Abstract
Disordered metabolic states, which are characterised by hypoxia and elevated levels of metabolites, particularly lactate, contribute to the immunosuppression in the tumour microenvironment (TME). Excessive lactate secreted by metabolism-reprogrammed cancer cells regulates immune responses via causing extracellular acidification, acting as an energy source by shuttling between different cell populations, and inhibiting the mechanistic (previously ‘mammalian’) target of rapamycin (mTOR) pathway in immune cells. This review focuses on recent advances in the regulation of immune responses by lactate, as well as therapeutic strategies targeting lactate anabolism and transport in the TME, such as those involving glycolytic enzymes and monocarboxylate transporter inhibitors. Considering the multifaceted roles of lactate in cancer metabolism, a comprehensive understanding of how lactate and lactate-targeting therapies regulate immune responses in the TME will provide insights into the complex relationships between metabolism and antitumour immunity.
Keywords: Lactate, Glycolytic enzymes, Monocarboxylate transporters, Cancer metabolism, Cancer immunity, Tumour microenvironment
1. Introduction
Cancer cells with aberrant metabolism consume large amounts of oxygen and nutrients, resulting in hypoxia, nutritional deficiency, and elevated levels of metabolic by-products in the tumour microenvironment (TME) [1]. Elevated metabolites in the TME, particularly lactate, facilitate the establishment of an immunosuppressive milieu that favours cancer cell growth and immune escape [2]. An increasing number of studies have demonstrated that lactate is not only an end product of glycolysis, but also an important regulator that participates in multiple signalling pathways in both normal and tumour tissues [3].
Excessive lactate production and rapid lactate transport in cancer cells depend primarily on the upregulation of hypoxia-inducible factor-1α (HIF-1α) and c-Myc [4,5]. Continuous activation of HIF-1α and c-Myc causes aberrant expression of multiple glycolytic enzymes and monocarboxylate transporters (MCTs), including lactate dehydrogenase A (LDHA), MCT1, and MCT4 [4]. Lactate in the TME not only induces lactic acidosis, but also shuttles among cell populations, including cancer cells, tumour-associated stromal cells, tumour-associated macrophages (TAMs), and tumour-infiltrating lymphocytes (TILs) [6].
Lactate is an evolutionarily conserved metabolite. The processes by which immune cells respond to this ancient molecule explain its modulatory effects on immune responses in the TME. In this review, we describe the current understanding of lactate-modulated immune responses and assess the recent literature to briefly summarise the therapeutic strategies that target lactate anabolism and transport in the TME.
2. Lactate in the TME
2.1. Excessive lactate secreted from cancer cells in the TME
Otto Warburg in the 1920s discovered distinct metabolic characteristics of cancer cells, including excessive glucose uptake and preferential production of lactate, even in the presence of sufficient oxygen [7]. Under normal circumstances, pyruvate from glycolysis is completely oxidised in the mitochondria via oxidative phosphorylation (OXPHOS) to produce ATP and CO2 [8]. Normal cells produce lactate only under hypoxic conditions through anaerobic glycolysis. The process of lactate production in cancer cells, even in an oxygen-rich environment, is referred to as aerobic glycolysis or the Warburg effect [9]. Lactate produced by cancer cells is exported into the extracellular space via MCTs to avoid their intracellular acidification, but this causes an increase in lactate levels in the TME [10], which has been associated with poor prognosis [11]. Below, we discuss how cancer cells upregulate lactate levels in the TME by producing and secreting excessive lactate.
One of the most significant characteristics of the TME is hypoxia, which leads to increased levels of reactive oxygen species (ROS) and enhanced tumour invasion, as well as chemotherapy resistance via the activation of nuclear factor kappa B (NF-κB), nuclear factor erythroid 2, and HIF-1α [12]. HIF-1α was initially identified as an important factor in response to hypoxic conditions [13]; posttranslational modifications play a critical role in its regulation. Hypoxia inhibits several critical enzymes that participate in the posttranslational modification of HIF-1α, including prolyl hydroxylase domain proteins (PHDs), the von Hippel-Lindau tumour suppressor protein (VHL), and HIF-1α subunit inhibitor HIF1AN (also termed FIH1). Hypoxia thus inhibits HIF-1α protein degradation and facilitates HIF-1α transcriptional activation [14]. Additionally, the HIF-1α pathway can be activated via hypoxia-independent mechanisms that cause pseudohypoxia, even when oxygen is sufficient. For example, loss-of-function and somatic mutations in the VHL gene or inactivation of VHL gene expression facilitate HIF-1α protein stabilisation and accumulation, even under normoxia [15]. In normoxia, loss of p53 protein function causes HIF-1α protein to accumulate by interrupting mouse double minute 2 homologue-mediated HIF-1α protein degradation [16]. In addition, activation of the PI3K/AKT/mTOR signalling pathway and elevated ROS levels are involved in the activation of HIF-1α signalling [14]. Under inflammatory conditions, multiple pro-inflammatory cytokines including IL-1β and TNF-α, can induce the expression of HIF-1α protein by activating HIF-1 DNA binding [17], whereas PI3K inhibitors interrupt cytokine-induced expression of HIF-1α mRNA [18]. In addition to cytokines, other non-hypoxic mediators such as growth factors, as well as vasoactive hormones such as vascular endothelial growth factor, angiotensin II, thrombin, and endothelin, can induce the expression of HIF-1α by activating the p42/p44-MAPK or PI3K/p70S6K/mTOR pathways [19], [20], [21]. Other proinflammatory mediators such as nitric oxide (NO) can induce the accumulation of HIF-1α under normoxia [22]. In macrophages, the production of NO is controlled in part by the differential expression of HIF-1α and HIF-2α, which are induced by Th1 cytokines during M1 polarisation and Th2 cytokines during M2 polarisation, respectively [23]. Furthermore, viral proteins, including hepatitis B virus X protein and Epstein-Barr virus latent membrane protein 1, can induce the accumulation of HIF-1α protein under normoxia [24,25].
HIF-1α and c-Myc are two critical transcriptional factors that maintain high glycolytic activity in cancer cells [26]. HIF-1α and c-Myc in cancer cells upregulate lactate production through multiple mechanisms. First, they can enhance pyruvate production by accelerating two of the three rate-limiting steps in glycolysis involving hexokinase 2 and fructose-2,6-bisphosphate [27]. Second, they can suppress pyruvate dehydrogenase-mediated pyruvate mitochondrial metabolism by inducing pyruvate dehydrogenase kinase-1, which phosphorylates pyruvate dehydrogenase and inhibits its activity [28]. Third, they can activate lactate dehydrogenase-5 (LDH-5) and inhibit LDH-1. There are five different combinations of four subunits for LDH tetramers: LDHA4, LDHB4, LDHA3LDHB, LDHA2LDHB2, and LDHALDHB3. LDH-5 (LDHA4) promotes the conversion of pyruvate to lactate, whereas LDH-1 (LDHB4) plays the opposite role. Both HIF-1α and c-Myc induce the expression of LDHA gene and inhibit the expression of LDHB gene, which further enhances the activity of LDH-5 and decreases the activity of LDH-1, causing an increase in lactate production [29].
High glycolytic activity has been associated with the activation of the mechanistic (previously ‘mammalian’) target of rapamycin (mTOR) pathway cascade in cancer cells. The mTOR protein is an evolutionarily conserved serine/threonine protein kinase and a member of the PI3K-related protein kinase family [30]. It can form two distinct catalytic complexes: mTOR complex 1 (mTORC1) and mTORC2. It senses and integrates diverse environmental signals to control cell growth and proliferation, acting as a critical regulatory node in the cellular metabolism network [30]. Activation of the mTOR pathway increases expression of HIF-1α and c-Myc to upregulate expression of glucose transporter 1 (GLUT1) and glycolytic enzymes, such as hexokinase 2 and pyruvate kinase muscle isoform 2 (PKM2), thereby facilitating lactate production in cancer cells [30]. Upregulated PKM2 also stimulates mTORC1 in cancer cells through a positive feedback loop [31].
In addition to aerobic glycolysis, cancer cells generate lactate from glutamine catabolism, which is referred to as glutaminolysis [32]. Cancer cells can increase glutamine uptake and promote the conversion of glutamine to glutamate via glutaminase, which is also regulated by c-Myc [33]. Glutamate subsequently enters the mitochondria and participates in the tricarboxylic acid (TCA) cycle in the form of α-ketoglutarate, which is converted to malate and then transported into the cytoplasm, providing pyruvate for lactate production [34]. In addition to providing sufficient ATP for tumour growth, glycolysis and glutaminolysis generate intermediates that are important carbon sources for anabolic pathways and antioxidant stress in cancer cells, including NADPH production, nucleotide synthesis, and purine-pyrimidine synthesis, as well as amino-acid, fatty-acid, and sterol synthesis.
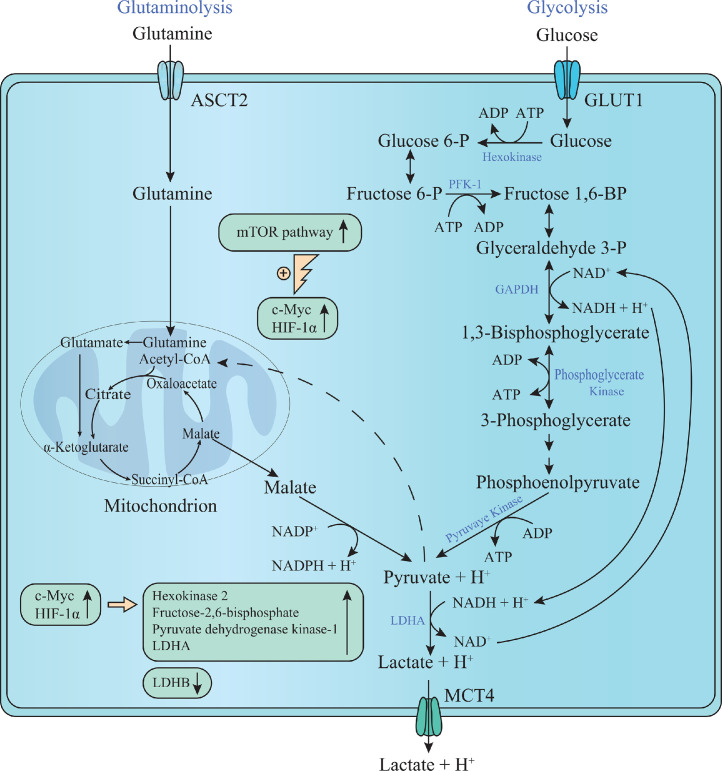
In addition to the production of excessive lactate, cancer cells induce the c-Myc-mediated high expression of MCT1 (also termed solute carrier 16A1 or SLC16A1) and the HIF-1α-mediated high expression of MCT4 (also termed solute carrier 16A3 or SLC16A3), facilitating the export of lactate into the extracellular TME to avoid intracellular acidification [35]. In summary, cancer cells increase lactate levels in the TME via excessive production and rapid export of lactate (Fig. 1).
Fig. 1.
Lactate from aerobic glycolysis and glutaminolysis in cancer cells. Aerobic glycolysis in cancer cells is efficient because of the cytoplasmic regeneration of NAD+ from NADH by LDHA without participating in mitochondrial electron transport chain. Without this process, deficiency of NAD+ pool limits glycolysis of cancer cells, then decreasing the rate of glycolysis at the GAPDH-mediated step. The continuous activation of HIF-1α, c-Myc, and the mTOR pathway induces aberrant expression of multiple glycolytic enzymes, thus facilitating aerobic glycolysis in cancer cells. ASCT2, alanine/serine/cysteine transporter 2; GLUT1, glucose transporter 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HIF-1α, hypoxia-inducible factor 1α; PFK-1, phosphofructokinase-1; MCT4, monocarboxylate transporter 4; mTOR, mechanistic target of rapamycin; LDHA, lactate dehydrogenase A. This figure is created by Pei Zhang, Zi-Hao Wang, and Qiong Zhou. All authors confirm originality of it and retain copyright to it.
2.2. Lactate shuttling in the TME
Lactate is an energy-rich signalling molecule that shuttles between cells under both physiological and pathological conditions. In normal skeletal muscle, slow-twitch fibres take up lactate produced by the glycolysis of fast-twitch fibres [36]. Astrocytes are also glycolytic and produce adequate lactate that is imported by adjacent neurons [37]. In an inflammatory environment, glycolytic synovial fibroblasts secrete lactate via MCT4, while CD4+ T cells take up lactate from inflamed joints via sodium-coupled monocarboxylate transporter 2 (SMCT2; also termed solute carrier 5A12 or SLC5A12), causing lactate-induced inhibition of T-cell motility and preferential differentiation into pro-inflammatory CD4+ T-cell subsets [38], [39], [40].
In the TME, cancer cells are metabolically heterogeneous, depending on their intratumoral spatial localizations [4]. Using dynamic contrast-enhanced magnetic resonance imaging, Hensley et al. showed that enrichments of 13C in the more well-perfused superior regions of human lung tumours were significantly lower than those in the inferior regions [41]. In addition, Hensley et al. infused mice bearing subcutaneous tumours with [2-13C]lactate and found that 13C was enriched in multiple metabolites, including glutamate, malate, and citrate, indicating that lactate is an important carbon source for lung tumours. Similarly, Faubert et al. infused mice bearing lung tumours with [13C]lactate, [13C]pyruvate, or [13C]alanine and found that after infusion with [13C]lactate, 13C was enriched in tumour lactate and intermediates in the TCA cycle, relative to 3-phosphoglycerate [42]. Furthermore, Faubert et al. constructed tumour-bearing mouse models with MCT1-deficient lung cancer cell lines and found that enrichment of 13C in tumour metabolites was significantly reduced, demonstrating that MCT1 is critical for lactate influx in cancer cells. Consistent with these studies, Hui et al. demonstrated that circulating lactate was the predominant source of TCA cycle intermediates in mice with lung tumours, while the contribution of glutamine exceeded that of lactate in pancreatic cancer [43]. In summary, cancer cells far from blood vessels are under hypoxic conditions and obtain energy mainly through glycolysis while producing excessive lactate, which is exported into the TME via MCT4 [44]. Cancer cells that are close to blood vessels are normoxic and can oxidise lactate for ATP synthesis via MCT1-mediated lactate influx. This lactate metabolic symbiosis occurs not only in cancer cells, but also in cancer-associated fibroblasts (CAFs) and tumour-associated endothelial cells [45], [46], [47]. For example, CAFs with an aerobic glycolytic metabolic phenotype produce and release high amounts of lactate via MCT4, while adjacent cancer cells with oxidative ability can take up lactate via MCT1 and oxidise it [48]. In angiogenic endothelial cells, MCT1-mediated lactate uptake induces ROS-dependent NF-κB activation independently of HIF-1α activation to upregulate the production of vascular endothelial growth factor (VEGF) [45]. Lactate metabolic symbiosis also occurs among tumour-infiltrating regulatory T (Treg) cells and TAMs, which will be discussed below. Altogether, lactate shuttling between cell populations is a prominent feature of the TME and is essential for tumorigenesis, and tumour progression and metastasis [49].
2.3. Modulation of immune response by lactate in the TME
2.3.1. T cells
Elevated lactate suppresses the antitumour activity of T cells by increasing the accumulation of H+ and maintaining low pH in the TME. Extracellular acidification suppresses the functions of CD8+ T lymphocytes (CTLs), while neutralisation of acidic TME and proton-pump inhibitors can reverse the suppression of antitumour immunity and improve immunotherapy [50]. Mechanistically, lactic acidosis can impair the functions of CTLs by inhibiting T-cell receptor-triggered activation of the p38 and JNK/c-JUN pathways, which are important for IFN-γ production. Lactic acidosis also induces T-cell apoptosis by reducing the levels of nicotinamide adenine dinucleotide (NAD(+)), which is crucial for the expression of the focal adhesion kinase family interacting protein of 200 kDa [51,52]. Additionally, lactic acidosis may drive the redistribution of perinuclear lysosomes to cellular peripheries, thus suppressing mTORC1 activity in immune cells by separating lysosomal mTORC1 from its activator, small GTPase Ras homologue enriched in brain [53]. Furthermore, lactate can modulate CD4+ T-cell polarisation and reduce the percentage of the antitumoral T-helper 1 subset by inducing SIRT1-mediated deacetylation/degradation of T-bet transcription factor [54].
Generally, quiescent human CD8+ and CD4+ T cells express low levels of MCT1 and MCT4 [55]. After T-cell activation via the binding of CD28 to the B7 receptor, the metabolic pattern of T cells is transformed from OXPHOS to glycolysis, leading to the remarkable upregulation of MCT1 and delayed induction of MCT4 [56]. In the TME, activated T cells not only have to compete with tumour cells for glucose, but also must avoid intracellular acidification via MCT-mediated lactate transmission. However, the high levels of tumour-derived lactate in the TME prevent activated T cells from secreting lactate because of the lactate transmembrane concentration gradient [4]. This causes an endogenous lactate accumulation, which hampers the antitumour activity of effector T cells.
2.3.2. Natural killer cells and natural killer T cells
Elevated lactate levels not only directly constrain the cytolytic functions of natural killer (NK) cells, but also indirectly restrain NK cells by increasing the number of myeloid-derived suppressor cells [57]. Moreover, lactate inhibits the activation of nuclear factor of activated T cells (NFAT) in NK cells, thus inhibiting their IFN-γ production [58]. Lactic acidosis can repress the lipid biosynthesis and antitumour activities of tumour-infiltrating invariant natural killer T cells by reducing the expression of peroxisome proliferator-activated receptor γ (PPARγ) [59,60].
2.3.3. Monocytes and dendritic cells
Lactic acidosis not only induces the differentiation of monocytes into dendritic cells with an immunosuppressive phenotype [61,62], but also promotes their differentiation into macrophages with an inflammatory protumour phenotype [63]. However, exorbitant lactate levels in the TME may also retard the differentiation of monocytes into dendritic cells [6]. G-protein-coupled receptor 81 (GPR81, also termed hydroxycarboxylic acid receptor 1 or HCAR1) is expressed on the surface of antigen-presenting cells in the TME; tumour-derived lactate can activate GPR81 to inhibit the expression of MHC-II, playing a paracrine role [4]. GPR81 on the surfaces of plasmacytoid dendritic cells (pDCs) can sense lactate and activate calcineurin phosphatase signalling, leading to an increase in free cytosolic Ca2+ and the inhibition of pDC activation and type I IFN production [64]. Additionally, MCT1-mediated lactate influx partially contributes to the inhibition of pDC activation [64].
2.3.4. Macrophages
Elevated lactate levels are essential for maintaining the pro-tumoral activities of TAMs. Lactic acidosis can suppress the function of M1 macrophages by reducing the expression of IL-6, iNOS, and CCL2 [6]. Lactate can also inhibit the expression of ATP6V0d2 in TAMs to promote tumour progression [65]. Lactate-induced M2 macrophage polarisation involves the stabilisation of HIF-1α and activation of the ERK-STAT3 signalling pathway and G-protein-coupled receptor 132 (GPR132) [50,66]. GPR132 is expressed at high levels on the surface of macrophages and plays an important role in the induction of the macrophage pro-inflammatory phenotype. Extracellular lactate sensed by GPR132 induces cyclic AMP (cAMP) and hence inducible cAMP early repressor (ICER), thus upregulating the expression of arginine-metabolizing enzyme arginase 1 (ARG1), VEGF, and HIF-1α and mediating the conversion of macrophages to a pro-angiogenic phenotype [47]. Moreover, intracellular lactate can cause epigenetic modifications via NAD(+)-independent histone deacetylase inhibition and histone lysine residue lactylation in macrophages [67,68]. In the TME, levels of histone lactylation have been associated with the production of oncogenic factors by M2 macrophages [68].
2.3.5. Treg cells
Tumour-infiltrating Treg cells can take up lactate to maintain their suppressive functions by increasing the expression of FOXP3 and MCT1 [46,69]. High expression of FOXP3 reprograms Treg cell metabolism by inhibiting c-Myc and glycolysis, enhancing OXPHOS, and increasing NAD(+) oxidation, thus making Treg cells more adaptable in the low-glucose and high-lactate TME [69]. Additionally, MCT1-mediated lactate influx and intracellular lactate metabolism are important for tumour-infiltrating Treg cells to sustain their suppressive activity, while high glucose levels dampen their function and stability [46].
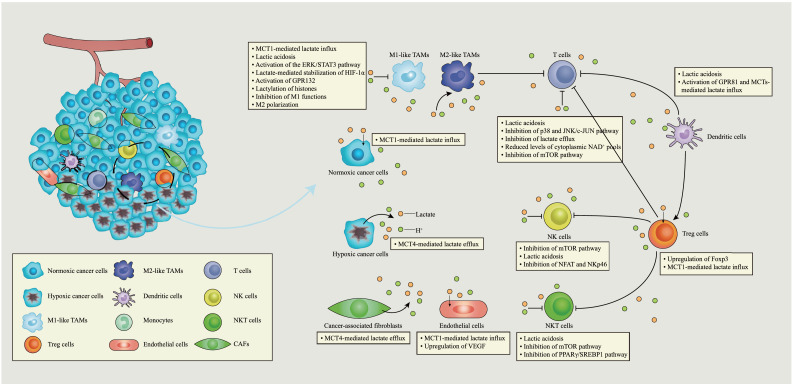
In summary, lactate modulates immune responses in the TME via both H+-dependent and lactate-dependent pathways. Lactic acidosis, lactate-mediated intracellular signal transduction, and histone modification are all involved in this regulation (Fig. 2).
Fig. 2.
Immune modulation by lactate in the tumour microenvironment. The tumour microenvironment (TME) is filled with multiple cell populations, including tumour, stromal, and immune cells, as well as vascular endothelial cells. In the TME, tumour cells consume most of the nutrients and secrete excessive lactate into the extracellular microenvironment, resulting in acidosis, angiogenesis and immunosuppression. Lactate also modulates the metabolism of innate and adaptive immune cells, by inhibiting the functions of CD8+ T cells, natural killer (NK) cells, natural killer T (NKT) cells and dendritic cells. By contrast, lactate favours FOXP3+ regulatory T (Treg) cells sustaining their immunosuppressive functions in the acidic environment. Additionally, lactate potentiates the M2 polarization of alternatively activated macrophages, promoting angiogenesis and tumorigenesis. Summarily, lactate plays a pro-oncogenic role in the TME. CAFs, cancer-associated fibroblasts; ERK, extracellular regulated protein kinases; FOXP3, forkhead box protein 3; GPR132, G-protein-coupled receptor 132; GPR81, Gi-protein-coupled receptor 81; HDAC, histone deacetylase; HIF-1α, hypoxia-inducible factor 1α; mTOR, mechanistic target of rapamycin; MCT, monocarboxylate transporter; NFAT, nuclear factor of activated T cells; PPARγ, peroxisome proliferator-activated receptor γ; STAT3, signal transducer and activator of transcription 3; SREBF1, sterol regulatory element-binding transcription factor 1; TAMs, tumour-associated macrophages; VEGF, vascular endothelial growth factor. Zi-Hao Wang and Qiong Zhou design this figure. All authors confirm originality of it and retain copyright to it.
2.4. Targeting lactate anabolism and transport therapies
2.4.1. Targeting lactate anabolism
Upregulation of LDHA serves as a robust indicator of poor prognosis in patients with diverse malignancies, whereas the loss of human LDHA protein induces only relatively mild exertional myopathy [29, 35]. Therefore, targeting LDHA is considered a safe therapeutic strategy. LDHA inhibitors that function via multiple mechanisms have been tested for efficacy in preclinical experiments (Table 1). Oxamate, which functions as a pyruvate‐competitive LDHA inhibitor, has been reported to inhibit gastric cancer cell proliferation [70,71]. NADH‐competitive LDHA inhibitors, including gossypol (also termed AT-101), 3-dihydroxy-6-methyl-7-(phenylmethyl)-4-propylnaphthalene-1-carboxylic acid (FX11), and quinoline 3‐sulfonamides, have also been shown to suppress cancer cell proliferation and cancer progression [72], [73], [74], [75], [76], [77], [78], [79]. N‐hydroxyindole (NHI), a pyruvate and NADH-competitive LDHA inhibitor, inhibits tumour proliferation [77,80]. Other LDHA inhibitors, including galloflavin and GNE‐140, have also been reported to inhibit tumour proliferation [81], [82], [83], [84], [85]. However, the clinical utility of LDHA inhibitors may be limited due to their non‐selective toxicity or complex interactions with other cellular components.
Table 1.
Lactate dehydrogenase (LDH) and monocarboxylate transporter (MCT) inhibitors.
| Inhibitors | Targets | Cancer types | References |
|---|---|---|---|
| Oxamate |
LDHA |
Gastric cancer cells; cervical cancer cells; leukemia cells; lung cancer cells | [70, 71, 114, 115] |
| Gossypol |
LDHA |
Melanoma cells; lung cancer cells; breast cancer cells; cervical cancer cells; leukemia cells; glioma cells; adrenal cancer cells | [[72], [73], [74], [75], [76], 116] |
| FX11 |
LDHA |
B‐lymphoma cells; pancreatic cancer cells; papillary thyroid carcinoma cells |
[77, 78, 117, 118] |
| Quinoline 3‐sulfonamides |
LDHA/LDHB |
Hepatocellular carcinoma cells |
[79] |
| NHI |
LDHA/LDHB |
Pancreatic ductal adenocarcinoma cells; cervical cancer cells; mesothelioma cells |
[77, 80, 119, 120] |
| Galloflavin |
LDHA |
Breast cancer cells; hepatocellular carcinoma cells |
[81], [82], [83] |
| GNE‐140 | LDHA | Pancreatic cancer cells | [84, 85] |
| 7ACC2 |
MCT1 |
Cervix cancer cells; pharynx squamous cell carcinoma cells; breast cancer cells; pancreatic adenocarcinoma cells |
[91, 92] |
| AR-C155858 |
MCT1/2 |
Breast cancer cells; cervix cancer cells; leukemic cells | [93, 94] |
| AZD3965 |
MCT1/2 |
Breast cancer cells; small cell lung cancer cells; colorectal cancer cells | [93, 95, 96] |
| BAY-8002 | MCT1/2 | Colorectal cancer cells | [97] |
| CHC |
MCT1/4 |
Cervix cancer cells; pharynx squamous cell carcinoma cells; breast cancer cells; colorectal cancer cells; prostate cancer cells; osteosarcoma cells; renal cell carcinoma cells | [[87], [88], [89], 121] |
| DIDS |
MCT1/4 |
Colorectal cancer cells; lung cancer cells | [87, 122] |
| Lonidamine | MCT1/4 | DB-1 melanoma cells | [123] |
| Phloretin |
MCT1/4 |
Breast cancer cells; lung cancer cells | [124] |
| pCMBS | MCT1/4 | Colorectal cancer cells | [125, 126] |
| Quercetin |
MCT1/4 |
Colorectal cancer cells; glioma cells; lung cancer cells |
[87, 122, 127] |
| Simvastatin |
MCT1/4 |
Lung cancer cells; breast cancer cells; prostate cancer cells; ovarian cancer cells; cervix cancer cells | [122, 128] |
Abbreviations: FX11, 3-dihydroxy-6-methyl-7-(phenylmethyl)-4-propylnaphthalene-1-carboxylic acid; NHI, N‐hydroxyindole; 7ACC2, 7-(N-benzyl-N-methylamino)-2-oxo-2H-chromene-3-carboxylic acid; CHC, α-cyano-4-hydroxycinnamate; DIDS, 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid disodium salt hydrate; pCMBS, p-chloromercuribenzene sulfonate.
2.4.2. Targeting lactate transport
Lactate metabolic symbiosis of cancer cells depends on MCT4-mediated lactate efflux and MCT1-mediated lactate influx [45]. Elevated levels of MCT1 and MCT4 have also been reported in multiple human malignancies [86]. Considering the central roles of MCT1 and MCT4 in lactate shuttling, targeting MCT-mediated lactate metabolic symbiosis may be beneficial for cancers that are resistant to conventional chemotherapy (Table 1). Mechanistically, normoxic cancer cells compete with hypoxic cancer cells for glucose under MCT1 inhibition, thereby resulting in the apoptosis of hypoxic cancer cells that mainly survive on glucose. Under MCT4 inhibition, hypoxic cancer cells cannot secrete excessive lactate over time, leading to intracellular acidosis and decreased survival.
To date, multiple MCT inhibitors have been shown to be effective in preclinical trials. For example, classic MCT1/MCT4 inhibitors, including α-cyano-4-hydroxycinnamate (CHC) and phloretin, have been reported to be effective in cervical cancer, pharynx squamous cell carcinoma, breast cancer, colorectal cancer, prostate cancer, osteosarcoma, and renal cell carcinoma cells [35,[87], [88], [89]]. Other MCT inhibitors, including p-chloromercuribenzenesulphonate, 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid, lonidamine, quercetin, and simvastatin, have demonstrated therapeutic potential for multiple cancers [90]. However, the earliest identified MCT inhibitors, such as CHC, phloretin, and quercetin, have been found to be non-selective against MCT1/MCT2/MCT4 isoforms, which limits their clinical application [90].
Newer MCT inhibitors have higher selectivity for individual MCT isoforms than first-generation MCT inhibitors. For example, 7-(N-benzyl-N-methylamino)-2-oxo-2H-chromene-3-carboxylic acid (7ACC2) is a potent inhibitor of MCT1 and mitochondrial pyruvate transport that has been reported to be effective in multiple human cancer types [91,92]. Other novel small-molecule inhibitors, including AR-C155858, AZD3965, and BAY-8002, have demonstrated potent MCT1-inhibitory and immunomodulatory activities [93], [94], [95], [96], [97]. Moreover, most of these MCT1 inhibitors are also active against MCT2 [90]. Cryo-electron microscopy has revealed that MCT1 has an ‘outward-open’ structure in the presence of BAY-8002 or AZD3965 and an ‘inward-open’ structure when complexed with 7ACC2 [98]. A phase I clinical trial of AZD3965 in patients with diffuse large B-cell lymphoma (DLBCL) and Burkitt lymphoma (BL) has been completed [99]. Preliminary results indicate that AZD3965 can be administrated to patients with solid tumours at a maximum tolerated daily oral dose of 20 mg [100]. For DLBCL and BL, AZD3965 can be safely administered to patients at 10 mg twice daily. Urinalyses showed that AZD3965 therapy increased the excretion of ketone bodies and lactate. In a patient who showed a confirmed complete response lasting 15 months, reduced 18F-fluorodeoxyglucose (FDG) uptake by the tumour was observed on [18F] FDG-PET on the third day of the first cycle. Further studies are urgently needed to determine the safe and biologically active doses of AZD3965.
In addition to directly targeting cancer cells, MCT inhibitors may also target immune cells in the TME. For example, tumour-infiltrating Treg cells and TAMs require MCT1-mediated lactate uptake to maintain their immunosuppressive and tumour-promoting functions [46,47]. Deletion of MCT1 in Treg cells prevents their accumulation in the hypoxic and lactate-rich TME, creating an environment conducive to antitumour immunity [46]. Theoretically, MCT1 inhibitors may exert their therapeutic effects by directly inhibiting tumour growth and repressing the functions of tumour-infiltrating Treg cells and TAMs. Conversely, MCT inhibition does not appear to compromise human CD4+ and CD8+ T-cell function [55]. Therefore, elucidating the roles of MCTs in energy metabolism and immunomodulation in the TME is crucial for improving cancer therapeutic strategies.
2.4.3. Targeting the mTOR pathway
Given the important roles of the mTOR pathway in lactate metabolism, combined therapy with mTOR inhibitors and lactate metabolism/transport inhibitors should be considered to improve antitumour immunity. Water-soluble rapamycin analogues (temsirolimus and everolimus), ATP-competitive mTOR inhibitors (MLN0128, PP242, AZD2014 and AZD8055), and dual PI3K/mTOR inhibitors (NVP-BEZ235, LY3023414, SAR245409, XL765, PQR309, XH00230381967, SN20229799306, GSK2126458, and PKI-587) have been used to treat multiple cancers [101]. Although the antitumour effect of mTOR inhibitor monotherapy alone was limited, the combination of mTOR inhibition and other treatments, such as anti-PD-1 antibody or metformin, had synergistic antitumour activity [102,103]. These synergistic effects of targeting mTOR pathway and inhibiting glycolysis have also been reported in several cancers, including lymphoma, leukaemia, and colorectal cancer [104,105]. However, further studies are needed to explore the therapeutic efficacy of combined therapy with lactate inhibitors and other metabolically targeted drugs, such as mTOR inhibitors.
3. Conclusions
The combination of glycolytic cancer cells and CAFs increases lactate levels in the TME. This elevated lactate can modulate immune responses to maintain immunosuppression in the TME via both H+-mediated and lactate-mediated pathways. Hence, therapeutic targeting to prevent this increase is regarded as a potent therapeutic strategy for metabolically reprogrammed cancers [6]. However, lactate-targeted monotherapy has limited therapeutic efficacy because of its off-target effects. Therefore, combining lactate targeting with other therapies, such as mTOR inhibitors, epidermal growth factor receptor (EGFR) inhibitors, anaplastic lymphoma kinase (ALK) inhibitors, anti-PD-1/PD-L1 therapy, anti-CTLA-4 therapy, anti-HER-2 therapy, and anti-VEGF therapy, may be an alternative therapeutic strategy. For example, preclinical trials have shown that combined therapy with the MCT1 inhibitor AZD3965 and anti-PD-1 therapy reduces the infiltration of exhaustive PD-1+ Tim-3+ T cells in solid tumours and improves antitumour immunity [106]. Another preclinical study has indicated that combining angiogenesis inhibitors (blocking the VEGF signalling pathway) with AZD3965 inhibits tumour growth and reduces both blood perfusion and hypoxia in tumour tissues [107]. Although studies on the combination of MCT1 inhibitors and tyrosine kinase inhibitors (TKIs) are limited, one preclinical trial has demonstrated that AZD3965 significantly inhibits cell proliferation and motility in TKI-sensitive and TKI-resistant non-small cell lung cancer cells, suggesting that combination therapies have great potential [108].
In addition to lactate targeting, targeting other metabolic pathways, including fatty acid, amino acid, one-carbon, and polyamine metabolism, are promising therapeutic regimens. For example, several fatty acid synthase (FASN) inhibitors, including cerulenin, C75, and epigallocatechin gallate, have been developed and have been reported to be effective in preclinical studies [109]. Additionally, polyamine metabolism therapy, such as the ornithine decarboxylase (ODC) inhibitor difluoromethylornithine, has been shown to have therapeutic efficacy in preclinical and phase I clinical trials [110], [111], [112]. Serine metabolism therapy, such as phosphoglycerate dehydrogenase (PHGDH) inhibitors, has also been reported to be effective in preclinical trials [113]. However, few studies have focused on how targeting metabolic pathways alters the metabolism of immune cells in the TME. Therefore, further studies are urgently needed to identify new targeted drugs that can modulate immune responses in the TME more efficiently and selectively.
3.1. Outstanding questions
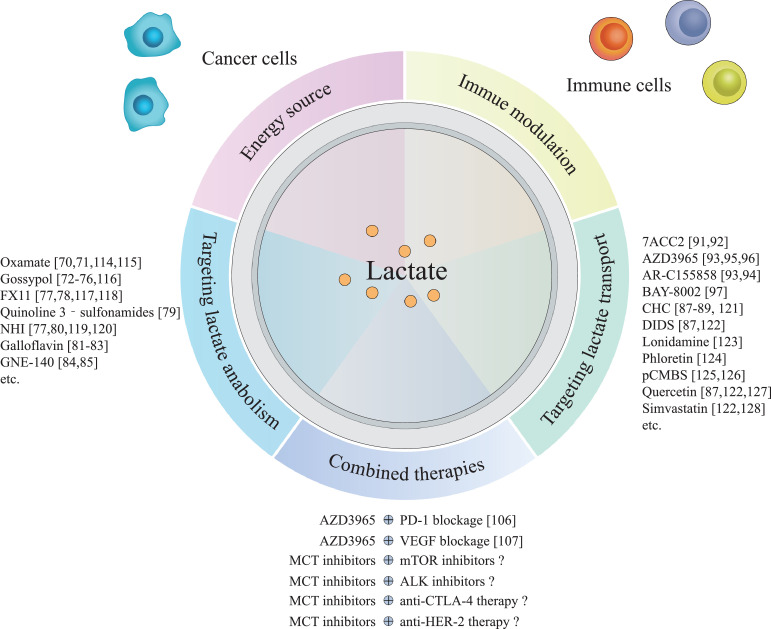
Accumulating data have revealed that lactate metabolism is an important aspect of aberrant cancer metabolism, with regions of heterogeneous lactate metabolism existing in individual tumours. Cancer cells in less-perfused regions prefer to utilise glucose and secrete excessive lactate, while those in highly perfused regions use lactate as their primary carbon source. In addition to serving as an energy source for tumours, lactate plays a critical role in immune regulation within the TME. Elevated lactate levels not only suppress the antitumour effects of immune cells such as CD4+ T cells, CD8+ T cells, NK cells, and NKT cells, but also favour immunosuppressive cells such as Treg cells. Numerous therapeutic strategies targeting lactate anabolism and transport have been developed to exploit the key role of lactate in cancer metabolism and immune modulation. Although small-molecule MCT inhibitors such as AZD3965 have demonstrated promising efficacy, these therapeutic strategies still face many challenges, and further studies are needed to improve them (Fig. 3).
Fig. 3.
Outstanding questions in lactate metabolism. This picture depicts the multi-faceted roles of lactate in the tumour microenvironment and provides a description of lactate-targeting therapies. This figure has not been published previously. Originality of it is confirmed by all authors.
Briefly, the challenges in lactate-targeting therapies include: (1) how to reduce or eliminate the off-target effects of lactate-targeting metabolism and transport inhibitors; (2) how lactate-targeting therapies regulate immunometabolism in the TME; (3) few clinical trials are designed to assess the therapeutic efficacy of combined therapy with lactate inhibitors and other targeted drugs such as mTOR inhibitors, FASN inhibitors, ODC inhibitors, PHGDH inhibitors, EGFR inhibitors, ALK inhibitors, anti-PD-1/PD-L1 therapy, anti-CTLA-4 therapy, anti-HER-2 therapy, and anti-VEGF therapy.
3.2. Search strategy and selection criteria
References for this review were identified by searching PubMed and Google Scholar databases for relevant articles preferentially published between 2012 and 2021 using the search terms ‘lactate’ or ‘lactic acid’ or ‘glycolytic enzymes’ or ‘lactate transporters’ and ‘cancer’ or ‘tumour immunity’ or ‘immune response’. Articles which explain well-known concepts were considered regardless of their date of publication.
Contributors
Q Zhou provided the concept. ZH Wang and P Zhang researched data and wrote the manuscript. Q Zhou, XP Yang and WB Peng reviewed and edited the manuscript.
Declaration of Competing Interest
All authors declare that they have no competing interests.
Acknowledgments
We would like to thank Editage for English language editing. National Natural Science Foundation of China under Grant No.81973990, No. 81900096, and No. 81770090 (to Qiong Zhou).
Contributor Information
Xiang-Ping Yang, Email: yangxp@hust.edu.cn.
Qiong Zhou, Email: zhouqiongtj@126.com.
References
- 1.Sahai E. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20(3):174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ngwa V.M. Microenvironmental metabolism regulates antitumor immunity. Cancer Res. 2019;79(16):4003–4008. doi: 10.1158/0008-5472.CAN-19-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks G.A. The science and translation of lactate shuttle theory. Cell Metab. 2018;27(4):757–785. doi: 10.1016/j.cmet.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Brown T.P., Ganapathy V. Lactate/GPR81 signaling and proton motive force in cancer: role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol Ther. 2020;206 doi: 10.1016/j.pharmthera.2019.107451. [DOI] [PubMed] [Google Scholar]
- 5.Masoud G.N., Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5(5):378–389. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Certo M. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat Rev Immunol. 2021;21(3):151–161. doi: 10.1038/s41577-020-0406-2. [DOI] [PubMed] [Google Scholar]
- 7.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 8.Ashton T.M. Oxidative phosphorylation as an emerging target in cancer therapy. Clin Cancer Res. 2018;24(11):2482–2490. doi: 10.1158/1078-0432.CCR-17-3070. [DOI] [PubMed] [Google Scholar]
- 9.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parks S.K., Pouysségur J. Targeting pH regulating proteins for cancer therapy-progress and limitations. Semin Cancer Biol. 2017;43:66–73. doi: 10.1016/j.semcancer.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Sun S. Lactic acid: no longer an inert and end-product of glycolysis. Physiology. 2017;32(6):453–463. doi: 10.1152/physiol.00016.2017. [DOI] [PubMed] [Google Scholar]
- 12.Semenza G.L. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 13.Wang G.L., Semenza G.L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A, 1993;90(9):4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi Y. Hypoxia/pseudohypoxia-mediated activation of hypoxia-inducible factor-1α in cancer. Cancer Sci. 2019;110(5):1510–1517. doi: 10.1111/cas.13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaelin W.G., Jr. Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2002;2(9):673–682. doi: 10.1038/nrc885. [DOI] [PubMed] [Google Scholar]
- 16.Ravi R. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14(1):34–44. [PMC free article] [PubMed] [Google Scholar]
- 17.Hellwig-Bürgel T. Interleukin-1beta and tumor necrosis factor-alpha stimulate DNA binding of hypoxia-inducible factor-1. Blood. 1999;94(5):1561–1567. [PubMed] [Google Scholar]
- 18.Westra J. Regulation of cytokine-induced HIF-1alpha expression in rheumatoid synovial fibroblasts. Ann N Y Acad Sci. 2007;1108:340–348. doi: 10.1196/annals.1422.035. [DOI] [PubMed] [Google Scholar]
- 19.Feldser D. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999;59(16):3915–3918. [PubMed] [Google Scholar]
- 20.Richard D.E., Berra E., Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1alpha in vascular smooth muscle cells. J Biol Chem. 2000;275(35):26765–26771. doi: 10.1074/jbc.M003325200. [DOI] [PubMed] [Google Scholar]
- 21.Pagé E.L. Induction of hypoxia-inducible factor-1alpha by transcriptional and translational mechanisms. J Biol Chem. 2002;277(50):48403–48409. doi: 10.1074/jbc.M209114200. [DOI] [PubMed] [Google Scholar]
- 22.Sandau K.B., Fandrey J., Brüne B. Accumulation of HIF-1alpha under the influence of nitric oxide. Blood. 2001;97(4):1009–1015. doi: 10.1182/blood.v97.4.1009. [DOI] [PubMed] [Google Scholar]
- 23.Takeda N. Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010;24(5):491–501. doi: 10.1101/gad.1881410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakisaka N. Epstein-Barr virus latent membrane protein 1 induces synthesis of hypoxia-inducible factor 1 alpha. Mol Cell Biol. 2004;24(12):5223–5234. doi: 10.1128/MCB.24.12.5223-5234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moon E.J. Hepatitis B virus X protein induces angiogenesis by stabilizing hypoxia-inducible factor-1alpha. Faseb j. 2004;18(2):382–384. doi: 10.1096/fj.03-0153fje. [DOI] [PubMed] [Google Scholar]
- 26.Gordan J.D.C.B., Thompson, M.C. Simon HIF, c-Myc sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 2007;12(2):108–113. doi: 10.1016/j.ccr.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clem B. Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther. 2008;7(1):110–120. doi: 10.1158/1535-7163.MCT-07-0482. [DOI] [PubMed] [Google Scholar]
- 28.Dang C.V. Therapeutic targeting of cancer cell metabolism. J Mol Med. 2011;89(3):205–212. doi: 10.1007/s00109-011-0730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng Y. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 2018;7(12):6124–6136. doi: 10.1002/cam4.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mossmann D., Park S., Hall M.N. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Cancer. 2018;18(12):744–757. doi: 10.1038/s41568-018-0074-8. [DOI] [PubMed] [Google Scholar]
- 31.Sun Q. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci U S A, 2011;108(10):4129–4134. doi: 10.1073/pnas.1014769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeBerardinis R.J. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A, 2007;104(49):19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhutia Y.D. Amino Acid transporters in cancer and their relevance to "glutamine addiction": novel targets for the design of a new class of anticancer drugs. Cancer Res. 2015;75(9):1782–1788. doi: 10.1158/0008-5472.CAN-14-3745. [DOI] [PubMed] [Google Scholar]
- 34.Wise D.R. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A, 2008;105(48):18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doherty J.R., Cleveland J.L. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123(9):3685–3692. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gastin P.B. Energy system interaction and relative contribution during maximal exercise. Sports Med. 2001;31(10):725–741. doi: 10.2165/00007256-200131100-00003. [DOI] [PubMed] [Google Scholar]
- 37.Magistretti P.J. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209(Pt 12):2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- 38.Haas R. Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PLoS Biol. 2015;13(7) doi: 10.1371/journal.pbio.1002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pucino V. Lactate buildup at the site of chronic inflammation promotes disease by inducing CD4(+) T cell metabolic rewiring. Cell Metab. 2019;30(6) doi: 10.1016/j.cmet.2019.10.004. 1055-1074.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Z. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J Exp Med. 2013;210(10):2119–2134. doi: 10.1084/jem.20130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hensley C.T. Metabolic heterogeneity in human lung tumors. Cell, 2016;164(4):681–694. doi: 10.1016/j.cell.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faubert B. Lactate metabolism in human lung tumors. Cell. 2017;171(2) doi: 10.1016/j.cell.2017.09.019. 358-371.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hui S. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551(7678):115–118. doi: 10.1038/nature24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilde L. Metabolic coupling and the Reverse Warburg Effect in cancer: Implications for novel biomarker and anticancer agent development. Semin Oncol. 2017;44(3):198–203. doi: 10.1053/j.seminoncol.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Payen V.L. Monocarboxylate transporters in cancer. Mol Metab. 2020;33:48–66. doi: 10.1016/j.molmet.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watson M.J. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature. 2021;591(7851):645–651. doi: 10.1038/s41586-020-03045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kes M.M.G. Oncometabolites lactate and succinate drive pro-angiogenic macrophage response in tumors. Biochim Biophys Acta Rev Cancer. 2020;1874(2) doi: 10.1016/j.bbcan.2020.188427. [DOI] [PubMed] [Google Scholar]
- 48.Martinez-Outschoorn U.E., Lisanti M.P., Sotgia F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin Cancer Biol. 2014;25:47–60. doi: 10.1016/j.semcancer.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Ferguson B.S. Lactate metabolism: historical context, prior misinterpretations, and current understanding. Eur J Appl Physiol. 2018;118(4):691–728. doi: 10.1007/s00421-017-3795-6. [DOI] [PubMed] [Google Scholar]
- 50.Ippolito L. Lactate: A metabolic driver in the tumour landscape. Trends Biochem Sci. 2019;44(2):153–166. doi: 10.1016/j.tibs.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 51.Xia H. Suppression of FIP200 and autophagy by tumor-derived lactate promotes naïve T cell apoptosis and affects tumor immunity. Sci Immunol. 2017;2(17) doi: 10.1126/sciimmunol.aan4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mendler A.N. Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. Int J Cancer. 2012;131(3):633–640. doi: 10.1002/ijc.26410. [DOI] [PubMed] [Google Scholar]
- 53.Walton Z.E. Acid Suspends the Circadian Clock in Hypoxia through Inhibition of mTOR. Cell. 2018;174(1) doi: 10.1016/j.cell.2018.05.009. 72-87.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Comito G. Lactate modulates CD4(+) T-cell polarization and induces an immunosuppressive environment, which sustains prostate carcinoma progression via TLR8/miR21 axis. Oncogene. 2019;38(19):3681–3695. doi: 10.1038/s41388-019-0688-7. [DOI] [PubMed] [Google Scholar]
- 55.Renner K. Restricting glycolysis preserves T cell effector functions and augments checkpoint therapy. Cell Rep. 2019;29(1) doi: 10.1016/j.celrep.2019.08.068. 135-150.e9. [DOI] [PubMed] [Google Scholar]
- 56.Frauwirth K.A. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16(6):769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 57.Husain Z., Seth P., Sukhatme V.P. Tumor-derived lactate and myeloid-derived suppressor cells: linking metabolism to cancer immunology. Oncoimmunology. 2013;2(11):e26383. doi: 10.4161/onci.26383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brand A. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016;24(5):657–671. doi: 10.1016/j.cmet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 59.Fu S. Impaired lipid biosynthesis hinders anti-tumor efficacy of intratumoral iNKT cells. Nat Commun. 2020;11(1):438. doi: 10.1038/s41467-020-14332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xie D., Zhu S., Bai L. Lactic acid in tumor microenvironments causes dysfunction of NKT cells by interfering with mTOR signaling. Sci China Life Sci. 2016;59(12):1290–1296. doi: 10.1007/s11427-016-0348-7. [DOI] [PubMed] [Google Scholar]
- 61.Erra Díaz F. Extracellular acidosis and mTOR inhibition drive the differentiation of human monocyte-derived dendritic cells. Cell Rep. 2020;31(5) doi: 10.1016/j.celrep.2020.107613. [DOI] [PubMed] [Google Scholar]
- 62.Nasi A. Dendritic cell reprogramming by endogenously produced lactic acid. J Immunol. 2013;191(6):3090–3099. doi: 10.4049/jimmunol.1300772. [DOI] [PubMed] [Google Scholar]
- 63.Paolini L. Lactic acidosis together with GM-CSF and M-CSF induces human macrophages toward an inflammatory protumor phenotype. Cancer Immunol Res, 2020;8(3):383–395. doi: 10.1158/2326-6066.CIR-18-0749. [DOI] [PubMed] [Google Scholar]
- 64.Raychaudhuri D. Lactate induces pro-tumor reprogramming in intratumoral plasmacytoid dendritic cells. Front Immunol. 2019;10:1878. doi: 10.3389/fimmu.2019.01878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu N. Lactate inhibits ATP6V0d2 expression in tumor-associated macrophages to promote HIF-2α-mediated tumor progression. J Clin Invest. 2019;129(2):631–646. doi: 10.1172/JCI123027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen P. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci U S A, 2017;114(3):580–585. doi: 10.1073/pnas.1614035114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Latham T. Lactate, a product of glycolytic metabolism, inhibits histone deacetylase activity and promotes changes in gene expression. Nucleic Acids Res. 2012;40(11):4794–4803. doi: 10.1093/nar/gks066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang D. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574(7779):575–580. doi: 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Angelin A. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 2017;25(6) doi: 10.1016/j.cmet.2016.12.018. 1282-1293.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao Z. Oxamate-mediated inhibition of lactate dehydrogenase induces protective autophagy in gastric cancer cells: involvement of the Akt-mTOR signaling pathway. Cancer Lett. 2015;358(1):17–26. doi: 10.1016/j.canlet.2014.11.046. [DOI] [PubMed] [Google Scholar]
- 71.Papaconstantinou J., Colowick S.P. The role of glycolysis in the growth of tumor cells. II. The effect of oxamic acid on the growth of HeLa cells in tissue culture. J Biol Chem. 1961;236:285–288. [PubMed] [Google Scholar]
- 72.Rani R., Kumar V. Recent update on human lactate dehydrogenase enzyme 5 (hLDH5) inhibitors: a promising approach for cancer chemotherapy. J Med Chem. 2016;59(2):487–496. doi: 10.1021/acs.jmedchem.5b00168. [DOI] [PubMed] [Google Scholar]
- 73.Shelley M.D. Stereo-specific cytotoxic effects of gossypol enantiomers and gossypolone in tumour cell lines. Cancer Lett. 1999;135(2):171–180. doi: 10.1016/s0304-3835(98)00302-4. [DOI] [PubMed] [Google Scholar]
- 74.Flack M.R. Oral gossypol in the treatment of metastatic adrenal cancer. J Clin Endocrinol Metab. 1993;76(4):1019–1024. doi: 10.1210/jcem.76.4.8473376. [DOI] [PubMed] [Google Scholar]
- 75.Bushunow P. Gossypol treatment of recurrent adult malignant gliomas. J Neurooncol. 1999;43(1):79–86. doi: 10.1023/a:1006267902186. [DOI] [PubMed] [Google Scholar]
- 76.Van Poznak C. Oral gossypol in the treatment of patients with refractory metastatic breast cancer: a phase I/II clinical trial. Breast Cancer Res Treat. 2001;66(3):239–248. doi: 10.1023/a:1010686204736. [DOI] [PubMed] [Google Scholar]
- 77.Granchi C. Discovery of N-hydroxyindole-based inhibitors of human lactate dehydrogenase isoform A (LDH-A) as starvation agents against cancer cells. J Med Chem. 2011;54(6):1599–1612. doi: 10.1021/jm101007q. [DOI] [PubMed] [Google Scholar]
- 78.Le A. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A, 2010;107(5):2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Billiard J. Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells. Cancer Metab. 2013;1(1):19. doi: 10.1186/2049-3002-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maftouh M. Synergistic interaction of novel lactate dehydrogenase inhibitors with gemcitabine against pancreatic cancer cells in hypoxia. Br J Cancer. 2014;110(1):172–182. doi: 10.1038/bjc.2013.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Manerba M. Galloflavin (CAS 568-80-9): a novel inhibitor of lactate dehydrogenase. ChemMedChem. 2012;7(2):311–317. doi: 10.1002/cmdc.201100471. [DOI] [PubMed] [Google Scholar]
- 82.Farabegoli F. Galloflavin, a new lactate dehydrogenase inhibitor, induces the death of human breast cancer cells with different glycolytic attitude by affecting distinct signaling pathways. Eur J Pharm Sci. 2012;47(4):729–738. doi: 10.1016/j.ejps.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 83.Fiume L. Galloflavin prevents the binding of lactate dehydrogenase A to single stranded DNA and inhibits RNA synthesis in cultured cells. Biochem Biophys Res Commun. 2013;430(2):466–469. doi: 10.1016/j.bbrc.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 84.Ždralević M. Double genetic disruption of lactate dehydrogenases A and B is required to ablate the "Warburg effect" restricting tumor growth to oxidative metabolism. J Biol Chem. 2018;293(41):15947–15961. doi: 10.1074/jbc.RA118.004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boudreau A. Metabolic plasticity underpins innate and acquired resistance to LDHA inhibition. Nat Chem Biol. 2016;12(10):779–786. doi: 10.1038/nchembio.2143. [DOI] [PubMed] [Google Scholar]
- 86.Kobayashi M. Transport function, regulation, and biology of human monocarboxylate transporter 1 (hMCT1) and 4 (hMCT4) Pharmacol Ther. 2021;226 doi: 10.1016/j.pharmthera.2021.107862. [DOI] [PubMed] [Google Scholar]
- 87.Amorim R. Monocarboxylate transport inhibition potentiates the cytotoxic effect of 5-fluorouracil in colorectal cancer cells. Cancer Lett. 2015;365(1):68–78. doi: 10.1016/j.canlet.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 88.Zhao Z. Downregulation of MCT1 inhibits tumor growth, metastasis and enhances chemotherapeutic efficacy in osteosarcoma through regulation of the NF-κB pathway. Cancer Lett. 2014;342(1):150–158. doi: 10.1016/j.canlet.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 89.Miranda-Gonçalves V. Lactate increases renal cell carcinoma aggressiveness through sirtuin 1-dependent epithelial mesenchymal transition axis regulation. Cells. 2020;9(4) doi: 10.3390/cells9041053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pérez-Escuredo J. Monocarboxylate transporters in the brain and in cancer. Biochim Biophys Acta. 2016;1863(10):2481–2497. doi: 10.1016/j.bbamcr.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Draoui N. Antitumor activity of 7-aminocarboxycoumarin derivatives, a new class of potent inhibitors of lactate influx but not efflux. Mol Cancer Ther. 2014;13(6):1410–1418. doi: 10.1158/1535-7163.MCT-13-0653. [DOI] [PubMed] [Google Scholar]
- 92.Corbet C. Interruption of lactate uptake by inhibiting mitochondrial pyruvate transport unravels direct antitumor and radiosensitizing effects. Nat Commun. 2018;9(1):1208. doi: 10.1038/s41467-018-03525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guan X., Rodriguez-Cruz V., Morris M.E. Cellular uptake of MCT1 inhibitors AR-C155858 and AZD3965 and their effects on MCT-mediated transport of L-lactate in murine 4T1 breast tumor cancer cells. Aaps j. 2019;21(2):13. doi: 10.1208/s12248-018-0279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saulle E. Targeting lactate metabolism by inhibiting MCT1 or MCT4 impairs leukemic cell proliferation, induces two different related death-pathways and increases chemotherapeutic sensitivity of acute myeloid leukemia cells. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.621458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Polański R. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin Cancer Res. 2014;20(4):926–937. doi: 10.1158/1078-0432.CCR-13-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beloueche-Babari M. MCT1 inhibitor AZD3965 increases mitochondrial metabolism, facilitating combination therapy and noninvasive magnetic resonance spectroscopy. Cancer Res. 2017;77(21):5913–5924. doi: 10.1158/0008-5472.CAN-16-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Quanz M. Preclinical efficacy of the novel monocarboxylate transporter 1 inhibitor BAY-8002 and associated markers of resistance. Mol Cancer Ther. 2018;17(11):2285–2296. doi: 10.1158/1535-7163.MCT-17-1253. [DOI] [PubMed] [Google Scholar]
- 98.Wang N. Structural basis of human monocarboxylate transporter 1 inhibition by anti-cancer drug candidates. Cell. 2021;184(2) doi: 10.1016/j.cell.2020.11.043. 370-383.e13. [DOI] [PubMed] [Google Scholar]
- 99.Halford S.E.R. Phase I expansion study of the first-in-class monocarboxylate transporter 1 (MCT1) inhibitor AZD3965 in patients with diffuse large B-cell lymphoma (DLBCL) and Burkitt lymphoma (BL) Journal of Clinical Oncology. 2021;39(15_suppl) 3115-3115. [Google Scholar]
- 100.Halford S.E.R. A first-in-human first-in-class (FIC) trial of the monocarboxylate transporter 1 (MCT1) inhibitor AZD3965 in patients with advanced solid tumours. J Clin Oncol. 2017;35(15_suppl) 2516-2516. [Google Scholar]
- 101.Hua H. Targeting mTOR for cancer therapy. J Hematol Oncol. 2019;12(1):71. doi: 10.1186/s13045-019-0754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li H. Programmed cell death-1 (PD-1) checkpoint blockade in combination with a mammalian target of rapamycin inhibitor restrains hepatocellular carcinoma growth induced by hepatoma cell-intrinsic PD-1. Hepatology. 2017;66(6):1920–1933. doi: 10.1002/hep.29360. [DOI] [PubMed] [Google Scholar]
- 103.Vitali E. Metformin and everolimus: a promising combination for neuroendocrine tumors treatment. Cancers (Basel) 2020;12(8) doi: 10.3390/cancers12082143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu R.H. Synergistic effect of targeting mTOR by rapamycin and depleting ATP by inhibition of glycolysis in lymphoma and leukemia cells. Leukemia. 2005;19(12):2153–2158. doi: 10.1038/sj.leu.2403968. [DOI] [PubMed] [Google Scholar]
- 105.Xing B.C. Synergistically suppressive effects on colorectal cancer cells by combination of mTOR inhibitor and glycolysis inhibitor, Oxamate. Int J Clin Exp Pathol. 2018;11(9):4439–4445. [PMC free article] [PubMed] [Google Scholar]
- 106.Huang T. Tumor-targeted inhibition of monocarboxylate transporter 1 improves T-cell immunotherapy of solid tumors. Adv Healthc Mater. 2021;10(4) doi: 10.1002/adhm.202000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ademi H. Targeting neovascularization and respiration of tumor grafts grown on chick embryo chorioallantoic membranes. PLoS One. 2021;16(5) doi: 10.1371/journal.pone.0251765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang C.Y. Inhibition of alternative cancer cell metabolism of EGFR mutated non-small cell lung cancer serves as a potential therapeutic strategy. Cancers. 2020;12(1) doi: 10.3390/cancers12010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Röhrig F., Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. 2016;16(11):732–749. doi: 10.1038/nrc.2016.89. [DOI] [PubMed] [Google Scholar]
- 110.Casero R.A., Jr., Murray Stewart T., Pegg A.E. Polyamine metabolism and cancer: treatments, challenges and opportunities. Nat Rev Cancer. 2018;18(11):681–695. doi: 10.1038/s41568-018-0050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bassiri H. Translational development of difluoromethylornithine (DFMO) for the treatment of neuroblastoma. Transl Pediatr. 2015;4(3):226–238. doi: 10.3978/j.issn.2224-4336.2015.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Saulnier Sholler G.L. A phase I trial of DFMO targeting polyamine addiction in patients with relapsed/refractory neuroblastoma. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0127246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang M., Vousden K.H. Serine and one-carbon metabolism in cancer. Nat Rev Cancer. 2016;16(10):650–662. doi: 10.1038/nrc.2016.81. [DOI] [PubMed] [Google Scholar]
- 114.Yu H. Targeting lactate dehydrogenase A (LDHA) exerts antileukemic effects on T-cell acute lymphoblastic leukemia. Cancer Commun. 2020;40(10):501–517. doi: 10.1002/cac2.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qiao T. Inhibition of LDH-A by oxamate enhances the efficacy of Anti-PD-1 treatment in an NSCLC humanized mouse model. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.632364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Flak D.K. AT101-loaded cubosomes as an alternative for improved glioblastoma therapy. Int J Nanomedicine. 2020;15:7415–7431. doi: 10.2147/IJN.S265061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mohammad G.H. Targeting pyruvate kinase M2 and lactate dehydrogenase A is an effective combination strategy for the treatment of pancreatic cancer. Cancers (Basel) 2019;11(9) doi: 10.3390/cancers11091372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hou X. LDHA induces EMT gene transcription and regulates autophagy to promote the metastasis and tumorigenesis of papillary thyroid carcinoma. Cell Death Dis. 2021;12(4):347. doi: 10.1038/s41419-021-03641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ruhnau J. Synergisms of genome and metabolism stabilizing antitumor therapy (GMSAT) in human breast and colon cancer cell lines: a novel approach to screen for synergism. BMC Cancer. 2020;20(1):617. doi: 10.1186/s12885-020-07062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li Petri G. Impact of hypoxia on chemoresistance of mesothelioma mediated by the proton-coupled folate transporter, and preclinical activity of new anti-LDH-A compounds. Br J Cancer. 2020;123(4):644–656. doi: 10.1038/s41416-020-0912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sonveaux P. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118(12):3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Izumi H. Monocarboxylate transporters 1 and 4 are involved in the invasion activity of human lung cancer cells. Cancer Sci. 2011;102(5):1007–1013. doi: 10.1111/j.1349-7006.2011.01908.x. [DOI] [PubMed] [Google Scholar]
- 123.Nancolas B. The anti-tumour agent lonidamine is a potent inhibitor of the mitochondrial pyruvate carrier and plasma membrane monocarboxylate transporters. Biochem J. 2016;473(7):929–936. doi: 10.1042/BJ20151120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Singh S.V. Metformin induced lactic acidosis impaired response of cancer cells towards paclitaxel and doxorubicin: role of monocarboxylate transporter. Biochim Biophys Acta Mol Basis Dis. 2021;1867(3) doi: 10.1016/j.bbadis.2020.166011. [DOI] [PubMed] [Google Scholar]
- 125.Wenzel U. Activation of mitochondrial lactate uptake by flavone induces apoptosis in human colon cancer cells. J Cell Physiol. 2005;202(2):379–390. doi: 10.1002/jcp.20129. [DOI] [PubMed] [Google Scholar]
- 126.Köpnick A.L., Geistlinger K., Beitz E. Cysteine 159 delineates a hinge region of the alternating access monocarboxylate transporter 1 and is targeted by cysteine-modifying inhibitors. Febs J. 2021 doi: 10.1111/febs.16024. [DOI] [PubMed] [Google Scholar]
- 127.Albatany M., Meakin S., Bartha R. The Monocarboxylate transporter inhibitor Quercetin induces intracellular acidification in a mouse model of Glioblastoma Multiforme: in-vivo detection using magnetic resonance imaging. Invest New Drugs. 2019;37(4):595–601. doi: 10.1007/s10637-018-0644-3. [DOI] [PubMed] [Google Scholar]
- 128.Lee Z.W. Intracellular hyper-acidification potentiated by hydrogen sulfide mediates invasive and therapy resistant cancer cell death. Front Pharmacol. 2017;8:763. doi: 10.3389/fphar.2017.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]