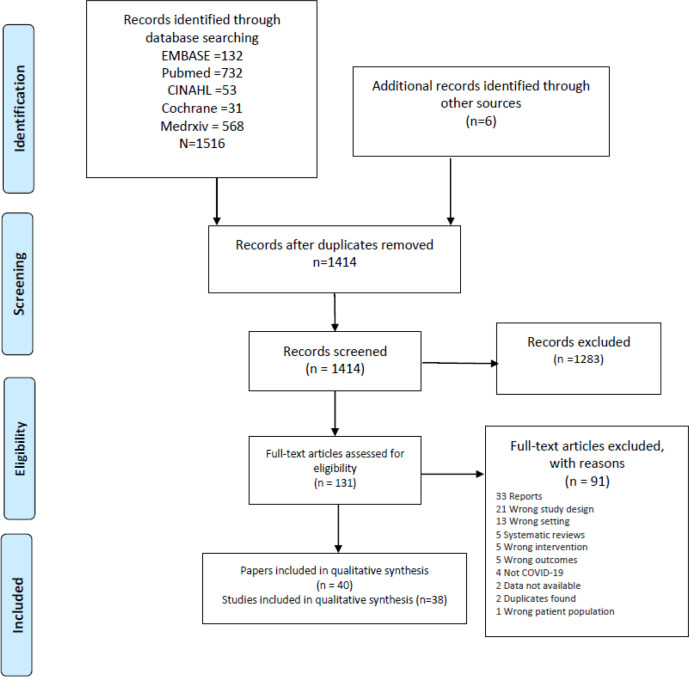
We identified 1414 articles and 131 full-text articles were selected for review. After an evaluation against our inclusion criteria, 38 studies (40 papers) are included in this systematic review (figure 1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart.
Study characteristics
Geographically we report evidence from 11 countries; the majority (20 studies) are from USA17–36 and UK.37–41 We report evidence from Canada,42–44 France,45 46 Hong Kong,47 48 Belgium,49 Germany,50 Ireland,51 Japan,52 Korea53 and Spain54 (table 1).
Table 1.
Characteristics of studies including infection control measures
| Study ID | Country | Study design | Setting | Population | Intervention/infection control strategy | Outcome groups | Primary outcome measure | Secondary outcome |
| Abrams et al17 | USA | Cross-sectional | Nursing homes | Nursing homes across 30 US states (n=9395 nursing homes) N=6446 facilities without COVID-19 cases; n=2949 facilities with COVID-19 cases |
Nursing homes characteristics associated with COVID-19 outbreaks | Facilities | Prevalence of COVD-19 | Estimates on the relationship of nursing home characteristics and documented COVID-19 cases |
| Arons et al18 | USA, King County, Washington | Cross-sectional cohort | Nursing home facility | Residents N=89 N=76 participated in point-prevalence testing |
PPE (eye protection, gown, gloves, face masks); mass testing | Residents, staff | COVID-19 prevalence, testing, symptoms, hospitalisation, mortality | |
| Blackman et al 19 | USA | Cross-sectional | Skilled nursing facility | A 150-bedded skilled nursing facility. Single story building with four units | Employee and visitor screening on entry; visitor restrictions; review of PPE and infection control in the building; use of heat maps in a facility to track staff and residents’ symptoms | Residents, staff | COVID-19 prevalence, testing, mortality | |
| Borras-Bermejo et al54 | Spain | Cross-sectional cohort | Nursing homes | N=69 nursing homes in Barcelona N=3214 residents and N=2655 staff |
Surveillance testing programme for COVID-19 in nursing homes; introduction of restrictions for visitors | Residents, staff | COVID-19 prevalence, testing, symptoms | |
| Brainard et al37 | England, Norfolk | Retrospective cohort | Care homes | N=248 care homes | Statistical modelling assessing detection of COVID-19 infection relative to PPE availability and impact of staffing by non-care workers | Facilities | Descriptive data and statistical modelling for COVID-19, staffing levels, access to PPE | |
| Brown et al42 | Canada, Ontario | Retrospective cohort | Nursing homes | N=623 nursing homes N=78 607 residents |
Impact of home crowding on COVID-19 infection and mortality using nursing home crowding index score | Residents, facilities | COVID-19 incidence, modelling mortality | Facility characteristics, overcrowding and transmission |
| Burton et al38 | Scotland | Cross-sectional cohort | Nursing homes | N=189 nursing homes included and data for 109 homes (57.7%) for older people reported, representing 5227 beds (89.5% of total beds in 189 care homes) | Surveillance data to understand the evolution of COVID-19 following outbreaks and care home characteristics in one health board | Facilities, residents | COVID-19 outbreaks, mortality | Facility characteristics associated with transmission |
| Dora et al20 | USA, California | Cross-sectional | Veterans Affairs Greater Los Angeles Healthcare System | N=3 skilled nursing facilities (n=150 long term beds) N=99 residents (95% male, age range 50–100 years) N=136 staff Visitors |
Three point-prevalence surveys; visitor restrictions (initially all visitors screened, then no visitors permitted into buildings); staff screening; hand hygiene, droplet and contact precautions; cohorting | Residents, staff | COVID-19 prevalence, symptoms, mortality | |
| Dutey-Magni et al39 | UK (England, Scotland and Northern Ireland) | Cohort | Long-term care facilities | N=8713 resident’s health records Daily counts of infection in 9339 residents and for 11 604 staff across 179 LTCF |
The home testing programme was introduced for all staff and residents in Four Seasons Healthcare Group (representing 9% of all long-term care beds). All tested at least once | Residents, staff and facilities | Cumulative incidence of COVID-19, Kaplan-Meier estimates mortality and symptoms | |
| Eckardt et al21 | USA, Florida | Cross-sectional cohort | Long-term care | 120-bedded long-term care facility | PPE; staff and visitor screening; visitor restrictions; distancing of residents; cohorting exposed residents; point-prevalence testing | Residents, staff | COVID-19 prevalence | |
| Feaster and Goh22 | USA, Pasadena | Cross-sectional cohort | Long-term care homes | Residents and staff (n=1093) of LTCF (n=9) N=608 residents (age 78±13.3 years; n=332 female) N=485 staff (age 41.8±13.3 years; n=249 female) |
Mass surveillance testing | Residents, staff | COVID-19 prevalence, symptoms | |
| Fisman et al43 | Canada, Ontario | Cohort | Long-term care facilities | N=269 total individuals who died of COVID-19 in Ontario to 11 April 2020, and n=83 individuals who died of COVID-19 in Ontario LTCF to 7 April 2020. Denominators are not available for long-term care residents approximated as the total number of long-term care facility beds in Ontario (79 498), assuming complete occupancy. Median beds 120 (9–543) | Surveillance data analysed to evaluate the risk of death and identification of risk factors for prevention strategies | Residents, staff, facilities | COVID-19–specific mortality incidence rate ratios (IRRs) of long-term care residents were calculated with community-living Ontarians older than 69 years as the comparator group | |
| Graham et al23 | England | Cross-sectional cohort | Four nursing homes in London, England | N=4 nursing homes. N=394 residents (37.6% male, median age 83 years (IQR 15), 75.4% white) N=596 staff |
Mass surveillance testing; isolation of infected residents | Residents, staff | COVID-19 prevalence, symptoms, mortality. Multivariable logistic regression of presenting symptoms in those who had an available test | |
| Guery et al45 | France, Nantes | Cross-sectional cohort | Nursing home | N=136 staff (age 39 years (IQR 27–48.5), n=112 female) | Surveillance testing of staff following confirmed index case | Staff | COVID-19 prevalence, symptoms | |
| Hand et al24 | USA, Louisiana | Cross-sectional cohort | Long-term care facility | Long-term care facility provides services for up to 130 residents: report on 20 resident cases | Outbreak surveillance after 20 cases reported. Adherence to standard droplet precautions for symptomatic residents | Residents, facilities | Prevalence of Coronavirus NL63 symptoms, hospitalisations, mortality | |
| Harris et al25 | USA, Virginia | Cross-sectional cohort | Long-term care facility | N=41 of 48 residents (median age 75 years (44–104), 52.1% female (25/48). 60.4% white (29/48)) N=7 staff |
Following an outbreak, response developed for the management of residents and the use of telemedicine. Early identification of residents for escalation of care; monitoring and treating patients safe to remain in a facility; care coordination—bidirectional; daily needs assessment related to technology, infection control and staff well-being | Residents, staff | COVID-19 prevalence, mortalities, comorbidities, telemedicine consultations | |
| Heung et al47 | Hong Kong | Cross-sectional cohort | Residential care home | N=90 residents N=32 staff N=67/90 residents participated; n=7 (10%) aged 65–75 years, n=32 (48%) 76–85 years, n=28 (42%) >85 years; n=53 (79%) females. Staff 26/32 participated; n=18 (69%) aged 31–50 years, n=8 (31%) >50 years; 85% females; 54% nursing care role, 46% assistance in daily activities |
Surveillance screening in a residential care home with the introduction of infection control precautions: droplet and contact precautions | Resident, staff, facilities | Seroprevalence of SARS-CoV antibodies. Symptoms, transmission and mortality | |
| Ho et al48 | Hong Kong | Cross-sectional cohort | Nursing home | N=7 residents, staff, visitors in one nursing home (n=4 females aged in their 60s–90s; n=3 males aged in their 20s–80s) | Proposed intervention for future management. Community-based outreach teams led by geriatricians, nurses to closely monitor nursing home residents discharged from hospital | Residents, staff, visitors, facilities | Descriptive data on seven cases, the onset of illness, transmission and outcome including mortality | |
| Hoxha et al49 | Belgium | Cross-sectional cohort | Long-term care facilities | Reporting for 2074 of 2500 invited facilities; 280 427 COVID-19 tests. 51% residents (N=142 100) and 49% staff (N=138 327) |
Mass testing | Residents and staff | COVID-19 prevalence, symptoms, characteristics associated with positive test outcome | |
| Iritani et al52 | Japan | Cross-sectional cohort | Across long-term care hospitals/facilities, general medical/welfare facilities and non-medical/welfare facilities | 381 clusters with 3786 infected cases accounting for 23.9% of 15 852 cases | Following government recommendation suspension or restricting temporary use of LTCF in areas where infection prevalent | Facilities | Descriptive data on clusters reported, mortality data | |
| Kennelly et al51 | Ireland | Cross-sectional cohort | Nursing homes | Nursing home residents in three community health organisations in Ireland (N=28 nursing homes). Represents 2043 residents and 2303 beds | Mass surveillance testing; post-testing programme | Staff, residents, facilities | COVID-19 prevalence, symptoms, clinical outcomes, including mortality | Characteristics of facilities associated with transmission |
| Kim53 | Korea (South) | Cross-sectional cohort | Nursing home | N=142 nursing home residents N=85 healthcare workers and caregivers working in one facility |
Procedures identified to reduce transmission of COVID-19 following confirmed case in a staff member | Facilities | Data on the preparedness of the facility to reduce transmission. | |
| Kimball et al26 | USA, King County, Washington | Cross-sectional cohort | Long-term care skilled nursing facility | Nursing home. N=82 residents; 76/82 (92.7%) underwent symptom assessment and testing; three (3.7%) refused testing |
Surveillance testing; PPE; hand hygiene; visitor restrictions; staff screening; daily resident symptom assessments; isolation of positive residents | Residents | COVID-19 prevalence and symptoms | |
| Klein et al50 | Germany, Hamburg | Cross-sectional | Residential care facility | N=60 resident and report from eight deceased residents | Mass testing; PPE; resident cohorting; visitor restrictions | Residents | COVID-19 prevalence and symptoms, management | |
| Lennon et al27 | USA, Massachusetts | Cross-sectional cohort | Skilled facilities, nursing homes and assisted living facilities | N=366 skilled nursing facilities N=32 480 residents and staff tested once, and 6.7% tested subsequently. N=16 966 residents (mean age 82±13; 65% female). N=15 514 staff (mean age 45±15; 76% female) |
Mass testing and recording of symptoms, comparison of viral levels | Residents, staff | COVID-19 prevalence, symptoms | |
| Louie et al28 | USA, San Francisco | Cohort | Three skilled nursing facilities and one assisted living facility | N=431 residents and staff tested as part of initial surveillance Follow-up testing of n=303 asymptomatic cases |
Mass surveillance testing; restrictions on visitors and non-essential staff; increased monitoring/screening of people entering/residing in a facility | Residents, staff | COVID-19 prevalence, hospitalisations, fatalities, management | |
| McMichael et al29 | USA, King County, Washington | Cross-sectional cohort | Skilled nursing facility | N=167 N=101 residents (median aged 83 (51–100), n=32 (31.7%) male, n=69 (68.3%) female) N=50 healthcare personnel (median age 43.5 (21–79), n=12 (24%) males, n=38 (76%) female) N=16 visitors (median age 72.5 (52–88), n=11 (68.7%) male, n=5 (31.2%) females) |
Mass surveillance testing; contact tracing; quarantine of exposed persons; isolation of confirmed and suspected cases; on-site enhancement of PPE/infection prevention and control | Residents, staff, visitors, facilities | COVID-19 prevalence, symptoms, mortality, hospitalisations, management | |
| Office for National Statistics40 | England | Cross-sectional cohort | Care homes providing care for older residents and those with dementia only | N=9081 care homes for people aged 65 years and older—representing 292 301 residents (95% CI 293 168 to 293 434) and 441 498 staff N=5126 homes participated (56%) |
Prevalence of COVID-19 in residents and staff. Factors associated with higher levels of infection | Residents, staff, facilities | COVID-19 prevalence in residents aged 65 years and older and employees | |
| Patel et al30 | USA, Illinois | Cross-sectional cohort | Nursing home (150 bedded unit) | N=127 residents. 9% (n=11) single occupancy rooms, 91% (n=116) double occupancy rooms |
Mass surveillance testing; screening of staff and visitors; visitor restrictions; cohorting of residents; PPE | Residents, staff, facilities | COVID-19 prevalence, symptoms, hospitalisations and survival rates, management | |
| Quicke et al31 | USA, Colorado | Longitudinal cohort | Five skilled nursing facilities | N=454 staff | Weekly surveillance nasopharyngeal swabs tests were collected | Staff | COVID-19 prevalence and incidence, symptoms and information on genomic epidemiology | |
| Quigley et al32 | USA, 29 states | Cross-sectional cohort | Nursing homes | N=56 nursing homes from 29 states: midwest (30%), west (25%), northeast (23%), south (22%) | Reported on preparedness for COVID-19, testing, supplies and staffing levels | Facilities | Preparedness of nursing home facilities during COVID-19 to reduce transmission | |
| Roxby et al33 | USA, Seattle, Washington | Cross-sectional cohort | Assisted living community older adults | Older aged residents and staff in an assisted living community. N=80 residents (mean age 86 years (range, 69–102); n=62 (77%) female). N=62 staff (mean age 40.0±15; n=42 (68%) female). N=83 private apartments, n=45 independent, n=38 assisted living |
Mass testing; resident cohorting/isolation; PPE; staff screening; visitor screening; additional hand hygiene stations | Residents, staff | COVID-19 prevalence and symptoms | |
| Sacco et al46 | France, Maine-et-Loire | Cross-sectional cohort | Nursing home | N=87 residents (age 87.9±7.2; 71% female) N=92 staff (age 38.3±11.7; 89% female) |
Mass testing; PPE; visitor restrictions; hand hygiene; resident isolation | Residents, staff, facilities | COVID-19 prevalence and case fatality rates. Resident’s clinical signs and symptoms obtained from retrospective chart audit | |
| Sanchez et al34 | USA, Detroit | Time series cohort | Skilled nursing facilities | N=26 skilled nursing facilities N=2773 residents’ tests reported at baseline (median age 72 years (IQR 64–82 years)); n=2218 1st follow-up; n=637 2nd follow-up |
Two point-prevalence surveys; follow-up in 12 facilities following PPE guidelines; resident cohorting | Residents, facilities | COVID-19 prevalence, hospitalisations and deaths preintroduction and postintroduction of testing | |
| Stall et al44 | Canada, Ontario | Retrospective cohort | Nursing homes | N=623 nursing homes (n=75 676 residents); 360/623 (57.7%) for-profit homes, 162/623 (26.0%) non-profit, 101/623 (16.2%) municipal homes. Mean number residents: n=113.2 (for profit); n=119.6 (non-profit); n=101 (municipal) |
Impact of profit status at the level of a home rather than a resident. Using data from the Ontario Ministries of Health and Long-Term Care as part of the province’s emergency ‘modelling table’ | Facilities, residents, and staff | Descriptive data on outbreaks and mortality rate. Nursing home COVID-19 outbreaks (at least one resident case), COVID-19 outbreak sizes (total number of confirmed resident cases among homes with outbreaks), and the total number of COVID-19 resident deaths (among homes with outbreaks). Outbreaks in staff reported. Death rates for residents | Facility characteristics including nursing home profit status (for profit, non-profit, or municipal) associated with transmission |
| Stow et al41 | England | Longitudinal ecological study | Care home units from 46 local authority areas in England | N=460 care home units N=6464 residents |
Use of National Early Warning Score (NEWS) for identification of at-risk/surveillance to reduce mortality | Residents | Descriptive data NEWS surveillance on reducing mortality. Time-series comparison with Office for National Statistics weekly reported registered deaths of care home residents and COVID-19 was the underlying cause of death, and all other deaths (excluding COVID-19) up to 10 May 2020 | |
| Telford et al35 | USA State of Georgia (Fulton County and City of Atlanta) | Cross-sectional cohort | Nursing homes | N=28 nursing homes. N=5671 participants; n=2868 (50.6%) residents, n=2803 (49.4%) staff |
Mass surveillance testing of staff and residents | Residents, staff | COVID-19 prevalence, hospitalisations, and deaths | |
| Unruh et al36 | USA States New Jersey, New York, Connecticut | Case study | Nursing homes with ≥100 beds | N=1162 nursing home facilities | Nursing home characteristics associated with mortality rates | Facilities | Mortality data. Predicted probabilities with logistic regression, independent variables compared on characteristics of facilities |
Study setting is presented as defined in the original study.
LTCF, long-term care facilities; PPE, personal protective equipment.
Infection control measures
Twenty studies report the nature of LTCFs related to outbreaks and transmission of COVID-19 infection (table 2).17 24 29 30 32 34 36–40 42–44 46–48 51–53 Thirty studies (table 318–30 33–35 38–44 46–51 54); report evidence of measures to reduce transmission of COVID-19 in long-term residential care facilities for residents, 25 studies (table 418–23 25 27–31 33 35 39 40 43–49 51 54); report evidence for employee outcomes, and two studies report evidence for visitors (table 5).29 48
Table 2.
COVID-19 outcomes related to the nature of long-term care facilities
| Study | Facilities | Outcomes |
| Abrams et al17 | Facilities | Average number of cases was 19.8 (range 1–256). New Jersey (88.6%, OR 7.16) and Massachusetts (78.0%, OR 4.36) had a higher number of affected facilities. Probability of having a COVID-19 case: Facility size (relative to small): large OR=6.52; medium OR=2.63. Location (relative to rural): urban OR=3.22. % African American residents (relative to low %): greater % OR=2.05. Nursing home chain status (relative to non-chain status): chain status OR=0.89. States were significantly related to the probability of having COVID-19 case. Outbreak size associations: Facility size (relative to small facility size): large=−15.88; medium=−10.8 (percentage point change). For-profit status (relative to non-profit status)=1.88. State. Medicaid dependency, ownership, five-star rating and prior infection violation were not significantly related to COVID-19 cases. |
| Brainard et al37 | Facilities | Risk of infection: Facility employee numbers (relative to <10 workers): 11–20 non-care workers HR=6.502 (95% CI 2.614 to 16.17); 21–30 non-care workers HR=9.870 (95% CI 3.224 to 30.22); >30 non-care workers HR=18.927 (95% CI 2.358 to 151.90). Predictors of spread and increase in cases per unit after 5 April risk increased 1.0347 (95% CI 1.02 to 1.05) p<0.001, reduced availability of PPE for eye protection increased risk 1.6571 (95% CI 1.29 to 2.13) p<0.001, PPE for facemasks 1.2602 (95% CI 1.09 to 1.46) p=0.002, count of care workers employed 1.0379 (95% CI 1.02 to 1.05) p<0.001 count of nurses employed (in bands of 0–10,11–20, 21–30 and 31+) 1.1814 (95% CI 1.13 to 1.24) p<0.001. |
| Brown et al42 | Facilities | Incidence in high crowding index homes was 9.7% vs 4.5% in low crowding index homes (p<0.001), while COVID-19 mortality was 2.7% vs 1.3%. Likelihood of COVID-19 introduction did not differ (31.3% vs 30.2%, p=0.79). After adjustment for a regional nursing home, and resident covariates, the crowding index remained associated with increased risk of infection (RR=1.72, 95% CI 1.11 to 2.65) and mortality (RR=1.72, 95% CI 1.03 to 2.86). Simulations suggested that converting all 4-bed rooms to 2-bed rooms would have averted 988 (18.9%) infections of COVID-19 and 271 (18.7%) deaths. |
| Burton et al38 | Facilities | Significant associations between the presence of an outbreak and number of beds (OR per 20-bed increase 3.50), a history of multiple outbreaks (OR 3.76) and regulatory risk assessment score (OR high-risk vs low 2.19). However, in the adjusted analysis, only number of beds (OR per 20-bed increase 3.50, 95% CI 2.06 to 5.94 per 20-bed increase). |
| Dutey-Magni et al39 | Facilities | COVID-19 outbreak recorded in 121 of 179 facilities (67.6%). Large LTCF had greater rates of infection (aHR=1.8 (95% CI 1.4 to 2.4) for LTCF with ≥70 beds versus <35 beds. The adjusted HR for confirmed infection was 2.5 times (95% CI 1.9 to 3.3) greater in LTCF with 0.85–1 resident per room vs LTCF with 0.7–0.85 resident per room. A 10-percentage point increase in the bed to staff ratio was associated with a 23% increase in infection (aHR=1.23 (95% CI 1.17 to 1.31)). |
| Fisman et al43 | Facilities | COVID-19 cases higher in for-profit operators 165/361 (45.7%) compared with charitable 18/57 (31.6%). |
| Hand et al24 | Facilities | Residents noted to share rooms, walk throughout the facility and spent time in shared areas (eg, gym, dining rooms and recreational rooms). Because all case-patients had visited the gym at the facility for recreation or physical therapy before becoming ill, environmental cleaning of this area was performed. |
| Heung et al47 | Facilities | 67 of 90 residents participated. 26 of 32 staff participated. Two residents and one staff member were positive during the outbreak. None of the remaining participants was positive for SARS-CoV antibodies. Residents were aged 65+ years, 79% were female, 93% were ambulant, 90% did activities with others, 79% went out. Review of residents who died: resident A transferred from the hospital and was chair bound and dependent with care needs. Resident B was chair bound and had not left home or had visitors. She was brought to a shared sitting room during mealtimes. This was only time residents A and B were located near each other. One resident shared a room with patient B and tested positive. Staff C was a domestic worker, and contact was via clinical waste in resident A room. Low seroprevalence attributed to precautionary measures taken in the facility to reduce droplet and prevent contact transmission. Risks noted of SARS via fomites possible. |
| Ho et al48 | Facilities | 3 residents positive for SARS. 1 employee positive for SARS. 3 visitors positive for SARS. The index case was a single resident who was infected during a hospital stay, returned to the LTCF, and the virus spread to another six people. Transmission of the virus occurred due to lack of isolation rooms in nursing homes, lack of restricted movement of other patients and relatives, lack of infection control precautions, lack of knowledge among staff. |
| Iritani et al 52 | Facilities | Larger cluster sizes in long term care hospitals/facilities were significantly positively associated with higher morbidity (ρ=0.336, p=0.006) and higher mortality (ρ=0.317, p=0.009). Multivariate logistic regression showed larger cluster size (OR=1.077, 95% CI 1.017 to 1.145) and larger cluster number (OR=2.019, 95% CI 1.197 to 3.404) associated with mortality. |
| Kennelly et al51 | Facilities | Outbreak recorded in 75.0% (21/28) of facilities—four public and seventeen private. During the study period, 40.1% of residents in 21 nursing homes with outbreaks had a laboratory diagnosis of COVID-19. Correlation between the proportion of symptomatic staff and number of residents with confirmed/suspected COVID-19 (ρ=0.81). No significant correlation between the proportion of asymptomatic staff and number of residents with confirmed/suspected COVID-19 (ρ=0.18 p=0.61). |
| Kim53 | Facilities | After the management of the outbreak, there were no more infected persons. All patients and employees tested negative 14 days from the start of quarantine. |
| McMichael et al29 | Facilities | 28 February 2020, four cases COVID-19 identified in county. One person identified as index case from facility A. Staff roles for confirmed cases reported: therapists, nurses, nurse assistants, health information manager, physician and case manager. Paper reports that 30 facilities in county had confirmed cases and provides detail on the first 9 (facilities A to I). Facility A shared staff with another facility and two resident transfers from facility A. Surveillance reported inadequate PPE, training, infection control practices, lack of documentation signs and symptoms, working in unfamiliar facilities or sharing staff. On 10 March 2020, the governor of Washington implemented mandatory screening of healthcare workers and visitor restrictions for all licensed nursing homes and assisted living facilities including screening, testing, policies around visiting, excluding symptomatic staff, close monitoring of residents, testing, training and PPE. Monitoring of staff absences. |
| Office for National Statistics40 | Facilities | For each additional member of infected staff working at the care home, the odds of resident infection increase by 11%, that is, OR=1.11 (95% CI 1.1 to 1.11). Care homes using bank or agency nurses or carers most or every day more likely to have cases in residents (OR=1.58, 95% CI 1.5 to 1.65) compared with those who never use bank or agency staff. Residents in care homes outside of London had a lower chance of infection, except West Midlands (OR=1.09, 95% CI 1.0 to 1.17). Homes where staff receive sick pay are less likely to have resident cases (OR=0.82 to 0.93, 95% CI 7% to 18%), compared with homes where no sick leave. For each additional infected resident at a home, the odds of staff infection increase by 4% OR=1.04 (95% CI 4% to 4%). Care homes using bank or agency staff most or every day OR=1.88 (95% CI 1.77 to 2.0) compared with homes not using. Homes where staff regularly work elsewhere (most or every day) increase odds (OR=2.4, 95% CI 1.92 to 3.0) compared with homes who never work elsewhere. Staff at homes outside London had higher odds of COVID-19 infection. |
| Patel et al30 | Facilities | First resident unwell 9 March, female aged in her 60s with cough and fever. Hospitalised 11 March and tested positive COVID-19 13 March. 14 residents who were positive developed symptoms over 30-day follow-up. 21% (n=7) confirmed cases lived in single occupancy rooms. 55% (n=18) were in a double room with another confirmed case, and 24% (n=8) were in a double room with a resident who was negative 15 March. Screening visitors and staff for symptoms, restricting visiting hours from 6 March. No visitor access from 12 March. Universal masking of all staff and residents from 14 March. 15−19 March on-site team implemented assessment of symptoms, resident cohorting. Staff testing positive isolated and return 7 days or after 72 hours of symptoms resolving. Education and training to staff in facility A infection control, PPE, vital signs. |
| Quigley et al32 | Facilities | For-profit=67.86%, non-profit=26.79% and government-owned=5.36%. 37.5% were part of a chain. 54% have COVID-19 plans. All had staff training for COVID-19 and 100% processes to restrict/ limit visitors. 29% conducted COVID-19 simulation training. Communication with local Public Health—96% and 68% linked to local hospital referral. 66% reported access to COVID-19 tests—available for all residents and 53% of staff. 72% reported inadequate PPE supplies. 83% expected staff shortages. Solutions for staff included staff volunteer for more shifts (55%), non-clinical staff used (45%). 19% reported they would use agency staff. |
| Sacco et al46 | Facilities | Restrictions on residents from 16 March—social distancing, remain in single rooms, no communal dining or group activities. No visitors since 10 March, individual walks outside only in the presence of one staff member. Mail and packages stored 24 hours before being delivered to residents. Enhanced hygiene and cleaning. Staff had permanent face masks and additional hand hygiene. |
| Sanchez et al34 | Facilities | Of the 12 facilities in the final survey, 8 had implemented cohorting in a dedicated COVID-19 unit before first follow-up. 4 remaining initiating cohorting after receiving results. 4 facilities did not assign dedicated personnel to care for residents with COVID-19 due to staff shortages. Final survey census 80 residents (range 36–147). 373 of 1063 (35%) had received positive results first follow-up. |
| Stall et al44 | Facilities | Adjusted modelling odds of COVID-19 outbreak associated with for-profit status aOR 1.01 (95% CI 0.64 to 1.57), municipal aOR 0.83 (95% CI 0.45 to 1.54). Model 2+ Health Region aOR 2.02 (95% CI 1.20 to 3.38) population <10 000 rural aOR 0.27 (95% CI 0.13 to 0.58); and model 3+ home characteristics. Number of residents (unit of 50) aOR 1.38 (95% CI 1.18 to 1.61), older design aOR 1.55 (95% CI 1.01 to 2.38), chain ownership vs single home aOR 1.47 (95% CI 0.86 to 2.51) and staff (full time equivalent/bed ratio aOR 1.98 (95% CI 0.39 to 9.97). The extent of a COVID-19 outbreak with profit aRR 1.83 (95% CI 1.18 to 2.84) vs municipal aRR 0.60 (95% CI 0.28 to 1.30) compared with non-profit. Health Region aRR 1.65 (95% CI 1.02 to 2.67), older design standards aRR (95% CI 1.27 to 2.79), chain ownership aRR 1.84 (95% CI 1.08 to 3.15) and staff/bed ratio aRR 0.73 (95% CI 0.10 to 5.35). Deaths accounted for 6.5% of all residents in for-profit homes vs 5.5% in non-profit vs 1.7% municipal LTCF. For-profit associated with total COVID-19 deaths aRR 1.78, (95% CI 1.03 to 2.07). Adjusted model increased risk of death with for-profit aRR 0.82 (95% CI 0.44 to 1.54), older design facilities aRR 2.08 (95% CI 1.28 to 3.36) and chain ownership aRR 1.89, (95% CI 1.00 to 3.59). Number of active residents was protective aRR 0.81 (95% CI 0.70 to 0.95)/50 beds. |
| Unruh et al36 | Facilities | 184 nursing homes (15.8%) had 6 or more COVID-19 deaths. Deaths associated with Medicaid patients (quintile 5: 8.6 PP greater probability vs quintile 1). Patients with higher ADL scores (2.6 (95% CI 1.4 to 3.8) PP, p<0.001), more total beds (0.1 (95% CI 0.0 to 0.1) PP, p<0.001), higher occupancy (0.3 (95% CI 0.1 to 0.5) PP, p<0.009), for-profit status (4.8 (95% CI 0.8 to 8.8) PP, p=0.019). Comparing states: higher mortality in those with Medicaid (quintile 5: 6.1 (95% CI 0.0 to 12.1) PP, p=0.048). Not significant for other states. More direct care hours per patient day associated with lower COVID-19 deaths. All states (−4.8 (95% CI −9.4 to−0.03) PP, p<0.04). |
ADL, activities of daily living; aHR, adjusted HR; aOR, adjusted OR; aRR, adjusted relative risk; LTCF, long-term care facility; PP, percentage points; PPE, personal protective equipment.
Table 3.
Resident-specific outcomes of strategies implemented in nursing homes
| Study | Interventions | Prevalence | Mortality | Other outcomes |
| Arons et al18 | Mass testing (two point-prevalence surveys) PPE |
48/76 (63%) across two surveys, 17/48 typical symptoms, 4/48 atypical symptoms, 3/48 asymptomatic, 24/48 presymptomatic 57/89 through point-prevalence, clinical evaluation, post mortem |
15/57 (26%) | Common symptoms: fever (71%), cough (54%), malaise (42%) Estimated doubling time: 3.4 days (95% CI 2.5 to 5.3) |
| Blackman et al19 | PPE Symptom screening Visitor restrictions |
12 positive cases, 2 awaiting results, 47 symptomatic residents | 3 COVID-19-related deaths | |
| Borras-Bermejo et al54 | Mass testing Visitor restrictions |
768/3214 (23.9%), 486 (69.5% of those with symptom information) were asymptomatic | 2624 of all residents reported symptoms in the previous 14 days | |
| Brown et al42 | Facility characteristics | 5218/78607 (6.6%) | 1452/5218 (27.8%) | |
| Burton et al38 | Facility characteristics | 403 deaths recorded in care homes | 472 excess deaths in care homes with an outbreak (399 COVID-19-related) | |
| Dora et al20 | Mass testing (three point-prevalence surveys) Symptom screening Visitor restrictions Hand hygiene, contact precautions Cohorting |
19/96 (19.8%) across three surveys, 5/19 symptomatic, 8/19 presymptomatic, 6/19 asymptomatic | 1/19 (5.3%) | Symptoms: fever (58%), myalgia (58%), cough (47%), dyspnoea (32%), nausea (32%) Oxygen therapy required for 4/8 presymptomatic, 4/5 symptomatic cases |
| Dutey-Magni et al39 | Mass testing | 951/9339 (10.2%) | 526/951 (55.3%) | 2075/9339 (22.2%) experienced infection symptoms |
| Eckardt et al21 | Mass testing (three point-prevalence surveys) PPE Symptom screening Visitor restrictions Cohorting |
Survey 1: 5/105 (4.8%) Survey 2: 4/86 (4.7%) Survey 3: 1/85 (1.2%) |
||
| Feaster and Goh22 | Mass testing | 408/582 (49.5%), 202/408 (49.5%) symptomatic 237/332 (71.4%) female residents positive, 121/237 (51.1%) asymptomatic 171/250 (68.4%) male residents positive, 81/171 (47.4%) asymptomatic |
||
| Fisman et al43 | Facility characteristics | 83/79498 (0.1%) | IRR (COVID-19-related death in LTCF residents)=13.1 (95% CI 9.9 to 17.3) compared with community-living adults older than 69 years | |
| Graham et al23 | Mass testing (two point-prevalence surveys) Cohorting |
Survey 1: 126/313 (40%), 72/126 (57.1%) symptomatic, 50 typical symptoms, 22 atypical symptoms, 54/126 (42.9%) asymptomatic Survey 2: 5/176 (2.8%) |
53/131 (40.4%) | Increased risk of death: men (48% of deaths vs 34% in those who survived; whole group 38% male, p=0.02); the trend for median age to be greater among those who died (p=0.058) Increased odds of COVID-19 positive: new onset anorexia (OR=3.74, 95% CI 1.5 to 9.8); cough and/or shortness of breath (OR=3.72, 95% CI 1.8 to 7.8); fever, altered mental state/behaviour, diarrhoea not associated with positive test |
| Hand et al24 | Symptom screening Hand hygiene, contact precautions |
20/130 residents suspected cases, 13/20 tested 7/13 (54%) tested positive; 6/7 required hospitalisation |
3/7 (42.9%) | No new cases identified after 18 November 2017 |
| Harris et al25 | Facility characteristics | 41/48 (85.4%) 18/48 residents hospitalised, 11/18 returned to facility from hospital |
6/48 (12.5%) | 13/48 (27.1%) of residents received telemedicine consultations |
| Heung et al47 | Hand hygiene, contact precautions | 2 residents were positive during the outbreak, 0/67 residents positive for SARS-CoV antibodies on screening | 2/67 reported symptoms | |
| Ho et al48 | PPE Cohorting |
3 residents positive | 2/3 (66.7%) | |
| Hoxha et al49 | Mass testing | 5390/142100 (3.8%), 4059/5390 (75.3%) asymptomatic | Infection odds: Women compared with men OR=1.2 (95% CI 1.1 to 1.2); symptomatic compared with asymptomatic OR=8.5 (95% CI 8.0 to 9.0) | |
| Kennelly et al51 | Mass testing Facility characteristics |
710/1741 (40.1%), 54/1741 (3.1%) residents were suspected COVID-19, 193/710 (27.2%) asymptomatic, 396/710 (55.8%) had recovered by the completion of surveillance period | 183/710 (25.8%) | Non-COVID-19 mortality rate similar between outbreak and non-outbreak NHS (5.1% vs 4%, p=0.4) |
| Kimball et al26 | Mass testing (three point-prevalence surveys) PPE Symptom screening Visitor restrictions Hand hygiene, contact precautions Cohorting |
23/76 (30.3%), 10/23 symptomatic (8/10 typical symptoms, 2/10 atypical symptoms), 3/23 asymptomatic, 10/23 presymptomatic | Symptoms: fever (61.5%), malaise (46.2%), cough (38.5), Presymptomatic mean interval from testing to symptom onset was 3 days |
|
| Klein et al50 | Mass testing PPE Visitor restrictions Cohorting |
39/60 (65%) | 8/39 (20.5%) | Symptoms: exhaustion, loss of appetite, dysphagia, fever, cough, colds, diarrhoea |
| Lennon et al27 | Mass testing | 2654/16966 (15.5%), 1692/2654 (63.8%) asymptomatic, 699/2654 (26.3%) symptomatic, (263/2654 symptom data missing) | ||
| Louie et al28 | Mass testing Symptom screening Visitor restrictions |
214/431 (49.7%) residents and healthcare workers, 128/214 (59.8%) symptomatic (78/128 were residents), 86/214 (40.2%) asymptomatic Additional 156 asymptomatic residents subsequently tested: 63/156 COVID-19 positive |
12/78 (15.4%) symptomatic residents died | 22/78 (28.2%) symptomatic residents hospitalised |
| McMichael et al29 | Mass testing PPE Cohorting |
101/118 (58.6%) | 34/101 (33.7%) | 55/101 (54.5%) hospitalised; (37/101 no data on hospitalisation status) |
| Office for National Statistics40 | Mass testing Facility characteristics |
19.9% (95% CI 18.5 to 21.3) in homes with a confirmed outbreak 10.7% (95% CI 10.1 to 11.3) in all homes |
15606 across all homes | Odds of resident infection: each additional infected staff member at a home OR=1.11 (95% CI 1.0 to 1.17) Homes using bank or agency nurses most or all days OR=1.58 (95% CI 1.5 to 1.65) compared with homes never using these staff Homes outside of London had lower infection chance, except West Midlands (OR=1.09, 95% CI 1.0 to 1.17) Homes where staff receive sick pay OR=0.82–0.93 (95% CI unknown) |
| Patel et al30 | Mass testing Symptom screening Visitor restrictions Cohorting |
33/118 (28.0%), 19/33 (58%) symptomatic (8 typical symptoms, 4 atypical symptoms, 10 both typical and atypical symptoms); 1/33 (3%) presymptomatic, 13/33 (39%) asymptomatic | 10/35 (28.6%) (5/10 symptomatic) 30-day survival=71% (95% CI 52 to 83) |
1/91 negative residents reported symptoms 35/90 negative asymptomatic residents developed symptoms during 30-day surveillance, 2/35 COVID-19 positive on re-testing 13/35 COVID-19 residents hospitalised |
| Roxby et al33 | Mass testing Symptom screening Visitor restrictions Hand hygiene, contact precautions Cohorting |
Survey 1: 3/80 (3.8%), 1/3 reported resolved cough and loose stool during the preceding 14 days Survey 2: 1/77 (1.3%) |
All residents clinically stable 14 days after second test 21 days after the test, all cases continued their usual state of health |
|
| Sacco et al46 | Mass testing PPE Visitor restrictions Hand hygiene, contact precautions Cohorting |
41/87 (47.1%) 3/41 asymptomatic |
11/41 (27%) All-cause mortality: 13% (95% CI 7.2 to 21.2), compared with 3% for the same period during the previous 5 years |
Incidence rate for residents=1.54 per 100 person-days 14/87 (16.1%) residents hospitalised |
| Sanchez et al34 | Mass testing (two point-prevalence surveys) Cohorting |
Survey 1: 716/2218 (32.3%), 344/716 (48%) symptomatic Survey 2: 115/637 (18.1%), 5/115 (4%) symptomatic Total surveillance period: 1207/2773 (44%) |
287/2773 (24%) | 446/2773 (37%) hospitalised |
| Stall et al44 | Facility characteristics | 5218/75676 (6.9%) 3599/5218 (69.0%) for-profit home residents 1239/5218 (23.7%) non-profit home residents 380/5218 (7.3%) municipal home residents |
1452/5218 (27.8%) 989/3599 (27.5%) for-profit home 368/1239 (29.7%) non-profit home 95/380 (25.0%) municipal home |
|
| Stow et al41 | Facility characteristics | 1532 COVID-19-related deaths | Highest correlation of increased NEWS and deaths observed for a 2-week lag (r=0.82, p<0.05) Above baseline measures of high respiratory rate (r=0.73, p<0.05 for a 2-week lag) and low oxygen saturation (r=0.8, p<0.05 for a 2-week lag) appear to follow the pattern of COVID-19 and non-COVID-19 deaths |
|
| Telford et al35 | Mass testing (15 facilities in response to outbreak, 13 facilities as prevention) | 821/2868 (28.6%) Response group: 804/1703 (47.2%) Preventive group: 17/1133 (1.5%) (p<0.0001) |
Response group: 131/804 (16.3%) Preventive group: 3/17 (17.6%) |
Response group: 171/804 (21.3%) residents hospitalised Preventive group: 5/17 (29.4%) residents hospitalised |
IRR, incidence risk ratio; LTCF, long-term care facility; NEWS, National Early Warning Score; PPE, personal protective equipment.
Table 4.
Staff-specific outcomes of strategies to reduce transmission
| Study | Interventions | Prevalence | Mortality | Other outcomes |
| Arons et al18 | Mass testing PPE |
26/51 (51.0%) 17/26 (65%) were nursing staff, 9/26 (35%) had roles that provided care/therapies across multiple units |
0/26 hospitalised | |
| Blackman et al19 | PPE Symptom screening Visitor restrictions |
26 staff members absent from work due to sickness | ||
| Borras-Bermejo et al54 | Mass testing Visitor restrictions |
403/2655 (15.2%), 144/403 (35.7%) asymptomatic | 1772/2665 (66.7%) staff reported fever or respiratory symptoms in the preceding 14 days | |
| Dora et al20 | Mass testing (three point-prevalence surveys) Symptom screening Visitor restrictions Hand hygiene, contact precautions Cohorting |
8/136 (6%) 4/8 (50%) asymptomatic 3/8 nursing staff 5/8 licensed vocational nurses |
||
| Dutey-Magni et al39 | Mass testing | 585/11604 (5.0%) | 1892/11604 (16.3%) reported symptoms | |
| Eckardt et al21 | Mass testing (three point-prevalence surveys) PPE Symptom screening Visitor restrictions Cohorting |
Survey 1: 10/176 (5.7%), 10/10 (100%) asymptomatic Survey 2: 5/175 (2.9%), 5/5 (100%) asymptomatic Survey 3: 1/173 (0.6%), 1/1 (100%) asymptomatic |
||
| Feaster and Goh22 | Mass testing | 223/356 (62.6%), 55/223 (24.7%) asymptomatic | Infection prevalence higher in staff with direct resident contact (150/219, 68.5%) compared with staff with no direct resident contact (25/52, 48.1%) | |
| Fisman et al43 | Facility characteristics | Infection among LTCF staff was associated with death among residents with a 6-day lag (adjusted IRR for death per infected staff member, 1.17; 95% CI 1.11 to 1.26) and a 2-day lag (relative increase in risk of death per staff member with infection, 1.20; 95% CI 1.14 to 1.26) | ||
| Graham et al23 | Mass testing (two point-prevalence surveys) Cohorting |
3/70 (4.3%) 3/3 (100%) asymptomatic |
Staff absence due to sickness/self-isolation between 1 March and 1 May elevated relative to background level (215.9% increase, 95% CI 80 to 352) | |
| Guery et al45 | Mass testing | 3/136 (2.2%) 1/3 (33.3%) asymptomatic 1/3 (33.3%) presymptomatic 1/3 (33.3%) symptomatic |
||
| Harris et al25 | Facility characteristics | 7 staff COVID-19 positive prior to intervention 0 further staff positive after intervention implemented |
||
| Heung et al47 | Hand hygiene, contact precautions | 1 staff member SARS-CoV positive during outbreak (a domestic worker) 0/26 staff positive for SARS-CoV antibodies |
||
| Ho et al48 | PPE Cohorting |
1 staff member SARS positive | 1/1 (100%) | |
| Hoxha et al49 | Mass testing | 2953/138327 (2.1%) 2185/2953 (74.0%) asymptomatic |
||
| Kennelly et al51 | Mass testing Facility characteristics |
675 staff COVID-19 positive 159/675 (23.6%) asymptomatic |
Proportion of symptomatic staff correlated with number of residents with confirmed/suspected COVID-19, ρ=0.81 (p<0.001) | |
| Lennon et al27 | Mass testing | 624/15514 (4.1%) 487/624 (78.0%) asymptomatic 40/624 (6.4%) symptomatic |
||
| Louie et al28 | Mass testing Symptom screening Visitor restrictions |
214/431 (49.7%) residents and staff COVID-19 positive 86/214 asymptomatic 128/214 symptomatic (50/128 were healthcare workers) Additional asymptomatic staff testing: 23/147 (15.6%) staff COVID-19 positive |
0/50 symptomatic healthcare workers hospitalised | |
| McMichael et al29 | Mass testing PPE Cohorting |
50 staff COVID-19 positive | 0/50 (0%) | 3/50 (6%) hospitalised Staff roles for confirmed cases: therapists, nurses, nurse assistants, health information manager, physician, case manager |
| Office for National Statistics40 | Mass testing Facility characteristics |
Estimated 6.9% (95% CI 5.9% to 7.9%) staff COVID-19 positive across homes that reported an outbreak | Odds of staff infection: for each additional infected resident, staff infection OR=1.04 (95% CI 1.04 to 1.04) Care homes using bank or agency staff most or every day OR=1.88 (95% CI 1.77 to 2.0) compared with homes not using these staff Homes where staff work in other homes most or every day OR=2.4 (95% CI 1.92 to 3.0) compared with homes where staff never work elsewhere Staff at homes outside London had higher odds of COVID-19 infection |
|
| Patel et al30 | Mass testing Symptom screening Visitor restrictions Cohorting |
19/42 (45.2%) 11/19 symptomatic (57.9%) 8/19 (42.1%) asymptomatic |
||
| Quicke et al31 | Mass testing (five point-prevalence surveys) | Site A: all staff uninfected Site B: low prevalence in week 1, weeks 2–5 no infections detected, week 6 increase in cases Site C: initial infection prevalence was lower (6.9%), and the incidence declined to zero by week 3 Site D: 22.5% of workers at site D had prevalent infections at the start of the study and incidence was high initially (12.2 per 100 workers per week), declining over time Site E: low prevalence in week 1 saw an increase in cases in subsequent weeks |
||
| Roxby et al33 | Mass testing Symptom screening Visitor restrictions Hand hygiene, contact precautions Cohorting |
2/62 (3.2%) (1 worked in dining facilities, 1 was a health aide) 2/2 (100%) symptomatic |
||
| Sacco et al46 | Mass testing PPE Visitor restrictions Hand hygiene, contact precautions Cohorting |
22 staff COVID-19 positive 9/22 (40.1%) asymptomatic |
0/22 (0%) | Staff incidence: care givers=0.48/100 person-days Non-care givers with resident contact=0.36/100 person-days Non-care givers with no resident contact=0.04/100 person-days |
| Stall et al44 | Facility characteristics | Outbreak involving staff and residents' for-profit homes 59/360 and staff only 44/360 Non-profit homes staff only 18/162. Municipal homes=outbreak staff only 16/101 |
||
| Telford et al35 | Mass testing (15 facilities in response to outbreak, 13 facilities as prevention) | 264/2803 (9.4%) Response group: 249/264 (94.3%) Preventive group: 15/264 (5.7%) (d) Prevalence: response group 12.8% vs preventive group 1.7%, p<0.0001 |
1/264 (0.4%) Response group: 0/249 (0%) Preventive group: 1/15 (6.7%) |
16/264 (6.1%) hospitalised Response group: 15/249 (6.0%) hospitalised Preventive group: 1/15 (6.7%) hospitalised 15/249 |
IRR, incidence risk ratio; LTCF, long-term care facility.
Table 5.
Visitor-specific outcomes following the implementation of strategies
| Study | Interventions | Prevalence | Mortality | Other outcomes |
| Ho et al48 | PPE Cohorting |
3 visitors SARS positive | 0/3 (0%) | |
| McMichael et al29 | Mass testing PPE Cohorting |
16 visitors COVID-19 positive | 1/16 (6.2%) | 8/16 (50%) hospitalised Underlying conditions: hypertension (2/8, 12.5%); cardiac disease (3/8, 18.8%); renal disease (2/8, 12.5%); obesity (3/8, 18.8%), pulmonary disease (2/8, 12.5%) |
PPE, personal protective equipment.
A variety of infection control measures are described (tables 1 and 3–5) including: mass testing/point-prevalence testing (22 studies18 20–23 26–31 33–35 39 40 45 46 49–51 54), use of PPE (10 studies18 19 21 26 29 30 33 46 48 50), screening of residents, staff or visitors for symptoms (8 studies19–21 24 26 28 30 33), restrictions on visitor entry (10 studies19–21 26 28 30 33 46 50 54), hand hygiene and contact and droplet precautions (6 studies20 24 26 33 46 47) and cohorting/isolation of residents (11 studies20 21 23 26 29 30 33 34 46 48 50). Thirteen studies examined characteristics of LTCF and their association with COVID-19 infection and risk.17 25 32 36–38 40–44 52 53
Morbidity and mortality
Morbidity and mortality results from included studies are presented for residents (table 3), staff (table 4) and visitors (table 5). Prevalence of COVID-19 infection was reported in 29 studies, including prevalence in residents (27 studies18–30 33–35 39 40 42 44 46–51 54) and staff (22 studies18 20–23 25 27–31 33 35 39 40 45–49 51 54), with two studies reporting absolute case numbers in visitors.29 48 Prevalence rates ranged from 3.8% in a sample of 2074 LTCF49 and 1.2% in the third point-prevalence survey at a single facility21 to 85.4% in a single facility that implemented a telemedicine service to limit transmission.25 Staff prevalence ranged from 0.6% in a point-prevalence survey in a single facility21 to 62.6% in a group of nine LTCF.22 One study reported 16 COVID-19 positive visitor cases,29 while a study that examined SARS infection following an outbreak in a Hong Kong facility reported three positive visitor cases.48
The symptom status (symptomatic/presymptomatic/asymptomatic, typical/atypical symptoms) of participants was reported in 16 studies, with resident and staff symptom status reported in 1518–20 22 23 26–28 30 33 34 46 49 51 54 and 13 studies,20–23 27 28 30 33 45 46 49 51 54 respectively. No studies reported symptom status of visitors. The proportion of COVID-19 positive residents presenting with symptoms ranged from 26.3%20 27 to 59.8% (a sample of both residents and healthcare workers).28 Asymptomatic cases in residents were reported in 13 studies,18 20 22 23 26–28 30 33 46 49 51 54 with proportions of COVID-19 positive residents presenting with no symptoms varying from 2.4%46 to 75.3%.49 Among COVID-19 positive staff, the proportion of symptomatic cases ranged from 6.4%27 to 100%,33 and asymptomatic cases ranged from 23.6%51 to 100%.21 23
Mortality results were reported in 22 studies, including information on mortality of residents (22 studies18–20 23–25 28–30 34 35 38–44 46 48 50 51), staff (4 studies29 35 46 48) and visitors (2 studies29 48). Mortality rates in COVID-19 positive residents ranged from 5.3%20 to 55.3%.39 One study reported a 66.7% death rate in residents who tested positive for the SARS virus.48 A study examining the mortality risk in Ontario LTCF reported a death rate of 0.1% across all residents.43 Across the three studies which presented mortality results in COVID-19 positive staff, mortality rates were 0%.29 35 46 One study presenting mortality rates in a nursing home following a SARS outbreak reported one death of a member of staff.48 Mortality rates reported in visitors in two studies was 0%48 and 6.2%,29 respectively.
Characteristics of LTCFs on COVID-19 transmission
Numerous facility-specific characteristics were linked with risk of COVID-19 cases (table 2). These include size of LTCF17 38 39 52; staffing levels and/or use of agency care staff29 32 37 39 40 44 51; part of larger chain of organisations and/or for profit status17 32 36 43 44 51; and related staffing, crowding, or availability of single rooms.24 30 40 42 44 46–48
Quality review
The quality ratings of included studies are presented in online supplemental table 2. Overall quality of evidence in this review is considered low based on MMAT assessment criteria.
Discussion
Evidence in this review indicates the impact of COVID-19 on LTCF, demonstrating the vulnerability of this setting in 11 countries. A novel outcome highlights the characteristics of LTCF associated with COVID-19 outbreaks, in addition to reporting the prevalence rates of COVID-19 and associated mortality and morbidity for residents, staff and visitors. A variety of measures were implemented in LTCF, of which many were instigated locally by facility managers, and others through agile public health policy. Due to the rapid nature of introducing public health measures though, the evidence base does not facilitate an evaluation of the effects of these measures individually. Mass testing of residents with or without staff testing was the primary measure used to reduce transmission of COVID-19. This provides objective evidence of infection rates in facilities, and enables application of subsequent measures, including isolation of residents who are infected with re-designation of specific staff to care for them. Repeated point-prevalence testing allows facilities to grasp the spread of the virus along with the impact of their mitigation strategies.
Further measures implemented in facilities echoed public health recommendations to the broader community to limit the spread of the virus. These included guidance on hand hygiene, contact and droplet precautions, and restricting staff, including agency workers, to working in only one facility.55 Restricting visitor access to facilities was implemented generally to reduce the likelihood of introducing COVID-19 into LTCF, assessing body temperature and symptom screening of staff and visitors on entry.
The prevalence of COVID-19 infection varied throughout included studies, with no distinct pattern emerging between prevention strategies and infection prevalence. Similarly, the mortality rate varied widely among studies and prevention measures. However, patterns emerged regarding associations between facility characteristics and the risk of a COVID-19 outbreak and spread. Sepulveda et al report the disproportionately higher risk of contracting COVID-19 for residents of LTCF, calculating a 12-country average mortality rate of 2772 per 100 000 LTCF residents compared with 122 per 100 000 for community dwelling older persons.56 This represented an average 24.2-fold higher rate of death (range 14.2 (Germany) to 73.7 (Canada)). Higher LTCF mortality rates in Canada (78.4% compared with the Organisation for Economic Co-operation and Development (OECD) 12 country average of 43.7%) are explained by poorer services in care facilities and includes limited staffing and funding.56
Evidence identified the facility size/number of beds was significantly associated with the probability of having a COVID-19 case, and the resulting size of an outbreak. For example, in a sample of 30 US nursing homes, the probability of having a COVID-19 case was increased in medium and large facilities compared with small facilities,17 while in 121 UK homes reporting an outbreak, facilities with ≥70 beds had 80% greater infection rates than facilities with <35 beds.39 A sample of 623 Canadian nursing homes demonstrated facilities with a high crowding index had more infections and deaths than those with a low crowding index. Simulations conducted suggested nearly 20% of infections and deaths may have been averted by converting all four-bed rooms into two-bed rooms.42 Similarly, facilities with a greater number of employees, staff who work in multiple facilities and an increased number of infected staff, were also more likely to experience a COVID-19 outbreak.37 40 51 However, facilities where staff receive sick leave were shown to be less likely to have positive cases.40 Reduced availability of PPE predicted the spread and increase in case number in facilities,37 while for-profit status of facilities was commonly identified as increasing the odds of case outbreaks relative to non-profit status.17 32 36 43 44
Rapid development of COVID-19 vaccines was recognised in early March 2020.57 Lurie et al note previous success in the development of H1N1 vaccination, and similarly the challenges for SARS, Ebola and Zika vaccines.57 The speed of developments is acknowledged, and Public Health England report that at the end of February 2021 up to 5900 deaths were averted in people aged 80 years and older, with over 200 deaths prevented in those aged 7–79 years.58 Montano advises that an accelerated pace of vaccine developments may not lead to total eradication of the virus, citing smallpox as the only virus that has been eliminated worldwide.59 Given this, the transmission reduction measures highlighted in the present review are of crucial importance for the continued management of COVID-19 in LTCF.
Quality review
The quality of evidence in this review is technically low, primarily reported from observational studies, expert opinion, reporting of outbreaks and describing the process and management (online supplemental table 2). Factors associated with lower quality of evidence include the reliance on self-reporting of symptoms, recall bias, use of datasets which may be incomplete and use of convenience sampling. However, confirmation of COVID-19 in the majority of studies was via laboratory testing. We did not remove any study following our review of quality and the evidence is consistent with real-time reporting of data to learn from outbreaks. Papers included from MEDRXIV pre publishing repository are acknowledged; however, as papers were subsequently published in peer review journals we reviewed accordingly. The Institute of Medicine60 advocates for early detection of epidemics, effective communication to the public and promotion of research and development for strategic planning.
Limitations in the review process
A key strength of this review is that it addresses a knowledge gap and has collated evidence from a broad methodological base to report the measures to reduce transmission of COVID-19 in LTCF and reports characteristics of facilities.
Due to the heterogeneity of included studies, meta-analysis was not performed, while the descriptive nature of studies prevents identification of a causative relationship between measures and outcomes. We acknowledge that while a summary of facility characteristics and COVID-19 outcomes are presented, insufficient evidence is available to statistically evaluate and summarise the relationship between individual measures to prevent COVID-19 transmission and thus further research studies are required to elucidate this. Despite this, the systematic approach to this review has identified the scope of interventions implemented in LTCF to reduce COVID-19 transmission.
Publication bias was minimised with inclusion of prepublished evidence, follow-up contacts with authors for early reporting and through the inclusion of observational study designs. Most studies reported are in English, we translated papers from German and Spanish as part of the assessment and review. Outbreak reports include convenience samples or smaller cohorts of residents in LTCF with limited data reported in brief reports and letters. However, real-time reporting of outbreaks provides immediate evidence and shared understanding advocated by the Institute of Medicine.60
Evidence in this review builds on publications from Salcher-Konrad et al,61 a report from WHO,62 and an Irish Expert Panel review,55 furthermore, data on the role of facilities in the transmission of COVID-19 are presented.
Conclusion
This novel, rapid review summarises the evidence base to date identifying specific factors for consideration as part of preparedness plans to reduce transmission of COVID-19 outbreaks in LTCF. Future research should incorporate methodologically robust study designs with longer follow-up to assess the impact on reducing transmission.
Supplementary Material
Footnotes
Twitter: @katef224, @doclach5195
KF and LM contributed equally.
Contributors: CCK, KF and LM designed the study; KF and DS developed the search strategy; DS conducted the literature search; KF and LM screened titles and full texts to select studies, and extracted data; LM, EL, KF and CCK conducted quality ratings; KF, LM, DS, EL, EC, CCK interpreted and synthesised data; KF, LM, DS, EL, EC, CCK were involved in writing. All authors have approved the final version of the manuscript. CCK acts as the guarantor for this work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: CCK was a member of an expert panel investigating COVID-19 in nursing homes in Ireland.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.European Centre for Disease Prevention and Control (ECDC) . Timeline of ECDC’s response to COVID-19, 2020. Available: https://www.ecdc.europa.eu/en/covid-19/timeline-ecdc-response [Accessed 04 Oct 2020].
- 2.World Health Organization . Coronavirus disease 2019 (COVID-19) situation report – 94, 2020. [Google Scholar]
- 3.Nussbaumer-Streit B, Mayr V, Dobrescu AI, et al. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochrane Database Syst Rev 2020;4:Cd013574. 10.1002/14651858.CD013574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Centre for Disease Prevention and Control (ECDC) . Risk assessment: outbreak of acute respiratory syndrome associated with a novel coronavirus, Wuhan, China. Stockholm: ECDC, 2020. https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-cluster-pneumonia-cases-caused-novel-coronavirus-wuhan2020 [Google Scholar]
- 5.World Health Organization . Statement on the second meeting of the International health regulations (2005) emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV. Geneva: WHO, 2020. https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) [Google Scholar]
- 6.Meng X, Deng Y, Dai Z, et al. COVID-19 and anosmia: a review based on up-to-date knowledge. Am J Otolaryngol 2020;41:102581–81. 10.1016/j.amjoto.2020.102581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Centre for Disease Prevention and Control (ECDC) . COVID-19 situation update worldwide, as of week 12, 2021. Available: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases [Accessed 08 Apr 2021].
- 8.European Centre for Disease Prevention and Control (ECDC) . Surveillance of COVID-19 at longterm care facilities in the EU/EEA. technical report 2020.
- 9.ECDC Public Health Emergency Team, Danis K, Fonteneau L, et al. High impact of COVID-19 in long-term care facilities, suggestion for monitoring in the EU/EEA, may 2020. Euro Surveill 2020;25:2000956. 10.2807/1560-7917.ES.2020.25.22.2000956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller AL, McNamara MS, Sinclair DA. Why does COVID-19 disproportionately affect older people? Aging 2020;12:9959–81. 10.18632/aging.103344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.United Nations . Policy brief: the impact of COVID-19 on older persons, 2020. [Google Scholar]
- 12.World Health Organization . Policy brief: the impact of COVID-19 on older persons, 2020. [Google Scholar]
- 13.Frazer K, Mitchell L, Stokes D. Systematic review of measures to protect older people in long term care facilities from COVID 19. Prospero: International prospective register of systematic reviews, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong QN, Fàbregues S, Bartlett G, et al. The mixed methods appraisal tool (MMAT) version 2018 for information professionals and researchers. Education for Information 2018;34:285–91. 10.3233/EFI-180221 [DOI] [Google Scholar]
- 16.Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (swim) in systematic reviews: reporting guideline. BMJ 2020;368:l6890. 10.1136/bmj.l6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrams HR, Loomer L, Gandhi A, et al. Characteristics of U.S. nursing homes with COVID-19 cases. J Am Geriatr Soc 2020;68:1653–6. 10.1111/jgs.16661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 2020;382:2081–90. 10.1056/NEJMoa2008457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackman C, Farber S, Feifer RA, et al. An illustration of SARS-CoV-2 dissemination within a skilled nursing facility using heat maps. J Am Geriatr Soc 2020;68:2174–8. 10.1111/jgs.16642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dora AV, Winnett A, Jatt LP, et al. Universal and Serial Laboratory Testing for SARS-CoV-2 at a Long-Term Care Skilled Nursing Facility for Veterans - Los Angeles, California, 2020. MMWR Morb Mortal Wkly Rep 2020;69:651–5. 10.15585/mmwr.mm6921e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckardt P, Guran R, Hennemyre J, et al. Hospital affiliated long term care facility COVID-19 containment strategy by using prevalence testing and infection control best practices. Am J Infect Control 2020;48:1552–5. 10.1016/j.ajic.2020.06.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feaster M, Goh Y-Y. High proportion of asymptomatic SARS-CoV-2 infections in 9 long-term care facilities, Pasadena, California, USA, April 2020. Emerg Infect Dis 2020;26:2416–9. 10.3201/eid2610.202694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham NSN, Junghans C, Downes R, et al. SARS-CoV-2 infection, clinical features and outcome of COVID-19 in United Kingdom nursing homes. J Infect 2020;81:411–9. 10.1016/j.jinf.2020.05.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hand J, Rose EB, Salinas A, et al. Severe respiratory illness outbreak associated with human coronavirus NL63 in a long-term care facility. Emerg Infect Dis 2018;24:1964–6. 10.3201/eid2410.180862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris DA, Archbald-Pannone L, Kaur J, et al. Rapid Telehealth-Centered response to COVID-19 outbreaks in Postacute and long-term care facilities. Telemed J E Health 2021;27:102–6. 10.1089/tmj.2020.0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and Presymptomatic SARS-CoV-2 Infections in Residents of a Long-Term Care Skilled Nursing Facility - King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep 2020;69:377–81. 10.15585/mmwr.mm6913e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lennon NJ, Bhattacharyya RP, Mina MJ. Comparison of viral levels in individuals with or without symptoms at time of COVID-19 testing among 32,480 residents and staff of nursing homes and assisted living facilities in Massachusetts. medRxiv 2020:2020.07.20.20157792. [Google Scholar]
- 28.Louie JK, Scott HM, DuBois A, et al. Lessons from Mass-Testing for coronavirus disease 2019 in long-term care facilities for the elderly in San Francisco. Clinical Infectious Diseases 2021;72:2018–20. 10.1093/cid/ciaa1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMichael TM, Currie DW, Clark S, et al. Epidemiology of covid-19 in a long-term care facility in King County, Washington. N Engl J Med 2020;382:2005–11. 10.1056/NEJMoa2005412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel MC, Chaisson LH, Borgetti S, et al. Asymptomatic SARS-CoV-2 infection and COVID-19 mortality during an outbreak investigation in a skilled nursing facility. Clinical Infectious Diseases 2020;71:2920–6. 10.1093/cid/ciaa763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quicke K, Gallichote E, Sexton N. Longitudinal surveillance for SARS-CoV-2 RNA among asymptomatic staff in five Colorado skilled nursing facilities: epidemiologic, virologic and sequence analysis. medRxiv 2020:2020.06.08.20125989. [Google Scholar]
- 32.Quigley DD, Dick A, Agarwal M, et al. COVID-19 preparedness in nursing homes in the midst of the pandemic. J Am Geriatr Soc 2020;68:1164–6. 10.1111/jgs.16520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roxby AC, Greninger AL, Hatfield KM, et al. Outbreak investigation of COVID-19 among residents and staff of an independent and assisted living community for older adults in Seattle, Washington. JAMA Intern Med 2020;180:1101–5. 10.1001/jamainternmed.2020.2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez GV, Biedron C, Fink LR, et al. Initial and Repeated Point Prevalence Surveys to Inform SARS-CoV-2 Infection Prevention in 26 Skilled Nursing Facilities - Detroit, Michigan, March-May 2020. MMWR Morb Mortal Wkly Rep 2020;69:882–6. 10.15585/mmwr.mm6927e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Telford CT, Onwubiko U, Holland D, et al. Mass screening for SARS-CoV-2 infection among residents and staff in Twenty-eight long-term care facilities in Fulton County, Georgia. medRxiv 2020:2020.07.01.20144162. 10.1101/2020.07.01.20144162 [DOI] [Google Scholar]
- 36.Unruh MA, Yun H, Zhang Y, et al. Nursing home characteristics associated with COVID-19 deaths in Connecticut, new Jersey, and new York. J Am Med Dir Assoc 2020;21:1001–3. 10.1016/j.jamda.2020.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brainard J, Rushton S, Winters T, et al. Introduction to and spread of COVID-19-like illness in care homes in Norfolk, UK. J Public Health 2021;43:228–35. 10.1093/pubmed/fdaa218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burton JK, Bayne G, Evans C, et al. Evolution and effects of COVID-19 outbreaks in care homes: a population analysis in 189 care homes in one geographical region of the UK. Lancet Healthy Longev 2020;1:e21–31. 10.1016/S2666-7568(20)30012-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dutey-Magni PF, Williams H, Jhass A. Covid-19 infection and attributable mortality in UK long term care facilities: cohort study using active surveillance and electronic records (March-June 2020). Age and Ageing 2021:afab060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Office for National Statistics . Impact of coronavirus in care homes in England: 26 may to 19 June 2020. they Vivaldi study, 2020. [Google Scholar]
- 41.Stow D, Barker RO, Matthews FE. National early warning scores (news / NEWS2) and COVID-19 deaths in care homes: a longitudinal ecological study. medRxiv 2020:2020.06.15.20131516. [Google Scholar]
- 42.Brown KA, Jones A, Daneman N, et al. Association between nursing home crowding and COVID-19 infection and mortality in Ontario, Canada. JAMA Intern Med 2021;181:229–36. 10.1001/jamainternmed.2020.6466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisman DN, Bogoch I, Lapointe-Shaw L, et al. Risk factors associated with mortality among residents with coronavirus disease 2019 (COVID-19) in long-term care facilities in Ontario, Canada. JAMA Netw Open 2020;3:e2015957–e57. 10.1001/jamanetworkopen.2020.15957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stall NM, Jones A, Brown KA, et al. For-Profit long-term care homes and the risk of COVID-19 outbreaks and resident deaths. CMAJ 2020;192:E946–55. 10.1503/cmaj.201197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guery R, Delaye C, Brule N, et al. Limited effectiveness of systematic screening by nasopharyngeal RT-PCR of Medicalized nursing home staff after a first case of COVID-19 in a resident. Med Mal Infect 2020;50:748–50. 10.1016/j.medmal.2020.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sacco G, Foucault G, Briere O, et al. COVID-19 in seniors: findings and lessons from mass screening in a nursing home. Maturitas 2020;141:46–52. 10.1016/j.maturitas.2020.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heung LCL, Li T, Mak SK. Prevalence of subclinical infection and transmission of severe acute respiratory syndrome (SARS) in a residential care home for the elderly. Hong Kong Med J 2006;12:201–7. [PubMed] [Google Scholar]
- 48.Ho WW, Hui E, Kwok TC, et al. An outbreak of severe acute respiratory syndrome in a nursing home. J Am Geriatr Soc 2003;51:1504–5. 10.1046/j.1532-5415.2003.514841.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoxha A, Wyndham-Thomas C, Klamer S, et al. Asymptomatic SARS-CoV-2 infection in Belgian long-term care facilities. Lancet Infect Dis 2021;21:e67. 10.1016/S1473-3099(20)30560-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein A, Edler C, Fitzek A, et al. Der erste COVID-19-Hotspot in einer Hamburger Senioreneinrichtung. Rechtsmedizin 2020;30:325–31. 10.1007/s00194-020-00404-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kennelly SP, Dyer AH, Noonan C, et al. Asymptomatic carriage rates and case fatality of SARS-CoV-2 infection in residents and staff in Irish nursing homes. Age Ageing 2021;50:49–54. 10.1093/ageing/afaa220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iritani O, Okuno T, Hama D, et al. Clusters of COVID-19 in long-term care hospitals and facilities in Japan from 16 January to 9 may 2020. Geriatr Gerontol Int 2020;20:715–9. 10.1111/ggi.13973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim T. Improving preparedness for and response to coronavirus disease 19 (COVID-19) in long-term care hospitals in Korea. Infect Chemother 2020;52:133. 10.3947/ic.2020.52.2.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borras-Bermejo B, Martínez-Gómez X, San Miguel MG, et al. Asymptomatic SARS-CoV-2 infection in nursing homes, Barcelona, Spain, April 2020. Emerg Infect Dis 2020;26:2281–3. 10.3201/eid2609.202603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelleher CC, Doherty B, Donnelly P. COVID-19 nursing homes expert panel. examination of measures to 2021. Report to the Minister for Health 2020. [Google Scholar]
- 56.Sepulveda ER, Stall NM, Sinha SK. A comparison of COVID-19 mortality rates among long-term care residents in 12 OECD countries. J Am Med Dir Assoc 2020;21:1572–4. 10.1016/j.jamda.2020.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. N Engl J Med 2020;382:1969–73. 10.1056/NEJMp2005630 [DOI] [PubMed] [Google Scholar]
- 58.Public Health England . Impact of COVID-19 vaccines on mortality in England December 2020 to February 2021. London, 2021. [Google Scholar]
- 59.Montano M. Pressing questions and challenges in the HIV-1 and SARS-CoV-2 Syndemic. AIDS Res Hum Retroviruses 2021;37:589–600. 10.1089/aid.2021.0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Institute of Medicine (IOM) . Learning from SARS: Preparing for the Next Disease Outbreak: Workshop Summary. In: Knobler S, Mahmoud A, Lemon S, eds. Learning from SARS: preparing for the next disease outbreak: workshop summary. Washington (DC: National Academies Press (US), Copyright © 2004, National Academy of Sciences, 2004. [PubMed] [Google Scholar]
- 61.Salcher-Konrad M, Jhass A, Naci H. COVID-19 related mortality and spread of disease in long-term care: a living systematic review of emerging evidence. medRxiv 2020:2020.06.09.20125237. [Google Scholar]
- 62.World Health Organization . Preventing and managing COVID-19 across long-term care services: policy brief. Geneva, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-047012supp001.pdf (143.4KB, pdf)
bmjopen-2020-047012supp002.pdf (68KB, pdf)
Data Availability Statement
No data are available.