Abstract
The B-cell lymphocyte kinase (Blk) is a src-family protein tyrosine kinase specifically expressed in B-lineage cells of mice. The early onset of Blk expression during B-cell development in the bone marrow and the high expression levels of Blk in mature B cells suggest a possible important role of Blk in B-cell physiology. To study the in vivo function of Blk, mice homozygous for the targeted disruption of the blk gene were generated. In homozygous mutant mice, neither blk mRNA nor Blk protein is expressed. Despite the absence of Blk, the development, in vitro activation, and humoral immune responses of B cells to T-cell-dependent and -independent antigens are unaltered. These data are consistent with functional redundancy of Blk in B-cell development and immune responses.
Activation of B cells by various ligands is accompanied by activation of src-family protein tyrosine kinases (PTKs). Cross-linking of the B-cell receptor (BCR) leads to the activation of src-family PTKs Blk, Fyn, and Lyn (22). In addition, Lyn can be activated by antibody-mediated cross-linking of CD19 and, to a lesser extent, of RP-105 (3), whereas Fyn is part of the interleukin-5 receptor signal-transducing complex (2, 26). Activation of src-family PTKs precedes and is probably required for the activation of PTK Syk (13, 21), which belongs to the ZAP-70/Syk family of PTKs and is essential for pre-BCR and BCR-mediated B-cell development in the bone marrow (5). The src-family PTKs also trigger the phosphorylation and activation of the Tec-homologous kinase Btk, which plays a critical role in B-cell survival (1) and antigen-induced B-cell activation (7, 23).
The role of src-family PTKs in B-cell function in vivo remains largely elusive. A deficiency in Lyn decreases the threshold for BCR-mediated B-cell activation but renders B cells unresponsive to antibody-mediated cross-linking of RP-105 (3). Abnormal signalling properties of Lyn-deficient B-lineage cells do not significantly affect B-cell development in the bone marrow but are probably responsible for an autoimmune disease associated with high titers of anti-DNA and anti-nuclear antibodies in the blood of the mutant mice (4, 9, 18). The deficiency in Fyn has no significant effect on B-cell development and activation, with the exception of causing diminished B-cell responses to interleukin-5 (2, 26). In contrast to Lyn and Fyn, which are expressed in cells of different hematopoietic lineages, Blk is the only src-family PTK specifically expressed in B-lineage cells of mice (6). The expression of Blk starts at the late pro-B-cell, early pre-B-cell stage of B-cell development and remains constantly high at later stages of B-cell maturation (24). These data, as well as the induction of malignant transformation of B-cell progenitors by the expression of constitutively active Blk (16), suggest a possible involvement of Blk in the control of B-lineage cell differentiation and proliferation. On the other hand, suppression of the surface immunoglobulin M (IgM)-mediated apoptosis of B-lymphoma cells by Blk antisense oligonucleotides points to a role for Blk in negative selection of B cells (25). To define the role of Blk in B-cell development and activation, we have analyzed B-cell development and function in Blk-deficient mice.
MATERIALS AND METHODS
Construction of the blk targeting vector.
The fragment of the blk gene containing a part of exon 8 (which encodes amino acids (aa) 285 to 311), intron 8, and a part of exon 9 (encoding aa 312 to 333) was amplified by PCR from C57BL/6 genomic DNA and used as a short arm of homology. Primers 5′ CTG CAG CAT GAG AGG CTG GTT CG 3′ (aa 285 to 292; direct PCR primer) and 5′ GTC AAT CAG CCT TGG AAG GGA C 3′ (aa 327 to 333; reverse PCR primer) were used for exons 8 and 9, respectively. The short arm of homology was cloned into the XhoI site of plasmid pTV-0 (B. Walter and A. Tarakhovsky, unpublished data). This plasmid carries both the neomycin resistance (Neor) and the herpes simplex virus thymidine kinase (HSV TK) genes. The polylinker containing ClaI, NotI, XbaI, and XhoI is located 5′ of the Neor gene, while a polylinker containing BamHI, HpaI, NheI, and SalI is positioned 3′ of the Neor gene. The 8.3-kb HindIII-BglII fragment corresponding to the region from exon 1′ to intron 7 of the murine blk gene (6) (long arm of homology) was cloned in the HpaI site of pTV-0.
Generation of mice harboring the blk mutation.
The ClaI-linearized DNA of the blk targeting construct (pTV-0/Blk) was transfected by electroporation into E14-1.1 cells followed by selection in the presence of G418 (300 μg/ml) and ganciclovir (2 μM) as described previously (12). The DNA of doubly resistant embryonic stem (ES) cells was digested with BamHI and tested for the presence of the targeted blk allele by Southern blot analysis with the HindIII-BamHI 1.5-kb DNA fragment of intron 9 as a probe (see Fig. 1A). This probe recognizes 8- and 4-kb DNA fragments of endogenous and mutated blk loci, respectively (see Fig. 1A). The presence of a single copy of the integrated targeting vector was confirmed by Southern blot analysis with the Neor gene as a probe. ES cell clones heterozygous for the blk mutation were injected into CB20 blastocysts, and the resulting chimeras were crossed to CB20 mice in order to identify the most efficient germ line-transmitting chimeric mice. The germ line-transmitting chimeras were crossed to 129/Sv mice, and heterozygous mice carrying the blk mutation were identified by Southern blot hybridization and intercrossed to produce homozygous offspring on the 129/Sv background. Age- and sex-matched 129/Sv and blk−/− mice were used in the experiments. Most of the experiments were done with 6- to 8-week-old mice. To facilitate the typing of blk+/− and Blk−/− mice, a PCR strategy was developed. The blk wild-type allele was specifically amplified with a primer located in exon 8 (5′ ATG TCA CCG GAA GCT TTC C 3′; aa 270 to 275) and a reverse primer that hybridizes to a sequence in exon 9 (5′ A CCT GCT ACC TTC ATC GGT C 3′, aa 320 to 326). The blk mutant allele cannot be amplified with this pair of oligonucleotides since the sequence information of exon 8 encoding aa 252 to 284 is missing. The blk mutant allele was detected by using a primer complementary to a Neor gene sequence (5′ TAG CCG AAT AGC CTC TCC AC 3′; nucleotides 786 to 805) and the primer hybridizing to exon 9 described above. The annealing temperature was 60°C, and the Mg2+ concentration was 2 mM. The PCR products obtained were 1.2 and 1.5 kb for the wild-type and mutant blk alleles, respectively.
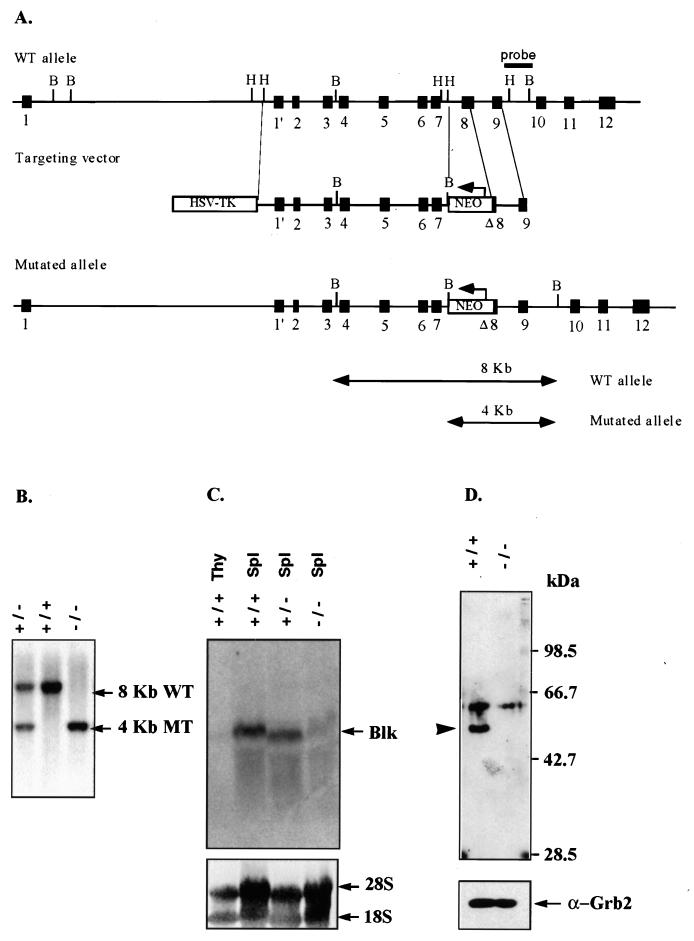
FIG. 1.
Targeted disruption of the murine blk gene by homologous recombination. (A) Schematic representation of the blk genomic locus, with the targeting construct shown below. The arrows indicate the direction of transcription of the Neor and HSV TK genes. The positions of blk exons are shown as boxes. The indicated external probe recognizes 8 and 4 kb of the BamHI-digested DNA of the wild-type and targeted blk genes, respectively. (B) Southern blot analysis of the blk mutation in mice. The tail DNA isolated from the wild-type 129/Sv, blk+/−, and blk−/− mice was digested with BamHI and analyzed as described above. Arrows indicate the position of the DNA fragments corresponding to the wild-type (8-kb) (WT) or targeted (4-kb) blk (MT) alleles. (C and D) Blk mRNA and protein expression in blk−/− mutant mice. Total RNA was prepared from lysates of splenic B cells and thymocytes of the wild-type 129/Sv, blk+/−, and blk−/− mice. (C) blk RNA expression was analyzed by Northern blotting with a blk cDNA probe which contains the entire blk coding sequence (6). (D) Blk protein expression was analyzed by Western blotting of B-cell lysates with rabbit polyclonal antibody that recognizes the unique N-terminal domain of Blk plus the SH3 and SH2 domains. The position of Blk is marked by an arrowhead. Protein loading was controlled by immunoblot staining with anti-Grb2 antibody.
Cell staining and flow cytometry.
Single-cell suspensions were prepared from different lymphoid organs and incubated for 10 min at 106 cells/20 μl on ice in staining buffer (phosphate-buffered saline [PBS] containing 0.5% bovine serum albumin [BSA] and 0.01% NaN3) with optimal amounts of fluorescein isothiocyanate-, phycoerythrin-, or biotin-conjugated antibodies. The following monoclonal antibodies were purchased from Pharmingen (San Diego, Calif.): S7 (anti-CD43), B3B4 (anti-CD23), and Ly1 (anti-CD5). The following antibodies were prepared: RA3-6B2 (anti-B220), R33-24.12 (anti-IgM), 1.3-5 (anti-IgD), and Cfo-1 (anti-Thy1.2). Flow cytometric analysis was performed on a FACScan cytometer (Becton Dickinson & Co., Mountain View, Calif.).
Analysis of B-cell proliferation and upregulation of activation markers.
Splenic B cells were purified by depletion of non-B cells on MACs columns (Miltenyi Biotec, Bergisch Gladbach, Germany) with anti-CD43 antibody coupled to magnetic beads (Miltenyi Biotec) as described previously (17). The purity of B cells was controlled by fluorescence-activated cell sorter analysis, and the preparations of B cells of 95% purity were used. B cells were stimulated with goat anti-IgM antibody (2.5 μg/ml) (Dianova, Hamburg, Germany), anti-CD40 antibody (0.6 μg/ml) (Pharmingen), and IL-4 (25 U/ml) (Genzyme Corp., Boston, Mass.). The analysis of cell proliferation and upregulation of activation markers was performed as described previously (3, 14).
Analysis of protein expression and tyrosine phosphorylation.
For the analysis of protein expression, cells were lysed in lysis buffer (10% glycerol, 1% Triton X-100, 20 mM Tris-HCl [pH 8.0], 150 mM NaCl, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml). The lysates equivalent to 5 × 106 cells were loaded onto a sodium dodecyl sulfate (SDS)–10% polyacrylamide gel, and the separated proteins were electrotransferred to a Hybond nitrocellulose filter (Amersham) by semidry method. After being subjected to blocking with PBS–0.5% BSA–0.1% Tween 20, the filter was incubated first with a rabbit polyclonal antibody that recognizes the unique domain of Blk plus the SH3 and SH2 domains and then with a horseradish peroxidase-conjugated goat anti-rabbit IgG (Amersham) and developed with the enhanced chemiluminescence system (Amersham). RNA was analyzed by Northern blot analysis (20) using a blk cDNA probe (6). This probe (2,094 bp) contains the entire Blk coding sequence. For the analysis of tyrosine phosphorylation of whole-cell lysates and specific substrates downstream of Blk, purified B cells were suspended in RPMI supplemented with 2% fetal calf serum and stimulated with 20 μg of F(ab′)2 fragment of goat anti-mouse IgM per ml for the indicated time (see Fig. 3) at 37°C. After centrifugation, cells were lysed in lysis buffer containing 1% Nonidet P-40. Whole-cell lysates corresponding to 5 × 105 cells were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) (10% polyacrylamide). The rest of the lysates (representing 2.5 × 106 cells) were incubated with either anti-Syk (a generous gift from C. A. Lowell), anti-phospholipase C-γ2 (PLCγ2) (Santa Cruz, Santa Cruz, Calif.), or anti-Grb2 (Transduction Laboratories) antibodies for 1 h and then with protein A-Sepharose (Pharmacia) for 30 min. Sepharose beads were washed three times with lysis buffer and subjected to SDS-PAGE. The proteins were transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.), incubated with PY99 antiphosphotyrosine antibody (Santa Cruz), and detected with the Supersignal System (Pierce, Rockford, Ill.). For the analysis of protein tyrosine phosphorylation, purified B cells were suspended in serum-free RPMI 1640 for 1 h and then stimulated for 15 min at 37°C with 15 μg of goat anti-mouse IgM per ml at a density of 5 × 107 cells/ml. The cells were pelleted by centrifugation and then lysed in lysis buffer containing 1% Nonidet P-40. The cell lysate was clarified by centrifugation for 10 min at 12,000 × g. Aliquots of supernatants were incubated for 2 h with 20 μg of bead-immobilized glutathione S-transferase–Blk SH2 domain fusion protein or 10 μg of bead-immobilized antiphosphotyrosine monoclonal antibody 4G10. The beads were collected by centrifugation and washed four times with lysis buffer. The pellets were boiled in SDS-PAGE loading buffer, and the protein was fractionated by electrophoresis through an SDS–8% polyacrylamide gel. The protein was transferred to nitrocellulose. Phosphotyrosine-containing proteins were detected by immunoblotting with 4G10 antibody, and the membrane-bound antibody was detected by enhanced chemiluminescence.
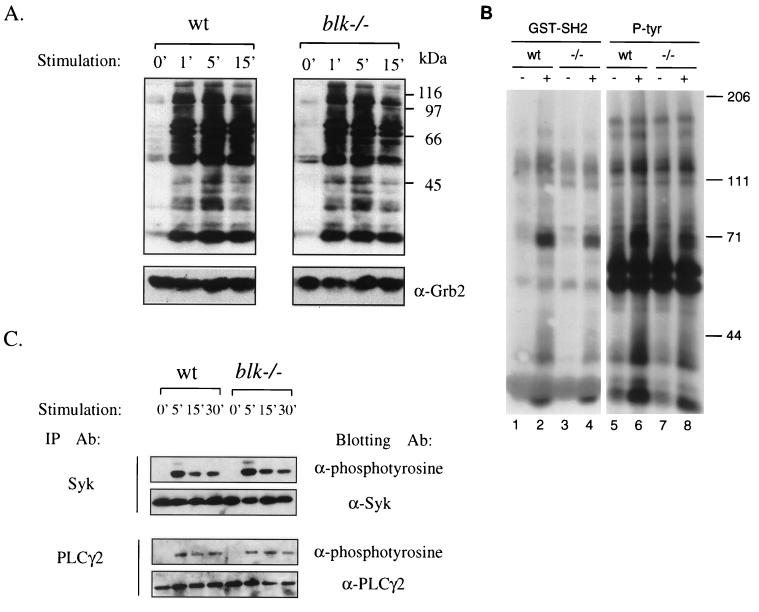
FIG. 3.
Anti-IgM-induced protein tyrosine phosphorylation in B cells. Purified splenic B cells were stimulated with medium alone or goat anti-IgM at 37°C for the indicated periods, and cell lysates were prepared. (A) Whole-cell lysates were resolved on by SDS-PAGE and transferred to polyvinylidene difluoride membranes, and phosphorylation of the transferred proteins was determined by incubation of membranes with antiphosphotyrosine antibody PY99. (B) Cell lysates of unstimulated (lanes 1, 3, 5, and 7) or anti-IgM-treated (lanes 2, 4, 6, and 8) wild-type control (wt) and Blk-deficient (−/−) purified splenic B cells were precipitated either with the glutathione S-transferase–Blk SH2 domain fusion protein (lanes 1 to 4) or with the antiphosphotyrosine monoclonal antibody 4G10 (lanes 5 to 8). Precipitated proteins were fractionated by SDS-PAGE and transferred to nitrocellulose, and phosphotyrosine-containing proteins were detected by immunoblotting with the 4G10 antibody by using the enhanced chemiluminescence system. Positions of molecular mass markers and their apparent sizes (in kilodaltons) are indicated on the right. (C) Whole-cell lysates were immunoprecipitated with anti-Syk and anti-PLCγ2 antibodies, the immunoprecipitates were resolved by SDS-PAGE, and the phosphorylation of the immunoprecipitated proteins was analyzed by immunoblotting with antiphosphotyrosine antibody PY99. Equal protein loading was verified by stripping and reprobing the immunoblots with the indicated antibodies.
Immune response to T-cell-independent and T-cell-dependent antigens.
Wild-type 129/Sv and Blk-deficient mice were immunized intraperitoneally (i.p.) with 5 μg of the T-cell-independent antigen NP25-Ficoll in PBS or 100 μg of the alum-precipitated T-cell-dependent antigen 4-hydroxy-3-nitrophenylacetyl–chicken γ-globulin conjugate (NP15-CG) (three to five mice per group). For the analysis of secondary immune responses, the NP15-CG-immunized mice were reimmunized i.p. 21 days after primary immunization. The concentration of NP-specific antibodies in serum at different time points were measured by an enzyme-linked immunosorbent assay. The assay was performed by coating plastic plates with NP15-BSA (10 μg/ml), and serial serum dilutions were applied onto the plate. Bound antibodies were revealed by using biotinylated antibodies specific for a particular isotype as described previously (19).
RESULTS AND DISCUSSION
Generation of Blk-deficient mice.
To inactivate the blk gene, E14-1.1 ES cells were transfected with the targeting construct shown in Fig. 1A. In this vector, the core portion of exon 8 of the blk gene is replaced by a neomycin resistance (Neor) gene, thereby disrupting the sequence encoding the essential part of the kinase domain of Blk. Separate clones of E14-1.1 ES cells which carried the disrupted blk gene were used to generate heterozygous (blk+/−) and homozygous (blk−/−) mutant mice (Fig. 1B). The blk mRNA expression levels in the purified splenic blk−/− B cells were below the detection limit of the Northern hybridization analysis (Fig. 1C). The RNA species carrying exons 1 to 4 of the blk gene could be detected by reverse transcription-PCR analysis of the RNA isolated from blk−/− B cells (data not shown). However, these RNAs were unable to give rise to a truncated Blk polypeptide(s) recognized by the anti-Blk antibody directed against the N-terminal portion of Blk (Fig. 1D). Furthermore, the truncated Blk proteins were not found in purified CD19+ bone marrow B-lineage cells or in purified splenic B cells activated by anti-IgM antibody alone or in combination with IL-4 (or CD40 in combination with IL-4) (data not shown). Collectively, these data show that the targeted modification of the blk gene leads to Blk deficiency in blk−/− mice.
Development of B and T cells is unaltered in Blk-deficient mice.
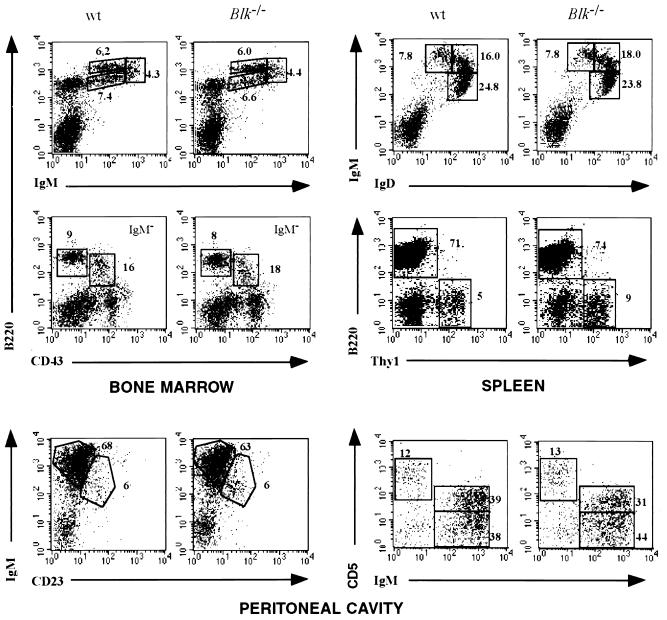
To analyze the potential influence of Blk deficiency on B-cell development and maturation, B-lineage cells from bone marrow, spleen, lymph nodes, and peritoneum of Blk-deficient mice and control 129/Sv mice were analyzed by flow cytometry. The total cell numbers and the frequency of B cells in spleen and lymph nodes were the same in Blk-deficient and control animals (data not shown). The bone marrow compartments of Blk-deficient and control mice were similar in terms of total cell number or the proportion of the pro-B cells (Ig− B220low CD43+), pre-B cells (Ig− B220low CD43−), immature B cells (IgMlo/hi B220high CD43−), and recirculating B cells (IgMlo IgDhi B220high CD43−). As in the 129/Sv control mice, about 95% of the splenic peripheral B cells expressed surface Ig containing κ chains (data not shown). The proportion of immature IgMhi IgDlo and mature IgMlo IgDhi B cells was the same in the spleen and lymph nodes of Blk-deficient and control mice (Fig. 2). Furthermore, the expression levels of surface proteins such as major histocompatibility complex class II, CD19, and CD23 were similar in wild-type and Blk-deficient splenic B cells (data not shown). Peritoneal B-1 (IgMhi B220low CD23) cells, including CD5-positive B-1a lymphocytes, were present at the same frequencies in the peritoneal cavities of control and Blk-deficient mice (Fig. 2). Collectively, these data demonstrate that development of B cells in the bone marrow and their maturation in the peripheral lymphoid organs are not dependent on Blk.
FIG. 2.
Lymphocyte populations in Blk-deficient mice. Flow cytometric analysis of bone marrow cells, splenocytes, thymocytes, and peritoneal cavity cells in 8-week-old wild-type 129/Sv mice and Blk-deficient mice is shown. Numbers indicate the percentage of gated cellular subpopulations within the lymphocyte population.
The exclusive expression of Blk in B cells has been challenged by the report on Blk expression in human thymocytes (10). Although the lack of Blk mRNA and protein expression in mouse thymocytes does not support these data (Fig. 1C), a possible effect of Blk deficiency on T-cell development was investigated. The thymuses of Blk-deficient and control mice were of equal size, and the ratios of CD4 and CD8 cells in the thymuses and spleens of Blk-deficient mice were the same as in 129/Sv control mice (data not shown). We also did not detect any difference in T-cell receptor αβ, CD3ɛ, heat-stable antigen, and CD69 expression in splenic T-cell and thymocyte subpopulations from control and Blk-deficient mice (data not shown).
Protein tyrosine phosphorylation.
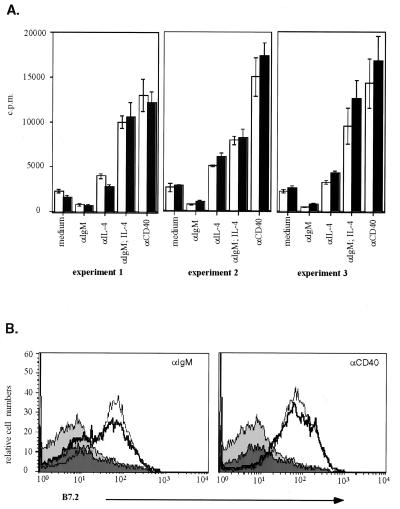
The role of Blk in BCR-induced signaling was addressed by the analysis of surface IgM-mediated tyrosine phosphorylation of intracellular proteins in purified splenic B cells. The patterns of phosphoproteins in whole-cell lysates of unstimulated and anti-IgM-treated 129/Sv control and Blk-deficient B cells were very similar (Fig. 3A). Since a deficiency of Blk could have specifically affected the phosphorylation of Blk-associated proteins, the phosphorylation of proteins which bind to the SH2 domain of Blk (Blk-SH2) was specifically analyzed. Similar to the proteins of whole-cell lysates, the phosphorylation of Blk-SH2-binding proteins was unaffected by the absence of Blk (Fig. 3B). Moreover, the anti-IgM-induced phosphorylation of known components of the BCR-dependent signaling chain such as Syk and PLCγ2 was similar in the wild-type and Blk-deficient splenic B cells (Fig. 3C). The lack of obvious changes in the pattern of the anti-IgM-induced protein tyrosine phosphorylation in the Blk-deficient B cells suggests a functional redundancy of Blk in BCR-induced B-cell activation. Indeed, antibody-mediated cross-linking of surface IgM on Blk-deficient cells led to upregulation of CD86 (B7.2) and major histocompatibility complex class II on the cell surface (data not shown) as well as to proliferation of mutant cells at levels similar to those of control cells (Fig. 4). The magnitudes of the proliferative responses of Blk-deficient and control splenic B cells to various amounts of anti-IgM were similar as well (data not shown). These data show that the Blk deficiency does not alter the threshold for anti-IgM-induced B-cell proliferation. The src-family PTKs are implicated in signal transduction mediated by B-cell-expressed surface receptor proteins such as CD38 and, to lesser extent, RP-105 (3). However, activation of Blk-deficient splenic B cells by anti-CD38 or anti-RP-105 is not impaired (3). Furthermore, proliferative responses of Blk-deficient cells to triggers of innate responses such as lipopolysaccharide or CG-rich oligonucleotides (11) are also unaltered (data not shown).
FIG. 4.
B-cell activation in vitro. (A) Proliferative responses of B cells. The amount of [3H]thymidine incorporated into the DNA of stimulated purified splenic B cells isolated from 129/Sv control (white bars) and Blk-deficient (black bars) mice is shown. All analyses were done in triplicate. (B) Upregulation of surface CD86 (B7.2). Histograms show the surface expression levels of CD86 (B7.2) on purified splenic B cells isolated from 129/Sv (thin line, light grey area) or Blk-deficient (thick line, dark grey area) mice. Cells were incubated with medium in the absence (filled area) or presence (line) of stimuli.
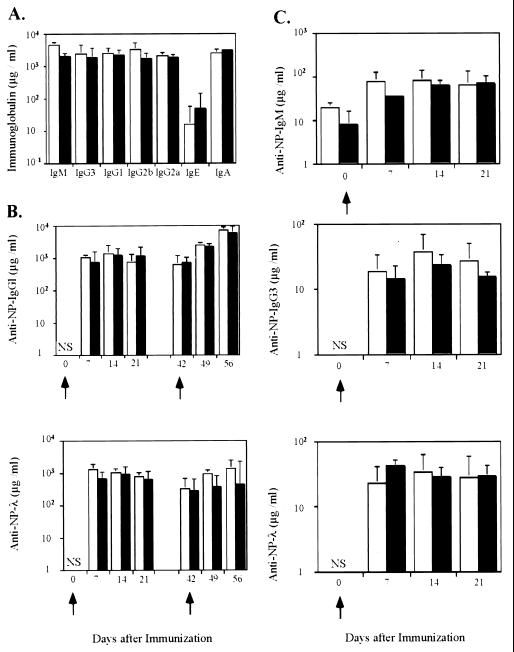
Blk-deficient mice respond efficiently to T-cell-dependent and independent antigens.
To assess the response of Blk-deficient mice to environmental antigens, the concentrations of immunoglobulins of various isotypes in the sera of mutant mice were determined. Immunoglobulins of various isotypes were present in the sera of Blk-deficient mice at levels similar to those seen in control mice (Fig. 5A). To test whether Blk-deficient B cells are able to mount an antibody response upon intentional immunization, Blk-deficient mice were immunized with the T-cell-dependent antigen NP-CG (8) and the T-cell-independent antigen NP-Ficoll (15). The concentration of hapten-binding antibodies was determined at different time points after immunization. In Blk-deficient mice, the primary response to NP, measured on days 7, 14, and 21 after immunization with NP-CG, was similar to that in control mice (Fig. 5B). Furthermore, secondary anti-hapten responses in Blk-deficient and control mice did not differ significantly (Fig. 5B). For the T cell-independent immunogen, both Blk-deficient and control mice mounted a humoral immune response at similar levels (Fig. 5C).
FIG. 5.
Serum immunoglobulin isotypes in unimmunized and immunized Blk-deficient mice. (A) Serum immunoglobulin isotypes in unimmunized control (white bars) and Blk-deficient (black bars) mice. (B) NP-specific antibodies in sera of 129/Sv and Blk-deficient mice immunized with the T-cell-dependent antigen NP-CG. Concentrations of NP-specific IgG1 (upper panel) and λ-bearing (lower panel) antibodies in sera of 129/Sv (open bars) and Blk-deficient (black bars) mice were determined at different times after immunization with NP-CG (primary response). For the analysis of the secondary immune responses, mice of the same groups were reimmunized i.p. 21 days after the primary immunization and the titers of antibodies were determined at different times thereafter. (C) NP-specific antibodies in sera of 129/Sv (open bars) and Blk-deficient (black bars) mice immunized with NP-Ficoll. The titers of NP-specific IgM, IgG3, and λ-bearing antibodies were determined on days 7, 14, and 21 after immunization with NP-Ficoll. Geometric mean values and standard errors of the mean obtained from individual sera of three to five mice per group are shown. NS, not significant.
Concluding remarks.
The experiments described here failed to reveal any defect in the immune system of Blk-deficient mice. Neither B-cell development nor B-cell responses in vitro and in vivo were altered by the lack of Blk. Although Blk is the only known B-cell-specific src-family PTK, our data suggest that Blk has no unique function in B-cell signaling and that other src-family PTKs expressed in Blk-deficient B cells can compensate for the lack of Blk. Our preliminary results on unaltered development and activation of Blk/Fyn doubly deficient B cells point to a key role of Lyn in src-family PTK-mediated B-cell functions in vivo. Analysis of Blk/Lyn doubly deficient and Blk/Fyn/Lyn triply deficient mice may elucidate the role of Blk and other src-family PTKs in B-cell signaling.
ACKNOWLEDGMENTS
We are grateful to C. A. Lowell (UCSF) for the gift of anti-Syk antibody and to D. Kitamura (Tokyo Science University) for the gift of anti-BASH antibody. We thank S. Irlenbusch and C. Uthoff-Hachenberg for technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft through SFB 243. I.M. is supported by a Graduiertenkolleg fellowship from the Deutsche Forschungsgemeinschaft. K.S. is supported by an EMBO long-term fellowship. S.D. is supported by the Howard Hughes Medical Institute and the National Cancer Institute.
G. Texido and I.-H. Su contributed equally to this work.
REFERENCES
- 1.Anderson J S, Teutsch M, Dong Z, Wortis H H. An essential role for Bruton's tyrosine kinase in the regulation of B-cell apoptosis. Proc Natl Acad Sci USA. 1996;93:10966–10971. doi: 10.1073/pnas.93.20.10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appleby M W, Kerner J D, Chien S, Maliszewski C R, Bondada S, Perlmutter R M, Bondadaa S. Involvement of p59fynT in interleukin-5 receptor signaling. J Exp Med. 1995;182:811–820. doi: 10.1084/jem.182.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan V W, Mecklenbräuker I, Su I, Texido G, Leitges M, Carsetti R, Lowell C A, Rajewsky K, Miyake K, Tarakhovsky A. The molecular mechanism of B cell activation by toll-like receptor protein RP-105. J Exp Med. 1998;188:93–101. doi: 10.1084/jem.188.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan V W, Meng F, Soriano P, DeFranco A L, Lowell C A. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 5.Cheng A M, Rowley B, Pao W, Hayday A, Bolen J B, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378:303–306. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 6.Dymecki S M, Niederhuber J E, Desiderio S V. Specific expression of a tyrosine kinase gene, blk, in B lymphoid cells. Science. 1990;247:332–336. doi: 10.1126/science.2404338. [DOI] [PubMed] [Google Scholar]
- 7.Fluckiger A C, Li Z, Kato R M, Wahl M I, Ochs H D, Longnecker R, Kinet J P, Witte O N, Scharenberg A M, Rawlings D J. Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J. 1998;17:1973–1985. doi: 10.1093/emboj/17.7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Förster I, Rajewsky K. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur J Immunol. 1987;17:521–528. doi: 10.1002/eji.1830170414. [DOI] [PubMed] [Google Scholar]
- 9.Hibbs M L, Tarlinton D M, Armes J, Grail D, Hodgson G, Maglitto R, Stacker S A, Dunn A R. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 10.Islam K B, Rabbani H, Larsson C, Sanders R, Smith C I. Molecular cloning, characterization, and chromosomal localization of a human lymphoid tyrosine kinase related to murine Blk. J Immunol. 1995;154:1265–1272. [PubMed] [Google Scholar]
- 11.Krieg A M. An innate immune defense mechanism based on the recognition of CpG motifs in microbial DNA. J Lab Clin Med. 1996;128:128–133. doi: 10.1016/s0022-2143(96)90004-9. [DOI] [PubMed] [Google Scholar]
- 12.Kühn R, Rajewsky K, Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 13.Kurosaki T, Takata M, Yamanashi Y, Inazu T, Taniguchi T, Yamamoto T, Yamamura H. Syk activation by the Src-family tyrosine kinase in the B cell receptor signaling. J Exp Med. 1994;179:1725–1729. doi: 10.1084/jem.179.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leitges M, Schmedt C, Guinamard R, Davoust J, Schaal S, Stabel S, Tarakhovsky A. Immunodeficiency in protein kinase c β-deficient mice. Science. 1996;273:788–791. doi: 10.1126/science.273.5276.788. [DOI] [PubMed] [Google Scholar]
- 15.Maizels N, Lau J C, Blier P R, Bothwell A. The T-cell independent antigen, NP-ficoll, primes for a high affinity IgM anti-NP response. Mol Immunol. 1988;25:1277–1282. doi: 10.1016/0161-5890(88)90042-9. [DOI] [PubMed] [Google Scholar]
- 16.Malek S N, Dordai D I, Reim J, Dintzis H, Desiderio S. Malignant transformation of early lymphoid progenitors in mice expressing an activated Blk tyrosine kinase. Proc Natl Acad Sci USA. 1998;95:7351–7356. doi: 10.1073/pnas.95.13.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miltenyi S, Müller W, Weichel W, Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11:231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- 18.Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 19.Rickert R C, Rajewsky K, Roes J. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature. 1995;376:352–355. doi: 10.1038/376352a0. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook T, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 21.Saouaf S J, Mahajan S, Rowley R B, Kut S A, Fargnoli J, Burkhardt A L, Tsukada S, Witte O N, Bolen J B. Temporal differences in the activation of three classes of non-transmembrane protein tyrosine kinases following B-cell antigen receptor surface engagement. Proc Natl Acad Sci USA. 1994;91:9524–9528. doi: 10.1073/pnas.91.20.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sefton B M, Taddie J A. Role of tyrosine kinases in lymphocyte activation. Curr Opin Immunol. 1994;6:372–379. doi: 10.1016/0952-7915(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 23.Takata M, Kurosaki T. A role for Bruton's tyrosine kinase in B cell antigen receptor-mediated activation of phospholipase C-γ2. J Exp Med. 1996;184:31–40. doi: 10.1084/jem.184.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wasserman R, Li Y S, Hardy R R. Differential expression of the blk and ret tyrosine kinases during B lineage development is dependent on Ig rearrangement. J Immunol. 1995;155:644–651. [PubMed] [Google Scholar]
- 25.Yao X R, Scott D W. Antisense oligodeoxynucleotides to the blk tyrosine kinase prevent anti-mu-chain-mediated growth inhibition and apoptosis in a B-cell lymphoma. Proc Natl Acad Sci USA. 1993;90:7946–7950. doi: 10.1073/pnas.90.17.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yasue T, Nishizumi H, Aizawa S, Yamamoto T, Miyake K, Mizoguchi C, Uehara S, Kikuchi Y, Takatsu K. A critical role of Lyn and Fyn for B cell responses to CD38 ligation and interleukin 5. Proc Natl Acad Sci USA. 1997;94:10307–10312. doi: 10.1073/pnas.94.19.10307. [DOI] [PMC free article] [PubMed] [Google Scholar]