Abstract
The circadian clock evolved in diverse organisms to integrate external environmental changes and internal physiology. The clock endows the host with temporal precision and robust adaptation to the surrounding environment. When circadian rhythms are perturbed or misaligned, as a result of jet lag, shiftwork or other lifestyle factors, adverse health consequences arise, and the risks of diseases such as cancer, cardiovascular diseases or metabolic disorders increase. Although the negative impact of circadian rhythm disruption is now well established, it remains underappreciated how to take advantage of biological timing, or correct it, for health benefits. In this Review, we provide an updated account of the circadian system and highlight several key disease areas with altered circadian signalling. We discuss environmental and lifestyle modifications of circadian rhythm and clock-based therapeutic strategies, including chronotherapy, in which dosing time is deliberately optimized for maximum therapeutic index, and pharmacological agents that target core clock components and proximal regulators. Promising progress in research, disease models and clinical applications should encourage a concerted effort towards a new era of circadian medicine.
In 1729, Jean-Jacques de Mairan documented that leaves of mimosa plants open and close with a daily rhythm, even in constant darkness. Such self-sustained rhythms suggested a so-called free-running, intrinsic biological timer as opposed to a passive response to environmental changes. Different Drosophila mutants with altered periodicity showed mutations that were mapped to the same locus, which was subsequently named period. The later cloning of the gene1,2, followed by the demonstration of negative-feedback regulation in Drosophila3, was ultimately proven to be a Nobel Prize-worthy discovery. We now have detailed knowledge of the genetic and molecular make-up of the biological timer in virtually all kingdoms of life, from cyanobacteria to humans4. It is generally believed that the biological timer, called circadian (from Latin circa, meaning ‘approximately’, and dies, meaning ‘day’) clock, has evolved independently in a convergent process to adapt to and use the daily geophysical cycle for maximum survival and competitive advantage. The endogenous period length is constrained by the natural environmental cycles dictated by Earth’s rotation, and remarkably complex regulatory mechanisms have evolved to couple internal and external rhythms to ensure precise periodicity4. Clocks are readily responsive to a broad range of entraining signals, or so-called zeitgebers (German for ‘time givers’), that determine or readjust the phase or period of the circadian rhythm. In addition to light, which is the dominant zeitgeber for photosensitive clocks, other external (such as feeding and exercise)5 or intracellular (temperature)6 cues can reset, or entrain, the endogenous rhythms.
Zeitgebers.
A German word (meaning ‘time giver’), from Zeit (meaning ‘time’) and Geber (meaning ‘giver’). It refers to any external or intracellular cues that can reset or entrain an organism’s biological rhythms to the 24-hour day–night cycle of Earth.
Proper circadian rhythms confer a myriad of cell growth and survival advantages. Mirroring results from laboratory hamsters7, lesions of the suprachiasmatic nucleus (SCN) — the master pacemaker in the hypothalamus — in wild rodents shorten their lifespan8. Although circadian disruption does not lead to acute death in animal models or humans, short-term and long-term adverse effects on fitness and health can result from circadian disturbances9. The repertoire of clock-targeting therapeutic strategies now extends beyond the traditional chronotherapeutic applications10. In this Review, we will provide an up-to-date account of circadian timing systems in mammals, highlight several diseases closely linked with clock mechanisms and review interventional strategies and therapeutic pipelines that target the clock.
The circadian rhythm in mammals
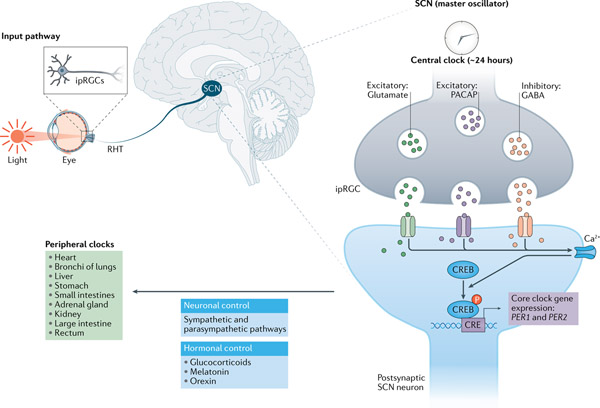
A circadian system consists of three components: inputs, the oscillator and outputs4. This basic design principle operates at two levels, systemic and cellular4. At the systemic scale (FIG. 1), light is a major input signal for the circadian system and resets the master pacemaker, the SCN11. A defining feature of the SCN is the robust networking among its oscillator cells compared with that in peripheral tissues12. For example, ex vivo SCN culture can sustain the rhythmicity of reporter expression for more than 1 year13, while brain regions outside the SCN show a loss or dampened rhythmicity in 120 hours14. The main function of the SCN is to coordinate other brain areas and peripheral tissues throughout the body by neural and hormonal signals15. Furthermore, the SCN also regulates the circadian secretion of diffusible endocrine signals, such as melatonin from the pineal gland, and glucocorticoids and catecholamines from the adrenal cortex, via the hypothalamic–pituitary–adrenal axis16,17.
Fig. 1 |. Hierarchical organization of the mammalian clock system.
The central pacemaker is located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus. In the input pathway to the SCN, light is received by the intrinsically photosensitive retinal ganglion cells (ipRGCs) expressing melanopsin, which sends electric signals to the SCN through the retinohypothalamic tract (RHT)203. Neurotransmitters released by ipRGCs, such as excitatory glutamate and pituitary adenylate cyclase-activating polypeptide (PACAP), cause membrane depolarization in postsynaptic SCN neurons. Changes in Ca2+ and cAMP levels, in turn, induce phosphorylation of cAMP-response element (CRE)-binding protein (CREB) and expression of immediate early genes including the core clock genes, PER1 and PER2, thereby resetting SCN cellular oscillators240. Meanwhile, the inhibitory neurotransmitter γ-aminobutyric acid (GABA) decreases the sensitivity of non-image-forming behaviours at low light levels241. The SCN neurons are tightly coupled and control peripheral clocks in other brain regions and throughout the body via neuronal and hormonal signals.
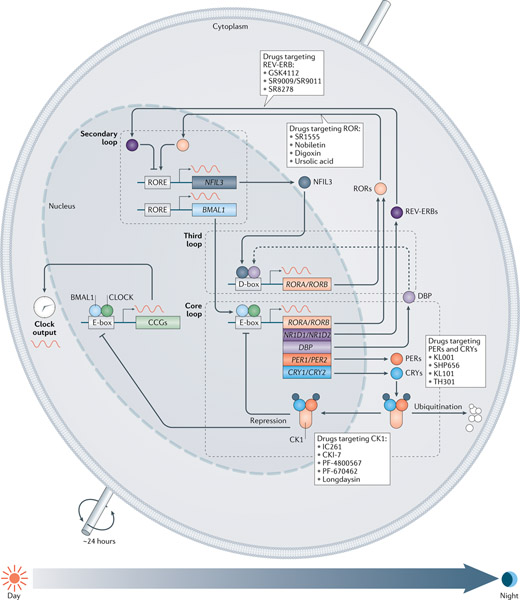
At the cellular level, the ubiquitous, cell-autonomous molecular oscillator (FIG. 2) is composed of interconnected negative-feedback loops4,15,18. In mammals, the transcription factors circadian locomotor output cycles kaput (CLOCK)19 and neuronal PAS domain-containing protein 2 (NPAS2) form heterodimers with aryl hydrocarbon receptor nuclear translocator-like protein 1 (ARNTL; also known as BMAL1)20 to drive the expression of the genes encoding period circadian protein homologues 1, 2 and 3 (PER1, PER2 and PER3) and cryptochromes 1 and 2 (CRY1 and CRY2) via direct binding to the E-box enhancer element in the daytime18. In the late afternoon or evening, PER and CRY proteins heterodimerize (and also interact with casein kinase 1δ (CK1δ) and CK1ε), translocate into the nucleus and interact with CLOCK and BMAL1, thus suppressing their transcriptional activity18. In the meantime, the protein levels of PER1, PER2, CRY1 and CRY2 also decline by polyubiquitination and subsequent degradation via specific E3 ligase complexes (β-TrCP and FBXL3 (REFS21–23), respectively). With the gradual relief of this negative-feedback repression, CLOCK–BMAL1 transcription activity can be restored, and a new cycle will be resumed the next morning. This core feedback cycle takes approximately 24 hours. Two families of nuclear receptors function to stabilize the core loop and govern output transcription in a distinct phase. REV-ERBα and REV-ERBβ24 (encoded by NR1D1 and NR1D2, respectively) and RAR-related orphan receptors α, β and γ (RORα, RORβ and RORγ)25, which are also direct targets of CLOCK–BMAL1, antagonistically regulate BMAL1 by competitively binding to ROR/REV-ERB-response elements (RORE) and cause an antiphase oscillation between BMAL1 and PER2. A third transcriptional loop involves the proline and acidic amino acid-rich basic leucine zipper (PAR-bZIP) proteins (DBP, TEF and HLF) and the repressor E4 promoter-binding protein 4 (E4BP4; also known as NFIL3)15, which competitively bind to the D-boxes, and are driven by the CLOCK–BMAL1 loop or the REV-ERB–ROR loop, respectively.
Fig. 2 |. The cell-autonomous core components of the circadian oscillator govern the ~24-hour cycle of gene expression.
The oscillator consists of positive-feedback and negative-feedback loops that intersect via transcriptional and post-transcriptional regulatory mechanisms4. In the core loop, circadian locomotor output cycles kaput (CLOCK) and aryl hydrocarbon receptor nuclear translocator-like protein 1 (BMAL1, encoded by ARNTL) heterodimerize to drive expression of period circadian protein homologue (PER) and cryptochrome (CRY) genes via the E-box elements in the morning. PER and CRY proteins subsequently form a repressive complex to inhibit CLOCK and BMAL1 transactivation. The stability of the PER and CRY proteins is regulated by parallel E3 ubiquitin ligase pathways21–23. In the secondary, or stabilization, loop two subfamilies of nuclear receptors, REV-ERBα and REV-ERBβ (encoded by NR1D1 and NR1D2, respectively) as repressors and RAR-related orphan receptors (RORs) α, β and γ as activators, antagonistically regulate BMAL1 and other target genes at the ROR/REV-ERB-response element (RORE) promoter elements at night-time24. Another key circadian promoter element is the D-box, activated by the proline and acidic amino acid-rich basic leucine zipper (PAR-bZIP) proteins (such as D-box binding PAR bZIP transcription factor; DBP) and repressed by E4 promoter-binding protein 4 (E4BP4; also known as NFIL3)15. Together, clock-controlled genes (CCGs) are transcriptionally regulated by the three loops via activation of the E-box, RORE and D-box elements in their gene promoter regions. Various ligands (PER/CRY, ROR and REV-ERB ligands) and small molecules (casein kinase 1 (CK1) inhibitors) have been found to regulate core clock components and circadian functions.
Together, these three interlocking feedback loops can drive the expression of a large number of target genes by binding to the cis elements (E-box, RORE and D-box) in their promoters and enhancer elements15 (FIG. 2). These genes, called ‘clock-controlled genes’, are highly tissue specific, and exhibit markedly different distributions of circadian phases in the two different tissues26,27. Moreover, in mice, an examination of 12 tissues revealed that 43% of all genes oscillate in at least one tissue28. Remarkably, increased profiling to 64 tissues and brain regions in male baboons showed that the proportion of protein-coding oscillatory genes in at least one tissue is more than 80%, establishing an overwhelming prevalence of circadian control of gene expression29. The tissue-specific rhythmic gene expression is jointly controlled by both central and local circadian clocks.
Circadian gene regulation is a complex, temporally orchestrated process that involves not only the circadian factors mentioned above but also a growing list of secondary or cell type-specific transcription factors, transcription co-regulators and epigenetic activities4. The mechanistic basis for tissue-specific circadian expression is emerging30,31. For example, hepatocyte nuclear factor 4α (HNF4A) transcript is rhythmically regulated in the mouse liver and modulates circadian rhythms through repression of CLOCK–BMAL1 activity30. Furthermore, transcriptomic analysis of islet cells revealed the circadian expression of genes involved in insulin secretion31. Epigenetic regulation is also an active area of research, and histone modifications, chromatin remodelling and topological organization have significant impact on the regulation of both E-box and RORE elements4,32. Recent evidence has suggested that, besides transcriptional regulation, post-transcriptional and post-translational mechanisms also have major regulatory roles in rhythmic physiology in all organisms21,33,34.
Melatonin and orexin signalling
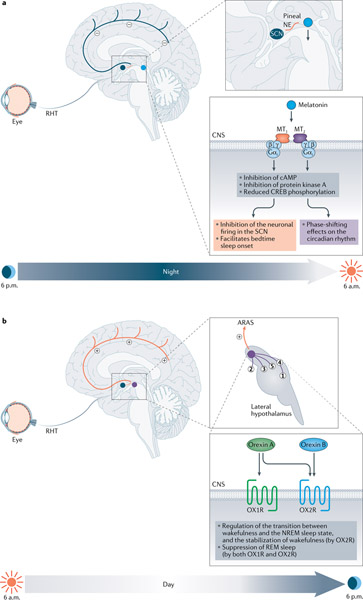
The sleep–wake cycle is the most profound circadian rhythm and also a tightly orchestrated physiological process regulated by both sleep-promoting and arousal centres in the brain35. In all mammals, either nocturnal or diurnal, the rhythmic secretion of melatonin (5-methoxy-N-acetyltryptamine) is modulated solely by the input of light and the circadian clock in the SCN, not by physical activity36. During the day, when the SCN is activated by light input from the retina, melatonin production is inhibited. When light conditions turn low, the SCN triggers a neural output signal to induce the synthesis and release of melatonin36. Unlike its sleep-promoting effects in humans, melatonin administration failed to promote sleep in nocturnal animals37 owing to the different mechanisms for circadian regulation of sleep. Therefore, melatonin levels are high at night in both diurnal (humans) and nocturnal species even though they have opposite activity periods. As an important endocrine signal that conveys the circadian information from the SCN to the peripheral tissues, melatonin functions mainly through the activation of two G-protein-coupled receptors, melatonin receptor 1 (MTNR1A; also known as MT1 receptor) and melatonin receptor 2 (MTNR1B; also known as MT2 receptor), which differ in terms of tissue distribution and the regulatory effects on sleep and circadian rhythms38,39. The MT1 receptor is predominantly expressed in the SCN and is crucial for the inhibition of neuronal firing in the SCN, and thereby facilitates bedtime sleep onset38. Besides its direct effect on sleep, melatonin also exerts a phase-shifting function in the circadian rhythm, through MT2 receptors in the SCN38,40,41 (FIG. 3a). Notably, this phase-shifting effect of melatonin on circadian rhythms is thought to be independent of the modulation of circadian gene transcription in the SCN, given that injection of melatonin in rats did not result in immediate changes in the expression of circadian genes — such as Per1, Per2, Per3, Cry1 and Bmal1 — in the SCN42. However, some circadian rhythm oscillators are potential targets of melatonin. In an experimental mouse model of jet lag, both adaptation of PER1 and CRY1 to the new light–dark cycle in the SCN and the re-entrainment of locomotor activity rhythm were more profound in melatonin-proficient mice with intact MT2 receptors43. Melatonin injections not only induced a phase advance in the Nr1d1 mRNA expression rhythm in rats but also phase-shifted Bmal1 mRNA expression, suggesting a link, although perhaps not direct, between melatonin and the core molecular circadian clock44. Collectively, melatonin is not only an important physiological sleep regulator but also serves as a time cue to synchronize the circadian physiological and behavioural rhythms, such as sleep–wake and locomotor activity, respectively.
Fig. 3 |. Melatonin and orexin signalling in circadian rhythm.
a | Melatonin promotes sleep at night: the nightly release of noradrenaline (NE; in orange) stimulated by the suprachiasmatic nucleus (SCN) induces melatonin synthesis in the pineal gland (blue circle)36. Melatonin functions through activation of two G-protein-coupled receptors, melatonin receptor 1 (MT1) and melatonin receptor 2 (MT2) mainly in the SCN38,39. By interacting with pertussis toxin-sensitive Gαi, MT1 receptor can inhibit cAMP-response element-binding protein (CREB) phosphorylation, thus is crucial for the suppression (minus symbols) of neuronal firing, whereas MT2 receptor exerts a phase-shifting effect38,40,41 and shortens the circadian period of bioluminescence in SCN explant cultures from Clock/+ mutant mice242. b | Orexin promotes wakefulness during daytime. The SCN receives signals from light through the retinohypothalamic tract (RHT) and activates orexinergic neurons (purple circle) in the lateral hypothalamic area46. Activated orexinergic neurons release orexin A and orexin B and project signals by binding to orexin receptor 1 (OX1R) and orexin receptor 2 (OX2R) in various neurons located throughout the central nervous system (CNS): (1) the locus coeruleus; (2) the tuberomammillary nucleus; (3) the dorsal raphe and median raphe nuclei; (4) the laterodorsal tegmental nucleus; and (5) the pedunculopontine tegmental nucleus47. Together, these activating neurons constitute the ascending reticular activating system (ARAS) and directly activate (plus symbols) the cortex, thus promoting wakefulness. Furthermore, OX2R has a major role in stabilization of wakefulness and suppression of sleep. NREM sleep, non-rapid-eye-movement sleep; REM sleep, rapid eye movement sleep.
Phase advance.
A phase shift in the circadian rhythm whereby the bedtime and wake-up time will move earlier in the day.
Orexins (orexin A and orexin B), also known as hypocretins, are a pair of excitatory neuropeptides that share 46% homology45. The levels of orexin A and orexin B, produced in orexinergic neurons located in the lateral hypothalamus, oscillate with the daily rhythm and peak in the awake phase under the control of the SCN circadian clock46. Orexins promote arousal and wakefulness by binding to the G-protein-coupled receptors orexin receptor 1 (OX1R) and orexin receptor 2 (OX2R), which are widely and differentially distributed. They are expressed at high levels mainly throughout the central nervous system (FIG. 3b) and at relatively low levels in the peripheral organs (adrenal glands, testes and jejunum)47. Activation of orexinergic neurons gives rise to a rapid transition from sleep (non-rapid-eye-movement (NREM) sleep or rapid eye movement (REM) sleep) to wakefulness48. During this process, OX2R has a major role in stabilization of wakefulness by reducing the transition between the NREM sleep state and wakefulness, whereas OX1R and OX2R both contribute to suppression of REM sleep48. Furthermore, recent studies have started to show a reciprocal regulation between the SCN clock cells and the orexinergic neurons. For example, in mice, orexin was found to predominantly suppress expression of PER1-enhanced green fluorescent protein (GFP) in clock cells in the SCN via presynaptic modulation of GABAergic signalling in the daytime and direct activation of leak K+ currents in the evening49. Thus, although orexin did not directly affect the clock phase, it may dampen the SCN control of the circadian rhythm by acutely and temporarily suppressing clock cells in SCN.
Non-rapid-eye-movement sleep.
Also known as quiescent sleep, with little or no eye movement, rare dreaming and less muscle paralysation than in rapid eye movement sleep.
Rapid eye movement sleep.
Also known as paradoxical sleep, a unique phase of sleep in mammals and birds characterized by rapid eye movement, low muscle tone throughout the body and vivid dreaming sometimes.
Circadian rhythm signalling in diseases
There are several ways in which diseases are associated with circadian components or rhythms. First, various symptoms or disease onsets display clear circadian preference. Among the best-known examples are the early morning peak of myocardial infarction and stroke (due to rapid rise of blood pressure) and night-time exacerbation of asthma and other inflammatory diseases17,50,51. Some people experiences seasonal and circadian clusters of excruciating headache attacks, with remarkable circadian precision of headache episodes (similar time each day, within minutes)52. Furthermore, in mouse models, sundown syndrome, which is often observed in patients with mid-stage Alzheimer disease, is characterized by significant behavioural perturbations in late afternoon and evening53. Secondly, besides circadian onset of certain diseases, different disease states and natural ageing correlate with altered circadian rhythms, including the alternation in the expression of circadian genes, sleep–wake cycles and rhythms of key biomarkers such as melatonin and cortisol9,54. Although disease-related circadian dysfunctions are often complex, certain disorders display a primary circadian dysfunction. For example, sleep disorders and jet lag are associated mainly with phase dissociation (in this case, also known as dissociation of circadian rhythm and light), whereas ageing, neurodegenerative diseases and metabolic disease all seem to be affected by attenuated or disrupted circadian rhythms5,54–56. Particularly, patients with Alzheimer disease initially exhibit out-of-sync sleep patterns and sleep fragmentations; as the disease progresses, the loss of sleep patterns becomes the primary driver for institutionalization56. Lastly, human genetic and genomic studies have uncovered direct links between gene mutations and circadian-related diseases. In addition to the mutations associated with familial sleep disorders, genome-wide association studies have also shown polymorphisms in circadian genes that correlate with diseases. For example, mutations in MTNR1B correlated strongly with elevated fasting glucose levels and type 2 diabetes risk in a cohort study57,58. In the following subsections, we describe several disease areas with significant research progress on circadian relevance.
Phase dissociation.
In the context of this Review, the dissociation of intrinsic circadian rhythm and the environmental light–dark cycle, as in jet lag and sleep disorders.
Circadian genes and sleeping disorders
Circadian components have important roles in the regulation of sleep timing59,60. Several human mutations have been associated with circadian sleep disorders, most notably in genes encoding PERs and casein kinases that phosphorylate PERs before degradation60. For example, mutations in PER2 (PER2-S662G) and CSNK1D (CK1δ-T44A) have been identified in familial advanced sleep phase syndrome, in which patients show significant circadian period shortening and early bedtime60. Studies of mouse models expressing these mutant forms validated the causality in the disease and revealed an important role of a PER phosphorylation cascade in gating sleep timing61. More recently, a missense mutation in CRY2 was identified in a human family with familial advanced sleep phase syndrome with marked morning preference and early melatonin onset, and the same mutation led to shortened periods in a mouse model62. Genetically engineering the mutant human CRY2 gene in mice led to elevated binding of CRY-targeting E3 ligase FBXL3, resulting in the degradation of CRY2 that is responsible for the period shortening62,63. Delayed sleep phase syndrome is the most common circadian sleep disorder in humans64, with an average bedtime onset in the early morning (around 3 a.m.). Previously, variants of human PER3 gene were found to correlate with delayed sleep phase syndrome65. More recent studies in a family with delayed sleep phase syndrome revealed an A-to-C mutation in a splice site following exon 11 of the CRY1 gene66. The mutant allele led to exon 11 skipping, producing a more potent CRY1 form (CRY1Δ11) that interacts with CLOCK and BMAL1 with greater affinity than the wild-type protein and consequently greater inhibitory activity.
Delayed sleep phase syndrome.
A circadian sleep disorder in which a person’s sleep is delayed by 2 hours or more beyond what is considered a conventional bedtime, thus causing difficulty in waking up in the morning.
Ischaemia and reperfusion injury
Ischaemia and reperfusion injury typically occurs as obstruction of blood flow to an organ in a wide range of diseases, including myocardial infarction, ischaemic stroke, liver injury and acute kidney injury67,68. Once the arterial obstruction is alleviated, reperfusion of the ischaemic tissue occurs, which can cause an even stronger inflammatory response and additional collateral tissue injury68. During ischaemia, the organ becomes profoundly hypoxic and hypoxia-inducible transcription factors are stabilized68,69. In many instances, this stabilization of hypoxia-inducible transcription factors enables the ischaemic tissues to adapt to limited oxygen availability — for example by transcriptionally enhancing the glycolytic capacity or by attenuating hypoxia-driven inflammation70,71. Daytime variations of the severity of ischaemia and reperfusion injury could be linked to an interaction between circadian rhythm and hypoxia signalling72,73. Initial evidence comes from studies of Per2- and Hif1a-mutant mice that show an abolished circadian rhythmicity of HIF1α following Per2 deletion74. Per2−/− mice have significantly larger myocardial infarct sizes and are not protected by ischaemic preconditioning74 — an experimental strategy that alleviates myocardial injury and which is regulated by hypoxia signalling75. Different molecular strategies have been attempted to enhance circadian rhythm signalling for the treatment of ischaemia and reperfusion injury in animal models, including agonists of purinergic receptors — such as the A2B adenosine receptor74,76 — intense light therapy and blue light therapy74,77,78. Intense light therapy is associated with higher cardiac PER2 levels, increased glycolytic capacity and significantly attenuated myocardial infarct sizes in a murine model of myocardial ischaemia and reperfusion injury74. In mice with endothelial-specific deletion of Per2, light therapy may function to promote endothelial barrier function via light-enhanced Hif1a-mediated transcription of glycolytic genes78. Similarly, in mice, carbon monoxide can protect the kidneys from ischaemia and reperfusion injury through a mechanism involving purinergic receptors, including the A2B adenosine receptor, and PER2 (REF.76). Given these observations, clinical studies that investigate pharmacological targeting of circadian signalling as therapy for ischaemia and reperfusion injury seem justified. This could be particularly promising in the field of perioperative medicine, in which patients who are undergoing elective surgery can be pretreated with circadian therapeutics before an operation to attenuate subsequent ischaemia and reperfusion injury. For example, intense light therapy before cardiac surgery could be used as an intervention to provide cardioprotection from ischaemia and reperfusion injury.
Ischaemic preconditioning.
An experimental technique (usually via repeated short episodes of ischaemia via coronary artery occlusion) to increase tolerance to the loss of blood supply, and thus oxygen, if there is a subsequent prolonged insult.
The severity of ischaemia and reperfusion injury has been linked to the molecular clock79. Most studies — but not all — report larger infarct sizes or a higher incidence of heart failure following myocardial infarction in the early morning rather than at later times in the day80,81. However, daytime variations of perioperative myocardial injury may occur in patients undergoing cardiac surgery82. In a cohort study of 596 patients who underwent surgical aortic valve replacement in either the morning or the afternoon, patients with afternoon surgery had lower incidences of major adverse cardiac events in a 500-day follow-up period82. In addition, in 88 patients randomly assigned to morning surgery (n = 44) or afternoon surgery (n = 44), patients assigned to afternoon aortic valve replacement surgery had a lower level of myocardial injury marker as assessed by perioperative cardiac troponin T release (with an estimated ratio for afternoon to morning of 0.79)82. Therefore, myocardial ischaemia and reperfusion injury likely has a circadian rhythm pattern, and optimization of surgery clock time based on the circadian rhythmicity of susceptibility to ischaemia and reperfusion injury will have to be further evaluated in clinical studies, as well as pharmacological approaches to enhance circadian rhythm signalling during ischaemia and reperfusion.
Cancer
Since the early twentieth century, studies have indicated the potential connections between mammalian circadian rhythms and cell division cycles. In 1917, Droogleever Fortuyn-van Leijden described difficulty in observing mitosis in growing tissues from a cat particularly late at night83. As of today, epidemiological and genetic studies link disruption of circadian clock functions to increased risk of several types of cancer. In the past decade, it has become clear that circadian clock components influence cell growth and transformation in a cell-autonomous manner. Earlier studies showed circadian regulation of cell proliferation and DNA damage repair84. For example, partial hepatectomy in mice led to liver regeneration marked by a night-time control of mitosis, through a mechanism by which CLOCK and BMAL1 directly regulated expression of Wee1 mRNA85. In addition, PER1 interacts with ataxia telangiectasia mutated (ATM) serine/threonine kinase proteins and regulates checkpoint kinase 2 (CHK2)-dependent DNA damage response, which may be an evolutionarily conserved mechanism86. Furthermore, mice with an in-frame deletion of Per2 showed a predisposition to radiation-induced tumorigenesis, and dysregulated expression of multiple genes involved in cell cycle and DNA damage response, including Myc, which is directly controlled by BMAL1 (REF.87).
More recent studies have further delineated a causal role of circadian disruption in tumorigenesis. A chronic jet lag paradigm (8-hour phase shift) led to reduced lifespan in mice, dysregulated gene expression and dysfunctional metabolism in the liver, which eventually led to hepatocellular carcinoma (HCC)88. The bile acid pathway is closely involved in this process, as disruption of farnesoid X receptor increased jet lag-induced HCC, whereas loss of the tumour promoter constitutive androstane receptor (CAR) inhibited hepatocarcinogenesis88. Furthermore, gene expression analysis of HCC tumour specimens by use of the program CYCLOPS showed a largely normal oscillation of core clock genes, in contrast to significant alteration of genes involved in the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway, apoptotic pathways and metabolic output89. Another mouse study found that both experimental jet lag and disruption of Per2 and Bmal1 exacerbate lung tumorigenesis90. CRY2, as a component of the FBXL3–E3 ligase complex, also promotes ubiquitination and degradation of MYC in primary mouse embryonic fibroblast by interacting with MYC phosphorylated on Thr58 (REF.91). In human cancer cell lines, such as SW480 and A549, overexpression of CRY2 reduces MYC protein levels and inhibits cell growth91. However, in some instances, circadian genes may also aggravate cancer progression. For example, Cry1- and Cry2-double-knockout mice showed inhibition of age-adjusted tumour development and extended lifespan in the absence of tumour suppressor p53 (REF.92). In addition, a recent functional small interfering RNA screen showed that Clock and Bmal1 are necessary factors for murine acute myeloid leukaemia cell growth in vitro and in vivo93. Furthermore, genetic knockout or knockdown of BMAL1 and CLOCK in patient-derived glioblastoma stem cells significantly impedes cell growth in vitro and inhibits intracranial tumour formation in mice in vivo94. Finally, the impact of polymorphisms of the circadian rhythm genes on cancer development is highly dependent on the specific polymorphism and types of cancer95. From the findings taken together, clock genes have disparate roles in different types of cancer, and further studies are required to investigate how the distinct circadian phenotypes converge on tumorigenesis.
Cancer or cancer-causing processes can reciprocally impact circadian rhythms. Gamma irradiation, a common mutagen, was found to phase-advance mouse wheel-running behaviour when administered in vivo96. Moreover, although lung adenocarcinoma has little impact on the core clock, it strongly alters the circadian transcriptome and metabolome in the liver, resulting in disrupted metabolic homeostasis of tumour-bearing mice97. A recent study further illustrated a reciprocal control of MYC on circadian rhythms. Specifically, MYC induces REV-ERB gene expression via the consensus E-box on its promoter, which subsequently dampens the expression and oscillation of BMAL1 in neuroblastoma cell lines, and alters cell metabolism, including glucose metabolism and glutaminolysis in U2OS cells98. Although it is an attractive idea that a healthy circadian clock or robust circadian rhythms facilitate tumour suppression, the specific contribution of individual components may vary depending on the cancer type and genetic background, and additional mechanistic studies will be important to further define the molecular roles of circadian rhythm signalling in tumorigenesis.
Metabolism and metabolic disorders
Energy metabolism is tightly linked with the natural daily rhythms of sleep–fasting and wake–feeding–activity cycles. Epidemiological evidence supports a greater risk of metabolic syndrome, including increased body mass index and insulin resistance, in shift workers with sleep deprivation99. Small-cohort human studies in laboratory circadian misalignment (recurring 28-hour ‘days’ or shiftwork) settings have shown acute effects of circadian desynchronization on metabolic fitness, such as dysregulated levels of glucose, insulin and leptin in circulation100, and diminished resting metabolic rate101 (BOX 1).
Box 1. Circadian parameters on disease progression.
Numerous studies show that alterations of cardinal circadian parameters — period, phase and amplitude — are not only risk factors for diseases but are also closely related to disease progression. The difference between peak and trough values is the amplitude, and the time interval between two peaks or two troughs is called the ‘period’. The phase is defined as the timing of a reference event relative to a fixed activity (such as the beginning of daytime). Shiftwork, characterized by circadian misalignment (mainly phase misalignment), serves as a good example. Prevalent health problems can arise from this chronic circadian rhythm disruption, including sleep–wake and metabolic disorders, increased risk of several cancers (such as breast cancer) and cardiovascular disease100,175,243. In addition to phase shift, experimental animal models103 and epidemiological data244 also indicate a pivotal role of dampened circadian amplitude in metabolic disorders. ClockΔ19/Δ19 mice — which have a metabolic syndrome of hyperlipidemia, hepatic steatosis, hyperglycaemia and hypoinsulinaemia — show reduced levels of expression of circadian genes and diurnal feeding rhythm103. Furthermore, circadian dysregulation and cancer progression are also interdependent. Disturbance of circadian rhythm either locally or systemically is associated with reduced DNA repair, increased cancer cell proliferation and growth in vitro245, and higher cancer incidence and poor prognosis in vivo175,246. In mice, both physiological disruption (experimental jet lag) and genetic disruption (mutations in Per2 and Bmal1) of the circadian machinery result in decreased survival and lung tumour growth and progression90. In addition, the tumour itself and its concomitant symptoms — such as acute or chronic pain, discomfort, or anxiety — may gradually disrupt a patient’s sleep, which in turn can result in disruption of endocrine and immunological rhythms formerly linked with cancer progression247,248. Circadian rhythm disturbances, in addition to being common symptoms of many neurodegenerative disorders, might also exacerbate the progression of disease. Recently, several longitudinal studies showed that attenuated amplitude and phase shifts of rest–activity rhythm even precede the clinical symptoms of Alzheimer disease and are associated with increased dementia risk, and that long daytime sleeping precedes the risk of Parkinson disease249.
Small-molecule modulators targeting clock components are emerging as powerful tools for understanding mechanisms of clock-related disorders as well as developing putative therapeutic strategies. For example, stabilization of period circadian protein homologues (PERs) by casein kinase 1 (CK1) inhibitors201 or cryptochrome (CRY) by small-molecule circadian modulators194, respectively, could lengthen the circadian period. Moreover, the CRY stabilizer KL001 lengthens the period and reduces the amplitude192. In turn, several selective inhibitors of glycogen synthase kinase 3β (GSK3β) can shorten the circadian period by reducing phosphorylation of both PER2 and CRY2 (REF.187). Early induction of Per1/Per2 via the cAMP/cAMP-response element-binding protein (CREB) signalling pathway is involved in acute phase resetting in mammals187. Thus, both cAMP-inducing compounds and inhibitors of the cAMP-catabolizing enzyme phosphodiesterase 4 could result in phase delay by immediate Per1/Per2 induction187. In addition, with regard to the complexity of the factors affecting clock amplitude regulation, many small molecules show modifying capability in more than one parameter. For example, clock amplitude-enhancing small molecules could enhance reporter oscillatory amplitude and cause period shortening in vitro250. Nobiletin, a naturally occurring compound, and an RAR-related orphan receptor (ROR) agonist, could enhance clock amplitude and show robust Clock-dependent metabolic protection in diabetic mice188. In conclusion, with the increasing understanding of the circadian parameters in disease progression, restoring or enhancing clock function by small molecules might improve clock output physiology, thus alleviating many clock-related diseases.
Phase delay.
A phase shift in the circadian rhythm whereby the bedtime and wake-up time will move later in the day.
Mirroring these human studies, circadian disruption in mice, via either genetic or environmental means, elicits metabolic disorders102. ClockΔ19/Δ19-mutant mice with severely dampened circadian wheel-running rhythms, are hyperglycaemic, with exaggerated food intake during the rest period, and are susceptible to body weight gain with a high-fat diet103. Similarly, when challenged with a high-fat diet, Bmal1-null mice gained more weight than wild-type mice104. As the predominant zeitgeber, light, when administered at night, led to increased food intake during the rest period, accompanied by body weight gain and impaired glucose homeostasis in mice105.
Circadian wheel-running rhythms.
When rodents have free access to a running wheel, voluntary use of this wheel is active during the night and inactive during the day to generate a specific circadian rhythm, which serves as a particularly reliable and convenient measure of the output of the master circadian clock.
The strong link between clock function and metabolic disorders has prompted vigorous efforts to understand the underlying molecular and cellular mechanisms using animal models106. The levels of NAD+, as a crucial metabolite for redox balance and a key cofactor for NAD-dependent protein deacetylase sirtuin 1 (SIRT1) and poly(ADP-ribose) polymerase 1 (PARP1), are well known to fluctuate substantially during the circadian cycle107,108. CLOCK and BMAL1 directly target the promoter of nicotinamide phosphoribosyltransferase (NAMPT) — an essential enzyme during NAD+ biosynthesis that catalyses nicotinamides and 5′-phosphoribosylpyrophosphate to nicotinamide mononucleotide — via its E-box element109. SIRT1, in turn, acts on clock protein substrates, including BMAL1 and PER2, completing a regulatory loop between the clock and metabolism109,110.
The clock controls metabolism in a tissue-specific manner28,29,111. CLOCK and BMAL1 are critically required for insulin secretion from pancreatic β-cells112, consistent with an over-representation of genes required for insulin secretion in the pancreatic circadian transcriptome in mice31. CLOCK and BMAL1 bind to distal promoter enhancers, colocalizing with the master transcription factor PDX1 for β-cells31, indicating a mechanism whereby the circadian machinery cooperates with local lineage factors to define tissue-specific metabolism. Circadian metabolic regulation has also been studied in other metabolic tissues, such as liver, muscle and adipose tissues106. Liver mass and cell size fluctuate as a function of the circadian cycle in mice, which closely follows cyclic ribosome assembly and protein accumulation113.
The hypothalamus, as an energy centre in the brain, plays an important part in the circadian regulation of energy homeostasis16. The SCN projects directly or indirectly to key regulatory centres, including the arcuate nucleus, the paraventricular nucleus, the lateral hypothalamic area and the dorsomedial hypothalamus35,114,115. Many of these regulatory centres express key neurochemicals and neuropeptides important for systemic metabolism. For example, arcuate nucleus neurons express orexigenic peptides such as neuropeptide Y (NPY) and agouti-related peptide (AGRP), as well as anorexigenic peptides such as pro-opiomelanocortin (POMC) and cocaine and amphetamine-regulated transcript (CART)116,117. Furthermore, the SCN, arcuate nucleus and ventromedial hypothalamus also form a functional axis with the dorsomedial hypothalamus to control thermogenesis, sleep, corticosteroid secretion, wakefulness and food-anticipatory behaviour35,115. SCN neurons also innervate the lateral hypothalamic area, which contains neurons that express orexin, which promotes wakefulness and energy expenditure118. Consequently, disruption of orexin or its receptor in mice leads to narcolepsy and an increased risk of diet-induced obesity, consistent with the elevated body mass index in patients with narcolepsy119,120. Taken together, these are the many examples of how circadian rhythm signalling impacts metabolism and metabolic disorders. Targeting circadian rhythm signalling pharmacologically, by alteration in sleep–wake rhythmicity, or feeding timing and behaviour is a promising approach to alter metabolic outcomes in humans.
Immune functions and sepsis
Inflammation serves as a natural defence mechanism against invading pathogens121. Given the high energy cost, the downside of collateral tissue damage, and the risk of sepsis and tissue injury, it is important to circumvent uncontrolled or unresolved inflammatory responses122–124. The circadian clock controls the timing and duration of expression of proinflammatory cytokines, such as interleukin 6 (IL-6), tumour necrosis factor (TNF), IL-17 and growth-regulated α-protein (CXCL1), as well as other aspects of inflammation or innate immune responses125,126. Mice display rhythmic sensitivity to potentially lethal challenges of bacterially derived endotoxins127 and are least able to respond to challenges just before (approximately 2 hours) activity onset. However, when mice go into the active phase, when inflammatory and injurious risks are greater, several aspects of inflammatory responses, including chemoattractant levels, leukocyte trafficking, phagocytosis and proinflammatory cytokines, are upregulated128,129. Moreover, the clock governs rhythmic haematopoietic cell homing to the bone marrow and leukocyte recruitment to skeletal muscle, partly through control of oscillatory expression of the intercellular adhesion molecule 1 gene (Icam1) and the chemokine gene Ccl2 (REF.128).
BMAL1 is a key circadian regulator of innate immunity. Mice with myeloid-specific deletion of Bmal1 showed greater susceptibility to sepsis, accompanied by elevated nuclear factor-κB (NF-κB) activity and the proinflammatory miR-155 (REF.130). In Ly6Chi monocytes, BAML1 rhythmically recruits a transcription co-repressor, polycomb repressive complex 2 (PRC2), to inhibit chemokine gene expression, which results in oscillatory trafficking to the inflammatory sites131. Moreover, BMAL1 regulates expression of the chemokine CXCL5 in epithelial club (Clara) cells, partly via regulation of binding of the glucocorticoid receptor to the CXCL5 promoter, which in turn controls neutrophil trafficking to the mouse lungs132. Other circadian proteins also have important roles. For example, REV-ERBα regulates transcription of inflammatory genes in macrophages, and mice deficient in REV-ERBα expression fail to control rhythmic IL-6 secretion following lipopolysaccharide challenge129. Therefore, the clock serves as a master gatekeeper of innate immunity, optimizing immune outcomes and the subsequent resolution and repair to avoid an exaggerated response and sepsis shock.
Lipopolysaccharide challenge.
An experimental technique to elicit inflammation by administering lipopolysaccharide via intraperitoneal injection to mice.
Recent studies have established that adaptive immune cells are subjected to circadian control125. Several clock components play a critical part in lymphocyte development. Nuclear receptor RORγt (also known as RORγ2, an isoform of RORγ, which has a different, shorter N terminus) is a master regulator for the development of IL-17-producing T helper cells (TH17 cells), an important immune cell type for autoimmunity133. NFIL3 serves as a repressor of RORγt, thus functioning in a new circadian loop to regulate TH17 cell differentiation134. Similarly to innate immunity, trafficking and tissue recruitment of lymphocytes are controlled by the circadian clock. For example, both T cells and B cells oscillate in number in circulation, peaking during the rest phase135. Glucocorticoids, which are regulated by the central clock in the brain, serve as cell-extrinsic signals to govern the expression of the chemokine CXCR4, leading to the diurnal oscillation of circulating T cells in mice136. In addition, CLOCK and BMAL1 regulate the rhythmic expression of Toll-like receptor 9 (TLR9) via non-canonical E-box elements, and circadian rhythms of TLR9 strongly influence septic severity and immunization response137. For example, the septic severity in a mouse model of caecal ligation and puncture was elevated when the caecal ligation and puncture procedure was performed in entrained animals at zeitgeber time 19 (peak of TLR9 expression in the spleen) compared with zeitgeber time 7 (nadir TLR9 expression). Molecular pathways of circadian rhythm signalling could be targeted in patients with inflammatory or infectious diseases, for example in the context of critical illness such as sepsis138. Observational studies of windowed rooms and real-time use of ambient light in critical care units indicate that physiological light–dark patterns may support recovery from critical illness139.
Caecal ligation and puncture.
A commonly used preclinical rodent model to study sepsis via ligation and perforation of the caecum, resulting in polymicrobial infection and systemic inflammation.
Zeitgeber time.
A standardized 24-hour notation for the phase in an entrained circadian cycle with reference to environmental regularities or zeitgebers.
Ageing and circadian rhythms
Besides its role in disease pathogenesis, the molecular clock output gained increasing attention from studies that focused on the physiological impact, such as in natural ageing. There has been growing evidence of the interaction between natural ageing and the alternations in circadian rhythmicity54. For example, aged mice maintain behaviour and cellular circadian rhythmicity in epidermal and muscle stem cells, but aged muscle stem cells show a reprogrammed oscillating transcriptome140. This reprogrammed oscillating transcriptome in aged mice is rewired to control expression of genes implicated in stress responses, rather than homeostasis. Similarly, ageing reprogrammes the circadian output in mouse liver, resulting in significantly reduced oscillation in protein acetylation141. In both scenarios, calorie restriction could reverse the ageing-related reprogramming, indicating a potential favourable impact of calorie restriction. Furthermore, from a therapeutic point of view, treatment with the NAD+ precursor nicotinamide riboside deacetylates PER2 at Lys680 to improve genome-wide BMAL1 chromatin binding142. The modulation of circadian rhythm by NAD+ precursors restores the ageing-related reprogramming of circadian rhythmicity in the liver of old mice. Therefore, reprogramming of the circadian output associated with natural ageing can potentially be targeted by behaviour modification with calorie restriction and therapeutic interventions such as NAD+ precursors.
Circadian rhythm genes also have a profound impact on the ageing process. A study using transgenic mice with modifications in critical circadian rhythm genes indicated that circadian rhythm genes affect the lifespan and contribute to ageing-related disease143. For instance, Bmal1-deficient mice show premature ageing indicated by reduced lifespan, with more than 90% of animals dying within 1 year, whereas wild-type mice have an average lifespan of 28 months143. Bmal1 deficiency also results in ageing-related skin and eye disaese accompanied by increased levels of reactive oxygen species in the kidney and spleen143. Moreover, SIRT1 and PER2 regulate each other reciprocally in circadian rhythm and ageing in mouse liver and human hepatocytes144. SIRT1 is a key NAD+-dependent deacetylase, and the absence of Sirt1 leads to overexpression of Per2 via acetylation of histone H4 on the promoter. On the other hand, Per2 overexpression reduces Sirt1 expression in mouse liver, causing increased levels of senescence markers, including the genes encoding phosphoenolpyruvate carboxykinase (Pck1) and glucose 6-phosphatase (G6pc)144. Finally, previous studies indicated that disruption of the sleep–wake cycle and disruption of circadian rhythm are risk factors for ageing-related diseases such as Alzheimer disease145. In summary, the relationship between ageing and the circadian rhythm is truly intricate, and direct targeting of the circadian rhythm genes could potentially reduce or slow down ageing-related disaese.
Targeting circadian rhythm in disease
Circadian pharmacology
Circadian changes in drug pharmacokinetics, including absorption, distribution, metabolism and excretion, are well documented10. The term ‘chronotherapy’ usually refers to the timed dosing of drugs to maximize therapeutic index10. Various cardiovascular parameters display strong circadian rhythms17,50. In humans, blood pressure starts to rise before wakening in the early morning, then peaks soon afterwards. However, 40% of patients with essential hypertension (without known causes) are non-dippers, which means that their blood pressure does not drop during night-time. Thus, several chronotherapies, such as bedtime intake of antihypertensive drugs — for example angiotensin-converting enzyme inhibitors or angiotensin receptor blocker — have shown increased cardiovascular efficacies in hypertensive patients by improving the nocturnal blood pressure profile50,146. In rodent models, 35 anticancer agents display a twofold to tenfold variation in tolerability in mice or rats when given at different times of the day10. These studies have motivated clinical trials in patients with cancer10,147, including assessing a combination therapy in which doxorubicin administered at 6 a.m., with cisplatin given 12 hours later, markedly increased survival of patients (a fourfold survival advantage) with advanced-stage ovarian cancer compared with patients who were given the same dose of the drugs at 6 p.m.147. The cytotoxic effects of cyclophosphamide correlated with the functional status of the CLOCK–BMAL1 complex, and the ablation of CRYs rendered tumours more resistant to the drug compared with tumours in wild-type mice148. Chronotherapy with glucocorticoid receptor agonists has also been extensively applied to asthma51, and dosing of prednisone in the afternoon (3 p.m.) showed a much-increased efficacy in the management of night-time symptoms compared with prednisone administered in the morning or later in the evening149.
Circadian rhythm sleep–wake disorders
Insomnia.
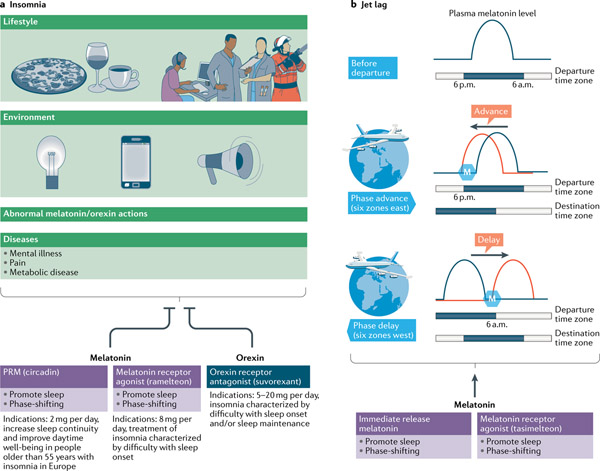
Approximately 25% of Americans develop insomnia each year, and up to 10% of them progress towards chronic insomnia, which is frequently associated with anxiety, depression, higher risk of cardiovascular disease, physical health problems and poor quality of life150. Owing to the close association between melatonin deficit in pathological conditions and natural ageing, and increased insomnia prevalence, a simple melatonin replacement therapy, as a chronobiotic, was first believed to reduce insomnia151,152. A randomized, double-blind placebo trial involving 791 adult outpatients (aged 18–80 years) with primary insomnia showed that prolonged-release melatonin (such as Circadin) reduced subjective sleep latency only in patients older than 65 years, demonstrating its specific efficacy in the older adult group but not in other age groups151. Particularly, Circadin, 2 mg per day orally, was recommended to increase sleep continuity and improve daytime wellness in insomnia disorder in adults aged 55 years or older in Europe152,153 (FIG. 4a). As an indispensable component of the melatonergic signalling system, melatonin agonists have been studied extensively as pharmaceutical approaches for treatment of insomnia. Ramelteon, a high-affinity MT1 and MT2 receptor agonist (with an affinity approximately ten times higher than that of melatonin), has been approved for treating insomnia disorders characterized by difficulties in sleep onset in the United States and Japan153 (FIG. 4a; TABLE 1). However, the effects of both exogenous melatonin and ramelteon on total sleep duration and maintenance are not so clear154. Notably, mice with deletion of Mtnr1a have a selective disruption of REM sleep and an increase of NREM sleep, whereas Mtnr1b-knockout mice have a selective disruption of NREM sleep155. These results highlight the opposing effects of the two receptors, which partially explain why melatonin or ramelteon only reduces sleep latency, without affecting total sleep time or sleep architecture. Thus, discovery of selective melatonin receptor agonists may promote the development of novel and more efficacious therapeutic agents.
Fig. 4 |. Pharmacological interventions for insomnia and jet lag.
a | Changes in lifestyle, the environment and health conditions and abnormal action of orexin or/and melatonin could all trigger insomnia150. According to its sleep-promoting effect, melatonin and its agonists have therapeutic potential to treat insomnia. Circadin, a commercially available prolonged-release form of melatonin (PRM) has been licensed on the basis of improved nightly sleep quality for treating insomnia disorder in adults older than 55 years in Europe152,153. Circadin has a similar affinity for melatonin receptors. Conversely, ramelteon is a melatonin receptor agonist and its affinity for melatonin receptor 1 (MT1 receptor) is eight times higher than that for melatonin receptor 2 (MT2 receptor), thus exerting a stronger inhibition of the neuronal firing in the suprachiasmatic nucleus to facilitate bedtime sleep onset38,153. Finally, suvorexant, which is a dual orexin receptor antagonist, attenuates orexin receptor 2 (OX2R)-mediated cortical arousal and enhances sleep maintenance and onset associated with orexin receptor 1 (OX1R), respectively158. b | Melatonin and its receptor agonists can be used in the treatment of jet lag disorder. The key to treating jet lag is the phase response of melatonin. Therefore, tasimelteon, a melatonin agonist with higher affinity for the phase-shifting MT2 receptor, has been shown to result not only in a significant shift in endogenous circadian rhythms but also an improvement in sleep initiation and maintenance166. The timing of melatonin administration is of the essence. Melatonin can phase-advance the physiological and behavioural circadian rhythm when administered in the late biological afternoon. Conversely, when administered in the early biological morning, melatonin delays the circadian clock41,168,170,171. Circadian status can be approximately predicted by habitual sleep timing in the adapted departure time zone, and the plasma melatonin level (blue curve) can be used to define circadian phase for the timing of melatonin administration. The adaptation to the destination time zone before transmeridian travel could be achieved as follows172: for eastward travel across six time zones, administration of melatonin (blue hexagon) in the afternoon at departure can advance circadian phase (left-shifted plasma melatonin level, red curve); for westward travel across six time zones, administration of melatonin (blue hexagon) in the early morning can delay circadian phase (right-shifted plasma melatonin level, red curve).
Table 1 |.
Examples of current clinical trials involving drug molecules
| Drug | Status | Diseases | Clinical trial identifier | Disease indications | Parameters |
|---|---|---|---|---|---|
| Melatonin signalling | |||||
| Melatonin | Phase III | Obesity; prediabetic state | NCT02681887 | Night-shift work, blindness, myocardial syndrome, migraine, metabolic syndrome, ICU sleep disruption, dialysis, epilepsy, nocturnal enuresis, DSPS, non-24-hour sleep–wake disorder, jet lag, Smith–Magenis syndrome | Metabolic parameters, sleep, mood, insulin and β-cell function, blood coagulation, migraine attack frequency, seizure, incontinence |
| Phase II | Migraine | NCT00849511 | |||
| Phase III | Sleep problems; haemodialysis | NCT00388661 | |||
| Phase II | Epilepsy | NCT00965575 | |||
| Tasimelteon | Phase II | Smith–Magenis Syndrome | NCT02231008 | ||
| Phase III | Circadian rhythm alterations | NCT02231008 | |||
| Phase III | Non-24-hour sleep–wake disorder | NCT01430754 | |||
| Phase II | Jet lag disorder | NCT03291041 | |||
| Ramelteon | Phase II | Sleep disorders; circadian rhythm | NCT00593736 | ||
| Orexin signalling | |||||
| Modafinil | Phase IV | Bipolar disorder | NCT01965925 | Bipolar disorder, long-term shiftwork, sleep disorder, Alzheimer disease | Sleep, circadian rhythms |
| Armodafinil (CEP 10953) | Phase III | Excessive sleepiness; shiftwork sleep disorder | NCT00080288 | ||
| Lemborexant | Phase II | Irregular sleep–wake rhythm disorder | NCT03001557 | ||
| Suvorexant | Phase IV | Shiftwork sleep disorder | NCT02491788 | ||
| Combined or other signalling | |||||
| Vitamin B12 | Not applicable | Sleep disorders; circadian rhythm | NCT00120484 | Night owl or DSPS | Sleep and circadian rhythm |
| Methylselenocysteine | Not applicable | Breast cancer; prostate cancer | NCT01611038 | Night-shift work | Circadian rhythm and oestrogen receptor B levels |
| Melatonin and meal timing | Not applicable | Obesity; cardiometabolic diseases | NCT03490864 | Obesity, chronic insomnia, DSPS | Sleep, circadian rhythm |
| Melatonin and light therapy | Phase IV | DSPS | NCT00834886 | ||
| Ramelteon and multicomponent behavioural therapy | Phase IV | Insomnia | NCT01180855 | ||
DSPS, delayed sleep phase syndrome; ICU, intensive care unit.
Chronobiotic.
An agent that is able to influence, directly or indirectly, the phase and/or the period of the body clock.
In turn, dual orexin receptor antagonists can enhance sleep maintenance and onset by attenuating cortical arousal and reducing the threshold of sleep state transitions associated with OX2R and OX1R inhibition, respectively156,157. Suvorexant, an orally active dual orexin receptor antagonist, was first approved by the FDA in 2014 for the treatment of insomnia marked by difficulty with sleep onset and/or sleep maintenance158 (FIG. 4a; TABLE 1). Similarly to natural sleep, the preservation of night-time arousability to salient cues159 and daytime cognitive performance160 following administration of dual orexin receptor antagonists in experimental animals (monkey and rat, respectively), suggests further advantages over conventional therapies such as benzodiazepines. From the findings taken together, suvorexant is more effective in treating sleep maintenance insomnia than sleep-onset difficulties.
Jet lag and shiftwork.
Mistimed circadian rhythms due to rapid travel across multiple time zones, together with the sleep deprivation from long flights, are the main contributing factors for jet lag symptoms161. The combination of hypnotic and phase-shifting properties of melatonin makes it an ideal drug to treat jet lag162. As discussed in the previous section, different melatonin receptors have different distributions and functions. Notably, animal studies revealed that melatonin phase-shifts the wheel-running activity rhythms, and this effect is diminished by the MT2 receptor antagonist 4P-PDOT41. In a jet lag model, re-entrainment after the phase advance was significantly slower in mice lacking functional MT2 receptors than in melatonin-proficient mice with intact MT2 receptors43. Thus, the MT2 receptor38,40,41 is believed to be the main mediator of the re-entrainment effect of melatonin on the SCN163,164 and the locomotor activity165. As the key to treating jet lag is to mimic the phase-shifting properties of melatonin, many studies have focused on phase-specific activation of melatonin receptors. Administration of tasimelteon, a melatonin receptor agonist with a higher affinity for the MT2 receptor, resulted in a significant shift in endogenous circadian rhythms and an improvement in sleep initiation and maintenance in phase II (n = 39) and phase III (n = 412) trials involving healthy volunteers exposed to experimentally induced jet lag166 (FIG. 4b; TABLE 2). The recent determination of the crystal structure of melatonin receptors167 has led to the discovery of two MT1 receptor-selective inverse agonists (UCSF7447 and UCSF3384)168, which, when administered at subjective dusk, advanced the onset of the running-wheel activity in mice in constant darkness. Furthermore, their effects on circadian phase advance were eliminated in Mtnr1a-knockout mice but not in Mtnr1b-knockout mice, indicating the impact of the MT1 receptor in the phase-shifting function of melatonin168. Therefore, it is still unclear whether the MT1 receptor is more important than the MT2 receptor for the phase-shifting effects of melatonin under constant darkness, and clinical trials are needed to investigate their effectiveness of specific melatonin receptor agonists in real-life jet lag situations.
Table 2 |.
Preclinical studies of small-molecule modulators targeting clock components and key regulators
| chemical modulators | Mechanism of action | Physiological effects | Refs |
|---|---|---|---|
| CRY1/CRY2 | |||
| KL001 and CRY-stabilizing derivative (SHP656, KL101 and TH301) | Stabilize CRY, lengthen period, reduce amplitude | Improvement of glucose tolerance in obese mice, inhibition of glioblastoma stem cell proliferation in vitro, inhibition of glioblastoma tumour growth in vivo, enhancers of brown adipocyte differentiation | 94,194,196,197 |
| 2-Ethoxypropanoic acid (KS15) | Inhibits CRY, activates E-box transcription, reduces amplitude | Inhibition of breast cancer cell growth | 231 |
| REV-ERBs | |||
| GSK4112 | Enhances REV-ERB and NCOR peptide interaction | Inhibition of gluconeogenesis and inflammatory response in primary cells | 129,179,189 |
| SR9009, SR9011 | Derived from GSK4112, they are selective agonists for REV-ERB that alter circadian behaviour, clock gene expression (BMAL1, PERI, and PER2) and expression of some glioblastoma stem cell markers (for example, OLIG2 and SOX2) | Improvement of glucose homeostasis in obese mice, promotion of wakefulness, anxiolytic, inhibition of glioblastoma stem cell proliferation | 94,181,183 |
| SR8278 | GSK4112-derived antagonist that increases expression of REV-ERB target genes (for example, BMAL1, PCK1 and G6PC1) in cells | Anxiety attenuation and amelioration of myocardial injury | 82,189 |
| RORs | |||
| Nobiletin | Agonist, enhances amplitude and lengthens period | Metabolic homeostasis improvement in obese/diabetic mice; broad efficacies with regard to inflammation and atherosclerosis | 188,232,233 |
| Neoruscogenin | Agonist promoting ROR interaction with NCOA2/TIF2, activates BMAL1 expression | Activation of hepatic expression of ROR metabolic target genes | 234 |
| SR1001 | Derived from T0901317 with high inverse agonist activity and selectivity for RORα and RORγt | Inhibition of TH17 cell differentiation and autoimmunity | 193 |
| SR2211, SR1555, digoxin, ursolic acid, ML209 | RORγ inverse agonist | Inhibition of TH17 cell differentiation | 189,191,192 |
| SR3335 | RORα inverse agonist | Reduction of blood glucose level in obese mice | 235 |
| SR1078 | RORα agonist | Induction of apoptosis and inhibition of hepatoma cell growth | 236 |
| CK1 kinases | |||
| CKI-7, IC261, D4476, PF-670462, PF-4800567 longdaysin, LH846, compounds 1–3 among others | Inhibitors, lengthen period | Pharmacological inhibition of CK1, significantly lengthen circadian rhythms mainly by nuclear retention of PER2. CK1 is broadly involved in various pathophysiologies, including familial sleep and mood disorders | 199–201,237 |
| ADORA2B | |||
| BAY 60-6583 | ADORA2B agonist, PER2 stabilization | Enhancement of adenosine signalling and cardioprotection against myocardial ischaemia | 74,238 |
| PER2 | |||
| Lithium | Increased transcription of PER2, lengthens period and enhances amplitude of PER2 rhythms | Treatment of bipolar disorder associated with circadian rhythm disruptions | 239 |
For additional clock-modulating compounds, see REFS5,189,192,237. ADORA2B, A2B adenosine receptor; CK1, casein kinase 1; CRY, cryptochrome; NCOR, nuclear receptor co-repressor; PER2, period circadian protein homologue 2; ROR, RAR-related orphan receptor; TH17 cell, interleukin-17-producing T helper cell.
Most importantly, the timing of melatonin administration is critical for optimal therapeutic effects169 (FIG. 4b). Melatonin phase-advances the physiological and behavioural circadian rhythm when administered in the late biological afternoon, whereas it delays the circadian clock when administered in the early biological morning170,171. Circadian status predicted by the timing of habitual sleep in the adapted departure time zone can be used to manage melatonin administration and begin shifting the circadian system in the desired direction even before a flight172: to delay the circadian system before westward travel, melatonin should be taken 1 hour after rising from bed (around 6 a.m.); to advance the circadian system before eastward travel, melatonin should be taken 6.5 hours before bed (around 6 p.m.). Similarly, melatonin could be used to shift the timing of the circadian system to the destination time zone during/after flights172. Although not approved for the treatment of jet lag, melatonin is still used to reduce jet lag symptoms following transmeridian flights161.
Similarly to jet lag, circadian misalignment also triggers shiftwork disorder173. Chronic circadian misalignment in shift workers can result in serious sleep disruption and a variety of associated health issues, including increased risk of several cancers (such as breast cancer), metabolic syndrome (prone to weight gain) and cardiovascular disease100,174,175. There is low-quality evidence that melatonin is associated with increased sleep length after a night shift but not with reduced sleep latency time176. Limitations exist in that most trials were small with a duration of 1 week or less176. Besides melatonin, other phase-shifting chemicals might also be useful for promoting circadian adaptation to environmental changes. Administration of sildenafil, an inhibitor of cGMP-specific phosphodiesterase 5, significantly accelerates the entrainment of locomotor activity rhythms to the new light–dark cycle in hamsters177. In addition, a recent study showed that pharmacological perturbation of RuvB-like ATPase 2 (RUVBL2) by the adenosine derivative cordycepin shifts the circadian phase both in human cells and mouse tissue, and rapidly entrains the circadian phase in a mouse jet lag model. This phase-shifting effect is the result of disassembly of interaction between RUVBL2 and BMAL1, thereby leading to the release of repression of clock gene transcription178. Furthermore, the treatment of shiftwork focuses on combating the reduced alertness during night shifts. For instance, modafinil (200 mg) and armodafinil (150 mg), the dopamine reuptake inhibitors which indirectly activate the release of orexin and histamine, were associated with increased alertness and reduced sleepiness in shift workers. However, these drugs were also reported to lead to headache and nausea176. Therefore, the long-term benefits and adverse effects of melatonin and new-found phase-shifting compounds, in combination with wake-promoting medications, need to be further evaluated by clinical studies in patients with shiftwork disorder.
Therefore, for the fatigued flyer or shift worker in a state of internal and external desynchrony, the best strategy may still be the combination of timed medication, sleep and timed light treatment161.
Targeting the circadian machinery
In addition to manipulating circadian output rhythms including feeding–fasting and sleep–wakefulness, direct manipulation of the core oscillator is an exciting new dimension to circadian medicine. Recent studies have aimed to develop candidate drug molecules targeting core components or the molecular oscillator (FIG. 2; TABLE 2).
Drugs targeting REV-ERBs.
REV-ERBα is a major modulator of the circadian expression of BMAL1, and the secondary feedback loop in the clock cycle. Ligands targeting REV-ERBs have been assessed in a broad range of disease models (TABLE 2). For example, GSK4112 — a small-molecule agonist of nuclear receptor co-repressor 1 (NCOR1) of REV-ERBα179 — showed inhibitory effects against lipopolysaccharide induction of IL-6, CXCL11, CXCL6 and CCL2 in human monocyte-derived macrophages and the leukaemia-derived cell line THP1 (REFS129,180). Subsequently, two pyrrole compounds, SR9009 and SR9011 (agonists of REV-ERBα), were derived, and showed strong in vivo activities, including metabolic regulation, anticancer and anxiolytic effects181–183. Mice treated with these compounds experienced acutely suppressed wheel-running behaviour and improved metabolic homeostasis in diet-induced obesity181. The compounds (SR9009 and/or SR9011) have also been assessed in other metabolic settings, including enhancement of mitochondrial content in skeletal muscle and exercise endurance in mice184. In addition, SR9009 and SR9011 display selective cytotoxicity with regard to leukaemia and several solid tumours with different tumorigenesis drivers182. SR9009 down-regulated key autophagy-related mRNAs in primary hepatic stellate cells, suggesting an inhibitory role in autophagy185, a clock-regulated process that cancer cells use to meet heightened metabolic requirements. SR9011 also displayed anxiolytic effects183, consistent with genetic evidence showing enhanced anxiety in Nr1d2-knockout mice. In comparison, SR8278, a REV-ERB antagonist, attenuated anxiety when administered to mouse ventral midbrain186 and ameliorated myocardial injury in an isolated heart system when administered at the activity onset82, indicating beneficial roles of REV-ERB inhibition. Together, these studies highlight a versatile yet complex role of targeting REV-ERBs in different diseases.
Drugs targeting RORs.
A few studies have also investigated compounds that enhance circadian amplitude, dubbed ‘clock amplitude-enhancing small molecules’187. One of the best-studied clock amplitude-enhancing small molecules is the natural flavonoid nobiletin, which was found to activate RORs directly in mice188, reaffirming a key role of the secondary stabilization loop in circadian amplitude regulation. Indeed, nobiletin potentiated circadian wheel-running activity and clock protein oscillation, reduced body weight and restored robust energy homeostasis in diet-induced obesity mice and db/db diabetic mice188. Other ROR modulators also have roles in metabolic diseases189. An inverse agonist of RORs, SR1555, was found to enhance energy expenditure and ameliorate obesity in a diet-induced obesity mouse model190. These results are reminiscent of the shared anxiolytic efficacies of SR9011 and SR8278, suggesting plasticity of circadian modulation against diseases. In addition to circadian amplitude-enhancing and metabolic regulatory effects, drugs targeting RORs can modulate numerous autoimmune disorders189,191. Furthermore, with canonical ligand-binding domains shared by nuclear receptors, RORs are bound by various endogenous and synthetic ligands189. For instance, digoxin and ursolic acid, as RORγt inverse agonists, ameliorated autoimmune disorders including arthritis and encephalomyelitis via suppression of TH17 cell differentiation191,192. Consistently, several optimized RORα/RORγ ligands attenuated expression of downstream cytokines and strongly alleviated autoimmune disease symptoms in mice (such as in animal models of multiple sclerosis and experimental autoimmune encephalomyelitis)192,193. Finally, the regulatory role of RORγt in TH17 cell differentiation is governed by a circadian pathway that involves REV-ERBs and BMAL1 (REF.134), which supports the notion that these drugs modulate the circadian system against immune diseases. In conclusion, the data obtained from the in vivo ROR synthetic ligand studies are extremely encouraging, specifically for the regulation of circadian rhythms, metabolism and immunity. However, how these ligands affect the pathophysiology of the diseases has yet to be extensively studied.
Drugs targeting PERs and CRYs.
Several chemical screens have found that compounds that affect levels of PERs and CRYs can alter circadian periods. For example, a CRY1- and CRY2-stabilizing carbazole compound, KL001, was identified in a cell-based screen to lengthen the period and reduce the amplitude194 (TABLE 2). Furthermore, evolutionarily conserved domains in both CRY1 and CRY2 can be targeted as binding surfaces for drug development. Mammalian CRY proteins belong to the evolutionarily conserved photolyase protein family, and their photolyase homology region functions as a low-affinity FAD-binding pocket63. As illustrated by the compound KL001, which competes with the FBXL3 carboxy terminus at the FAD-binding pocket to stabilize CRYs195, even such a relatively shallow, suboptimal binding surface can mediate functional binding with chemical ligands. Besides circadian period-regulating function, KL001 derivative also has a role in metabolic regulation by normalizing glucose tolerance in diet-induced obesity mice196. Moreover, combination of KL001 and the REV-ERB agonist SR9011 inhibits cell cycle progression and reduces the self-renewal potential of patient-derived glioblastoma stem cells94. Notably, SHP656, one of the most promising KL001 derivatives, specifically inhibits the growth of patient-derived glioblastoma stem cells in vitro at a concentration of 2–30 μM, while having an insignificant impact on differentiated glioblastoma cells or non-malignant epilepsy-derived neural cells94. SHP656 also shows improved in vivo safety and increased in vivo stability, and significant efficacy in the treatment of glioblastoma by reducing tumour growth and prolonging mouse survival94. Although the evolutionarily conserved features of CRY domains and the CRY FAD-binding pockets offer potential as targets for in silico screening or derivation of known ligands, this high sequence conservation between homologous proteins makes it particularly challenging to develop isoform-selective compounds. Recently, KL101 and TH301 were identified to stabilize CRY1 and CRY2, respectively, which is dependent on the disordered carboxy-terminal region outside the pockets, leading to enhancement of brown adipocyte differentiation in mice197. Finally, structural studies of clock proteins have pinpointed interaction hotspots with disproportionally high binding energy. For example, the PER–CRY crystal structure revealed several regulatory interaction surfaces on the CRY-binding domain of PER198, particularly in a region that competes with FBXL3 to bind to the extreme carboxy-terminal surface on CRY.
Drugs targeting CK1.
CK1 kinases are excellent pharmacological targets as they have a pivotal role in phosphorylating core clock proteins18. Although early inhibitors — including IC261 and CKI-7 — were only moderately active and selective, a specific inhibitor of CK1ε, PF-4800567, was derived to confer an inhibitory activity greater than 20-fold over CK1δ199. This ‘super’ strong inhibition has shown additional therapeutic promises in mice. For example, in mice rendered arrhythmic either by constant light exposure or by deletion of vasoactive intestinal polypeptide receptor 2 (Vipr2−/− mice), the CK1δ-specific inhibitor PF-670462 was able to induce behavioural rhythms200, suggesting that a pharmacological agent that targets CK1δ can be tested in patients with familial advanced sleep phase syndrome or animal models. Moreover, from high-throughput chemical screening, a novel compound called ‘longdaysin’, which is a purine derivative, was discovered to lengthen the circadian period in cell culture in vitro and in zebrafish in vivo by targeting CKIδ, CKIα and ERK2 (REF.201). Longdaysin could potentially serve as another promising novel compound for the treatment of circadian rhythm disorders.
Environmental and lifestyle modifications
Environmental and lifestyle regimens are known to alter circadian timing and various clinical trials are ongoing to test their effects on health (TABLE 3). Blue light is a dominant zeitgeber, and the pervasive night-time light exposure in modern societies interferes with neuroendocrine circuits to perturb sleep and dampen rhythms of melatonin and cortisol5. Conversely, bright light therapy, commonly conducted by exposure to broad-spectrum bright light (2,000–10,000 lux) for 1–3 hours in the morning, can improve sleep–wake cycles, mood and cognitive functions in patients with sleep or seasonal affective disorders202,203. In particular, in intensive care units — where patients suffer more from circadian arrhythmia due to both external (environmental) and internal (medical) factors — several randomized clinical trials showed that morning bright light can reduce delirium prevalence and shorten delirium episodes139. Therefore, safe, non-invasive chronotherapeutic interventions, such as light therapy, may be effective in maintaining and resynchronizing circadian rhythmicity and improve the short-term and long-term outcomes.
Table 3 |.
Clinical trials involving behavioural and environmental modifications
| Modifications | Disease indications | Parameters | Examples |
|---|---|---|---|
| Light therapy | Stroke, ageing, Parkinson disease, Alzheimer disease, mood disorders, metabolic disease, night shift, PTSD, traumatic brain injury, sepsis, cancer-related fatigues | Circadian rhythms in sleep and blood parameters, cognition, activity, fatigue, depression and PTSD scores, inflammation markers | NCT02186392 |
| NCT02502045 | |||
| NCT02769858 | |||
| NCT01855126 | |||
| NCT01048294 | |||
| NCT01747811 | |||
| Time-restricted feeding | Obesity, metabolic syndrome, shiftwork | Body weight, metabolic homeostasis, cardiometabolic parameters | NCT02204735 |
| NCT03527290 | |||
| Sleep intervention | Night-shift work, traumatic brain injury | Sleep quality | NCT02838082 |
| NCT02609373 | |||
| Scheduled activity | Diabetes, Alzheimer disease | Sleep, glucose homeostasis | NCT03553524 |
| NCT01920672 | |||
| Combination: including sleep, light, exercise and lithium | Night-shift work, HIV-related fatigue, mood disorders, ICU stay | Circadian phase, sleep, metabolic parameters, mood rating, clock gene expression | NCT01284140 |
| NCT01767181 | |||
| NCT02126007 | |||
| NCT01799733 | |||
| NCT03405493 | |||
| NCT01431573 |
ICU, intensive care unit; PTSD, post-traumatic stress disorder.
A number of lifestyle modifications, including timed meals, sleep and activity, have been investigated for their safety and their efficacy to achieve optimal circadian health5. Exciting advancements have been reported in recent years with regard to feeding regimens. For example, time-restricted feeding (TRF), characterized by food access for 8–9 hours during the active phase, has been found to exert strong preventive and therapeutic effects in metabolic disorders204,205. In mouse studies in which the overall caloric intake is largely constant between TRF and ad libitum feeding, considerable metabolic benefits, including energy balance, insulin sensitivity and reduced hepatic steatosis, were observed in the TRF group, independent of diet or initial body weight205. There was also a legacy effect in which benefits were maintained when TRF was applied for only 5 days a week and ad libitum feeding was applied for the remaining 2 days, mimicking the workdays and weekends in a human lifestyle. Furthermore, TRF showed the capacity to not only stabilize but also reverse the progress of metabolic diseases in mice with pre-existing obesity and type 2 diabetes205. Consistent with the notion that eating during the inactive phase diminishes energy expenditure and exacerbates weight gain, pilot human studies also showed that when total intake is maintained at a similar level, participants consuming a larger portion during breakfast showed a strong improvement in metabolic homeostasis in comparison with the calorie-rich dinner group206. In a randomized, crossover clinical study, an early TRF regimen, involving a 6-hour feeding period earlier in the day, was found to increase key insulin sensitivity and improve β-cell function in prediabetic men207. Conversely, patients with night-eating syndrome are prone to obesity and type 2 diabetes208. Such energy imbalance as a result of abnormally timed food consumption may explain metabolic dysregulation from nocturnal sleep deprivation or late sleep. In a human study in which a 5-day sleep deprivation was implemented to mimic a workweek, an increase in energy expenditure was observed in study participants, which led to more exaggerated energy intake, resulting in energy storage and bodyweight gain209.
Besides TRF, temporally controlled activity has also been studied in laboratory and clinical settings. Studies in hamsters showed that stimulated activity during the rest phase caused phase advance in activity onset the following day210, perhaps attributable to acute clock gene differential regulation in the SCN211. In aged mice, scheduled wheel activity also enhanced behavioural rhythmic amplitude and entrainment212. Likewise, in humans, exercise and activity can reset circadian melatonin rhythms213, and afternoon exercise has been found to improve sleep quality in older people214. Thus, it is conceivable that scheduled sleep routines can also improve circadian rhythms. Indeed, in a mouse model of Huntington disease, induction of sleep by alprazolam (a benzodiazepine) during the inactive period improved cognition and mobility215.
Human rhythm determination
Intrinsic circadian rhythms in humans are slightly over 24 hours, and manifest themselves as morning or evening chronotypes. Several questionnaires have been developed to assess human chronotypes216. For example, the self-assessment Morningness–Eveningness Questionnaire has shown usefulness in determining chronotypes and uncovering outliers by evaluating the circadian variation of oral temperature. People with morning types have a higher daytime temperature and lower post peak temperature than people with evening types217. The Munich Chronotype Questionnaire refined the method to distinguish workdays versus rest days, and collected detailed information on actual schedules of sleep and activity, as well as time spent outdoors218. Electronic devices can also monitor behavioural rhythms, including wrist actigraphy sensors and mobile phone apps219,220. Physiological markers that can be routinely assessed in the clinic include circulating hormones (melatonin and cortisol), core body temperature and midpoint sleep. As surrogates, human fibroblast primary cultures221 and hair follicle cells222 serve as minimally invasive in vitro systems to report behavioural rhythms. In clinical settings, common difficulties in assessing behavioural rhythms accurately include lack of information on the time of sample collection or obtaining a single time point — rather than a longitudinal — biopsy. To overcome these challenges, innovative statistical methods can assign circadian time or phase for human-derived samples, including blood metabolites223, gene expression in tumours89 and post-mortem brain samples224.
Chronotypes.
Characterized by individual differences in the timing of sleep–wake schedules, biological parameters (such as core body temperature, melatonin and cortisol) and cognitive performance (such as attention).
Circadian time.
A notation for the phase in a circadian cycle that represents an organism’s endogenous circadian clock, without reference to any environmental regularities or zeitgebers.
Most of the 662 currently registered clinical trials evaluating circadian-related compounds in different phases of the pipeline involve small-size cohorts and simply investigate circadian rhythms as an observational readout. Behavioural and physiological parameters, including sleep, activity, and melatonin and cortisol, are most commonly used to assess circadian rhythms. In some cases, expression of clock genes was also determined by use of skin biopsy samples. Among those that involve circadian intervention, the predominant regimens are bright light therapy, timed food intake and melatonin therapy (TABLES 1, 3). A meta-analysis showed that light therapy was effective in treating circadian rhythm sleep disorders, specifically insomnia225. Nevertheless, most studies are small to medium size. The timing of food intake showed a more profound influence on the peripheral circadian rhythms than the timing of light226. In addition, melatonin therapy seems to be an encouraging treatment for sleep problems, including insomnia and other wake–sleep disorders176,227. However, the beneficial effects of melatonin supplements were often minor in humans, and there is a lack of high-quality, randomized controlled trials of melatonin therapy in circadian and sleep disorders published in the literature169. Other agents include orexin signalling modulators and vitamin B (TABLE 1). Although there are no registered clinical trials ongoing for agents that target the core clock factors, small human studies have been reported for some natural compounds. For example, a Japanese study of six cohorts of older patients revealed a preventive effect on the progression of the cognitive impairment associated with Alzheimer disease following prolonged intake of citrus extracts rich in the clock modulator nobiletin228. Given a causal role of perturbation in sleep and circadian rhythms in amyloid aggregation and cognitive abnormalities229, it will be interesting to investigate whether circadian enhancement can alleviate symptoms of Alzheimer disease in larger cohorts.
Conclusions
The omnipresent circadian control permeates physiology and pathophysiology. This current growing interest in circadian rhythm signalling could propel discoveries of new circadian therapeutics and their functions and mechanisms in preclinical and clinical settings. Although existing studies in disease models illustrate the promising potential of targeting circadian rhythms as a therapeutic strategy, they also highlight the complex or even seemingly contradictory effects. These results are consistent with the notion that owing to integrated circadian feedback regulation, there may be an interventional range, in either direction, to manipulate circadian clocks and alter pathophysiology in a chronic setting. Importantly, although the levels of some circadian modulators are determined solely by light input, melatonin, as a typical representative, could show the opposite physiological impact: sleep-promoting effects in humans and awakeness-promoting effects in nocturnal animals. Thus, the difference in diurnal (humans) and nocturnal (rodents) species needs to be taken into careful consideration during circadian rhythm research.
Extending the preclinical efficacy of pharmacological manipulation of circadian rhythms to clinical trials with highly validated compounds — by themselves or in combination with existing drugs — will offer exciting insight to guide further mechanistic investigation and drug development efforts. With the remarkable elucidation of circadian expression of most protein targets for drugs29, a re-evaluation of circadian pharmacology for desired drugs, as well as discovering behaviour changes to modify the circadian output, will be a critical step for bringing circadian rhythm signalling into routine clinical practice. Besides serving as a treatment for circadian rhythm disorders, therapeutic targeting of circadian rhythm genes and modifying the circadian outputs could potentially be of benefit in other diseases. For instance, as demonstrated in a recent study of the relevance of daytime during cardiac surgery82, a straightforward chronotherapeutic application can markedly modify therapeutic outcomes and disease prognosis. Therapeutic targeting of the circadian rhythm pathway could also be beneficial in intensive care units, as patients in intensive care units often experience disruption in the circadian rhythm230, although more studies are required to dissect the mechanism and impact of circadian disruption. Overall, we believe that the progress made in this area — which was recognized with the Nobel Prize in Physiology or Medicine in 2017 for discoveries on how internal clocks govern human biology — indicates that it is a timely and exciting challenge to harness the power of timing in disease intervention and drug discovery for the treatment of human diseases.
Acknowledgements
This work was in part supported by US National Institute of Health grants R01HL154720, R01DK122796, R01DK109574, R01HL133900 and Department of Defense Grant W81XWH2110032 to H.K.E, an American Thoracic Society unrestricted grant, American Heart Association grant 19CDA34660279, American Lung Association grant CA-622265, the Center for Clinical and Translational Sciences pilot project award 1UL1TR003167–01 and a Parker B. Francis Fellowship to X.Y., and National Natural Science Foundation of China grants 81201448 and 81972312 and Natural Science Foundation of Hunan Province grant 2018JJ3736 to W.R. The authors thank S.-H. Yoo and Z. Chen for their help with the initial draft of the manuscript. The authors acknowledge Y. Wang and K. Wallen for assisting with manuscript editing.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1. Reddy P et al. Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell 38, 701–710 (1984). This landmark article reports the discovery of the period (per) locus in Drosophila melanogaster by molecular mapping of chromosome aberrations.
- 2. Bargiello TA, Jackson FR & Young MW Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature 312, 752–754 (1984). This article demonstrates that introduction of a DNA fragment from a per+ fly encoding a 4.5-kb poly(A)+ RNA (later known as per) in a per0 (arrhythmic) fly can restore its circadian rhythmicity.
- 3. Hardin PE, Hall JC & Rosbash M Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 343, 536–540 (1990). This landmark article identifies a feedback loop through which the cyclic per RNA level is regulated by its own protein activity.
- 4.Takahashi JS Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet 18, 164–179 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroeder AM & Colwell CS How to fix a broken clock. Trends Pharmacol. Sci 34, 605–619 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buhr ED, Yoo SH & Takahashi JS Temperature as a universal resetting cue for mammalian circadian oscillators. Science 330, 379–385 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurd MW & Ralph MR The significance of circadian organization for longevity in the golden hamster. J. Biol. Rhythm 13, 430–436 (1998). [DOI] [PubMed] [Google Scholar]
- 8.DeCoursey PJ Survival value of suprachiasmatic nuclei (SCN) in four wild sciurid rodents. Behav. Neurosci 128, 240–249 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Bass J & Lazar MA Circadian time signatures of fitness and disease. Science 354, 994–999 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Levi F & Schibler U Circadian rhythms: mechanisms and therapeutic implications. Annu. Rev. Pharmacol. Toxicol 47, 593–628 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Moore RY & Eichler VB Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 42, 201–206 (1972). [DOI] [PubMed] [Google Scholar]
- 12.Liu AC et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell 129, 605–616 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamazaki S & Takahashi JS Real-time luminescence reporting of circadian gene expression in mammals. Methods Enzymol. 393, 288–301 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abe M et al. Circadian rhythms in isolated brain regions. J. Neurosci 22, 350–356 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mohawk JA, Green CB & Takahashi JS Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci 35, 445–462 (2012). This important review summarizes the hierarchy of circadian oscillators that function at the cellular, tissue and systems levels.
- 16.Liu AC, Lewis WG & Kay SA Mammalian circadian signaling networks and therapeutic targets. Nat. Chem. Biol 3, 630–639 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Paschos GK & FitzGerald GA Circadian clocks and vascular function. Circ. Res 106, 833–841 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko CH & Takahashi JS Molecular components of the mammalian circadian clock. Hum. Mol. Genet 15 (Suppl 2), R271–R277 (2006). [DOI] [PubMed] [Google Scholar]
- 19. Vitaterna MH et al. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 264, 719–725 (1994). This landmark research work discovers Clock by studies of N-ethyl-N-nitrosourea mutation and map position. The combination of both methods provides a generally applicable approach to study complex behaviour in mammals.
- 20.Bunger MK et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103, 1009–1017 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallego M & Virshup DM Post-translational modifications regulate the ticking of the circadian clock. Nat. Rev. Mol. Cell Biol 8, 139–148 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Siepka SM et al. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell 129, 1011–1023 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoo SH et al. Competing E3 ubiquitin ligases govern circadian periodicity by degradation of CRY in nucleus and cytoplasm. Cell 152, 1091–1105 (2013). This important article finds that CRY ubiquitination is regulated by the balance and cellular compartmentalization of the competing E3 ligases encoded by Fbxl21 and Fbxl3.
- 24.Preitner N et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110, 251–260 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Ueda HR et al. A transcription factor response element for gene expression during circadian night. Nature 418, 534–539 (2002). [DOI] [PubMed] [Google Scholar]
- 26. Panda S et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109, 307–320 (2002). This landmark article finds that most cycling transcripts are regulated in a tissue-specific manner in the SCN and the liver. Major biological processes and rate-limiting steps in these pathways are regulated by the core oscillator components.
- 27.Storch KF et al. Extensive and divergent circadian gene expression in liver and heart. Nature 417, 78–83 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Zhang R, Lahens NF, Ballance HI, Hughes ME & Hogenesch JB A circadian gene expression atlas in mammals: implications for biology and medicine. Proc. Natl Acad. Sci. USA 111, 16219–16224 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mure LS et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 359, eaao0318 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qu M, Duffy T, Hirota T & Kay SA Nuclear receptor HNF4A transrepresses CLOCK:BMAL1 and modulates tissue-specific circadian networks. Proc. Natl Acad. Sci. USA 115, E12305–E12312 (2018). This article demonstrates that the transcriptional activity of the CLOCK–BMAL1 heterodimer can be transrepressed by nuclear receptor HNF4A, which defines a novel feedback loop in tissue-specific mammalian oscillators.
- 31.Perelis M et al. Pancreatic beta cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science 350, aac4250 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papazyan R, Zhang Y & Lazar MA Genetic and epigenomic mechanisms of mammalian circadian transcription. Nat. Struct. Mol. Biol 23, 1045–1052 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo SH et al. Period2 3′-UTR and microRNA-24 regulate circadian rhythms by repressing PERIOD2 protein accumulation. Proc. Natl Acad. Sci. USA 114, E8855–E8864 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green CB Circadian posttranscriptional regulatory mechanisms in mammals. Cold Spring Harb. Perspect. Biol 10, a030692 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saper CB, Scammell TE & Lu J Hypothalamic regulation of sleep and circadian rhythms. Nature 437, 1257–1263 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Wurtman RJ, Axelrod J & Fischer JE Melatonin synthesis in the pineal gland: effect of light mediated by the sympathetic nervous system. Science 143, 1328–1329 (1964). [DOI] [PubMed] [Google Scholar]
- 37.Fisher SP, Foster RG & Peirson SN The circadian control of sleep. Handb. Exp. Pharmacol 217, 157–183 (2013). [DOI] [PubMed] [Google Scholar]
- 38. Liu C et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19, 91–102 (1997). This study provides critically important insights into the molecular basis for two distinct, mechanistically separable effects of melatonin on SCN functions.
- 39.Pandi-Perumal SR et al. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog. Neurobiol 85, 335–353 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Hunt AE, Al-Ghoul WM, Gillette MU & Dubocovich ML Activation of MT2 melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am. J. Physiol. Cell Physiol 280, C110–C118 (2001). [DOI] [PubMed] [Google Scholar]
- 41.Dubocovich ML, Yun K, Al-Ghoul WM, Benloucif S & Masana MI Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J. 12, 1211–1220 (1998). [DOI] [PubMed] [Google Scholar]
- 42.Poirel VJ et al. Contrary to other non-photic cues, acute melatonin injection does not induce immediate changes of clock gene mRNA expression in the rat suprachiasmatic nuclei. Neuroscience 120, 745–755 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Pfeffer M, Rauch A, Korf HW & von Gall C The endogenous melatonin (MT) signal facilitates reentrainment of the circadian system to light-induced phase advances by acting upon MT2 receptors. Chronobiol. Int 29, 415–429 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Agez L, Laurent V, Pevet P, Masson-Pevet M & Gauer F Melatonin affects nuclear orphan receptors mRNA in the rat suprachiasmatic nuclei. Neuroscience 144, 522–530 (2007). [DOI] [PubMed] [Google Scholar]
- 45. Sakurai T et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585 (1998). This article demonstrates that by binding and activating the two orphan G-protein-coupled receptors in the rat brain, orexins could stimulate food consumption, suggesting its pivotal role in the feedback to the feeding behaviour.
- 46.Deboer T et al. Convergence of circadian and sleep regulatory mechanisms on hypocretin-1. Neuroscience 129, 727–732 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Xu TR, Yang Y, Ward R, Gao L & Liu Y Orexin receptors: multi-functional therapeutic targets for sleeping disorders, eating disorders, drug addiction, cancers and other physiological disorders. Cell. Signal 25, 2413–2423 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Mieda M et al. Differential roles of orexin receptor-1 and -2 in the regulation of non-REM and REM sleep. J. Neurosci 31, 6518–6526 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belle MD et al. Acute suppressive and long-term phase modulation actions of orexin on the mammalian circadian clock. J. Neurosci 34, 3607–3621 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsimakouridze EV, Alibhai FJ & Martino TA Therapeutic applications of circadian rhythms for the cardiovascular system. Front. Pharmacol 6, 77 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sundar IK, Yao H, Sellix MT & Rahman I Circadian molecular clock in lung pathophysiology. Am. J. Physiol. Lung Cell. Mol. Physiol 309, L1056–L1075 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burish MJ, Chen Z & Yoo SH Cluster headache is in part a disorder of the circadian system. JAMA Neurol. 75, 783–784 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bedrosian TA & Nelson RJ Sundowning syndrome in aging and dementia: research in mouse models. Exp. Neurol 243, 67–73 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Hood S & Amir S The aging clock: circadian rhythms and later life. J. Clin. Invest 127, 437–446 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stubblefield JJ et al. Temporal control of metabolic amplitude by nocturnin. Cell Rep. 22, 1225–1235 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Musiek ES, Xiong DD & Holtzman DM Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp. Mol. Med 47, e148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karamitri A, Renault N, Clement N, Guillaume JL & Jockers R Minireview: toward the establishment of a link between melatonin and glucose homeostasis: association of melatonin MT2 receptor variants with type 2 diabetes. Mol. Endocrinol 27, 1217–1233 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prokopenko I et al. Variants in MTNR1B influence fasting glucose levels. Nat. Genet 41, 77–81 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takahashi JS, Hong HK, Ko CH & McDearmon EL The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet 9, 764–775 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones CR, Huang AL, Ptacek LJ & Fu YH Genetic basis of human circadian rhythm disorders. Exp. Neurol 243, 28–33 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu Y et al. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell 128, 59–70 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hirano A et al. A cryptochrome 2 mutation yields advanced sleep phase in humans. eLife 5, e16695 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xing W et al. SCF(FBXL3) ubiquitin ligase targets cryptochromes at their cofactor pocket. Nature 496, 64–68 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sack RL et al. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep 30, 1484–1501 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Archer SN et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep 26, 413–415 (2003). [DOI] [PubMed] [Google Scholar]
- 66.Patke A et al. Mutation of the human circadian clock gene CRY1 in familial delayed sleep phase disorder. Cell 169, 203–215 e13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zarbock A et al. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 313, 2133–2141 (2015). This multicentre randomized clinical trial demonstrates that remote ischaemic preconditioning significantly reduced acute kidney injury and uses of renal replacement therapy in high-risk patients undergoing cardiac surgery, representing a simple and promising strategy to improve postoperative outcomes.
- 68. Eltzschig HK & Eckle T Ischemia and reperfusion — from mechanism to translation. Nat. Med 17, 1391–1401 (2011). This review discusses the molecular and immunological consequences of ischaemia and reperfusion and potential strategies that could be used as innovative therapies for treating patients with tissue inflammation due to this condition.
- 69.Koeppen M et al. Hypoxia-inducible factor 2-alpha-dependent induction of amphiregulin dampens myocardial ischemia-reperfusion injury. Nat. Commun 9, 816 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eltzschig HK & Carmeliet P Hypoxia and inflammation. N. Engl. J. Med 364, 656–665 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eltzschig HK, Bratton DL & Colgan SP Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat. Rev. Drug Discov 13, 852–869 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peek CB et al. Circadian clock interaction with HIF1alpha mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab. 25, 86–92 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu Y et al. Reciprocal regulation between the circadian clock and hypoxia signaling at the genome level in mammals. Cell Metab. 25, 73–85 (2017). [DOI] [PubMed] [Google Scholar]
- 74. Eckle T et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat. Med 18, 774–782 (2012). This important study identifies a functional role for extracellular adenosine signalling via adenosine receptor A2B to promote Per2 stabilization, causing a metabolic switch providing cardioprotection against ischaemia. This study is among the first reports to demonstrate a functional interaction between hypoxia-inducible factor and circadian rhythm signalling, and the potential use of light therapy as a mechanism to dampen myocardial ischaemia and reperfusion injury.
- 75.Eckle T, Kohler D, Lehmann R, El Kasmi KC & Eltzschig HK Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation 118, 166–175 (2008). [DOI] [PubMed] [Google Scholar]
- 76. Correa-Costa M et al. Carbon monoxide protects the kidney through the central circadian clock and CD39. Proc. Natl Acad. Sci. USA 115, E2302–E2310 (2018). This important study indicates a cellular signalling mechanism whereby carbon monoxide modulates purinergic responses involving increased CD39 ectonucleotidase expression and Per2 to protect against kidney ischaemia and reperfusion injury. These findings support therapeutic use of carbon monoxide to treat ischaemia and reperfusion injury in association with organ transplantation, stroke or myocardial infarction.
- 77.Yuan D et al. Blue light reduces organ injury from ischemia and reperfusion. Proc. Natl Acad. Sci. USA 113, 5239–5244 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oyama Y et al. Intense light-mediated circadian cardioprotection via transcriptional reprogramming of the endothelium. Cell Rep. 28, 1471–1484 e11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crnko S, Du Pre BC, Sluijter JPG & Van Laake LW Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol 16, 437–447 (2019). [DOI] [PubMed] [Google Scholar]
- 80.Muller JE et al. Circadian variation in the frequency of onset of acute myocardial infarction. N. Engl. J. Med 313, 1315–1322 (1985). [DOI] [PubMed] [Google Scholar]
- 81.Bulluck H et al. Circadian variation in acute myocardial infarct size assessed by cardiovascular magnetic resonance in reperfused STEMI patients. Int. J. Cardiol 230, 149–154 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Montaigne D et al. Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbalpha antagonism: a single-centre propensity-matched cohort study and a randomised study. Lancet 391, 59–69 (2018). This landmark article demonstrates that perioperative myocardial injury is transcriptionally orchestrated by the circadian clock in patients undergoing aortic valve replacement with improved patient outcomes in the afternoon.
- 83.Fortuyn-van Leijden CE Some observations on periodic nuclear division in the cat. KNAB 19, 38–44 (1917). [Google Scholar]
- 84.Canaple L, Kakizawa T & Laudet V The days and nights of cancer cells. Cancer Res. 63, 7545–7552 (2003). [PubMed] [Google Scholar]
- 85.Matsuo T et al. Control mechanism of the circadian clock for timing of cell division in vivo. Science 302, 255–259 (2003). [DOI] [PubMed] [Google Scholar]
- 86.Gery S et al. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol. Cell 22, 375–382 (2006). [DOI] [PubMed] [Google Scholar]
- 87. Fu L, Pelicano H, Liu J, Huang P & Lee C The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111, 41–50 (2002). This important article finds that Per2 acts as a tumour suppressor by regulating DNA damage-responsive pathways, indicating that the circadian clock could also play an important part in the response to unpredicted hazards that are detrimental to genomic material.
- 88.Kettner NM et al. Circadian homeostasis of liver metabolism suppresses hepatocarcinogenesis. Cancer Cell 30, 909–924 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anafi RC, Francey LJ, Hogenesch JB & Kim J CYCLOPS reveals human transcriptional rhythms in health and disease. Proc. Natl Acad. Sci. USA 114, 5312–5317 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Papagiannakopoulos T et al. Circadian rhythm disruption promotes lung tumorigenesis. Cell Metab. 24, 324–331 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huber AL et al. CRY2 and FBXL3 cooperatively degrade c-MYC. Mol. Cell 64, 774–789 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ozturk N, Lee JH, Gaddameedhi S & Sancar A Loss of cryptochrome reduces cancer risk in p53 mutant mice. Proc. Natl Acad. Sci. USA 106, 2841–2846 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Puram RV et al. Core circadian clock genes regulate leukemia stem cells in AML. Cell 165, 303–316 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dong Z et al. Targeting glioblastoma stem cells through disruption of the circadian clock. Cancer Discov. 9, 1556–1573 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morales-Santana S et al. An overview of the polymorphisms of circadian genes associated with endocrine cancer. Front. Endocrinol 10, 104 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oklejewicz M et al. Phase resetting of the mammalian circadian clock by DNA damage. Curr. Biol 18, 286–291 (2008). [DOI] [PubMed] [Google Scholar]
- 97.Masri S et al. Lung adenocarcinoma distally rewires hepatic circadian homeostasis. Cell 165, 896–909 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Altman BJ et al. MYC disrupts the circadian clock and metabolism in cancer cells. Cell Metab. 22, 1009–1019 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Knutson KL, Spiegel K, Penev P & Van Cauter E The metabolic consequences of sleep deprivation. Sleep Med. Rev 11, 163–178 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Scheer FA, Hilton MF, Mantzoros CS & Shea SA Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl Acad. Sci. USA 106, 4453–4458 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Buxton OM et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med 4, 129ra43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bass J & Takahashi JS Circadian integration of metabolism and energetics. Science 330, 1349–1354 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Turek FW et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308, 1043–1045 (2005). This study identifies the important role of the circadian gene Clock in mammalian energy balance.
- 104.Shi SQ, Ansari TS, McGuinness OP, Wasserman DH & Johnson CH Circadian disruption leads to insulin resistance and obesity. Curr. Biol 23, 372–381 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fonken LK et al. Light at night increases body mass by shifting the time of food intake. Proc. Natl Acad. Sci. USA 107, 18664–18669 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reinke H & Asher G Crosstalk between metabolism and circadian clocks. Nat. Rev. Mol. Cell Biol 20, 227–241 (2019). [DOI] [PubMed] [Google Scholar]
- 107.Ramsey KM et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324, 651–654 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakahata Y, Sahar S, Astarita G, Kaluzova M & Sassone-Corsi P Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324, 654–657 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nakahata Y et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134, 329–340 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peek CB et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 342, 1243417 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yeung J et al. Transcription factor activity rhythms and tissue-specific chromatin interactions explain circadian gene expression across organs. Genome Res. 28, 182–191 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marcheva B et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466, 627–631 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sinturel F et al. Diurnal oscillations in liver mass and cell size accompany ribosome assembly cycles. Cell 169, 651–663 e14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sanchez-Lasheras C, Konner AC & Bruning JC Integrative neurobiology of energy homeostasis-neurocircuits, signals and mediators. Front. Neuroendocrinol 31, 4–15 (2010). [DOI] [PubMed] [Google Scholar]
- 115.Huang W, Moynihan Ramsey K, Marcheva B & Bass J Circadian rhythms, sleep, and metabolism. J. Clin. Invest 121, 2133–2141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yi CX et al. Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus. Endocrinology 147, 283–294 (2006). [DOI] [PubMed] [Google Scholar]
- 117.Hill JW Gene expression and the control of food intake by hypothalamic POMC/CART neurons. Open Neuroendocrinol. J 3, 21–27 (2010). [PMC free article] [PubMed] [Google Scholar]
- 118.Mieda M & Yanagisawa M Sleep, feeding, and neuropeptides: roles of orexins and orexin receptors. Curr. Opin. Neurobiol 12, 339–345 (2002). [DOI] [PubMed] [Google Scholar]
- 119.Hara J et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30, 345–354 (2001). [DOI] [PubMed] [Google Scholar]
- 120.Mahoney CE, Cogswell A, Koralnik IJ & Scammell TE The neurobiological basis of narcolepsy. Nat. Rev. Neurosci 20, 83–93 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Iwasaki A & Medzhitov R Toll-like receptor control of the adaptive immune responses. Nat. Immunol 5, 987–995 (2004). [DOI] [PubMed] [Google Scholar]
- 122. Serhan CN & Savill J Resolution of inflammation: the beginning programs the end. Nat. Immunol 6, 1191–1197 (2005). This comprehensive review summarizes the mechanisms of an active, coordinated program for inflammation resolution which may bring profound advances in therapies aimed at terminating persistent inflammation.
- 123.Ehrentraut H et al. CD73+ regulatory T cells contribute to adenosine-mediated resolution of acute lung injury. FASEB J. 27, 2207–2219 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Serhan CN et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med 196, 1025–1037 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Man K, Loudon A & Chawla A Immunity around the clock. Science 354, 999–1003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Adrover JM et al. A neutrophil timer coordinates immune defense and vascular protection. Immunity 51, 966–967 (2019). [DOI] [PubMed] [Google Scholar]
- 127.Halberg F, Johnson EA, Brown BW & Bittner JJ Susceptibility rhythm to E. coli endotoxin and bioassay. Proc. Soc. Exp. Biol. Med 103, 142–144 (1960). [DOI] [PubMed] [Google Scholar]
- 128.Scheiermann C et al. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 37, 290–301 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gibbs JE et al. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Natl Acad. Sci. USA 109, 582–587 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Curtis AM et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc. Natl Acad. Sci. USA 112, 7231–7236 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nguyen KD et al. Circadian gene Bmal1 regulates diurnal oscillations of Ly6Chi inflammatory monocytes. Science 341, 1483–1488 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gibbs J et al. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat. Med 20, 919–926 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jetten AM Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl. Recept. Signal 7, e003 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Yu X et al. TH17 cell differentiation is regulated by the circadian clock. Science 342, 727–730 (2013). This landmark study reports how the molecular circadian clock directly regulates the differentiation of TH17 cells in the intestine via the transcription factor NFIL3, which suggest that both nutrition and light are important environmental factors that directly regulate the immune response.
- 135.Scheiermann C, Gibbs J, Ince L & Loudon A Clocking in to immunity. Nat. Rev. Immunol 18, 423–437 (2018). [DOI] [PubMed] [Google Scholar]
- 136.Shimba A et al. Glucocorticoids drive diurnal oscillations in T cell distribution and responses by inducing interleukin-7 receptor and CXCR4. Immunity 48, 286–298 e6 (2018). [DOI] [PubMed] [Google Scholar]
- 137.Silver AC, Arjona A, Walker WE & Fikrig E The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity 36, 251–261 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McKenna H, van der Horst GTJ, Reiss I & Martin D Clinical chronobiology: a timely consideration in critical care medicine. Crit. Care 22, 124 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Oldham MA, Lee HB & Desan PH Circadian rhythm disruption in the critically ill: an opportunity for improving outcomes. Crit. Care Med 44, 207–217 (2016). [DOI] [PubMed] [Google Scholar]
- 140.Solanas G et al. Aged stem cells reprogram their daily rhythmic functions to adapt to stress. Cell 170, 678–692 e20 (2017). [DOI] [PubMed] [Google Scholar]
- 141.Sato S et al. Circadian reprogramming in the liver identifies metabolic pathways of aging. Cell 170, 664–677 e11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Levine DC et al. NAD+ controls circadian reprogramming through PER2 nuclear translocation to counter aging. Mol. Cell 78, 835–849 e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV & Antoch MP Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 20, 1868–1873 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang RH et al. Negative reciprocal regulation between Sirt1 and Per2 modulates the circadian clock and aging. Sci. Rep 6, 28633 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wu H, Dunnett S, Ho YS & Chang RC The role of sleep deprivation and circadian rhythm disruption as risk factors of Alzheimer’s disease. Front. Neuroendocrinol 54, 100764 (2019). [DOI] [PubMed] [Google Scholar]
- 146.Hermida RC et al. Chronotherapy improves blood pressure control and reduces vascular risk in CKD. Nat. Rev. Nephrol 9, 358–368 (2013). [DOI] [PubMed] [Google Scholar]
- 147.Kobayashi M, Wood PA & Hrushesky WJ Circadian chemotherapy for gynecological and genitourinary cancers. Chronobiol. Int 19, 237–251 (2002). [DOI] [PubMed] [Google Scholar]
- 148.Gorbacheva VY et al. Circadian sensitivity to the chemotherapeutic agent cyclophosphamide depends on the functional status of the CLOCK/BMAL1 transactivation complex. Proc. Natl Acad. Sci. USA 102, 3407–3412 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Griffett K & Burris TP The mammalian clock and chronopharmacology. Bioorg. Med. Chem. Lett 23, 1929–1934 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Medalie L & Cifu AS Management of chronic insomnia disorder in adults. JAMA 317, 762–763 (2017). [DOI] [PubMed] [Google Scholar]
- 151.Wade AG et al. Nightly treatment of primary insomnia with prolonged release melatonin for 6 months: a randomized placebo controlled trial on age and endogenous melatonin as predictors of efficacy and safety. BMC Med. 8, 51 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wilson S et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders: an update. J. Psychopharmacol 33, 923–947 (2019). [DOI] [PubMed] [Google Scholar]
- 153.Williams WP 3rd, McLin DE 3rd, Dressman MA & Neubauer DN Comparative review of approved melatonin agonists for the treatment of circadian rhythm sleep-wake disorders. Pharmacotherapy 36, 1028–1041 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kuriyama A, Honda M & Hayashino Y Ramelteon for the treatment of insomnia in adults: a systematic review and meta-analysis. Sleep Med. 15, 385–392 (2014). [DOI] [PubMed] [Google Scholar]
- 155.Comai S, Ochoa-Sanchez R & Gobbi G Sleep-wake characterization of double MT1/MT2 receptor knockout mice and comparison with MT1 and MT2 receptor knockout mice. Behav. Brain Res 243, 231–238 (2013). [DOI] [PubMed] [Google Scholar]
- 156.Gotter AL et al. Orexin 2 receptor antagonism is sufficient to promote NREM and REM sleep from mouse to man. Sci. Rep 6, 27147 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Morairty SR et al. Dual hypocretin receptor antagonism is more effective for sleep promotion than antagonism of either receptor alone. PLoS ONE 7, e39131 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Coleman PJ, Gotter AL, Herring WJ, Winrow CJ & Renger JJ The discovery of suvorexant, the first orexin receptor drug for insomnia. Annu. Rev. Pharmacol. Toxicol 57, 509–533 (2017). [DOI] [PubMed] [Google Scholar]
- 159.Tannenbaum PL et al. Inhibition of orexin signaling promotes sleep yet preserves salient arousability in monkeys. Sleep 39, 603–612 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Uslaner JM et al. Orexin receptor antagonists differ from standard sleep drugs by promoting sleep at doses that do not disrupt cognition. Sci. Transl. Med 5, 179ra44 (2013). [DOI] [PubMed] [Google Scholar]
- 161.Arendt J Approaches to the pharmacological management of jet lag. Drugs 78, 1419–1431 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Arendt J & Skene DJ Melatonin as a chronobiotic. Sleep Med. Rev 9, 25–39 (2005). [DOI] [PubMed] [Google Scholar]
- 163.McArthur AJ, Hunt AE & Gillette MU Melatonin action and signal transduction in the rat suprachiasmatic circadian clock: activation of protein kinase C at dusk and dawn. Endocrinology 138, 627–634 (1997). [DOI] [PubMed] [Google Scholar]
- 164.McArthur AJ, Gillette MU & Prosser RA Melatonin directly resets the rat suprachiasmatic circadian clock in vitro. Brain Res. 565, 158–161 (1991). [DOI] [PubMed] [Google Scholar]
- 165.Pfeffer M, Korf HW & Wicht H Synchronizing effects of melatonin on diurnal and circadian rhythms. Gen. Comp. Endocrinol 258, 215–221 (2018). [DOI] [PubMed] [Google Scholar]
- 166.Rajaratnam SM et al. Melatonin agonist tasimelteon (VEC-162) for transient insomnia after sleep-time shift: two randomised controlled multicentre trials. Lancet 373, 482–491 (2009). [DOI] [PubMed] [Google Scholar]
- 167.Stauch B et al. Structural basis of ligand recognition at the human MT1 melatonin receptor. Nature 569, 284–288 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Stein RM et al. Virtual discovery of melatonin receptor ligands to modulate circadian rhythms. Nature 579, 609–614 (2020). This important article identifies several MT1 receptor-selective inverse agonists from structure-based screens of diverse, ultralarge libraries. These chemotypes could phase-advance the mouse circadian clock, therefore illuminating new in vivo pharmacology.
- 169.Riha RL The use and misuse of exogenous melatonin in the treatment of sleep disorders. Curr. Opin. Pulm. Med 24, 543–548 (2018). [DOI] [PubMed] [Google Scholar]
- 170.Lewy AJ, Ahmed S, Jackson JM & Sack RL Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol. Int 9, 380–392 (1992). [DOI] [PubMed] [Google Scholar]
- 171.Burgess HJ, Revell VL & Eastman CI A three pulse phase response curve to three milligrams of melatonin in humans. J. Physiol 586, 639–647 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Roach GD & Sargent C Interventions to minimize jet lag after westward and eastward flight. Front. Physiol 10, 927 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wickwire EM, Geiger-Brown J, Scharf SM & Drake CL Shift work and shift work sleep disorder: clinical and organizational perspectives. Chest 151, 1156–1172 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Green CB, Takahashi JS & Bass J The meter of metabolism. Cell 134, 728–742 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wegrzyn LR et al. Rotating night-shift work and the risk of breast cancer in the nurses’ health studies. Am. J. Epidemiol 186, 532–540 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liira J et al. Pharmacological interventions for sleepiness and sleep disturbances caused by shift work. Cochrane Database Syst. Rev CD009776 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Agostino PV, Plano SA & Golombek DA Sildenafil accelerates reentrainment of circadian rhythms after advancing light schedules. Proc. Natl Acad. Sci. USA 104, 9834–9839 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ju D et al. Chemical perturbations reveal that RUVBL2 regulates the circadian phase in mammals. Sci. Transl. Med 12, eaba0769 (2020). This important study demonstrates that pharmacological perturbation of RUVBL2 by the adenosine derivative cordycepin shifts the circadian phase both in human cells and in mouse tissue and rapidly entrains the circadian phase in a mouse jet lag model. This phase-shifting effect is the result of disassembly of interaction between RUVBL2 and BMAL1, thereby leading to the release of repression of clock gene transcription.
- 179.Grant D et al. GSK4112, a small molecule chemical probe for the cell biology of the nuclear heme receptor Rev-erbalpha. ACS Chem. Biol 5, 925–932 (2010). [DOI] [PubMed] [Google Scholar]
- 180.Trump RP et al. Optimized chemical probes for REV-ERBalpha. J. Med. Chem 56, 4729–4737 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Solt LA et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 485, 62–68 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sulli G et al. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 553, 351–355 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Banerjee S et al. Pharmacological targeting of the mammalian clock regulates sleep architecture and emotional behaviour. Nat. Commun 5, 5759 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Woldt E et al. Rev-erb-alpha modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat. Med 19, 1039–1046 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Thomes PG, Brandon-Warner E, Li T, Donohue TM Jr. & Schrum LW Rev-erb agonist and TGF-beta similarly affect autophagy but differentially regulate hepatic stellate cell fibrogenic phenotype. Int. J. Biochem. Cell Biol 81, 137–147 (2016). [DOI] [PubMed] [Google Scholar]
- 186.Chung S et al. Impact of circadian nuclear receptor REV-ERBalpha on midbrain dopamine production and mood regulation. Cell 157, 858–868 (2014). [DOI] [PubMed] [Google Scholar]
- 187.Chen Z, Yoo SH & Takahashi JS Small molecule modifiers of circadian clocks. Cell. Mol. Life Sci 70, 2985–2998 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.He B et al. The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab. 23, 610–621 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kojetin DJ & Burris TP REV-ERB and ROR nuclear receptors as drug targets. Nat. Rev. Drug Discov 13, 197–216 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chang MR et al. Antiobesity effect of a small molecule repressor of RORgamma. Mol. Pharmacol 88, 48–56 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Huh JR et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature 472, 486–490 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chen Z, Yoo SH & Takahashi JS Development and therapeutic potential of small-molecule modulators of circadian systems. Annu. Rev. Pharmacol. Toxicol 58, 231–252 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Solt LA et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 472, 491–494 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hirota T et al. Identification of small molecule activators of cryptochrome. Science 337, 1094–1097 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nangle S, Xing W & Zheng N Crystal structure of mammalian cryptochrome in complex with a small molecule competitor of its ubiquitin ligase. Cell Res. 23, 1417–1419 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Humphries PS et al. Carbazole-containing sulfonamides and sulfamides: discovery of cryptochrome modulators as antidiabetic agents. Bioorg. Med. Chem. Lett 26, 757–760 (2016). [DOI] [PubMed] [Google Scholar]
- 197.Miller S et al. Isoform-selective regulation of mammalian cryptochromes. Nat. Chem. Biol 16, 676–685 (2020). [DOI] [PubMed] [Google Scholar]
- 198.Nangle SN et al. Molecular assembly of the period-cryptochrome circadian transcriptional repressor complex. eLife 3, e03674 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Walton KM et al. Selective inhibition of casein kinase 1ϵ minimally alters circadian clock period. J. Pharmacol. Exp. Ther 330, 430–439 (2009). [DOI] [PubMed] [Google Scholar]
- 200.Meng QJ et al. Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes. Proc. Natl Acad. Sci. USA 107, 15240–15245 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hirota T et al. High-throughput chemical screen identifies a novel potent modulator of cellular circadian rhythms and reveals CKIalpha as a clock regulatory kinase. PLoS Biol. 8, e1000559 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Barion A & Zee PC A clinical approach to circadian rhythm sleep disorders. Sleep Med. 8, 566–577 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.LeGates TA, Fernandez DC & Hattar S Light as a central modulator of circadian rhythms, sleep and affect. Nat. Rev. Neurosci 15, 443–454 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Asher G & Sassone-Corsi P Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell 161, 84–92 (2015). [DOI] [PubMed] [Google Scholar]
- 205.Chaix A, Zarrinpar A, Miu P & Panda S Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 20, 991–1005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Jakubowicz D, Barnea M, Wainstein J & Froy O High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 21, 2504–2512 (2013). [DOI] [PubMed] [Google Scholar]
- 207.Sutton EF et al. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 27, 1212–1221 e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Colles SL, Dixon JB & O’Brien PE Night eating syndrome and nocturnal snacking: association with obesity, binge eating and psychological distress. Int. J. Obes 31, 1722–1730 (2007). [DOI] [PubMed] [Google Scholar]
- 209.Markwald RR et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl Acad. Sci. USA 110, 5695–5700 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mrosovsky N & Salmon PA A behavioural method for accelerating re-entrainment of rhythms to new light-dark cycles. Nature 330, 372–373 (1987). [DOI] [PubMed] [Google Scholar]
- 211.Maywood ES, Mrosovsky N, Field MD & Hastings MH Rapid down-regulation of mammalian period genes during behavioral resetting of the circadian clock. Proc. Natl Acad. Sci. USA 96, 15211–15216 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Welsh D, Richardson GS & Dement WC Effect of running wheel availability on circadian patterns of sleep and wakefulness in mice. Physiol. Behav 43, 771–777 (1988). [DOI] [PubMed] [Google Scholar]
- 213.Buxton OM, Lee CW, L’Hermite-Baleriaux M, Turek FW & Van Cauter E Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am. J. Physiol. Regul. Integr. Comp. Physiol 284, R714–R724 (2003). [DOI] [PubMed] [Google Scholar]
- 214.Reid KJ et al. Aerobic exercise improves selfreported sleep and quality of life in older adults with insomnia. Sleep Med. 11, 934–940 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Pallier PN et al. Pharmacological imposition of sleep slows cognitive decline and reverses dysregulation of circadian gene expression in a transgenic mouse model of Huntington’s disease. J. Neurosci 27, 7869–7878 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hofstra WA & de Weerd AW How to assess circadian rhythm in humans: a review of literature. Epilepsy Behav. 13, 438–444 (2008). [DOI] [PubMed] [Google Scholar]
- 217.Horne JA & Ostberg O A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol 4, 97–110 (1976). [PubMed] [Google Scholar]
- 218.Roenneberg T et al. Epidemiology of the human circadian clock. Sleep Med. Rev 11, 429–438 (2007). [DOI] [PubMed] [Google Scholar]
- 219.Ancoli-Israel S et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 26, 342–392 (2003). [DOI] [PubMed] [Google Scholar]
- 220.Gill S & Panda S A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 22, 789–798 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Pagani L et al. The physiological period length of the human circadian clock in vivo is directly proportional to period in human fibroblasts. PLoS ONE 5, e13376 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Akashi M et al. Noninvasive method for assessing the human circadian clock using hair follicle cells. Proc. Natl Acad. Sci. USA 107, 15643–15648 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kasukawa T et al. Human blood metabolite timetable indicates internal body time. Proc. Natl Acad. Sci. USA 109, 15036–15041 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chen CY et al. Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc. Natl Acad. Sci. USA 113, 206–211 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.van Maanen A, Meijer AM, van der Heijden KB & Oort FJ The effects of light therapy on sleep problems: a systematic review and meta-analysis. Sleep Med. Rev 29, 52–62 (2016). [DOI] [PubMed] [Google Scholar]
- 226.Johnston JD Physiological responses to food intake throughout the day. Nutr. Res. Rev 27, 107–118 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Liu J et al. MT1 and MT2 melatonin receptors: a therapeutic perspective. Annu. Rev. Pharmacol. Toxicol 56, 361–383 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Seki T et al. Nobiletin-rich citrus reticulata peels, a kampo medicine for Alzheimer’s disease: a case series. Geriatr. Gerontol. Int 13, 236–238 (2013). [DOI] [PubMed] [Google Scholar]
- 229.Kondratova AA & Kondratov RV The circadian clock and pathology of the ageing brain. Nat. Rev. Neurosci 13, 325–335 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Telias I & Wilcox ME Sleep and circadian rhythm in critical illness. Crit. Care 23, 82 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Jang J et al. The cryptochrome inhibitor KS15 enhances E-box-mediated transcription by disrupting the feedback action of a circadian transcription-repressor complex. Life Sci. 200, 49–55 (2018). [DOI] [PubMed] [Google Scholar]
- 232.Shinozaki A et al. Potent effects of flavonoid nobiletin on amplitude, period, and phase of the circadian clock rhythm in PER2::LUCIFERASE mouse embryonic fibroblasts. PLoS ONE 12, e0170904 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Mulvihill EE, Burke AC & Huff MW Citrus flavonoids as regulators of lipoprotein metabolism and atherosclerosis. Annu. Rev. Nutr 36, 275–299 (2016). [DOI] [PubMed] [Google Scholar]
- 234.Helleboid S et al. The identification of naturally occurring neoruscogenin as a bioavailable, potent, and high-affinity agonist of the nuclear receptor RORalpha (NR1F1). J. Biomol. Screen 19, 399–406 (2014). [DOI] [PubMed] [Google Scholar]
- 235.Kumar N et al. Identification of SR3335 (ML-176): a synthetic RORalpha selective inverse agonist. ACS Chem. Biol 6, 218–222 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Byun JK et al. Retinoic acid-related orphan receptor alpha reprograms glucose metabolism in glutamine-deficient hepatoma cells. Hepatology 61, 953–964 (2015). [DOI] [PubMed] [Google Scholar]
- 237.He B & Chen Z Molecular targets for small-molecule modulators of circadian clocks. Curr. Drug Metab 17, 503–512 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Eltzschig HK, Bonney SK & Eckle T Attenuating myocardial ischemia by targeting A2B adenosine receptors. Trends Mol. Med. 19, 345–354 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Li J, Lu WQ, Beesley S, Loudon AS & Meng QJ Lithium impacts on the amplitude and period of the molecular circadian clockwork. PLoS ONE 7, e33292 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Welsh DK, Takahashi JS & Kay SA Suprachiasmatic nucleus: cell autonomy and network properties. Annu. Rev. Physiol 72, 551–577 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Sonoda T et al. A noncanonical inhibitory circuit dampens behavioral sensitivity to light. Science 368, 527–531 (2020). This important article indicates that the inhibitory neurotransmitter GABA released by a subpopulation of intrinsically photosensitive retinal ganglion cells decreases the sensitivity of non-image-forming behaviours at low light levels.
- 242.Shimomura K et al. Genetic suppression of the circadian Clock mutation by the melatonin biosynthesis pathway. Proc. Natl Acad. Sci. USA 107, 8399–8403 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Knutsson A, Jonsson BG, Akerstedt T & Orth-Gomer K Increased risk of ischaemic heart disease in shift workers. Lancet 328, 89–92 (1986). [DOI] [PubMed] [Google Scholar]
- 244.Panda S Circadian physiology of metabolism. Science 354, 1008–1015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Savvidis C & Koutsilieris M Circadian rhythm disruption in cancer biology. Mol. Med 18, 1249–1260 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kelleher FC, Rao A & Maguire A Circadian molecular clocks and cancer. Cancer Lett. 342, 9–18 (2014). [DOI] [PubMed] [Google Scholar]
- 247.Vgontzas AN & Chrousos GP Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol. Metab. Clin. North Am 31, 15–36 (2002). [DOI] [PubMed] [Google Scholar]
- 248.Sephton S & Spiegel D Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain Behav. Immun 17, 321–328 (2003). [DOI] [PubMed] [Google Scholar]
- 249.Leng Y, Musiek ES, Hu K, Cappuccio FP & Yaffe K Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 18, 307–318 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Chen Z et al. Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proc. Natl Acad. Sci. USA 109, 101–106 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]