Abstract
Cancer-induced muscle wasting, i.e. cachexia, is associated with different types of cancer such as pancreatic, colorectal, lung, liver, gastric and esophageal. Cachexia affects prognosis and survival in cancer, and it is estimated that it will be the ultimate cause of death for up to 30% of cancer patients. Musculoskeletal alterations are known hallmarks of cancer cachexia, with skeletal muscle atrophy and weakness as the most studied. Recent evidence has shed light on the presence of bone loss in cachectic patients, even in the absence of bone-metastatic disease. In particular, we and others have shown that muscle and bone communicate by exchanging paracrine and endocrine factors, known as myokines and osteokines. This review will focus on describing the role of the most studied myokines, such as myostatin, irisin, the muscle metabolite β-aminoisobutyric acid, BAIBA, and IL-6, and osteokines, including TGF-β, osteocalcin, sclerostin, RANKL, PTHrP, FGF23, and the lipid mediator, PGE2 during cancer-induced cachexia. The interplay of muscle and bone factors, together with tumor-derived soluble factors, characterizes a complex clinical scenario in which musculoskeletal alterations are amongst the most debilitating features. Understanding and targeting the “secretome” of cachectic patients will likely represent a promising strategy to preserve bone and muscle during cancer cachexia thereby enhancing recovery.
Keywords: Muscle, bone, cancer, cachexia, myokines, osteokines
Impact statement
The concept that muscle and bone communicate not only mechanically, but also by exchanging soluble biochemical factors has triggered new investigations in musculoskeletal research. The idea that not just tumor-derived factors, but also the interaction of secreted myokines and osteokines can induce musculoskeletal loss paves the way to the development of new therapeutics. This review summarizes the most recent progress in the study of bone and muscle-derived factors in a setting of cancer cachexia. We highlight that soluble factors initially thought to derive exclusively from bones and muscles can instead be released also by tumors, suggesting a new concept of the trifecta of cancer-bone-muscle crosstalk. Lastly, we suggest the idea that future personalized therapeutic interventions should target not just the cancer soluble factors, but also bone and muscle to mitigate the negative effects of this triple “secretome” in order to improve outcomes and enhance retention and recovery of musculoskeletal function.
Cancer cachexia
Cachexia is defined as “ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment”. 1 This condition is a comorbidity of cancer and other chronic diseases such as heart failure, chronic kidney disease, as well as chronic obstructive pulmonary disease, and is known to impair physical function, alter quality of life, reduce tolerance to anticancer therapy, and shorten patients’ survival. 2 It is estimated that the prevalence of cachexia in the cancer population varies from 50% up to 80% depending on stage of the disease and tumor type. As such, cachexia is particularly common in pancreatic, esophageal, gastric, colorectal, lung, and liver cancer patients. 3 Moreover, it is estimated that cachexia will be the ultimate cause of death for up to 30% of all cancer patients. 4
The etiology of cachexia is influenced by a complex interplay between the tumor burden and the consequent immune/inflammatory response of the host body. As such, several tumor-derived and host-derived humoral factors participate in the organ damage characterizing this syndrome. 5 New evidence from ours and other groups clearly shows the detrimental role of anticancer treatments (e.g. chemotherapy) in the pathogenesis of cachexia.6,7 The progression of cachexia is a continuum composed of three stages of clinical relevance: pre-cachexia, cachexia, and refractory cachexia. Not all patients experience all phases and the progression depends on different individual factors. 1 This classification is critically important for the clinical management of cancer patients. Based on this, the identification of biomarkers of pre-cachexia is crucial to design clinical interventions targeting muscle and bone that, when administered in its earliest stages, may be able to correct this condition.
Features of cachexia
Some of the principal systemic features of cachexia are reduced food intake, altered energy balance, metabolic abnormalities, and imbalance between anabolism and catabolism. These systemic derangements affect several tissues and organs, thus contributing to defining cachexia as a multi-organ syndrome. Among the most important drivers of cachexia are the systemic inflammatory response, the presence of high levels of tumor-derived factors, and the exacerbated protein catabolism that characterizes tumor metabolism per se. 4
Systemic inflammation appears to be a hallmark of the majority of cancer patients, and proinflammatory cytokines, including IL-6, TNF-α, IFN-γ, and IL-1β are found chronically elevated in blood, peripheral tissues, and the central nervous system of subjects with cancer cachexia. 8 Pro-inflammatory cytokines can be produced by both the immune system and by tumor cells. Interestingly, the presence of tumor cells stimulates the body’s innate immune system to produce an acute phase response, characterized by the production of acute phase proteins such as C-reactive protein, which in turn can negatively impact muscle mass, thus playing a critical role in the pathogenesis of cachexia. 9 Cytokines and chemokines are known to act in both paracrine and endocrine manners and together can generate a negative energy balance, altered metabolism, and a dramatic imbalance between anabolism and catabolism. 10 Moreover, it has been shown that inflammatory mediators can affect the central nervous system, thus promoting the occurrence of anorexia, 11 which, along with altered food taste, early satiety, pain and nausea, contributes to the reduction of food intake and to overall malnutrition, 4 thereby leading to the progressive body wasting that characterizes cachectic cancer patients.
Amongst the most relevant features of cancer cachexia are severe skeletal muscle loss and weakness. Skeletal muscle atrophy is due to an imbalance between protein synthesis and degradation rates that together determine a negative nitrogen balance. 12 During cancer cachexia, the increased skeletal muscle protein breakdown is primarily due to the hyperactivation of different proteolytic systems such as the ubiquitin-proteasome, Ca2+-dependent calpains, caspases, and autophagy-lysosome systems. 13 However, whether alterations of protein synthesis rates also play a role in cancer-induced muscle wasting remains unknown. In this regard, we recently showed that impaired ribosomal production can determine anabolic deficit in cancer cachexia, although the causes of such impairment remain partially unclear. 14 In contrast, a reduced muscle regenerative process has also been found to contribute to muscle atrophy in a cancer setting. 13
Fatigue and weakness (i.e. reduction of muscle strength and endurance) often accompany skeletal muscle atrophy and are both complications that severely compromise quality of life. The drivers of these functional impairments are not clearly defined. Interestingly, in combination with tumor burden, anticancer cytotoxic agents appear to play a detrimental role in muscle mass and function, and it has been shown that chemotherapy muscle toxicity can persist for long periods of time, even after tumor remission. In this regard, proper assessment of body composition (i.e. the proportion between lean and fat mass in a body) is becoming crucially important in choice of cancer treatment, especially considering that lower lean mass in cancer patients frequently associates with discontinuation, dose limitation of the therapy, and poor survival. 15
While the alterations of skeletal muscle consequent to the onset of cancer are extensively studied, on the contrary, the bone loss occurring during cancer cachexia is poorly understood. Bone is the preferential site of metastasis for several types of malignancies, including breast and prostate cancer, and is a fairly common event in melanoma, lung, colorectal, and thyroid cancers. Cancer metastases can dramatically affect quality of life and ultimately reduce survival in cancer patients since once cancer has entered bone it is more difficult to treat. 16 Cancer metastases to bone, as well as anticancer agents are known to increase osteoclast number and activity, thus leading to reduced bone mass by enhanced bone resorption and formation of osteolytic lesions. 16 These events increase the risk of fracture, pain, and hypercalcemia. 16
Interestingly, bone loss can occur also in the absence of bone metastases. Indeed, the incidence of vertebral fracture was found five times higher in women with breast cancer without bone metastases. 17 Similarly, cervical cancer patients were found to present lower bone mineral density in the absence of bone metastases, 18 whereas 40% of patients affected by non-small lung cancer without tumor dissemination to bone were shown to present with osteoporosis and osteopenia. 19 Several preclinical models for the study of cancer support the idea that bone alterations can occur in the absence of bone metastases. We showed that mice bearing the ES-2 ovarian cancer or the MC-38 colorectal cancer present with severe bone loss along with the development of skeletal muscle atrophy and weakness.20,21 In addition, we also previously showed that routinely administered chemotherapy regimens can affect bone tissue and, for this reason, may play a critical role in driving the harmful musculoskeletal alterations that affect cancer patients.22,23
Bone and muscle: A mutual interaction
Bone and muscle both derive from the paraxial mesoderm, 24 share common mesenchymal precursors, and develop synchronously during embryonic development. 25 Together, they are the most abundant tissues in the whole body. With the contribution of tendons, ligaments, joints, vascular and nervous systems, they constitute the support and the locomotion apparatus for the organism. While muscle contraction loads the bone, hence improving bone strain leading to maintenance or new bone formation, in contrast, immobility, and muscle atrophy induces bone loss due to unloading of bone. 26 It is well known that exercise is essential to maintain a healthy musculoskeletal system. However, different types of exercise have distinctive effects on bone and muscle. For example, endurance exercise, such as running, stimulates oxidative fibers can be associated with lower bone mineral density, BMD, compared with the resistance exercise, such as lifting weights, that increases muscle mass and is associated with higher BMD. 27 Dogma was that the mechanical interaction was the only interaction between the two tissues. More recently, several studies have suggested that muscle and bone can interact also in a biochemical and endocrine manner, in both physiological and pathological conditions. 28 Based on this idea, bone and skeletal muscle can function as secretory organs that can release paracrine and endocrine factors named “osteokines” and “myokines”, respectively. This new concept of biochemical signaling is becoming an important area of study and the balance between myokines and osteokines likely plays a critical role in the maintenance of a healthy musculoskeletal system.
One example of the importance of bone and muscle interaction is in fracture healing. Using a murine open tibia fracture model in which skeletal muscle tissue was simultaneously damaged, faster bone recovery and a superior quality of the repair were observed. 29 The same observation can be appreciated also in human open tibia fractures. 26 A possible explanation of this event is that the muscle flaps adjacent to the bone fracture produce factors that improve bone repair. 26 Moreover, skeletal muscle stem cells have also been shown to act like osteoprogenitors, thus improving bone healing. 30 Overall, these findings show that access of skeletal muscle, i.e. “muscle flaps”, to the fracture location can improve the fracture outcome. 31 Another example of bone-muscle communication was shown by using a murine model of osteogenesis imperfecta, a genetic connective tissue disorder characterized by “brittle bone” more susceptible to fractures. Surprisingly these mice also develop skeletal muscle weakness in spite of the absence of any muscle pathology. The investigators suggest that abnormal bone can release factors that compromise skeletal muscle function.26,32
The role of myokines in muscle-bone crosstalk
Myostatin
Myostatin (GDF-8) is a well-known myokine and member of the transforming growth factor (TGF) β superfamily. It was first described in double-muscled cattle, characterized by dramatically increased muscle mass due to a mutation in the myostatin gene. 33 Myostatin is a negative regulator of skeletal muscle mass; high levels induce muscle atrophy, whereas lower or lack of expression induces muscle hypertrophy in not only animals, but also in humans. Myostatin binds the activin receptor type-2 resulting in the phosphorylation of Smad2 and Smad3 leading to downstream signaling to inhibit the Akt-TORC1 anabolic pathway to ultimately impact muscle differentiation. 34 The skeletal muscle atrophy and adipose tissue wasting induced by overexpression of myostatin were the first indication of a potential role in the pathogenesis of cancer cachexia. 35 Several preclinical models of cancer cachexia, such as the Yoshida AH‐130 hepatoma in rats and the C26 adenocarcinoma in mice, were shown to have increased myostatin levels in skeletal muscle, consistent with severe skeletal muscle wasting.36,37 High levels of myostatin were also found to be elevated in the skeletal muscle of cancer patients. 38 However, the actual function of myostatin in human cancer-associated cachexia remains less clear.
Not only does myostatin have a negative effect on muscle, but it also has negative consequences on bone. Myostatin deficiency was associated with increased osteogenic differentiation of bone marrow-derived mesenchymal stem cells, as well as with increased bone mass and strength. 39 More recently, it was shown that myostatin inhibits osteoblast differentiation by directly reducing the osteocyte-derived production of exosomal miR-218. 40 Myostatin was also found able to enhance the action of RANKL on osteoclast formation, both in vitro and in vivo. 41 In elderly subjects, the levels of mature myostatin were found to negatively correlate with BMD and positively with markers of bone resorption. 42
Other members of the TGF-β superfamily such as activin are associated with muscle and bone alterations during cachexia. We recently showed that treatment with antagonists to the activin receptor type-2B (ACVR2B) was able to improve the cachectic phenotype. Indeed, the administration of the ACVR2B/Fc soluble receptor decoy was able to preserve body weight, bone mass, skeletal muscle mass, and strength both in models of chemotherapy and metastatic colorectal cancer-induced cachexia.22,43
Irisin
Irisin is the cleavage product of the transmembrane protein fibronectin type III domain-containing protein 5 (FNDC5) and was first described as released from skeletal muscle after exercise. 44 This protein can increase oxidative metabolism, promote myogenesis, and increase skeletal muscle mass. 45 Due to its potent action on regulating the browning of white adipose tissue, hence leading to reduced body weight and reduced adipose accumulation, a role for irisin in the regulation of obesity has also been intensely investigated. 46 In a model of atrophy induced by denervation, irisin was able to improve muscle mass by affecting myogenic signaling. 47 Interestingly, cortical bone mass was also modestly increased by the treatment with a low-irisin dose. 48 In post-menopausal women with osteoporosis, as well as in patients with diabetes, liver and heart disease, irisin levels negatively correlated with the risk of fractures. 49 However, on the contrary, work from other groups showed that global FNDC5 knock-out mice show reduced RANKL levels in the circulation, consistent with increased trabecular bone. 50 Also, irisin was found to increase the expression of the negative bone regulator sclerostin, in MLO-Y4 osteocyte cultures and in vivo, thus possibly stimulating bone catabolism. 50 Based on these opposing observations, the role of irisin on bone regulation remains controversial.
In a model of gastric cancer-induced cachexia, the expression of FNDC5 was increased in adipose tissue, as were high circulating levels of irisin. 51 Moreover, irisin levels were found increased in the serum of patients suffering from cardiac cachexia, along with changes in the expression of markers of heart failure. 52 Interestingly, irisin protein expression was also recently shown to be highly expressed in lung, liver, and gastrointestinal tumors, 53 and in the tumor of cachectic patients, irisin levels were higher than cancer patients with stable body weight.54–56 On the other hand, the levels of irisin were reduced in colorectal and breast cancer patients compared to normal subjects.57,58 Again, as in bone metabolism, the role of irisin in cancer cachexia remains unclear.
Interleukin-6
Interleukin-6 (IL-6) is a pleiotropic proinflammatory cytokine. The main producer and regulator of this cytokine is the adipose tissue, but also other cells and tissues such as immune cells, hepatocytes and neoplastic cells can secrete IL-6 59. IL-6 plays an important role in the immune and acute phase responses. 59 The role of IL-6 as a myokine was first described by Pedersen and collaborators by showing evidence that IL-6 is a product of the skeletal muscle contraction, and that its levels are regulated by duration and intensity of the contraction. 60 On this regard, in exercised human muscle, the IL-6 receptor was described as increased, suggesting a autocrine role of IL-6 on muscle. 61 The signaling of IL-6 on skeletal muscle improves insulin-stimulated glucose uptake as well as fatty acid oxidation via AMPK. 62 This cytokine has also an important role in muscle stem cell-mediated hypertrophy. Indeed, it has been shown that IL-6 is produced by growing myofibers, whereas IL-6 deficiency reduces muscle hypertrophy by inhibiting muscle stem cell proliferation and fusion with preexisting myofibers. 63 A recent study by Chowdhury et al. reported that the bone-derived protein, osteocalcin, enhanced the effects muscle-derived IL-6 to enhance exercise capacity. They also showed that IL-6 from muscle induced osteoblasts to send signals to osteoclasts which is turn were responsible for the release of osteocalcin from bone. Therefore, bone and muscle crosstalk can occur through the release of osteocalcin from bone due to the action of Il-6 produced by contracted muscle. 64 However, high, pathologic levels IL-6 are able to directly inhibit osteoblast maturation and differentiation both in vivo and in vitro.65,66 High IL-6 levels were also described in association with increased osteoclastogenesis and bone loss, 62 and IL-6 released by apoptotic osteocytes was found to improve the adhesion of osteoclast precursors, thus enhancing bone resorption. 67
The role of IL-6 in cancer-induced cachexia is extensively described. Several murine preclinical models for the study of cancer cachexia showed elevated levels of IL-6 in the circulation.20,43,68 In human cancer cachexia, elevated IL-6 levels are predictors of body weight loss, 69 as well as a negative prognostic factor especially in cachectic lung cancer patients. 70 In cancer, IL-6 is often directly produced by cancer cells, and both IL-6 levels and body mass return to normal values when IL-6-producing tumors are removed from cachectic mice. 71 Similarly, the use of specific neutralizing antibodies against IL-6 was effective in reducing muscle protein hypercatabolism and preserving muscle mass. 72 The mechanisms of IL-6-dependent muscle atrophy are well characterized. In particular, IL-6 has been shown to drive muscle atrophy during cancer cachexia by activating the JAK/STAT3 pathway, thereby stimulating muscle protein degradation and the activation of an acute phase response in skeletal muscle. 9
β-aminoisobutyric acid
β-aminoisobutyric acid (BAIBA), a novel small molecule metabolite, has been described to participate in several bone and muscle processes. BAIBA was first described as being produced by skeletal muscle during exercise in humans and rodents via the regulation of the transcription factor PGC-1α. 73 BAIBA promotes the browning of white adipose tissue by increasing the expression of specific genes such as uncoupling proteins, and improves glucose tolerance and enhances hepatic fatty acid β-oxidation. 73 In skeletal muscle, L-BAIBA has an autocrine function and was found to improve insulin resistance and inflammation, and to stimulate fatty acid β-oxidation through the regulation of the AMP and PPARδ pathways. 74 BAIBA was also shown to improve muscle contraction and strength in a sex-dependent manner. 45 The L/S enantiomer of BAIBA is a natural catabolite of valine and the D/R enantiomer is produced from thymine. Normally, one enantiomer is active and the other inactive, although there are examples of both enantiomers being concurrently active. In the first study to test the different potential functions of L and D BAIBA, L-BAIBA was found to play a role in the maintenance of bone mass by protecting osteocytes from reactive oxygen species. 75 Trabecular bone loss resulting from hindlimb unloading was attenuated along with osteocyte cell death in mice receiving drinking water supplemented with L-BAIBA. 75 L-BAIBA exerts its function by binding the receptor Mas-related G-protein receptor type D. 75 In osteocytes, the expression of this receptor is reduced with aging, and this could explain the involvement of this pathway in the osteoporotic process. 75 The ability of L-BAIBA to increase muscle function and maintain bone volume suggests the potential use of this molecule for the detrimental effects of cancer cachexia on musculoskeletal health.
Osteokines in muscle-bone crosstalk
Transforming growth factor β
Cancer invasion of bone activates the latent bone transforming growth factor β (TGF-β) leading to the release of active TGF-β, which through a vicious cycle stimulates tumor growth, cancer cell invasion, and more bone destruction. 76 In addition to the devastating effects of this autocrine factor on bone, TGF-β released from the bone matrix was also described to induce muscle weakness by decreasing Ca2+-induced muscle force production. Breast, lung and prostate metastatic cancer, as well as multiple myeloma are potent activators of TGF-β leading to not only devasting effects on bone but also on muscle. 77 Moreover, body mass, skeletal muscle atrophy, and weakness were improved by blocking TGF-β signaling by using the TGF-β receptor I kinase inhibitor SD-208 or the bone-targeting bisphosphonate zoledronic acid. 77 Using a similar approach, treatment with the bisphosphonate pamidronate was found to reduce bone loss and improve muscle atrophy in pediatric burn patients, 78 and we recently demonstrated that pamidronate exerts its beneficial effect in pediatric burn patients by reducing the release of TGF-β by the bone matrix. 79
Osteocalcin
Osteocalcin (Ocn) is a non-collagenous protein involved in bone mineralization. Ocn is produced by mature osteoblasts and stored in the bone matrix. 28 The carboxylated form of Ocn has affinity for hydroxyapatite, and its release from bone into the circulation as an undercarboxylated bioactive form is due to osteoclast-mediated pH decrease on the bone surface. 28 Ocn acts in target tissues by binding to the Gprc6a receptor, and primarily impacts the regulation of glucose uptake and energy metabolism. 80 It has been shown that Ocn levels are increased in both humans and murine models after aerobic exercise.64,81 In this regard, Ocn was shown to play an important role in the adaptation to exercise. Indeed, mice with deletion of Ocn or its receptor Gprc6a displayed reduced exercise capacity and reduced muscle mass, accompanied by enhanced uptake of energy substrates, including glucose and fatty acid by myofibers. 80 Interestingly, Ocn levels were reduced with aging in both mice and humans, and exogenous administration of Ocn was able to restore exercise capacity and muscle mass in old mice and increase exercise performance in young animals.80,81 Whether Ocn plays a role in cancer cachexia remains to be elucidated. These findings highlight the potential role of Ocn/Gprc6a to improve muscle atrophy and weakness associated with cancer and other chronic conditions.
Sclerostin and Wnts
Sclerostin is a glycoprotein predominantly expressed by mature osteocytes, although also some tumors were found to be a source of this factor. 82 Sclerostin is an antagonist of the Wnt/β-catenin signaling pathway and considered to be the most important bone-derived negative regulator of bone mass and osteoblast differentiation. 83 Interestingly, high sclerostin levels were found associated with lower muscle mass, but not lower bone mass in female and male subjects. 84 In another study, low skeletal muscle mass index was correlated with higher serum levels of sclerostin in hemodialysis patients. 85 Moreover, type 2 diabetic patients undergoing high-intensity interval training exercise showed improved muscle mass, along with reduced levels of circulating sclerostin. 86 Hesse et al. showed that tumor-derived sclerostin inhibits bone formation in breast cancer-induced cachexia, hence suggesting sclerostin as an important driver of both bone and muscle loss. 82 Interestingly, treatment with anti-sclerostin antibodies in breast cancer-bearing mice not only was able to improve bone mass, but also reduced skeletal muscle atrophy by acting on the NF-kB pathway and on the differentiation process. More importantly, in this study, sclerostin inhibition also improved muscle strength, reduced tumor mass, and prolonged survival in tumor-bearing mice. 82 These data provide evidence that sclerostin directly contributes to maintain muscle homeostasis and its levels may be used as predictors of low skeletal muscle mass. Of note, it was recently shown that sclerostin can be produced and released by C2C12 and primary myoblasts, and the conditioned media derived from muscle cells was able to inhibit the differentiation of 2T3 osteogenic cells. 87 These findings were further confirmed by evidence that sclerostin is also produced in vivo by muscle, regardless of age, muscle type, or phenotype (i.e. glycolytic vs. oxidative). 87 Altogether, these observations would seem to suggest that also muscle-derived sclerostin can affect bone mass. 87
Whereas sclerostin is an inhibitor of the Wnt/β-catenin signaling pathway, the Wnts are agonists of this pathway and are also made by bone cells. Brotto et al. showed that osteocyte-derived Wnt3a was able to play a role in muscle-bone crosstalk. Specifically, Wnt3a released in the conditioned media of MLO-Y4 osteocyte-like cells stimulated C2C12 myoblast differentiation, as well as improved muscle contractility ex vivo by modulating intracellular Ca2+ signaling. 88 Also Wnt7a, another member of the Wnt family of ligands, was shown to improve muscle atrophy induced by the cachexiogenic C26 colon adenocarcinoma cells, both in vitro and in vivo, mainly by reactivating the AKT/mTOR anabolic pathway and by stimulating muscle stem cell differentiation. 89 Therefore, the Wnts appear to be positive regulators of both bone and muscle in contrast to sclerostin.
Rank/RANKL/OPG
Receptor activator of nuclear factor β ligand, RANKL, is the most important regulator of bone resorption. Though made by immune cells, in bone the production of RANKL is carried out mostly by late osteoblasts and osteocytes. 90 RANKL binds its receptor, RANK, on bone monocytes/macrophage osteoclast precursors inducing fusion and activation into bone resorptive osteoclasts. The activity of RANKL is regulated by its decoy receptor osteoprotegerin (OPG), that binds to RANKL and inhibits its osteoclastogenic activity. 90 The ratio and relative abundance of each determine the degree of bone resorption/bone formation.
Interestingly, RANK receptor was found also in skeletal muscle and in C2C12 myotubes, thereby suggesting a regulatory role of the RANK/RANKL/OPG axis on skeletal muscle. It was subsequently discovered that in denervated fast-twitch fibers, the RANK/RANKL pathway affects skeletal muscle function by modulating SERCA activity and Ca2+ storage. 91 RANK/RANKL muscle levels were found elevated in mdx mice, a murine model of Duchenne muscular dystrophy, and the use of neutralizing antibodies against RANKL was able to improve muscle histology and function. 92 The anti-human RANKL antibody (i.e. Denosumab) is an FDA-approved drug for the treatment of bone loss in patients who have risk of bone fracture. Bonnet et al. showed that the treatment of osteoporotic post-menopausal women with Denosumab not only improved BMD, but also increased appendicular lean mass and muscle handgrip strength. 93 Furthermore, alterations of the RANKL/OPG balance using OPG−/− mice were sufficient to cause bone loss, as well as atrophy and weakness in fast-twitch myofibers. 92 Though developed for the treatment of osteoporosis, anti-RANKL therapies clearly have potential to benefit muscle in addition to bone with regard to cancer cachexia
Parathyroid hormone-related protein
Parathyroid hormone-related protein (PTHrP) is highly expressed in the lactating breast and in the placenta to insure calcium uptake but has also been shown to be produced by osteoblasts.94,95 Abnormal expression in tumors is well described resulting in hypercalcemia. In cancer, this factor is an important driver of cancer-induced osteolysis and calcium release. 96 In particular, hypercalcemia due to elevated PTHrP can induce neuromuscular symptoms and muscle weakness. 97 Onuma et al. were the first to show that in a model of cancer cachexia, treatment with anti-PTHrP antibody was able to reduce hypercalcemia, as well as restore locomotor activity, along with attenuation of body weight loss, fat wasting, and skeletal muscle atrophy. 98 PTHrP was also shown to stimulate browning of the white adipose tissue (WAT), hence inducing the thermogenic program in mice implanted with Lewis lung carcinomas (LLC). 99 Conversely, in vivo neutralization of PTHrP was able to prevent WAT browning and reduce energy wasting, as well as improve weight loss and skeletal muscle wasting in tumor hosts. 99 Interestingly, it was shown that LLC tumors are able to release extracellular vesicles (EVs) containing PTHrP, and this event induces lipolysis in 3T3-L1 adipocytes in vitro. 100 In in vivo conditions, the same EVs-containing PTHrP were found to be important drivers of fat loss and WAT browning in LLC bearers. 100 Altogether, these observations support PTHrP as a new target to counteract muscle, WAT, and bone loss during cancer cachexia.
Fibroblast growth factor 23
Fibroblast growth factor 23 (FGF23) was the first osteocyte-derived hormone described to have a systemic regulatory function. 28 The most important role of FGF23 is the regulation of phosphate reabsorption by the kidney. 101 FGF23 has negative effects on cardiac muscle by inducing left ventricular hypertrophy; 102 however, even though FGF23 receptors are expressed in skeletal muscle, ex vivo treatment with recombinant FGF23 did not significantly alter muscle function.28,103 Further studies are needed to elucidate a direct role of FGF23 in skeletal muscle homeostasis other than phosphate uptake by muscle.
Prostaglandin E2
Prostaglandin E2 (PGE2), an arachidonic acid metabolite, secreted by mechanically stimulated osteocytes was described to improve myogenic differentiation in primary myoblasts. 104 This molecule is produced by various cell types, including osteocytes, when stimulated by fluid-flow stress or under bone loading. 104 For example, it is estimated that osteocytes produce 100-fold more PGE2 when compared to the muscle tissue. 28 Regardless of the source of the PGE2, a recent study showed increased activity of 15-PDGH, an enzyme that degrades PGE2, in aging skeletal muscle, suggesting a critical role of PGE2. In 24- to 28-month-old mice muscle, specific inhibition of 15-PGDH using adeno-associated virus containing the short hairpin RNA to 15-PGDH resulted in increased PGE2 to levels similar to those found in young mice and this was sufficient to preserve muscle mass and function. 105 The question remains as to the major source of PGE2 targeting muscle in young animals, an autocrine source or from a distant organ-such as bone.
Conclusions
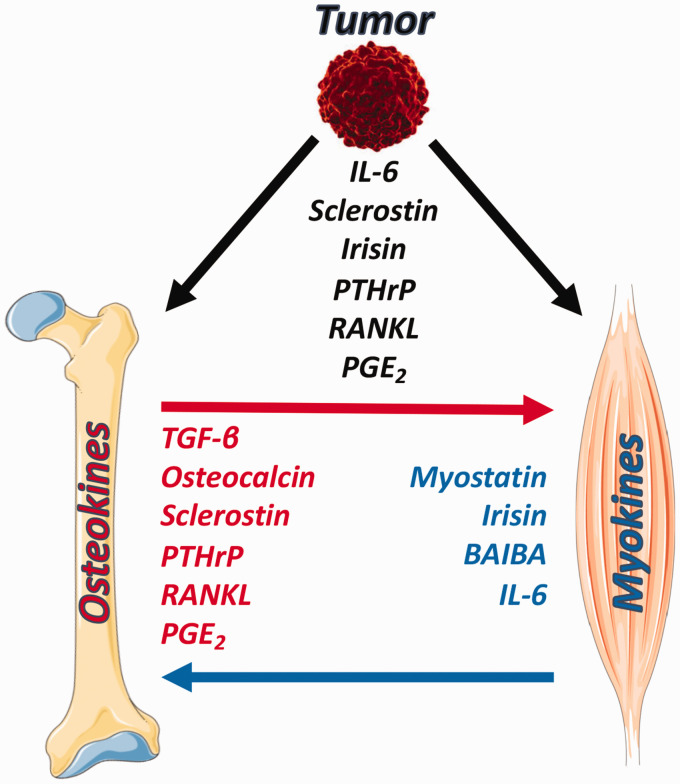
Skeletal muscle and bone have a long-lasting relationship that starts with embryogenesis and, through a harmonized development, reaches completion in the adult organism. Together bone and muscle share the decline that characterizes aging, resulting in the loss of bone and muscle known as osteoporosis and sarcopenia, respectively. The two tissues are regulated in tandem and can be concomitantly compromised in different pathological conditions. The reasons for this close association not only include the mechanical interactions but also the biochemical crosstalk. In this review, we emphasized the importance of the communication between muscle and bone during cancer cachexia. We summarized some of the most studied myokines and osteokines that are known to be directly involved in the musculoskeletal pathologies associated with cancer cachexia, representing a clinical scenario in which the biochemical exchanges between muscle and bone are complicated by the ability of the tumor to produce and release factors normally made by bone or muscle to target and compromise bone and muscle function (Figure 1). Given that different tumors are known to secrete soluble factors and to affect muscle and bone tissue homeostasis, we propose that personalized therapeutic interventions targeting myokines and osteokines should be taken into account and combined with routinely used anticancer strategies to better preserve bone and muscle in a context of cancer cachexia.
Figure 1.
Schematic representation of the bone-muscle crosstalk in a context of cancer cachexia. Osteokines and myokines contribute to the bone-muscle derangements associated with tumor burden. Some of the factors produced by muscle and bone can be released also from the tumor, thus complicating the bone-muscle interaction. Images adapted from Servier Medical Art (https://smart.servier.com). (A color version of this figure is available in the online journal.)
Footnotes
AUTHORS’ CONTRIBUTIONS: FP, LFB, and AB conceived the contents of the review; FP and AB wrote the review; LFB edited the paper. All authors have read and agreed to the published version of the manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Indiana Center for Musculoskeletal Health, by the Department of Surgery and the Department of Otolaryngology—Head & Neck Surgery at Indiana University School of Medicine, and by grants from National Institute of Health (NIH NIA PO1 AGO39355 to L.F.B.), V Foundation for Cancer Research (V2017-021 to A.B.), and American Cancer Society (Research Scholar Grant 132013-RSG-18–010-01-CCG to A.B.).
ORCID iD: Andrea Bonetto https://orcid.org/0000-0002-3235-1871
References
- 1.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011; 12:489–95 [DOI] [PubMed] [Google Scholar]
- 2.von Haehling S, Anker MS, Anker SD. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: facts and numbers update 2016. J Cachexia Sarcopenia Muscle 2016; 7:507–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freire PP, Fernandez GJ, de Moraes D, Cury SS, Dal Pai-Silva M, Dos Reis PP, Rogatto SR, Carvalho RF. The expression landscape of cachexia-inducing factors in human cancers. J Cachexia Sarcopenia Muscle 2020; 11:947–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 2012; 16:153–66 [DOI] [PubMed] [Google Scholar]
- 5.Tisdale MJ. Pathogenesis of cancer cachexia. J Support Oncol 2003; 1:159–68 [PubMed] [Google Scholar]
- 6.Barreto R, Mandili G, Witzmann FA, Novelli F, Zimmers TA, Bonetto A. Cancer and chemotherapy contribute to muscle loss by activating common signaling pathways. Front Physiol 2016; 7:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coletti D. Chemotherapy-induced muscle wasting: an update. Eur J Transl Myol 2018; 28:7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seelaender M, Batista M, Jr., Lira F, Silverio R, Rossi-Fanelli F. Inflammation in cancer cachexia: to resolve or not to resolve (is that the question?). Clin Nutr 2012; 31:562–6 [DOI] [PubMed] [Google Scholar]
- 9.Bonetto A, Aydogdu T, Kunzevitzky N, Guttridge DC, Khuri S, Koniaris LG, Zimmers TA. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS One 2011; 6:e22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Argiles JM, Busquets S, Stemmler B, Lopez-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 2014; 14:754–62 [DOI] [PubMed] [Google Scholar]
- 11.Laviano A, Meguid MM, Rossi-Fanelli F. Cancer anorexia: clinical implications, pathogenesis, and therapeutic strategies. Lancet Oncol 2003; 4:686–94 [DOI] [PubMed] [Google Scholar]
- 12.DeWys WD. Pathophysiology of cancer cachexia: current understanding and areas for future research. Cancer Res 1982; 42:26s–721s [PubMed] [Google Scholar]
- 13.Penna F, Ballaro R, Beltra M, De LS, Garcia CL, Costelli P. The skeletal muscle as an active player against cancer cachexia. Front Physiol 2019; 10:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HG, Huot JR, Pin F, Guo B, Bonetto A, Nader GA. Reduced rDNA transcription diminishes skeletal muscle ribosomal capacity and protein synthesis in cancer cachexia. FASEB J 2021; 35:e21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pin F, Couch ME, Bonetto A. Preservation of muscle mass as a strategy to reduce the toxic effects of cancer chemotherapy on body composition. Curr Opin Support Palliat Care 2018; 12:420–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fornetti J, Welm AL, Stewart SA. Understanding the bone in cancer metastasis. J Bone Miner Res 2018; 33:2099–113 [DOI] [PubMed] [Google Scholar]
- 17.Kanis JA, McCloskey EV, Powles T, Paterson AH, Ashley S, Spector T. A high incidence of vertebral fracture in women with breast cancer. Br J Cancer 1999; 79:1179–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung YC, Yeh LS, Chang WC, Lin CC, Kao CH. Prospective study of decreased bone mineral density in patients with cervical cancer without bone metastases: a preliminary report. Jpn J Clin Oncol 2002; 32:422–4 [DOI] [PubMed] [Google Scholar]
- 19.Dumanskiy YV, Syniachenko OV, Stepko PA, Taktashov GS, Chernyshova OY, Stoliarova OY. The state of bone metabolism in lung cancer patients. Exp Oncol 2018; 40:136–9 [PubMed] [Google Scholar]
- 20.Pin F, Barreto R, Kitase Y, Mitra S, Erne CE, Novinger LJ, Zimmers TA, Couch ME, Bonewald LF, Bonetto A. Growth of ovarian cancer xenografts causes loss of muscle and bone mass: a new model for the study of cancer cachexia. J Cachexia Sarcopenia Muscle 2018; 9:685–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huot JR, Pin F, Essex AL, Bonetto A. MC38 tumors induce musculoskeletal defects in colorectal cancer. Int J Mol Sci 2021; 22:1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barreto R, Kitase Y, Matsumoto T, Pin F, Colston KC, Couch KE, O'Connell TM, Couch ME, Bonewald LF, Bonetto A. ACVR2B/Fc counteracts chemotherapy-induced loss of muscle and bone mass. Sci Rep 2017; 7:14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Essex AL, Pin F, Huot JR, Bonewald LF, Plotkin LI, Bonetto A. Bisphosphonate treatment ameliorates Chemotherapy-Induced bone and muscle abnormalities in young mice. Front Endocrinol 2019; 10:809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pourquie O. Vertebrate somitogenesis. Annu Rev Cell Dev Biol 2001; 17:311–50 [Database [DOI] [PubMed] [Google Scholar]
- 25.Land C, Schoenau E. Fetal and postnatal bone development: reviewing the role of mechanical stimuli and nutrition. Best Pract Res Clin Endocrinol Metab 2008; 22:107–18 [DOI] [PubMed] [Google Scholar]
- 26.Brotto M, Bonewald L. Bone and muscle: interactions beyond mechanical. Bone 2015; 80:109–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scofield KL, Hecht S. Bone health in endurance athletes: runners, cyclists, and swimmers. Curr Sports Med Rep 2012; 11:328–34 [DOI] [PubMed] [Google Scholar]
- 28.Bonewald L. Use it or lose it to age: a review of bone and muscle communication. Bone 2019; 120:212–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harry LE, Sandison A, Paleolog EM, Hansen U, Pearse MF, Nanchahal J. Comparison of the healing of open tibial fractures covered with either muscle or fasciocutaneous tissue in a murine model. J Orthop Res 2008; 26:1238–44 [DOI] [PubMed] [Google Scholar]
- 30.Schindeler A, Liu R, Little DG. The contribution of different cell lineages to bone repair: exploring a role for muscle stem cells. Differentiation 2009; 77:12–8 [DOI] [PubMed] [Google Scholar]
- 31.Liu R, Schindeler A, Little DG. The potential role of muscle in bone repair. J Musculoskelet Neuronal Interact 2010; 10:71–6 [PubMed] [Google Scholar]
- 32.Gentry BA, Ferreira JA, McCambridge AJ, Brown M, Phillips CL. Skeletal muscle weakness in osteogenesis imperfecta mice. Matrix Biol 2010; 29:638–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci U S A 1997; 94:12457–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol 2009; 296:C1258–70 [DOI] [PubMed] [Google Scholar]
- 35.Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. Induction of cachexia in mice by systemically administered myostatin. Science 2002; 296:1486–8 [DOI] [PubMed] [Google Scholar]
- 36.Costelli P, Muscaritoli M, Bonetto A, Penna F, Reffo P, Bossola M, Bonelli G, Doglietto GB, Baccino FM, Rossi Fanelli F. Muscle myostatin signalling is enhanced in experimental cancer cachexia. Eur J Clin Invest 2008; 38:531–8 [DOI] [PubMed] [Google Scholar]
- 37.Bonetto A, Penna F, Minero VG, Reffo P, Bonelli G, Baccino FM, Costelli P. Deacetylase inhibitors modulate the myostatin/follistatin axis without improving cachexia in tumor-bearing mice. Curr Cancer Drug Targets 2009; 9:608–16 [DOI] [PubMed] [Google Scholar]
- 38.Aversa Z, Bonetto A, Penna F, Costelli P, Di Rienzo G, Lacitignola A, Baccino FM, Ziparo V, Mercantini P, Rossi Fanelli F, Muscaritoli M. Changes in myostatin signaling in non-weight-losing cancer patients. Ann Surg Oncol 2012; 19:1350–6 [DOI] [PubMed] [Google Scholar]
- 39.Hamrick MW, Shi X, Zhang W, Pennington C, Thakore H, Haque M, Kang B, Isales CM, Fulzele S, Wenger KH. Loss of myostatin (GDF8) function increases osteogenic differentiation of bone marrow-derived mesenchymal stem cells but the osteogenic effect is ablated with unloading. Bone 2007; 40:1544–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin Y, Peng Y, Zhao W, Pan J, Ksiezak-Reding H, Cardozo C, Wu Y, Divieti Pajevic P, Bonewald LF, Bauman WA, Qin W. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: a novel mechanism in muscle-bone communication. J Biol Chem 2017; 292:11021–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dankbar B, Fennen M, Brunert D, Hayer S, Frank S, Wehmeyer C, Beckmann D, Paruzel P, Bertrand J, Redlich K, Koers-Wunrau C, Stratis A, Korb-Pap A, Pap T. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat Med 2015; 21:1085–90 [DOI] [PubMed] [Google Scholar]
- 42.Wu LF, Zhu DC, Wang BH, Lu YH, He P, Zhang YH, Gao HQ, Zhu XW, Xia W, Zhu H, Mo XB, Lu X, Zhang L, Zhang YH, Deng FY, Lei SF. Relative abundance of mature myostatin rather than total myostatin is negatively associated with bone mineral density in Chinese. J Cell Mol Med 2018; 22:1329–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huot JR, Pin F, Narasimhan A, Novinger LJ, Keith AS, Zimmers TA, Willis MS, Bonetto A. ACVR2B antagonism as a countermeasure to multi-organ perturbations in metastatic colorectal cancer cachexia. J Cachexia Sarcopenia Muscle 2020; 11:1779–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481:463–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colaianni G, Storlino G, Sanesi L, Colucci S, Grano M. Myokines and osteokines in the pathogenesis of muscle and bone diseases. Curr Osteoporos Rep 2020; 18:401–7 [DOI] [PubMed] [Google Scholar]
- 46.Huh JY, Dincer F, Mesfum E, Mantzoros CS. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int J Obes 2014; 38:1538–44 [DOI] [PubMed] [Google Scholar]
- 47.Reza MM, Subramaniyam N, Sim CM, Ge X, Sathiakumar D, McFarlane C, Sharma M, Kambadur R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat Commun 2017; 8:1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P, Lu P, Sartini L, Di Comite M, Mori G, Di Benedetto A, Brunetti G, Yuen T, Sun L, Reseland JE, Colucci S, New MI, Zaidi M, Cinti S, Grano M. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci U S A 2015; 112:12157–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polyzos SA, Anastasilakis AD, Efstathiadou ZA, Makras P, Perakakis N, Kountouras J, Mantzoros CS. Irisin in metabolic diseases. Endocrine 2018; 59:260–74 [DOI] [PubMed] [Google Scholar]
- 50.Kim H, Wrann CD, Jedrychowski M, Vidoni S, Kitase Y, Nagano K, Zhou C, Chou J, Parkman VA, Novick SJ, Strutzenberg TS, Pascal BD, Le PT, Brooks DJ, Roche AM, Gerber KK, Mattheis L, Chen W, Tu H, Bouxsein ML, Griffin PR, Baron R, Rosen CJ, Bonewald LF, Spiegelman BM. Irisin mediates effects on bone and fat via alphaV integrin receptors. Cell 2018; 175:1756–68.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Us Altay D, Keha EE, Ozer Yaman S, Ince I, Alver A, Erdogan B, Canpolat S, Cobanoglu U, Mentese A. Investigation of the expression of irisin and some cachectic factors in mice with experimentally induced gastric cancer. QJM 2016; 109:785–90 [DOI] [PubMed] [Google Scholar]
- 52.Kalkan AK, Cakmak HA, Erturk M, Kalkan KE, Uzun F, Tasbulak O, Diker VO, Aydin S, Celik A. Adropin and irisin in patients with cardiac cachexia. Arq Bras Cardiol 2018; 111:39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang D, Tan X, Tang N, Huang F, Chen Z, Shi G. Review of research on the role of irisin in tumors. Onco Targets Ther 2020; 13:4423–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Castro Gs Correia-Lima J, Simoes E, Orsso Ce Xiao J, Gama Lr Gomes Sp Goncalves DCCosta RGF, Radloff K, Lenz U, Taranko Ae Bin Fc Formiga Fb de Godoy LGL, de Souza Rp Nucci LHA, Feitoza M, de Castro CC, Tokeshi F, Alcantara PSM, Otoch Jp Ramos AF, Laviano A, Coletti D, Mazurak Vc Prado Cm, Seelaender M. Myokines in treatment-naive patients with cancer-associated cachexia. Clin Nutr 2020. doi: 10.1016/j.clnu.2020.10.050 [DOI] [PubMed] [Google Scholar]
- 55.Nowinska K, Jablonska K, Pawelczyk K, Piotrowska A, Partynska A, Gomulkiewicz A, Ciesielska U, Katnik E, Grzegrzolka J, Glatzel-Plucinska N, Ratajczak-Wielgomas K, Podhorska-Okolow M, Dziegiel P. Expression of irisin/FNDC5 in cancer cells and stromal fibroblasts of non-small cell lung cancer. Cancers 2019; 11:1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gaggini M, Cabiati M, Del Turco S, Navarra T, De Simone P, Filipponi F, Del Ry S, Gastaldelli A, Basta G. Increased FNDC5/irisin expression in human hepatocellular carcinoma. Peptides 2017; 88:62–6 [DOI] [PubMed] [Google Scholar]
- 57.Zhu H, Liu M, Zhang N, Pan H, Lin G, Li N, Wang L, Yang H, Yan K, Gong F. Serum and adipose tissue mRNA levels of ATF3 and FNDC5/irisin in colorectal cancer patients with or without obesity. Front Physiol 2018; 9:1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Provatopoulou X, Georgiou GP, Kalogera E, Kalles V, Matiatou MA, Papapanagiotou I, Sagkriotis A, Zografos GC, Gounaris A. Serum irisin levels are lower in patients with breast cancer: association with disease diagnosis and tumor characteristics. BMC Cancer 2015; 15:898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol 1993; 54:1–78 [DOI] [PubMed] [Google Scholar]
- 60.Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund Pedersen B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol 2000; 529(Pt 1):237–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keller C, Steensberg A, Hansen AK, Fischer CP, Plomgaard P, Pedersen BK. Effect of exercise, training, and glycogen availability on IL-6 receptor expression in human skeletal muscle. J Appl Physiol 2005; 99:2075–9 [DOI] [PubMed] [Google Scholar]
- 62.Severinsen MCK, Pedersen BK. Muscle-organ crosstalk: the emerging roles of myokines. Endocr Rev 2020; 41:594–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Serrano AL, Baeza-Raja B, Perdiguero E, Jardi M, Munoz-Canoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 2008; 7:33–44 [DOI] [PubMed] [Google Scholar]
- 64.Chowdhury S, Schulz L, Palmisano B, Singh P, Berger JM, Yadav VK, Mera P, Ellingsgaard H, Hidalgo J, Bruning J, Karsenty G. Muscle-derived interleukin 6 increases exercise capacity by signaling in osteoblasts. J Clin Invest 2020; 130:2888–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peruzzi B, Cappariello A, Del Fattore A, Rucci N, De Benedetti F, Teti A. c-Src and IL-6 inhibit osteoblast differentiation and integrate IGFBP5 signalling. Nat Commun 2012; 3:630. [DOI] [PubMed] [Google Scholar]
- 66.Kaneshiro S, Ebina K, Shi K, Higuchi C, Hirao M, Okamoto M, Koizumi K, Morimoto T, Yoshikawa H, Hashimoto J. IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. J Bone Miner Metab 2014; 32:378–92 [DOI] [PubMed] [Google Scholar]
- 67.Cheung WY, Simmons CA, You L. Osteocyte apoptosis regulates osteoclast precursor adhesion via osteocytic IL-6 secretion and endothelial ICAM-1 expression. Bone 2012; 50:104–10 [DOI] [PubMed] [Google Scholar]
- 68.Huot JR, Novinger LJ, Pin F, Bonetto A. HCT116 colorectal liver metastases exacerbate muscle wasting in a mouse model for the study of colorectal cancer cachexia. Dis Model Mech 2020; 13:dmm043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carson JA, Baltgalvis KA. Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc Sport Sci Rev 2010; 38:168–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pettersen K, Andersen S, Degen S, Tadini V, Grosjean J, Hatakeyama S, Tesfahun AN, Moestue S, Kim J, Nonstad U, Romundstad PR, Skorpen F, Sorhaug S, Amundsen T, Gronberg BH, Strasser F, Stephens N, Hoem D, Molven A, Kaasa S, Fearon K, Jacobi C, Bjorkoy G. Cancer cachexia associates with a systemic autophagy-inducing activity mimicked by cancer cell-derived IL-6 trans-signaling. Sci Rep 2017; 7:2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Strassmann G, Fong M, Kenney JS, Jacob CO. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest 1992; 89:1681–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.White JP, Puppa MJ, Sato S, Gao S, Price RL, Baynes JW, Kostek MC, Matesic LE, Carson JA. IL-6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the ApcMin/+ mouse. Skelet Muscle 2012; 2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roberts LD, Bostrom P, O'Sullivan JF, Schinzel RT, Lewis GD, Dejam A, Lee YK, Palma MJ, Calhoun S, Georgiadi A, Chen MH, Ramachandran VS, Larson MG, Bouchard C, Rankinen T, Souza AL, Clish CB, Wang TJ, Estall JL, Soukas AA, Cowan CA, Spiegelman BM, Gerszten RE. Beta-aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 2014; 19:96–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jung TW, Hwang HJ, Hong HC, Yoo HJ, Baik SH, Choi KM. BAIBA attenuates insulin resistance and inflammation induced by palmitate or a high fat diet via an AMPK-PPARdelta-dependent pathway in mice. Diabetologia 2015; 58:2096–105 [DOI] [PubMed] [Google Scholar]
- 75.Kitase Y, Vallejo JA, Gutheil W, Vemula H, Jahn K, Yi J, Zhou J, Brotto M, Bonewald LF. Beta-aminoisobutyric acid, l-BAIBA, is a muscle-derived osteocyte survival factor. Cell Rep 2018; 22:1531–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guise TA. The vicious cycle of bone metastases. J Musculoskelet Neuronal Interact 2002; 2:570–2 [PubMed] [Google Scholar]
- 77.Waning DL, Mohammad KS, Reiken S, Xie W, Andersson DC, John S, Chiechi A, Wright LE, Umanskaya A, Niewolna M, Trivedi T, Charkhzarrin S, Khatiwada P, Wronska A, Haynes A, Benassi MS, Witzmann FA, Zhen G, Wang X, Cao X, Roodman GD, Marks AR, Guise TA. Excess TGF-beta mediates muscle weakness associated with bone metastases in mice. Nat Med 2015; 21:1262–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Borsheim E, Herndon DN, Hawkins HK, Suman OE, Cotter M, Klein GL. Pamidronate attenuates muscle loss after pediatric burn injury. J Bone Miner Res 2014; 29:1369–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pin F, Bonetto A, Bonewald LF, Klein GL. Molecular mechanisms responsible for the rescue effects of pamidronate on muscle atrophy in pediatric burn patients. Front Endocrinol 2019; 10:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mera P, Laue K, Wei J, Berger JM, Karsenty G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol Metab 2016; 5:1042–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mera P, Laue K, Ferron M, Confavreux C, Wei J, Galan-Diez M, Lacampagne A, Mitchell SJ, Mattison JA, Chen Y, Bacchetta J, Szulc P, Kitsis RN, de Cabo R, Friedman RA, Torsitano C, McGraw TE, Puchowicz M, Kurland I, Karsenty G. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab 2016; 23:1078–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hesse E, Schroder S, Brandt D, Pamperin J, Saito H, Taipaleenmaki H. Sclerostin inhibition alleviates breast cancer-induced bone metastases and muscle weakness. JCI Insight 2019; 5:e125543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Atkins GJ, Rowe PS, Lim HP, Welldon KJ, Ormsby R, Wijenayaka AR, Zelenchuk L, Evdokiou A, Findlay DM. Sclerostin is a locally acting regulator of late-osteoblast/preosteocyte differentiation and regulates mineralization through a MEPE-ASARM-dependent mechanism. J Bone Miner Res 2011; 26:1425–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim JA, Roh E, Hong SH, Lee YB, Kim NH, Yoo HJ, Seo JA, Kim NH, Kim SG, Baik SH, Choi KM. Association of serum sclerostin levels with low skeletal muscle mass: the Korean Sarcopenic Obesity Study (KSOS). Bone 2019; 128:115053. [DOI] [PubMed] [Google Scholar]
- 85.Medeiros MC, Rocha N, Bandeira E, Dantas I, Chaves C, Oliveira M, Bandeira F. Serum sclerostin, body composition, and sarcopenia in hemodialysis patients with diabetes. Int J Nephrol 2020; 2020:4596920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ghardashi-Afousi A, Davoodi M, Hesamabadi BK, Asvadi-Fard M, Bigi MAB, Izadi MR, Gaeini AA. Improved carotid intima-media thickness-induced high-intensity interval training associated with decreased serum levels of dkk-1 and sclerostin in type 2 diabetes. J Diabetes Complicat 2020; 34:107469. [DOI] [PubMed] [Google Scholar]
- 87.Magaro MS, Bertacchini J, Florio F, Zavatti M, Poti F, Cavani F, Amore E, De Santis I, Bevilacqua A, Reggiani Bonetti L, Torricelli P, Maurel DB, Biressi S, Palumbo C. Identification of sclerostin as a putative new myokine involved in the muscle-to-bone crosstalk. Biomedicines 2021; 9:71 [ 10.3390/biomedicines9010071][Mismatch] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang J, Romero-Suarez S, Lara N, Mo C, Kaja S, Brotto L, Dallas SL, Johnson ML, Jahn K, Bonewald LF, Brotto M. Crosstalk between MLO-Y4 osteocytes and C2C12 muscle cells is mediated by the wnt/beta-catenin pathway. JBMR Plus 2017; 1:86–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmidt M, Poser C, von Maltzahn J. Wnt7a counteracts cancer cachexia. Mol Ther Oncolytics 2020; 16:134–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ono T, Hayashi M, Sasaki F, Nakashima T. RANKL biology: bone metabolism, the immune system, and beyond. Inflamm Regen 2020; 40:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dufresne SS, Dumont NA, Boulanger-Piette A, Fajardo VA, Gamu D, Kake-Guena SA, David RO, Bouchard P, Lavergne E, Penninger JM, Pape PC, Tupling AR, Frenette J. Muscle RANK is a key regulator of Ca2+ storage, SERCA activity, and function of fast-twitch skeletal muscles. Am J Physiol Cell Physiol 2016; 310:C663–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hamoudi D, Marcadet L, Piette Boulanger A, Yagita H, Bouredji Z, Argaw A, Frenette J. An anti-RANKL treatment reduces muscle inflammation and dysfunction and strengthens bone in dystrophic mice. Hum Mol Genet 2019; 28:3101–12 [DOI] [PubMed] [Google Scholar]
- 93.Bonnet N, Bourgoin L, Biver E, Douni E, Ferrari S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J Clin Invest 2019; 129:3214–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kovacs CS, Lanske B, Hunzelman JL, Guo J, Karaplis AC, Kronenberg HM. Parathyroid hormone-related peptide (PTHrP) regulates fetal-placental calcium transport through a receptor distinct from the PTH/PTHrP receptor. Proc Natl Acad Sci U S A 1996; 93:15233–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martin TJ. Osteoblast-derived PTHrP is a physiological regulator of bone formation. J Clin Invest 2005; 115:2322–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Soki FN, Park SI, McCauley LK. The multifaceted actions of PTHrP in skeletal metastasis. Future Oncol 2012; 8:803–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician 2003; 67:1959–66 [PubMed] [Google Scholar]
- 98.Onuma E, Tsunenari T, Saito H, Sato K, Yamada-Okabe H, Ogata E. Parathyroid hormone-related protein (PTHrP) as a causative factor of cancer-associated wasting: possible involvement of PTHrP in the repression of locomotor activity in rats bearing human tumor xenografts. Int J Cancer 2005; 116:471–8 [DOI] [PubMed] [Google Scholar]
- 99.Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, Spiegelman BM. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 2014; 513:100–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu W, Xiong H, Ru Z, Zhao Y, Zhou Y, Xie K, Xiao W, Xiong Z, Wang C, Yuan C, Shi J, Du Q, Zhang X, Yang H. Extracellular vesicles-released parathyroid hormone-related protein from lewis lung carcinoma induces lipolysis and adipose tissue browning in cancer cachexia. Cell Death Dis 2021; 12:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bergwitz C, Juppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med 2010; 61:91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Touchberry CD, Green TM, Tchikrizov V, Mannix JE, Mao TF, Carney BW, Girgis M, Vincent RJ, Wetmore LA, Dawn B, Bonewald LF, Stubbs JR, Wacker MJ. FGF23 is a novel regulator of intracellular calcium and cardiac contractility in addition to cardiac hypertrophy. Am J Physiol Endocrinol Metab 2013; 304:E863–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Avin KG, Vallejo JA, Chen NX, Wang K, Touchberry CD, Brotto M, Dallas SL, Moe SM, Wacker MJ. Fibroblast growth factor 23 does not directly influence skeletal muscle cell proliferation and differentiation or ex vivo muscle contractility. Am J Physiol Endocrinol Metab 2018; 315:E594–E604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mo C, Zhao R, Vallejo J, Igwe O, Bonewald L, Wetmore L, Brotto M. Prostaglandin E2 promotes proliferation of skeletal muscle myoblasts via EP4 receptor activation. Cell Cycle 2015; 14:1507–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Palla AR, Ravichandran M, Wang YX, Alexandrova L, Yang AV, Kraft P, Holbrook CA, Schurch CM, Ho ATV, Blau HM. Inhibition of prostaglandin-degrading enzyme 15-PGDH rejuvenates aged muscle mass and strength. Science 2021; 371:eabc8059. [DOI] [PMC free article] [PubMed] [Google Scholar]